Patents
Literature
58results about "Nitro/nitroso group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of bismuth nitrate and iron nitrate as nitrification agent in aromatic compound nitrification
InactiveCN1854114AChemically stableLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationNitrateNitrate agent
Owner:北京清华紫光英力化工技术有限责任公司
Nitration of activated aromatics in microreactors
InactiveCN101400628AHigh yieldHigh mass fluxChemical/physical/physico-chemical microreactorsNitro/nitroso group formation/introductionMicroreactorNitration
The invention relates to the nitration of aromatic or heteroaromatic compounds, wherein an activated aromatic or heteroaromatic compound and a nitrating agent, in the presence, if desired, of a solvent, are mixed intensely in a microreactor, and wherein the proportion of the nitrating agent to the activated aromatic or heteroaromatic compound, the concentration of nitrating agent in the reaction mixture, and the temperature are selected at levels such that the nitration begins autocatalytically, and wherein the nitration product is obtained after leaving the microreactor and, if desired, after an after-reaction time outside the microreactor.
Owner:LONZA LTD
Process for monitoring and controlling nitrating processes with the aid of an online spectrometer
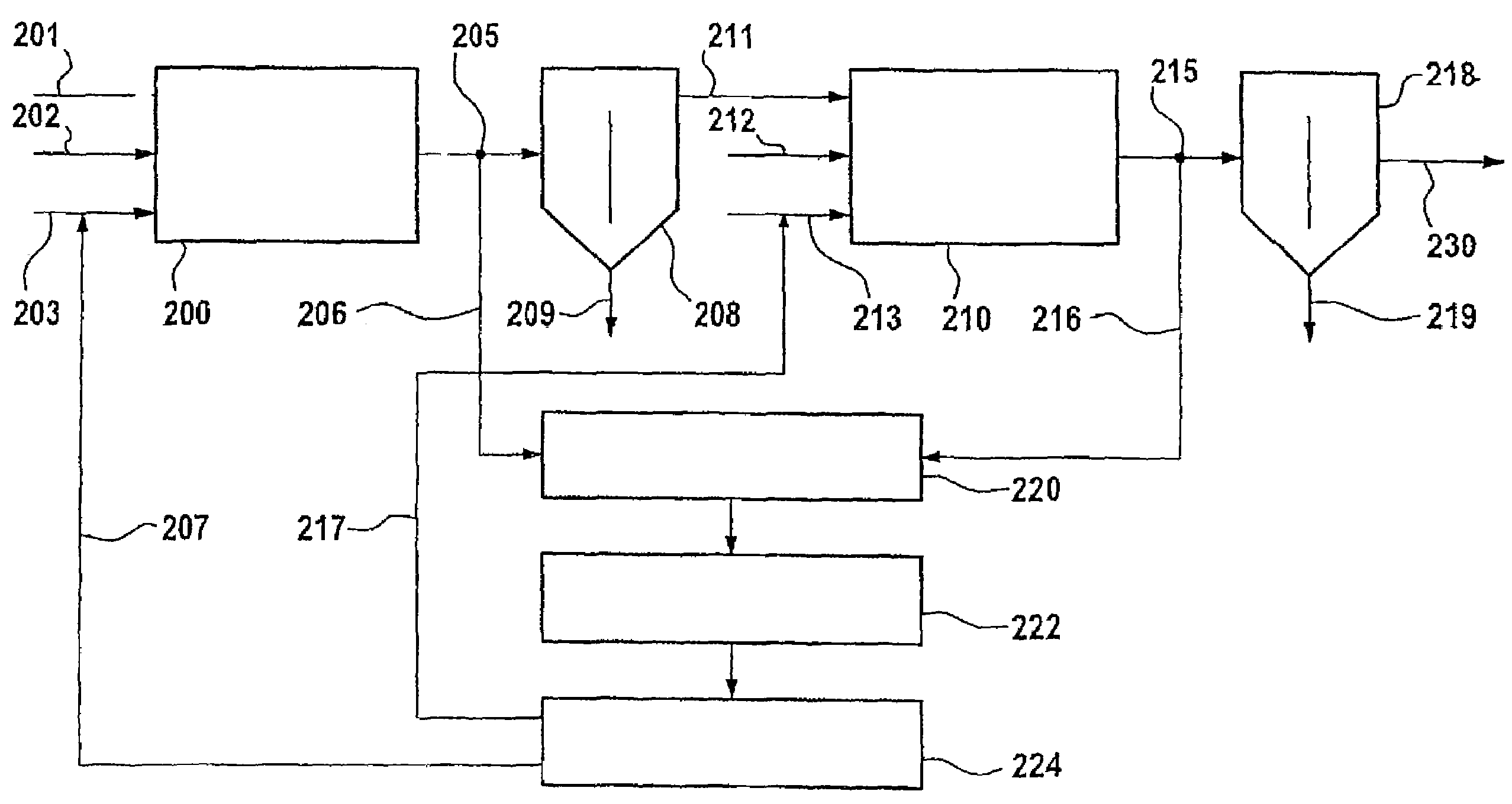
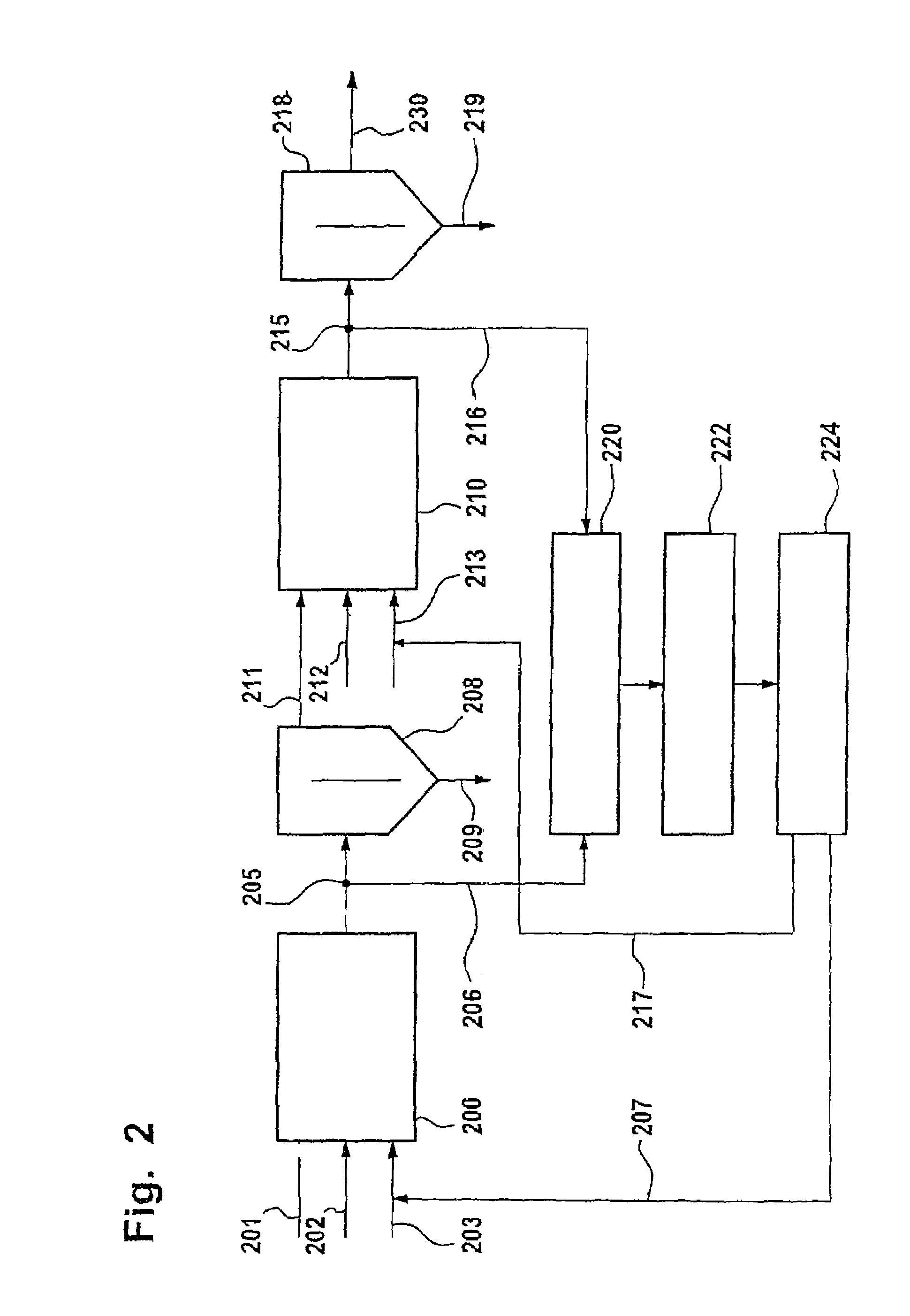
InactiveUS7227147B2Reduce excessRadiation pyrometryMaterial analysis by optical meansNitrationComputer-aided
A process for monitoring and / or controlling a nitrating process, having the following steps:measuring inline infrared spectra of nitric acid content in a reaction mixture stream downstream of the nitration reaction, preferably near-infrared spectra,evaluating the measured spectra by means of a computer-assisted, matrix-specific calibration model for the purpose of determining the content of nitric acid,transmitting the results of spectrometric examination to a process control system,inputting the results of spectrometric examination for the purpose of specifying the content of nitric acid in the acid phase into a regulator (224) for control of the metering (207,217) of nitric acid into a nitrating reactor.
