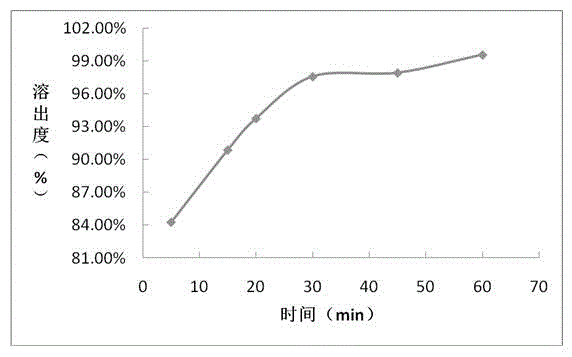
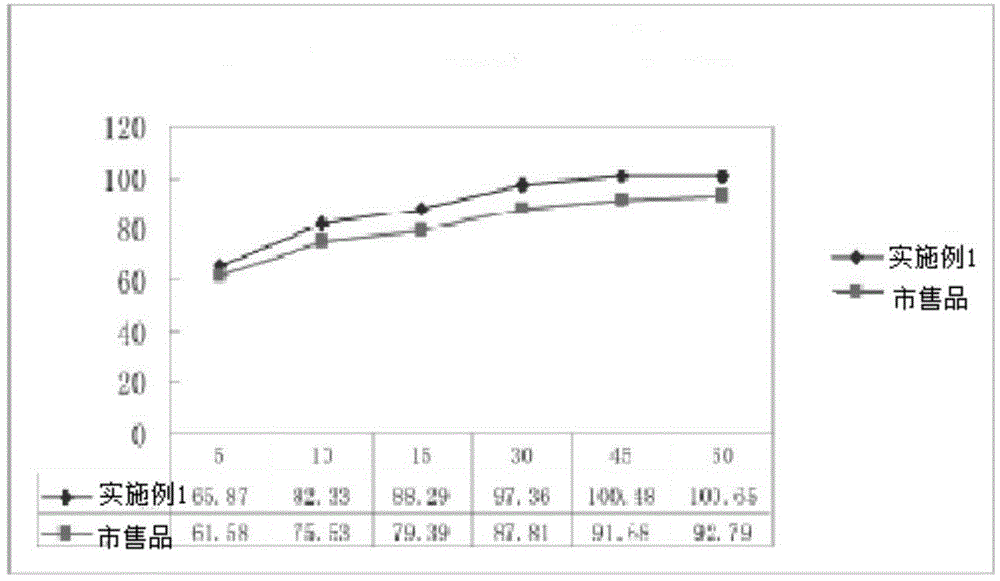
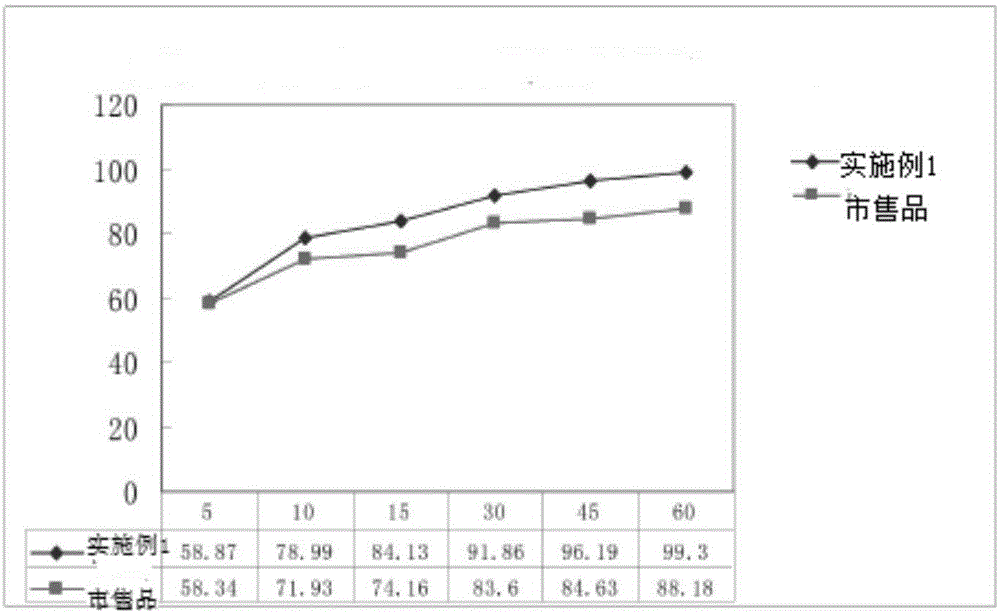
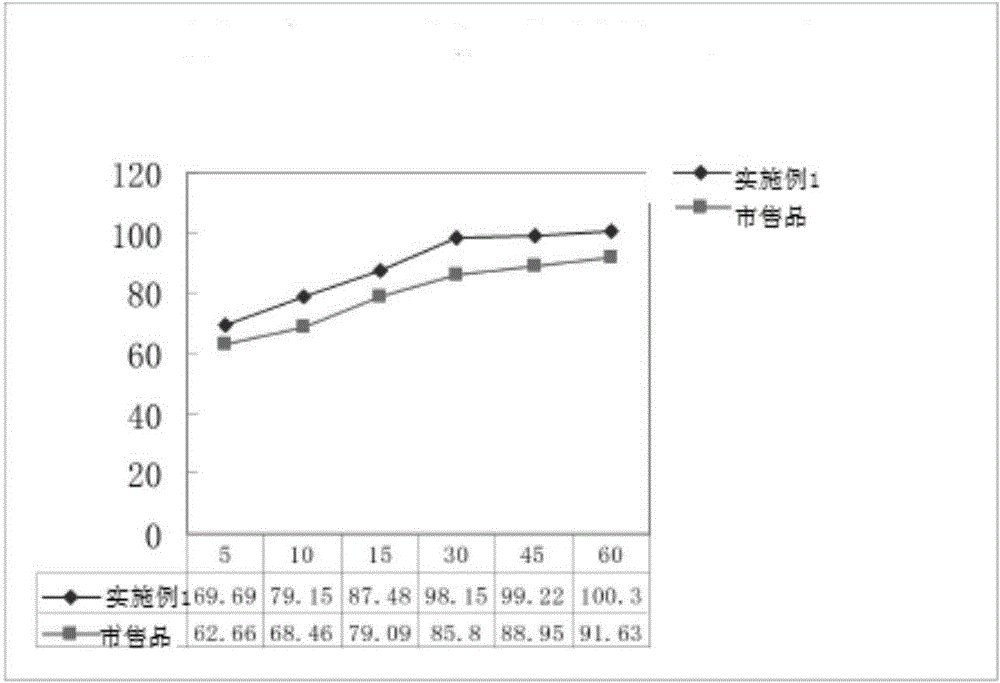
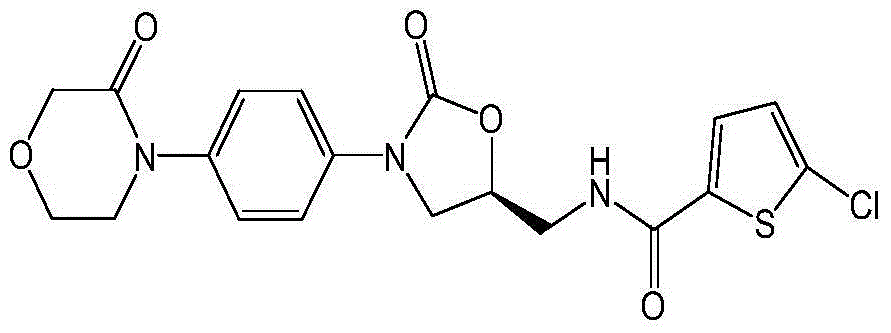
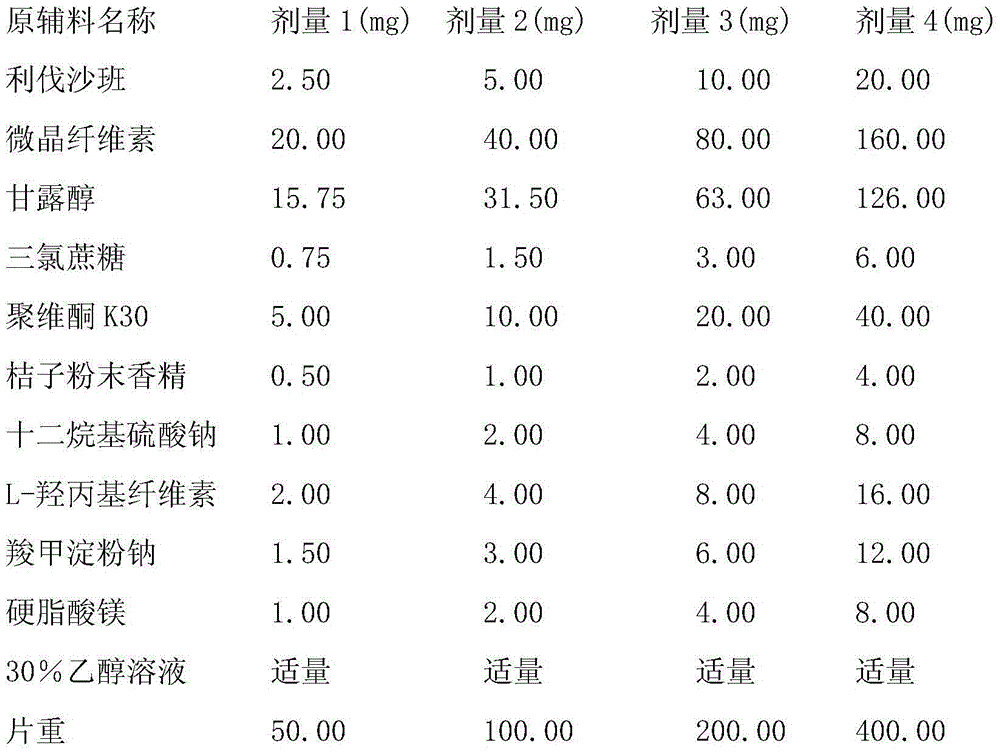
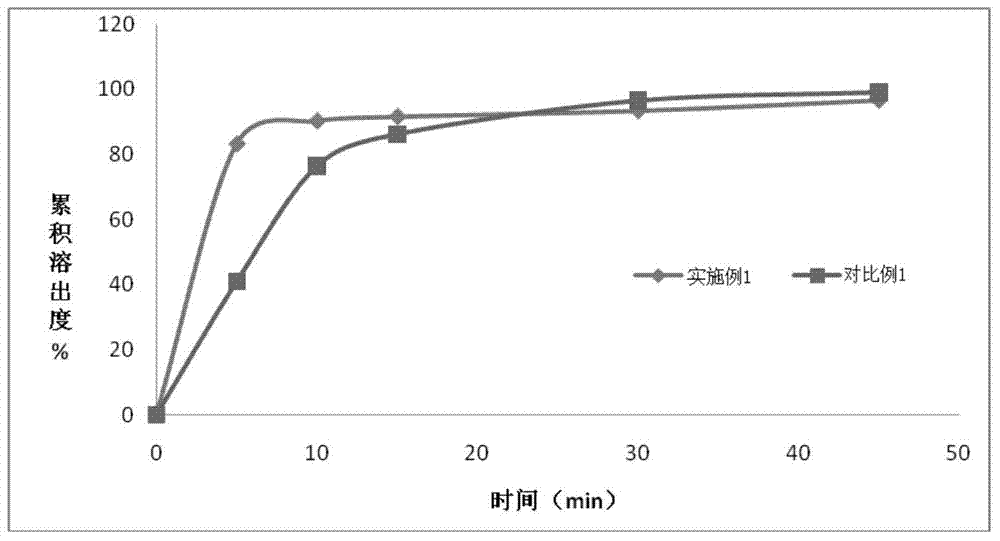
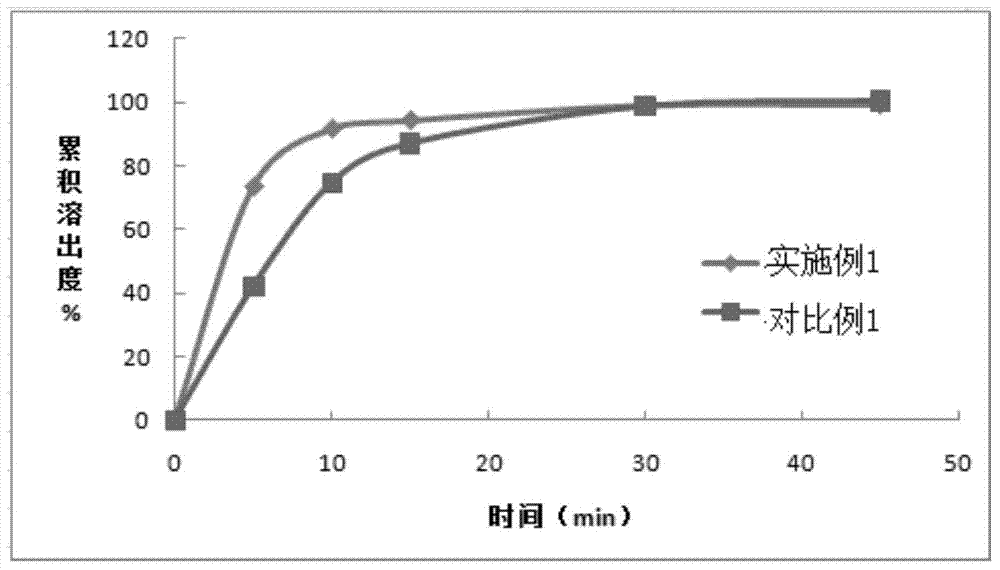
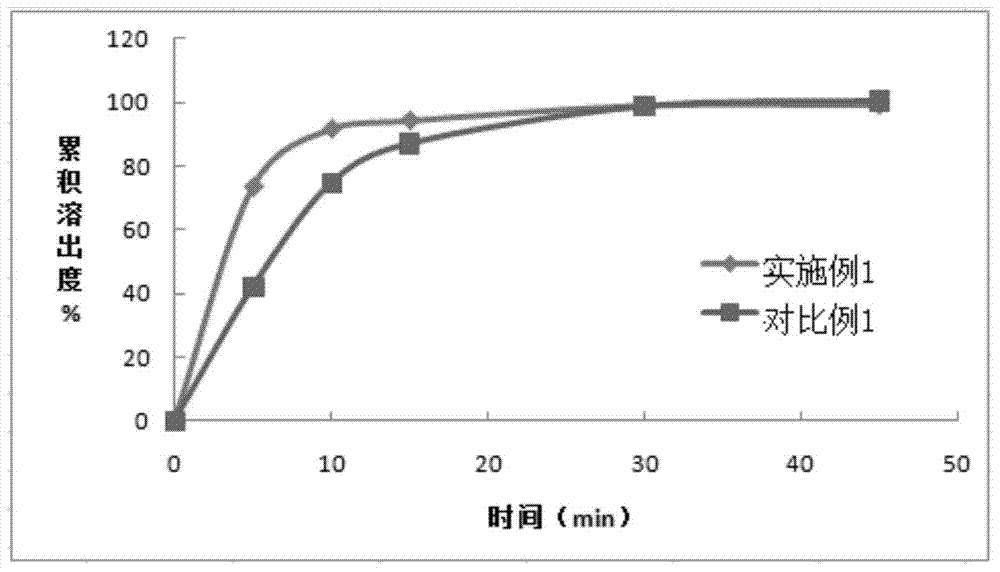
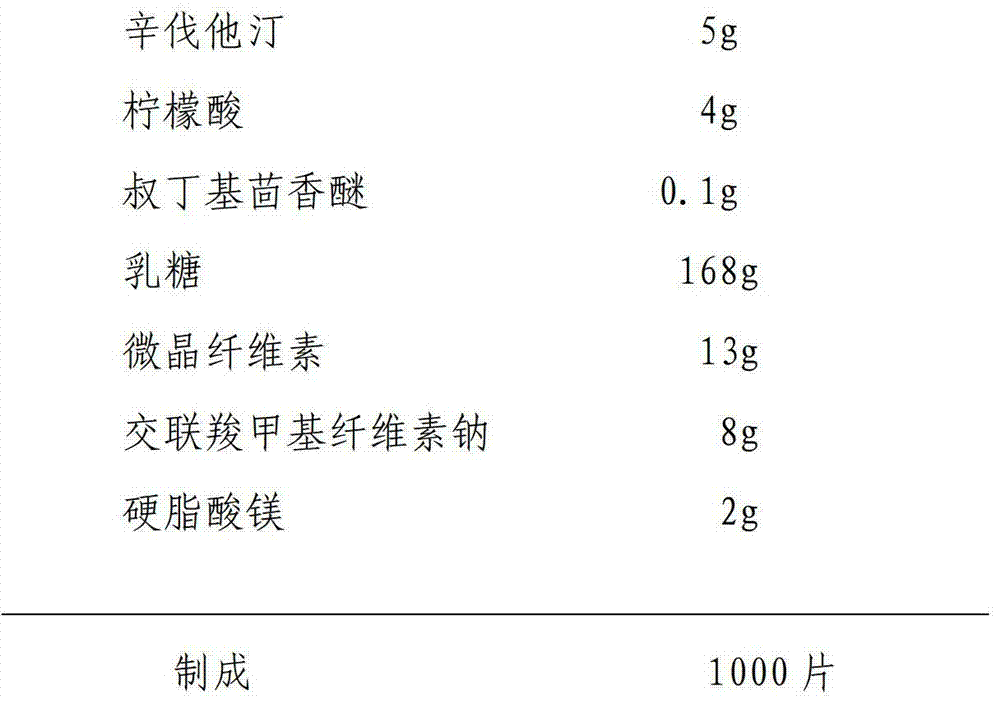
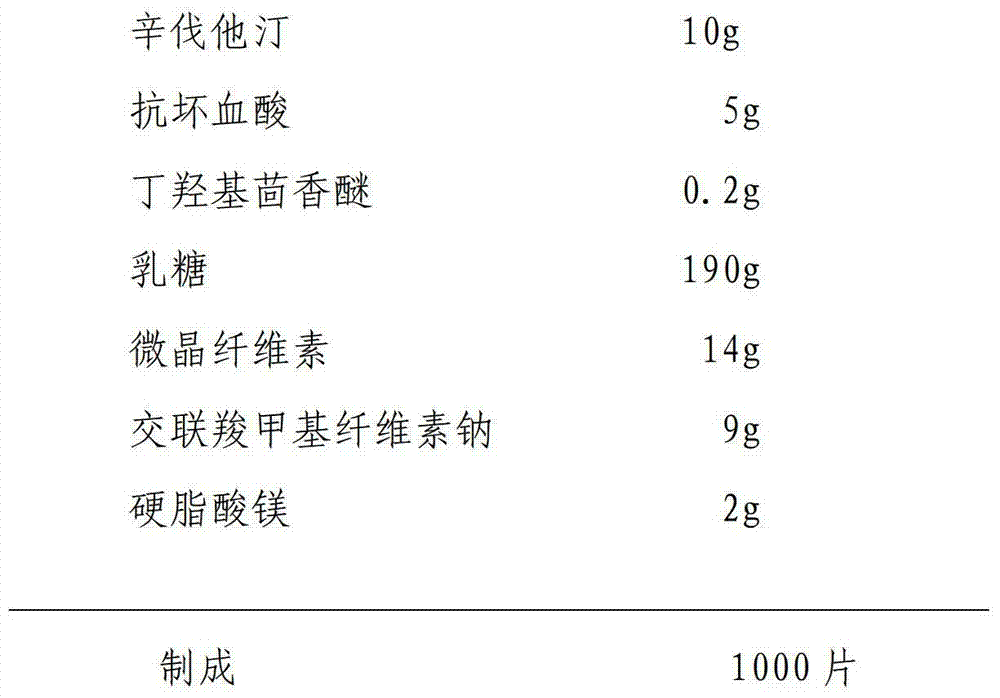
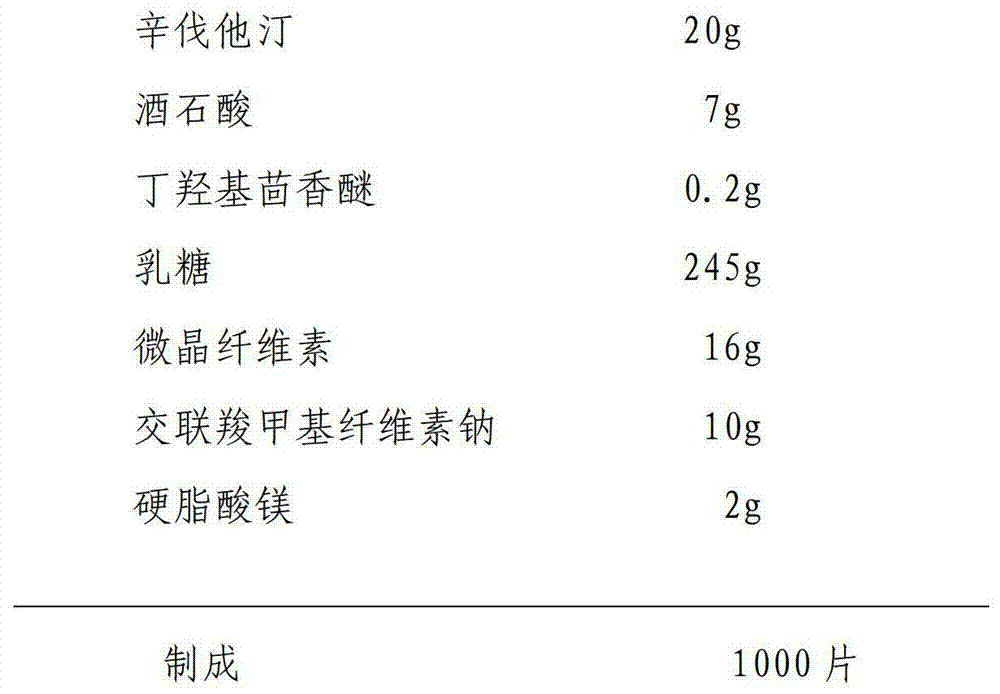
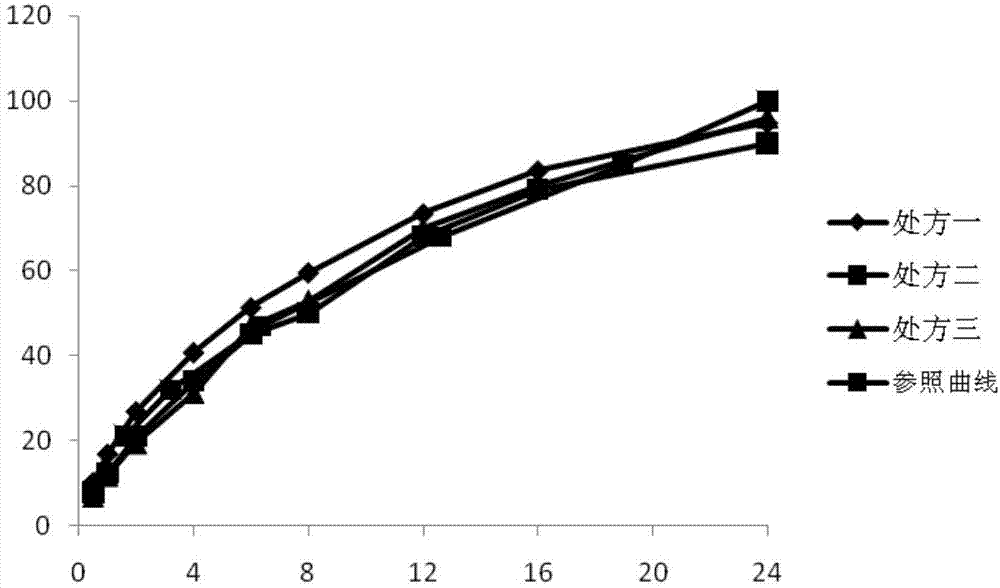
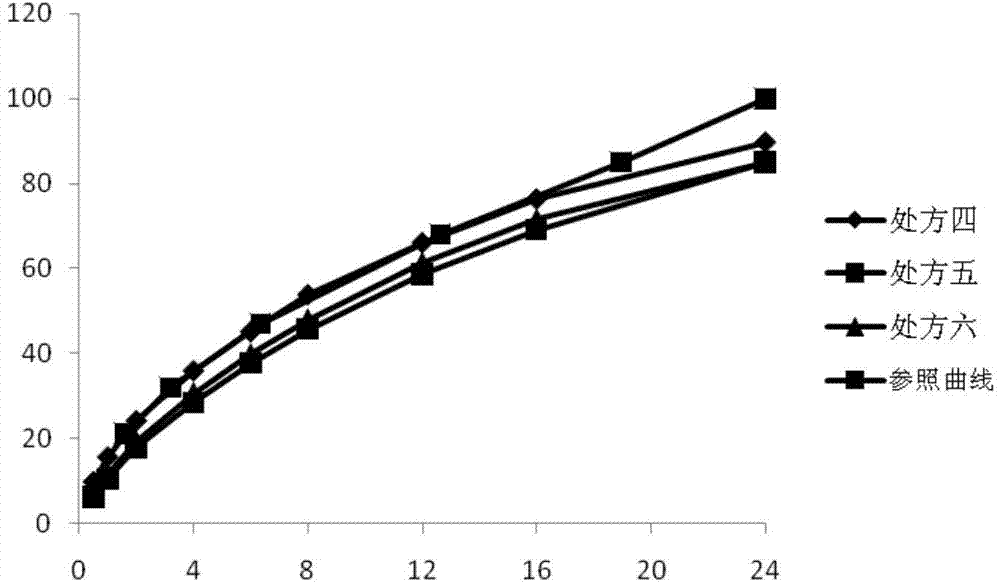
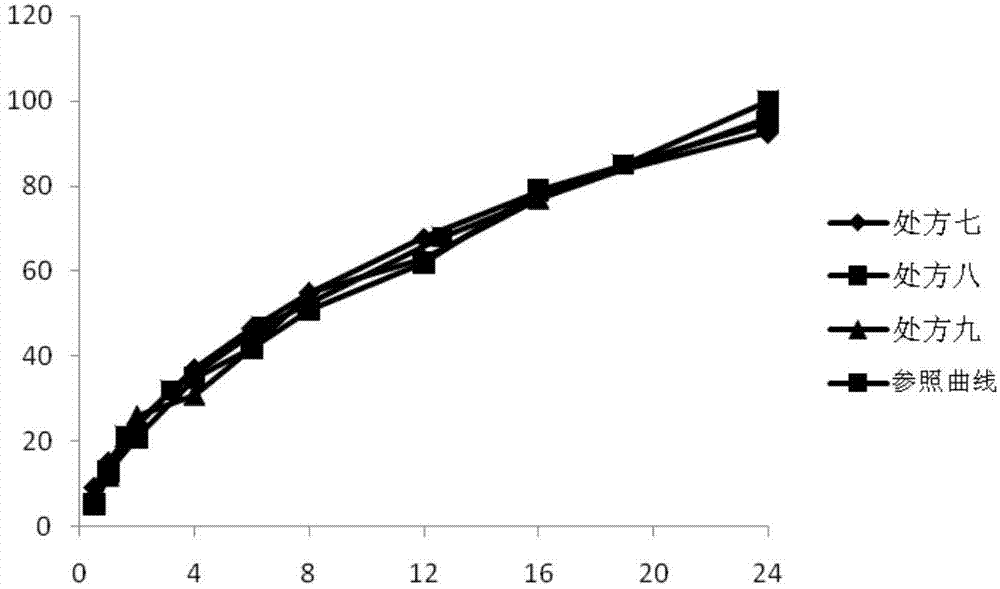
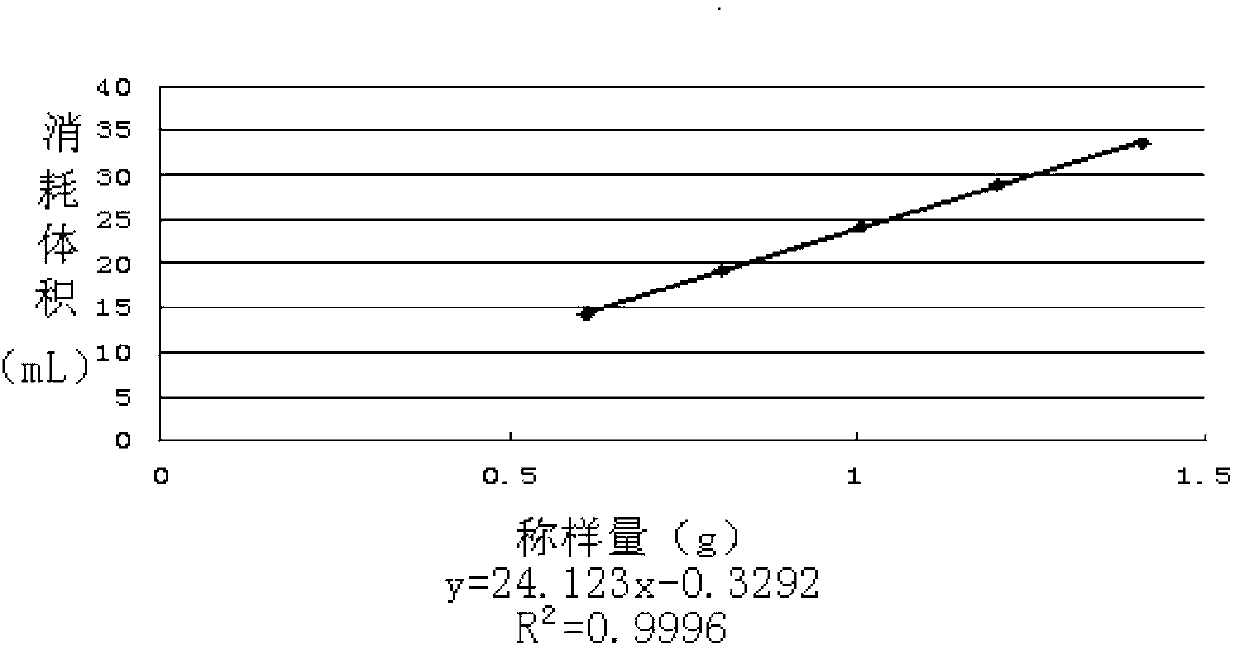
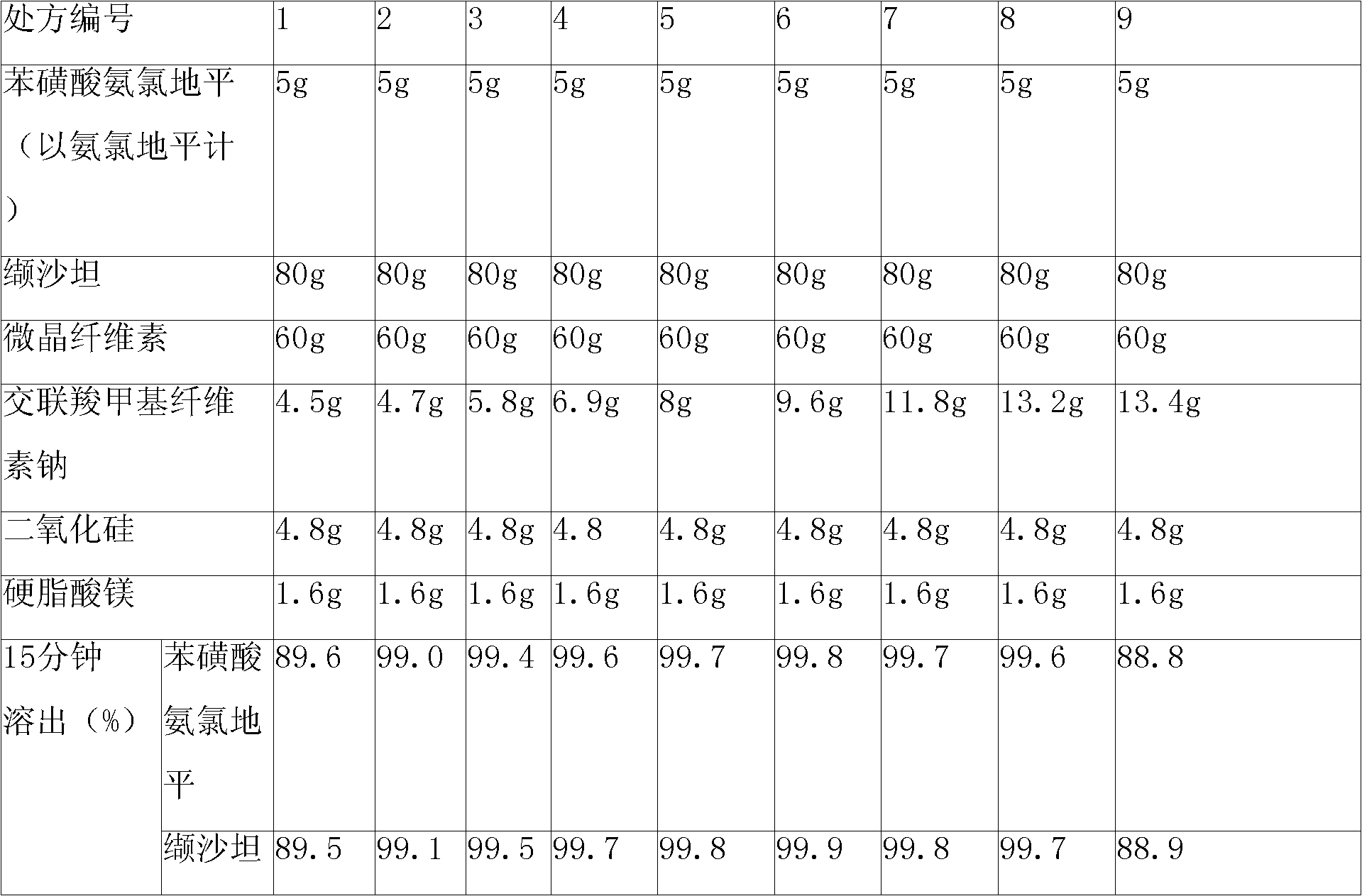Patents
Literature
629 results about "Croscarmellose sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
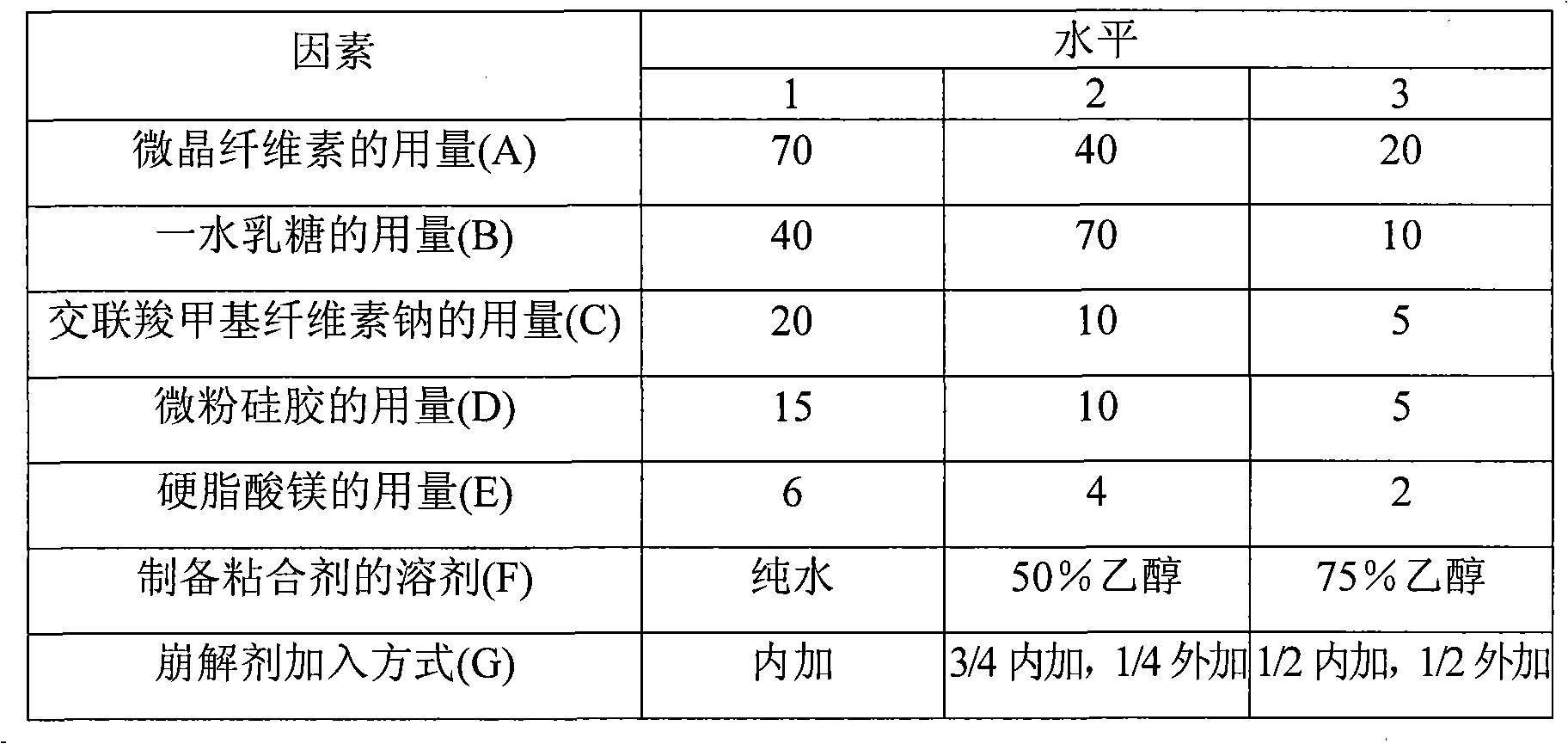
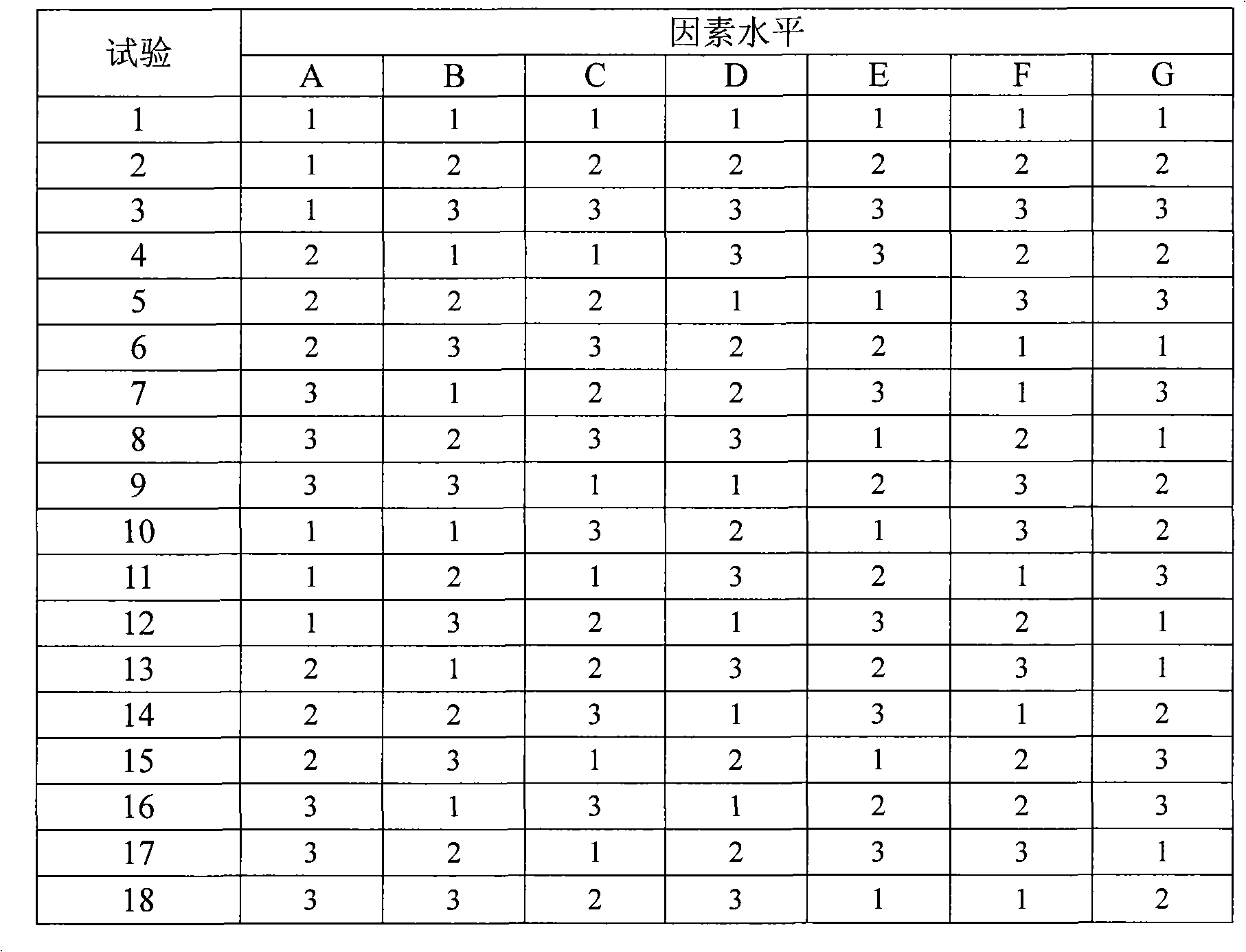
Sodium croscarmellose is an internally cross-linked sodium carboxymethylcellulose for use as a superdisintegrant in pharmaceutical formulations. E468 is the E number of crosslinked sodium carboxymethyl cellulose, used in food as an emulsifier.
Compound danshen oral disintegrant tablet and its preparation method
The present invention relates to a compound salvia oral disintegrant tablet capable of being disintegrated quickly in oral cavity to release medicine. It composition includes the salvia root extract, notoginseng total saponin, borneol and excipient, and its disintegration time is within 40 sec, in which the excipient includes