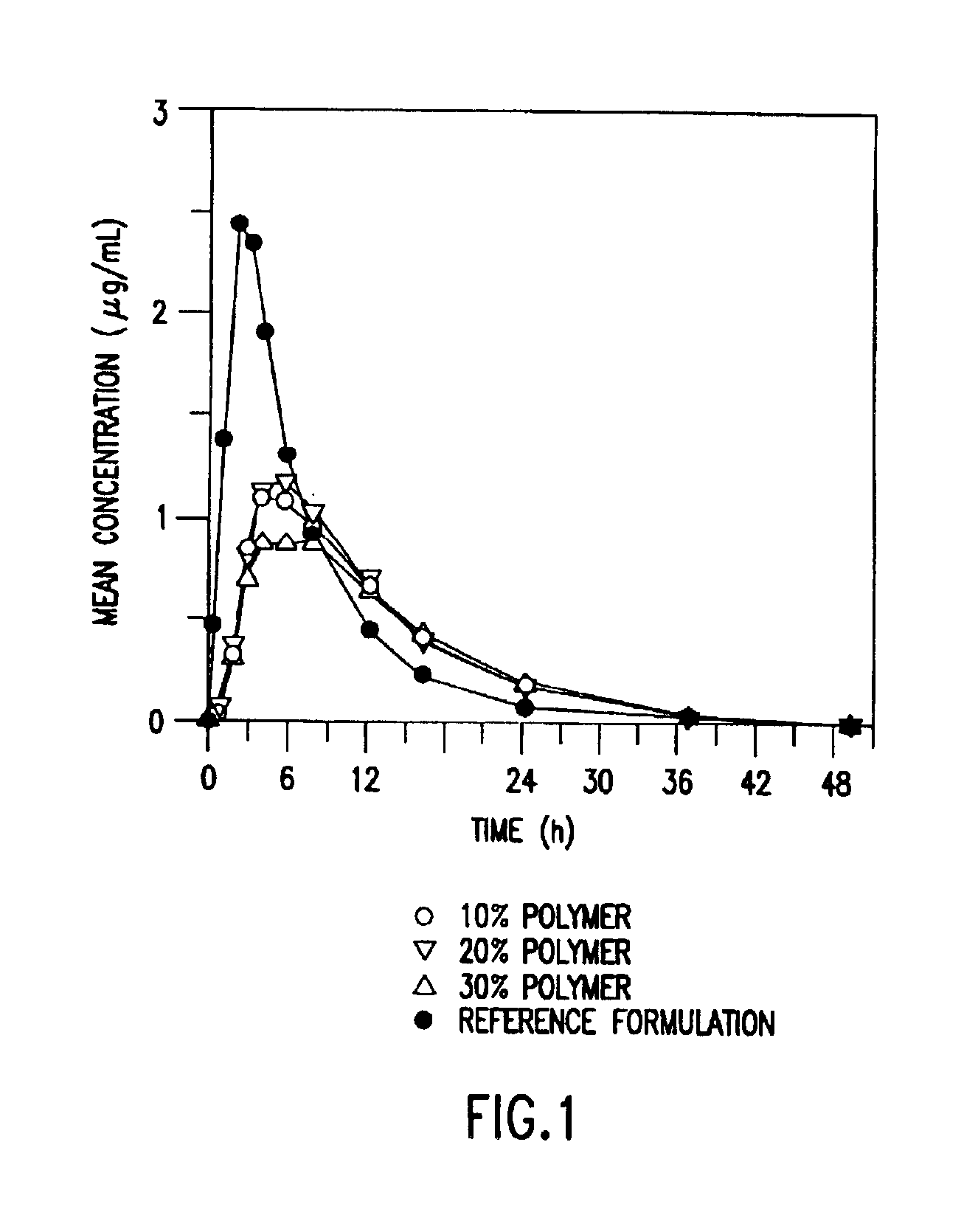
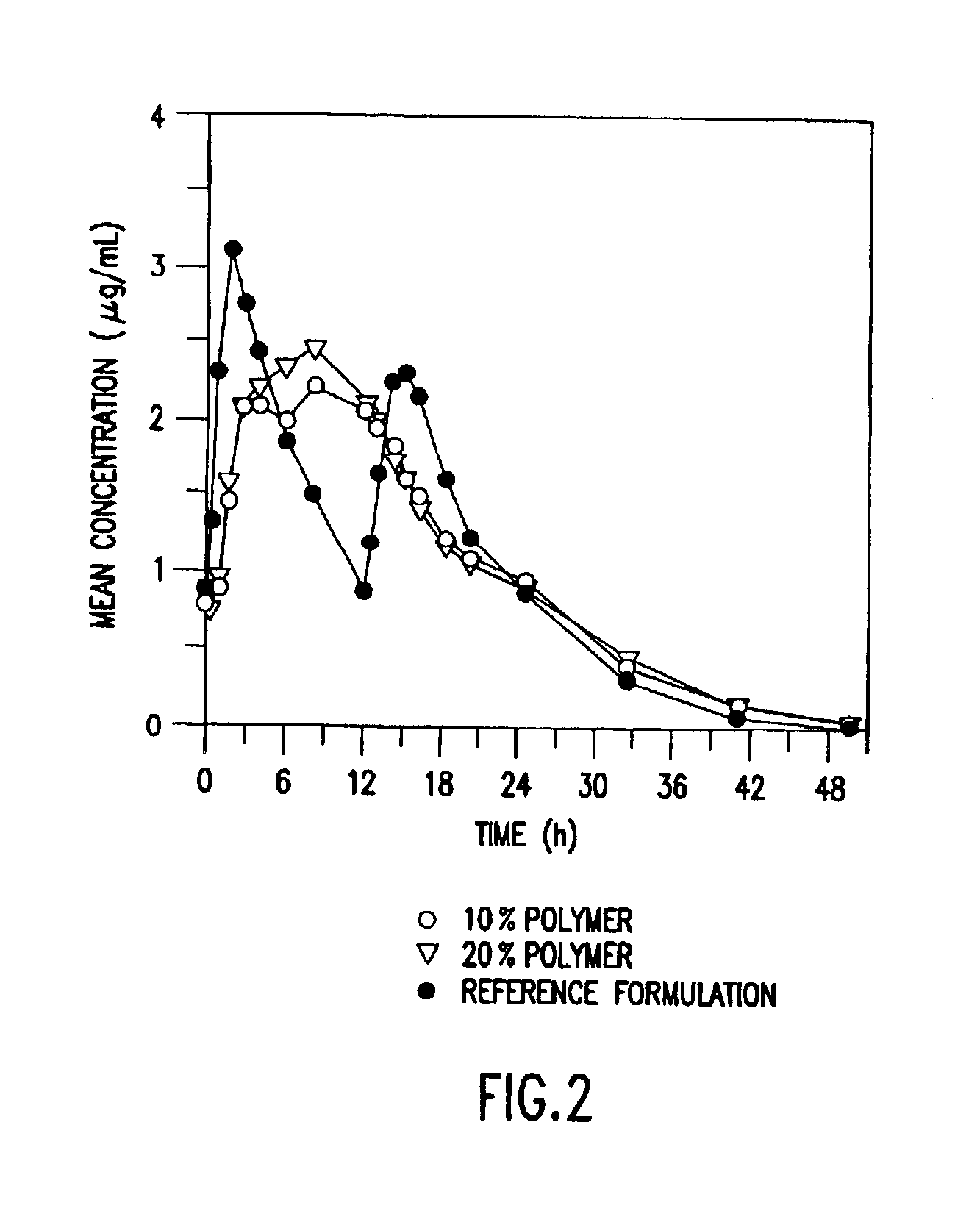
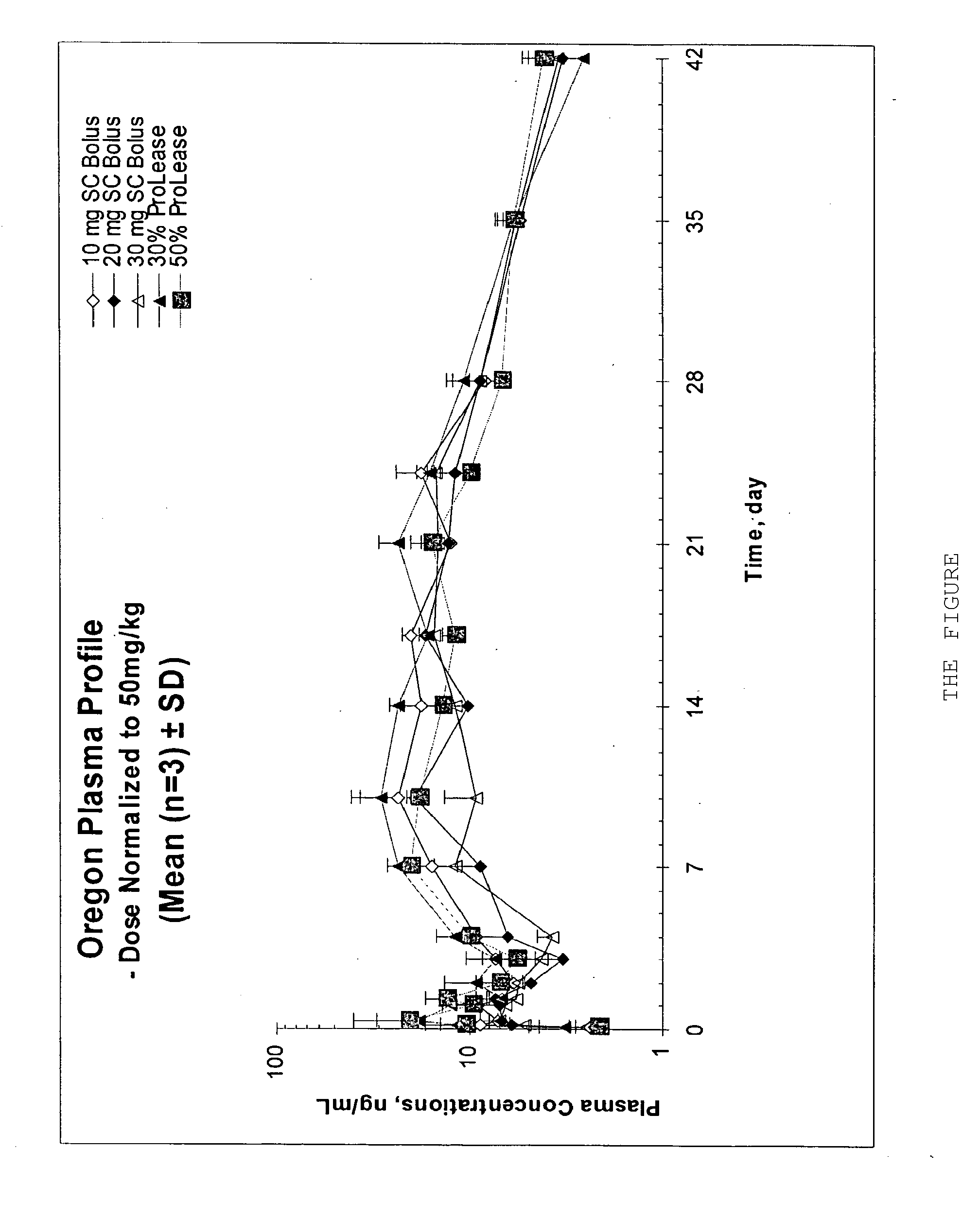
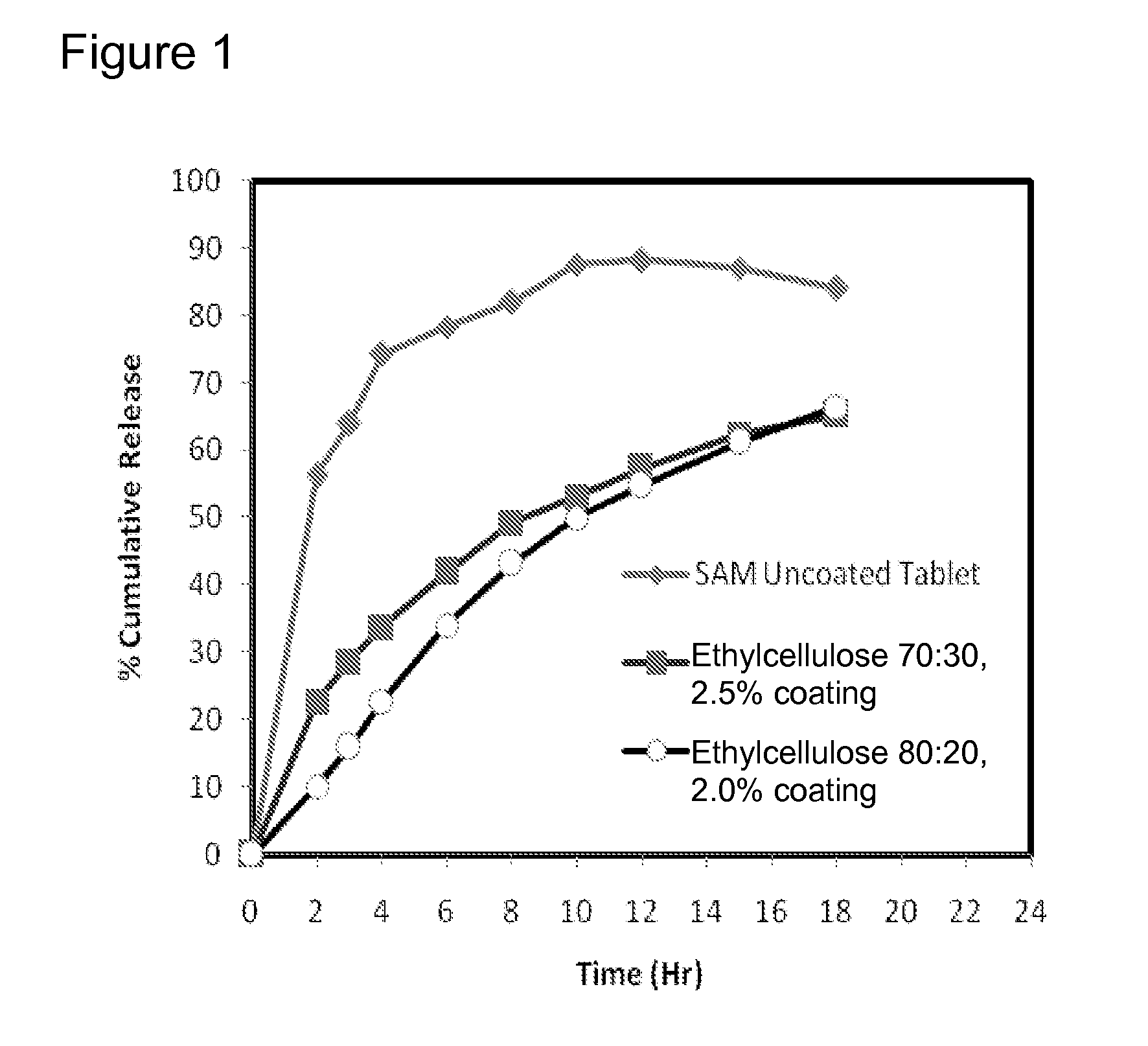
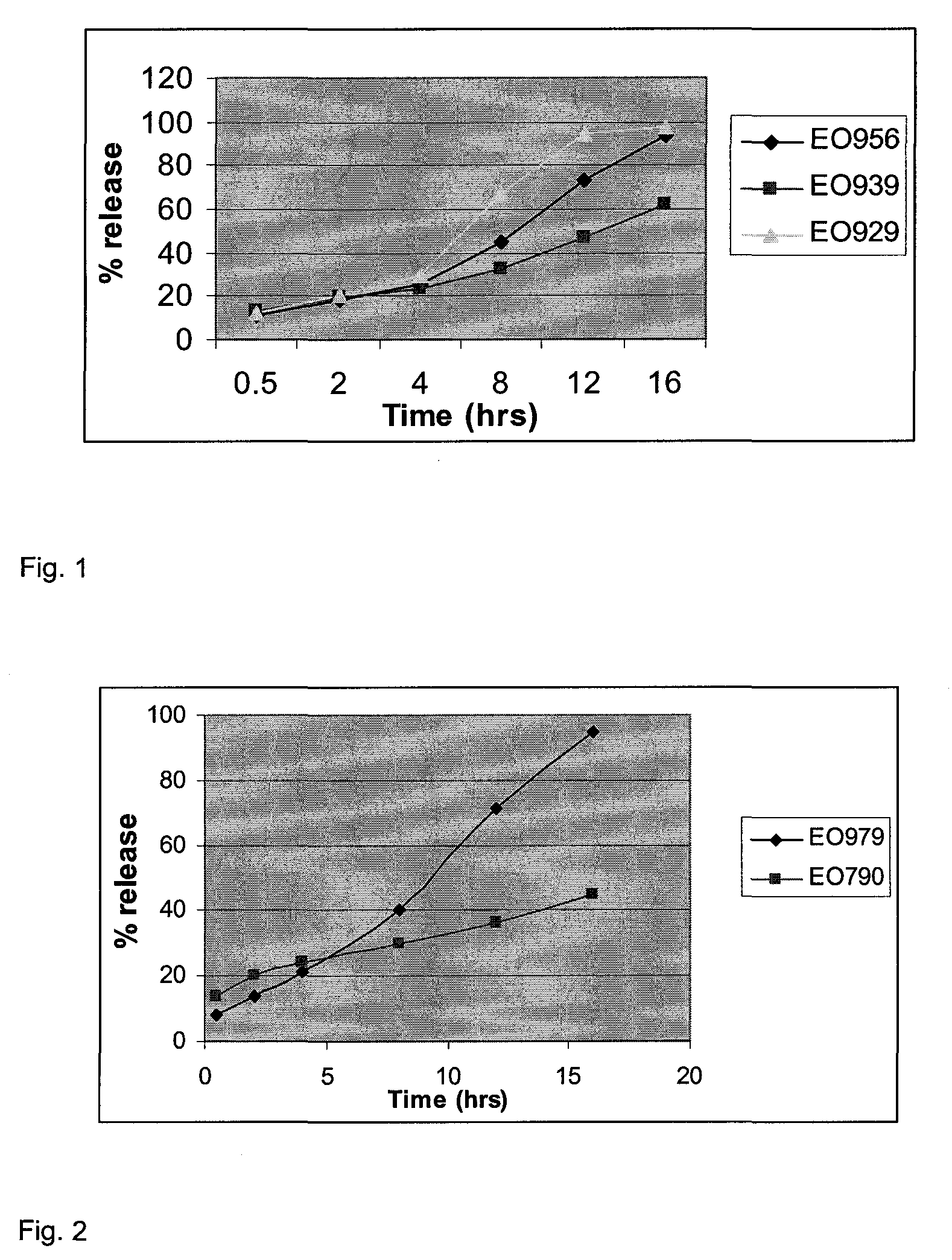
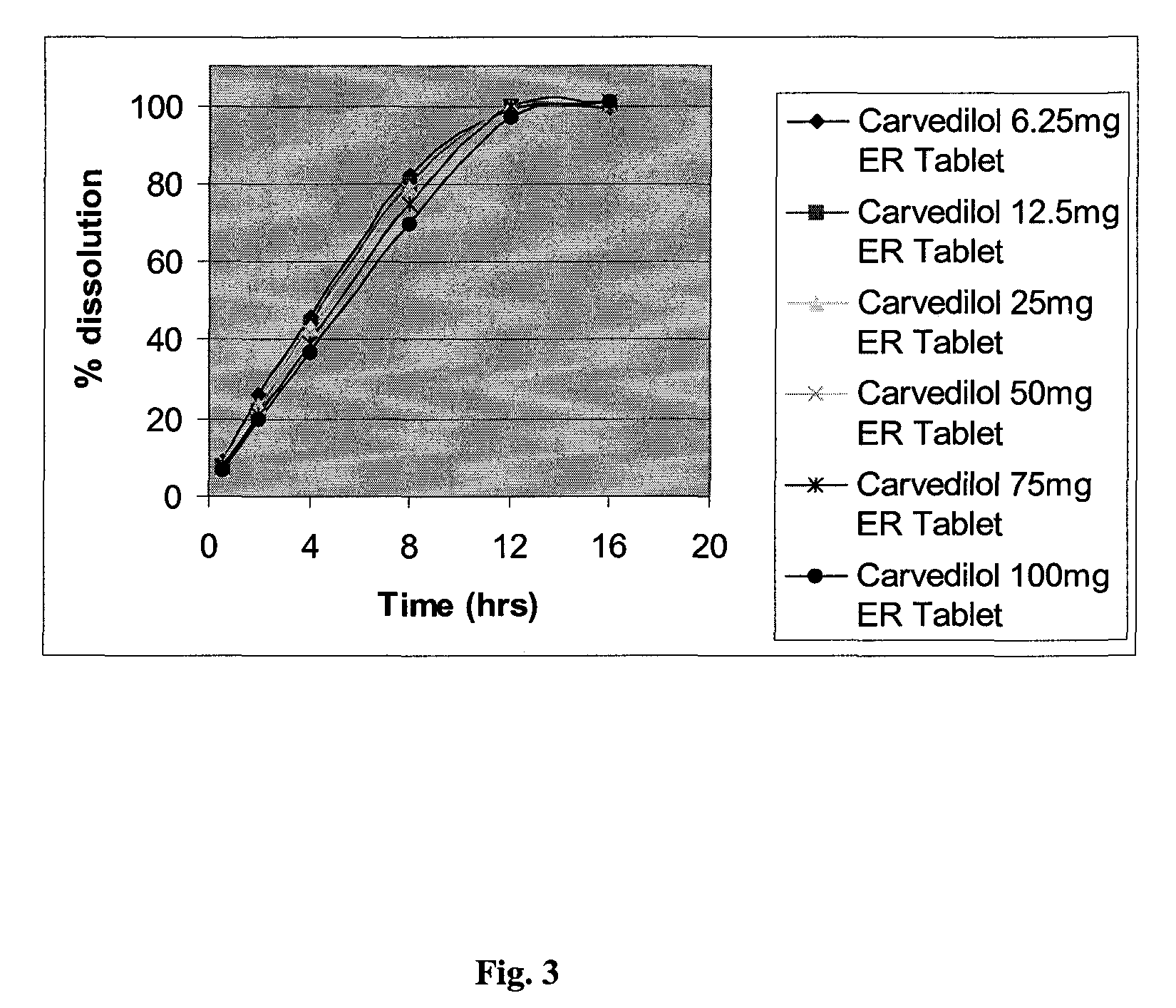
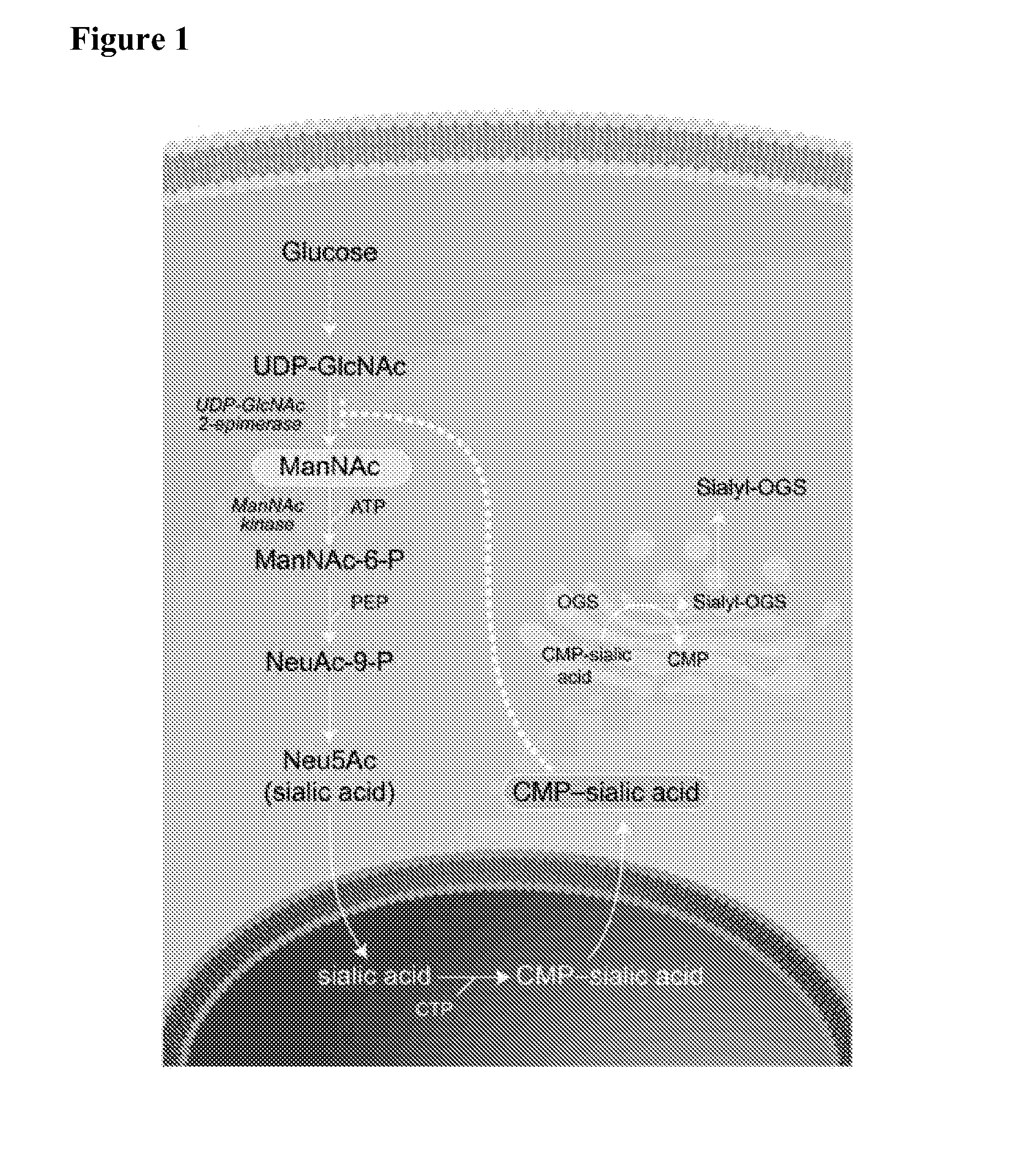
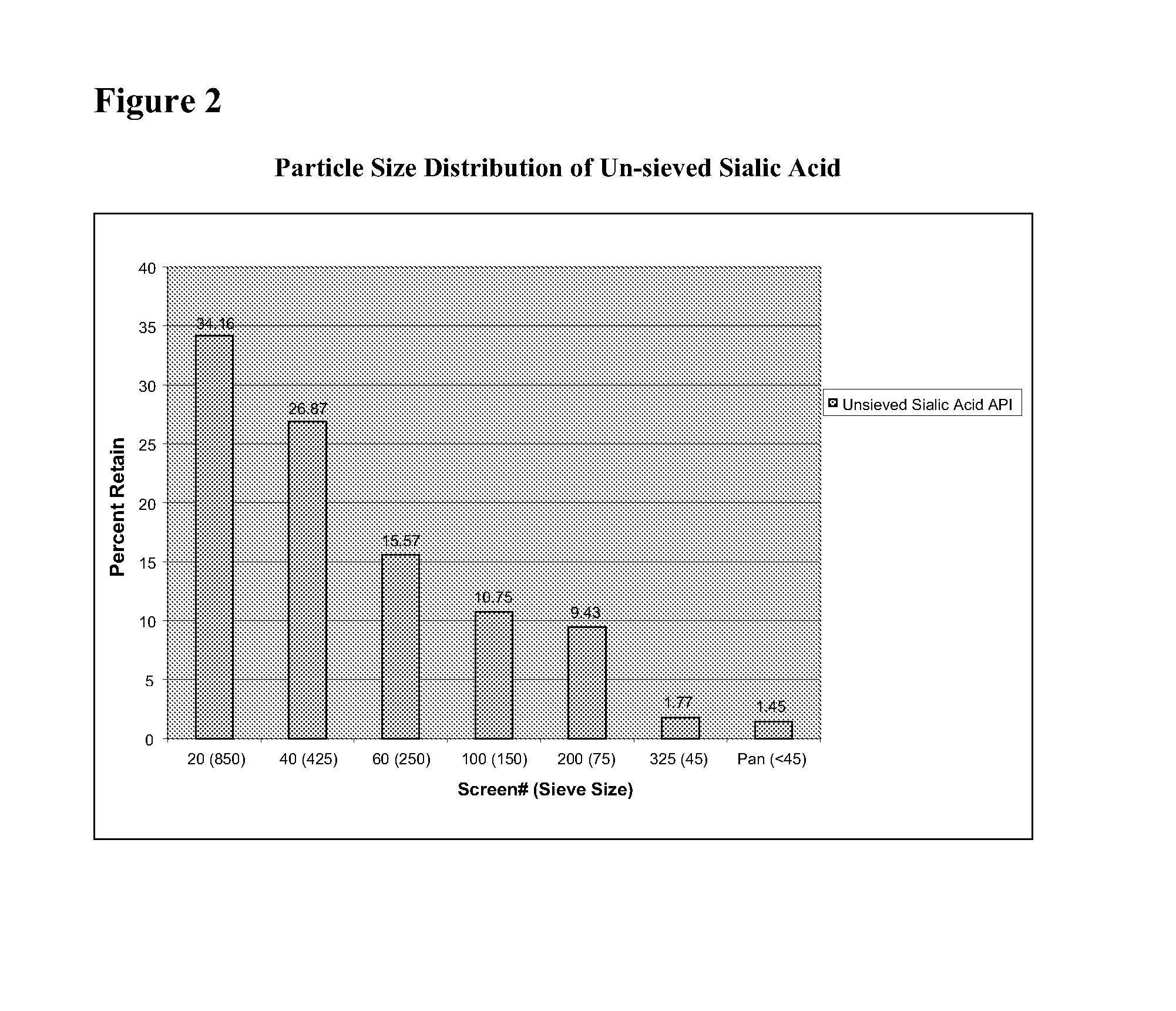
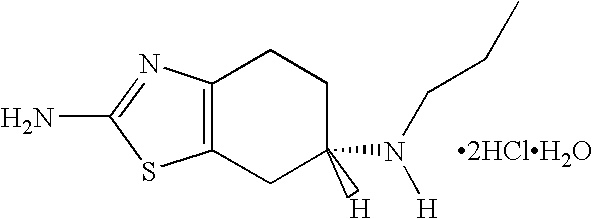
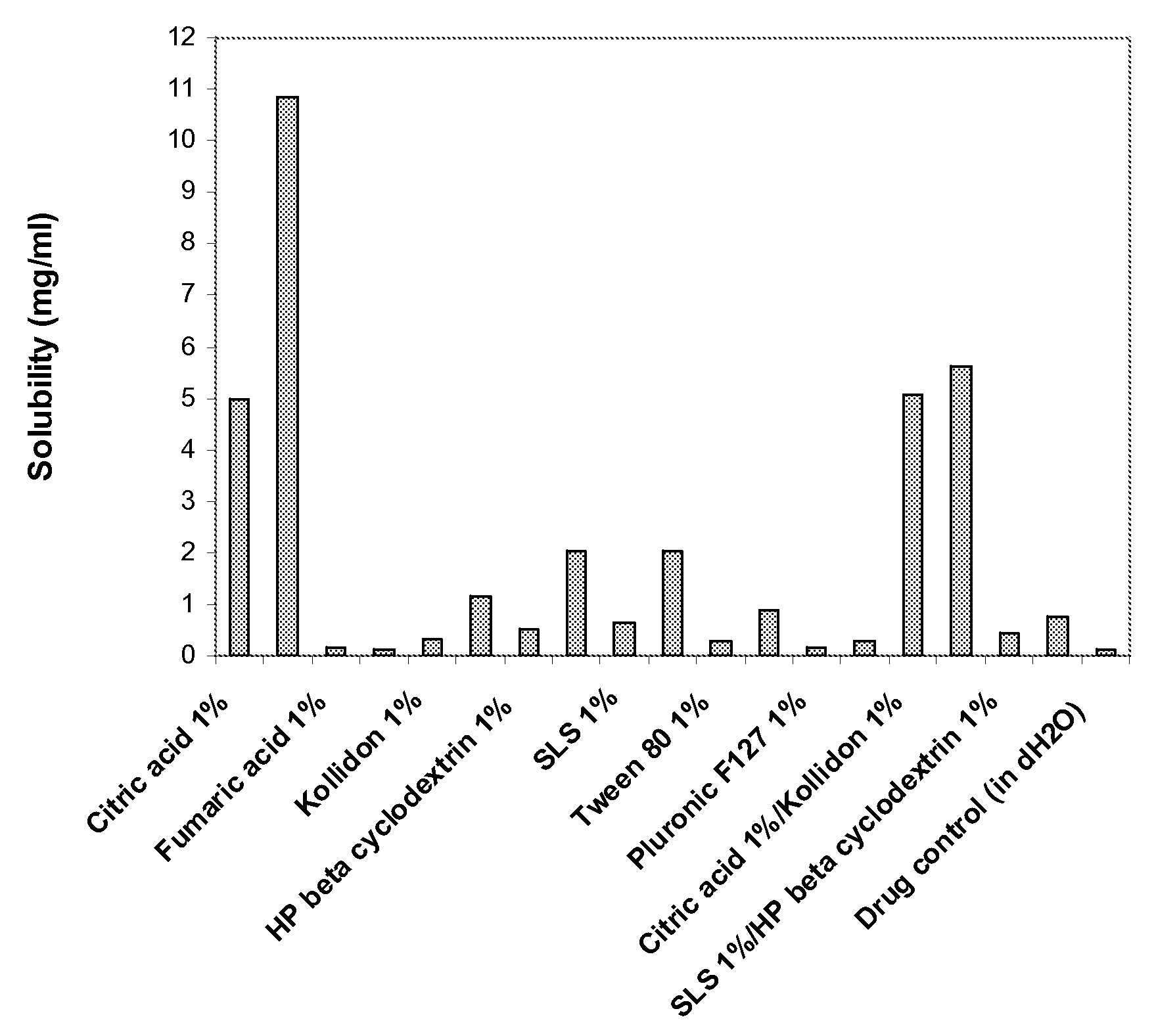
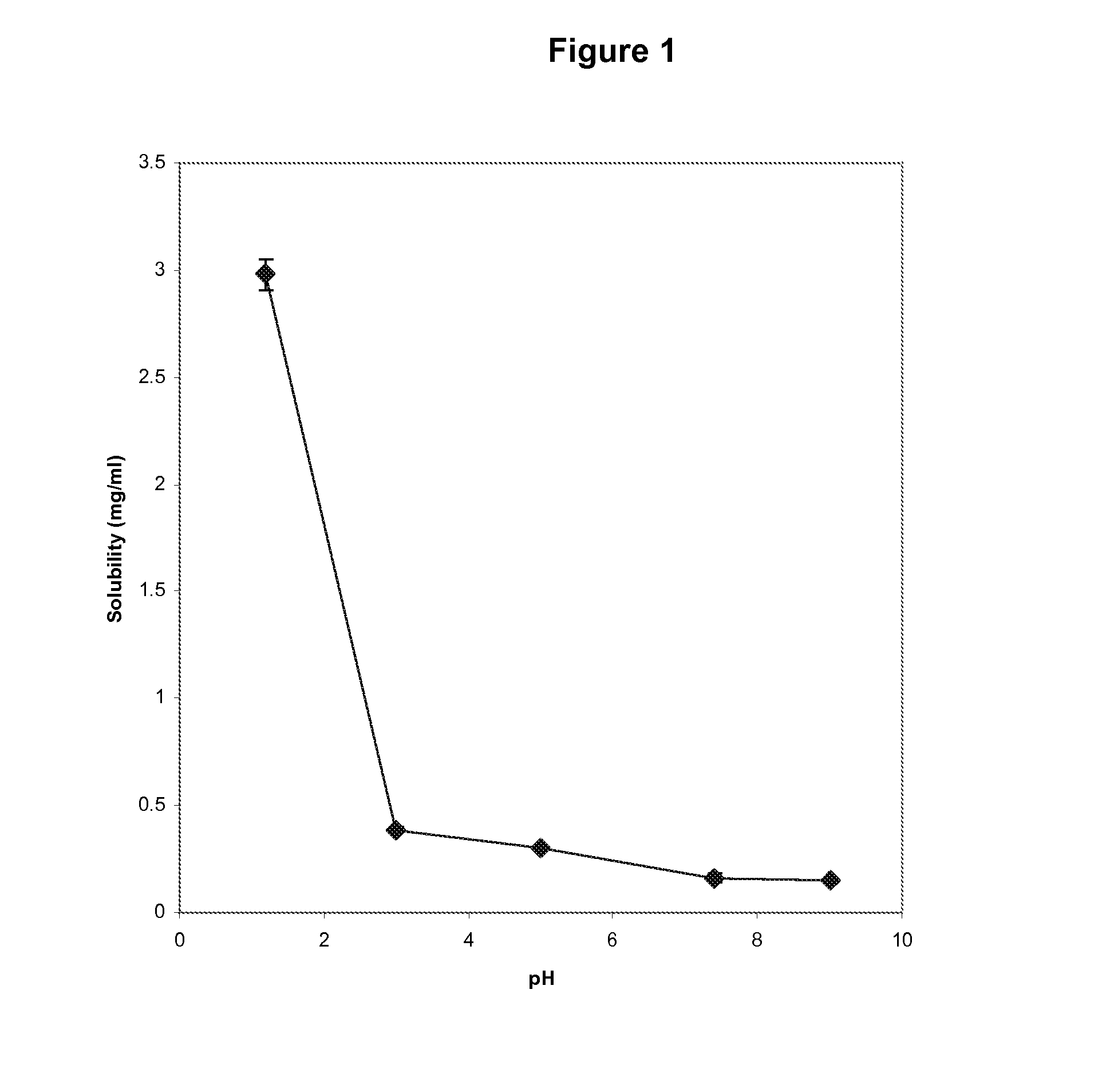
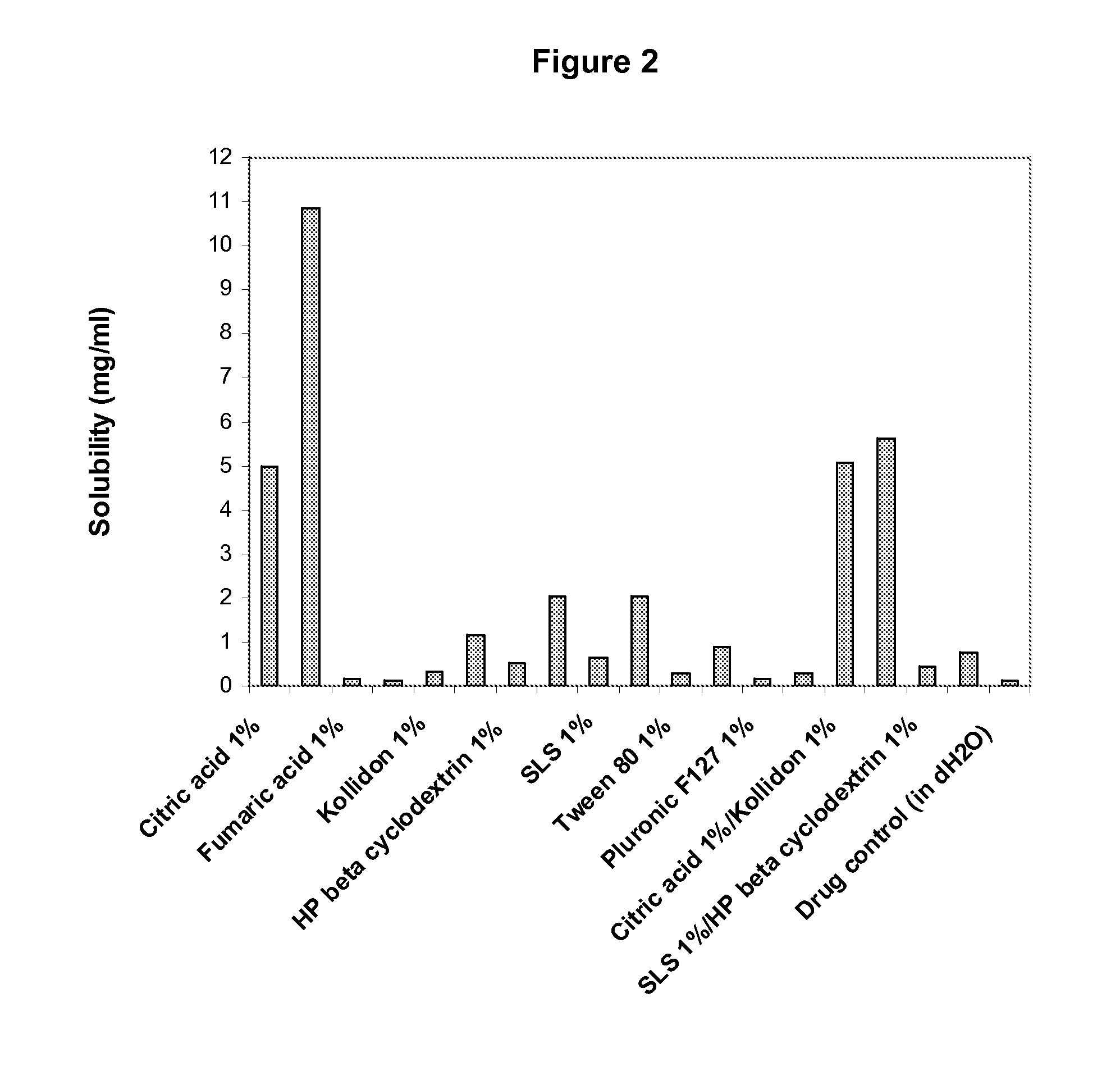
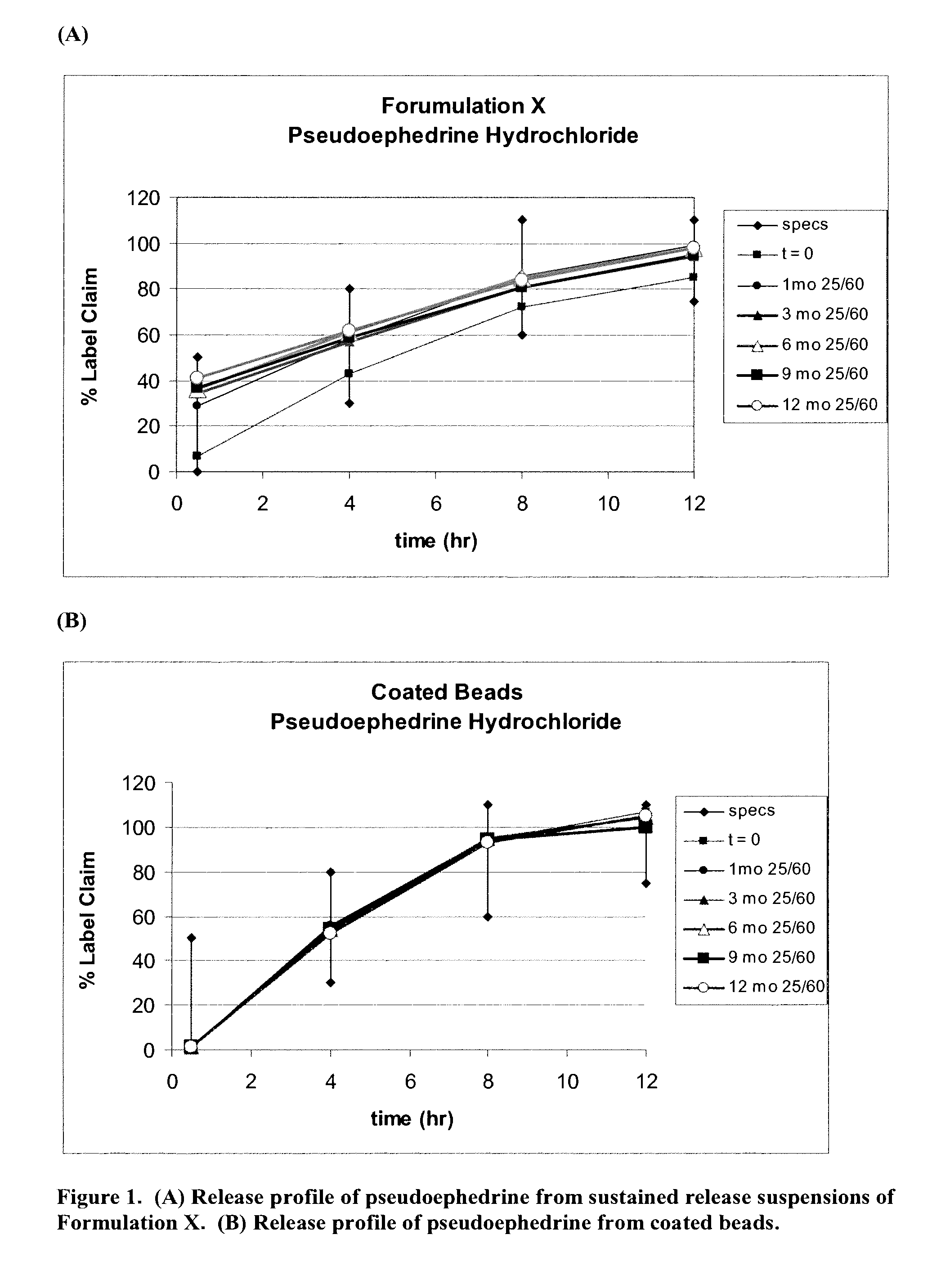
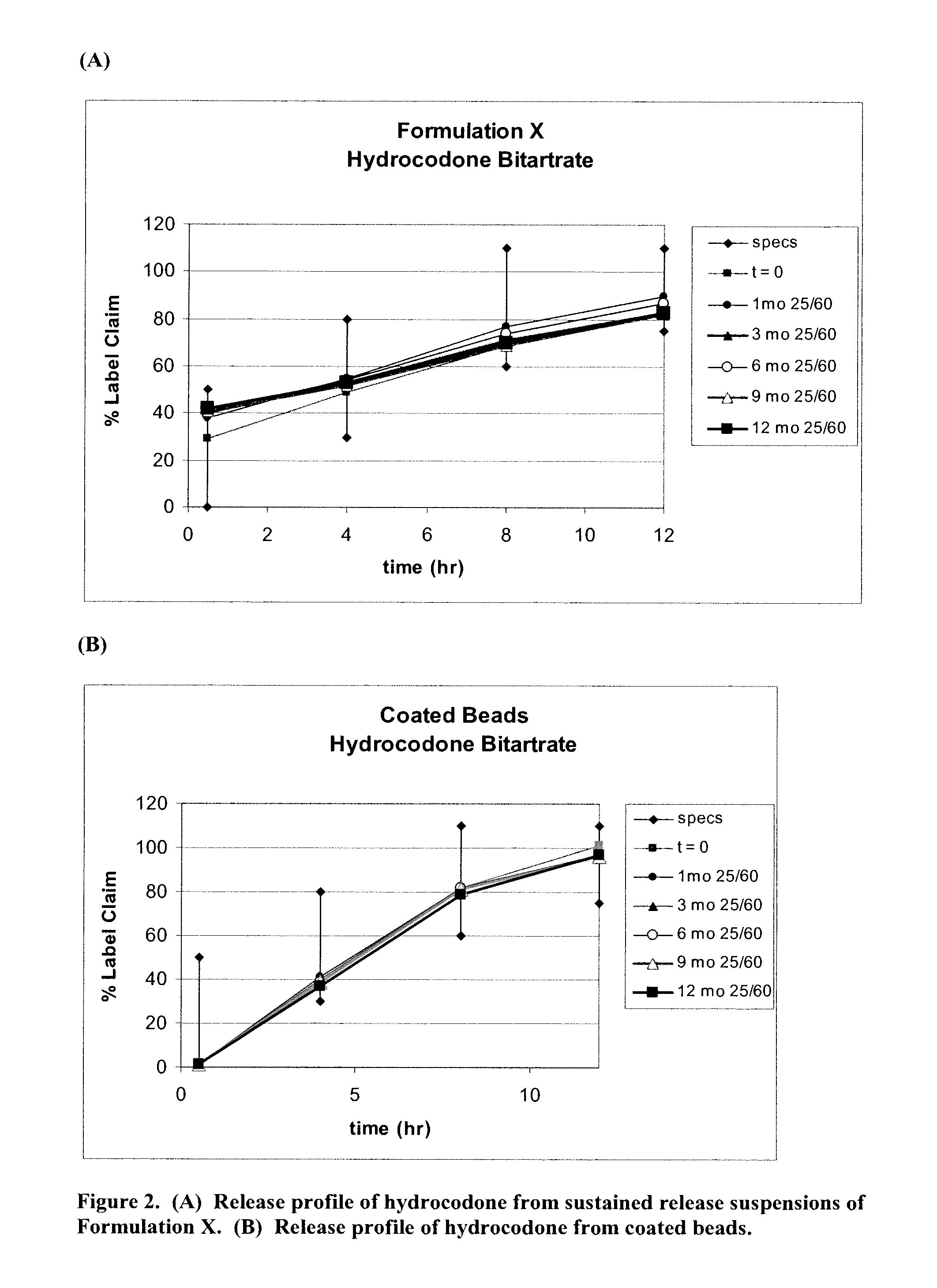
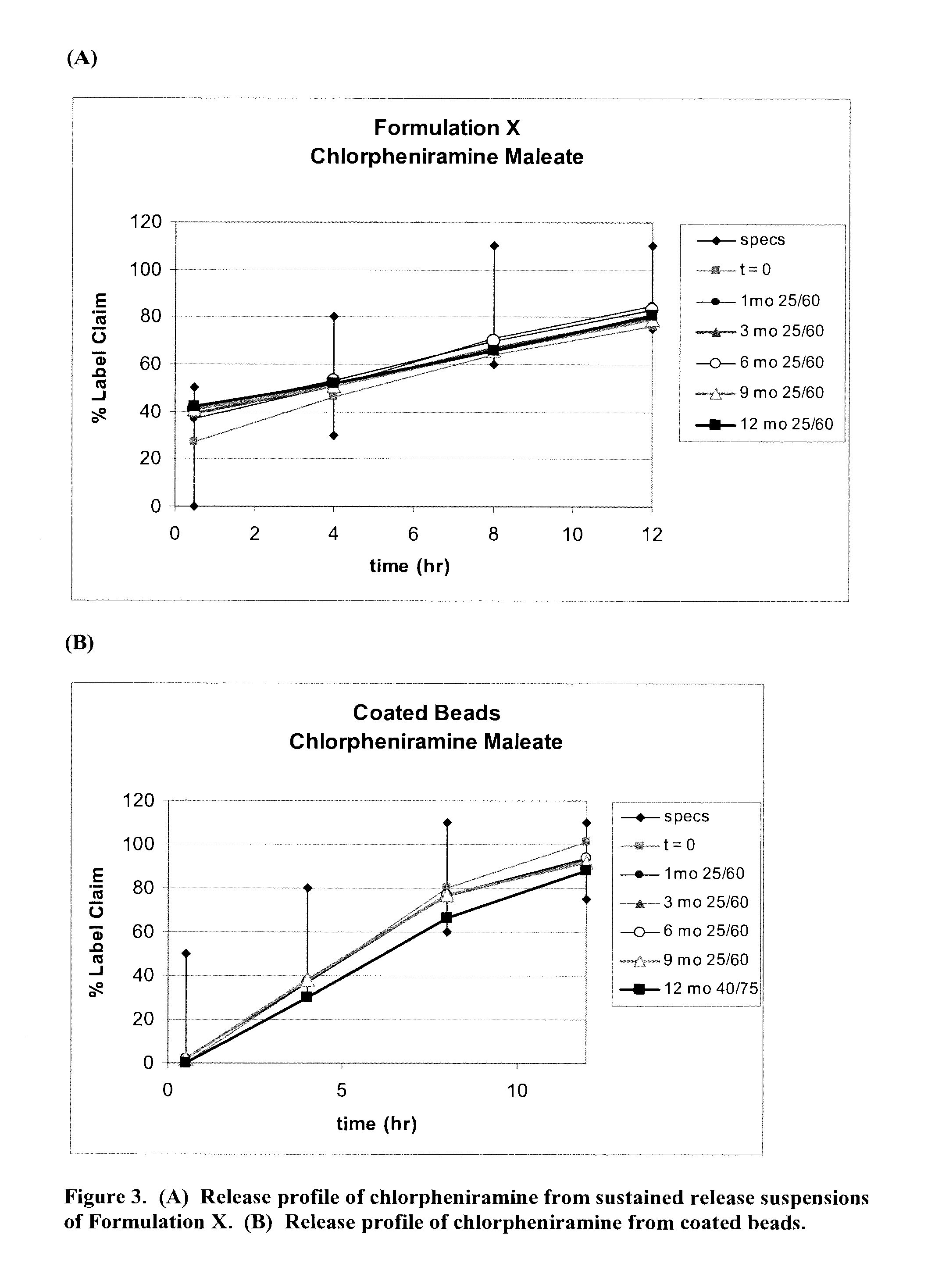
Patents
Literature
104 results about "Extended Release Formulations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
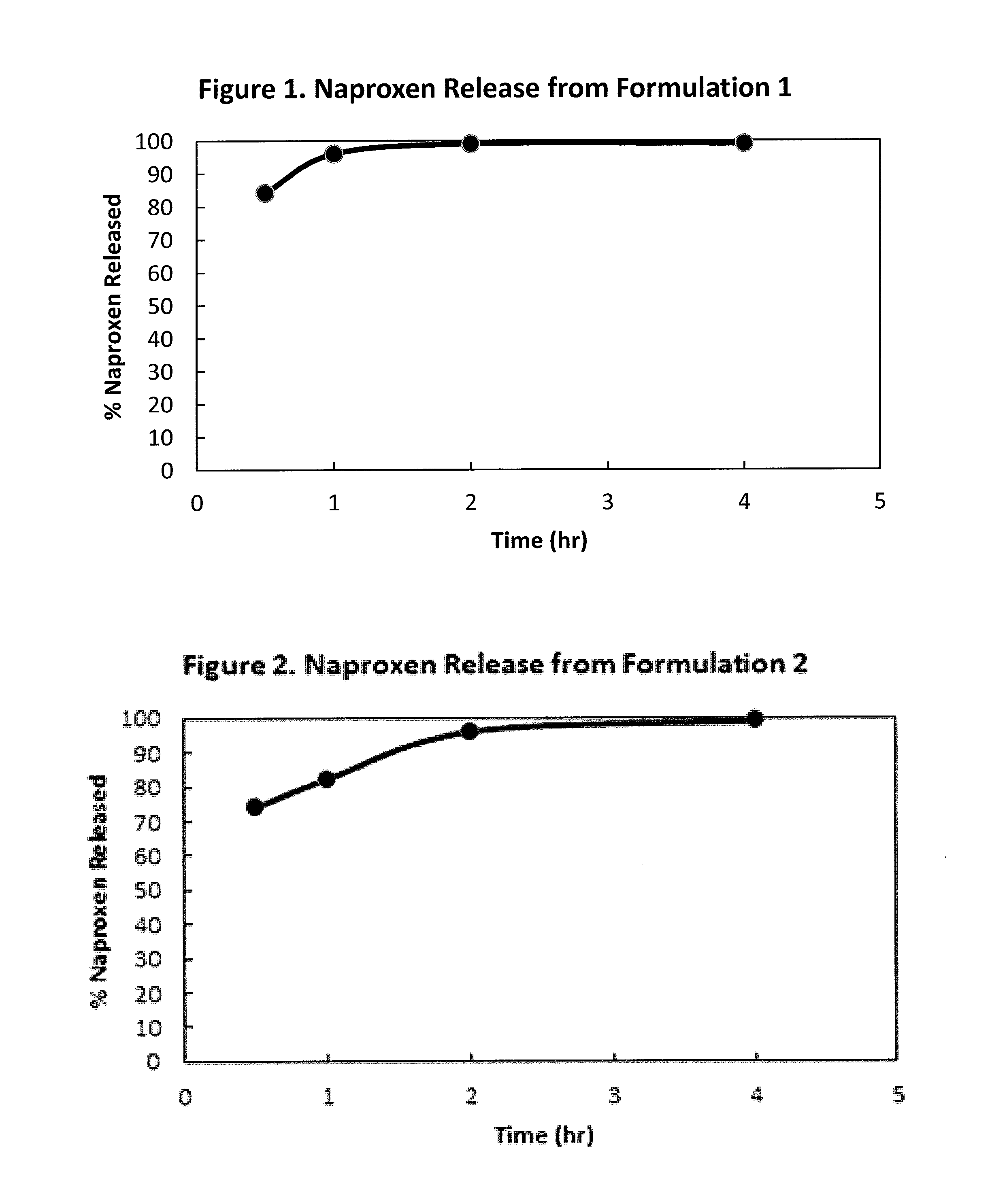
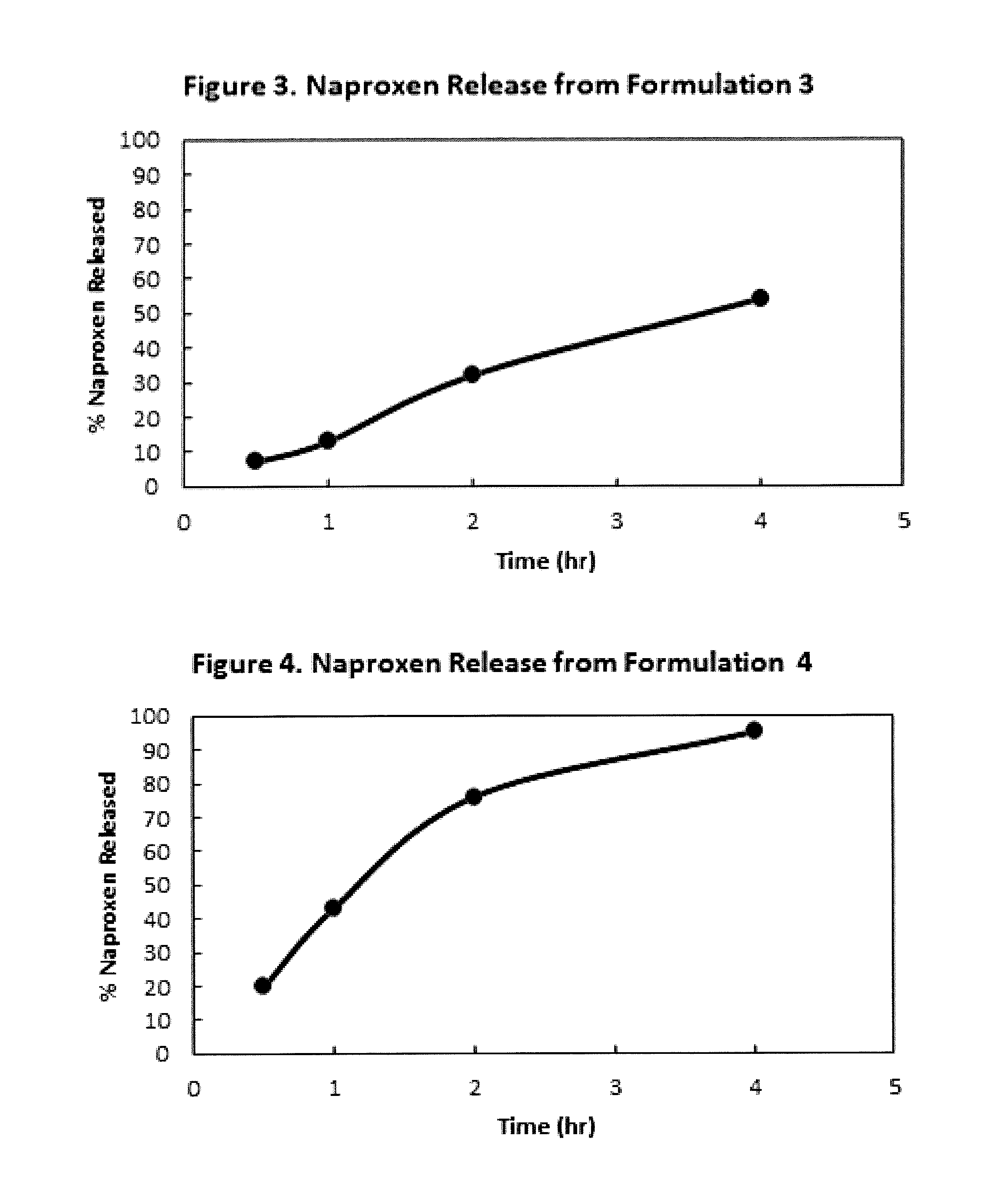
Extended-release formulations are available for several AEDs, and their number is increasing steadily. This article will appraise the scientific rationale for and discuss the value of extended-release AED formulations in the management of seizure disorders.
