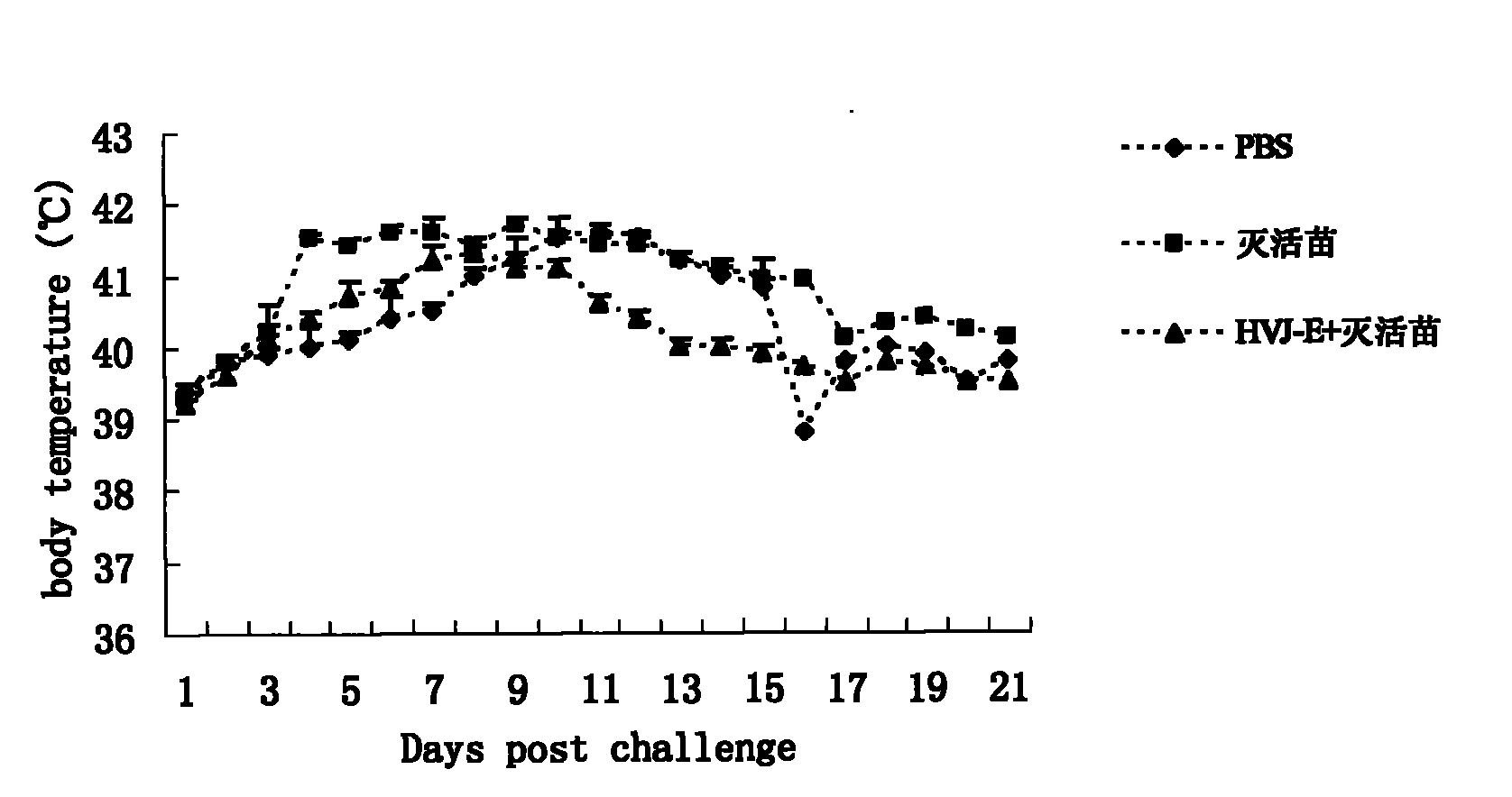Patents
Literature
101 results about "Sendai virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is a negative sense, single-stranded RNA virus of the family Paramyxoviridae, a group of viruses featuring, notably, the genera Morbillivirus and Rubulavirus. SeV is a member of genus Respirovirus. The virus was isolated in Sendai (Japan) in the early 1950s. Since then, it has been actively used in research as a model pathogen. The virus is infectious for many cancer cell lines (see below) and has oncolytic properties demonstrated in animals model and natural form of cancers. SeV ability to fuse eukaryotic cells, was used to produce hybridoma cells capable of manufacturing monoclonal antibodies in large quantities.
Recombinant sendai virus
InactiveUS20050266566A1Efficient systemSsRNA viruses negative-senseSugar derivativesViral replicationGenetics manipulation
A method for regenerating Sendai virus particles by transfecting the Sendai virus genome to a host expressing all genes for the initial viral replication has been developed, enabling the genetic manipulation of Sendai virus and effective utilization of said virus as the vector.
Owner:DNAVEC RES
Paramyxovirus-derived RNP
InactiveUS20070009949A1Small genome sizeLow costSsRNA viruses negative-senseMicrobiological testing/measurementNegative strandLipofectamine
A functional RNP containing negative-strand single-stranded RNA derived from Sendai virus, which has been modified so as not to express at least one envelope protein, has been successfully prepared. An RNP comprising a foreign gene is prepared and inserted into a cell with the use of a cationic liposome, thereby successfully expressing the foreign gene.
Owner:KITAZATO KAIO +9
Recombinant Sendai virus
InactiveUS7101685B2Improve expression levelReduce distanceSsRNA viruses negative-senseSugar derivativesViral replicationGenetics manipulation
A method for generating Sendai virus particles by transfecting the Sendai virus genome to a host expressing all genes for the initial viral replication has been developed, enabling the genetic manipulation of Sendai virus and effective utilization of said virus as the vector.
Owner:DNAVEC RES
Method of intracellular sustained-release of drug and preparations
InactiveUS6875448B1Efficient introductionEfficiency of introduction is very lowPowder deliveryMicroencapsulation basedBiologyNiosome
A substance of interest is contained in nanospheres which are then encapsulated in fusogenic liposomes to prepare transport carriers that allow physiologically active substances, especially those having high molecular weight such as proteins and genes, to be introduced into cells efficiently and which permit the introduced active substance to be released in the cell at controlled rate. The fusogenic liposomes are prepared by conferring the fusogenic capability of Sendai virus to known liposomes.
Owner:CHUGAI PHARMA CO LTD
Colloidal gold test strip and test strip card for detecting IgM antibody, and preparation and detection method
InactiveCN105259345AThe detection process is fastImprove efficiencyBiological testingParainfluenza virus antigenIgm antibody
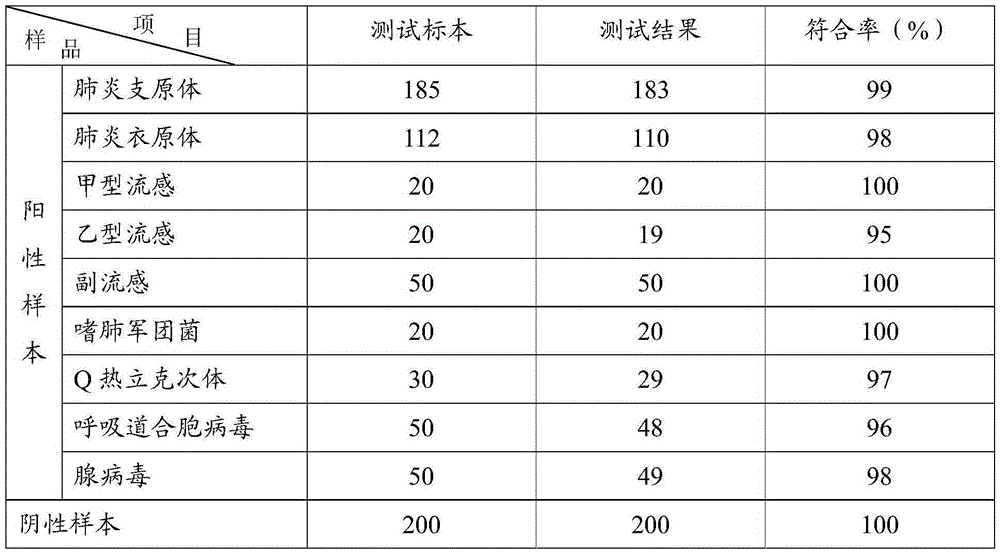
The invention provides a colloidal gold test strip for detecting an IgM antibody. The IgM antibody is a specific IgM antibody for nine respiratory tract infection pathogens, and the colloidal gold test strip comprises a sample pad, a conjugate pad, a nitrocellulose film and a water absorption pad which are attached to a polyvinyl chloride base plate in sequence; the conjugate pad is a glass fiber film wrapped with a rabbit-anti-human IgM antibody-colloidal gold conjugate; the nitrocellulose film is wrapped with 9 detection lines and 1 quality control line in sequence; the 9 detection lines are respectively a mycoplasma pneumoniae recombined antigen, a chlamydia pneumoniae recombined antigen, an influenza a virus antigen, an influenza B virus antigen, a sendai virus antigen, a legionella pneumophila antigen, a Coxiella burnetii antigen, a respiratory syncytial virus antigen and an adenovirus antigen, and the quality control line is a second antibody. The invention further provides a colloidal gold test strip card comprising the colloidal gold test strip and a colloid gold kit, a preparation method of the colloidal gold test strip card, and a method for realizing detection by adopting the colloidal gold test strip, the test strip card or the kit.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Recombinant sendai virus vector for introducing exogenous genes to airway epithelia
InactiveUS7314614B1Not affect infection efficiencySlightly affectedSsRNA viruses negative-senseBiocideDiseaseGene transfer
Provided are a recombinant Sendai virus vector for introducing exogenous genes to airway epithelia and a method for introducing exogenous genes using the vector. The recombinant Sendai virus vector enables efficient gene transfer to native mucus-layered airway epithelial cells by briefly contacting the vector with the cells. Furthermore, the vector can introduce genes to not only apical surfaces but also submucosal glands where CFTR primarily expresses. The vector can thus be used for gene therapy of CF, a CFTR-deficient disease.
Owner:DNAVEC RES
Vectors for generating pluripotent stem cells and methods of producing pluripotent stem cells using the same
ActiveUS20120214240A1Promotes reprogrammingEliminate riskSsRNA viruses negative-senseSugar derivativesValineViral vector
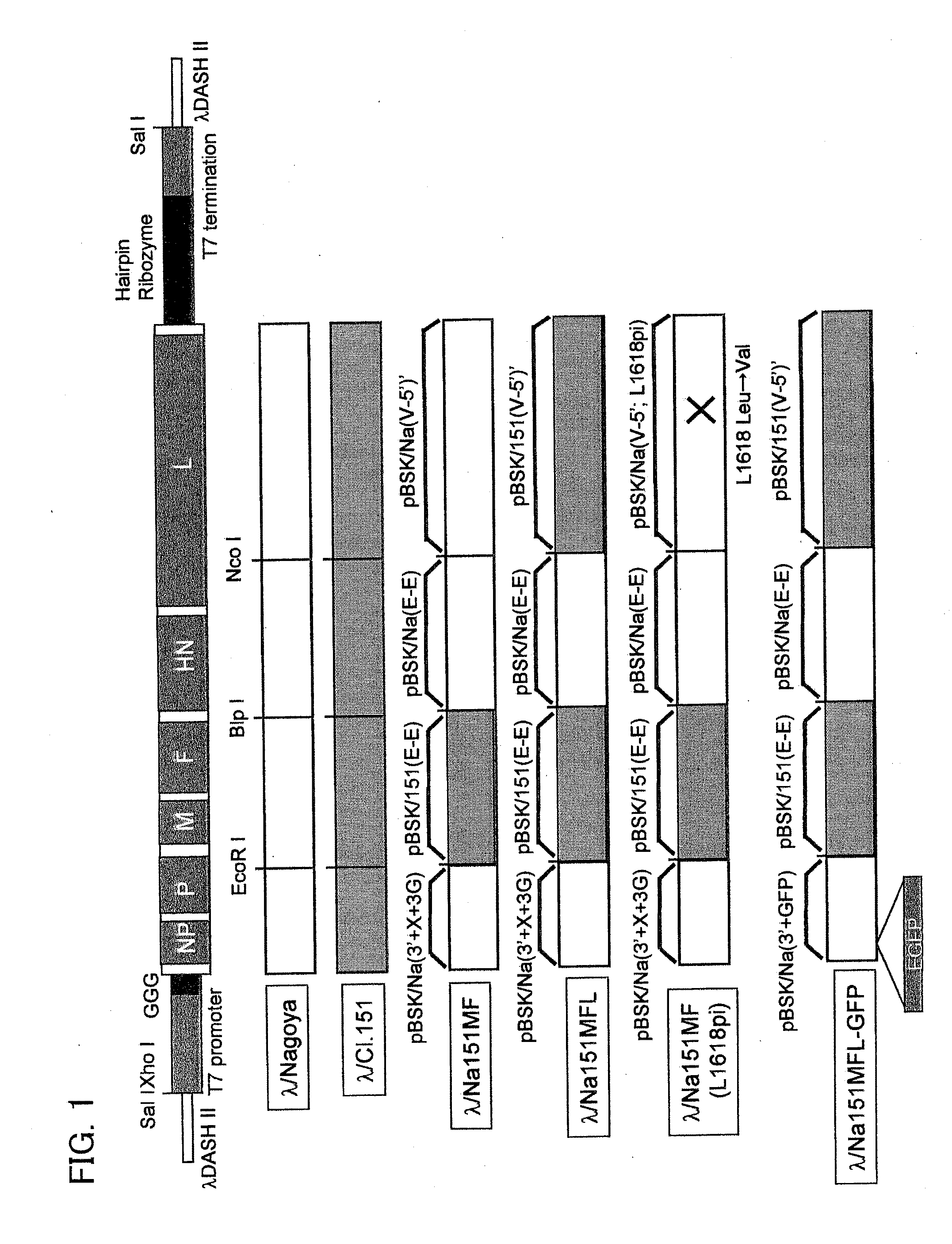
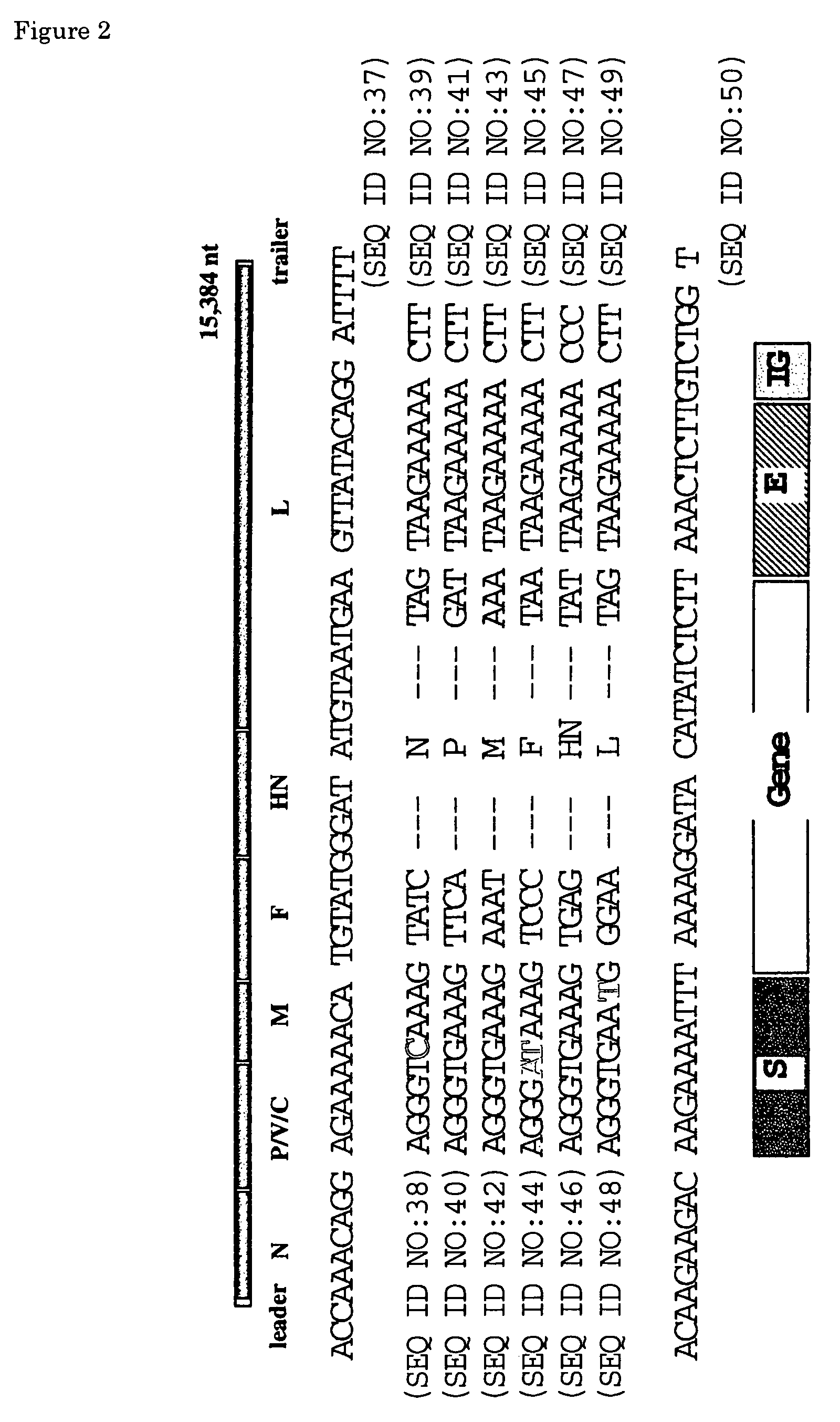
A reprogramming gene-loaded Sendai viral vector comprising Sendai virus genes and reprogramming genes, wherein the Sendai virus genes include an NP gene, P / C gene, M gene, F gene, HN gene and L gene, wherein each of the M gene, the F gene and the FIN gene is from a Sendai virus strain Cl.151-derived gene and wherein at least one of the M gene, the F gene and the HN gene is functionally deleted and the L gene encodes the amino-acid sequence of the L protein in which the amino-acid residue at position 1618 is valine and a method of producing the same.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Antiviral preparations obtained from a natural cinnamon extract
The present application provides a natural aqueous extract obtainable from a cinnamon bark (Cinnamon sp.) which has antiviral activity against enveloped viruses including influenza A, Parainfluenza (Sendai) virus and HSV-1 viruses, as well as in vivo activity in inhibition of Influenza A and Parainfluenza viruses. The present application also concerns a method for the extraction of said cinnamon extract and applications thereof.
