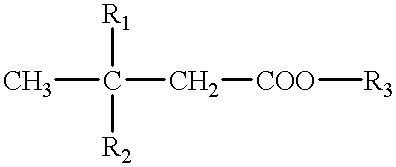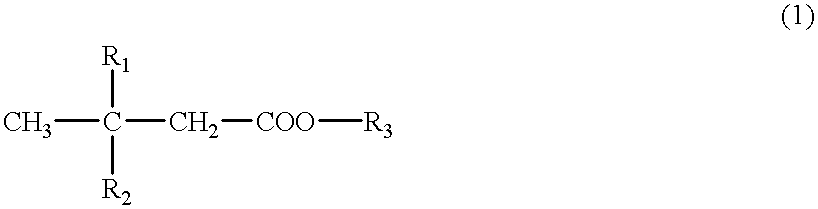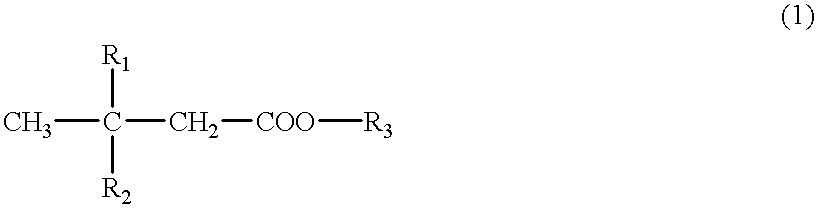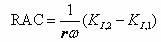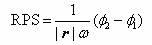Patents
Literature
223 results about "Cerebral edema" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain.
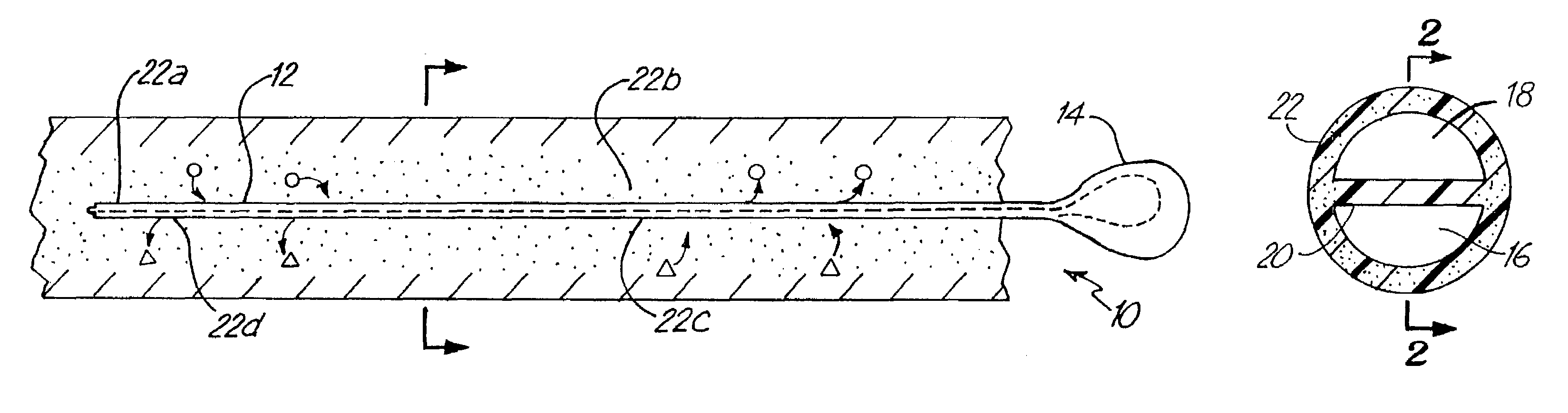
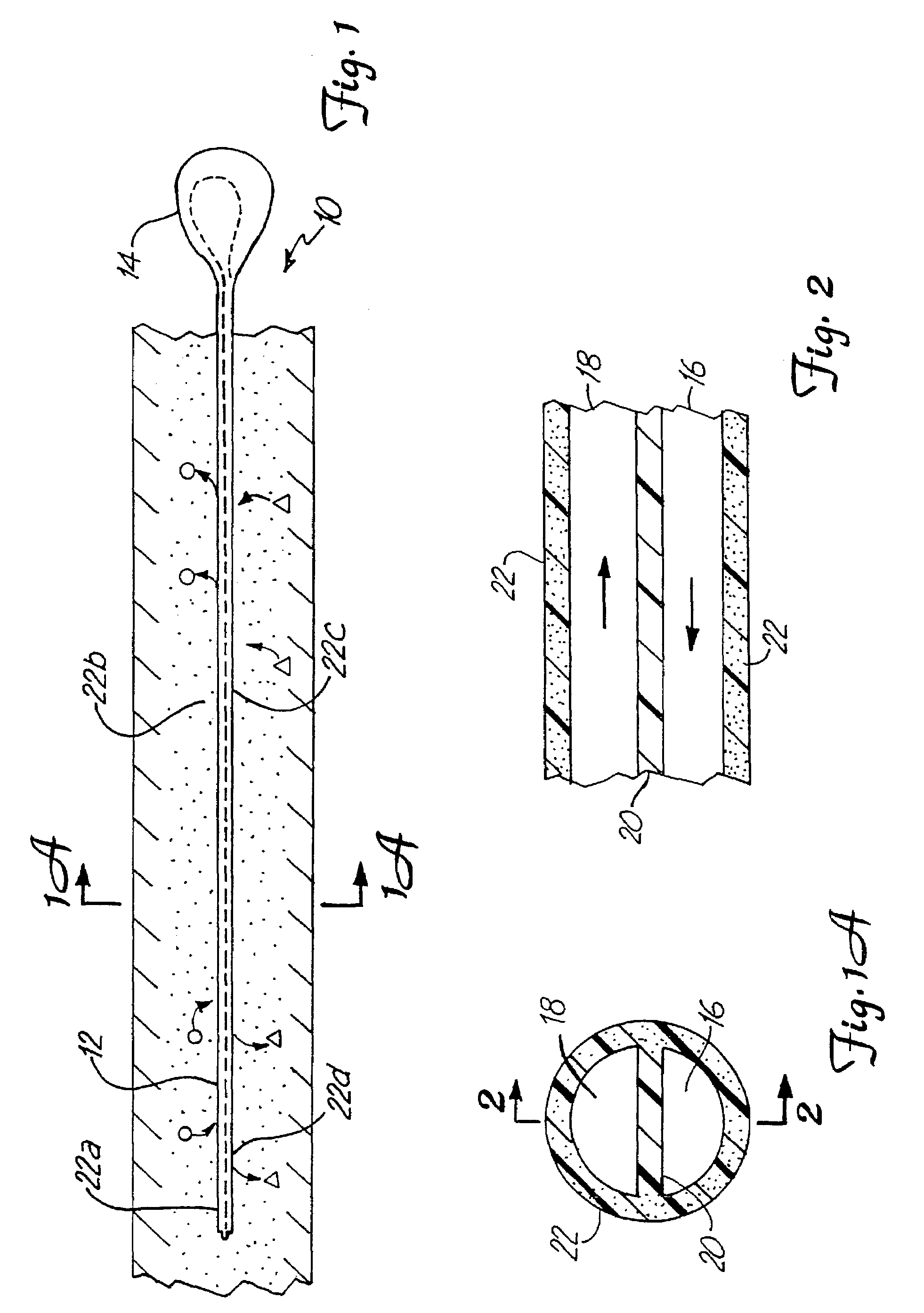
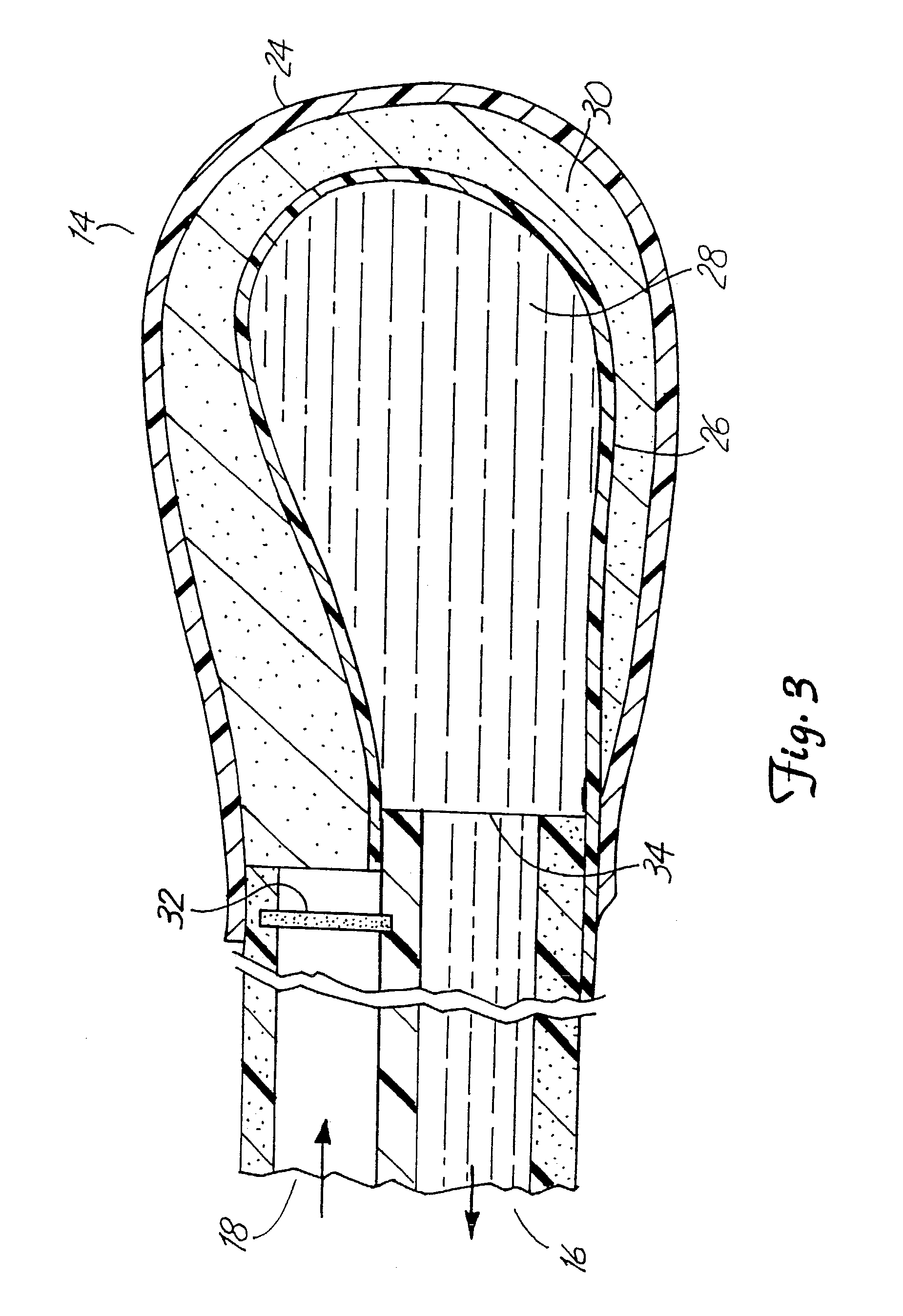
System for treating tissue swelling
InactiveUS6942634B2Easy to separateIncrease concentrationSemi-permeable membranesWound drainsUltrafiltrationBiology
Owner:TWIN STAR MEDICAL
Method and system for treating tissue swelling
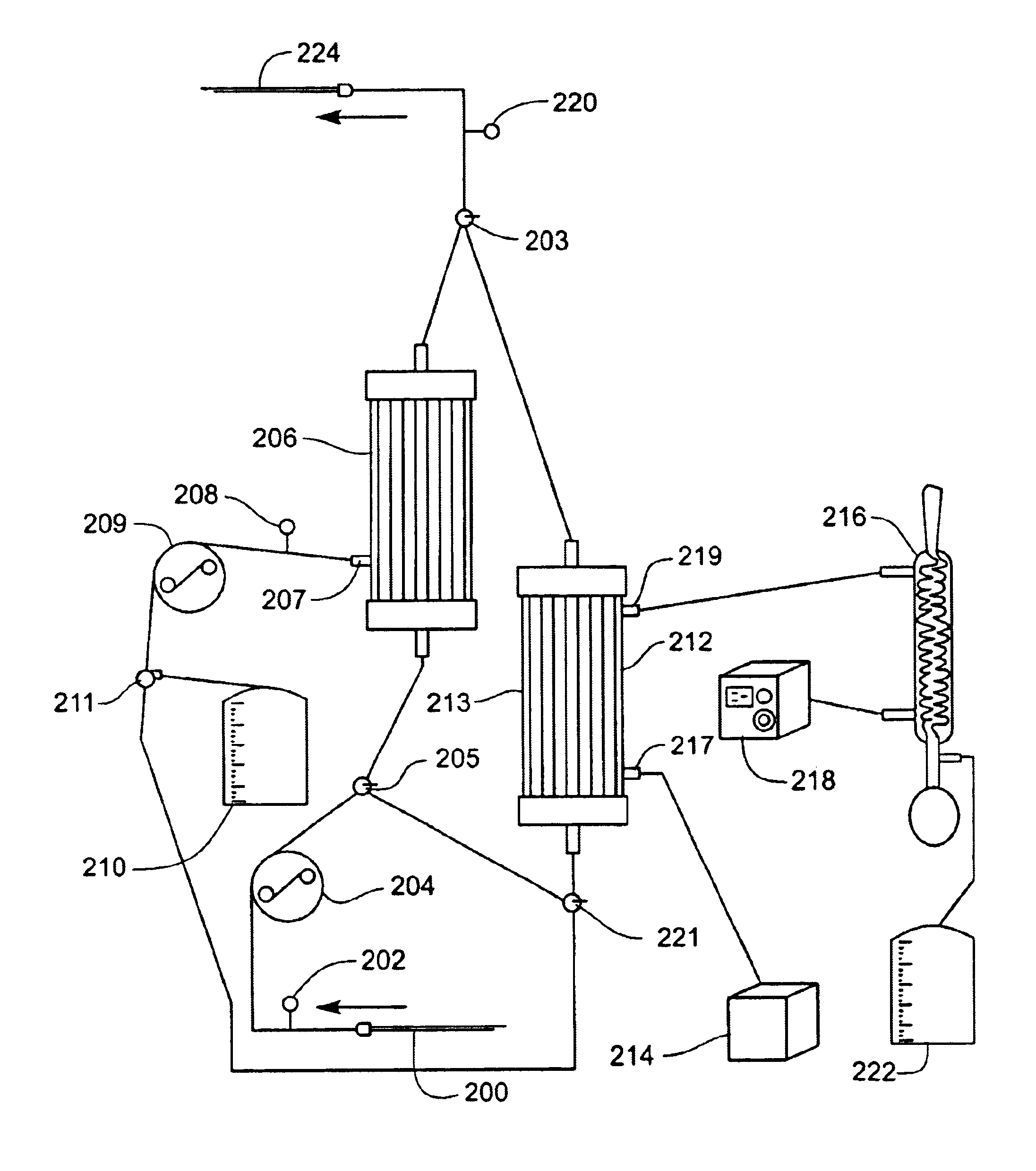
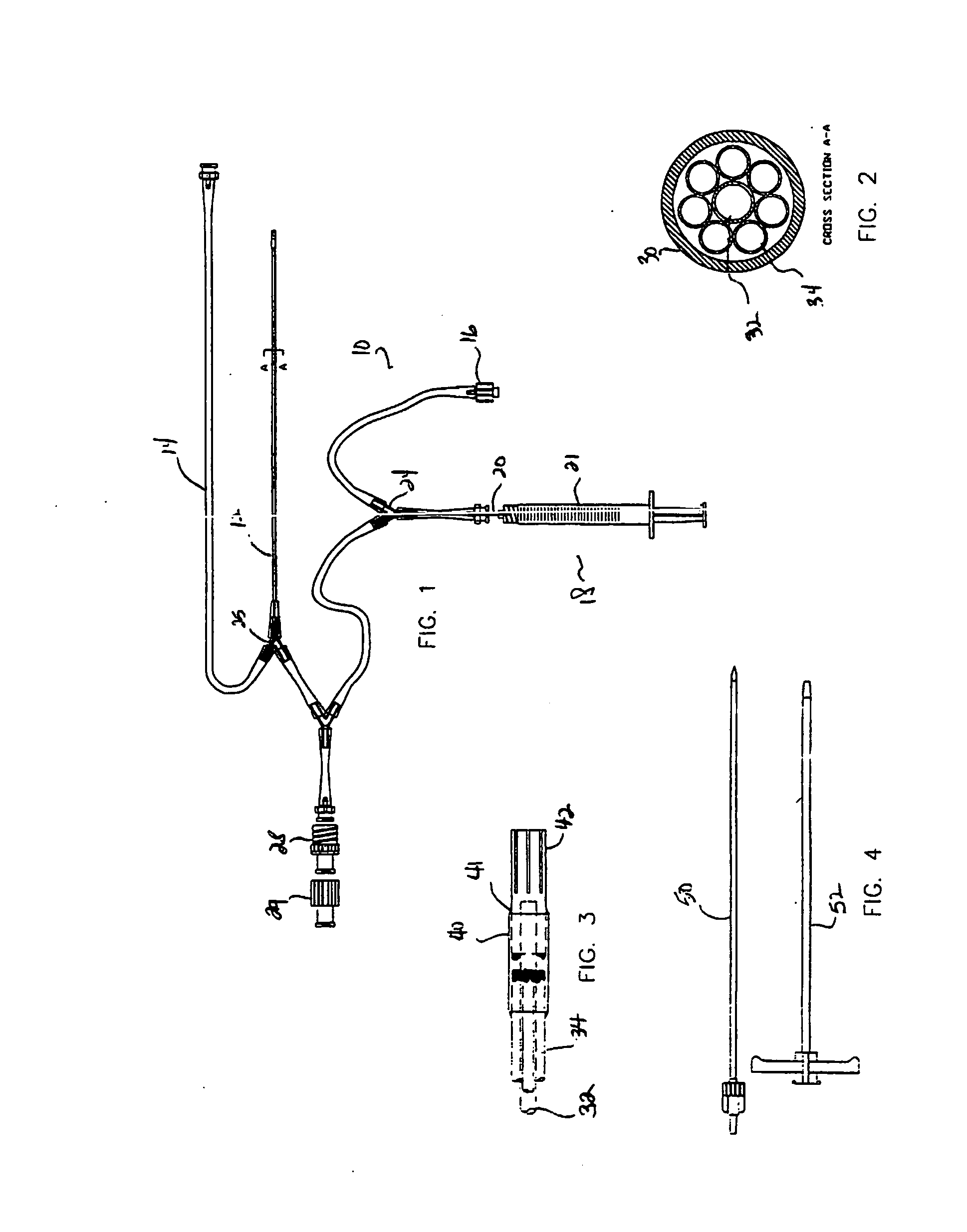
A system and related methods and components for treating a tissue site exhibiting interstitial hypertension, including tissue swelling, and particularly swelling associated with cerebral edema, compartment syndrome, and congestive heart failure, by the use of water removal therapy, in order to remove only water from biological fluids. Included also is a system for such use that incorporates one or more monitors, optionally in addition to the use of water removal therapy. By removing only water, all other biologic agents, including essentially all solutes and formed blood elements (such as cells) are increased in concentration in the remaining bodily fluid(s). WRT can be applied to several clinical conditions in which there is an excess of water, and is ideally used in an extracorporeal fashion, in combination with other functions and related components as well, including ultrafiltration.
