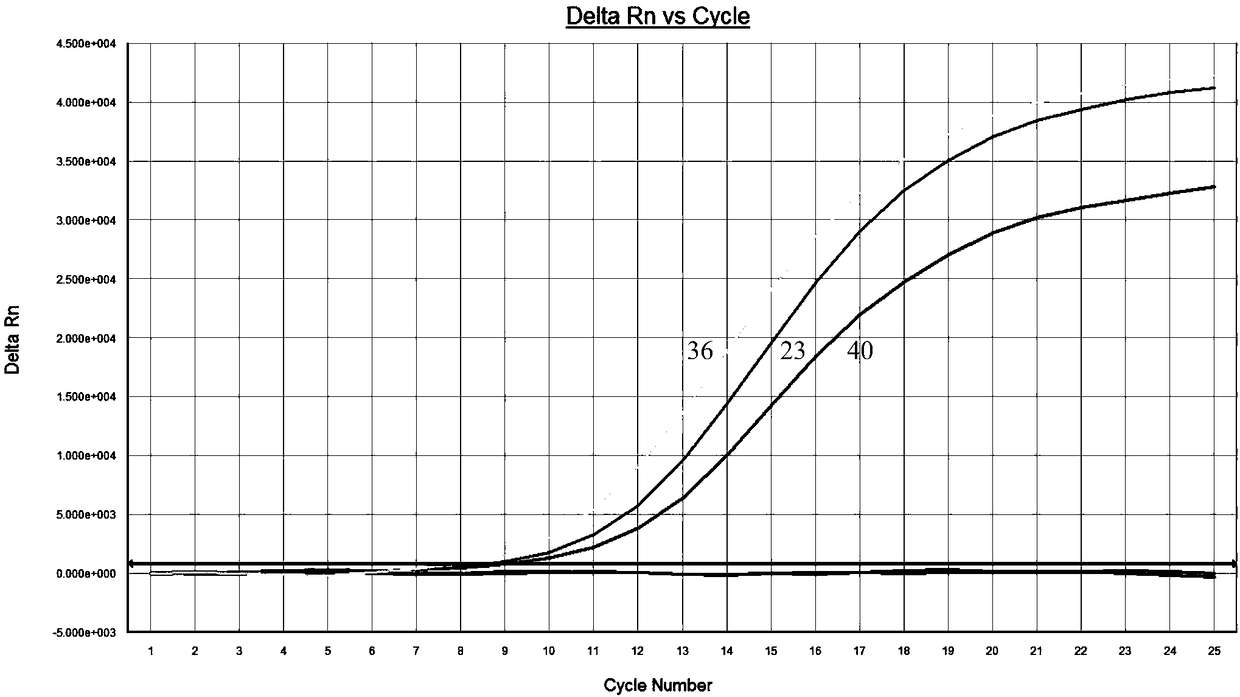
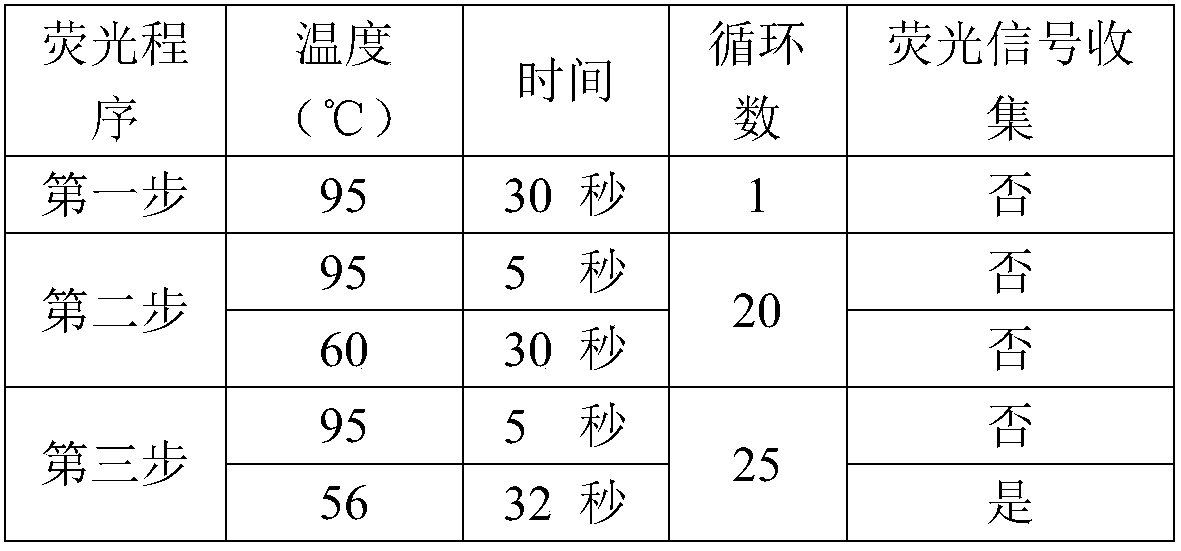
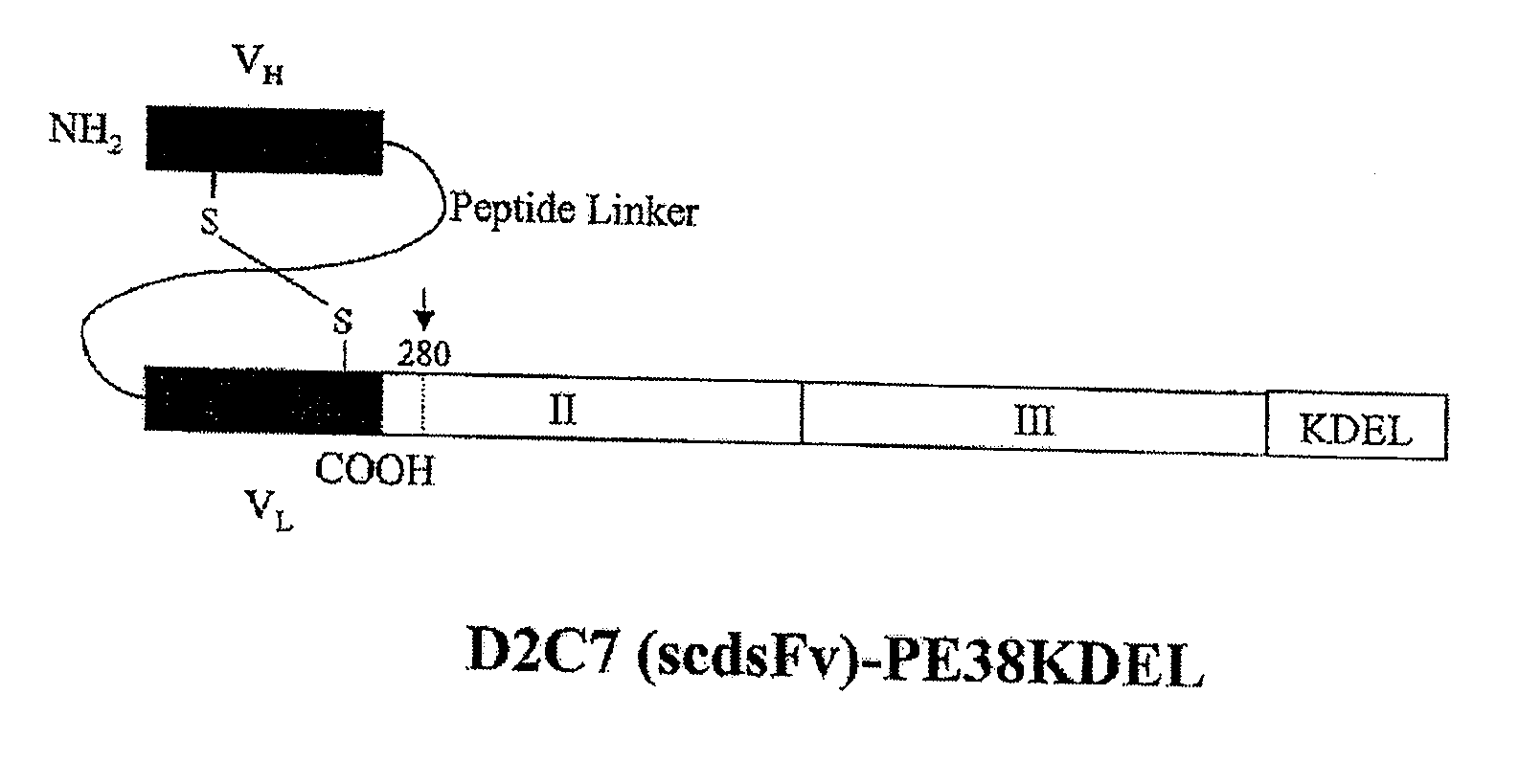
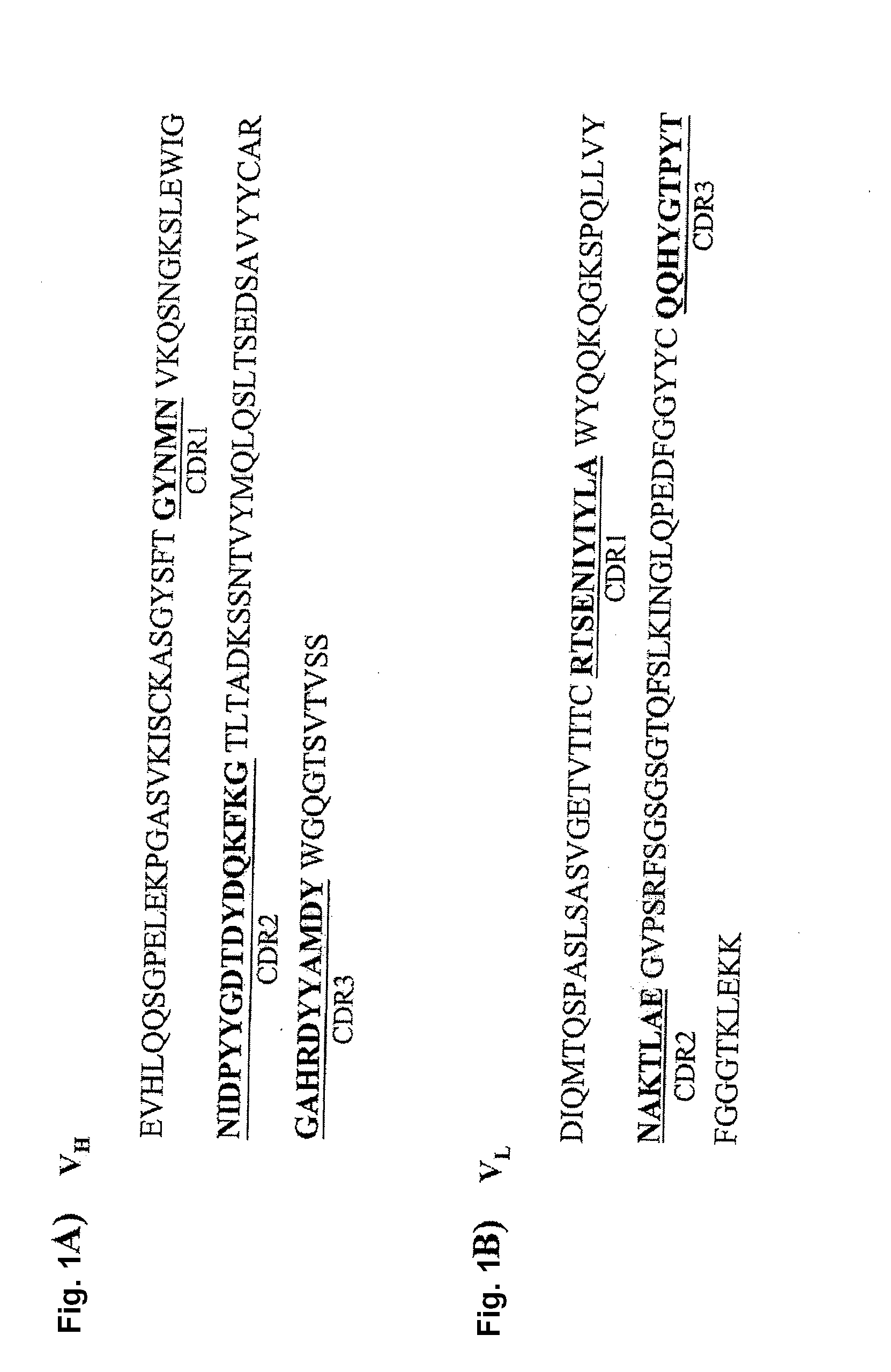
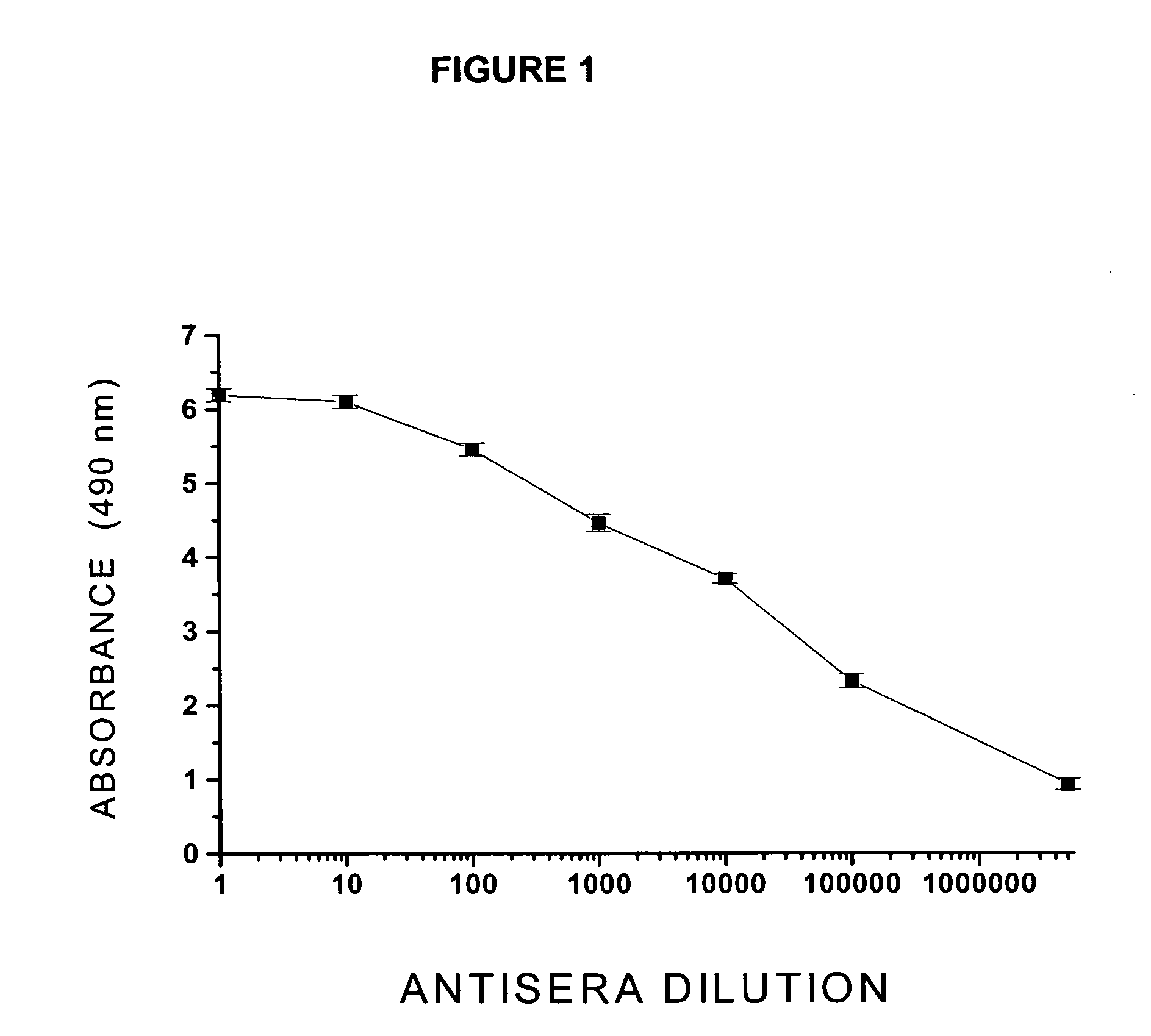
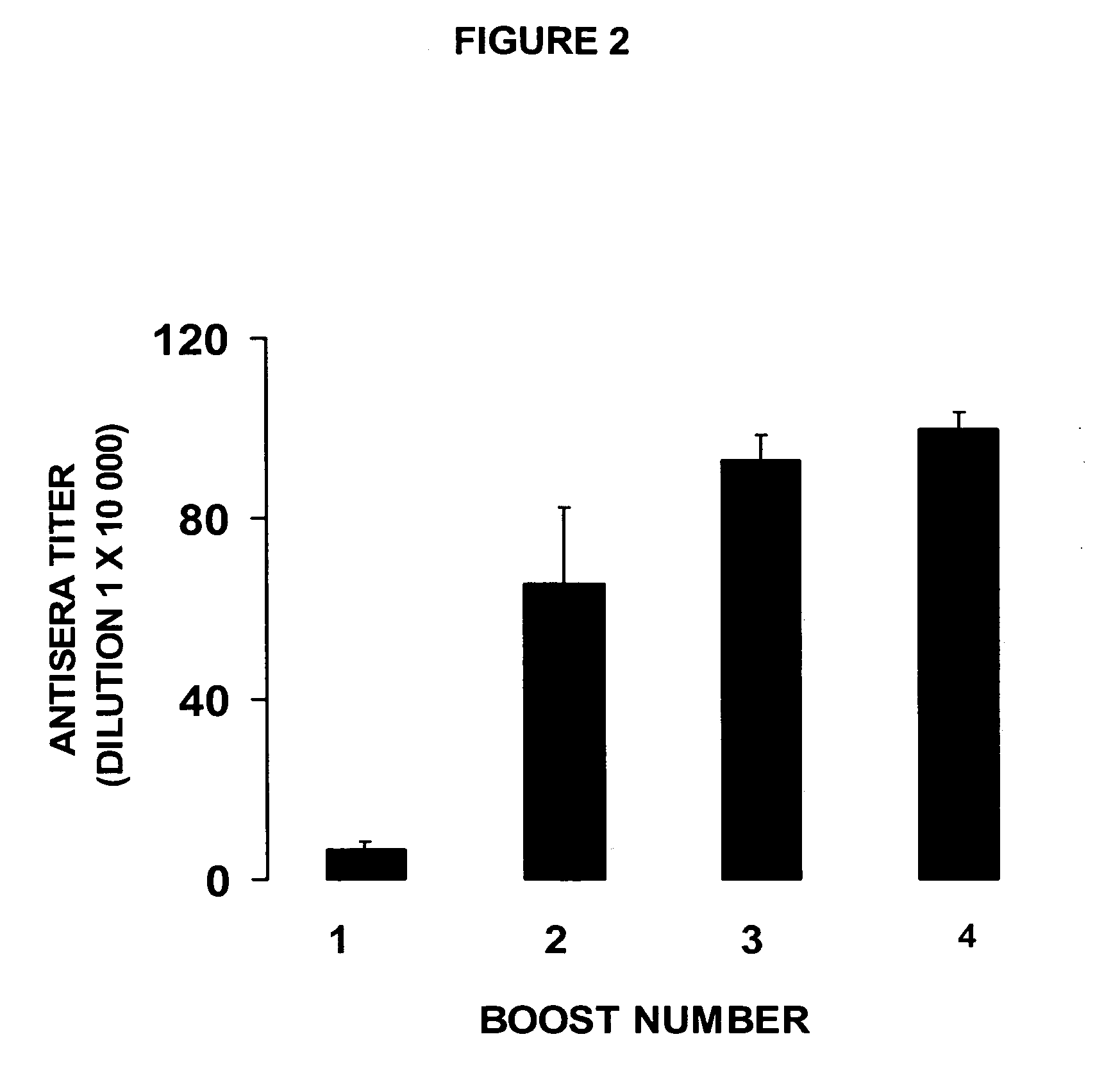
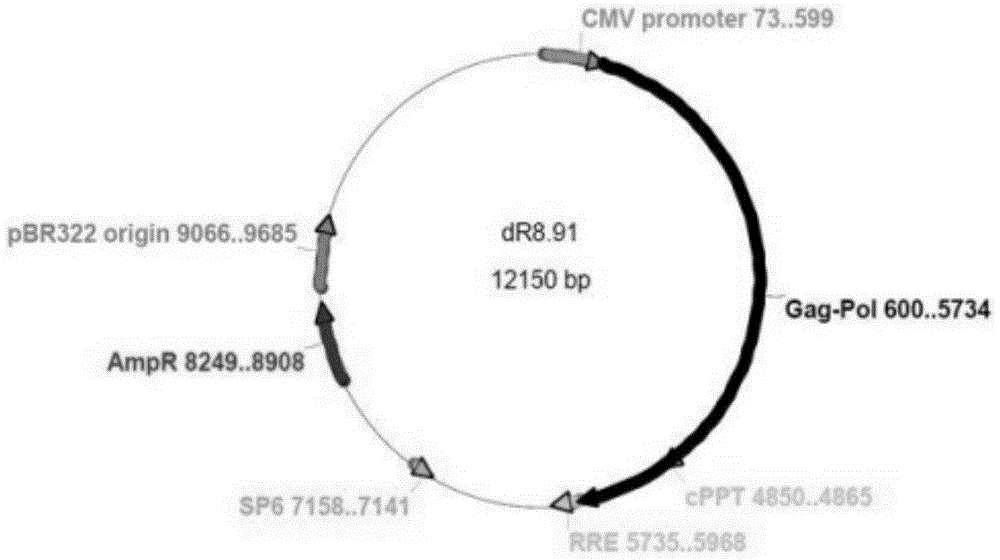
Patents
Literature
108 results about "Preclinical research" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preclinical Research. Preclinical is the step between discovery research and clinical trials. Because clinical trials are so expensive, preclinical research is an important step in selecting only those drug candidates that have the greatest chance of success. This work involves testing drug candidates in cell lines and in animal models...
