Application of total saponins of bacopa monnieri (L.) wettst. in preparation of medicaments for resisting cerebral ischemia
A technology of Bacopa monnieri and total saponins, applied in the field of traditional Chinese medicine extracts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the preparation of Bacopa monnieri total saponins
[0038] Take 10kg of Bacopa monniera medicinal material and add 1200kg of 40-70% ethanol to soak for 60min, decoct for 120min, repeat three times, combine the extract, filter to remove precipitation; Wash with deionized water to remove water-soluble impurities. Use 5 times the column volume of 40% ethanol, 5 times the column volume of 75% ethanol to elute successively, collect the 75% ethanol eluate; take the 75% ethanol eluate, evaporate the ethanol at low temperature until there is no alcohol smell, and concentrate to dryness , Dry under reduced pressure at 80°C for 24h to constant weight to obtain 70g of total saponins of Bacopa monnieri as a solid.
[0039] Bacopaside I, Bacoside A3, Bacopaside II, BacopasaponinC isomer and Bacopasaponin C five monomer standard preparations are prepared by liquid phase:
[0040]Take an appropriate amount of the total saponins of Bacopa monniera, add chromatographic p...
Embodiment 2
[0049] Embodiment 2, the application of total saponins of Bacopa monnieri in the preparation of medicines for the treatment of cerebral ischemic injury
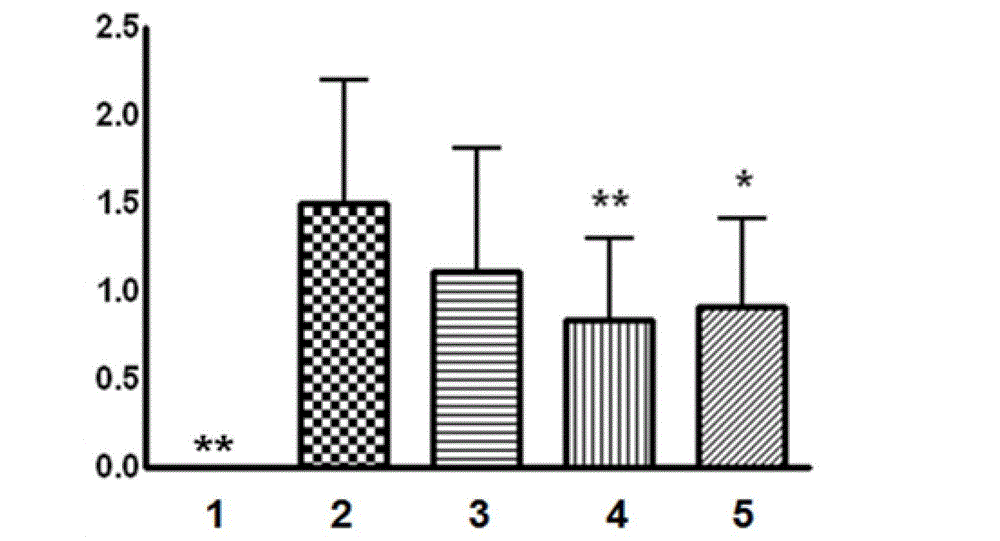
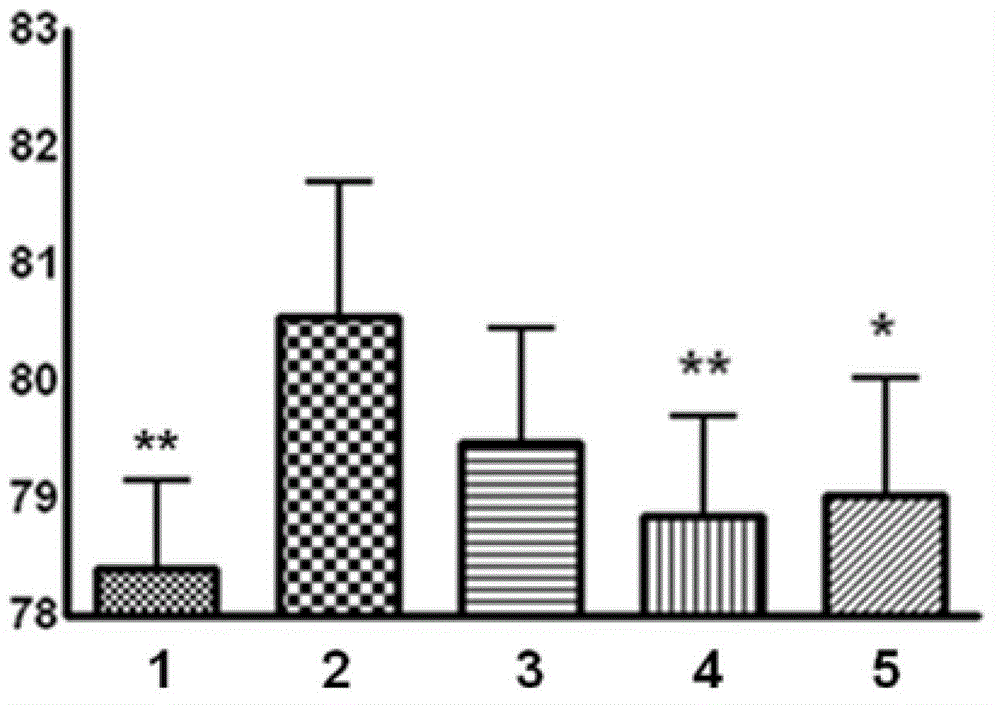
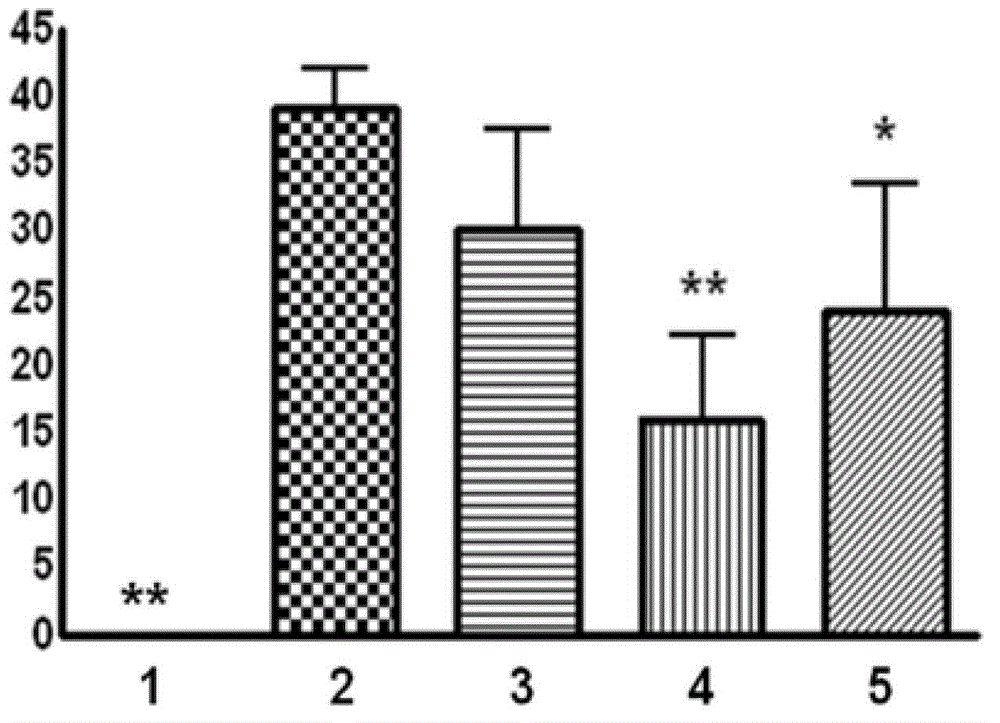
