Patents
Literature
96results about How to "Good substrate adaptability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
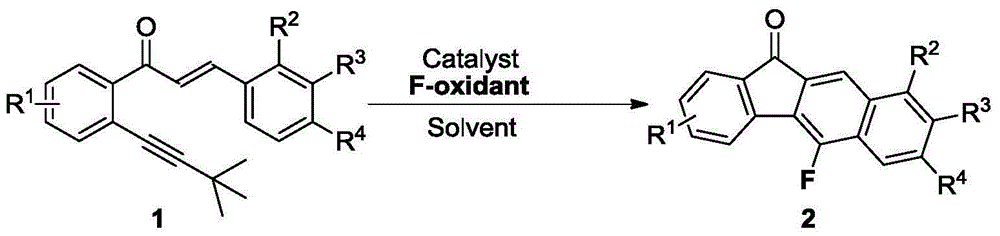
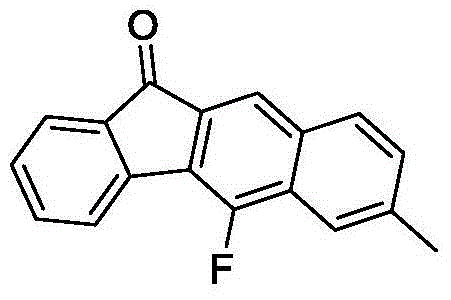
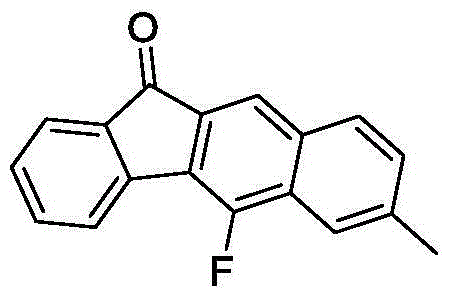
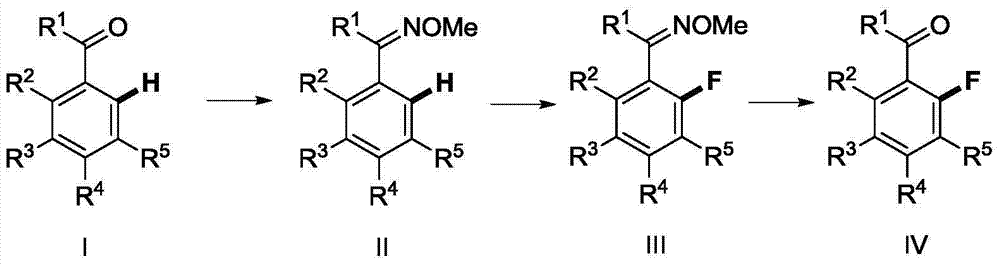
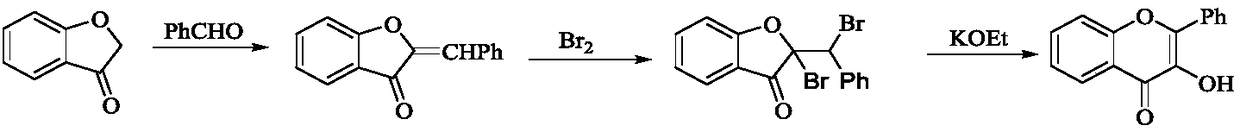
Method for synthesizing fluorofluorenone compound
ActiveCN104892387AGood substrate adaptabilityAchieve aromatization/fluorinationOrganic compound preparationCarbonyl compound preparationOrganic solventFluoride
The invention discloses a method for synthesizing a fluorofluorenone compound. the method is characterized in that a o-t-butyl ethynyl chalcone compound as shown in the formula 1 is used as a raw material to react in an organic solvent under the action of a copper catalyst and a fluorine oxidizer at the temperature of 25-60 DEG C for 0.5-6 h; and a reaction liquid undergoes separation and purification to prepare the fluorofluorenone compound as shown in the formula 2. The method has advantages of simple reaction steps, good substrate universality, mild reaction condition, safety and environmental protection, etc. According to the method, various fluoride-free nonaromatic chalcones are directly used as raw materials and cheap copper is used as a catalyst. The method is a new route for synthesizing various fluorofluorenone compounds containing a substituent group.
Owner:ZHEJIANG UNIV OF TECH
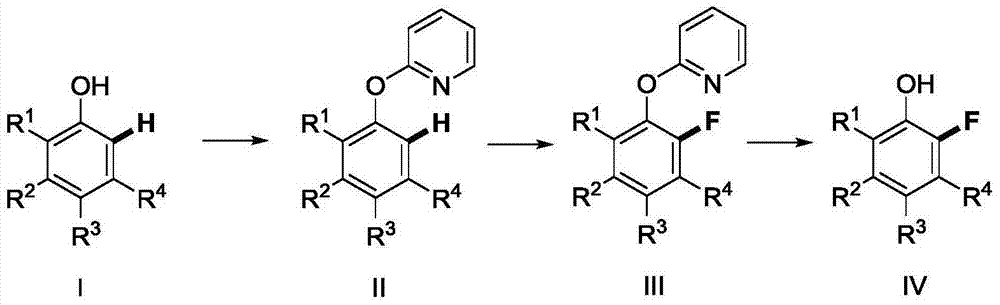
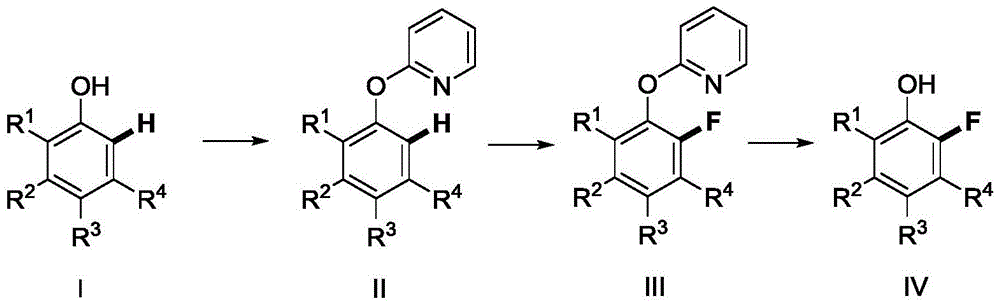
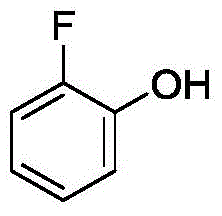
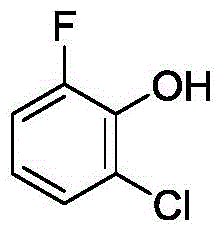
Method for synthetizing 2-fluoro phenol compound
ActiveCN104844399AWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationHydroxy group formation/introductionOrganic solventOrtho position
The present invention provides a method for synthetizing a 2-fluoro phenol compound shown in a formula IV. The phenol compound shown in the formula I is prepared into a 2-pyridine oxygroup arene compound shown in a formula II through an Ullmann reaction, the 2-pyridine oxygroup arene compound shown in the formula II is mixed with a palladium catalyst, a fluorinating reagent, an additive and an organic solvent, the mixture is stirred under the temperature of 30-160 DEG C to perform a fluorination reaction to obtain an ortho-position fluoridated 2-pyridine oxygroup arene compound shown in a formula III, and the ortho-position fluoridated 2-pyridine oxygroup arene compound shown in the formula III is prepared into the 2-fluoro phenol compound shown in the formula IV through the action of alkali. The method provided by the present invention has the advantages of mild reaction conditions, simplicity in operations, good substrate adaptability, high fluorination selectivity and the like. The 2-fluoro phenol compound is shown in the figure below.
Owner:ZHEJIANG UNIV OF TECH
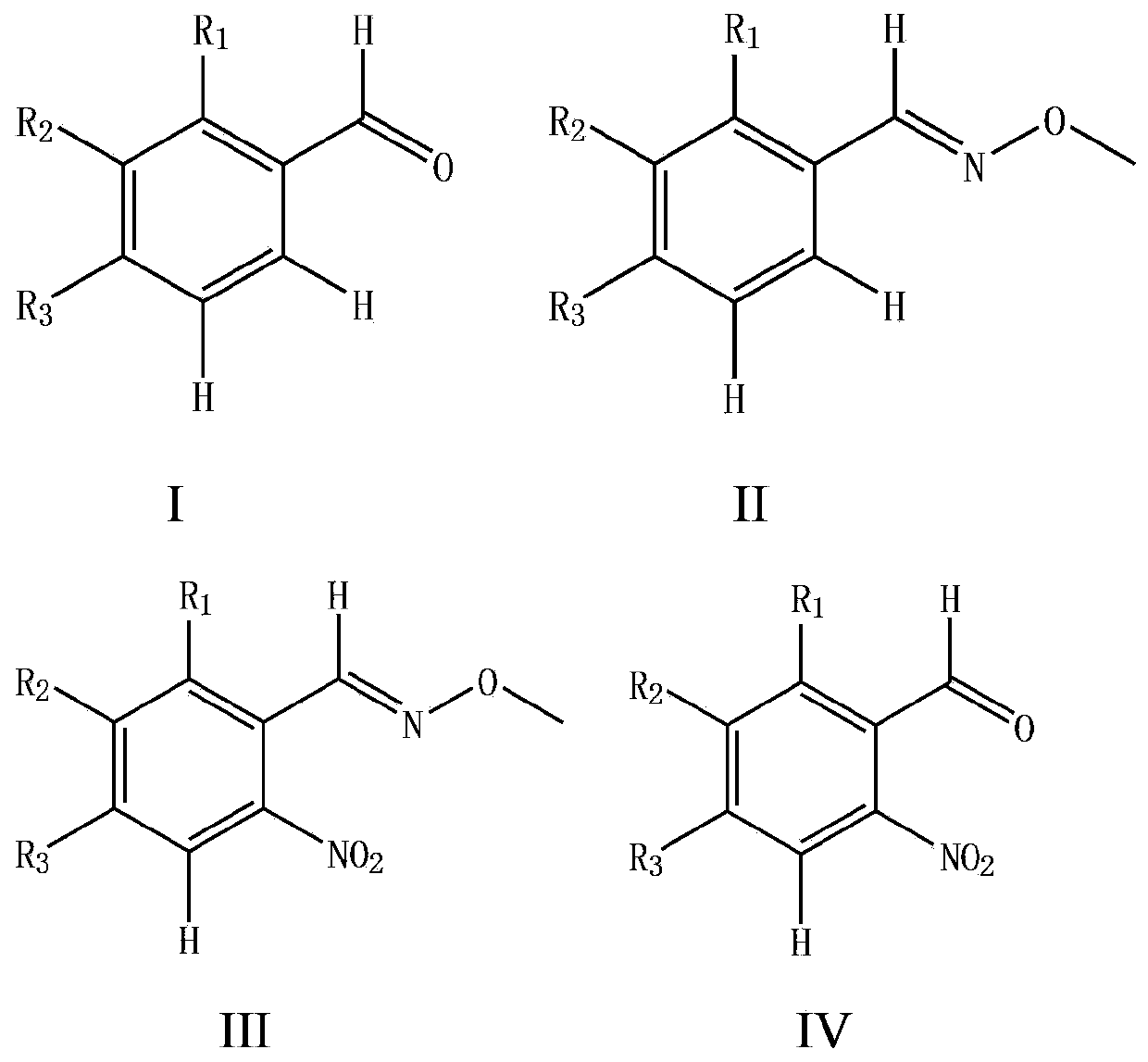
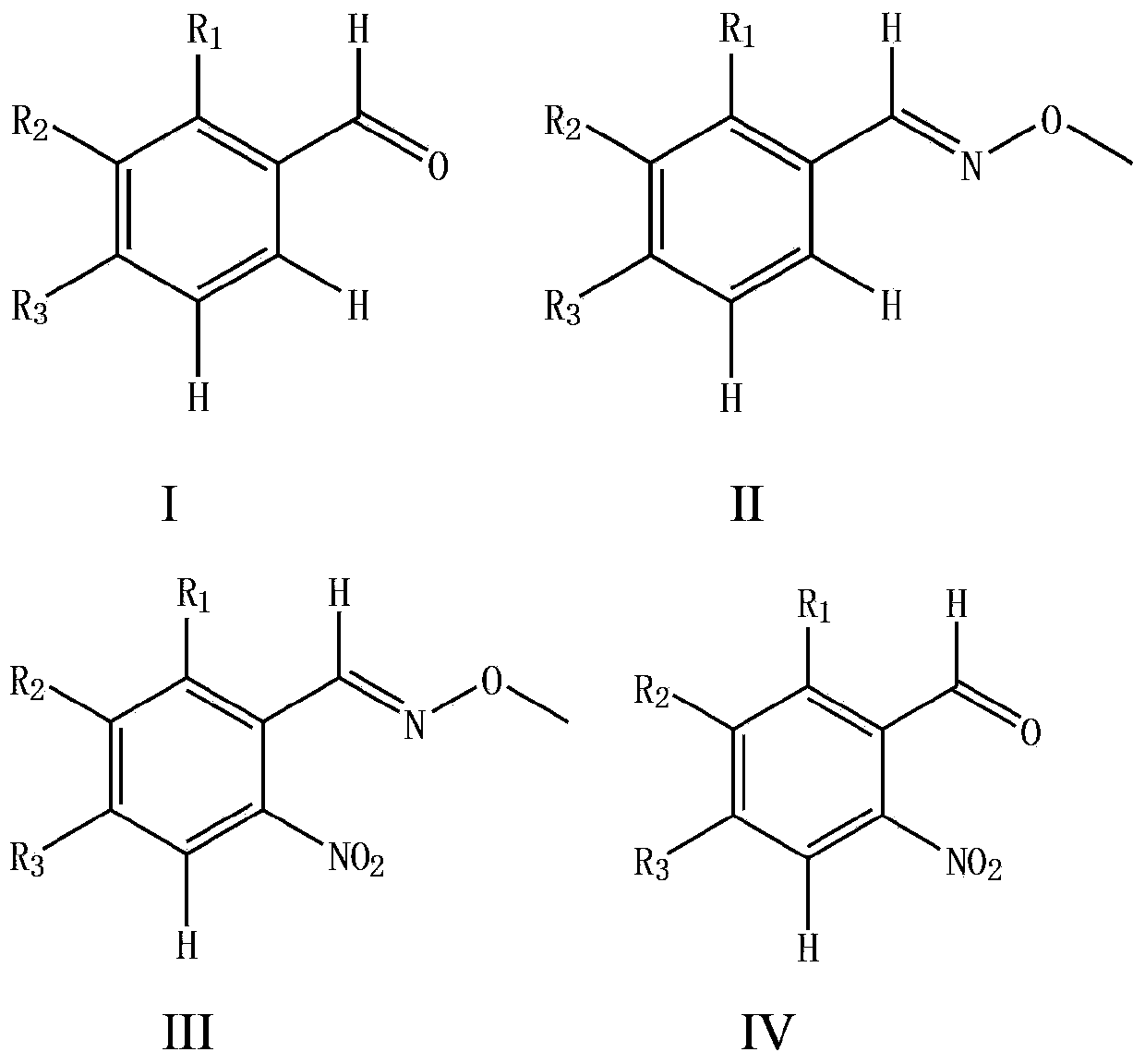
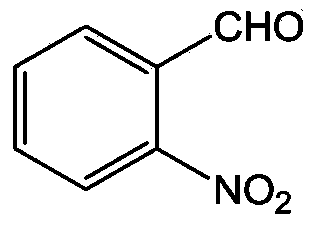
Synthesizing method for o-nitrobenzaldehyde compound
ActiveCN103467300AHigh yieldLow priceOrganic chemistryOrganic compound preparationOrganic acidBenzene
The invention provides a preparation method for an o-nitrobenzaldehyde compound. The method directly taking benzaldehyde compounds as starting raw materials comprises the following steps: firstly, converting a formyl group into an O-methyl oximido; secondly, taking divalent palladium salt as a catalyst, and realizing the carbon-hydrogen bond activation single nitration reaction on an o-position of an oximido under the condition that both an oxidant and a nitration agent exist; finally, removing the O-methyl oximido by using strong organic acid to obtain the o-nitrobenzaldehyde compound. The nitration method provided by the invention has the advantage of specificity in the o-position of a nitration position, the reaction process is safe and environment-friendly, the substrate is excellent in adaptability, and various substituents can realize o-position nitration; various benzaldehyde is directly taken as raw materials, so that the reaction steps are simple, and the synthesizing method is a novel route for synthesizing various o-nitrobenzaldehyde compounds containing substituents.
