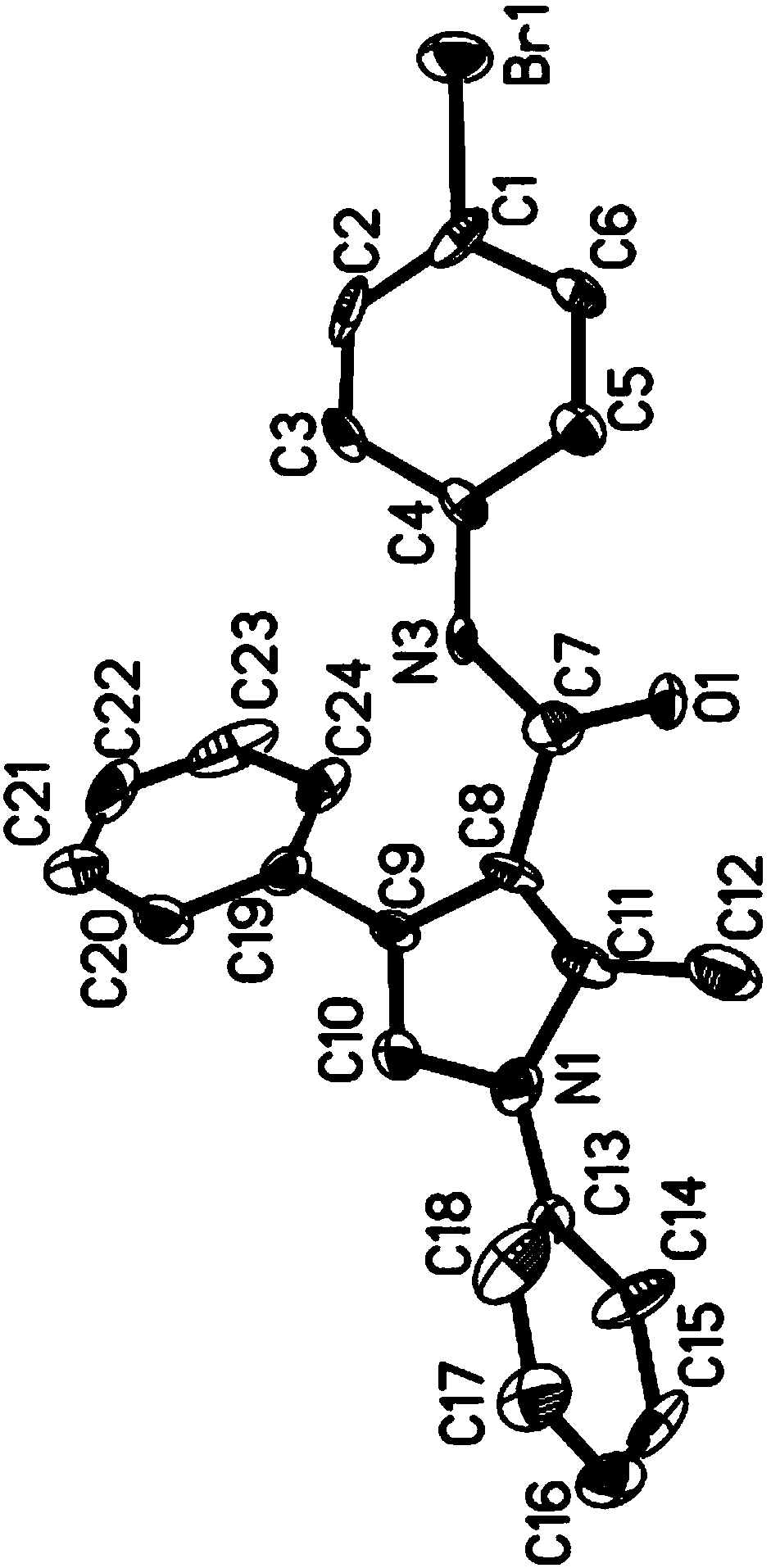
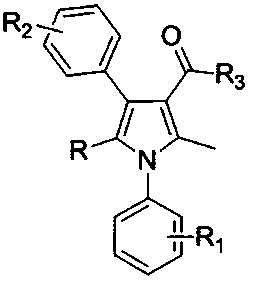
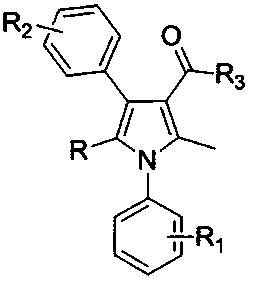
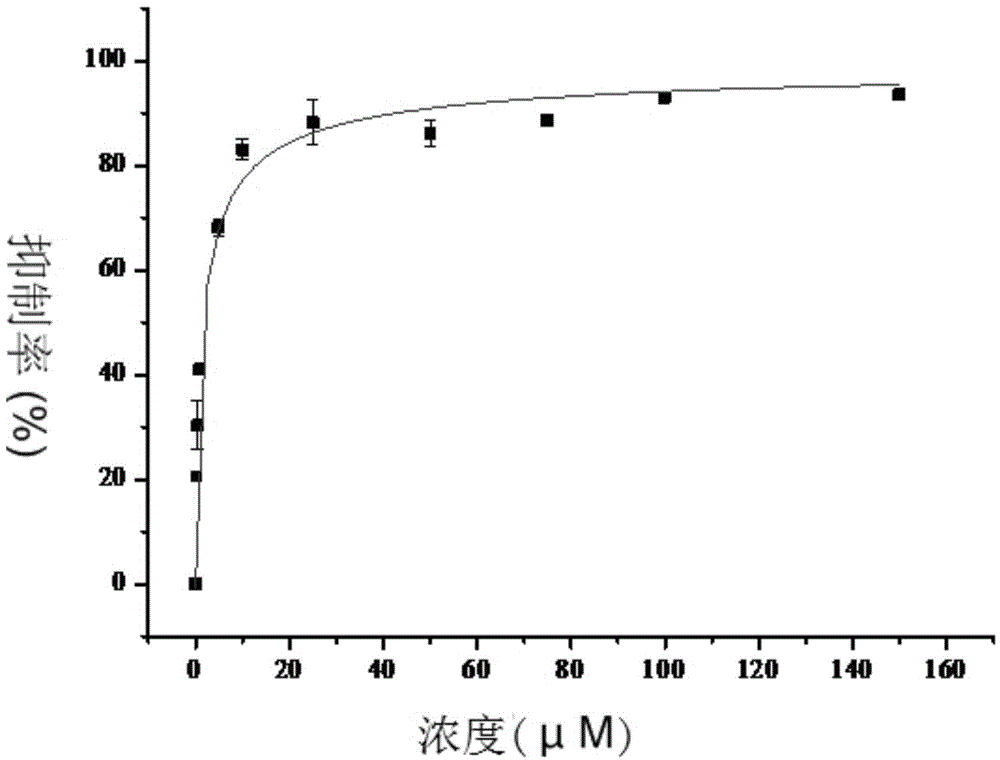
Patents
Literature
92 results about "Nitrostyrol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antimicrobial Composition and Method
InactiveUS20080249136A1Improve performanceImprove securityAntibacterial agentsBiocideSodium PyrithioneNitrostyrol
An antimicrobial composition containing a cationic polymer having limited antimicrobial activity (such as a hydrophobically-modified quaternary ammonium cellulose ether) and an antimicrobial compound (such as one or more compounds selected from the group consisting of diiodomethyl-para-tolylsulfone, ortho-phenylphenol, sodium pyrithione, zinc pyrithione, 3-iodo-2-propynylbutylcarbamate, 2-methyl-4-isothiazolin-3-one, 1,2-benzisothiazolin-3-one, 2-n-octyl-4-isothiazolin-3-one, 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride, 2-(4-thiazolyl)-benzimidazole, β-bromo-β-nitrostyrene, 2,4,4′-trichloro-2-hydroxyphenyl ether, chloroxylenol, chlorocresol, para-tert-amylphenol, N-(4-chlorophenyl)-N′-(3,4 dichlorophenyl)-urea, and para-hydroxybenzoic acid esters). The growth of microorganisms (such as Pseudomonas aeruginosa) can be inhibited by exposing the microorganism to such a composition.
Owner:DOW GLOBAL TECH LLC
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
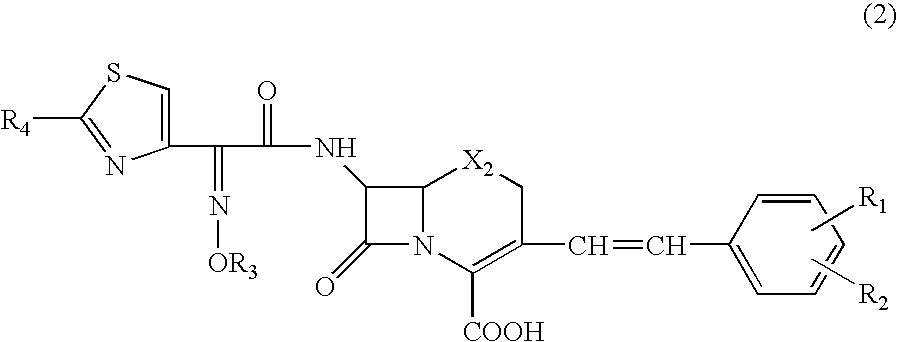
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
Method for preparing beta-nitrostyrolene and derivatives thereof
ActiveCN103497082AEasy to useEasy to operateOrganic compound preparationOrganic chemistry methodsOrganic solventNitrostyrol
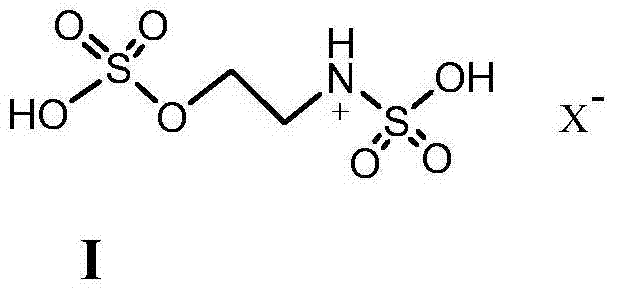
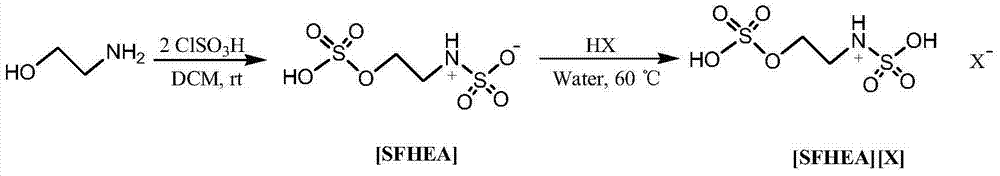
The invention discloses a method for preparing beta-nitrostyrolene and derivatives thereof. The method comprises the following steps: with novel multiple acidic ionic liquid prepared by utilizing ethanol amine as a raw material as a catalyst, carrying out a Henry condensation reaction on a nitroparaffin substance and an aromatic aldehyde substance under a normal pressure heating condition, and dewatering to obtain corresponding beta-nitrostyrolene and derivatives thereof, wherein the ionic liquid can be repeatedly used for multiple times. The method is simple to operate, high in yield, good in repeatability of a catalytic reaction system and free of any organic solvent, thereby having a good industrial prospect.
Owner:湖州达立智能设备制造有限公司
Antimicrobial composition and method
InactiveCN101291581AGrowth inhibitionAntibacterial agentsCosmetic preparationsSodium PyrithioneNitrostyrol
Owner:DOW GLOBAL TECH LLC
Method for preparing beta-nitrostyrolene compound
InactiveCN105152935AHigh yieldMild reaction conditionsOrganic chemistryOrganic compound preparationNitrostyrolMicrowave
The invention discloses a method for preparing beta-nitrostyrolene compounds. The method uses cinnamic acid compounds and cupric nitrate as raw materials, the raw materials are heated in acetonitrile and finally the beta-nitrostyrolene compounds are obtained through decarboxylation coupling. The cupric nitrate not only is used as a reactant, but also is used as transition metal catalyst. The reaction does not need the participation of ligands, acid, alkali, oxidant and the like, and does not need microwave heating, either. The preparation method has the advantages that the raw materials are low-cost and easy to obtain, the reaction system is mild, the operation is simple, the yield is high and the industrialization prospect is good.
