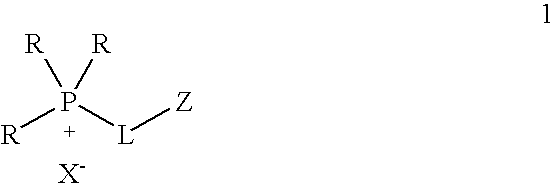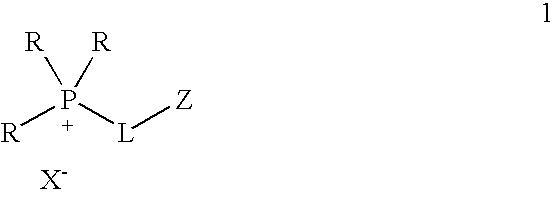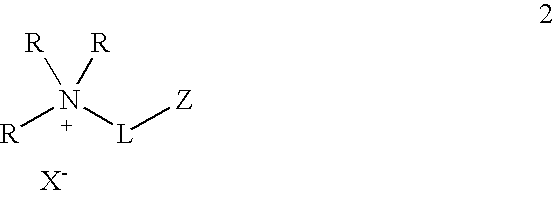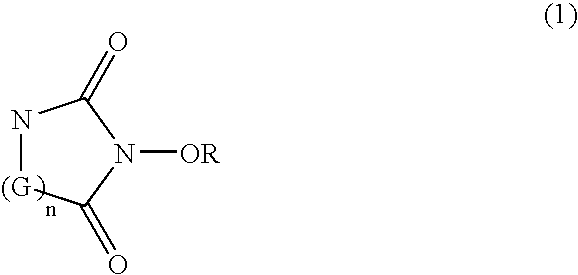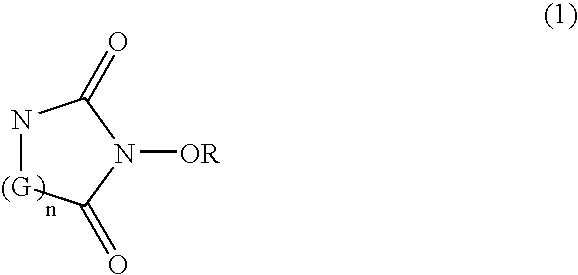Patents
Literature
1102results about "Nitro compound preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Functionalized ionic liquids, and methods of use thereof
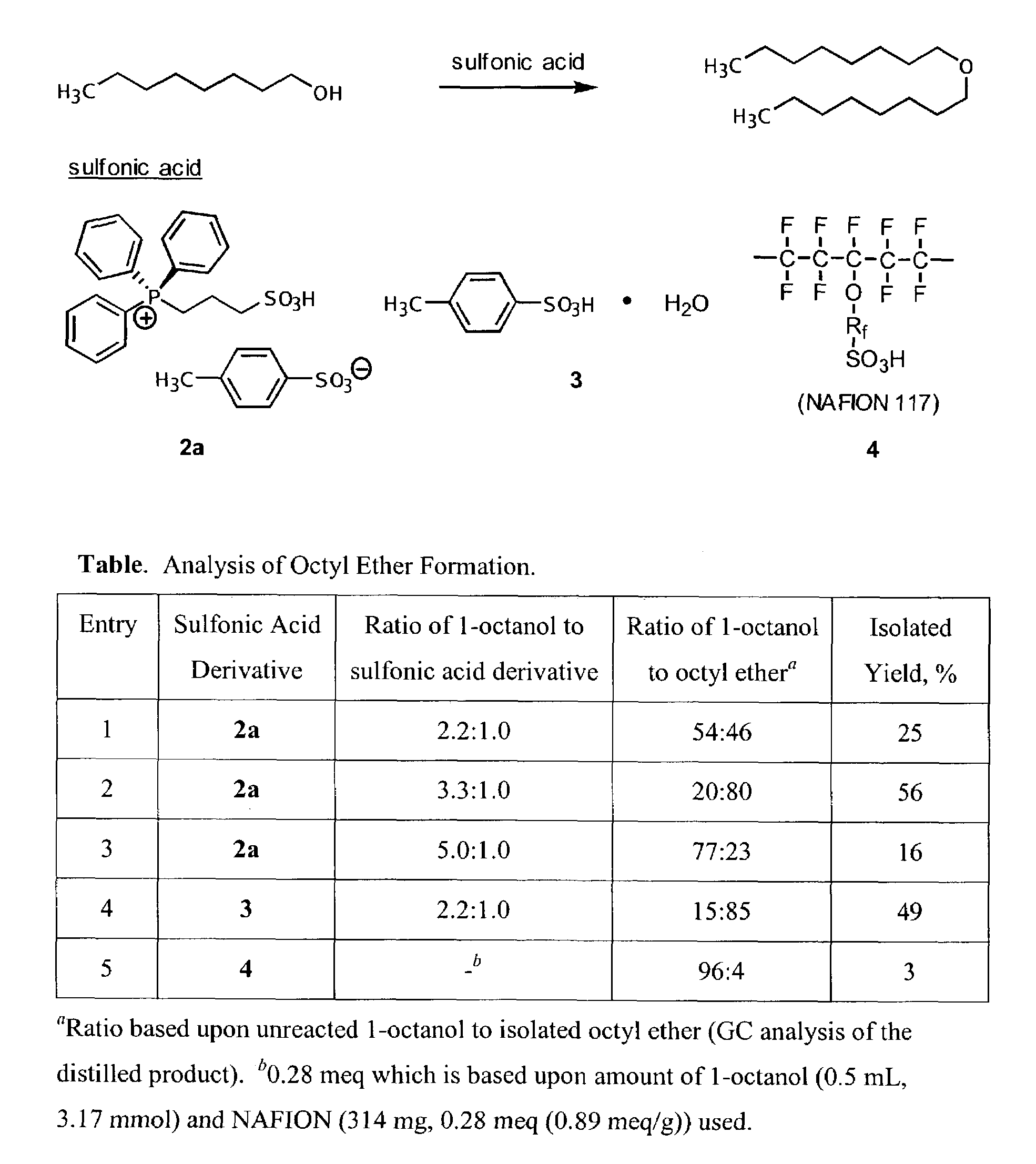
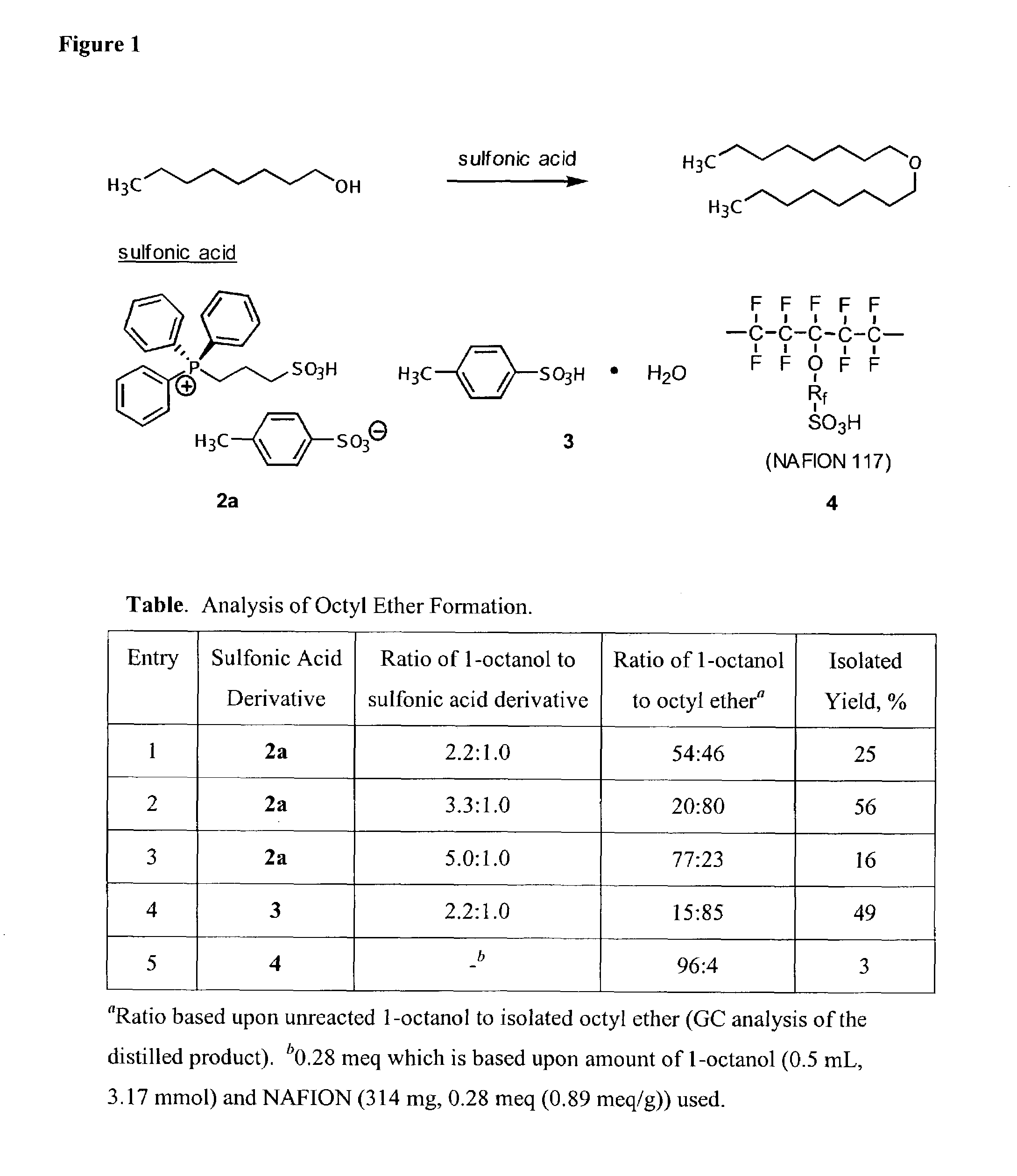
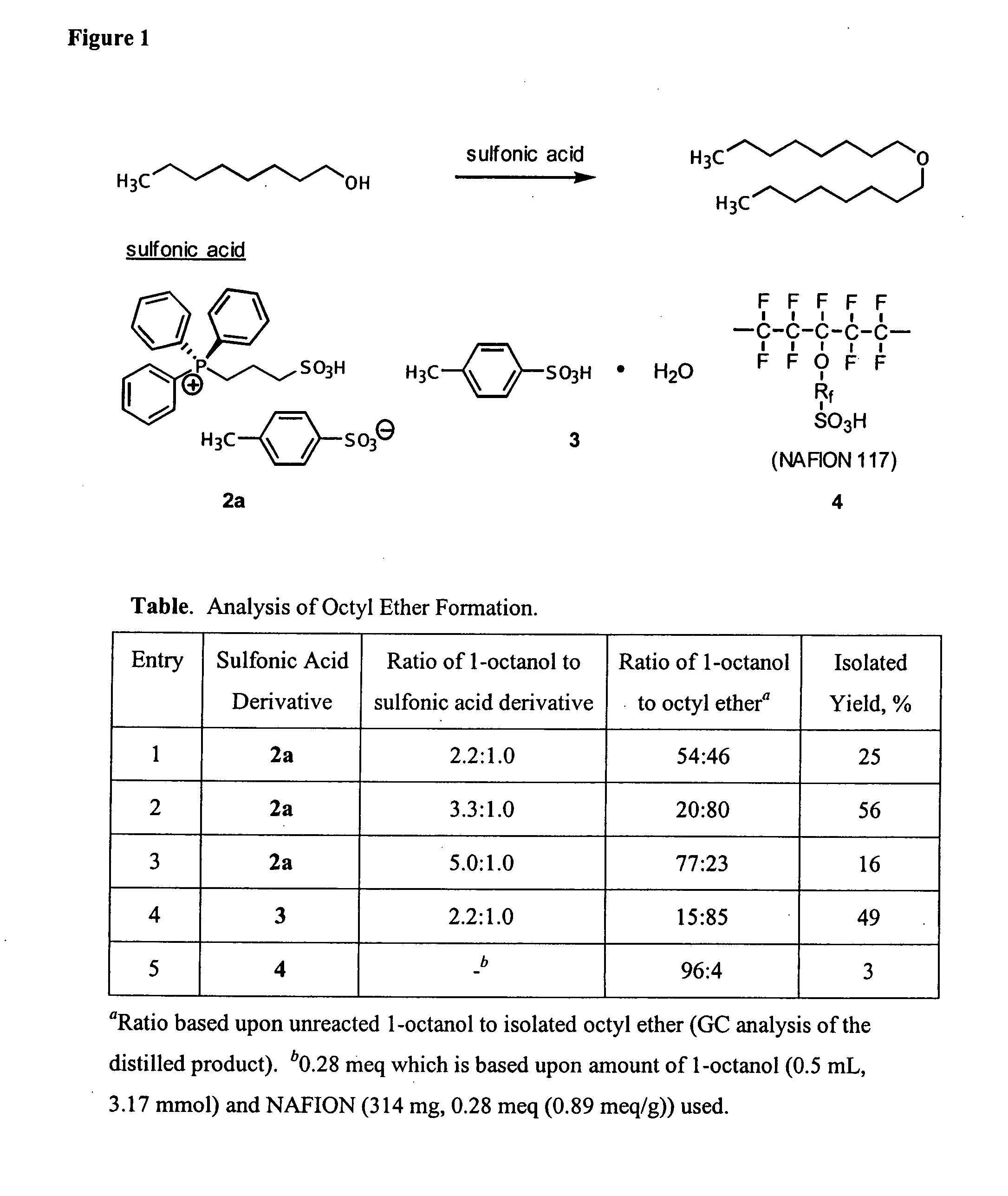
One aspect of the present invention relates to ionic liquids comprising a pendant Bronsted-acidic group, e.g., a sulfonic acid group. Another aspect of the present invention relates to the use of an ionic liquid comprising a pendant Bronsted-acidic group to catalyze a Bronsted-acid-catalyzed chemical reaction. A third aspect of the present invention relates to ionic liquids comprising a pendant nucleophilic group, e.g., an amine. Still another aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to catalyze a nucleophile-assisted chemical reaction. A fifth aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to remove a gaseous impurity, e.g., carbon dioxide, from a gas, e.g., sour natural gas.
