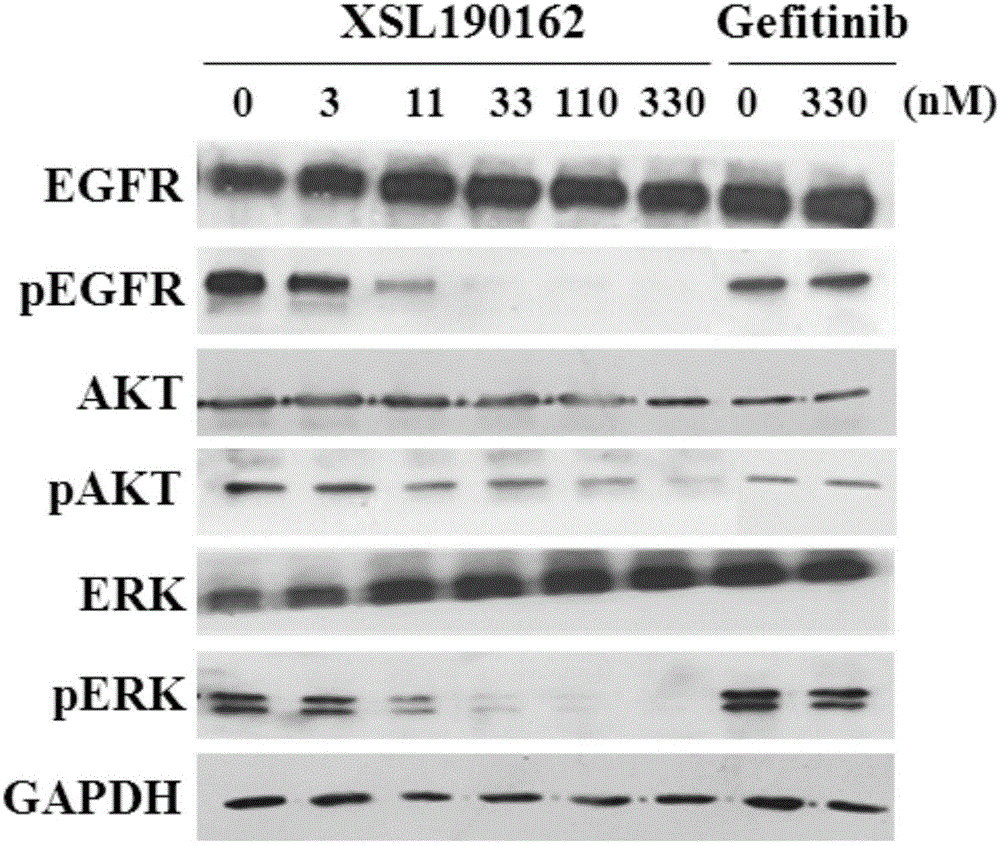
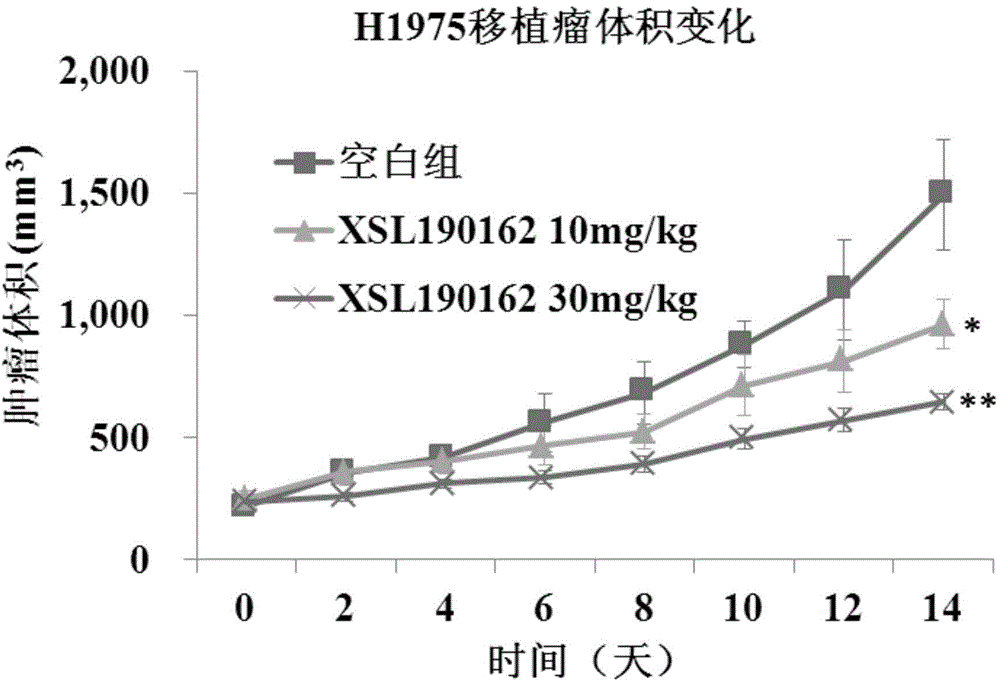
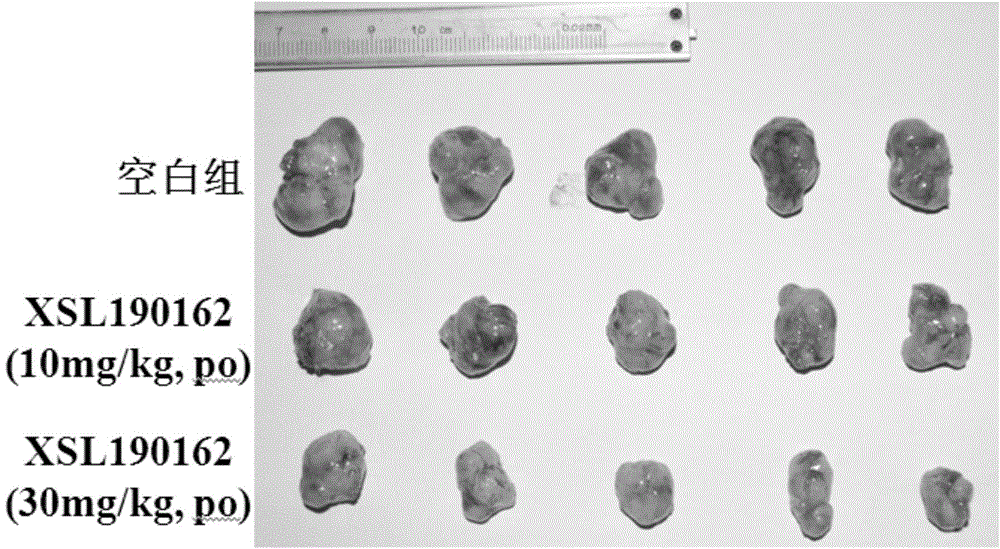
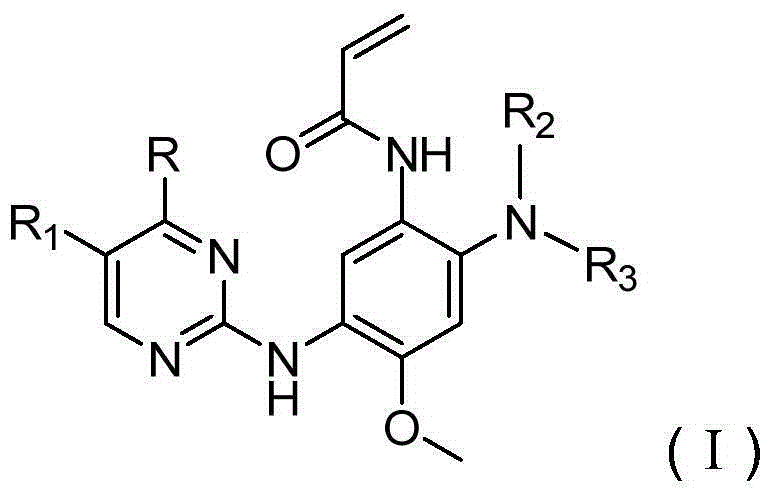
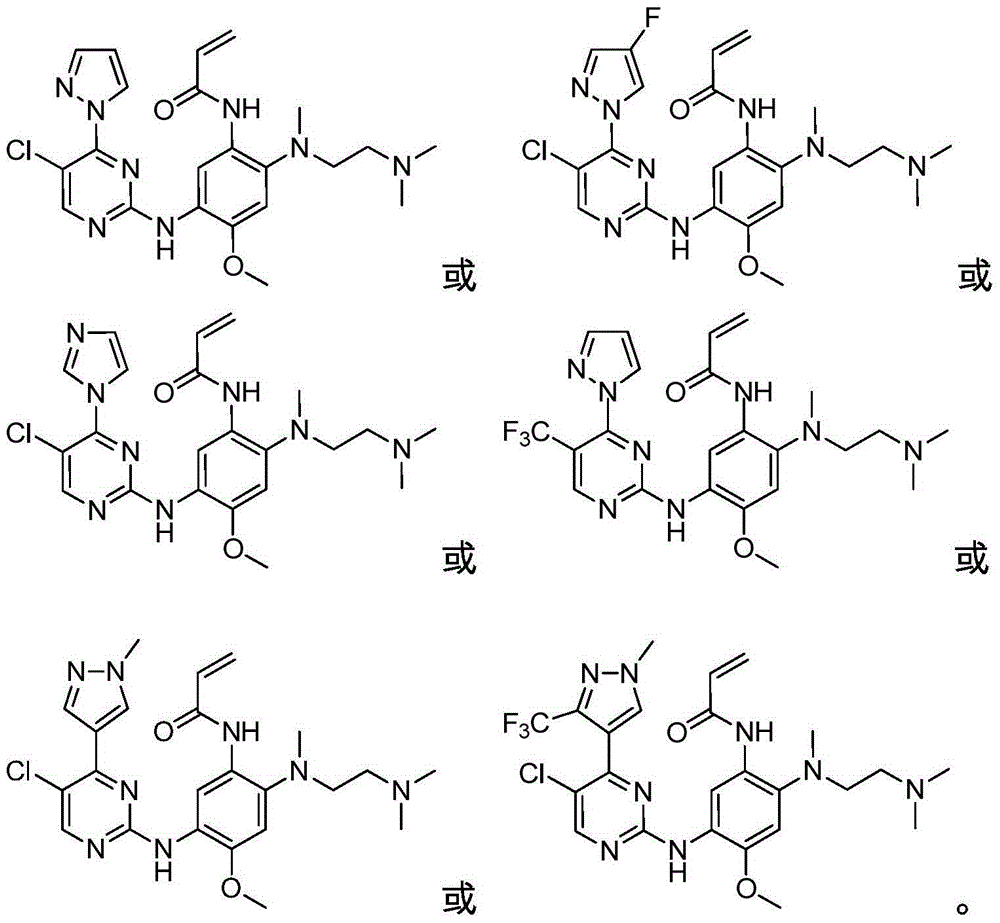
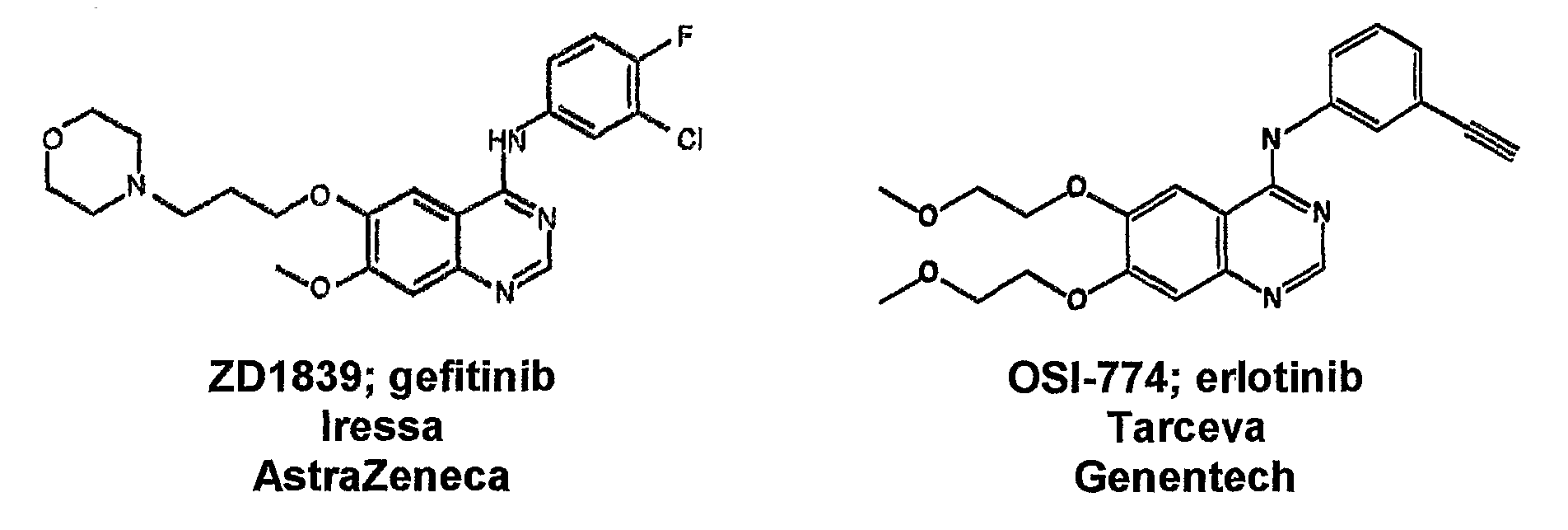
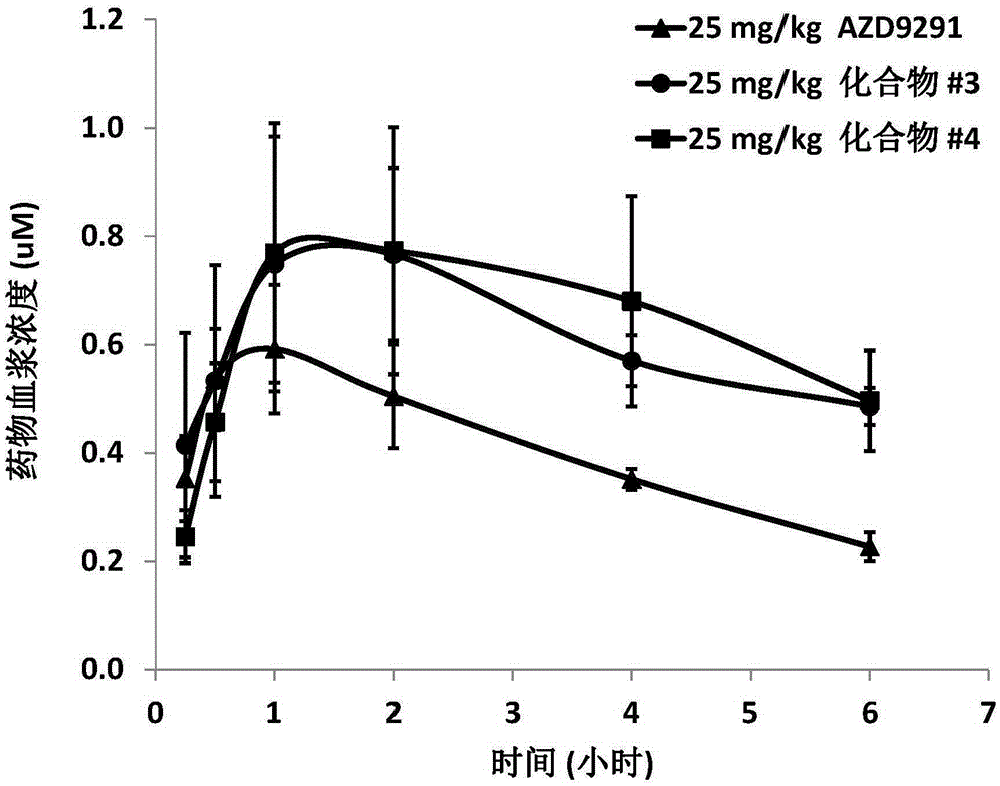
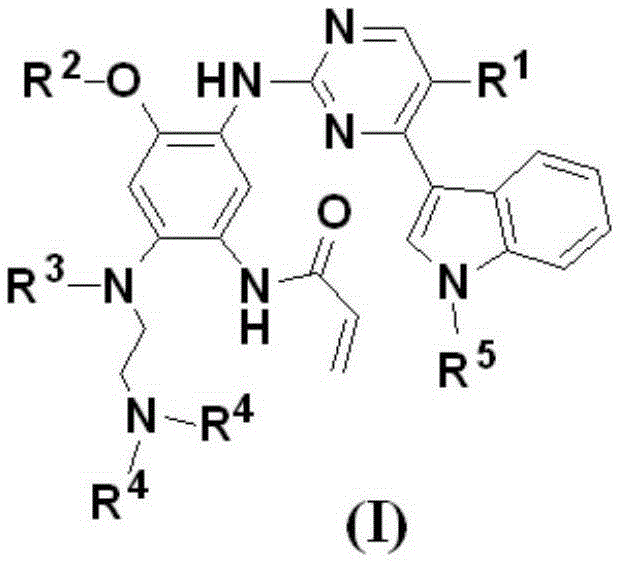
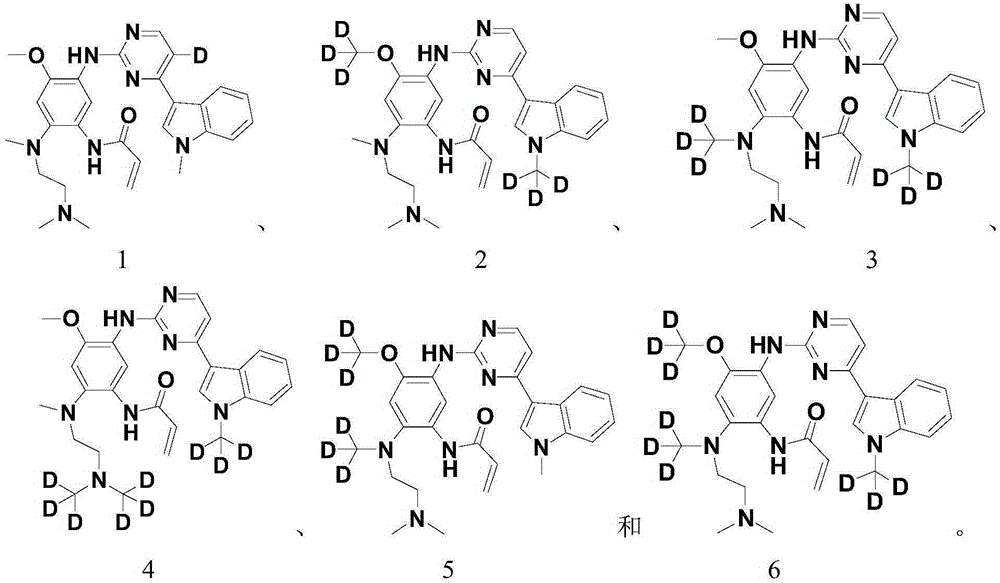
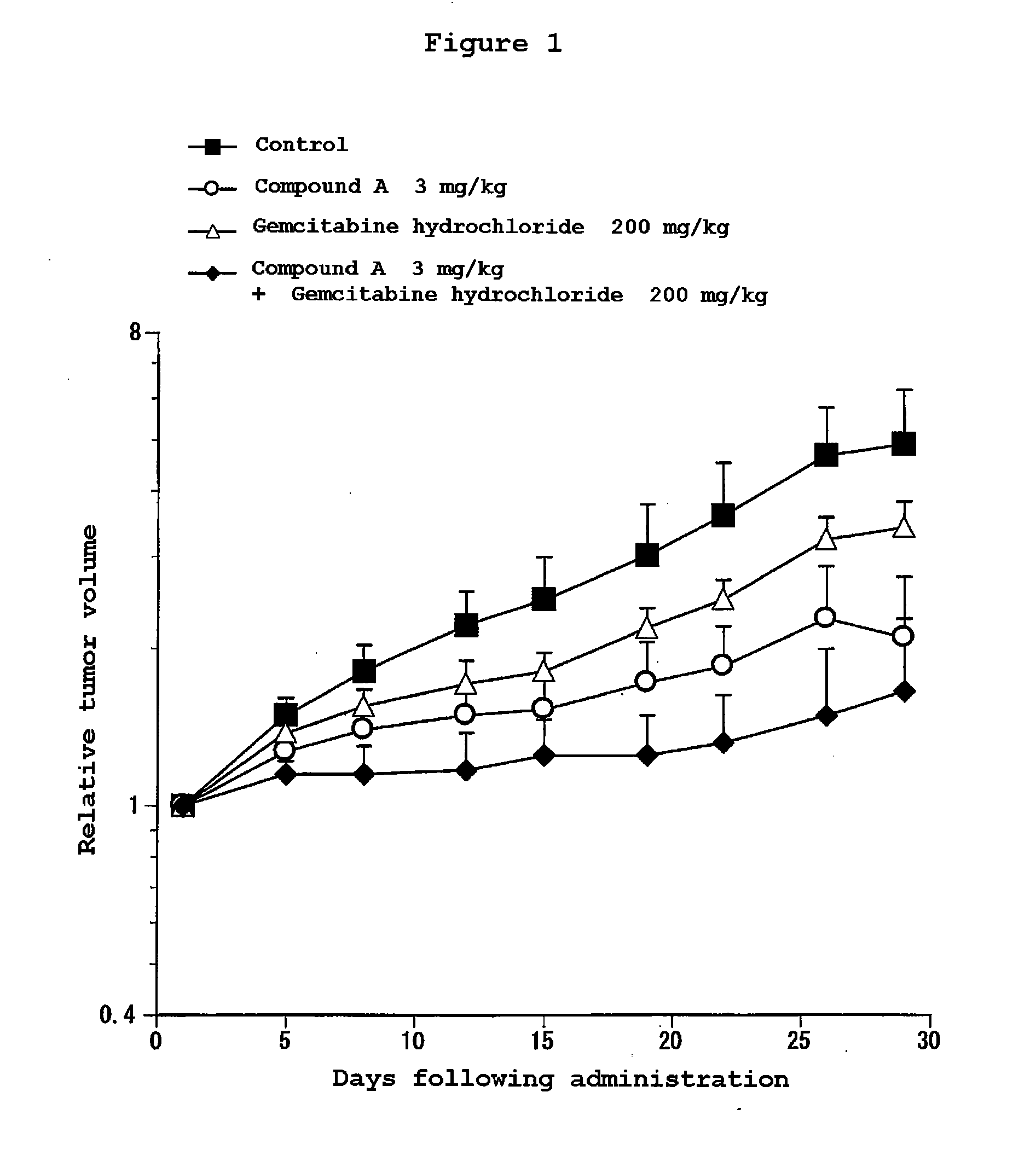
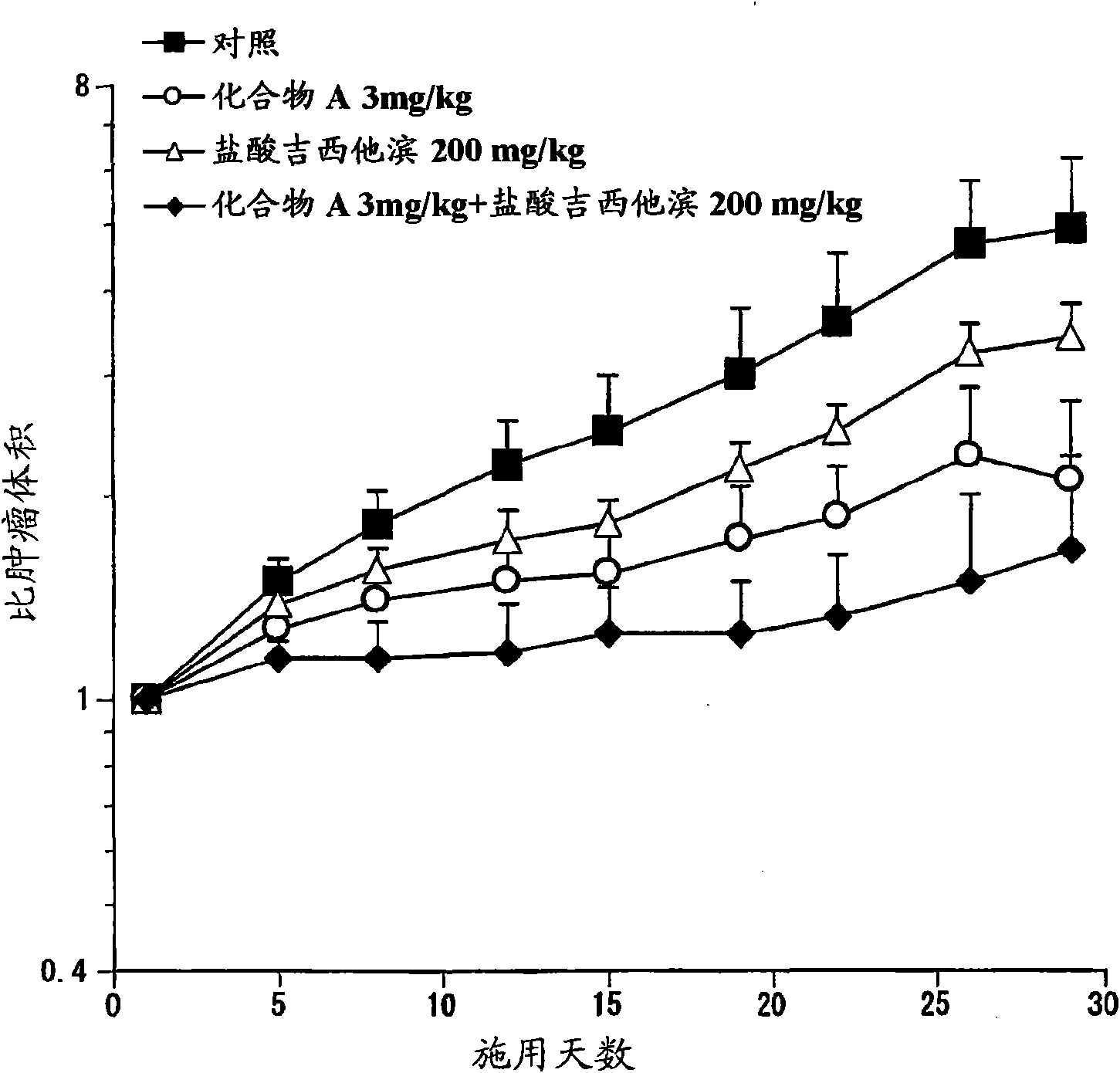
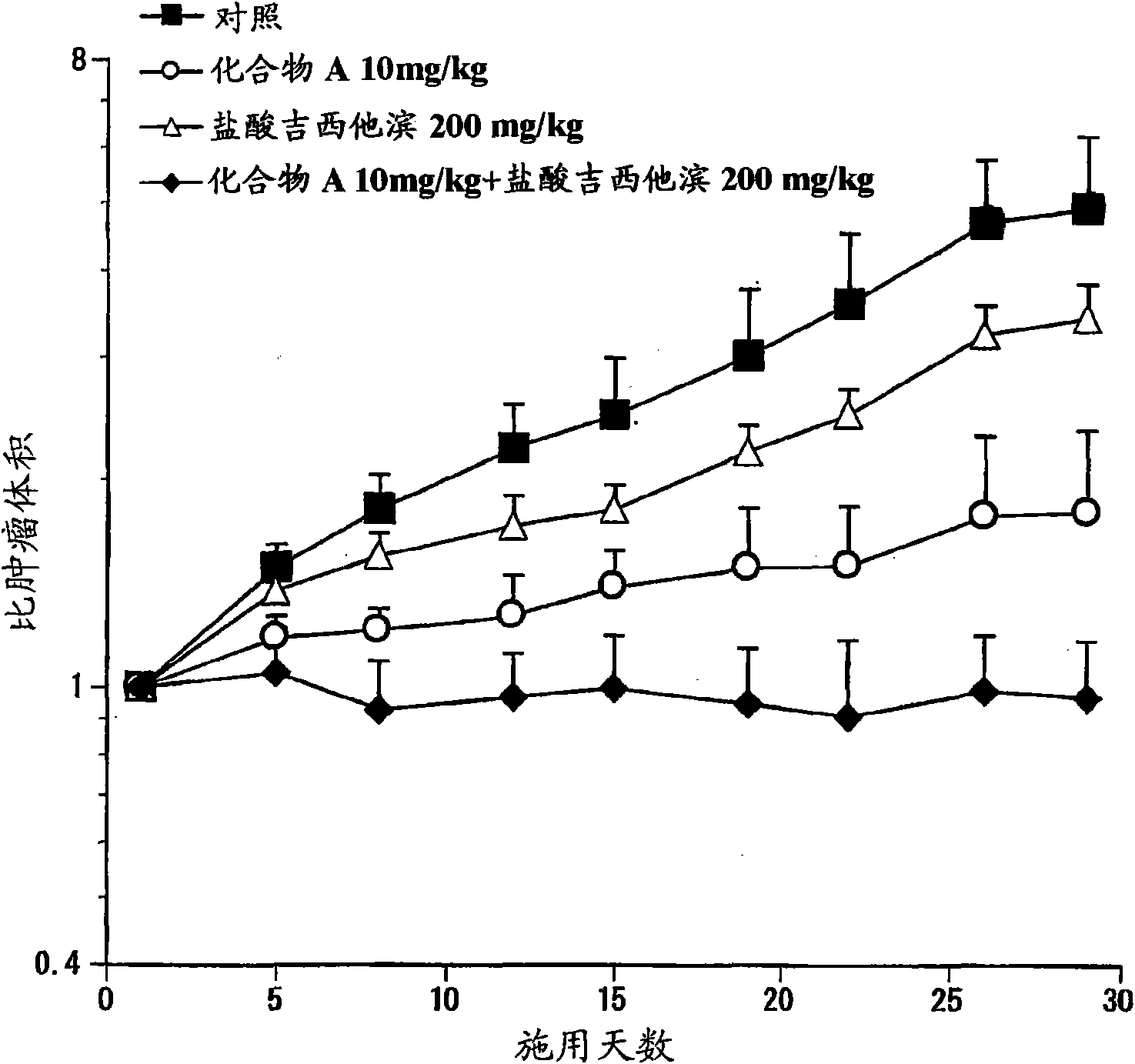
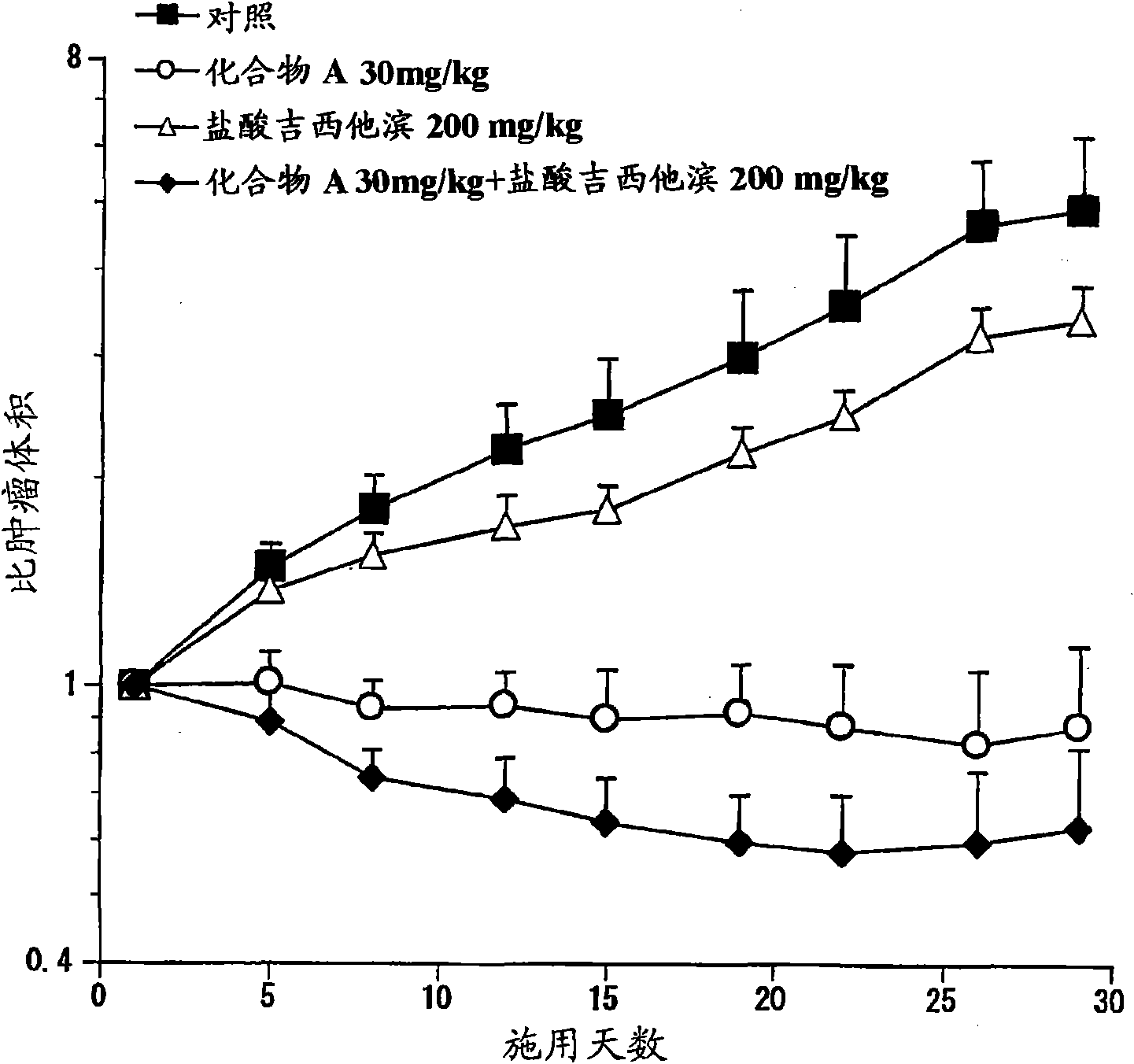
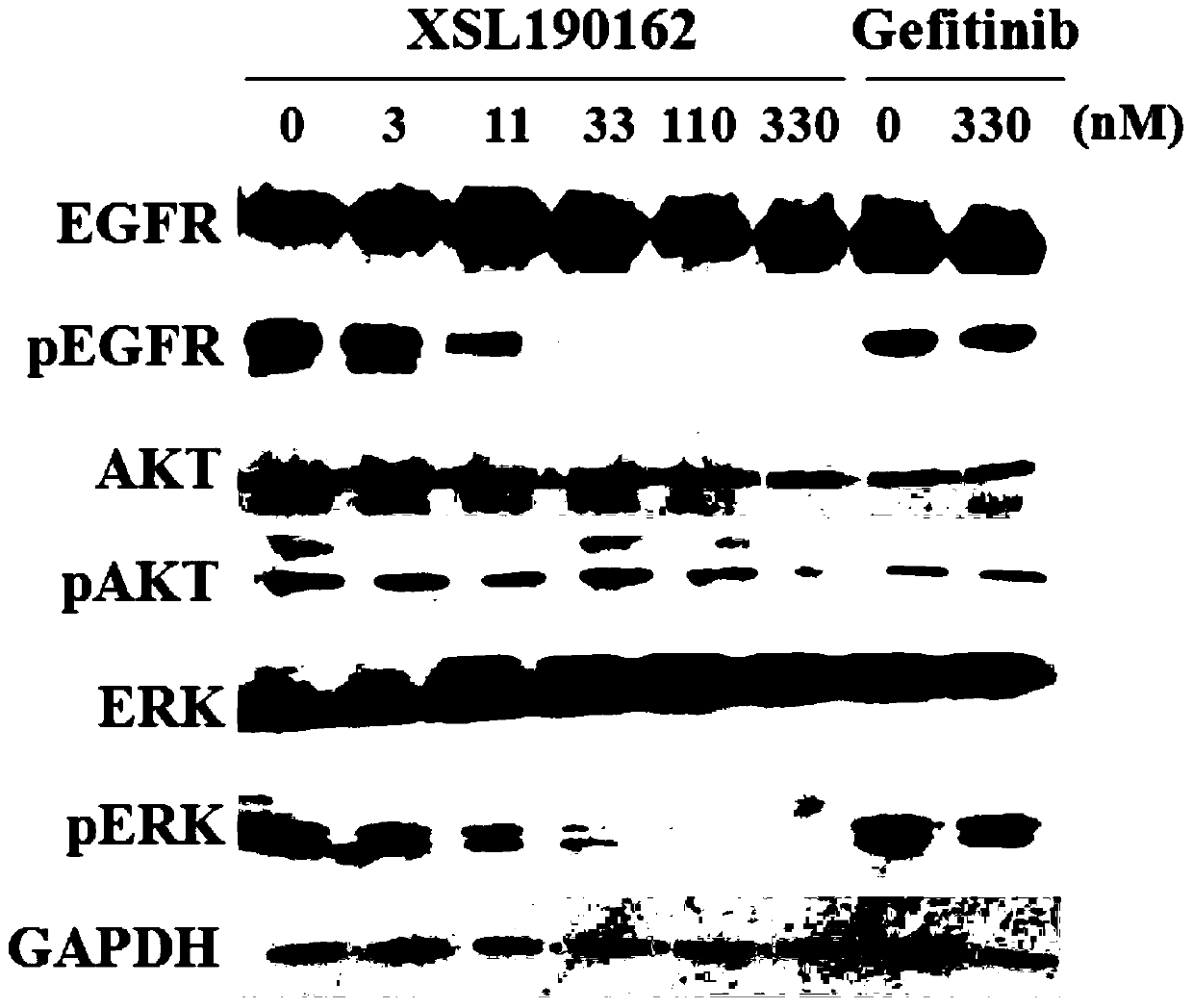
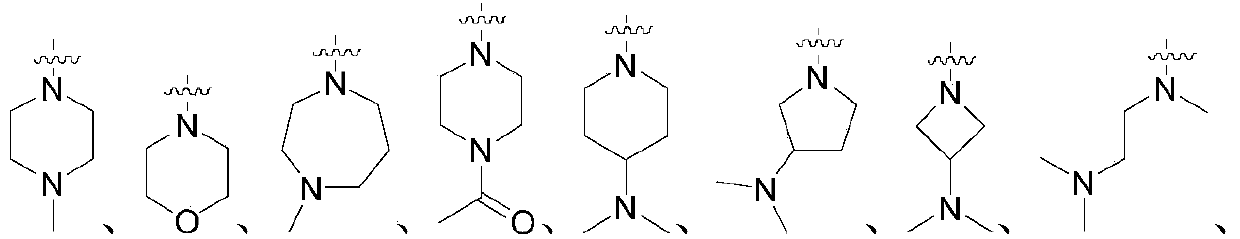
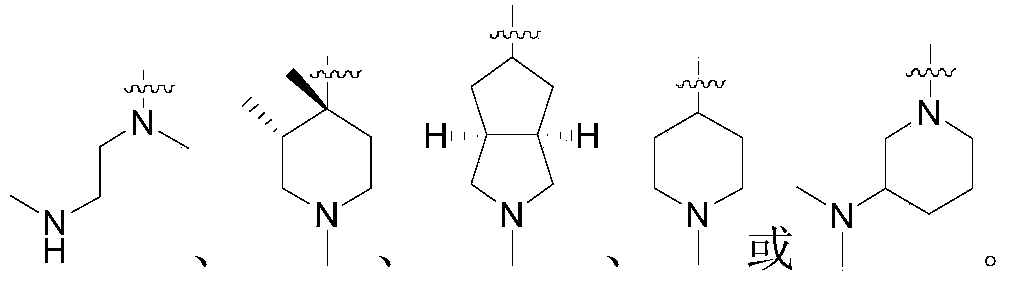
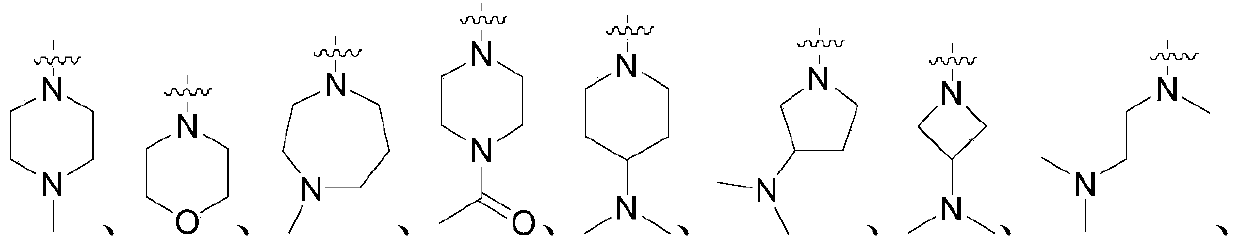
Patents
Literature
233 results about "Erlotinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Erlotinib is used to treat lung and pancreatic cancer.
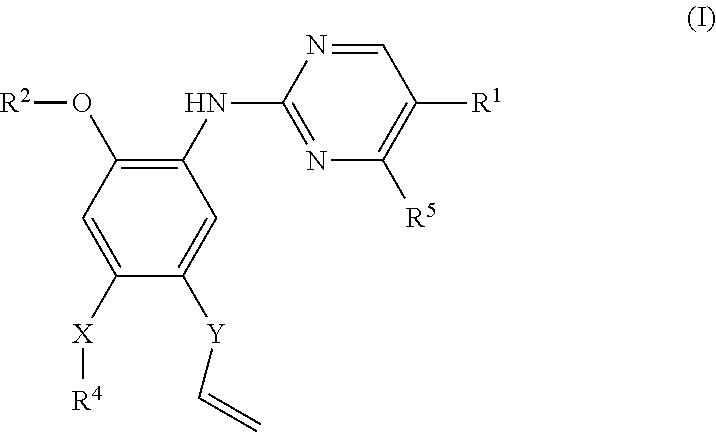
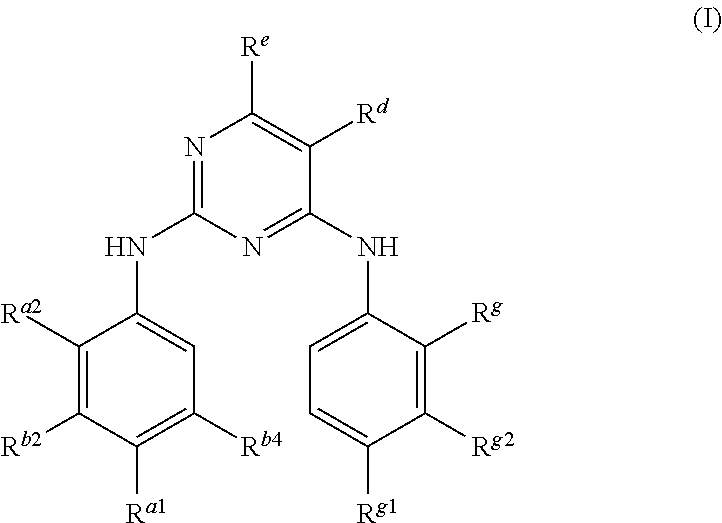
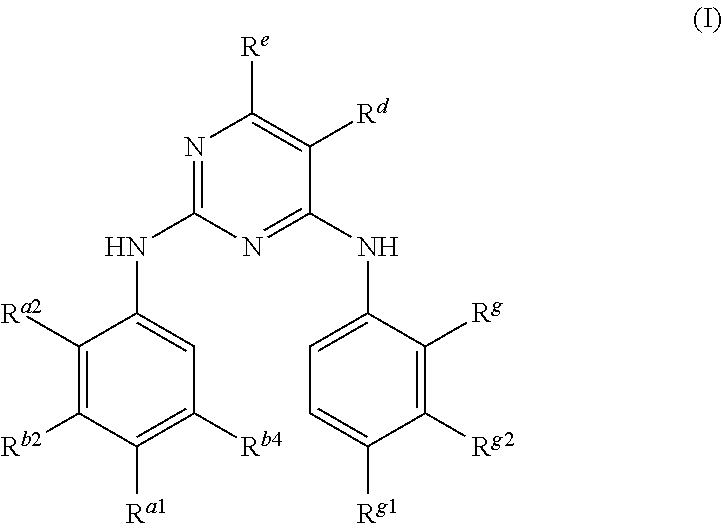
Pyrimidoheterocyclic compound, medicinal composition and application thereof
ActiveCN104418860ASelectiveGood pharmacokinetic propertiesOrganic active ingredientsOrganic chemistryErlotinibMedicine
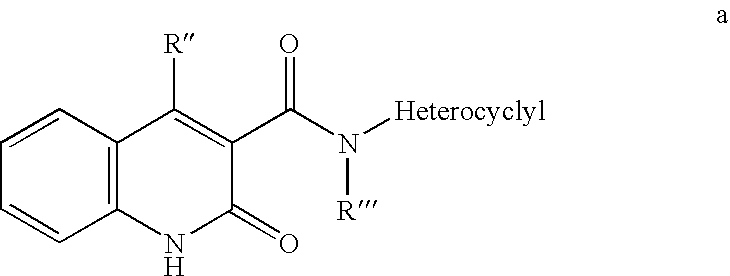
