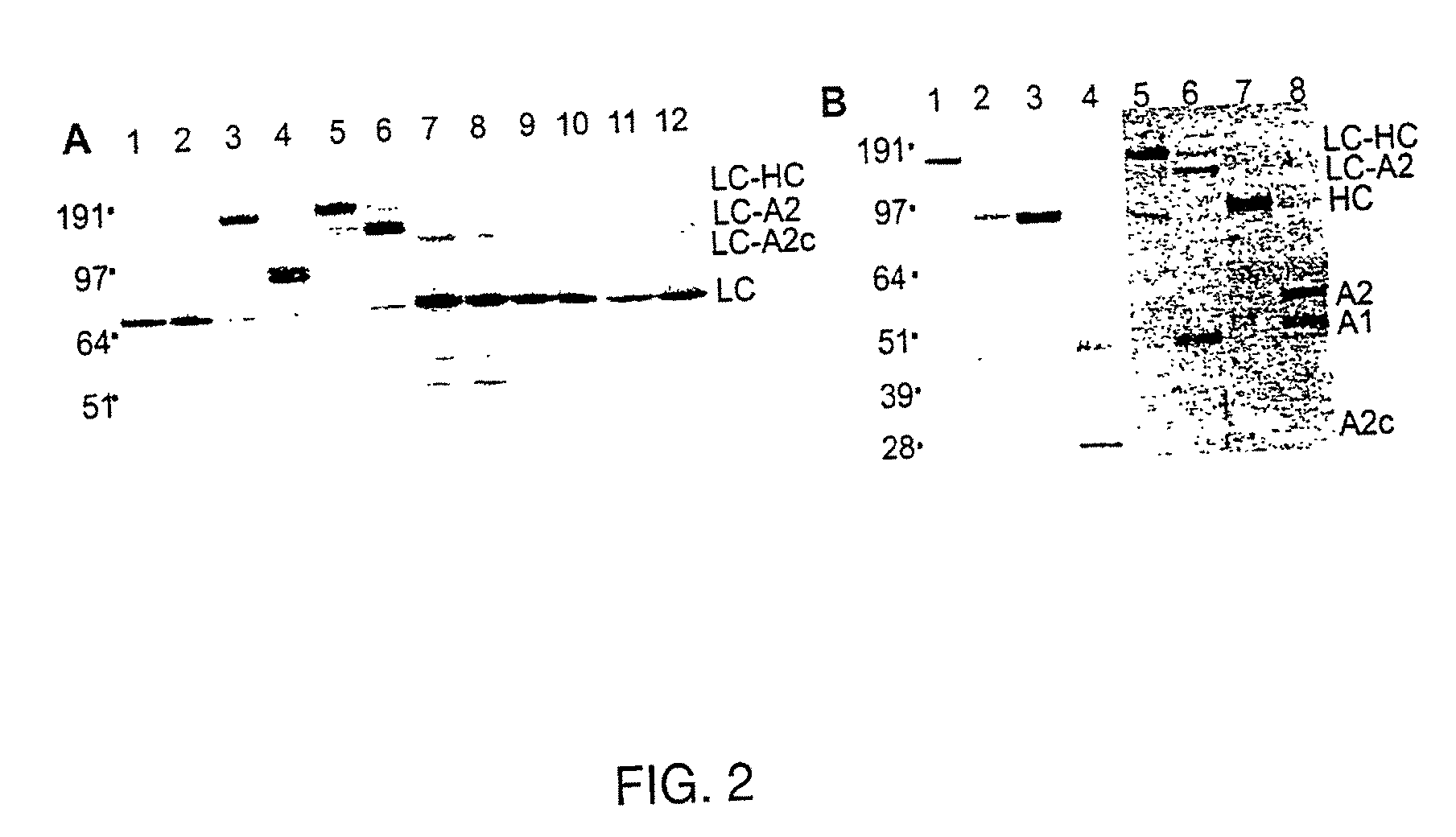
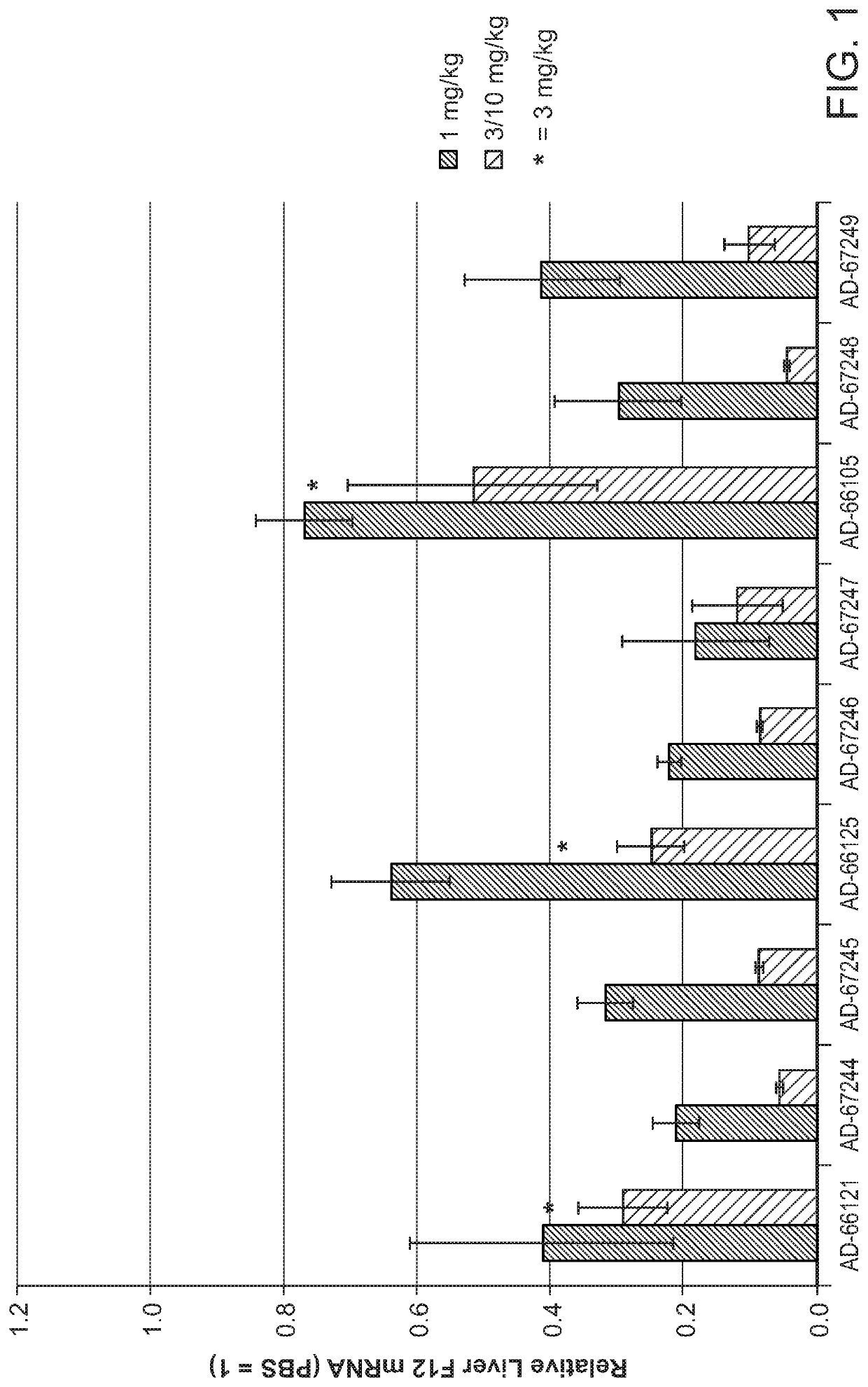
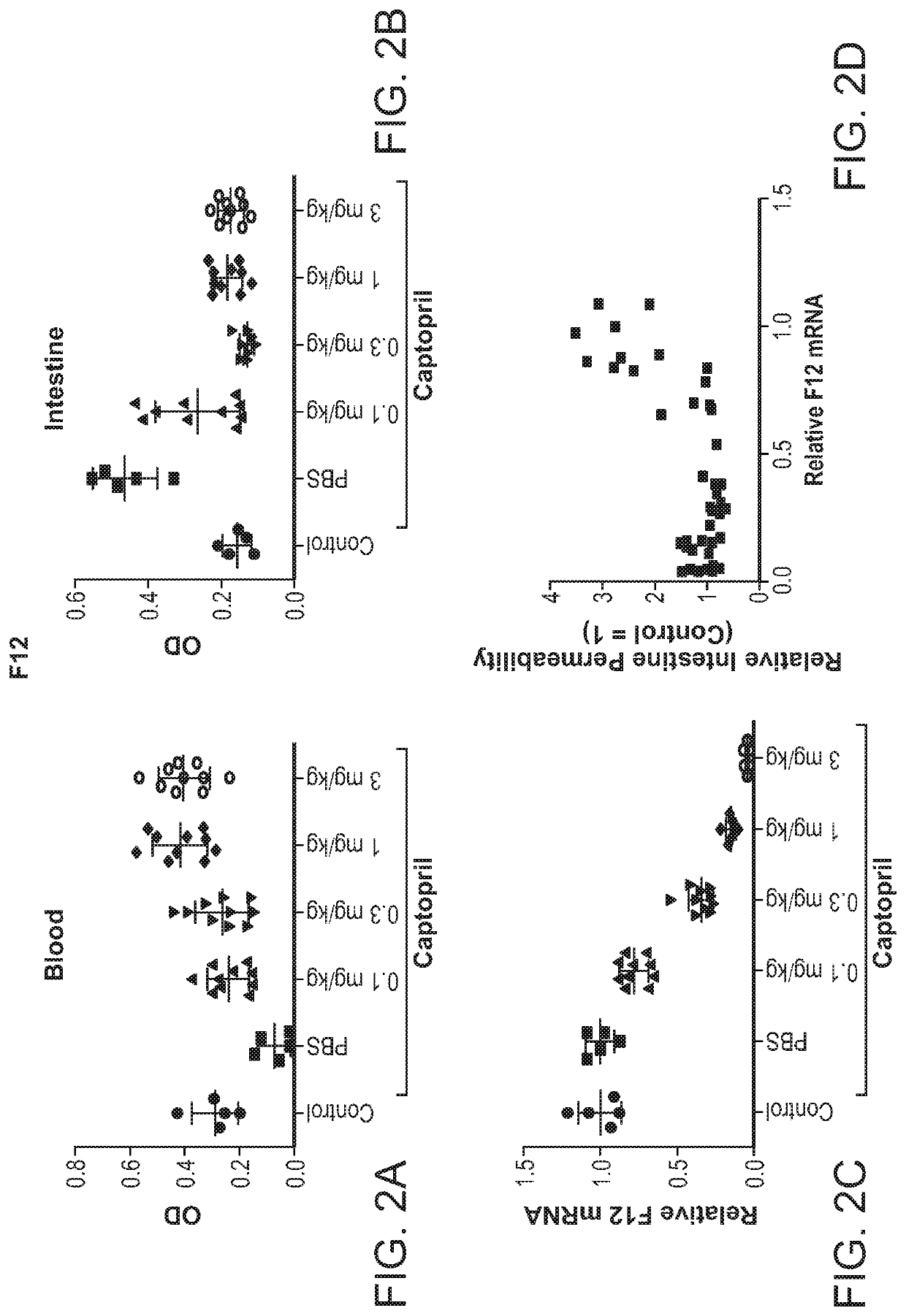
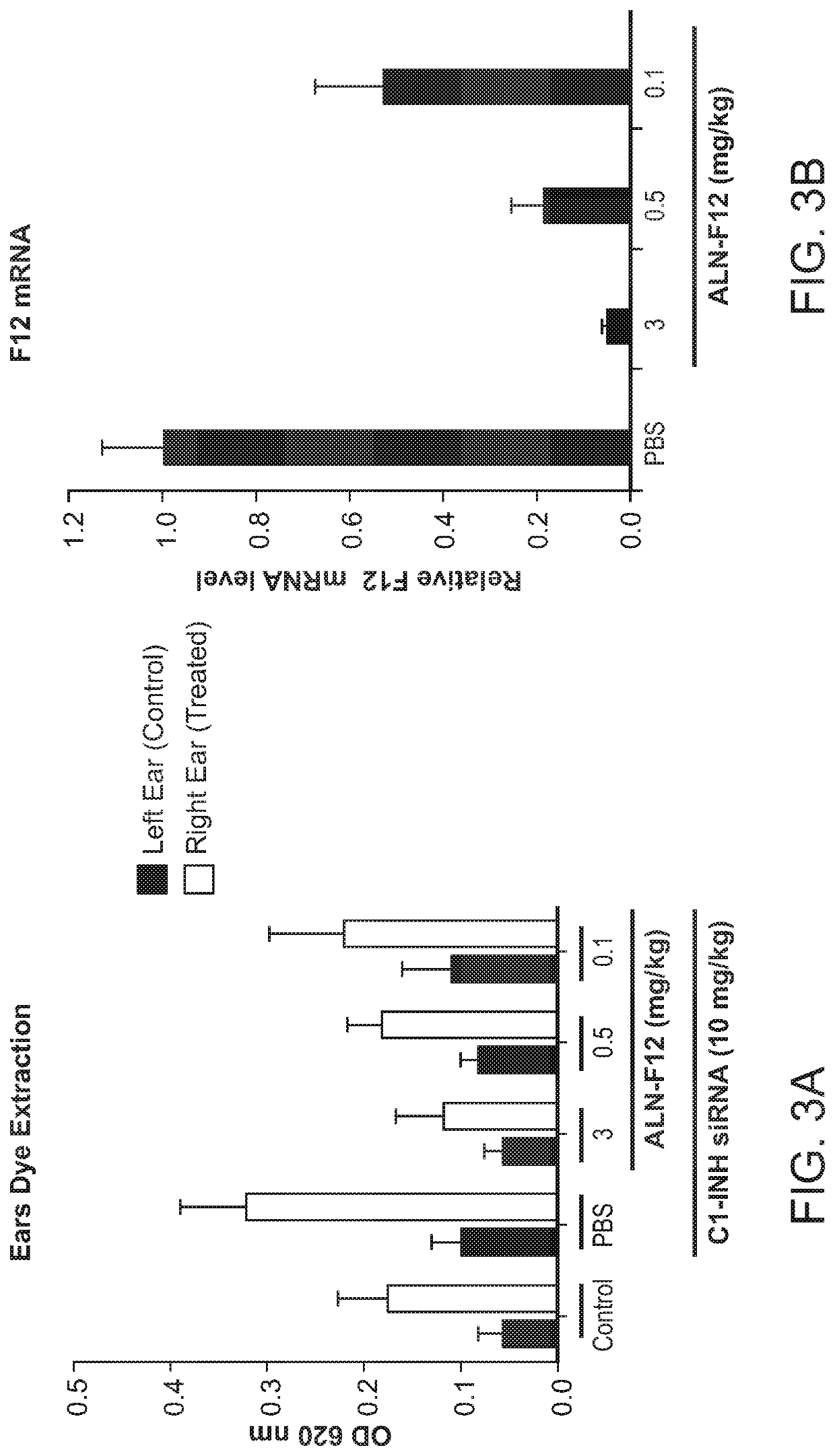
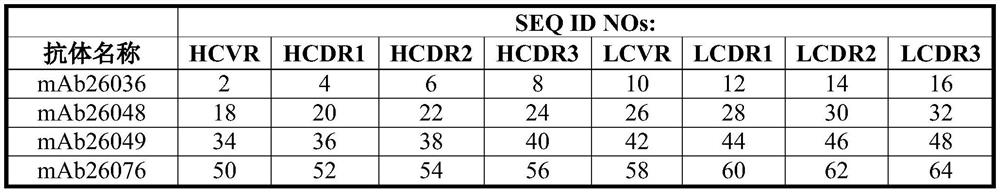
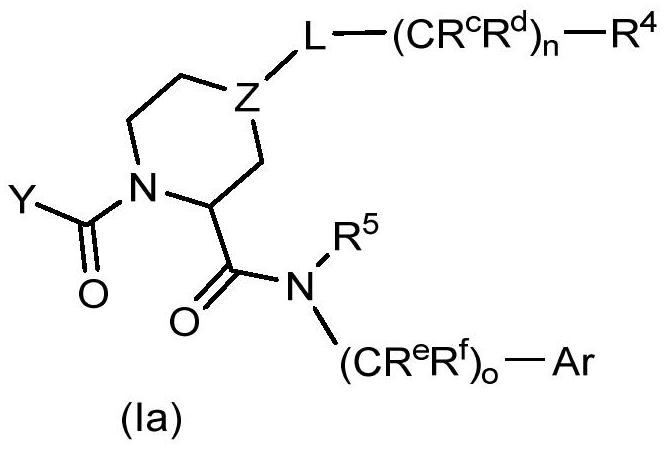
Patents
Literature
34 results about "Factor XII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
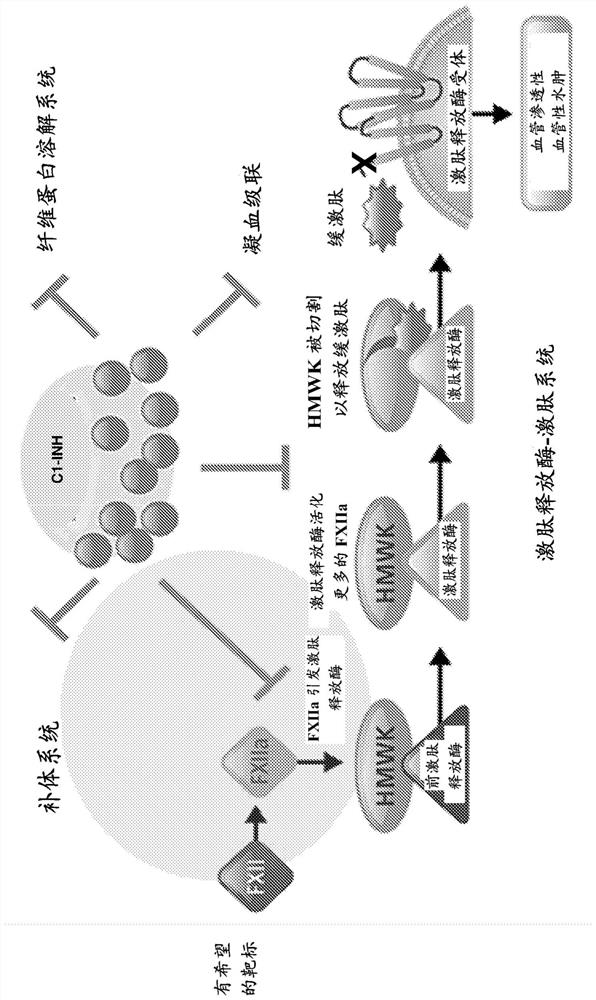
Coagulation factor XII, also known as Hageman factor, is a plasma protein. It is the zymogen form of factor XIIa, an enzyme (EC 3.4.21.38) of the serine protease (or serine endopeptidase) class. In humans, factor XII is encoded by the F12 gene.
