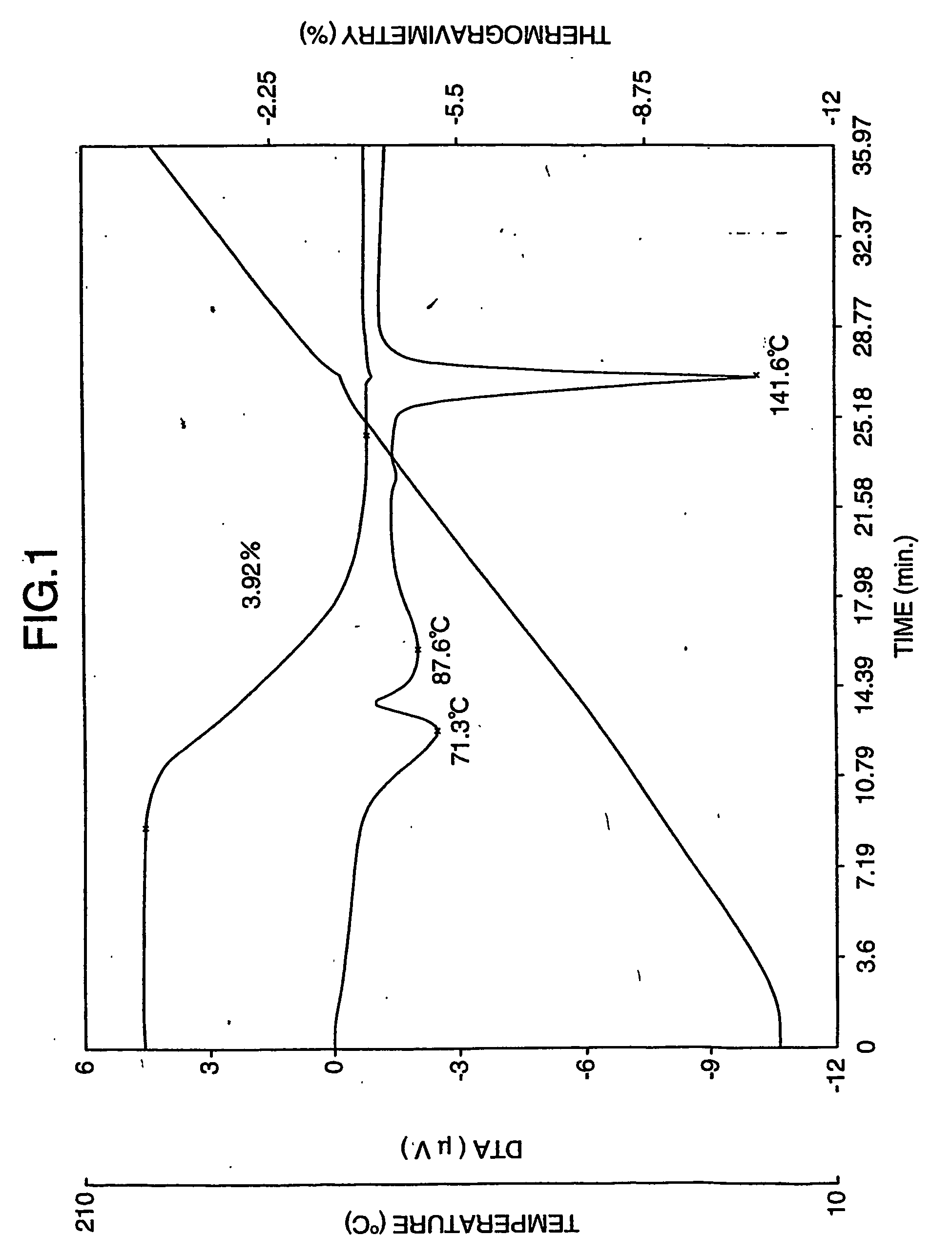
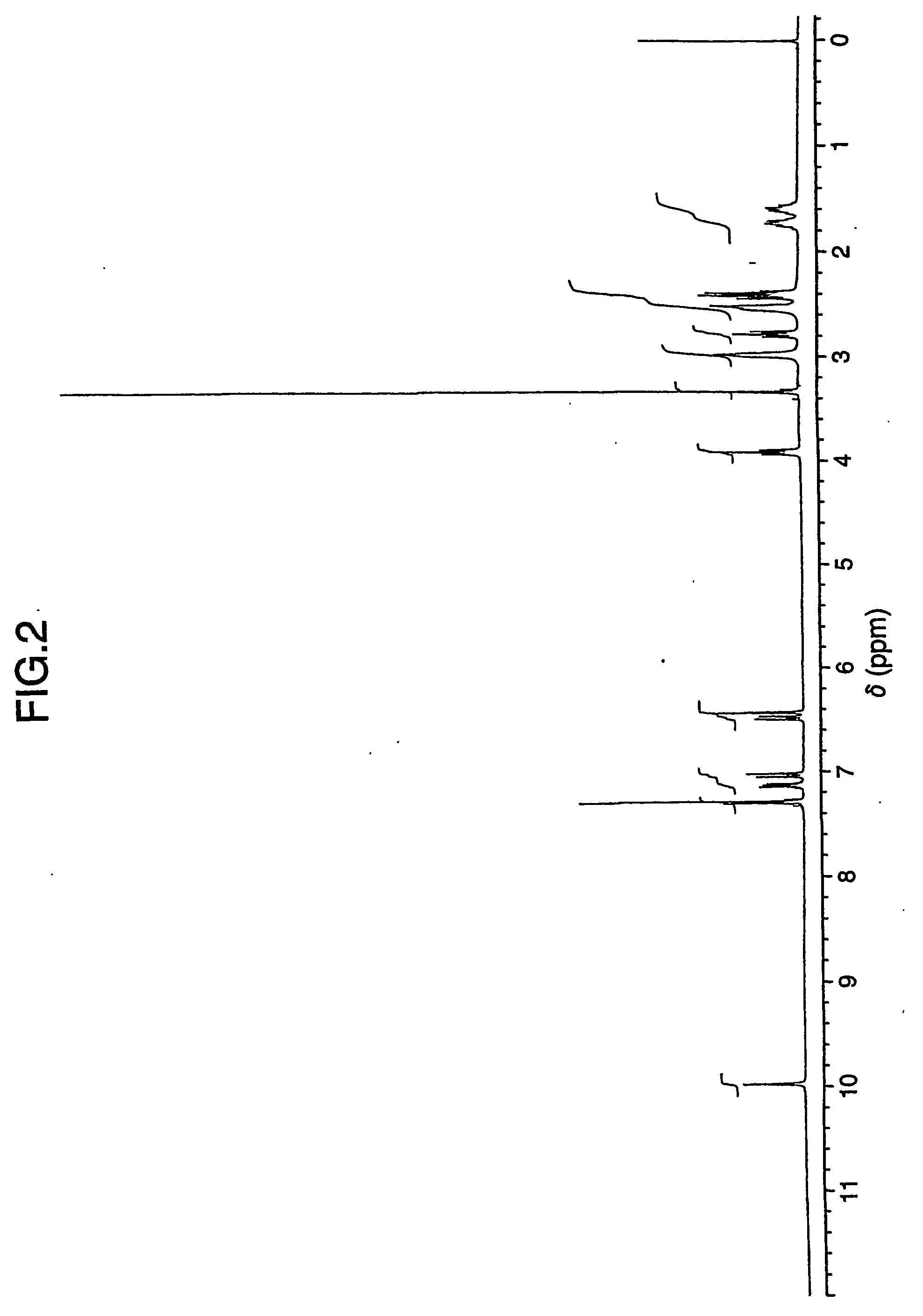
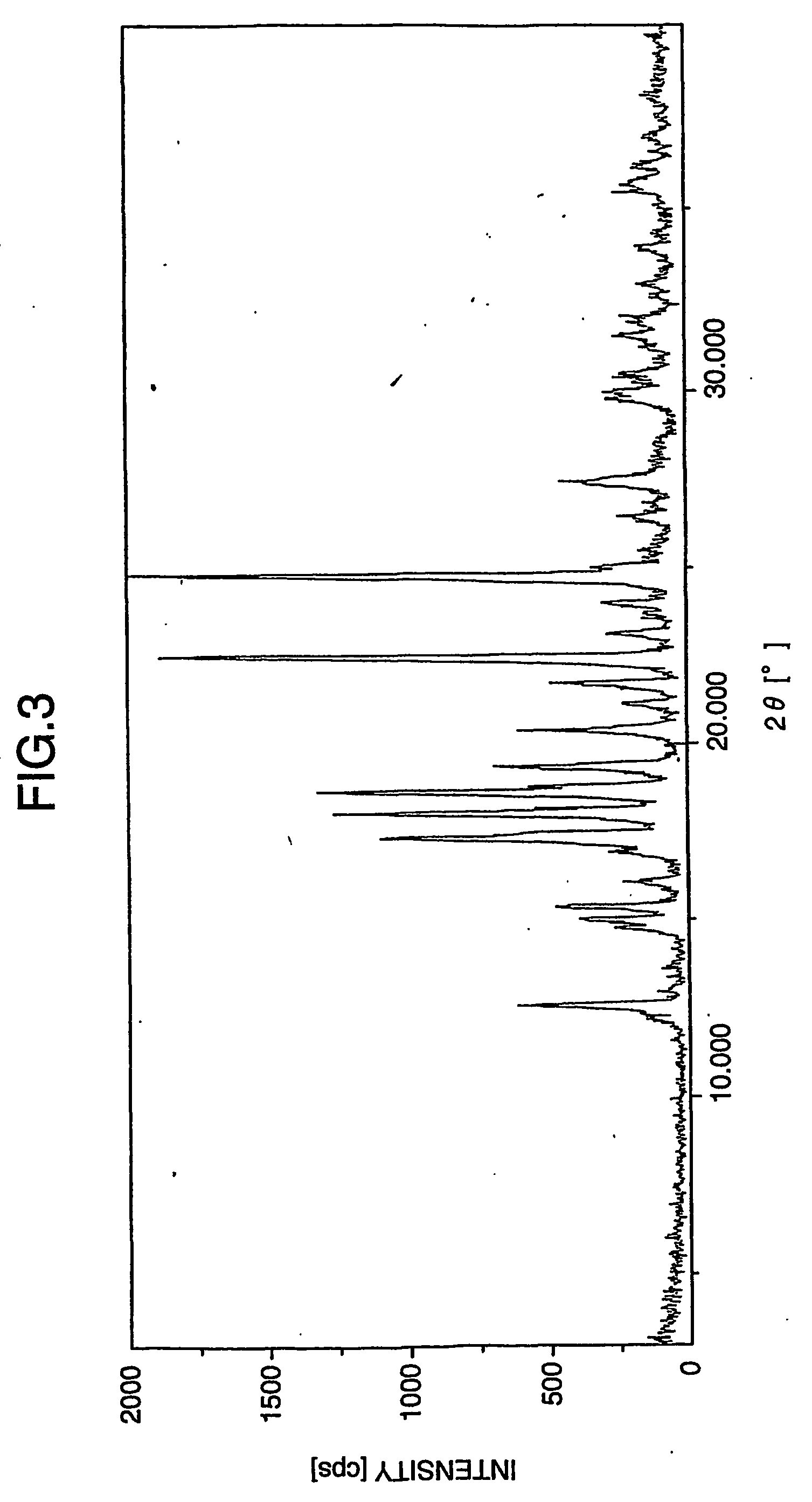
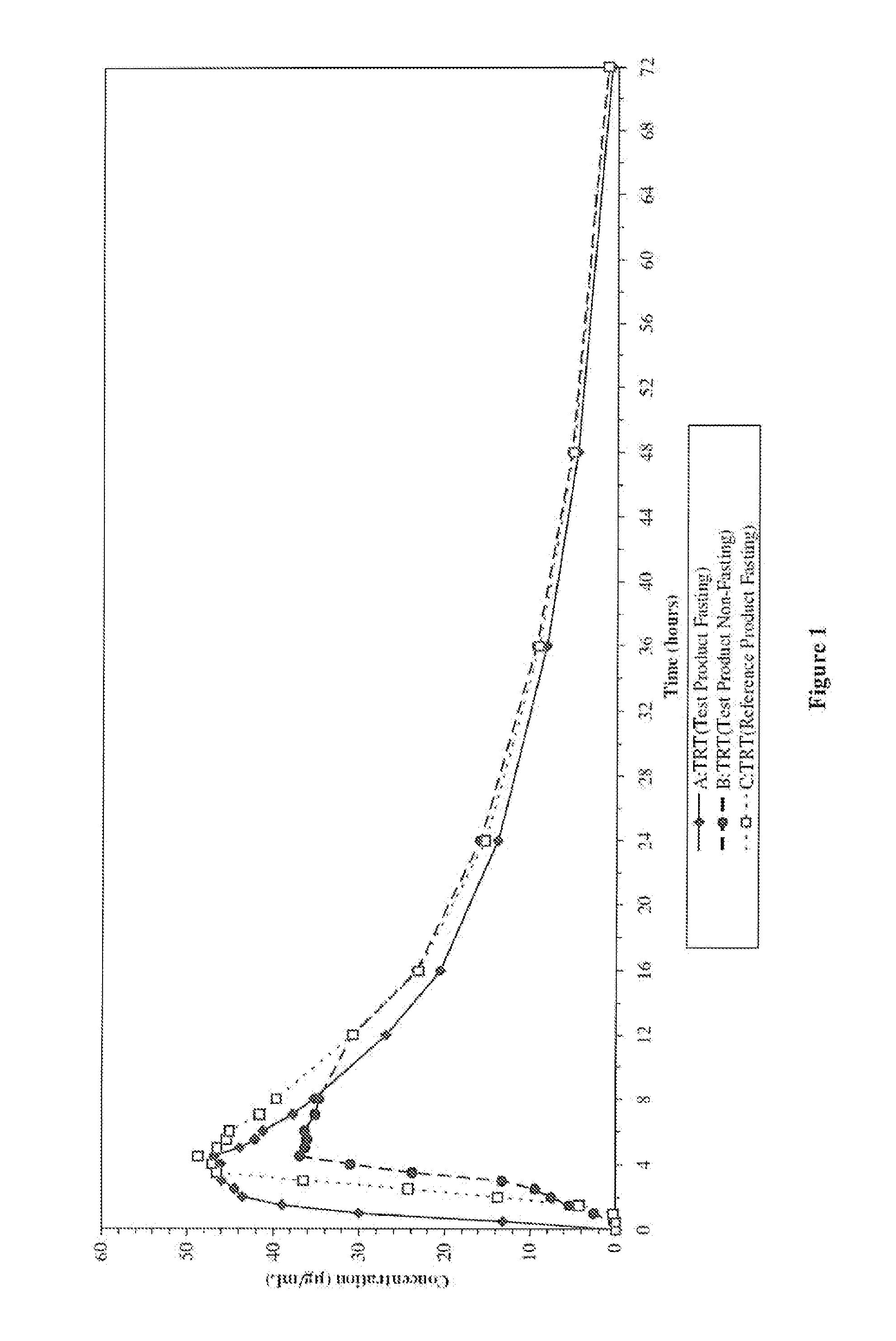
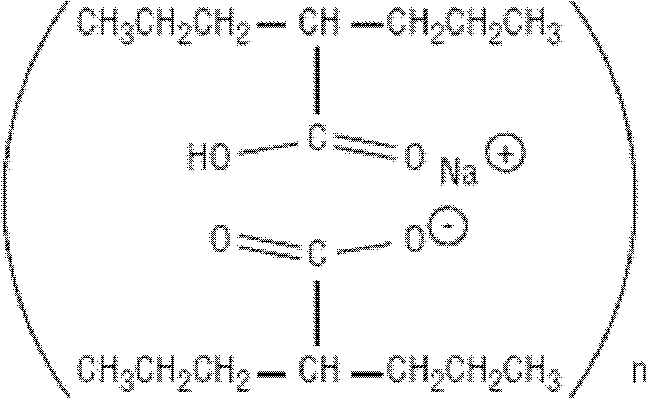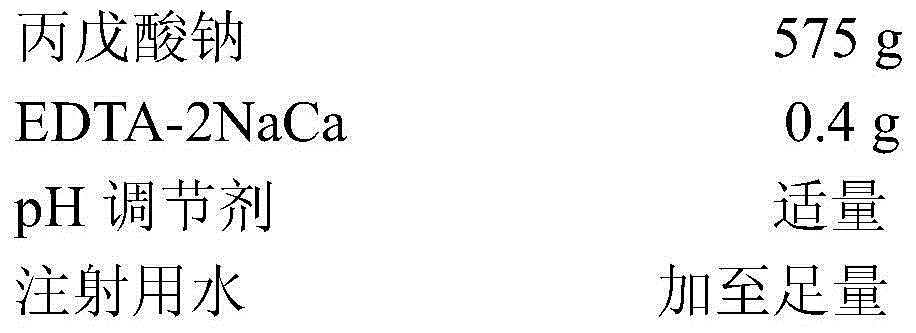Patents
Literature
63 results about "Divalproex Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
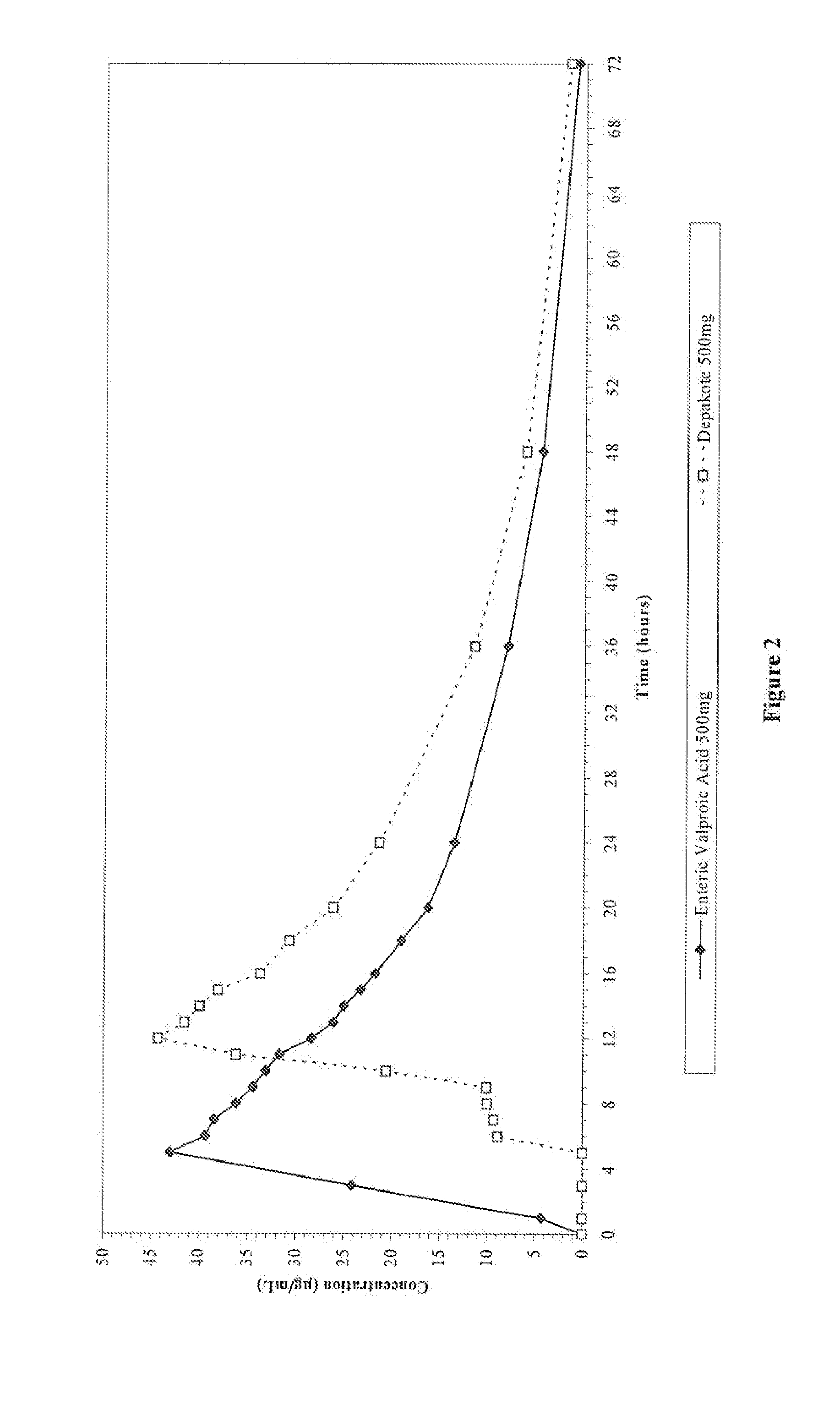
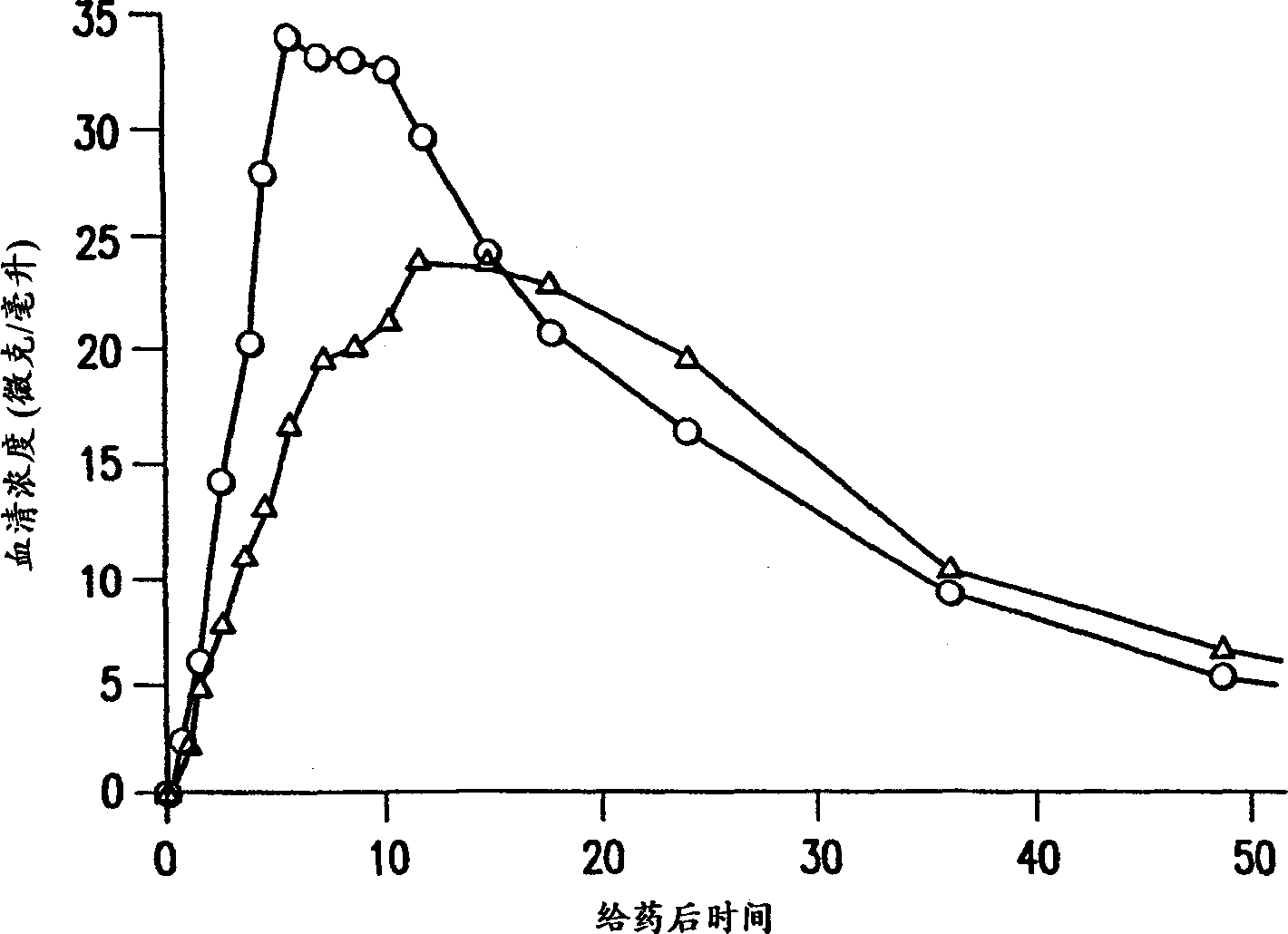
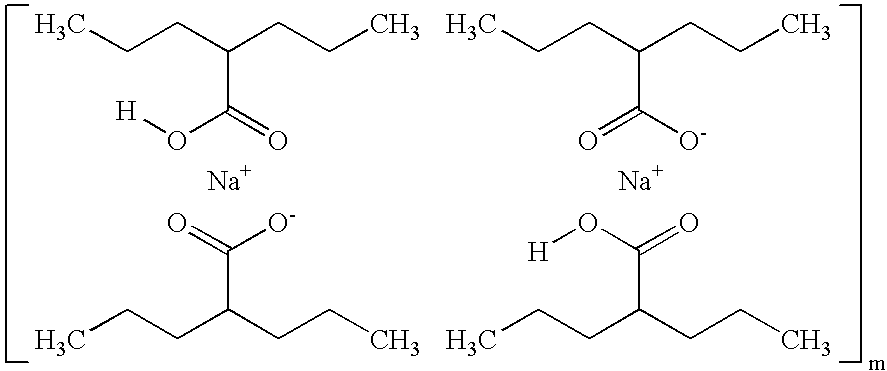
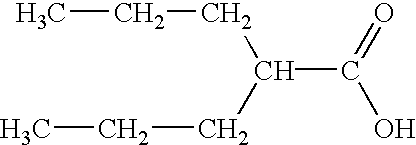
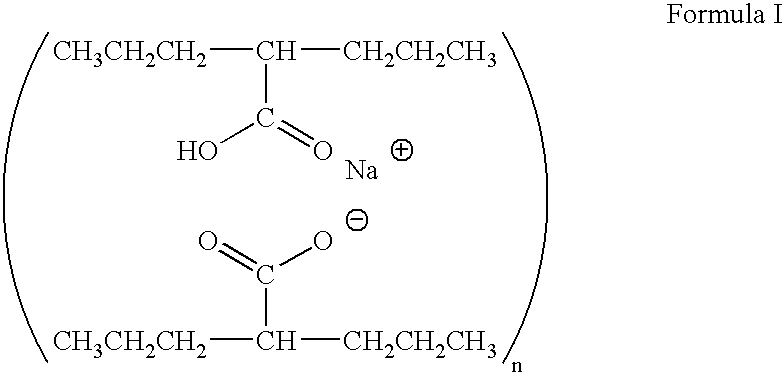