The invention discloses a pyrimidoheterocyclic compound represented as the formula (I), or a pharmaceutically acceptable salt or a stereisomer thereof, or a prodrug molecule thereof. The pyrimidoheterocyclic compound can effectively inhibit growth of various tumor cells and has an inhibiting effect on EGFR protease. The pyrimidoheterocyclic compound can be used for preparing an anti-tumor drug, and can overcome drug resistance caused by medicines in the prior art, such as gefitinib, erlotinib and the like, has a selectivity on wild non-small cell lung cancer and is excellent in pharmacokinetic property.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
3-(2-pyrimidine amino) phenyl acrylic amide compound and application thereof
ActiveCN104788427AAvoid drug resistanceGrowth inhibitionOrganic active ingredientsOrganic chemistryErlotinibDrug resistance
The present invention discloses a 3-(2-pyrimidine amino) phenyl acrylic amide compound in the structure of formula (I) or a pharmaceutically acceptable salt or a stereisomer or a prodrug molecule. The 3-(2-pyrimidine amino) phenyl acrylic amide compound or the pharmaceutically acceptable salt can effectively inhibit the growth of various tumor cells, and inhibit EGFR (epidermal growth factor receptor), HER (human epidermal receptor2) family other protease, can be used in the preparation of antitumor drugs, and can overcome the drug resistance induced by gefitinib, erlotinib and the like.
