Patents
Literature
42 results about "Angioedema" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition presenting as severe swelling under the skin surface.
Fusion molecules and treatment of IgE-mediated allergic diseases
InactiveUS20030082190A1Negatively expressionFind utility in the treatmentSenses disorderFungiLate phaseVaccination
The invention concerns bifunctional fusion molecules for the treatment of IgE-mediated allergic conditions and FcepsiRI-mediated autoimmune conditions. The invention provides a new therapeutic approach for the treatment of both acute and late-phase allergic responses due to ingestion, inhalation, cutaneous and parenteral exposure to allergens, responses including asthma, allergic rhinitis, atopic dermatitis, severe food allergies, chronic urticaria and angioedema, as well as anaphylactic reactions due to exposures such as bee stings or penicillin allergy. In addition, the invention provides for a novel, safer and more efficacious form of allergy vaccination.
Owner:RGT UNIV +1
Fusion molecules and treatment of IgE-mediated allergic diseases
The invention concerns bifunctional fusion molecules for the treatment of IgE-mediated allergic conditions and FcεRI-mediated autoimmune conditions. The invention provides a new therapeutic approach for the treatment of both acute and late-phase allergic responses due to ingestion, inhalation, cutaneous and parenteral exposure to allergens, responses including asthma, allergic rhinitis, atopic dermatitis, severe food allergies, chronic urticaria and angioedema, as well as anaphylactic reactions due to exposures such as bee stings or penicillin allergy. In addition, the invention provides for a novel, safer and more efficacious form of allergy vaccination.
Owner:RGT UNIV OF MINNESOTA +1
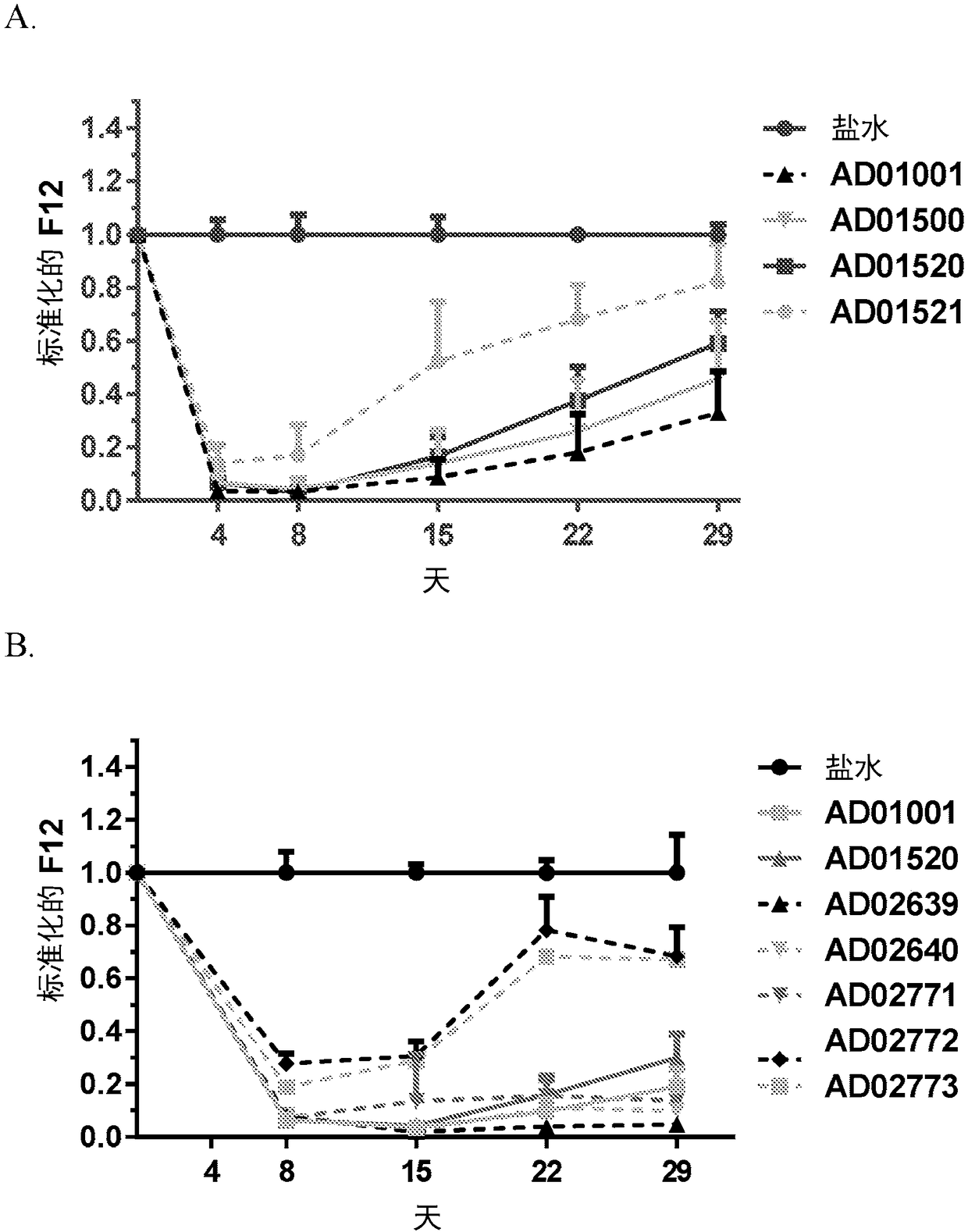
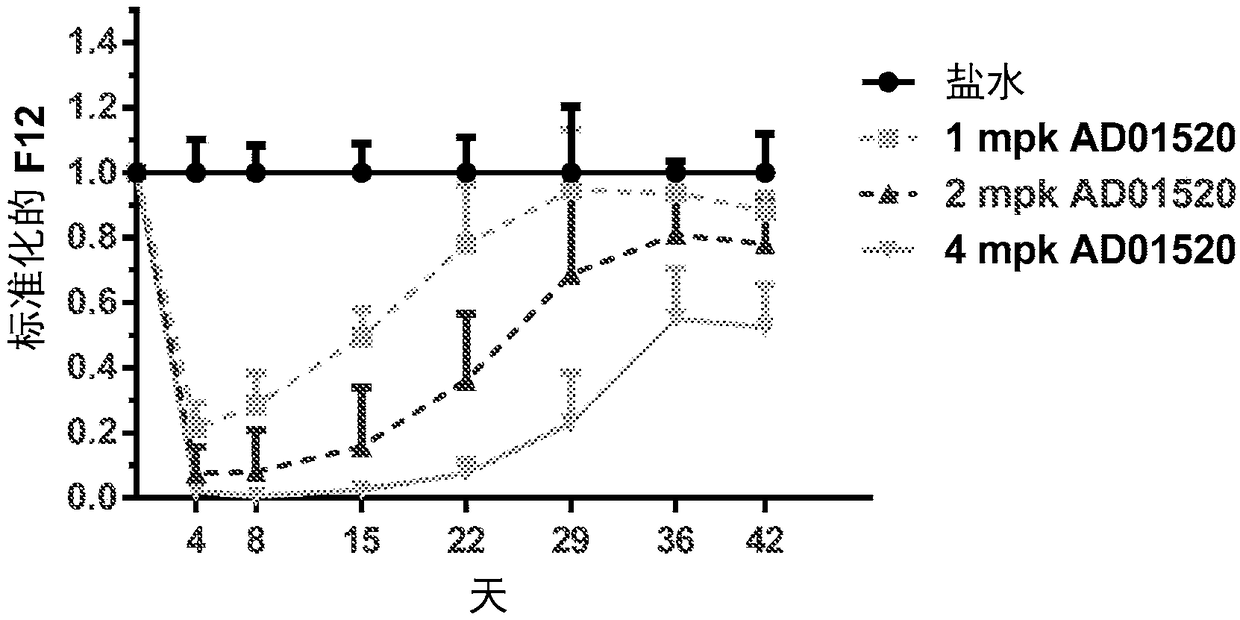
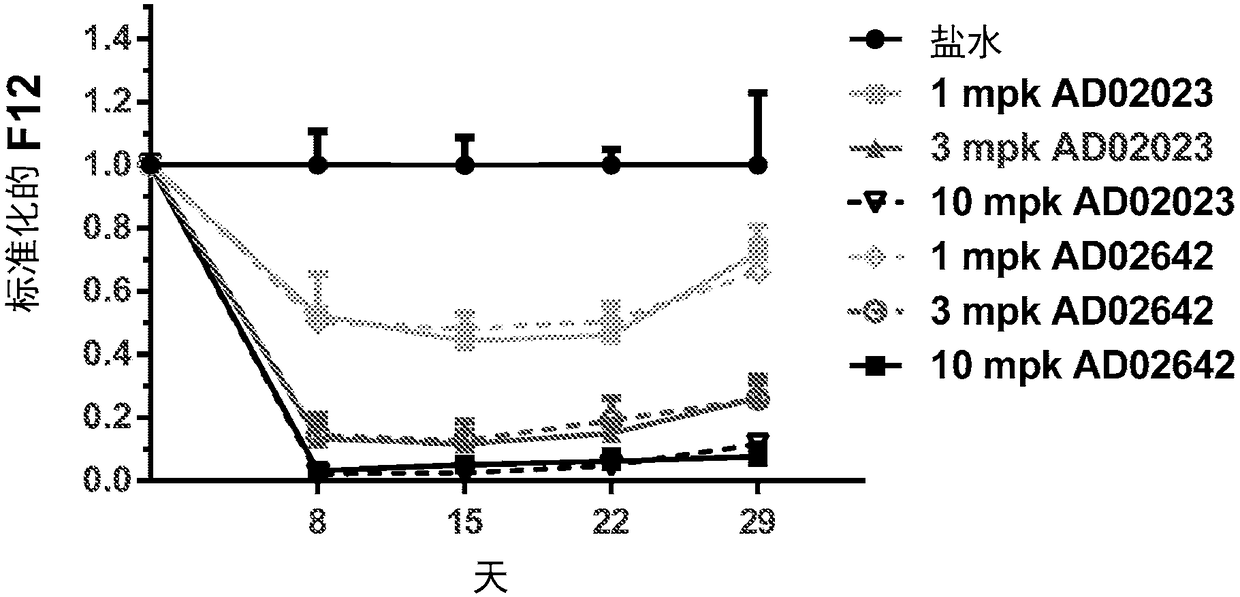
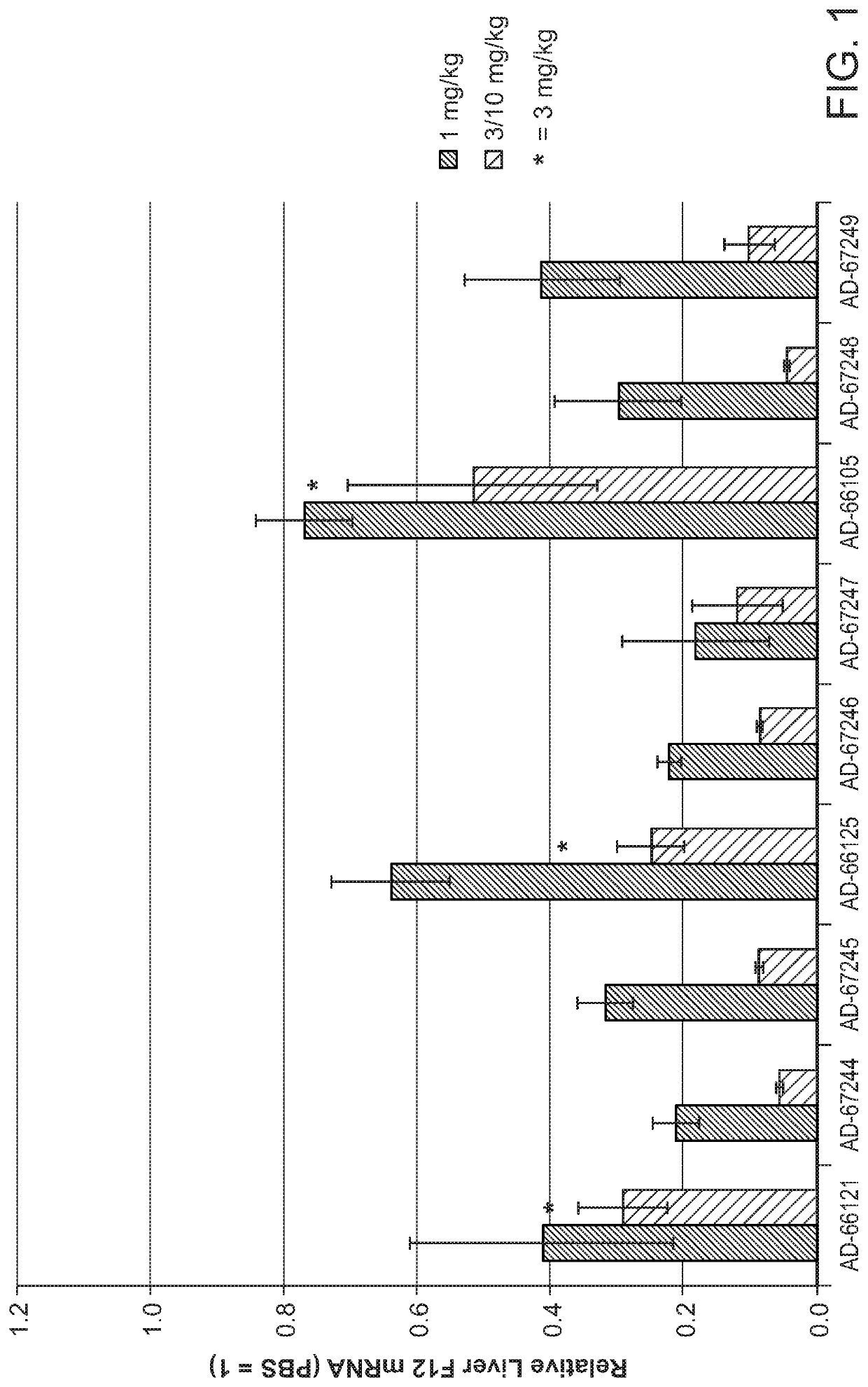
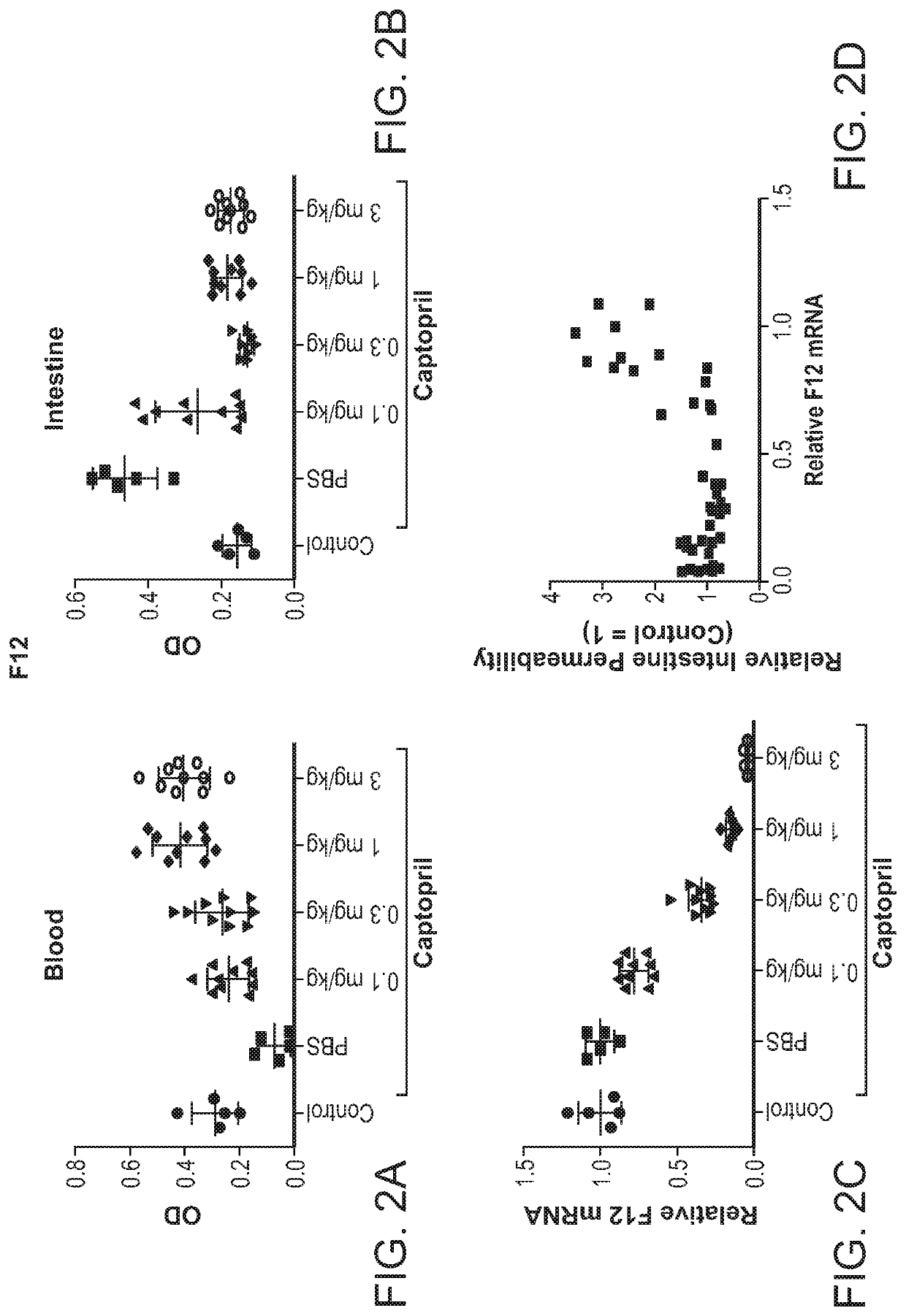
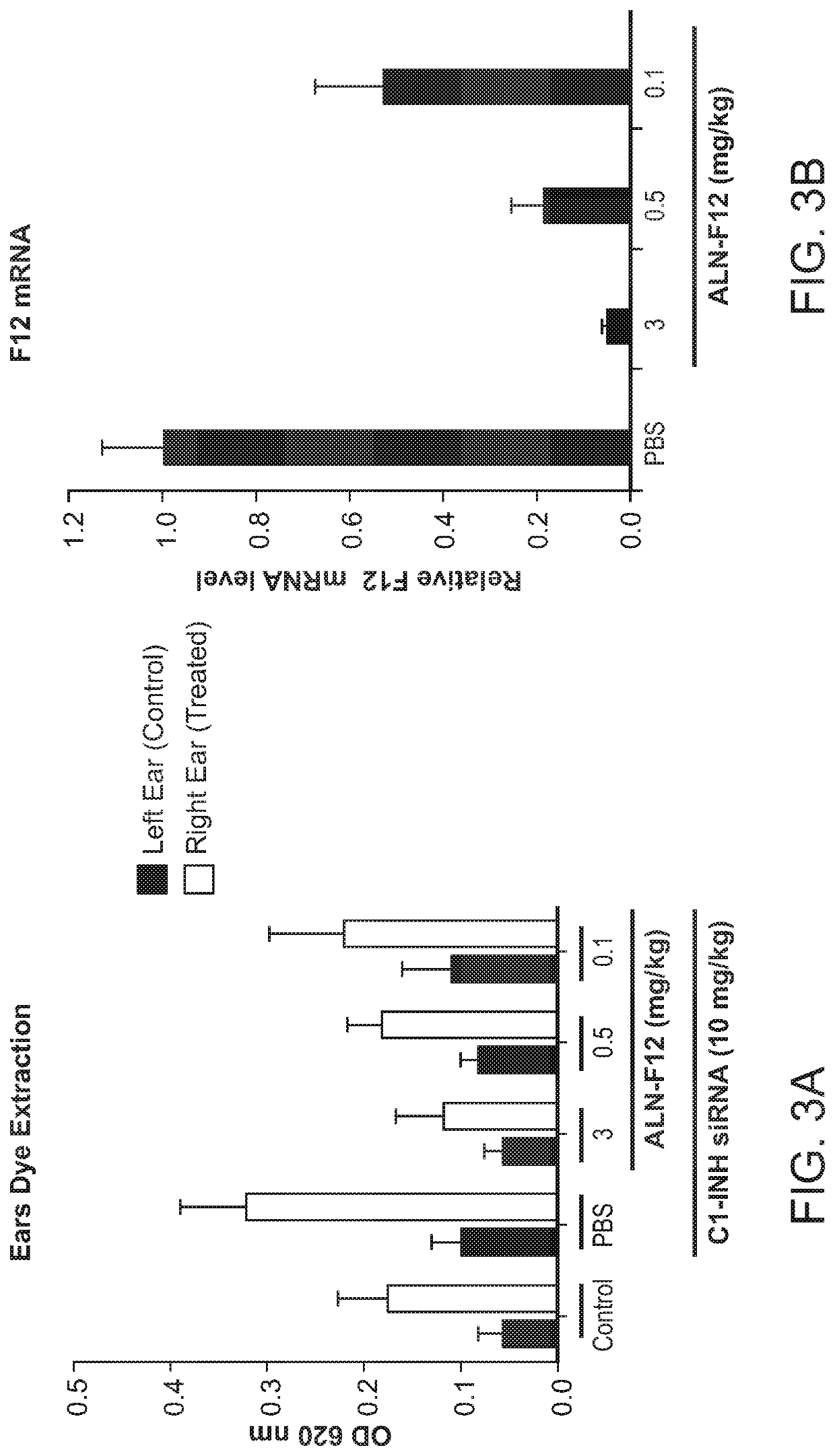
Compositions and methods for inhibiting gene expression of factor XII
ActiveCN108064313AOrganic active ingredientsMicrobiological testing/measurementDiseaseHereditary angioedema
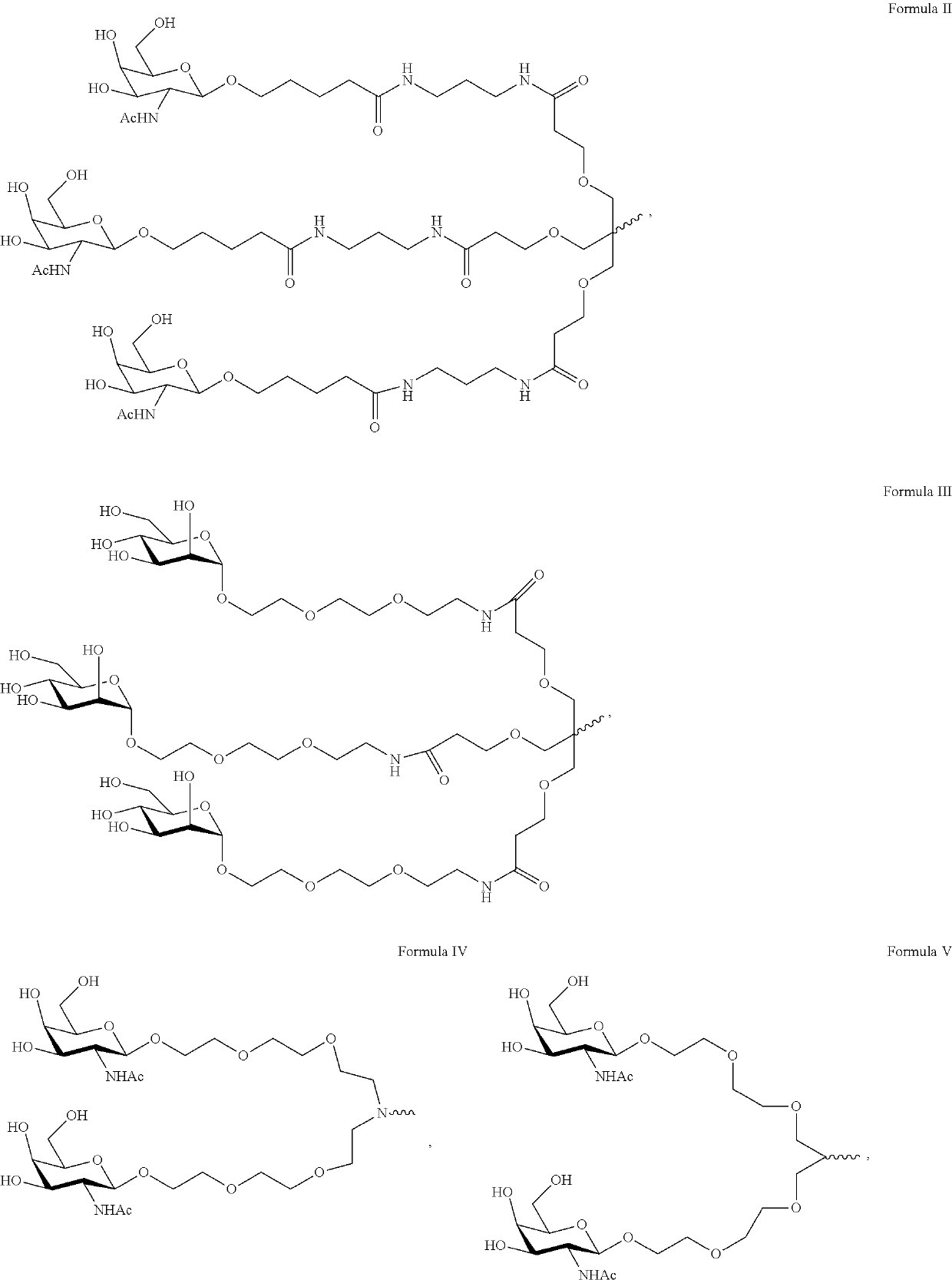
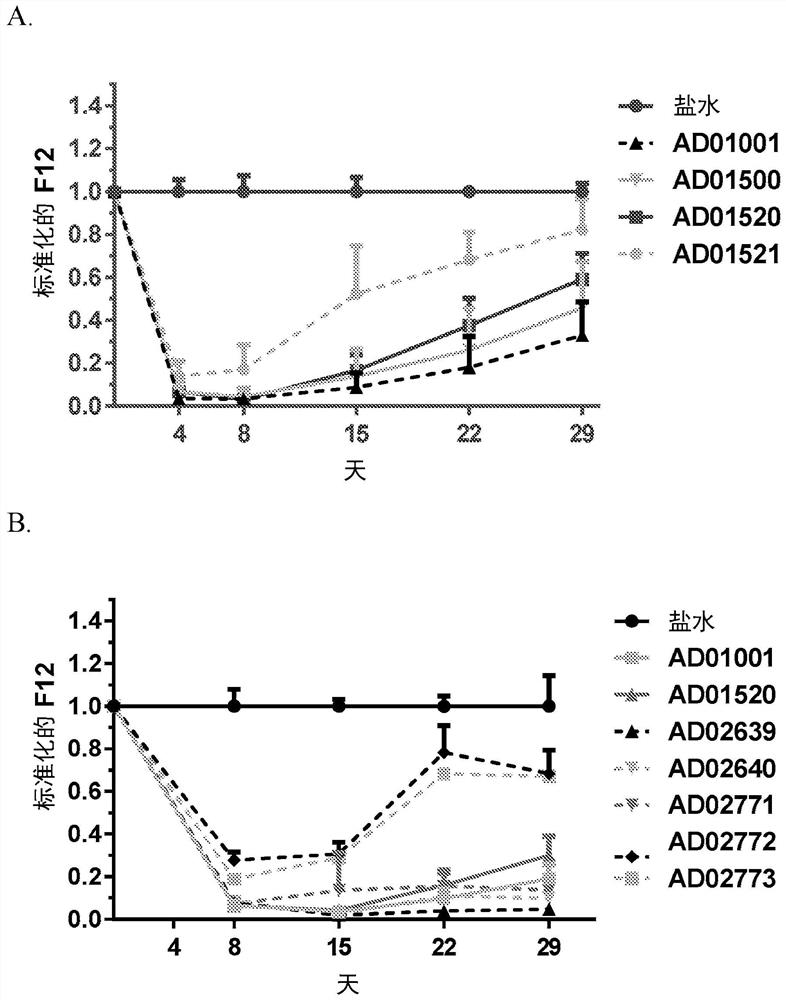
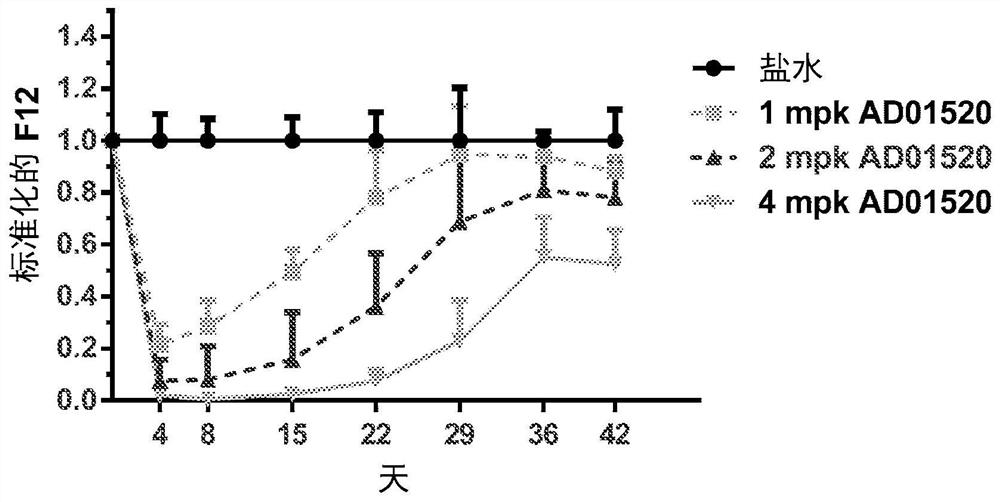
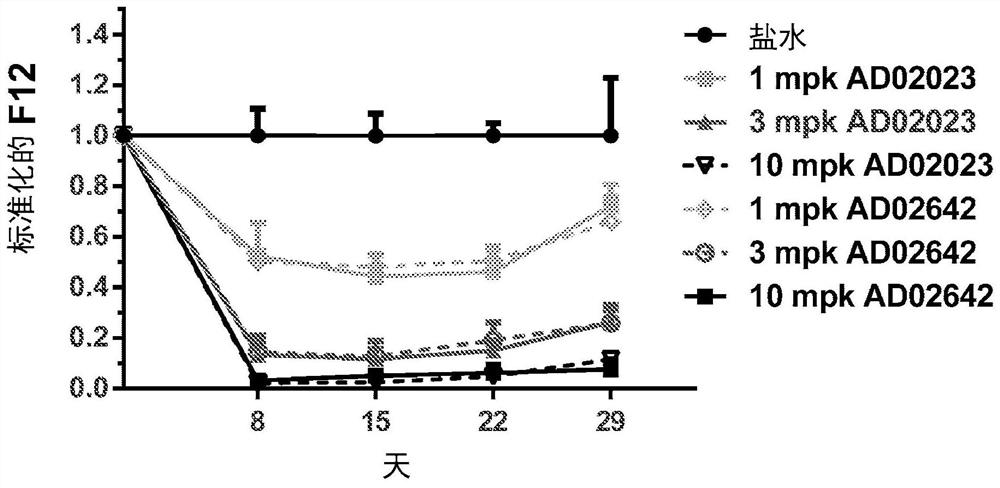
