Nitration method for synthesizing dinitrotoluene in one step, and microchannel reactor
A technology of microchannel reactor and dinitrotoluene, which is applied in the preparation of nitro compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of high production cost, long reaction time, unsafety, etc., and achieve material saving Cost, process safety and continuity, and the effect of increasing space-time productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
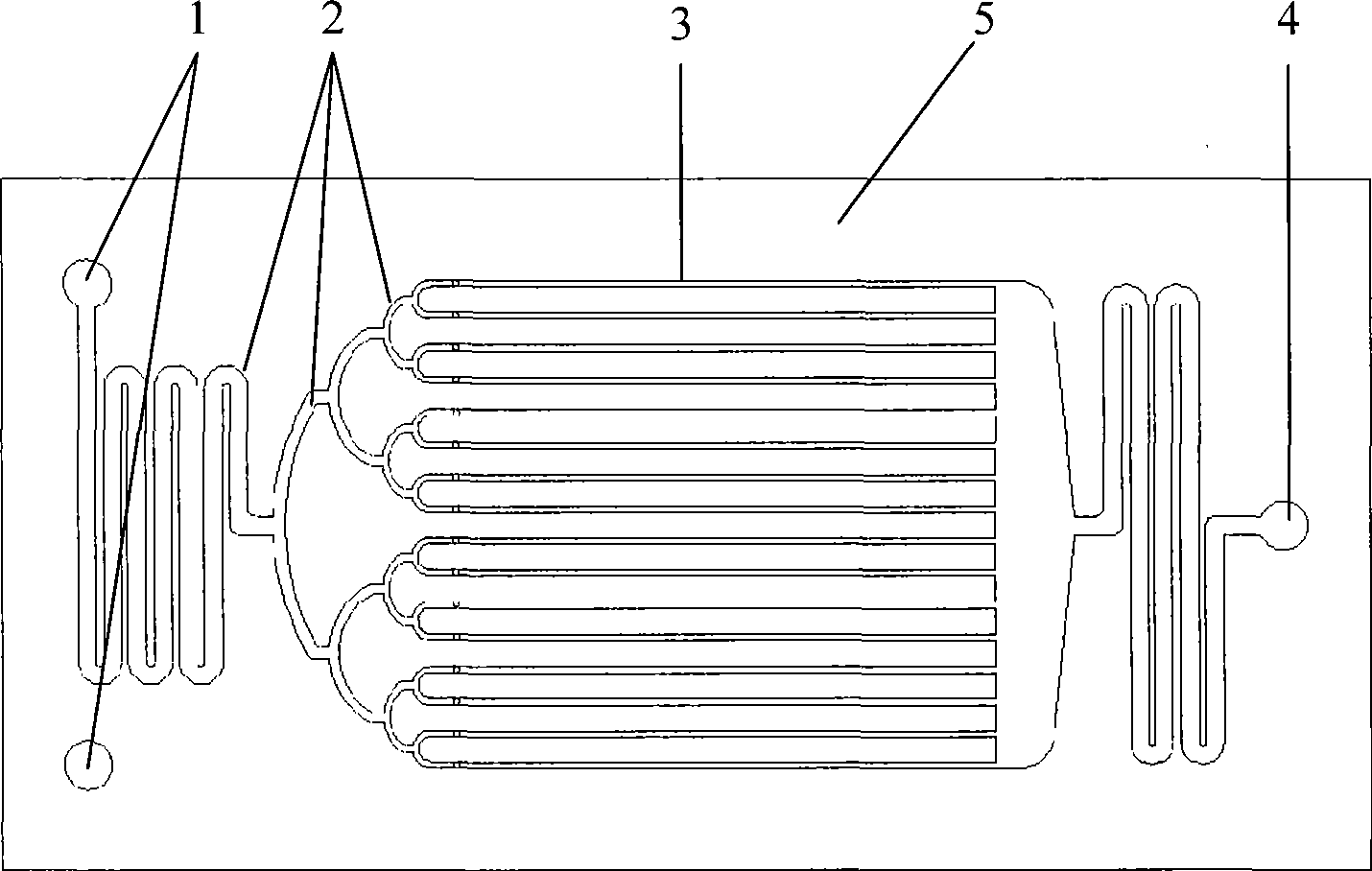
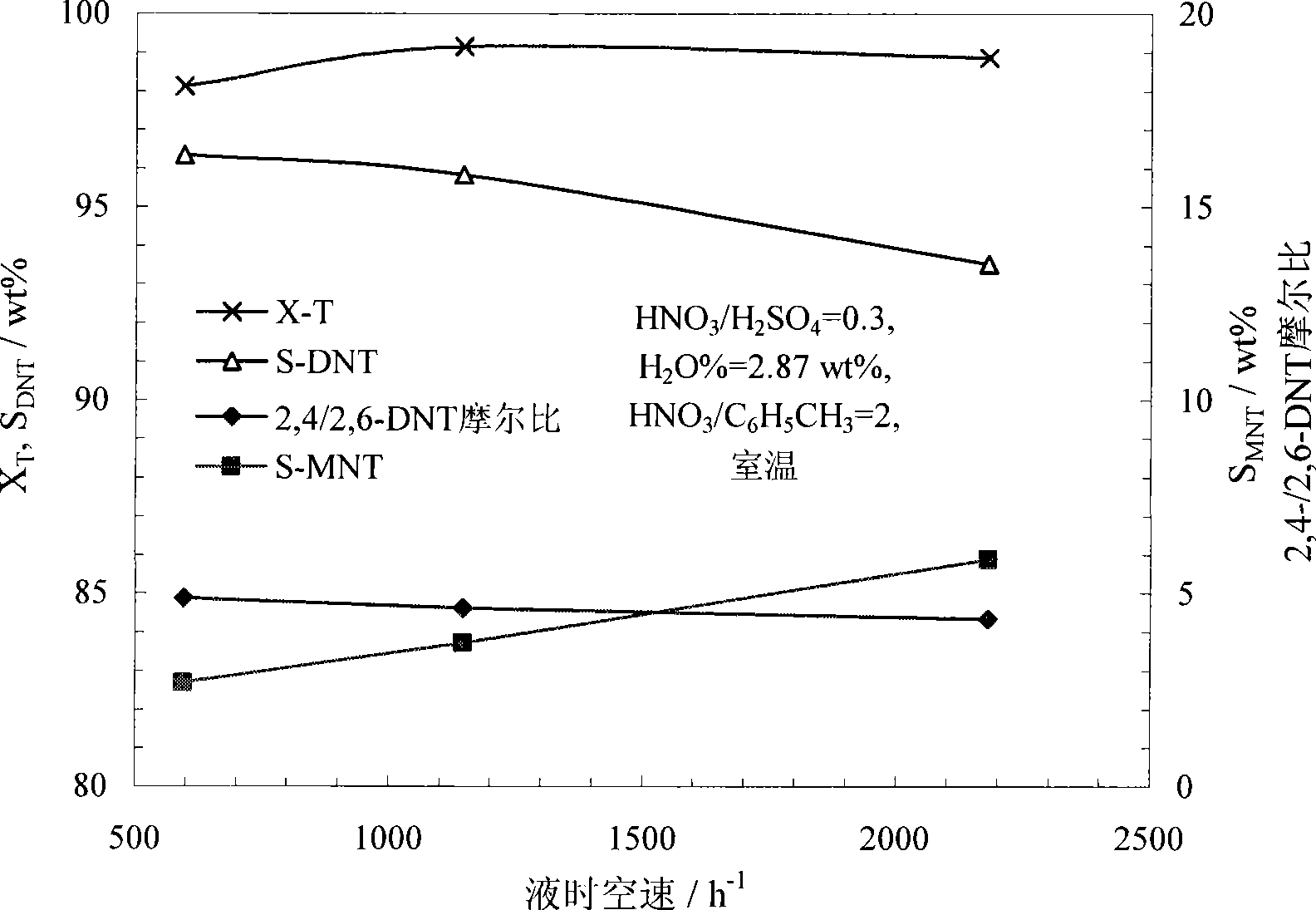
[0062] Use anhydrous nitric acid (≥98%) and concentrated sulfuric acid (>95%) to prepare a mixed acid with a molar ratio of nitric acid and sulfuric acid of 0.3 and a water concentration of 2.87%. During the preparation of mixed acid, the temperature can be controlled below 35°C. Under normal temperature conditions, the mixed acid and toluene were continuously pumped into the microreactor ( figure 2 The reactor shown) and react in the micro reaction channel. Control the molar ratio of nitric acid and toluene to 2.0, and the liquid hourly space velocity of the reactor is 600~2000h -1 . The reaction product flows out of the reactor continuously, and is statically layered in the separator to separate the acid solution, and the organic phase is washed with water, alkali and water until neutral. The reaction result is attached image 3 . image 3 The result shows that under the microchannel reactor used in this implementation and reaction condition, improving space velocity i...
Embodiment 2
[0064]The process and the reactor used are the same as in Example 1, the molar ratio of nitric acid to sulfuric acid is changed to 0.5, the molar ratio of nitric acid to toluene is 2.15, the reactor is operated at normal temperature, and the liquid hourly space velocity in the reactor is 500~2000h -1 . The implementation results are attached Figure 4 Show. Compared with Example 1, the concentration of nitric acid increases, the selectivity of DNT decreases by 11-17%, the selectivity of MNT increases, and the conversion rate of toluene remains unchanged.