Owner:PHARMA SHANGHAI
Methods and Compositions for Detecting a Drug Resistant Egfr Mutant
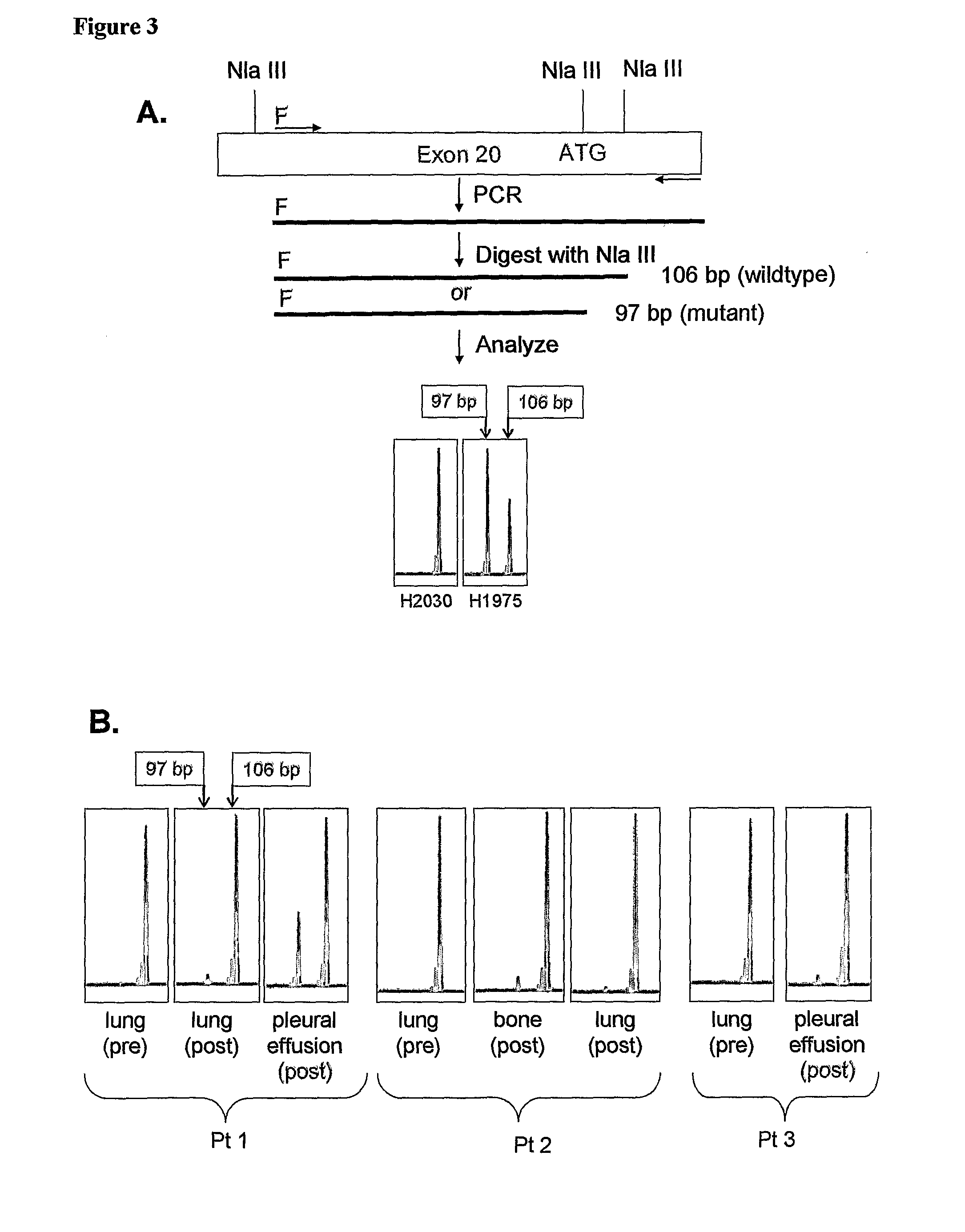
Polymerase chain reaction primers and methods directed to detecting the EGFR mutant C→T at the position corresponding to base 2369 of EGFR cDNA. The invention provides a PCR primer that hybridizes under suitable PCR conditions to a polynucleotide sequence 5′ in each respective strand to a mutation of an EGFR gene that encodes a substitution of threonine by methionine at position 790 of the EGFR polypeptide. The invention also provides a PCR primer hybridizes to a sequence that includes a mutant T at the position corresponding to base 2369 of EGFR cDNA but not to a second EGFR polynucleotide containing a wild type C. The invention provides several methods and kits for detecting a mutant epidermal growth factor receptor (EGFR) gene in a sample comprising probing the sample with a means for selectively detecting a nucleotide sequence comprising a mutant T at the position corresponding to base 2369 of EGFR cDNA, and identifying that the base at said position is T. These methods and kits are also useful to predict resistance to the therapeutic effects of gefitinib or erlotinib in a subject suffering from or suspected of having a cancer.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compounds for immunopotentiation
InactiveUS20100226931A1Reduce capacityLow production costSsRNA viruses positive-senseSnake antigen ingredientsPyridazineErlotinib
Methods of stimulating an immune response and treating patients responsive thereto with 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-diones, staurosporine analogs, derivatized pyridazines, chromen-4-ones, indolinones, quinazolines, nucleoside analogs, and other small molecules are disclosed. In a preferred embodiment benzopyrimidine derivatives such as ZD-6474, MLN-518, lapatinib, gefitinib or erlotinib are used.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Pentadeuteropyridine compounds, and preparation method, pharmaceutical compositions and uses thereof
ActiveCN105237515AImprove securityStrong inhibitory activityOrganic active ingredientsOrganic chemistry methodsDiseaseErlotinib
The invention relates to pentadeuteropyridine compounds represented by the following formula (I) and pharmaceutically acceptable salts, stereoisomers, prodrugs and solvates thereof, and a preparation method, pharmaceutical compositions and uses thereof. The compounds can generate an inhibitory effect on variation forms of epidermal growth factor receptor (EGFR) protein kinase, thereby effectively inhibiting the growth of a variety of tumor cells; the compounds can be used for preparation of antitumor drugs, are used for treatment or prevention of a plenty of different cancers, and moreover, can overcome the drug resistance induced by conventional drugs gefitinib, erlotinib and other first-generation EGFR inhibitors. More specifically, the compounds can be used for preparation of drugs for treatment or prevention of diseases, obstacles, disorders or illness conditions mediated by certain variation-form epidermal growth factor receptors (such as L858R activated mutants, Exon19 deletion activated mutants, and T790M resistance mutants).
Owner:INVENTISBIO CO LTD +1
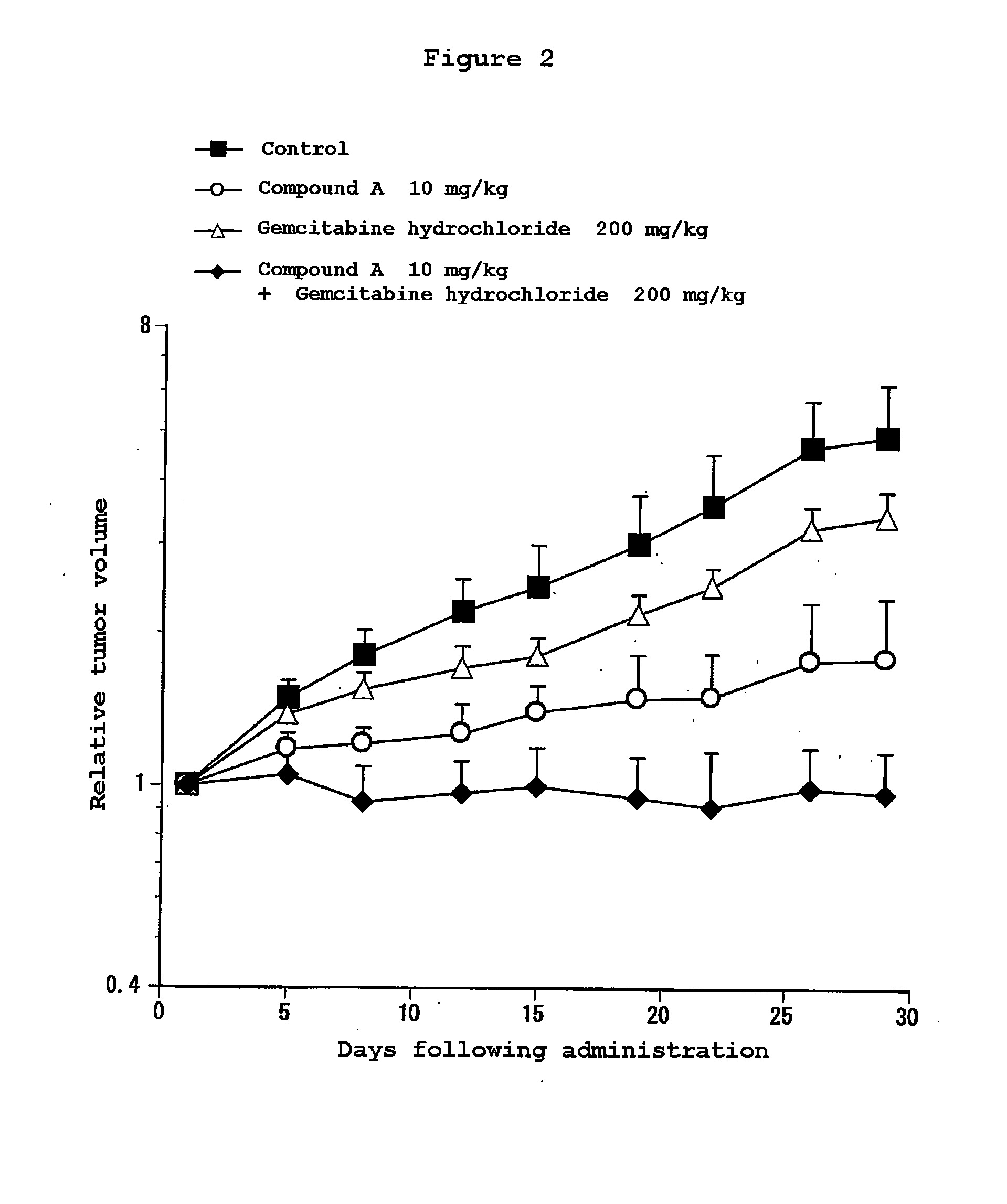
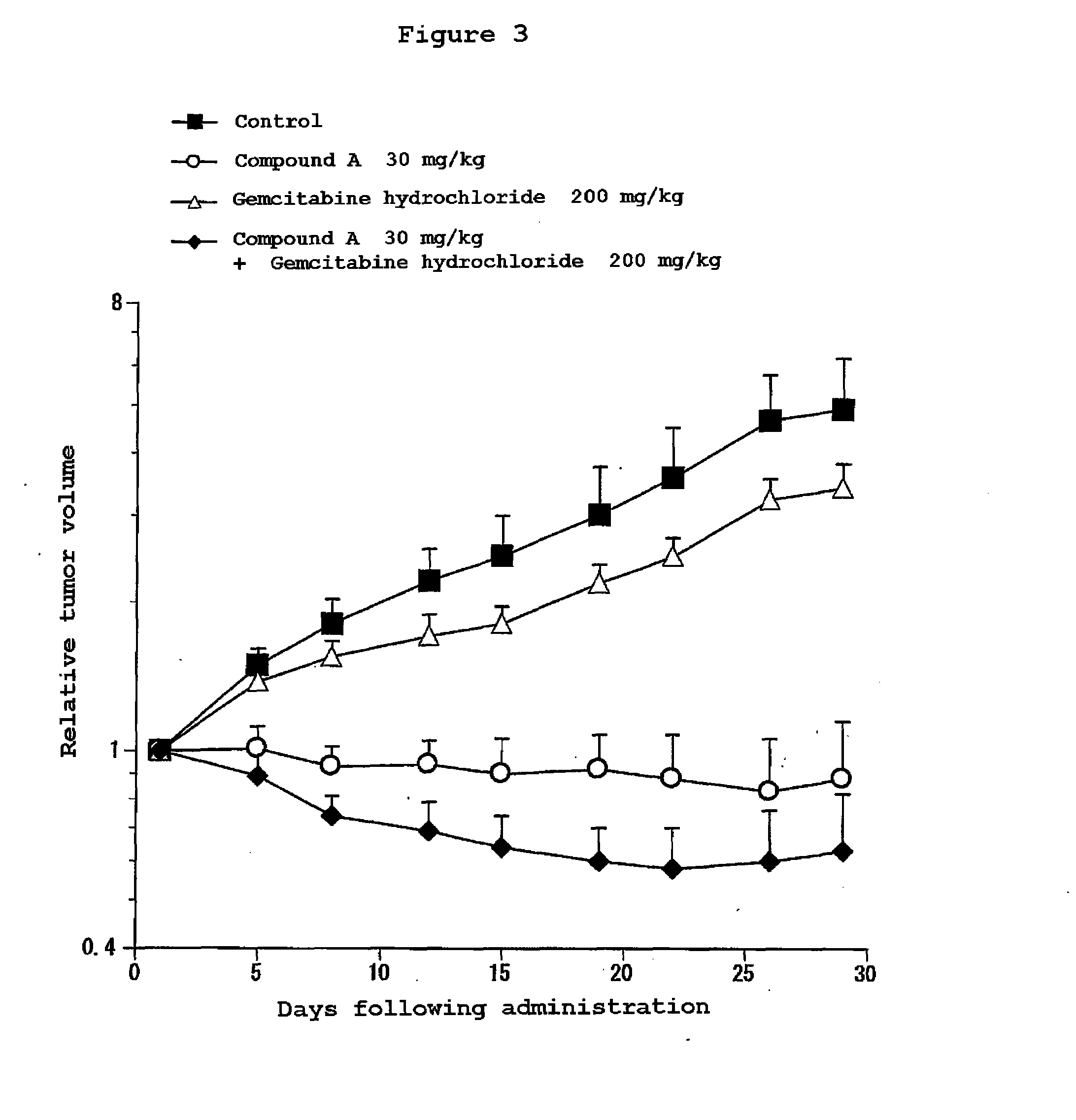
Composition for treatment of pancreatic cancer
Disclosed are a pharmaceutical composition having excellent antitumor activity, and a method for treating a cancer. Specifically, excellent antitumor activity is achieved when 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide or an analogous compound thereof, a pharmacologically acceptable salt thereof or a solvate thereof is used in combination with gemcitabine or erlotinib, a pharmacologically acceptable salt thereof or a solvate of any of them.