Owner:RAMOT AT TEL AVIV UNIV LTD
Persistently infective sendai virus vector
ActiveUS20100196993A1Low cytotoxicityExpression becomes sustainableSsRNA viruses negative-senseOrganic active ingredientsCytotoxicityViral vector
A persistently infective virus vector is produced by using a gene so modified as to encode an amino acid sequence including a valine substituted for an amino acid residue at position-1618 in the amino acid sequence for an L protein of a persistently non-infective Sendai virus. A non-transmissible, persistently infective virus vector is also produced by defecting or deleting at least one of M gene, F gene, and HN gene. These virus vectors have no cytotoxicity, can achieve the sustained gene expression over a long period of time, is safe, and is therefore useful.
Owner:NAT INST OF ADVANCED IND SCI & TECH
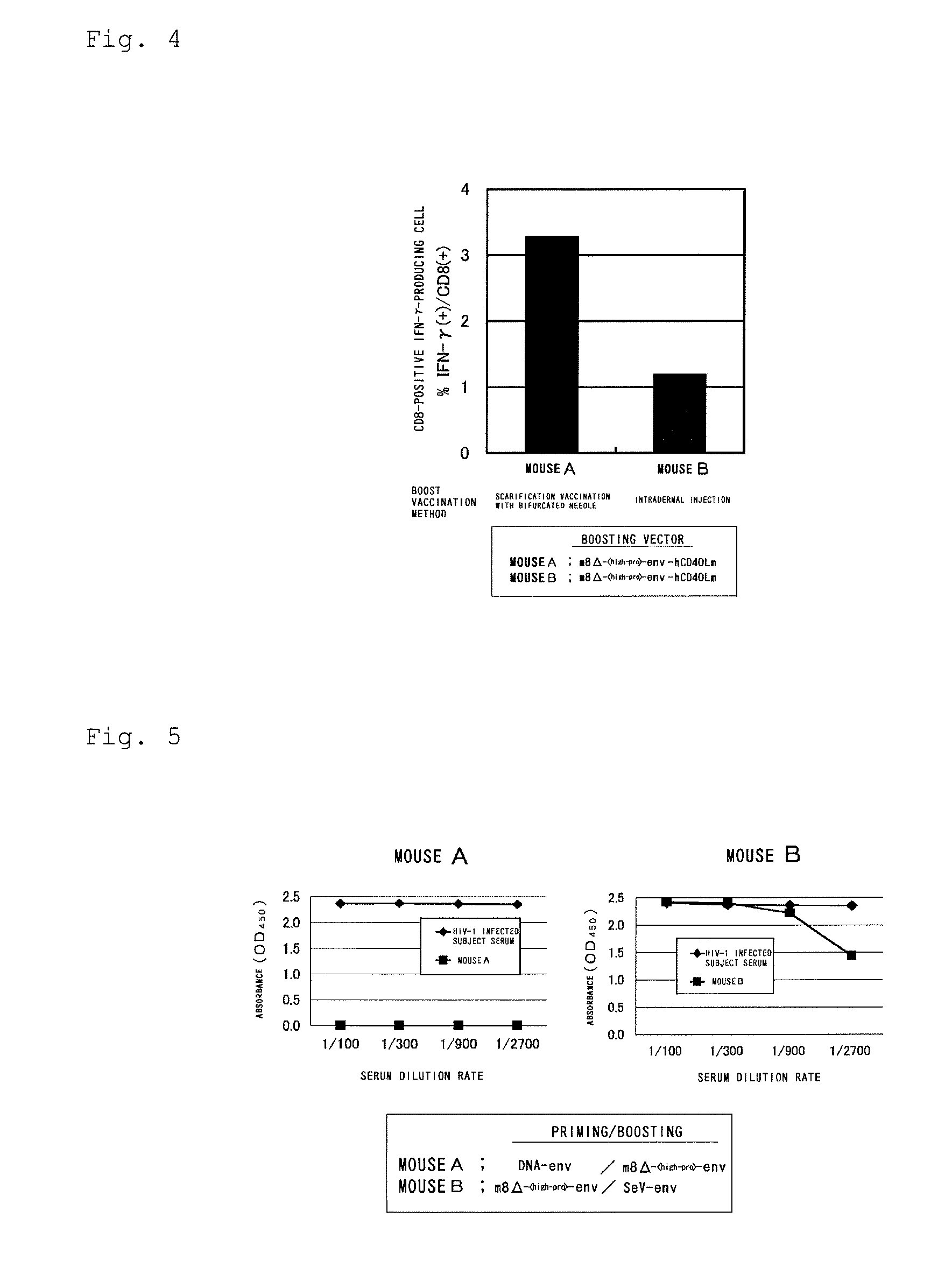
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveCN103189506APrevention is effectiveEffective treatmentSsRNA viruses negative-senseViral antigen ingredientsPathogenic microorganismPox Virus Vector
To provide a virus vector which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy. Provided is a virus vector for prime / boost vaccines, comprising the following components (a) and (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY
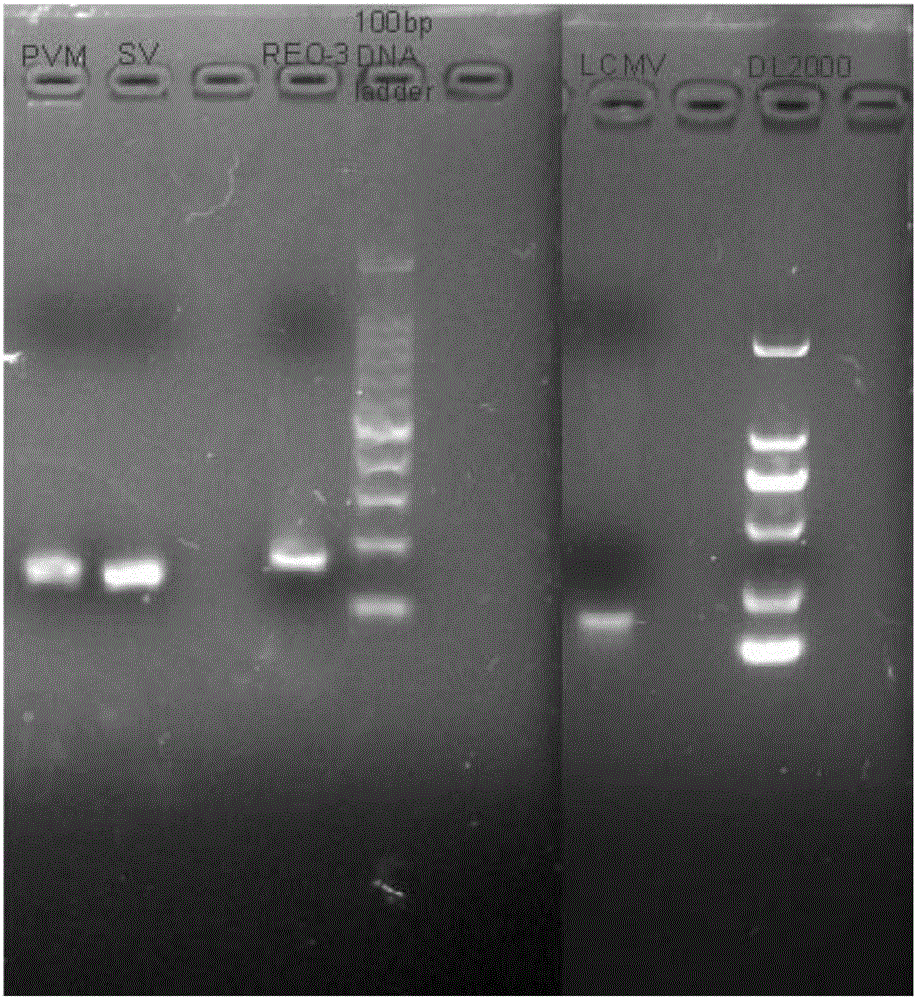
Multiple liquid phase gene chip method and reagent for rapidly detecting guinea pig LCMV, SV, PVM and Reo-3 viruses
ActiveCN106191311AAccurate detectionReduce dosageMicrobiological testing/measurementMicroorganism based processesBiotin-streptavidin complexFluorescence
The invention discloses a multiple liquid phase gene chip method and a reagent for rapidly detecting guinea pig LCMV, SV, PVM and Reo-3 viruses. The operation is simple. A target amplified fragment is acquired through PCR, and then the amplified product, fluorescent coding micro-balloon and streptavidin-phycoerythrin are hybridized, MFI value is read through a tester and different viruses are distinguished. According to the method provided by the invention, the rat lymphocyte choriomeningitis virus, sendai virus, rat pneumonia virus and Reo-3 virus can be accurately detected at the same time; the specificity is strong, the sensitivity is high and the repeatability is excellent; compared with the traditional detection method, the method has the advantages that different target molecules in a same sample can be detected, the detection flux is high, the sample dosage is less, the operation is simple and quick, the detection cost is greatly reduced and the detection efficiency is increased.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