Owner:ANHUI UNIV OF SCI & TECH
Preparation of efficient out-phase hydrogen bond donor MOF catalyst and application of catalyst
ActiveCN107540848AEasy to reachReduce self-aggregationOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNitrostyrolMetal
The invention provides a novel carbamido-containing metal-organic frame (MOF) material. The material is used as a hydrogen bond donor (HBD) catalyst, the hydrogen bond donor MOF catalyst can efficiently catalyze nitrostyrolene to react with Friedel-Crafts of indoles (F-C reaction), and very high reaction yield can be obtained at a very low catalyst loading capacity, and the catalyst can be recycled for multiple times. Moreover, compared with other catalysts, the catalyst has superhigh catalytic reaction activity.
Owner:HEFEI UNIV OF TECH
Method for preparing aminostyrene through catalytic hydrogenation of nitrostyrene
ActiveCN104974047AReduce dosageReduce pollutionOrganic compound preparationAmino compound preparationNitrostyrolHydroxylamine
The invention relates to a method for preparing aminostyrene through catalytic hydrogenation of nitrostyrene. According to the method, in environment-friendly solvents H2O, ethanol, n-heptane, H2O-ethanol or H2O-n-heptane, nitrostyrene is catalyzed by Pt / SnO2-Sb2O3 serving as a catalyst to have hydrogenation reaction at 35-120 DEG C to prepare aminostyrene. Pt / SnO2-Sb2O3 has high selectivity in generation of aminostyrene; in H2O, ethanol and n-heptane, the selectivity for aminostyrene is respectively greater than 97%, greater than 95% and greater than 96%; the reaction rate in H2O is highest and the yield of aminostyrene is greater than 97%; no accumulation of harmful intermediates such as phenyl hydroxylamine is generated in the reaction process; since Pt / SnO2-Sb2O3 almost has no catalytic activity for hydrogenation of aminostyrene, the yield of aminostyrene does not decrease when time goes by after the reactants are completely converted.
Owner:CHANGCHUN UNIV OF TECH
Fullerene pyrrolidine derivative and preparation method thereof
ActiveCN105669529AHigh synthetic yieldLow yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisNitrostyrol
The invention belongs to the technical field of chemical synthesis, particularly relates to a N-methyl-2-(4-nitrobenzene vinyl)-3,4-fullerene pyrrolidine derivative, and further discloses a preparation method of the fullerene pyrrolidine derivative. The method for preparing N-methyl-2-(4-nitrobenzene vinyl)-3,4-fullerene pyrrolidine comprises the steps that the existing 1,3-dipolar-cycloaddition reaction is utilized, methylbenzene is used as a solvent for a reaction, and the target product N-methyl-2-(4-nitrobenzene vinyl)-3,4-fullerene pyrrolidine is obtained. It is surprised to find that after the whole reaction system is cooled to indoor temperature, an organic solvent comprising trichloromethane, carbon disulfide and orthodichlorobenzene is added, the reaction continues to be carried out to the end, the synthetic yield of the product is far higher than that of the product obtained through the solvent only comprising methylbenzene, and the unexpected technical effect is obtained.
Owner:HUANGSHAN UNIV
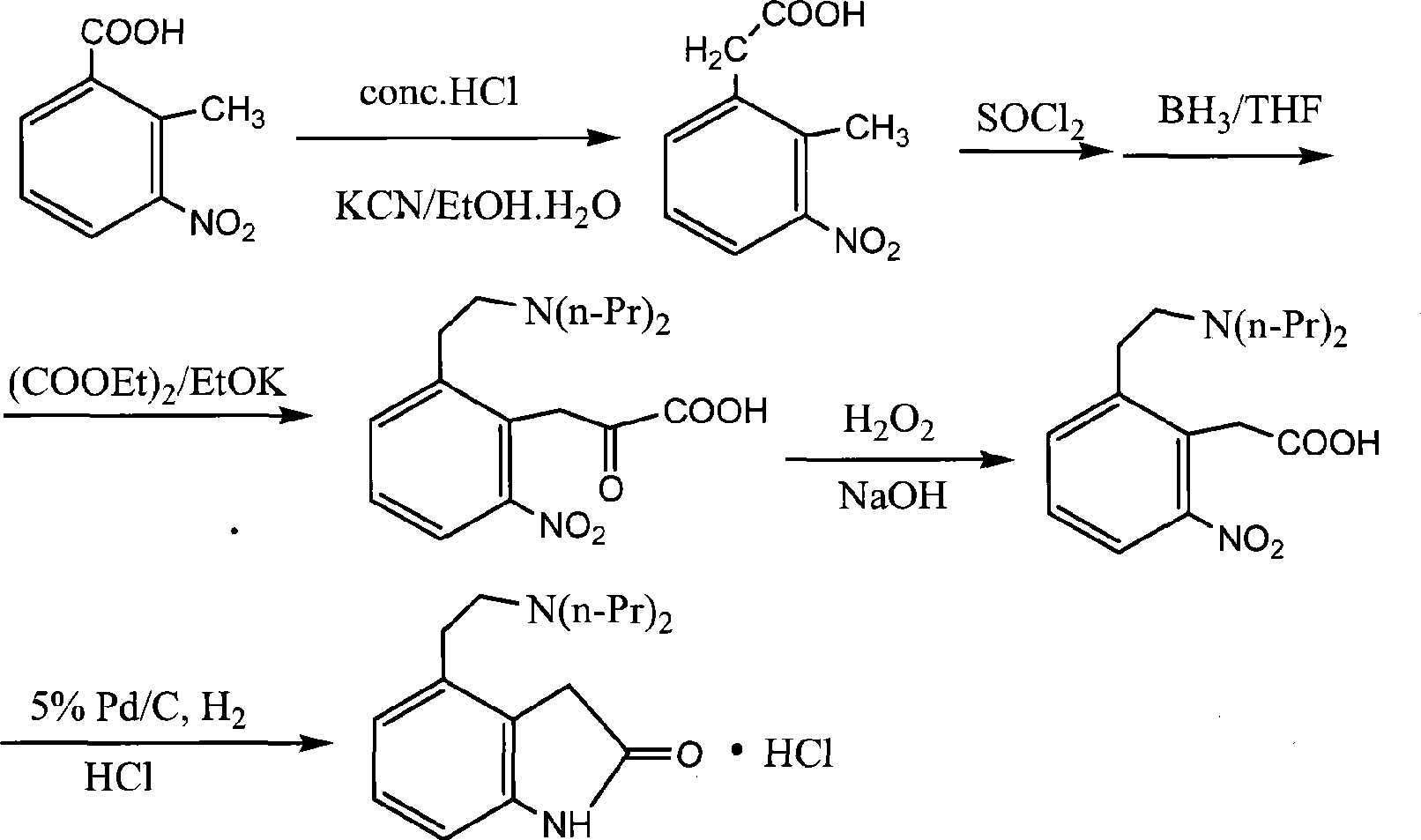
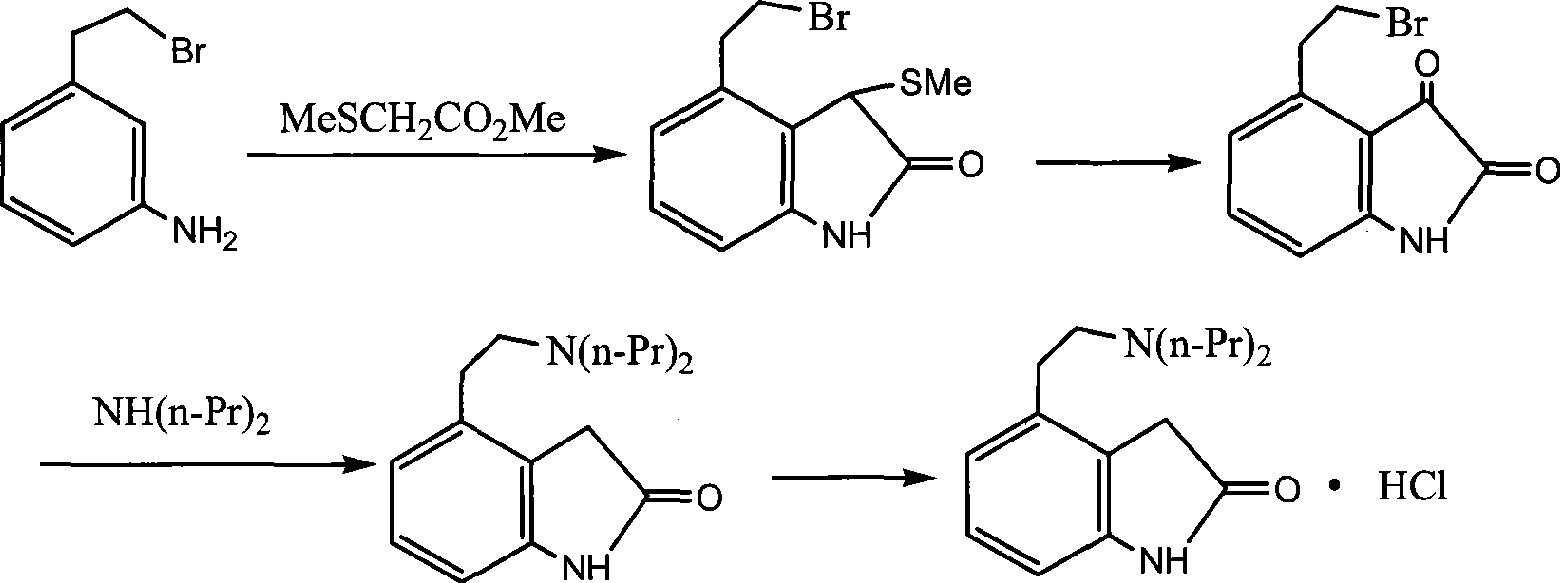
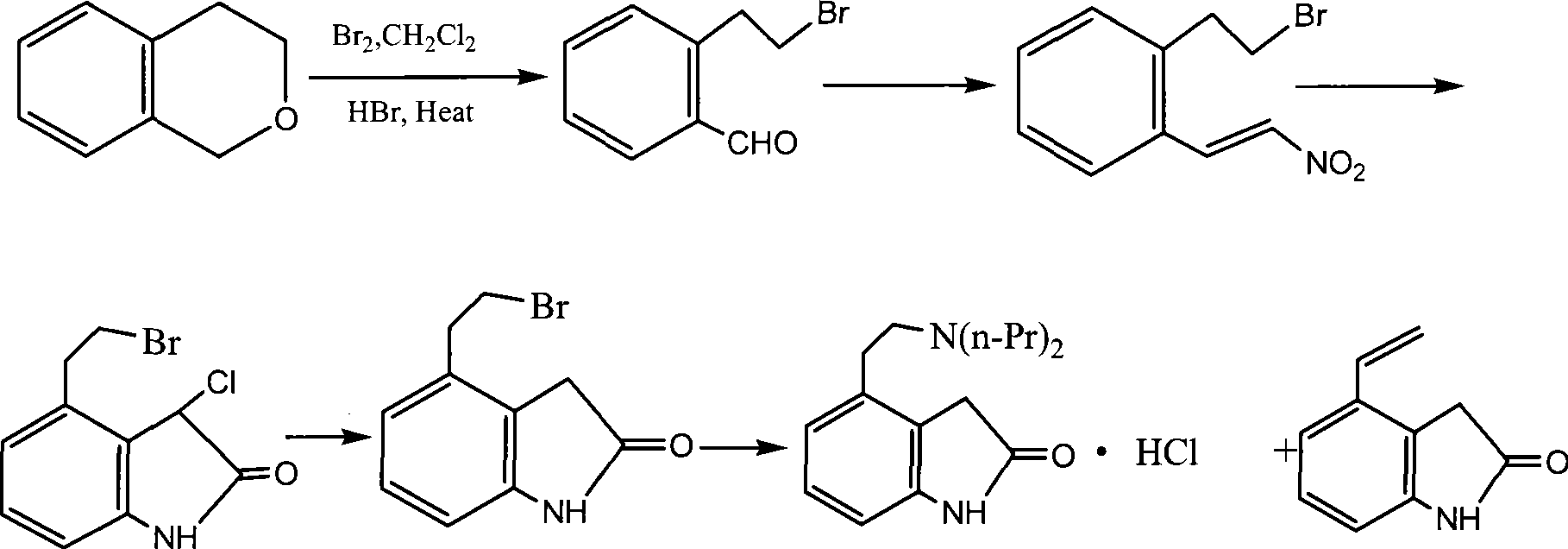
Preparation of ropinirole hydrochloride
InactiveCN101481347ARaw materials are easy to getLow costNervous disorderOrganic chemistryBenzaldehydeKetone
The invention relates to a method for preparing ropinirole hydrochloride, comprising the followings in turn: (1) beta-phenylethyl alcohol reacts with paraformaldehyde to produce isochroman; (2) the product obtained in step (1) reacts with bromide to produce 2-bromoethyl benzaldehyde; (3) the product obtained in step (2) reacts with nitromethane to produce 2-bromoethyl nitrostyrolene; (4) the product obtained in step (3) reacts with acetyl chloride to produce 4-bromoethyl-3-chloride-1, 3-dihydro-2H-indole-2-ketone; (5) 4-bromoethyl-1, 3- dihydro-2H-indole-2-ketone is obtained after catalytic hydrogenation of the product obtained in step (4); (6) 4-ethoxyl-1, 3-dihydro-2H-indole-2-ketone is obtained after acetylation and hydrolysis of the product obtained in step (5); (7) the product obtained in step (6) reacts with toluene sulfonic acid to produce toluene sulfonic acid-2-(2-oxygen-1, 3-dihydro-4-indole) ethyl ester; (8) the product obtained in step (7) and di-n-propylamine undergo reflux reaction in water and pH value is regulated by hydrochloric acid to 1-2 to obtain the ropinirole hydrochloride. In the method, the raw materials are easily acquired; the target products enjoy high selectivity and yield, thus being suitable for industrial production.