Embodiment 3
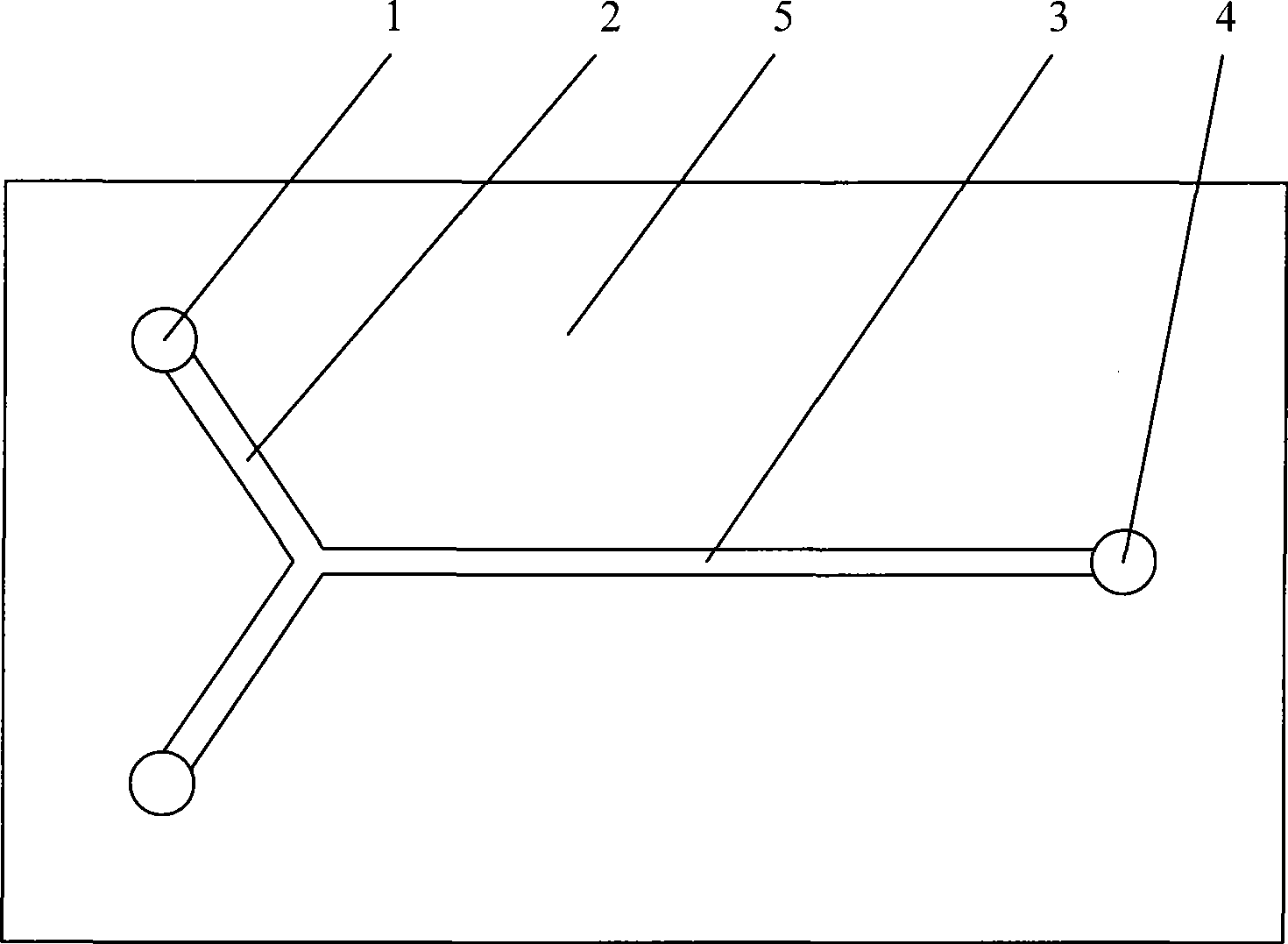
[0066] Process is with embodiment 1, and reactor uses attached figure 1 In the 'T'-shaped single-channel structure microreactor shown in , the molar ratio of nitric acid and sulfuric acid is 1.0, the mass concentration of water in the mixed acid is 2.87%, the molar ratio of nitric acid and toluene is 2.45, and the reaction is carried out at a space velocity of 4000-50000h -1 within. The implementation results are attached Figure 5 , the degree of toluene nitration conversion in the microreactor is almost not limited by the space velocity, the reaction residence time has little effect on the nitration conversion, and the toluene conversion rate is at a space velocity of 52870h -1 conditions can still be as high as 97.5% at 40000h -1 In a wide range of space velocity, the selectivity of main product DNT increased obviously, while the selectivity of MNT decreased. The ratio of the two major isomers of DNT, 2,4-DNT to 2,6-DNT, hardly changed in the wide space velocity range, b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com