Preparation of human umbilical cord mesenchymal stem cells wound surface smearing agent and storage application method
InactiveCN101451124APromote healingSolve source difficultiesMammal material medical ingredientsSkeletal/connective tissue cellsResuscitationUmbilical cord
The invention discloses a method for separating, purifying and culturing human umbilical mesenchymal stem cells and a method for preparing, storing and quickly preparing and applying a wound surface painting reagent of the human umbilical mesenchymal stem cells. The method comprises: firstly, separating, purifying and culturing the human umbilical mesenchymal stem cells; secondly, preparing a methyl cellulose composite culture medium; thirdly, preparing the wound surface painting reagent of the human umbilical mesenchymal stem cells; and fourthly, detecting the biological properties of the human umbilical mesenchymal stem cells. The method takes methyl cellulose as a substrate, mixes the human umbilical mesenchymal stem cells into the methyl cellulose, and generates the wound surface painting reagent of the human umbilical mesenchymal stem cells, and the wound surface painting reagent can be uniformly coated on the wound surface of the skin to form a coating. The invention performs preclinical researches such as animal toxicity test, pyrogen test and hypersensitive test, constructs a cryopreservation resuscitation method for human umbilical mesenchymal stem cells and a stem cell bank, and prepares for large-scale production and preparation of the wound surface painting reagent of the human umbilical mesenchymal stem cells and application of the wound surface painting reagent of the human umbilical mesenchymal stem cells.
Owner:戴育成
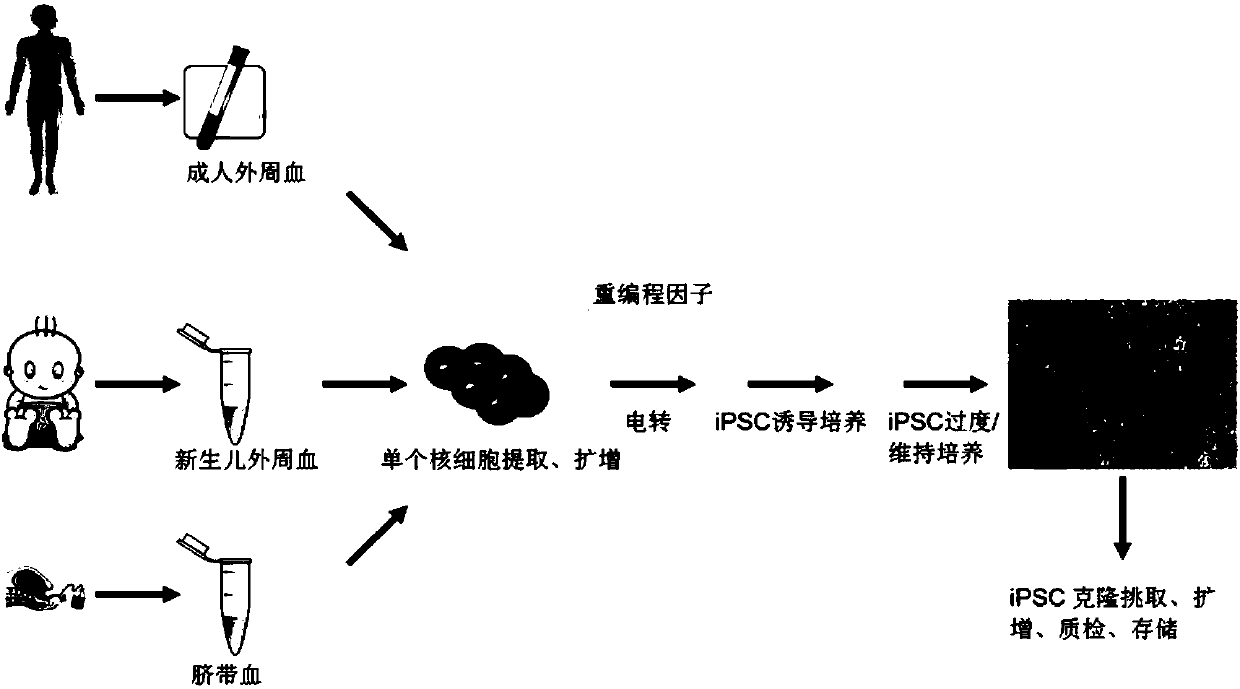
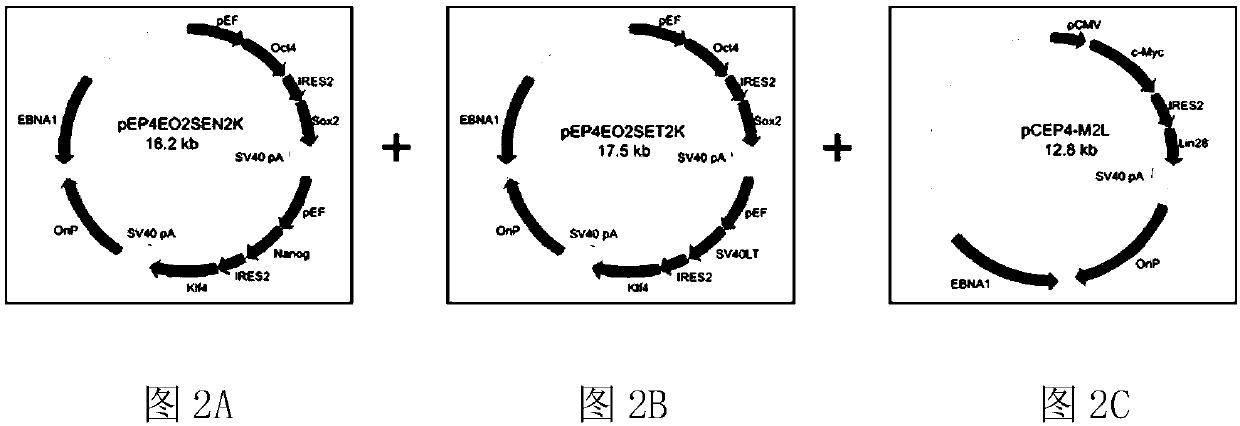
Efficient induced pluripotent stem cell reprogramming method for blood cells
PendingCN108085299ASample source is convenientAccumulation of genetic mutations is lowBlood/immune system cellsArtificially induced pluripotent cellsProgenitorRed blood cell
The invention belongs to the field of cells and in particular relates to an efficient induced pluripotent stem cell reprogramming method for blood cells. The method comprises the following steps: S1,extracting monocyte from a blood specimen, and performing selective culture with an amplification culture medium so as to obtain erythrocyte progenitor cells; S2, introducing a free carrier with at least one potentiality determinant factor into the obtained erythrocyte progenitor cells; S3, culturing the erythrocyte progenitor cells with the free carrier by using a pluripotent stem cell inductionculture medium, and performing induction in a feeding layer free system so as to obtain reprogrammed intermediate state cells; S4, after induction is completed, replacing the pluripotent stem cell induction culture medium in the step S3 by a pluripotent stem cell culture medium to maintain culture, thereby obtaining cells that potentiality determinant factor expression is vanished and expression of endogenous pluripotent genes POU5F1, NANOG, TRA-1-60 and TRA-1-81 is activated, namely the induced pluripotent stem cells. The method has the beneficial effects that pluripotent stem cells without endogenous gene components can be efficiently induced, and the method is applicable to preclinical study and clinical application.
Owner:安徽中盛溯源生物科技有限公司
Non-invasive detection method and kit for early screening bladder cancer by multi-gene combination
PendingCN108531594AHigh acceptanceImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMultiple Tumor Suppressor-1Non invasive
The invention discloses a non-invasive detection method and a kit for early screening a bladder cancer by multi-gene combination. The combined genes include APC, NID2 and p16, and specific methylationprimers are designed to detect three nucleotide sequences of target gene fragment methylation of the APC, the NID2 and the p16, so that early screening and auxiliary diagnosis of the bladder cancer are achieved. Methylation levels of three genetic markers of the APC, the NID2 and the p16 in urinary sediments are analyzed by a three-channel fluorescent quantitative PCR (polymerase chain reaction)method, and a positive or negative result of a sample is judged according to a reported CT (computed tomography) value. The non-invasive detection method can detect 90% of early bladder cancers, 64% of precancerous adenomas (larger than or equal to 1 centimeter) in preclinical study, specificity reaches 100%, invasiveness is completely omitted, the method only needs to collect urine of 50mL, and acceptability of throngs wearing no symptoms is high.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
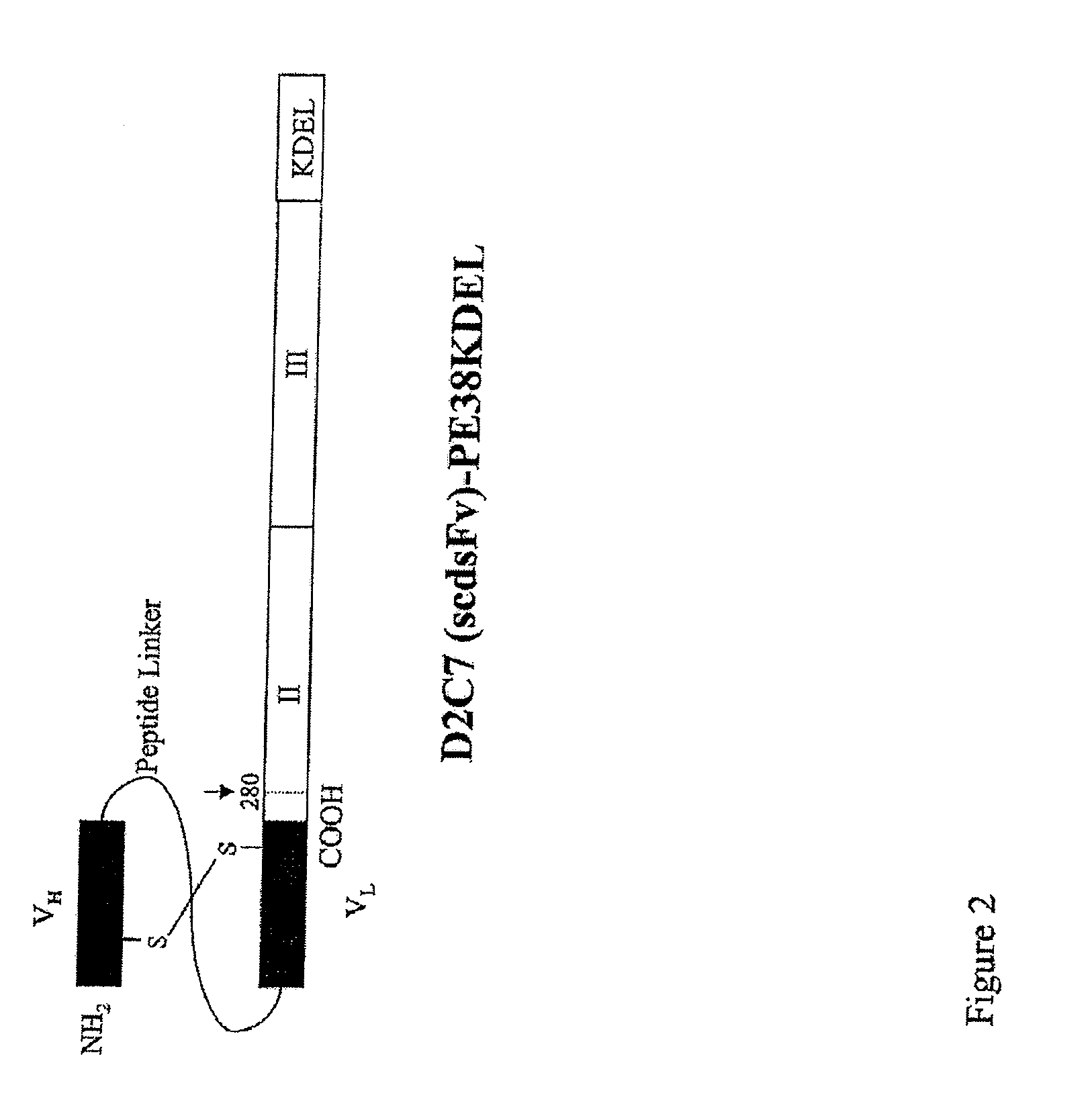
Dual Specific Immunotoxin for Brain Tumor Therapy
InactiveUS20130022598A1Polypeptide with localisation/targeting motifHybrid immunoglobulinsSingle-Chain AntibodiesAntiendomysial antibodies
We tested the in vitro and in vivo efficacy of a recombinant bispecific immunotoxin that recognizes both EGFRwt and tumor-specific EGFRvIII receptors. A single chain antibody was cloned from a hybridoma and fused to toxin, carrying a C-terminal peptide which increases retention within cells. The binding affinity and specificity of the recombinant bispecific immunotoxin for the EGFRwt and the EGFRvIII proteins was measured. In vitro cytotoxicity was measured. In vivo activity of the recombinant bispecific immunotoxin was evaluated in subcutaneous models and compared to that of an established monospecific immunotoxin. In our preclinical studies, the bispecific recombinant immunotoxin, exhibited significant potential for treating brain tumors.
