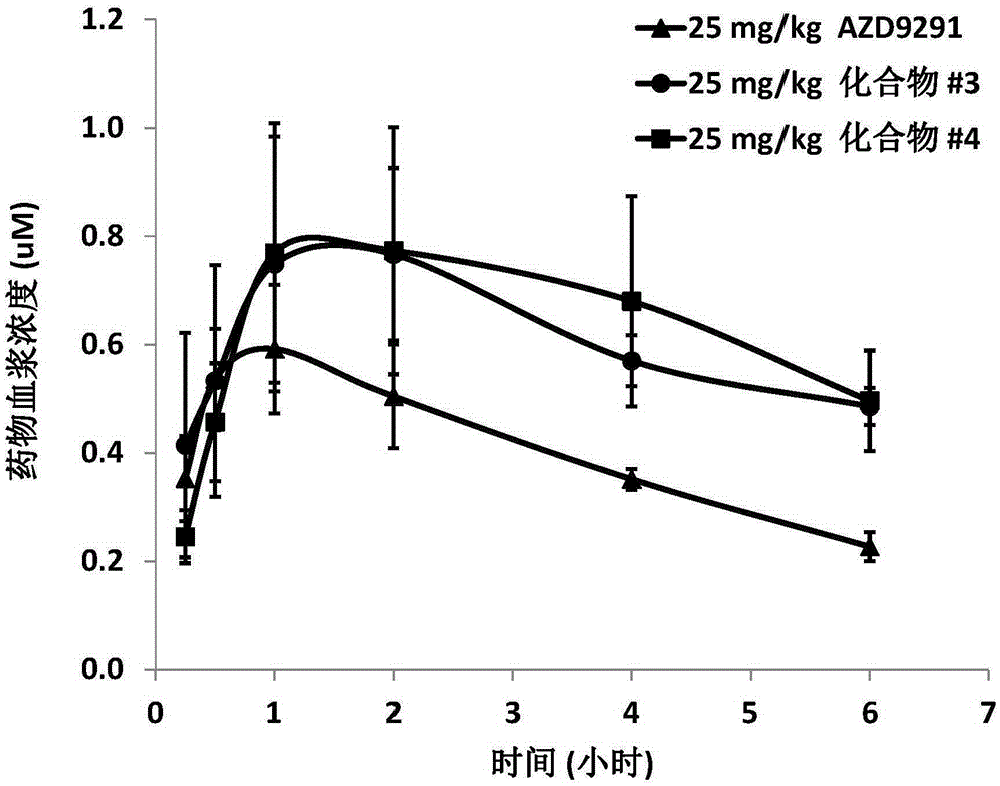
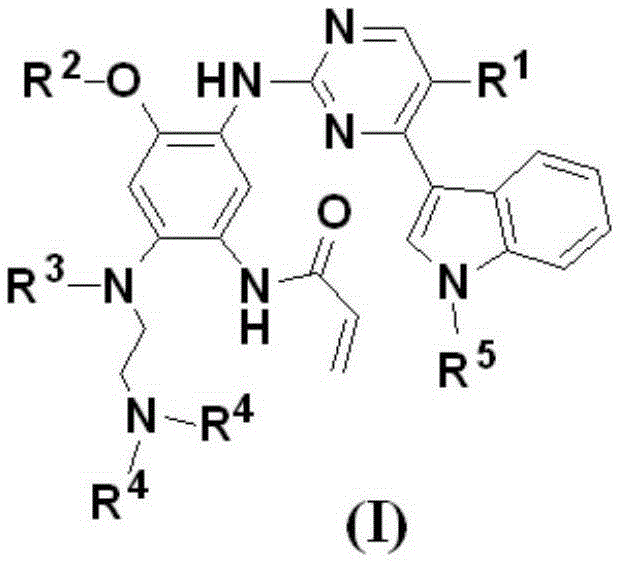
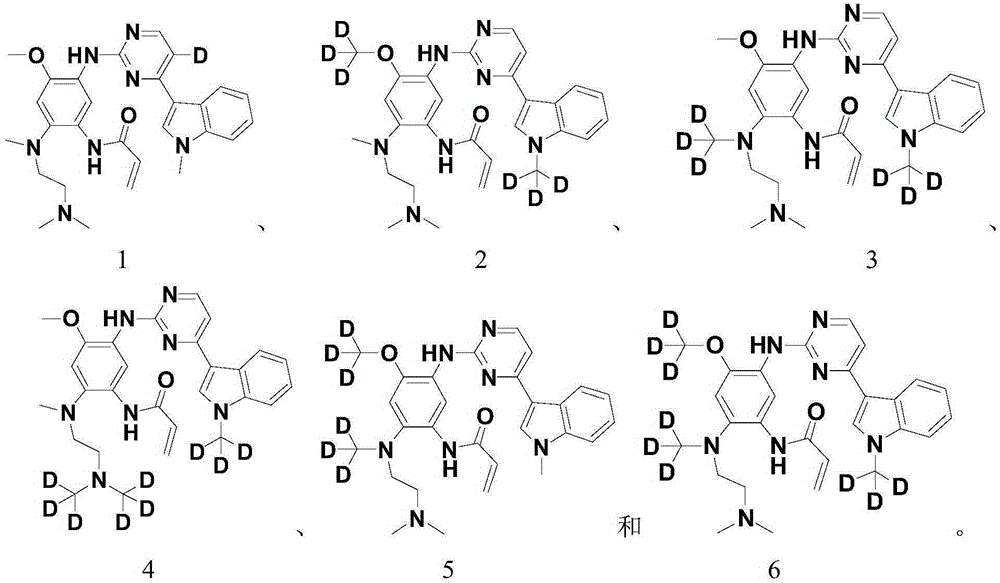
Pentadeuteropyridine compounds, and preparation method, pharmaceutical compositions and uses thereof
A compound and composition technology, applied in the field of chemical medicine, can solve problems such as poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065] 1. Synthesis of Intermediate 001-2
[0066]
[0067]Under nitrogen protection, 001-1 (100g, 708.5mmol) and 800mL sulfuric acid (H 2 SO 4 ), lower the temperature to 0°C, and add potassium nitrate (KNO 3 ) (71.6g, 708.19mmol), took 1h, and finally reacted overnight at room temperature (rt). After the reaction was over, 2000 mL of ice water was added to the three-neck flask to quench the reaction. The reaction mixture was adjusted to pH 10 with aqueous ammonia at low temperature and extracted 3 times with 1 liter (L) of dichloromethane (DCM). After the organic phases were combined, they were backwashed three times with 3000 mL of saturated brine, dried over anhydrous sodium sulfate, and spin-dried. The obtained crude product was subjected to silica gel column chromatography (eluent: ethyl acetate (EA):petroleum ether (PE)=1:4-1:1), and the eluent was spin-dried to obtain 79g (60%) 001-2, It is a yellow solid. LC-MS: 187.0.
[0068] 2. Synthesis of In...
Embodiment 2
[0091]
[0092] 1. Synthesis of intermediate 002-2
[0093]
[0094] Under nitrogen protection, add 002-1 (10g, 85.4mmol) and 100mL anhydrous THF to a 250mL three-necked flask in sequence, cool down to 0°C, add sodium hydrogen (NaH) (63%, dispersed in mineral oil) in batches (3.47g, 144.6mmol) for 5min, then reacted at 0°C