Hemostatic compositions, devices and methods
InactiveUS7094428B2Lower Level RequirementsReduce concentrationPowder deliveryFactor VIIFactor VIIaBiopolymer
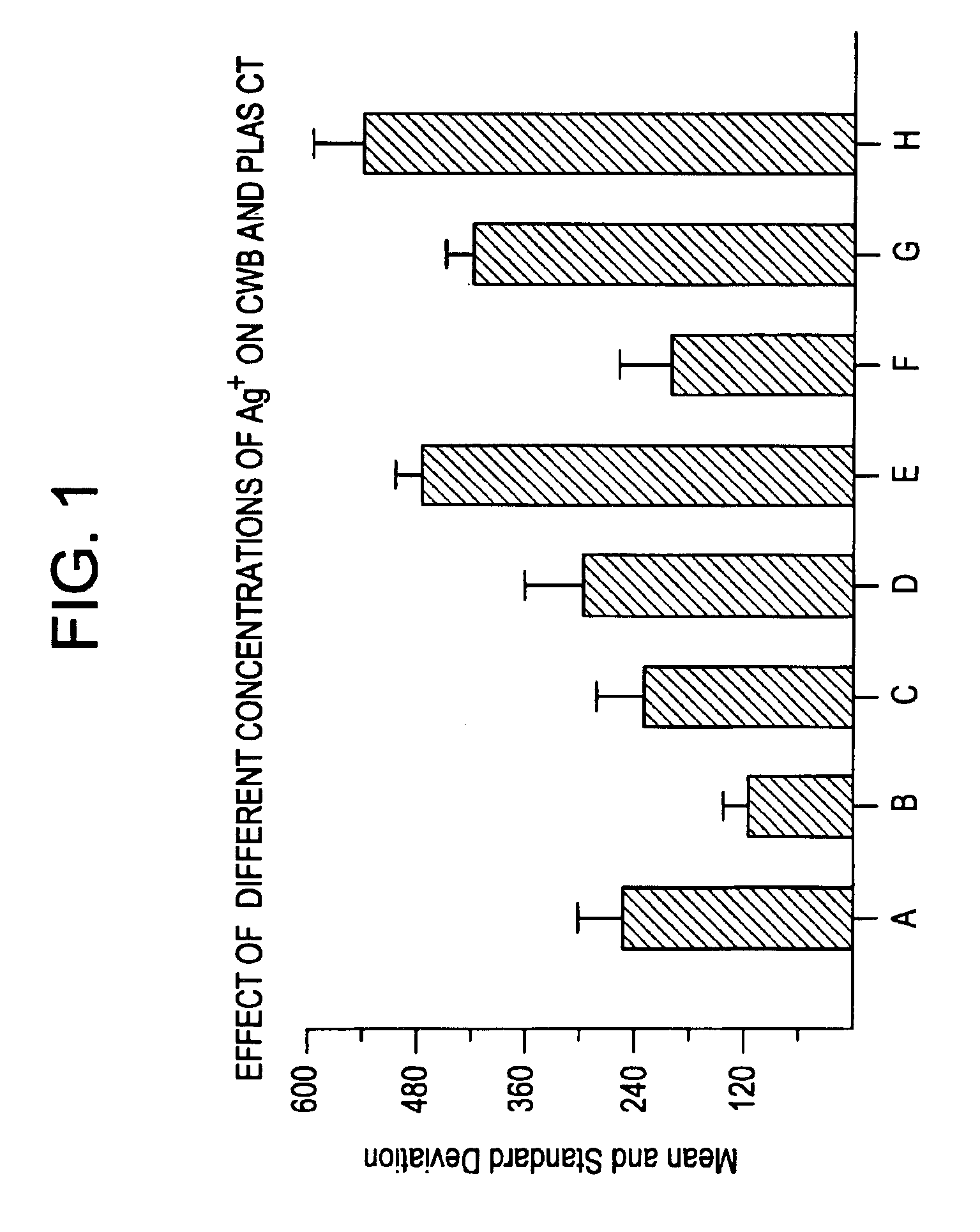
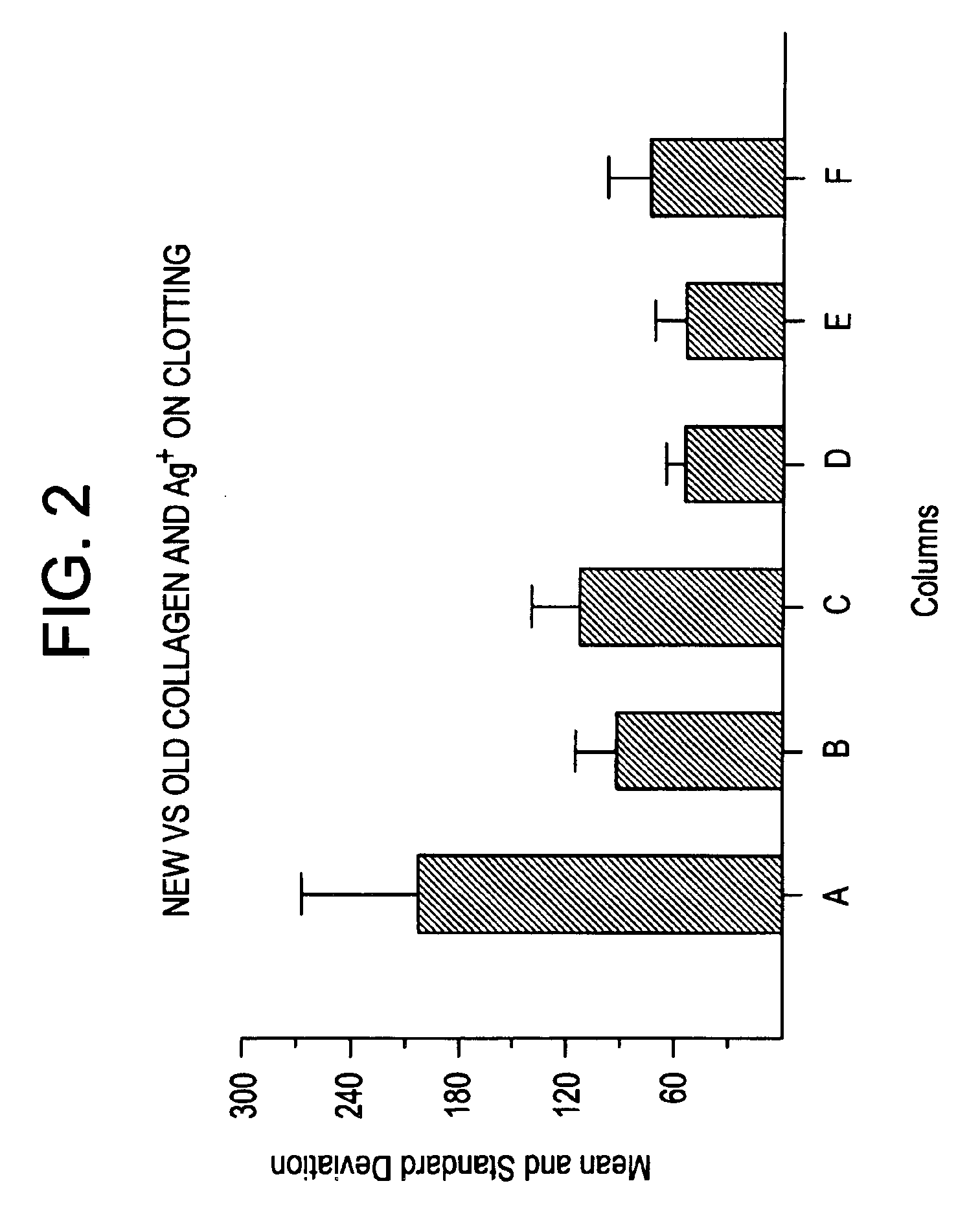
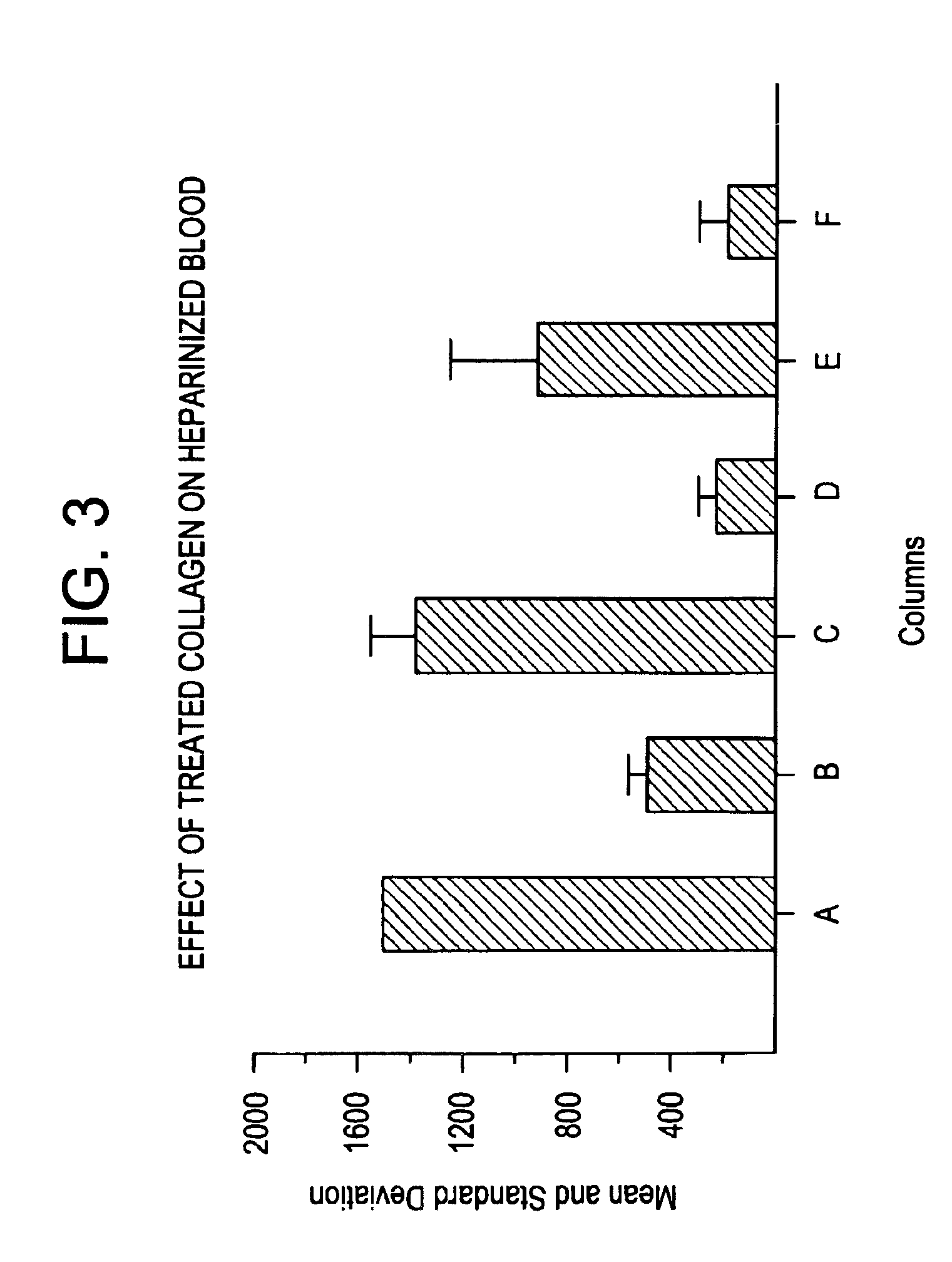
A hemostatic composition which comprises at least one procoagulant metal ion, such as silver (I) or mercury (II), and at least one procoagulant biopolymer, such as collagen, thrombin, prothrombin, fibrin, fibrinogen, heparinase, Factor VIIa, Factor VIII, Factor IXa, Factor Xa, Factor XII, von Willebrand Factor, a selectin, a procoagulant venom, a plasminogen activator inhibitor, glycoprotein IIb-IIIa, a protease, or plasma. The composition in the form of a paste, dough, glue, liquid, lyophilized powder or foam, may be provided, for application to a wound. A hemostatic device is also described which comprises a hemostatic composition as described above. The device may be in the form of, for example, a plug, bandage, gauze, cloth, tampon, membrane or sponge. Methods are also provided for prophylaxis or treatment of bleeding at a site by application to the site of the composition or device as described.
Owner:RUTGERS THE STATE UNIV
Compositions and methods for inhibiting gene expression of factor XII
ActiveCN108064313AOrganic active ingredientsMicrobiological testing/measurementDiseaseHereditary angioedema
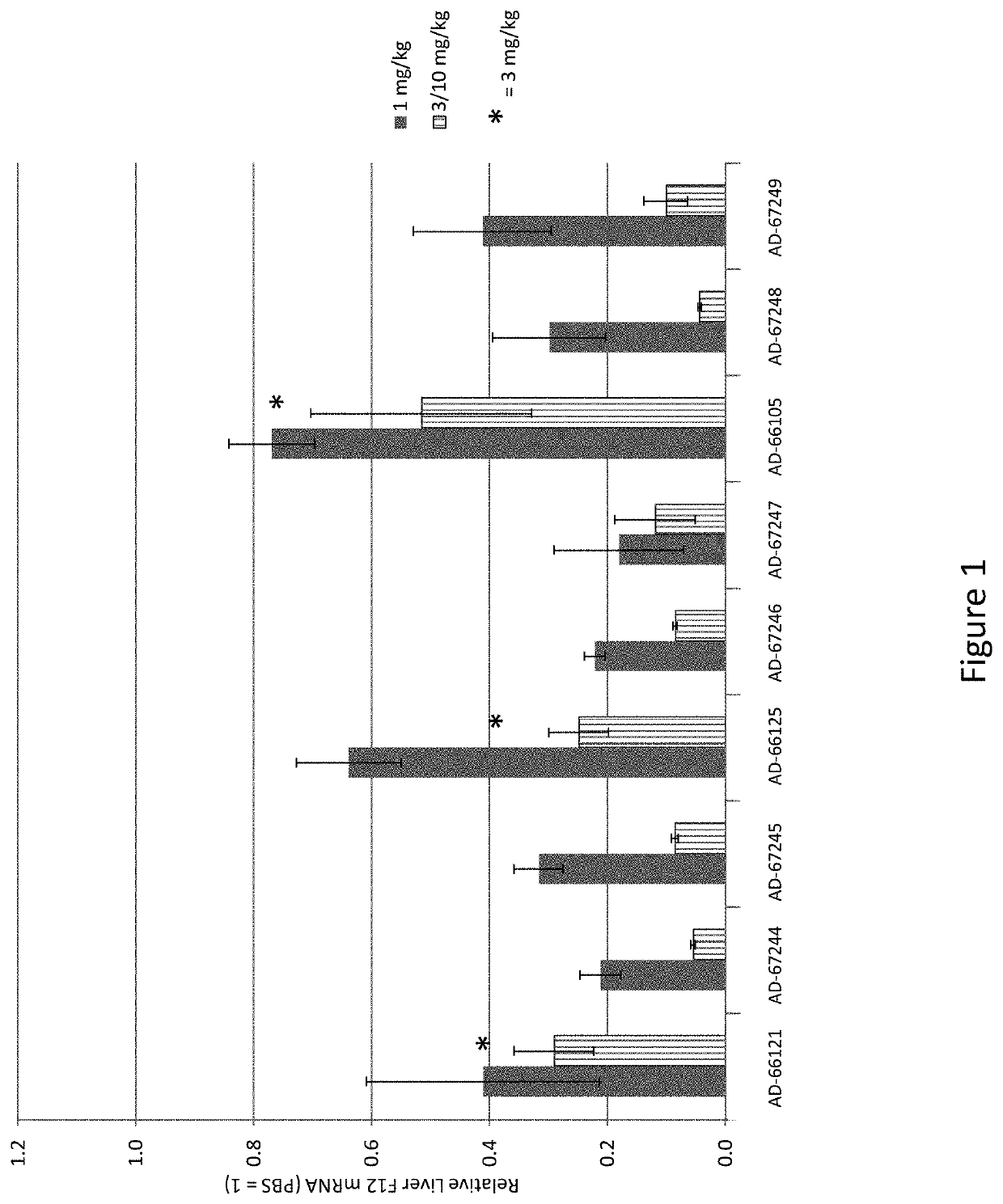
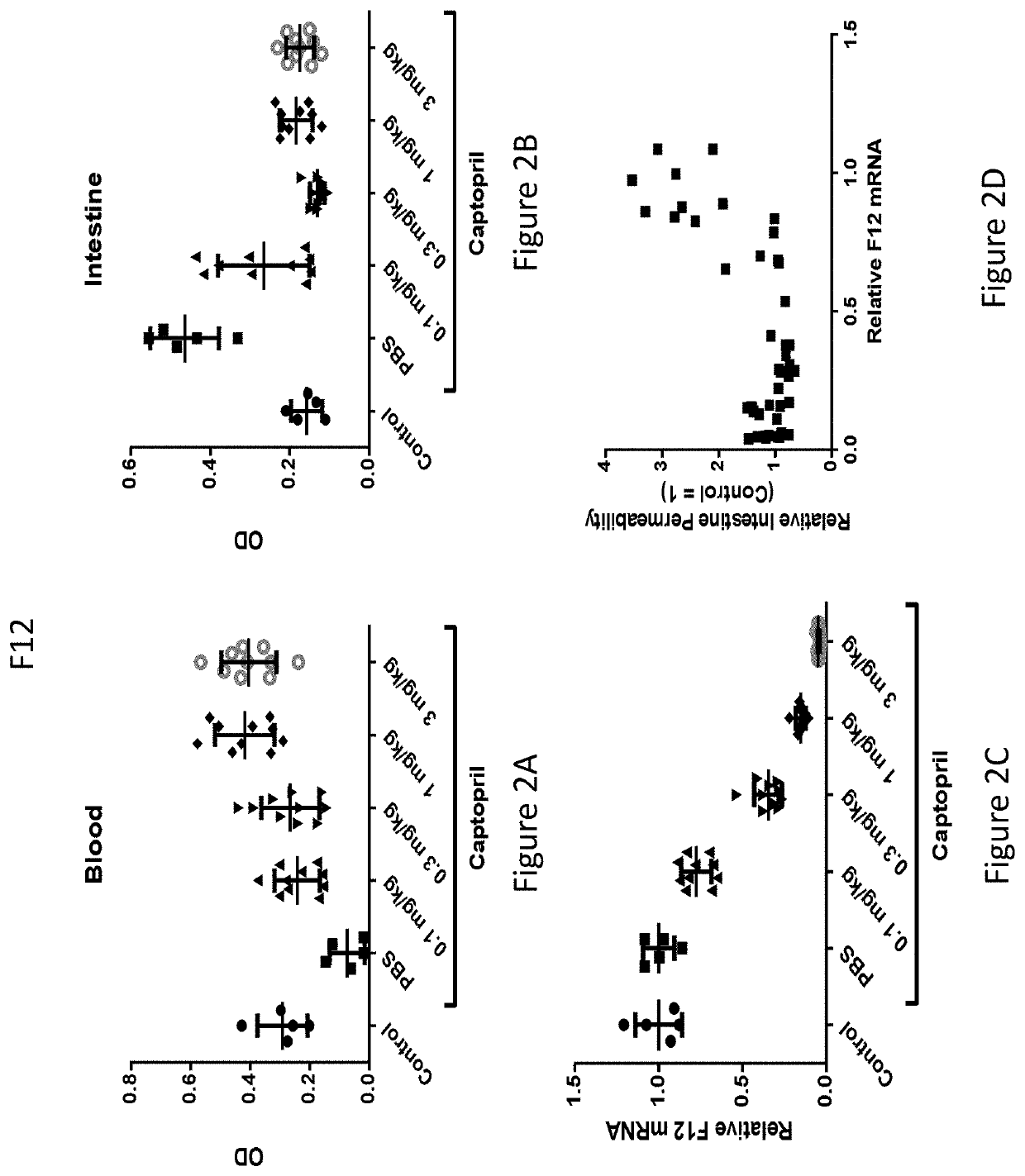
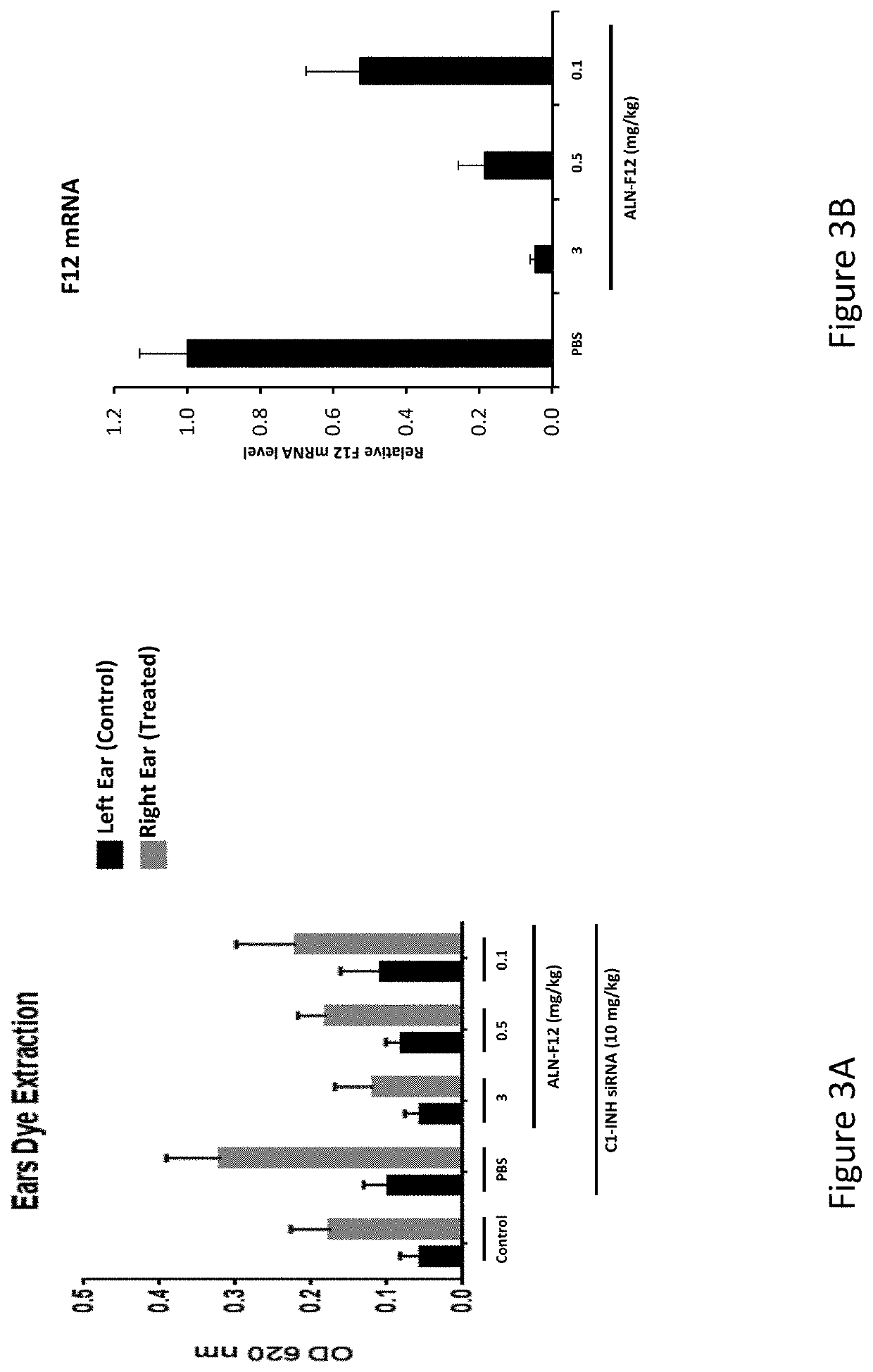
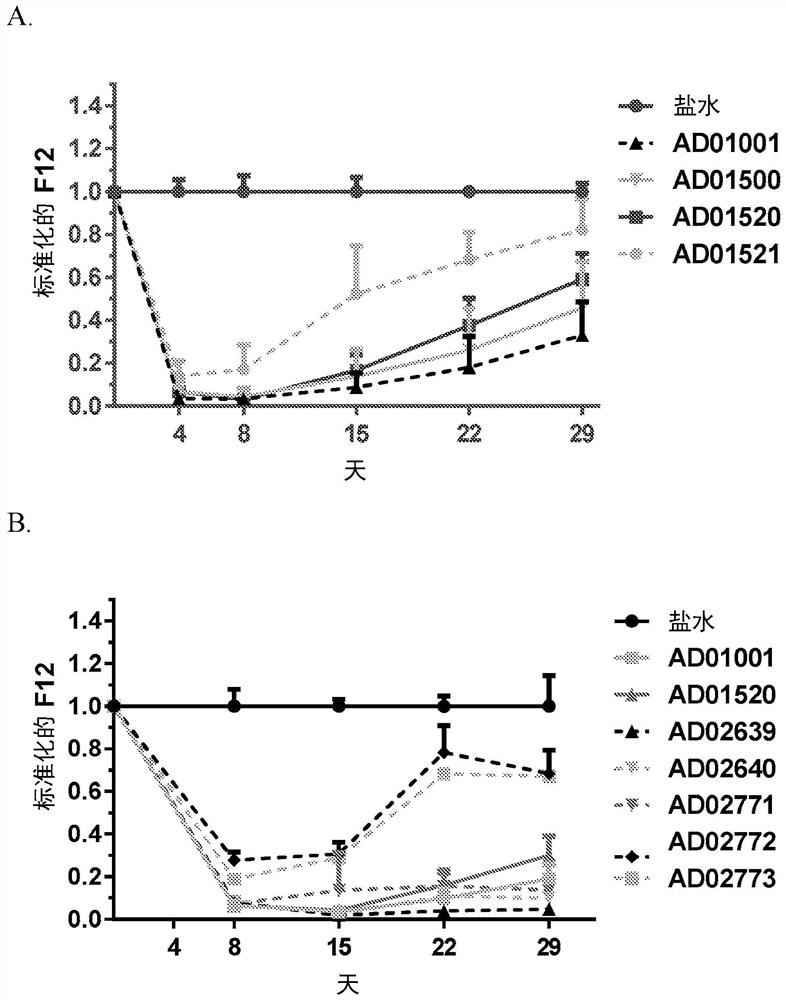
RNA interference (RNAi) triggers for inhibiting the expression of Factor XII (F12) gene through the mechanism of RNA interference are described. Pharmaceutical compositions comprising one or more F12RNAi triggers together with one or more excipients capable of delivering the RNAi trigger(s) to a liver cell in vivo are also described. Delivery of the F12 RNAi trigger(s) to liver cells in vivo provides for inhibition of F12 gene expression and treatment of angioedema, including hereditary angioedema (HAE) and venous thromboembolism (VTE), and diseases associated with angioedema.