Owner:UNIV OF SOUTH ALABAMA
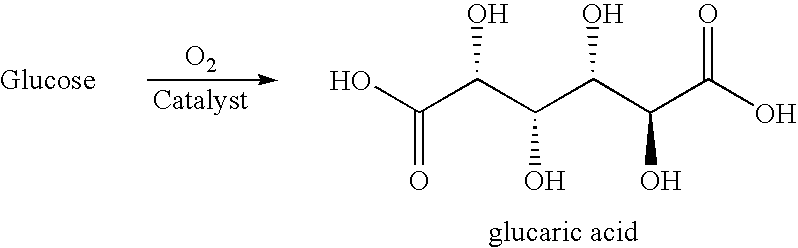
Production of adipic acid and derivatives from carbohydrate-containing materials
ActiveUS8669397B2Low costLactams preparationCarboxylic acid nitrile preparationCatalytic oxidationHydrodeoxygenation
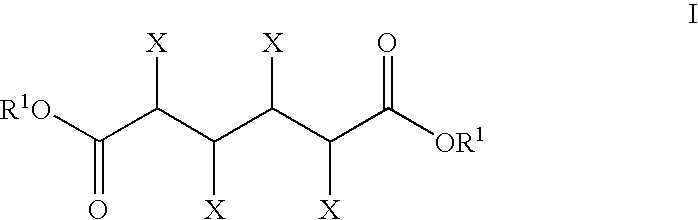
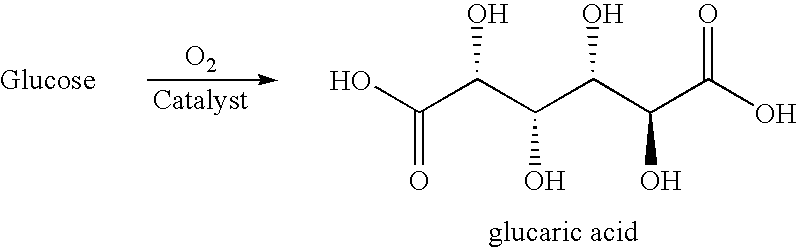
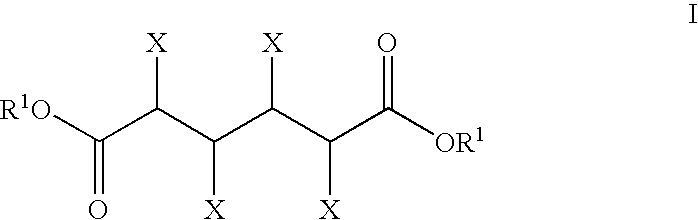
The present invention generally relates to processes for the chemocatalytic conversion of a glucose source to an adipic acid product. The present invention includes processes for the conversion of glucose to an adipic acid product via glucaric acid or derivatives thereof. The present invention also includes processes comprising catalytic oxidation of glucose to glucaric acid or derivative thereof and processes comprising the catalytic hydrodeoxygenation of glucaric acid or derivatives thereof to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Microchannel with internal fin support for catalyst or sorption medium
InactiveUS7220390B2Fast thermal swingFaster cycle timeCombination devicesMethane captureEngineeringSorption
This invention relates to an apparatus, comprising: at least one process microchannel having a height, width and length, the height being up to about 10 mm, the process microchannel having a base wall extending in one direction along the width of the process microchannel and in another direction along the length of the process microchannel; at least one fin projecting into the process microchannel from the base wall and extending along at least part of the length of the process microchannel; and a catalyst or sorption medium supported by the fin.
Owner:VELOCYS CORPORATION
Method for undergoing chlorobenzene nitration reaction by using micro-channel reactor
ActiveCN102432471APrevent leakageAvoid dangerNitro compound preparationTemperature controlChlorobenzene
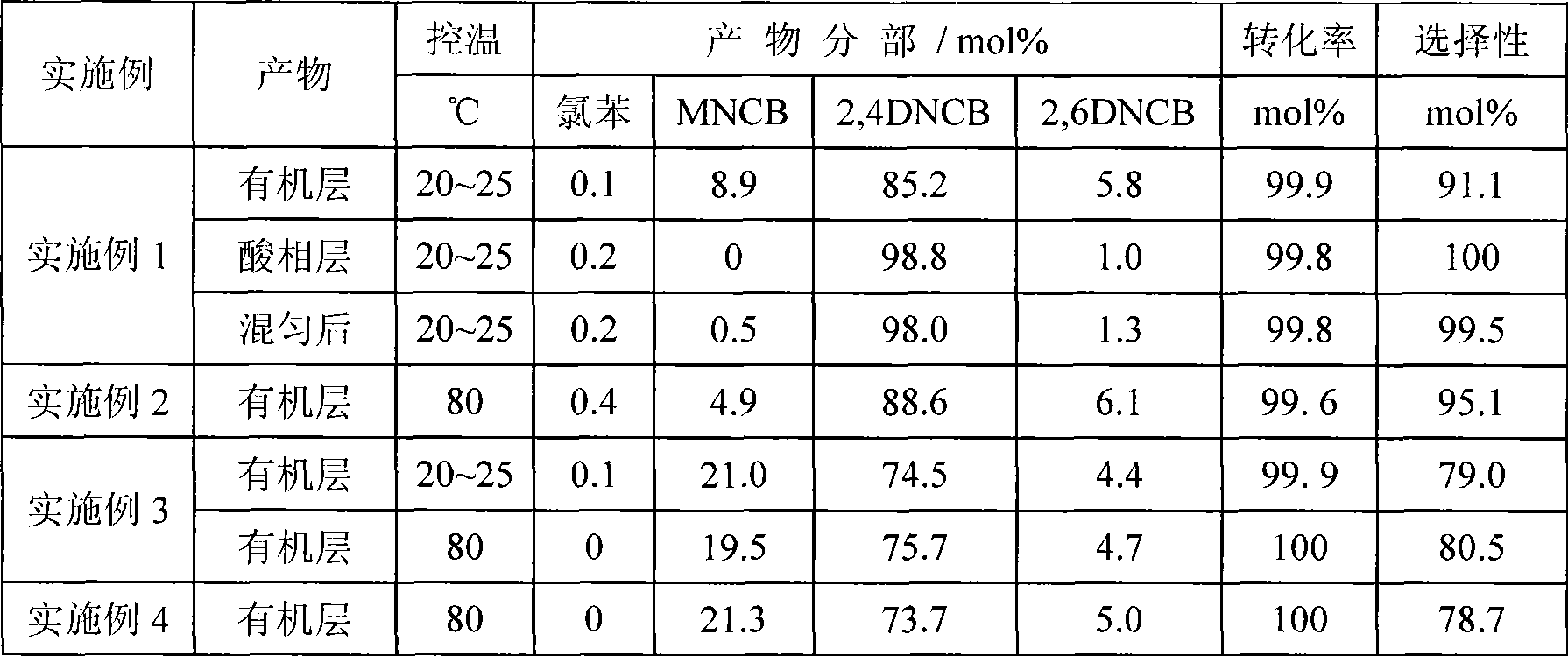
The invention relates to a method for undergoing a chlorobenzene nitration reaction by using a micro-channel reactor, belonging to the technical field of application of organic synthesis. In the method, nitric acid, sulfuric acid, water and chlorobenzene are taken as initial reaction raw materials, and processes such as mixed acid preparation, mixed acid and chlorobenzene preheating, mixed acid and chlorobenzene reacting and the like are completed in a micro-channel reactor system. In the reaction, nitro-sulfuric mixed acid is taken as a nitrating agent, the effective concentration of sulfuric acid in the mixed acid is 50-90 percent, the molar ratio of the nitric acid to the sulfuric acid in the mixed acid is 1:1-1:10, the molar ratio of the chlorobenzene to the nitric acid is 1:1.0-1:2.0, the reaction temperature is 50-100 DEG C, and the reaction time is 30-120 seconds. The chlorobenzene transformation ratio is up to 97 percent, the selectivity of nitrochlorobenzene serving as a product is over 96.5 percent, and the ratio of ortho-para nitrochlorobenzene is over 0.6. A strengthened mixed micro-channel reactor adopted in the invention is particularly suitable for undergoing a continuous nitration reaction, and has the characteristics of stable temperature control and safe process.
Owner:CHANGZHOU UNIV
Process for working up the waste water obtained in the preparation of dinitrotoluene
ActiveUS6936741B2Easy to transportReduce pointsLiquid degasificationOrganic compound preparationWash waterWastewater
The present invention relates to a process for working up or treating aqueous waste waters which are formed during the nitration of toluene to dinitrotoluene with nitrating acid. These aqueous waste waters containing acidic wash water and alkaline wash water from the dinitrotoluene washing step, and distillate from the sulfuric acid concentration step. The process comprises,a) combining the acidic and alkaline waste waters from the washing step and the aqueous distillate from the sulfuric acid concentration step such that the resulting mixture has a pH below 5,b) separating the aqueous and organic phases which are formed by phase separation,c) subjecting the aqueous phase from b) to an extraction step, whereind) the organic components contained in the aqueous phase from c) are extracted with toluene, ande) introducing the toluene phase enriched with the organic components into the toluene nitration.
Owner:COVESTRO DEUTSCHLAND AG
Production of Adipic Acid and Derivatives from Carbohydrate-Containing Materials
ActiveUS20100317823A1Increase costLow costCarboxylic acid nitrile preparationOrganic chemistry methodsCatalytic transformationCatalytic oxidation
The present invention generally relates to processes for the chemocatalytic conversion of a glucose source to an adipic acid product. The present invention includes processes for the conversion of glucose to an adipic acid product via glucaric acid or derivatives thereof. The present invention also includes processes comprising catalytic oxidation of glucose to glucaric acid or derivative thereof and processes comprising the catalytic hydrodeoxygenation of glucaric acid or derivatives thereof to an adipic acid product. The present invention also includes products produced from adipic acid product and processes for the production thereof from such adipic acid product.
Owner:ARCHER DANIELS MIDLAND CO
Dinitrochlorobenzene synthesis method and microreactor
InactiveCN101544568AProcess Safety ContinuitySimple structureNitro compound preparationRoom temperatureSynthesis methods
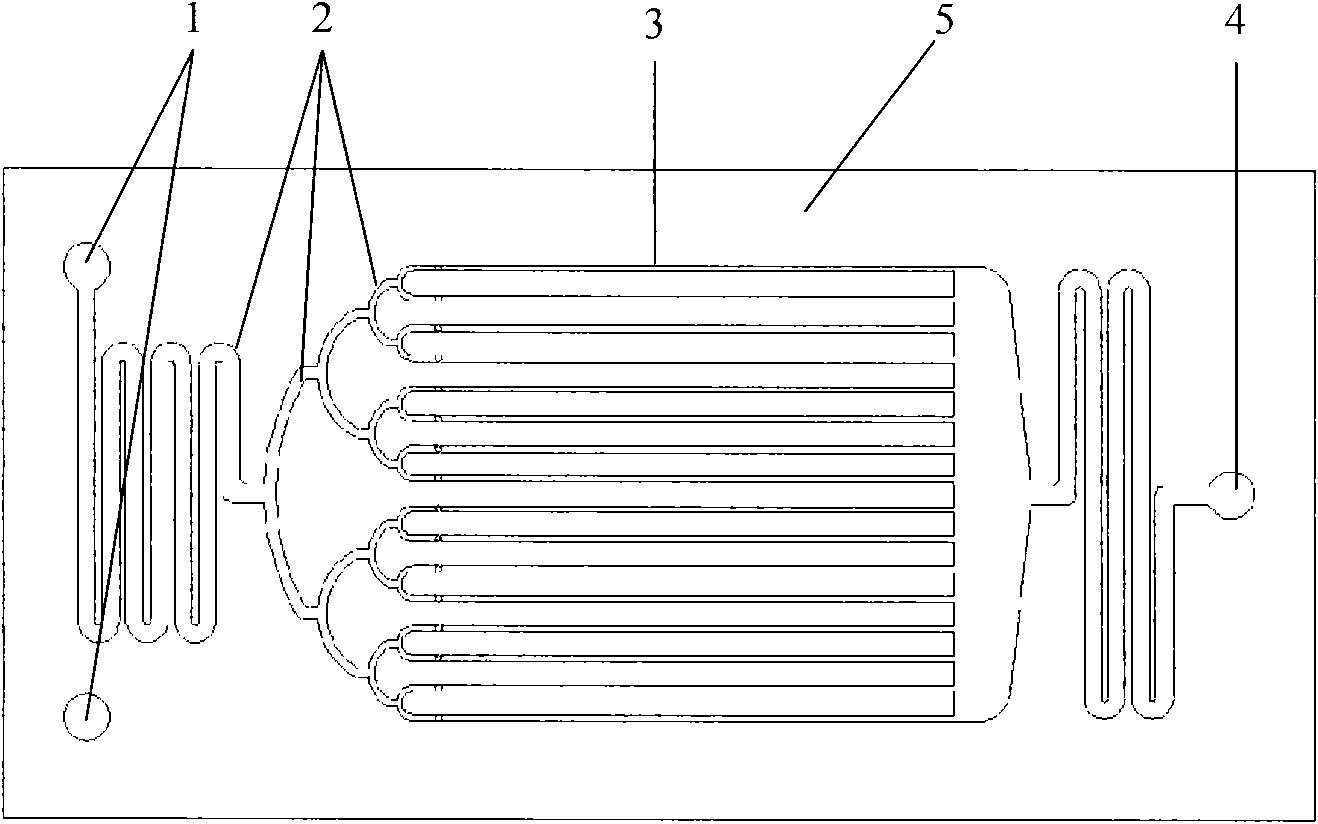
