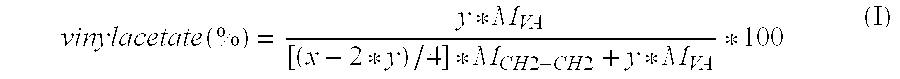Patents
Literature
118 results about "Progestin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A progestin (P) is a type of medication which is used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestins are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration.
Implantation rates after in vitro fertilization, treatment of infertility and early pregnancy loss with a nitric oxide donor alone or in combination with progesterone, and a method for contraception with nitric oxide inhibitors
InactiveUS6040340AImprove implantation rateSymptoms improvedHeavy metal active ingredientsBiocideEarly Pregnancy LossPhysiology
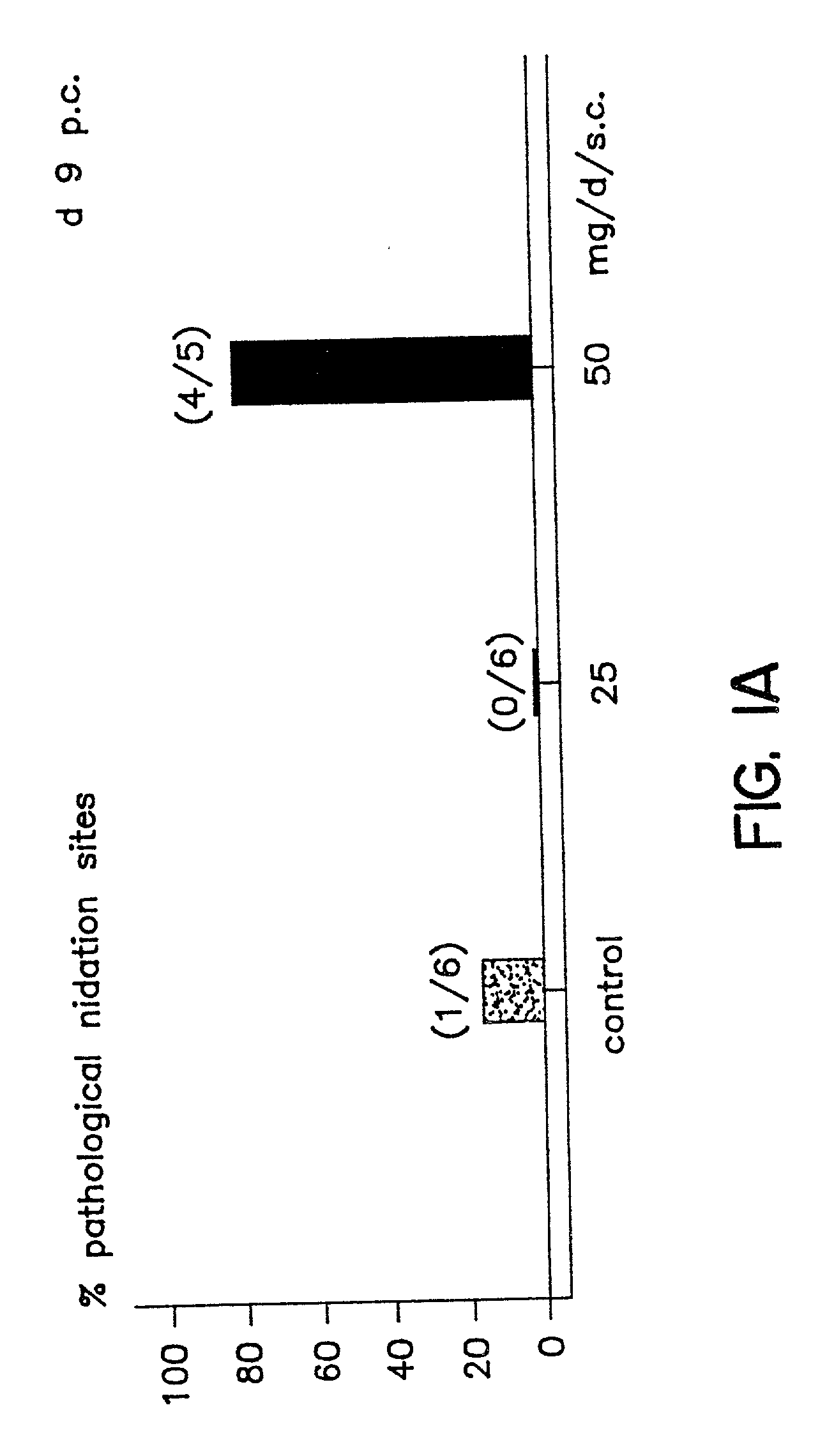
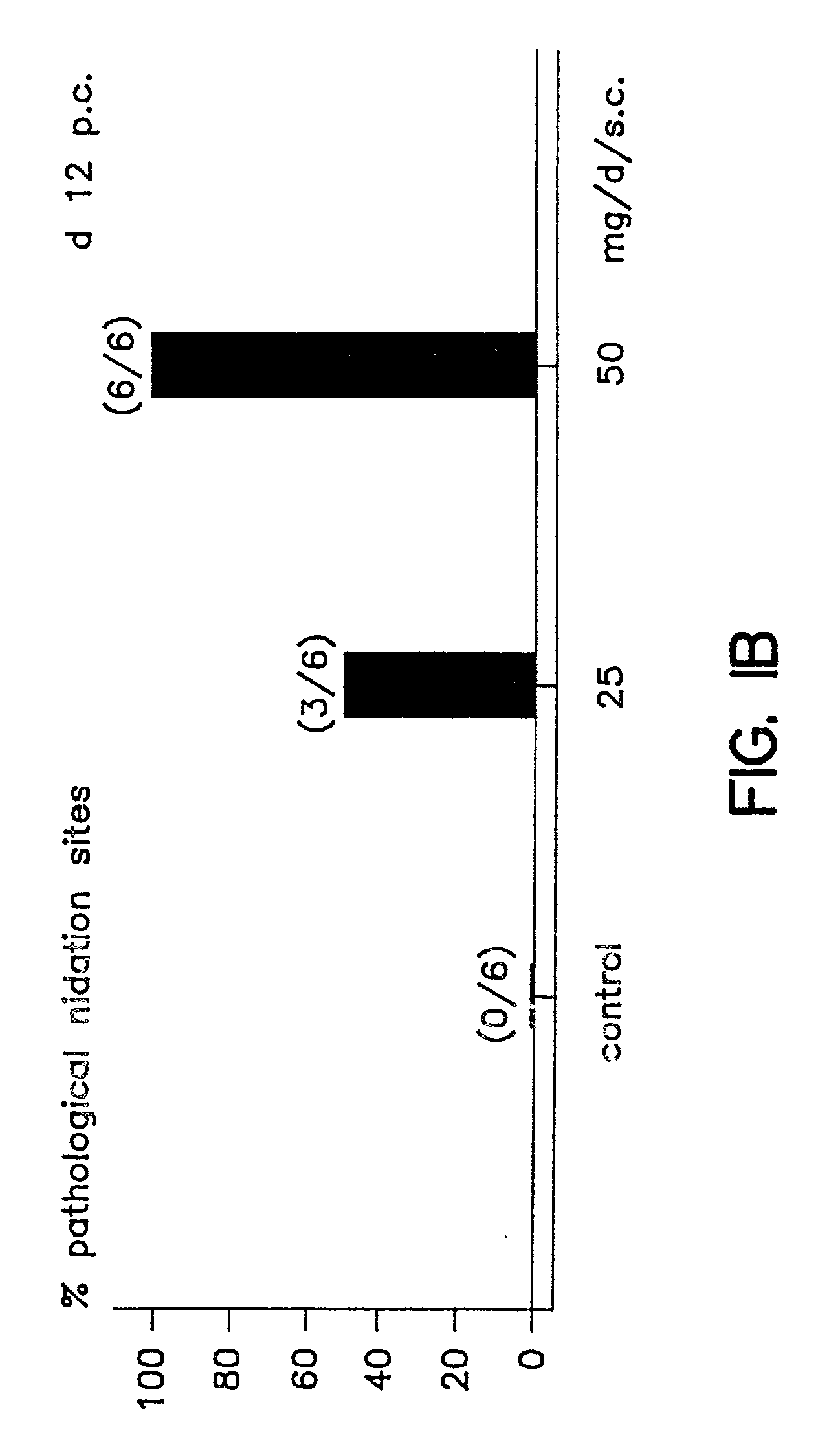
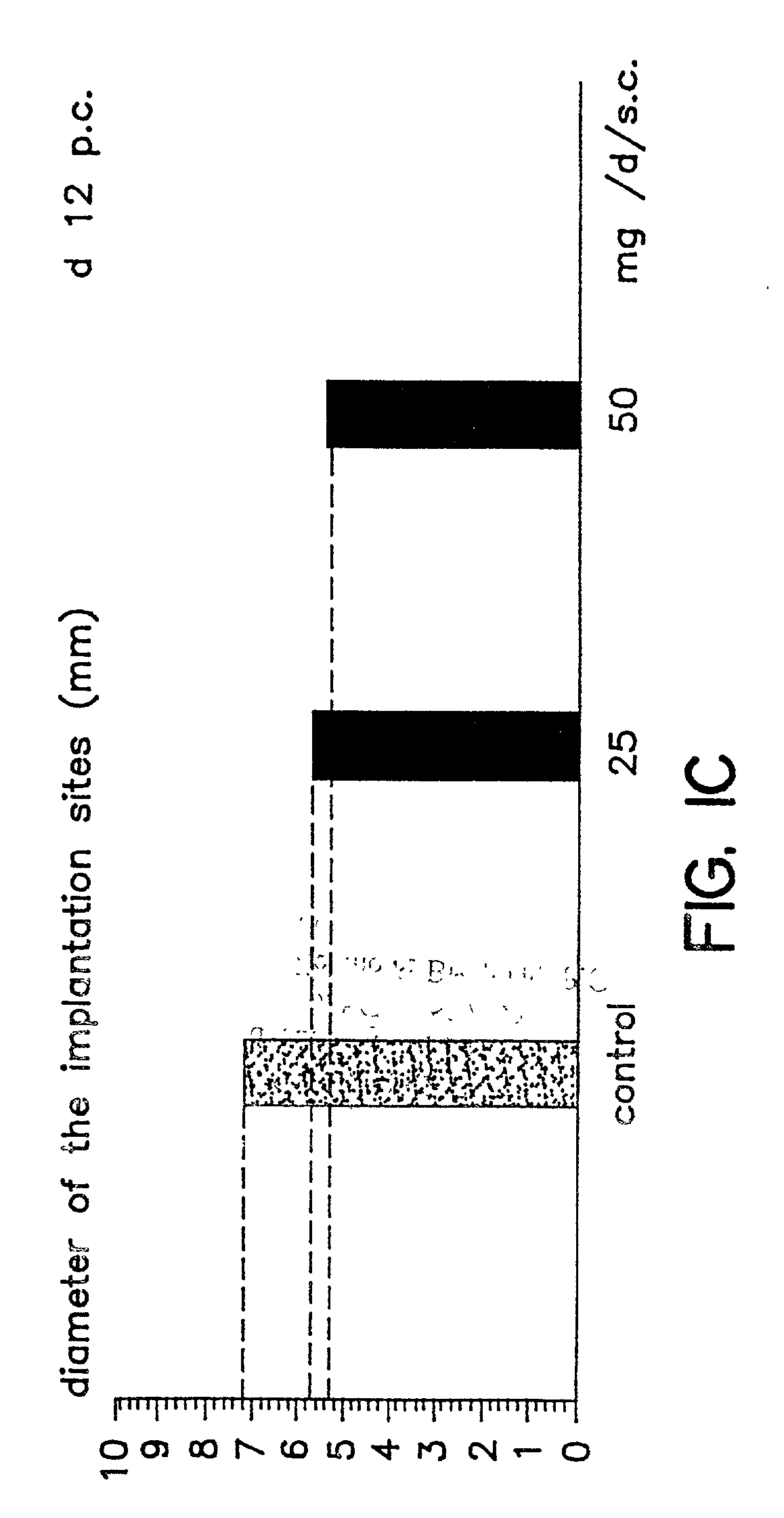
A method is provided for the improvement of implantation rates and / or pregnancy rates in a female mammal, comprising administering to a female mammal in whom pregnancy is desired an effective amount of (a) a nitric oxide synthase substrate, a nitric oxide donor, or both, optionally in combination with (b) a progestin, and, (c) optionally, in further combination with an estrogen. A method is also provided for fertility control for a female mammal, comprising administering to a female mammal in whom pregnancy is not desired and at risk for becoming pregnant an effective amount of nitric oxide synthase inhibitor in combination with an antiprogestin. Pharmaceutical compositions are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
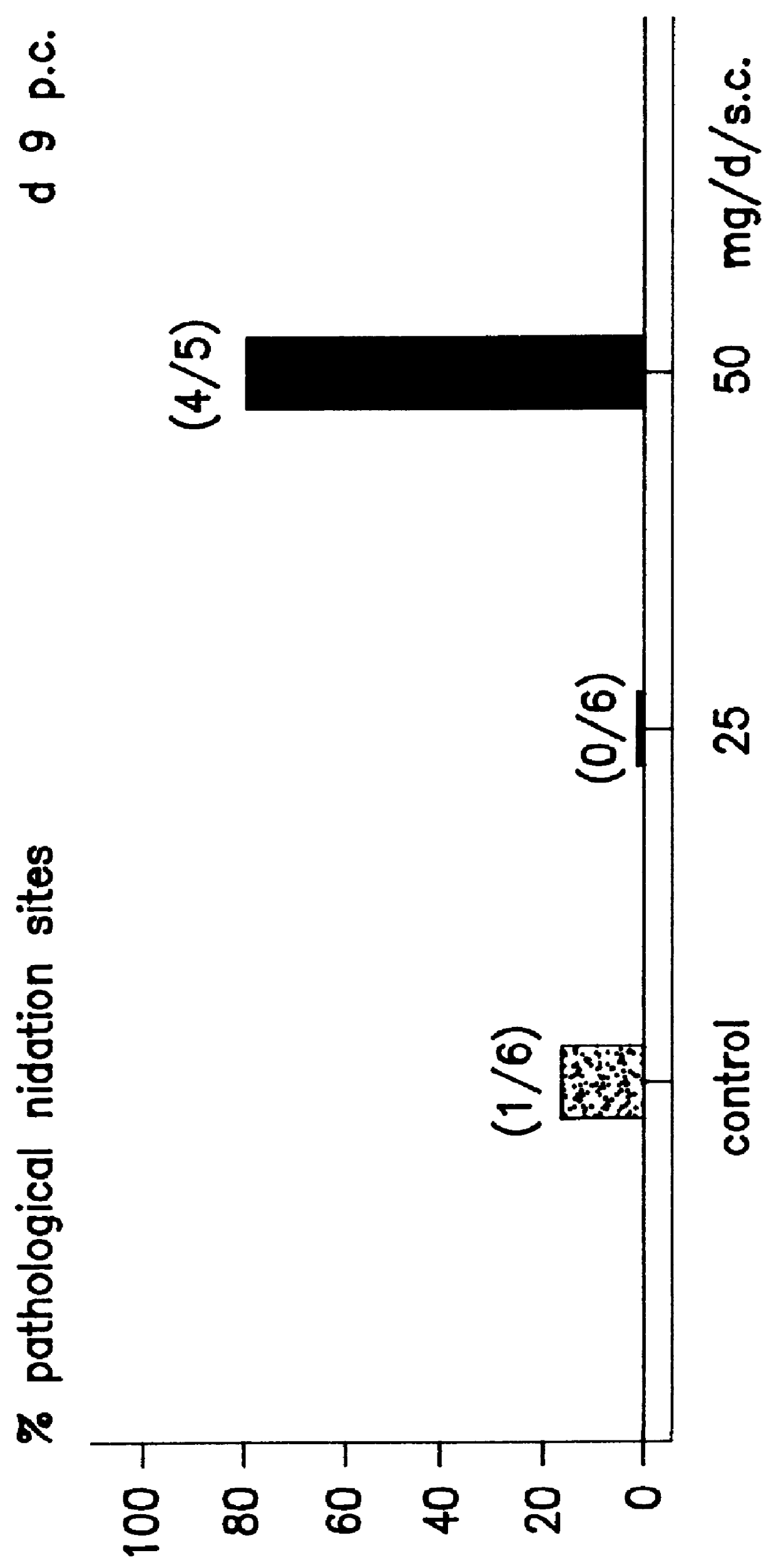
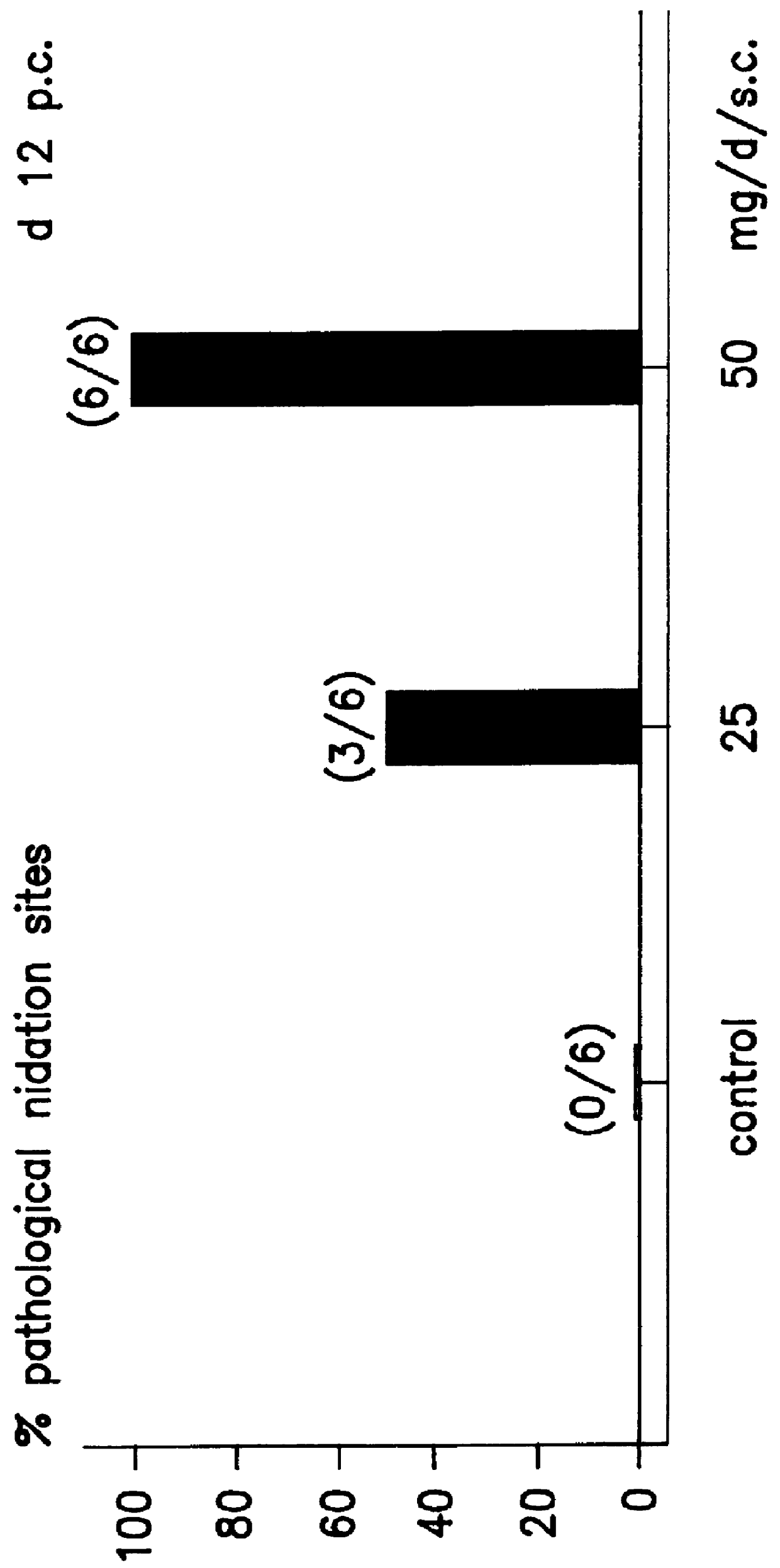
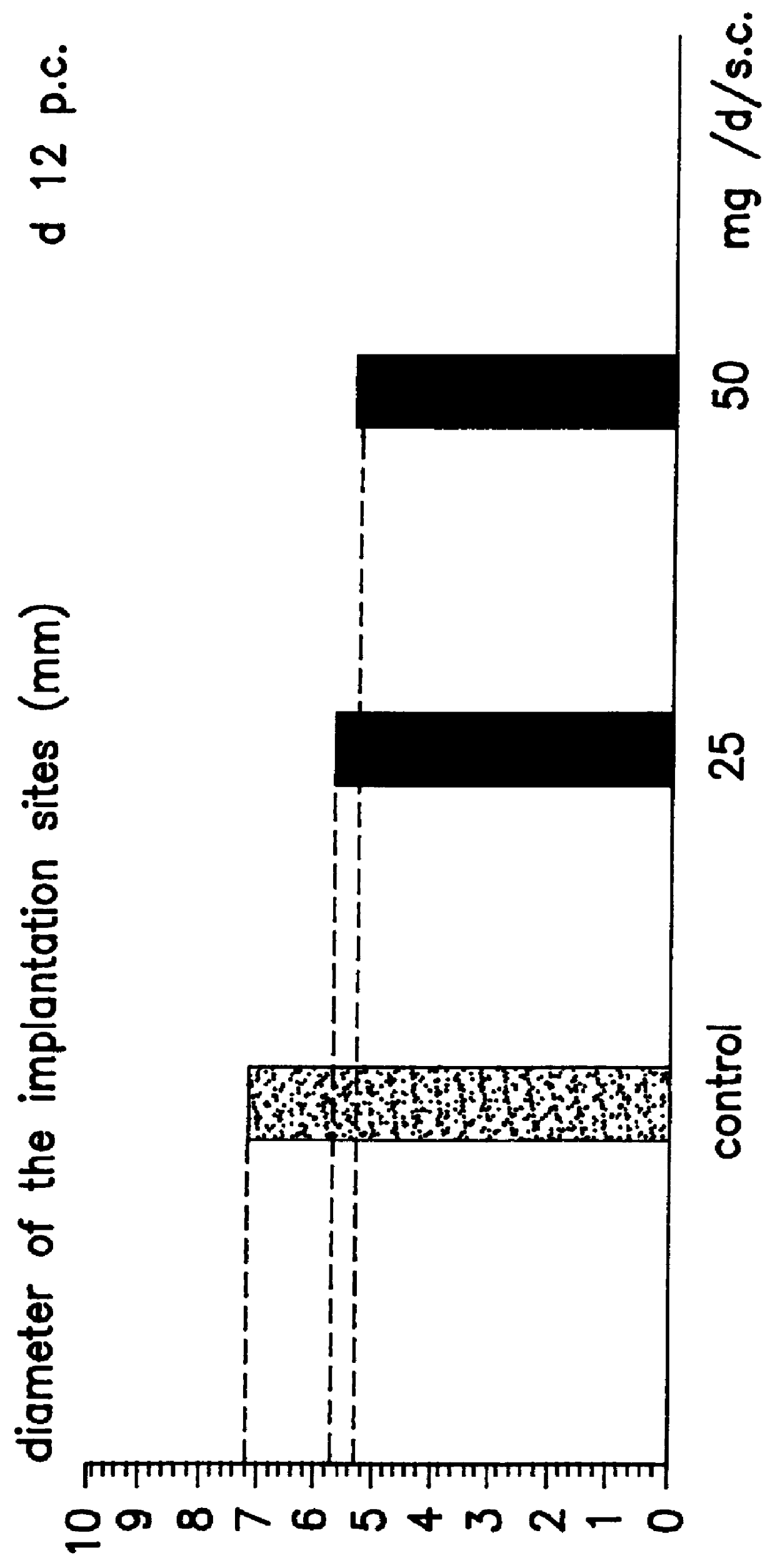
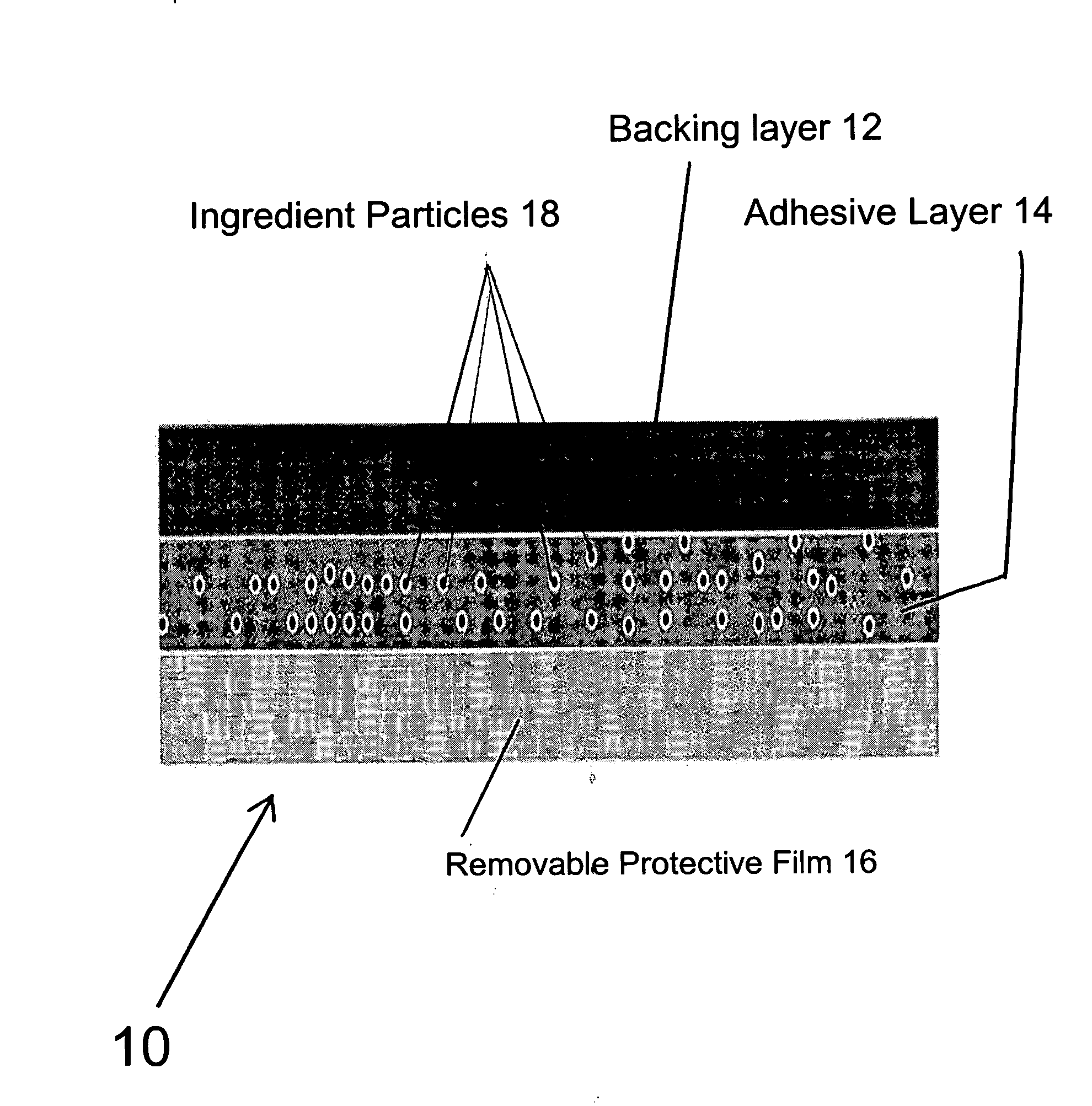
Transdermal pharmaceutical preparation with a progesterone A-specific ligand (PRASL) as active ingredient
InactiveUS20060134188A1Avoid reactionReduce riskBiocideOrganic active ingredientsTransdermal patchAdditive ingredient
