Patents
Literature
66 results about "Plasma derived" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Plasma-derived concentrates are made from human blood which is donated by healthy volunteers and screened for safety. These products have been used since the 1970s.
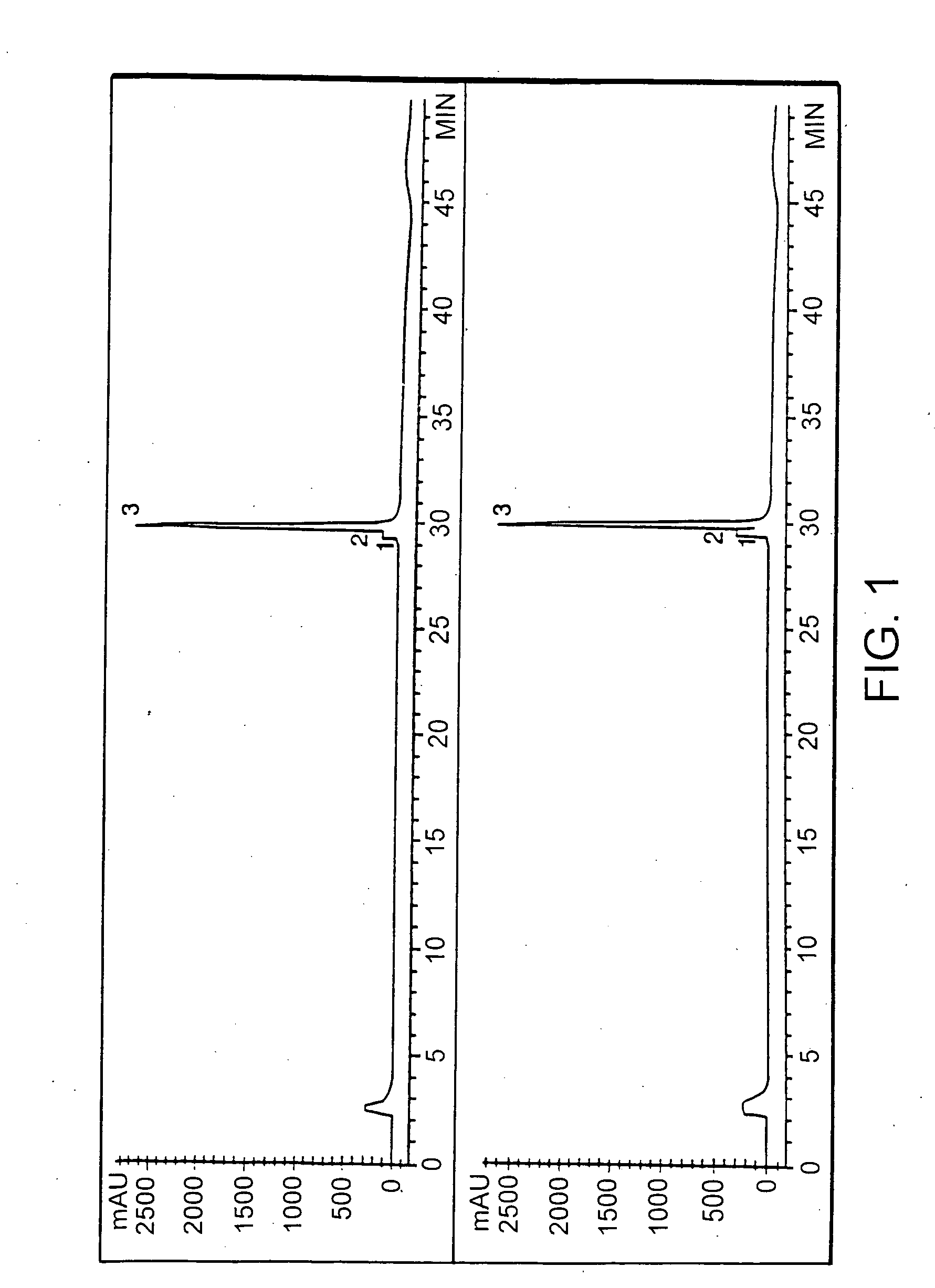
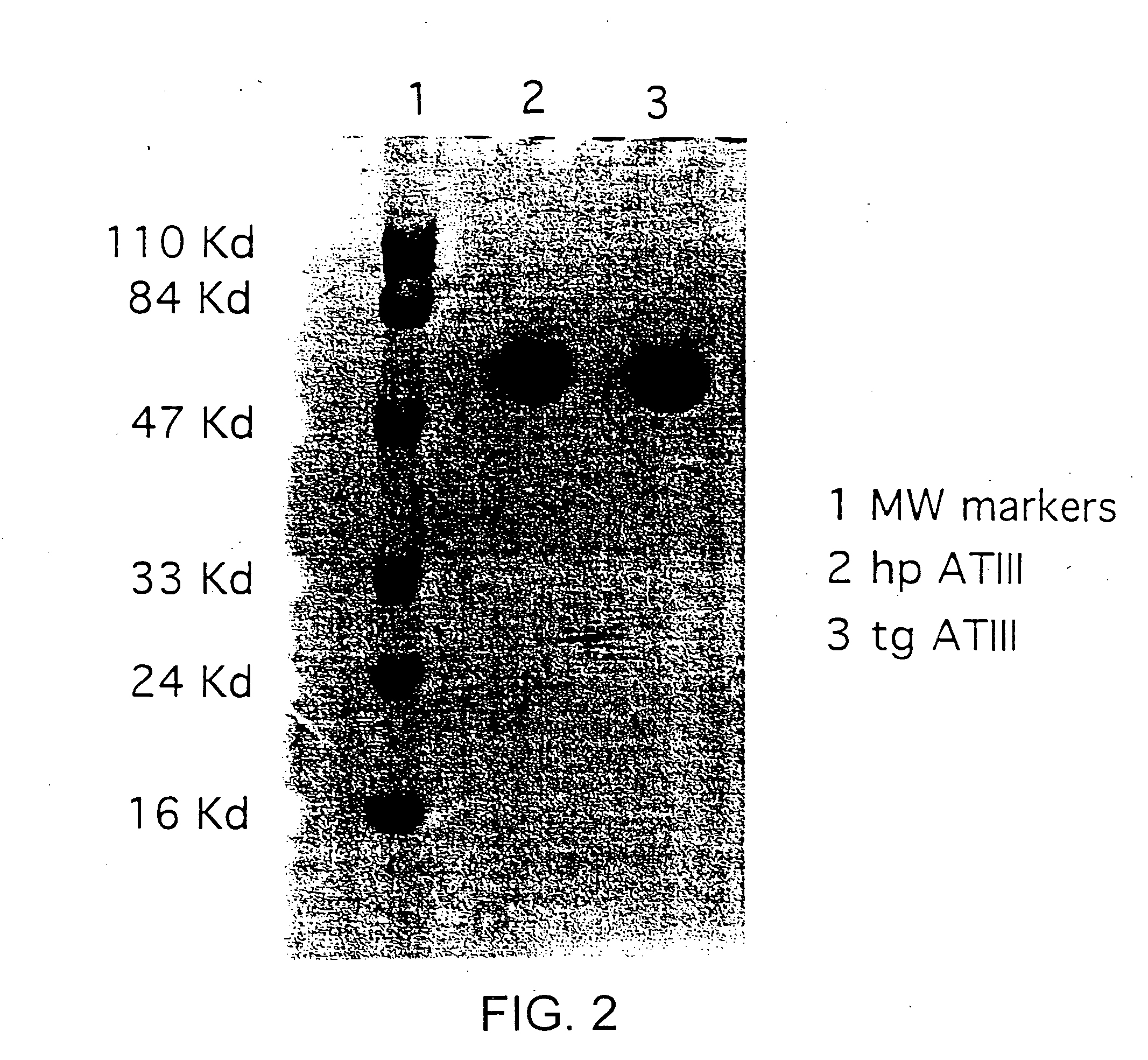
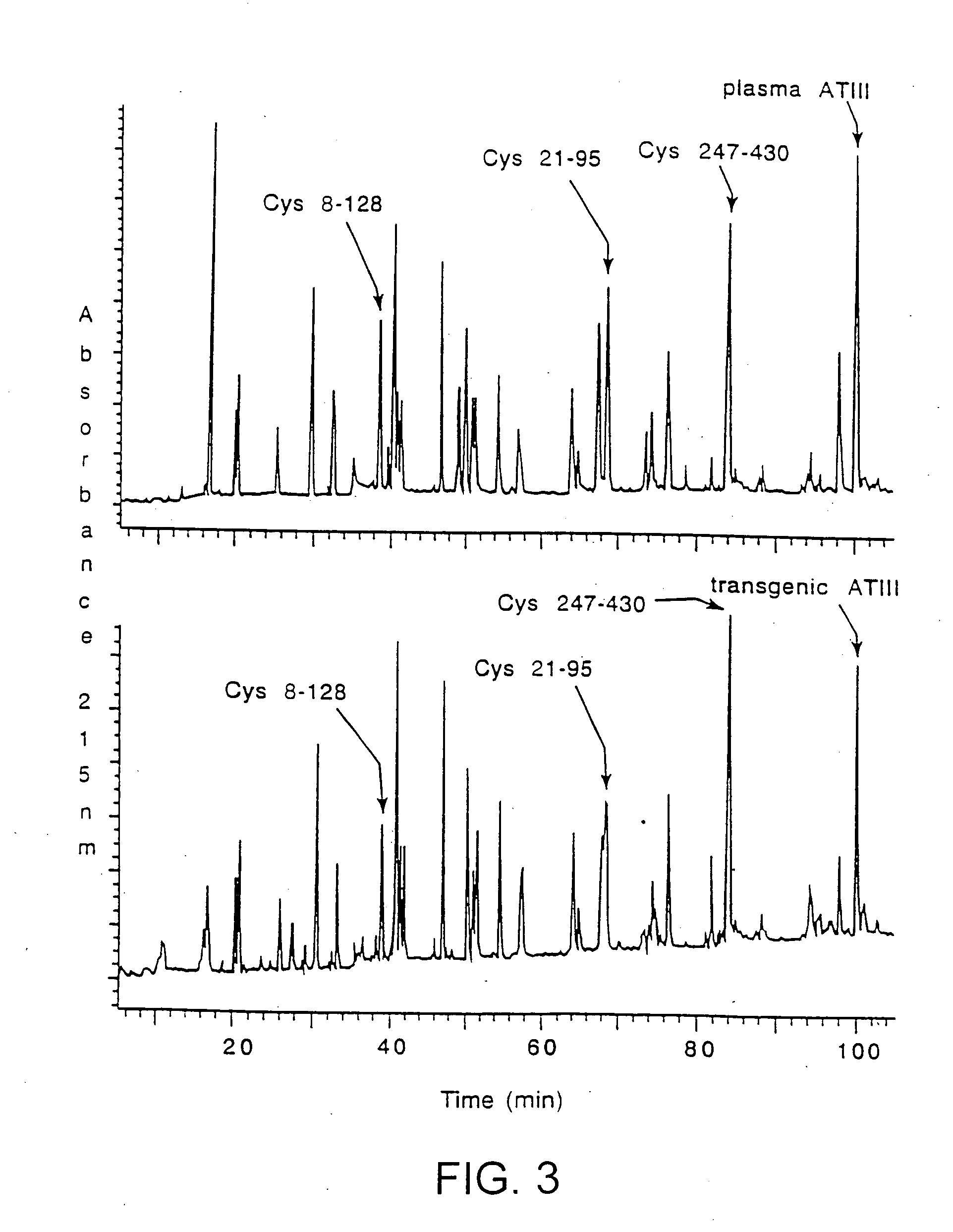
Treatments using transgenic goat produced antithrombin III
InactiveUS7019193B2Improve clearance ratePeptide/protein ingredientsHydrolasesPlasma derivedMonosaccharide composition
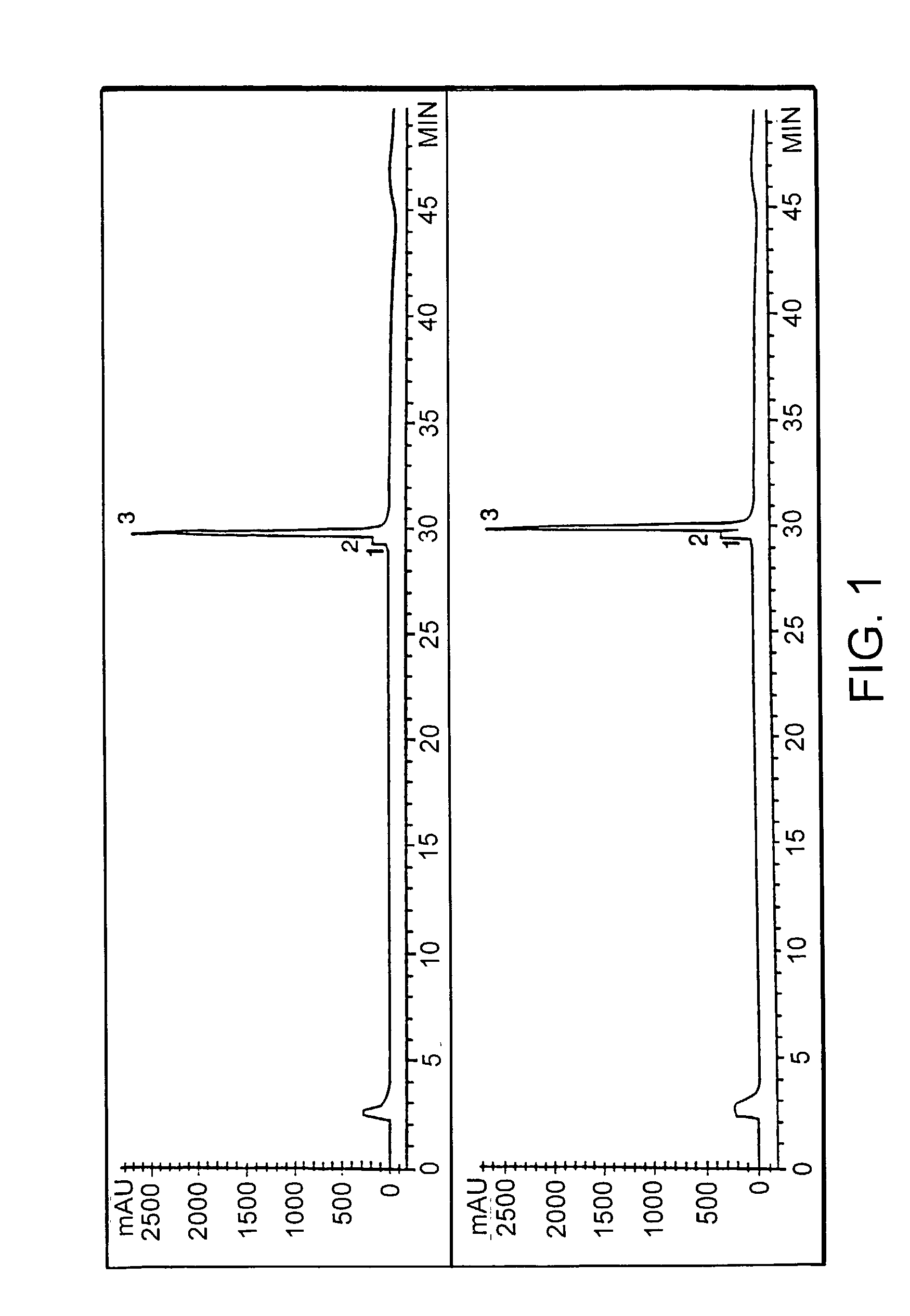
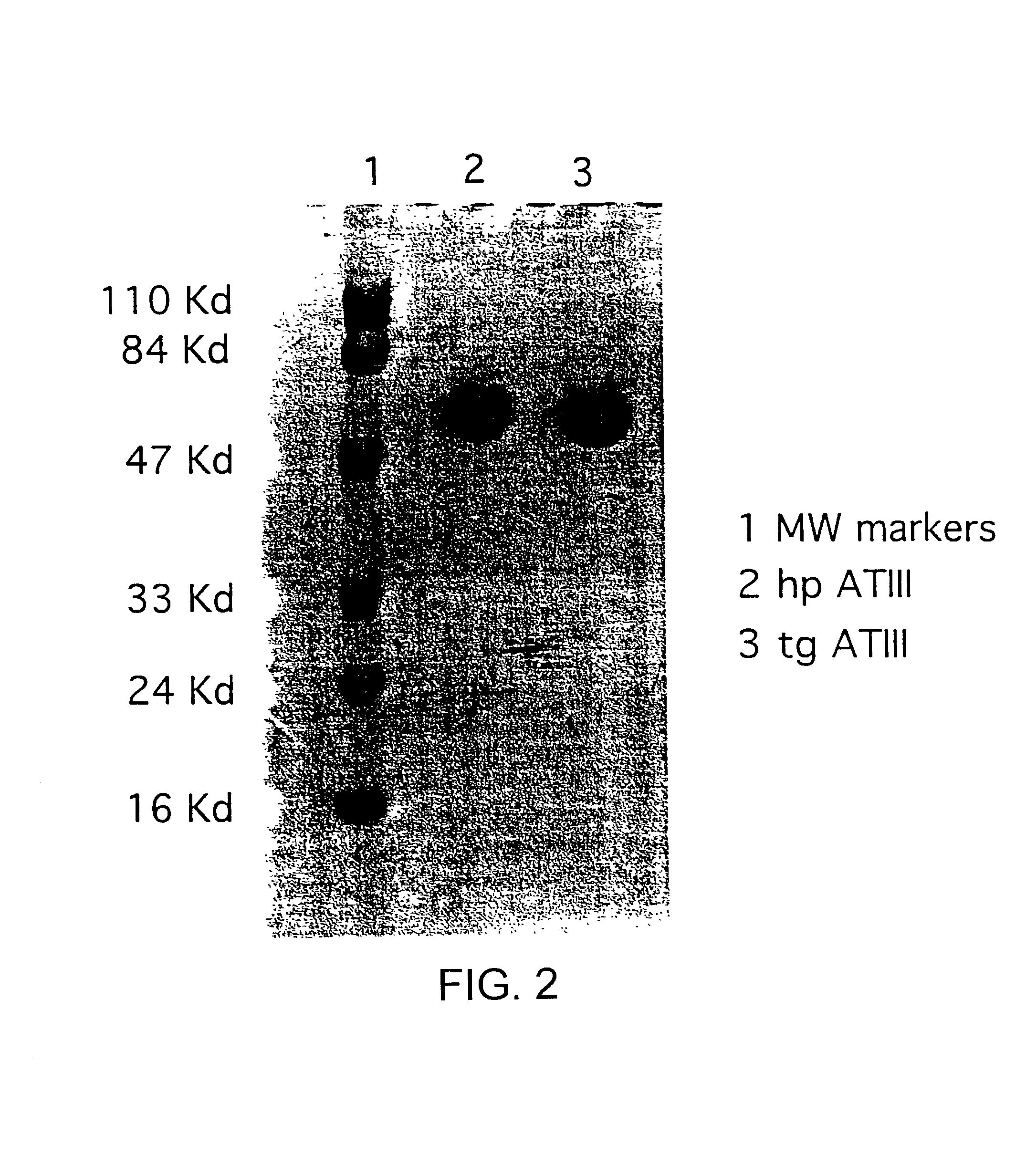
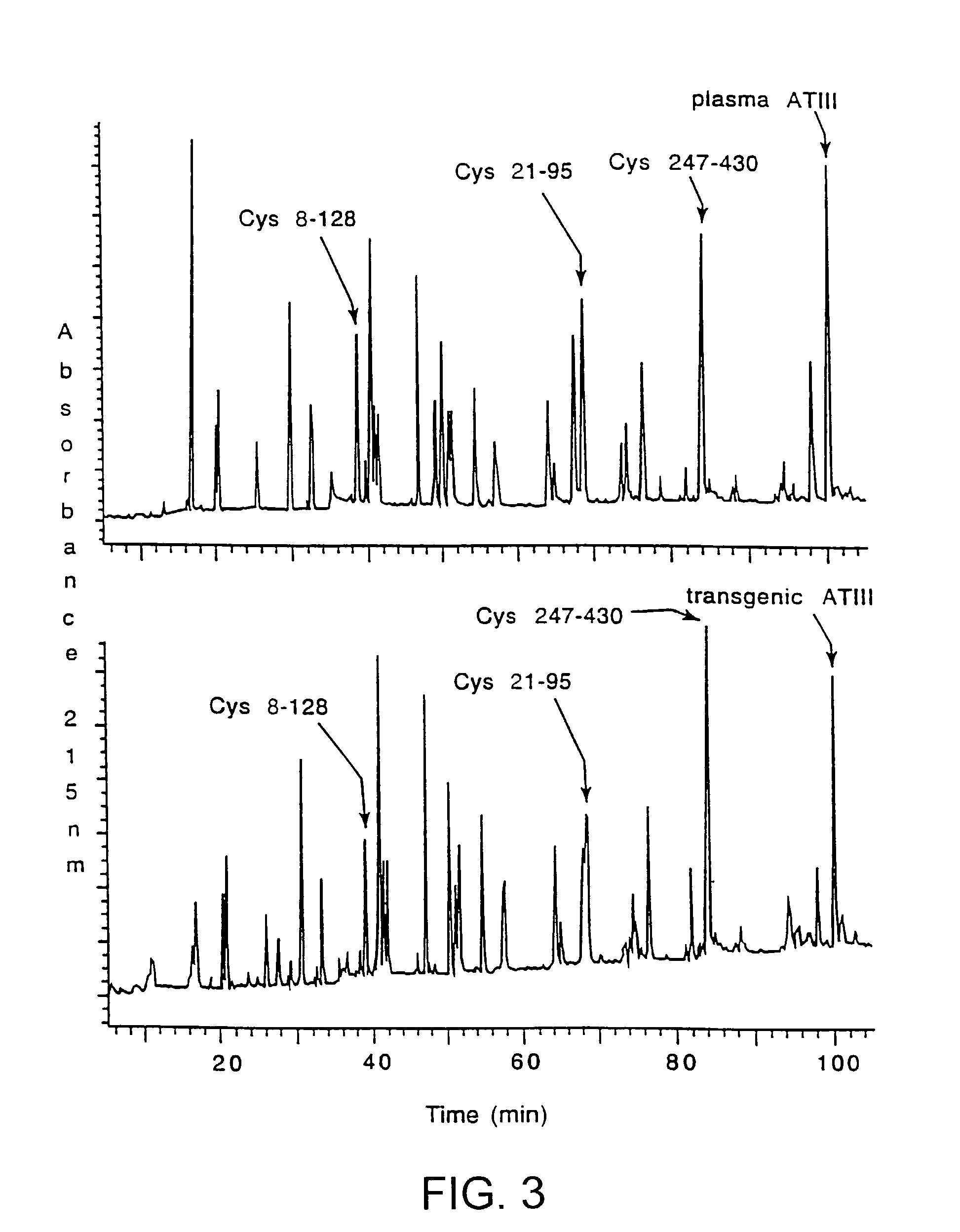
This invention relates to transgenically produced human Antithrombin III (tgATIII). The human ATIII produced by the transgenic process of the present invention has a monosaccharide composition which comprises N-acetylgalactosamine (GalNAc) along with fucose, N-acetylglucosamine, galactose, mannose, and N-acetylneuraminic acid / N-glycolyneuraminic acid. The monosaccharide composition differs with that of plasma derived ATIII (phATIII). It has been found that tgATIII has an increased clearance rate when compared to phATIII.