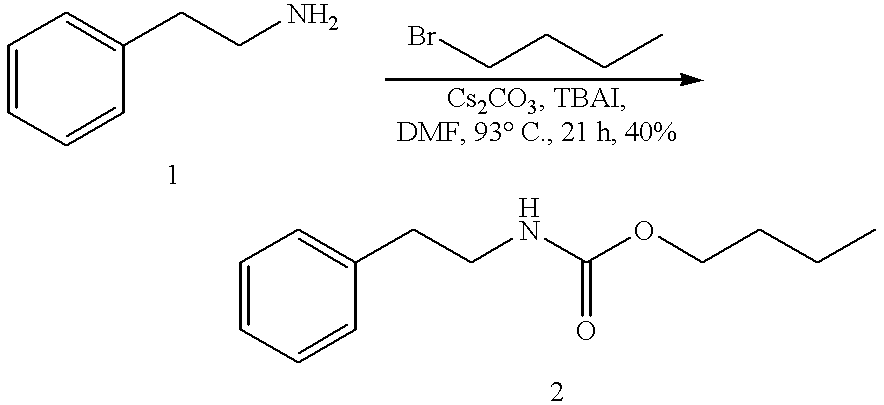
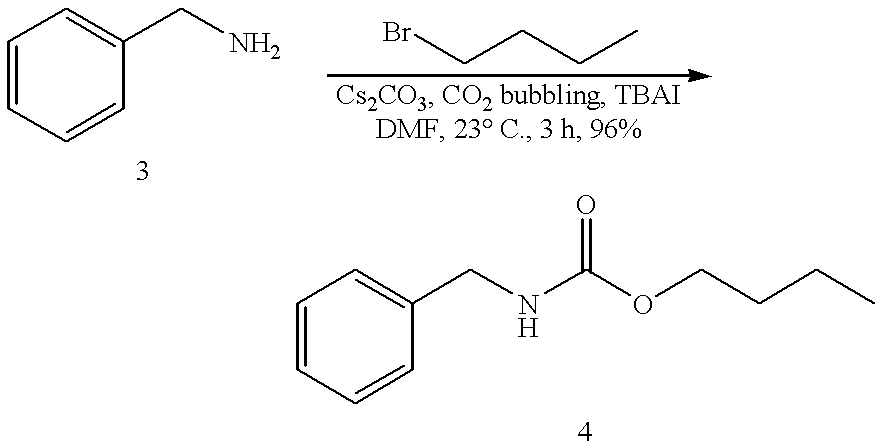
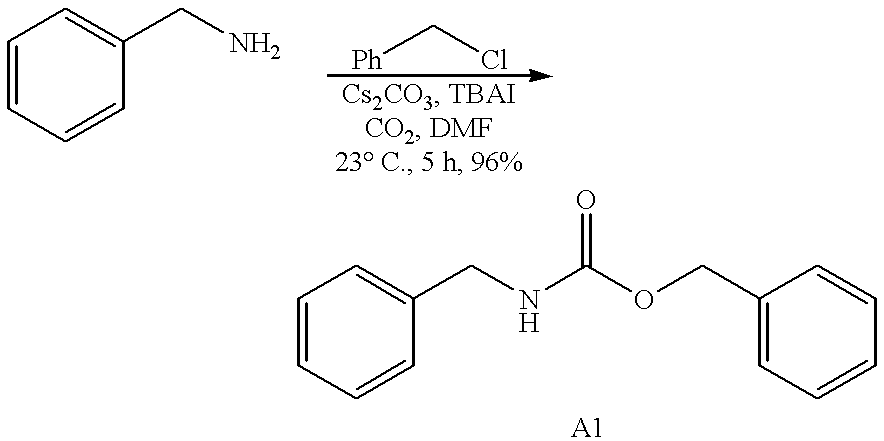
Efficient carbamate synthesis
InactiveUS6399808B1Simple and efficientPreserve the enantiomeric purity of the starting materialsGroup 4/14 element organic compoundsCarbamic acid derivatives preparationHydrogenSilylene
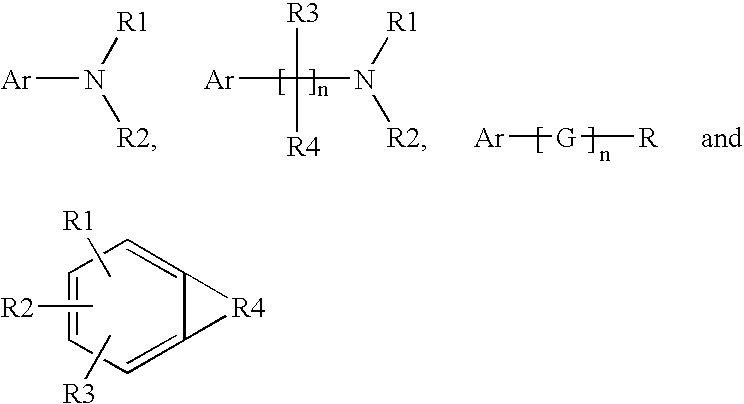
A method of preparing carbamates of the general formula RR'-N-CO2-R'' by reacting an amine with carbon dioxide and an organic electrophile in an anhydrous solvent in the presence of a cesium base, whereby carbamate synthesis is accomplished in good yield at mild temperatures, either in solution or on a solid support, and wherein R, R', and R'' are hydrogen, alkyl of 1-18 carbon atoms, silyl, phenyl, benzyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, allyl, or heterocycle, which are widely used as industrial products, and as intermediates in organic synthesis.
Owner:SOUTH FLORIDA UNIVESITY OF
Process for the preparation of toluenediamines by catalytic hydrogenation of dinitrotoluenes
The present invention relates to a process for the preparation of toluenediamine, in which dinitrotoluene is reacted with hydrogen in the presence of a catalyst. The dinitrotoluene required by this process has a content of carbon dioxide, in either physically dissolved or chemically bonded form, of not more than 0.175 mol %, based on the molar amount of the dinitrotoluene.
Owner:COVESTRO DEUTSCHLAND AG
Palladium-catalyzed ortho-orientation nitrification method of aza calixarene compounds
InactiveCN102372532AHigh chemoselectivityHigh regional selectivityOrganic-compounds/hydrides/coordination-complexes catalystsOximes preparationPtru catalystOrtho position
The invention discloses a palladium-catalyzed ortho-orientation nitrification method of aza calixarene compounds, relating to a nitrification method, comprising the following steps: successively adding the aza calixarene compounds, palladium catalyst, nitrated reagent, oxidizing agent and reaction solvent in a sealed pressure container, controlling the pressure between 1 mbar and 100 bar, heatingthe mixture for 6-72 hour in an oil bath with the reaction temperature of 30-200 DEG C to obtain a product of the ortho-orientation nitrification. According to the invention, the method is carried out under neutral conditions, and has no generation of exhaust gas and waste water; directional mononitration is only carried out on the ortho-position of the nitrogen containing substituents without being influenced from other substituents on aromatic nucleus, and the chemo-selectivity and region-selectivity are good; the functional groups and the substrate have good adaptability, and the directional ortho-position nitration of a plurality of aromatic nucleus containing various substituents and a plurality of aromatic nucleus containing various nitrogen containing oriented groups can all be realized; and pre-functionalized aromatic compounds are not needed.
Owner:ZHEJIANG UNIV OF TECH
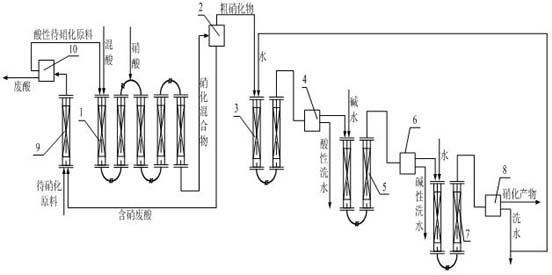
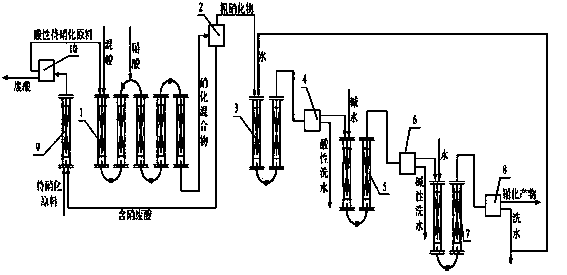
Production method of nitro compounds by tubular continuous nitrification reaction
ActiveCN102432410AEnhanced mass transferImprove heat transfer performanceNitro/nitroso group formation/introductionNitro compound preparationNitro compoundChemical reaction
The invention relates to a production method of a chemical reaction process, and particularly relates to a production method of nitro compounds by tubular continuous nitrification reaction. The device comprises a tubular nitrification reactor, a tubular water-washing alkali-washing device, a nitrification mixture separator, and a water-washing alkali-washing separator. The nitrification raw materials and mixed acid flow, are mixed, react, and transfer heat in the tubular reactor; the separated nitro compounds are washed by water and alkali in a tubular manner to obtain qualified nitrification products; nitric acid is added from an inlet of the tubular nitrification reactor, or added respectively from the inlet and other parts of the tubular nitrification reactor. The production method for preparing nitro compounds by nitrification reaction provided by the invention realizes tubular continuous production, has high operation flexibility, high production efficiency, good product quality, few by-product, low energy consumption and raw material consumption, and safe and reliable production process. The invention is used for heat extraction nitrification and heat insulation nitrification.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY +1
Green nitrification method and application for phenolic compound
ActiveCN104987293AReduce pollutionLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationChromatographic separationOrganic synthesis
The invention discloses a green nitrification method and application for a phenolic compound, belonging to the technical field of organic synthesis. The method provided by the invention comprises the following steps: with the phenolic compound as a raw material, dissolving the phenolic compound into a solvent at room temperature, adding sodium nitrite, then dropwise adding hydrogen peroxide into the obtained reaction solution, adding water-soluble metalloporphyrin at room temperature and starting reaction, carrying out nitrification reaction under stirring, then carrying out extraction by using an organic solvent, and carrying out vacuum concentration and column chromatographic separation so as to obtain a target product. The nitrification method provided by the invention has the advantages of mild reaction conditions, no need of heating, convenient operation and easy treatment of products. The green nitrification reaction is suitable for the phenolic compound.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Mono-nitration of aromatic compounds via nitrate salts
InactiveUS20070255057A1Avoid isolationSimple processOrganic active ingredientsOrganic compound preparationNitrate saltsNitration
Owner:F HOFFMANN LA ROCHE INC
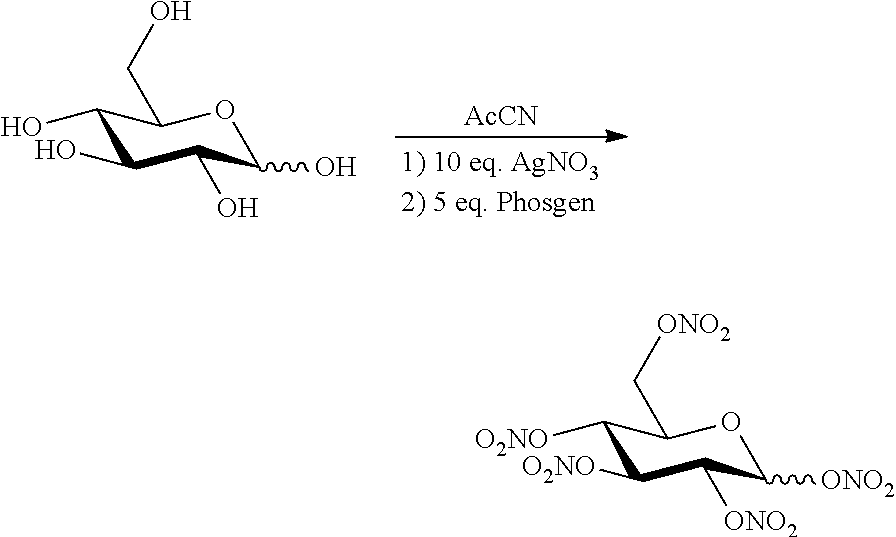
Novel Method for Directly Nitration of OH-, SH-and NHR-Functions in Organic Molecules by Means of in Situ Generated Carbonic Acid Dinitrate
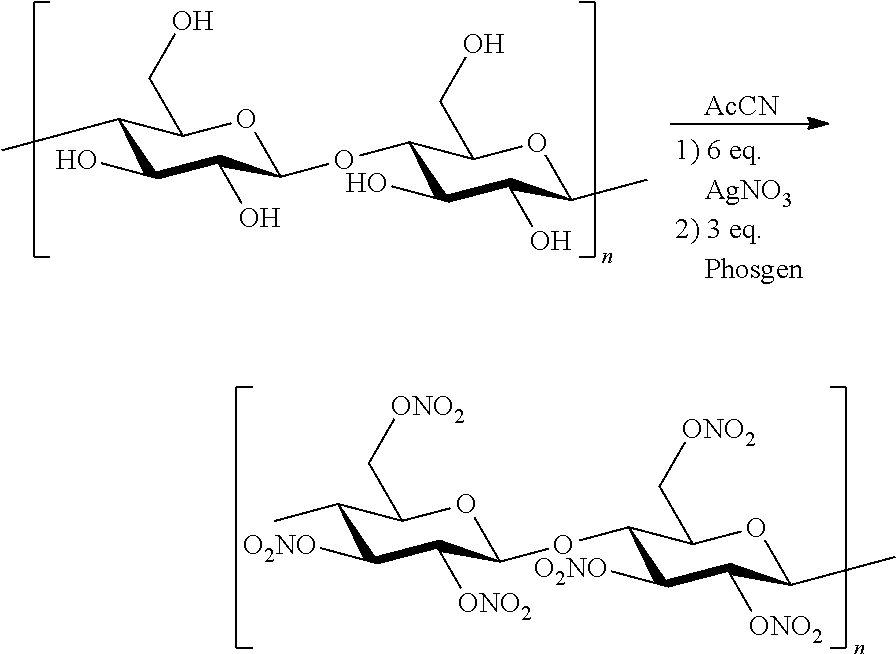
The invention relates to a nitration method having the following principles: a phosgene species is converted with two equivalent silver nitrates into a double-mixed anhydride of carbonic acid and nitric acid, known here as carbonic acid dinitrate (I). Said operation is carried out in situ, and the formed dinitrate decomposes spontaneously. In addition to carbon dioxide, nitrate ions and nitronium ions are formed, said ions comprising electrophiles which are necessary for nitration. The solution which is used is acetonitrile, and is insignificant if the alcohol species is dissolved or suspended. The necessary equivalent silver nitrates are introduced into the system and optionally heated or cooled to the desired temperature. Subsequently, the acid chloride is introduced slowly, drop by drop or slowly little by little. Phosgene, diphosgene, triphosgene and chloroformic acid ester can be used as carbonic acid dichloride and monochloride, and their thiocarbonic acid analogues. A brown colouration and precipitated silver chloride display the formation of the carbonic acid reactants, said brown colouration rapidly discolouring due to an immediate reaction of the nitronium ions with the substrate with is to be nitrated. Towards the end of the addition of phosgene, the brown colouration remains for longer and longer until it no longer disappears. Then, it is stirred for another hour at room temperature. In the event of high acid-sensitive educts, non-nucleophilic nitrogen bases such as DBU can be added to the system in order to intercept the formation of nitric acid.