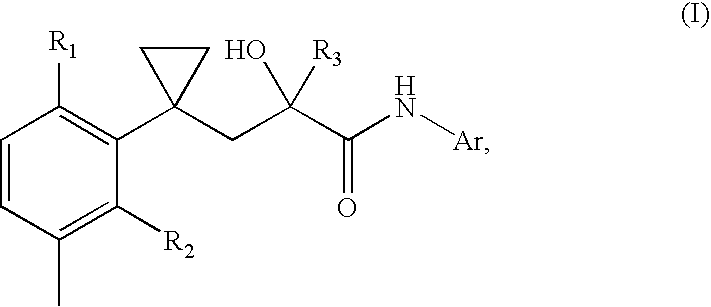
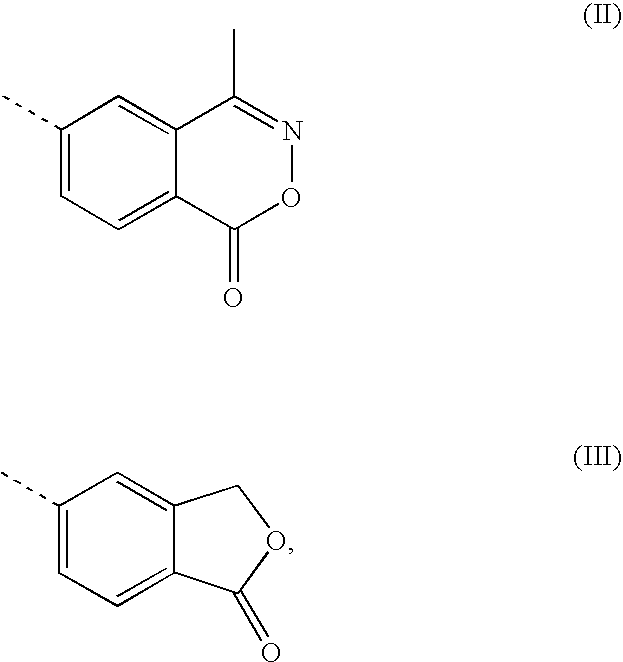
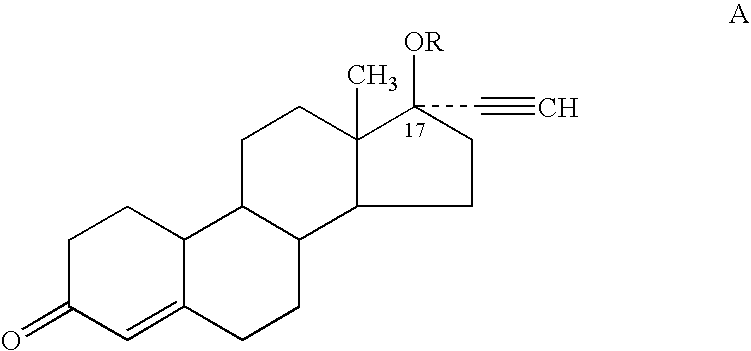
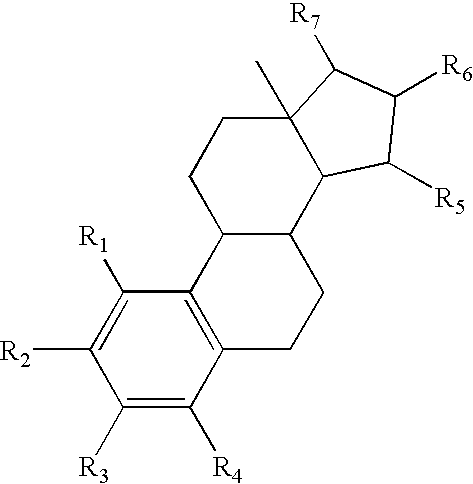
A transdermal patch for hormone therapy and fertility control has a backing layer, an effective-ingredient-containing adhesive layer adhering to the backing layer and a removable protective film. The adhesive layer includes a progestagenic effective ingredient and an estrogen in an adhesive matrix based on a silicone polymer, a polyisobutylene polymer (PIB), a polyacrylate polymer or a styrene block copolymer with butadiene or isoprene (SBS or SIS). The transdermal patch contains from 0.1 to 10%, based on a total weight of the adhesive matrix, of a progestagenic effective ingredient of formula I: wherein R1 and R2 each represent, independently of each other, H or F; R3 represents CH3 or CF3 and Ar is a group of formula II or III: or a pharmaceutically suitable derivative thereof.
Owner:SCHERING AG
Estrogen/serm and estrogen/progestin bi-layer tablets
The present invention is directed to bi-layer tablets comprising at least one estrogen in a first layer and a therapeutic agent in a second layer, and processes for their preparation.
Owner:WYETH LLC
Skin permeation enhancement composition for transdermal hormone delivery system
Owner:AGILE THERAPEUTICS
Methods of hormonal treatment utilizing ascending-dose extended cycle regimens
ActiveUS20080125402A1Reducing breakthrough bleedingBiocideOrganic active ingredientsObstetricsRegimen
The present invention provides ascending-dose extended cycle regimens in which a female is administered an estrogen and a progestin for a period of greater than 30 or 31 consecutive days, optionally followed by a hormone-free period or by a period of administration of estrogen. The disclosed regimens can be administered to a female to provide contraceptive and non-contraceptive benefits.
