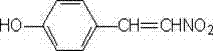
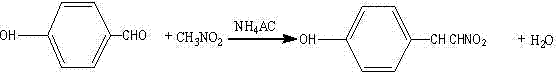Synthesis method of p-hydroxy-beta-nitrostyrene
A technology of nitrostyrene and a synthesis method, which is applied in the preparation of nitro compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of easy conversion, poor stability, large product loss, etc., and achieves the reaction cost. Low, simple post-processing, and the effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
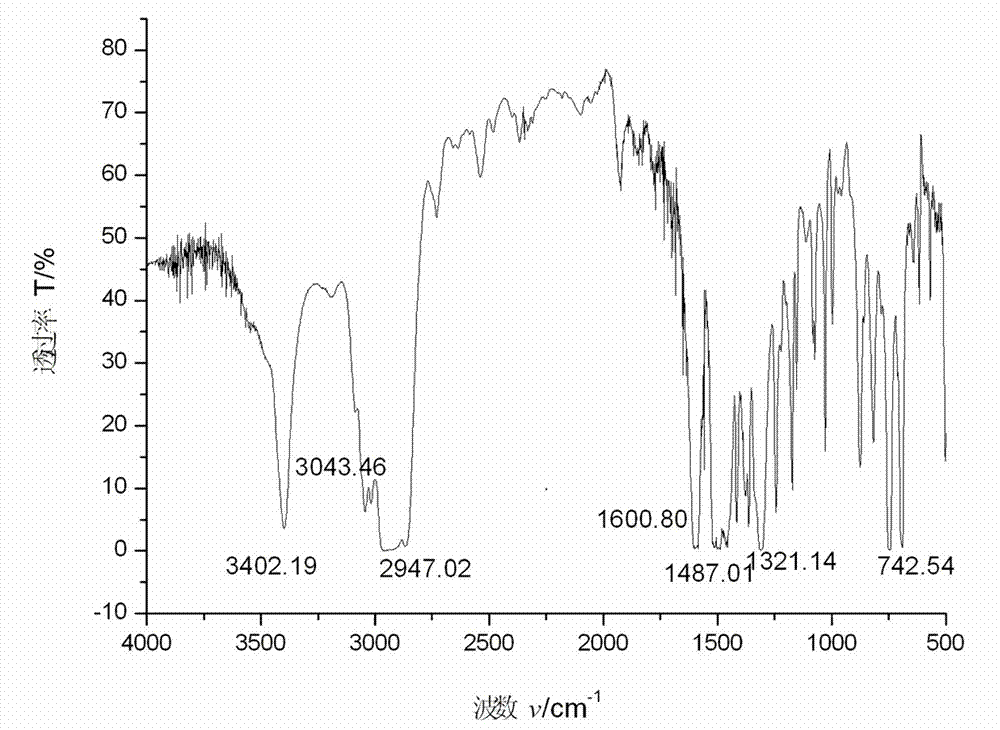
Image
Examples
Embodiment 1
[0026] Take 10mL of glacial acetic acid, add 0.02g of anhydrous hydroquinone, and let stand for 45 minutes.
[0027] Add 3.66g (0.03mol) of p-hydroxybenzaldehyde, 5.1mL (0.09mol) of nitromethane, the above-mentioned 10mL of glacial acetic acid, and 1g of ammonium acetate into a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, and stir Heated to 120°C for 1 hour, then added 0.39 g of ammonium acetate, and maintained at 120°C for 3 hours.
[0028] Change the reflux device to a distillation device, add 8 mL of toluene, distill the fraction after half an hour, and stop the reaction.
[0029] After the reaction was completed and cooled, the reactant was poured into an appropriate amount of ice water, and a yellow precipitate was precipitated. Without filtering, directly heat the system to 60-70°C, slowly add ethanol dropwise under stirring, until the precipitate is completely dissolved, then pour the solution into an appropriate amou...
Embodiment 2
[0033] Take 20mL of glacial acetic acid, add 0.04g of anhydrous hydroquinone, and let stand for 30 minutes.
[0034] Add 3.66g (0.03mol) of p-hydroxybenzaldehyde, 1.7mL (0.03mol) of nitromethane, the above-mentioned 20mL of glacial acetic acid, and 0.77g of ammonium acetate into a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser in sequence, Heat to 80°C for 1 hour under stirring, then add 0.39 g of ammonium acetate, and maintain 80°C for 3 hours.
[0035] Change the reflux device to a distillation device, add 8 mL of toluene, distill the fraction after half an hour, and stop the reaction.
[0036] After the reaction was completed and cooled, the reactant was poured into an appropriate amount of ice water, and a yellow precipitate was precipitated. Without filtering, directly heat the system to 60-70°C, slowly add ethanol dropwise under stirring, until the precipitate is completely dissolved, then pour the solution into an approp...
Embodiment 3
[0039] Take 15mL of glacial acetic acid, add 0.03g of anhydrous hydroquinone, and let stand for 45 minutes.
[0040]Add 3.66g (0.03mol) of p-hydroxybenzaldehyde, 5.1mL (0.09mol) of nitromethane, the above-mentioned 15mL of glacial acetic acid, and 0.26g of ammonium acetate into a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser in sequence, Heat to 100°C for 1 hour under stirring, then add 0.12 g of ammonium acetate, and maintain 100°C for 3 hours.
[0041] Change the reflux device to a distillation device, add 6 mL of toluene, distill the distillate after half an hour, and stop the reaction.
[0042] After the reaction was completed and cooled, the reactant was poured into an appropriate amount of ice water, and a yellow precipitate was precipitated. Without filtering, directly heat the system to 60-70°C, slowly add ethanol dropwise under stirring, until the precipitate is completely dissolved, then pour the solution into an app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com