Patents
Literature
5531 results about "Benzotriazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
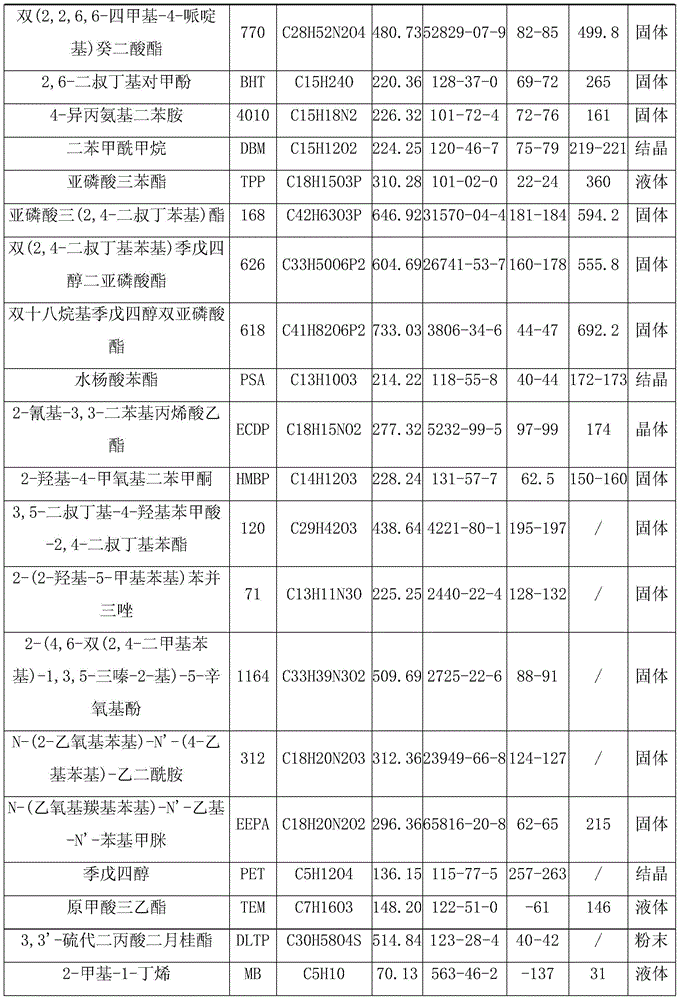
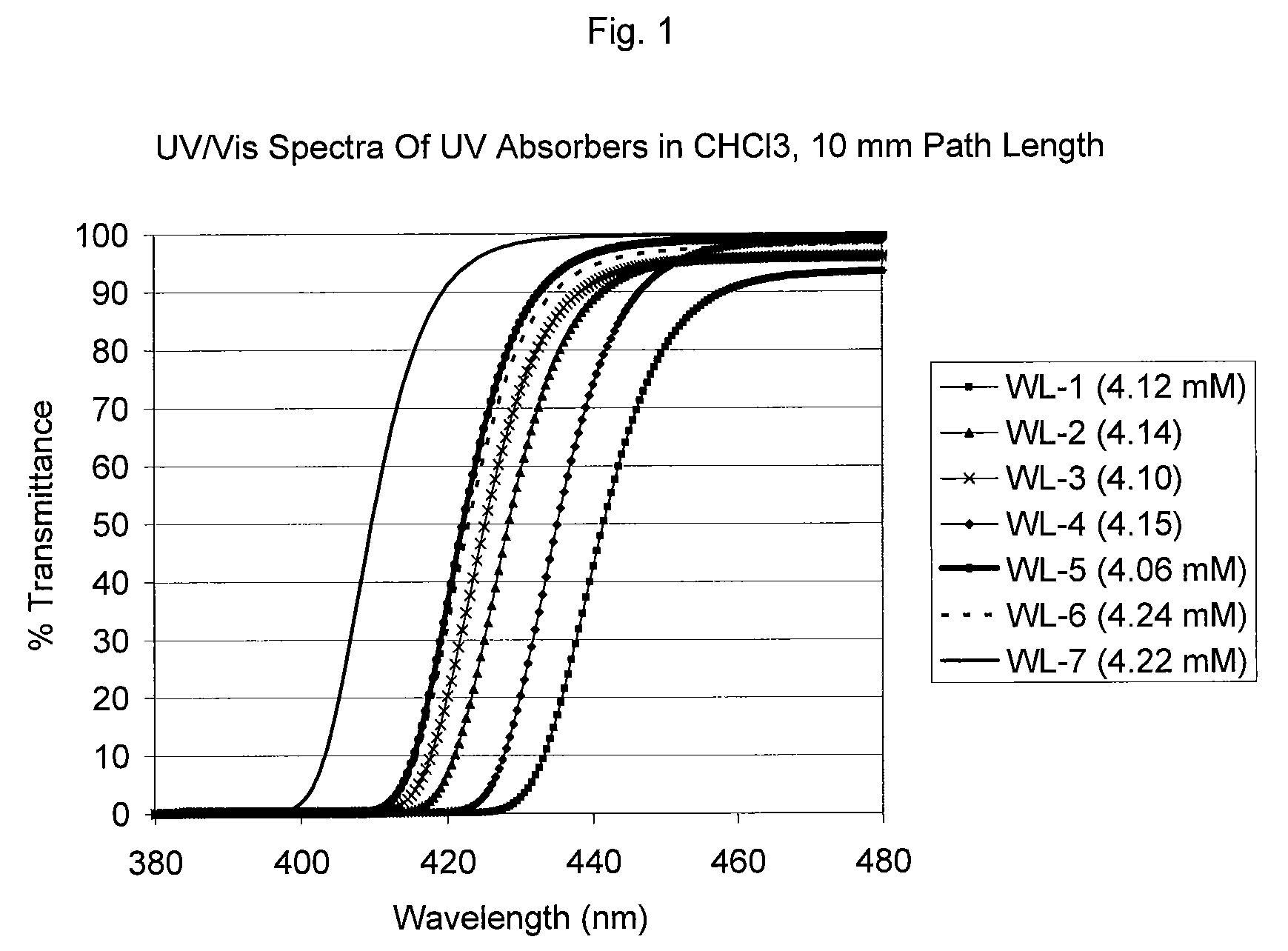
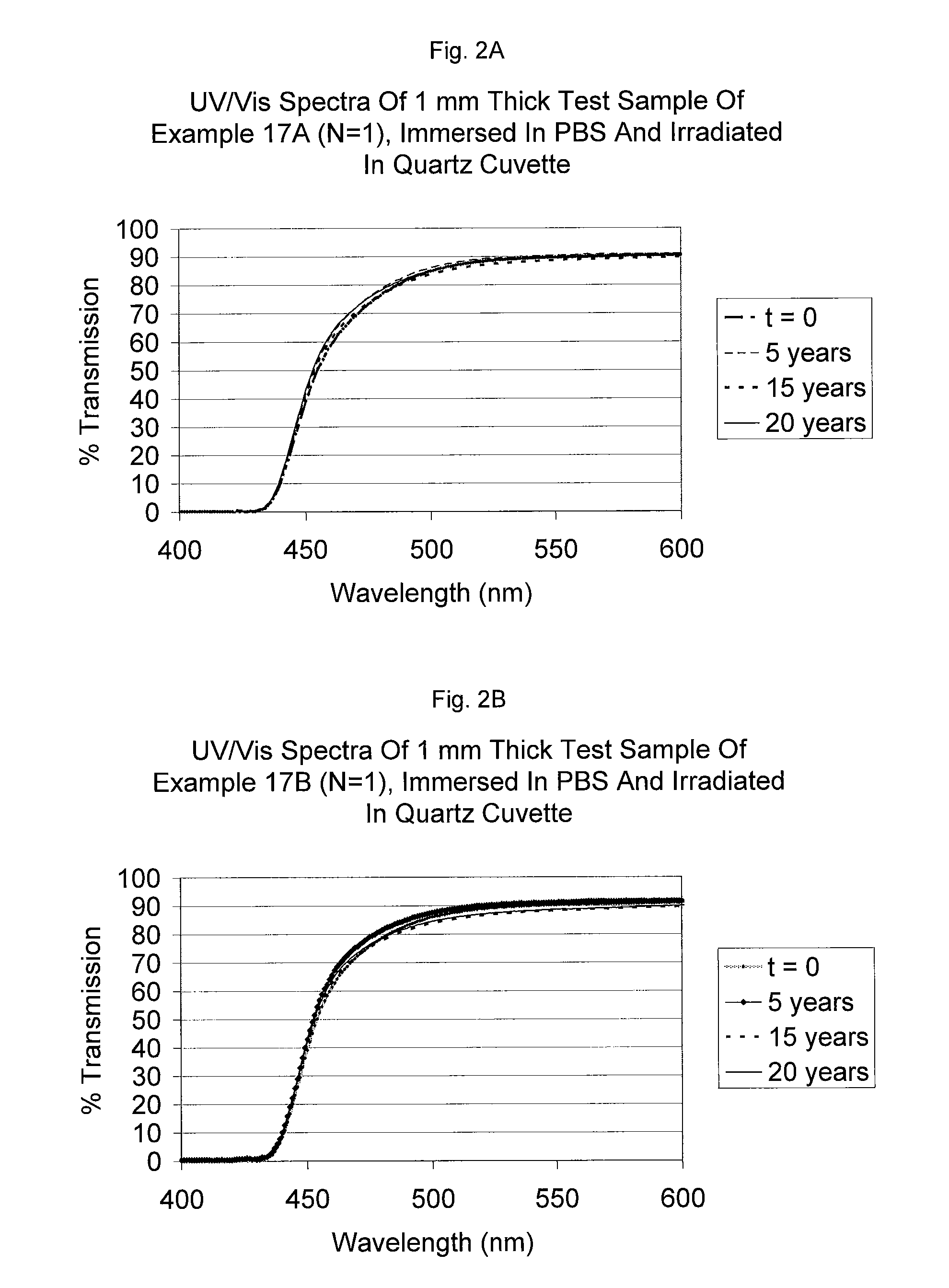
Benzotriazole (BTA) is a heterocyclic compound containing three nitrogen atoms, with the chemical formula C₆H₅N₃. This aromatic compound is colorless and polar and can be used in various fields.
Bloom-resistant benzotriazole UV absorbers and compositions stabilized therewith
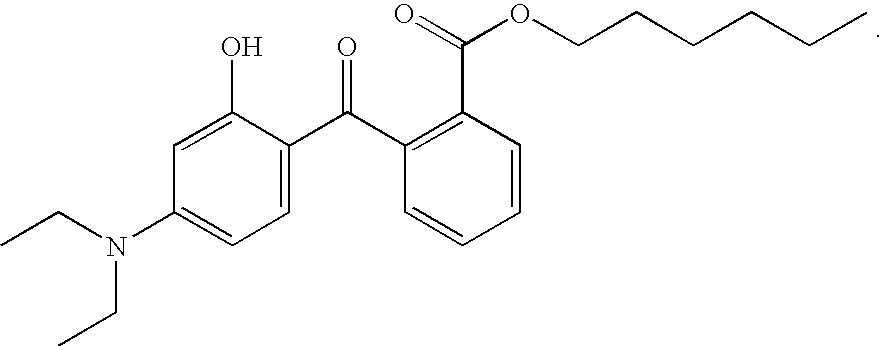
Benzotriazole UV absorbers substituted with a ultra long ester or amide moiety wherein the ester or amide group is a hydrocarbyl group of 25 to 100 carbon atoms or is a group of alkyl of 25 to 100 carbon atoms interrupted by 5 to 39 oxygen atoms and terminated with an omega-OH or an omega-OR group exhibit excellent stabilization efficacy while they concomitantly do not bloom when incorporated into polyolefin films. These benzotriazole UV absorbers also provide excellent protection to white, dyed, dipped, unscented and / or scented candle wax from discoloration and degradation.
Owner:CIBA SPECIALTY CHEM CORP
UV resistant naphthalate polyester articles
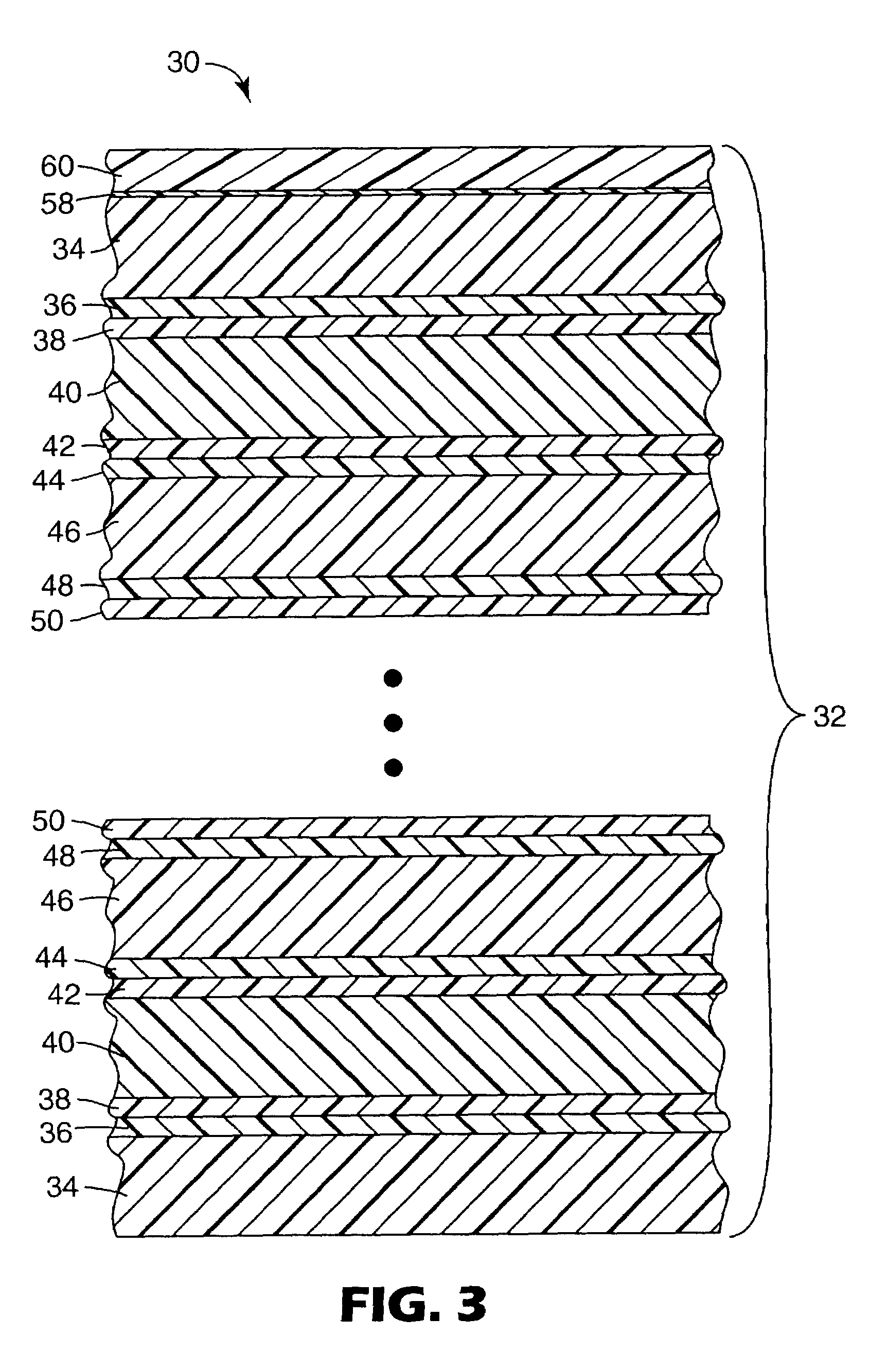
ActiveUS7153588B2Extend life of and protectReduce solubilitySynthetic resin layered productsPretreated surfacesPolyesterBenzene
Naphthalate polyester articles can be coated with polymerizable compositions containing a vinyl-functional crosslinkable film former, a large amount of benzotriazole and a copolymerizable monomer that solubilizes the benzotriazole. The cured compositions help protect the naphthalate polyester from UV exposure and other weathering effects.
