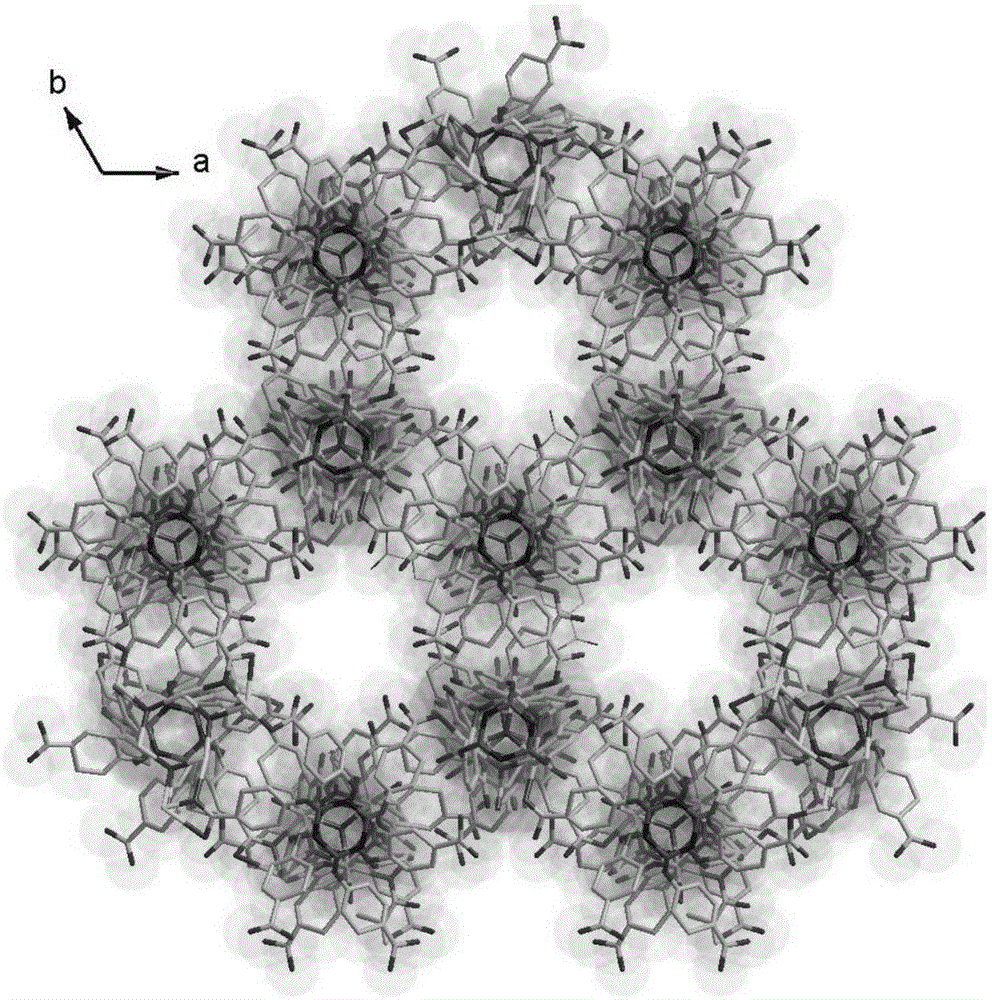
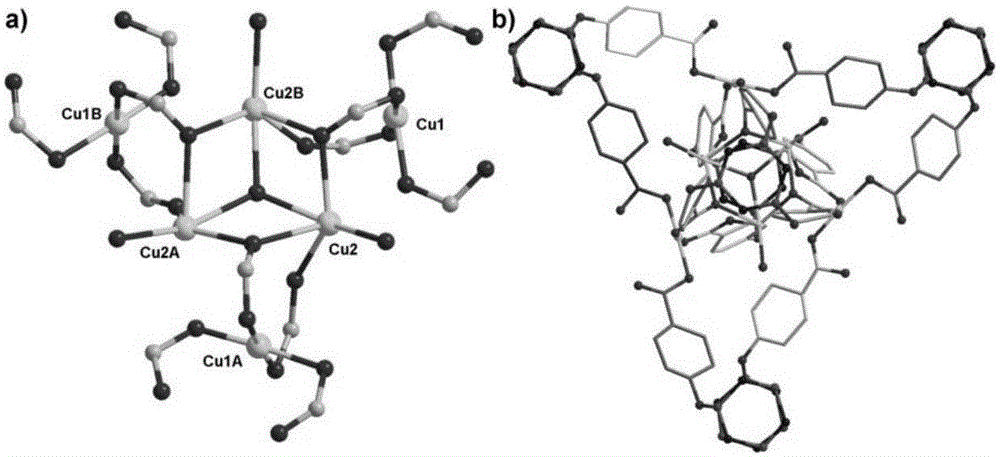
Copper-cyclotriphosphazene hexacarboxylic acid derivative coordination framework material and preparation and application thereof
A technology of cyclotriphosphazene hexacarboxylic acid and framework materials, which is applied in the direction of copper organic compounds, other chemical processes, alkali metal compounds, etc., and can solve the application occasions that do not have specific requirements for molecular arrangement and cannot form specific structures. Derivatives, structural arrangement random problems, etc., to achieve excellent adsorption performance, novel topology, and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 complex 1
[0045] Six-(4-carboxylic acid phenoxy group) cyclotriphosphazene preparation method is as follows:
[0046] First synthesize methyl p-hydroxybenzoate, weigh 6g of p-hydroxybenzoic acid with electronic balance, add in 100ml round bottom flask, then add about 50ml of methanol, drop about 15 drops of concentrated sulfuric acid (mass percentage concentration is 98%) , after refluxing at 70°C for 4h, a colorless transparent solution was obtained, the reaction device was removed, methanol was evaporated to dryness, and finally washed with distilled water to obtain 7g of white product (ie, methyl p-hydroxybenzoate), which was dried for later use, and the yield was about 93 %. Next, add 1.5g (0.0043mol) sublimated hexachlorocyclotriphosphazene (baked at 50°C for 5h), 6.5g (0.043mol) methyl p-hydroxybenzoate (dried), about 1g under Ar atmosphere Potassium carbonate (can be baked at 120°C for about 1-2 hours to ensure dryness), then a...
Embodiment 2
[0054] The preparation of embodiment 2 complex 2
[0055] The preparation method of hexa-(4-carboxylic acid phenoxy)cyclotriphosphazene is the same as in Example 1.
[0056] The synthetic preparation method of complex 2 is as follows:
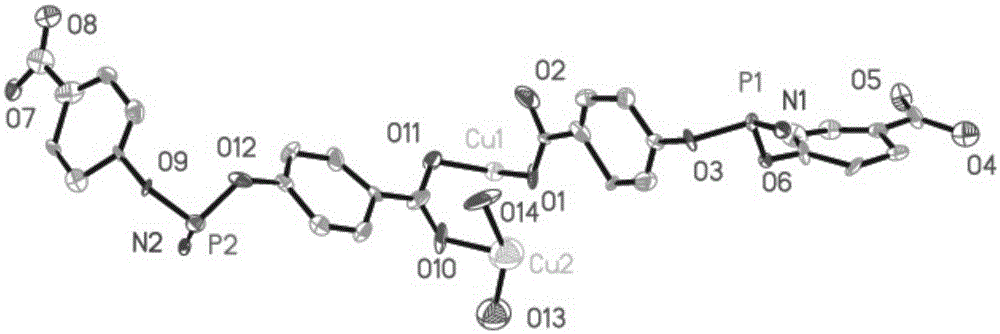
[0057] First weigh 20mg of hexa-(4-carboxyphenoxy)cyclotriphosphazene, 10mg of copper nitrate and 10mg of 4,4'-bipyridine, add 1.5mL of deionized water and 10mL of N,N'-dimethylformamide, After ultrasonic reaction for 5 minutes, add 5 drops of 2molL -1 nitric acid aqueous solution to obtain a solvothermal reaction precursor solution; put the precursor solution into a hydrothermal reaction kettle, and place the reaction kettle in a 100°C oven to react for 4 days, and then cool down at a rate of 5°C / h to obtain blue rod-shaped crystals , filtered and washed with appropriate deionized water to obtain the final product complex 2 (complex 2 is the framework material 2); the complex 2 has the following structure:
[0058]
[0059] The method fo...
Embodiment 3
[0065] The preparation of embodiment 3 complex 1
[0066](1) Ligand synthesis: In an argon atmosphere, add 1.5g of hexachlorocyclotriphosphazene, 6.5g of methyl p-hydroxybenzoate and 1g of potassium carbonate to 50ml of acetone, then stir for 5min, and heat to 60°C for reaction A mixed solution was formed in 20 hours; then, the acetone in the mixed solution was evaporated to dryness to obtain a white solid; then, the white solid was washed with distilled water, then filtered, and then the filter residue obtained by filtering was added to 40ml of ethyl acetate, stirred Spin dry after dissolving, promptly obtain six (4-formic acid methyl ester-phenoxy) cyclotriphosphazene; In 30mL methanol, add 5g six (4-formic acid methyl ester-phenoxy) cyclotriphosphazene, and 6g Sodium hydroxide was stirred and heated to reflux at 65° C. for at least 12 hours, and then the methanol was dried under reduced pressure to obtain solid I; then, 150 mL of distilled water was added to the solid I to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| Carbon dioxide adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com