RNA interference (RNAi) triggers for inhibiting the expression of Factor XII (F12) gene through the mechanism of RNA interference are described. Pharmaceutical compositions comprising one or more F12RNAi triggers together with one or more excipients capable of delivering the RNAi trigger(s) to a liver cell in vivo are also described. Delivery of the F12 RNAi trigger(s) to liver cells in vivo provides for inhibition of F12 gene expression and treatment of angioedema, including hereditary angioedema (HAE) and venous thromboembolism (VTE), and diseases associated with angioedema.
Owner:ARROWHEAD RES CORP
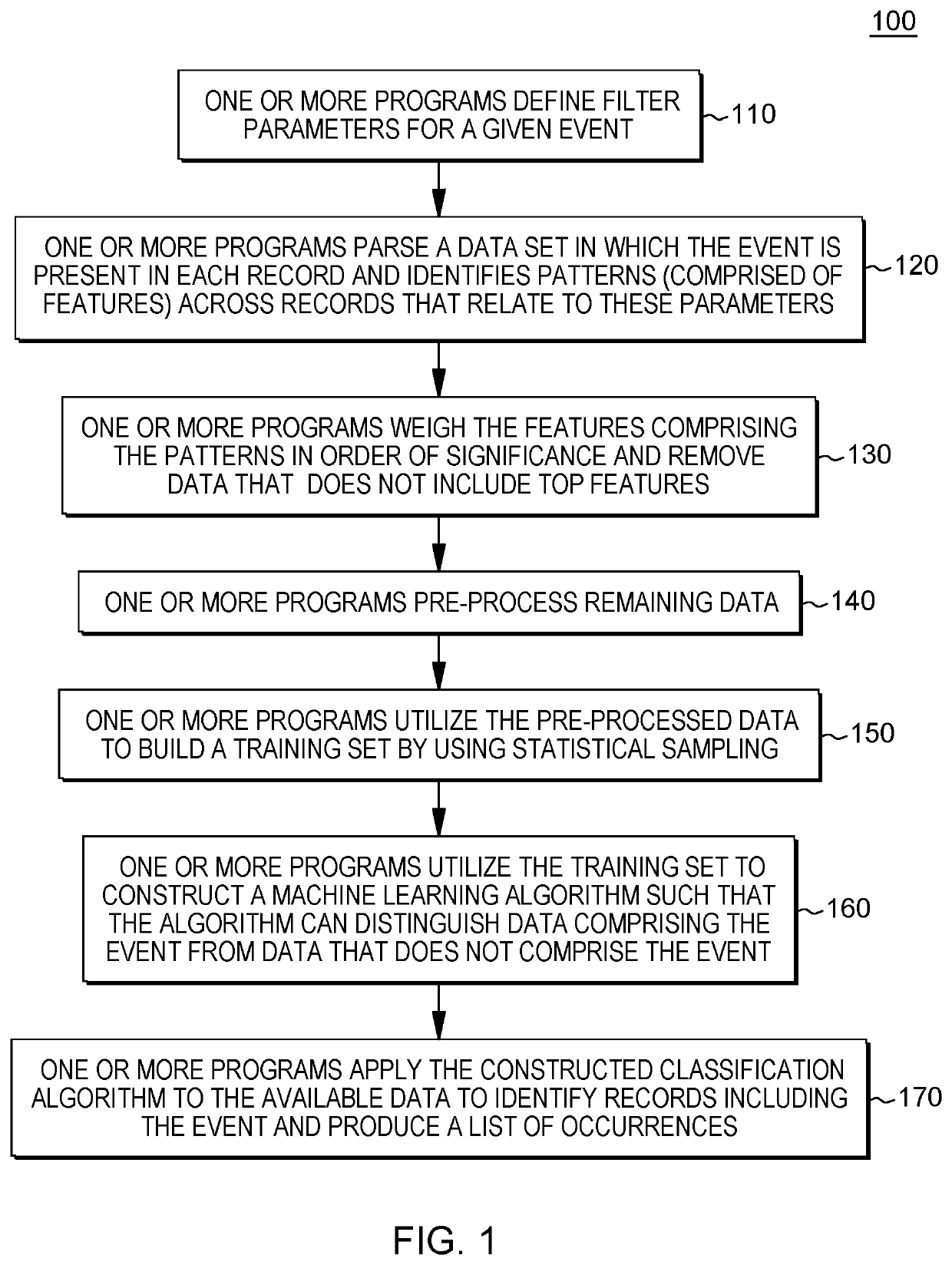
Machine-learning based query construction and pattern identification for hereditary angioedema
PendingUS20210193320A1Maximize efficiencyImprove accuracyMedical data miningDatabase distribution/replicationData setPatient data
A method, computer program product, and system identifying a probability of a medical condition in a patient. The method includes a processor obtaining data set(s) related to a patient population diagnosed with a medical condition and based on a frequency of features in the data set(s), identifying common features and weighting the common features based on frequency of occurrence in the data set(s) to generate mutual information. The processor generates pattern(s) including a portion of the common features to generate a machine learning algorithm(s). The processor compiles a training set of data to use to tune the machine learning algorithm(s). The processor dynamically adjusts common features in the pattern(s) such that the machine learning algorithm(s) can distinguish patient data indicating the medical condition from patient data not indicating the medical condition. The processor applies the machine learning algorithm(s) to data related to the undiagnosed patient, to determine the probability.
Owner:HVH PRECISION ANALYTICS LLC
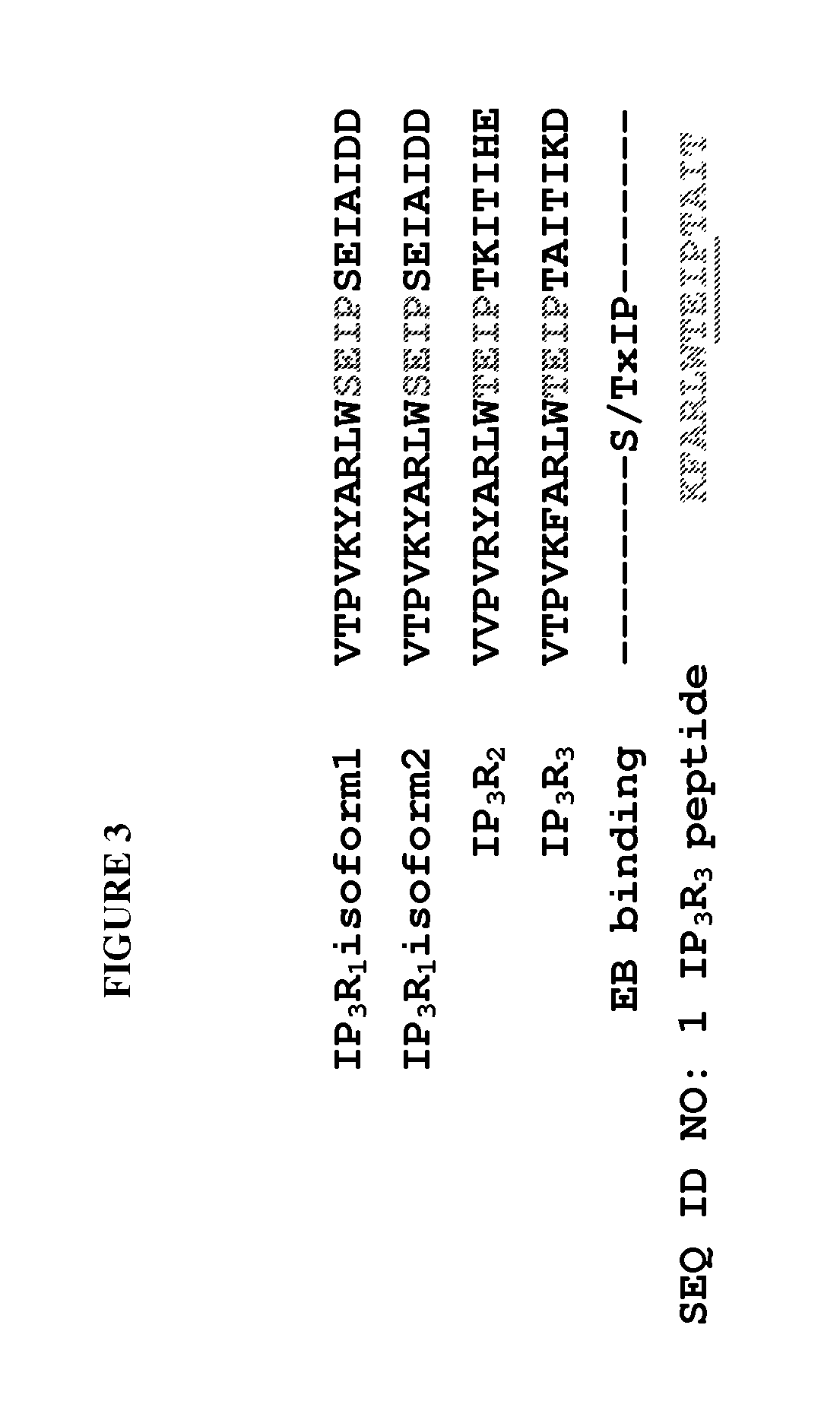
Peptide compositions and methods for treating lung injury, asthma, anaphylaxis, angioedema, systemic vascular permeability syndromes, and nasal congestion
ActiveUS8912139B2Inflammatory-induced hyper-permeability of endothelial barrierImprove breathabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsALI - Acute lung injuryInositol
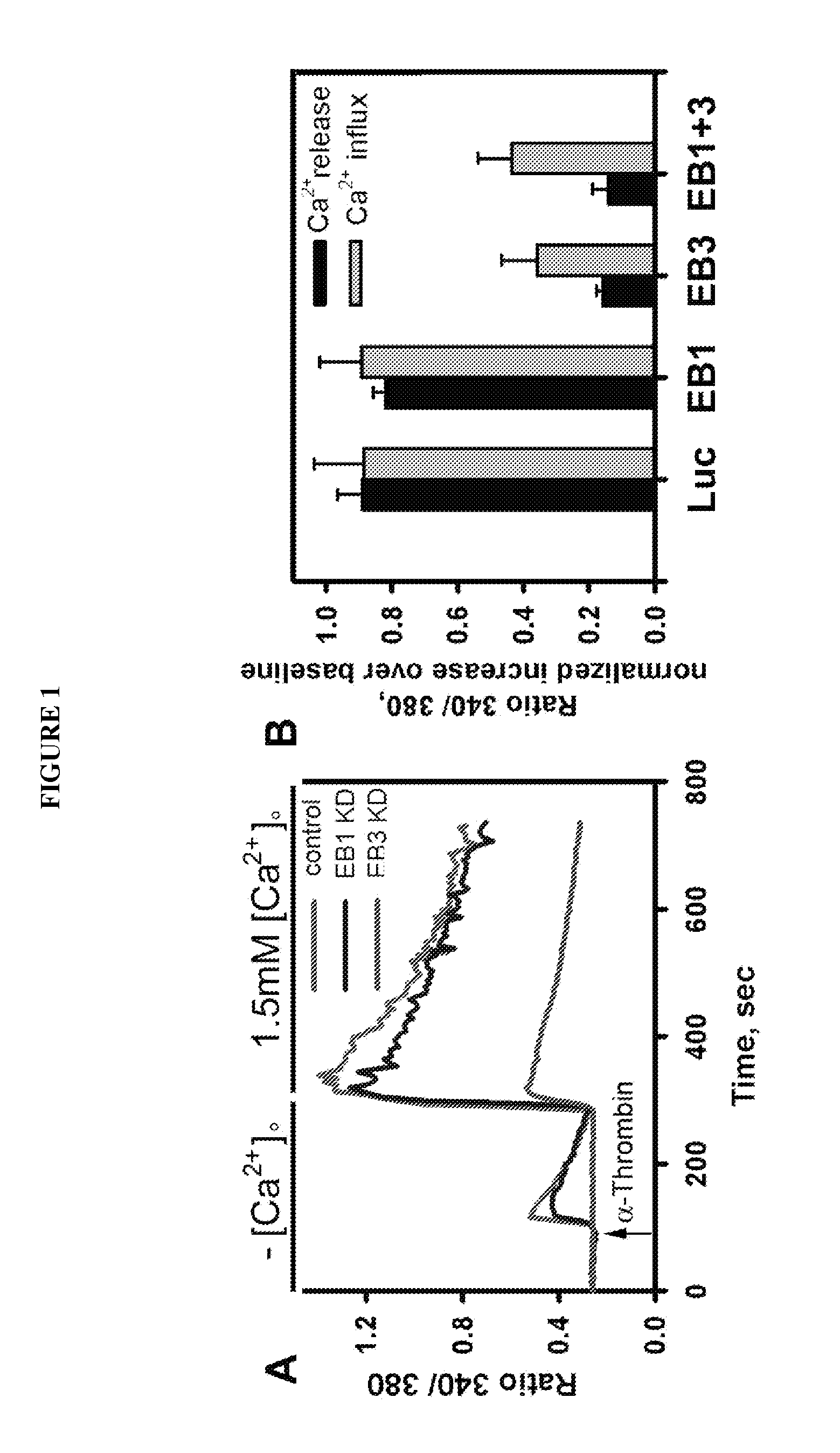
