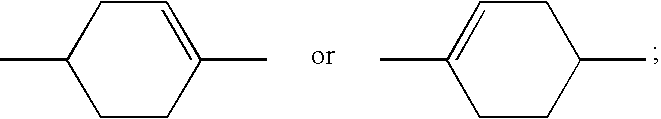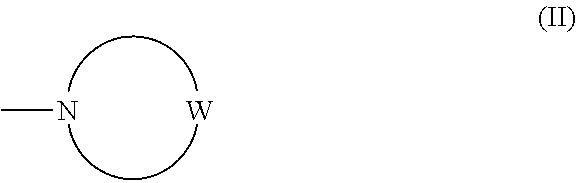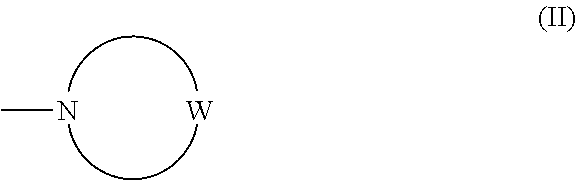Patents
Literature
657 results about "Pyridazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyridazine is a heterocyclic organic compound with the molecular formula (CH)₄N₂. It contains a six-membered ring with two adjacent nitrogen atoms, and is aromatic. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other (CH)₄N₂ rings, pyrimidine and pyrazine.
Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors
ActiveUS7750007B2Inhibitory activityBiocideOrganic chemistryProtein kinase inhibitor activityPTK Inhibitors
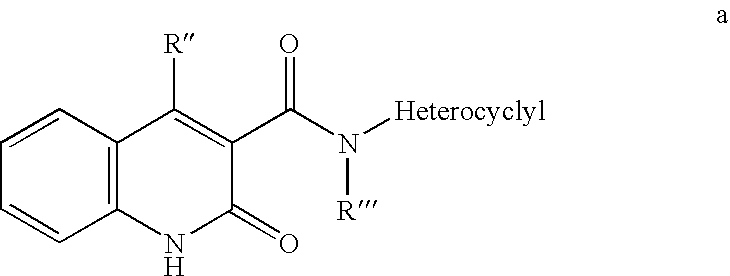
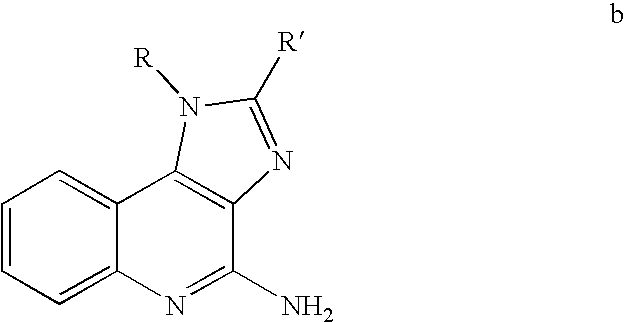
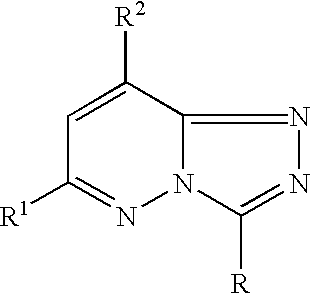
The present invention provides protein kinase inhibitors comprising imidazo[1,2-b]pyridazine and pyrazolo[1,5-a]pyrimidine compounds of the following structure (I) and (II):or a stereoisomer, prodrug or pharmaceutically acceptable salt thereof, wherein R, R1, R2 and X are as defined herein. Compositions and methods for using the same in the treatment of cancer and other Pim kinase-associated conditions are also disclosed.
Owner:SUMITOMO PHARMA ONCOLOGY INC
Active matrix displays having high contrast values
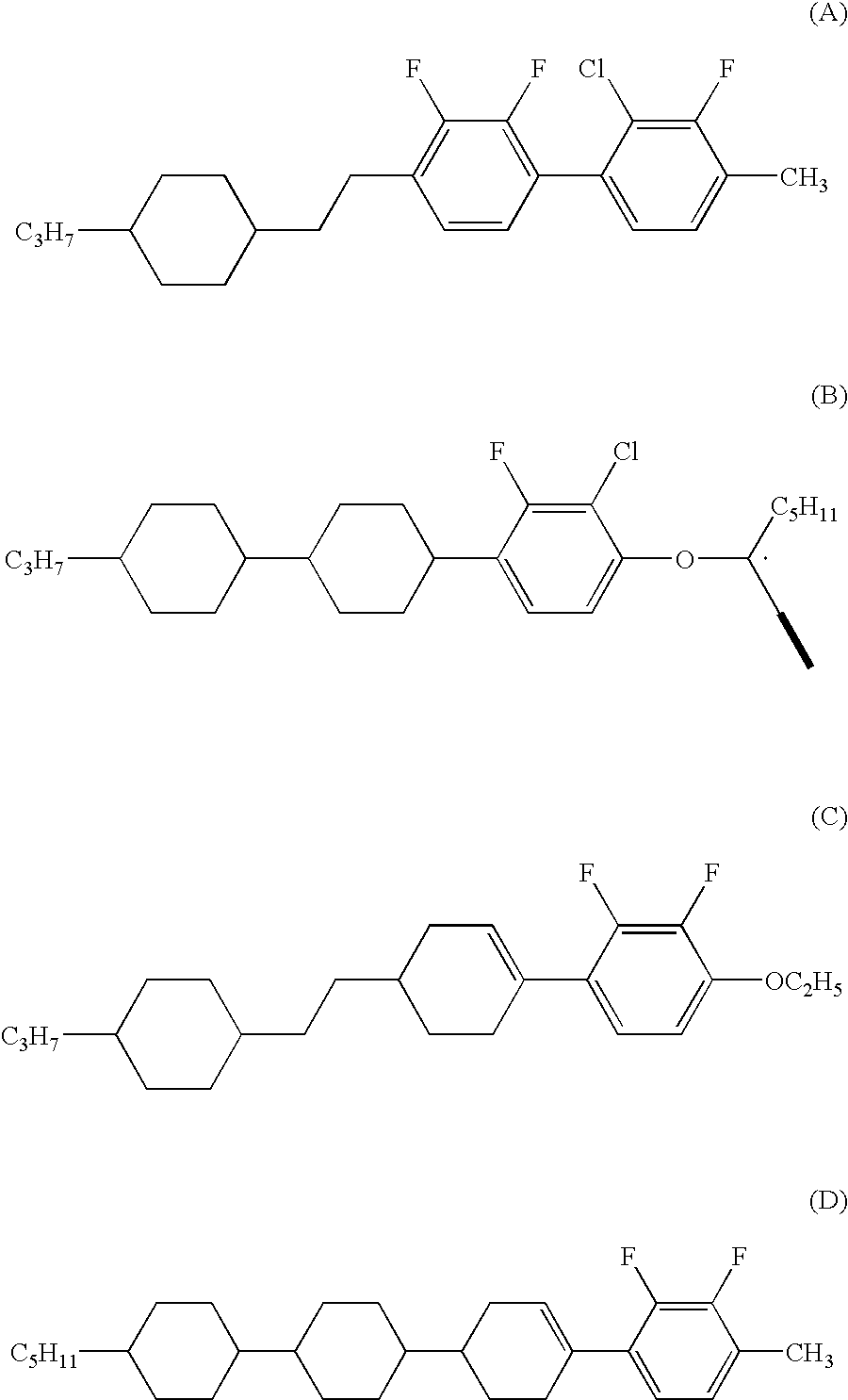
InactiveUS6482479B1High maximum transmissionIncrease contrastLiquid crystal compositionsOrganic chemistryPyridazineActive matrix
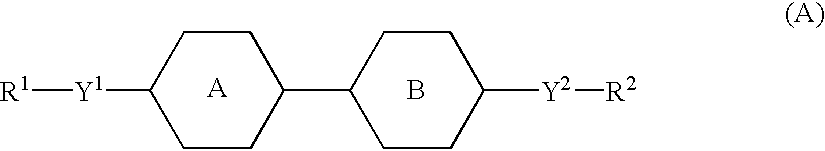
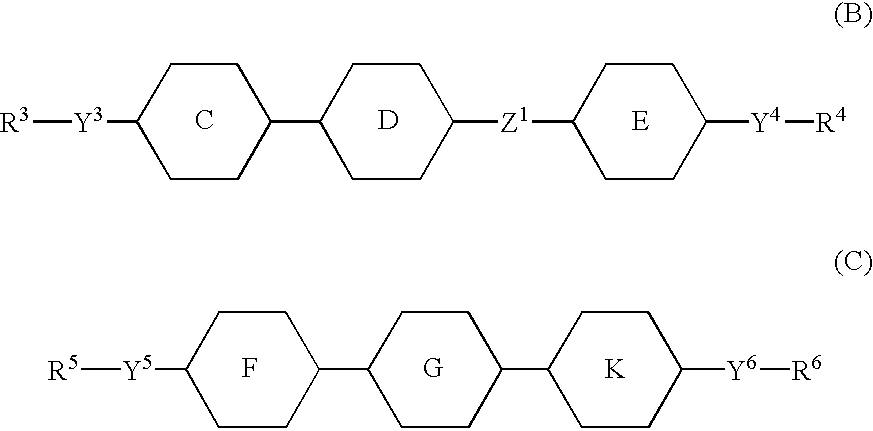
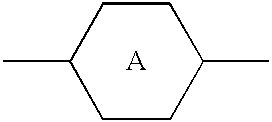
An active matrix display comprises a chiral smectic liquid crystal mixture which has the phase sequence I-N*-SmC*, a spontaneous polarization in the operating temperature range of <40 nC / cm2 and a pitch of >10 mum at at least one temperature in the nematic or cholesteric phase and comprises at least one compound each from at least two of the substance classes (A), (B) and (C) and one or more compounds from substance class (D):(A): compounds comprising two rings which are directly linked to one another and are selected from phenylene-1,4-diyl, pyrimidine-2,5-diyl, pyridine-2,5-diyl and pyridazine-2,5-diyl with the proviso that at least one of these rings is a nitrogen heterocycle;(B): compounds comprising three rings selected from phenylene-1,4-diyl, two of the rings being directly linked to one another and the third ring being linked to one of the other two rings via an -OC(=O)- or -C(=O)-group, with the proviso that at least one of the three rings is fluorophenylene-1,4-diyl or ortho-difluorophenylene-1,4-diyl;(C): compounds comprising three rings which are directly linked to one another and are selected from phenylene-1,4-diyl, pyrimidine-2,5-diyl, pyridine-2,5-diyl and pyridazine-2,5-diyl with the proviso that at least one of these rings is a nitrogen heterocycle;(D): compounds comprising mesogenic groups suitable as components of liquid crystal mixtures.
Owner:MERCK PATENT GMBH
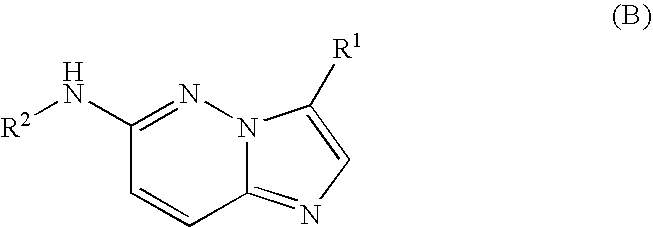
6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS
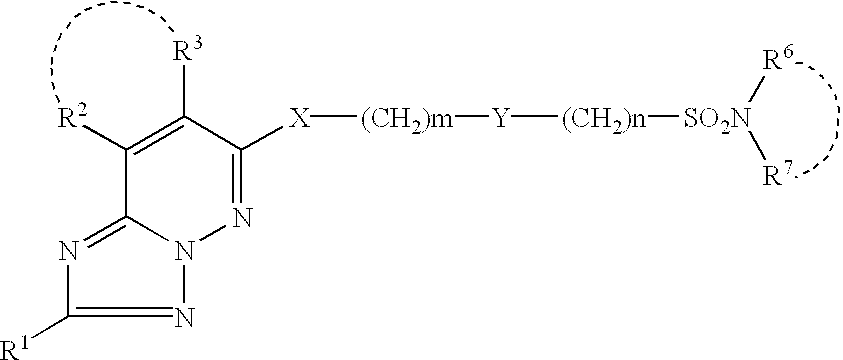
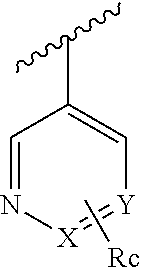
Methods for using 6-aminoimidazo[1,2-b]pyridazine analogs are disclosed herein to treat rho kinase-mediated diseases or rho kinase-mediated conditions, including controlling intraocular pressure and treating glaucoma, are disclosed. Ophthalmic pharmaceutical compositions useful in the treatment of eye diseases such as glaucoma, and additionally useful for controlling intraocular pressure, the compositions comprising an effective amount of 6-aminoimidazo[1,2-b]pyridazine analogs, are disclosed herein.