Owner:SYNOVO
Method for synthesizing beta-iodo-nitroolefin compound
InactiveCN106083505AHigh yieldMild reaction conditionsNitro/nitroso group formation/introductionNitro compound preparationNitroalkeneNitrite
The invention provides a method for synthesizing a beta-iodo-nitroolefin compound as shown in a formula (III). The method comprises: mixing substituted alkyne shown in a formula (I), an iodine source and tert-butyl nitrite shown in a formula (II) in a solvent for completely reacting to obtain reaction liquid, and after reacting for 4 to 6h at a temperature of 25 to 80 DEG C, processing the reaction liquid to obtain the beta-iodo-nitroolefin compound, wherein the solvent is tetrahydrofuran, acetonitrile, acetone or 1,2-dichloroethane, and a ratio of quantities of fed substances, i.e., the substituted alkyne shown in the formula (I), the iodine source and the tert-butyl nitrite shown in the formula (II), is 1 to (0.5 to 1) to (2 to 5). The method provided by the invention is high in substrate adaptability and mild in reaction condition, does not relate to use of an oxidizing agent and a metal catalyst, is safe and environmental-friendly, is simple in reaction operation, and is more beneficial to application in medicine synthesis. The formulas are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
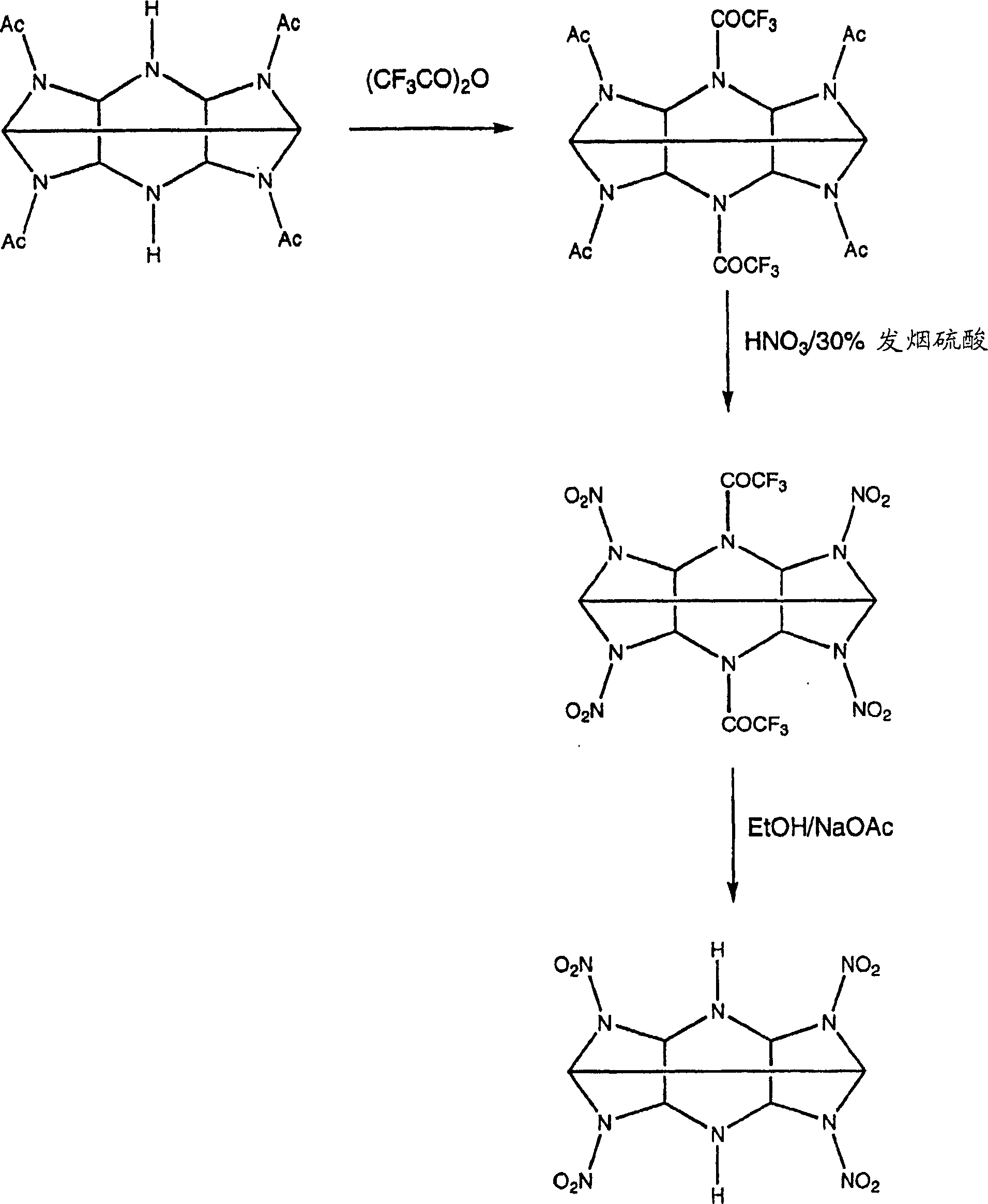
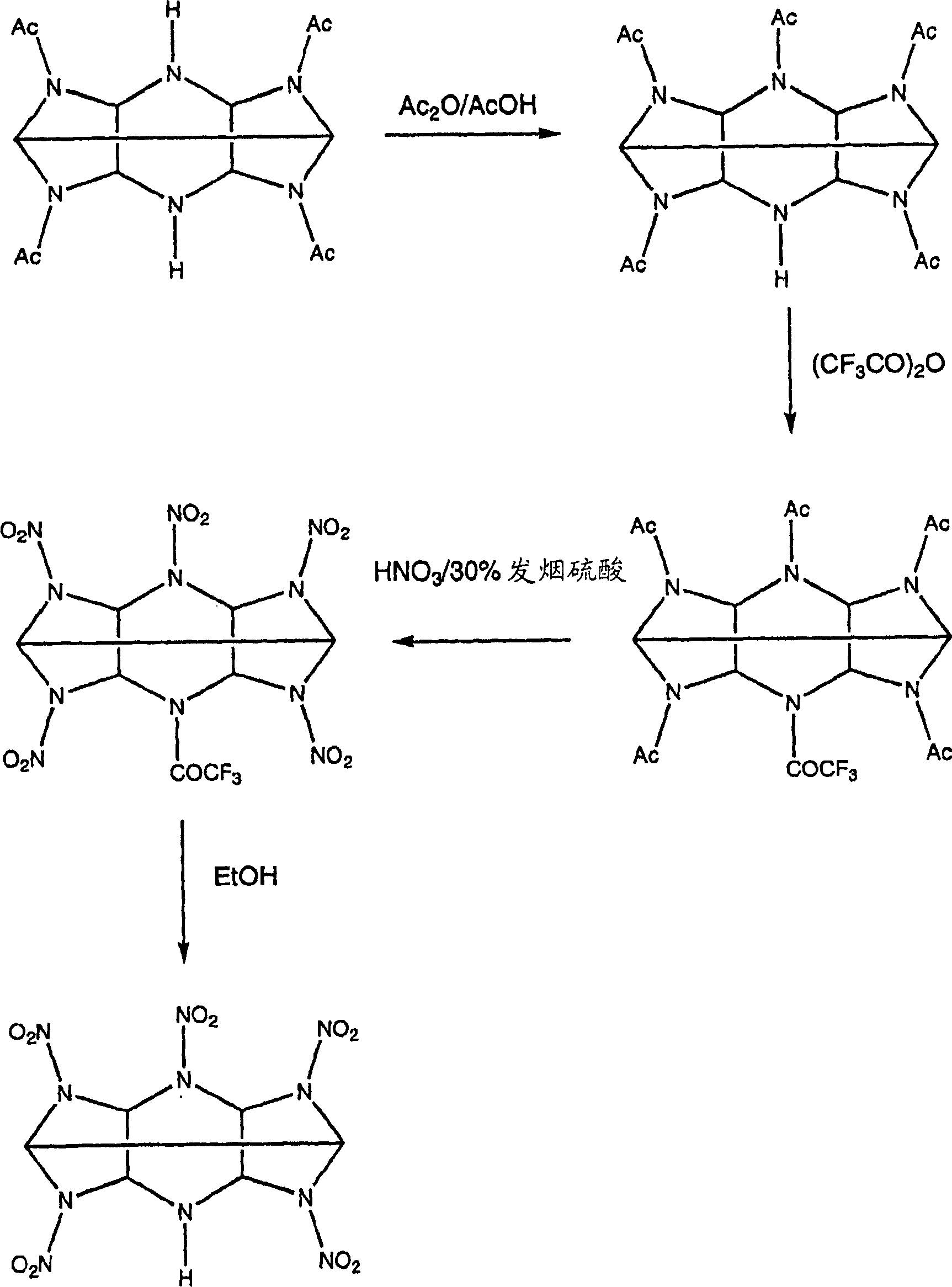
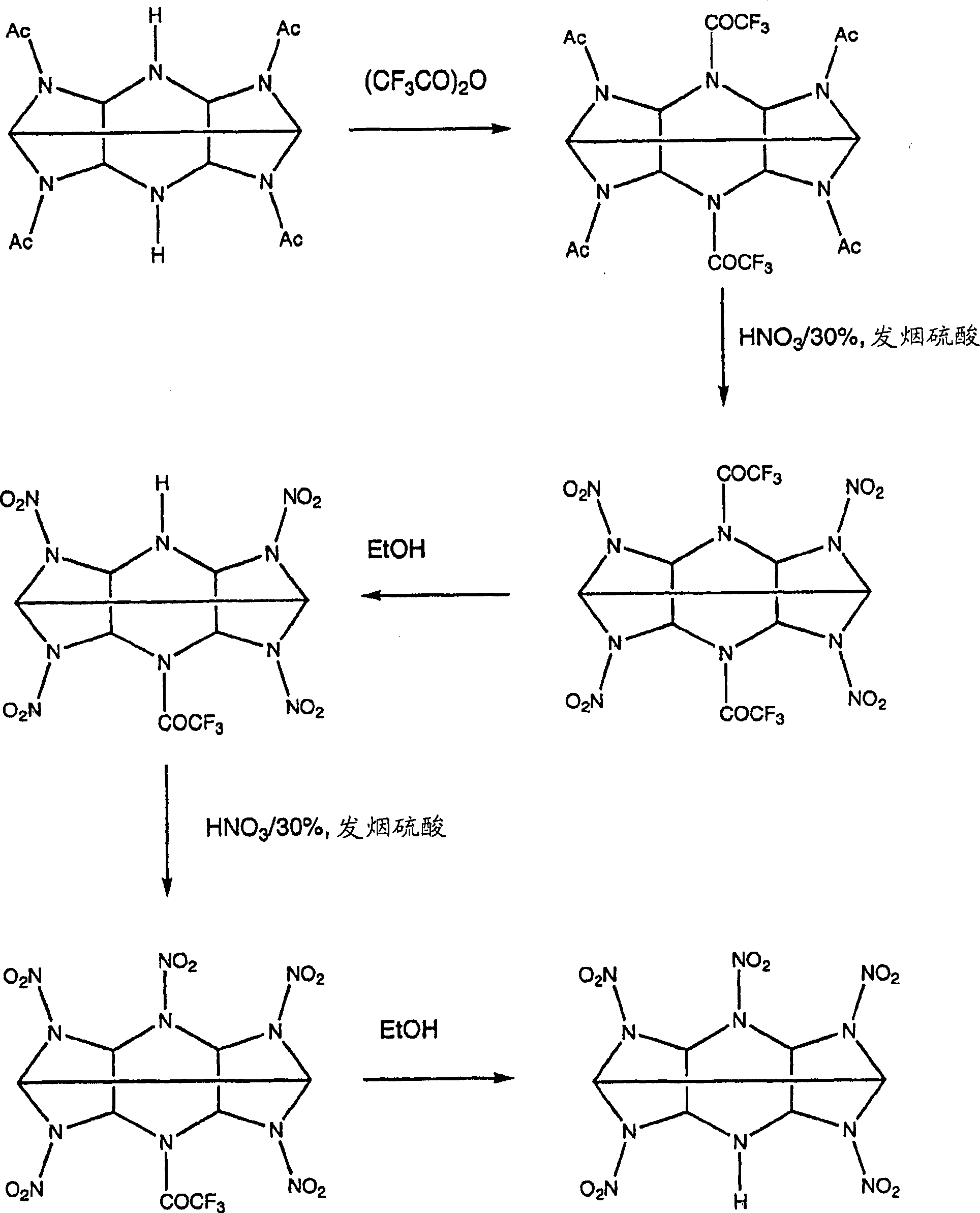
Mono amine and diamine derivatives of cl-20
InactiveCN1753849ANitrated acyclic/alicyclic/heterocyclic amine explosive compositionsNitro/nitroso group formation/introductionHEXADiamine
Owner:THE SEC OF STATE FOR DEFENCE IN HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Process for preparing aromatic amines
ActiveUS7122701B2Reduce the amount of wasteEnhances their economic viabilityAmino compound purification/separationOrganic compound preparationAromatic hydrocarbonAromatic amine
Aromatic amines are produced from aromatic hydrocarbons bya) reacting the aromatic hydrocarbon(s) with a mixture of nitric acid and sulfuric acid to generate a two-phase reaction mixture,b) separating the reaction mixture into an aqueous acid phase and an organic phase containing the nitroaromatic compoundsc) washing the organic phase to purify the nitroaromatic compound(s),d) hydrogenating the nitroaromatic compound(s) in the presence of a catalyst to produce the aromatic amine(s) and water of reaction, ande) separating the water of reaction formed in step d) from the aromatic amine(s),in which the water of reaction separated in step e) is used to wash the organic phase containing the nitroaromatic compounds in step c).
Owner:COVESTRO DEUTSCHLAND AG
Method for synthesizing nitrosoureas antineoplastic drugs through stannic chloride-sodium nitrite system