Owner:EISIA R&D MANAGEMENT CO LTD
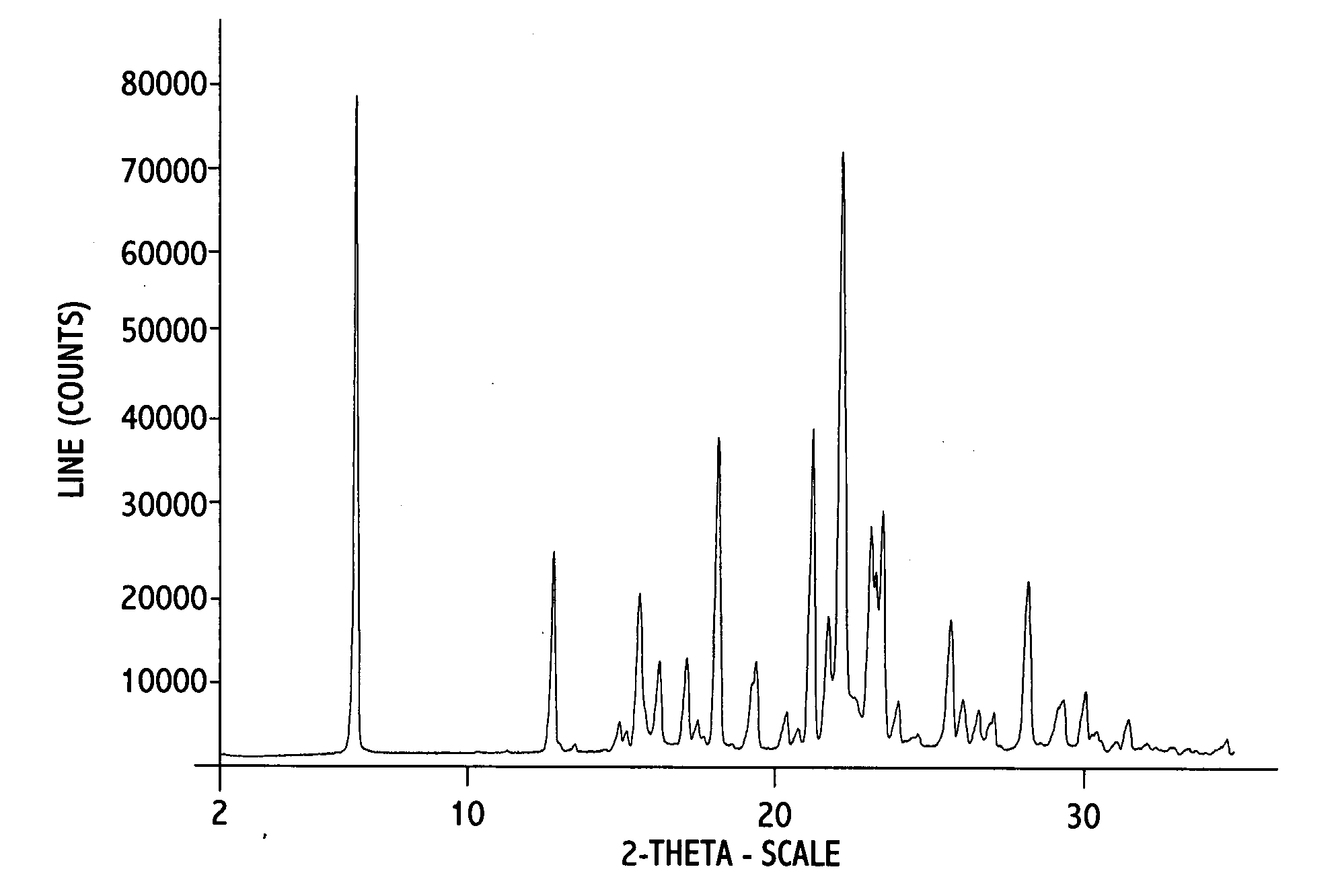
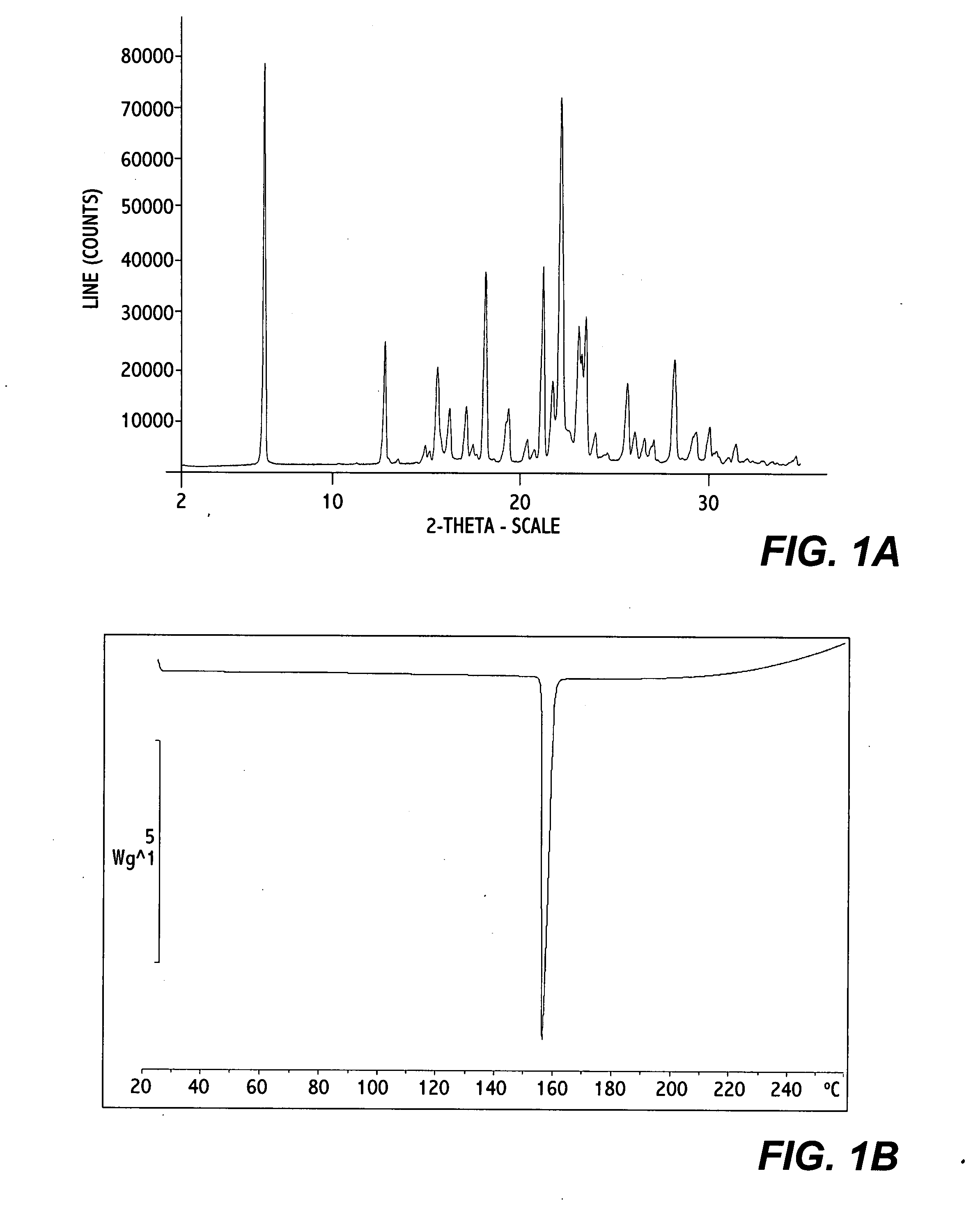
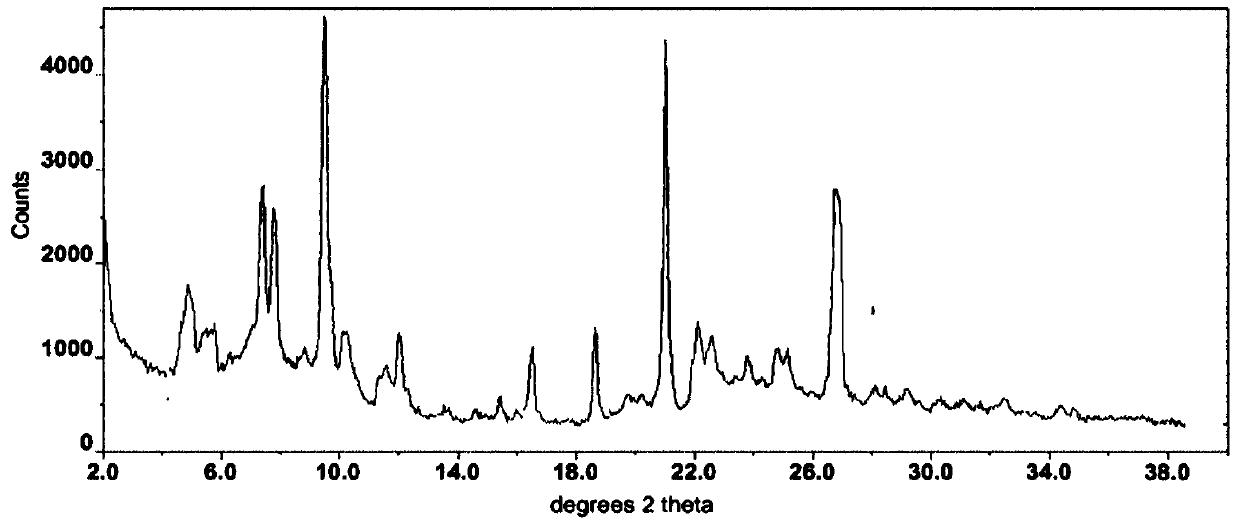
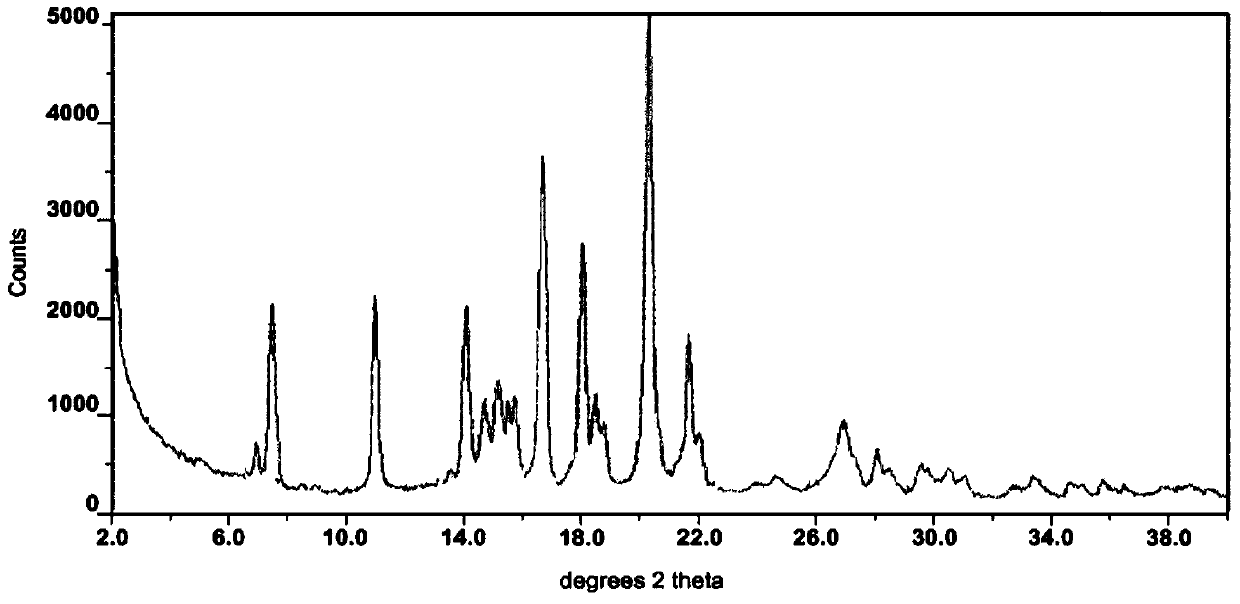
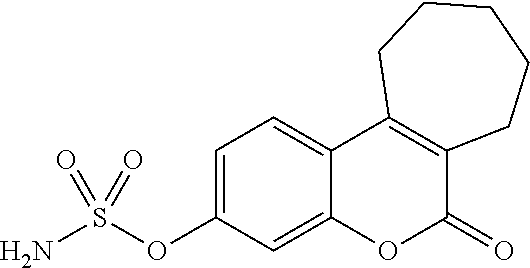
Crystalline erlotinib
Crystalline Forms of erlotinib are made. The crystalline materials are useful as pharmaceutical active agents in treating various cancers as well as in forming erlotinib salts.
Owner:SYNTHON BV
4- fragrant amido quinazoline derivatives and method of manufacturing the same and application in pharmacy thereof
The invention relates to a 4-aromatic aminoquinazoline compounds with general formula of (I) or a receivable salt pharmaceutically and the application in preparing the drugs for treating the tumour. Wherein, R1, R2 are hydrogen, halogen, nitryl, amino, alkyl of C1-6, alkylamino of C1-6 or alkoxy of C1-6, or the group replaced by the alkylamino on the terminal of the alkyl of C1-6, alkylamino and alkoxy; X is N,O or S; Ar is aromatic ring or heteroaromatic groups replaced by the ester of C5 to C8. The invention can inhibit the increase of the tumour cell obviously and has obvious inhibited effect on the tyrosine kinase of the cell, and has activity equal to that of the positive drugs Gefitinib and Erlotinib and even exceeds the positive control.
Owner:SOUTHEAST UNIV
Composition for treatment of pancreatic cancer
Disclosed are a pharmaceutical composition having excellent antitumor activity, and a method for treating a cancer. Specifically, excellent antitumor activity is achieved when 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7- methoxy-6-quinolinecarboxamide or an analogous compound thereof, a pharmacologically acceptable salt thereof or a solvate of any of them is used in combination with gemcitabine or erlotinib, a pharmacologically acceptable salt thereof or a solvate of any of them.
Owner:EISIA R&D MANAGEMENT CO LTD
Pyrimidoheterocyclic compound and its pharmaceutical composition and application
ActiveCN104418860BGood pharmacokinetic propertiesSelectiveOrganic active ingredientsOrganic chemistryErlotinibTumor cells
The invention discloses a pyrimidoheterocyclic compound represented as the formula (I), or a pharmaceutically acceptable salt or a stereisomer thereof, or a prodrug molecule thereof. The pyrimidoheterocyclic compound can effectively inhibit growth of various tumor cells and has an inhibiting effect on EGFR protease. The pyrimidoheterocyclic compound can be used for preparing an anti-tumor drug, and can overcome drug resistance caused by medicines in the prior art, such as gefitinib, erlotinib and the like, has a selectivity on wild non-small cell lung cancer and is excellent in pharmacokinetic property.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Pyrimido diazepine compound as well as pharmaceutical composition and application thereof
ActiveCN103374000AGrowth inhibitionOvercome drug resistanceOrganic active ingredientsOrganic chemistryErlotinibMedicine
The invention discloses a 5,8-dioxo-pyrimido[4,5-e][1,4]diazepine compound of a structural formula (1) or a pharmaceutically acceptable salt or stereoisomer or prodrug molecule thereof. The 5,8-dioxo-pyrimido[4,5-e][1,4]diazepine compound has the effects of effectively inhibiting the growth of various tumor cells and the generation of the EGFR (epidermal growth factor receptor), can be used for preparing anticancer medicaments and can overcome the resistance of the existing medicaments including the gefitinib, the erlotinib and the like.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Inhalation-type pharmaceutical composition for the treatment of lung cancer and preparation method thereof
ActiveUS9763946B2Promote absorptionGood curative effectPowder deliveryOrganic active ingredientsSide effectErlotinib
The present invention provides an inhalation-type pharmaceutical composition for lung cancer and preparation method thereof, comprising a first gas and an atomized medicine. The first gas comprises hydrogen. The gas volume concentration of hydrogen in the inhalation-type pharmaceutical composition is between 2 to 96%. The atomized medicine is selected from a group comprising cisplatin, docetaxel, etoposide, gefitinib, erlotinib, and any combination thereof. The inhalation-type pharmaceutical composition of the present invention can remove harmful radicals in the body of the patient through the use of hydrogen while also increases the absorption effect of the medicine for the patient by using an atomized medicine. At the same time, because the use of the small amount of the vaporized pharmaceutical liquid can indirectly reduce the side effects on the user.
Owner:LIN HSIN YUNG
2-aminopyrimidine compounds as well as pharmaceutical compositions and applications thereof
ActiveCN105601573ANovel structureGrowth inhibitionOrganic chemistryAntineoplastic agentsErlotinibWild type
The invention discloses 2-aminopyrimidine compounds as well as pharmaceutical compositions and applications thereof. The structure of the 2-aminopyrimidine compounds is shown in the formula I, definitions of R1, R2, R3, R4, R5, X, Y, Z and W in the formula are shown in the specification and the claim. The compounds can effectively inhibit growths of a plurality of tumor cells, generate inhibition effects for EGFR and IGF1R protease, and is used for preparing antitumor drugs; the compounds can overcome drug resistance which is induced by prior medicaments Gefitinib and Erlotinib and the like, has selectivity for tumor, especially wild type non-small cell lung cancers, and has good pharmacokinetics property.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +2
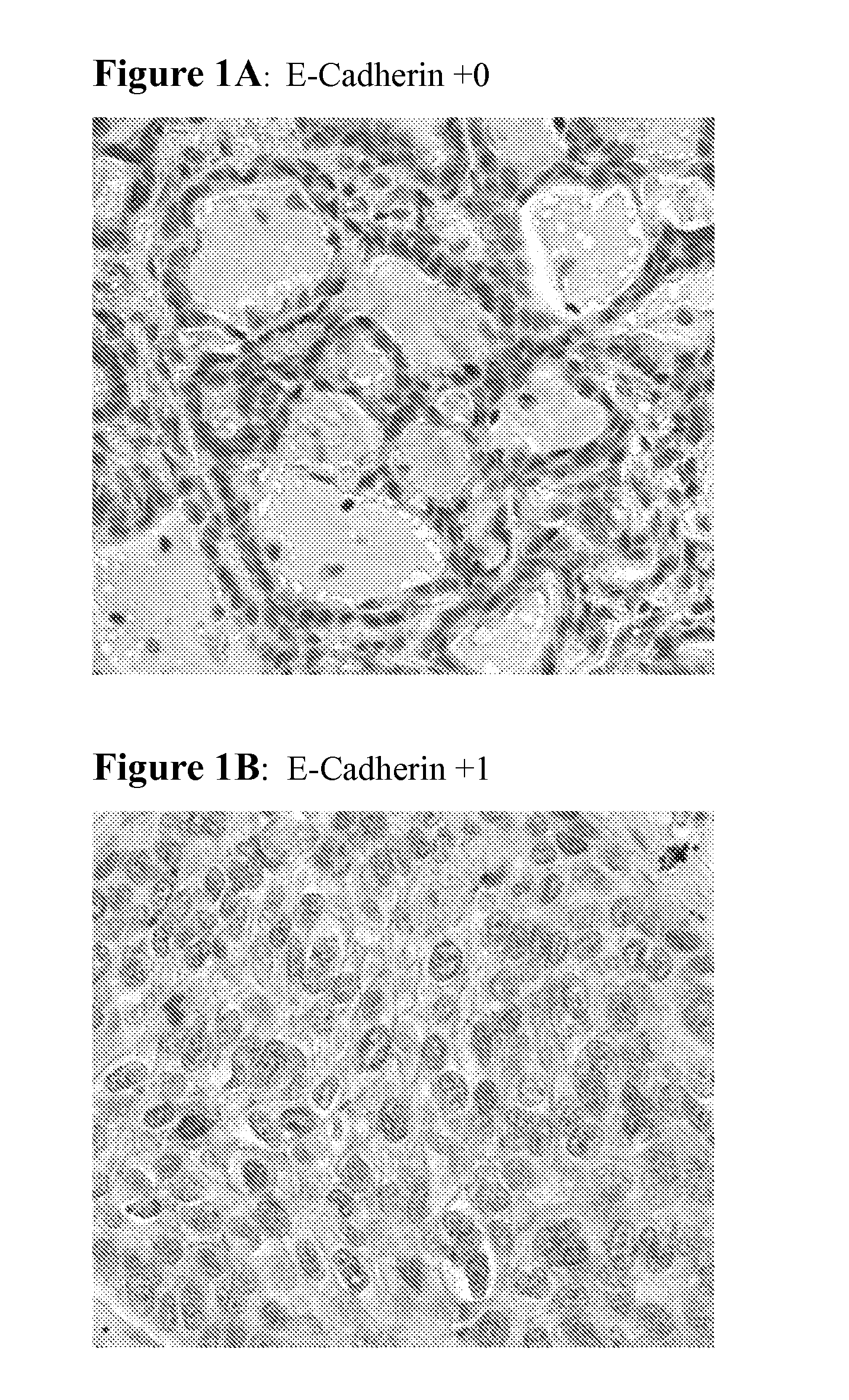
Biological markers predictive of Anti-cancer response to epidermal growth factor receptor kinase inhibitors
InactiveUS20120142028A1Long survival progression free survivalEffectiveness of treatmentDisease diagnosisAntineoplastic agentsEpidermal Growth Factor Receptor KinaseErlotinib
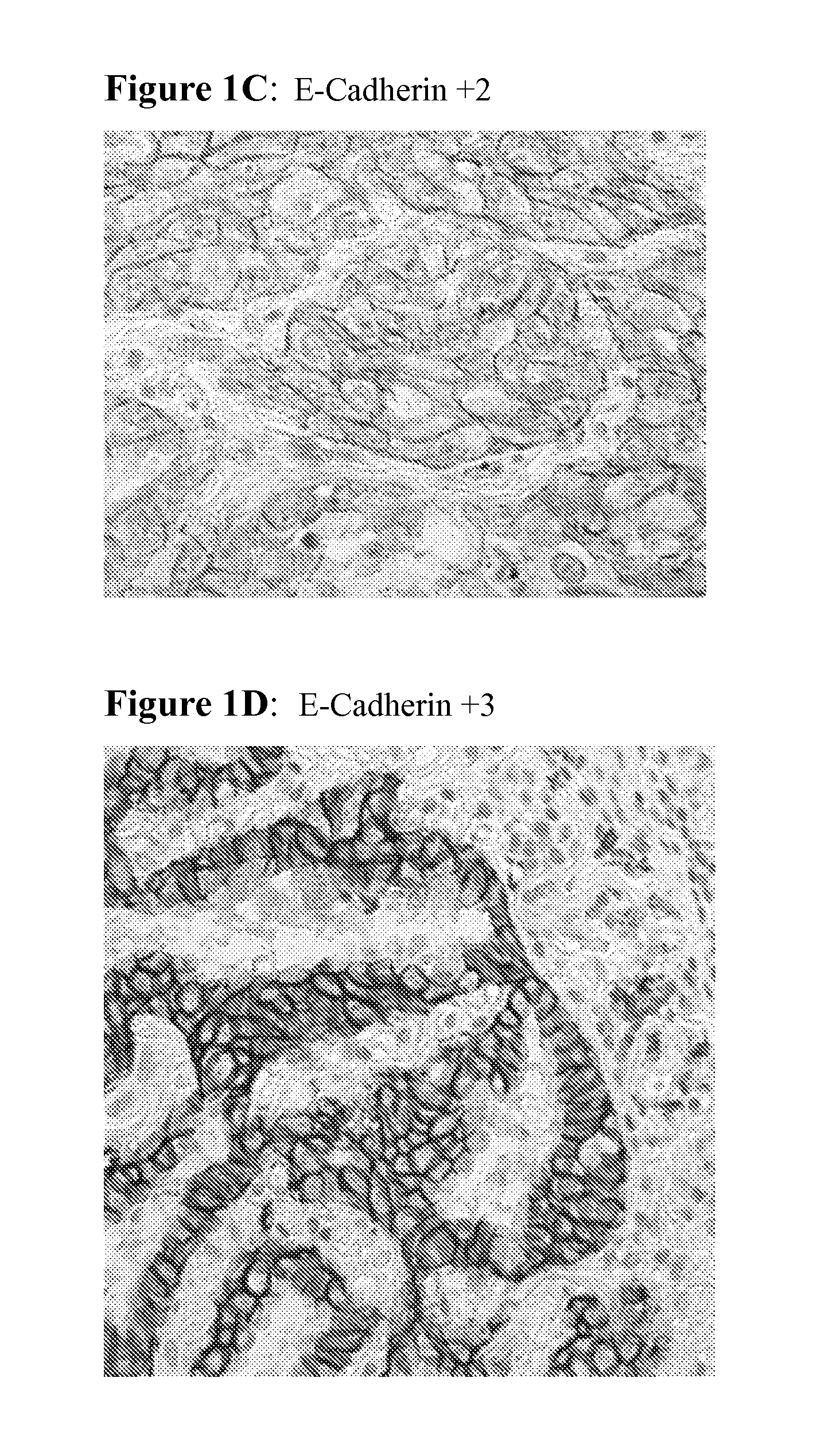
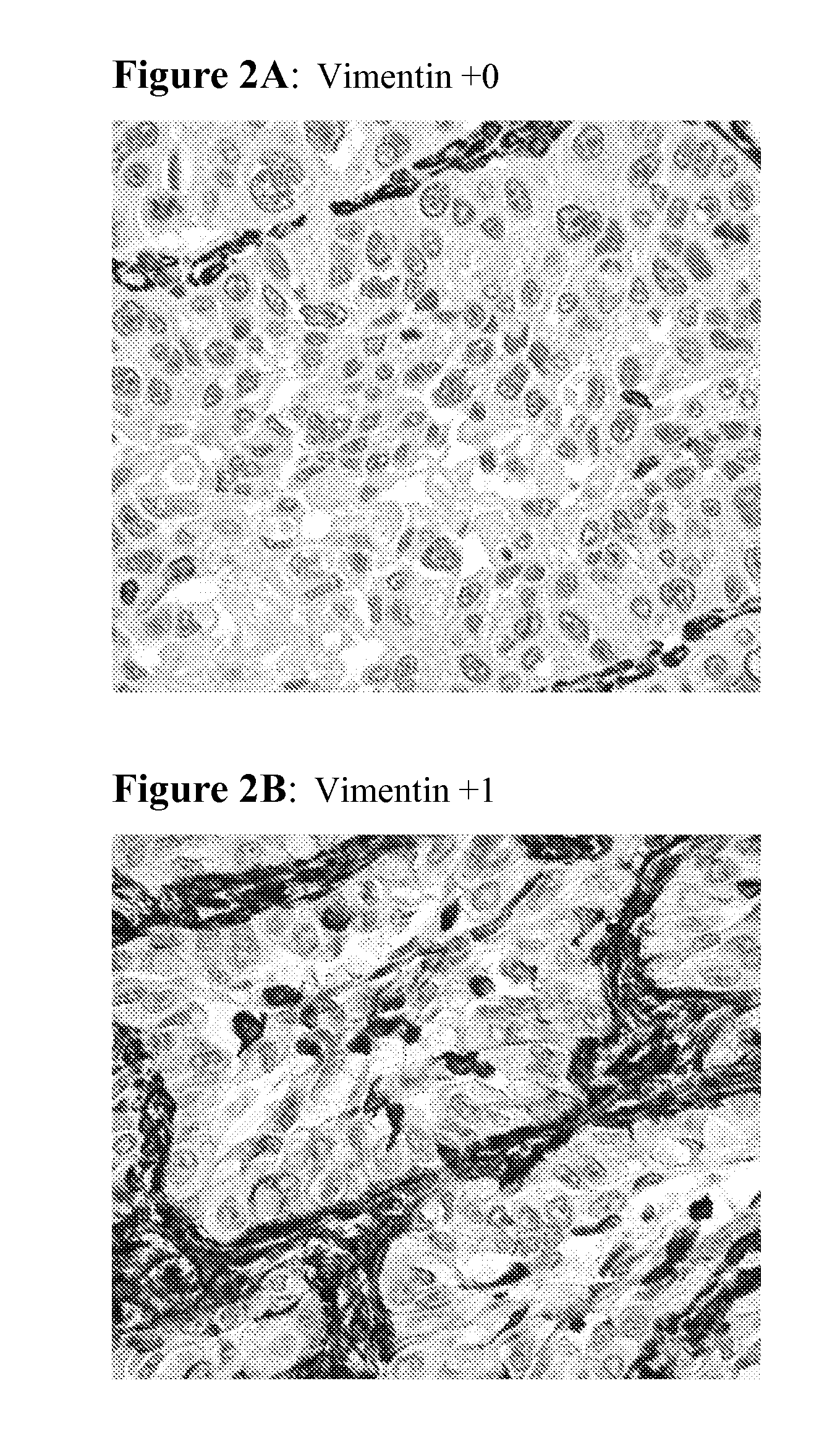
The present invention provides diagnostic methods for predicting the effectiveness of treatment of a cancer patient with an EGFR kinase inhibitor. These methods are based on the surprising discovery that the effectiveness of treatment with an EGFR kinase inhibitor is predicted by whether a patient's tumor cells express a high or a low level of the biomarkers vimentin and E-cadherin, such that patients whose tumors express a high level of at least one of the biomarkers vimentin and E-cadherin have a longer overall survival and progression free survival than patients whose tumors express a low level of both vimentin and E-cadherin. The present invention further provides a method for treating tumors or tumor metastases in a patient, comprising the steps of diagnosing a patient's likely responsiveness to an EGFR kinase inhibitor by assessing whether tumor cells express a high level of at least one of the biomarkers vimentin and E-cadherin, and administering to said patient a therapeutically effective amount of an EGFR kinase inhibitor (e.g. erlotinib), particularly when effectiveness of the inhibitor is predicted.