Owner:TWIN STAR MEDICAL
Cerebral function improving agents
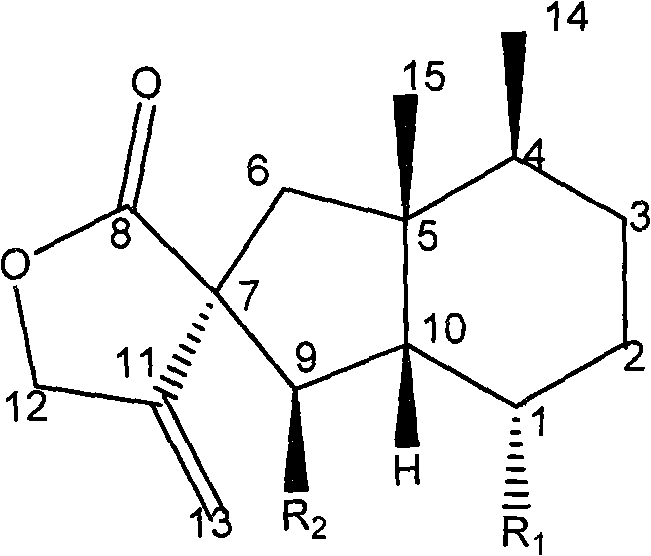
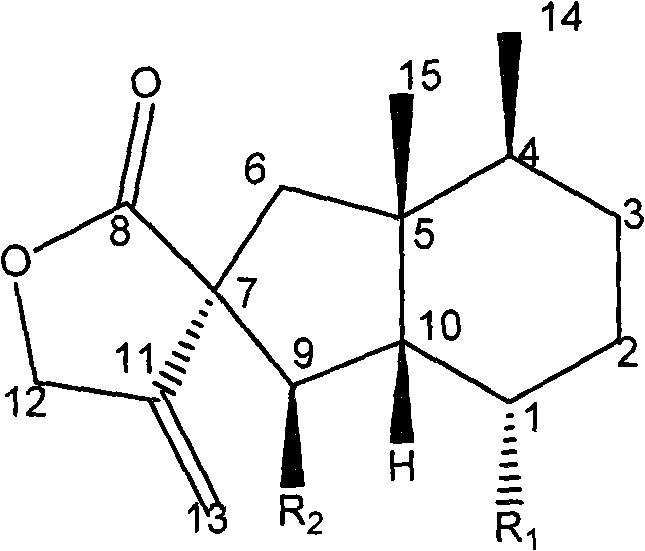
A cerebral function improving agent containing as the active ingredient a compound represented by the following formula:wherein R2 represents a hydrogen atom when R1 is a hydroxyl group;or R1 and R2 in combination represent an oxo group;R3 represents a hydrogen atom, an alkali metal, or a monohydric,dihydric or trihydric alcohol residue;which may be an oligomer composed of 2-10 molecules when R1 represents a hydroxyl group and R2 and R3 represent hydrogen atoms.The agent supresses cerebral edema, protects cerebral function, activates cerebral metabolisms and reduces the extent of cerebral infarction.
Owner:BTG INT LTD
Intrathecal and intratumoral superantigens to treat malignant disease
InactiveUS20060052295A1Improve effectivenessStrong specificityPeptide/protein ingredientsSnake antigen ingredientsDiseaseAbnormal tissue growth
The presence of tumor nodules in organs often results in serious clinical manifestations and the permeation by cancer cells of sheaths surrounding organs often produces clinical manifestations of pleural effusion, ascites or cerebral edema. The present invention addresses this problem by providing a method for treating tumors comprising (a) intratumoral administration of a superantigen and / or (b) intrathecal or intracavitary administration of a superantigen directly into the sheath. Intratumoral superantigen results in significant and sustained reduction of the tumor size. Intrathecal administration produces significant sustained reduction of the fluid accumulation associated with clinical improvement and prolonged survival. Useful superantigen compositions for intrathecal and intratumoral injection include tumoricidally effective homologues, fragments and fusion proteins of native superantigens. Also disclosed is combined therapy that includes intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs.
Owner:JENQUEST
System and method for site specific therapy
InactiveUS7717871B2Optimize allocationLower the volumeDialysis systemsMedical devicesEngineeringPhysical therapy
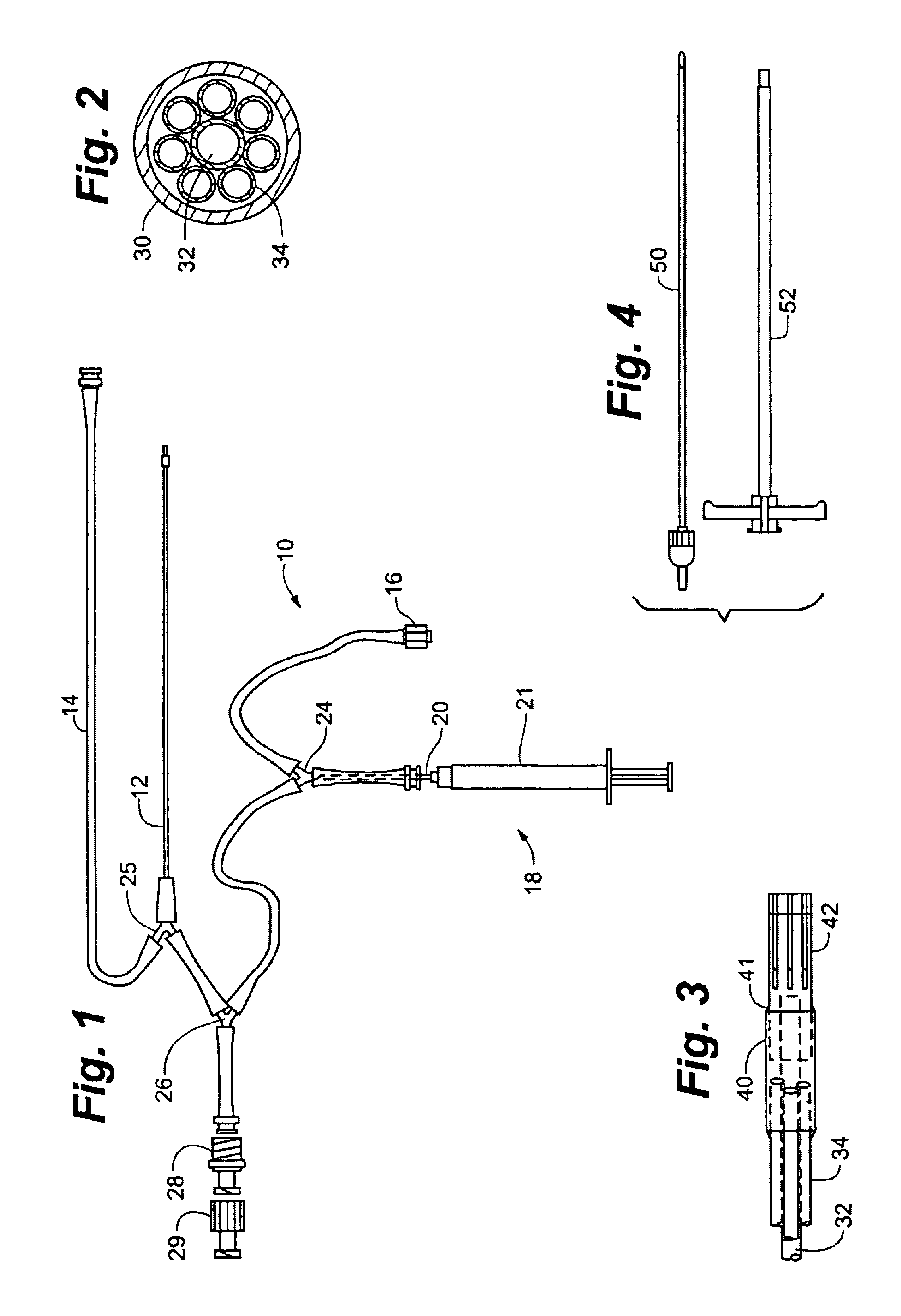
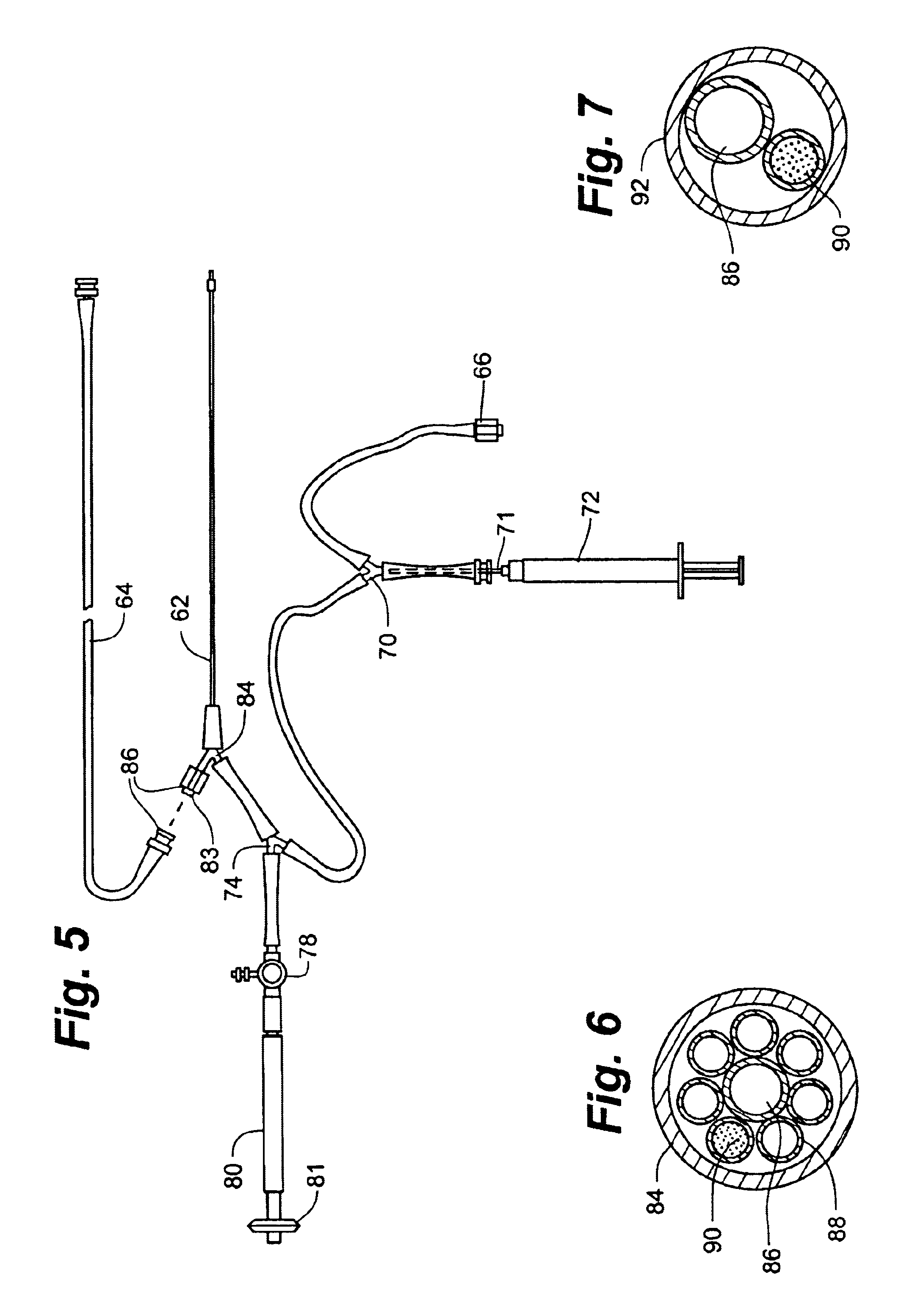
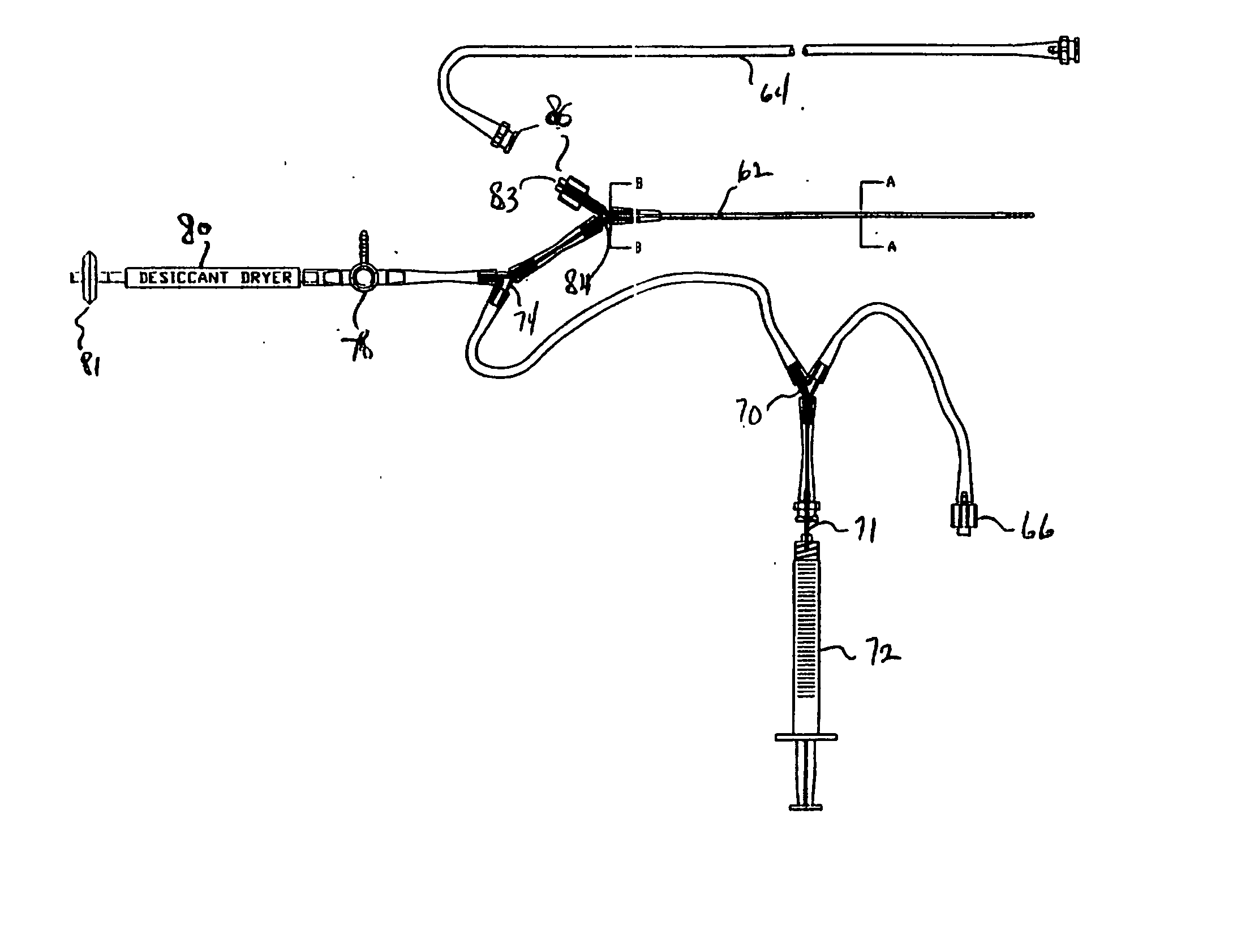
A system, including catheter apparatus, and related method for performing site specific therapy. The catheter apparatus can include one or more semipermeable microcatheters for use in performing site specific microdialysis. The system and method are particularly suited for use in addressing cerebral edema by affecting the osmolar relationship between fluids making up the brain tissue. Also disclosed is an apparatus having a delivery / recovery mechanism in the form of a pump reservoir and one or more catheters in the form of semipermeable microcatheters, for use in delivering and / or recovering fluid to and / or from a tissue site or for performing tissue engineering outside of the body. The apparatus can be used in a method to perform site specific microtherapy, including for the treatment of avascular necrosis, compartment syndrome, cerebral edema, and to improve skin flap survival in the course of reconstructive surgery.