InactiveCN102964193ALow costEasy to removeUrea derivatives preparationSugar derivativesAlkaneAfter treatment
The invention belongs to the field of chemistry, and particularly relates to a method for synthesizing nitrosoureas antineoplastic drugs through a stannic chloride-sodium nitrite system, and the method is used for solving the technical problems of low yield and complicated after-treatment of a conventional synthetic method. The technical scheme adopted by the invention is as follows: the method for synthesizing the nitrosoureas antineoplastic drugs through the stannic chloride-sodium nitrite system comprises the following steps: a, adding stannic chloride, precursors of nitrosoureas antineoplastic drugs and nitrous acid to chloralkane, and stirring; b, filtering to remove insoluble substances, and washing the filtrate by using a saturated weak alkaline solution, and then drying; and c, removing chloralkane from the dried filtrate, and recrystallizing the obtained solid, so as to obtain the nitrosoureas antineoplastic drugs. The invention provides a novel high-yield method for synthesizing nitrosoureas antineoplastic drugs.
Owner:ASTATECH CHENGDU PHARMA
Process for the preparation of toluenediamines by catalytic hydrogenation of dinitrotoluenes
The present invention relates to a process for the preparation of toluenediamine, in which dinitrotoluene is reacted with hydrogen in the presence of a catalyst. The dinitrotoluene required by this process has a content of carbon dioxide, in either physically dissolved or chemically bonded form, of not more than 0.175 mol %, based on the molar amount of the dinitrotoluene.
Owner:COVESTRO DEUTSCHLAND AG
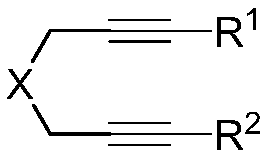
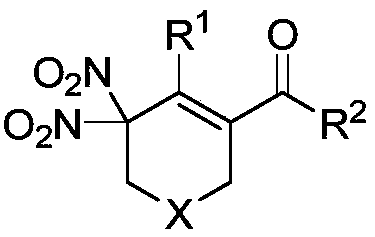
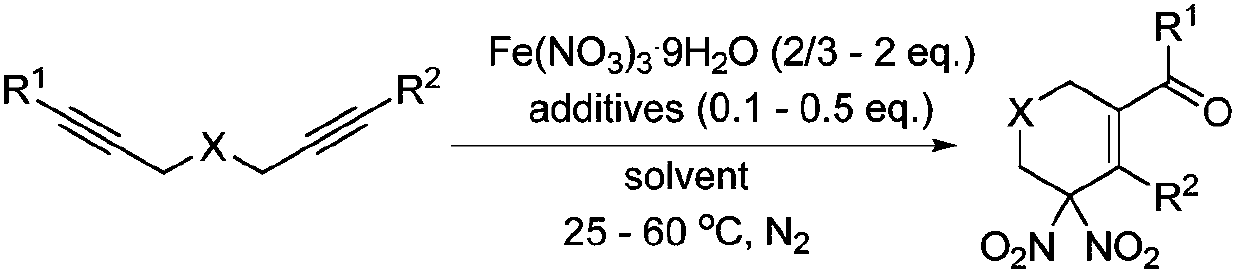
Method of using nitrate hydrate to prepare bisphosphonate nitro compound
InactiveCN109574913AHas Nitric Oxide Release CapabilitiesReduce dosageNitro/nitroso group formation/introductionNitro compound preparationNitro compoundNitrate
The invention discloses a method of using nitrate hydrate to prepare a bisphosphonate nitro compound. The method includes: in an organic solvent, taking nitrate hydrate as a nitro source, an oxygen-containing compound as an additive and 1, 6-diyne as a substrate for nitration-cyclization-oxidation tandem reaction, and performing aftertreatment after reaction is finished to obtain the bisphosphonate nitro compound. The compound is synthesized by direct nitration of 1, 6-diyne; the method is simple, and the nitrate hydrate is used as the nitro source, so that the compound is low in cost, easy toobtain, safe and stable. The method can be used for synthesizing a series of bisphosphonate nitro compounds, and nitric oxide release ability and drug activity of synthesized products can be furtherstudied.
