Preparation method of amoxicillin and clavulanate potassium tablets
A technology of potassium amoxicillin and clavulanate tablets and potassium clavulanate, which is applied in the direction of medical preparations containing active ingredients, pill delivery, and pharmaceutical formulations, and can solve the problem of unsatisfactory tablet appearance, fluidity and Poor compressibility, capping and other problems, to achieve the effect of reducing dosage, reducing production cycle and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (a) Weigh 136g of amoxicillin, 34g of potassium clavulanate, 32g of microcrystalline cellulose (P112), 2g of croscarmellose sodium (ADS), 2g of micropowder silica gel, and 2g of magnesium stearate, totaling 205g
[0018] (b) After amoxicillin is granulated, it is mixed with potassium clavulanate at a mass ratio of 4:1 to form the main ingredient.
[0019] (c) The auxiliary materials micropowder silica gel, croscarmellose sodium (ADS) and microcrystalline cellulose are added and mixed in equal amounts, and then added into the mixer with the same amount of main ingredients and mixed for 36-70 minutes.
[0020] During the whole process, the moisture content of the intermediate mixed powder is controlled to be less than or equal to 9.0%, and the marked content is controlled at 95.0% to 115.0%.
[0021] (d) Compressing the mixed powder into tablets.
[0022] Finally, the plain tablet is film-coated as usual. Packaging: Double aluminum packaging, the thickness of the bottom...
Embodiment 2
[0025] Weigh 136 g of amoxicillin, 34 g of potassium clavulanate, 30 g of microcrystalline cellulose (P112), 3 g of croscarmellose sodium (ADS), 3 g of micropowder silica gel, and 2 g of magnesium stearate, totaling 205 g.
[0026] Refer to Example 1 for the specific preparation method.
Embodiment 3
[0038] Product Stability Test
[0039] Adopt the quality comparison of the manufactured product of the inventive method and the manufactured product of prior art:
[0040] (See Table 1 for details)
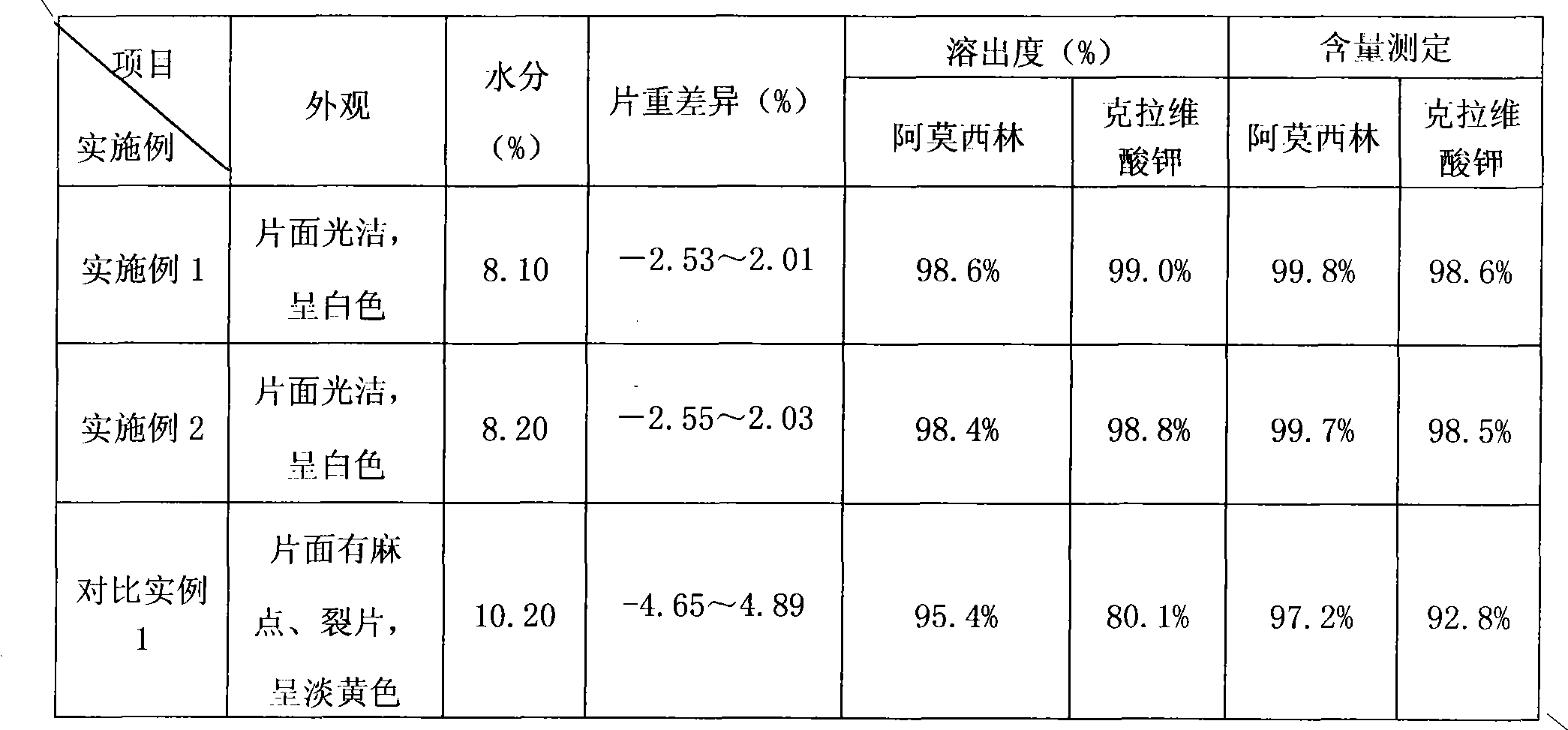
[0041] Table 1
[0042]
[0043] It can be seen that the quality of the amoxicillin-clavulanate potassium tablet prepared by the method of the present invention is obviously due to the prior art.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com