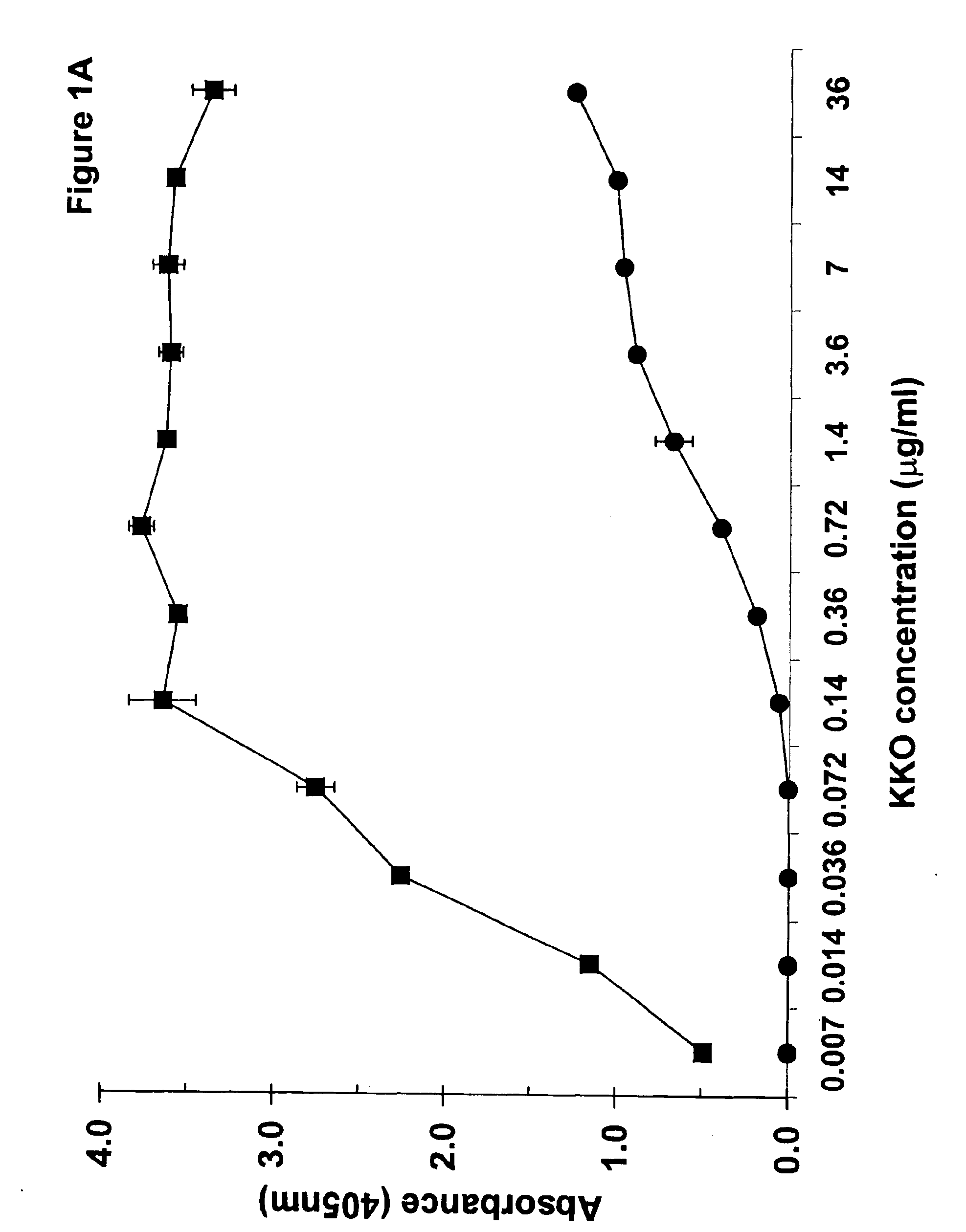
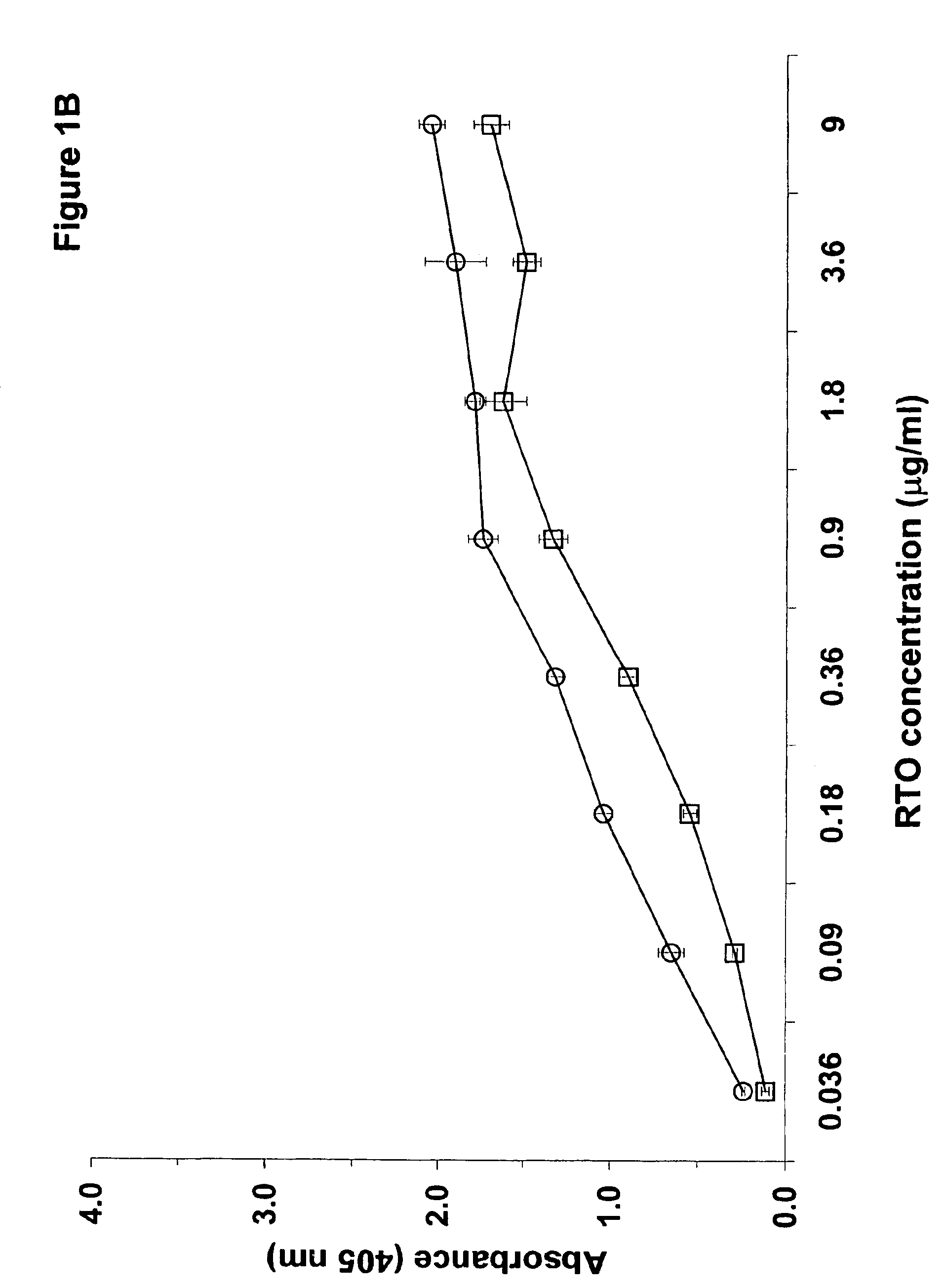
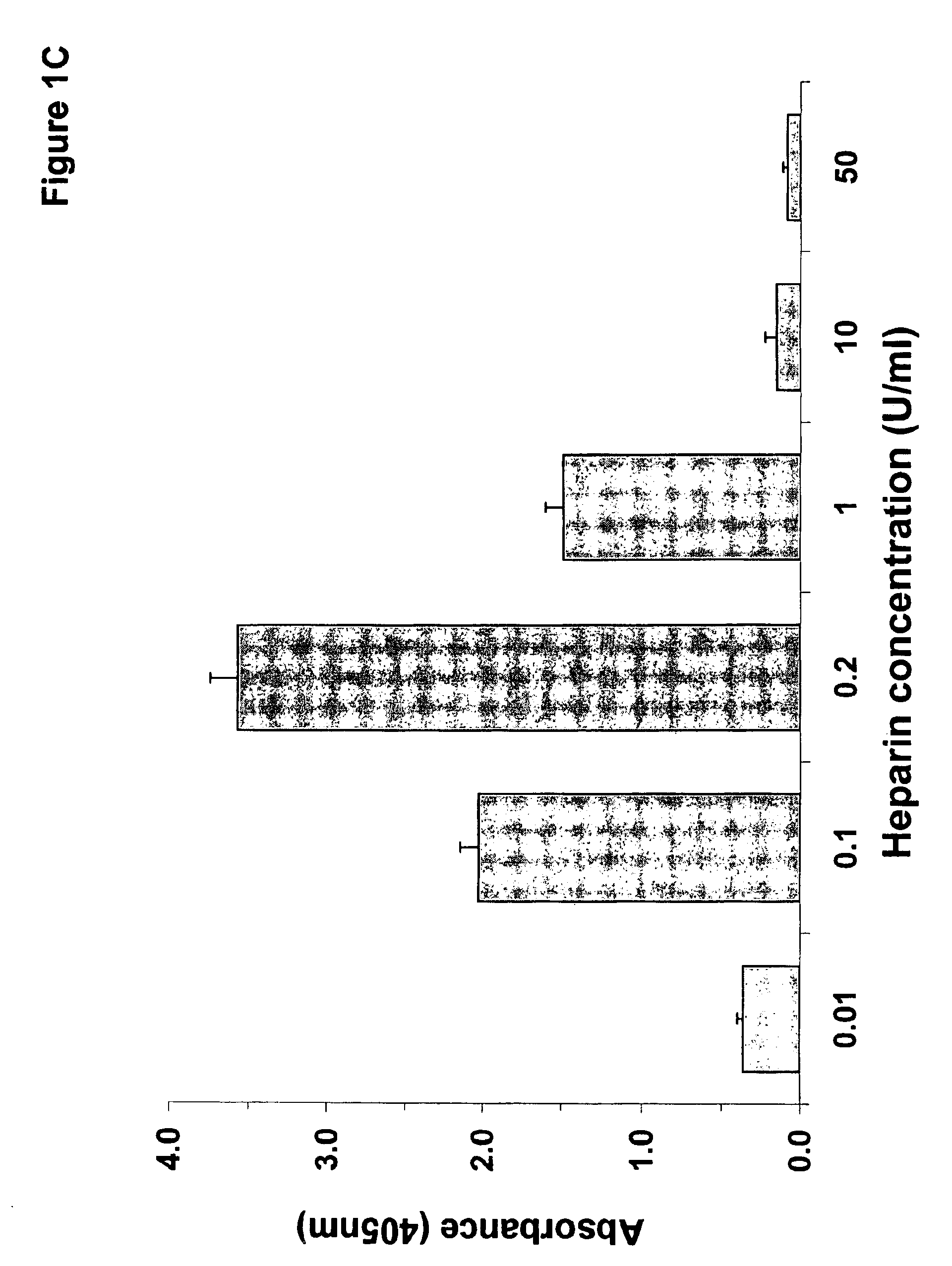
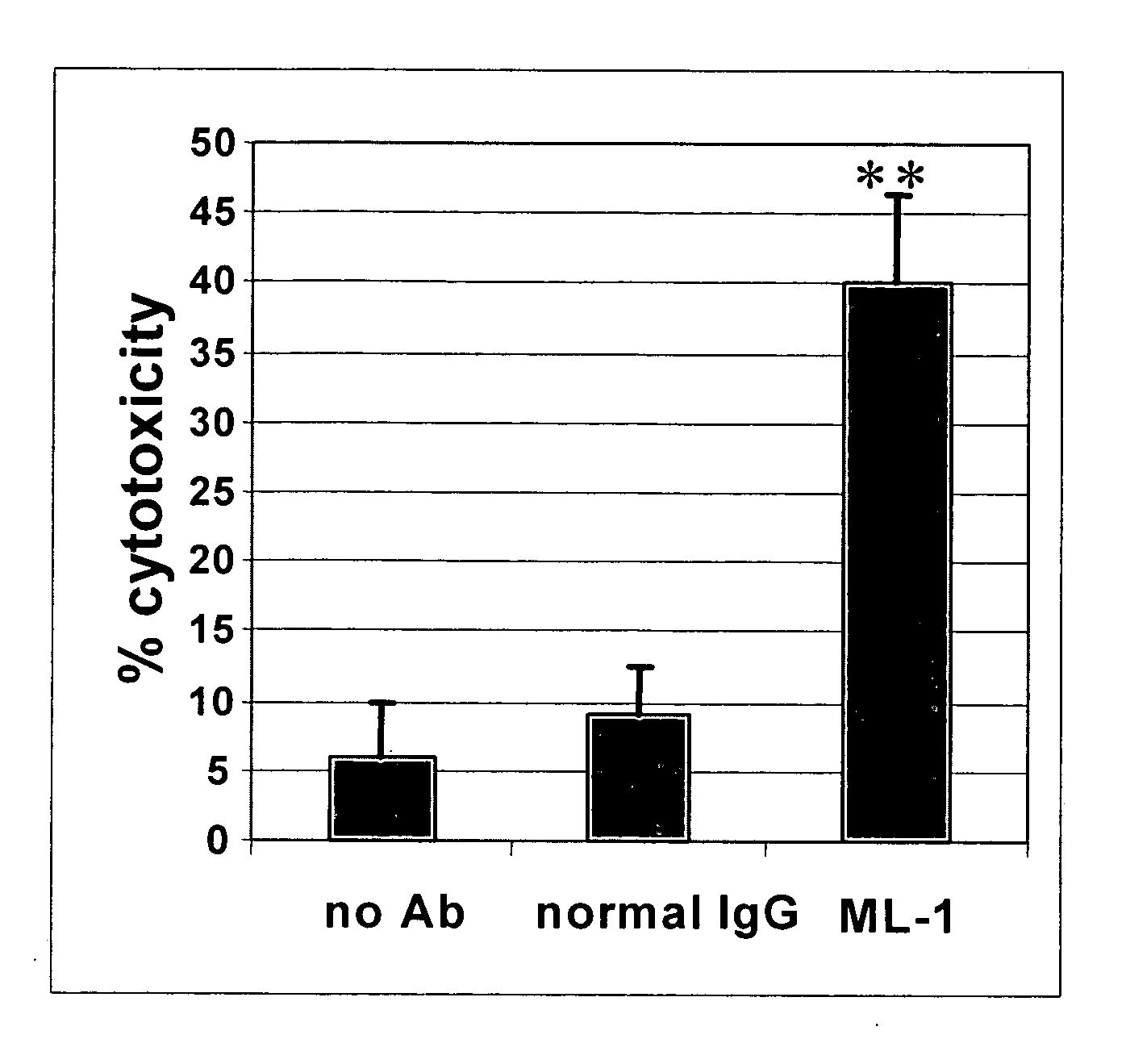
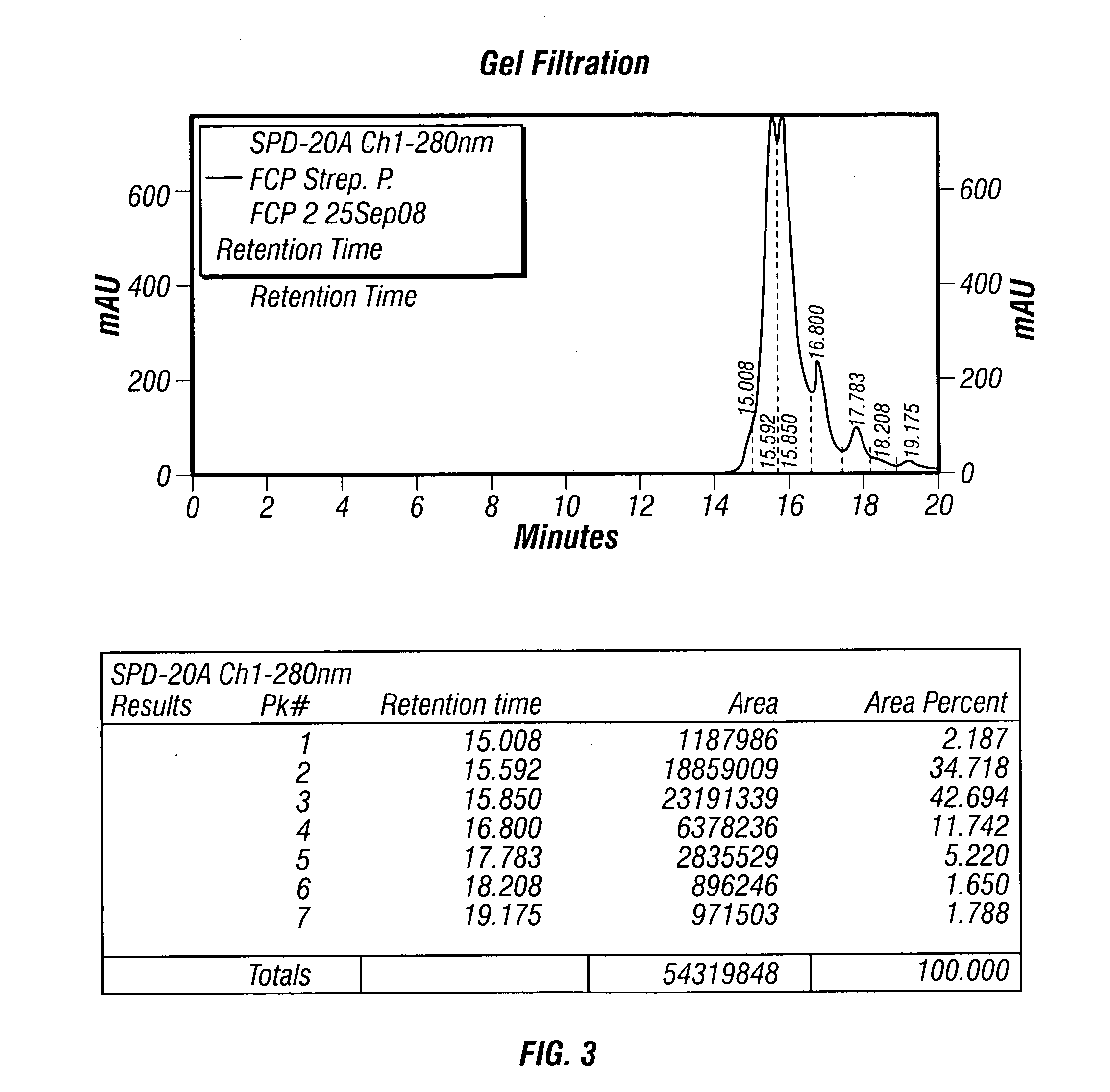
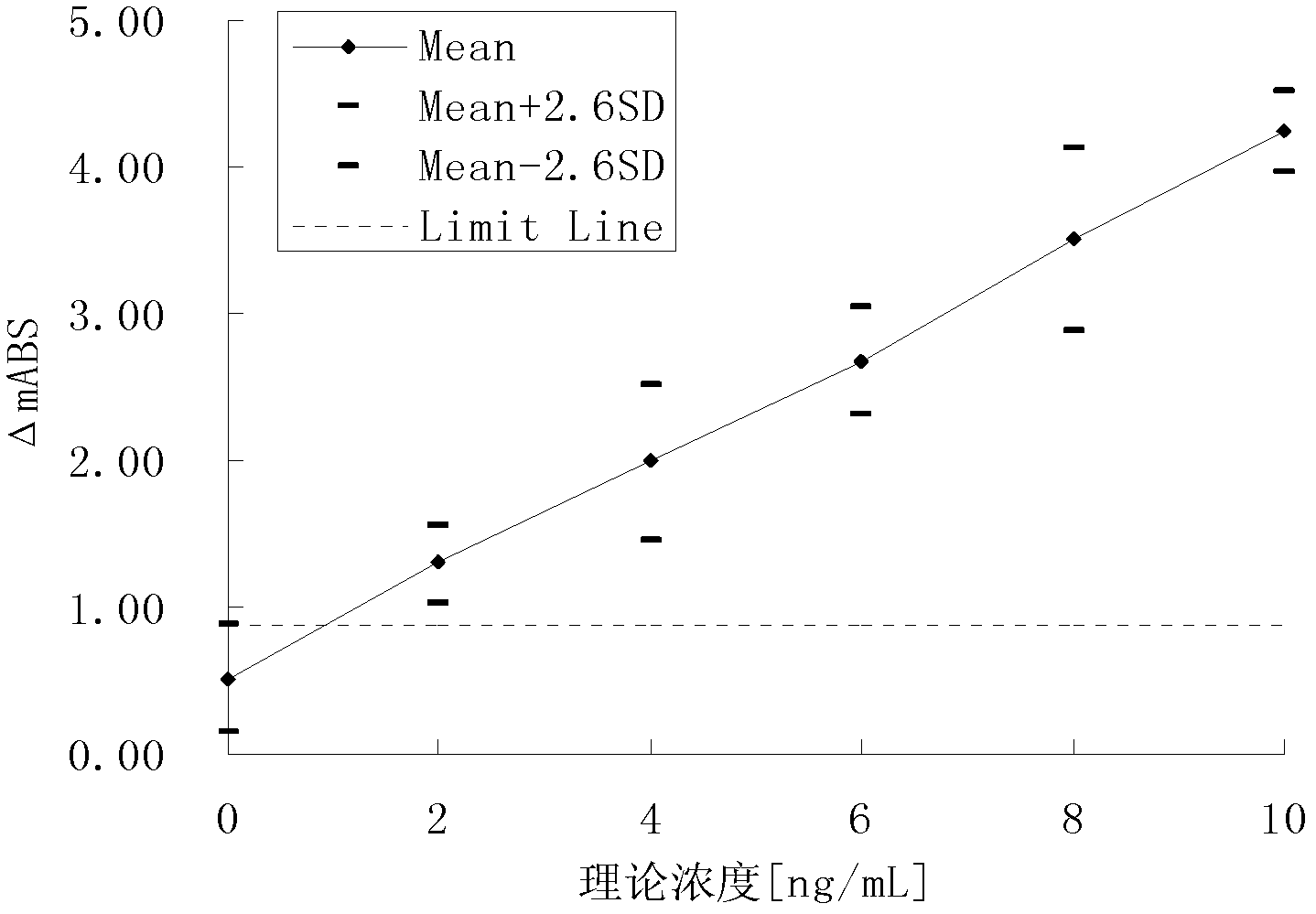
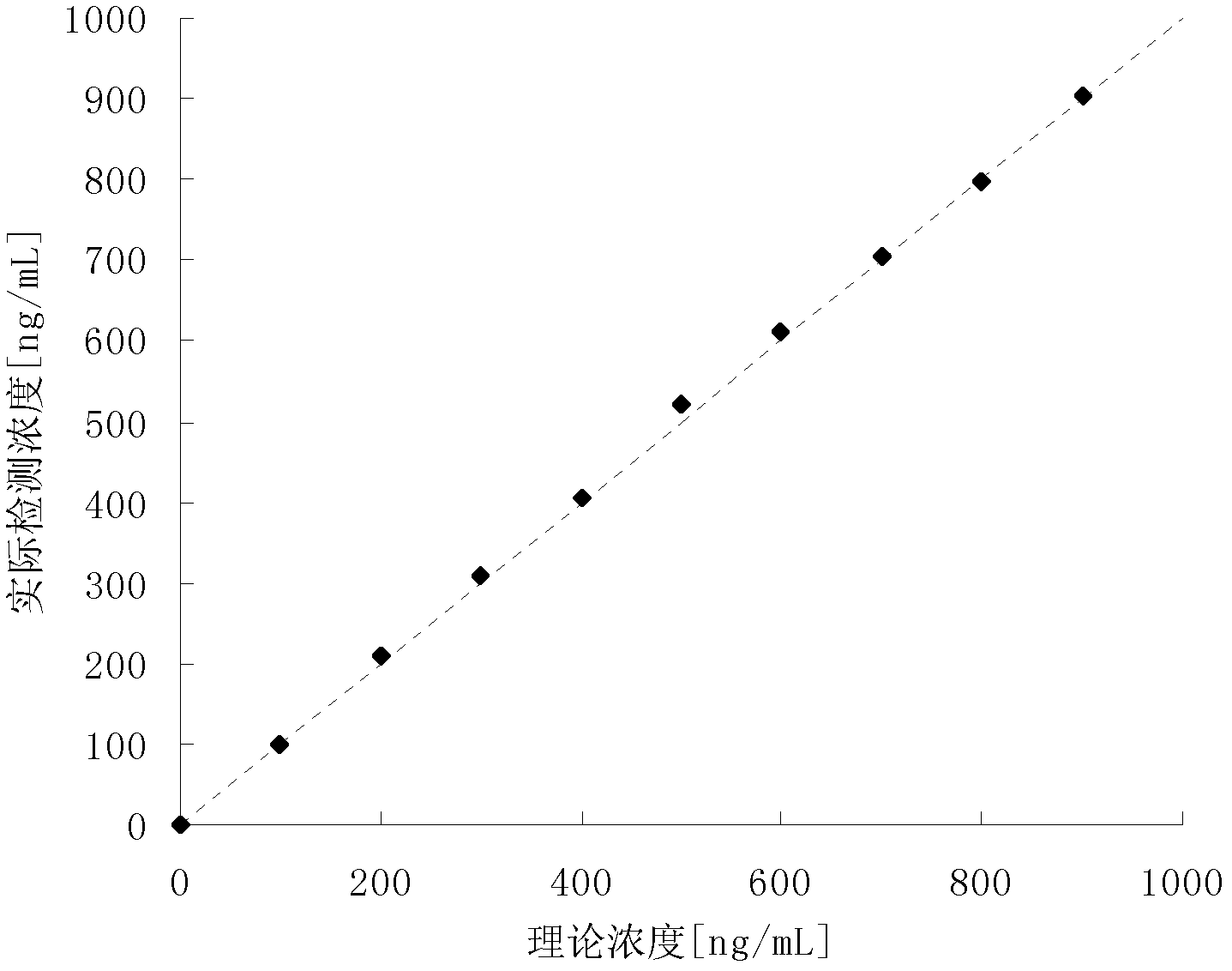
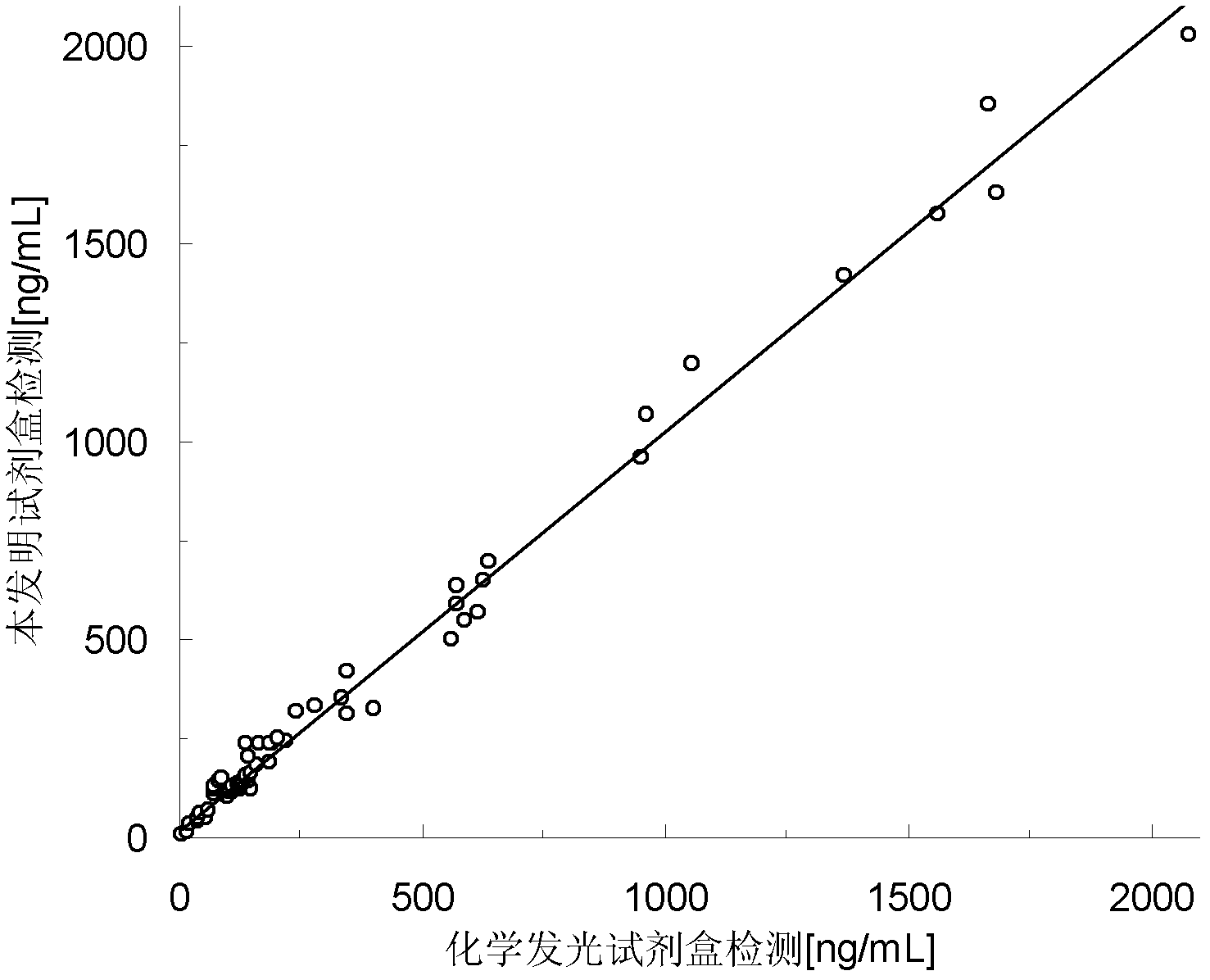
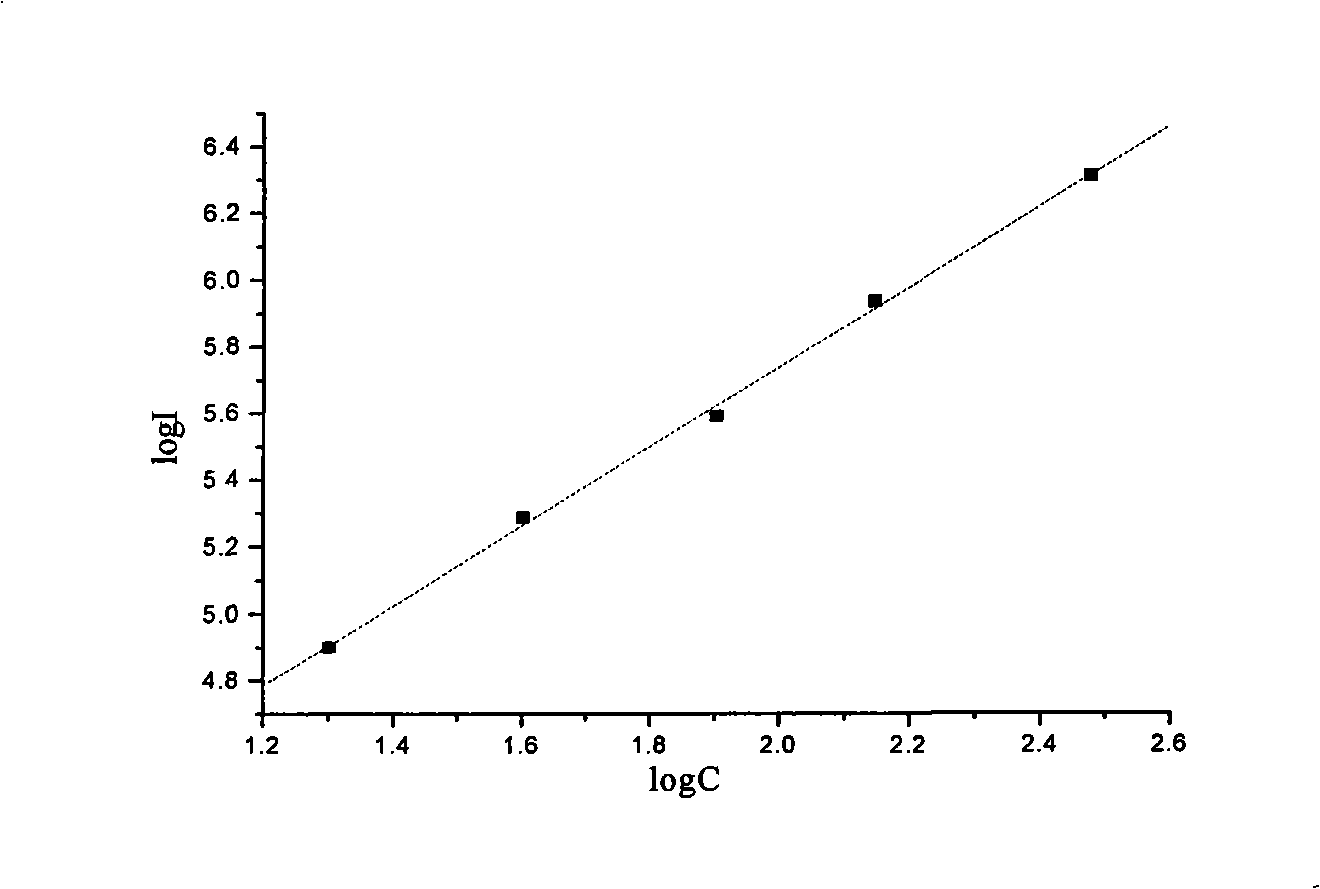
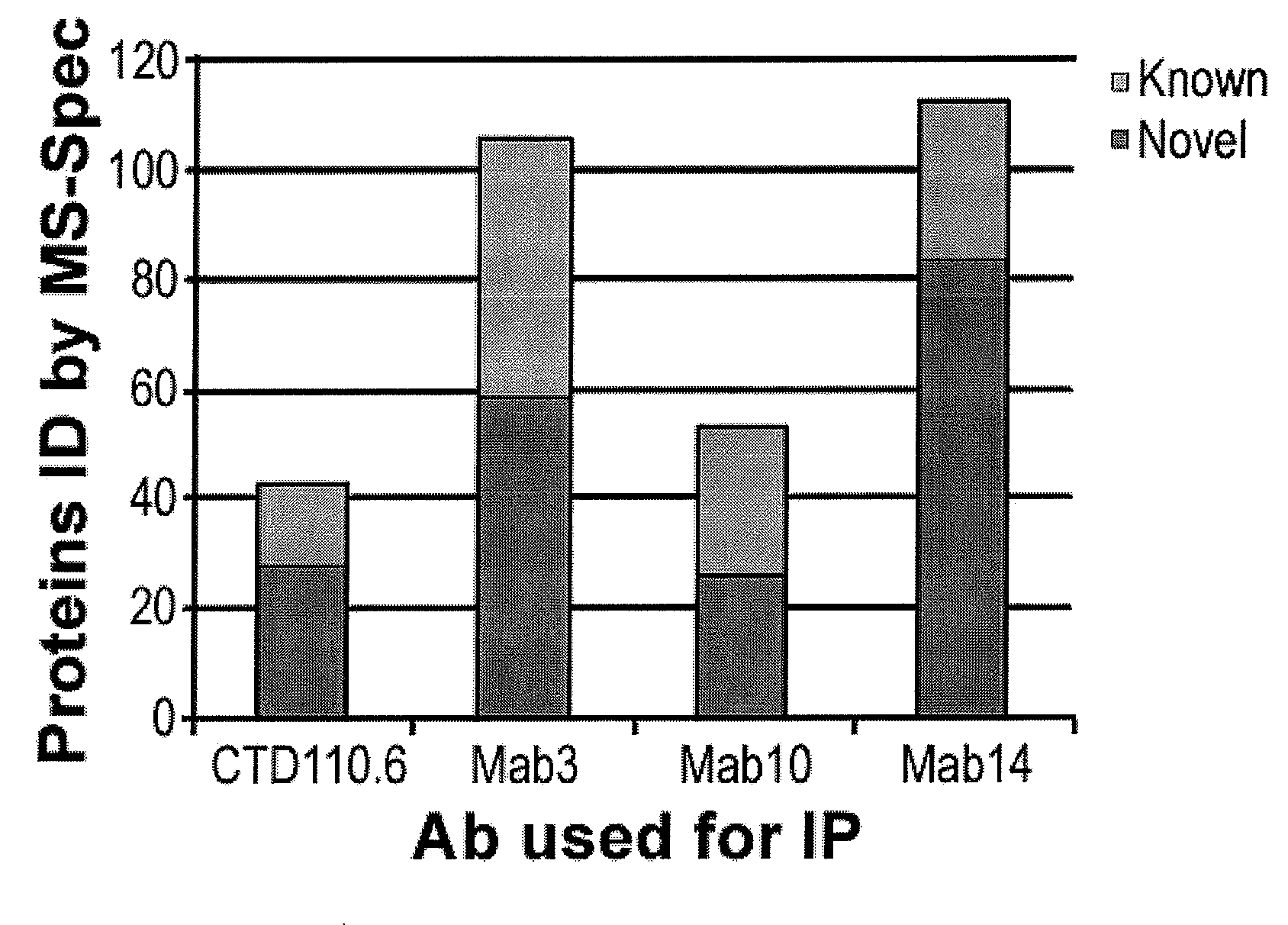
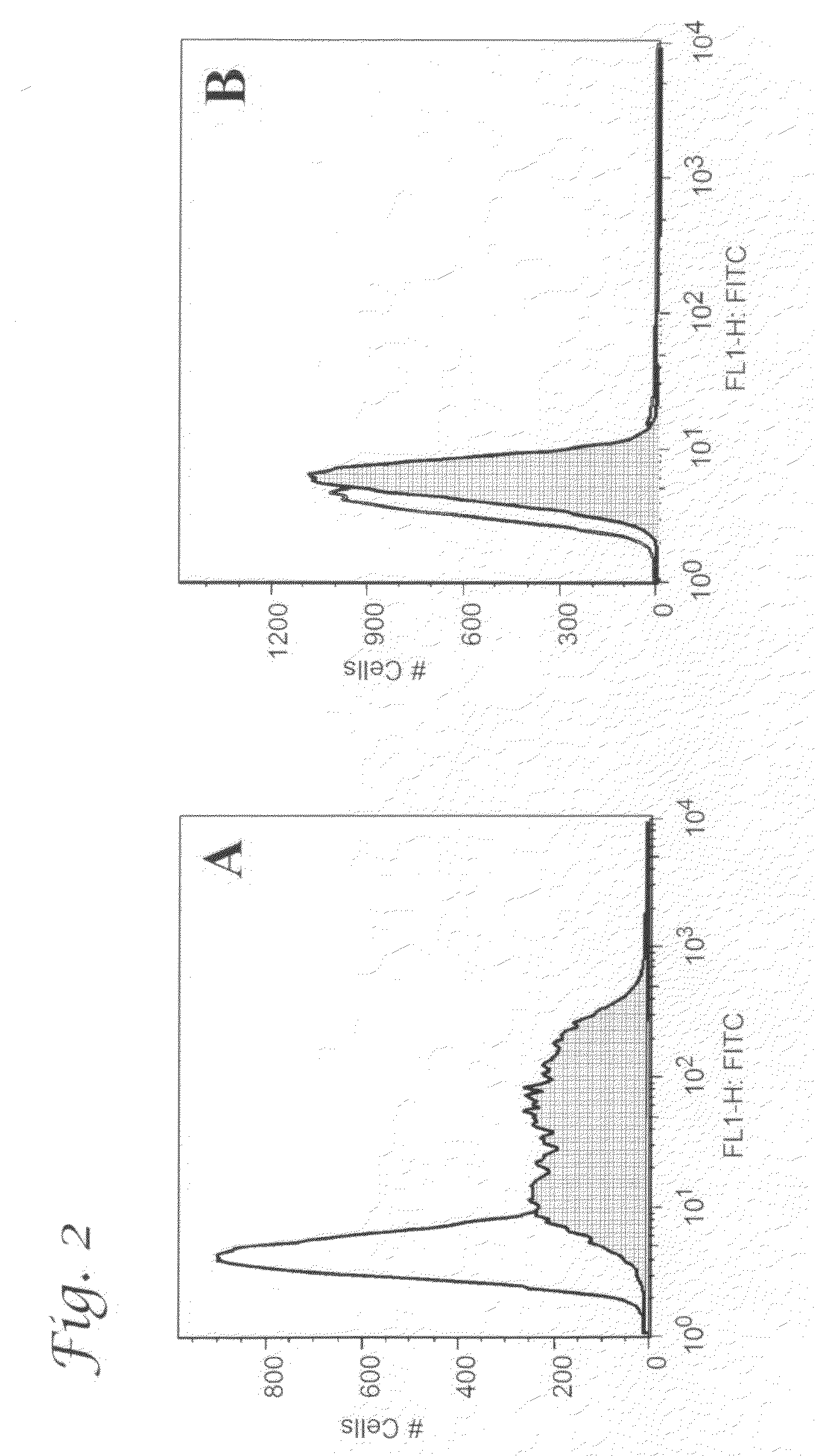
Patents
Literature
2903 results about "Polyclonal antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
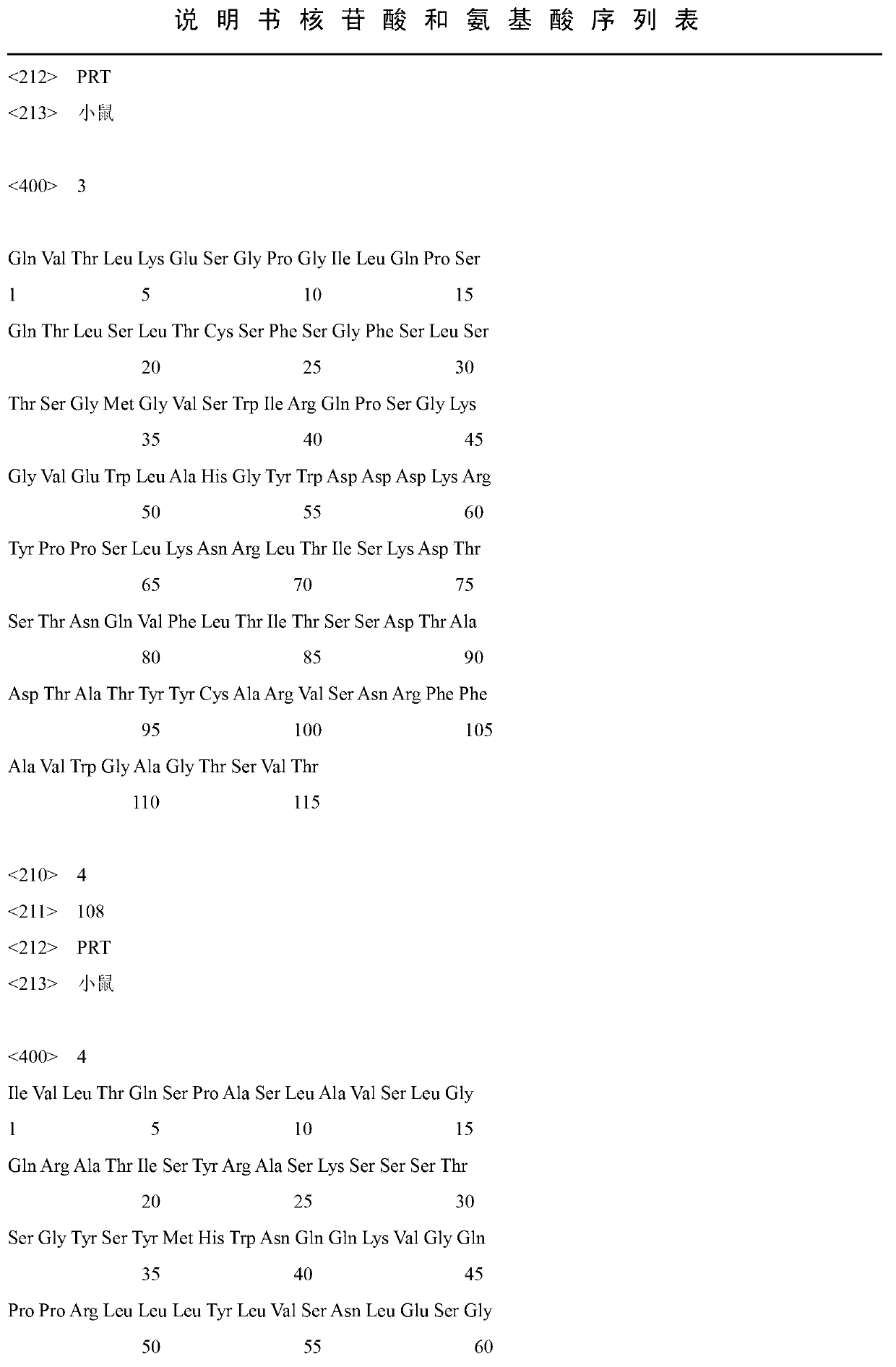
Polyclonal antibodies (pAbs) are antibodies that are secreted by different B cell lineages within the body (whereas monoclonal antibodies come from a single cell lineage). They are a collection of immunoglobulin molecules that react against a specific antigen, each identifying a different epitope.
Polyclonal antibody composition for treating allergy
InactiveUS6849259B2Efficient removalPotential clinical advantageImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
Compositions and methods useful for the diagnosis and treatment of heparin induced thrombocytopenia/thrombosis
InactiveUS6964854B1Animal cellsImmunoglobulins against cytokines/lymphokines/interferonsHeparin antibodyMammal
The invention includes compositions, kits and methods comprising a monoclonal antibody which shares key functional properties with the polyclonal antibodies which participate in the pathogenesis of heparin induced thrombocytopenia / thrombosis (HIT / HITT) in a mammal. The monoclonal antibody of the invention preferentially binds with a PF4 / heparin complex relative to the binding of the antibody with PF4 or heparin alone. The monoclonal antibody of the invention also binds specifically with PF4 in a complex with other glycosaminoglycans besides heparin, and also activates platelets. The monoclonal antibody of the invention is useful in methods for diagnosing and treating HIT / HITT in a mammal. A humanized version of the monoclonal antibody of the invention is also included, along with a process for humanizing the monoclonal antibody of the invention.