Owner:OSI PHARMA LLC
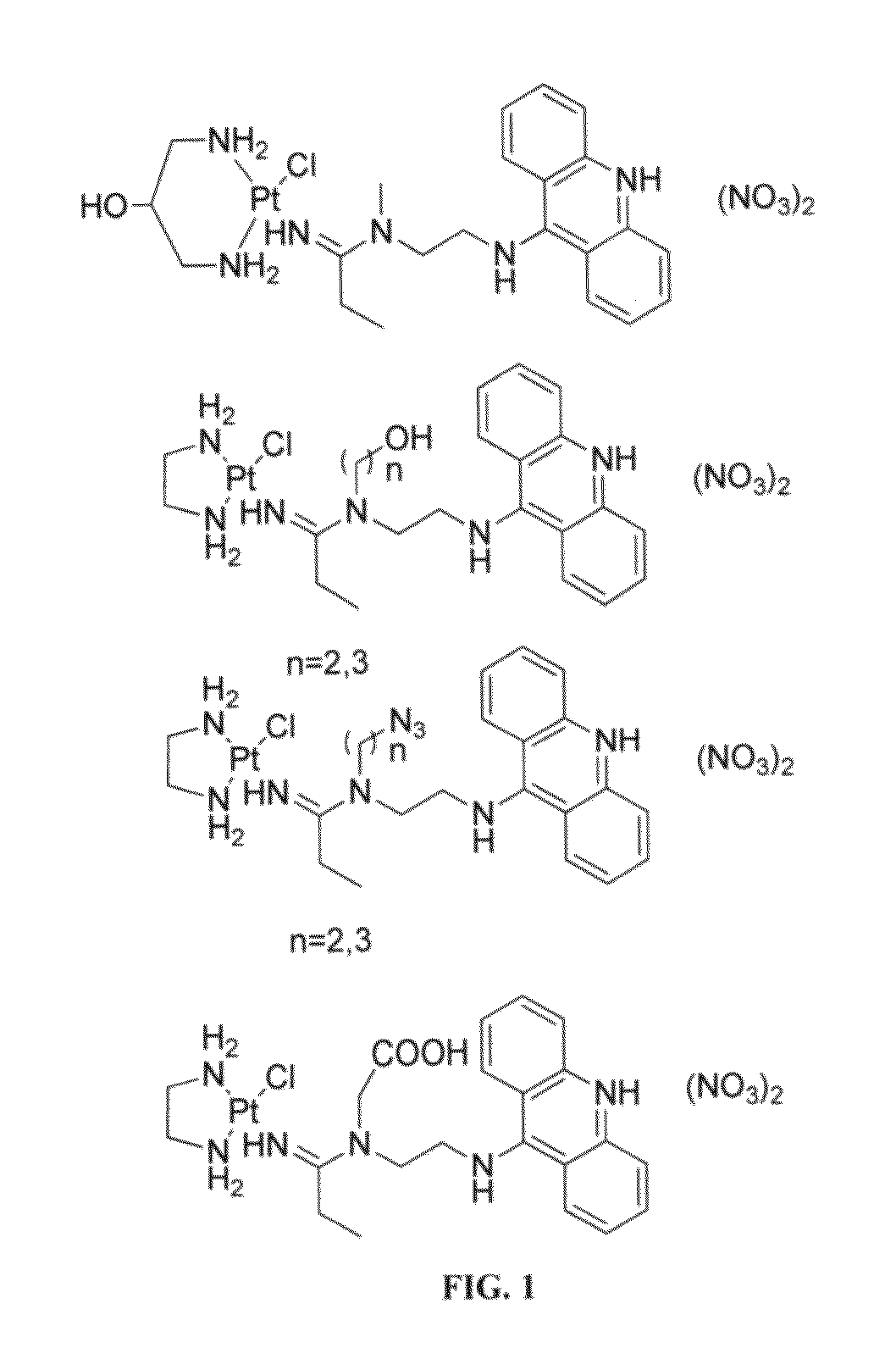
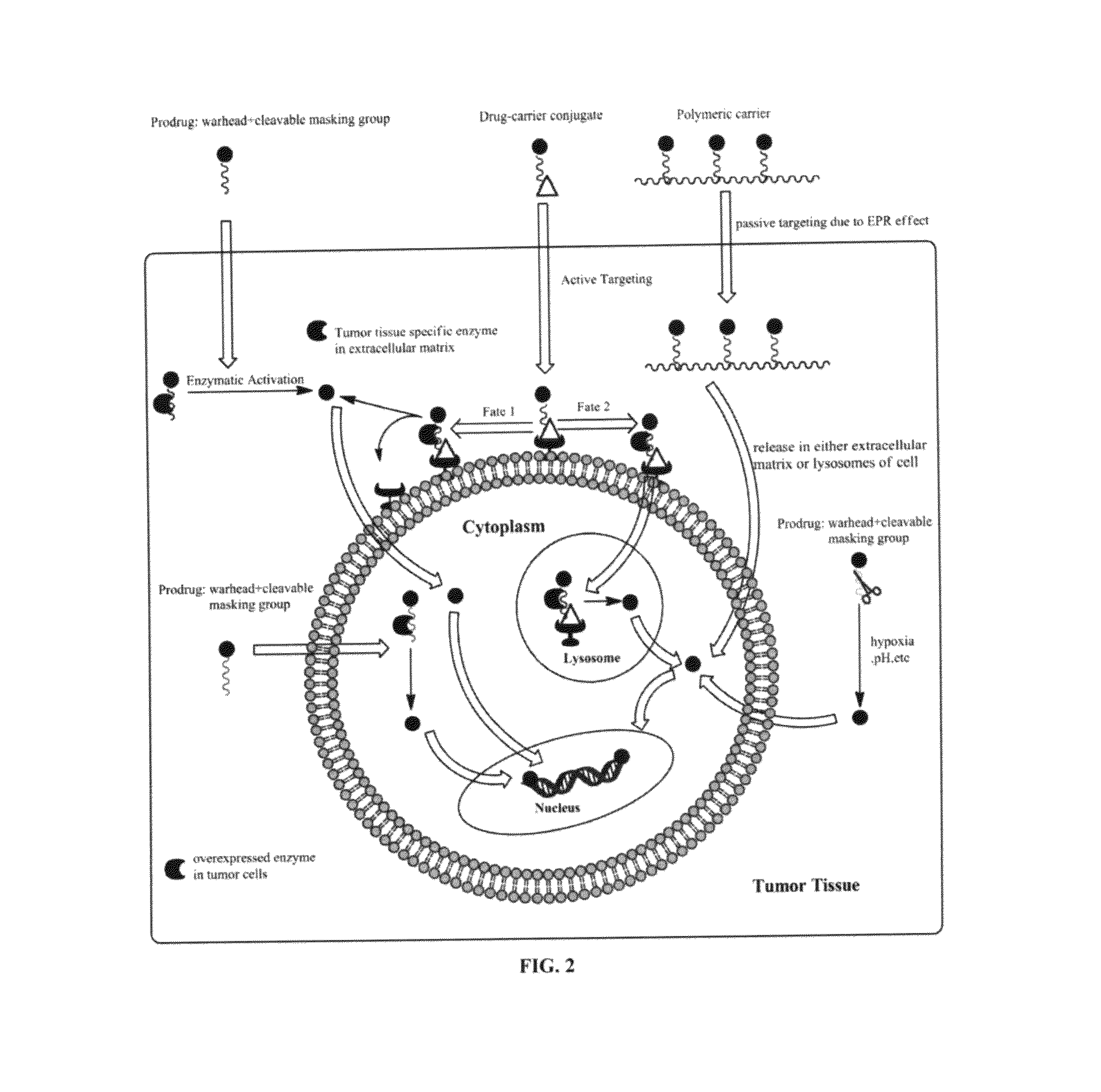
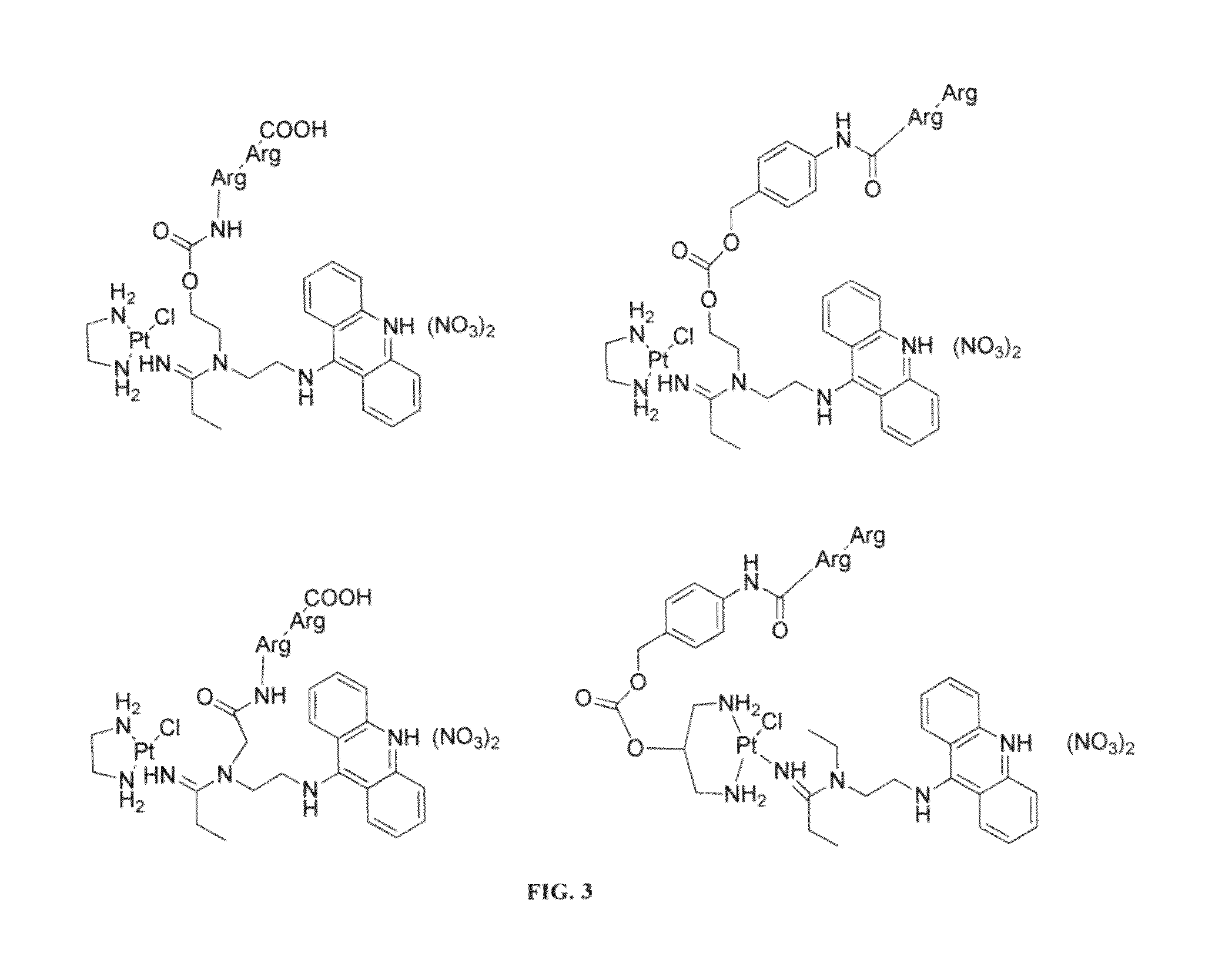
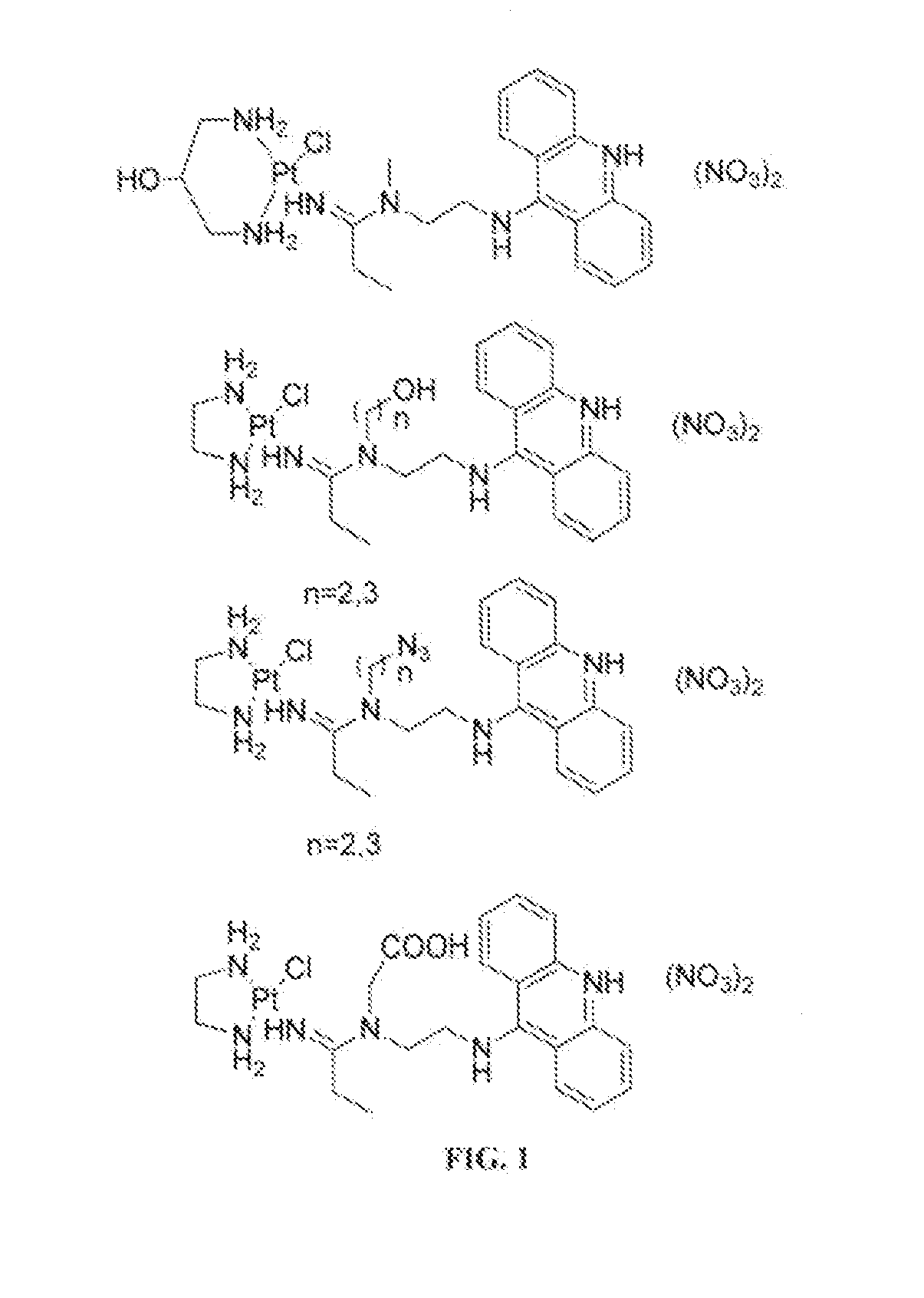
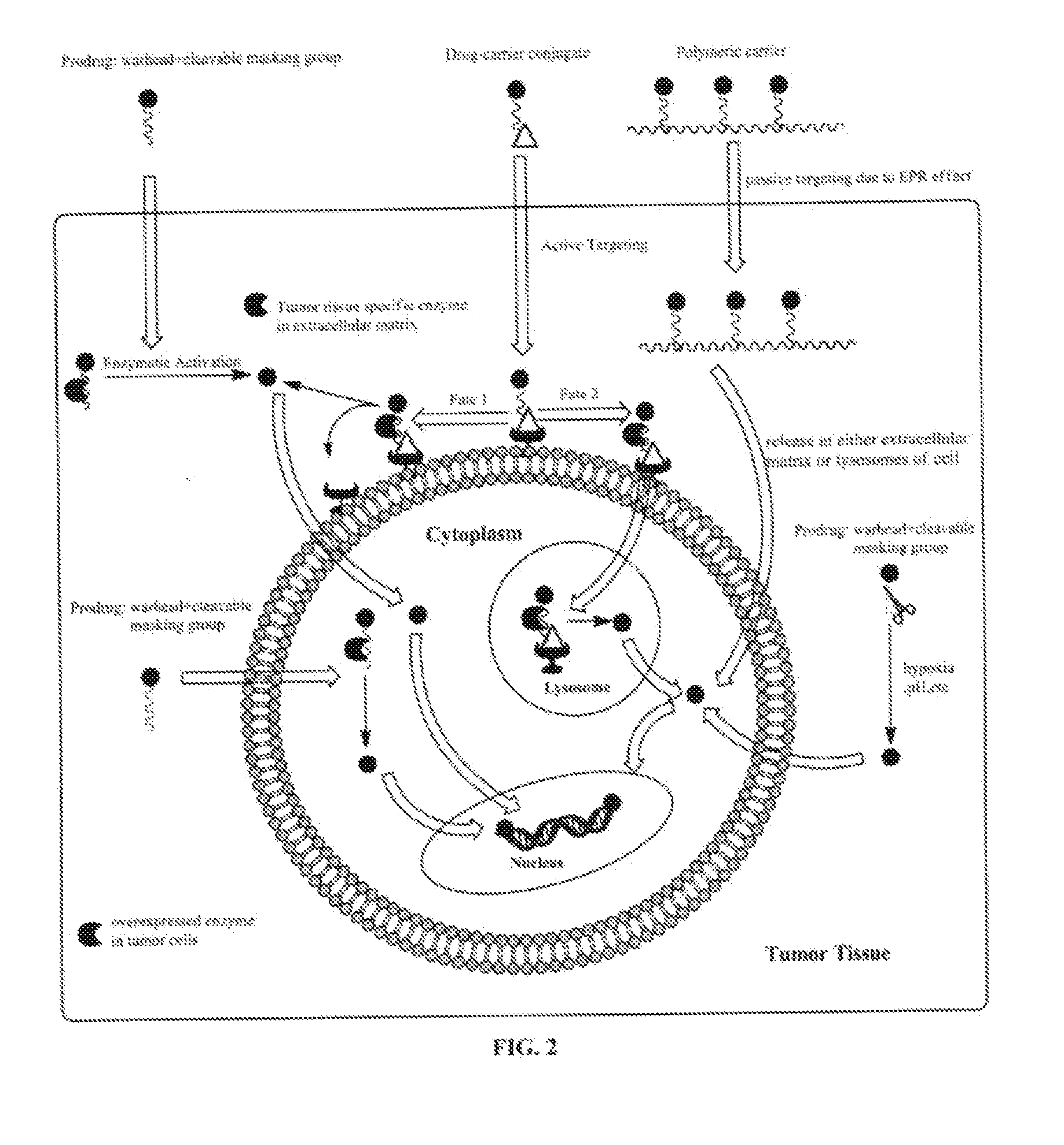
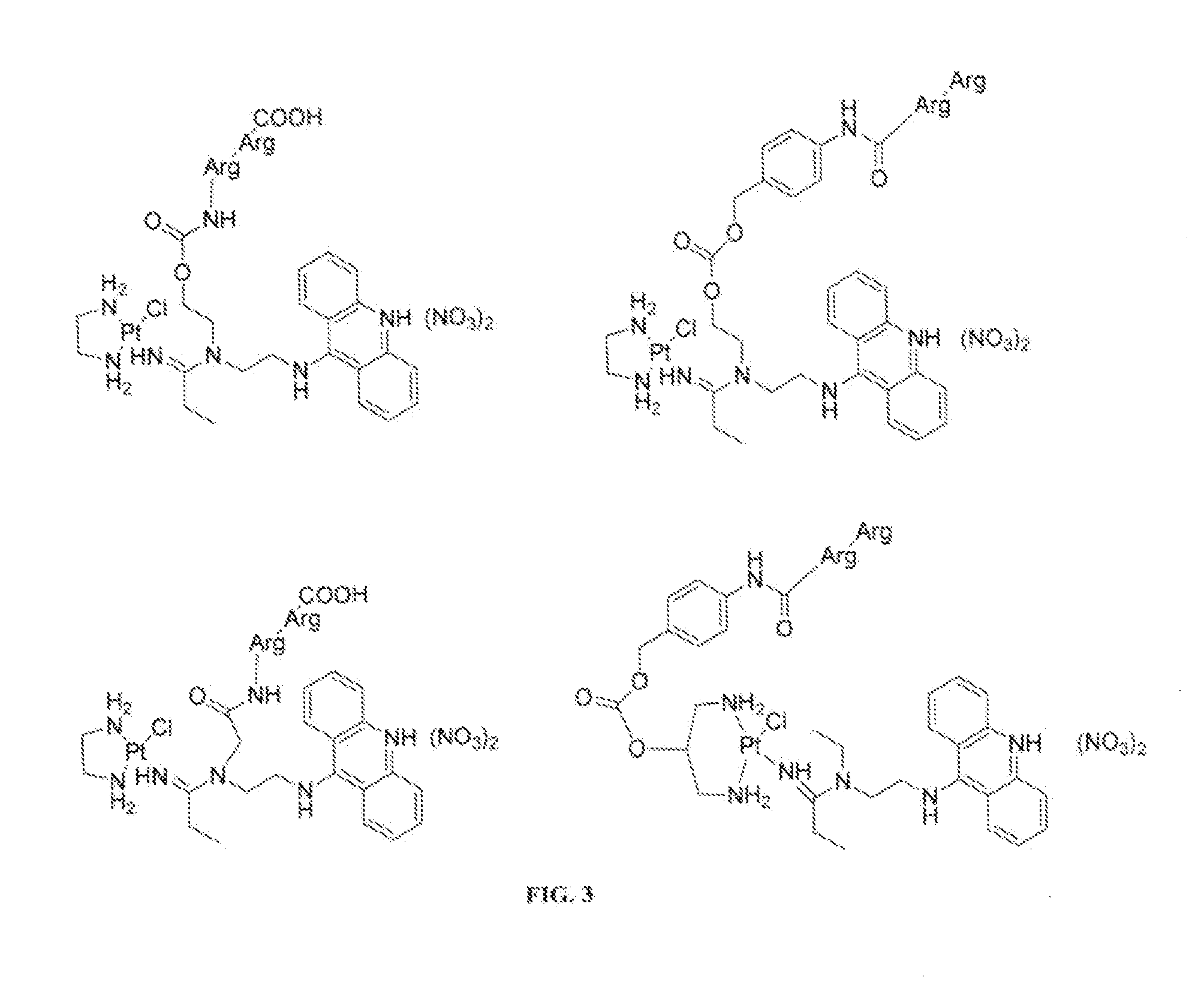
Targeted delivery and prodrug designs for platinum-acridine anti-cancer compounds and methods thereof
ActiveUS9090640B2Not induce DNA cross-linksStrong cytotoxicityOrganic active ingredientsPlatinum organic compoundsErlotinibCancer cell
Acridine containing cisplatin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective cisplatin compounds to the nucleus in cancer cells is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C1-6alkylene-phenyl-NH—C(O)—R15, folic acid, αvβ3 integrin RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazoles, oxazoles, erlotinib, and / or mixtures thereof; wherein R15 is a peptide.