Owner:ALCON RES LTD
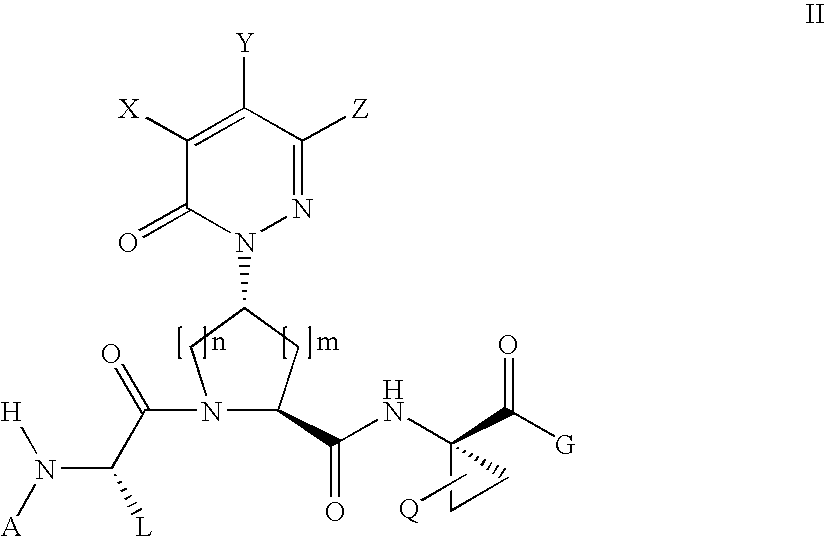
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
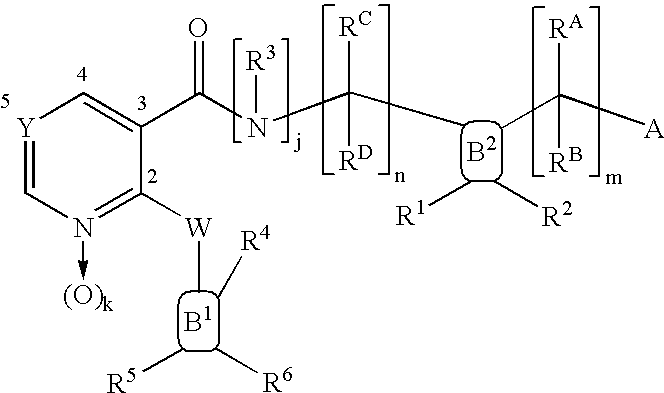
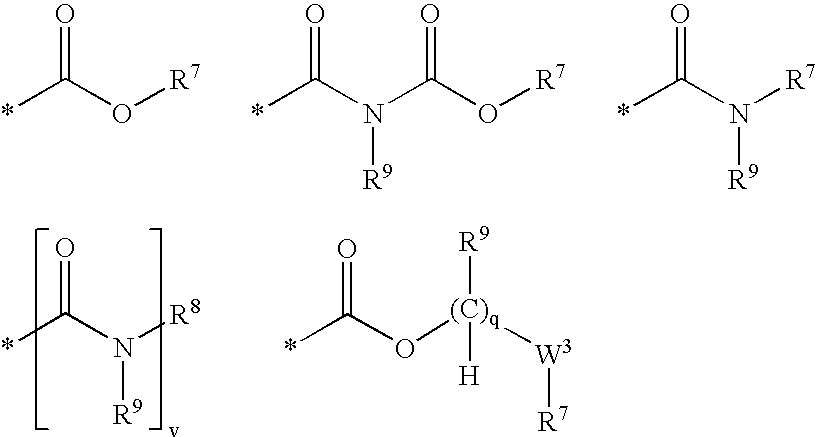
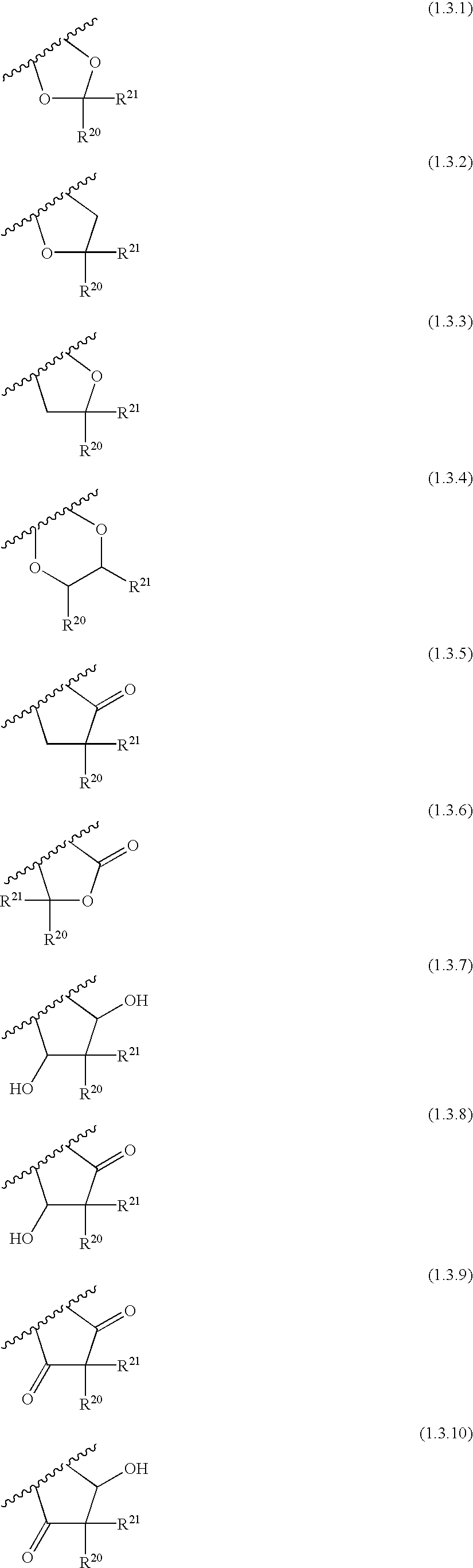
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Fused heterocycles as lck inhibitors
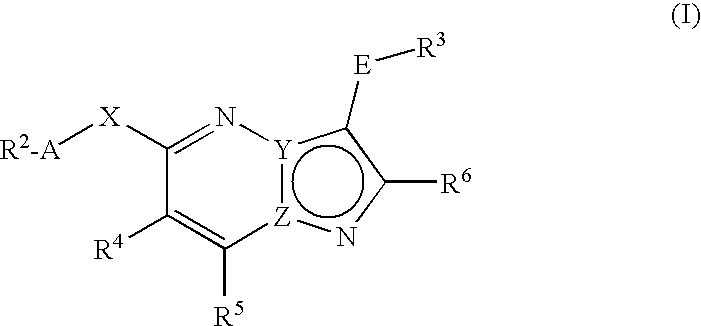
There is provided fused heterocycles of imidazopyridazine or pyrazolopyrimidine derivative represented by the formula (I), which have excellent Lck inhibitory activity and are useful for a medicament particularly an immunosuppressive agent.[wherein one of Y and Z is C atom, and the other is N atom; —X— is —N(R1)— or the like, —R1 represents hydrogen or the like, -A- represents bond or the like,—R2 is cycloalkyl, aryl or the like, -E- is bond or the like, —R3 is aryl, aromatic heterocycle or the like, —R4, —R5 and —R6 are the same or different, each being hydrogen or the like.]
Owner:ASTELLAS PHARMA INC
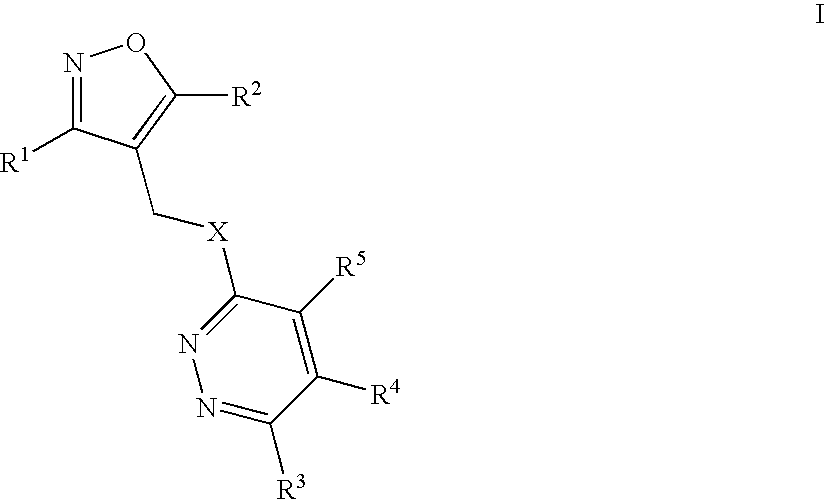
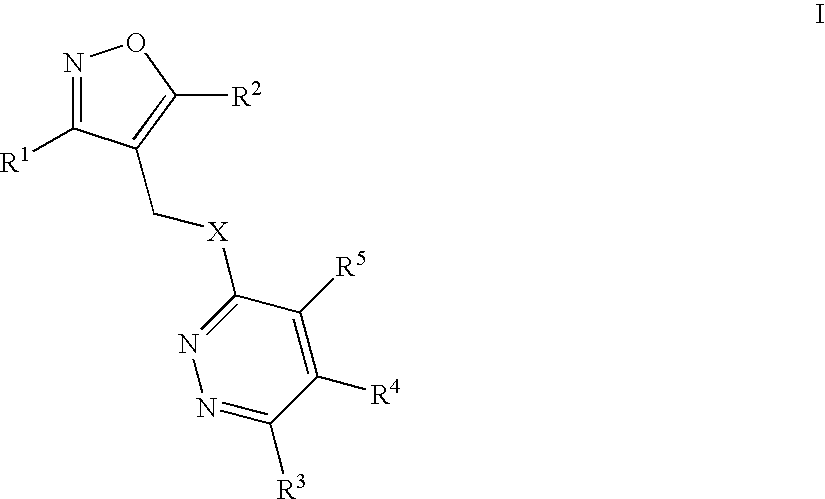
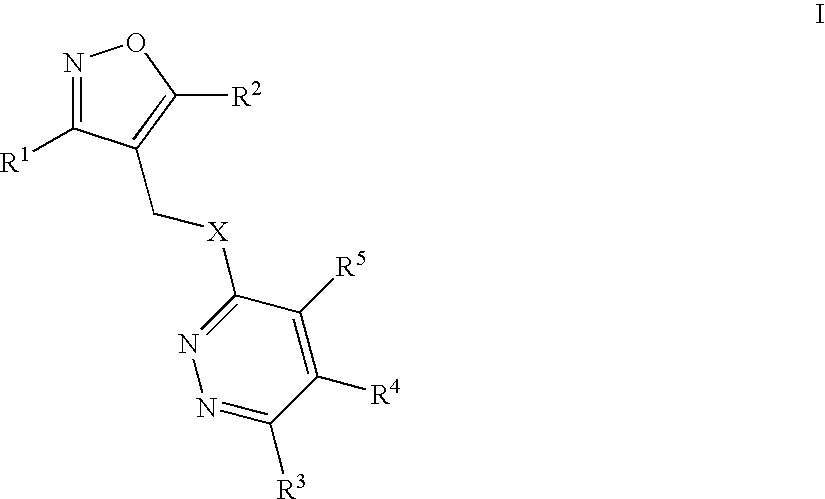
Isoxazolo-pyridazine derivatives
The invention relates to isoxazolo-pyridazine compounds, in particular those of formula I as described above and to a pharmaceutically acceptable salts thereof, having affinity and selectivity for the GABA A α5 receptor binding site, their manufacture, pharmaceutical compositions containing them and their use as cognitive enhancers or for the treatment of cognitive disorders like Alzheimer's disease.
