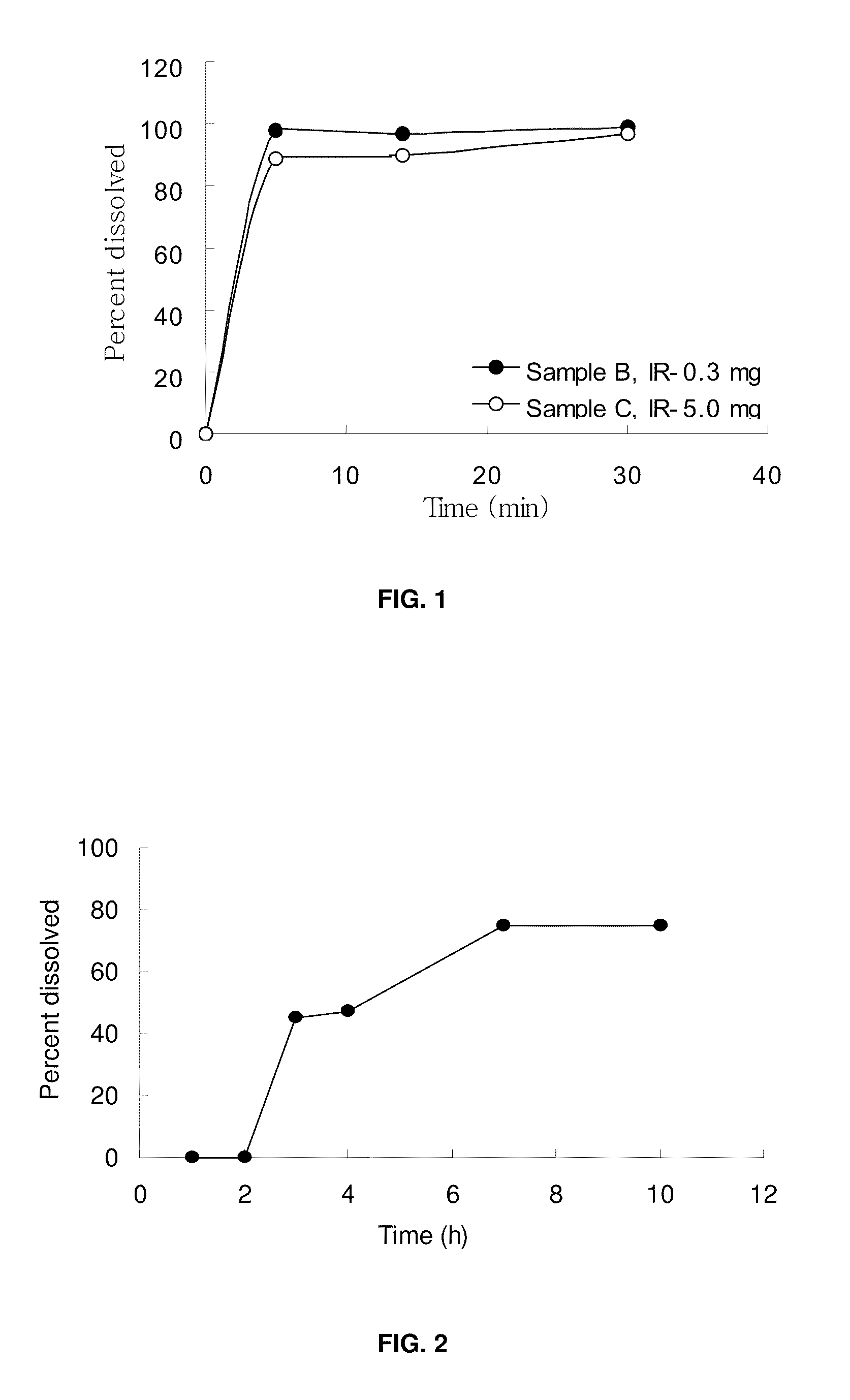
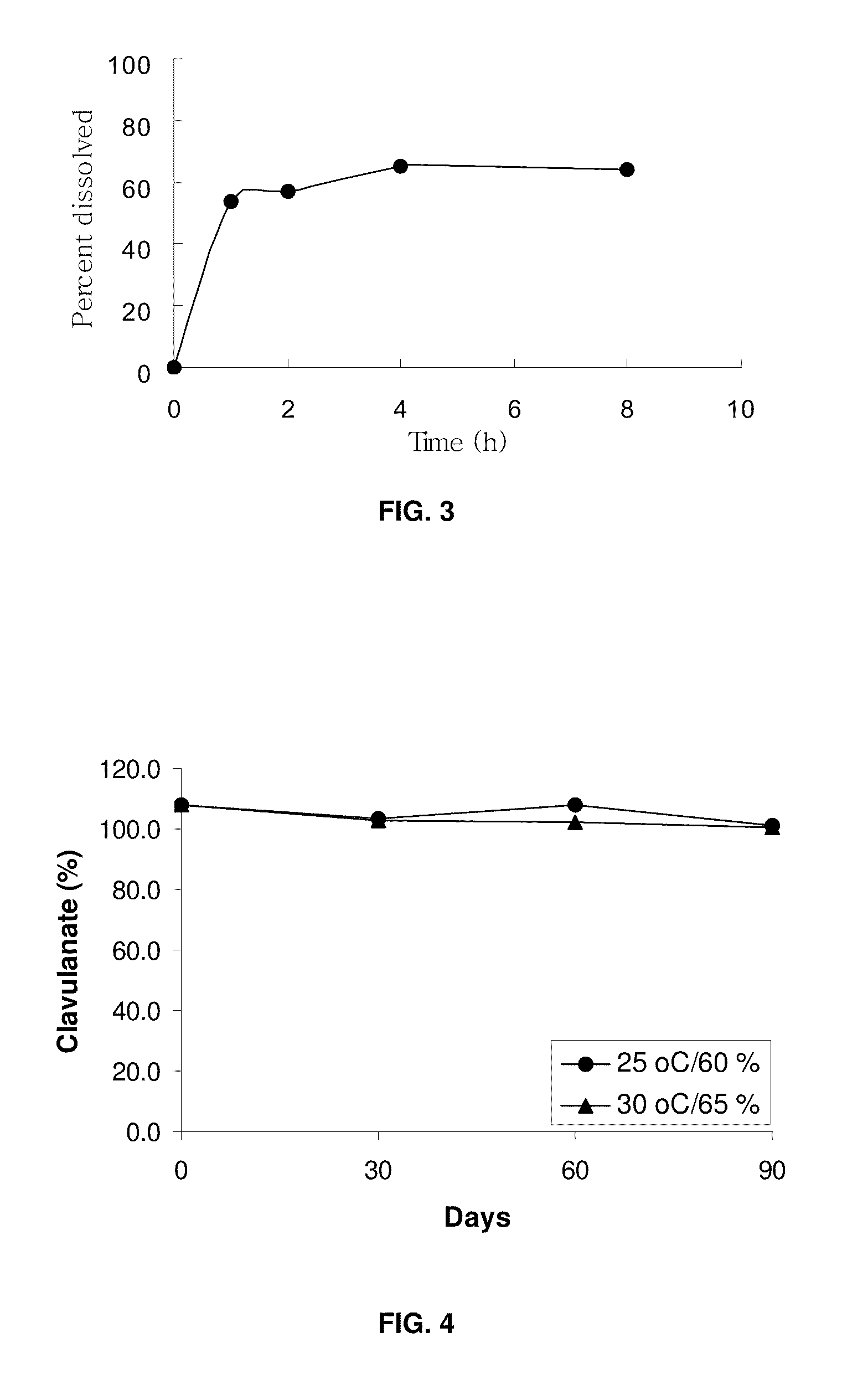
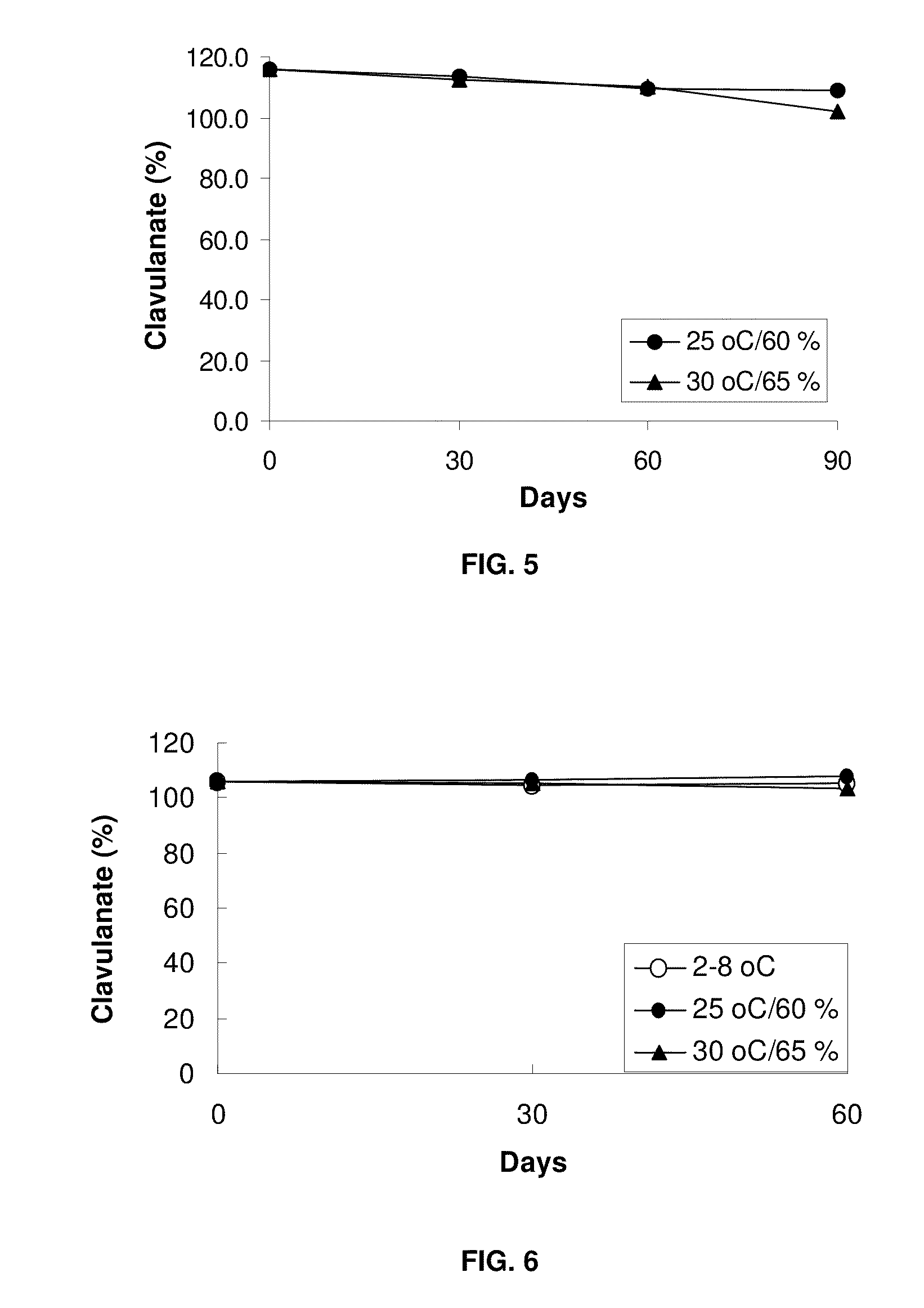
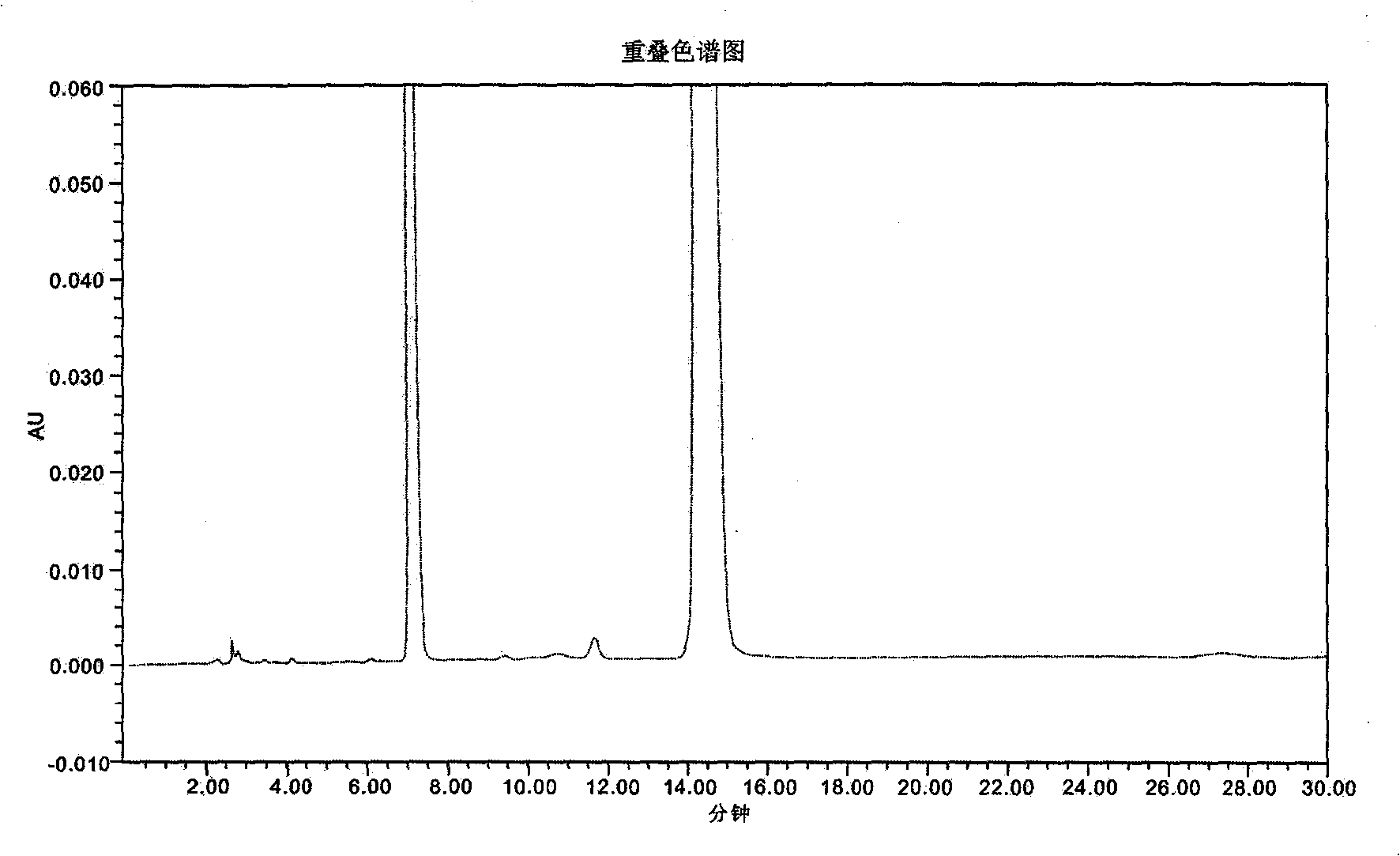
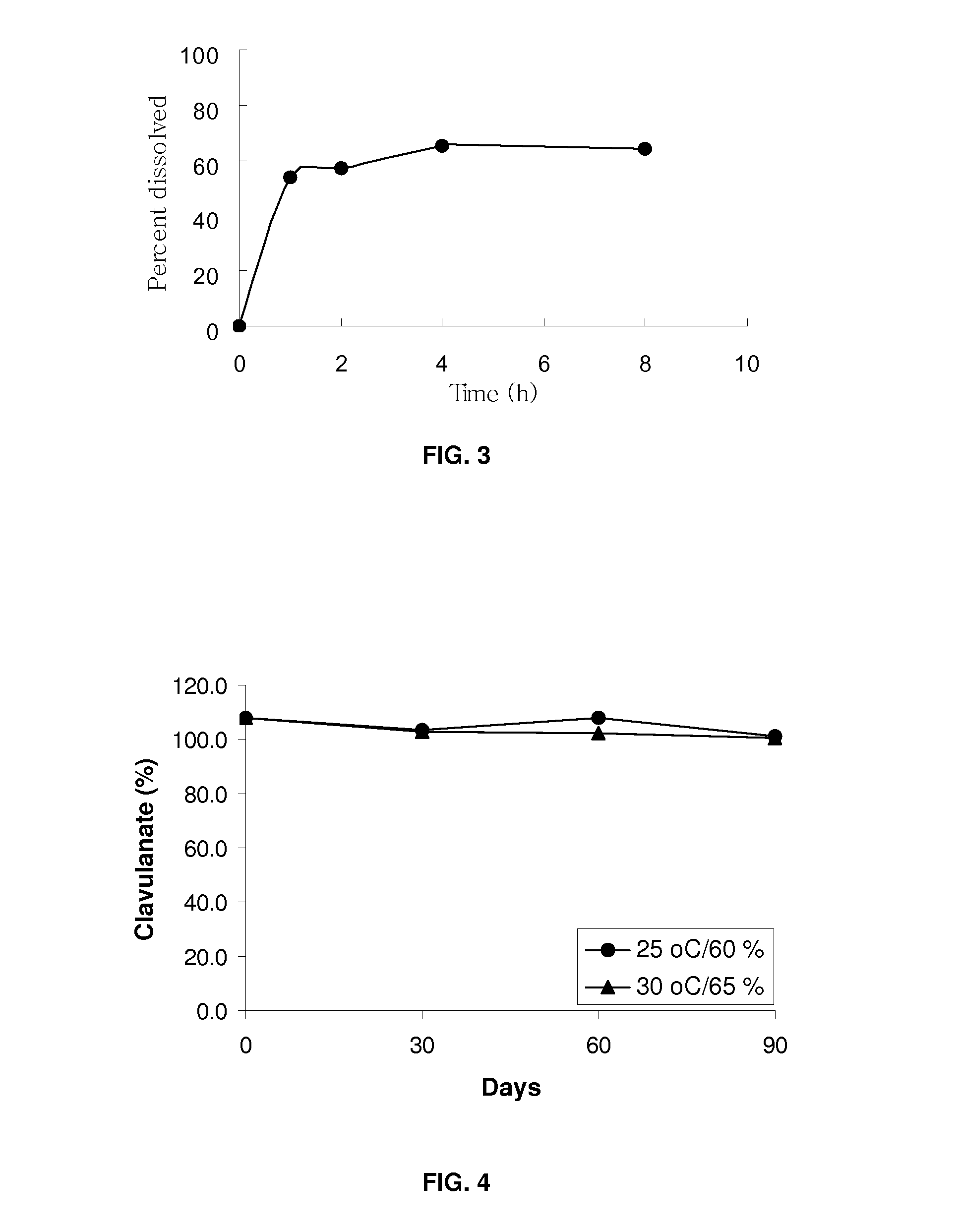
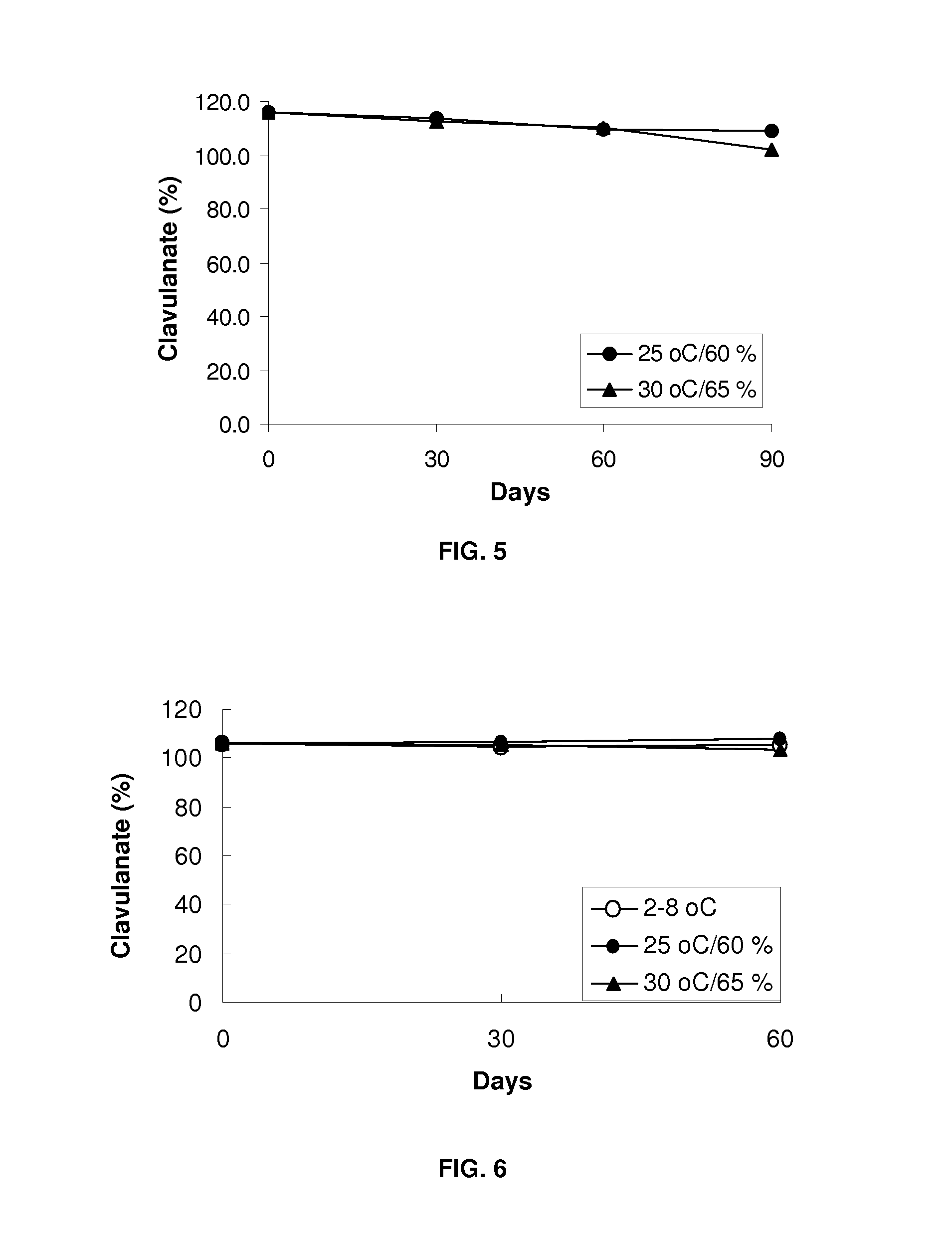
Patents
Literature
144 results about "Clavulanic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
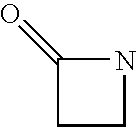
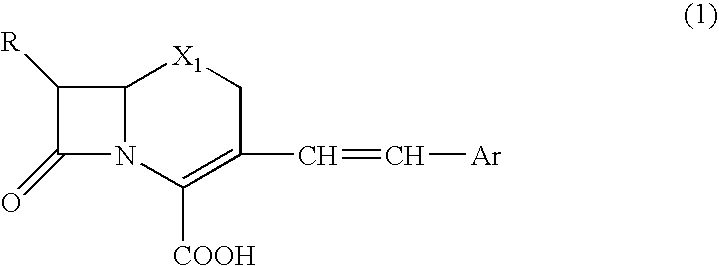
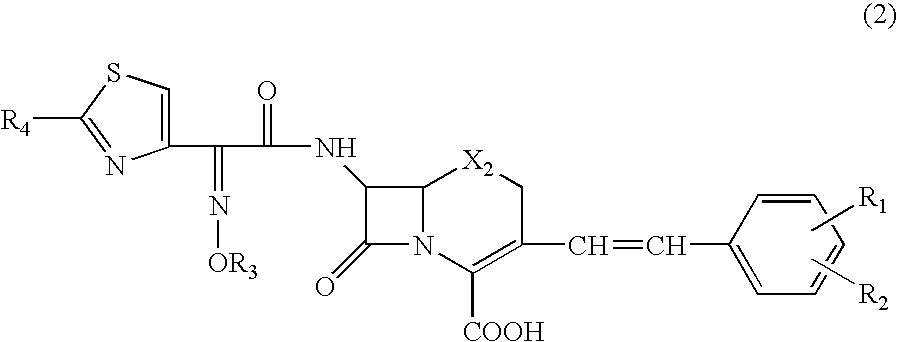
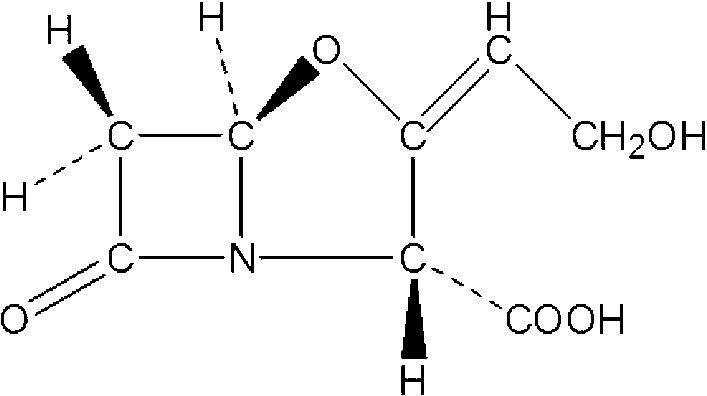
Clavulanic acid is a β-lactam drug that functions as a mechanism-based β-lactamase inhibitor. While not effective by itself as an antibiotic, when combined with penicillin-group antibiotics, it can overcome antibiotic resistance in bacteria that secrete β-lactamase, which otherwise inactivates most penicillins.
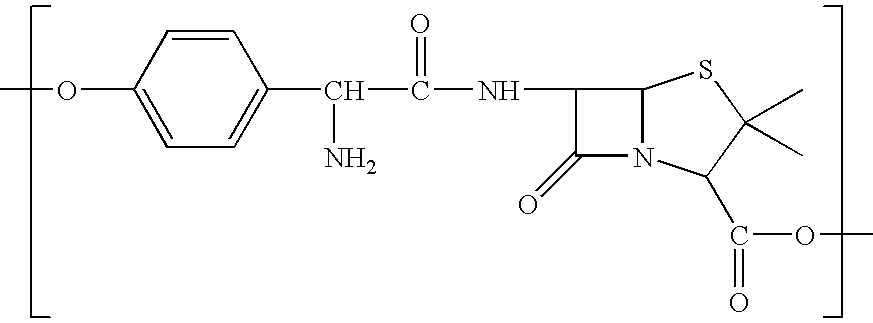