Owner:GTC BIOTHERAPEUTICS INC
Anti-rhesus d recombinant polyclonal antibody and methods of manufacture
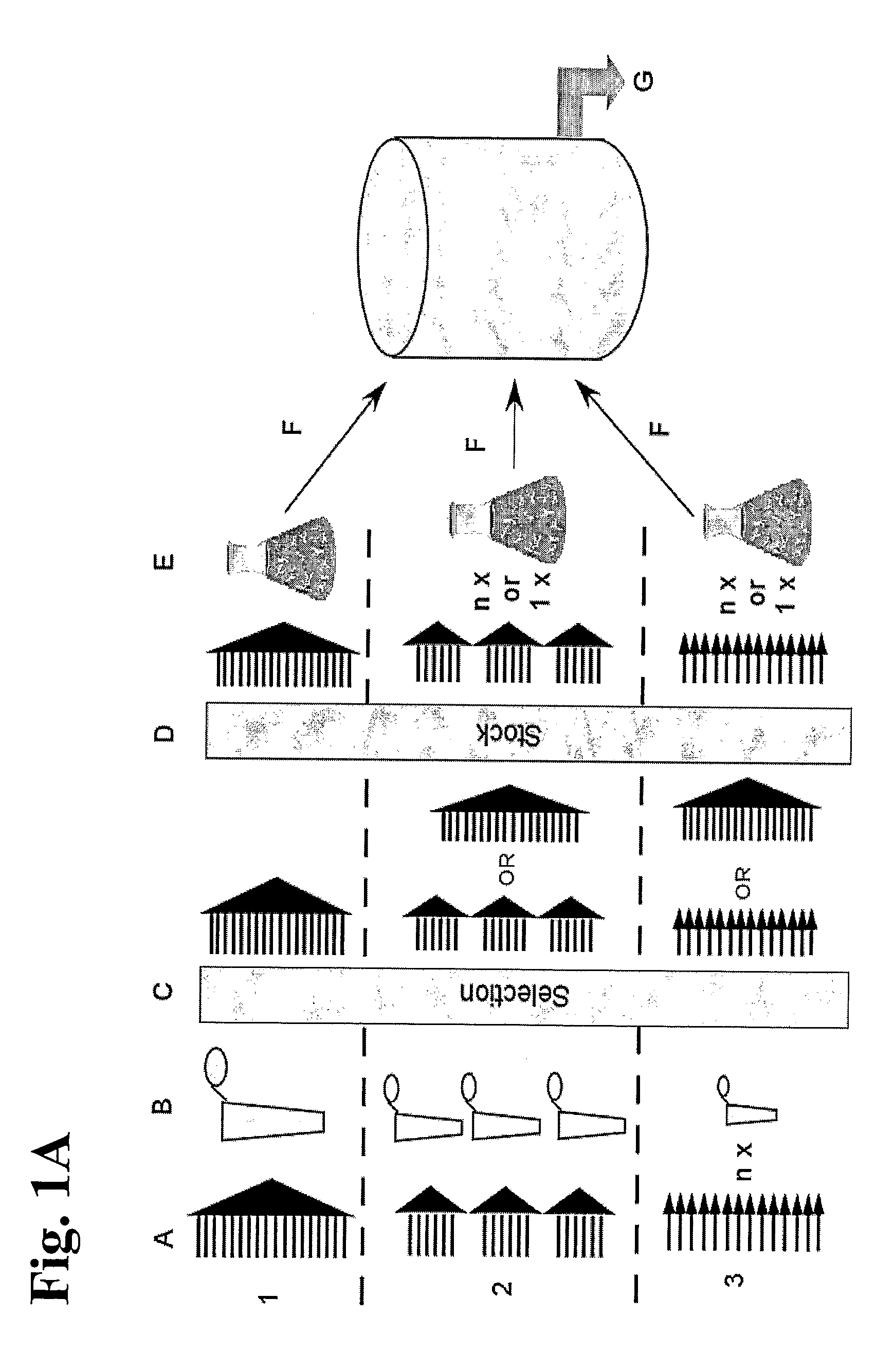
InactiveUS20090017017A1Reduce concentrationImmunoglobulins against blood group antigensLibrary screeningPlasma derivedAntibody expression
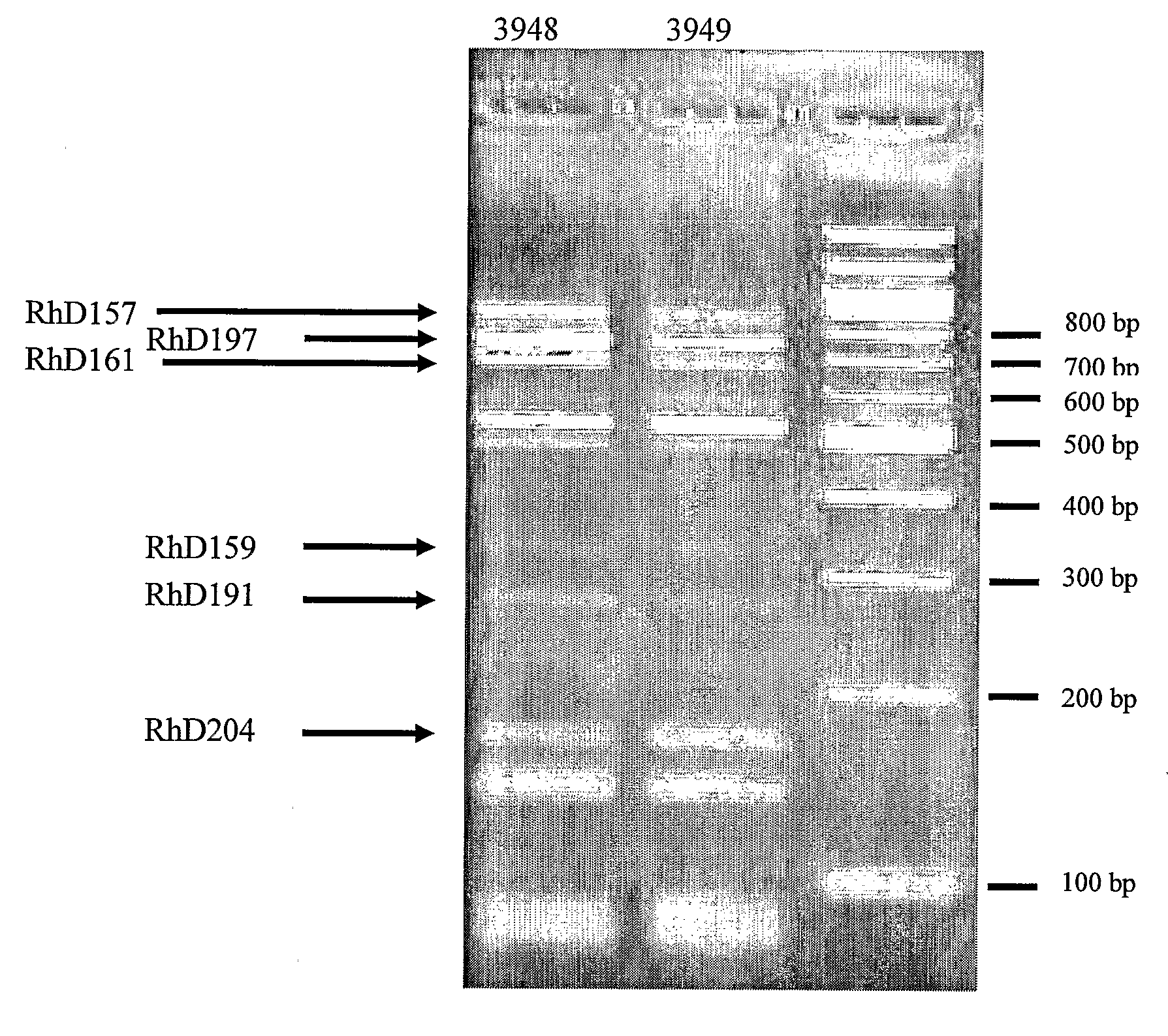
The invention relates to a method for manufacturing an anti-RhD recombinant polyclonal antibody composition (anti-RhD rpAb). The method comprises obtaining a collection of cells transfected with a library of anti-RhD antibody expression vectors, wherein each cell in the collection is capable of expressing from a VH and VL comprising nucleic acid segment, one member of the library, which encodes a distinct member of anti-RhD recombinant polyclonal antibody composition and which is located at the same site in the genome of individual cells in said collection. The cells are cultured under suitable conditions for expression of the recombinant polyclonal antibody, which is obtained from the cells or culture supernatant. The nucleic acid segments encoding the anti-RhD rpAb is introduced into the cells by transfection with a library of vectors for site-specific integration. The present method is suitable for manufacturing anti-RhD rpAb, thereby making available a superior replacement of plasma-derived prophylactic and therapeutic immonoglobulin products.
Owner:SYMPHOGEN AS
Method for manufacturing recombinant polyclonal proteins
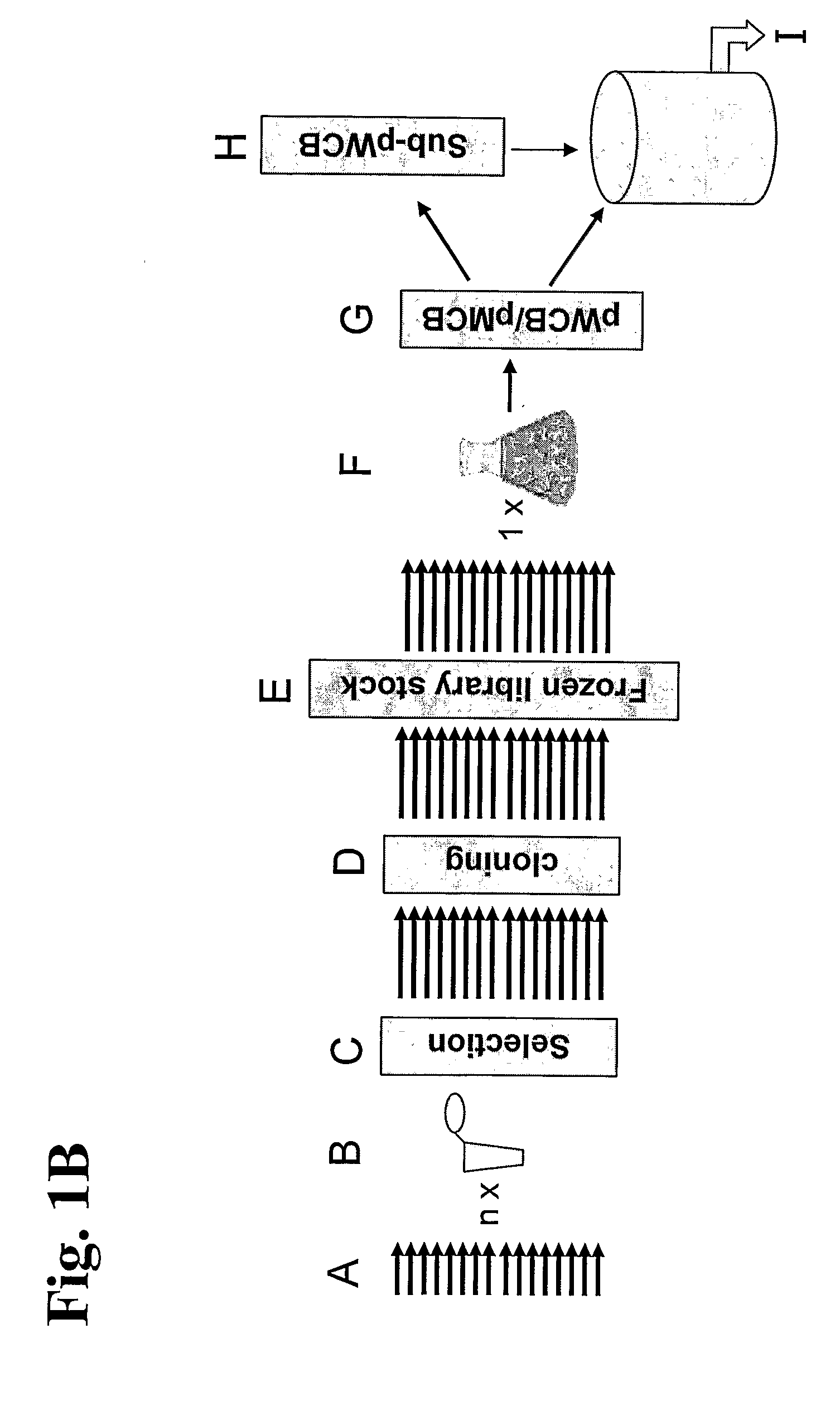
InactiveUS20060275766A1Minimizing unwanted batch-to-batch variationImmunoglobulins against blood coagulation factorsPeptide librariesPresent methodPlasma derived
Owner:SYMPHOGEN AS
Apparatus and method for oxidation and stabilization of polymeric materials
ActiveUS20090263295A1From normal temperature solutionsLiquid separation by electricityFiberCross-link
An apparatus for treating polymeric materials comprises a treatment chamber adapted to maintain a selected atmosphere; a means for supporting the polymeric material within the chamber; and, a source of plasma-derived gas containing at least one reactive oxidative species whereby the polymer is stabilized and cross linked through exposure to the oxidative species in the chamber at a selected temperature. The polymer may be directly exposed to the plasma, or alternatively, the plasma may be established in a separate volume from which the reactive species may be extracted and introduced into the vicinity of the polymer. The apparatus may be configured for either batch-type or continuous-type processing. The apparatus and method are especially useful for preparing polymer fibers, particularly PAN fibers, for later carbonization treatments.
Owner:REMAXCO TECH LLC +1
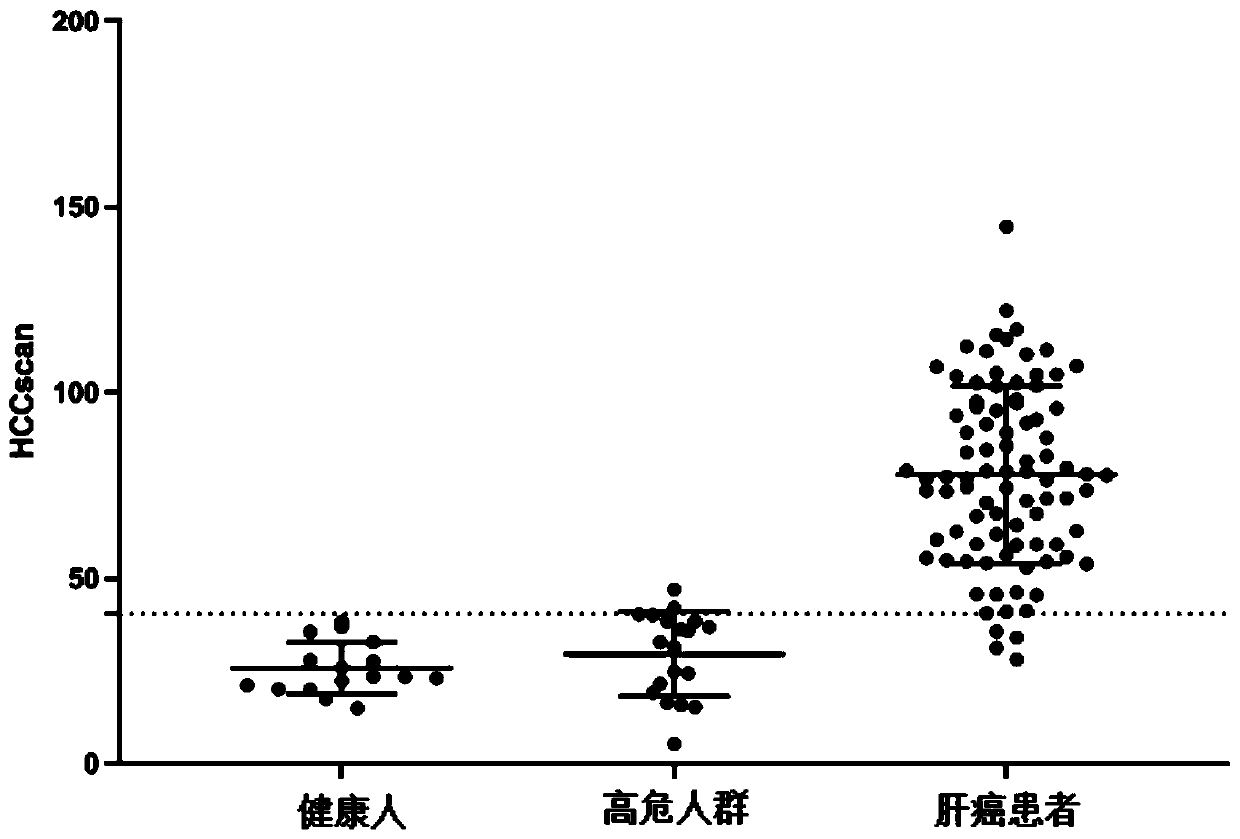
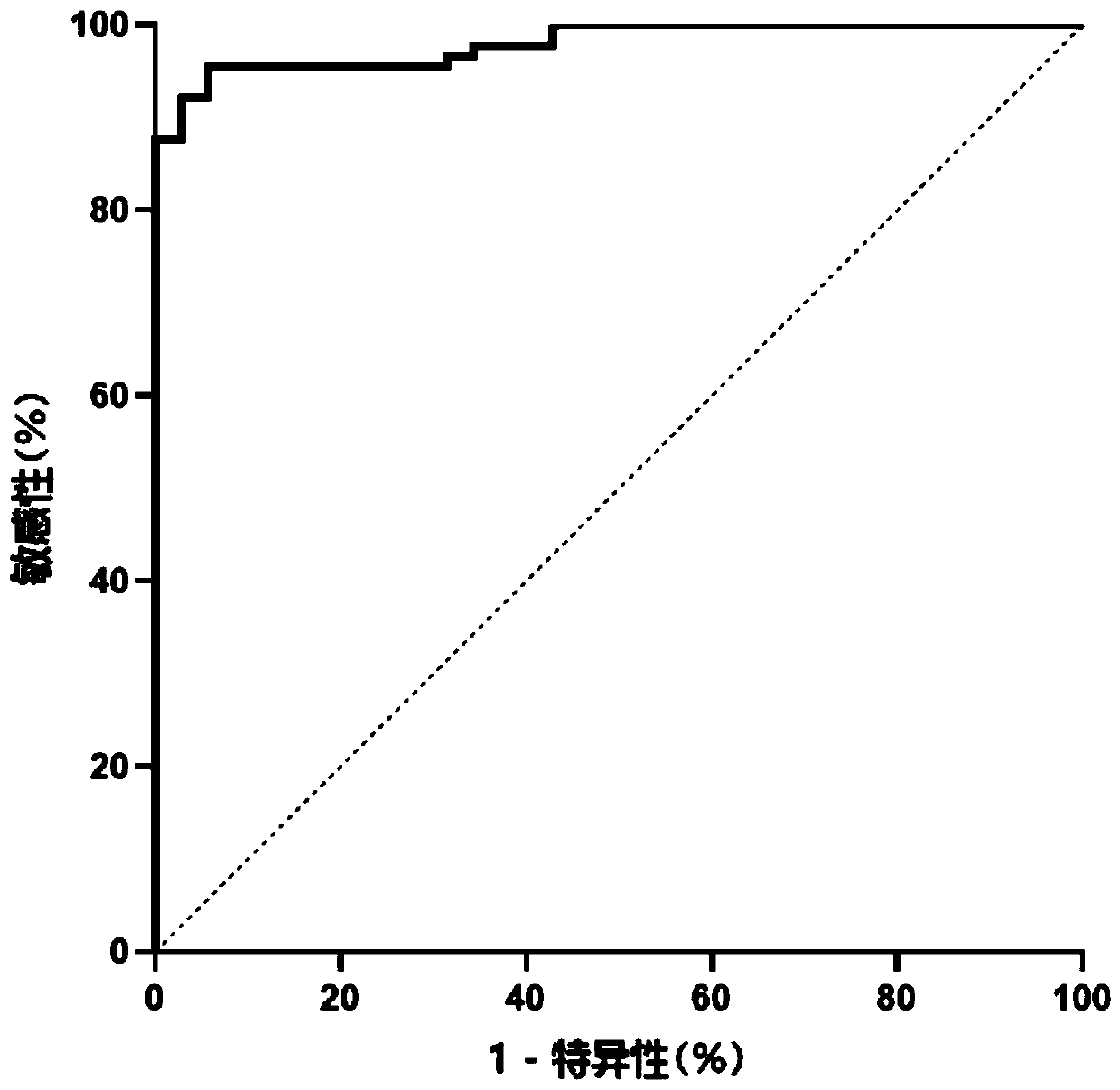
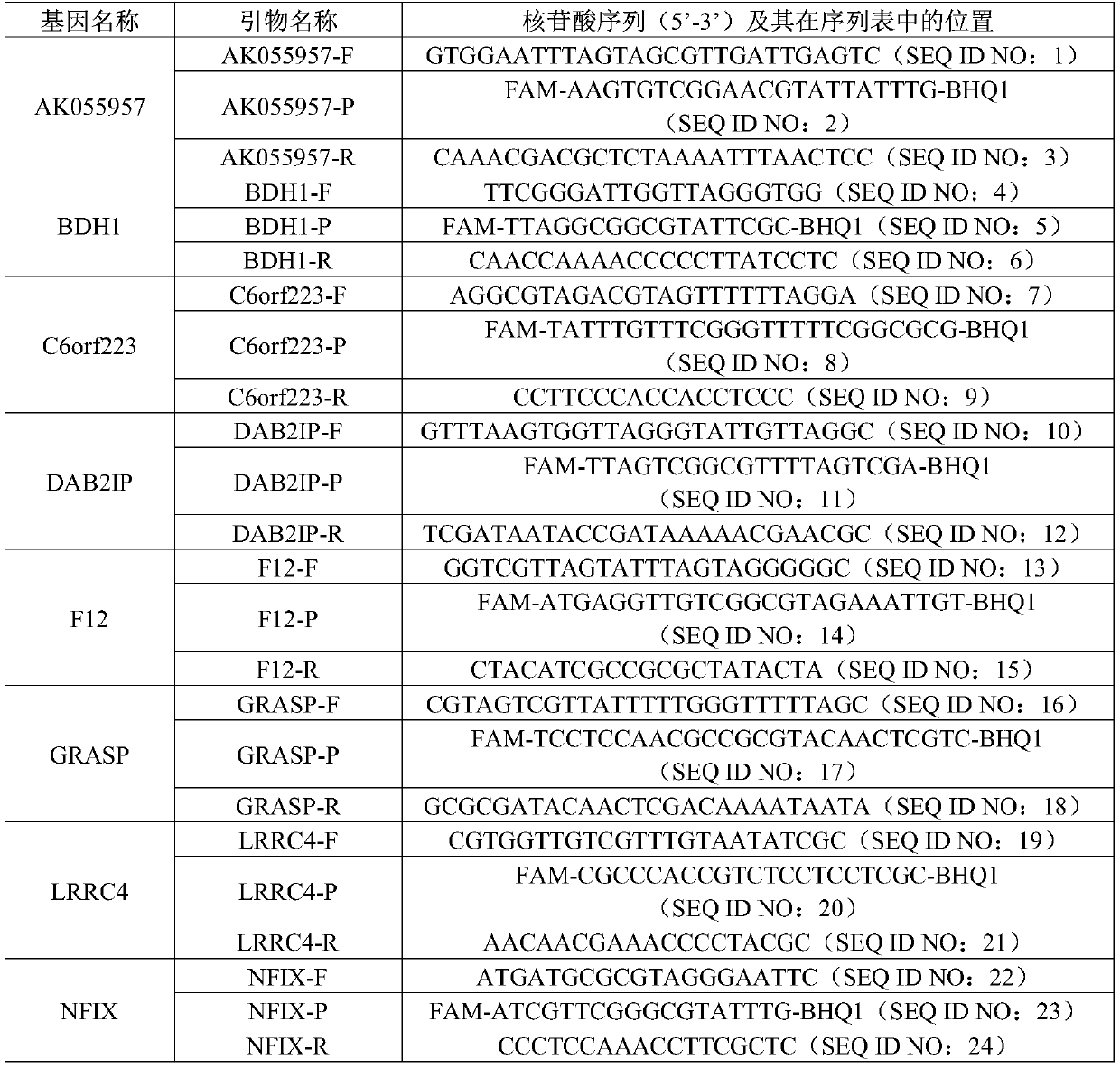
Combination marker used for liver cancer detection and application thereof
ActiveCN110904225AAvoid biopsyFully reflect the whole pictureMicrobiological testing/measurementProteomicsAdenylate kinaseNuclear factor I
The invention discloses a combination marker used for liver cancer detection and application thereof. The combination marker used for liver cancer detection comprises the respective methylation levelsof adenylate kinase 055957 (AK055957), 3-hydroxybutyrate dehydrogenase 1 (BDH1), chromosome 6 open reading frame 223 (C6orf223), disabled homolog 2-interacting protein (DAB2IP), human coagulation factor XII (F12), Golgi reassembly stacking protein (GRASP), leucine-rich repeat-containing protein 4 (LRRC4), nuclear factor I / X (NFIX), 5-oxoprolinase (ATP-hydrolysing) (OPLAH), recombinant protein tyrosine phosphatase F interacting protein 1 (PPFIA1), Pleckstrin homology and SEC7 domain-containing protein 4 (PSD4) and T-box 15 (TBX15). Experiments prove that whether the genome DNA or plasma circulating free DNA (cfDNA) of to-be-detected liver tissue comes from patients with liver cancer or patients without liver cancer can be judged by detecting the combination marker, and accuracy is relatively high; and the combination marker has significant application value.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Silicon dot forming method and apparatus
InactiveUS20070158182A1Easy to getUniform particle sizeCellsRadiation applicationsSputteringHydrogen
A silicon sputter target is arranged in a silicon dot forming chamber, and a silicon dot formation target substrate is arranged in the chamber. Plasma is formed from a sputtering gas (typically a hydrogen gas) supplied into the chamber, and chemical sputtering is effected on the target with the plasma thus formed to form silicon dots on the substrate S. Optionally, with the plasma formed from a hydrogen gas and a silane-containing gas at a plasma emission intensity ratio (Si(288 nm) / Hβ) of 10.0 or lower, the silicon dots are formed on the substrate S. The silicon dots are terminally treated with the plasma derived from a terminally treating gas such as an oxygen gas.
