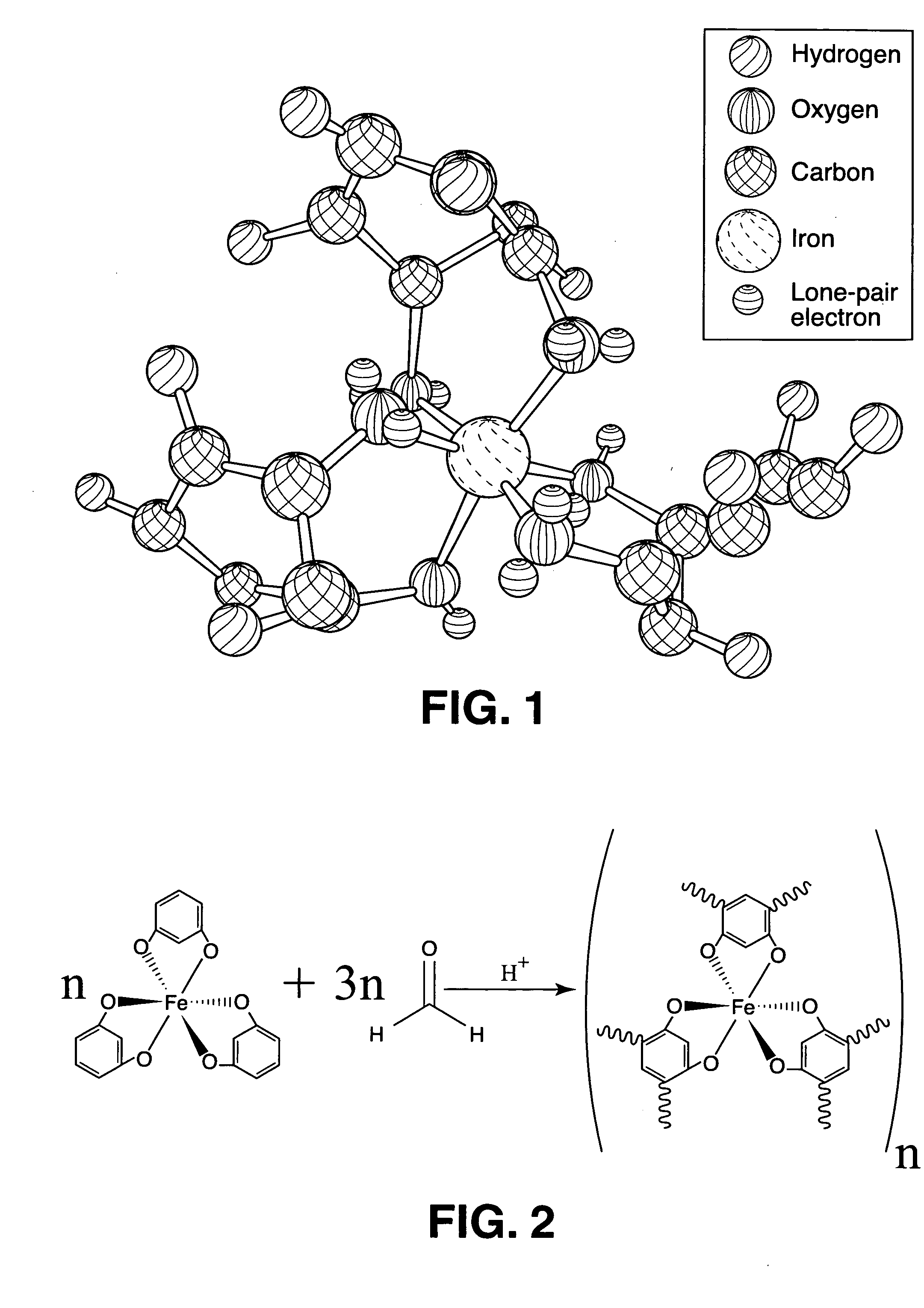
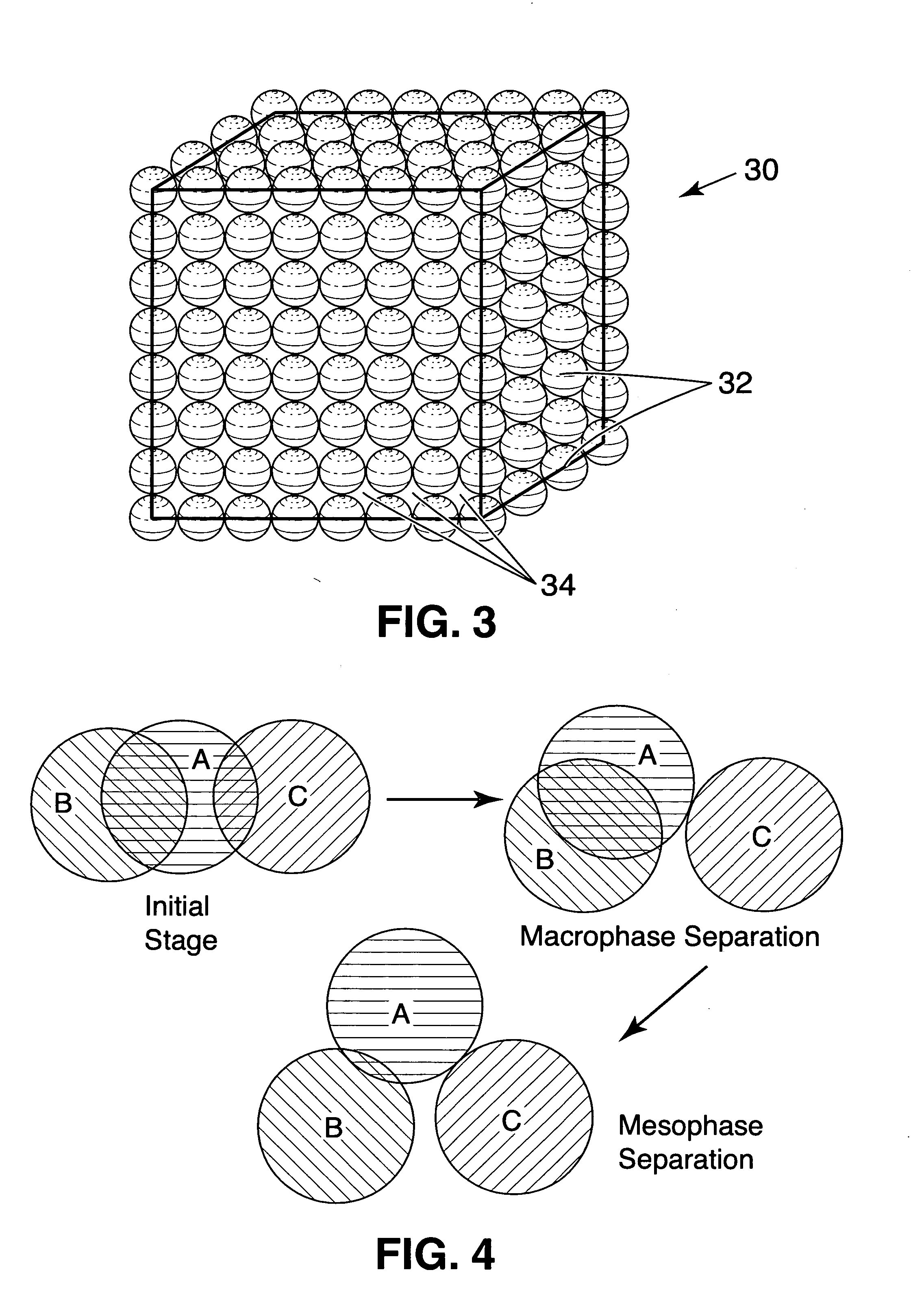
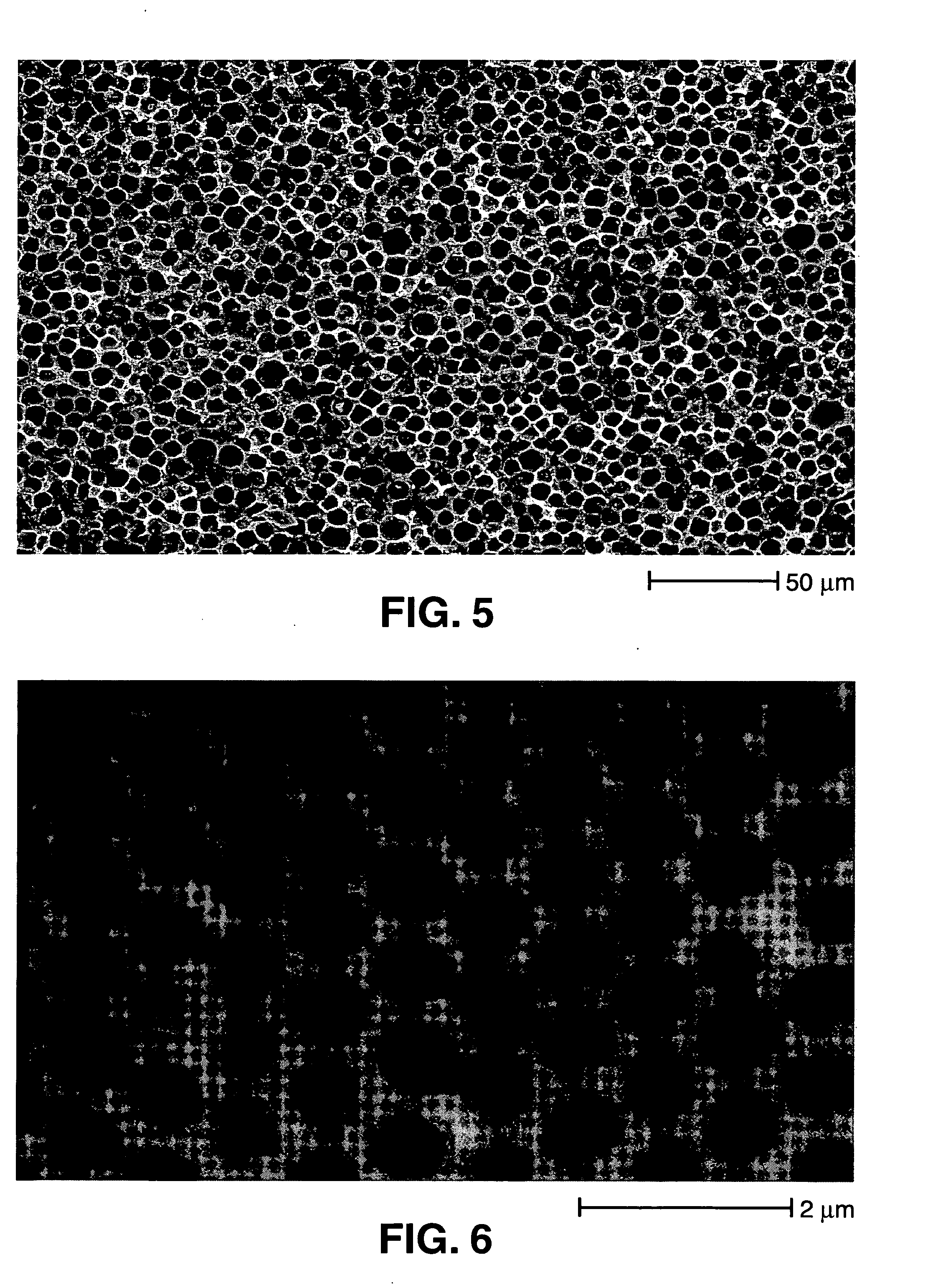
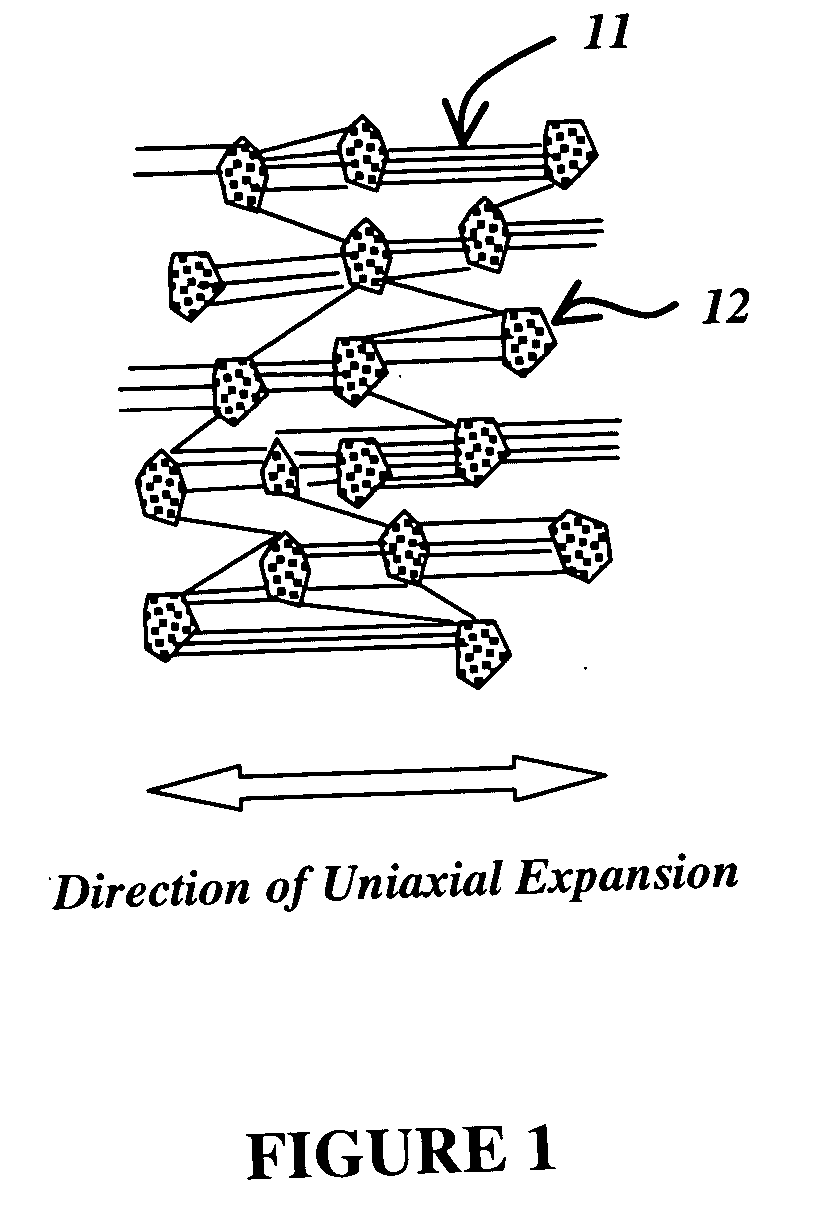
Patents
Literature
17238results about "Alkali metal oxides/hydroxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
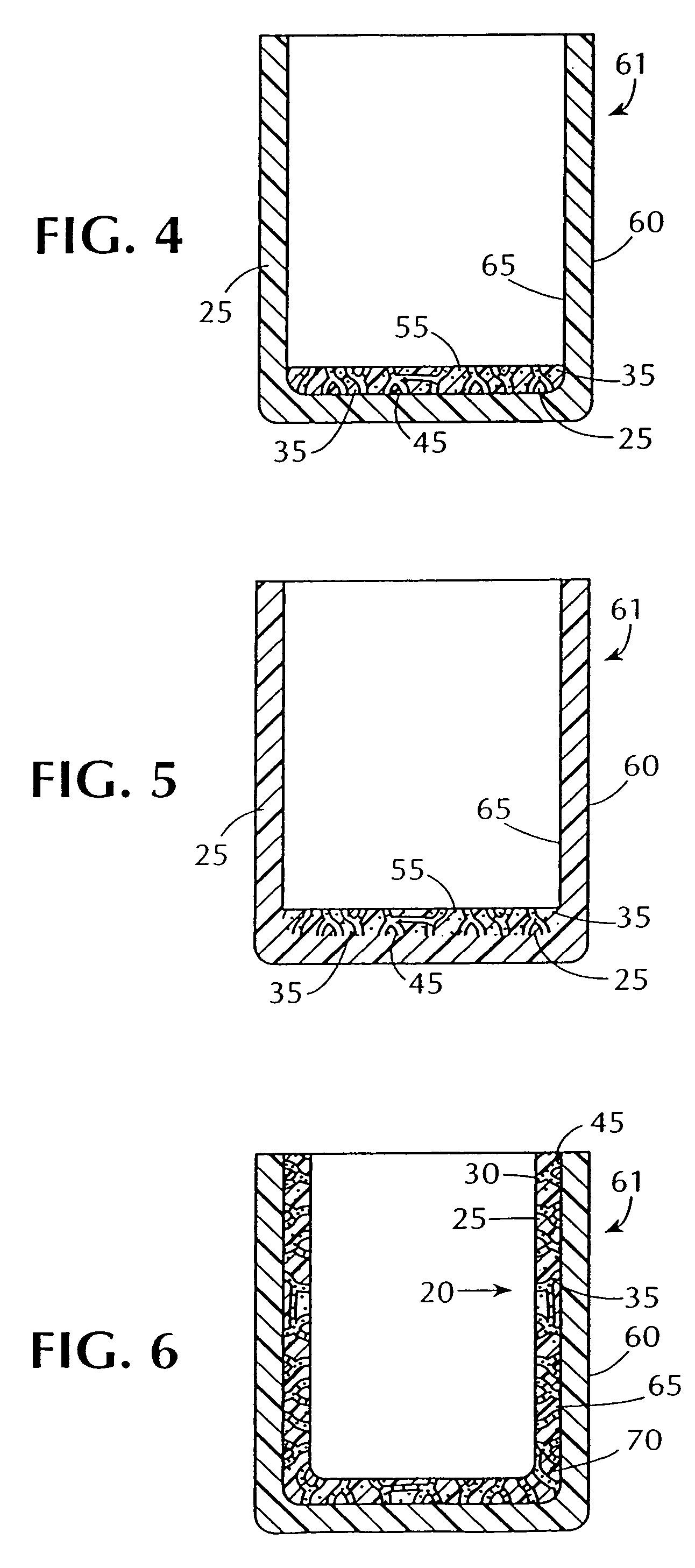
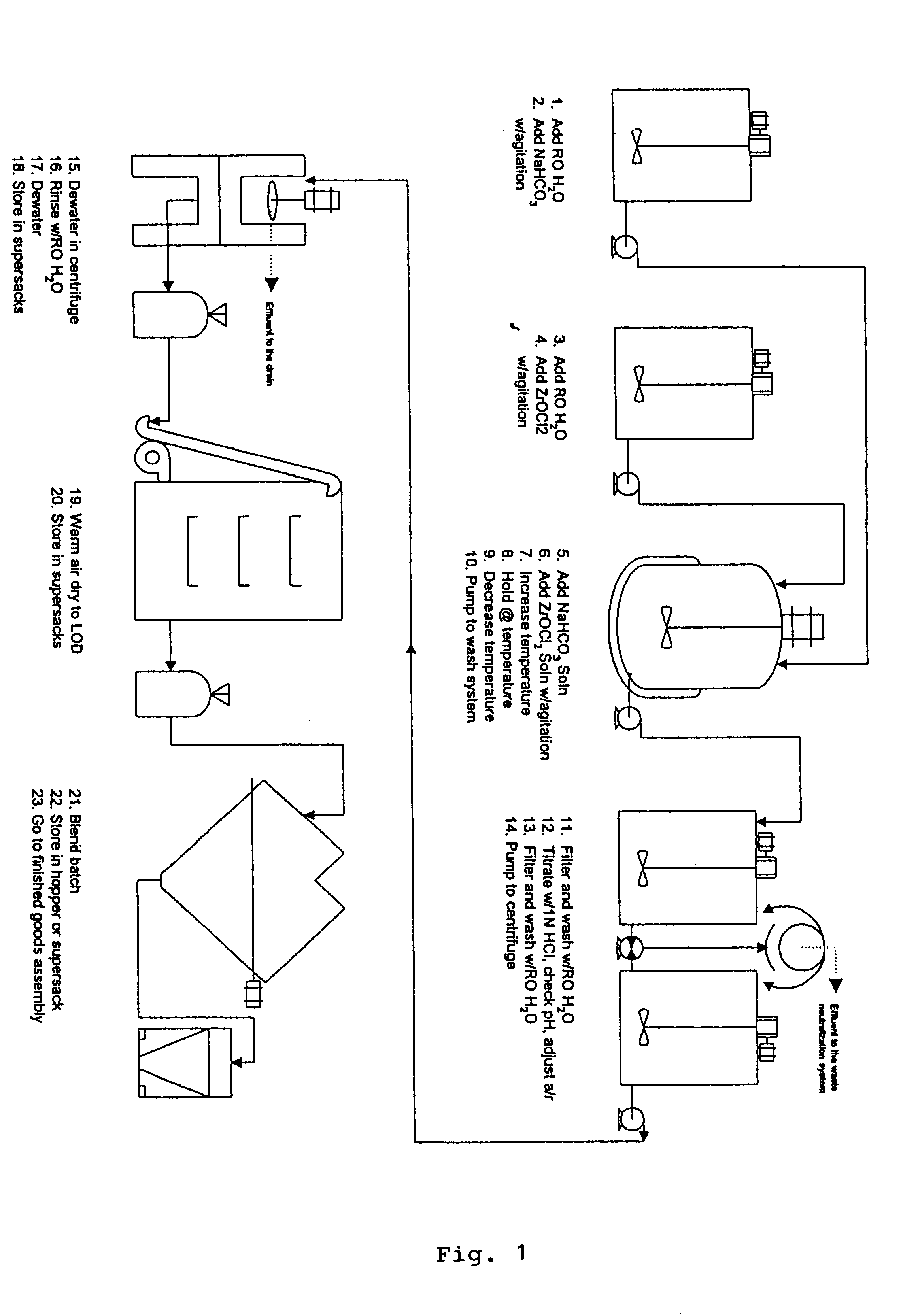
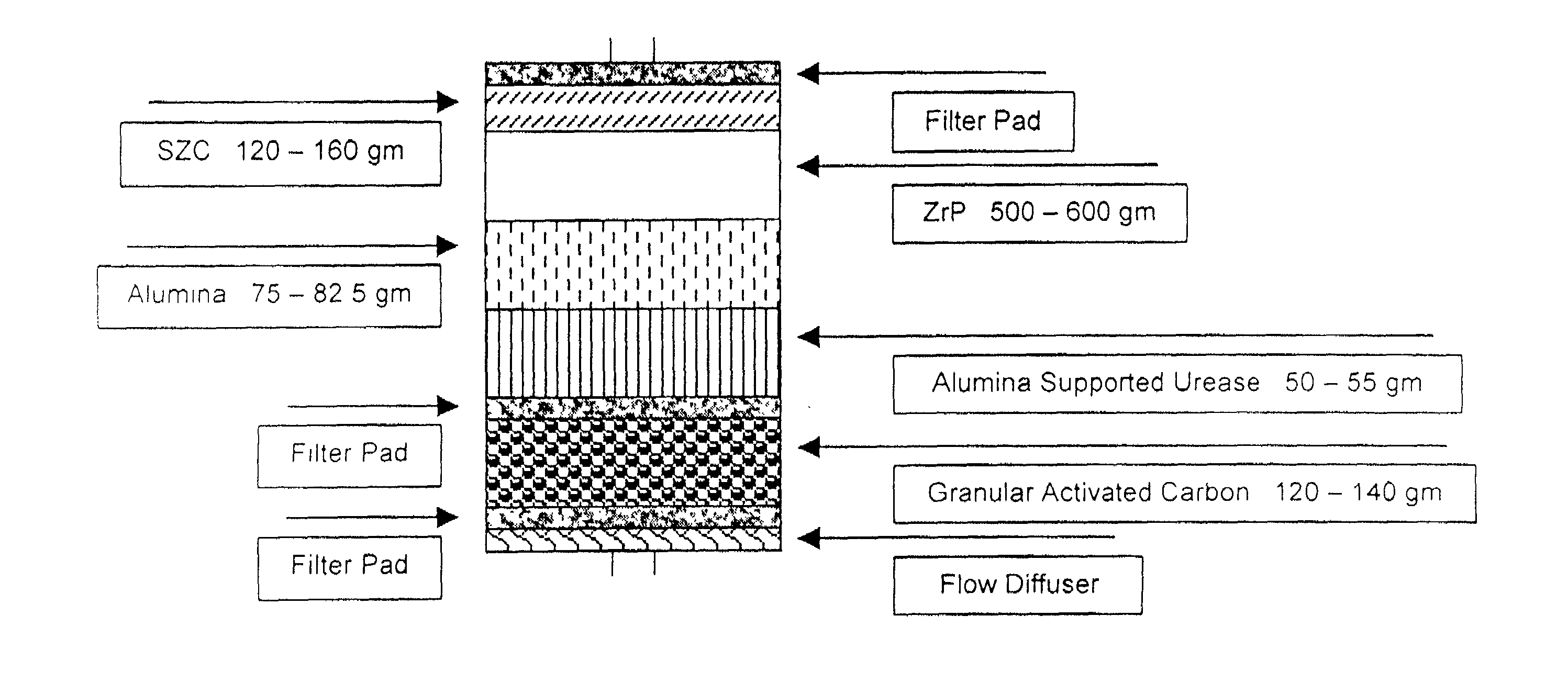
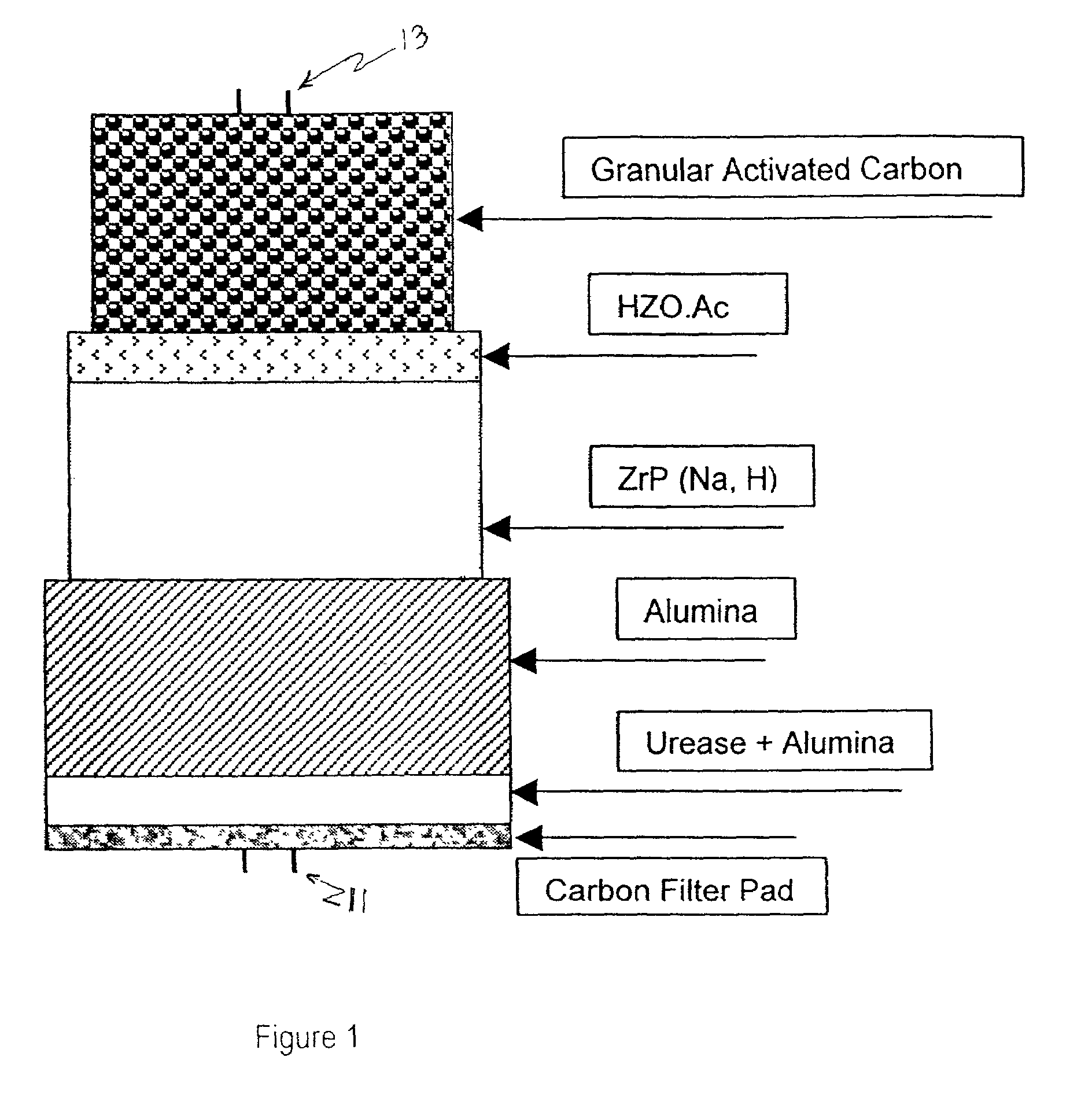
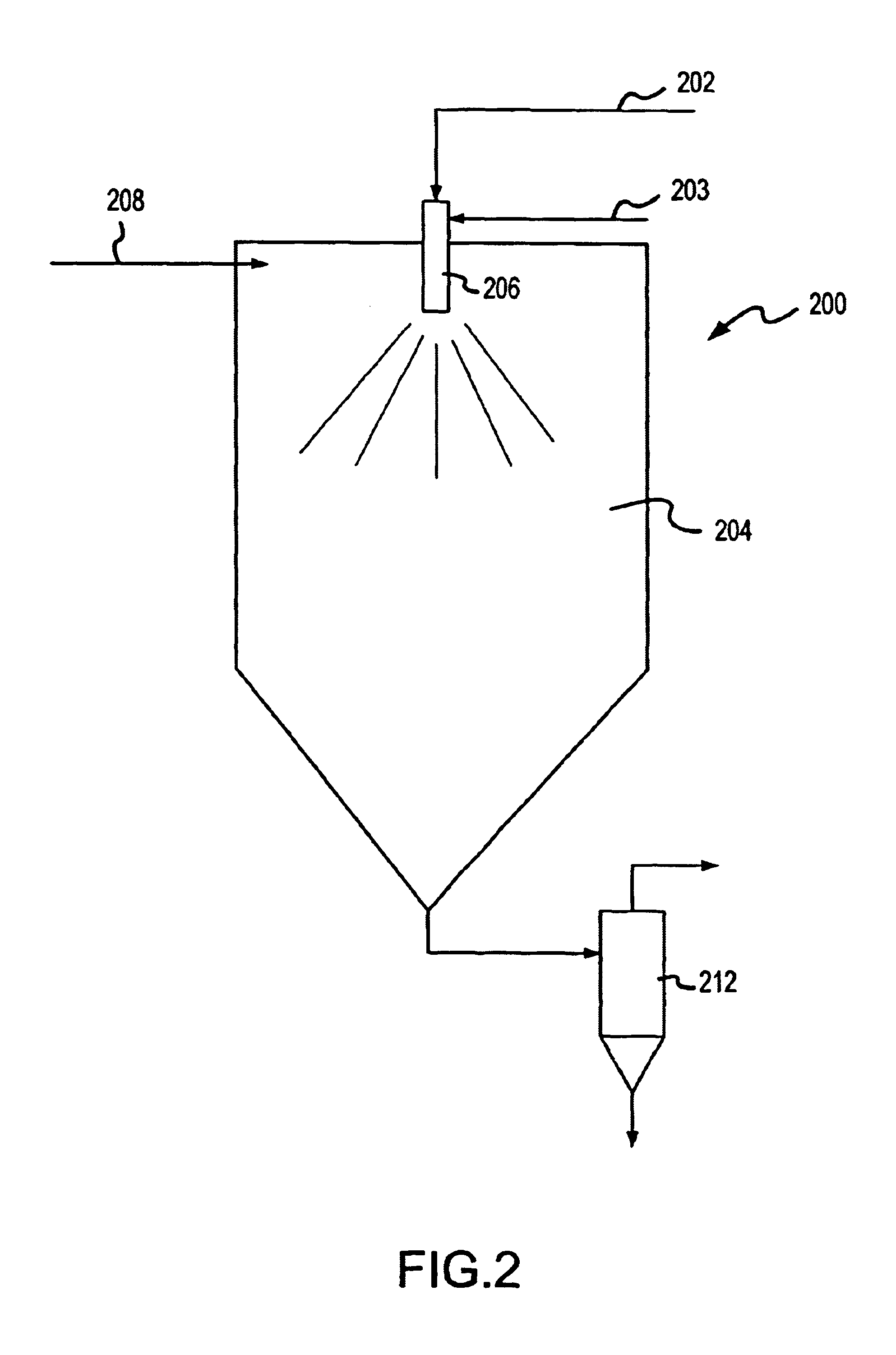
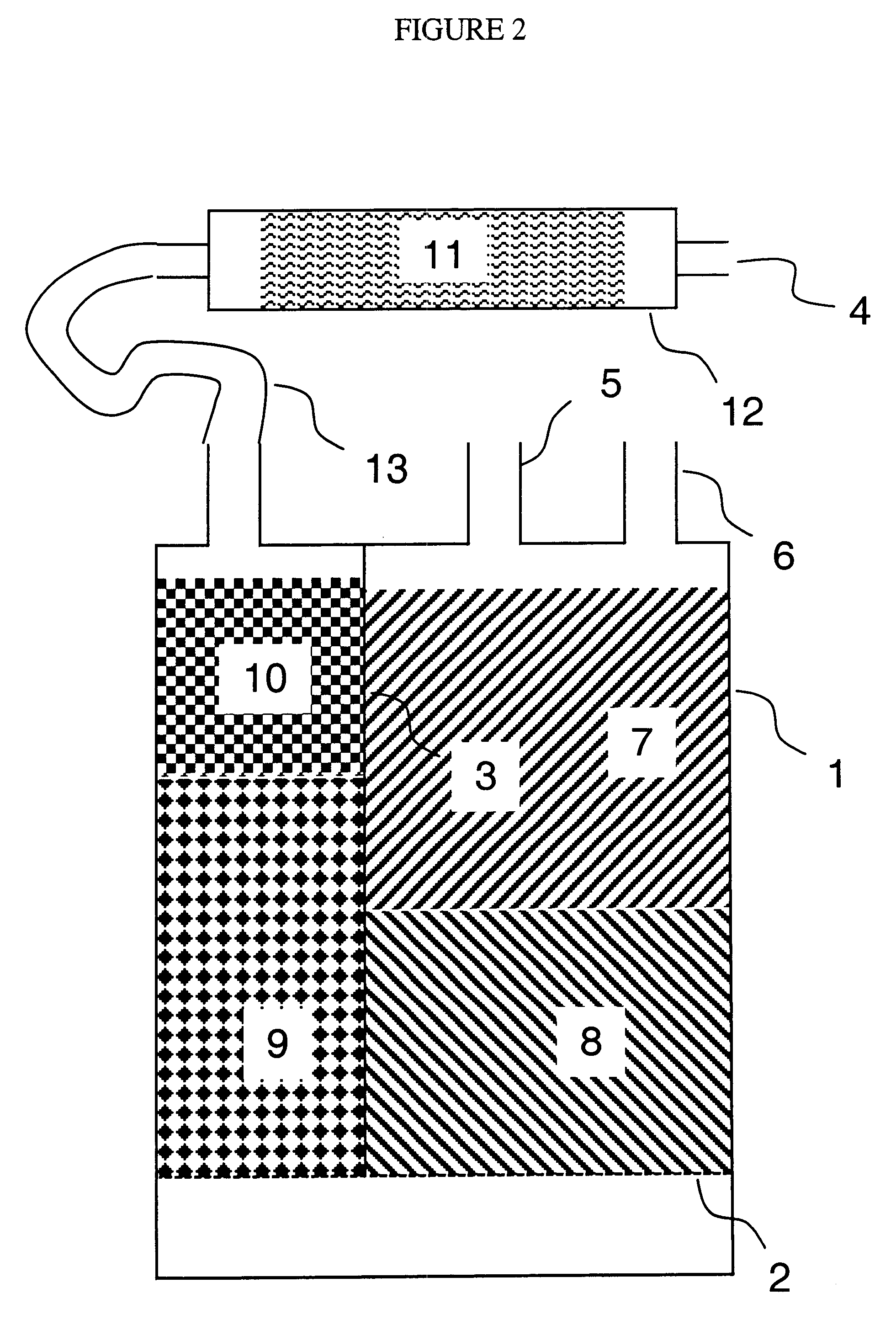
Cartridges useful in cleaning dialysis solutions
InactiveUS20020112609A1Avoid a lotSolve the blockageIsotope separationLoose filtering material filtersHemodialysis membraneChemistry