The invention relates to a one-step method for synthesizing dinitrochlorobenzene from chlorobenzene, and a microchannel reactor. The method comprises the steps of taking chlorobenzene and mixed acid of nitric acid / sulfuric acid (n / s) as initial reaction materials, inputting material flow synchronously into a microreactor through a metering pump and finishing mixed mass transfer and reaction in a reaction channel. The substance ratio of n to s in the reactive mixed acid is 0.1 to 0.5, and the mass ratio of water is less than 10 percent. The microchannel reactor adopts a stainless steel flat-plate structure which comprises fixed pressing-sealing plates, a microchannel plate, a raw-material inlet, a product outlet, as well as a temperature-control pore channel and a thermal-couple measurement-control jack which are positioned on two sealing plates. The method is continuously operated under a technological condition with strong nitrating acid, and is safe in process. The selectivity of the dinitrochlorobenzene in the reactor at an operation temperature between normal temperature and 80 DEG C is more than 99.5 mol percent, wherein the selectivity of 2,4 dinitrochlorobenzene is more than 98 mol percent.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthetic method and special equipment for nitrobenzene
InactiveCN101613285AHigh selectivityImprove transfer characteristicsNitro compound preparationBenzeneNitration
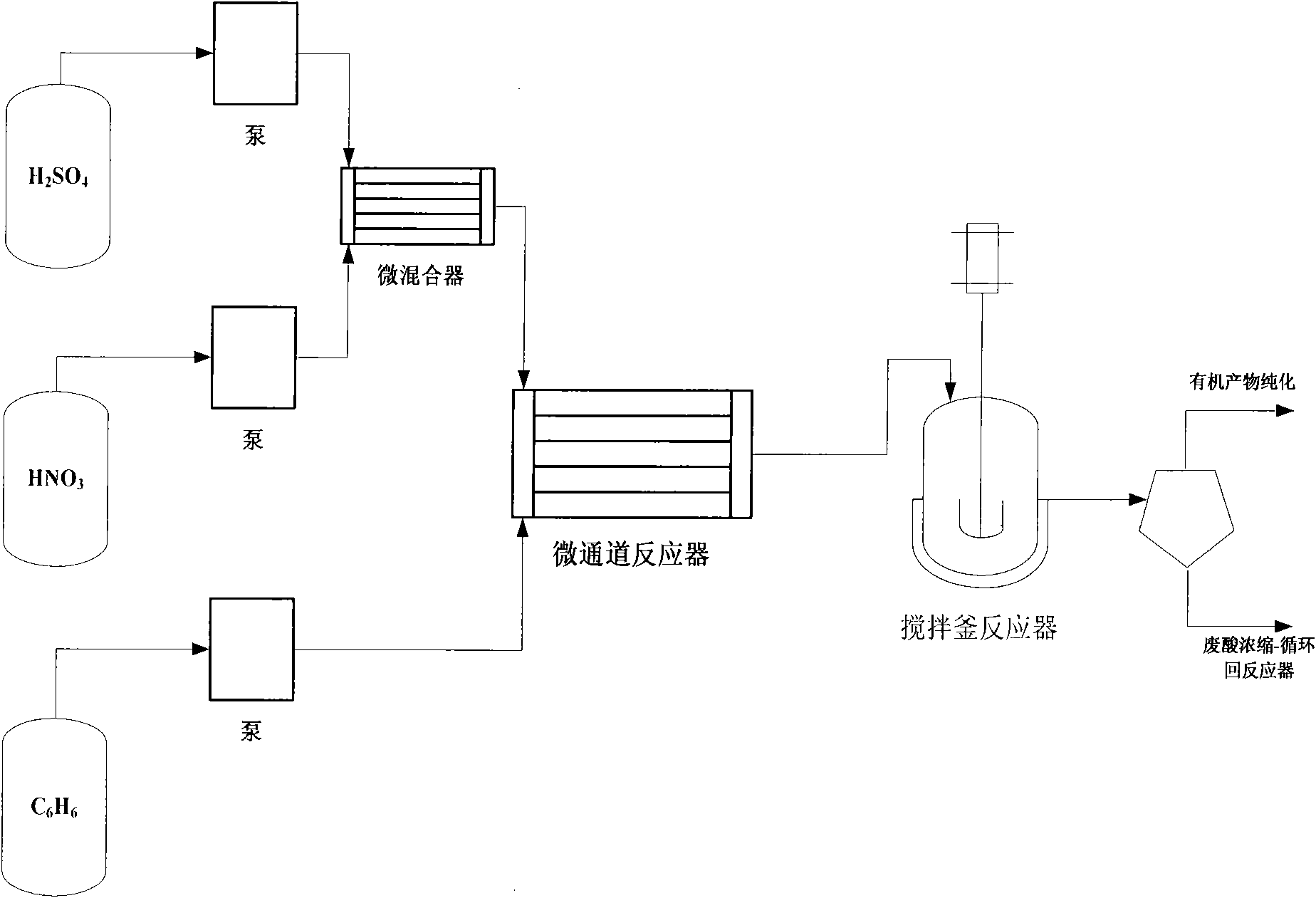
The invention relates to a combined nitrification method for synthesizing nitrobenzene and a combined device of a micro-reactor and a stirring reactor. During the synthesis, benzene and mixed acid of nitric acid and sulfuric acid are taken as initial materials, are conveyed to two inlets of the micro-reactor through a metering pump respectively, are premixed in micro-channels, and perform partialnitration reaction at the same time; and a first-stage nitration product with high dispersion state is produced at an outlet of the micro-reactor. The first-stage nitration product continuously flowsinto a stirred tank nitration reactor to continue to react. The method and the device perform continuous operation under the condition of the mixed acid with high water content and have safe process,the conversion rate of the benzene is 99.85 percent (mol), and the selectivity of a product, namely the nitrobenzene is 99.8 percent (mol).
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for the production of nitrobenzene
ActiveUS7326816B2Simple and economical for productionHigh purityElectrolysis componentsOrganic compound preparationBenzeneElectrophoresis
Nitrobenzene is produced and then purified using an acidic wash, an alkaline wash, a neutral wash, subjecting a dispersion formed in the neutral wash to electrophoresis to separate water and benzene from the nitrobenzene and recover purified nitrobenzene.
Owner:COVESTRO DEUTSCHLAND AG
Functionalized ionic liquids, and methods of use thereof
InactiveUS20080112866A1Group 5/15 element organic compoundsCarboxylic acid esters preparationChemical reactionSide chain
One aspect of the present invention relates to ionic liquids comprising a pendant Bronsted-acidic group, e.g., a sulfonic acid group. Another aspect of the present invention relates to the use of an ionic liquid comprising a pendant Bronsted-acidic group to catalyze a Bronsted-acid-catalyzed chemical reaction. A third aspect of the present invention relates to ionic liquids comprising a pendant nucleophilic group, e.g., an amine. Still another aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to catalyze a nucleophile-assisted chemical reaction. A fifth aspect of the present invention relates to the use of an ionic liquid comprising a pendant nucleophilic group to remove a gaseous impurity, e.g., carbon dioxide, from a gas, e.g., sour natural gas.
Owner:UNIV OF SOUTH ALABAMA
Nitration method for synthesizing dinitrotoluene in one step, and microchannel reactor
ActiveCN101544567AHigh selectivityIncrease space-time productivityProductsReagentsMass ratioNitration
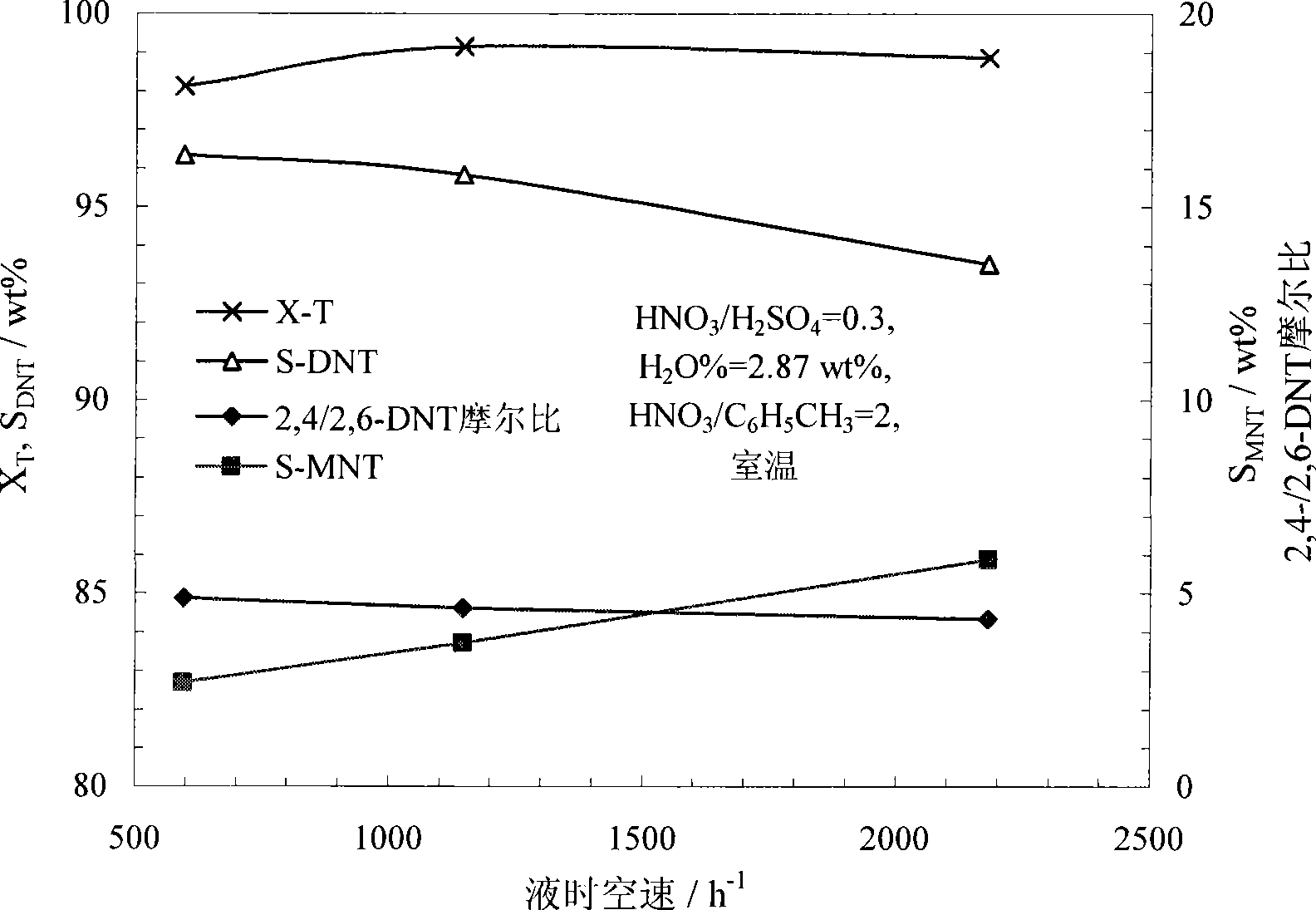
The invention relates to one-step dinitrotoluene synthesis in a microchannel reactor. The method comprises the steps of taking toluene and mixed acid of nitric acid / sulfuric acid (n / s) as initial reaction materials, inputting material flow synchronously into a microreactor through a metering pump and finishing mixed mass transfer and reaction in a reaction channel. The substance ratio of n to s in the reactive mixed acid is 0.1 to 1.0, and the mass ratio of water is less than 15 percent. The liquid hourly space velocity in the reactor is 500 to 50,000 h<-1>. The microchannel reactor adopts a stainless steel flat-plate structure which comprises fixed pressing-sealing plates, a microchannel plate, a raw-material inlet, a product outlet, as well as a temperature-control pore channel and a thermal-couple measurement-control jack which are positioned on two sealing plates. The method is continuously operated under a technological condition with medium / strong nitrating acid, and is safe in process. The conversion rate of the toluene in the reactor at an operation temperature between normal temperature and 80 DEG C is higher than 98 percent, and the yield of dinitrotoluene is higher than 95 percent, wherein the ratio of two main isomer components, namely 2,4-dinitrotoluene and 2,6-dinitrotoluene, is more than 4 and can be nearly 5 under optimum conditions.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for continuous preparing diruitro methylbenzele and apparatus thereof
ActiveCN1793106AEnsure and improve qualityReduce quota consumptionNitro compound preparationTolueneSpent acid
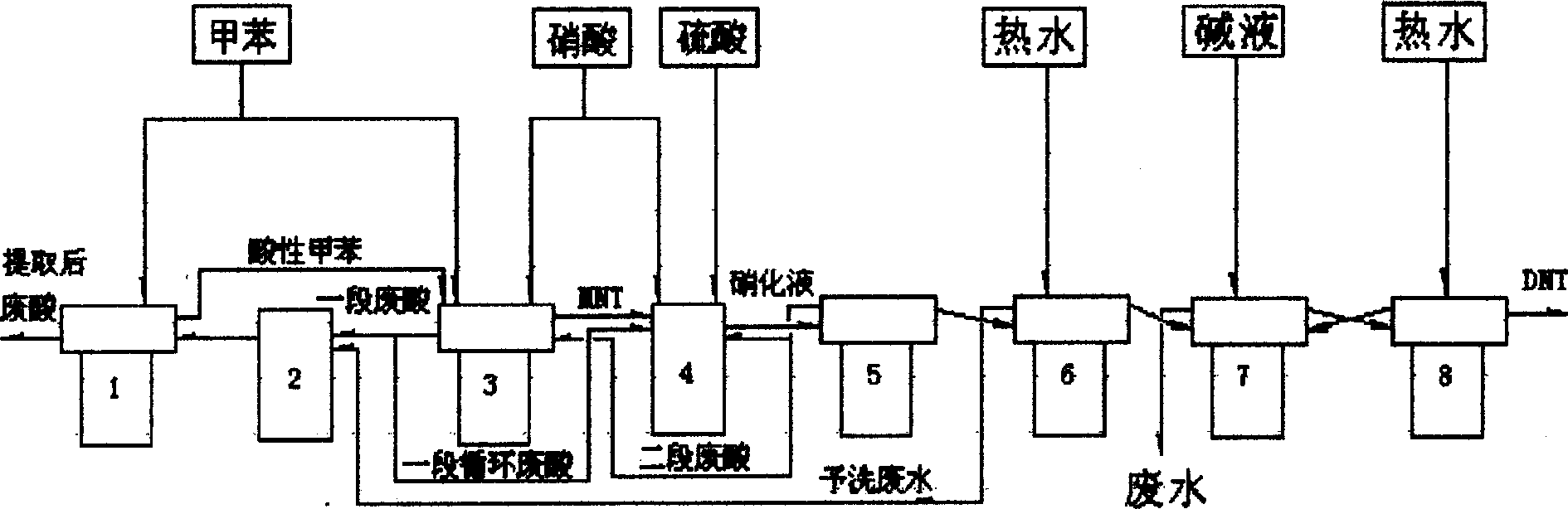
The invention relates to a method to continuously produce dinitrotoluene and the device that is suited to the method. The method adopts two steps to make dinitrotoluene and organic phase and spent acid phase are separated. The acid phase is cycling used in the first and the second stage; toluene, nitric acid and mixture acid are added into the center of draft tube of reactor synchronously, mixing fully, cycling in the center and outside of the draft tube; after fully reacting, the taking gravity settling separation in the separator where spent acid cycling device, stop cycling device, and disc type separating device are installed.