Owner:F HOFFMANN LA ROCHE INC
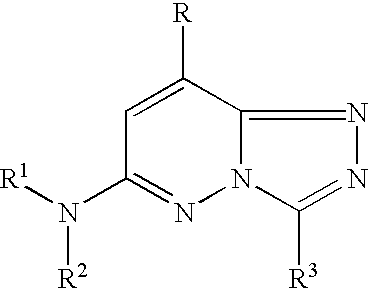
Nitrogen-containing heterocyclic compound
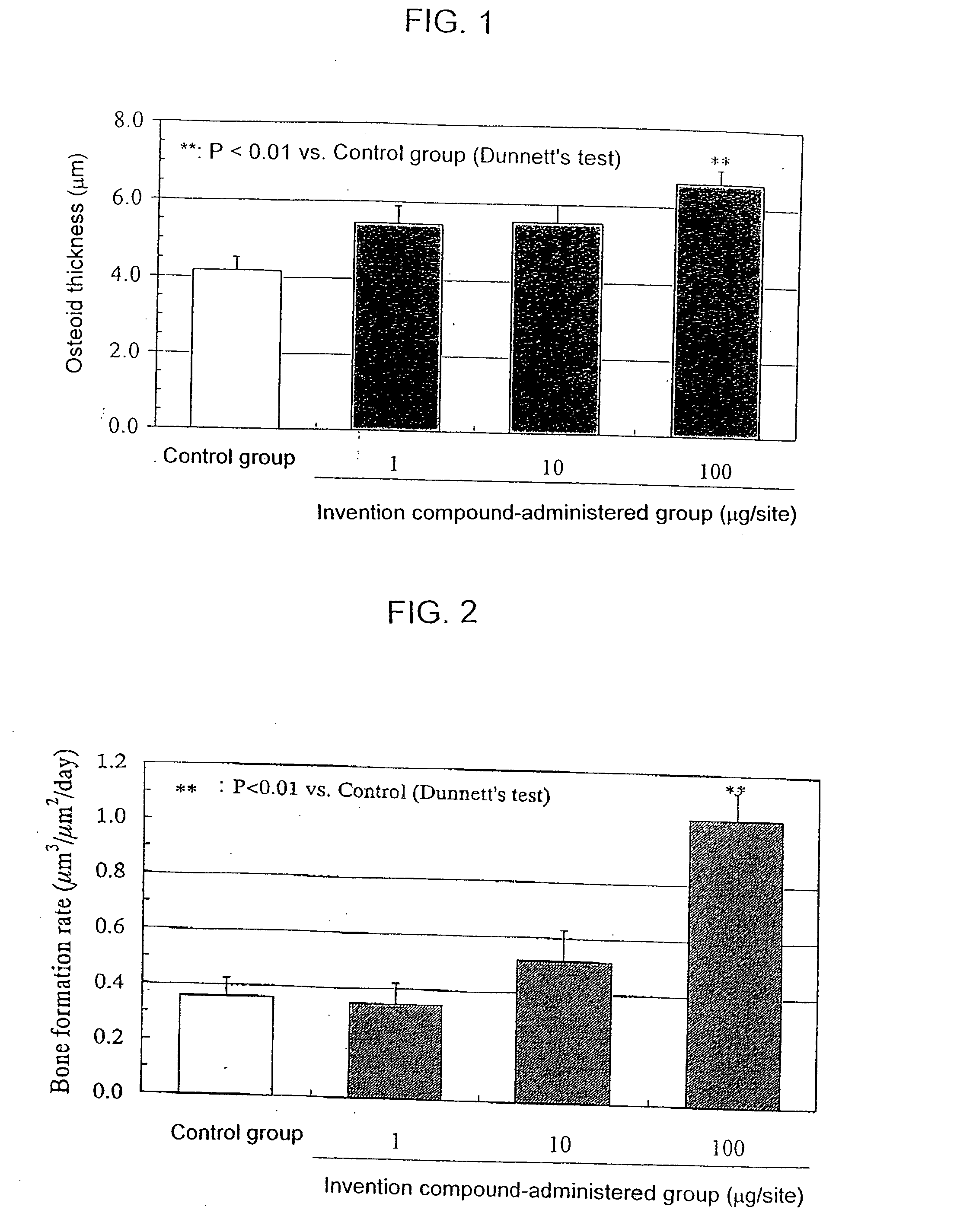
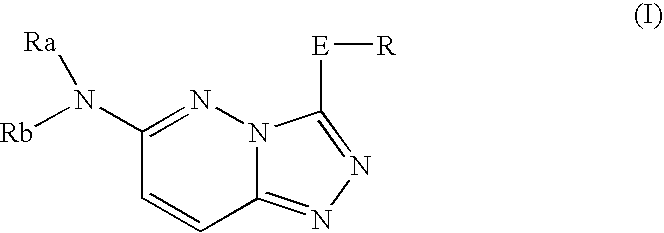
InactiveUS20050261297A1Excellent bone formation-stimulating effectIncrease ratingsBiocideOrganic chemistryPyridazineOsteoblast
As a result of an effort made by us for the purpose of developing a therapeutic agent having a bone formation-stimulating effect by promoting the functions of osteoblasts, the present inventors discovered that a certain nitrogen-containing heterocyclic compound exhibits a potent bone formation-stimulating effect on the osteoblast and thus can serve as an excellent prophylactic or therapeutic agent against a metabolic bone disease, whereby establishing the present invention. Thus, the present invention provides a 3,6-disubstituted 1,2,4-triazolo[4,3-b]pyridazine compound or a pharmaceutically acceptable salt thereof as well as a pharmaceutical composition comprising such a compound and a pharmaceutically acceptable carrier, especially a bone-forming agent.
Owner:ASTELLAS PHARMA INC
1,4-disubstituted pyridazine analogs there of and methods for treating SMN-deficiency-related conditions
Owner:NOVARTIS AG
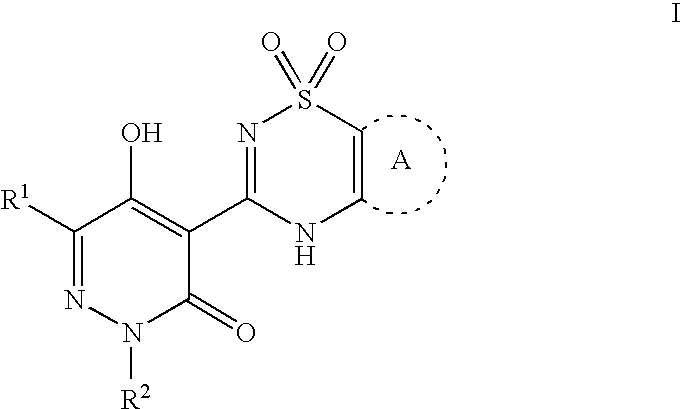
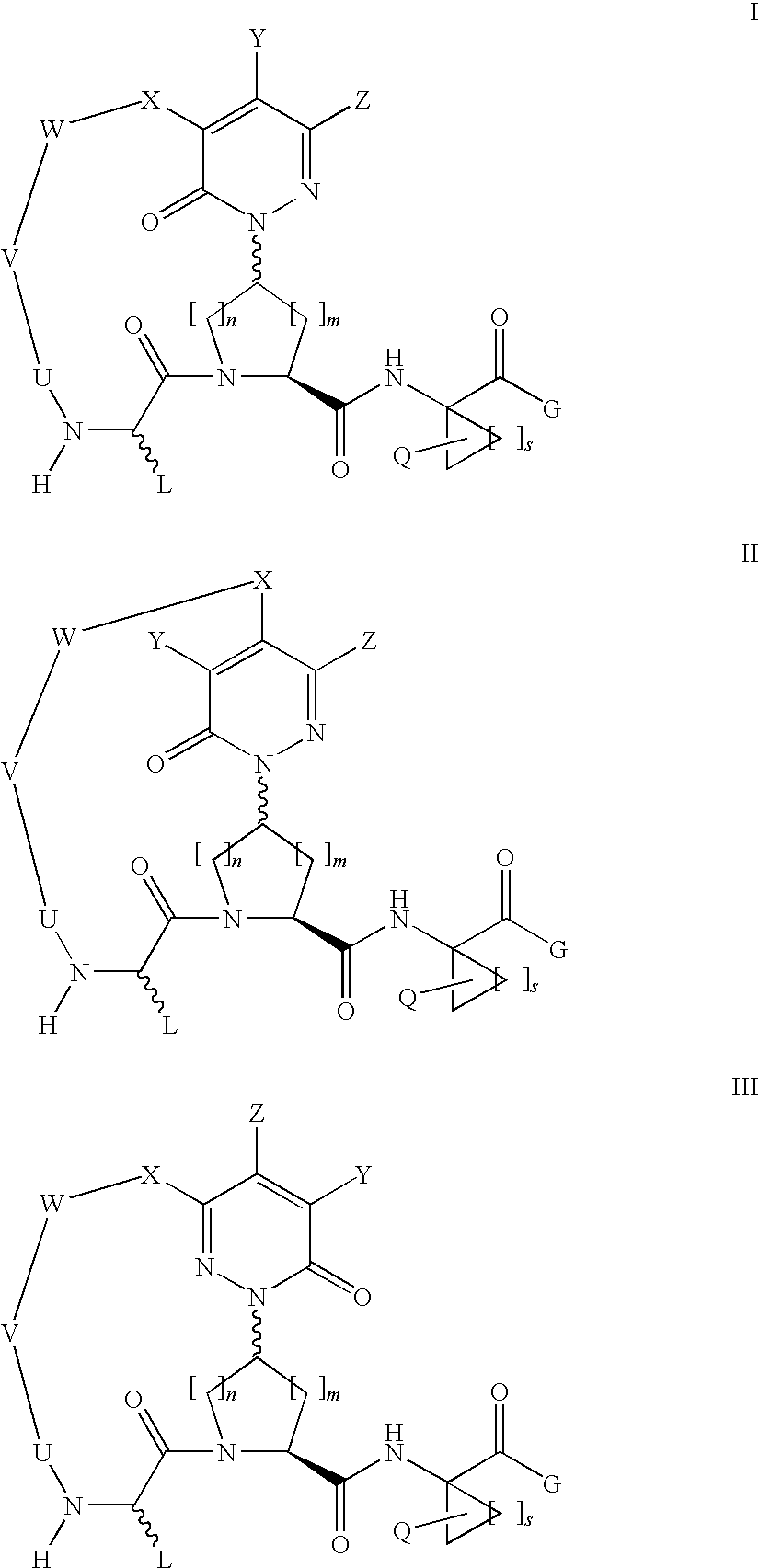
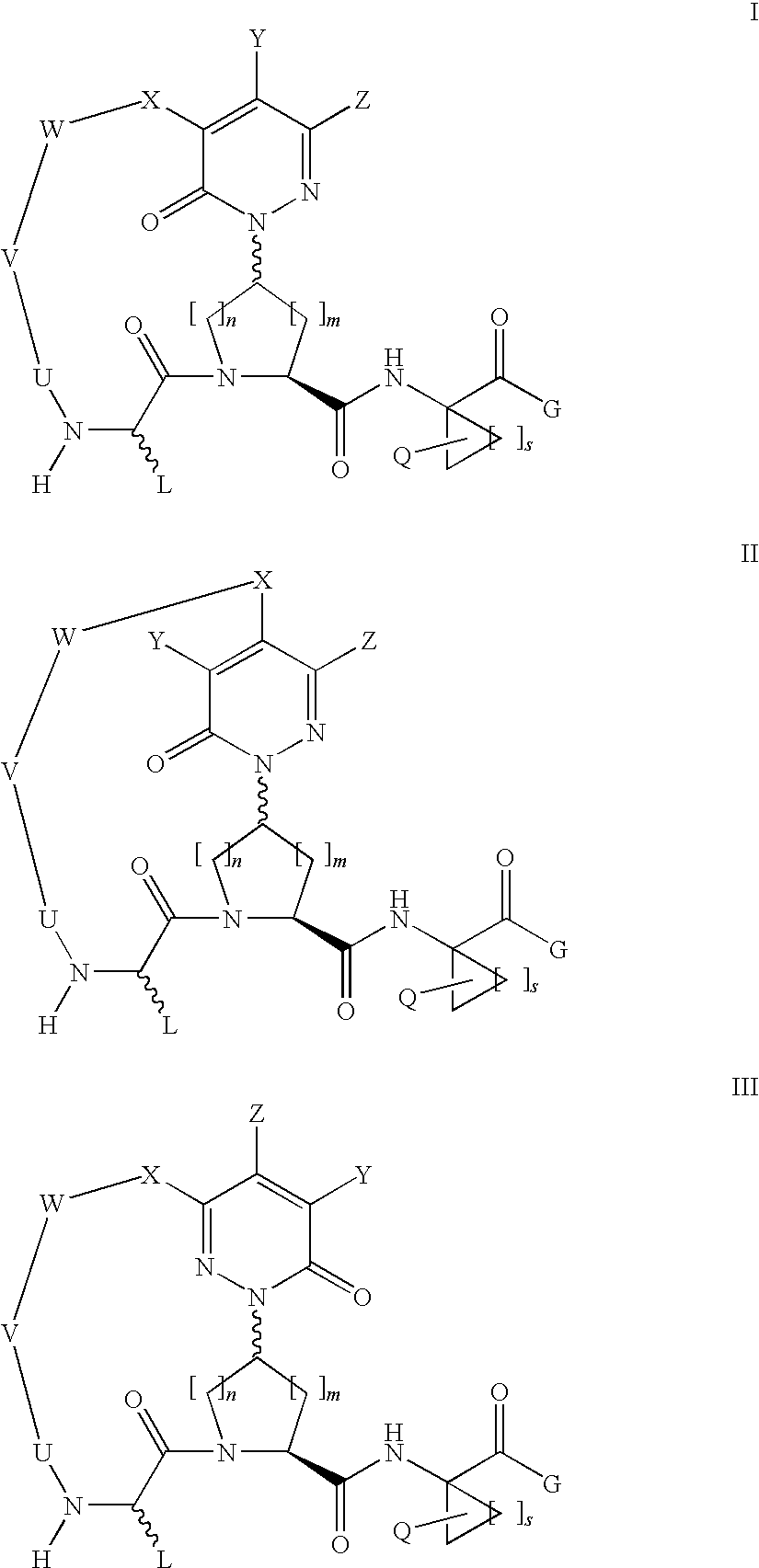
Macrocyclic, pyridazinone-containing hepatitis c serine protease inhibitors
The present invention relates to compounds of Formula I, II, or III, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising a compound of the present invention.
Owner:ENANTA PHARM INC
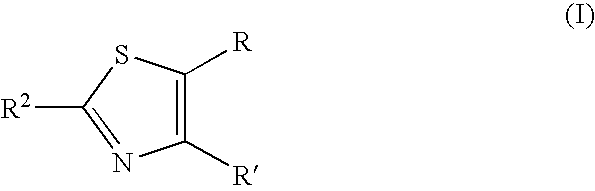
Thiazole derivative and pharmaceutical use thereof