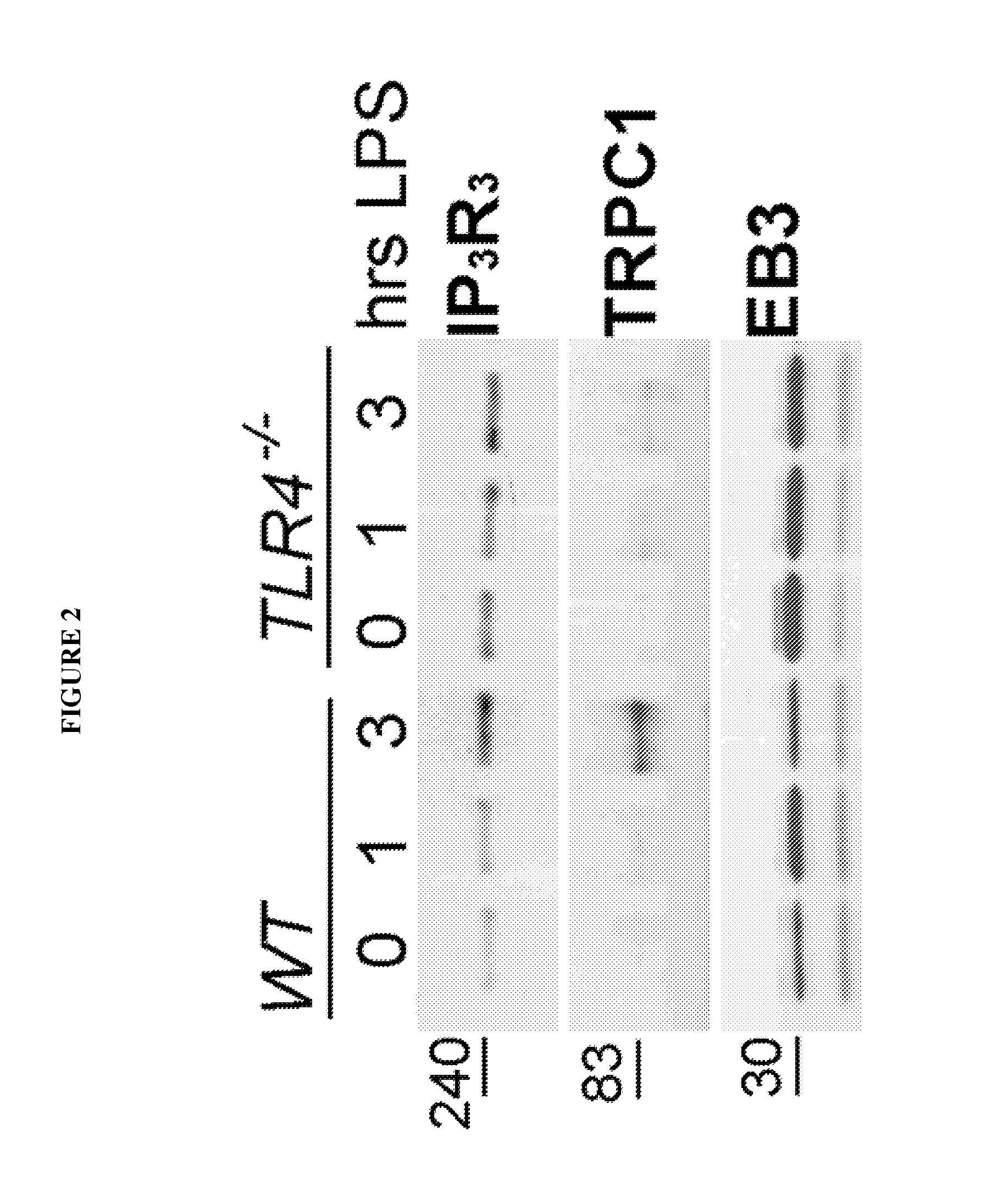
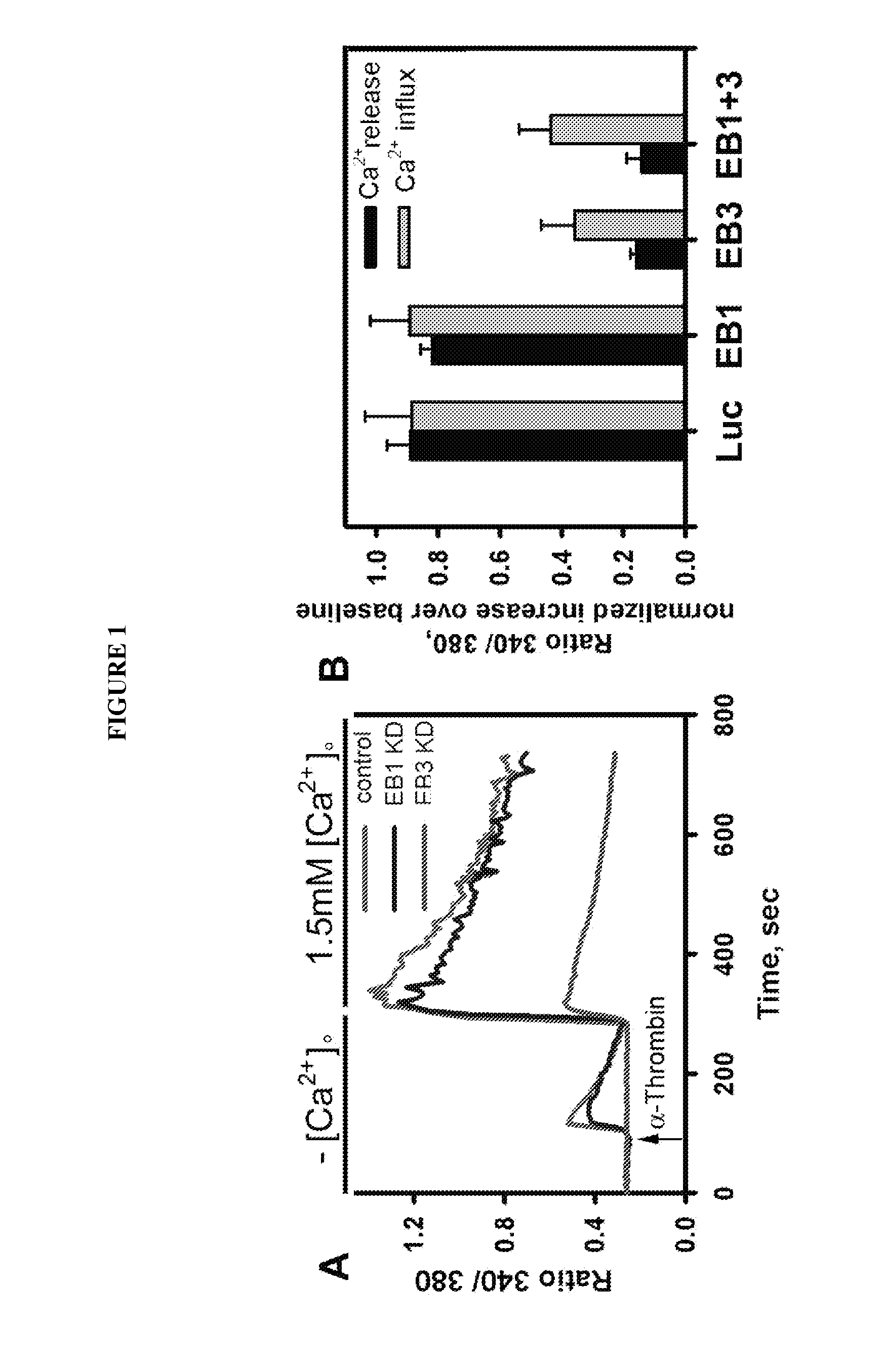
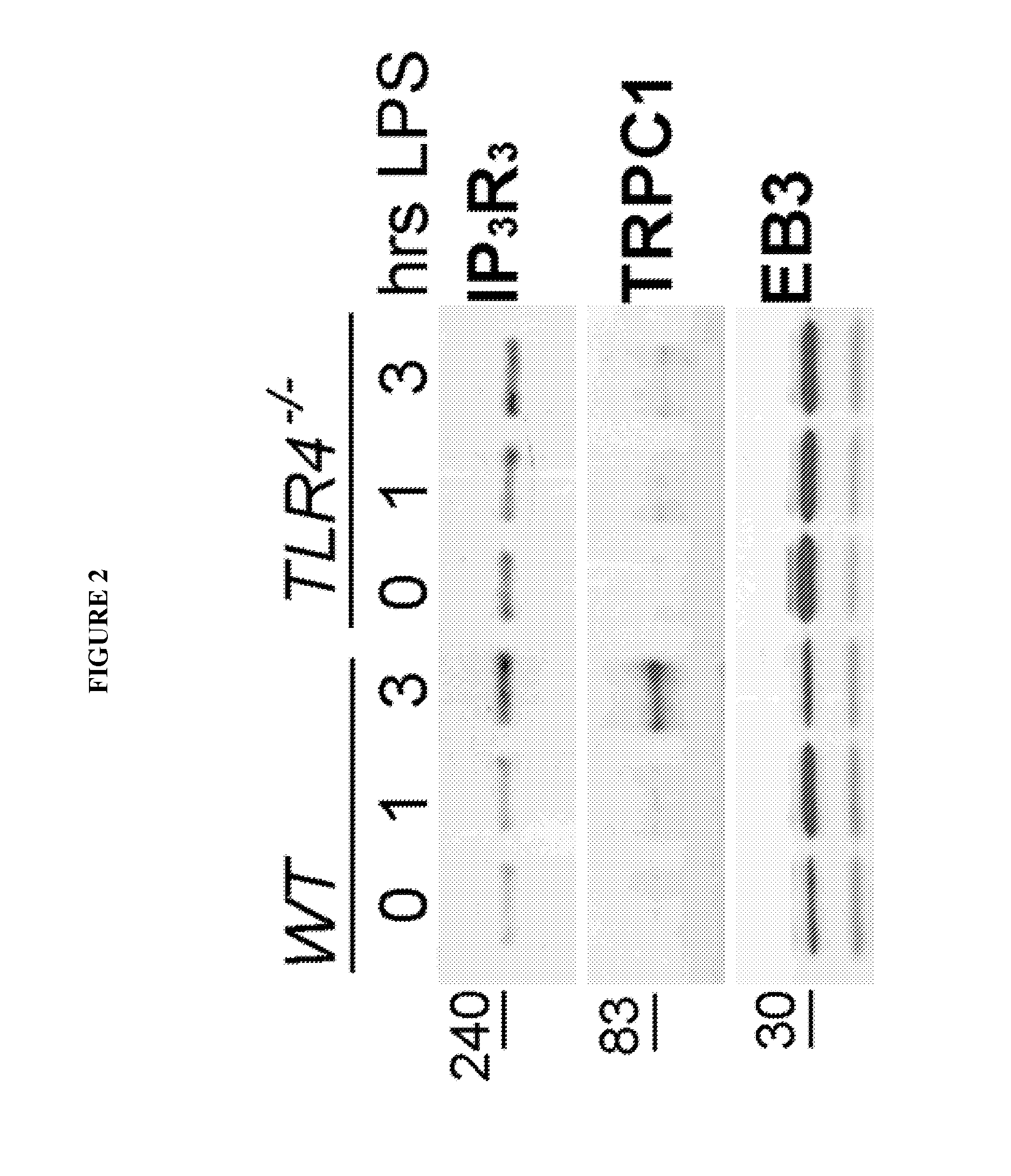
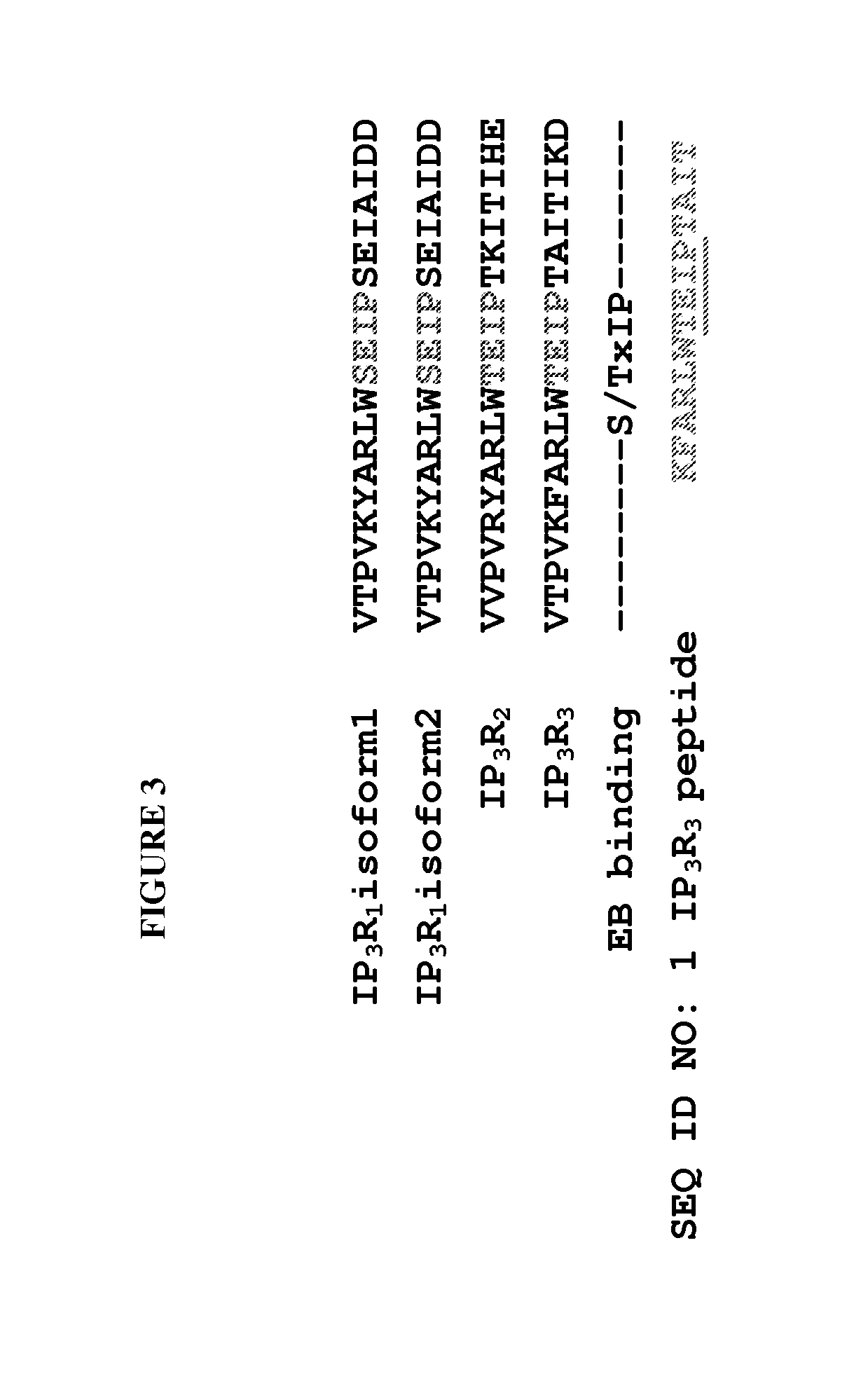
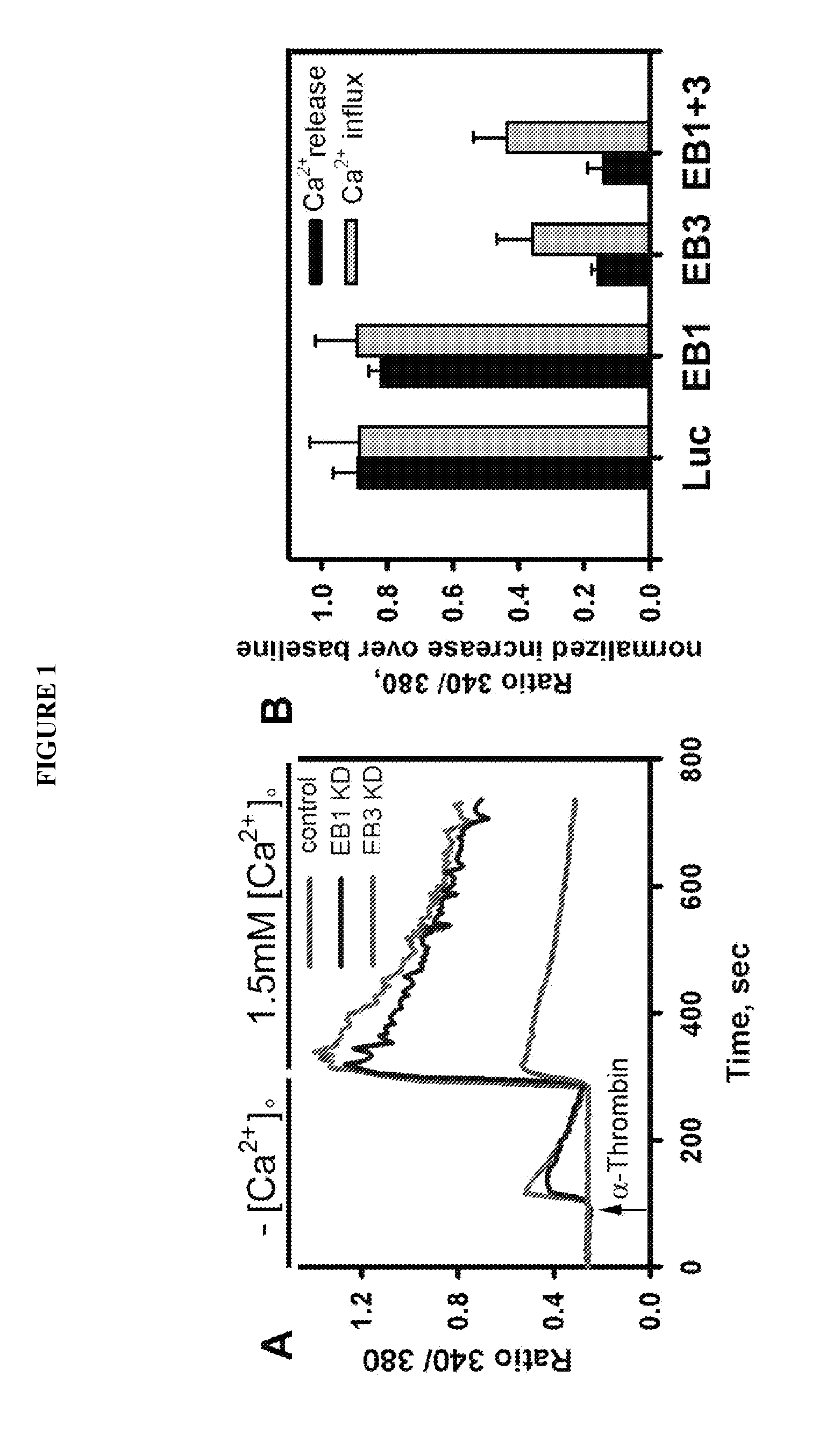
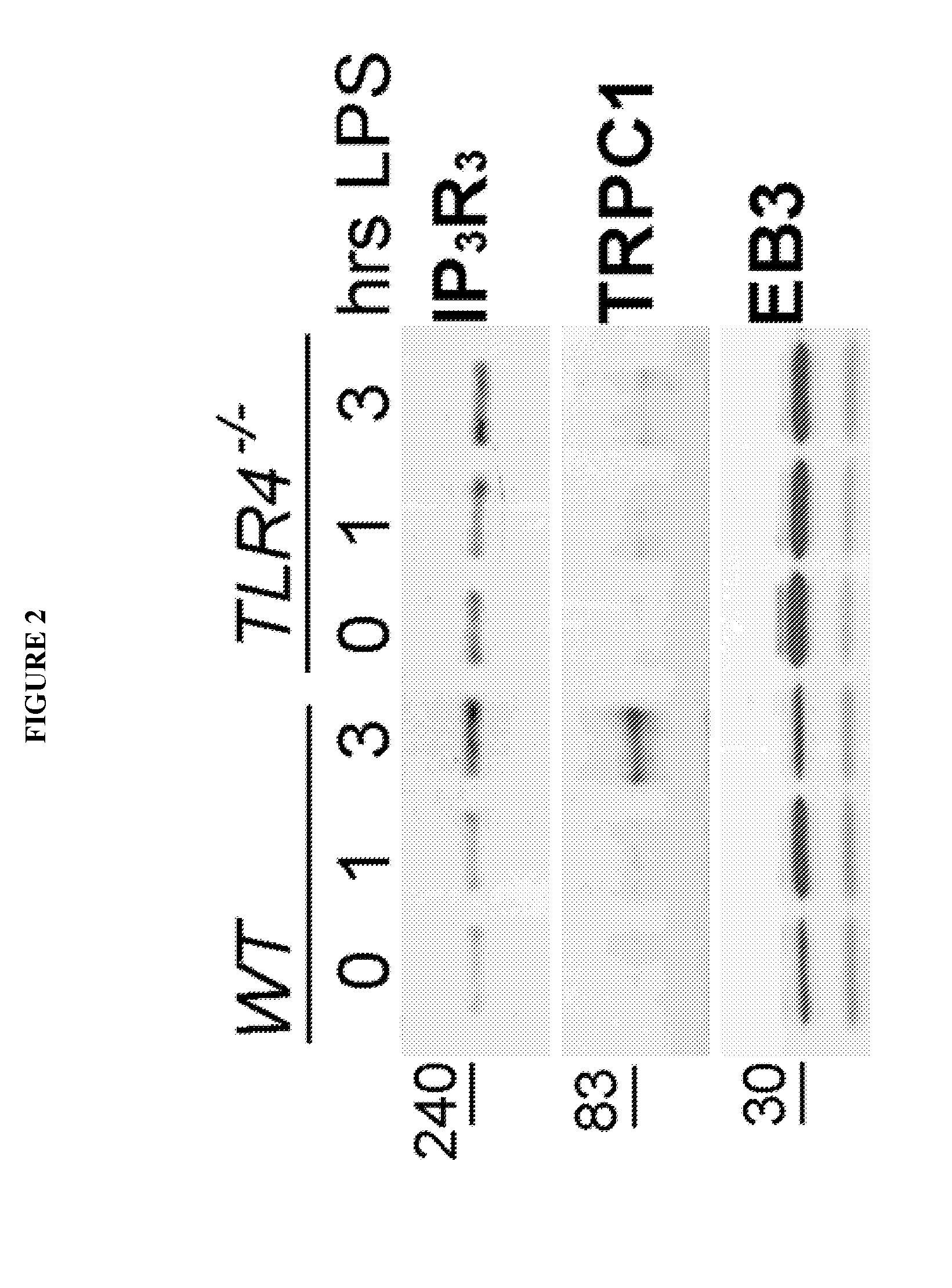
Provided herein are peptide inhibitors of the interaction between End Binding Protein 3 (EB3) and Inositol 1,4,5-Trisphosphate Receptor Type 3 (IP3R3). Also provided are methods and materials for treating lung injury, including acute lung injury, which may include hyperpermeability of lung vessels, vascular leakage, the development of edema, asthma, anaphylaxis, angioedema, systemic vascular permeability syndromes, and nasal congestion.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Nerve block medicinal composition
InactiveCN103877099AAvoid damageReduce toxicityAnaestheticsHeterocyclic compound active ingredientsGlucocorticoidAllergy
The invention relates to a nerve block medicinal composition. The nerve block medicinal composition consists of the following components in parts by volume: 10 parts of 0.75 percent ropivacaine, 2 parts of glucocorticoid and 13 parts of 0.9 percent normal saline. The nerve block medicinal composition has the advantages that dexamethasone is added into a formula, so that injuries to nerve roots and toxic reactions of local anesthetics in a puncturing process can be reduced; dexamethasone has the effects of resisting allergy, resisting angioedema and reducing the toxic reactions of local anesthetics, so that the nerve block success rate is increased, and toxic reactions are reduced.
Owner:王寿世
METHODS FOR TREATING OR PREVENTING CONTACT-ACTIVATION PATHWAY-ASSOCIATED DISEASES USING iRNA COMPOSITIONS TARGETING FACTOR XII (HAGEMAN FACTOR) (F12)
InactiveUS20200208150A1Reduce depositionCardiovascular disorderDNA/RNA fragmentationDiseaseContact activation
The present invention relates to methods of use of RNAi agents, e.g., double stranded RNAi agents, targeting a Factor XII (Hageman Factor (F12) gene, for treating subjects having a contact activation pathway-associated disease, such as a thrombophilia or hereditary angioedema (HAE), methods for preventing at least one symptom in a subject having a contact activation pathway-associated disease, such as a thrombus formation or an angioedema attack, and RNAi agents targeting an F12 gene, for use in the methods of the invention.