Owner:ARROWHEAD RES CORP
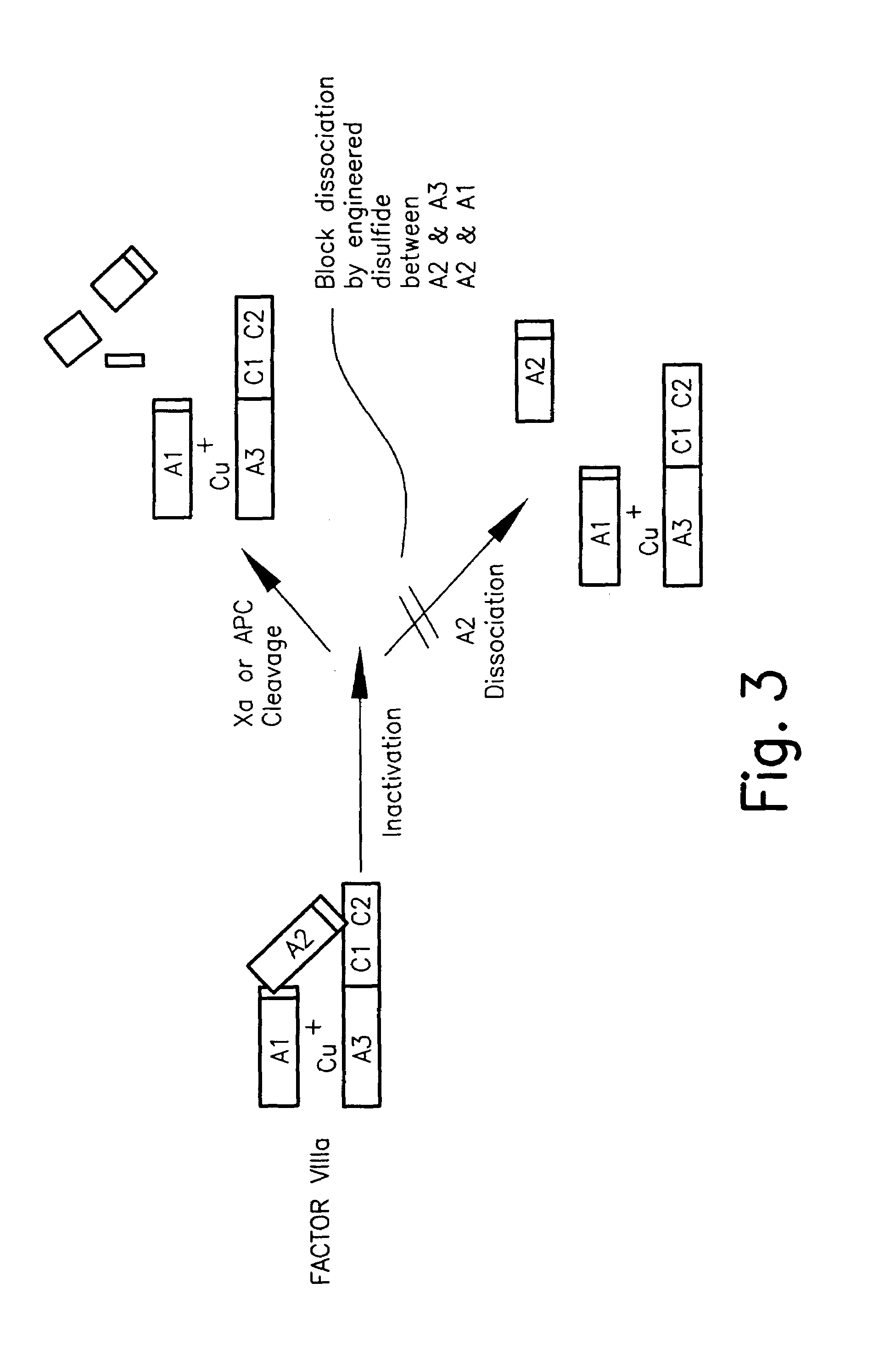
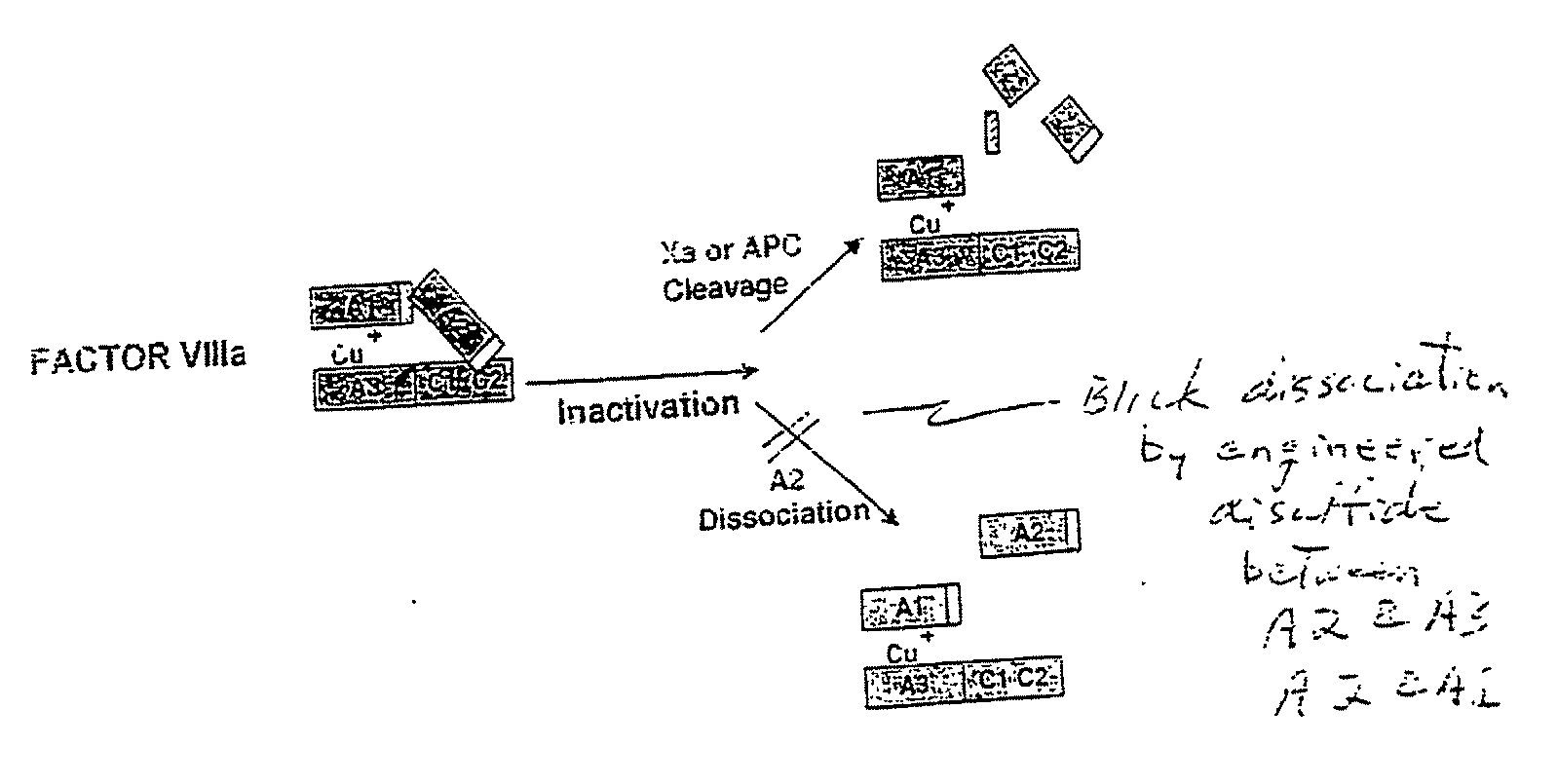
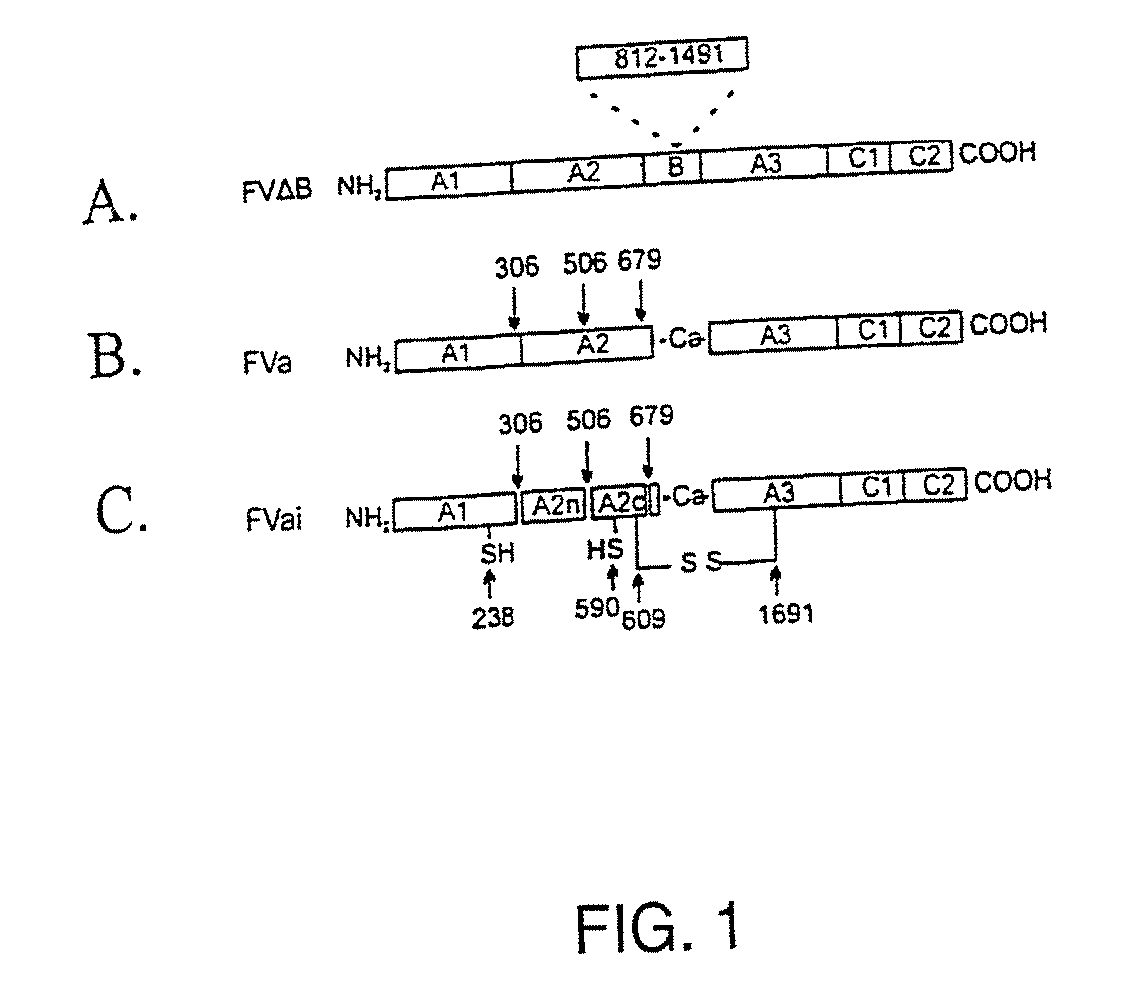
Stabilized proteins with engineered disulfide bonds
InactiveUS7205278B2High retention rateAvoidance of undesired activityOrganic active ingredientsFungiADAMTS ProteinsBlood plasma
The present invention relates to methods of introducing one or more cysteine residues into a polypeptide which permit the stabilization of the polypeptide by formation of at least one bond, preferably a disulfide bond, between different domains of the polypeptide. The invention also relates to polypeptides containing such introduced cysteine residue(s), nucleic acids encoding such polypeptides and pharmaceutical compositions comprising such polypeptides or nucleic acids. The invention also relates to vectors, viral particles and host cells containing such nucleic acids, and methods of using them to produce the polypeptides of the invention. Exemplified polypeptides include plasma proteins, including hepatocyte growth factor activator and plasma hyaluronin binding protein, as well as blood coagulation factors, such as Factor VIII, Factor V, Factor XII and prothrombin.
Owner:THE SCRIPPS RES INST
Target spot for treating septicemia
InactiveCN102600471AImprove living conditionsLow side reaction rateAntibacterial agentsGenetic material ingredientsAspirinSide effect
The invention discloses a target spot for treating septicemia, belonging to the technical field of treatment of septicemia. The target spot is Factor XI or Factor XII, and can block a Factor XI or Factor XII expression in a septicemia patient body so as to treat septicemia. According to the invention, an antibody-14E11for blocking the Factor XI expression is a novel medicine for treating septicemia after the APC (aspirin compound tablet), and due to the special position of Factor XI on a clotting cascade chain, the occurrence rate of side effects should be obviously lower than that of the APC.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Inhibitory Anti-factor xii/xiia monoclonal antibodies and their uses
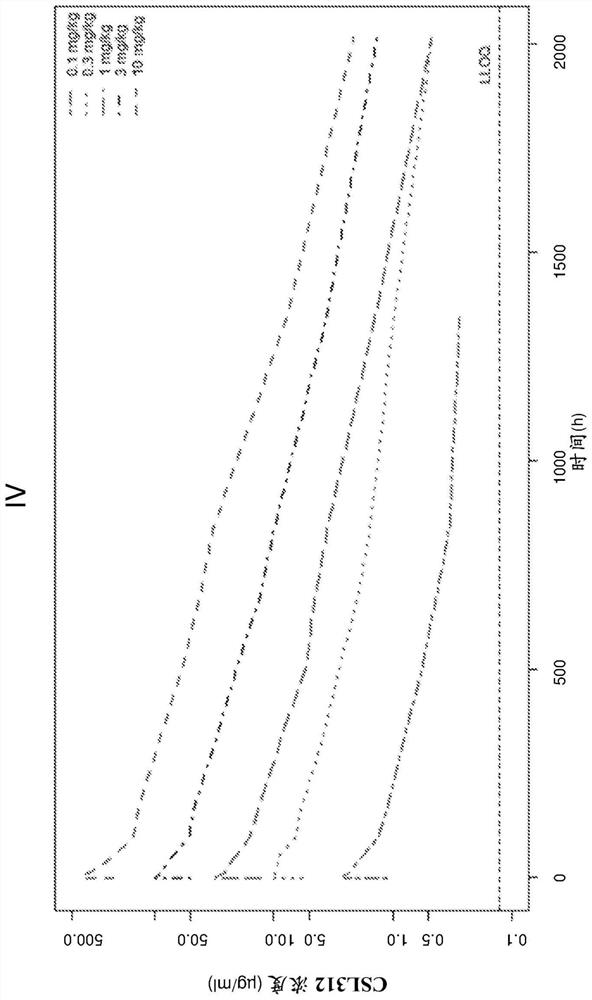
ActiveUS20140199361A1Inhibiting amidolytic activityHigh binding affinityAntibacterial agentsSenses disorderFactor XIIAnti-factor XII
Owner:CSL LTD +1
Adeno-associated virus mediated delivery of c1ei as a therapy for angioedema
ActiveUS20160347822A1Genetic material ingredientsProtease inhibitorsNucleic acid sequencingAngioneurotic oedema
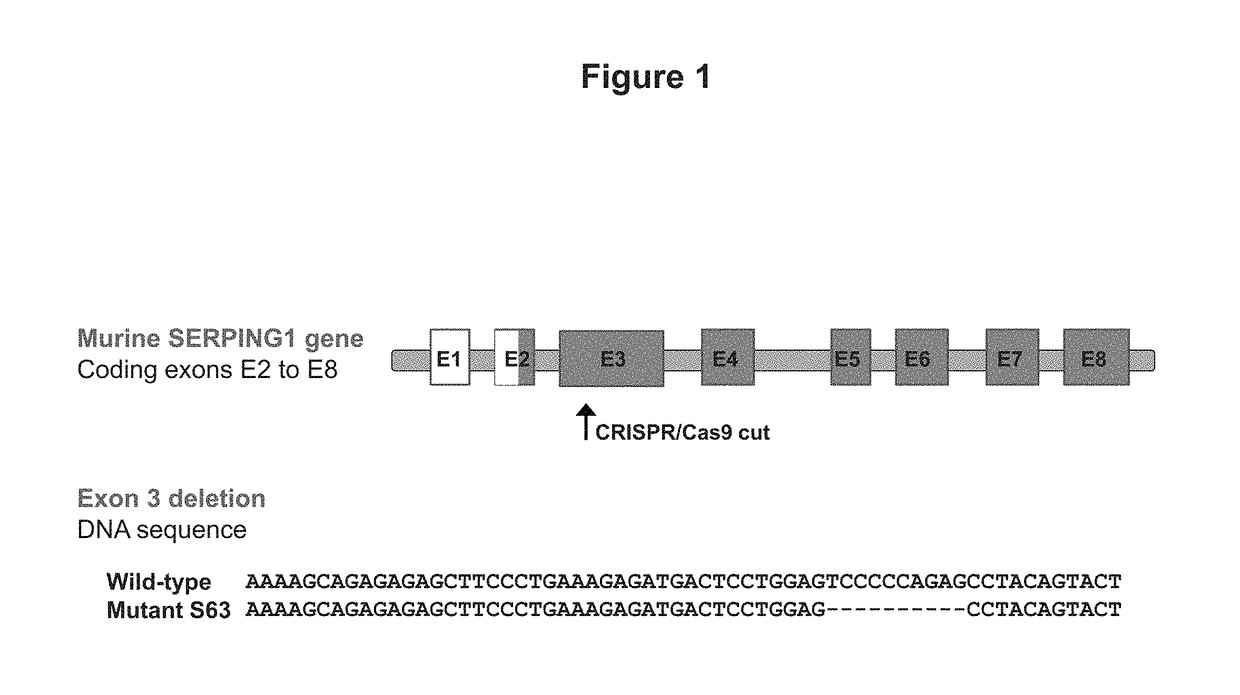
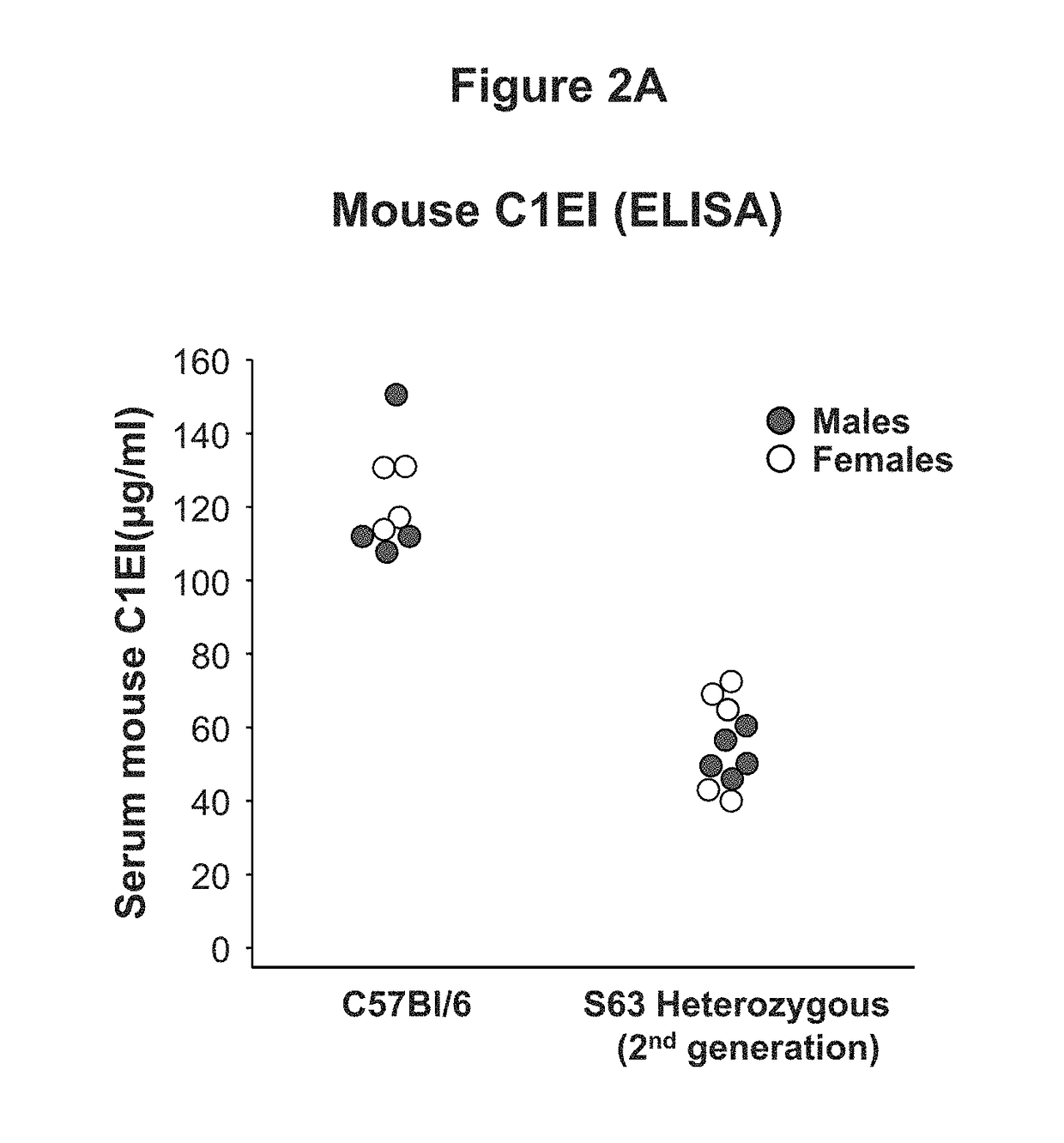
This invention is directed to a vector which comprises a promoter operably linked to a nucleic acid sequence encoding the human C1 esterase inhibitor or Factor XII. The invention is also directed to a composition comprising the vector and a method of using the vector to treat or prevent hereditary angioedema.