Owner:GANSU YINGUANG CHEM IND GRP CO LTD
Oxidation process using microchannel technology and novel catalyst useful in same
InactiveUS7226574B2Maximize contactMethane captureChemical/physical/physico-chemical microreactorsContact timeCarbon oxide
Owner:VELOCYS INC
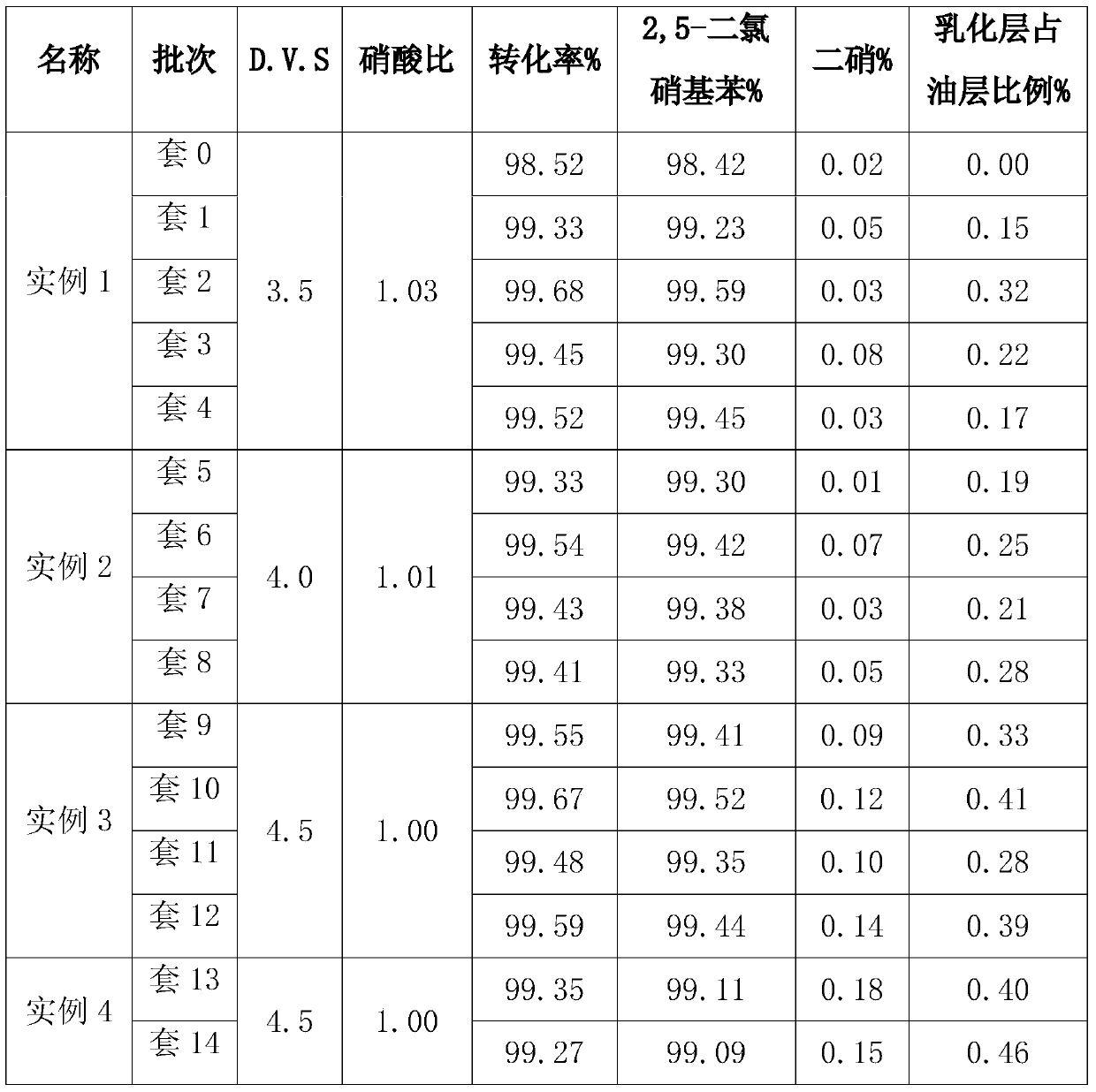
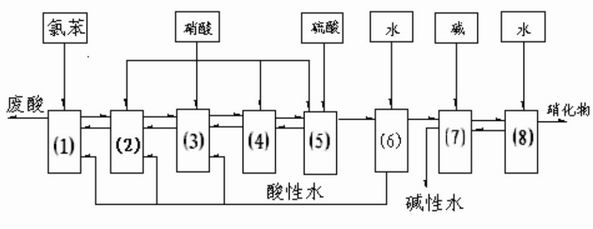
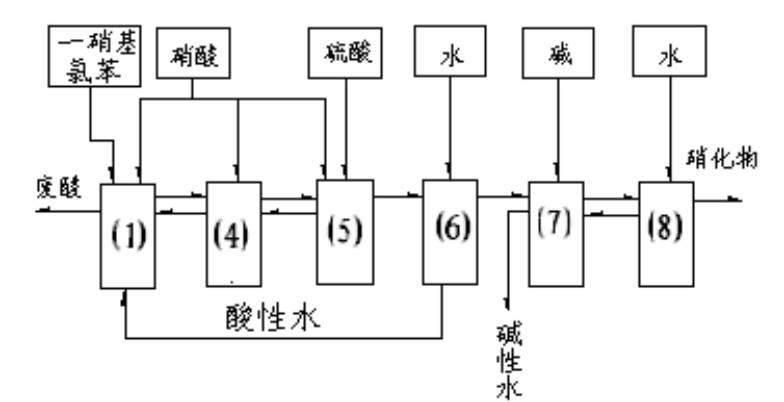
Method capable of recycling acids for preparing 2,5-dichloronitrobenzene (DCNB) through continuous nitration
ActiveCN104649910AReduce labor intensitySimple processNitro compound preparationContinuous reactorEmulsion
The invention relates to a production method for preparing 2,5-dichloronitrobenzene (DCNB) through continuous nitration, which particularly realizes the recycling of waste acids in the preparing process. The invention provides a technological program capable of recycling acids for preparing 2,5-dichloronitrobenzene (DCNB) through the continuous nitration, which is characterized in that sulfuric acid and nitric acid are prepared into a mixed acid, the mixed acid and paradichlorobenzene are simultaneously fed into a three-stages kettle (ring) type continuous reactor to carry out nitration reaction, a nitration reaction solution discharged form the third-stage kettle (ring) type continuous reactor is stood for layering so as to obtain a nitrifying oil layer and a nitrifying waste acid, and the nitrifying oil layer is subjected to alkali cleaning, water washing and light component removal so as to obtain a 2,5-DCNB finished product; paradichlorobenzene is added into the nitrifying waste acid to extract organic matters in the acid layer, residual HNO3 in the acid layer is consumed, after the extraction is completed, the obtained product is layered so as to obtain an extraction oil layer and an extracted residual waste acid, the extraction oil layer is used for next-batch nitrification, the extracted residual waste acid after being concentrated and nitric acid are prepared into the waste acid for next-batch nitrification, a small amount of emulsion layer is produced in the process of recycling, and the emulsion layer after being combined with the nitrifying oil layer is subjected to alkali cleaning and water washing.
Owner:JIANGSU YANGNONG CHEM GROUP +2
Method for continuously preparing dinitrochlorobenzene
ActiveCN102070457AIncrease profitIncrease production capacityNitro compound preparationNitrateChlorobenzene
The invention discloses a method for continuously preparing dinitrochlorobenzene. Multiple dinitration reactors are connected in series for reaction, and mononitrochlorobenzene is continuously added to the first dinitration reactor, and continuously flows through all the dinitration reactors and flows out; a mixed acid nitrating agent is prepared from 75-85% of sulfuric acid, 2-7% of nitric acid and 5-15% of water; the reaction temperature is 40-95 DEG C; and each dinitration reactor is provided with a separation device for separating an organic phase from an inorganic phase, and the inorganic phase is returned into the reactor, thereby keeping the inorganic phase and the organic phase in the required proportion. In the invention, the dinitrochlorobenzene is prepared by continuous reactions, and the reactions are continuously carried out and sequentially completed; chlorobenzene is used for extracting nitrates and nitric acid in an acid phase, thereby lowering the content of the nitric acid in the acid phase and recycling the nitrates in the acid phase; by the invention, the dinitrochlorobenzene can be continuously prepared from two raw materials (chlorobenzene and p-nitrochlorobenzene) by using one set of devices; and the invention has the advantages of low raw material consumption and high production capacity.
Owner:LIANYUNGANG DIPU CHEM
Process of producing nitrobenzether aminobenzether amidobenzether from chlorobenzene
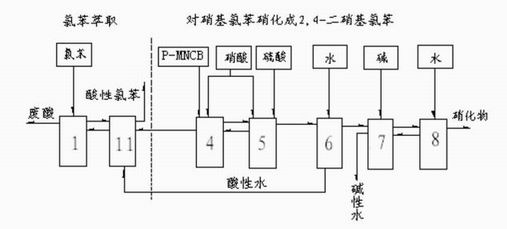
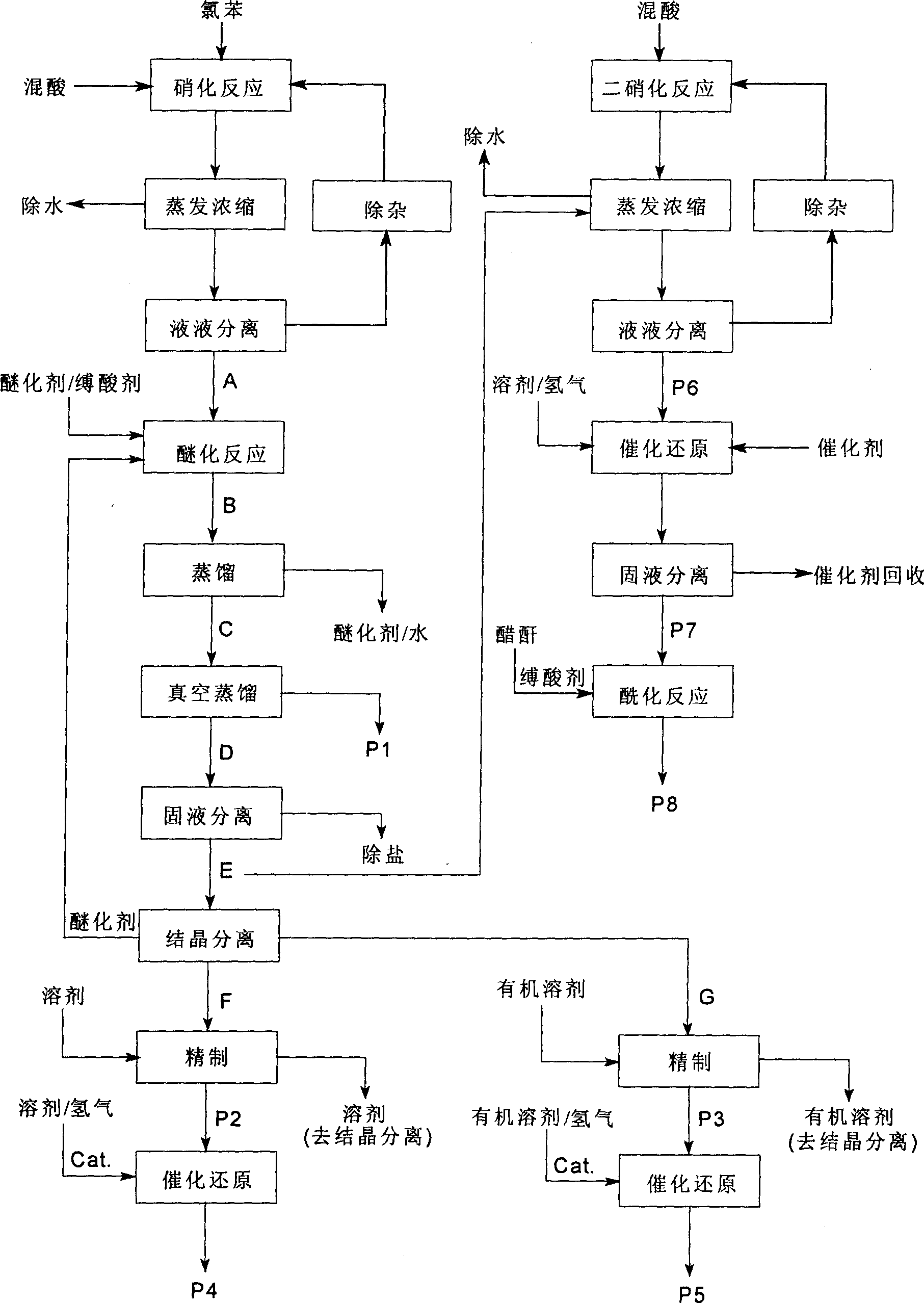
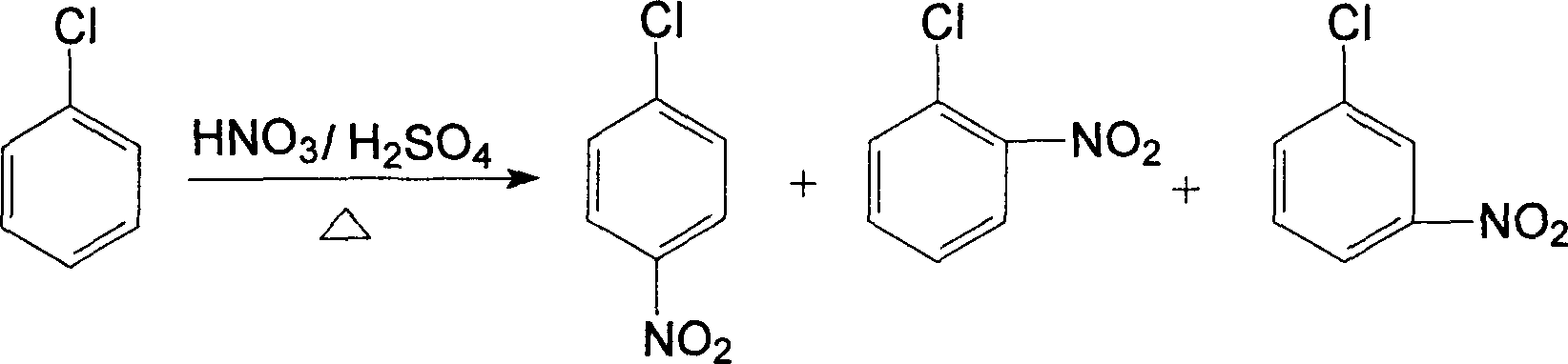
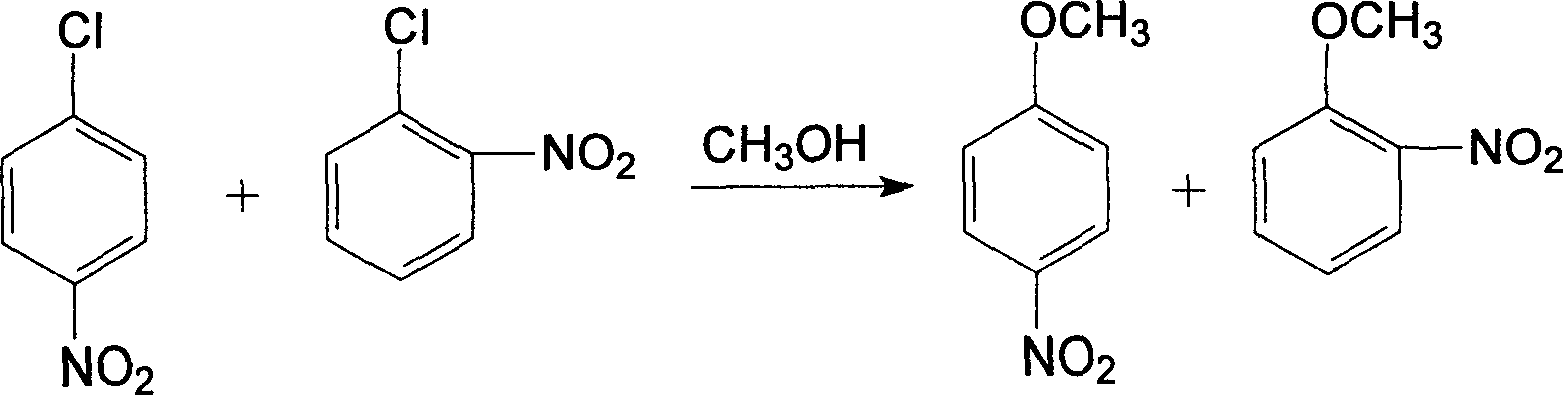
InactiveCN1861562AAvoid conditionsAvoid investment in equipmentOrganic compound preparationCarboxylic acid amides preparationO-nitrochlorobenzeneChloride salt
A process for preparing nitrophenylether, aminophenylether and amidophenylether between chlorobenzene and acid mixture removing water, liquid-liquid separation to obtain organic phase consisting of o-, p- and meta-nitro chlorobenzene compounds, etherifying said mixture, recovering etherifying agent, vacuum distilling, separating and refining meta-nitro chlorobenzene, removing chloride salt generated in etherification, crystallizing separation, recrystallizing p- and o-nitro phenylether compounds, catalytic hydrogenating reaction, nitrifying reaction to obtain 2,4-drinitro phenylether, catalytic hydroreducing reaction to obtain 2,4-diamino phenylether, and aceylating reaction to obtain 2-amino-4-acetylamino phenylether.