InactiveUS20050004134A1High reactivityImprove solubilityNervous disorderOrganic chemistryArylHydrogen
A thiazole derivative of the formula (I): wherein R is a 1-optionally substituted-6-oxo-1,6-dihydro-3-pyridazinyl, R′ is an optionally substituted phenyl, and R2 is hydrogen, a group of the formula (i): wherein R4 is hydrogen, lower alkyl or lower alkenyl, and R5 is hydrogen, optionally substituted lower alkyl, acyl, cyclo(lower)alkyl, lower alkenyl, optionally substituted aryl or heterocyclic, or a group of the formula (ii): wherein X is oxygen or sulfur, R8 is hydrogen or lower alkyl, R9 is hydrogen, optionally substituted lower alkyl, cyclo(lower)alkyl, lower alkoxy or mono- or di-lower alkylamino or R8 and R9 may combine together to form optionally substituted saturated N-containing heterocyclic, or a salt thereof.
Owner:ASTELLAS PHARMA INC
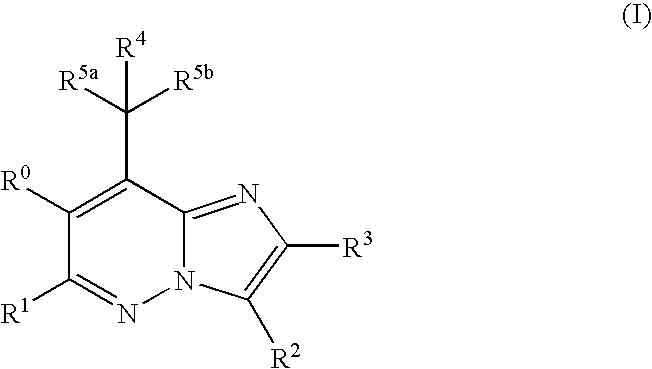
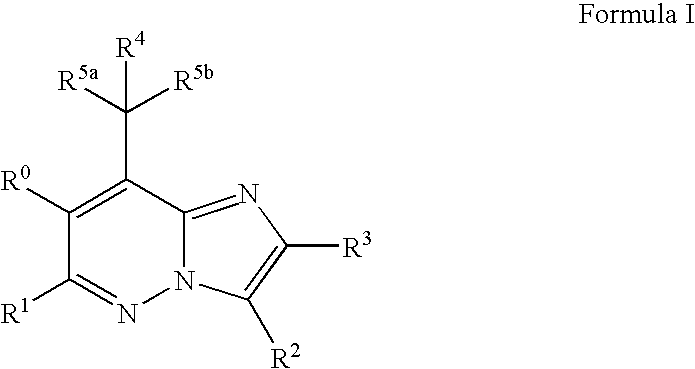
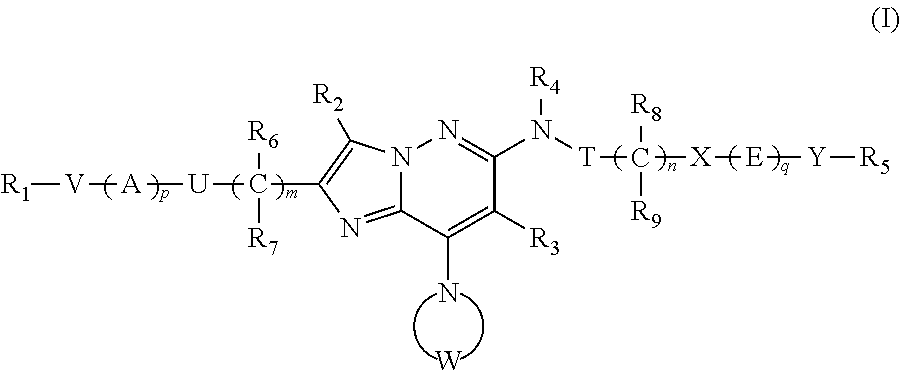
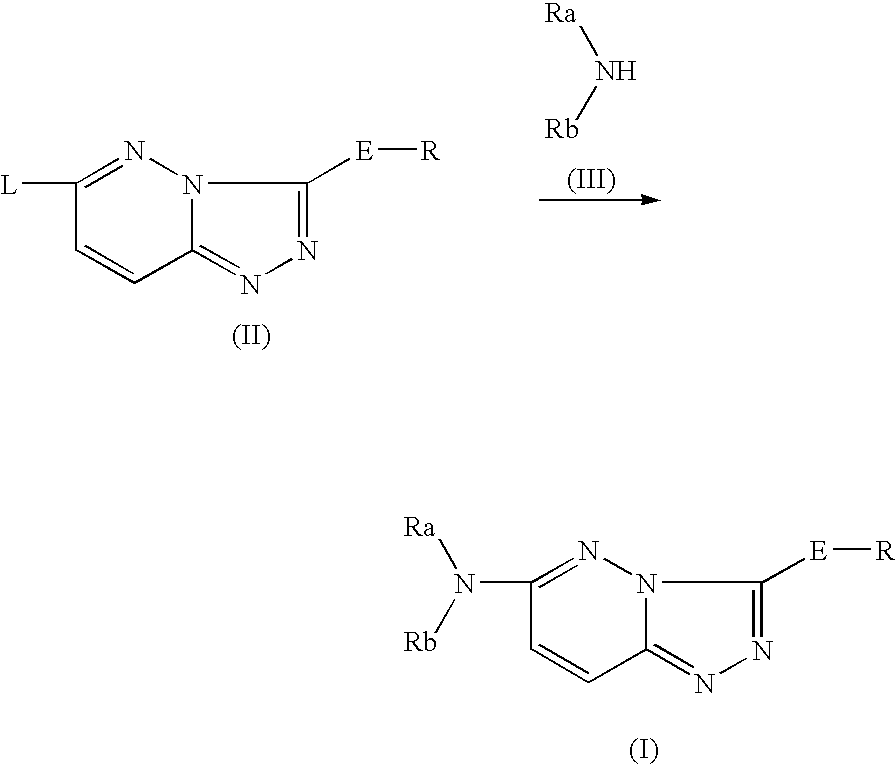
Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors
ActiveUS9187489B2Potent ROS kinase enzyme activity inhibitory effectInhibit cell growthOrganic active ingredientsOrganic chemistryPyridazineKinase
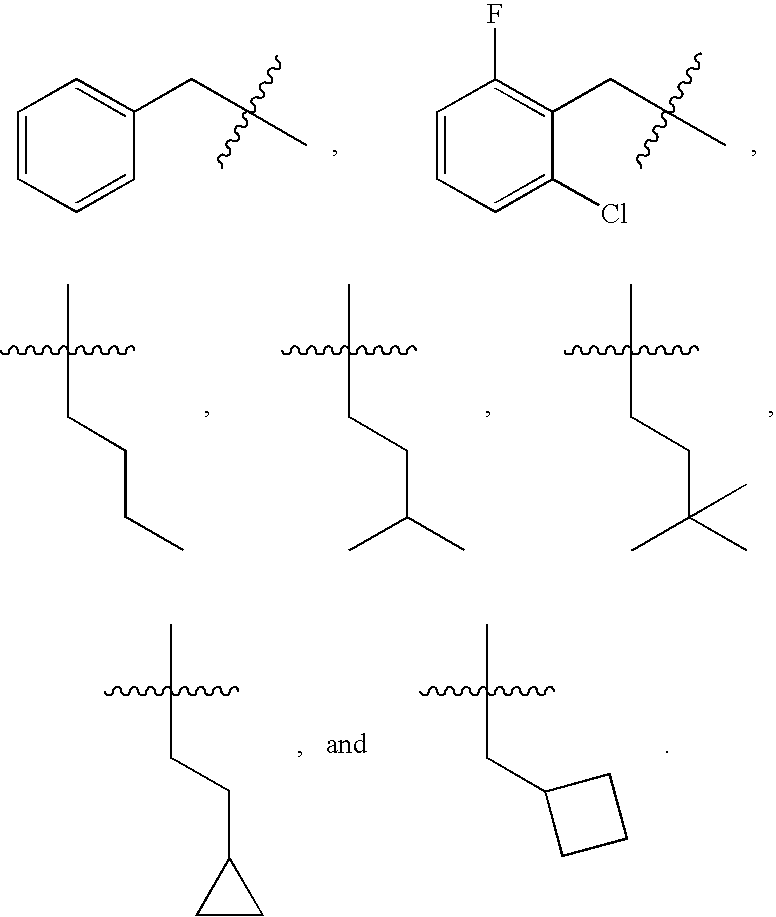
The present invention is intended to provide a compound or a pharmacologically acceptable salt thereof which is useful in the treatment of a tumor through its ROS1 kinase enzyme activity inhibitory effect and NTRK kinase enzyme inhibitory effect. The present invention provides a compound having an imidazo[1,2-b]pyridazine structure represented by the general formula (I) or a pharmacologically acceptable salt thereof, and a pharmaceutical composition comprising the compound. In the formula, R1, G, T, Y1, Y2, Y3, and Y4 are as defined herein.
