Divalproex sodium medicine composition with improved oral absorptivity
The technology of sodium divalproex and composition is applied in the field of sodium divalproex pharmaceutical composition and preparation thereof, which can solve the problems of poor particle fluidity, low release degree, stickiness and the like, and achieves improved bioavailability The effect of improving the release degree in vitro and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
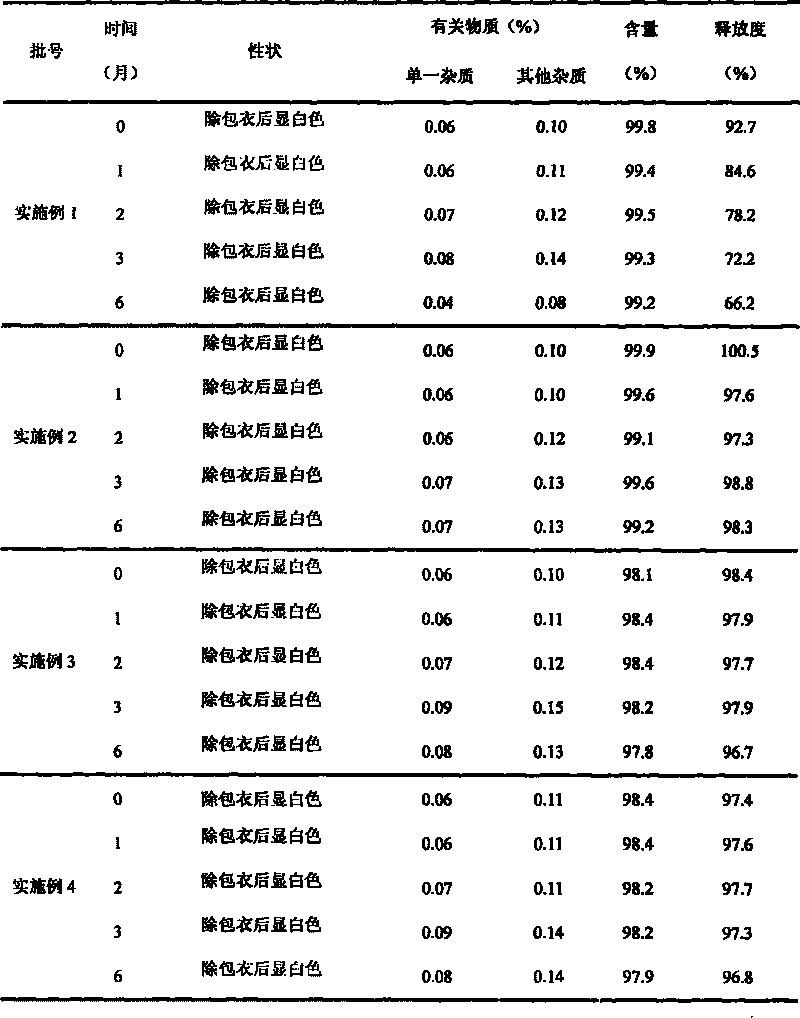
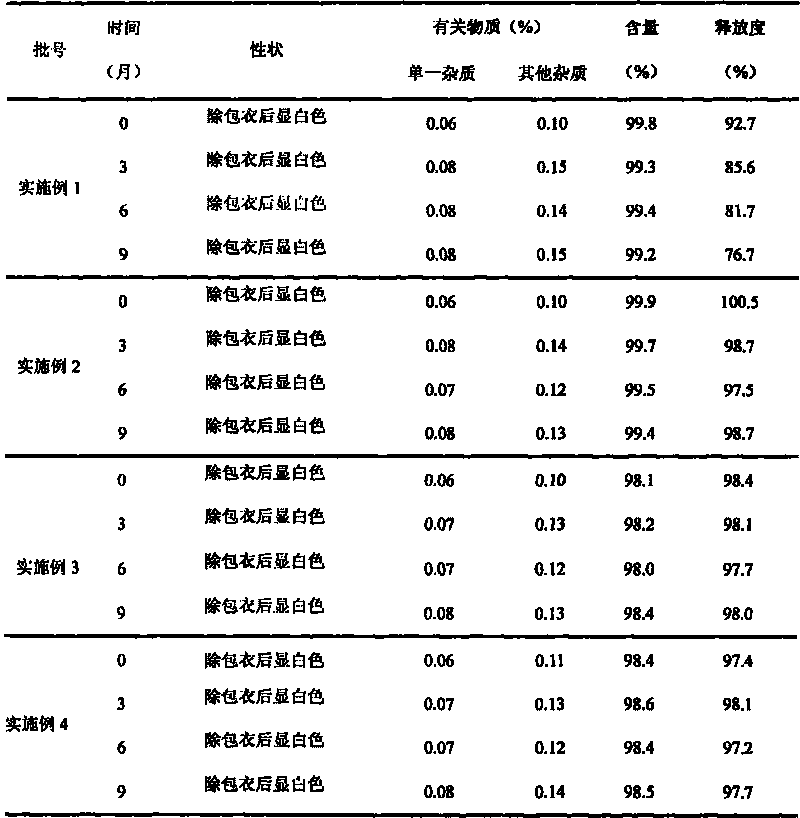
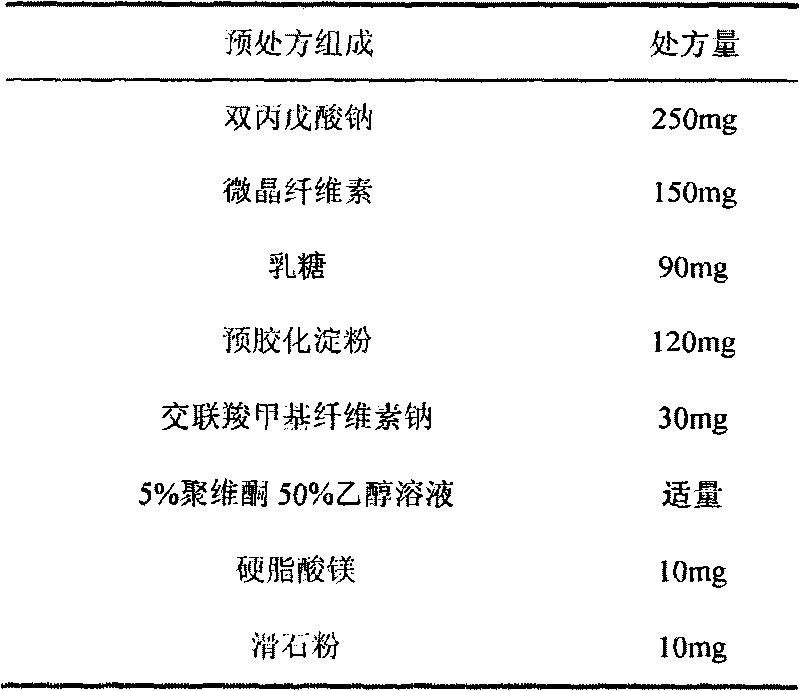
[0089] Embodiment 1 (prior art)
[0090] (1) Prescription
[0091] Chip composition
[0092] Divalproex Sodium 250g
[0093] Croscarmellose Sodium 30g
[0094] Microcrystalline Cellulose 150g
[0095] Lactose 90g
[0096] 120g pregelatinized starch
[0099] 5% povidone absolute ethanol appropriate amount
[0100] Coating Composition
[0101] Coating powder (enteric-coated type) 72.8g
[0102] 80% ethanol solution 728ml
[0103]
[0104] Makes 1000 pieces
[0105] (2) Preparation method
[0106] 1) Pulverize divalproex sodium, sieve, and set aside; sieve microcrystalline cellulose, lactose, croscarmellose sodium, pregelatinized starch, magnesium stearate, and talcum powder, respectively, and set aside;
[0107] 2) Weigh the raw and auxiliary materials according to the prescription amount respectively, and set aside;
[0108] 3) The uniformly mixed raw and auxiliary m...
Embodiment 2
[0112] (1) Prescription
[0113] Composition 1
[0114] Divalproex Sodium 250g
[0115] Beta Cyclodextrin 125g
[0116] Composition 2
[0117] Croscarmellose Sodium 20g
[0118] Microcrystalline Cellulose 100g
[0119] Lactose 120g