Owner:STC UNM
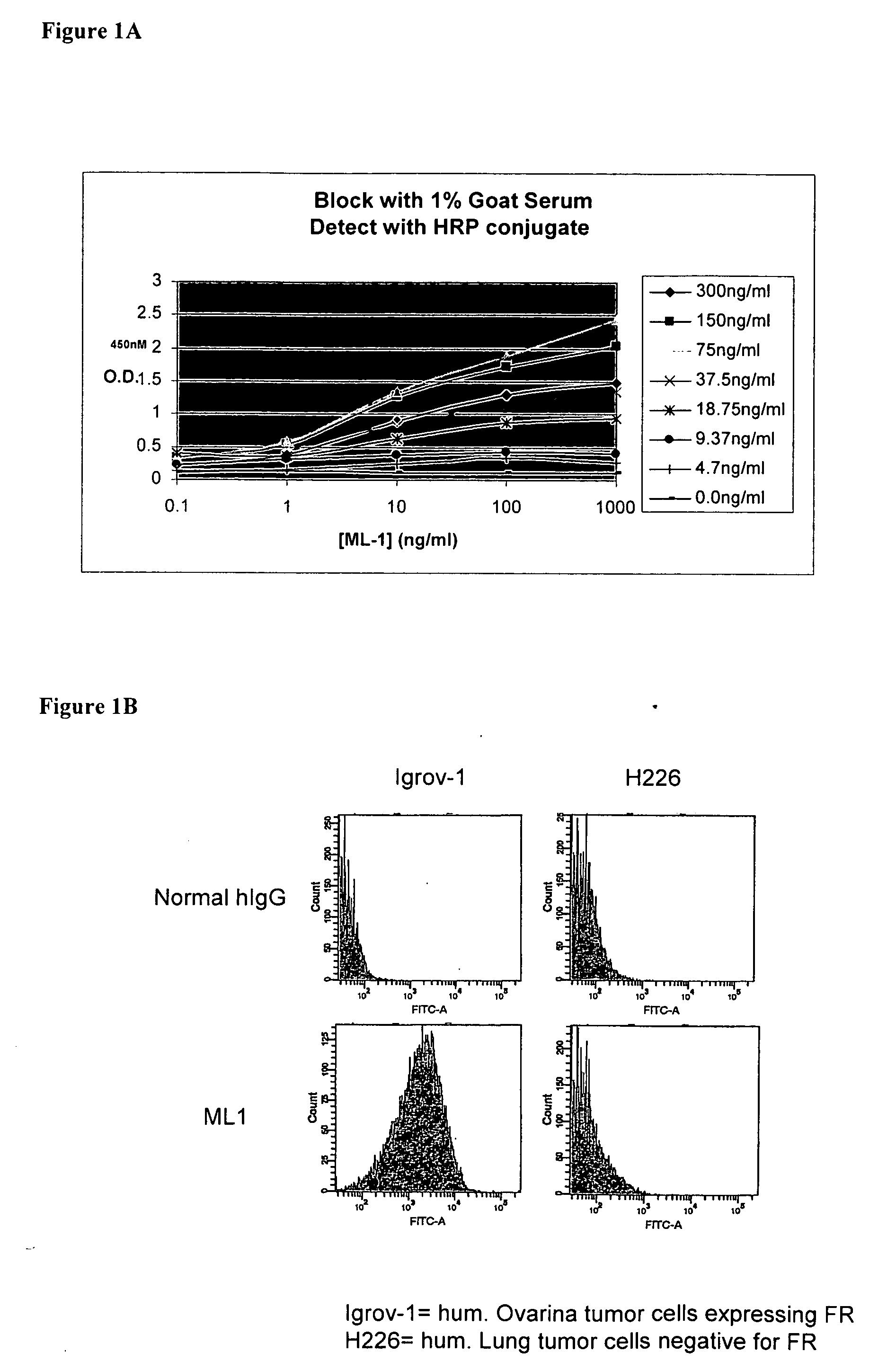
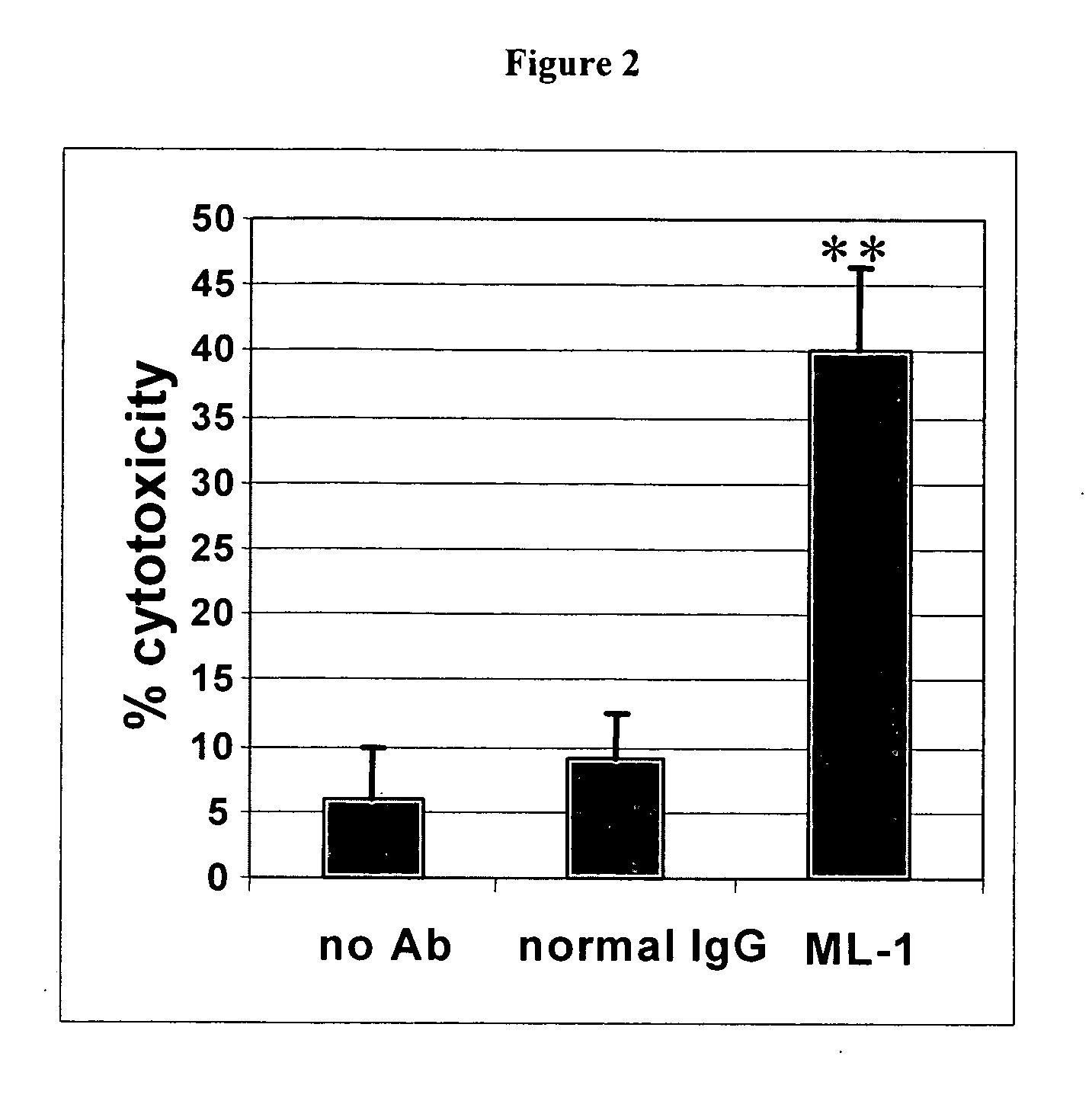
Antibodies with immune effector activity and that internalize in folate receptor alpha-positive cells
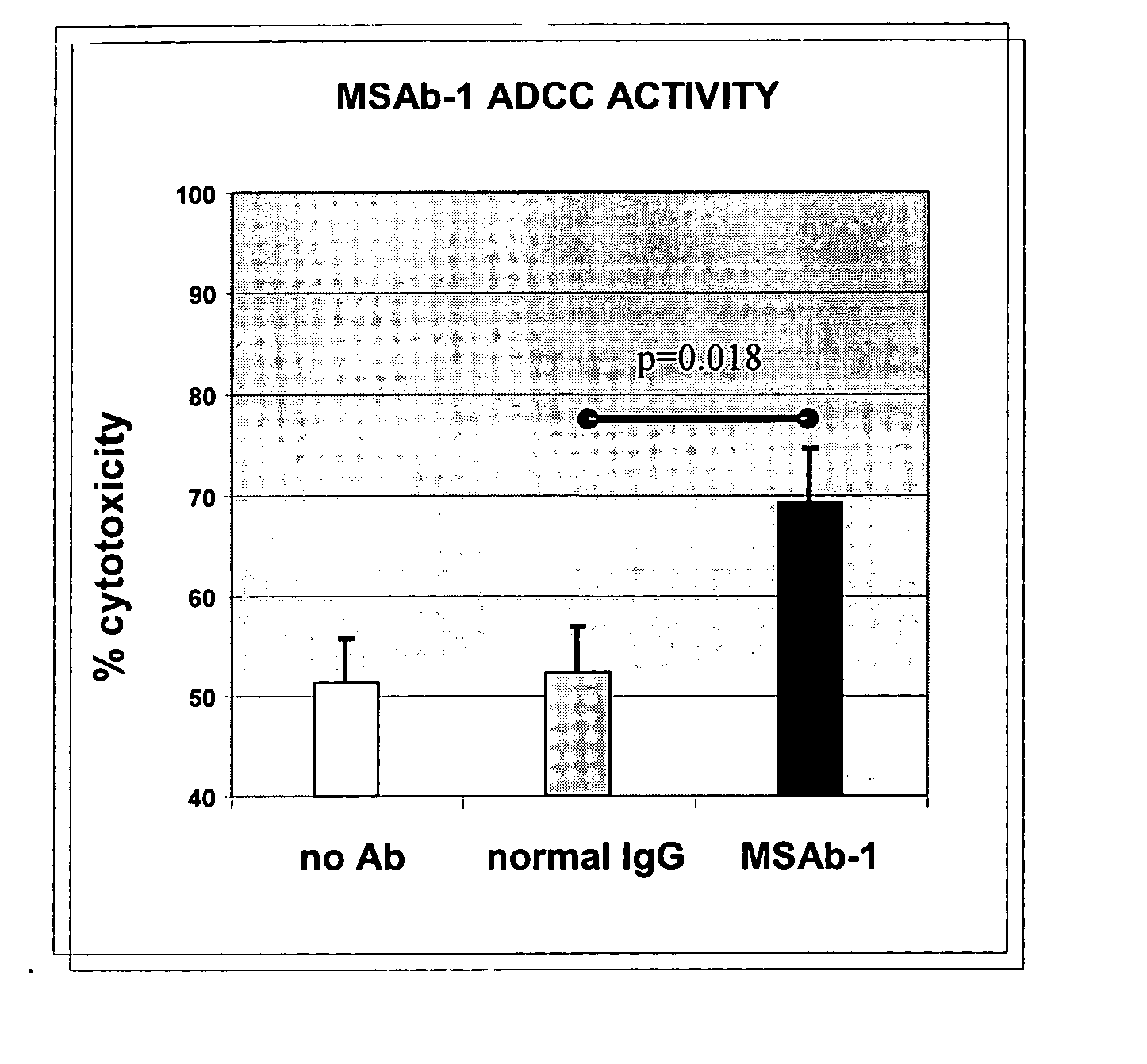
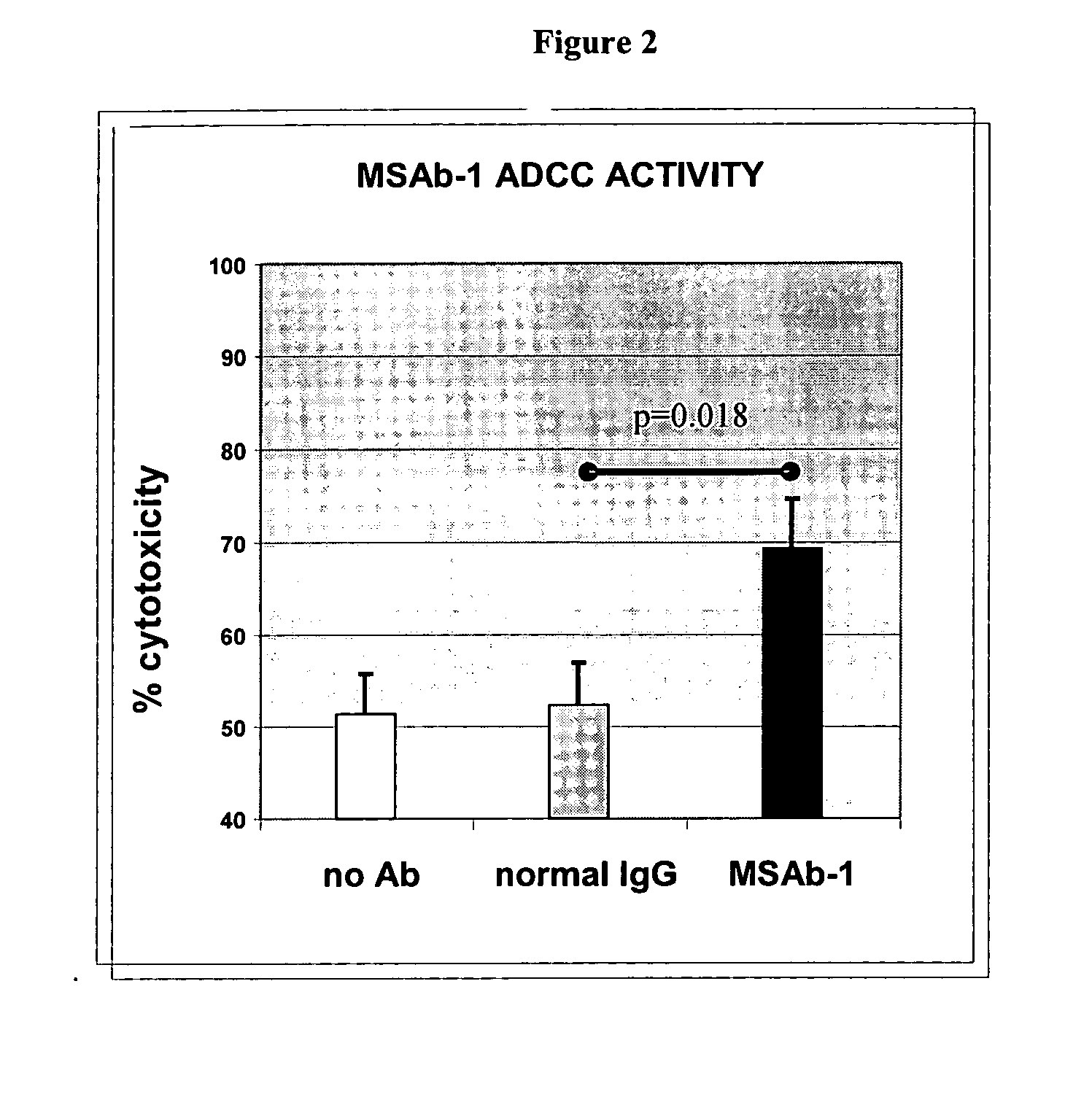
InactiveUS20060239910A1Improve anti-tumor activityEnhanced antibody-dependent cellular cytotoxicityOrganic active ingredientsNanomedicineDrug specific IgEFolate Receptor Alpha
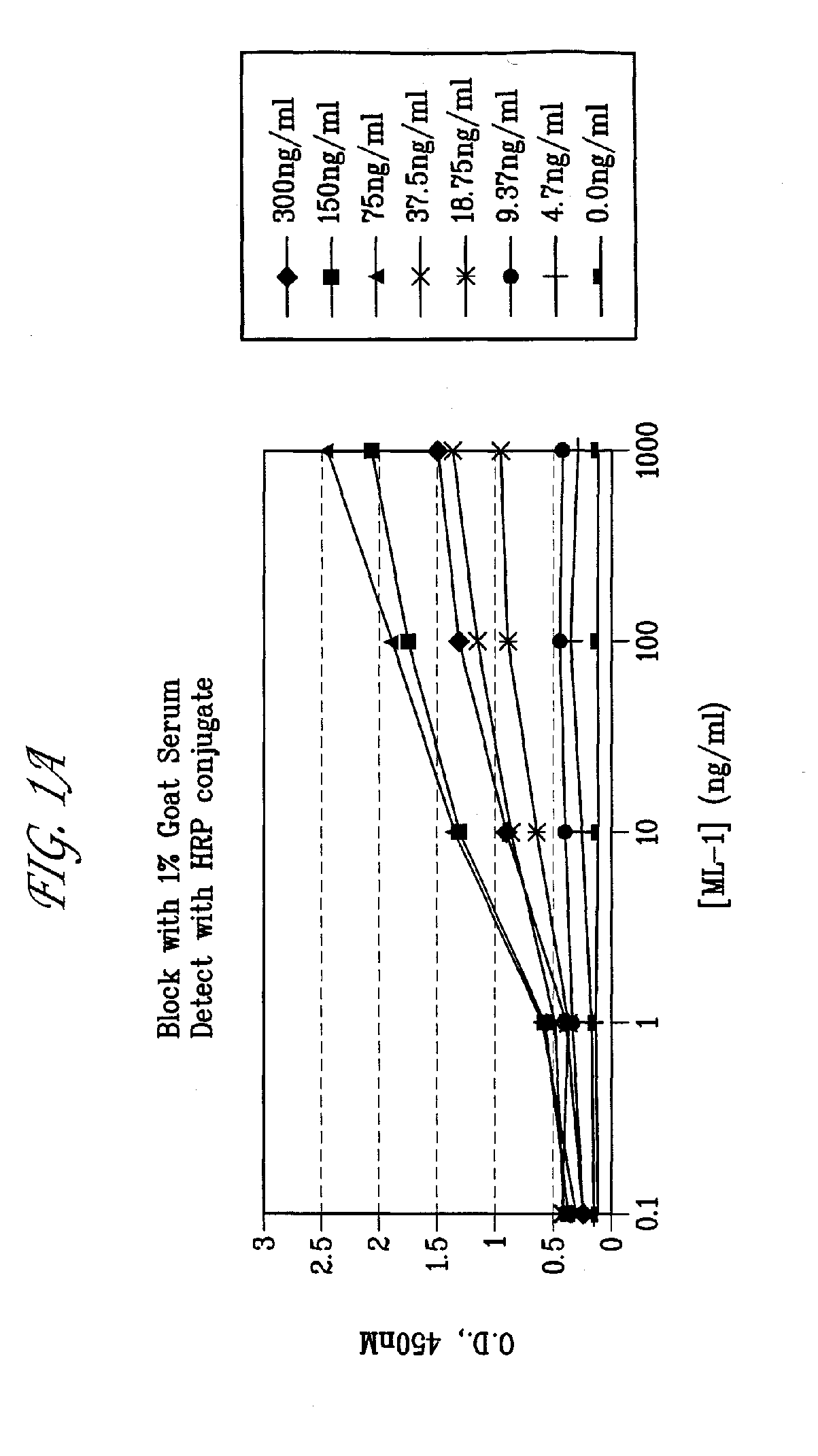
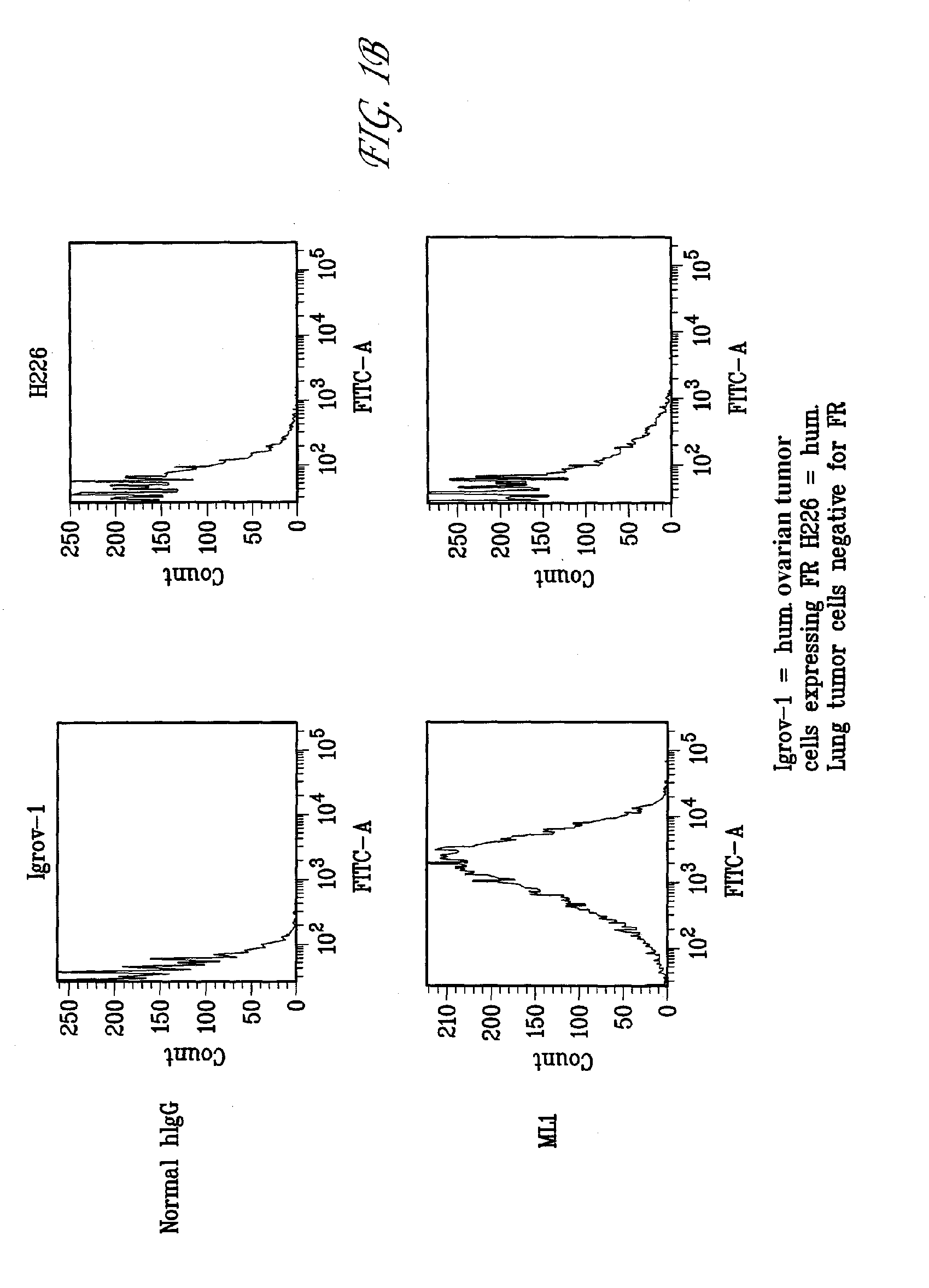
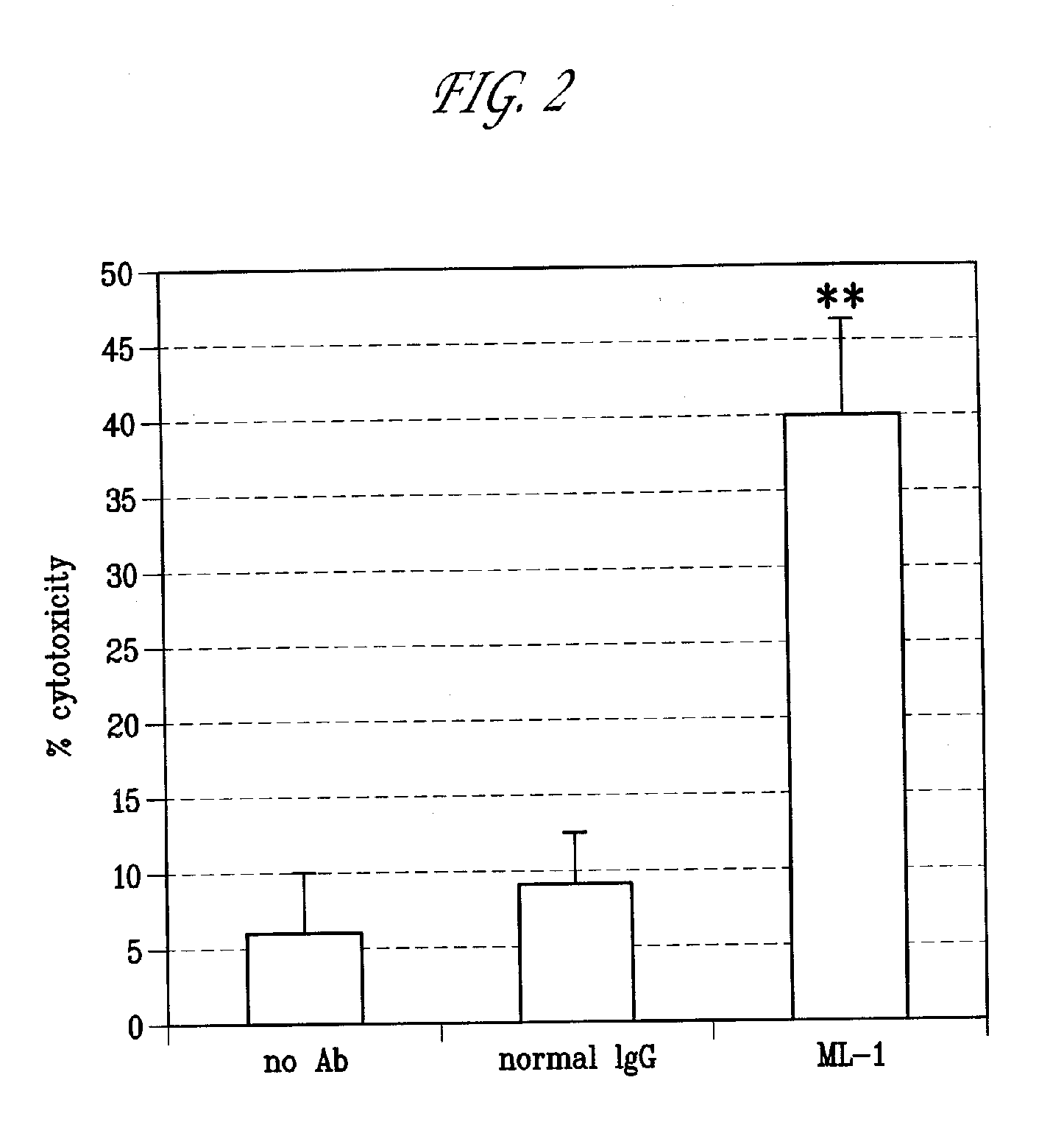
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
Recombinant polyclonal antibody for treatment of respiratory syncytial virus infections
InactiveUS20100040606A1Reduce the possibilityMinimizing developmentSsRNA viruses negative-senseSugar derivativesEpitopeProtein G
Disclosed are novel polyclonal antibodies, which target respiratory syncyticilal virus (RSV), and novel high affinity antibody molecules reactive with RSV. The polyclonal antibodies may comprise antibody molecules which are reactive with both RSV protein F and RSV protein G, and preferably the polyclonal antibodies target a variety of epitopes on these proteins. The single antibody molecules of the invention are shown to exhibit affinities which provide for dissociation constants as low as in the picomolar range. Also disclosed are methods of producing the antibodies of the invention as well as methods of their use in treatment for RSV infection.