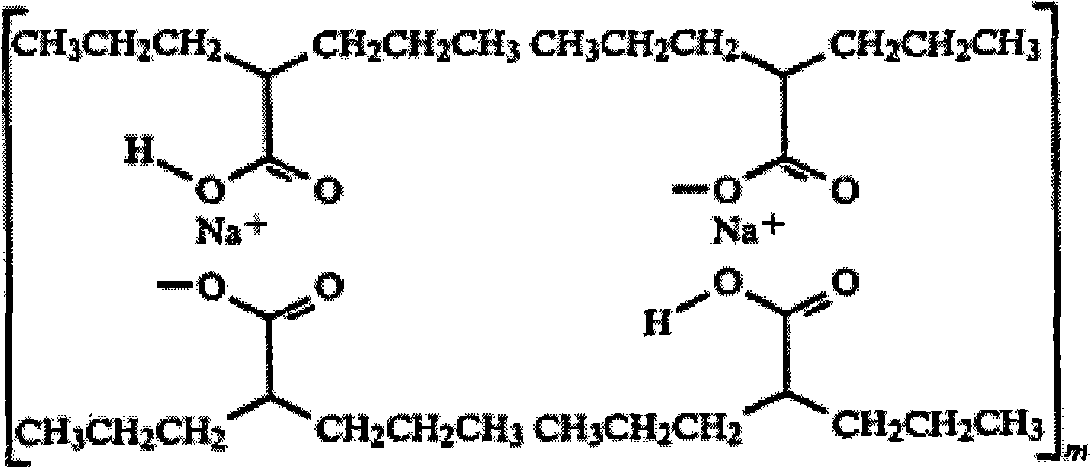
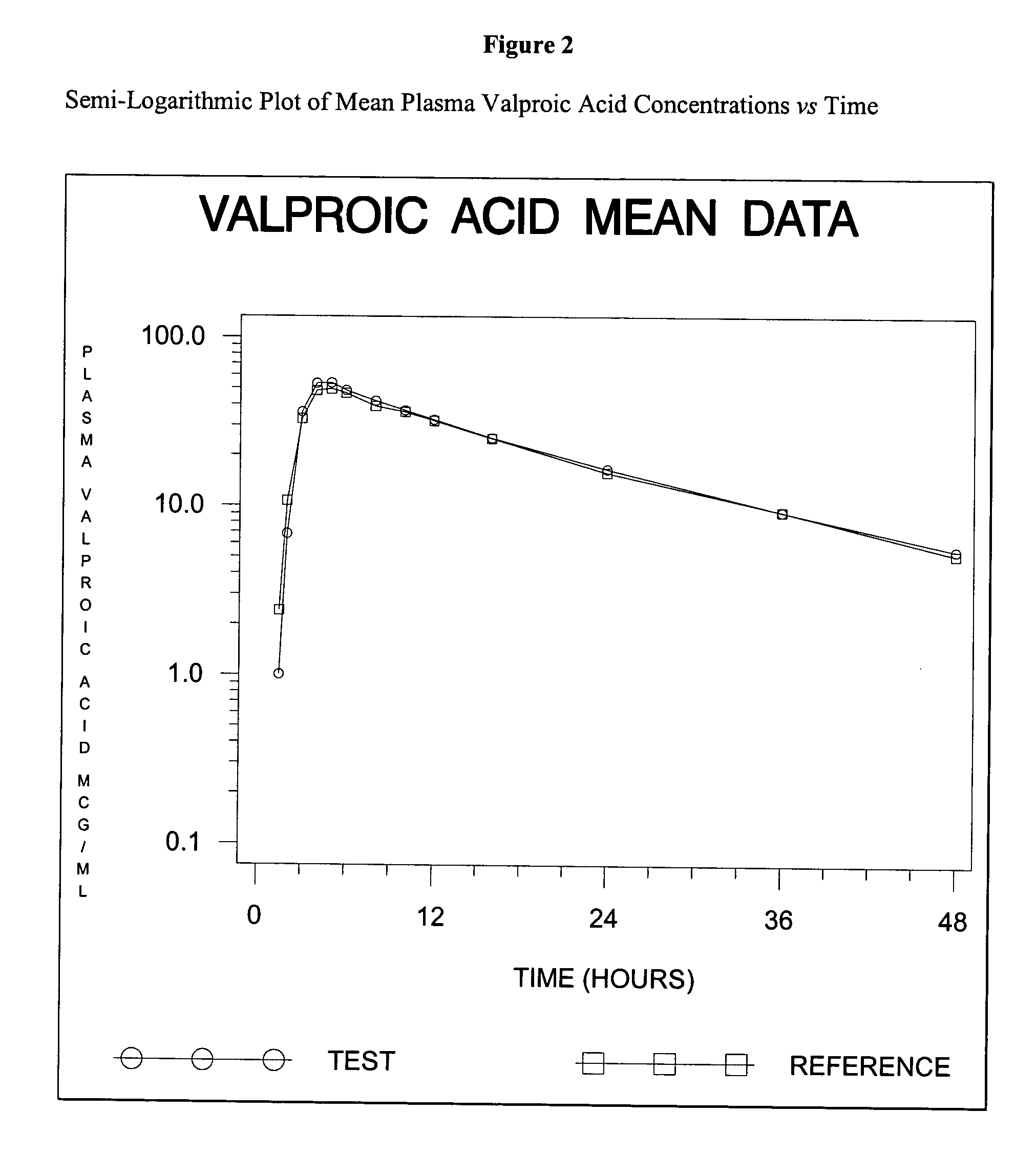
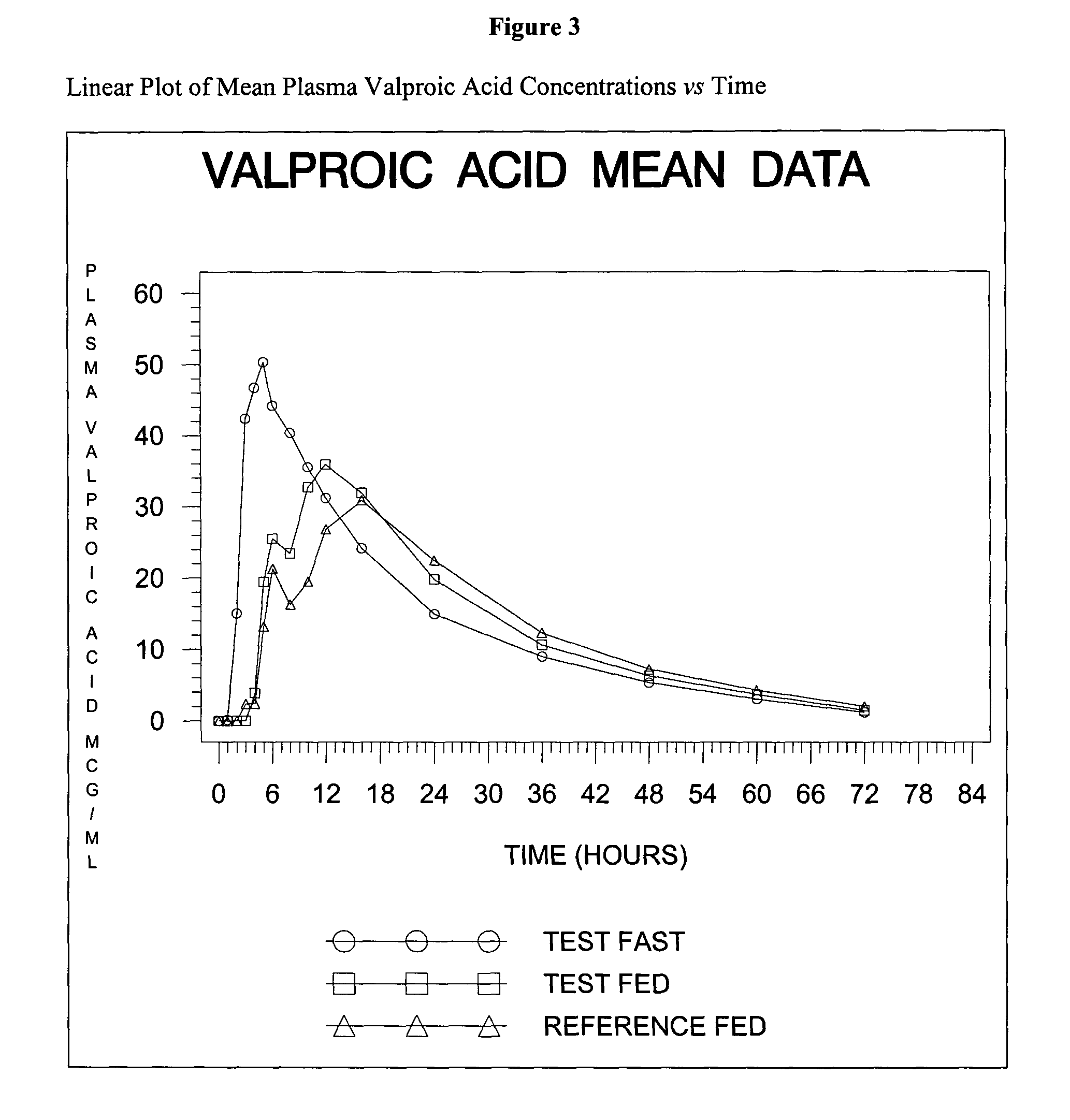
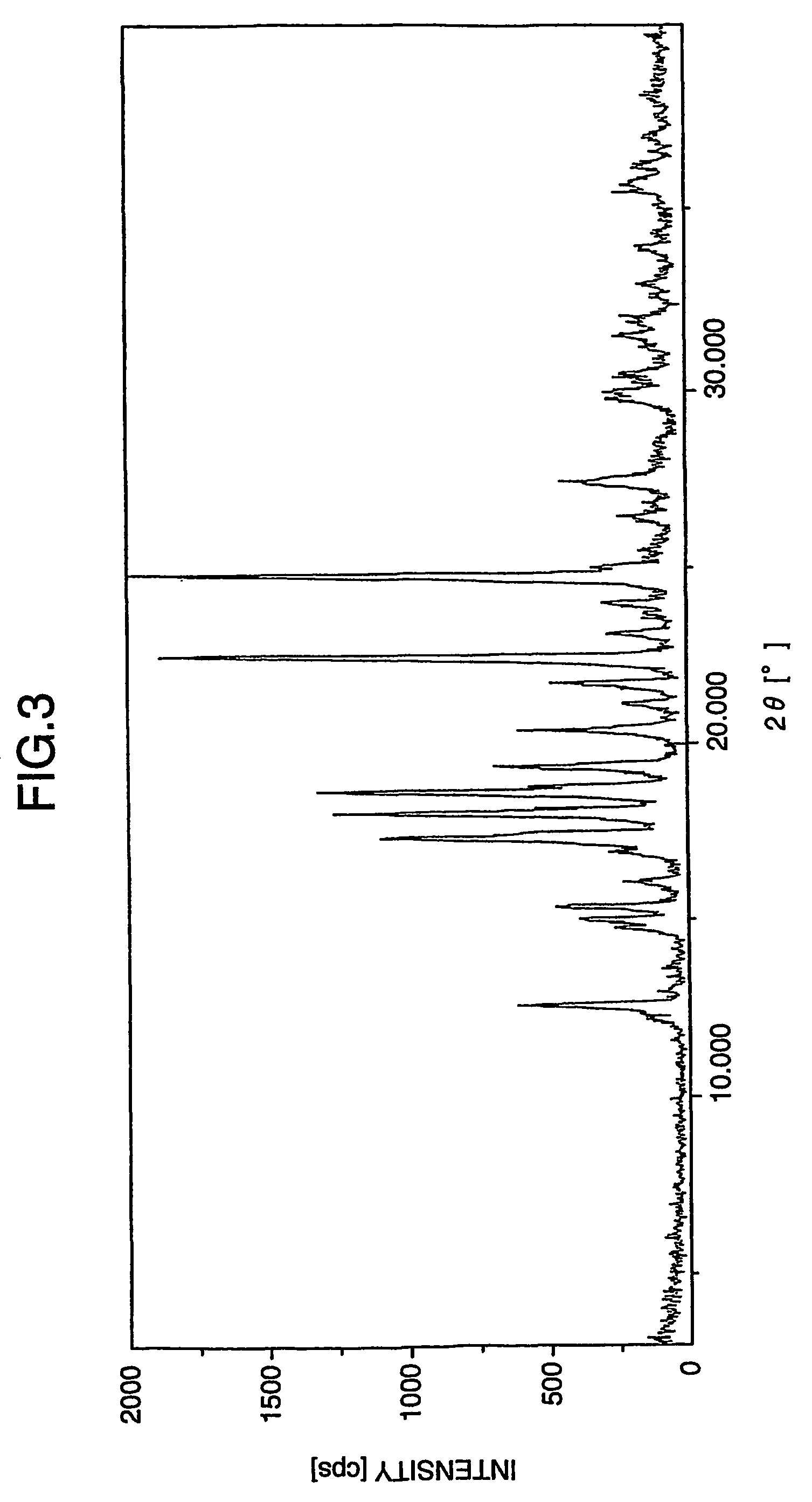
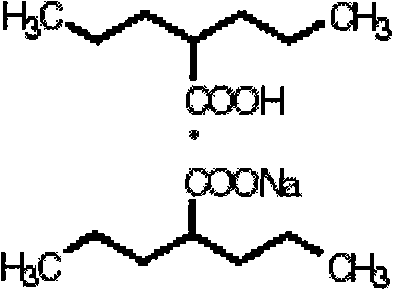
Valproate semisodium or divalproex sodium consists of a compound of sodium valproate and valproic acid in a 1:1 molar relationship in an enteric coated form. Its chief use in medicine is as a treatment for bipolar disorder, epilepsy and in the prevention of migraines.
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Divalproex sodium tablets
Owner:ANDRX LABS
Divalproex sodium tablets
A process for preparing divalproex sodium tablets is provided. The process comprises preparing a neutralized divalproex sodium solution by combining divalproex sodium, having a sodium valproate and a valproic acid moiety, with an aqueous solvent and a base, e.g., sodium hydroxide, the base being in sufficient amount to ensure neutralization of the valproic acid moiety of the divalproex sodium. The neutralized divalproex sodium solution is sprayed onto a pharmaceutically acceptable carrier, and processed to obtain divalproex sodium tablets.