Owner:TEVA WOMENS HEALTH
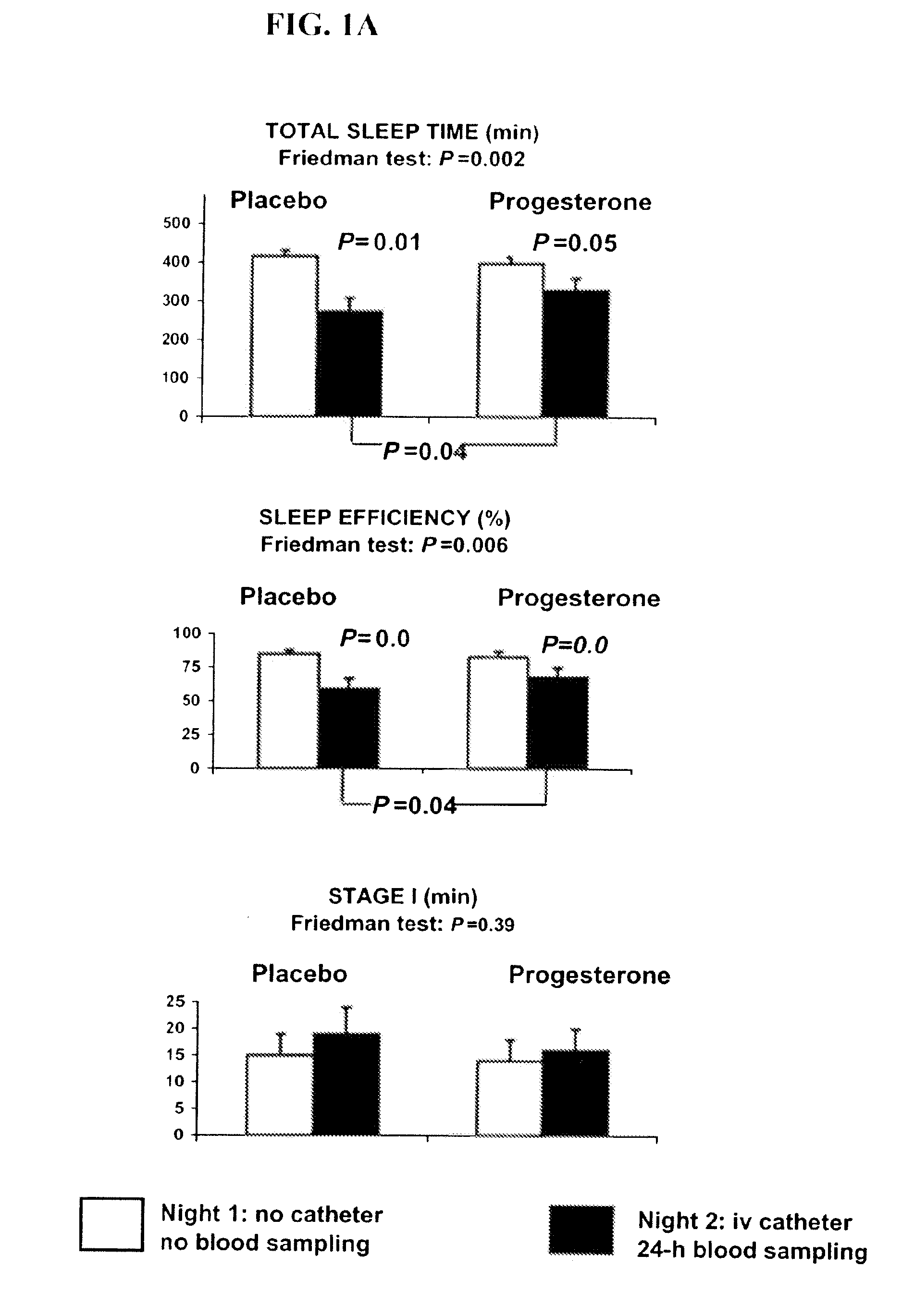
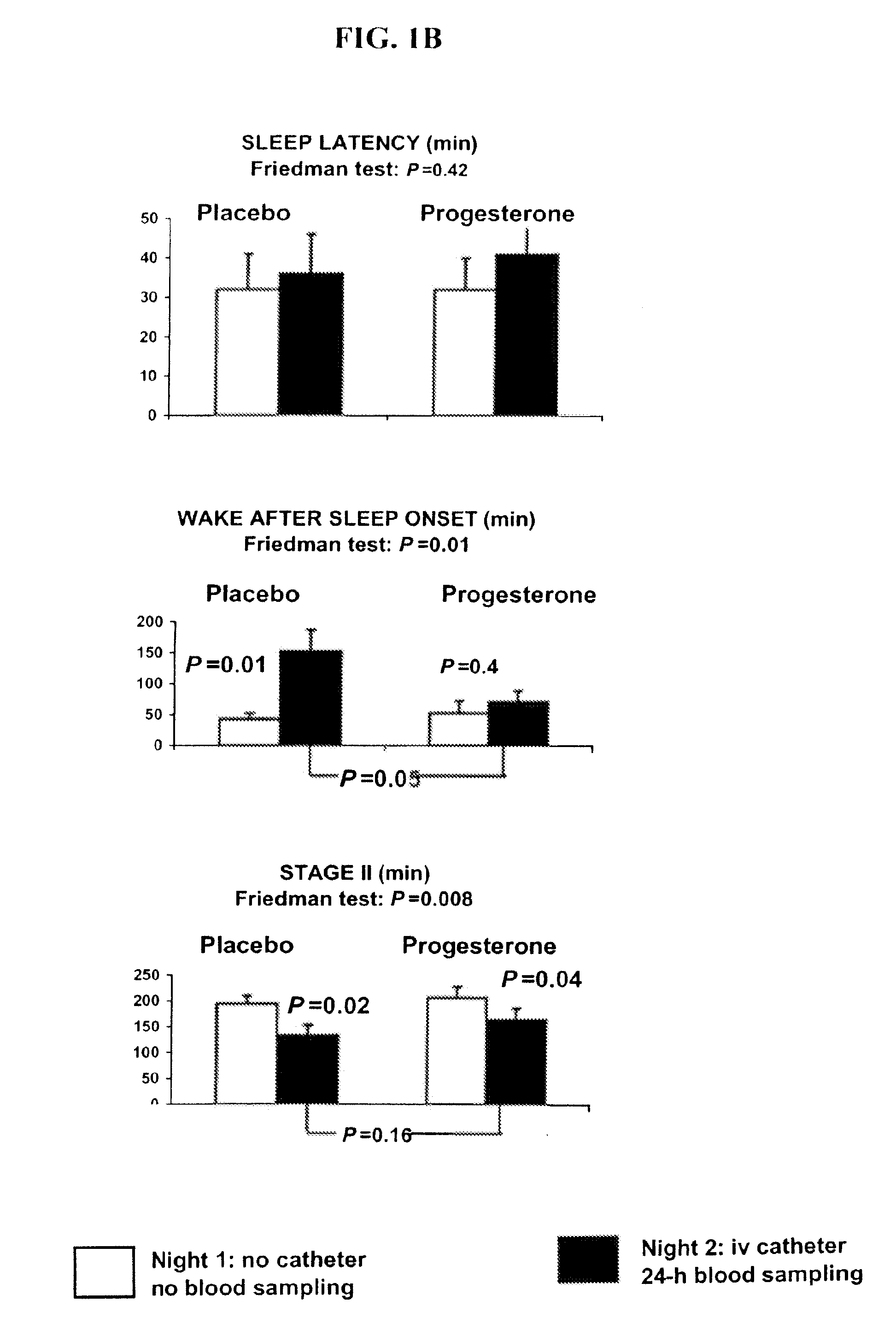
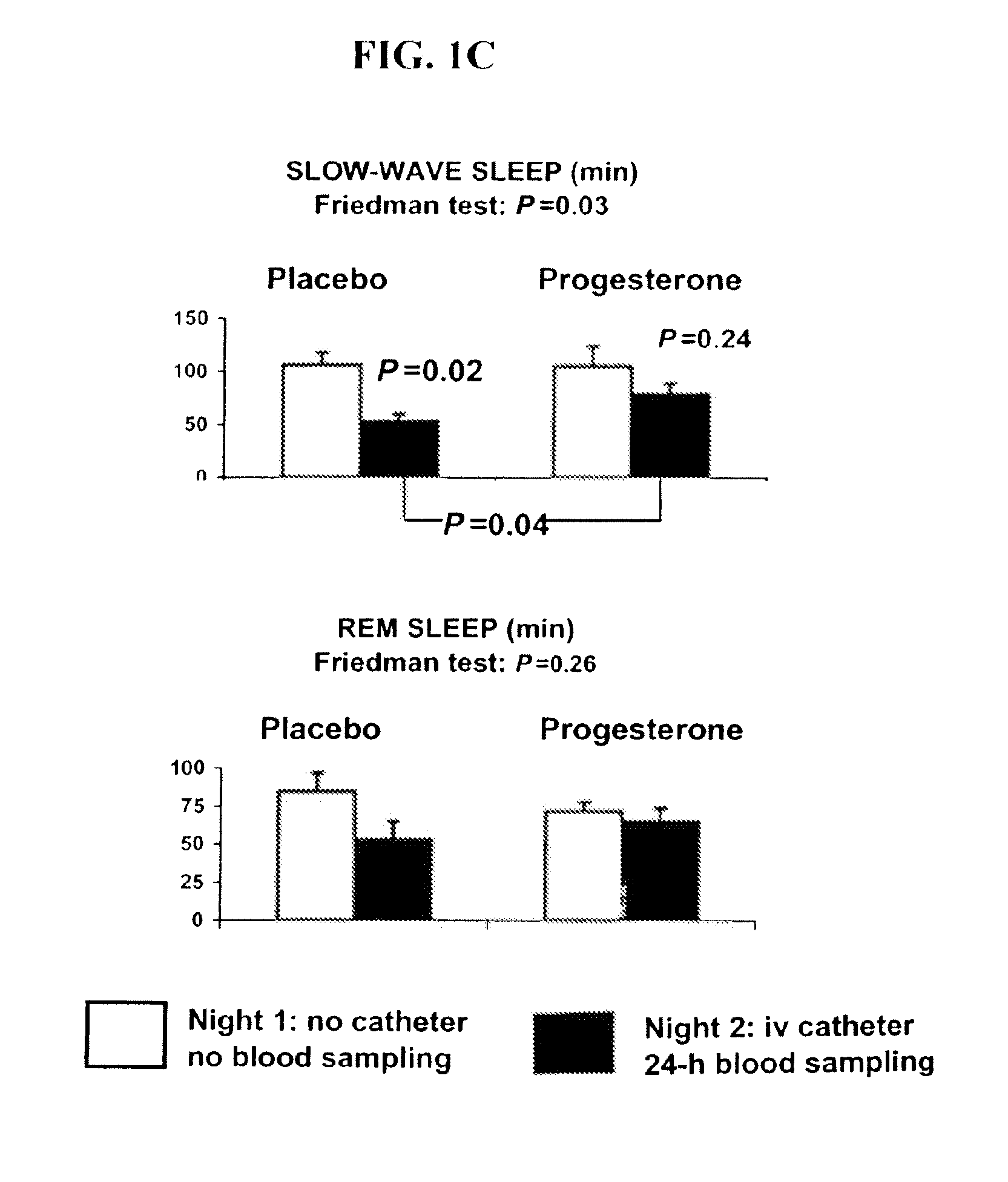
Progesterone treatment for improving sleep quality
ActiveUS20120302535A1Improve sleep qualityReduce wake up timeOrganic active ingredientsNervous disorderSleep patternsPhysiology
Described herein are therapeutic methods using progesterone and progesterone analogs to improve sleep quality. The methods may be particularly useful to treat subject with perturbed sleep patterns, such as subjects who suffer from mid-sleep period awakenings.
Owner:BESINS HEALTHCARE LUXEMBOURG (LU)
Implantation rates after in vitro fertilization, and treatment of infertility and early pregnancy loss with a nitric oxide donor or substrate alone or in combination with progesterone, and a method for contraception with nitric oxide inhibitors in combination with antiprogestins or other agents
InactiveUS20020169205A1Improve implantation rateIncrease implantationBiocidePeptide/protein ingredientsEarly Pregnancy LossPhysiology
A method is provided for the improvement of implantation rates and / or pregnancy rates in a female mammal, comprising administering to a female mammal in whom pregnancy is desired an effective amount of (a) a nitric oxide synthase substrate, a nitric oxide donor, or both, optionally in combination with (b) a progestin, and, (c) optionally, in further combination with an estrogen. A method is also provided for fertility control for a female mammal, comprising administering to a female mammal in whom pregnancy is not desired and at risk for becoming pregnant an effective amount of nitric oxide synthase inhibitor in combination with an antiprogestin. Pharmaceutical compositions are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Hormonal contraceptive product
The present invention relates to a hormonal contraceptive product having two hormonal components, an estrogen and a gestagen, and a method for the continuous suppression of the menstrual cycle by uninterrupted administration of the product of the invention.
Owner:HESCH ROLF DIETER
Methods for the modulation of brain progestagen signaling in the prevention and treatment of neurological disorders and neurodegenerative diseases
InactiveUS20100028360A1Promoting neurodegenerationImprove cognitive abilityBiocideOrganic active ingredientsNeurogenesisPhysiology
The present invention relates to methods for modulating progestagen signaling for treating neurological disorders or neurodegenerative disease, or preventing or delaying its onset in individuals deemed by competent observation and testing to be susceptible thereto. Progestagens can be administered to elevate serum and brain levels of progestagens and induce neurogenesis. Progestagen therapy may prevent some of the neurodegenerative and cognitive changes associated with developmental and aging associated neurological disorders and neurodegenerative diseases. Progestagen therapy together with suppression of GnRH, kisspeptin, LH and / or FSH signaling also may be used for treating neurological disorders or neurodegenerative diseases. The invention also relates to methods for inhibiting or delaying blastulation during embryogenesis, and neurogenesis during embryogenesis, fetal, neonatal, childhood, puberty or adult life. Blocking progestagen, estrogen and / or opioid signaling with receptor antagonists will inhibit neurogenesis. The invention also relates to using progestagens in vitro to induce neurogenesis in embryonic or adult stem cells.
Owner:ATWOOD CRAIG STEPHEN
Hormonal contraceptive product
InactiveUS20030073673A1Improve reliabilityAvoid developmentOrganic active ingredientsBiocideObstetricsMenstrual cycle
The present invention relates to a hormonal contraceptive product having two hormonal components, an estrogen and a gestagen, and a method for the continuous suppression of the menstrual cycle by uninterrupted administration of the product of the invention.
Owner:WYETH LLC
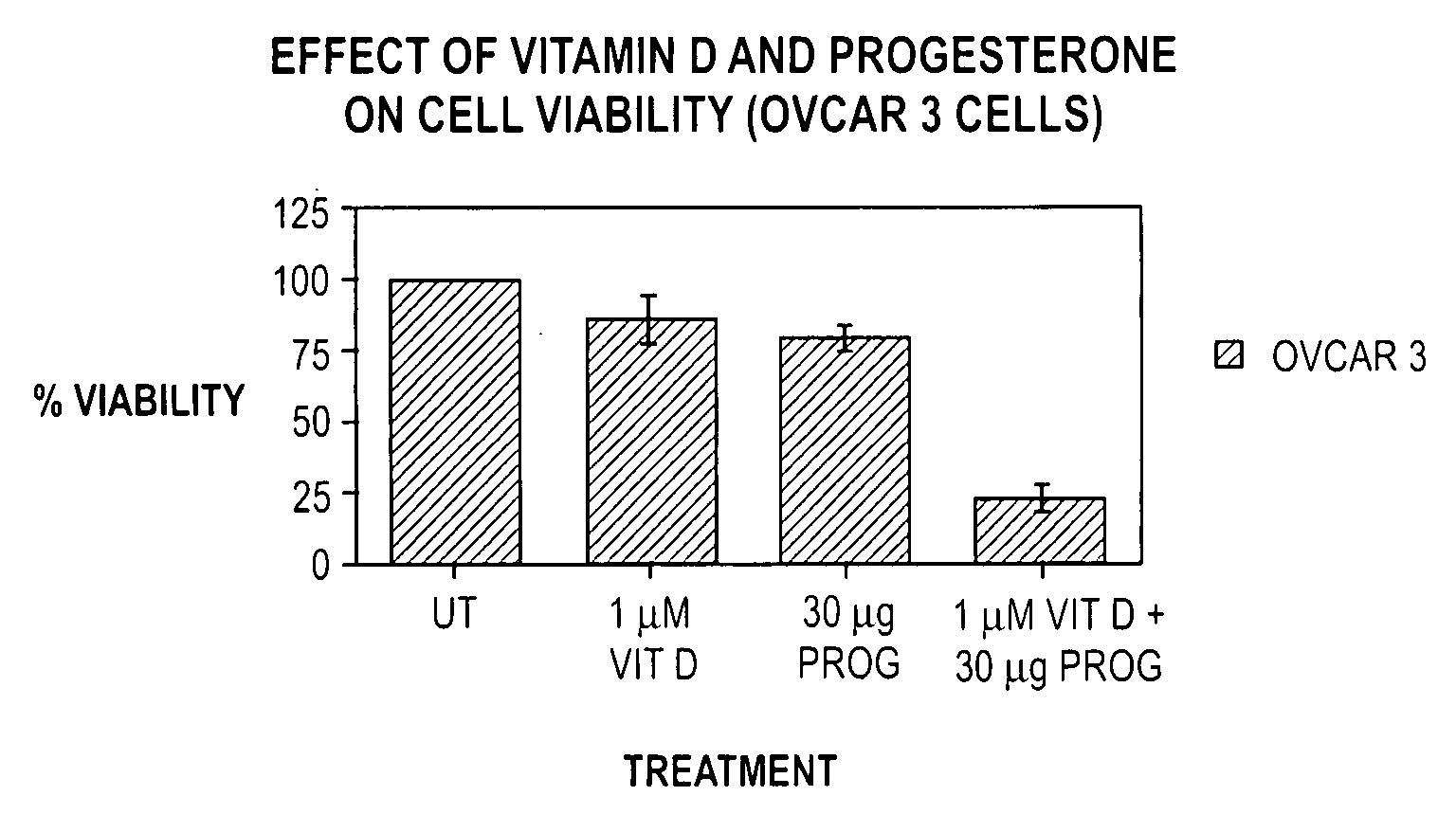
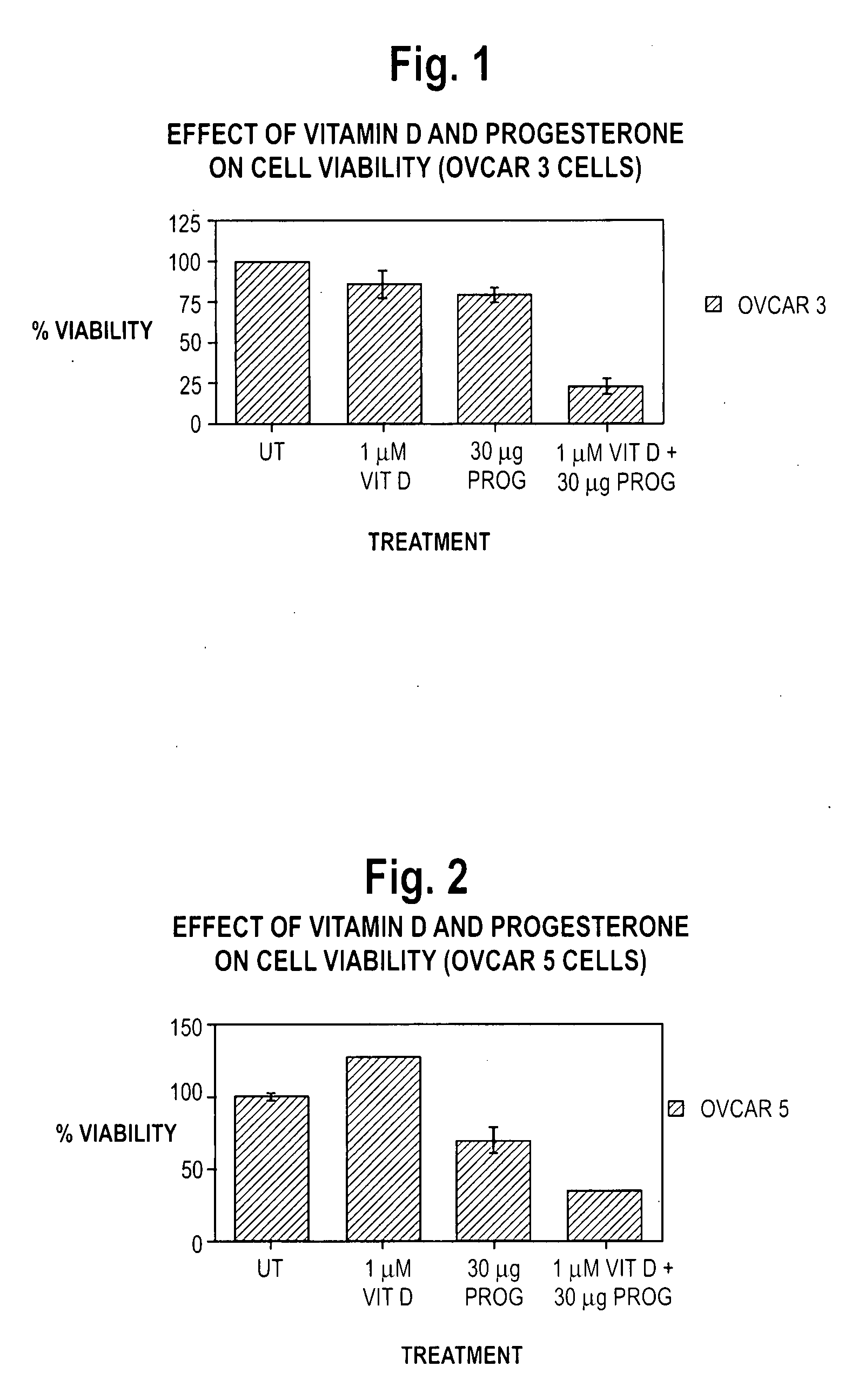
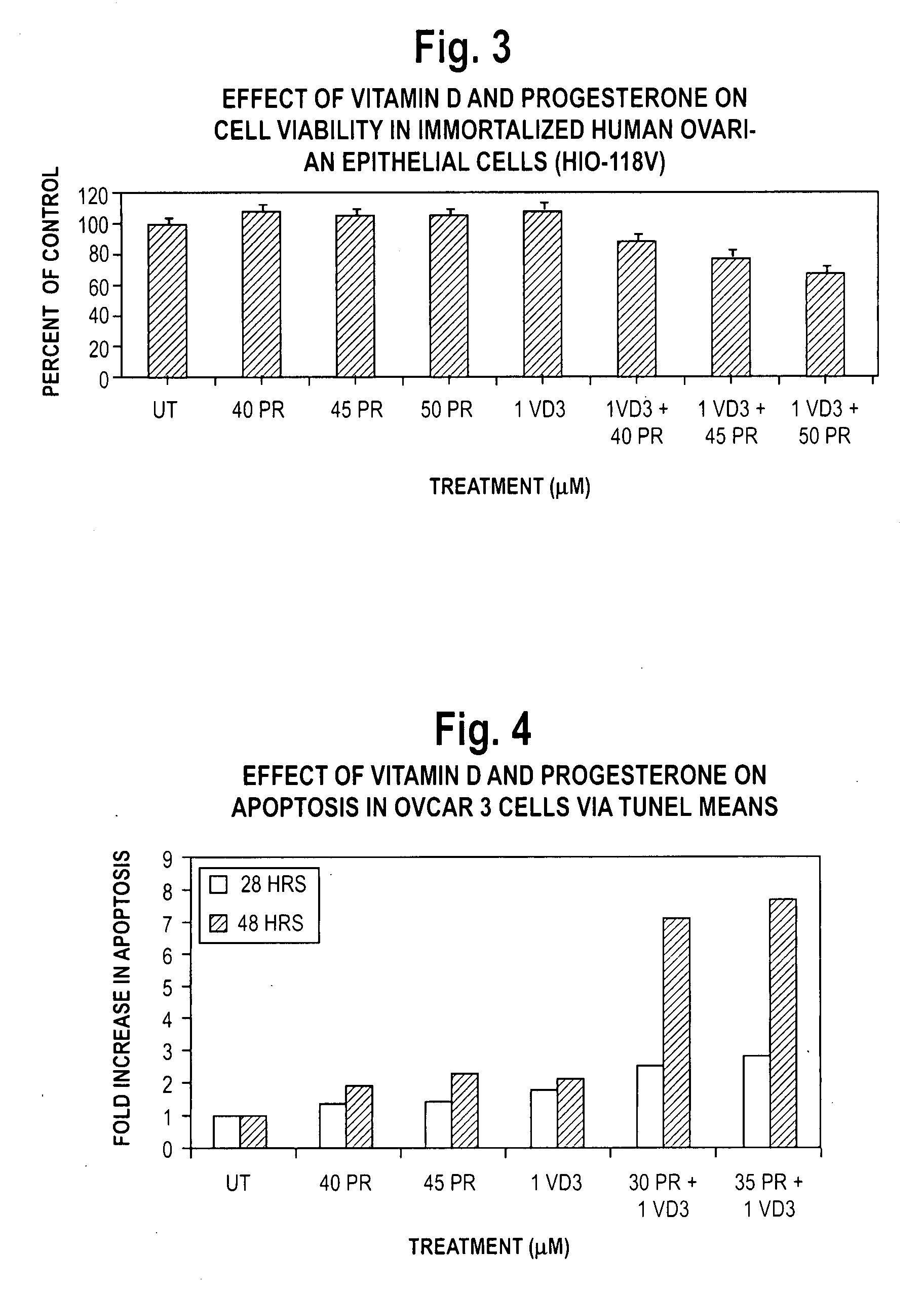
Pharmaceutical products containing hormones and a 25-hydroxy vitamin d compound
InactiveUS20080312197A1Maximum safetyGood effectBiocideOrganic active ingredientsMedicineVitamin D+Metabolites
The present invention relates to pharmaceutical products containing progestins in combination with the 25 hydroxy Vitamin D compounds. The 25 hydroxy Vitamin D compounds are preferably administered with the progestins. In OC and HRT regimens, the 25 hydroxy Vitamin D compounds can be administered daily, or on a non-daily basis, and if so, preferably when the progestin dosages are the highest in the cycle.