filling agent, disintegrant, glidant and corrective. Its filling agent is of microcrystalline cellulose, its dose is 40%-90% of total weight of the prescription, and the disintegrant is selected from sodium carboxymethylstarch, cross-linked carboxymethylcellulose sodium, cross-linked polyvinylpyrrolidone and low-substituted hydroxypropyl cellulose, and its dose is 5%-25% of total weight of prescription.
Owner:BEIJING BOERDA BIO TECH DEV
Orally disintegrating tablets and process for obtaining them
InactiveUS20060165781A1High dissolution rateImprove compression performancePill deliveryPharmaceutical non-active ingredientsMANNITOL/SORBITOLOrally disintegrating tablet
The tablets comprise: at least 59.5% spray-dried mannitol; active ingredient below or equal to 10%, where at least 90% in weight of the active ingredient has a particle size below 100 μm; microcrystalline cellulose 10-18%, with an average particle size of 50 μm and where at least 99% in weight of microcrystalline cellulose has a particle size below 250 μm; sodium croscarmellose 14%; and a lubricant agent 0.5-2%; where, unless specified otherwise, the percentages are expressed in weight of the total weight of the tablet. And also a process comprising: sieving and mixing of components except for the lubricant agent; mixing of all components; and direct compression of the final mixture. The tablets of the invention give lower disintegration times as well as good perception on the tongue after disintegration, and overcome the problem of insufficient mechanical resistance for packaging and transport operations.