Owner:TWIN STAR MEDICAL
Use of timosaponin for preparing medicine for preventing and treating brain apoplexy
InactiveCN1451384AReduce edemaReduce volumeOrganic active ingredientsNervous disorderReperfusion injuryCerebral edema
An use of common timosaponin in preparing medicine or food for preventing and treating cerebral apoplexy is disclosed.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
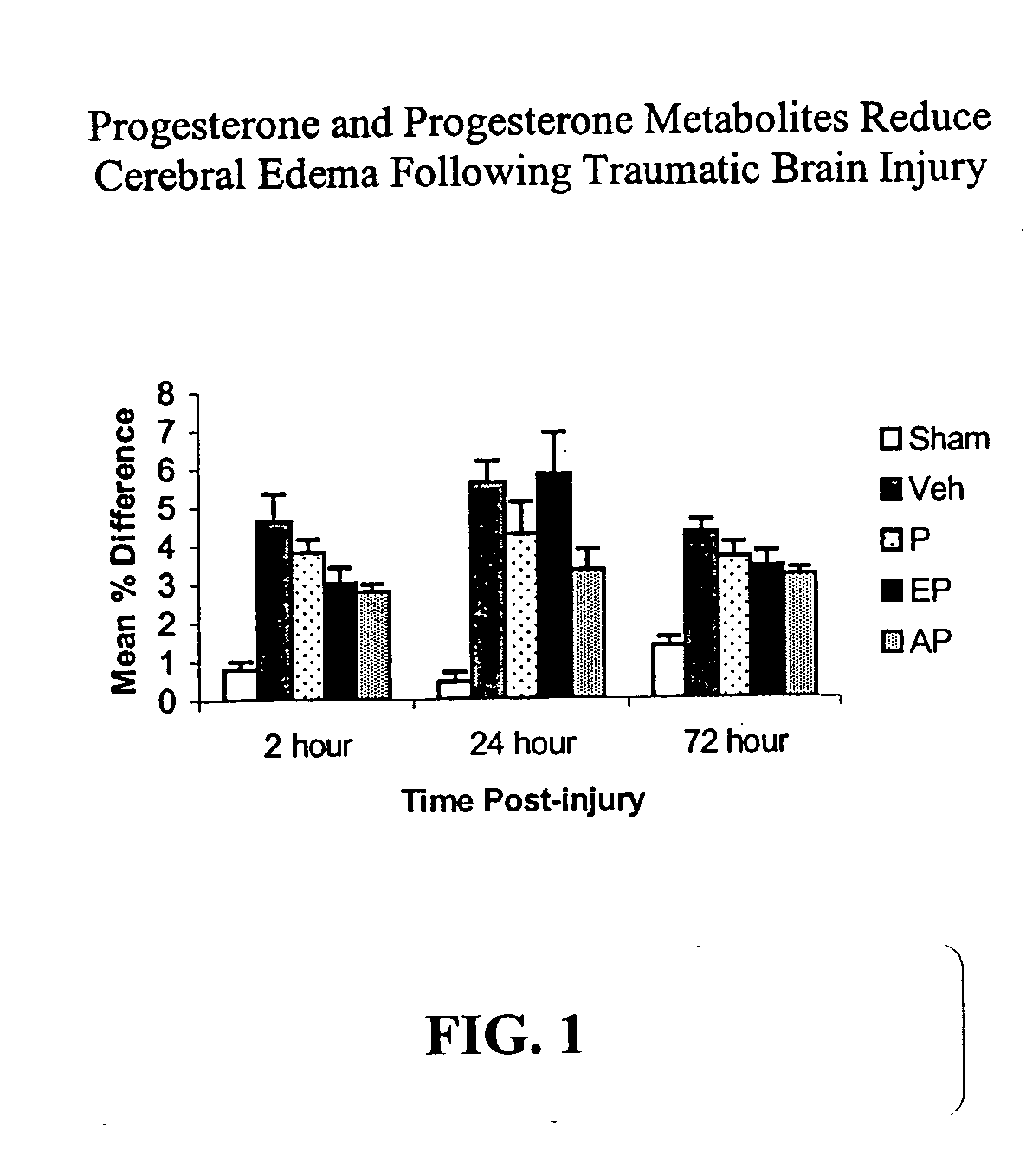
Methods for the treatment of a traumatic central nervous system injury
InactiveUS20050187188A1Reducing cerebral edema and inflammatory responseAvoid damageBiocideOrganic active ingredientsMetaboliteBrain traumas
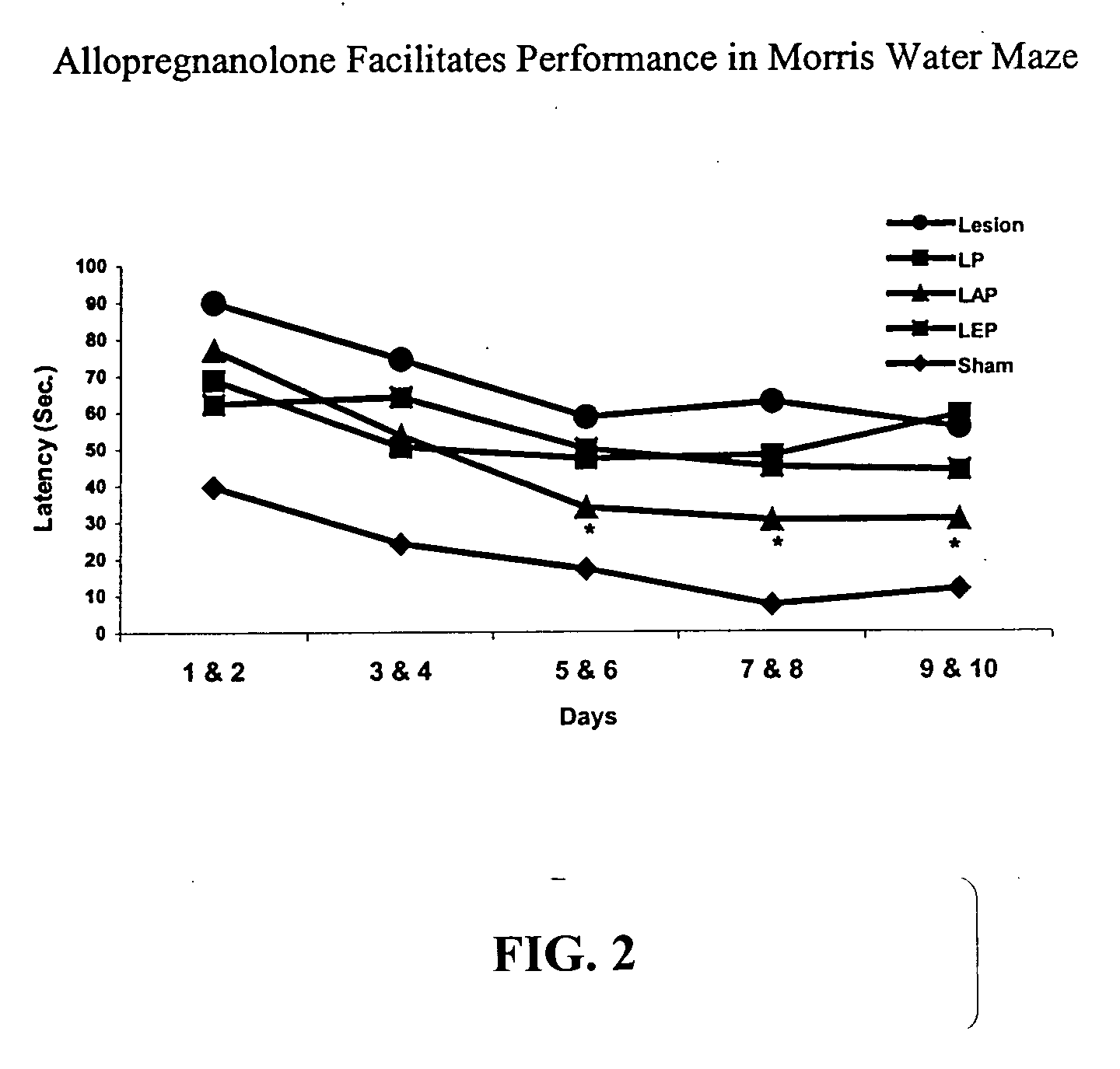
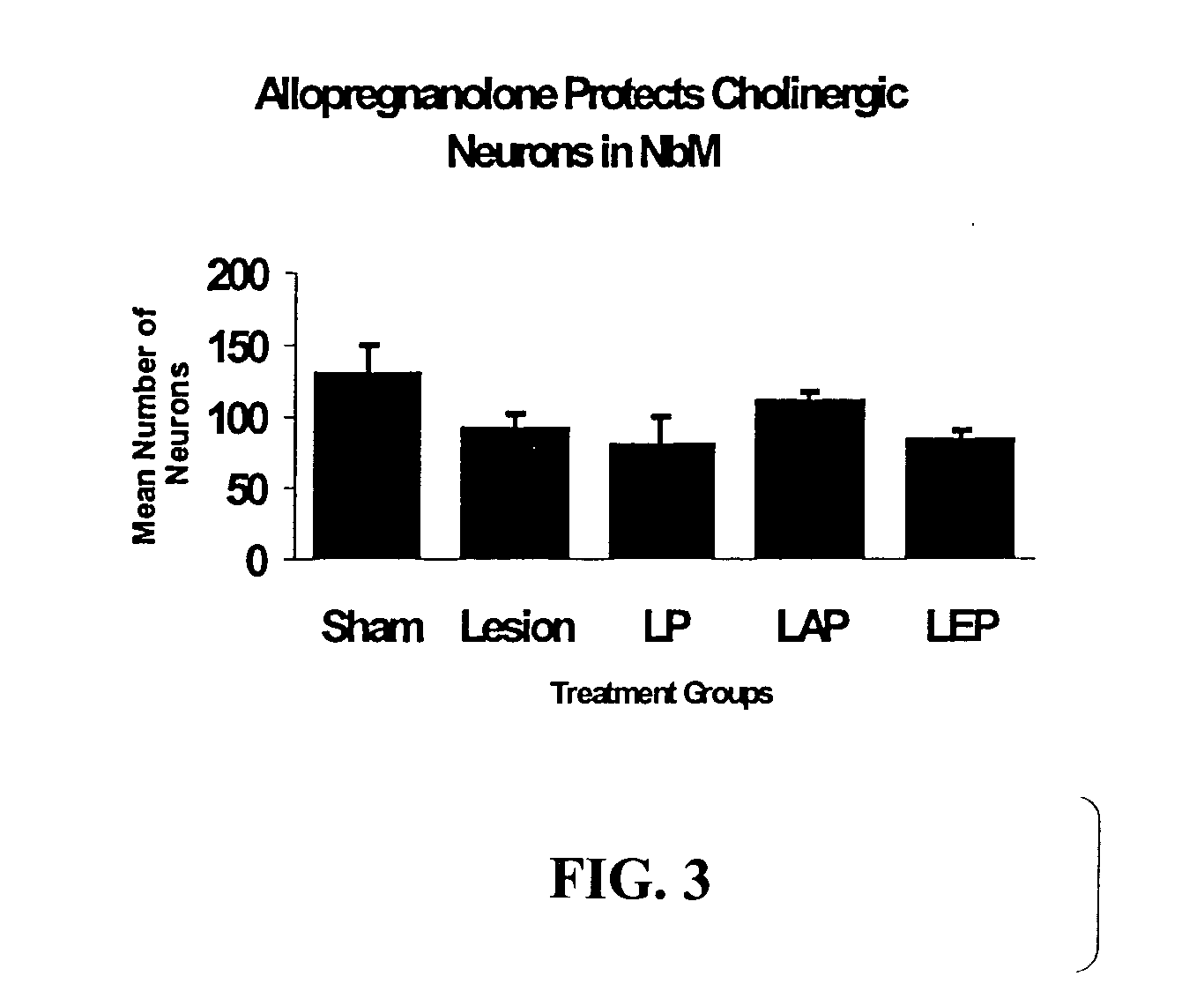
The present invention provides methods for conferring a neuroprotective effect on a population of cells in a subject following a traumatic injury to the central nervous system. Specifically, the methods of the invention provide for the administration of a progestin or progestin metabolite following a traumatic brain injury. The progestin or progestin metabolite is administered at therapeutically effective concentrations that produce a neuroprotective effect (i.e., a decrease in the loss of neuronal activity) and reduces and / or prevents the various physiological events leading to neurodegeneration, such as, cerebral edema and the immune / inflammatory response.
Owner:EMORY UNIVERSITY
Medicine for acute ischemic apoplexy and its preparing method
A Chinese medicine in the form of injection for treating the acute stage of ischemic apoplexy and cerebral embolism is prepared from 3 Chinese-medicinal materials; red sage root, red peony root and grass-leaved sweetflag rhizome. Its advantage is high curative effect.
