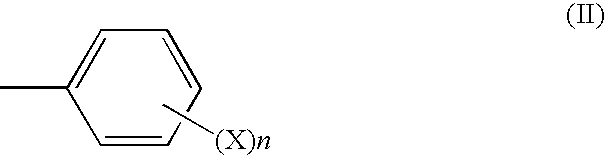Patents
Literature
384 results about "Tranexamic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
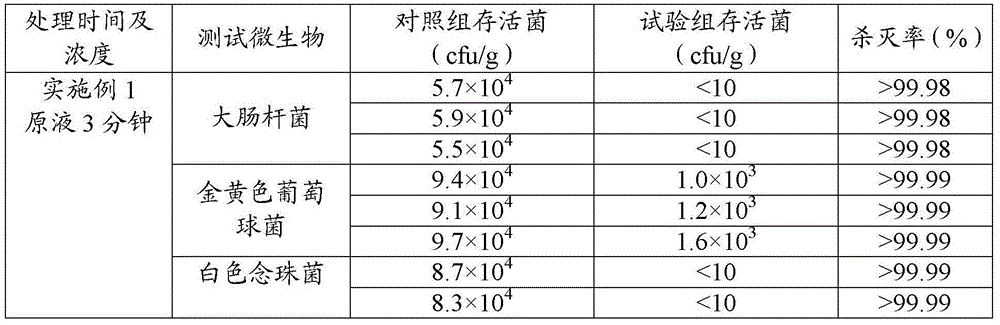
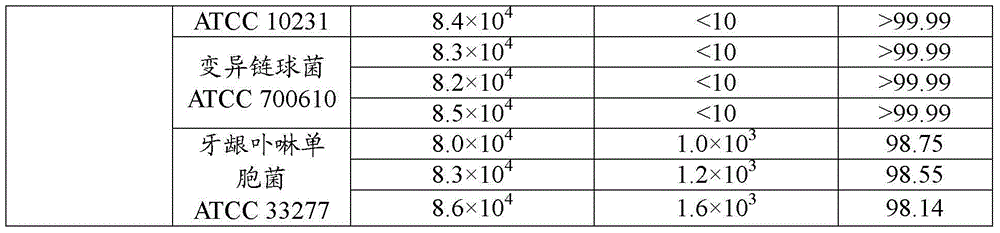
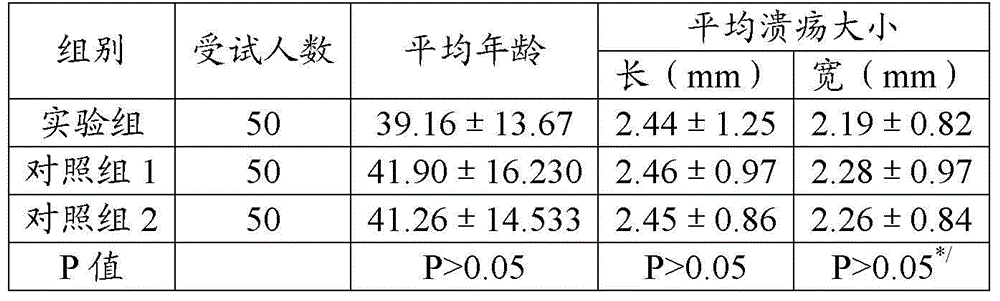
This medication is used short-term in people with a certain type of bleeding disorder (hemophilia) to prevent and reduce bleeding from having a tooth pulled (extraction). It is also used in people with other high-risk bleeding conditions to control bleeding at such times as after surgery or an injury, during heavy nosebleeds, or during heavy menstrual bleeding.
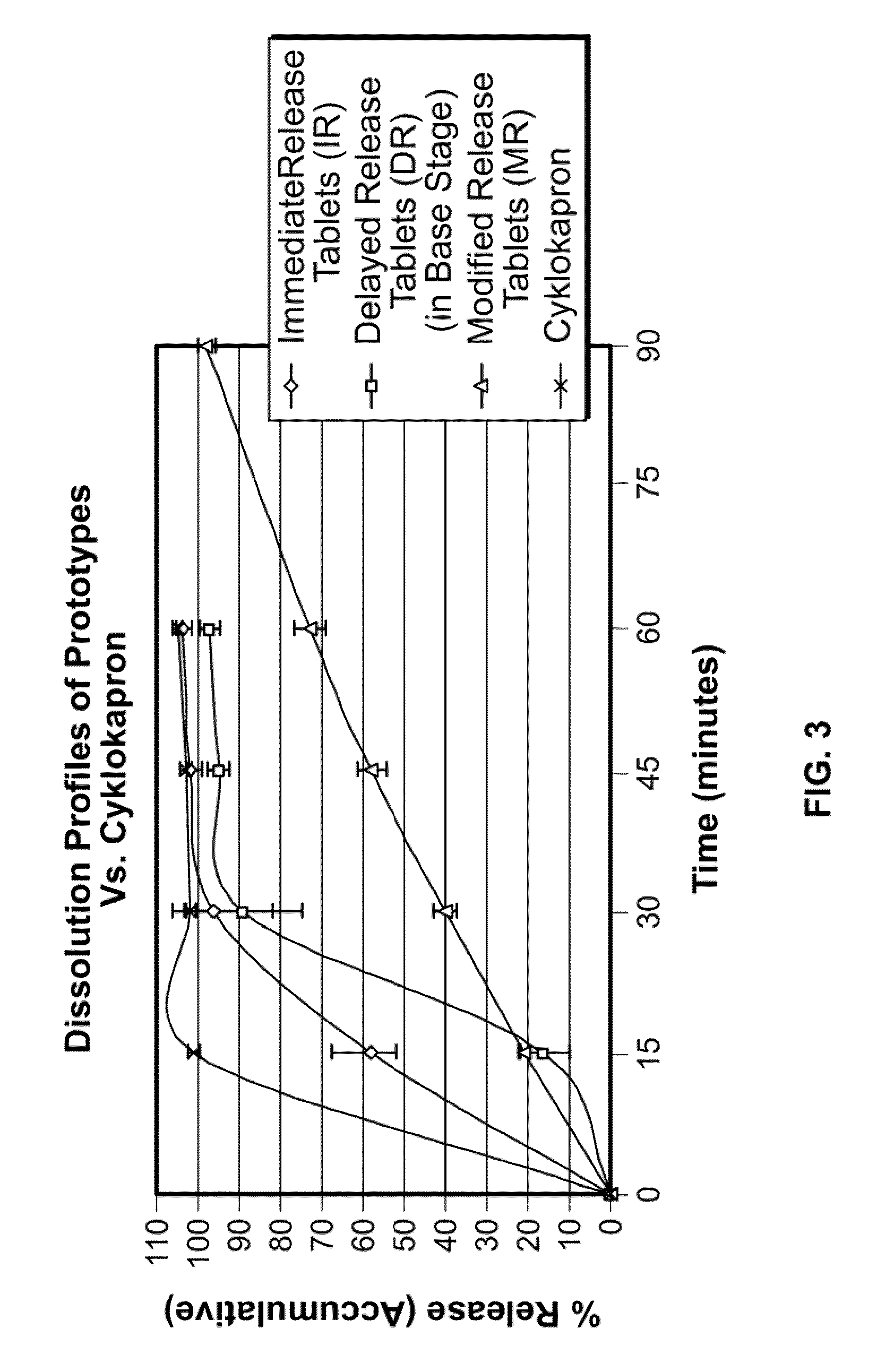
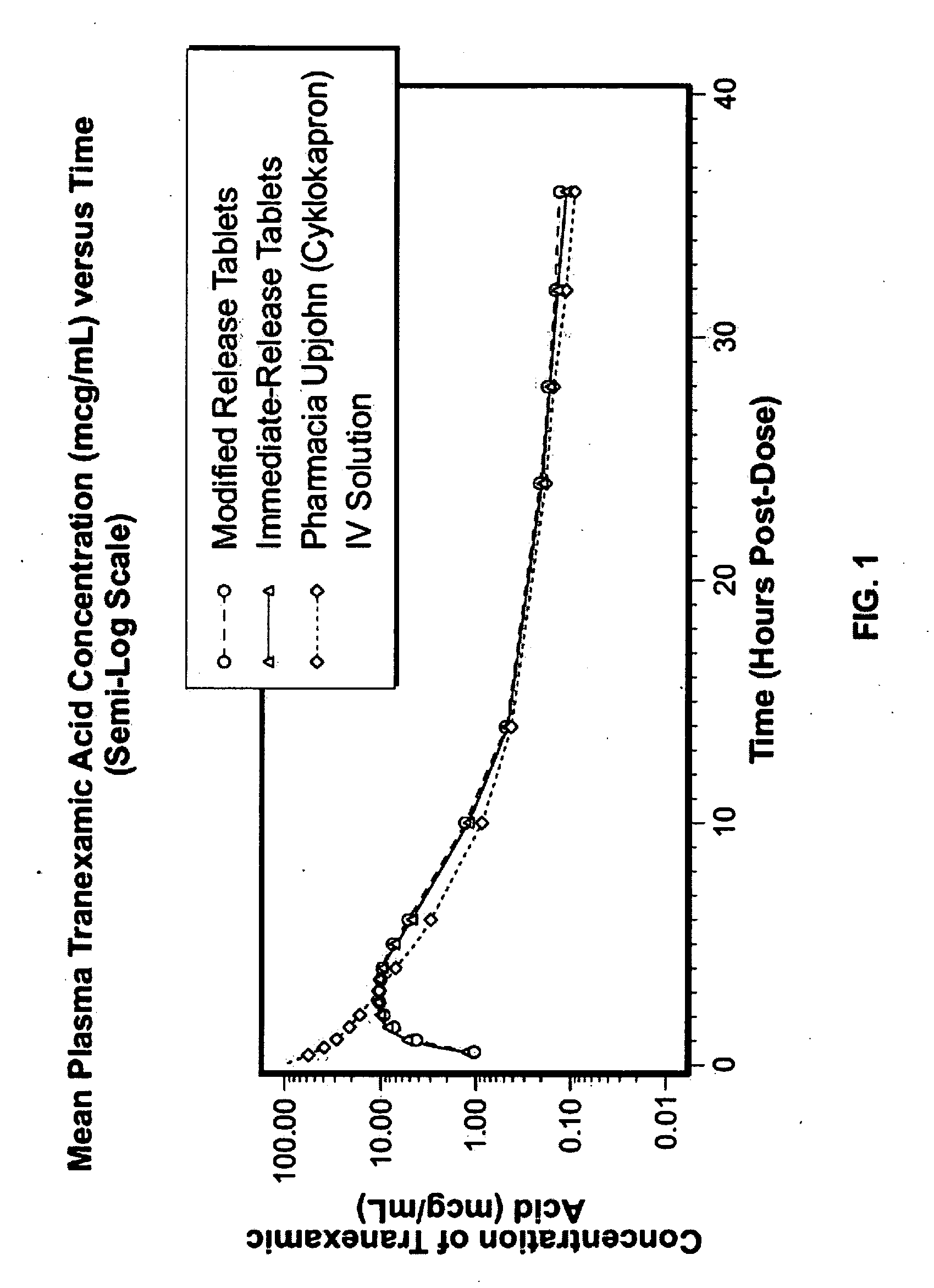
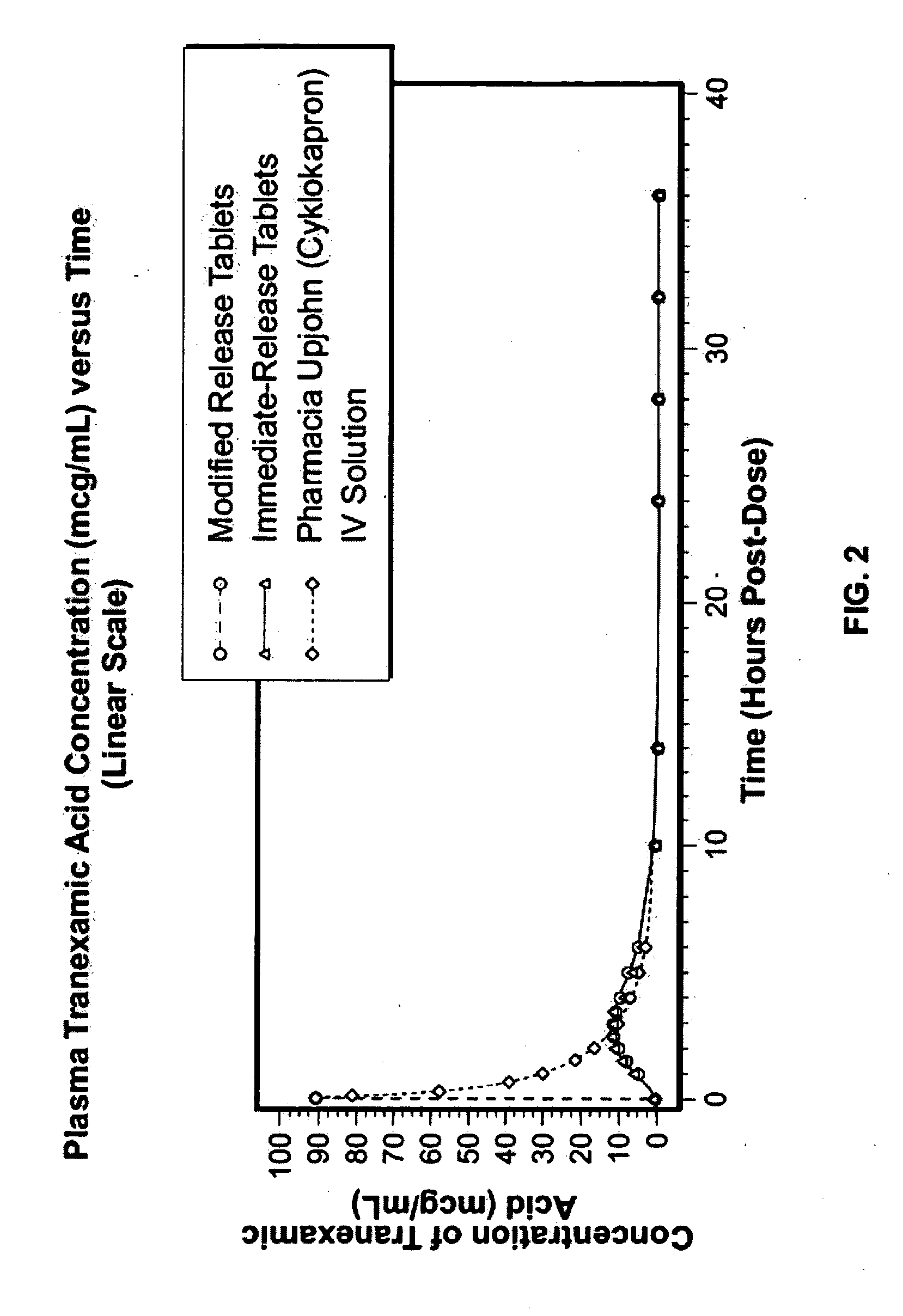
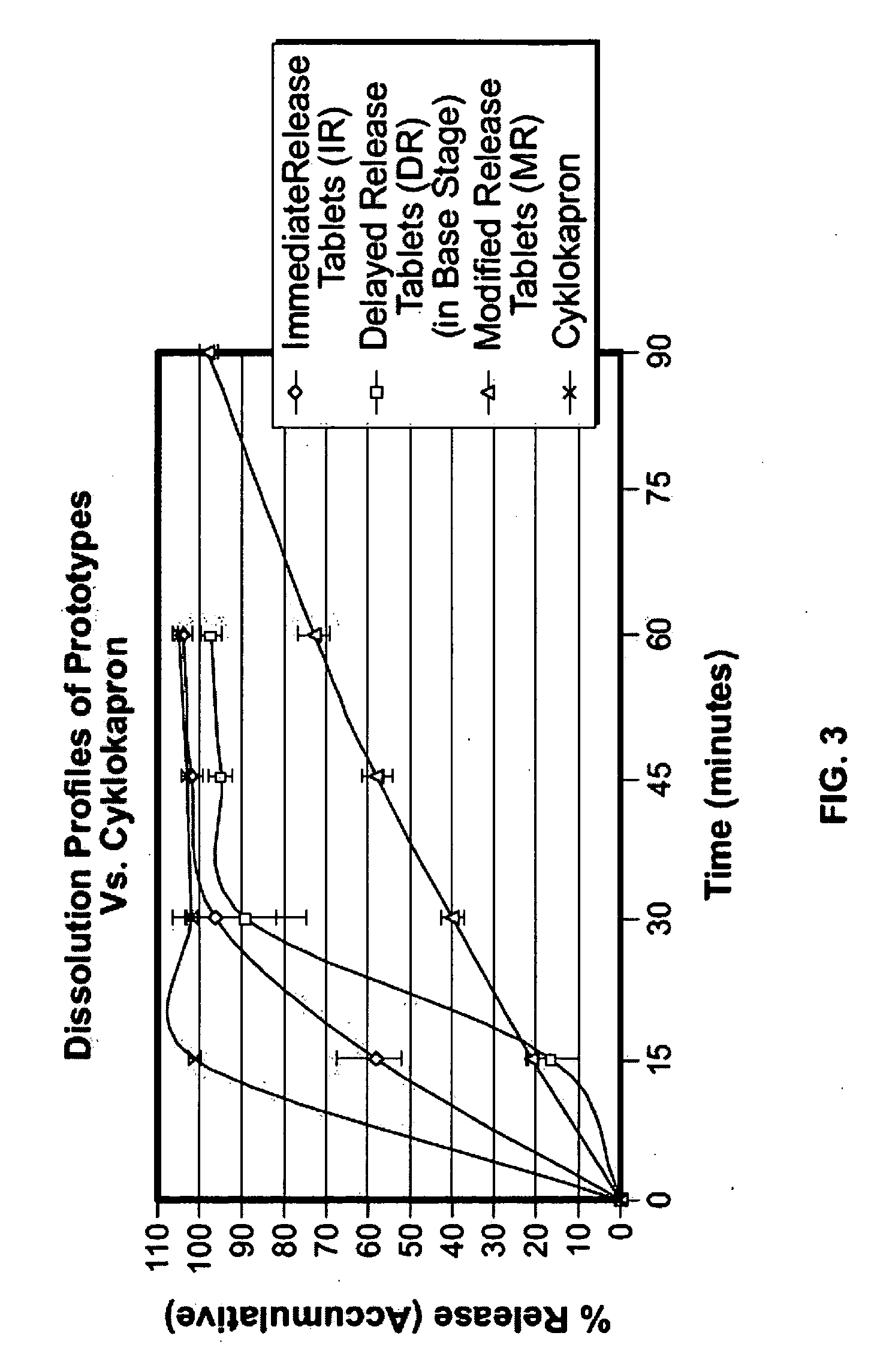
Tranexamic acid formulations with reduced adverse effects
InactiveUS20090017114A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePowder deliveryIntestinal structureSide effect
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:FERRING BV
Skin age multi-effect silk mask liquid
InactiveCN105640845AStrong anti agingEfficient hydrationCosmetic preparationsToilet preparationsFacial skinSodium hyaluronate