Owner:DAIICHI SANKYO CO LTD
Compounds for immunopotentiation
InactiveUS20100226931A1Reduce capacityLow production costSsRNA viruses positive-senseSnake antigen ingredientsPyridazineErlotinib
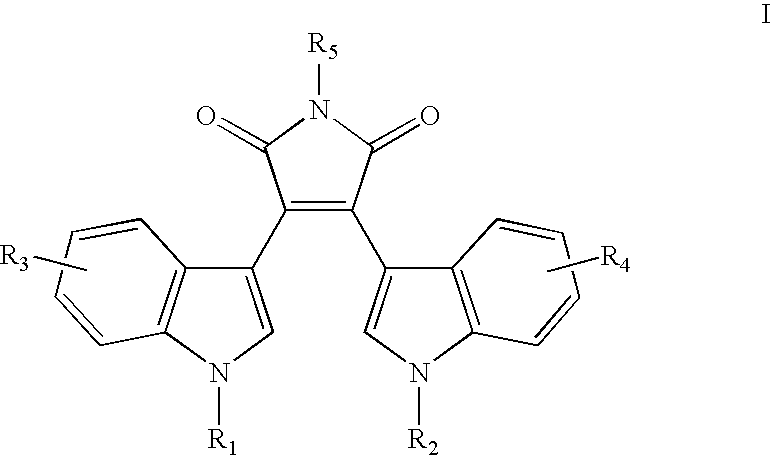
Methods of stimulating an immune response and treating patients responsive thereto with 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-diones, staurosporine analogs, derivatized pyridazines, chromen-4-ones, indolinones, quinazolines, nucleoside analogs, and other small molecules are disclosed. In a preferred embodiment benzopyrimidine derivatives such as ZD-6474, MLN-518, lapatinib, gefitinib or erlotinib are used.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
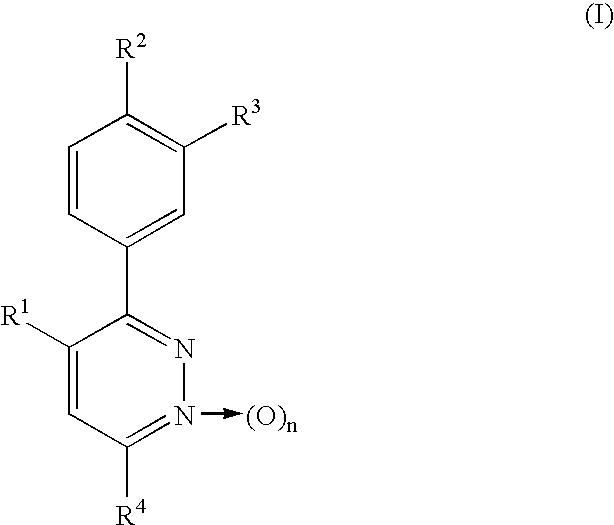
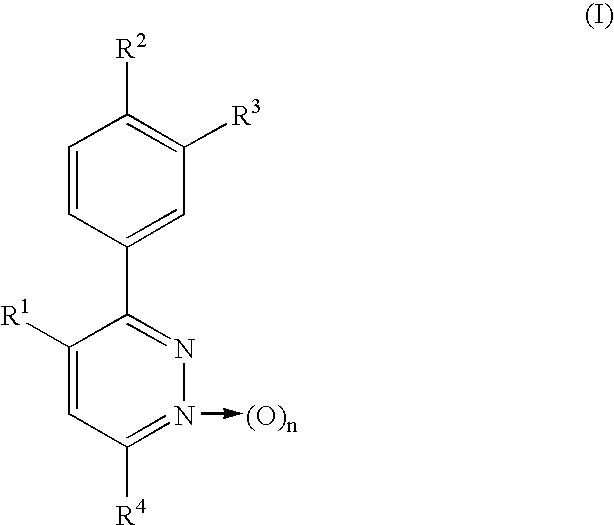
Phenylpyridazine compounds and medicines containing the same
InactiveUS6664256B1Excellent inhibitory activityStrong inhibitory activityBiocideOrganic chemistryIschemic diseaseWhite blood cell
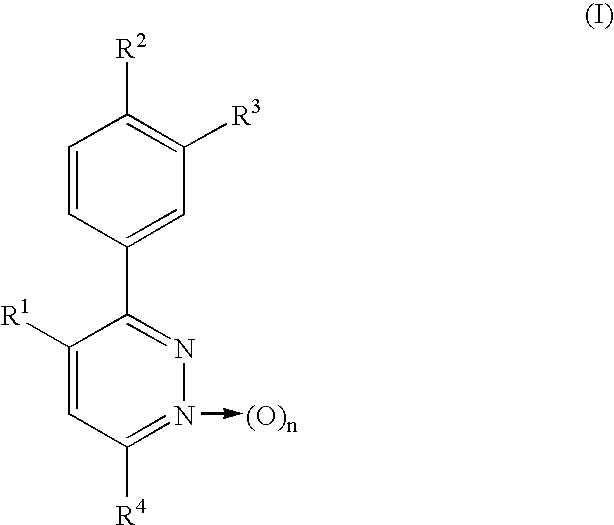
Phenylpyridazine compounds represented by the following formula (I): ##STR1## are provided, wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4, and n are as defined herein having excellent inhibitory activity against interleukin-1.beta. production, and useful in the treatment of prevention of diseases caused by stimulation of interleukin-1.beta. production, such as immune system diseases, inflammatory diseases, and ischemic diseases.
Owner:KOWA CO LTD
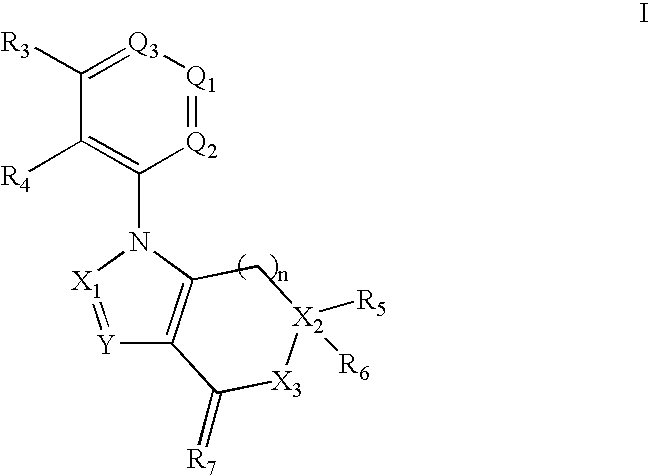
Benzene, Pyridine, and Pyridazine Derivatives
Disclosed are compounds and pharmaceutically acceptable salts of Formula Iwherein A, Q1, Q2, Q3, R31, and R41 are as defined herein. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer, inflammation, arthritis, angiogenesis, or the like. Also disclosed are pharmaceutical compositions comprising compounds of the invention and methods of treating the aforementioned conditions using such compounds.
Owner:SERENEX INC
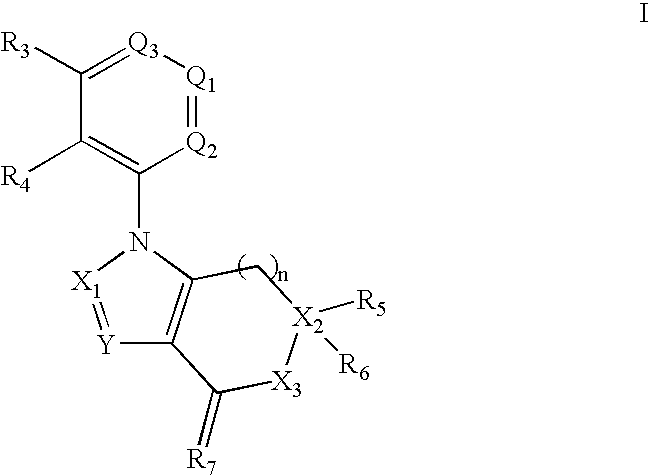
Substituted imidazo[1,2b]pyridazine compounds as trk kinase inhibitors
Compounds of formula (I): in which R1, R2, R3, R4, X, Y and n have the meanings given in the specification, are inhibitors of Trk kinases and are useful in the treatment of diseases which can be treated with a Trk kinase inhibitor. The formula (I) is shown in the description.
Owner:ARRAY BIOPHARMA
Carboxamide Derivatives and Use Thereof
The present disclosure provides substituted pyridyl-, pyrimidinyl-, pyrazinyl-, pyridazinyl-, and triazinyl-based carboxamides of Formula I-A: R10 Z-HET-E I-A and the pharmaceutically acceptable salts and solvates thereof, wherein Z, HET, R10 and E are defined as set forth in the specification. The present disclosure is also directed to the use of compounds of Formula I-A to treat a disorder responsive to the blockade of sodium channels. Compounds of the present disclosure are especially useful for treating pain.
Owner:PURDUE PHARMA LP
Imidazopyridazine compounds
The present invention relates to novel substituted imidazo[1,2-b]pyridazine compounds of Formula (I): pharmaceutical compositions thereof, and the use such compounds as corticotropin releasing factor 1 (CRF1) receptor antagonists in the treatment of psychiatric disorders and neurological diseases.