Owner:WARNER CHILCOTT IBERIA
Compositions Containing Ibrutinib
InactiveUS20160287594A1Easy to sprinkleOrganic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
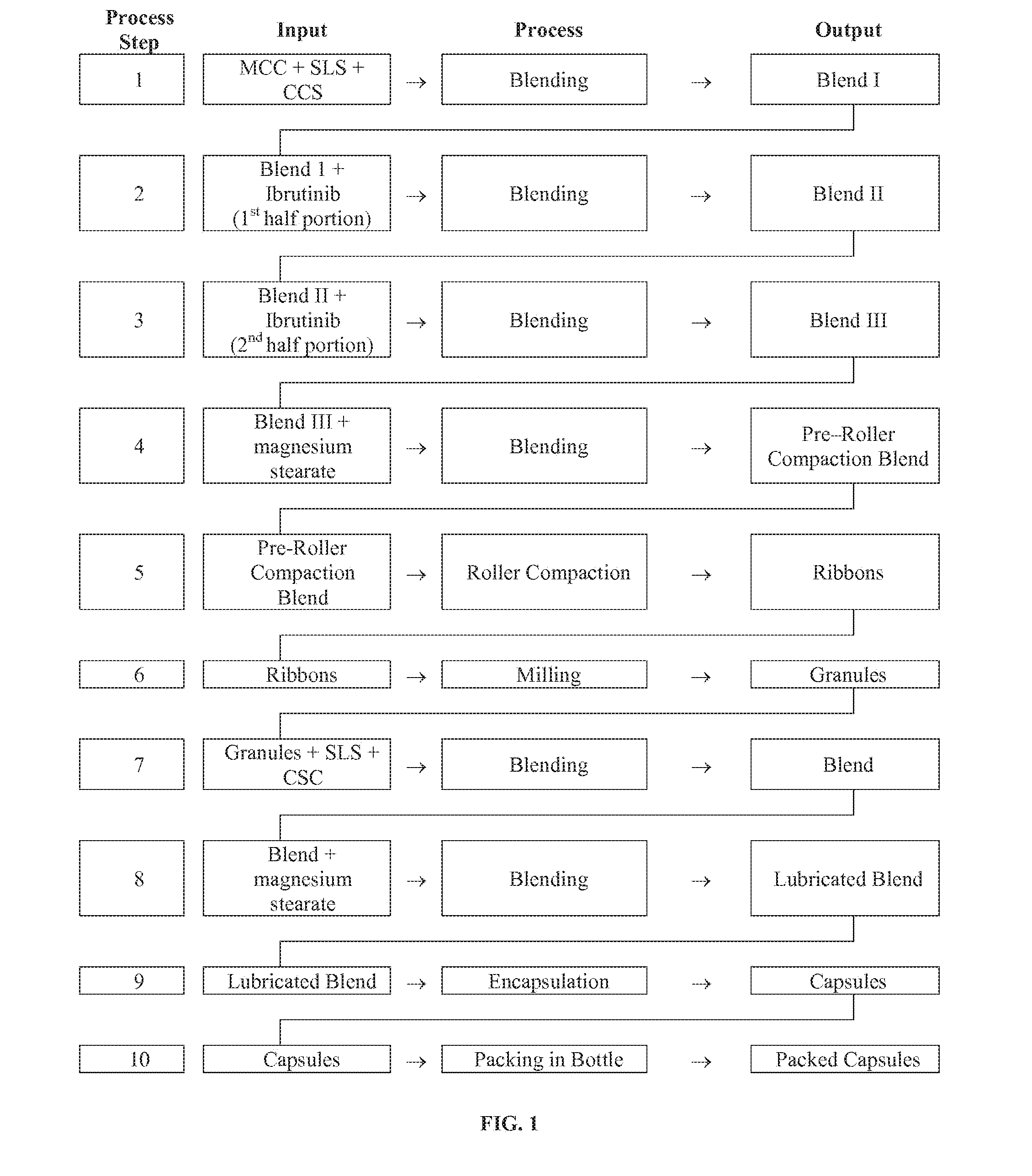
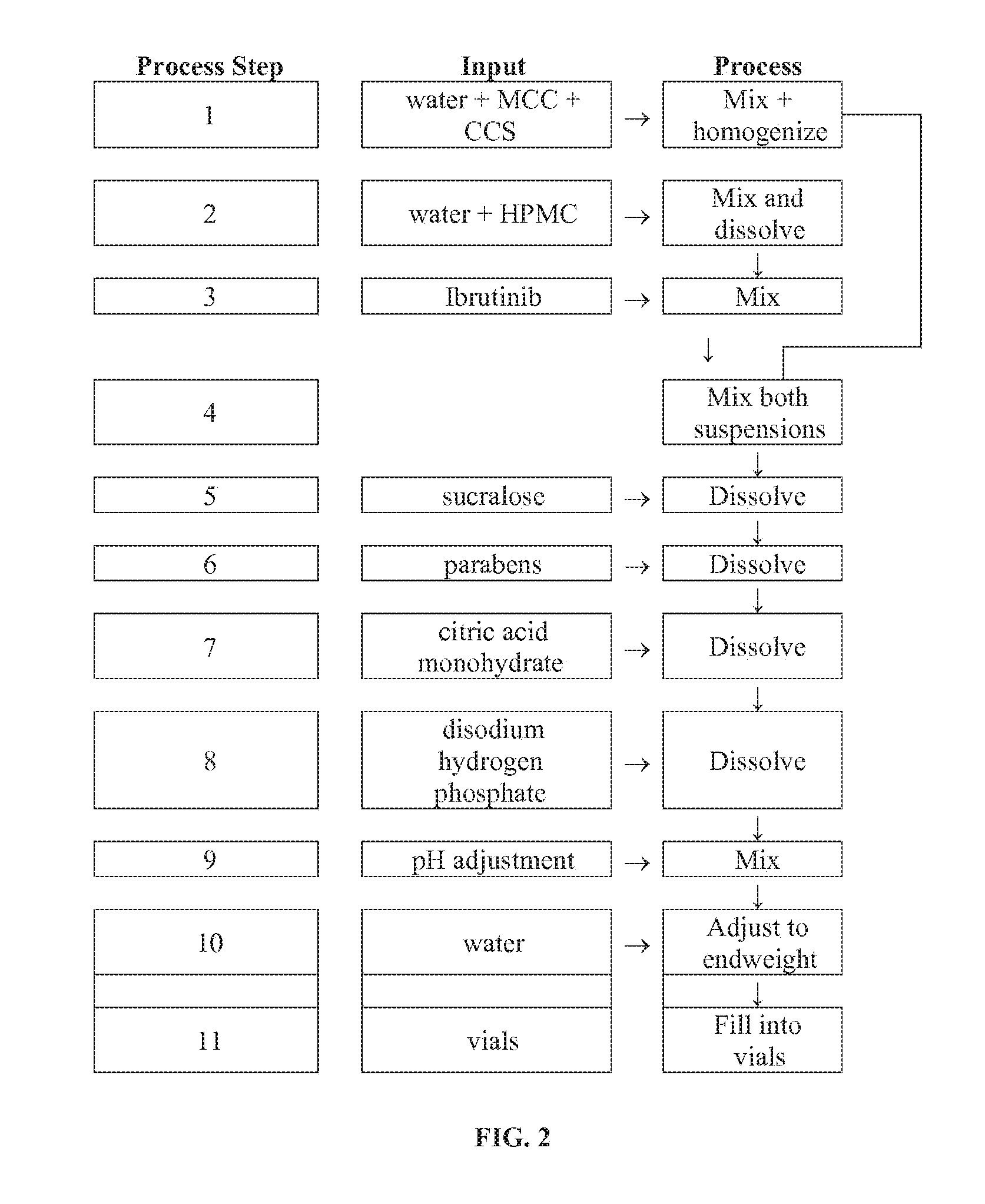
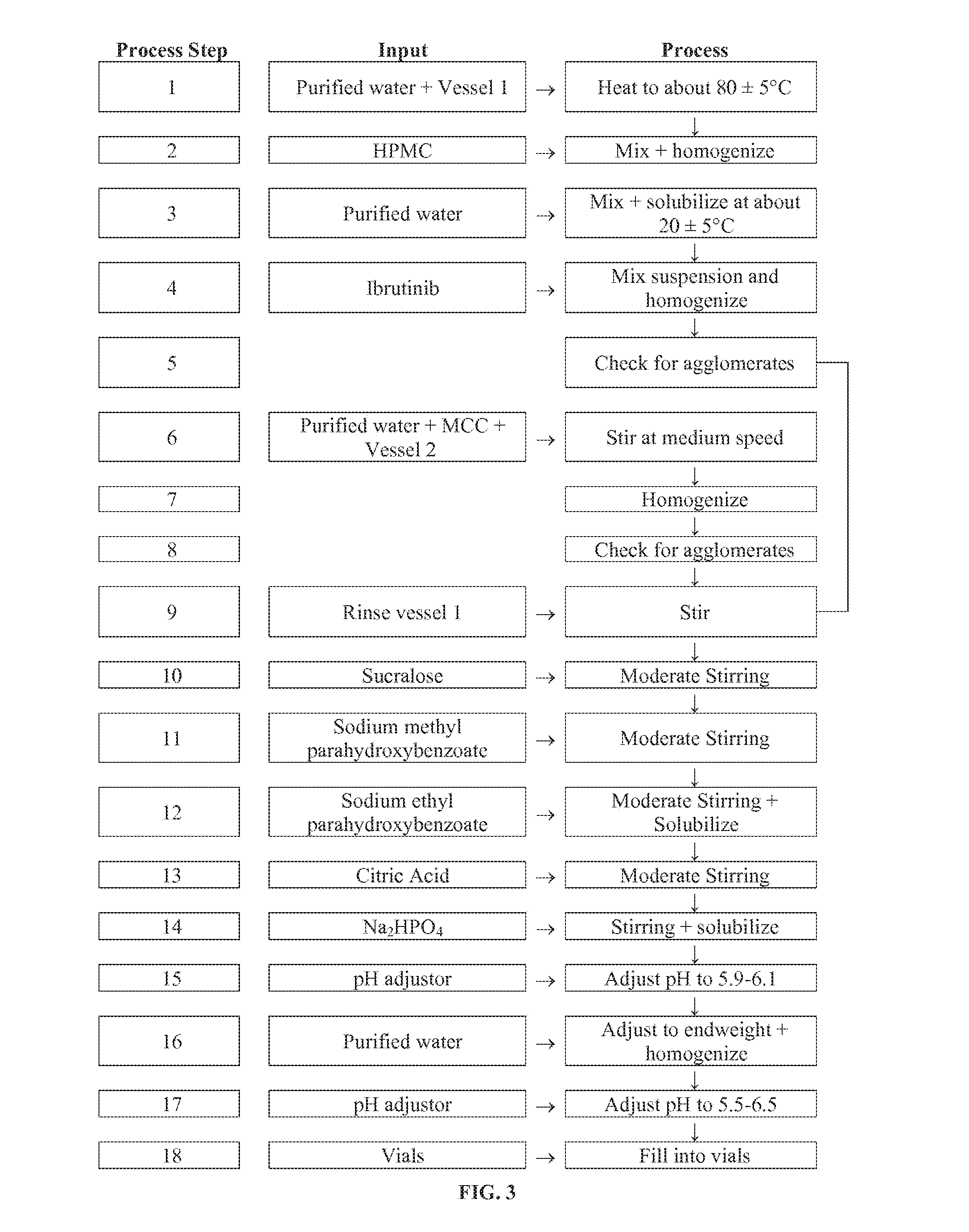
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Pharmaceutical composition containing Amlodipine besilate and valsartan and preparation method thereof
InactiveCN101647797ALess investmentSimple production processPharmaceutical product form changeDrageesValsartanMedicine
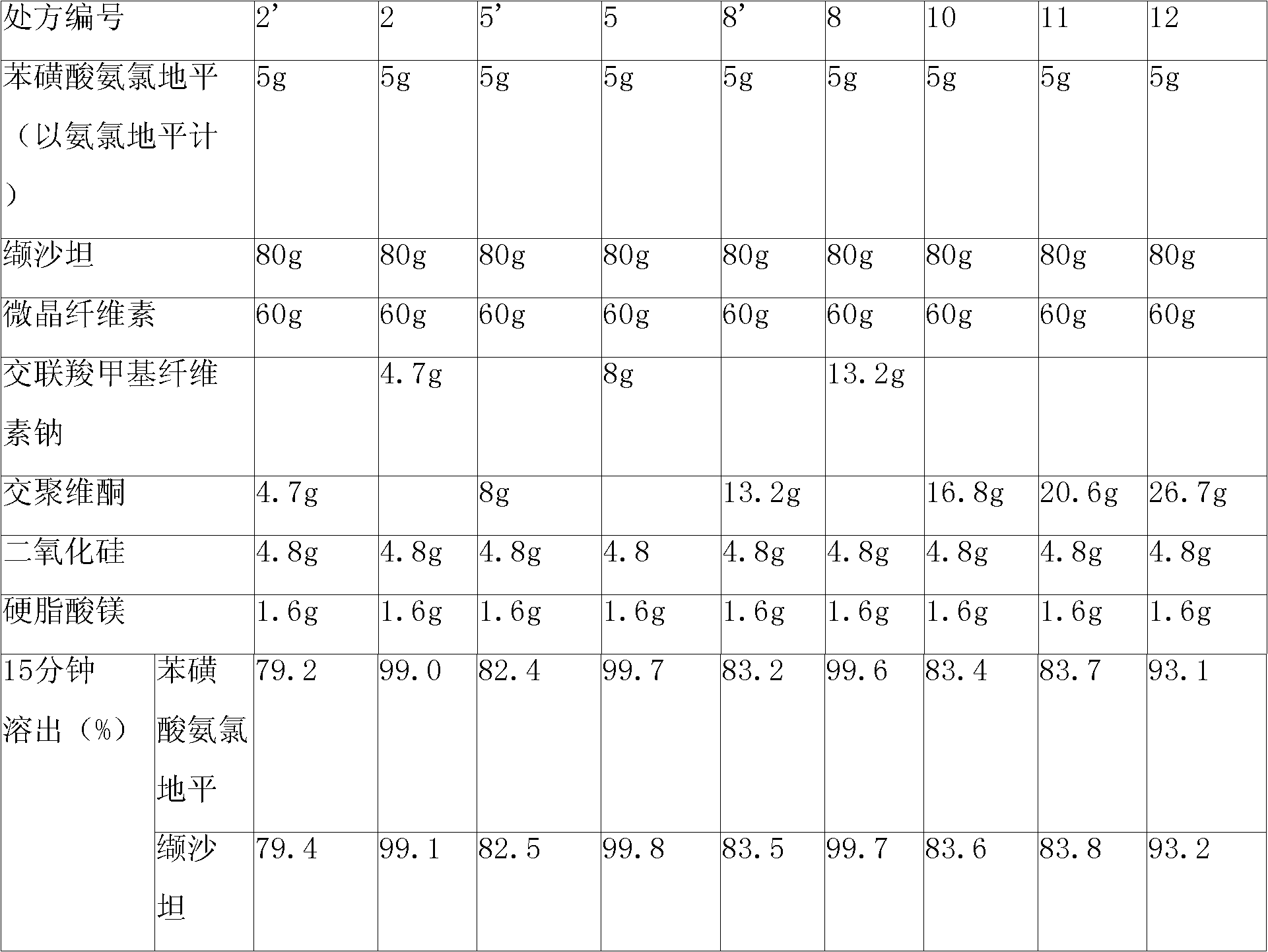
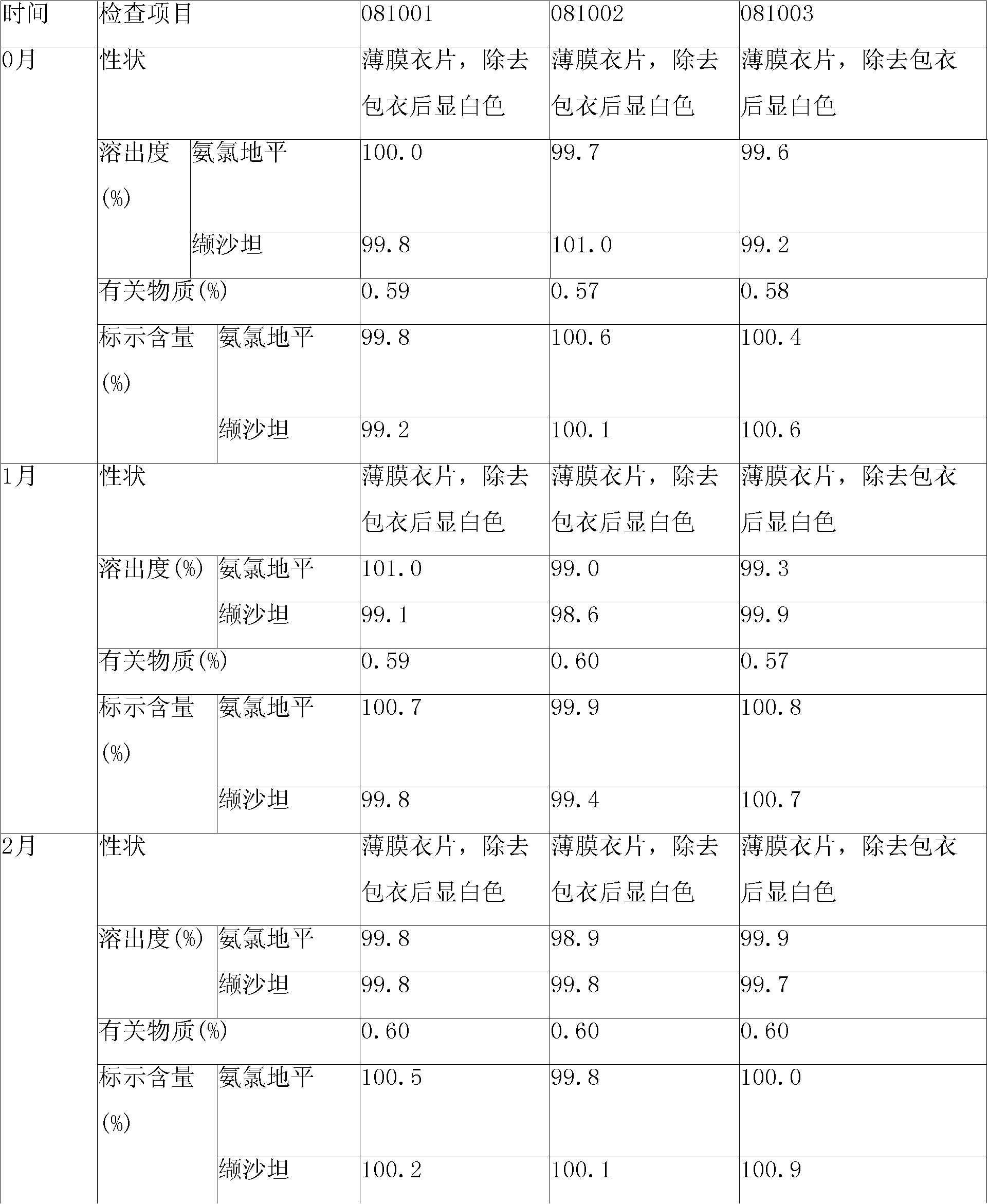
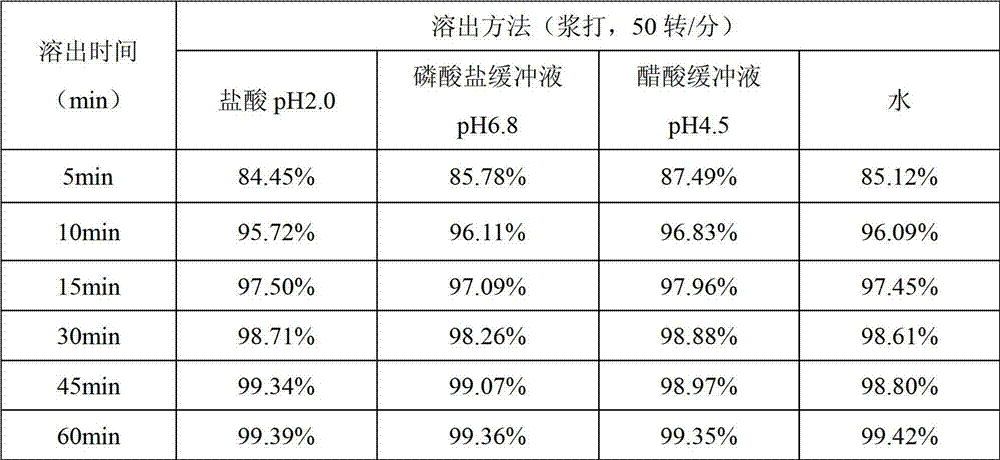
The invention relates to a pharmaceutical composition containing Amlodipine besilate and valsartan and a preparation method thereof. The pharmaceutical composition is prepared from the following ingredients in parts by weight: 5 parts of Amlodipine besilate, 80 parts of valsartan, 60 parts of microcrystalline cellulose, 4.7-13.2 parts of croscarmellose sodium, 4.8 parts of silicon dioxide and 1.6parts of magnesium stearate. The pharmaceutical composition is prepared by using a direct powder compression technique. The invention can reach the dissolution rate of more than 90% by using less disintegrating agent, and has advantages of good stability and faster disintegrating. The preparation method of the invention has simpler production process, reduces the investment