Antibiotic polymers
InactiveUS7396527B2Reduce frequencyReducing and eliminating riskPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsPolyesterPolyamide
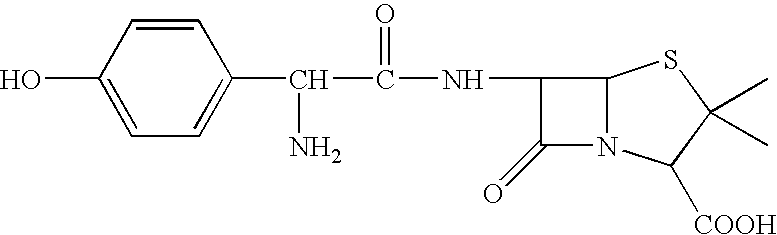
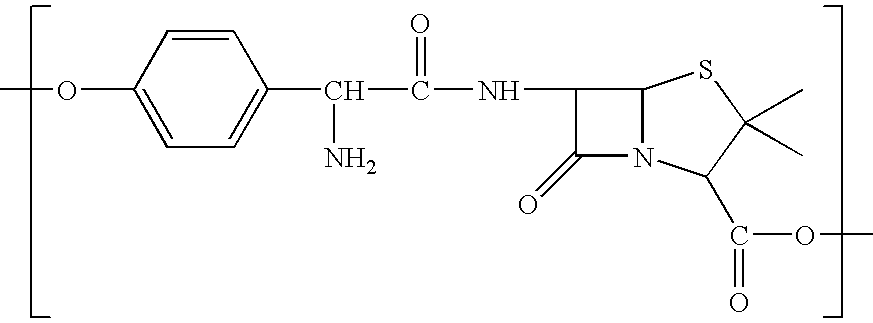
Polymers (i.e. polyesters, polyamides, polythioesters, polyanhydrides, or a mixture thereof) which degrade hydrolytically to provide a combination of a beta-lactam antibiotic (e.g., amoxicillin) and a beta-lactamase inhibitor (e.g., clavulanic acid) (or a pharmaceutically acceptable salt thereof) are provided. Methods of producing these polymers, intermediates useful for preparing these polymers, and methods of using these polymers to deliver a combination of a beta-lactam antibiotic and a beta-lactamase inhibitor (or a pharmaceutically acceptable salt thereof) to a host are also provided.