Owner:CHANGZHOU JIASEN CHEM +1
Synthesis system, rubber chemical substance for tires, synthetic rubber for tires, and pneumatic tire
ActiveUS20130090445A1Reduce the amount requiredEfficient synthesisMolecular sieve catalystsMolecular sieve catalystAlcoholAniline
The present invention provides a synthesis system that can synthesize aniline and / or styrene efficiently, a synthesis system that can synthesize butadiene (1,3-butadiene) efficiently, a rubber chemical for a tire which is synthesized from the aniline obtained by the synthesis system, a synthetic rubber for a tire which is synthesized from the styrene and / or butadiene obtained by the synthesis systems, and a pneumatic tire produced using the rubber chemical for a tire and / or the synthetic rubber for a tire. The present invention relates to a synthesis system for synthesizing aniline and / or styrene from an alcohol having two or more carbon atoms via an aromatic compound.
Owner:SUMITOMO RUBBER IND LTD
Use of bismuth nitrate and iron nitrate as nitrification agent in aromatic compound nitrification
InactiveCN1854114AChemically stableLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationNitrateNitrate agent
Owner:北京清华紫光英力化工技术有限责任公司
Process for the manufacture of nitrated hydrocarbons
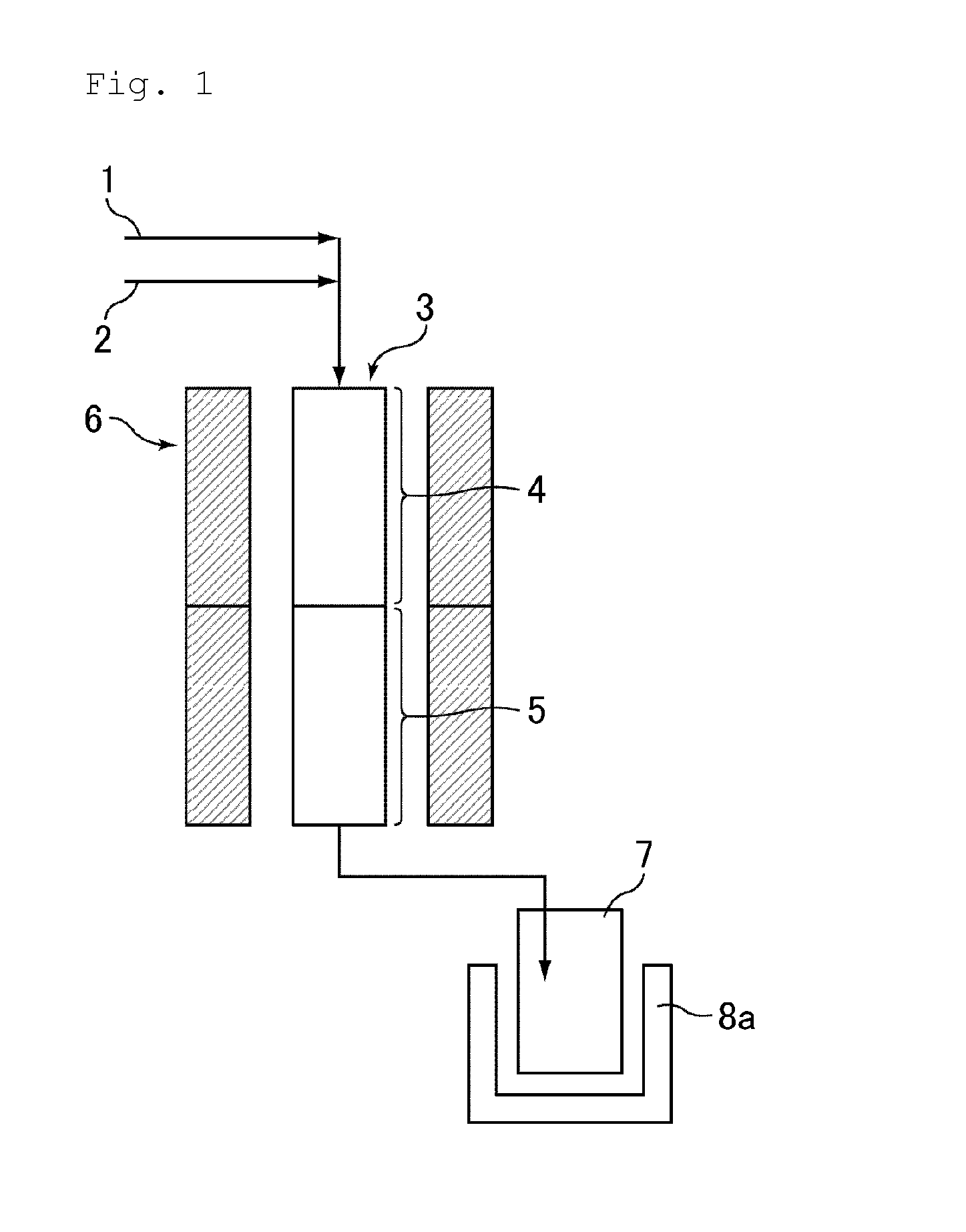
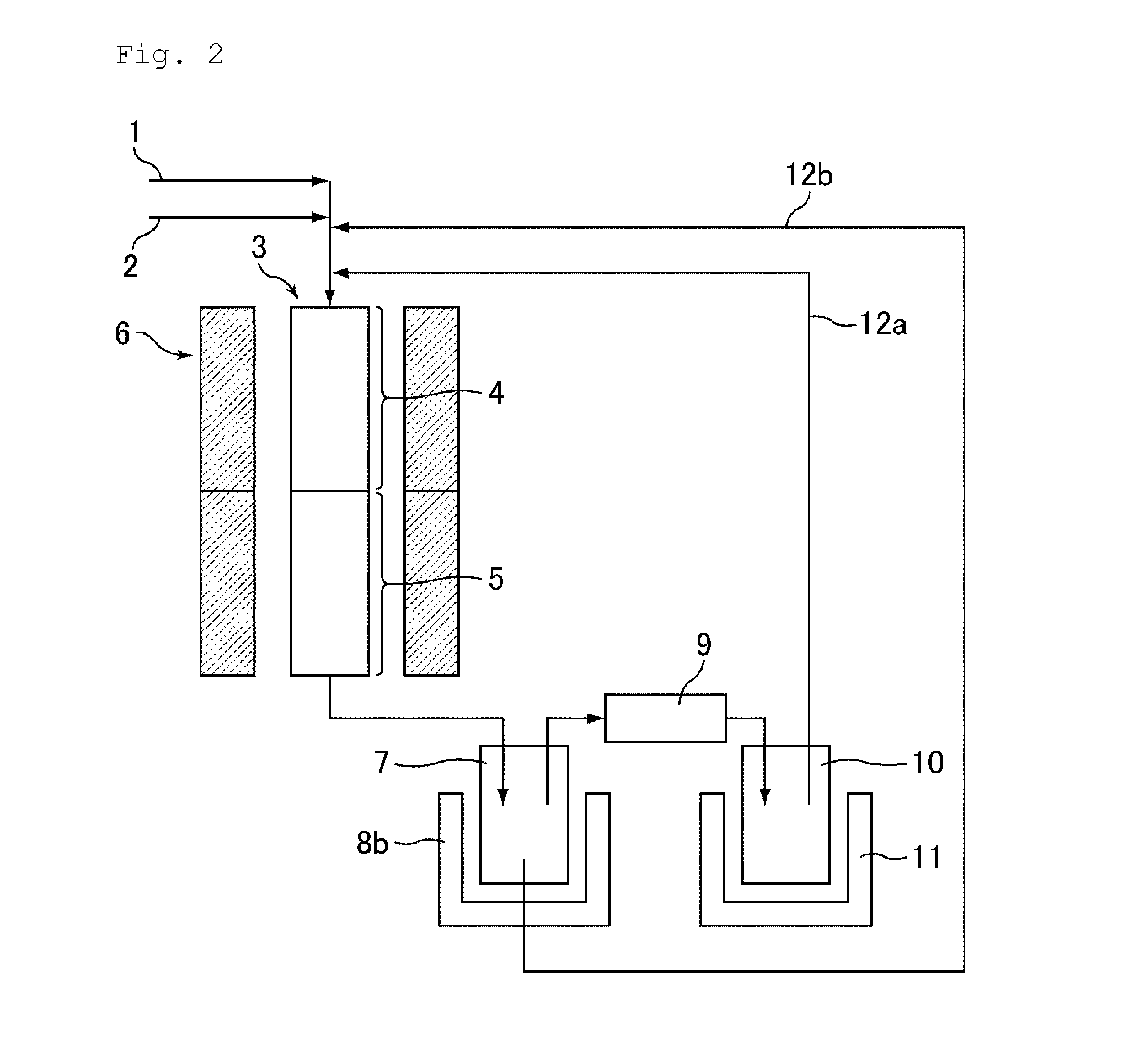
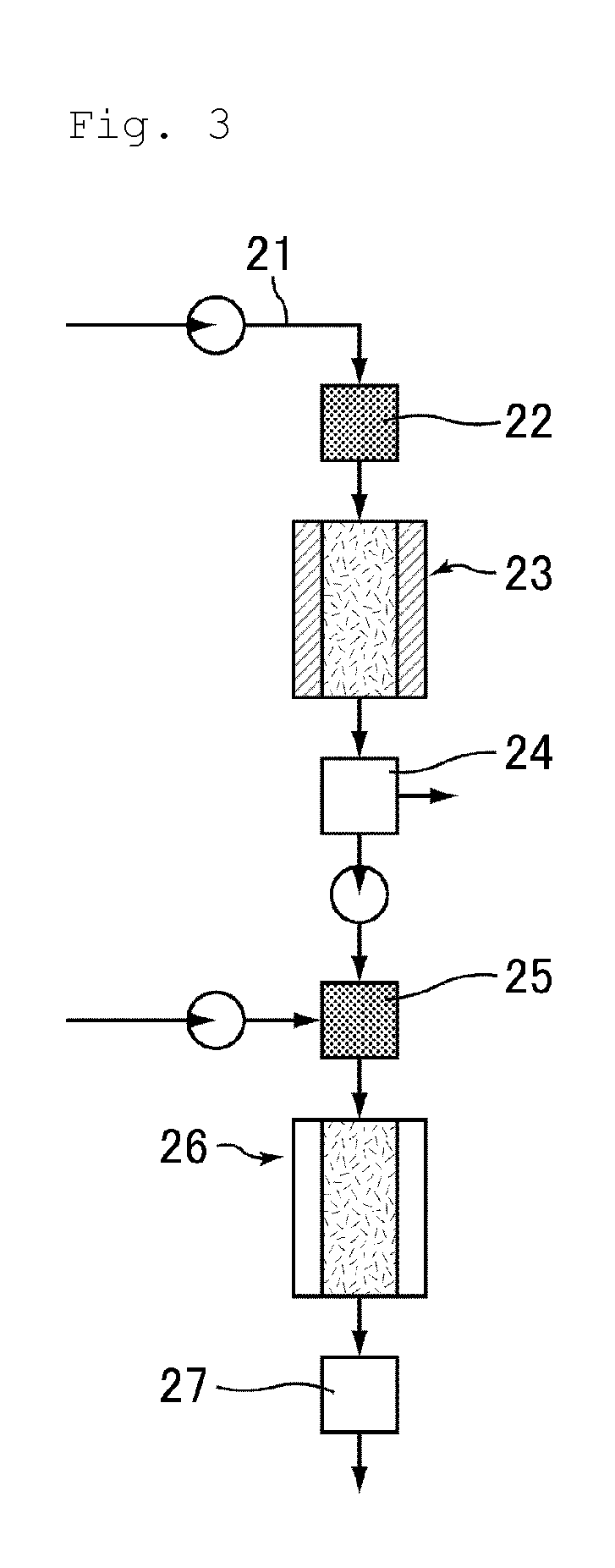
Provided is a process for making nitrated hydrocarbons by reacting aqueous nitric acid with a hydrocarbon feedstock and a carboxylic acid under specific reaction conditions.