Owner:RODRIGUEZ GUSTAVO C
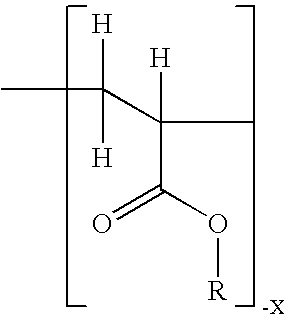
Non-steroidal progesting
InactiveUS7388006B2Suitable for useBiocideOrganic chemistryPR - Progesterone receptorOral contraceptive drug
The present invention relates to non-steroidal progestins of the general formula (I)whereinR1 and R2 are independently of each other —H or —F,R3 is —CH3 or —CF3, andAr isor a pharmaceutically acceptable derivative or analogue thereof. These progestins are suitable for selectively modulating progesterone receptor mediated effects in different target tissues, particularly in uterine tissue versus breast tissue. Therefore, the progestins of the present invention, optionally in combination with estrogens, may be used for contraception (in particular in estrogen-free oral contraceptives), hormone replacement therapy and the treatment of gynecological disorders. The present invention furthermore relates to methods for selectively modulating progesterone receptor mediated effects in different target tissues or organs.
Owner:BAYER SCHERING PHARMA AG
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:BAYER SCHERING PHARMA AG
Methods of Hormonal Treatment Utilizing Ascending-Dose Extended Cycle Regimens
InactiveUS20070111975A1Reducing breakthrough bleedingBiocideOrganic active ingredientsGynecologyRegimen
The present invention provides ascending-dose extended cycle regimens in which a female is administered an estrogen and a progestin for a period of greater than 30 or 31 consecutive days, optionally followed by a hormone-free period or by a period of administration of estrogen. The disclosed regimens can be administered to a female to provide contraceptive and non-contraceptive benefits.
Owner:TEVA WOMENS HEALTH
Treatment for dry eye using testosterone and progestagen
InactiveUS20100016264A1Minimizes and avoids systemic treatmentAvoid disadvantagesOrganic active ingredientsBiocideOcular surfaceProgestin
The present invention comprises a composition and methods for treating eye conditions using a composition having a therapeutically effective amount of a progestagen, or a therapeutically effective amount of a progestagen with a testosterone; and pharmaceutically acceptable carrier, wherein the composition is applied to the palpebral part of the eye and / or ocular surface.
Owner:CONNOR CHARLES G +1
Combination contraceptive, kits that contain the latter, and a method that uses the latter
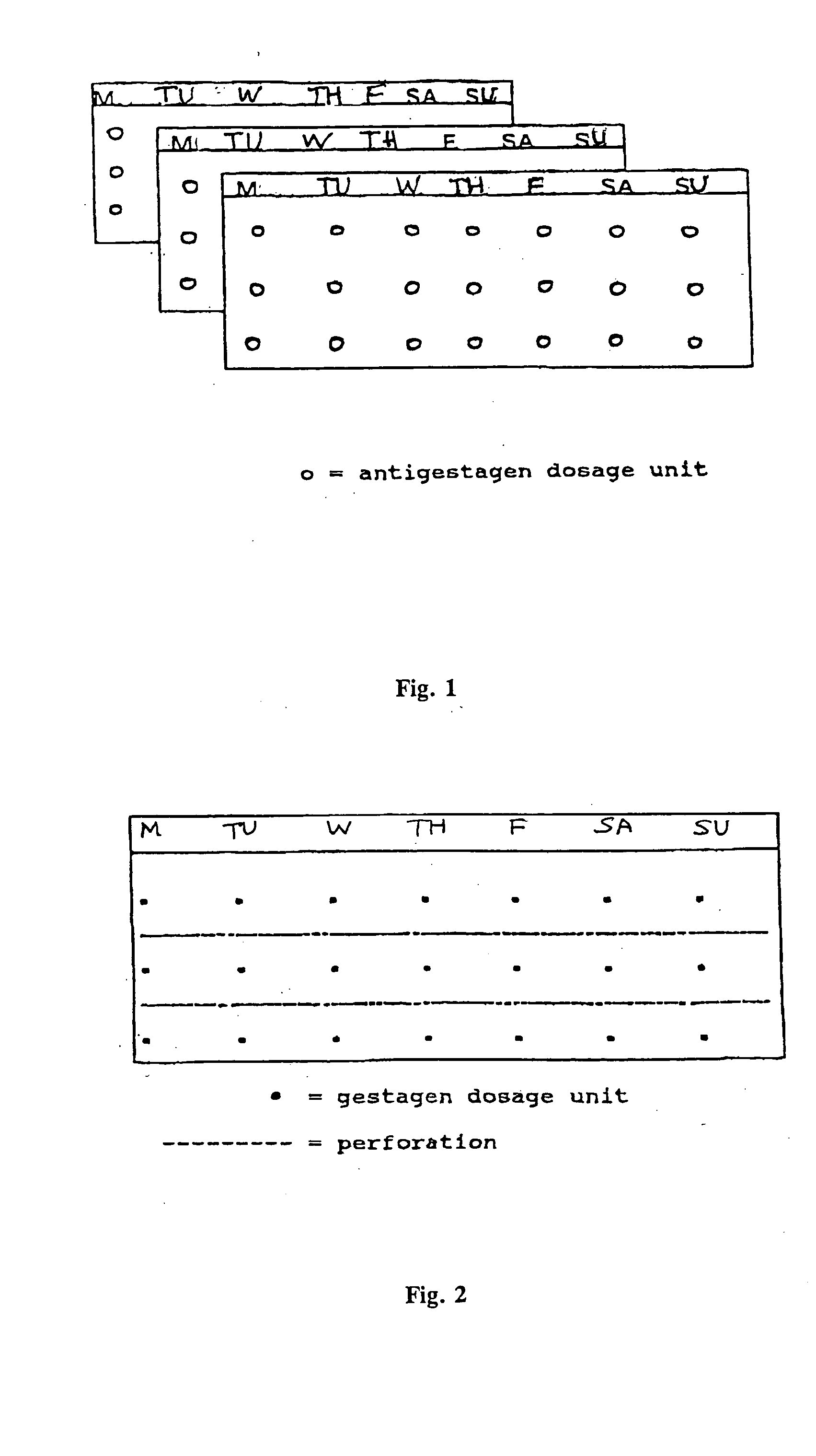
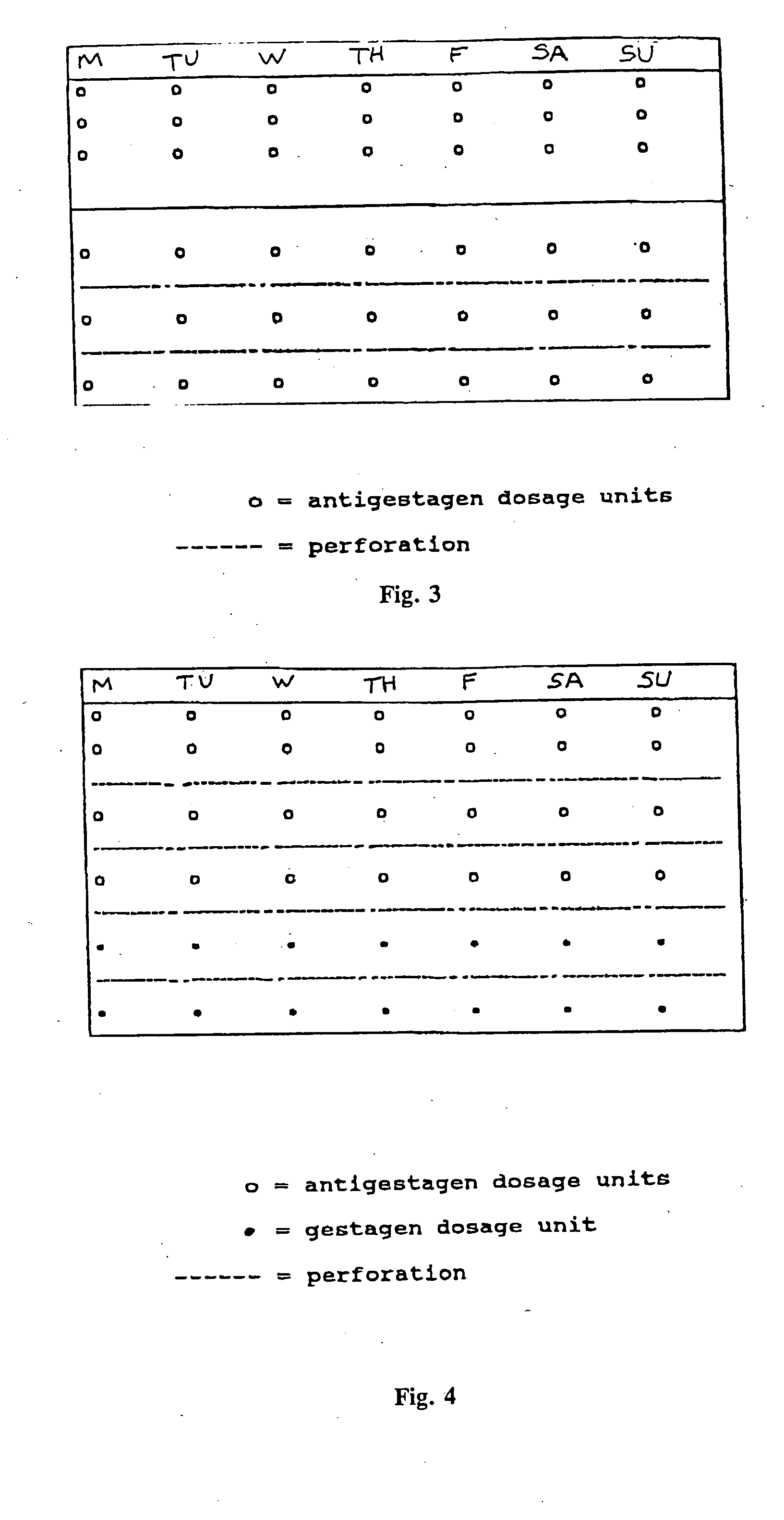
This invention relates to a multi-phase combination preparation that contains at least 28 daily dosage units: with a first phase of at least 21 initial daily dosage units, containing a competitive progesterone antagonist in a dosage which inhibits ovulation during the first above-named phase; and a second phase of 5 to 28 daily dosage units, in which each dosage unit of this second phase contains a gestagen, as well as a corresponding package (contraceptive kit) that contains this combination preparation and a contraceptive method which uses the combination preparation above.