Owner:RUTGERS THE STATE UNIV
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
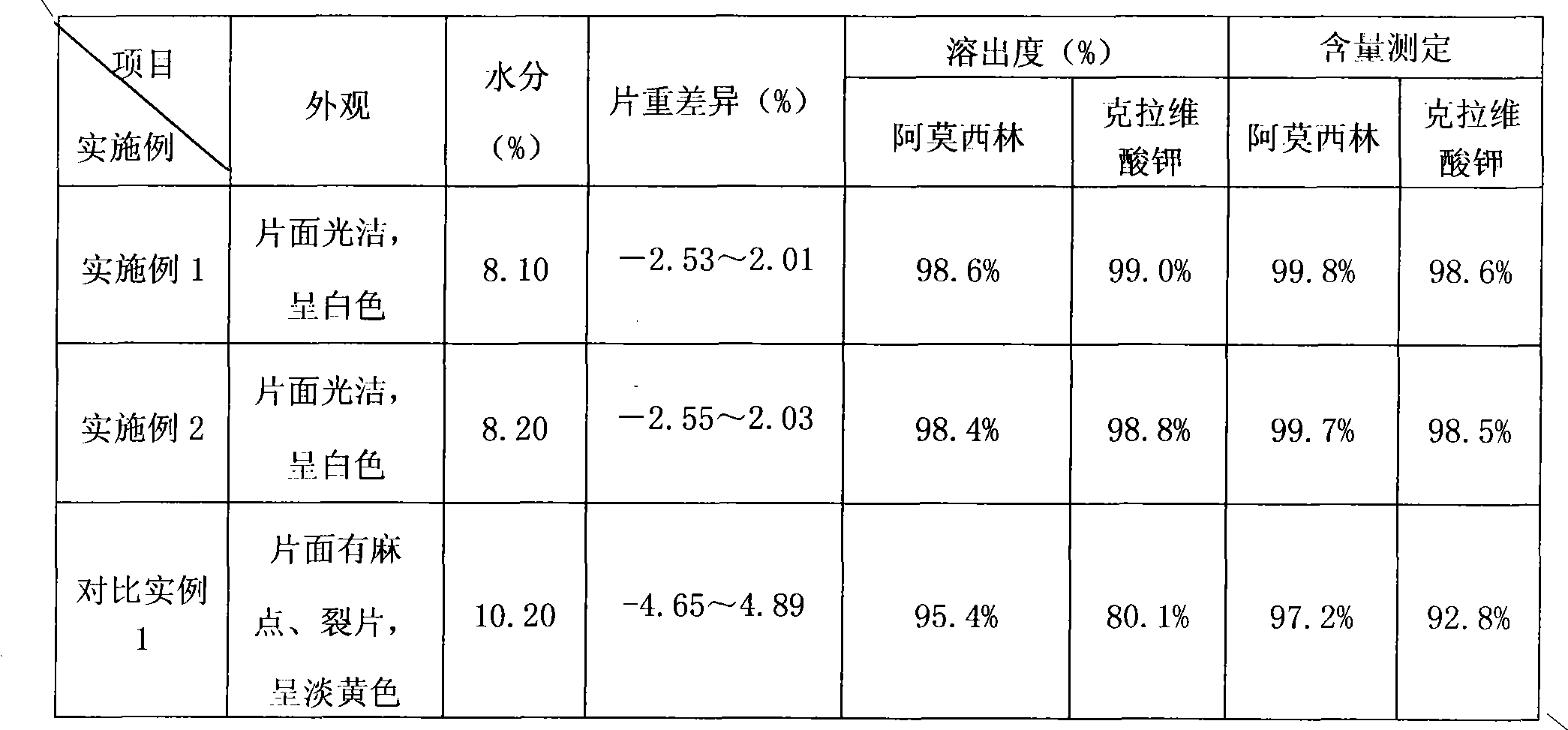
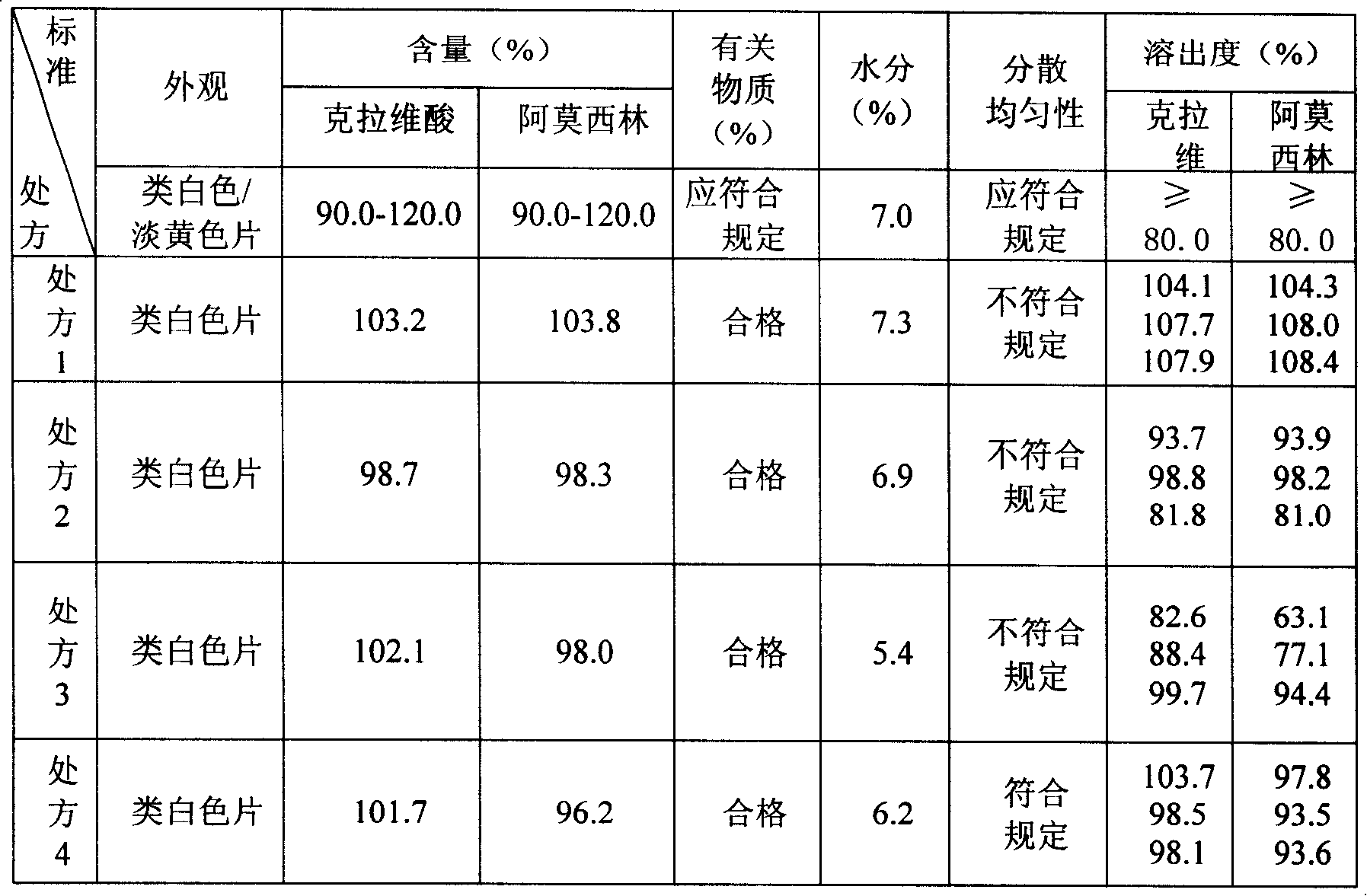
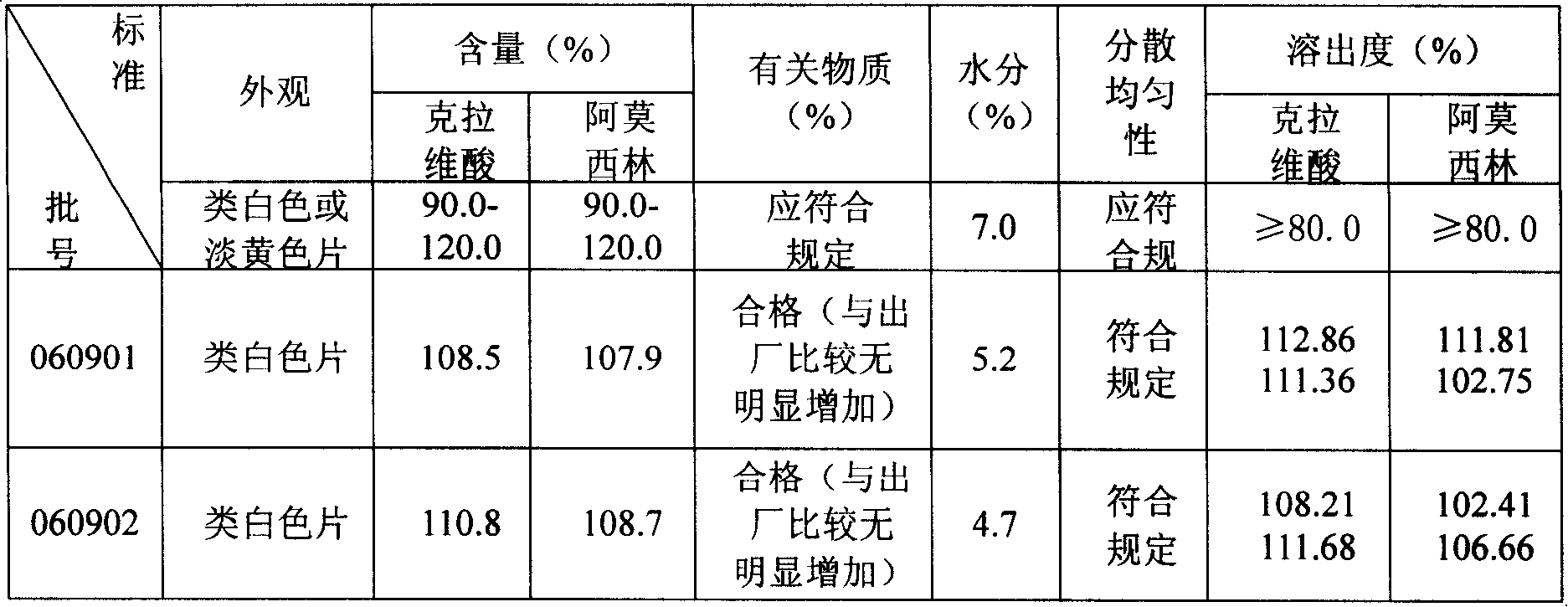
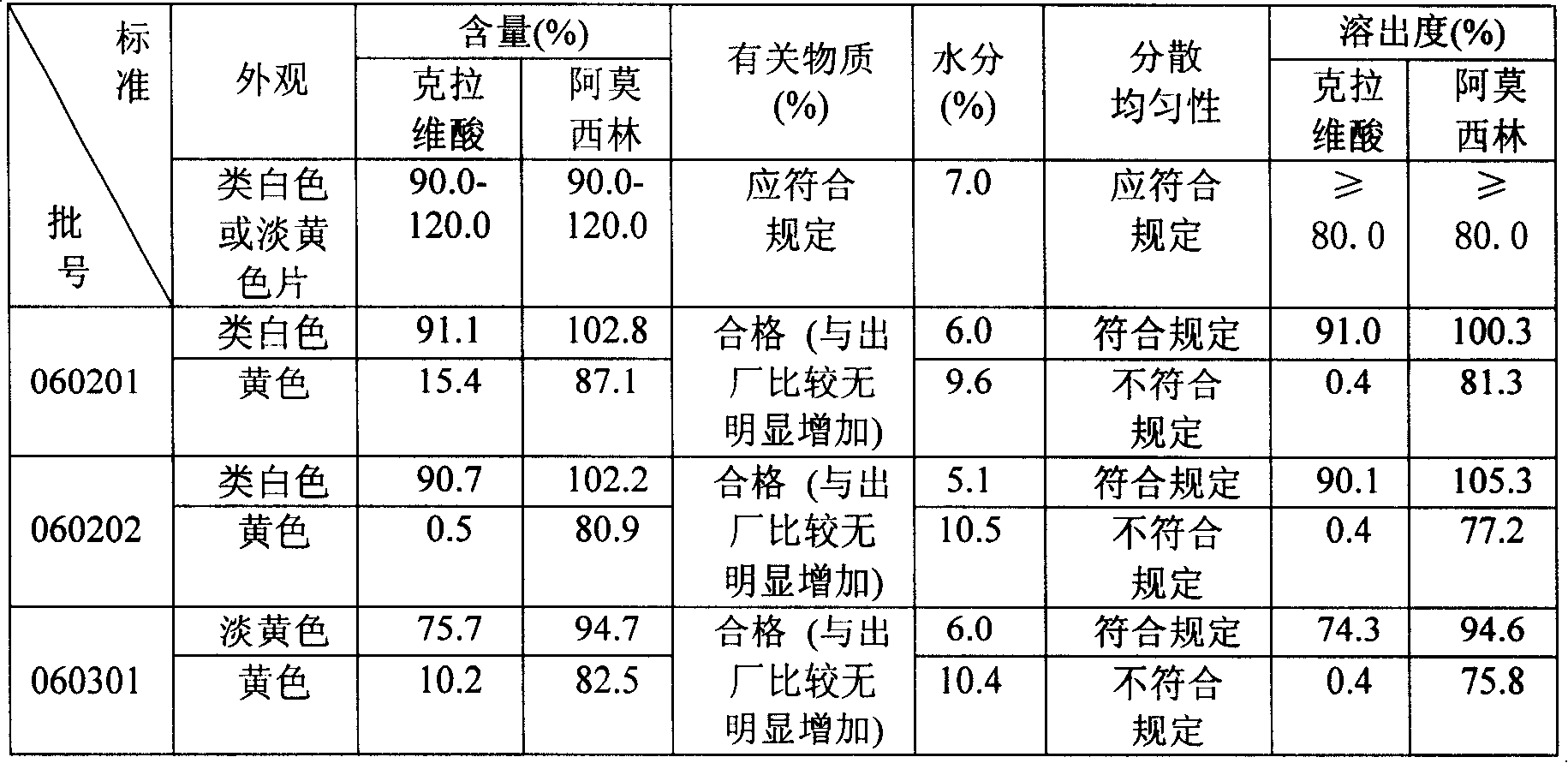
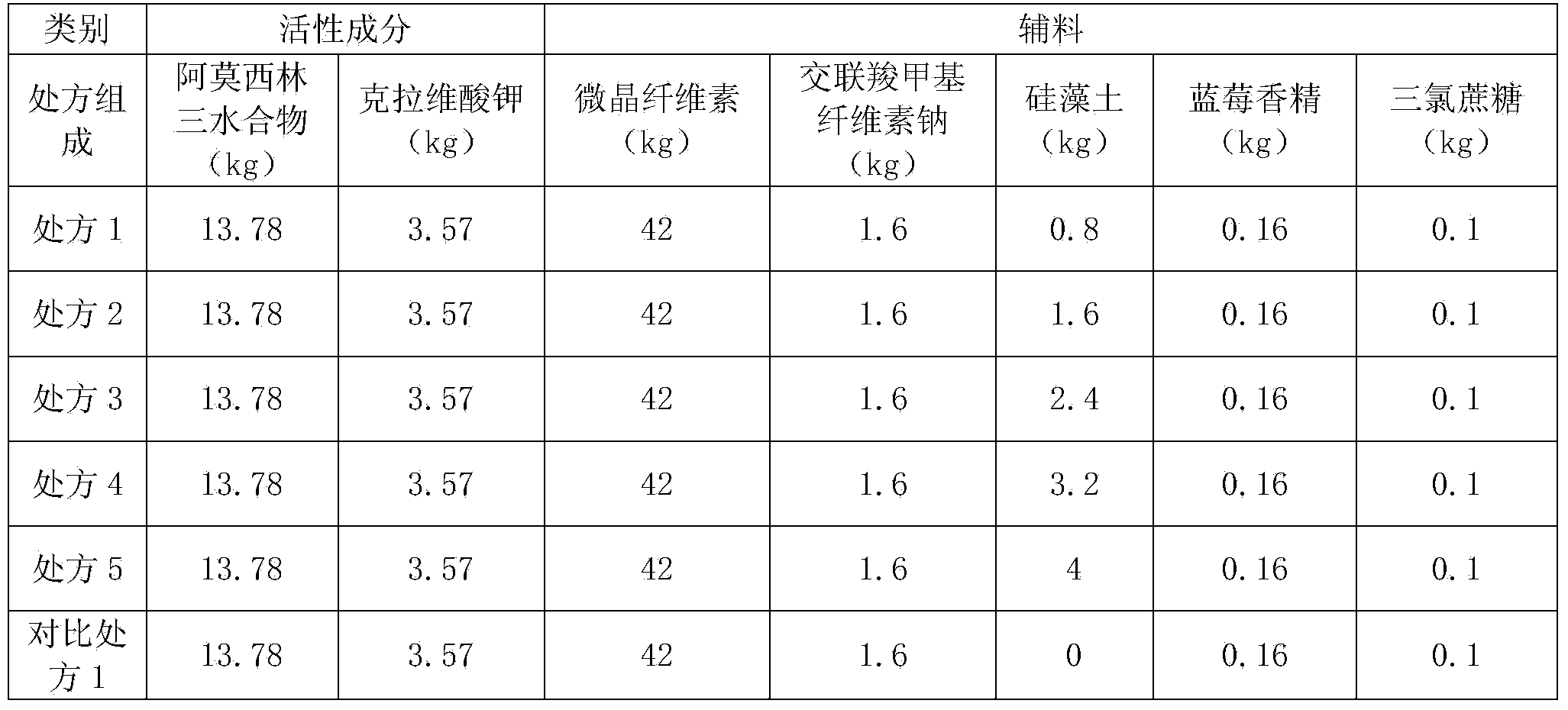
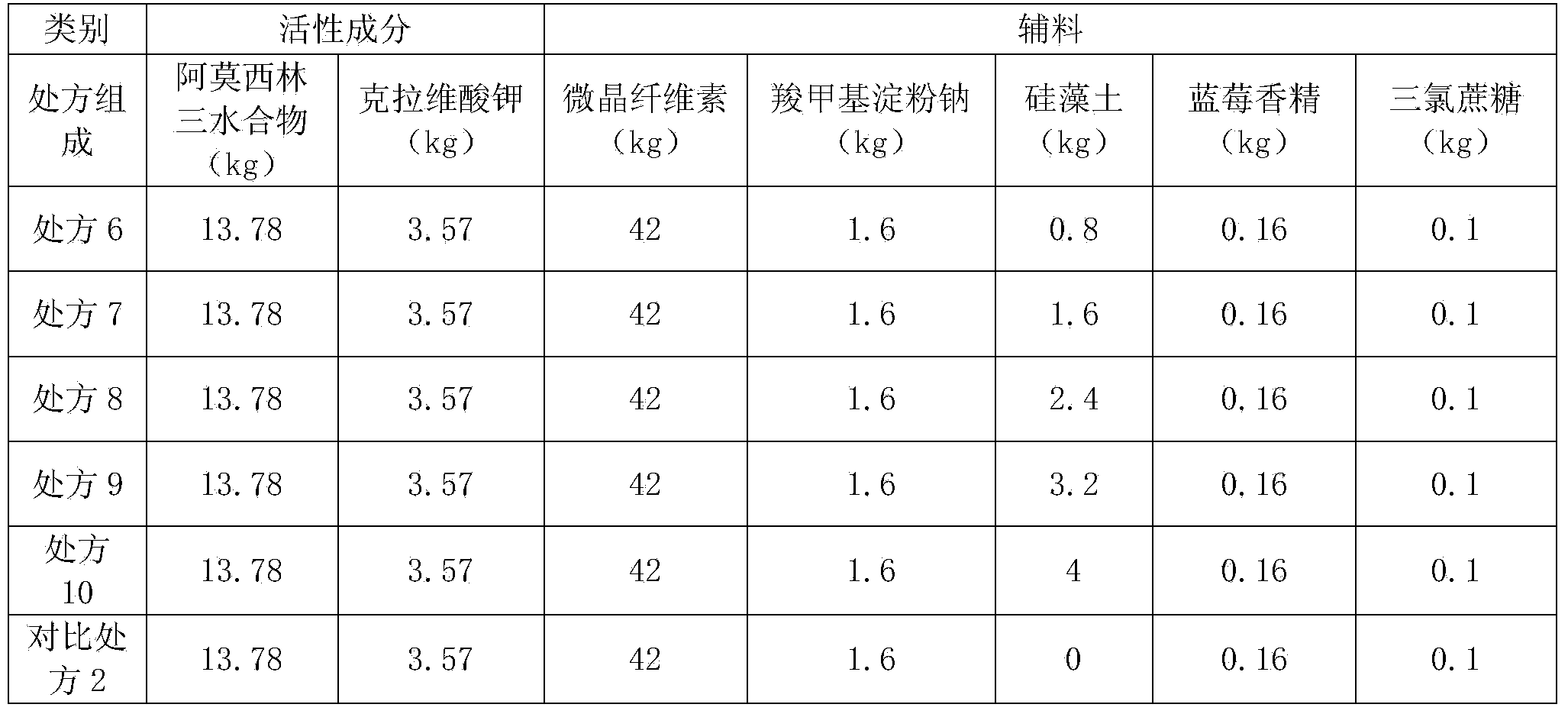
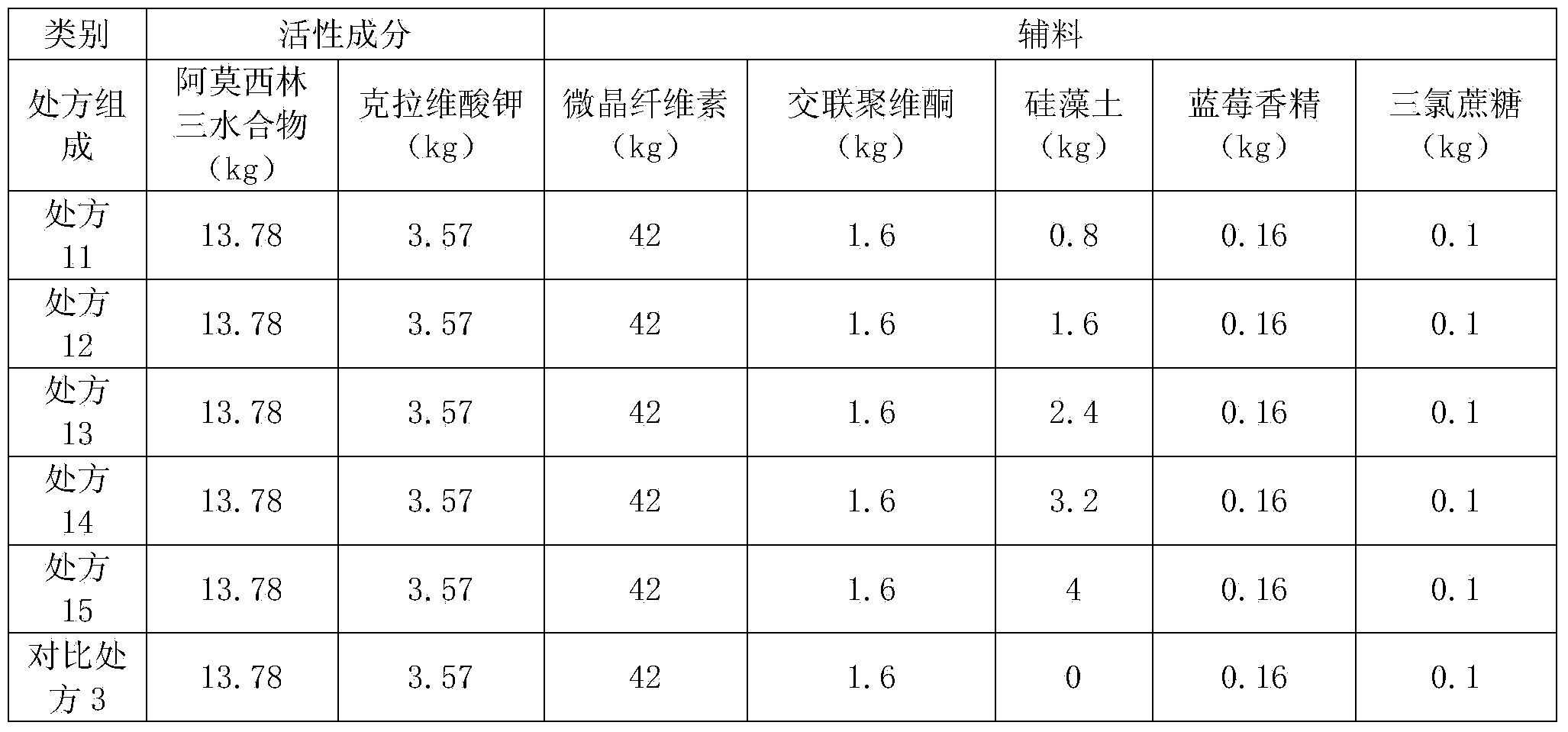
Preparation method of amoxicillin and clavulanate potassium tablets
InactiveCN101897701AReduce exposure timeGuaranteed stabilityAntibacterial agentsPharmaceutical product form changeMass ratioDissolution
The invention discloses a preparation method of amoxicillin and clavulanate potassium tablets, comprising the following steps: (a) weighing the following main and auxiliary materials: 120-135 parts of amoxicillin, 40-45 parts of clavulanate potassium, 27-32 parts of microcrystalline cellulose, 1-3 parts of croscarmellose sodium (ADS), 1-3 parts of superfine silica powder and 2-4 parts of magnesium stearate; (b) after pelletizing amoxicillin, mixing the pelletized amoxicillin with clavulanate potassium according to the mass ratio of 4:1 to form the main materials; (c) uniformly mixing the auxiliary materials including superfine silica powder, ADS and microcrystalline cellulose by the method of increment by equal quantity and throwing the main materials and the auxiliary materials into a mixer by the method of increment by equal quantity to be mixed for 36-70min; and (d) tabletting the mixed powder. The products prepared by the method have bright, clean and beautiful appearances and stable content and dissolution.
Owner:NORTH CHINA PHARMA COMPANY
Reagent composition for detecting beta-lactamase, detection kit and detection method
InactiveCN1729299AMicrobiological testing/measurementBiological material analysisCarboxylic acidCephem
It is intended to provide a reagent composition for detecting beta-lactamase which contains 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido)-3-cephem-4-carboxylic acid or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxyimino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid as a substrate for detecting beta-lactamase together with at least one beta-lactamase inhibitor selected from among clavulanic acid, aztreonam, ethylenediaminetetraacetic acid and cloxacillin and by which beta-lactamase can be quickly and easily identified at a high sensitivity. Moreover, a detection kit containing the above detection reagent composition and a method of detecting beta-lactamase comprising contacting a liquid specimen containing the subject to be analyzed with the above composition are provided.
Owner:昭和药品化工股份有限公司 +1
Antibiotic compound recipe comprising piperacillin
The invention involves antibiotic compound recipe containing piperacillin, sulbactam or clavulanic acid, ion chelator which can inhibit the formation of particles chelator, the recipe can be further added in buffering ingredient as stability system, the characteristics of the recipe is that it can be prepared to stable pharmaceutical solutions, and with aminoglycoside antibiotics in the same container are re-prepared to drugs used for complex anti-microbial infection.
