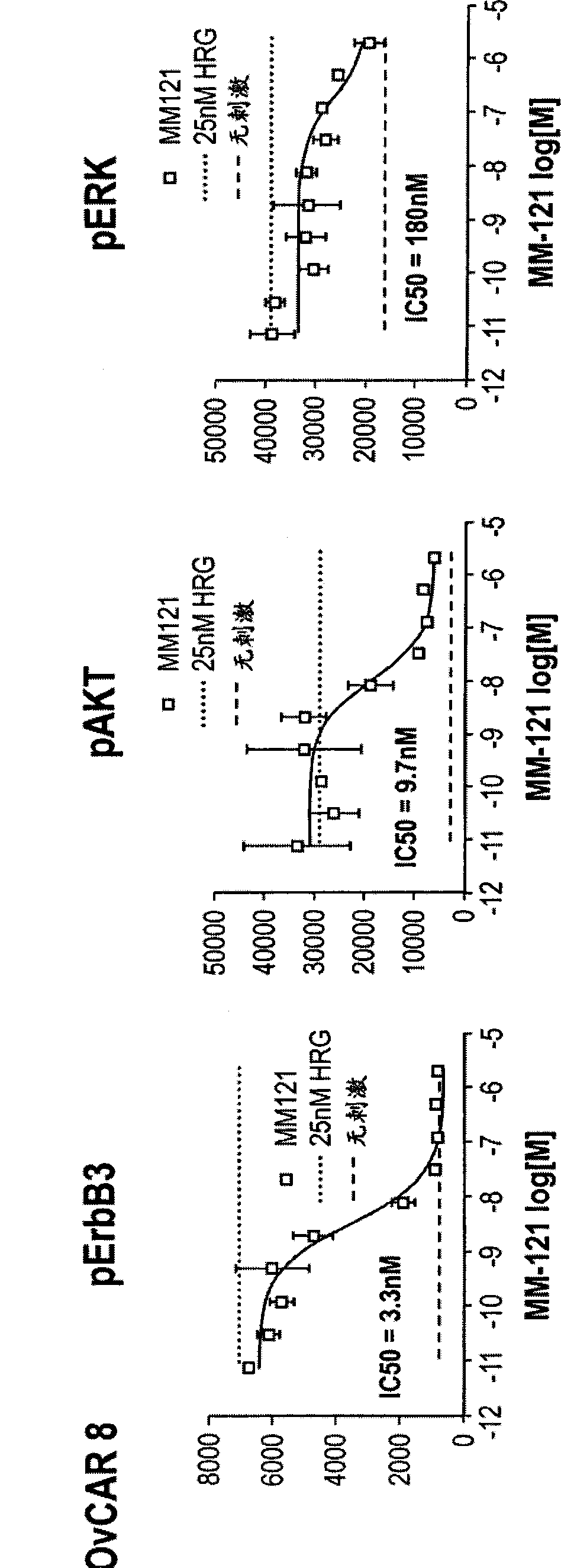
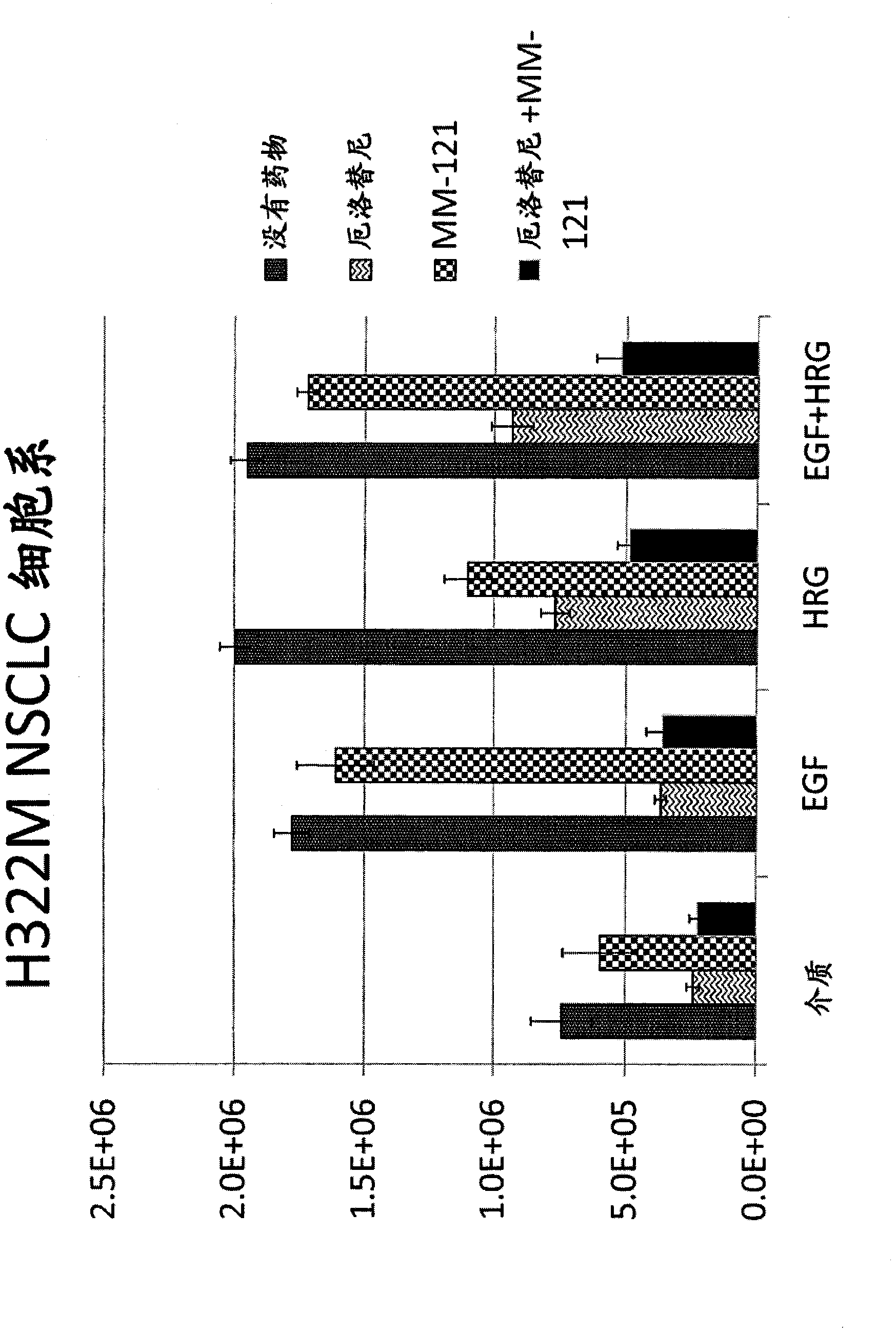
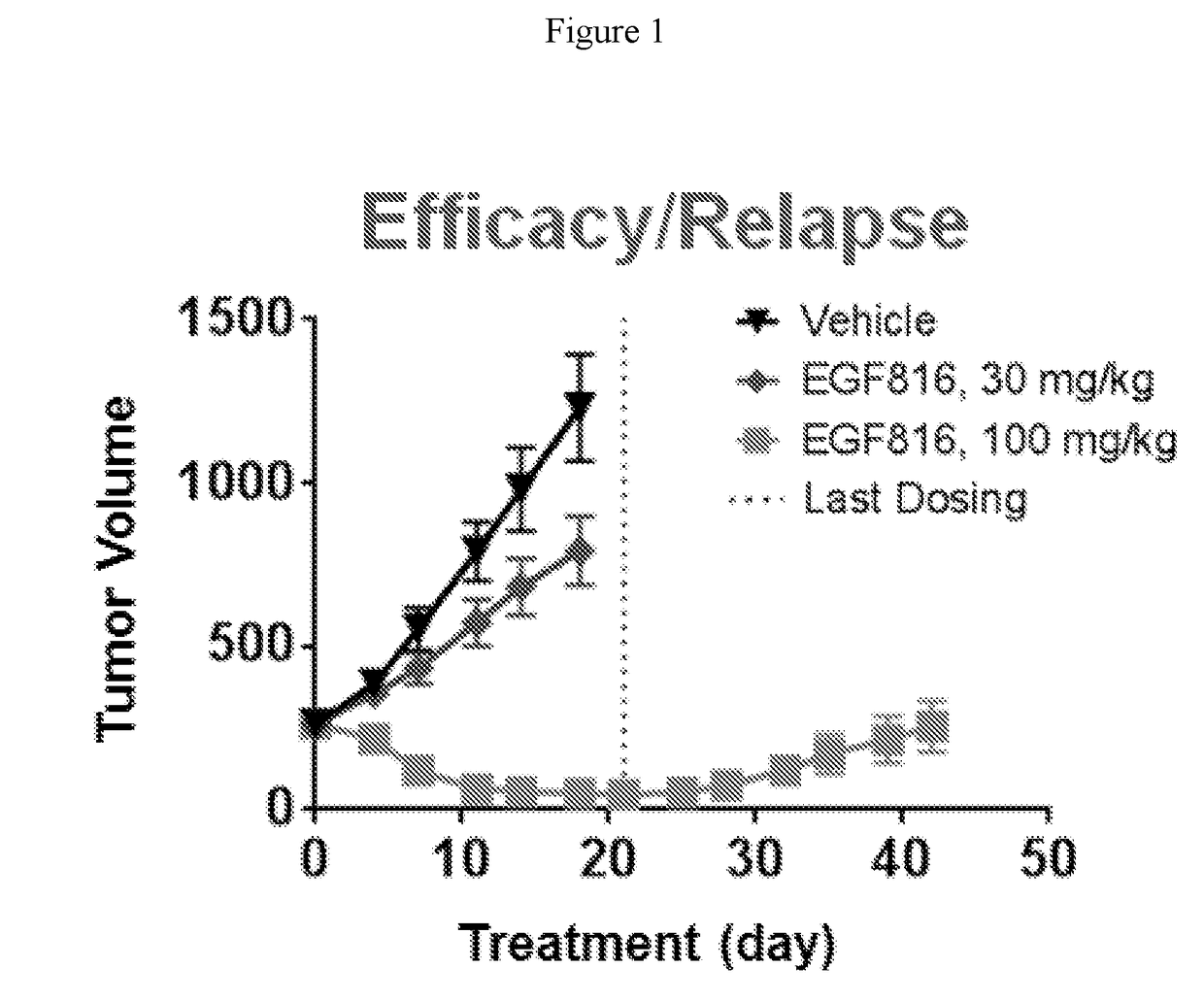
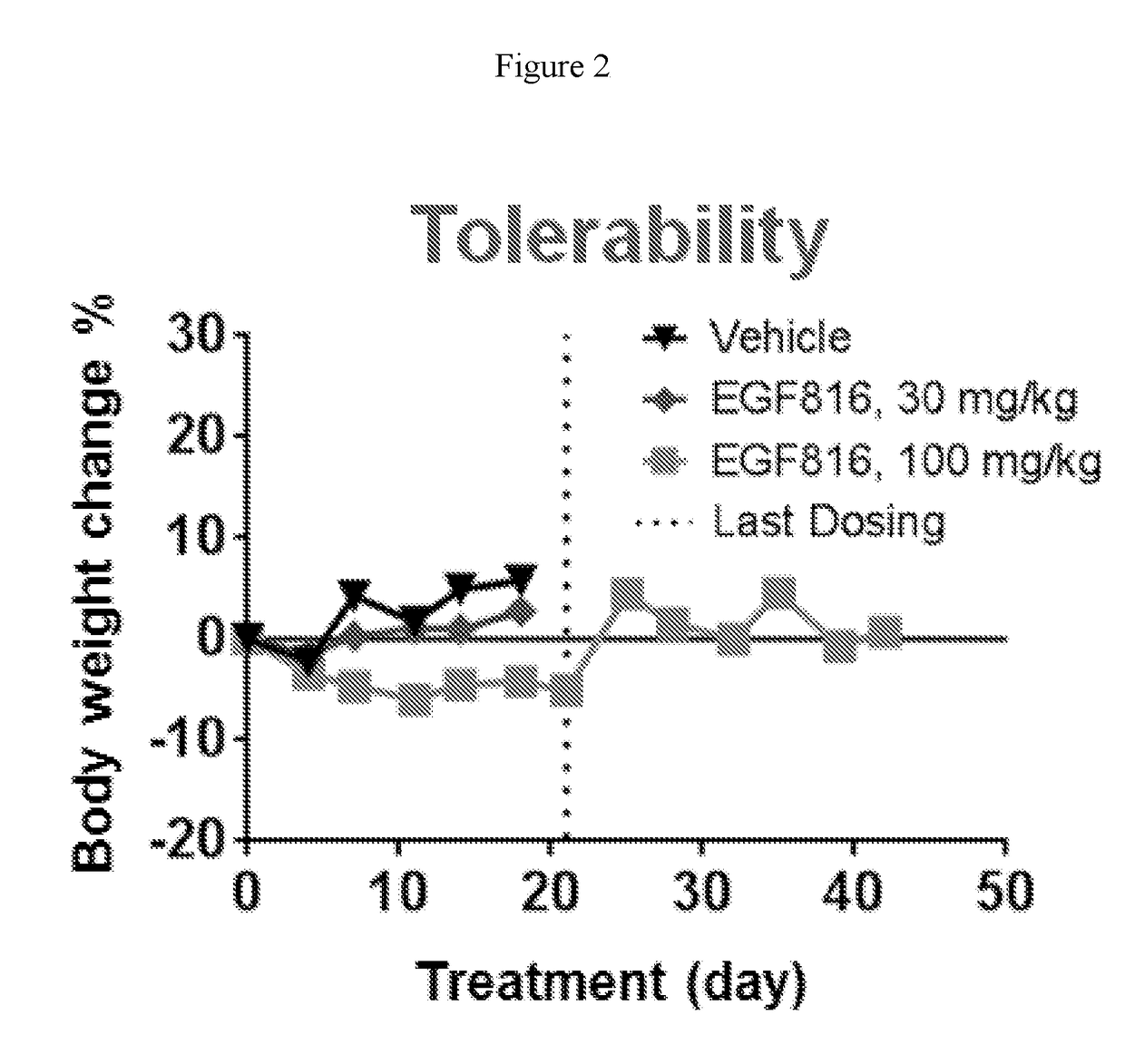
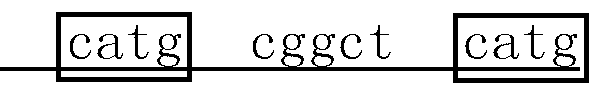Patents
Literature