Owner:ALNYLAM PHARMA INC
Evaluation, assays and treatment of PKAL-mediated disorders
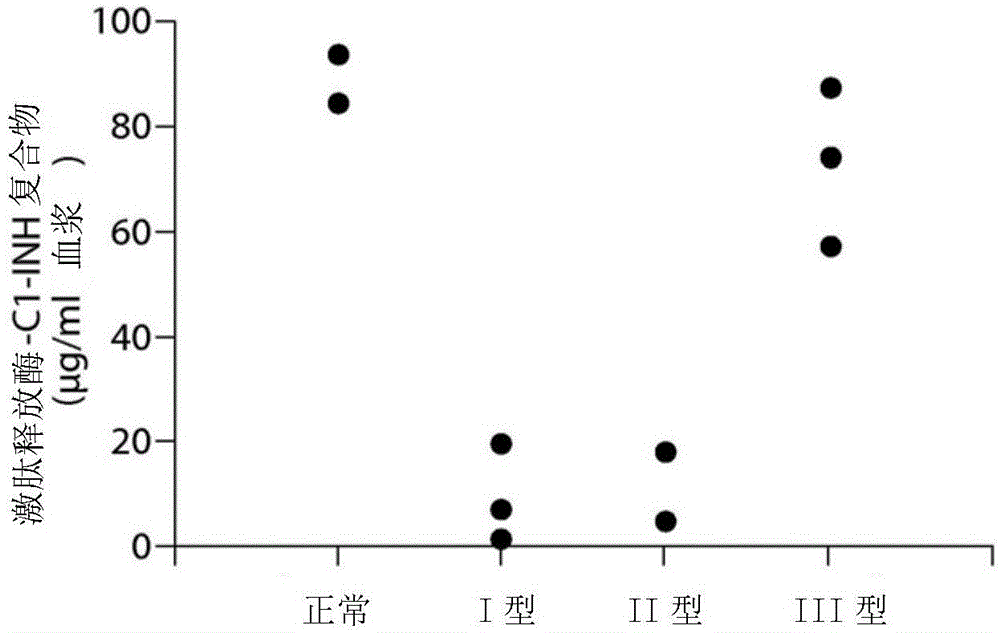
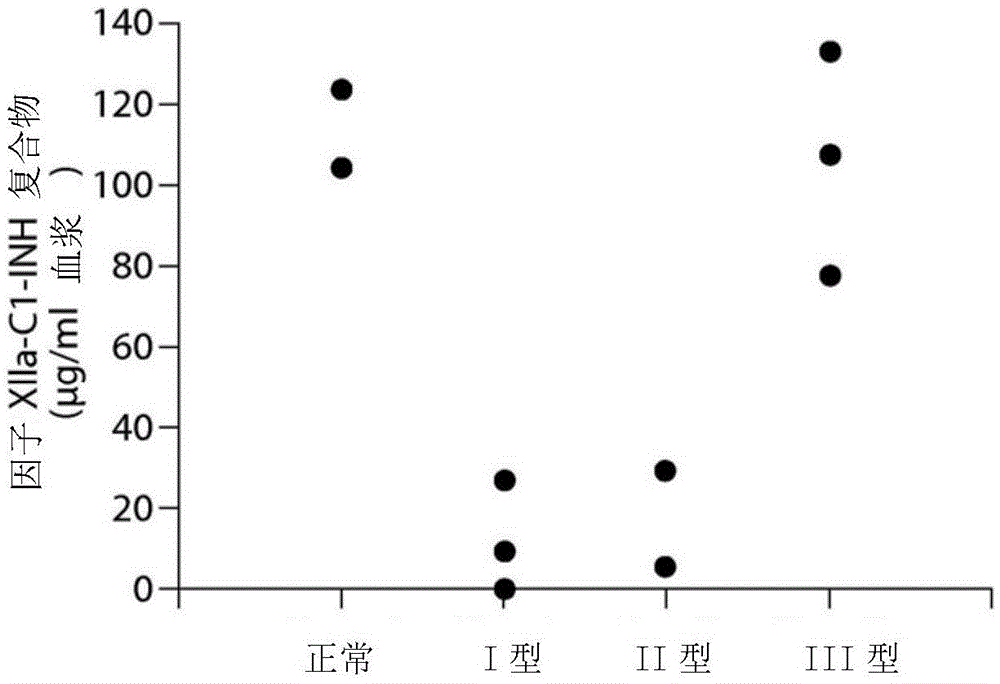
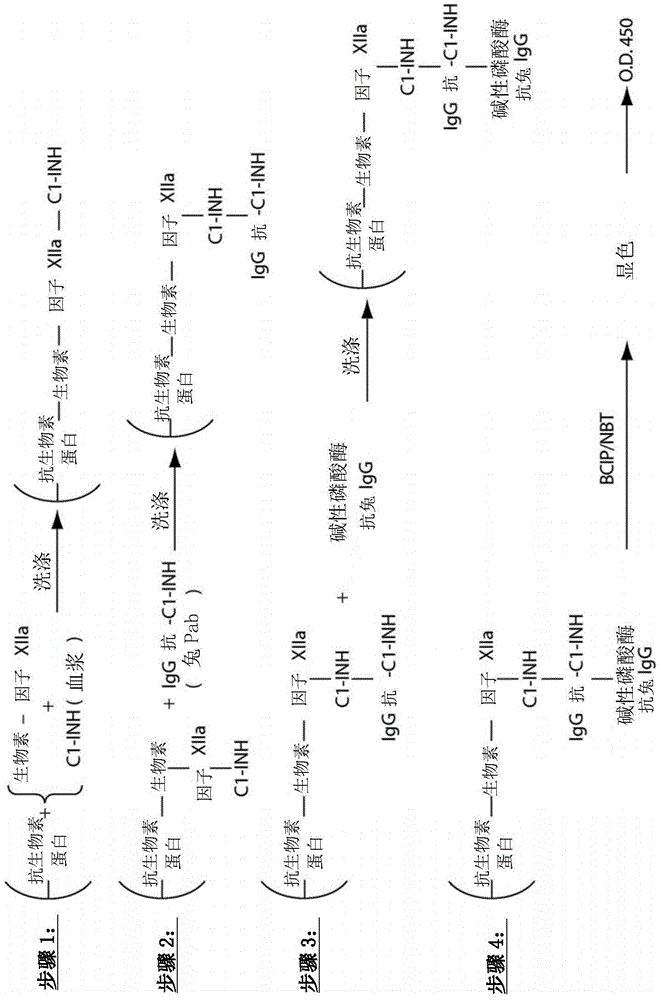
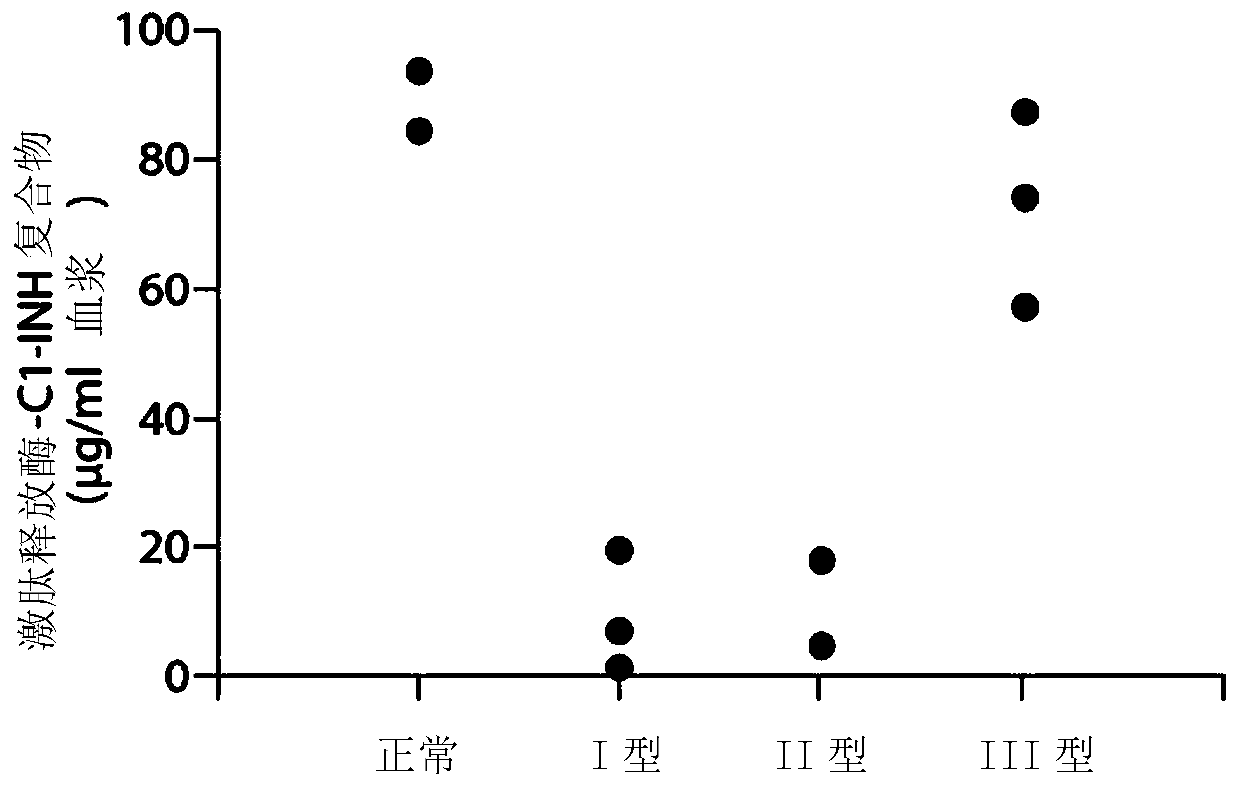
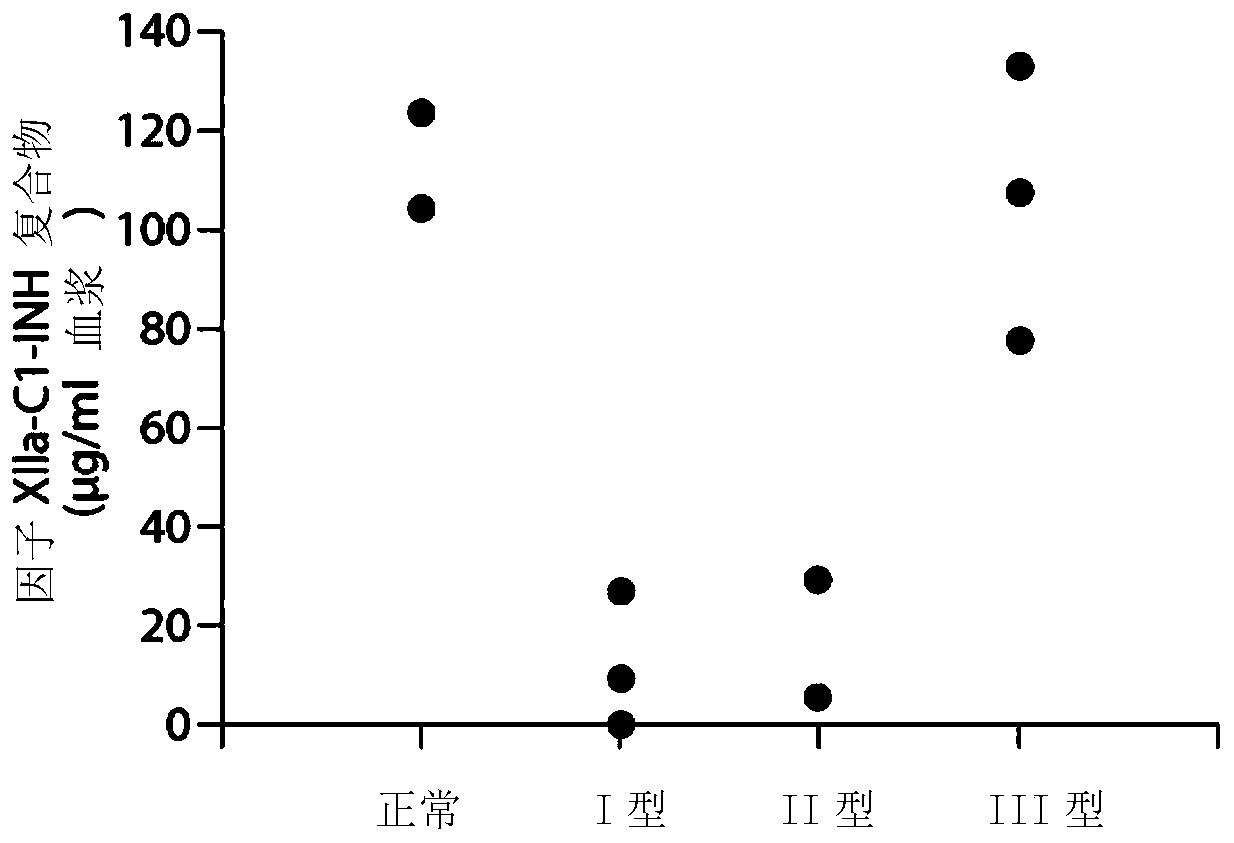
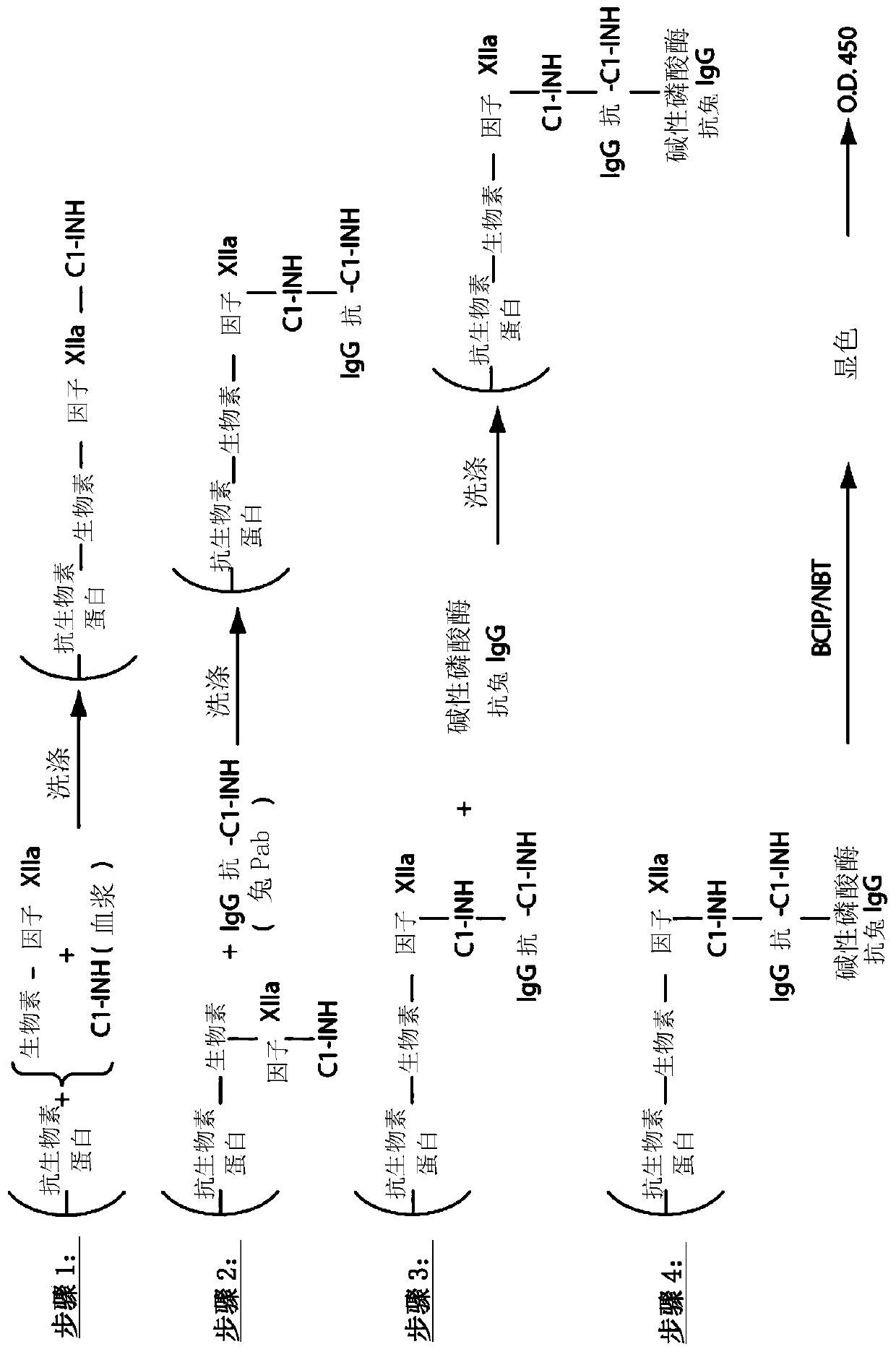
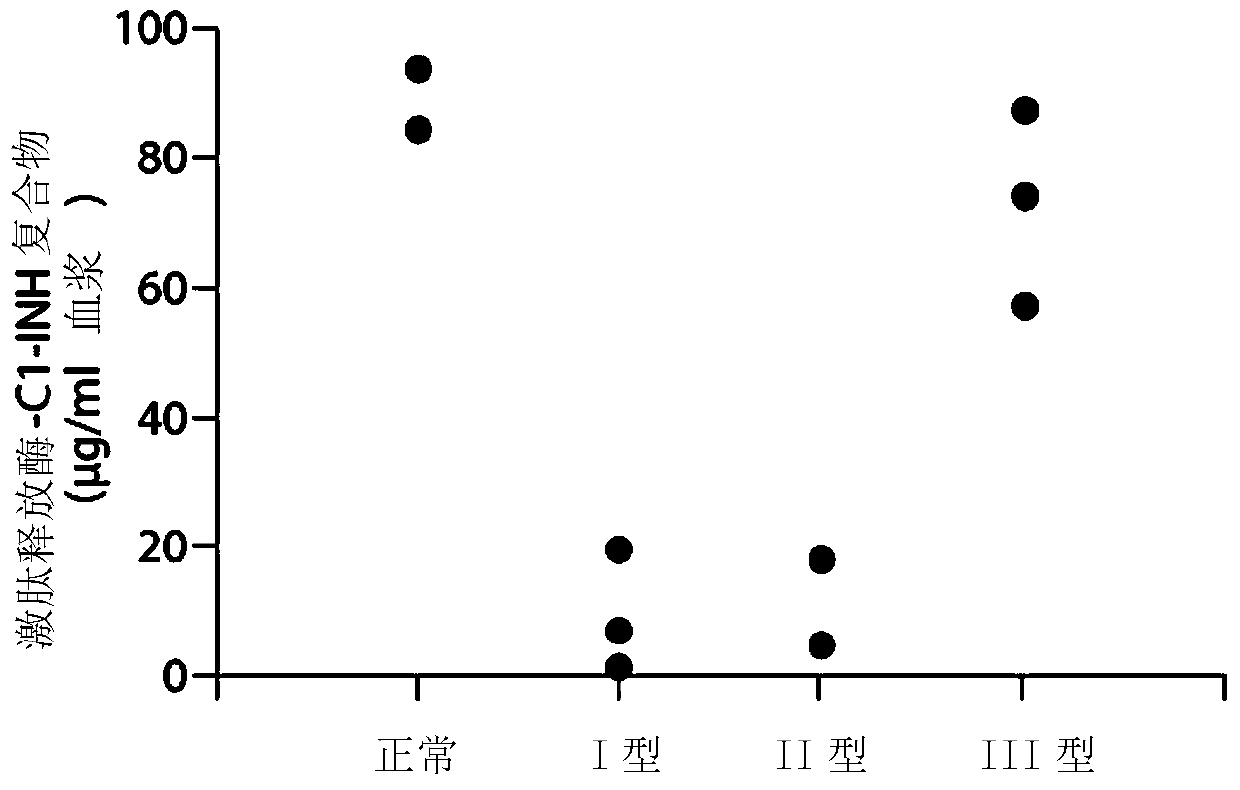
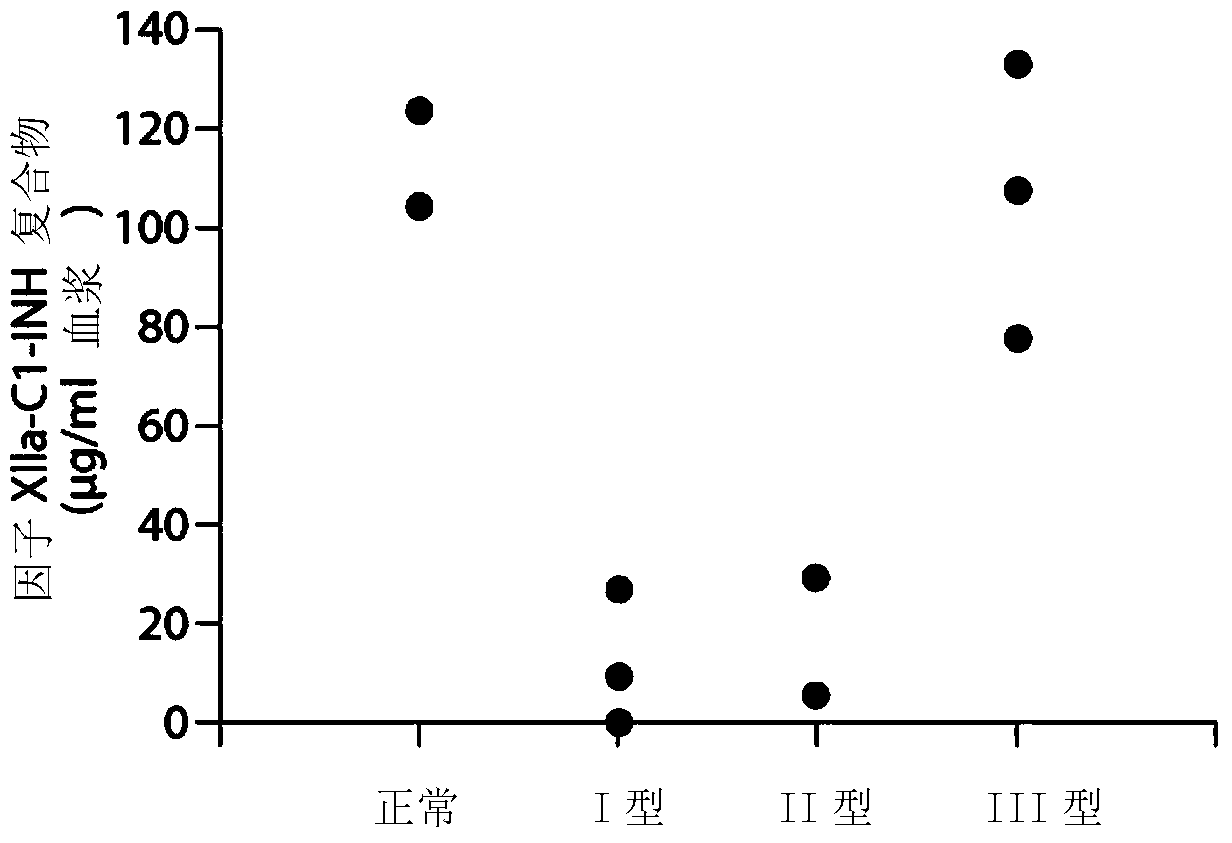
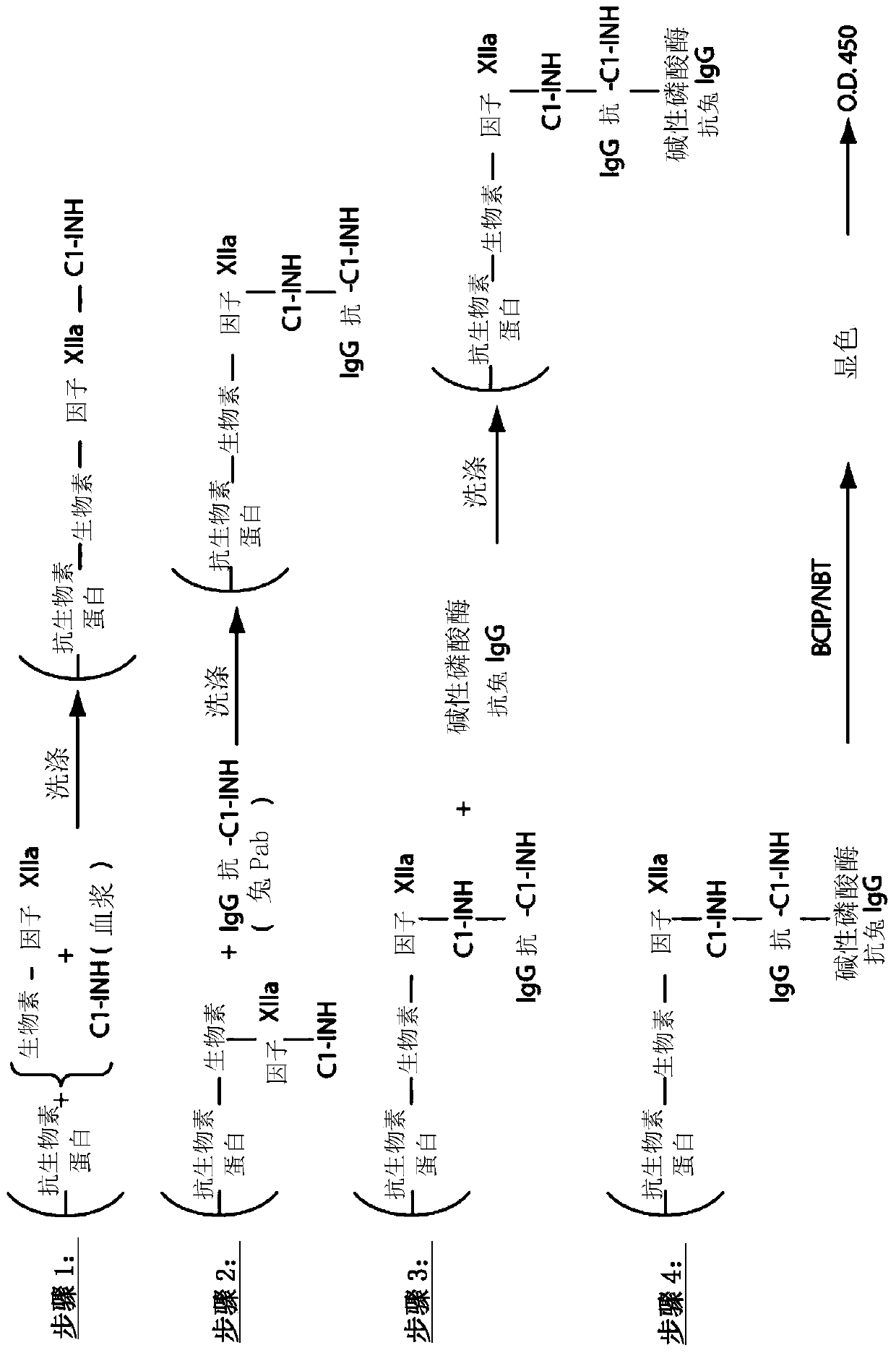
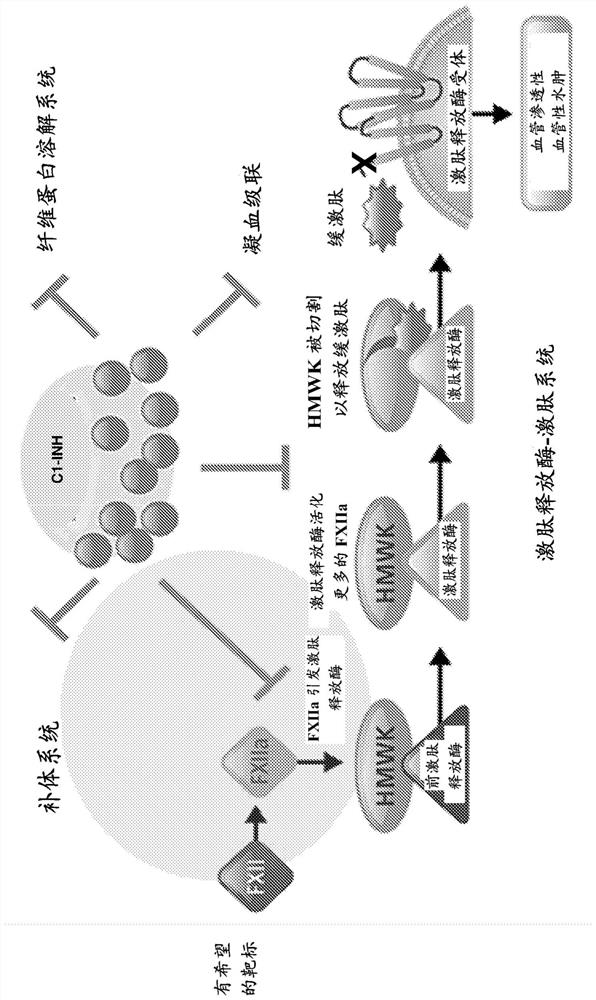
The invention provides assay methods of detecting plasma protease CI inhibitor (Cl-INH) that binds plasma kallikrein, Factor XII, or both, and uses thereof for identifying subjects at risk for or suffering from a pKal-mediated or bradykinin-mediated disorder. Provided methods permit analysis of patients with plasma kallikrein-mediated angioedema (KMA), or other diseases mediated by pKal useful in the evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
Evaluation, assays and treatment of PKAL-mediated disorders
The invention relates to evaluation, assays and treatment of PKAL-mediated disorders. The invention provides assay methods of detecting plasma protease CI inhibitor (Cl-INH) that binds plasma kallikrein, factor XII, or both, and uses thereof for identifying subjects at risk for or suffering from a pKal-mediated or bradykinin-mediated disorder. Provided methods permit analysis of patients with plasma kallikrein-mediated angioedema (KMA), or other diseases mediated by pKal useful in the evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