Owner:ガンゾウ ヘメイ ファーマスーティカル カンパニー リミテッド
Antibiotic polymers
InactiveUS20050100526A1Reduce frequencyReducing and eliminating riskPharmaceutical non-active ingredientsSynthetic polymeric active ingredientsPolyesterPolyamide
Polymers (i.e. polyesters, polyamides, polythioesters, polyanhydrides, or a mixture thereof) which degrade hydrolytically to provide a combination of a beta-lactam antibiotic (e.g., amoxicillin) and a beta-lactamase inhibitor (e.g., clavulanic acid) (or a pharmaceutically acceptable salt thereof) are provided. Methods of producing these polymers, intermediates useful for preparing these polymers, and methods of using these polymers to deliver a combination of a beta-lactam antibiotic and a beta-lactamase inhibitor (or a pharmaceutically acceptable salt thereof) to a host are also provided.
Owner:RUTGERS THE STATE UNIV
Amoxicillin clavulanate potassium 4:1 dispersible tablet and production technology thereof
InactiveCN101190217AFast disintegrationImprove dispersion uniformityAntibacterial agentsPill deliveryAmoxicillin-clavulanate potassiumPotassium
The invention provides an amoxillin-potassium clavulanatein 4:1 dispersible tablet. The dispersible tablet adopts lactose, crystallite fibrin and crystal mannitol as thinner, and adopts crosslinked polyvinyl pyrrolidone (XL type) as disintegrant and also adopts micro-powder silica gel and magnesium stearate as lubricant. The dispersible tablet of the invention adopts the lactose and mannitol with better fluidity, which is good for directly pressing the powder into tablet. The invention also provides a technique of the dispersible tablet: subsidiary substances are dried for 12 to 16 hours at the temperature of 80 to 100 DEG C; the two-time tablet pressing technique is adopted and the crystallite fibrin is added both internally and externally. The environment-humidity is less than 28 percent, and the environment-temperature is below 30 DEG C in the whole preparation process. The method of the invention thoroughly dries the subsidiary substances and the process environment-humidity is controlled, so as to guarantee the stability of the product; the mixing between the subsidiary substances and the medicines, the internal and external adding method is adopted, so as to enhance the disintegrating speed of the dispersible tablet; by adopting the two-time tablet-pressing method, the dispersing equality and hardness of the dispersible tablet can be improved.
Owner:SHANGHAI NEW ASIATIC PHARMA MINHANG
Clavulanate formulation for neuroprotection and treatment of neurodegenerative disorders
InactiveUS20100255099A1Preventing cell lossAvoid cell deathBiocideOrganic active ingredientsImmediate releaseBULK ACTIVE INGREDIENT
The present invention generally relates to use of a stable solid pharmaceutical compositions that includes a clavulanate as the pharmaceutically active ingredients in an immediate-release or an extended-release solid dosage form. The composition can be used in a method of treating a neurodegenerative disease, providing neuroprotection, or preventing neuronal cell loss or death. Exemplary neurodegenerative diseases include Parkinson's disease, Alzheimer's disease and multiple sclerosis.