Owner:ZHEJIANG UNIV
Environment-friendly bionic catalytic nitration method for phenolic compound
ActiveCN106045803AReduce pollutionLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationOrganic synthesisFiltration
The invention discloses an environment-friendly bionic catalytic nitration method for a phenolic compound and belongs to the technical field of organic synthesis. The method provided by the invention comprises the steps of dissolving the phenolic compound, which serves as a raw material, in a solvent at normal temperature, adding sodium nitrite into the solution, then, dropwise adding hydrogen peroxide into the reaction solution, adding metal-doped Al-MCM-41 molecular sieves into the reaction solution at normal temperature so as to start a reaction, carrying out stirring so as to carry out a nitration reaction, and then, carrying out suction filtration, organic solvent extraction, depressurized concentration and column chromatography separation, thereby obtaining a target product. According to the nitration method disclosed by the invention, the reaction conditions are mild, heating is not required, the operation is convenient, and the product is easy to treat. The nitration method is applicable to an environment-friendly bionic catalytic nitration reaction for phenolic compounds.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Synthesizing method of alpha, beta unsaturated nitroolefin derivative
ActiveCN107032939AThe process steps are simpleEase of industrial productionNitro/nitroso group formation/introductionNitro compound preparationOne pot reactionFerroporphyrin
The invention discloses a synthesizing method of an alpha, beta unsaturated nitroolefin derivative. The synthesizing method includes: allowing an alkene compound to have one-pot reaction in a tert-butyl hydroperoxide system containing tetra-arylated ferroporphyrin (III) and halogenated ammonium salt to generate the alpha, beta unsaturated nitroolefin derivative. By the method, the alpha, beta unsaturated nitroolefin derivative with high E three-dimensional selectivity is synthesized under mild reaction conditions in a high-yield manner.
Owner:YUANJIANG HUALONG CATALYST TECH
Synthesis method of substituted nitroaniline
InactiveCN103848706AReduce pollutionMild conditionsOrganic compound preparationCarboxylic acid amides preparationPhenacylNitration
The invention discloses a synthesis method of substituted nitroaniline, comprises the following steps: performing nitration on the substituted aniline and nitrite in a solvent at 20-80 DEG C to obtain the substituted nitroaniline, the structural formula of the substituted aniline is as shown in the specification, wherein R1 is p-Toluenesulfonyl, acetyl, t-butyloxycarbonyl, benzoyl, -COC(CH3)3, benzyl or methyl; R2, R3, R4, R5 and R6 are respectively and independently C1-C4 alkyl, C1-C4 alkoxy, halogen, H, NO2, OCF3 or aryloxy, at least one of R2, R4 and R6 is H, the nitration means that at least one of R2, R4 and / or R6 with value of H on the substituted aniline is substituted by NO2. The synthesis method disclosed by the invention is free from high-temperature and high-pressure, the condition is gentle, the operation is safer, the strong acid is avoided, the post-treatment is simple, and the pollution to the environment is small, and the harm is light.
Owner:LANZHOU UNIVERSITY
Process for nitrating aromatic hydrocarbons
InactiveCN1346823ANitro/nitroso group formation/introductionNitro compound preparationNitrogen oxidesNitrogen
Owner:BASF AG
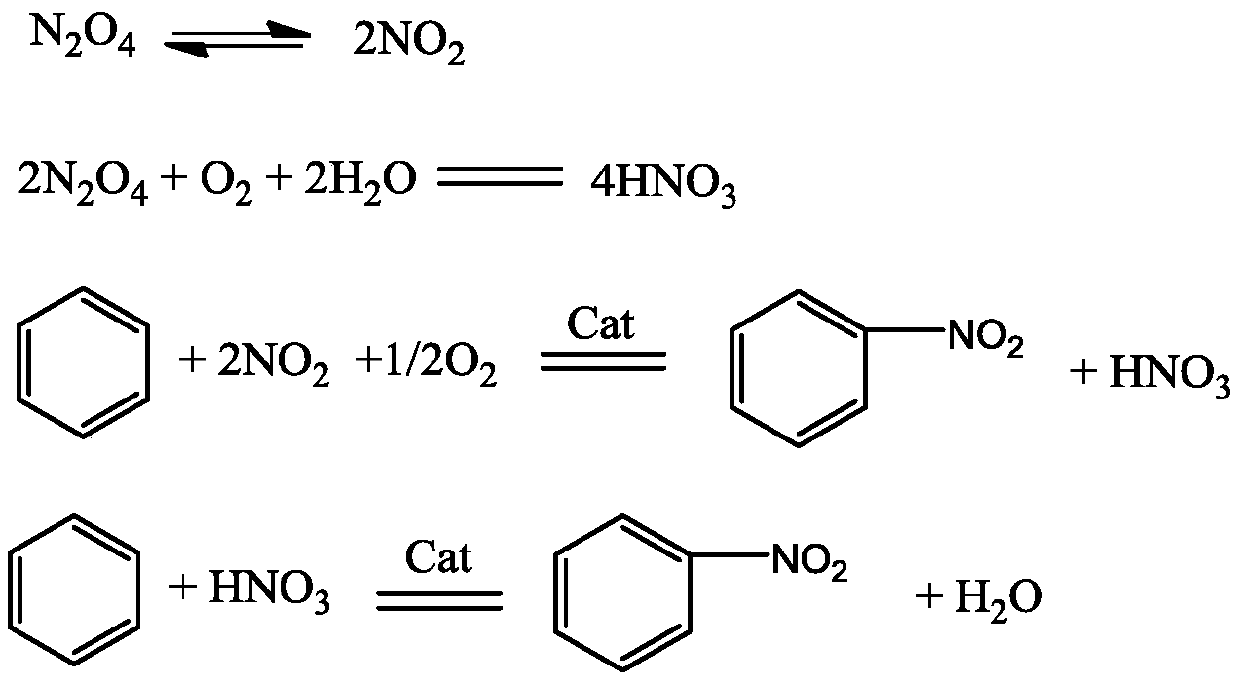
Nitration method of aromatic hydrocarbon compound
ActiveCN110511145AReduce the amount addedDosage controlNitro/nitroso group formation/introductionBulk chemical productionChemistrySulfuric acid
The invention relates to a nitration method of an aromatic hydrocarbon compound, in particular to a method for preparation of a nitro aromatic hydrocarbon compound by subjecting an aromatic hydrocarbon compound to nitration reaction with liquid dinitrogen tetroxide and oxygen-containing air flow in the presence of a solid acid catalyst and a small amount of sulfuric acid. And the method is a production process suitable for industrialization.
Owner:江苏方圆芳纶研究院有限公司
Organic-matter nitrosoation method with no production of nitric oxide waste gas
InactiveCN101712585ANo pollutionSave raw materialsAmino preparation from aminesOrganic compound preparationNitric oxide gasOrganic matter
The invention provides an organic-matter nitrosoation method with no production of nitric oxide waste gas, which belongs to the technical field of clean chemical processes and comprises nitrosoation reaction. The method is characterized in that the nitrosoation reaction is performed in closed space, and the highest pressure in the closed space is 0.4 to 0.6 MPa. The method prevents nitrous acid from being decomposed by sealing and pressurizing equipment so as to avoid producing nitric oxide waste gas. The method has the advantages of ensuring no emission of nitric oxide gas during production, producing no environmental pollution, saving raw material and reducing production cost.
Owner:陈学玺
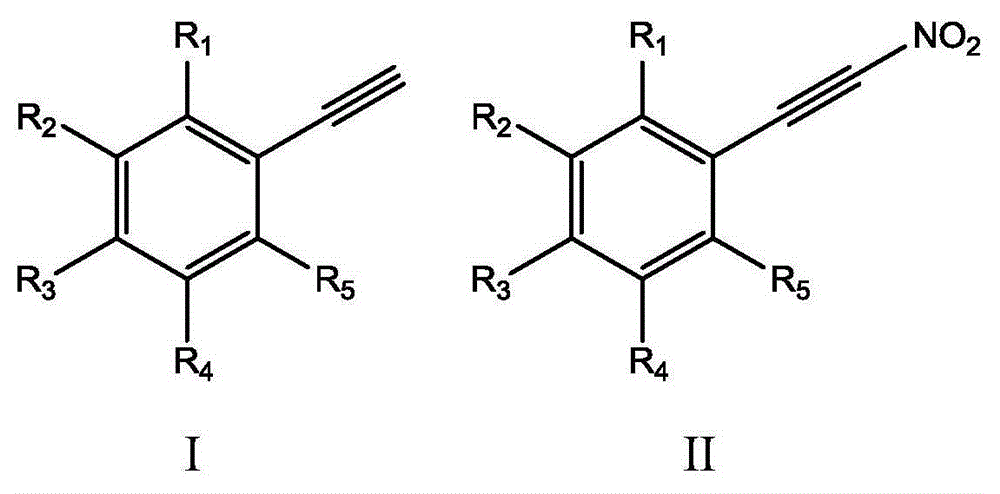
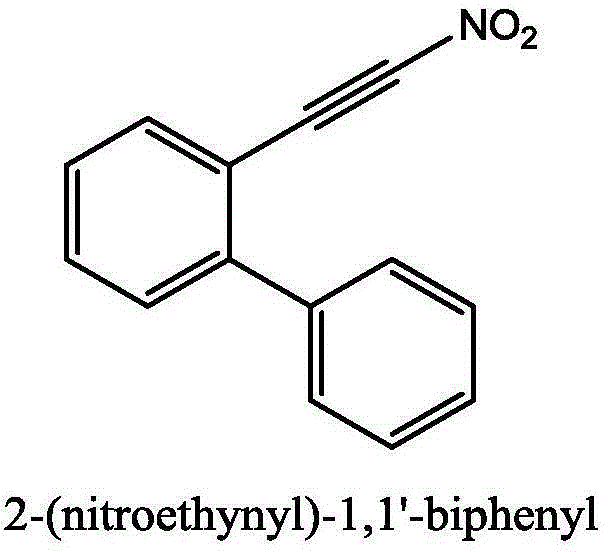
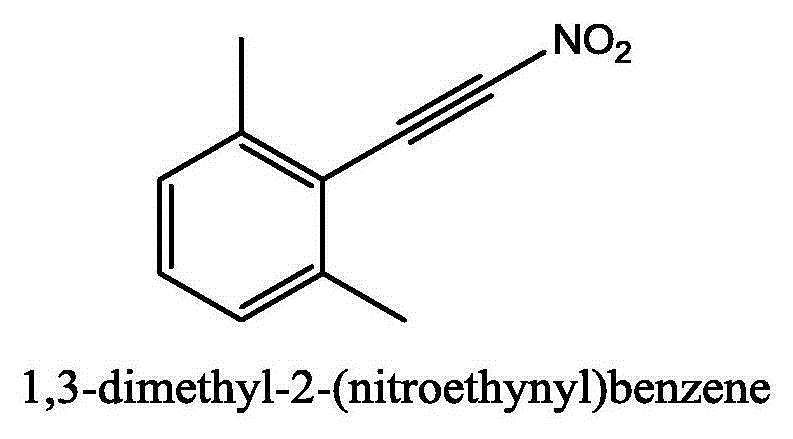
Method for synthesizing (nitroalkynyl)benzene compounds
ActiveCN106478327AAchieve nitrificationGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationBenzeneOrganic solvent
The invention provides a method for synthesizing (nitroalkynyl)benzene compounds shown in formula II, comprising: adding phenylacetylene compounds shown in formula I as materials, along with a nitrifying agent, into an organic solvent, hermetically reacting at 20-50 DEG C, performing TLC (thin layer chromatography) tracing until the reaction is over, and post-treating the reacted liquid to obtain (nitroalkynyl)benzene compounds shown in the formula II. The nitrifying method of the invention has the advantage of good nitrifying site specificity, nitrifying is carried out only on alkynyl, no nitrification products are produced on benzene rings, the reaction process is good in safety, environment-friendly and good in substrate adaptability, and it is possible to provide alkynyl nitrifying for various substituents.