Owner:BAYER SCHERING PHARMA AG
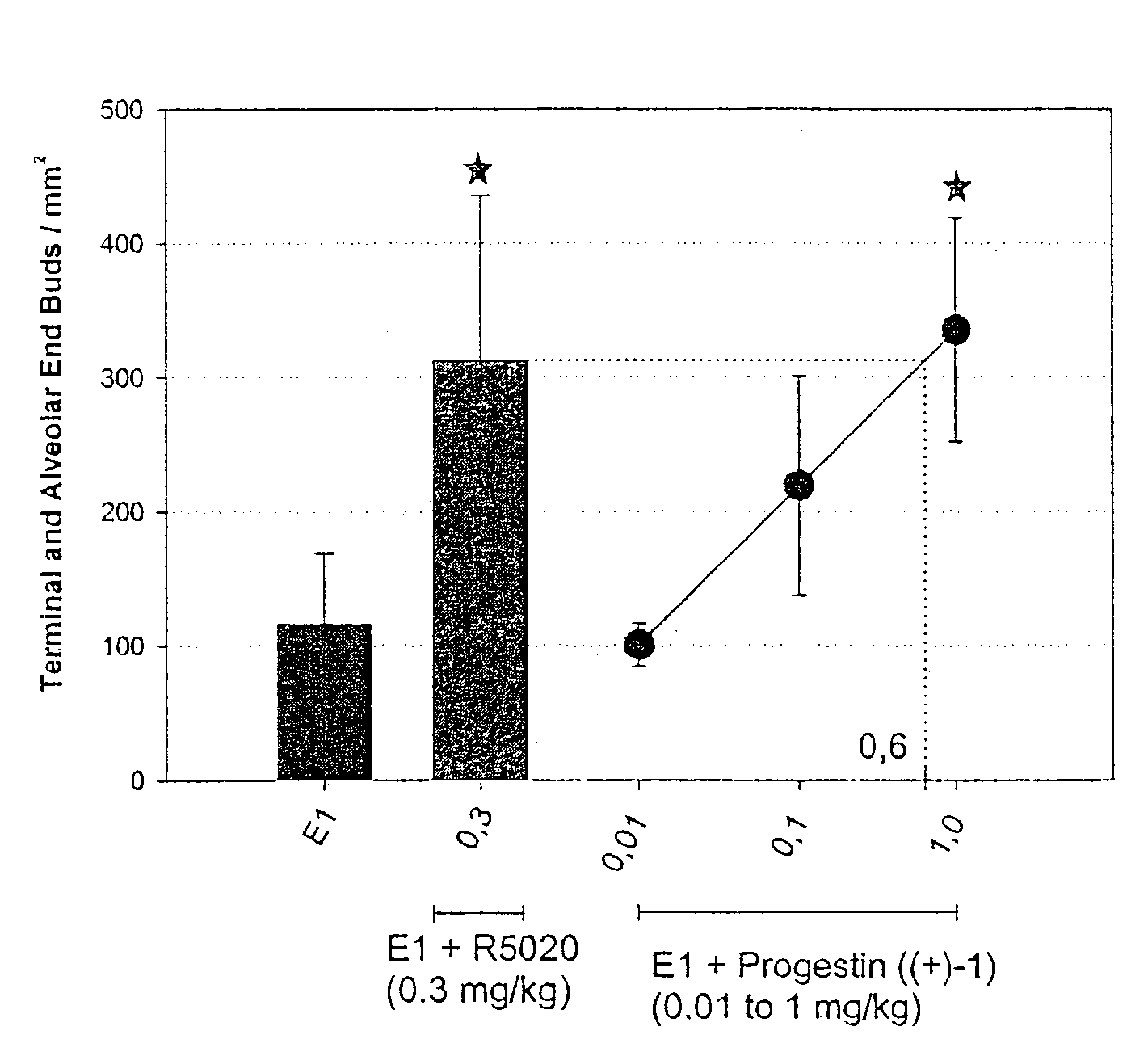
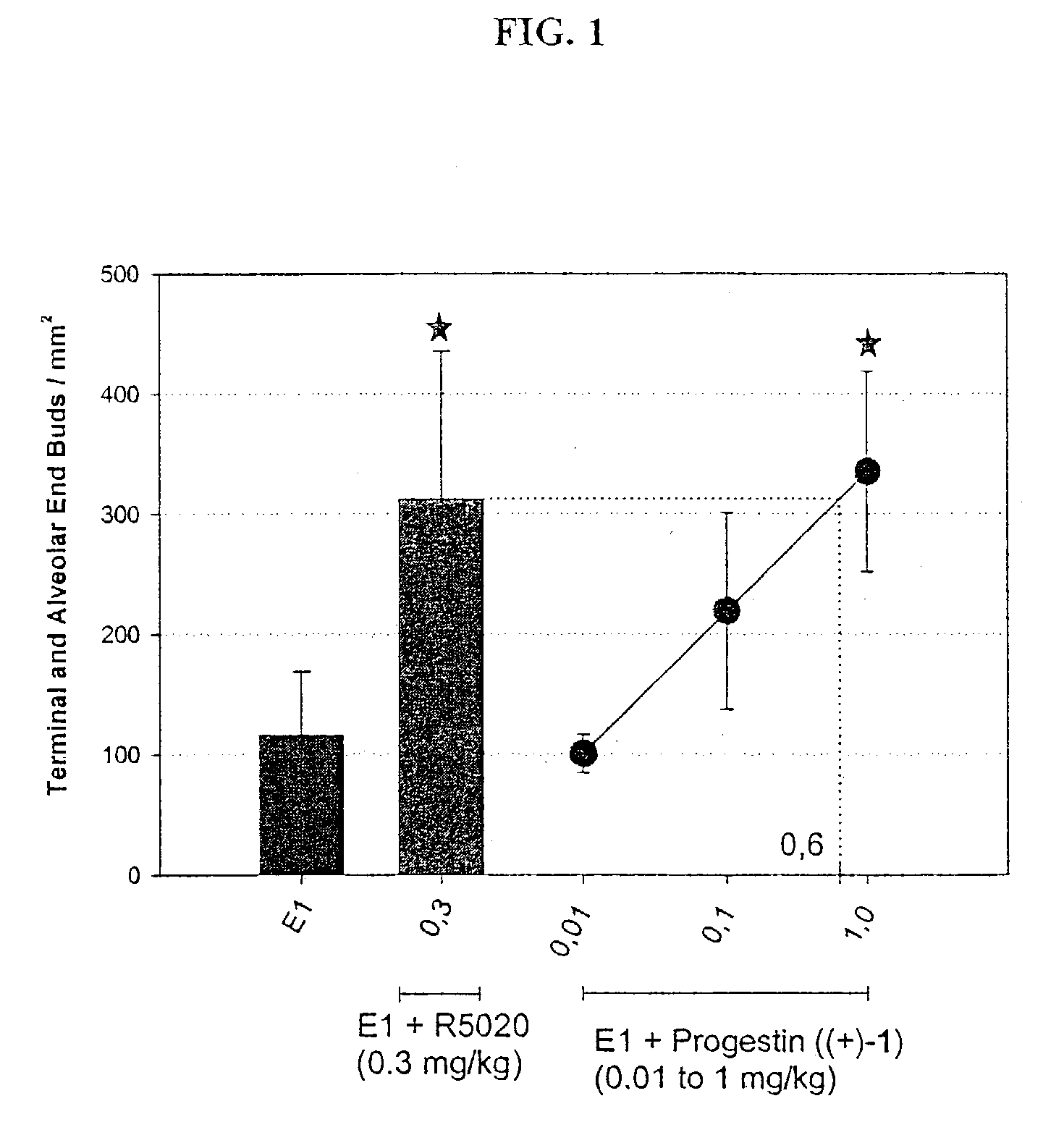
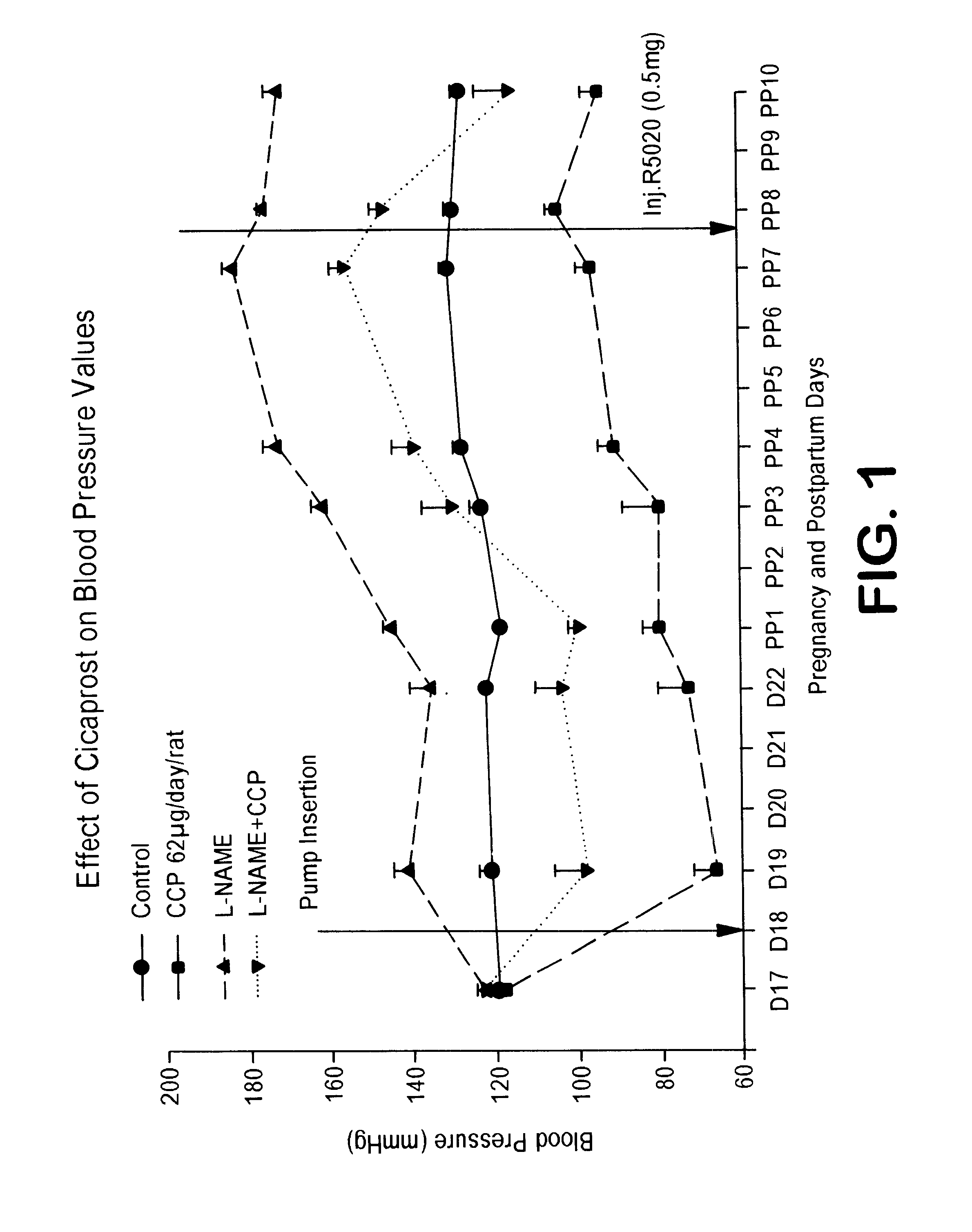
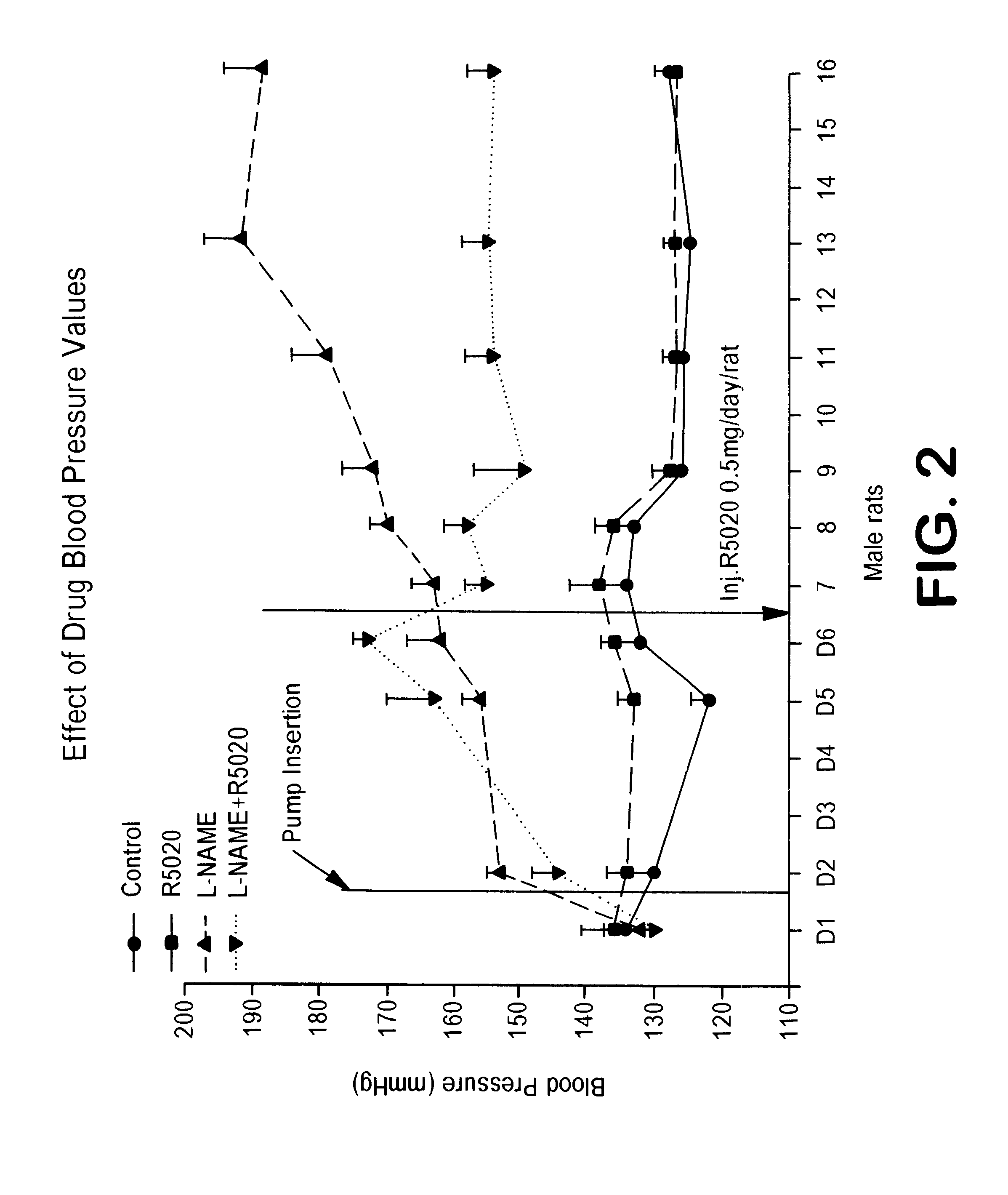
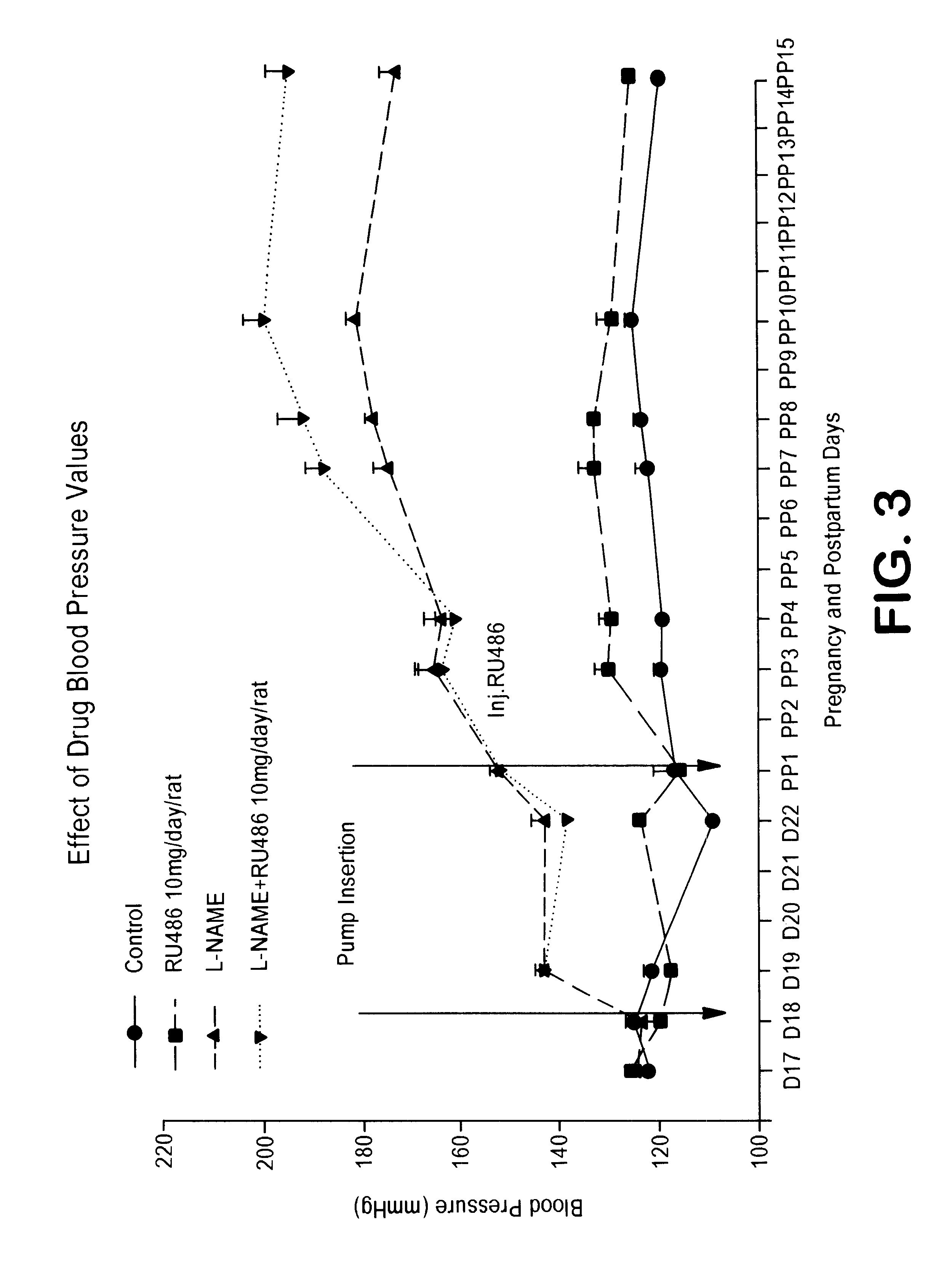
Combination of prostacyclin with an estrogen or progestin for the prevention and treatment of atherosclerotic vascular disease including preeclampsia and for the treatment of hypertension, and for hormone replacement therapy
Cardiovascular disease, including preeclampsia in pregnant women and hypertension in both women and men, are prevented or treated by administering thereto prostacyclin or a prostacyclin analog in combination with one or both of an estrogen and a progestin, which combination is also useful for HRT in peri- and post-menopausal women.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Combination progestin oral contraceptive regimen
InactiveUS20010020015A1Reducing breakthrough bleedingBiocideOrganic active ingredientsRegimenPhysiology
An oral contraception regimen which comprises sequentially administering two or more progestational agents exhibiting different effects on the human endometrium in combination with an estrogen. The invention is also directed to an extended use oral contraception regimen comprising the sequential administration of two or more progestational agents in combination with an estrogen.