Owner:REXAHN PHARMA INC
Beta-cyclodextrin / amoxicillin inclusion compound and its composition with clavulanic kalium and preparation thereof
InactiveCN1698604AImprove drug stabilityPromote dissolutionAntibacterial agentsHeterocyclic compound active ingredientsCellulosePolyethylene glycol
The invention provides a beta-cyclodextrin / amoxicillin inclusion compound medicinal composition which comprises amoxicillin and beta cyclodextrin by the weight ratio of 1:2.5-1:5. In a preferred embodiment, the composition also comprises clavulanate potassium, wherein the mass ratio of amoxicillin and clavulanate potassium is 14:1-2:1, the supplementary materials are selected from crystalline cellulose, maize starch, citric acid, talcum powder, sodium carboxymethylstarch, stearic acid, calcium stearate, magnesium stearate, amylopectin, lactose, mannitol, crosslinked povidone, talcum powder, polyethylene glycol 4000, and low substituted methylcellulose propylene glycol ether. The invention also discloses the preparing process.
Owner:NANJING J ONE MEDICAL TECH DEV
Beta- lactamase suppressing antibacterial compound drugs
InactiveCN1565457AHigh tissue contentWide distribution in the bodyAntibacterial agentsOrganic active ingredientsCompounding drugsCeftizoxime
The invention discloses a beta- lactamase suppressing antibacterial compound drugs, which comprises ceftizoxime, or cefodizime and beta-lactam enzyme inhibitor by the active acid weight ratio of 1-10:10-1, which are in the forms of alkali metal salts or free acid and assisting solvents, the beta-lactam enzyme inhibitor can be Tazobactam, or clavulanic acid, or tapazole or their derivatives.
Owner:张哲峰
Novel detection method of injection use compound amoxicillin sodium and clavulanate potassium
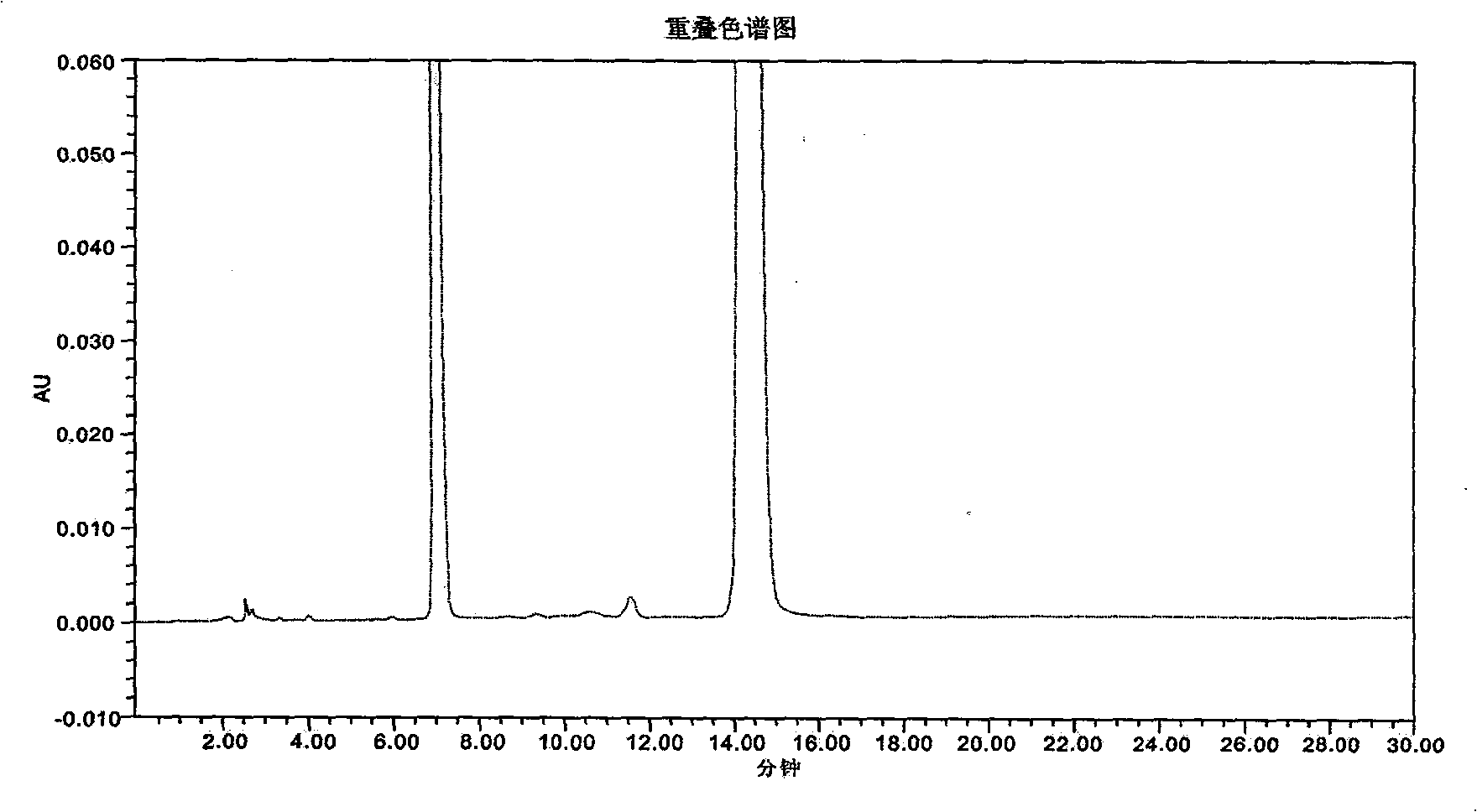
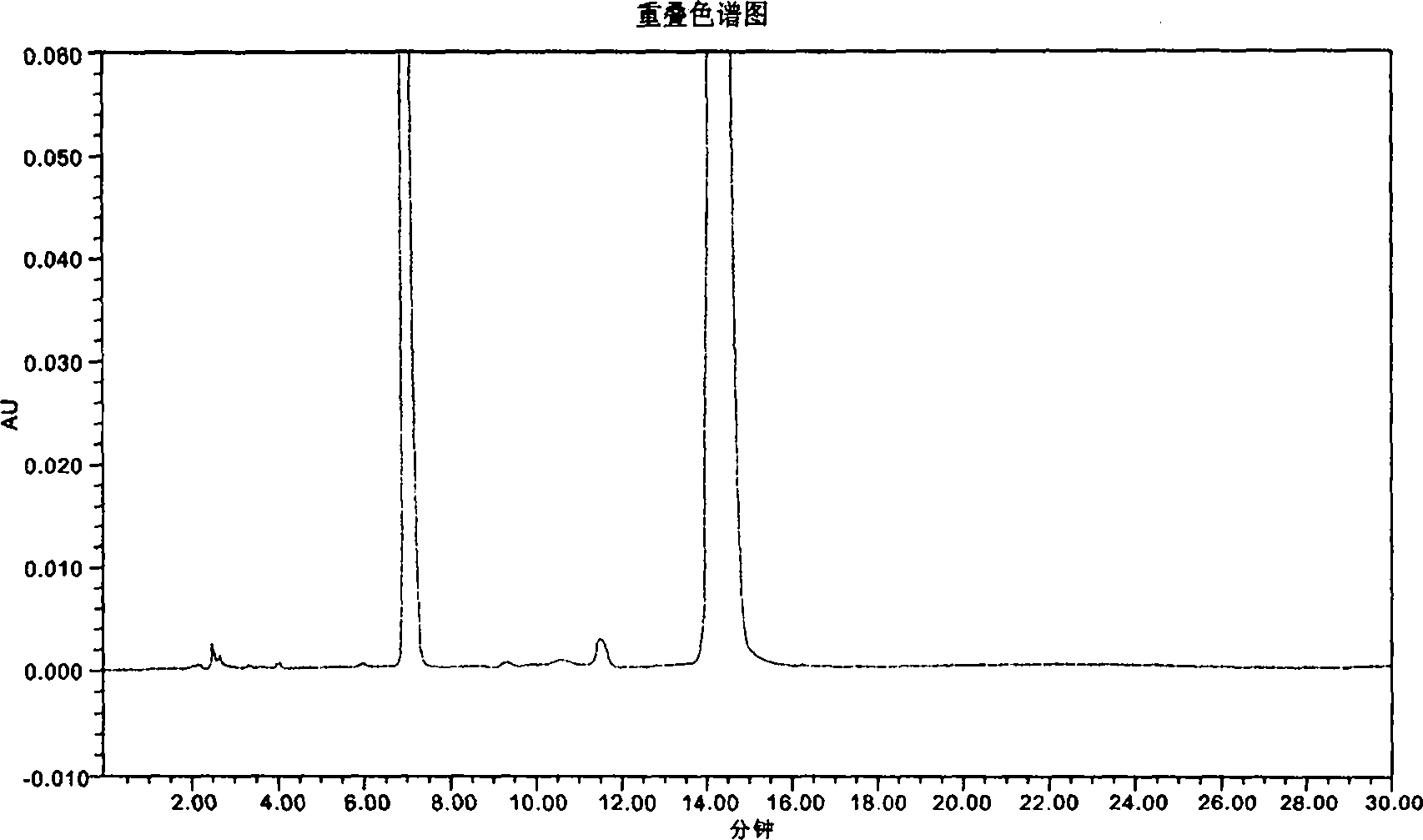
InactiveCN101776675AComponent separationAnalysis by thermal excitationAdditive ingredientAmoxicillin Sodium
The invention provides a novel high-efficiency liquid chromatography (HPLC) method capable of synchronously detecting the contents of two single ingredients and relevant impurities in injection use compound amoxicillin sodium and clavulanate potassium. Compared with the existing detection method, the invention has higher precision on the 7 / 1 compound preparation with relatively low clavulanic acid content, and has better separation degree on the injection preparation with higher purity requirement, in addition, the two ingredients of the amoxicillin and the clavulanic acid can not be mutually interfered or influenced in the detection process. The method has the advantages of simple operation, easy implementation, strong specificity, high sensitivity, large linear range, good stability and good repetitiveness, and can be used for detecting compound sterile injection preparations and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Pharmaceutical formulations comprising amoxicillin and clavulanante
InactiveCN1809348AAntibacterial agentsHeterocyclic compound active ingredientsCarboxymethylcellulose SodiumPharmaceutical formulation
Owner:GLAXO GRP LTD
Preparation method of clavulanic acid amine salt
ActiveCN105384758AReduce solubilityHigh extraction rateOrganic compound preparationOrganic chemistry methodsOrganic solventSalting out
The invention belongs to the technical field of pharmacy, and relates to a preparation method of clavulanic acid amine salt. The method comprises the following steps: (1) extraction of a clavulanic acid aqueous solution and concentration of extract liquor: acidizing the clavulanic acid aqueous solution, then adding a salting-out agent, and extracting by an organic solvent, thus obtaining the extract liquor containing clavulanic acid; nano-filtering and concentrating by using an organic solvent-resistant film, thus obtaining a clavulanic acid extraction concentrated solution; (2) preparation of the clavulanic acid amine salt: mixing the clavulanic acid extraction concentrated solution with an organic amine donor and a cosolvent, thus obtaining clavulanic acid amine salt crystals. The salting-out agent is introduced, and the extraction rate of the clavulanic acid is increased; the organic solvent-resistant roll film is innovatively adopted for nano-filtering and concentrating, so the energy consumption is lowered; the addition of the cosolvent can effectively reduce the content of various impurities in a final clavulanic acid amine salt product. Therefore, in the quality parameter aspects of the content, the impurities, light transmittance and the like, the clavulanic acid amine salt prepared by the preparation method is remarkably superior to clavulanic acid amine salt prepared by a conventional process.
Owner:SHANXI WEIQIDA PHARMA IND
Amoxicillin potassium clavulanate powder injection and preparation method thereof
InactiveCN102406614AAvoid the hidden danger of excessive moistureGood curative effectAntibacterial agentsPowder deliverySolubilityCurative effect
The invention discloses an amoxicillin potassium clavulanate powder injection which is prepared from the following raw materials in percentage by weight: 10% of amoxicillin, 2.5% of potassium clavulanate, 4% of cosolvent and the balance of soluble filler. The invention also discloses a preparation method of the amoxicillin potassium clavulanate powder injection. The test proves that the amoxicillin potassium clavulanate powder injection disclosed by the invention has the characteristics of stability in storage, convenience in transportation, good water solubility and the like and greatly improves the curative effect of the amoxicillin and potassium clavulanate, thereby being a novel broad-spectrum and efficient special powder injection for animals.
Owner:上海恒丰强生物技术有限公司
Composition containing amoxicillin and potassium clavulanate, and preparation method thereof
InactiveCN105919941ASolve the disadvantage of being easily destroyed quicklyAntibacterial agentsPowder deliveryInstabilityControllability
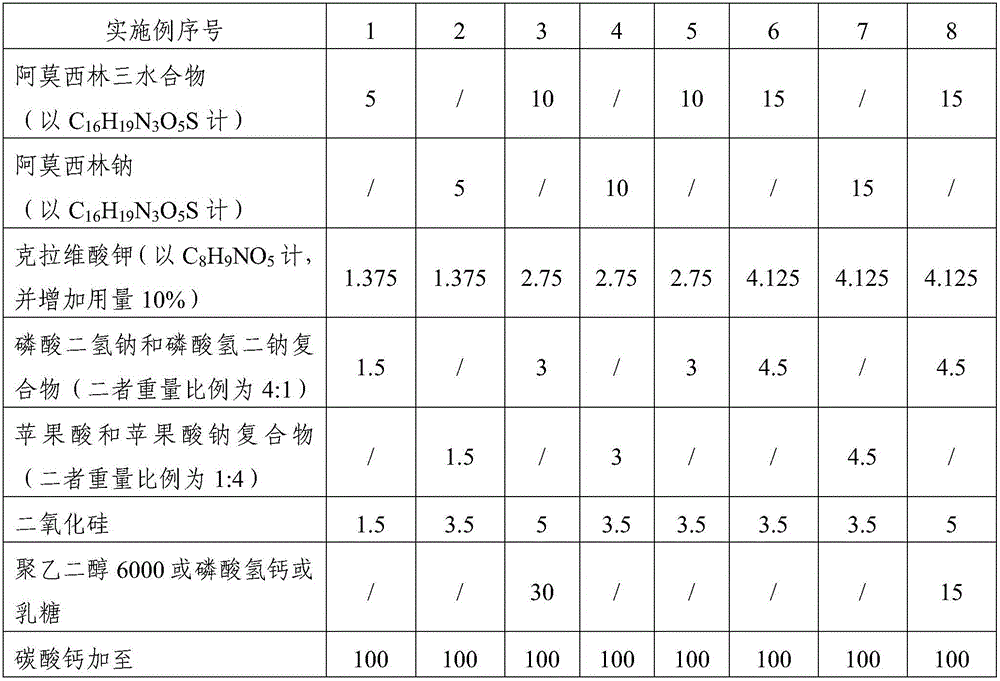
The invention relates to the field of a veterinary medicine preparation, and concretely provides a composition containing amoxicillin and potassium clavulanate, and a preparation method and a use method thereof. The composition is prepared from the following ingredients in parts by weight: 5 to 15 parts of amoxicillin, 1.375 to 4.125 parts of clavulanate, 1 to 5 parts of stabilizing agents and 1 to 5 parts of diluting agents. The problem of instability since the amoxicillin and the potassium clavulanate can easily absorb moisture in air is solved; the preparation process and the use method are simple; the addition of special equipment is not needed; all auxiliary ingredients are medical grade auxiliary materials; the cost is low; the materials can be easily obtained; no toxicity and no residue exist; and the requirements of safety, effectiveness and quality controllability on the veterinary medicine preparation can be met. The stability of the preparation is improved; the formula cost is also reduced; and the large-scale batch production can be realized.