Owner:ZHEJIANG UNIV OF TECH
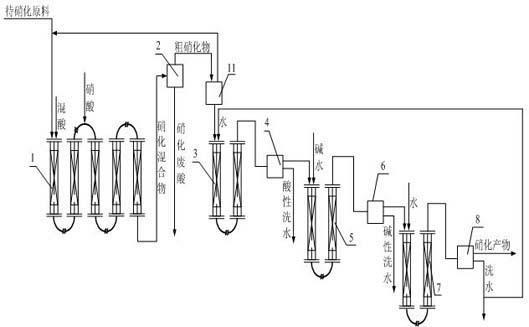
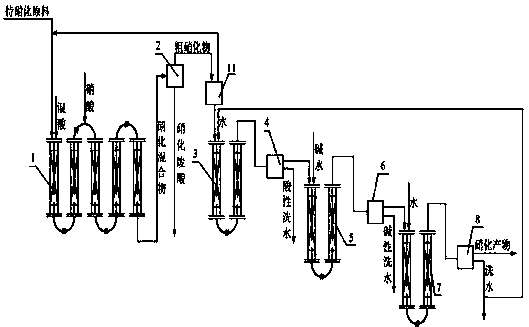
Production method of nitro compounds by tubular continuous nitrification reaction
ActiveCN102432410BSimple processLess investmentNitro/nitroso group formation/introductionNitro compound preparationNitro compoundChemical reaction
The invention relates to a production method of a chemical reaction process, and particularly relates to a production method of nitro compounds by tubular continuous nitrification reaction. The device comprises a tubular nitrification reactor, a tubular water-washing alkali-washing device, a nitrification mixture separator, and a water-washing alkali-washing separator. The nitrification raw materials and mixed acid flow, are mixed, react, and transfer heat in the tubular reactor; the separated nitro compounds are washed by water and alkali in a tubular manner to obtain qualified nitrification products; nitric acid is added from an inlet of the tubular nitrification reactor, or added respectively from the inlet and other parts of the tubular nitrification reactor. The production method for preparing nitro compounds by nitrification reaction provided by the invention realizes tubular continuous production, has high operation flexibility, high production efficiency, good product quality, few by-product, low energy consumption and raw material consumption, and safe and reliable production process. The invention is used for heat extraction nitrification and heat insulation nitrification.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY +1
A green biomimetic catalytic nitration method for phenolic compounds
ActiveCN106045803BReduce pollutionLess corrosiveNitro/nitroso group formation/introductionNitro compound preparationFiltrationOrganic synthesis
The invention discloses an environment-friendly bionic catalytic nitration method for a phenolic compound and belongs to the technical field of organic synthesis. The method provided by the invention comprises the steps of dissolving the phenolic compound, which serves as a raw material, in a solvent at normal temperature, adding sodium nitrite into the solution, then, dropwise adding hydrogen peroxide into the reaction solution, adding metal-doped Al-MCM-41 molecular sieves into the reaction solution at normal temperature so as to start a reaction, carrying out stirring so as to carry out a nitration reaction, and then, carrying out suction filtration, organic solvent extraction, depressurized concentration and column chromatography separation, thereby obtaining a target product. According to the nitration method disclosed by the invention, the reaction conditions are mild, heating is not required, the operation is convenient, and the product is easy to treat. The nitration method is applicable to an environment-friendly bionic catalytic nitration reaction for phenolic compounds.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
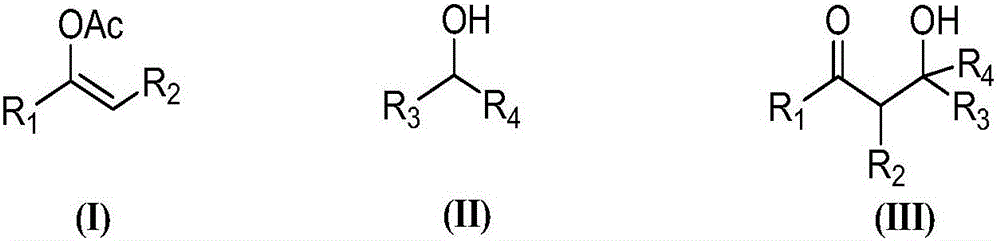
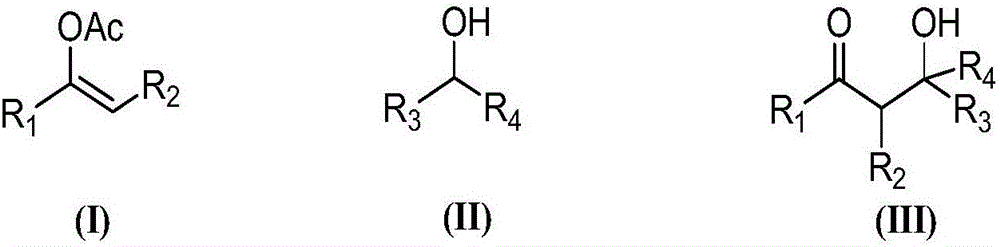
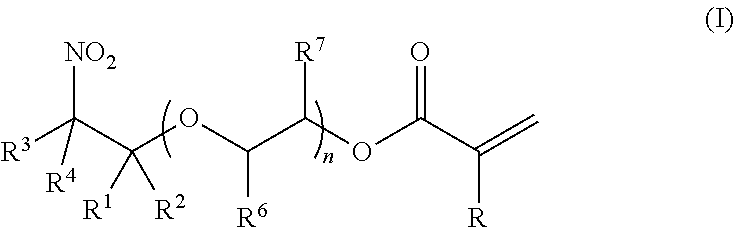
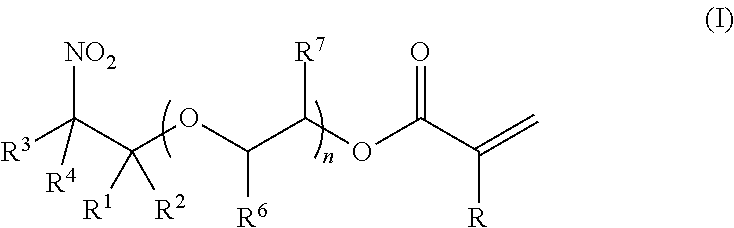
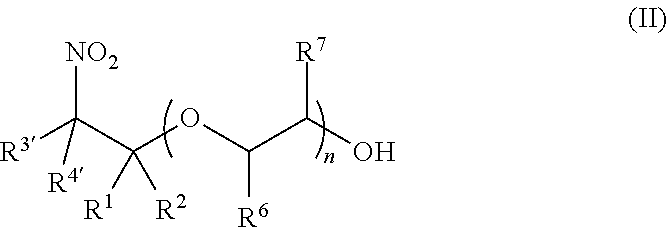
Synthesis of (2-nitro)alkyl (meth)acrylates via transesterification of (meth)acrylate esters
ActiveUS9670134B2Organic compound preparationCarboxylic acid ester formation/introductionTransesterificationFree Radical Suppression
Provided is a process for making (2-nitro)alkyl (meth)acrylate compounds of formula I: wherein n, R, R1, R2, R3, R4, R6, R7, and n are as defined herein, by a transesterification reaction between a nitroalcohol compound and a (meth)acrylate compound in the presence of a transesterification catalyst and a free radical inhibitor.
Owner:ANGUS CHEM CO
Nitration reaction stabilizer and application thereof
InactiveCN104262072ASimple componentsEasy to makeNitro/nitroso group formation/introductionEpoxyNitration
The invention provides a nitration reaction stabilizer and application thereof. The stabilizer comprises isooctyl dimethyl dithioglycolate tin, butyl epoxy stearate, barium stearate and trioctyl phenyl phosphite. The stabilizer can prevent explosive products from generation in the nitration reaction so as to avoid the resulting dangerous accidents, and is a stable composition with excellent stability.
