Mono amine and diamine derivatives of cl-20
A technology of derivatives and products, applied in the field of synthesis of CL-20 derivatives, can solve problems such as inability to generate diamine derivatives
Inactive Publication Date: 2006-03-29
THE SEC OF STATE FOR DEFENCE IN HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
View PDF4 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, applicants were unable to generate dia
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
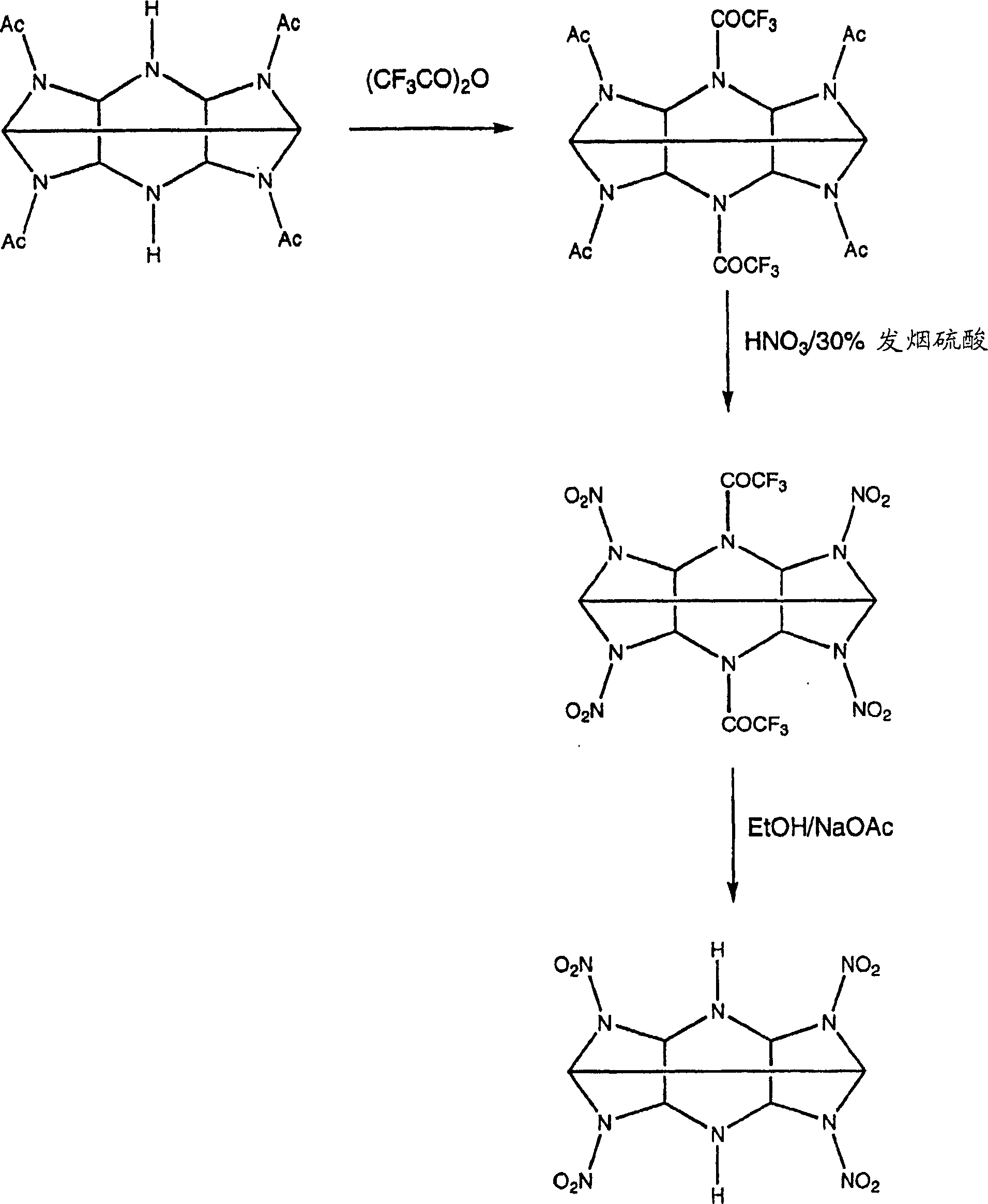
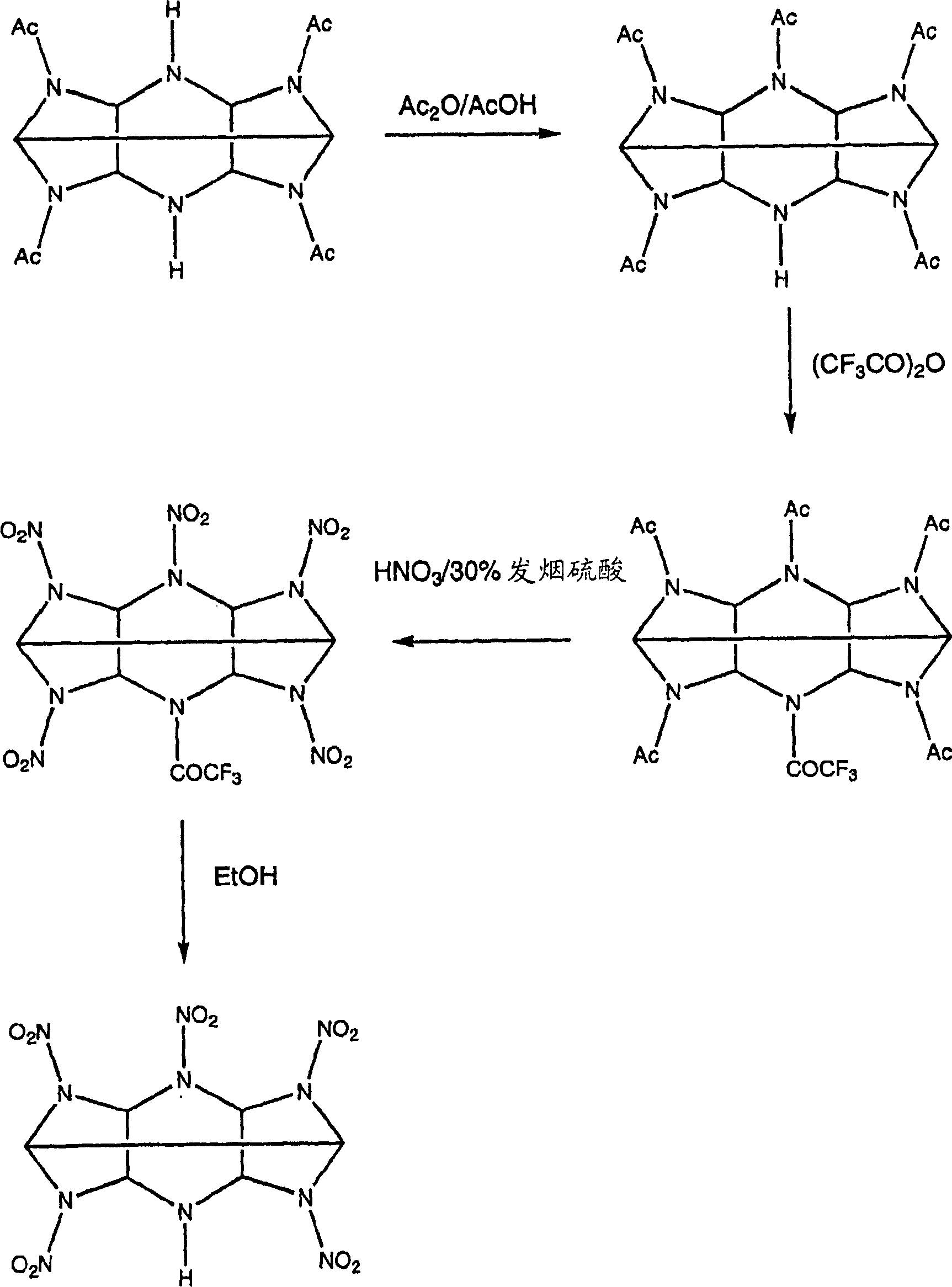
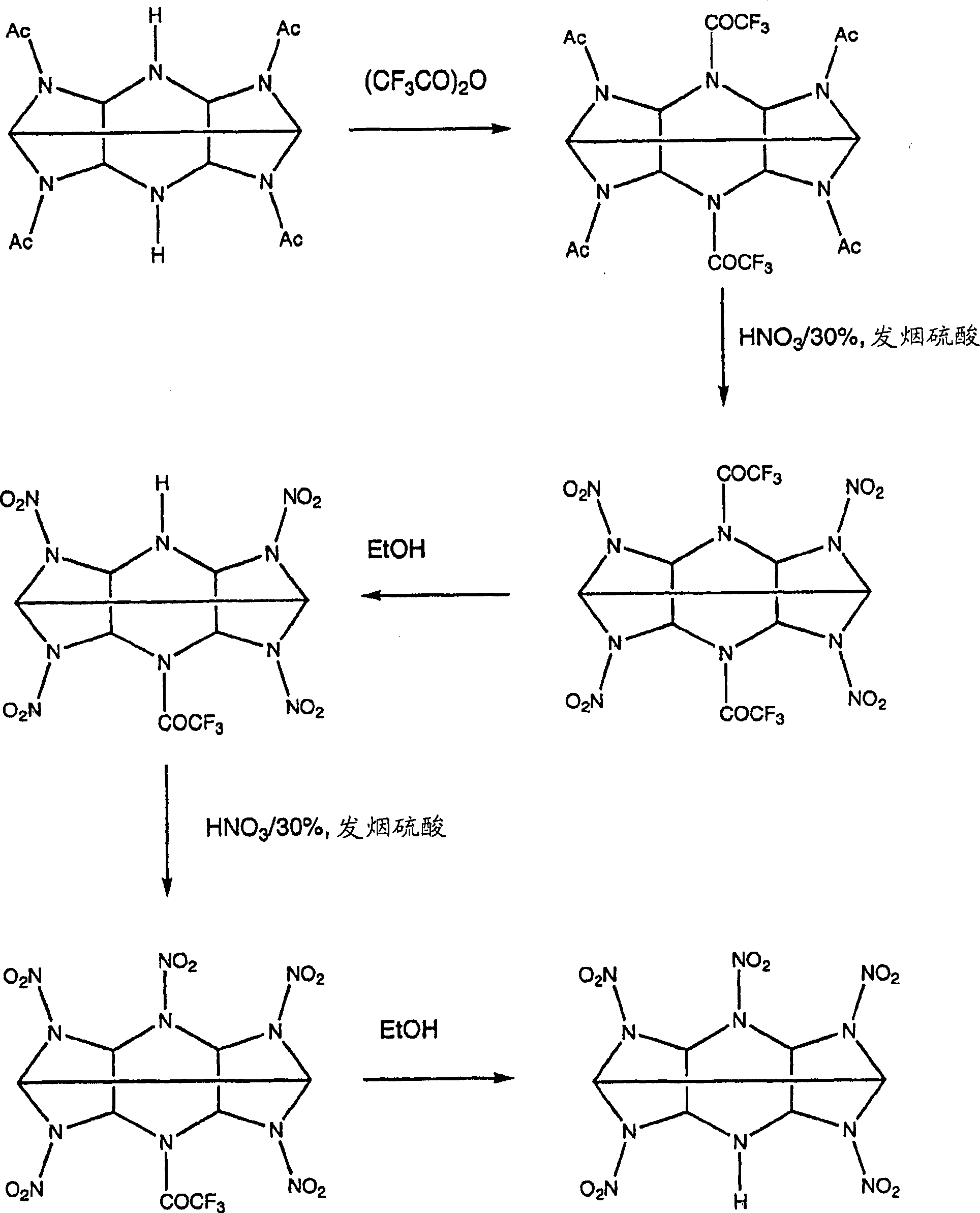
The invention describes the synthesis of novel mono-amine and di-amine derivatives of hexa-nitro-hexaazaisohexawurtzitane (CL-20). The synthesis is effected by the novel use of fluoroacylating compounds to protect the secondary amine groups