Owner:RENAL SOLUTIONS
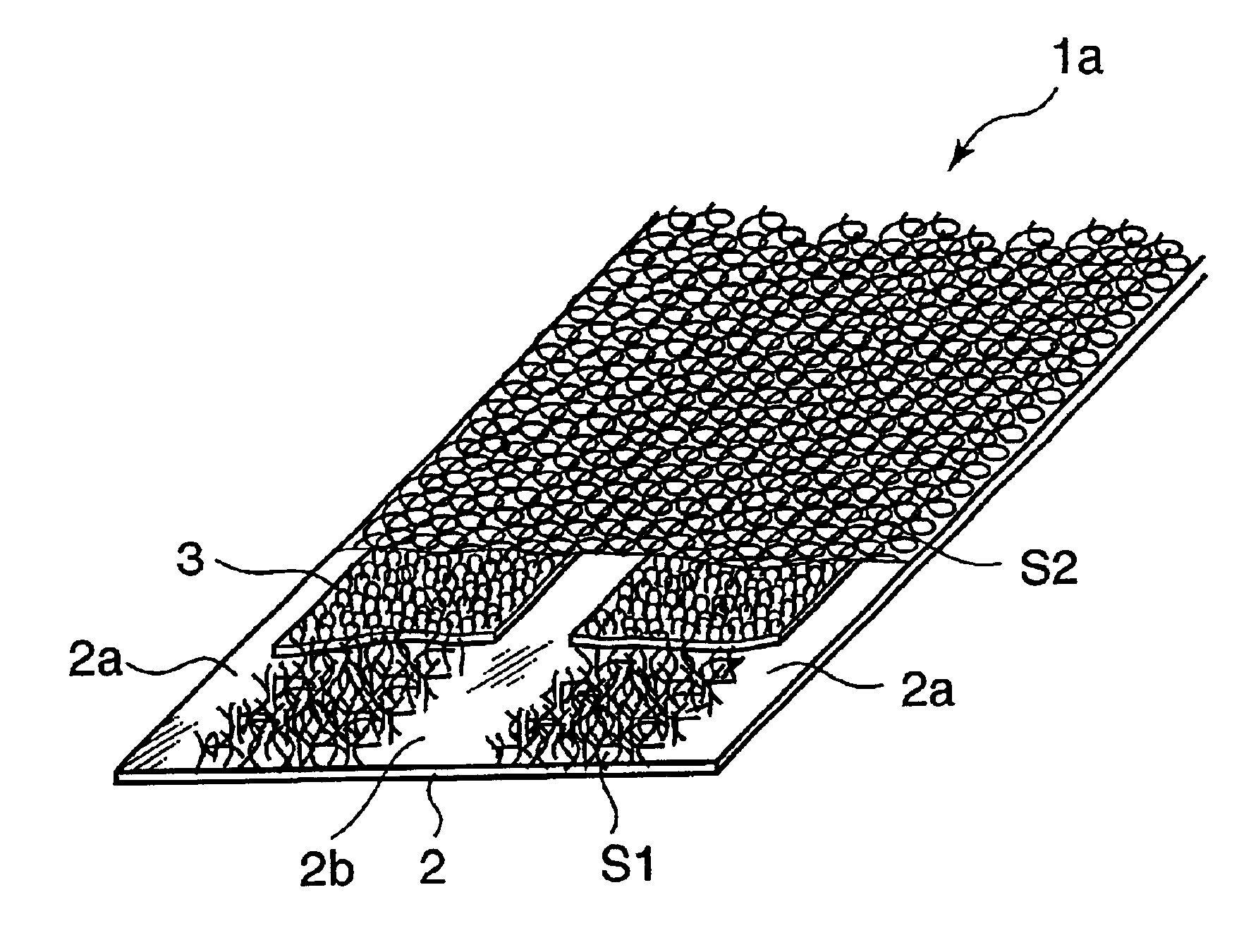
Ultra-thin absorbing sheet body, disposable absorbent article provided with ultra-thin absorbing sheet body and production device for ultra-thin absorbing sheet body
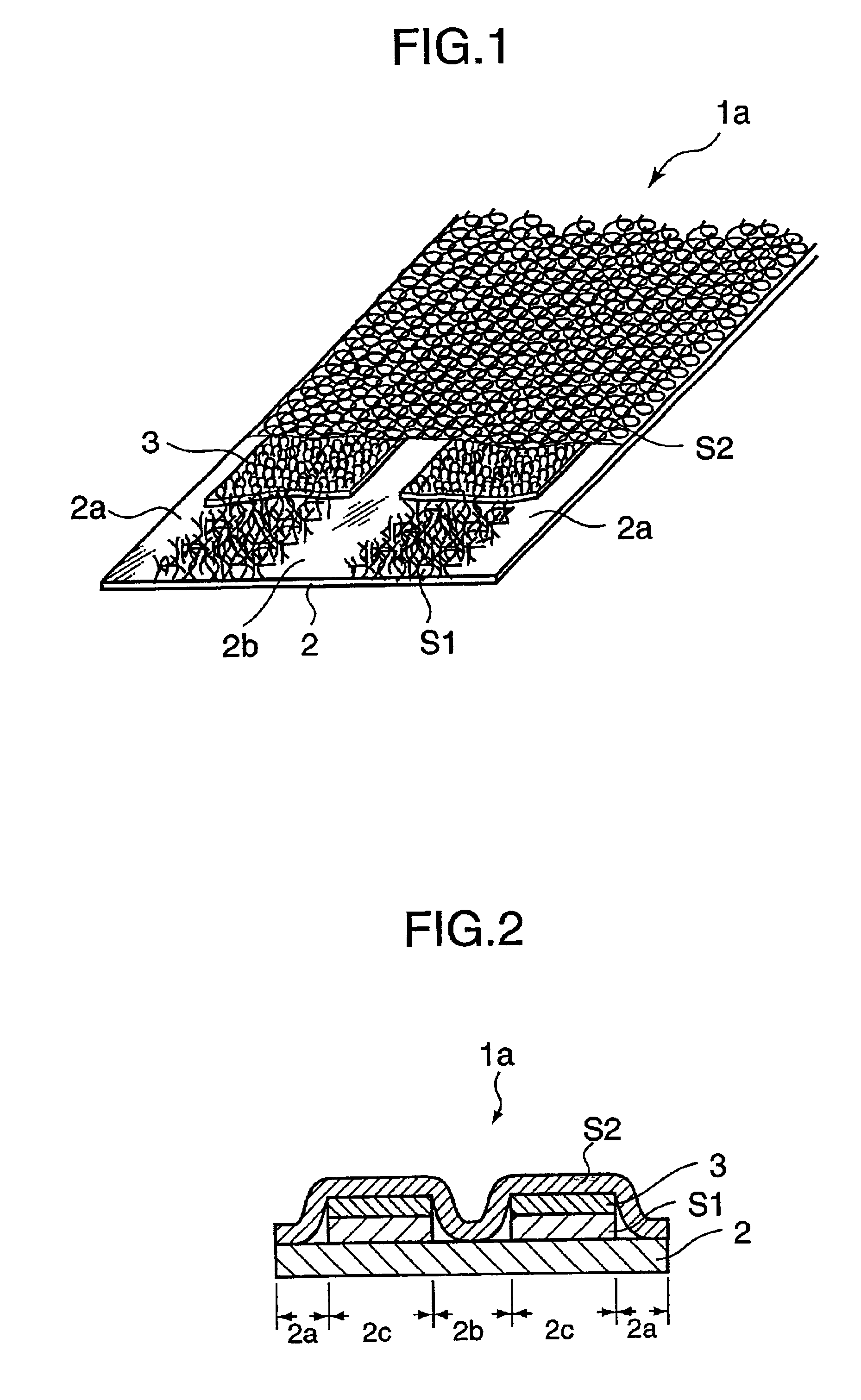
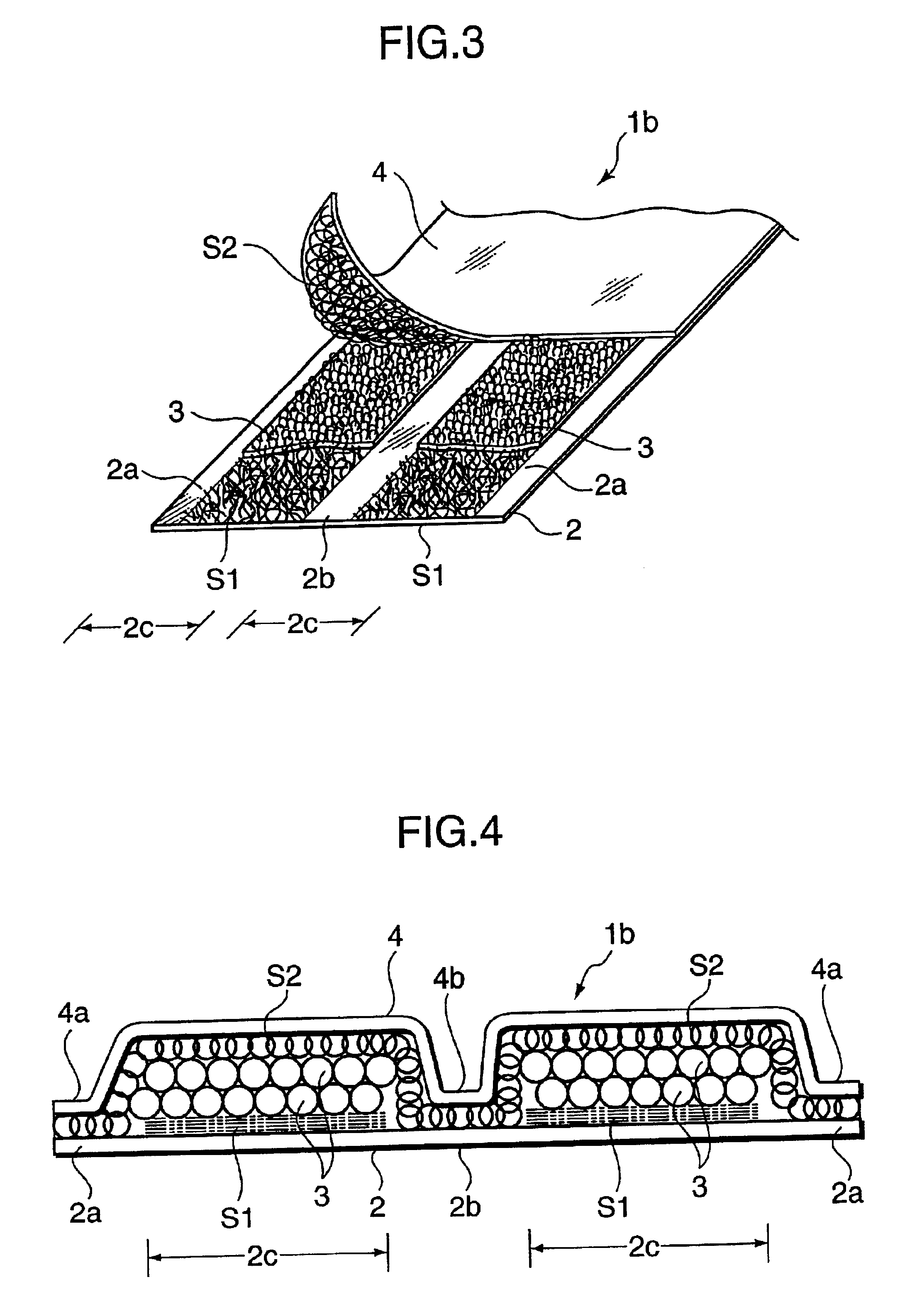
In an ultra-thin absorbent sheet member 1a in which an absorbent polymer powder 3 is adhered to one surface of a first nonwoven fabric 2 by a hotmelt adhesive such that absorbent polymer powder present areas 2c and absorbent polymer powder absent areas 2a, 2b exist; the absorbent polymer powder absent areas are present at opposite widthwise ends (2a) of the ultra-thin absorbent sheet member and at least one position (2b) between the opposite ends; the absorbent polymer powder 3 is bonded to the first nonwoven fabric 2 by first hotmelt adhesive layers S1 formed on an upper side of the first nonwoven fabric 2 and on a lower side of the absorbent polymer powder 3 and a second hotmelt adhesive layer S2 formed to cover upper sides of the absorbent polymer powder present areas 2c and the absorbent polymer powder absent areas 2a, 2b; and the first hotmelt adhesive layer S1 and the second hotmelt adhesive layer S2 are both made of an aggregate of linear hotmelt adhesive pieces.