Owner:王晓伟
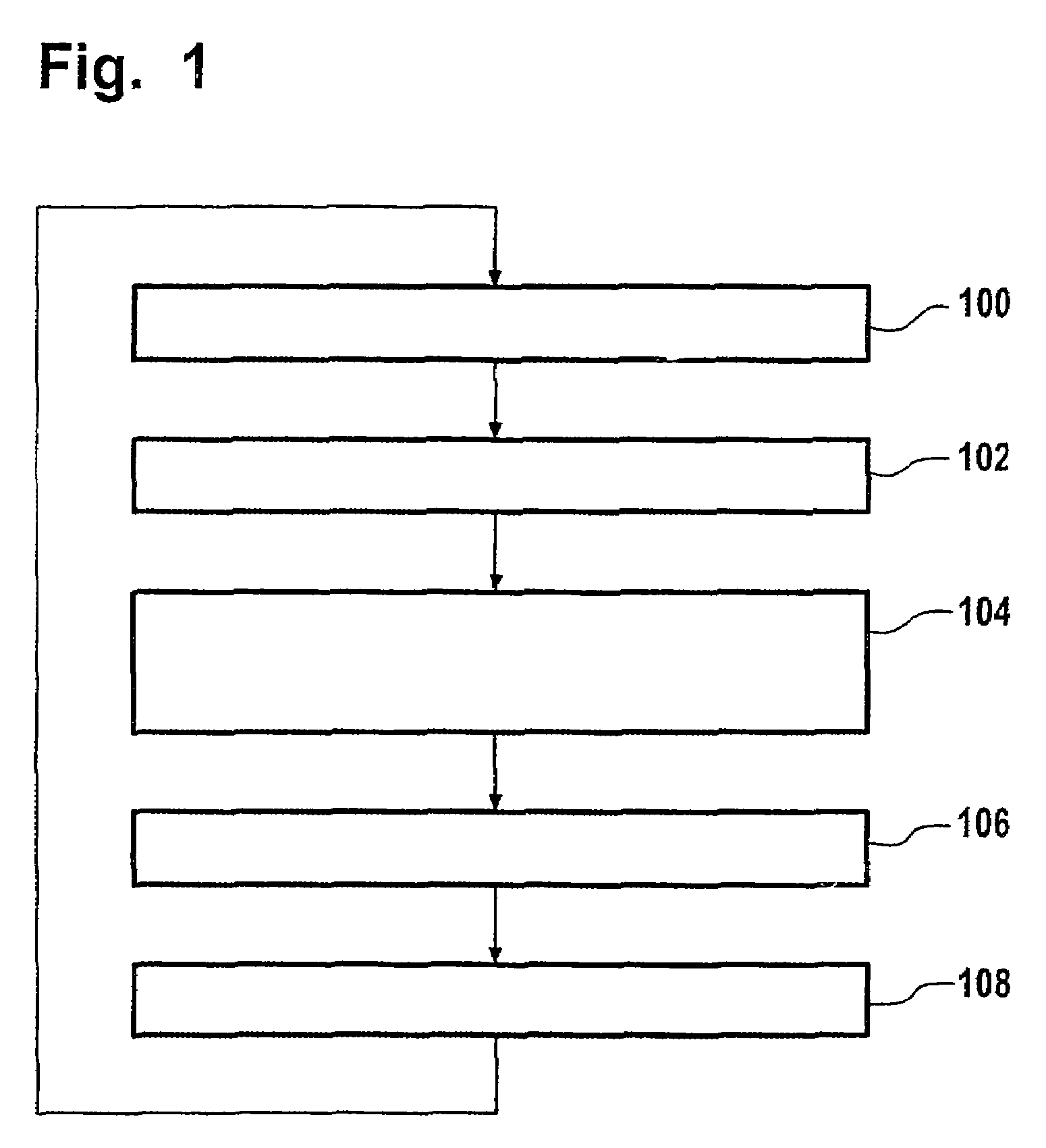
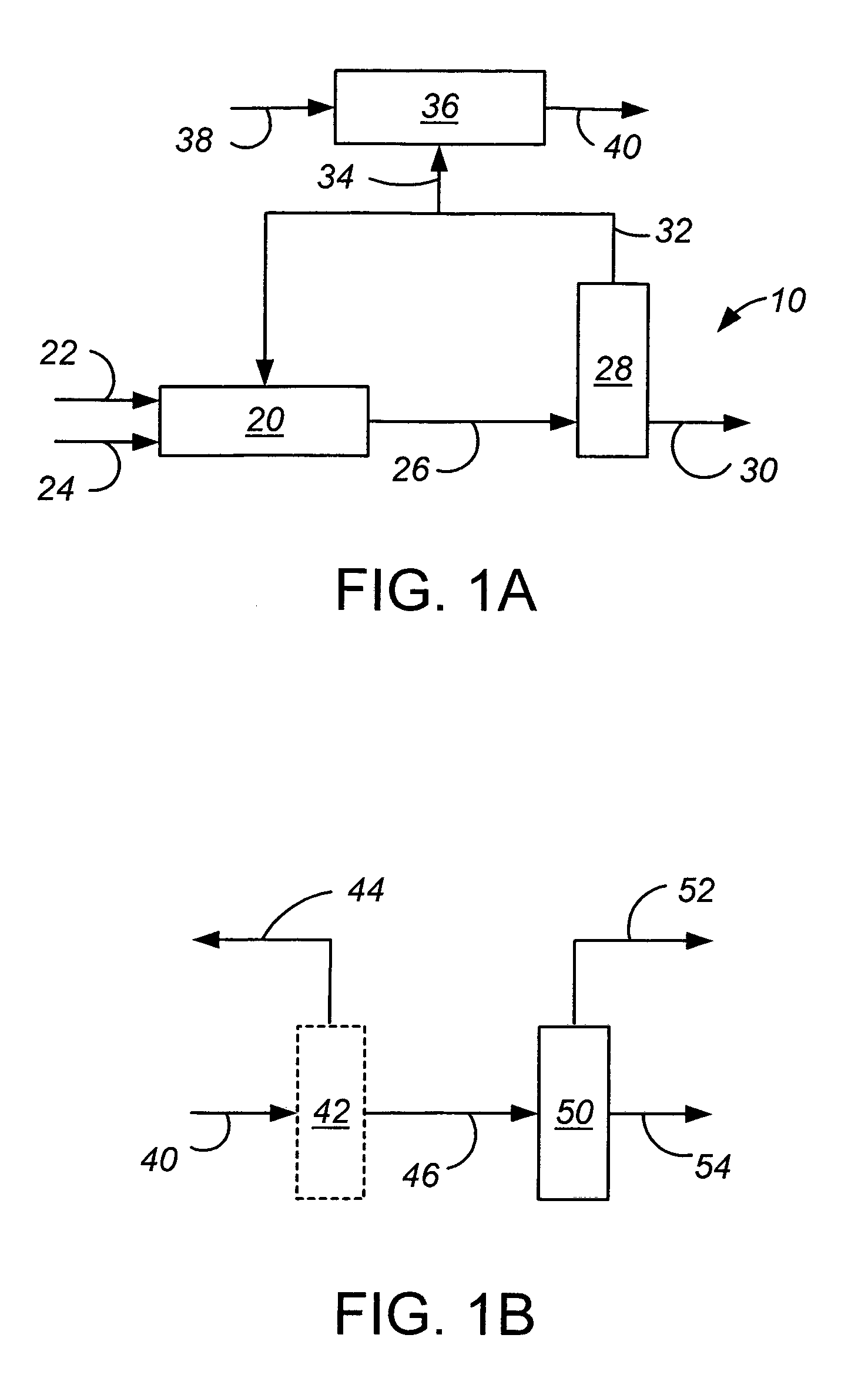
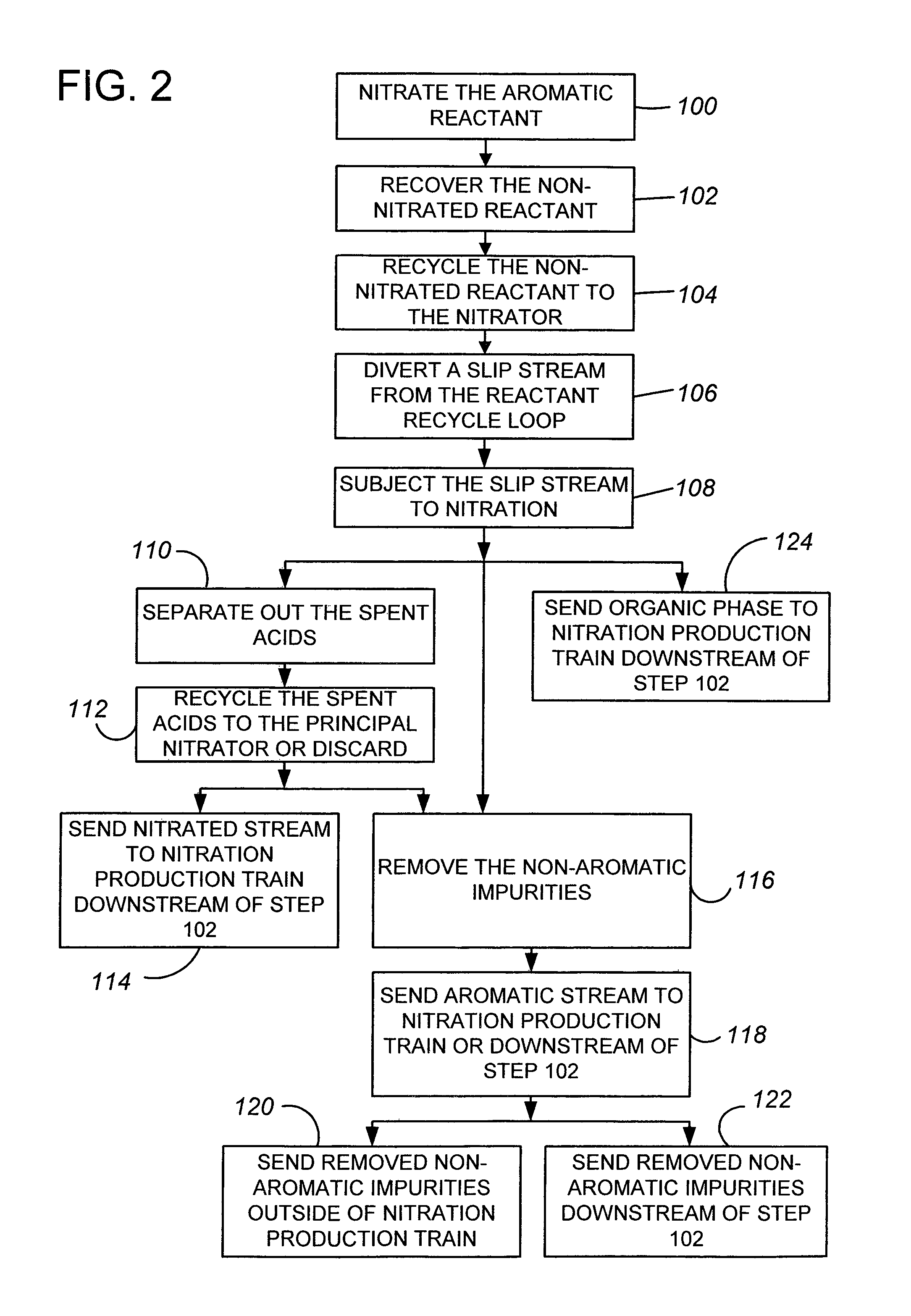
Removal of non-aromatic impurities from a nitration process
A method and apparatus for removing non-aromatic impurities from non-nitrated aromatic reactant in a nitration production process, in which process an aromatic reactant is nitrated (100) to produce a nitrated aromatic product using a molar excess of the aromatic reactant, and non-nitrated aromatic reactant is recovered (102) from the produced nitrated aromatic product and is recycled (104) for use in the nitration production process. A portion of the removed excess non-nitrated aromatic reactant is diverted (106) and subjected to nitration (108). The nitrated stream may be further processed by separating out the spent acids (110) and the non-aromatic impurities (116). These streams may be sent (114, 118) to a suitable location in the nitration production train.