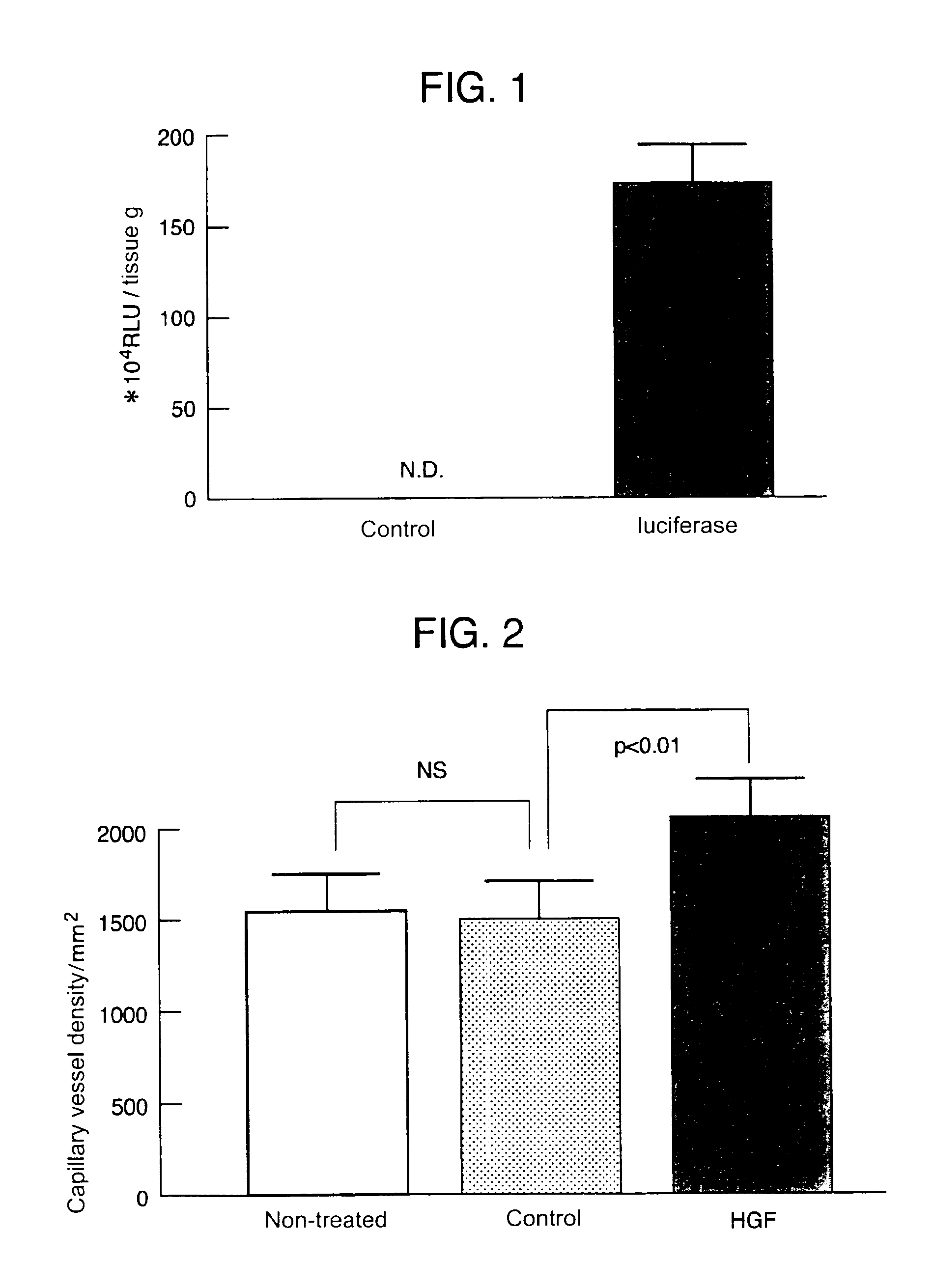
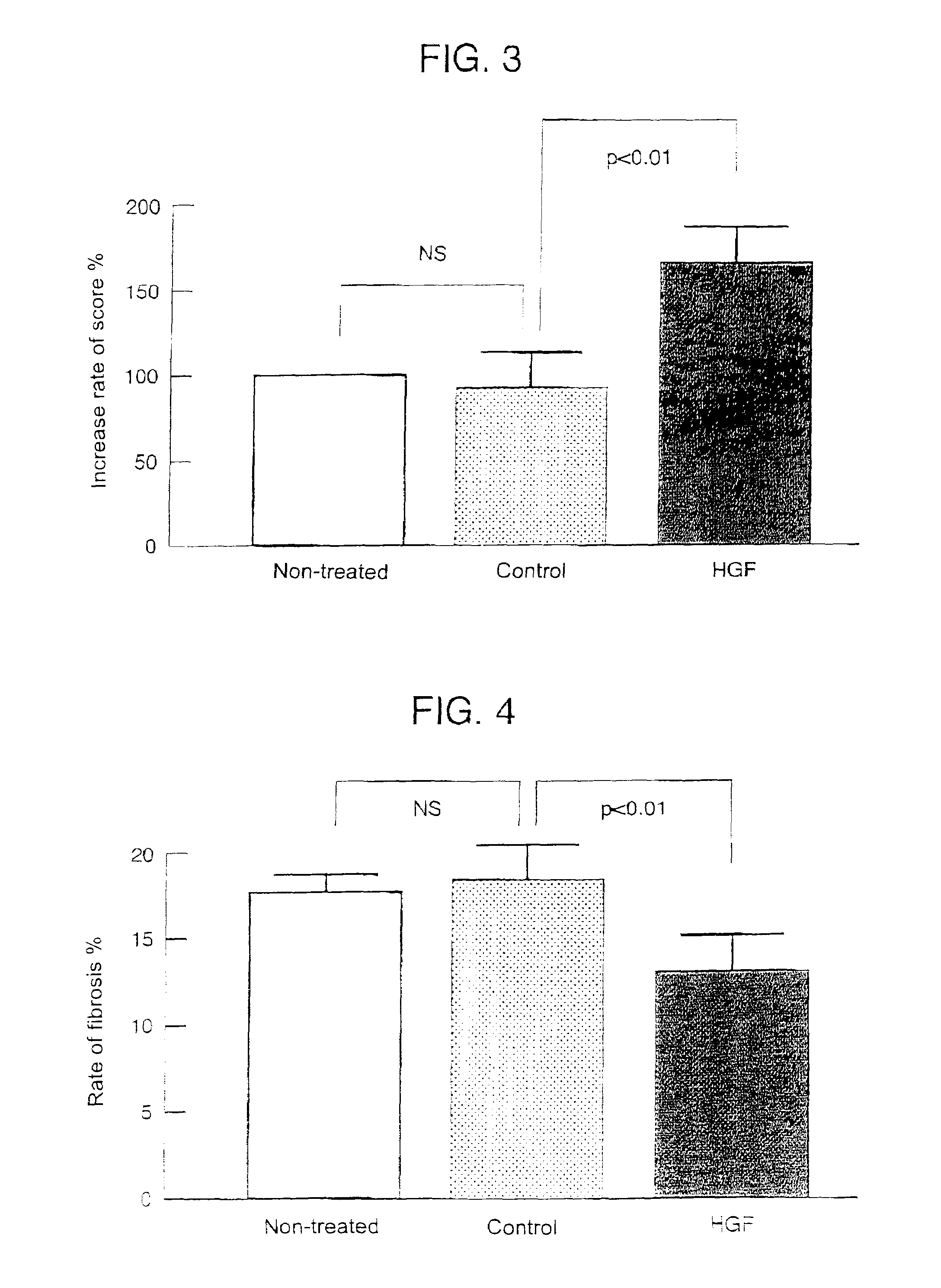
Gene therapy for cardiomyopathy
InactiveUS6989374B1The result is validTreat myocardiopathy efficientlySugar derivativesPeptide/protein ingredientsAngiogenesis growth factorFibrosis
This invention enables the repair of cardiac function by noninvasive administration of an HGF gene in the form of Sendai virus (HVJ)-liposome into the affected cardiac muscle, thereby inducing angiogenesis of the cardiac muscle layer and repressing fibrosis.
Owner:ANGES MG INC
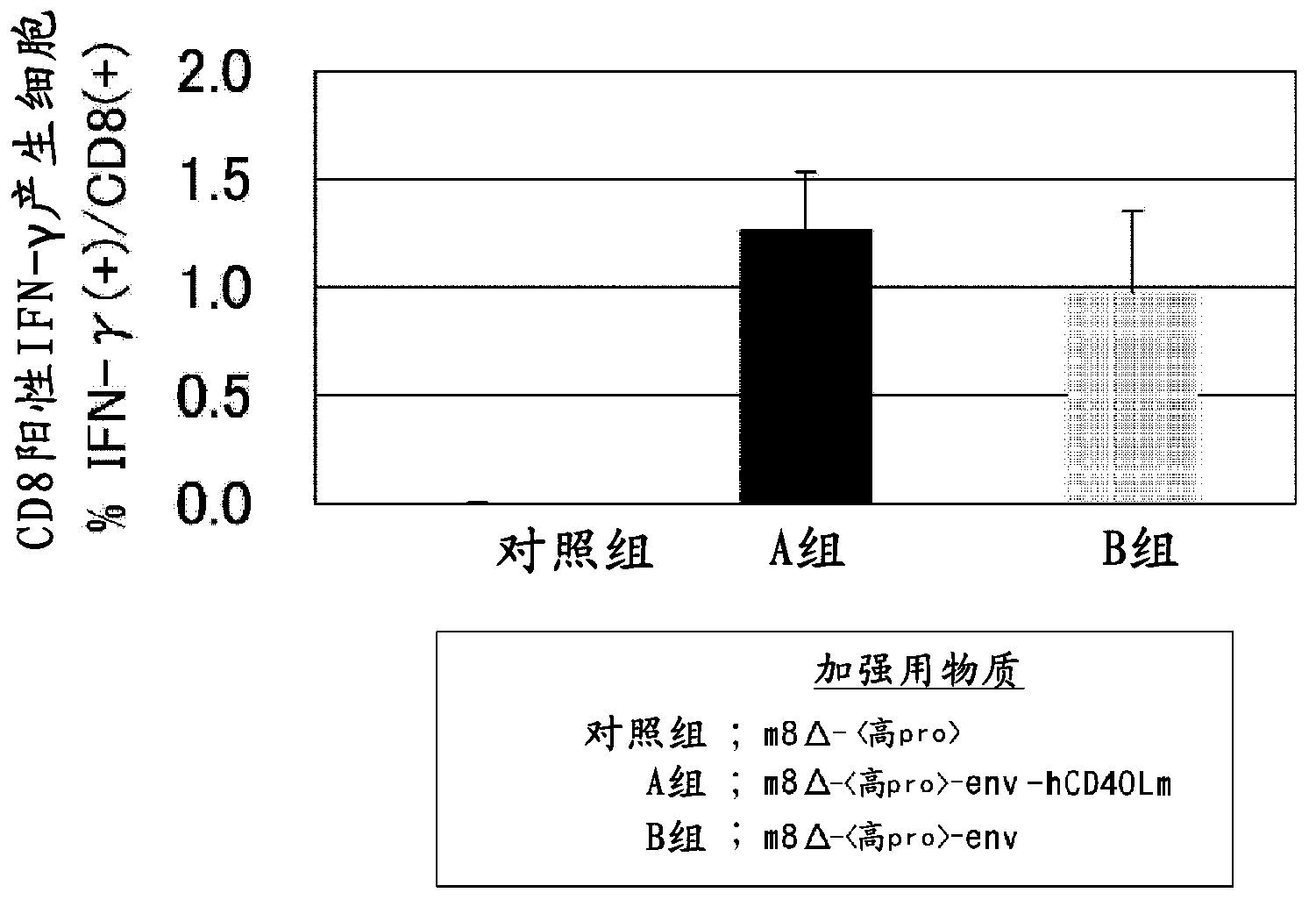
Modified sendai virus vaccine and imaging vector
ActiveUS20140186397A1Easy to trackEasy rescueSsRNA viruses negative-senseVirus peptidesViral VaccineIn vivo
The present invention relates to a Sendai virus or recombinant Sendai virus vector. In particular the present invention provides methods, vectors, formulations, compositions, and kits for a modified Enders strain Sendai viral vector. An immunogenic vector can be used in any in vitro or in vivo system. Moreover, some embodiments include vectors for imaging virus growth, location and transmission.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Use of sendai virus as a human parainfluenza vaccine
InactiveUS20060110740A1Safety managementAvoid infectionSsRNA viruses negative-senseViral antigen ingredientsHealth riskImmunogenicity
Immunogenic compositions to protect against human parainfluenza (hPIV) virus infection are provided. The compositions comprise an unmodified Sendai virus that can be safely administered to human subjects, particularly young children, to protect against symptoms and health risks associated with hPIV infection.
Owner:HURWITZ JULIA +3
Composition for inducing pluripotent stem cell, and use thereof
ActiveUS20130210150A1Improve efficiencyImprove induction efficiencySsRNA viruses negative-senseSugar derivativesGene deliveryInduced pluripotent stem cell
The present invention provides Sendai virus vectors in which genes that encode reprograming factors for inducing pluripotent stem cells are incorporated in a specific order, compositions comprising these vectors for gene delivery to be used in the induction of pluripotent stem cells, and uses thereof. Incorporation of the KLF gene, OCT gene, and SOX gene in a specific order into a single Sendai virus vector successfully and significantly increased the efficiency of pluripotent stem cell induction. Loading multiple reprogramming factors into a single vector can further increase the induction efficiency of pluripotent stem cells while reducing the number of necessary vectors.