Abuse Resistant and Extended Release Formulations and Method of Use Thereof
InactiveUS20090082466A1Reducing solvent extraction efficiencyReduce filtration efficiencyBiocidePharmaceutical non-active ingredientsOpioid abuseChemical toxicity
The present invention is in the field of oral, abuse resistant pharmaceutical compositions of opioids, extended release pharmaceutical compositions of opioids and extended release abuse resistant pharmaceutical compositions of opioids and the use thereof for the treatment of pain. The present invention is also directed to extended release pharmaceutical compositions and the use thereof for preventing or minimizing the risk of opioid abuse and / or opioid toxicity from either intentional or unintentional tampering. The present invention is further directed at a method of preventing or minimizing the risk of opioid abuse and / or opioid toxicity from either intentional or unintentional tampering.
Owner:RELMADA THERAPEUTICS
Extended release formulations of erythromycin derivatives
Disclosed is a pharmaceutical composition for extended release of an erythromycin derivative in the gastrointestinal environment. The composition comprises an erythromycin derivative and a pharmaceutically acceptable polymer so that, when ingested orally, the composition induces statistically significantly lower Cmax in the plasma than an immediate release composition of the erythromycin derivative while maintaining bioavailability and minimum concentration substantially equivalent to that of the immediate release composition of the erythromycin derivative upon multiple dosing. The compositions of the invention have an improved taste profile and reduced gastrointestinal side effects as compared to those for the immediate release composition.
Owner:ABBVIE INC
Tranexamic acid formulations with reduced adverse effects
InactiveUS20090017114A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePowder deliveryIntestinal structureSide effect
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:FERRING BV