Owner:ORTHO MCNEIL PHARM INC
Preparation method for progestin
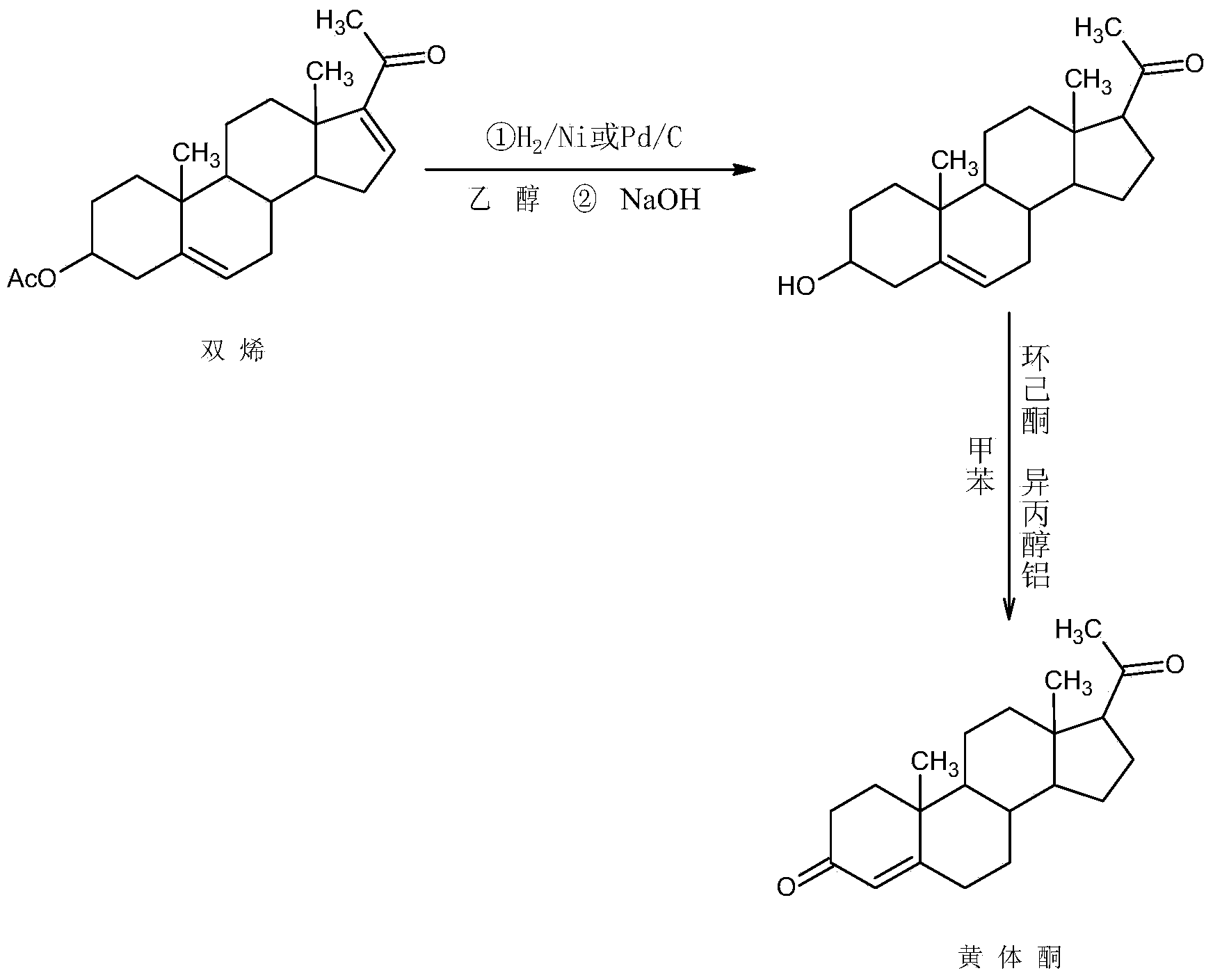
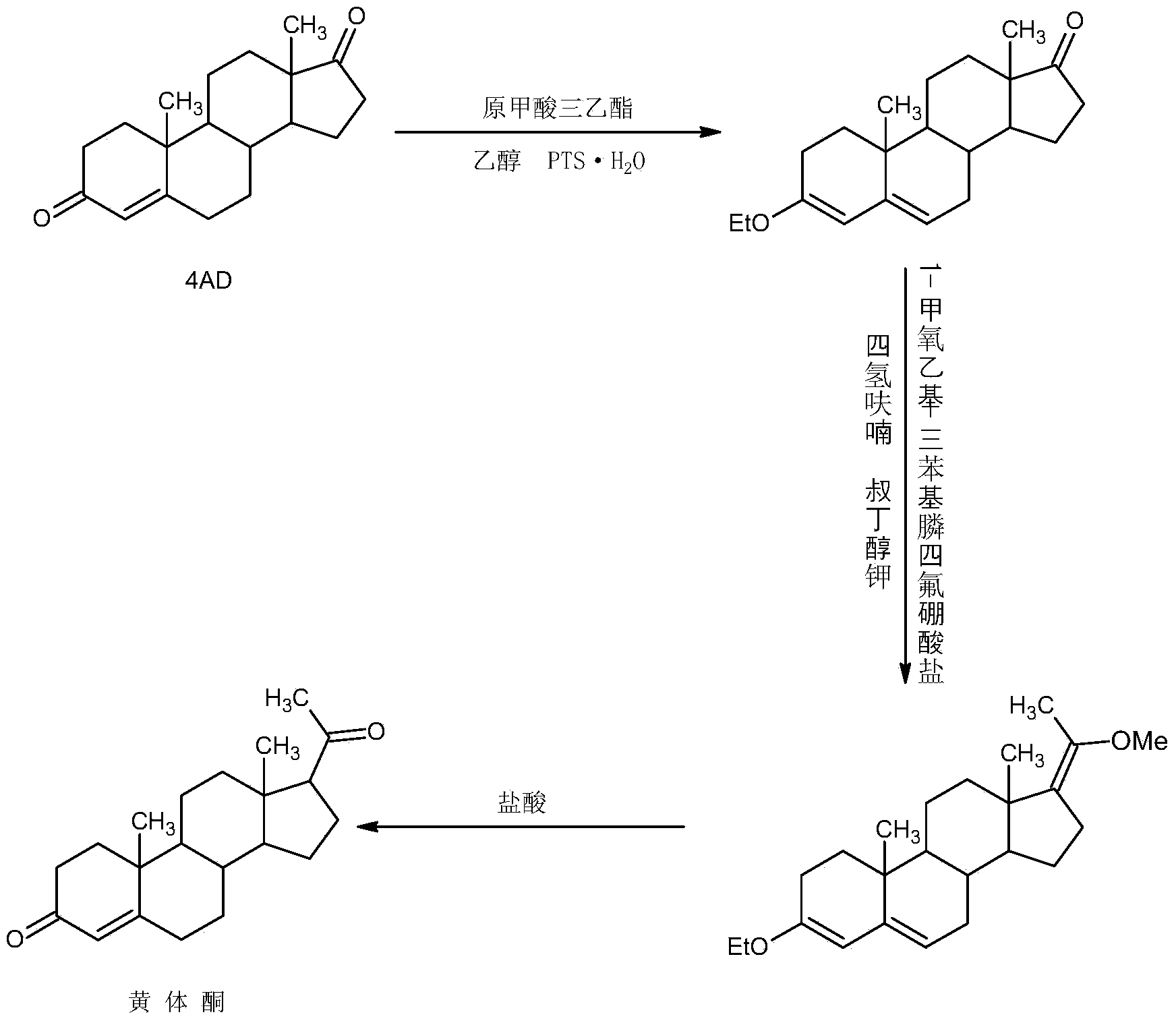
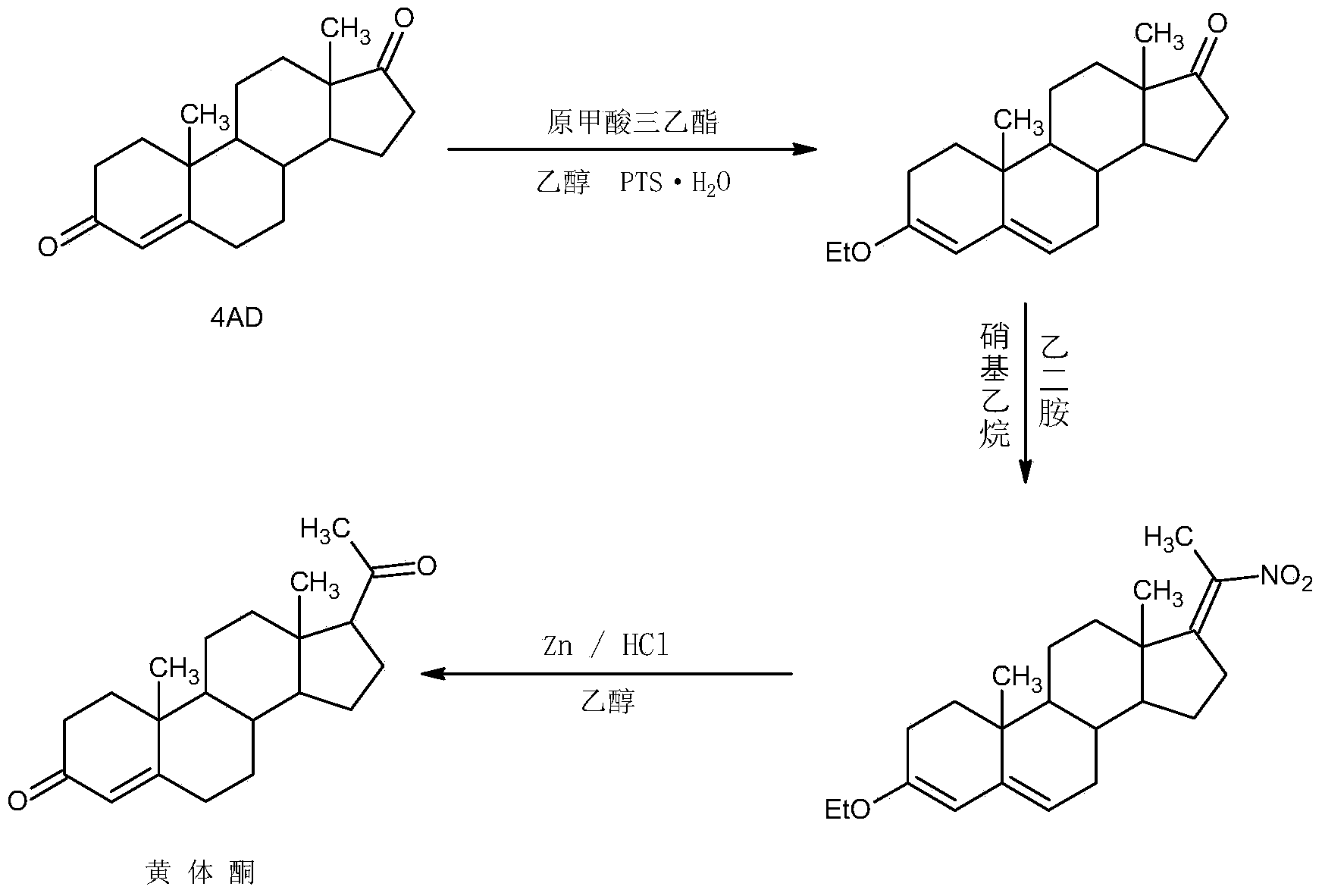
ActiveCN104262442AWide variety of sourcesProcess economy and environmental protectionSteroidsEthylenediamineKetone
The invention relates to a preparation method for progestin. 4-androstenedione is used as a raw material. The preparation method comprises the following steps: A, etherate is synthetized, wherein the 4-androstenedione and triethyl orthoformate perform an acid catalyzed reaction in organic solvents of dichloromethane, low-carbon alcohol and the like to obtain the etherate 3-ethoxy-androstane-3, 5-diolefin-17-ketone; B, a nitro substance is synthetized, wherein the etherate in the organic solvents and nitroethane perform 17-bit addition under the catalysis of ethylenediamine to obtain the nitro substance 3-ethoxy-20-nitro-pregnane-3, 5, 17 (20)-triene; and C, the progestin is synthetized, wherein the nitro substance is reduced by zinc powder in organic solvents of acetic acids, low-carbon alcohol and the like, acid hydrolysis is performed, so that semi-finished products of the progestin are obtained, the semi-finished products of the progestin are decolored and refined by alcohol and activated carbon to obtain the progestin, the content of HPLC is more than 99.5%, the melting point is 128-131 DEG C, and the total yield of synthetized weight is 83-87%. When the method disclosed by the invention is used for producing the progestin, the yield is high, the degree of purity is good, the quality is stable, the solvent recovering rate is high, and the method is economic and environment-friendly.
Owner:HUNAN KEREY BIOTECH
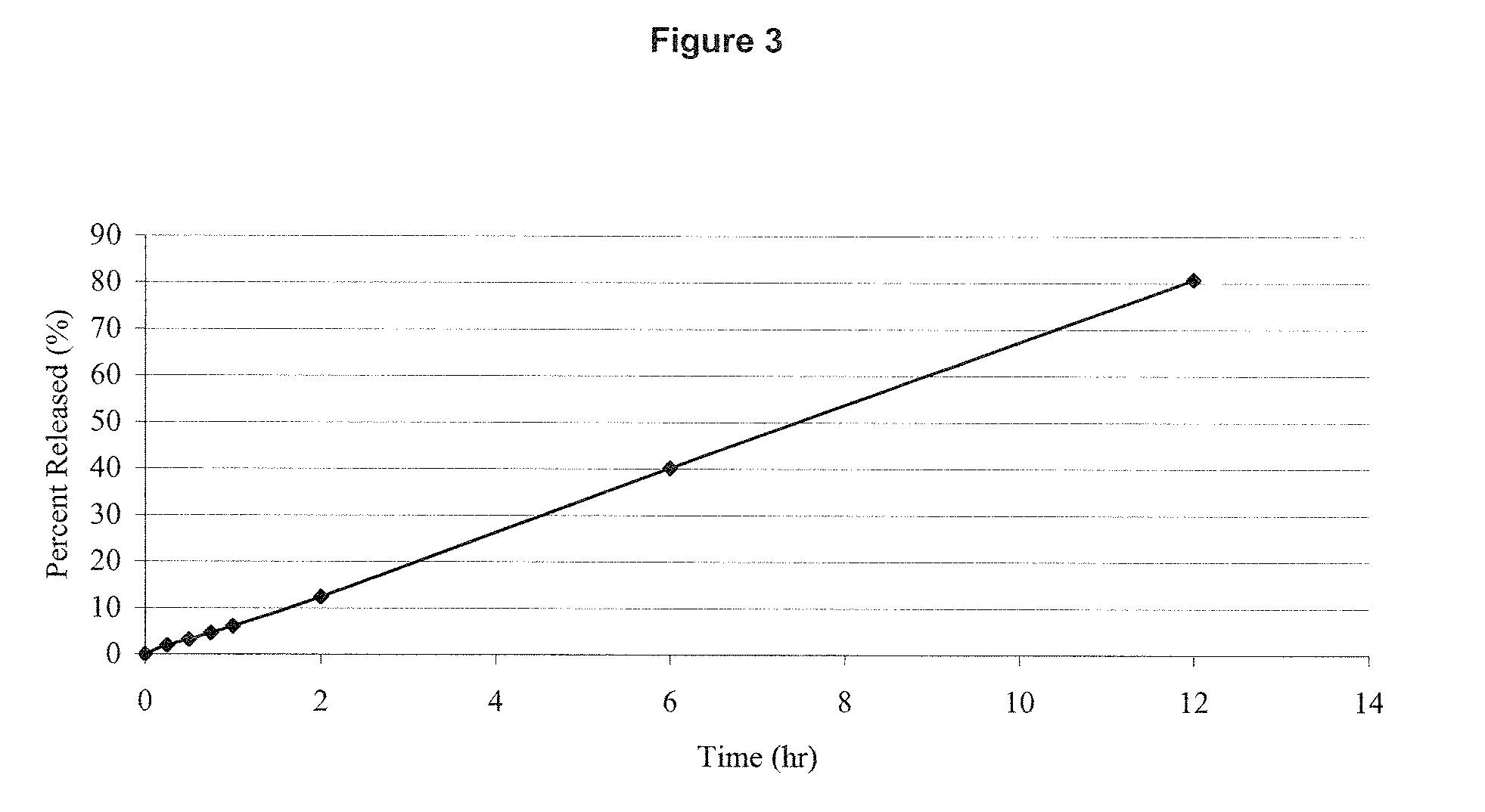
Preparation method of progestin sustained-release gel for treating threatened abortion
InactiveCN103239389AOrganic active ingredientsPharmaceutical delivery mechanismProgesteronesThreatened Abortions
The invention relates to a progestin sustained-release gel for treating threatened abortion. The progestin sustained-release gel is prepared by the following steps: preparing micronized progestin emulsion; and dispersing the emulsion in a temperature-sensitive body gel matrix to form the progestin temperature-sensitive sustained-release gel.