of corresponding equipment and plants and saves the production cost; the tablet produced by using direct powder compression technique has faster disintegrating and is helpful to improve the dissolving of pharmaceutical; through detection, the tablet prepared by using the method is dissolved out by more than 90% within 15 min.
Owner:HAINAN JINRUI PHARMA CO LTD
Atorvastatin calcium tablet and preparation method thereof
ActiveCN102920675AHigh dissolution rateImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsFiller ExcipientHardness
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet consists of the following components in parts by mass: 7.22 parts of main medicine atorvastatin calcium, 84.55 parts of filler, 6 parts of disintegrating agent croscarmellose sodium, 1.33 parts of adhesive hydroxy propyl cellulose, 800.4 parts of cosolvent polysorbate and 0.5 part of lubricating agent magnesium stearate, wherein the filler comprises the following raw materials in parts by mass: 22.01 parts of calcium carbonate, 21.87 parts of milk sugar and 40.67 parts of microcrystalline cellulose. The atorvastatin calcium tablet has the characteristics of short disintegrating time, fast dissolving-out speed, high bioavailability and small particle diameter, and is convenient to take. Furthermore, the hardness of the tablet can reach 60-70N, so that the tablet is hardly broken, and therefore, the packing and transporting costs are reduced, and the industrialized popularization of the tablet is easily realized.