Owner:WAKE FOREST UNIV
Erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivatives, and preparation method and application thereof
InactiveCN104387412AIncrease intakeEnhanced selective uptakeEnergy modified materialsGroup 3/13 element organic compoundsTumor targetErlotinib
The invention discloses erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivatives, and a preparation method and application thereof. According to the invention, erlotinib which is a listed small-molecule inhibitor is used as a tumor target and is covalently connected to a 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene photosensitizer capable of being used for photodynamic therapy by the click reaction so as to obtain third-generation anti-tumor photosensitizers capable of being used for targeted therapy, namely, a double-erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivative and a single-erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivative. Meanwhile, by using the erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivatives as research objects and respectively using human body hepatoma carcinoma cells HepG2 and HELF (Human Embryonic Lung Fibroblast) as tested cell strains, research on in vitro anti-tumor activity of the erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivatives is developed, a prodrug suitable for molecular targeted therapy is screened out and the foundation is laid for application of the erlotinib modified 4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene derivatives to targeted therapy of cancers. A synthetic method of the derivatives is simple; raw materials of the derivatives are easy to obtain and low in cost, have few side effects and high yield, are easy to purify and are beneficial to industrial production.
Owner:FUZHOU UNIV
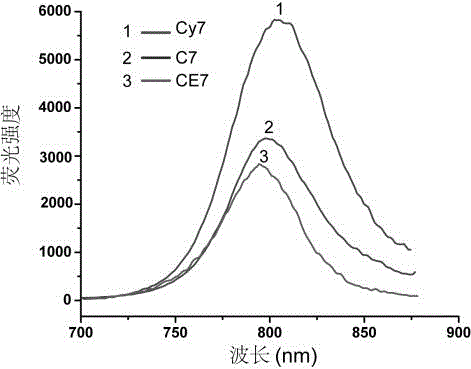
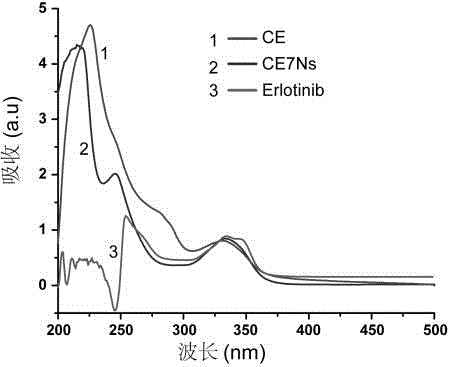
Erlotinib-Cy7-chitosan polymer with tumor targeting
InactiveCN106832059AOvercome the defect of poor solubilityGood biocompatibilityOrganic active ingredientsPhotodynamic therapySolubilityChemical synthesis
The invention discloses an Erlotinib-Cy7-chitosan polymer with tumor targeting. According to the Erlotinib-Cy7-chitosan polymer, lung-cancer molecular targeted medicine Erlotinib and the heptamethine cyanine dye Cy7 are connected to chitosan modified by the chemical structure through click chemistry, and a chitosan derivative (CE7) is obtained. According to the Erlotinib-Cy7-chitosan polymer, chemical synthesis is simple and easy to carry out, the polymer CE7 can be self assembled to form nanometer particle CE7Ns, the nontoxic and high-biocompatibility characteristics of chitosan are reserved, the water solubility and the bioavailability of the Erlotinib are greatly improved, the toxic and side effects of medicine are reduced, near-infrared fluorescence imaging and photodynamic therapy can also be carried out, and the therapy effect is improved.
Owner:FUZHOU UNIV
Targeted Delivery and Prodrug Designs for Platinum-Acridine Anti-Cancer Compounds and Methods Thereof
ActiveUS20140193334A1Strong cytotoxicityInhibition is effectiveOrganic active ingredientsBiocideCancer cellErlotinib
Acridine containing cisplatin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective cisplatin compounds to the nucleus in cancer cells is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C1-6alkylene-phenyl-NH—C(O)—R15, folic acid, αvβ3 integrin RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazoles, oxazoles, erlotinib, and / or mixtures thereof; wherein R15 is a peptide.
Owner:WAKE FOREST UNIV
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
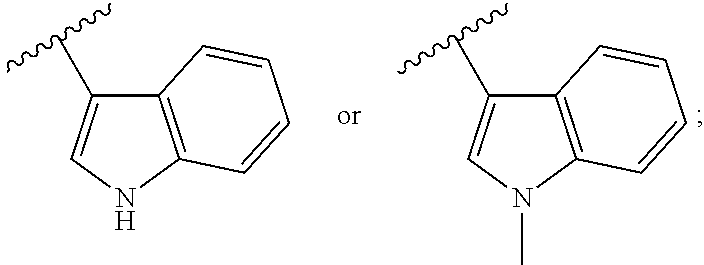
Pyrimidine or pyridine compounds, preparation method therefor and pharmaceutical uses thereof
ActiveUS20170355696A1Strong inhibitory activityReduce inhibitionOrganic active ingredientsOrganic chemistryDiseaseActivating mutation
The present invention disclosed a class of pyrimidine or pyridine compounds, pharmaceutically acceptable salts, stereoisomers, prodrugs and solvates thereof, preparation method therefor and pharmaceutical compositions and pharmaceutical uses thereof. The compounds can inhibit the variants of EGFR (Epidermis Growth Factor Receptor) proteinases, and therefore can inhibit the growth of a variety of tumor cells effectively. The compounds can be used to prepare antitumor drugs, used for the treatment, combined therapy or prevention of various different cancers. The compounds can overcome the drug resistance induced by the existing first-generation EGFR inhibitors such as gefitinib, erlotinib and so on. Particularly, the compounds can be used to prepare drugs for treating or preventing diseases, disturbances, disorders or conditions mediated by epidermis growth factor receptor variants (such as L858R activated mutants, Exon19 deletion activated mutants and T790M resistant mutants).
Owner:INVENTISBIO CO LTD
Preparation method of quinazoline derivate
InactiveCN102321032AReduce pollutionRaw materials are cheap and easy to getOrganic chemistrySide effectErlotinib
The invention discloses a preparation method of a quinazoline derivate. Compared with the prior art, the method disclosed by the invention has the advantages that raw materials are cheap and easy to obtain, the reaction condition is mild, side effects are fewer, the yield is high, the pollution to the environment is small, and the method is suitable for industrial production and provides a new way for preparing erlotinib.
Owner:SHANGHAI CHANGLIN CHEM TECH
Methods and compositions for predicting resistance to anticancer treatment
InactiveUS20140296248A1Reduce expressionBiocideMicrobiological testing/measurementCancer cellReceptor tyrosine kinase inhibitor
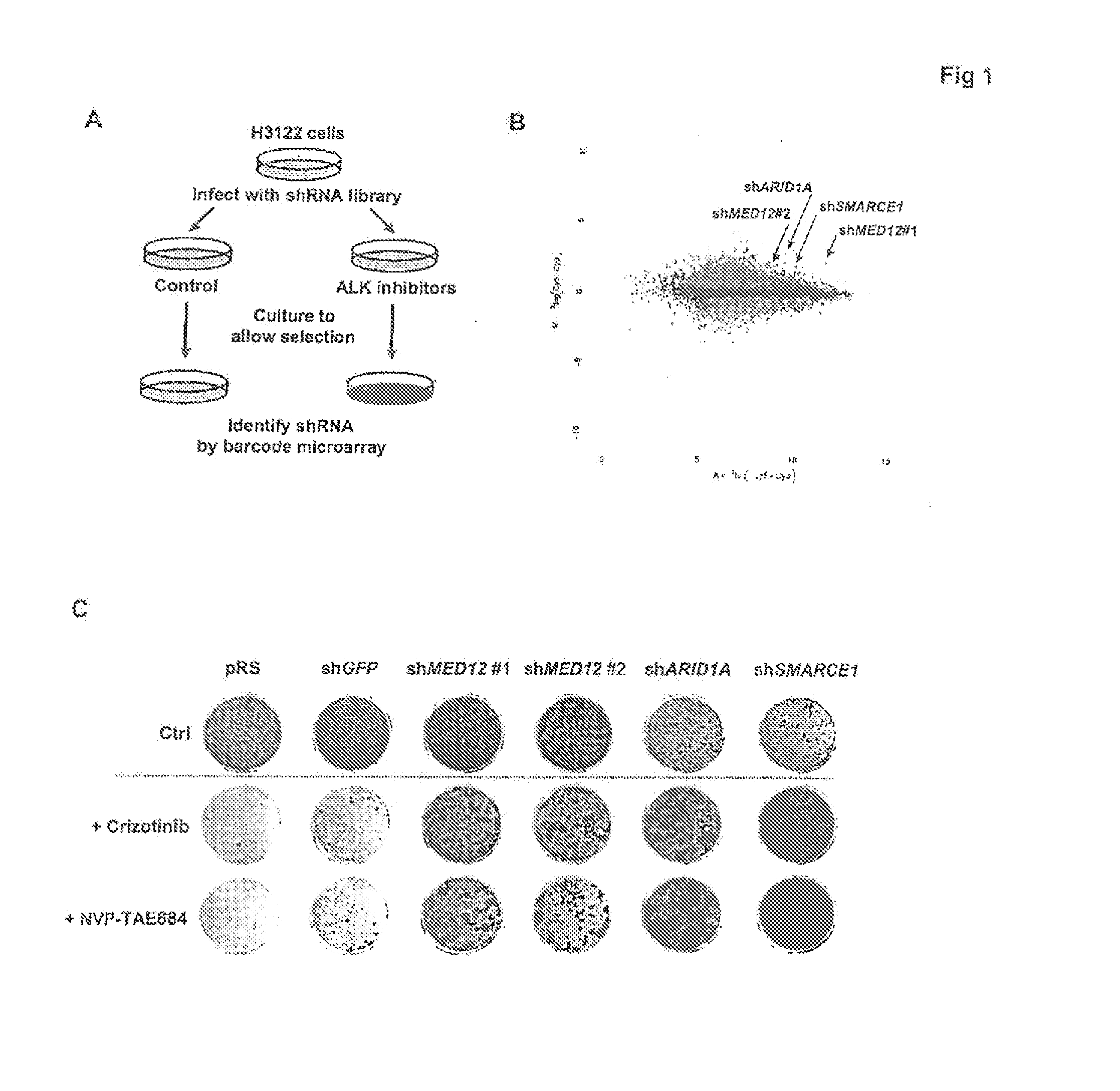
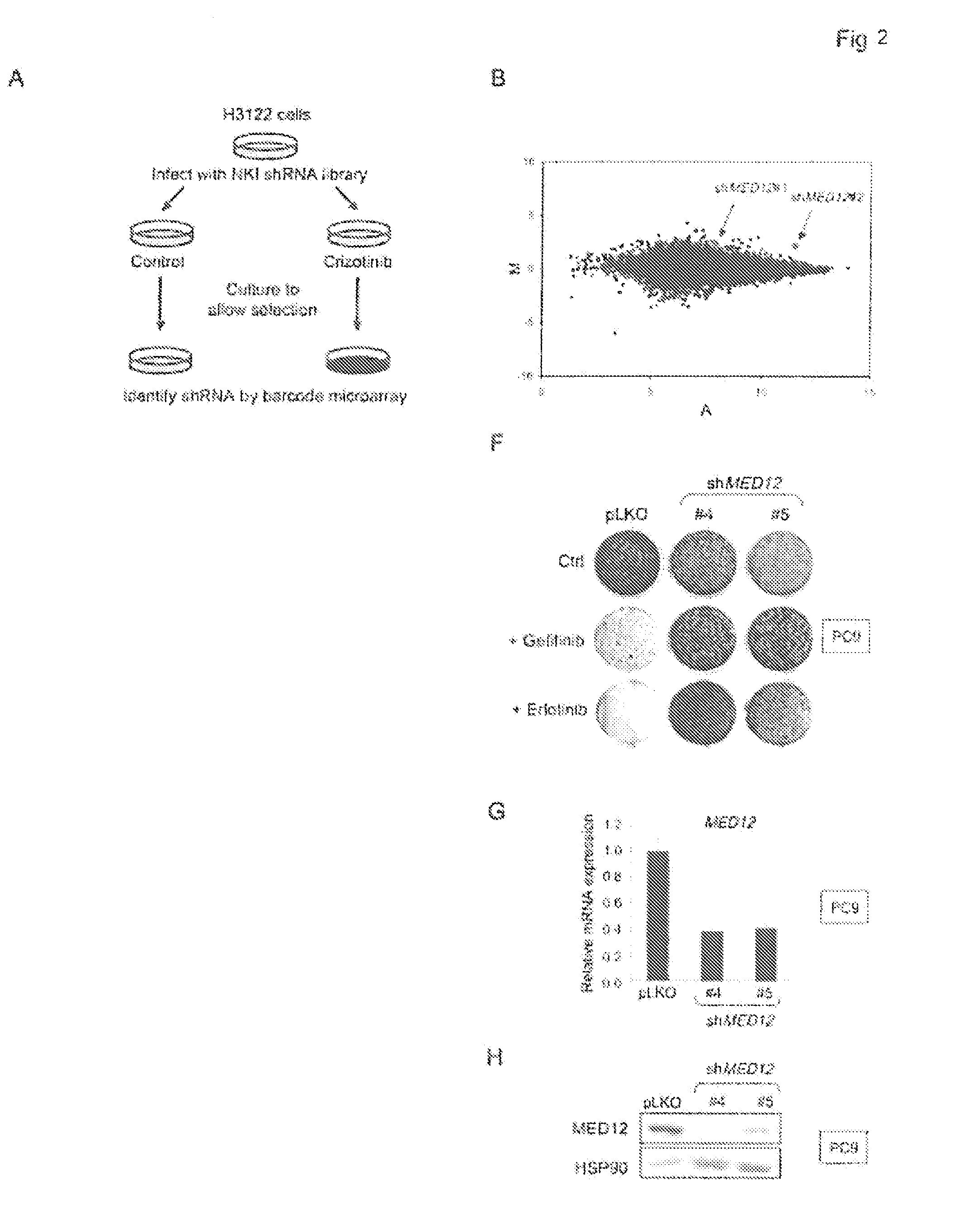
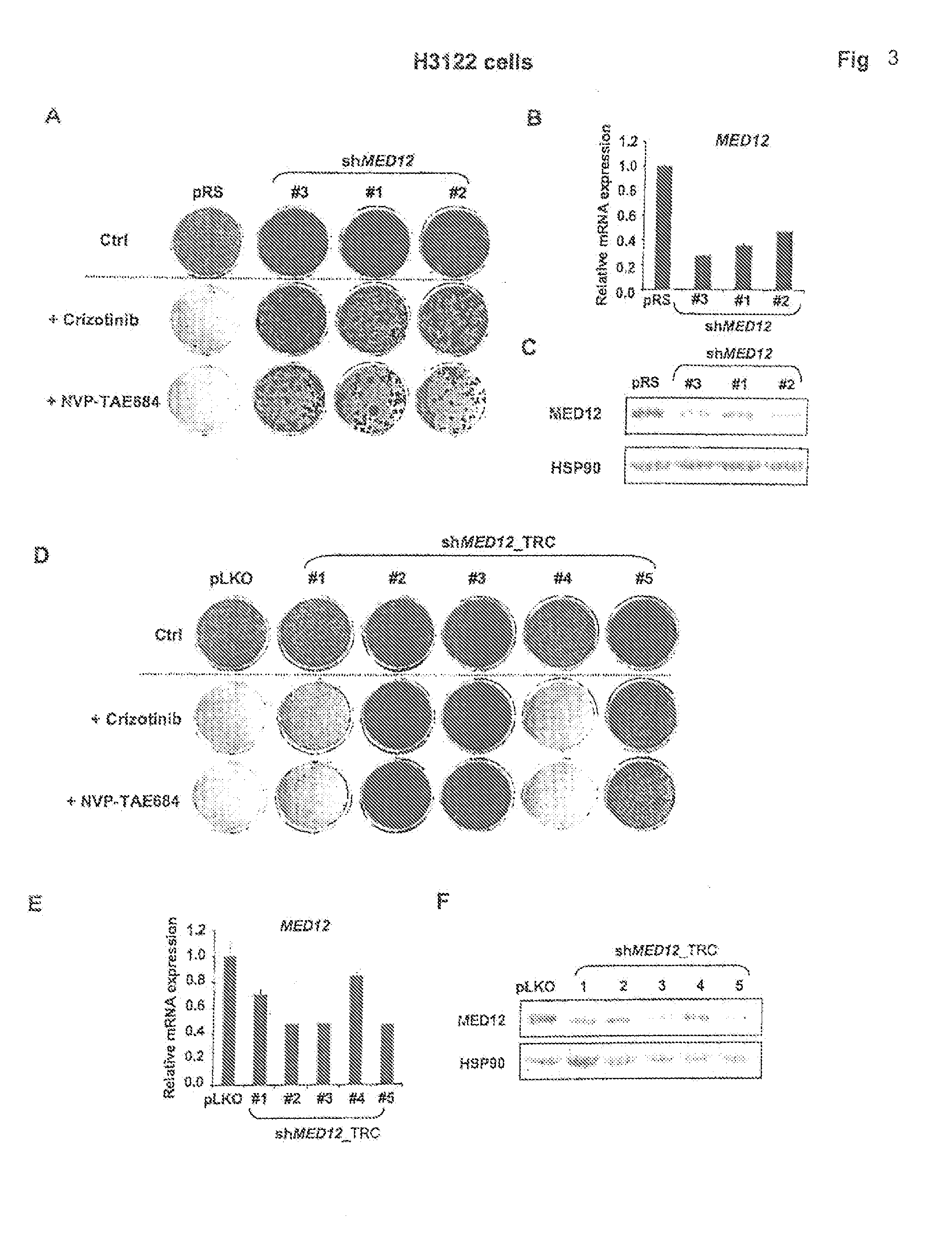
The instant application provides methods and related compositions pertaining to the identification of resistance to anticancer treatment in a patient. In a particular embodiment, the invention provides biomarkers for the identification of resistance to anticancer treatment in a lung cancer patient, wherein a reduced expression of a MEDIATOR and / or SW1 / SNF complex gene in the lung cancer cells of the patient indicates that the lung cancer cells in the patient may be resistant to treatment with a receptor tyrosine kinase inhibitor, such as gefitinib and / or erlotinib. In some embodiments, the invention relates to methods and related compositions for predicting resistance to anticancer treatment by detecting the expression levels of one or more TGF-beta pathway nucleic acids and / or proteins.