Owner:CORNELL UNIVERSITY
Inhibitory anti-factor XII/XIIA monoclonal antibodies and their uses
ActiveUS9518127B2Antibacterial agentsImmunoglobulins against blood coagulation factorsAntiendomysial antibodiesFactor XII
Owner:CSL LTD +1
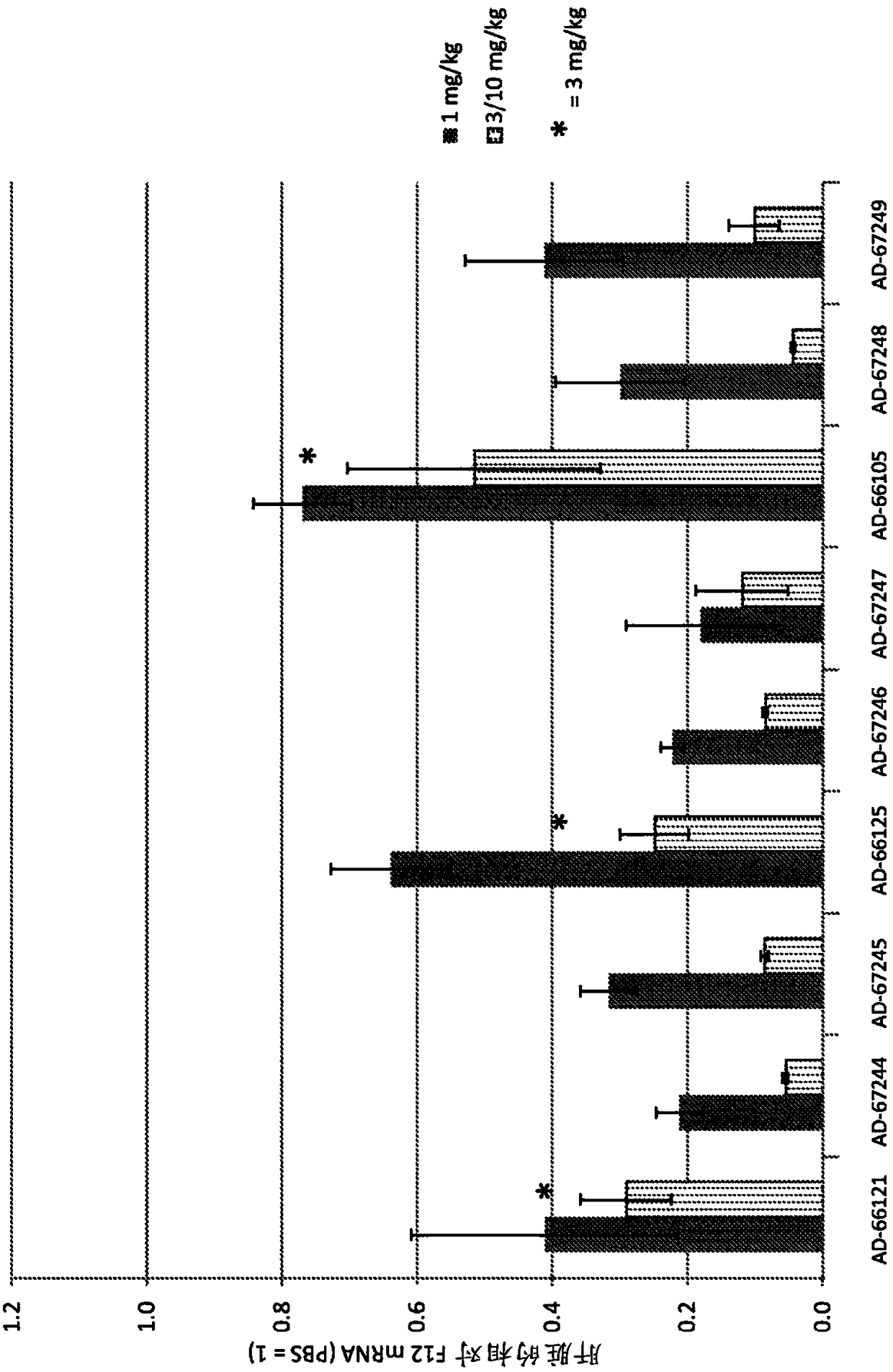
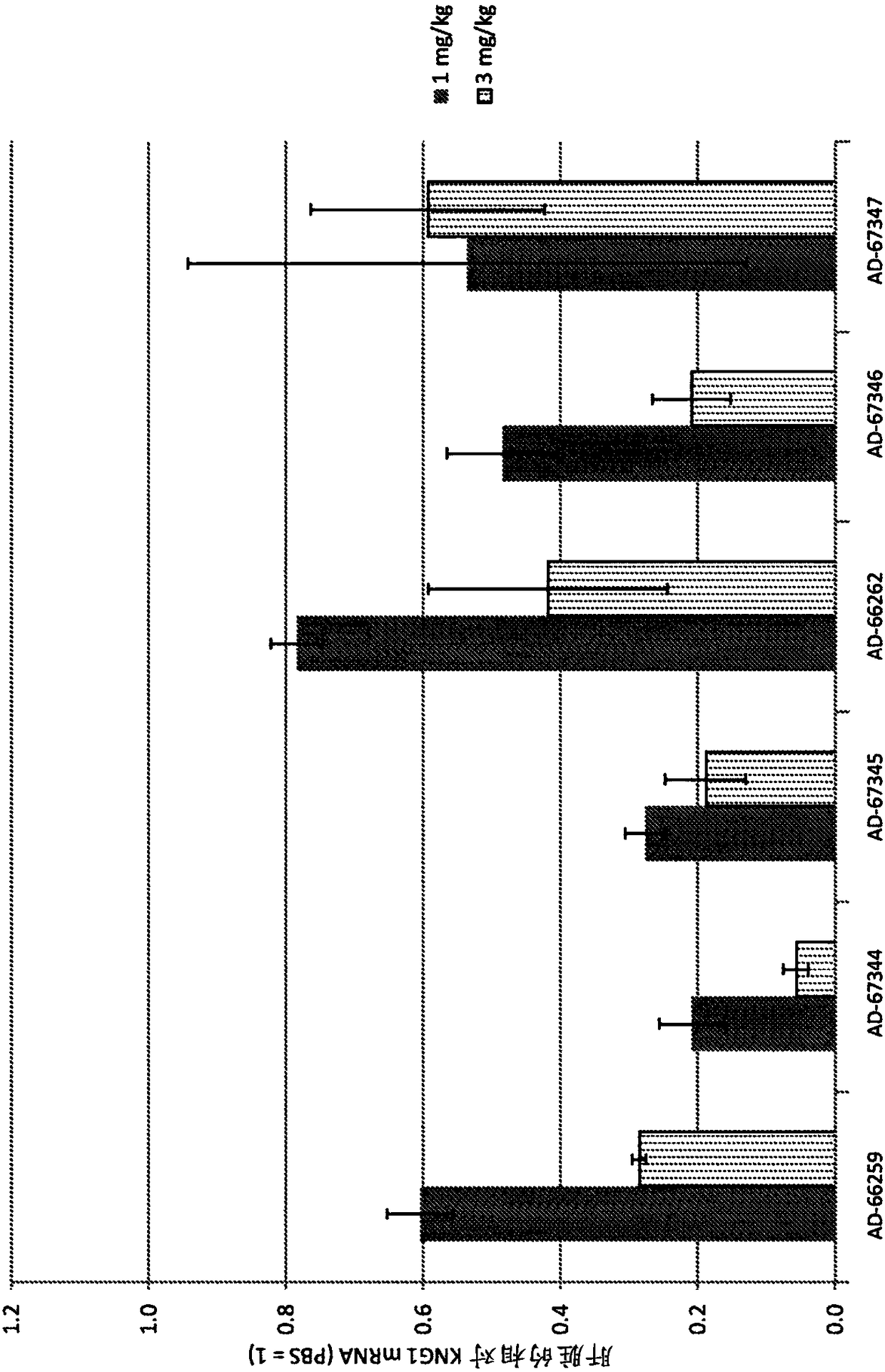
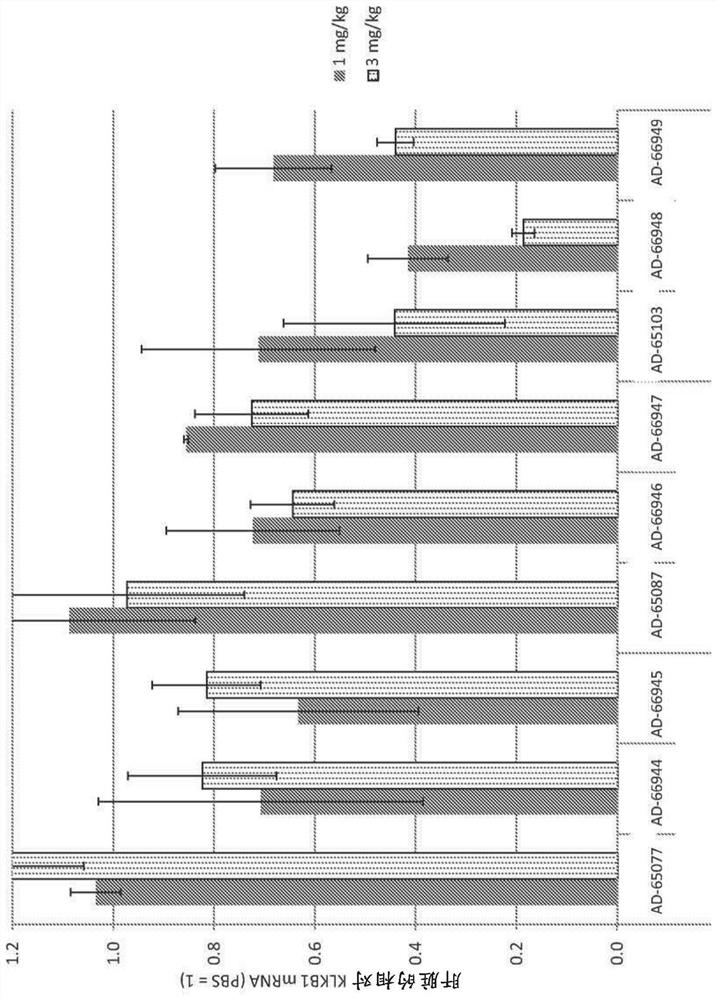
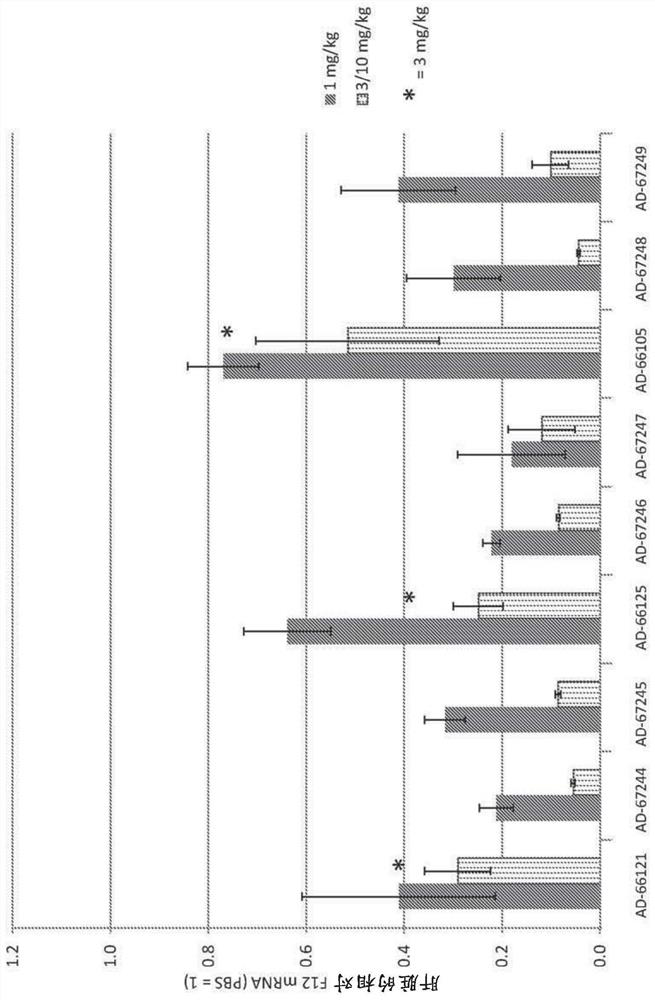
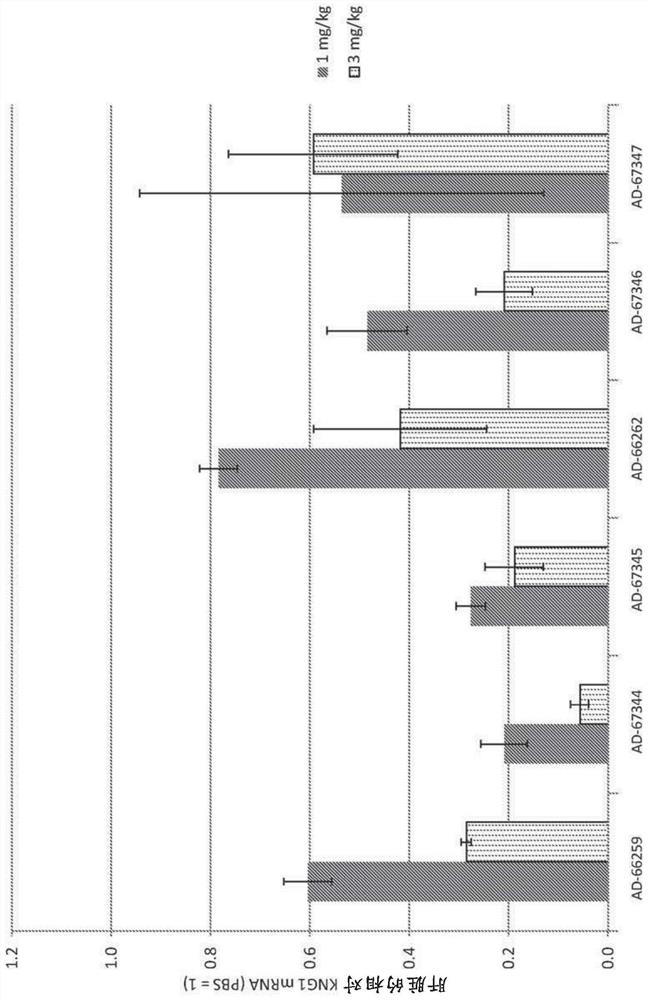
Factor xii (hageman factor) (F12), kallikrein b, plasma (fletcher factor) 1 (KLKB1), and kininogen 1 (KNG1) irna compositions and methods of use thereof
ActiveCN108271386AOrganic active ingredientsMicrobiological testing/measurementContact activationKinin
The present invention relates to RNAi agents, e.g., double stranded RNAi agents, targeting the Kallikrein B, Plasma (Fletcher Factor) 1 (KLKB1) gene, the Factor XII (Hageman Factor (F12) gene, or theKininogen 1 (KNG1) gene, and methods of using such RNAi agents to inhibit expression of a KLKB1 gene, an F12 gene, and / or a KNG1 gene, and methods of treating subjects having an hereditary angioedema(HAE) and / or a contact activation pathway-associated disorder.
Owner:ALNYLAM PHARM INC
Stabilized Proteins with Engineered Disulfide Bonds
InactiveUS20070276128A1High retention rateAvoidance of undesired activityFactor VIIFungiCysteine thiolateBlood plasma
The present invention relates to methods of introducing one or more cysteine residues into a polypeptide which permit the stabilization of the polypeptide by formation of at least one bond, preferably a disulfide bond, between different domains of the polypeptide. The invention also relates to polypeptides containing such introduced cysteine residue(s), nucleic acids encoding such polypeptides and pharmaceutical compositions comprising such polypeptides or nucleic acids. The invention also relates to vectors, viral particles and host cells containing such nucleic acids, and methods of using them to produce the polypeptides of the invention. Exemplified polypeptides include plasma proteins, including hepatocyte growth factor activator and plasma hyaluronin binding protein, as well as blood coagulation factors, such as Factor VIII, Factor V, Factor XII and prothrombin.
Owner:THE SCRIPPS RES INST
METHODS FOR TREATING OR PREVENTING CONTACT-ACTIVATION PATHWAY-ASSOCIATED DISEASES USING iRNA COMPOSITIONS TARGETING FACTOR XII (HAGEMAN FACTOR) (F12)
InactiveUS20200208150A1Reduce depositionCardiovascular disorderDNA/RNA fragmentationDiseaseContact activation
The present invention relates to methods of use of RNAi agents, e.g., double stranded RNAi agents, targeting a Factor XII (Hageman Factor (F12) gene, for treating subjects having a contact activation pathway-associated disease, such as a thrombophilia or hereditary angioedema (HAE), methods for preventing at least one symptom in a subject having a contact activation pathway-associated disease, such as a thrombus formation or an angioedema attack, and RNAi agents targeting an F12 gene, for use in the methods of the invention.