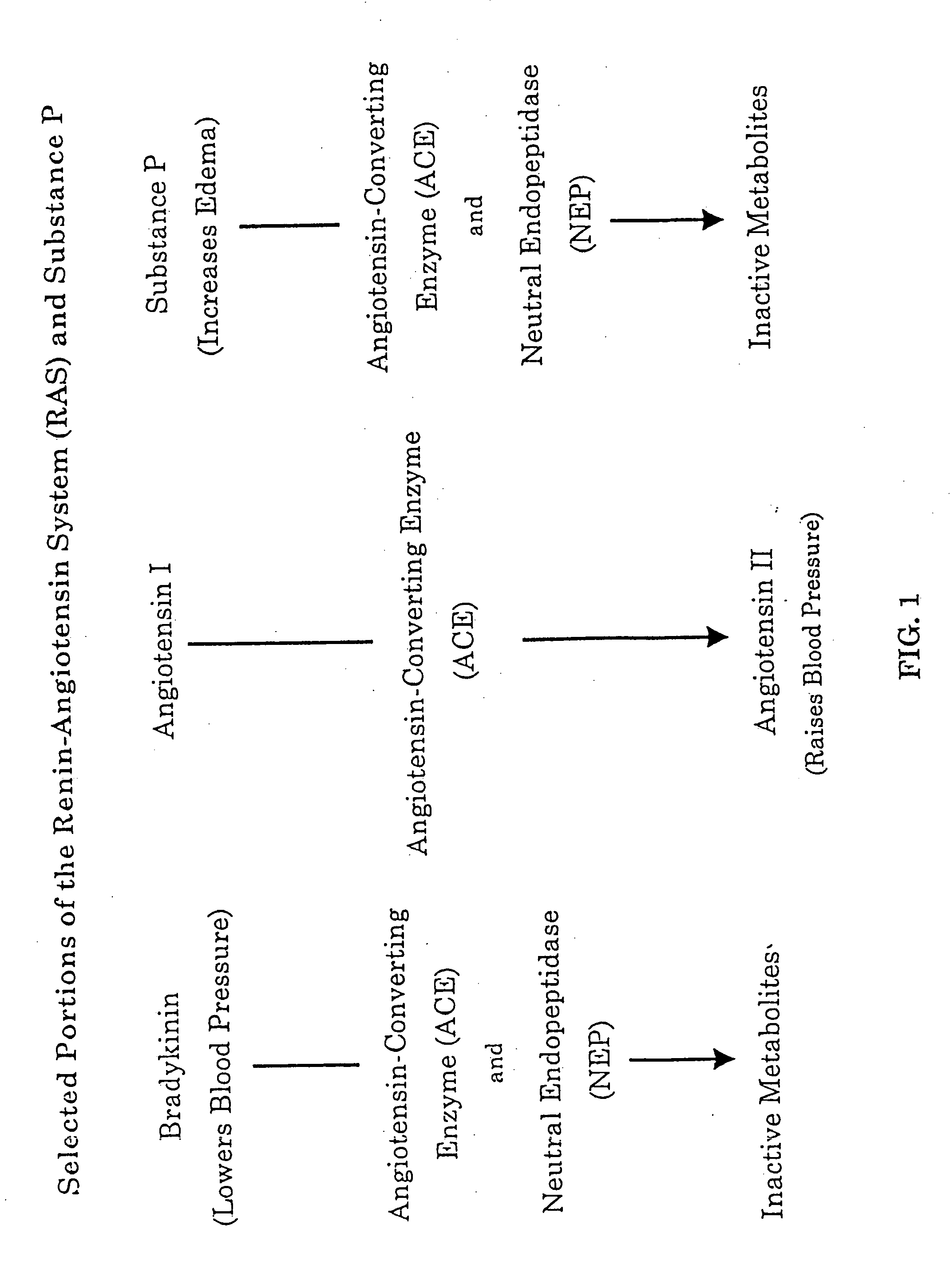
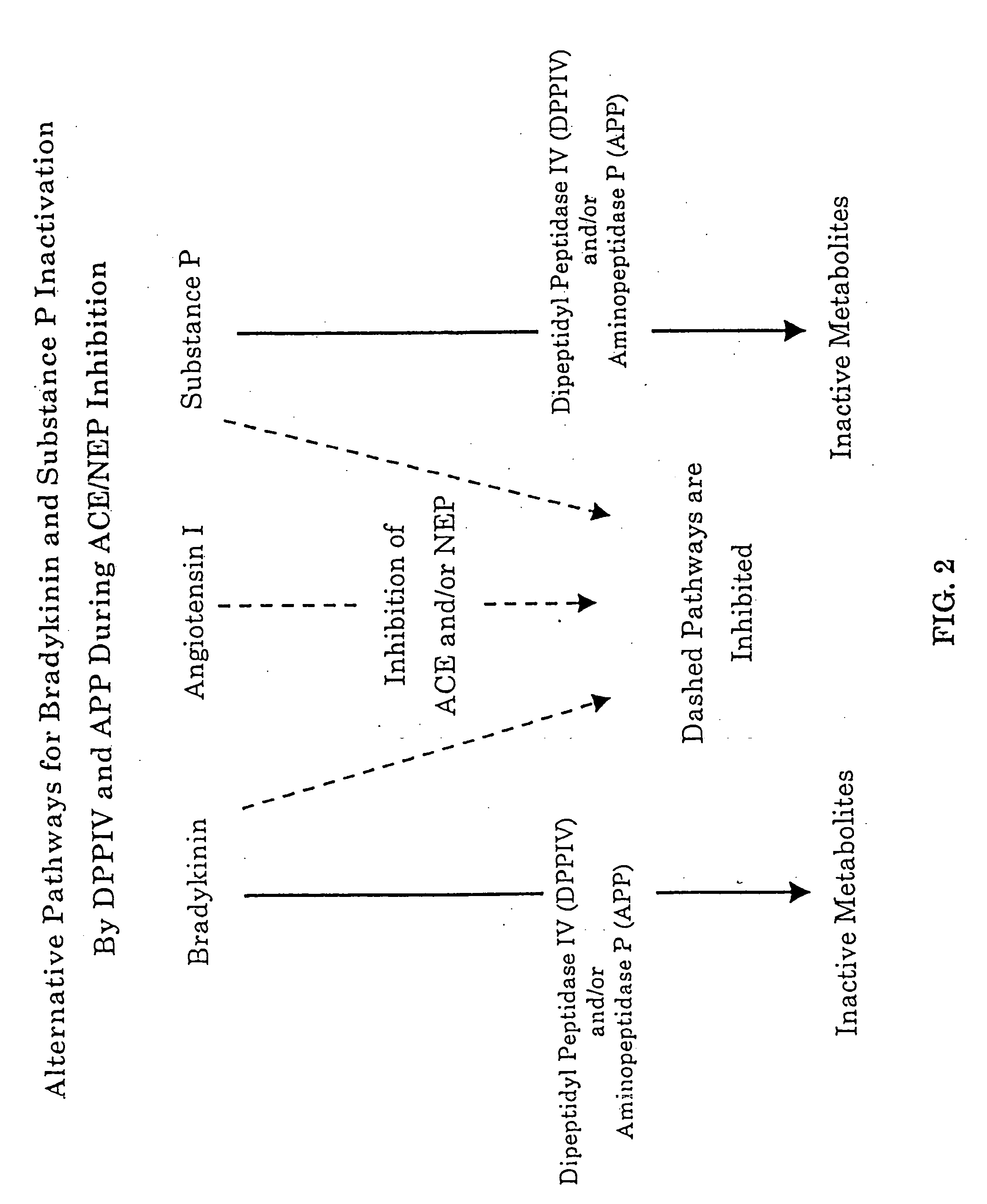
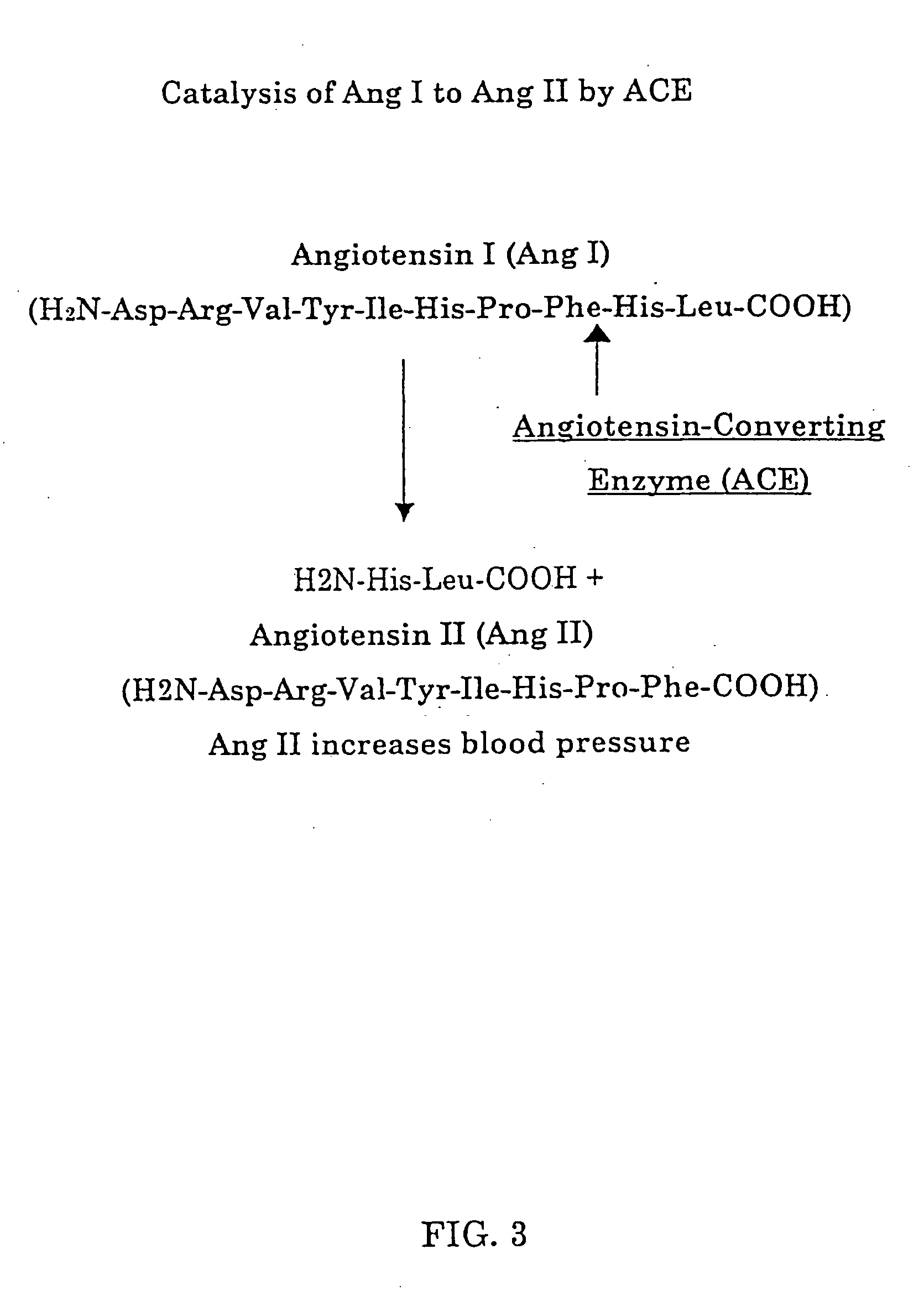
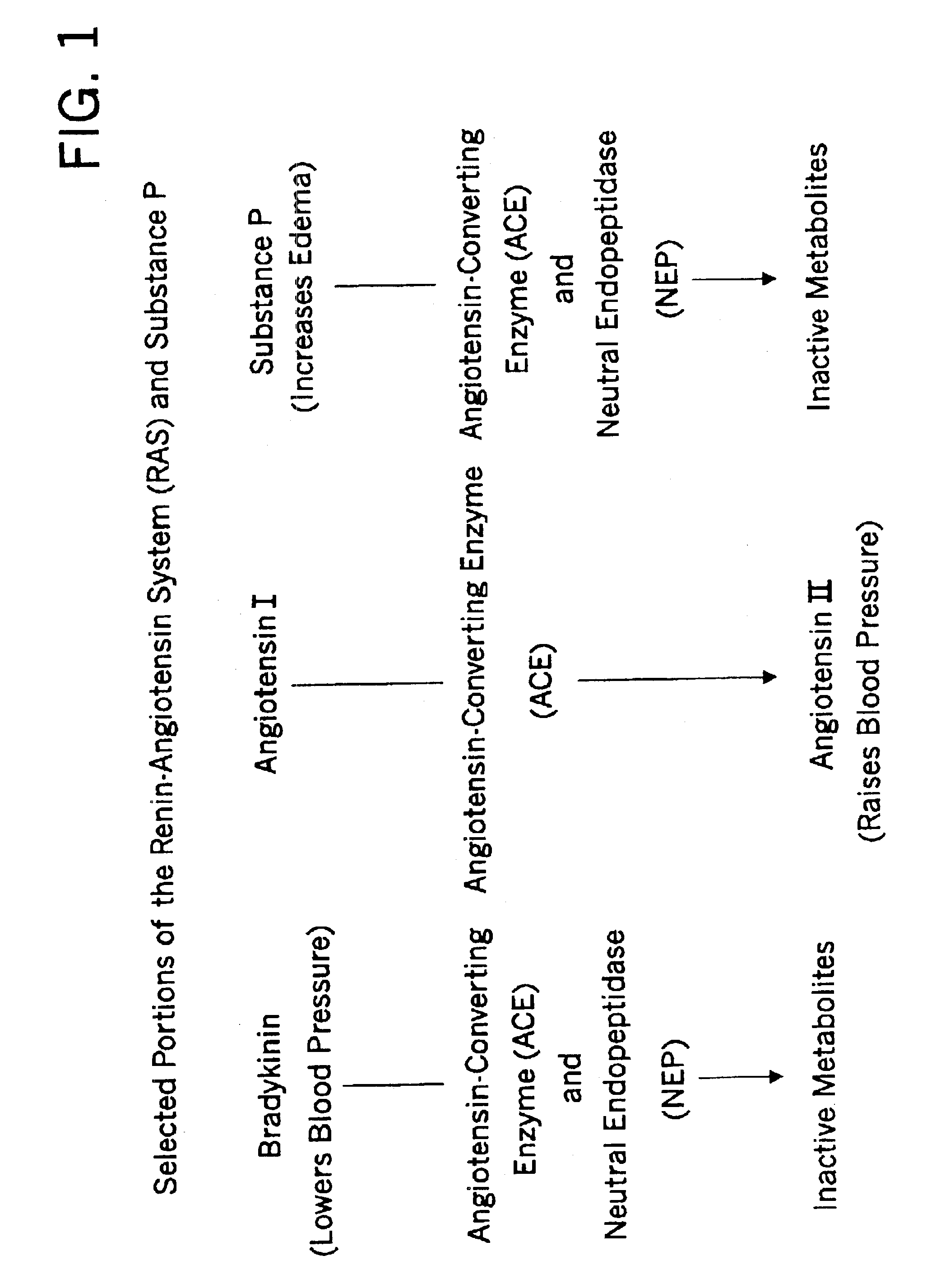
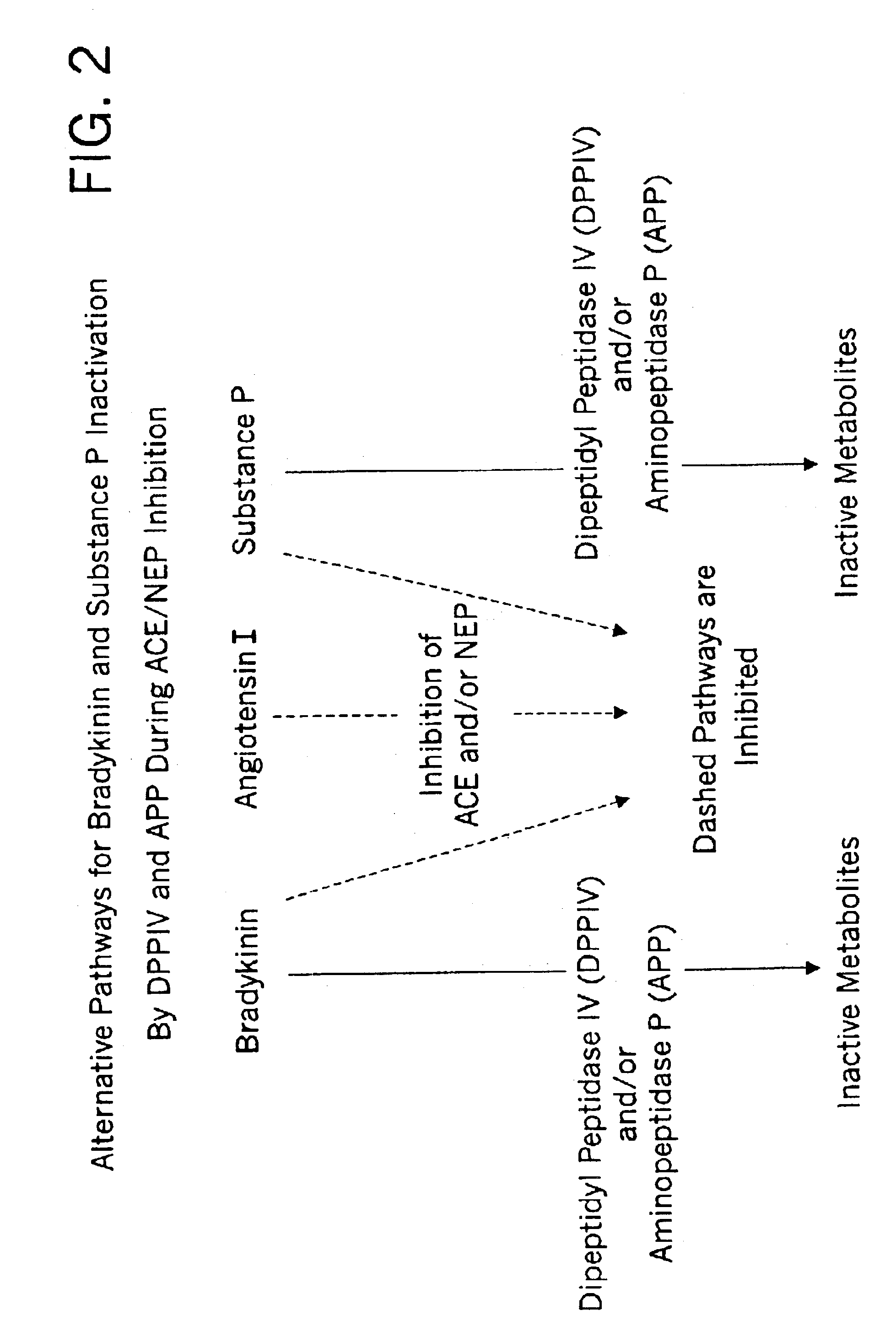
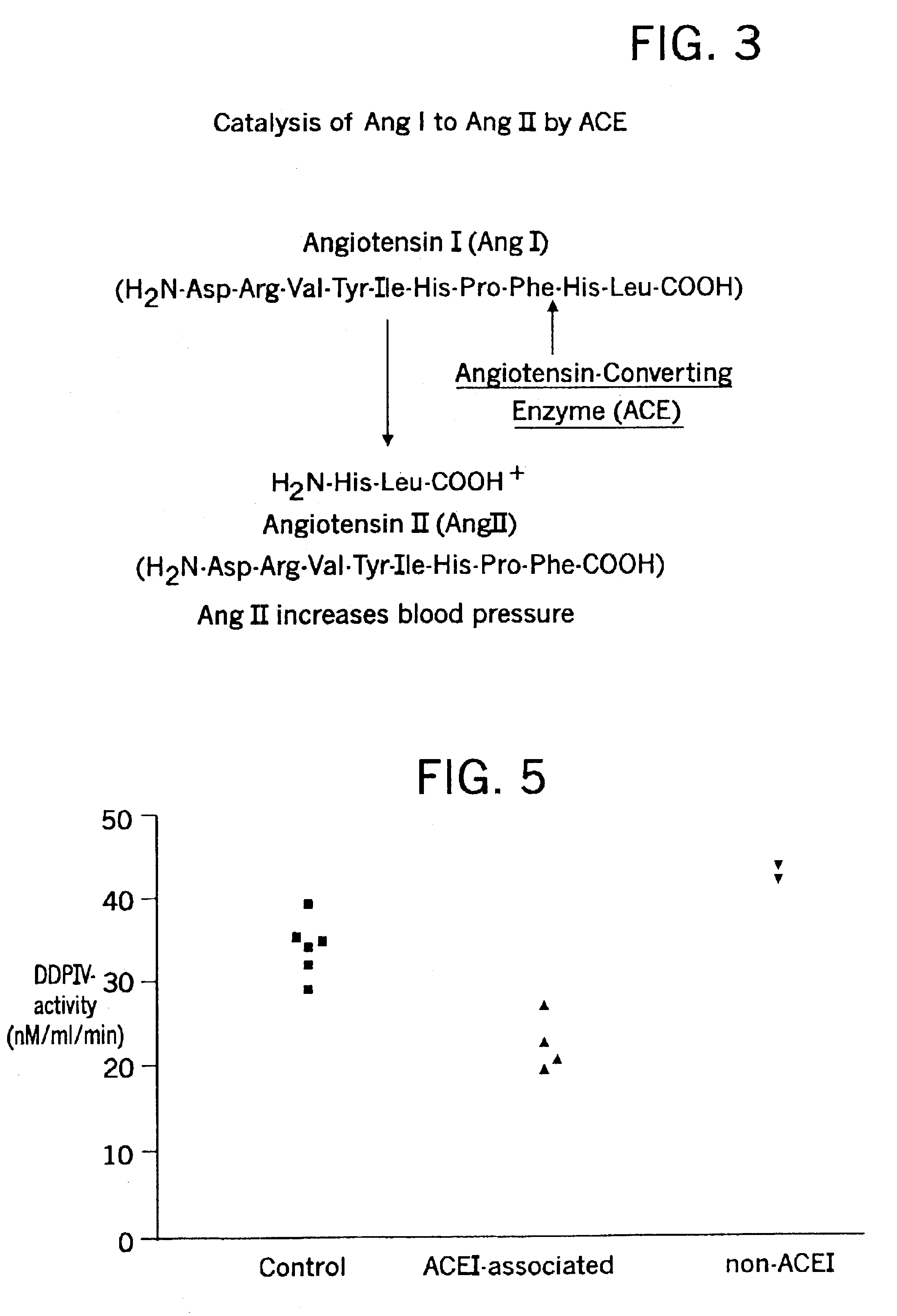
Biological markers and diagnostic tests for angiotensin converting enzyme inhibitor and vasopeptidase inhibitor-associated angioedema
InactiveUS20050181468A1Microbiological testing/measurementDipeptidyl peptidaseVasopeptidase inhibitor
Deficiencies in certain physiological pathways are linked with ACE or vasopeptidase inhibitor associated angioedema. Additionally, detection and / or measurement of dipeptidyl peptidase IV (DPP IV) enzyme activity and aminopeptidase P (APP) enzyme activity is a predictor of this risk. The present invention provides biological markers, diagnostic tests, and pharmaceutical indications that are useful in the diagnosis and treatment of angioedema and in the marketing and safety of certain medications. This ability can be important for the treatment of a subject that is in need of or are taking an angiotensin-converting enzyme (ACE) inhibitor and / or a vasopeptidase inhibitor (combined ACE and neutral endopeptidase (NEP) inhibitor), which are commonly used in the treatment of hypertension (high blood pressure), diabetes, and cardiac and renal diseases.