The invention discloses skin age multi-effect silk mask liquid which is prepared by mixing hydrosolvent, humectant, skin conditioner, preservative, thickener and ph regulator. The humectant comprises glycerin, propylene glycol, butylene glycol, maltooligosyl glucoside, hydrogenated starch hydrolysate, glyceryl polymethacrylate, PVMMA copolymer, sodium hyaluronate, betaine and baobab pulp extract, and the skin conditioner comprises nicotinamide, Herba portulacae extract, polyquaternium-51, soybean polypeptide, blackberry leaf extract, purple coneflower extract, Aloe vera extract, European horse chestnut extract, nonapeptide-1, tranexamic acid, Herba centella extract, ceramide 1, palmitoyl pentapeptide-4, oligopeptide-1, carnosine and dipotassium glycyrrhizinate. The mask liquid prepared by applying the component ratio can effectively realize efficacy of moisturizing and whitening facial skin, is free of irritation to the facial skin and has good whitening effect.
Owner:韩玉逍
Skin lightening composition
InactiveUS20060142382A1Good effectGood whitening effectBiocideCosmetic preparationsEngineeringTranexamic acid
A composition which comprises (i) tranexamic acid or a salt thereof, (ii) L-cysteine, a derivative thereof or a salt thereof and, as occasion demands, (iii) L-ascorbic acid, a derivative thereof or a salt thereof.