Owner:太仓浦源医药原料有限公司
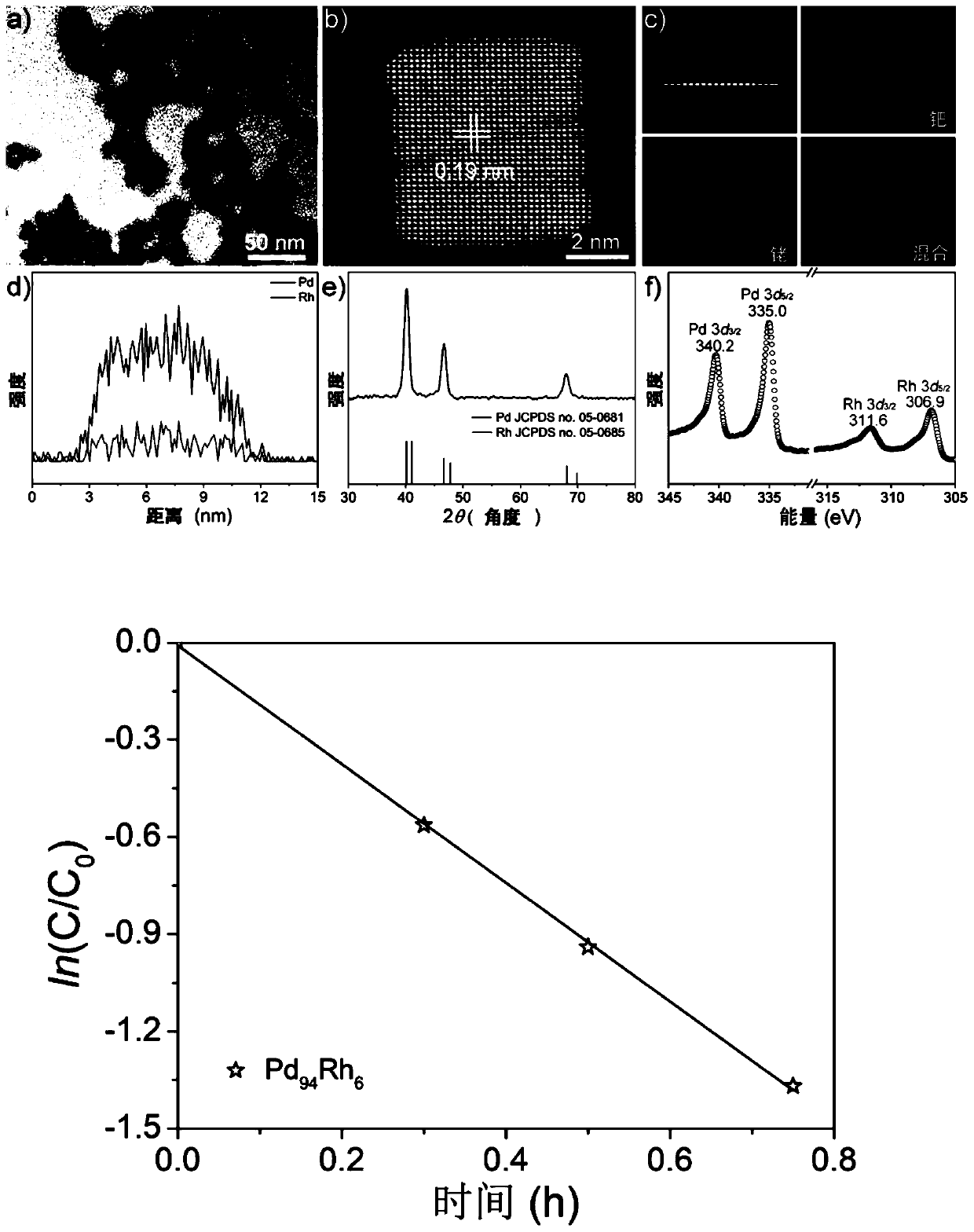
Pd-Rh nanocatalyst, and preparation method and application thereof
ActiveCN110201665AHigh catalytic activityImprove catalytic performanceOrganic chemistryOrganic compound preparationNano catalystNitrostyrol
The invention provides a preparation method of a Pd-Rh nanocatalyst. The preparation method comprises the following steps: a palladium source solution, a rhodium source solution, an aqueous solution of potassium iodide and polyvinylpyrrolidone are mixed and dissolved in a solvent to obtain a mixture, and the mixture is heated, cooled, centrifuged and washed to obtain the Pd-Rh nanocatalyst, wherein the palladium source is disodium tetrachloropalladate, and the rhodium source is sodium hexachlororhodate (III). An element doping process is adopted to dope a palladium nanocrystal with a rhodium atom in order to construct the nanocatalyst, and a unique 3-nitrostyrene adsorption structure different from the Pd nanocrystal is obtained through the doping of the nanocatalyst with the rhodium atom,so hydrogenation of a nitro group is difficult, thereby the selectivity to carbon-carbon double bonds is improved. The Pd-Rh nanocatalyst of the invention has the advantages of good catalytic activity, high selectivity of double bond hydrogenation of 3-nitrostyrene, and stable catalytic performance.
Owner:UNIV OF SCI & TECH OF CHINA
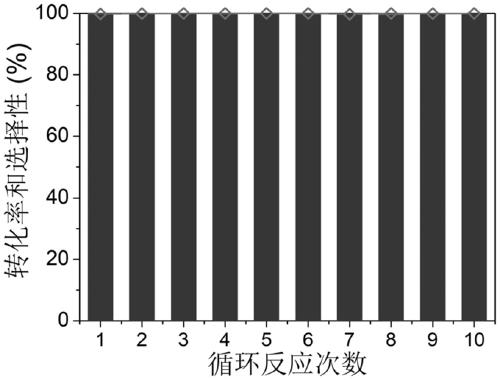
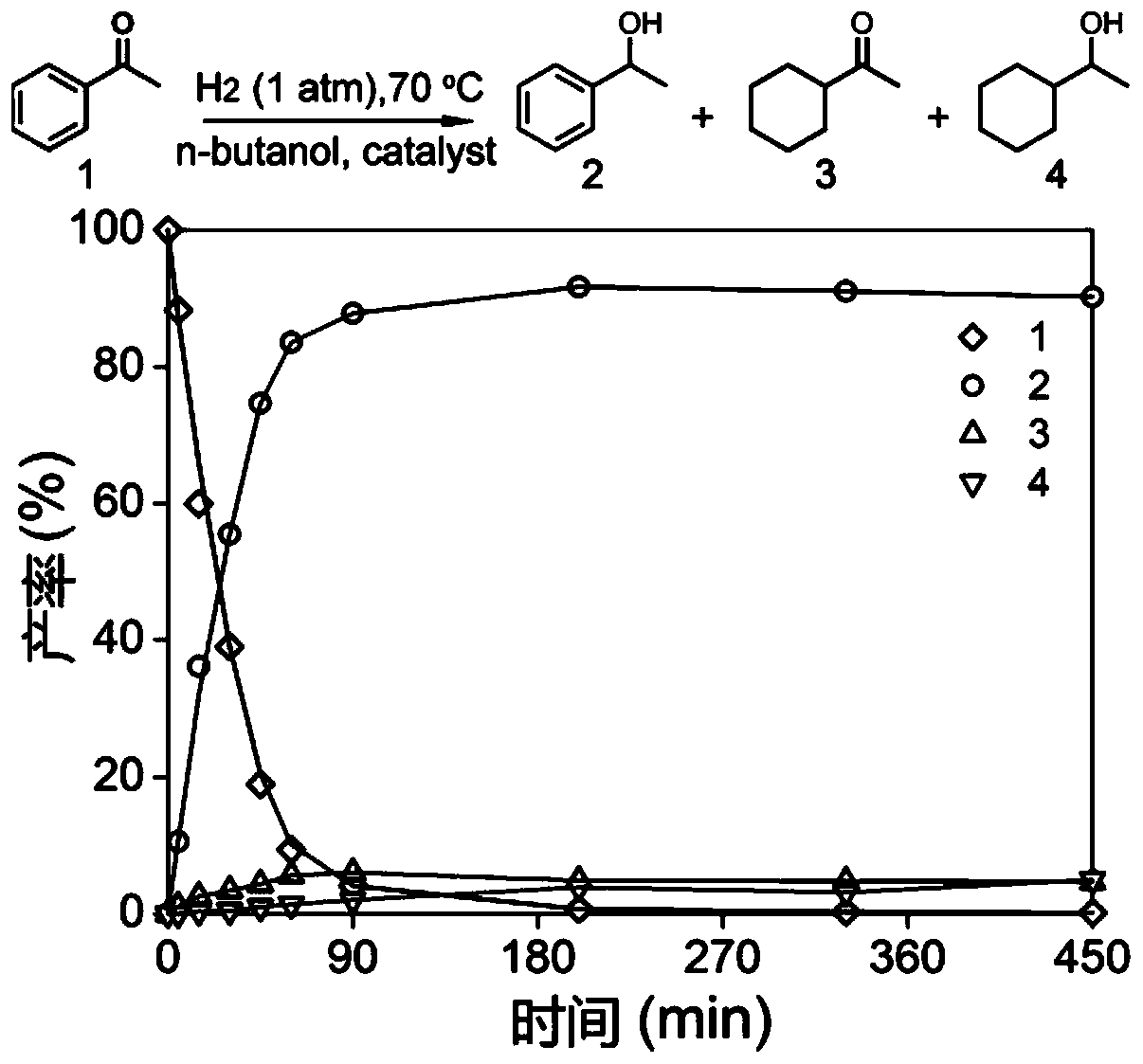
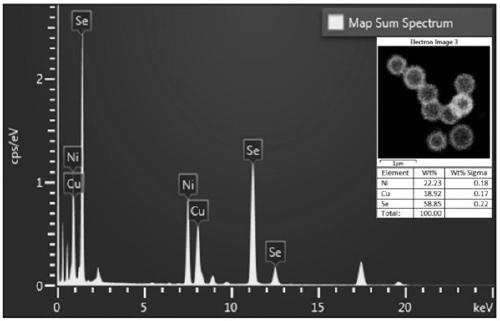
Sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material and preparation method and application thereof
ActiveCN109621988AGood catalytic hydrogenation performancePhysical/chemical process catalystsOrganic compound preparationNitrostyrolP-Nitroaniline
The invention provides a sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material and a preparation method and application thereof. Copper nitrate trihydrateand nickel nitrate hexahydrate are dissolved into a mixing solvent of ethylene glycol and water, urea and polyvinylpyrrolidone are added, a hydrothermal reaction is performed, and the hollow sea urchin shaped precursor structure is formed; the precursor is dispersed into deionized water, a sodium hydrogen selenide solution is added into the precursor dispersing solution drowse, the precursor is adopted as a template, the hydrothermal reaction is performed, and the sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material is obtained. Compared with theprior art, the sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material is successfully prepared for the first time through the method which is mild in reaction condition and simple and easy to popularize, the prepared sea urchin shaped hollow structure nickel, copper and selenium ternary nanometer catalytic material can catalyze a hydrogenation reactionof p-nitrophenol, p-nitro-styrene and paranitroaniline, and the material has the good catalytic hydrogenation performance.