Owner:徐州宏阳新材料科技股份有限公司
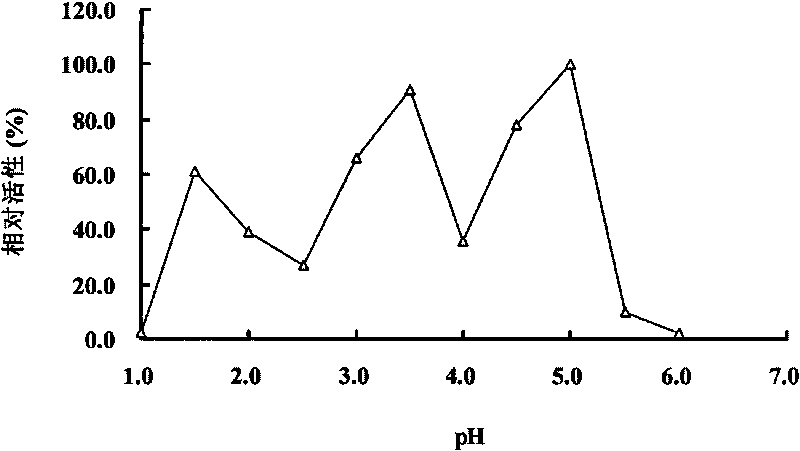
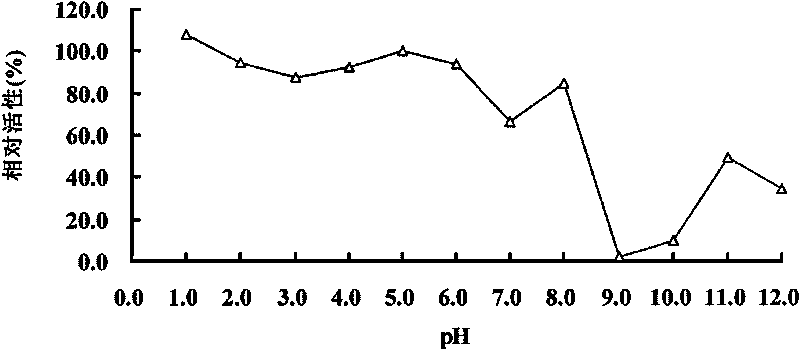
Acidophil Beta-glucanase GLU7A and gene and application thereof
ActiveCN101748108AGood substrate adaptabilityImprove digestive energyFungiBacteriaBiotechnologyCellulose
The invention relates to the field of genetic engineering, in particular to an acidophil Beta-glucanase GLU7A and gene and application thereof. The invention provides a glucanase GLU7A from acidophil bispora Bisporasp, and the amino acid sequence thereof is shown as SEQIDNO.1, and the invention provides a genome of coding the glucanase and cDNA coding gene glu7A. The invention obtains an acidophil glucanase which has the optimum pH value of 1.5-5.0, simultaneously remains high enzymatic activity in acid environment, and resists protease hydrolysis. In addition, the glucanase not only hydrolyzes Beta-1, 3-1, 4-glucanase, but also can hydrolyze cellulose. Due to the properties, the acidophil Beta-glucanase GLU7A can be applied in the industries of food, feedstuff and beer.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Bi-component corrosion-proof dope
InactiveCN100526406CEasy curingStrong adhesionAnti-corrosive paintsEpoxy resin coatingsEpoxyPolyamide
A titanium-nickel nanometer alloy powder contains A and B components; A components consist of epoxy-resin, titanium-nickel nanometer alloy powder, antirust pigment, filler corrosion stabilizer and anti-sagging accessory; B components consist of composite curing agent with cashew nut shell modified phenolic amine curing agent and polyamide epoxy curing agent; the proportion of A and B is 10:1. It has excellent adhesion and physical performance and better oil, water, acid-alkali and salt fog resistances and mating ability. It can be used as universal base coat and used for various substrate materials such as steel, aluminum and stainless steel etc.
Owner:中国人民解放军海军装备技术研究所
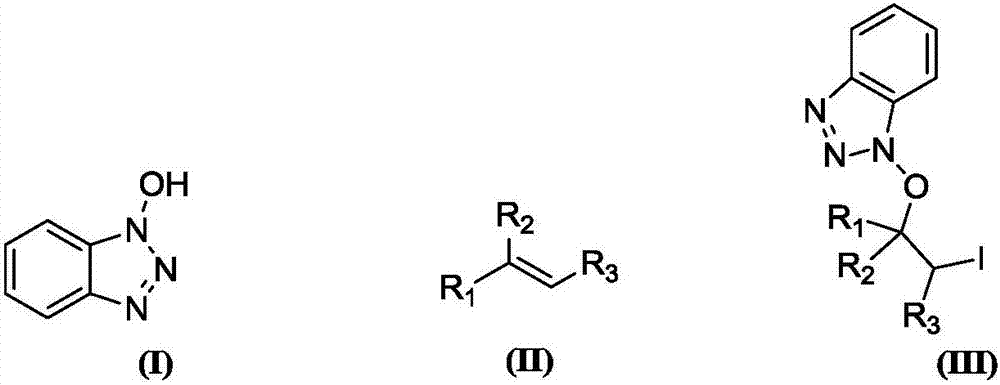
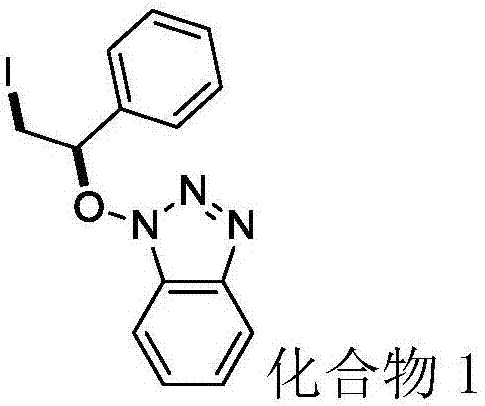
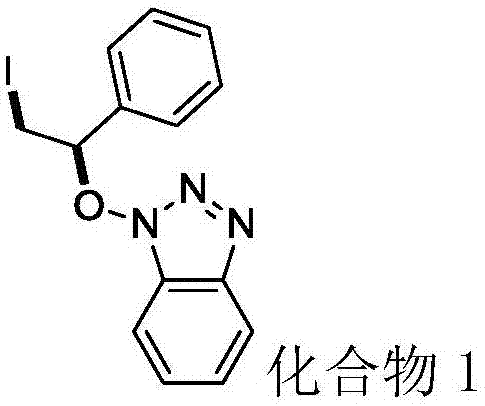
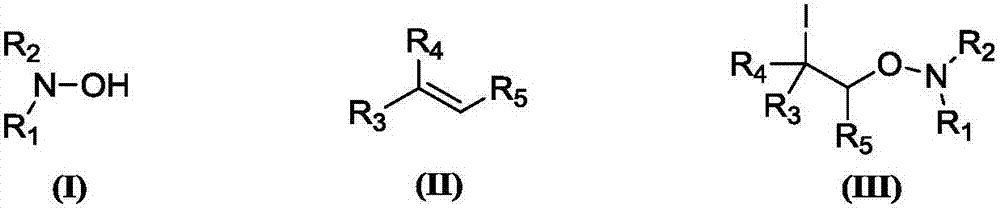
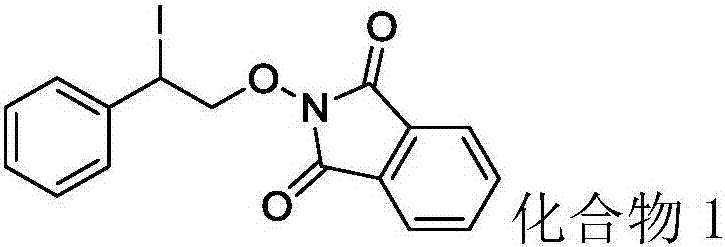
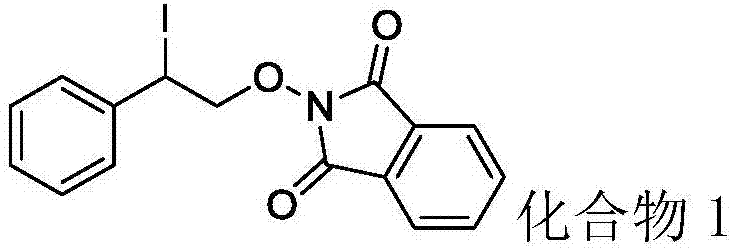
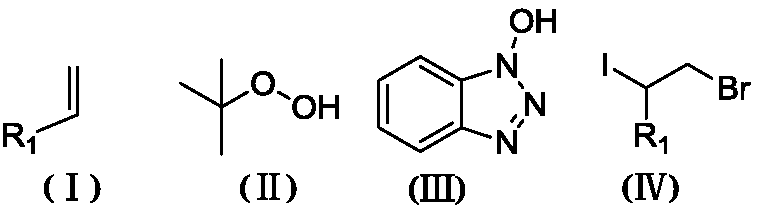
Synthesis method of beta-iodo-N-alkoxy benzotriazole compounds
ActiveCN107382884AMild reaction conditionsAchieve synthesisOrganic chemistrySynthesis methodsMetal catalyst
The invention provides a synthesis method of beta-iodo-N-alkoxy benzotriazole compounds shown as a formula (III). According to the method, N-hydroxybenzotriazole shown as a formula (I), olefin compounds shown as a formula (II), an iodine source and an oxidizing agent are mixed into a polar solvent to react for 2 to 8h at 0 to 80 DEG C; after the reaction is completed, reaction liquid is obtained; reaction liquid is subjected to post treatment to obtain the beta-iodo-N-alkoxy benzotriazole compounds. The addition mole ratio of the N-hydroxybenzotriazole shown as the formula (I) to the olefin compounds shown as the formula (II) to the iodine source to the oxidizing agent is 1:(0.5 to 5):(0.5 to 1):(1 to 5). The reaction conditions are mild; safety and environment protection are realized; the substrate applicability is high; no metal catalyst participates; the operation is simple; the application to medicine synthesis is facilitated. The formulas (I), (II) and (III) are shown in the specification.