Owner:DUKE UNIV
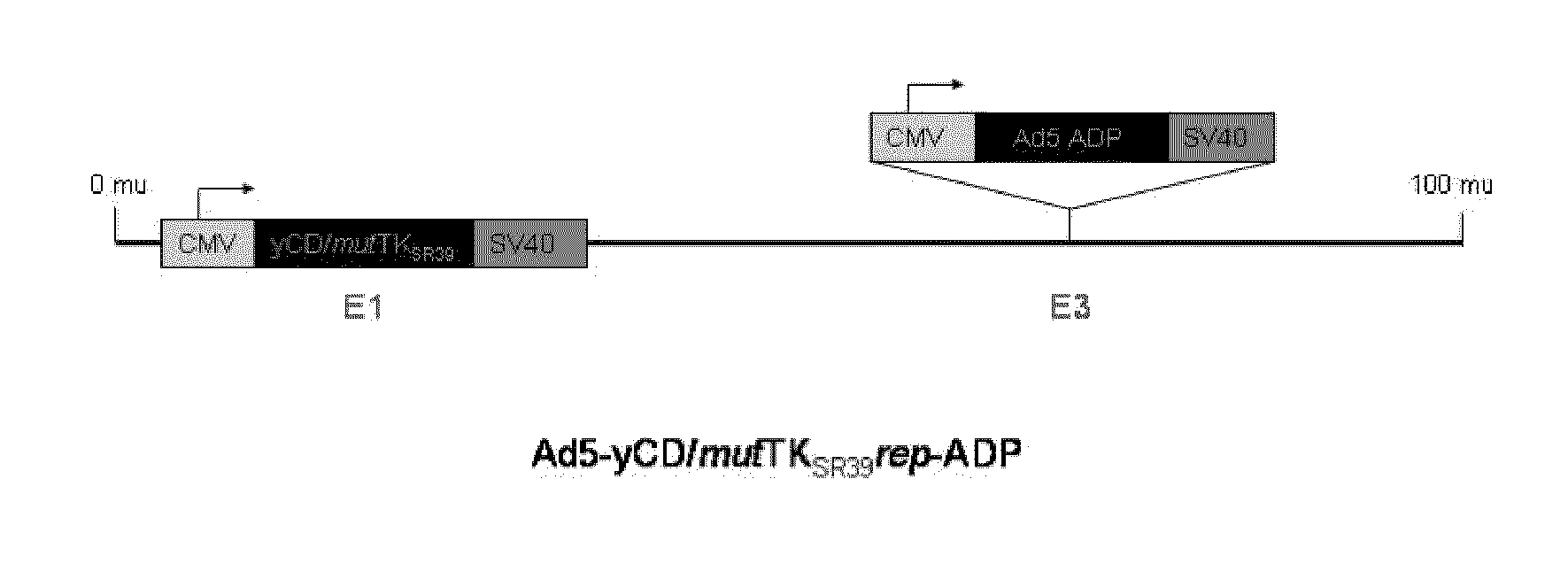
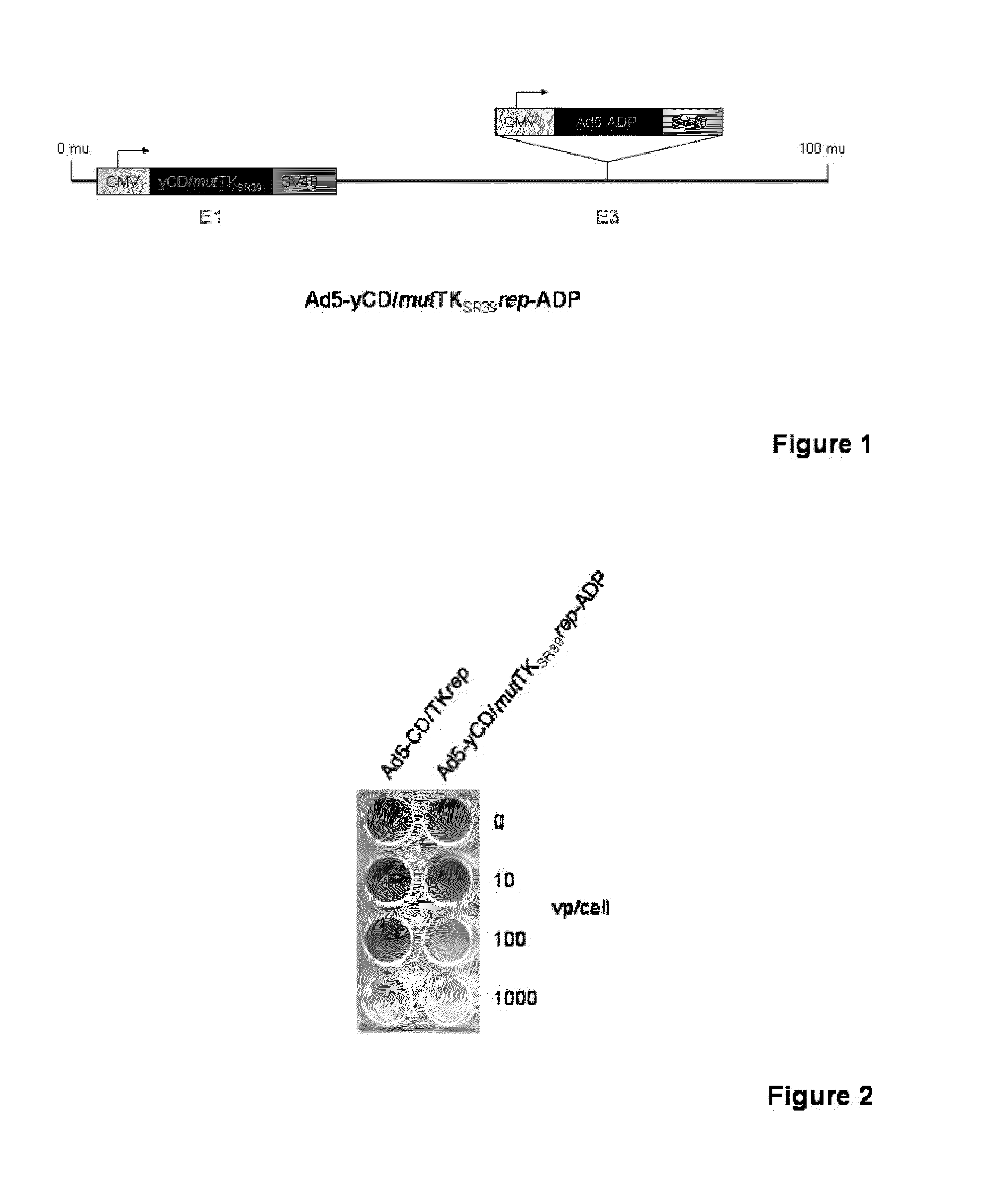
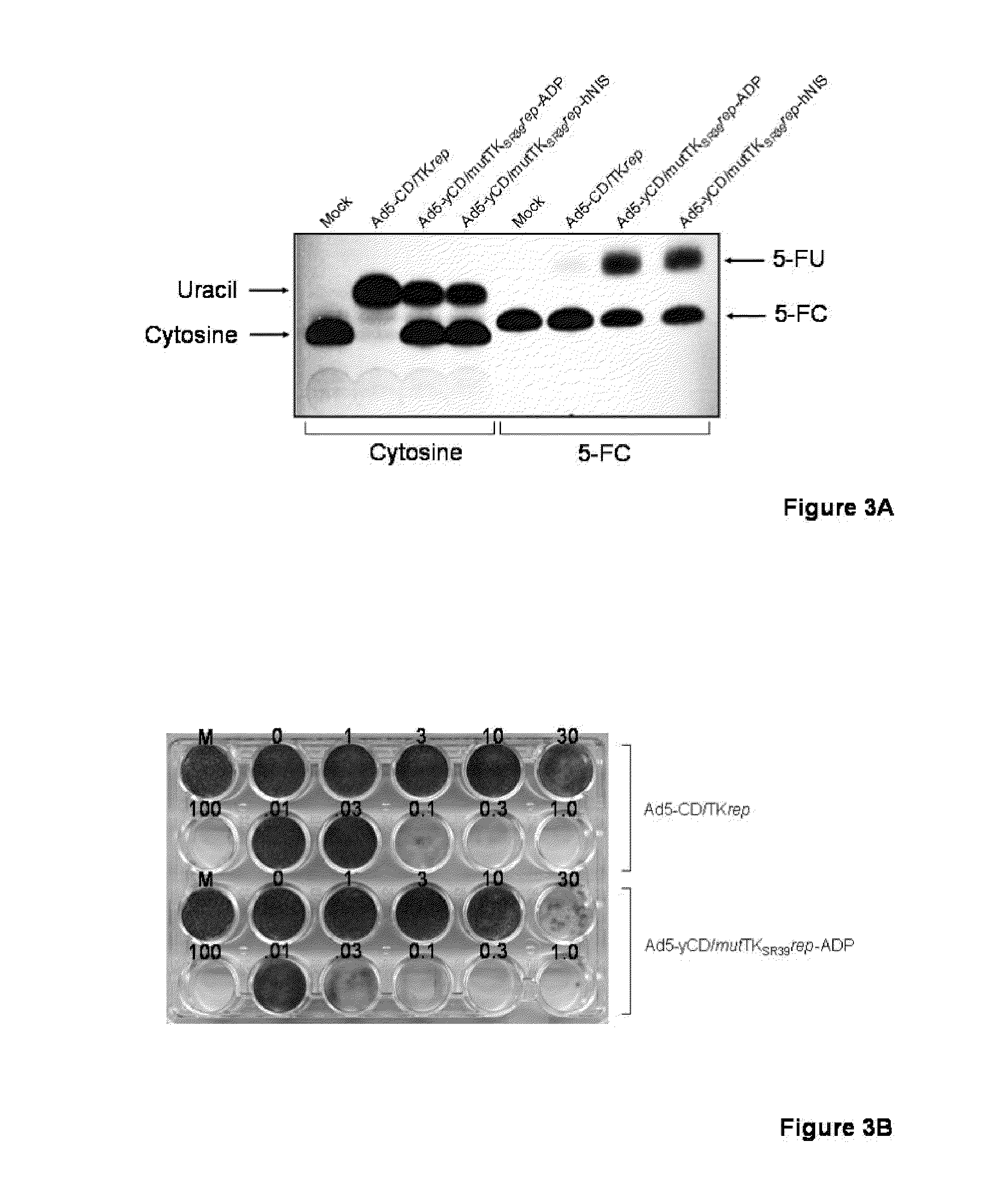
Methods and compositions for cancer therapy using a novel adenovirus
Owner:HENRY FORD HEALTH SYST
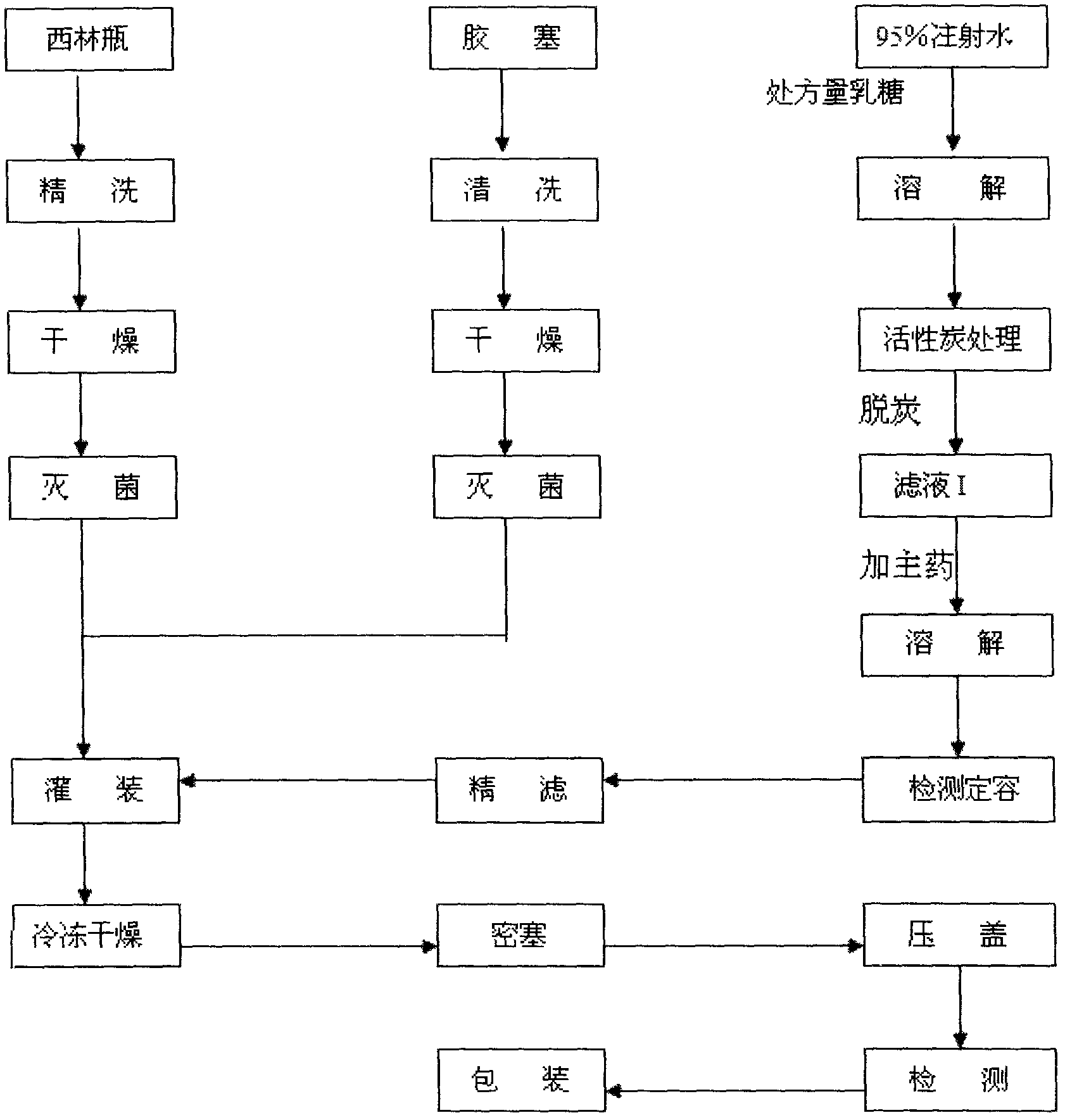
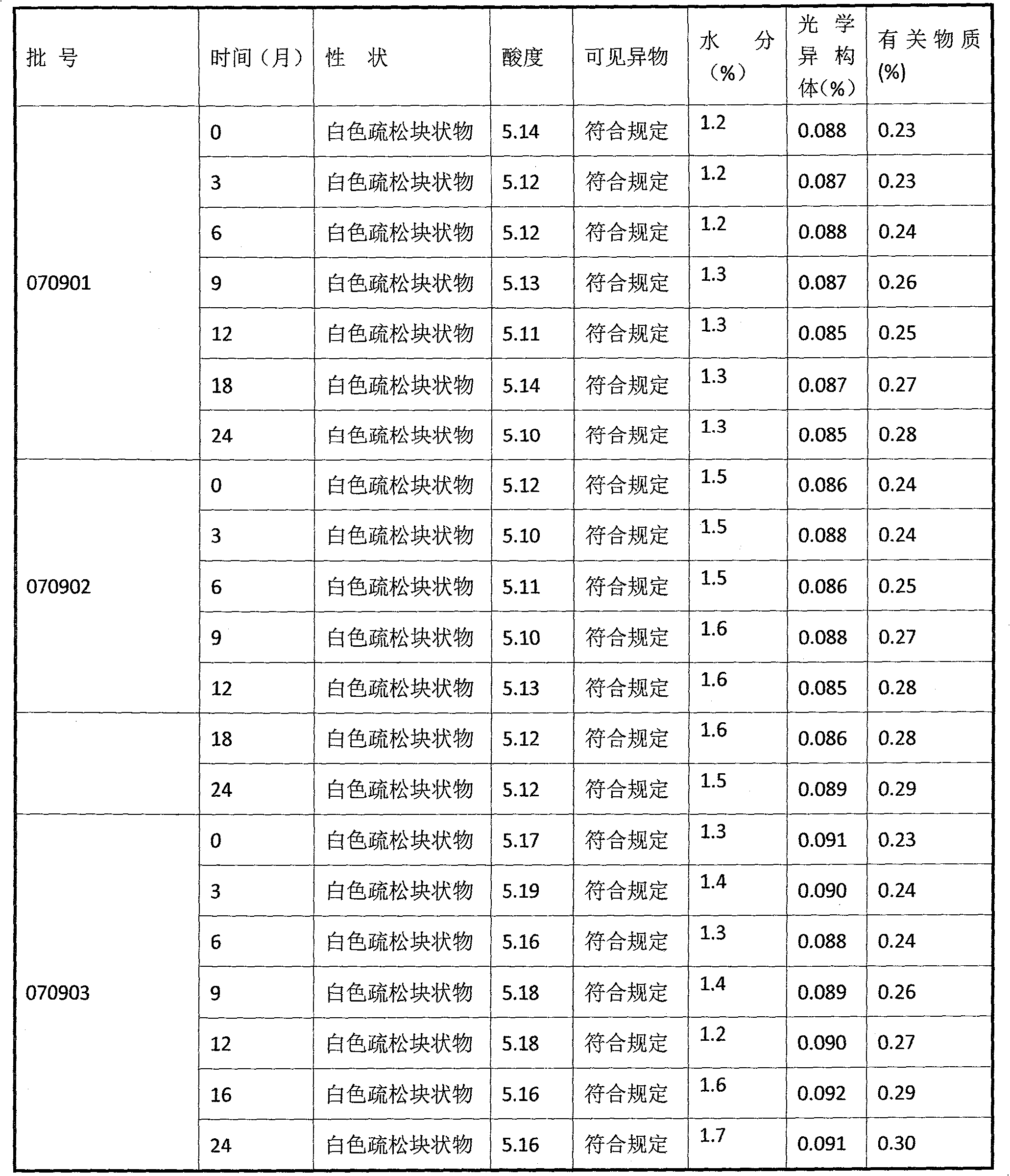
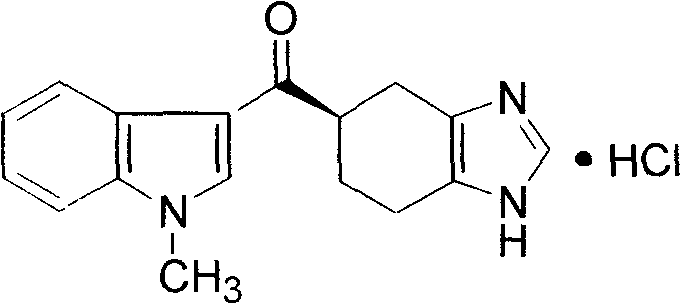
Ramosetron hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN103211771AEasy to shapeLoose appearancePowder deliveryOrganic active ingredientsTreatment effectFreeze-drying
The invention discloses a ramosetron hydrochloride freeze-dried powder injection and a preparation method thereof. Under illumination, the solution of the existing ramosetron hydrochloride has poor stability, poordrug effects and high drug adverse effects; and the solids of the existing ramosetron hydrochloride have good stability. Therefore, the ramosetron hydrochloride freeze-dried powder injection improves product stability and safety. A preclinical research shows that an antagonistic activity of ramosetron hydrochloride to 5-HT3 acceptors is stronger and more stable than that of the existing 5-HT3 acceptor antagonist; and ramosetron hydrochloride has stronger effect of treatment on chemotherapeutic drug-caused emesis. Therefore, ramosetron hydrochloride is clinically used for preventing and treating digestive tract symptoms such as nausea and emesis caused by cancer-resistant drugs.