Methods for Administering Aripiprazole
ActiveUS20090143403A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:OTSUKA PHARM CO LTD
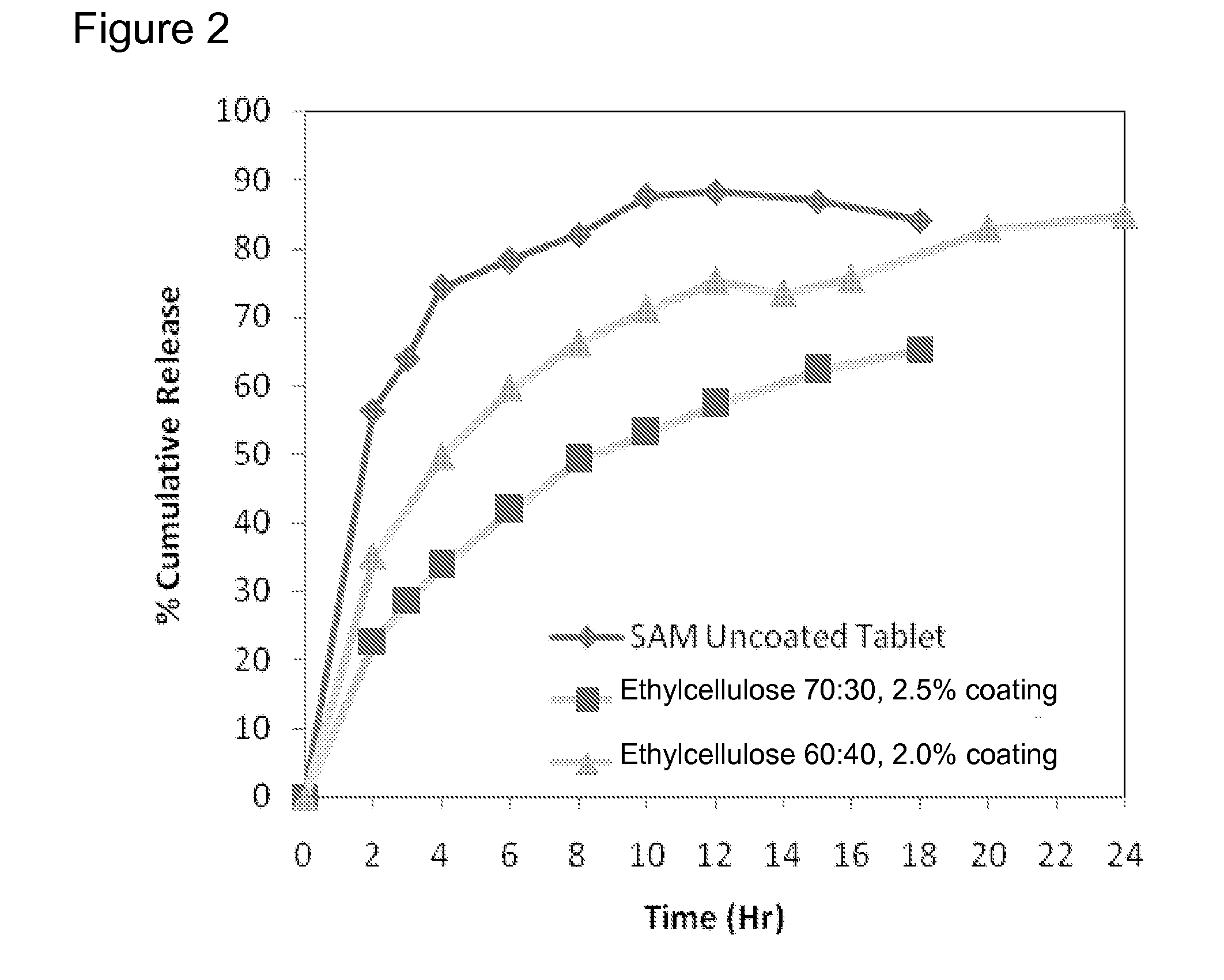
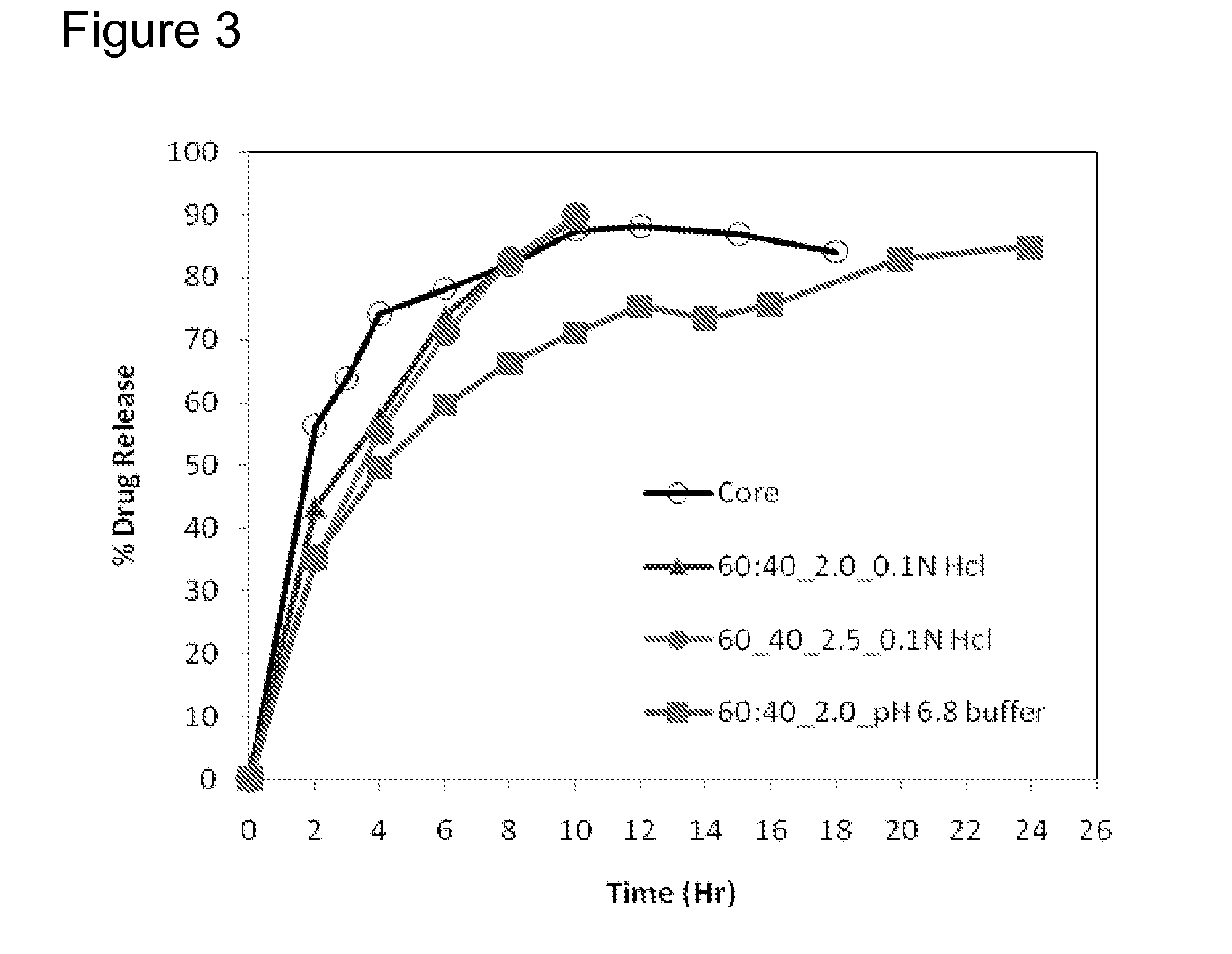
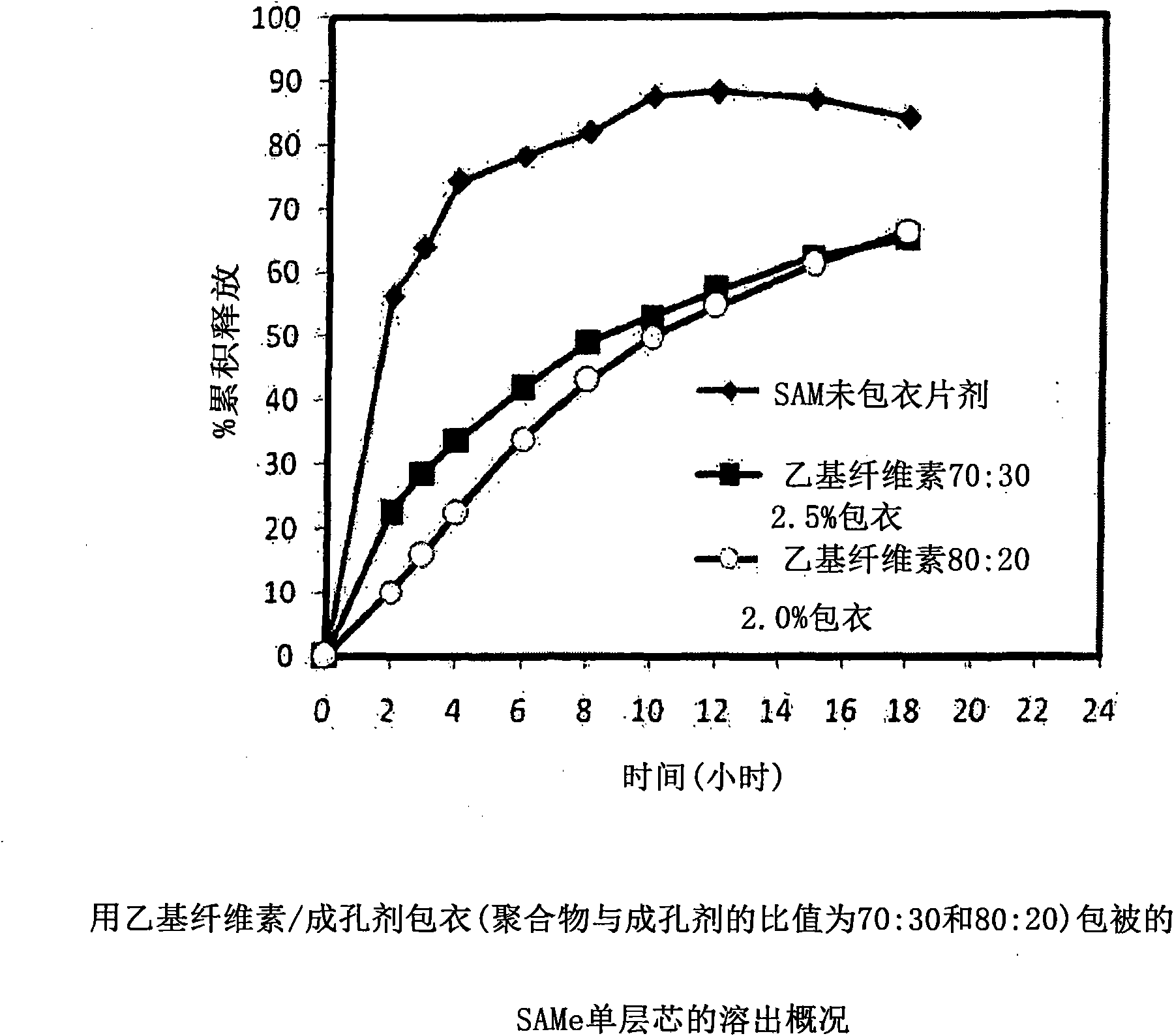
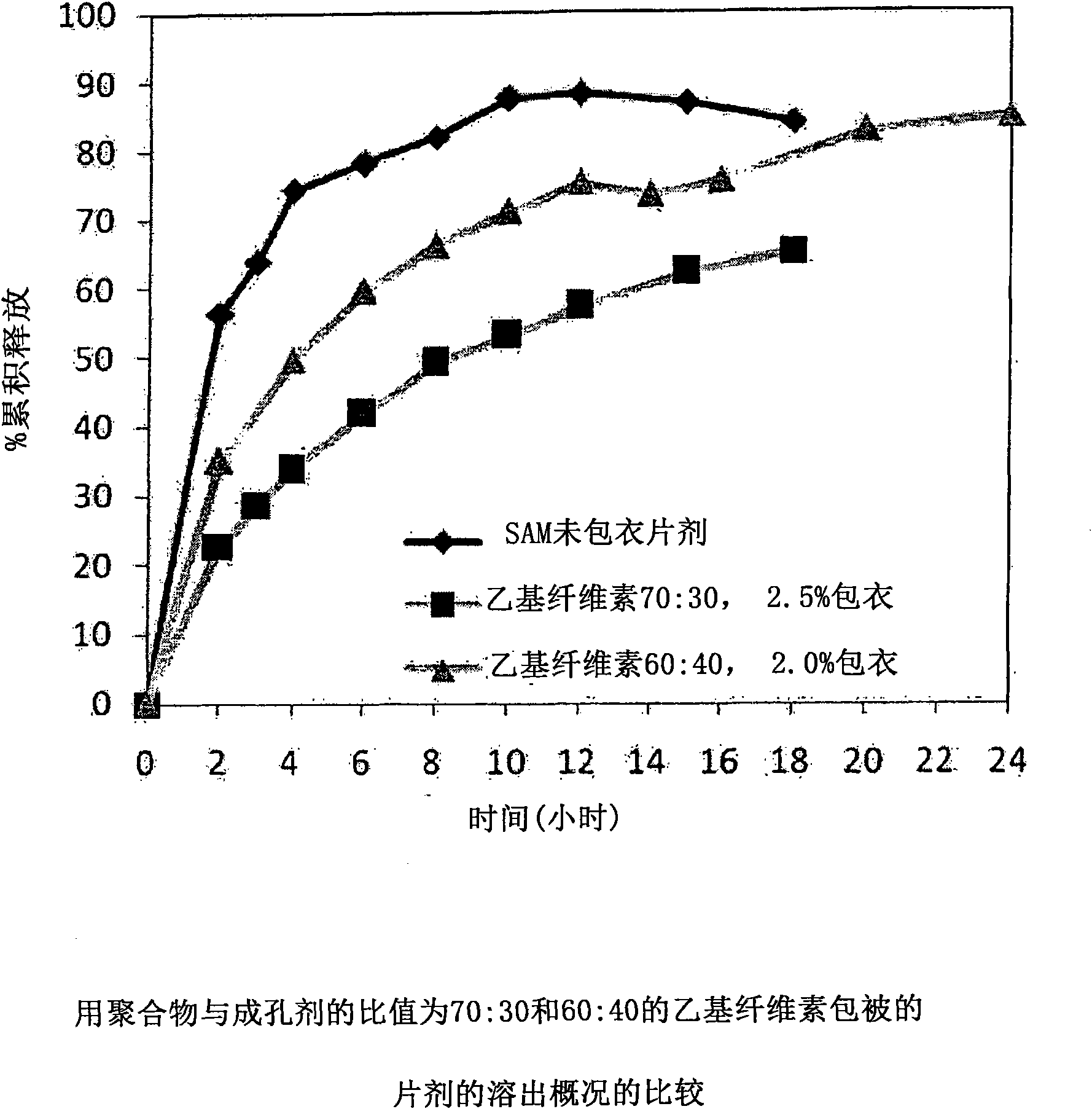
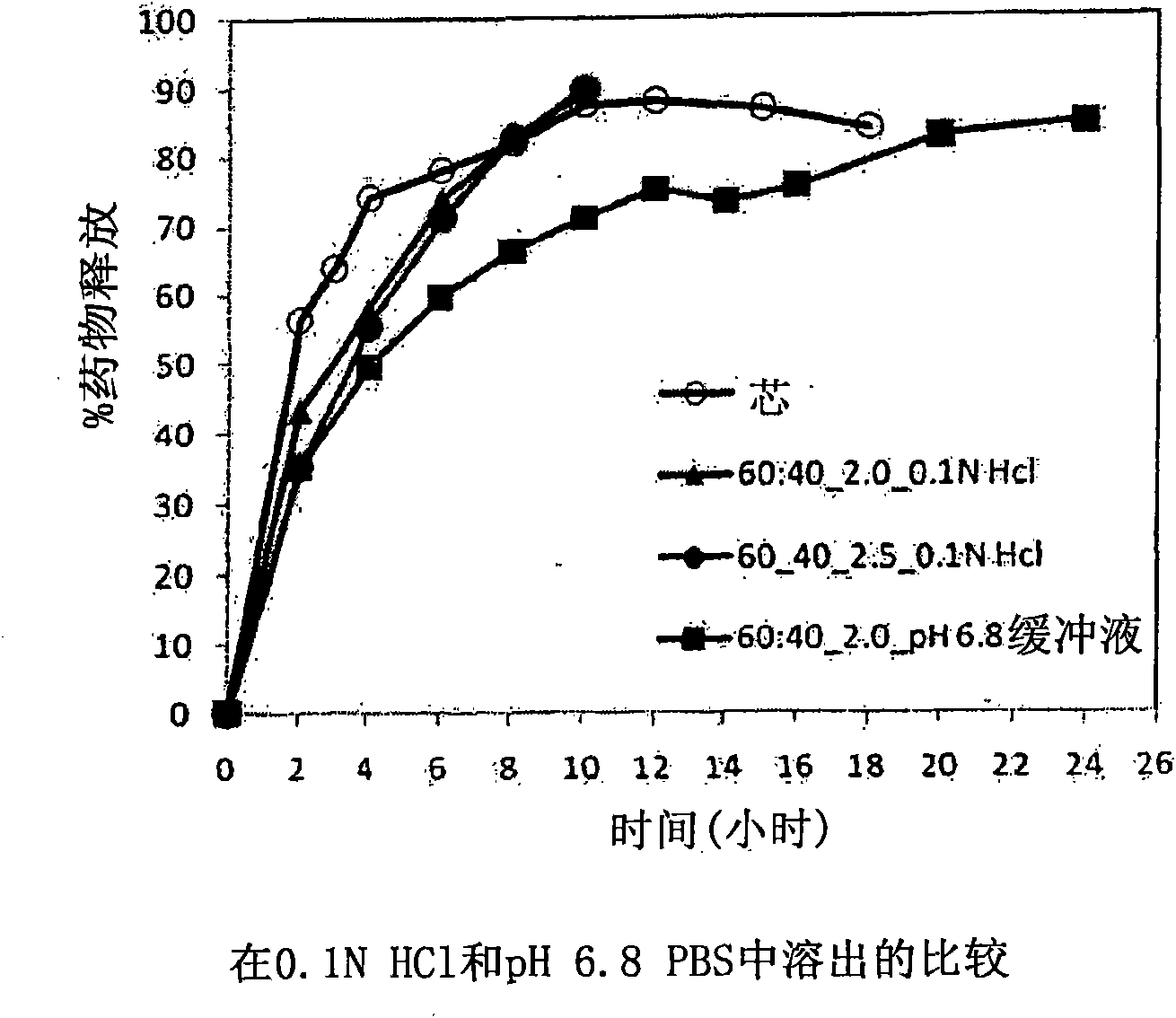
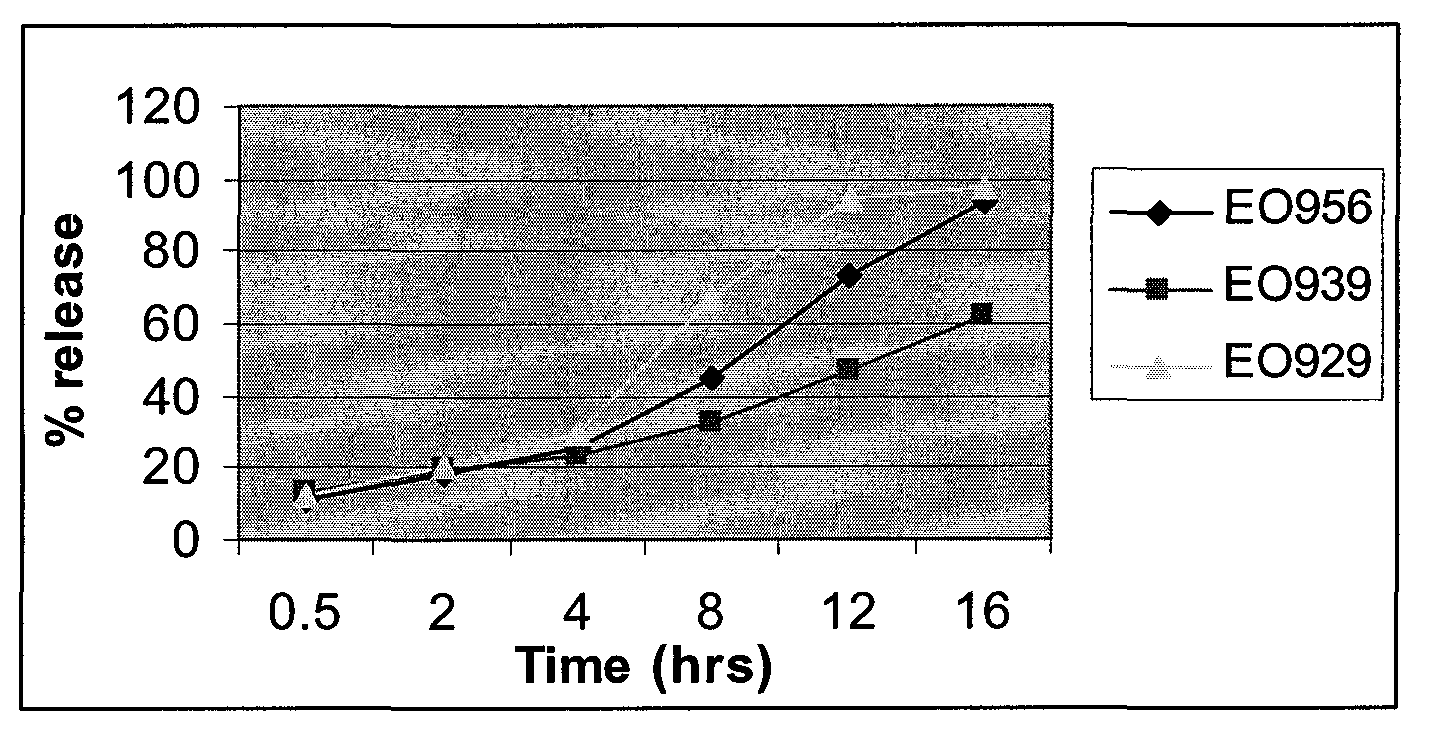
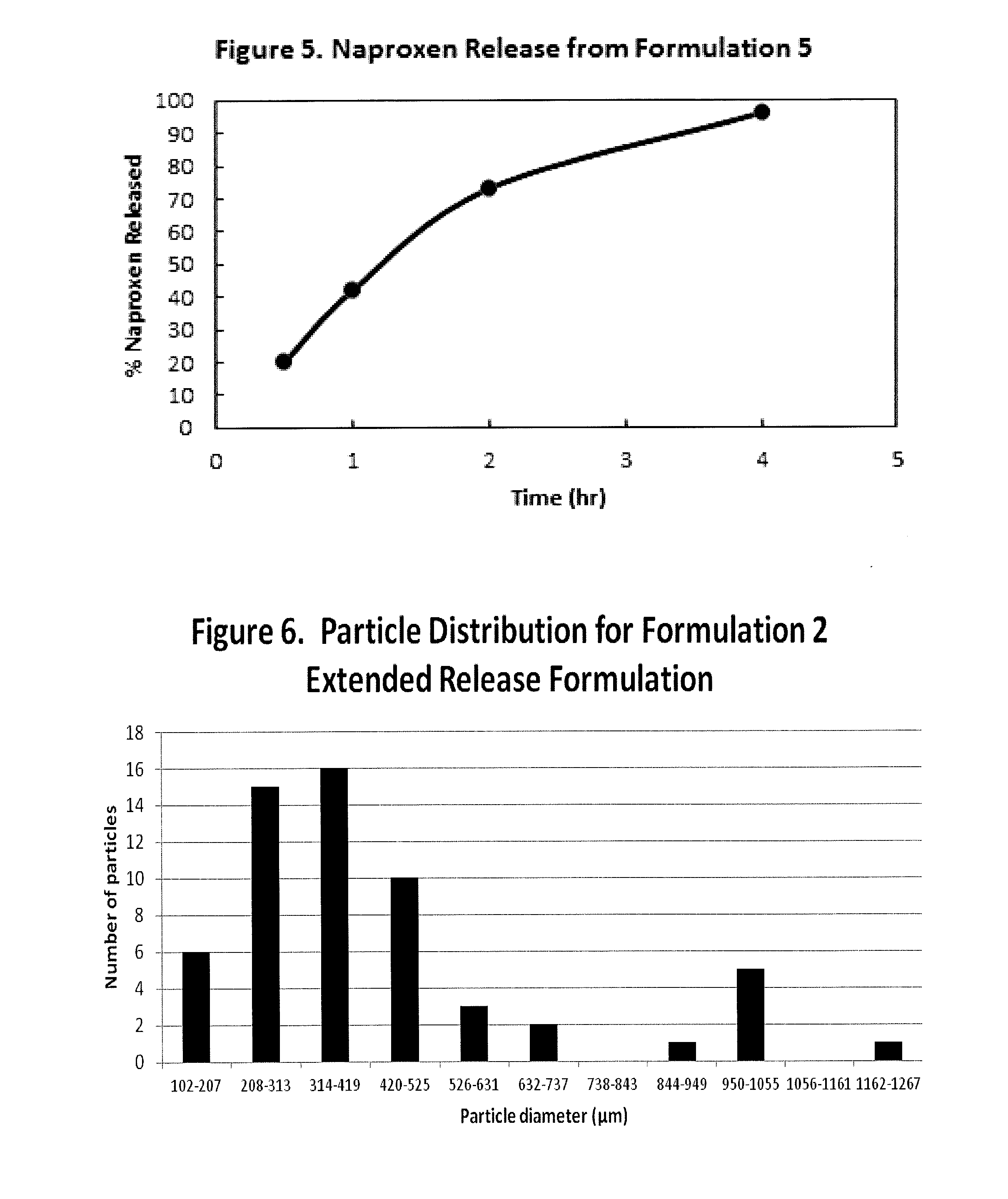
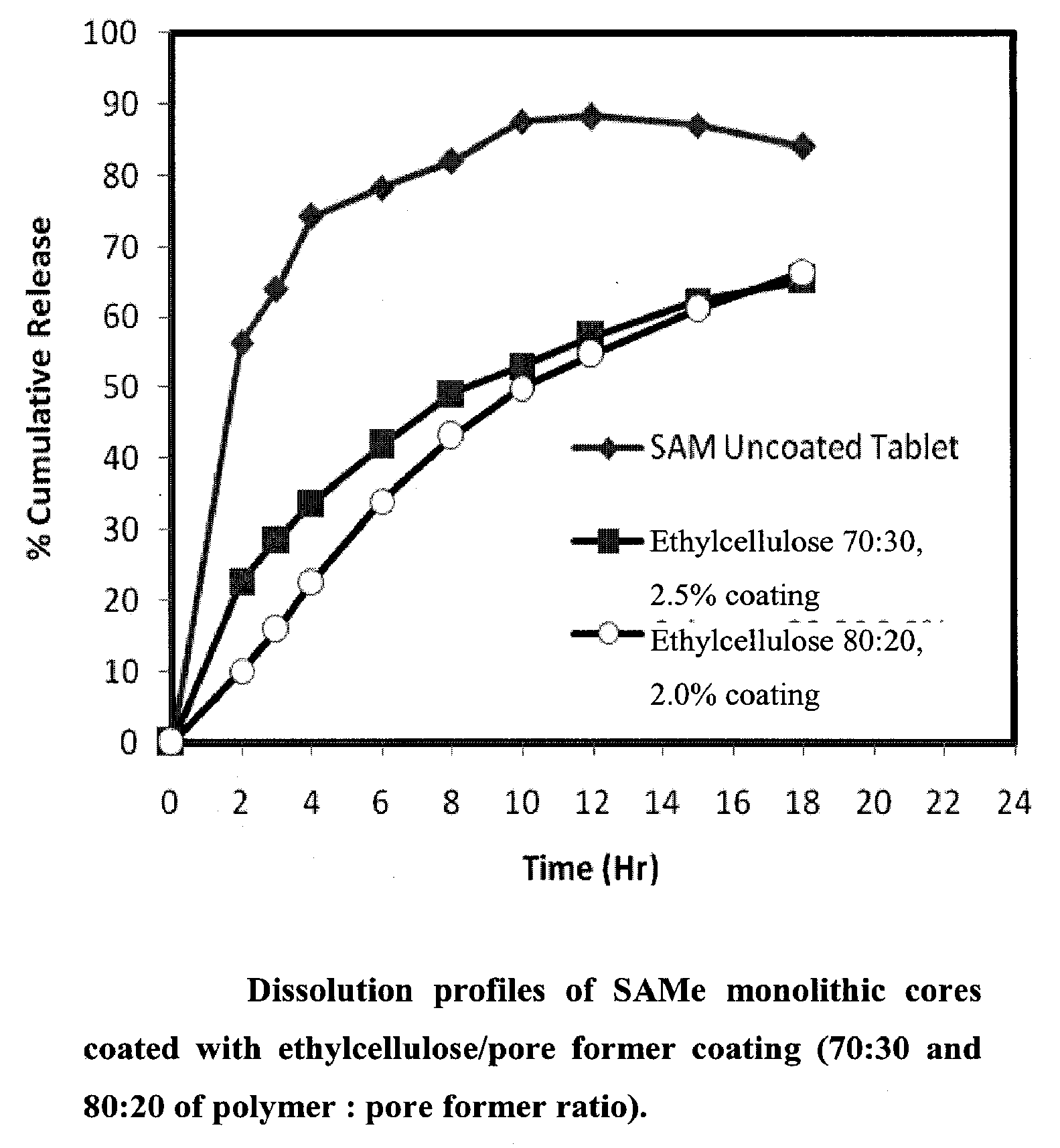
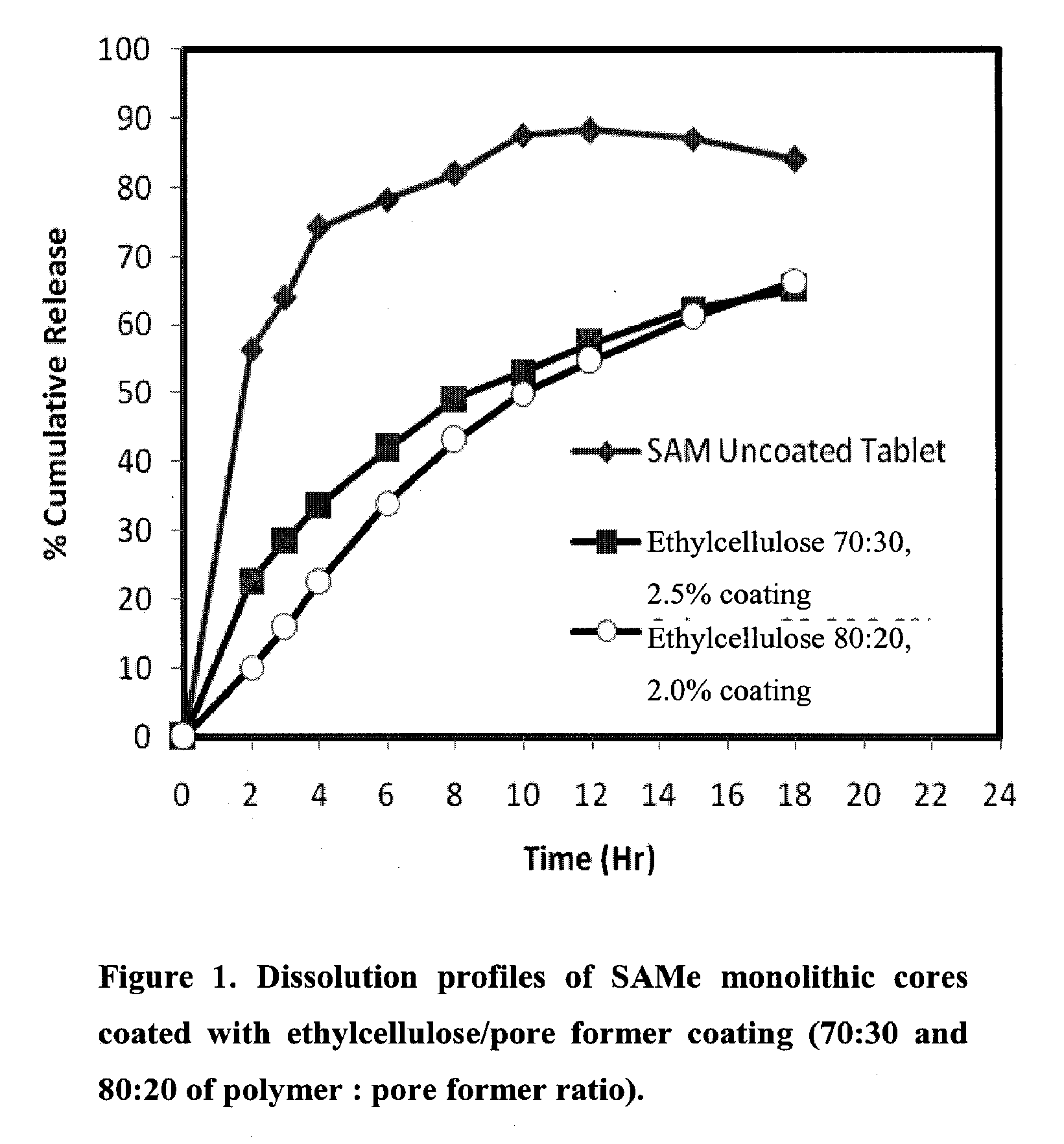
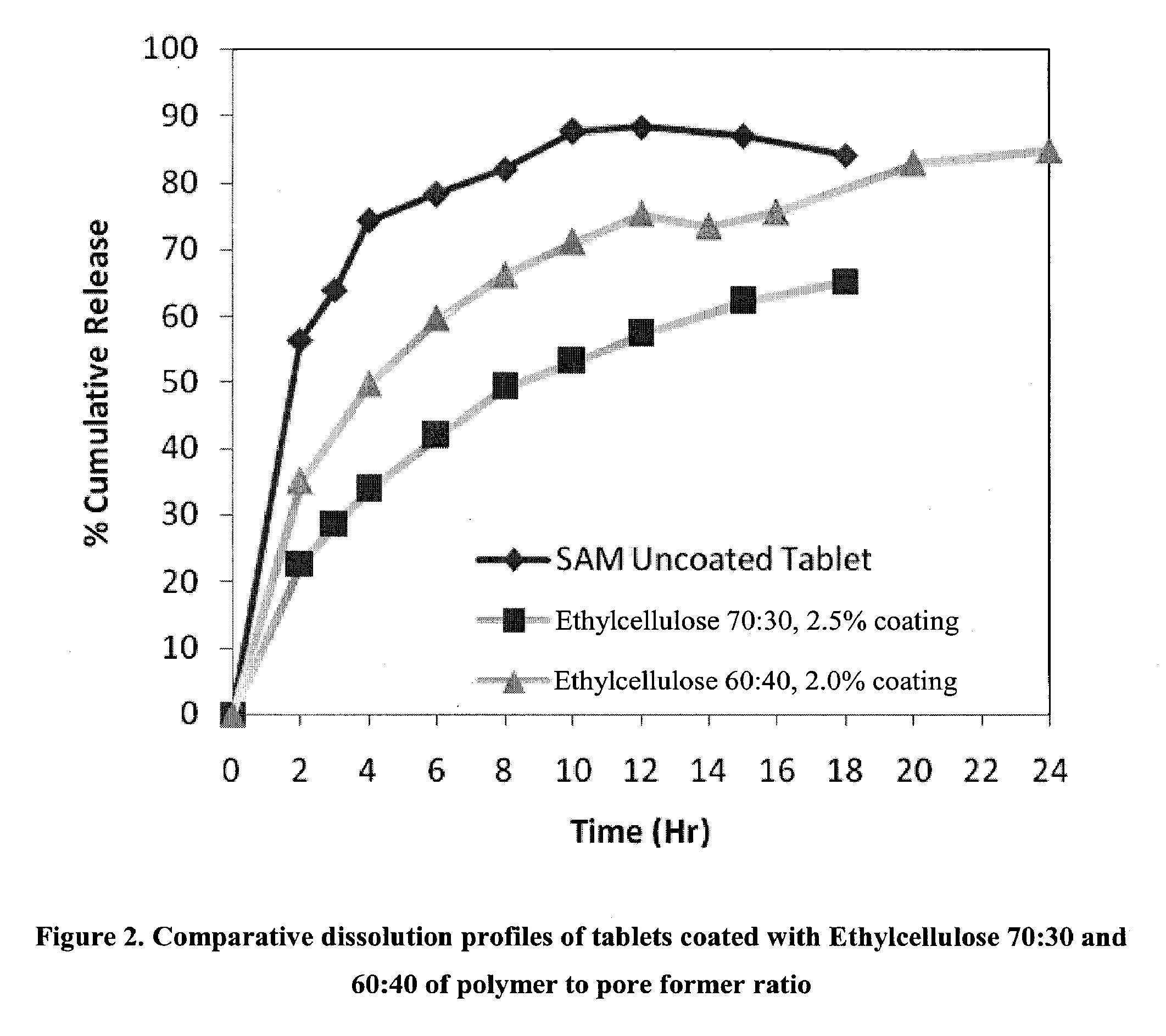
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
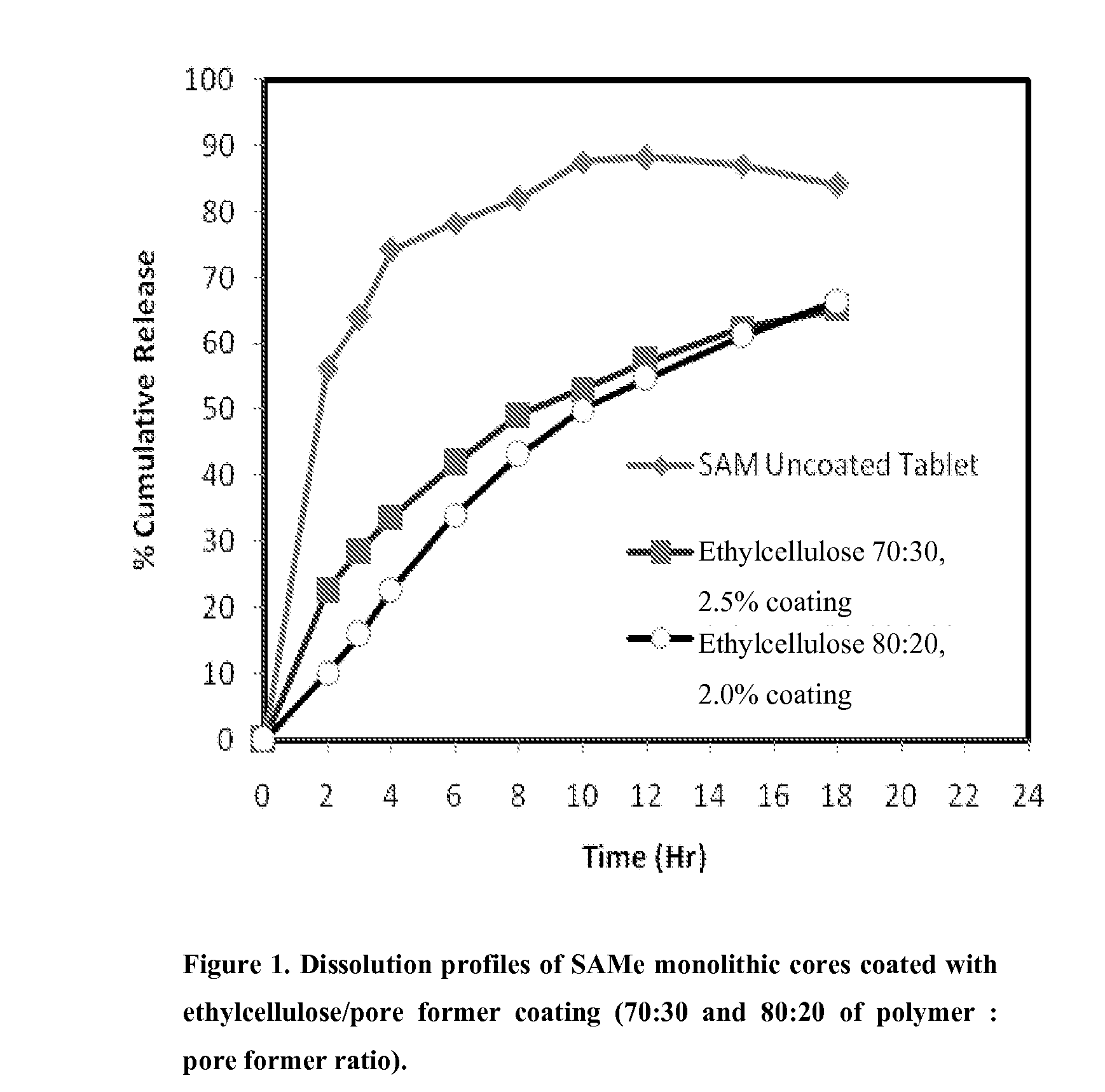
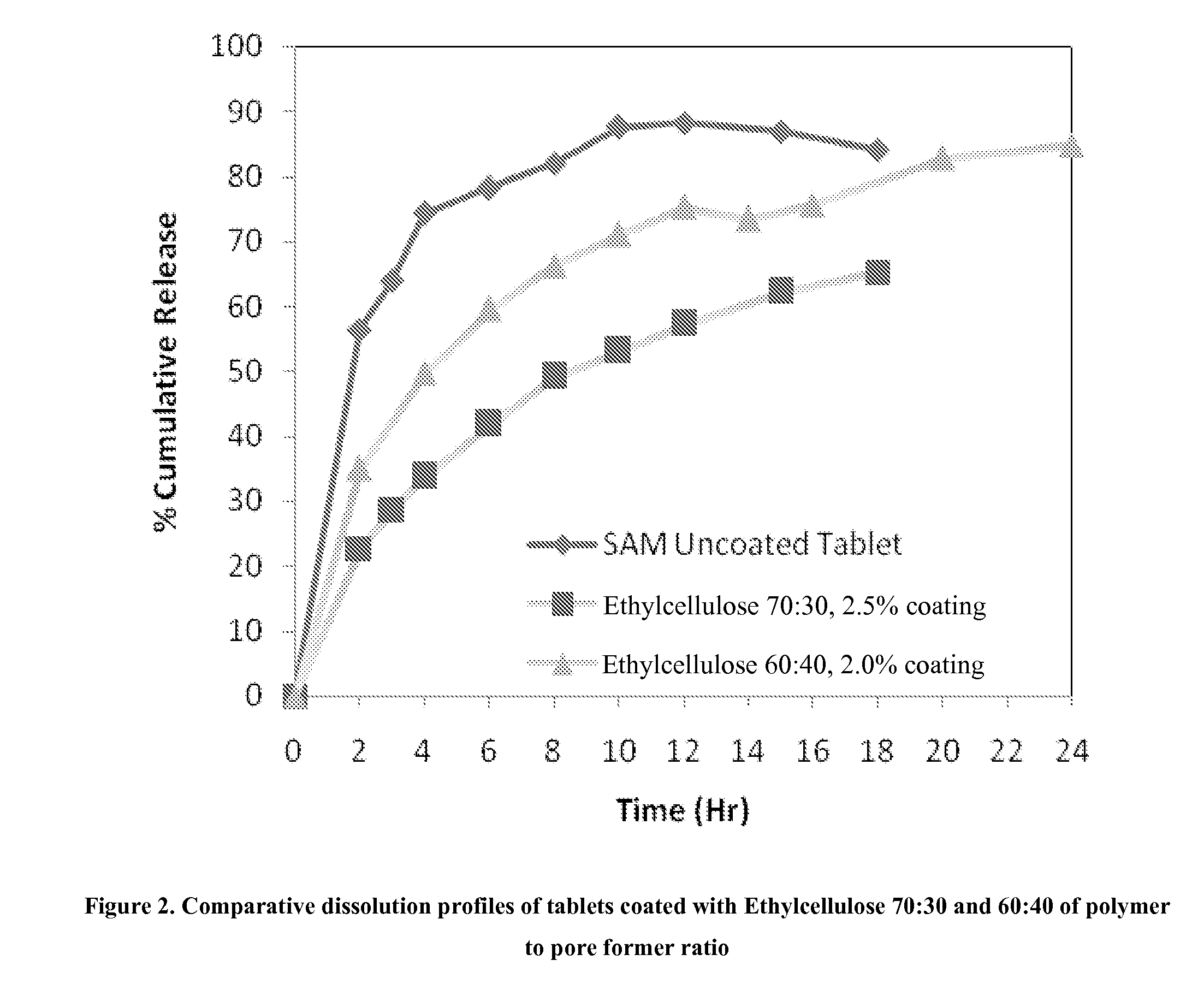
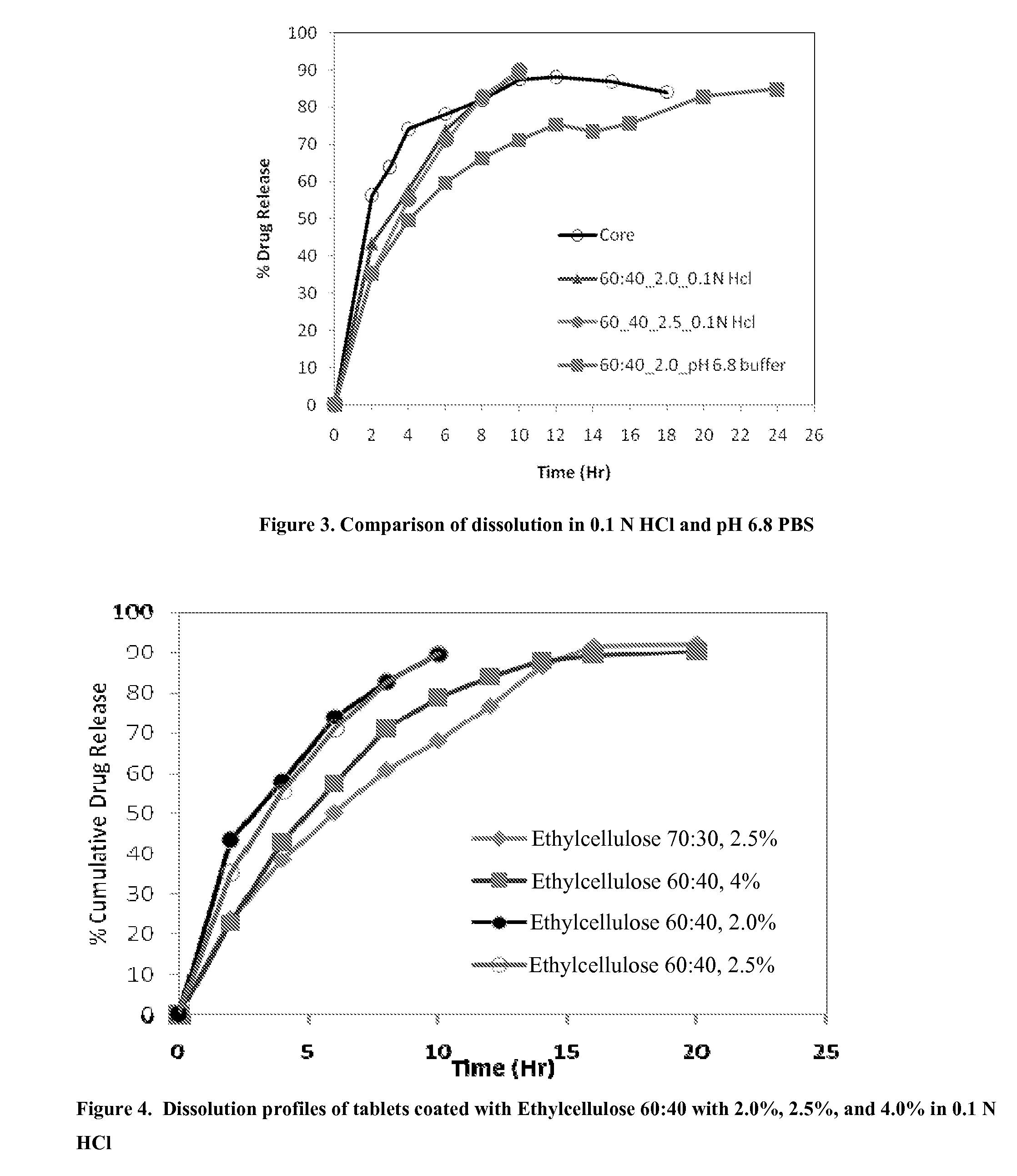
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Tranexamic acid formulations with reduced adverse effects
InactiveUS20050025825A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocideOrganic active ingredientsIntestinal structureNausea sickness
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:XANODYNE PHARMACEUTICALS INC +1
