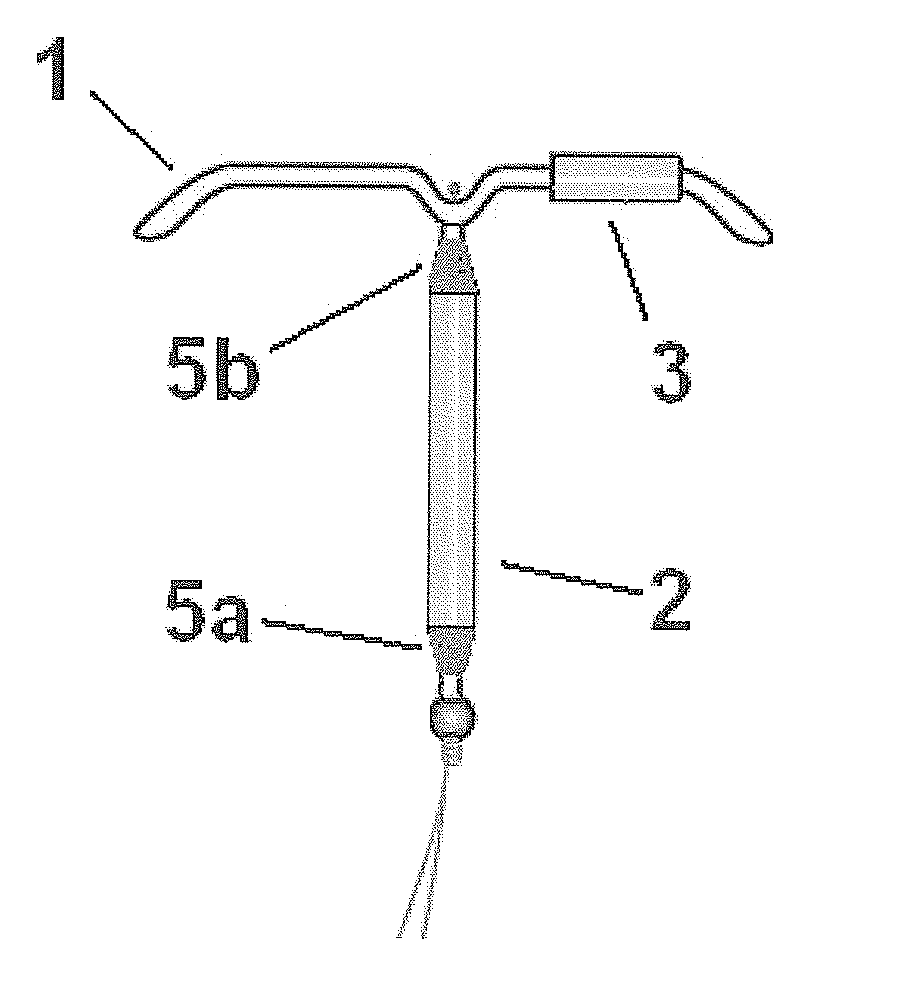
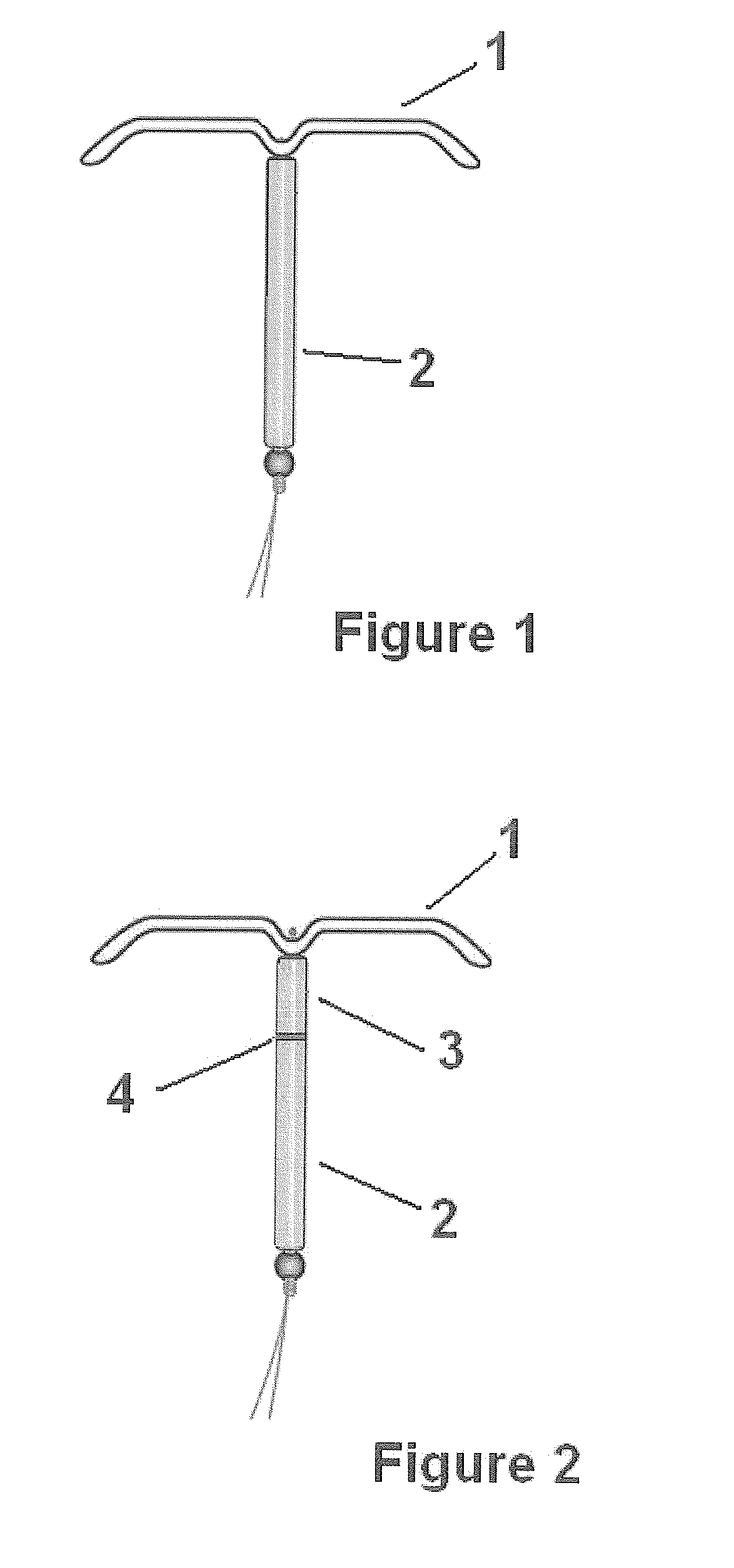
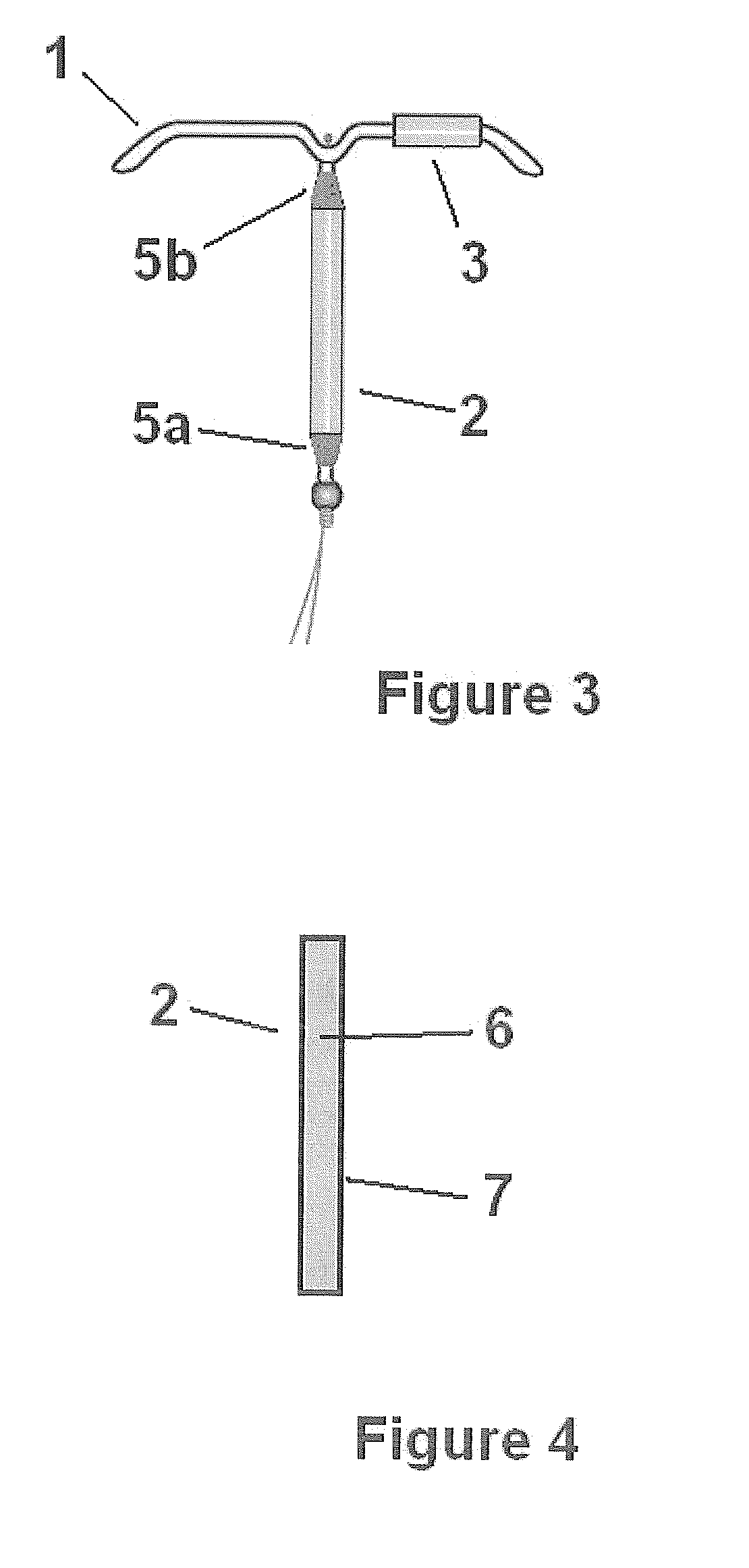
Intrauterine delivery system for contraception
a delivery system and intrauterine technology, applied in the field of intrauterine delivery system for contraception, can solve the problems of reducing the success rate of medical treatment, reluctance to prescribe the required high oral dosage, and general lack of evidence-based approaches, and achieves stable amenorrhea, low side effects or related complications, and high success rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089]Core Preparation
[0090]45 parts by weight of levonorgestrel, 10 parts by weight of tranexamic acid and 50 parts by weight of poly(dimethylsiloxane-co-vinylmethylsiloxane) and 1.2 parts by weight of dichlorobenzoylperoxide-polydimethylsiloxane paste (50% of dichlorobenzoylperoxide) were mixed with a 2-roll mill. The mixture was extruded to a tube-like form with a wall thickness of 0.8 mm and outer diameter of 2.8 mm and cured by heat at +150° C. for 15 minutes, during which crosslinking took place. The crosslinked core was cut into 24 mm length.