Owner:STICHTING HET NEDERLANDS KANKER INSTIUUT ANTONI VAN LEEUWENHOEK ZIEKENHUIS
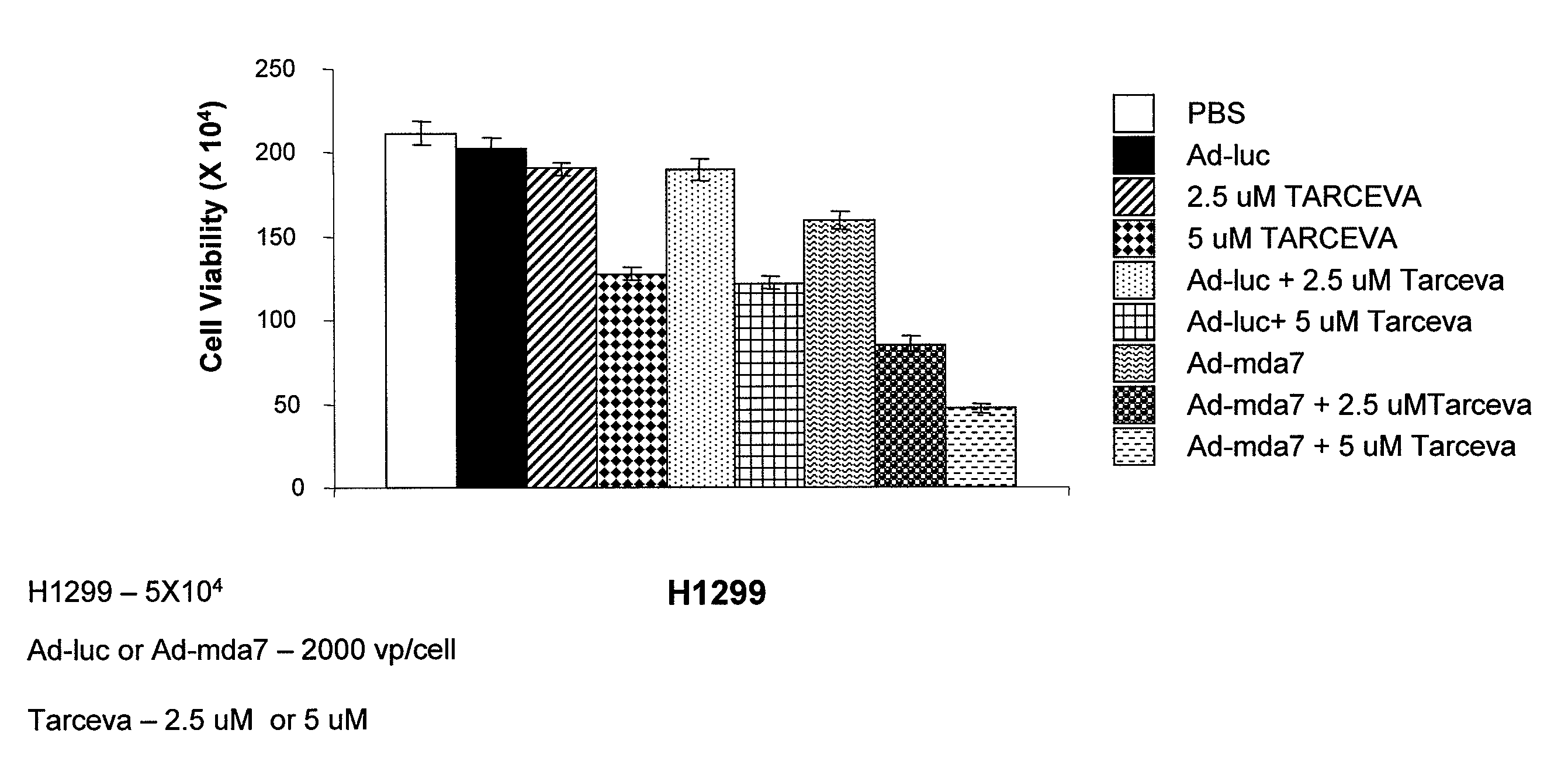
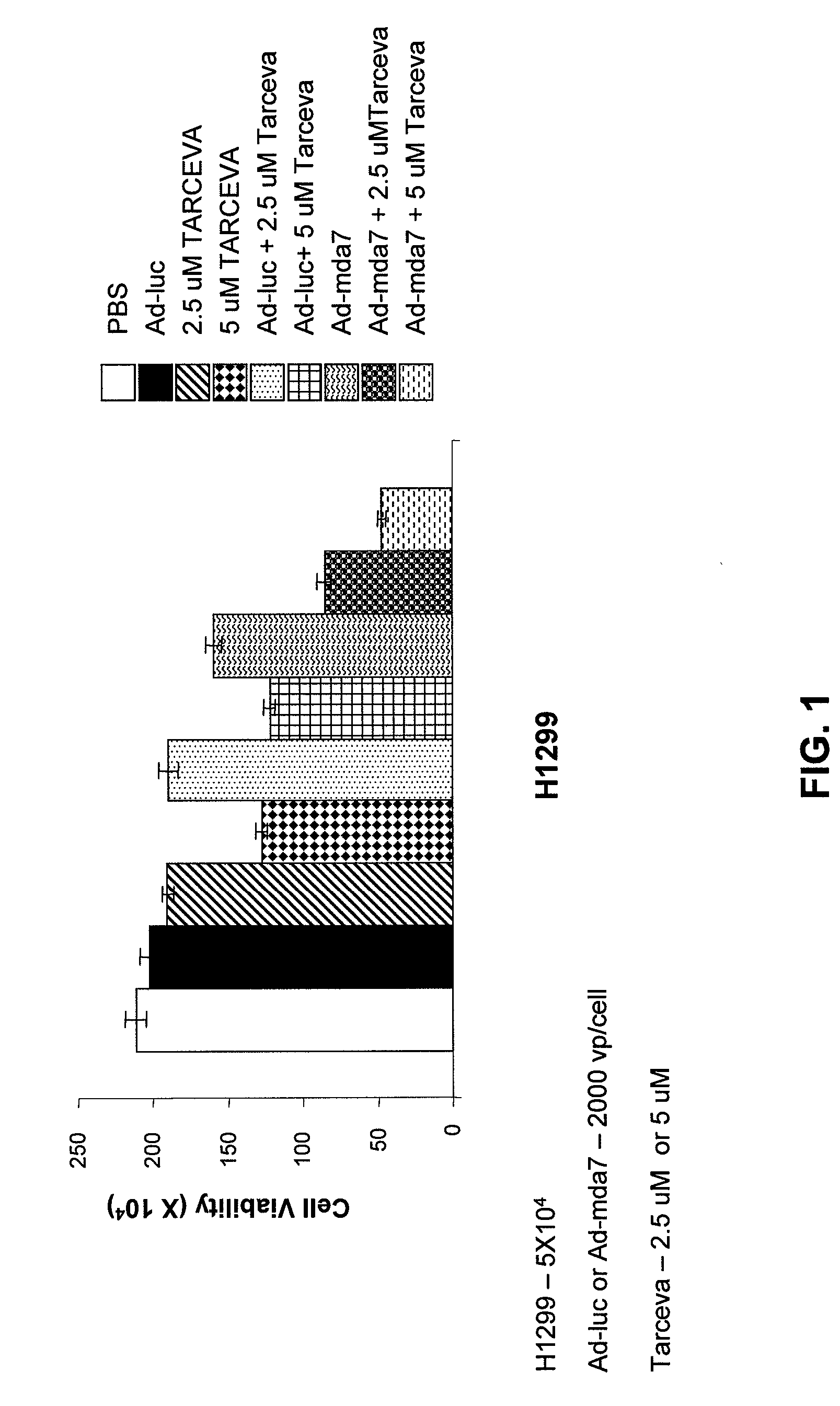
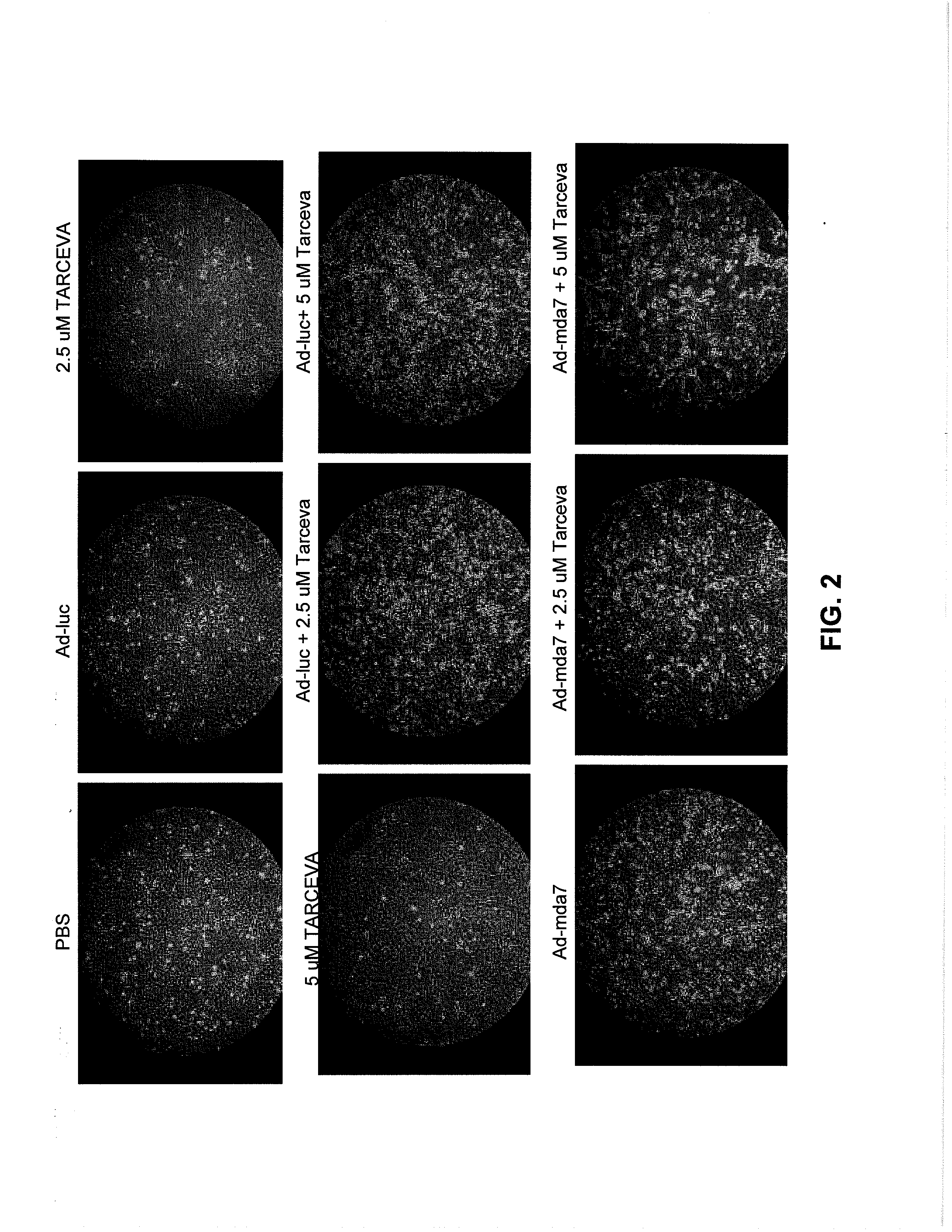
Compositions and Methods Involving MDA-7 for the Treatment of Cancer
InactiveUS20070281041A1Good treatment effectBiocideHeavy metal active ingredientsErlotinibTyrosine-kinase inhibitor
The present invention concerns methods and compositions involving MDA-7 protein or an MDA-7-encoding nucleic acid and an EGFR inhibitor for the treatment of cancer. In certain embodiments, the invention specifically concerns a small molecule tyrosine kinase inhibitor, for example, erlotinib, as the EGFR inhibitor.
Owner:INTROGEN THERAPEUTICS INC +1
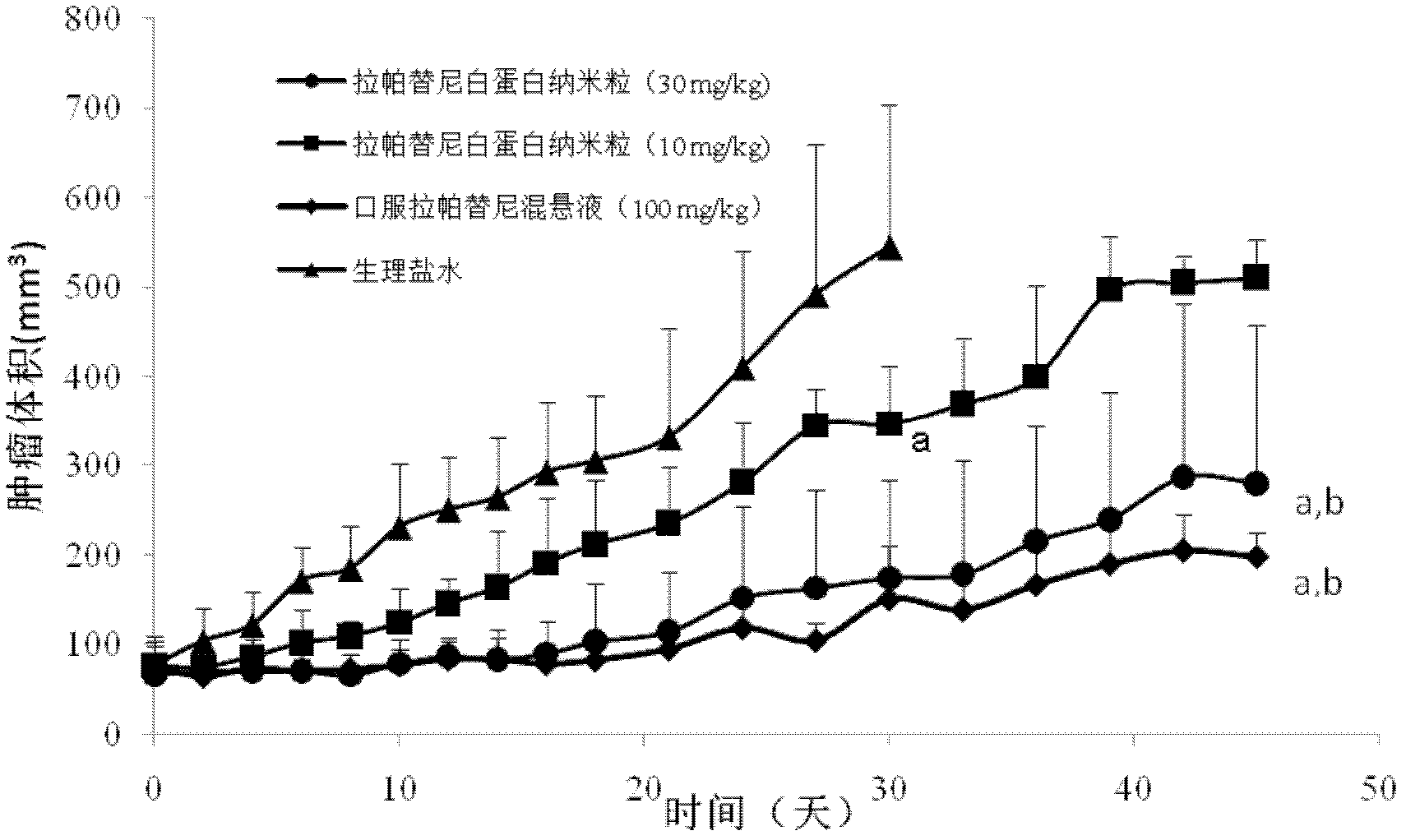
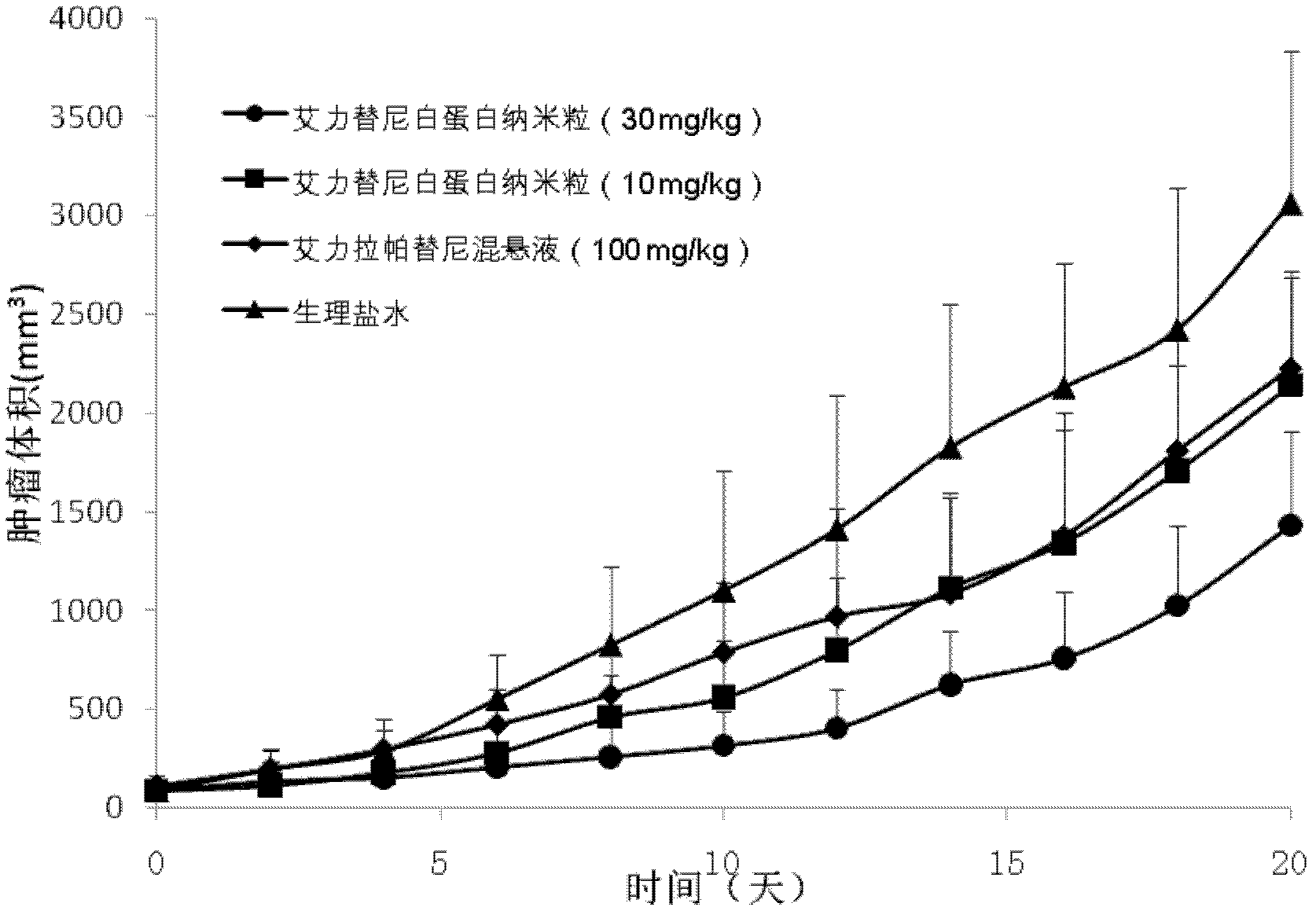
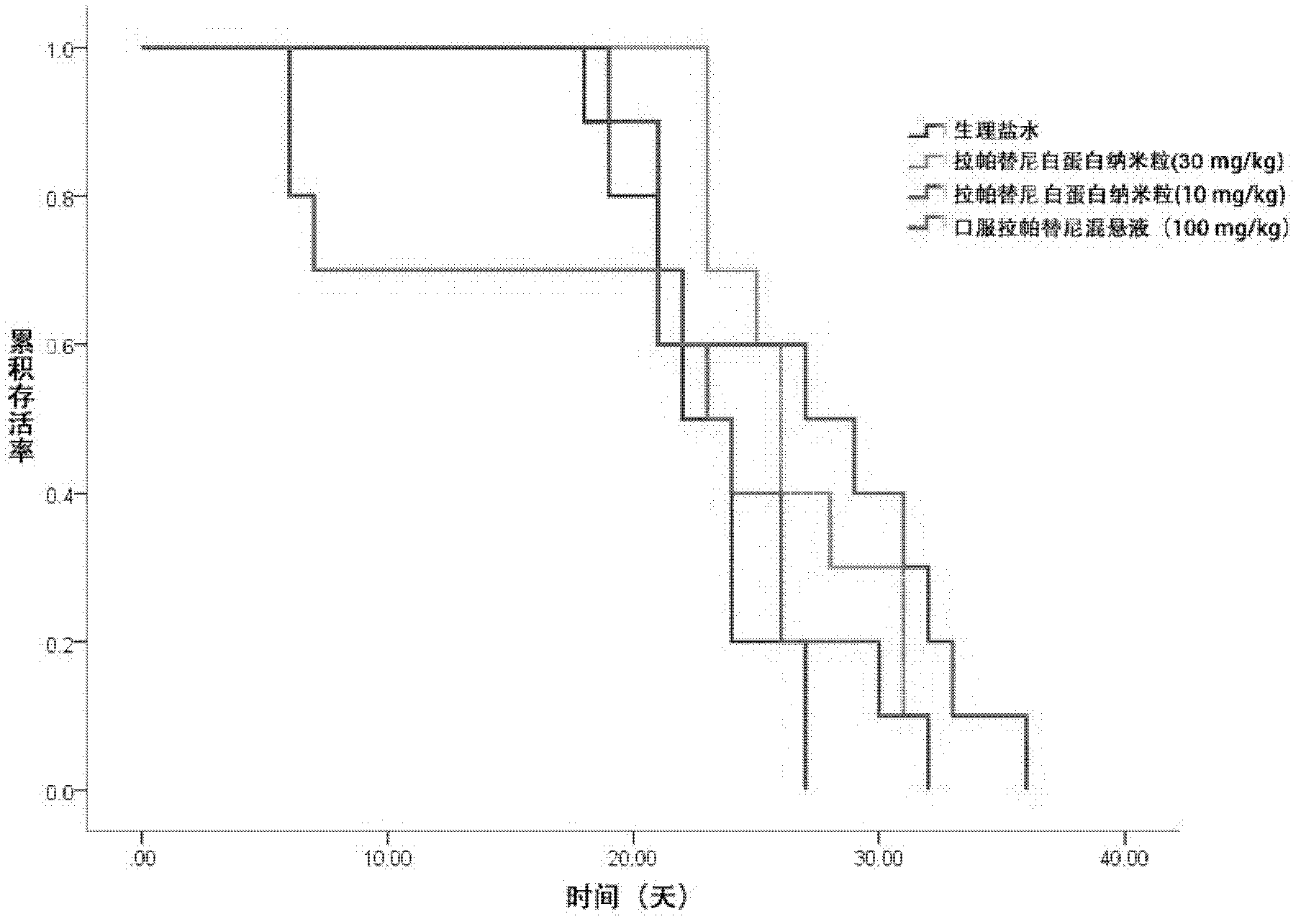
Albumin nanometer particle preparation for soluble injection and preparation method of albumin nanometer particle preparation
InactiveCN102626393AImprove permeabilityEnhanced retention effectPowder deliveryMacromolecular non-active ingredientsErlotinibPhospholipid
The invention belongs to the field of pharmaceutical preparation and relates to an albumin nanometer particle preparation for soluble injection. The albumin nanometer particle, which is formed by combining albumin, indissolvable erlotinib drugs and adding phospholipid for scattering and stabilizing, is prepared into the albumin nanometer particle preparation for soluble injection. The enhancing permeation and detaining effect of nanometer particle to tumor are utilized by the albumin nanometer particle preparation provided by the invention, so that more drugs are passively targeted and gathered on a tumor tissue and the anti-tumor effect is increased. Meanwhile, the bioavailability of injection mode is high, the albumin nanometer particle preparation for soluble injection has a passive targeting function and the dosage is greatly reduced, so that the concentration of the drugs in non-target part is efficiently reduced, the reducing of the toxic side effect of the drugs is boosted and the clinical application prospect is excellent.