Owner:3M INNOVATIVE PROPERTIES CO
Lubricating oil for bearing
ActiveUS20060019840A1Improve performanceLiquid carbonaceous fuelsAdditivesGallic acid esterPHENOL LIQUID
Disclosed herein is a lubricating oil for bearings comprising (a) a diester represented by General Formula (1) wherein R1 and R2 are the same or different, and each represents a C3-C17 linear alkyl group; A represents a C2-C10 linear alkylene group or A represents a branched alkylene group consisting of a linear alkylene group, the linear alkylene group being the principal chain, and one or more alkyl groups (branches) bonded to the linear alkylene group, wherein the total number of carbon atoms of the linear alkylene group and the one or more alkyl groups is 3 to 10; with the proviso that when A is a branched alkylene group and has two or more alkyl groups, the two or more alkyl groups are not bonded to the same carbon atom; or a mixture of the diester with an additional base oil and (b) at least one member selected from the group consisting of phenol-based antioxidants and amine-based antioxidants, and optionally containing (c) at least one member selected from the group consisting of phosphorus-based compounds and aliphatic linear monocarboxylic acids, and further optionally containing (d) at least one member selected from the group consisting of benzotriazole-based compounds and gallic acid-based compounds.
Owner:NEW JAPAN CHEM CO
Barrier polishing liquid and chemical mechanical polishing method
InactiveUS20070181534A1Sufficient rateSufficient polishing ratePigmenting treatmentOther chemical processesOrganic acidColloidal silica
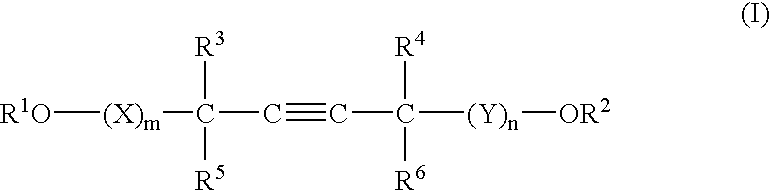
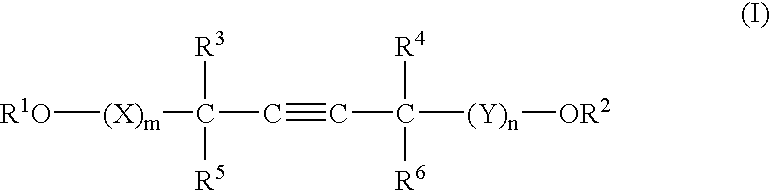
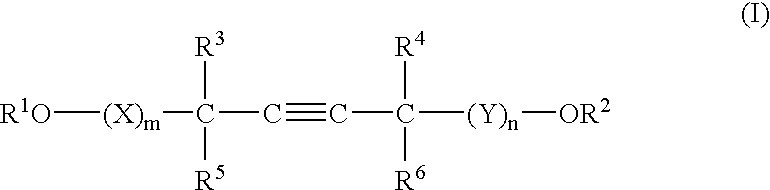
A barrier polishing liquid is provided that includes (a) a nonionic surfactant represented by Formula (I) below, (b) at least one type of organic acid selected from the group consisting of an aromatic sulfonic acid, an aromatic carboxylic acid, and a derivative thereof, (c) colloidal silica, and (d) benzotriazole or a derivative thereof.(In Formula (I), R1 to R6 independently denote a hydrogen atom or an alkyl group having 1 to 10 carbons, X and Y independently denote an ethyleneoxy group or a propyleneoxy group, and m and n independently denote an integer of 0 to 20.) There is also provided a chemical mechanical polishing method that includes supplying the barrier polishing liquid to a polishing pad on a polishing platen at a flow rate per unit area of a semiconductor substrate per unit time of 0.035 to 0.25 mL / (min·cm2), and polishing by making the polishing pad and a surface to be polished move relative to each other while they are in a contacted state.