Owner:SHANGHAI ZHAOHUI PHARMA
Drug delivery system comprising a tetrahydroxylated estrogen for use in hormonal contraception
ActiveUS7871995B2Improve reliabilityReliable efficacyOrganic active ingredientsBiocidePresent methodPhysiology
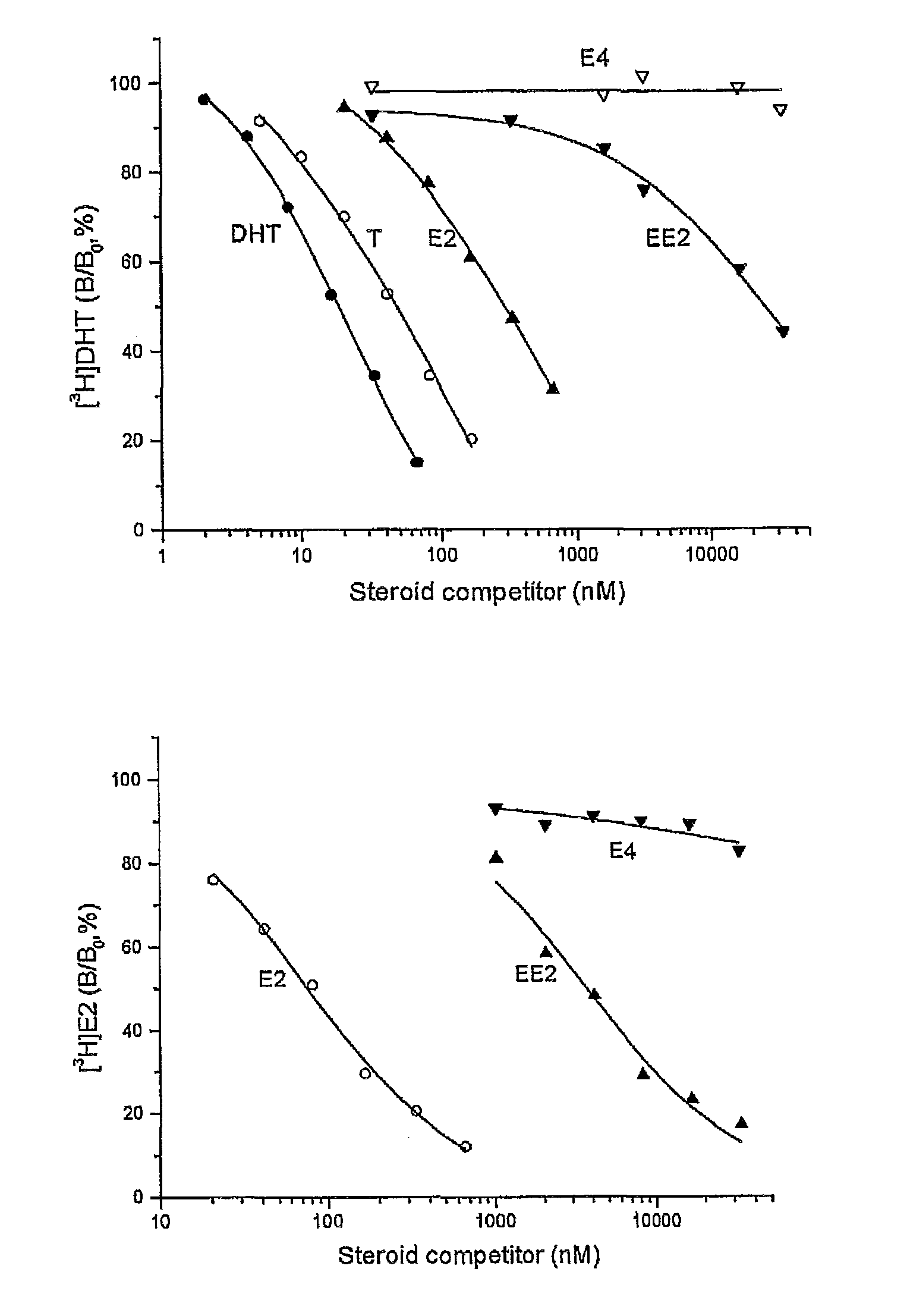
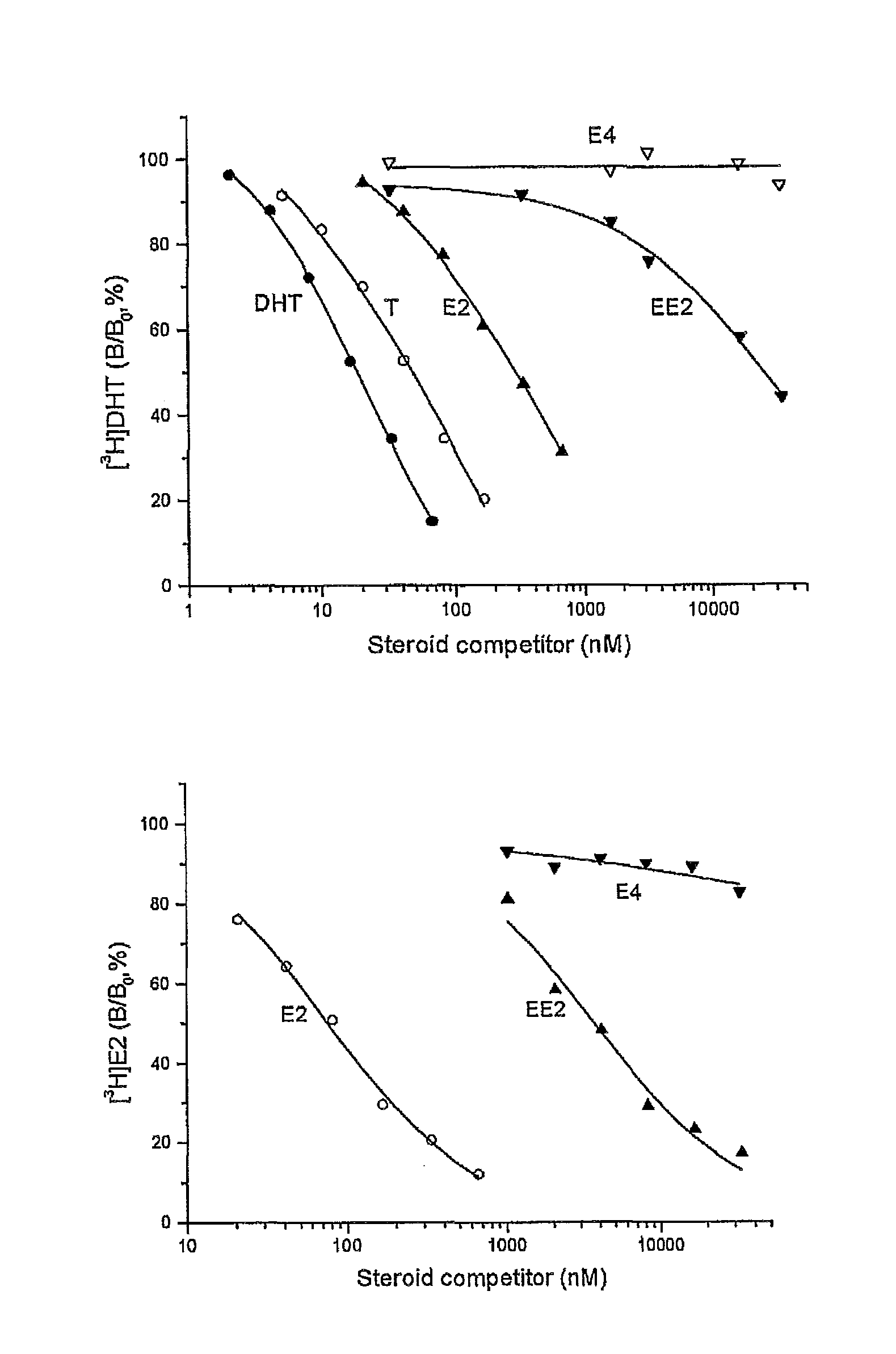
A method of contraception in mammalian females, which method comprises the parenteral or rectal administration of an estrogenic component and a progestogenic component to a female of childbearing capability in an amount effective to inhibit ovulation, wherein the estrogenic component is selected from the group consisting of substances represented by the following formula (1)in which R1, R2, R3, R4 independently are a hydrogen atom, a hydroxyl group or an alkoxy group with 1-5 carbon atoms; each of R5, R6, R7 is a hydroxyl group; and no more than 3 of R1, R2, R3, R4 are hydrogen atoms; precursors capable of liberating a substance according to the aforementioned formula when used in the present method; and mixtures of one or more of the aforementioned substances and / or precursors. Another aspect of the invention concerns a drug delivery system for parenteral or rectal administration that contains the aforementioned estrogenic component and a progestogenic component, said drug delivery system being selected from the group consisting of suppositories, systems for intravaginal delivery, inhalers, nasal sprays and transdermal delivery systems.
Owner:ESTETRA SRL
Drug delivery systems (WAFER) for pediatric use
InactiveUS20120207836A1Improve complianceUnpleasant taste is effectivelyPowder deliveryBiocideAlkaline earth metalWater soluble
The present invention describes drug delivery compositions in the form of thin water-soluble films (wafers), which contain particles that comprise at least one active ingredient—which is not an estrogen and / or a progestin and / or an alkaline earth metal salt of 5-methyl-(6S)-tetrahydrofolate—and at least one protective agent. The protective agent provides effective taste-masking of the active ingredient due to limited release of the active ingredient in the mouth. The active ingredient is hence not absorbed via the buccal route, but rather via the enteral (per-oral) route. The particles contained in the wafer provided by the present invention have a particle size of below 40 μm thereby resulting in an acceptable sensation in the mouth while dissolving. Such wafers are especially suitable for pediatric use.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Method for inducing high-yield dairy cow to regularly ovulate and increasing conception rate
InactiveCN106137448APromote estrusOvercome the serious loss and other problemsAnimal reproductionMilk cow'sEmbryo
The invention provides a method for inducing a high-yield dairy cow to regularly ovulate and increasing the conception rate. The method includes the steps that 1, 35-40 days after the high-yield dairy cows calve, a progestational hormone device is embedded into a dairy cow vagina for the first time; 2, the progestational hormone device is taken out after being embedded for 14-16 days; 3, prostaglandin is injected; 4, 2 days after the prostaglandin is injected, an ovulation promoting hormone is injected; 5, 12-16 hours after the ovulation promoting hormone is injected, artificial insemination is carried out; 6, on the sixth to eighth day after artificial insemination, the progestational hormone device is embedded again, and the further embedding time ranges from 14-16 days. According to the method, the development and ovulation time of follicles in the ovary of the dairy cow can be regulated, detection of oestrus is not needed, artificial insemination is directly carried out, fetus protection medicine is adopted, and the survival rate of embryos is increased. The defects that oestrus of the high-yield dairy cow is not easy to observe is overcome, the oestrus rate and the conception rate of the dairy cow can be increased, the calving interval of the high-yield dairy cow is shortened, breeding benefits of the high-yield dairy cow are increased, and production management of a dairy cow farm is convenient.
Owner:HEBEI UNIV OF ENG
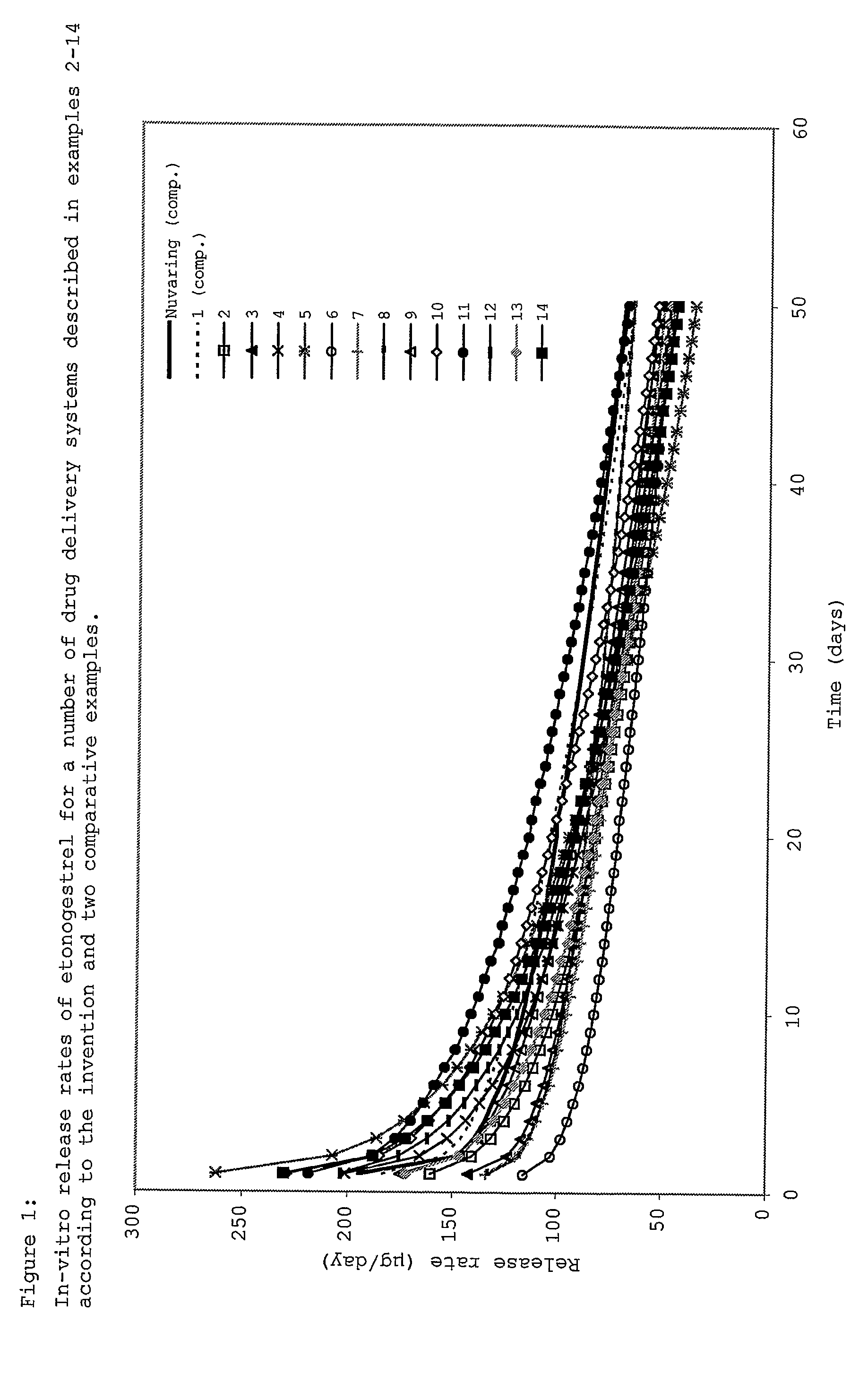
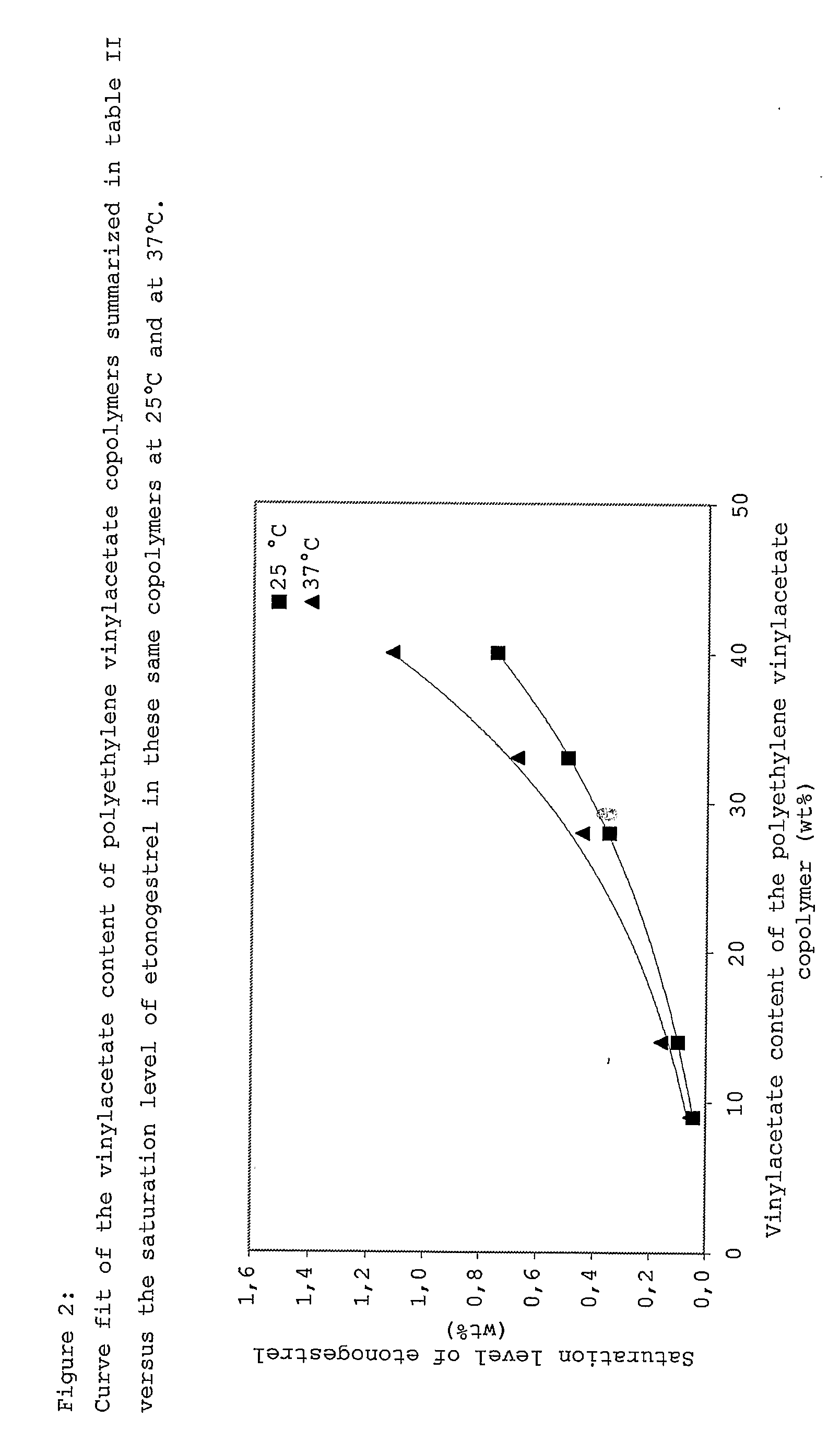
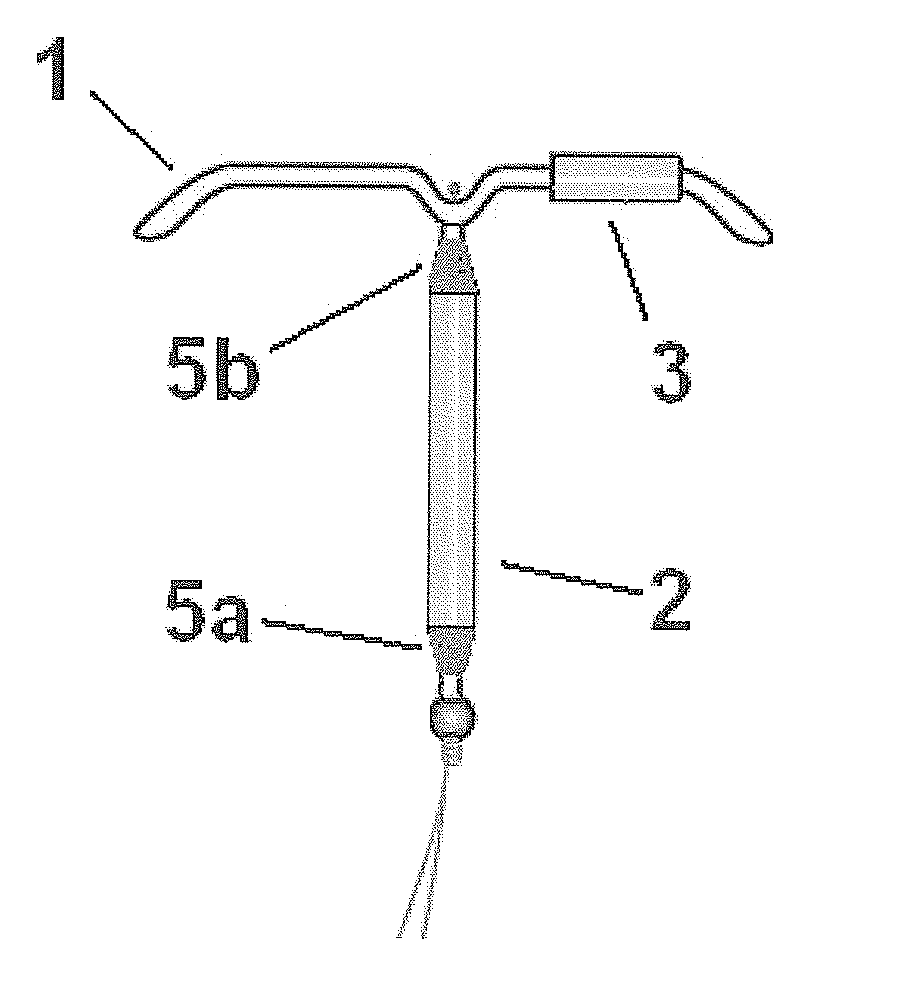
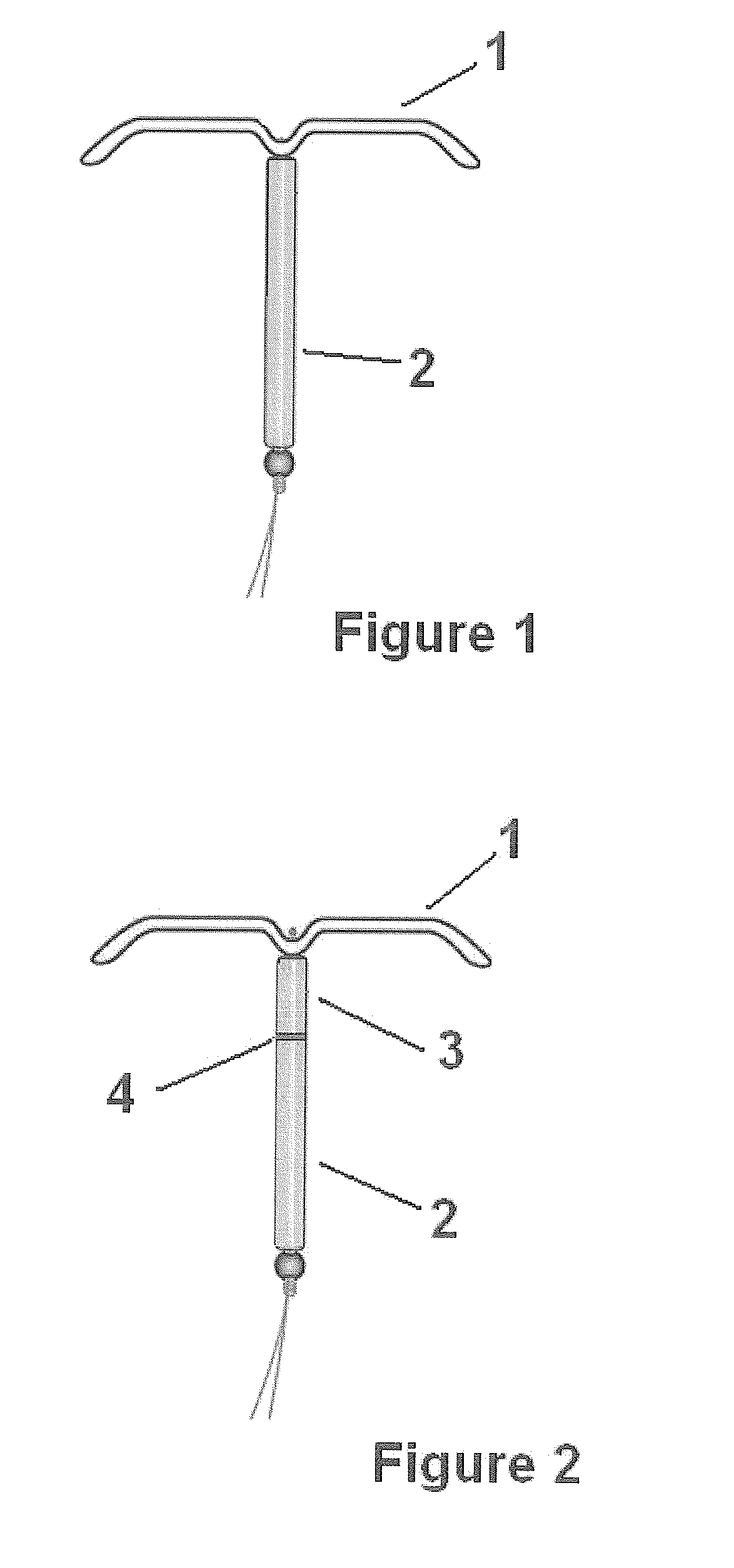
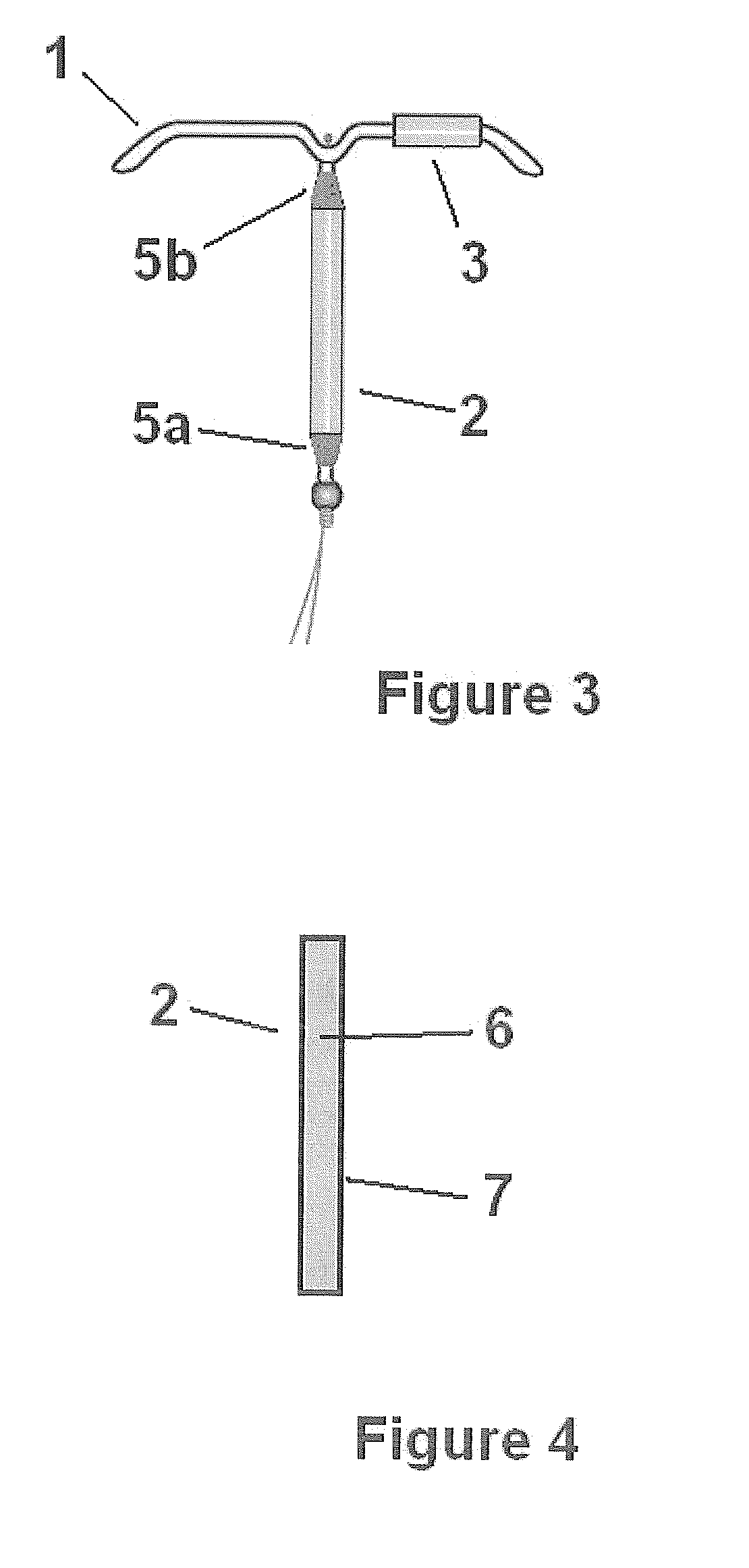
Drug delivery system based on polyethylene vinylacetate copolymers
ActiveUS20070141102A1Easy to prepareOrganic active ingredientsPowder deliveryPolyethylene vinyl acetateDisease
Disclosed herein is a drug delivery system consisting of one or more compartments and comprising a progestogenic compound dissolved in a thermoplastic polyethylene vinylacetate copolymer. Also disclosed are a contraceptive kit or hormone-replacement therapy kit comprising the drug delivery system, and a combination preparation that simultaneously provides contraception and treatment of a sexually transmitted disease comprising the drug delivery system.
Owner:MERCK SHARP & DOHME BV
Intrauterine delivery system for contraception
InactiveUS20110146693A1High success rateStable amenorrheaOrganic active ingredientsBiocideControl releaseEndometrium