Owner:ALNYLAM PHARMA INC
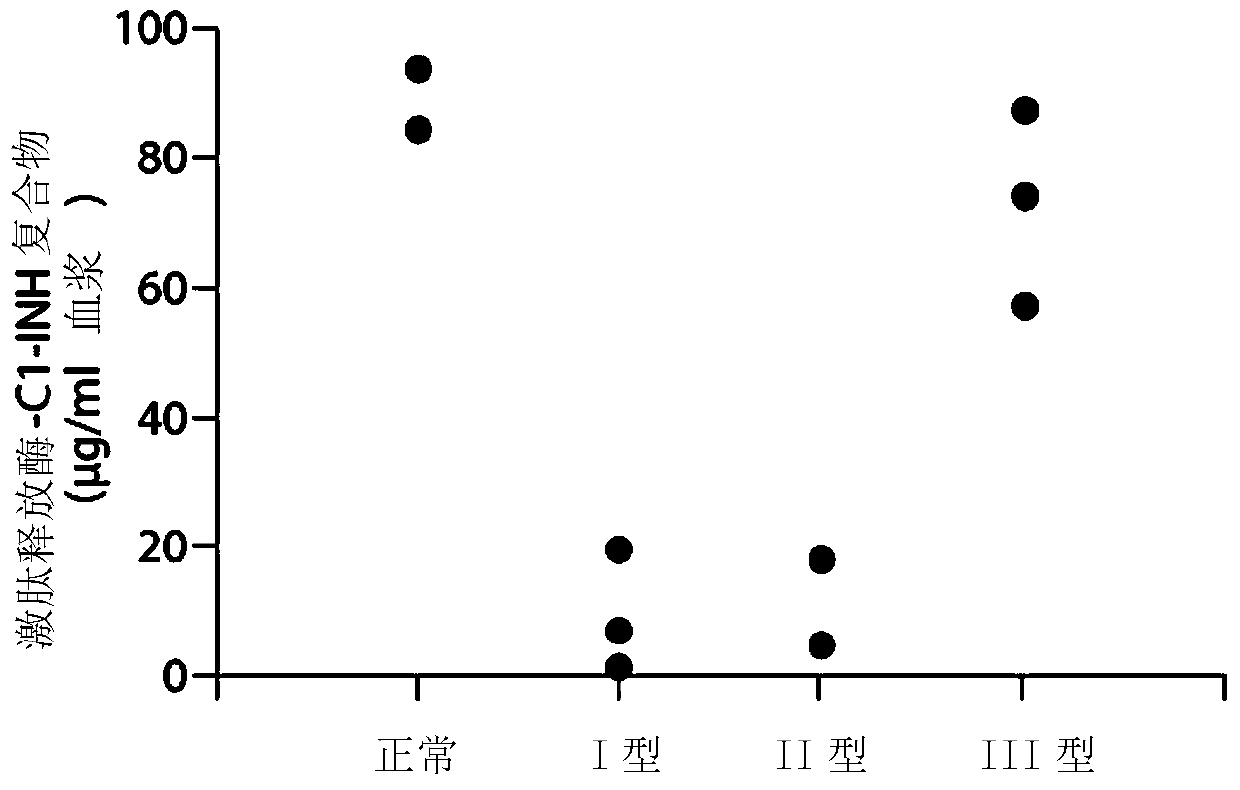
Evaluation, assays and treatment of PKAL-mediated disorders
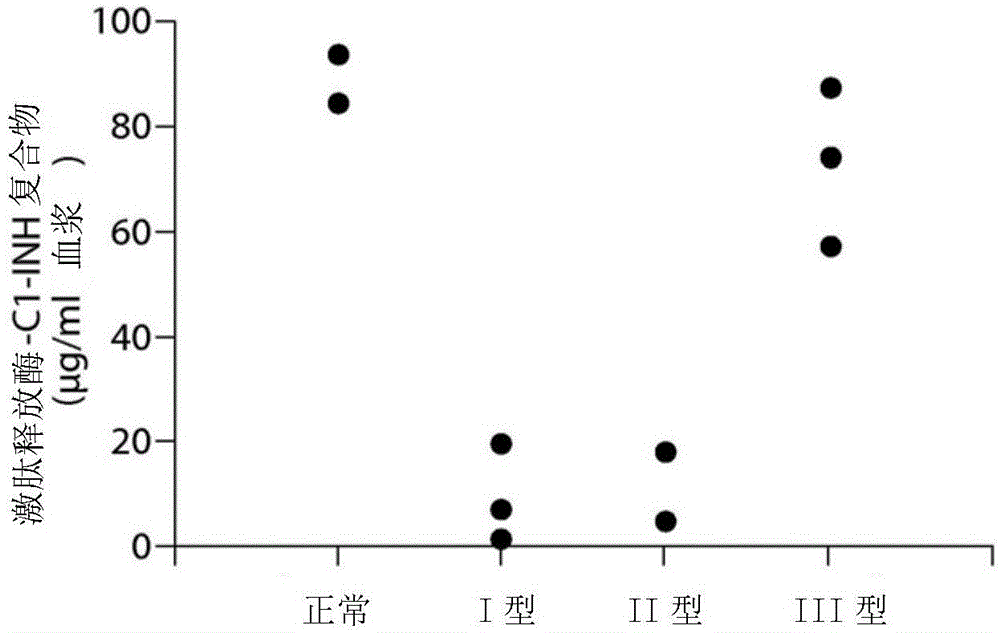
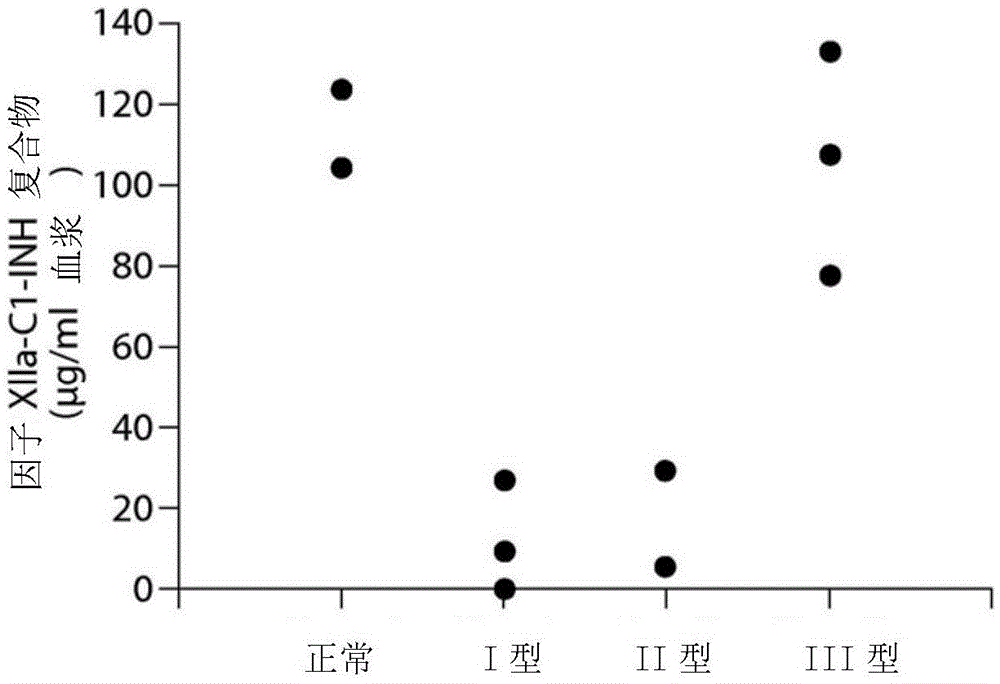
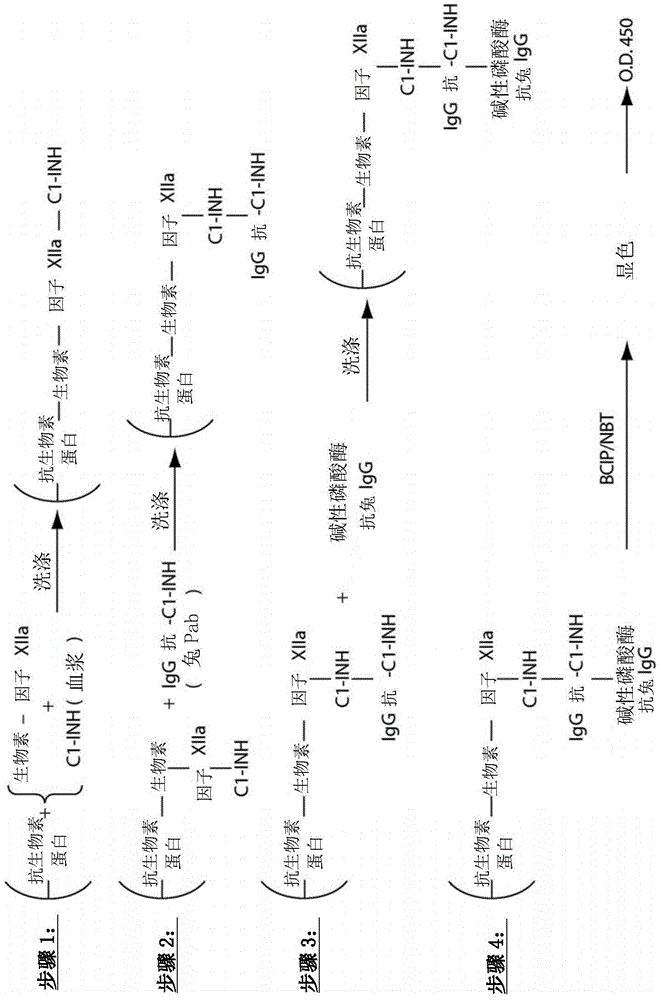
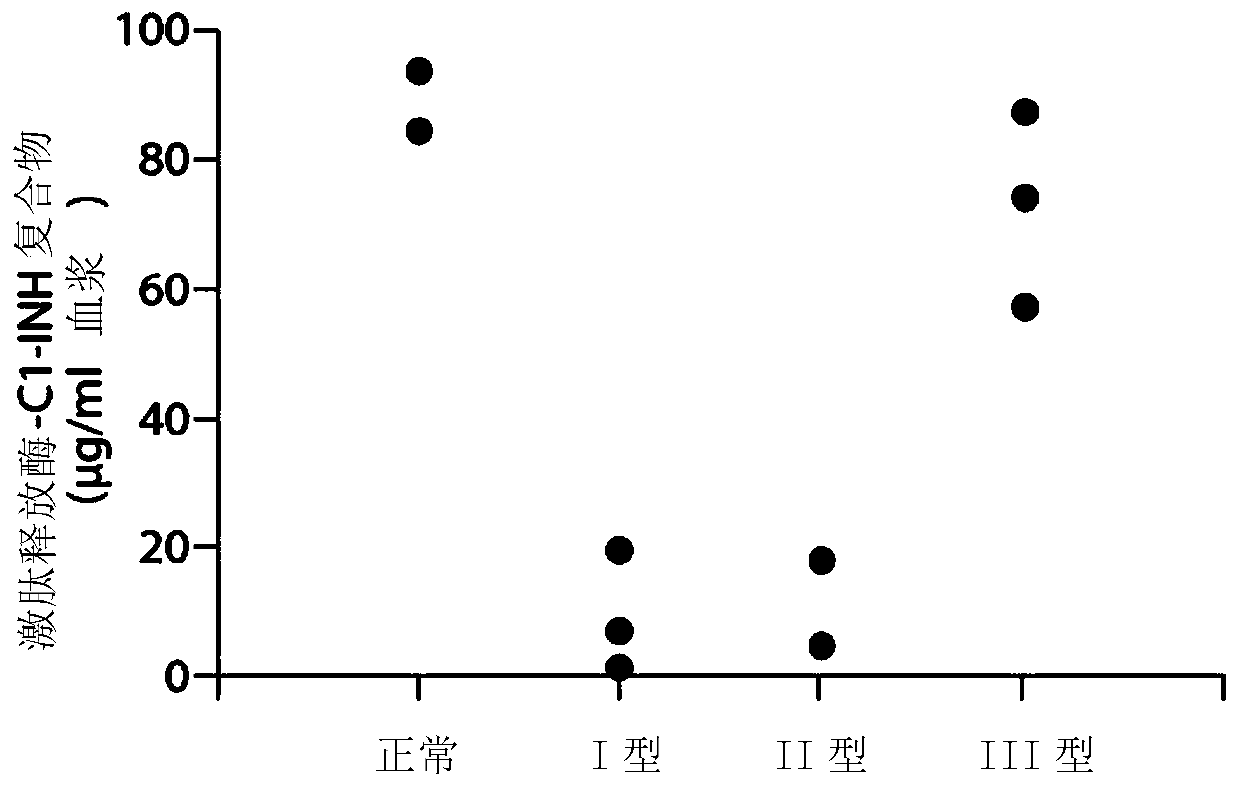
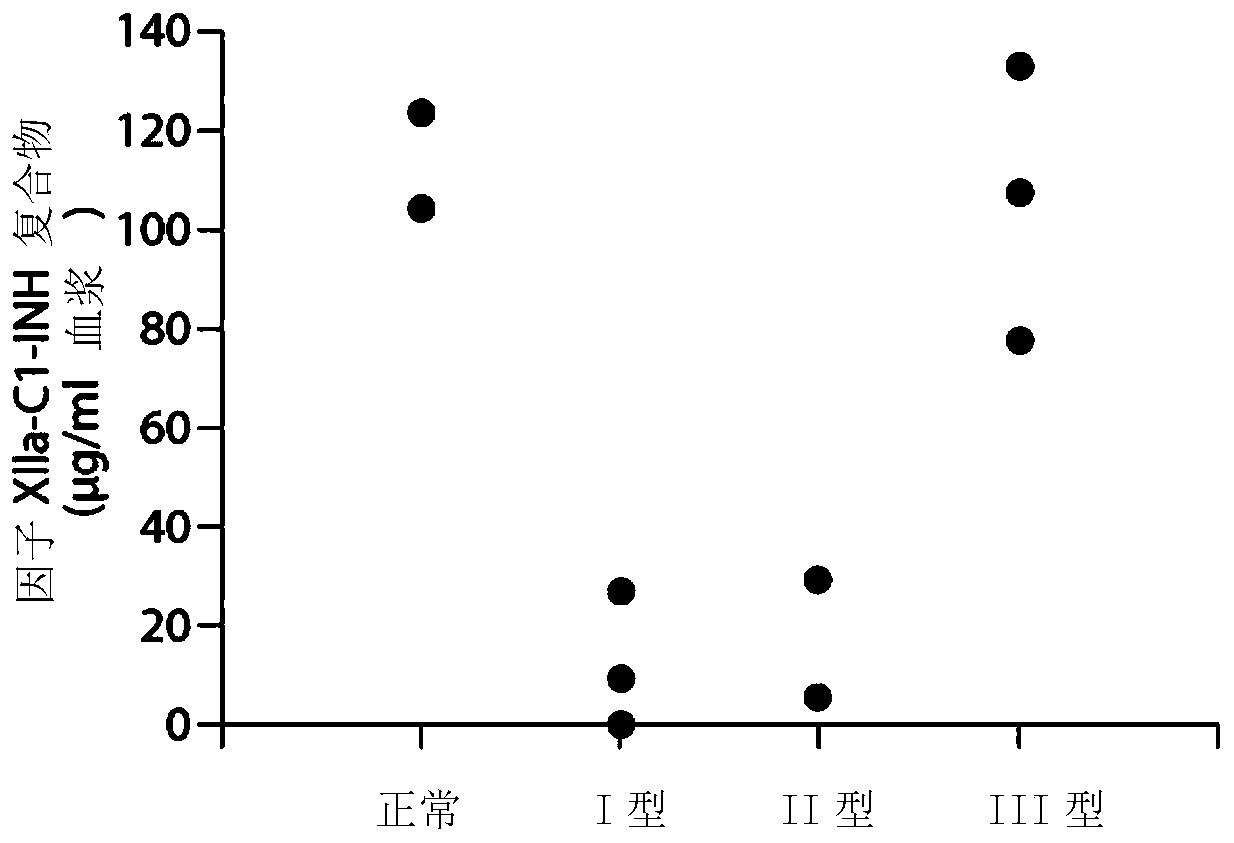
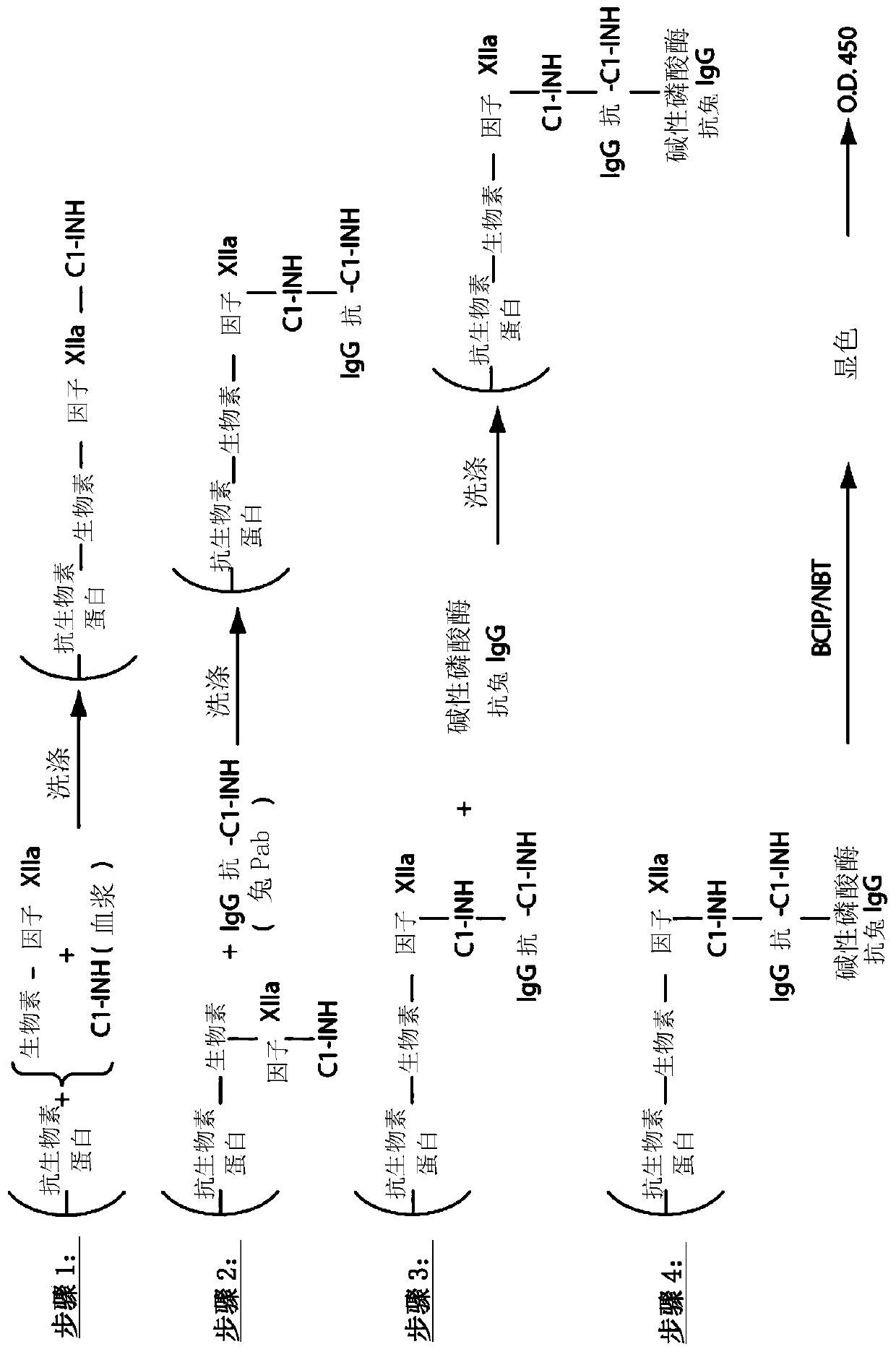
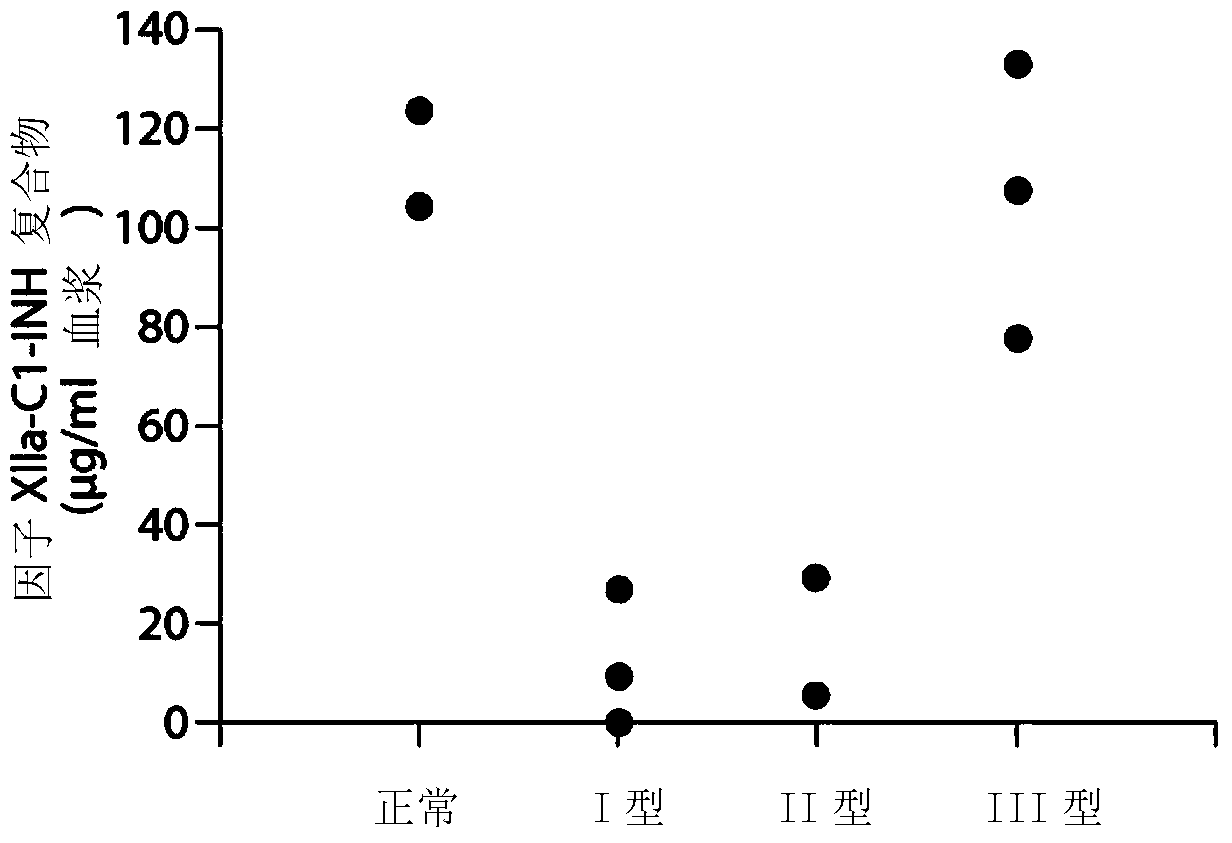
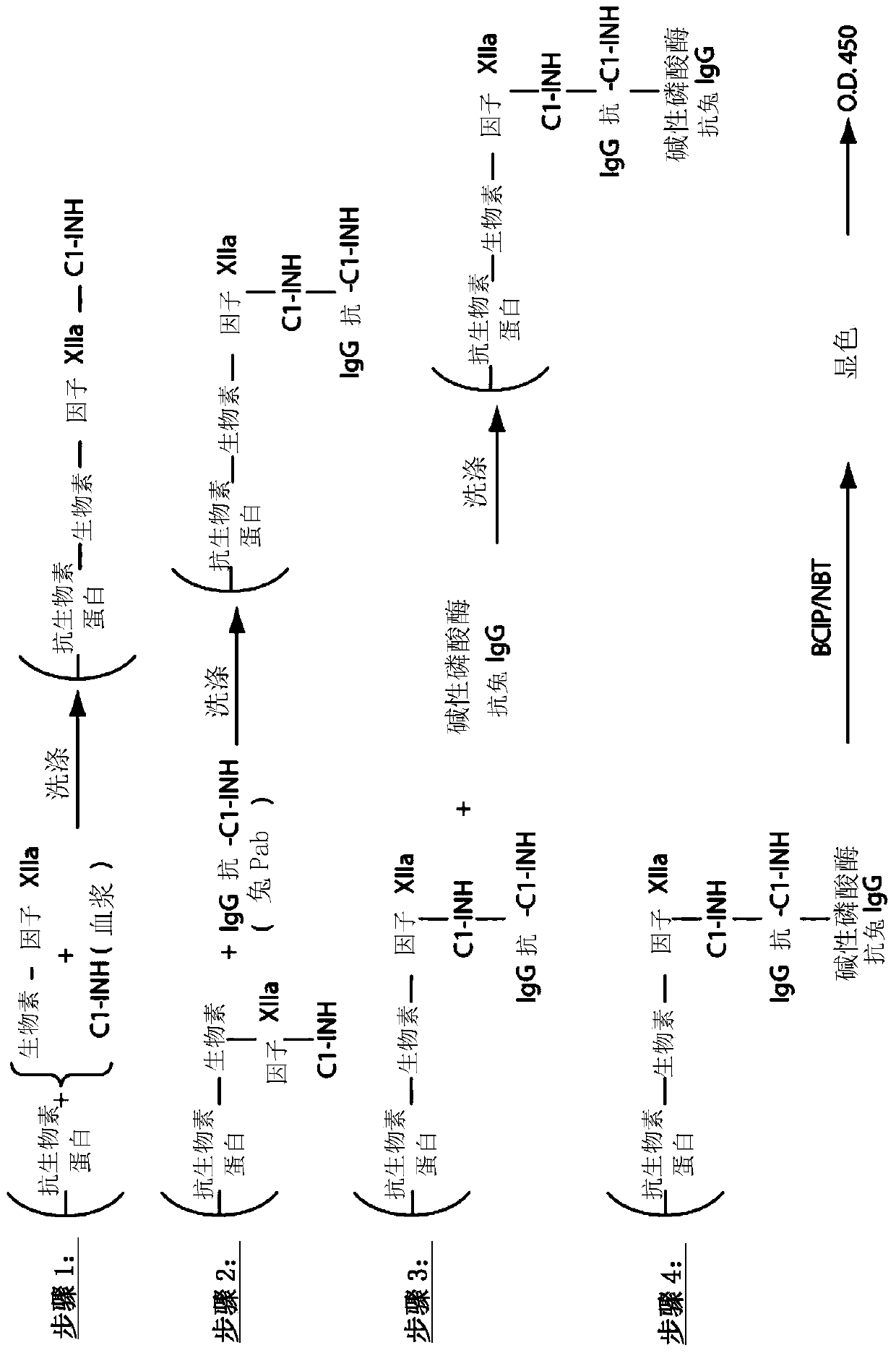
The invention provides assay methods of detecting plasma protease CI inhibitor (Cl-INH) that binds plasma kallikrein, Factor XII, or both, and uses thereof for identifying subjects at risk for or suffering from a pKal-mediated or bradykinin-mediated disorder. Provided methods permit analysis of patients with plasma kallikrein-mediated angioedema (KMA), or other diseases mediated by pKal useful in the evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
Evaluation, assays and treatment of PKAL-mediated disorders
The invention relates to evaluation, assays and treatment of PKAL-mediated disorders. The invention provides assay methods of detecting plasma protease CI inhibitor (Cl-INH) that binds plasma kallikrein, factor XII, or both, and uses thereof for identifying subjects at risk for or suffering from a pKal-mediated or bradykinin-mediated disorder. Provided methods permit analysis of patients with plasma kallikrein-mediated angioedema (KMA), or other diseases mediated by pKal useful in the evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
Adeno-associated virus mediated delivery of C1E1 as a therapy for angioedema
ActiveUS10214731B2Genetic material ingredientsProtease inhibitorsHereditary angioedemaAngioneurotic oedema
This invention is directed to a vector which comprises a promoter operably linked to a nucleic acid sequence encoding the human C1 esterase inhibitor or Factor XII. The invention is also directed to a composition comprising the vector and a method of using the vector to treat or prevent hereditary angioedema.