Owner:NISSIN ELECTRIC CO LTD
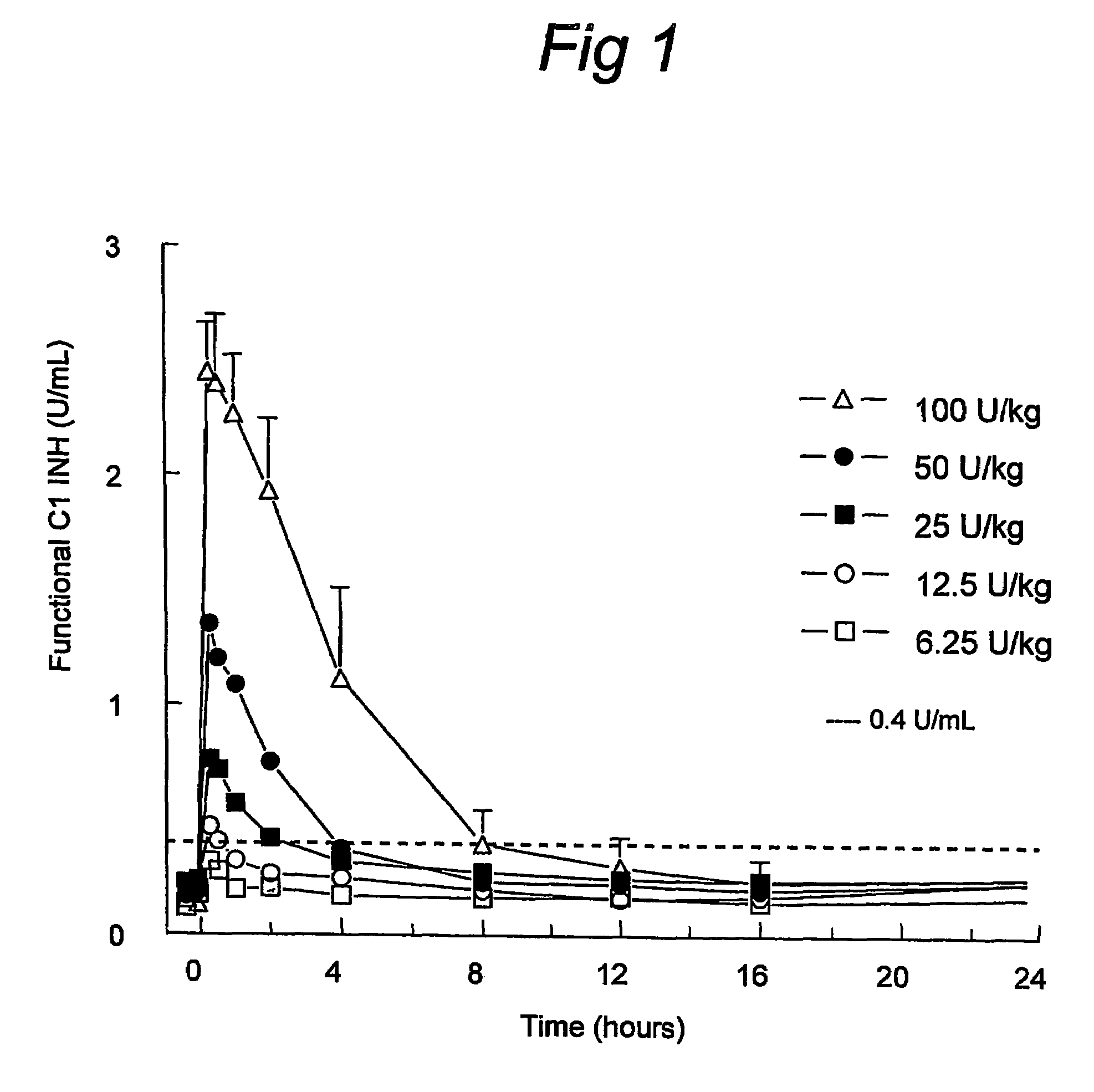
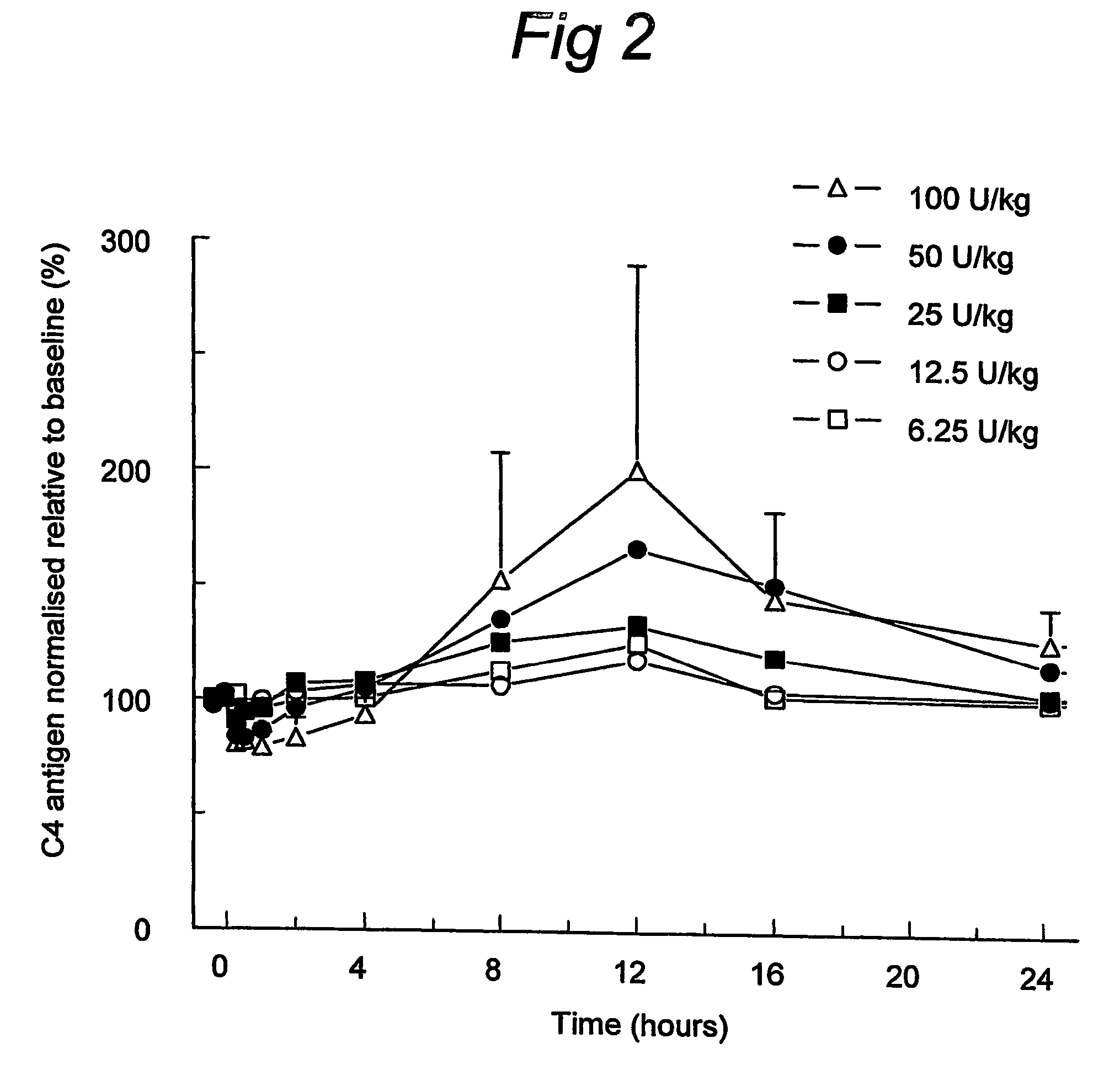
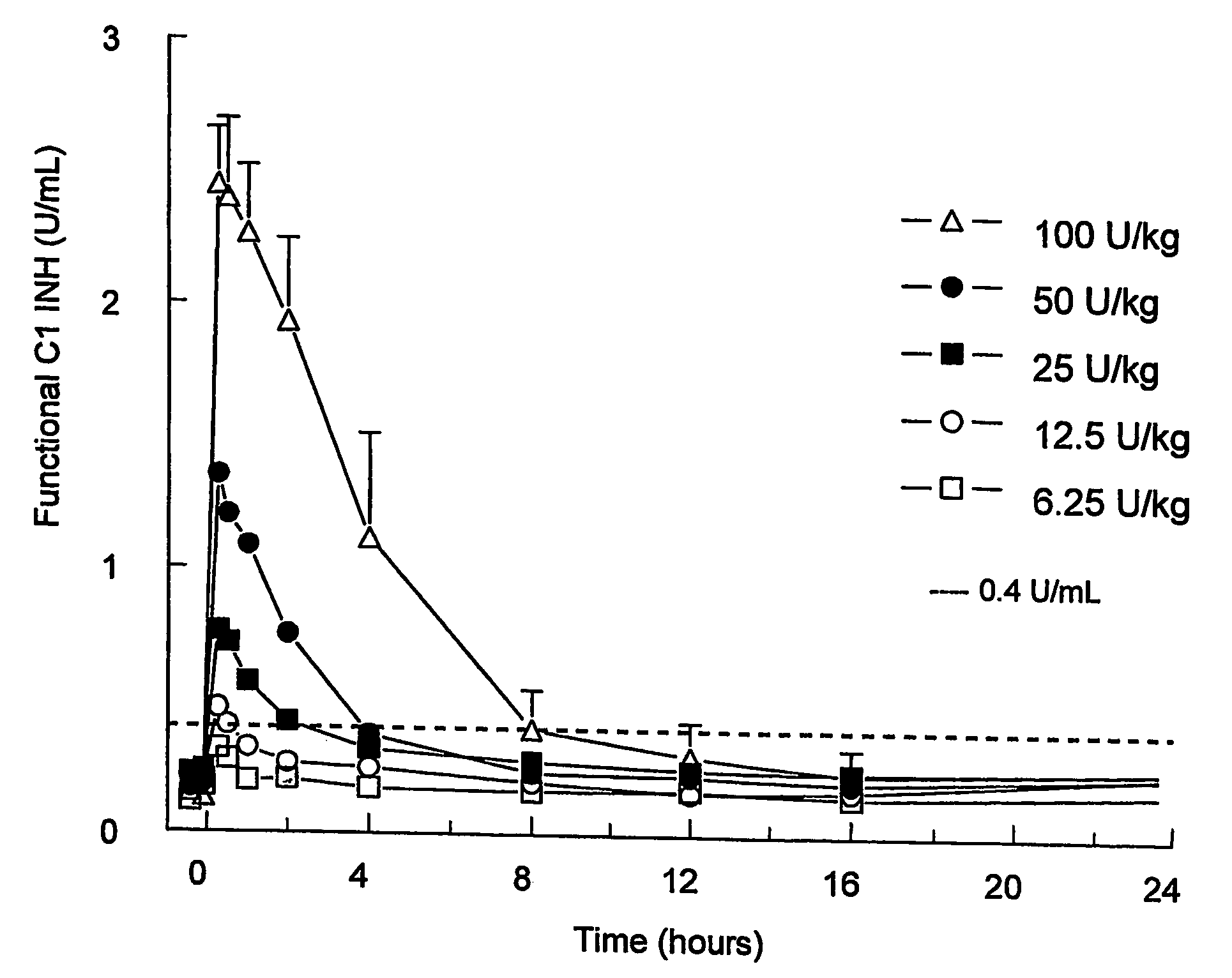
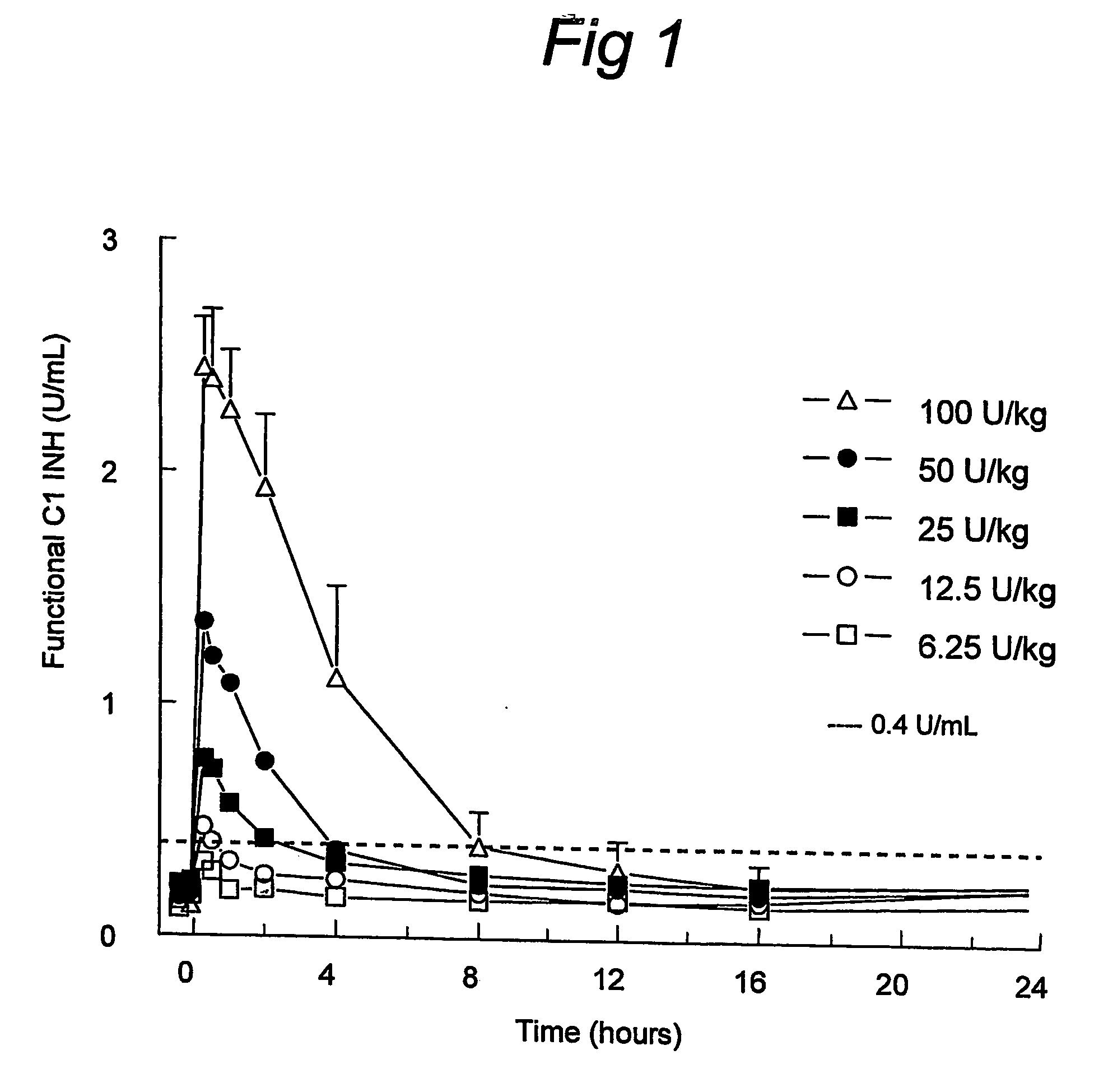
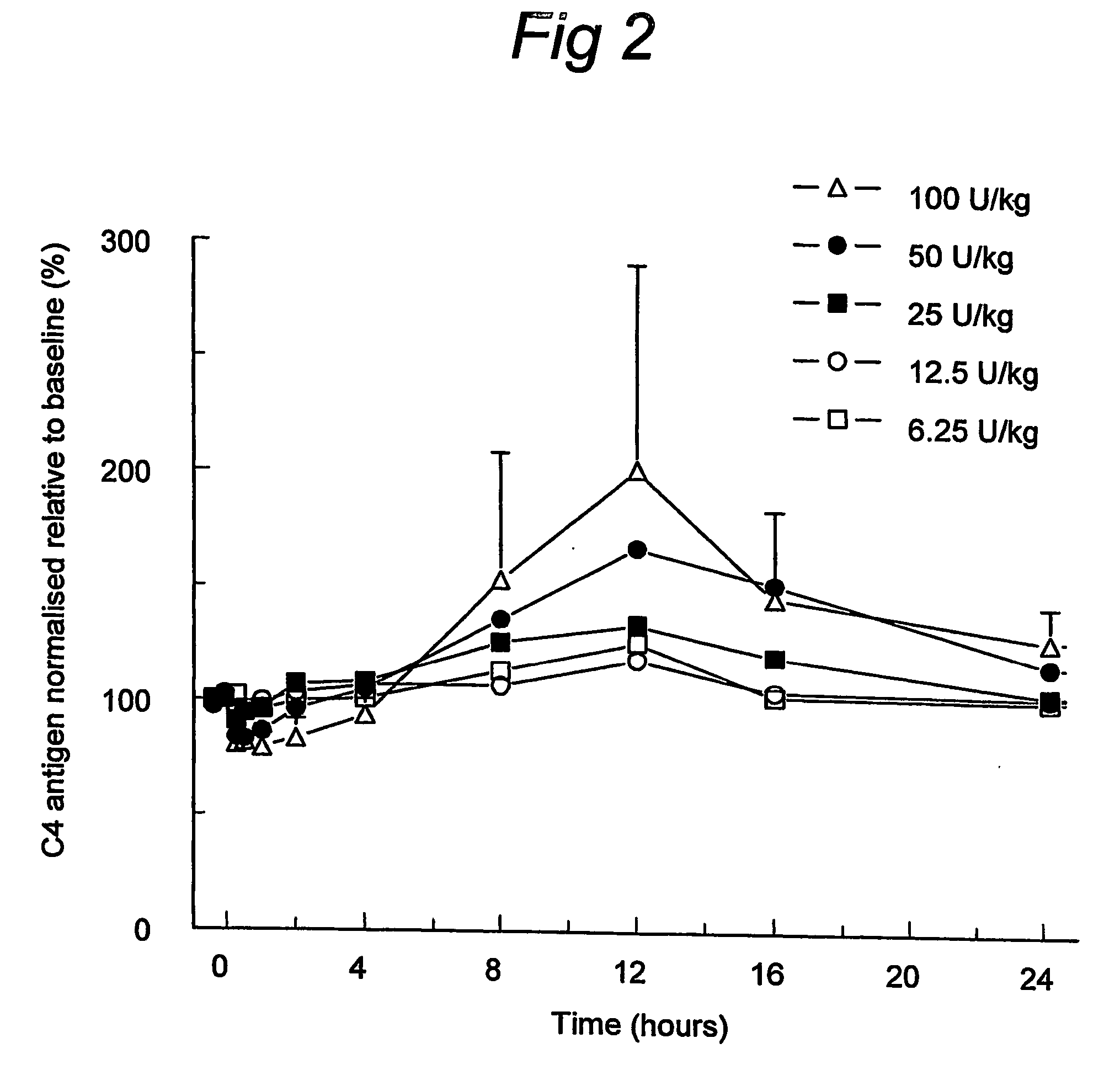
C1 inhibitor with short half-life transient treatment
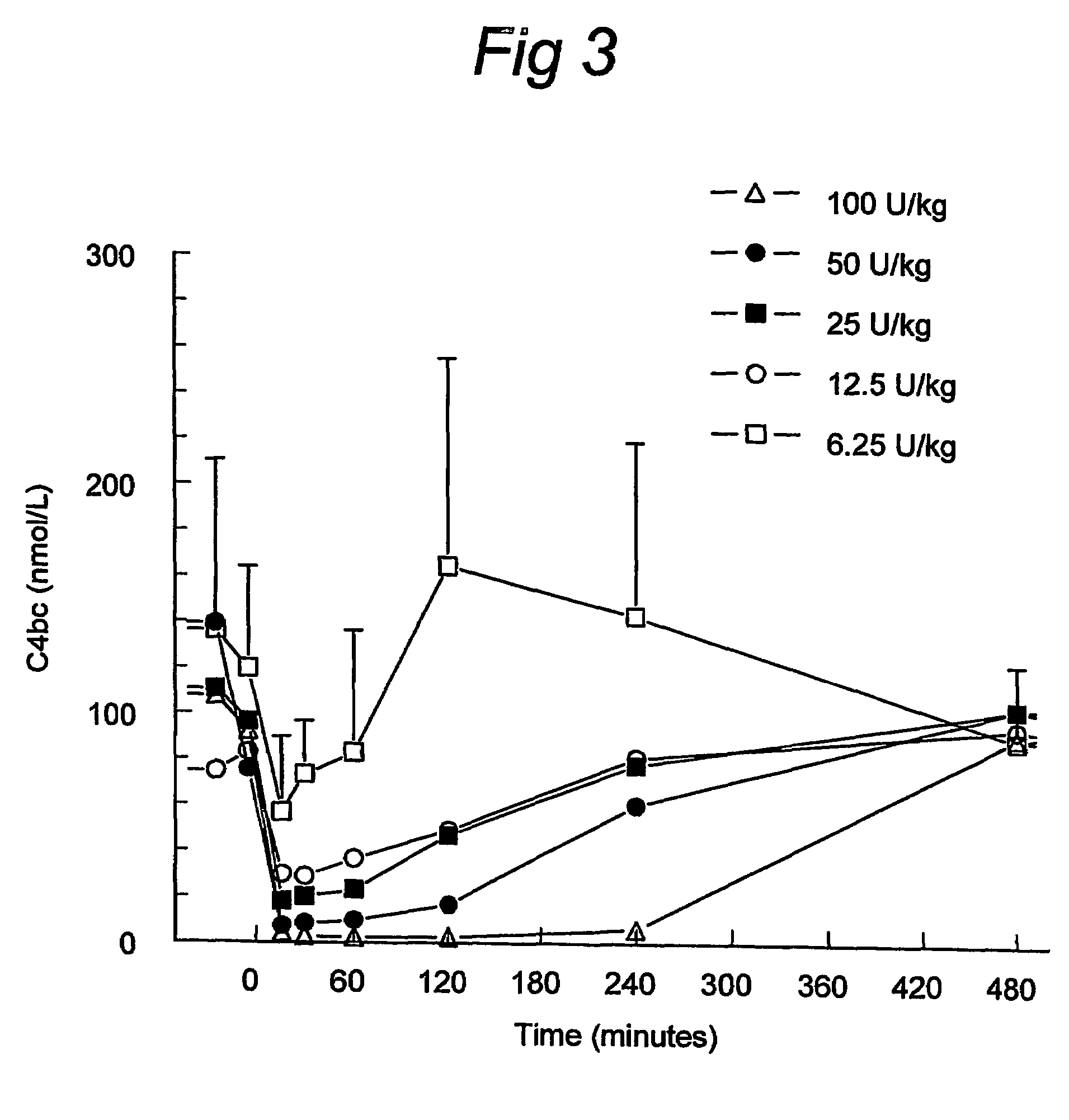
ActiveUS7544853B2Long exposureAvoid detrimental side-effectsPeptide/protein ingredientsAntipyreticPlasma derivedHalf-life
Owner:PHARMING INTPROP BV
Porous, film, wiring structure, and method of forming the same
InactiveUS6873052B2Low dielectric constantRetain sufficient mechanical strengthSemiconductor/solid-state device detailsSolid-state devicesPlasma derivedOrganic compound
An organic-inorganic hybrid film is deposited on a substrate by introducing, into a vacuum chamber, a gas mixture of a silicon alkoxide and an organic compound and generating a plasma derived from the gas mixture. Then, a hydrogen plasma process is performed with respect to the organic-inorganic hybrid film by introducing, into the vacuum chamber, a gas containing a reducing gas and generating a plasma derived from the gas. As a result, an organic component in the organic-inorganic hybrid film eliminates therefrom and numerous fine holes are formed in hollow portions from which the organic component has eliminated, whereby a porous film composed of the organic-inorganic hybrid film is obtained.