Owner:HUNAN TAIGU BIOLOGICAL VETERINARY DRUG CO LTD
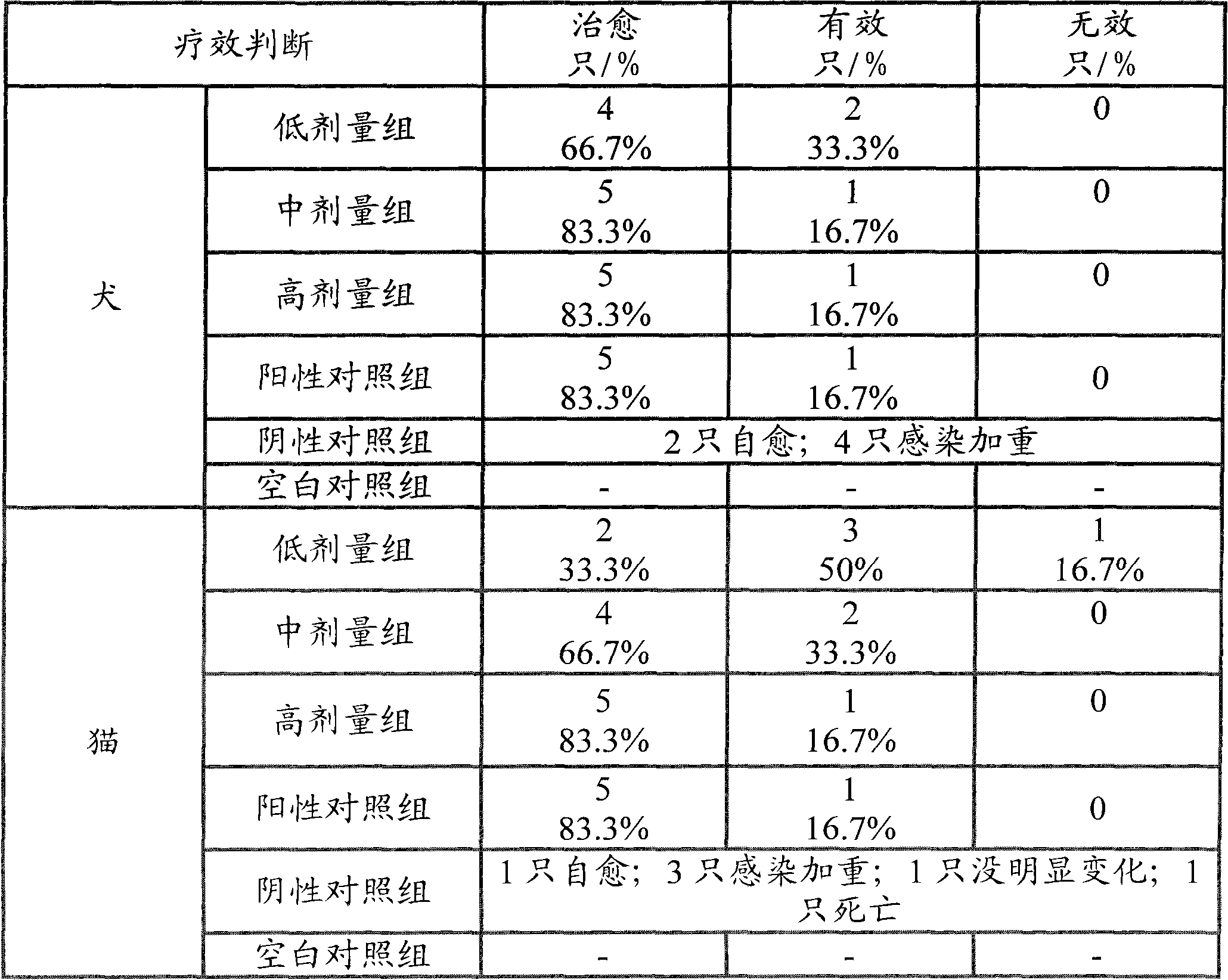
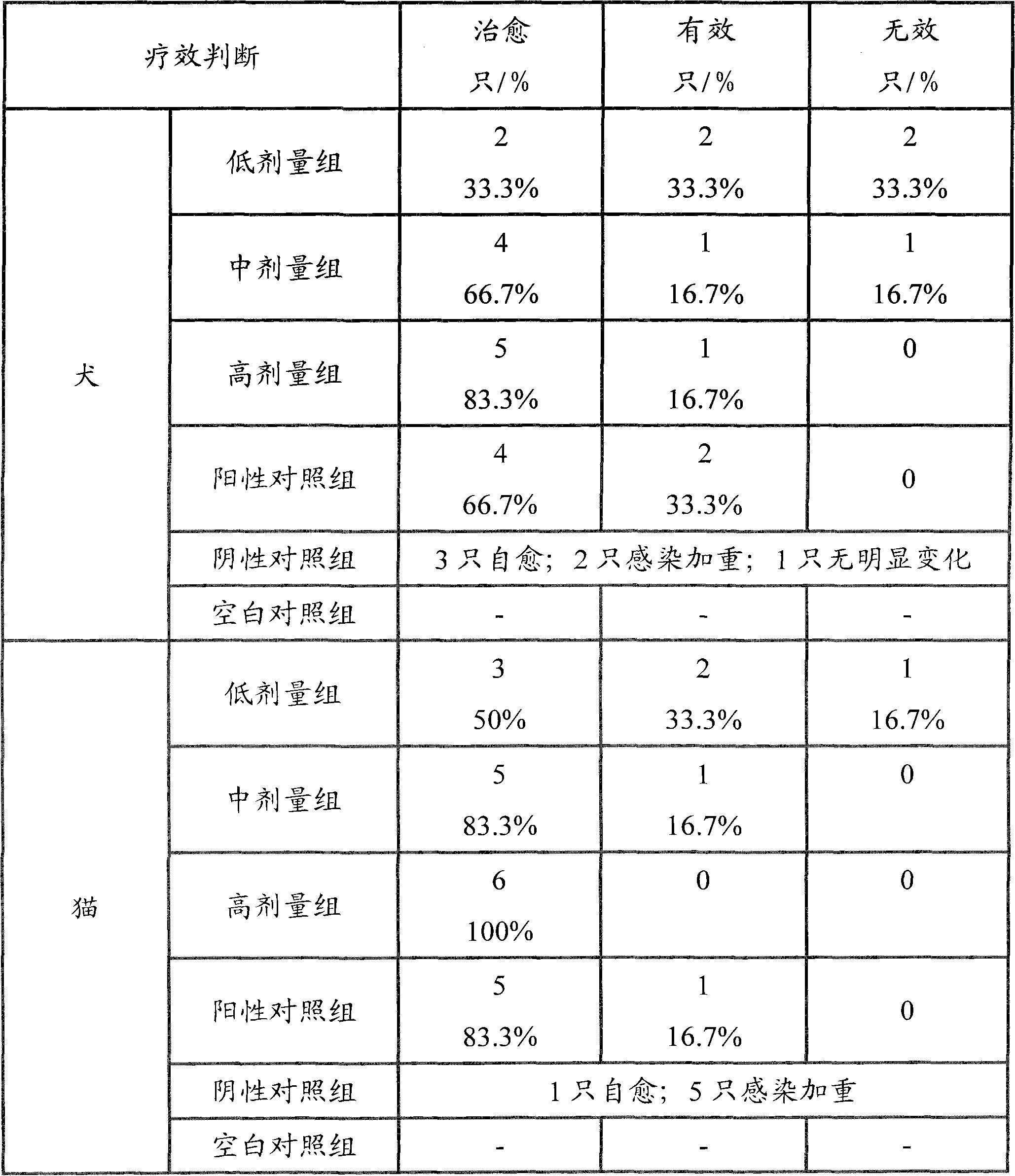
Amoxicillin and potassium clavulanate tablet for dogs and cats, and its preparation method and application
InactiveCN102240285AEasy to useGood treatment effectDigestive systemAntiinfectivesDiluentInfective disorder
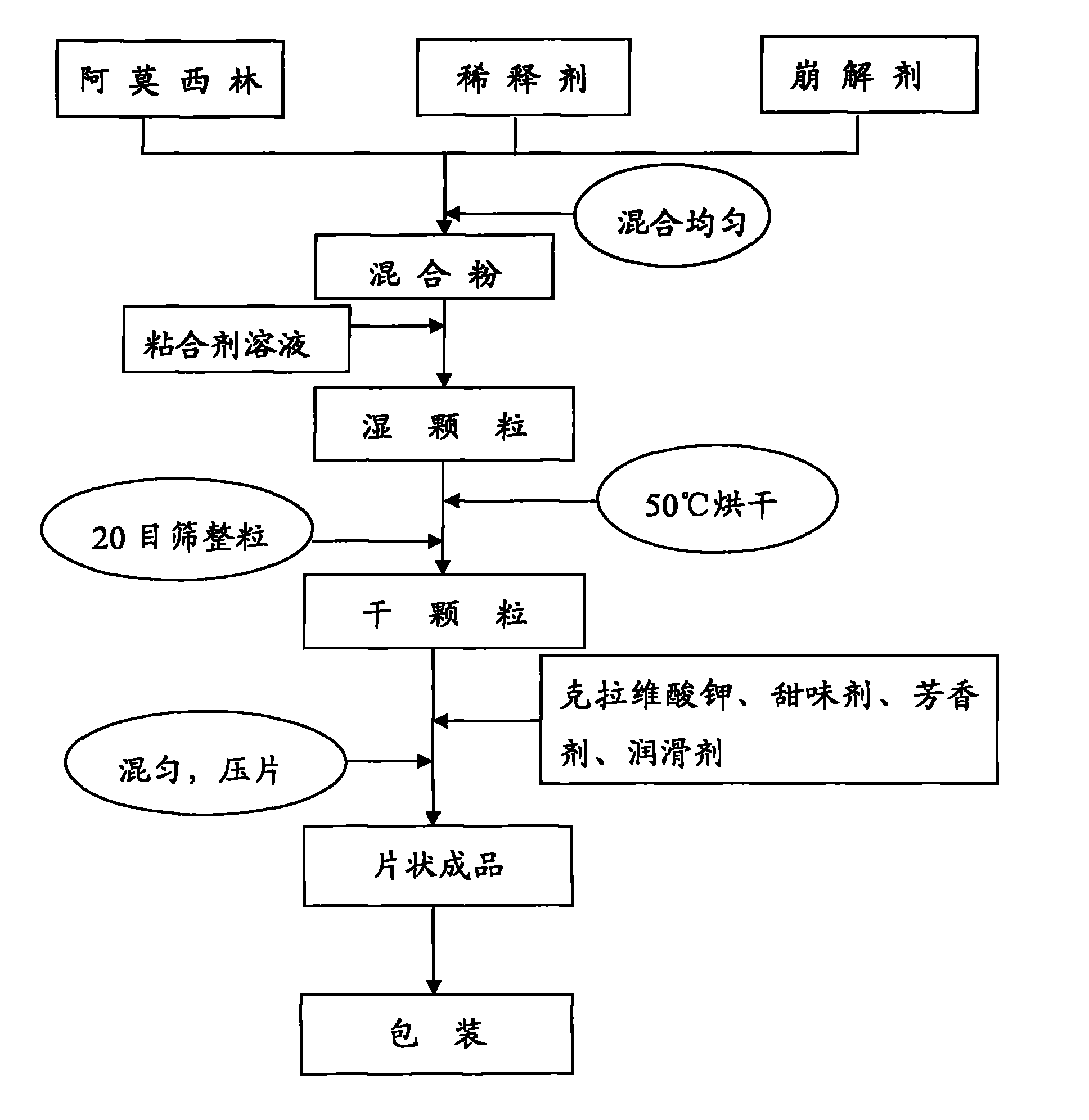
The invention relates to an amoxicillin and potassium clavulanate tablet for dogs and cats, and its preparation method and application; the tablet is mainly used for treating and preventing infectious diseases of dogs and cats. According to the invention, the problem of control scope for environmental temperature and humidity in production of the tablet is solved, accessories that guarantee the stability of the tablet are obtained through screening, and a package form that ensures stabilization of the tablet is employed. The tablet is prepared by granulating amoxicillin in advance and then mixing amoxicillin granules with potassium clavulanate for tabletting, which guarantees the quality of tabletting on one hand and reduces the possibility of decomposition of potassium clavulanate on the other hand. The amoxicillin and potassium clavulanate tablet for dogs and cats comprises, by weight, 5 to 45% of amoxicillin, 1 to 20% of potassium clavulanate, 50 to 90% of a diluent, 1 to 10% of a disintegrating agent, 1 to 5% of an adhesive, 0.1 to 3% of a sweetener, 0.2 to 2% of an aromatic and 0.5 to 3% of a lubricant, wherein the carrier for potassium clavulanate is microcrystalline cellulose. The preparation method mainly comprises the steps of pulverizing, granulating, drying, size grading, granule mixing, tabletting and packaging.
Owner:NANJING SBEED BIOTECH
Antiseptic composition of mezlocillin
InactiveCN1485035AImprove antibacterial propertiesHigh antibacterial activityAntibacterial agentsHeterocyclic compound active ingredientsSolubilitySemisynthetic penicillin
An antibiotic composition of meloxine, which is composed of meloxine andª‰-lactamase inhibitor at the weight ratio of 1-10í†10-1 based on the amount of active acids. meloxine is in the form of the alkali salt of meloxine or in the form of its free acid and solubility promoter; theª‰-lactamase inhibitor is clavulanic acid or tazobactam or their derivatives. Besides mixing and preparing beforehand,the composition could be prepared by mixing meloxine andª‰-lactamase inhibitor proportionally in clinical applications, then is administered, ormeloxine andª‰-lactamase inhibitor are administered independently. meloxine andª‰-lactamase inhibitor are synergetic, which could solve the problem of meloxine resistance in clinical applications.