Owner:ZHEJIANG YATAI PHARMA
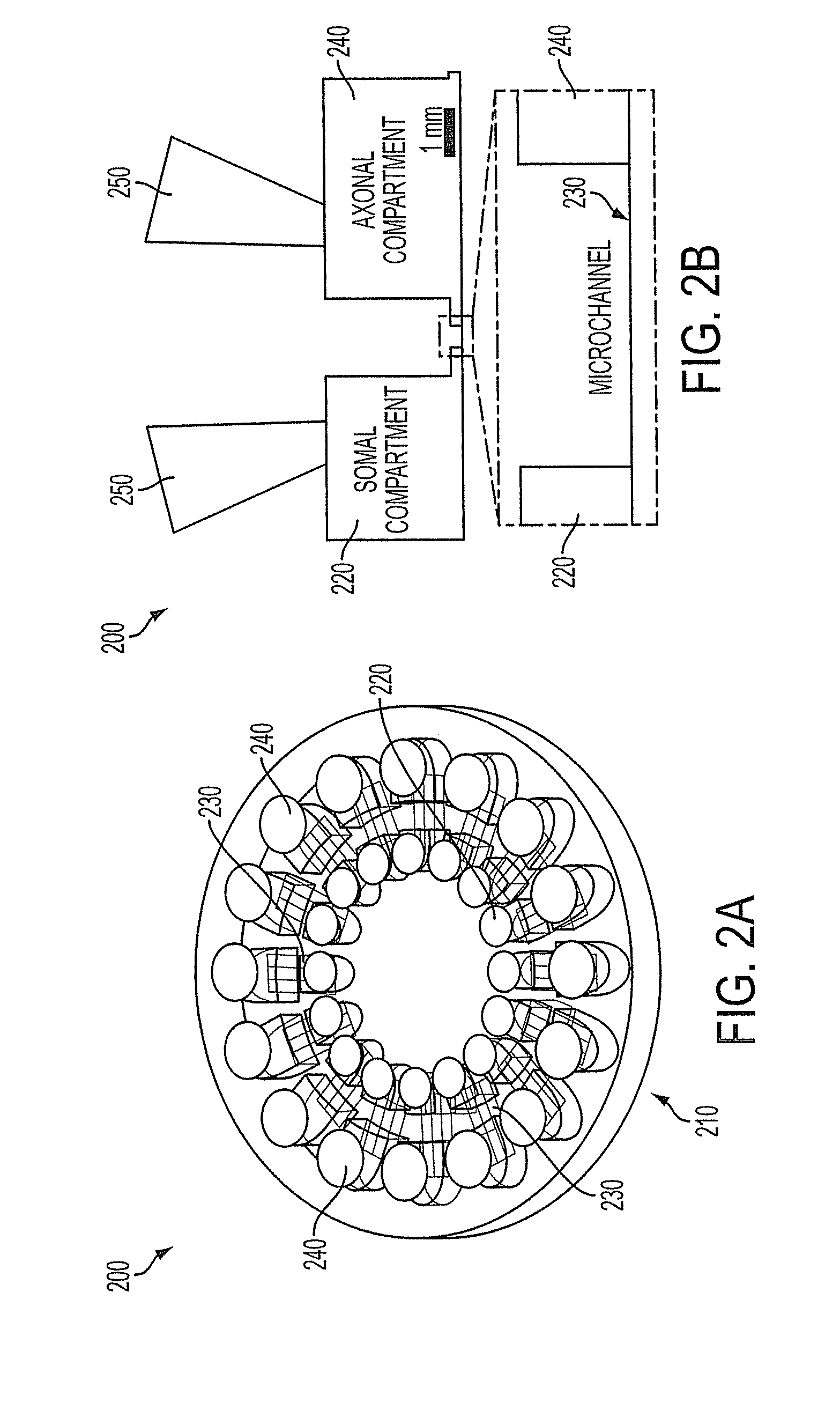
Compartmentalized Nerve Culture System
InactiveUS20100323434A1Bioreactor/fermenter combinationsBiological substance pretreatmentsClinical researchToxicology studies
The present invention relates generally to a compartmentalized nerve culture system. The compartmentalized nerve culture system has numerous applications including isolation of axons and cell bodies. The present invention has broad application for basic and pre-clinical research including, but not limited to, use in neuroscience, neuronal culture systems, co-culture systems, drug screening, morphological studies, and toxicology studies.
Owner:YANG IN HONG
Efficient mesenchymal stem cell culture solution without serum component
InactiveCN109402050APromote growthGuaranteed feasibilitySkeletal/connective tissue cellsCell culture active agentsIfn alphaHuman platelet
The invention relates to the technical field of biology, in particular to a mesenchymal stem cell culture solution. The mesenchymal stem cell culture solution is prepared from the following components: human platelet lysate, mycillin (Pen Strep), long-acting glutamine, a chemotactic factor XCL1, a chemotactic factor CCL3, a heat shock protein (HSP70), a telomerase inhibitor IFN-alpha 2b and a basal culture medium DMEM. The components of the stem cell culture solution are wide in source, cultured mesenchymal stem cells are high in purity, quick in proliferation and good in dryness, and the mesenchymal stem cell culture solution is suitable for extracorporeal large-scale culture to conduct preclinical study and related clinical study.
Owner:沈阳中心血站
Cell strain MSCs for overexpression of Nrf2 gene as well as preparation method and application of cell strain MSCs
InactiveCN104877967AStrong anti-apoptotic propertiesMicrobiological testing/measurementUnknown materialsEnzyme digestionHuc mscs
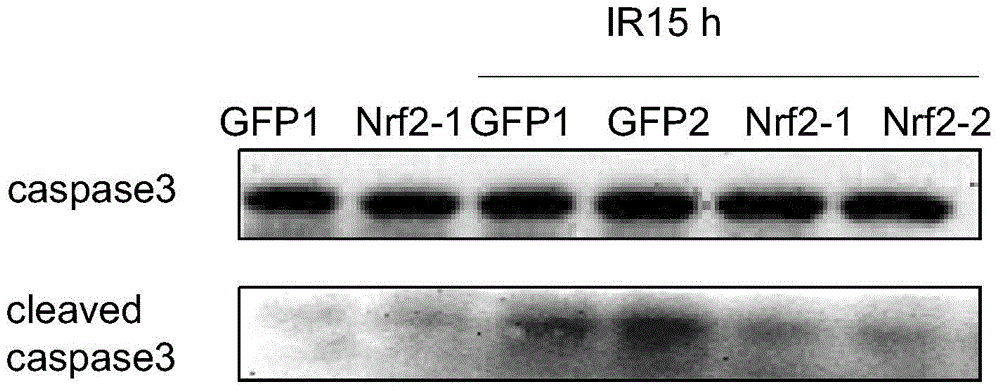
The invention discloses a cell strain MSCs for overexpression of an Nrf2 gene as well as a preparation method and application of the cell strain MSCs. The preparation method comprises the following steps: performing PCR amplification on a human-derived Nrf2 gene ORF; performing purification and double enzyme digestion on the PCR product obtained in the step (1), then connecting the PCR product with the framework plasmid pLV-CMV-XbaI-BamHI-GFP to construct an Nrf2 slow virus recombinant vector, and after conversion, selecting clone and determining positive clone; sequencing the positive clone, and after no mutation is determined, performing amplification massively; leading the recombinant vector and an auxiliary plasmid in a 293 FT cell to obtain a virus; using the virus to infect the 3-5 th generation hUC-MSCs cells so as to obtain the cell strain MSCs for overexpression of the Nrf2 gene. According to the invention, the overexpression of Nrf2 in MSCs is stably performed to increase the cell activity, and a higher anti-apoptosis feature is also realized under the anoxic and oxidative stress conditions; Nrf2-MSCs with the treatment level amount is obtained in vitro and can serve as an excellent tool cell for preclinical study of MSCs and can be used for preparing a medicine for enhancing preclinical study of MSCs transplanting.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Microwave reactor
InactiveUS20110189056A1High microwave field uniformityEnergy based chemical/physical/physico-chemical processesElectric/magnetic/electromagnetic heatingField uniformityMicrowave
A microwave reactor for processing a flow of a mixture, said reactor comprising a reaction chamber having an unpressurized interior and a reaction block disposed within the interior of the reaction chamber, with at least one antenna disposed within the interior of the chamber; and at least one generator of electromagnetic radiation connected to the antenna so that the flow may circulate through said reaction block, and the generator generates a radiation that is uniformly and homogeneously propagated in the chamber and is evenly absorbed by the mixture. The present invention provides embodiments of microwave reactors capable of processing reaction volumes normally found in early stage drug development and pre-clinical studies, as well as embodiments sufficient for commercial production, the designs disclosed incorporate process controls as well as high microwave field uniformity. The reactor may process batches, or may be a flow through design, or a ‘stop flow’ design whereby flow is admitted, a batch is processed and the flow is re-started.
Owner:UPSCALE HLDG
Sodium citrate injection for tube-enveloping and method for preparing the same
InactiveCN101112627ASignificant double control effectDefinite curative effectSurgeryPharmaceutical containersPharmacological actionPreclinical research
The present invention relates to a formula of the tube-sealing solution sodium citrate injection and the preparation method and the usage. The in vitro central venous catheter simulation device designed by the present invention has simple equipment, low cost and good simulation, which provides the test device for the in vitro screening of the tube-sealing solution for the pre-clinical studies of the tube-sealing solution. The screened tube-sealing solution has the dual-function of the prevention and treatment of the blood coagulation and anti-infection, the pharmacological effect is stronger, the efficacy is accurate, the nature is stable; furthermore, the present invention has safety, low toxicity, controllable quality, direct use and better effect, which can overcome the two difficulties of blood coagulation and infection in the sealing tube, avoid the adverse effects of antibiotics to the patients and have significant advantages compared with the similar products. In addition, the sources of the raw materials are rich, the preparation process is simple, the feasibility is strong, so the present invention is applicable to large-scale production and easy to popularize. The demand of the blood dialysis is increasing along with the increasing number of the patients with the medium and end-stage renal failure year by year; therefore the present invention can generate tremendous social and economic benefits in a short period.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Multiple-target effects of isoflavone derivative and its application in improvement of learning and memory
ActiveCN104095849ARegulating neuroinflammationImprove learning and memory functionOrganic active ingredientsNervous disorderDamage repairDisease injury
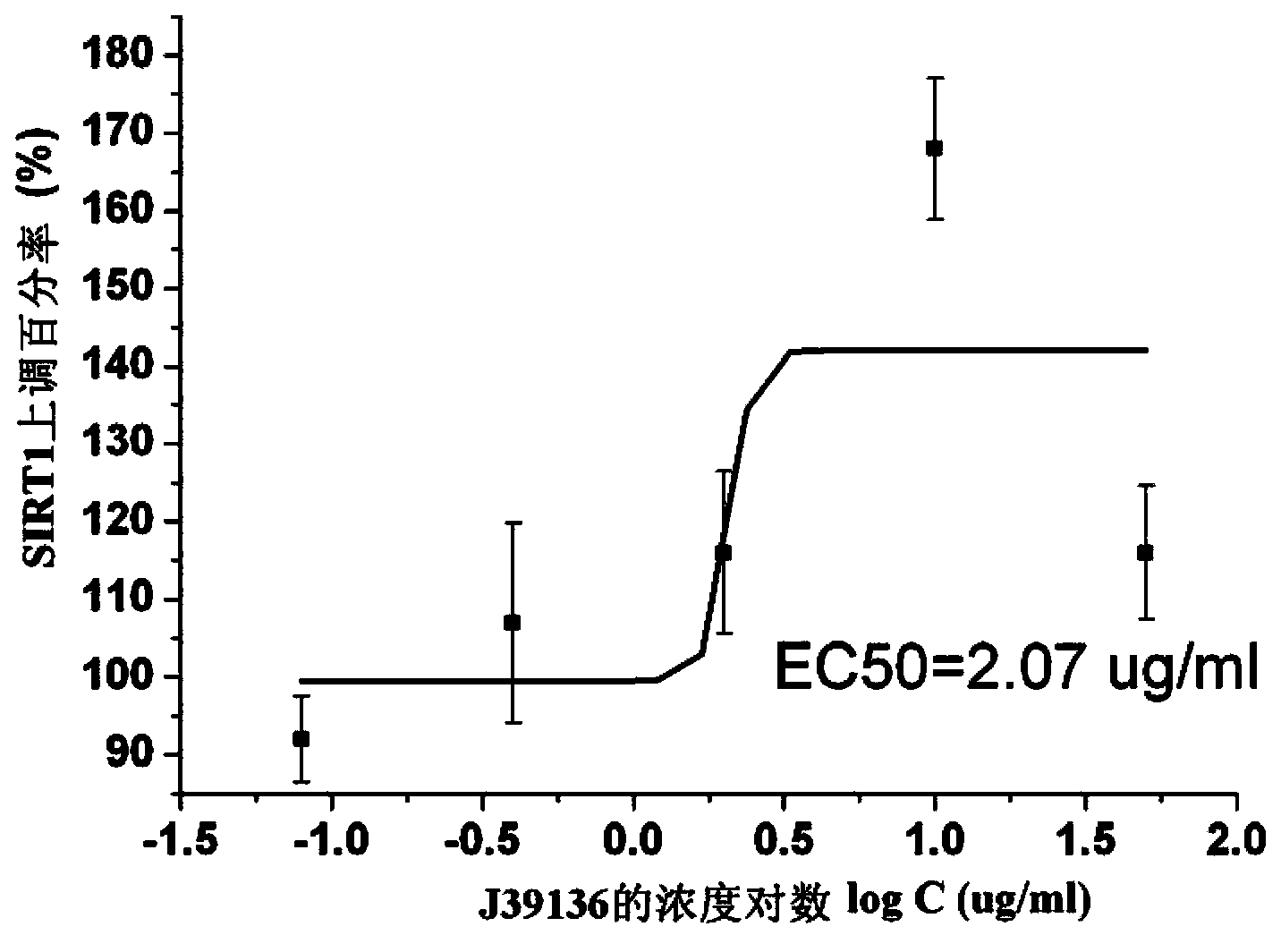
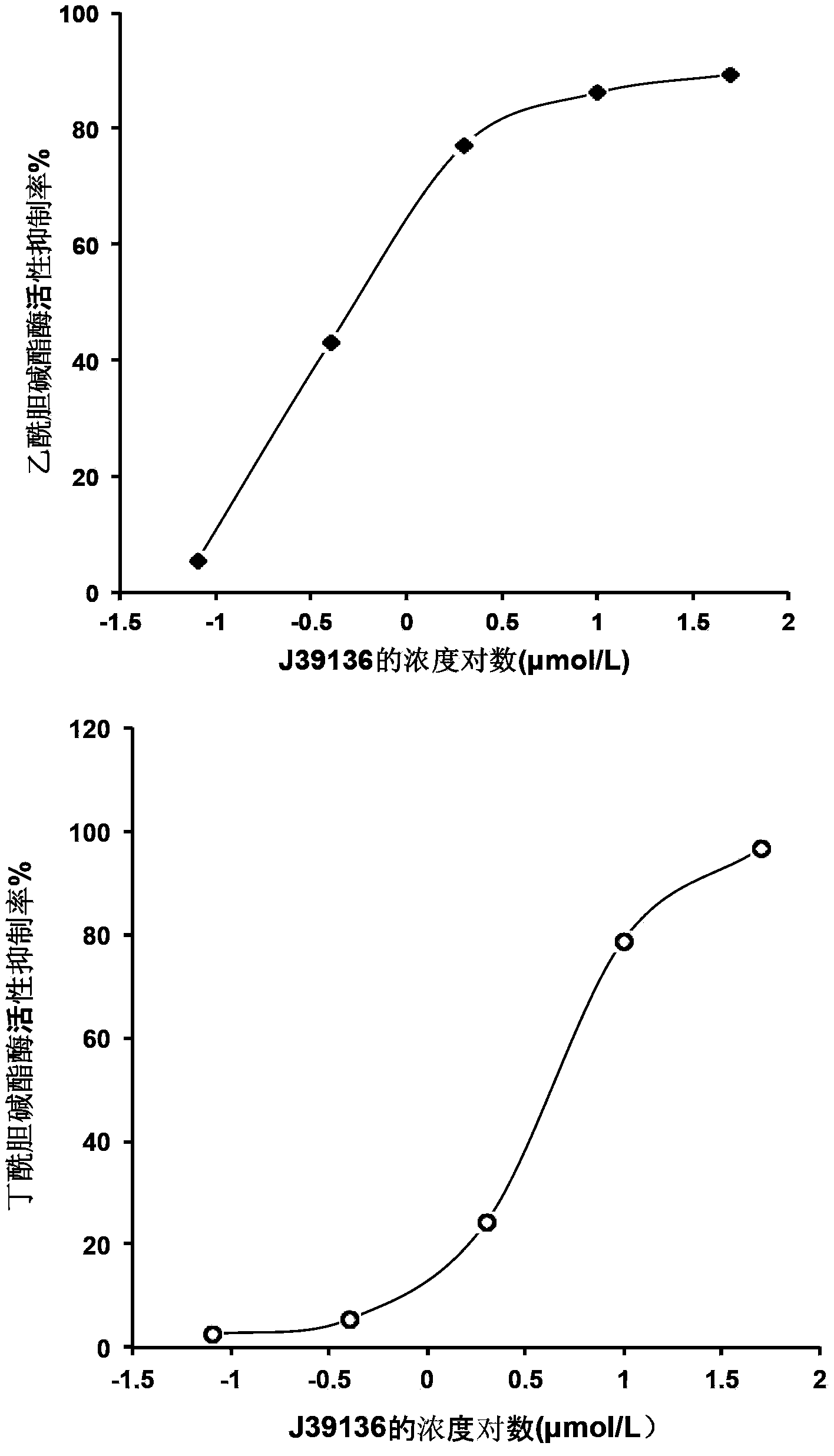
The invention discloses multiple-target effects of an isoflavone derivative and its application in improvement of learning and memory. Specifically, the invention discloses that a compound J39136 has the effects of: upregulating the expression of SIRT1 protein with damage repair and neuroprotective effects; inhibiting the activity of acetylcholinesterase and butyrylcholinesterase, and dose-dependently inhibiting the acetylcholinesterase activity of cells under a safe dose; protecting nerve cells, inhibiting abnormal expression of beta-APP, and reducing Abeta1-42 secretion; and regulating neuroinflammation. Animal experimental results prove that J39136 has low toxicity, can penetrate the blood-brain barrier, and can significantly improve scopolamine caused mouse dementia and strengthen learning and memory functions. Preclinical study results prompt that J39136 is expected to become the drug for prevention and / or treatment of learning and memory disorder and Alzheimer's disease.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Ph-weighted MRI using fast amine chemical exchange saturation transfer (CEST) imaging
ActiveUS20180164393A1Easy to optimizeReduce scan timeDiagnostic recording/measuringAnalysis using nuclear magnetic resonanceMetaboliteNon invasive
A pH-weighted chemical exchange saturation transfer (CEST) magnetic resonance imaging (MRI) method and system are provided that works by indirectly measuring the NMR signal from amine protons found on the backbones of amino acids and other metabolites, which resonate at a frequency of +2.8-3.2 ppm with respect to bulk water protons. The technique uses a modified magnetization transfer radiofrequency saturation pulse for the generation of image contrast. A train of three 100 ms Gaussian pulses at high amplitude (6 uT) or Sinc3 pulses are played at a particular frequency off-resonance from bulk water prior to a fast echo planar imaging (EPI) readout, with one full image acquired at each offset frequency. This non-invasive pH-weighted MRI technique does not require exogenous contrast agents and can be used in preclinical investigations and clinical monitoring in patients with malignant glioma, stroke, and other ailments.