Owner:GK BRIDGE 1
Method for Manufacturing Recombinant Polyclonal Proteins
InactiveUS20090136498A1Minimizing unwanted batch-to-batch variationUnwanted variationPeptide/protein ingredientsAntipyreticPresent methodProtein composition
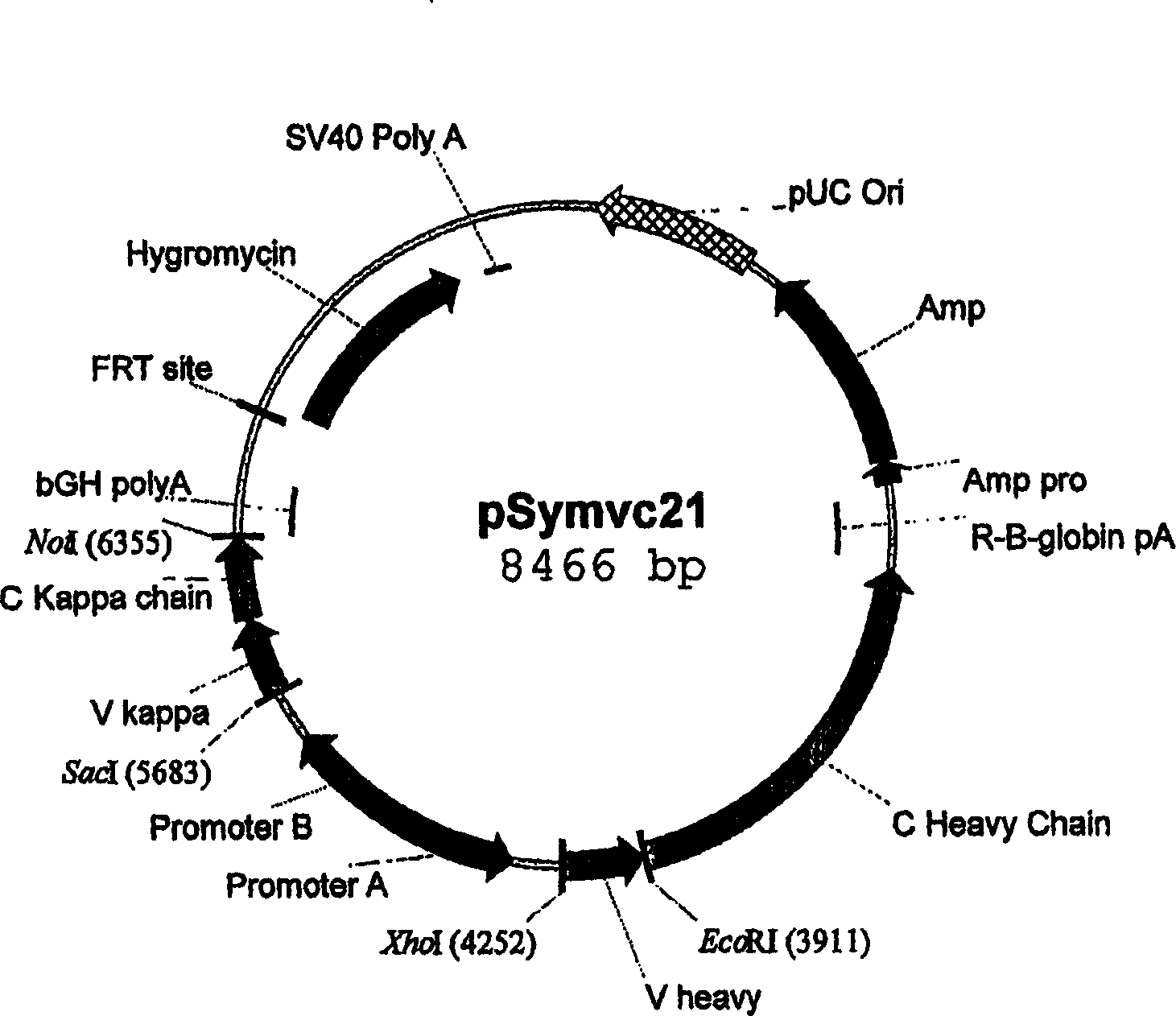
The invention relates to a method for manufacturing a recombinant polyclonal protein composition, in particular a recombinant polyclonal antibody composition. The method comprises obtaining a collection of cells transfected with a library of variant nucleic acid sequences, wherein each call in the collection is transfected with and capable of expressing one member of the library, which encodes a distinct member of a polyclonal protein that binds a particular antigen and which is located at the same single site in the genome of individual cells in said collection, wherein said nucleic acid sequence is not naturally associated with said cell in the collection. The cells are cultured under suitable conditions for expression of the polyclonal protein, which is obtained from the cells or culture supernatant. The nucleic acid sequence id introduced into the cells by transfection with a library of vectors for site-specific integration. The present method is suitable for manufacturing recombinant polyclonal antibodies, thereby making available a superior replacement of plasma-derived therapeutic immunoglobulin products.
Owner:SYMPHOGEN AS
C1 inhibitor with short half-life transient treatment
ActiveUS20070185011A1Long exposureAvoid detrimental side-effectsPeptide/protein ingredientsAntipyreticPlasma derivedMedicine
The present invention relates to the use of a C1 inhibitor (C1INH) with shorter half-life than plasma-derived C1INH for the preparation of a medicament for the transient treatment of an individual. It relates to both therapeutic and prophylactic treatment. The method of the invention allows for the administration of C1INH at certain therapeutic levels for a concise pre-determined time span. Pharmaceutical compositions based on C1INH with shorter half-lives may be used both in situations where transient treatment is merely and advantage. The advantage of the use according to the invention is that an individual is not exposed to C1INH for longer than required, since the levels of the C1INH more rapidly subsides after administration has stopped. In contrast, levels of plasma-derived C1INH would remain elevated for a prolonged period of time.
Owner:PHARMING INTPROP BV
Purification process for large scale production of Gc-globulin, the Gc-globulin produced hereby, a use of Gc-globulin and a Gc-globulin medicinal product
InactiveUS20050020816A1High yieldHigh purityPeptide/protein ingredientsMammal material medical ingredientsPlasma derivedPurification methods
A purification process for large-scale production of Gc-globulin is described. The source of Gc-globulin is preferably a crude plasma fraction but can be any solution, suspension or supernatant containing Gc-globulin, e.g., a milk product, colostrum or a fermentation broth. The Gc-globulin can be plasma-derived or produced by a genetic modified organism. The process includes two key elements: purification by series of ion exchange chromatography steps, and performing at least two virus-reduction steps. A diagnostic method to measure the free Gc-globulin in a patient blood sample, a use of Gc-globulin in medicine and the preparation of a Gc-globulin medicinal product is also provided. The product can be used in therapy for patients with circulatory disorders and complications, i.e., where it is contemplated that patients would benefit from the administration of Gc-globulin.
Owner:STATENS SERUM INST
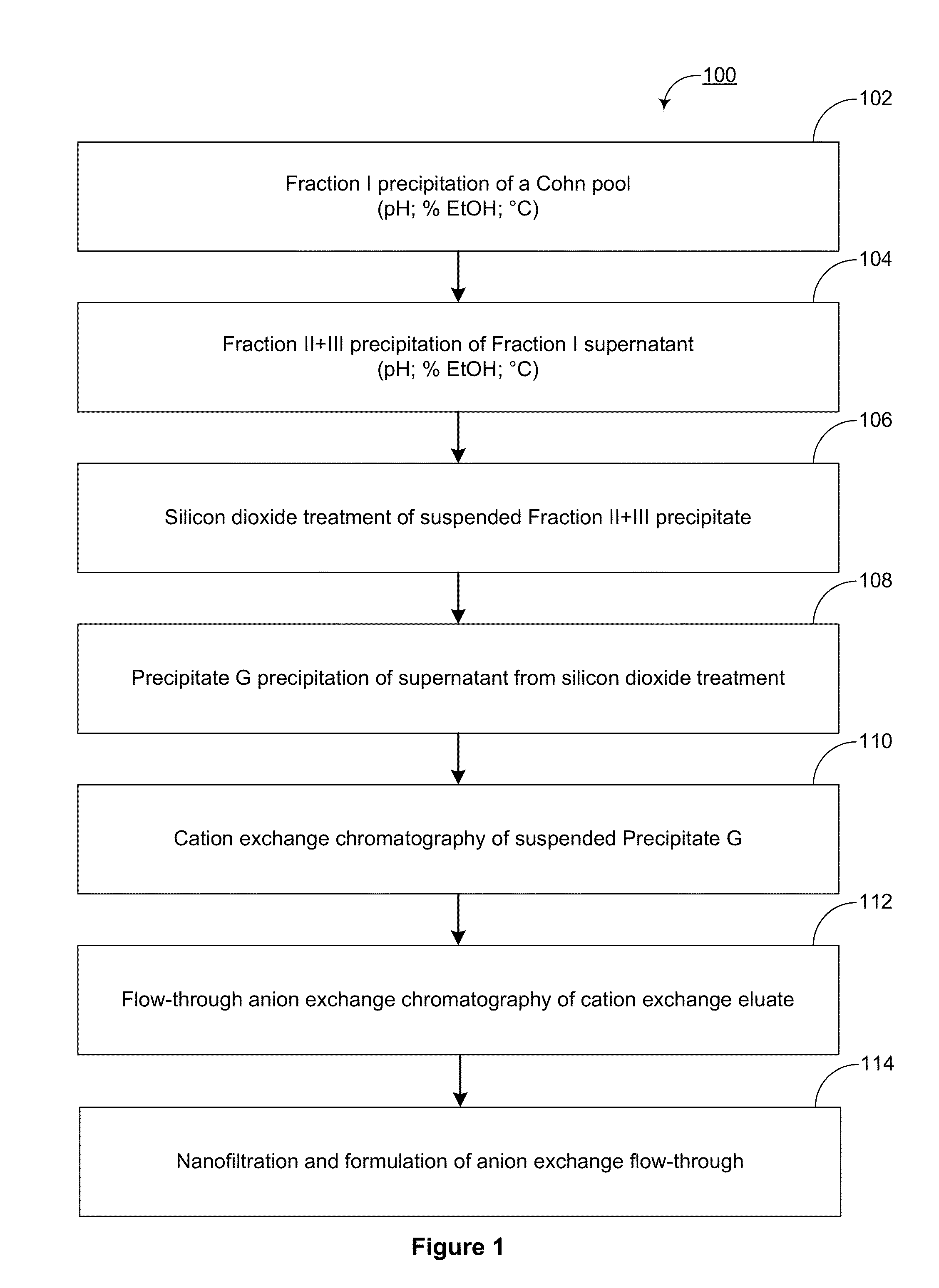
Removal of serine proteases by treatment with finely divided silicon dioxide
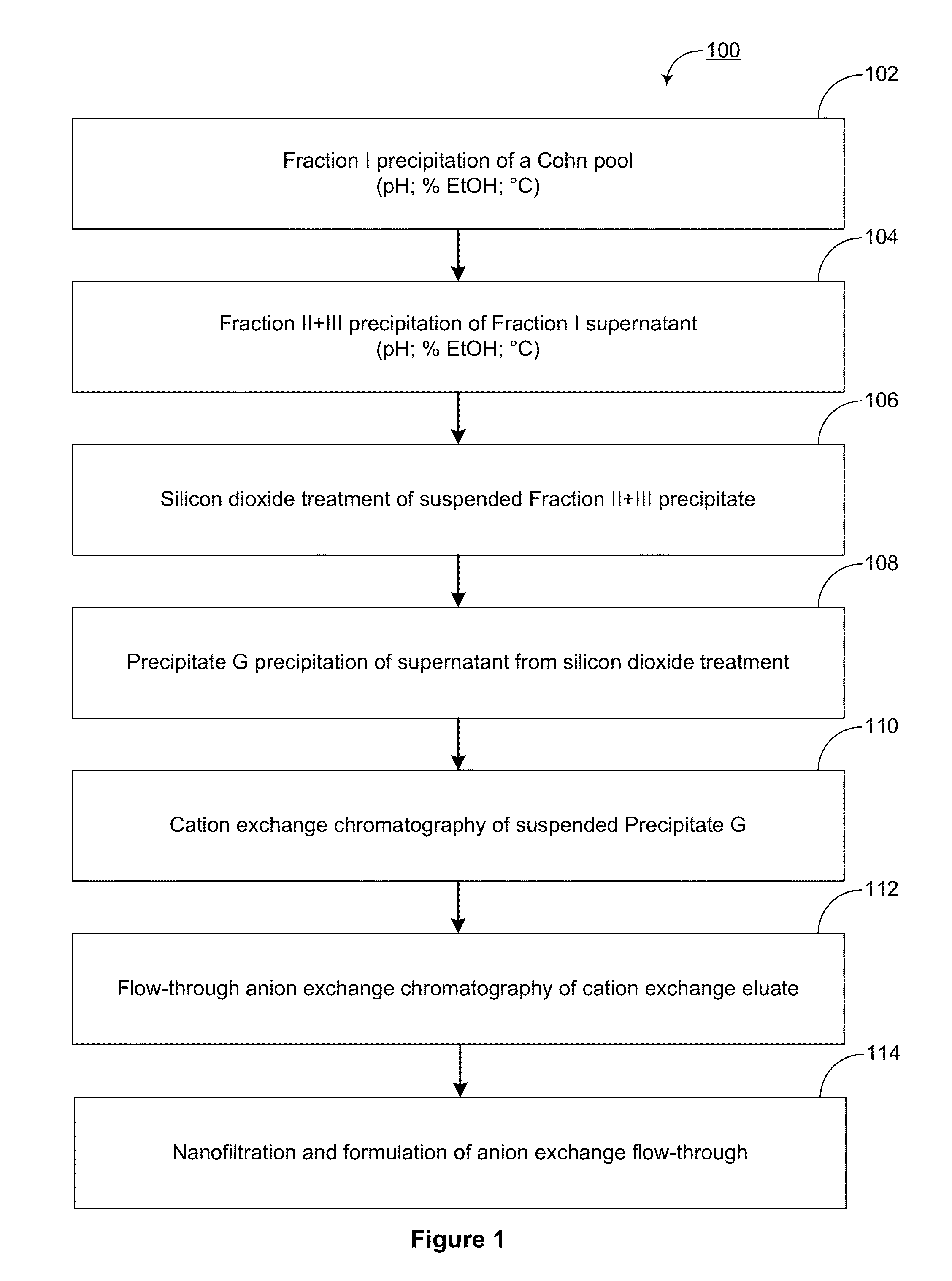
The present invention provides novel methods for reducing the serine protease and / or serine protease zymogen content of a plasma-derived protein composition. Also provided are methods for manufacturing plasma-derived protein compositions having reduced serine protease and\or serine protease zymogen content. Among yet other aspects, the present invention provides aqueous and lyophilized compositions of plasma-derived proteins having reduced serine protease and / or serine protease zymogen content. Yet other aspects include methods for treating, managing, and / or preventing a disease comprising the administration of a plasma-derived protein composition having a reduced serine protease or serine protease zymogen content.
Owner:TAKEDA PHARMA CO LTD
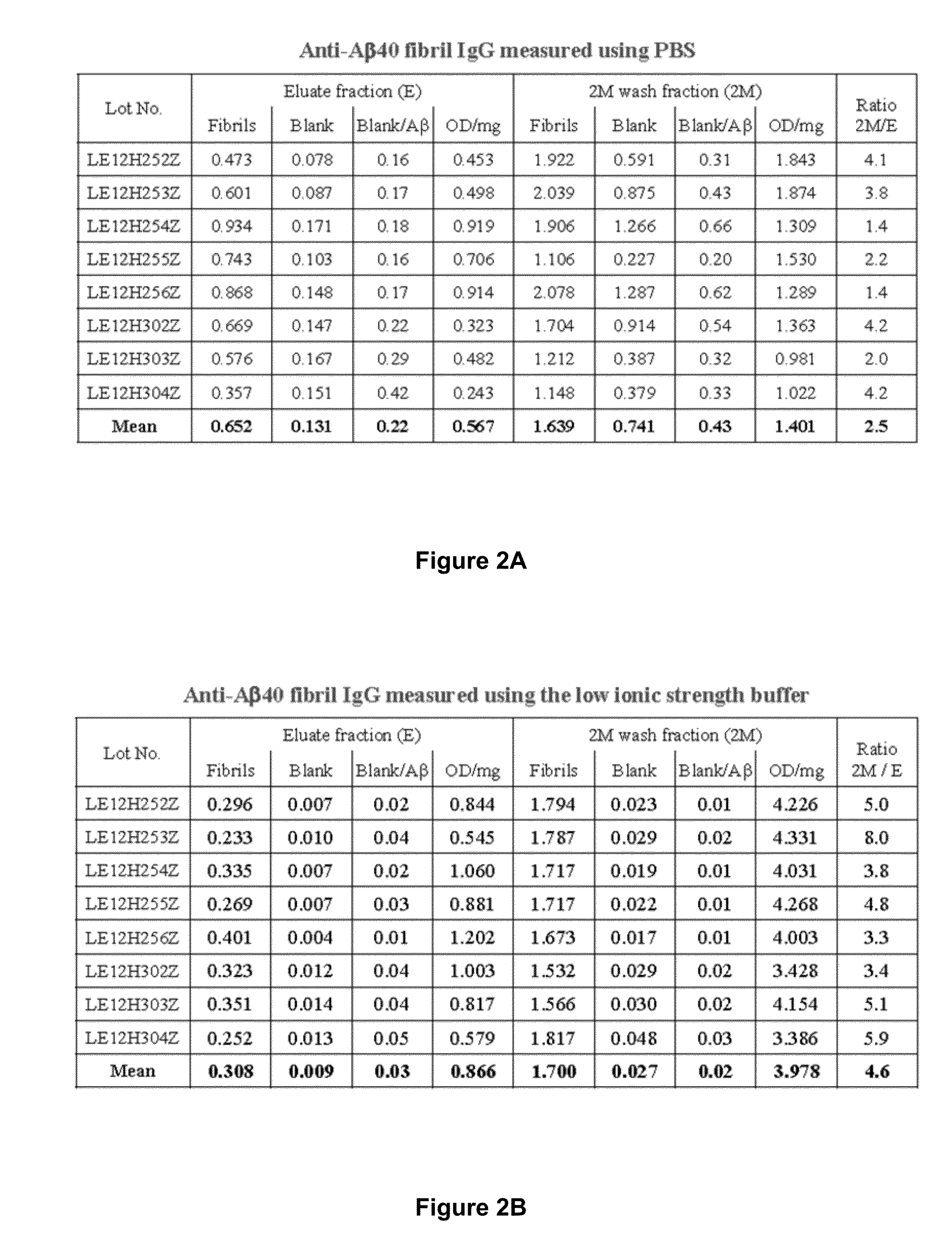
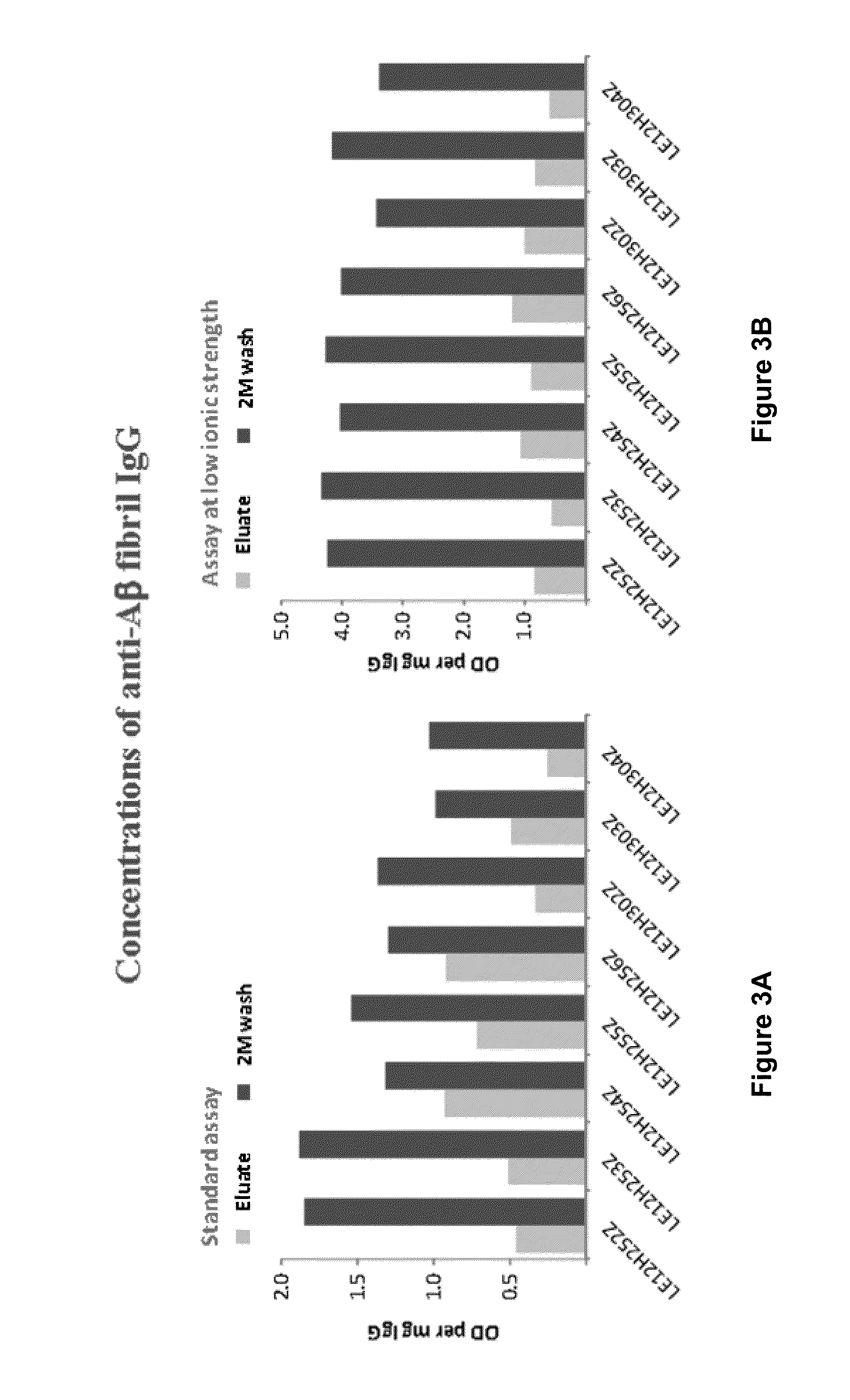
Methods to produce a human plasma-derived igg preparation enriched in brain disease-related natural iggs
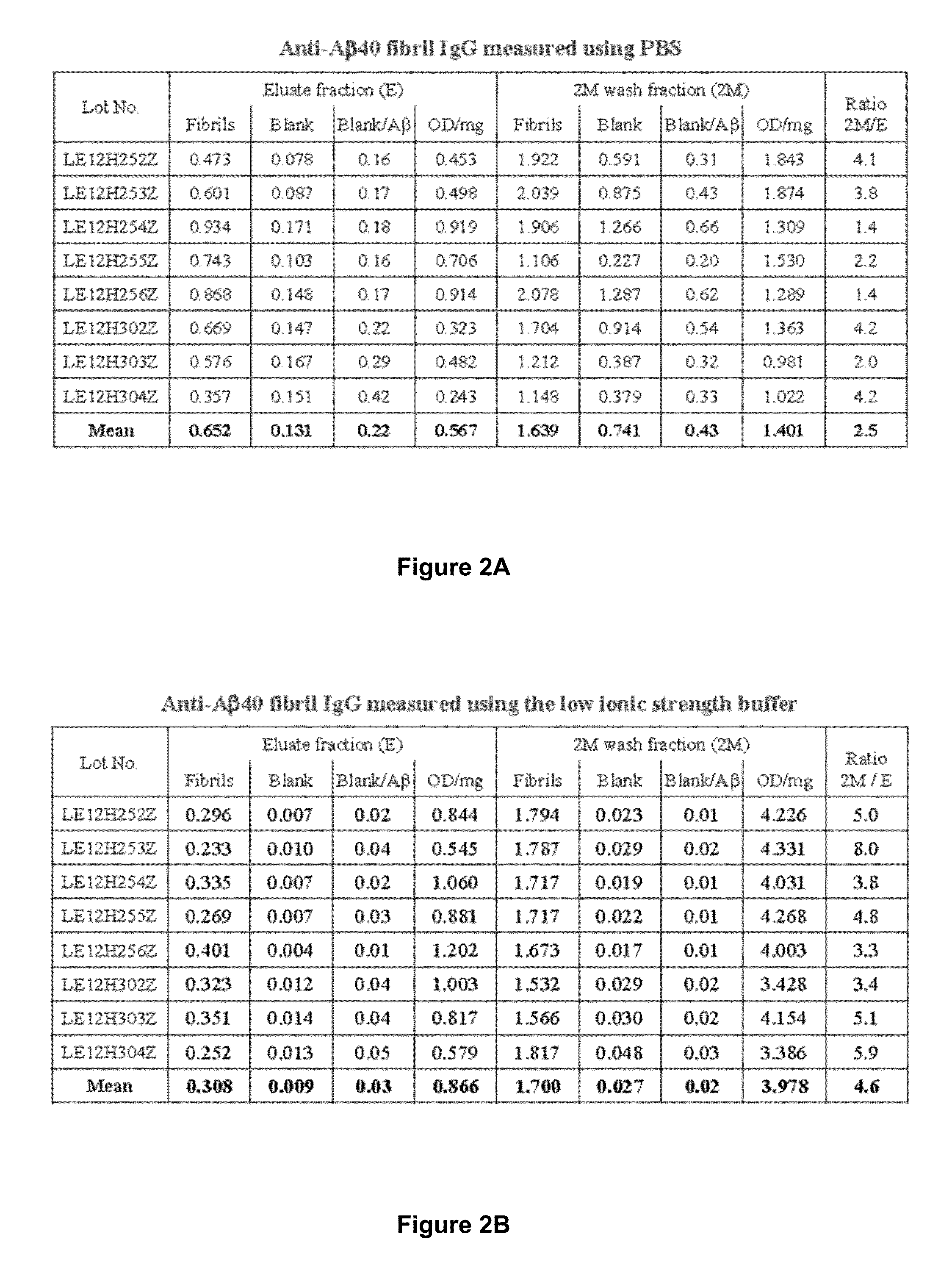
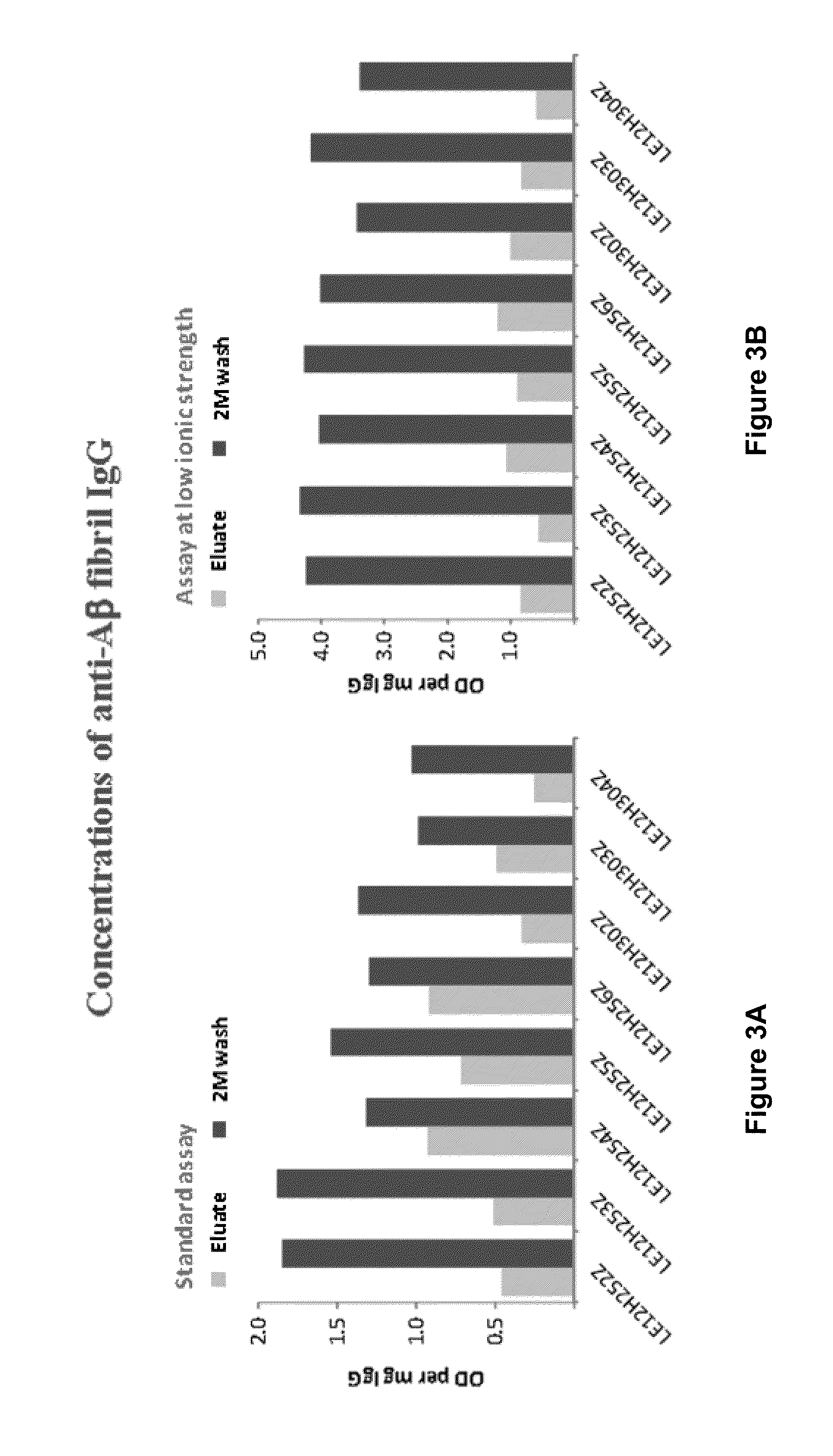
The present invention provides, among other aspects, methods for the manufacture of plasma-derived immunoglobulin G compositions highly enriched for anti-brain disease related protein antibodies (e.g., anti-Aβ, anti-RAGE, and anti-α-synuclein antibodies). Advantageously, the methods provided do not affect the manufacturing processes or capabilities for producing plasma-derived IgG therapeutics. Plasma-derived IgG compositions that are highly enriched for anti-brain disease related protein antibodies (e.g., anti-Aβ, anti-RAGE, and anti-α-synuclein antibodies), as also provided here. Methods for the treatment of brain diseases and disorders by administration of plasma-derived IgG compositions highly enriched for anti-brain disease related protein antibodies (e.g., anti-Aβ, anti-RAGE, and anti-α-synuclein antibodies), are also provided.