Owner:ANHUI NORMAL UNIV
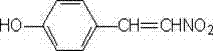
Synthesis method of p-hydroxy-beta-nitrostyrene
InactiveCN103497108AIncrease profitEliminate oxygenOrganic compound preparationNitro compound preparationNitrostyrolAntioxidant
The invention discloses a synthesis method of p-hydroxy-beta-nitrostyrene, which comprises the following step: synthesizing p-hydroxybenzaldehyde and nitromethane used as raw materials into p-hydroxy-beta-nitrostyrene by using ammonium acetate as a catalyst and glacial acetic acid as a solvent, wherein an antioxidant anhydrous hydroquinone is added to protect the raw material p-hydroxybenzaldehyde from oxidation, and toluene is added in the later reaction period to take the generated water out of the reaction system, so that the reaction proceeds in the positive reaction direction. The method has the advantages of low reaction cost, high product yield, simple after-treatment and the like, and the yield of the hydroxy-beta-nitrostyrene can reach higher than 92%.
Owner:ZHONGBEI UNIV
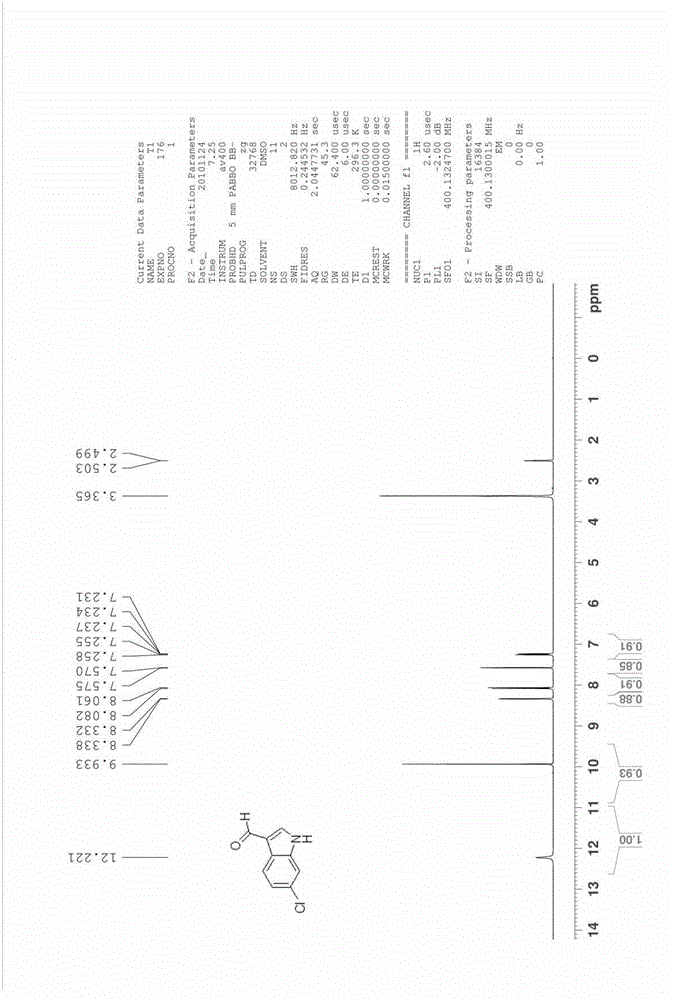
Method for preparing substituted indole-3-methanal compound
The invention relates to the field of organic synthesis and medical intermediate preparation and especially relates to a method for preparing a substituted indole-3-methanal compound. The method comprises the following steps that N,N-dimethylformamidodimethylacetal or N,N-methylformamidodiethylacetal and substituted 2-nitrotoluene undergo a reflux reaction to produce beta-dimethylamino-2-nitrostyrene; beta-dimethylamino-2-nitrostyrene and a mixed solution of hydrazine and alcohol undergo a reaction to produce a substituted indole; and the substituted indole, dimethylformamide (DMF) and phosphorus oxyhalogen undergo a reaction to produce the substituted indole-3-methanal compound. Yields of all processes of the method provided by the invention are more than 90%. A total yield of the method is more than 80%.
Owner:上海泰坦科技股份有限公司
Synthetic method for 4-amino-3-phenylbutyric acid hydrochloride
ActiveCN104402746ASimple processHigh yieldOrganic compound preparationAmino-carboxyl compound preparationNitrostyrolEthyl acetate
A provided synthetic method for 4-amino-3-phenylbutyric acid hydrochloride comprises the steps: preparing ethyl benzoylacetate, preparing nitroethenylbenzene, preparing an addition product, and processing to obtain the product. The synthetic method is an integral technology for synthesizing 4-amino-3-phenylbutyric acid hydrochloride. The method is simple in technology, high in yield and relatively low in cost, and mainly solves the problems that an initial raw material used in the prior art is high in price, operation steps are tedious and a production process is large in pollution.
Owner:SHAANXI JIAHE PHYTOCHEM
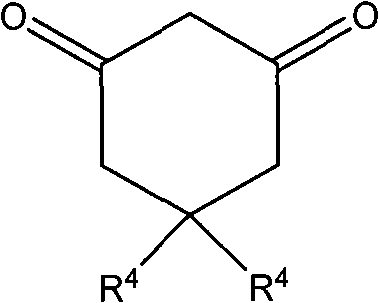
Synthesis method of 2-nitro-3-aryl-2,3,5,7-tetrahydrobenzofuran-4-one derivative
The invention discloses a synthesis method of 2-nitro-3-aryl-2,3,5,7-tetrahydrobenzofuran-4-one derivatives, comprising the following steps: adopting one of beta-nitrostyrolene, beta-nitrofurylethylene, beta-nitrothienylethylene, beta-nitropyrrylethylene or beta-nitropyridylethylene or one of derivative thereof and 1,3-macrocyclicdiketone as reactants to react at 30-70 DEG C and preparing the 2-nitro-3-aryl-2,3,5,7-tetrahydrobenzofuran-4-one derivative. In the method of the invention, the raw materials are cheap, accessible and various, the synthesized products have various types and the products can be used as potential molecules with biological activity and important key intermediates; the invention adopts ethanol as reaction medium so as to reduce the pollution, shorten the reaction time, simplify the reaction process and the post-treatment process and reduce the production cost. The method of the invention has the advantages that the regioselectivity and the stereoselectivity are good, the yield is high, the addition reaction and the cyclization reaction can be realized by one-pot method and the process is simple.
Owner:SUZHOU UNIV +1
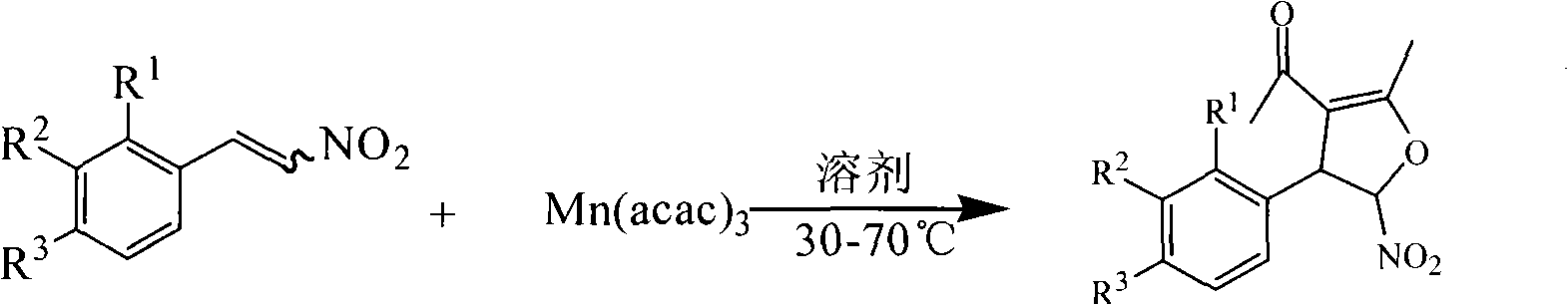
Method for synthesis of 5-nitryl-4, 5-dihydrofuran derivant
The invention discloses a synthetic method of 5-nitryl-4, 5-dihydrofuran derivates, comprising: taking one of Beta-nitrostyrene with a substituent on a benzene ring, Beta-nitrofuran ethylene with a substituent on a furan ring, Beta-nitrothiophene ethylene with a substituent on a thiophene ring, Beta-nitropyrrole ethylene with a substituent on a pyrrol ring and Beta-nitropyridine ethylene with a substituent on a pyridine ring as well as manganic acetyl acetonate (III) as reactants, and anhydrous alcohol as a reaction solvent, and preparing the 5-nitryl-4, 5-dihydrofuran derivates by the reaction at a temperature of between 30 and 70 DEG C. The raw materials used in the method are low-priced and can be obtained easily, and have a plurality of species, and the synthesized products can serve as potential molecules having the biological activity and key intermediates; and the method utilizes ethanol as a reaction medium, so as to reduce the pollution, shorten the reaction time, simplify the reaction operation and post treatment process, and reduce production costs. The method has advantages of good selectivity and high yield.
