Use of anti-factor XII antibodies for treating or preventing hereditary angioedema
An angioedema, -FXII technology, applied in the direction of anti-coagulation factor immunoglobulin, antibody, anti-animal/human immunoglobulin, etc., can solve the problem that the effective treatment or prevention of anti-factor XII mAb is not clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
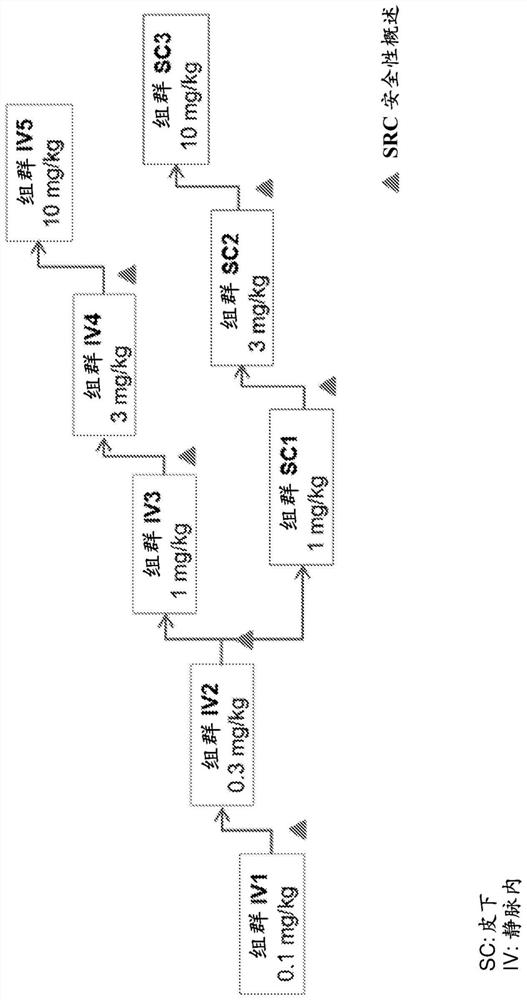
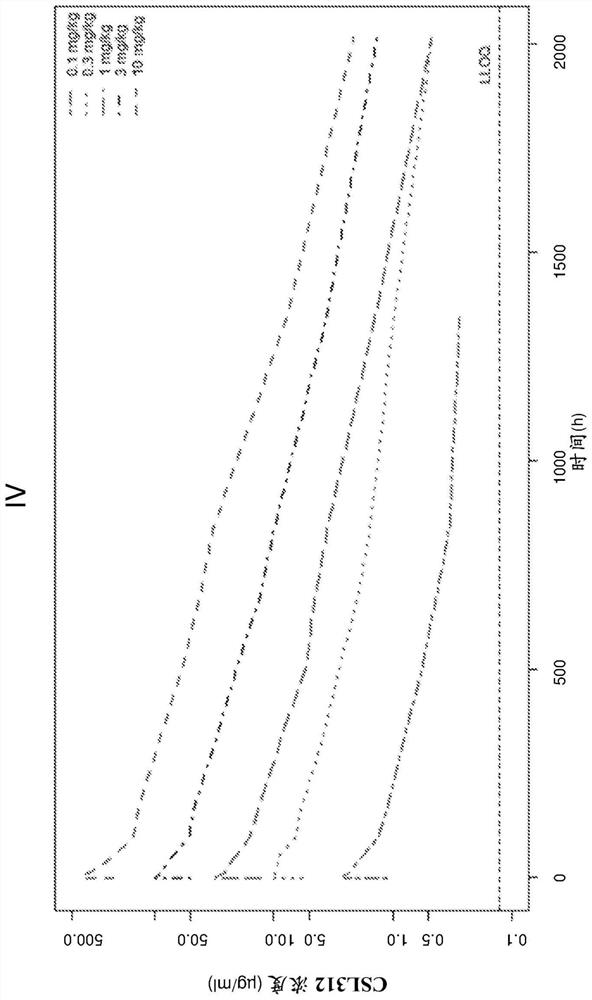
Examples
Embodiment Construction
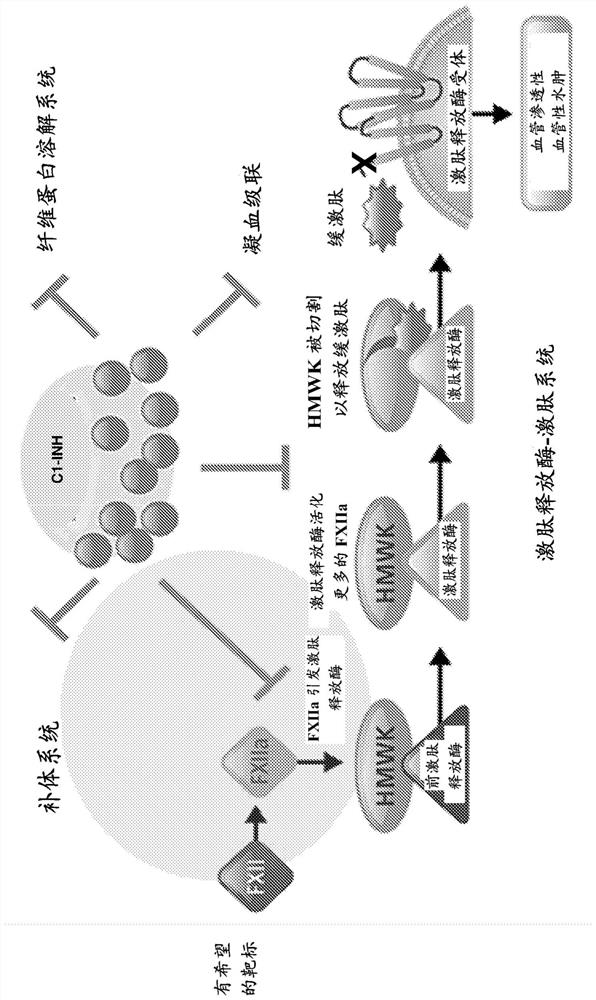
[0032] According to the invention, "anti-FXII antibodies" bind and inhibit the activated form of FXII, ie FXIIa-beta (beta-factor XIIa), but also bind FXII and FXIIa (alpha-factor XIIa).
[0033] An "antibody" in its broadest sense is a polypeptide comprising an immunoglobulin variable region that specifically recognizes an epitope on an antigen. The term "antibody" also includes antibody fragments that maintain the ability to bind FXIIa and FXII. Preferred antigen-binding fragments are Fab fragments, Fab' fragments, F(ab') 2 Fragments, Fv fragments, single chain antibodies, single chain Fv fragments, disulfide stabilized Fv proteins or dimers of single chain Fv fragments. Also included in the present invention are antibodies that are chimeric antibodies, humanized antibodies, murine antibodies or bispecific antibodies. Methods for producing these fragments and antibodies are well known in the art (see, eg, Harlow and Lane: Antibodies, A Laboratory Manual, Cold Spring Harbor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com