Owner:TAKEDA PHARMA CO LTD
Platelet-enriched plasma, as well as preparation method and application thereof
ActiveCN106754681ALow costScalable productionCulture processSkeletal/connective tissue cellsPlasma derivedMedical waste
The invention provides platelet-enriched plasma, as well as a preparation method and application thereof. According to the preparation method of the platelet-enriched plasma, the platelet-enriched plasma is prepared by taking medical waste white membrane layer cells as raw materials. Compared with the traditional preparation method of platelet-enriched plasma derived from whole blood, the preparation method of the platelet-enriched plasma has the advantages that the raw materials can be obtained cheaply and massively, so that preparation cost is greatly reduced and preparation scale is enlarge. In addition, the method provided by the invention integrates the steps of separating platelet-enriched plasma and activating platelet into one step, and the platelet-enriched plasma prepared by the method can be directly used.
Owner:李众利
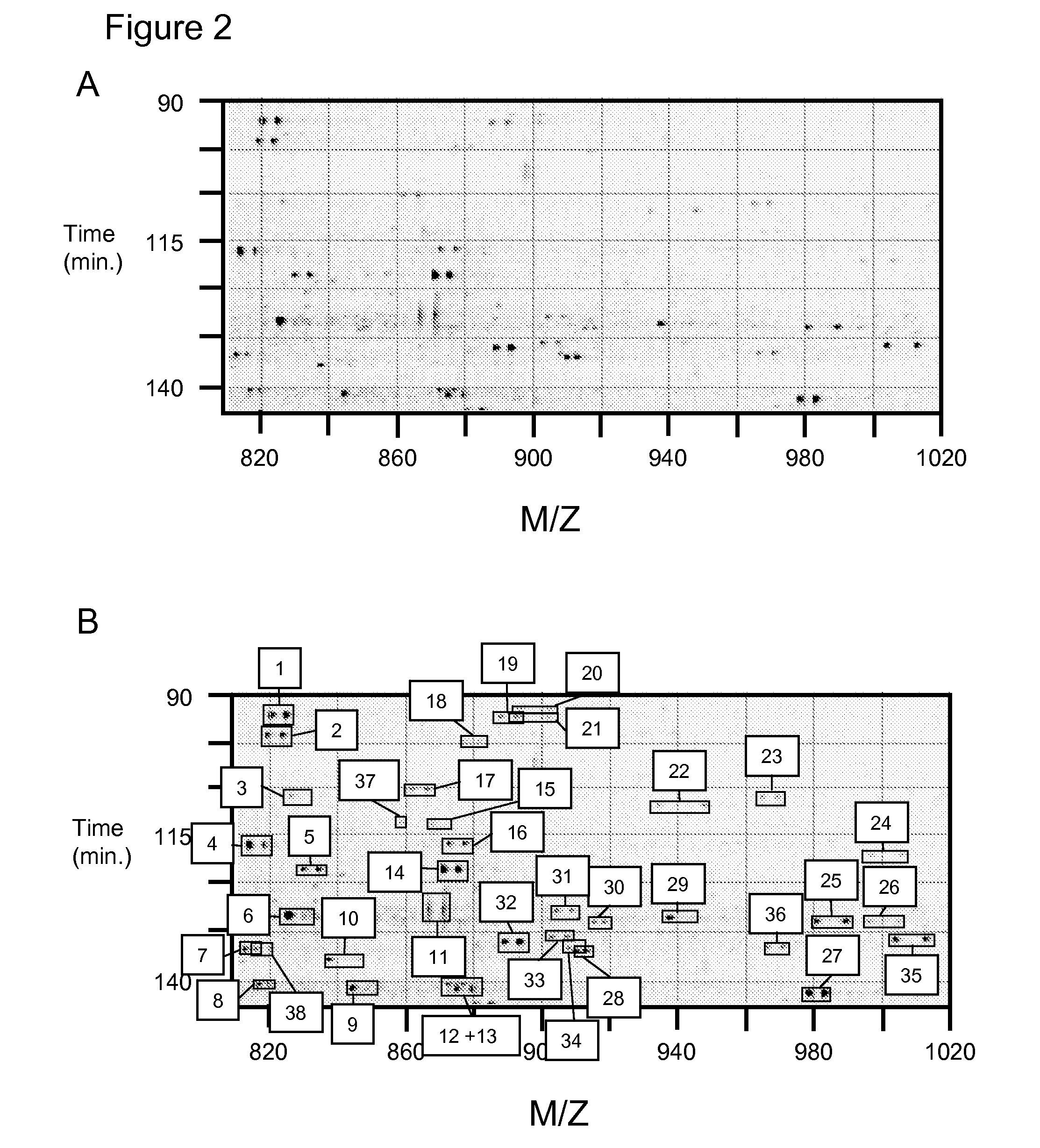
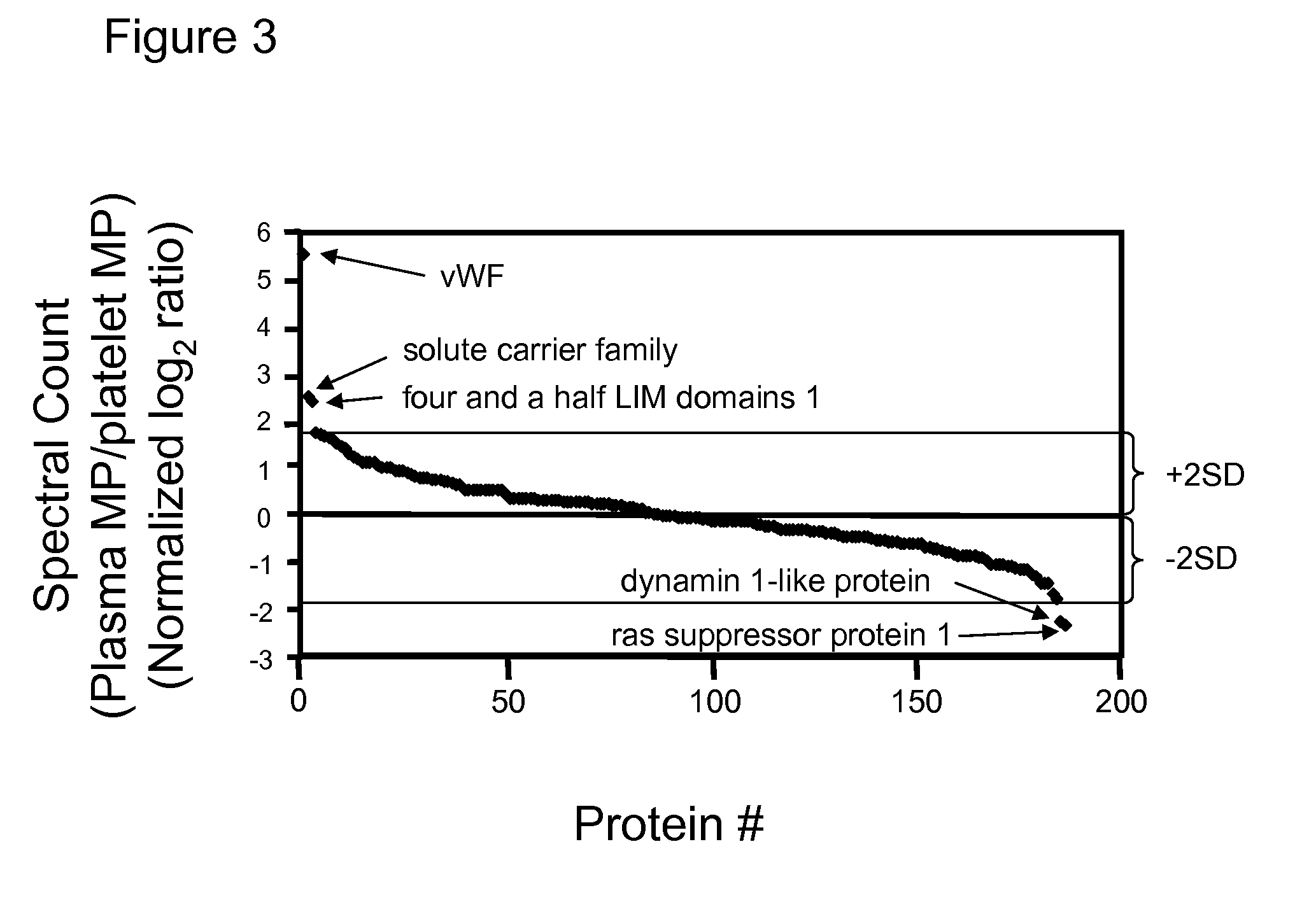
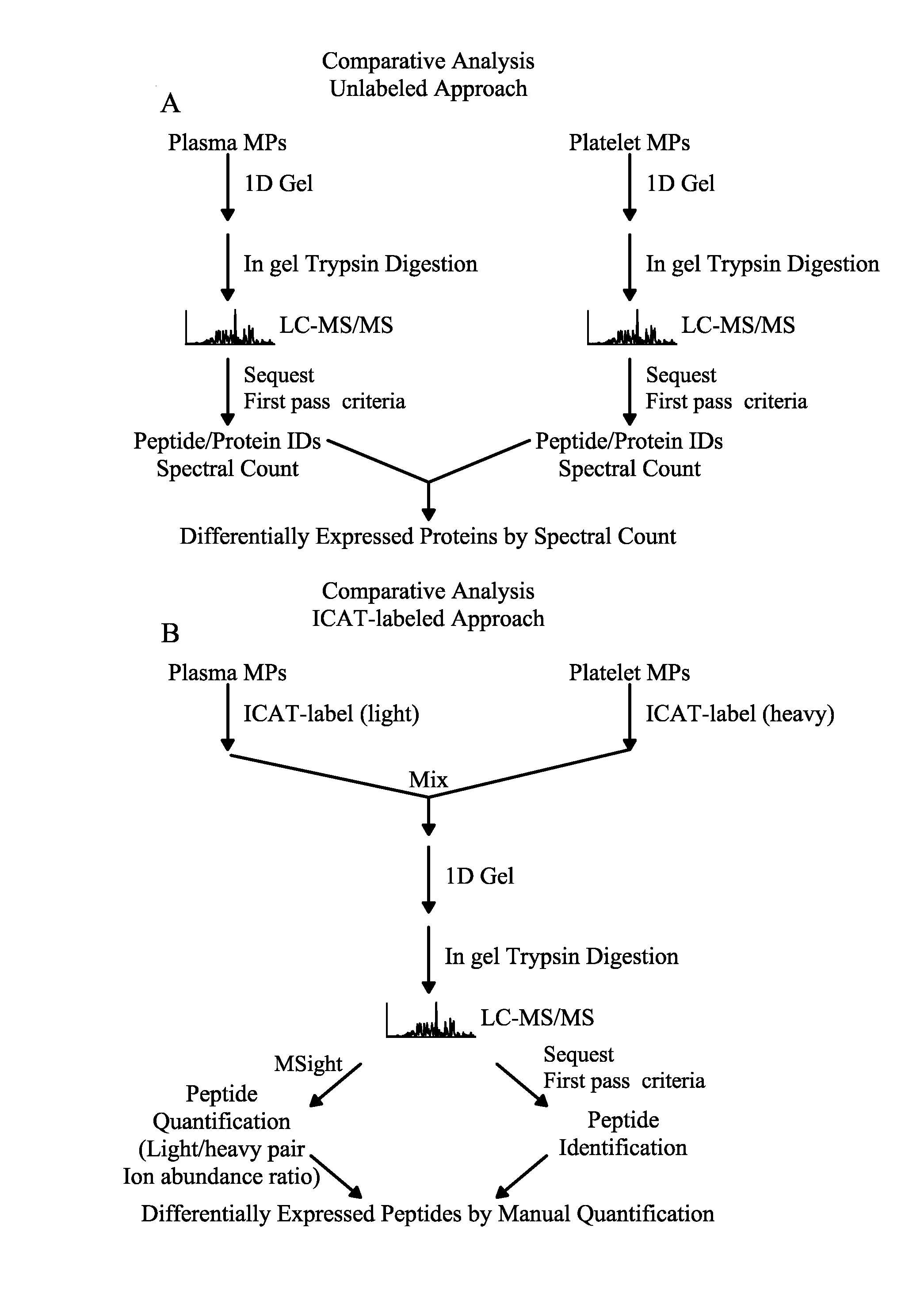
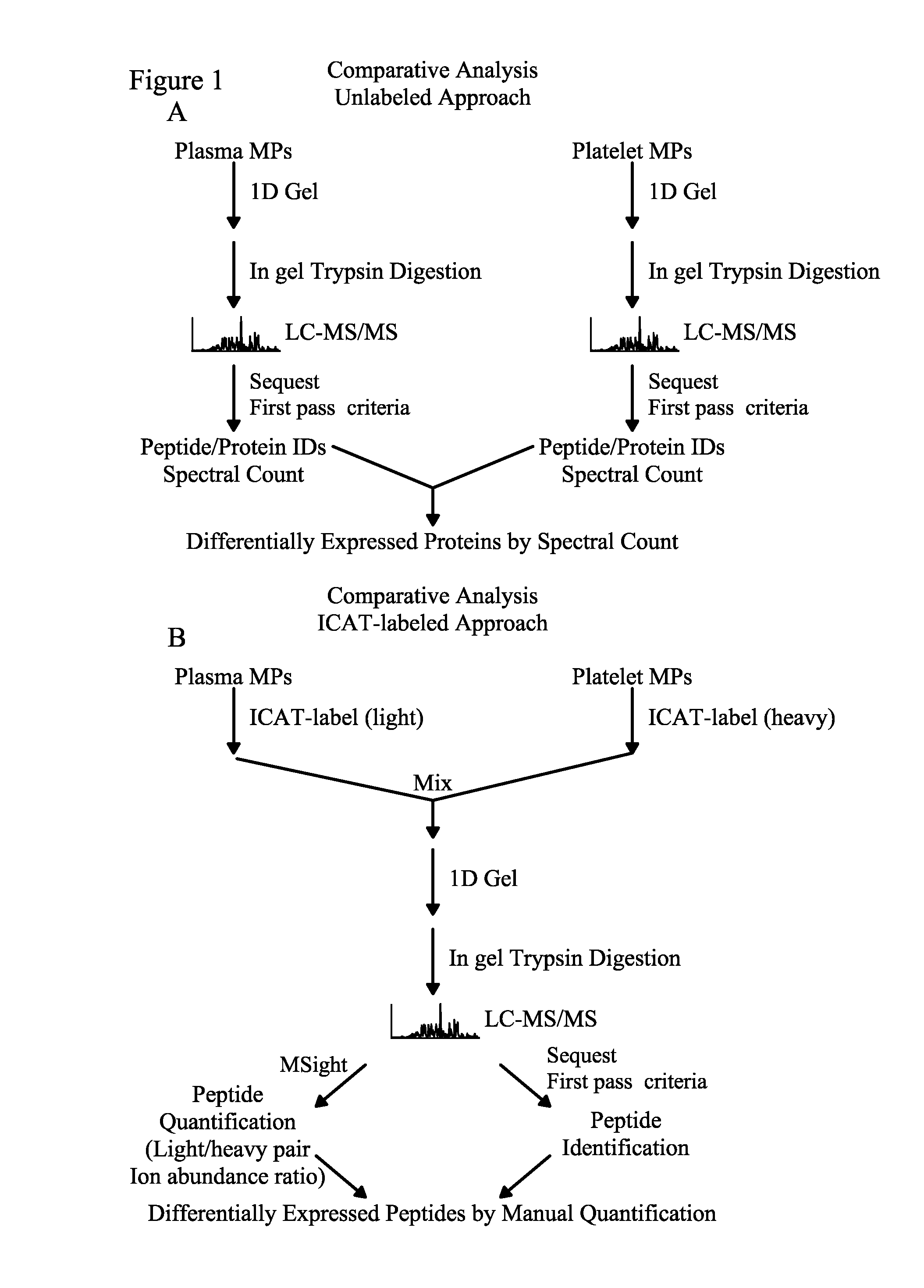
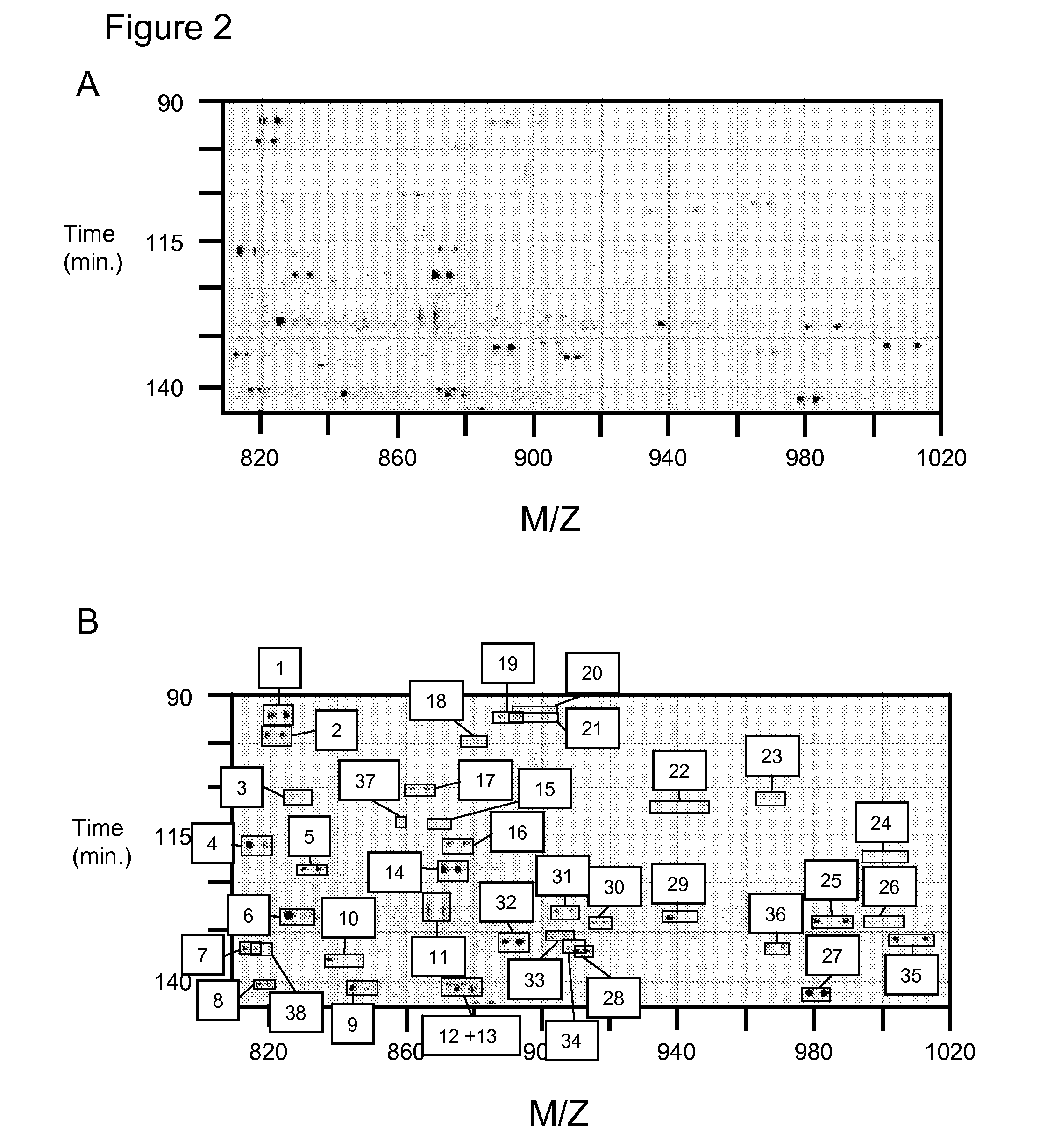
Methods for identifying and analyzing biomarkers from plasma-derived microparticles
Owner:MICROPARTICLE PROTEOMICS LLS
Methods of using a fixed dose of a clotting factor
ActiveUS20160296607A1Low variabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDosing regimen
The present invention provides methods of administering a clotting factor by a fixed dosing regimen; methods of reducing, ameliorating, or preventing one or more symptoms of a bleeding disease or disorder; and a kit comprising a dotting factor useful for a fixed dosing regimen. While plasma-derived and recombinant clotting factor products allow hemophilia patients to live longer and healthier, hemophilia still remains one of the most costly and complex conditions to manage.
Owner:BIOVERATIV THERAPEUTICS INC
Removal of serine proteases by treatment with finely divided silicon dioxide
ActiveUS20130195897A1Peptide/protein ingredientsSolid sorbent liquid separationZymogenPlasma derived
The present invention provides novel methods for reducing the serine protease and / or serine protease zymogen content of a plasma-derived protein composition. Also provided are methods for manufacturing plasma-derived protein compositions having reduced serine protease and\or serine protease zymogen content. Among yet other aspects, the present invention provides aqueous and lyophilized compositions of plasma-derived proteins having reduced serine protease and / or serine protease zymogen content. Yet other aspects include methods for treating, managing, and / or preventing a disease comprising the administration of a plasma-derived protein composition having a reduced serine protease or serine protease zymogen content.