Owner:ANGUS CHEM CO
Method for manufacturing nitrobenzole
ActiveCN101012169AReduce consumptionHigh purityOrganic compound preparationNitro compound preparationBenzeneElectrophoresis
Owner:COVESTRO DEUTSCHLAND AG
Recovery of nitrating acid mixtures from nitration processes
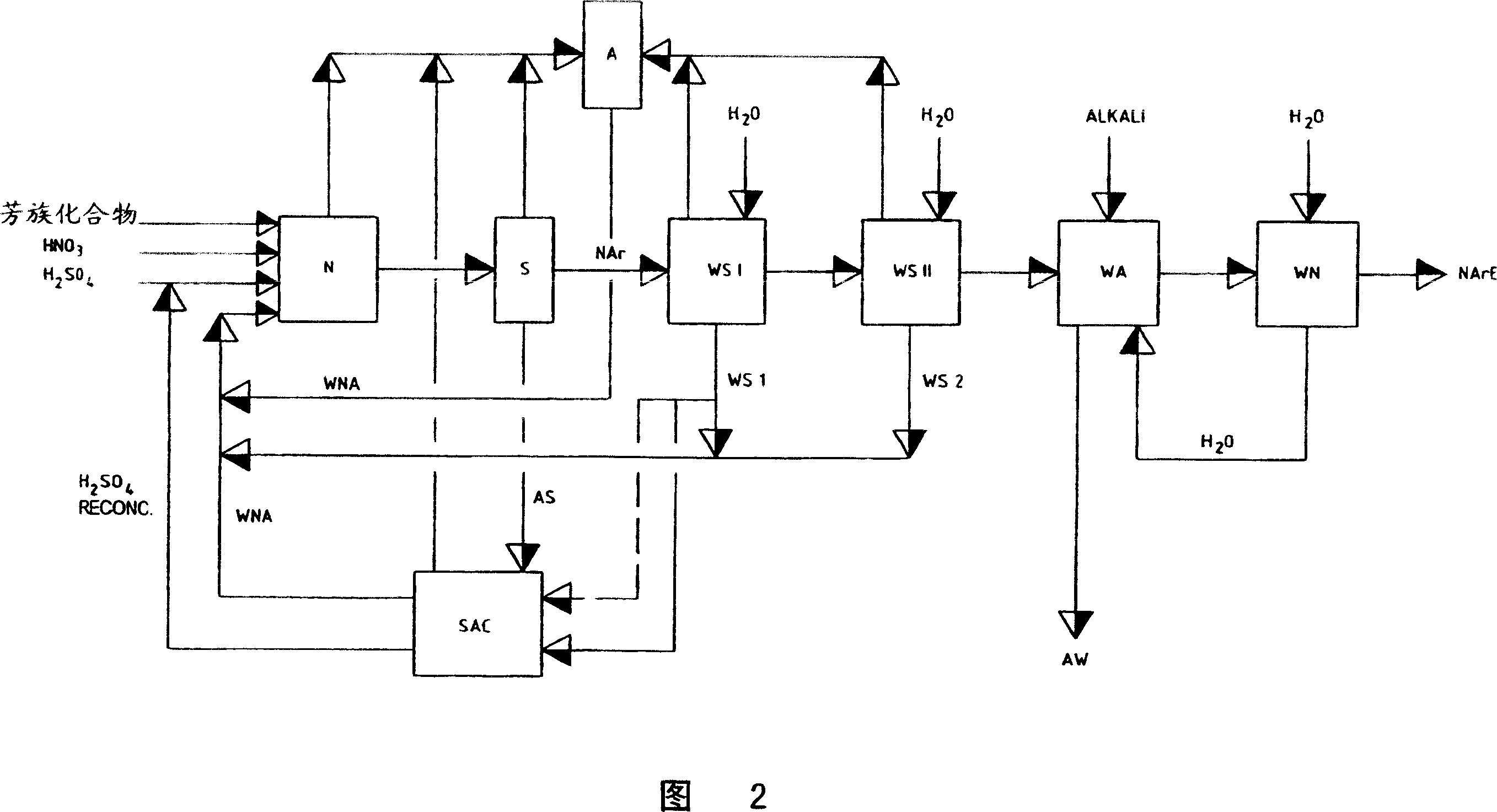
ActiveUS7470826B2Avoid repetitionOrganic compound preparationNitro compound preparationHigh concentrationWastewater
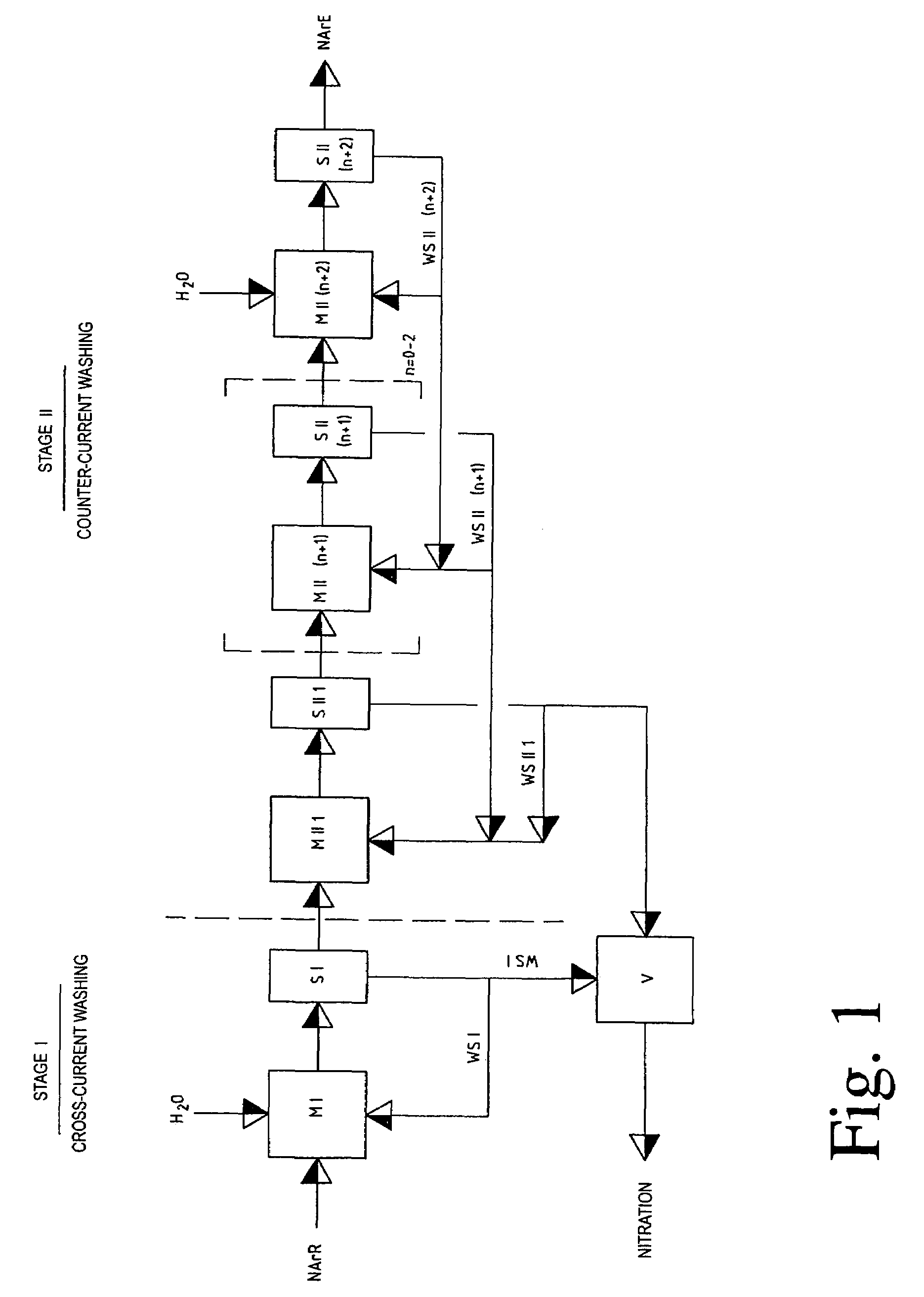
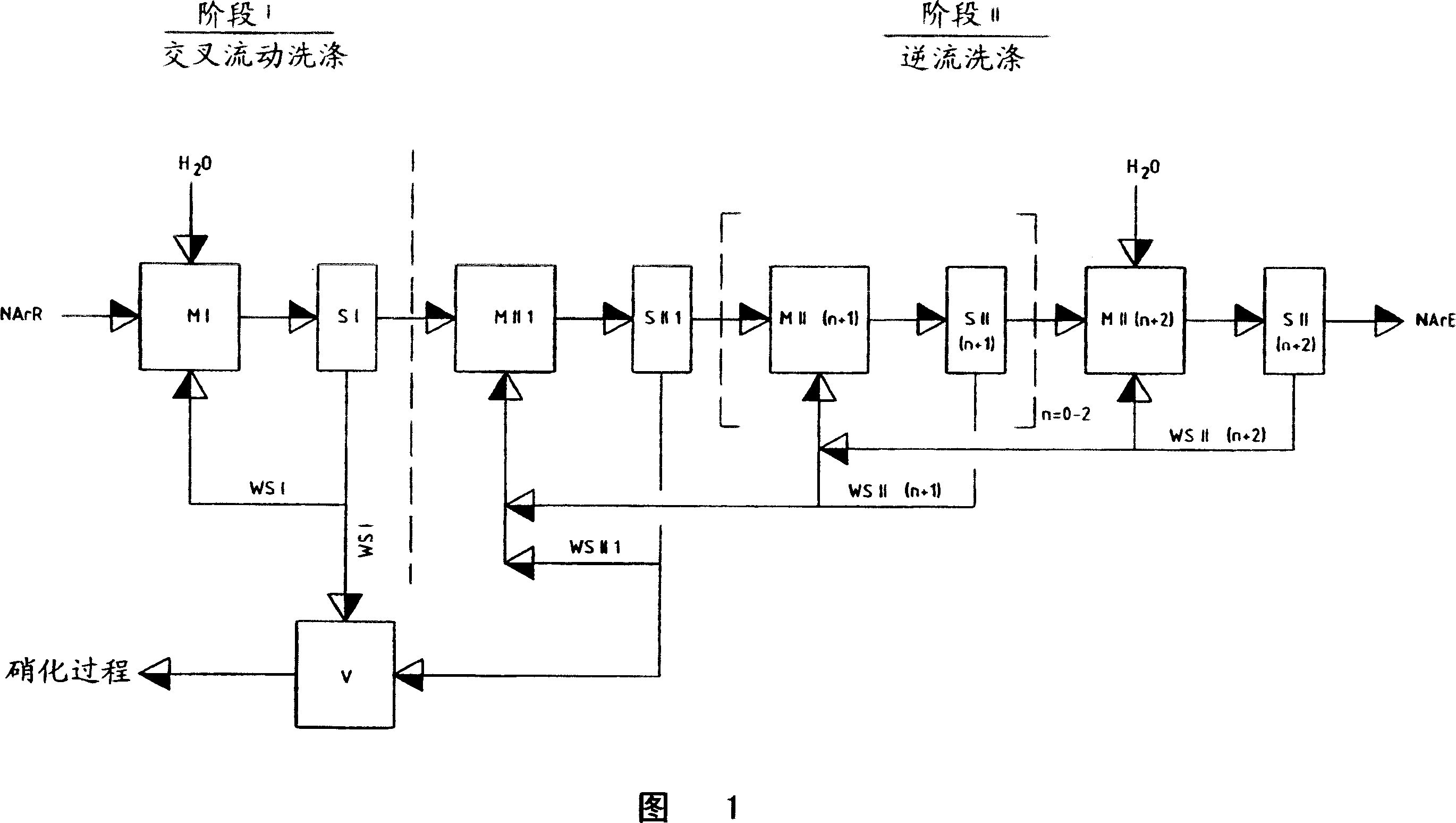
The invention relates to a process for removing and recovering nitrating acid mixtures, in particular nitric acid, sulphuric acid and oxides of nitrogen, from the nitrated crude products occurring in the nitration of nitratable aromatic compounds after the nitrating acid has been separated off, by acidic scrubbing by means of a multistage extraction process, the extraction process comprising a cross-current extraction with downstream countercurrent extraction. The process permits essentially complete recovery of the abovementioned acids, including the oxides of nitrogen, in high concentrations, so that they can be recycled to the nitration and no longer pollute the wastewater.
Owner:JOSEF MEISSNER
Sol-gel derived porous microcomposite of perfluorinated ion-exchange polymer and metal oxide
Porous microcomposites have been prepared from perfluorinated ion-exchange polymer and metal oxides such as silica using the sol-gel process. Such microcomposites possess high surface area and exhibit extremely high catalytic activity.