Owner:TOOYOO EIZAI
Desiccant entrained polymer
The present invention includes a composition having a co-continuous interconnecting channel morphology. These co-continuous interconnecting channels are predominately occupied with a polymer and particles that control the percolation through the composition. The particles are composed of a material such as an absorbing agent, releasing agent and / or activation agent. The polymer composition may be used to form a desired shaped article such as plug type inserts and liners for closed containers, or it may be formed into a film, sheet, bead or pellet.
Owner:CSP TECH NORTH AMERICA
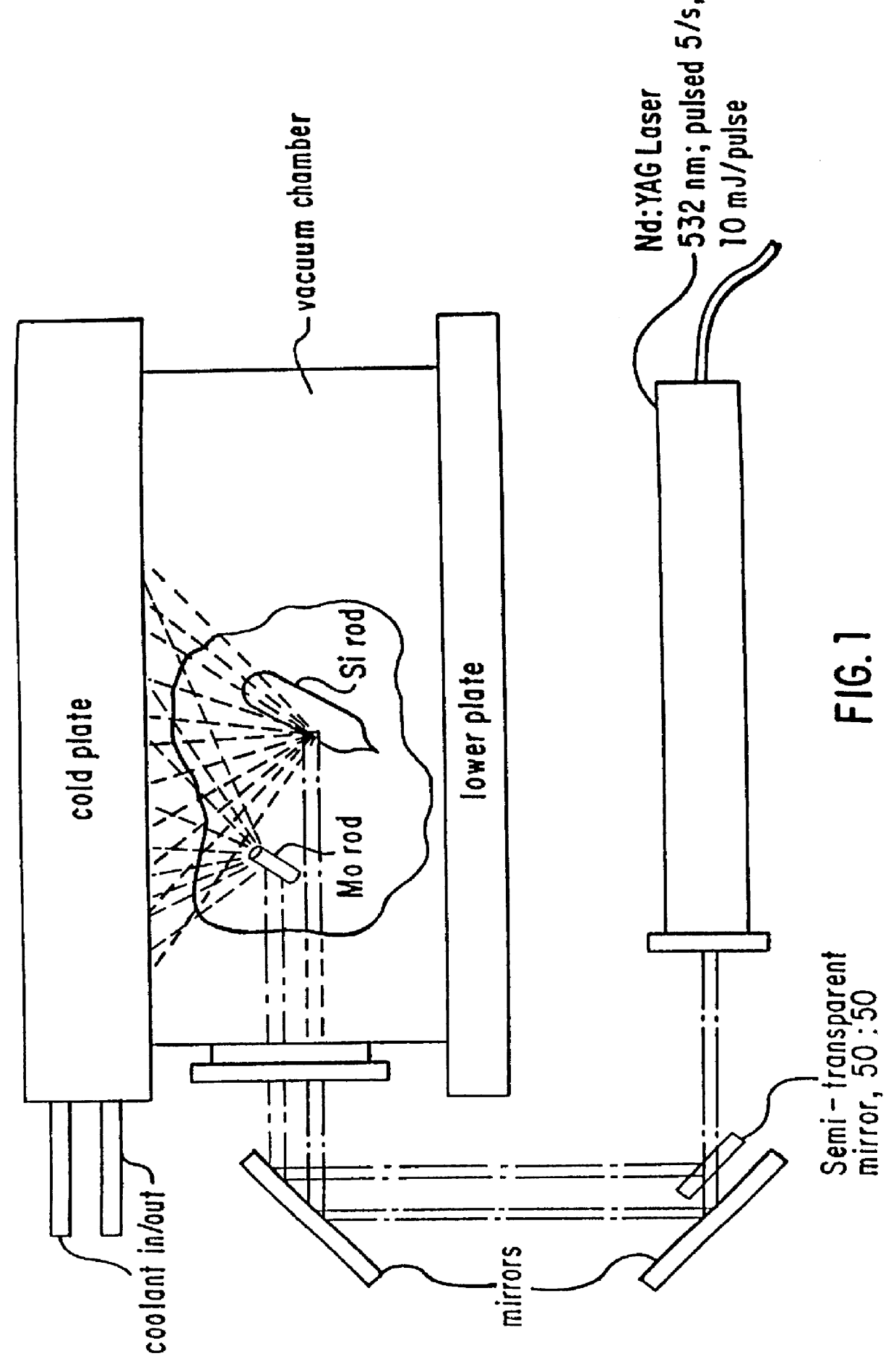
Nanoparticles of silicon oxide alloys
Nanoparticles of silicon oxide alloys (i.e., oxides of SiMo, SiPt, and SiAl) are produced by laser vaporization of a silicon target and a target of a metal (i.e., Mo, Pt, or Al), in an oxygen containing atmosphere in a diffusion cloud chamber, where the target metal vapors aggregate into novel three-dimensional porous web structures. The structures have a homogeneous composition with a uniform ratio of silicon to the metal.
Owner:VIRGINIA COMMONWEALTH UNIV
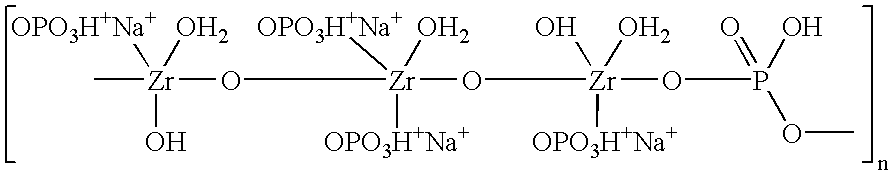
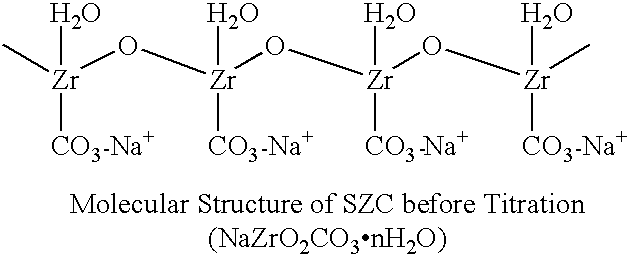
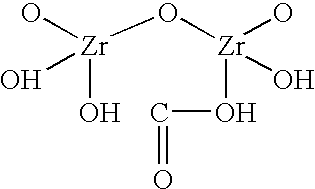
Sodium zirconium carbonate and zirconium basic carbonate and methods of making the same
InactiveUS6627164B1Quality improvementImprove adsorption capacityHeavy metal active ingredientsOther chemical processesSufficient timeFiltration
A method of making sodium zirconium carbonate is described which involves forming a mixture of zirconium oxychloride with soda ash and then heating at a sufficient temperature and for a sufficient time to form the sodium zirconium carbonate. Subsequent washing and filtration steps can further form parts of this process. A novel sodium zirconium carbonate is further described which contains from about 2 wt % to about 5 wt % Na<+>; from about 44 wt % to about 50 wt % ZrO2; from about 12 wt % to about 18 wt % CO3<2->; and from about 32 wt % to about 35 wt % H2O.
Owner:RENAL SOLUTIONS
Cartridges useful in cleaning dialysis solutions
InactiveUS7033498B2Avoid a lotSolve the blockageZirconium compoundsLoose filtering material filtersMedicineHemodialysis membrane
Owner:RENAL SOLUTIONS
Electrocatalyst powders, methods for producing powders and devices fabricated from same
Electrocatalyst powders and methods for producing electrocatalyst powders, such as carbon composite electrocatalyst powders. The powders have a well-controlled microstructure and morphology. The method includes forming the particles from an aerosol of precursors by heating the aerosol to a relatively low temperature, such as not greater than about 400° C.
Owner:CABOT CORP
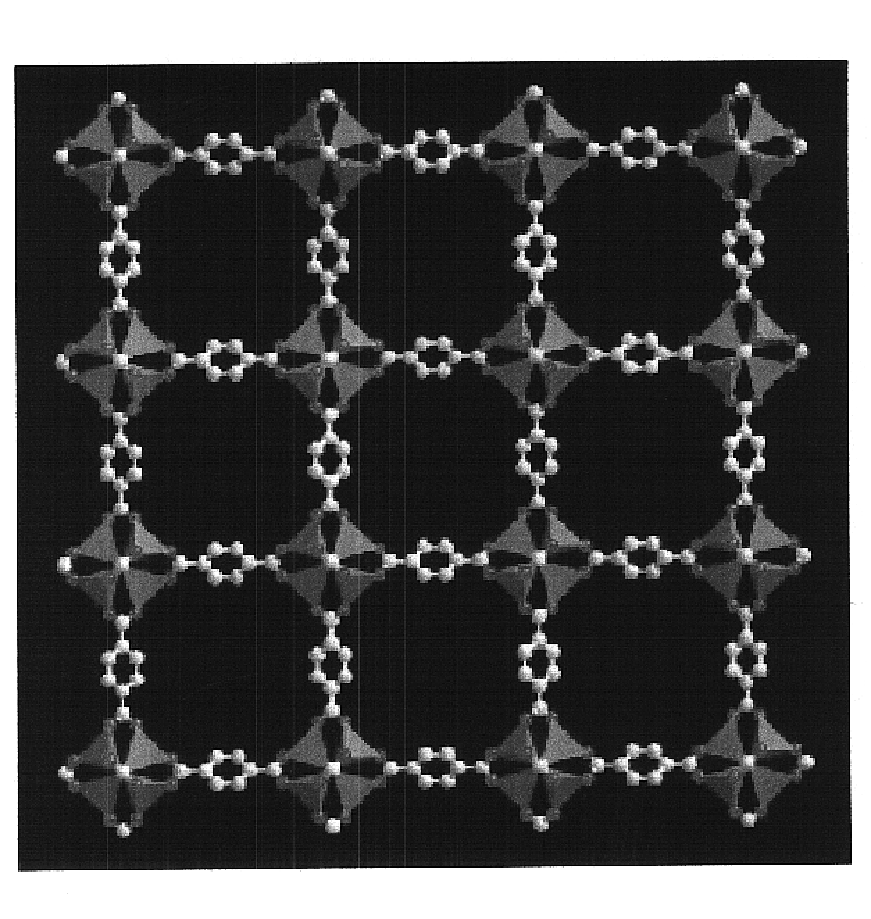
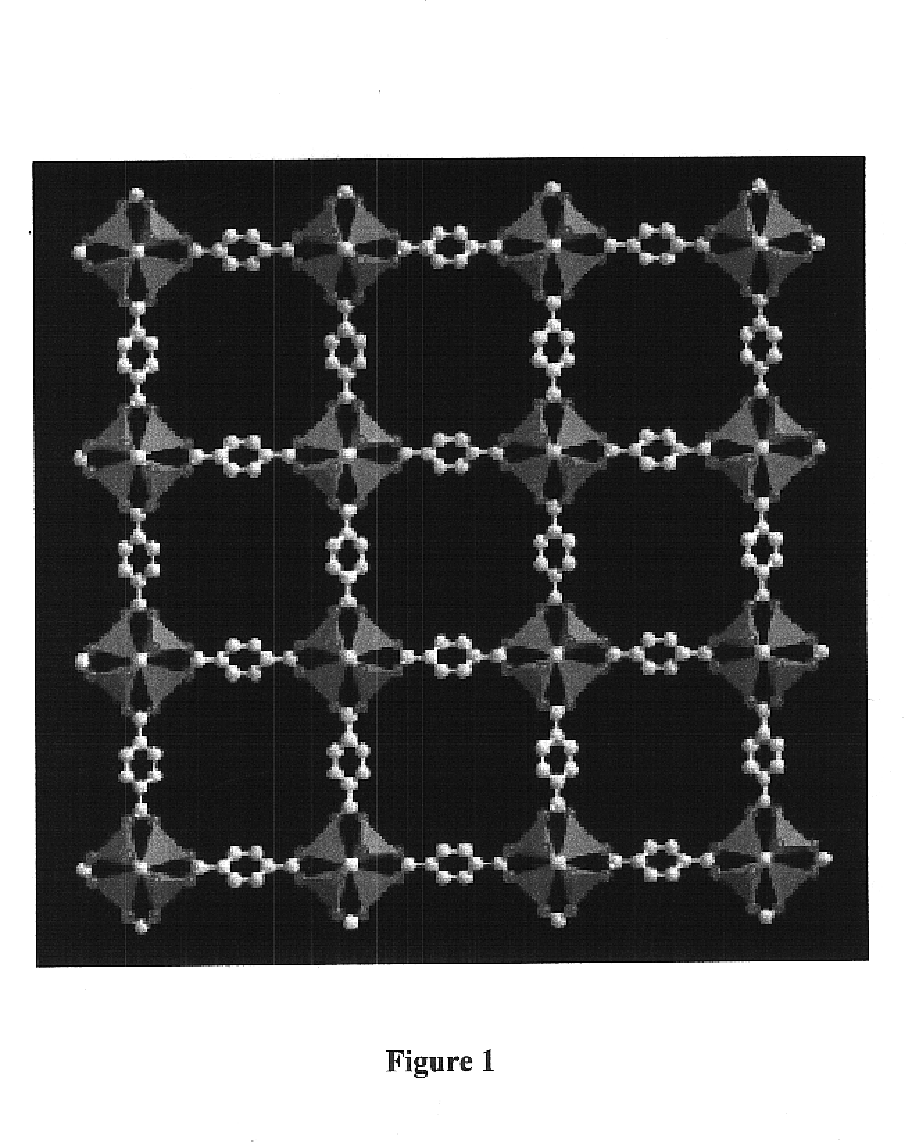
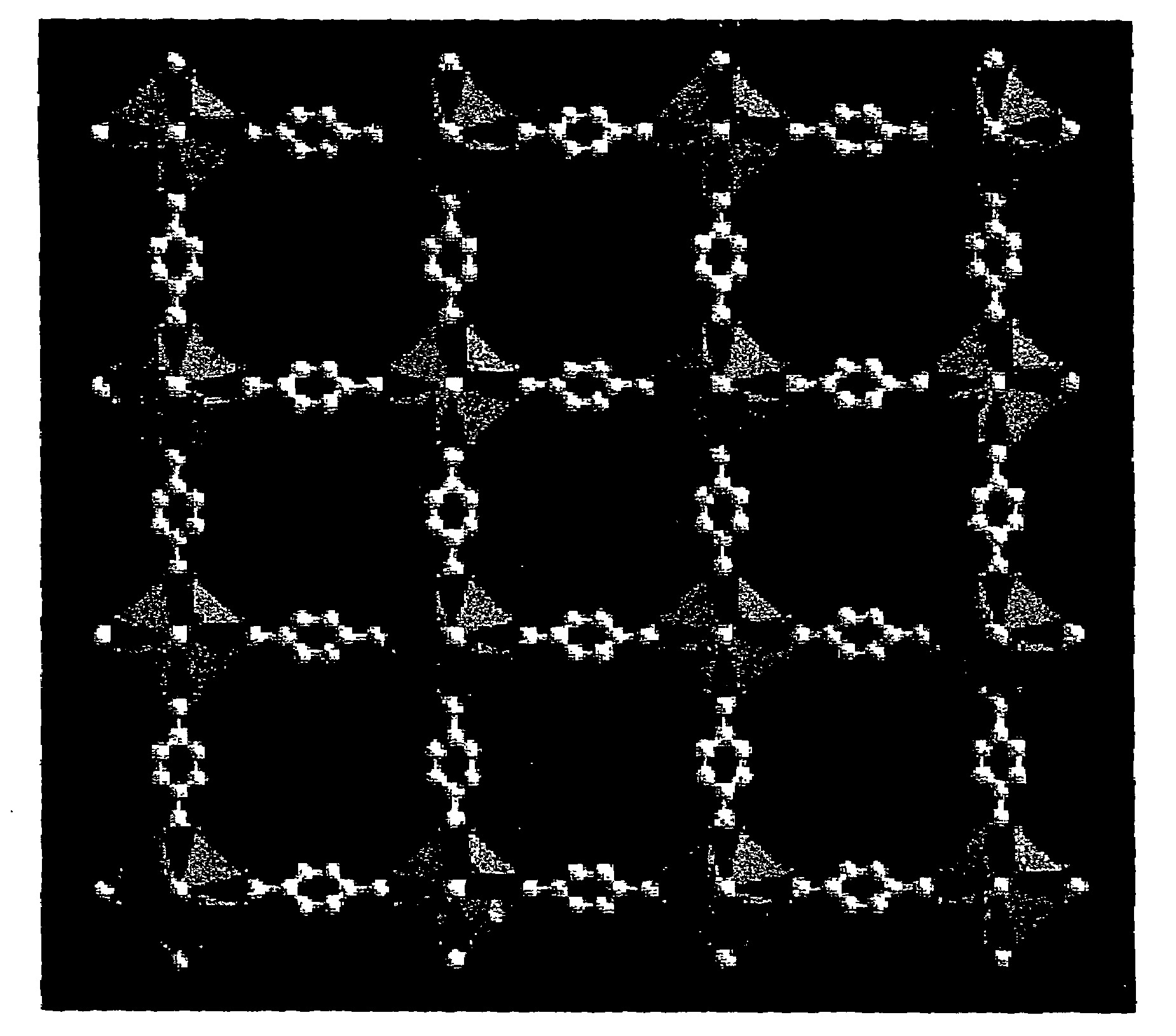
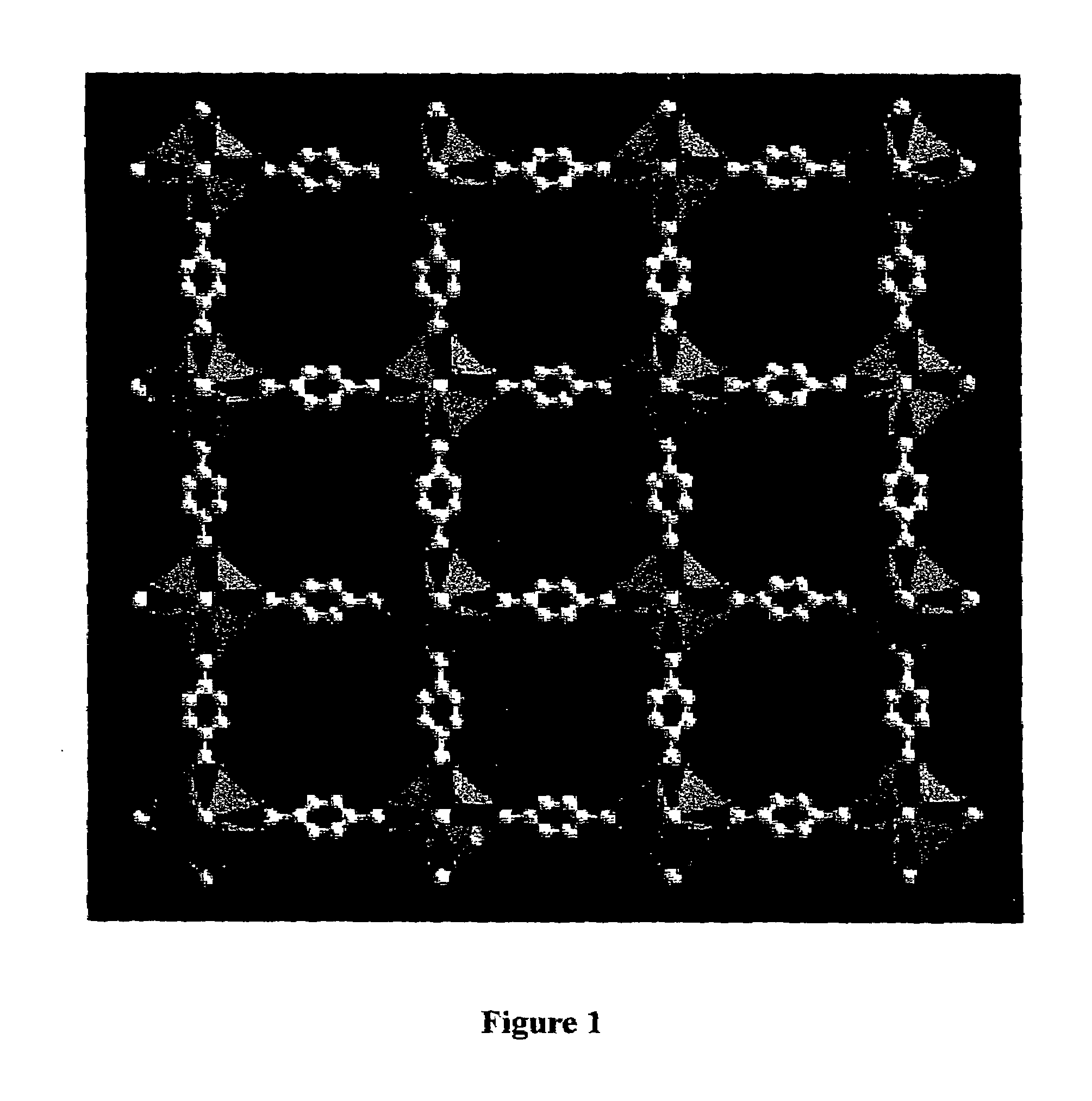
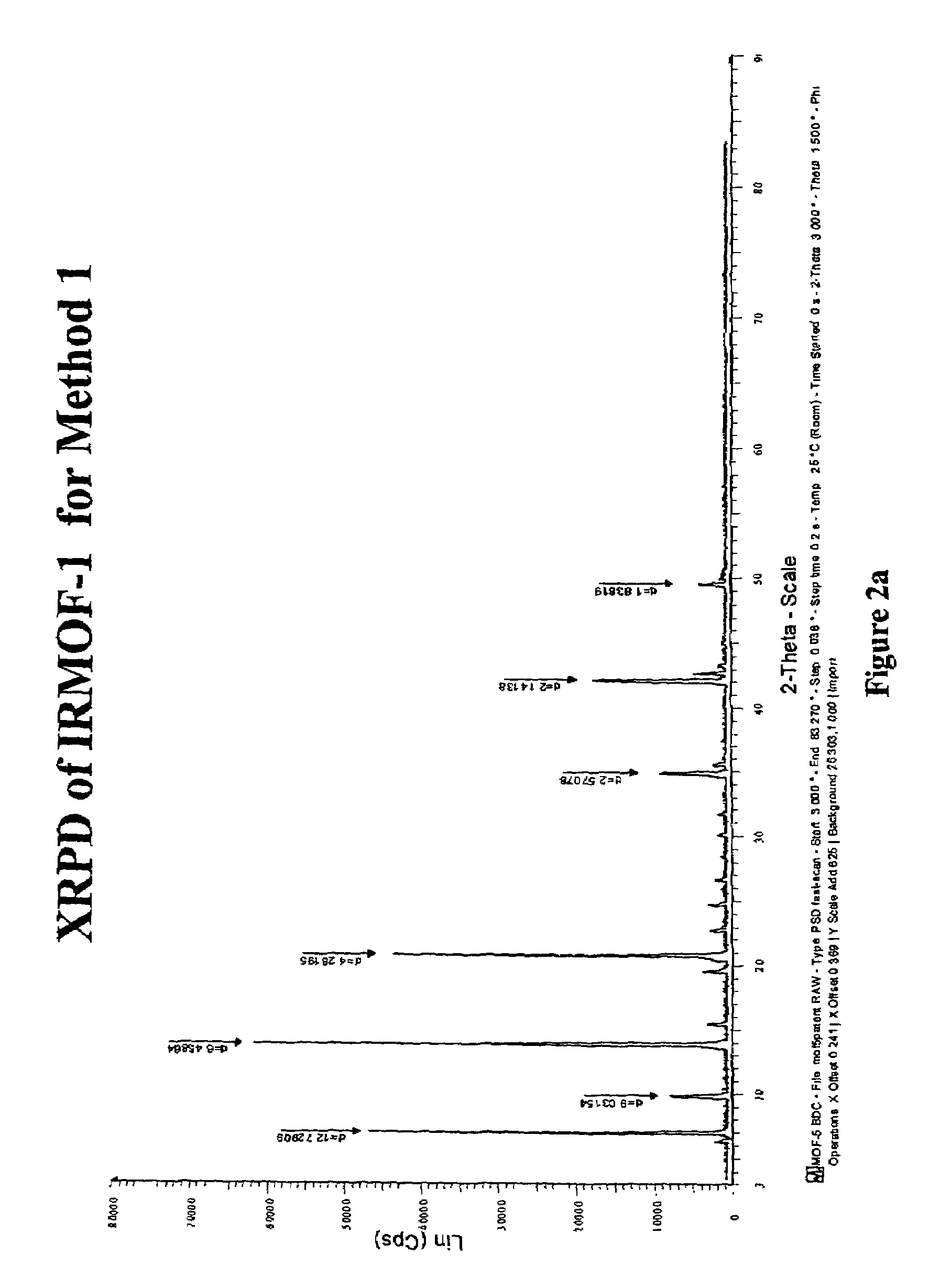
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS6930193B2High methane storage capacityIncrease storage capacityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic linkingSystems design
An isoreticular metal-organic framework (IRMOF) and method for systematically forming the same. The method comprises the steps of dissolving at least one source of metal cations and at least one organic linking compound in a solvent to form a solution; and crystallizing the solution under predetermined conditions to form a predetermined IRMOF. At least one of functionality, dimension, pore size and free volume of the IRMOF is substantially determined by the organic linking compound.
Owner:RGT UNIV OF MICHIGAN
Conversion of petroleum residua to methane
InactiveUS6955695B2Eliminate needReduce usageThermal non-catalytic crackingElectrolysis componentsParticulatesGas phase
This invention discloses improvements on previous inventions for catalytic conversion of coal and steam to methane. The disclosed improvements permit conversion of petroleum residua or heavy crude petroleum to methane and carbon dioxide such that nearly all of the heating value of the converted hydrocarbons is recovered as heating value of the product methane. The liquid feed is distributed over a fluidized solid particulate catalyst containing alkali metal and carbon as petroleum coke at elevated temperature and pressure from the lower stage and transported to the upper stage of a two-stage reactor. Particulate solids containing carbon and alkali metal are circulated between the two stages. Superheated steam and recycled hydrogen and carbon monoxide are fed to the lower stage, fluidizing the particulate solids and gasifying some of the carbon. The gas phase from the lower stage passes through the upper stage, completing the reaction of the gas phase.
Owner:PETRO2020
Method for reducing emissions from evaporative emissions control systems
InactiveUS6540815B1Loss in working capacityEmission reductionGas treatmentNon-fuel substance addition to fuelHigh concentrationSorbent