Owner:TAKEDA PHARMA CO LTD
Transgenically produced antithrombin III
InactiveUS20080176786A1Peptide/protein ingredientsAntipyreticMonosaccharide compositionPlasma derived
This invention relates to transgenically produced human Antithrombin III (tgATIII). The human ATIII produced by the transgenic process of the present invention has a monosaccharide composition which comprises N-acetylgalactosamine (GalNAc) along with fucose, N-acetylglucosamine, galactose, mannose, and N-acetylneuraminic acid / N-glycolyneuraminic acid. The monosaccharide composition differs with that of plasma derived ATIII (phATIII). It has been found that tgATIII has an increased clearance rate when compared to phATIII.
Owner:GTC BIOTHERAPEUTICS INC
Method for manufacturing recombinant polyclonal proteins
InactiveCN1723283AAvoid ethicsAvoid clinical problemsCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsPresent methodProtein composition
The invention relates to a method for manufacturing a recombinant polyclonal protein composition, in particular a recombinant polyclonal antibody composition. The method comprises obtaining a collection of cells transfected with a library of variant nucleic acid sequences, wherein each cell in the collection is transfected with and capable of expressing one member of the library, which encodes a distinct member of a polyclonal protein that binds a particular antigen and which is located at the same single site in the genome of individual cells in said collection, wherein said nucleic acid sequence is not naturally associated with said cell in the collection. The cells are cultured under suitable conditions for expression of the polyclonal protein, which is obtained from the cells or culture supernatant. The nucleic acid sequence id introduced into the cells by transfection with a library of vectors for site-specific integration. The present method is suitable for manufacturing recombinant polyclonal antibodies, thereby making available a superior replacement of plasma-derived therapeutic immunoglobulin products.
Owner:SYMPHOGEN AS
Purification process for large scale production of Gc-globulin, the Gc-globulin produced hereby, a use of Gc-globulin and a Gc-globulin medicinal product
InactiveUS6921808B2High yieldHigh purityPeptide/protein ingredientsMammal material medical ingredientsPurification methodsPlasma derived
A purification process for large-scale production of Gc-globulin is described. The source of Gc-globulin is preferably a crude plasma fraction but can be any solution, suspension or supernatant containing Gc-globulin, e.g., a milk product, colostrum or a fermentation broth. The Gc-globulin can be plasma-derived or produced by a genetic modified organism. The process includes two key elements: purification by series of ion exchange chromatography steps, and performing at least two virus-reduction steps. A diagnostic method to measure the free Gc-globulin in a patient blood sample, a use of Gc-globulin in medicine and the preparation of a Gc-globulin medicinal product is also provided. The product can be used in therapy for patients with circulatory disorders and complications, i.e., where it is contemplated that patients would benefit from the administration of Gc-globulin.
Owner:STATENS SERUM INST
Preparation method and application of specific antibody containing milk or serum produced by novel coronavirus immunized cows
InactiveCN111961132AMake up the limitEffective neutralizationSerum immunoglobulinsMilk immunoglobulinsPlasma derivedMilk cow's
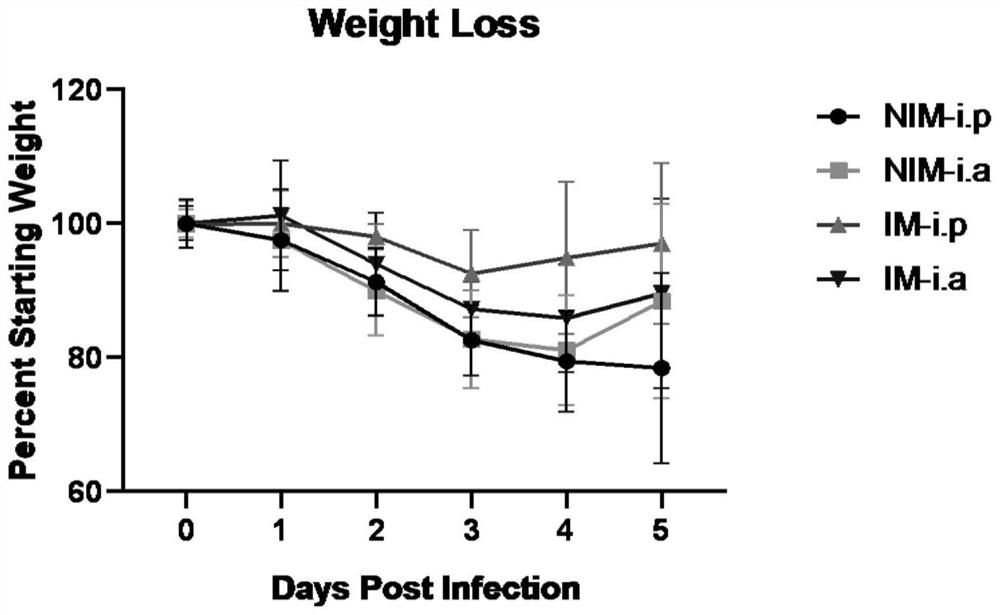
The invention belongs to the field of biotechnology, and discloses a preparation method and application of specific antibody containing milk or serum produced by novel coronavirus immunized cows. Theinactivated novel coronavirus hCov-19 / Wuhan / ZY38-1 / 2020 is used for immunizing dairy cows, and a large amount of immune milk and serum can be obtained. Both the immune milk and the serum contain highcontent of antibodies, viruses can be effectively neutralized and stopped from invading cells, and the significant anti-SARS-CoV-2 virus effect is achieved; after Igs protein purified from immune milkis injected into mice, the mortality of novel coronavirus infected mice can be significantly reduced, the influence of novel coronavirus infection on weight loss of the mice is reduced, and the content of viruses in lung tissue of the mice is reduced. The immunized milk and serum can be used as sources of antibodies for preparations for preventing and treating novel coronavirus pneumonia, and thelimitation of the sources of plasma for rehabilitation of novel coronavirus pneumonia.
Owner:HUAZHONG AGRI UNIV +2
Anti-rhesus D recombinant polyclonal antibody
InactiveUS8198415B2Reduce concentrationImmunoglobulins against blood group antigensLibrary screeningPlasma derivedPresent method
The invention relates to a method for manufacturing an anti-RhD recombinant polyclonal antibody composition (anti-RhD rpAb). The method comprises obtaining a collection of cells transfected with a library of anti-RhD antibody expression vectors, wherein each cell in the collection is capable of expressing from a VH and VL comprising nucleic acid segment, one member of the library, which encodes a distinct member of anti-RhD recombinant polyclonal antibody composition and which is located at the same site in the genome of individual cells in said collection. The cells are cultured under suitable conditions for expression of the recombinant polyclonal antibody, which is obtained from the cells or culture supernatant. The nucleic acid segments encoding the anti-RhD rpAb is introduced into the cells by transfection with a library of vectors for site-specific integration. The present method is suitable for manufacturing anti-RhD rpAb, thereby making available a superior replacement of plasma-derived prophylactic and therapeutic immunoglobulin products.
Owner:SYMPHOGEN AS
Methods for preparing hepatitis b immunoglobulin derived from plasma
ActiveUS20180140699A1High purityImprove stabilityCation exchanger materialsOrganic anion exchangersThrombusHuman hepatitis-B immunoglobulin
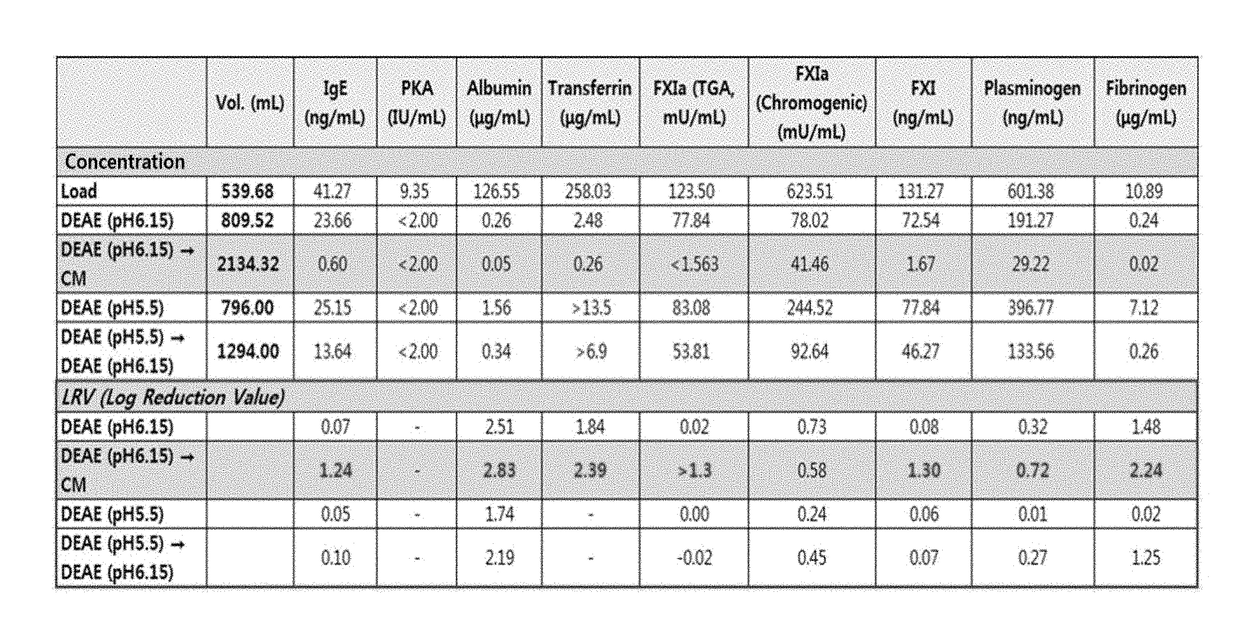
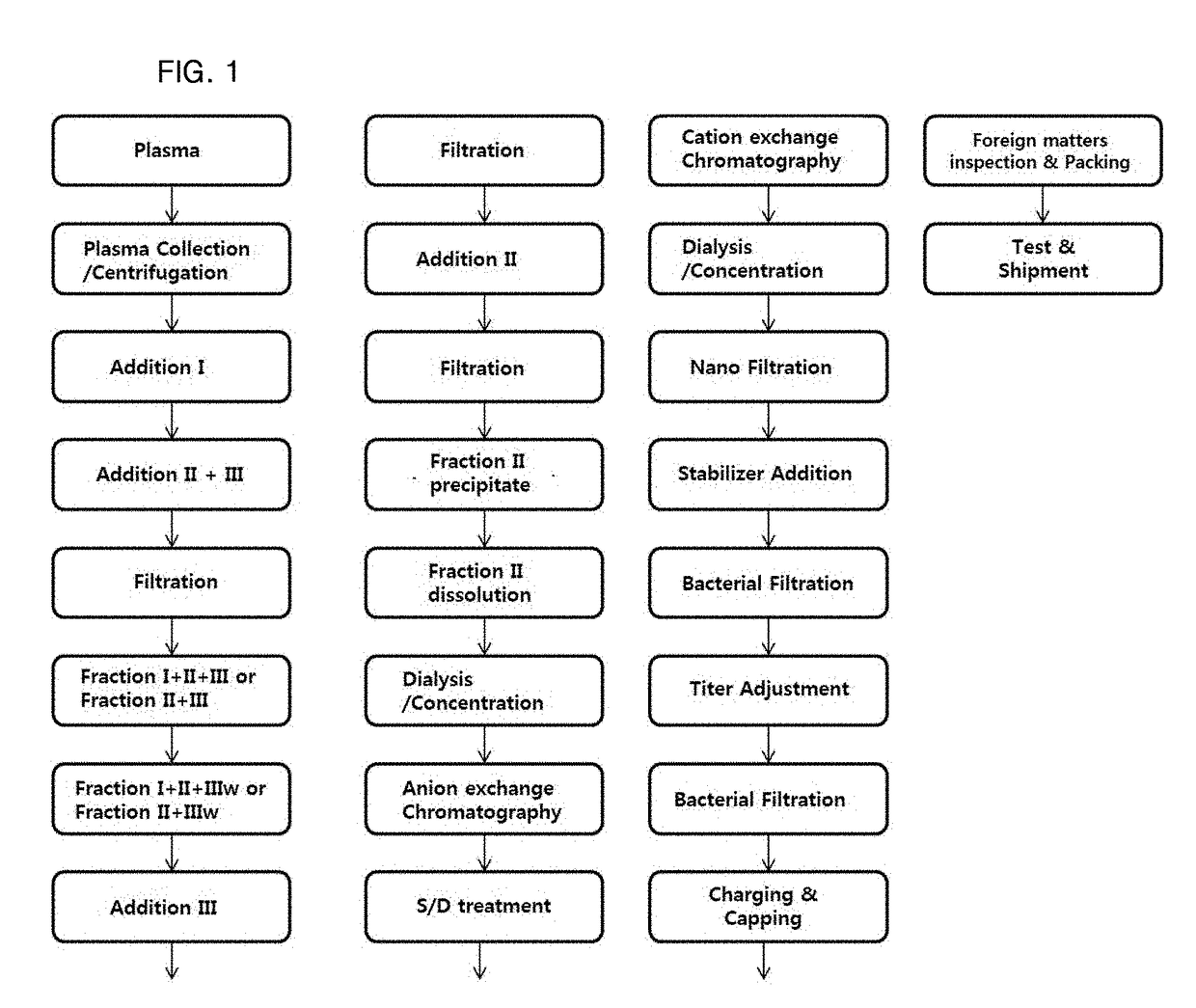
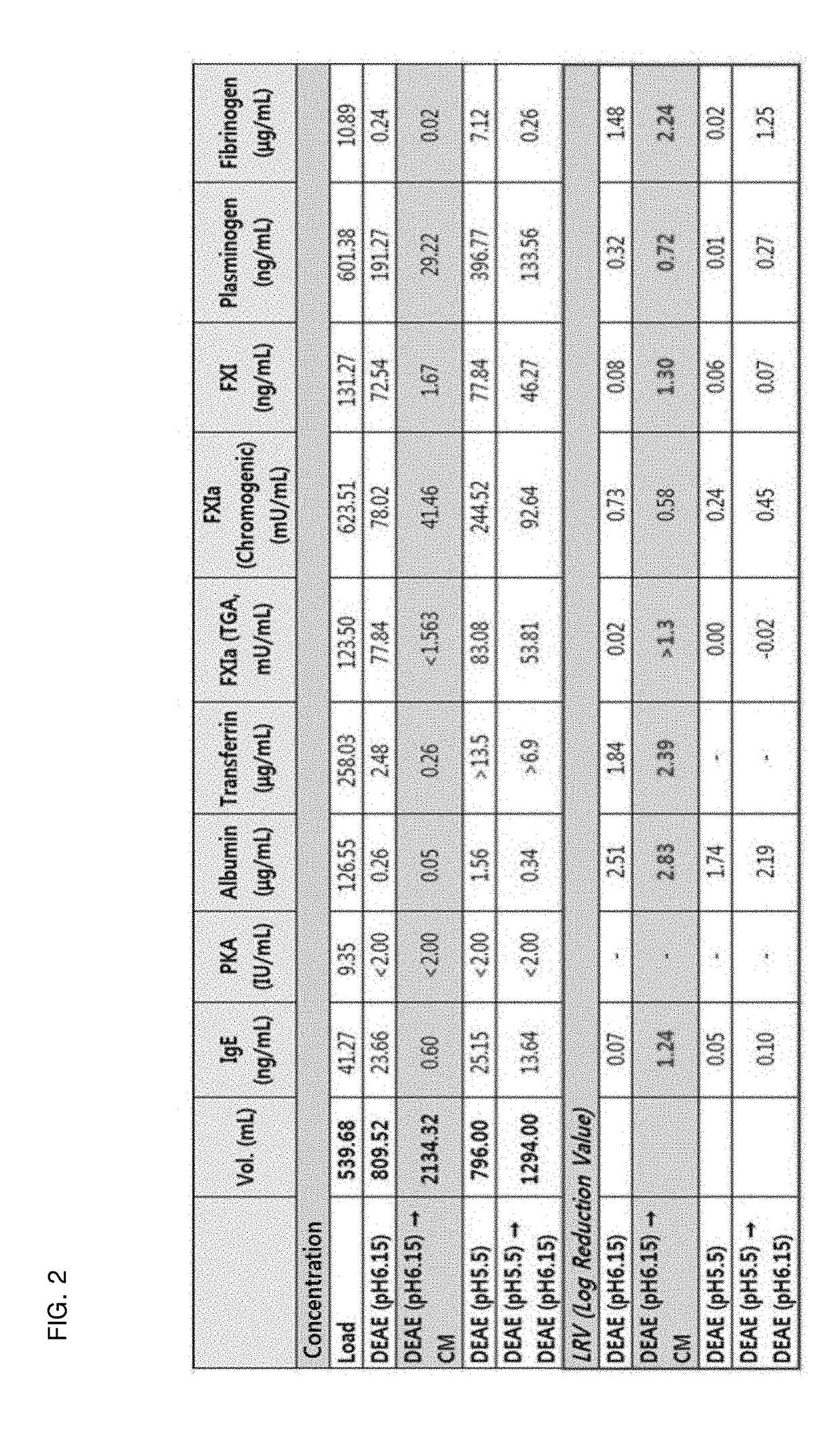
The present invention relates to a preparation method of a human plasma-derived hepatitis B immunoglobulin preparation. More specifically, the present invention relates to a preparation method of a human plasma-derived hepatitis B immunoglobulin preparation characterized in that plasma protein fraction II (fraction II) containing human hepatitis B immunoglobulin is dialysis concentrated and then thrombus-producing materials and impurities formed during processes are removed by anion exchange resin and cation exchange resin purification techniques. By using the preparation method of the human plasma-derived hepatitis B immunoglobulin preparation according to the present invention, the efficiency of removing impurities and thrombus-producing materials is increased and a polymer content is maintained, whereby it is possible to produce human hepatitis B immunoglobulin with stable and improved quality.
Owner:GREEN CROSS HLDG
Methods for identifying and analyzing biomarkers from plasma-derived microparticles
InactiveUS20080171312A1Peptide/protein ingredientsElectrophoretic profilingPlasma derivedMicroparticle
Owner:MICROPARTICLE PROTEOMICS LLS
Aged rat brain vascular endothelial cell culture fluid
InactiveCN103045534AHigh purityPromote divisionArtificial cell constructsVertebrate cellsInsulin-like growth factorPlasma derived
The invention relates to an aged rat brain vascular endothelial cell culture fluid. On the basis of each 100 ml of DMEM (Dulbecco Modified Eagle Medium) culture medium, components including plasma derived serum (PDS), penicillin-streptomycin, L-glutamine, gentamicin, heparin, an alkaline fiber growth factor, an endothelial cell growth replenishing factor, vitamin C, a vascular endothelial growth factor, an insulin-like growth factor-1 and an endothelium growth factor are added. According to the aged rat brain vascular endothelial cell culture fluid, the PDS is adopted for replacing FBS (Fetal Bovine Serum), beef calf serum or horse serum in the traditional culture fluid to ensure that the purity of brain vascular endothelial cells is increased by 30 percent; and the vitamin C and puromycin are added to ensure that the brain vascular endothelial cells grow more rapidly and purely.
Owner:SHANDONG UNIV
Use of a chromatography substrate for reducing the amount of adamts13 in a solution derived from plasma
InactiveUS20100193440A1Reduce decreaseReduce the amount requiredFactor VIIPeptide/protein ingredientsGlycinePlasma derived
An ion-exchange chromatography support for reducing the ADAMTS13 amount present in a plasma-derived solution containing human von Willebrand factor. The support includes a large-pore, vinyl polymer-type resin bearing DEAE groups, and a buffer including trisodium citrate, sodium chloride, calcium chloride, glycine and lysine.
Owner:LFB BIOTECH
Recombinant human c1 esterase inhibitor and uses thereof
PendingUS20180334493A1Cost-effective and reliable manufacturingSafer effective treatmentPowder deliverySenses disorderDiseasePlasma derived
The present invention provides, among other things, methods and compositions for treating complement mediated disease. In some embodiments, recombinant human C1 esterase inhibitor proteins having similar or longer half-life than native plasma-derived human C1 esterase inhibitor, and methods of making the same are provided. In some embodiments, the invention provides a method for administering an effective amount of a recombinant human C1 esterase inhibitor protein to an individual who is suffering from or susceptible to a complement-mediated disease such that at least one symptom or feature of said complement-mediated disease is prevented and / or reduced in intensity, severity, or frequency.