Owner:ELI LILLY & CO
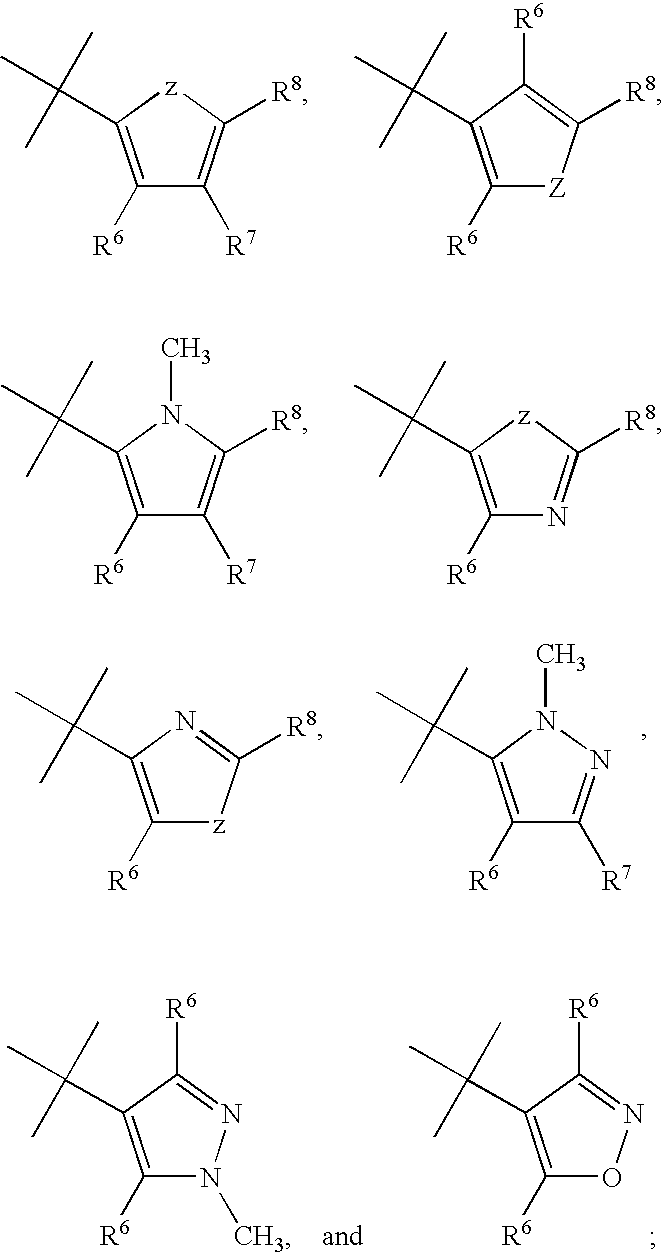
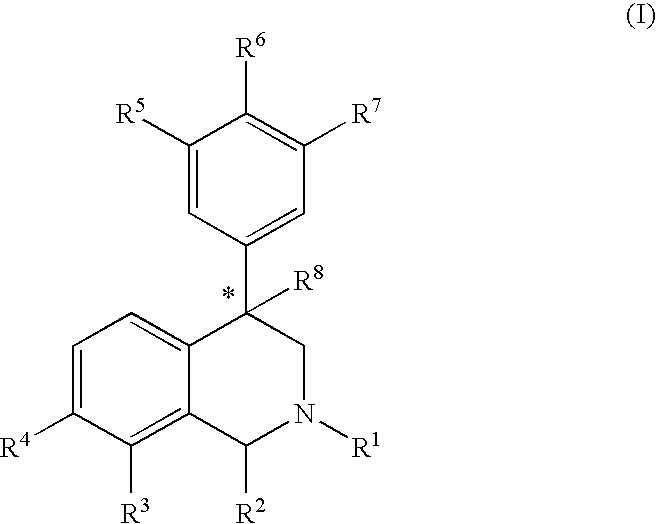
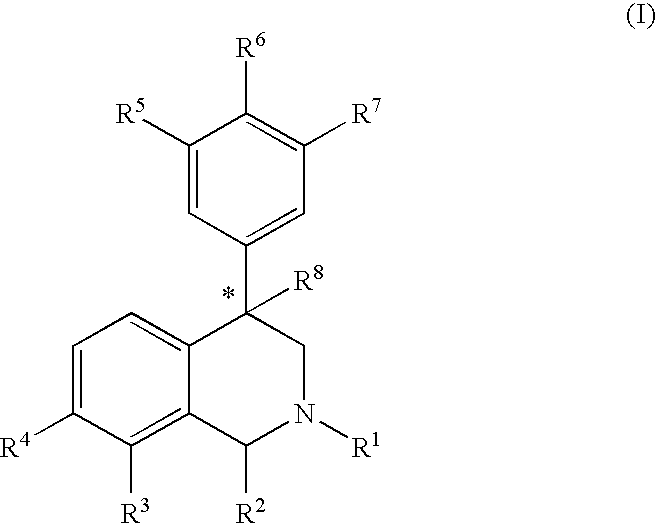
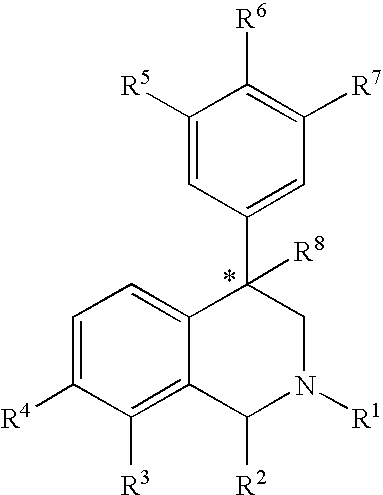
Aryl and heteroaryl substituted tetrahydroisoquinolines and use thereof
Provided herein are compounds of the formula (I): wherein R1-R8 are as described herein, R4 being phthalazinyl, pyrazinyl, pyridazinyl, or quinoxalinyl. Such compounds are particularly useful in the treatment of a disorder which is created by or is dependent upon decreased availability of serotonin, norepinephrine, or dopamine.
Owner:ALBANY MOLECULAR RESEARCH INC
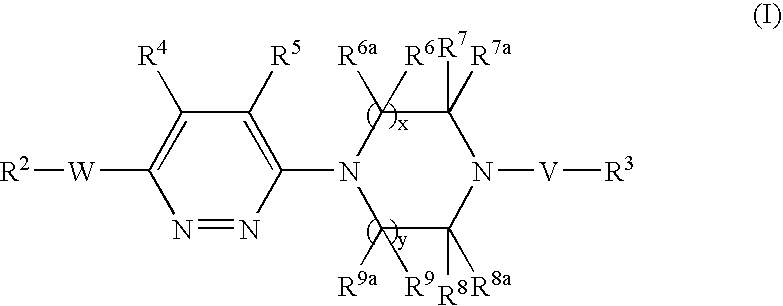
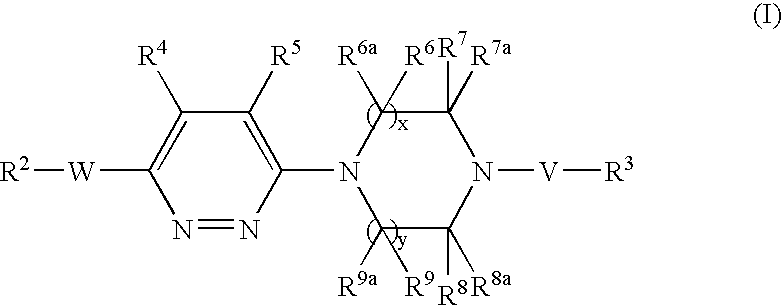
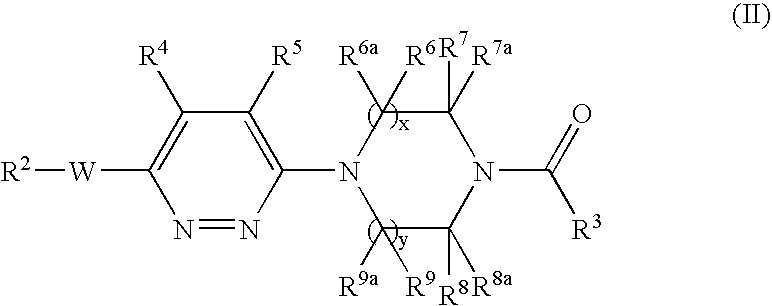
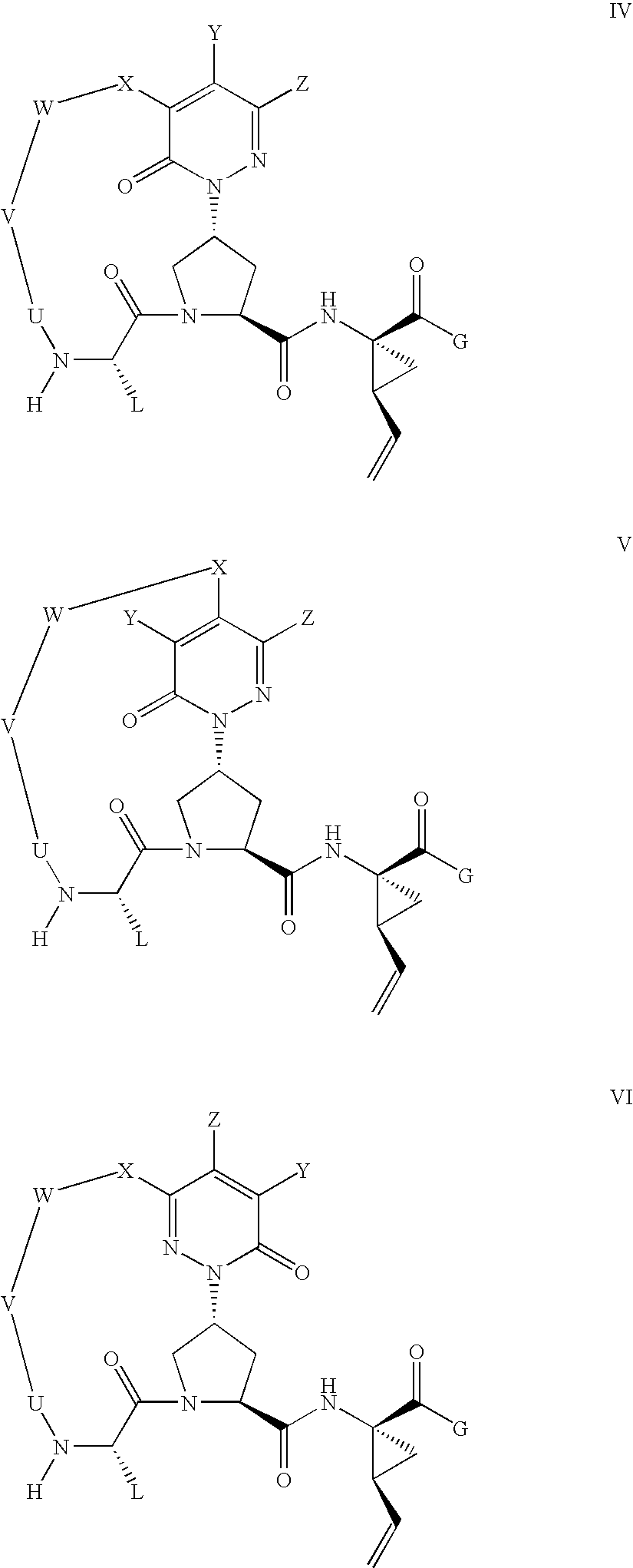
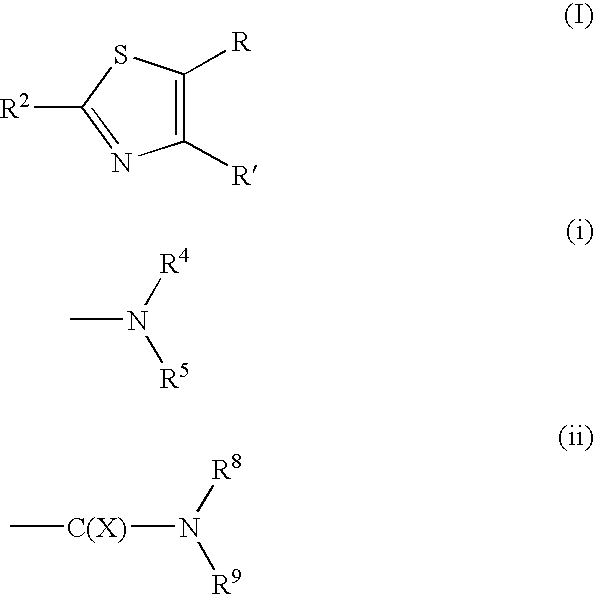
Pyridazine derivatives and their use as therapeutic agents
InactiveUS20050065143A1Lower blood lipid levelsEffective to modulate triglyceride levelBiocideOrganic chemistryDisease causeStereochemistry
Methods of treating an SCD-mediated disease or condition in a mammal, preferably a human, are disclosed, wherein the methods comprise administering to a mammal in need thereof a compound of formula (I): where x, y, W, V, R2, R3, R4, R5, R6, R6a, R7, R7a, R8, R8a, R9 and R9a are defined herein. Pharmaceutical compositions comprising the compounds of formula (I) are also disclosed.