Disclosed is a method for sharply reducing diurnal breathing loss emissions from automotive evaporative emissions control systems by providing multiple layers, or stages, of adsorbents. On the fuel source-side of an emissions control system canister, high working capacity carbons are preferred in a first canister (adsorb) region. In subsequent canister region(s) on the vent-side, the preferred adsorbent should exhibit a flat or flattened adsorption isotherm on a volumetric basis and relatively lower capacity for high concentration vapors as compared with the fuel source-side adsorbent. Multiple approaches are described for attaining the preferred properties for the vent-side canister region. One approach is to use a filler and / or voidages as a volumetric diluent for flattening an adsorption isotherm. Another approach is to employ an adsorbent with the desired adsorption isotherm properties and to process it into an appropriate shape or form without necessarily requiring any special provision for dilution. The improved combination of high working capacity carbons on the fuel source-side and preferred lower working capacity adsorbent on the vent-side provides substantially lower diurnal breathing emissions without a significant loss in working capacity or increase in flow restriction compared with known adsorbents used in canister configurations for automotive emissions control systems.
Owner:INGEVITY SOUTH CAROLINA
Catalytic Gasification Process with Recovery of Alkali Metal from Char
ActiveUS20090169448A1Quantity minimizationThermal non-catalytic crackingMuffle furnacesPhysical chemistryAlkali metal
Processes are described for the extraction and recovery of alkali metal from the char that results from catalytic gasification of a carbonaceous material. Among other steps, the processes of the invention include a hydrothermal leaching step in which a slurry of insoluble particulate comprising insoluble alkali metal compounds is treated with carbon dioxide and steam at elevated temperatures and pressures to effect the conversion of insoluble alkali metal compounds to soluble alkali metal compounds. Further, processes are described for the catalytic gasification of a carbonaceous material where a substantial portion of alkali metal is extracted and recovered from the char that results from the catalytic gasification process.
Owner:SURE CHAMPION INVESTMENT LTD
Catalytic Gasification Process with Recovery of Alkali Metal from Char
ActiveUS20090169449A1Quantity minimizationThermal non-catalytic crackingMuffle furnacesParticulatesSlurry
Processes are described for the extraction and recovery of alkali metal from the char that results from catalytic gasification of a carbonaceous material. Among other steps, the processes of the invention include a hydrothermal leaching step in which a slurry of insoluble particulate comprising insoluble alkali metal compounds is treated with carbon dioxide and steam at elevated temperatures and pressures to effect the conversion of insoluble alkali metal compounds to soluble alkali metal compounds. Further, processes are described for the catalytic gasification of a carbonaceous material where a substantial portion of alkali metal is extracted and recovered from the char that results from the catalytic gasification process.
Owner:SURE CHAMPION INVESTMENT LTD
Positively charged membrane
InactiveUS6780327B1High rateHigh charge densityCation exchanger materialsIon-exchanger regenerationPorous substrateFiltration
The present invention provides a positively charged microporous membrane having a protein binding capacity about 25 mg / ml or greater comprising a hydrophilic porous substrate and a crosslinked coating that provides a fixed positive charge to the membrane. The present invention further provides a positively charged microporous membrane comprising a porous substrate and a crosslinked coating comprising pendant cationic groups. The membranes of the present invention find use in a variety of applications including ion-exchange chromatography, macromolecular transfer, as well as detection, filtration and purification of biomolecules such as proteins, nucleic acids, endotoxins, and the like.
Owner:PALL CORP
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Nanosize electropositive fibrous adsorbent
InactiveUS6838005B2Improve the immunityEfficient precipitationIon-exchanger regenerationLoose filtering material filtersFiberParticulates
Aluminum hydroxide fibers approximately 2 nanometers in diameter and with surface areas ranging from 200 to 650 m2 / g have been fount to be highly electropositive. When dispersed in water they are able to attach to and retain electronegative particles. When combined into a composite filter with other fibers or particles they can filter bacteria and nano size particulates such as viruses and colloidal particles at high flux through the filter. Such filters can be used for purification and sterilization of water, biological, medical and pharmaceutical fluids, and as a collector / concentrator for detection and assay of mirobes and viruses. The alumina fibers are also capable of filtering sub-micron inorganic and metallic particles to produce ultra pure water. The fibers are suitable as a substrate for growth of cells. Macromolicules such as proteins may be separated from each other based on their electronegative charges.