Owner:VANDERBILT UNIV
Compositions and Uses of Amidine Derivatives
ActiveUS20170266172A1Organic active ingredientsDispersion deliveryOrganic solventHereditary angioedema
Use of a compound of formula (I): wherein A, X, Y, R1 and R2 as defined herein, in treating hereditary angioedema is disclosed. A composition containing the compounds, a polar organic solvent or a mixture thereof; and optionally a co-solvent, is also disclosed.
Owner:BIOCRYST PHARM INC
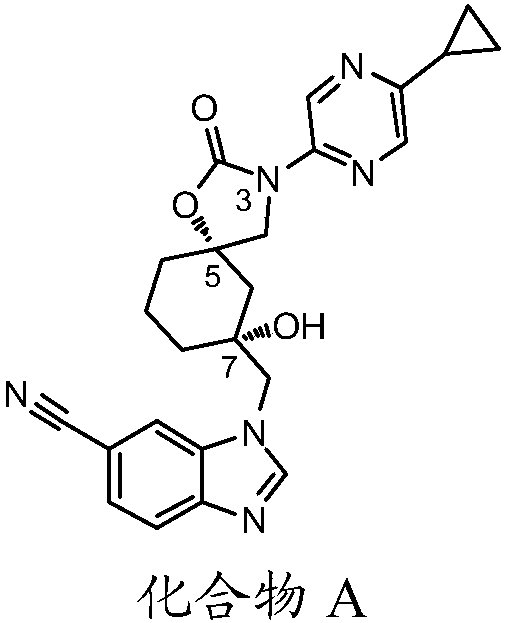
Trpv4 antagonist
The present invention relates to a novel compound useful as a TRPV4 antagonist, specifically the compound 1-(((5S,7R)-3-(5-cyclopropylpyrazin-2-yl)-7-hydroxy-2-oxo-1-oxa-3-azaspiro[4.5]decan-7-yl)methyl)-1H-benzo[d]imidazole-6-carbonitrile, pharmaceutically acceptable salts thereof and pharmaceutical compositions containing the compound. The compound of the invention can be useful in the treatmentof a disease state selected from: atherosclerosis, disorders related to vasogenic edema, postsurgical abdominal edema, ocular edema, cerebral edema, local and systemic edema, fluid retention, sepsis,hypertension, inflammation, bone related dysfunctions and congestive heart failure, pulmonary disorders, chronic obstructive pulmonary disorder, ventilator induced lung injury, high altitude inducedpulmonary edema, acute respiratory distress syndrome, acute lung injury, pulmonary fibrosis, sinusitis / rhinitis, asthma, cough; including acute cough, sub-acute cough and chronic cough, pulmonary hypertension, overactive bladder, cystitis, pain, motor neuron disorders, genetic gain of function disorders, cardiovascular disease, renal dysfunction, stroke, glaucoma, retinopathy, endometriosis, pre-term labor, dermatitis, pruritus, pruritus in liver disease, diabetes, metabolic disorder, obesity, migraine, pancreatitis, tumor suppression, immunosuppression, osteoarthritis, crohn's disease, colitis, diarrhea, intestinal irregularity (hyperreactivity / hyporeactivity), fecal incontinence, irritable bowel syndrome (IBS), constipation, intestinal pain and cramping, celiac disease, lactose intolerance, and flatulence.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
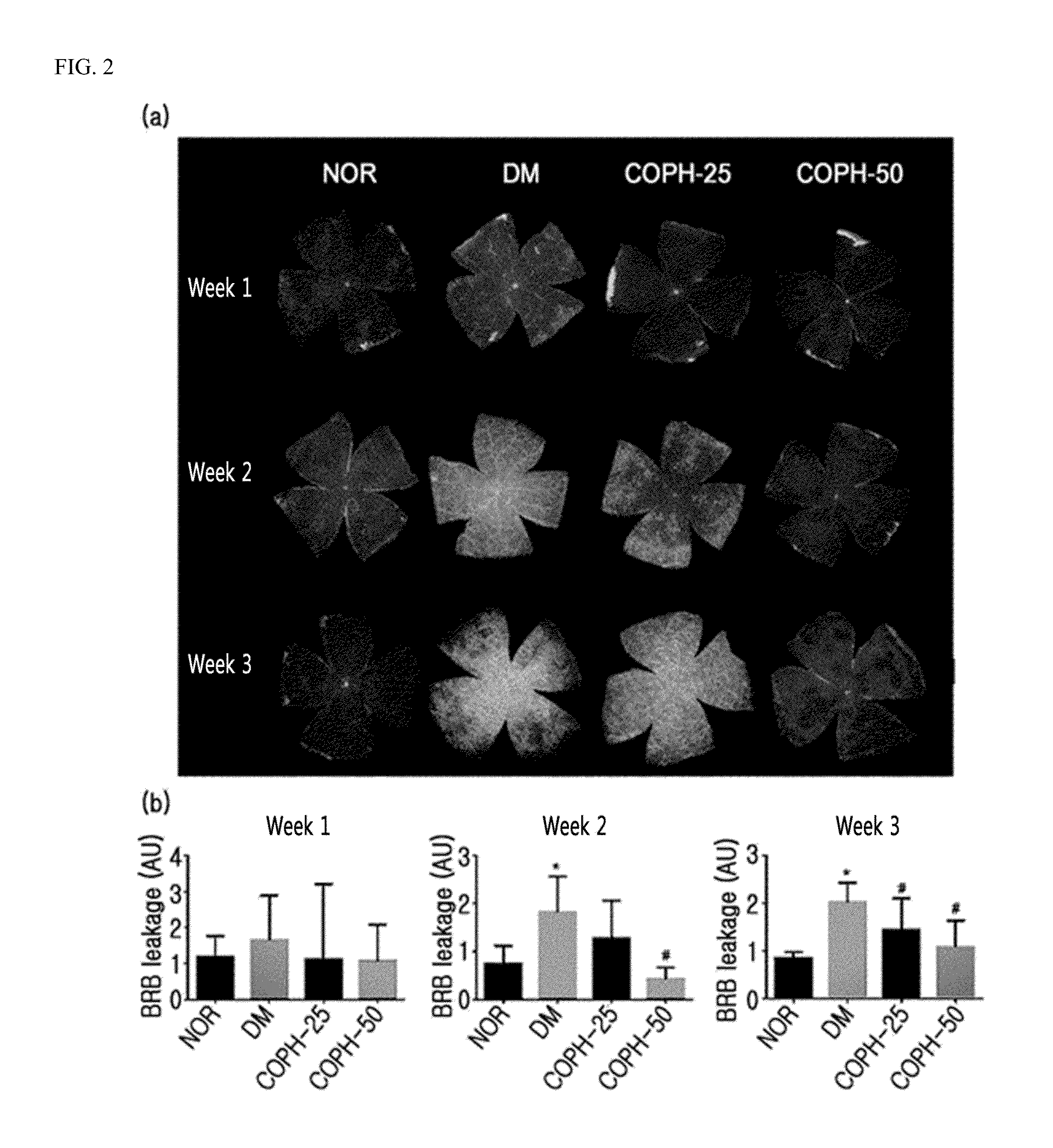
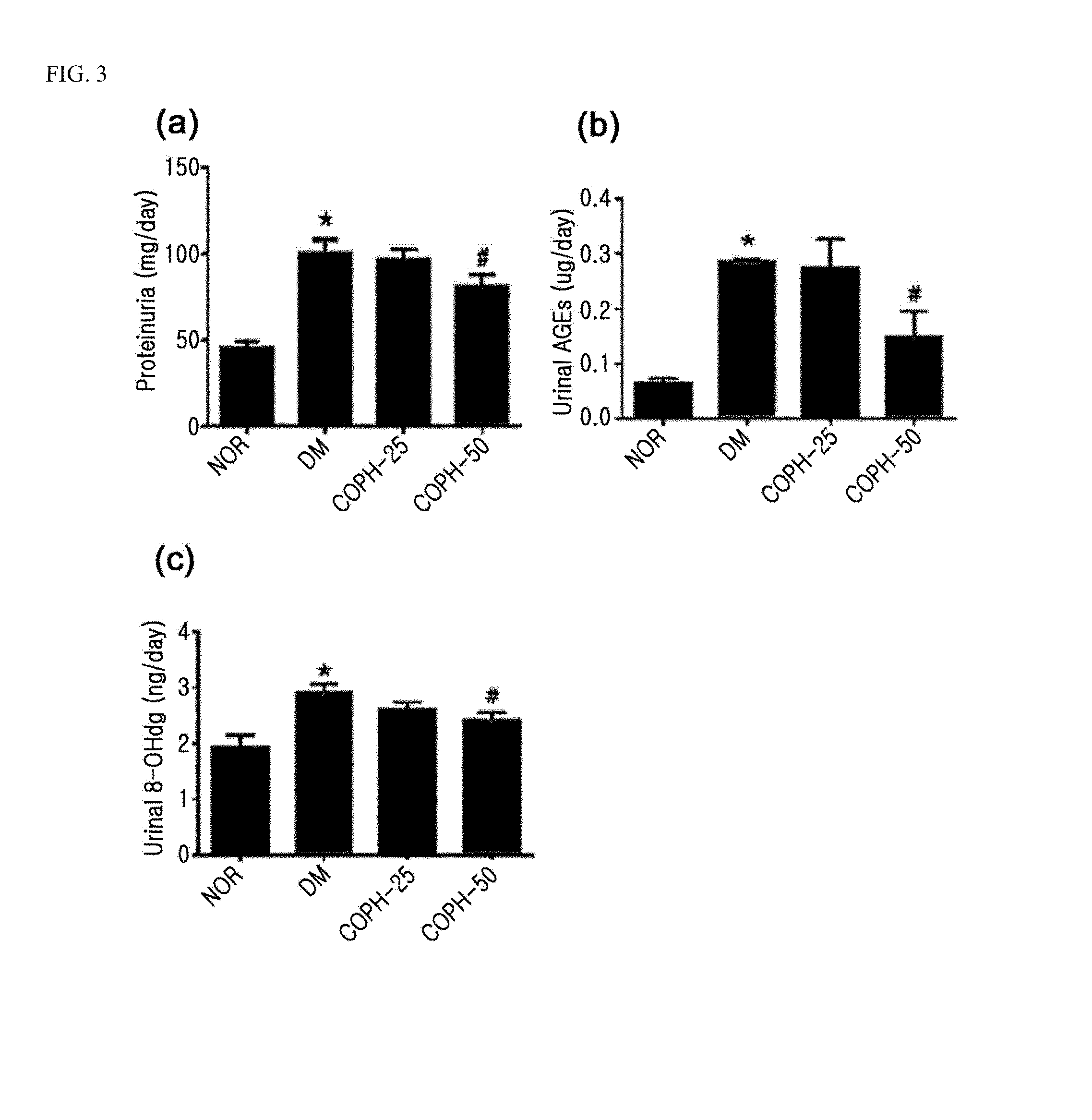
Pharmaceutical composition and functional food comprising natural extracts for preventing or treating diabetic complications or angiodema
ActiveUS20150343005A1Inhibition effectTreatment complicationsBiocideOrganic active ingredientsAdditive ingredientGrape seed
The present invention relates to a pharmaceutical composition and a health functional food composition for prevention, treatment or amelioration of diabetic complications or angioedema containing a mixed extract of Hedera helix leaves and Coptis chinensis as an active ingredient. More specifically, the present invention relates to a pharmaceutical composition and a health functional food composition for prevention, treatment or amelioration of diabetic complications or angioedema further containing an extract of Rheum palmatum, Puerariae radix, Ginkgo leaves, Cassiae semen, blueberry, bilberry, raspberry, or grape seeds as an active ingredient, in addition to the mixed extract of Hedera helix leaves and Coptis chinensis.
Owner:KOREA INST OF ORIENTAL MEDICINE
Methods for identifying contraindications to angiotensin converting enzyme inhibitor and/or vasopeptidase inhibitor treatment
InactiveUS6887679B2Reduced activityMicrobiological testing/measurementDipeptidyl peptidaseBiomarker (petroleum)
Deficiencies in certain physiological pathways are linked with ACE or vasopeptidase inhibitor associated angioedema. Additionally, detection and / or measurement of dipeptidyl peptidase IV (DPP IV) enzyme activity and aminopeptidase P (APP) enzyme activity is a predictor of this risk. The present invention provides biological markers, diagnostic tests, and pharmaceutical indications that are useful in the diagnosis and treatment of angioedema and in the marketing and safety of certain medications. This ability can be important for the treatment of a subject that is in need of or are taking an angiotensin-converting enzyme (ACE) inhibitor and / or a vasopeptidase inhibitor (combined ACE and neutral endopeptidase (NEP) inhibitor), which are commonly used in the treatment of hypertension (high blood pressure), diabetes, and cardiac and renal diseases.
Owner:VANDERBILT UNIV
Peptide compositions and methods for treating lung injury, asthma, anaphylaxis, angioedema, systemic vascular permeability syndromes, and nasal congestion
ActiveUS20140213505A1Inflammatory-induced hyper-permeability of endothelial barrierImprove breathabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBlood vesselInositol
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Compositions and uses of amidine derivatives
Owner:BIOCRYST PHARM INC
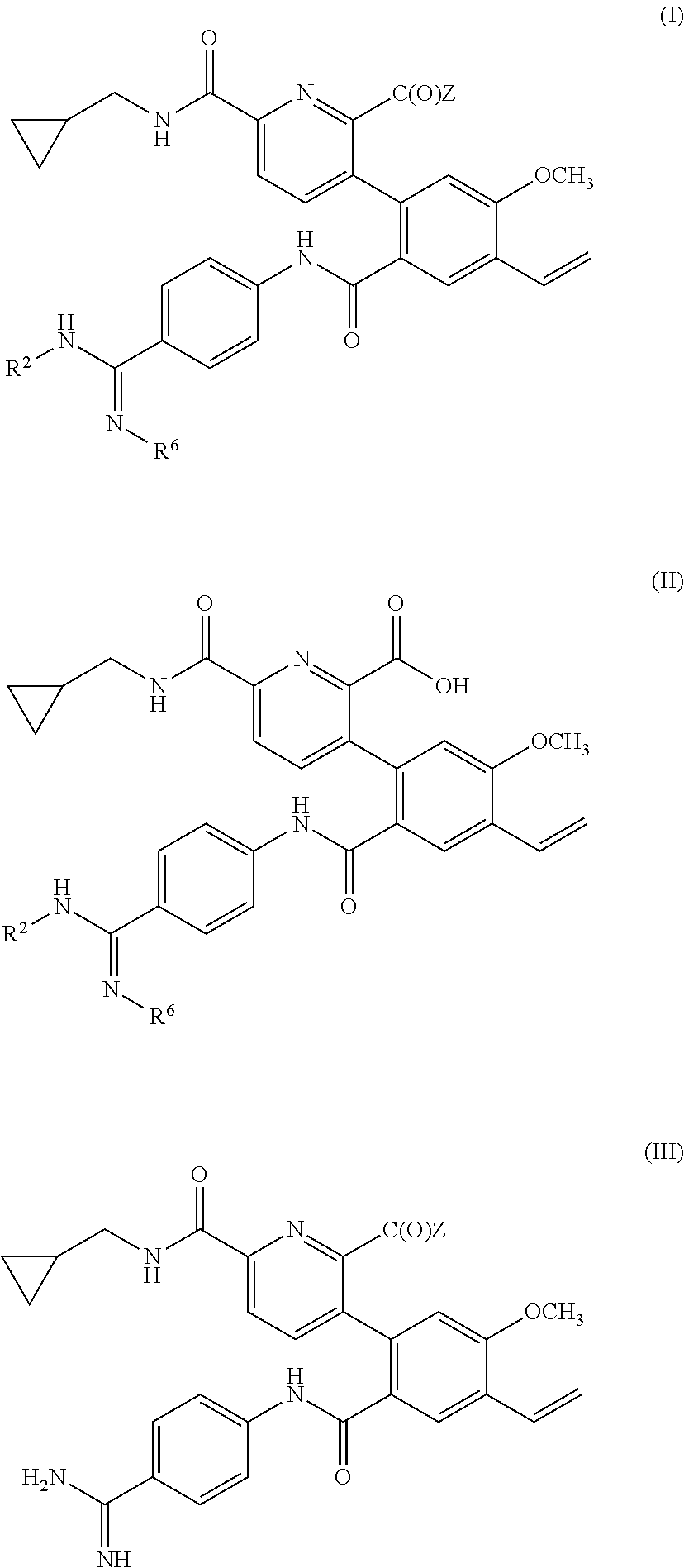
Prodrugs of kallikrein inhibitors
Disclosed are compounds of formula I, II, and III, and pharmaceutically acceptable salts thereof, which are inhibitors of kallikrein. Also provided are pharmaceutical compositions comprising such a compound, and methods involving use of the compounds and compositions in the treatment and prevention of acquired or hereditary angioedema, or other diseases and conditions characterized by aberrant kallikrein activity.
Owner:BIOCRYST PHARM INC
Peptide compositions and methods for treating lung injury, asthma, anaphylaxis, angioedema, systemic vascular permeability syndromes, and nasal congestion
ActiveUS20140155314A1Inflammatory-induced hyper-permeability of endothelial barrierImprove breathabilityPolypeptide with localisation/targeting motifCell receptors/surface-antigens/surface-determinantsNoseALI - Acute lung injury
Provided herein are peptide inhibitors of the interaction between End Binding Protein 3 (EB3) and Inositol 1,4,5-Trisphosphate Receptor Type 3 (IP3R3). Also provided are methods and materials for treating lung injury, including acute lung injury, which may include hyperpermeability of lung vessels, vascular leakage, the development of edema, asthma, anaphylaxis, angioedema, systemic vascular permeability syndromes, and nasal congestion.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Application of Sola A in ischemic brain damage
InactiveCN106580956AExtended treatment windowDelay progressOrganic active ingredientsNervous disorderMedicineNeuron
The invention discloses an application of Sola A in ischemic brain damage. Specifically, the invention discloses (I) the usage of the Sola A in producing a pharmaceutical composition used to prevent and / or treat ischemic brain damage and / or (II) the function of the Sola A in preparing a pharmaceutical composition used to protect ischemic neuron. According to experiments, Sola A can effectively control the development of acute brain death, reduce infarct size and slow down neuronal edema and angioedema. The Sola A also plays an positive part in protecting neurological functions for a long time.
Owner:李佩盈
Pharmaceutical composition and functional food comprising natural extracts for preventing or treating diabetic complications or angiodema
The present invention relates to a pharmaceutical composition and a health functional food composition for prevention, treatment or amelioration of diabetic complications or angioedema containing a mixed extract of Hedera helix leaves and Coptis chinensis as an active ingredient. More specifically, the present invention relates to a pharmaceutical composition and a health functional food composition for prevention, treatment or amelioration of diabetic complications or angioedema further containing an extract of Rheum palmatum, Puerariae radix, Ginkgo leaves, Cassiae semen, blueberry, bilberry, raspberry, or grape seeds as an active ingredient, in addition to the mixed extract of Hedera helix leaves and Coptis chinensis.
Owner:KOREA INST OF ORIENTAL MEDICINE
Application of 5α-androsta-3β, 5,6β-triol and their analogues in preventing or treating altitude sickness caused by hypobaric hypoxia
ActiveCN105012313BNeuroprotectiveOrganic active ingredientsNervous disorderNeuronal degenerationHypobaric hypoxia
The invention provides applications of 5α-androsta-3β, 5,6β-triol and analogs thereof in preparing medicines for treating or preventing altitude sickness caused by hypobaric hypoxia, thereby providing a novel method for the prevention and treatment of altitude sickness. drug. Studies have shown that 5α-androsta-3β, 5,6β-triol treatment can effectively reduce the angioedema of cynomolgus monkey brain tissue caused by hypobaric hypoxia, reduce the increase of brain water content, and protect neurons caused by hypobaric hypoxia Degeneration injury, which can improve the neurological dysfunction caused by hypobaric hypoxia, for the prevention or treatment of altitude sickness.