Owner:ZHEJIANG UNIV OF TECH
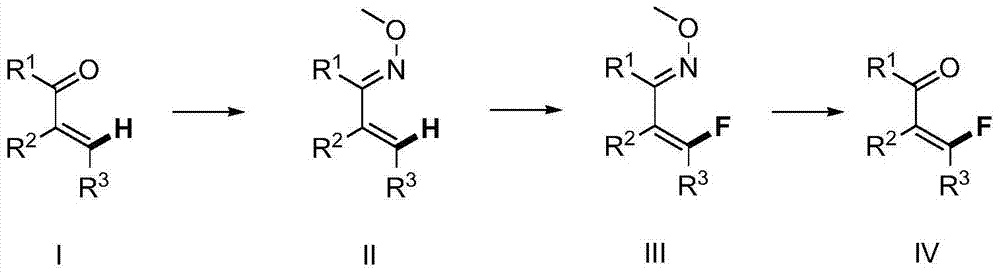
Method for synthesizing beta-fluoro-alpha,beta-unsaturated ketene compounds
ActiveCN103922909AHigh derivative valueMild reaction conditionsOximes preparationCarbonyl compound preparation by hydrolysisPalladium catalystHigh selectivity
The invention provides a method for synthesizing beta-fluoro-alpha,beta-unsaturated ketene compounds, which comprises the following steps: converting alpha,beta-unsaturated ketene compounds into corresponding carbonyl oxime ether compounds, mildly implementing sp2 alkenyl hydrocarbon chain direct fluoridation of high-selectivity beta- position in the presence of a palladium catalyst, a fluoridation reagent and additives, and finally, rehydrolyzing oxime ethers under the action of acid to obtain the beta-fluoro-alpha,beta-unsaturated ketene compounds. The fluoridation method has the advantages of mild reaction conditions, high substrate adaptability, high fluoridation selectivity and the like, is simple to operate, and has higher application research value.
Owner:ZHEJIANG UNIV OF TECH
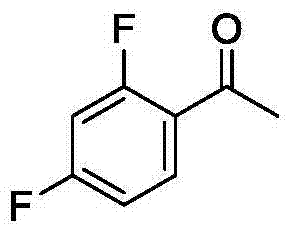
Method for synthesizing 2-fluoroarylcarbonyl compounds
ActiveCN103922904AWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationCarbonyl compound preparation by hydrolysisArylOrtho position
The invention provides a method for synthesizing 2-fluoroarylcarbonyl compounds, which comprises the following steps: converting arylcarbonyl compounds into corresponding carbonyl oxime ether compounds, mildly implementing aryl hydrocarbon chain direct fluoridation of high-selectivity oximido substituent group ortho-position in the presence of a palladium catalyst, a fluoridation reagent and additives, and finally, rehydrolyzing oxime ethers under the action of acid to obtain the 2-fluoroarylcarbonyl compounds. The fluoridation method has the advantages of mild reaction conditions, high substrate adaptability, high fluoridation selectivity and the like, is simple to operate, and has higher application research value.
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of beta-iodo-N-alkoxyamine compounds
ActiveCN107382821AMild reaction conditionsAchieve synthesisOrganic chemistryPolyamine CompoundHydroxylamine
The invention provides a synthesis method of beta-iodo-N-alkoxyamine compounds shown as a formula (III). Substituted hydroxylamine shown as a formula (I), olefin compounds shown as a formula (II), an iodine source and an oxidizing agent are mixed into a polar solvent to completely react to obtain reaction liquid; after the reaction for 2 to 12 hours at 20 to 120 DEG C, the reaction liquid is subjected to post treatment to obtain the beta-iodo-N-alkoxyamine compounds. The addition mole ratio of the substituted hydroxylamine shown as the formula (I) to the olefin compounds shown as the formula (II) to the iodine source to the oxidizing agent is 1:(5 to 15):(0.5 to 1):(1 to 5). The reaction conditions are mild; safety and environment protection are realized; the substrate applicability is high; no metal catalyst participates; the operation is simple; the application to medicine synthesis is facilitated. The formulas (I), (II) and (III) are shown in the specification.
Owner:ZHEJIANG UNIV OF TECH
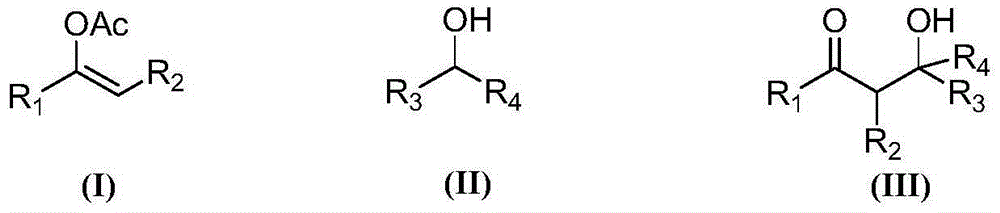
Synthesis method for beta-hydroxy-ketone compound
InactiveCN105001070AAchieve synthesisGood substrate adaptabilityOrganic compound preparationCarbonyl compound preparation by condensationAlcoholSynthesis methods
The invention provides a synthesis method for a beta-hydroxy-ketone compound shown in a formula (III). The method comprises the steps that substitute vinyl acetate shown in a formula (I), a substitute alcohol compound shown in a formula (II) and an oxidizing agent are mixed to obtain reaction liquid, and the reaction liquid reacts for 2-12 hours at the temperature of 20 DEG C-120 DEG C, and then is treated to obtain the beta-hydroxy-ketone compound. The method is safe and environmentally friendly, and is a new path for synthesizing the beta-hydroxy-ketone compound containing various substituents, the substrate adaptability is good, and the reaction operation is easy.
Owner:上海汉亭化学有限公司
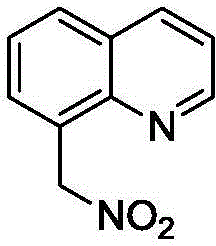
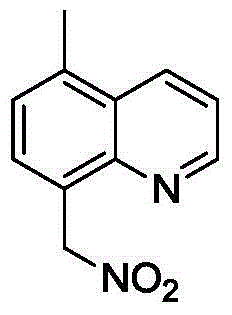
Method for synthesizing 8-(nitro methyl) quinoline compounds
ActiveCN104860880AAchieve nitrificationGood substrate adaptabilityOrganic chemistryOrganic solventNitration
The invention provides a method for synthesizing 8-(nitro methyl) quinoline compounds. 8-methylquinoline compounds are taken as a raw material and are added into an organic solvent together with a catalyst and a nitration reagent, the mixture is sealed and heated to 80-130 DEG C for a reaction, the reaction is traced with TLC (thin-layer chromatography) until the reaction ends, a reaction liquid is subjected to aftertreatment, and the 8-(nitro methyl) quinoline compounds shown in the formula II are obtained. The nitration method has the advantage of specifity of the nitration position, nitration is only performed on methyl, no nitration products are generated on a benzene ring, the reaction process is safe and environment-friendly, the substrate adaptability is good, and methyl nitration of all substituent groups can be realized; 8-methylquinoline is directly taken as a raw material, the reaction steps are simple, and the method is a new way for synthesizing 8-(nitro methyl) quinoline compounds containing substituent groups.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 3-acyl screw ring dibenzosuberenone compound
The invention belongs to the field of pharmaceutical and organic intermediate synthesis, and concretely relates to a preparation method of 3-acyl screw ring dibenzosuberenone compound. An alkyne amidecompound as shown in a formula II and acyl chloride as shown in a formula III are reacted in an organic solvent under illumination with the existence of a photocatalyst [Ir(ppy)3] and alkali so as toobtain the 3-acyl screw ring dibenzosuberenone compound as shown in the formula I as the description.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing 1-naphthol phosphine oxide ligand
ActiveCN110183488ASimple stepsMild reaction conditionsGroup 5/15 element organic compoundsOrganic solventSulfur
The invention discloses a method for preparing a 1-naphthol phosphine oxide ligand. The method comprises the following steps: uniformly mixing a sulfur ylide compound, a phenylethyny phosphine oxide derivative, a catalyst, a Lewis acid and an organic solvent, and reacting in nitrogen at a temperature of 60-100 DEG C for 6-16 hours, thereby obtaining the finished 1-naphthol phosphine oxide ligand of a structural formula as shown in the specification. According to the method disclosed by the invention, the 1-naphthol phosphine oxide ligand that is difficultly prepared in the conventional synthetic method can be obtained, the step of the method is simple, and the 1-naphthol phosphine oxide ligand can be constructed by one step only. Moreover, the method is mild in reaction condition, excellent in substrate adaptability and high in yield, and can synthesize the 1-naphthol phosphine oxide ligand of multiple different substituent groups.
Owner:FOSHAN UNIVERSITY
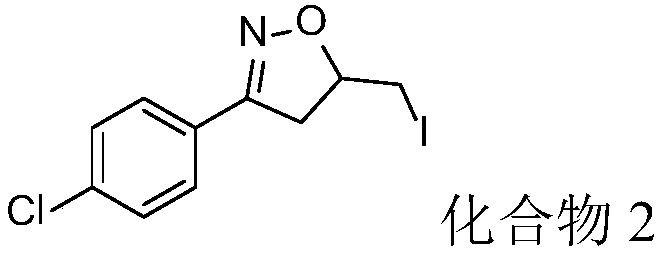
Synthesis method for iodoisoxazoline compound
InactiveCN109293593AAtom economy is highEasy to operateOrganic chemistrySynthesis methodsMetal catalyst
The invention discloses a synthesis method for an iodoisoxazoline compound shown as formula (II). The method includes: dissolving ketoxime shown as formula (I), an iodine source and an oxidant mixedlyin a solvent, carrying out reaction for 7-15h at 25DEG C-60DEG C in the presence of an inert gas, and subjecting the obtained reaction liquid to post-treatment so as to obtain the isoxazoline compound shown as formula (II). The method provided by the invention directly takes ketoxime as the raw material for intramolecular cyclization reaction. The whole process has no need for a metal catalyst, avoids metal residue, and has high atom economy, also the reagents are cheap and environment-friendly, and the reaction operation is simple, therefore the method is more beneficial to application in pharmaceutical synthesis.
Owner:ZHEJIANG UNIV OF TECH
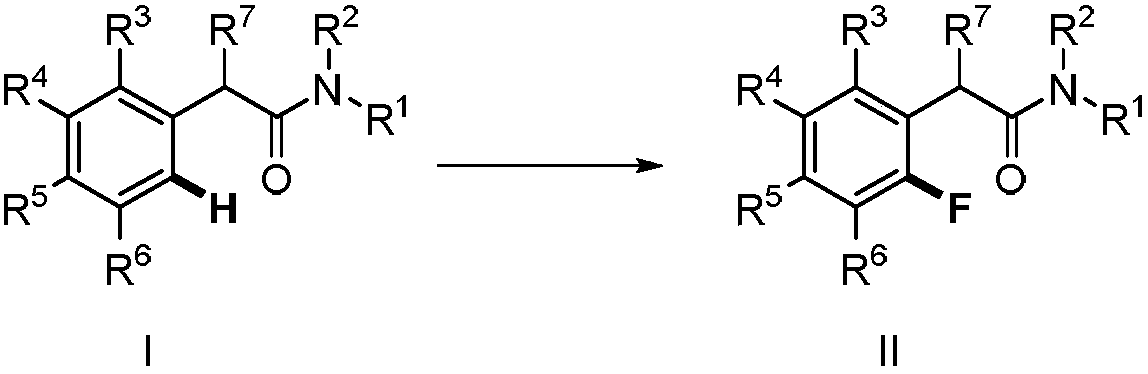
Method for synthesizing 2-fluoro-N-substituted aryl acetamide compound
The invention provides a method for synthesizing a 2-fluoro-N-substituted aryl acetamide compound. The method comprises the following steps that a N-substituted aryl acetamide compound reacts at 20 to150 DEG C under the condition of existence of palladium catalysts, fluorinated reagents and additives; TLC tracking detection is performed until the reaction is thoroughly performed; through aftertreatment, the compound shown as a formula II is obtained; the amide substituent ortho-position high-selectivity aryl hydrocarbon bond direct fluorination can be mildly realized. The method has the advantages that the reaction conditions are mild; the operation is simple; the substrate applicability is high; the fluoridation selectivity is high, and the like. High application study values are realized.