Owner:ARGONIDE CORP
Device for concentrating and purifying macromolecules
InactiveUS6837995B1Analysis using chemical indicatorsPreparing sample for investigationFiltrationChemistry
A device for concentrating and / or purifying macromolecules in liquids is disclosed wherein the concentration / purification is accomplished by a membrane separating concentration and filtration chambers, the membrane and chambers being compressively held in fluid-tight relationship by sliding engagement with a sleeve-like housing. A method for assembling such a device is also disclosed.
Owner:VIVASCI
Adsorption/separation method and a medium for adsorption/separation
InactiveUS6428707B1Increase productionImprove productivityChromatographic cation exchangersCation exchanger materialsChemistrySeparation method
A method for adsorption of a substance from a liquid sample on a fluidized bead or stirred suspension, in which the beads used comprise a base matrix and exhibit a structure having affinity to the substance, characterized in that the structure is covalently bound to the base matrix via an extender. Populations of beads in which the beads contain a filler incorporated in a base matrix and an extender are also described.
Owner:GE HEALTHCARE BIOPROCESS R&D
Robust carbon monolith having hierarchical porosity
InactiveUS20050169829A1Maintain good propertiesOther chemical processesCarbon preparation/purificationMaterials scienceMonolith
Owner:UNIV OF TENNESSEE RES FOUND +1
Flue gas purification process using a sorbent polymer composite material
ActiveUS20050019240A1Easy to fixImprove removal efficiencyGas treatmentNitrogen compoundsSorbentFluoropolymer
This invention provides a process of removing sulfur oxides, mercury vapor, and fine particulate matters from industrial flue gases that contain such pollutants. The pollutants are removed by modules, which contain microporous adsorbent (i.e., sorbent) material that is held within a polymer matrix. The preferred polymers are fluoropolymers. The composite material that contains the microporous absorbent material held within a polymer matrix removes sulfur oxides by converting them into high concentration sulfuric acids. It also removes mercury vapor by chemically adsorbing the mercury into the matrix. It also removes fine particulate matters by surface filtration. The sulfuric acid that is produced inside the composite material is automatically expelled onto the external surfaces of the composite material and is drained into an acid reservoir together with the fine particulate matters which are washed from the external surfaces of the composite material by the constant dripping of the sulfuric acid along the external surfaces of the composite material.
Owner:WL GORE & ASSOC INC
Thermosensitive polymer carriers having a modifiable physical structure for biochemical analysis, diagnosis and therapy
The invention relates to thermosensitive polymers which contain magnetic and / or metallic colloids and whose physical structure can be altered through magnetic induction or through the supply of energy. The invention also relates to processes for the production of such thermosensitive polymers, and the use of such polymers for diagnostic and therapeutic purposes.
Owner:MAGNAMEDICS
Solid ion conductor which has a garnet-like crystal structure and has the stoichiometric composition L7+XAXG3-XZr2O12
ActiveUS8658317B2Easily employedImprove ionic conductivityFinal product manufactureCell electrodesElectrical conductorCrystal structure
The invention is directed to a solid ion conductor which has a garnet-like crystal structure and has the stoichiometric composition L7+xAxG3−xZr2O12, whereinL is in each case independently a monovalent cation,A is in each case independently a divalent cation,G is in each case independently a trivalent cation,0≦x≦3 andO can be partly or completely replaced by divalent or trivalent anion.
Owner:BASF AG
Adsorbent sheet material for parallel passage contactors
ActiveUS7077891B2Maximize capacityImprove efficiencyMaterial nanotechnologyOther chemical processesParticulatesSorbent
An adsorbent material fabricated into a reinforcement-free, self-supported coherent thin sheet and configured for use as a parallel passage contactor element in adsorption / separation applications with gases and liquids is disclosed. The adsorbent sheet material is obtained by enmeshing fine adsorbent particulates in a polymer binder. Particulates include but are not limited to carbon particles, inorganic oxides particles, or ceramic particles, or synthetic polymer resin particles. The adsorbent sheet advantageously contains a large volume percentage of active adsorbent particles. The parallel passage contactor device fabricated from the adsorbent sheet material is characterized by minimal mass transfer resistance and better separation efficiency expressed as height equivalent to a theoretical plate, while it maintains most of the adsorptive properties of the starting particulates, and can be used in gas separation applications with short adsorption cycles, such as rapid pressure swing adsorption, rotary concentrators, rapid electric swing adsorption.
Owner:AIR PROD & CHEM INC
Preparation method and applications of porous metal organic skeleton material
ActiveCN104370820AGood application effectEasy to operateOther chemical processesGroup 8/9/10/18 element organic compoundsMetal-organic frameworkSolvent
The invention relates to a preparation method of a porous metal organic skeleton material. The porous metal organic skeleton material comprises at least a metal ion and at least an organic compound that can be matched with the metal ion. The material can be prepared by dissolving metal compounds, organic ligand, and sustained-released alkali into a solvent and carrying out direct water (solvent) thermal synthesis at a certain temperature under a certain pressure so as to obtain the porous metal organic skeleton material. The operation of the provided preparation method is simple, the preparation method is suitable for massive industrial production and is suitable for the synthesis of porous metal organic skeleton membrane, and the provided porous metal organic skeleton material is used to absorb, separate, and store gas.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
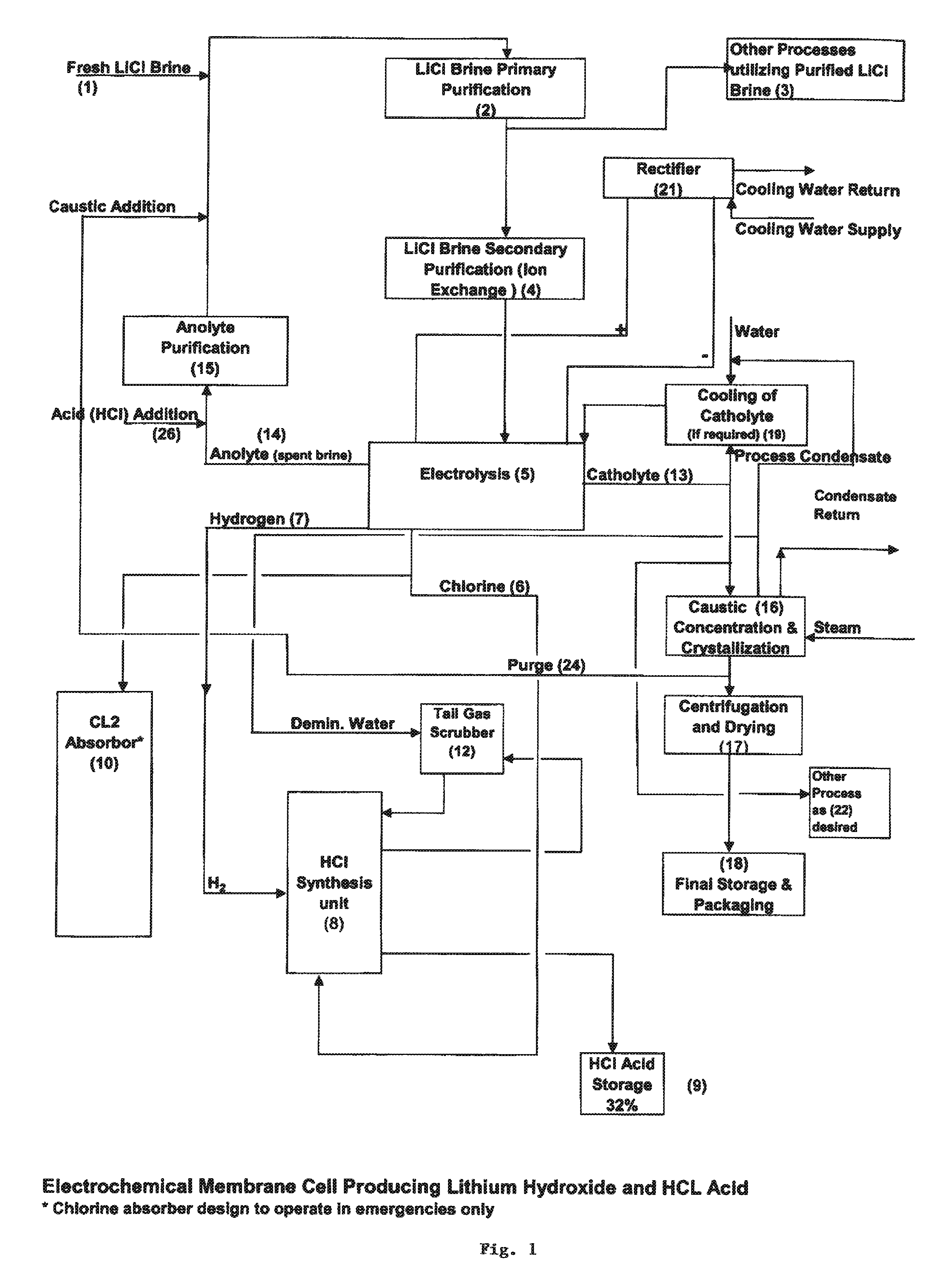
Method of making high purity lithium hydroxide and hydrochloric acid
InactiveUS20110044882A1Simple and economical processEasy to convertElectrolysis componentsEnergy inputElectrolysisIon exchange
The present invention relates to a process for producing high purity lithium hydroxide monohydrate, comprising following steps: concentrating a lithium containing brine; purifying the brine to remove or to reduce the concentrations of ions other than lithium; adjusting the pH of the brine to about 10.5 to 11 to further remove cations other than lithium, if necessary; neutralizing the brine with acid; purifying the brine to reduce the total concentration of calcium and magnesium to less than 150 ppb via ion exchange; electrolyzing the brine to generate a lithium hydroxide solution containing less than 150 ppb total calcium and magnesium, with chlorine and hydrogen gas as byproducts; producing hydrochloric acid via combustion of the chlorine gas with excess hydrogen and subsequent scrubbing of the resultant gas stream with purified water, if elected to do so; and concentrating and crystallizing the lithium hydroxide solution to produce lithium hydroxide monohydrate crystals.
Owner:ROCKWOOD LITHIUM INC
Use of absorbent materials to separate water from lipophilic fluid
InactiveUS6855173B2Safe separationEfficient and cost-effectiveOrganic detergent compounding agentsIon-exchanger regenerationEmulsionAbsorbent material
The present invention relates to the use of absorbent materials for separating water from an emulsion comprising water and lipophilic fluid. The methods, systems, and compositions of the present invention expose the emulsion to absorbent materials such that water is absorbed out of the emulsion in order to facilitate the recovery of the lipophilic fluid.
Owner:THE PROCTER & GAMBLE COMPANY
Odor controlling article including a visual indicating device for monitoring odor absorption
The present invention relates to a visual indicating device and an article for controlling odors, in particular foot, garbage, basement, cooking, pet, tobacco, feces and urine odors. The article comprises a visual indicating agent that is color sensitive to the odor, and optionally, an odor absorbing agent. The visual indicating agent changes color when the article has been exposed to a sufficient amount of odor to saturate the article. The indicating agent may be applied in differing concentrations to two or more zones so as to indicate to a user of the article how much of the odor absorbing capacity has been used, or conversely, how much of the odor absorbing capacity remains. Suitable visual indicating agents that change color in response to odors are also described. The article for controlling odors may be a disposable odor absorbing sheet, air freshening product, diaper, undergarment pad, face mask, air filtration device, sanitary napkin, tampon, panty shield or incontinence pad.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Porous inorganic/organic hybrid monolith materials for chromatographic separations and process for their preparation
InactiveUS7250214B2Extended service lifeReduce back pressureComponent separationIon-exchanger regenerationChromatographic separationNew materials
Novel materials for chromatographic separations, processes for their preparation, and separation devices containing the chromatographic materials. In particular, the novel materials are porous inorganic / organic hybrid monolith materials, which desirably may be surface modified, and which offer more efficient chromatographic separations than that known in the art.