of acylated precursors to CL-20 against nitrolysis. In so doing the mono-amine and di-amine derivatives of CL-20 are rendered and which in turn may be subsequently utilised as intermediates to generate further novel derivatives with differing physical and chemical properties to the parent compound. Formula (I), wherein:- X=H, and Y=H or NO2.
Description
technical field [0001] The present invention relates to the synthesis of CL-20 derivatives. Background technique [0002] The explosive 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane, known as CL-20, is a explosives, but it is too sensitive for some applications. In its pure form it is very susceptible to damage and fragmentation, releasing CL-20 powder and dust which may cause an accidental explosion. [0003] To reduce the likelihood of such incidents, the crystals of the explosive are coated with a binder. The binder makes it possible to shape the explosive composition into the desired shape, thereby reducing sensitivity. However, in some cases, the interaction between the explosive and the binder was weak and the coating was easily detached from the CL-20 crystals. [0004] One solution is to mix CL-20 with a less sensitive but still explosive compound to reduce the sensitivity of the mixture. CL-20 has been mixed with dinitrotetraoxadiazacyclododecane (...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C06B25/34C07B43/02C07C201/08C07D487/22C07D487/18C07D255/00C07D241/00C07D235/00
CPCC07D487/22C07D487/18C06B25/34Y02P20/55
Inventor 彼得·戈尔丁阿利斯泰尔·J·麦克奎史安东尼·约翰·贝拉米
Owner THE SEC OF STATE FOR DEFENCE IN HER BRITANNIC MAJESTYS GOVERNMENT OF THE UK OF GREAT BRITAIN & NORTHERN IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com