Owner:NORAM INT LTD
Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one
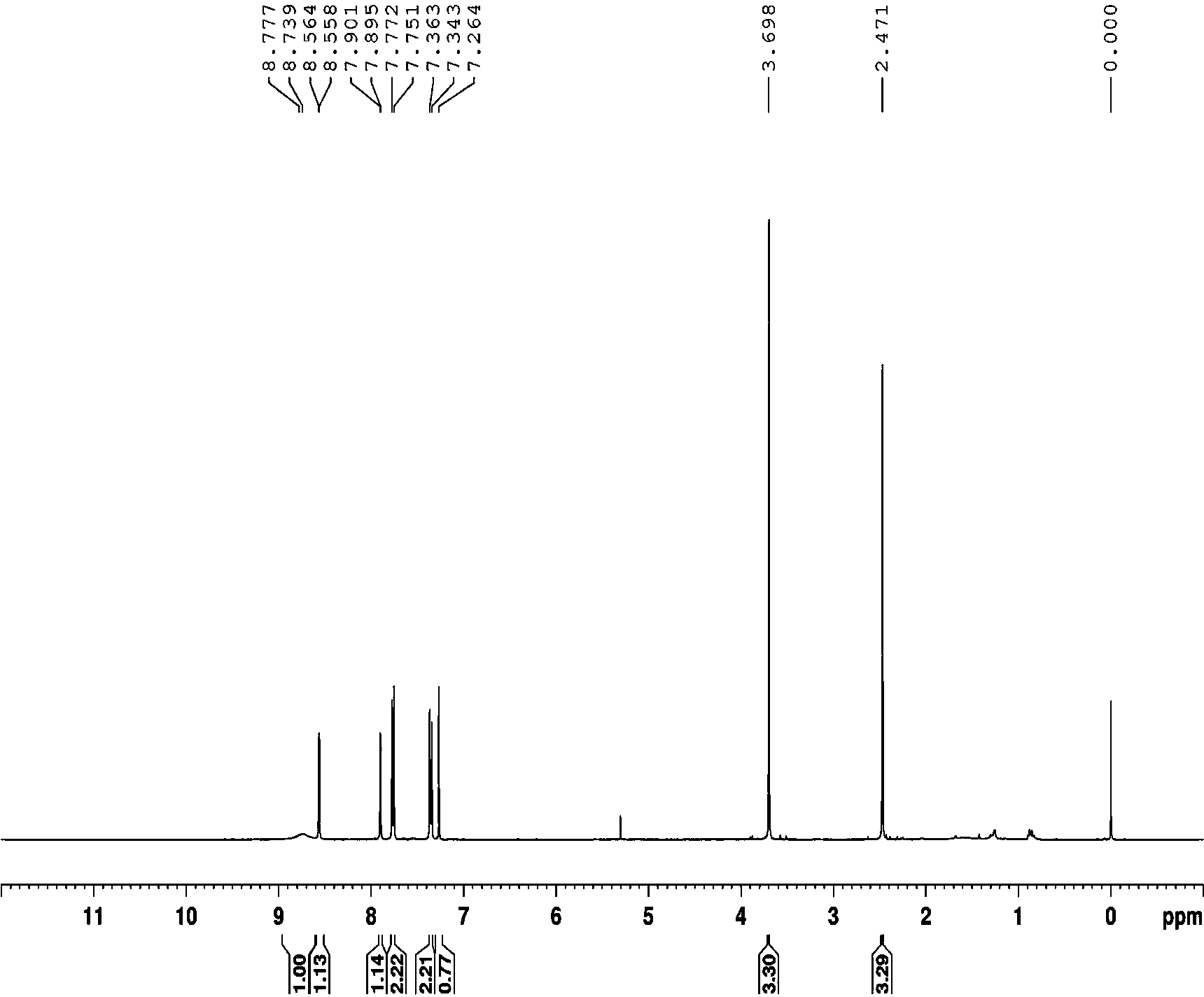
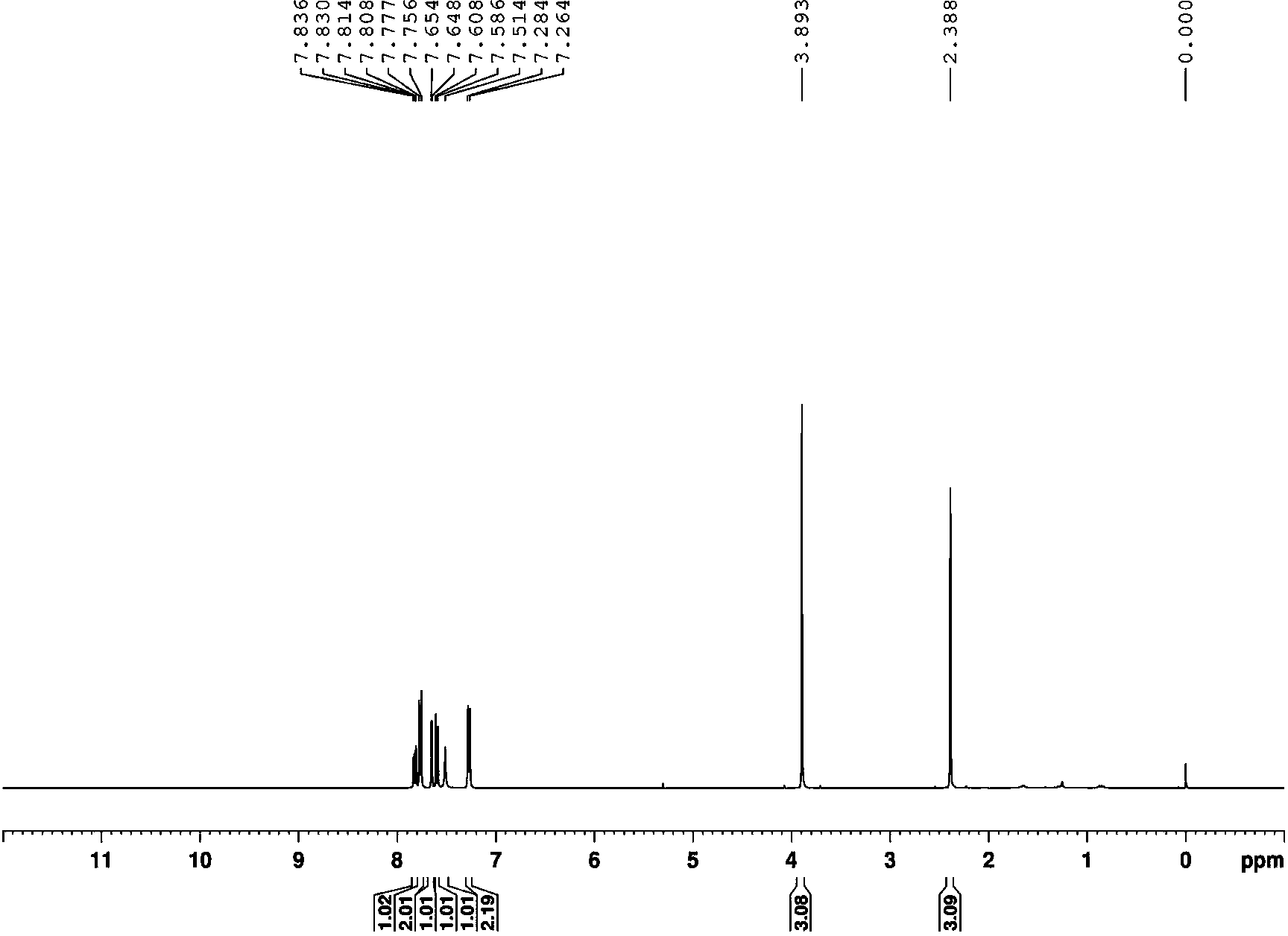
InactiveUS20180134643A1Easy to separateEfficient separationOrganic compounds purification/separation/stabilisationNervous disorderEnantiomerKetone
A process to isolate a compound of Formula (2a) or a salt or solvate thereof, comprising a) reacting a mixture of diastereoisomers of Formulae (2a, 2b) with a basic heterocyclic-aldehyde compound and an optically active amine in the presence of a base; and b) separating the compound of Formula (2a) from the product of step a) by acid extraction. The compound of Formula (2a) may be produced with an enantiomeric excess of 98%. Compounds of Formula (2a) are useful intermediates in a process to prepare a bicyclic γ-amino tetrazole derivative of Formula (I) which finds utility in treating neuropathic pain and disorders of the central nervous system.
Owner:NOVASSAY
Liquid phase nitration of aromatics using solid acid catalyst
ActiveUS20160145191A1High activityHigh selectivityMolecular sieve catalystsChemical industryNitrationSolid acid
The present invention discloses an improved process for the liquid phase nitration of aromatic compounds catalyzed by WO3 supported on mesoporous silica support, at low temperature, with high conversion and selectivity.
Owner:COUNCIL OF SCI & IND RES
Popular searches
Carbonic/haloformic acid esters preparation Amino-hyroxy compound preparation Nitric acid ester preparation Saccharide with acyclic radicals Organic chemistry methods Group 5/15 element organic compounds Sulfonic acid amide preparation Liquid-gas reaction processes Preparation from chlorides Carbonyl compound separation/purification
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

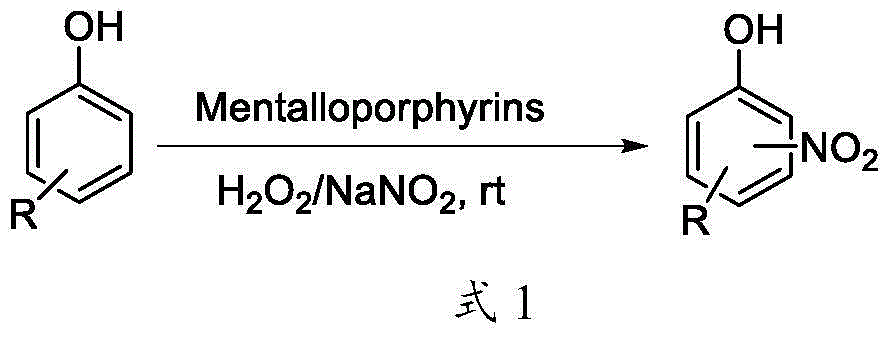


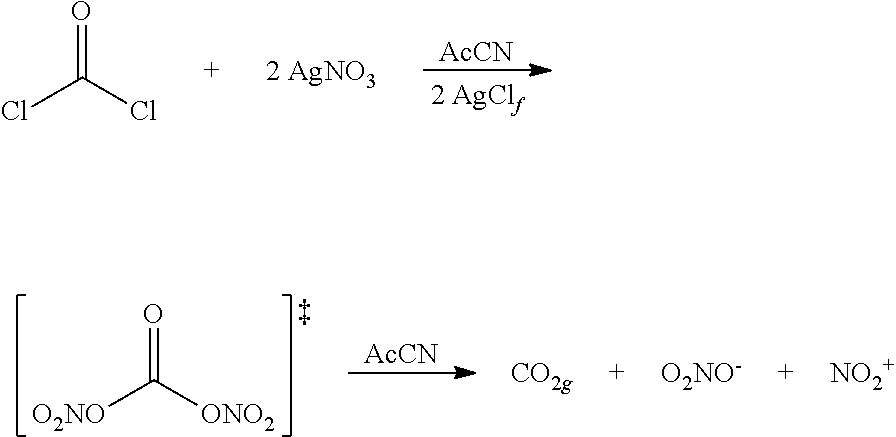
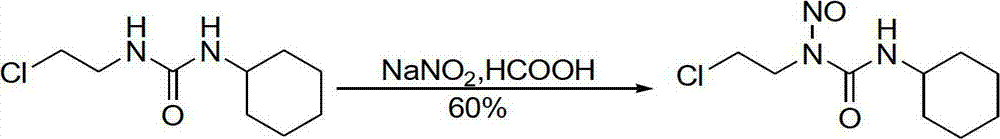
![Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one](https://images-eureka.patsnap.com/patent_img/0037f2f4-c78a-456d-a183-764fbfb325cc/US20180134643A1-20180517-C00001.png)
![Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one](https://images-eureka.patsnap.com/patent_img/0037f2f4-c78a-456d-a183-764fbfb325cc/US20180134643A1-20180517-C00002.png)
![Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one Separation of enantiomers of 3-ethylbicyclo[3.2.0]hept-3-en-6-one](https://images-eureka.patsnap.com/patent_img/0037f2f4-c78a-456d-a183-764fbfb325cc/US20180134643A1-20180517-C00003.png)