Owner:HENAN RUNHONG PHARMA
Compositions containing micronized tanaproget prepared by wet granulation
Compositions, preferably pharmaceutical compositions, containing micronized tanaproget, or pharmaceutically acceptable salt thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, butylated hydroxyanisole, povidone, and magnesium stearate, are provided. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefor. Additional uses include stimulation of food intake.
Owner:WYETH LLC
Dispersible tablet containing cefixime and preparation method thereof
The invention belongs to the technical field of pharmaceutical preparation, and in particular relates to a cefixime dispersible tablet and a method for preparing the same. The method comprises the following steps: firstly, weighing cefixime, starch, microcrystalline cellulose, partially cross-linked polyvinylpyrrolidone, partially cross-linked sodium carboxymethyl cellulose, and partially low substituted-hydroxypropyl cellulose, and mixing the components evenly; secondly, dripping 5 percent polyvinylpyrrolidone K30 ethanol solution into the mixture prepare a soft material, and performing granulating through a sieve of between 18 and 24 meshes; thirdly, drying wet particles at a temperature of between 50 and 80 DEG C, palletizing the particles through the sieve of between 18 and 24 meshes, adding the remaining cross-linked polyvinylpyrrolidone, the remaining cross-linked sodium carboxymethyl cellulose, and the remaining low substituted-hydroxypropyl cellulose, magnesium stearate and superfine silica gel powder into the mixture to be mixed evenly, and tabletting the mixture to obtain the required cefixime dispersible tablet. The cefixime dispersible tablet has the characteristics of rapid disintegration with water, even dispersion, high dissolution, and convenient taking and carrying around.
Owner:BEIJING TRADE STAR MEDICAL TECH
Preparation method of xylitol liver-protecting tablet
InactiveCN101744865AEffective preventionEffective therapeuticAnthropod material medical ingredientsHydroxy compound active ingredientsCross-linkPropolis
The invention relates to a preparation method of a xylitol liver-protecting tablet, belonging to the technical field of medical health care. The formula of the xylitol liver-protecting tablet comprises the following raw materials in proportion: 15-45 parts of kudzu root extract, 2-20 parts of glycyrrhizic acid, 5-35 parts of propolis powder, 20-60 parts of xylitol, 1-10 parts of low-substituted hydroxypropyl cellulose, 1-10 parts of cross-linked sodium carboxymethyl cellulose and 0.5-5 parts of magnesium stearate; and a coating agent comprises 0.5-40 parts of hydroxypropyl methylcellulose, 1-30 parts of polyethylene glycol 6000, 0.5-40 parts of talcum powder, 0.5-30 parts of titanium pigment, 0.5-30 parts of iron oxide brown and 10-75 parts of maltitol. The finished product of the xylitol liver-protecting tablet is prepared by the processing steps of crushing, mixing, tabletting, coating and the like. In the invention, by using the xylitol as a main raw material and adding partial auxiliary materials, the xylitol liver-protecting tablet which is a health food and has auxiliary protection effect on chemical liver injury is prepared. The xylitol liver-protecting tablet can effectively prevent and treat fatty liver; and after patients suffering from the fatty liver take the xylitol liver-protecting tablet, severe fatty liver can be relieved into moderate fatty liver, and moderate fatty liver can be relieved into mild fatty liver. Thus, the xylitol liver-protecting tablet has obvious benefit to improve liver functions of human bodies and has wide market prospects.
Owner:FUTASTE PHARM CO LTD
Preparation for composition of vitamin and mineral matter
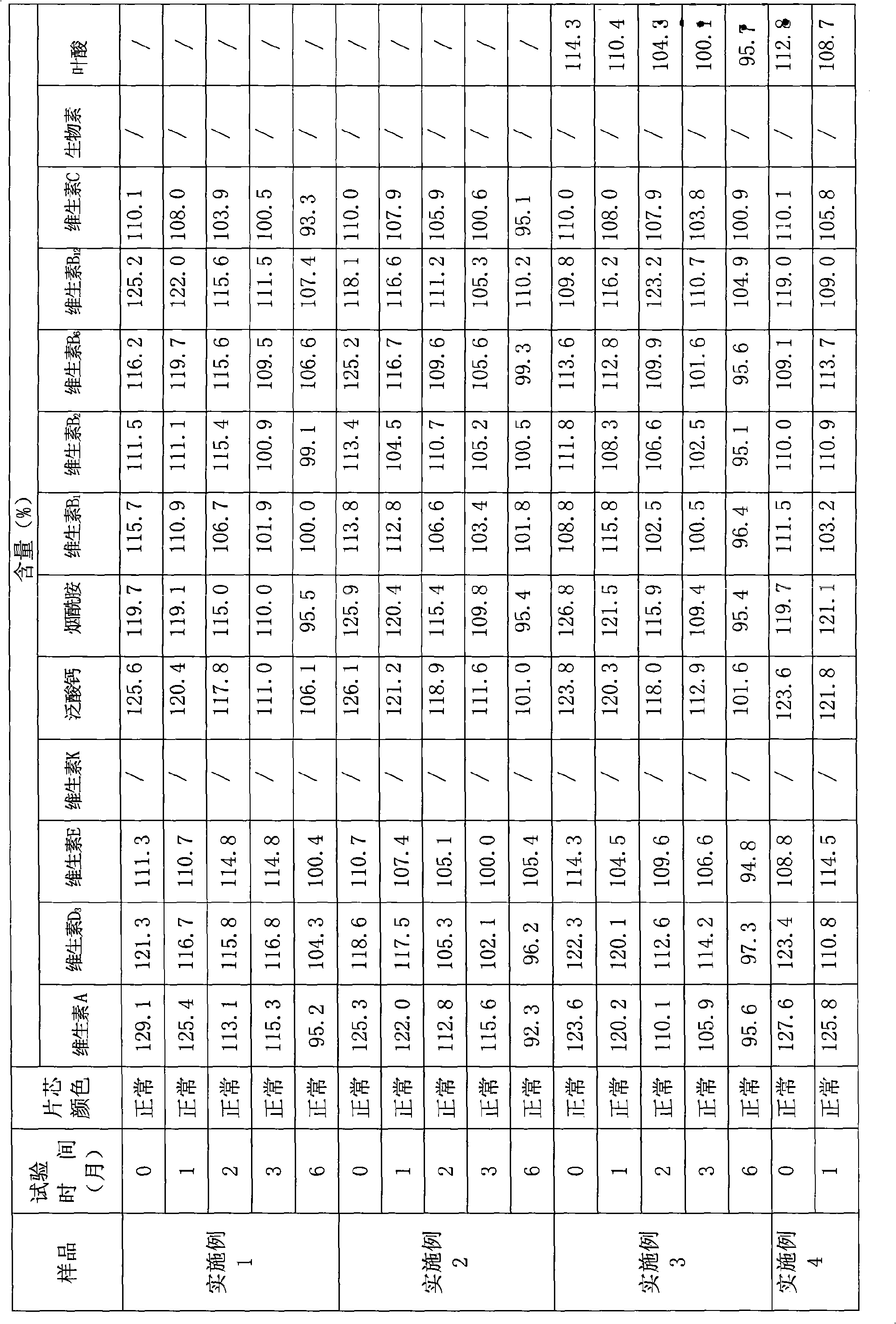
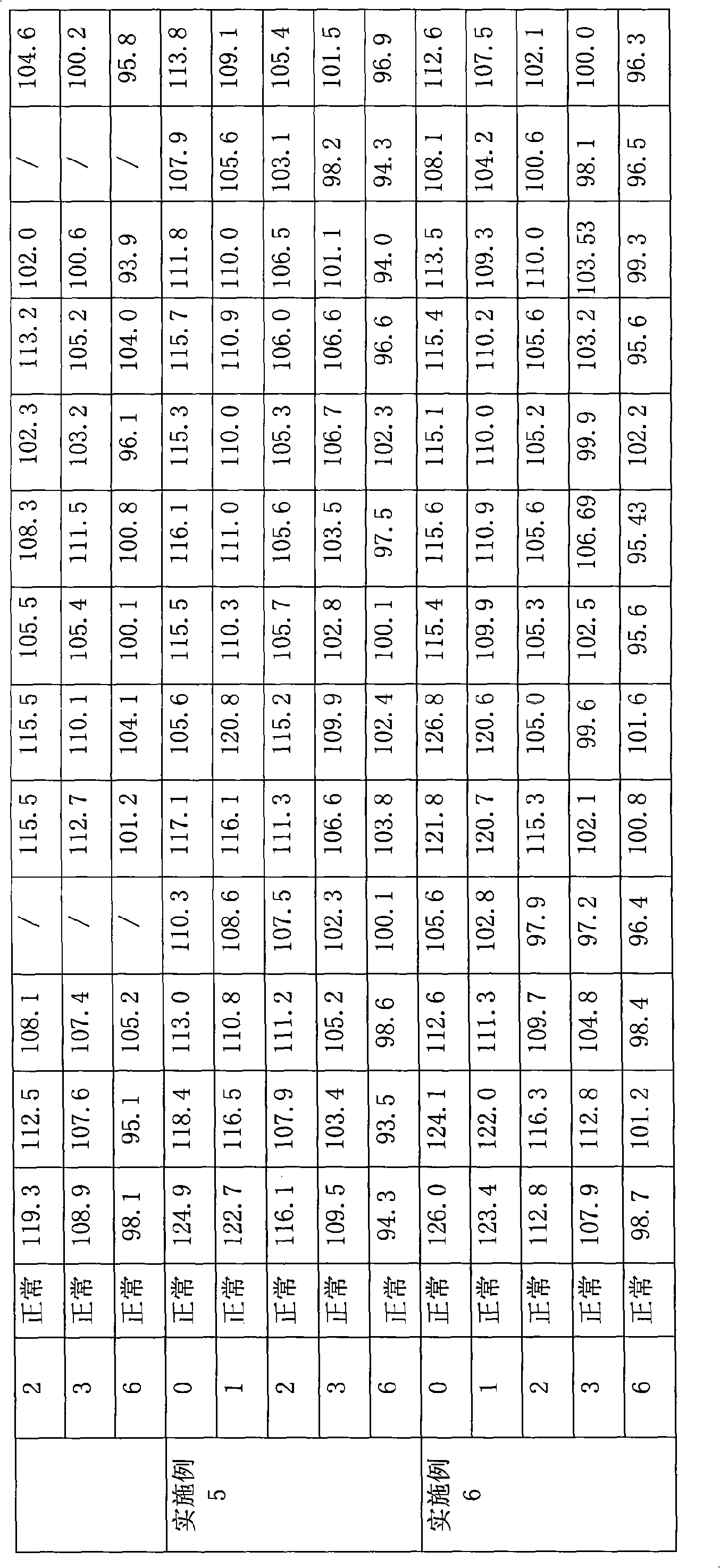
ActiveCN101401809AImprove stabilityNo significant change in colorMetabolism disorderInorganic active ingredientsBeta-CarotenePhosphate
The invention provides a process for preparing a composition of vitamins and minerals, which contains the following components in each 1,000 tablets: 1 to 5 x 10<6>IU of vitamin A, 0 to 2 grams of beta-carotene, 0.75 to 4 x 10<5>IU of vitamin D, 0 to 30 milligrams of vitamin K, 0.5 to 5 grams of vitamin B1, 0.5 to 5 grams of vitamin B2, 0.25 to 1 gram of vitamin B6, 0.5 to 2 milligrams of vitamin B12, 25 to 100 grams of vitamin C, 0 to 0.4 grams of folic acid, 0 to 30 milligrams of biotin, 5 to 15 grams of vitamin E, 5 to 15 grams of nicotinamide, 2.5 to 10 grams of calcium pantothenate, 0 to 50 grams of heavy choline bitartrate, 0 to 100 milligrams of iodine, 0 to 25 grams of L-lysine salt, 0 to 50 grams of inositol, 5 to 10 grams of potassium, 5 to 15 grams of iron, 0.5 to 1 grams of copper, 0 to 50 milligrams of selenium, 0 to 120 grams of magnesium, 0 to 2 grams of manganese, 0.25 to 15 grams of zinc, 0 to 20 milligrams of chromium, 0 to 25 milligrams of molybdenum, 163 to 558 grams of bicalcium phosphate, 0 to 600 grams of calcium carbonate, 50 to 100 grams of starch, 30 to 60 grams of lactose, 100 to 200 grams of microcrystalline cellulose, 20 to 50 grams of dextrin, 15 to 50 grams of cross-linked sodium carboxymethyl cellulose, 0 to 30 grams of low substituted hydroxypropyl cellulose, 8 to 16 grams of tartaric acid, 5 to 20 grams of talcum powder, 8 to 12 grams of superfine silica gel powder, and 6 to 10 grams of magnesium stearate. The process is characterized in that the vitamin C adopts powder coating, and the coating material is methyl acrylate copolymer, preferably a mixture of a methacrylic acid / ethyl acrylate (1 to 1) copolymer and an ethyl acrylate / methyl methacrylate (2 to 1) copolymer, wherien the weight ratio of mixture is between 1 to 4 and 4 to 1. The composition of the vitamins and the minerals has simple preparation process and convenient operation, and can improve the stability of the vitamins.
Owner:HANGZHOU MINSHENG HEALTHCARE CO LTD
Irbesartan and hydrochlorothiazide pharmaceutical composition and preparation method thereof
ActiveCN101327213AGood auxiliary effectGood quality and stabilityOrganic active ingredientsPharmaceutical non-active ingredientsLACTITOL MONOHYDRATEAdditive ingredient
The invention relates to a medicinal composite of Irbesartan hydrochlorothiazide. The medicinal composite is composed of 150 portions of Irbesartan, 12.5 portions of hydrochlorothiazide, 20 portions to 60 portions of microcrystalline cellulose, 20 portions to 60 portions of lactose monohydrate, 15 portions to 25 portions of crosslinked sodium carboxymethyl cellulose, 1 portion to 10 portions of hydroxypropylmethyl cellulose, 2 portions to 7 portions of SiliciiDoxydum, 1 portion to 3 portions of magnesium stearate. The major medicinal ingredients are mixed with part of the crosslinked sodium carboxymethyl cellulose and the microcrystalline cellulose, and the mixture is crushed down and then added with the lactose monohydrate for mixing; the mixture obtained from the former step is added with 50 percent of ethanol solvent containing 2 percent of hydroxypropylmethyl cellulose for even mixing, then the mixture is screened, made into integral grains which are then dried; the dry grains are mixed with the SiliciiDoxydum, the magnesium stearate and the residual crosslinked sodium carboxymethyl cellulose, and the mixture then undergoes tabletting and coating so as to obtain the medicinal composite. The prescription of the medicinal composite of the invention is reasonable, the quality is stable and reliable, and the medicinal composite has a satisfactory dissolution rate.