The invention relates to a method for contraception and for reducing menstrual problems and inducing amenorrhea, wherein an intrauterine delivery device is used for the controlled release of a combination of progestogen or a drug having a progestogenic activity and at least one therapeutically active substance capable of preventing or suppressing abnormal and / or irregular endometrial bleeding over a prolonged period of time.
Owner:BAYER OY
Combination preparation for contraception based on natural estrogens
InactiveUS6884793B2Improves cycle bleeding behaviorMinimizes and prevents side effectOrganic active ingredientsBiocidePhysiologyPlacebo
The combination preparation for contraception includes from 2 to 4 first stage daily dosage portions each including an effective amount of at least one natural estrogen as sole active ingredient, from 16 to 22 second stage daily dosage portions each including an effective amount of a combination of at least one natural estrogen and at least one natural or synthetic gestogen as active ingredient; from 2 to 4 third stage daily dosage portions each including an effective amount of at least one natural estrogen as sole active ingredient; and from 2 to 4 final stage daily dosage portions containing a pharmaceutically acceptable placebo. The estrogen may be estradiol, an estradiol compound that is metabolized to estradiol when taken into the body, a conjugated equine estrogen or a phytoestrogen. The natural or synthetic gestogen can be natural progesterone or a synthetic gestogens, such as medroxyprogesterone acetate.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
In-vitro cultivating matural process of immatural ovocyte in ovarium organized block
The extracorporeal culture and mature method of immature oocyte from ovary tissue block includes selecting marrow mesenchyme stem cell MSC of female mouse as the feeder cell, compounding MSC culture liquid and agar and covering the culture rack, adding HCG in 3 IU / ml, and adhering the ovary tissue block on the rack to culture. In the co-culture system, estradiol, progestin and luteotopin contents are increased obviously, and after culturing, there is mature oocyte discharged. The method is significant in surviving rear endangered species of animals, breeding fine variety of animal, cell biological research of embryo stem cell, etc.
Owner:ANHUI PROVINCIAL HOSPITAL
Compositions and methods for non-toxic delivery of antiprogestins
InactiveUS20130018027A1Avoid liver toxicityPotent antiprogestational activityOrganic active ingredientsAntipyreticHormone dependenceTreatment field
The subject matter of the instant invention is pertinent to the field of treatment of hormone-dependent conditions. New compounds and methods for treating these conditions are disclosed. Embodiments of the instant invention disclose methods for treating endometriosis, dysmenorrhea, breast cancer, uterine fibroids and endometrial hyperproliferation.
Owner:APTALIS PHARMA
Intravaginal drug delivery device
InactiveUS20110236462A1Antibacterial agentsOrganic active ingredientsIntravaginal administrationActive ingredient
Described herein is an intravaginal drug delivery system. In an embodiment the intravaginal drug delivery system includes a progestin and estrogen compound, and releases the active ingredients in a fixed physiological ratio over a prolonged period of time to produce a contraceptive state in a female.
Owner:EVESTRA
Enzyme immunoassay method for progestational hormone in excrement of sows and method for detecting oestrous cycle of sow
InactiveCN103175960ANo harmSimple equipment requirementsColor/spectral properties measurementsBiological testingEstriolFeces
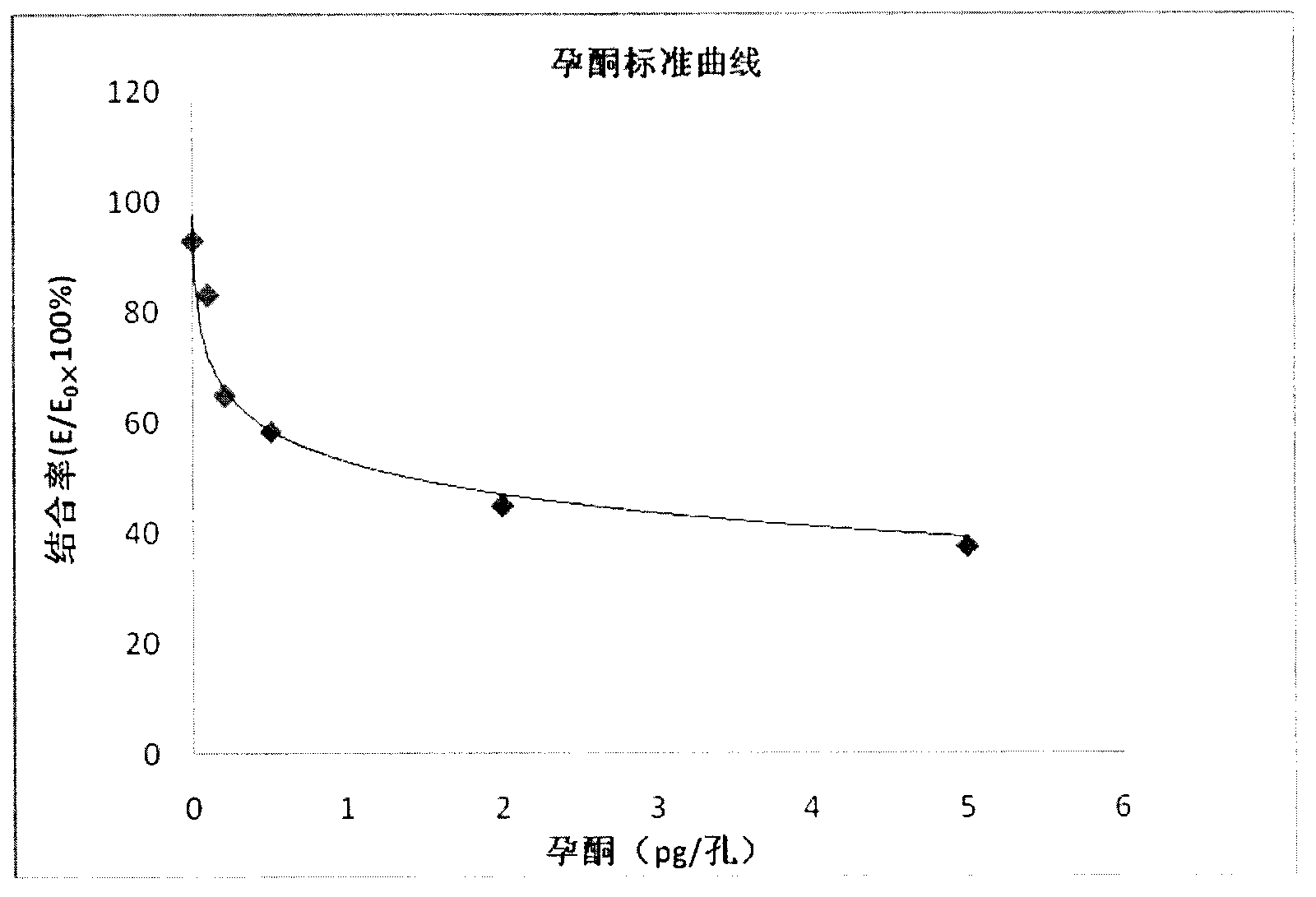
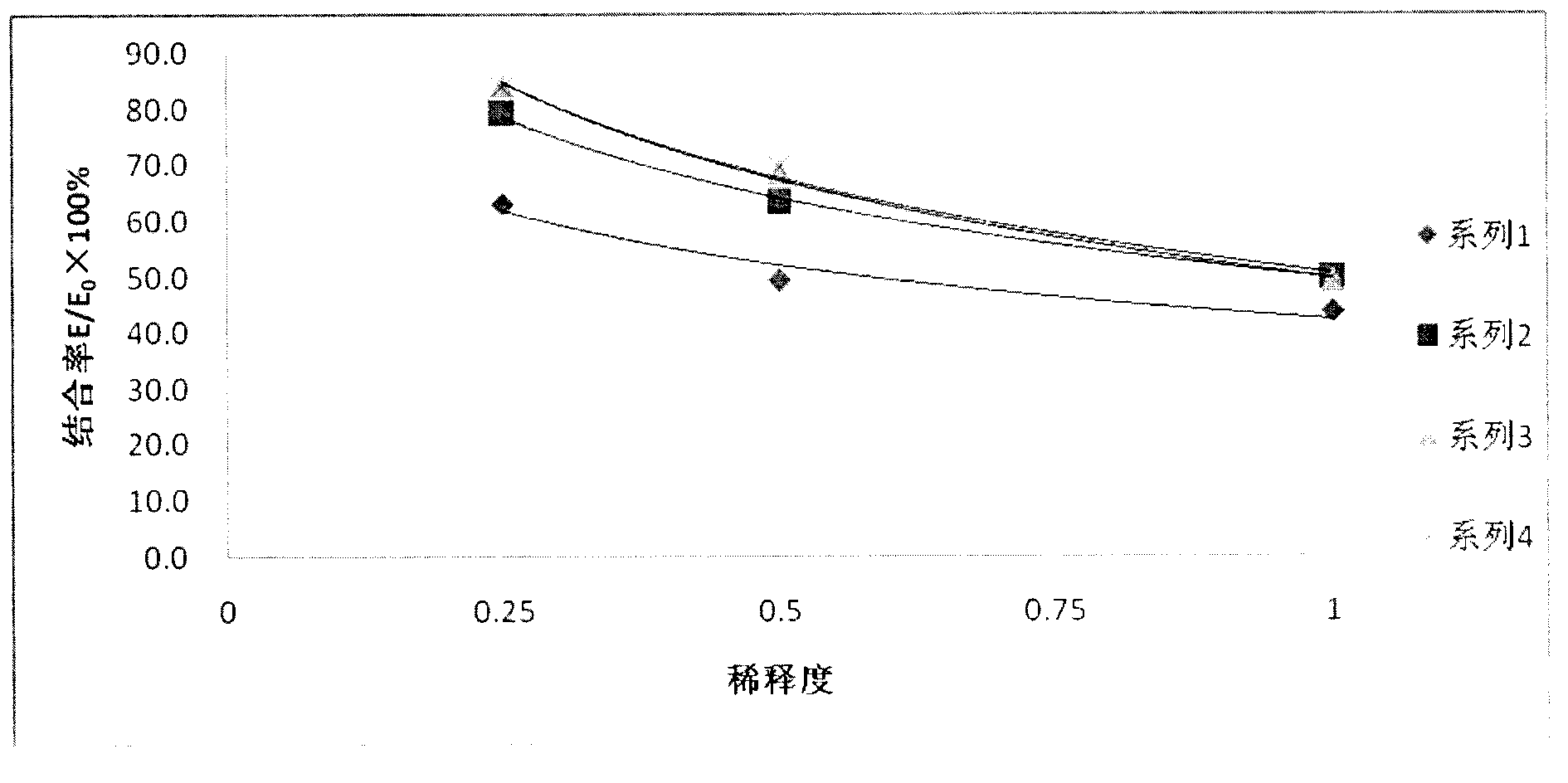
The invention discloses an enzyme immunoassay method for progestational hormone in excrement of sows. The method comprises the steps of: (1) sample collection: collecting the excrement of the sows, weighing and dissolving 0.5 g of excrement of the sows into 20 ml of measuring buffer liquid, carrying out centrifuging, taking and placing supernatant at 4 DEG C to be detected; and (2) measuring content of progestational hormone in a sample by utilizing competitive ELISA (enzyme-linked immuno sorbent assay): measuring according to an ELISA process. According to the enzyme immunoassay method for the progestational hormone in the excrement of the sows, the detection sensitivity of progesterone reaches 0.2 ng / ml or 8 ng / g; the recovery rate of the progesterone is 95.78%; inter-assay and intra-assay variable coefficients are respectively 6.8% (n is equal to 9) and 11.23% (n is equal to 8); the specificity of the progesterone is that the cross reaction rates of the progesterone to estradiol, estriol and oestrone are all less than 0.01%, and the cross reaction rate of the progesterone to testosterone is 0.35%; the stability is good; the required sample is easy to collect, animals are not injured, a measured result is reliable, and a method for measuring blood of the animals can be replaced to monitor a series of reproductive activities of the animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com