Owner:SUZHOU UNIV
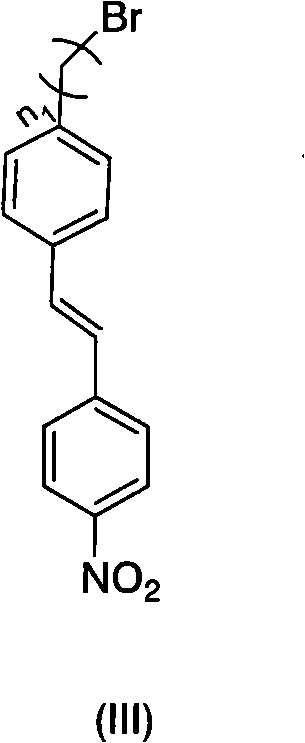
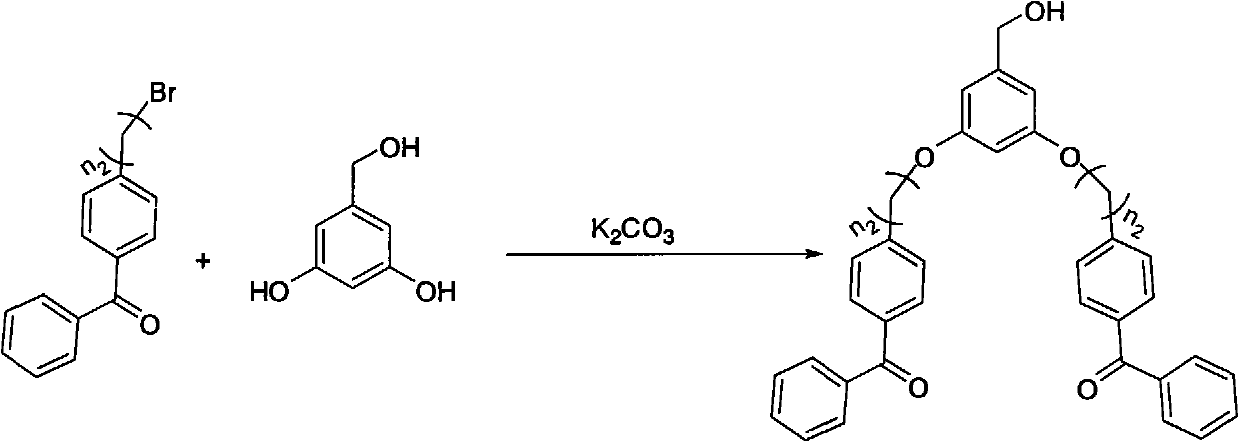
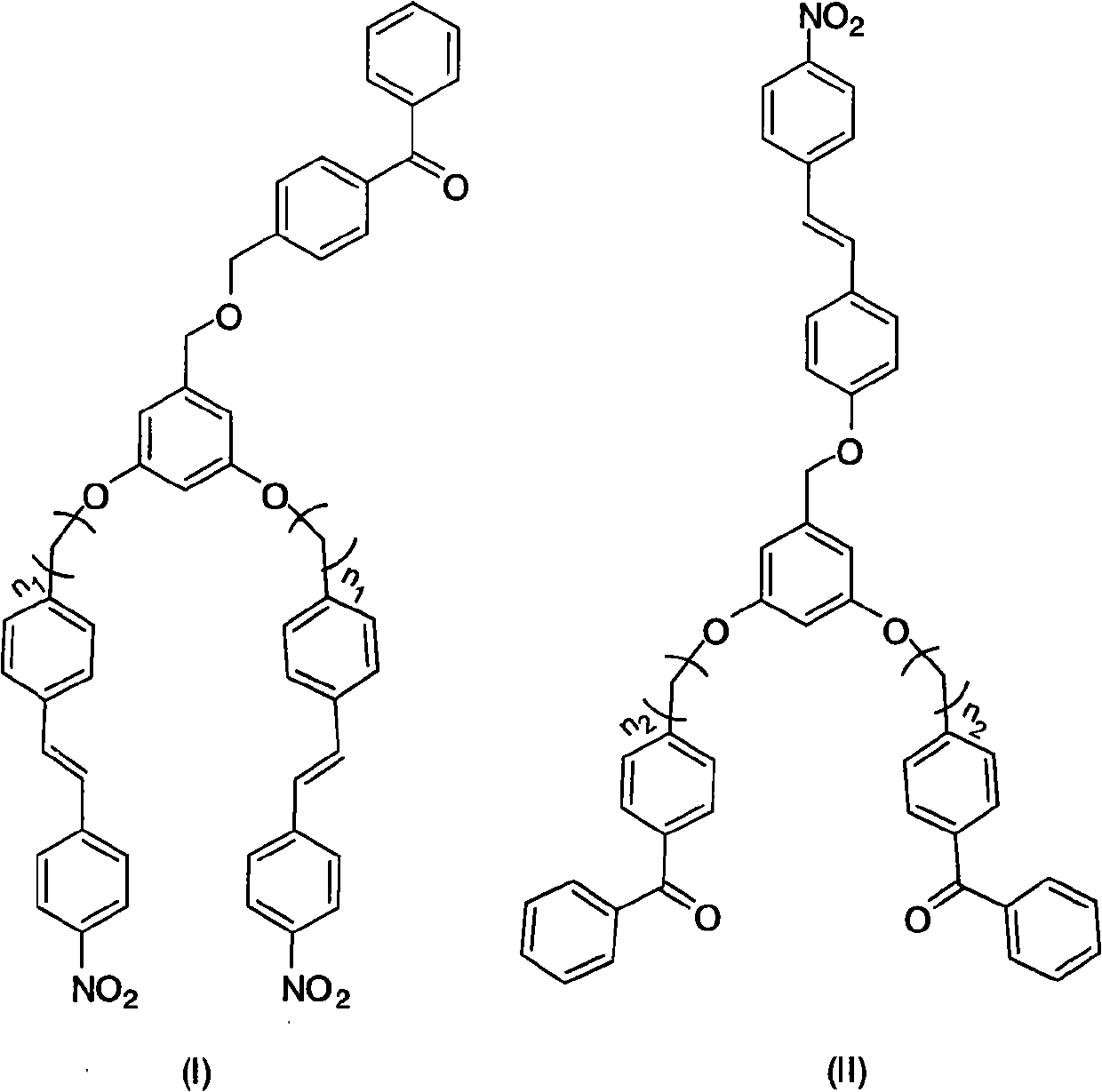
Benzophenone fragment and p-nitrodiphenylethene fragment-containing tree like visible light photoinitiator and synthesis and application thereof
InactiveCN101942046AImprove photoinitiation efficiencyCapable of initiating visible lightOrganic chemistryOrganic compound preparationBenzeneNitrostyrol
The invention relates to a benzophenone fragment and p-nitrodiphenylethene fragment-containing tree like visible light photoinitiator and synthesis and application thereof, belonging to the field of photoinitiators. The tree like visible light photoinitiator comprises the typical chemical structural general formulae shown in the description, wherein n1 and n2 in molecular structural formulae (I) and (II) represent 1-6, wherein in the molecular structural formula (I), the (I) is prepared through reacting 4-(4'-bromohydrocarbylstyyl) nitrobenzene with 3,5-dihydroxylbenzyl alcohol to obtain a product of 3,5-di((4'-nitrobenzenevinyl)benzene alkoxide)benzyl alcohol, and then reacting the 3,5-di((4'-nitrobenzenevinyl)benzene alkoxide)benzyl alcohol with 4-bromomethylbenzophenone under alkaline conditions; and in the molecular structural formula (II), the (II) is prepared through reacting 4-bromohydrocarbylbenzophenone with 3,5-dihydroxylbenzyl alcohol to obtain a product of 3,5-di((4'-benzoyl)benzene alkoxide)benzyl alcohol, and then reacting the 3,5-di((4'-benzoyl)benzene alkoxide)benzyl alcohol with 4-(4'-hydroxylstyryl)nitrobenzene under alkaline conditions. The benzophenone fragment and p-nitrodiphenylethene fragment-containing tree like visible light photoinitiator has the maximum absorption in a visible light region and can be used as the photoinitiator to form a photosensitive system together with a triethanolamine auxiliary agent and used for visible light polymerization of alkene monomers in a solution or used as photocuring materials.
Owner:CHONGQING UNIV
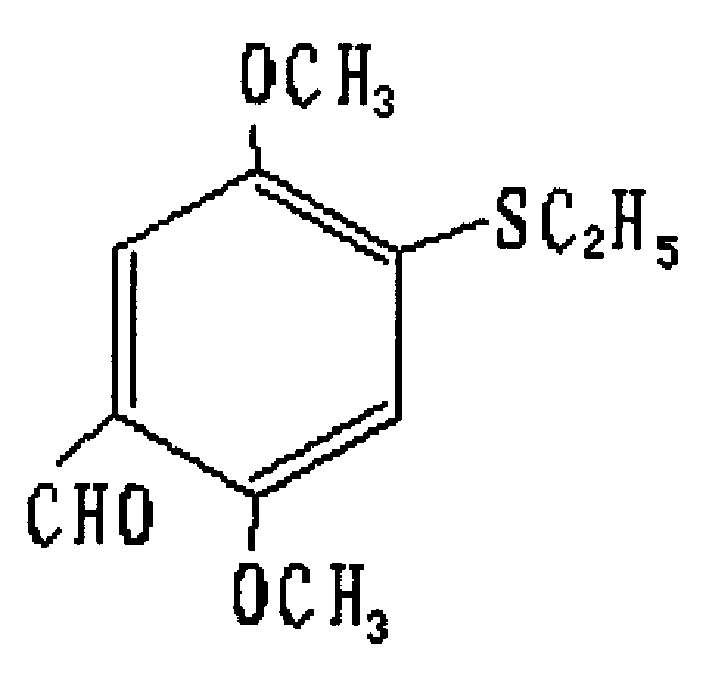
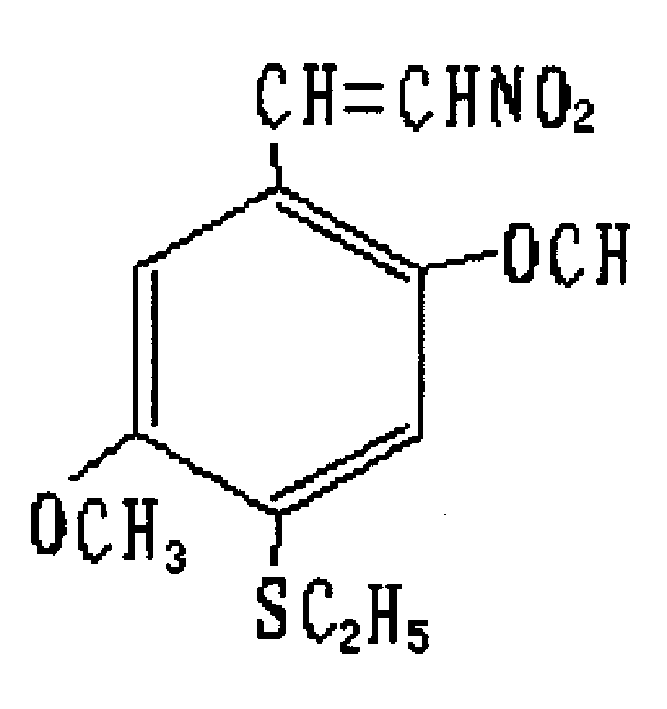
2,5-dimethoxy-4-ethyl sulfide-beta-nitrostyrolene and preparation process and use thereof
The present invention relates to 2,5-dimethoxy-4-ethylthio-beta-nito-styrene and its method for preparing the above-mentioned compound. Said invention provides its structure formula and uses 2.5-dimethoxybenzenesulfonyl chloride as raw material, and adopts the following steps: using zinc powder to make reductino under the acidic condition at 70-80 deg.C, reduced pressure distilling to obtain 2,5-dimethoxythiophenol, making it and bromoethane undergo the process of condensation reaction under the alkaline condition at 70-80 deg.C, reduced pressure distilling to obtain 2.5-dimethoxyethyl phenylsulfide, making it and phosphorus oxychloride and dimethyl formamide produce reaction at 70-80 deg.C to obtain 2,5-dimethoxy-4-ethylthiobenzaldehyde.