Owner:XENON PHARMACEUTICALS INC
Condensed pyridazine derivatives, their production and use
A condensed pyridazine derivative which is useful as a pharmaceutical composition for preventing or treating allergic skin diseases such as contact dermatitis, pruritus, dried dermatitis, acute urticaria and prurigo.
Owner:TAKEDA PHARMA CO LTD
Pharmaceutical composition
InactiveUS20050220881A1High dissolution rateImproves dissolution and dissolution rateBiocidePowder deliveryPolyethylene-polypropylene glycolPharmacology
The present invention provides a composition including an association complex of a pharmaceutical composition and one or more polyethylene-polypropylene glycol block co-polymers (poloxamers). The pharmaceutical composition may include a member selected from the group consisting of (a) an association complex of an eprosartan composition including eprosartan or a pharmaceutically acceptable salt of eprosartan and (b) the non-zwitterionic compound 2-(7-chloro-5-methyl-4-oxo-3-phenyl-4,5 dihydro-3H-pyridazino (4,5-b)indol-1-yl)-N,N-dimethylacetamide (NZ).
Owner:BVM HLDG
Chlorofluorobenzene liquid crystal compound, liquid crystal composition and liquid crystal display device
ActiveUS20080075891A1Liquid crystal compositionsOrganic compound preparationCrystallographyPyridazine
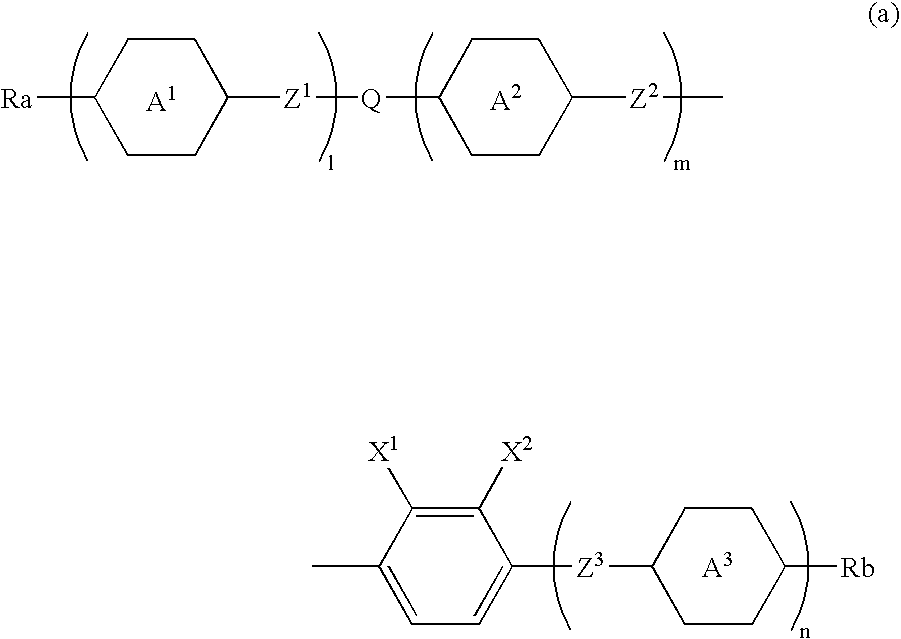
A liquid crystal compound selected from a group of compounds represented by formula (a): wherein Ra and Rb are each independently alkyl having 1 to 10 carbons, alkoxy having 1 to 9 carbons, alkoxyalkyl having 2 to 9 carbons, alkenyl having 2 to 10 carbons, alkenyloxy having 2 to 9 carbons, fluoroalkyl having 1 to 10 carbons or fluoroalkoxy having 1 to 9 carbons; ring A1, ring A2 and ring A3 are each independently trans-1,4-cyclohexylene, trans-1,3-dioxane-2,5-diyl, trans-tetrahydropyran-2,5-diyl, 1,4-phenylene, 2-fluoro-1,4-phenylene, 2,3-difluoro-1,4-phenylene, 2,5-difluoro-1,4-phenylene, 2,6-difluoro-1,4-phenylene, pyridine-2,5-diyl, 6-fluoropyridine-2,5-diyl or pyridazine-2,5-diyl; Z1, Z2 and Z3 are each independently a single bond, —(CH2)2—, —CH═CH—, —C≡C—, —CH2O, —OCH2—, —COO—, —OCO—, —OCF2—, —CF2O—, —CH2CH2OCF2— or —CF2OCH2CH2—; l, m and n are each independently 0, 1 or 2, provided that l+m+n is 0, 1, 2 or 3; one of X1 and X2 is fluorine, and the other is chlorine; Q is and atoms comprising the compound each may be an isotope thereof.
Owner:JNC PETROCHEM +1
Imidazopyridazine compounds
InactiveUS20120323002A1High selectivityImprove medicinal effectAntibacterial agentsOrganic active ingredientsDiseasePharmacology
Owner:AJINOMOTO CO INC
Pyridazinones, the preparation and the use thereof
InactiveUS20110112061A1High activitySignificant effectBiocideOrganic chemistryMedicineHepatic Cancers
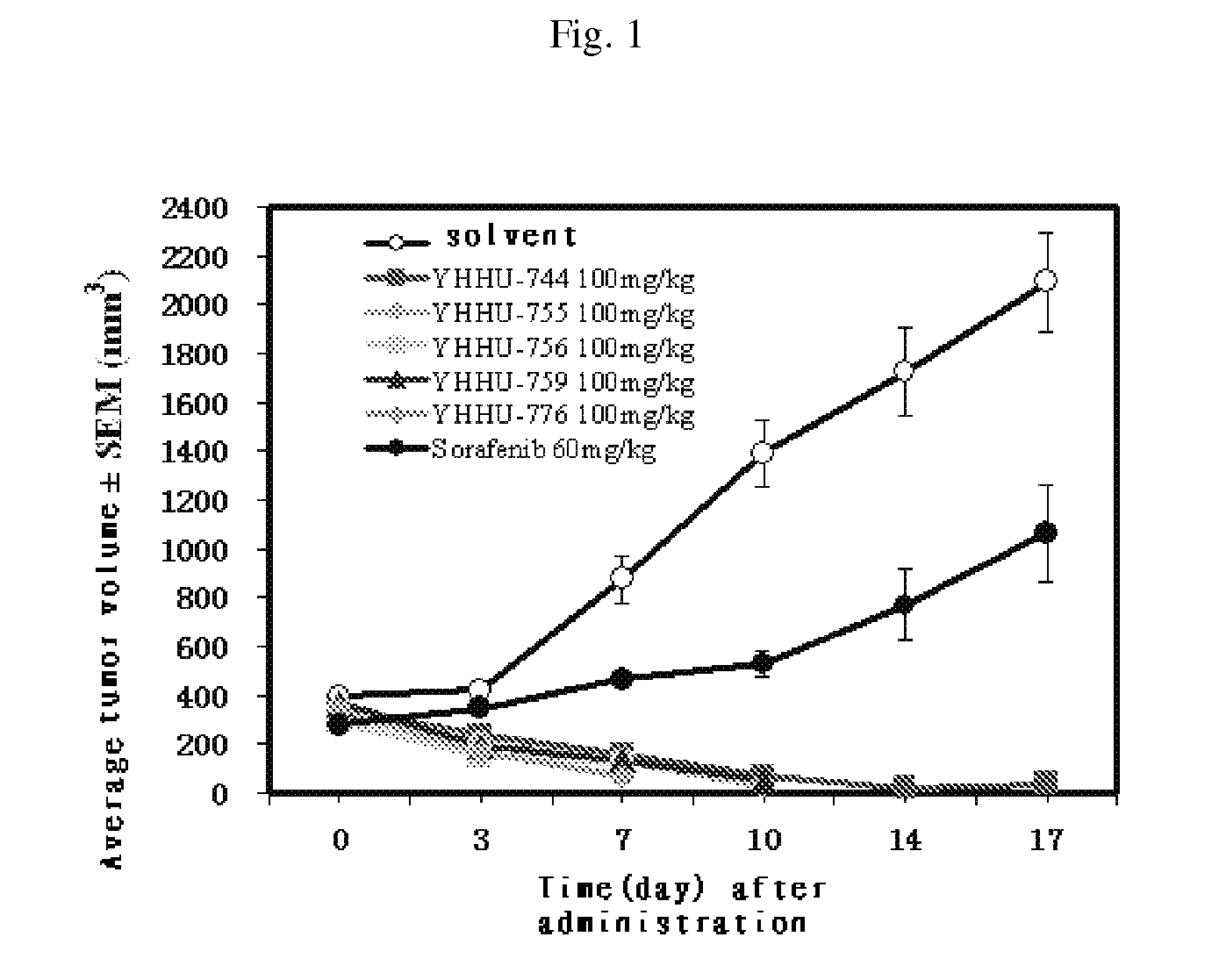
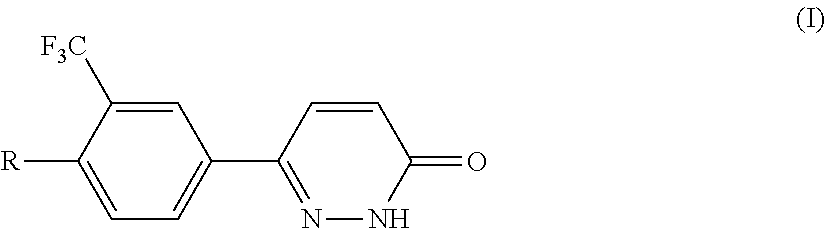
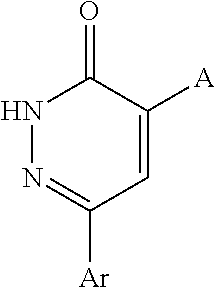
The present invention relates to a class of pyridazinones of formula I, which comprises 6-[3-(trifluoromethyl)phenyl]pyridazin-3(2H)-one as a mother nucleus, the preparation method thereof and the use thereof in manufacturing medicaments against tumors, especially liver cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
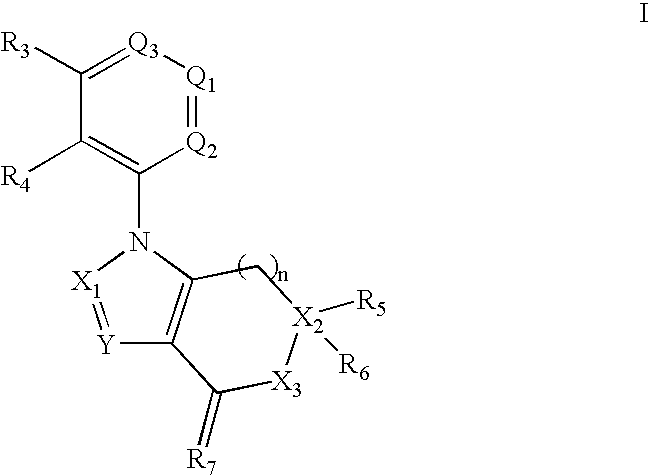
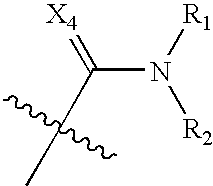
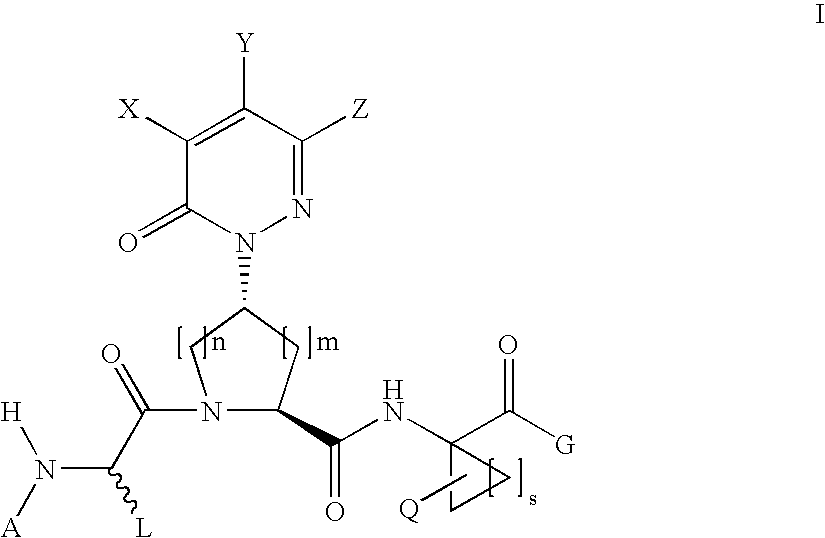
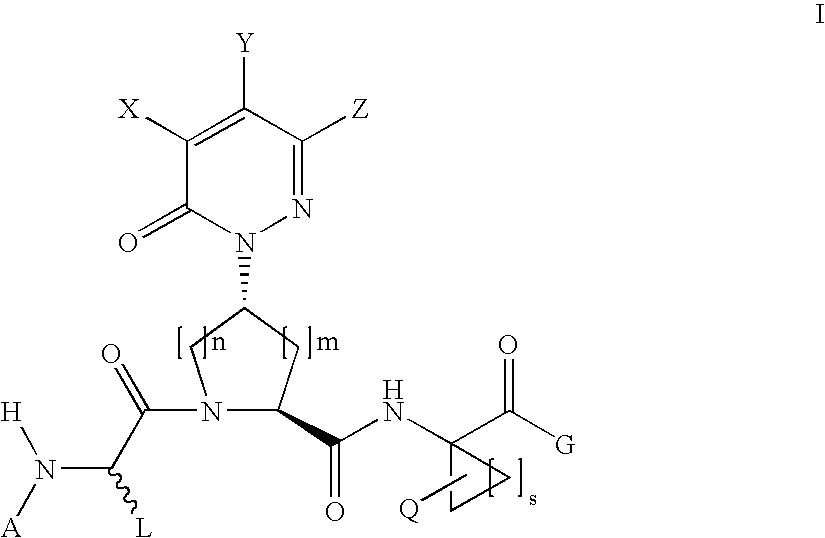
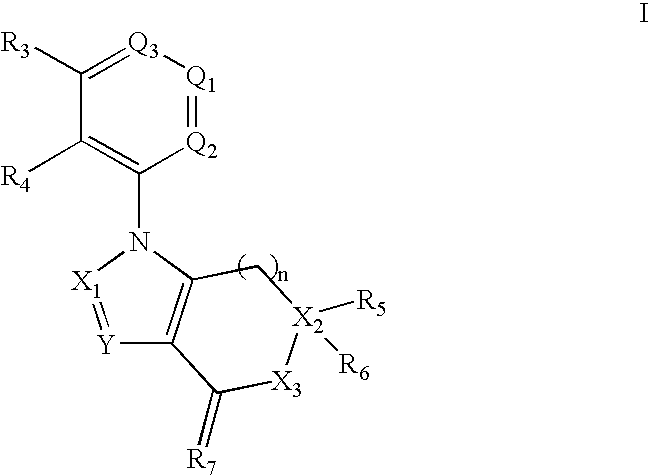
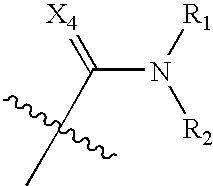
Benzene, pyridine, and pyridazine derivatives