Owner:DAIICHI PHARMA CO LTD
Tranexamic acid formulations with reduced adverse effects
InactiveUS20050025825A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocideOrganic active ingredientsIntestinal structureNausea sickness
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:XANODYNE PHARMACEUTICALS INC +1
Cosmetic and preparation method thereof
InactiveCN104107159ADelicate skinBrighten skin colourCosmetic preparationsToilet preparationsSkin colorHydroxyethyl cellulose
The invention discloses a cosmetic and a preparation method thereof. The cosmetic is prepared from five components of A, B, C, D and E. The cosmetic is composed of thirty raw materials including deionized water, hydroxyethyl cellulose, allantoin, carbopol U20, propanediol, 1,3-butanediol, sodium hyaluronate, an aloe vera extract, a natto extract, asiaticoside, vitamin C, bisabolol, an amino acid humectant, D-panthenol, VC ethyl ether, nicotinamide, glabridin, tranexamic acid, ceramide, algal polysaccharide, glucan, dipotassium glycyrrhizinate, an epidermal growth factor (EGF), triethanolamine, isothiazolinone, essence, squalane, jojoba oil, vitamin E oil and crystal sparkling pearl powder. The cosmetic has the functions of making skins fine and smooth, brightening the skins, repairing sensitive skins, controlling oil, toning skins and modifying skin blemishes, has no side effect or no dependence, and does not threaten health of a human body.
Owner:陈海
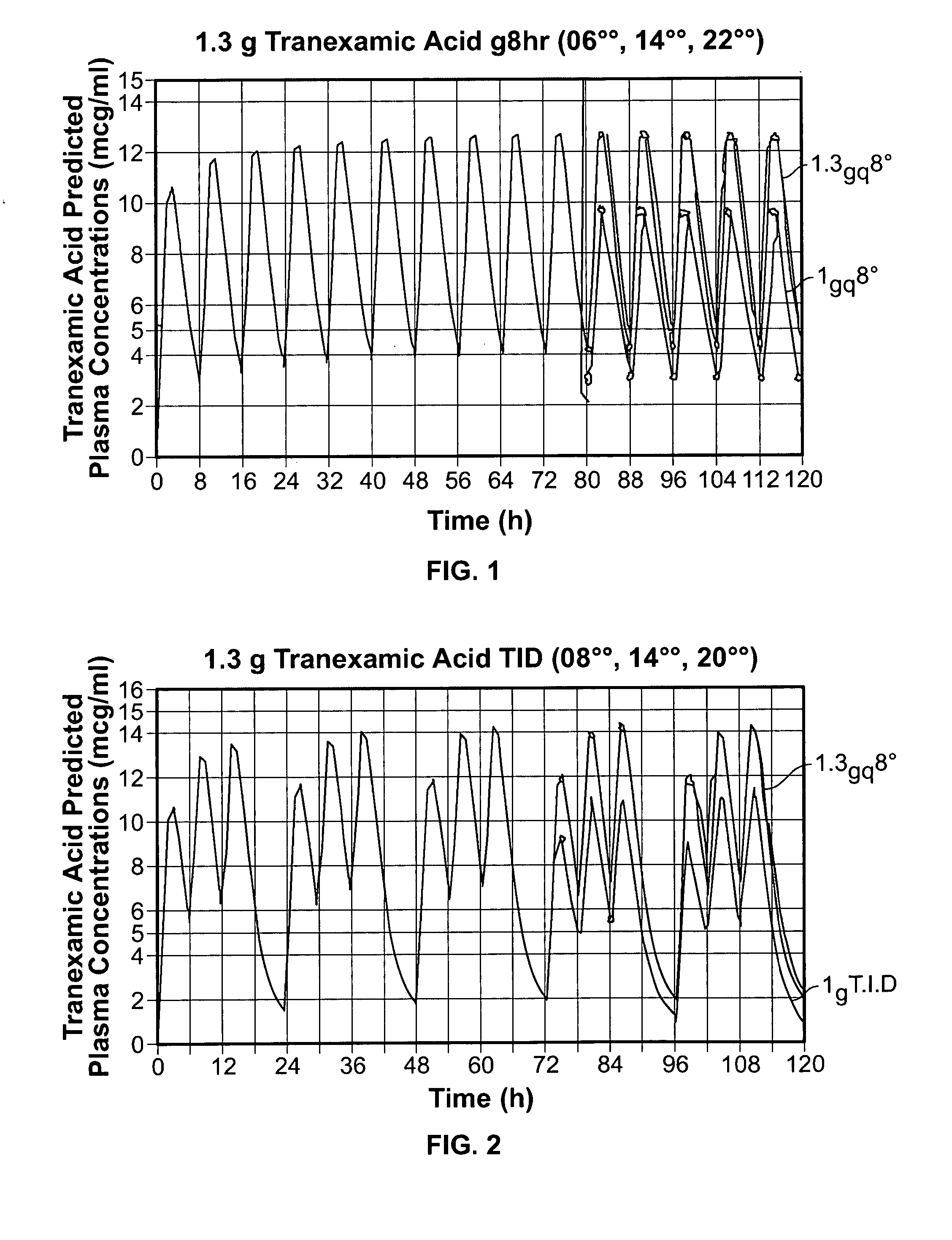
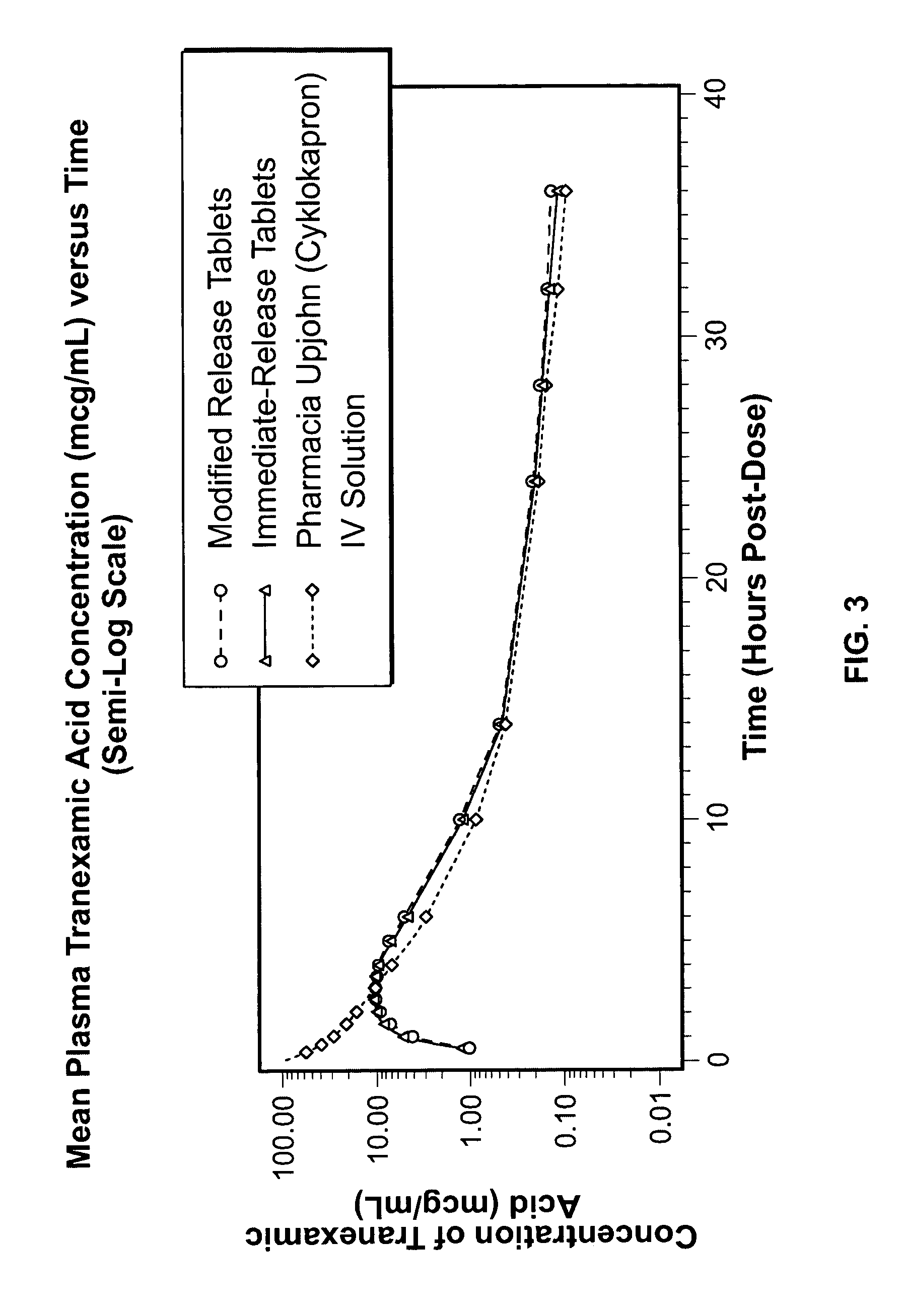
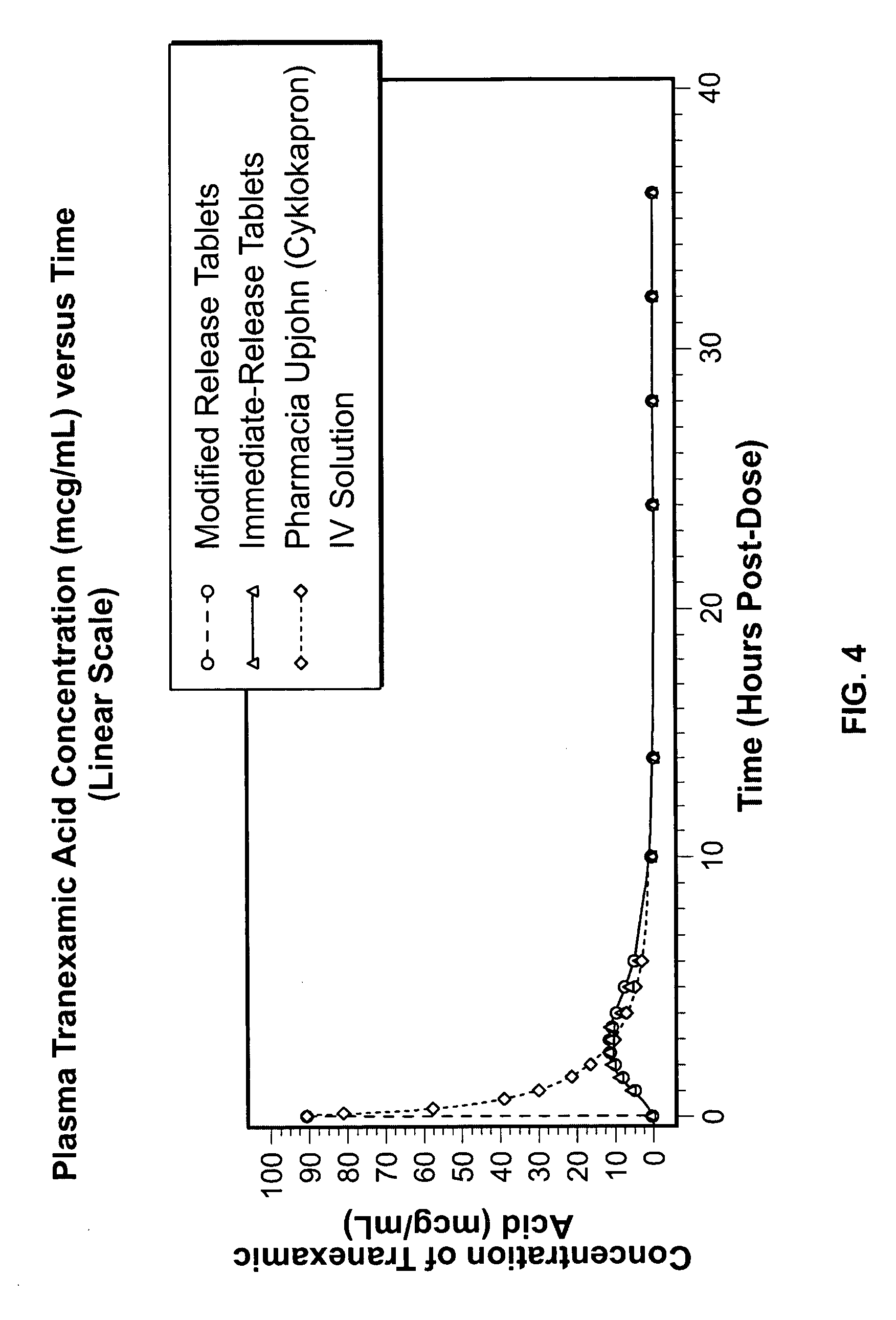
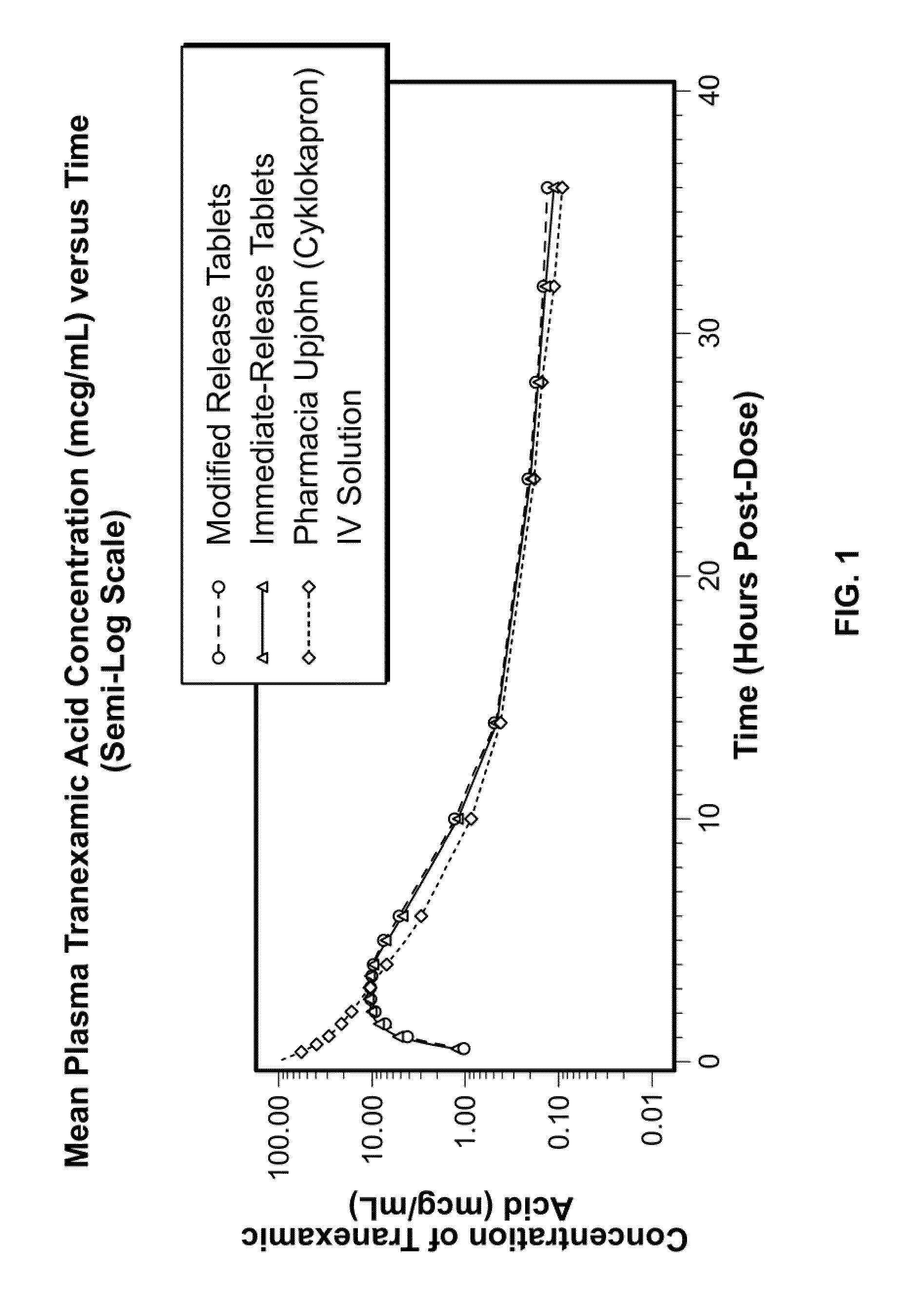
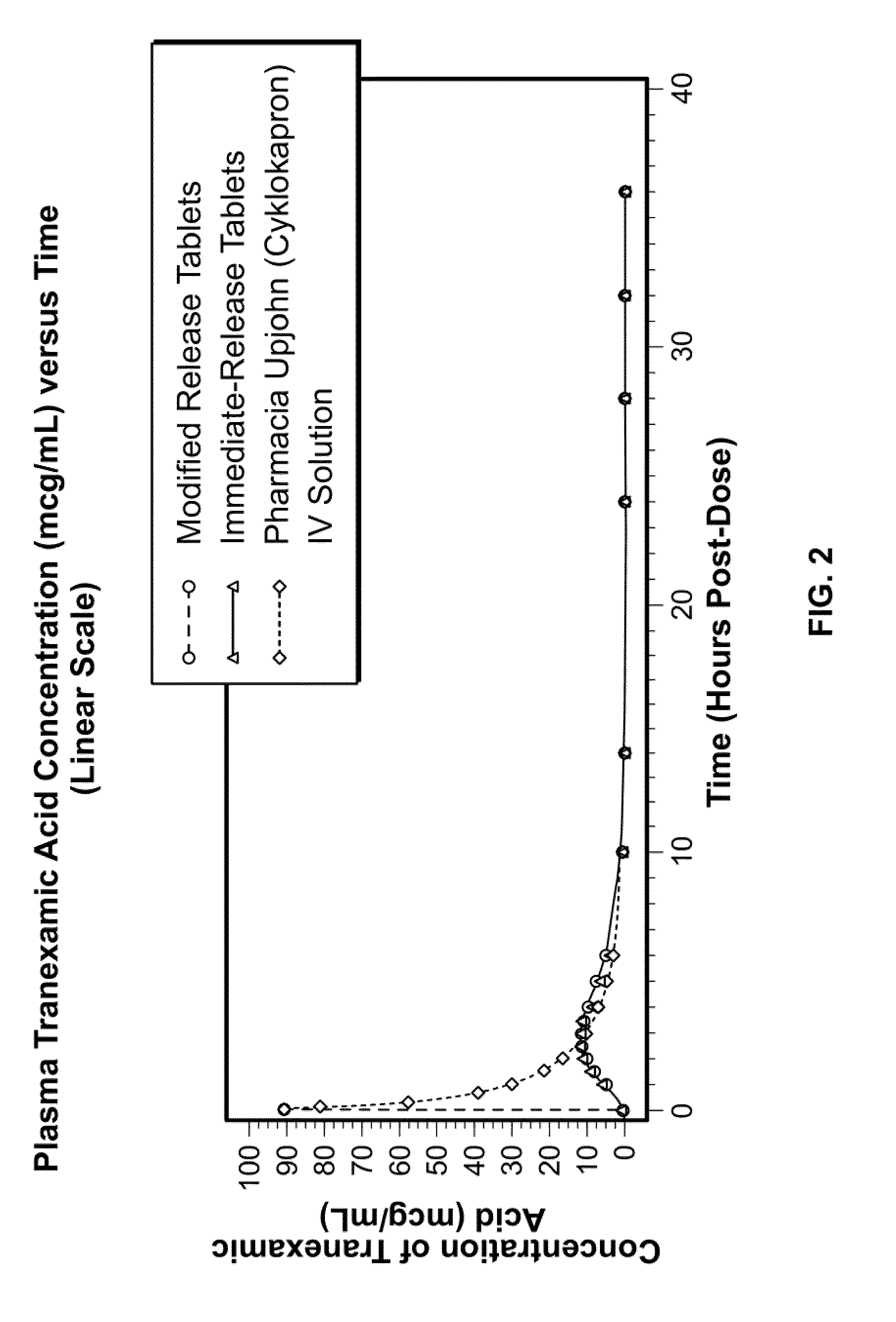
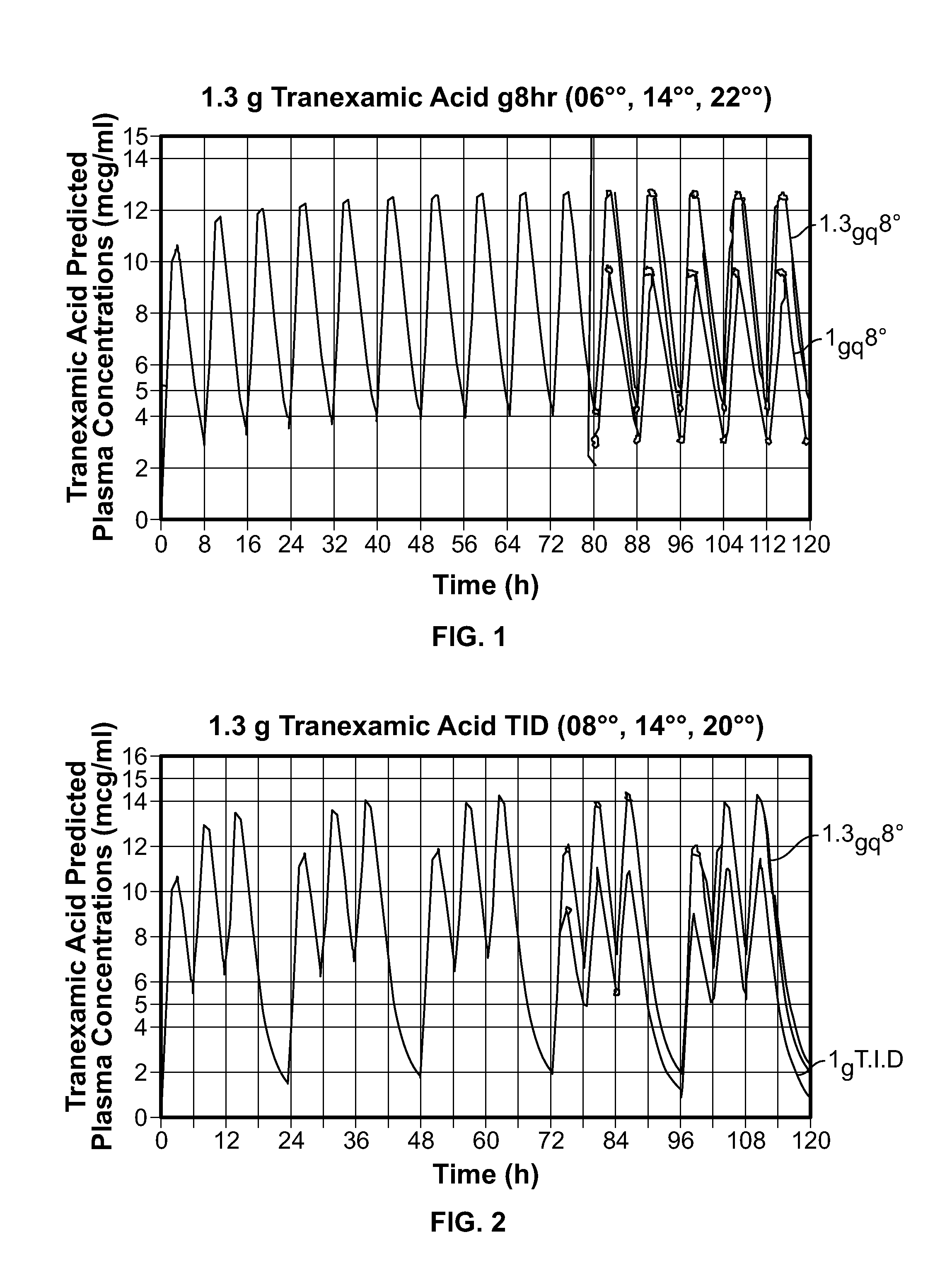
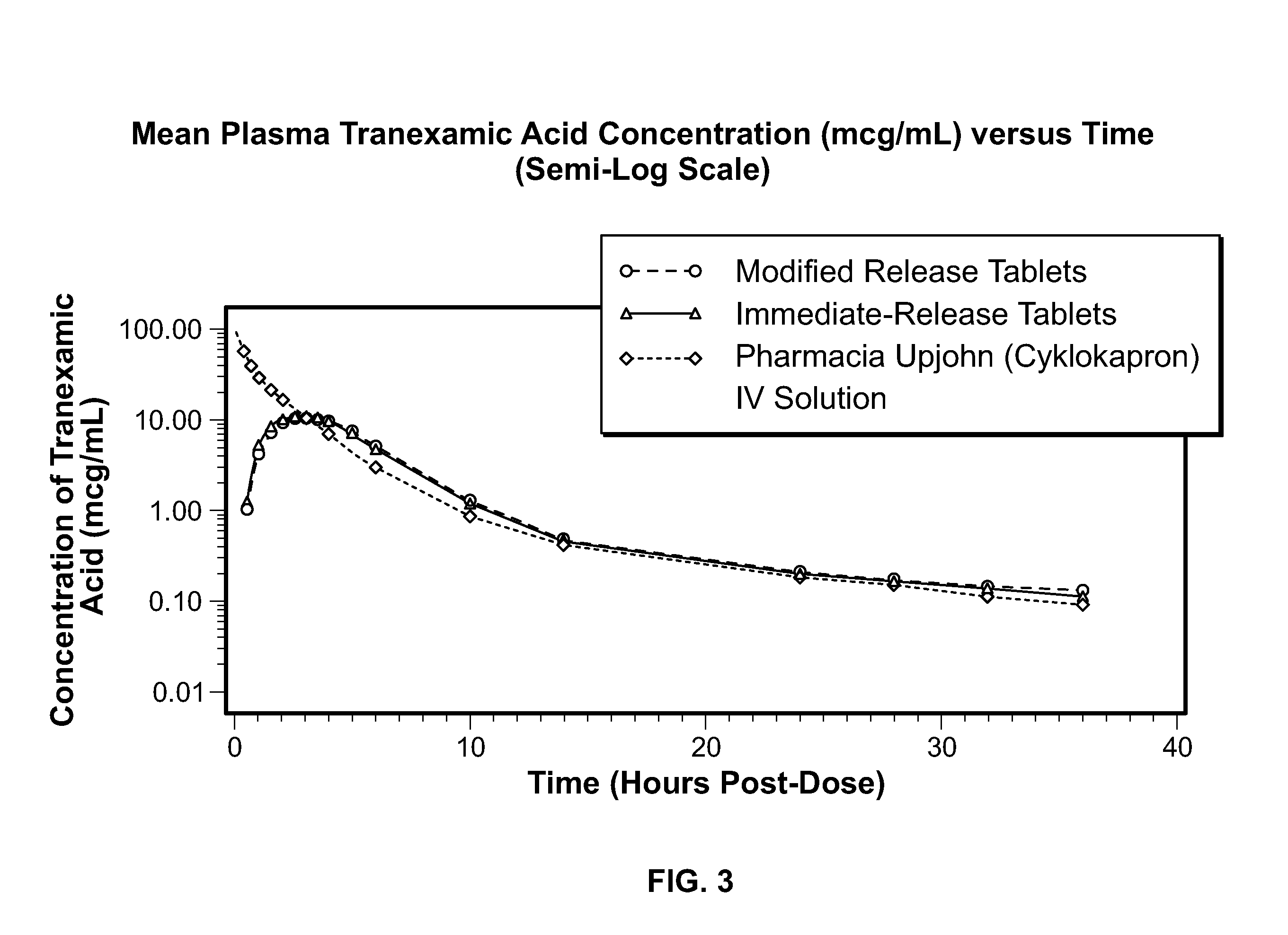
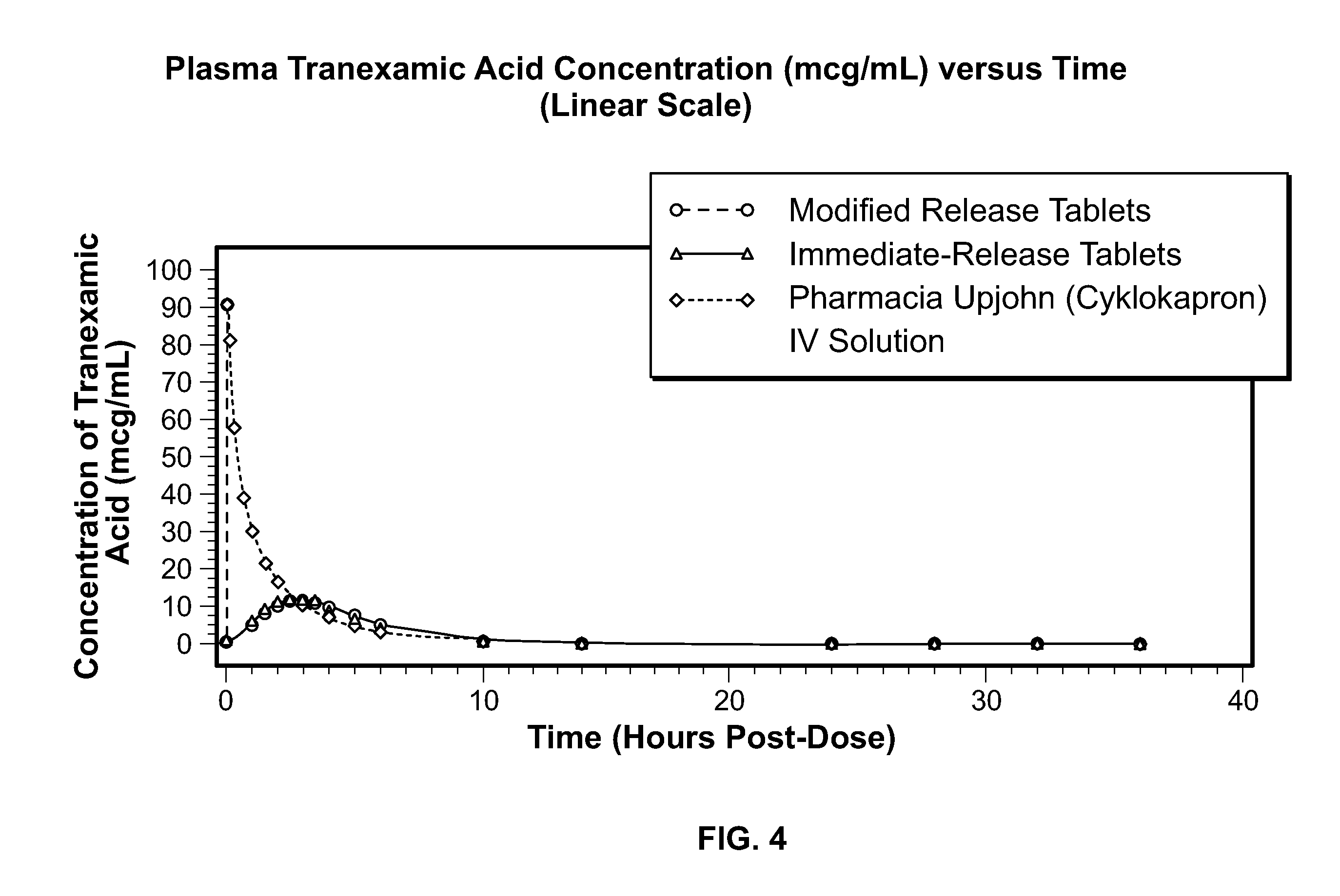
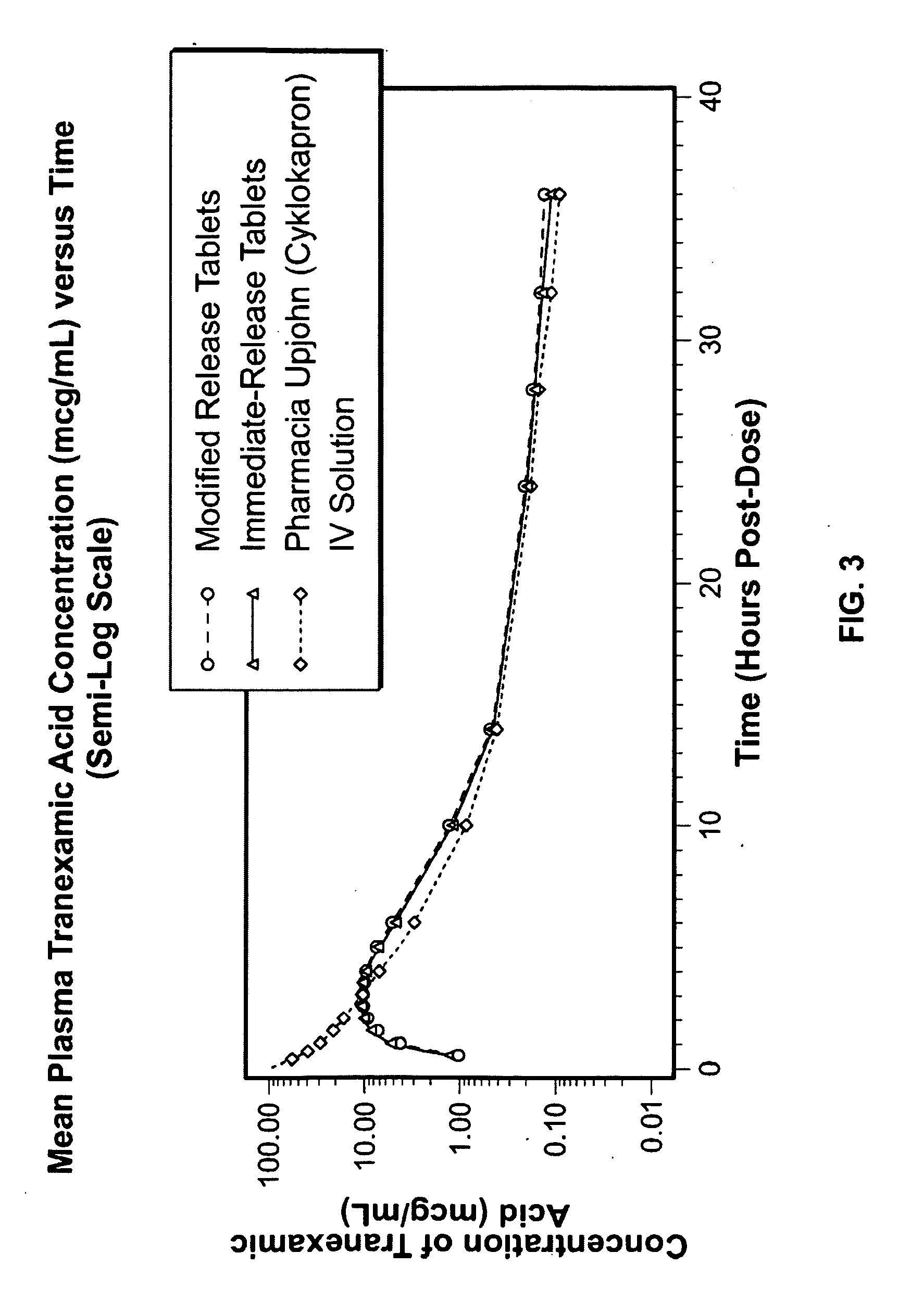
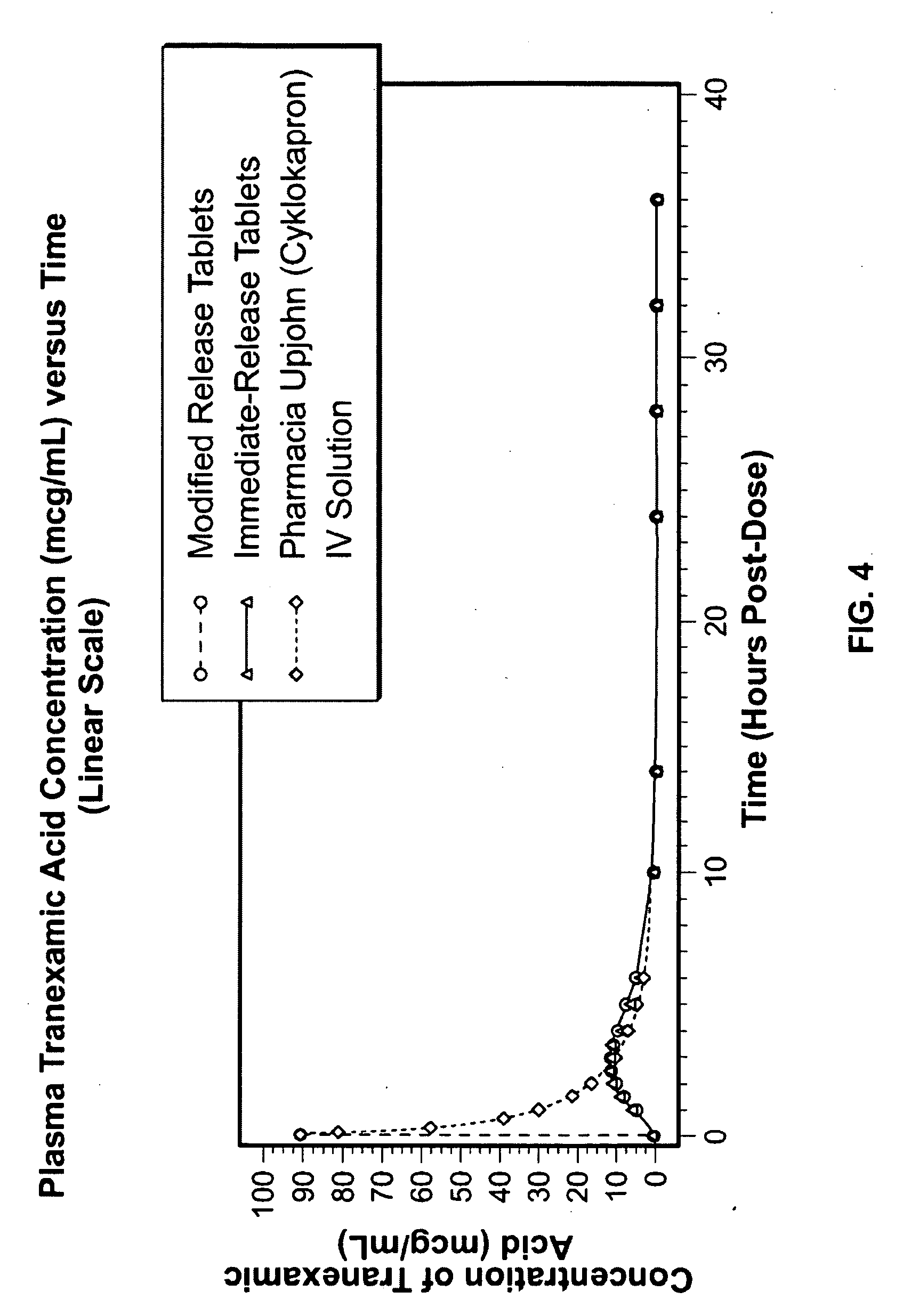
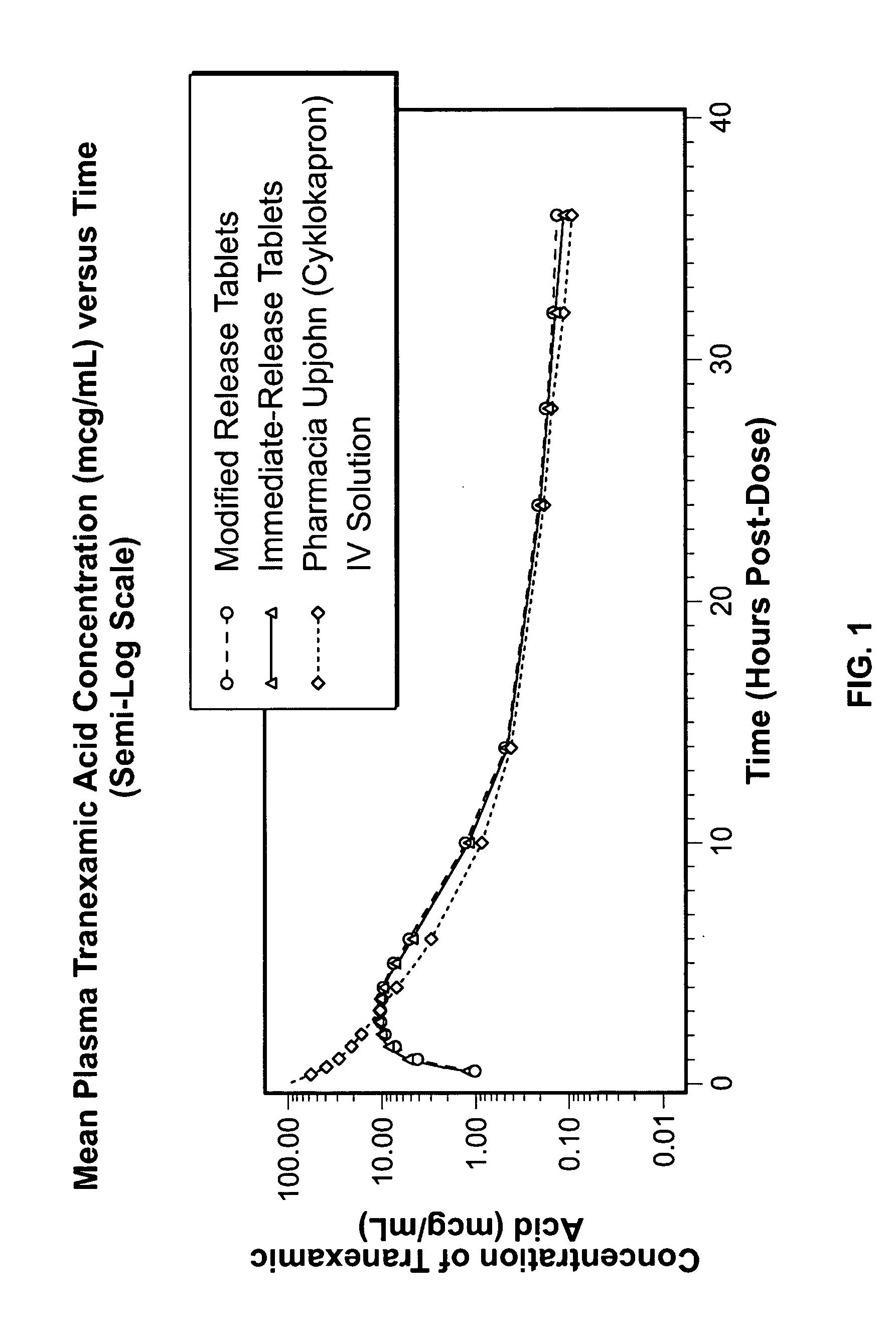
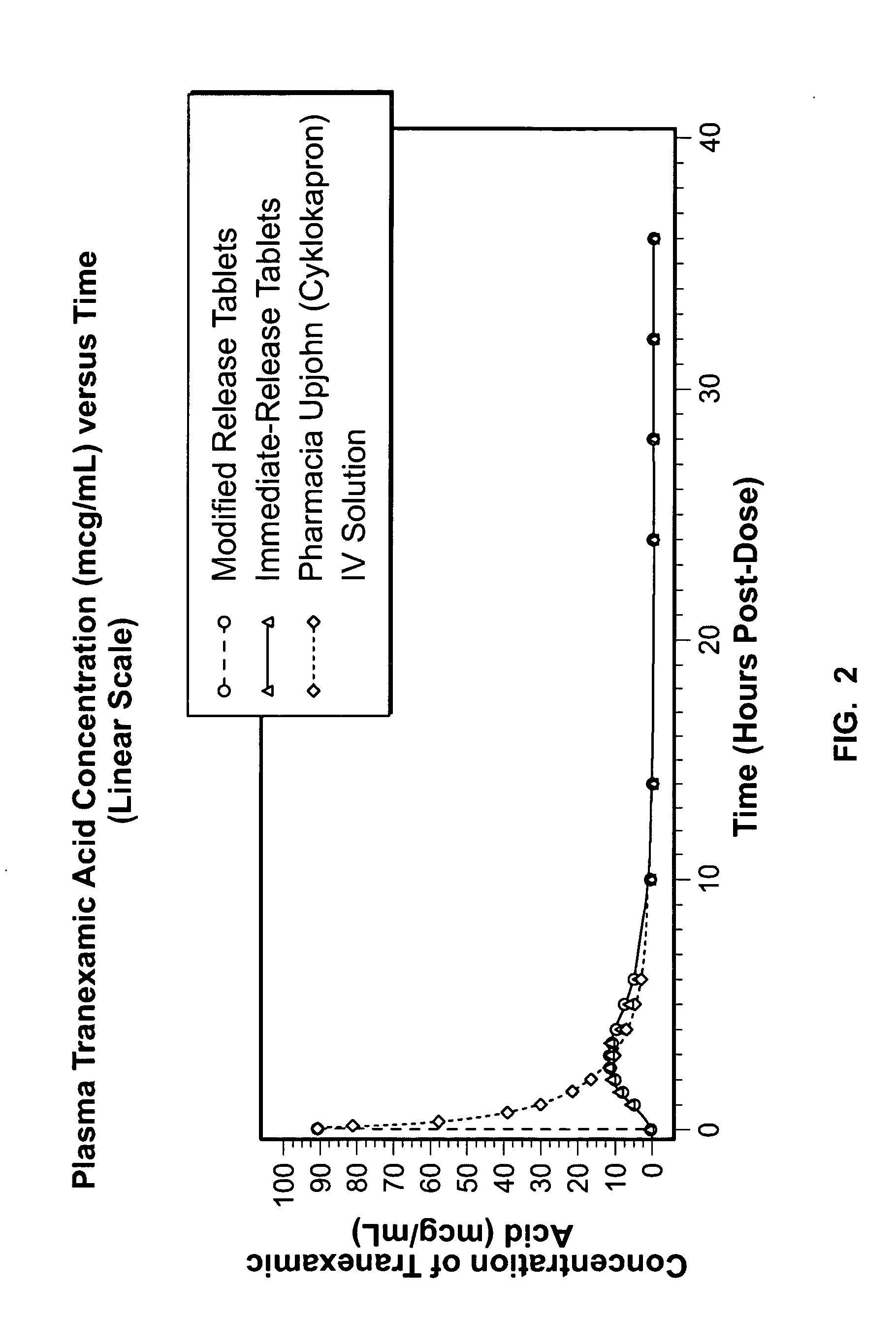
Tranexamic acid formulations
InactiveUS20050244495A1Minimize and eliminate undesirable gastrointestinal side effectReduce concentrationBiocidePill deliveryTranexamic acidPharmacology
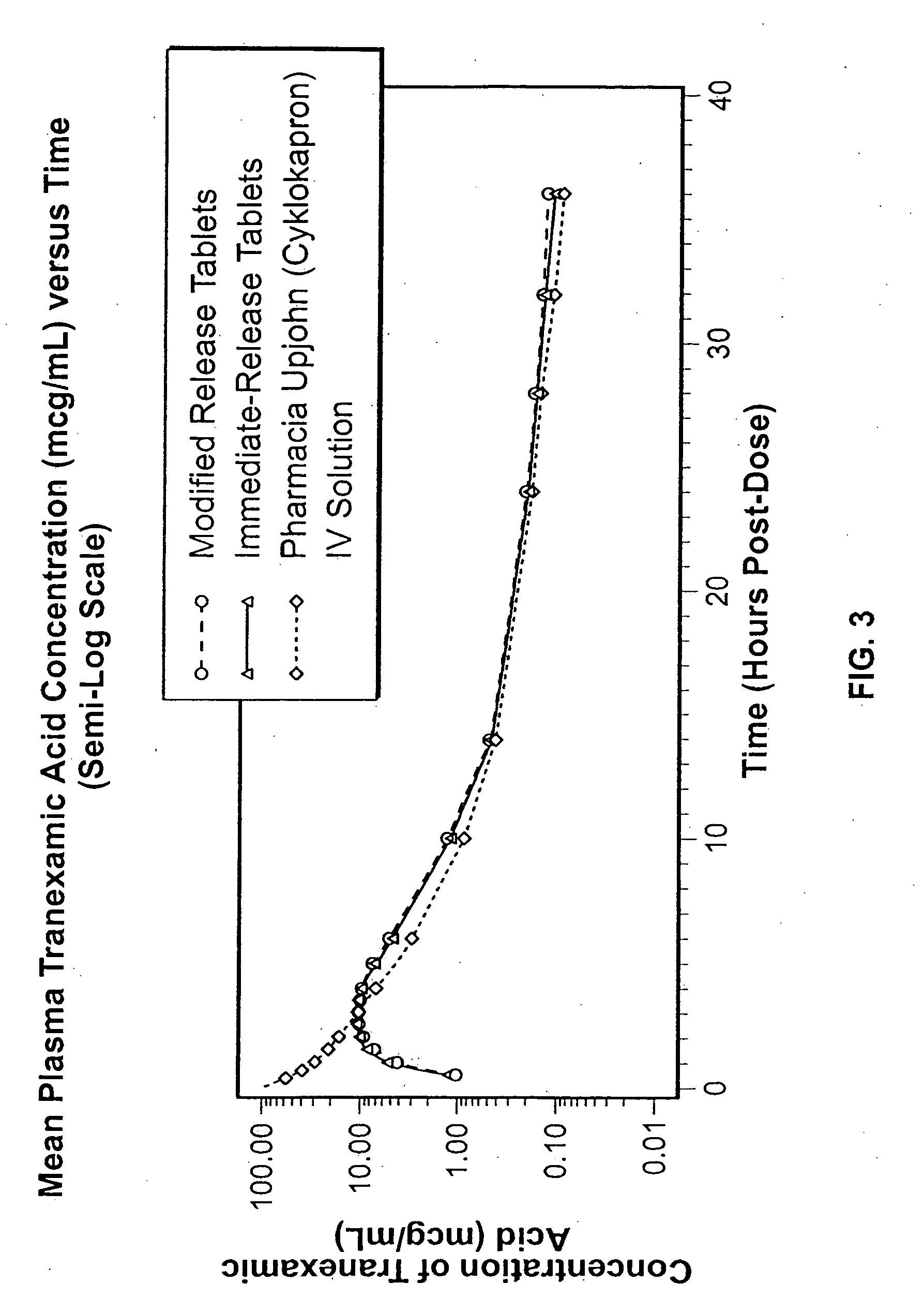
Disclosed are modified release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC +1
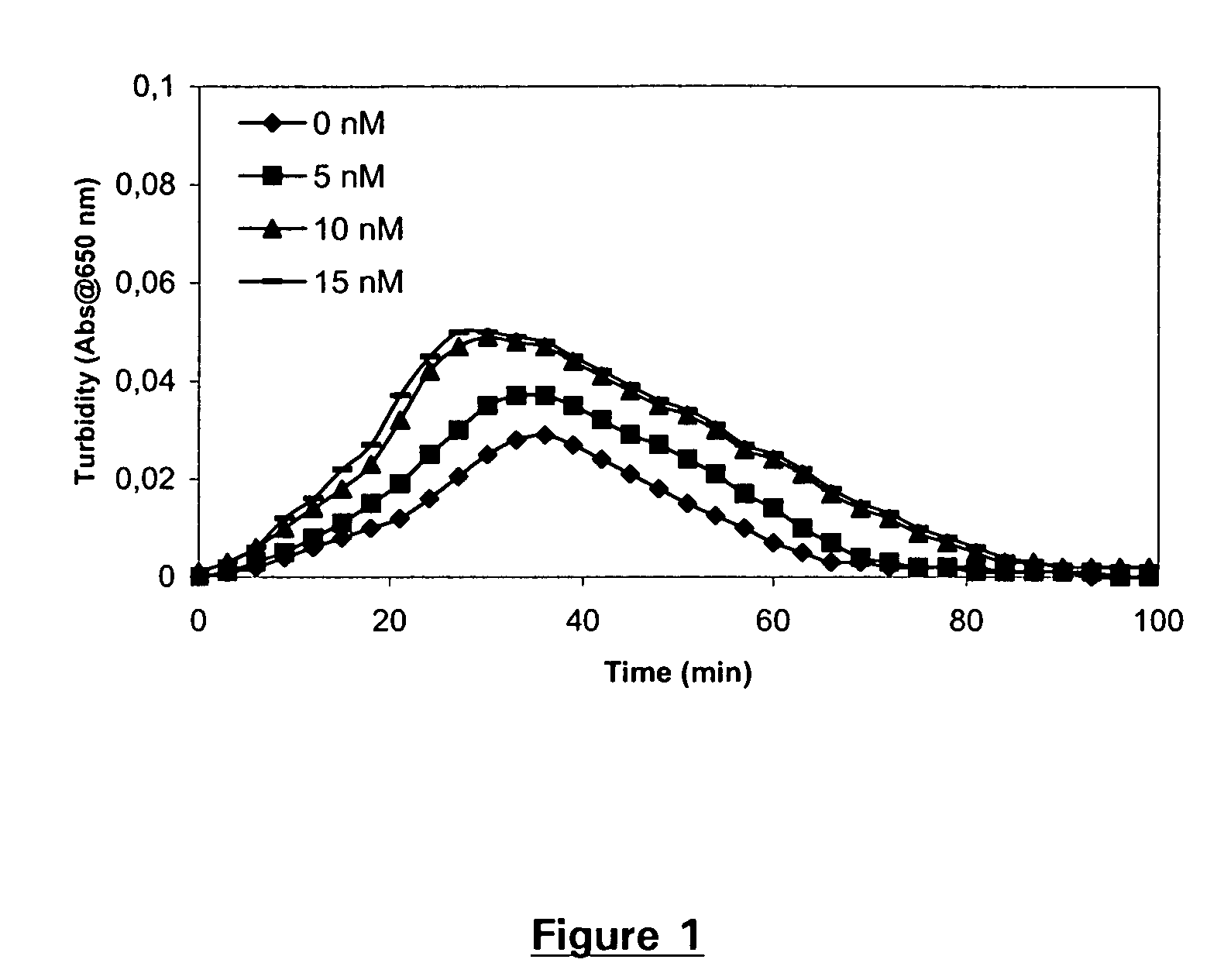
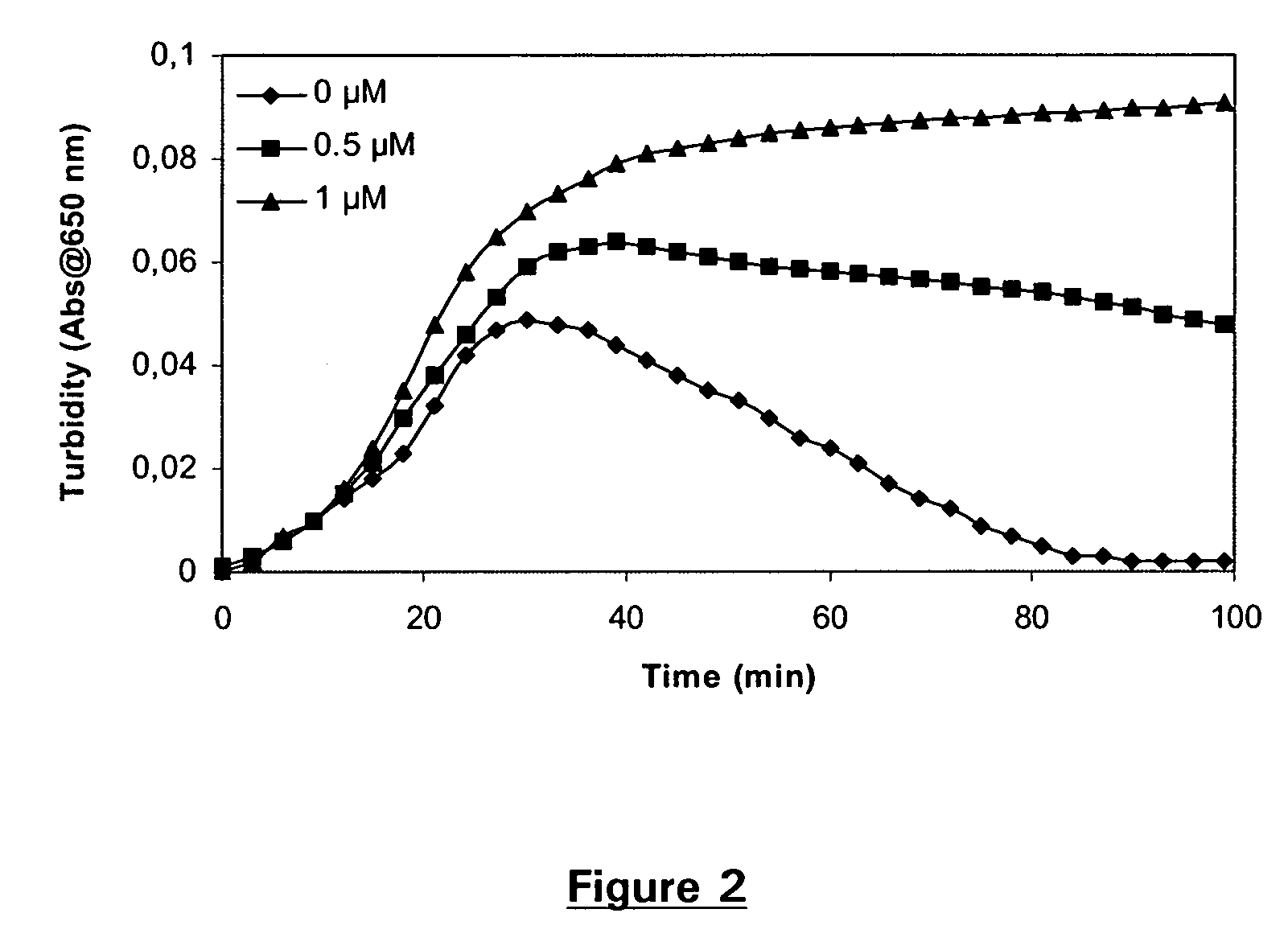
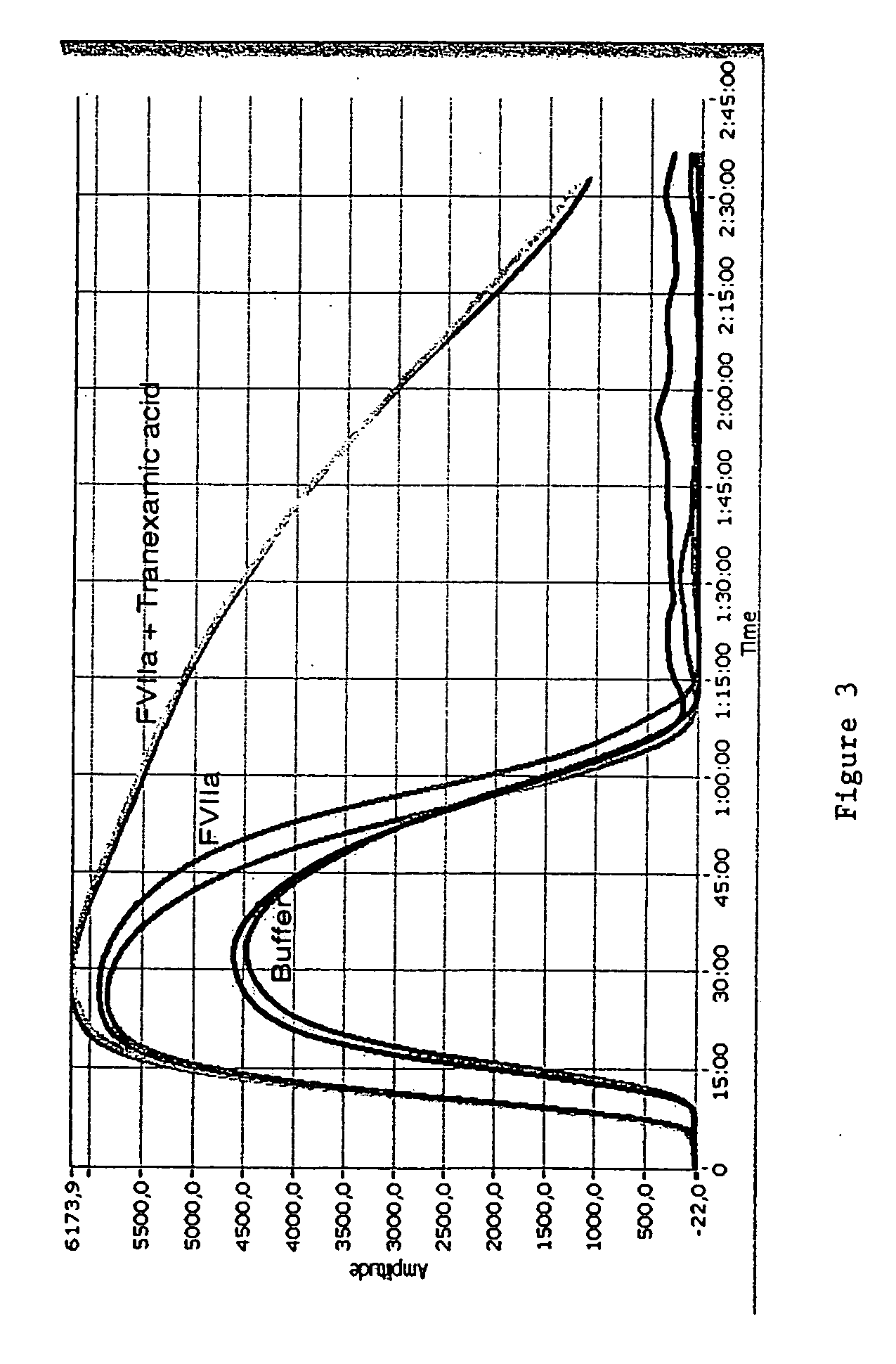