Owner:WATERS TECH CORP
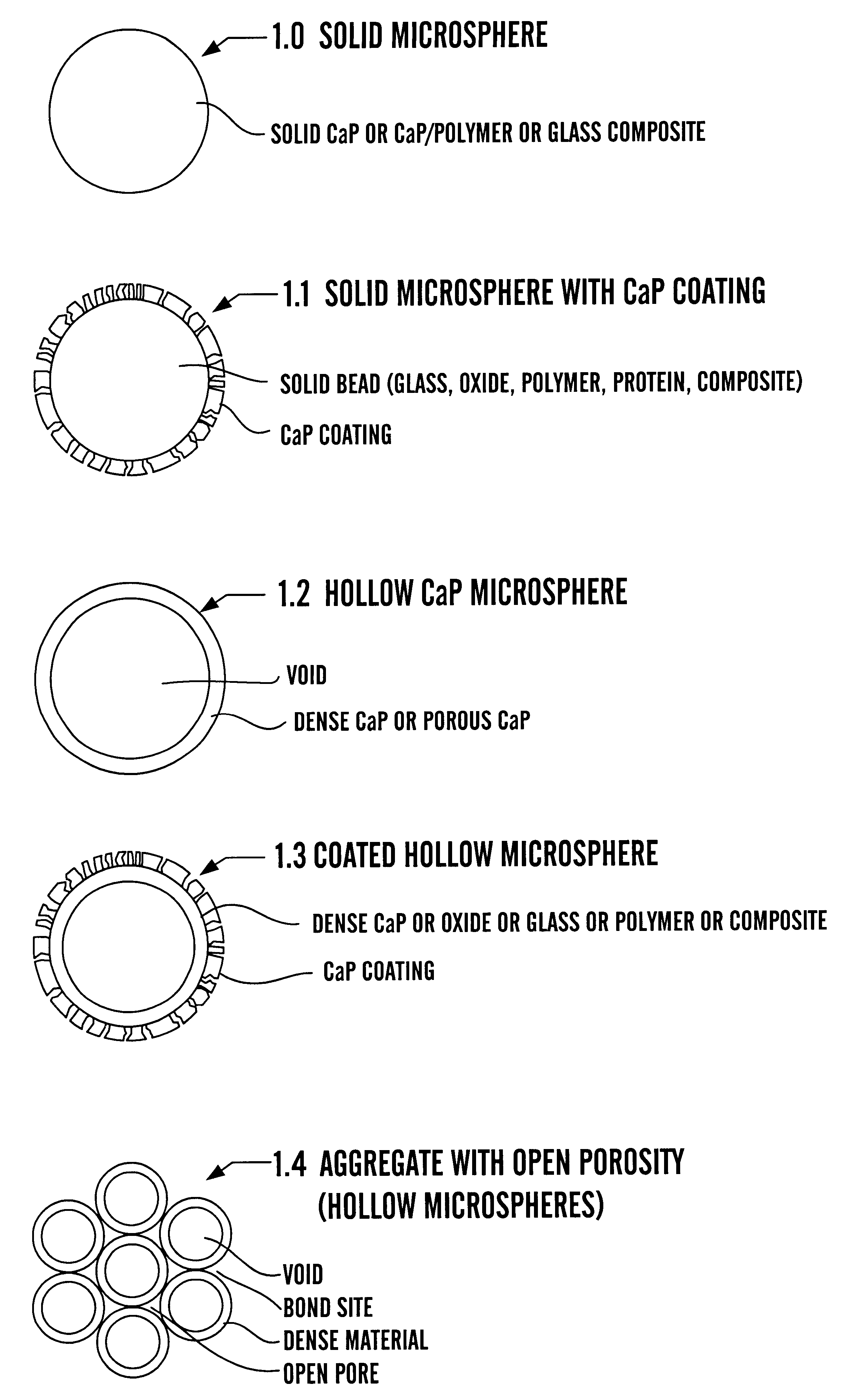
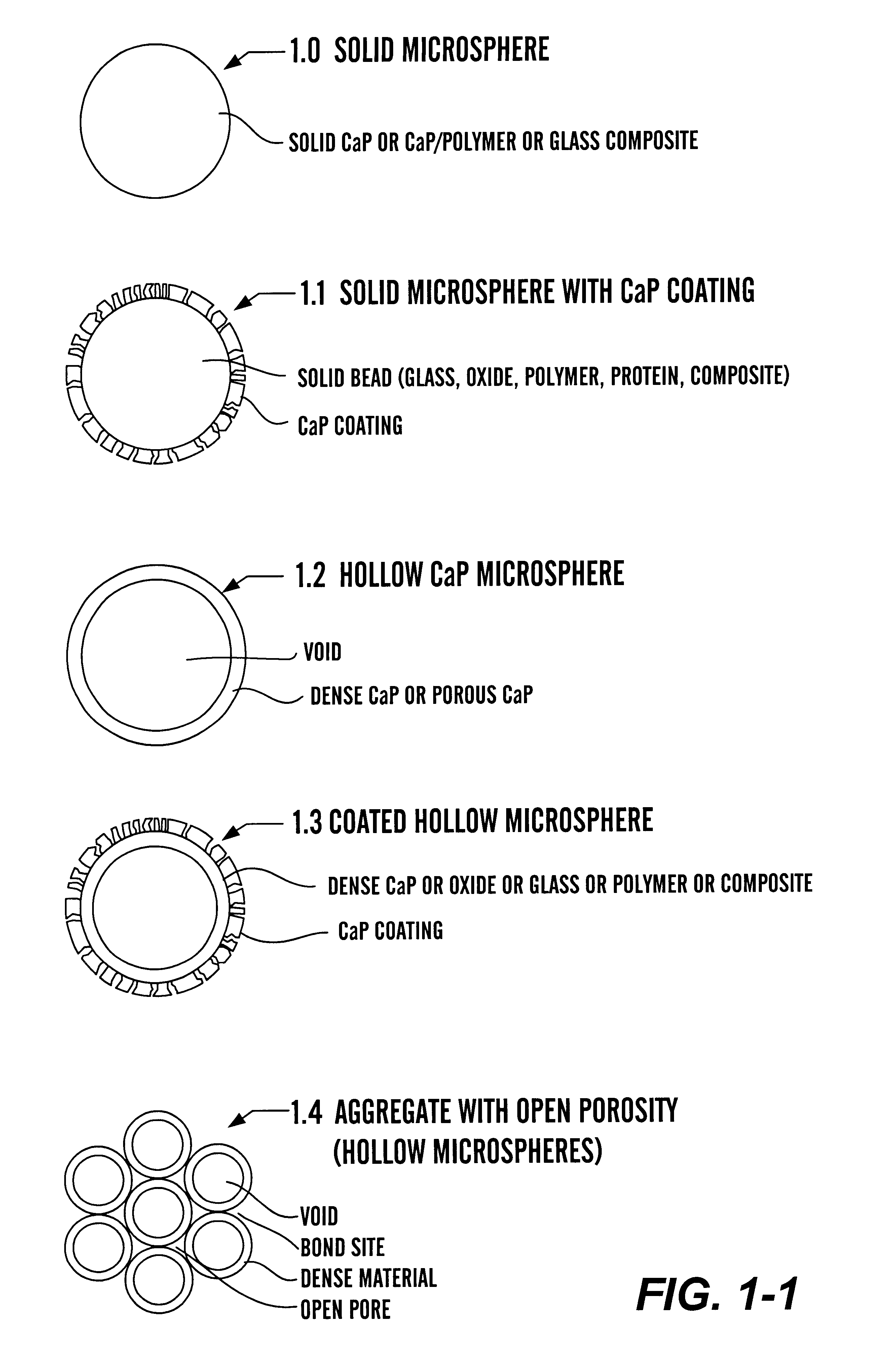
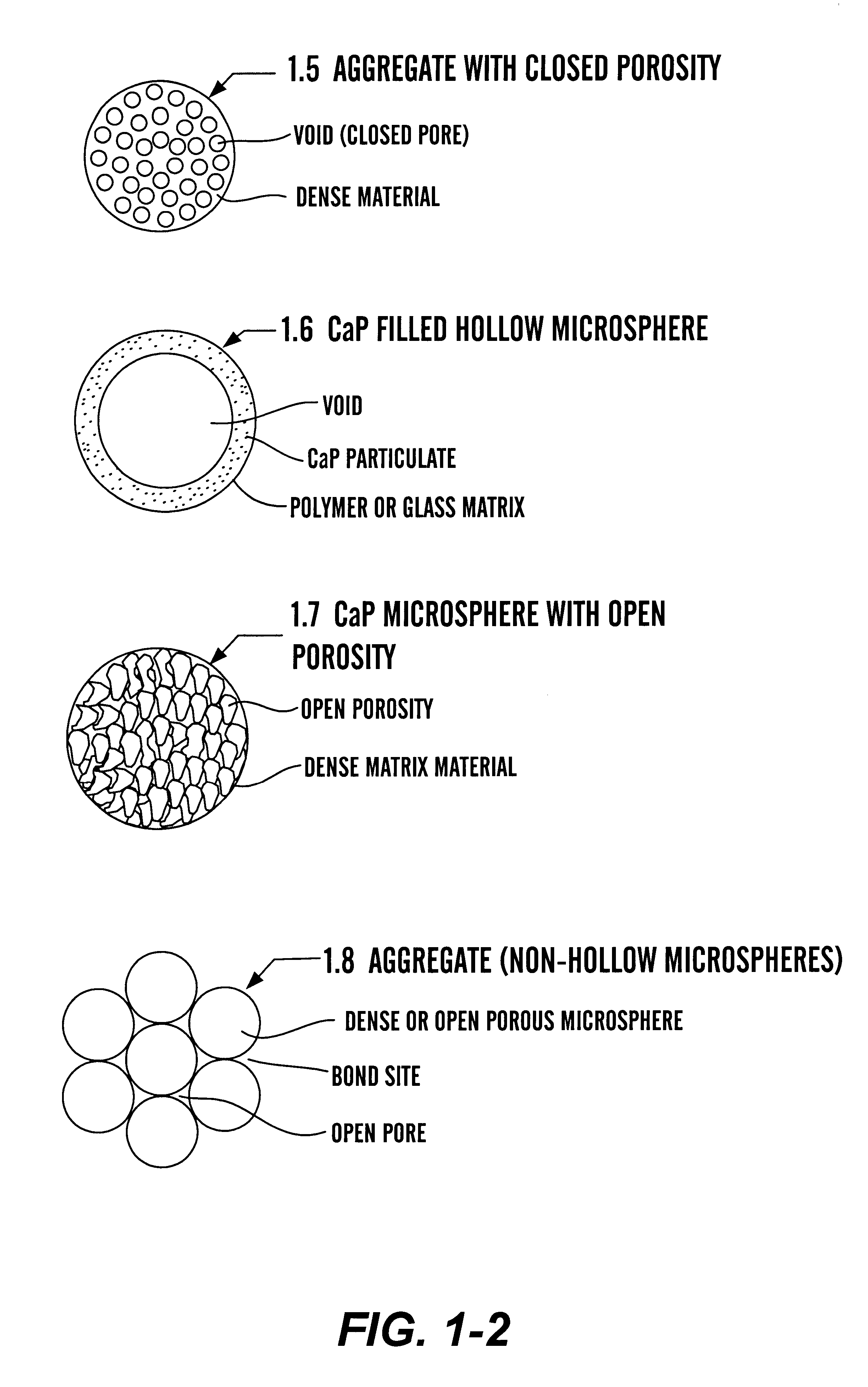
Calcium phosphate microcarriers and microspheres
The present invention provides calcium phosphate-based (CaP) microcarriers and their use, for example, in cell culturing systems, chromatography, and implantable biomedical materials.
Owner:CAP BIOTECH
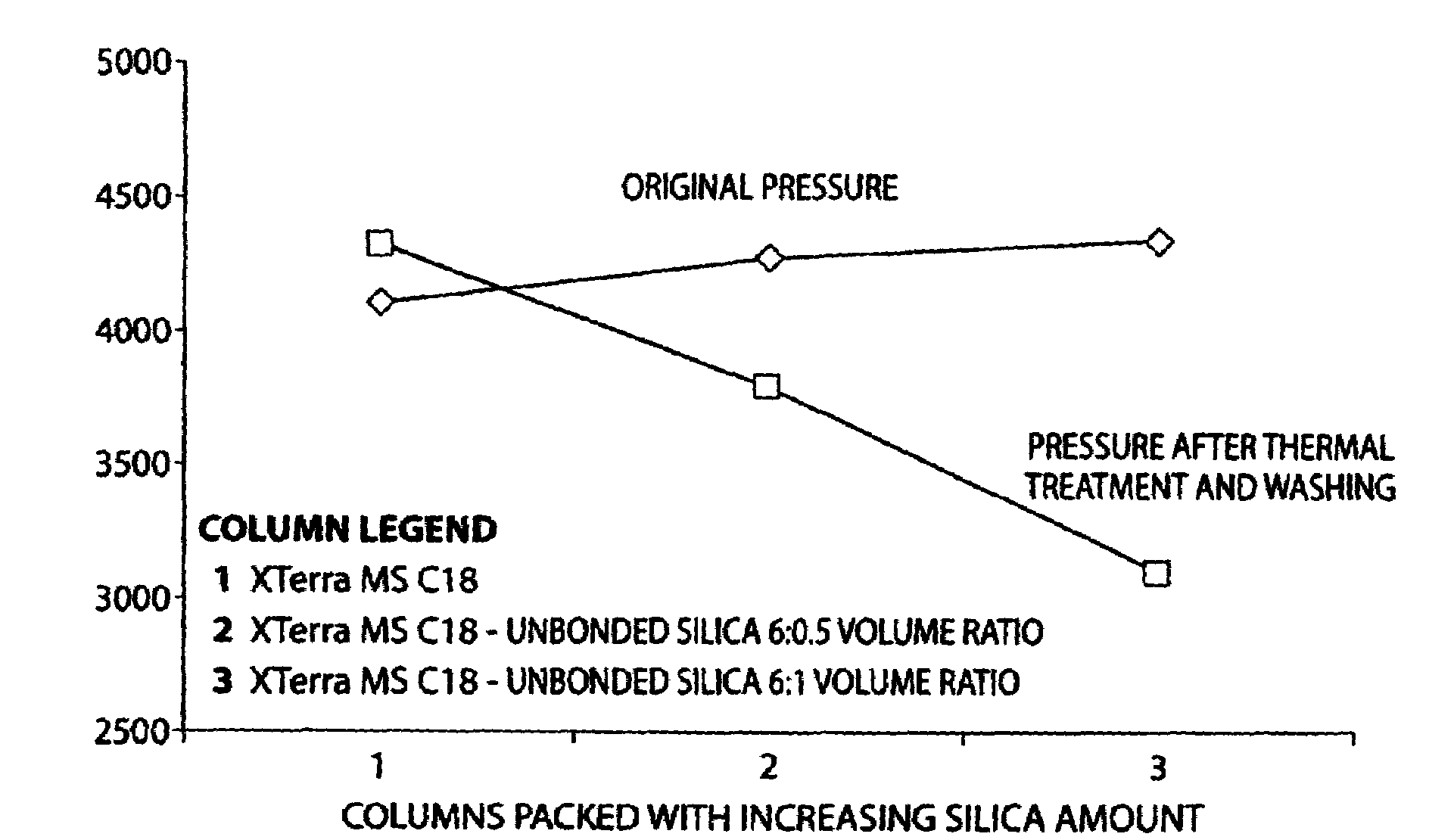
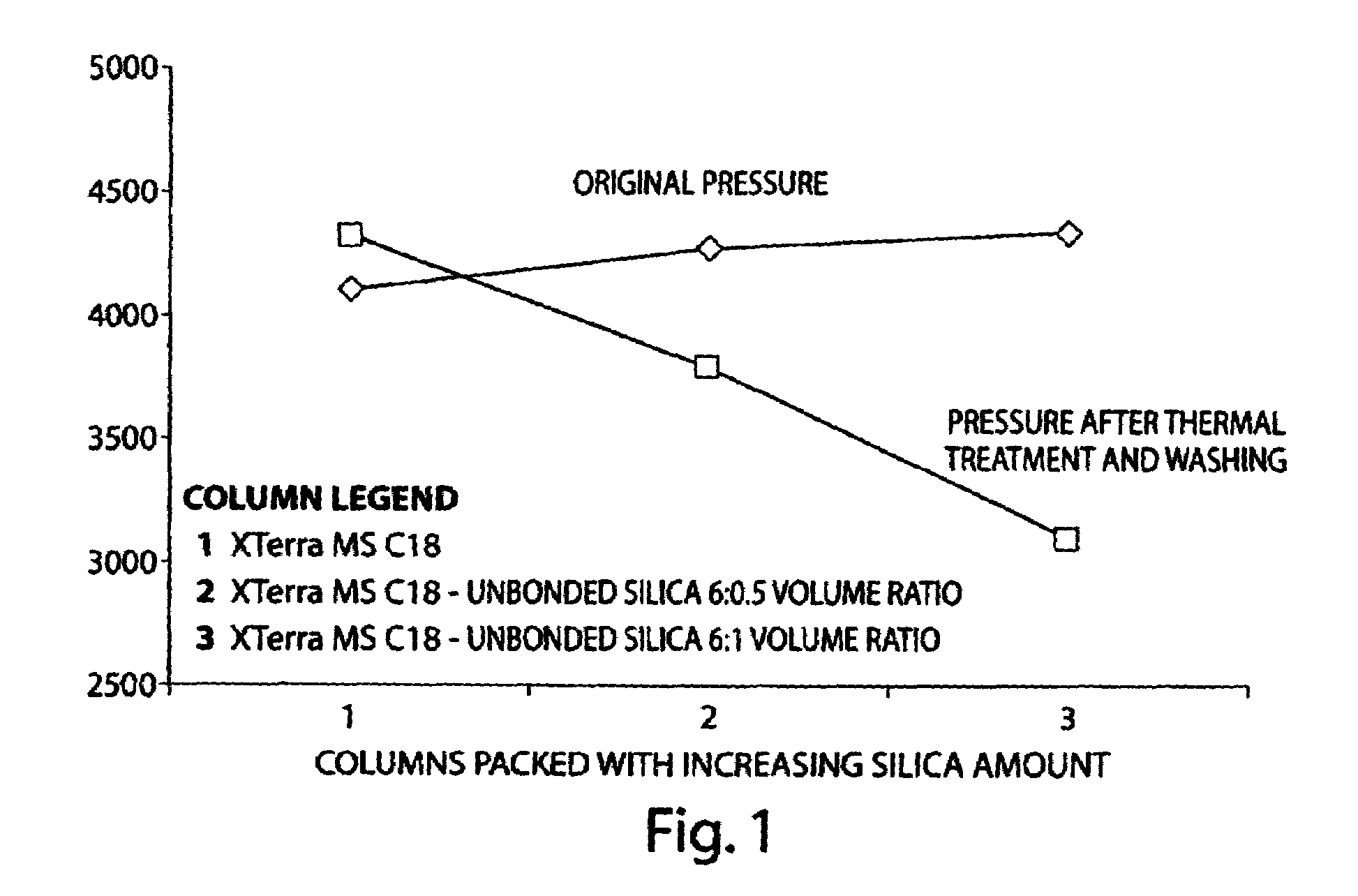
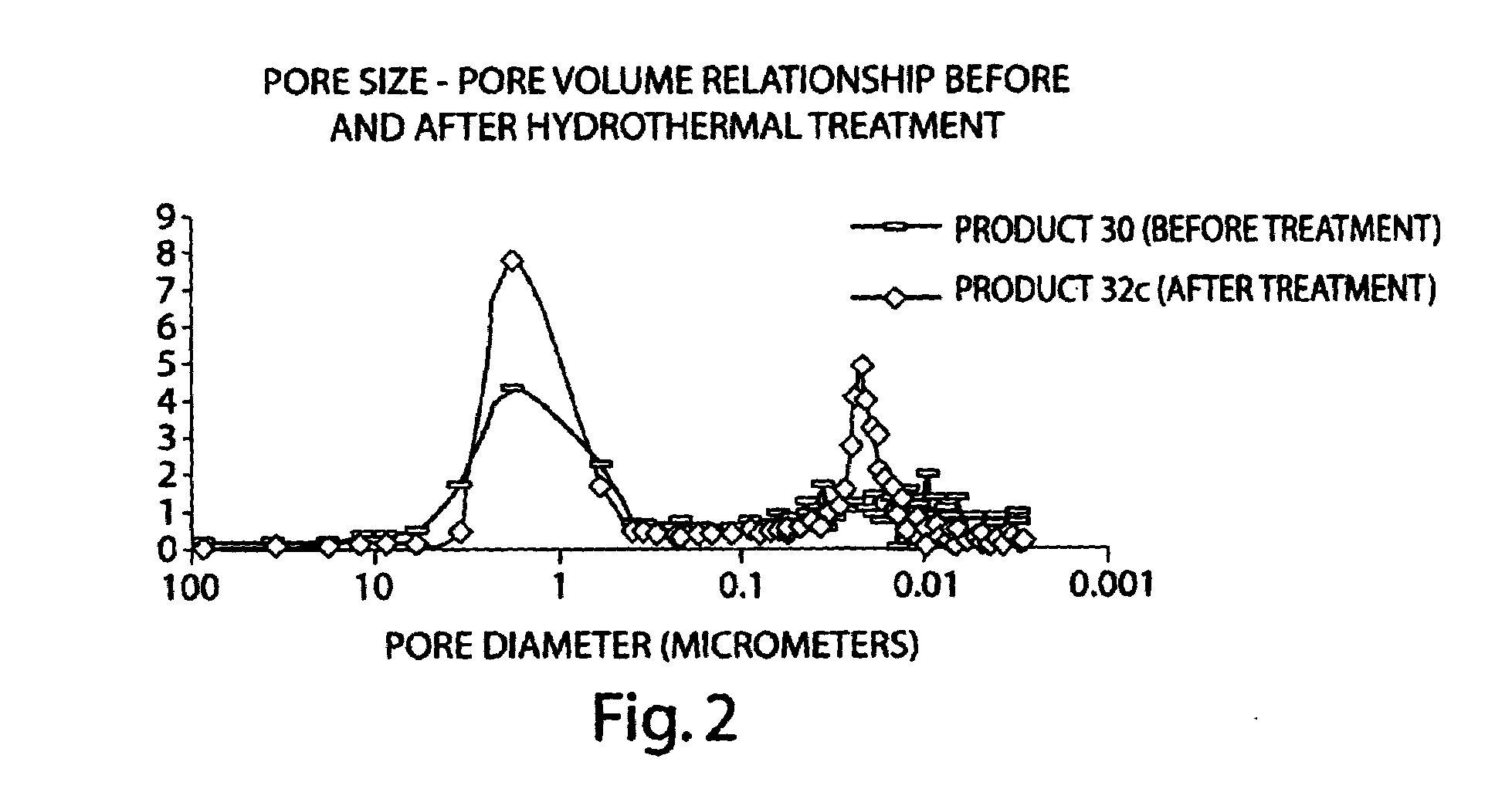
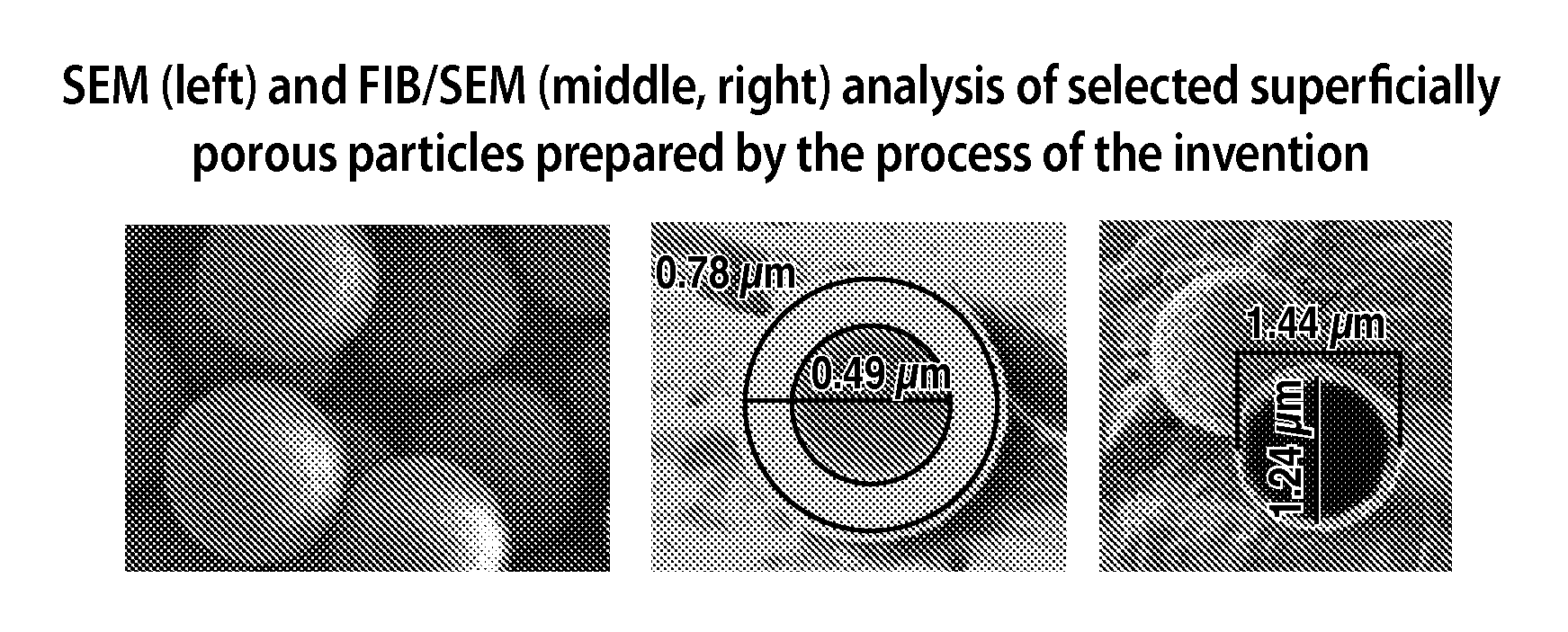
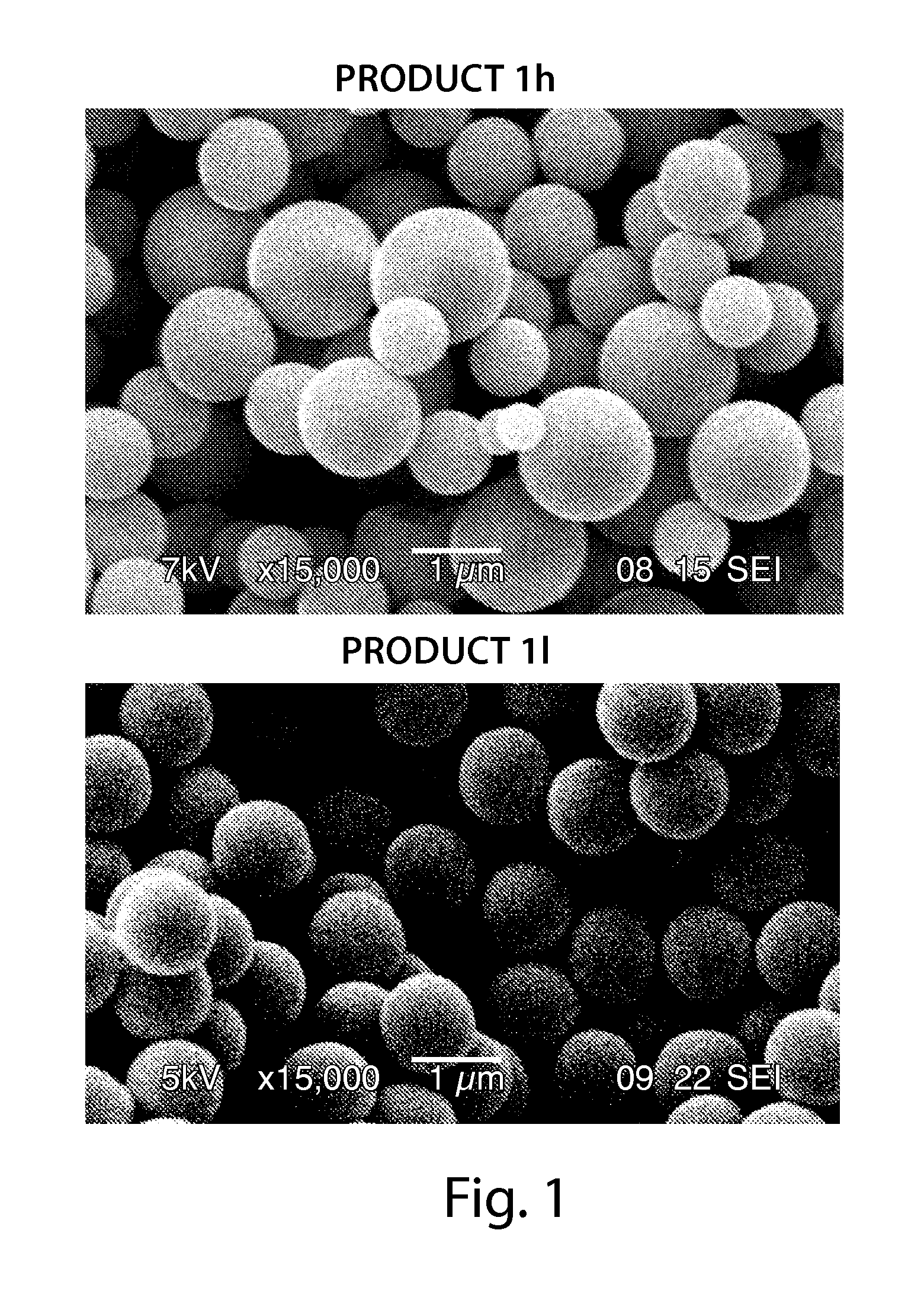
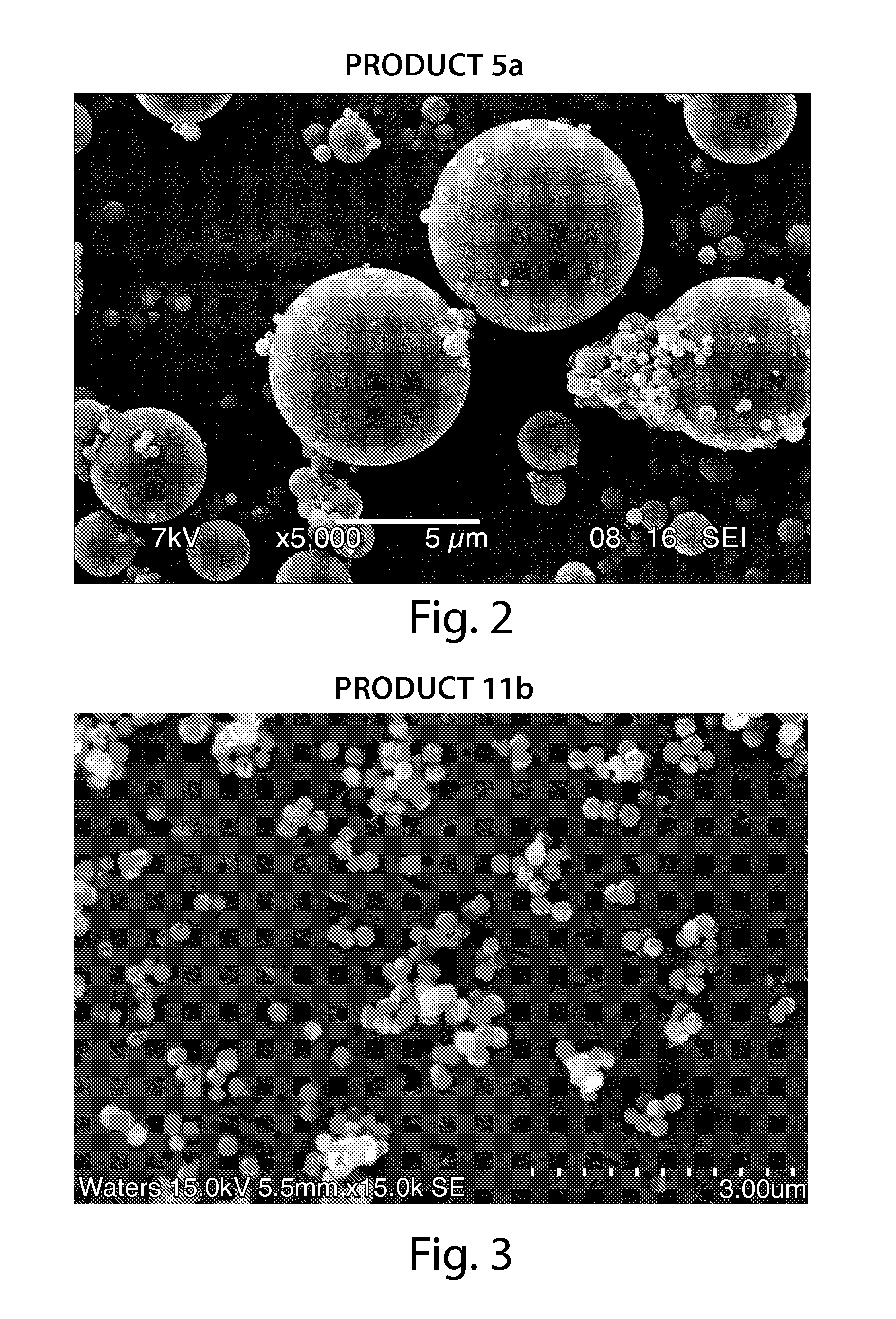
Superficially porous materials comprising a substantially nonporous core having narrow particle size distribution; process for the preparation thereof; and use thereof for chromatographic separations
InactiveUS20130112605A1Prevent fine generationInhibit aggregationIon-exchanger regenerationPretreated surfacesChromatographic separationHybrid material
Novel chromatographic materials for chromatographic separations, columns, kits, and methods for preparation and separations with a superficially porous material comprising a substantially nonporous core and one or more layers of a porous shell material surrounding the core. The material of the invention is comprised of superficially porous particles and a narrow particle size distrution. The material of the invention is comprised of a superficially porous monolith, the substantially nonporous core material is silica; silica coated with an inorganic / organic hybrid surrounding material; a magnetic core material; a magnetic core material coated with silica; a high thermal conductivity core material; a high thermal conductivity core material coated with silica; a composite material; an inorganic / organic hybrid surrounding material; a composite material coated with silica; a magnetic core material coated with an inorganic / organic hybrid surrounding material; or a high thermal conductivity core material coated with an inorganic / organic hybrid surrounding material.
Owner:WATERS TECH CORP
Reactive compositions for fluid treatment
InactiveUS6861002B2Easy to disassembleImprove efficiencyPerfluorocarbons/hydrofluorocarbons captureLoose filtering material filtersCystFiltration
A method and device for the chemical conversion, filtration and / or purification of fluids water or other solutions containing microbiological and chemical contaminants, such as fluids containing arsenic, chlorine, bacteria, viruses, and cysts, where the fluid is passed through a purification material composed of fluid treatment carbon, metal phosphates, metal oxides, reduced metals, metal silicates, metal sulfates, metal carbonates, metal hydroxides, or combinations thereof. The material may be included in a fixed binder matrix.
Owner:WATERVISIONS INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com