Owner:WUHAN UNIV
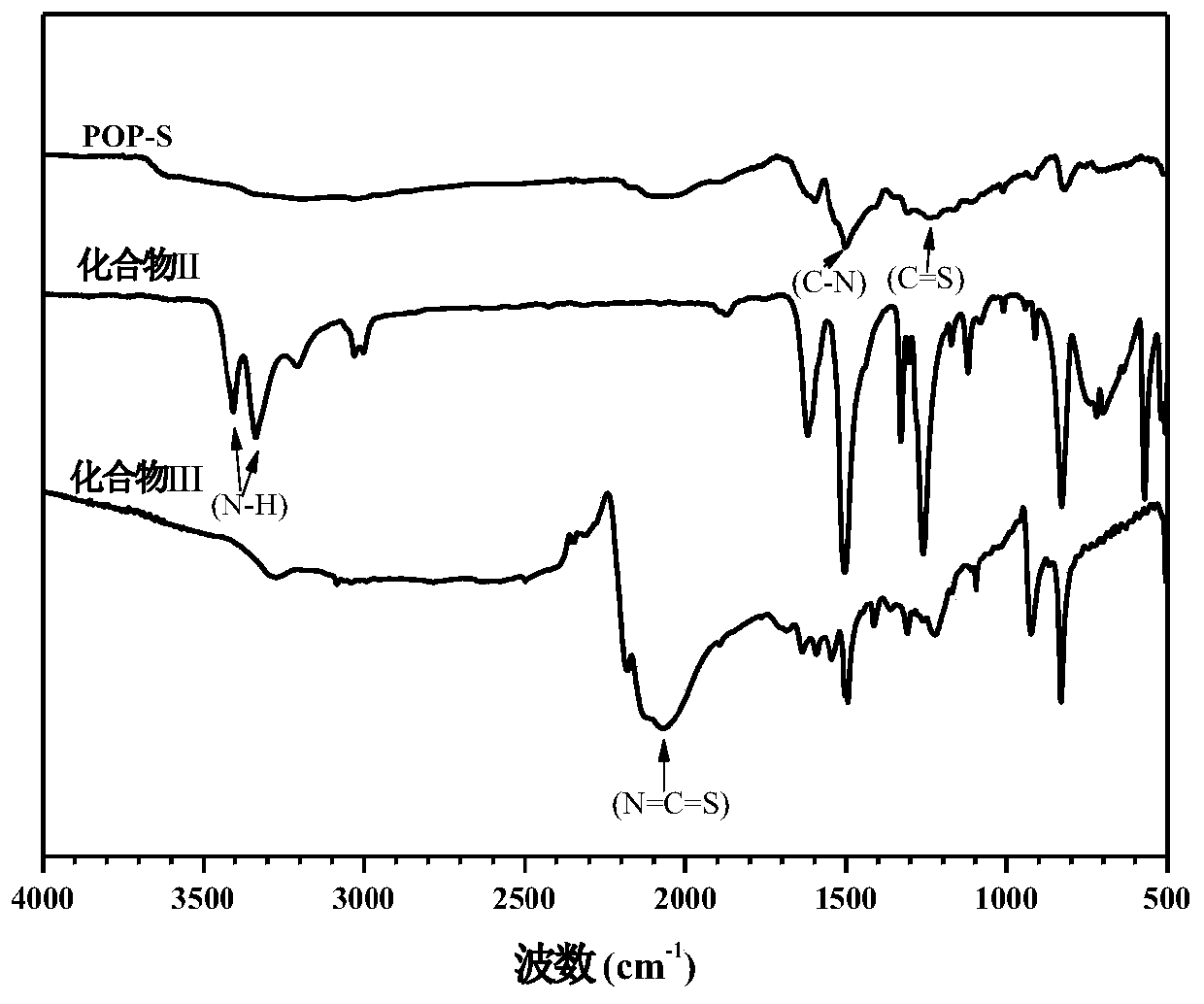
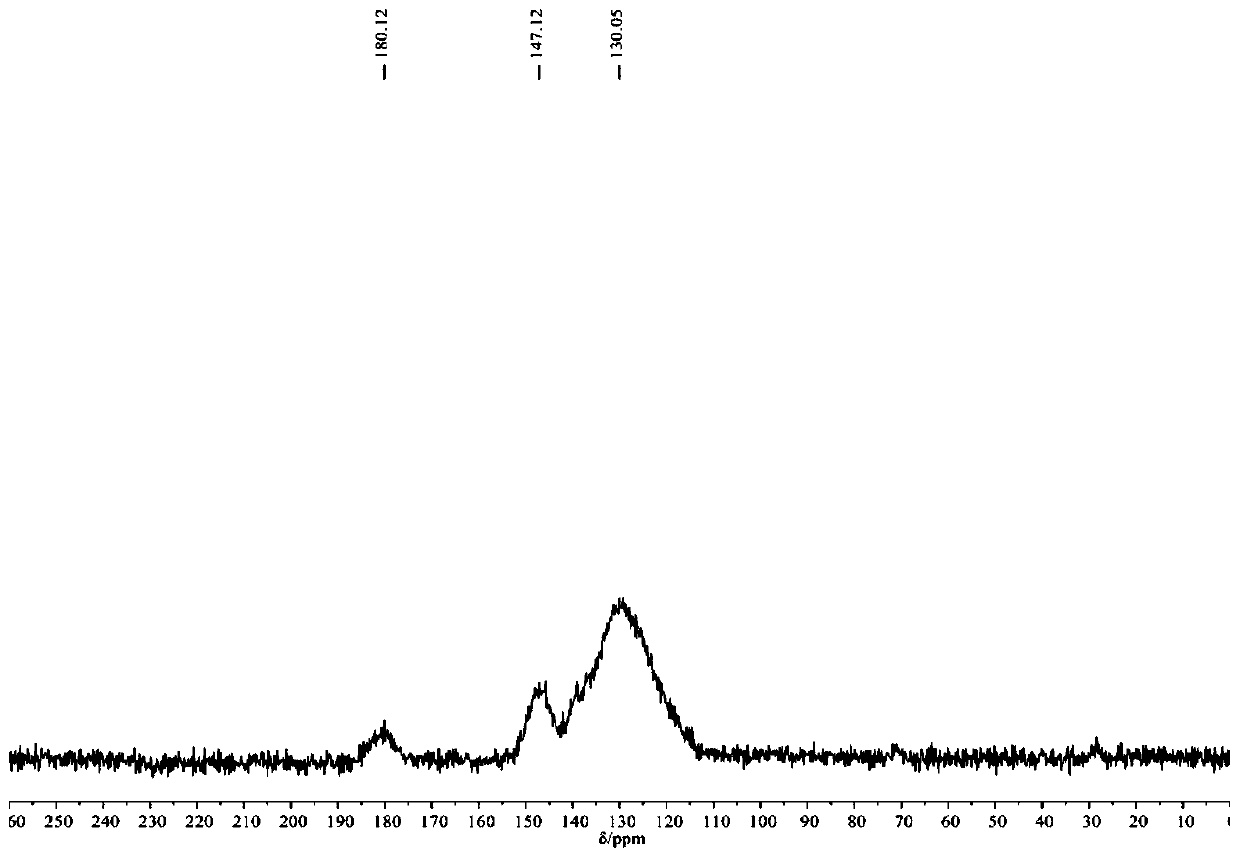
Porous organic polymer linked by thiourea structure as well as preparation method and application of porous organic polymer
ActiveCN110078888ANovel structureHigh catalytic efficiencyOrganic chemistryOrganic compound preparationNitrostyrolThiourea
The invention discloses a porous organic polymer linked by a thiourea structure as well as a preparation method and an application of the porous organic polymer. The preparation method comprises the following steps: adding a compound II and a compound III to an organic solvent under argon or nitrogen atmosphere, and uniformly dispersing the compound II and the compound III by ultrasonic at room temperature; after freezing and deoxidizing, heating the mixture to 60-120 DEG C for a reaction for 24-72 hours; then, cooling the reaction product to room temperature, adding acetone to a reaction solution, performing stirring for 1-2 hours, and performing filtering, washing and drying to obtain the porous organic polymer POP-S linked by the thiourea structure. The synthesized porous organic polymer catalytic material linked by the thiourea structure has more thiourea structures in unit pore channels, can efficiently catalyze Michael reaction between beta-nitrostyrene and diethyl malonate on the basis of dihydrogen bond interaction of thiourea in POP-S as a catalytic active center, and has good solvent tolerance, stability and recyclability.
Owner:SOUTH CHINA UNIV OF TECH
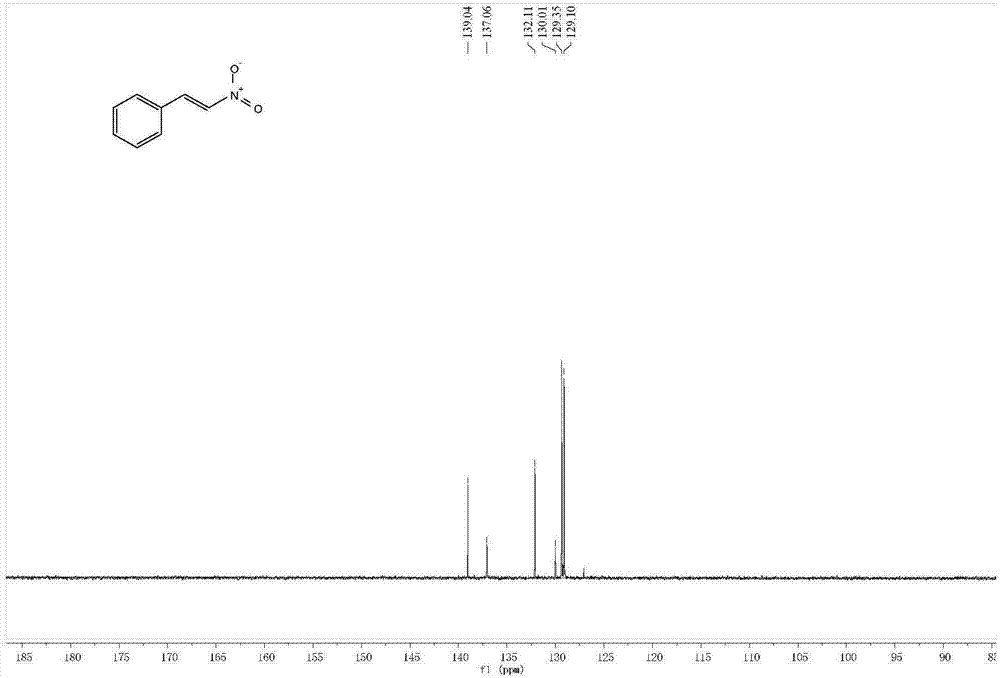
Synthesis method of (E)-beta-nitrostyrene
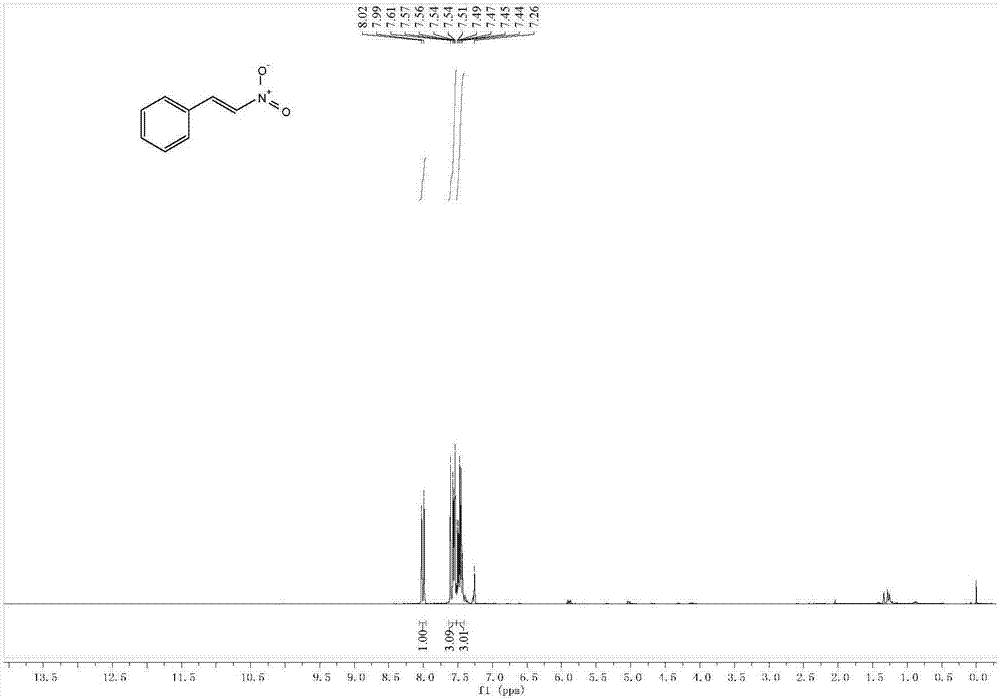
ActiveCN107098811AThe process steps are simpleEase of industrial productionNitro compound preparationNitrostyrolSynthesis methods
The invention discloses a synthesis method of (E)-beta-nitrostyrene. In the method, the (E)-beta-nitrostyrene is produced through a one-pot reaction to styrene in a system comprising tetraaryl ferric porphyrin (III), ammonium halide salt and tert-butyl hydroperoxide. The method can synthesize the (E)-beta-nitrostyrene, which has high E-stereoscopic selectivity, under gentle reaction conditions at high yield.