Owner:ANDRX LABS
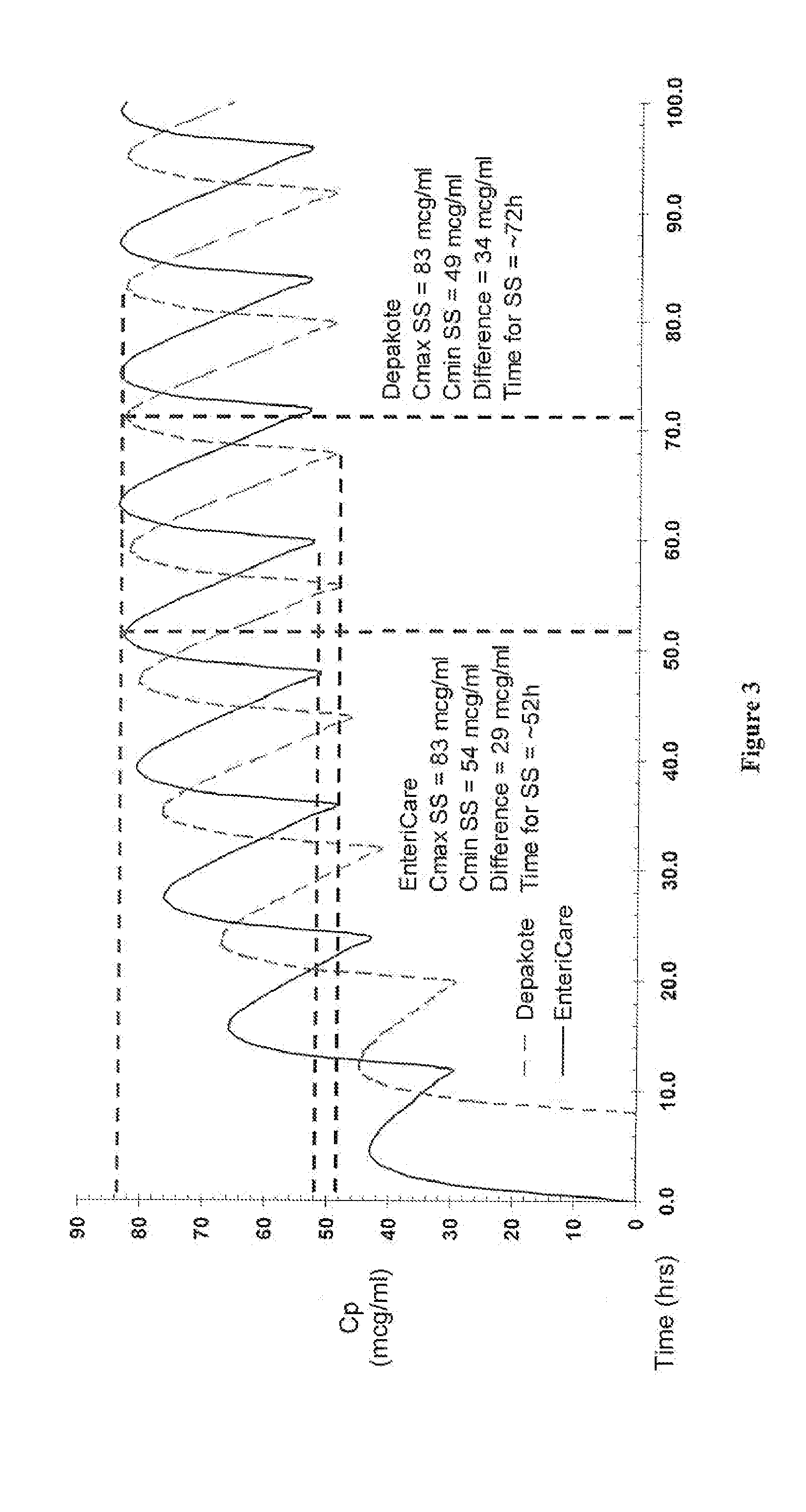
Sustained release neutralized divalproex sodium
InactiveUS20050276848A1Good lookingEasy to operateBiocideAnimal repellantsDivalproex SodiumDosage form
The present invention is directed to sustained release oral dosage forms comprising neutralized divalproex sodium, methods of manufacturing the dosage forms, and methods of treatment with the dosage forms.
Owner:ANDRX
Enteric valproic acid
An enteric valproic acid soft gelatin capsule, in which the enteric polymer is a component of the capsule shell rather than a coating, has been developed. The fill material comprises valproic acid or divalproex sodium and, optionally, one or more pharmaceutically acceptable excipients such as corn oil. The capsule shell is prepared from a mass comprising a film-forming polymer, an acid insoluble polymer, an aqueous solvent, and optionally a plasticizer. Suitable film-forming polymers include gelatin. Suitable acid-insoluble polymers include acrylic-acid / methacrylic acid copolymers. The acid-insoluble polymer is present in an amount from about 8% to about 20% by weight of the wet gel mass. The weight ratio of acid-insoluble polymer to film-forming polymer is from about 25% to about 50%. The aqueous solvent is water or an aqueous solution of alkalis such as ammonia or diethylene amine or hydroalcoholic solutions of the same. Suitable plasticizers include glycerin and triethylcitrate. The enteric soft gelatin capsule does not require an enteric coating and thus is not susceptible to the processing problems associated with enteric coated dosage forms. Enteric valproic acid soft gelatin capsules may be smaller in size and thus easier to swallow than currently available enteric coated tablets due to the presence of fewer ingredients, as well as smaller amounts of ingredients in the capsule shell.
Owner:PATHEON SOFTGELS INC
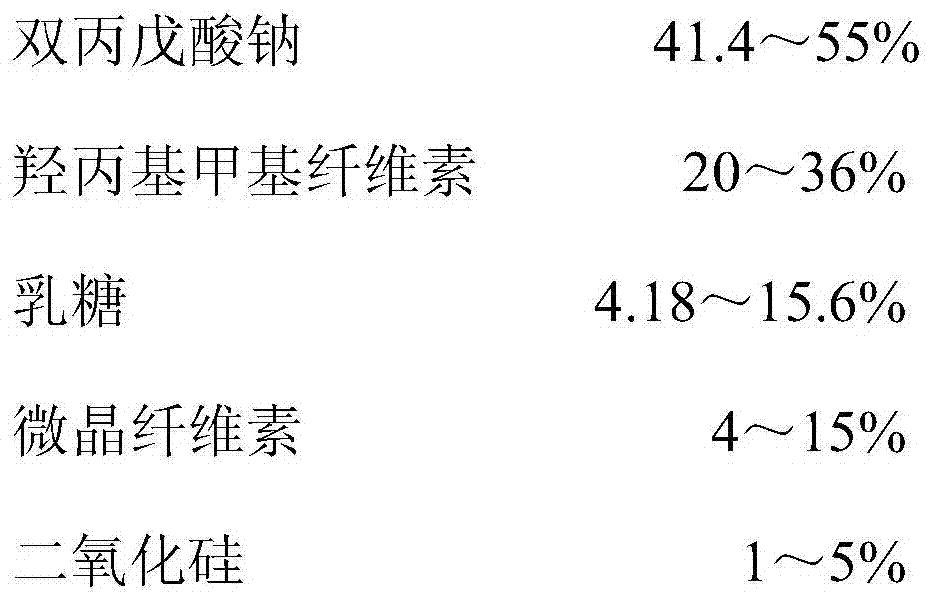
Controlled release formulation of divalproex sodium
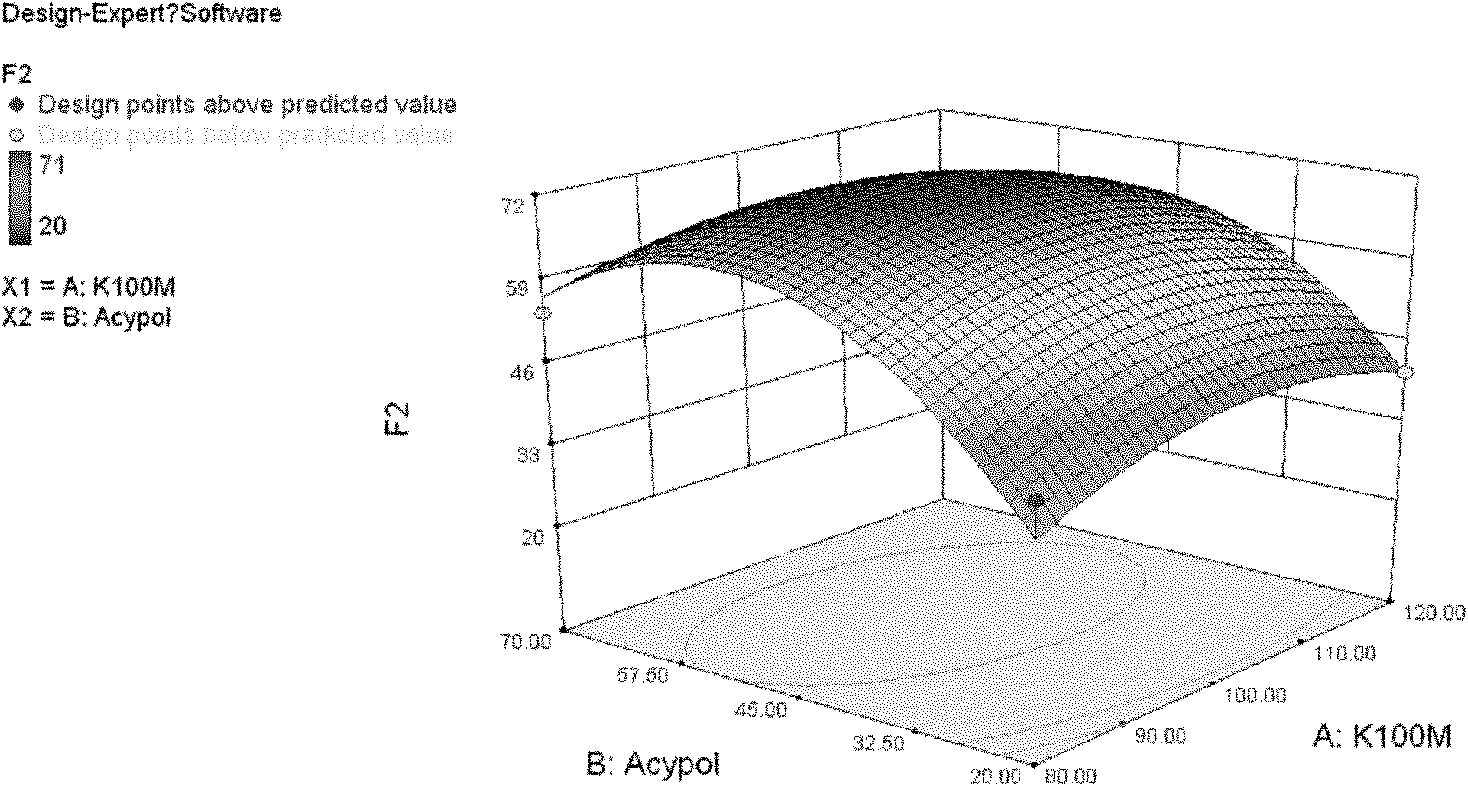
A controlled release tablet formulation which permits once daily dosing in the treatment of epilepsy comprises from about 50 weight percent to about 55 weight percent of an active ingredient selected from the group consisting of valproic acid, a pharmaceutically acceptable salt or ester of valproic acid, divalproex sodium, and valpromide; from about 20 weight percent to about 40 weight percent hydroxypropyl methylcellulose; from about 5 weight percent to about 15 weight percent lactose, from about 4 weight percent to about 6 weight percent microcrystalline cellulose, and from about 1 weight percent to about 5 weight percent silicon dioxide having an average particle size ranging between about 1 micron and about 10 microns; all weight percentages based upon the total weight of the tablet dosage form. Also disclosed are pre-tableting granular formulations, methods of making the granular formulations and tablets, and a method of treating epilepsy employing the controlled release tablet formulations of the invention.