for 30min, then added deuteroiodomethane (14.9g, 102.6mmol) dropwise at 0°C for 5min, and finally warmed to room temperature and reacted overnight. After the reaction was completed, 100 mL of ice water was added to the reaction mixture to quench the reaction, and then extracted three times with 100 mL of ethyl acetate (EA). The organic phases were combined, backwashed once with 100 mL saturated brine, dried over anhydrous sodium sulfate, and spin-dried. The obtained crude product was subjected to silica gel column chromatography (eluent: EA:PE=1:20-1:10), and the obtained eluate was spin-dried to obtain 9.8 g of 002-2 (86%) as a light white solid. LC...
Embodiment 3
[0124]
[0125] 1. Synthesis of intermediate 003-1
[0126]
[0127] Under nitrogen protection, 002-4 (1.0g, 4.05mmol), 001-2 (756mg, 4.06mmol), 20mL of isopropanol and p-toluenesulfonic acid (839mg, 4.87mmol) were sequentially added to a 100mL single-necked bottle, oil The bath was heated to 105°C and reacted for 5h. After the reaction, the reaction mixture was cooled to room temperature and filtered. The filter cake was washed 3 times with 100 mL of isopropanol, then 3 times with 100 mL of acetonitrile, and dried to obtain 1.0 g of 003-1 (62%) as a yellow solid. LC-MS: 397.1.
[0128] 2. Synthesis of intermediate 003-3
[0129]
[0130] Under nitrogen protection, 003-2 (90g, 611.7mmol), THF (1500mL), PPh 3 (176.4g, 672.6mmol) and CD 3 OD (22.5 g, 642.9 mmol). The temperature was lowered to 0°C, diethyl azodicarboxylate (DEAD) (117 g, 671.8 mmol) was added dropwise, and the addition was completed within 1 h. React overnight at room temperature. After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com