Owner:FUDAN UNIV
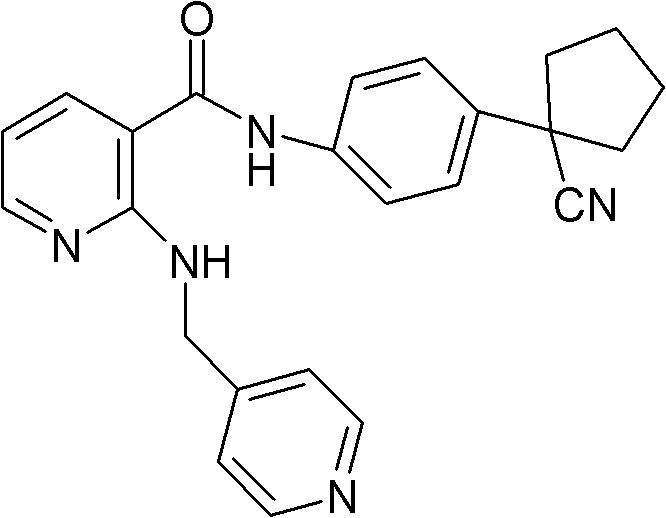
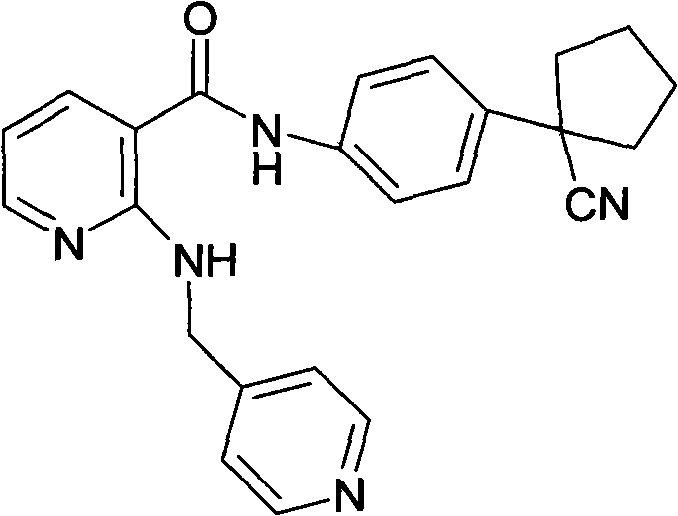
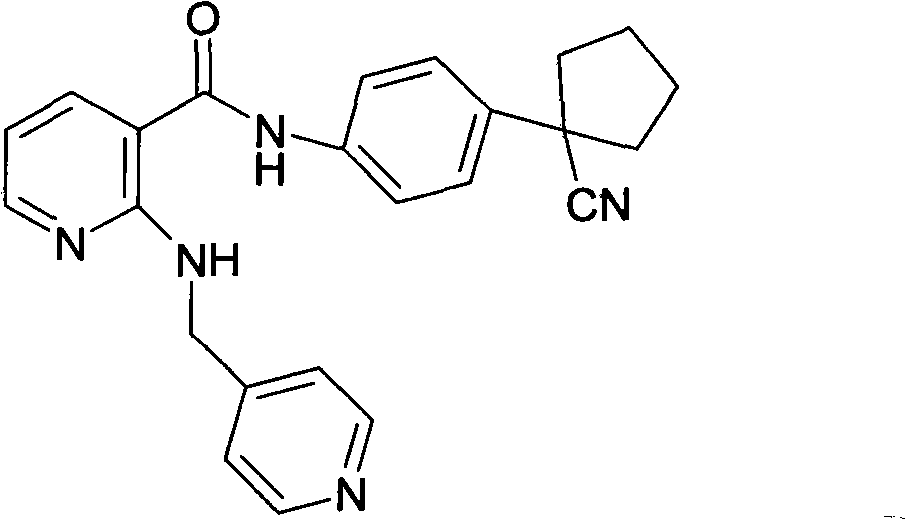
Drug composition for curing tumour diseases
The invention discloses a drug composition for curing tumour diseases, which comprises fixed dose of N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide or pharmaceutically acceptable salt of the N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide and erlotinib or pharmaceutically acceptable salt of the erlotinib or comprises fixed dose of N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide or pharmaceutically acceptable salt of the N-(4-(1-cyanocyclopentyl) phenyl)-2-(4-picolyl) amino-3-pyridinecarboxamide and gefitinib or pharmaceutically acceptable salt of the gefitinib. A method for preparing the drug composition and an application of the drug composition in preparing drugs for curing tumour diseases are further provided.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method of treatment of EGFR inhibitor toxicity
InactiveUS20110190244A1Reduce severityGood curative effectBiocideSenses disorderErlotinibTyrosine-kinase inhibitor
The invention provides a method of treating and / or preventing a toxicity associated with epidermal growth factor receptor (EGFR) inhibitor therapy in a subject, the method comprising administering to the subject an effective amount of a steroid sulfatase (STS) inhibitor. The toxicity may be ocular toxicity; or dermatologic toxicity, such as papulopustular rash. The EGFR inhibitor may be selected from the group consisting of: a small molecule; an antibody or derivative or fragment thereof; another agent that targets the extracellular or intracellular domain of the EGFR, such as a tyrosine kinase inhibitor selected from the group consisting of: erlotinib; gefitinib; lapatinib; and any combination thereof. The EGFR inhibitor may also be antibody selected from the group consisting of: cetuximab; panitumumab; and any combination thereof.Preferably the STS inhibitor is selected from the group consisting of: alternative STS substrates; reversible STS inhibitors; and irreversible STS inhibitors; and any combination thereof. A preferred STS inhibitor is the irreversible nonsteroidal STS inhibitor STX64.In some embodiments, the subject receiving EGFRI therapy has a cancer comprising cells that express wildtype k-ras and / or wildtype b-raf. In other embodiments, the cancer may be hormone-dependent. Cancers that may be treated with EGFRI therapy include colorectal cancer and non-small cell lung cancer.
Owner:PETER MACCALLUM CANCER INST
Erlotinib-phthalocyanine conjugate as molecule-targeting anticancer photosensitizer
ActiveCN103341166AImprove targetingEnhance photodynamic activityEnergy modified materialsAntineoplastic agentsPhotosensErlotinib
The invention discloses an erlotinib-phthalocyanine conjugate as a molecule-targeting anticancer photosensitizer and its preparation method and use. Erlotinib structure units with alcoxyl long-chains are introduced to the periphery of a metal phthalocyanine large-ring so that amphipathy, biocompatibility and photosensitizer targeting are improved. The erlotinib-phthalocyanine conjugate is not aggregated easily, is conducive to cell uptake ratio improvement, has a single structure, has no isomers and can be purified easily. The erlotinib-phthalocyanine conjugate can improve photosensitizer targeting in photodynamic therapy and improve photosensitizer activity in photodynamic therapy. The preparation method has simple processes, less side reactions and a high yield, adopts easily available raw materials, has a low cost and is conducive to industrial production.
Owner:FUZHOU UNIV
Methods for Inhibiting Cell Proliferation in EGFR-Driven Cancers
InactiveUS20140024620A1Easy to measureReduce riskBiocideOrganic active ingredientsErlotinibTyrosine-kinase inhibitor
The invention features a method for treating patients who have an EGFR-driven cancer, which is, or has become, refractory to a tyrosine kinase inhibitor, such as eriotinib and gefitinib, by administering a compound of formula (I) to the patient. The invention also features treating EGFR-driven cancers having an EGFR mutation identified herein.
Owner:ARIAD PHARMA INC
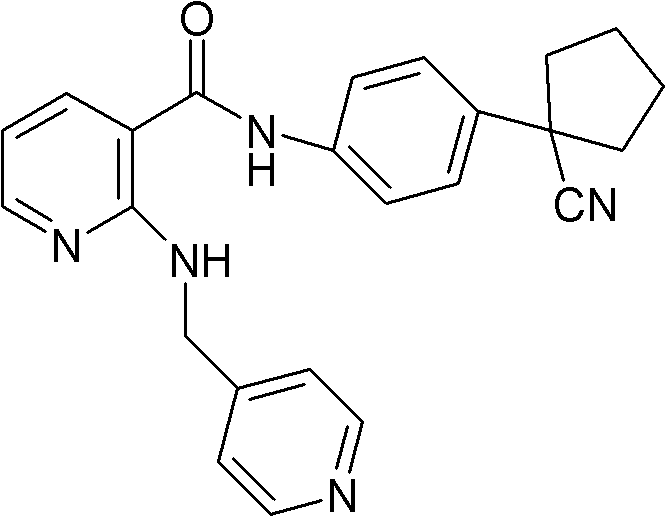
Pharmaceutical composition for treating tumor diseases
The invention discloses a pharmaceutical composition for treating tumor diseases, a preparation method of the pharmaceutical composition and application of the pharmaceutical composition in preparing the medicines for treating tumor diseases, wherein the pharmaceutical composition consists, in a fixed dosage, of N-[4-(1-cyan cyclopentyl)phenyl]-2-(4-picolyl) amidogen-3-pyridinecarboxamide or pharmaceutically acceptable salts thereof and angiostatin or pharmaceutically acceptable salts thereof, or N-[4-(1-cyan cyclopentyl)phenyl]-2-(4-picolyl) amidogen-3-pyridinecarboxamide or pharmaceutically acceptable salts thereof and gefitinib or pharmaceutically acceptable salts thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
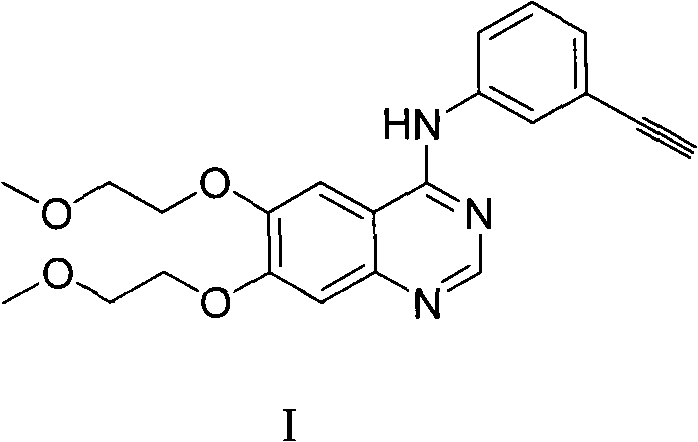
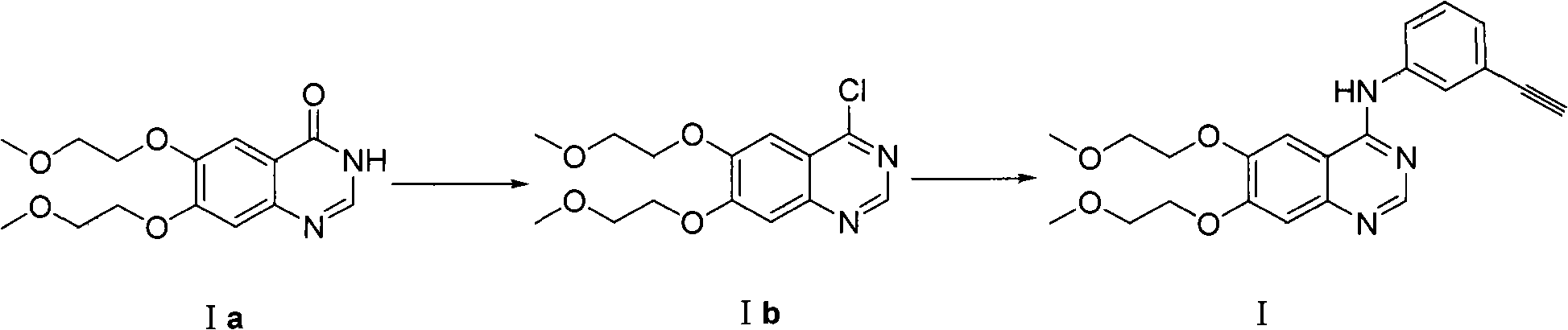
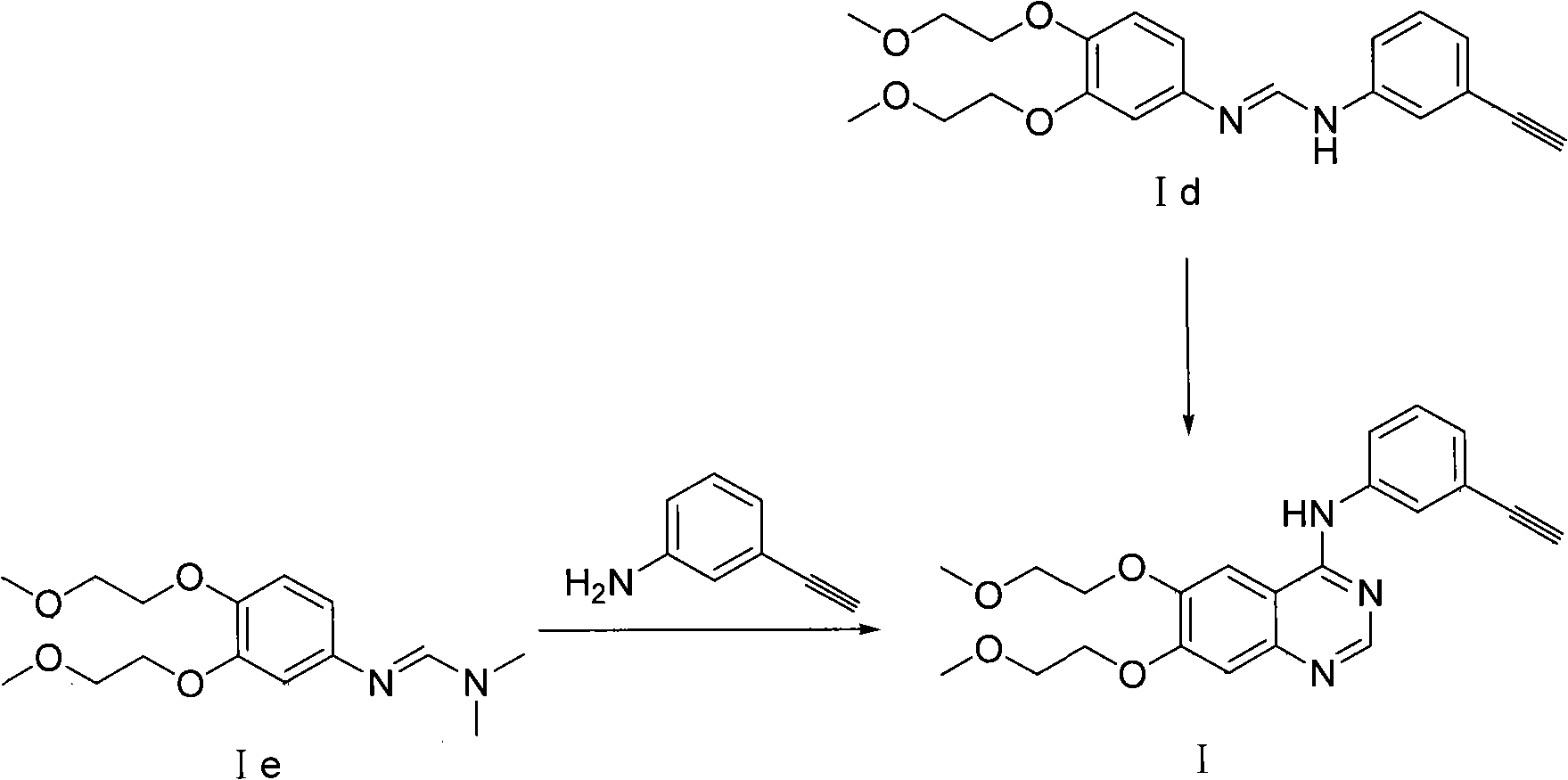
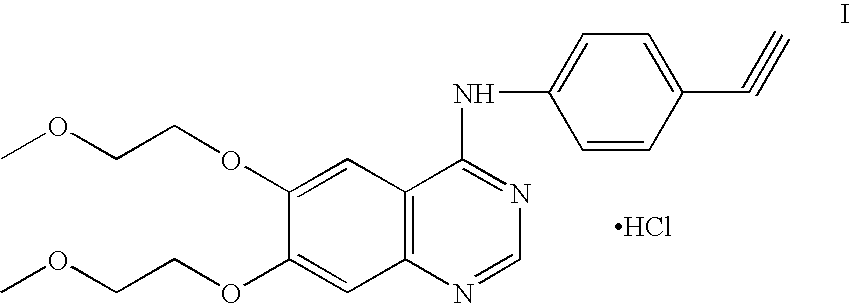
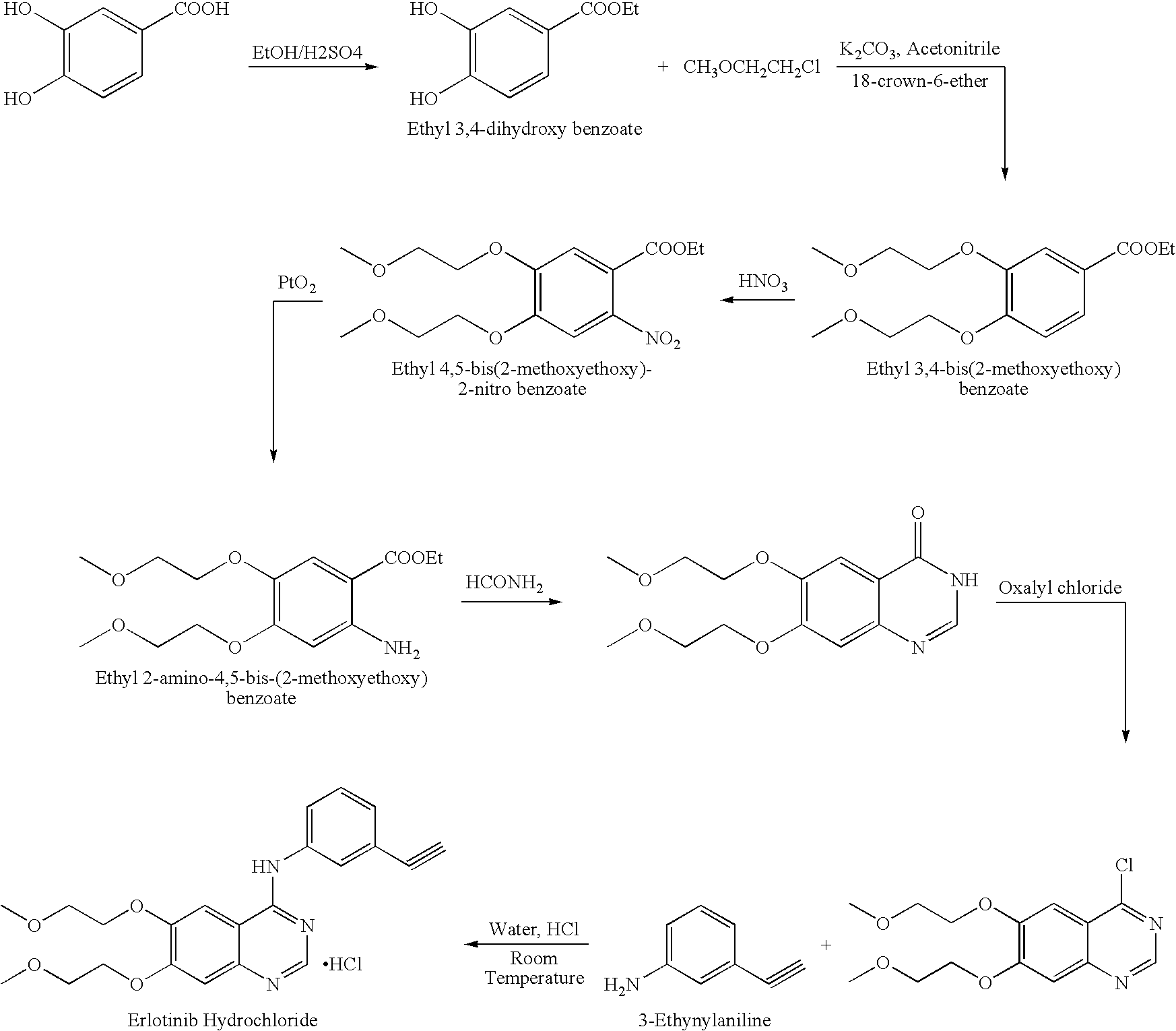
Process for Preparation of Erlotinib and its Pharmaceutically Acceptable Salts
InactiveUS20100094004A1Simple and economical commercial productionSimple and economical processOrganic chemistryDrug compositionsErlotinibQuinazoline
A process for the preparation of a salt of N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine comprising reacting a 4˜halo-6,7-bis(2-methoxyethoxy) quinazoline with 3-aminophenyl acetylene or an acid salt thereof under acidic conditions to form the corresponding acid salt of N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine, the process optionally further comprising converting the acid salt of N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine to N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine.
Owner:CIPLA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com