Owner:ABBOTT LAB INC
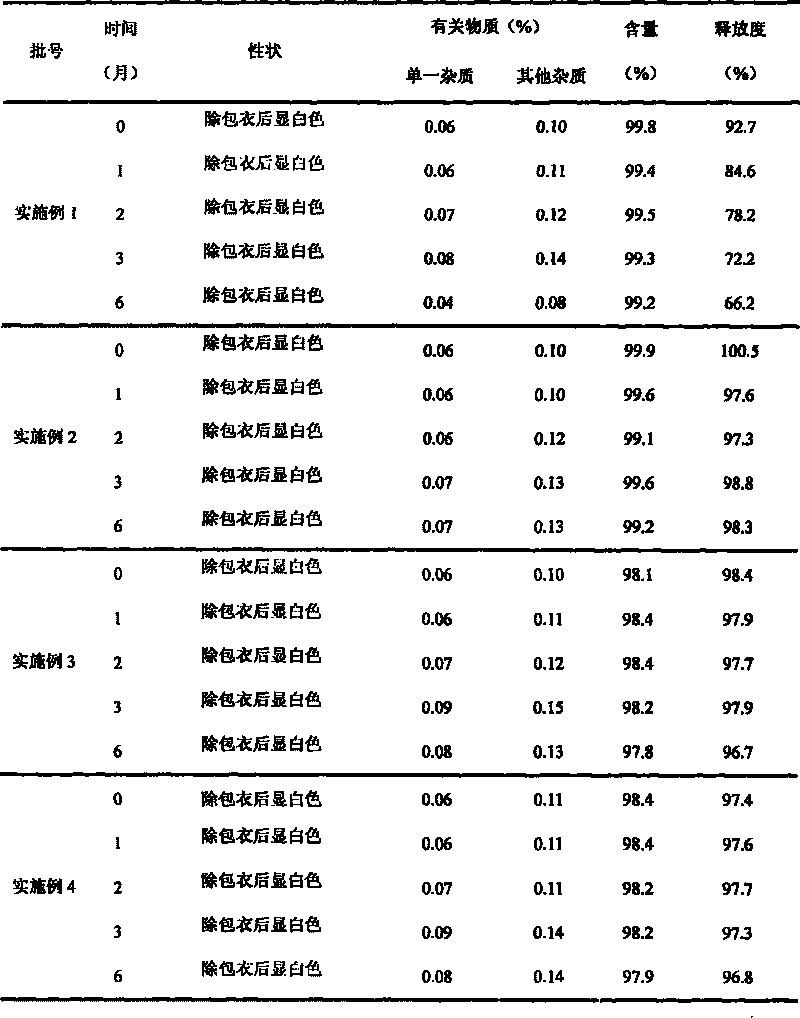
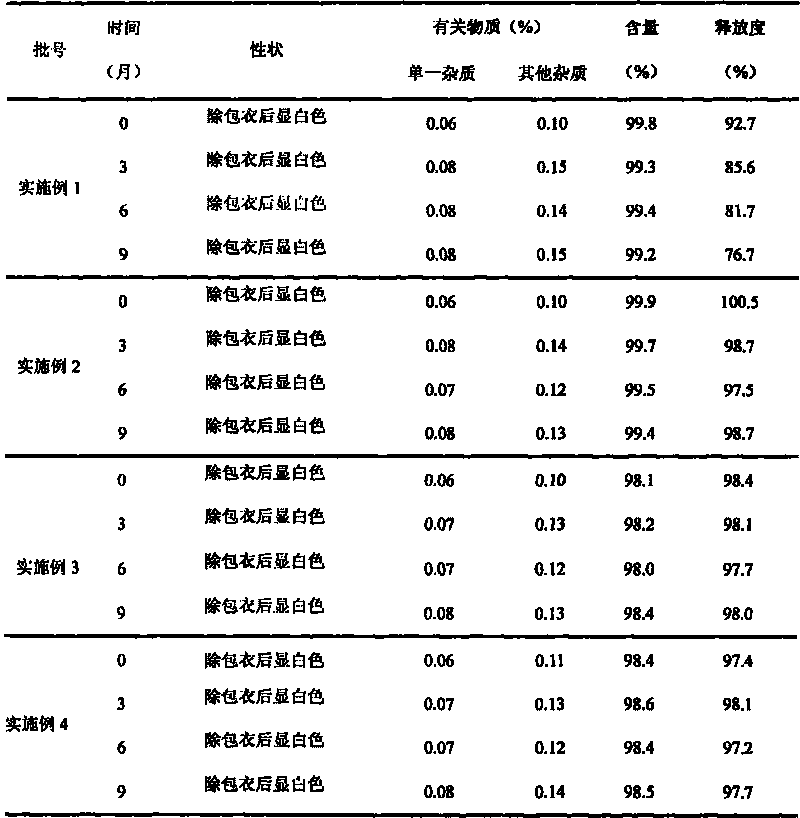
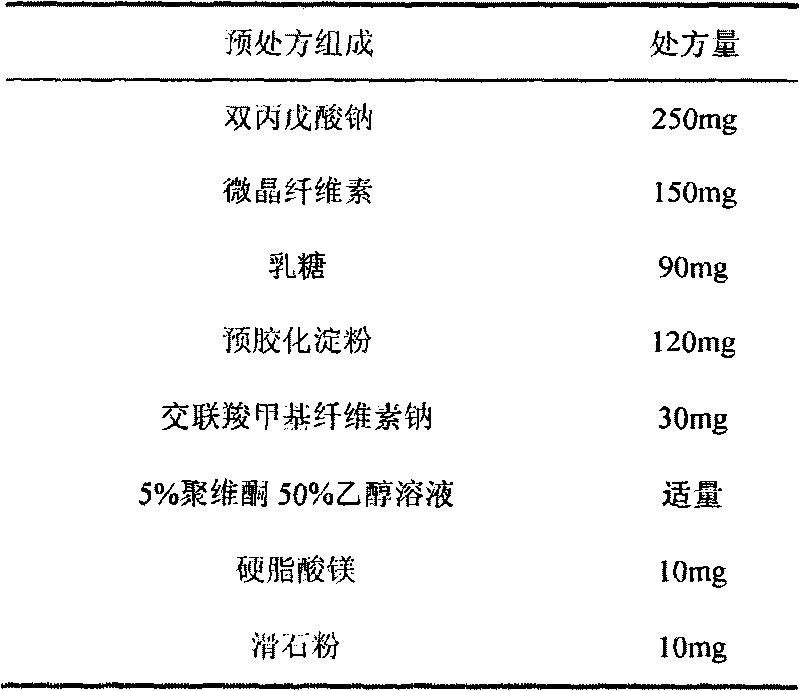
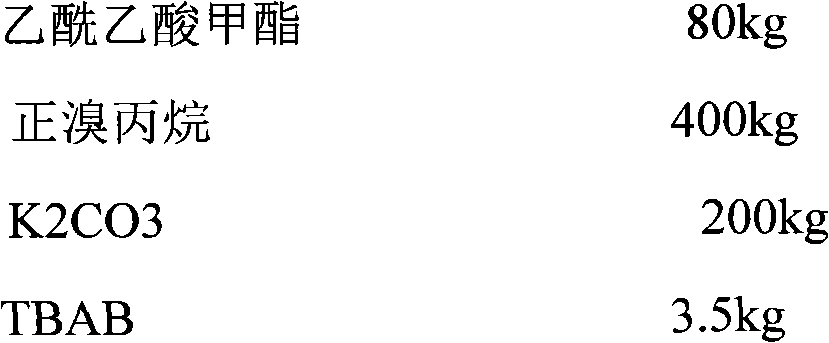
Divalproex sodium sustained release tablets and preparation method thereof
ActiveCN102138911AReduce dosageGood correlation between in vitro and in vivoNervous disorderPharmaceutical delivery mechanismMedicineDiluent
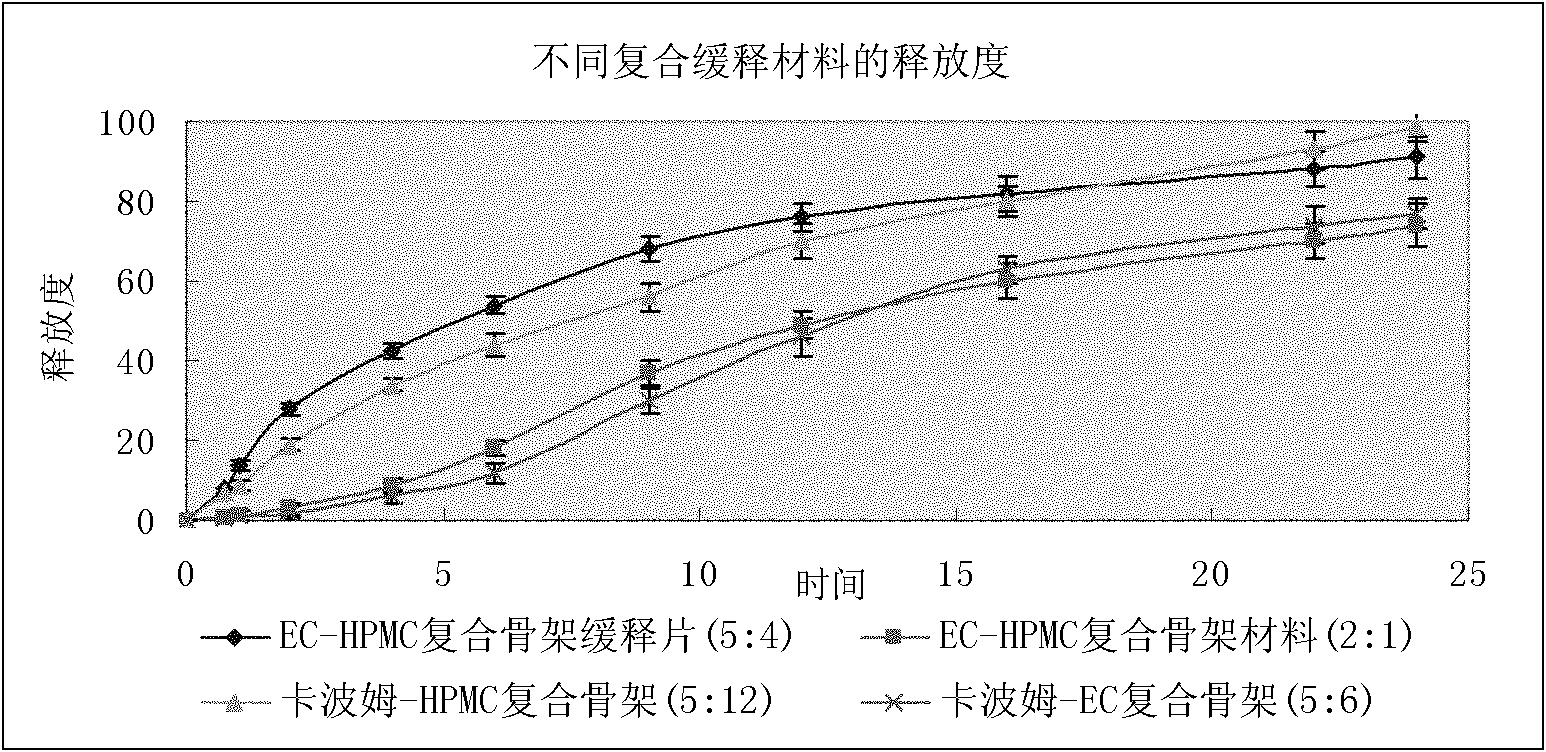
The invention belongs to the field of medicinal preparations, and particularly relates to divalproex sodium sustained release tablets and a preparation method thereof. The sustained release table comprises divalproex sodium, a retarding agent, a diluent, a disintegrant, a binder, a pH regulating agent, a lubricant, an antiadherent and the like.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Divalproex sodium medicine composition with improved oral absorptivity
InactiveCN101693113AMature technologyEasy to operateNervous disorderInorganic non-active ingredientsTraditional medicineDivalproex Sodium
The invention relates to a medicine composition containing divalproex sodium, which can be applied to patients safely, not only can improve the wettability and the flowability of the divalproex sodium, but also can improve the absorptivity of the divalproex sodium in gastrointestinal tracts. Particularly, the invention relates to a medicine composition containing amorphous divalproex sodium and betacyclodextrin. The preparation method of the medicine composition comprises the following steps: grinding, drying and pulverizing the divalproex sodium and the betacyclodextrin to obtain fine powder of 80-100 meshes; mixing with proper assistant materials and making into granules, and then tabletting and coating to obtain the medicine composition. The divalproex sodium medicine composition not only can improve release in vitro to a greater degree, thereby improving bioavailability, but can effectively treat epilepsia and vesania and prevent cephalagra; in addition, the divalproex sodium medicine composition can be taken orally and conveniently, can cover odor, and can be disintegrated and absorbed quickly and carried conveniently.
Owner:TIANJIN HANKANG PHARMA BIOTECH
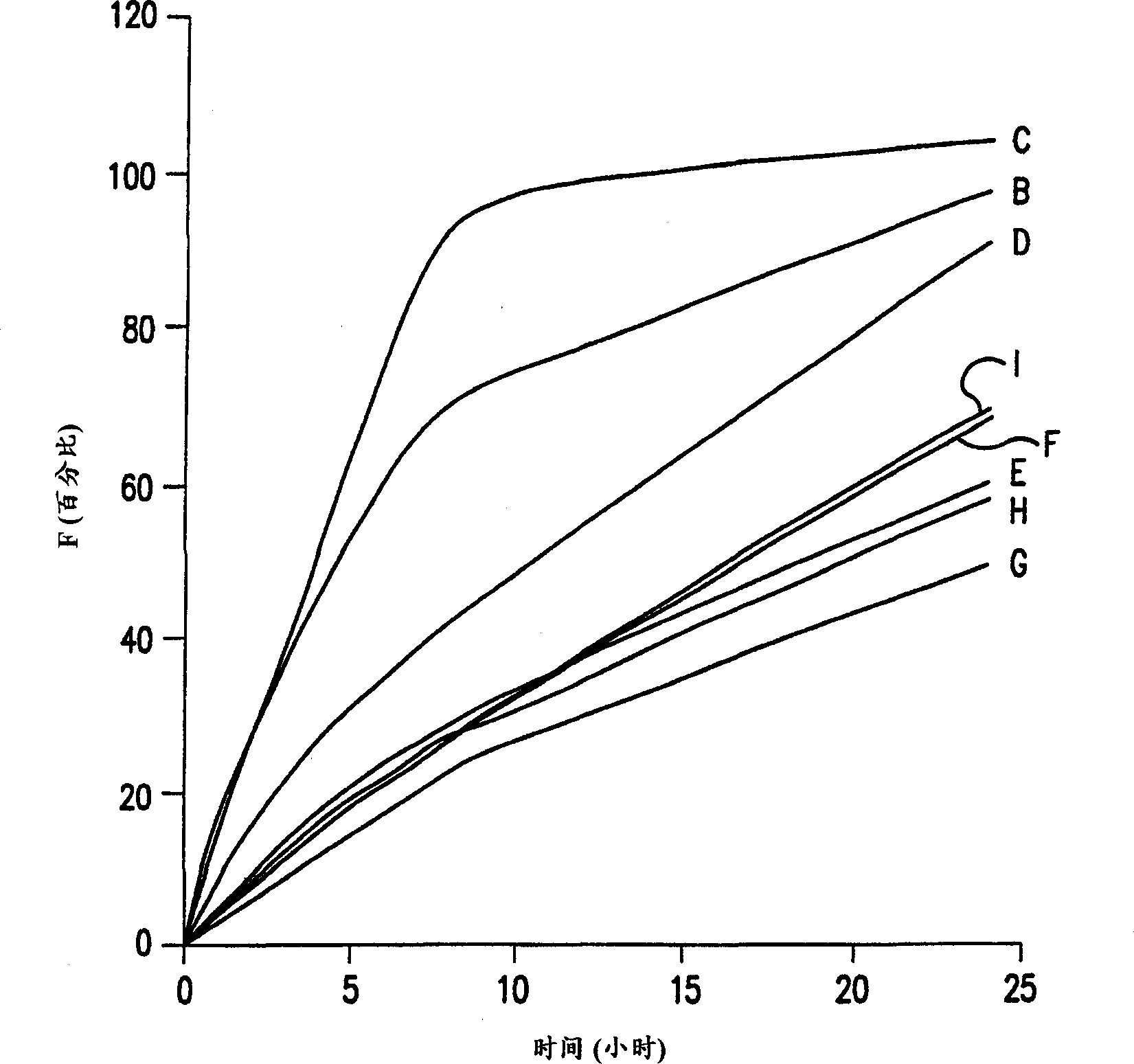
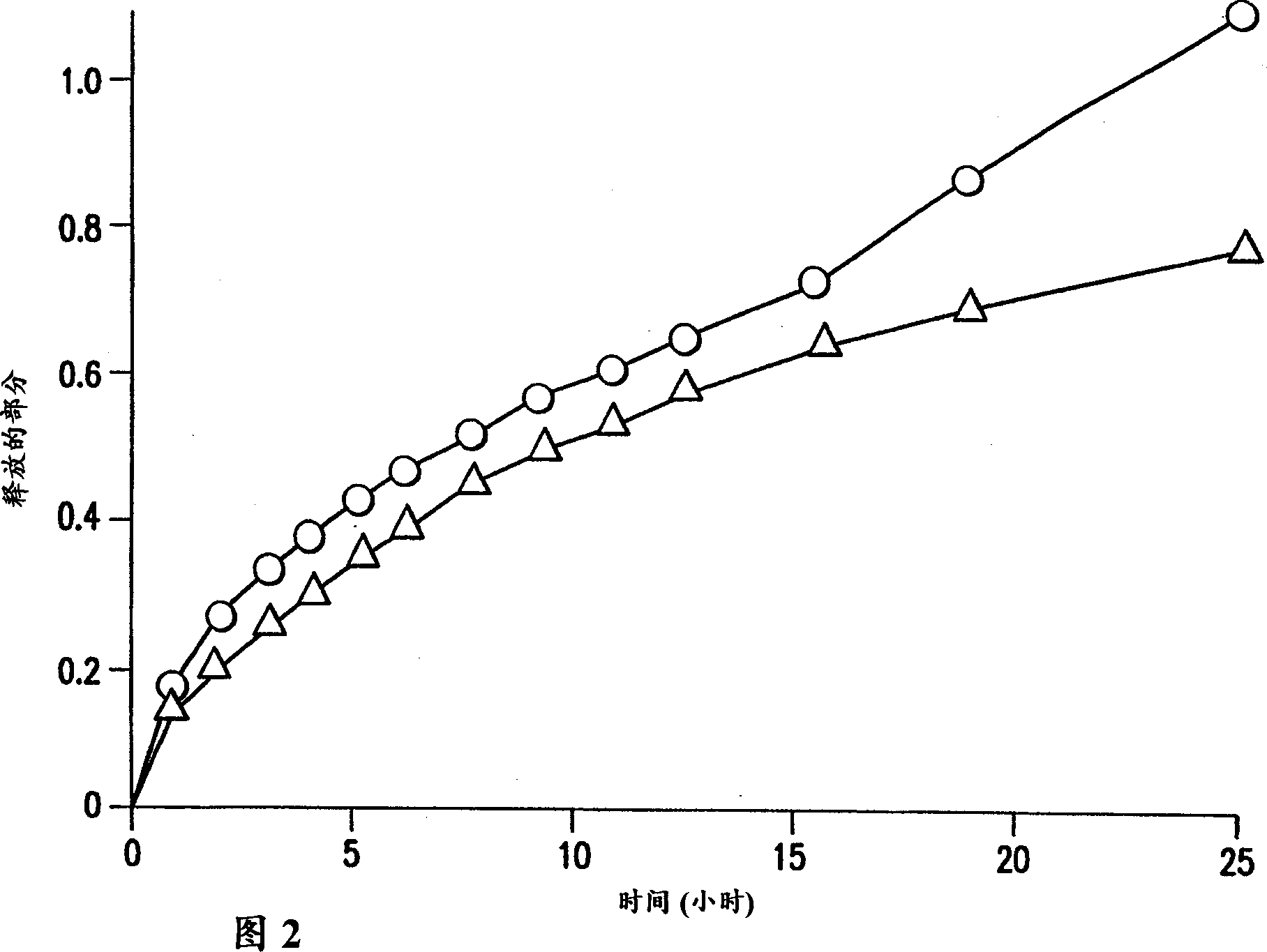
Extended release pharmaceutical compositions of divalproex sodium
The present invention relates to a sustained release preparation comprising valproic acid and the pharmaceutical acceptable salt, ester or amide, or divalproex sodium, and a preparation method of the sustained release preparation.