[0091]Preparation of the Delivery System
[0092]The core was swollen in cyclohexane and pulled over the IUS body. Cyclohexane was allowed to evaporate.
example 2
[0093]Core Preparation
[0094]50 parts by weight of levonorgestrel, 50 parts by weight of poly(dimethylsiloxane-co-vinylmethylsiloxane) and 1.2 parts by weight of dichlorobenzoylperoxide-polydimethylsiloxane paste (50% of dichlorobenzoylperoxide) were mixed with a 2-roll mill. The mixture was extruded to a tube-like form with a wall thickness of 0.8 mm and outer diameter of 2.8 mm and cured by heat at +150° C. for 15 minutes, during which crosslinking took place. The crosslinked core was cut into 15 mm length.
[0095]Second core was prepared in a similar manner by using 10 parts by weight of danazol in place of levonorgestrel. The crosslinked core was cut into 8 mm length.
[0096]Membrane Preparation
[0097]99 parts of silica-filled poly(dimethylsiloxane-co-vinylmethylsiloxane), 10 ppm Pt-catalyst (of the reaction species) and 0.03 parts of inhibitor (ethynyl cyclohexanol) and approximately 0.6 parts of poly(hydrogenmethylsiloxane-co-dimethylsiloxane) crosslinker were mixed in a 2-roll mill...
example 3
[0098]Core Preparation
[0099]54 parts of commercial poly(dimethylsiloxane-co-vinylmethylsiloxane), 45.5 parts by weight of levonorgestrel, 0.4 parts of poly(hydrogenmethylsiloxane-co-dimethylsiloxane) crosslinker, 0.02 parts of ethynyl cyclohexanol inhibitor and 10 ppm of Pt-catalyst (of the reaction species) in vinyl-methyl-siloxane were mixed in a kneating mill. The mixture was extruded to a tube-like form with a wall thickness of 0.7 mm and cured by heat at +115° C. for 30 minutes and cooled.
[0100]Second core was prepared in a similar manner by using 79.5 parts of commercial poly(dimethylsiloxane-co-vinylmethylsiloxane) and in place of levonorgestrel 20 parts by weight of mefenamic acid.
[0101]Membrane Preparation
[0102]9 parts of α,ω-divinylether terminated poly(ethylene oxide)-b-poly(dimethylsiloxane) multiblock copolymer (PEO-b-PDMS), 89 parts of silica-filled poly(dimethylsiloxane-co-vinylmethylsiloxane), 10 ppm Pt-catalyst (of the reaction species), 0.03 parts inhibitor (ethyny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com