Owner:EI DU PONT DE NEMOURS & CO
Recovery of the nitration acid mixtures from nitration processes
ActiveCN1951802AAvoid repetitionOrganic compound preparationSulfur-trioxide/sulfuric-acidProduction lineNitration
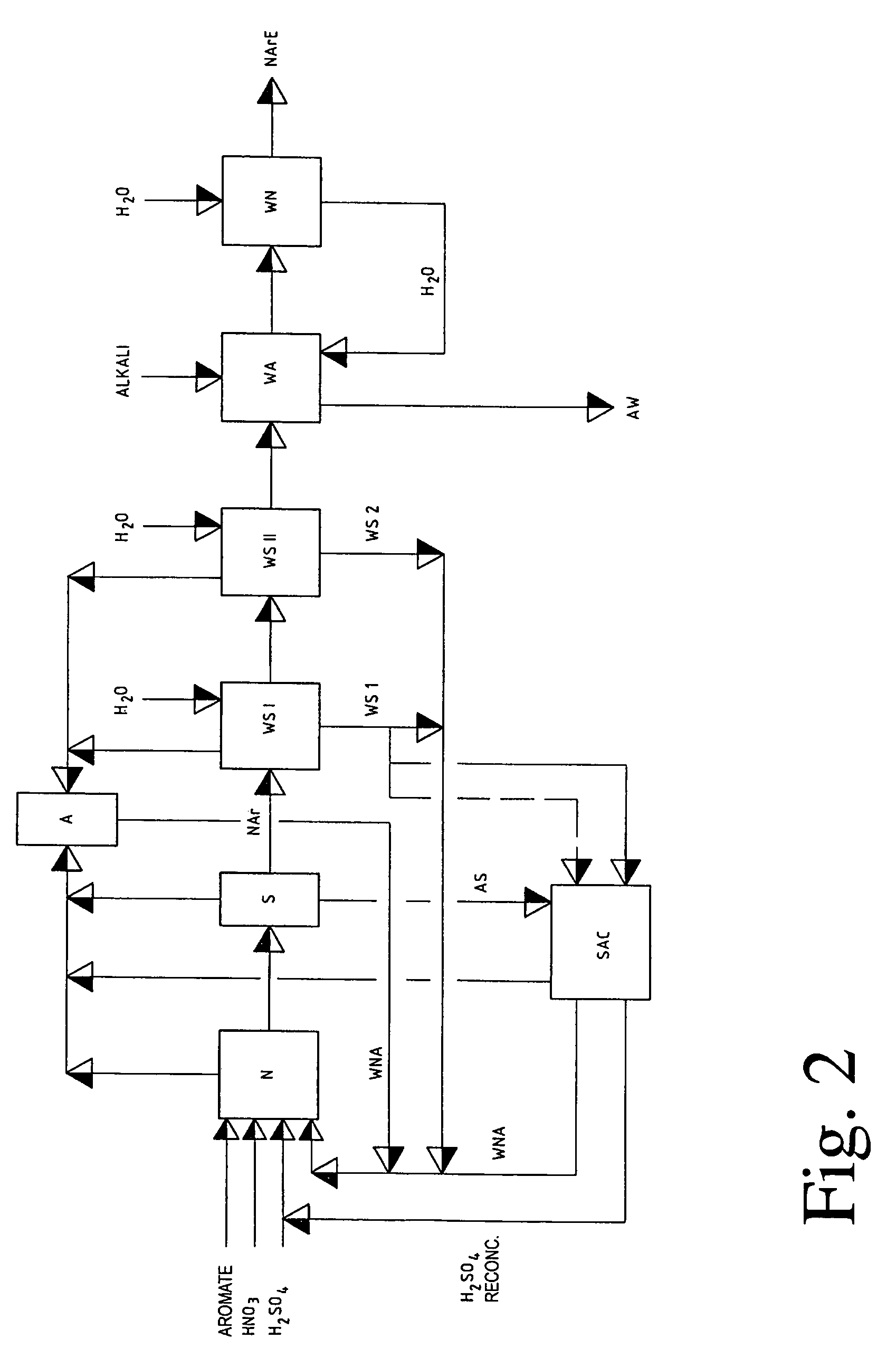
Removing and recovering nitrating acid mixtures of nitric acid, sulphuric acid and oxides of nitrogen, from the nitrated crude products occurring in the nitration of nitratable aromatic compounds, after the nitrating acid has been separated off, by a multistage extraction process involves one stage of a cross-flow extraction and another stage of a countercurrent extraction. An independent claim is also included for production plant for nitrating nitratable aromatic compounds with subsequent purification of the nitrated products, including removal and recovery of nitrating acid mixtures of sulphuric acid, nitric acid and oxides of nitrogen, comprises: (a) a nitrating unit for nitrating nitratable aromatic compounds, having at least one appropriate reaction containers for carrying out the nitration reactions; and (b) arranged in the production line downstream of the nitrating unit, a unit for carrying out acidic scrubbing by units of extraction and is comprising a cross-flow extraction unit for carrying out acidic scrubbing of nitrated crude products by units of cross-flow extraction, and in the production line downstream of the cross-flow extraction unit, a countercurrent extraction unit for carrying out acidic scrubbing of the nitrated crude products, scrubbed beforehand by units of cross-flow extraction, by units of countercurrent extraction.
Owner:JOSEF MEISSNER
Process for the manufacture of nitropropanes
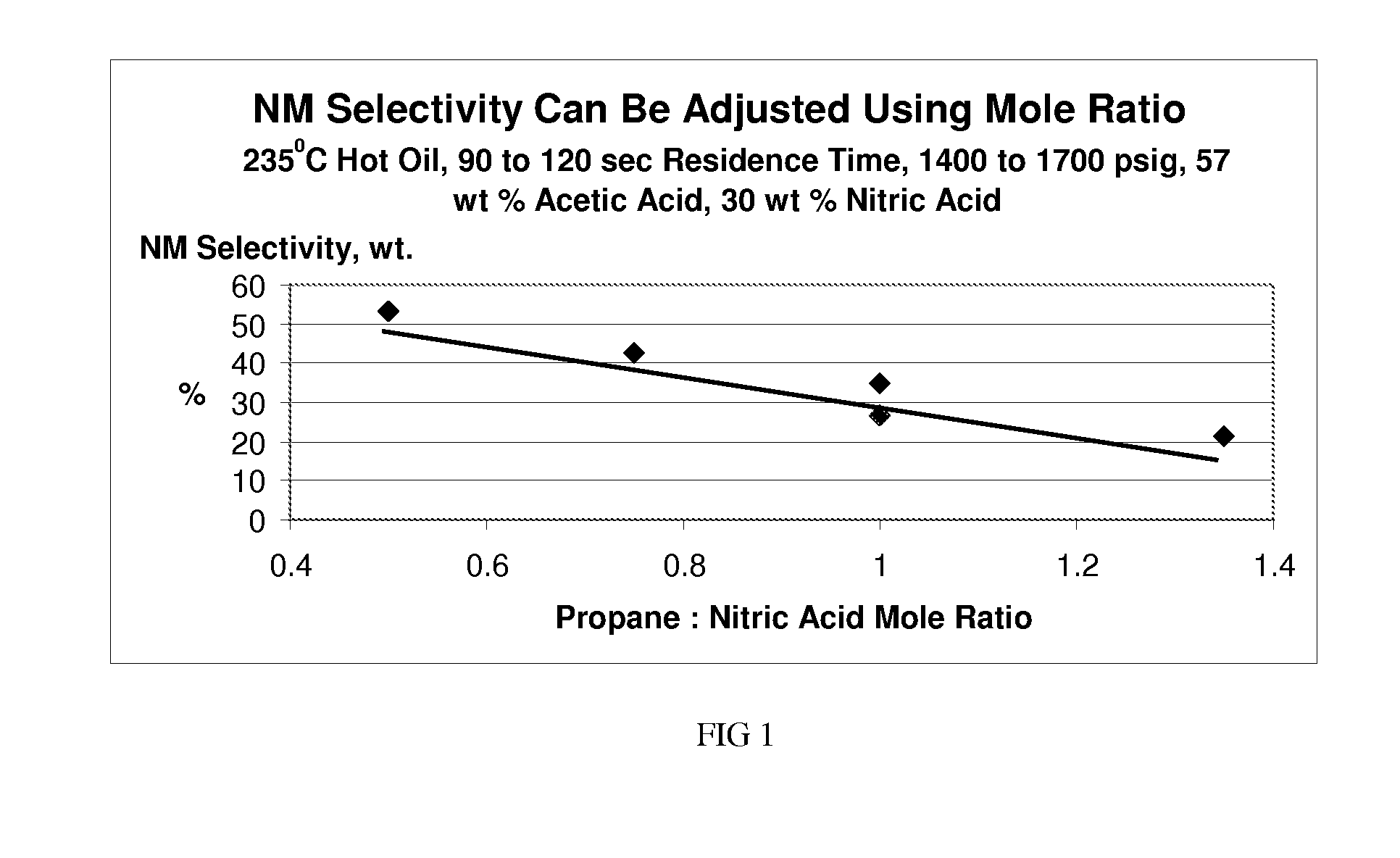
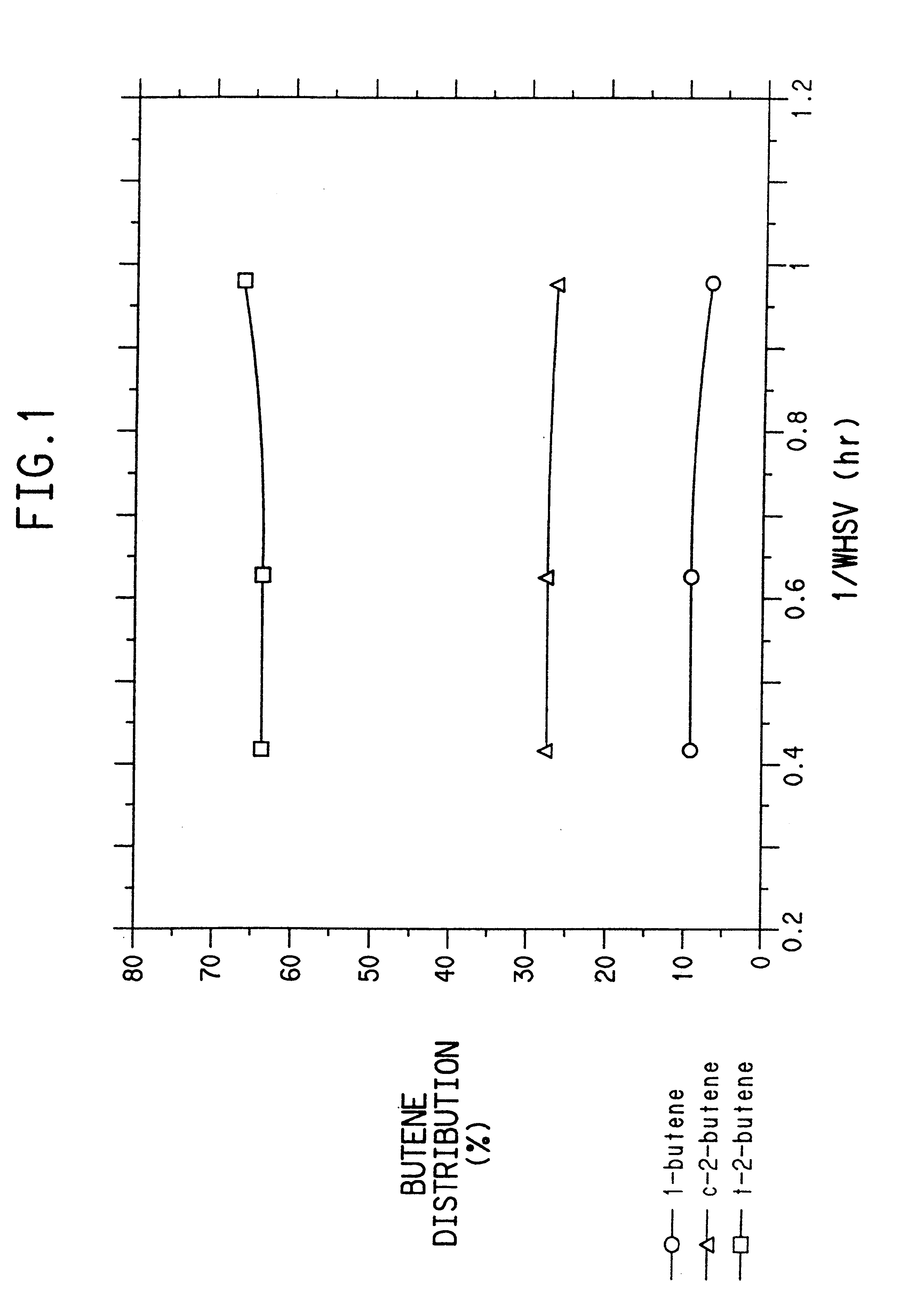
ActiveUS20110028731A1Increase conversionsHigh yieldAmino compound purification/separationOrganic compound preparationNitrationPropane
Provided is a process for the formation of 2-nitropropane and / or 2,2-dinitropropane by the nitration of propane with dilute nitric acid.