Owner:张晴龙
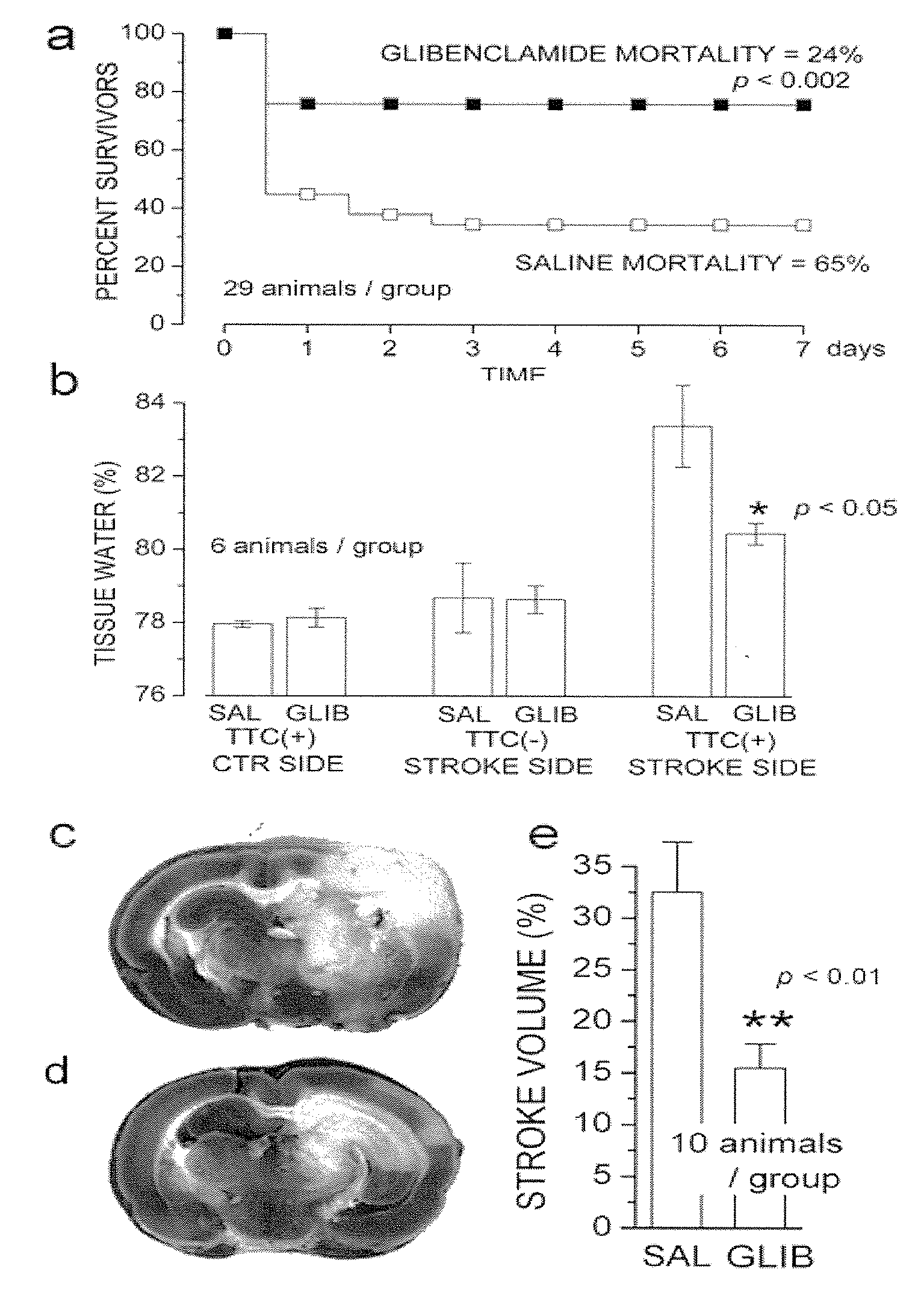
Therapeutic Agents Targeting the NCCA-ATP Channel and Methods of Use Thereof
ActiveUS20090130083A1Expanding Therapeutic WindowAvoid depolarizationBiocideNervous disorderAbnormal tissue growthAntagonist
The present invention is directed to therapeutic compositions targeting the NCCa-ATP channel of an astrocyte, neuron or capillary endothelial cell and methods of using same. More specifically, agonists and antagonists of the NCCa-ATP channel are contemplated. The therapeutic compositions are used to treat cancer, more specifically, a metastatic brain tumor, wherein a tumor-brain barrier is present. Such treatments are contemplated in combination with conventional anti-cancer therapies. Alternatively, the compositions are used to prevent cell death and to treat cerebral edema that result from ischemia, due to interruption of blood flow, to tissue trauma or to increased tissue pressure.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
System and method for site specific therapy
InactiveUS20100286586A1Optimize allocationLower the volumeWound drainsDialysis systemsFlap survivalReconstructive surgery
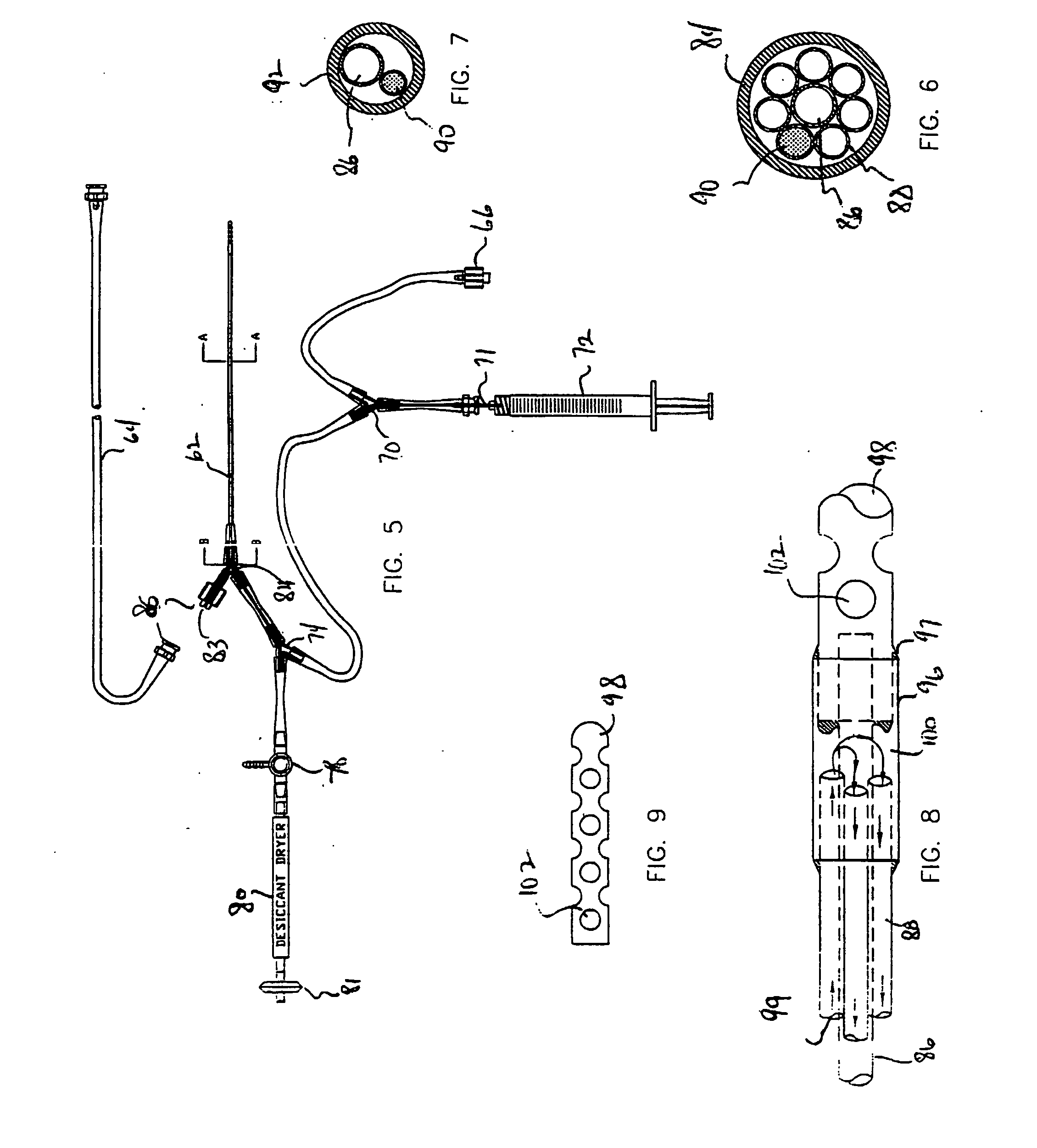
A system, including catheter apparatus, and related method for performing site specific therapy. The catheter apparatus can include one or more semipermeable microcatheters for use in performing site specific microdialysis. The system and method are particularly suited for use in addressing cerebral edema by affecting the osmolar relationship between fluids making up the brain tissue.Also disclosed is an apparatus having a delivery / recovery mechanism in the form of a pump reservoir and one or more catheters in the form of semipermeable microcatheters, for use in delivering and / or recovering fluid to and / or from a tissue site or for performing tissue engineering outside of the body. The apparatus can be used in a method to perform site specific microtherapy, including for the treatment of avascular necrosis, compartment syndrome, cerebral edema, and to improve skin flap survival in the course of reconstructive surgery.
Owner:TWIN STAR MEDICAL
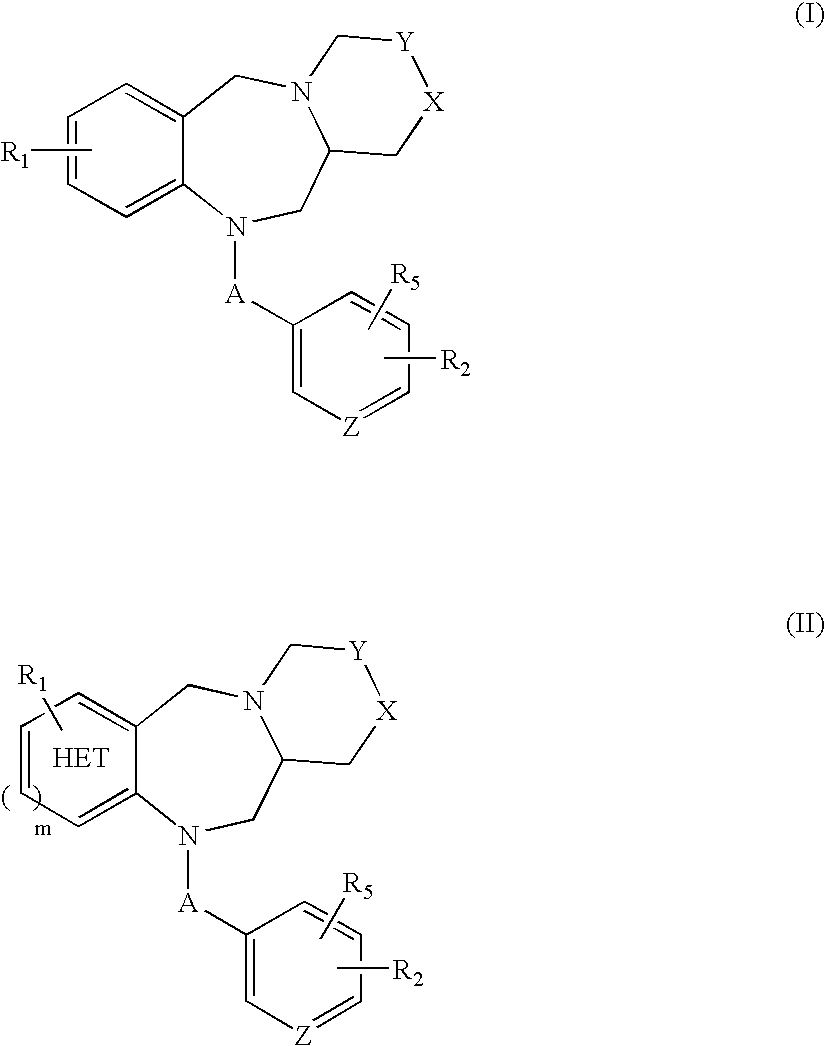
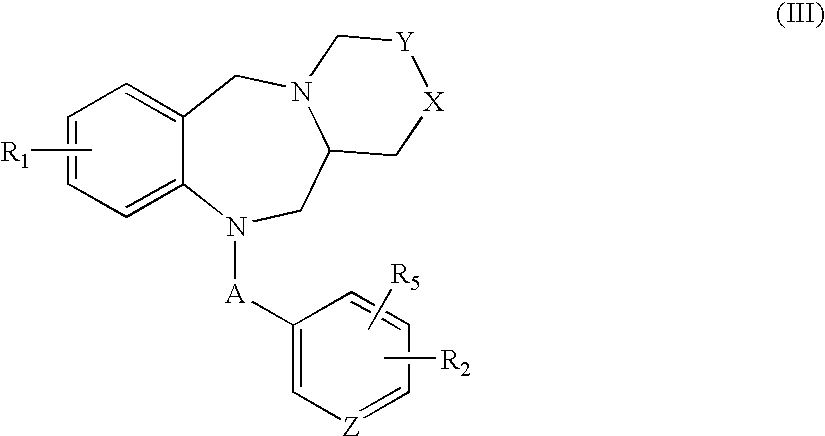
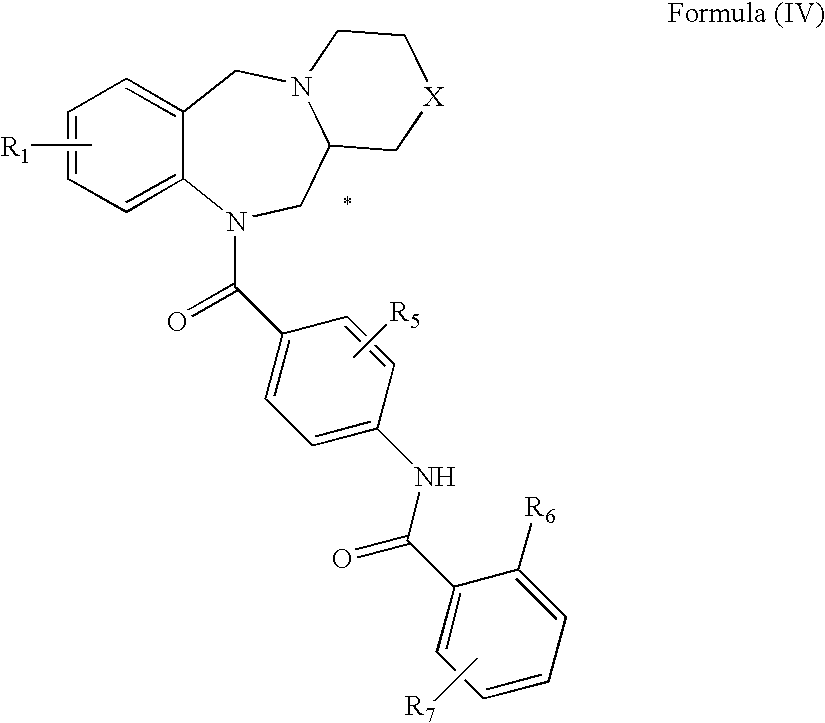
Tricyclic benzodiazepines as vasopressin receptor antagonists
The invention is directed to tricyclic benzodiazepines useful as vasopressin receptor antagonists for treating conditions involving increased vascular resistance and cardiac insufficiency. Pharmaceutical compositions comprising tricyclic benzodiazepines of the present invention and methods of treating conditions such as hypertension, congestive heart failure, cardiac insufficiency, coronary vasospasm, cardiac ischemia, liver cirrhosis, renal vasospasm, renal failure, cerebral edema and ischemia, stroke, thrombosis, or water retention are also disclosed.
Owner:ORTHO MCNEIL PHARM INC
Chinese medicine composition for treating cerebral hemorrhage and its prepn
InactiveCN1772052APromote repairPromote nerve functionAnthropod material medical ingredientsCardiovascular disorderDiseaseCerebral hemorrhages
The present invention is one kind of Chinese medicine composition for treating cerebral hemorrhage and is preparation process. The Chinese medicine composition consists of effective components from motherwort, giant knotweed, leech, gentian, and buffalo horn or rhinoceros horn; and may be prepared into medicine powder, tincture, capsule, tablet, micro pill and other forms. The Chinese medicine composition has the function of promoting blood circulation to disperse blood clots, and can alleviate hemorrhagic apoplexy cerebral edema, promote the absorption of hematoma and disease focus repair, improve the ischemic and anoxic state of nerve tissue around hematoma, protect damaged brain cell and promote restoring of the nerve function.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Composition and method to prevent and treat brain and spinal cord injuries
InactiveUS20060057065A1Reduce productionLow COPBiocideNervous disorderSubarachnoid spaceCerebral edema
The cerebrospinal fluid (CSF) contains low concentration of albumin and insulin because of the blood-CSF barrier. This is the major reason for cerebral edema and the resultant blood perfusion deficit when brain or spinal cord is injured. A composition and method for treating brain and spinal cord are provided. The composition includes magnesium, colloidal osmotic agent, insulin and ATP in artificial CSF. The method includes withdrawing a volume of cerebrospinal fluid from the subarachnoid space and infusing the invented composition.