Owner:CORNELL UNIVERSITY
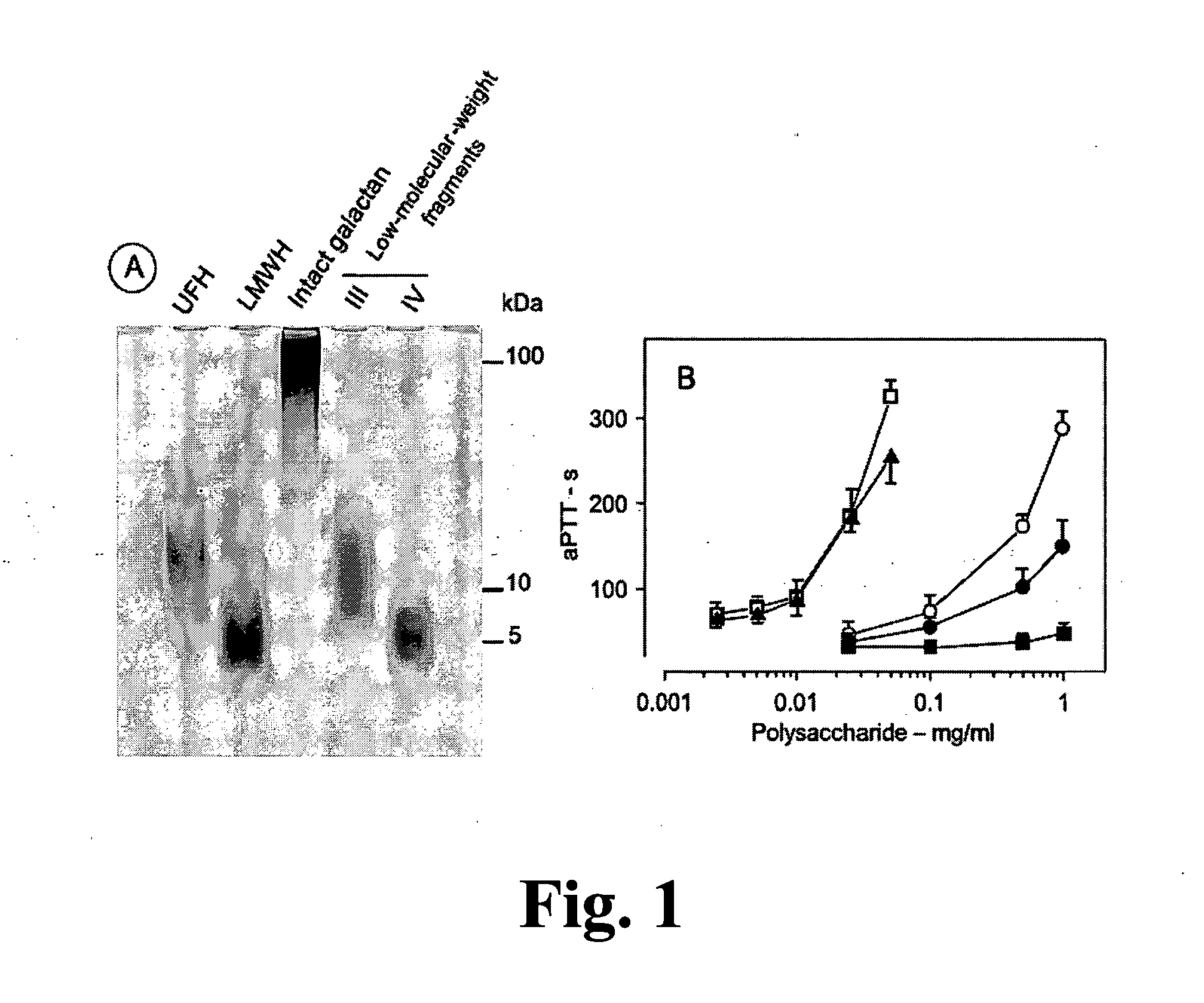
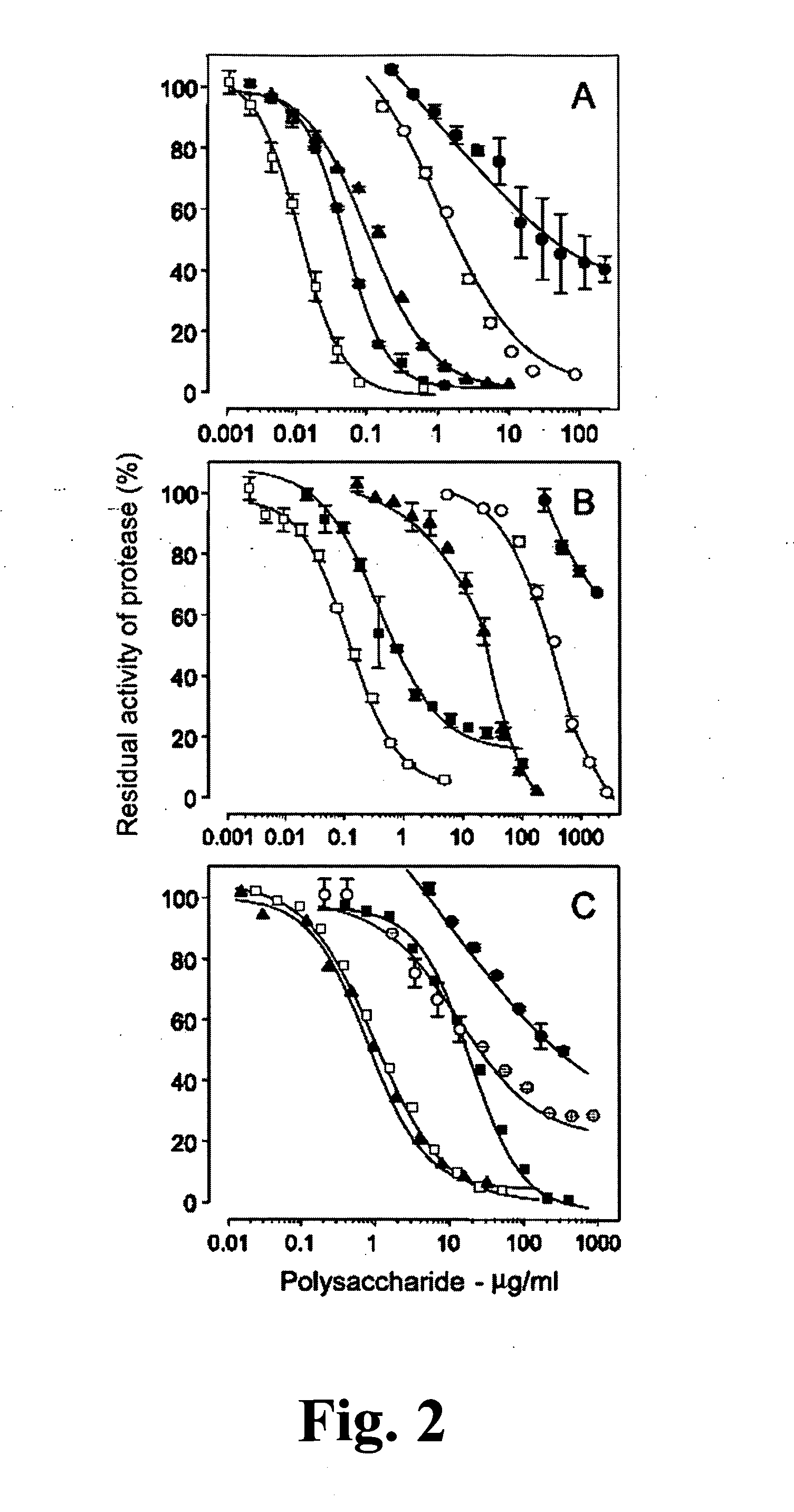
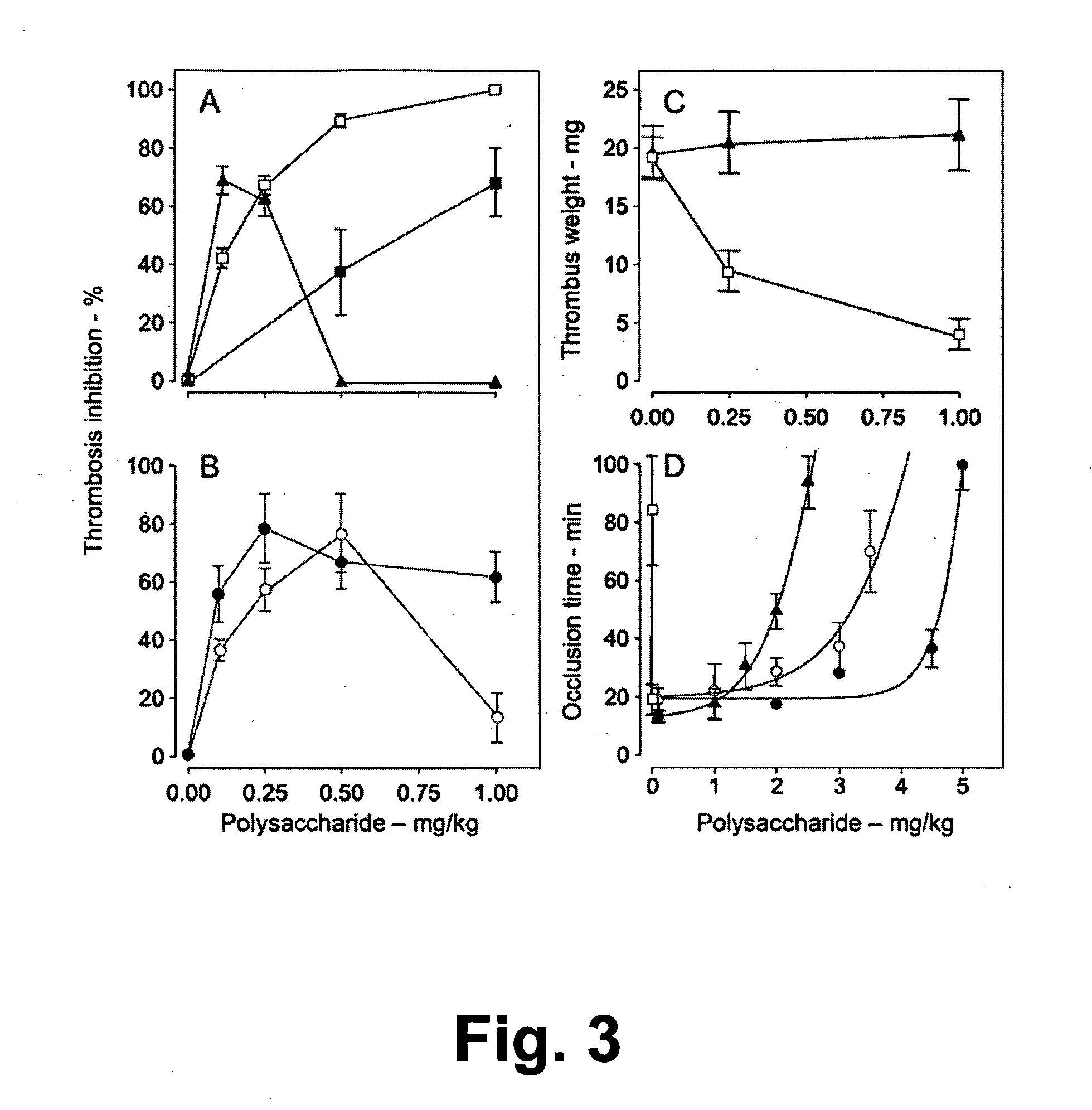
Sulfated Galactans With Antithrombotic Activity, Pharmaceutical Composition, Method for Treating or Prophylaxis of Arterial or Venous Thrombosis, Method of Extraction and Use Thereof
InactiveUS20110263527A1Inhibits platelet aggregationUnwanted effectEsterified saccharide compoundsOrganic active ingredientsSulfationMedicine
The present invention relates to low molecular weight sulfated galactans, obtained from algae, particularly genus Botryocladia, preferably species Botryocladia occidentallis, which have no effect on the factor XII activation of the clotting cascade, having antithrombotic heparinoid activity. The present invention also refers to a pharmaceutical composition comprising said sulfated galactans and the use thereof, as heparin substitute, in the treatment or prophylaxis of arterial or venous thrombosis in humans and animals. Furthermore, the present invention provides a method of extraction of the said sulfated galactans.