Owner:GUANGZHOU CELLPROTEK PHARMA
Treatment of hereditary angioedema
PendingCN114126612AInhibition of lysisInhibition of activationOrganic active ingredientsNervous disorderKininHereditary angioedema
Owner:KALVISTA PHARMA
Markers for hereditary angioedema and their application
ActiveCN113311056BReduce expressionEasy to detectMaterial analysis by electric/magnetic meansDisease diagnosisHereditary angioedemaBiology
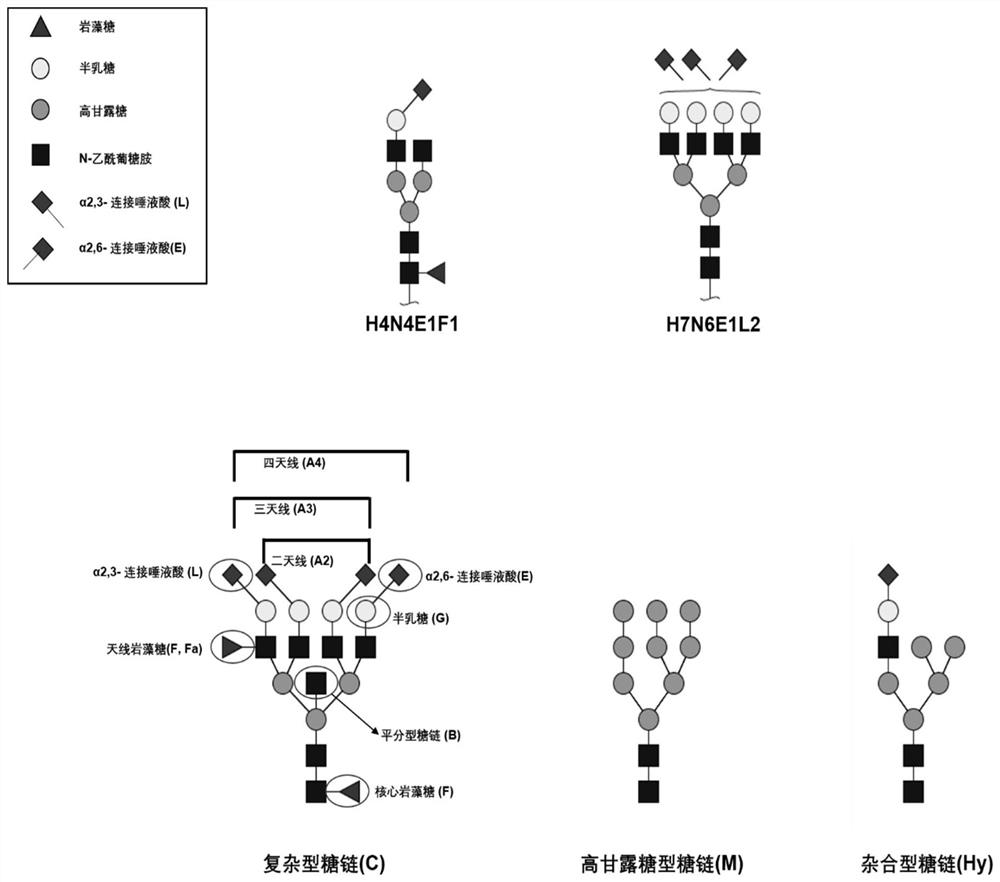
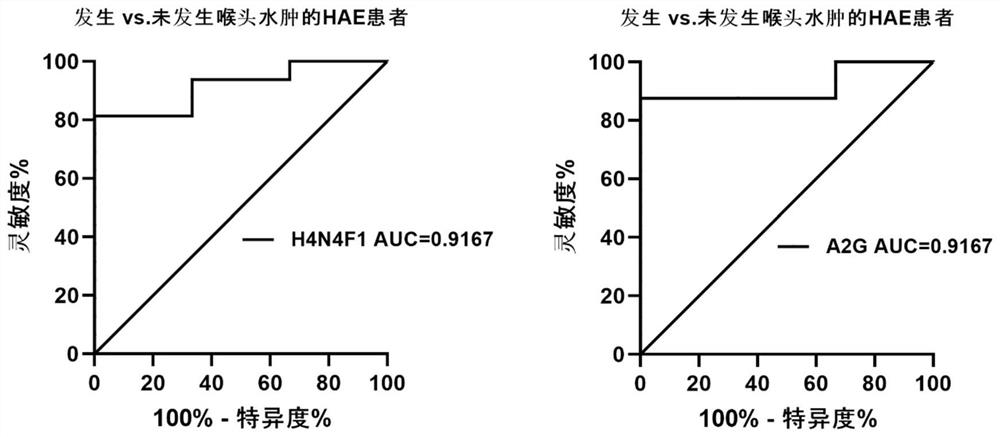
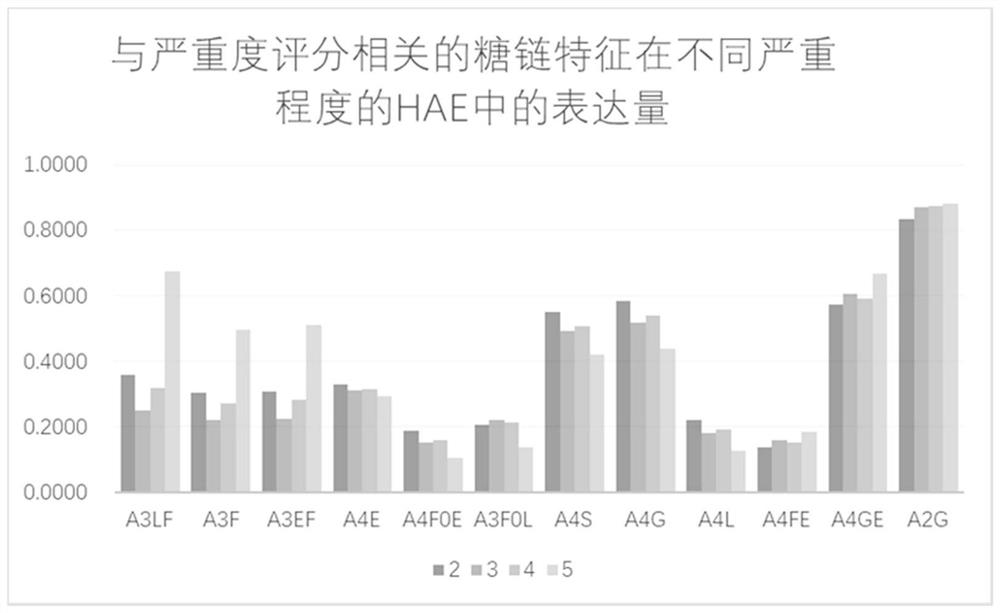
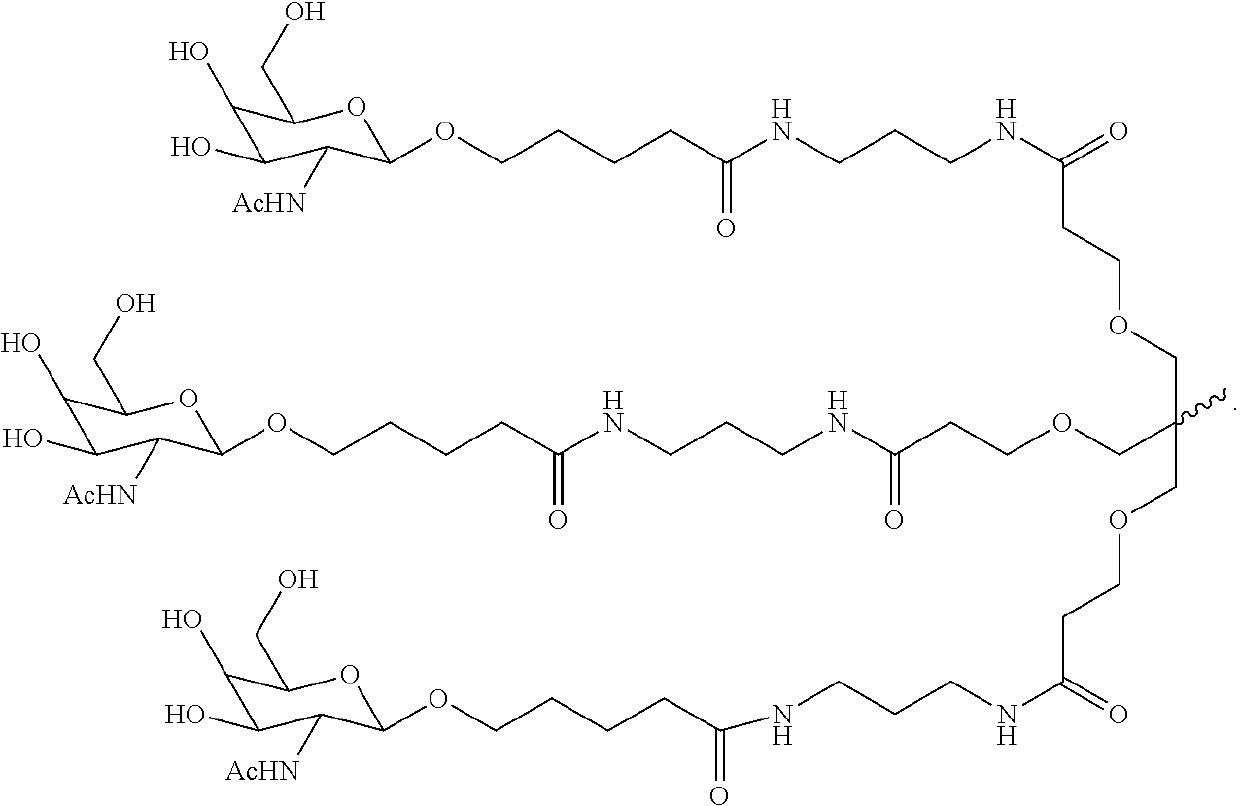
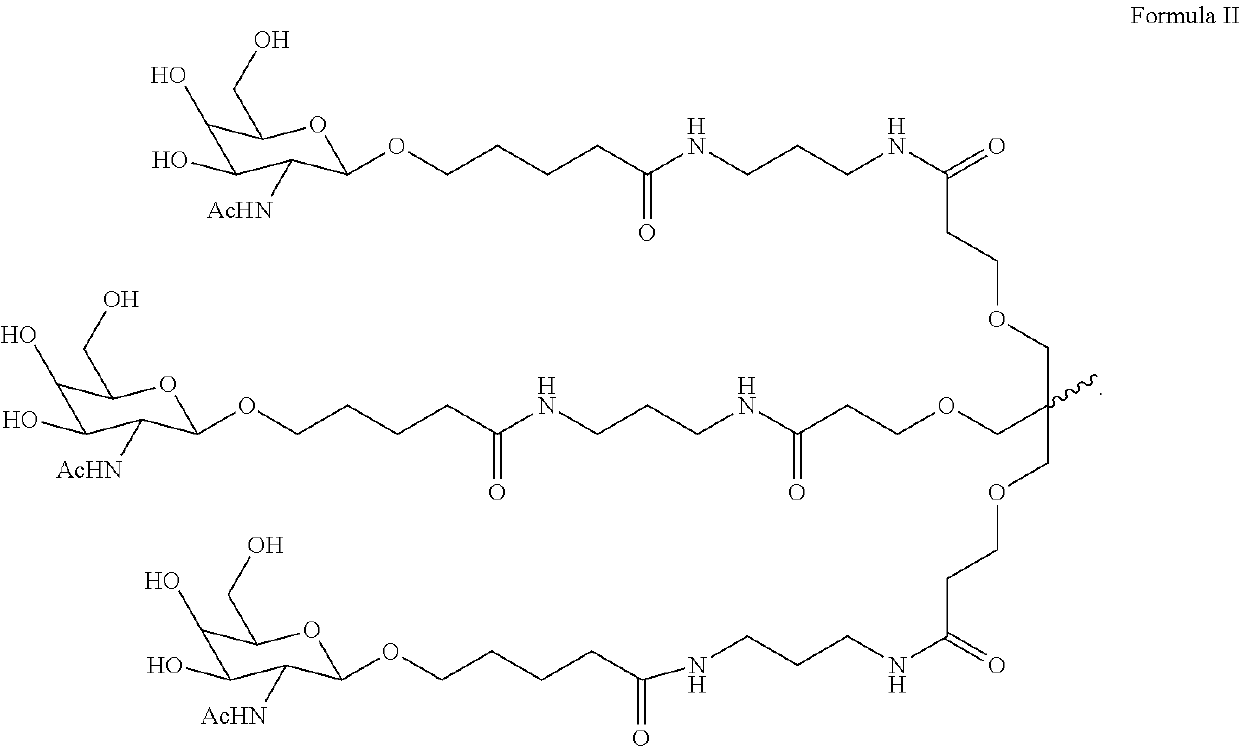
The invention relates to the technical field of medical detection, in particular to markers for hereditary angioedema and applications thereof. The present invention provides markers for hereditary angioedema including N-sugar chain markers H4N6F1E1 and / or CA4. The expression levels of the N-sugar chain markers provided by the present invention are significantly different in healthy people, patients with hereditary angioedema, and patients with hereditary angioedema who develop laryngeal edema, severe abdominal pain, and patients with different degrees of severity, and can be used for hereditary angioedema The diagnosis of edema and the prediction of laryngeal edema, severe abdominal pain and the severity of hereditary angioedema have the advantages of convenient detection, short time consumption, specificity, high sensitivity and high accuracy, and can be used in practice for genetic Diagnosis of acute angioedema, stratification of patients' clinical symptoms, guidance of prevention and treatment, prediction and prognosis evaluation.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Polynucleotide agents targeting factor XII (hageman factor) (F12) and methods of use thereof
ActiveUS10883107B2Organic active ingredientsSugar derivativesMalignant essential hypertensionEnd stage renal disease
Owner:ALNYLAM PHARMA INC
Compositions and methods for inhibiting gene expression of factor XII
RNA interference (RNAi) initiators for inhibiting the expression of Factor XII (F12) gene by the RNA interference mechanism are disclosed. Also disclosed are pharmaceutical compositions comprising one or more F12 RNAi initiators and one or more excipients capable of delivering the RNAi initiators to hepatocytes. Delivery of the F12 RNAi initiator to hepatocytes in vivo provides inhibition of F12 gene expression and treatment of angioedema, including hereditary angioedema (HAE), venous thromboembolism (VTE), and diseases associated with angioedema.
Owner:ARROWHEAD PHARMA INC
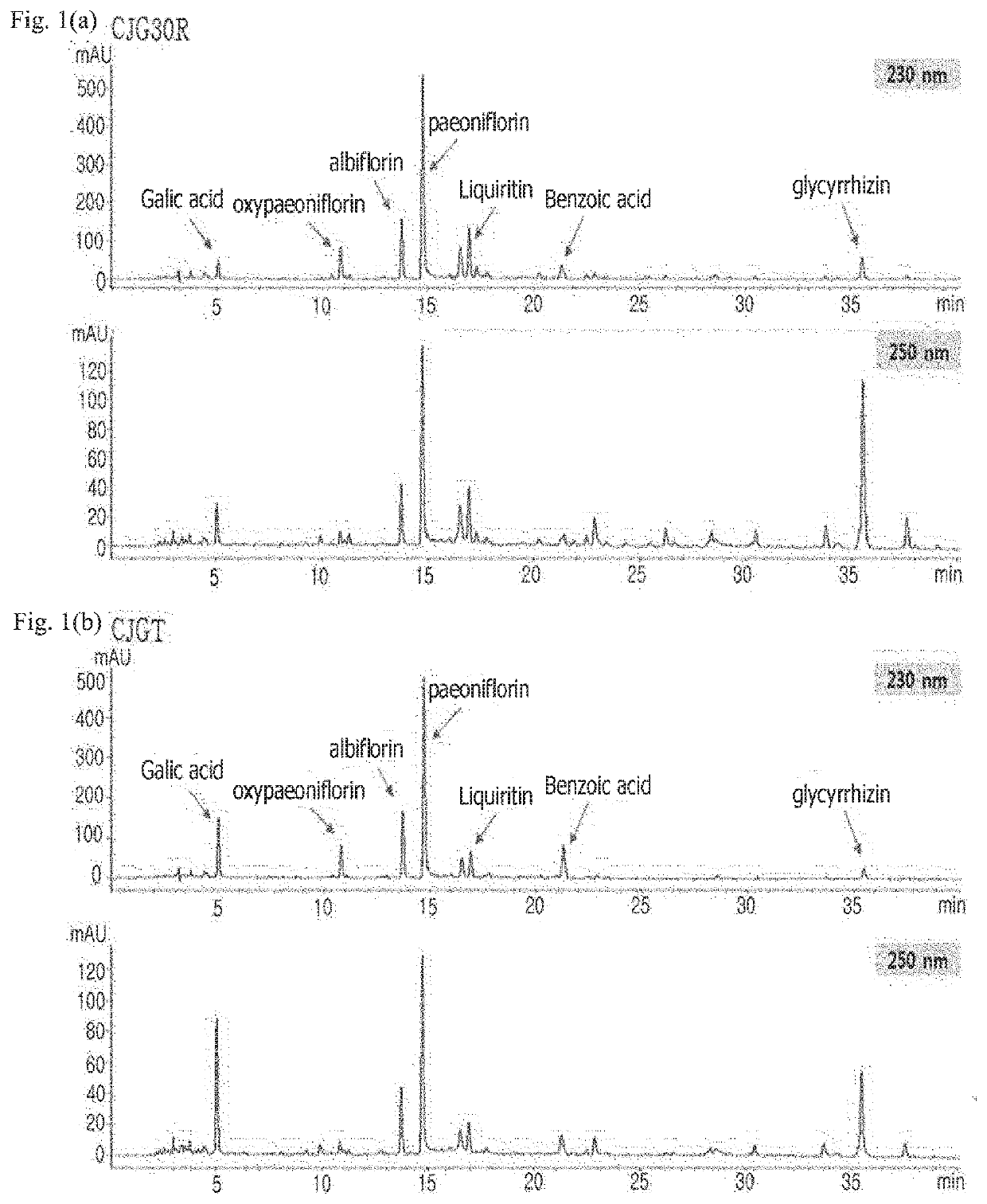
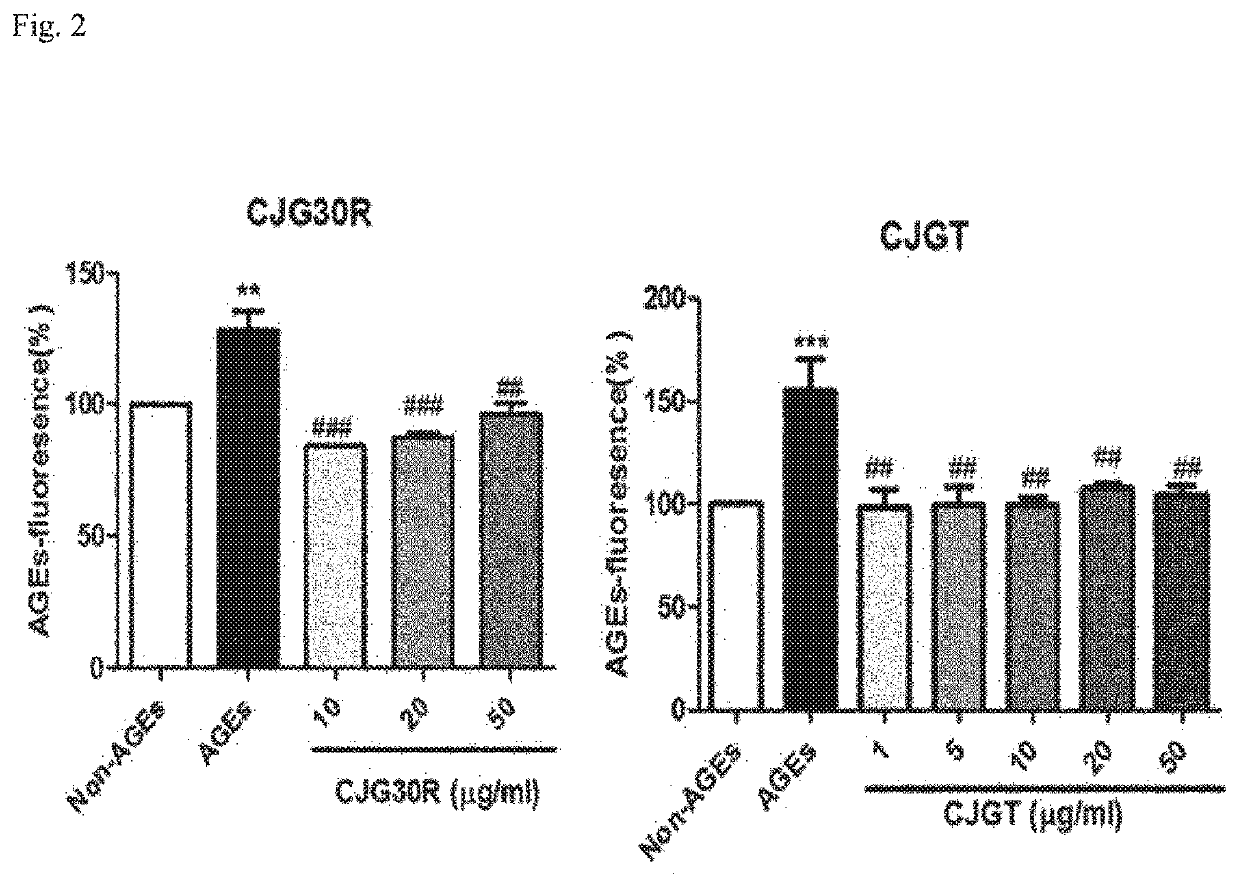
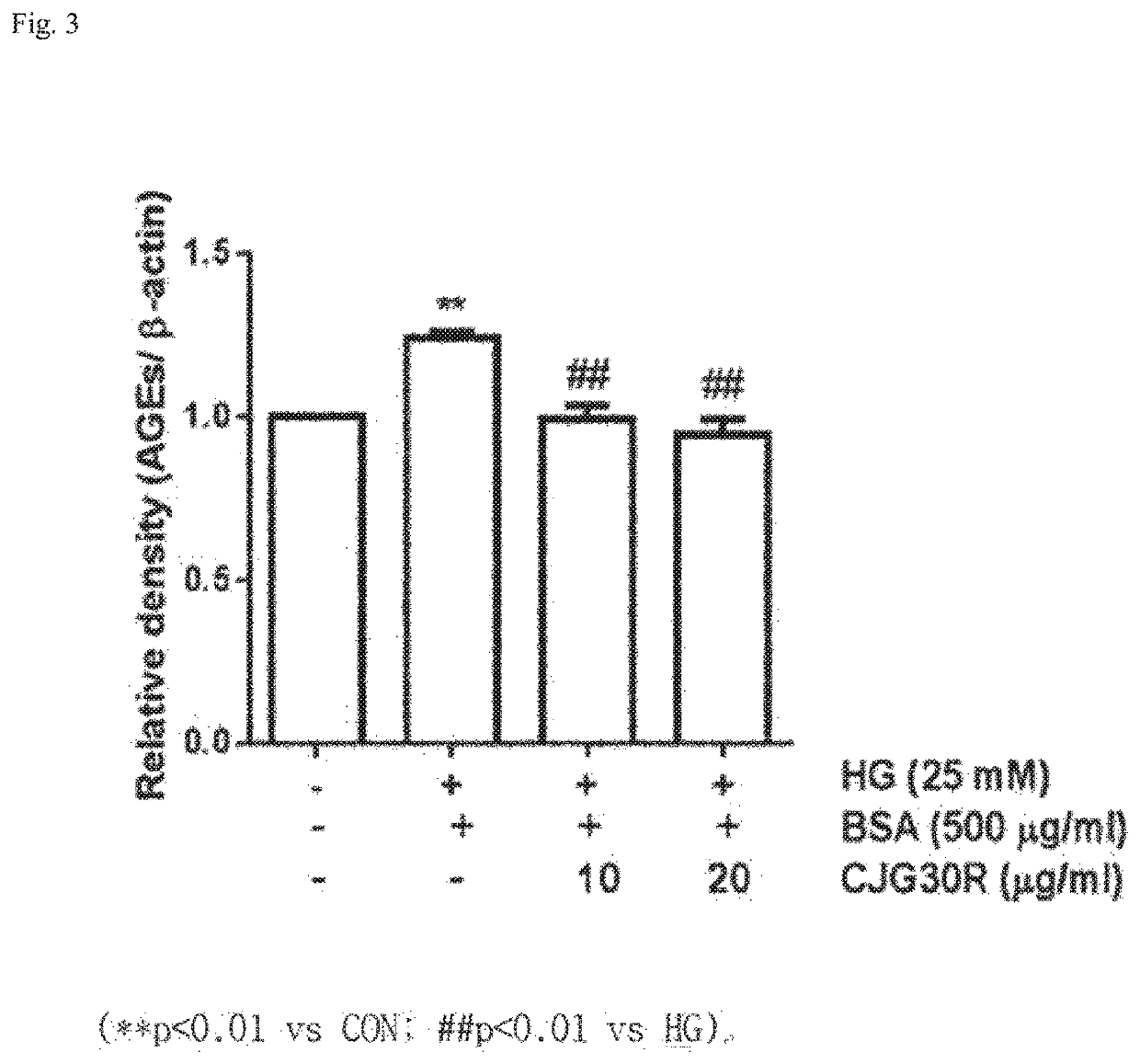
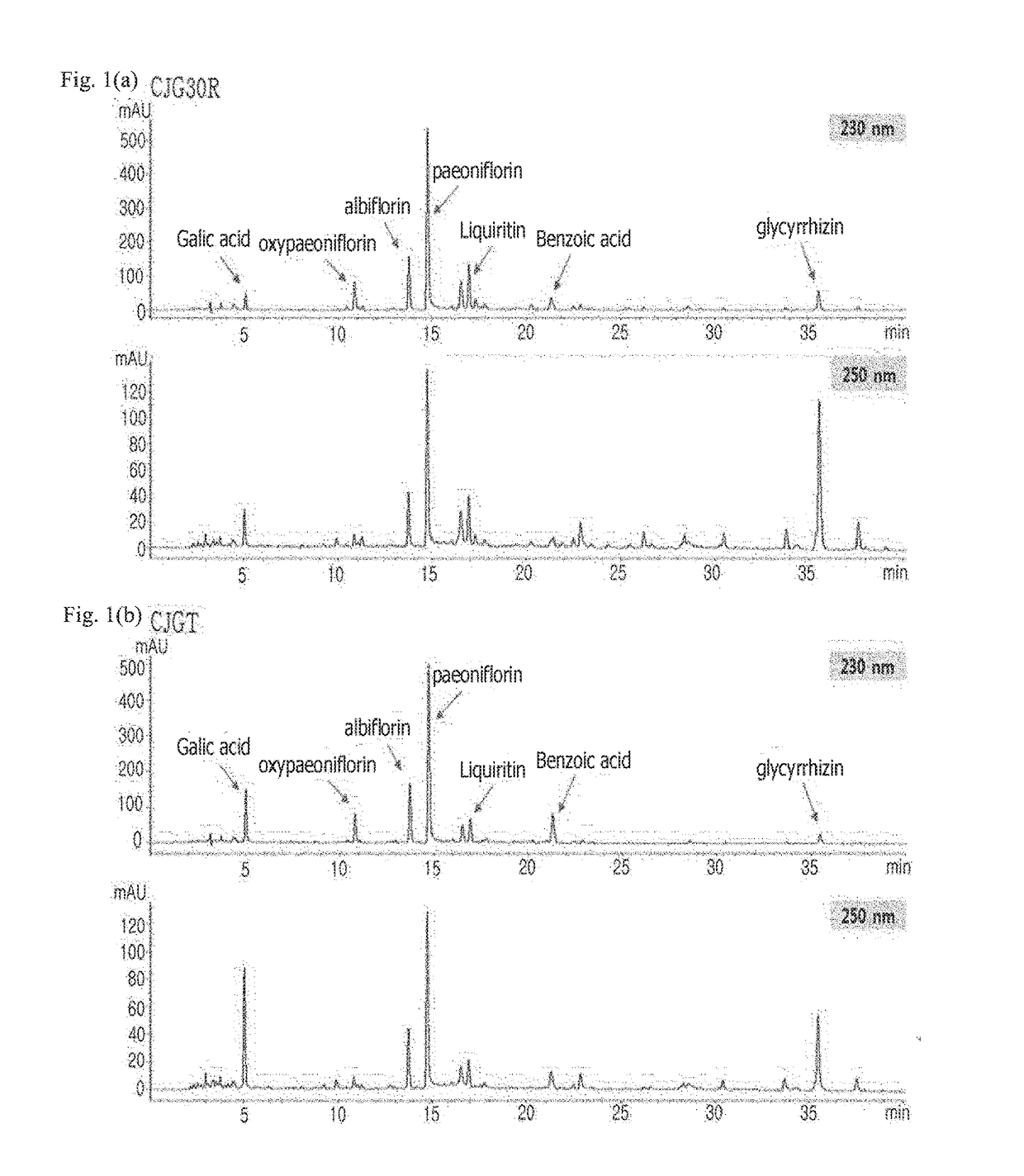
Pharmaceutical composition for preventing or treating angioedema, containing extract of peony root or mixture of peony root and licorice as active ingredient
ActiveUS10537603B2Preventing and ameliorating angioedemaDry macular degenerationCardiovascular disorderPlant ingredientsPAEONIA ExtractMacula lutea degeneration
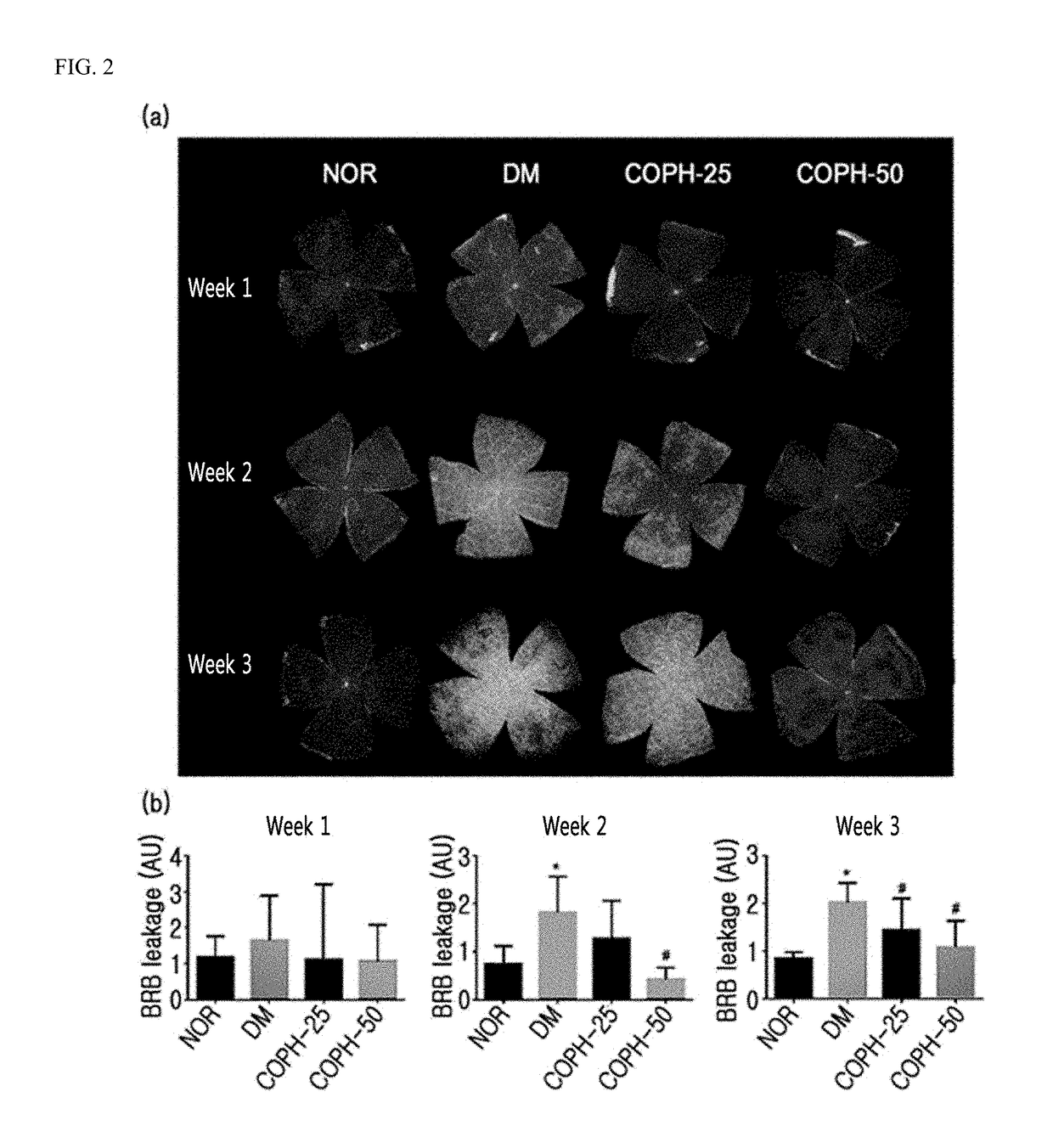
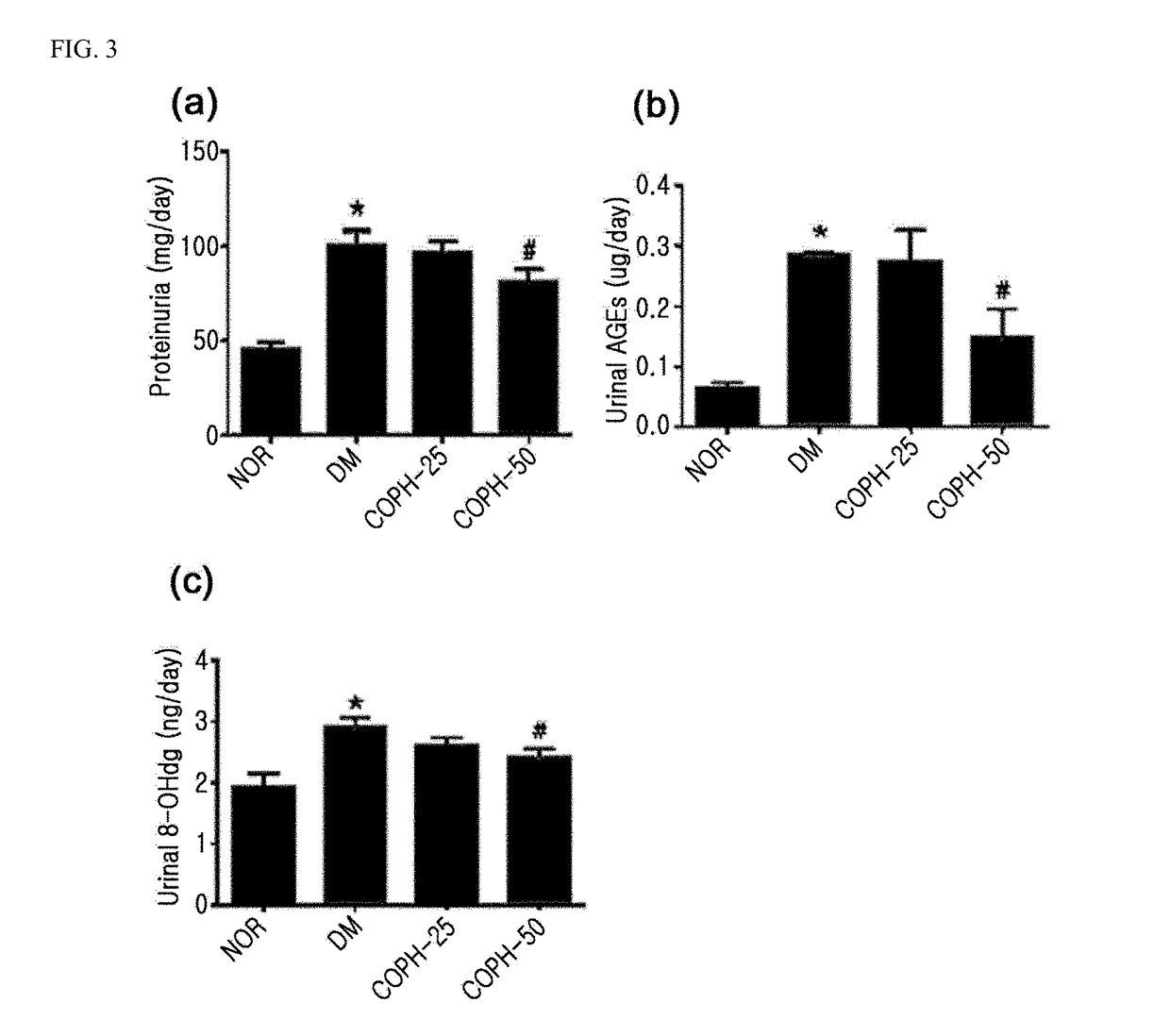
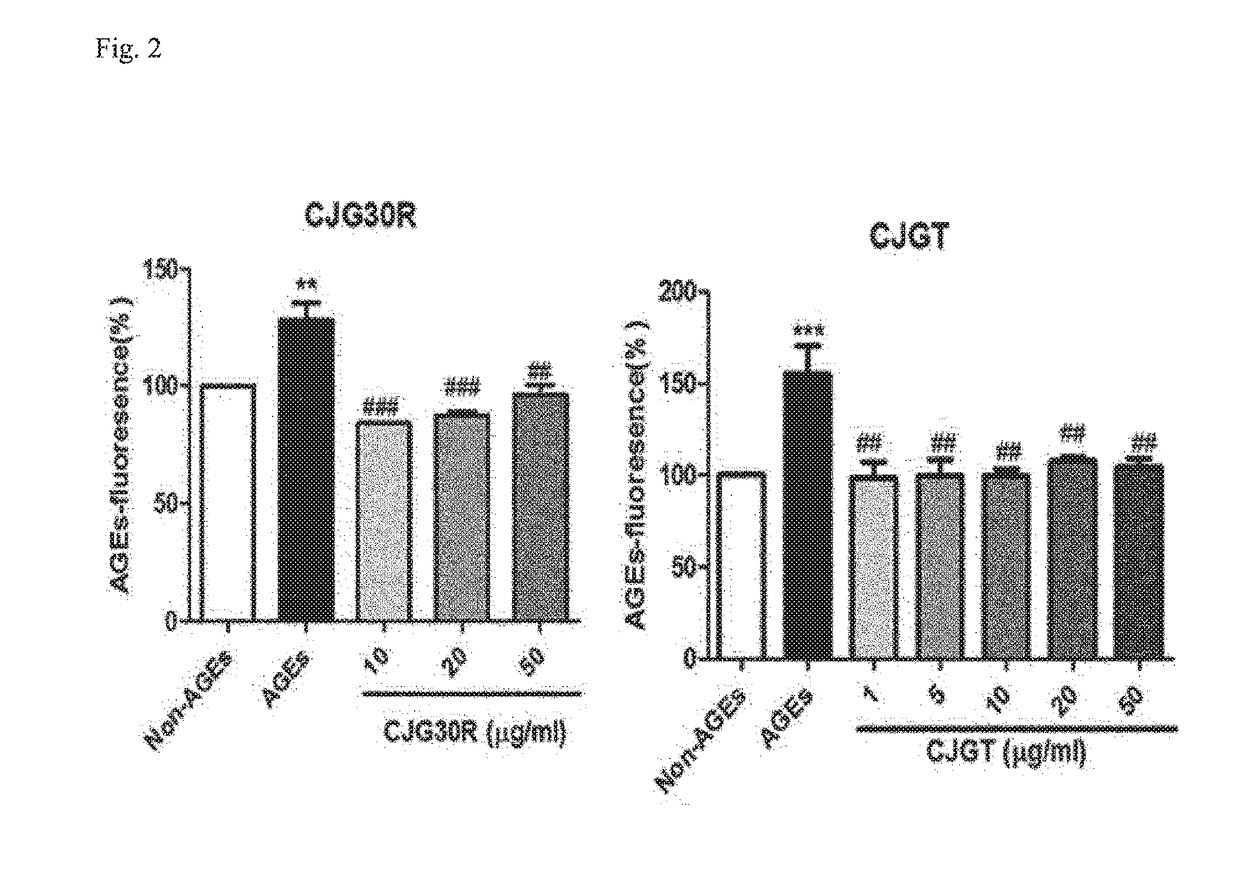
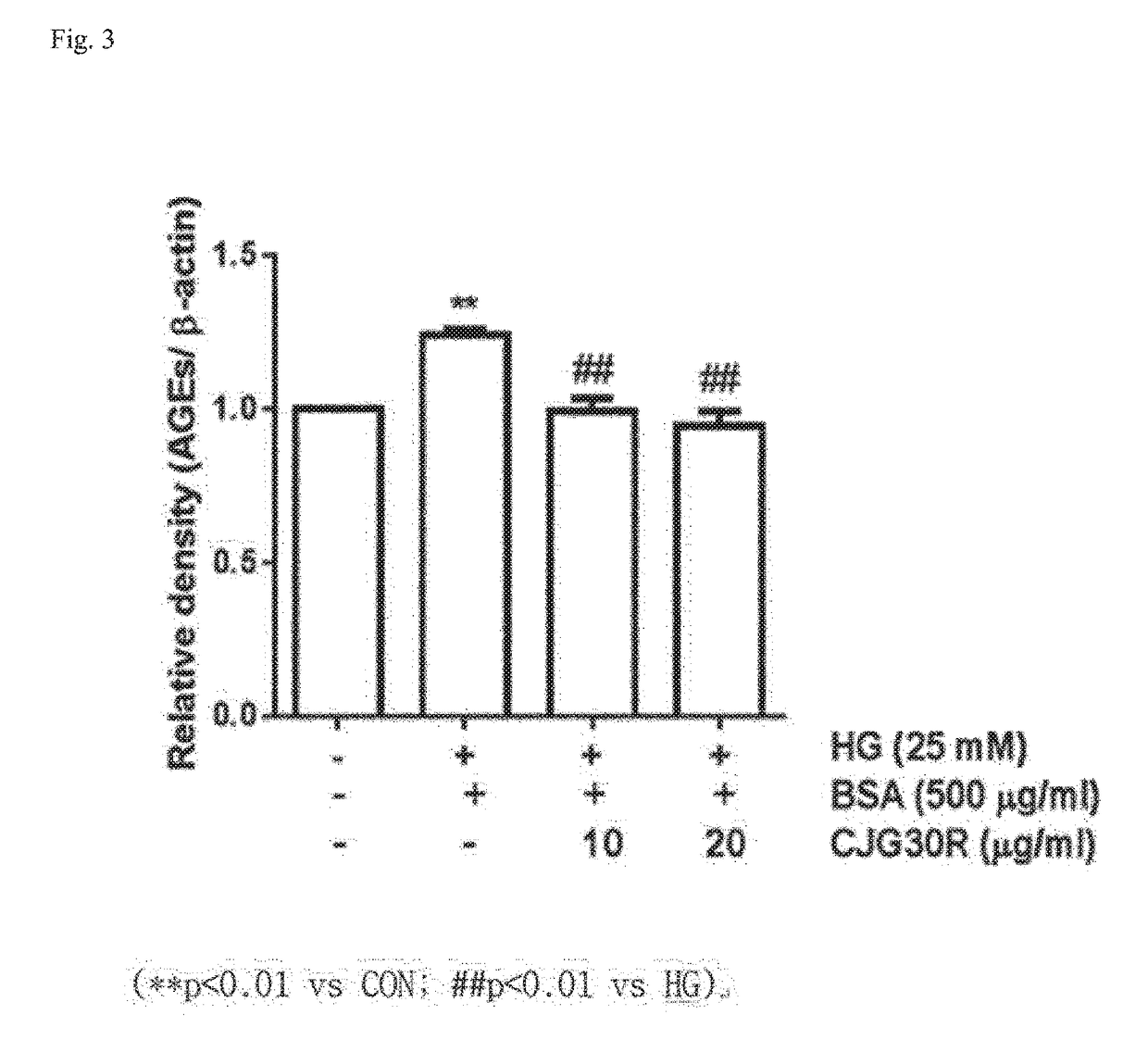