Owner:RGT UNIV OF CALIFORNIA
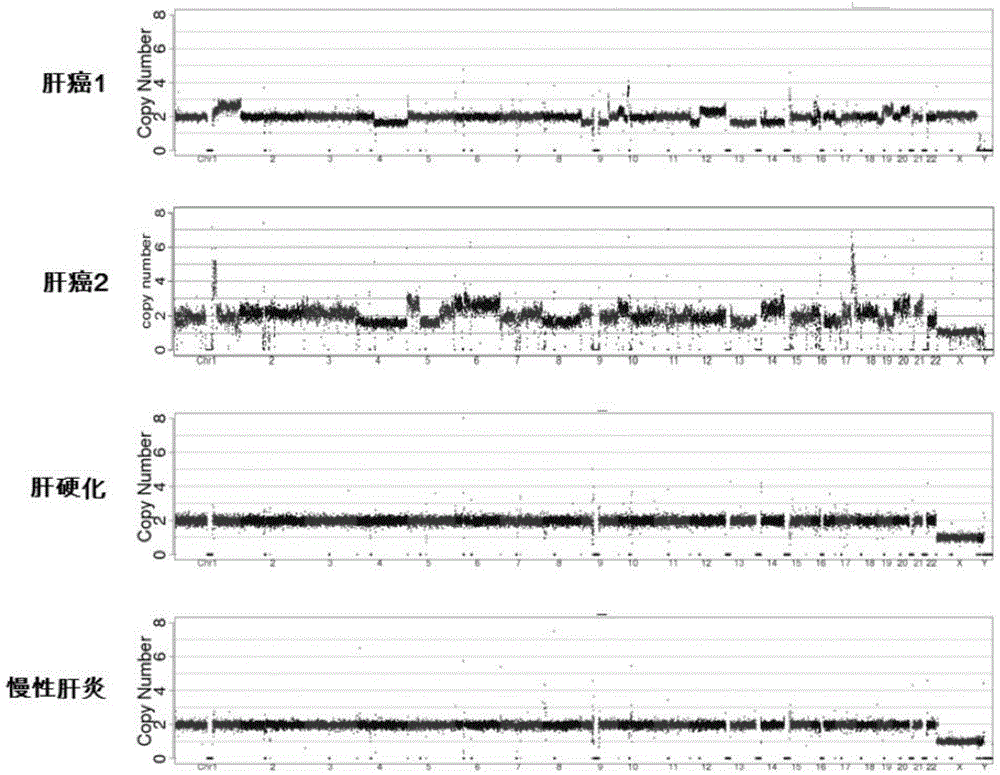
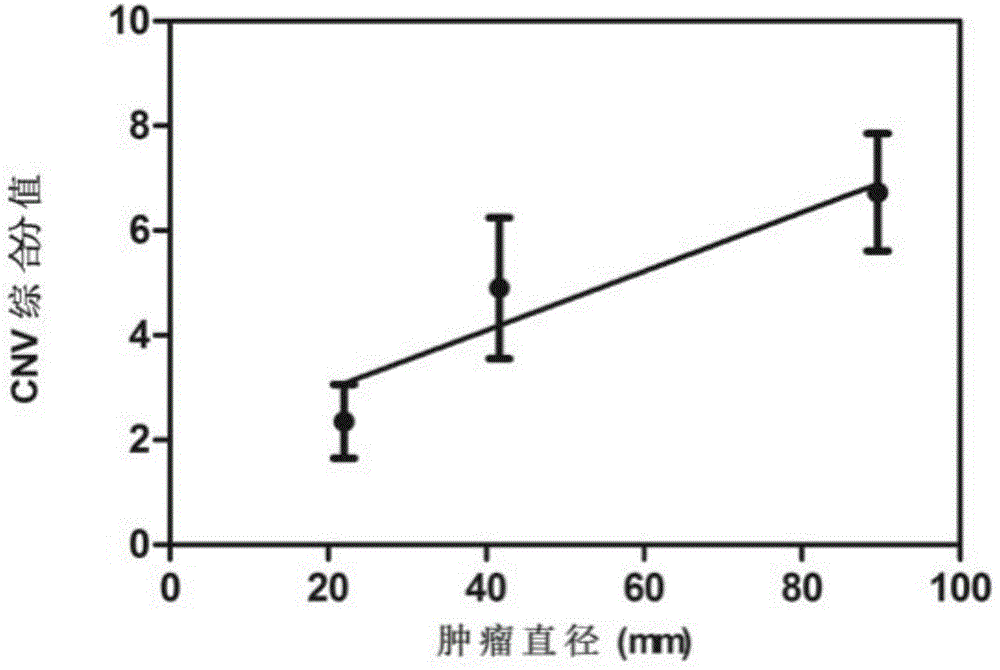
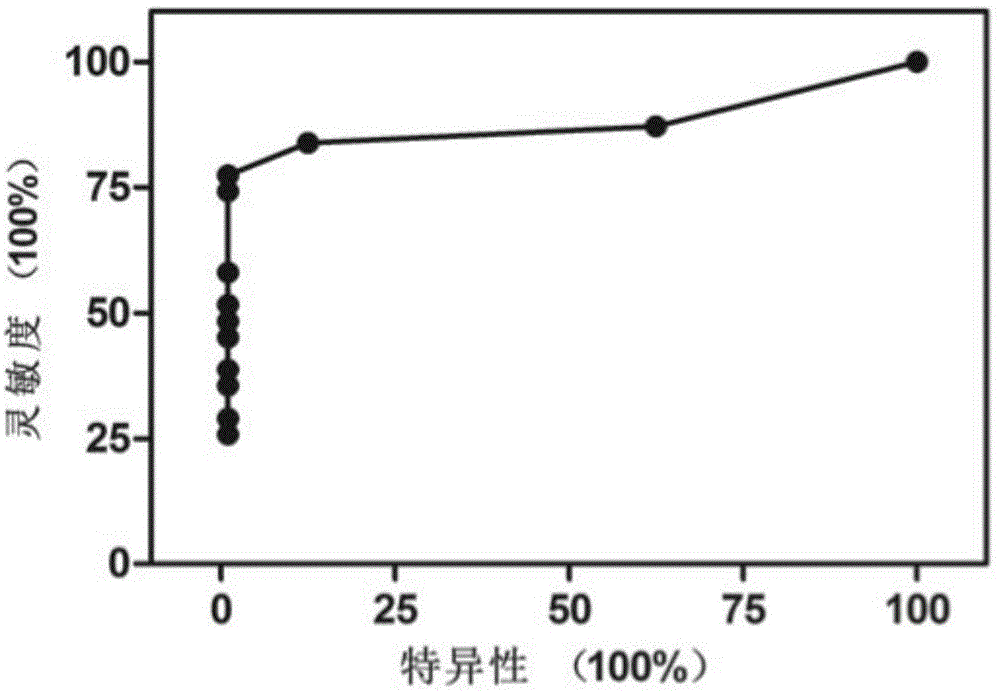
Noninvasive human liver cancer early detection and differential diagnosis method and system
InactiveCN104313136AMicrobiological testing/measurementLibrary creationGenomic sequencingBlood plasma
The invention discloses a noninvasive human liver cancer early detection and differential diagnosis method which comprises the following steps: 1) extracting free DNA of plasma from in-vitro plasma, and constructing a genomic sequencing library; 2) sequencing the DNA, measuring the DNA sequence of the genome, and performing sequence alignment; 3) by taking different genome sequence lengths as basic analysis units, performing chromosome copy number variation CNV analysis, and constructing chromosome CNV graphs to observe whether visual observable copy number variation exists; and 4) by taking the different genome sequence lengths as basic analysis units, performing Z-score analysis of chromosome copy number variation CNV. The detection method disclosed by the invention can be used for early detection and differential diagnosis of liver cancer and is used for preclinical study or clinical detection.
Owner:YIKONGENOMICS
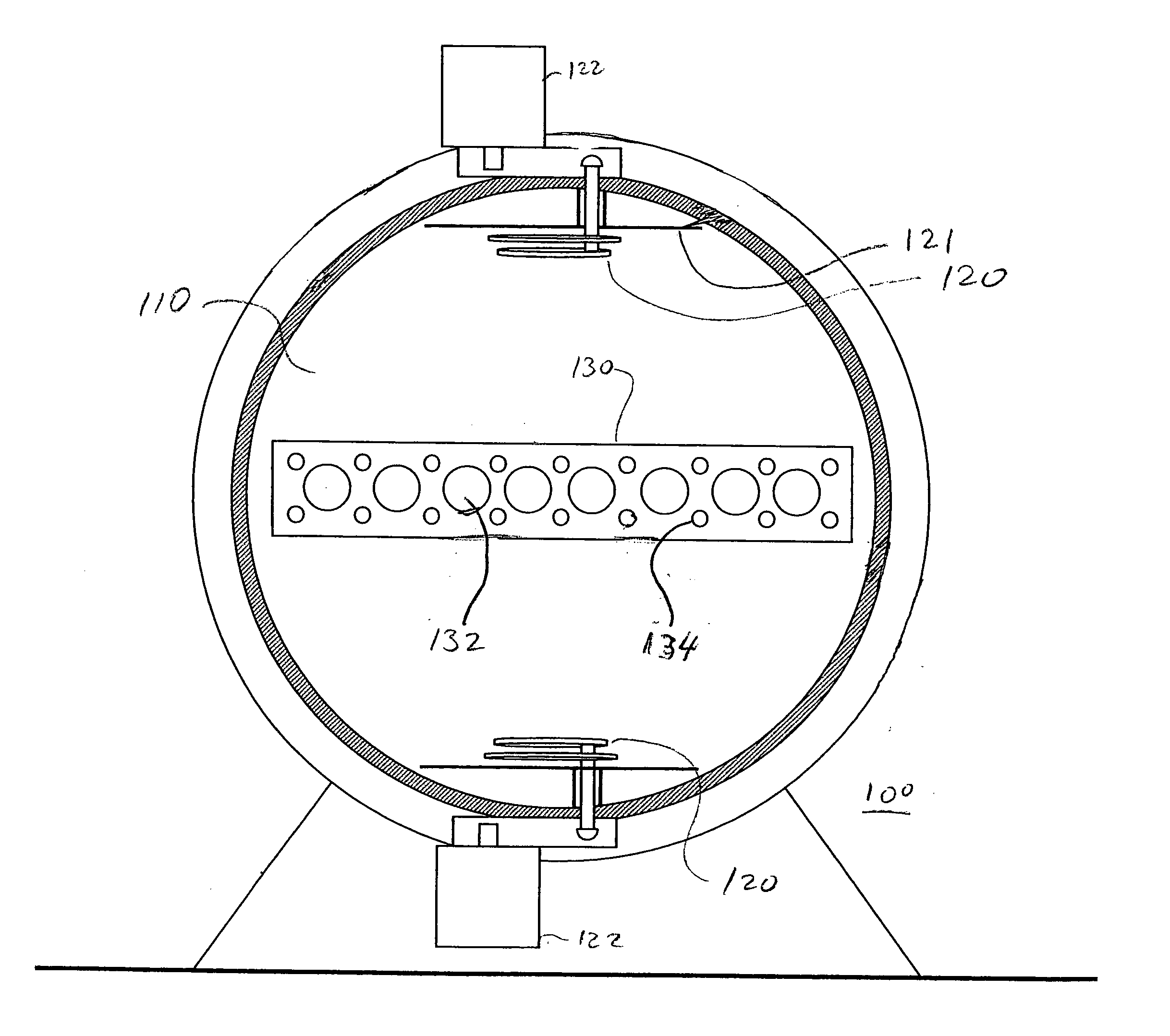
Small animal integrated radiotherapy system fusing computed tomography (CT) and positron emission tomography (PET) bimodal image guidance
PendingCN107626048APrecise positioningAccurate radiotherapyComputerised tomographsTomographyAnatomical structuresX-ray
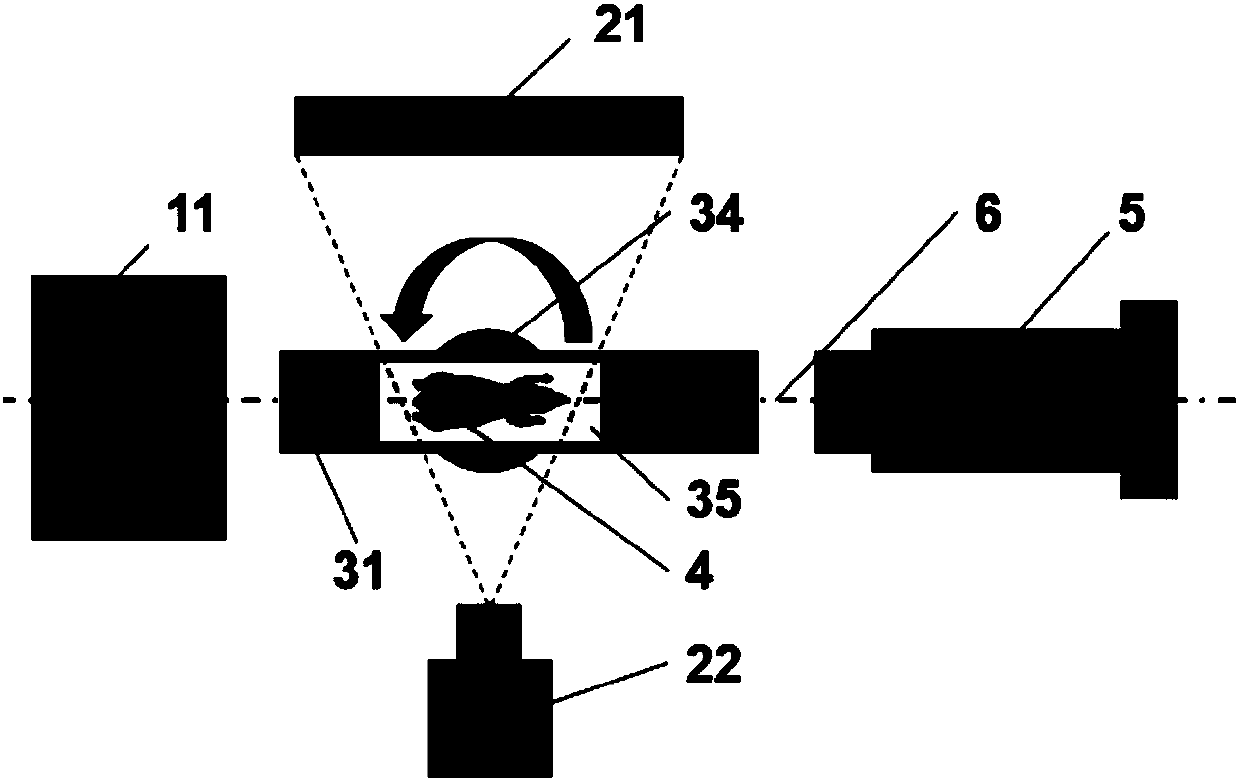
The present invention provides a small animal integrated radiotherapy system fusing CT and PET bimodal image guidance, and belongs to the detection imaging equipment technology field. The system comprises a PET imaging system, an X-ray CT imaging system, an animal bed system, a to-be-detected object, a radiotherapy system, a data acquisition system, a control system and a computer, integrates theadvantages of the CT and PET bimodal imaging, and utilizes a high-resolution anatomical structure image of the CT imaging and the molecular image information provided by the PET imaging to position atumor accurately. The system is an integrated system of the image guidance and the radiotherapy, realizes the accurate radiotherapy of the tumor, at the same time, carries out the monitoring and evaluation of the molecular level on a tumor radiotherapy effect, thereby providing an efficient preclinical research platform for the tumor radiotherapy research, establishing a bridge for the animal experiment and the clinical transformation, and accelerating the clinical transformation progress.
Owner:DALIAN UNIV OF TECH
Methods and Compositions for Generating Stable Transfected Cells
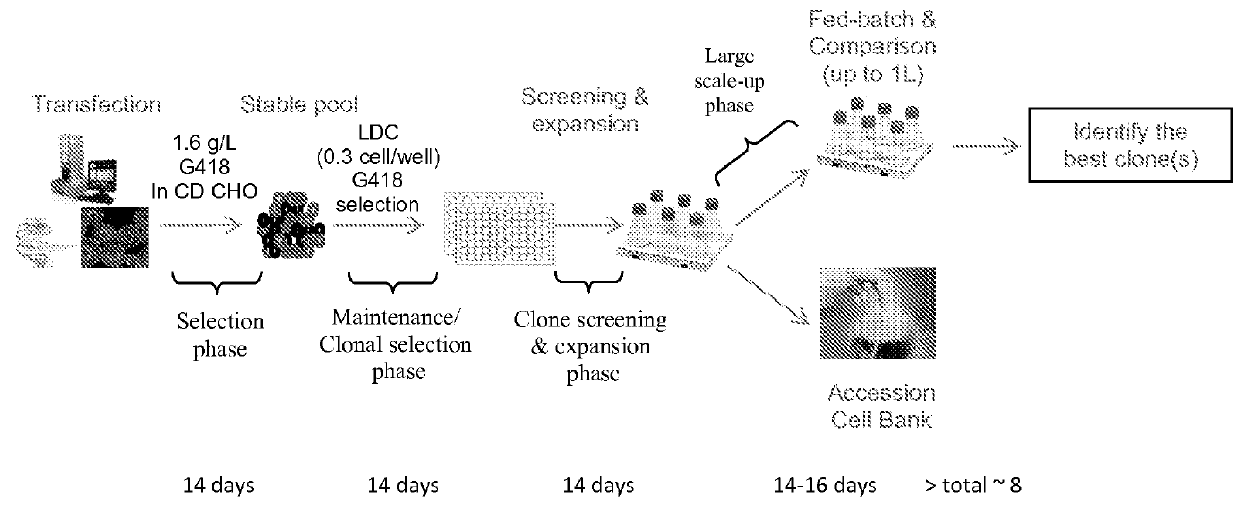
Methods and compositions are provided involving high producing cell lines. Embodiments concern efficient methods for screening for such cell lines and for creating such cell lines. These cell lines can be used to create large amounts of protein. To quickly generate large quantity of recombinant proteins or vaccines for both pre-clinical study and clinical trials, almost all drug development will face the same challenging obstacle of rapidly generating a high stable producer. Developing and identifying a stable cell line is a critical part of biopharmaceutical development.