Owner:TAKEDA PHARMA CO LTD
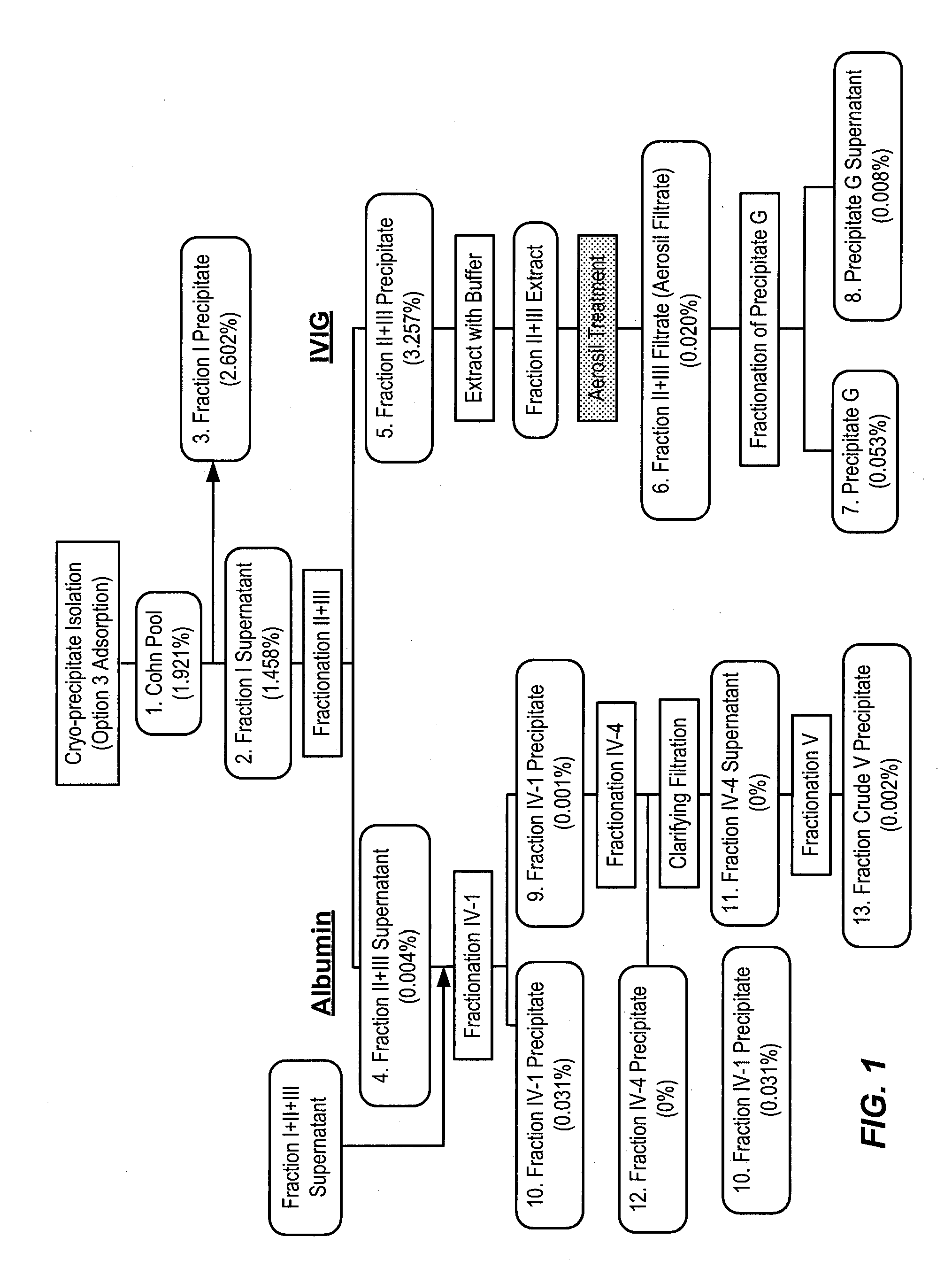
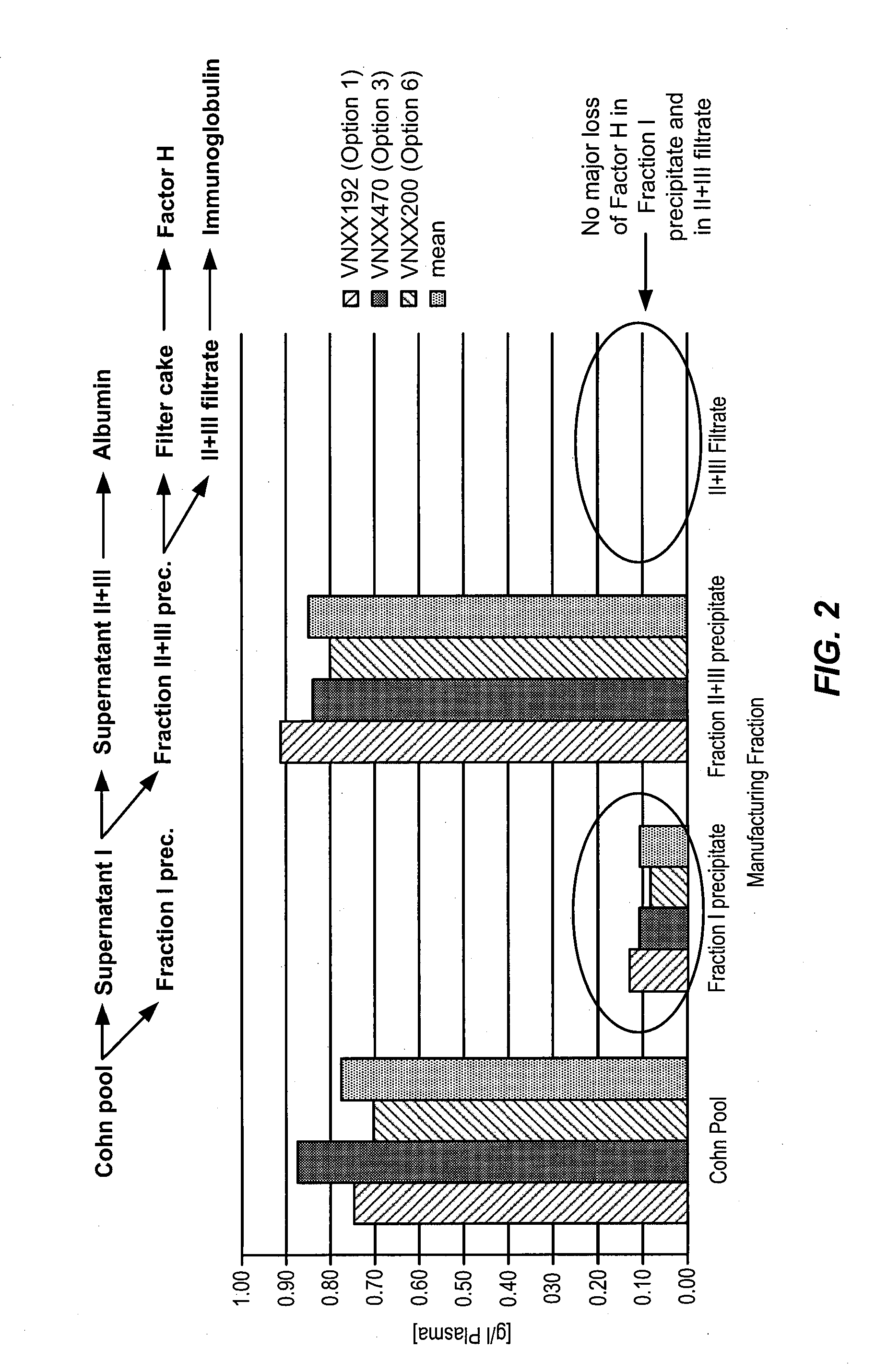
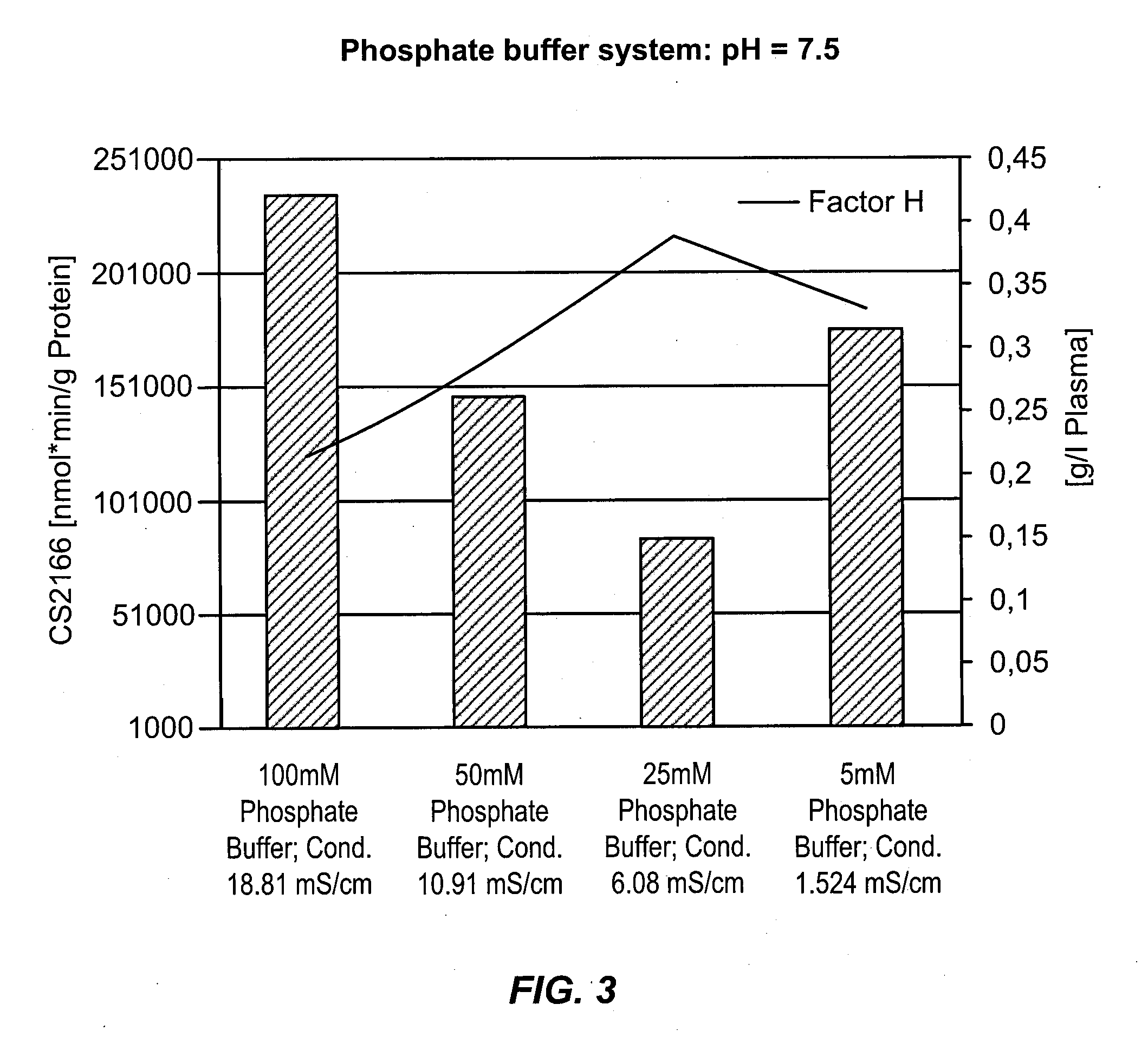
Isolation of factor h from fraction i paste
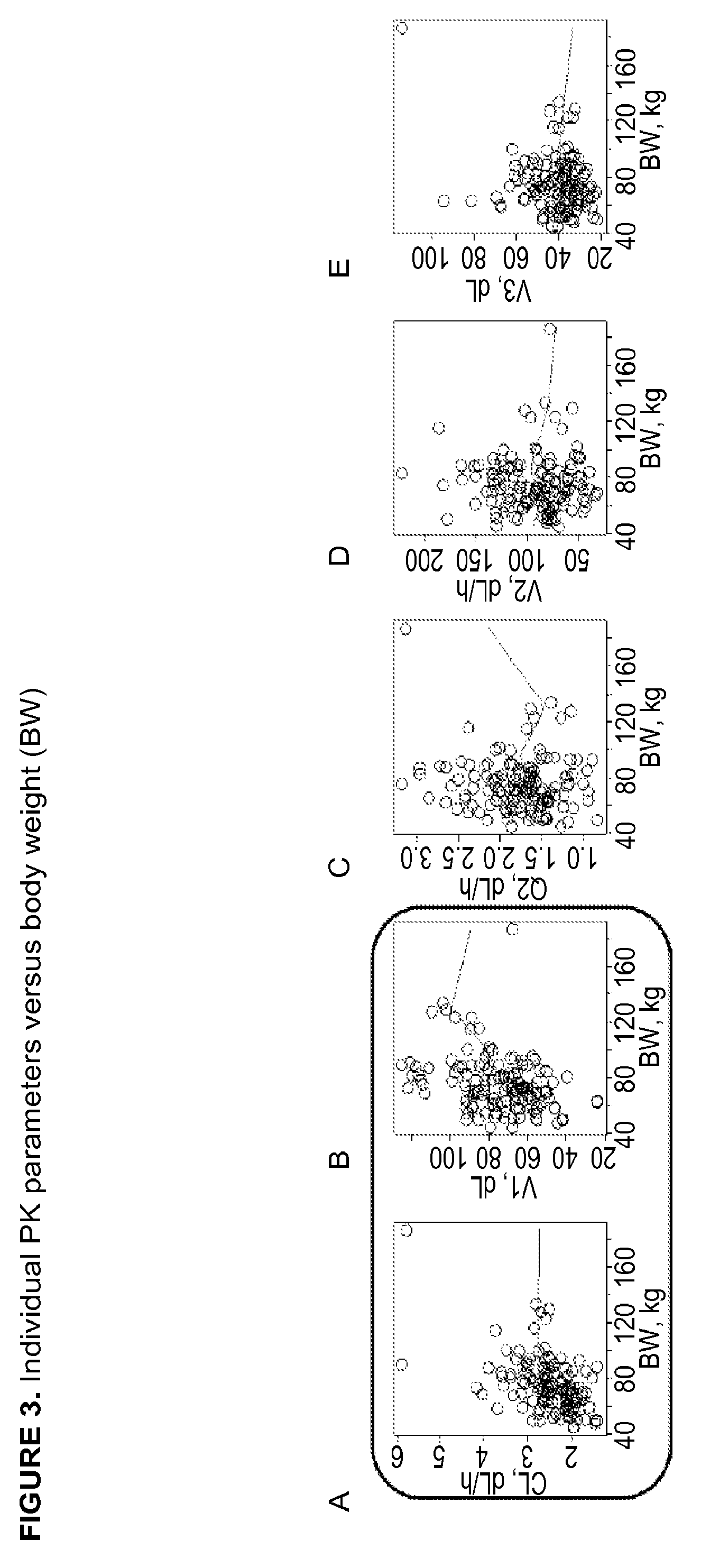
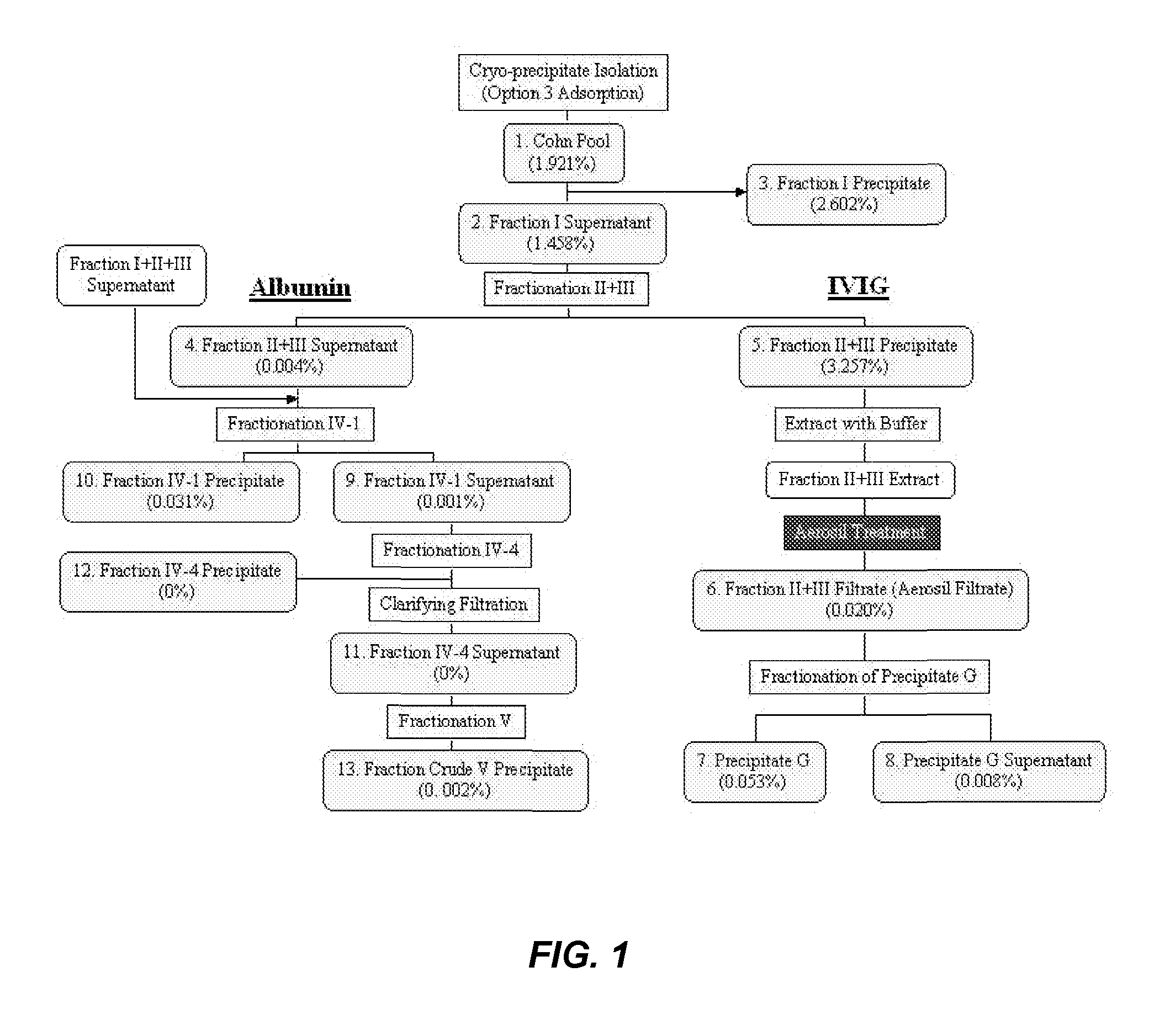
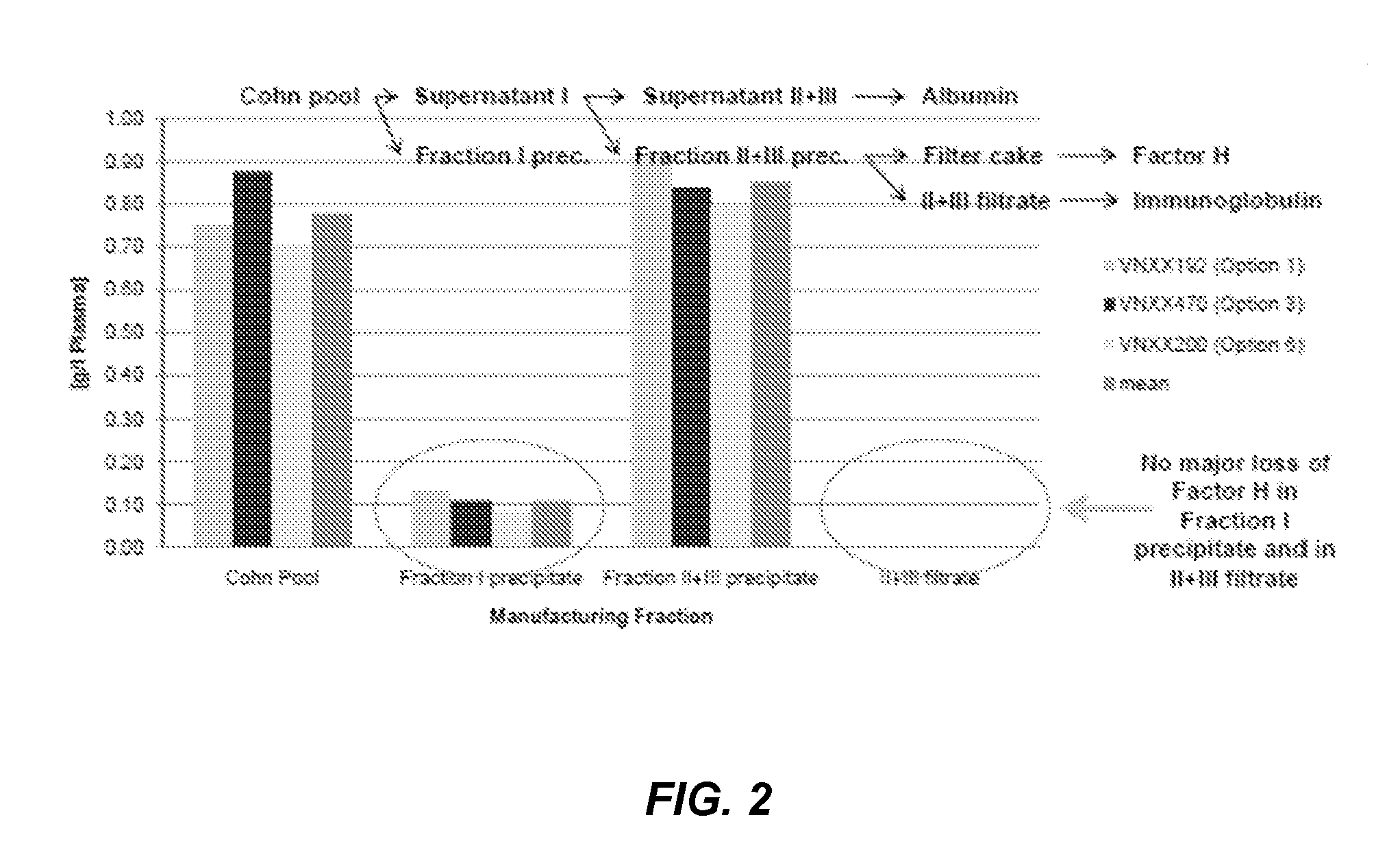
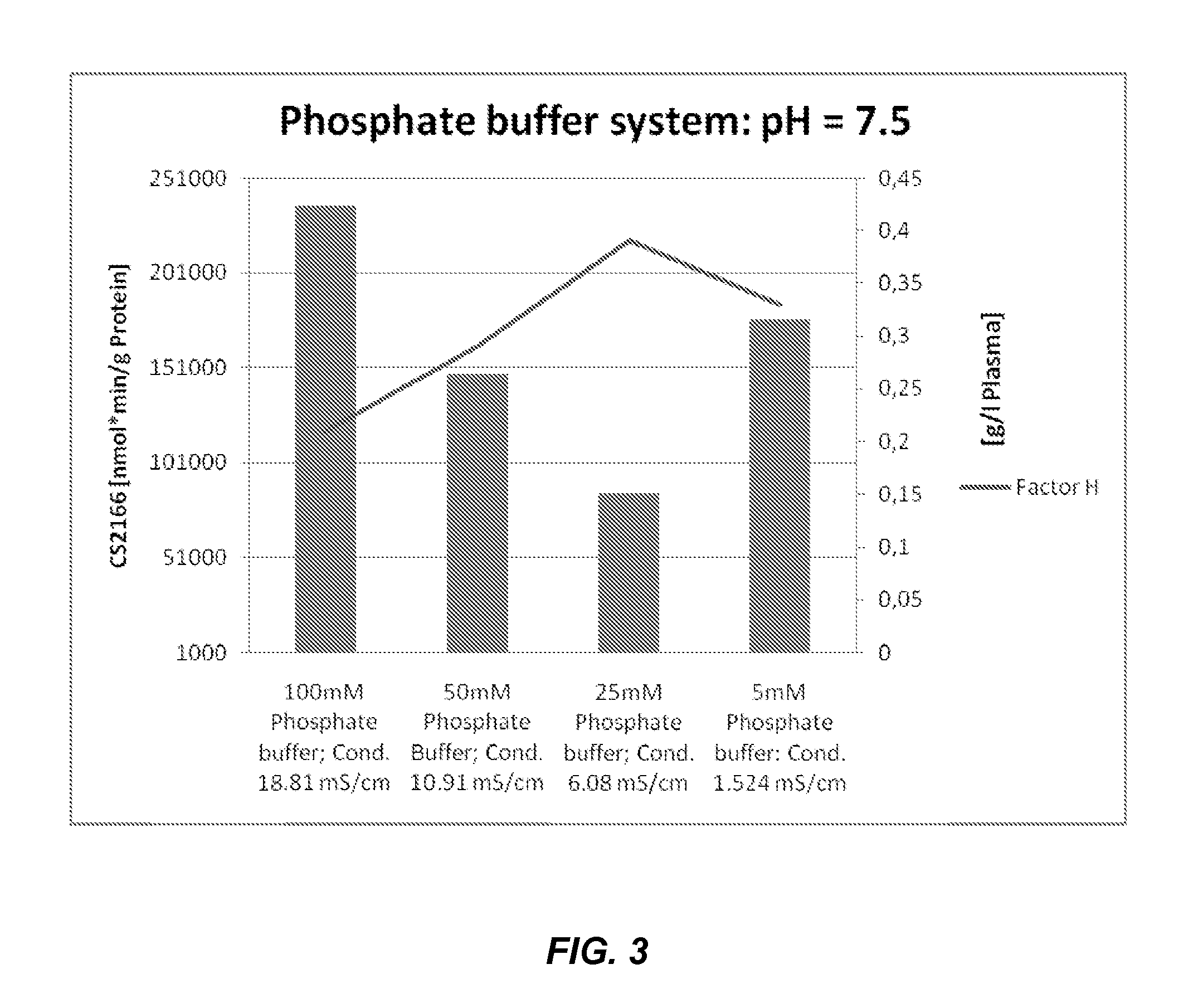
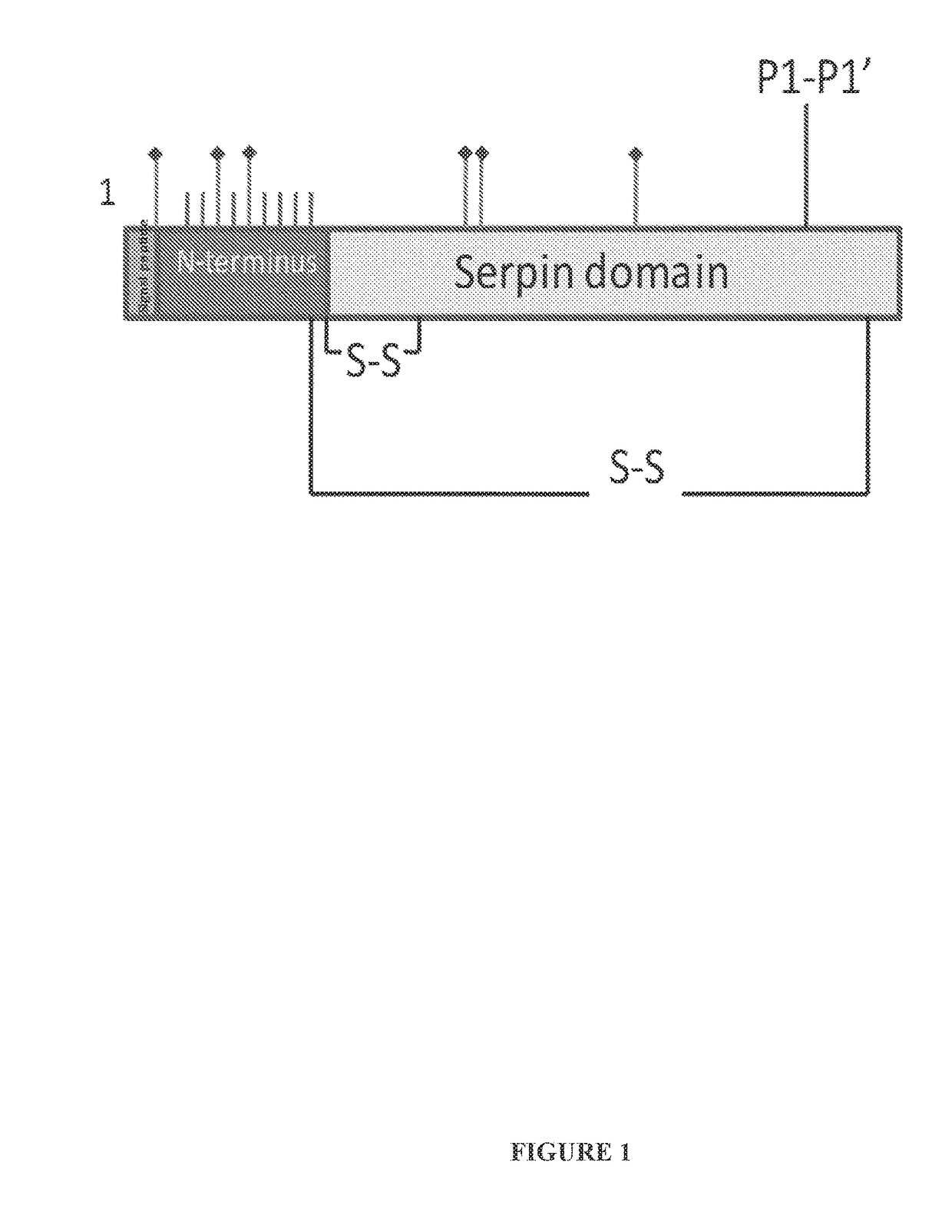
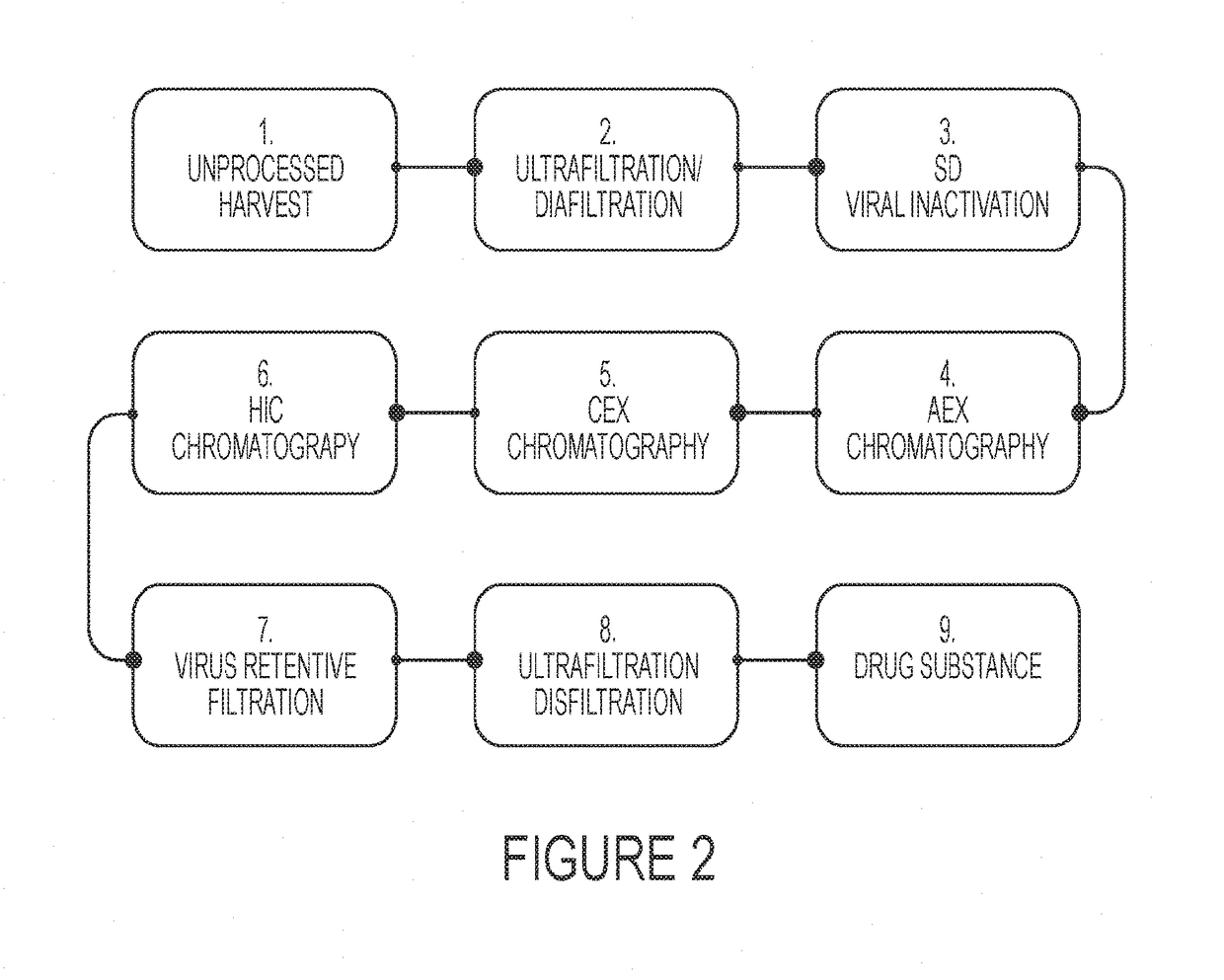
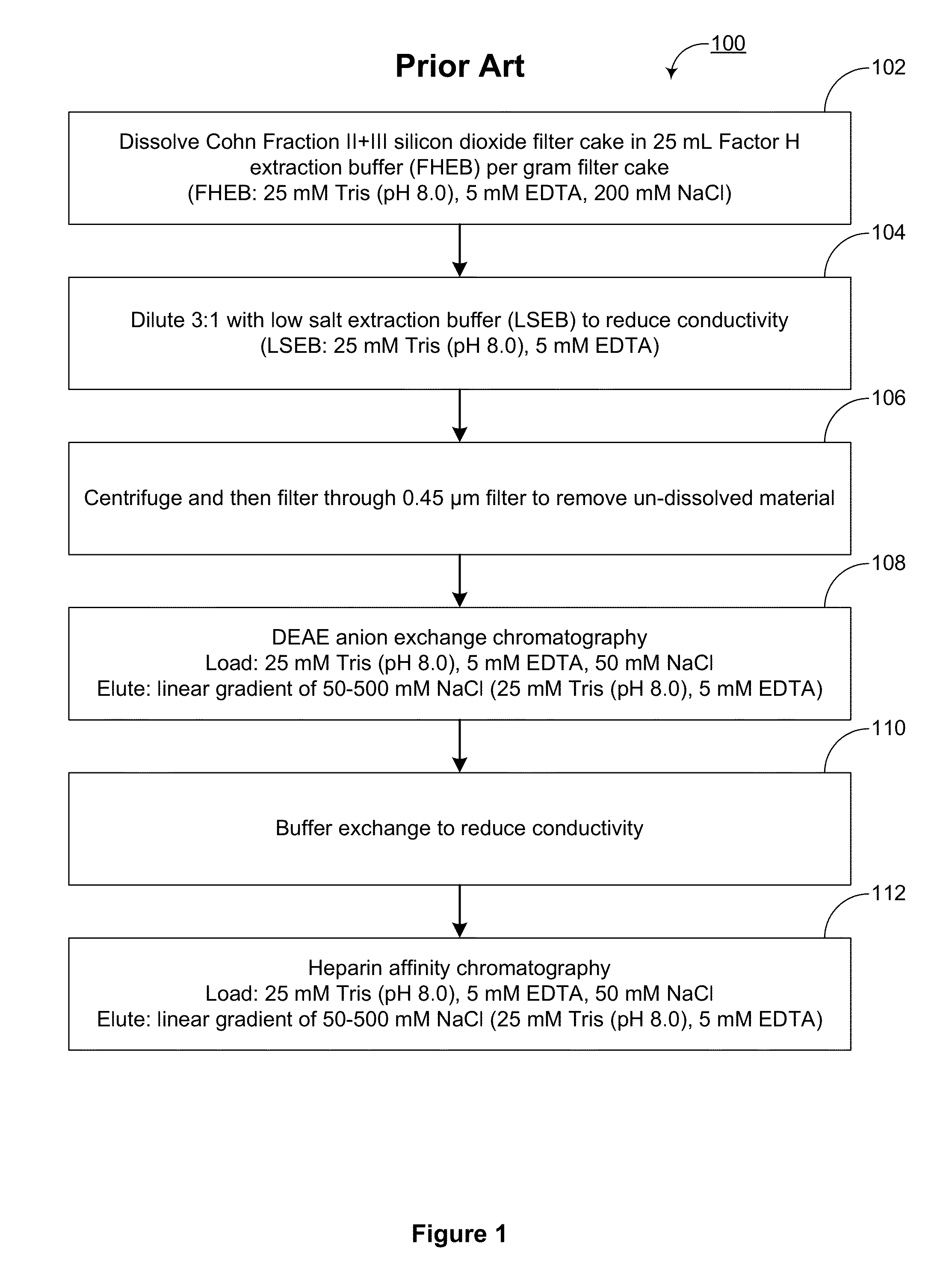
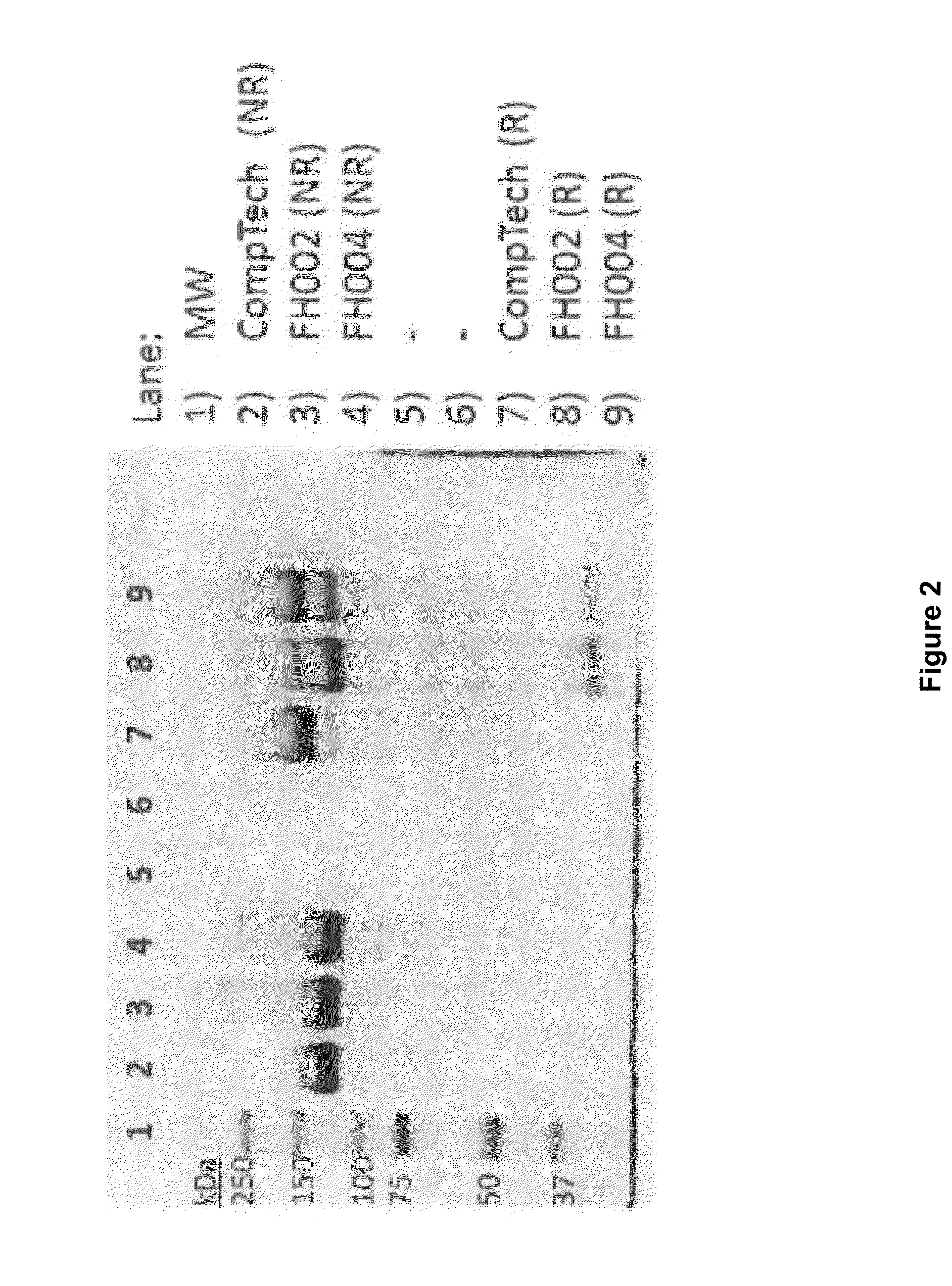
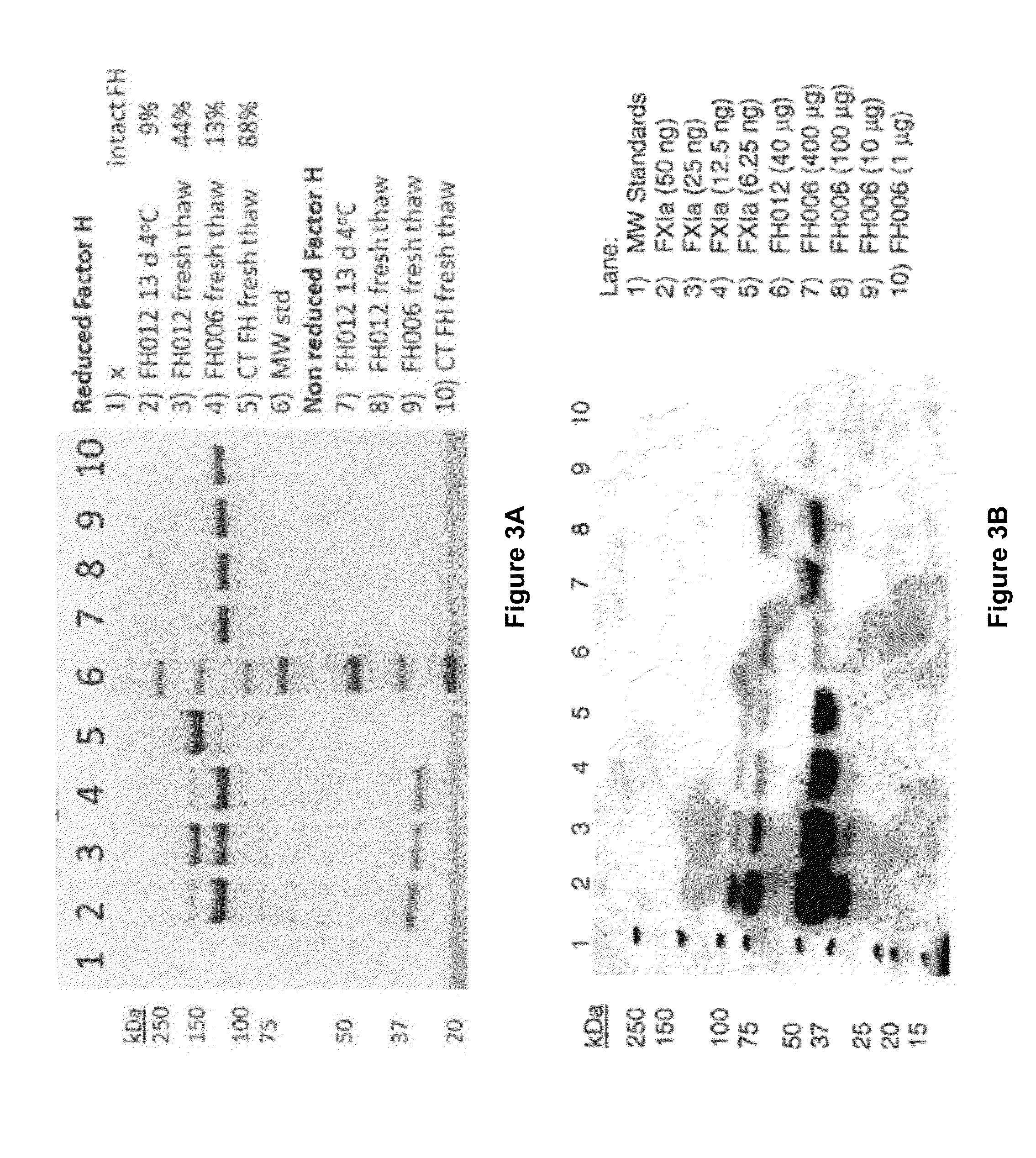
Among other aspects, the present disclosure provides methods for preparing enriched compositions of plasma-derived Factor H from fractions formed during the manufacturing processes of established plasma-derived therapeutic compositions. Specifically, methods are provided for the isolation of Factor H from Fraction precipitates commonly discarded during the manufacture of commercial IgG therapeutics. Advantageously, the Factor H compositions prepared according to these methods have improved proteolytic profiles and reduced amidolytic activity.
Owner:BAXTER HEALTHCARE SA +1
Methods to produce a human plasma-derived igg preparation enriched in brain disease-related natural iggs
The present invention provides, among other aspects, methods for the manufacture of plasma-derived immunoglobulin G compositions highly enriched for anti-brain disease related protein antibodies (e.g., anti-β, anti-RAGE, and anti-α-synuclein antibodies). Advantageously, the methods provided do not affect the manufacturing processes or capabilities for producing plasma-derived IgG therapeutics. Plasma-derived IgG compositions that are highly enriched for anti-brain disease related protein antibodies (e.g., anti-β, anti-RAGE, and anti-α-synuclein antibodies), as also provided here. Methods for the treatment of brain diseases and disorders by administration of plasma-derived IgG compositions highly enriched for anti-brain disease related protein antibodies (e.g., anti-β, anti-RAGE, and anti-α-synuclein antibodies), are also provided.