Owner:SYMPHOGEN AS
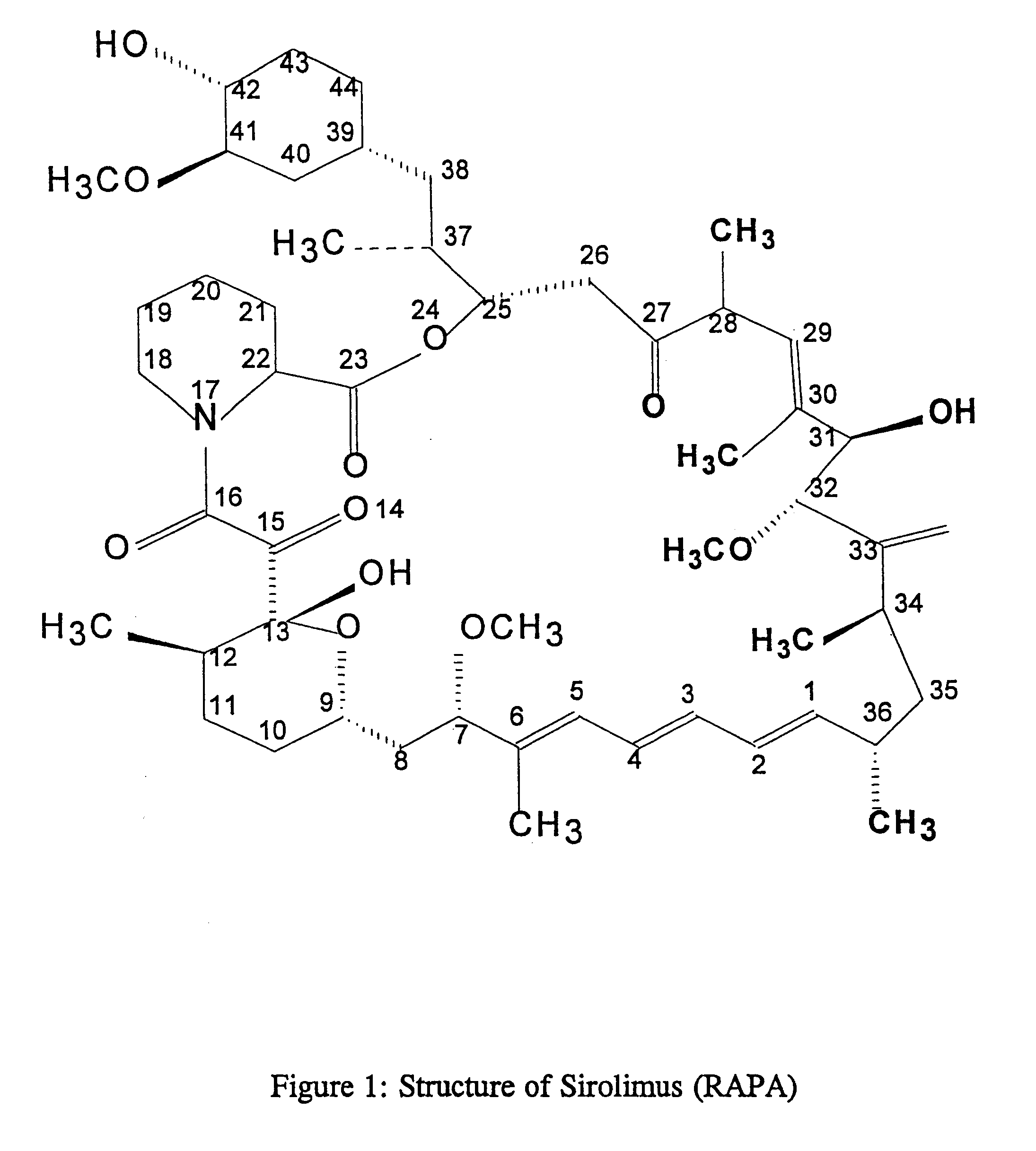
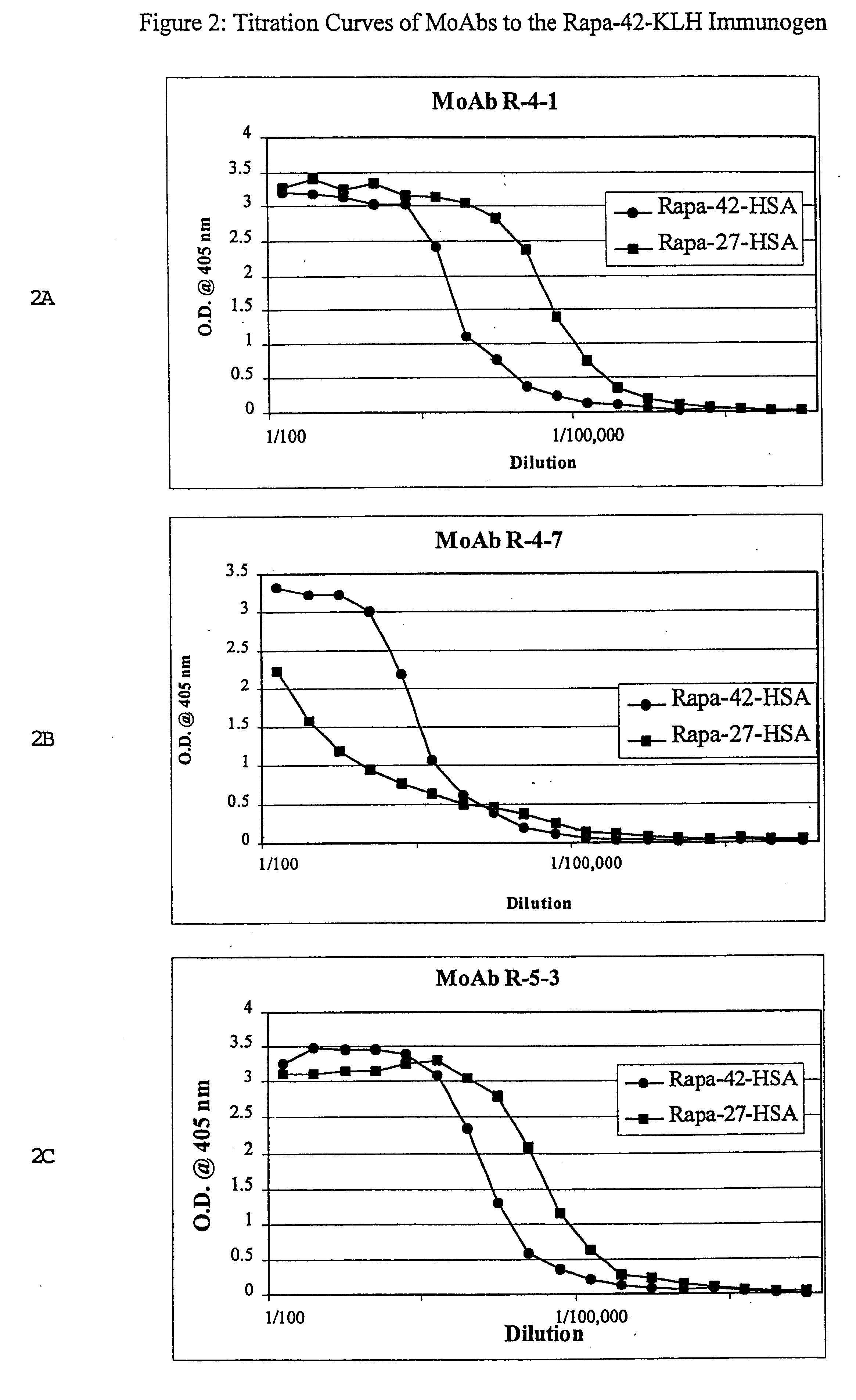
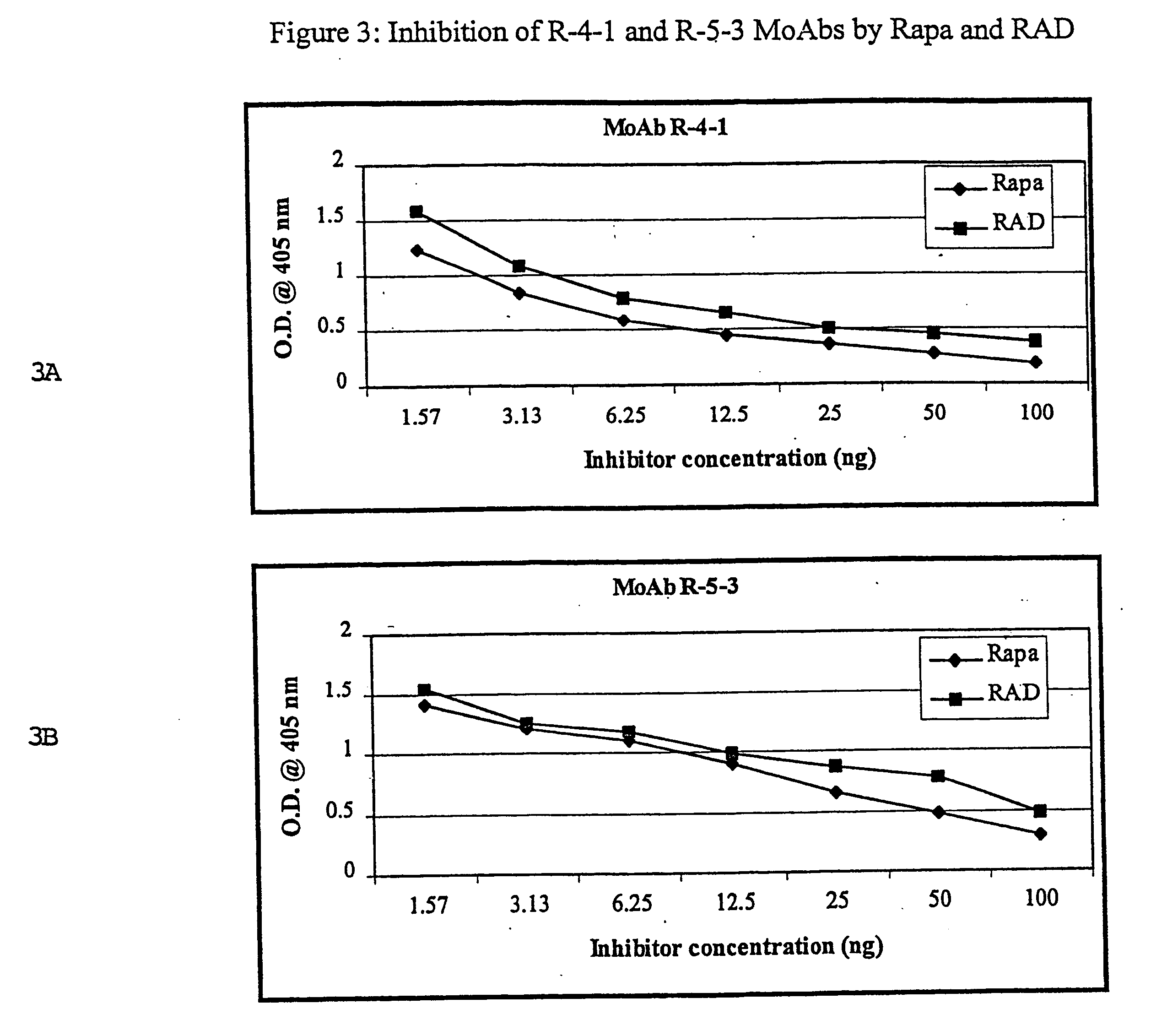
Method for production of antibodies to specific sites of rapamycin
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
Anti-orthopoxvirus recombinant polyclonal antibody
InactiveUS20080069822A1Increased activationGood removal effectAnimal cellsMicrobiological testing/measurementGenus OrthopoxvirusPolyclonal antibodies
Disclosed is an anti-orthopoxvirus recombinant polyclonal antibody comprising distinct members which in union are capable of binding at least three orthopoxvirus related antigens, a pharmaceutical composition comprising the antibody, and a method for its production. Also disclosed is a polyclonal cell line capable of producing the recombinant polyclonal antibody as therapeutic methods utilizing the polyclonal antibody. Finally, the invention also pertains to a method for screening for useful VH and VL pairs useful when preparing the polyclonal antibody.
Owner:SYMPHOGEN AS
Kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay
ActiveCN102628864AStrong specificityImprove accuracyColor/spectral properties measurementsBiological testingLatex particlePHA granule
The invention relates to a kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay. Specifically, the kit for determining the heart-type fatty acid binding protein comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 contains a reaction promoter, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; the reagent R2 contains latex particles with binding of anti-heart-type fatty acid binding protein monoclonal antibody and polyclonal antibody, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; and the calibrator contains an antiseptic, an electrolyte, a stabilizing agent, a heart-type fatty acid binding protein pure product and a buffer. By the complex coating method of latex particles with the monoclonal antibody and the polyclonal antibody, high sensitivity and wide linear range of the kit are guaranteed. Simultaneously, the kit also has advantages of high accuracy, good repeatability, strong singularity, easy operation and the like, and is applicable to an automatic biochemical analyzer which is commonly used in clinic.
Owner:BEIJING STRONG BIOTECH INC
Fluorescent microsphere immunity chromatography test paper for detecting food-borne pathogenic microbe
InactiveCN101464464AExpand the scope ofIncrease varietyMaterial analysisPathogenic microorganismGlass fiber
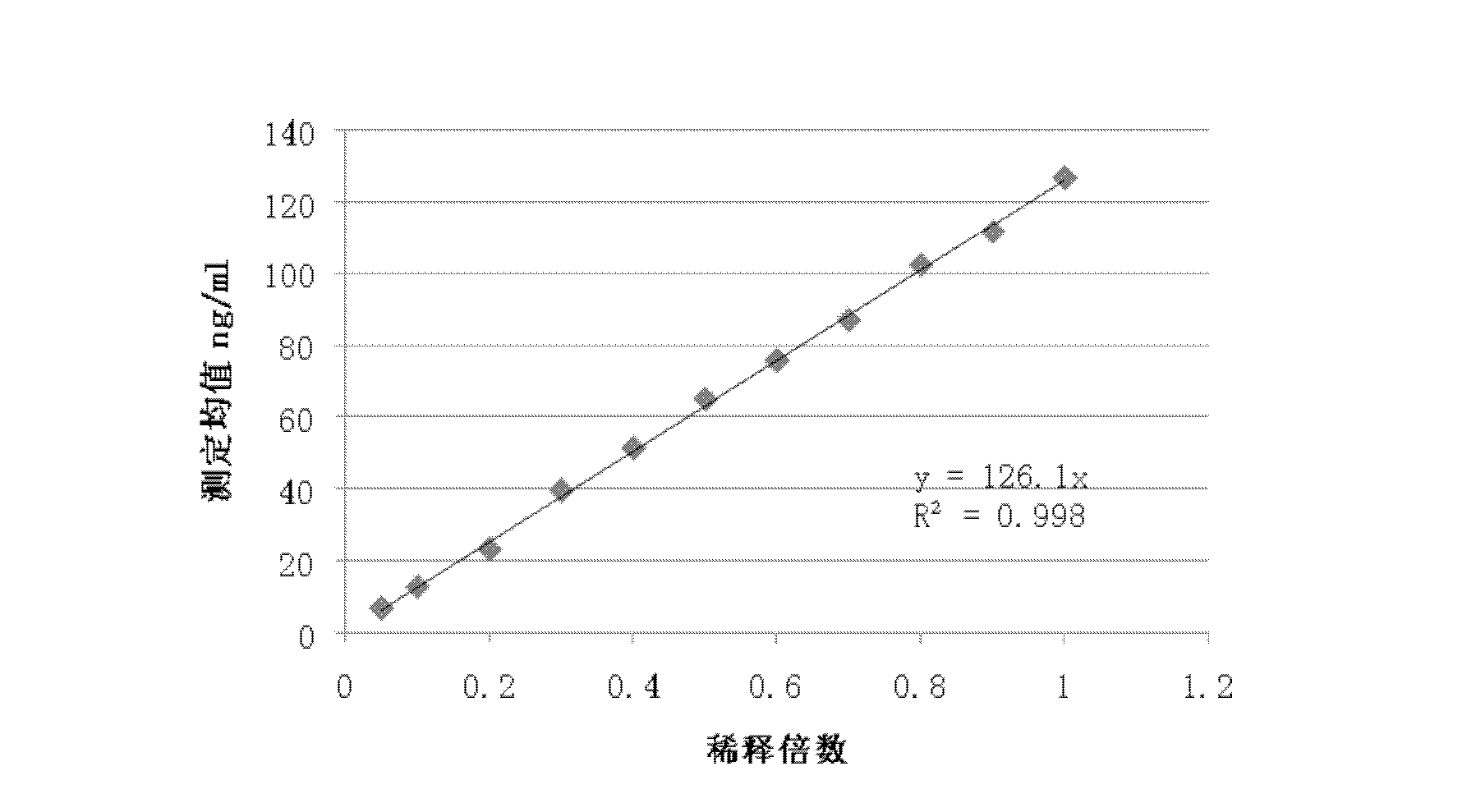
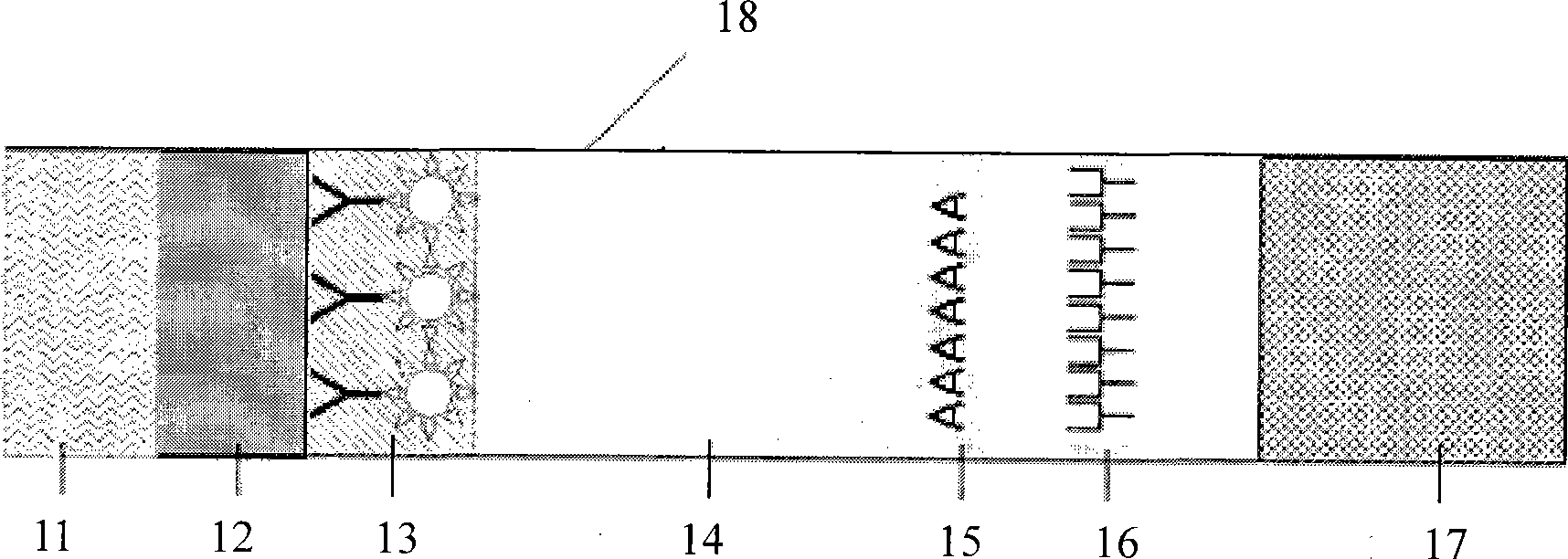
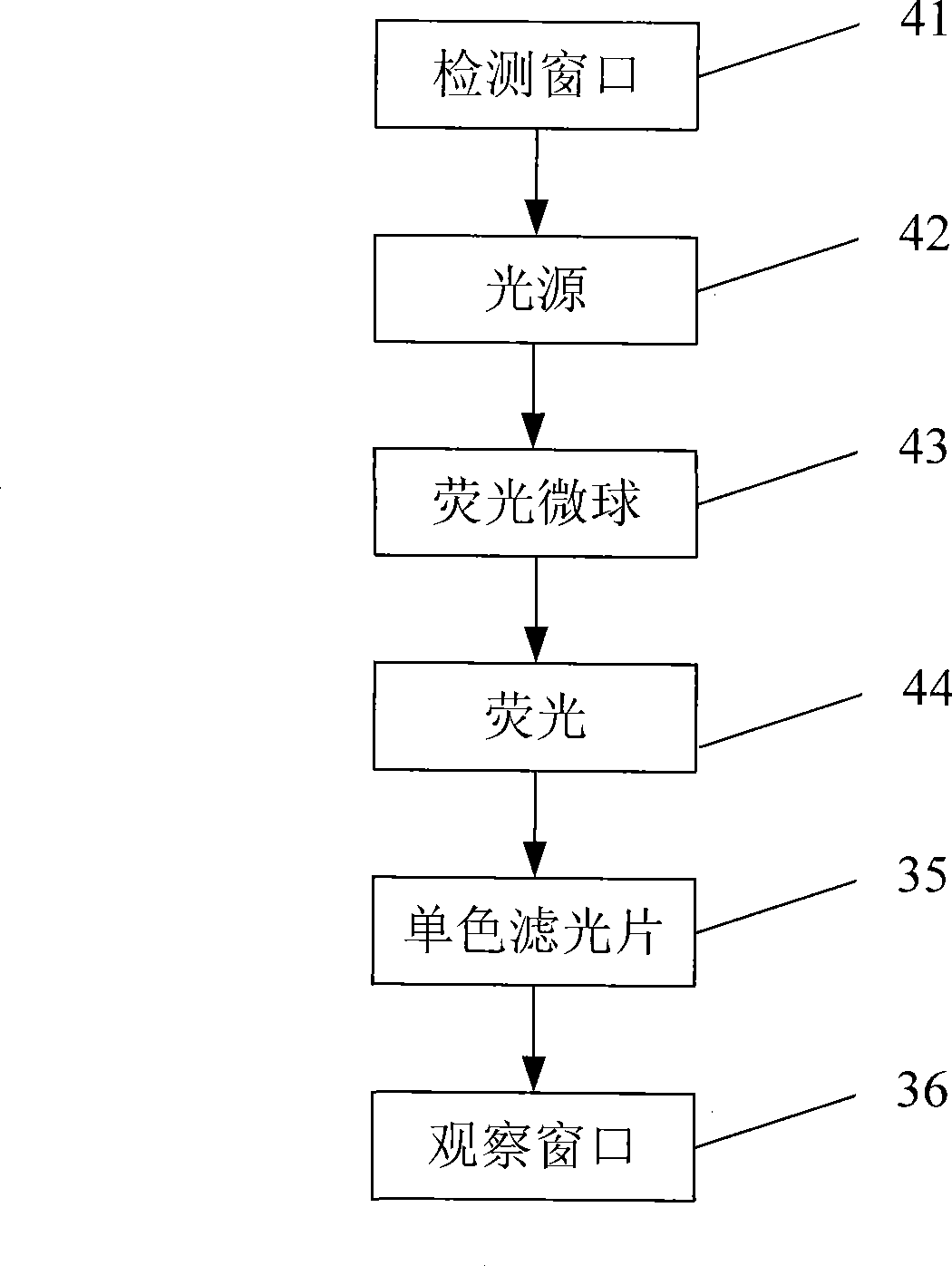
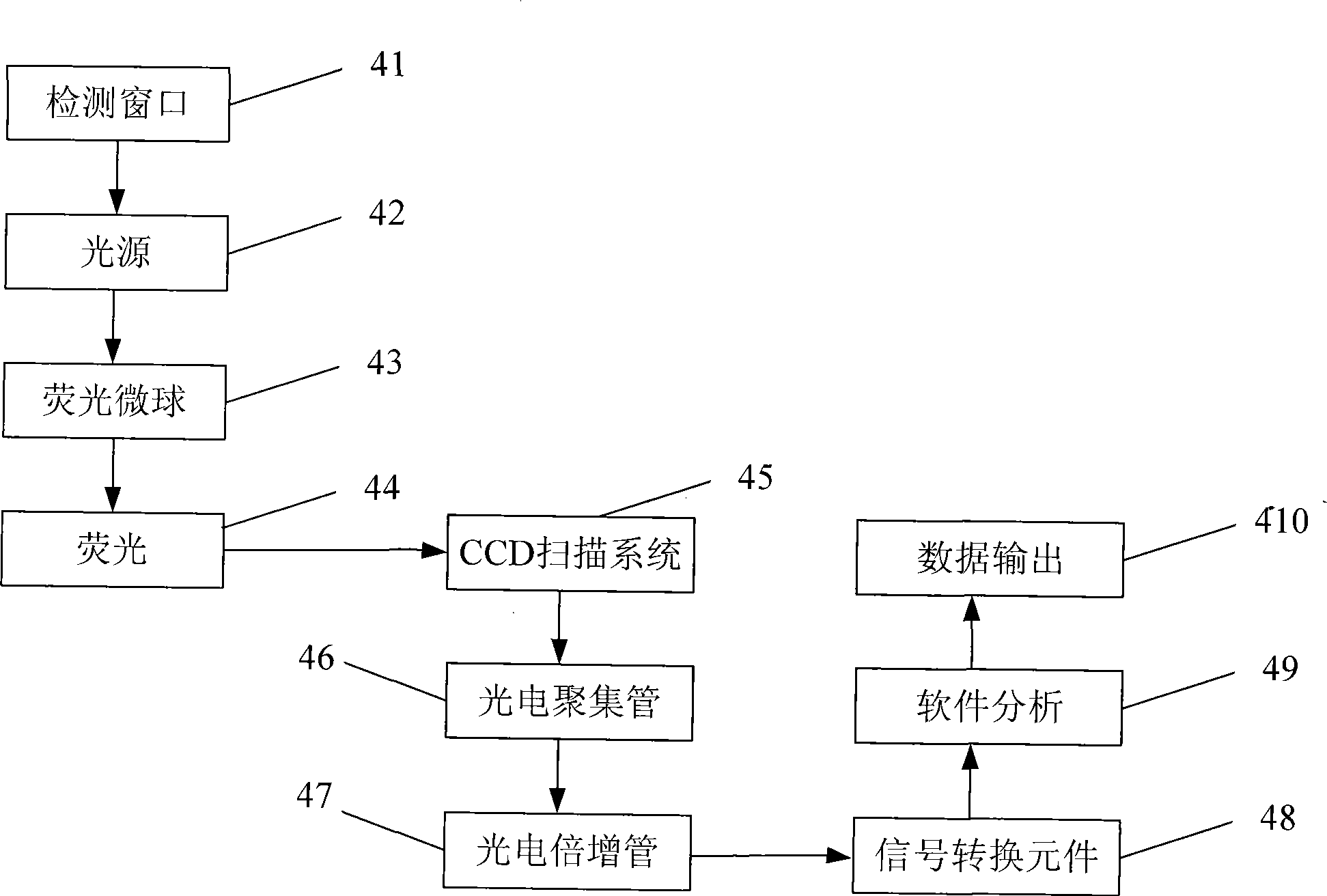
The invention discloses a fluorescent microsphere immunity-chromatography test strip for detecting food-borne pathogenic microorganisms in a rapid, specific, qualitative and quantitative manner and a preparation method thereof. Filter paper, a sample pad, a glass fiber membrane spray-painted by fluorescent microsphere marked food-borne pathogenic microorganism monoclonal antibodies or polyclonal antibodies, a nitrocellulose membrane and drinking paper are stuck to an underplate orderly in a related joint manner; the food-borne pathogenic microorganism monoclonal antibodies or the polyclonal antibodies are coated on the nitrocellulose membrane and are used as a detection area; and anti-mouse antibodies are coated on the nitrocellulose membrane and used as a quality control area. The test strip has the preparation steps as follows: (1) samples to be detected are processed; (2) the nitrocellulose membrane is prepared; (3) a fluorescent microsphere pad is prepared; (4) the test strip is assembled; and during the detection process, the CCD scanning technique is utilized to collect all emission spectrums, and then the emission spectrums are analyzed through a fluorescence analyzer and relevant software after accumulation and multiplication, so that the quantitative detection is realized. The fluorescent microsphere immunity-chromatography test strip and the preparation method thereof are mainly used for qualitative and quantitative detection of all the food-borne pathogenic microorganisms in food safety inspection.