Owner:FUJIFILM CORP
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
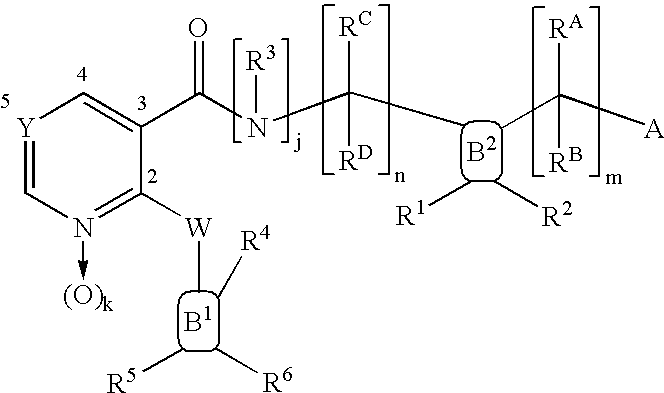
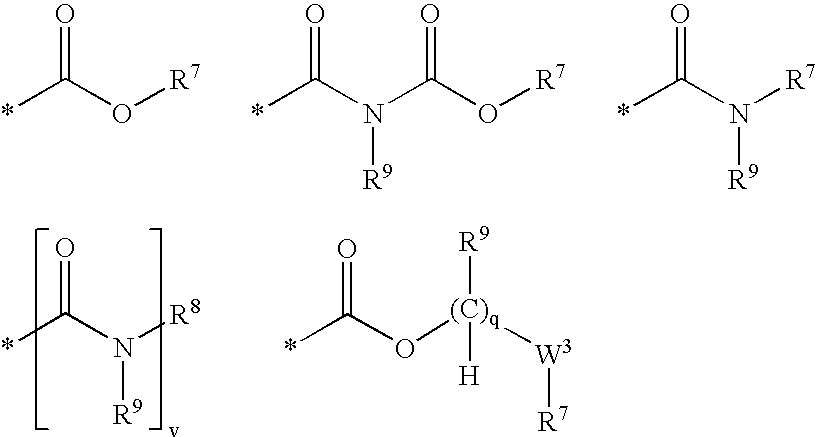
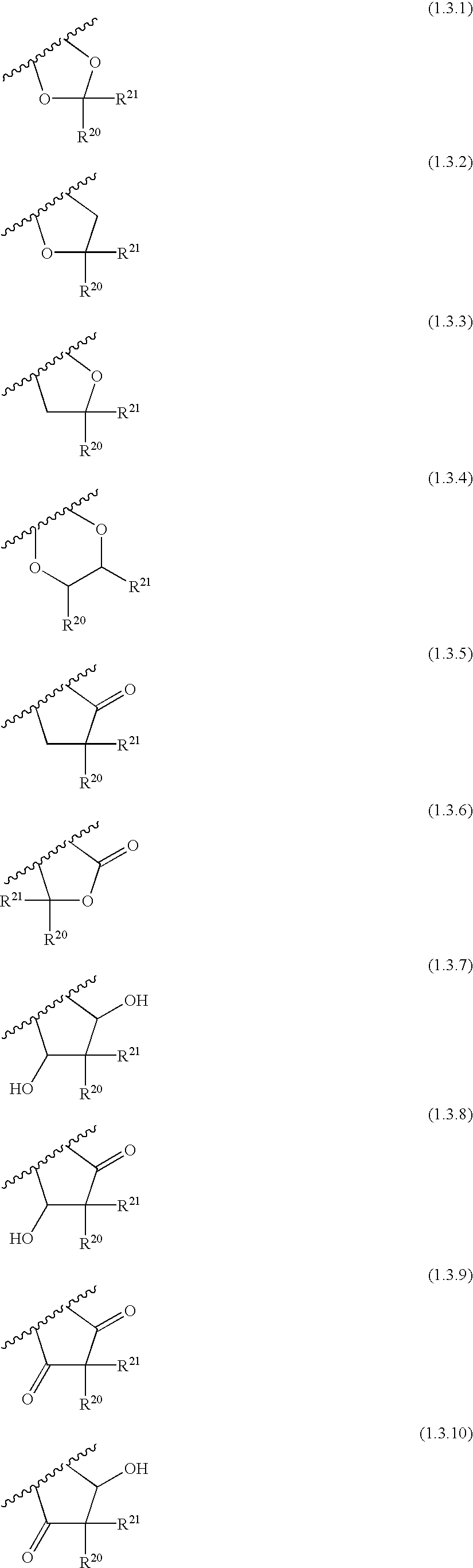
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Electrolyte composition and treatment for electrolytic chemical mechanical polishing
Owner:APPLIED MATERIALS INC
Composite lithium-base grease and method for making same
The present invention provides composite lithium-based lubricating grease and a preparation method thereof. The composite lithium-based lubricating grease consists of thickener, base oil and additive. The thickener consists of 12-hydroxy stearic acid lithium dibasic acid and / or lithium borate, and the mol ratio is 1 ®U 0.1 to 1 ®U 0.1to 1. The base oil is mineral oil or poly-alpha-olefin synthesis oil. The additive consists of organic amine compound antioxidant, benzotriazole and barium petroleum sulfonate or barium dinonylnaphthalene sulfonate anti-corrosion additive and rust protection agent, dialkyl dithiocarbamate or dibenzyl disulfide and sulfurized olefin cottonseed oil extreme pressure antiwear additive and nanometer copper powder repairing additive. The contents of the thickener and the base oil are respectively 6 percent to 18 percent and 82 percent to 94 percent according to the weight percentage. The lubricating grease has the multi-effect performances of high dripping point, good high-temperature performance and low-temperature performance, chemical invariability, colloid invariability, antiwear extreme pressure performance etc., and especially has the repairing function towards a damaged bearing.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
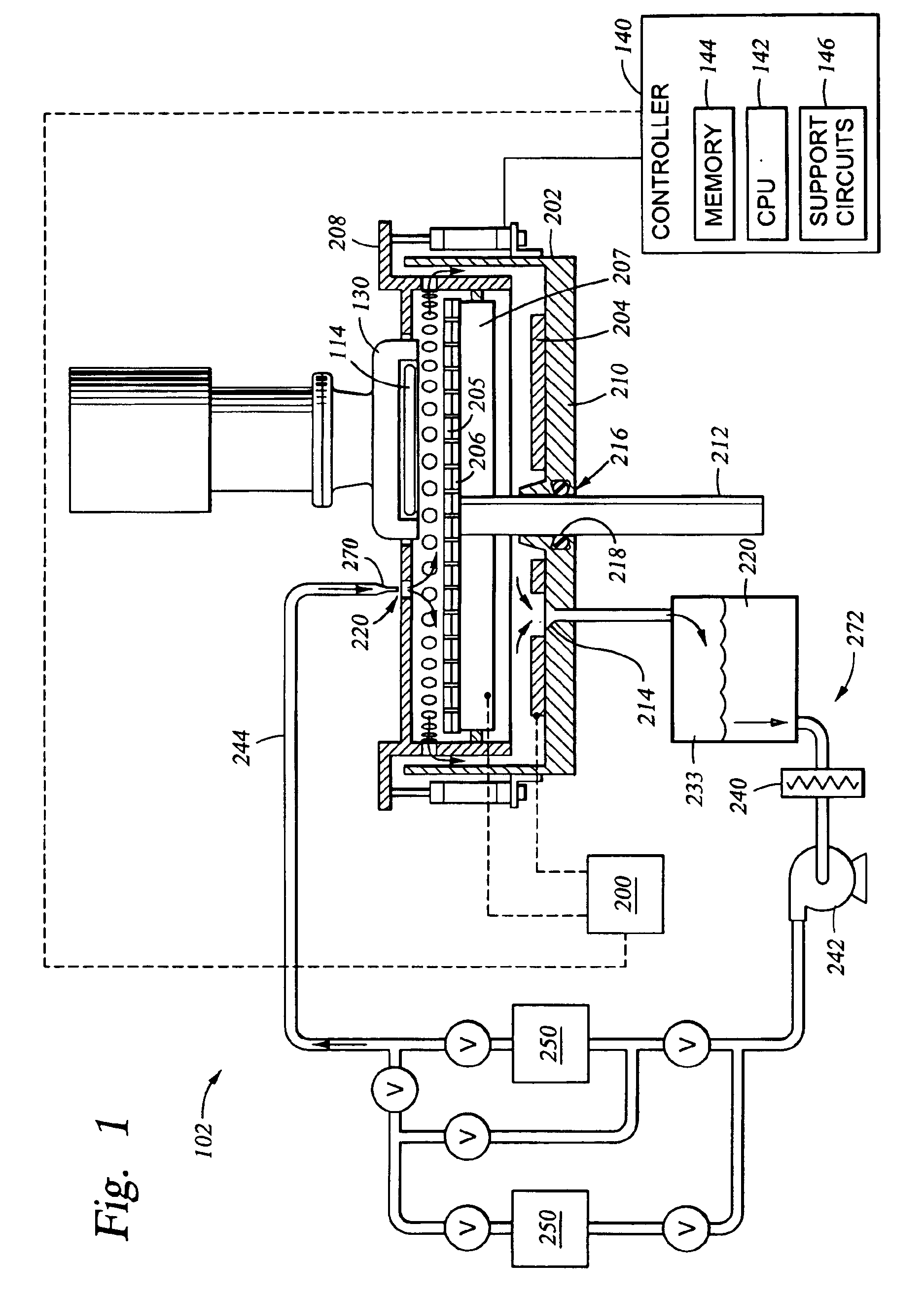
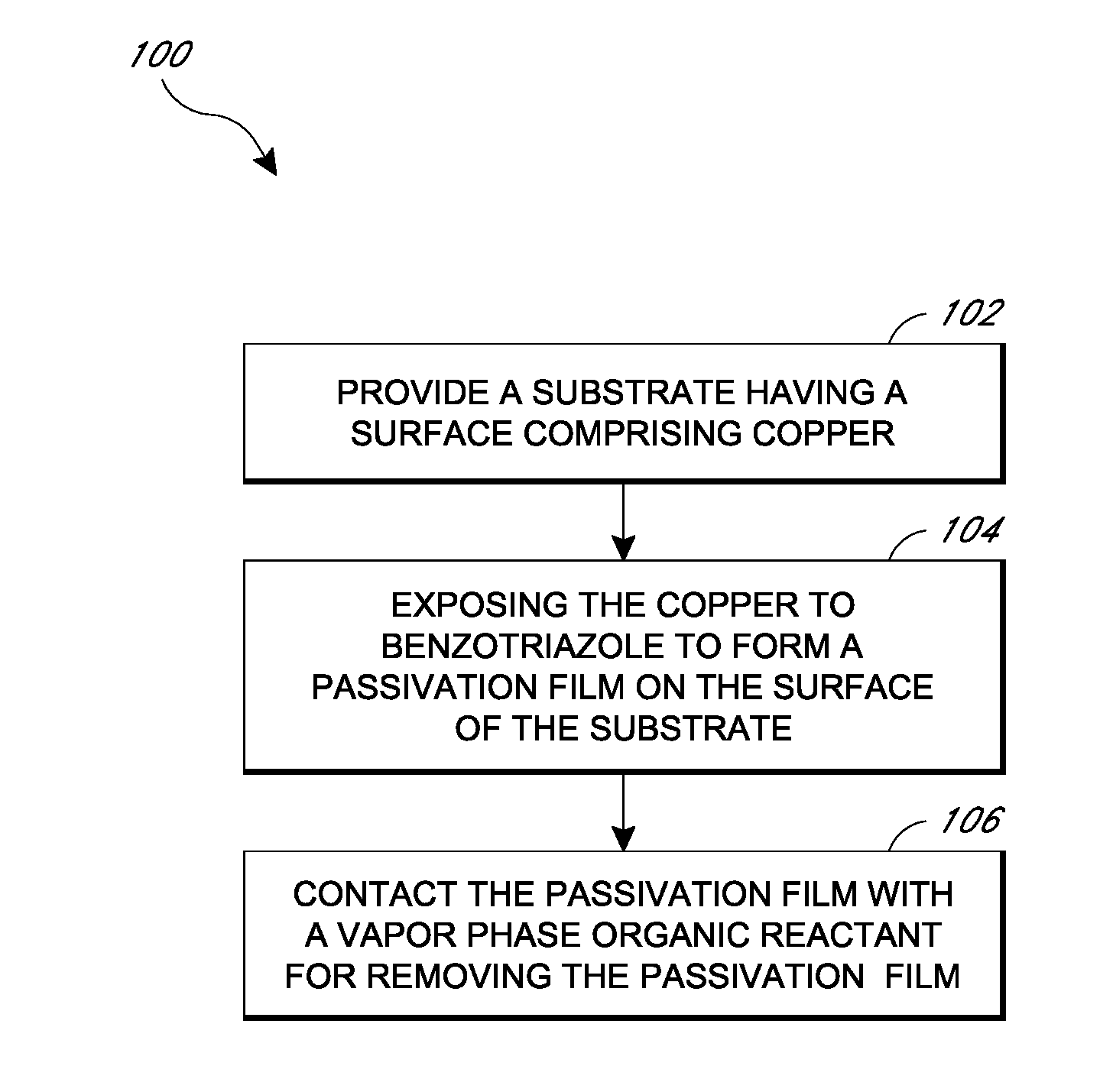
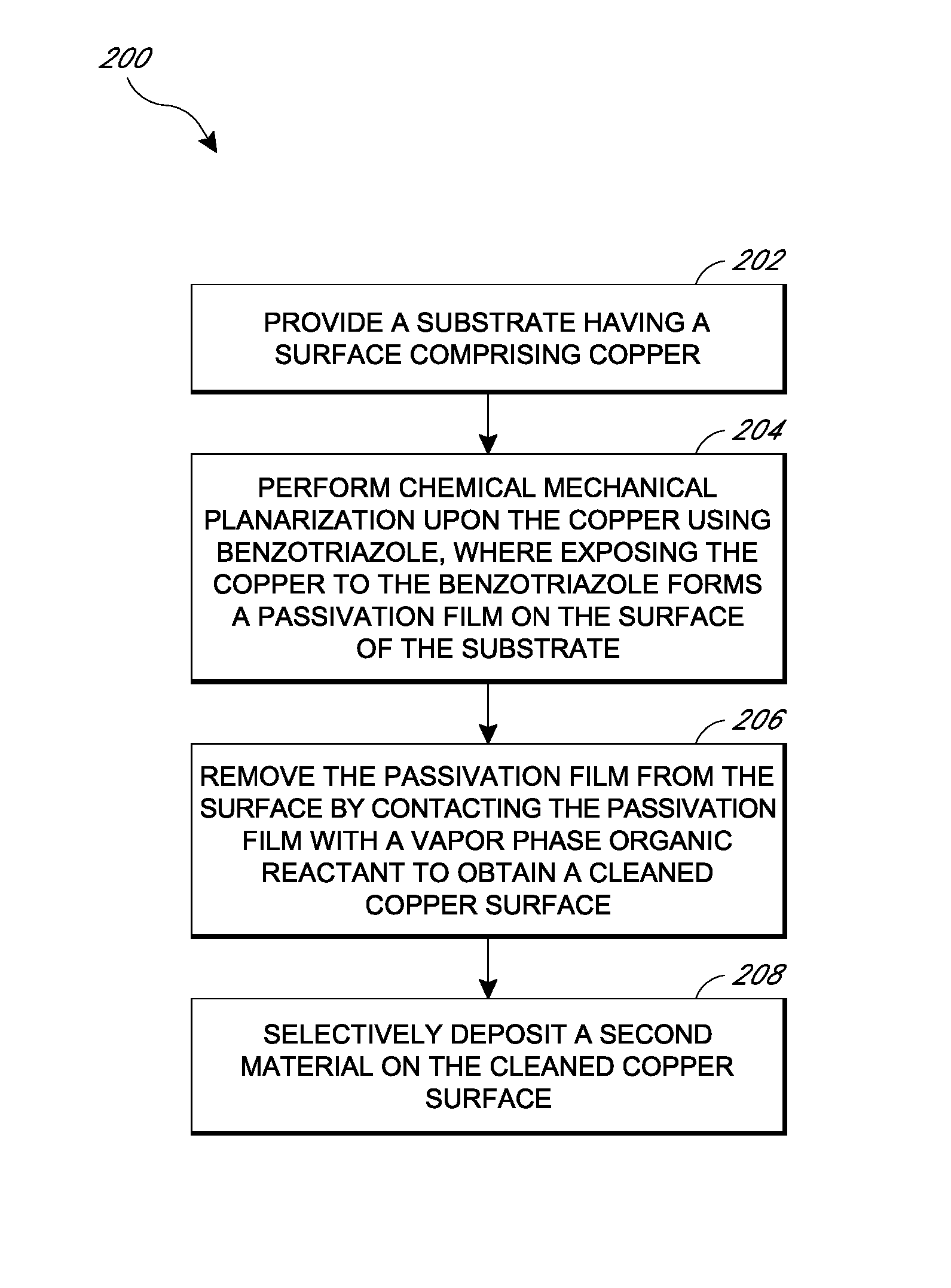
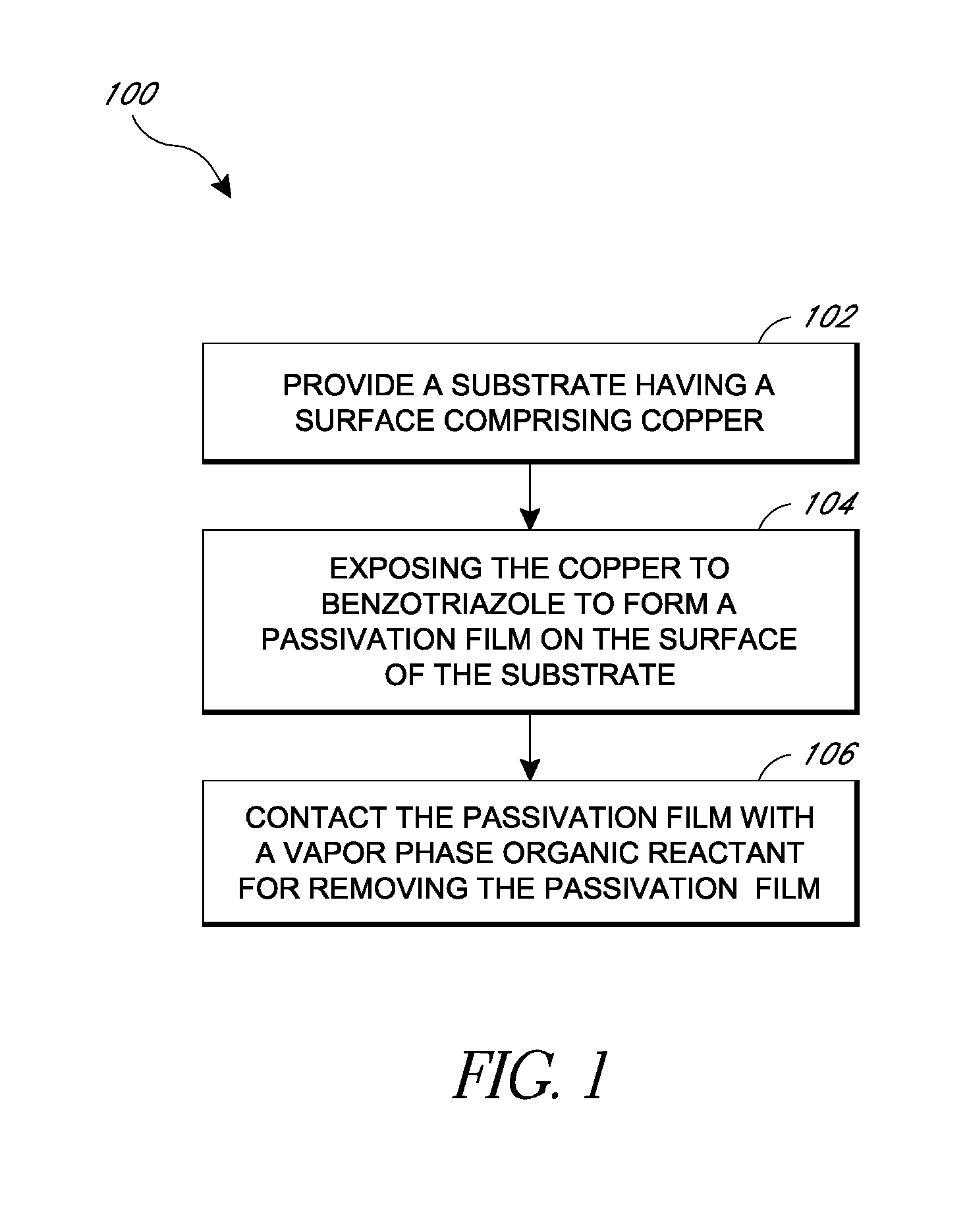
Removal of surface passivation
Methods for removing a passivation film from a copper surface can include exposing the passivation film to a vapor phase organic reactant, for example at a temperature of 100° C. to 400° C. In some embodiments, the passivation film may have been formed by exposure of the copper surface to benzotriazole, such as can occur during a chemical mechanical planarization process. The methods can be performed as part of a process for integrated circuit fabrication. A second material can be selectively deposited on the cleaned copper surface relative to another surface of the substrate.