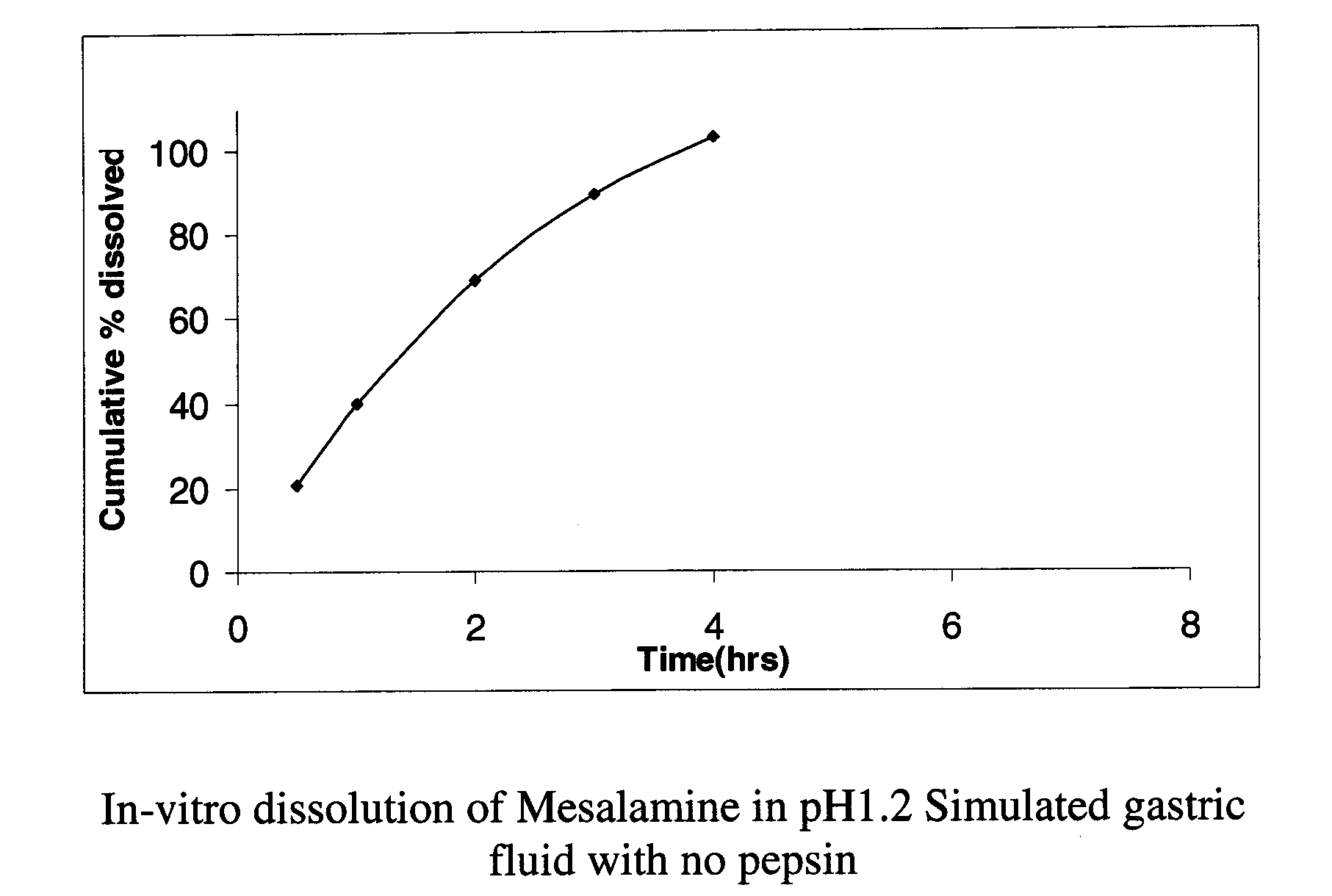
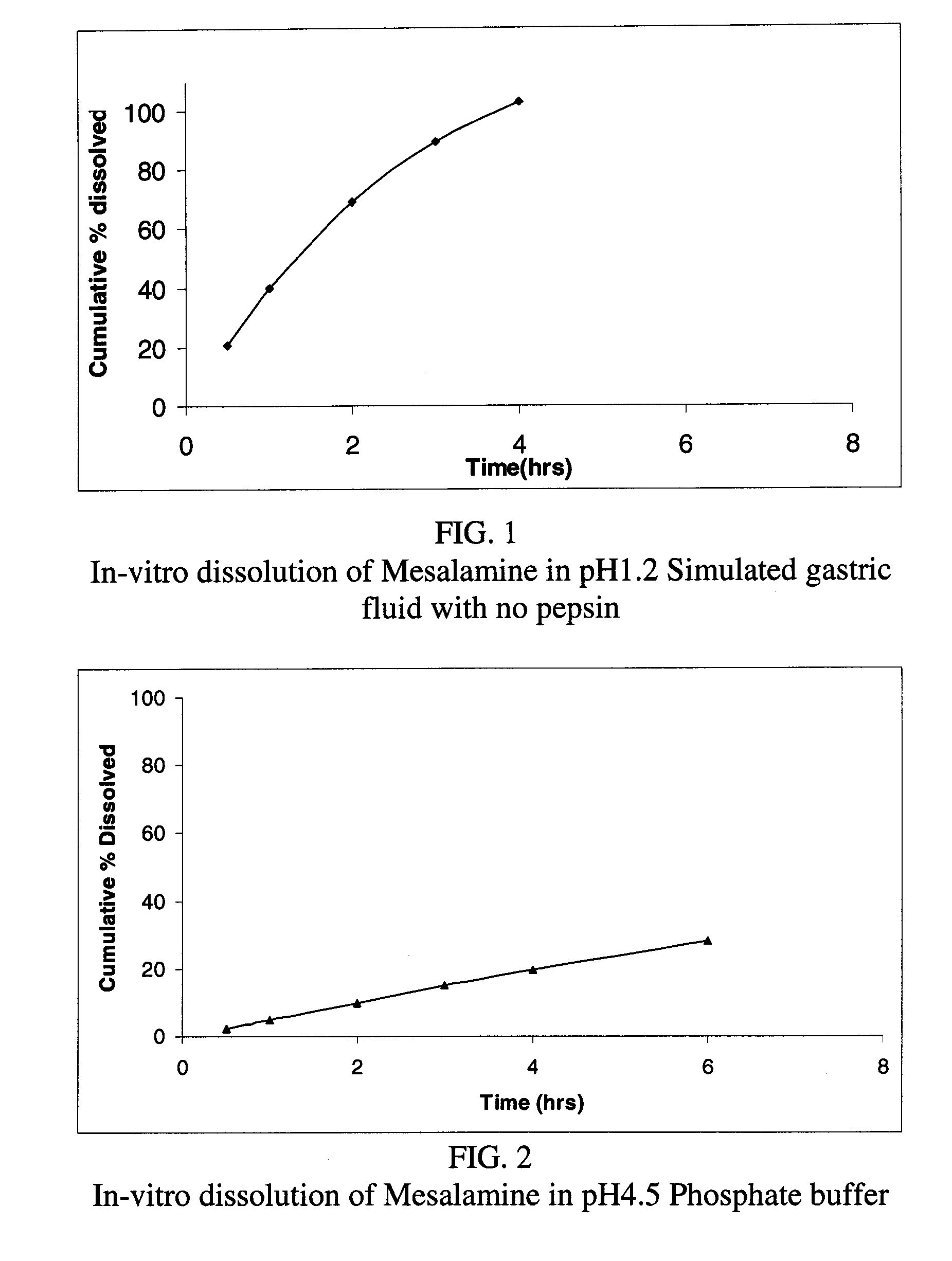
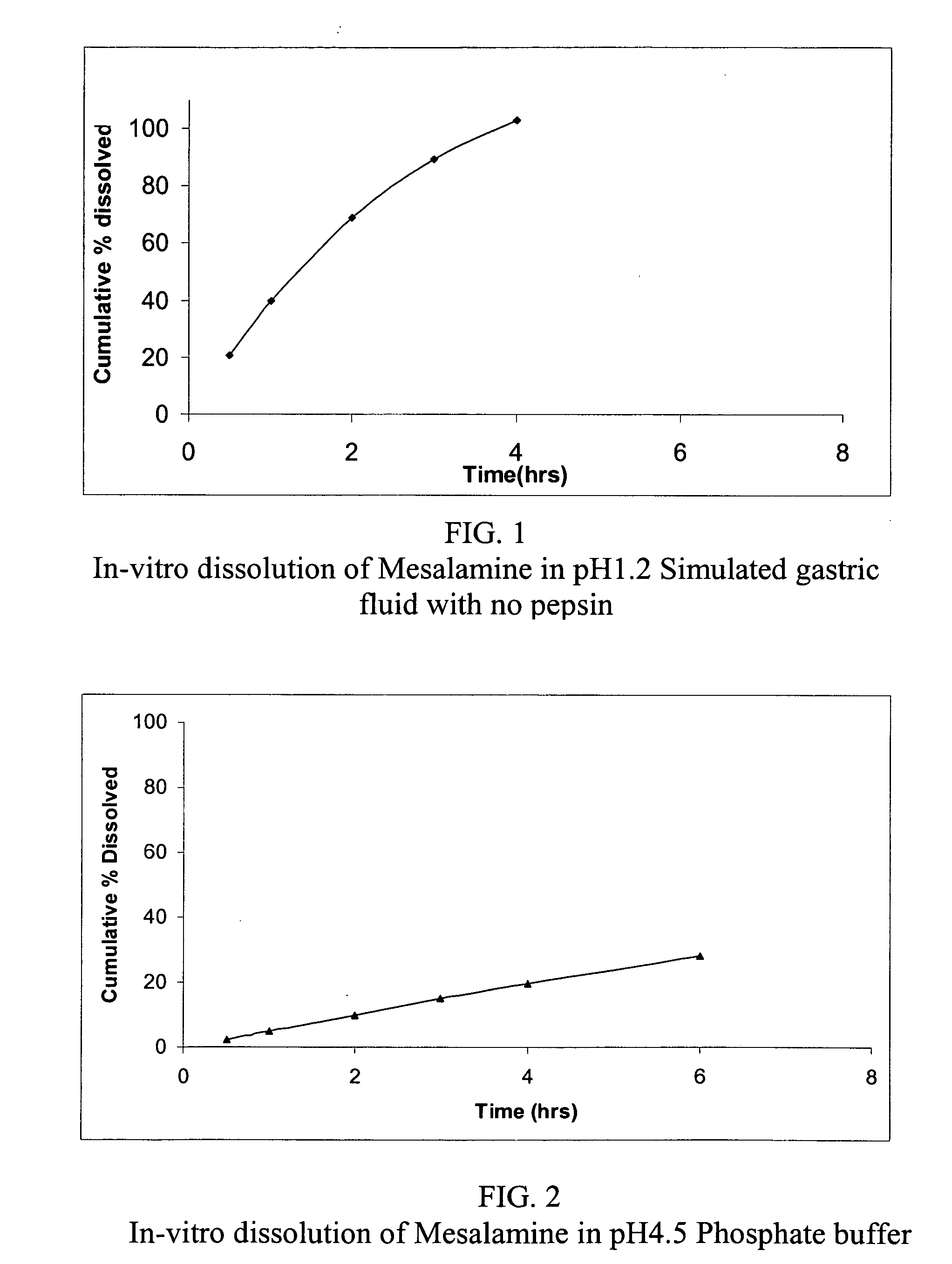
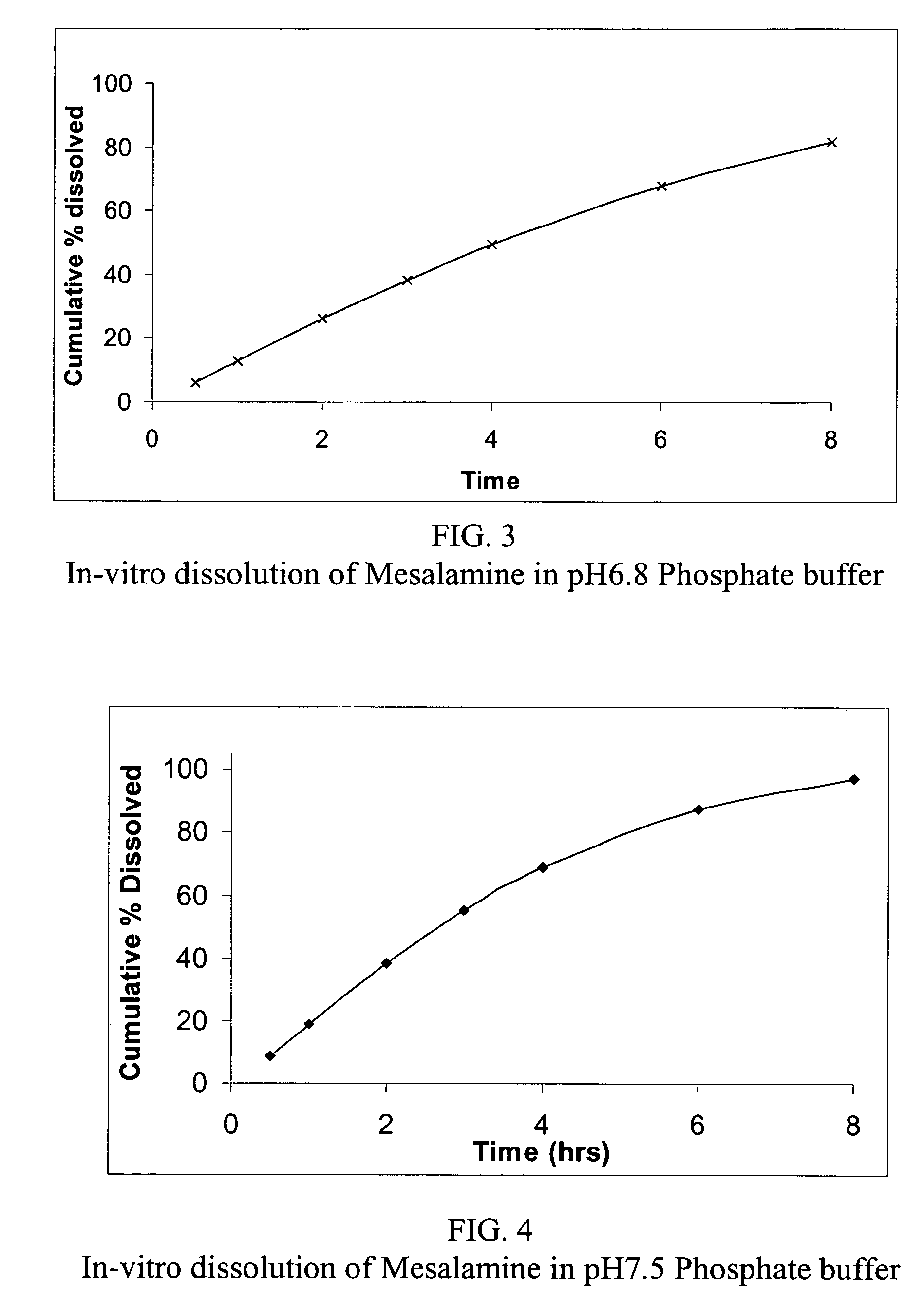
Modified release formulations of Anti-irritability drugs
InactiveUS20090017110A1Provide flexibilityBiocideOrganic active ingredientsFda approvalAnti allergic drug
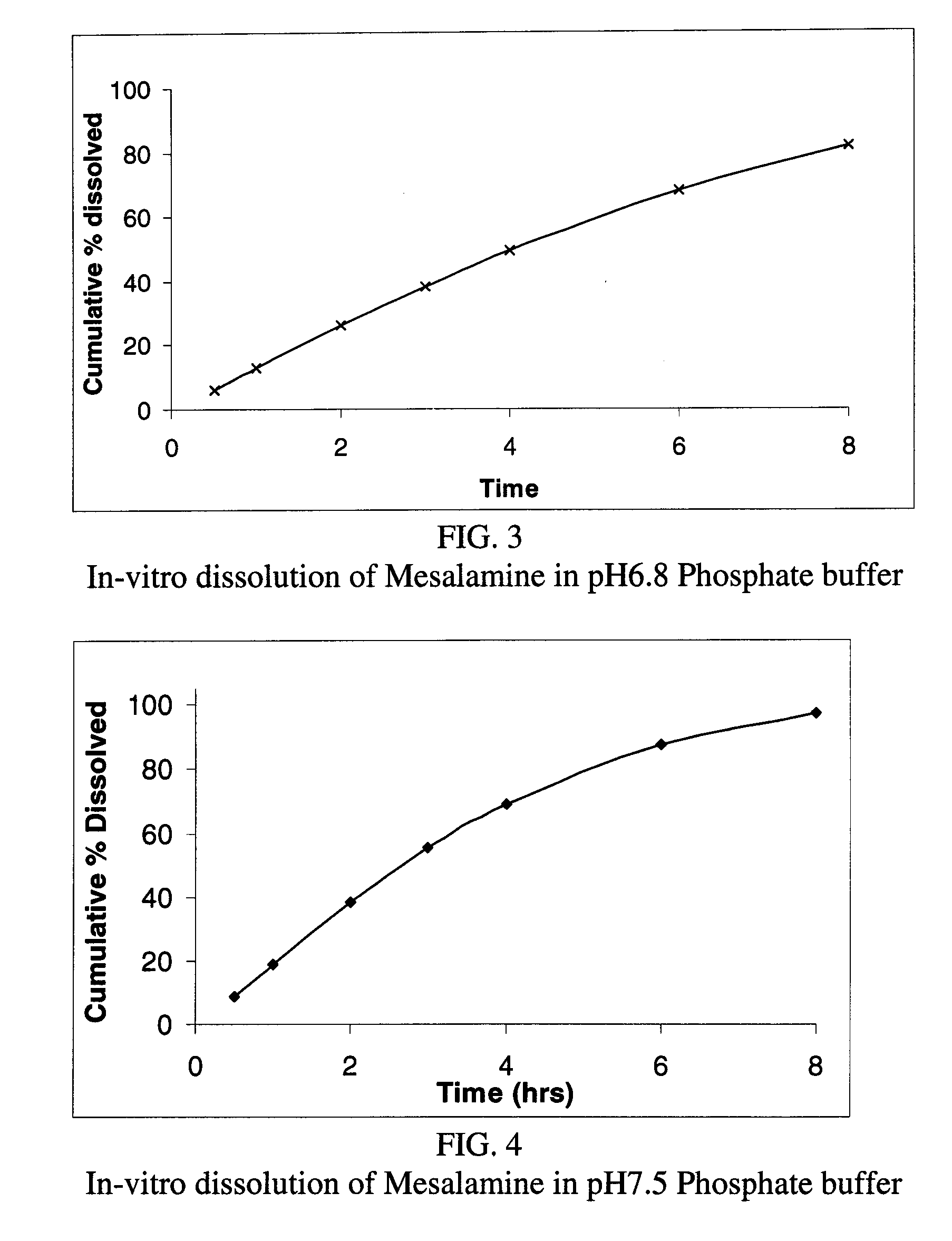
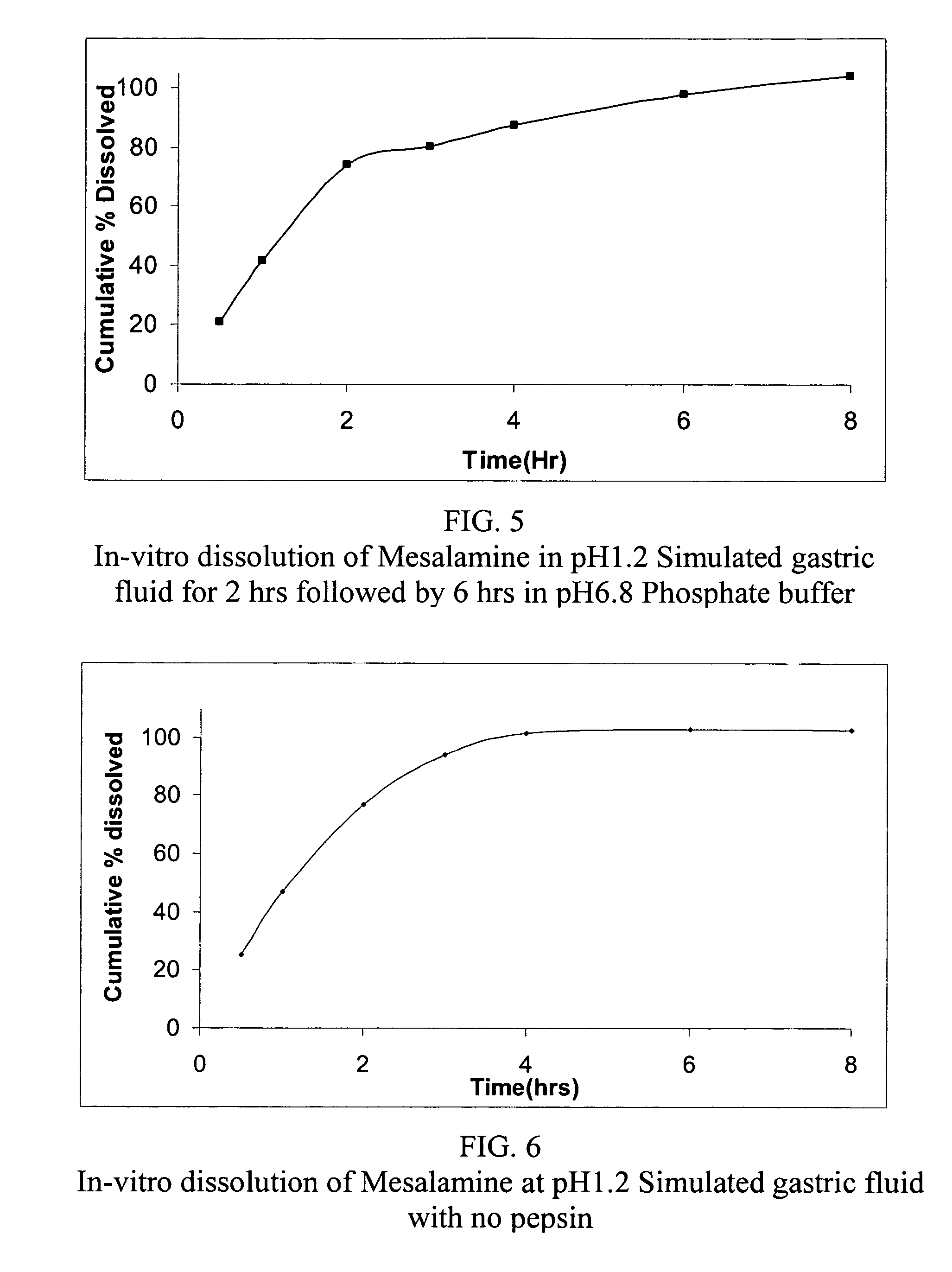
Modified or extended release formulations containing mesalamine compounds and associated methods are disclosed and described. In some aspects, such formulations may be substantially bioequivalent to known FDA approved mesalamine formulations such as PENTASA®.
Owner:CAPRICORN PHARMA INC
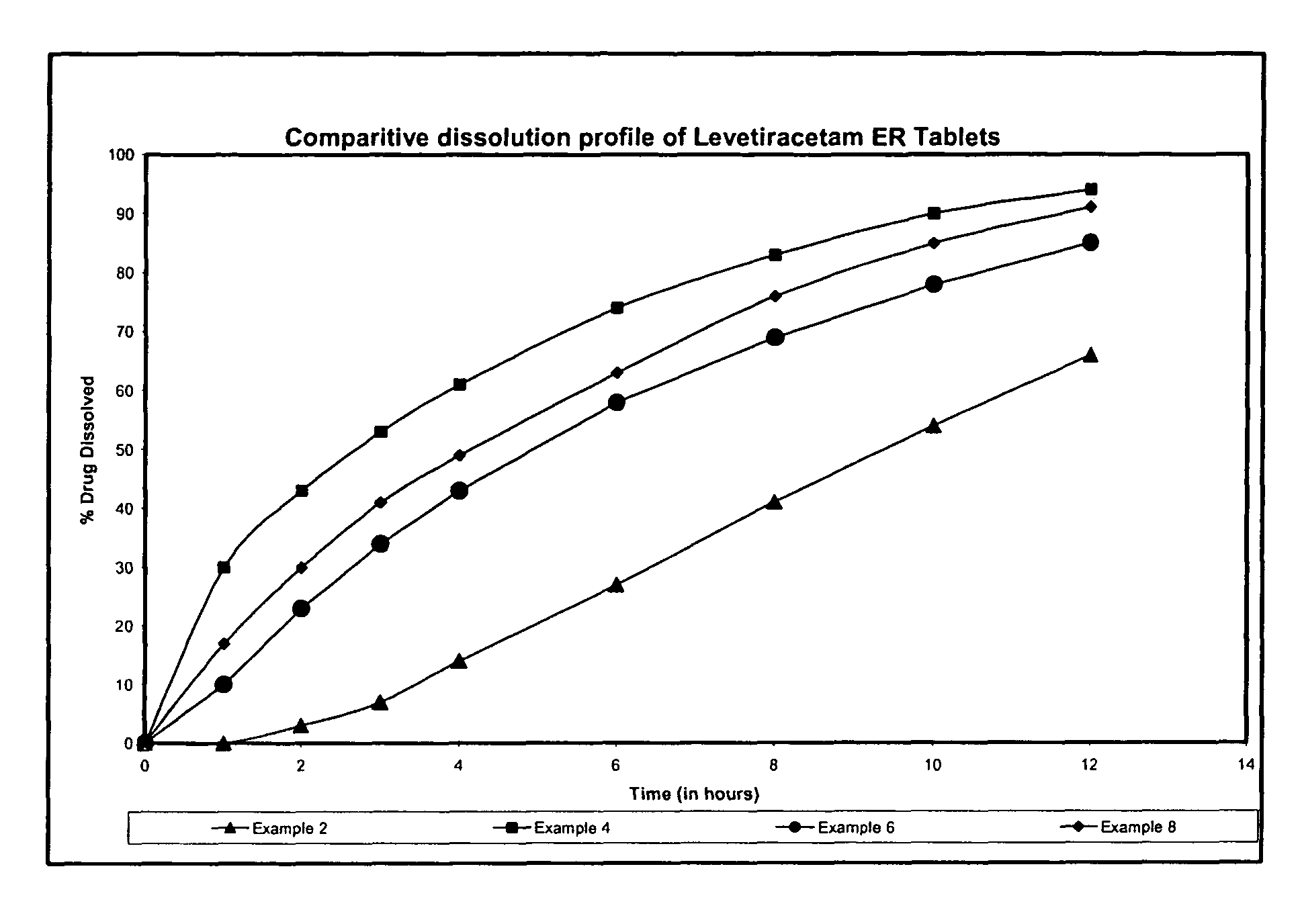
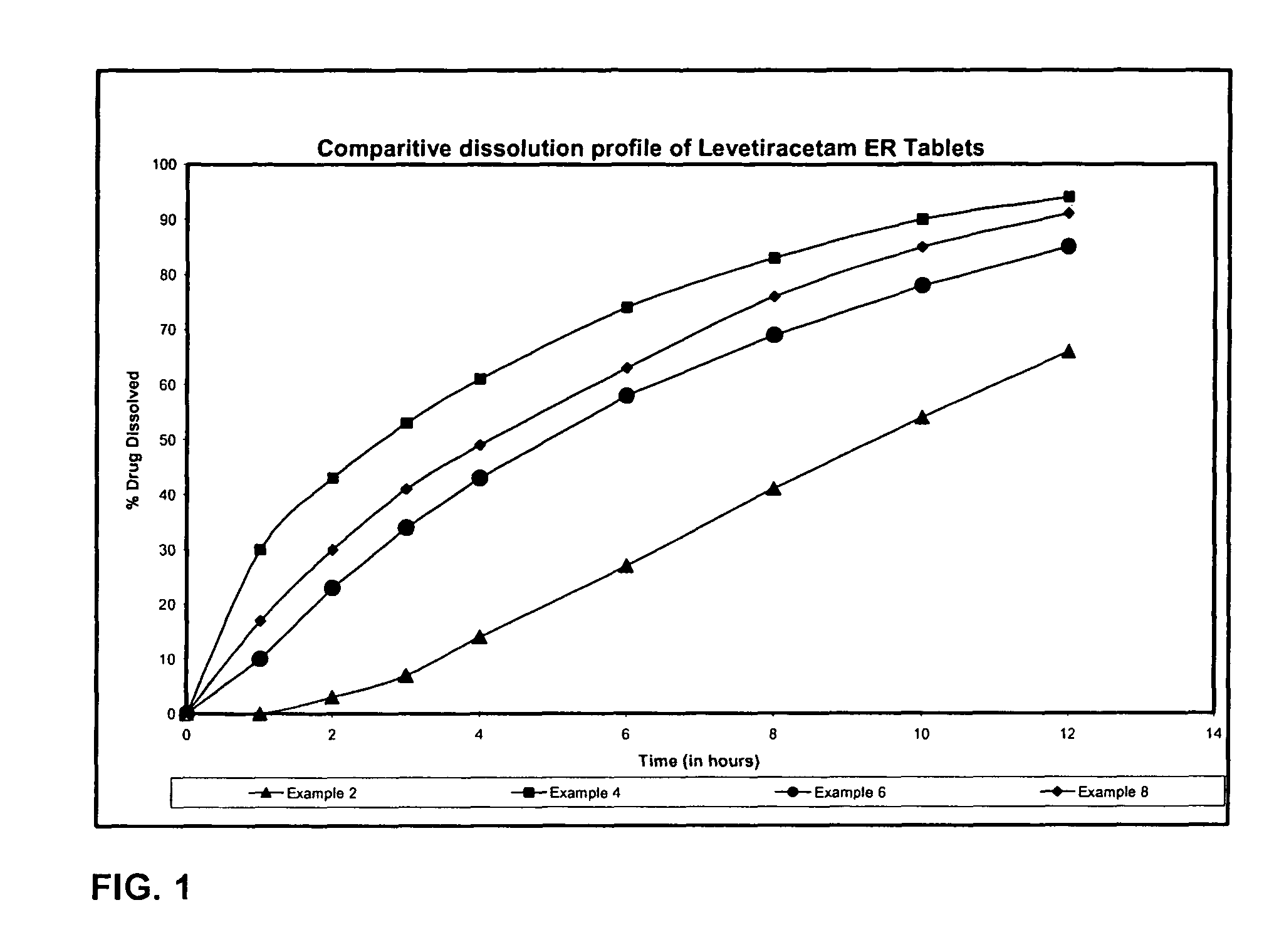
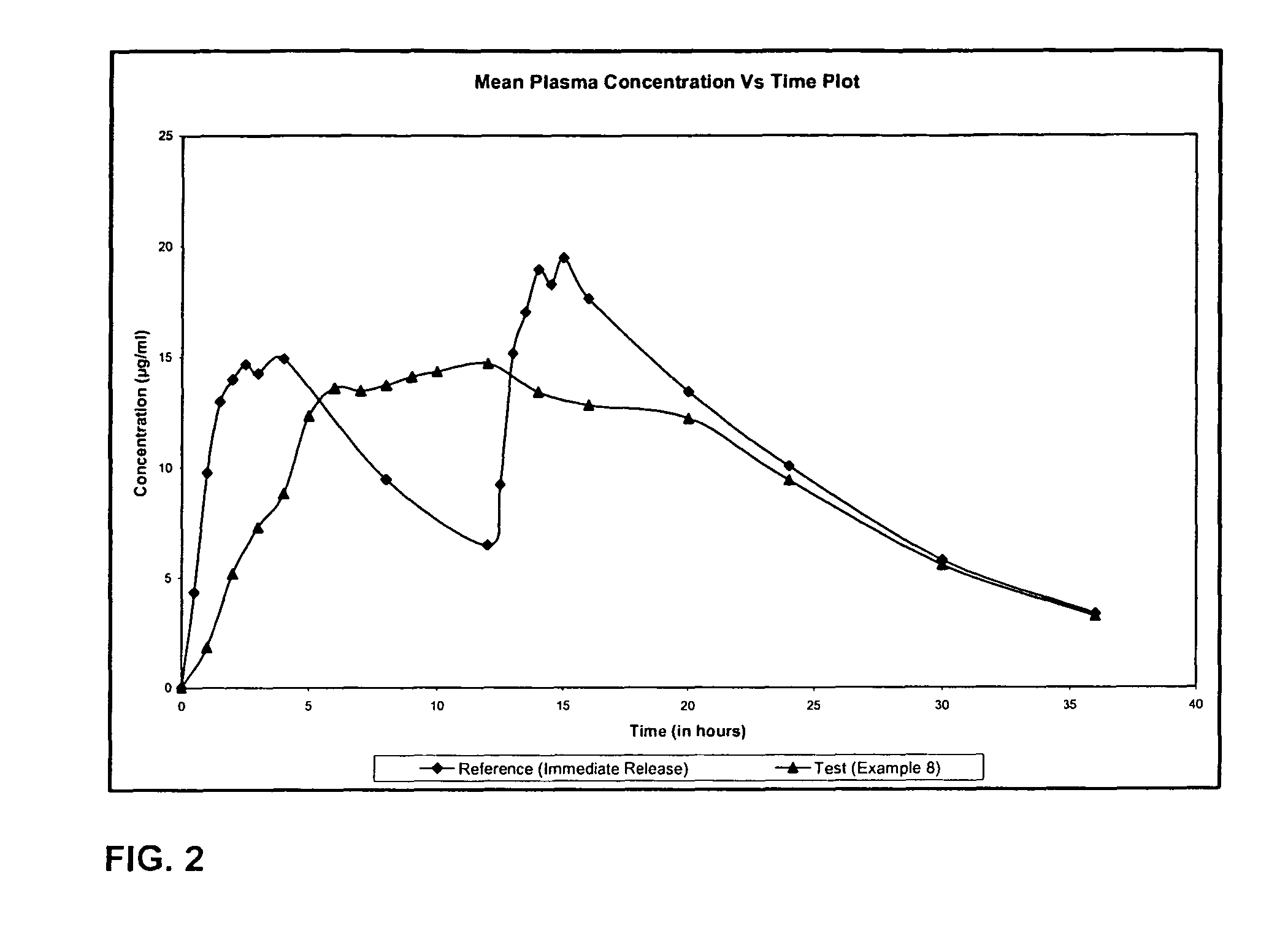
Extended release formulation of Levetiracetam
ActiveUS7863316B2Reduced inter subject variabilityBiocideNervous disorderWater dispersibleFOOD EFFECT
Owner:UCB PHARMA SA
Tranexamic acid formulations with reduced adverse effects
ActiveUS20060127476A1Minimize and eliminate undesirable gastrointestinal side effectMinimize and prevent dissolutionBiocidePeptide/protein ingredientsNausea sicknessPatient compliance
Tranexamic acid formulated in an oral dosage form with at least one agent that decreases tranexamic acid release in the stomach. Such formulations minimize nausea, vomiting, and other adverse gastric effects that may accompany tranexamic acid therapy, for example, to treat heavy menstrual bleeding. One embodiment is an extended release formulation with waxes, polymers, etc. that prevent a bolus release of tranexamic acid in the stomach. An alternative embodiment is a delayed release formulation with polymers that prevent release of tranexamic acid in the acid environment of the stomach and delay its release until the formulation reaches the less acid environment of the intestines. Such formulations enhance patient compliance with therapy because adverse effects of tranexamic acid therapy are reduced.