Owner:WANG YANMING
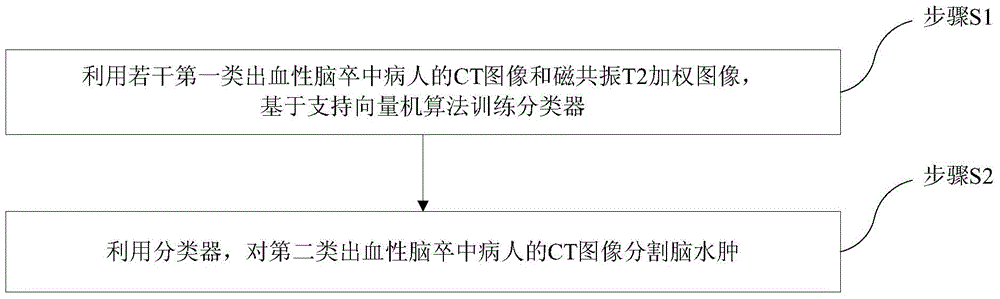
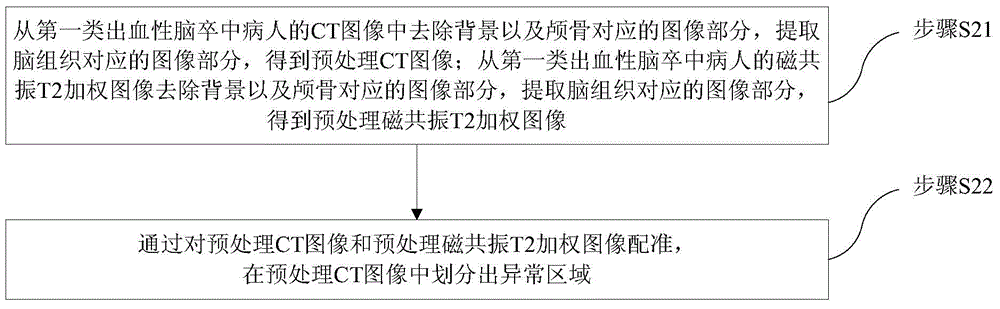
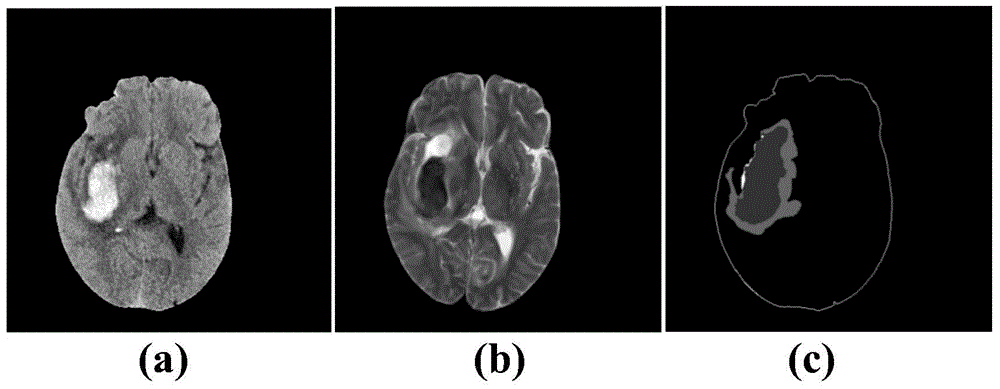
Encephaledema segmentation method and system based on support vector machine algorithm
The invention provides an encephaledema segmentation method and system based on a support vector machine algorithm, which are applied to the medical diagnosis technology field. The encephaledema segmentation method comprises steps of using a plurality of CT images and a plurality of magnetic resonance T2 weighted images of patients suffering from first type hemorrhagic cerebral apoplexy to train a classifier based on the support vector machine algorithm, using the classifier to perform encephaledema segmentation on CT images of patients suffering from the second type hemorrhagic cerebral apoplexy, wherein the patients suffering from the first type hemorrhagic cerebral apoplexy have the CT images and the magnetic resonance T2 weighted images; and the patients suffering from the second type hemorrhagic cerebral apoplexy only have the CT images and do not have the magnetic resonance T2 weighted images. The invention utilizes few patients suffering from the hemorrhagic cerebral apoplexy having the CT images and the magnetic resonance T2 images to perform combined modeling, through studying, establishes a classifier which can identify the encephaledema on the CT from the CT image characteristics, can be applied to the patients who suffer from the hemorrhagic cerebral apoplexy and have the CT images and have no magnetic resonance T2 weighted images, and obtains the higher encephaledema segmentation accuracy.
Owner:SHENZHEN INST OF ADVANCED TECH
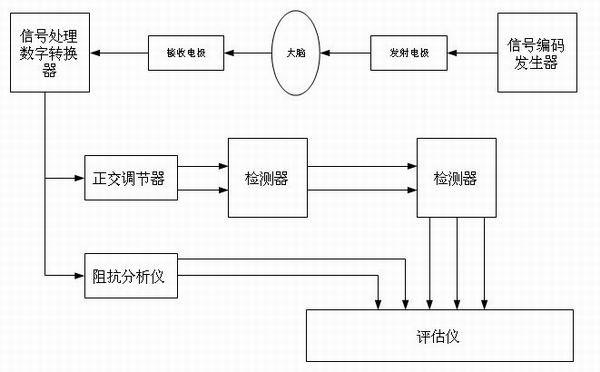
Device for monitoring hydrocephalus and encephaledema
ActiveCN102525458AImprove medical safetyDiagnostic recording/measuringSensorsDigital converterSignal encoding
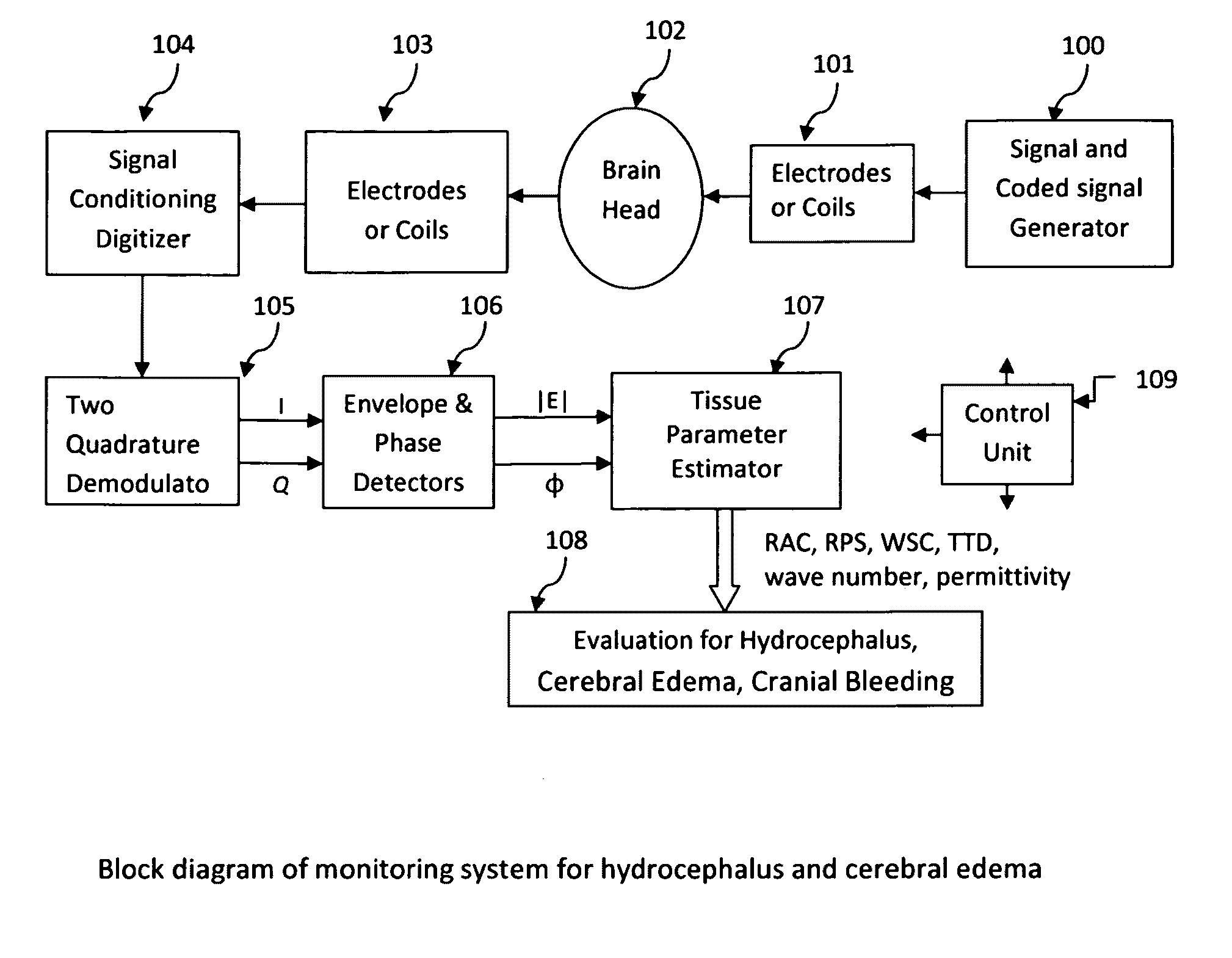
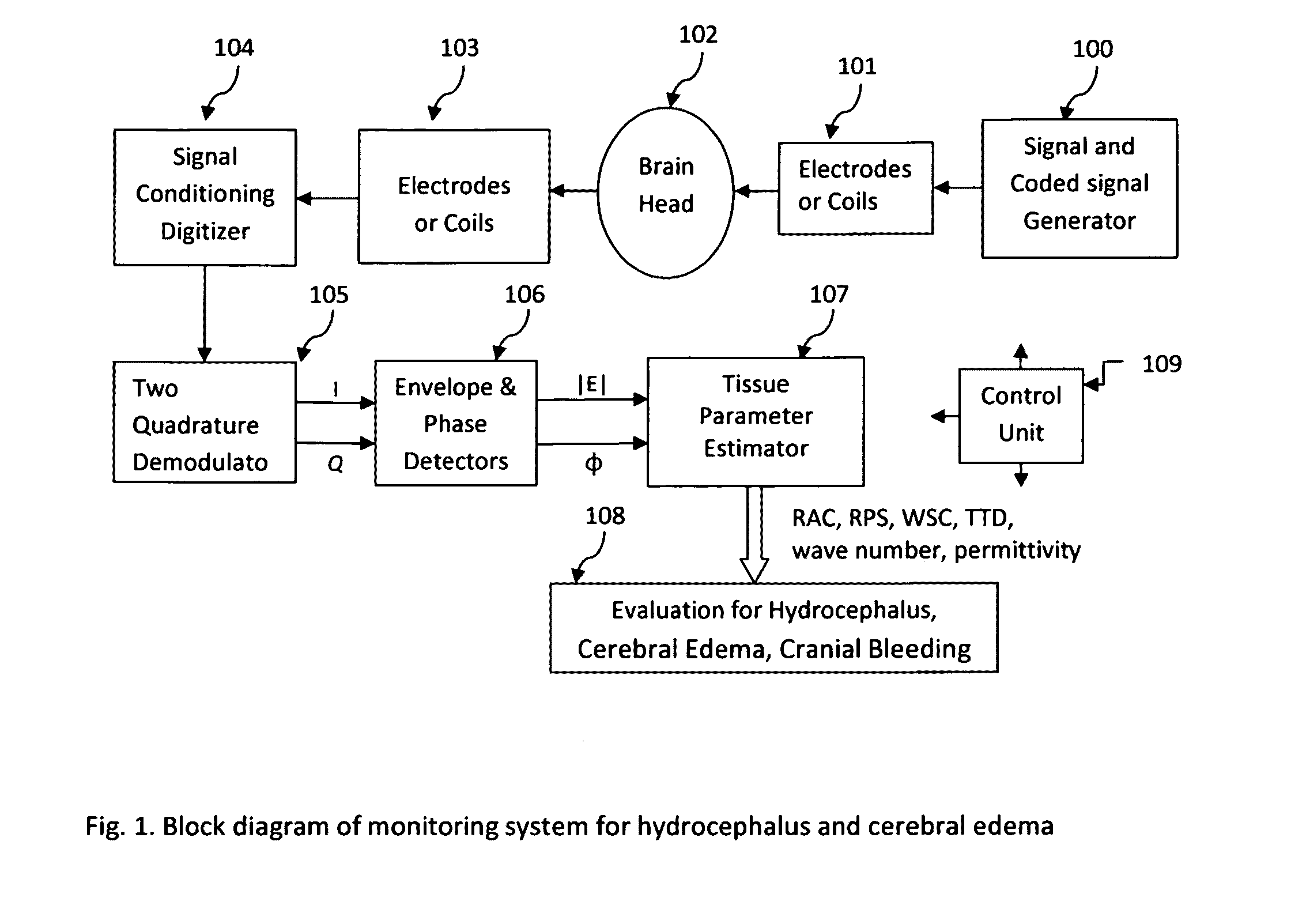
The invention relates to a device for monitoring hydrocephalus and encephaledema. The device comprises a signal encoding generator, an emitting electrode, a receiving electrode, a signal processing digital converter, an orthogonality regulator, a detector, a parameter evaluator, an impedance analysis instrument and an evaluation instrument. After passing through the human brain, electromagnetic waves change, by comparing attenuation coefficients, relative phase shifts, spreading time differences and complex wave values K of the electromagnetic waves before and after the electromagnetic waves enter the human brain, the specific conditions of the hydrocephalus and encephaledema be can evaluated, the method is different from the traditional invasion detection method, the device can be used for realizing 24-hour monitoring, so that the medical safety in treatment of hydrocephalus and encephaledema is obviously improved.
Owner:CHONG QING BORN FUKE MEDICAL EQUIP CO LTD
Compositions and treatment method for brain and spinal cord injuries
InactiveUS20040142905A1Effective treatment and preventionOrganic active ingredientsBiocideSubarachnoid spacePreventing injury
Owner:ONYX OPTICS
Oral medicament for curing craniocerebral injury associated with hydrocephalus
InactiveCN101279016AResponse improvedLow costBlood disorderPlant ingredientsSalvia miltiorrhizaAdemetionine
The invention relates to an internal medicine for curing cerebral edema caused by craniocerebral injury, which pertains to traditional Chinese medicine preparation. The internal medicine of the invention is characterized in that: the effective components of the internal medicine comprises the raw pharamaceutical materials with the following parts by weight: 15-45 parts of Indian buead, 10-24 parts of polyporus, 10-24 parts of largehead atractylodes rhizome, 15-45 parts of water plaintain, 8-18 parts of twotooth achyranthes root, 15-45 parts of motherwort herb, 15-45 parts of raidx astragali, 8-18 parts of rhubarb, 8-18 parts of peach seed, 8-18 parts of rhizomaligustici chuanxiong, 10-36 parts of radix salivae miltiorrhizae, 8-18 parts of safflower, 8-18 parts of red paeony root, 10-24 parts of kudzuvine root, 8-18 parts of thinleaf milkwort root-bark and 8-18 parts of drug sweetflag rhizome. The preparation method of the internal medicine comprises the steps: all the pharamaceutical raw materials (except rhubarb) are mixed in a casserole, appropriate amount of water is added for pickling, the boiling by strong fire is firstly carried out, the rhubarb is added, the liquid medicine is filtered after half an hour, the boiling by small fire is carried out, and the liquid medicine is filtered; the two liquid medicines are mixed and cooled. The method of administration is that: the internal medicine is orally taken three times a day for conscious patients, and is injected by using a gastric tube three times a day for narcose patients. The cure rate of the composition of the invention for curing traumatic brain edema is 96%, and the effective rate is 100%.
Owner:赵青菊 +1
Traditional Chinese medicine composition for treating cardiovascular and cerebrovascular diseases
ActiveCN103735584ASignificant effectQuick resultsAnthropod material medical ingredientsCardiovascular disorderFormularyCoronary heart disease
The invention discloses a traditional Chinese medicine composition for treating cardiovascular and cerebrovascular diseases, which is prepared from the following bulk drugs: leech, lumbricus, scorpio and pseudo-ginseng. The traditional Chinese medicine composition disclosed by the invention accords with the theories of traditional Chinese medicine pharmacology of removing stasis, promoting new and self-quenching wind through blood circulation; in addition, by utilizing the traditional Chinese medicine composition formula, the traditional Chinese medicine composition disclosed by the invention is capable of promoting circulation, removing stasis, removing blood stasis, clearing hematoma, stopping rehaemorrhagia, reducing intracranial pressure, eliminating encephaledema, adjusting whole-body blood, resisting stress reaction and improving microcirculation and has the unique curative effects to perennial serious hypertension, cerebral hemorrhage, stroke, coronary heart disease and cerebral thrombosis.