Owner:ASM IP HLDG BV
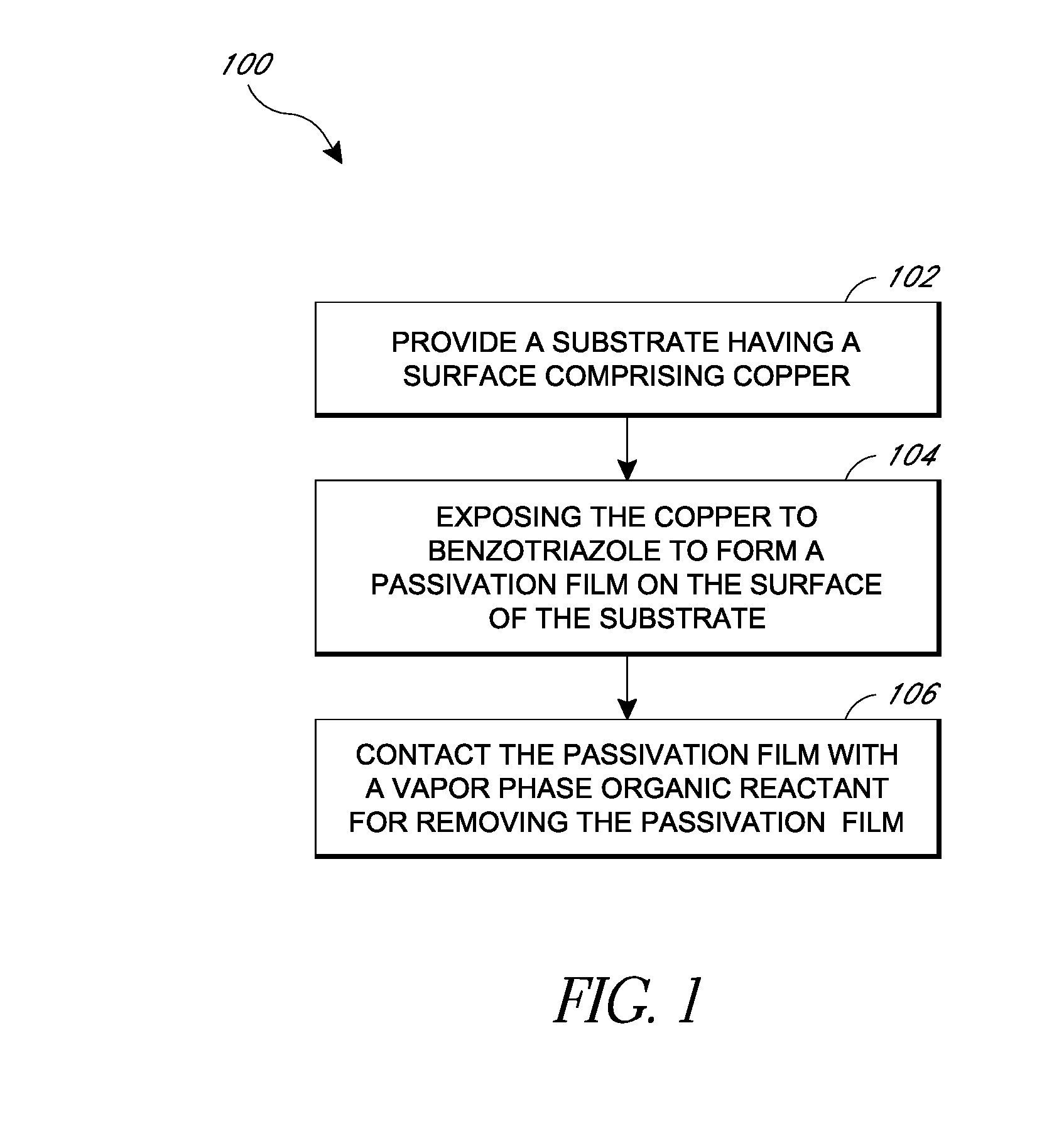
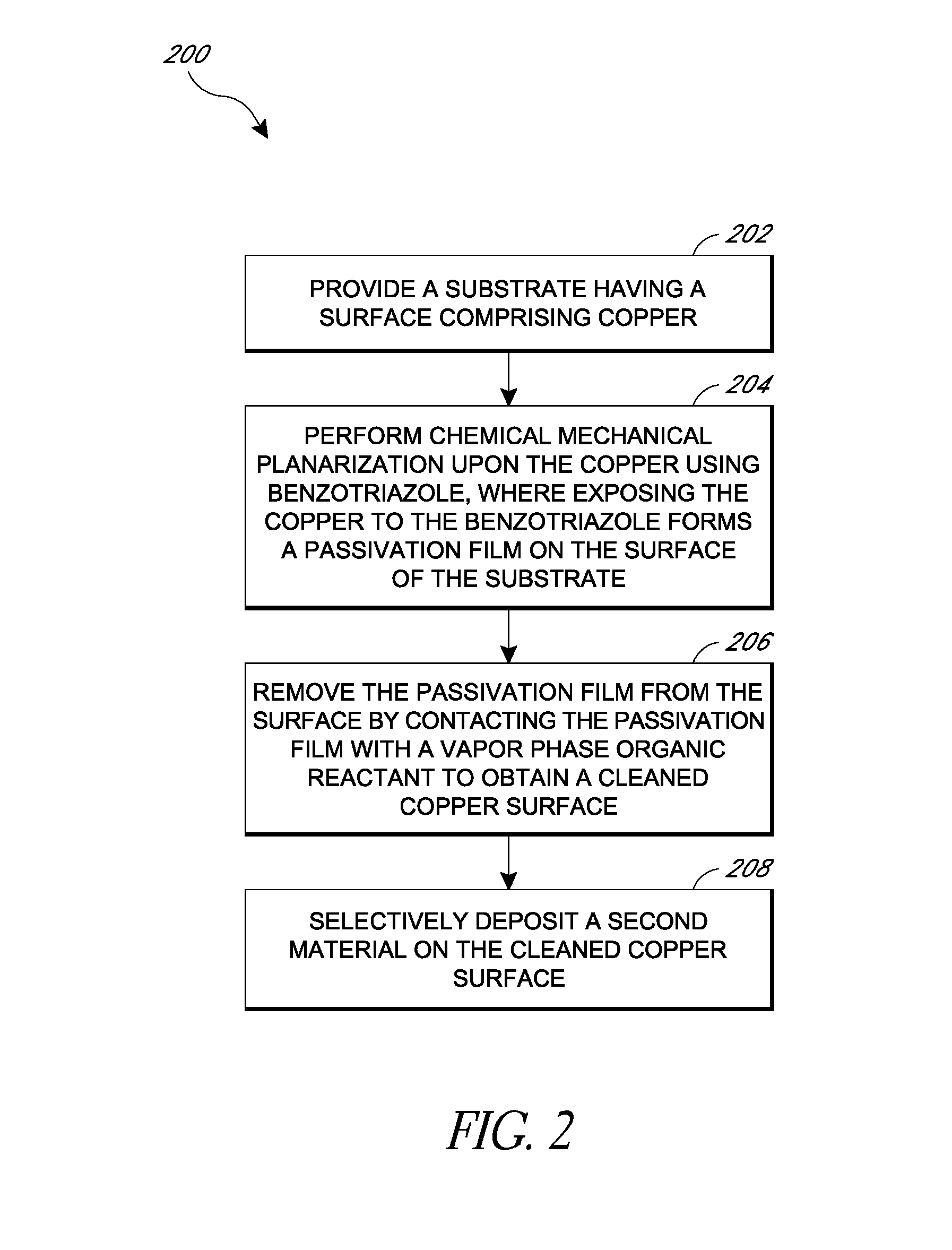
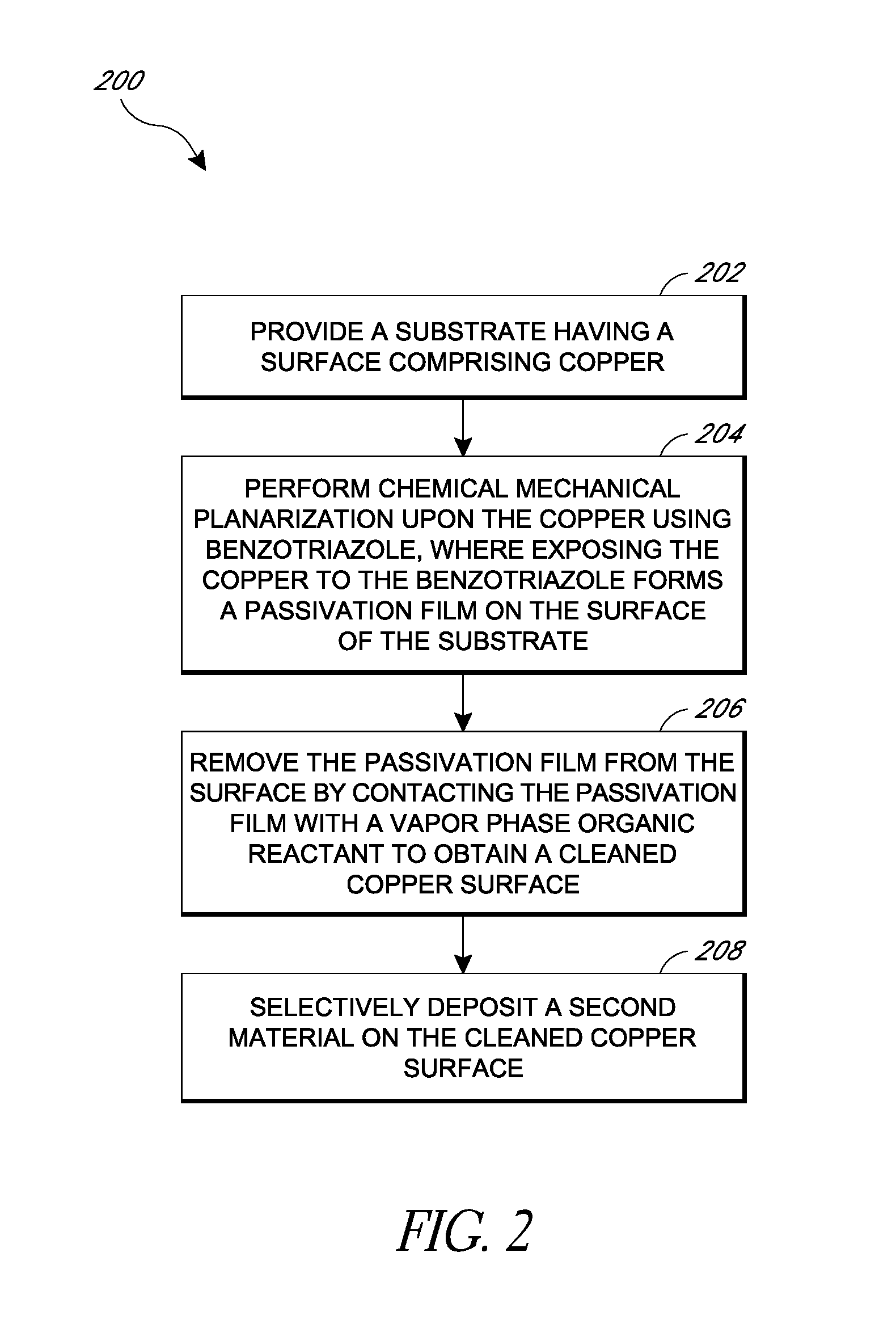
Removal of surface passivation
Methods for removing a passivation film from a copper surface can include exposing the passivation film to a vapor phase organic reactant, for example at a temperature of 100° C. to 400° C. In some embodiments, the passivation film may have been formed by exposure of the copper surface to benzotriazole, such as can occur during a chemical mechanical planarization process. The methods can be performed as part of a process for integrated circuit fabrication. A second material can be selectively deposited on the cleaned copper surface relative to another surface of the substrate.
Owner:ASM IP HLDG BV
Ultraviolet light filter element
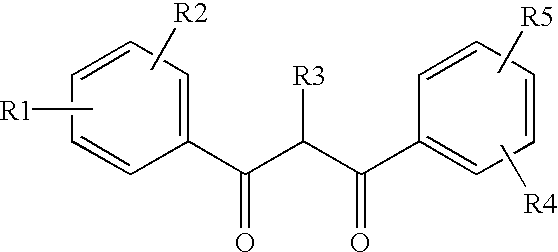
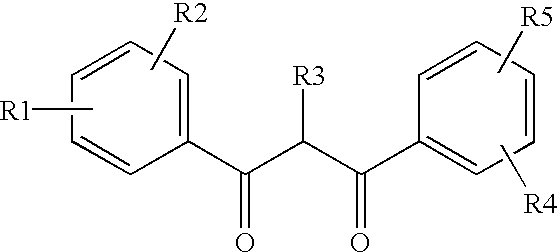
InactiveUS6872766B2Increased durabilityAvoid lightLiquid crystal compositionsOther chemical processesThio-Display device
Ultraviolet light absorbing polymer film, coating, or molded article UV filter elements are described which comprise a polymer phase having molecularly dispersed therein a) a first ultraviolet absorbing dibenzoylmethane compound of formula (I) where R1 through R5 are each independently hydrogen, halogen, nitro, or hydroyxl, or further substituted or unsubstituted alkyl, alkenyl, aryl, alkoxy, acyloxy, ester, carboxyl, alkyl thio, aryl thio, alkyl amine, aryl amine, alkyl nitrile, aryl nitrile, arylsulfonyl, or 5-6 member heterocylce ring groups, and b) a second ultraviolet light absorbing compound which absorbs ultraviolet light at a wavelength for which the first compound is deficient at absorbing. In particular embodiments, the second ultraviolet light absorbing compound may comprise a hydroxyphenyl-s-triazine, hydroxyphenylbenzotriazole, formamidine, benzoxazinone, or benzophenone compound. In a specific embodiment of the invention, the above UV absorbing compounds are employed in cellulose acetate film for the fabrication of a protective film for polarizers for use in display applications.
Owner:EASTMAN KODAK CO
Polishing liquid
ActiveUS20100167547A1InhibitionHigh rateOther chemical processesDecorative surface effectsColloidal silicaDevice material
A polishing liquid for a chemical mechanical polishing of a semiconductor device includes (a) a carboxylic acid compound having one or more carboxy groups, (b) colloidal silica particles having a ζ potential of −10 mV to −35 mV when used in the polishing liquid, (c) a benzotriazole derivative, (d) an anionic surfactant, and (e) an oxidizing agent, and the polishing liquid has a pH of from 5.0 to 8.0.
Owner:FUJIFILM CORP
Cosmetic and dermatological photoprotective formulations with a content of hydroxybenzophenones, triazine derivatives and/or benzotriazole derivatives
InactiveUS20050008587A1Harmful effectReduced activityCosmetic preparationsToilet preparationsBenzotriazoleTriazine derivative
A photoprotective cosmetic or dermatological composition which comprises a triazine derivative and / or a benzotriazole derivative and also a hydroxybenzophenone.