Pharmaceutical composition comprising factor VII polypeptides and tranexamic acid
The present invention relates to compositions comprising factor VII or a factor VII-related polypeptide and tranexamic acid, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Tranexamic acid formulations with reduced adverse effects
InactiveUS20090214644A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocideOrganic active ingredientsTranexamic acidAdverse effect
Owner:FERRING BV
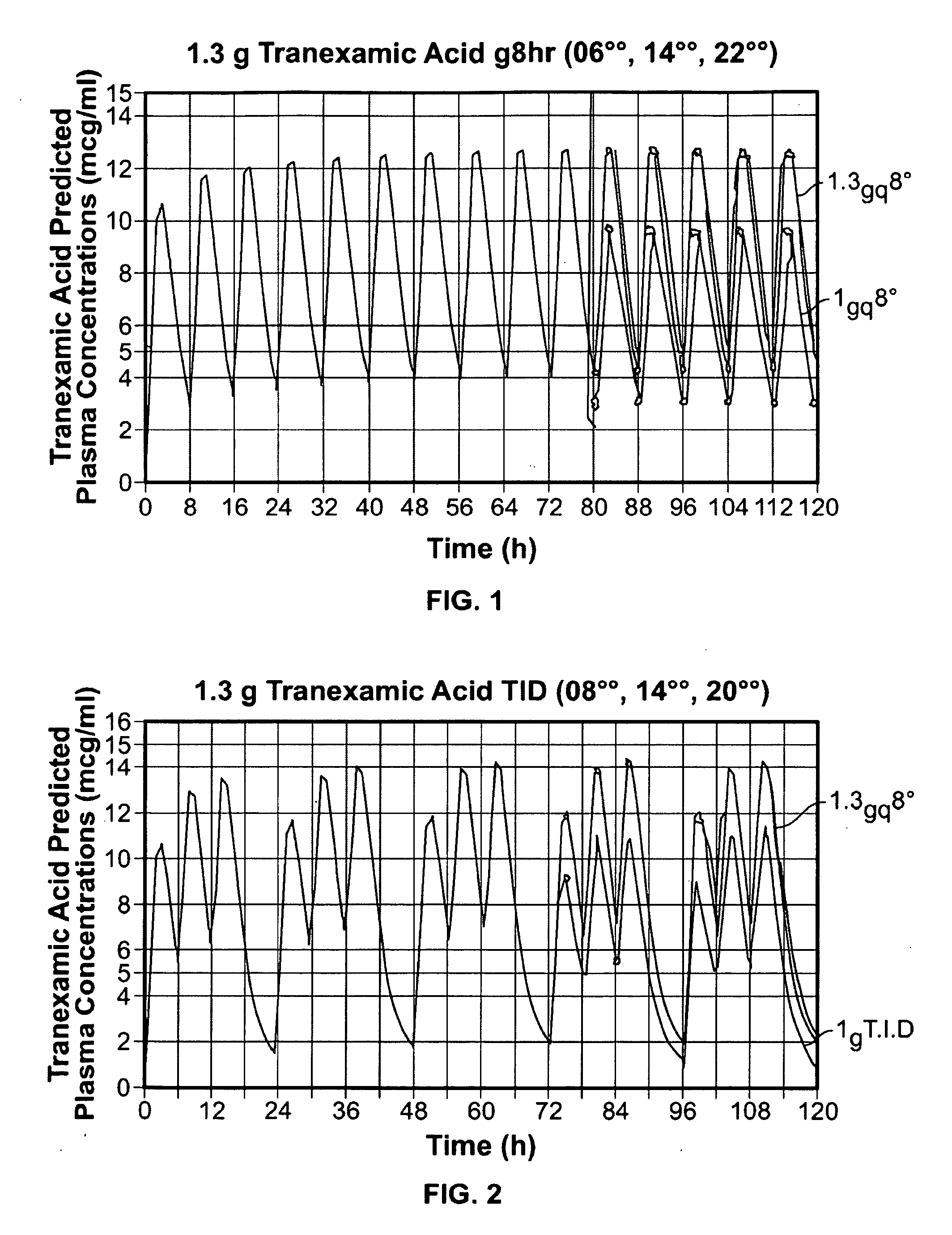
Tranexamic Acid Formulations
ActiveUS20100143468A1Minimize and eliminate undesirable gastrointestinal side effectReduce concentrationBiocideNervous disorderTranexamic acidPharmacology
Disclosed are modified release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC
Tranexamic acid formulations
InactiveUS20090215898A1Less side effectsFew adverse effectBiocideOrganic active ingredientsImmediate releaseTranexamic acid
Disclosed are immediate release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC
Tranexamic acid formulations
ActiveUS20090209646A1Minimize and eliminate undesirable gastrointestinal side effectReduce concentrationBiocidePeptide/protein ingredientsTranexamic acidPharmacology
Disclosed are modified release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC
Preparation method of polypeptide silk mask
InactiveCN105878048AEfficient hydrationEfficient AntioxidantCosmetic preparationsToilet preparationsCentella asiatica extractBetaine
The invention discloses a preparation method of a polypeptide silk facial mask, which comprises the steps of fully mixing polypeptide powder and silk protein nutrient solution and cooling. Polypeptide powder is mainly composed of aloe vera, carnosine, oligopeptide, soluble collagen, 2‑o‑ethyl ascorbic acid, betaine, trehalose, niacinamide, allantoin; the protein nutrient solution of silk is mainly composed of water, glycerin, Propylene glycol, butylene glycol, malto-oligosaccharide glucoside, hydrogenated starch hydrolyzate, glycerol polymethacrylate, PVM / MA copolymer, carbomer, sodium hyaluronate triethanolamine, baobab pulp extract, tranexamic acid , Centella asiatica extract, dipotassium glycyrrhizate and other skin conditioners or moisturizers. Through the combination of polypeptide powder and silk protein liquid, it can effectively give fresh nutrition to the skin, promote the skin repair process, strengthen the nutrient absorption capacity, deeply nourish, smooth and rejuvenate the skin, and make the skin elastic.
