Method for continuously preparing dinitrochlorobenzene
A technology of dinitrochlorobenzene and mononitrochlorobenzene is applied in the field of preparation of dinitrochlorobenzene, and can solve the problems of unguaranteed safety and product quality, difficulty in large-scale continuous production, and low product purity. , to achieve the effect of expanding production capacity, reducing raw material consumption and reducing labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
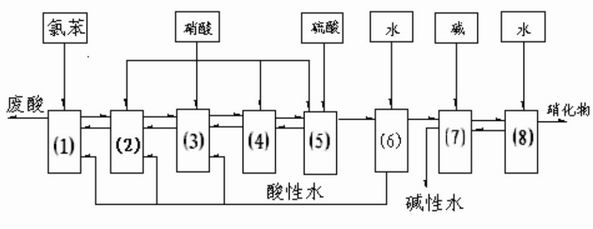
[0043] Such as figure 1 As shown, according to the method described in this patent, wherein reactors 1, 2, 3, 4, 5, 6, 7, and 8 all have separation devices. The control conditions of the inorganic phase of each reactor and the average residence time of the organic phase are shown in Table 1 below. The total amount of chlorobenzene, the total amount of nitric acid, and the total amount of sulfuric acid are added in the ratio of chlorobenzene:total amount of nitric acid:total amount of sulfuric acid=1:1.2:1.5.
[0044]
[0045] Table 1
[0046]
[0047] The first step is that chlorobenzene and nitric acid react to generate mononitrochlorobenzene, and mixed acid is formed into 74% sulfuric acid, 4% nitric acid, 18% water in the reaction; The second step is that the mononitrochlorobenzene that reaction generates continues and Nitric acid reacts to generate dinitrochlorobenzene, and the mixed acid standard composition in the reaction is 79% sulfuric acid, 5% nitric acid, and ...
Embodiment 2
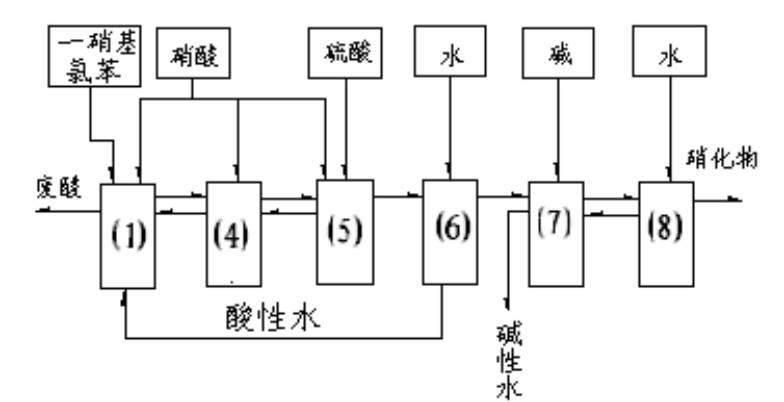
[0055] Such as figure 2 As shown, according to the flow process described in this patent, wherein reactors 1, 4, and 5 all have separation devices. The total amount of mononitrochlorobenzene, total amount of nitric acid, and total amount of sulfuric acid are added in the ratio of mononitrochlorobenzene: total amount of nitric acid: total amount of sulfuric acid = 1: (0.4~0.5): (1.0~1.3). The control conditions of the inorganic phase and the average residence time of the organic phase of each reactor are shown in Table 3 below, and the purification conditions of water washing and alkali washing are the same as in Example 1.
[0056]
[0057] table 3
[0058]
[0059] The product quality is as shown in Table 4, and the product yield: 96.5%. :
[0060] Table 4
[0061] components
Embodiment 3
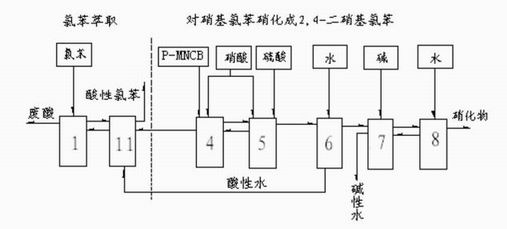
[0063] Such as image 3 As shown in the process flow, p-nitrochlorobenzene is used as raw material to prepare 2,4 dinitrochlorobenzene, and nitrate and nitric acid in the inorganic phase are extracted with chlorobenzene. Chlorobenzene continuously passes through two reactors in series to extract dinitrochlorobenzene in the inorganic phase flowing out of the first dinitrolation reactor, and then p-nitrochlorobenzene passes through two dinitrolation reactors to make dinitrochlorobenzene Nitrochlorobenzene is washed with water, alkali and water to make finished products. Reactors 1, 11, 4, 5, 6, 7, and 8 all have separation devices. Mononitrochlorobenzene: total amount of nitric acid: total amount of sulfuric acid mass ratio=1:0.41~0.45:0.9~1.2. The parameters of the inorganic phase of each reactor and the average residence time of the organic phase are shown in Table 5, and the purification conditions of water washing and alkali washing are the same as in Example 1.
[0064] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com