Owner:周宇
Ticarcillin sodium clavulanic acid potassium freeze-dried powder as well as preparation and preparation method thereof
ActiveCN102058583AAvoid safety hazardsImprove uniformityAntibacterial agentsPowder deliveryTicarcillin sodiumFreeze-drying
The invention discloses ticarcillin sodium clavulanic acid potassium freeze-dried powder. The freeze-dried powder is prepared from the following components in percentage by weight: 75.2-96.4 percent of ticarcillin and 2.70-7.50 percent of clavulanic acid, wherein the bulk density of the freeze-dried powder is between 0.4 g / ml and 0.85 g / ml, and the repose slope is between 25 degrees and 40 degrees. The invention also provides a preparation method of the ticarcillin sodium clavulanic acid potassium freeze-dried powder, which has the advantages of no potential safety hazard, good product equality, less production link, high safety in clinical use, and the like and is particularly suitable for industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +2
Beta-cyclodextrin / amoxicillin inclusion compound and its composition with clavulanic kalium and preparation method thereof
InactiveCN100391456CImprove drug stabilityPromote dissolutionAntibacterial agentsMacromolecular non-active ingredientsCellulosePolyethylene glycol
Owner:NANJING J ONE MEDICAL TECH DEV
Preparations of effervescent formulations comprising second and third generation cephalosporin and uses thereof
The invention relates to effervescent pharmaceutical dosage forms including cefdinir as the active agent, and their preparation. The invention also relates to effervescent formulations including ceftibuten and / or its pharmaceutically acceptable salts, hydrates, solvates, esters, amorphous and crystal forms and / or a combination thereof. The invention also relates to pharmaceutical compositions including (Z)-3-Carboxymethyl-7-(2-(2-furyl)-2-methoxyiminoacetylamino)-3-sefem-4-carboxylic acid which is named cefuroxime axetil or any pharmaceutically acceptable derivative thereof, and the use of these compositions in the treatment of bacterial infections. Lastly, the invention relates to pharmaceutical formulations including a third generation cephalosporin together with clavulanic acid and / or derivatives thereof as the active agents.
Owner:MAHMUT BILGIC
Method for purifying clavulanic acid from fermentation liquor
ActiveCN102838624AMembrane pore diameter is largeStrong anti-pollutionOrganic chemistryEmulsionCentrifugation
The invention discloses a method for purifying clavulanic acid from fermentation liquor. The method comprises the steps of performing ultrafiltration treatment on the fermentation liquor and removing solids and partial coloring matters in the fermentation liquor to obtain penetrating fluid A; performing first stage nanofiltration treatment on the penetrating fluid A and removing proteins and surplus coloring matters in the penetrating fluid A to obtain penetrating fluid B; performing second stage nanofiltration treatment on the penetrating fluid B and removing partial water in the penetrating fluid B to obtain intercepted concentrated liquor C; and performing extraction separation on the concentrated liquor C to prepare purification liquid of the clavulanic acid. Solid bodies, the proteins, sugars and the coloring matters in the fermentation liquor can be effectively removed through the ultrafiltration treatment and the first stage nanofiltration treatment, so that the concentrated liquor C can be easily layered in a subsequent extraction separation process, excessive emulsion can be avoided, high speed centrifugation treatment is not needed, and investment cost of equipment can be greatly reduced.
Owner:安徽普朗膜技术有限公司
Antibiotic composition
InactiveCN1709263AHigh antibacterial activityPreservation of antimicrobial activityOrganic active ingredientsActive componentCefmenoxime
The present invention discloses an antibiotic composition. It includes the following components: cefmenoxime or cefmenoxime salt or hydrate of cefmenoxime salt or cefmenoxime ester or precursor medicine of cefmenoxime as active component, and beta-lactamase inhibitor as inhibitor; the described beta-lactamase inhibitor is clavulanic acid or sulbactam or tazobactam or clavulanic acid medicinal salt or its hydrate or precursor medicine of clavulanic acid or sulbactam medicinal salt or its hydrate or tazobactam medicinal salt or its hydrate or tazobactam ester or precursor medicine of tazobactam. The mixing ratio of both cefmenoxime and inhibitor (by weight portion) is 1:0.1-99.
Owner:黄文豪
Bactericide for gray mold of actinidia chinensis
The invention provides a bactericide for gray mold of actinidia chinensis. The bactericide is prepared from the following components by weight: 400-600g of deionized water, 4-6g of emulsifier, 15-25g of chloroisobromine cyanuric acid, 2-4g of cyrtomium rhizome extract, 2-4g of perilla extract, 2-4g of cypress bark extract, 2-4g of derris trifoliata extract, 4-6g of matrine, 4-6g of amoxicillin, 3-5g of clavulanic acid, 2-4g of tigecycline, 4-6g of alpha-cypermethrin, 3-5g of compound sodium nitrophenolate, 2-4g of benzoic acid, 2-4g of copper sulfate, 2-4g of tobramycin, 2-4g of anthocyanin from purple sweet potatoes and 2-4g of ethylparaben. The bactericide is prepared by the steps of heating the deionized water to 50-65 DEG C, putting the rest components into the deionized water, conducting continuous stirring for about 10 minutes, and conducting cooling to normal temperature to obtain the bactericide. By adopting the bactericide, gray mold of actinidia chinensis can be effectively prevented and controlled, and germs can be killed. The bactericide cannot be remained in a plant body under a normal application technical condition, and has small influence on the natural environment and other organisms.
Owner:GUANGDE YUANYE FRUIT GROWING FAMILY FARM
Drug composition containing cefazolin and beta-lactamase inhibitor
InactiveCN1424039AAddressing drug resistanceHigh activityAntibacterial agentsHeterocyclic compound active ingredientsCefazolinAntibacterial action
An antibacterial composite medicine contains ancef or its salt and beta-lactamase inhibitor (tazobactam or clavulanic acid) in Wt ratio of (1-20):(1-5). Its advnatage is high synergistic antibacterial action.
Owner:YOUCARE PHARMA GROUP +1
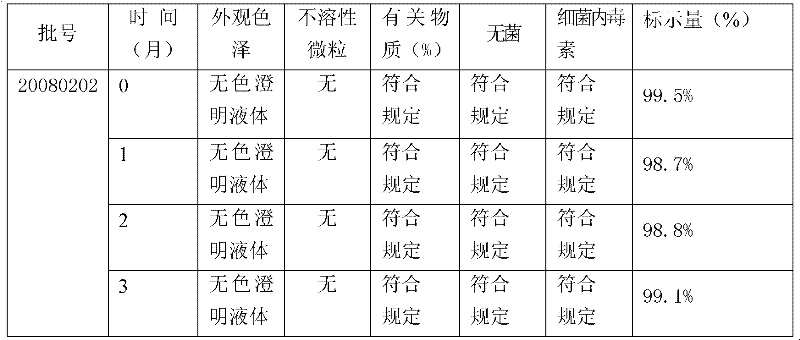
Injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium
ActiveCN103271925AEnhanced inhibitory effectGood resolubilityAntibacterial agentsOrganic active ingredientsGlycineMANNITOL/SORBITOL
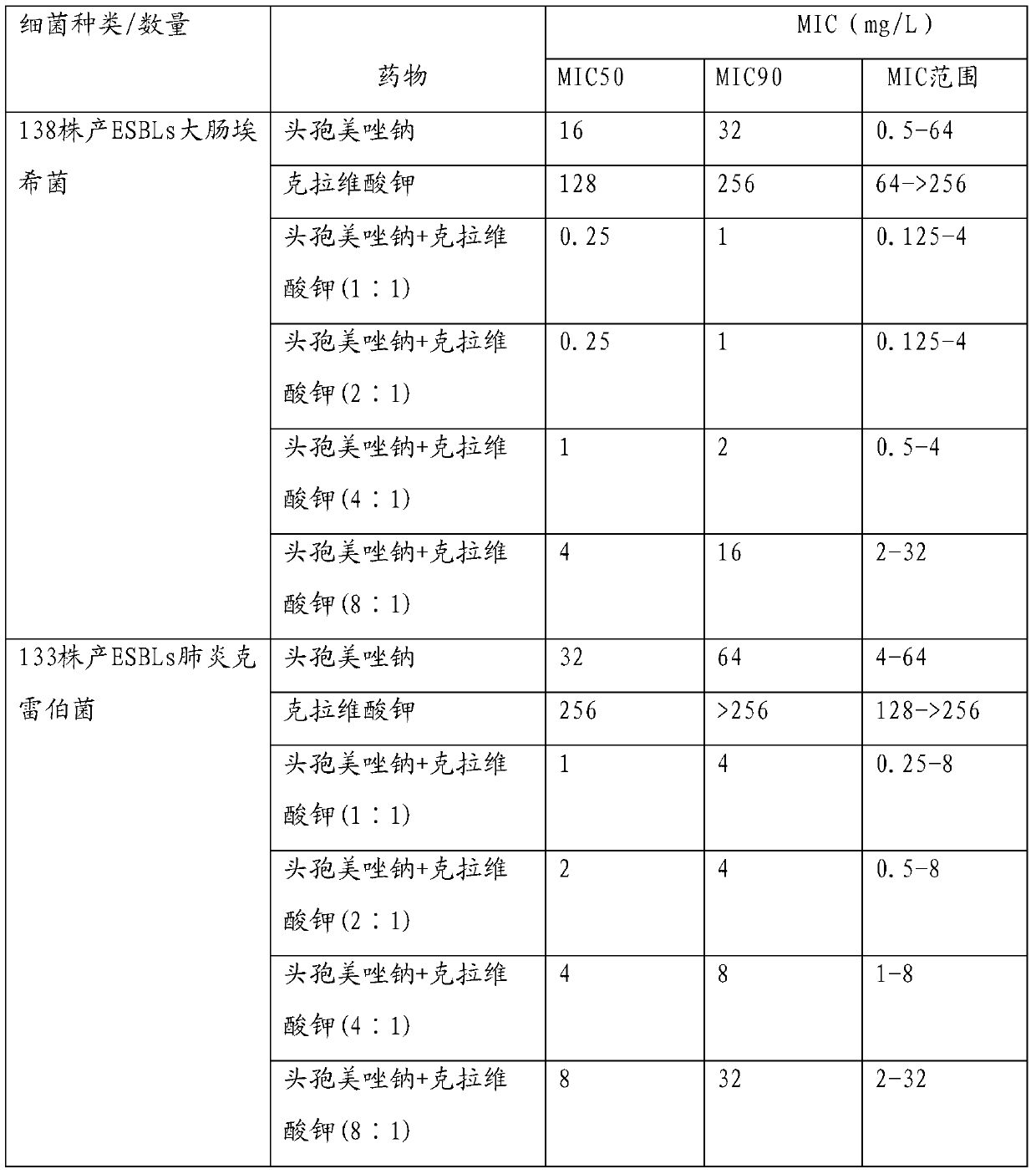
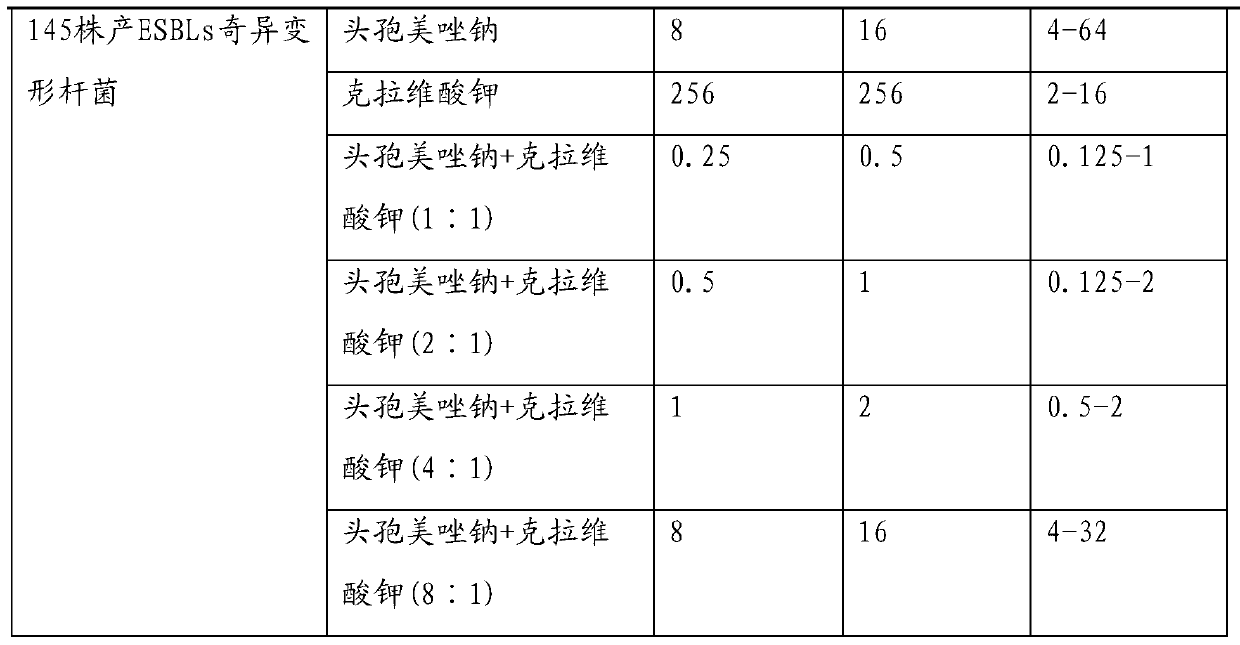
The invention provides an injection-use pharmaceutical composition comprising cefmetazole sodium and clavulanate potassium, and the pharmaceutical composition has a cooperatively antibacterial effect. The pharmaceutical composition comprises the following ingredients: in parts by weight, 1-4 parts of the cefmetazole sodium; 1-2 parts of the clavulanate potassium; 2-6 parts of polyalkylcyanoacrylate; 0.2-0.5 parts of L-arginine; 0.5-1 parts of PVP; 0.5-1 parts of mannitol; 0.5-1 parts of glycine; 1-2 parts of a tween. The pharmaceutical composition has the advantages of high stability, good redissolubility, simple preparation processes, a good inhibitory effect on ESBLs-producing escherichiacoli, klebsiellapeumoniae, and proteusmirabilis, and a definite curative effect, can reduce the dosage of a single prescription preparation and shorten the treatment cycle, and has broad market prospects.
Owner:FUAN PHARM (GRP) CO LTD +1
Antibiotic medicament containing amoxicillin nano granule and potassium clavulanate
InactiveCN101214244AHigh antibacterial activityAvoid drug resistanceAntibacterial agentsPowder deliveryButyl cyanoacrylateDistilled water
The present invention discloses an antibiotic medicine which contains amoxicillin nano-particles and clavulanate potassium and is made from 0.2 percent to 0.4 percent of amoxicillin, 0.02 percent to 0.25 percent of the clavulanate potassium, 0.5 percent to 2 percent of carrier, 0.5 percent to 2 percent of stabilizing agent, 0.5 percent to 2 percent of surfactant and 93 percent to 98.5 percent of distilled water according to the following method that: carrier monomers and the surfactant are dissolved in the distilled water completely; the amoxicillin is added; pH value is adjusted to be 2 to 3; butyl cyanoacrylate is added while being stirred by magnetic force and is stirred continuously for 5 to 12 hours, the pH value is adjusted to be 6 to 7, flaxen amoxicillin poly butyl cyanoacrylate nano-particle colloid solution is obtained; the clavulanate potassium is added by the proportion of the amoxicillin and the clavulanate potassium of 2 ®U 1 to 10 ®U 1 to obtain the antibiotic medicine which not only has simple preparation method but also has the advantages of wide spectrum, high efficiency, being targeted, slow-released, safe, etc.