[0120] 40g pregelatinized starch
[0121] Talc powder 10g
[0123] 5% povidone absolute ethanol solution appropriate amount
[0124] Coating Composition
[0125] Coating powder (enteric-coated type) 81g
[0126] 80% ethanol solution 810ml
[0127] (2) Preparation method
[0128] 6) Put divalproex sodium and beta-cyclodextrin in a mortar, add a small amount of water to grind into a paste, dry below 50°C, grind into 80-100 mesh fine powder, and set aside;
[0129] 7) Sieve microcrystalline cellulose, lactose, croscarmellose sodium, pregelatinized starch, talcum powder, and magnesium stearate respectively, and set aside;
[0130] 8) Add the excipient 2) of the prescription amount into 1), mix evenly, and ...
Embodiment 3
[0134] (1) Prescription
[0135] Composition 1
[0136] Divalproex Sodium 250g
[0137] Beta Cyclodextrin 250g
[0138] Composition 2
[0139] Croscarmellose Sodium 30g
[0140] Microcrystalline Cellulose 60g
[0141] Lactose 80g
[0142] 30g pregelatinized starch
[0143] Talc powder 10g
[0145] 5% povidone absolute ethanol solution appropriate amount
[0146] Coating Composition
[0147] Coating powder (enteric-coated type) 79.2g
[0148] 80% ethanol solution 792ml
[0149] (2) Preparation method
[0150] 1) Put divalproex sodium and beta-cyclodextrin in a mortar, add a small amount of water and grind them into a paste, dry below 50°C, grind them into 80-100 mesh fine powder, and set aside;
[0151] 2) Sieve microcrystalline cellulose, lactose, croscarmellose sodium, pregelatinized starch, talcum powder, and magnesium stearate respectively, and set aside;
[0152] 3) Add the excipient 2) of the prescription amount into 1), mix e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com