Owner:ZHEJIANG UNIV OF TECH
Benzodihydropyrone derivative and preparation method thereof
The invention discloses a benzodihydropyrone derivative and a preparation method thereof. The method includes: adopting a phenoxy-containing alkynone compound as the reaction raw material, taking goldtrichloride as the catalyst, using chlorobenzene as the solvent, and employing 2, 6-dichloropyridine nitrogen oxide as the oxidant, carrying out reaction at 55DEG C-65DEG C for 2h-3h to obtain the benzodihydropyrone derivative. Specifically, the alkynone compound has a chemical structural formula shown as A, and the benzodihydropyrone derivative has a chemical structural formula shown as B; R1 isselected from C1-C12 alkyl, substituted aryl, aromatic heterocycle or thick ring; R2 is selected from hydrogen atom, C1 C12 alkyl, various substituent aryl, aromatic heterocyclic or condensed ring;R2 is selected from hydrogen atom, C1-C12 alkyl, various substituted aryls, aromatic heterocycles or thick rings; the formula A is shown as the specification; and the formula B is shown as the specification. According to the method provided by the invention, the product has the advantages of easy separation, high yield, good substrate adaptability, simple reaction process and great application potential.
Owner:NORTHWEST A & F UNIV
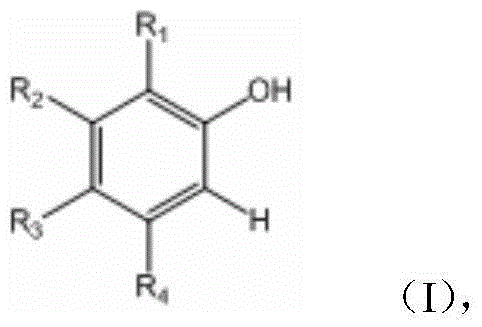
A kind of method of synthesizing 2-fluorophenol compound
ActiveCN104844399BWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationHydroxy group formation/introductionOrganic solventOrtho position
The present invention provides a method for synthetizing a 2-fluoro phenol compound shown in a formula IV. The phenol compound shown in the formula I is prepared into a 2-pyridine oxygroup arene compound shown in a formula II through an Ullmann reaction, the 2-pyridine oxygroup arene compound shown in the formula II is mixed with a palladium catalyst, a fluorinating reagent, an additive and an organic solvent, the mixture is stirred under the temperature of 30-160 DEG C to perform a fluorination reaction to obtain an ortho-position fluoridated 2-pyridine oxygroup arene compound shown in a formula III, and the ortho-position fluoridated 2-pyridine oxygroup arene compound shown in the formula III is prepared into the 2-fluoro phenol compound shown in the formula IV through the action of alkali. The method provided by the present invention has the advantages of mild reaction conditions, simplicity in operations, good substrate adaptability, high fluorination selectivity and the like. The 2-fluoro phenol compound is shown in the figure below.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing 2-bromo-1-iododihalide by one-pot process
InactiveCN108503500AMild reaction conditionsGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationEnvironmental resistanceOrganic solvent
The invention discloses a method for synthesizing 2-bromo-1-iododihalide represented by the formula (IV). The method comprises the following steps: mixing an olefin compound represented by formula (I)with an iodine source, tert-butyl hydroperoxide represented by formula (II) and N-hydroxybenzotriazole represented by formula (III) in an organic solvent, and completely reacting the obtained solution at a temperature from room temperature to 50 DEG C to obtain a reaction solution A; and adding acetyl bromide to the reaction solution A, completely reacting the obtained solution at room temperature to obtain a reaction solution B, and post-treating the reaction solution B to obtain the 2-bromo-1-iododihalide. The method has the advantages of safety, environmental protection, no generation of waste gas, facilitation of application in pharmaceutical synthesis, mild reaction conditions, good substrate adaptability, and realization of corresponding 2-bromo-1-iododihalides from various substituent groups.
Owner:ZHEJIANG UNIV OF TECH
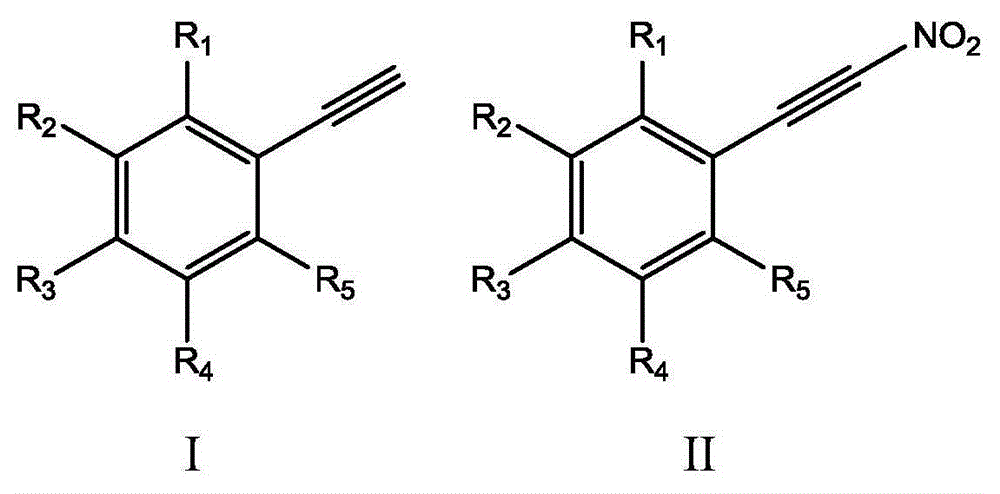
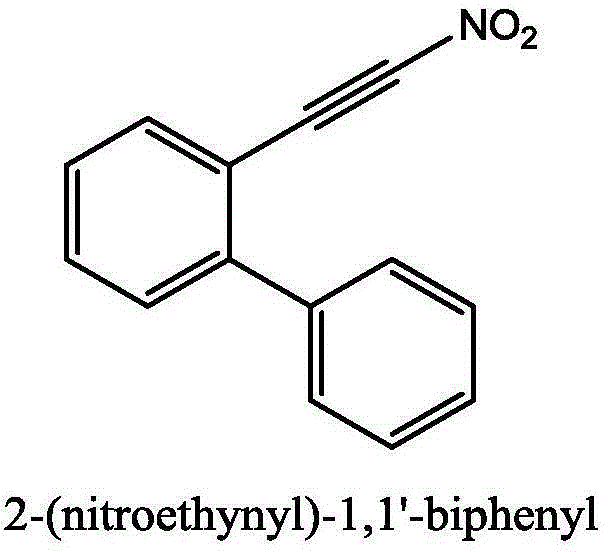
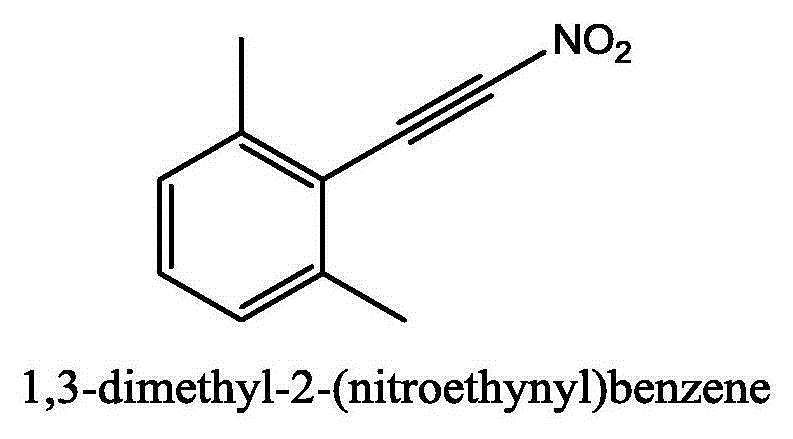
Method for synthesizing (nitroalkynyl)benzene compounds
ActiveCN106478327AAchieve nitrificationGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationBenzeneOrganic solvent
The invention provides a method for synthesizing (nitroalkynyl)benzene compounds shown in formula II, comprising: adding phenylacetylene compounds shown in formula I as materials, along with a nitrifying agent, into an organic solvent, hermetically reacting at 20-50 DEG C, performing TLC (thin layer chromatography) tracing until the reaction is over, and post-treating the reacted liquid to obtain (nitroalkynyl)benzene compounds shown in the formula II. The nitrifying method of the invention has the advantage of good nitrifying site specificity, nitrifying is carried out only on alkynyl, no nitrification products are produced on benzene rings, the reaction process is good in safety, environment-friendly and good in substrate adaptability, and it is possible to provide alkynyl nitrifying for various substituents.
Owner:ZHEJIANG UNIV OF TECH
Water-phase one-pot synthesis method of 3-flavonol and 3-flavonol derivative
InactiveCN109320488AStrong substrate adaptabilityGood substrate adaptabilityOrganic chemistryChemical synthesisSynthesis methods
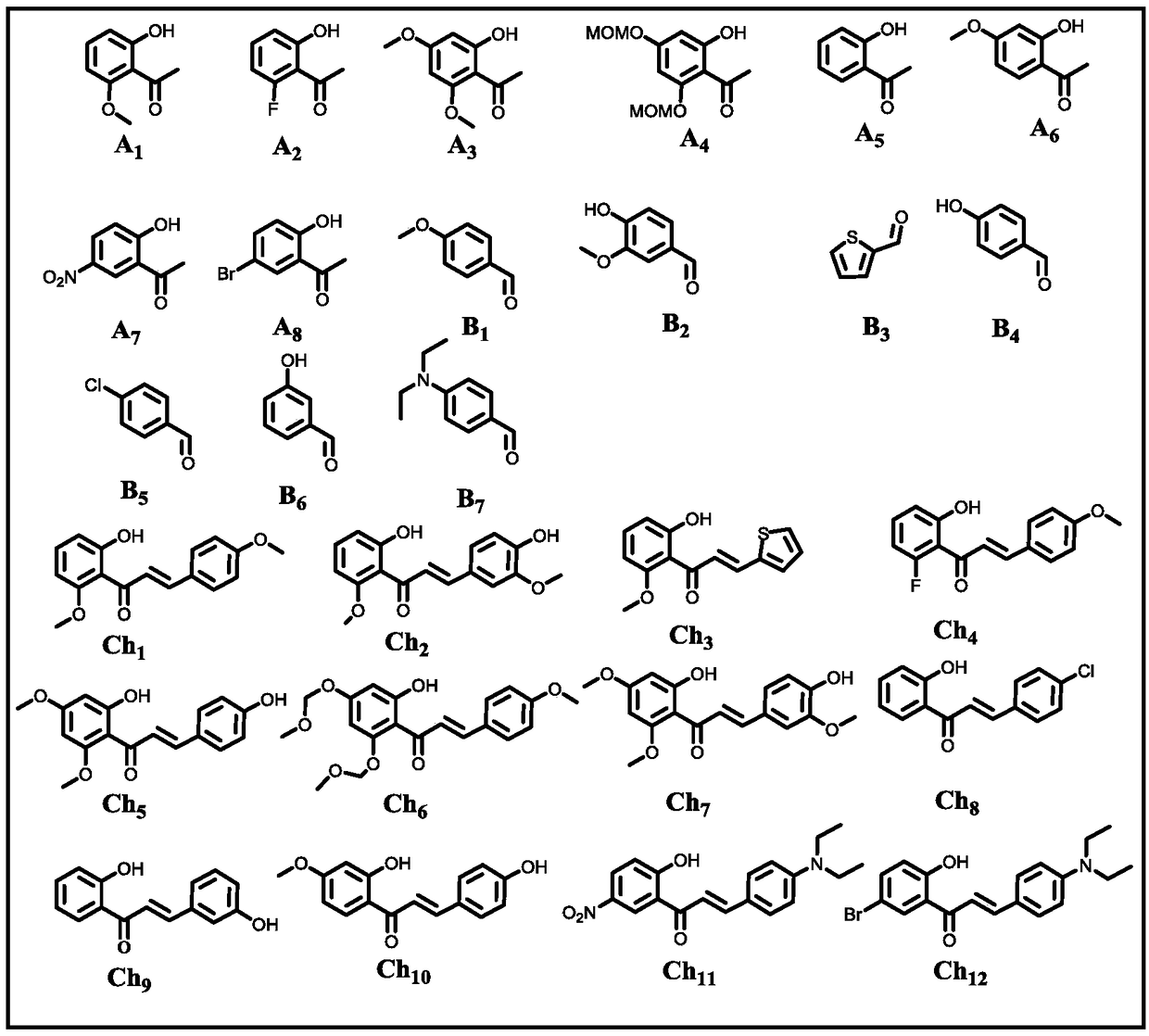
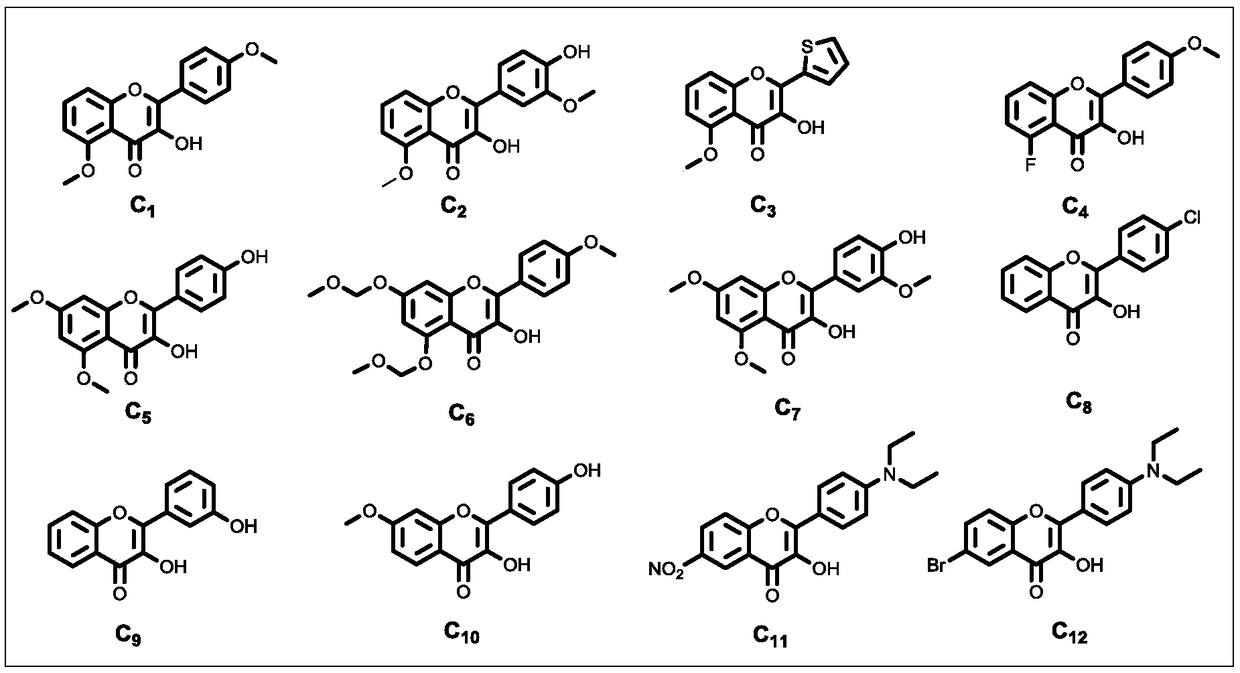
The invention belongs to the field of chemical synthesis, and particularly relates to a water-phase one-pot synthesis method of 3-flavonol and a 3-flavonol derivative. According to the method, 2-hydroxyacetophenone, 2-hydroxyacetophenone derivatives, benzaldehyde and benzaldehyde derivatives are used as reaction substrates, or 2-hydroxy-chalcone and hydroxy-chalcone derivatives are used as reaction substrates; water or ethanol water solution is used as a solvent; under the aerobic condition at 20 to 100 DEG C, reaction is performed to obtain the 3-flavonol and the 3-flavonol derivative. The invention provides a fire-new reaction mechanism, and develops a novel 3-flavonol synthesis method with the advantages of high efficiency, convenience, high speed and wide substrate adaptability. The invention also provides a fire-new 3-flavonol derivative synthesized by the novel method; important application values are realized in the field of medical care sanitation.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Method of catalyzing conversion of CO2 with alcohol amine bromide ionic liquid to synthesize cyclic carbonate compound
InactiveCN108707132AEasy to manufactureGood biocompatibilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholEthylene oxide
The invention discloses a method of catalyzing conversion of CO2 with alcohol amine bromide ionic liquid to synthesize a cyclic carbonate compound and belongs to the technical field of synthesis of cyclic carbonate compounds with catalysis of ionic liquids. The method is characterized in that: by taking the alcohol amine bromide ionic liquid as a catalyst, an ethylene oxide compound and CO2 are catalyzed at the atmospheric pressure and 90 DEG C to perform cycloaddition reaction to prepare the cyclic carbonate compound as a target product, and the alcohol amine bromide ionic liquid is repeatedly used after being recovered. In the method, the used alcohol amine bromide ionic liquid is simple and convenient to prepare and has good biocompatibility; the catalyst is high in catalytic activity and good in selectivity; and a reaction system does not have special requirements on a reaction vessel, and operation and the aftertreatment process of a catalytic system are relatively simple.