Owner:大庆三聚能源净化有限公司
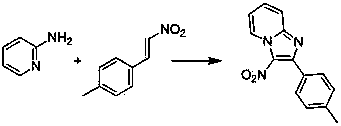
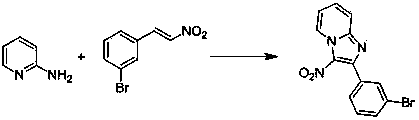
Simple method for synthesizing imidazo (1,2-a) pyridine derivatives
The invention relates to a simple method for synthesizing imidazo (1,2-a) pyridine derivatives. The method comprises the steps of: dissolving reactants including 2-aminopyridine or derivatives thereof and beta-nitrostyrolene or derivatives thereof into a solvent, and preparing the imidazo (1,2-a) pyridine at 25DEG C-150DEG C by taking iodine and pyridine as catalysts and t-butylhydroperoxide as an oxidant, wherein the mole ratio of 2-aminopyridine or derivatives thereof to beta-nitrostyrolene or derivatives thereof to iodine to t-butylhydroperoxide to pyridine is 1:(0.9-1.5):(0.1-0.5):(1-4):(0.3-1). The simple method uses low-cost and easily available reaction raw materials and catalysts, is short in technological process, mild in reaction conditions, and easy for the expansion of the product structure; the target product can be obtained in one step through the implemented serial reaction; the catalyst is a nonmetal system, thus having metal residue risk. The method is high-efficiency, economical and environmentally-friendly synthesis route.
Owner:JINTAN DEPEI CHEM
Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition
InactiveCN104610267AReaction conditions are green and environmentally friendlyLow costOrganic chemistryNitrostyrolNitrobenzene
The invention belongs to the technical field of organic chemistry, and particularly relates to a method for efficiently synthetizing pyrazol[1,5-c]-quinazoline skeleton compounds under no catalytic condition. The structure of the compounds is represented and confirmed with 1H NMR, 13 C NMR, MS and other methods. The method disclosed by the invention comprises the following steps: reacting 1,3-dipolar quinazoline dipoles obtained from o-nitrobenzaldehyde and a series of compounds of triethyl orthoformate with beta-nitrostyrolene under the condition of no any catalysts based on DMSO as a solvent at the temperature of 110 DEG C to generate a series of pyrazol[1,5-c]-quinazoline derivatives. By adopting the method, the pyrazol[1,5-c]-quinazoline compounds can be efficiently prepared. The method disclosed by the invention is mild in reaction conditions and simple to oeprate, the cost is greatly lowered with comparison to the cost of previous reaction between the 1,3-dipolar quinazoline dipoles and terminal alkyne, the reaction conditions are environmentally friendly, the production purity is high, separation and purification are convenient, the method is suitable for large-scale preparation, and the pyrazol[1,5-c]-quinazoline skeleton compounds have excellent application prospect in new drug research and development since the structure of quinazoline compounds has broad-spectrum biological activity.
Owner:JIANGXI NORMAL UNIV
Method for synthesizing multi-substituted pyrrole derivatives
The invention relates to a synthetic method of multi-substituted pyrrole derivatives. The synthetic method adopts an aromatic primary amine derivative shown in formula II, beta-nitrostyrolene shown informula III and derivatives thereof and 1,3-dicarbonyl compound shown in formula IV as raw materials, and LAuCl and AgOTf as catalysts to react at 20 to 50 DEG C, thus obtaining the multi-substitutedpyrrole derivatives. According to the method, by selecting the catalysts, the reaction of the multi-substituted pyrrole derivatives at a low temperature and even at normal temperature is realized, the product yield is high, the practicability is high, the reaction condition is easy to control and implement, and the synthesis of the multi-substituted pyrrole derivatives is performed in a safer environment.
Owner:南京医科大学康达学院
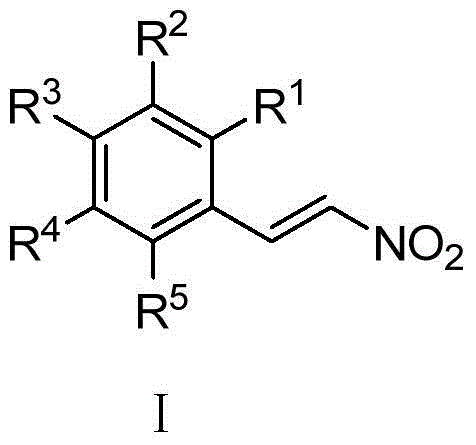
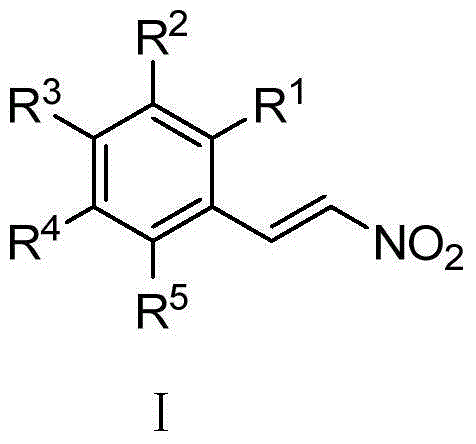
New application of nitro-styrene derivatives in inhibition of fructose-1,6-bisphosphatase
ActiveCN104146989AEnhanced inhibitory effectHydroxy compound active ingredientsMetabolism disorderFructoseNitrostyrol
The invention provides a new application of nitro-styrene derivatives having a structure shown in a general formula I, and a good effect of inhibiting fructose-1,6-bisphosphatase, with the lowest IC up to 0.36muM. When R1, R2, R3, R4 and R5 are hydrogen, R3 represents hydrogen, methoxyl, hydroxyl, borono, fluorine, chlorine, bromine, amino, phenyl, methyl, ethyl, isopropyl, tert-butyl, trifluoromethyl, amino, cyano, methylthio, benyloxyl, carboxyl or 2-nitroethenyl; when R1 and R2 are hydrogen, R3 is methoxyl, and R5 is nitro, R4 represents hydroxyl or methoxymethanoyl; when R1, R2 and R5 are hydrogen, R3 represents methyoxyl, and R4 represents methyoxyl or hydroxyl; when R1 and R4 are hydrogen and R5 is nitro, R2 and R3 represent hydroxyl; and when R2 is 2-nitroethenyl, R1, R3, R4 and R5 are hydrogen or hydroxyl.
Owner:HUAZHONG NORMAL UNIV
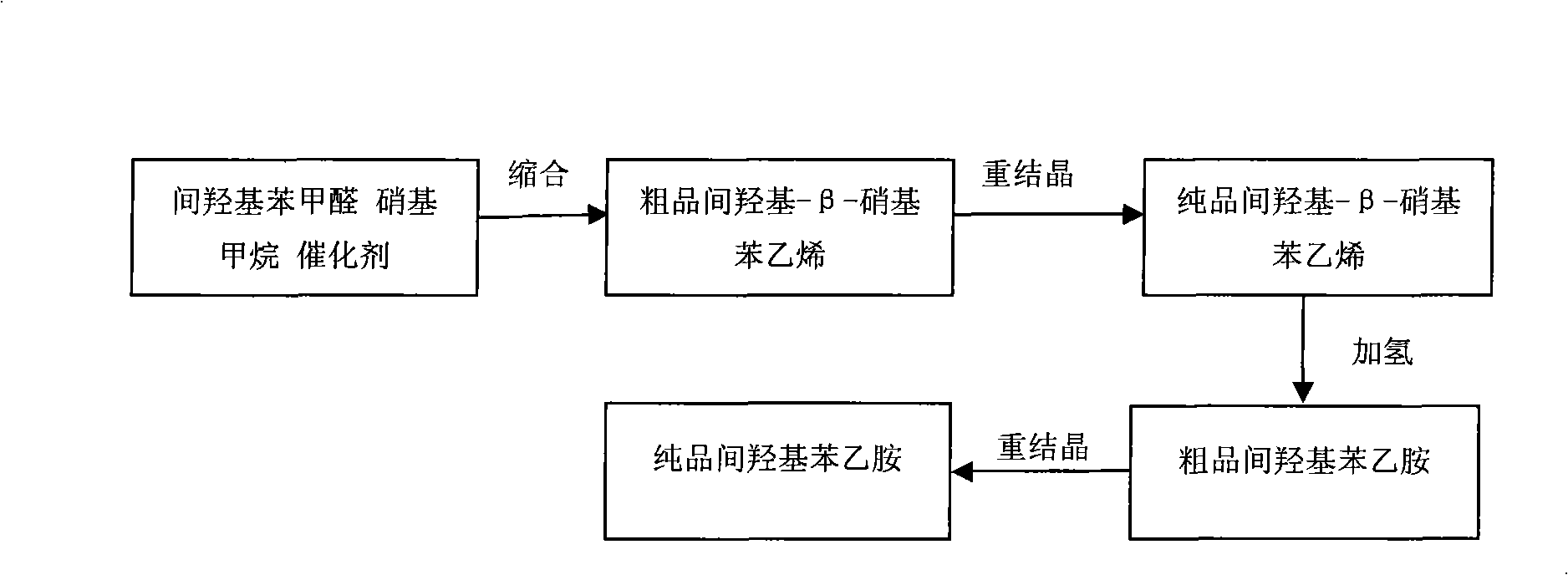
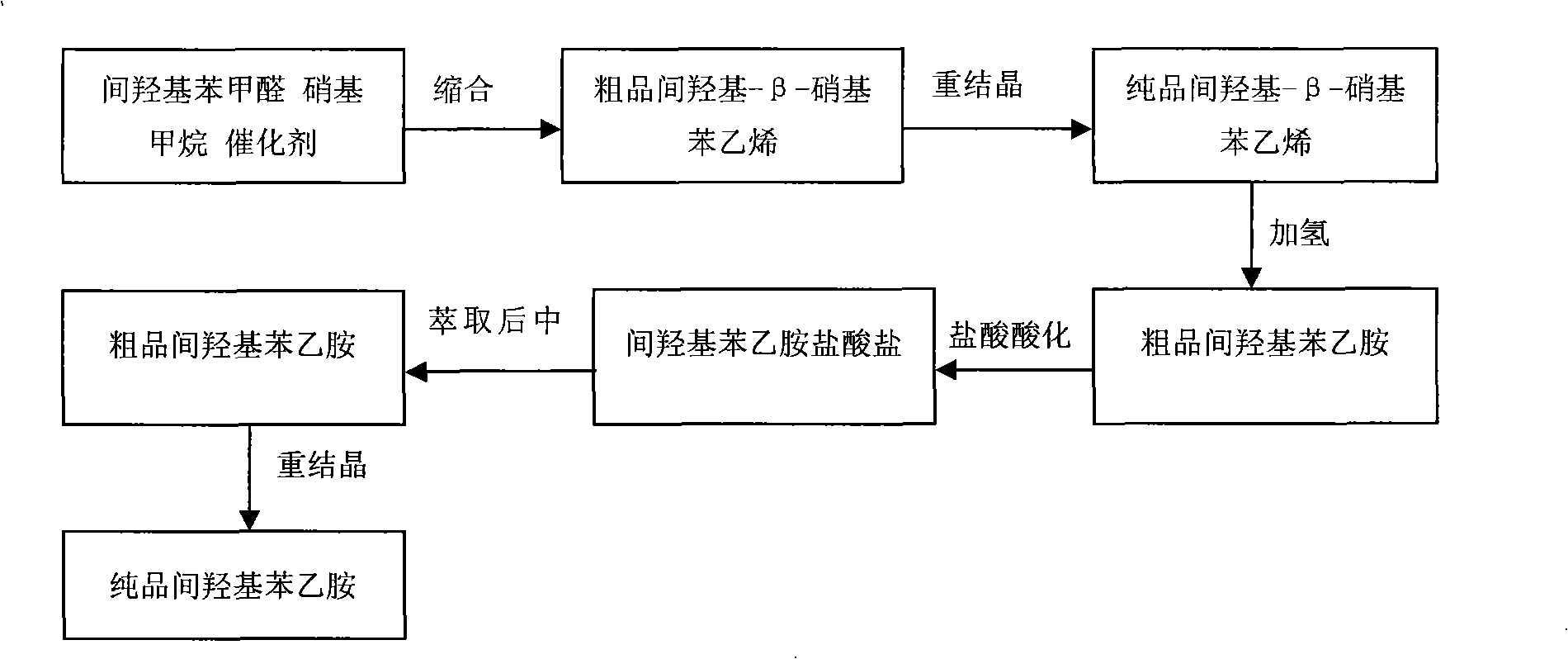
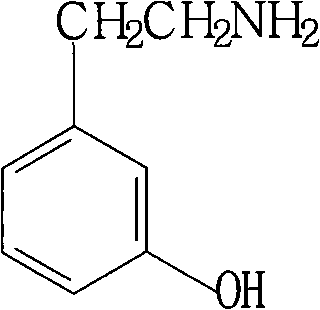
Method for synthesizing m-hydroxyphenylacetamide
InactiveCN101337898AHigh yieldReduce lossOrganic compound preparationAmino-hyroxy compound preparationIce waterNitrostyrol
The invention discloses a method for synthesizing m-hydroxy phenylethylamine from m-hydroxy benzaldehyde by condensation and catalytic hydrogenation. The method comprises the following steps: adding a solvent in m-hydroxy benzaldehyde, nitromethane and a catalyst, and carrying out condensation reaction in a reaction kettle; pouring the reactive solution in the precious step to an ice-water mixture to separate out yellow precipitate, and recrystallizing to obtain m-hydroxy-beta-nitrostyrene; and subjecting m-hydroxy-beta-nitrostyrene in the previous step to catalytic hydrogenation in a high-pressure kettle to obtain m-hydroxy phenylethylamine. The product synthesized by the method can meet the market requirements, that is to say, the color, the purity and the ignition residue of the product can meet the market requirements. The method has high yield, less experiment steps and low cost, and the organic solvent used in the entire synthesis process is nontoxic and cost-effective and can be recycled to reduce the solvent loss in industrial production. Liquid-phase catalytic hydrogenation reaction is selected to achieve easier recovery and higher yield of the product.