Owner:BEIERSDORF AG
High-antiwear cutting fluid
InactiveCN104277900AImprove anti-wear performanceLow costLubricant compositionLanthanum fluoridePolyethylene glycol
The invention discloses a high-antiwear cutting fluid, which comprises the following raw materials in parts by weight: 10-25 parts of soybean oil, 5-15 parts of rapeseed oil, 8-25 parts of 2-ethylhexyl oleate, 15-30 parts of fatty alcohol-polyoxyethylene ether, 3-8 parts of disodium sulphonatoacetate, 1-5 parts of copper / silica composite nano materials, 5-12 parts of citric acid-modified lanthanum fluoride nanoparticles, 20-40 parts of an imidazoline-ammonium salt corrosion inhibitor, 3-15 parts of glycerin, 2-15 parts of triethanolamine, 2-6 parts of borax, 0.5-1.5 parts of benzotriazole, 8-20 parts of polyethylene glycol and 30-50 parts of water. The high-antiwear cutting fluid is excellent in antiwear, cooling, lubrication, cleaning and antirust functions, and is low in cost and strong in stability.
Owner:CHAOHU GUANGFENG METAL PROD
New luminescent compositions and their uses
High quantum yield luminescent monomers, oligomers, and polymers, comprising benzotriazole repeating units and derivatives thereof have been discovered and utilized in optical devices and components therefor, including electroluminescent devices, light emitting devices, photoluminescent devices, organic light emitting diodes (OLEDs), OLED displays, sensors, and the like.
Owner:HITACHI CHEM CO LTD +1
Phosphorus-free corrosion and scale inhibitor
InactiveCN101607763AReduce dosagePlay a role in corrosion inhibitionTreatment using complexing/solubilising chemicalsChelationPrecipitation types
The invention relates to a phosphorus-free corrosion and scale inhibitor, which is prepared from sodium molybdate, zinc salt, citrate, triethanolamine, benzotriazole (BTA), polyaspartic acid (PASP), polyepoxysuccinic acid (PESA), AA / AMPS terpolymer, solid alkali and water. The phosphorus-free corrosion and scale inhibitor inhibits corrosion of metals by forming oxidization type and precipitation type films on the surfaces of the metals, has the effect of inhibiting scale through chelation and dispersive action on salts causing scale in cooling water, is non-toxic and phosphorus-free, has easily biodegradable major organic compositions, does not cause environmental pollution and is not limited by phosphorus in emission.
Owner:SHANGHAI WEILAI ENTERPRISE
Volatile rust preventive oil
InactiveCN102719302AInhibit corrosion and rustClean working environmentAdditivesGas phaseAntioxidant
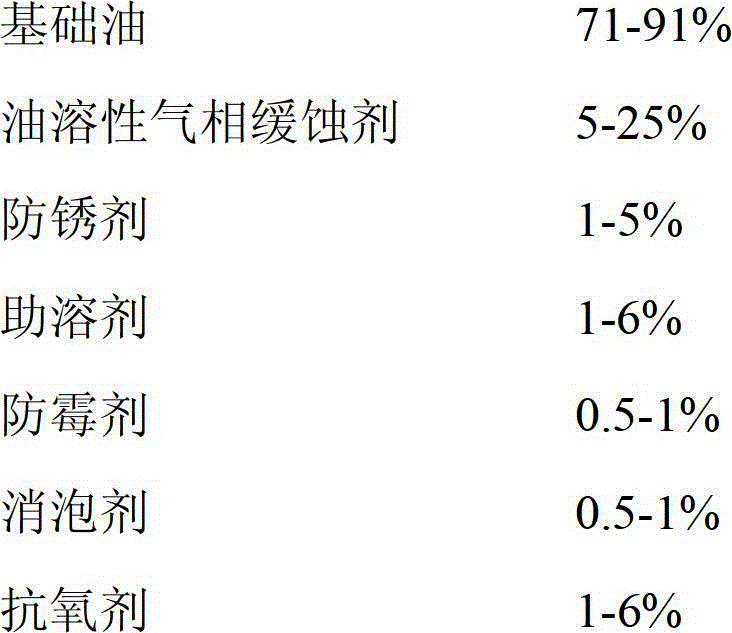
The invention discloses volatile rust preventive oil which comprises, by weight, 71-91% of base oil, 5-25% of an oil soluble volatile corrosion inhibitor, 1-5% of an antirusting agent, 1-6% of a cosolvent, 0.5-1% of a mildew-proof agent, 0.5-1% of an antifoaming agent and 1-6% of antioxidant, wherein the base oil is one of 500SN 46# machine oil, 32# machine oil, 600SN 150# machine oil and 100# machine oil; and the oil soluble volatile corrosion inhibitor is a mixture of four kinds of 2-heptadecenyl-imidalidine, octadecylamine, triazole tributylamine, dicyclohexylamine carbonate, dicyclohexylaminenitrite, benzotriazole, tert-butyl chromate, nephthenic soap, petroleum sodium sulfonate, sorbitan monooleate and stearic acid; and the antioxidant is sulfurphosphorousbutyloctyl zinc salt. The volatile rust preventive oil has good volatile rust preventive and corrosion resistant performances, metal which cannot be coated with rust preventive oil can achieve rust preventive protection, the operation is simple, and the cost is low.