Owner:DNAVEC CORP
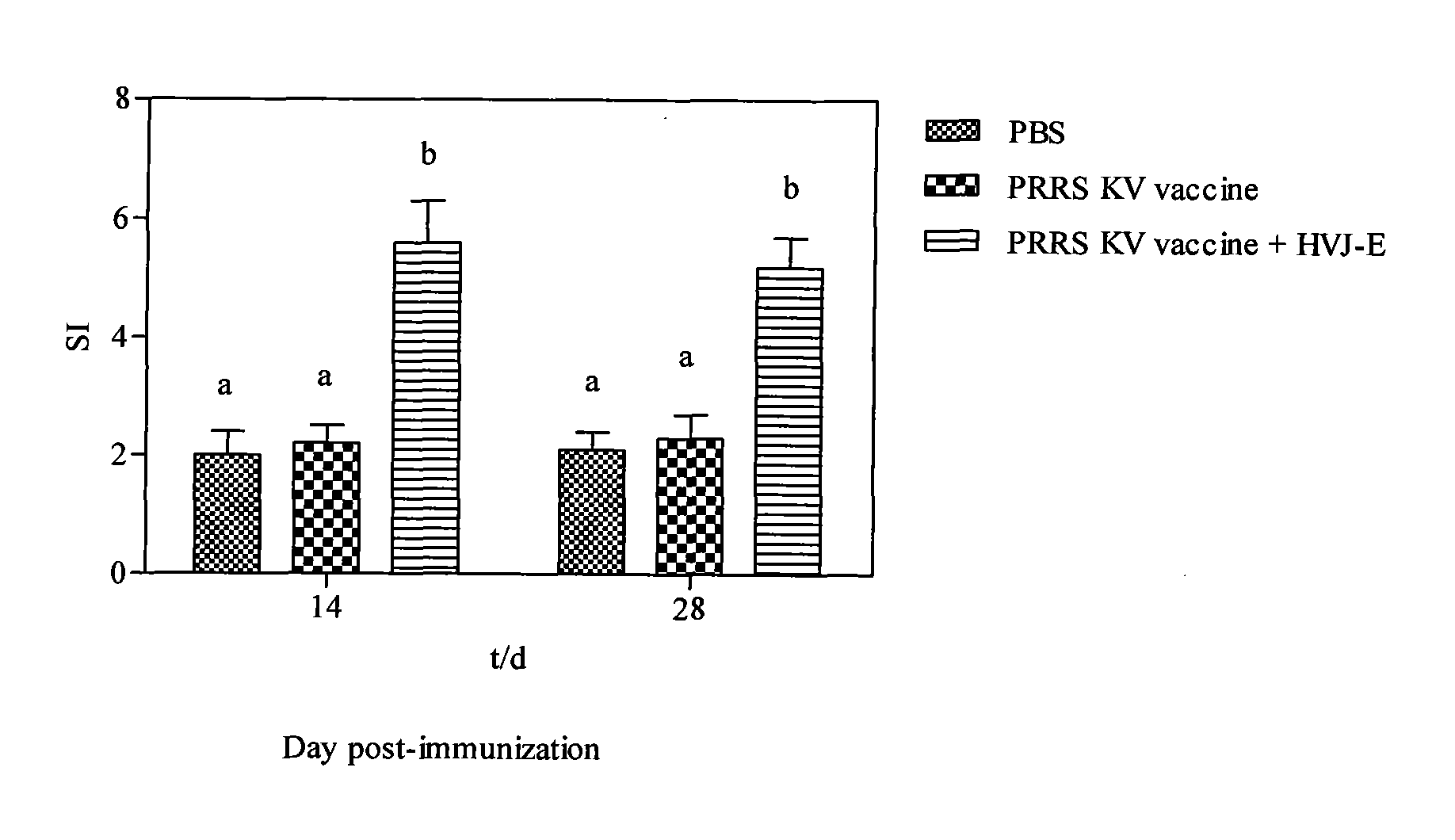
Adjuvant for improving effect of porcine reproductive and respiratory syndrome inactivated vaccine, preparation method thereof and application thereof
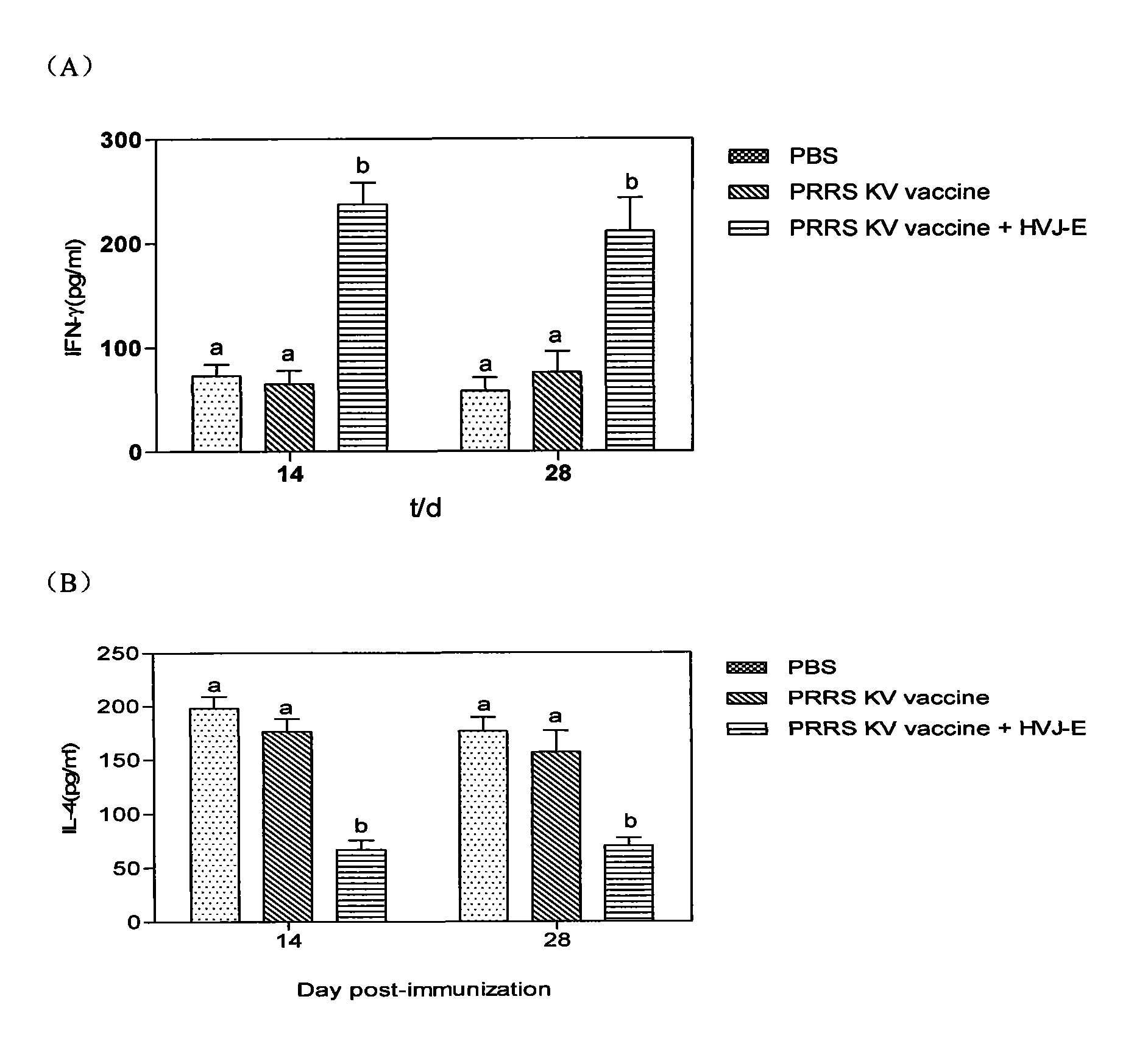
InactiveCN101940787AImprove the immunityImprove immunityViral antigen ingredientsAntiviralsAdjuvantClinical manifestation
The invention discloses an adjuvant for improving the effect of a porcine reproductive and respiratory syndrome (PRRSV) inactivated vaccine, a preparation method thereof and application thereof and relates to the preparation method and steps of Hemagglutinating virus of Japan envelope (HVJ-E) and application of a medicament for improving the effect of the PRRSV inactivated vaccine. The preparation method of adjuvant comprises the following steps of: breeding Hemagglutinating virus of Japan (HVJ) by using an SPF chick embryo, and then concentrating, purifying, counting and inactivating the HVJ to prepare the Hemagglutinating virus of Japan envelope (HVJ-E); immunizing PRRSV negative pigs with the HVJ-E and the PRRSV inactivated vaccine together, and setting a PBS contrast group and a PRRSV inactivated vaccine group at the same time, wherein compared with the contrast group, the HVJ-E and PRRSV inactivated vaccine group can obviously improve the response level of hunoral immunity and cellular immunity of the pigs; and after immunizing, expelling toxin by using a PRRSV virulent strain (JXA1), wherein the result shows that the clinical manifestations and the weight increasing of the HVJ-E and PRRSV inactivated vaccine group are better than those of the contrast group, and the generated viremiavirusemia rate, toxin expelling rate and virus distribution rate are obviously reduced when compared with the contrast group. The adjuvant of the invention has the advantages of convenient production and low cost and is suitable for large-scale production.
Owner:朱善元 +2
Virus vector for prime/boost vaccines, which comprises vaccinia virus vector and sendai virus vector
InactiveUS20130302367A1Avoid infectionEffective preventionSsRNA viruses negative-senseViral antigen ingredientsCowpox virusPrime boost
[Problem]To provide a set of virus vectors which can be used for producing a prime / boost vaccine that can activate both cellular immunity and humoral immunity and is effective on infections by pathogenic microorganisms and malignant tumors which are generally believed to be difficult to be treated by vaccine therapy.[Solution]Provided is a set of virus vectors for prime / boost vaccines, comprising the following virus vector (a) and virus vector (b): (a) a vaccinia virus vector which carries a gene encoding an immunogenic polypeptide in such a manner that the gene can be expressed; and (b) a Sendal virus vector which carries the gene encoding the immunogenic polypeptide in such a manner that the gene can be expressed.
Owner:HOKKAIDO UNIVERSITY +1
Multi-liquid-phase gene chip detection primer, kit and method for quickly distinguishing 5 respiratory pathogens of mouse
ActiveCN107312873AAvoid crossbreedingGuaranteed temperatureMicrobiological testing/measurementMicroorganism based processesBiotin-streptavidin complexFluorescence
The invention discloses a multi-liquid-phase gene chip detection primer, kit and method for quickly distinguishing 5 respiratory pathogens of a mouse. The multi-liquid-phase gene chip detection primer is easy to operate; a target amplified fragment is obtained through PCR (Polymerase Chain Reaction); an amplified product, fluorescence coded microspheres and streptavidin-phycoerythrin are hybridized; an MFI (Mean Fluorescence Intensity) value is read through a detector so as to distinguish different types of viruses. The method disclosed by the invention can detect a pneumonia virus, a hantaan virus, a sendai virus, a lymphocytic choriomeningitis virus and mycoplasma pulmonis of the mouse at the same time, and is high in specificity, high in sensitivity and high in repetitiveness. Compared with the conventional detection method, the method disclosed by the invention realizes simultaneous detection of various different target molecules in the same sample; the sample use amount is small; the operation is simple and quick; the detection cost can be greatly reduced.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
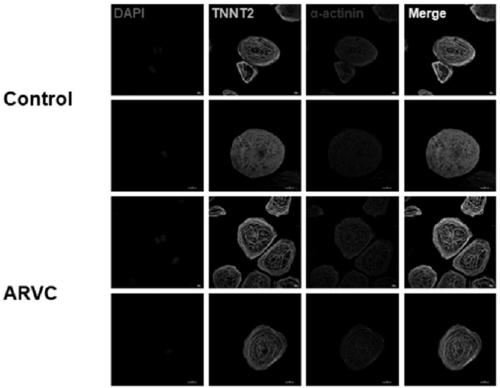
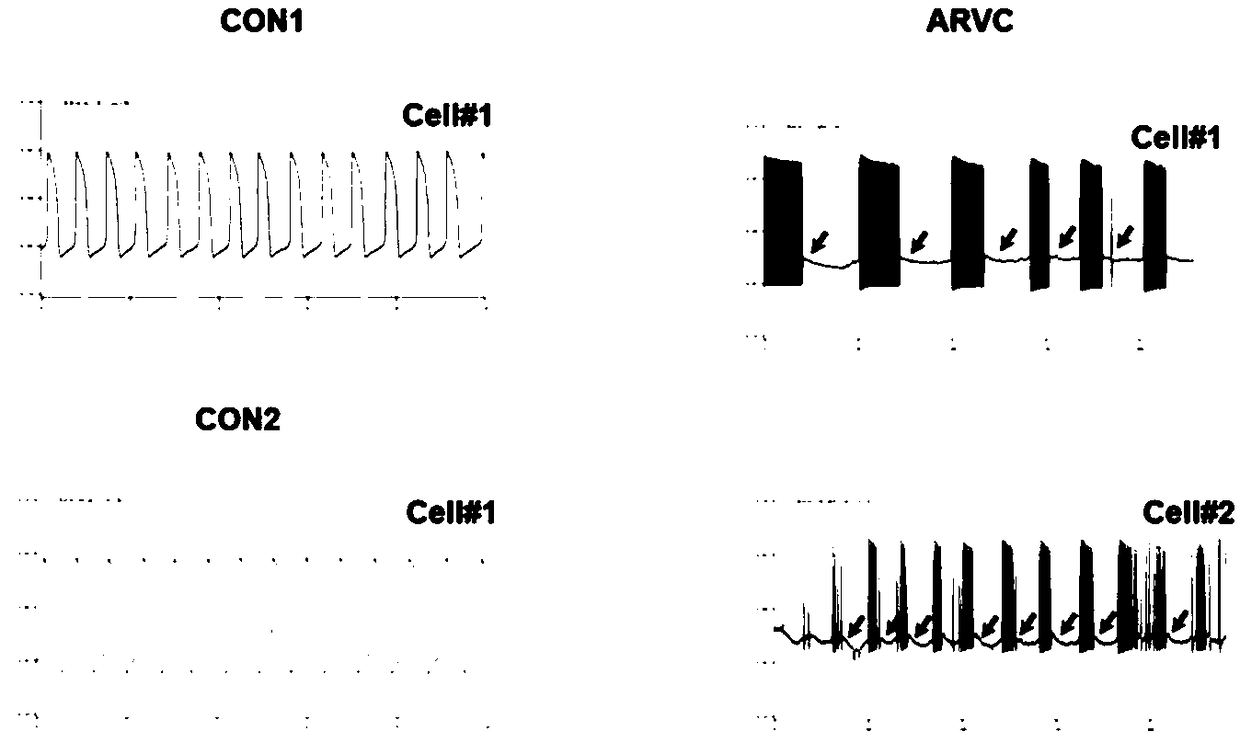
Method for establishing human arrhythmogenic right ventricular cardiomyopathy (ARVC) disease model
InactiveCN109402048AConforms to ARVC characteristicsCulture processSkeletal/connective tissue cellsRight ventricular cardiomyopathyDirected differentiation
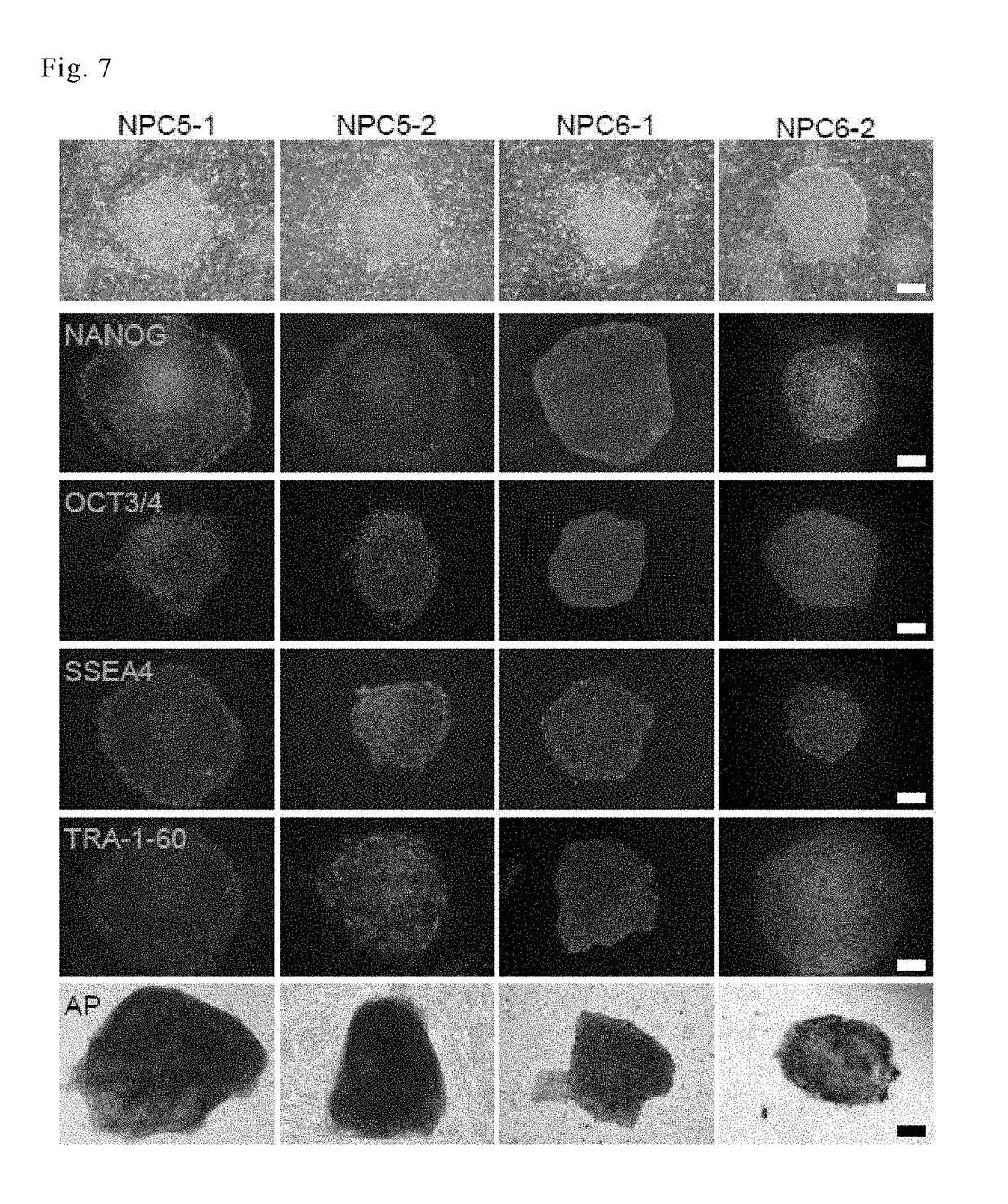
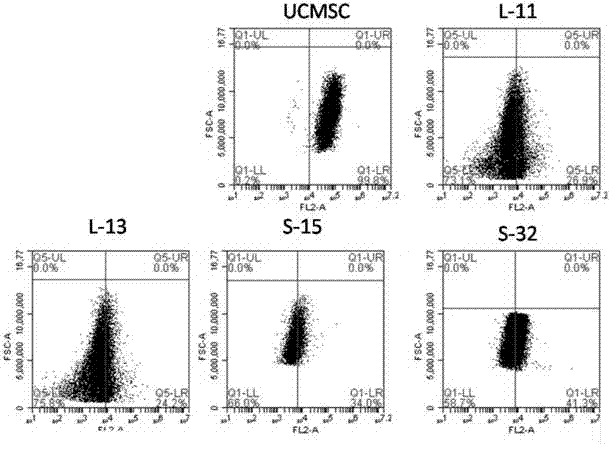
The invention discloses a method for establishing a human arrhythmogenic right ventricular cardiomyopathy (ARVC) disease model. The method comprises the steps that primary culture is carried out on the skin of a patient with the ARVC to obtain fibroblasts, and specific induced pluripotent stem cells (iPSC) of the patient with the ARVC are obtained by non-integrated Sendai virus induction, and thendirectionally differentiated into specific cardiomyocytes of the patient with ARVC so that a stable in-vitro cardiomyocyte disease model can be established. The method can establish the stable humanARVC model based on an iPSC technology; the morphology and function of the model are both in accordance with the pathological characteristics of the ARVC, and the model is suitable for the research ofARVC related pathological mechanisms and individualized drug screening.