Owner:HENAN NORMAL UNIV
Preparation method of palladium catalyzed 1,2-trans diaryl alkene
InactiveCN109809955AEasy to operateGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationSulfonateOrganic solvent
The invention discloses a preparation method of palladium catalyzed 1,2-trans diaryl alkene. The method comprises the following steps that under the effects of catalysts, cocatalysts and alkali, arylacrylic acid and aromatic esters p-toluene sulfonate take decarboxylation coupling reaction in an organic solvent; after the reaction is finished, the 1,2-trans diaryl alkene is obtained through posttreatment. The method has the advantages that through C-O bond fracture, the operation is simple; a stable palladium catalyst with low cost is used; the substrate applicability is high; the harsh reaction conditions and the addition of strong alkali are not needed; the trans 1,2-diaryl alkene can be generated at high selectivity.
Owner:SHAOXING UNIVERSITY
Method for synthesizing polysubstituted dihydrofuran
InactiveCN109810079AAtom economy is highEasy to operateOrganic chemistryMetal catalystRoom temperature
The invention discloses a novel method for synthesizing a polysubstituted dihydrofuran compound. The method for synthesizing the polysubstituted dihydrofuran compound comprises the following steps that an olefinic dicarbonyl compound shown in the formula (I), an iodine source and an oxidizing agent are mixed in a solvent, after reacting is performed at the room temperature for 8-12 h, a reaction solution is subjected to post-treatment to obtain a dihydrofuran compound shown in the formula (II); the ratio of the amount of feeding materials of the olefinic dicarbonyl compound shown in the formula (I) to the iodine source to the oxidizing agent is 1 to 0.5-1.1-2.5; and the oxidizing agent is tert-butyl hydroperoxide or hydrogen peroxide. The method for synthesizing the polysubstituted dihydrofuran compound directly uses the olefinic dicarbonyl compound as a raw material to carry out an intramolecular cyclization reaction; the whole process does not require a metal catalyst, the metal residue is avoided, the reaction operation is simple, the reaction can be carried out under mild conditions, a good yield is obtained, and the application of the polysubstituted dihydrofuran compound in pharmaceutical synthesis is further facilitated. (Please see the specification for the formulae).
Owner:ZHEJIANG UNIV OF TECH
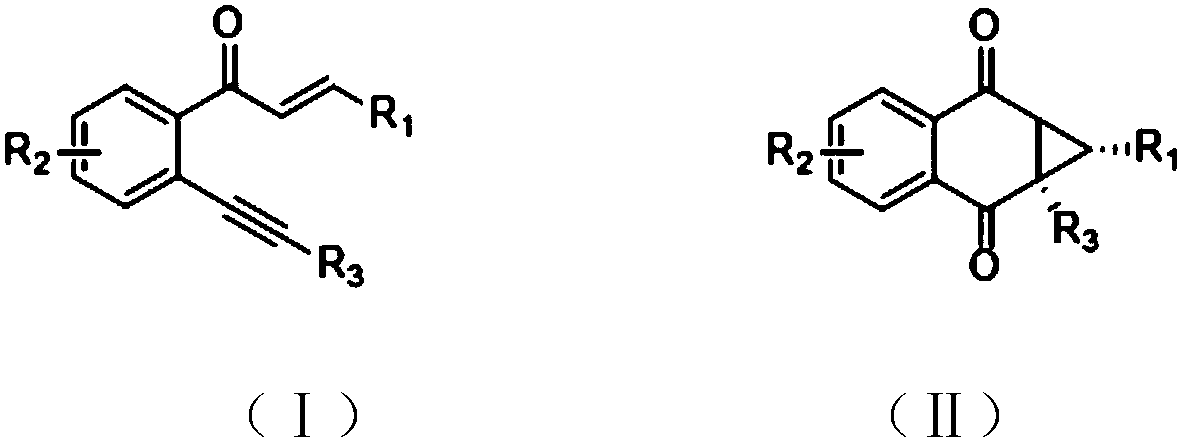
Method for synthesizing 1,4-naphthoquinone cyclopropane compound
ActiveCN109896944AReduce operational riskOxidative cyclizationCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsChalcone
The invention provides a method for synthesizing 1,4-naphthoquinone cyclopropane and a derivative thereof. The synthesis method is characterized by taking an o-alkynyl chalcone compound shown in a formula (I) as an initiator, taking ferric chloride or ferric nitrate as a catalyst, reacting for 0.1-1 hours at the temperature of 80 DEG C-120 DEG C in an organic solvent under the condition of takingdiiodine pentoxide as an oxidant, and performing separation and purification to prepare a corresponding target product. The synthesis method provided by the invention has the characteristics that theharm to environment is little; the reaction conditions are mild; the operation is convenient; and the like.
Owner:浙江工业大学上虞研究院有限公司 +1
Method for synthesizing 1- chlorine-2-dihalogenated iodine
InactiveCN108276244AMild reaction conditionsGood substrate adaptabilityCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventAcetyl chloride
The invention discloses a method for synthesizing 1-chlorine-2-dihalogenated iodine as shown in formula (IV). The method comprises the following steps: mixing an olefin compound as shown in formula (I), an iodine source, tert-butyl hydroperoxide as shown in formula (II) and N-hydroxybenzotriazole as shown in formula (III) into an organic solvent, and reacting completely from the room temperature to a temperature lower than 50 DEG C, thereby obtaining reaction liquor A; adding acetyl chloride into reaction liquor A, completely reacting at the room temperature, and performing post-treatment on reaction liquor B to obtain a 1-chlorine-2-dihalogenated iodine compound. The method is safe and environmentally friendly, does not generate waste gas, and is more beneficial for being applied to pharmaceutical synthesis. And reaction conditions are gentle, substrate adaptation is good, and various substituent groups also can realize corresponding 1-chlorine-2-iodine halogenation.(The formulas is shown in the description.).
Owner:ZHEJIANG UNIV OF TECH
Nucleic acid containing photosensitive unit and preparation method of nucleic acid
ActiveCN108484708AToxicHighly hazardousSugar derivativesSugar derivatives preparationEtherNitrobenzene
The invention provides nucleic acid containing a photosensitive unit and a preparation method thereof. The preparation method comprises the step of preparing to obtain 2-nitrobenzene methyl-(4,4-dimethoxy triphenyl)ether-5-(2-O-cyanoethyl-N,N-diisopropylphosphoramidite). Phosphoramidite can be used for preparing the nucleic acid containing the photosensitive unit. The phosphoramidite is adopted toprepare the nucleic acid containing the photosensitive unit; experiments show that the phosphoramidite has high reaction acvity, favorable substrate adaptability, sufficient chemical stability and favorable light responsiveness when being used for preparing; a simple and high-efficient option is provided for the functionalization of the nucleic acid; the method for preparing the nucleic acid containing the photosensitive unit is simplified; the sensitivity of photodegradation is improved; the preparation cost is reduced; the market application of preparing the nucleic acid containing the photosensitive unit is enlarged.
Owner:北京超精核酸科技有限公司
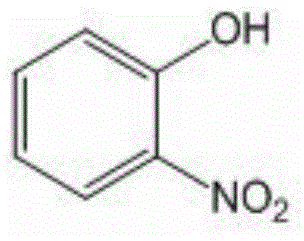
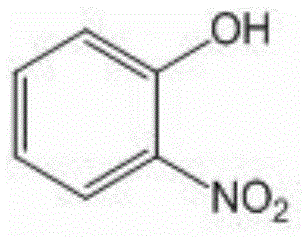
O-nitrophenol and its derivative synthesis method
ActiveCN105085275ASimple and fast operationGood substrate adaptabilityOrganic chemistryOrganic compound preparationSynthesis methodsStrong acids
The invention relates to an organic compound synthesis method. The organic compound synthesis method solves the problem that the existing o-nitrophenol synthesis method utilizes strong acid, produces environmental pollution and has long and complex steps. The invention provides an o-nitrophenol and its derivative synthesis method. The synthesis method comprises that a phenol compound is synthesized into 2-(phenoxy)pyridine, the product 2-(phenoxy)pyridine, a catalyst, tert-butyl nitrite and an organic solvent are orderly added into a sealed pressure-resistant container, the mixed materials are heated in an oil bath at a temperature of 50-100 DEG C and undergo a reaction for 10-30h to produce 2-(2-nitrophenyl)oxypyridine, and the 2-(2-nitrophenyl)oxypyridine is treated to form o-nitrophenol and its derivative. The synthesis method has simple processes and high efficiency.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Synthesis method of optically-active beta-amino ketones
ActiveCN109180606AHigh enantioselective excessMild reaction conditionsAntipyreticOrganic chemistry methodsMannich reactionOrganosolv
The invention discloses a synthesis method of optically-active beta-amino ketones represented by formula (I). The synthesis method is characterized in that benzothiazole imide represented by formula (II) and aromatic ketone compounds represented by formula (III) undergo an asymmetric Mannich reaction under the action of a cinchona-derived quaternary ammonium salt phase transfer catalyst represented by formula (IV) in a reaction medium composed of an organic solvent and an aqueous alkali solution to obtain the optically-active beta-amino ketones represented by formula (I). The synthesis methodhas the advantages of mild reaction conditions, simplicity in operation, good substrate adaptability and high atom utilization rate.