Owner:上海福岛新材料科技有限公司
Extreme pressure antiwear additive and preparation method and application thereof
InactiveCN102504913AModerate chemical activityGood oil solubilityGroup 5/15 element organic compoundsAdditivesSolubilityOil sludge
The invention provides an extreme pressure antiwear additive and a preparation method and application thereof; the extreme pressure antiwear additive has a general formula structure as shown in the specification, wherein R1, R2 and R3 in the formula is respectively alkyl groups, aryl groups or aralkyl of a C4-C20 linear chain or branched chain. The extreme pressure antiwear additive provided by the invention is formed by reacting phosphorus oxychloride, C1-C20 alcohols, organic amine and benzotriazole. The extreme pressure antiwear additive provided by the invention can be applied to lubrication oil and lubricating grease with 0.1-2.0wt% of recommended dosage. The extreme pressure antiwear additive provided by the invention has moderate chemical activity, good oil solubility, very good compatibility with other addition agents, no peculiar smell and good thermal oxidation stability, and can effectively reduce the abrasion of devices and oil sedimentation as well as generation of oil sludge. After applied to gear oil, the extreme pressure antiwear additive has excellent antiwear performance and thermal oxidation stability; and simultaneously the extreme pressure antiwear additive can be applied to a lubricating grease and automatic transmission liquid and hydraulic oil of automobiles.
Owner:天津市金岛润滑科技股份有限公司
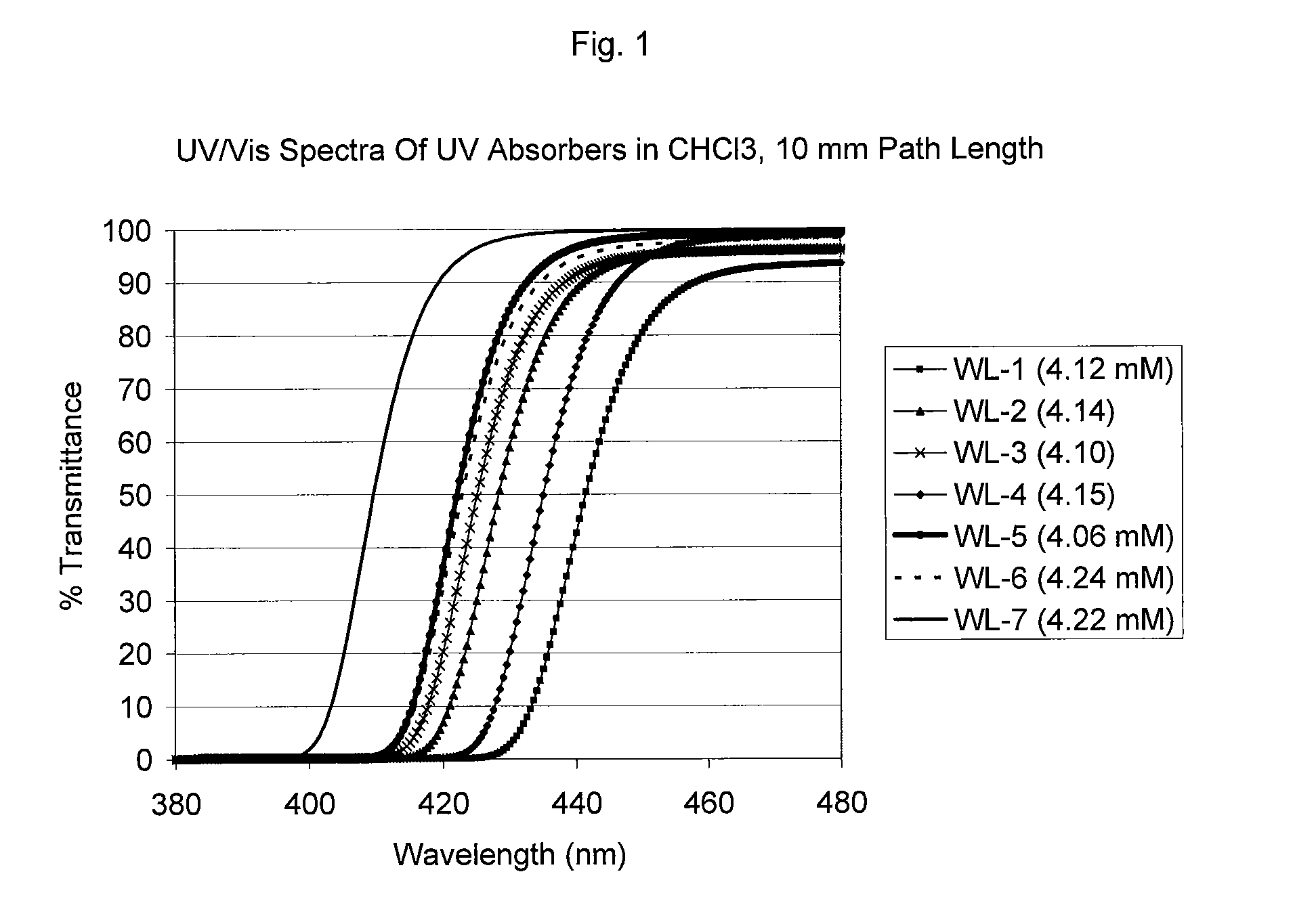
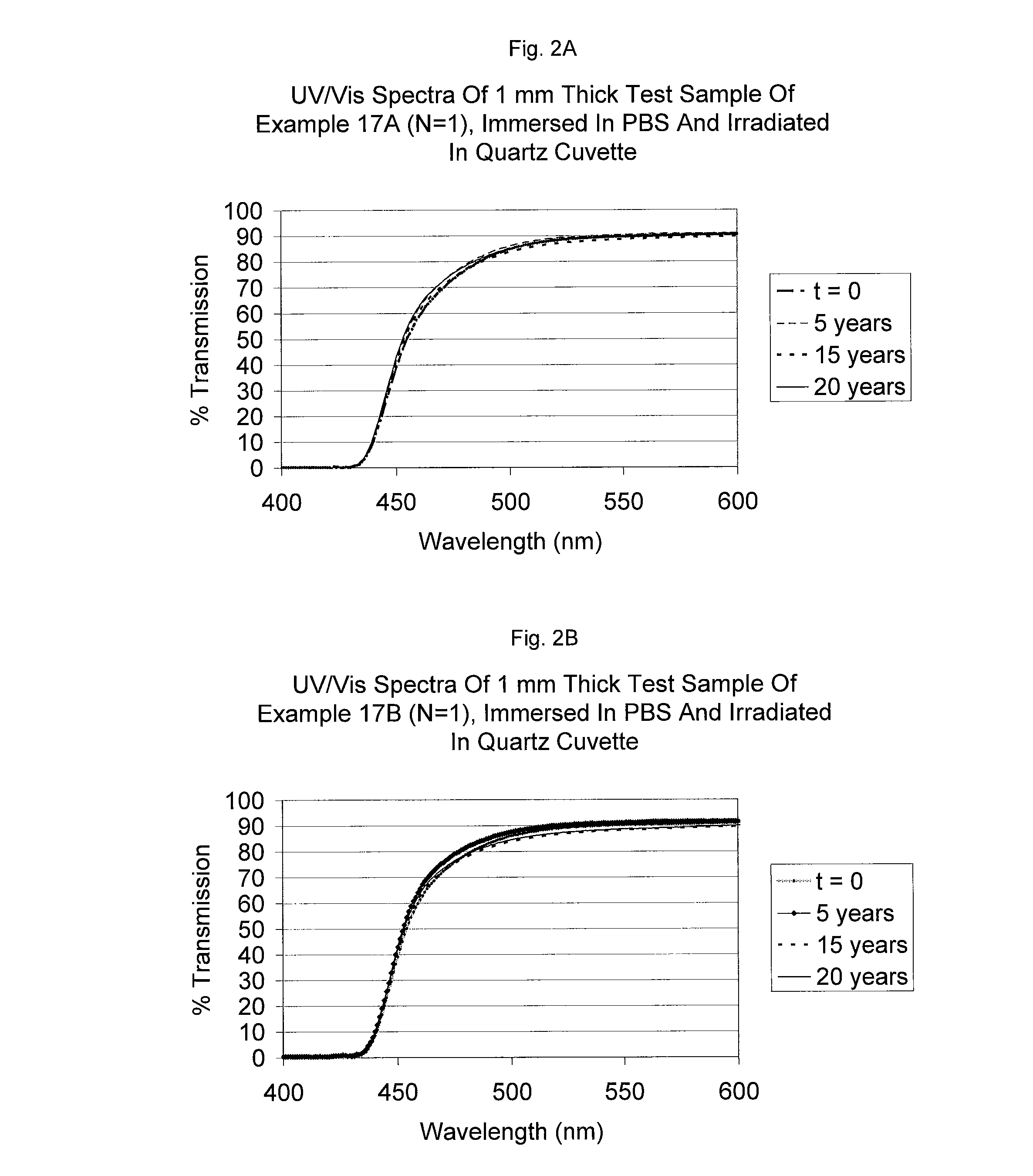
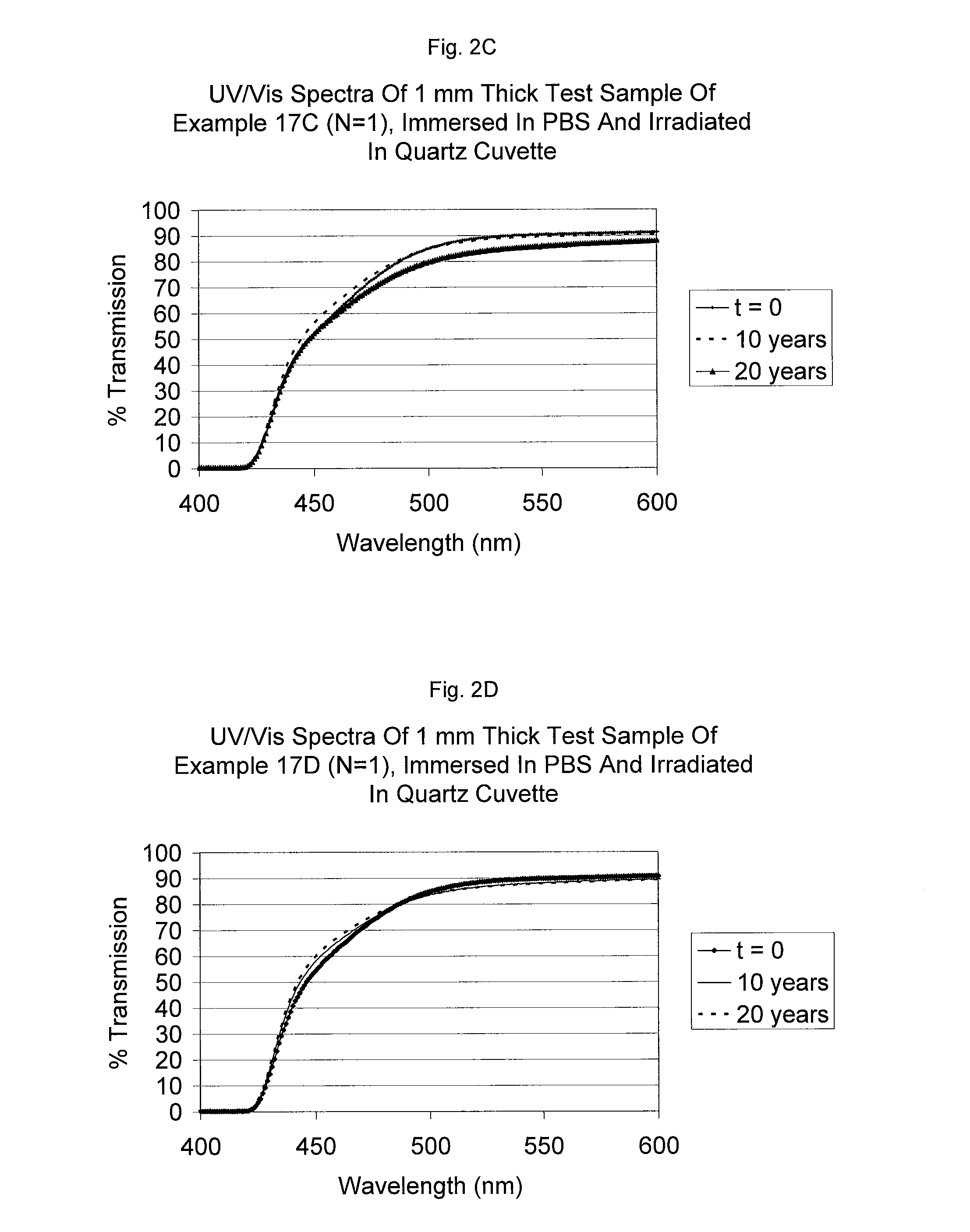
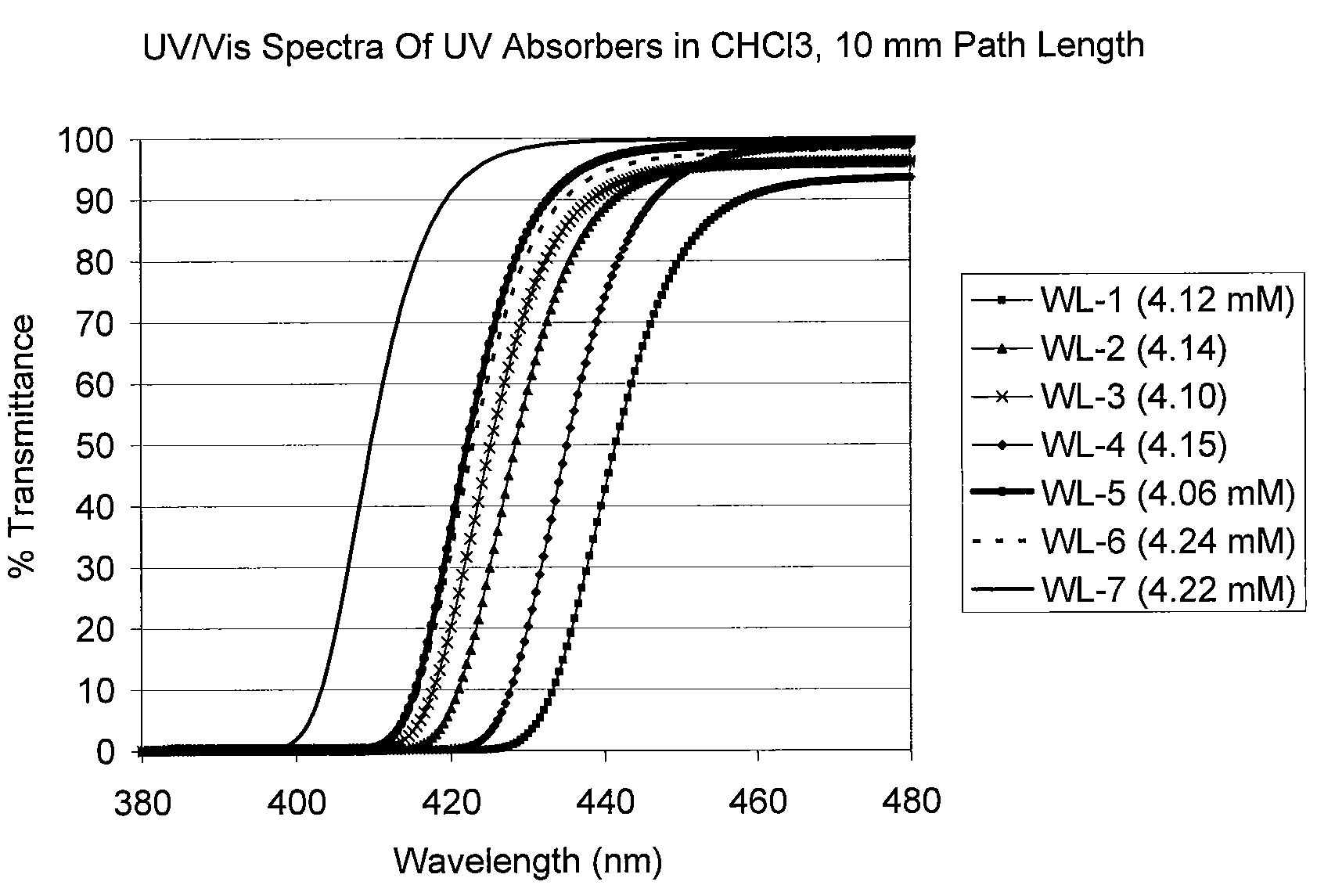
Uv/visible light absorbers for ophthalmic lens materials
Benzotriazole UV / Visible light-absorbing monomers are disclosed. The UV / Vis absorbers are particularly suitable for use in intraocular lens materials.
Owner:ALCON INC
UV/visible light absorbers for ophthalmic lens materials
Benzotriazole UV / Visible light-absorbing monomers are disclosed. The UV / Vis absorbers are particularly suitable for use in intraocular lens materials.
Owner:ALCON INC
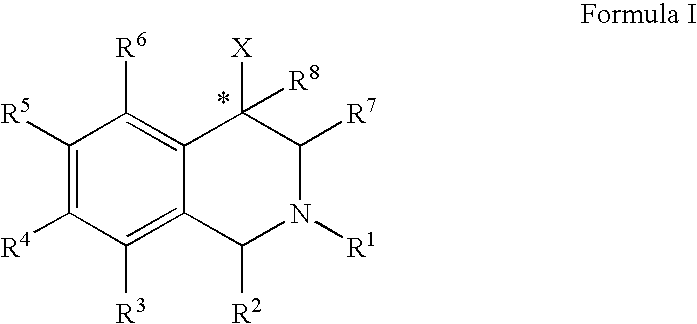
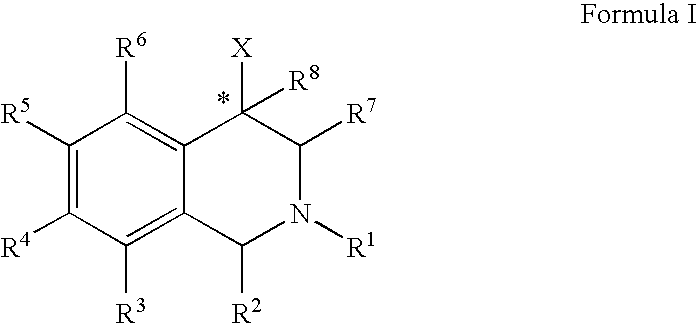
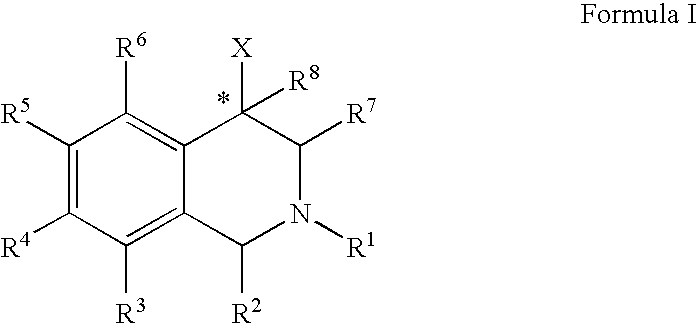
Aryl- and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
ActiveUS20060052378A1Good curative effectQuick effectBiocideNervous disorderBenzoxazoleChemical structure
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Outdoor weatherable photopolymerizable coatings
InactiveUS6974850B2Prolong lifeLimited solubilityOrganic chemistryLayered productsBenzeneBenzotriazole
Plastic articles can be coated with polymerizable compositions containing a vinyl-functional crosslinkable film former, a large amount of benzotriazole and a copolymerizable monomer that solubilizes the benzotriazole. The cured compositions help protect the article from UV exposure and other weathering effects.