Owner:ANGUS CHEM CO
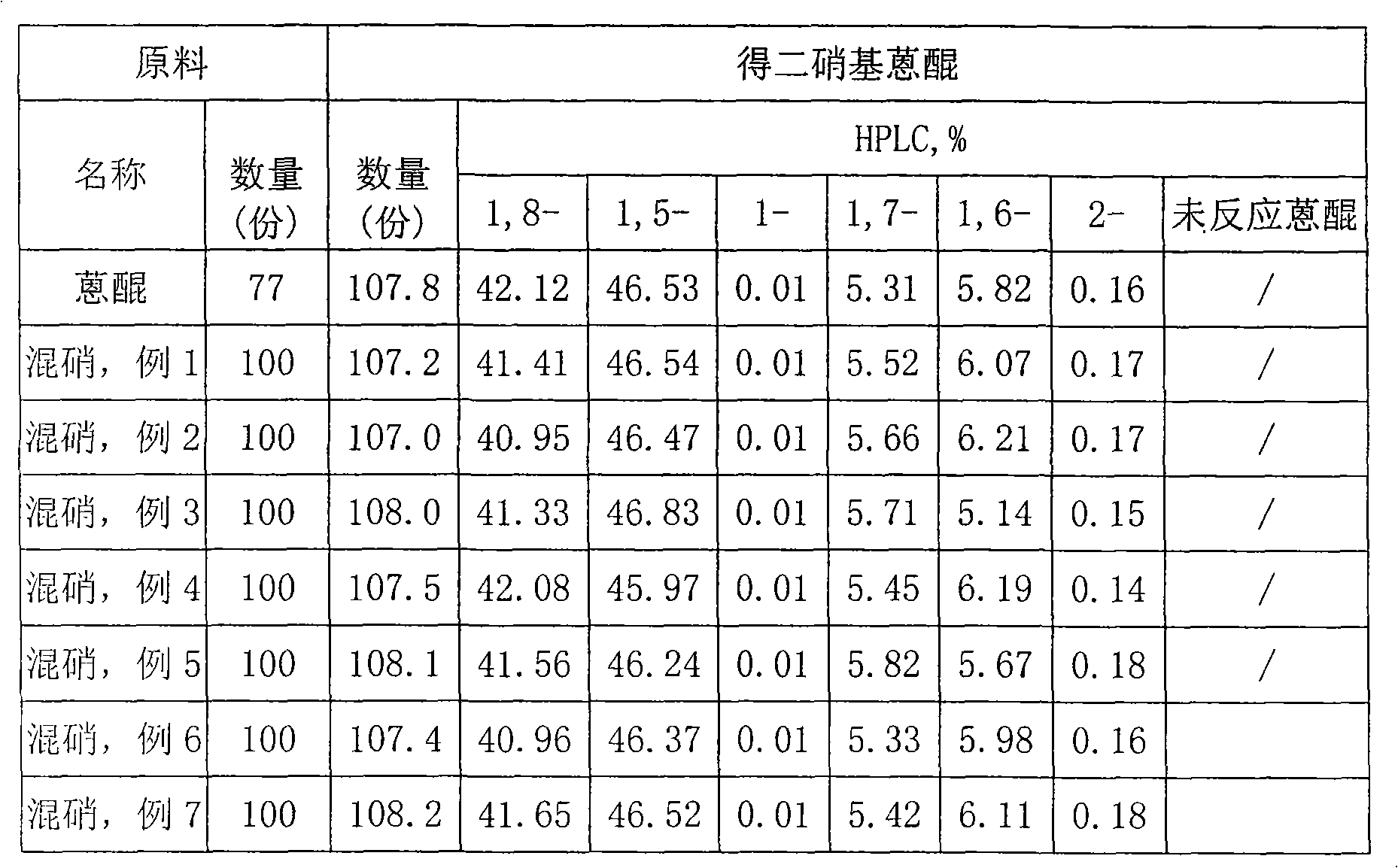
Method for treating waste residue for producing 1-aminoanthraquinone into raw material for producing 1,5(1, 8)-dinitroanthraquinone
InactiveCN101591249ASolve the problem of resource utilizationSimple processOrganic compound preparationNitro compound preparationEconomic benefitsNitration
The invention discloses a method for treating waste residue for producing 1-aminoanthraquinone into a raw material for producing 1,5(1, 8)-dinitroanthraquinone. The method treats and removes isomer containing beta-nitroanthraquinone in the waste residue for producing the 1-aminoanthraquinone to obtain mixed nitroanthraquinone (mixed niter for short), and the mixed niter mainly contains the 1,5(1, 8)-dinitroanthraquinone and the 1-aminoanthraquinone and can be used as the raw material for producing the 1,5(1, 8)-dinitroanthraquinone. The method comprises the following steps: crushing 100 portions by mass of dry waste residue for producing the 1-aminoanthraquinone into powder, adding 400 to 500 portions by mass of sulfuric acid or nitric acid into the powder, heating the mixture to between 50 and 65 DEG C, stirring the mixture for 1 to 2 hours, and filtering, washing and drying the mixture to obtain the mixed niter which can be directly used as the raw material for producing the 1,5(1, 8)-dinitroanthraquinone, wherein the 1,5(1, 8)-dinitroanthraquinone can be produced through dinitration reaction of anthraquinone according to the method of CN101423477A, and bulk commodity dye disperse blue 2BLN (C.I. disperse blue 56) is further produced. The method has simple process; and the obtained product has stable quality and obvious economic benefit.
Owner:JIANGSU JIHUA CHEMICAL CO LTD
Nitration of activated aromatics in microreactors
InactiveCN101400628AHigh yieldHigh mass fluxChemical/physical/physico-chemical microreactorsNitro/nitroso group formation/introductionMicroreactorNitration
The invention relates to the nitration of aromatic or heteroaromatic compounds, wherein an activated aromatic or heteroaromatic compound and a nitrating agent, in the presence, if desired, of a solvent, are mixed intensely in a microreactor, and wherein the proportion of the nitrating agent to the activated aromatic or heteroaromatic compound, the concentration of nitrating agent in the reaction mixture, and the temperature are selected at levels such that the nitration begins autocatalytically, and wherein the nitration product is obtained after leaving the microreactor and, if desired, after an after-reaction time outside the microreactor.
Owner:LONZA LTD
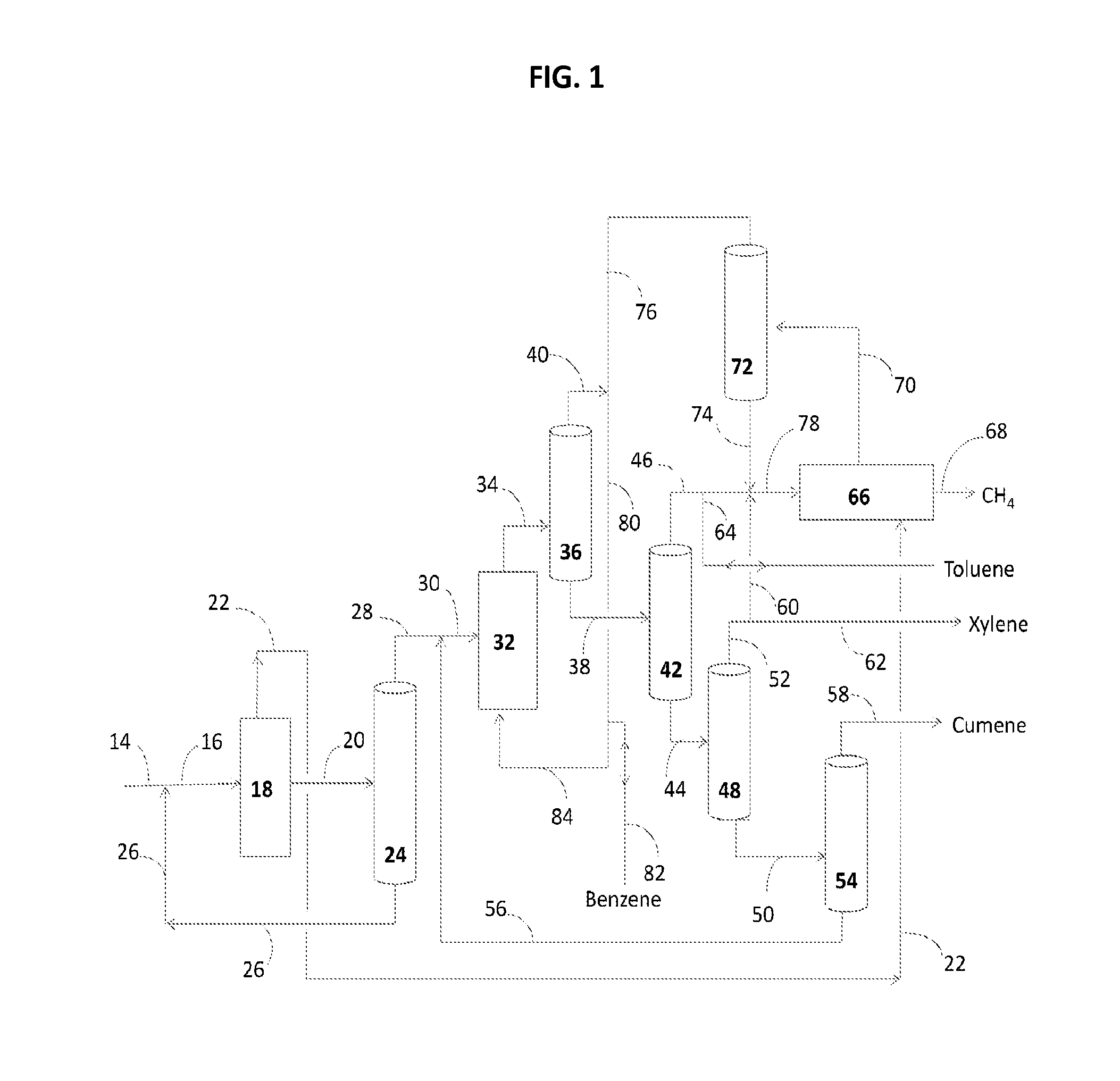
Production of renewable aromatic compounds
InactiveUS20130130345A1Hydrocarbon by isomerisationOrganic chemistry methodsBenzoic acidCyclohexanone
The invention provides a process for producing a variety renewable aromatic compounds such as benzene, toluene, xylenes, and cumene, as well as compounds derived from these including, for example, aniline, benzoic acid, cresol, cyclohexane, cyclohexanone, phenol and bisphenol A, toluene di-isocyanate, isophthalic acid, phthalic anhydride, terephthalic acid and dimethyl terephthalate. The invention also provides for renewable forms of these aromatic compounds.
Owner:JNF BIOCHEM
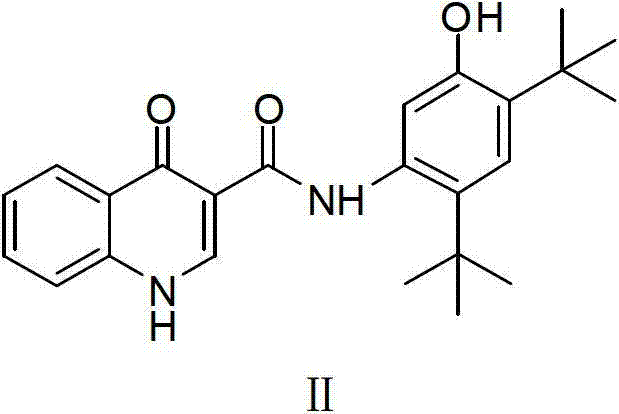
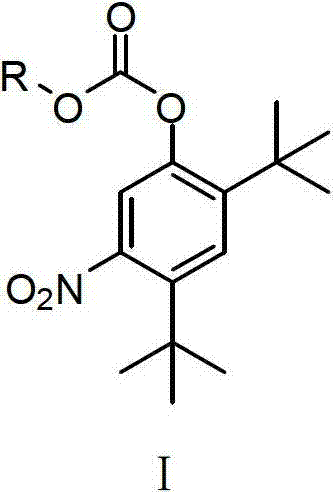
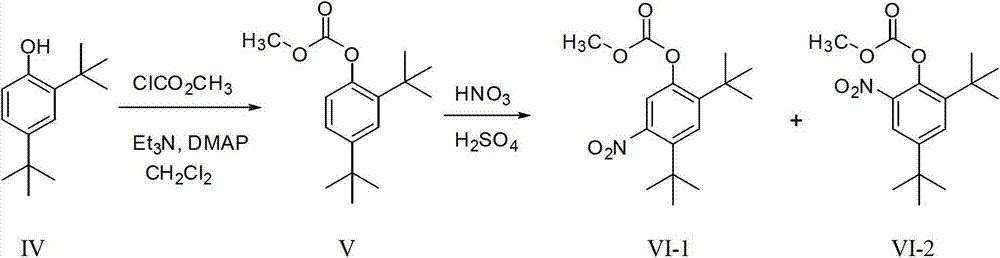
Preparing method for medicament midbody for treating cystic fibrosis
The invention relates to the field of pharmaceutical synthesis, and in particular relates to a preparation method for a medicament Ivacaftor for treating cystic fibrosis. The method is characterized by comprising the following steps of: protecting phenolic hydroxyl by using alkoxycarbonyl by using 2,4-ditertiary butyl phenol as an initial raw material; nitrifying and reducing phenolic hydroxyl; condensing the phenolic hydroxyl with 4-oxo-1,4-dihydroquinolines-3-formyl chloride to obtain carbonic acid (2,4-ditertiary butyl-5-(1,4-dihydro-4-oxoquinoline-3-formamide) phenylester ethyl ester; and finally removing alkoxycarbonyl under an alkali condition to obtain the Ivacaftor. The method can be used for overcoming multiple defects of the existing synthetic method, is simple and easily available in raw materials, convenient to experimentally operate and postprocess, high in yiled, good in product quality and suitable for scale production.
Owner:CHINA PHARM UNIV +1
2-methyl-3-methoxybenzoyl chloride synthesizing process
InactiveCN109384667AImprove separabilitySolve the problem of raw materialsOrganic compound preparationCarboxylic acid esters preparationO-XyleneSynthesis methods
The invention discloses a 2-methyl-3-methoxybenzoyl chloride synthesizing process. According to the present invention, low-cost o-xylene is used as a starting raw material, the product is synthesizedby using a conventional synthesis method comprising nitrification, esterification, reduction, diazotization, methylation, acyl chlorination and other steps, and the total yield is controlled at more than 65%; the esterification of the intermediate product improves the separation degree of the intermediate; the reaction solvent is added in the diazotization step, such that the process parameters are relatively easy to control, and the purity of the intermediate in the diazotization step is more than 96% so as to provide the guarantee for the quality of the subsequent product; and with the synthesis process, the product quality is stable and reliable, and the cost is low.
Owner:JIANGSU YONGAN CHEM CO LTD
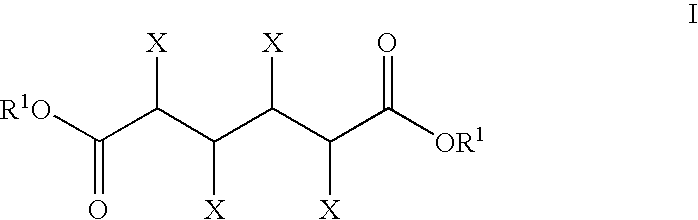
Catalyst comprising cyclic acylurea compounds and processes for production organic compounds with the same
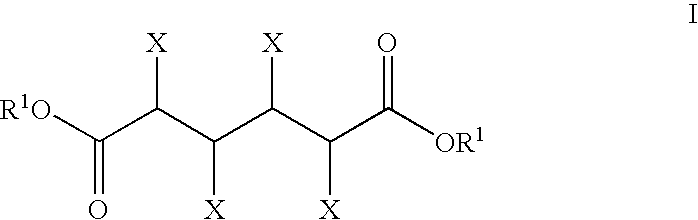
InactiveUS20050020439A1High selectivityHigh yieldPreparation by oxidation reactionsCarboxylic acid nitrile preparationSimple Organic CompoundsHydrogen atom
A catalyst of the invention includes a cyclic acylurea compound having a cyclic acylurea skeleton represented by following Formula (I): wherein R is a hydrogen atom or a hydroxyl-protecting group; n is 1 or 2; G is a carbon atom or a nitrogen atom, where two Gs are the same or different when n is 2. The catalyst may include the cyclic acylurea compound and a metallic compound in combination. In the presence of the catalyst, (A) a compound capable of forming a radical is allowed to react with (B) a radical scavenging compound and thereby yields an addition or substitution reaction product of the compound (A) and the compound (B) or a derivative thereof. This catalyst can produce an organic compound with a high selectivity in a high yield as a result of, for example, an addition or substitution reaction under mild conditions.
Owner:DAICEL CHEM IND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com