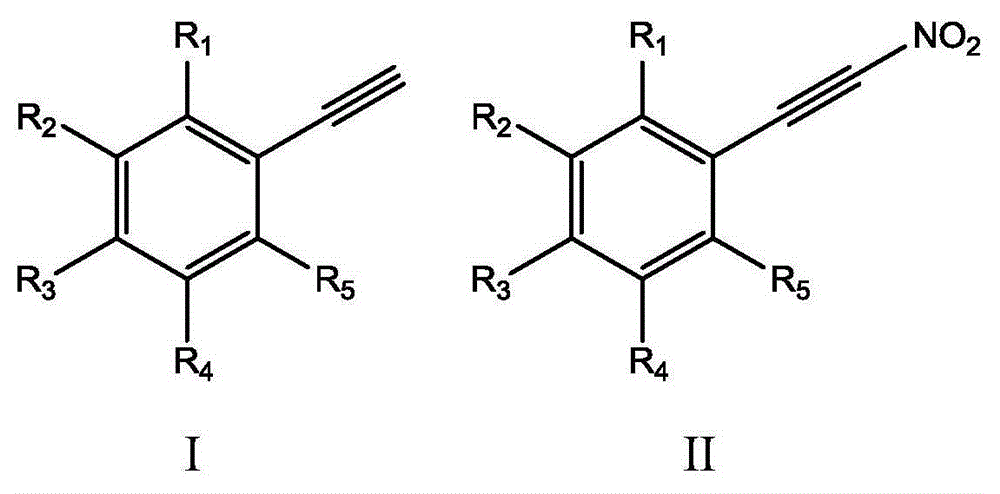
Method for synthesizing (nitroalkynyl)benzene compounds
A technology for nitroalkynyl and compound, applied in the field of preparation of benzene compounds, can solve the problems of difficult nitration reaction, low product yield and purity, difficult to obtain target product and the like, and achieves good selectivity, simple reaction steps, Good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
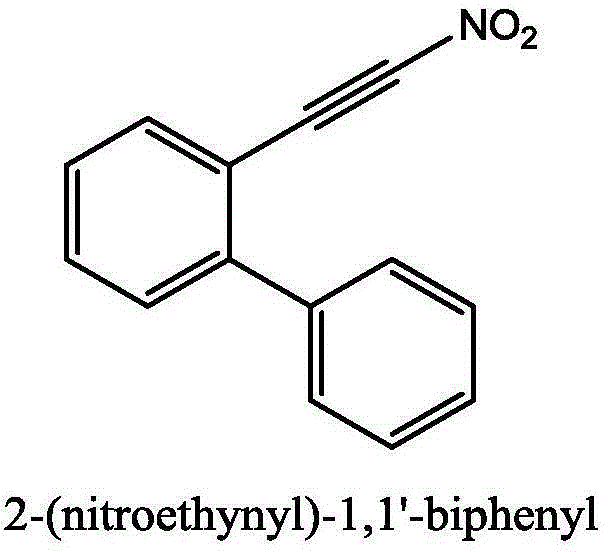
[0032] (1) Add 0.5mmol (89mg) of 2-phenylphenylacetylene, 1mmol (133mg) of N-chlorosuccinimide, 1mmol (154mg) of silver nitrite and 5ml of acetonitrile into a sealed pressure vessel with a volume of 25ml. The mixture was reacted at 25°C for 8 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluent, the eluent was distilled off to obtain 2-(nitroalkynyl)-1,1'biphenyl (78% yield).
[0033]
[0034] yellow solid; 1 H NMR (CDCl 3 ,500MHz):δ7.79(d,J=1.0Hz,1H),7.66(t,J=1.5Hz,1H),7.57(m,2H),7.47(m,5H),
Embodiment 2
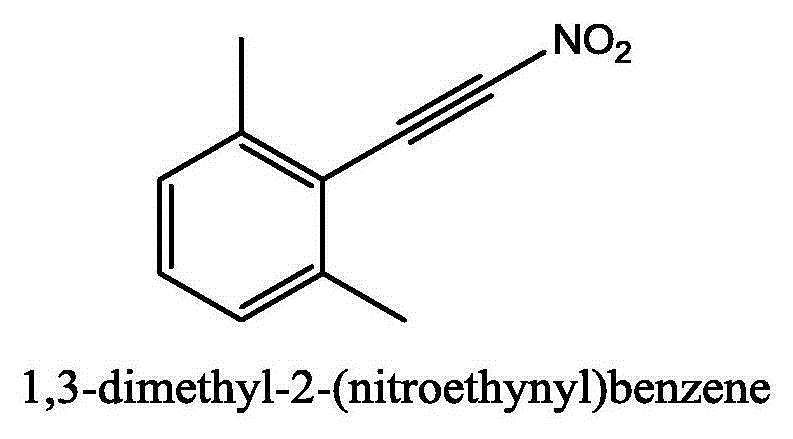
[0036] Add 0.5mmol (65mg) of 2,6-dimethylphenylacetylene, 0.5mmol (67mg) of N-chlorosuccinimide, 0.5mmol (77mg) of silver nitrite and 5ml of acetonitrile into a 25ml sealed pressure vessel in sequence middle. The mixture was reacted at 25°C for 8 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluent, eluent was distilled off to obtain 2,6-dimethyl-(nitroalkynyl)benzene (56% yield).
[0037]
[0038] white solid, 1 H NMR (CDCl 3 ,500MHz):δ7.35(t,J=7.5Hz,1H),7.13(d,J=7.5Hz,2H),6.17(s,6H).
Embodiment 3
[0040] Add 0.5mmol (80mg) of 3,5-dimethoxyphenylacetylene, 1mmol (133mg) of N-chlorosuccinimide, 1mmol (154mg) of silver nitrite and 4ml of acetonitrile into a sealed pressure vessel with a volume of 25ml. . The mixture was reacted at 40°C for 8 hours. After the TLC detection reaction finished, the reaction solution was diluted with dichloromethane, filtered to obtain the clear liquid, separated by column chromatography (eluent ratio: sherwood oil to ethyl acetate volume ratio 10:1), and collected the product containing Eluent, eluent was distilled off to obtain 1,3-dimethoxy-5-(nitroalkynylbenzene) (66% yield).
[0041]
[0042] white solid; 1 H NMR (CDCl 3 ,500MHz): δ6.76(d,J=2.5Hz,2H),6.66(d,J=2.0Hz,1H),3.81(s,6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com