Owner:谢国清
Optical detection of seizure, a pre-seizure state, and cerebral edema and optical fiber detection of the same
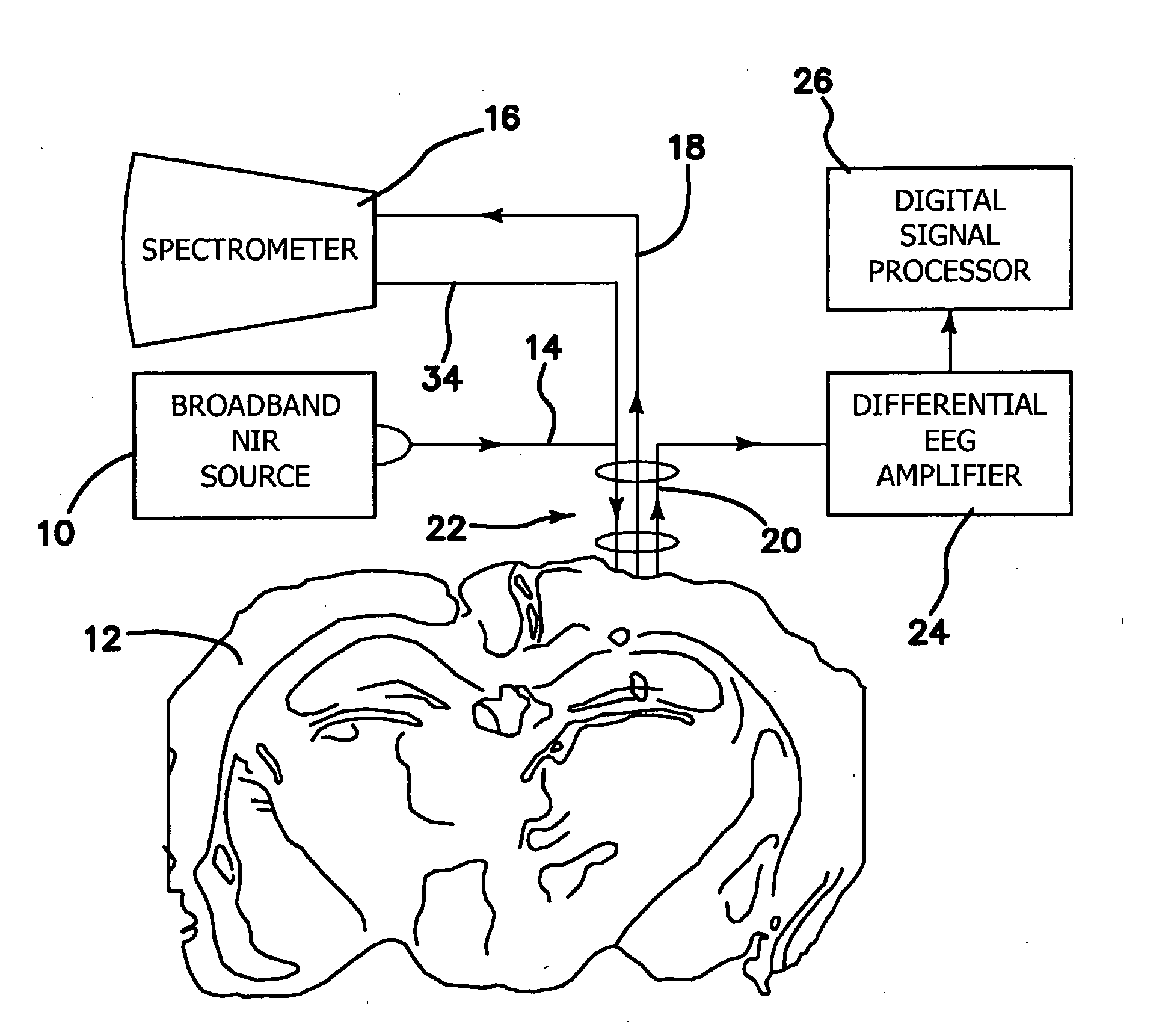
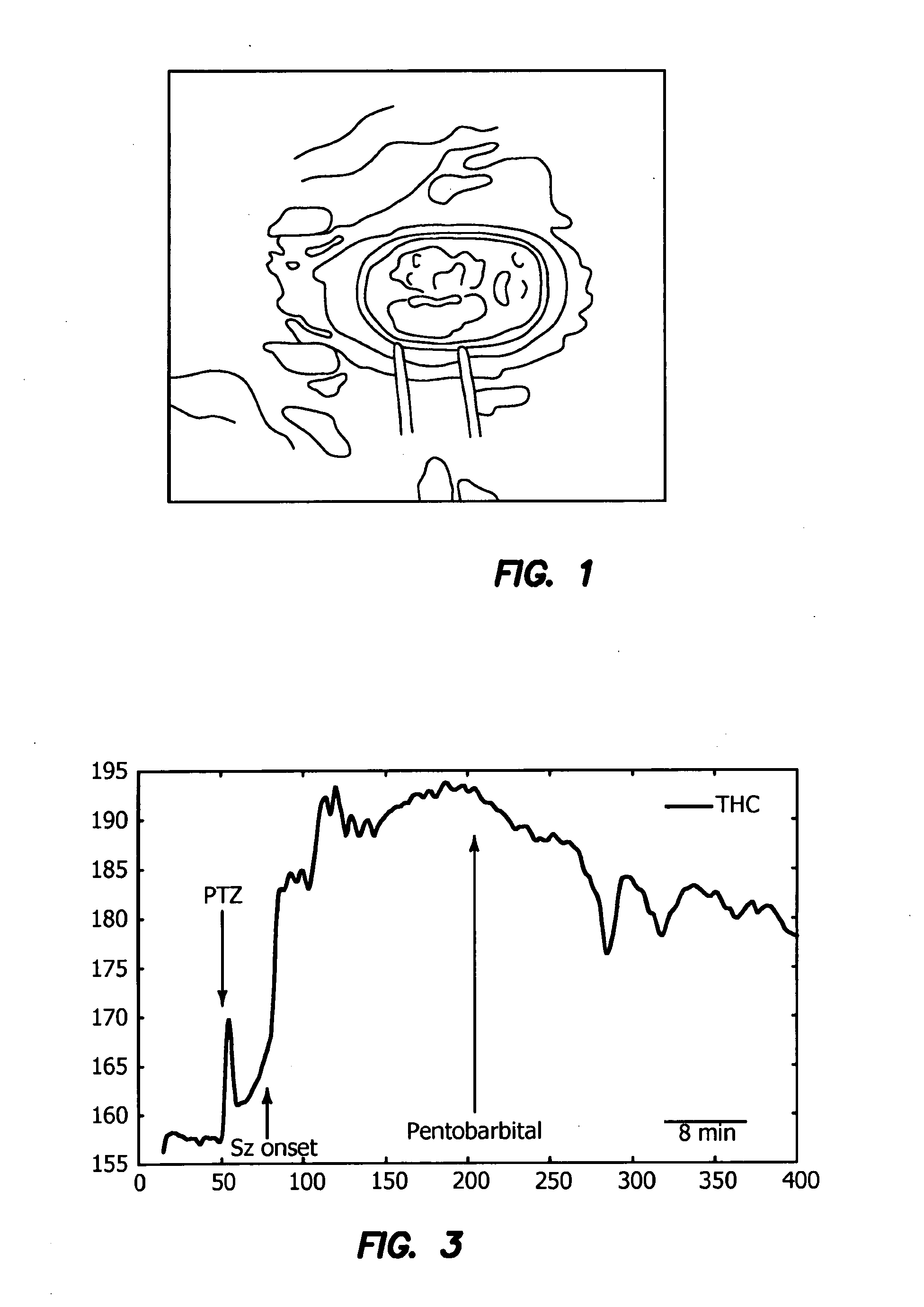
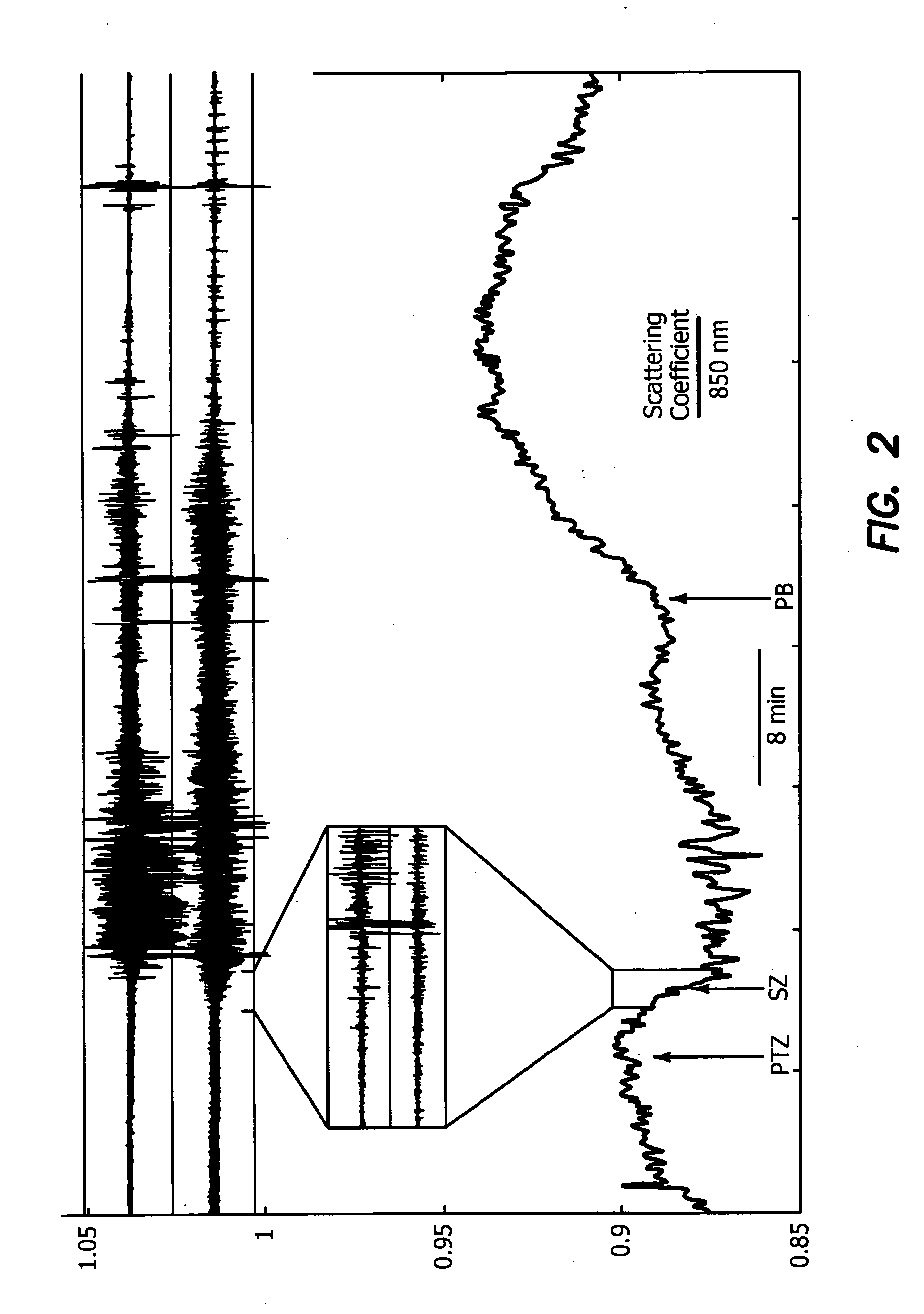
InactiveUS20090312646A1Degree of reductionReliable detectionDiagnostics using lightSensorsCerebral edemaSignal processing
A method for using optical parameters to monitor for a physiological event and / or a state prior to the physiological event includes the steps of: illuminating neural tissue with diagnostic light of a predetermined frequency at a predetermined location; detecting magnitude of optical scattering by neural tissue of the diagnostic light as a function of time; and determining a signature signal of the optical scattering of the diagnostic light before the physiological event in the neural tissue becomes clinically manifested. An apparatus includes a source of diagnostic light of a predetermined frequency for illuminating neural tissue at a predetermined location, a detector of optical scattering and / or optical absorption by neural tissue of the diagnostic light as a function of time, and a signal processor for determining a signature signal of the optical scattering and / or optical absorption of the diagnostic light before the physiological event becomes clinically manifested.