Owner:3M INNOVATIVE PROPERTIES CO
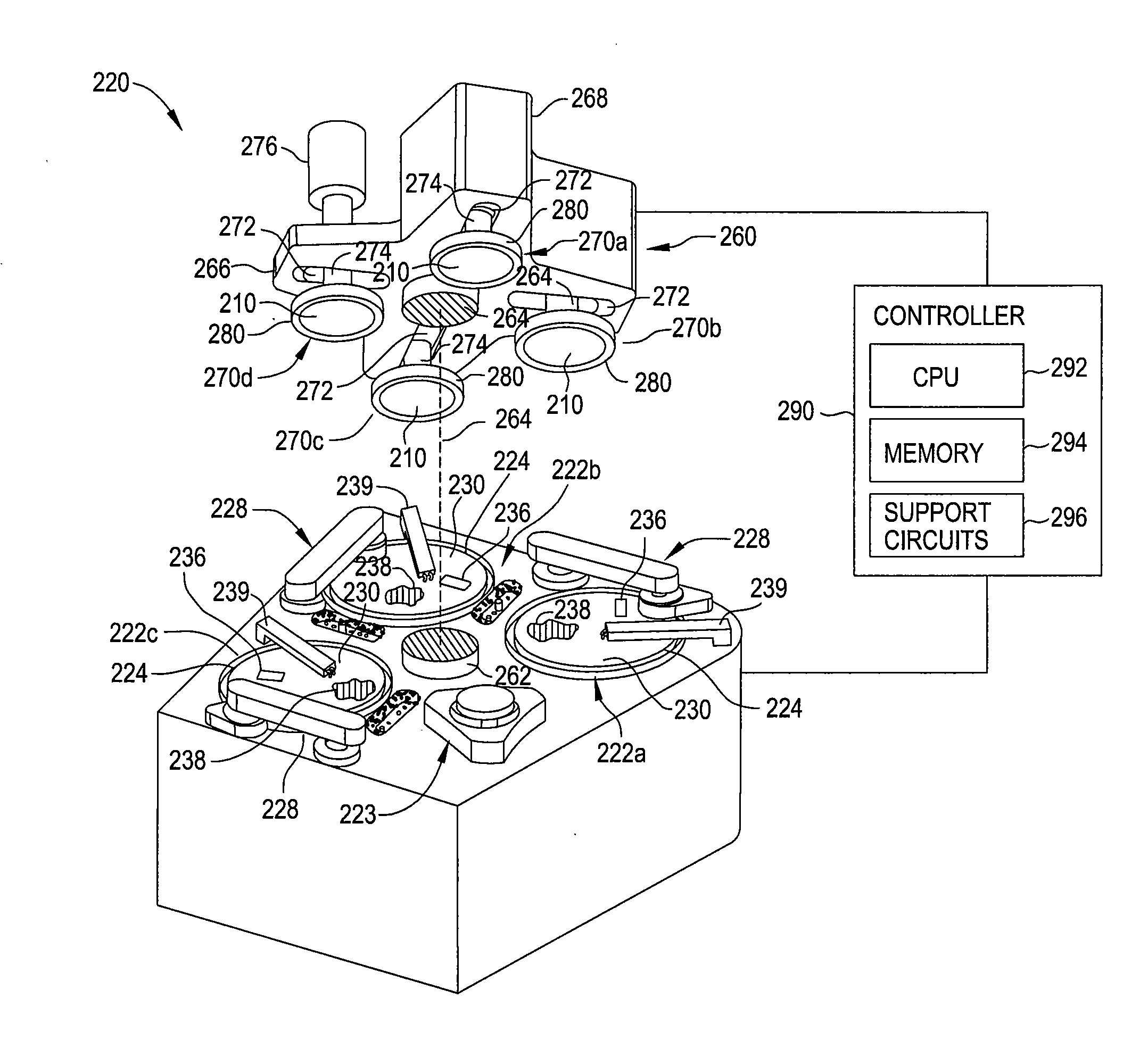
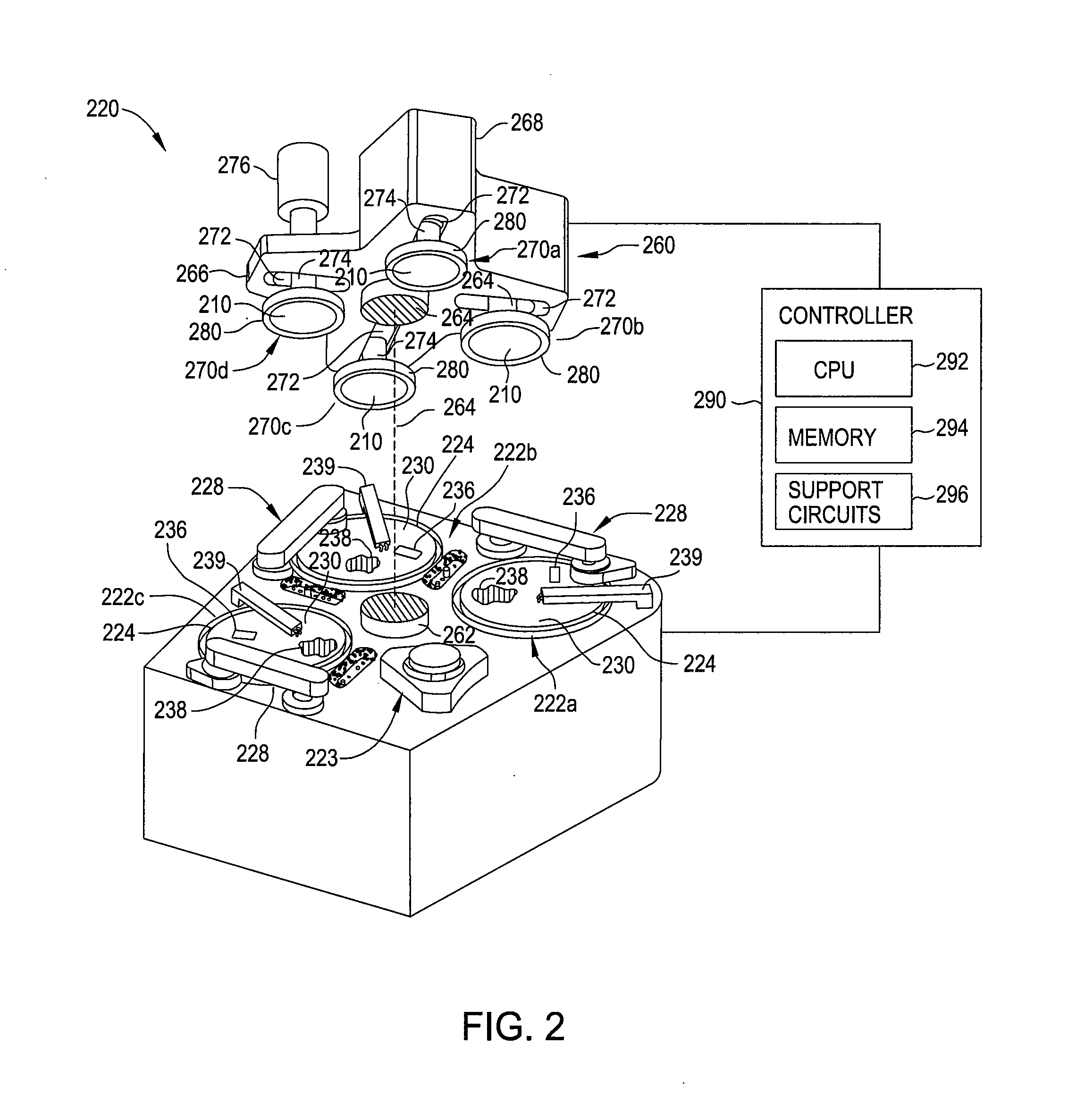
Slurry composition for gst phase change memory materials polishing
InactiveUS20100130013A1Other chemical processesSemiconductor/solid-state device manufacturingPhase-change memoryPolyethylene glycol
Owner:APPLIED MATERIALS INC
Optical film, polarizing plate and display
InactiveUS20060078754A1Easy to produceImprove production efficiencyElectric discharge tubesSynthetic resin layered productsCelluloseBenzene
A method for producing an optical film comprising steps of mixing a heated and molten cellulose ester having an acylated degree for from 2.5 to 2.9 and at least one of a UV absorbent having at least two benzotriazole skeletons and a UV absorbent having a weight average molecular weight of from 2,000 to 50,000 to form a mixture, and extruding the mixture to form a film.
Owner:KONICA MINOLTA OPTO
Methods and compositions for improving light-fade resistance and soil repellency of textiles and leathers
ActiveUS20050022313A1Easy to useImpart propertyDispersed particle filtrationPhysical treatmentAdditive ingredientChemical compound
One method includes applying to a post-manufactured textile material a liquid composition resulting from a combination of ingredients. The ingredients include one or more anti-fading compounds, one or more anti-soiling compounds, one or more silicon-based compounds, and one or more carrying media. One composition is a liquid composition resulting from a combination of ingredients, with the ingredients including a benzotriazole, a fluorocarbon, an organosiloxane, and odorless mineral spirits.
Owner:SCHEIDLER KARL J
Chemical deplating solution and deplating method suitable for removing tin-nickel coating on surface of brass
InactiveCN101775601AImprove the status quo of low dissolution rateImprove work efficiencyWater bathsTin
The invention discloses a deplating solution and a deplating method thereof for tin-nickel coating (commonly called plated scrap) on the surface of brass. The deplating solution comprises oxidizer, accelerator, complexing agent and inhibitor, and the deplating solution is prepared by adding water to the reagents, stirring, dissolving, then putting in a water bath and slowly heating. The oxidizer adopts 65-68% nitric acid, the accelerator adopts 36-38% hydrochloric acid, the complexing agent adopts citric acid and the inhibitor adopts benzotriazole. After being deplated by the deplating solution of the invention, the surface of the brass is bright, the color of the substrate is basically unchanged, and the brass has no pinholes and small corrosion amount.
Owner:HEFEI UNIV OF TECH
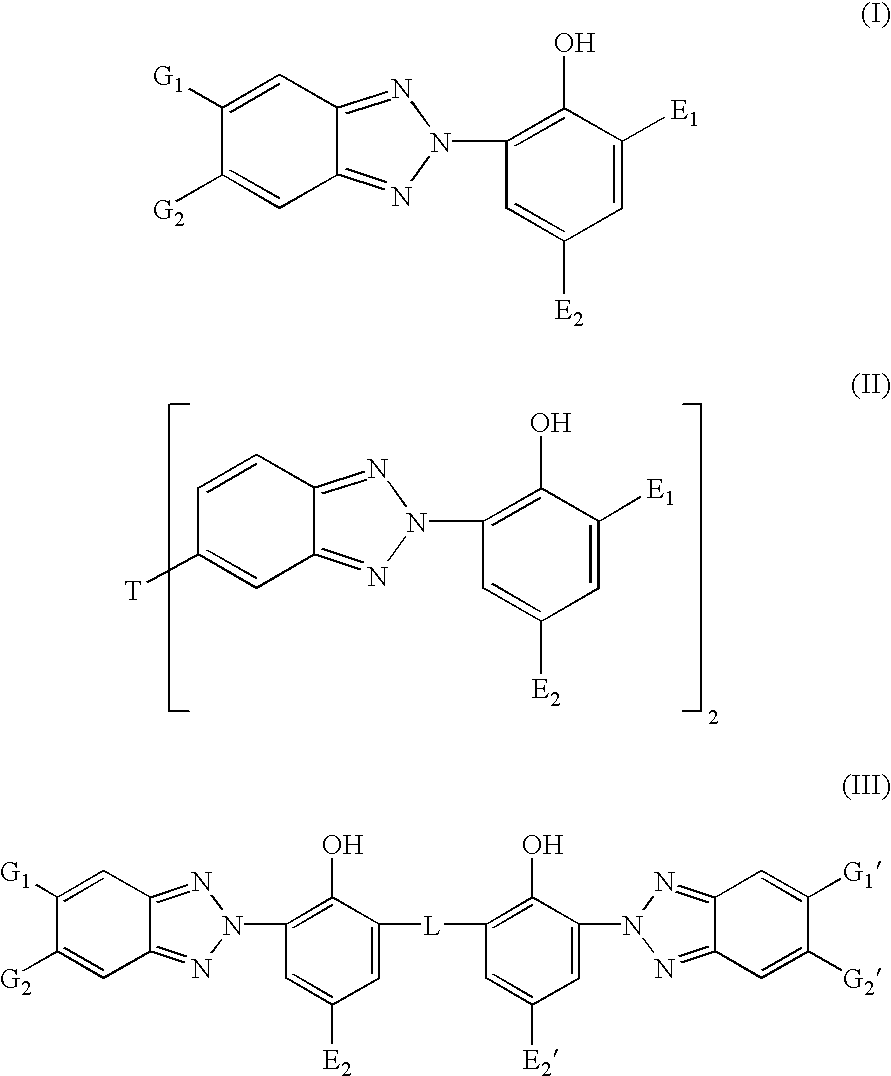
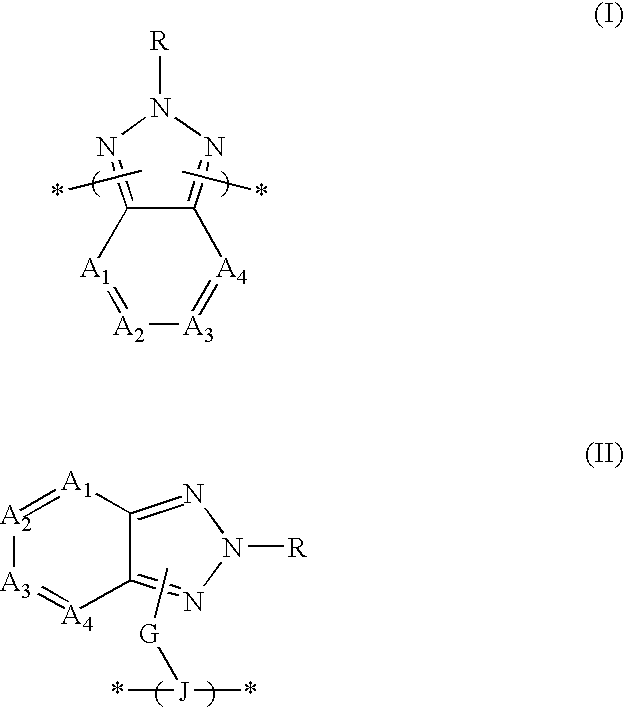
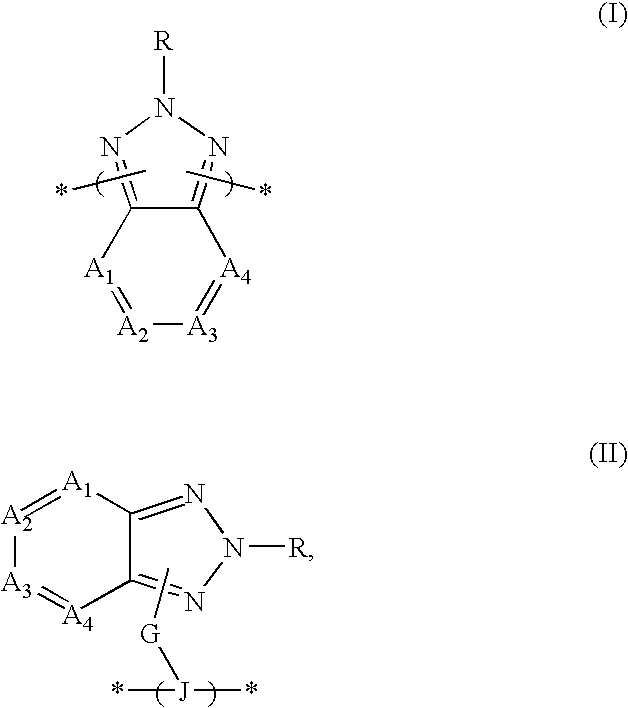
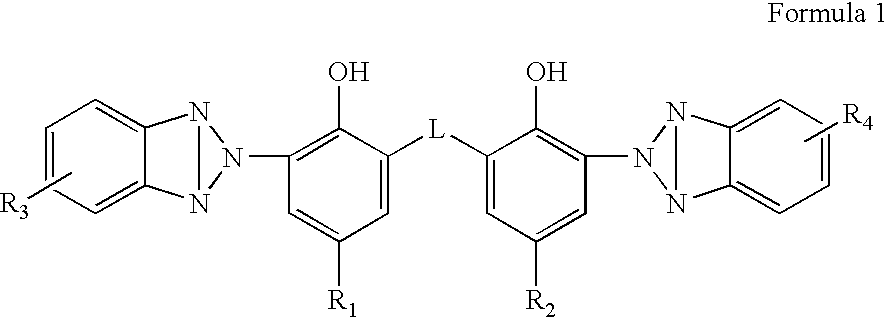
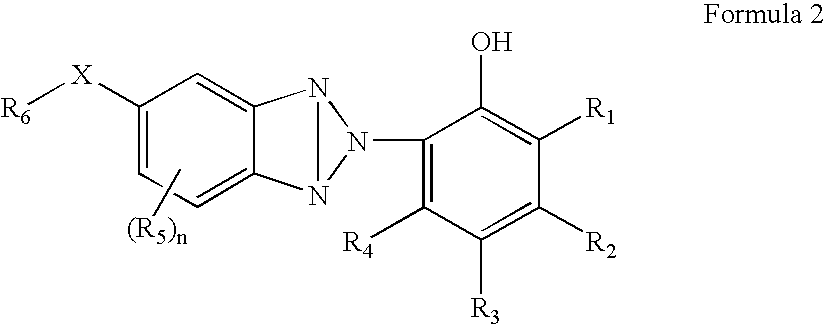
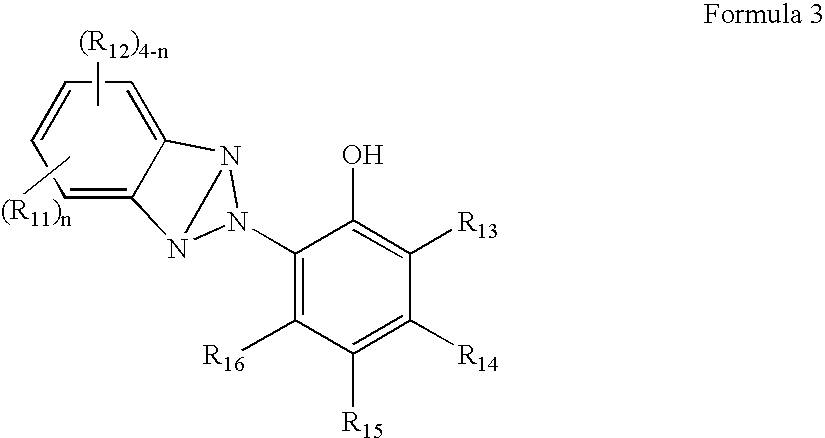
Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof
ActiveCN102050940AIdeal electron deficient materialEasy to processSolid-state devicesSemiconductor/solid-state device manufacturingSolubilityAlkyl transfer
The invention provides an organic semiconductor material containing 6-R group-[1, 2, 5] thiazole [3, 4-g] benzotriazole and an application thereof. Diazosulfide is used as parent, benzotriazole derivatives are formed by reduction and ring closure of nitrated diazosulfide, and finally, a halogenated 6-R group-[1, 2, 5] thiazole [3, 4-g] benzotriazole monomer is synthesized by performing alkylation reaction on an H of an N atom; and the monomer is reacted with a monomer containing an aromatic group under effect of metal catalyst to prepare the organic semiconductor material. Positions 1, 2, 5 and 6 of a benzene ring of the organic semiconductor material are respectively connected with alkyl triazole and thiazole, the two kinds of chemical groups have excellent electron attracting performance, and a formed ternary condensed ring has better planarity and conjugated bonds, and also has good dissolubility. The organic semiconductor material provided by the invention can be used as an active layer having application foreground to be applied in organic photoelectric devices.