Owner:ZHEJIANG UNIV
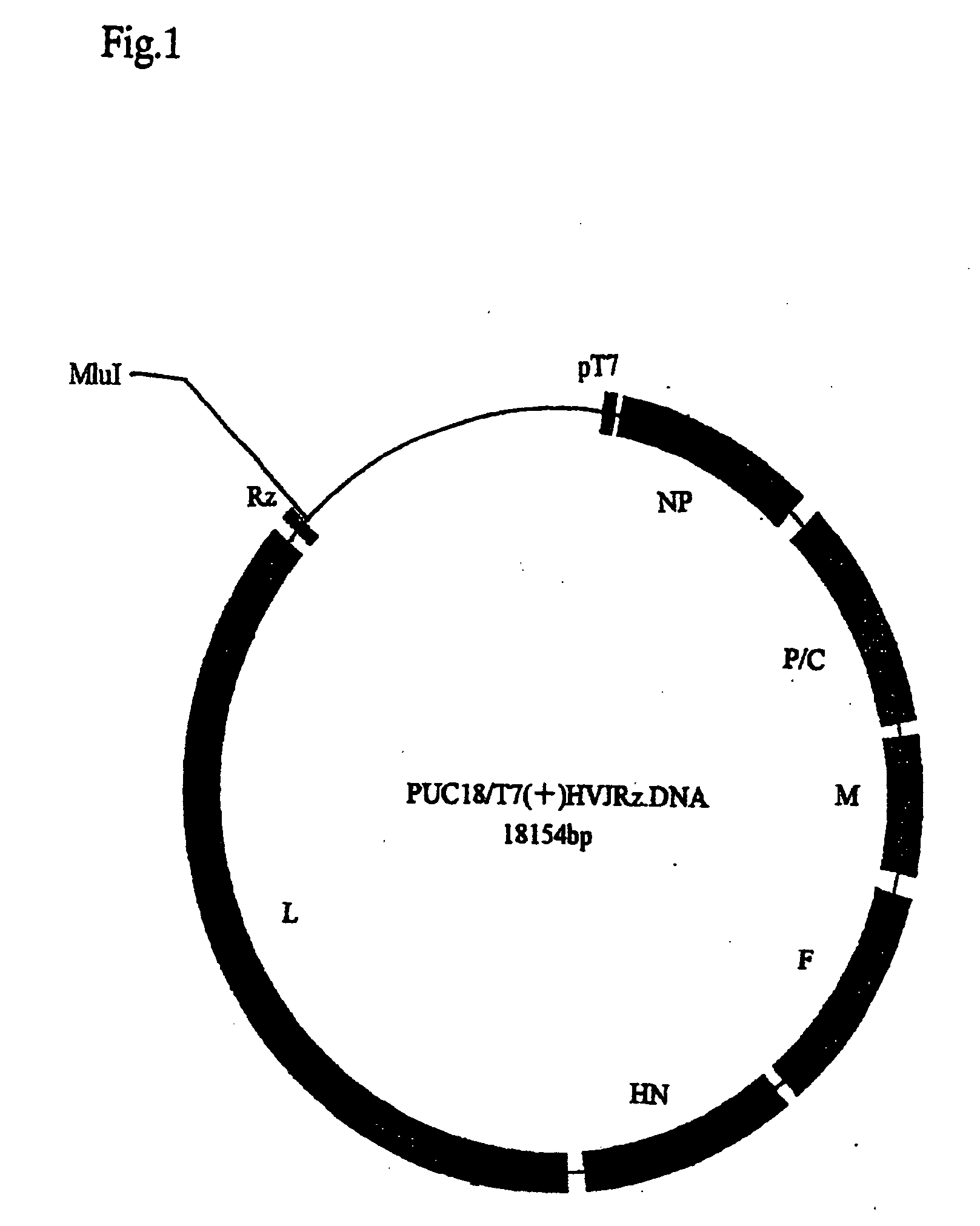
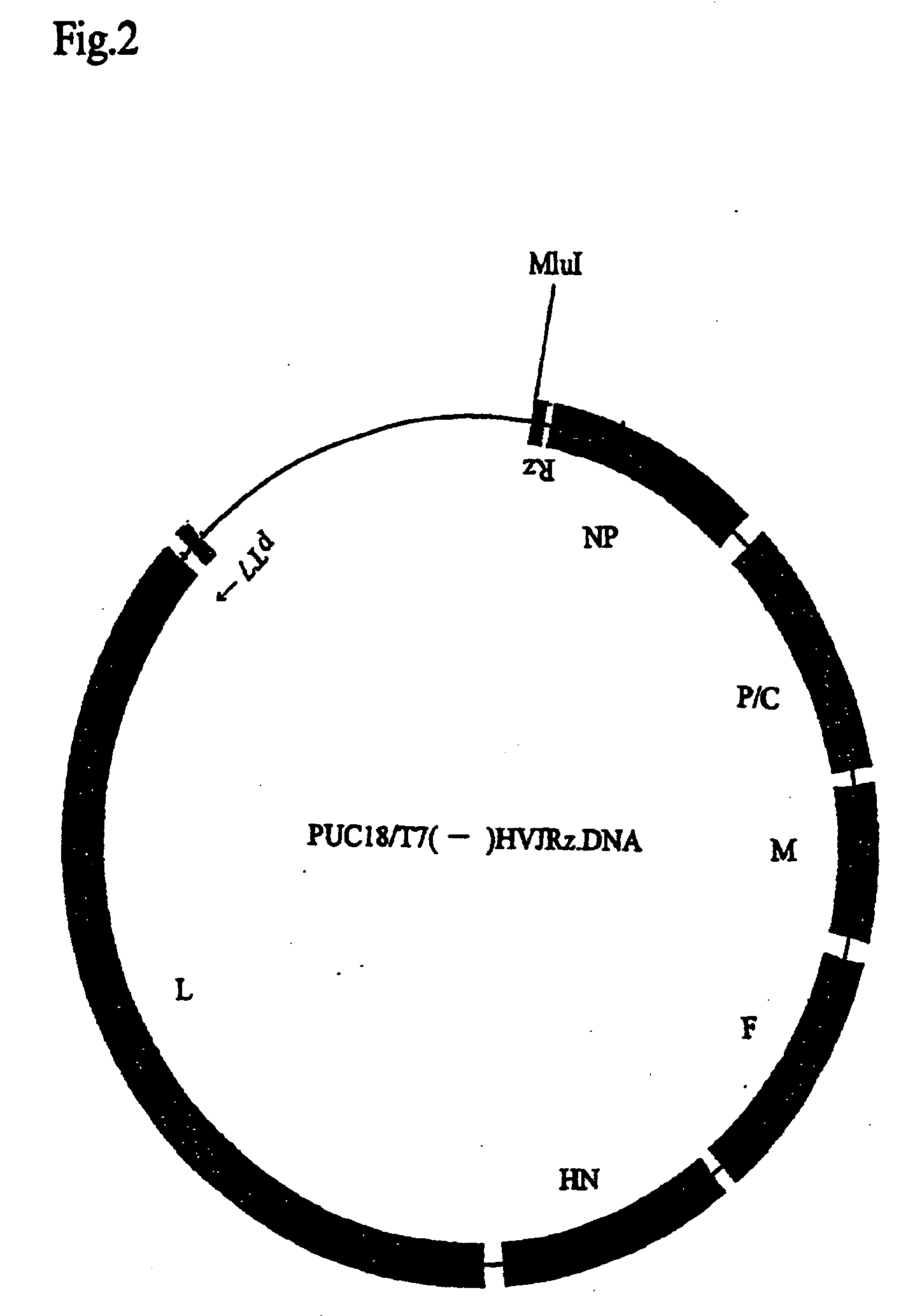
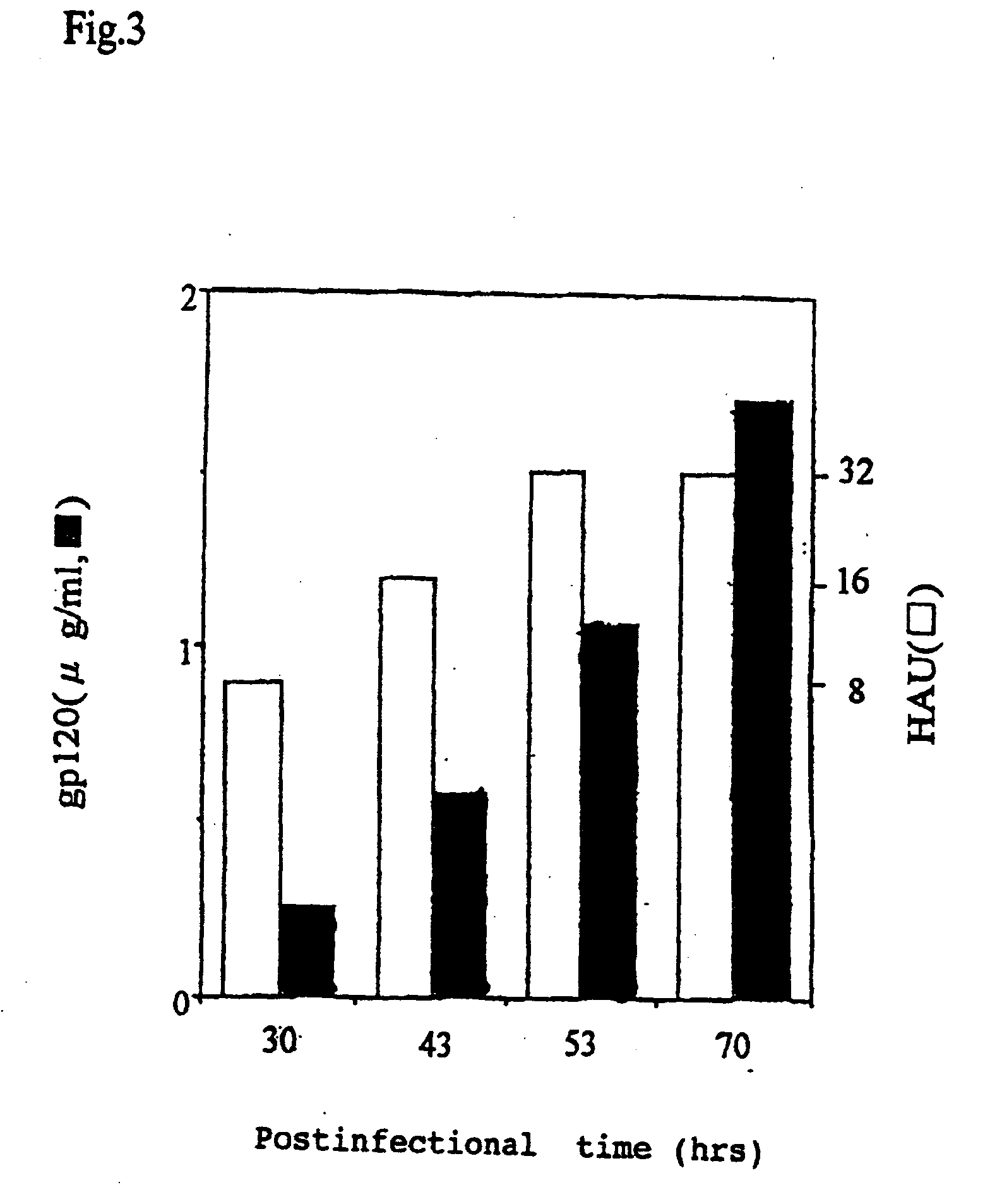
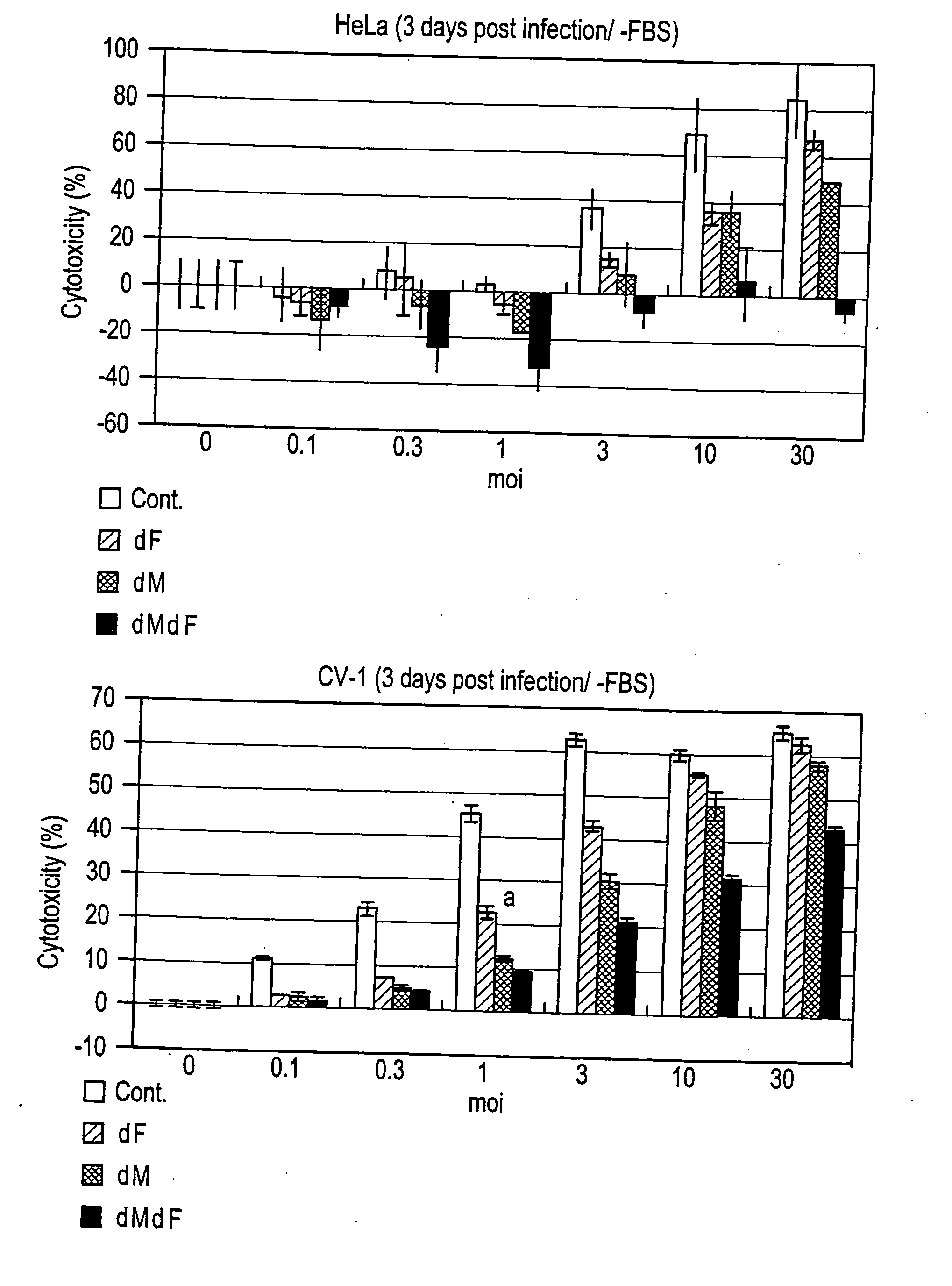
Paramyxorividae virus vector defective in envelope gene
InactiveCN1355851AHigh gene transfer efficiencyImprove securitySsRNA viruses negative-senseGenetic material ingredientsF proteinParamyxoviridae
Virus virions defective in F gene are successfully collected by using a Sendai virus genomic cDNA with deletion of F gene. Further, infectious viral particles defective in F gene are successfully constructed by using F-expression cells as helper cells. Also, virus virions defective in F gene and HN gene are successfully collected by using a virus genomic cDNA with deletion of both of F gene and HN gene. Further, infectious viral particles defective in F gene and HN gene are successfully produced by using F- and HN-expression cells as helper cells. A virus being defective in F gene and HN gene and having F protein is constructed by using F-expression cells as helper cells. Further, a VSV-G pseudo type virus is successfully constructed by using VSV-G-expression cells. Techniques for constructing these defective viruses contribute to the development of vectors of Paramyxoviridae usable in gene therapy.