Owner:DELTA DO PRATA
Modified serpins for the treatment of bradykinin-mediated disease
PendingUS20200010533A1Strong specificityReduce sensitivityPeptide/protein ingredientsGenetic material ingredientsDiseaseKinin
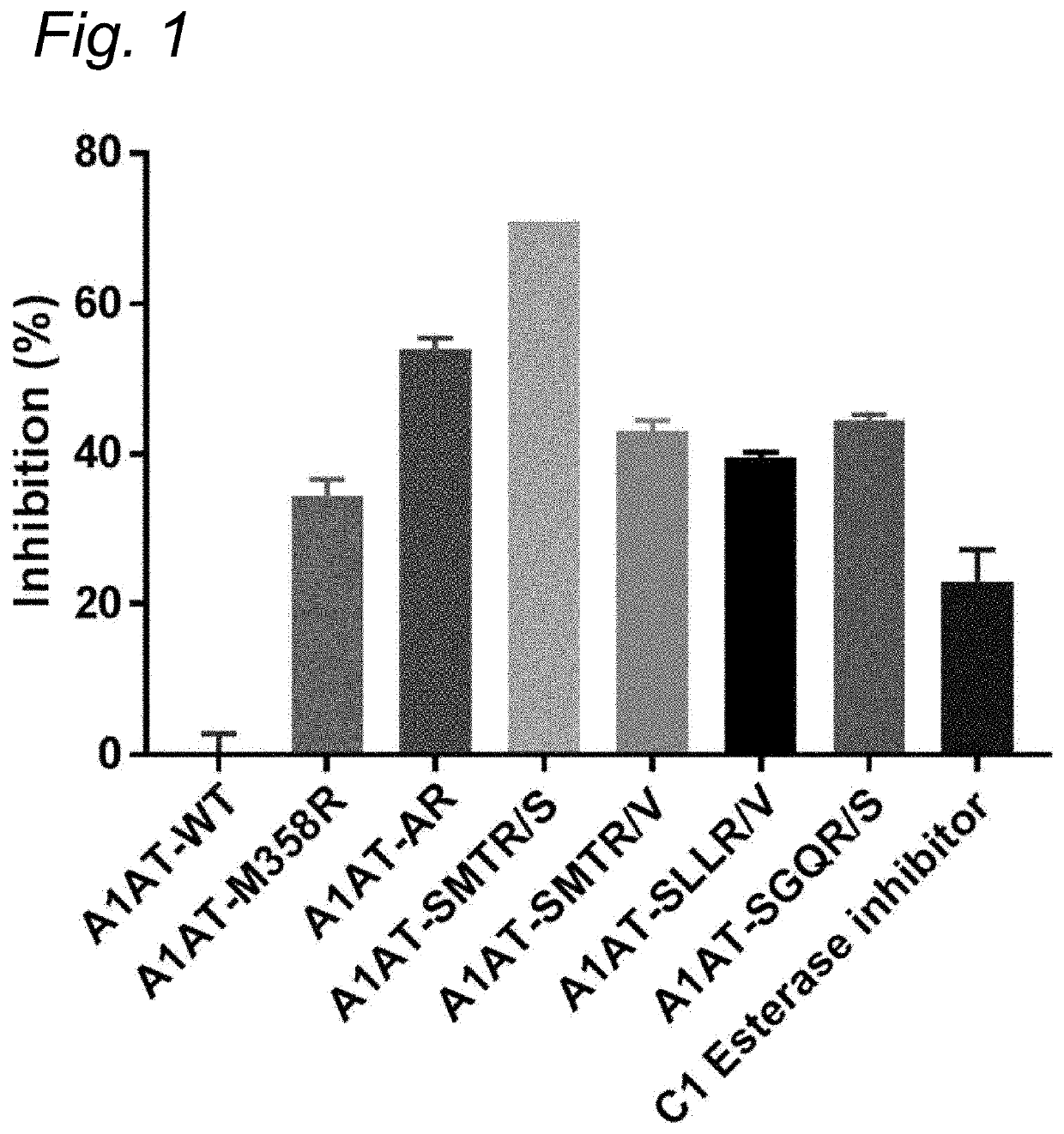
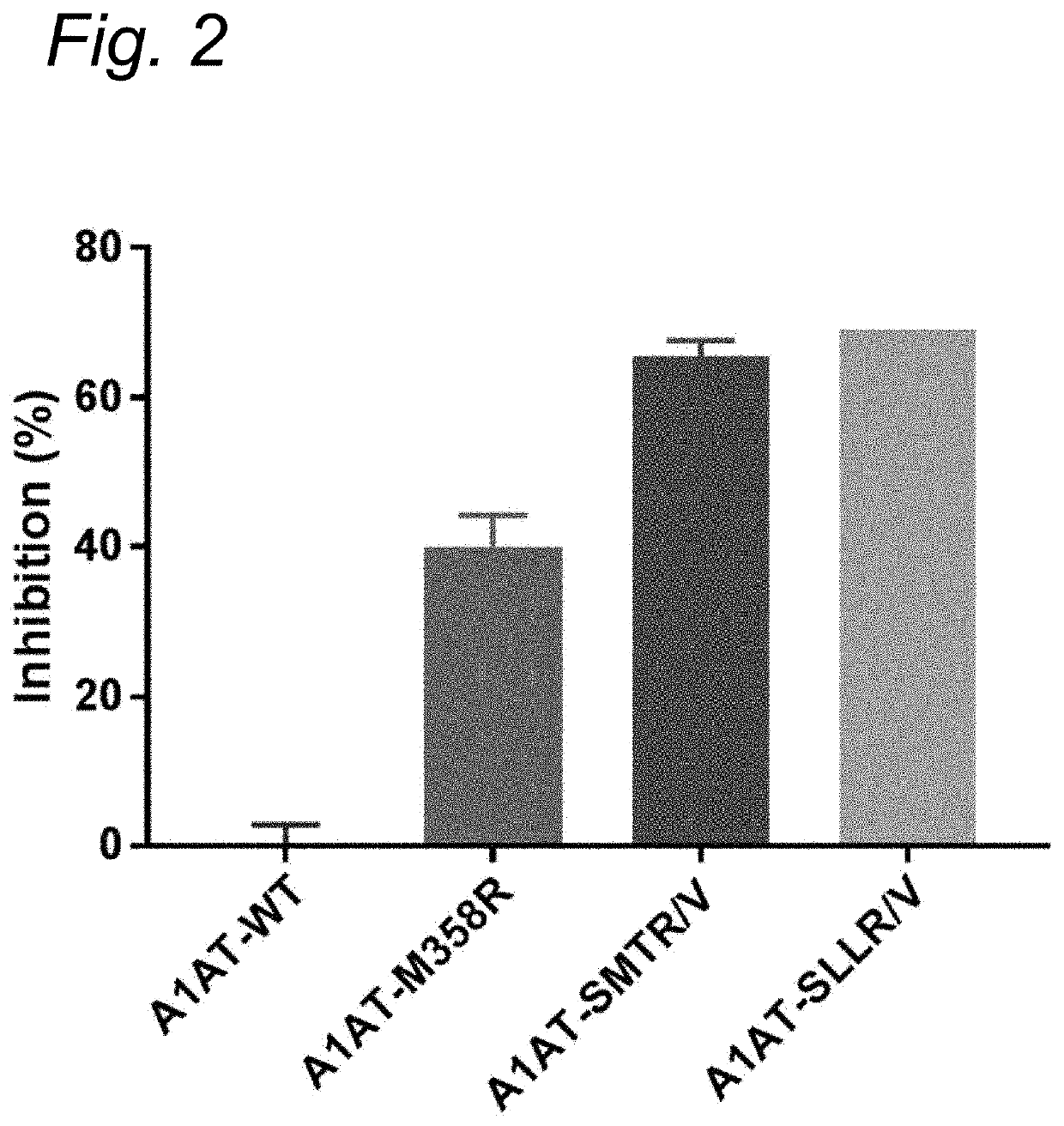
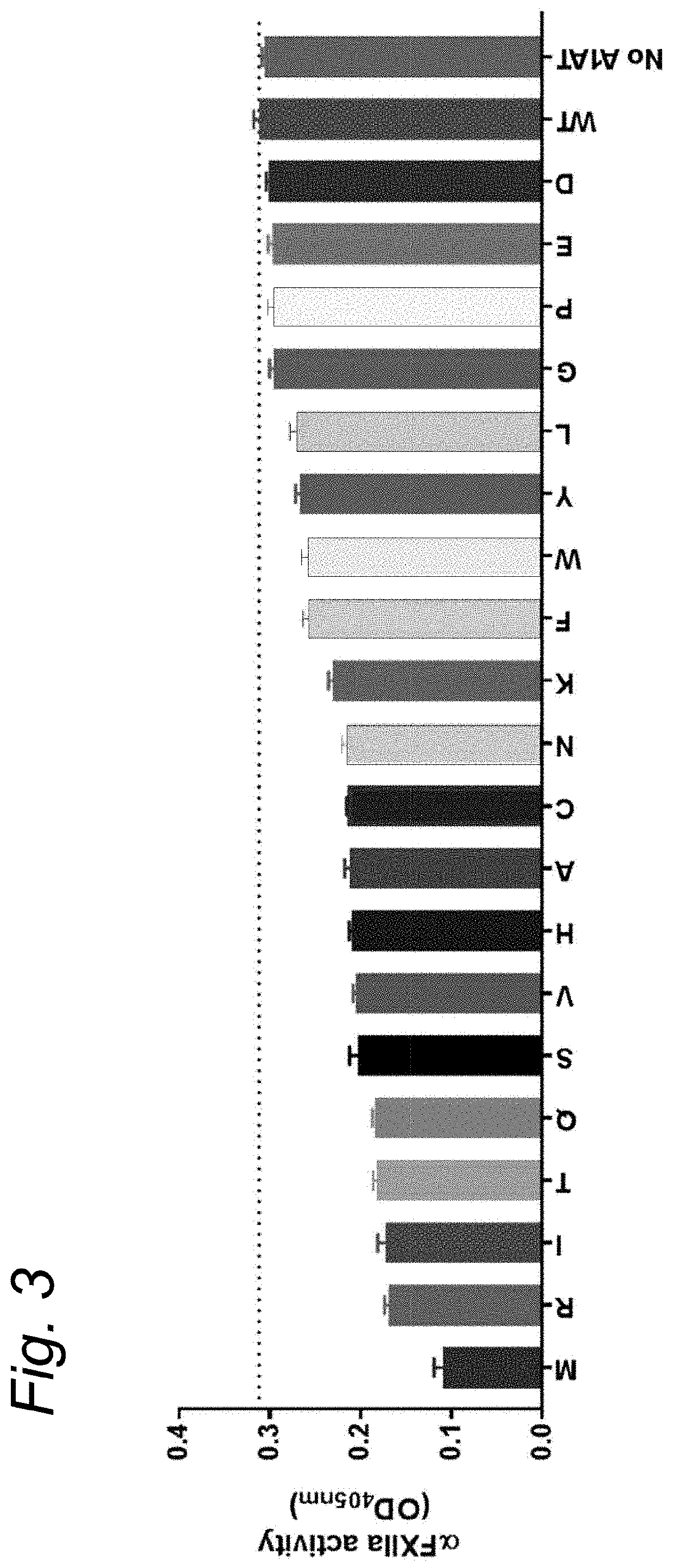
The present invention relates to modified serpins for use in the treatment of bradykinin-mediated diseases. The modified serine protease inhibitors (serpins) have mutations in one or more of the P4, P3, P2, P1 and P1′ residues of their reactive center loop, which mutations increase the serpin's inhibition of plasma kallikrein (PK) as compared to the corresponding unmodified serpin. The mutations in the modified serpins of the invention further ensure that serpins display substantially no inhibition of at least thrombin and activated protein C. A modified serpin of the invention further preferably shows increased inhibition of at least one of an active form of Factor XII (FXII) and plasmin as compared to the corresponding unmodified serpin, and, preferably, the serpin inhibits at least one of an active form of FXII and PK stronger than they are inhibited by C1 esterase inhibitor. Preferably the modified serpin is a modified α1-antitrypsin. The invention further pertains to nucleic acid molecule encoding the modified serpins of the invention, e.g. a gene therapy vector, and to pharmaceutical compositions comprising the modified serpins of the invention or such gene therapy vectors.
Owner:PRECLINICS GES FUER PRAEKLINISCHE FORSCHUNG
Anti-factor xii/xiia antibodies and uses thereof
PendingCN112437682AImmunoglobulins against blood coagulation factorsAmplifier with semiconductor-devices/discharge-tubesDiseaseAntiendomysial antibodies
The present invention provides monoclonal antibodies that bind to the Factor XII (FXII) protein, and methods of use thereof. In various embodiments of the invention, the antibodies are fully human antibodies that bind to FXII and to the activated form of FXII (FXIIa). In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing FXII activity, thus providing a means of treating or preventing a disease, disorder or condition associated with thrombosis in humans.
Owner:REGENERON PHARM INC
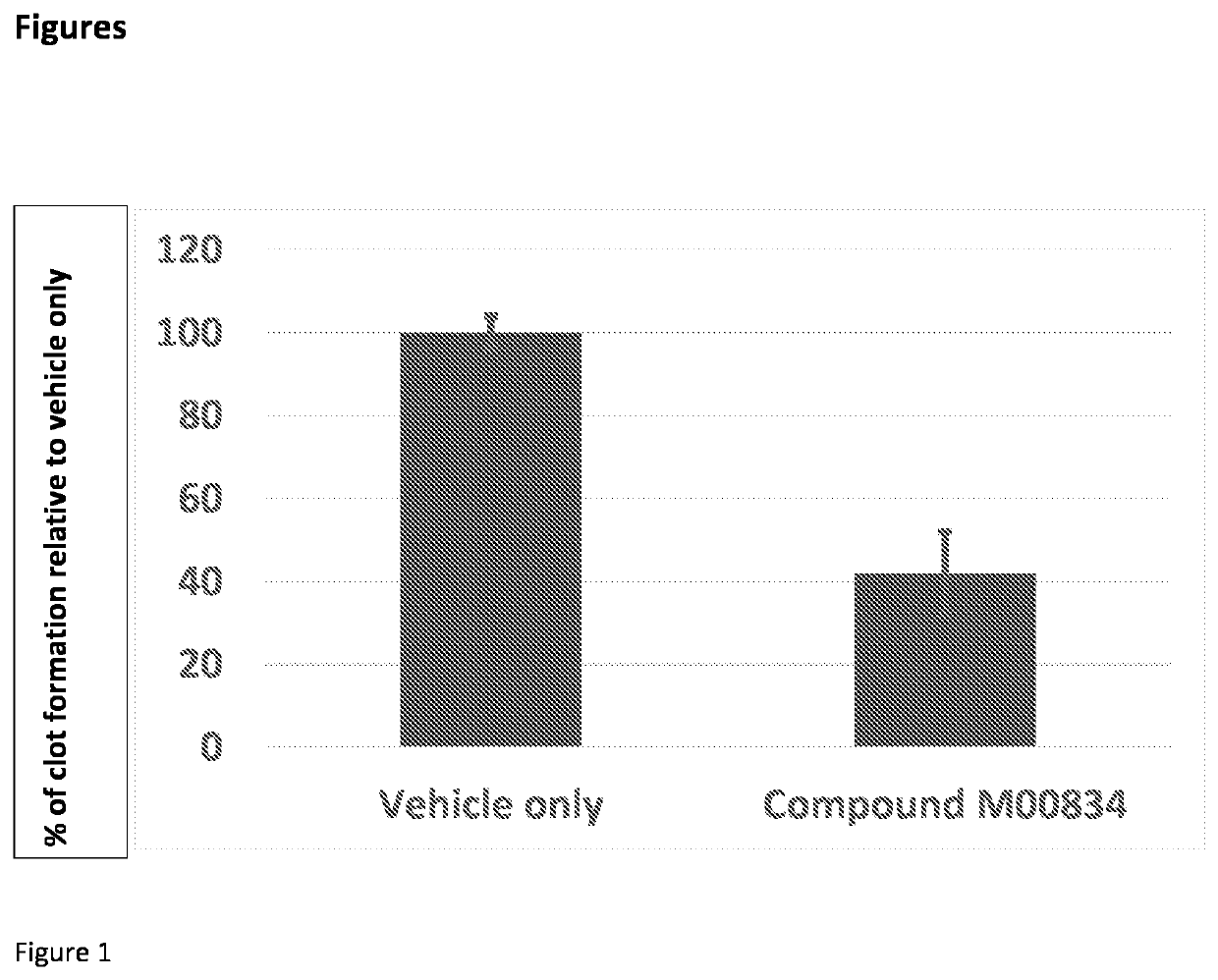
Factor xiia inhibitors
PendingCN112165938AIncreased anti-thrombotic efficacyIncreased bleeding riskOrganic chemistryBlood disorderFactor XIIa inhibitorAnticoagulant
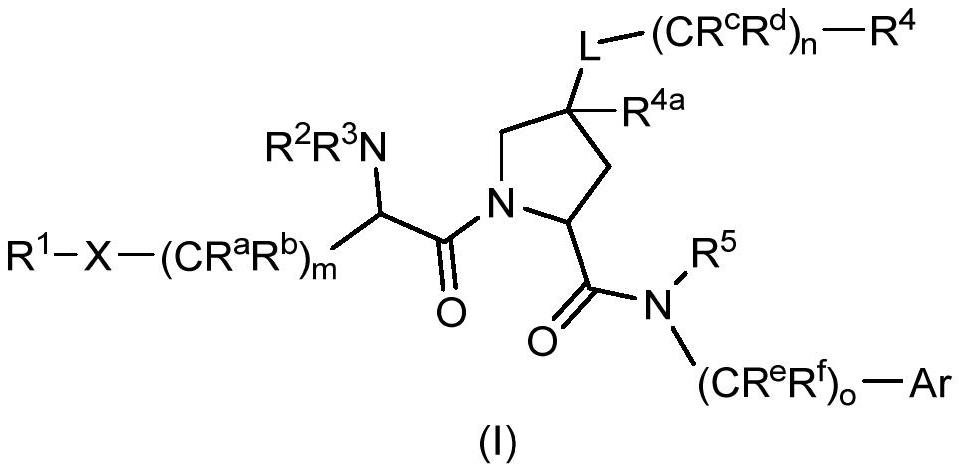
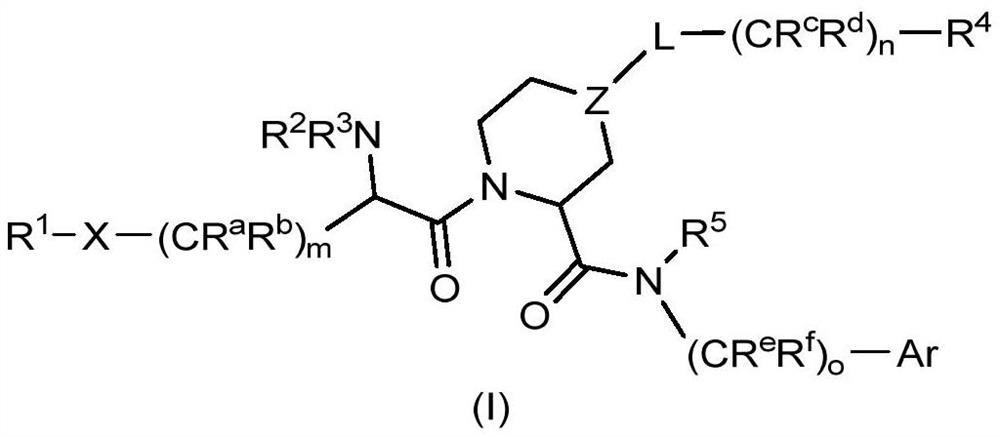
The invention relates to compounds of formula (I) and methods of treatment by using the compounds. The invention also relates to processes and methods for producing the compounds of the invention. Thecompounds of the invention are modulators of Factor XII (e.g. Factor XIIa). In particular, the compounds are inhibitors of Factor XIIa and may be useful as anticoagulants.
Owner:UNIV OF LEEDS
FACTOR XIIa INHIBITORS
This invention relates to compounds of formula (I). The compounds of formula (I) are modulators of Factor XII, specifically Factor XIIa. The compounds are inhibitors of Factor XIIa and may be useful as anticoagulants. The compounds of formula (I) may be used in methods of treatment (or prevention) of blood disorders related to bleeding or coagulation.
Owner:UNIV OF LEEDS
Polynucleotide agents targeting factor XII (hageman factor) (F12) and methods of use thereof
ActiveUS10883107B2Organic active ingredientsSugar derivativesMalignant essential hypertensionEnd stage renal disease
Owner:ALNYLAM PHARMA INC
Compositions and methods for inhibiting gene expression of factor XII
RNA interference (RNAi) initiators for inhibiting the expression of Factor XII (F12) gene by the RNA interference mechanism are disclosed. Also disclosed are pharmaceutical compositions comprising one or more F12 RNAi initiators and one or more excipients capable of delivering the RNAi initiators to hepatocytes. Delivery of the F12 RNAi initiator to hepatocytes in vivo provides inhibition of F12 gene expression and treatment of angioedema, including hereditary angioedema (HAE), venous thromboembolism (VTE), and diseases associated with angioedema.
Owner:ARROWHEAD PHARMA INC
Early detection of liver cancer based on F12 gene methylation
InactiveCN114438211AAccurate detectionNo painMicrobiological testing/measurementDNA/RNA fragmentationTissue sampleBlood plasma
The invention discloses a nucleic acid methylation detection method for early screening of liver cancer and a corresponding kit for liver cancer detection. A methylation gene detected by the kit contains an F12 (Coagation factor XII) gene, and a detected sample comprises a tissue sample and a plasma sample.
Owner:HANGZHOU NEW HORIZON HEALTH TECH CO LTD
Therapeutic factor xii antibody
PendingCN113423465AImmunoglobulins against blood coagulation factorsGeneral/multifunctional contrast agentsAntigen Binding FragmentImmune complex deposition
Monoclonal antibodies that bind, such as specifically bind, blood protein factor XII (FXII) are described. The monoclonal antibodies (including antigen-binding fragments thereof) are capable of forming immune complexes with human (FXII) and inhibiting (FXII) activity, resulting in safe anti-inflammatory and anti-thrombotic effects.