Owner:AMRING PHARM INC
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090197824A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Extended Release Formulation
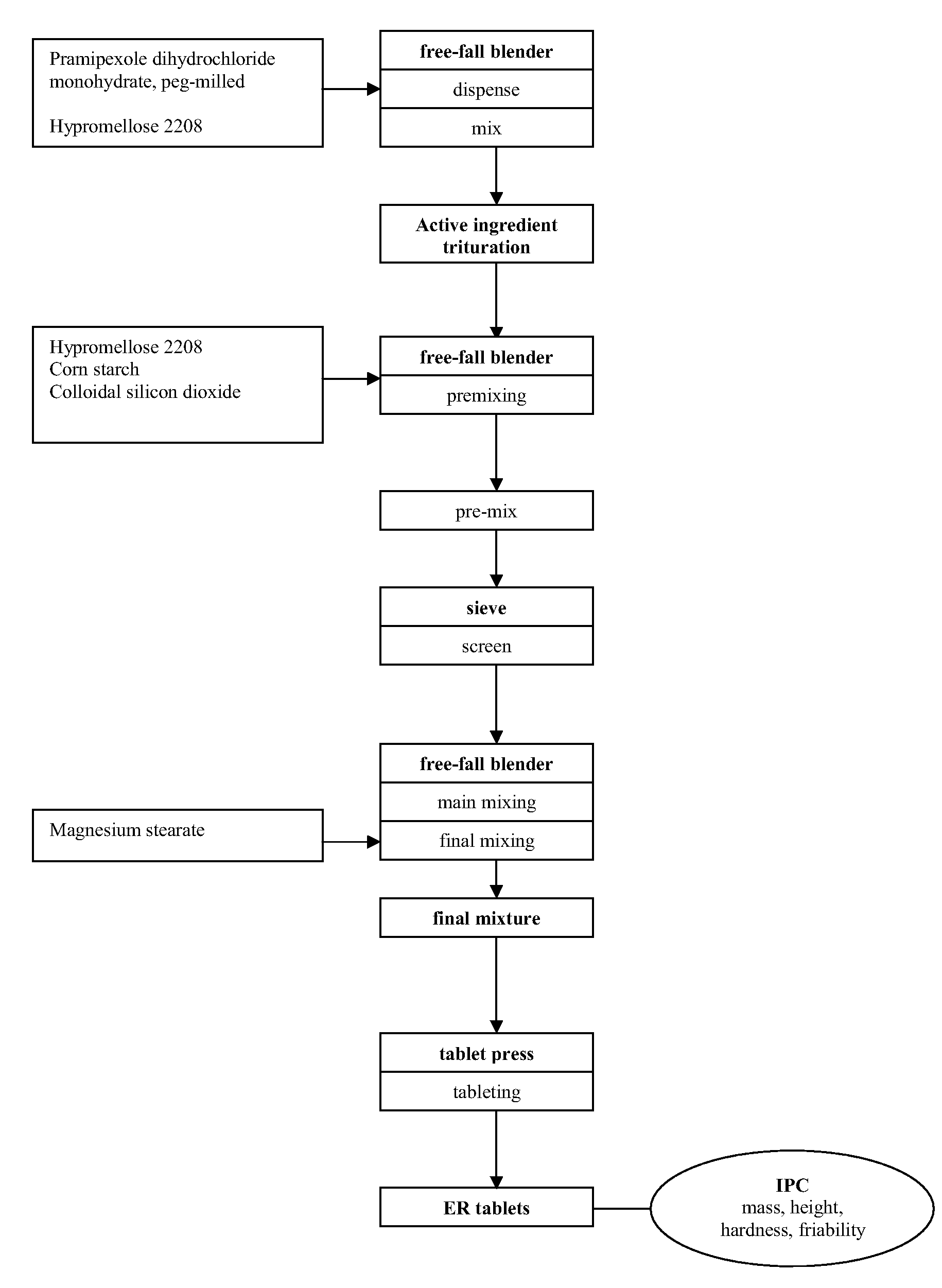
InactiveUS20090098202A1Steady state plasma concentrations of the drugEffective and tolerableBiocideOrganic active ingredientsPramipexolePharmacology
The invention is directed to an extended release formulation comprising pramipexole or a pharmaceutically acceptable salt thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
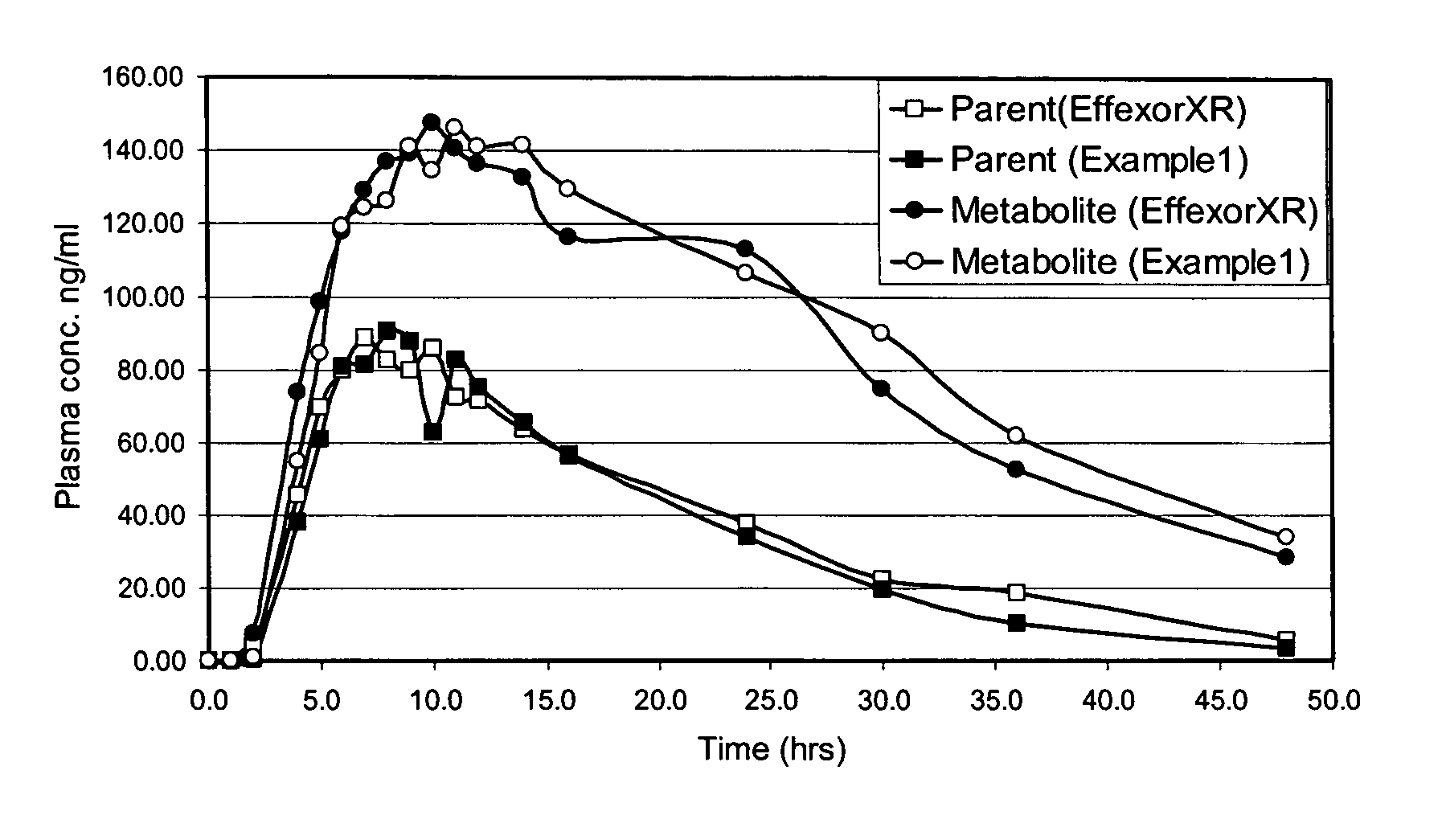
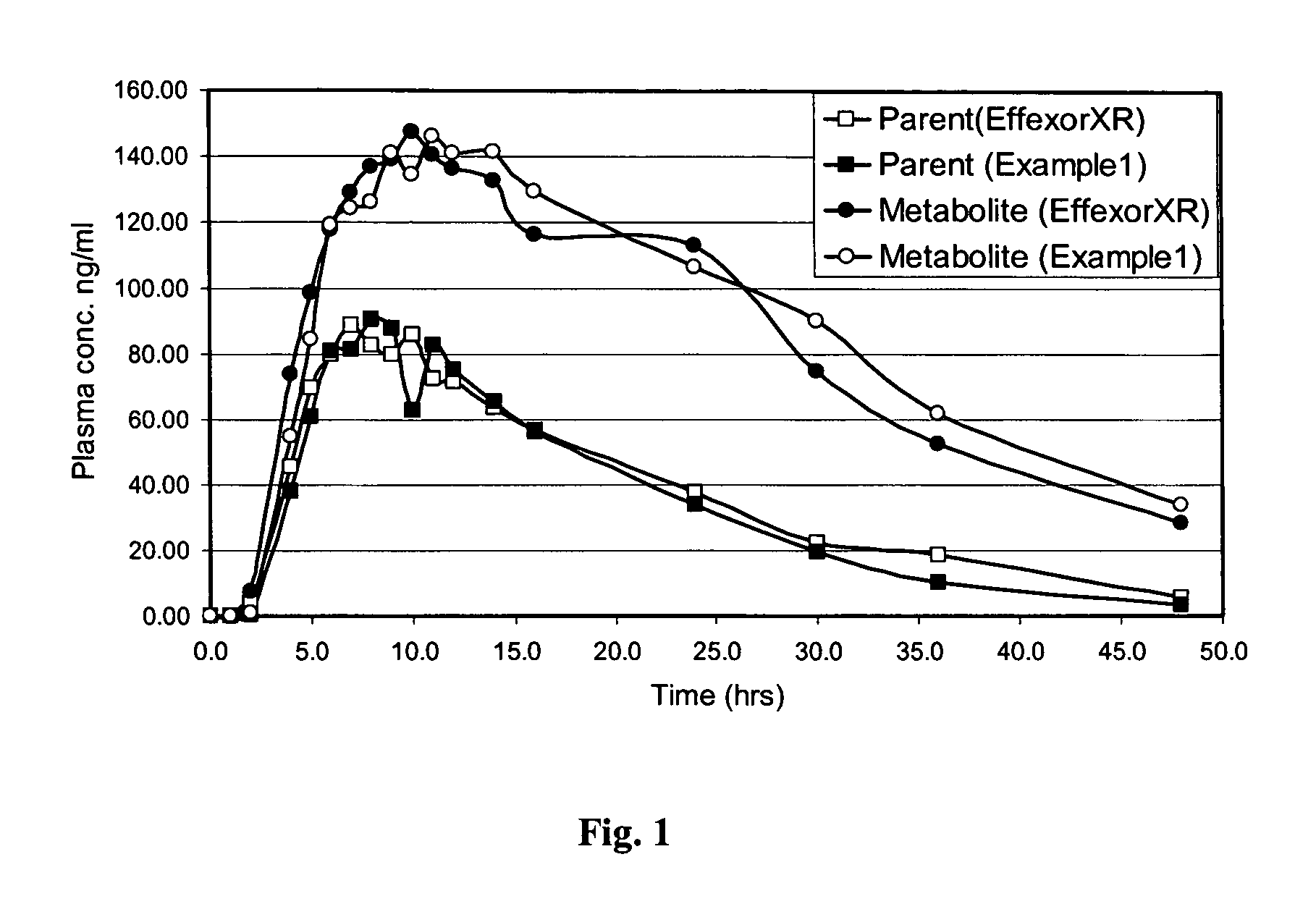
Extended release formulation of venlafaxine hydrochloride
InactiveUS20050169985A1Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Modified release formulations of anti-irritability drugs
Modified or extended release formulations containing mesalamine compounds and associated methods are disclosed and described. In some aspects, such formulations may be substantially bioequivalent to known FDA approved mesalamine formulations such as PENTASA®.
Owner:CAPRICORN PHARMA INC
Extended release formulations
The present invention relates to an extended release formulation containing a poorly water soluble active ingredient and to a method for preparing the formulation. The formulation contains a wax-based extended release material, which provides the extended release of the active ingredient.
Owner:TEVA PHARM USA INC
Extended release formulation of levetiracetam
ActiveUS20070092569A1Reduced inter subject variabilityBiocideNervous disorderWater dispersibleExtended release tablets
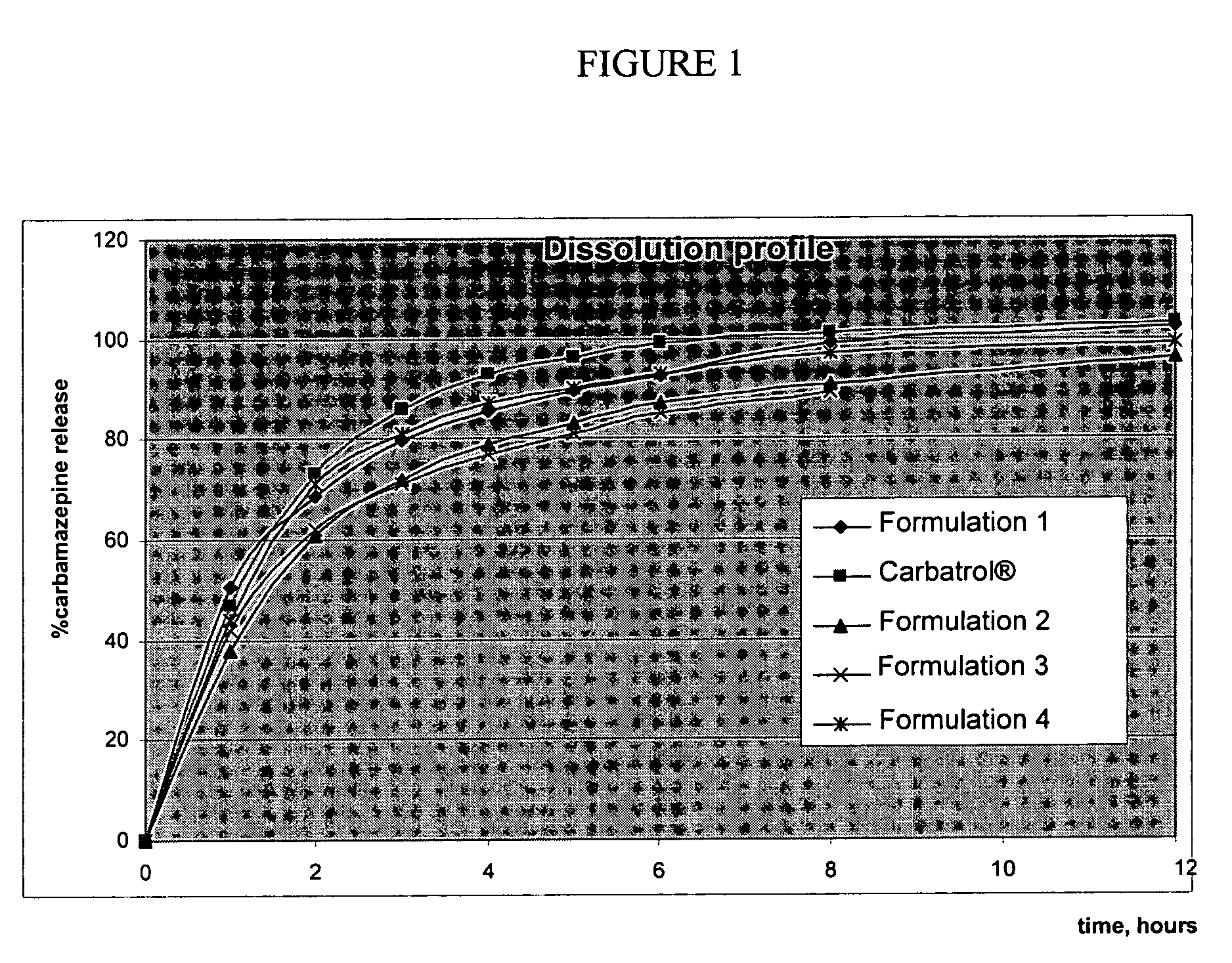
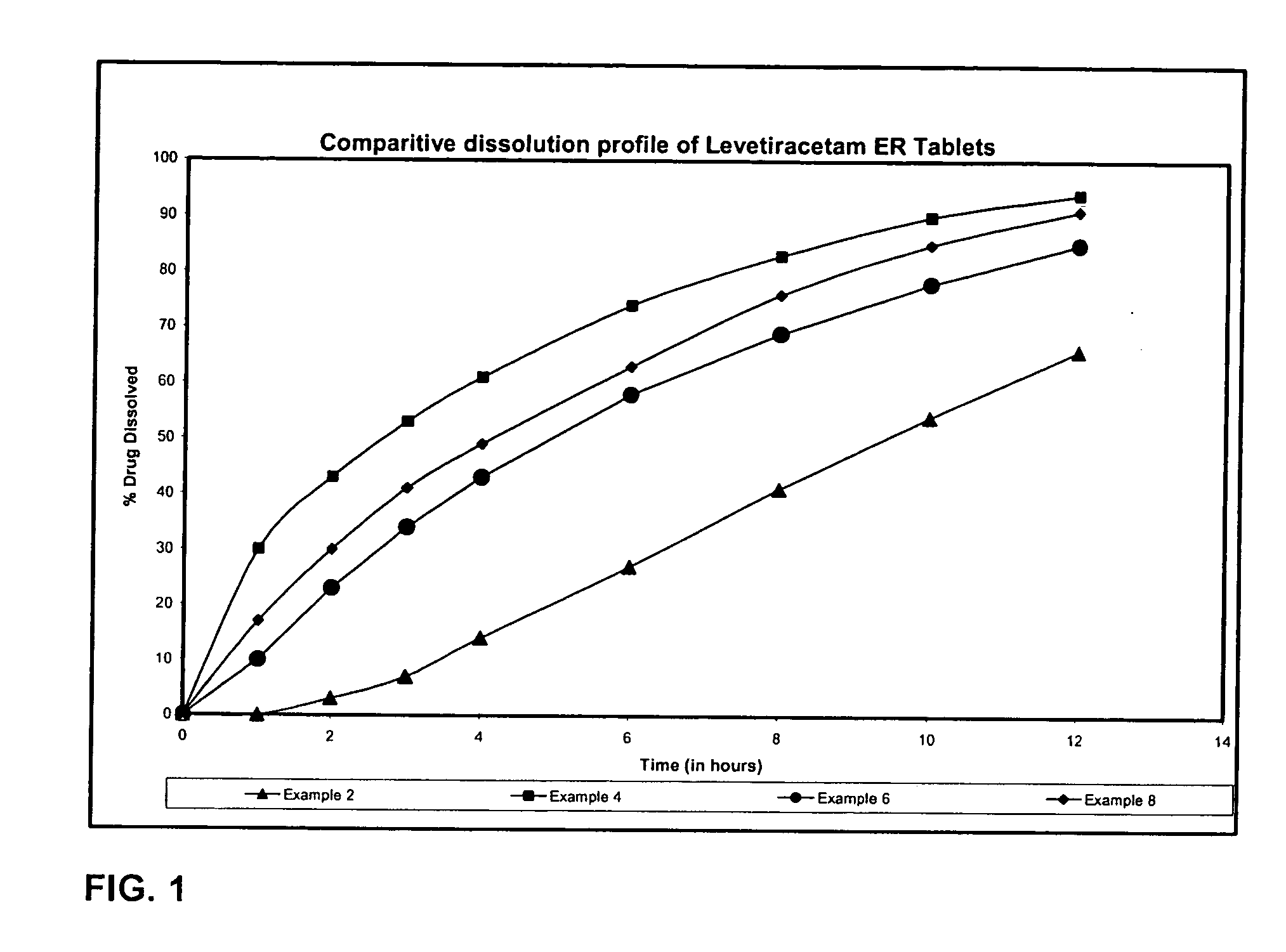
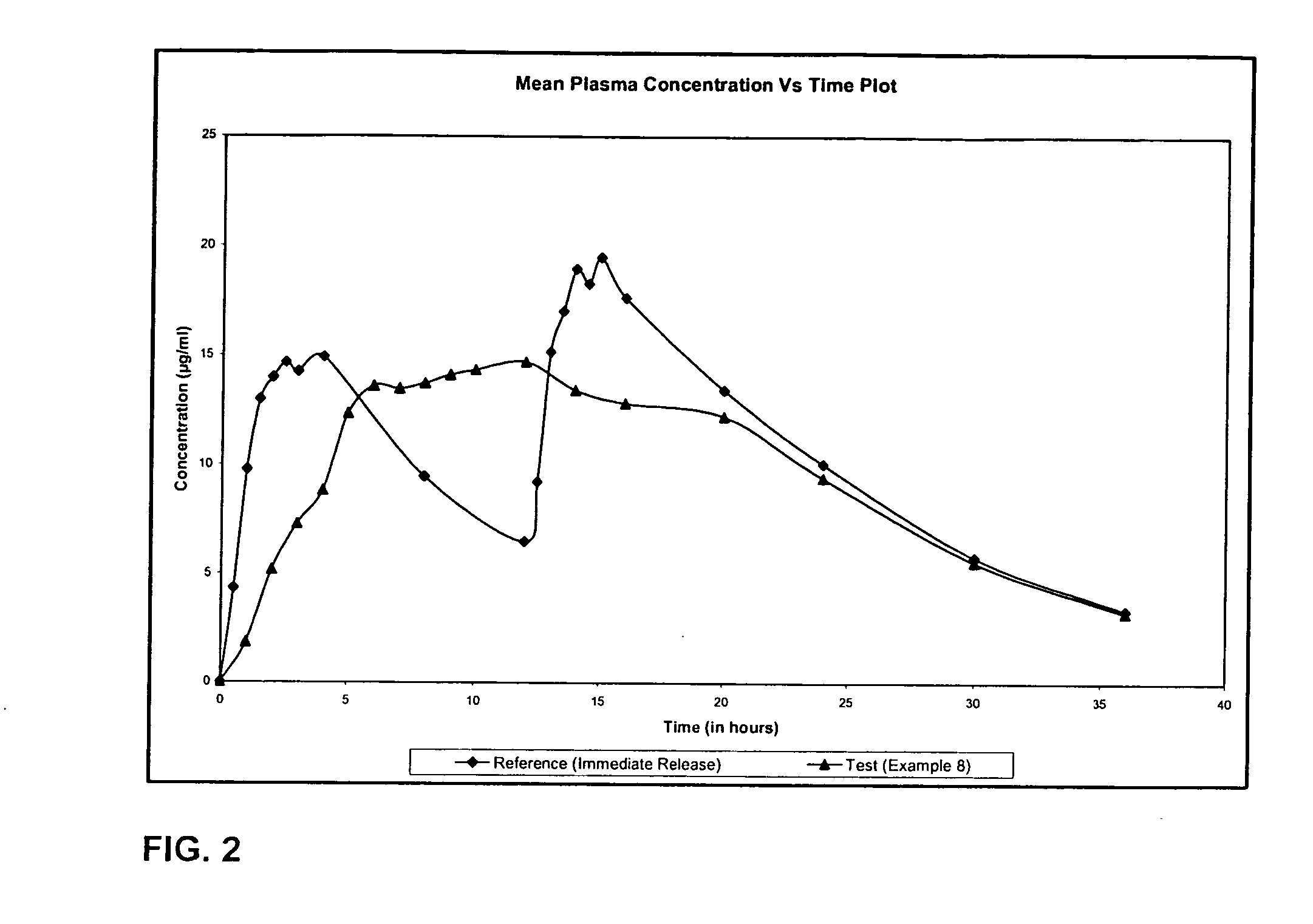
The present invention relates to extended release pharmaceutical compositions of Levetiracetam and processes for preparing the same. The extended release tablet of Levetiracetam is with a core comprising of Levetiracetam and water dispersible rate controlling polymer, and the tablet core is optionally functional coated comprising a combination of water non-dispersible and / or water dispersible polymer. It provides extended therapeutically effective plasma levels over a twenty four hour period with diminished incidences of neuropsychiatric adverse events by eliminating the troughs and peaks of drug concentration in a patient's blood plasma. The composition also exhibits no food effect.
Owner:UCB PHARMA SA
Extended release pharmaceutical formulations of s-adenosylmethionine
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. majorclinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Extended release formulations of carvedilol
InactiveUS20080138404A1Dissolve fastEnhancing rate and extent of releaseBiocidePill deliveryHydrophilic polymersCarvedilol
An improved controlled release dosage form for once-daily administration of carvedilol is described. The controlled release dosage form comprises a therapeutically effective amount of carvedilol and / or a pharmaceutically acceptable salt thereof; one or more hydrophilic polymers; one or more pharmaceutically acceptable excipients; and a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material or a combination of a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material.