Owner:ZHEJIANG UNIV OF TECH
Nucleic acid containing photosensitive unit, preparation method and application thereof
InactiveCN109879922AHigh reactivityHigh activitySugar derivativesSugar derivatives preparationChemical synthesisEnd-group
The invention provides a nucleic acid containing a photosensitive unit, a preparation method and an application thereof, which belong to the technical filed of chemical synthesis, and can solve the technical problems that the operation of the existing photoresponsive nucleic acid end group sulfhydrylation reagents is complex, the reaction is not mild, and the efficiency is poor. The nucleic acid containing the photosensitive unit provided by the technical scheme is as shown in a formula (I): wherein a value of n ranges from 1 to 15. The nucleic acid can be applied to the preparation of a thiolend group modifying reagent.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
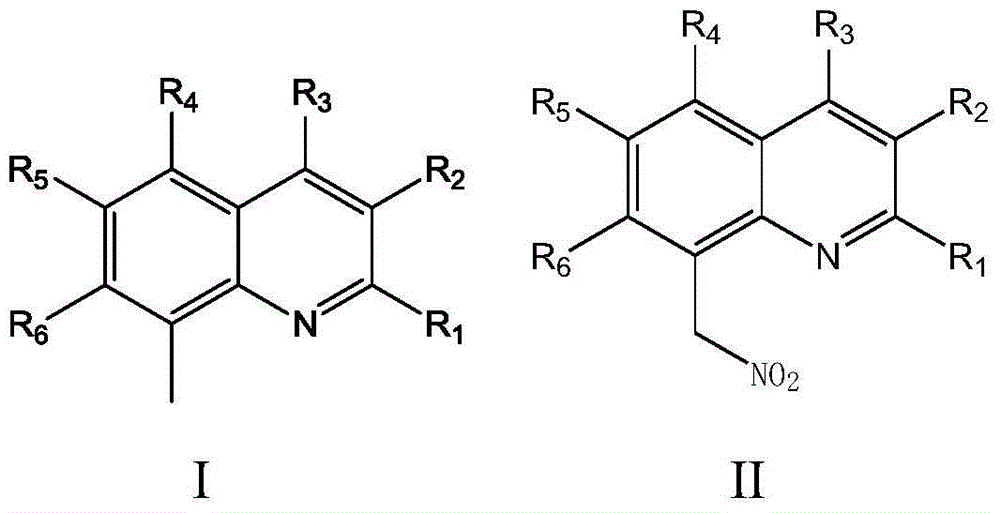
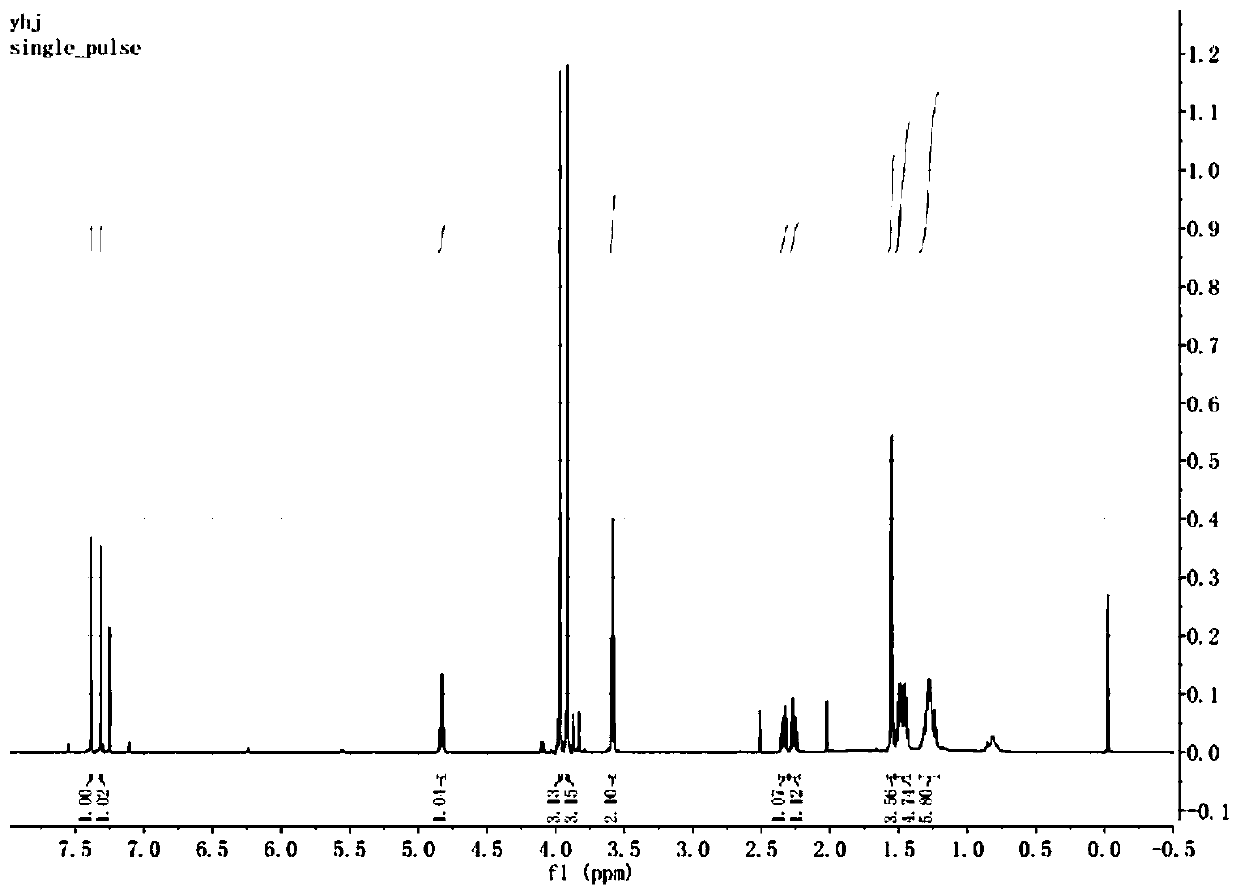
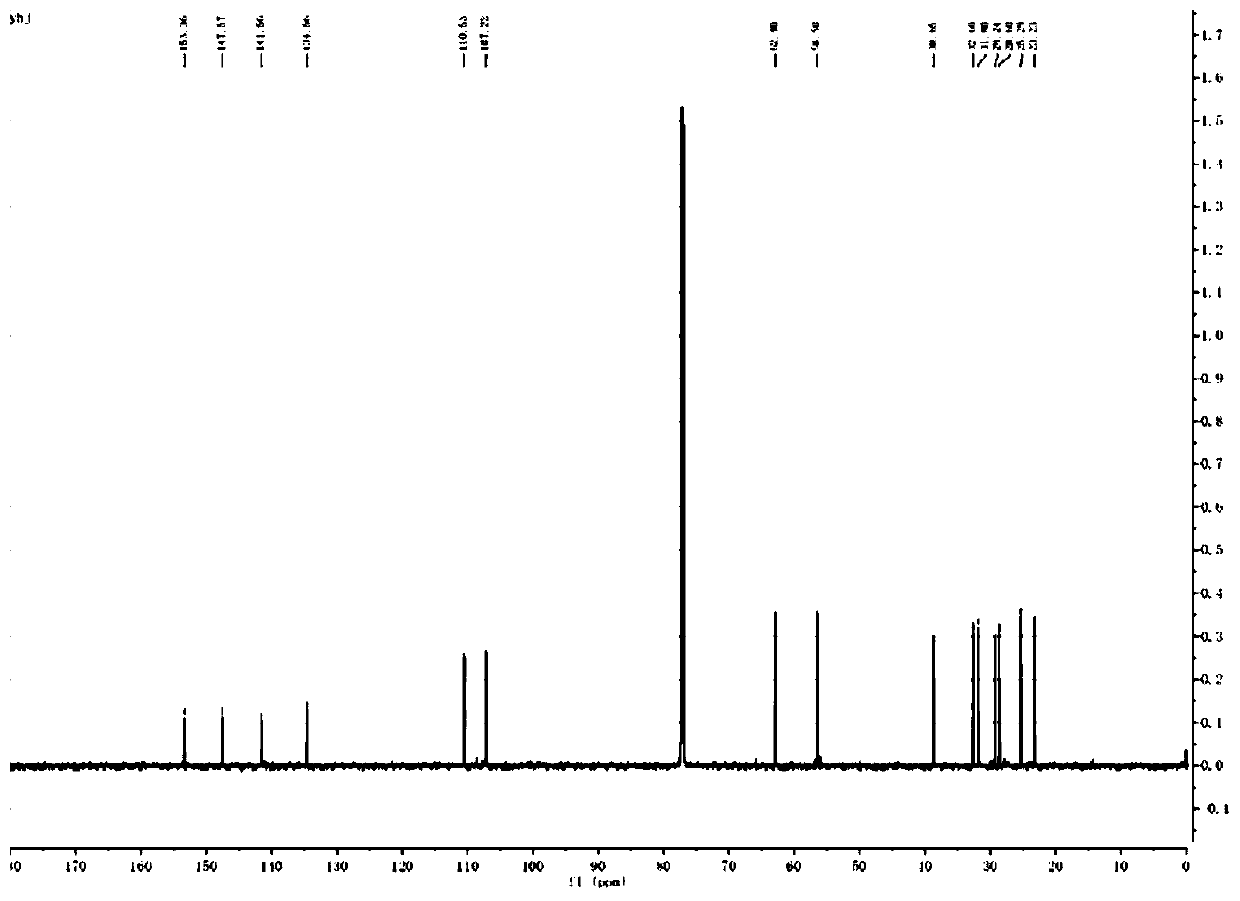
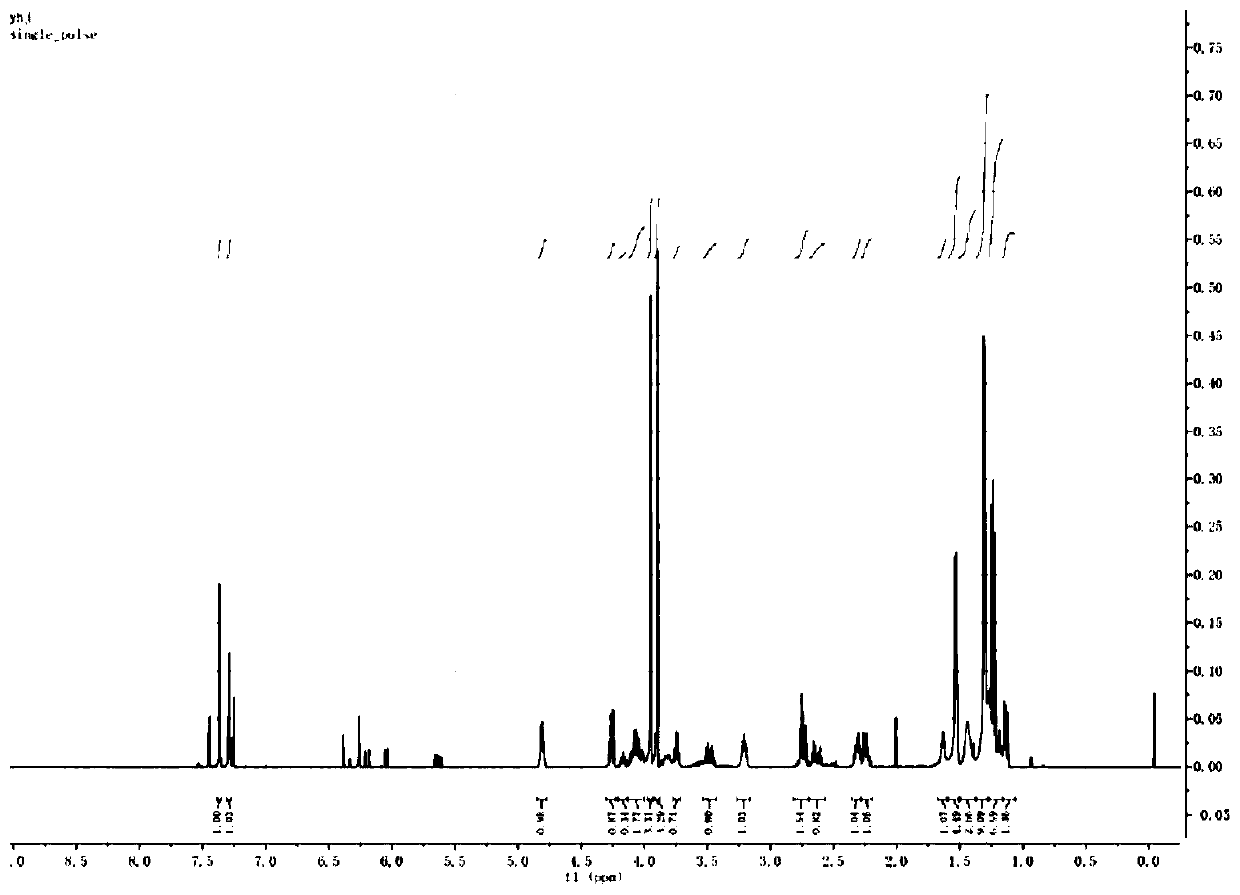
Imidazo[1,2-a]indole compound synthesis method