Owner:WUXI ZODOLABS BIOTECH
Anti-mesothelin antibodies
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and become internalized by mesothelin-positive cells and also induce an immune effector activity such as antibody dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to mesothelin expressing cells as well as eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to cells expressing the monoclonal antibodies, polyclonal antibodies, antibody derivatives, such as human, humanized, and chimeric monoclonal antibodies, antibody fragments, mammalian cells expressing the monoclonal antibodies, derivatives and fragments, and methods of treating cancer using the antibodies, derivatives and fragments.
Owner:EISAI INC
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
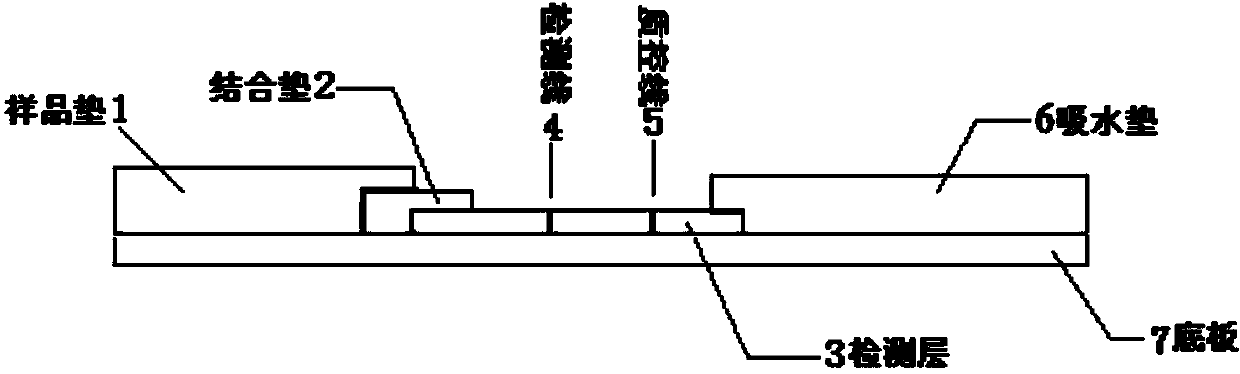
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
Monoclonal Antibodies That Specifically Block Biological Activity Of A Tumor Antigen
ActiveUS20090274697A1Sugar derivativesBiological material analysisAntibody fragmentsMonoclonal antibody
This invention relates to novel monoclonal antibodies that specifically bind to the alpha-folate receptor. In some embodiments, the antibodies inhibit a biological activity of folate receptor-α (FR-α). The antibodies are useful in the treatment of certain cancers, particularly cancers that have increased cell surface expression of the alpha-folate receptor (“FR-α”), such as ovarian, breast, renal, colorectal, lung, endometrial, or brain cancer. The invention also relates to cells expressing the monoclonal antibodies, antibody derivatives, such as chimeric and humanized monoclonal antibodies, antibody fragments, and methods of detecting and treating cancer using the antibodies, derivatives, and fragments.
Owner:EISAI INC
Recombinant anti-epidermal growth factor receptor antibody compositions
ActiveUS7887805B2Reduce exerciseReduction tendencyImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHuman cancerCancer cell
Owner:LES LAB SERVIER SA
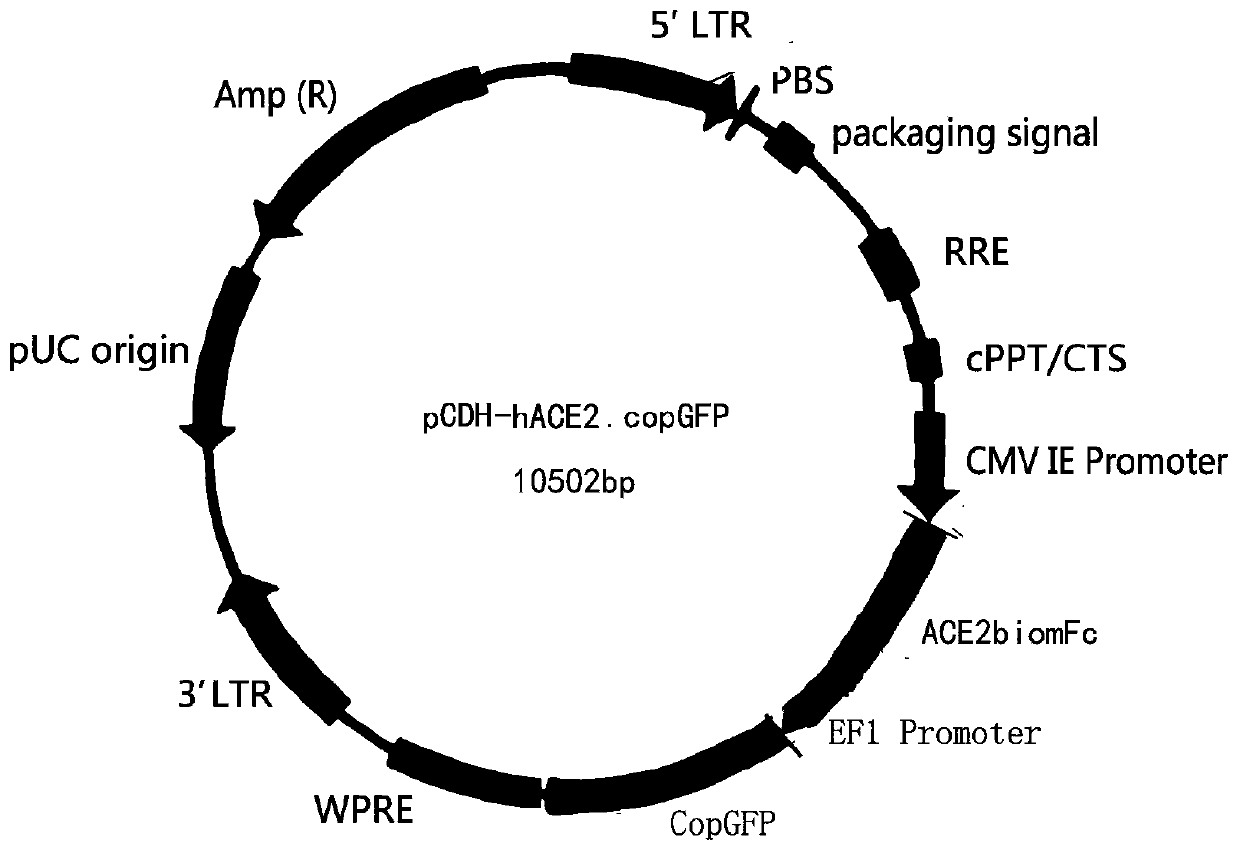
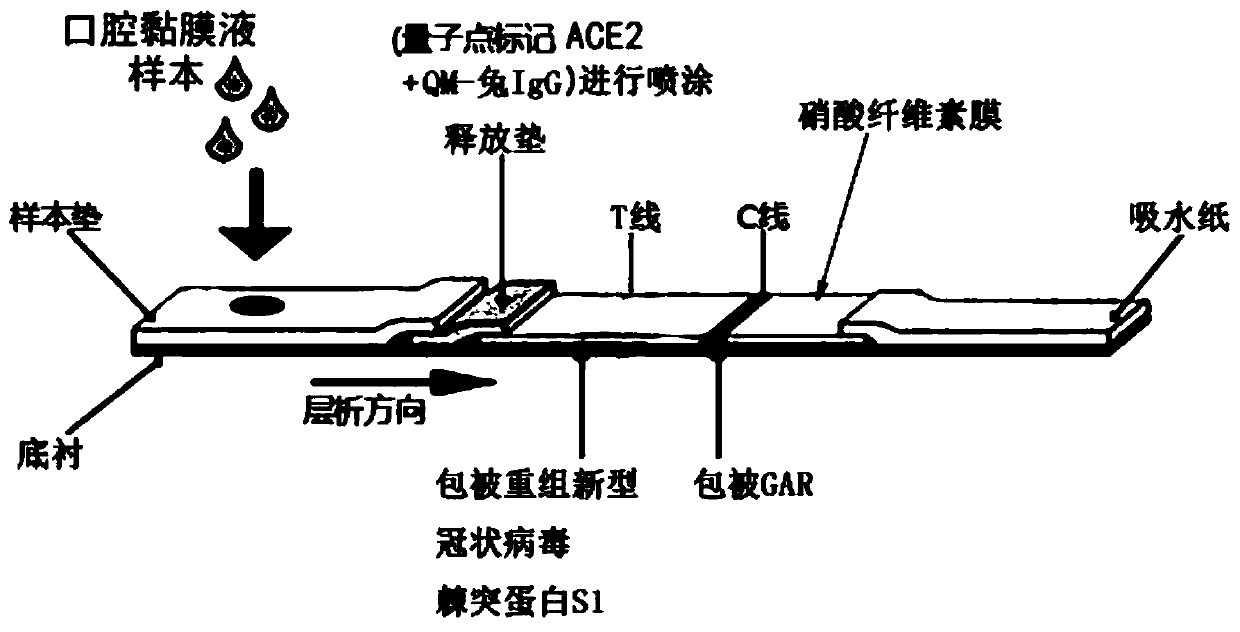
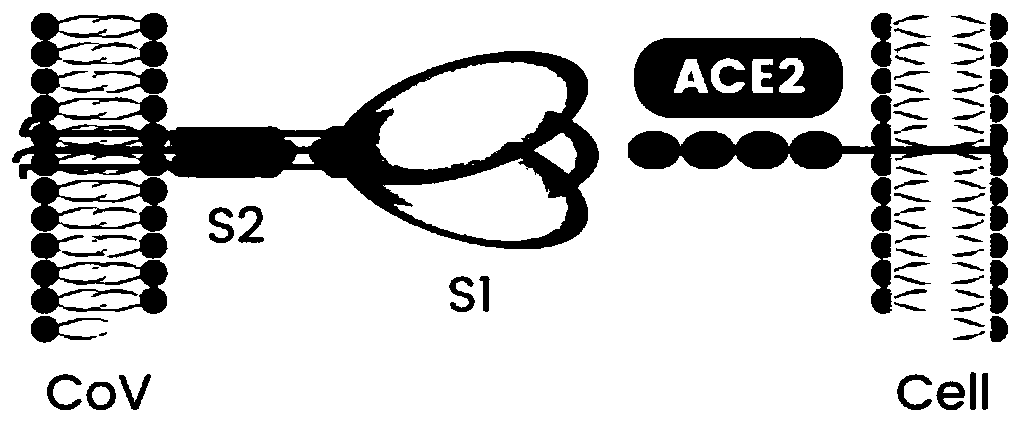
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
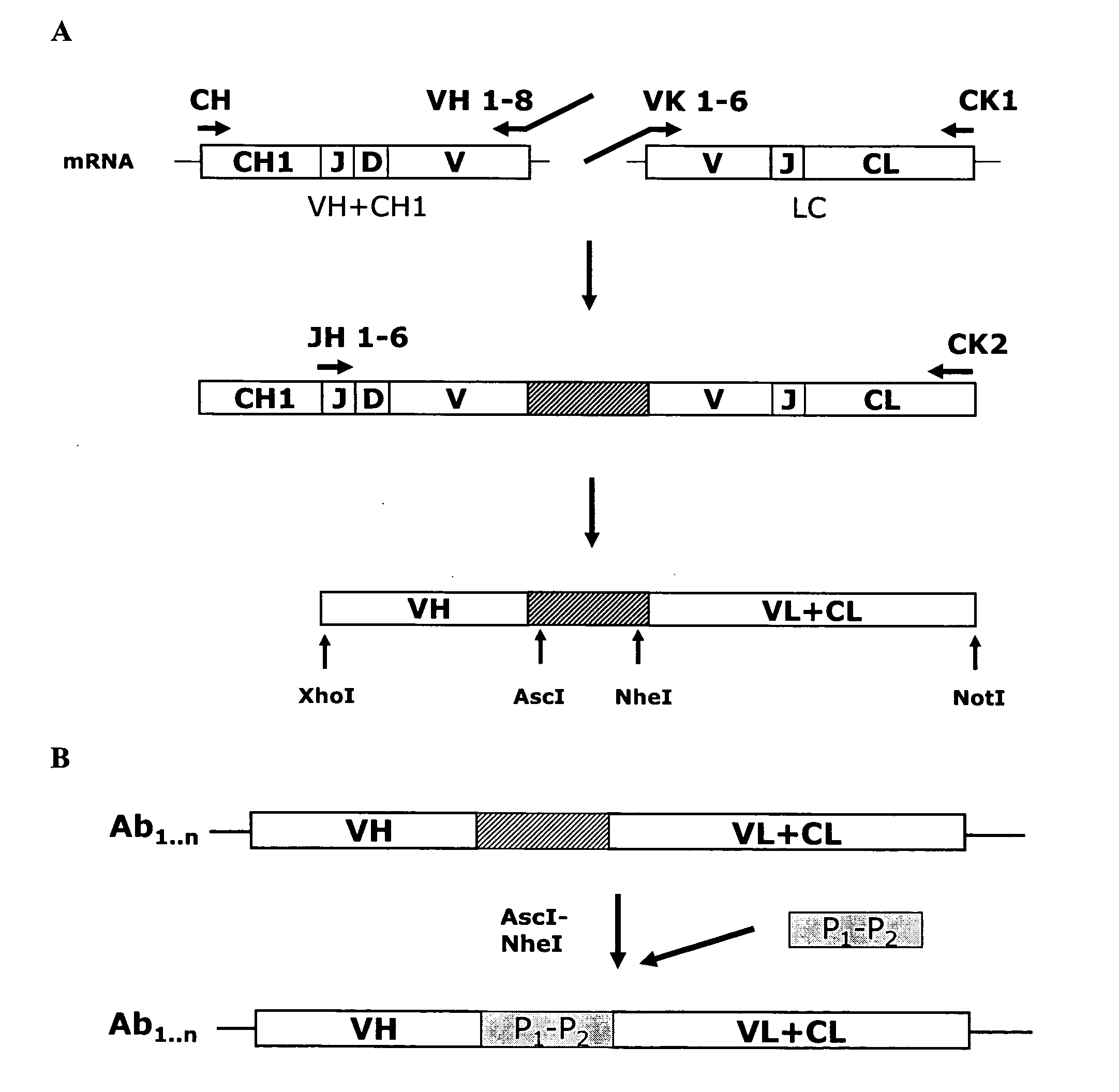
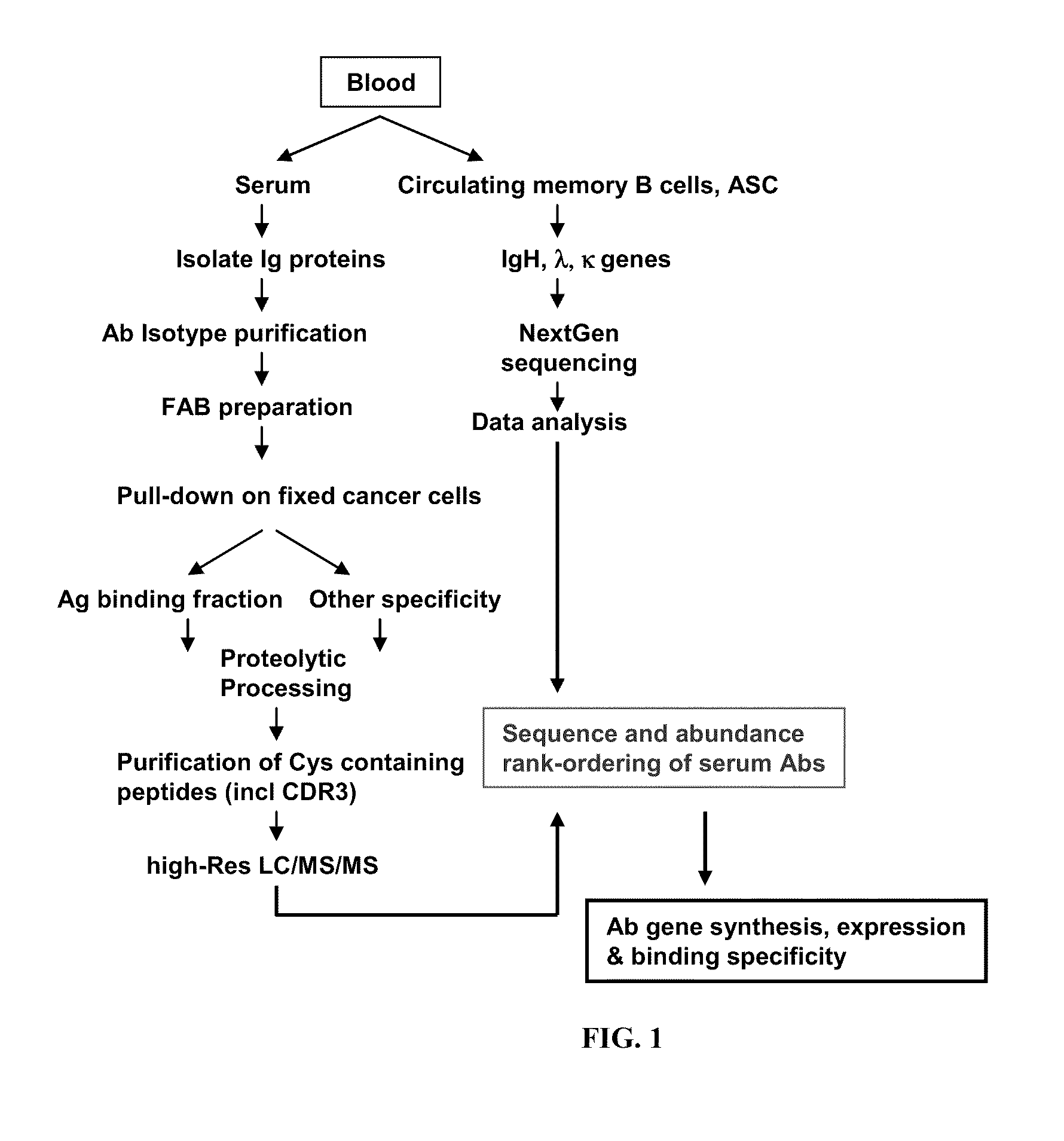
Rapid Isolation of Monoclonal Antibodies from Animals
ActiveUS20110312505A1Microbiological testing/measurementLibrary screeningSerum igeAntigen Binding Fragment
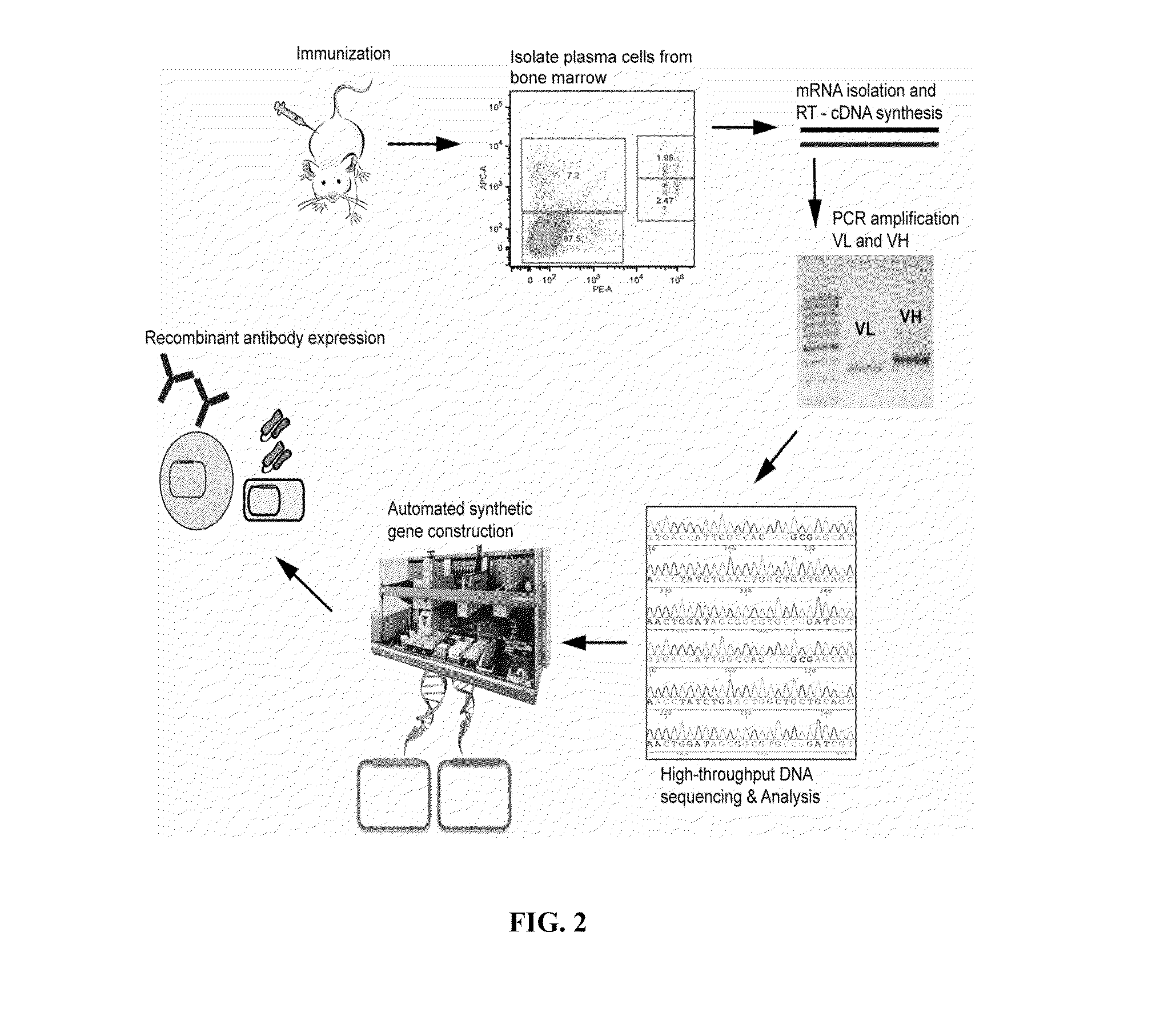
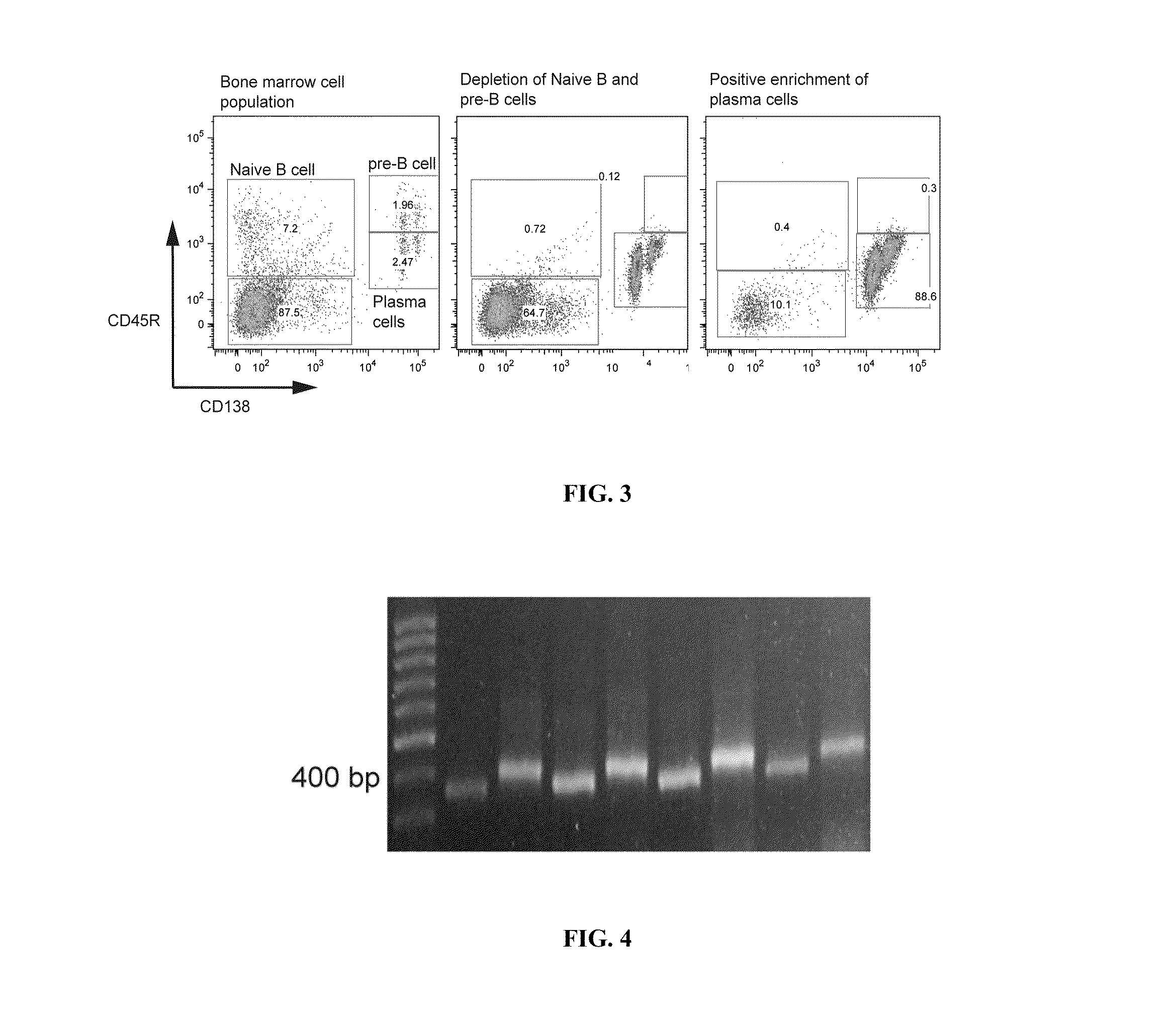
Methods and compositions for identification of candidate antigen-specific variable regions as well as generation of antibodies or antigen-binding fragments that could have desired antigen specificity are provided. For example, in certain aspects methods for determining amino acid sequences of serum antibody CDR and abundancy level are described. In some aspects, methods for determining nucleic acid sequences of antibody variable region sequences and frequency are provided. Furthermore, the invention provides methods for identification and generation of antibody or antigen-binding fragments that comprise highly-represented CDR.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
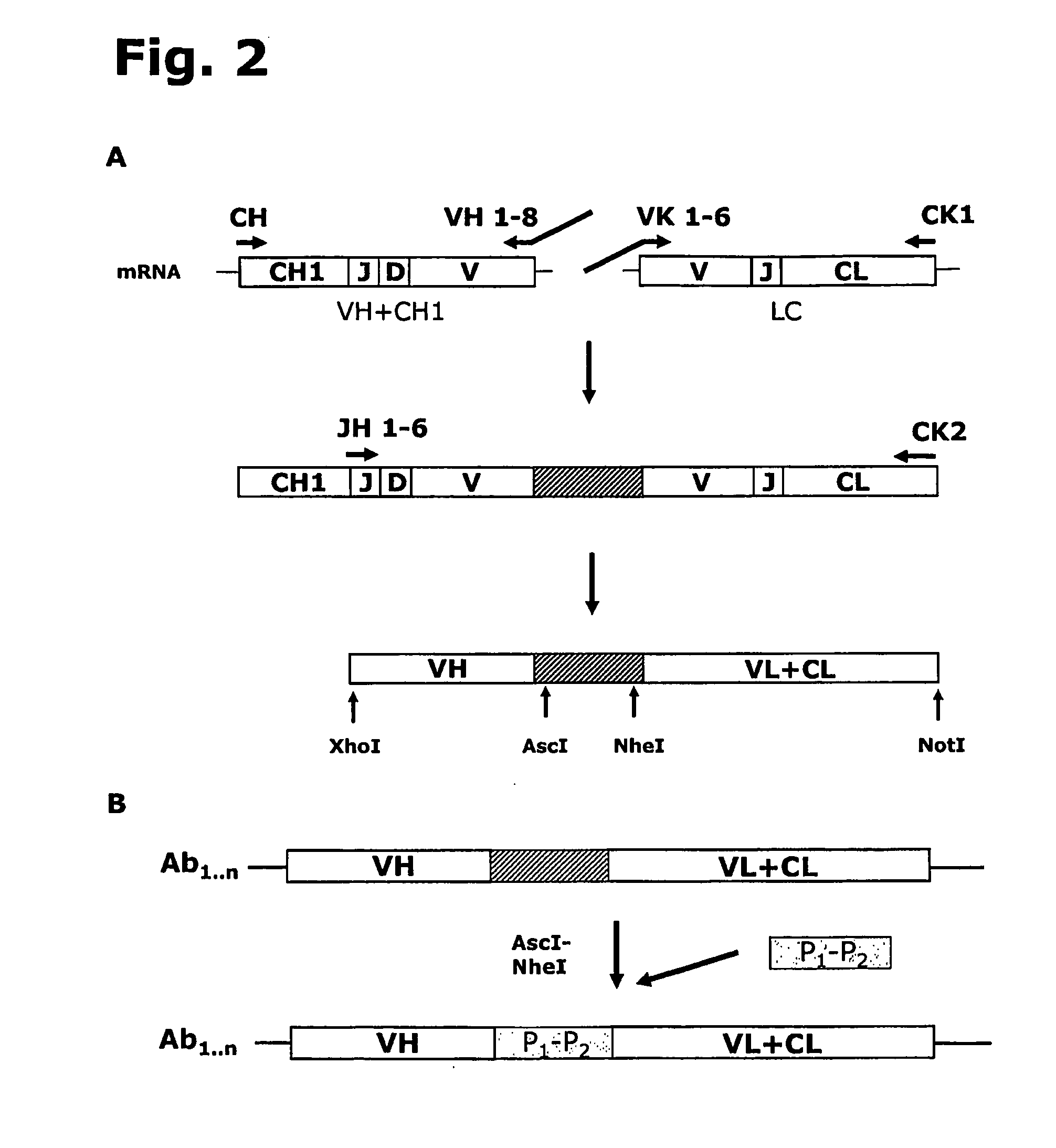
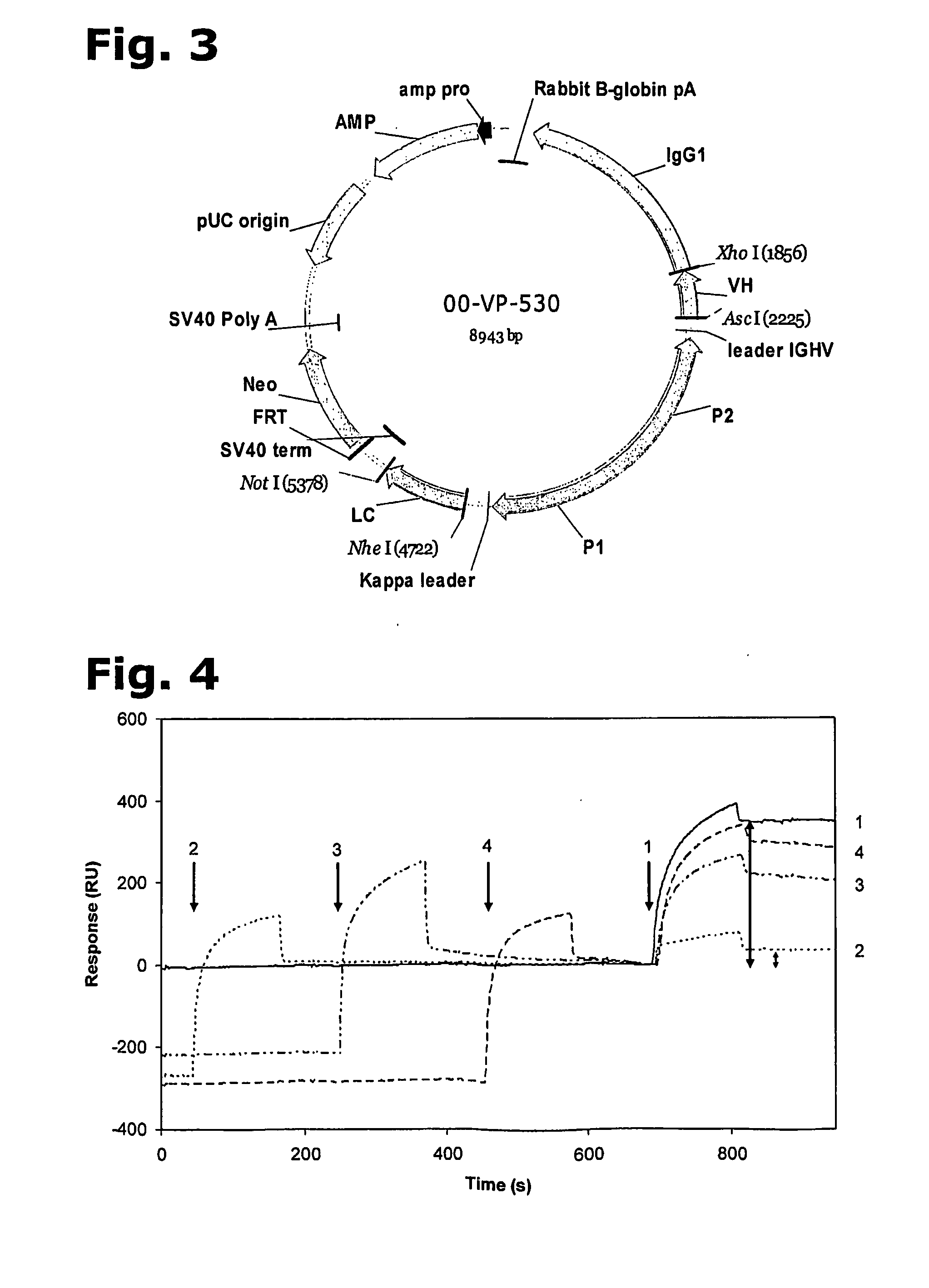
Affinity purified human polyclonal antibodies and methods of making and using them
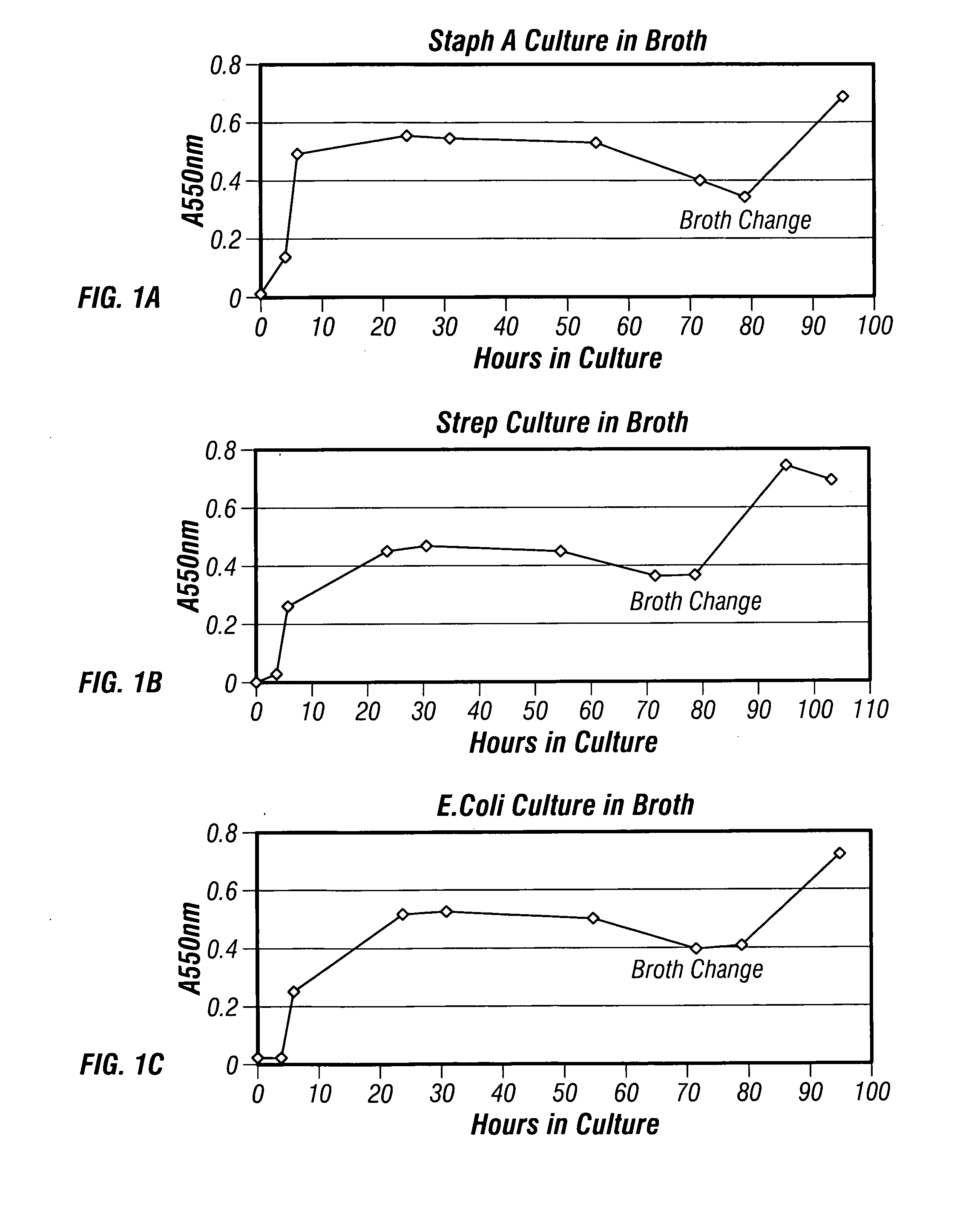
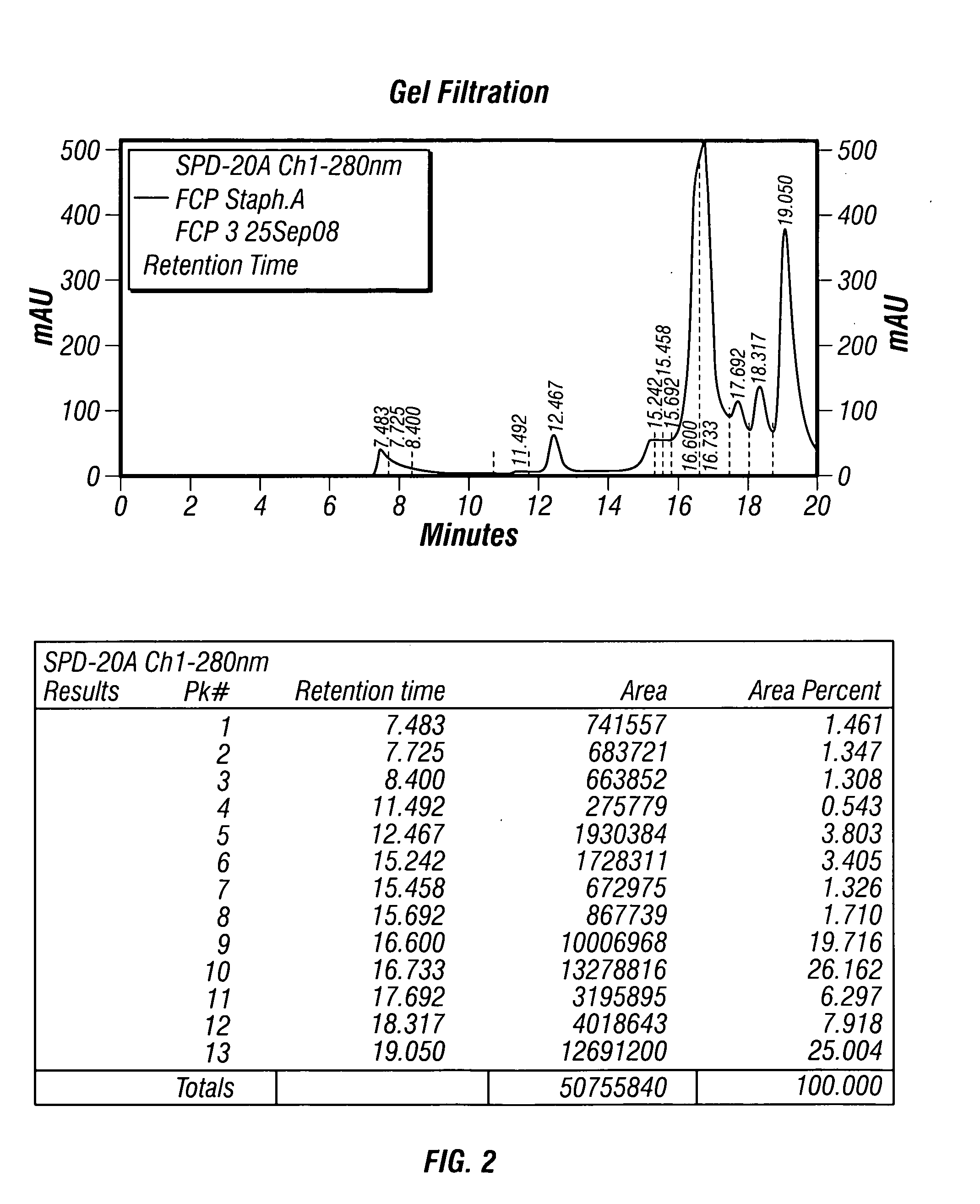
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
Vibrio parahemolyticus detection kit and detection method thereof
InactiveCN102586452AEfficient analysisHigh throughput processingMicrobiological testing/measurementVibrio parahemolyticusMicrobiology