Owner:RANBAXY LAB LTD
Divalproex sodium sustained-release tablet and preparation process thereof
The invention provides a method for preparing a divalproex sodium-containing sustained-release tablet for treating epilepsy. The sustained-release tablet contains divalproex sodium, a sustained-release material, a binder and a lubricant. The preparation method comprises the following steps of: mixing the divalproex sodium and the sustained-release material to obtain a mixture, adding the binder for wet granulation, drying, granulating, adding into other uniformly mixed auxiliary materials, and tabletting to obtain the divalproex sodium-containing sustained-release tablet. The sustained-release tablet can be sustainedly released for 24 hours, and is good in appearance, stable in performance and convenient to take.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Extended release formulation of divalproex sodium
InactiveCN1671363AAvoid stickinessNervous disorderInorganic non-active ingredientsDivalproex SodiumExtended Release Formulations
The present invention relates to an extended release pharmaceutical composition comprising valproic acid, a pharmaceutically acceptable salt, ester, or amide thereof or divalproex sodium.
Owner:RANBAXY LAB LTD
Sustained release dosage forms
InactiveUS20050276849A1Good water solubilityIncrease chanceGranular deliveryMicrocapsulesSustained release drugSustained Release Dose Form
The present invention is directed to sustained release neutralized divalproex sodium oral dosage forms, processes for preparing the same, and methods of treatment therewith.
Owner:ANDRX
Controlled release formulation of divalproex sodium
The present invention provides a controlled release dosage formulation comprising a) about 40% to about 80% of a valproic acid compound such as Divalproex Sodium and b) at least two, preferably hydrophilic, polymers each in an amount of less than about 20% of the dosage weight.
Owner:TEVA PHARM USA INC
Solid dosage forms of divalproex sodium
InactiveUS20020127277A1Limited solubilityEnhanced interactionPowder deliveryPill deliveryOrganic solventDivalproex Sodium
Owner:ABBOTT LAB INC
Compositions with reduced hepatotoxicity
Pharmaceutical compositions of hepatotoxic compounds are provided in which the hepatotoxicity of the compounds is mitigated by including quantities of nicotinamide and methionine in the composition. Folic acid also can be included to further mitigate the hepatotoxic effects. The hepatotoxic compounds can include acetaminophen, methotrexate, atorvastatin, simvastatin, niacin, flucanozole, divalproex sodium, and valproic acid.
Owner:WINSTON LAB
Divalproex pharmaceutical compositions
InactiveUS20080057118A1Improve efficiencyLess drug lossBiocideNervous disorderDrug compoundValproic Acid
Pharmaceutical compositions comprising a valproic acid drug compound, providing modified release of the drug.
Owner:DR REDDYS LAB LTD +1
Novel controlled release formulations of divalproex sodium
A controlled release formulation comprising less than about 40% of anti-epileptic drug, about 20% to about 50% of rate controlling polymer and silica having a particle size less than about 1 micron and specific surface area not less than 70 m2 / g, all weight percentages are based upon the total weight of the dosage form, manufactured under normal atmospheric conditions.
Owner:LUPIN LTD
Stabilized divalproex sodium coated granules, preparation method and solid preparation thereof
ActiveCN102188392AImprove liquiditySelf-lubricatingNervous disorderPill deliveryDivalproex SodiumCoating materials
The invention discloses stabilized divalproex sodium coated granules containing cores of divalproex sodium crystal granules and coatings made of coating material. The invention also discloses a preparation method of the coated granules and a solid preparation containing the divalproex sodium coated granules. The divalproex sodium coated granules are characterized in that: no caking occurs after long-term storage, and preparations are easily prepared by using the granules.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Medicine for treating dumps emotional handicap type disease
InactiveCN101081272AEasy to takeImprove compliancePowder deliveryNervous disorderDiseaseTypes diseases
The medicine for treating depression and other mental disturb diseases is prepared with chlorimipramine, sulpiride, sodium valproate, borneol and Chinese herbal medicine extractum prepared with nutagrass flatsedge rhizome, arisaema with bile, wild jujube seed and other Chinese medicinal materials. The medicine consists of chemically synthetic medicine and Chinese herbal medicine component, and has synergistic treating effect, less toxic side effect, and good patient's compliance.
Owner:张宝山
Divalproex sodium tablets