Owner:HAINAN JINRUI PHARMA
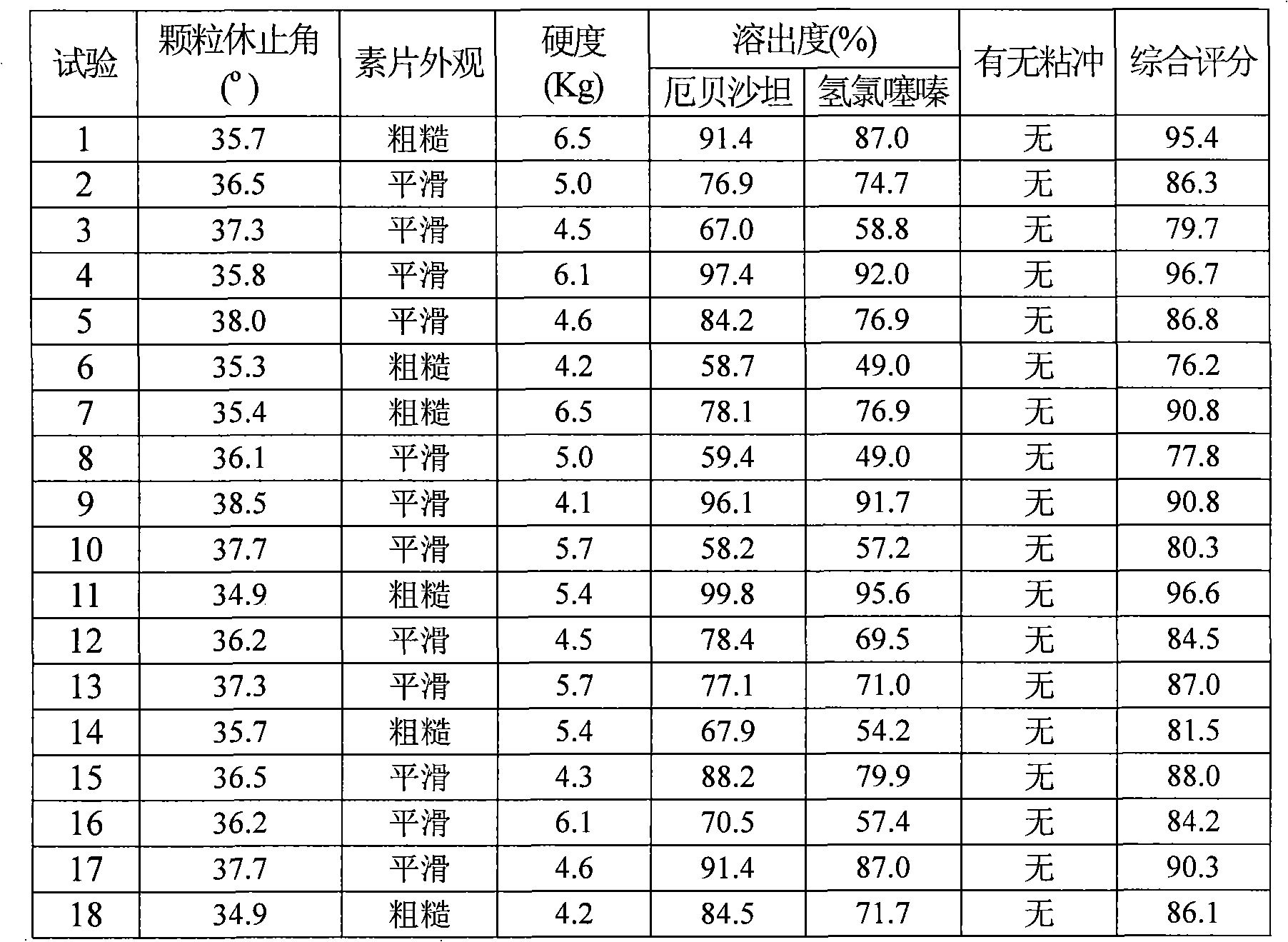
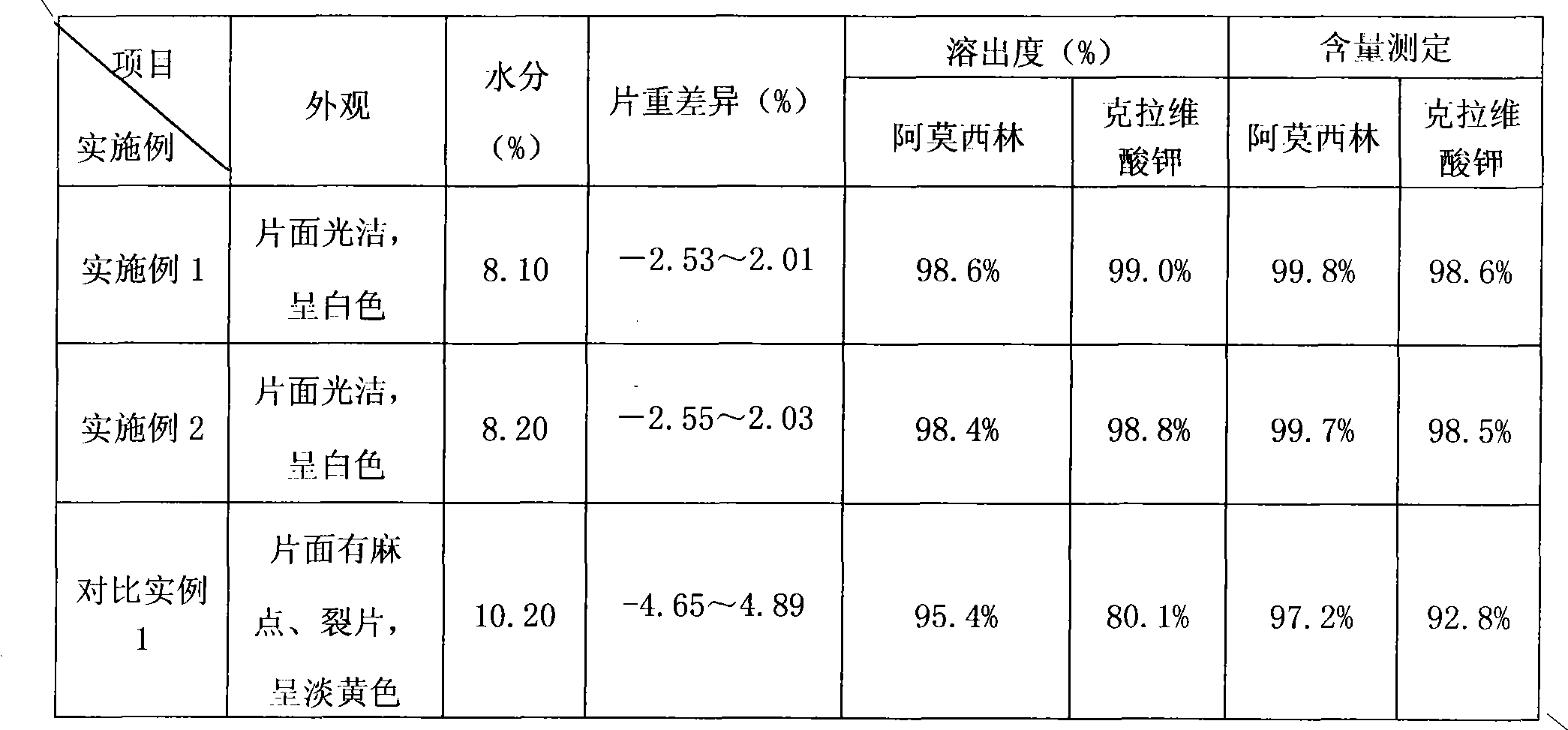
Preparation method of amoxicillin and clavulanate potassium tablets
InactiveCN101897701AReduce exposure timeGuaranteed stabilityAntibacterial agentsPharmaceutical product form changeMass ratioDissolution
The invention discloses a preparation method of amoxicillin and clavulanate potassium tablets, comprising the following steps: (a) weighing the following main and auxiliary materials: 120-135 parts of amoxicillin, 40-45 parts of clavulanate potassium, 27-32 parts of microcrystalline cellulose, 1-3 parts of croscarmellose sodium (ADS), 1-3 parts of superfine silica powder and 2-4 parts of magnesium stearate; (b) after pelletizing amoxicillin, mixing the pelletized amoxicillin with clavulanate potassium according to the mass ratio of 4:1 to form the main materials; (c) uniformly mixing the auxiliary materials including superfine silica powder, ADS and microcrystalline cellulose by the method of increment by equal quantity and throwing the main materials and the auxiliary materials into a mixer by the method of increment by equal quantity to be mixed for 36-70min; and (d) tabletting the mixed powder. The products prepared by the method have bright, clean and beautiful appearances and stable content and dissolution.
Owner:NORTH CHINA PHARMA COMPANY
Micronized tanaproget, compositions, and methods of preparing the same
The present invention provides compositions, desirably pharmaceutical compositions, containing micronized tanaproget. The compositions can also contain microcrystalline cellulose, croscarmellose sodium, anhydrous lactose, magnesium stearate, micronized edetate calcium disodium hydrous, and micronized sodium thiosulfate pentahydrate. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefor. Additional uses include stimulation of food intake.
Owner:WYETH LLC
Micronized tanaproget and compositions containing same
The present invention provides compositions, desirably pharmaceutical compositions, containing micronized tanaproget. The compositions can also contain microcrystalline cellulose, croscarmellose sodium, anhydrous lactose, and magnesium stearate; or can contain microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, povidone, and magnesium stearate. The compositions are useful in contraception and hormone replacement therapy and in the treatment and / or prevention of uterine myometrial fibroids, benign prostatic hypertrophy, benign and malignant neoplastic disease, dysfunctional bleeding, uterine leiomyomata, endometriosis, polycystic ovary syndrome, and carcinomas and adenocarcinomas of the pituitary, endometrium, kidney, ovary, breast, colon, and prostate and other hormone-dependent tumors, and in the preparation of medicaments useful therefore Additional uses include stimulation of food intake.
Owner:WYETH LLC
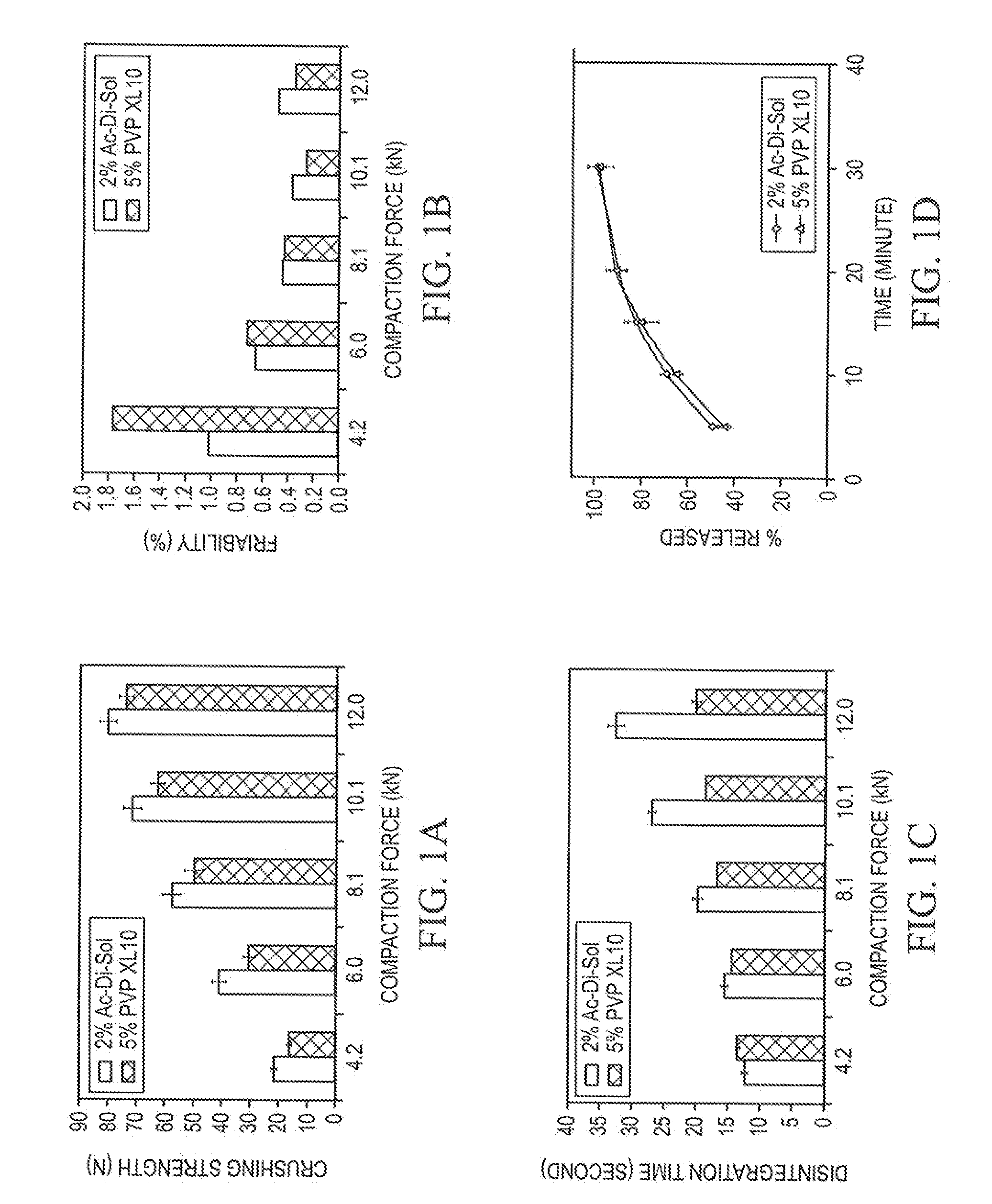
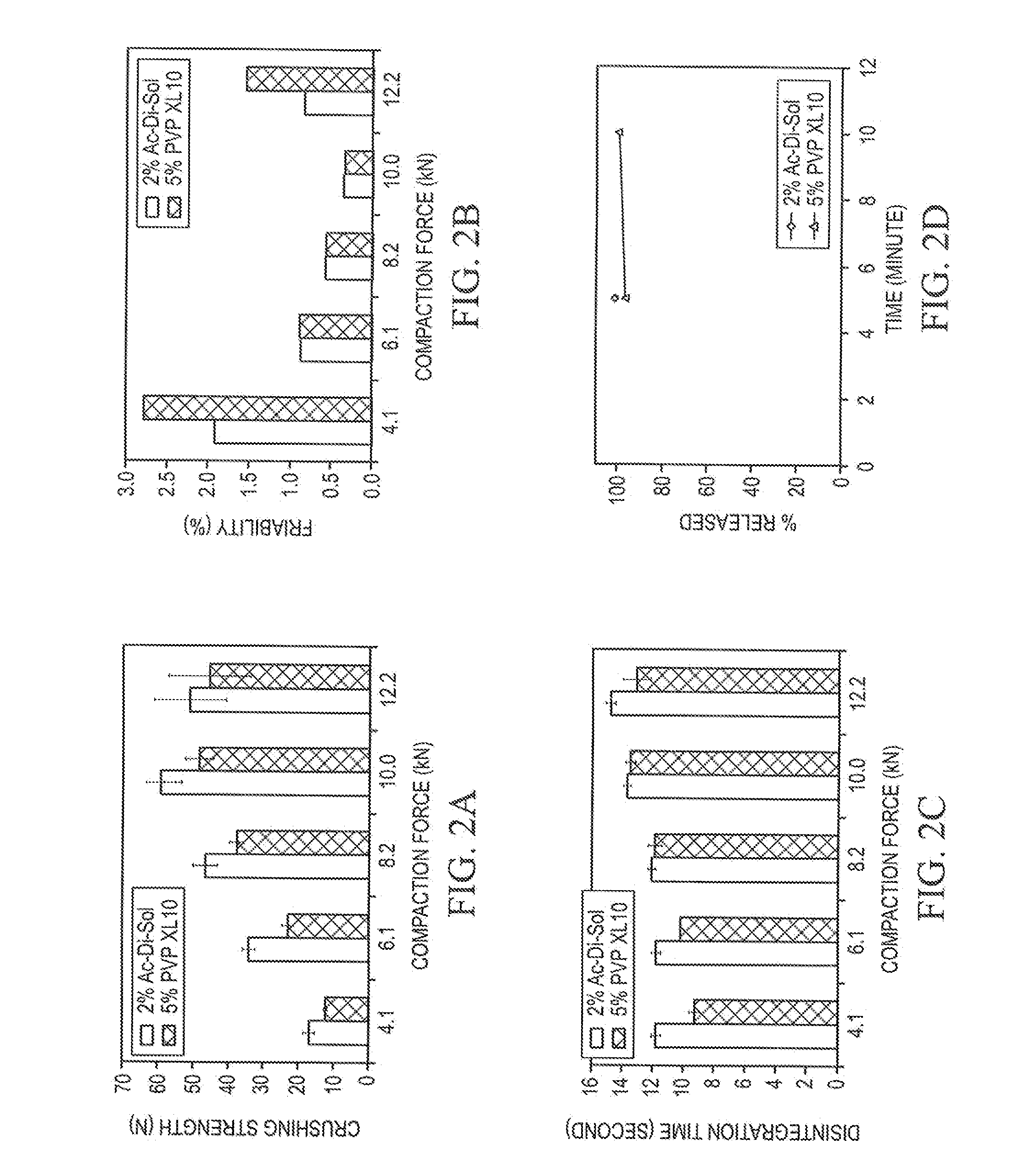
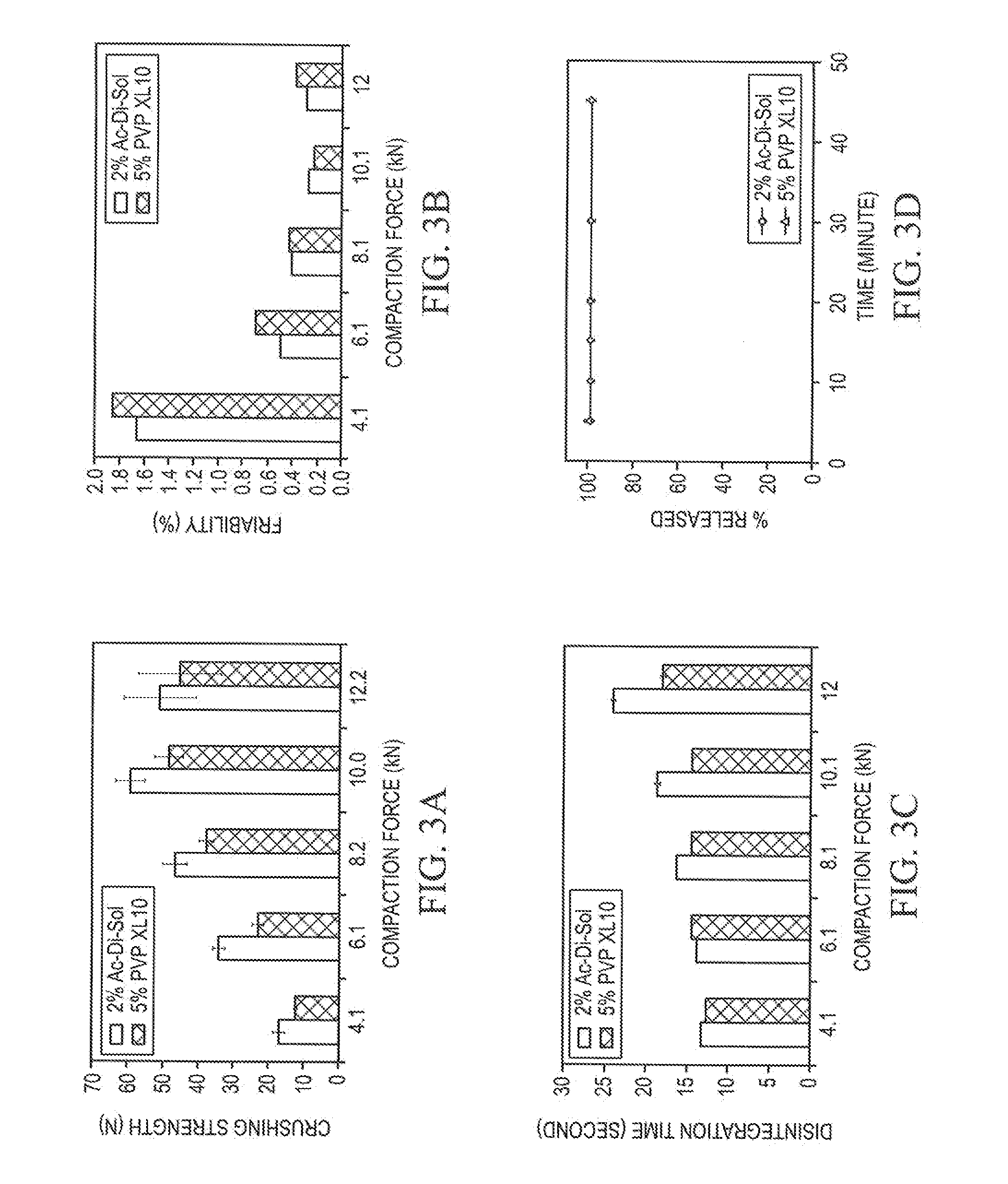
Stable Orally Disintegrating Tablets Having Low Superdisintegrant
The invention is directed to the functionality and performance of superdisintegrants in orally disintegrating tablets (ODT). The invention can be an aged direct compression ODT having between about 0.3% to about 2% (wt / wt) sodium croscarmellose relative to the total weight of the ODT, a polyol matrix, optionally a lubricant, and an active pharmaceutical or nutraceutical ingredient, in which after storage for four months the ODT has a disintegration time using an excess water test that is less than 30 seconds and a tensile strength greater than 0.5 MPa. The invention is also directed to a direct compression ODT, consisting essentially of about 0.5% to 2.0% sodium croscarmellose, from 0.1% to 2.0% lubricant, an API, up to 10% (wt / wt) microcrystalline cellulose, optionally one or more colorants, sweeteners, fragrances, flavor compounds, and / or flavor blockers, and the balance spray-dried mannitol.