Owner:DNAVEC RES
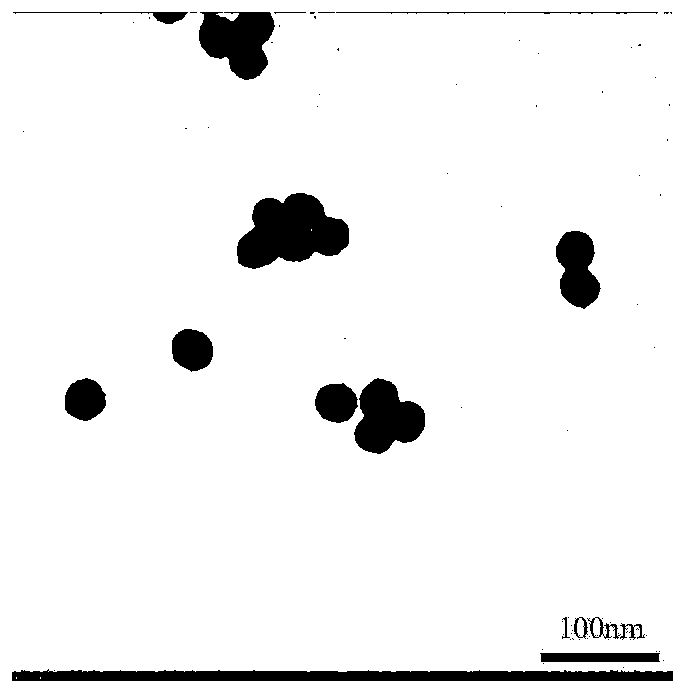
Method for synthesizing nano-preparations for visual guidance and combined immunotherapy of tumors by Gd:CuS mineralized Sendai virus
PendingCN110201005AEfficient guidanceSignificant T1-weighted magnetic resonance/photoacoustic dual-modal imaging capabilityEnergy modified materialsUnknown materialsSide effectDual mode
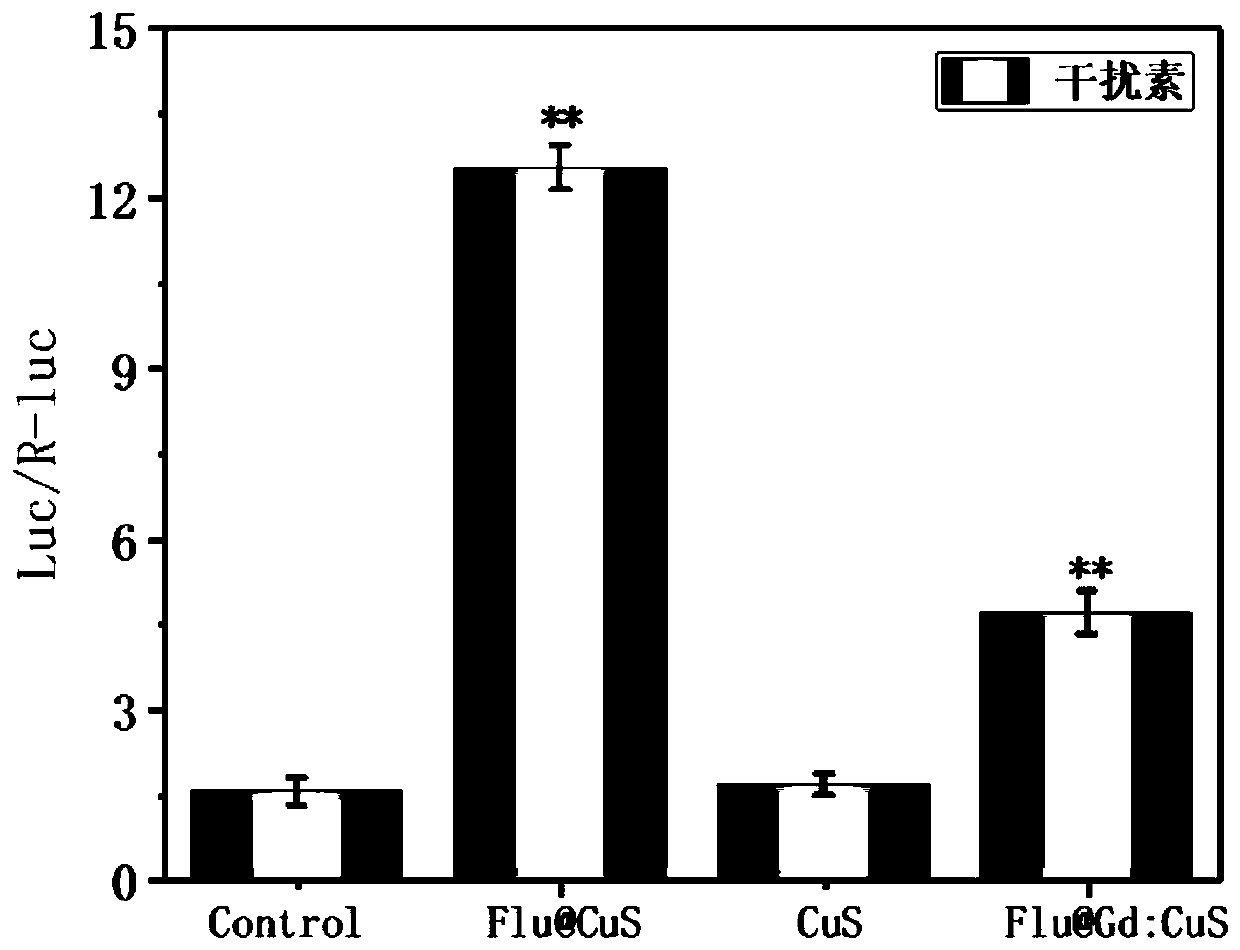
The invention discloses a method for successfully synthesizing nano-preparations for combined therapy of tumors by strategy biomimetic simulation of Gd:CuS mineralized Sendai virus (SeV@Gd:CuS). The method includes the steps: 1) preparation of a CuCl2.2HO2 aqueous solution; 2) preparation of a GdCl3.6H2O aqueous solution; and 3) one-pot synthesis of SeV@Gd:CuS nanoparticles. Sendai virus has a complex structure, can systemically activate the immune function of a body and causes all-round killing and clearance of tumors. The SeV@Gd:CuS nanoparticles have significant T1-weighted magnetic resonance / photoacoustic dual-mode imaging ability, have high photothermal conversion efficiency under near-infrared laser irradiation, can highly efficiently guide the follow-up photothermal therapy of tumors, have excellent biocompatibility and do not generate side effects.
Owner:TIANJIN UNIV
Paramyxoviruses comprising modified transcription start sequence
InactiveUS7144579B2Reduced expression levelLow cytotoxicitySsRNA viruses negative-senseSugar derivativesTranscription initiationGene type
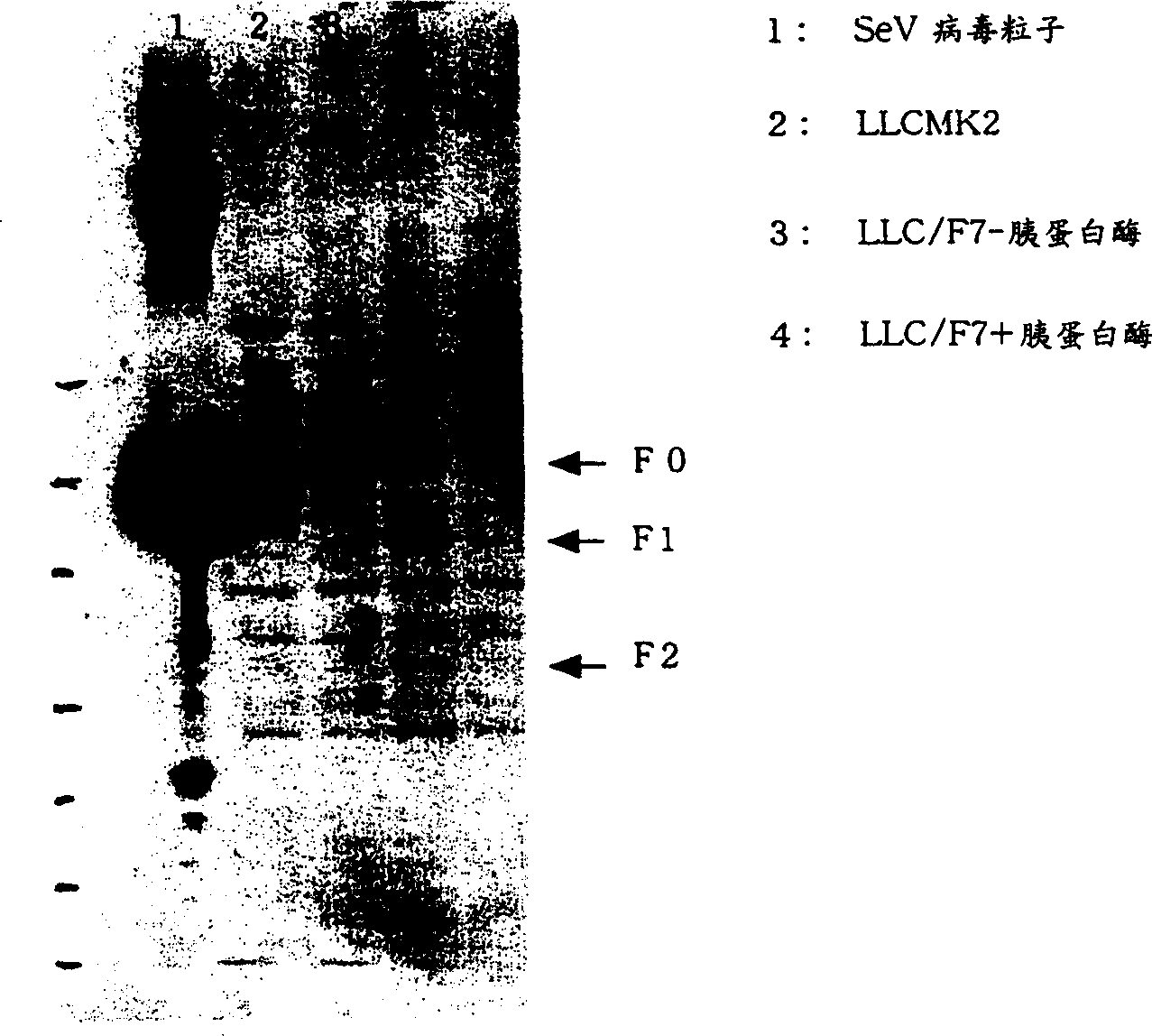
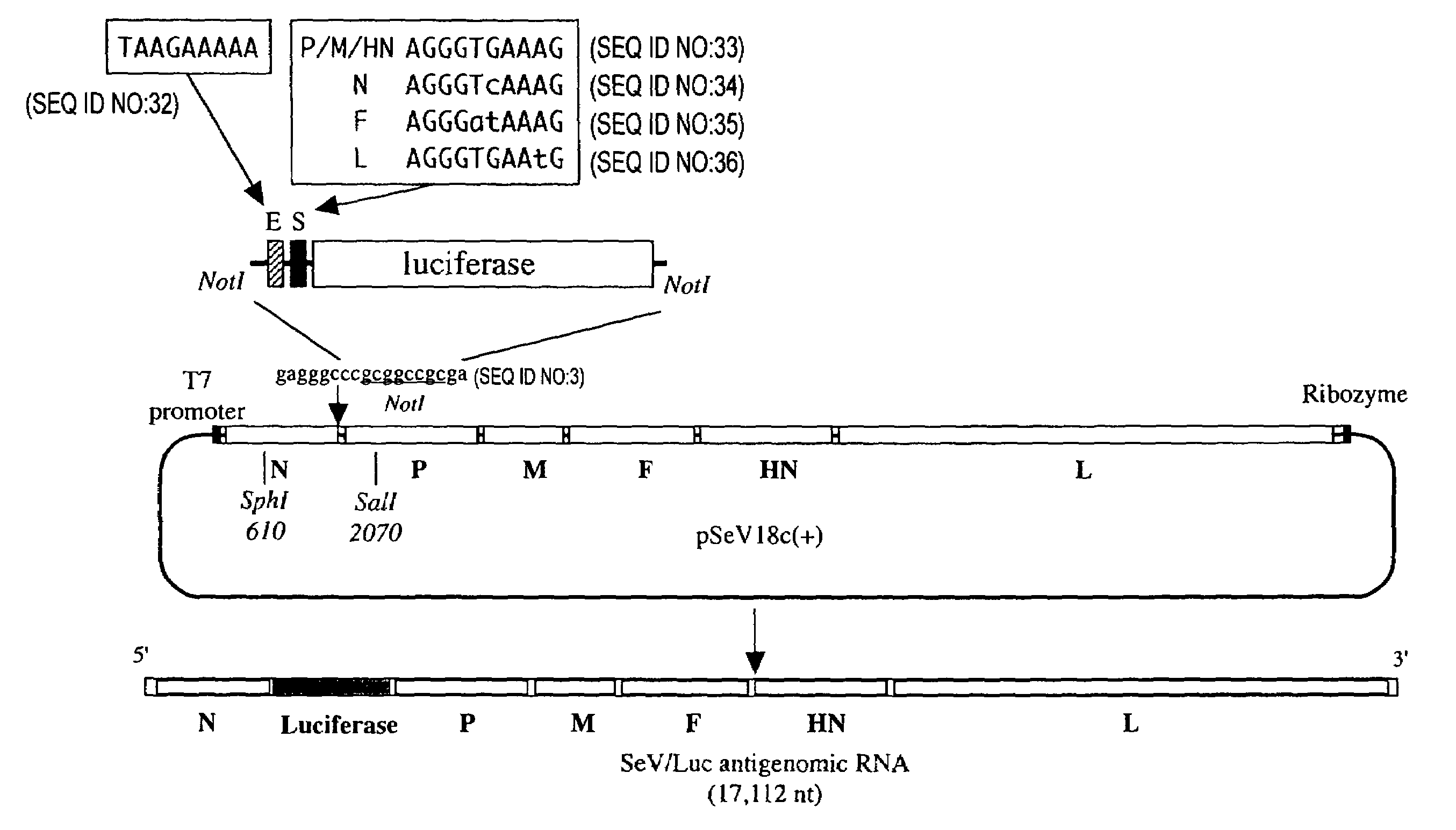
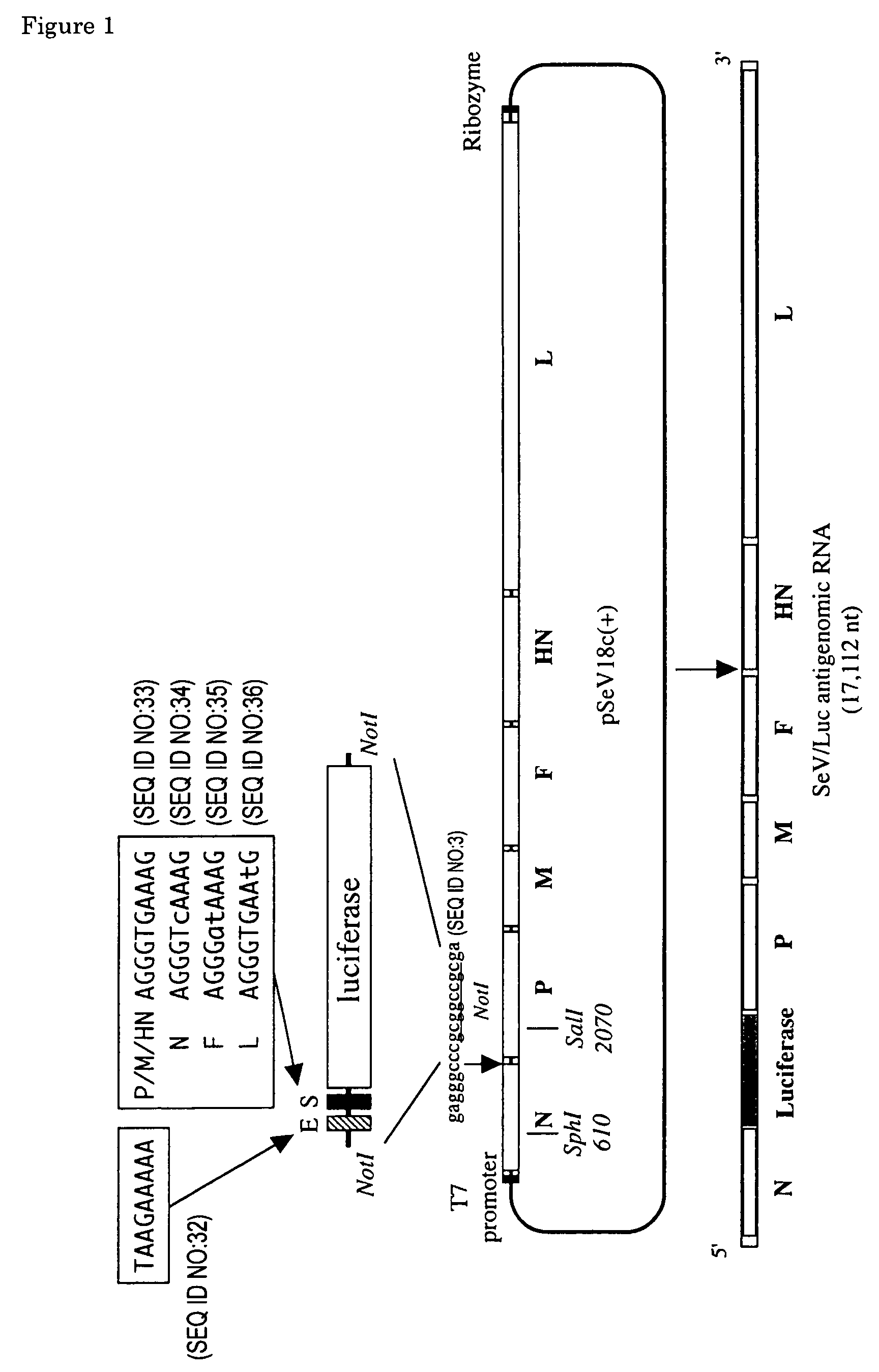
The present invention provides virus vectors of the family Paramyxoviridae in which the transcription start (S) sequence has been modified so as to modify the expression of genes located downstream thereof, a method for producing the vectors, and uses thereof. By measuring the transcription initiation efficiency of the S sequence of each gene carried by Sendai viruses (SeV), it was clarified that the S sequence of F gene has a significantly lower ability to promote transcription than the other three S sequences. When the S sequence of the F gene of wild type Sendai virus was substituted by the S sequence of the P / M / HN gene-type showing a high transcription initiation efficiency, the F gene of the resultant Sendai virus mutant and genes located downstream thereof show elevated expression levels. It was also revealed that this mutant proliferates more quickly than the wild type. The vectors of this invention are useful in elevating the expression of foreign genes and producing pharmaceutical compositions and vaccines. Furthermore, by lowering virus gene expression from virus vectors, it is possible to suppress transcription and / or replication and reduce cytotoxicity of the vector genome.