A process for preparing divalproex sodium tablets is provided. The process comprises preparing a neutralized divalproex sodium solution by combining divalproex sodium, having a sodium valproate and a valproic acid moiety, with an aqueous solvent and a base, e.g., sodium hydroxide, the base being in sufficient amount to ensure neutralization of the valproic acid moiety of the divalproex sodium. The neutralized divalproex sodium solution is sprayed onto a pharmaceutically acceptable carrier, and processed to obtain divalproex sodium tablets.
Owner:ANDRX
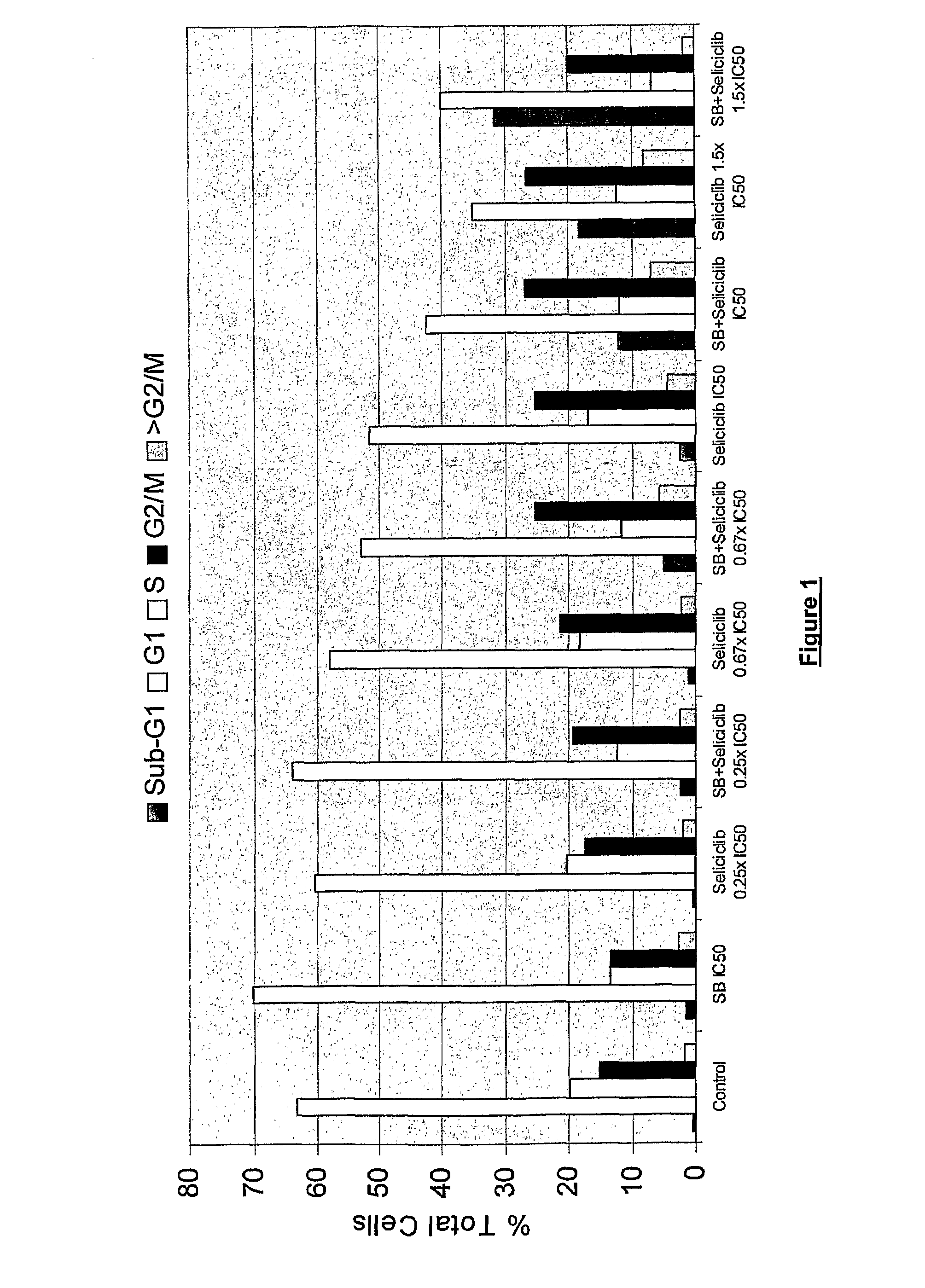
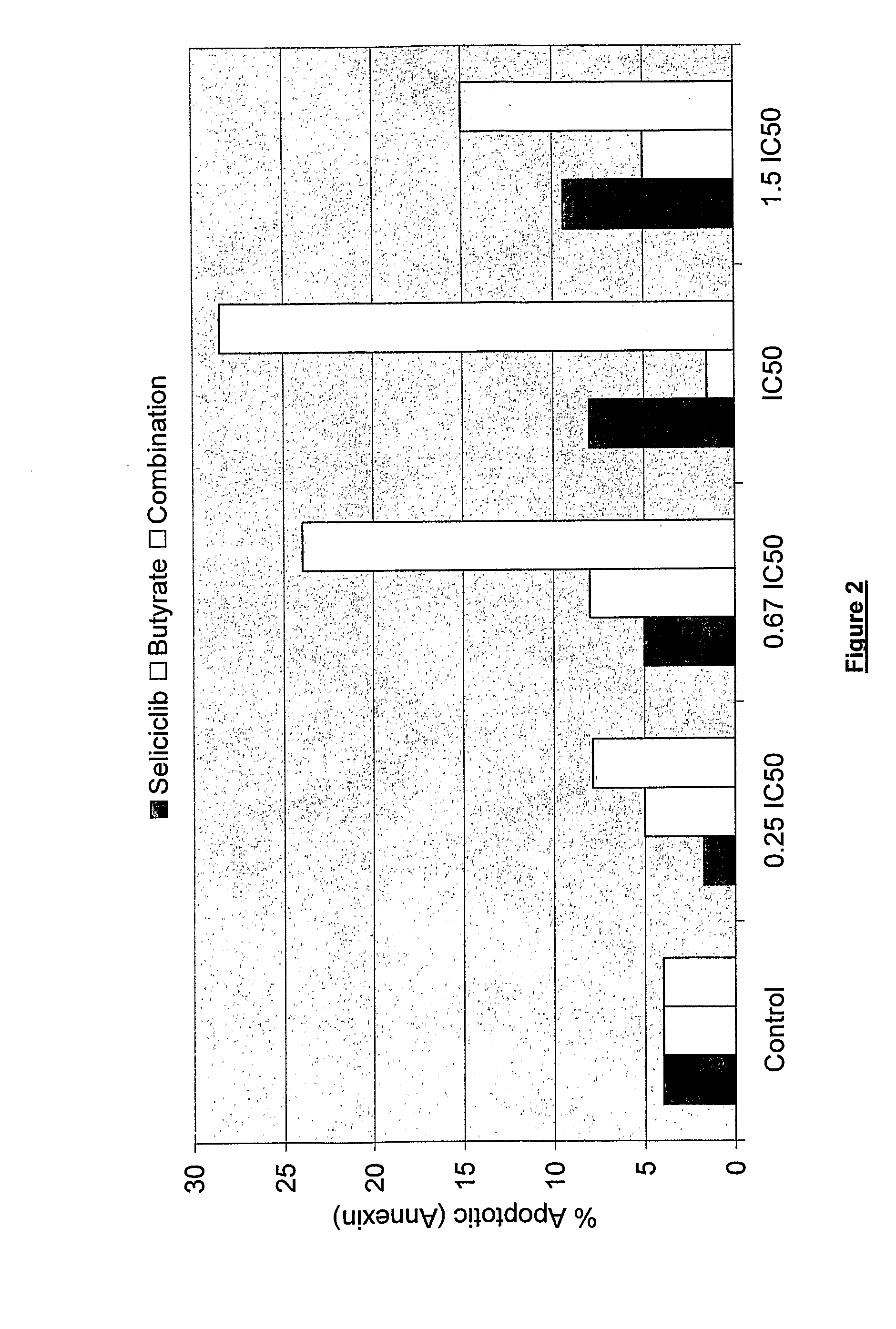
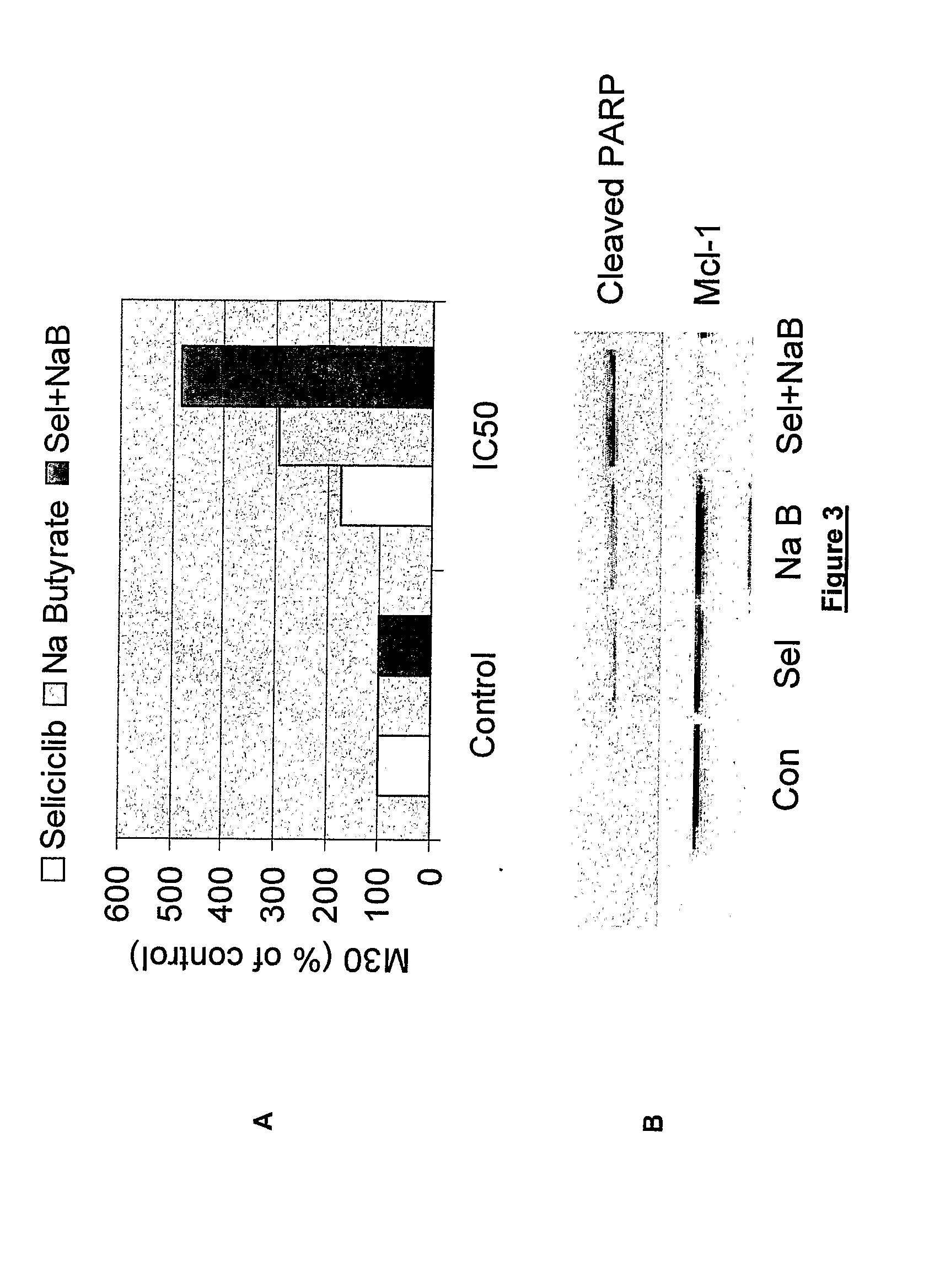
Combination of roscovitine and a hdca inhibitor to treat proliferative diseases
A first aspect of the invention relates to a combination comprising roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA). A second aspect of the invention relates to a pharmaceutical product comprising roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA) as a combined preparation for simultaneous, sequential or separate use in therapy. A third aspect of the invention relates to a method for treating a proliferative disorder, said method comprising simultaneously, sequentially or separately administering roscovitine, or a pharmaceutically acceptable salt thereof, and a HDAC inhibitor selected from sodium butyrate, or a prodrug thereof, suberoylanilide hydroxamic acid (SAHA), sodium valproate and trichostatin A (TSA) to a subject.
Owner:CYCLACEL
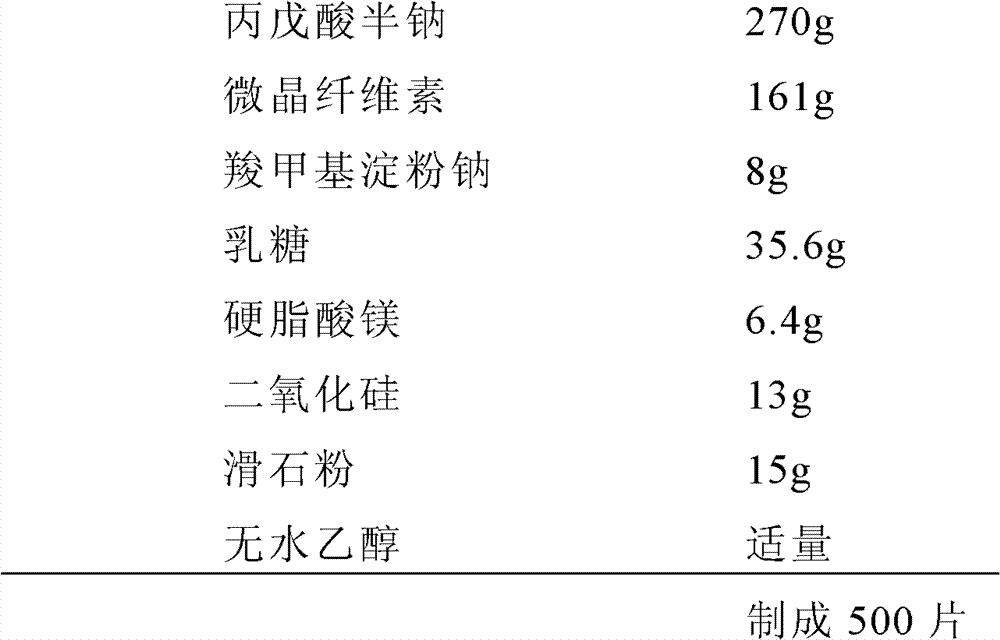
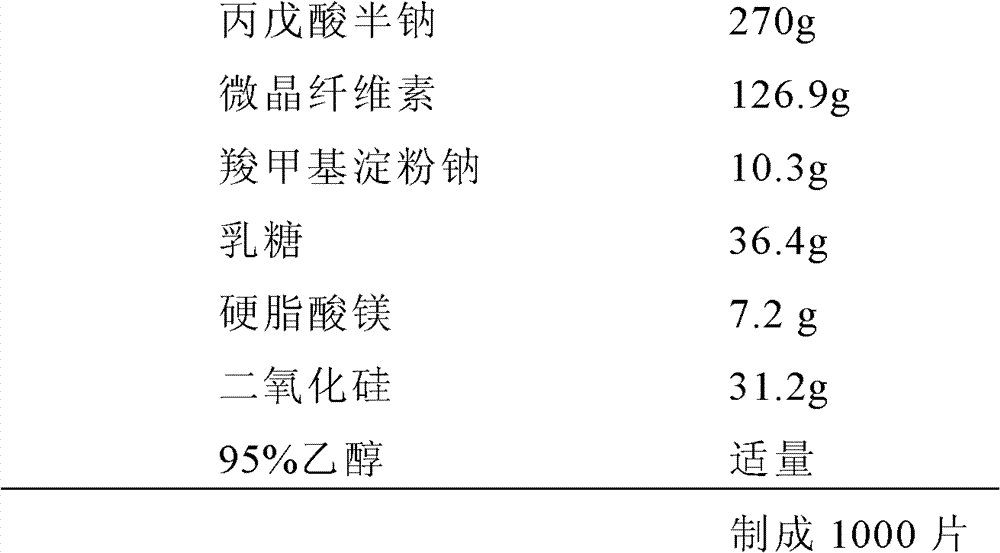
Valproate semisodium tablet and its preparation method
ActiveCN102895201AQuality improvementGood lookingNervous disorderPharmaceutical non-active ingredientsMedicineLactose
The invention provides a valproate semisodium tablet and its preparation method. The common valproate semisodium tablet comprises 50-67wt% of valproate semisodium, 25-35wt% of a microcrystalline cellulose, 5-9wt% of lactose, 1-3wt% of sodium carboxymethyl starch, 0.2-2wt% of magnesium stearate, and 1-10wt% of silica and / or talcum powder. The valproate semisodium tablet has the advantages of stable quality, no fractured, cracked or crushed tablets, and realization of the enteric-coated tablet release degree according with requirements of Chinese Pharmacopoeia 2005. The preparation method obviously overcomes the disadvantages comprising bad particle fluidity, sticking, tablet breaking and tablet appearance disqualification in present production technologies, has no special requirements on the production environment, enables the qualification rate of products to reach above 95%, and is suitable for large-scale industrialized production.
Owner:NEW FOUNDER HLDG DEV LLC +2
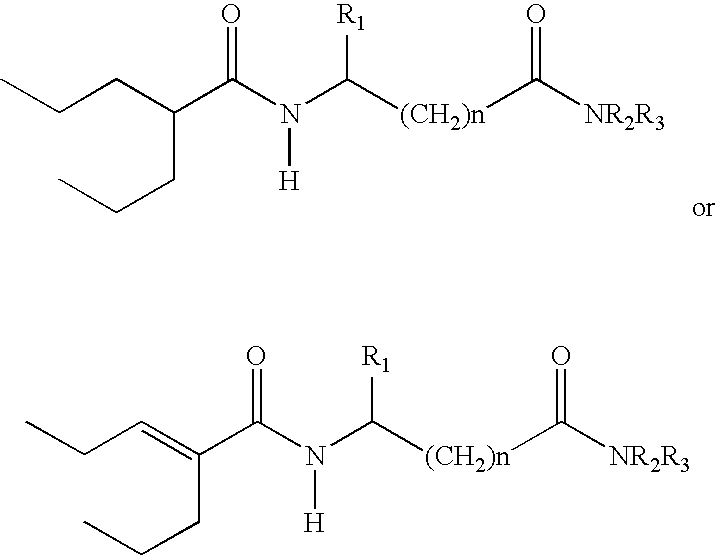
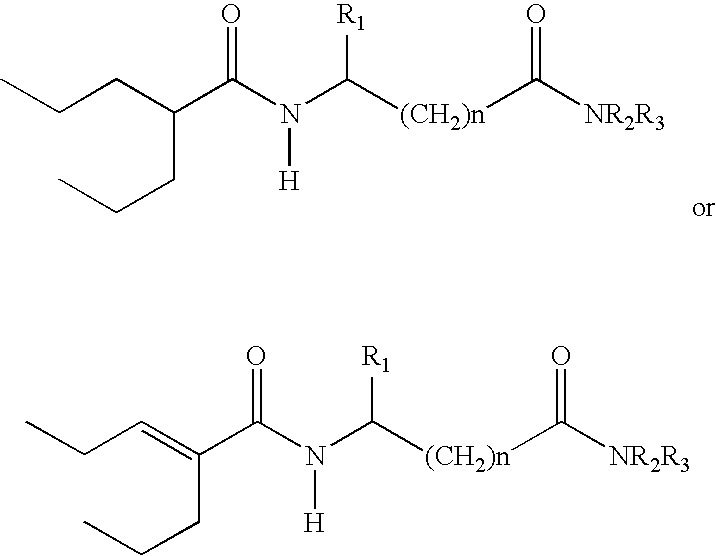
Sustained release formulation of N- (2-propylpentanoyl) glycinamide and related compounds
InactiveUS20040175423A1Maximum sustained actionHigh viscosityBiocidePill deliveryBULK ACTIVE INGREDIENTSustained Release Formulations
The subject provides a sustained release tablet comprising the following components: a) a uniform admixture of an active ingredient selected from the group consisting of valproic sodium acid, a pharmaceutically acceptable salt or ester of valproic acid, divalproex sodium, valpromide and a compound having the structure: wherein R1, R2, and R3 are independently the same or different and are hydrogen, a C1-C6 alkyl group, an aralkyl group, or an aryl group, and n is an integer which is greater than or equal to 0 and less than or equal to 3; and a binder, and b) a hydroxypropylmethyl cellulose, a process for manufacturing the tablet and a method of treating neuropathic pain, epilepsy, mania in bipolar disorder, a headache disorder, pain or of effecting pain prophylaxis in a subject.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
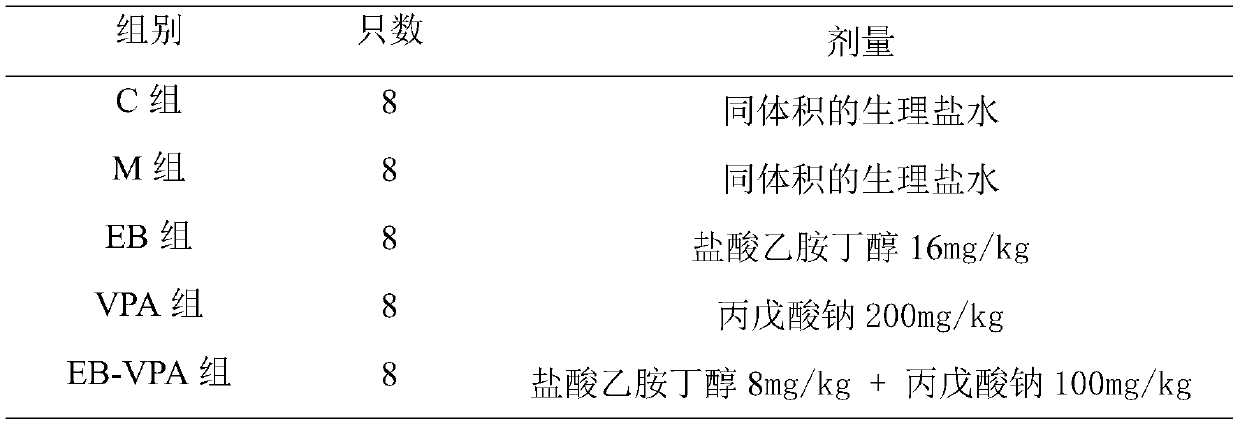
Pharmaceutical composition for treating intractable epilepsy and application thereof
ActiveCN109966277AHigh dependenceSmall toxicityNervous disorderAnhydride/acid/halide active ingredientsSide effectActive component
The invention discloses a pharmaceutical composition for treating intractable epilepsy and application thereof. The active components of the pharmaceutical composition comprise sodium valproate or divalproex sodium and ethambutol or medicinal salt thereof. The pharmaceutical composition has the advantages that the fact that low-dose ethambutol hydrochloride has an anti-epileptic effect is discovered for the first time, the ethambutol hydrochloride is combined with the sodium valproate or divalproex sodium to treat the intractable epilepsy, the pharmaceutical composition is evident in anti-epileptic effect, and the toxic and side effects of the pharmaceutical composition are lowered evidently.