The invention relates to a vibrio parahemolyticus detection kit. The vibrio parahemolyticus detection kit is characterized by comprising the following components: (1) an immune enrichment reaction system component, (2) a loop-mediated isothermal amplification (LAMP) system component and (3) a set of specific primers based on an LAMP technology of vibrio parahemolyticus tlh genes, wherein the specific primers comprise two outer primers F3 and B3, two inner primers FIP and BIP and two ring primers LF and LB. The detection method for the vibrio parahemolyticus detection kit comprises the following steps of: detecting the vibrio parahemolyticus by adopting a method of combining immune enrichment and the LAMP technology; preparing an immunomagnetic bead by adopting a vibrio parahemolyticus polyclonal antibody; preliminarily screening the vibrio parahemolyticus in an actual sample by adopting an immunology method; and designing an LAMP specific primer according to the species specificity gene of the vibrio parahemolyticus. According to the invention, molecular detection is carried out on a nucleic acid level, therefore, the condition of false positive or undetection of a simple immunology method or a molecular method is effectively avoided, and the invention is a new development direction of the quick detection of the vibrio parahemolyticus.
Owner:SHANGHAI OCEAN UNIV
Kit for determination of human alpha-fetoprotein content by latex-enhanced immunoturbidimetry
ActiveCN102662061AAdapt to the requirements of rapid high-throughput screeningStrong specificityBiological testingLatex particleAlpha-fetoprotein
The invention relates to a kit for determination of human alpha-fetoprotein content by a latex-enhanced immunoturbidimetry. The kit provided by the invention comprises an alpha-fetoprotein R1 reagent, an alpha-fetoprotein R2 reagent and an alpha-fetoprotein calibrator, wherein the alpha-fetoprotein R1 reagent comprises one or more electrolytes, a coagulation accelerator, one or more stabilizing agents, one or more surfactants, an antiseptic and a buffer solution; the alpha-fetoprotein R2 reagent comprises latex particles coated by polyclonal antibodies capable of resisting a human alpha-fetoprotein, one or more electrolytes, one or more stabilizing agents, one or more surfactants, an antiseptic and a buffer solution; and the alpha-fetoprotein calibrator comprises an antiseptic, one or more electrolytes, one or more stabilizing agents, alpha-fetoprotein antigens and a buffer solution. The kit has strong specificity and high sensitivity, is homogeneous and stable, can be used fast and conveniently and can be used for various biochemical analyzers.
Owner:BEIJING STRONG BIOTECH INC
Magnetic microparticle chemiluminescence enzyme immune analytic reagent kit for detecting saccharide antigen and its use method
InactiveCN101324579AQuantitative detection of carbohydrate antigen contentLow pre-processing requirementsMaterial analysisCarbohydrate antigenMicroparticle
The invention relates to a magnetic corpuscule chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigen and the application method thereof. The kit comprises FITC antibody-coated magnetic corpuscules; a marker solution prepared by mixing the FITC-marked carbohydrate antigen monoclonal antibody and the enzyme-marked carbohydrate antigen monoclonal antibody; a carbohydrate antigen standard sample solution; a concentrated washing solution; and a luminescent substrate solution, wherein carbohydrate antigen optionally adopts one of CA72-4, CA50, CA19-9, CA242, CA15-3, CA27-29 and CA125. The enzyme-marked antibody and the FITC-marked antibody are the monoclonal antibodies corresponding to the antigens. The FITC antibody coating the magnetic corpuscules adopts a polyclonal antibody or a monoclonal antibody. The marker solution is prepared by mixing an FITC-marked capture antibody working solution and an enzyme-marked antibody pair working solution by the volume ratio of 1:(1-3). Compared with the known kit for mensurating the carbohydrate antigen, the kit has the advantages of high flux, high sensitivity, wide linear range, rapidness, etc., and has a wide application prospect for the clinical inspection, etc.