Owner:DNAVEC RES
Primers and probe for detecting mouse Sendai virus and method thereof
ActiveCN102337358AImprove quality controlSimple and fast operationMicrobiological testing/measurementMicroorganism based processesMedicineNucleotide
The invention discloses primers and a probe for detecting mouse Sendai virus and a method thereof. The primers have a pair of oligonucleotides constituted by an upstream primer with the SEQ ID No. 1 (sequence identity number 1) in a sequence table and a downstream primer with the SEQ ID No. 2 in the sequence table, and the probe matched with the primers has a nucleotide sequence of the SEQ ID No. 3 in the sequence table. By using the method and a kit for detecting the mouse Sendai virus, the advantages of rapidness, simplicity, high sensitivity, strong specificity and high recovery rate are realized, the defects of being complex and time-consuming in the existing detection means, being long in period and having certain requirements for an operation laboratory are overcome, and the application prospects are great.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD
Nucleic Acid Construct
InactiveUS20070281356A1Enhancement of DNA releaseExpression efficiency is loweredPolypeptide with localisation/targeting motifVectorsNucleic acid transportGene delivery
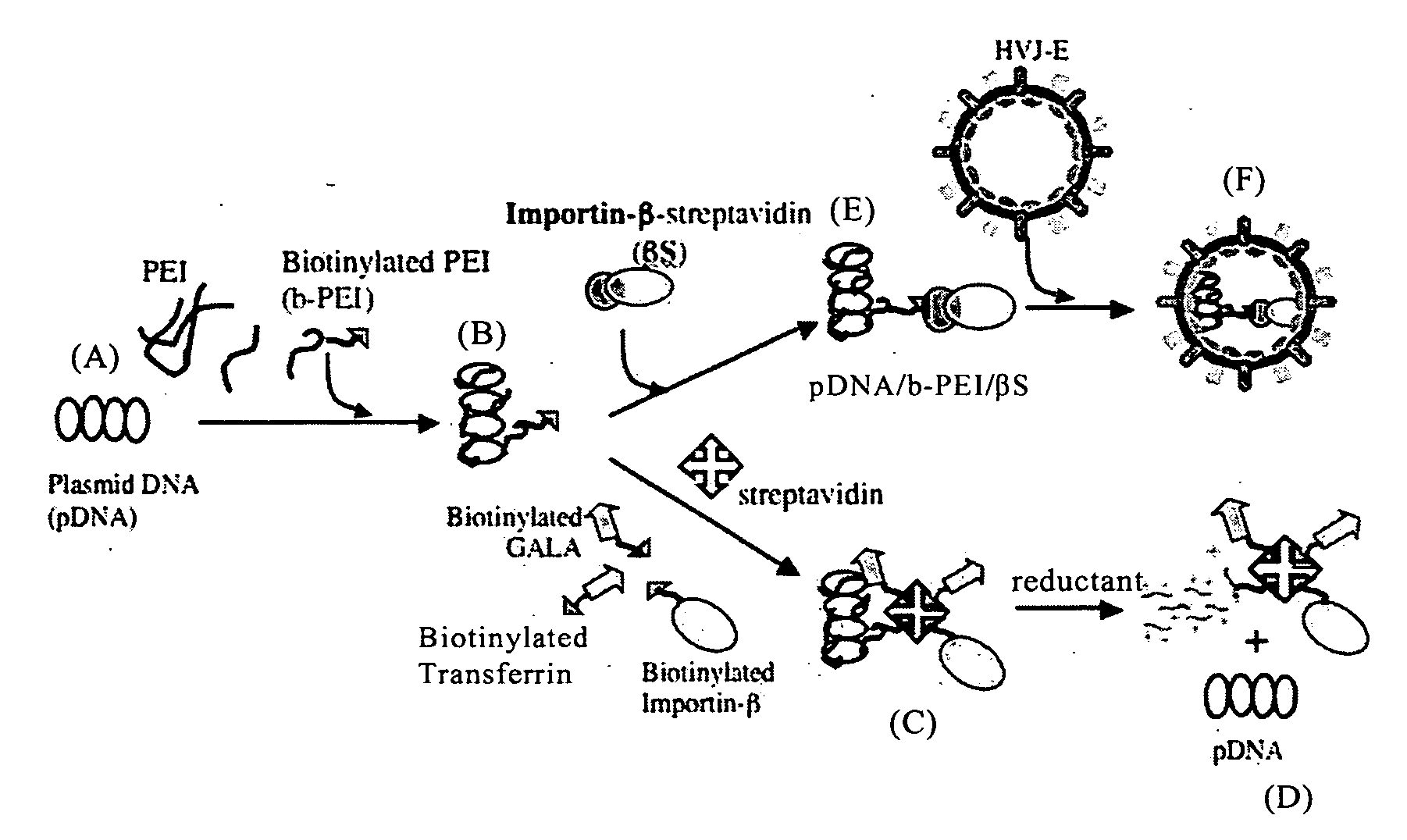
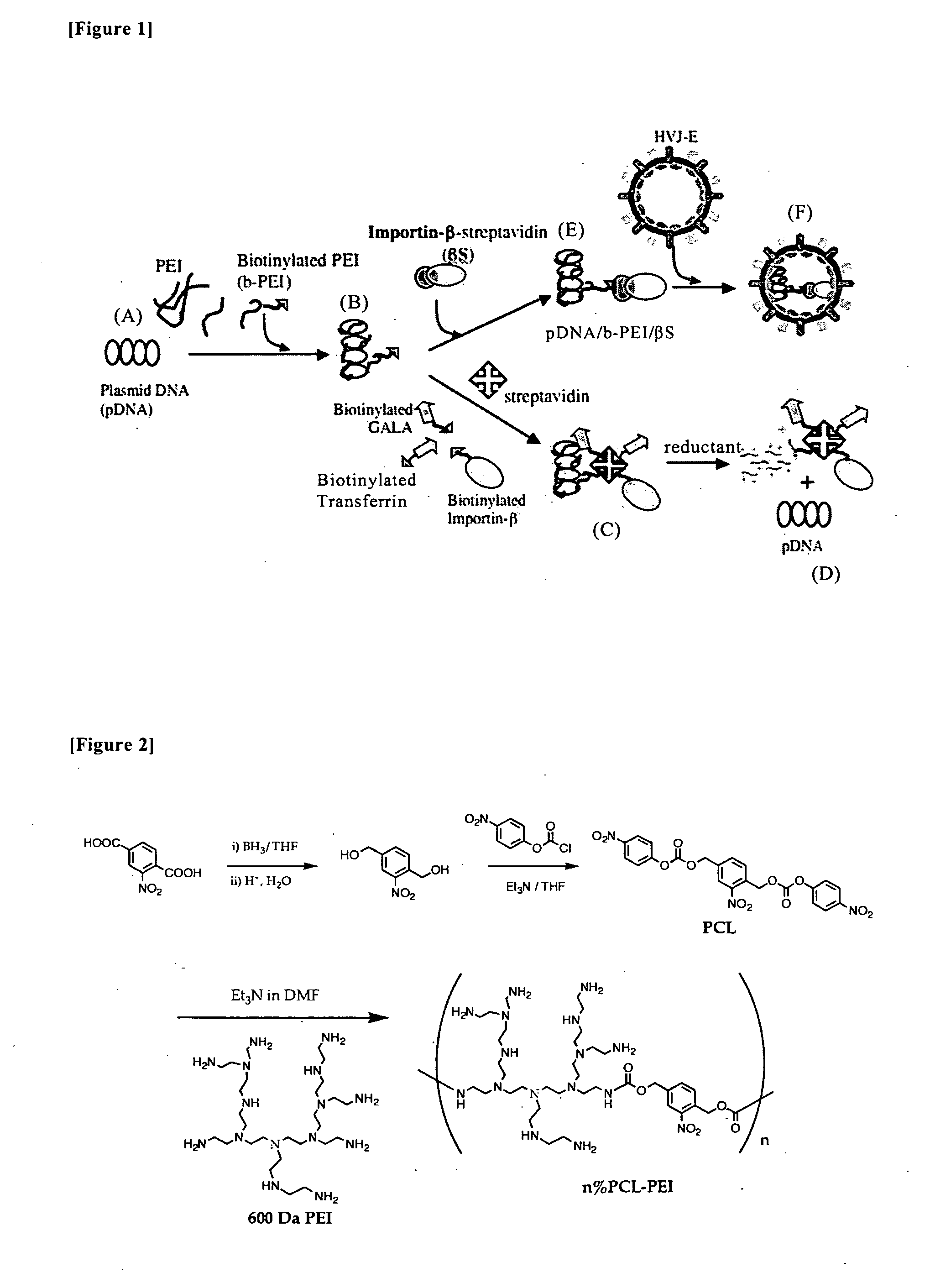
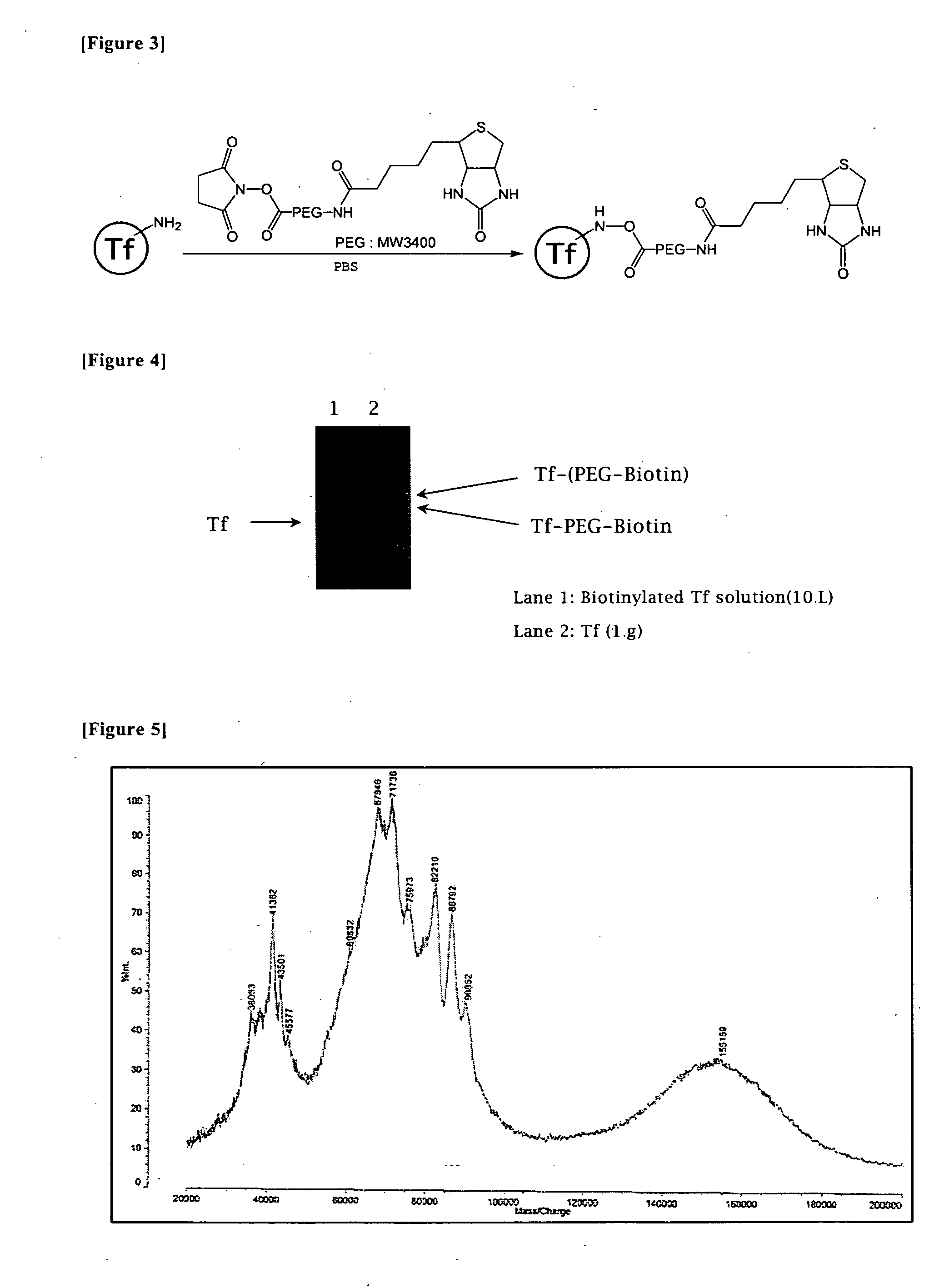
To provide a new technique by which efficient transfection is ensured in delivering a target gene into a cell, disclosed is a nucleic acid construct for nuclear import, which comprises a ternary complex consisting of a nucleic acid substance containing a gene to be delivered into the nucleus of a cell, an importin protein (for example, importin-β) capable of passing through the nuclear pore and involved in the nuclear transport, and a binding substance (for example, polyethyleneimine) bound to both of the nucleic acid substance and the importin protein. Nucleic acid transport from outside of a cell into the cell nucleus can be particularly promoted by administering the nucleic acid construct bound to a cell membrane receptor binding factor and / or a membrane fusing substance, or administering the nucleic acid construct encapsulated in a non-viral vector (for example, Sendai virus envelope) having cell membrane permeability and membrane fusing properties.