Owner:BIOVAIL LAB INT SRL
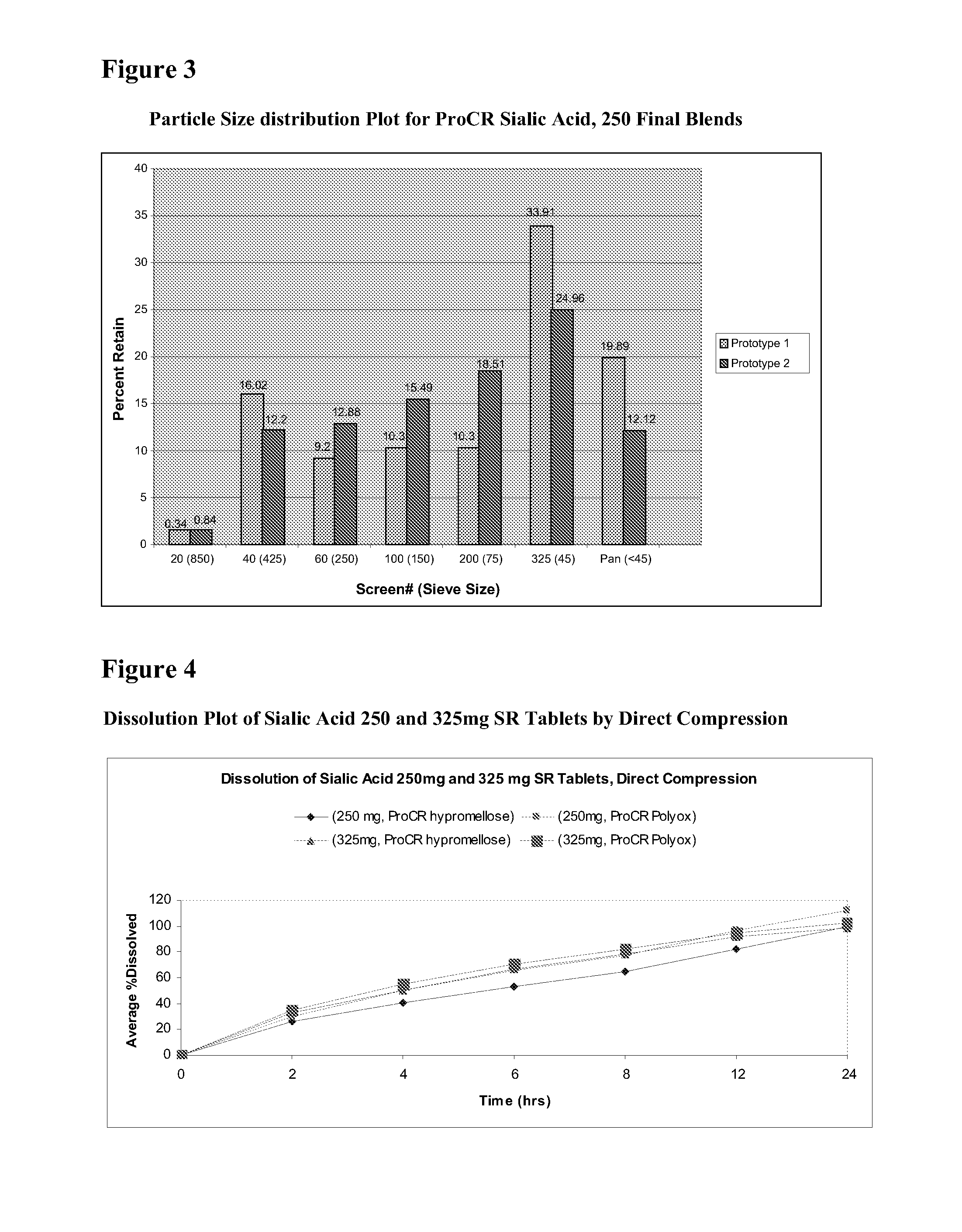
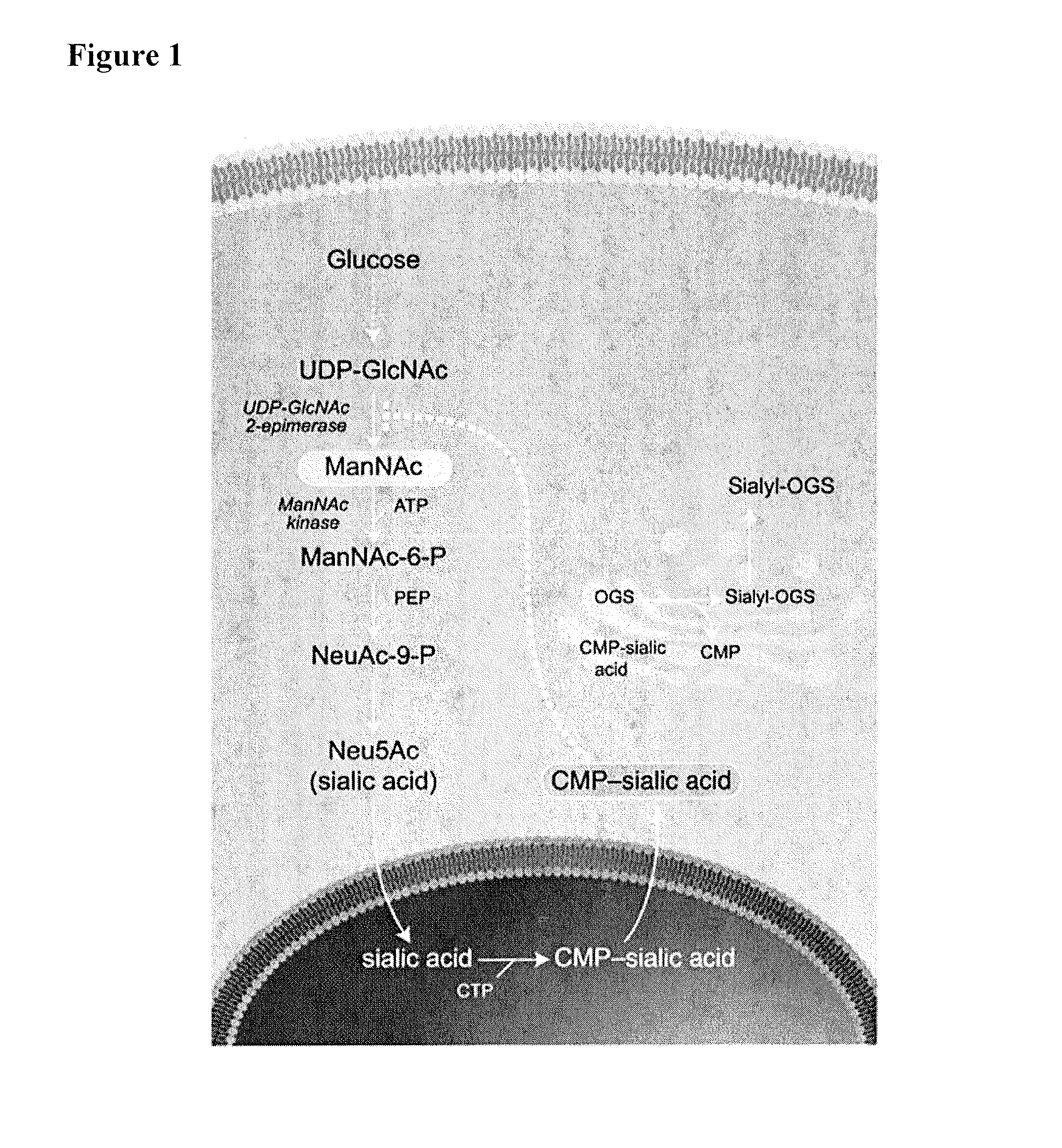
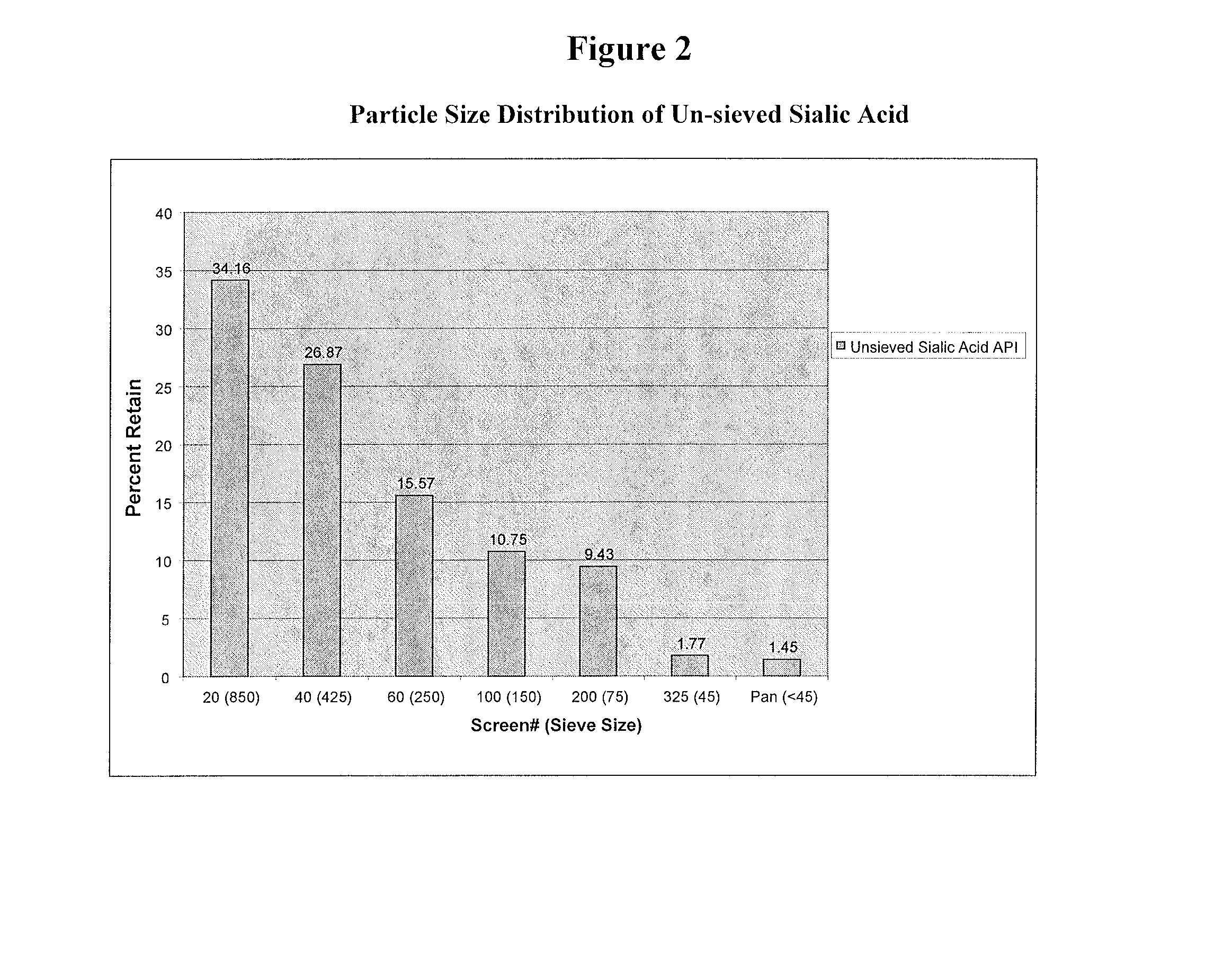
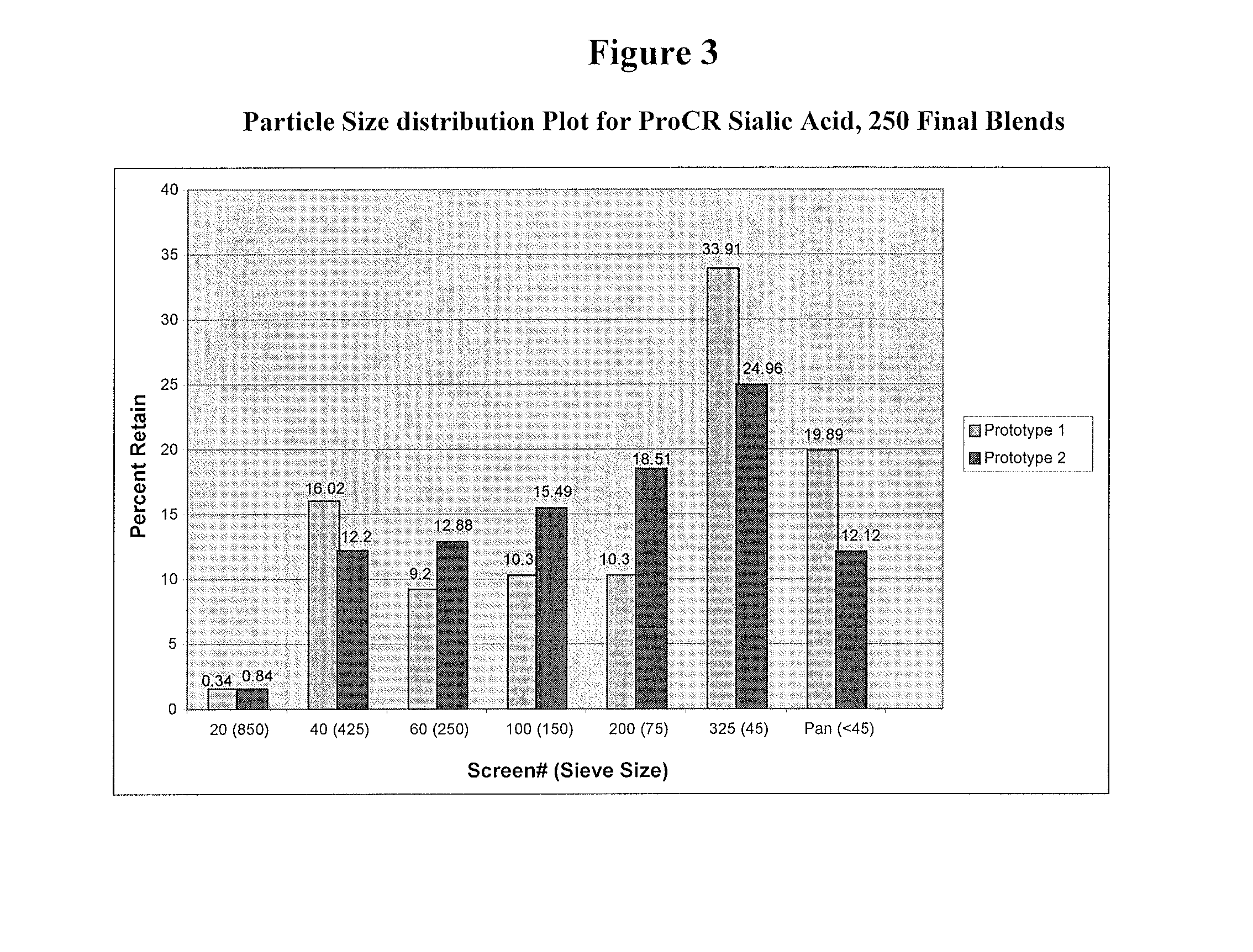
Methods and formulations for treating sialic acid deficiencies
The present invention relates to compositions and methods for treating sialic acid deficiencies comprising extended release formulations.
Owner:ULTRAGENYX PHARMA
Methods and formulations for treating sialic acid deficiencies
ActiveUS20130225513A1Improved absorption profileImproved profileBiocideMuscular disorderSialic acidMedicine
The present invention relates to compositions and methods for treating sialic acid deficiencies comprising extended release formulations.
Owner:ULTRAGENYX PHARMA
Extended release formulation of divalproex sodium
InactiveCN1671363AAvoid stickinessNervous disorderInorganic non-active ingredientsDivalproex SodiumExtended Release Formulations
The present invention relates to an extended release pharmaceutical composition comprising valproic acid, a pharmaceutically acceptable salt, ester, or amide thereof or divalproex sodium.
Owner:RANBAXY LAB LTD
Extended release formulation of pramipexole dihydrochloride
InactiveUS20060110454A1Reduce doseHighly photosensitiveBiocideOrganic active ingredientsMulti unitActive agent
An extended release composition of Pramipexole or a pharmaceutical acceptable salt thereof, wherein the active agent is coated on a non pareil inert core, the drug loaded core is further coated with a polymeric layer which enables the release of the active agent over an extended period and optionally the extended release pellets being further blended with suitable excipients and compressed into a multi unit tablet and processes for the preparation of the said composition.
Owner:ALEMBIC LTD
Enhanced formulations of lamotrigine
A once-a-day, extended-release formulation of lamotrigine, exhibiting a significantly similar release rate throughout the GI tract irrespective of the pH of the environment, is provided. The formulation comprises lamotrigine, an organic acid, a release enhancing polymer and a release controlling polymer. The use of the formulation for the treatment of the neurological disorders is also disclosed.
Owner:SUPERNUS PHARM INC
Extended release formulation of venlafaxine hydrochloride
InactiveUS7807195B2Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Extended release formulations of poorly soluble antibiotics
InactiveUS20060193908A1Improve wettabilityControl erosionBiocideCarbohydrate active ingredientsAntibiotic YMacrolide resistance
An extended release pharmaceutical compressed composition and dosage form comprising poorly water soluble macrolide antibiotic, surfactant and non-lipophilic, non-polymeric excipient is disclosed. The composition releases the macrolide antibiotic over an extended period of time, generally at least over 12 hours, even in the absence of a release rate-retarding polymer, release rate-retarding coating or release rate-retarding lipophilic excipient. The composition is suitable for once daily or twice daily oral administration for the treatment of many different types of bacterial infections. One embodiment of the compressed composition includes a drug-containing granular composition and a binding composition, wherein the two are mixed together and then compressed into a tablet or pill. The surfactant is in admixture with or coated onto the macrolide antibiotic, and it can be included in the granular composition and / or the binding composition. The non-polymeric, non-lipophilic excipient is included in the granular composition and / or the binding composition.
Owner:PHARMAFORM
Pharmaceutical Capsules Comprising Extended Release Dipyridamole Pellets
InactiveUS20090196935A1Improve bioavailabilityPowder deliveryOrganic active ingredientsDipyridamoleRisk stroke
The present invention is directed to pharmaceutical capsules comprising extended release formulations of dipyridamole, processes for preparing such dipyridamole extended release formulations and their use in the treatment of stroke.
Owner:BARR LAB
Orally administrable extended release pellet and tablet formulations of a highly water soluble compound
InactiveUS20070134315A1Suitable for oralOral convenienceBiocidePill deliveryOral medicationActive component
Pharmaceutical compositions comprising an extended release formulation of active compounds effective in the treatment of various pathological conditions are provided. More particularly, the invention provides methods of making and using extended release formulations comprising active compounds that present formulation challenges such as short biological half-life, instability, highly water soluble and / or high dose requirements. Specifically, orally administrable extended release pellet and tablet formulations of isovaleramide are preferred.
Owner:SUPERNUS PHARM INC
Long Acting Injectable Formulations
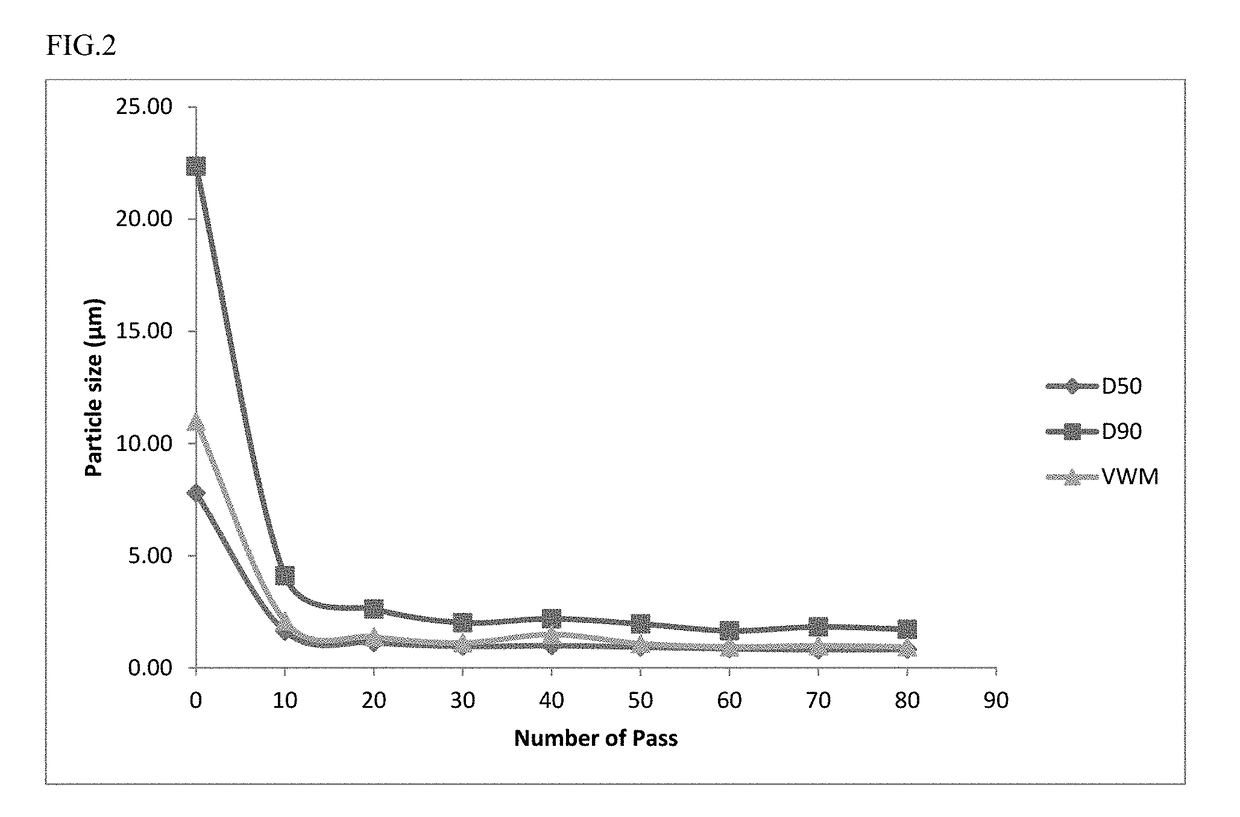
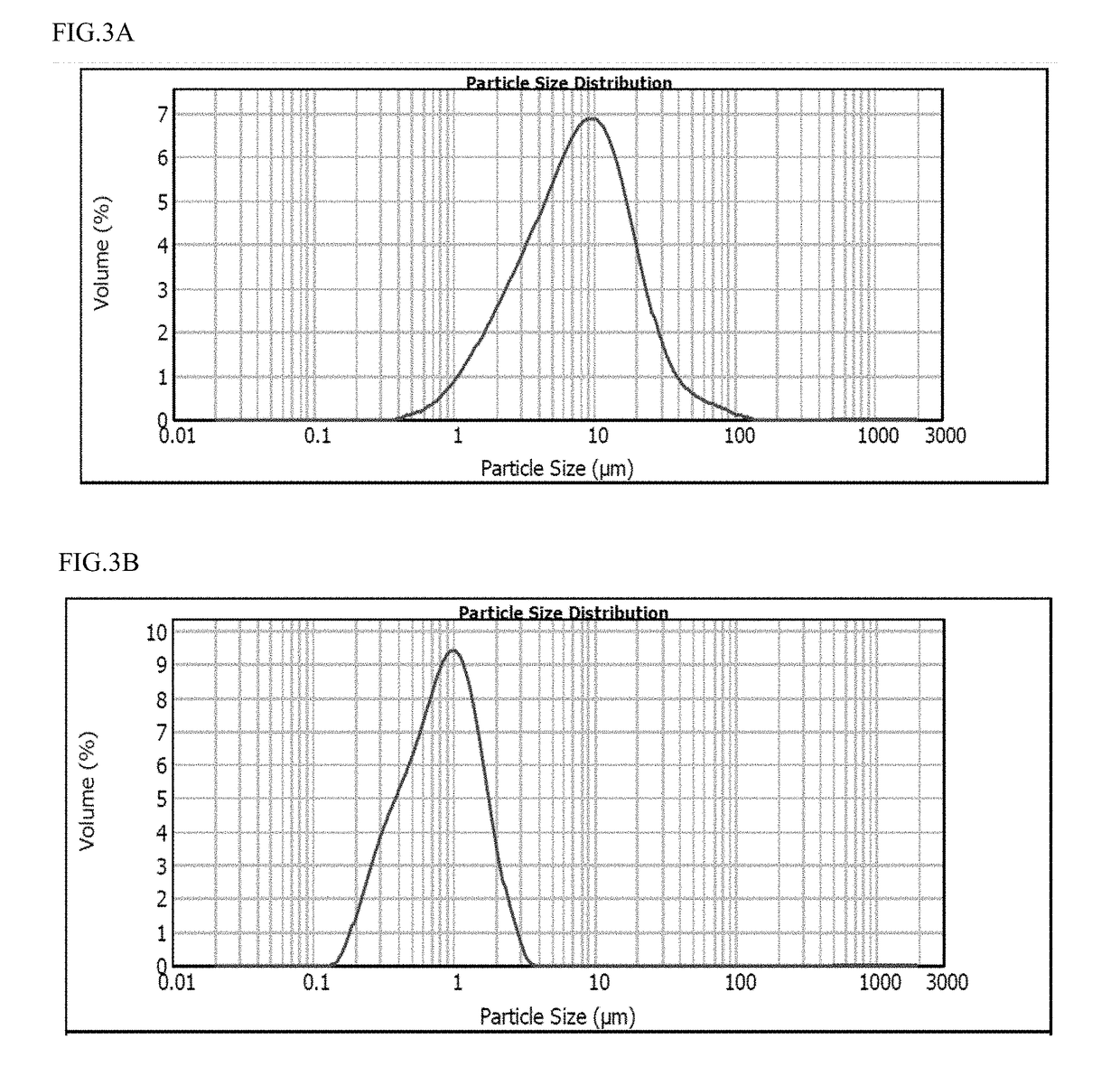
The invention relates to extended-release formulations comprising: (i) a poorly water-soluble active pharmaceutical ingredient; and (ii) a non-aqueous liquid vehicle comprising (a) a hydrophobic lipid comprising a glyceryl ester of a C6-C24 fatty acid, or (b) a hydrophilic organic compound selected from the group consisting of polyethylene glycol, propylene glycol, glycerin, and dimethylsulfoxide, or (c) a combination of (a) and (b), and (iii) an amphiphilic agent wherein the active pharmaceutical ingredient is dispersed as discrete particles having a D90 particle size of about 0.5 μm to about 25 μm in the formulation, and wherein the formulation is non-gelling and thixotropic with a viscosity of less than 10 poise at a shear rate of 10 / s at 25° C.
Owner:ABON PHARMA
Controlled Absorption Water-Soluble Pharmaceutically Active Organic Compound Formulation for Once-Daily Administration
The present disclosure provides a once-daily water-soluble pharmaceutically active formulation for oral administration. In certain embodiments, the composition comprises a watersoluble pharmaceutically active organic compound incorporated into a small particulate, each particulate having a core of the water-soluble pharmaceutically active organic compound or an acceptable salt thereof in reversible association with a pharmaceutically acceptable drug-binding polymer. The core of the composition being surrounded by an insoluble water permeable membrane that is capable of delaying the dissolution of the pharmaceutically active compound therewithin and providing for extended release of the pharmaceutically active compound. In some embodiments, the formulation of the invention are designed to extend release of the pharmaceutically active organic compound for about 3 hours to about 8 hours, thereby enabling preparation of an extended release formulation for any pharmaceutically active compound with a half-life of from about 16 hours to about 21 hours.
Owner:CLINSMART LLC
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20080206333A1Improved pharmacokinetic propertiesAct quicklyBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Compositions comprising an antihistamine, antitussive and decongestant in extended release formulations
The invention provides oral formulations for the treatment of cold and allergy symptoms. Each formulation combines an antihistamine, an antitussive, and / or a decongestant into one extended release composition. The invention further provides for methods of making and using such formulations, as well as for methods for preventing abuse or extraction of a single drug present in an oral extended release composition comprising two or more of an antihistamine, antitussive, and / or decongestant.
Owner:ATTKISSON ELIZABETH E
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com