Owner:RGT UNIV OF CALIFORNIA
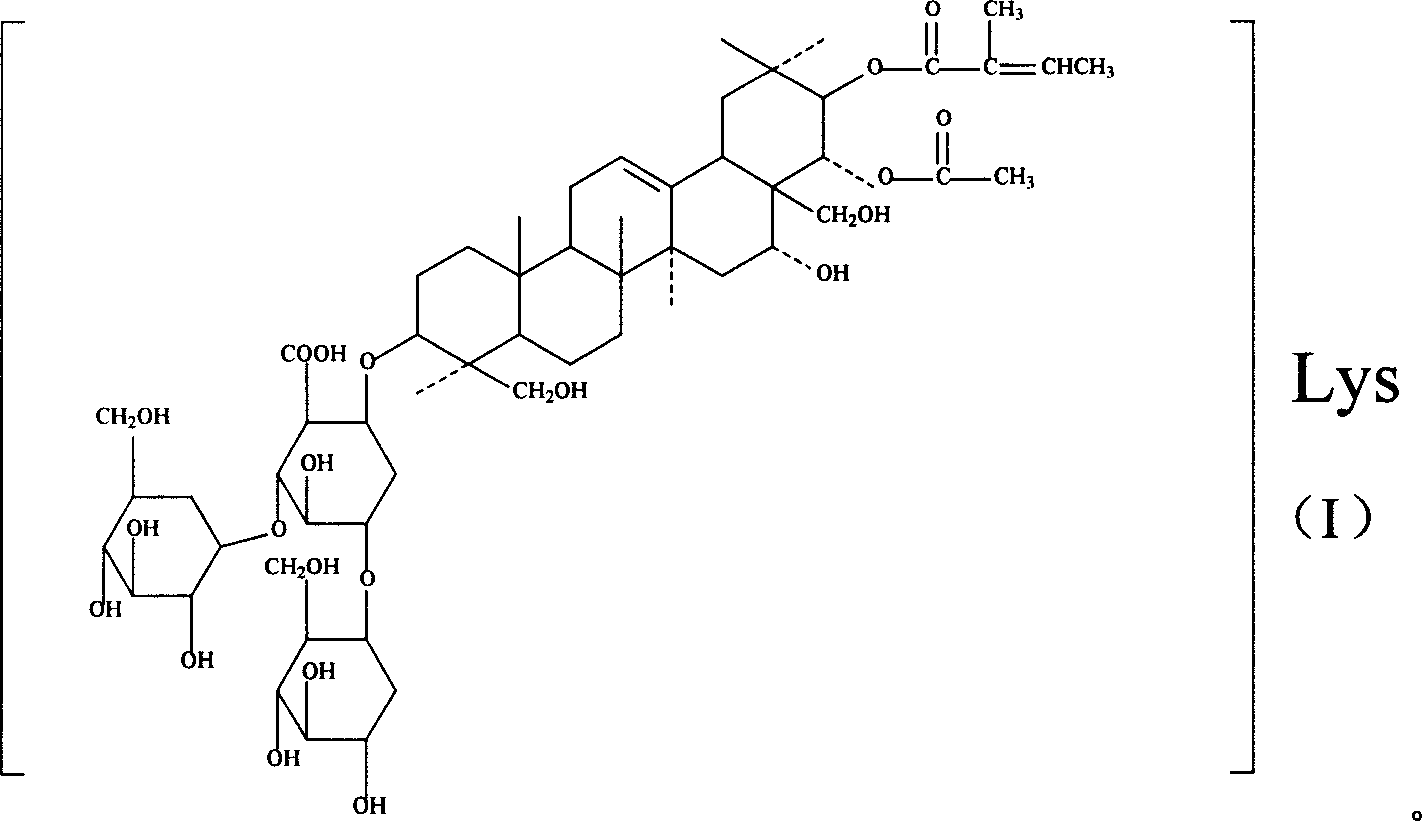
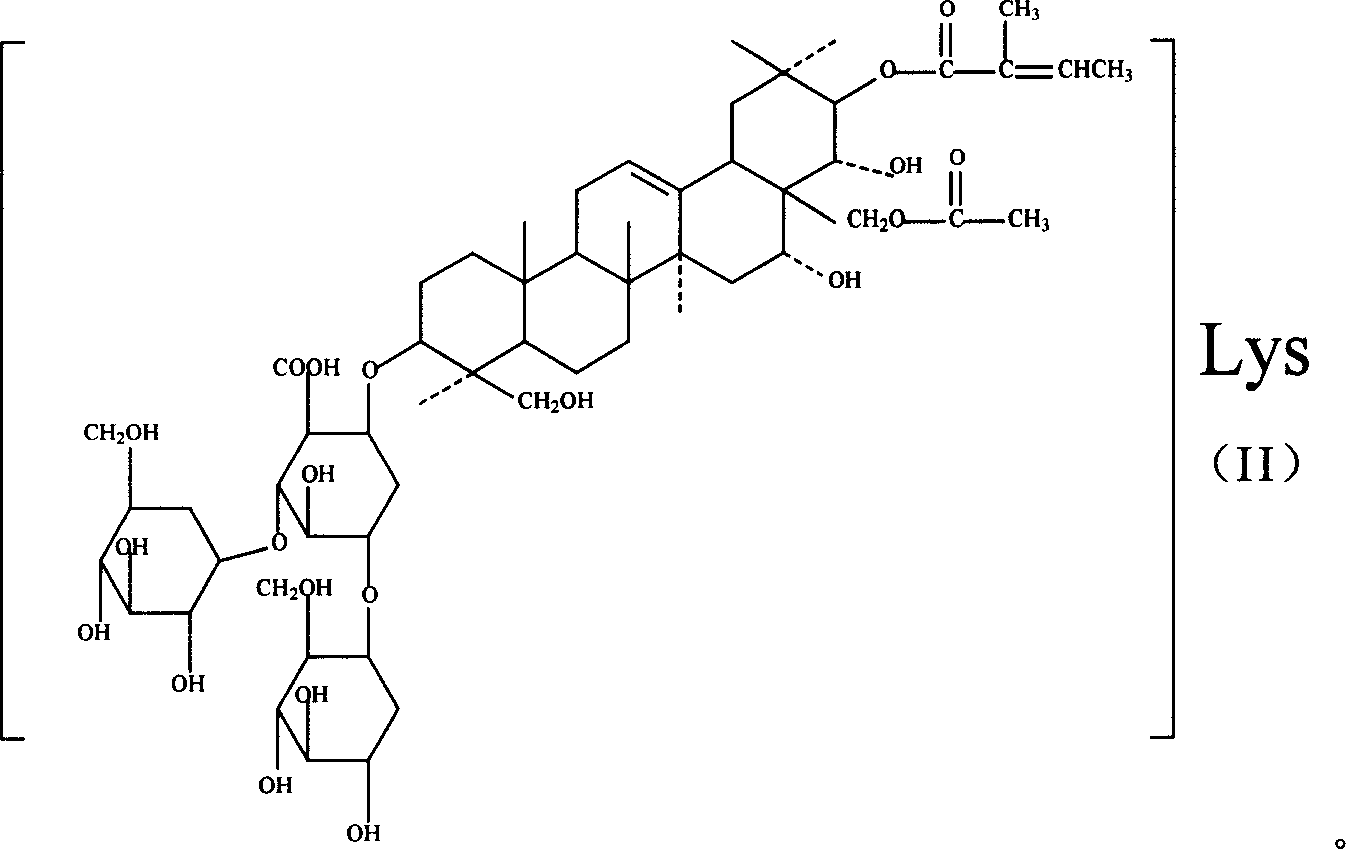
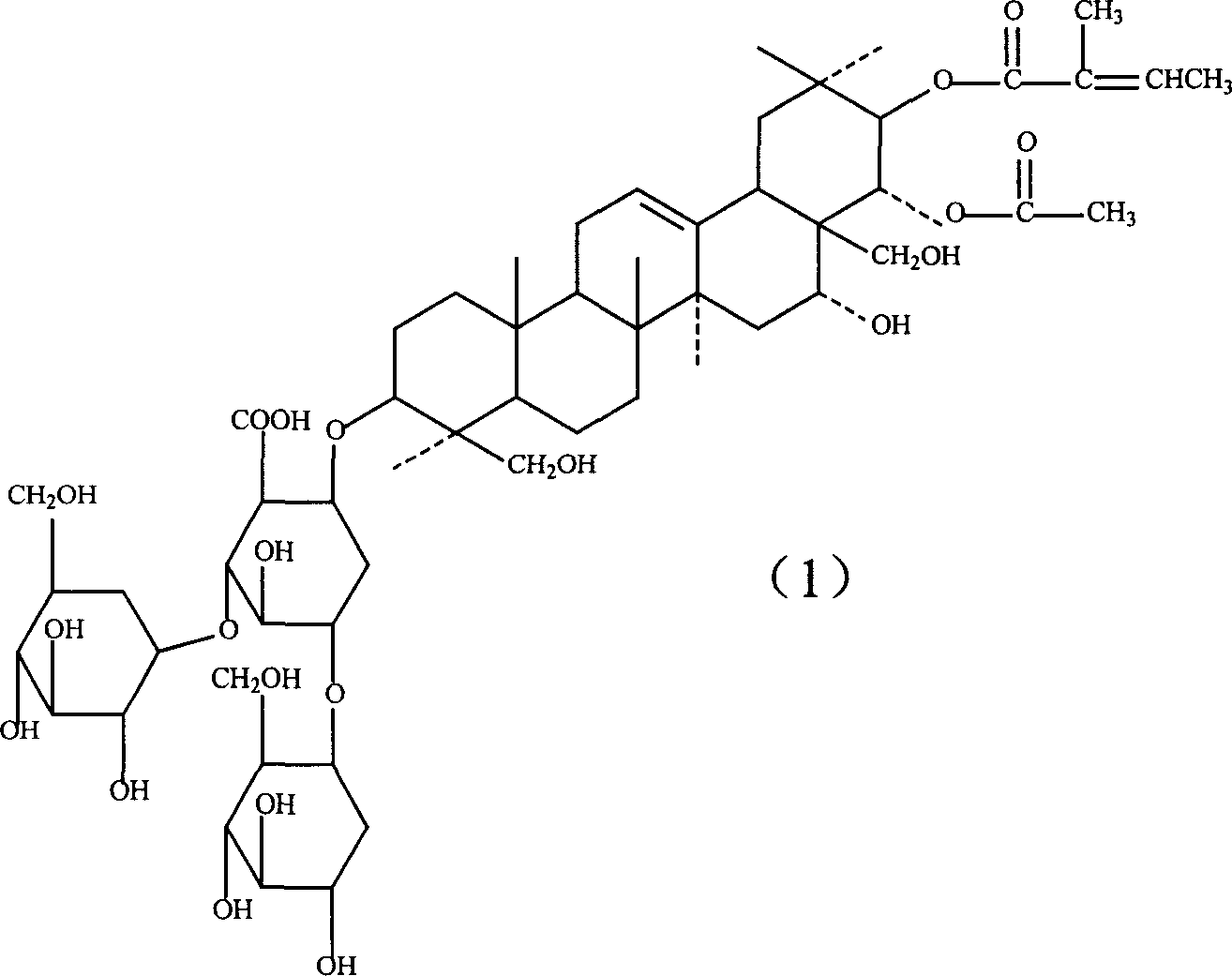
Lysine aescin saponin, its preparation and use
A lysine escin, its compound, production and use are disclosed. The compound consists of beta-escin lysine salt, isoescin lysine salt or their mixture and / or the other accepted carriers. It has better anti-inflammatory and anti-exudative performances, better suppressant for brain edema and medicinal safety. It can also improve blood circulation increase venous tension.
Owner:WUHAN AIMIN PHARMA
Chinese traditional medicine composition for treating hydrocephalus, cerebral edema and intracranial hypertension
InactiveCN1634385ASignificant diuretic effectEnhanced diuretic effectUnknown materialsDrug compositionsAdemetionineRhizome
The invention relates to a Chinese traditional medicine composition for treating hydrocephalus, cerebral edema and intracranial hypertension which comprises fish encephalolith, raw astragalus root, oriental water plantain rhizome, Poria cocos, plantain seeds, notoginseng, root of red rooted saliva, sweetgum fruit, grassleaved sweetflag rhizome, evodia rutaecarpa, and achyranthes and cyathula root.
Owner:谢静
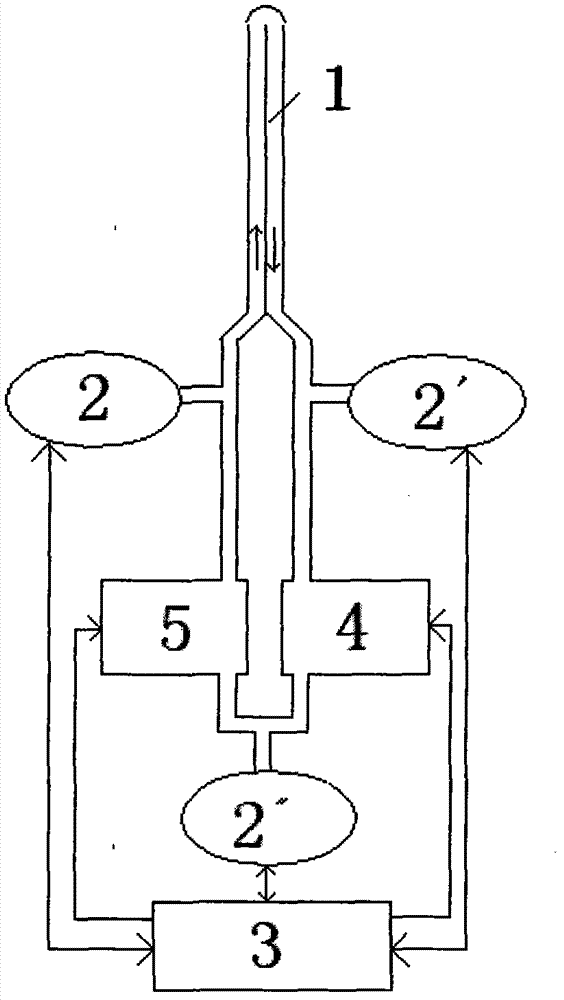
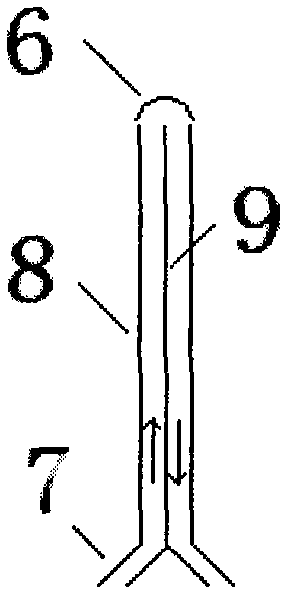
Brain fixed-point sub-hypothermia control device
InactiveCN102727338APrecise cooling positioningHigh speedTemperatue controlTherapeutic coolingCase fatality rateControl signal
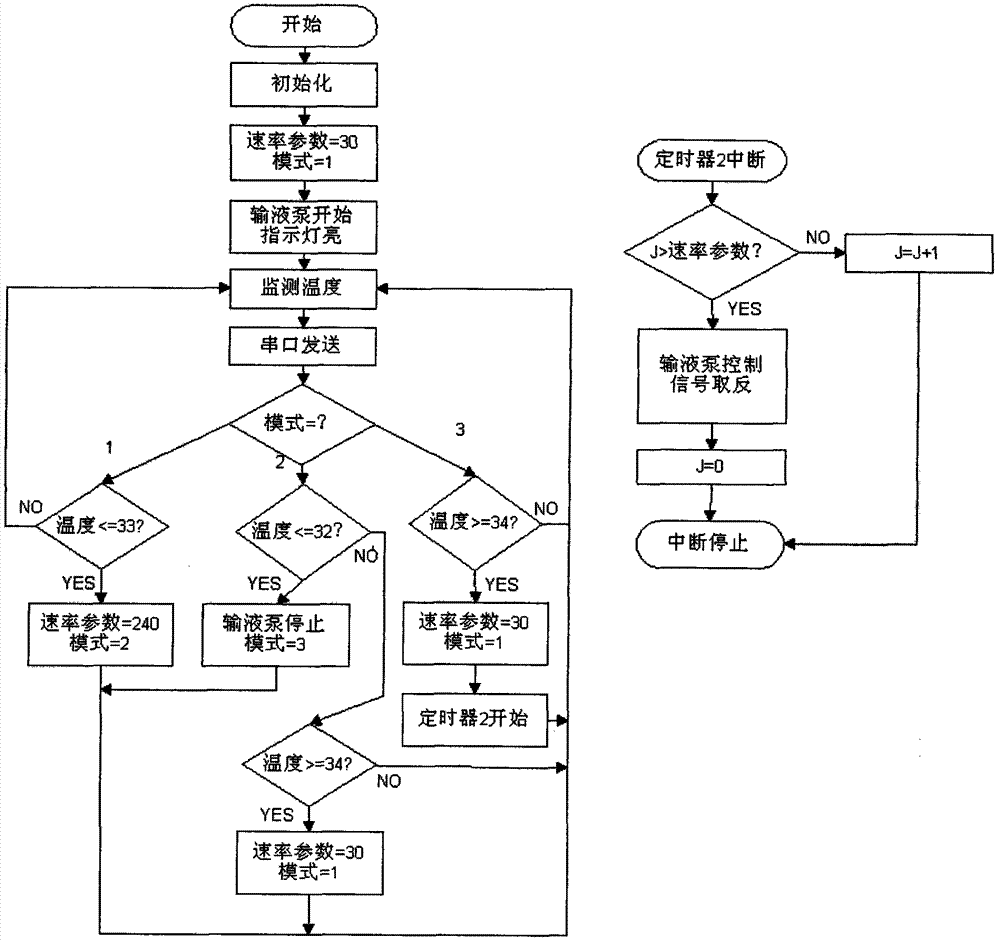
The invention relates to a brain fixed-point sub-hypothermia control device. The brain fixed-point sub-hypothermia control device comprises an intrusive guide tube, temperature detection elements, a control system, a liquid cooling device and a liquid pushing device, wherein the temperature detection elements (2, 2') are used for respectively monitoring the temperatures of an inlet and an outlet of the intrusive guide tube (1); the temperature detection element (2'') is used for monitoring the temperature of the output cooling liquid of the liquid cooling device (4); and the control system (3) is used for sending a control signal to start the liquid pushing device (5) to push the cooled liquid into the inlet of the intrusive guide tube at a certain speed. With the adoption of the brain fixed-point sub-hypothermia control device, the brain cooling part can be accurately positioned, the cooling process is timely and quick, and the sub-hypothermia state can be accurately and reliably maintained; in addition, acute and serious cerebral edema can be controlled, and conditions are created for promoting recovery of brain function, so that the brain fixed-point sub-hypothermia control device plays an important role in reducing fatality rate and disability rate.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Noninvasive monitoring hydrocephalus, cerebral edema, and intracranial bleeding using electromagnetic wave propagation properties
ActiveUS9456757B1High sensitivityStrong specificityCatheterIntracranial pressure measurementPermittivityNon invasive
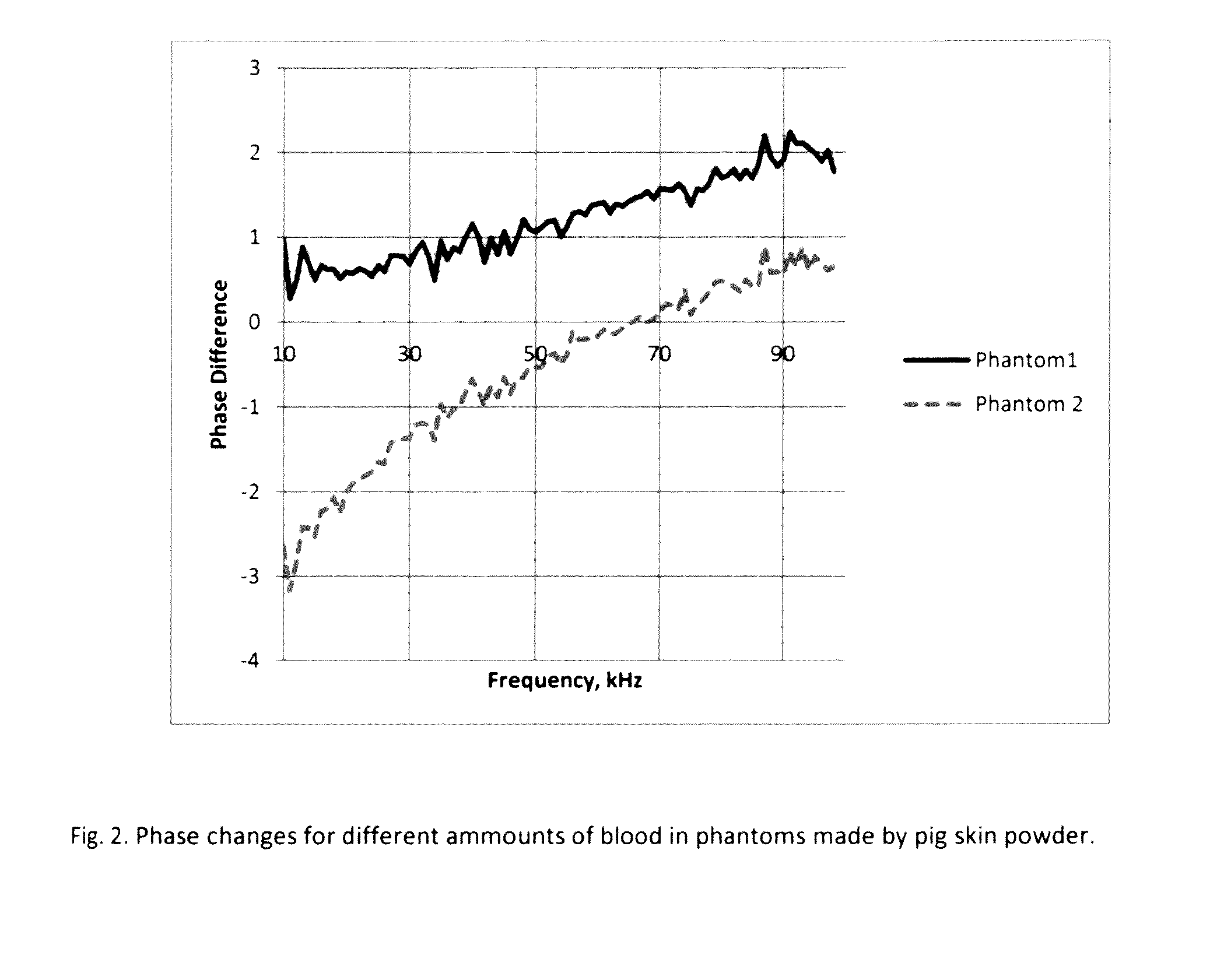
The present invention describes a system and methods to monitor hydrocephalus and cerebral edema in noninvasive or minimum invasive ways. The system monitors the changes of electromagnetic wave propagations in brain tissues changed by the tissue pathological statues. One of the tissue properties monitored is the tissue permittivity that determines the wave propagation velocity. By avoiding the tissue conductivity that has variations due to many different reasons including non-pathological factors, this approach has advantages of acquiring reliable pathological information of brain tissue and being independent to electrode properties and skin conditions. Several parameters are defined to quantitatively measure and assess hydrocephalus and cerebral edema: relative phase shift (RPS), travel-time difference (TTD), and change of relative wave velocity. The parameters are defined and normalized in distance and time for measuring relative changes for the monitoring applications. The method includes non-invasive and minimum invasive approaches. Amplitude modulated wave and coded waves are used to reduce interference and effectively detect small changes of tissue properties in preferred frequency ranges.