Owner:韩玉逍
Tranexamic acid formulations
Owner:XANODYNE PHARMACEUTICALS INC +1
Tranexamic acid formulations with reduced adverse effects
ActiveUS20060127476A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePeptide/protein ingredientsNausea sicknessPatient compliance
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:AMRING PHARM INC
Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates
Owner:LES LAB SERVIER
Medicinal sustained-release and hemostatic composition and preparation method thereof
InactiveCN101837143ARelease stabilityRapid hemostasisAbsorbent padsBandagesSocial benefitsHemostatic function
The invention discloses a medicinal sustained-release and hemostatic composition and a preparation method thereof, which belong to the technical field of novel hemostatic materials. The medicinal sustained-release and hemostatic composition comprises the composition of a medicament-loaded chitosan microsphere, chitosan and hyaluronic acid, wherein the medicament-loaded chitosan microsphere comprises medicinal grade chitosan and medicinal grade tranexamic acid. The novel hemostatic composition is prepared by using chitosan and the hyaluronic acid as a main hemostatic matrix, combining chitosan medicament sustained-release microsphere composite medicament, namely tranexamic acid, utilizing the special property of the chitosan with a hemostatic function and a medicament sustained-release function, and adopting the composition of the hyaluronic acid with good hemostatic function and high biocompatibility and the chitosan as the main matrix. The novel hemostatic composition has the advantages of stopping bleeding rapidly and releasing medicament / factors continuously and stably in a certain time, along with great economic and social benefits.
Owner:北京博恩康生物科技有限公司
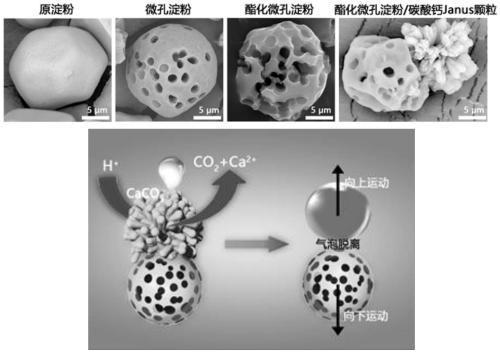
Preparation method for janus structure based rapid hemostatic agent having directional propulsion function
ActiveCN111135339AEffective hemostasisGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismJanus particlesDeep wounds
For defects that existing hemostasis research and products cannot intelligently perform self-propelling or cannot be driven to forward deep in the wound under the action of external field force, the invention provides a preparation method for a janus structure based rapid hemostatic agent having a directional propulsion function. The preparation method includes performing esterification on microporous starch to unidirectionally grow calcium carbonate particles so as to obtain esterified microporous starch / calcium carbonate Janus particles; fixedly assembling thrombin on the surfaces of the esterified microporous starch / calcium carbonate Janus particles to obtain esterified microporous starch / calcium carbonate Janus particles assembled with the thrombin; mixing the esterified microporous starch / calcium carbonate Janus particles assembled with the thrombin and protonated tranexamic acid powder so as to obtain the janus structure based rapid hemostatic agent having a directional propulsion function. Through the formation of the biphasic heterogeneous Janus structure, the unidirectional and intelligent self-propulsion of hemostatic starch can be realized by cooperating with the protonated tranexamic acid, so that rapid three-dimensional hemostasis on deep wounds, penetrating wounds and irregular wounds such as aortic / venous rupture can be realized.
Owner:SOUTHWEST UNIVERSITY
Skin care substrate with long acting moisturizing and whitening effects and preparation and application of skin care substrate
ActiveCN106309154AGood moisturizing effectGood skin whiteningCosmetic preparationsToilet preparationsALPHA-ARBUTINTremella
The invention belongs to the technical field of cosmetics, and discloses a skin care substrate with long acting moisturizing and whitening effects and preparation and application of skin care substrate. The skin care substrate is prepared from the following components in mass percent: 10%-20% of tremella polysaccharide, 5%-10% of glycosphingolipid, 10%-20% of saccharide isomerate, 5%-10% of nicotinamide, 3%-6% of alpha-arbutin, 3%-6% of tranexamic acid, 1%-3% of ADS-Oligonol and balance of water. The skin care substrate disclosed by the invention has obvious long acting moisturizing and skin whitening effects and can make the skin moisturized and bright white by persistent use.
Owner:GUANGDONG MARUBI BIOLOGICAL TECH CO LTD
Composition for Prevention or Alleviation of Pigmentation
InactiveUS20080260878A1Effectively prevent and improvePreventing and improving pigmentation of skinBiocideCosmetic preparationsArbutinAdenosine
A composition for the prevention or alleviation of pigmentation which can produce the higher effect of preventing or alleviating pigmentation. The composition for the prevention or alleviation of pigmentation comprises a combination of (A) at least one member selected from the group consisting of adenosine 5′-monophosphate and salts thereof with (B) at least one member selected from the group consisting of arbutin, ellagic acid, 4-alkylresorcinols, linoleic acid, tranexamic acid, salts of these, Chamomilla recuita extract, and Ubiquinone.
Owner:OTSUKA PHARM CO LTD
Moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition and preparation method thereof
InactiveCN105434211ABalance secretionReduce generationCosmetic preparationsToilet preparationsPhenolic content in teaTremella
The invention discloses a moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition. The composition is mainly prepared from the following raw materials: raspberry ketone glucoside, arbutin, matrine, fructo-oligosaccharide, tea tree oil, dipotassium glycyrrhizinate, an extracting solution of leaf of Tasmanian bluegum, tea polyphenol, a peony root extract, tremella polysaccharide, hyaluronic acid with low molecular weight, hyaluronic acid with high molecular weight, transparent xanthan gum, zinc gluconate, Carbomer 940, hydroxyethyl cellulose, 1,3-butanediol, nicotinamide, D-panthenol, tranexamic acid, vitamin C ethyl ether, triethanolamine, an emulsifier, water, and the like. The moisturizing, whitening, acne removing, grease controlling and relieving skin beautifying composition has the beneficial effects that the composition is rich in plant essence, is reasonable in proportioning and can achieve the effects of effectively comforting and smoothening pores, cleaning hair follicles, balancing grease excretion, promoting wound healing and ensuring no pockmarks after healing and low recurrence probability; besides, the composition is also rich in moisturizing ingredients, so that the skins can be hydrated and bright, fine lines can be reduced and smooth and fair skins can be restored after the composition is used for a long term.
Owner:马南行 +1
Tranexamic acid formulations
InactiveUS20080280981A1Few adverse effectBiocidePeptide/protein ingredientsTranexamic acidPharmacology
Owner:AMRING PHARM INC
Tranexamic acid formulations
ActiveUS20090048341A1Minimize and eliminate undesirable gastrointestinal side effectReduce concentrationBiocidePeptide/protein ingredientsTranexamic acidPharmacology
Disclosed are modified release oral tranexamic acid formulations and methods of treatment therewith.
Owner:AMRING PHARM INC
Whitening essence and preparation method thereof
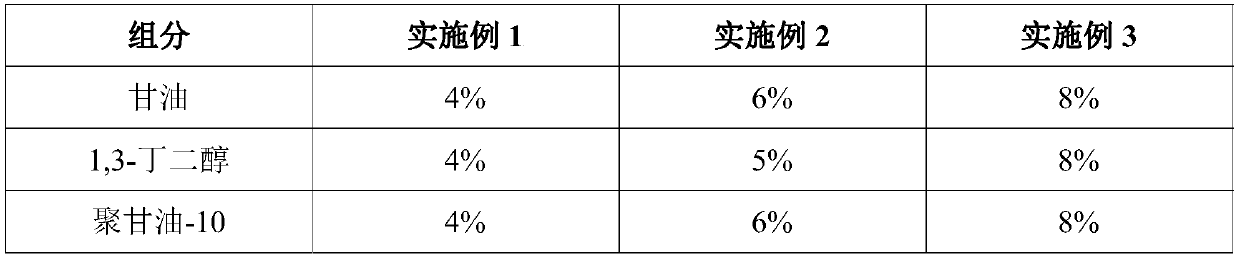
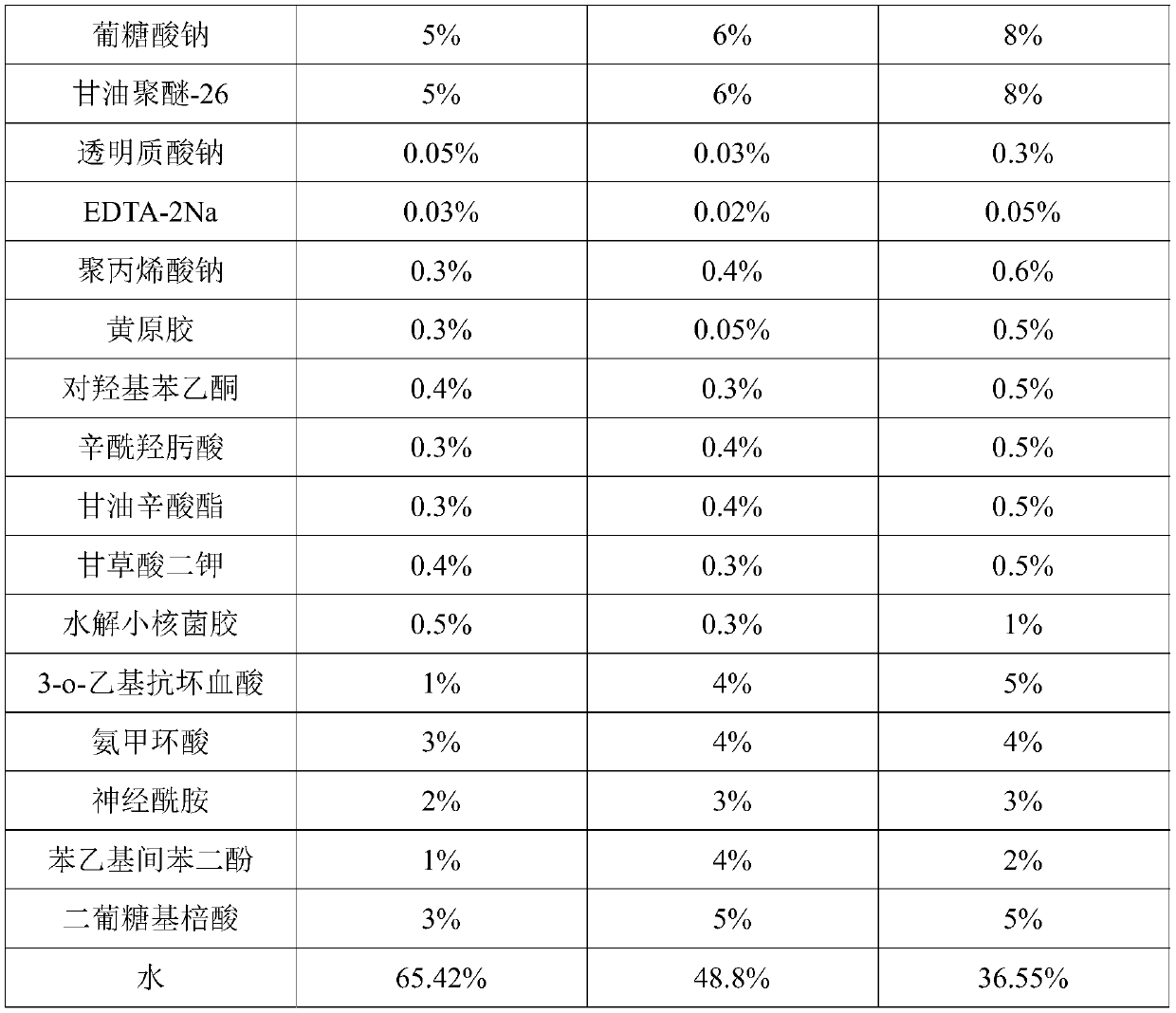
PendingCN111067818AInhibit transferPhotoprotectiveCosmetic preparationsToilet preparationsAcropustulosisButanediol
The invention provides whitening essence and a preparation method thereof. The whitening essence consists of the following components of glycerine, 1,3-butanediol, sodium hyaluronate, EDTA-2Na, polyglycerol-10, sodium polyacrylate, xanthan gum, hydroxyacetophenone, dipotassium glycyrrhizinate, hydrolyzed sclerotium gum, gluconic acid sodium salt, glycereth-26, caprylhydroxamic acid, glycerol caprylate, 3-o-ethyl ascorbic acid, tranexamic acid, ceramide, phenethyl resorcinol, diglucosyl gallic acid and water. The whitening essence disclosed by the invention can refrain conversion of tyrosine tomelanin before the tyrosine is in preparation of conversion to the melanin, so that the activity of the tyrosinase is also high, generation of more melanin cannot be caused, and transfer of skin melanin can also be restrained. Besides, the whitening essence has powerful antioxidant ability, has light protection effects on skin, can control dermatitis, and is a whitening product most comprehensivein action mechanism at present, so that whitening effects can be achieved.