Owner:JAPAN SCI & TECH CORP
Attenuated minus-stranded rna virus
InactiveCN101646768ASsRNA viruses negative-senseGenetic material ingredientsNegative strandCytotoxicity
Disclosed is attenuated minus-stranded RNA virus. It is found that the mutation of an amino acid residue at position-1214 in the amino acid sequence for Sendai virus L-protein (Y1214F) can inhibit thegenome replication activity and / or the genome transcription activity of the virus. It is also found that a cytotoxicity or immunological response can be reduced compared with the original one by deleting a specific gene from a viral genome.
Owner:DNAVEC CORP
Drug for the treatment of cholesterol accumulation disorders, and screening method for same
ActiveUS20170216342A1Effective therapyEasy screeningOrganic active ingredientsCompound screeningCholesterolScreening method
Owner:NAT UNIV CORP KUMAMOTO UNIV +1
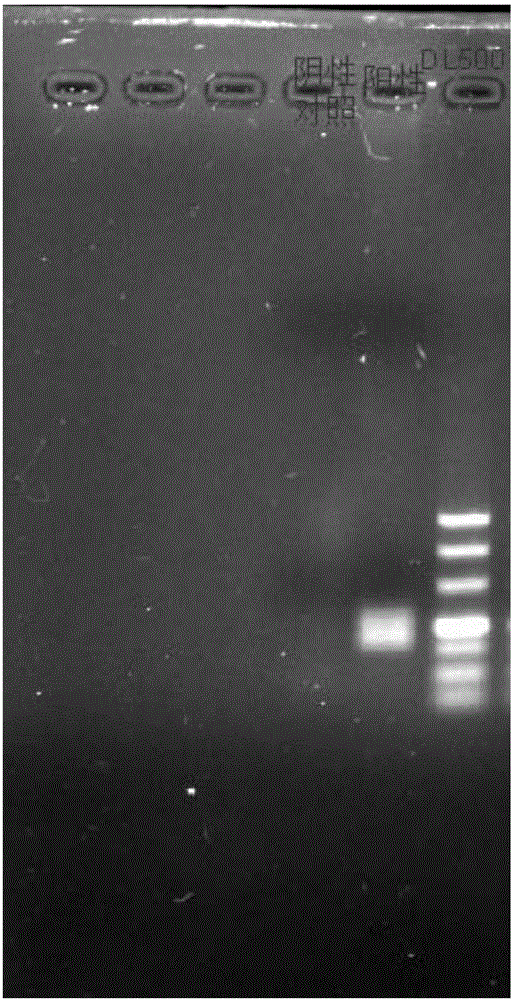
One-step fluorescent parting RT-PCR detection kit for sendai virus
InactiveCN108239676ARapid identificationAccurate identificationMicrobiological testing/measurementDNA/RNA fragmentationPositive controlFluorescence
The invention provides a one-step fluorescent parting RT-PCR detection kit for sendai virus. The kit comprises an RT-PCR reaction liquid, an RT-PCR enzyme mixture, a sendai virus parting primer probe,an internal reference, negative control, critical positive control and strong positive control. The kit can be used for carrying out one-step RT-PCR reaction directly on extracted sendai virus RNA and fluorescent parting detection on the sendai virus RNA in a sample, and preventing pollution by taking an internal reference gene sequence as internal control by means of a UNG enzyme. The one-step amplification method of the kit is simple, short in progress, simple to operate and pollution-preventative, and the detection result is high in specificity, high in sensitivity, clear in result and high in credibility. The kit can be used for fluorescent parting detection of type 1, type 2 and type 3 sendai viruses in a human nasopharyngeal swab sample.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
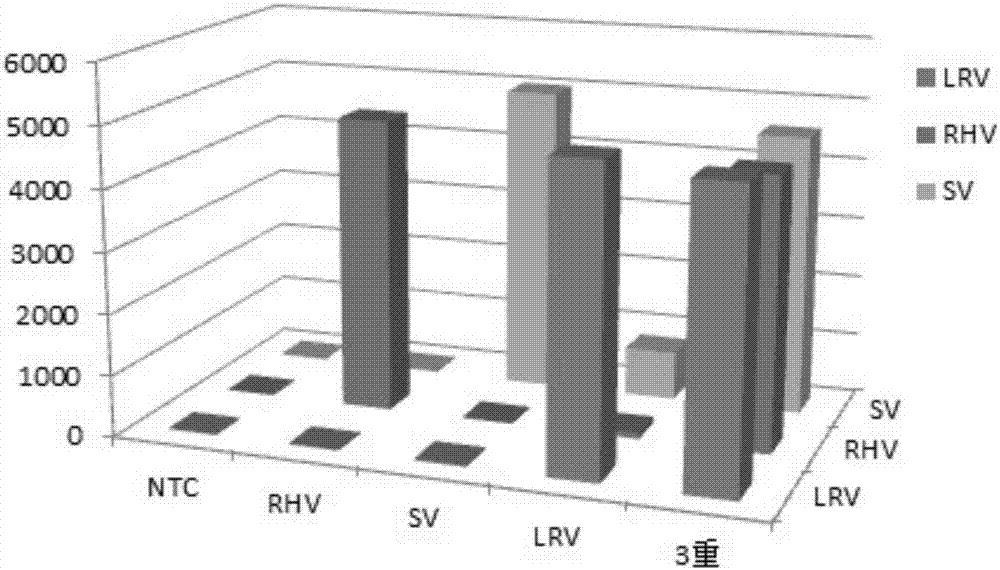
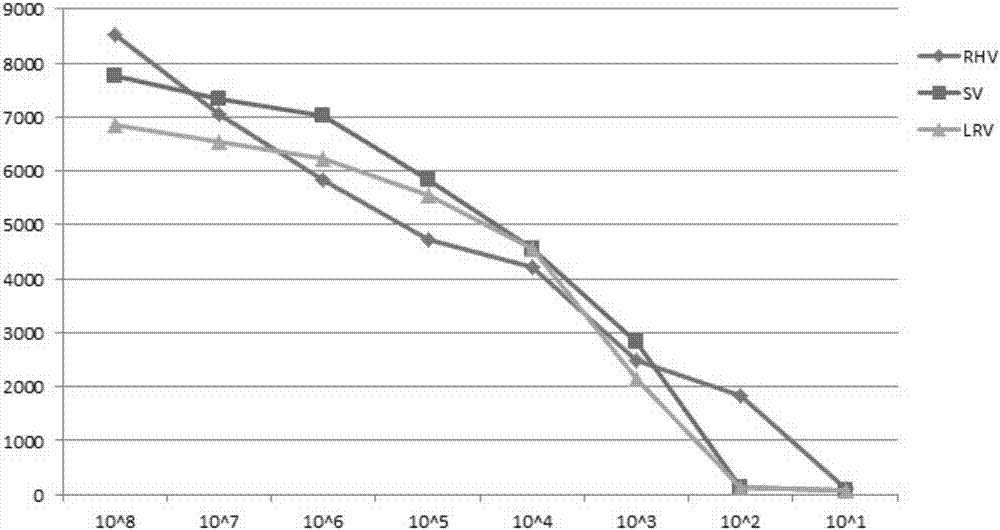
Multiplex fluoroimmunoassay method for rapidly distinguishing RHV (rabbit hemorrhagic disease), SV (sendai virus) and LRV (lapine rotavirus) and reagent
ActiveCN107326098AAccurate detectionReduce dosageMicrobiological testing/measurementMicroorganism based processesBiotin-streptavidin complexRotavirus RNA
The invention discloses a multiplex fluoroimmunoassay method for rapidly distinguishing RHV (rabbit hemorrhagic disease), SV (sendai virus) and LRV (lapine rotavirus) and a reagent. The fluoroimmunoassay method is simple in operation, a target amplification fragment is obtained through PCR, then an amplification product, fluorescence coded microspheres and streptavidin-phycoerythrin are subjected to hybridization, an MFI value is read through a detector, and different types of viruses are distinguished. The method can be used for accurately detecting the RHV, the SV and the LRV simultaneously and has high specificity and sensitivity and good repeatability; compared with a traditional detection method, the method can be used for detecting different target molecules in the same sample simultaneously, few samples are required, operation is simple and rapid, and the detection cost can be reduced greatly.
Owner:GUANGDONG LAB ANIMALS MONITORING INST
Method of utilizing rare earth up-conversion fluorescent particles to mark sendai virus envelope
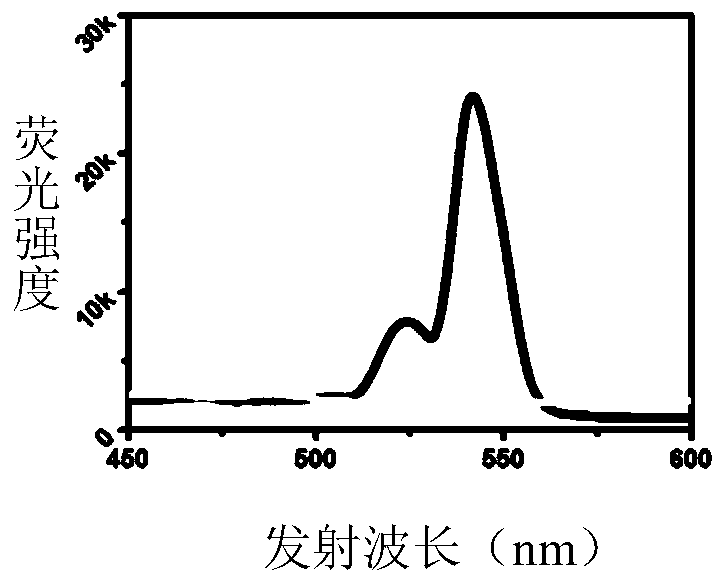
ActiveCN110846286AAchieving Marking EfficiencyEfficient expressionSsRNA viruses negative-senseVector-based foreign material introductionViral MarkersBinding site
The invention discloses a method of utilizing rare earth up-conversion fluorescent particles to mark sendai virus envelope. The method includes following steps: building efficient expression plasmid pCDH-BirA-AP-GPI containing biotin ligase (BirA), biotin binding site (APtag) and glycosylphatidylinositol cytomembrane anchoring sequence (GPI); synthesizing streptavidinized rare earth up-conversionfluorescent particles; preparing enveloped biotinylated sendai virus; utilizing the streptavidinized rare earth up-conversion fluorescent particles to mark the enveloped biotinylated sendai virus. Byadopting virus envelope marking technology, virus marking efficiency of 40-60% can be realized; by excited by near infrared light of 980nm, the rare earth up-conversion fluorescent particles can emitgreen fluorescence of 540nm, and observation can be realized under a confocal microscope.
Owner:TIANJIN UNIV
Reprogramming method for induced pluripotent stem cells
ActiveCN106916850AHigh reprogramming efficiencyLow immunogenicitySsRNA viruses negative-senseGenetically modified cellsVirus typeReprogramming
The invention relates to a reprogramming method for human induced pluripotent stem cells. The reprogramming method is more favorable for preclinical research and mainly employs Sendai virus-mediated infection of the human induced pluripotent stem cells and reprogramming. The reprogramming method provided by the invention has high reprogramming efficiency; and the obtained human induced pluripotent stem cells have the characteristics of low immunogenicity, high security and the like. Moreover, compared with reprogramming methods for lentivirus-mediated human induced pluripotent stem cells, the reprogramming method provided by the invention has the following advantage that the human induced pluripotent stem cells obtained by using the method is free of integration of exogenous genes and thus has higher safety.
Owner:ZONHON BIOPHARMA INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com