Owner:ZHENG YI
Uses of Petasites tricholobus franch extract in medicament preparation for preventing and controlling cardiovascular and cerebrovascular diseases
ActiveCN101297828APharmacologically activeHave cardio-cerebrovascular pharmacological activityOrganic active ingredientsOrganic chemistryDiseaseReperfusion injury
The invention relates to the field of medical technology, which discloses a new usage of a petasites tricholobus franch extract and the contained bakkenolide compound in the preparation of drugs for prevention and treatment for cardiovascular and cerebrovascular diseases. The biological activity tests show that, the petasites tricholobus franch extract and the contained bakkenolide compound can significantly reduce the encephalic necrosis percentage of the rats with local cerebral ischemia, improve the behavior score of the ischemic rats and alleviate volume of brain edema and cerebral infarction of the rats with ischemia reperfusion injury, thus prompting that the petasites tricholobus franch extract and the contained bakkenolide compound have significant protective effect on the cerebral ischemia injury. The petasites tricholobus franch extract and the contained bakkenolide compound can further significantly reduce the J-point displacement caused by ISO and the LDH level in plasma, significantly reduce the scope of myocardial ischemic myocardial infarction of the rats caused by coronary artery ligation and lower the LDH level in the plasma of the rats with the myocardial ischemia. As the petasites tricholobus franch extract and the contained bakkenolide compound have good effects on the prevention and the treatment for heart and brain ischemic diseases aspects, the petasitestricholobus franch extract and the contained bakkenolide compound can be used in the preparation of drugs for prevention and treatment for cardiovascular and cerebrovascular diseases, including coronary heart disease, cerebral ischemia, cerebral infarction (stroke), myocardial infarction and so on.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
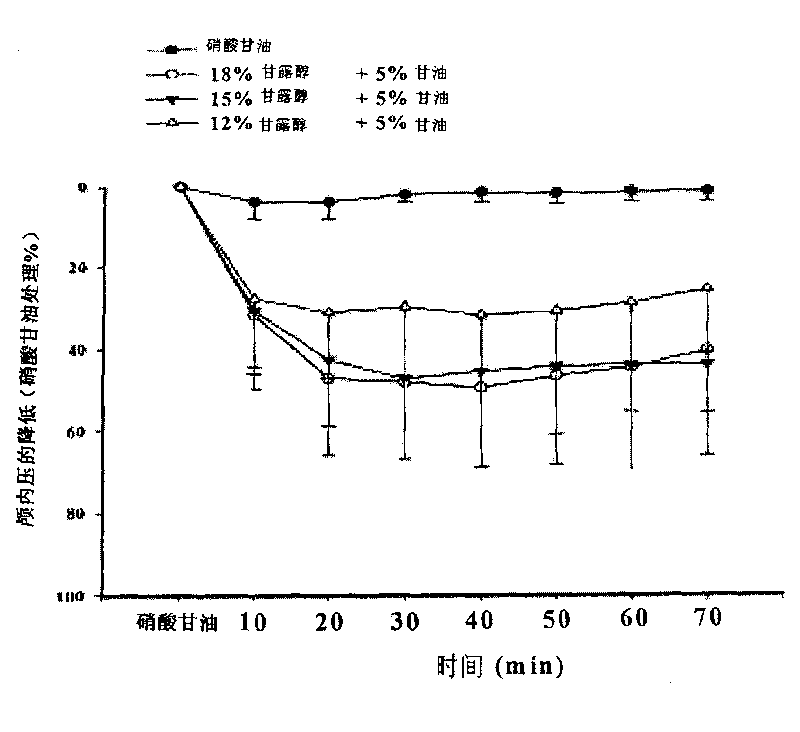
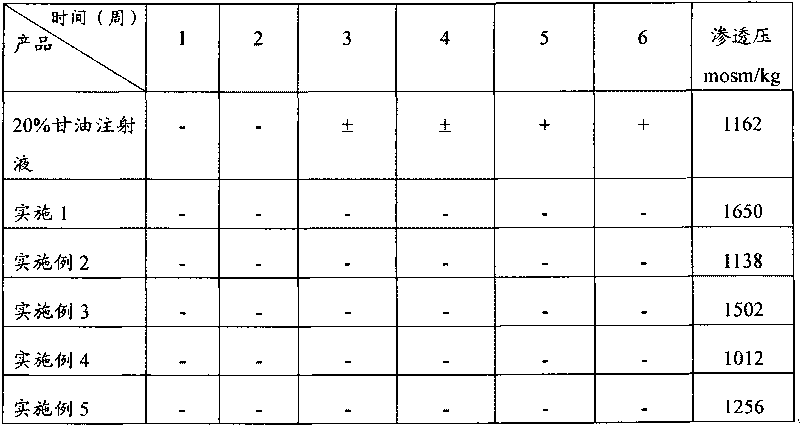
Injection of mannite and glycerol and preparation method thereof
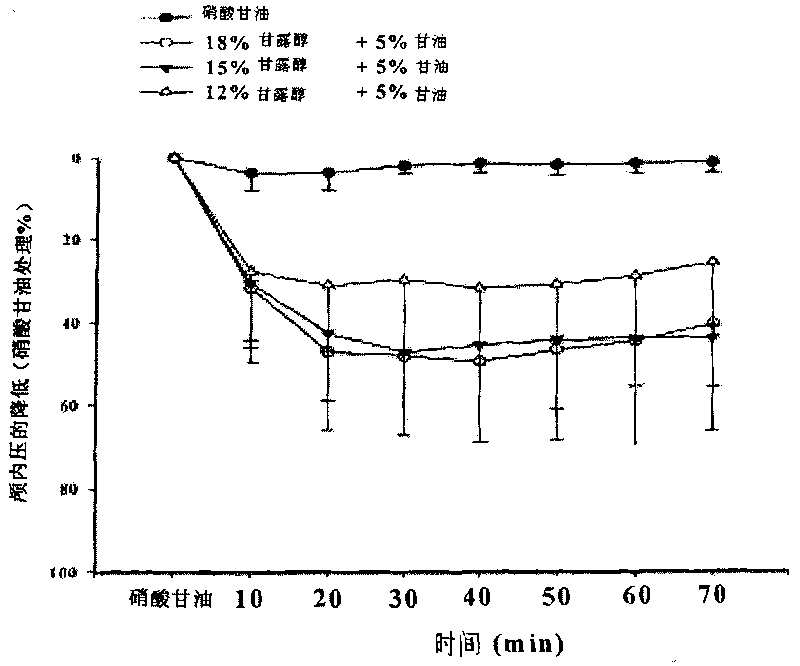
ActiveCN101732287AAvoid damageImproves and restores energy metabolismHydroxy compound active ingredientsPharmaceutical delivery mechanismDiseaseNeural cell
The invention discloses injection of mannite and glycerol, which contains mannite, glycerol and water for injection. Furthermore, the invention also discloses a method for preparing the injection, which comprises the following steps: taking the mannite and the glycerol, dissolving into the water for injection, filtering and filling. The injection of the mannite and the glycerol is clinically used for treating cerebrovascular diseases, brain trauma, cerebral tumors, intracranial inflammation and the diseases of acute and chronic intracranial hypertension, hydrocephalus and the like caused by other reasons. The injection of the mannite and the glycerol has the functions of lowering the intracranial pressure quickly and eliminating the hydrocephalus and can also avoid death because of acute renal failure caused by the injury of the kidney; the injection of the mannite and the glycerol suppresses the apoptosis of neural cells and achieves the function of protecting the brain; the function of lowering the intracranial pressure is stable and has long retention time; crystals occurring because of the change of the air temperature or in the processes of storage and transportation are eliminated; and the glycerol is used as a caloric agent and can improve and recover the energy metabolism of the important viscera of the heart, the brain, the kidney and the like during oxygen deficiency. The injection of the mannite and the glycerol is a very effective, stable and safe medicine for promoting urination and removing water clinically.
Owner:北京圣方达隆医药科技发展有限责任公司 +2
Method for establishing high altitude cerebral edema animal model
InactiveCN103535322AIncrease breathing rateEnhance oxygen consumptionAnimal husbandryMedicineCerebral edema
The invention discloses a method for establishing a high altitude cerebral edema animal model. The method for establishing the high altitude cerebral edema animal model comprises the following steps of selecting a plurality of rats, putting the rats in a low-pressure low-oxygen environment for two days with normal diets, continuously carrying out 6 times of swimming training on the rats every day for consecutive 4-6 days, maintaining the normal diets everyday, and establishing the high altitude cerebral edema animal model, wherein a method for the swimming training comprises the steps that the rats are put into a swimming pool to swim freely, the low-pressure low-oxygen environment is maintained, the rats rest for 15-25 minutes after the rats swim for 20-30 minutes every time, and then the rats are immediately made to proceed with the swimming training of a next time. The rats can be induced to generate cerebral edema symptoms in the low-pressure low-oxygen environment; multiple times of the swimming training is carried out on the rats in several consecutive days in the low-pressure low-oxygen environment condition, the breathing frequency and the oxygen consumption of the rats are enhanced in the swimming training process, the cerebral edema symptoms of the rats are made to become obvious by increasing the oxygen consumption of the rats, and it is detected in the subsequent detection that the positive rate of cerebral edemas is increased.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
Device to reduce brain edema by surface dialysis and cooling
We have developed a novel method of brain surface dialysis that reduces intracranial pressure and modifies movement of extracellular fluid in a rat model of brain injury. A chamber with a semipermeable membrane at the site of brain contact is perfused with a hyperosmolar solution (e.g. 15% dextran, inulin, hydroxyethyl starch). It is also capable of providing local brain cooling. In principle, osmotic forces draw water and small molecules from the injured brain into the dialysis chamber thereby reducing brain swelling. The dialysate does not move into the brain.
Owner:DEL BIGIO MARC RONALD +1
Application of total saponins of bacopa monnieri (L.) wettst. in preparation of medicaments for resisting cerebral ischemia
The invention provides an application of total saponins of bacopa monnieri (L.) wettst. in preparation of medicaments for resisting cerebral ischemia. The total saponins of the bacopa monnieri (L.) wettst. mainly comprise the following dammarane type triterpenoid saponin compounds: bacopaside I, bacoside A3, bacopaside II, bacopasaponin cisomer and bacopasaponin C. The invention further provides an application of the bacopaside I in preparation of a medicament for resisting cerebral ischemia. The experimental study shows that the total saponins of the bacopa monnieri (L.) wettst. and the bacopaside I have the effects of improving the behavioral symptoms caused by cerebral ischemia reperfusion injury, reducing the cerebral infarct volume and reducing the cerebral edema caused by cerebral ischemia of a rat. Therefore, the total saponins of the bacopa monnieri (L.) wettst. can be used for preparing the medicaments for preventing and treating cerebral ischemia. The medicaments for preventing and treating the cerebral ischemia are prepared from the total saponins of the bacopa monnieri (L.) wettst. or bacopaside I as the active component and medicinal accessories by adopting a conventional method. The invention further expands the function of the bacopaside I and the total saponins of the bacopa monnieri (L.) wettst. and provides the novel medicaments for preventing and treating the cerebral ischemia. The bacopaside I and the total saponins of the bacopa monnieri (L.) wettst. have high clinical application values.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Medicine for treating angiocardiopathy and cerebrovascular disease and its preparing method
InactiveCN1557403AReduce manufacturing costActive ingredients are clearPowder deliveryUnknown materialsDiseaseSide effect
The medicine for treating cardiac and cerebral vascular diseases is prepared with astragalus root and notoginseng as material, and is Chinese medicine injection, including injection liquid, transfusion liquid and freeze dried powder for injection, prepared through extraction, purification and addition of proper amount of medicinal supplementary material. The medicine has high stability and long effective period, and may be used in intramuscular injection, intravenous injection and intravenous instillation. Pharmacodynamic test shows that the medicine can protect brain against oxygen lack and cardiac muscle damage, raise anaerobic resistance, lower serum LDH and CK activity and reduce myocardial infarction range. The medicine is used in treating cardiac and cerebral vascular diseases and has fast acting, determined curative effect and no toxic side effect.
Owner:陈玲玲
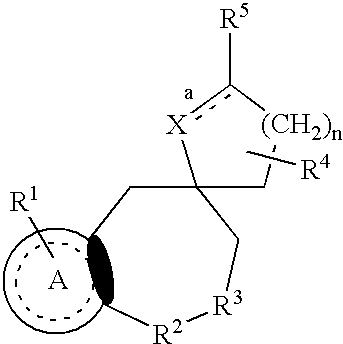
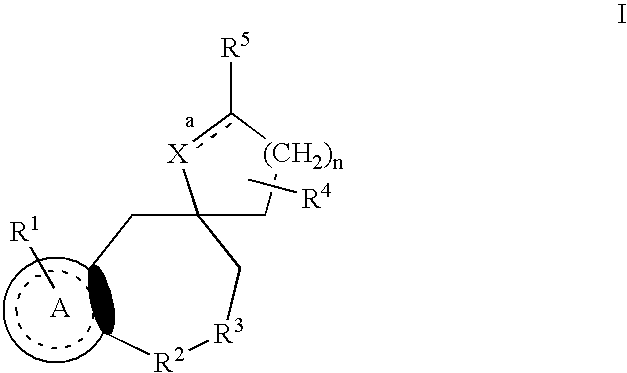
Nonpeptide substituted spirobenzoazepines as vasopressin antagonists
The invention is directed to nonpeptide substituted benzodiazepines of Formula I, wherein A, X, n, R1, R2, R3, R4, R5, a and b are as described in the specification, which are useful as vasopressin receptor antagonists for treating conditions associated with vasopressin receptor activity such as those involving increased vascular resistance and cardiac insufficiency. Pharmaceutical compositions comprising a compound of Formula I and methods of treating conditions such as hypertension, congestive heart failure, cardiac insufficiency, coronary vasospasm, cardiac ischemia, liver cirrhosis, renal vasospasm, renal failure, cerebral edema and ischemia, stroke, thrombosis, or water retention are also disclosed.
Owner:ORTHO MCNEIL PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com