Owner:TSINGHUA UNIV
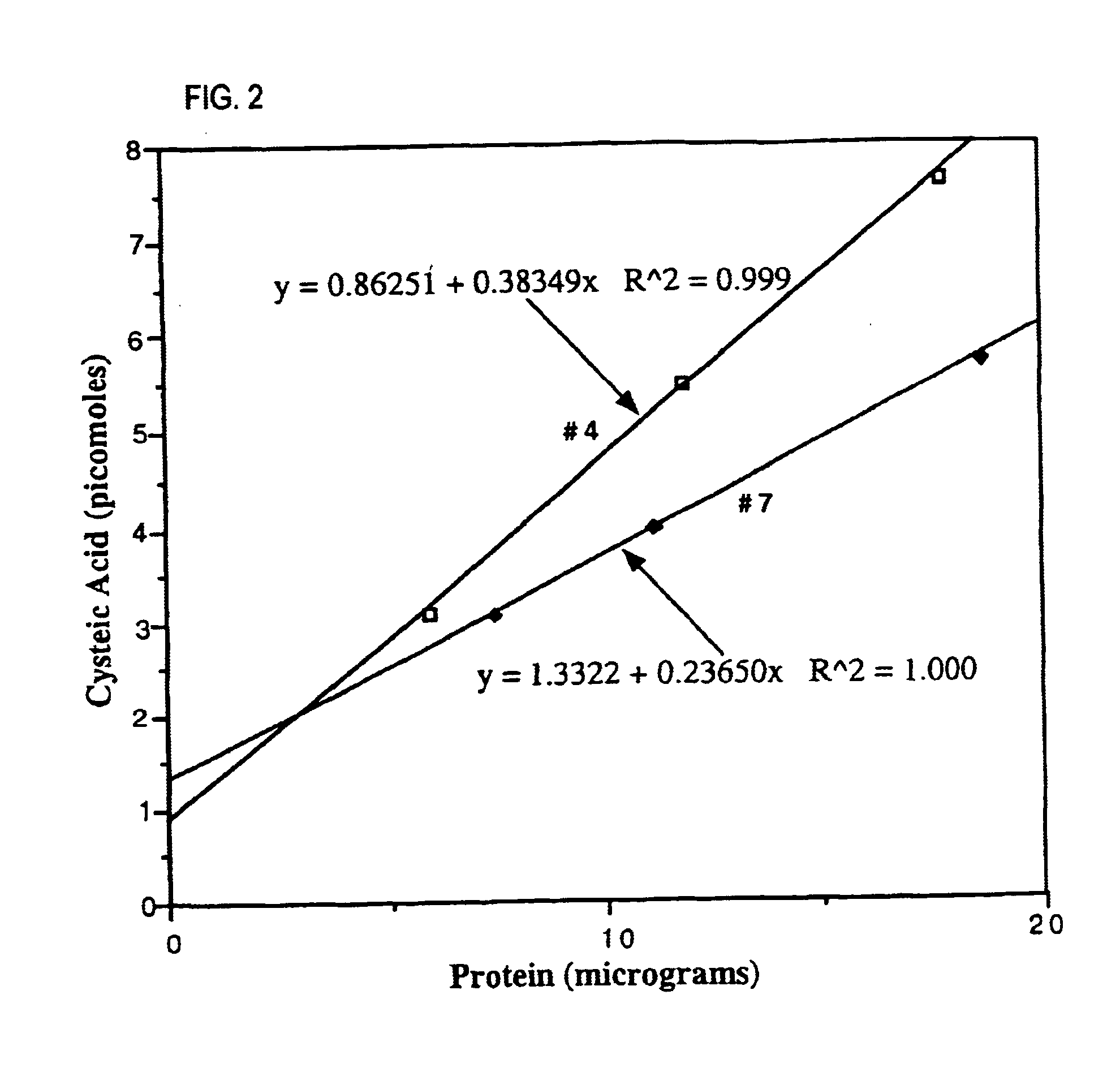
Biomarkers for oxidative stress
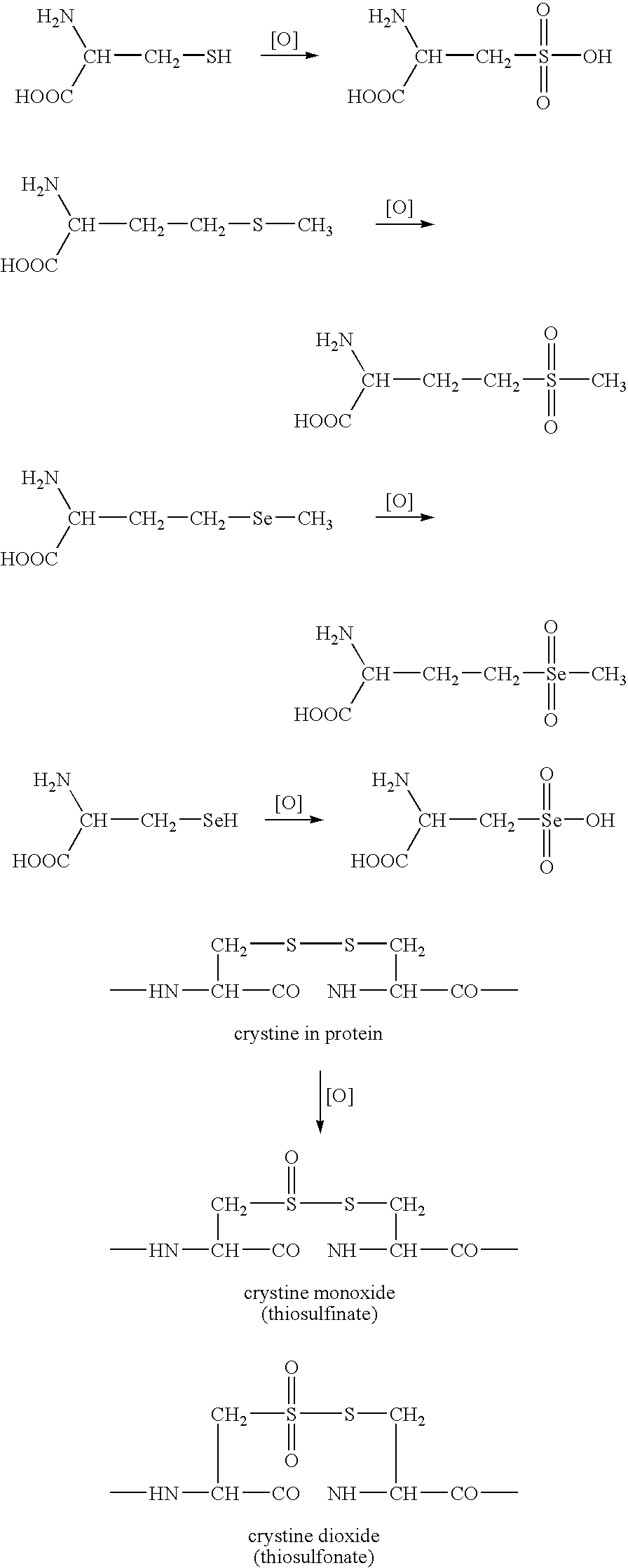
This invention related generally to methods of detecting and quantifying biomarkers of oxidative stress in proteins. The biomarker may be any amino acid that has undergone oxidation (or other modification, e.g. chloro-tyrosine, dityrosine). Emphasis is given herein on oxidized sulfur- or selenium-containing amino acids (SSAA). The biomarker of oxidative stress in proteins may be detected with an antibody that binds to oxidized amino acids, specifically oxidized sulfur- or selenium-containing amino acids. The antibody may be monoclonal or polyclonal. The presence of biomarker or amount of biomarker present in a sample may be used to aid in assessing the efficacy of environmental, nutritional and therapeutic interventions, among other uses.
Owner:EMORY UNIVERSITY
Digital immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 and preparation method thereof
InactiveUS20120034711A1Quick checkSimple procedureAnalysis using chemical indicatorsLamination ancillary operationsPaperboardBovine serum albumin
The present invention belongs to the field of biological detection. Multi-line immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 comprises a paperboard, wherein a water-absorbing pad, a detection pad, a gold-labeled pad and a sample pad are adhered sequentially on one surface of the paperboard from top to bottom, wherein each adjacent pads is overlapped and connected, the detection pad uses a nitrocellulose film as a backing pad, the nitrocellulose film is provided with a transverse control line, a test line I, a test line II and a test line III, wherein the control line is coated with a rabbit anti-mouse polyclonal antibody, and the test line I, test line II and test line III are coated with aflatoxin B1-bovine serum albumin conjugate (AFB1-BSA), respectively: and the gold-labeled pad is transversely coated with a nanogold-labeled anti-aflatoxin B1 monoclonal antibody. Said test strip is used for semi-quantitative detection of aflatoxin B1, and is characterized by quick detection, simple procedure and high sensitivity.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
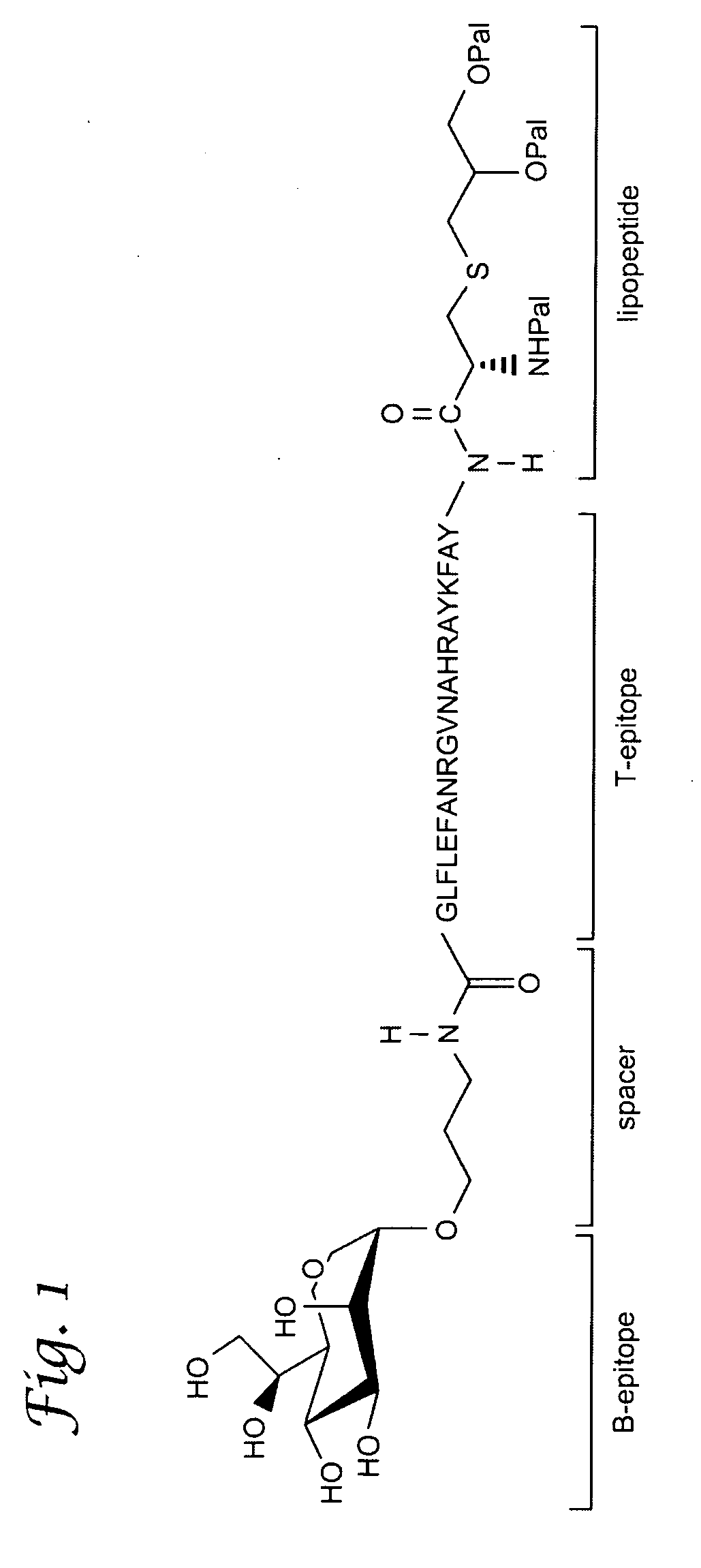
Glycolipopeptide and uses thereof
ActiveUS20090041836A1Reduce deliveryConvenience to mergeAntibacterial agentsVirusesPolyclonal antibodiesGlycolipid
A glycolipopeptide comprising a carbohydrate component, a peptide component and a lipid component, for use as a therapeutic or prophylactic vaccine. Also provided are monoclonal and polyclonal antibodies that recognize the glycolipopeptide of the invention, as well as uses thereof.
Owner:GEORGIA RESERACH FOUND INC UNIV OF
Method of detecting residue of small-molecule substance harmful to human body and a special kit
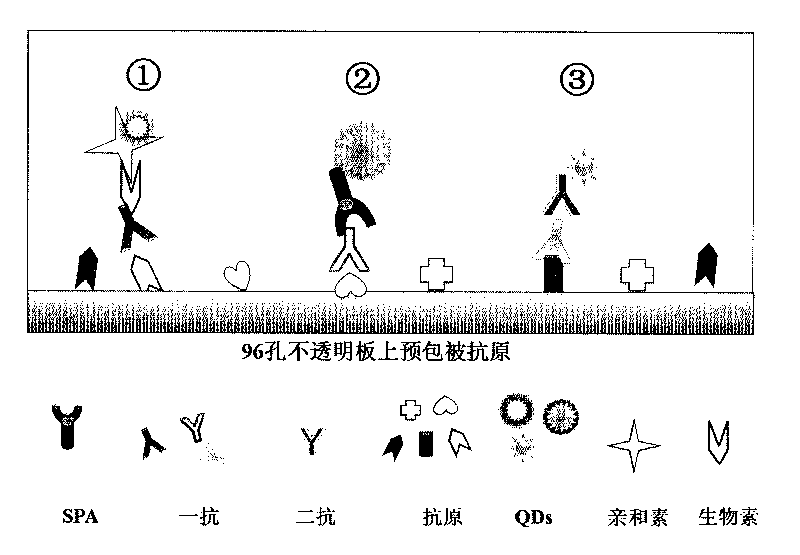
InactiveCN101762706AExcitation spectrum widthNarrow emission spectrumFluorescence/phosphorescenceHuman bodyBiology
The invention discloses a method of detecting the residue of small-molecule substances harmful to the human body and a special kit. The special kit comprises a non-transparent micro-porous plate and a light-emitting compound, wherein each hole of the non-transparent micro-porous plate is filled with a coat antigen which is simultaneously coated with three kinds of small-molecule substances. The invention makes full use of the multi-color marking function of QDs, establishes a novel kit for simple and rapid detection of the residue and a method thereof, and realizes the multi-color marking through indirect marking of polyclonal antibodies and monoclonal antibodies in the veterinary drug by coupling the QDs with different particle sizes and targets with functional groups (such as an amino) with specific surfaces. The method comprises: obtaining quantum dots with different fluorescent characteristics through separation and purification, namely, multi-color antibody markers, using the multi-color antibody markers as fluorescent probes, and establishing a reaction system for synchronous analysis of various antigen components of different kinds, thereby realizing the synchronous detection of multiple kinds of residues of the veterinary drug in animal food. Moreover, the method has the advantages of simple operation, high fluorescence intensity and long stabilization time.
Owner:CHINA AGRI UNIV
Immunofluorescence chromatography test paper for CRP (C-reaction protein)/SAA (Serum amyloid A protein) quantitative combined detection and preparation method of immunofluorescence chromatography test paper
InactiveCN105092861AAccurate detectionSolve the problem of CRP/SAA contentDisease diagnosisBiological testingImmunofluorescenceVenous blood
The invention discloses immunofluorescence chromatography test paper for CRP (C-reaction protein) / SAA (Serum amyloid A protein) quantitative combined detection, aiming at providing a test strip capable of realizing quantitative combined detection on the content of CRP / SAA in human peripheral blood and quantitative combined detection on the content of CRP / SAA in venous blood. The immunofluorescence chromatography test paper is technically characterized by comprising a box with a cover, wherein a detection test strip and a blood diluent bottle are arranged in the box, the detection test strip comprises a bottom lining, the bottom lining is provided with a nitrocellulose membrane, one end of the nitrocellulose membrane is connected with a fluorescent microsphere marked antibody fixation pad, the fluorescent microsphere marked antibody fixation pad is connected with a sample pad, the other end of the nitrocellulose membrane is connected with an absorption pad, the fluorescent microsphere marked antibody fixation pad is coated by a CRP monoclonal antibody and an SAA monoclonal antibody, a CRP detection line coated by a CRP monoclonal antibody, an SAA detection line coated by an SAA monoclonal antibody, and a quality control line coated by a goat-anti-mouse IgG polyclonal antibody are arranged on the nitrocellulose membrane in parallel. The immunofluorescence chromatography test paper belongs to the technical field of biological medicines.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
Antibodies With Immune Effector Activity And That Internalize In Folate Receptor Alpha-Positive Cells
This invention relates to the use of monoclonal and polyclonal antibodies that specifically bind to and have the ability in the alternative to become internalized by cells expressing folate receptor alpha (FRA) and to induce an immune effector activity such as antibody-dependent cellular cytotoxicity. The antibodies are useful in specific delivery of pharmacologic agents to FRA-expressing cells as well as in eliciting an immune-effector activity particularly on tumor cells and precursors. The invention is also related to nucleotides encoding the antibodies of the invention, cells expressing the antibodies; methods of detecting cancer cells; and methods of treating cancer using the antibodies.
Owner:EISAI INC
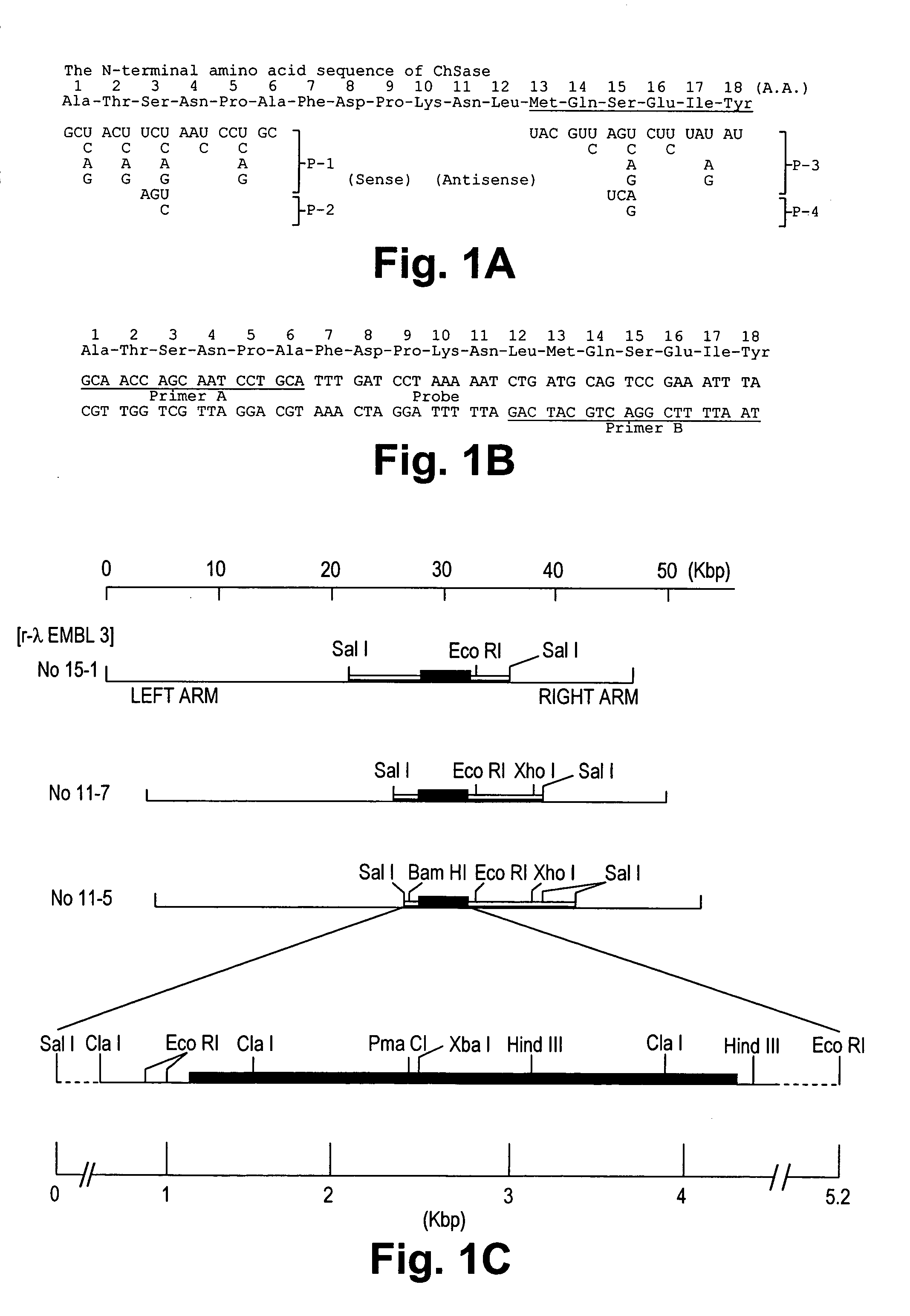
Gene encoding chondroitinase ABC and uses therefor
Nucleic acid sequences coding for the chondroitinase ABC gene and isolated chondroitinase ABE protein produced in a host cell transformed with a nucleic acid vector directing the expression of a nucleotide sequence coding for chondroitinase ABE protein described. Chondroitinase ABC prepared by chemical synthesis also described. Monoclonal and polyclonal antibodies which are specifically reactive with chondroitinase ABC protein are disclosed. The isolated chondroitinase ABC can be used in methods of treating intervertebral disc replacement, promoting neurite regeneration, and detecting galactosaminoglycans.
Owner:MARUHA NICHIRO
Models for vaccine assessment
ActiveUS20080008653A1Improve accuracyImprove predictabilityCompounds screening/testingCrankshaftsAdjuvantBiological Immunotherapy
The present invention is directed to methods for constructing and using in vivo and in vitro models of aspects of human immunity and, in particular, construction of a human immune system model for the testing of, for example, vaccines, adjuvants, immunotherapy candidates, cosmetics, drugs, biologics and other chemicals. The present invention comprises both in vivo and in vitro models of aspects of human immunity that are useful for assessing the interaction of substances with the immune system, and thus can be used to accelerate and improve the accuracy and predictability of, for example, vaccine, drug, biologic, immunotherapy, cosmetic and chemical development. The invention is also useful for the generation of human monoclonal and polyclonal antibodies.