ActiveCN110872295ARaw materials are easy to getGood substrate adaptabilityOrganic chemistryPtru catalystAromatization
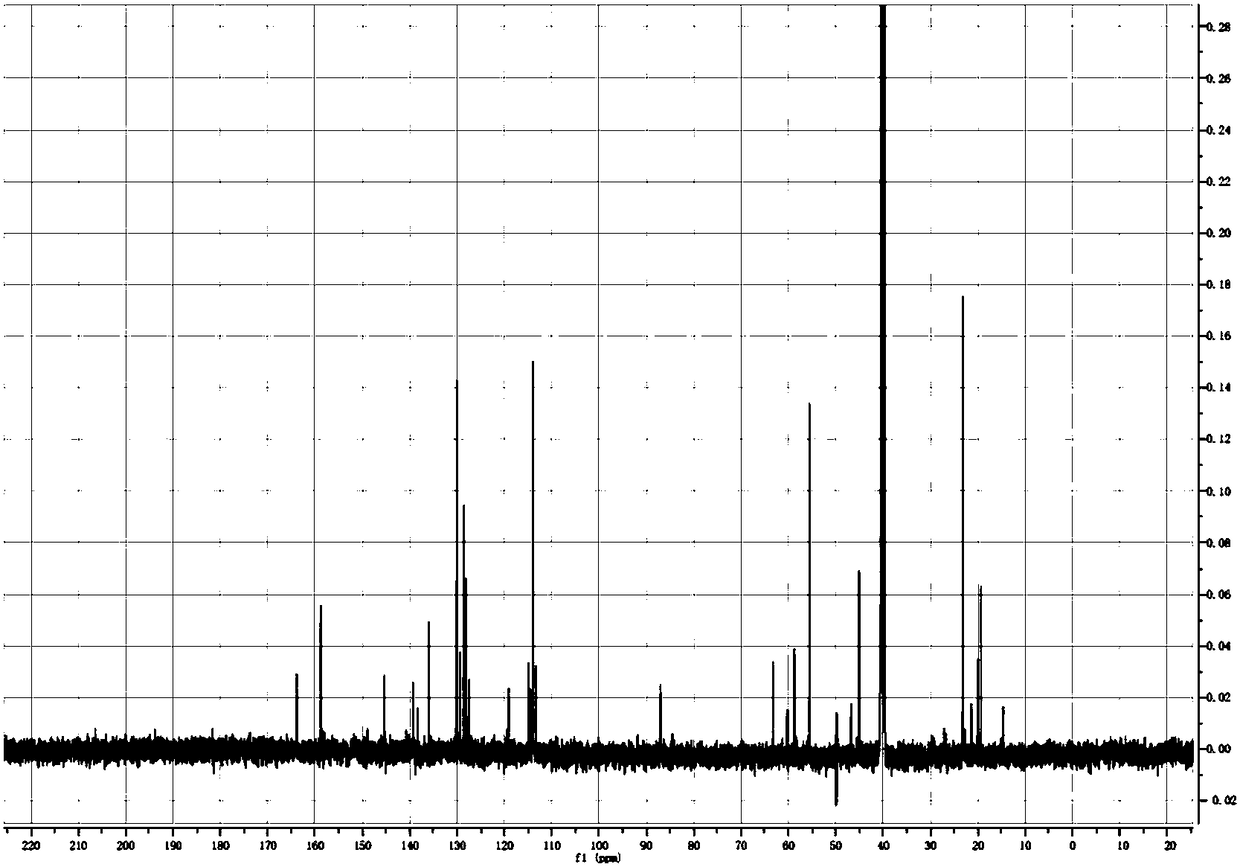
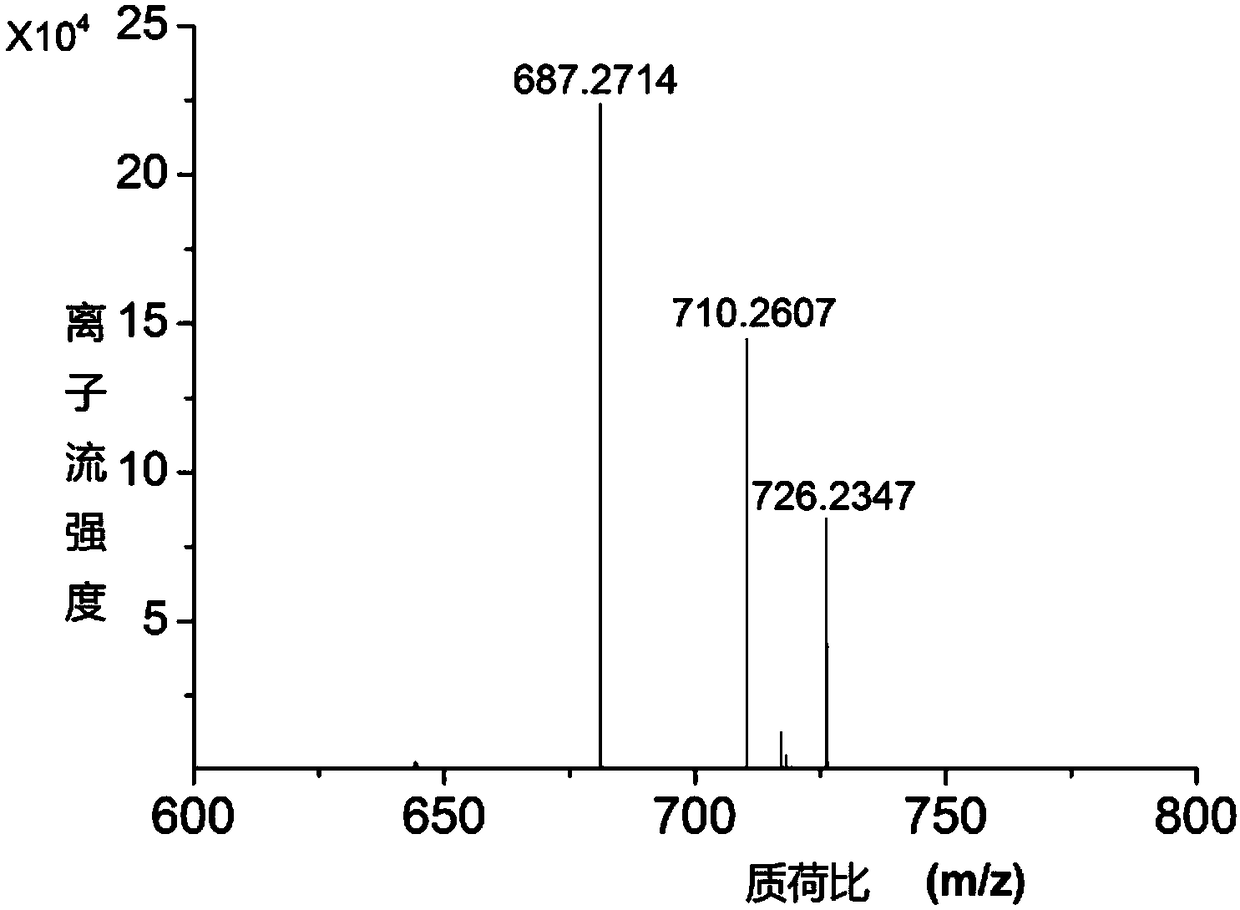
The invention discloses an imidazo[1,2-a]indole compound synthesis method, which comprises the following steps: under water-free and oxygen-free conditions, mixing a compound represented by a formula(I), a compound represented by a formula (II), 2-bromoallylamine and a solvent, adding a catalyst A, adding triethylamine in a dropwise manner, stirring at a room temperature for 0.5-3 hours, adding acatalyst B, an alkaline substance and a ligand into the reaction system, heating to 60-90 DEG C, reacting for 5-8 hours, and carrying out post-treatment to obtain the imidazo[1,2-a]indole compound represented by the formula (III). According to the invention, the method is safe and environment-friendly, and does not generate waste gas and wastewater; the raw materials are easy to obtain, the substrate adaptability is good, and aromatization of various substituents can be realized; reaction conditions are mild; reaction steps are simple; and the method is a new route for synthesizing imidazo[1,2-a]indole compounds containing various substituents.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