Owner:MAXCYTE
Humanized intestinal cancer precancerous lesion immortalized epithelial cell line and construction method and application thereof
ActiveCN111172114AProliferative activity in vitroValue-added activity did not change significantlyGastrointestinal cellsGenetically modified cellsOncologyViral vector
The invention relates to a humanized intestinal cancer precancerous lesion immortalized epithelial cell line and a construction method and application thereof. The construction method comprises the following steps: firstly, transfecting primarily separated human colorectal adenoma polyp epithelial cells by using an SV40 overexpression lentiviral vector, then performing screening by using puromycin, and finally amplifying the screened cells to obtain the humanized intestinal cancer precancerous lesion immortalized epithelial cell line. The humanized intestinal cancer precancerous lesion immortalized epithelial cell line constructed by the invention overcomes the problems that conventional adenoma polyp cannot be subjected to passage in vitro, is low in cell proliferation and poor in cell activity, and cannot meet the cell passage requirement. According to the invention, the humanized intestinal cancer precancerous lesion immortalized epithelial cell line is established for the first time, an important cell experiment tool is provided for developing an in-vitro experiment of intestinal cancer precancerous lesions, and preclinical researches such as new drug screening and drug effectcomponents are also convenient to develop.
Owner:YUEYANG INTEGRATED TRADITIONAL CHINESE & WESTERN MEDICINE HOSPITAL SHANGHAI UNIV OF CHINESE TRADITIONAL MEDICINE
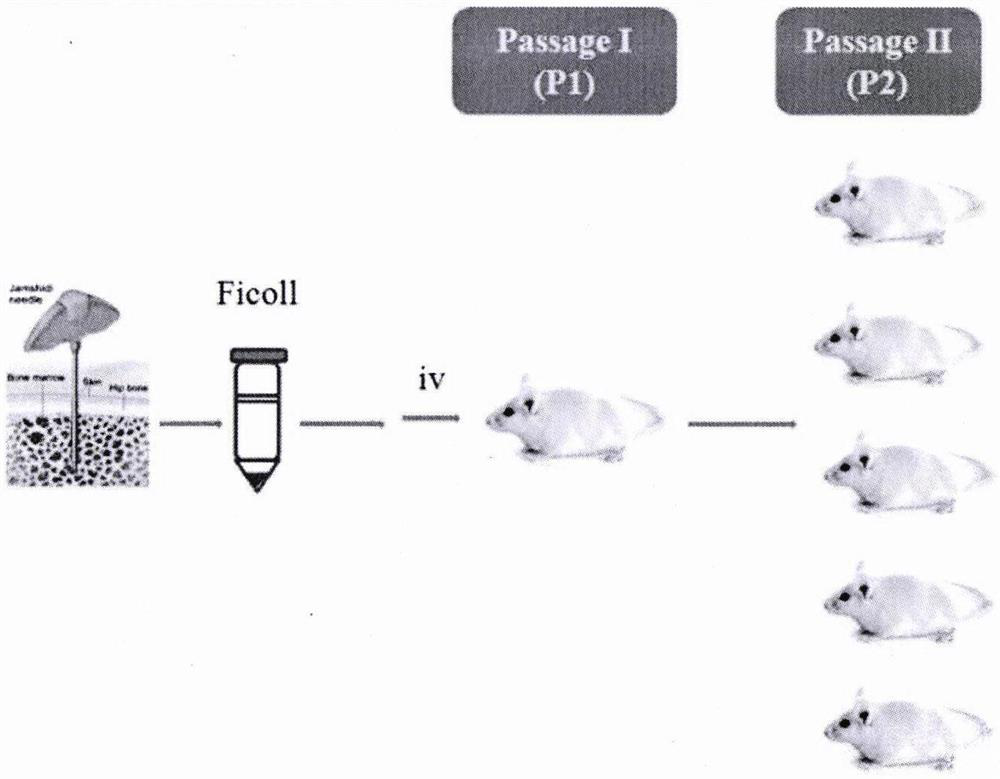
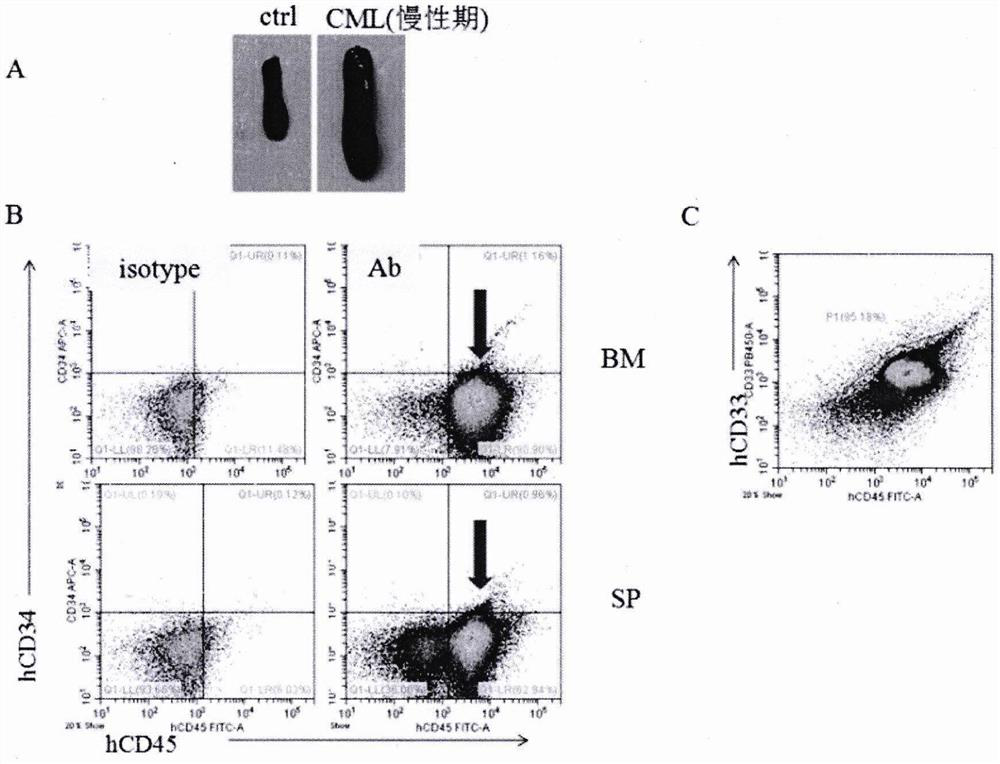
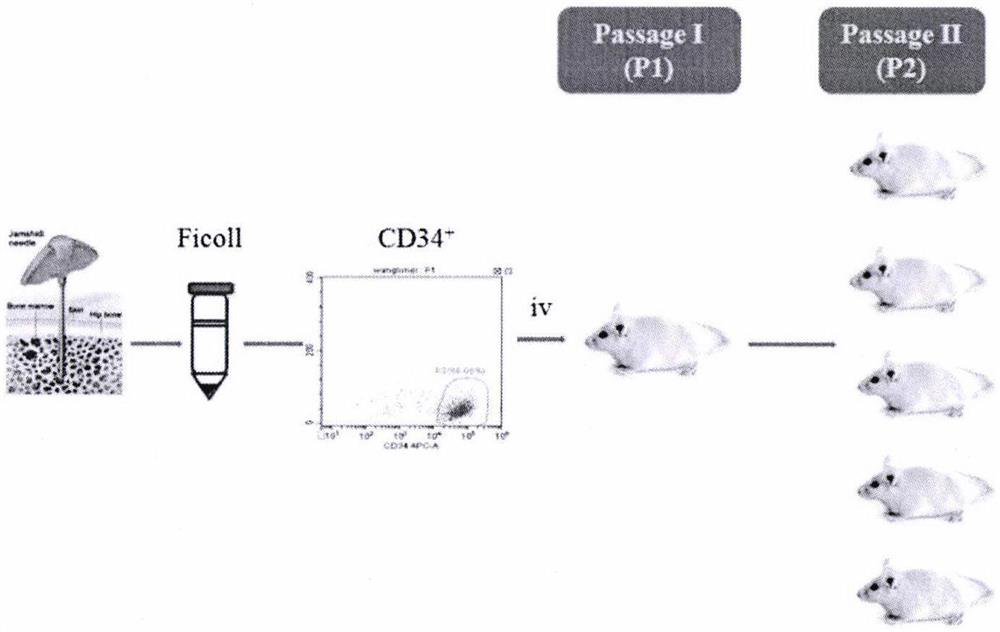
Method for establishing PDX model of chronic myeloid leukemia
The invention discloses a method for establishing a PDX model of chronic myeloid leukemia, and relates to the technical field of animal models. The method specifically comprises the steps of: screening CML clinic patients in a chronic phase and an acute transformation phase, separating mononuclear cells in bone marrow, transplanting the mononuclear cells into an NSG mouse radiated by a half lethaldose, and carrying out CD34 marking before transplantation aiming at the acute transformation phase so as to screen stem / progenitor cells. A user can judge whether the model is successfully established according to the implantation ratio of human-derived cells in peripheral blood after 4-16 weeks of the transplantation. Tumor growth characteristics and cytogenetic abnormality of a series of established CML PDX mouse models are same as those of sourcing patients, so that an important preclinical study model is provided for researching pathogenesis and convulsion mechanisms of CML and screeningtargets and small molecular drugs aiming at different stages of the CML, and the method has relatively good popularization and application values.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE +1
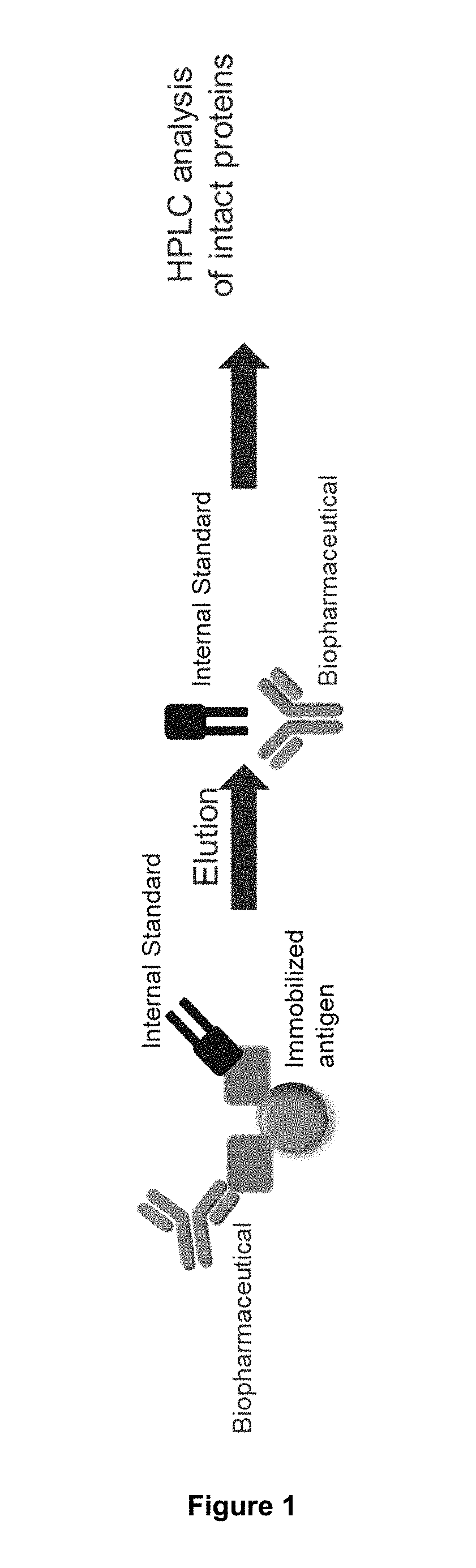
Methods of Mapping Protein Variants
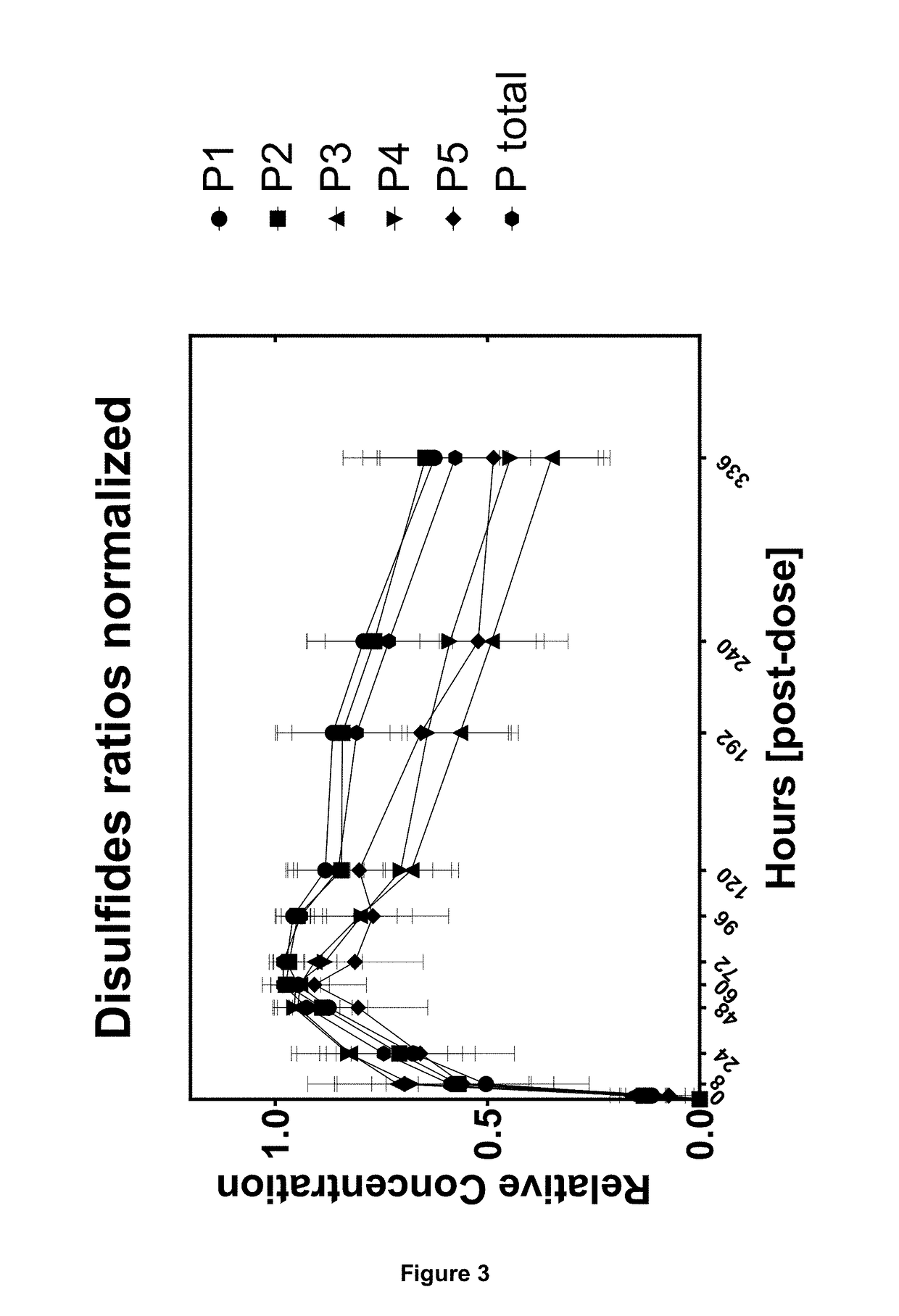
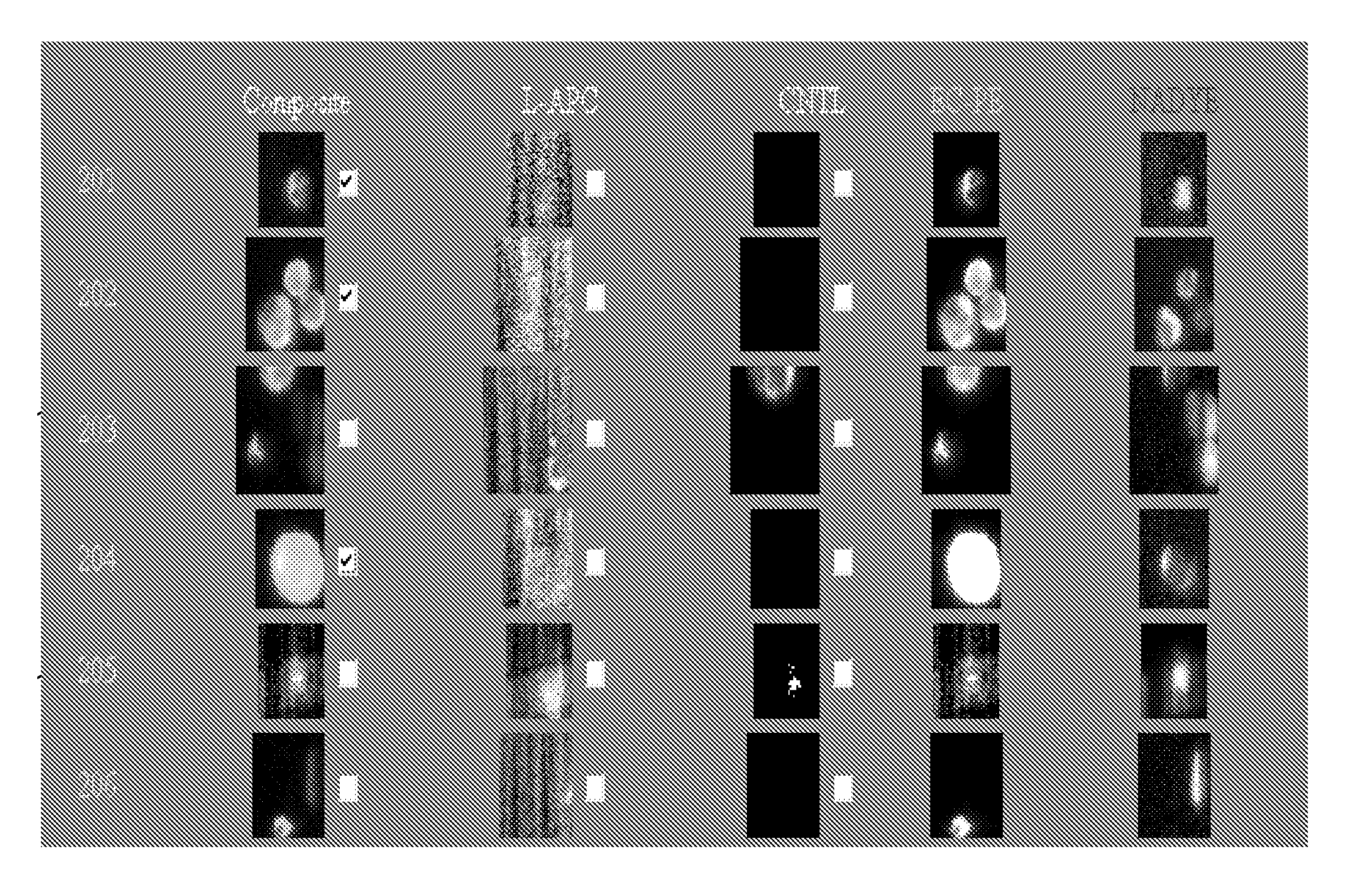
The present invention relates to a method for analysing protein variants of a recombinant protein of interest, such as antibodies or Fc-fusion proteins, in a liquid sample of a mammal. Specifically the method comprises a step of affinity purifying the recombinant protein of interest from the sample together with an internal standard, and analyzing the protein variants using an analytic separating method such as HPLC, capillary electrophoresis or MS. This method is particularly suited to measure pharmacokinetic parameters of a recombinant protein of interest, such as a biopharmaceutical, in a mammal in clinical or pre-clinical studies. It allows for the use of a small sample volume and the possibility to operate with high throughput, such as in a 96-well plate sample preparation. It also provides high sensitivity and allows analysis of protein variants individually.