Owner:OREGON HEALTH & SCI UNIV +1
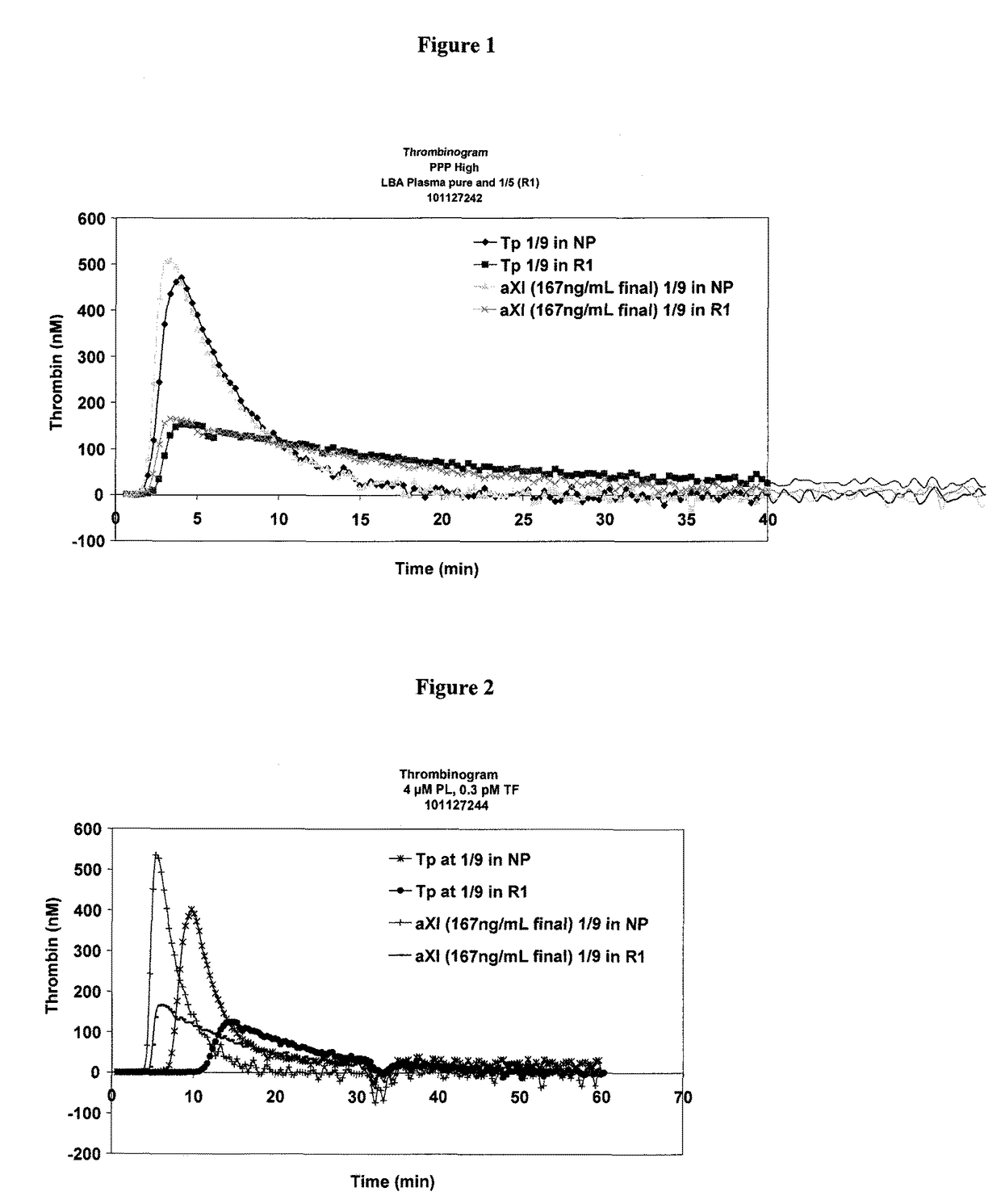
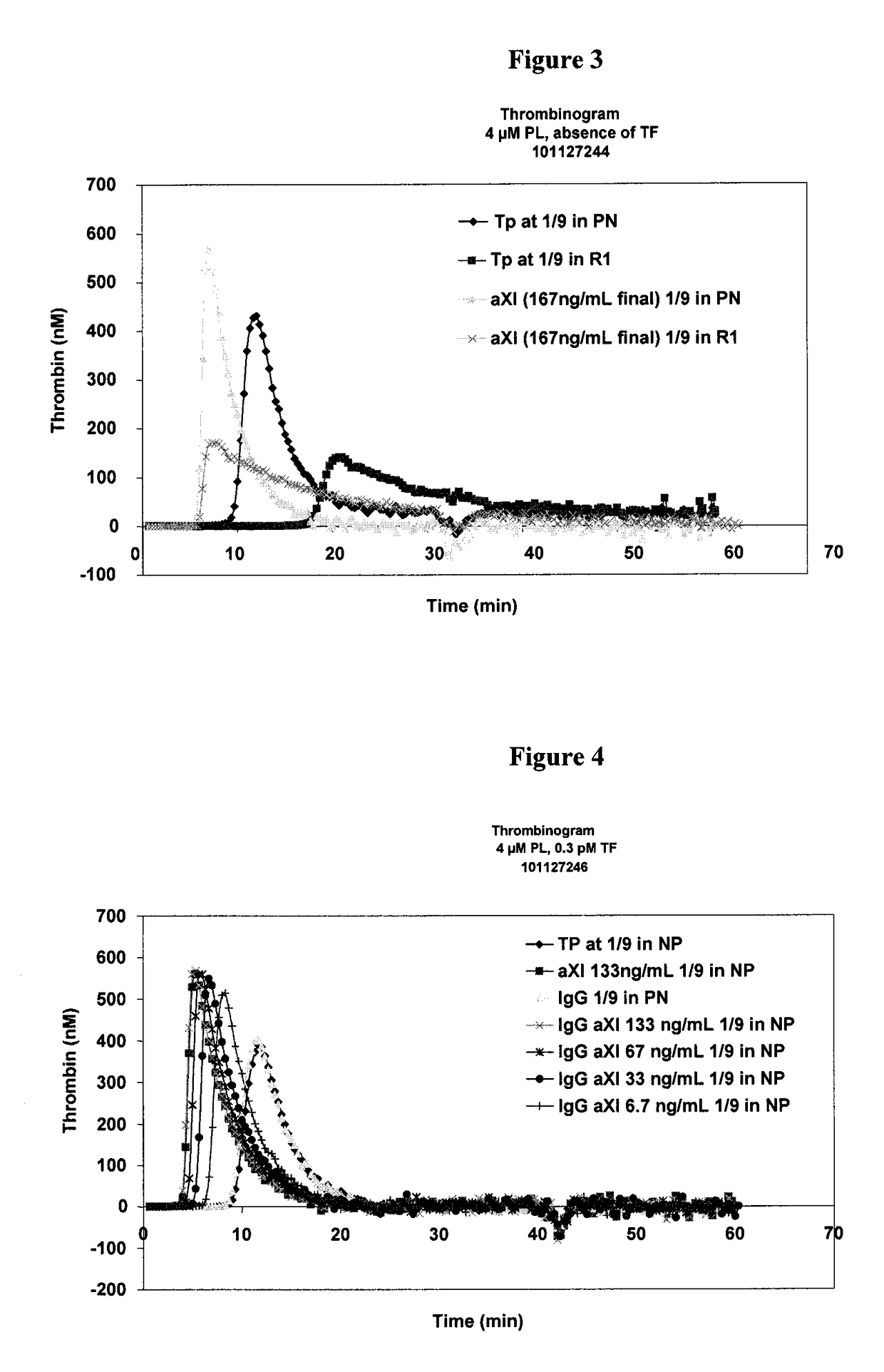
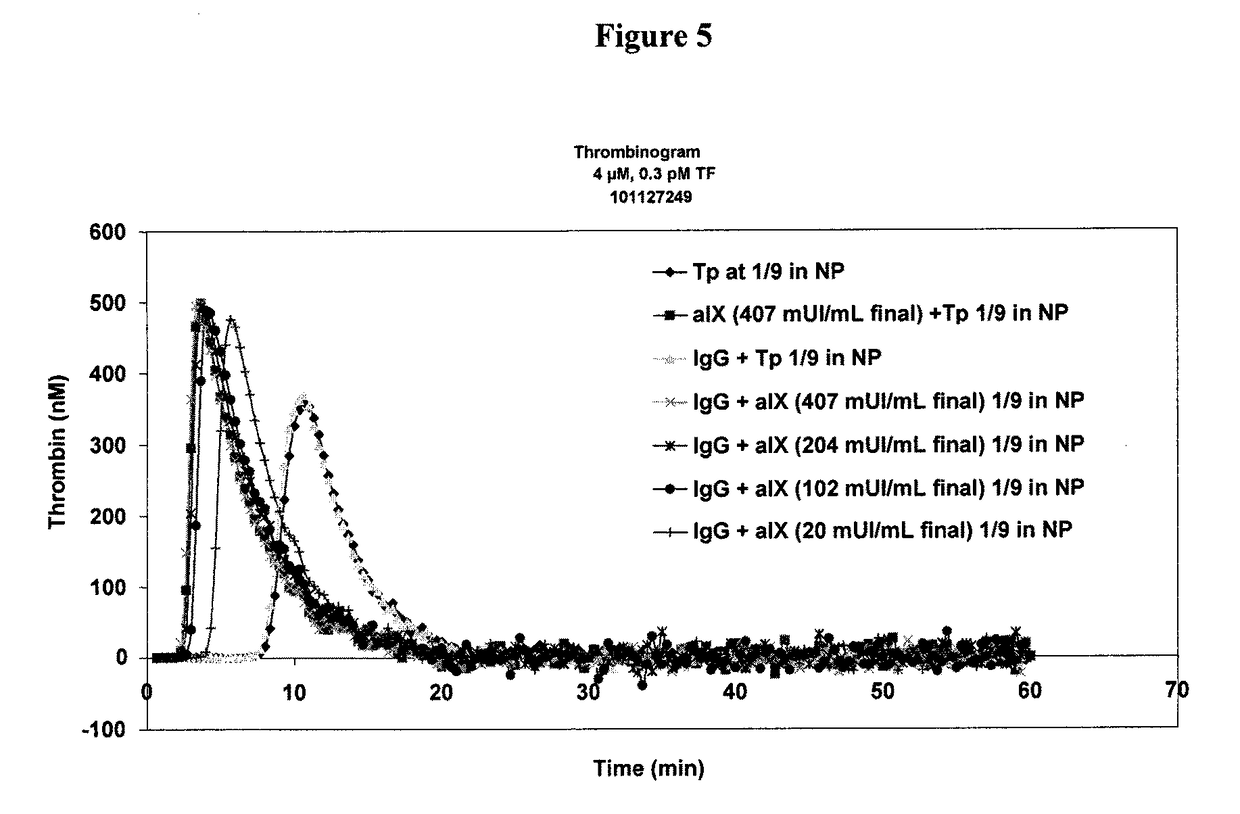
Determination of the thrombogenic power of human immunoglobulins
ActiveUS9891238B2Microbiological testing/measurementBiological material analysisFactor iiActivated factor XI
A kit for the determination of the thrombogenic power of human immunoglobulins contained in a biologically acceptable product. Also a process making it possible to determine the thrombogenic power linked to the presence of activated Factor XI and / or activated Factor IX and / or activated Factor XII, and / or activated Factor VII and / or activated Factor X in a sample capable of being administered to humans.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Assessment, determination and treatment of pkal-mediated disorders
The present invention provides assays for the detection of plasma protease C1 inhibitors (C1-INH) that bind plasma kallikrein, factor XII, or both, and their use to identify enzymes that are pKal-mediated or bradykinin-mediated. Risk of a disorder or use in a subject with a pKal-mediated or bradykinin-mediated disorder. The provided methods allow analysis of patients with plasma kallikrein-mediated angioedema (KMA) or other diseases mediated by pKal that can be used for evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
Use of anti-factor XII antibodies for treating or preventing hereditary angioedema
PendingCN114761437AReduce the risk of seizuresImmunoglobulins against blood coagulation factorsAntibody ingredientsAntiendomysial antibodiesHereditary angioedema
The present invention relates to an anti-FXII antibody for use in a method of treating or preventing hereditary angioedema (HAE) in a subject in which said antibody is administered subcutaneously to said subject.
Owner:シーエスエル イノベーション プロプライアタリー リミティド
Factor xii (Hagemann factor) (f12), kallikrein b, plasma (Fletcher factor) 1 (klkb1) and kininogen 1 (kng1) irna compositions and methods of use thereof
ActiveCN108271386BOrganic active ingredientsMicrobiological testing/measurementContact activationGenetics
The present invention relates to RNAi agents targeting kallikrein B, plasma (Fletcher factor) 1 (KLKB1) gene, factor XII (Hagemann factor) (F12) gene, or kininogen 1 (KNG1) gene, Such as double-stranded RNAi agents, and methods of using these RNAi agents to inhibit the expression of the KLKB1 gene, F12 gene, and / or KNG1 gene, and to treat patients with hereditary angioedema (HAE) and / or disorders associated with the contact activation pathway tester's method.
Owner:ALNYLAM PHARMA INC
METHODS FOR TREATING OR PREVENTING CONTACT-ACTIVATION PATHWAY-ASSOCIATED DISEASES USING iRNA COMPOSITIONS TARGETING FACTOR XII (HAGEMAN FACTOR) (F12)
InactiveUS20220267767A1Reduce depositionCardiovascular disorderDNA/RNA fragmentationDiseaseContact activation
The present invention relates to methods of use of RNAi agents, e.g., double stranded RNAi agents, targeting a Factor XII (Hageman Factor (F12) gene, for treating subjects having a contact activation pathway-associated disease, such as a thrombophilia or hereditary angioedema (HAE), methods for preventing at least one symptom in a subject having a contact activation pathway-associated disease, such as a thrombus formation or an angioedema attack, and RNAi agents targeting an F12 gene, for use in the methods of the invention.
Owner:ALNYLAM PHARMA INC
Compositions and methods for inhibiting gene expression of factor xii
Owner:ARROWHEAD RES CORP
FACTOR XIIa INHIBITORS
PendingCN112204023AOrganic active ingredientsOrganic chemistryFactor XIIa inhibitorHematological Diseases
This invention relates to compounds of formula (I). The compounds of formula (I) are modulators of Factor XII, specifically Factor XIIa. The compounds are inhibitors of Factor XIIa and may be useful as anticoagulants. The compounds of formula (I) may be used in methods of treatment (or prevention) of blood disorders related to bleeding or coagulation.
Owner:UNIV OF LEEDS
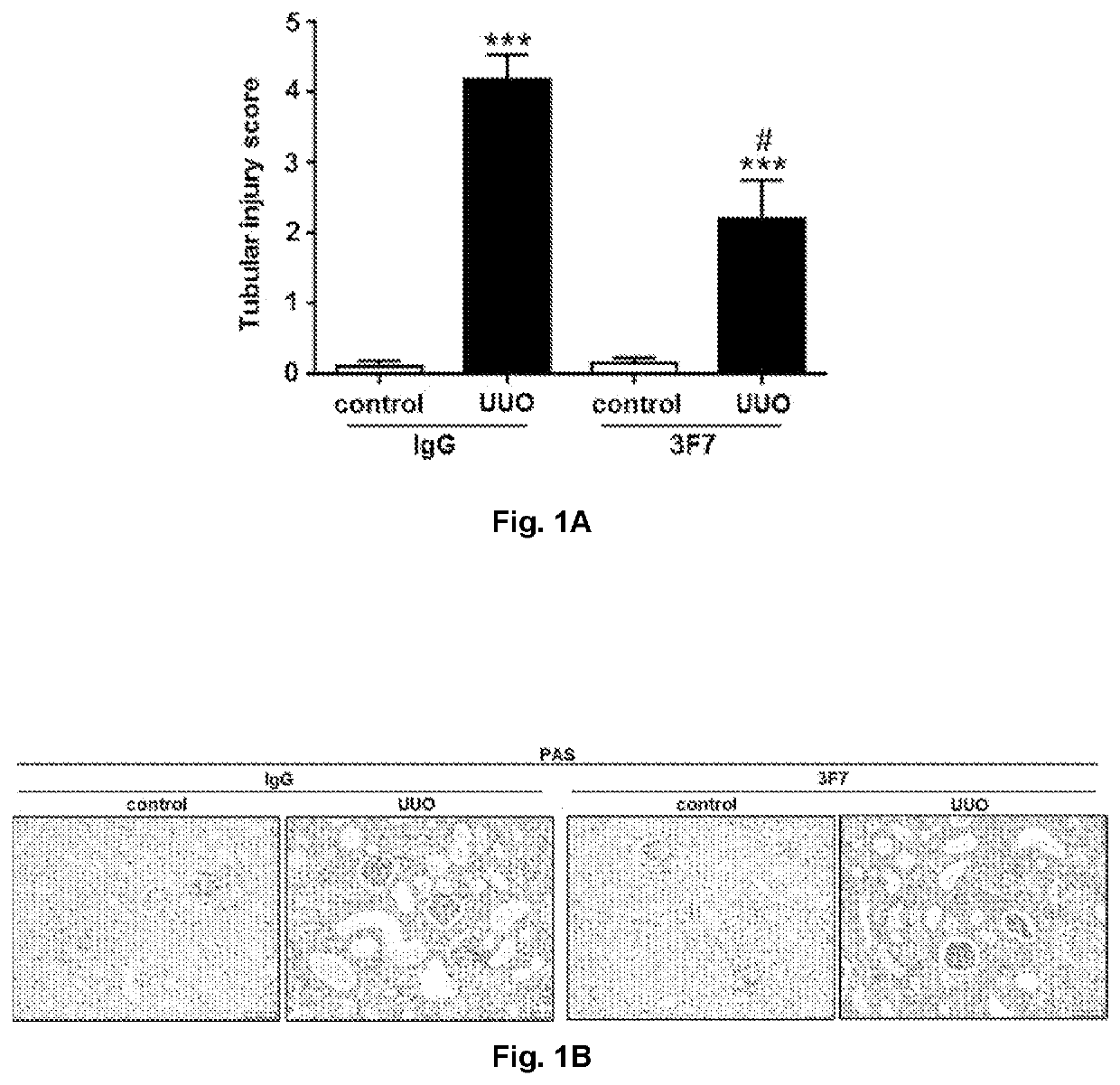
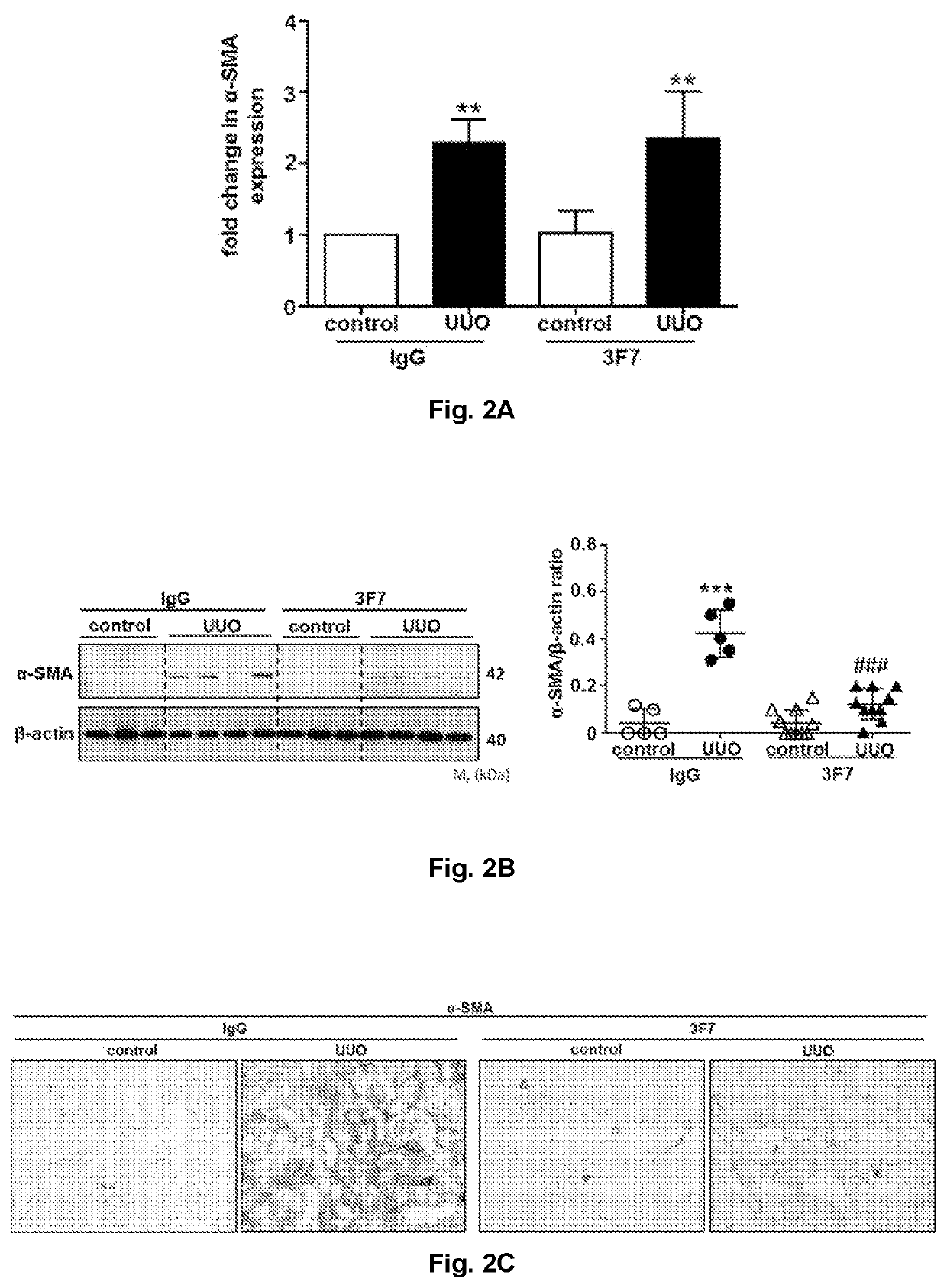
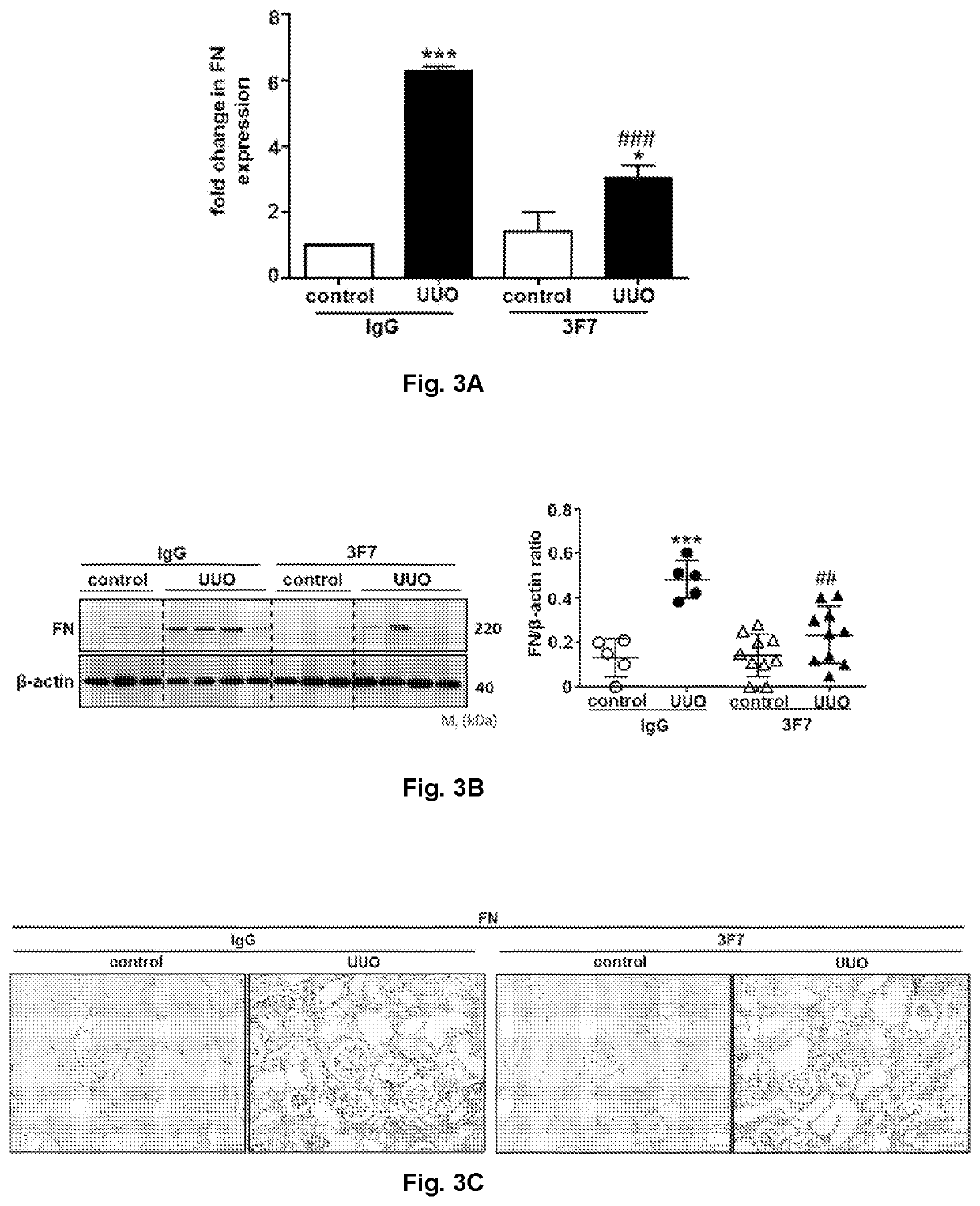
Use of a FXIIa-inhibitor in the treatment of renal fibrosis and/or chronic kidney disease
ActiveUS11505619B2Lower potentialInhibiting FXIIaImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntigenDisease
The invention relates to an inhibitor of Factor XII (FXII) for use in treating or preventing chronic kidney disease, renal fibrosis, glomerulosclerosis, renal scarring, ischemia / reperfusion injury in native or transplant kidneys and / or acute kidney injury, a kit for use in treating or preventing chronic kidney disease, renal fibrosis, glomerulosclerosis, renal scarring, ischemia / reperfusion injury in native or transplant kidneys, acute kidney injury, renal fibrosis as a result of rejection of a kidney transplant / allograft, and / or fibrosis of a kidney transplant / allograft as a result of rejection or recurrent underlying disease comprising one inhibitor of Factor XII, and an anti-Factor XII (FXII) antibody or antigen binding fragment thereof for use in treating or preventing chronic kidney disease, renal fibrosis, glomerulosclerosis, renal scarring, ischemia / reperfusion injury in native or transplant kidneys and / or acute kidney injury comprising one inhibitor of Factor XII.
Owner:CSL LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com