The present invention relates to a pharmaceutical composition for preventing or treating angioedema, containing as an active ingredient a peony root extract or an extract of a mixture of peony root and licorice. In particular, the extract of a mixture of peony root and licorice according to the present invention inhibits the excessive generation of advanced glycation end products, which may cause macular degeneration, inhibits blood-retinal barrier breakdown causing retinal edema in various animal models, protects or treats a subretinal region causing dry macular degeneration, inhibits angiogenesis causing wet macular degeneration, and thus can be usefully used as an active ingredient for the composition for preventing and treating angioedema including macular degeneration, macular edema, retinal edema, or varicose veins.
Owner:KOREA INST OF ORIENTAL MEDICINE
Pharmaceutical composition for preventing or treating angioedema, containing extract of peony root or mixture of peony root and licorice as active ingredient
ActiveUS20170209510A1Excessively generatedDry macular degenerationCardiovascular disorderPlant ingredientsAdditive ingredientGLYCYRRHIZA EXTRACT
The present invention relates to a pharmaceutical composition for preventing or treating angioedema, containing as an active ingredient a peony root extract or an extract of a mixture of peony root and licorice. In particular, the extract of a mixture of peony root and licorice according to the present invention inhibits the excessive generation of advanced glycation end products, which may cause macular degeneration, inhibits blood-retinal barrier breakdown causing retinal edema in various animal models, protects or treats a subretinal region causing dry macular degeneration, inhibits angiogenesis causing wet macular degeneration, and thus can be usefully used as an active ingredient for the composition for preventing and treating angioedema including macular degeneration, macular edema, retinal edema, or varicose veins.
Owner:KOREA INST OF ORIENTAL MEDICINE
Use of N-Phenyl-2-pyrimidineamine Derivativea Against Mast Cell-based Diseases Like Allergic Disorders
InactiveUS20100041666A1Organic active ingredientsNervous disorderAllergic dermatitisSIDS - Sudden infant death syndrome
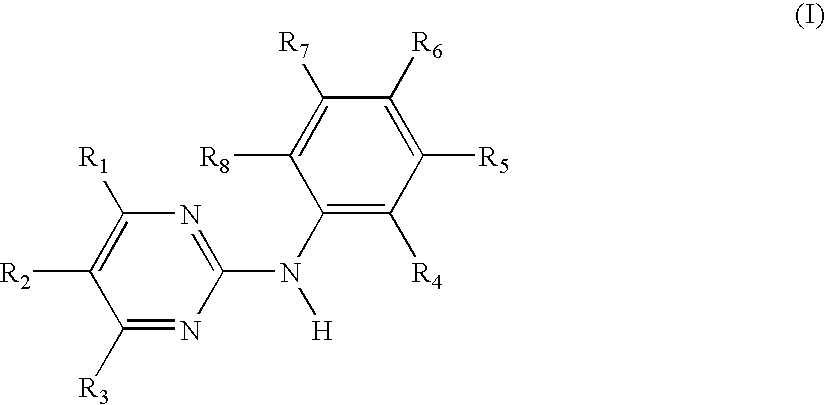
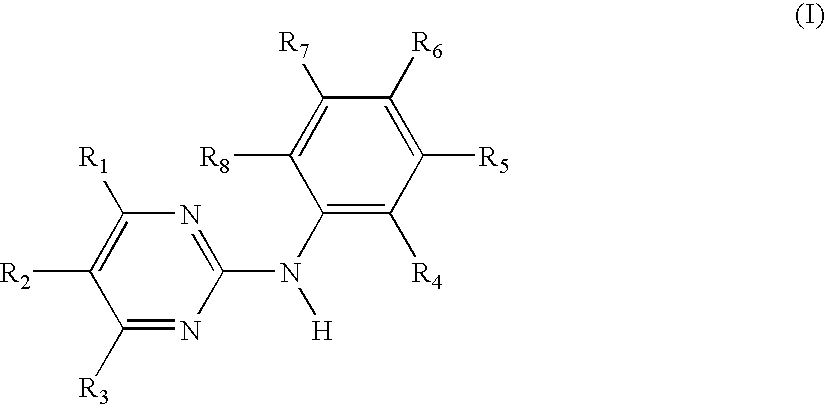
Use of the N-phenyl-2-pyrimidine-amine derivatives of formula I,in which the symbols and substituents have the meaning as given herein in free form or in pharmaceutically acceptable salt form in the manufacture of a pharmaceutical composition for the treatment of anergic rhinitis, allergic dermatitis, drug allergy or food allergy, angioedema, urticaria, sudden infant death syndrome, bronchopulmonary aspergillosis, multiple sclerosis or mastocytosis;
Owner:BARNETT STANLEY F +4
Assessment, determination and treatment of pkal-mediated disorders
The present invention provides assays for the detection of plasma protease C1 inhibitors (C1-INH) that bind plasma kallikrein, factor XII, or both, and their use to identify enzymes that are pKal-mediated or bradykinin-mediated. Risk of a disorder or use in a subject with a pKal-mediated or bradykinin-mediated disorder. The provided methods allow analysis of patients with plasma kallikrein-mediated angioedema (KMA) or other diseases mediated by pKal that can be used for evaluation and treatment.
Owner:TAKEDA PHARMA CO LTD
Use of anti-factor XII antibodies for treating or preventing hereditary angioedema
PendingCN114761437AReduce the risk of seizuresImmunoglobulins against blood coagulation factorsAntibody ingredientsAntiendomysial antibodiesHereditary angioedema
The present invention relates to an anti-FXII antibody for use in a method of treating or preventing hereditary angioedema (HAE) in a subject in which said antibody is administered subcutaneously to said subject.
Owner:シーエスエル イノベーション プロプライアタリー リミティド
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com