Owner:SOUTH CHINA UNIV OF TECH
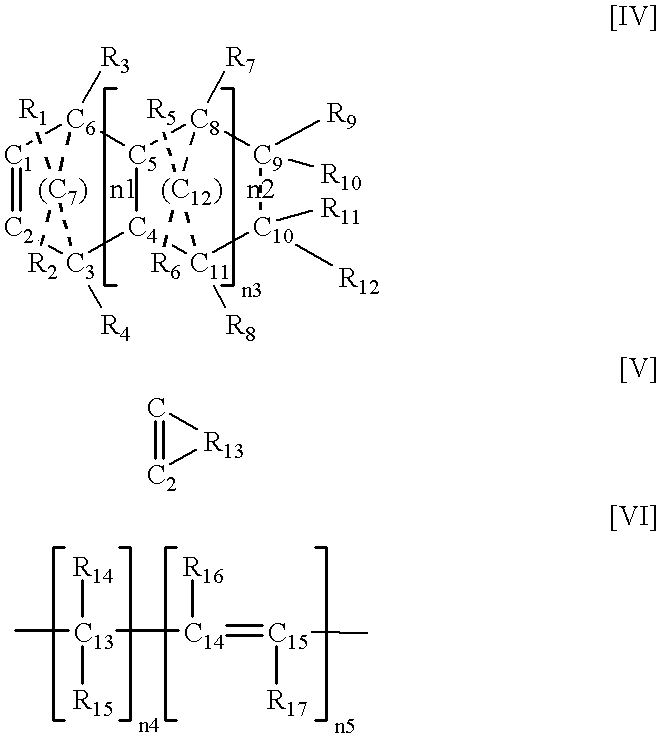
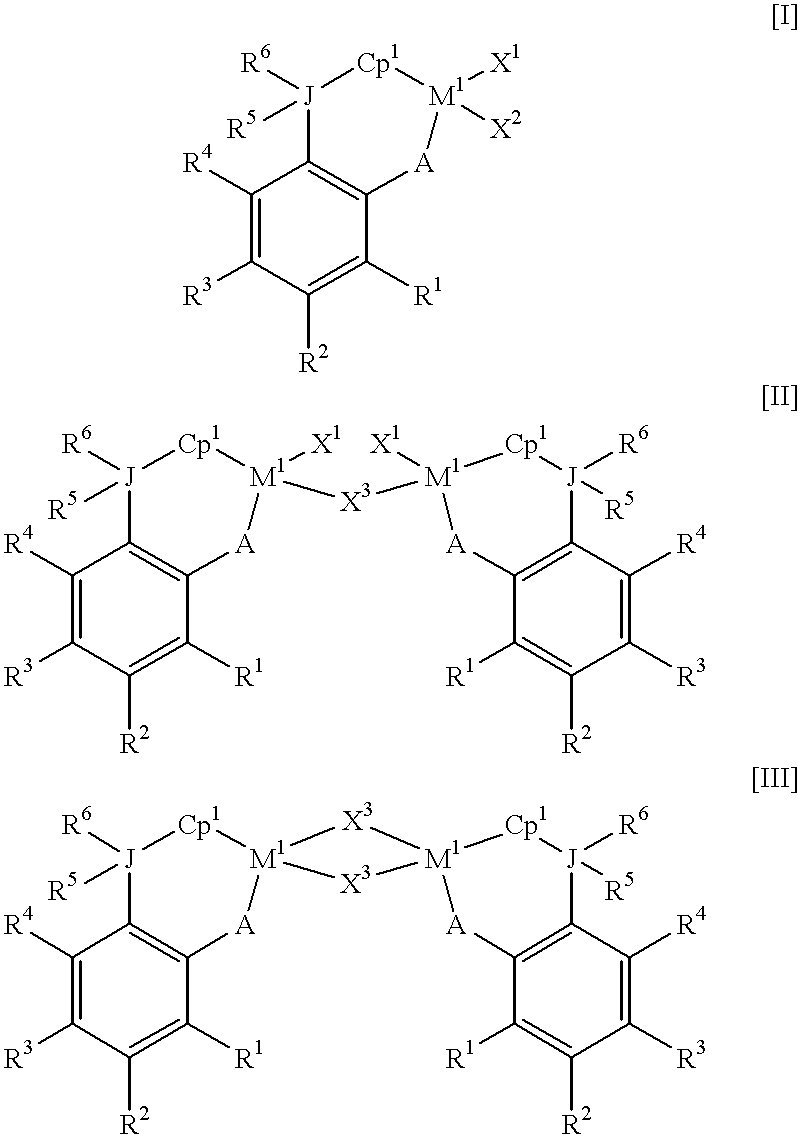
Olefin-based copolymer composition
InactiveUS20020072561A1Film/foil adhesivesSynthetic resin layered productsHindered amine light stabilizersBenzotriazole
An olefin-based copolymer composition comprising the following component (a) in combination with any one component selected from the following components (b) to (i): (a): an olefin-based copolymer obtained by copolymerizing two or more olefins, wherein the olefins are selected from the group consisting of ethylene, propylene and alpha-olefins having 4 to 20 carbon atoms, and the total number of carbon atoms of the two or more selected olefins is 6 or more; (b): a crystalline alpha-olefin-based polymer comprising an alpha-olefin having 3 or more carbon atoms; (c): the following component (c-1) and / or component (c-2), (c-1): a block copolymer comprising (cH) a polymer block containing a vinyl aromatic compound and (cS) a polymer block containing a conjugated diene compound, (c-2): a hydrogenated product of the block copolymer recited in (c-1); (d): the component (b) in combination with an isobutylene-isoprene copolymer rubber and / or halogenated isobutylene-isoprene copolymer rubber; (e): the component (b) in combination with a tackifier resin; (f): the component (b), a component (f-1) which is an ethylene-propylene (non-conjugated diene) random copolymer rubber, and a component (f-2) which is an inorganic filler; (g): a component (g-1) and / or (g-2), (g-1): a benzotriazole light stabilizer having a benzotriazole skeleton and having a molecular weight of 100 to 5,000, (g-2): a hindered amine light stabilizer having a piperidyl group in its molecular skeleton and having a molecular weight of about 200 or more; (h): the component (f-1) in combination with the component (b) and / or an ethylene-based polymer having an ethylene content of 90 mol % or more; and (i): the component (b) and the inorganic filler (f-2).
Owner:SUMITOMO CHEM CO LTD
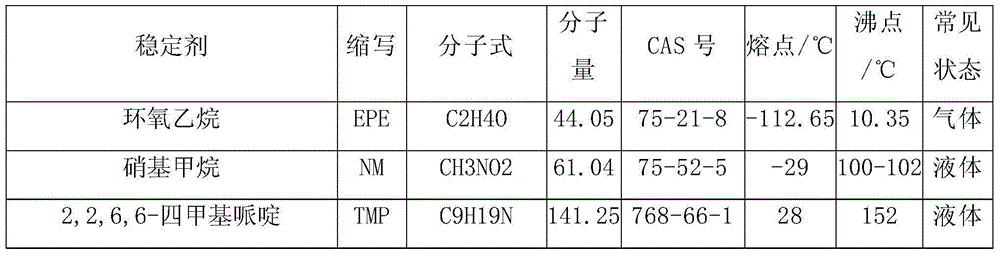
Composition containing HFC-161 and stabilizer
The invention discloses a composition containing HFC-161 and a stabilizer, wherein the stabilizer is selected from one or more of epoxy compound, nitro-compound, hindered amines, hindered phenols, secondary arylamines, beta-diketones, phosphite esters, salicylic acid esters, cinnamic acid esters, hydroxybenzophenones, hydroxybenzoic acids, hydroxylphenyl benzotriazoles, hydroxylphenyl triazines, oxanilides, formamidines, polyhydric alcohols, ortho-formates, organosulfur compounds, olefins, ethanol amines and fatty amines. The composition provided by the invention is capable of keeping a stable state in a long time under the coexistence of air or oxygen, and capable of being used as a heat transfer composition in the form of a liquid or a gas, a refrigerant, an aerosol propellant and the like.
Owner:SINOCHEM LANTIAN +2
Copper particle dispersing solution and method for producing conductive film using same
InactiveUS20160346838A1Improve conductivityTransportation and packagingMetal-working apparatusScreen printingLight irradiation
A copper particle dispersing solution obtained by dispersing fine copper particles having an average particle diameter of 1 to 100 nm, each of the fine copper particles being coated with an azole compound, such as benzotriazole, and coarse copper particles having an average particle diameter of 0.3 to 20 μm in a dispersing medium, such as ethylene glycol, so as to cause the total amount of the fine copper particles and coarse copper particles to be 50 to 90% by weight and so as to cause the ratio of the weight of the fine copper particles to the weight of the coarse copper particles to be in the range of from 1:9 to 5:5, is applied on a substrate by screen printing or flexographic printing to be preliminary-fired with vacuum drying, and then, fired with light irradiation by irradiating light having a wavelength of 200 to 800 nm at a pulse period of 100 to 3000 μm and a pulse voltage of 1600 to 3600 V, to form a conductive film on the substrate.
Owner:DOWA ELECTRONICS MATERIALS CO LTD
Multiple-effect type water-based synthesized cutting solution and preparation thereof
InactiveCN101323813AExtended service lifeImprove extreme pressure and anti-wearLubricant compositionWater basedCleansing Agents
The invention relates to a multi-effect water-based composite cutting / grinding fluid which consists of water-soluble oil, cleaning agent, extreme pressure antiwear agent, antirust, antibacterial agent, complexant, solubilizer and anstatic agent. The water-soluble oil is the mixture of aminoethyl alcohol and triethanolamine oleate, the cleaning agent is anionic surfactant or nonionic surfactant, the extreme pressure antiwear agent is one or two of additives A, B, C, D, E and F, the antirust is benzotriazole and sodium benzoate, the antibacterial agent is boric acid and borax, the complexant is EDTA and the solubilizer is urea. The cutting / grinding fluid with various improved performances is non-toxic and tasteless, is an environment-friendly product and has no harm to human body and the skin of users; the operating cycle is normally more than 6 months.
Owner:大连弘瑞化工有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
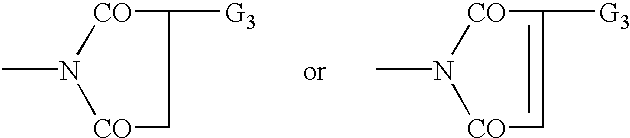
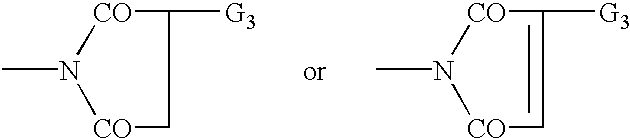



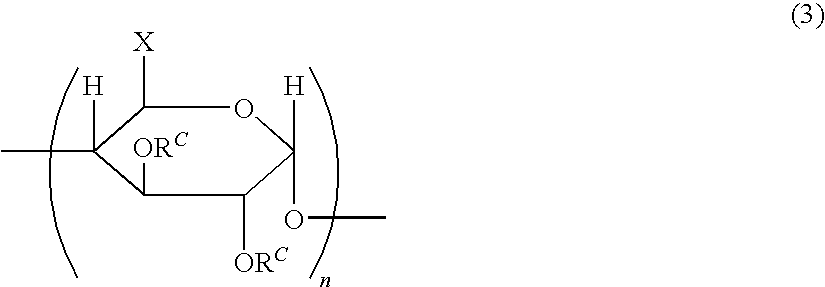




![Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof](https://images-eureka.patsnap.com/patent_img/63e990ec-e906-470a-909f-1f287dde0f0f/110128101030.PNG)
![Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof](https://images-eureka.patsnap.com/patent_img/63e990ec-e906-470a-909f-1f287dde0f0f/110128101033.PNG)
![Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof Organic semiconductor material containing 6-R group- [1, 2, 5] thiazole [3, 4-g] benzotriazole and application thereof](https://images-eureka.patsnap.com/patent_img/63e990ec-e906-470a-909f-1f287dde0f0f/110128101036.PNG)