Owner:FMC CORP
Voglibose dispersible tablet, capsule and method for preparing the same
InactiveCN101219127APromote dissolutionPromote absorptionOrganic active ingredientsMetabolism disorderSucroseLow-substituted hydroxypropylcellulose
The invention relates to a voglibose dispersible tablet and capsule and a preparation method thereof. The voglibose dispersible tablet comprises: 0.01-5 percent of voglibose, 1-99 percent of disintegrating agent, 0-98 percent of diluting agent; 0.5-20 percent of lubricant and fluidizer, 0.1-20 percent of bonding agent; wherein, the disintegrating agent is one or more selected from starch, modified starch, cellulose, microcrystalline cellulose, cross-linked polyvinyl pyrrolidone, sodium carboxymethyl starch, cross-linked sodium carboxymethyl cellulose, low-substituted hydroxypropyl cellulose, alginic acid and colloid magnesium aluminum silicate; the diluting agent is one or more selected from lactose, mannitol, sorbitol, sucrose, calcium sulfate, kaolin, dextrine and sodium chloride. The capsule preparation does not contain the bonding agent. The invention has the advantages that the voglibose dispersible tablet disintegrates swiftly and disperses evenly, the voglibose dispersible tablet can be disintegrated swiftly into fine particles and scattered evenly after being taken orally, which is beneficial for the dissolution and the absorption of the voglibose dispersible tablet with the short onset time. Disintegrated swiftly in three minutes; after being taken orally, the capsule shell quickly swells and splits, which conceals the discomfort caused by the capsule taken in mouth.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Cefixime oral disintegration tablet and its preparation method
ActiveCN1803138AShort disintegration timeGreat tasteAntibacterial agentsOrganic active ingredientsCelluloseMANNITOL/SORBITOL
The invention discloses an orally disintegrating cefixime tablet and its preparation, wherein the ingredients include (by weight ratio): cefixime 10.0-35.0%, crystalline cellulose 0-10%, lactose starch 0-35%, mannitol 35.0-59%, cross-linked sodium carboxymethylcellulose 4.0-15.0%, Kollidon 1.0-5.0%, sodium dodecylsulfate 0.01-1.0%, and silica gel powder 0.01-0.5%, the preparing process comprises the following steps, mixing the medicinal materials homogeneously, charging water, ethanol or their mixture, granulating and drying, mixing homogeneously with miropowdered silica gel and tabletting.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Hiliezdum fast-release tablet and preparing method thereof
InactiveCN1454600AGreat tasteHigh strengthOrganic active ingredientsNervous disorderCross-linkCellulose
The invention is a kind of quick-released tablet of shenshuai fruit elements and the manufacturing method. The tablet contains 10-30 % shenshuai fruit element extraction material, 2-5 % sputtering agent, 1.5-3%, the other is filter. The sputtering agent is cross-linked sodium cellulose glycolate, sodium carboxy methyl starch or the compound of them. The additive includes lubricant, flow aid, deodorizing agent. The filler is the compound of alditol and cellulose. The method used polyketone as adhesive, used alcohol or the fixed liquid of alcohol and water as humectant. The sputtering agent uses inner adding method or outer adding method, wet process to produce particles. The character of the invention lies in fast dilution, the medicine can be dissolved entirely in then minutes, it needn't sugar-coat, the troche has good taste, it has no obvious bitterness.
Owner:TSINGHUA UNIV
Simvastatin tablet and preparation method thereof
The invention discloses a simvastatin tablet and a preparation method thereof. The simvastatin tablet comprises an active ingredient simvastatin and pharmaceutical excipients, wherein the pharmaceutical excipients are spherical lactose, cross-linked sodium carboxymethyl cellulose, butylhydroxyanisole, hydroxypropyl methyl cellulose, silicon dioxide, magnesium stearate and film coating premix, which are added according to a specific mass ratio and process. The simvastatin and the pharmaceutical excipients are incompatible, and are prone to hydrolysis and oxidation, lactone bonds break to open loop to generate an active metabolite simvastatin hydroxy acid under the high-humidity condition, the intramolecular diene bond is subjected to a slow oxidative copolymerization reaction to generate a dimer or polymer under the high-temperature condition, and the preparation of a stable preparation is greatly difficult. In recent years, with the continuous disclosure of information, the difference between the quality standards of simvastatin tablets produced by different manufacturers is great, wherein the dissolution behavior difference is more significant, so that the situation that the simvastatin tablets have the same name but have different quality is very obvious. The prescription process determined by the study can continuously produce the simvastatin tablet at large scale, and the prepared simvastatin tablet has a good dissolution performance in various PH-value dissolution media, and keeps good stability in the long-term storage process.
Owner:DIAO GRP CHENGDU PHARMA
Sofosbuvir film coating tablet preparation and preparation method thereof
InactiveCN104546783AWell mixedImprove liquidityOrganic active ingredientsDigestive systemPolythylene glycolStearic acid
The invention discloses a sofosbuvir film coating tablet preparation. The preparation is prepared from the following active ingredients in percentage by mass: 25.0-40.0 percent of sofosbuvir, 2.0-8.0 percent of a disintegrating agent, 50.0-80.0 percent of a diluting agent and 0.5-1.5 percent of a lubricating agent, wherein the disintegrating agent is one or more of croscarmellose sodium, hydroxypropyl methylcellulose and sodium carboxymethyl starch, one or two of sodium carboxymethylcellulose and hydroxypropyl methylcellulose as first choice, and preferably sodium carboxymethylcellulose; the diluting agent is one or more of microcrystalline cellulose, mannitol, lactose and polyethylene glycol, one or two of microcrystalline cellulose and mannitol as first choice, and preferably microcrystalline cellulose; and the lubricating agent is one or more of magnesium stearate, talcum powder, aerosil and calcium stearate, one or two of magnesium stearate and aerosil, and preferably magnesium stearate. The preparation has the advantages of simple process, high yield, good stability and the like, and is easy for large-scale industrial production.
Owner:ANHUI YELLEN PHARMA
Atorvastatin calcium tablets and preparation method thereof
ActiveCN102138910AGood dispersionRapid dissolutionMetabolism disorderPill deliveryDissolutionLactose
The invention relates to atorvastatin calcium tablets and a preparation method thereof. The tablets comprise the following components in parts by weight: 1 part of atorvastatin calcium (in terms of atorvastatin), 1 part of croscarmellose sodium, 15 parts of lactose (80M), and 0.2 part of magnesium stearate. The preparation method comprises the following steps: screening the atorvastatin calcium and the croscarmellose sodium based on the amounts of the formula through a 120-meshed sieve respectively, and mixing uniformly; uniformly mixing the mixture with the lactose based on the amount of the formula according to a uniform progressive increasing method; and uniformly mixing the magnesium stearate based on the amount of the formula, and tabletting. The tablets have the advantages of stable quality, controllable related substances, good dispersibility and high dissolution speed.
Owner:LUNAN PHARMA GROUP CORPORATION
Ticagrelor tablets and preparation method thereof
ActiveCN104523640AIncrease dissolution ratePromote absorptionOrganic active ingredientsPharmaceutical non-active ingredientsMANNITOL/SORBITOLTicagrelor
The invention discloses ticagrelor tablets and a preparation method thereof and belongs to the technical field of medicines. According to the ticagrelor tablets disclosed by the invention, mannitol, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate are selected as auxiliary materials and are compounded with the raw material medicine ticagrelor tablets so as to obtain the ticagrelor tablets, and each auxiliary material and the raw material medicine are synergetic in the defined amount range, so that the dissolution rate of the prepared ticagrelor tablets is higher than that of the conventional commercially available tablets. Moreover, the ticagrelor tablets have the same dissolution behavior as the commercially available medicines, the ticagrelor tablets have good absorption effects, and the bioavailability of the ticagrelor tablets is improved. The ticagrelor tablets disclosed by the invention are small in impurity content and are stable in performance under high-temperature illumination conditions. The method for preparing the ticagrelor tablets disclosed by the invention is simple in process flow, easy to operate and implement and suitable for industrial popularization and application.
Owner:HENAN RUNHONG PHARMA
Complex acetaminophen vitamin C dispersion tablet and preparing method thereof
InactiveCN1507862AQuick effectDefinite curative effectOrganic active ingredientsAntipyreticSorbyl alcoholCross-link
The present invention relates to compound paracetamol vitamin C disperser tablet and its preparation method. It is formed from paracetamol, vitamin C, disintegrant, filling agent, correctives, adhesive and lubricating agent according to the ratio of 200-400:200-400:1-60:1-100:1-60:2-50:1-10. The disintegrant is carboxy methyl starch sodium, hydroxypropyl starch, low-substituted hydroxypropyl cellulose, calcium cellulose glycollate, cross-linked sodium cellulose glycollate, sodium lauryl sulfate, and the filling agent is lactose, mannitol, sorbyl alcohol, starch, modified starch, beta-dextrin, calcium sulfate dihydrate, microcrystal cellulose, and the corrective is wintergreen oil, peperitol, aspatan, citric acid, cane sugar, sterioside, the adhesive is polyvidone, hydroxypropyl cellulose, gelling starch and ethyl cellulose, and the lubricating agent is magnesium stearate, calcium stearate, polyglycol 6000 and talcum powder.
Owner:严洁
Preparation method of oral preparation containing rivaroxaban
ActiveCN104055743AGood reproducibilityOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseDissolution
The invention relates to an oral tablet containing rivaroxaban. The tablet is prepared by adopting a powder direct mixing and tabletting method. an inventor solves partial problems and solves the problems of pitted surface, sticking, loosening tablet and disintegration and dissolution rate and stability problems through addition and replacement of a proper additives and a screening experiment, so as to obtain a novel formula of rivaroxaban tablets and a preparation method of the rivaroxaban tablets by adopting the powder direct mixing and tabletting method. Each rivaroxaban tablet comprises the following components by weight: 5-20mg of rivaroxaban, 60-80mg of lactose, 4-6mg of mannitol, 2-4mg of croscarmellose sodium, 1-3mg of citric acid, 0.5-2mg of lauryl sodium sulfate, 3-5mg of carbomer, 3-5mg of povidone K90, 3-5mg of hydroxyl propyl cellulose, 0.5-2mg of magnesium stearate and 0.5-2mg of silicon dioxide.
Owner:JILIN BODA PHARMA
Apixaban tablet and preparation method thereof
ActiveCN104490841ADissolution rate is fastLow impurity contentOrganic active ingredientsPharmaceutical delivery mechanismCross-linkDissolution
The invention discloses an apixaban tablet and a preparation method thereof, and belongs to the technical field of medicines. The apixaban tablet consists of a tablet core and a coating, wherein the tablet core is composed of apixaban, a fiber-lactose compound, crosslinked carboxy methyl cellulose, lauryl sodium sulfate and magnesium stearate; various components in the tablet core are controlled within limited dosage ranges and a mutual synergistic effect is achieved, so that the dosage of the cross-linked sodium carboxymethyl cellulose as a disintegrating agent is reduced and the dissolution rate of the apixaban tablet is improved; therefore, the average dissolution rate within 10min is more than 90%, the dissolution rate is slightly affected by illumination, temperature and humidity, the performance is stable, and the apixaban tablet is low in contents of impurities. According to the preparation method of the apixaban tablet disclosed by the invention, the tablet core is prepared by a way of directly tabletting a powdery mixture, so that a granulation process is avoided, operation is simple and convenient, and the technological process is simple; and the apixaban, as a crude drug, is subjected to micronization treatment before the mixed powder is prepared, so that the dissolution rate of the apixaban tablet is accelerated.
Owner:HENAN RUNHONG PHARMA
Preparation method of simvastatin tablet
InactiveCN104224736ASmall particle sizeLarge specific surface areaOrganic active ingredientsMetabolism disorderPrillDissolution
The invention relates to a preparation method of a simvastatin tablet. The simvastatin tablet is composed of simvastatin, an acidic protecting agent, an antioxidant agent, a filling agent, a disintegrating agent and a lubricant. The preparation method disclosed by the invention comprises the following steps: carrying out low-temperature superfine grinding on a simvastatin raw material and lactose in a weight ratio of 1 to (1-10), so that the particle size is reduced and the specific surface area is increased, and then the raw material is changed from a lipophilic material into a hydrophilic material; uniformly mixing the obtained mixed material with the acidic protecting agent and the antioxidant agent, the rest of lactose, microcrystalline celluloses and crosslinked carboxymethyl cellulose sodium, granulating by using a wet method, and carrying out fluidized drying while controlling the water content of a finished product grains at 1-5%, so that the simvastatin tablet is prepared. The prescription process is simple and easy to control, and prepared preparation is good in content uniformity, high in dissolution rate, good in absorption, and high in bioavailability in comparison with other preparations. The preparation method of the simvastatin tablet disclosed by the invention is good in good in reproducibility, large in productivity, and stable and controllable in quality, and can effectively guarantee the effectiveness of drugs and the safety of drug application of patients.