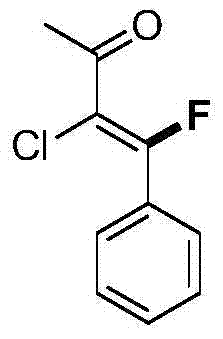
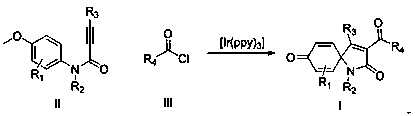

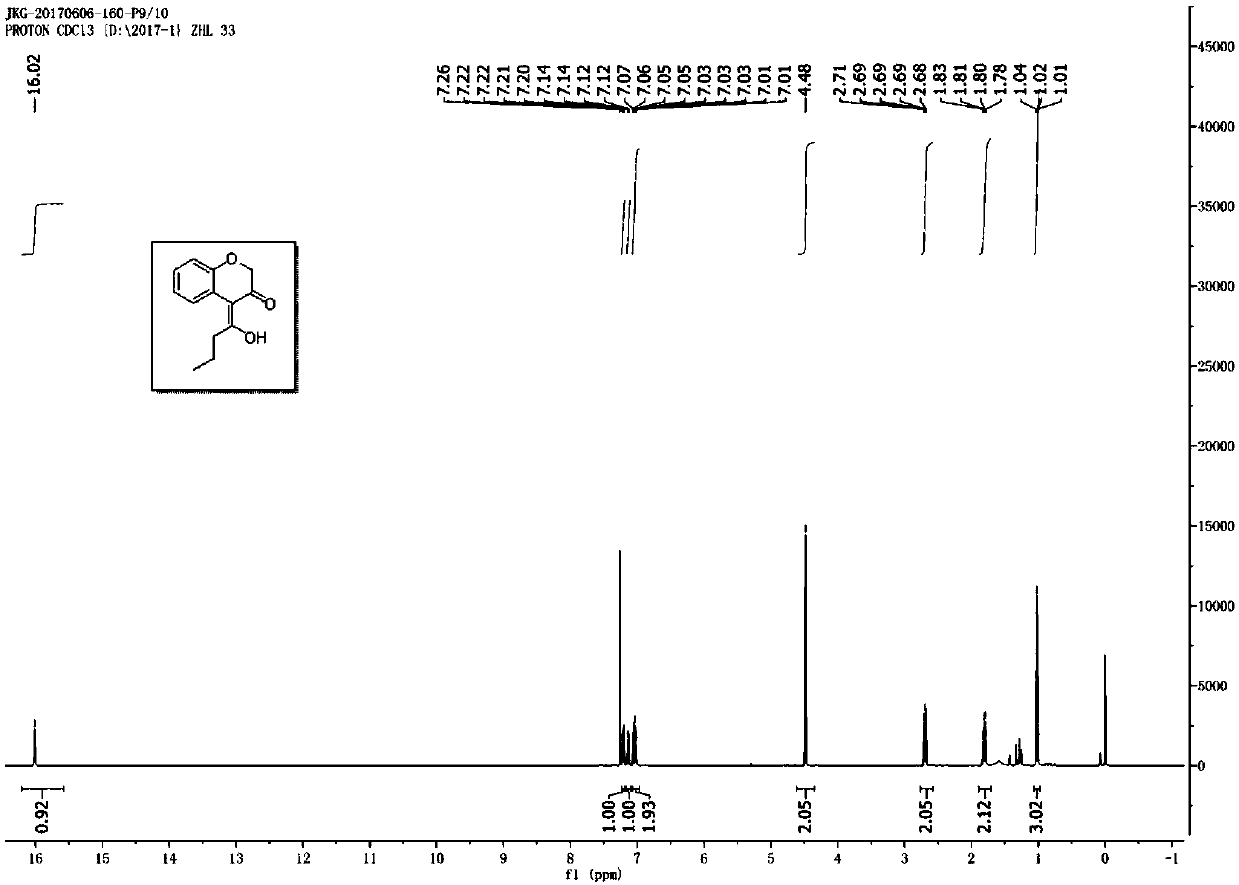
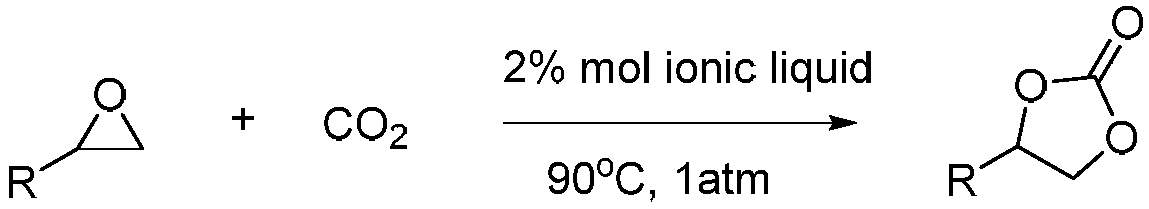






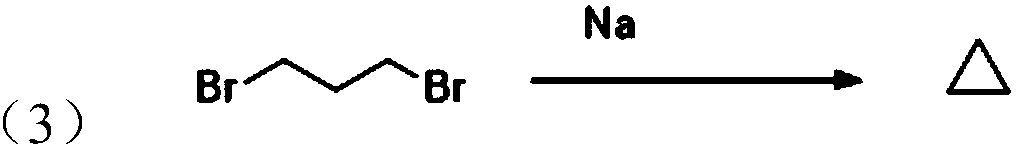
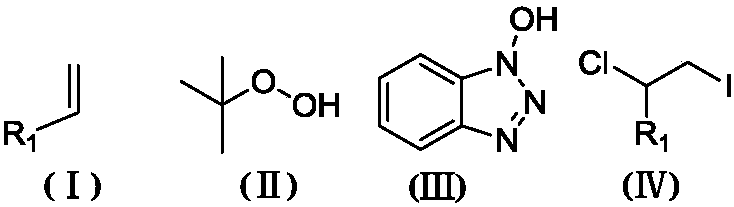
![Imidazo[1,2-a]indole compound synthesis method Imidazo[1,2-a]indole compound synthesis method](https://images-eureka.patsnap.com/patent_img/fdad12bd-b709-42e9-807c-d17c82ac5e51/FDA0001783385360000011.png)
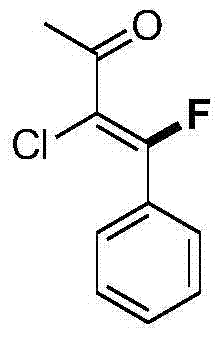
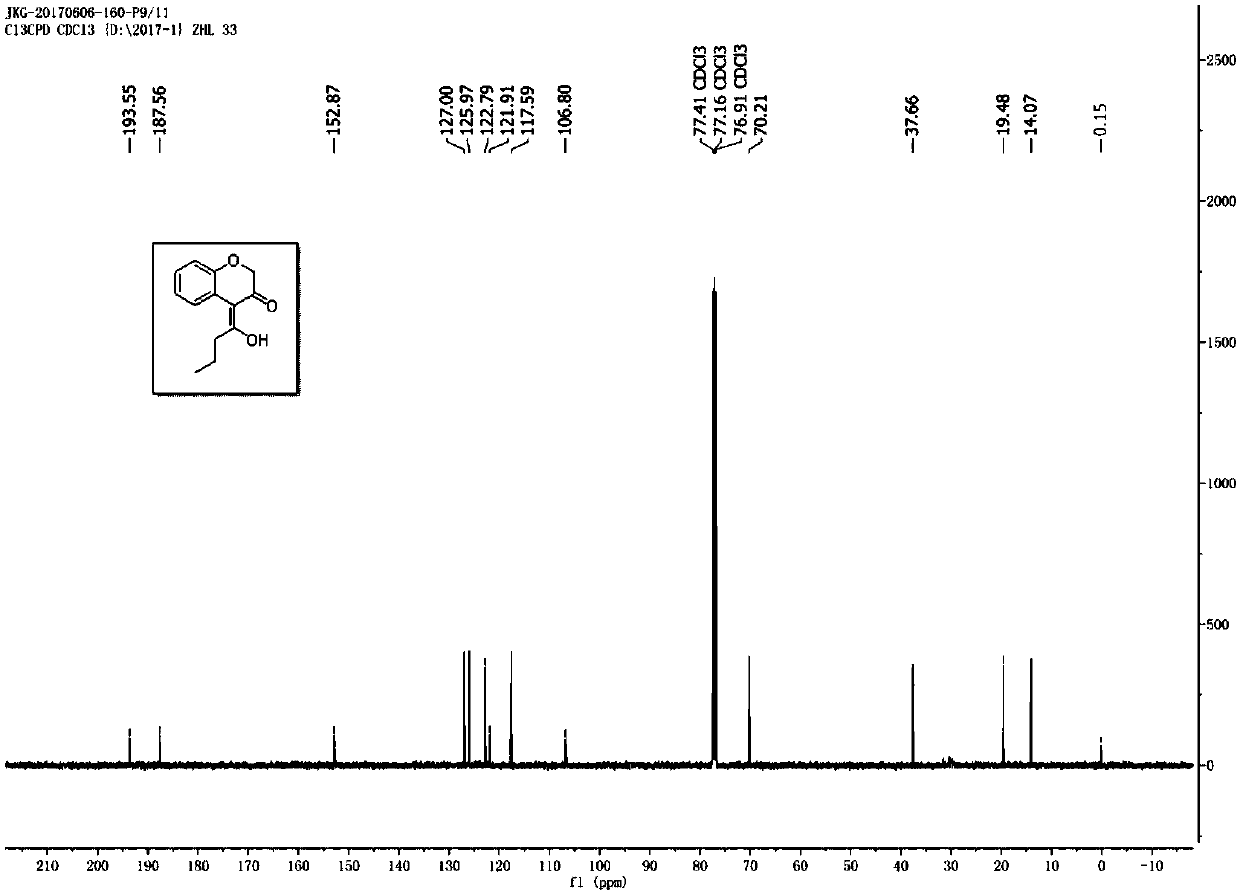
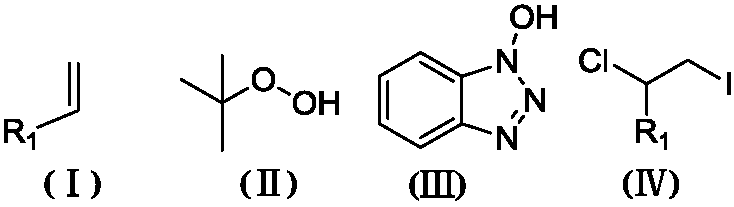
![Imidazo[1,2-a]indole compound synthesis method Imidazo[1,2-a]indole compound synthesis method](https://images-eureka.patsnap.com/patent_img/fdad12bd-b709-42e9-807c-d17c82ac5e51/BDA0001783385370000021.png)
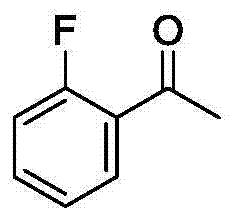
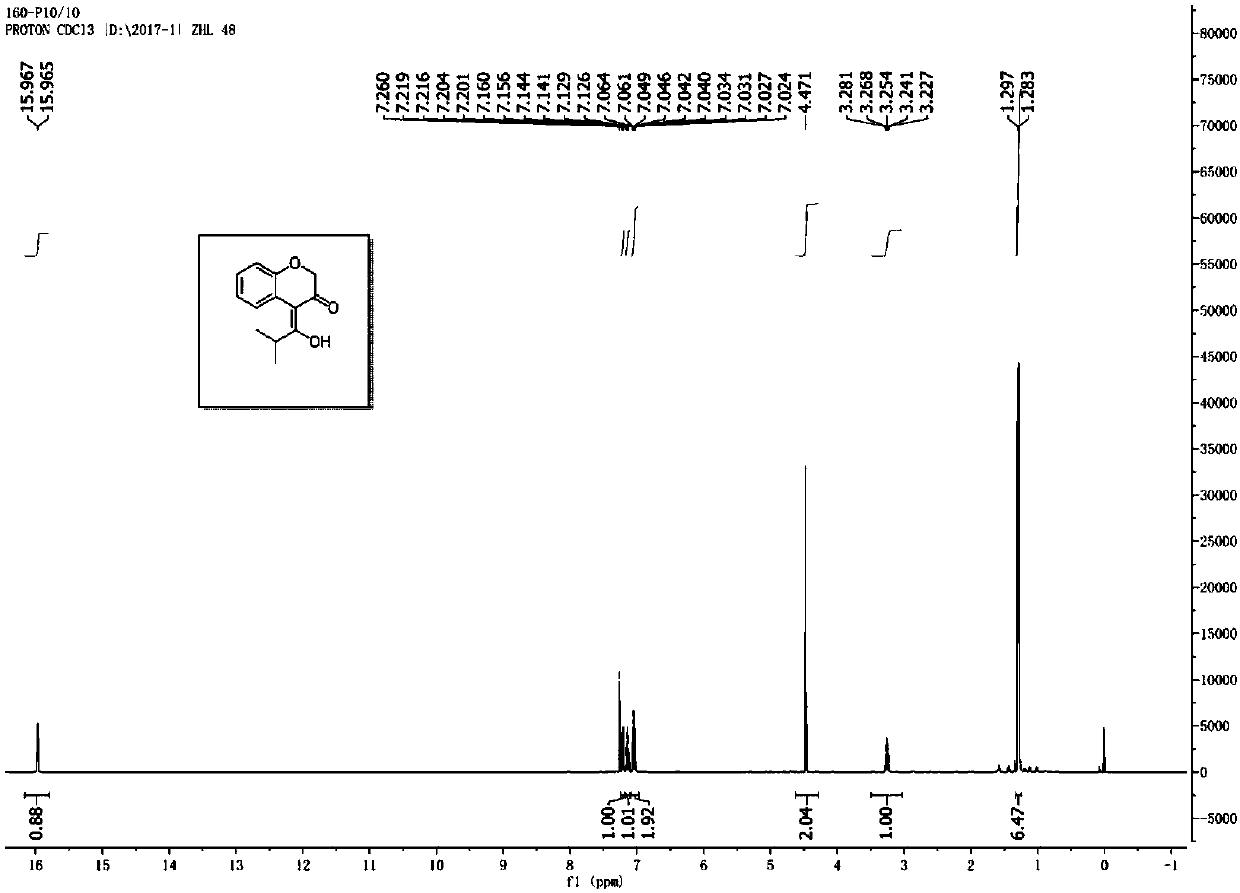
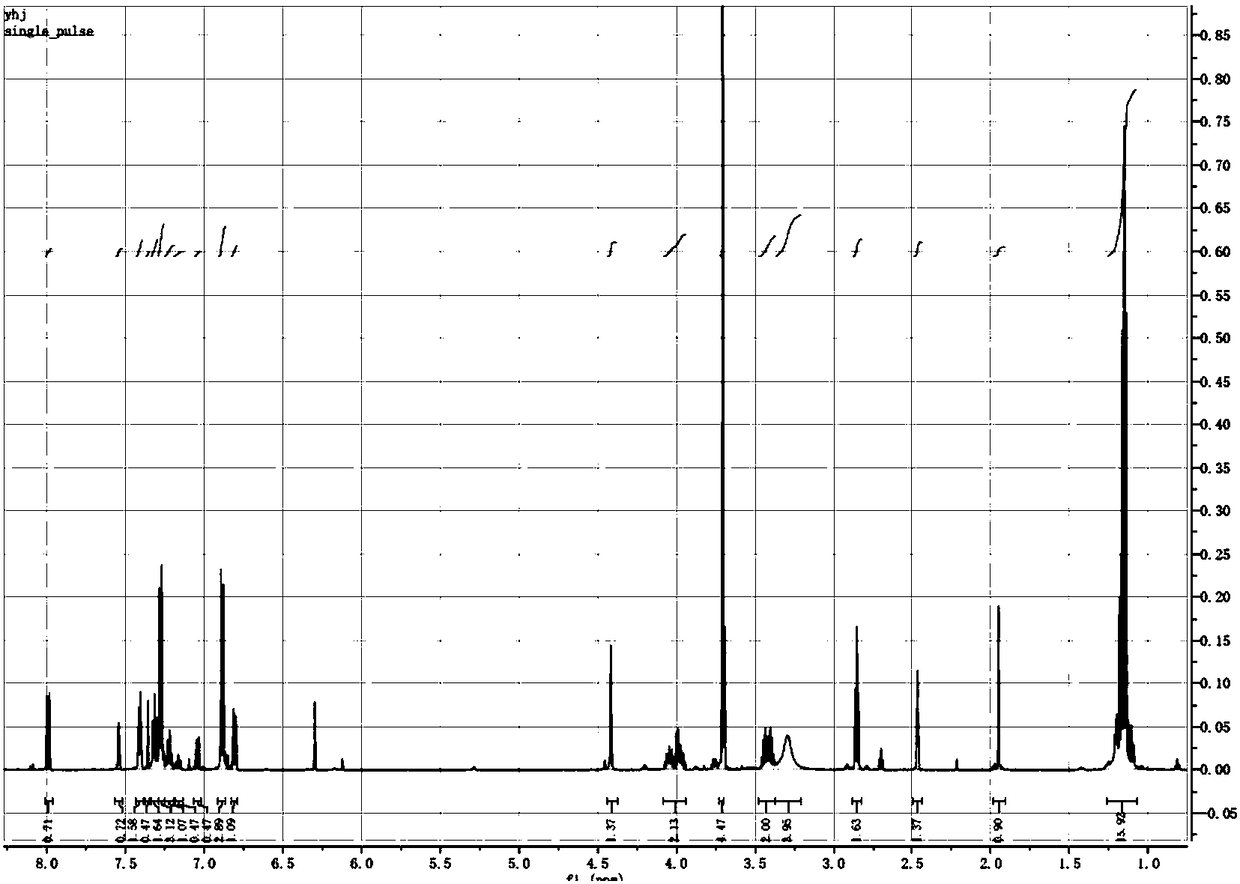
![Imidazo[1,2-a]indole compound synthesis method Imidazo[1,2-a]indole compound synthesis method](https://images-eureka.patsnap.com/patent_img/fdad12bd-b709-42e9-807c-d17c82ac5e51/BDA0001783385370000022.png)