Owner:TAKEDA PHARMA CO LTD
Antibody composition for prevention or treatment of mutant hepatitis b virus infection
ActiveUS20150166637A1Prevent and treat diseasePeptide/protein ingredientsDigestive systemPlasma derivedBinding site
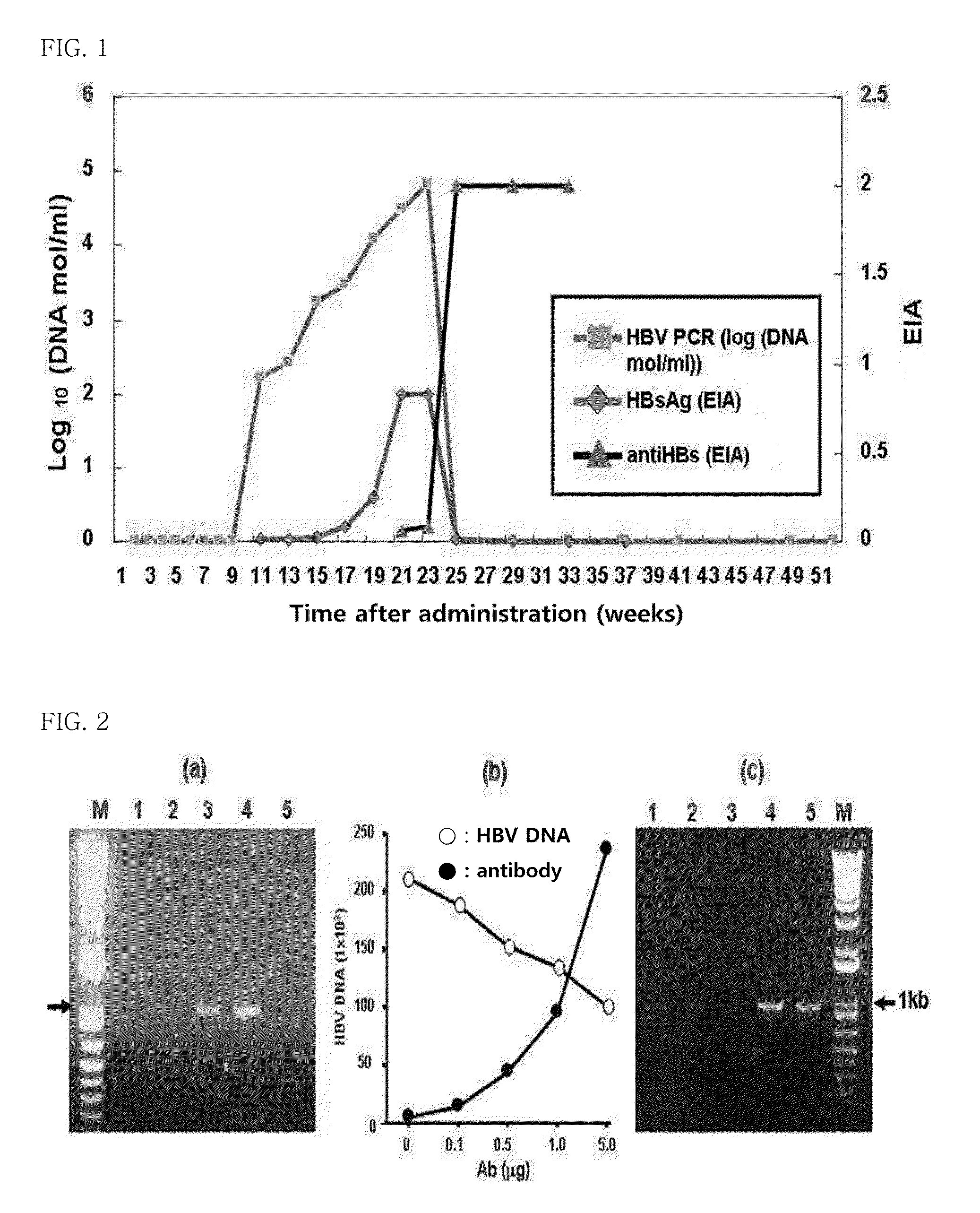
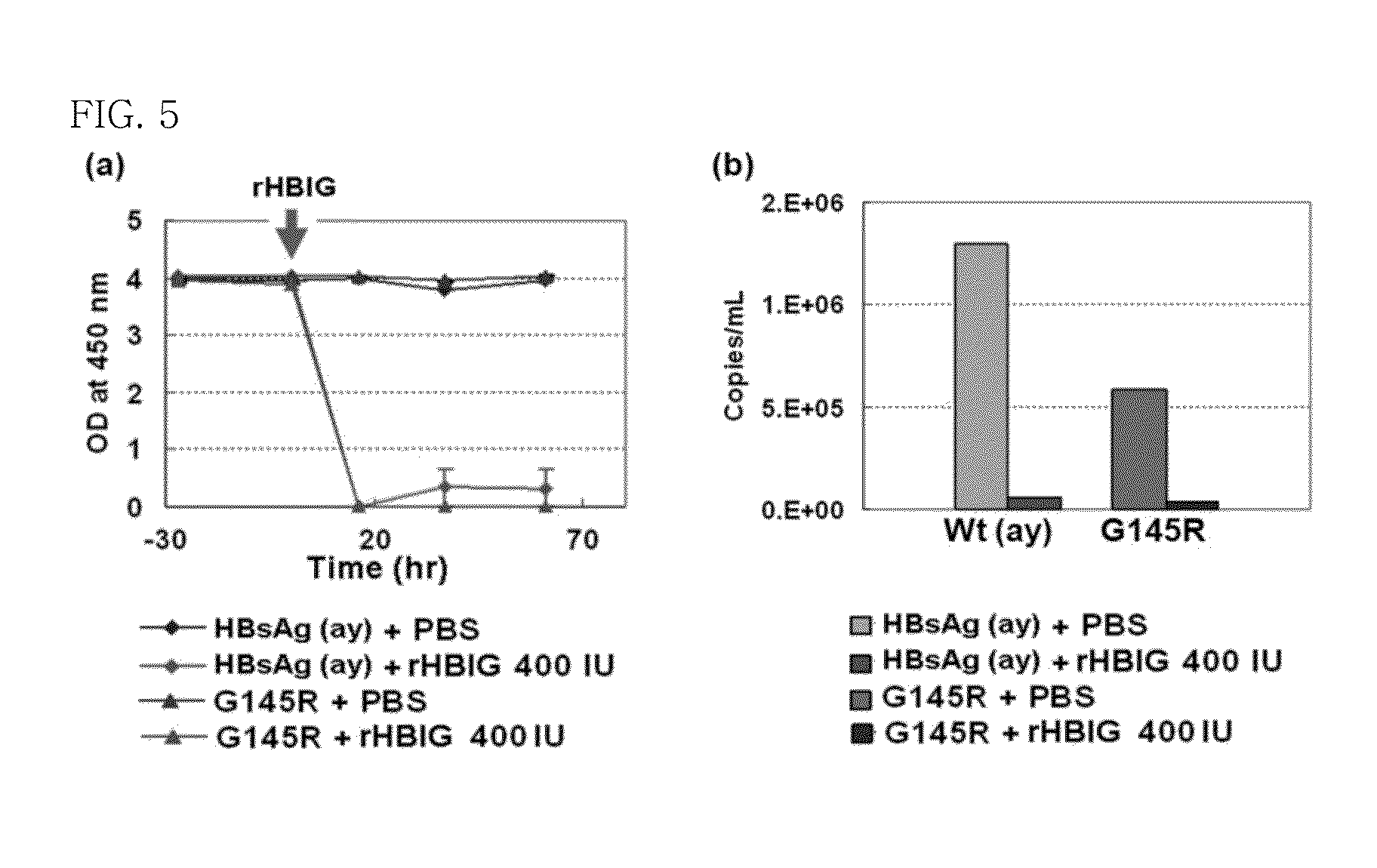
The present invention provides an antibody that binds to the surface antigen (HBsAg) of hepatitis B virus (HBV) to neutralize the hepatitis B virus. The surface antigen-binding site of the antibody was found to play a very important role in viral replication, and when a mutation in the site occurs, viral replication is significantly inhibited, and thus at least HBV virus cannot cause a mutation in the site.In the present invention, it was confirmed by the use of patient-derived virus that the antibody of the present invention binds to either YMDD mutant hepatitis B virus, produced by conventional viral replication inhibitors, or G145R HBsAg mutants to which plasma-derived HBIG (hepatitis B immunoglobulin) does not bind.In addition, the in vivo effect of the antibody of the present invention was examined using chimpanzees which are unique animal models for hepatitis B virus. As a result, it was found that the antibody has the effect of neutralizing even wild-type hepatitis B virus in the in vivo model. Thus, it can be seen that the antibody of the present invention has the ability to bind not only to wild-type hepatitis B virus, but also mutant hepatitis B viruses having a polymerase YMDD mutant and a surface antigen G145R mutation, as well as various mutant viruses derived from patients.Thus, the antibody of the present invention can be effectively used for the prevention or treatment of infections with not only wild-type hepatitis B virus, but also mutant hepatitis B viruses.
Owner:THE GREEN CROSS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com