Owner:哈药集团人民同泰医药股份有限公司
Medicine composition containing active ingredients of pregabalin
ActiveCN104840443APromote absorptionReduce the number of dosesOrganic active ingredientsNervous disorderTherapeutic effectCarvacryl acetate
The invention belongs to the field of medicinal preparations, and particularly relates to a pregabalin slow release medicine composition. The pregabalin slow release medicine composition comprises active ingredients and excipients, wherein the active ingredients are pregabalin or pharmaceutically acceptable salts of the pregabalin; the excipients comprise a matrix forming agent and a swelling agent; the matrix forming agent is a mixture of polyvinyl acetate and polyvinylpyrrolidone; the swelling agent is selected from one or any combination from croscarmellose sodium, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and polyoxyethylene. The medicine composition is suitable for oral administration once in each day; the number of medication times is reduced; the blood concentration peak-to-valley ratio is reduced; meanwhile, the proper swelling agent is selected, so that the gastric retention can be reached, and the problem of stability caused by peroxides can also be avoided; the product stability is improved; the therapy effect can be achieved through once medication in each day; the effect of the medicine composition is equivalent to that of quick release preparations. The medicine composition is manly used for treating epilepsy, neuropathic pain and the like.
Owner:QILU PHARMA HAINAN
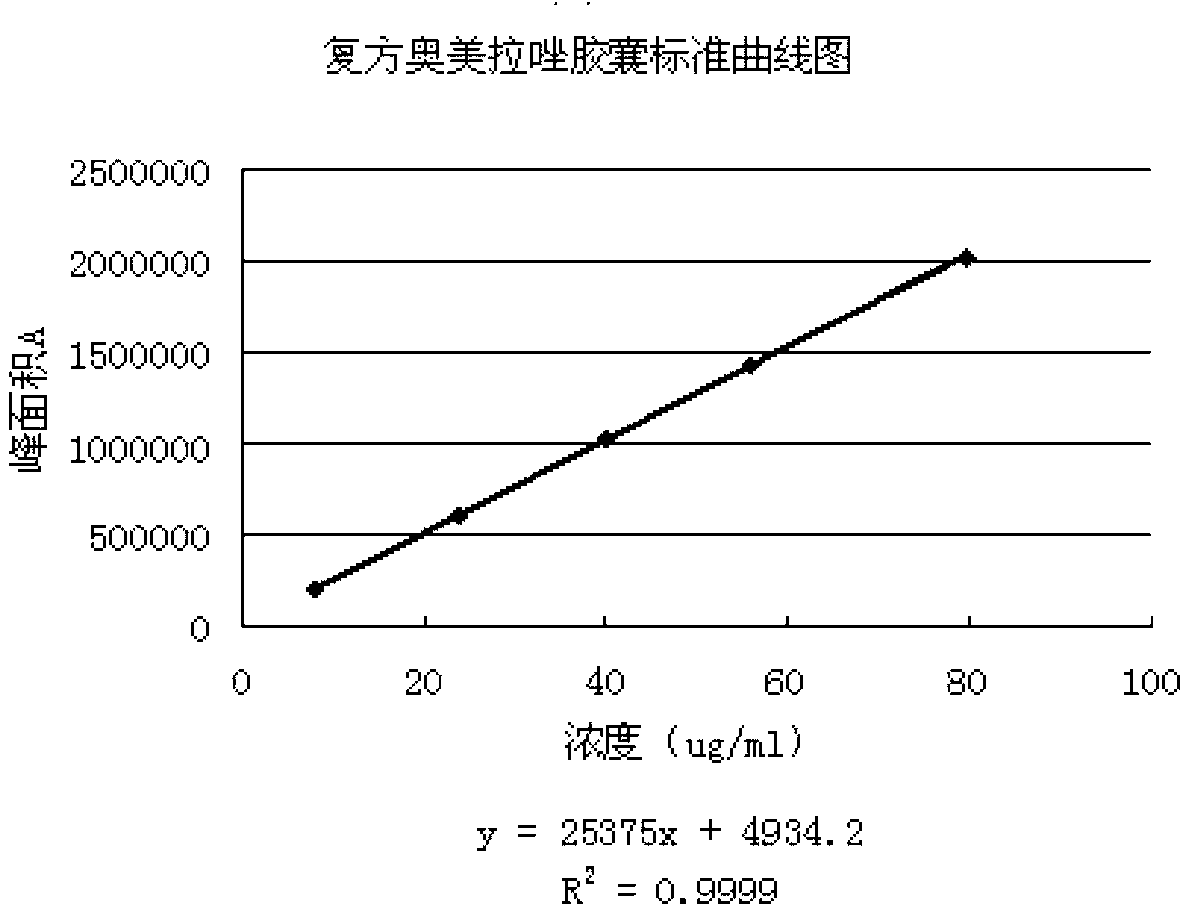
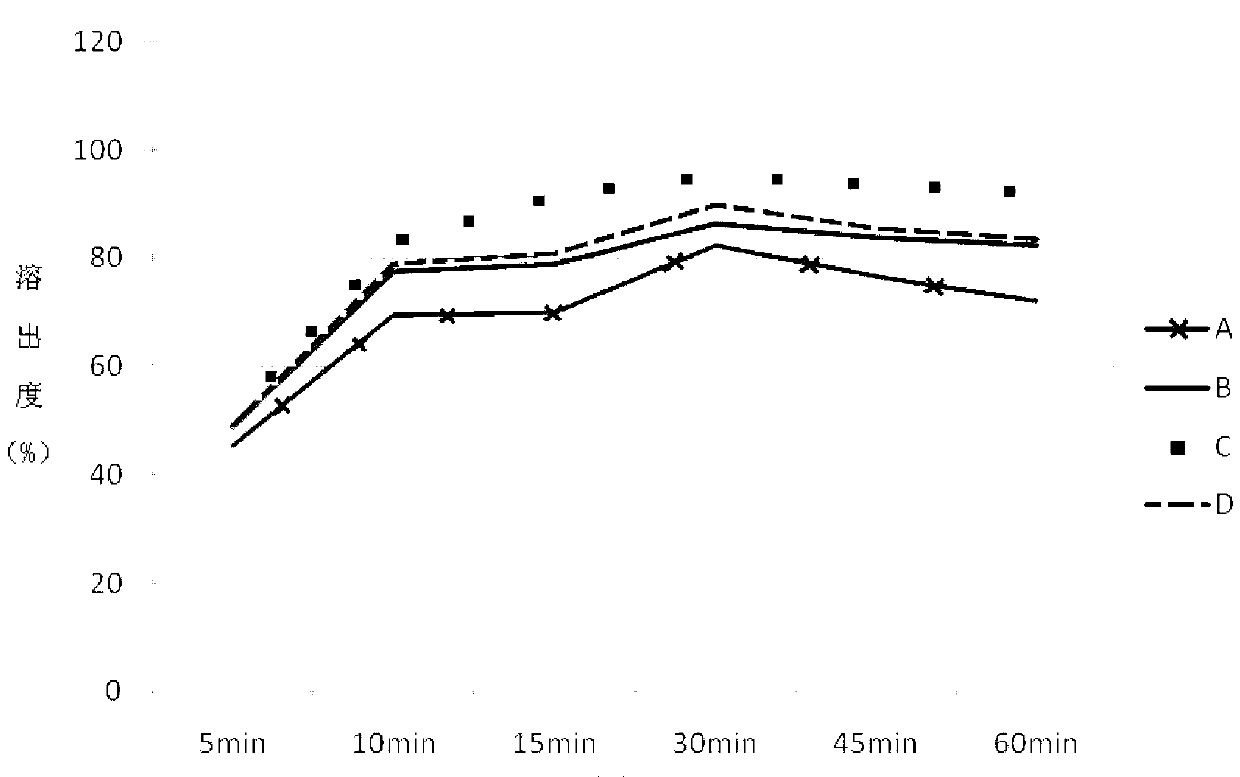
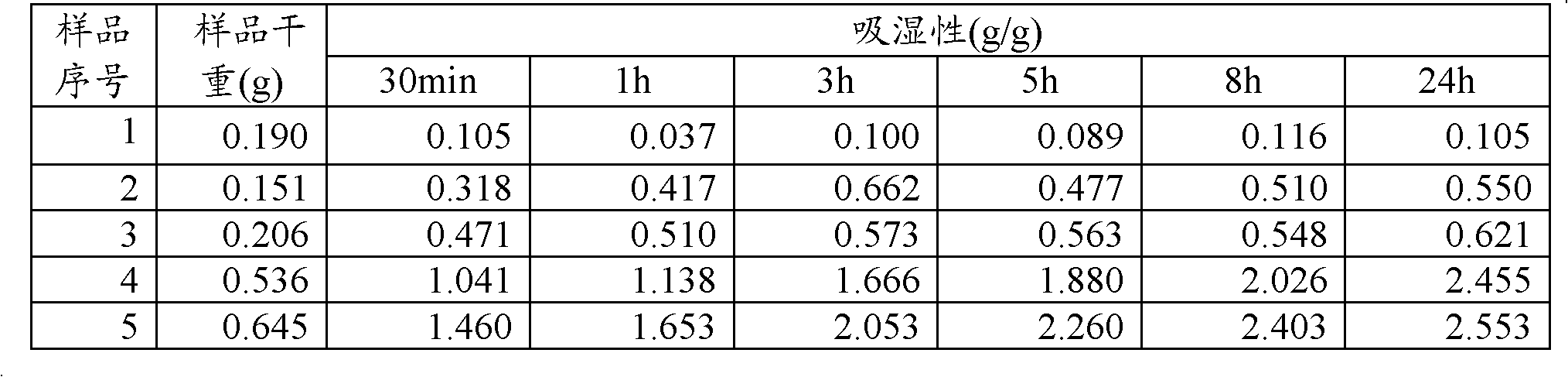
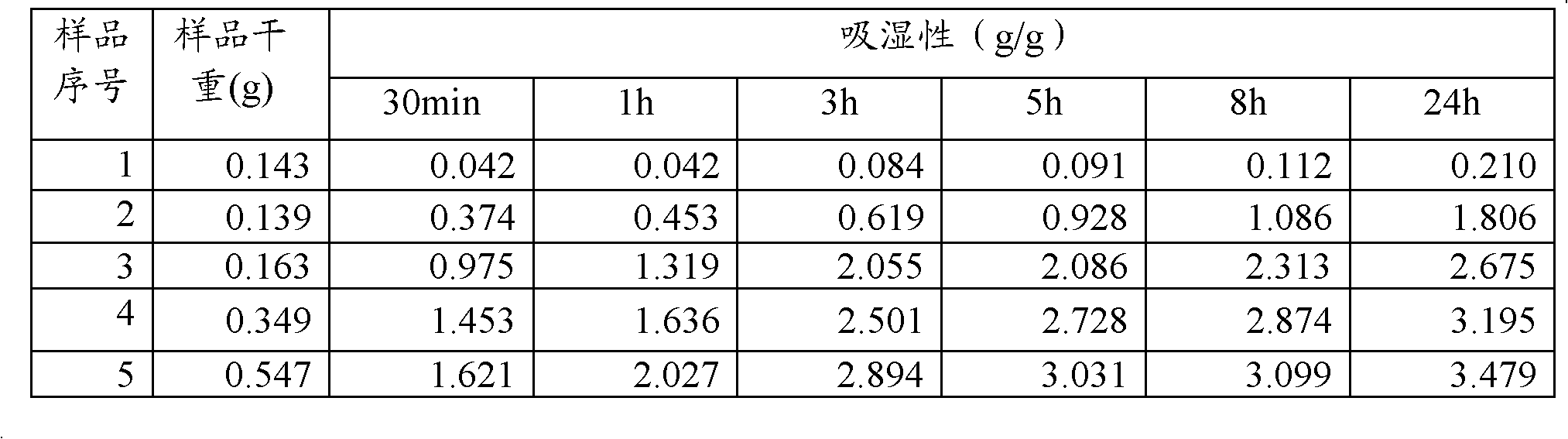
Compound omeprazole capsule, and preparation method and detection method thereof
ActiveCN103340892ASimple preparation processWon't breakOrganic active ingredientsComponent separationCross-linkSodium bicarbonate
The invention discloses a compound omeprazole capsule, and a preparation method and a detection method thereof. The capsule is prepared by: in parts by weight, 20 parts of omeprazole, 1100 parts of sodium bicarbonate, 30 parts of cross linked sodium carboxymethyl cellulose and proper amount of magnesium stearate; or the capsule is prepared by 40 parts of omeprazole, 1100 parts of sodium bicarbonate, 30 parts of cross linked sodium carboxymethyl cellulose and proper amount of magnesium stearate. Aiming at the deficiency of the prior art The formulation, the preparation technology and the detection method of the conventional compound omeprazole capsule are optimized, the compound omeprazole capsule of the invention exhibits more substantial curative effect for diseases comprising peptic ulcer, gastroesophageal reflux disease and the like caused by gastric hyperacidity; a systematic, integrated and effective component discrimination and content determination method is established, thereby the medicine quality is effectively controlled, and thus the clinic curative effect is guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Polyurethane porous membrane as well as preparation method and application thereof
InactiveCN102304281AShorten healing timePromote healingAbsorbent padsBandagesCarrageenanPorous membrane
The invention discloses a polyurethane porous membrane which comprises the following components by weight percent: 0.5%-75.0% of hydrophilic high polymer material and 99.5%-25.0% of polyurethane, wherein the hydrophilic high polymer material is at least one of carboxymethylcelluose sodium, crosslinked carboxymethylcelluose sodium, agaropectin, carrageenan and sodium alginate. According to the invention, the polyurethane porous membrane contains the hydrophilic high polymer material so that a wound percolate can be adsorbed in the porous membrane structure; and the polyurethane porous membrane absorbs the ejection of a wound tissue after being applied to the surface of a wound and prevents the naked hypodermis from being dried, thereby shortening the healing time of the wound, alleviating the pain of a patient and promoting the wound to well heal.
Owner:JIAXING UNIV
Method for preparing pharmaceutical excipient croscarmellose sodium from wood fiber
InactiveCN102295706AMeet the requirementsImprove performancePharmaceutical non-active ingredientsCarboxymethyl celluloseFiber
A method for preparing pharmaceutical excipient croscarmellose sodium from wood fiber, characterized in that the method steps are: a, cooling the mixed solution to below 30°C for use; b, alkalization reaction; c, etherification reaction ; d, cross-linking reaction; e, neutralization: add alkaline solution to adjust the pH value of the system, control the pH value of the material to 5.0-7.0, centrifugally dry, pulverize and sieve to obtain the pharmaceutical auxiliary material cross-linked carboxymethyl fiber Vegetarian Sodium. The technical effects of the invention are: the preparation of cross-linked sodium carboxymethyl cellulose as a medical auxiliary material from wood fiber is realized, the product cost is greatly reduced, the cross-linking conditions are mild and easy to control, and the compound has reliable performance and stable quality.
Owner:ANHUI SUNHERE PHARMA EXCIPIENTS
Direct compression process for cefuroxime axetil dispersible tablets
InactiveCN101703448AHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsDissolutionStearic acid
The invention relates to a direct compression process for cefuroxime axetil dispersible tablets, which is characterized in that: the following raw materials and auxiliary materials are sieved through a 40 mesh sieve and are mixed for thirty minutes in a mixing tank, and then the mixed powder materials are directly compressed by a high speed rotary tablet press to form the cefuroxime axetil dispersible tablets; and based on 125 grams of cefuroxime axetil, the raw materials and the auxiliary materials for producing 1000 dispersible tablets comprise 25 grams of cefuroxime axetil, 4 to 10 grams of stearic acid, 210 to 250 grams of microcrystalline cellulose, 28 to 35 grams of croscarmellose sodium, 2 to 4 grams of silicon dioxide and 2 to 4 grams of sodium dodecyl sulfate. The direct compression process for the cefuroxime axetil dispersible tablets improves the dissolution rate of the cefuroxime axetil dispersible tablets and improves the bioavailability and the healing effect of the medicament, wherein the dissolution rate reaches 99.5 percent at the time when the tablets dissolve for 45 minutes. The direct compression process simplifies the production process, shortens the production period and saves energy.
Owner:山东淄博新达制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com