Owner:HEXAL AG
Pre-clinical method for monitoring serial changes in circulating breast cancer cells in mice
InactiveUS20090117532A1Dead animal preservationMaterial analysisAbnormal tissue growthClinical study
The CellTracks® System provides a system to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. Pre-clinical studies of circulating tumor cells (CTC's) have been limited by the inability to repetitively monitor CTC's in animal models. The present invention provides a method to enumerate CTC's in blood samples obtained from living mice, using a protocol similar to an in vitro diagnostic system for quantifying CTC's in patients. Accordingly, this technology can be adapted for serial monitoring of CTC's in mouse xenograft tumor models of human breast cancer.
Owner:VERIDEX LCC
Novel coronavirus Delta mutant strain specific antibody and application thereof
ActiveCN114349855ASpeed up the R&D processHigh affinityViral antigen ingredientsBiological material analysisCoronavirus vaccinationImmunogenicity
The invention relates to the technical field of antibodies, in particular to a novel coronavirus Delta mutant strain specific antibody and application thereof. The invention provides an antibody specifically combined with a novel coronavirus Delta mutant strain, and the antibody can be used as a specific antibody for Delta mutant strain antigen detection, is used for identifying a novel coronavirus vaccine designed for the Delta mutant strain, and is used for quantitatively detecting the Spike protein content expressed by the vaccine. The antibody can be used for quality control of novel coronavirus vaccines and immunogenicity detection of clinical and preclinical research, or can be used as a quality control antibody for detection of protective antibodies in serum after vaccination.
Owner:百斯医学诊断科技(北京)有限公司
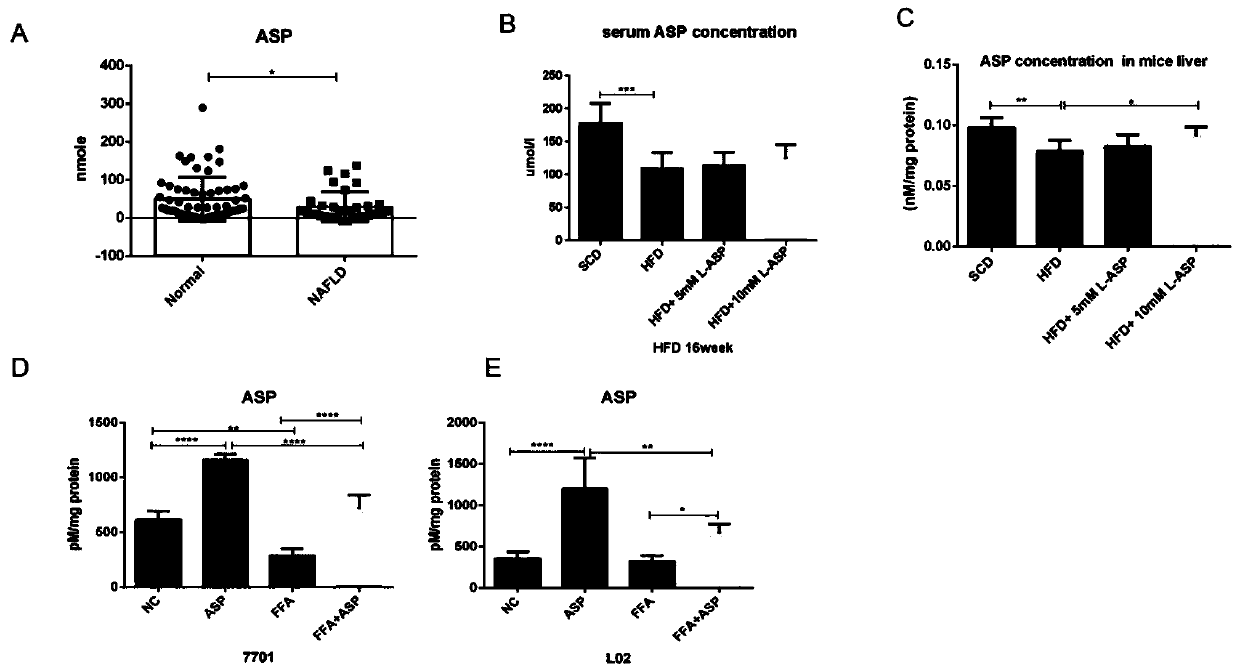
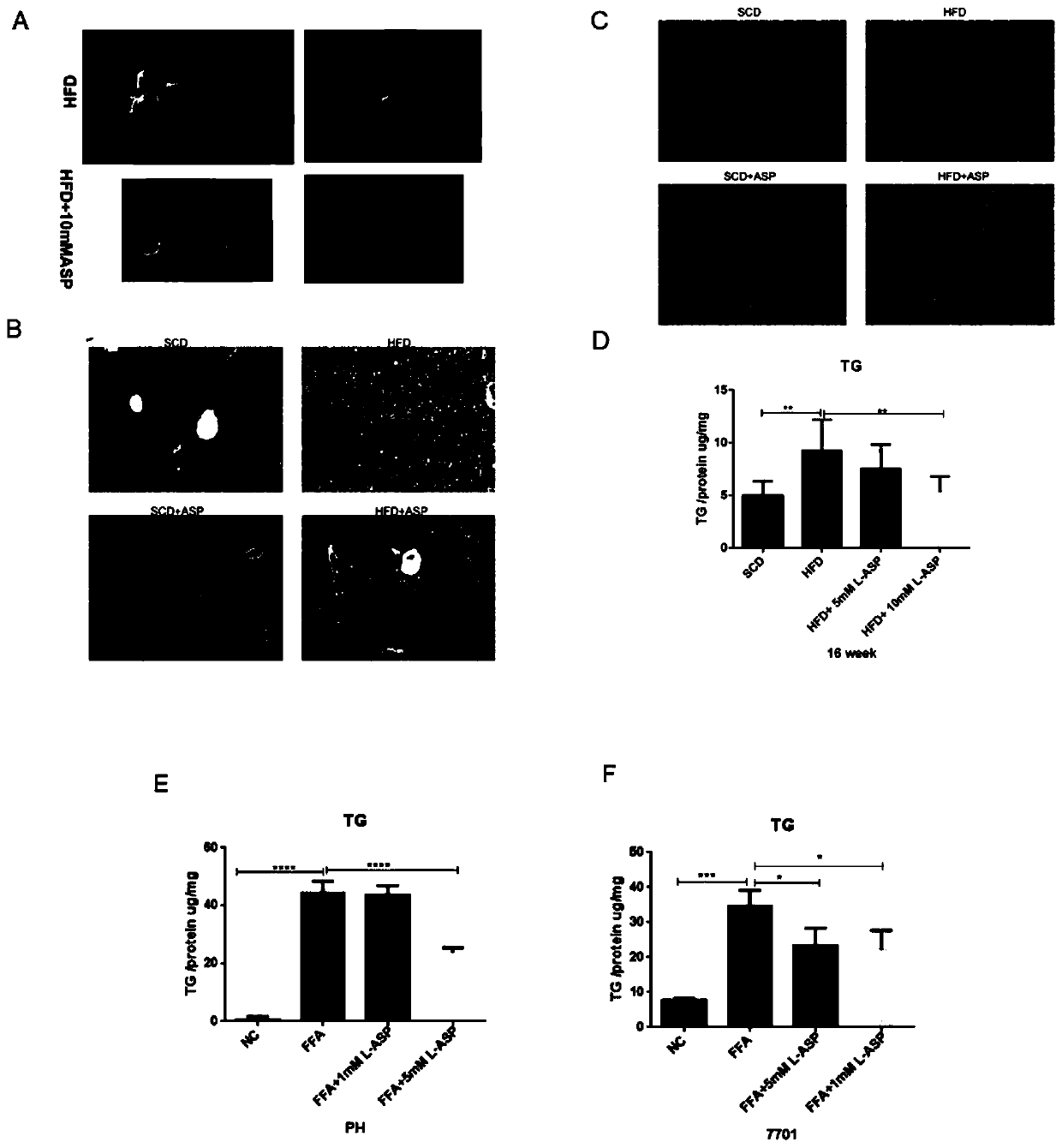
Application of aspartic acid in preparation of medicine for preventing and treating nonalcoholic fatty liver disease
The invention relates to an application of aspartic acid in preparation of a medicine for preventing and treating a nonalcoholic fatty liver disease (NAFLD). The invention explores for the first timean important biological effect of the aspartic acid in the occurrence and development of the NAFLD and the molecular mechanism, reveals the effect of aspartic acid metabolic abnormality in the development process of the NAFLD, and provides an important theoretical basis for aspartic acid intervention therapy; and through in-vivo in-vitro experiments, the invention clarifies for the first time themedicinal properties of the aspartic acid, establishes a system to evaluate the biological effectiveness and safety, and lays the foundation for subsequent standardized preclinical research of the medicine.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Preparation method of protein 4-1BBL
InactiveCN102174522AHigh sensitivityStrong specificityFermentationAnimals/human peptidesClinical researchGene engineering
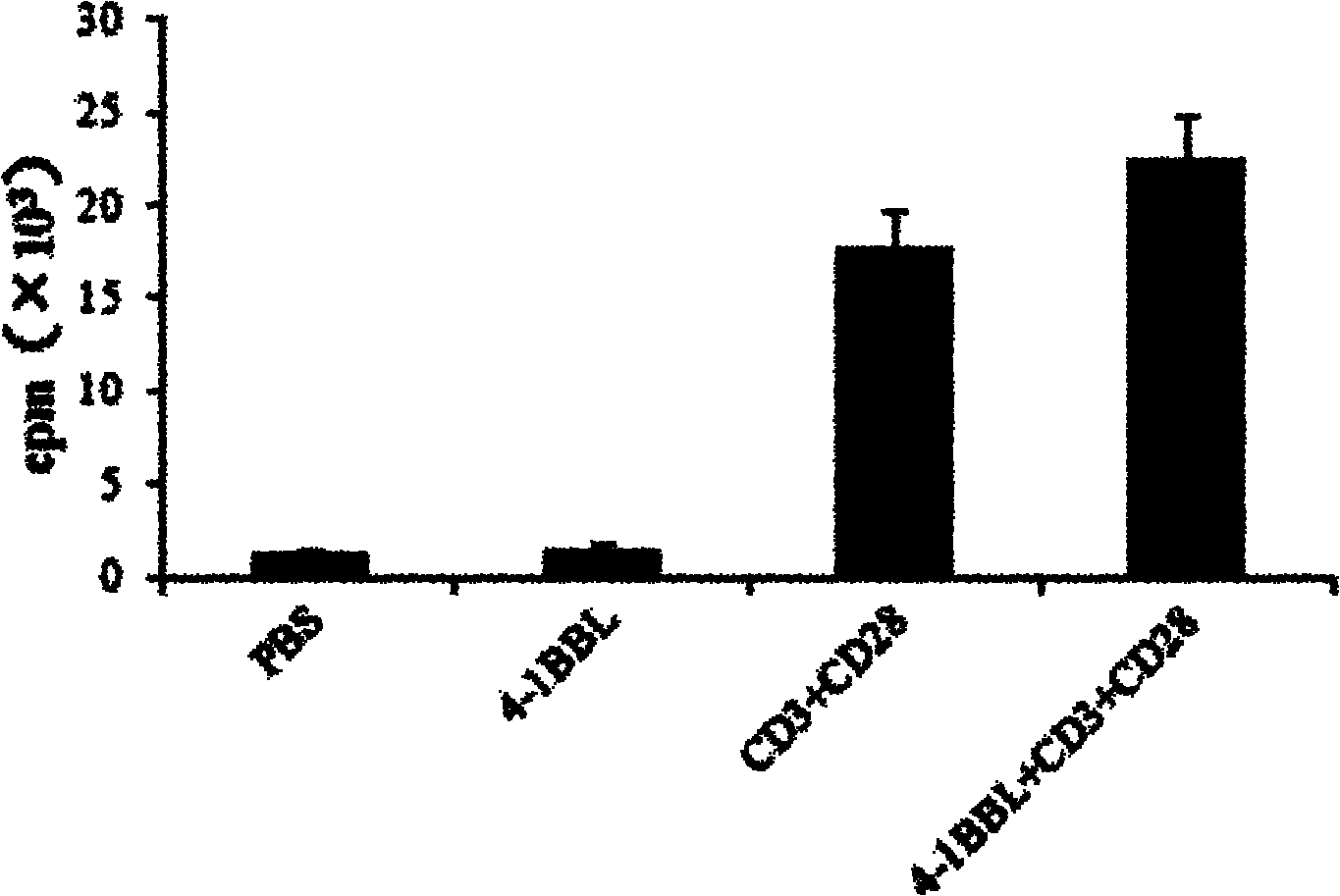
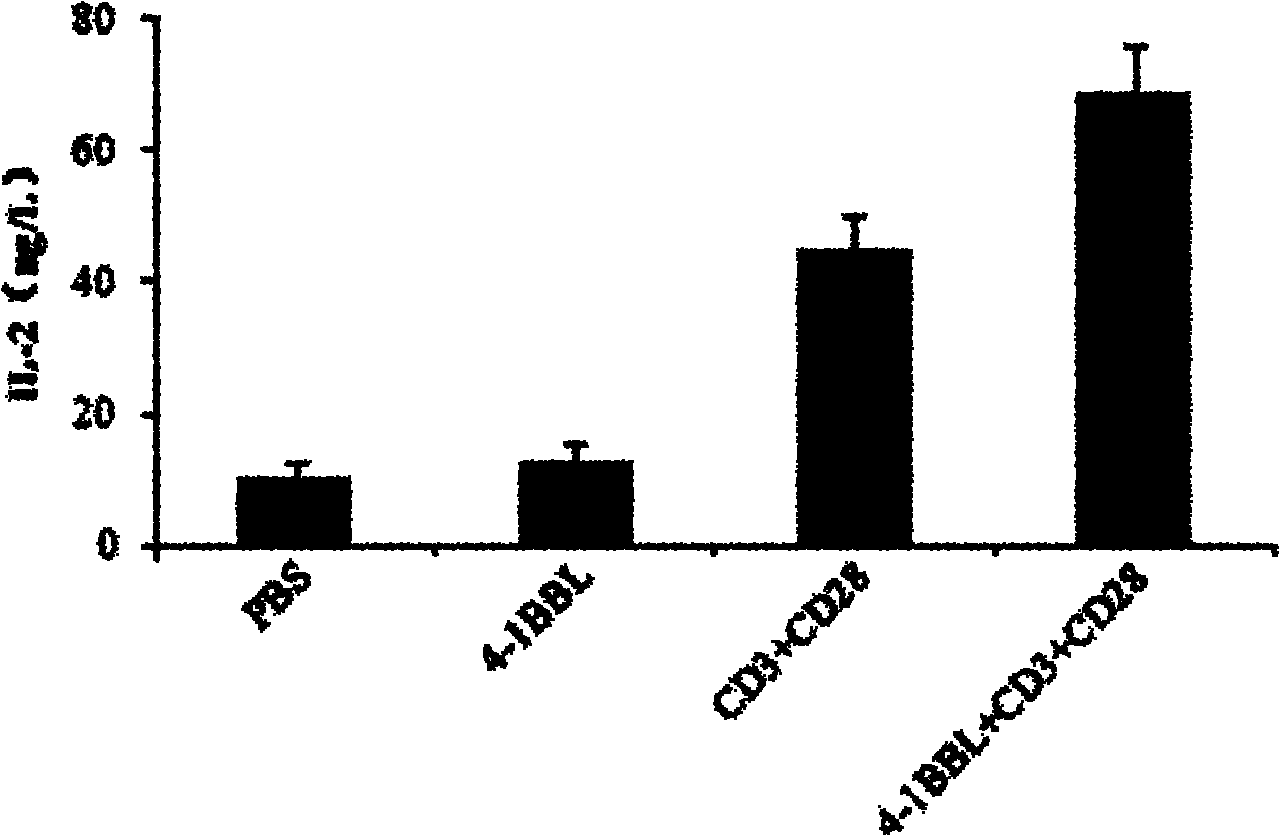
The invention provides a preparation method of a protein 4-1BBL. In the preparation method, a recombinant protein 4-1BBL containing 206 amino acid residues (Arg 104-Glu 309) is prepared from an extracellular-segment protein of mouse 4-1BBL (TNFSF9 (Tumor Necrosis Factor Receptor Superfamily 9), NP_033430.1) by using a gene engineering method. By adopting the preparation method provided by the invention, a large quantity of proteins 4-1BBL can be obtained, so that the yield of the protein is greatly improved, and the obtained extracellular-segment protein 4-1BBL can be applied to preparation of kits for the quantitative pre-clinical researches of the protein 4-1BBL or for medicinal purposes and has a wide application prospect.