Owner:广州市柏姿生物科技有限公司
Medicinal toothpaste composition and preparation method and application thereof
InactiveCN104644460AReduce inflammationShorten the course of the diseaseCosmetic preparationsAntipyreticOral ulcersToothpaste
The invention discloses a medicinal toothpaste composition which comprises the following raw materials in percentage by weight: 0.01%-0.05% of sodium guaiazulene sulfonate, 0.1%-0.5% of paeonol, 0.1-0.5w / w% of allantoin, 0.1-0.5w / w% of tranexamic acid, and toothpaste matrix on the basis of the total weight. In addition, the invention further discloses a preparation method of the medicinal toothpaste composition and applications in resisting bacteria, diminishing inflammation, easing pain, shortening the treatment course of dental ulcer, and promoting oral wound healing.
Owner:北京华素制药股份有限公司
Inhibitor for melanin, and cosmetic composition containing same
InactiveCN102821742APrevent or improve hyperpigmentationReduce stimulationCosmetic preparationsToilet preparationsNiacinamideBULK ACTIVE INGREDIENT
The present invention relates to an inhibitor for melanin which contains tranexamic acid and niacinamide as active ingredients for inhibiting the formation of melanin cells in the skin, and a cosmetic composition containing the inhibitor for melanin as an active ingredient for relieving liver spots, blemishes, freckles and inflammatory hyperpigmentation, improving skin tone and texture, and skin whitening.
Owner:AMOREPACIFIC CORP
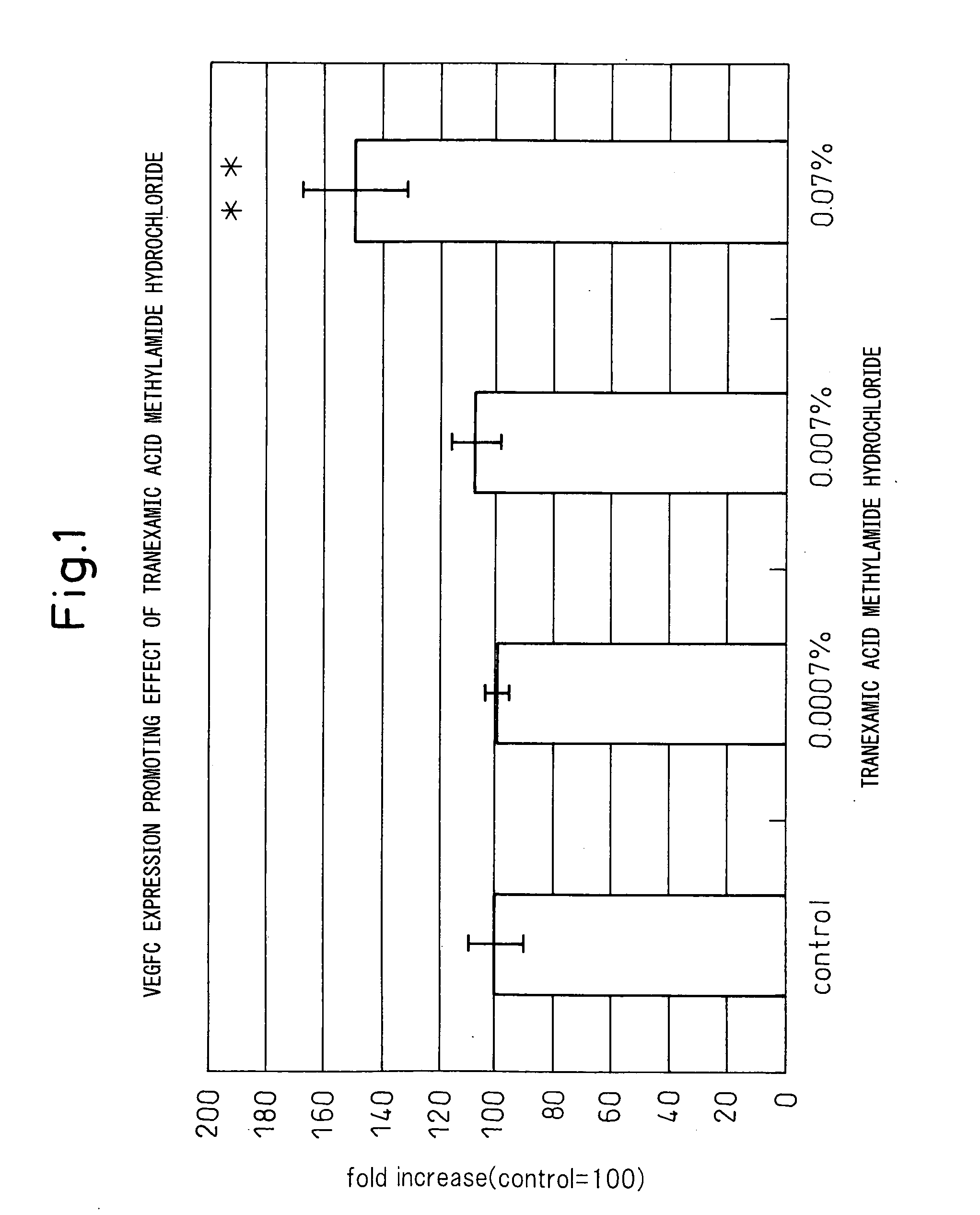
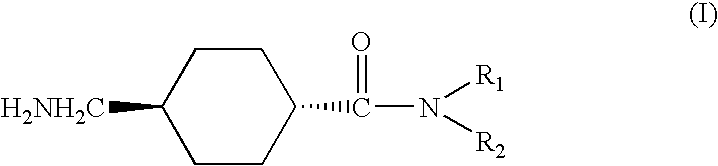
Vegfc production promoter
InactiveUS20100292509A1Promote activationPreventing or inhibiting bloating, lymphedema, wrinkle formationCosmetic preparationsOrganic active ingredientsStereochemistryTranexamic acid
A bloating ameliorant, a lymphatic vessel activator and a VEGFC production promoter comprising a tranexamic acid amide derivative and / or salt thereof.
Owner:SHISEIDO CO LTD
Toothpaste with hemostasis and gum protection functions and preparation method of toothpaste
The invention provides toothpaste with hemostasis and gum protection functions and a preparation method of the toothpaste. The toothpaste comprises, by weight, 0.03-1.0% of panax notoginseng extract, 30-55% of abrasives, 15-30% of wetting agents, 0.6-1.5% of adhesives, 2-2.7% of foaming agents and water. The preparation method includes the steps of mixing panax notoginseng saponins, tranexamic acid, the abrasives, the wetting agents, the adhesives and the water according to the weight percentage, conducting stirring and grinding for 25-40 minutes, conducting vacuum degassing, and obtaining a toothpaste body. By means of the combined application of the panax notoginseng extract and tranexamic acid, the toothpaste can have the enhanced hemostasis and gum protection functions.
Owner:昆明牙膏有限责任公司
Producing method of of tranexamic acid
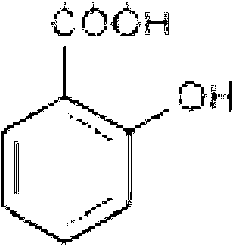
InactiveCN1524847AImprove product qualitySolve technical problems that have not been resolved for a long timeOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidTranexamic acid
The invention discloses a process for producing tranexamic acid, wherein p-aminomethyl benzoic acid raw material is hydrogenised, and the hydrogenization liquid is neutralized to pH7-7.5 through barium carbonate, after filtering, the solution is condensed to 10 gram molecule p-aminomethyl benzoic acid reaction liquid of 5l, then charge raw materials by the portion ratio of ammonia cyclohexyl-methane carboxyl acid sym-form liquid (V) : barium hydroxide (W) : water (W) : ethanol (V) = 1:0.5-1.5:2-3.5:1.5-3, elevating the temperature to 230-260 deg. C, the pressure being 2.9-4.8.0Mpa, reacting time being 7.5-10 hours, after reaction charging water and elevating the temperature to 60-70 deg. C, neutralizing through carbon dioxide till pH=6-7.5, filtering and adjusting pH=5.2-5.5 through sulfuric acid, stewing, charging right amount activated charcoal, boiling and decolorizing, filtering, filter cake washing, condensing, charging ethanol for recrystallization, cooling down to 8-12 deg. C, filtering, washing crystallization by ethanol, filter cake drying to obtain tranexamic acid.
Owner:HUNAN DONGTING PHARMA
Composition for improving skin color and whitening cosmetic
InactiveCN112618400APrevent pigmentationPrevent or improve skin pigment spotsCosmetic preparationsToilet preparationsChitosamineNicotinamide mononucleotide
The invention discloses a composition for improving skin color and a whitening cosmetic. The composition comprises tranexamic acid, acetyl chitosamine and beta-nicotinamide mononucleotide. The tranexamic acid, the acetyl chitosamine and the beta-nicotinamide mononucleotide are compounded, the beta-nicotinamide mononucleotide is combined with the tranexamic acid and the acetyl chitosamine, pigmentation caused by ultraviolet rays or other skin irritation can be inhibited, and skin pigmented spots related to pigmentation are effectively prevented or improved. The acetyl chitosamine can improve transdermal absorption of effective components such as the tranexamic acid and the beta-nicotinamide mononucleotide, has a whitening effect, and can achieve a synergistic whitening effect when being used together with the tranexamic acid and the beta-nicotinamide mononucleotide, so that the tranexamic acid, the acetyl chitosamine and the beta-nicotinamide mononucleotide can achieve whitening and brightening effects at a low concentration, and are stable, safe and non-irritant.
Owner:SHANDONG BLOOMAGE HYINC BIOPHARM CORP LTD
Medical sodium alginate gel microsphere and preparation method and application thereof
InactiveCN103239730AControl releaseReduce erosionAntibacterial agentsPharmaceutical non-active ingredientsWater insolubleMicrosphere
The invention provides a medical sodium alginate gel microsphere and a preparation method and application of the medical sodium alginate gel microsphere. The medical sodium alginate gel microsphere consists of a composite medicine carrier and a water-insoluble medicine; the medicine is coated with the composite medicine carrier; and the composite medicine carrier is an ion crosslinking agent-sodium alginate-divalent metal ion, wherein the ion crosslinking agent is 4-aminomethylbenzoic acid or tranexamic acid. The preparation method comprises the following steps of: (1) mixing ion crosslinking agent aqueous solution with divalent metal ion solution in the same volume to obtain composite solidifying liquid; (2) dispersing medicine powder or an agent into sodium alginate aqueous solution; uniformly mixing; dropwise adding the mixture into the composite solidifying liquid obtained in step (1) through a high-voltage static droplet generating device or a syringe needle, so that the mixture drops are solidified into spheres; and (3) dehydrating gel microspheres which are washed with the distilled water; and drying at normal temperature. The medical sodium alginate gel microsphere can be used for treating tuberculosis, endocrine disease and tumor, and also can be used for treating local acute hemorrhage and chronic errhysis.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates](https://images-eureka.patsnap.com/patent_img/3a4fef23-05ad-49cd-ac3b-ec925b692bd8/US20040059134A1-20040325-C00001.png)
![Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates](https://images-eureka.patsnap.com/patent_img/3a4fef23-05ad-49cd-ac3b-ec925b692bd8/US20040059134A1-20040325-C00002.png)
![Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates Process for the industrial synthesis of tetraesters of 5-[BIS(CARBOXYMETHYL)AMINO]-3-carboxymethyl-4-cyano-2-thiophenecarboxylic acid, and application to the synthesis of bivalent salts of ranelic acid and their hydrates](https://images-eureka.patsnap.com/patent_img/3a4fef23-05ad-49cd-ac3b-ec925b692bd8/US20040059134A1-20040325-C00003.png)