Disclosed are compounds and pharmaceutically acceptable salts of Formula Iwherein R1, R2, R3, R4, R5, R6, R7, n, Q1, Q2, Q3, Y, and X1-X4 are as defined herein. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer, inflammation, arthritis, angiogenesis, or the like. Also disclosed are pharmaceutical compositions comprising compounds of the invention and methods of treating the aforementioned conditions using such compounds.
Owner:ESANEX INC
Acyclic, pyridazinone-derived hepatitis c serine protease inhibitors
InactiveUS20090035267A1Inhibit serine protease activityInhibitory activityBiocidePeptide/protein ingredientsPyridazinePharmaceutical medicine
The present invention relates to compounds of Formula I, or pharmaceutically acceptable salts, esters, or prodrugs thereof, which can inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising a compound of the present invention.
Owner:ENANTA PHARM INC
Benzene, pyridine, and pyridazine derivatives
Disclosed are compounds and pharmaceutically acceptable salts of Formula I wherein R1, R2, R3, R4, R5, R6, R7, n, Q1, Q2, Q3, Y, and X1-X4 are as defined herein. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer, inflammation, arthritis, angiogenesis, or the like. Also disclosed are pharmaceutical compositions comprising compounds of the invention and methods of treating the aforementioned conditions using such compounds.
Owner:ESANEX
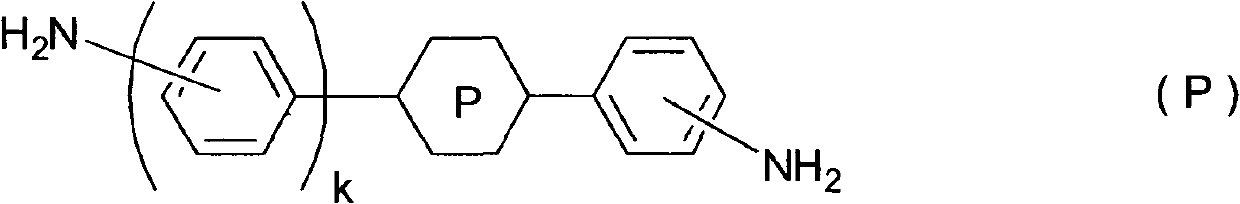
Liquid crystal aligning agent, liquid crystal aligning film and liquid crystal display unit
InactiveCN102154018AResidual DC is lowLiquid crystal compositionsNon-linear opticsCrystallographyLiquid-crystal display
The invention relates to a liquid crystal aligning agent, a liquid crystal aligning film and a liquid crystal display unit. The liquid crystal aligning film with less residual DC can be obtained through the liquid crystal aligning agent containing following polyamide acids which is obtained from diamines represented by the formula (P) or from reaction between mixture of diamines represented by the formula (P) and other diamines and tetracaboxylic dianhydride. Moreover, the liquid crystal display unit without burn-in can be obtained through the liquid crystal aligning film. Ring P is pyridine-2, 5-diyl, pyridazine-3,6-diyl, pyrimidine-2, 5-diyl or pyrazine-2, 5-diyl, and K equals 0 or 1, wherein when K equals 0, ring P is pyridine-2, 5-diyl, pyrimidine-2, 5-diyl, and the amino bonding position in the ring is 5.
Owner:JNC CORP +1
Pyridazinone-amides derivatives
Owner:MERCK PATENT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00001.png)
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00002.png)
![Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors Imidazo[1,2-beta]pyridazine and pyrazolo[1,5-alpha]pyrimidine derivatives and their use as protein kinase inhibitors](https://images-eureka.patsnap.com/patent_img/7d3e1e07-4aea-462e-858e-a93a49cbb310/US07750007-20100706-D00003.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00001.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00002.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00003.png)
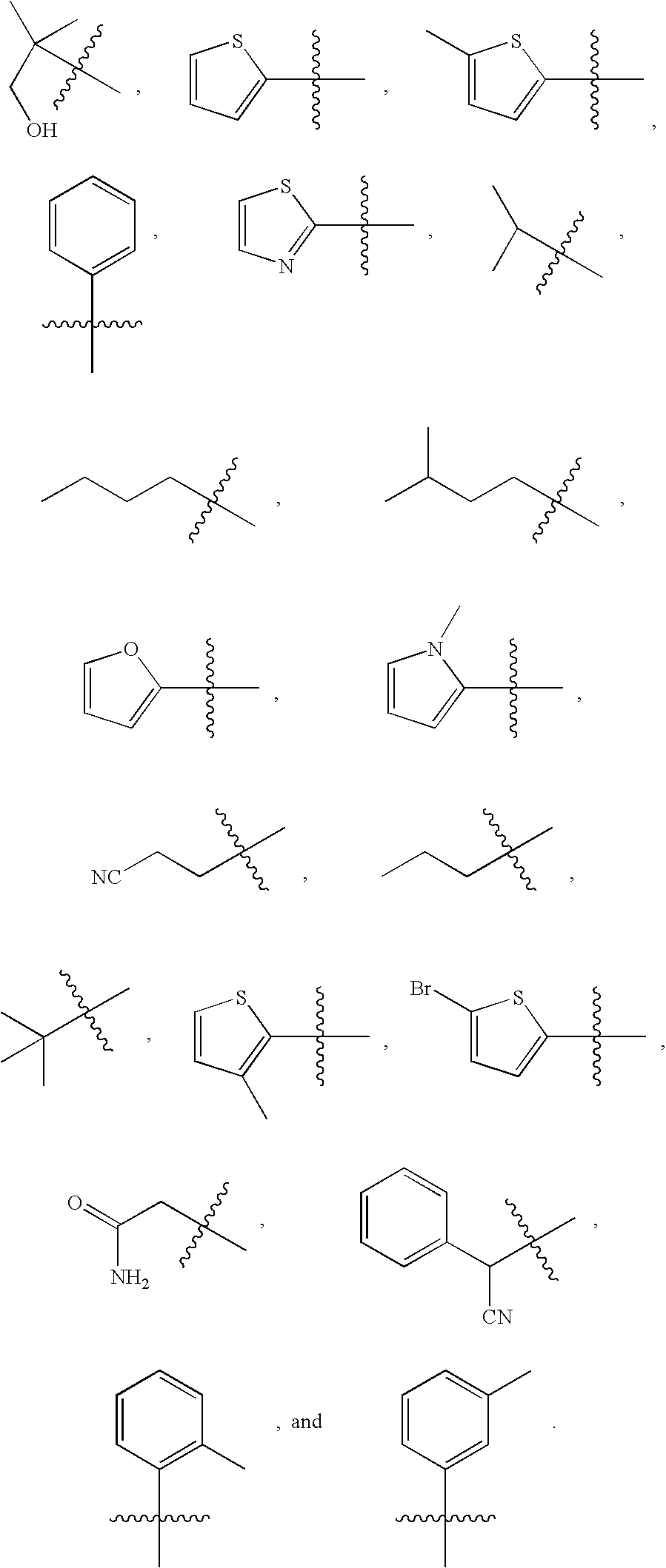
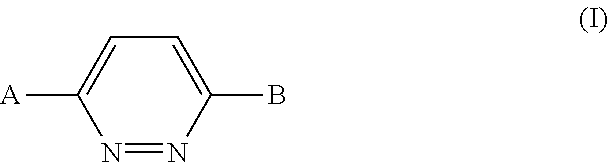
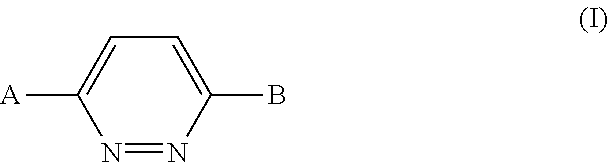
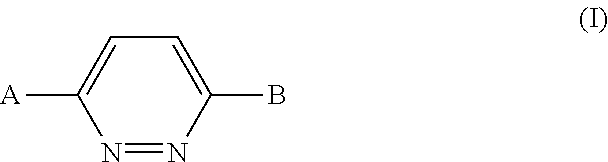

![Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors](https://images-eureka.patsnap.com/patent_img/87e6c50b-ff7e-4ff3-866e-b04bf55482cd/US09187489-20151117-C00001.PNG)
![Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors](https://images-eureka.patsnap.com/patent_img/87e6c50b-ff7e-4ff3-866e-b04bf55482cd/US09187489-20151117-C00002.PNG)
![Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors Imidazo[1,2-b]pyridazine derivatives as kinase inhibitors](https://images-eureka.patsnap.com/patent_img/87e6c50b-ff7e-4ff3-866e-b04bf55482cd/US09187489-20151117-C00003.PNG)