Owner:NANJING UNIV OF SCI & TECH
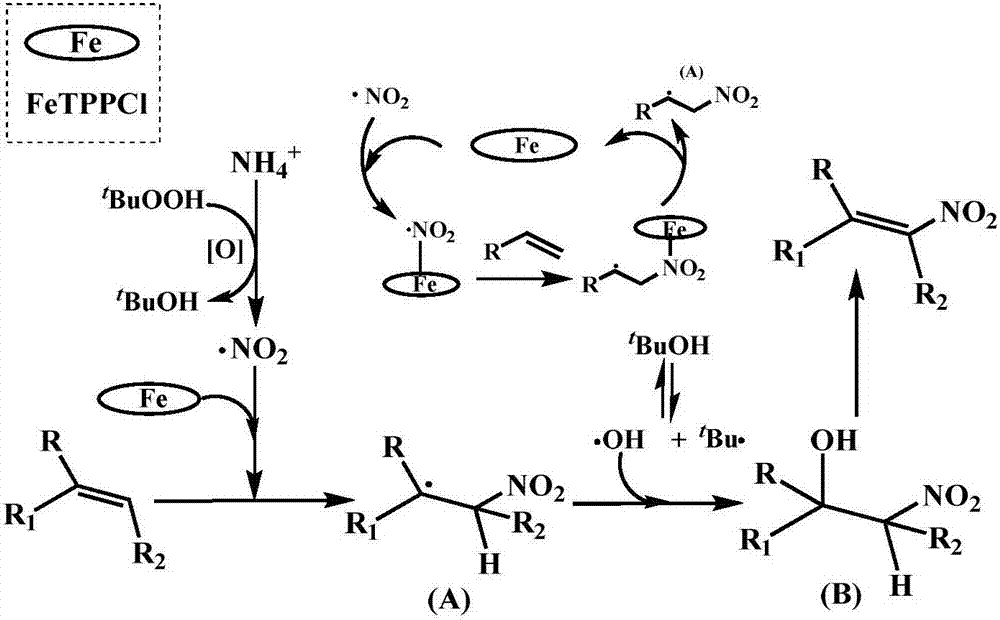
High-selectivity (E)-beta-nitrostyrene derivative one-pot synthesizing method
ActiveCN107417535AThe process steps are simpleEase of industrial productionOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationNitrostyrolTetra
The invention discloses a high-selectivity (E)-beta-nitrostyrene derivative one-pot synthesizing method. In air or sealed environment, styrene derivative generates one-pot reaction in a system containing tetra-acryl ferroporphyrin (III), ammonium iodide and tert-butyl hydroperoxide to generate (E)-beta-nitrostyrene derivative. The method achieves the purpose of synthesizing the beta-nitrostyrene derivative with high E three-dimensional selectivity in high yield under moderate reaction conditions.
Owner:东营睿港管道工程有限责任公司
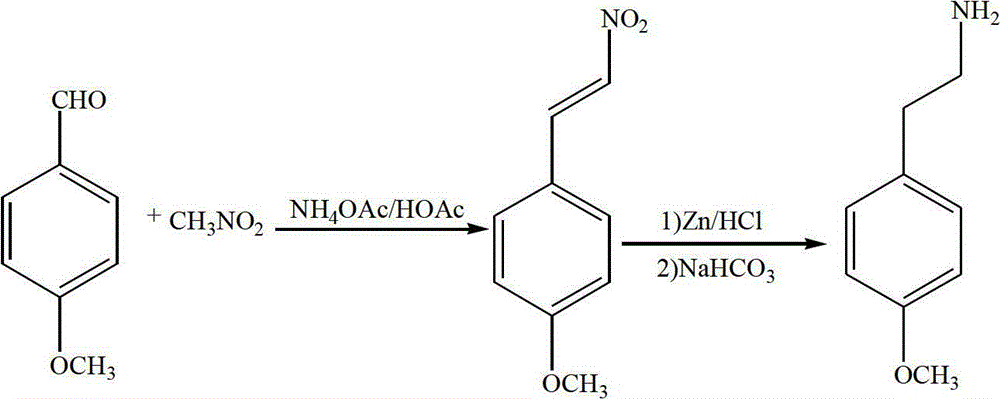
Preparation method of 4-methoxy-beta-phenylethylamine
InactiveCN102976958ALow costEasy to operateOrganic compound preparationAmino-hyroxy compound preparationSodium bicarbonateNitrostyrol
The invention discloses a preparation method of 4-methoxy-beta-phenylethylamine. The method comprises the following preparation steps: 1) reacting 4-methoxy benzaldehyde, nitromethane, ammonium acetate and glacial acetic acid to obtain 4-methoxy-beta-nitrostyrolene; and 2) stirring and reacting activated zinc powder, 4-methoxy-beta-nitrostyrolene and 30-32wt% of hydrochloric acid aqueous solution, distilling under reduced pressure after post-treatment to obtain 4-methoxy-beta-phenylethylamine. The method disclosed by the invention has the advantages of simple operation steps, low cost of the reducing agent, high reaction activity due to Zn powder activated by hydrochloric acid, short reaction time and high yield. The sodium bicarbonate solution is used for neutralizing in the post-treatment after the reduction reaction is ended and then 4-methoxy-beta-phenylethylamine is obtained.
Owner:张家港市大伟助剂有限公司
2,5-dimethoxy-4-ethyl benzaldehyde sulfide and preparation process and use thereof
The present invention relates to a 2,5-dimethoxy-4-ethylthiobenzaldehyde, its preparation method and application. Said invention provides its structure for mula and uses 2,5-methoxybenzenesulfonyl chloride as raw material, and adopts the following steps: using zinc powder to make reduction under the acidic condition at 70-80 deg.C, then reduced pressure distilling to obtain 2,5-dimethoxythiophenol, making the obtained 2,5-dimethoxythiophenol implement condensation reaction with bromoethane under the alkaline condition at 70-80 deg.C, reduced pressure distilling to obtain yellow oil liquor 2,5-dime thoxyethyl phenyl sulfide, making it and phosphorus oxychloride and dimethyl formamide react at 70-80 deg.C so as to obtain the white solid, i.e. required 2,5-dimethoxy-4-ethylthio-benzaldehyde.
Owner:WUHAN UNIV
New preparation method for 4-hydroxyl-beta-nitrostyrolene
Owner:SHANGHAI HUAJIN BIOTECH CO LTD
New preparation method of 3,4-dyhydroxyl-Beta-nitrostyrolene
The invention discloses a new preparation method of 3,4-dyhydroxyl-Beta-nitrostyrolene, and belongs to the technical field of medicine technologies. A technical problem to be solved relates to a moreadvanced new preparation method of the 3,4-dyhydroxyl-Beta-nitrostyrolene. The key point of the technical problem to be solved is a new preparation method of a compound.
Owner:SHANGHAI HUAJIN BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
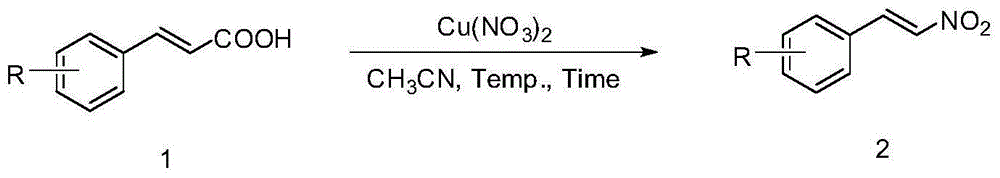
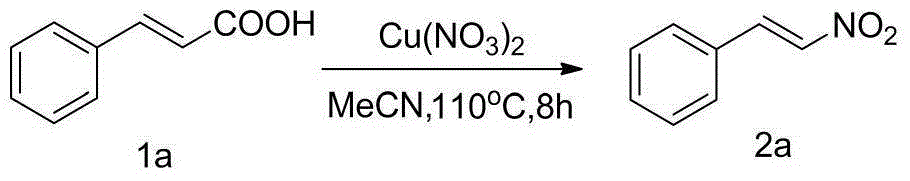
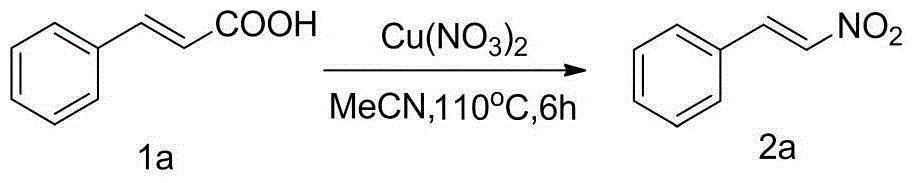


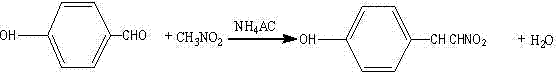


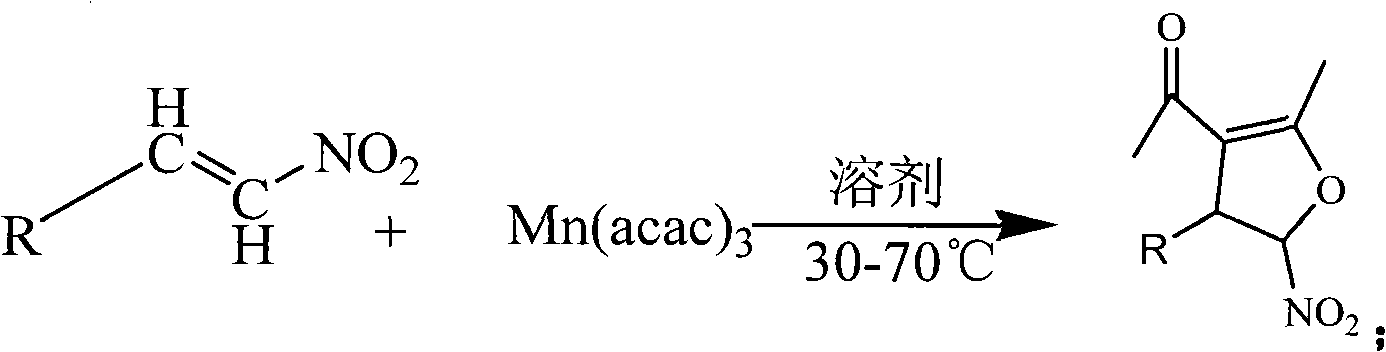
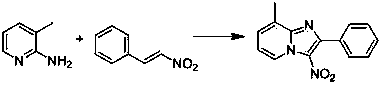
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/2015100642629100002DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE002.PNG)
![Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition Method for efficiently synthetizing 6-alkylpyrazol-[1,5-c]-quinazoline skeleton compounds under no catalytic condition](https://images-eureka.patsnap.com/patent_img/ef8c07ac-51ca-42e8-a02f-a605cd8029cc/DEST_PATH_IMAGE004.PNG)