Owner:AFFILIATED HOSPITAL OF JINING MEDICAL UNIV
Divalproex sodium sustained release pellets and preparation method thereof
InactiveCN104352445AReduce absorption rateStable absorptionNervous disorderGranular deliverySustained release pelletsAdhesive
The invention provides divalproex sodium sustained release pellets. The divalproex sodium sustained release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated by the coating layers; the medicine-containing pellets comprise 250mg of divalproex sodium, 70mg of hollow pellet cores, 60-110mg of filling agent, 18-68mg of lubricating agent and 2-10mg of adhesive; the coating layers comprise 45-225mg of Eudragit NE30D and 7-68mg of talcum powder. A preparation method of the divalproex sodium sustained release pellets comprises the following processes: 1. material preparation; 2. mixing; 3. preparation of the adhesive; 4. preparation of the pellets; 5. preparation of a coating agent; 6. coating; 7. filling; 8. aluminium-plastic packaging and preparation of finished products. The divalproex sodium sustained release pellets used for treating epilepsy and mania and the preparation method have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the divalproex sodium sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Double sodium valproate orally disintegrating tablets and preparation method thereof
InactiveCN101310710AReasonable formulaGood compatibilityNervous disorderPill deliveryOrally disintegrating tabletCross-linked polyethylene
The invention discloses a divalproex sodium orally disintegrating tablet and a preparation method thereof. The principal medicine of the orally disintegrating tablet is divalproex sodium and the accessories are microcrystalline cellulose, mannitol, lactose, polyacrylic resin II, cross linked polyvinylpyrrolidone, sodium cyclamate, menthol and aerosil. The divalproex sodium orally disintegrating tablet of the invention can effectively cure falling sickness and vesania, can prevent hemicrania and has the advantages of convenient taking, good taste, fast disintegration, quick absorption and high biological availability. The divalproex sodium orally disintegrating tablet provides convenience for old people, children or patients having difficulty in swallowing and people having inconvenience to get water.
Owner:QINGDAO UNIV OF SCI & TECH
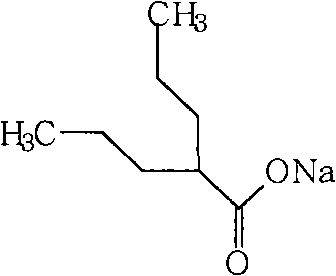
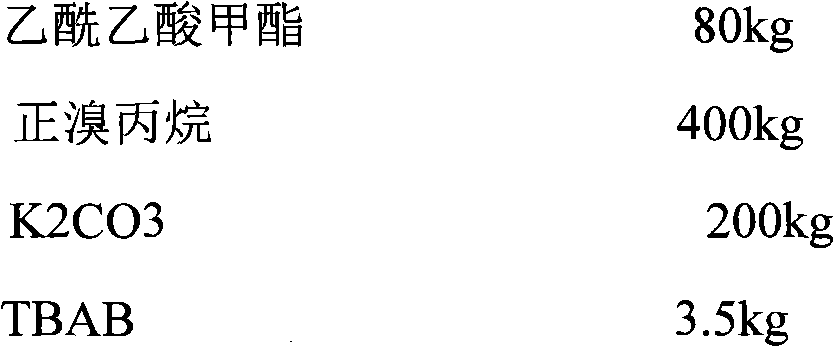
Sodium valproate and preparation process thereof
ActiveCN102579849AHigh yieldThe group is rational and orderlyNervous disorderAnhydride/acid/halide active ingredientsCelluloseSalvia miltiorrhiza
The invention relates to compound sodium valproate and a preparation process thereof. The sodium valproate is prepared by raw materials including sodium valproate 0.3 part, salvia miltiorrhiza 30 parts, pepper 25 parts, rhizoma nardostachyos 20 parts, gastrodia elata 12 parts and uncaria 10 parts by weight, and the sodium valproate is prepared into capsules, tablets, particles and other drug forms after pharmaceutical excipients such as starch or edible cellulose are added according to a common pharmacy method. A compound sodium valproate drug composition treats both principal and secondary aspect of epilepsia disease, is non-toxic harmless and free of side effect to human body, low in price, simple and convenient to manufacture, and has wide application prospect.
Owner:仁和堂药业有限公司
Sodium valproate injection
ActiveCN105326784AImprove stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsEthylenediamineAcetic acid
The invention provides a sodium valproate injection. The sodium valproate injection is composed of sodium valproate, a stabilizing agent, a pH regulating agent and water for injection, wherein EDTA (ethylene diamine tetraacetic acid)-2NaCa serves as the stabilizing agent. By adoption of the EDTA-2NaCa serving as the stabilizing agent, stability of the sodium valproate injection in preparation and storage is evidently superior to that of sodium valproate injections adopting other stabilizing agents, to be more specific, the sodium valproate injection in preparation and storage is small in pH value variation range, related substances are less increased, and active ingredients are less reduced. Therefore, safety of the sodium valproate injection can be further improved by adoption of the EDTA-2NaCa serving as the stabilizing agent.
Owner:SICHUAN CREDIT PHARMA
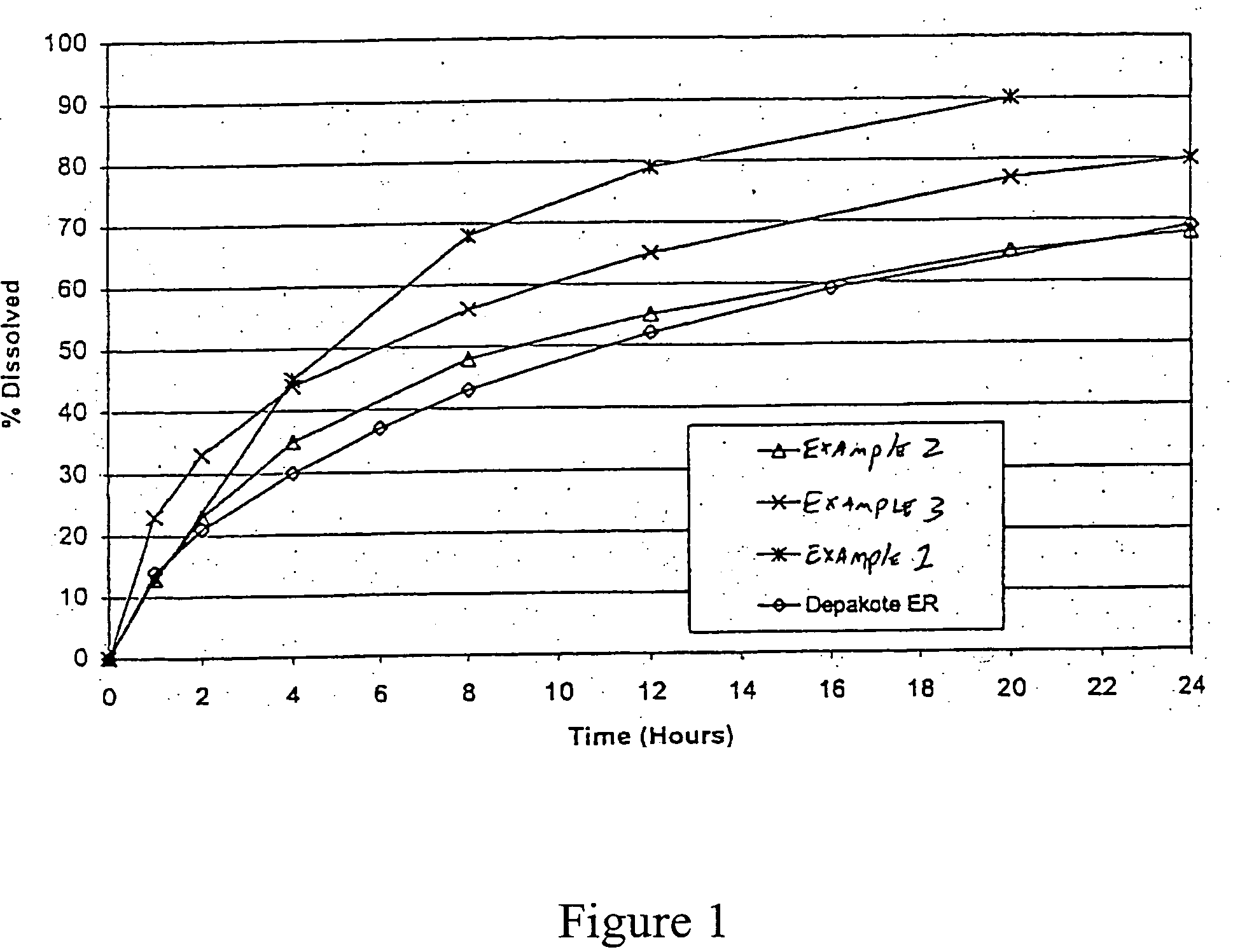
Divalproex sodium sustained release tablet
ActiveCN107028907ASolve sticking problemsUniform contentNervous disorderPill deliverySustained Release TabletMedicine
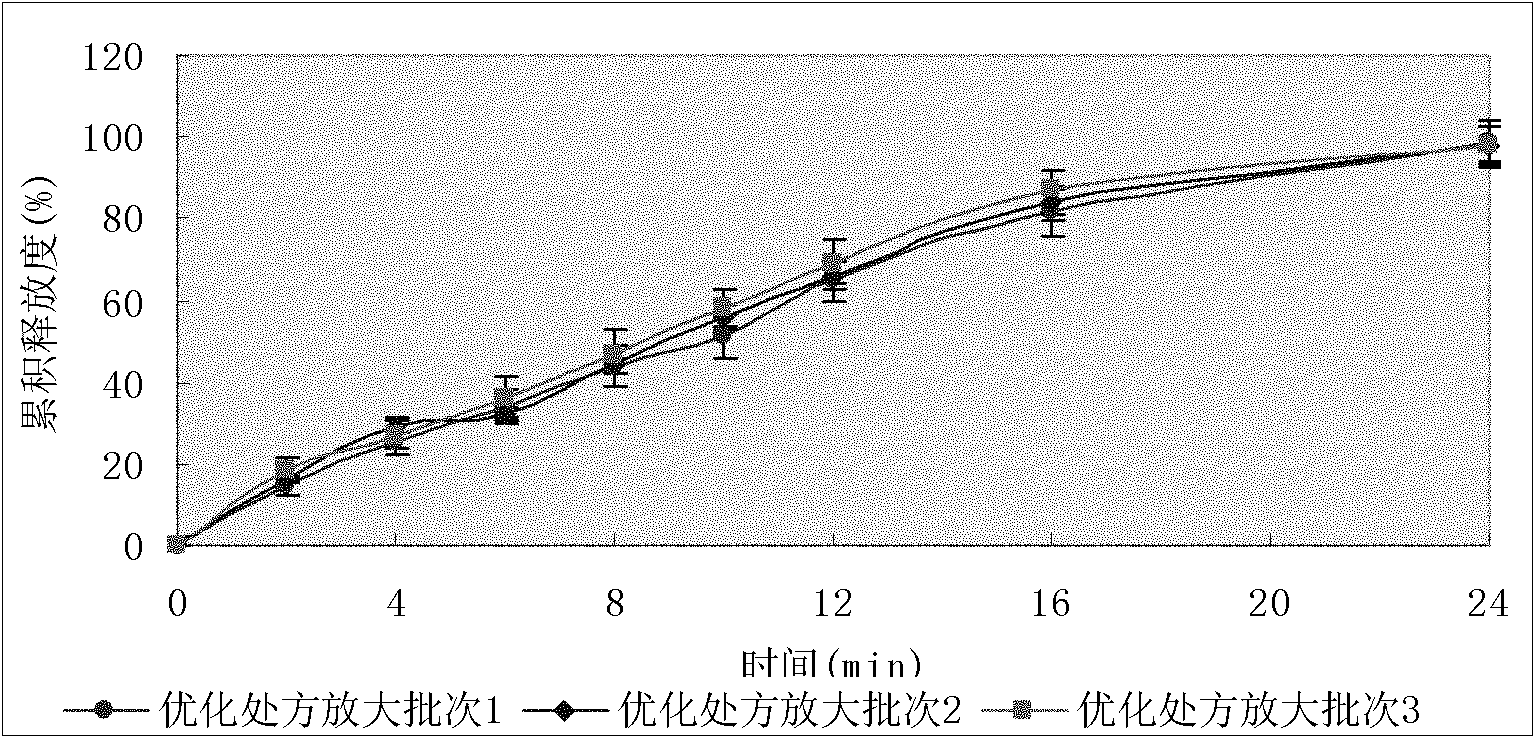
The invention discloses a divalproex sodium sustained release tablet. A process comprises the following steps: S1, preparing an auxiliary soft material; and S2, preparing a medicine-containing soft material, and preparing particles. The obtained particles are even and orderly in appearance, no sticky micelles are formed, the particles are moderate in size and have good mobility and compressibility, and the contents of the active ingredients are uniform, so that the quality of the medicine is stable and controllable. The divalproex sodium sustained release tablet prepared by adopting the particles has good in-vitro release, the release curve has high similarity with that of a post-market drug product, and the medicine can be effectively controlled to slowly release within 24h.
Owner:CHENGDU KANGHONG PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com