Owner:VIRGINIA COMMONWEALTH UNIV +1
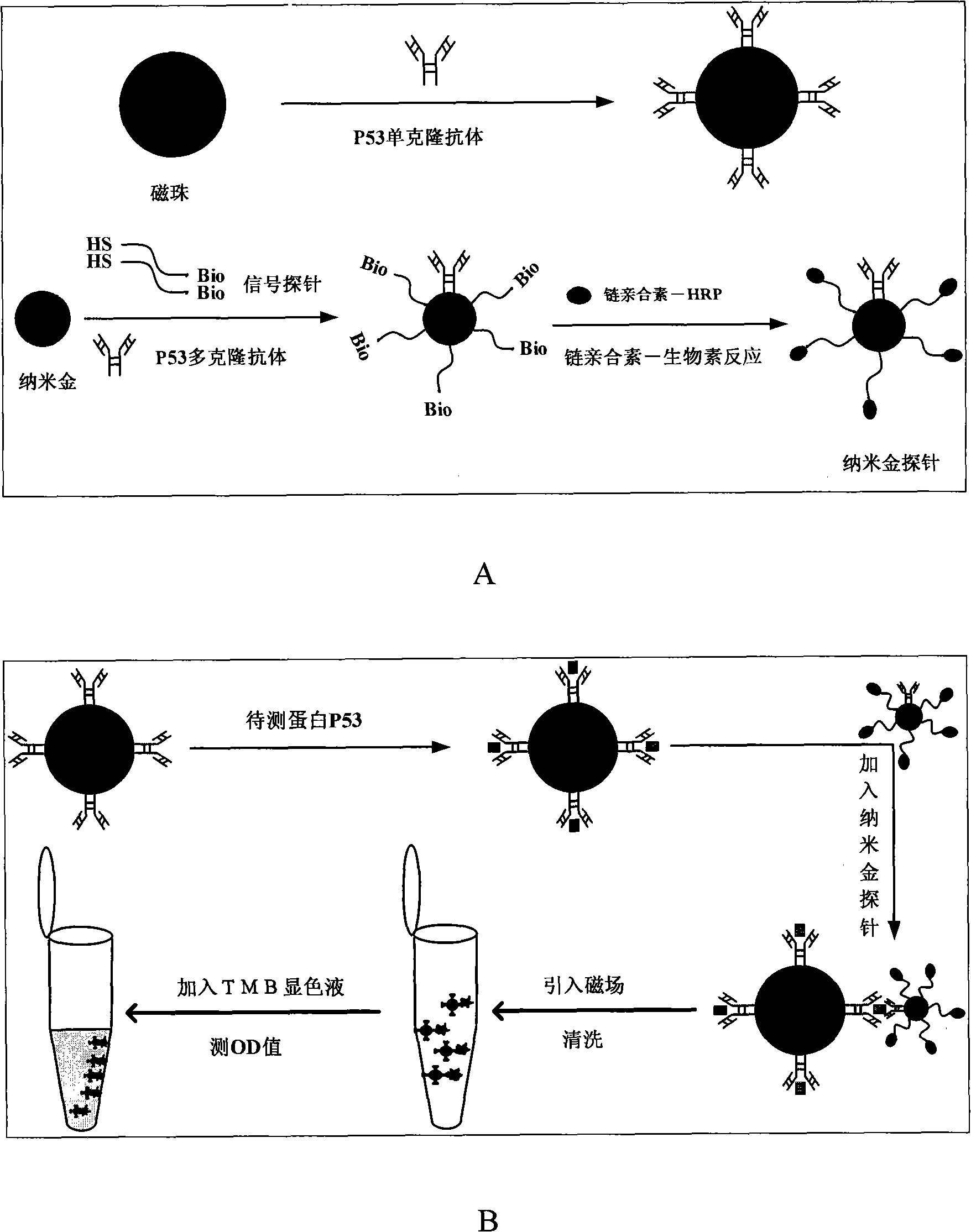
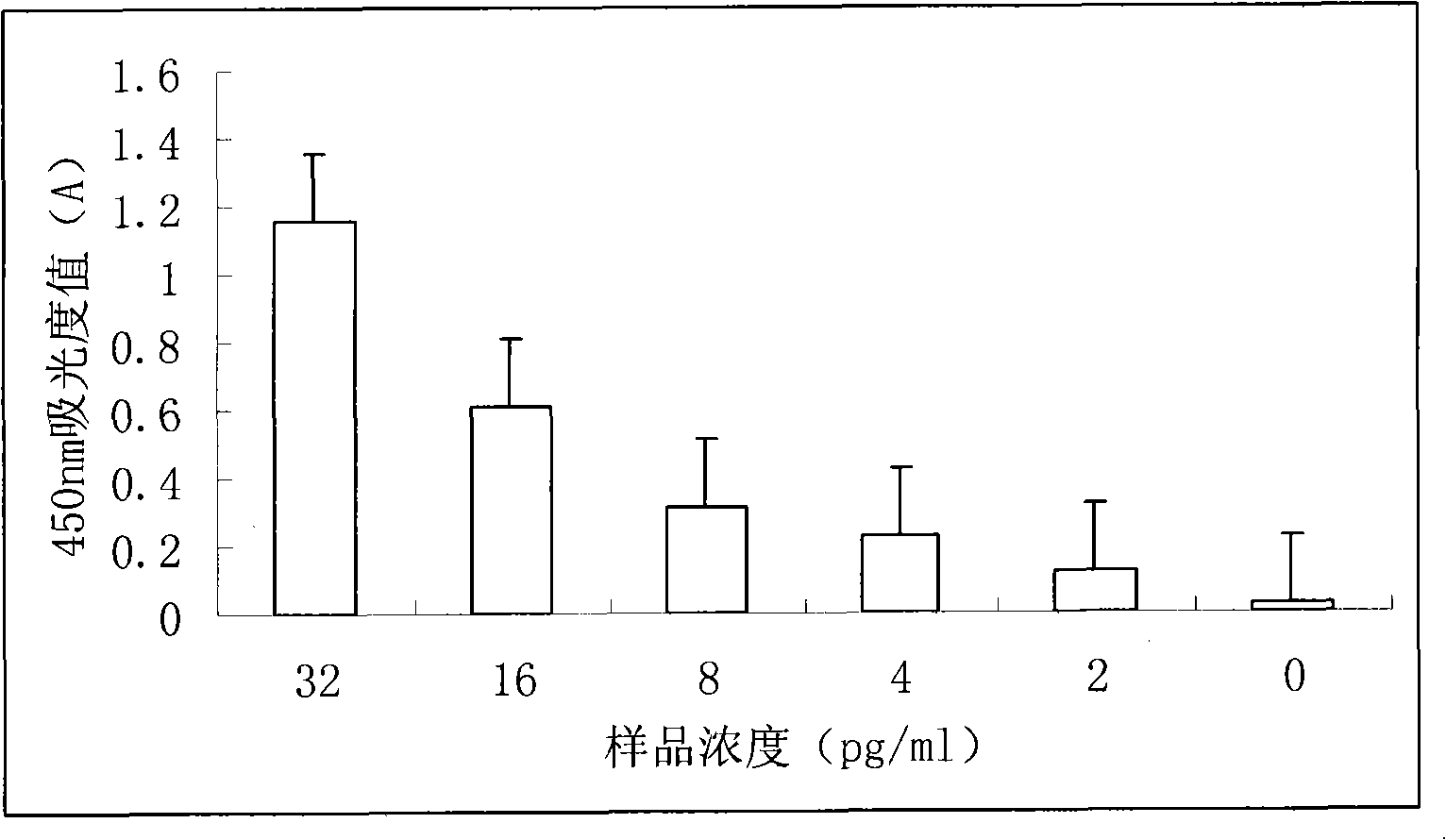
Method for determining minim proteins based on magnetic pearl and nano gold probe
InactiveCN101256191AHigh sensitivityEasy to detectBiological testingMagnetic beadMonoclonal antibody
The invention relates to a high-sensitivity trace of protein measuring method based on bead and nm metal probe, comprising: labelling the bead using monoclonal antibody of protein to measure; labelling the polyclonal antibody of protein to measure on nm metal probe and at the same time labelling a DNA probe with biotin label on the nm metal probe; labelling horseradish peroxidase on the DNA probe of nm metal probe by biotin-streptavidin reaction to form nm metal probe; mixing the labelled bead and nm metal probe and protein sample and incubating for a period of time at 37 degree; and then washing the nm metal probes which do not react; developing using TMB development system, therefore the protein can be quantificationally detected. The detection time can be reduced to 1-1.5 hours and the sensitivity can be pg / ml.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and application thereof
The invention relates to an aflatoxin B1 flow lag immunization time distinguishing fluorescence rapid-detection kit and an application thereof. The kit comprises a fluorescent test strip and a sample reaction bottle containing an europium-labeled anti-aflatoxin B1 monoclonal antibody lyophilized product, wherein the fluorescent test strip comprises a cardboard, a water absorption pad, a detection pad and a sample pad are sequentially pasted on one surface of the cardboard from top to bottom, adjacent pads are connected at the connection in an overlapping manner, the detection pad treats a cellulose nitrate membrane as a base pad, a transverse quality control line and a detection line are arranged on the cellulose nitrate membrane from top to bottom, the quality control line is coated with a rabbit anti-mouse polyclonal antibody, and the detection line is coated with an aflatoxin B1 bovine serum albumin conjugate; and the anti-aflatoxin B1 monoclonal antibody is secreted by a hybridoma cell strain having a preservation number of CCTCC NO.C201015. The kit can be used for the quantitative determination of the content of the aflatoxin B1, and has the advantages of simple operation, rapidness and high accuracy.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
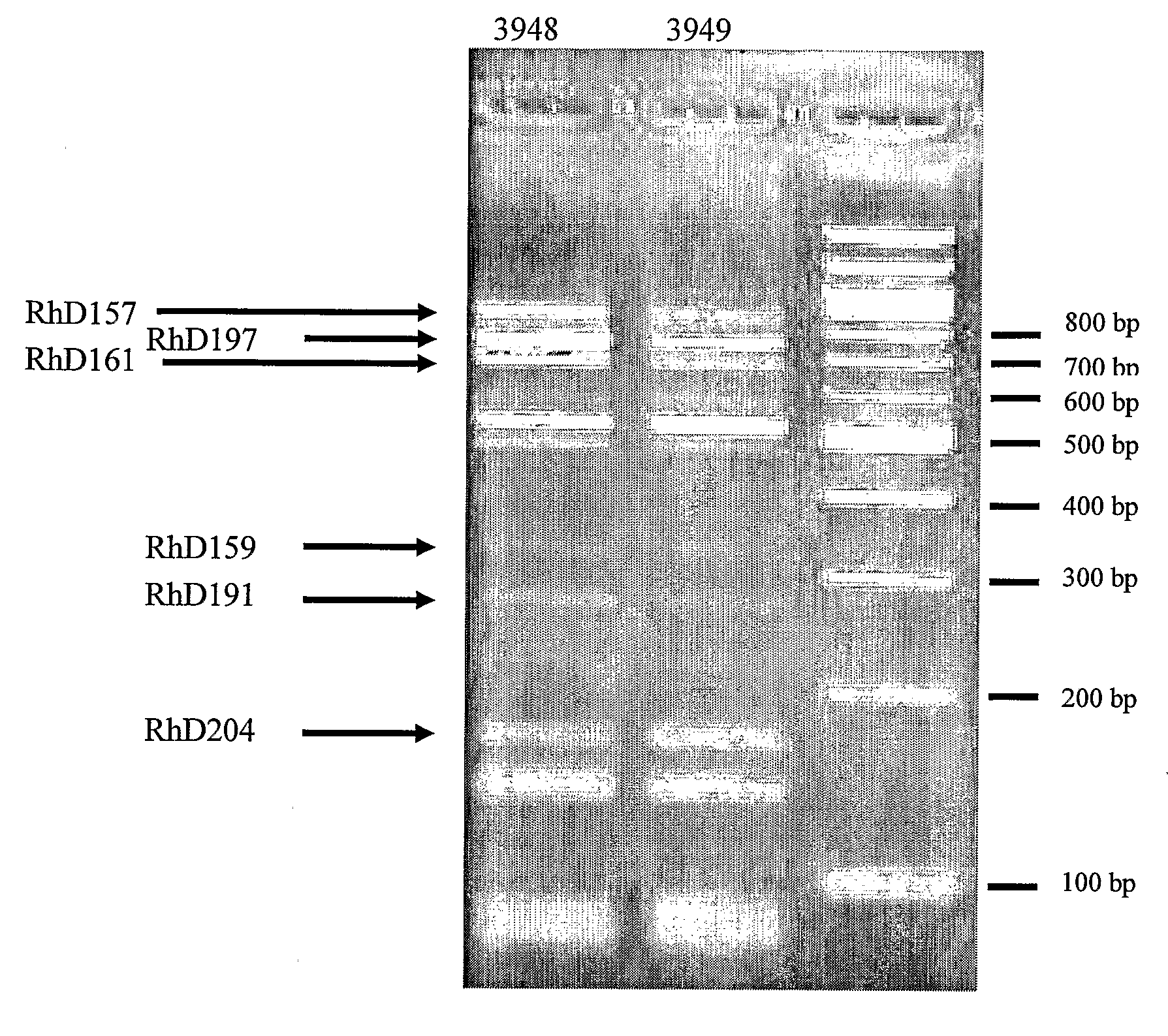
Anti-rhesus d recombinant polyclonal antibody and methods of manufacture
InactiveUS20090017017A1Reduce concentrationImmunoglobulins against blood group antigensLibrary screeningPlasma derivedAntibody expression
The invention relates to a method for manufacturing an anti-RhD recombinant polyclonal antibody composition (anti-RhD rpAb). The method comprises obtaining a collection of cells transfected with a library of anti-RhD antibody expression vectors, wherein each cell in the collection is capable of expressing from a VH and VL comprising nucleic acid segment, one member of the library, which encodes a distinct member of anti-RhD recombinant polyclonal antibody composition and which is located at the same site in the genome of individual cells in said collection. The cells are cultured under suitable conditions for expression of the recombinant polyclonal antibody, which is obtained from the cells or culture supernatant. The nucleic acid segments encoding the anti-RhD rpAb is introduced into the cells by transfection with a library of vectors for site-specific integration. The present method is suitable for manufacturing anti-RhD rpAb, thereby making available a superior replacement of plasma-derived prophylactic and therapeutic immonoglobulin products.
Owner:SYMPHOGEN AS
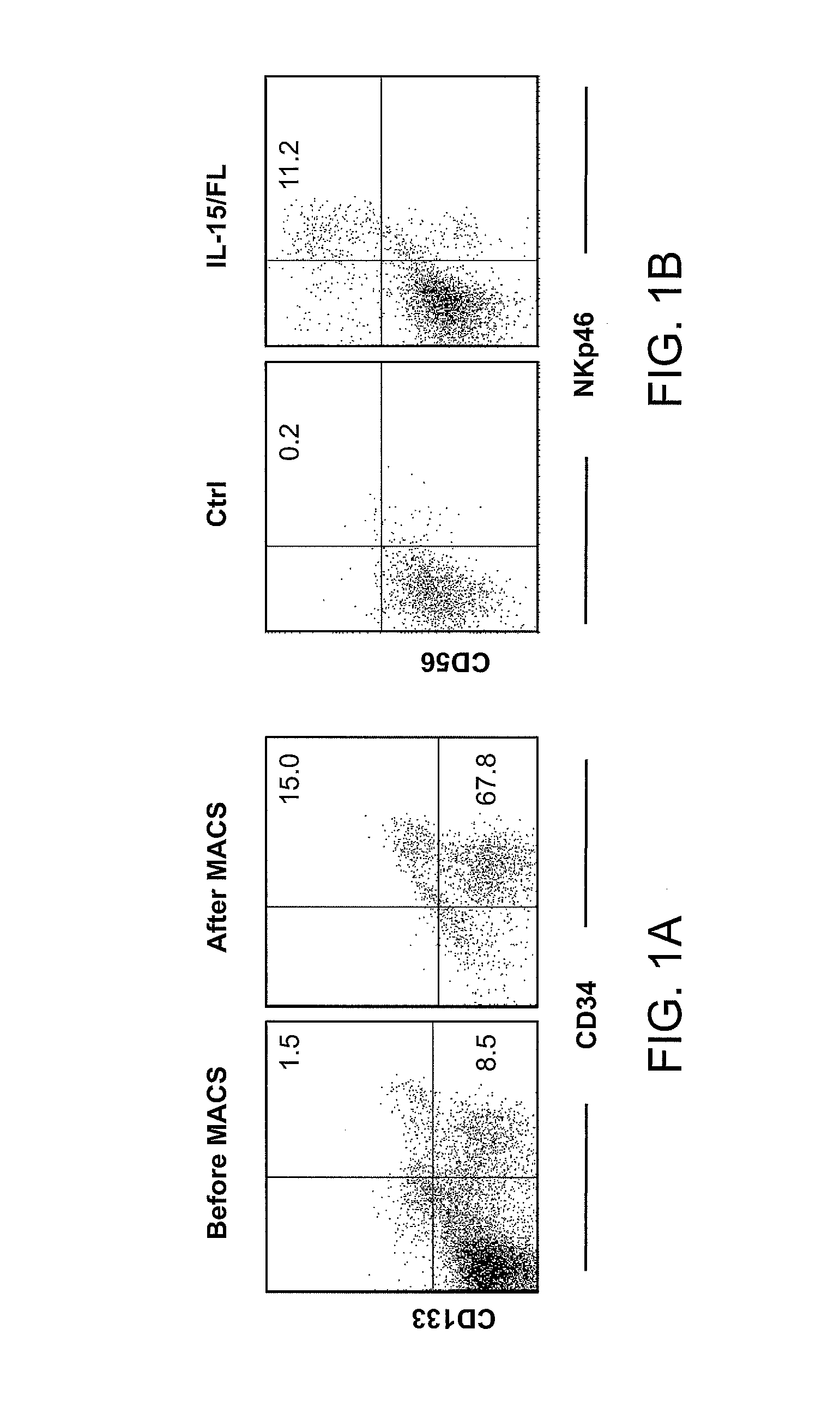
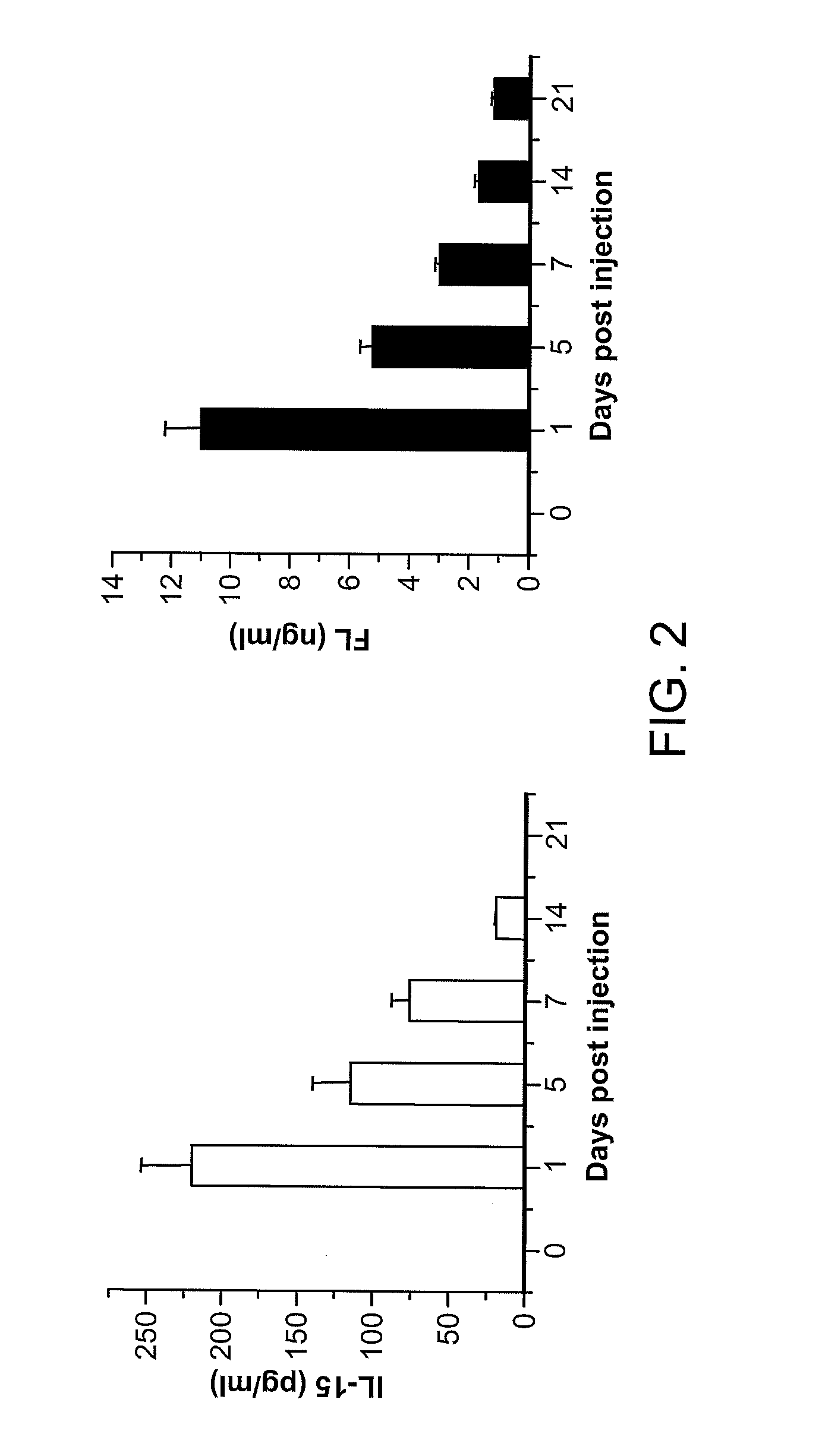
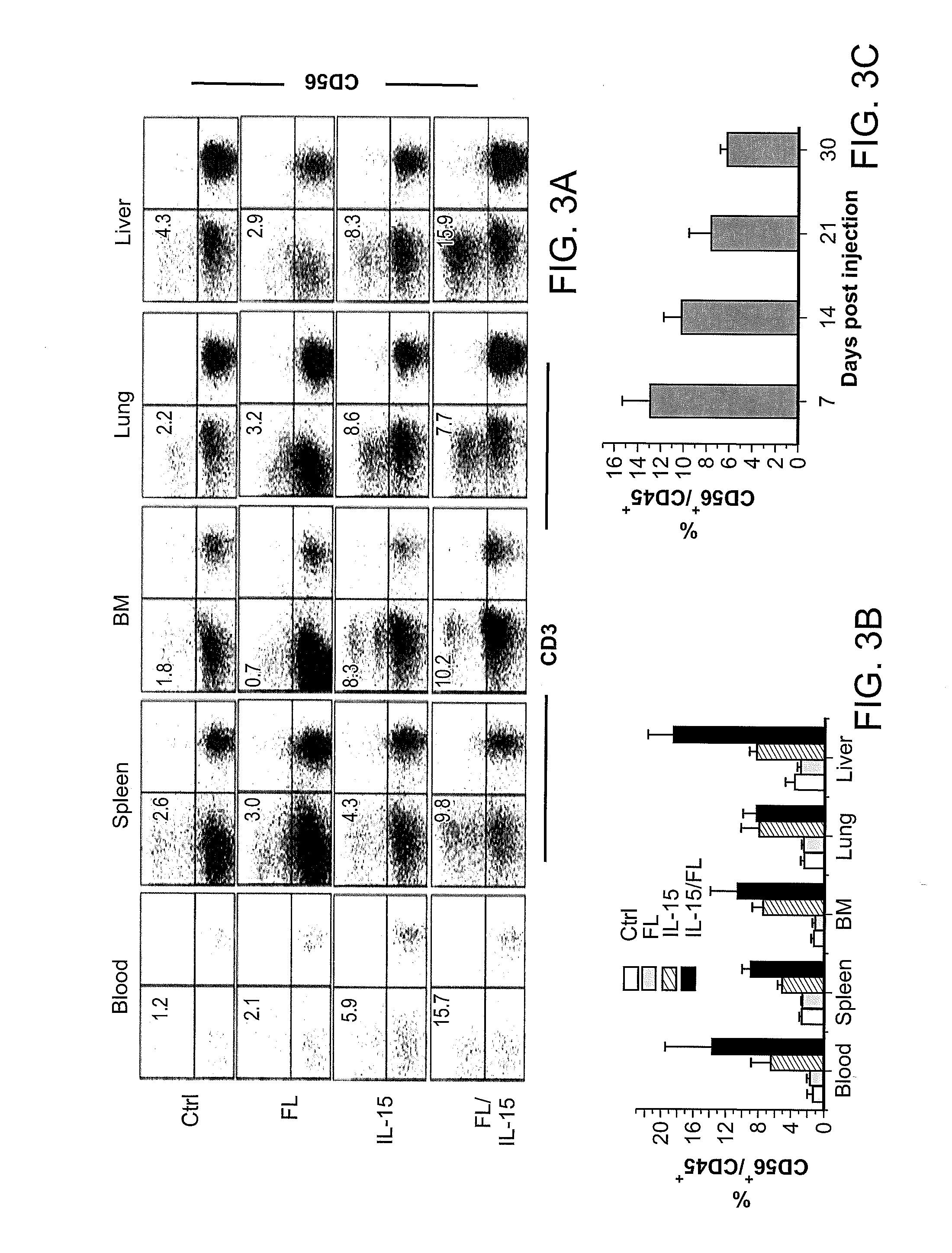
Methods Of Producing Humanized Non-Human Mammals
InactiveUS20120157667A1Reconstitution is increasedImprove the level ofAnimal cellsImmunoglobulins against cytokines/lymphokines/interferonsCell lineageMammal
Provided herein are methods of reconstituting functional human blood cell lineages in a non-human mammal comprising introducing human hematopoietic stem cells (HSCs) and nucleic acid encoding one or more human cytokines into an immunodeficient non-human mammal. The non-human mammal is maintained under conditions in which the nucleic acid is expressed and the human HSCs differentiate into functional human blood cell lineages in the non-human mammal, thereby reconstituting functional human blood cell lineages in the non-human mammal. Also provided are methods of producing human antibodies directed against an immunogen in a non-human mammal, hybridomas that secrete the monoclonal antibodies as well as antibodies (e.g., polyclonal antibodies; monoclonal antibodies) produced by the B cells and non-human mammals produced by the methods.
Owner:MASSACHUSETTS INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com