Owner:NORTHWEST A & F UNIV
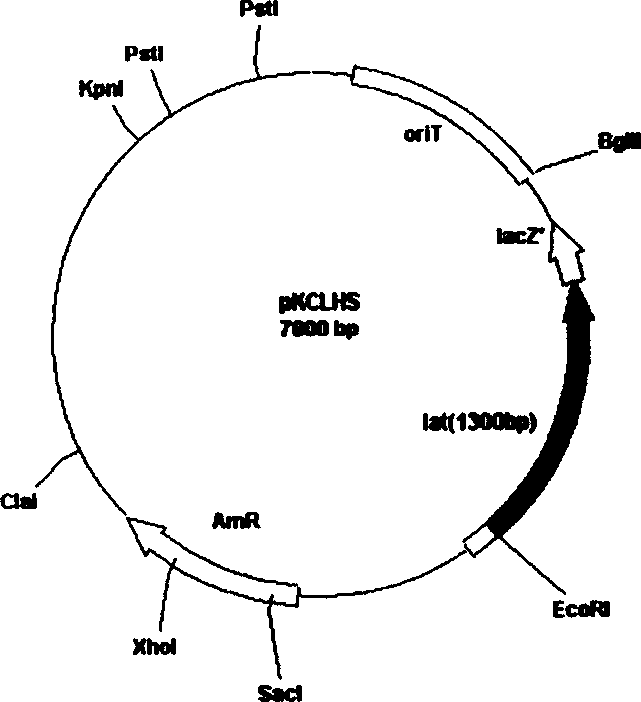
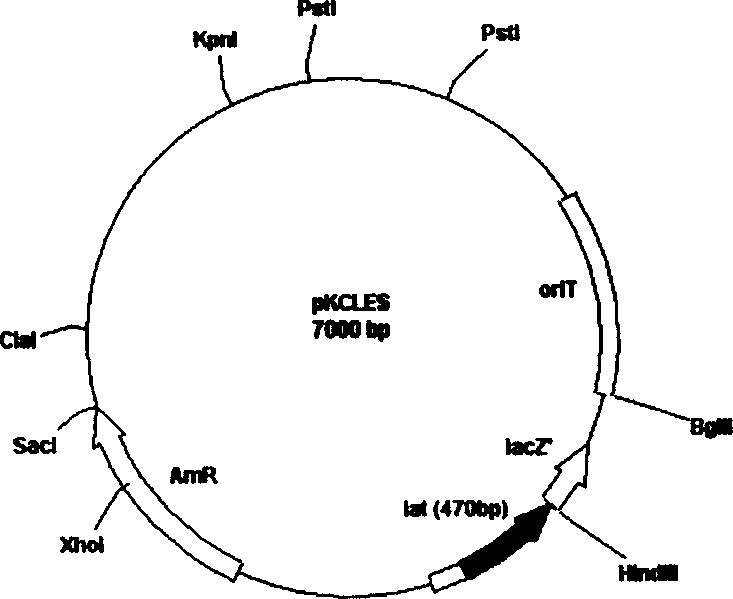
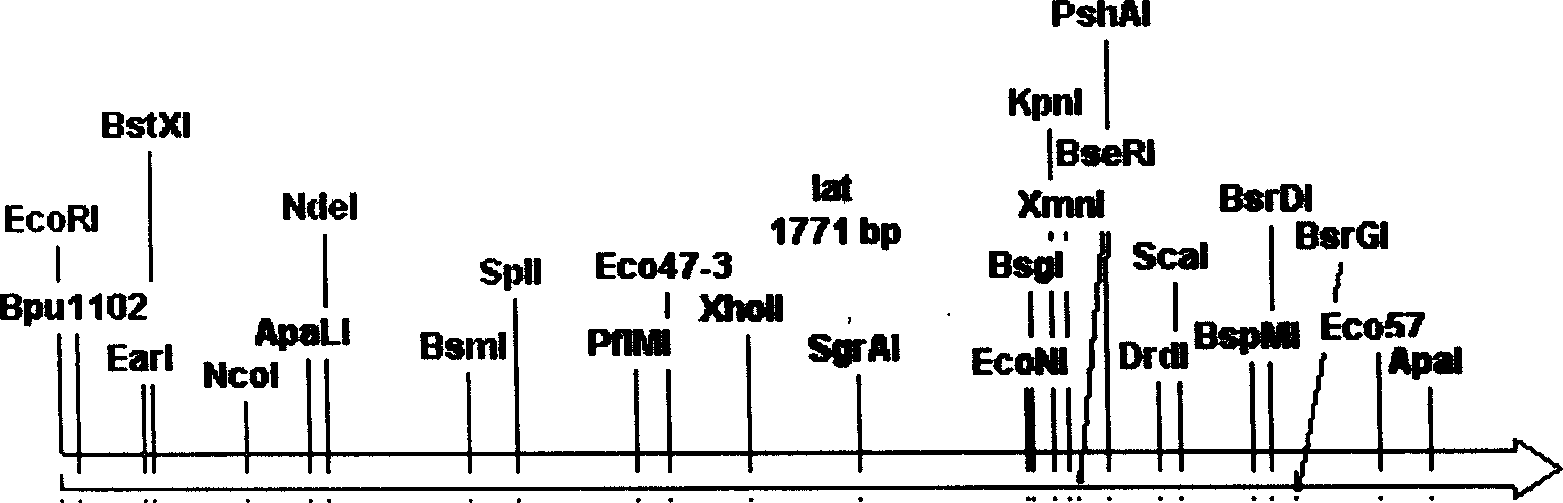
Plasmid with bar streptomycete Lat gene loss, derivative and constructing method thereof
InactiveCN1644700AIncrease productionPurposefulVector-based foreign material introductionStreptomycesReplicon
Plasmid with lat gene deletion of claviform streptomyces, its derivative and constructing method are disclosed. Upstream and downstream inducers are designed to in vitro amplify lat gene, constructing plasmid and derivative with single exchage and assembly, deletion mutating lat gene with influence on clavulanic acid yield, and constructing in colibacellus. The said plasmid contains a part of encoded lysine e-transaminase gene and duplication inducing points of plasmids of colibacillus and streptomyces, resistant selective marks of ambylan expressed in colibacillus and streptomyces, and moderate sensitive duplication sub-gene.
Owner:TIANJIN UNIV OF SCI & TECH
Amoxicillin and clavulanate potassium dispersible tablet
ActiveCN103768056ADisintegrates quicklyImprove stabilityAntibacterial agentsPharmaceutical non-active ingredientsNuclear chemistryDispersible tablet
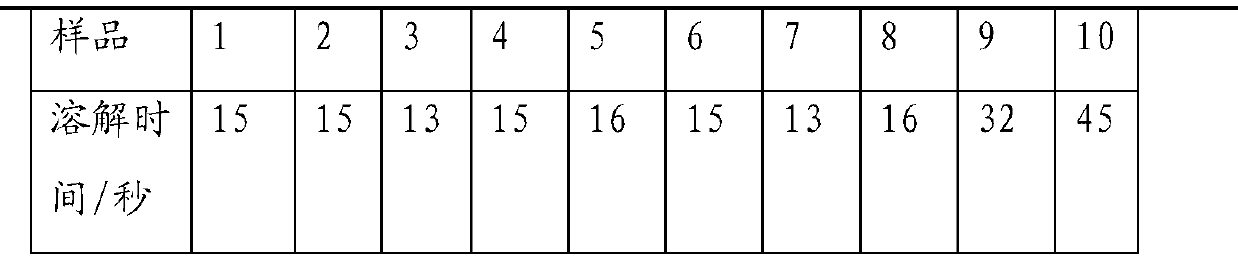
The invention discloses an amoxicillin and clavulanate potassium dispersible tablet applied to the field of a preparation of the amoxicillin and clavulanate potassium dispersible tablet. The dispersible tablet is prepared from the following components: amoxicillin trihydrate, clavulanate potassium, microcrystalline cellulose, a disintegrant, diatomite, a sweetener and an aromatic, wherein the disintegrant is selected from one of croscarmellose sodium, carboxymethyl starch sodium and polyvinylpolypyrrolidone; the disintegrant is preferably selected from croscarmellose sodium; the sweetener is selected from one of aspartame and sucralose; the aromatic is selected from one of a blueberry flavor and a lemon essence; the sweetener is preferably selected from sucralose; the aromatic is preferably selected from the blueberry flavor; the weight ratio of amoxicillin trihydrate to clavulanate potassium in the dispersible tablet is 4:1 based on amoxicillin and clavulanate; the dispersible tablet is prepared from powder by a direct compression method. The amoxicillin and clavulanate potassium dispersible tablet is rapid to disintegrate, still can be rapidly disintegrated at low water temperature, and is good in stability, good in mouthfeel, simple in prescription, good in mobility of mixed powder in the prescription, and not sticking in the tabletting process.
Owner:NORTHEAST PHARMA GRP SHENYANG SHIDE PHARMA
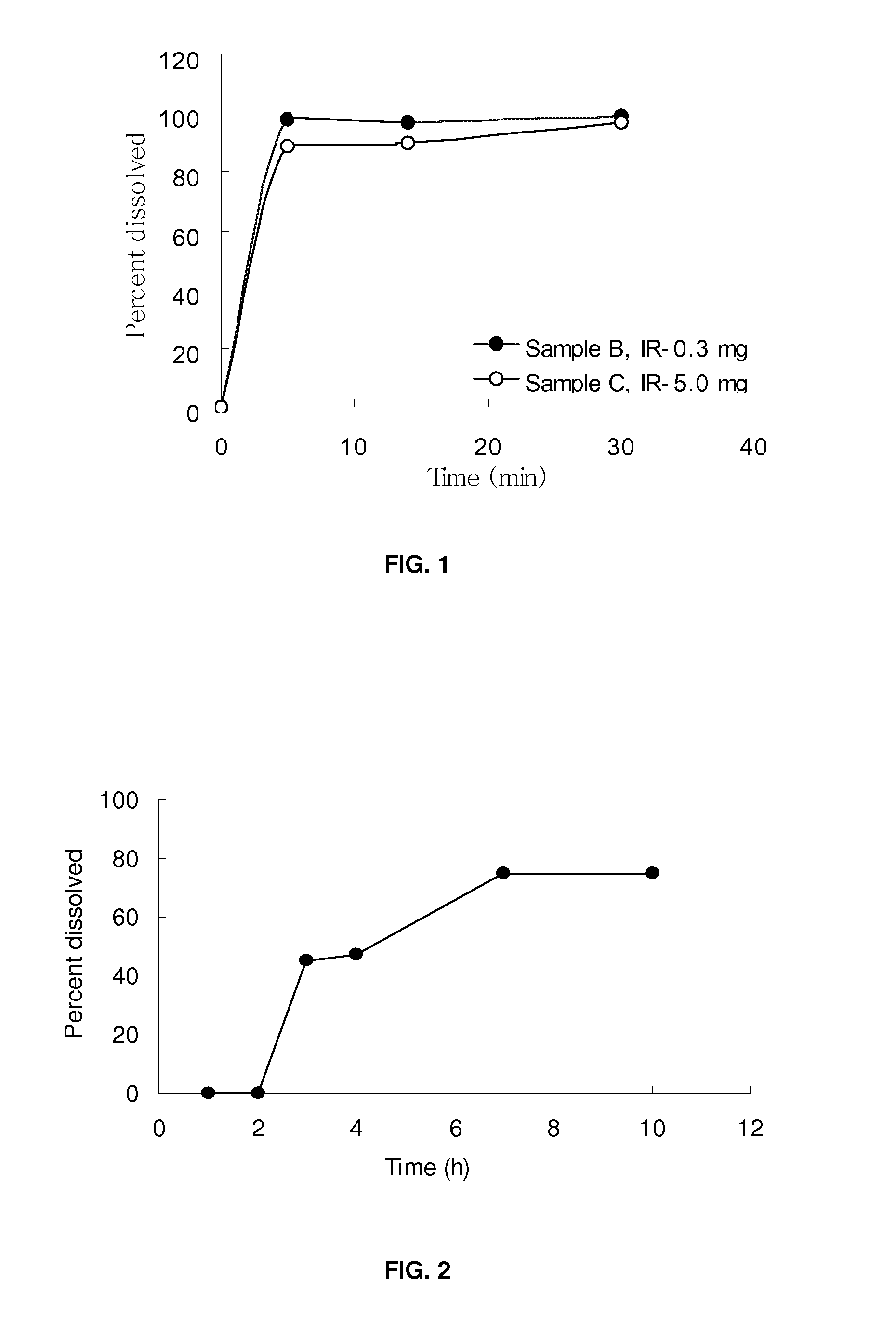
Pharmaceutical formulation of clavulanic acid
InactiveUS20090270358A1Process stabilityImprove stabilityBiocideCarbohydrate active ingredientsImmediate releaseBULK ACTIVE INGREDIENT
The present invention generally relates to stable pharmaceutical compositions, and methods of making and administering such compositions. In one aspect, the invention features stabilized pharmaceutical compositions that include pharmaceutically active ingredients such as potassium clavulanate or Clavitesse™, preferably in an immediate-release solid dosage form or an extended-release solid dosage form. Also provided are methods for making and using such immediate-release and stabilized compositions or extended-release and stabilized compositions.
Owner:REXAHN PHARMA INC
Liquid chromatography method for determining clavulanic acid related substances in amoxicillin and clavulanic acid potassium pharmaceutical composition
ActiveCN111239265AImprove accuracyEasy to controlComponent separationAmoxicillin-clavulanate potassiumFluid phase
The invention discloses a liquid chromatography method for determining clavulanic acid related substances in an amoxicillin and clavulanic acid potassium pharmaceutical composition. According to the method, octadecylsilane chemically bonded silica is used as a filler, an ammonium acetate aqueous solution is used as a mobile phase A, an ammonium acetate aqueous solution-acetonitrile is used as a mobile phase B, linear gradient elution is carried out, and the pH value of the ammonium acetate aqueous solution is 5.60-5.80. The method provided by the invention can be used for effectively detectingthe clavulanic acid impurities in the amoxicillin and clavulanic acid potassium pharmaceutical composition, is high in separation degree, simple and feasible, low in analysis cost, accurate and reliable in result, and convenient for controlling the product quality in the production and quality control process.
Owner:JIANGSU SIMCERE PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com