[0050] The present invention adopts the middle cerebral artery occlusion model of thread embolism to cause acute cerebral ischemia-reperfusion injury model in rats, and adopts the prevention and treatment administration method of modeling on the third day of administration and continuous administration for six days, from the cerebral infarction area, The degree of cerebral edema and animal behavior scores proved the therapeutic effect of total saponins of Bacopa monnieri on acute cerebral ischemia-reperfusion injury.
[0051] 1. Experimental materials and methods
[0052] 1) Animals and groups:
[0053] SD male rats, weighing 260-280 g, were provided by the Experimental Animal Center of the Second Military Medical University. The animals were randomly divided into 5 groups: (1) sham operation group; (2) model group; (3) tot...
Embodiment 3
[0069] The preparation of embodiment 3, Bacopaside I
[0070] Bacopa monnieri is pulverized into powder 10kg after removing impurities, dissolved with methanol-water volume ratio 9:1, filtered, 90% methanol equilibrated Mitsubishi Chemical chiral MCI column on the filtrate, then eluted with 90% methanol, evaporated to dryness Get samples. After decolorization, the sample is mixed with silica gel in equal proportions and then prepared under medium pressure. The medium pressure conditions are: pressure 25bar, flow rate 6ml / min, water phase 70%-10%, methanol 30%-90%, total time 6.5h, of which 0.5 h is the equilibration column time. The samples obtained in Central Asia were compared with the standard by spot plate (developing agent: ethyl acetate: methanol: water = 20:3.4:2.7) to find the desired point, collected and evaporated to dryness. The extract obtained above was dissolved in equal volumes of chloroform and methanol, and passed through a gel column. The purified Bacopasi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com