Owner:HANGZHOU NORMAL UNIVERSITY
Methods of Screening Compounds to Predict Toxicity and Residual Proliferative and Differentiation Capacity of the Lympho-Hematopoietic System
InactiveUS20080248503A1Faster assayMethod is fastMicrobiological testing/measurementBiological testingAtp contentState dependent
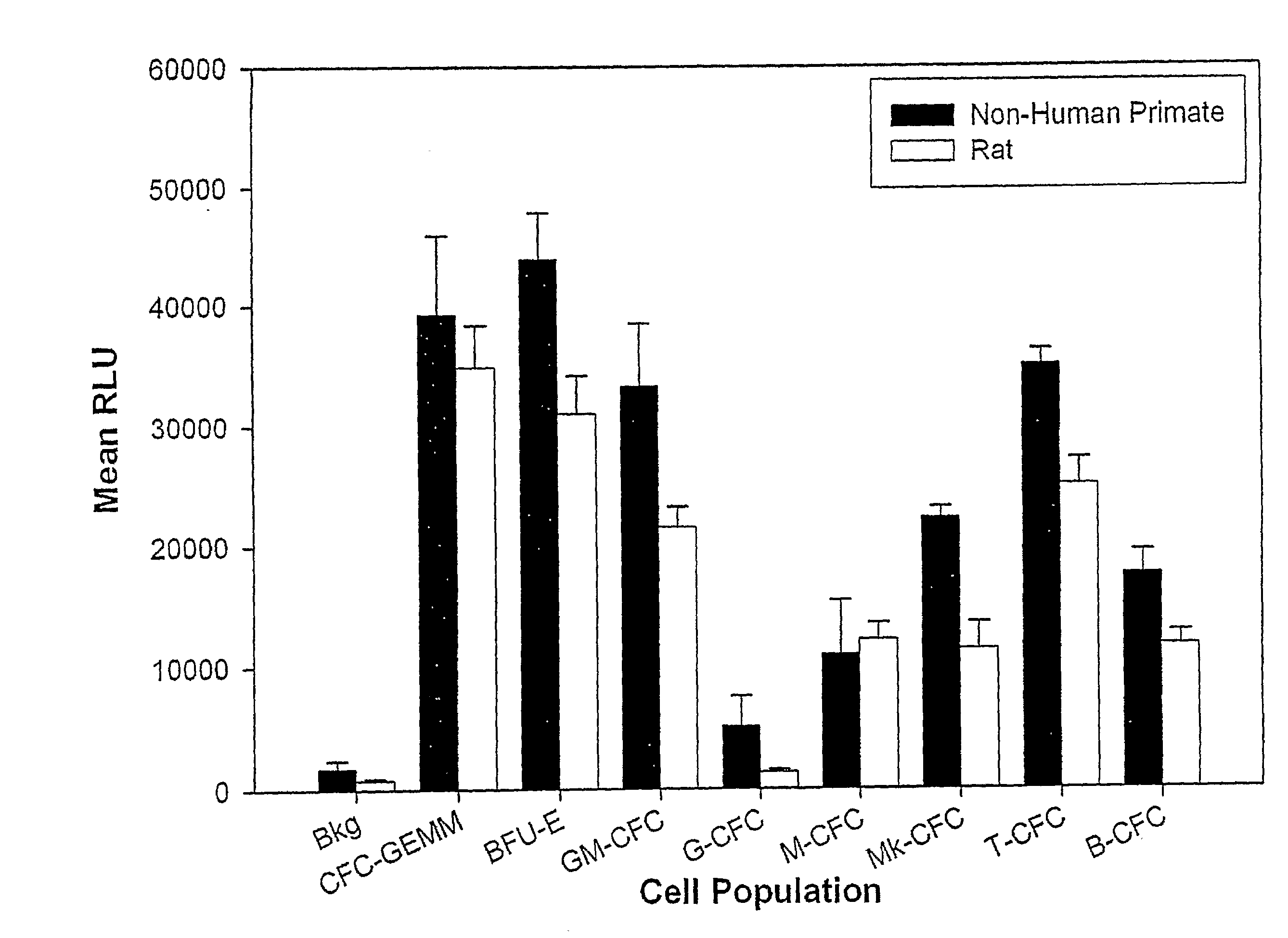
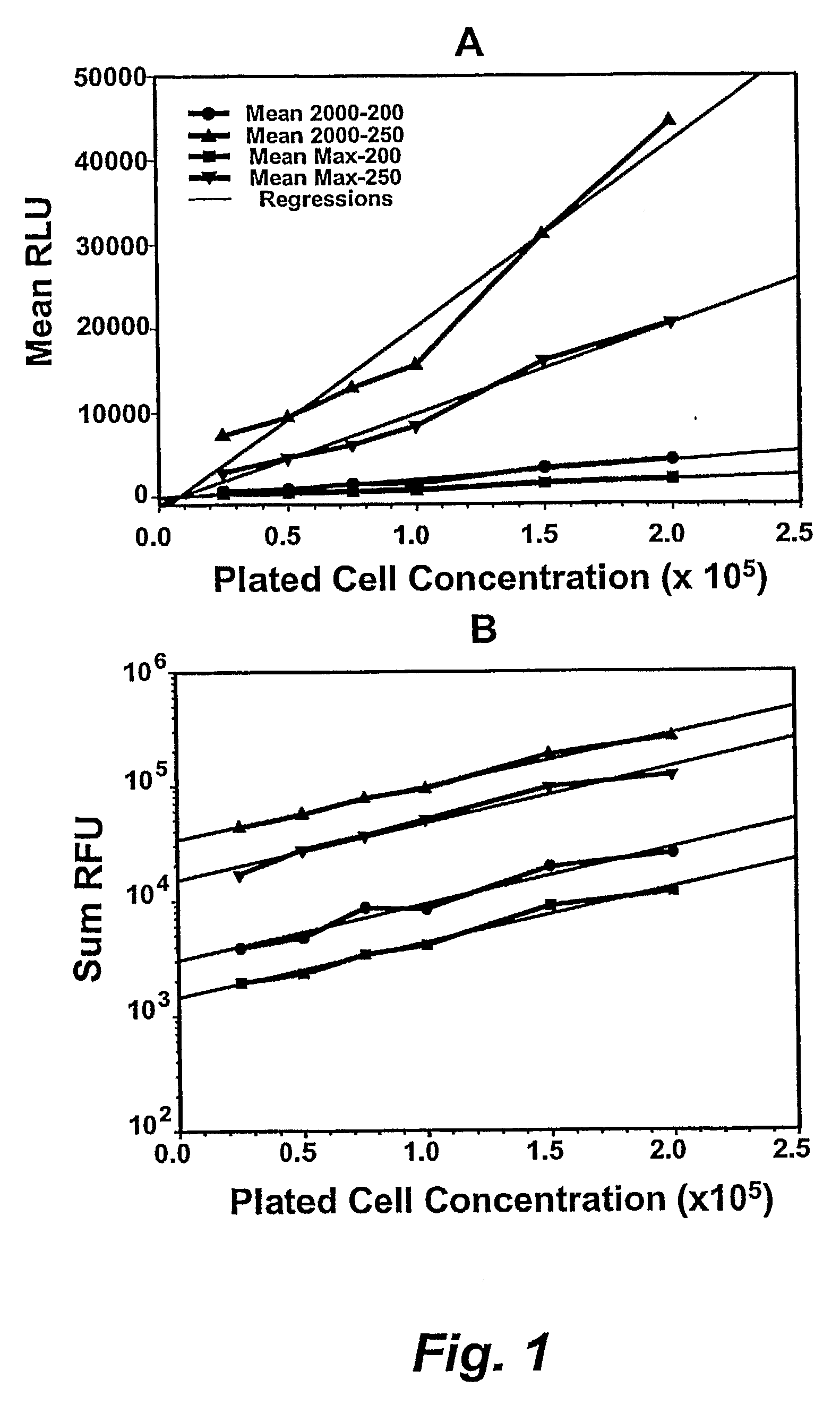
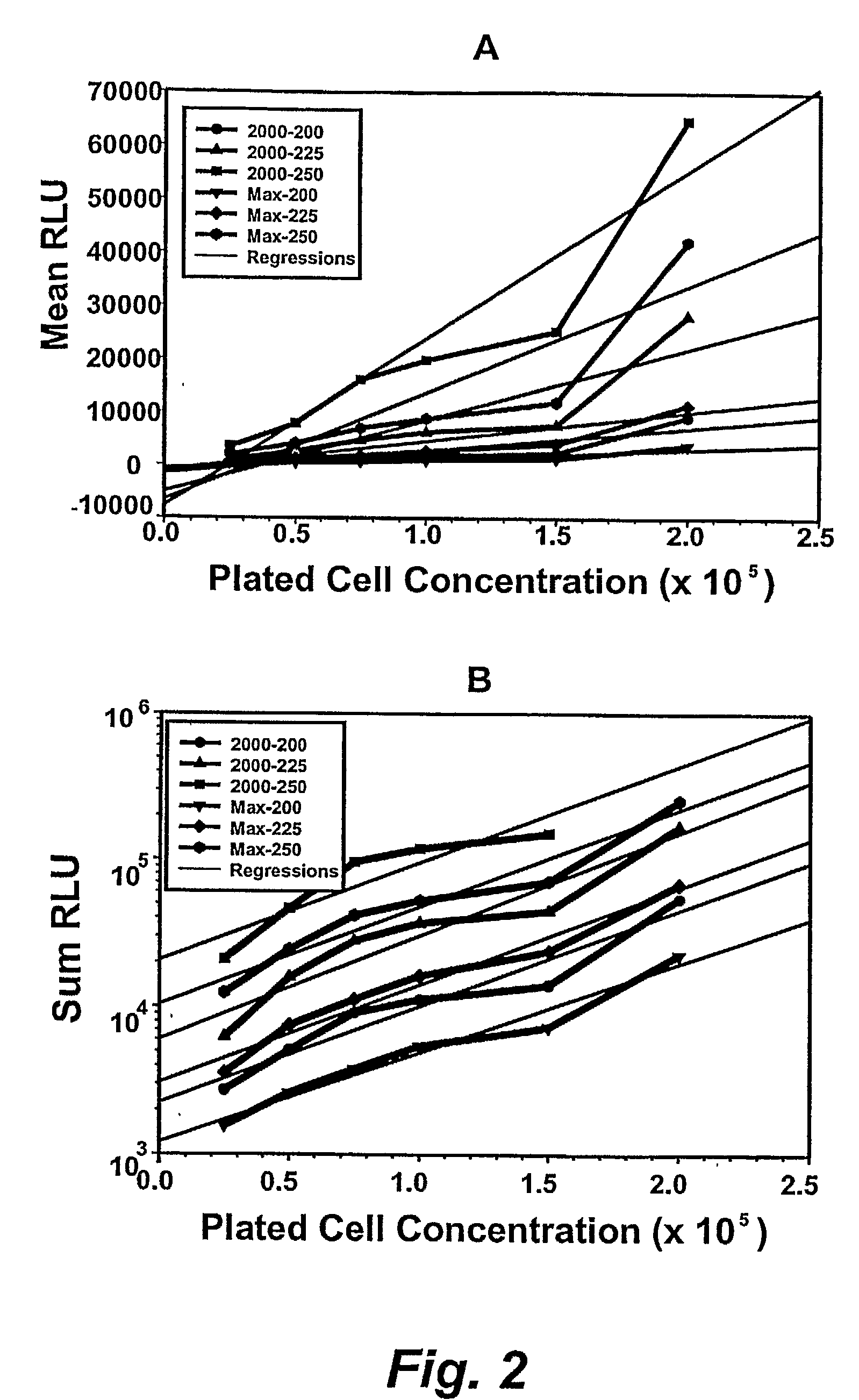
The present invention relates generally to kits that provide reagent mixes and instructions for the use thereof, in performing high-throughput assay methods that provide a method of screening compounds for cytotoxicity or other effects on target cell populations of the lymphohematopoietic system, including specific lineages. The methods measure the luminescent output derived from the intracellular ATP content of incubated target cells, and correlate the luminescence with the proliferative status of the cells. The methods may be used to predict the effect of virtually any compound on the lymphohematopoietic system and may be performed on multiple species simultaneously, thereby providing valuable information regarding potential cytotoxicity prior to preclinical studies and especially, patient clinical trials. The methods also provide the ability to screen compounds early in the drug development profile.
Owner:RICH
Kit for detecting excrement exfoliative cell DNA methylation state to analyze colorectal cancer
InactiveCN108753963AEasy to detectFacilitate early detectionMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationFeces
The invention discloses a kit for detecting an excrement exfoliative cell DNA methylation state to analyze colorectal cancer and specifically provides a kit for detecting colorectal cancer related PDX1 gene methylation from colorectal cancer excrement exfoliative cells and application thereof. According to the kit disclosed by the invention, a specific methylation primer is designed to detect a nucleotide sequence of PDX1 gene methylation to achieve screening and auxiliary diagnosis of colorectal cancer. A fluorescent quantitative PCR method is utilized to analyze a methylation level of a PDX1gene marker, and a positive result or a negative result of a sample can be judged according to a reported CT value. In clinical researches, absolute majority of colorectal cancer and most precancerous adenoma can be detected; 90% of early colorectal cancer can be detected, 64% of precancerous adenoma (larger than or equal to 1 centimeter) can be detected, and specificity is 100%; furthermore, wound is completely avoided, only 4.5 grams of excrement is needed, and acceptability by crowds without symptoms is high.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
Process For The Preparation And Use Of A Bivalent Vaccine Against Morphine-Heroine Addiction
ActiveUS20080241183A1Easy to solveMaintain curative effectNervous disorderBacteria peptidesHuman useVaccination
The structural design, preparative methods and chemical composition of two structural formulations of bivalent vaccines against morphine-heroin addiction (morphine-6-hemisuccinyl-EDC-TFCS-tetanus toxoid and 3-O-carboxymethylmorphine-EDC-TFCS-tetanus toxoid), are disclosed. These vaccines are suitable for human use in which they are capable of triggering the synthesis of polyclonal antibodies against morphine opiate and its structural analogue, heroin, through the repeated in vivo administration of these formulations, in active vaccination protocols, in pre-clinical studies in rodents. The active vaccination paradigm through which these immunogens trigger a humoral immune response consolidated with a long-term immunological memory, characterized by the presence of high titers of specific antibodies against these two drugs of abuse, is also disclosed. Furthermore, the present invention reveals the efficacy of these conjugate formulations for triggering a sustained immunoprotection against morphine and heroin addiction using an intravenous self-administration paradigm of these two opiate substances in the rodent. Finally, a discussion is also made on the potential future use of these immunoconjugates in active vaccination protocols for treating both morphine and heroin addiction in the humans.
Owner:INST NACIONAL DE PSIQUIATRIA RAMON DE LA FUENTE MUNIZ
MDS (myelodysplastic syndrome) transfection leukocyte line with capacity of stable expression of GFP (green fluorescent protein)
ActiveCN105670999ATo prevent lossUniform fluorescenceMicroorganism based processesPeptidesBiologyIndividual animal
The invention belongs to the field of microbial and animal cell lines and provides an MDS (myelodysplastic syndrome) transfection leukocyte line with capacity of stable expression of GFP (green fluorescent protein) as well as establishment method and an application of the MDS transfection leukocyte line. A human MDS transfection leukocyte line SKM-1 is taken as maternal cells and is transfected through lentiviruses carrying GFP genes, GFP-positive single cells are obtained with a limiting dilution method and cloned, cells after amplified culture are screened in a mouse body in a subcutaneous injection manner, tumor masses are separated after tumorigenesis and cultured continuously, and the MDS transfection leukocyte line SKM-1 / GFP with stable expression of GFP is obtained. The morphology, growth characteristics and the growth curve of the cell line have no difference with those of the maternal cells, and the cell line can express GFP stably, has tumorigenicity, can be further applied to establishment of a murine animal model and provides a platform for MDS, minimal residual disease and other preclinical study.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method of activating lvier stem cell
InactiveCN1548528APromote activationShorten the timeArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsRound cellBiology
The method of activating liver stem cell includes combined ethyl thioamino butyric acid and partial liver excision method, and conventional collagenase digesting and density gradient centrifugation method to separate oval liver cell. The method of the present invention can activate liver stem cell in short period without damage, and present invention makes it possible to provide great amount of cell for the preclinical research of liver stem cell and preclinical animal transplantation experiment.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Reprogramming method for induced pluripotent stem cells
ActiveCN106916850AHigh reprogramming efficiencyLow immunogenicitySsRNA viruses negative-senseGenetically modified cellsVirus typeReprogramming
The invention relates to a reprogramming method for human induced pluripotent stem cells. The reprogramming method is more favorable for preclinical research and mainly employs Sendai virus-mediated infection of the human induced pluripotent stem cells and reprogramming. The reprogramming method provided by the invention has high reprogramming efficiency; and the obtained human induced pluripotent stem cells have the characteristics of low immunogenicity, high security and the like. Moreover, compared with reprogramming methods for lentivirus-mediated human induced pluripotent stem cells, the reprogramming method provided by the invention has the following advantage that the human induced pluripotent stem cells obtained by using the method is free of integration of exogenous genes and thus has higher safety.
Owner:ZONHON BIOPHARMA INST
Method using salamander Oct4 to reprogram human blood cells into iPSC
ActiveCN108373998AWill not integrate stablyImprove induction efficiencyForeign genetic material cellsProgenitorReprogramming
The invention belongs to the field of cells and particularly relates to a method using salamander Oct4 to reprogram human blood cells into iPSC. The method includes the steps of S1, acquiring progenitor red blood cells; S2, importing a free vector containing a salamander Oct4 transcription factor and other transcription factors into the progenitor red blood cells obtained in S1; S3, subjecting theprogenitor red blood cells, containing the free vector, obtained in S2 to multipotential stem cell induction medium culture, and inducing into reprograming intermediate-state cells in a feed-layer-free system; S4, after complete induction, replacing the multipotential stem cell induction medium in S3 with multipotential stem cell culture medium to continue the culture so as to obtain the cells with disappeared salamander Oct4 transcription factor and other transcription factor expression and activated endogenous multipotential gene POU5F1, NANOG, TRA-1-60 and TRA-1-81 expression, wherein thecells are the iPSc. The method has the advantages that efficient induction can be performed to generate the hiPSC without exogenous gene components, and the method is applicable to preclinical study and clinical application.
Owner:安徽中盛溯源生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com