116 results about "T790M" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
T790M, also known as Thr790Met, is a gatekeeper mutation of the epidermal growth factor receptor (EGFR). The mutation substitutes a threonine (T) with a methionine (M) at position 790 of exon 20, affecting the ATP binding pocket of the EGFR kinase domain. Threonine is a small polar amino acid; methionine is a larger nonpolar amino acid. Rather than directly blocking inhibitor binding to the active site, T790M increases the affinity for ATP so that the inhibitors are outcompeted; covalent inhibitors such as neratinib can overcome this resistance.
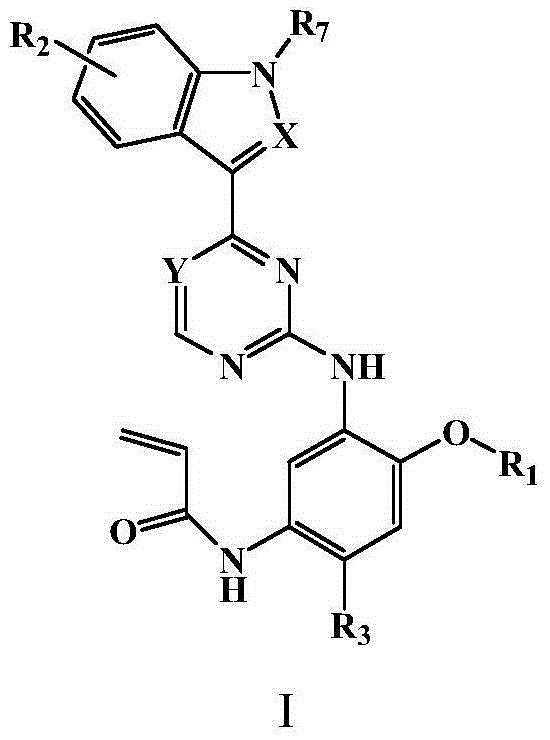
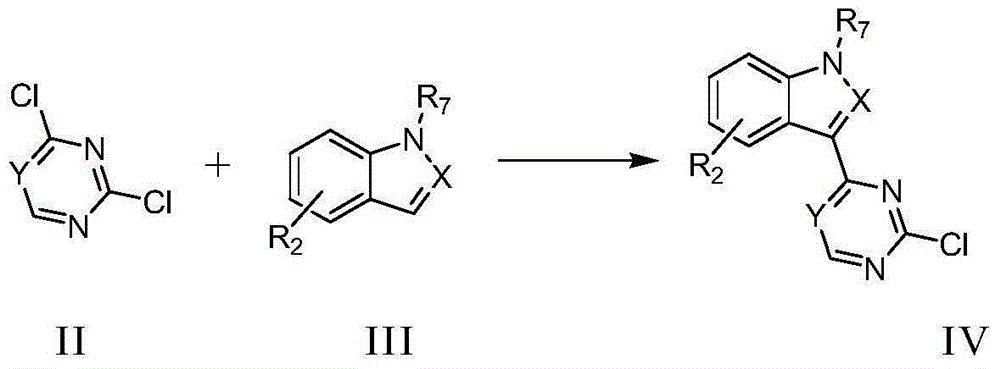
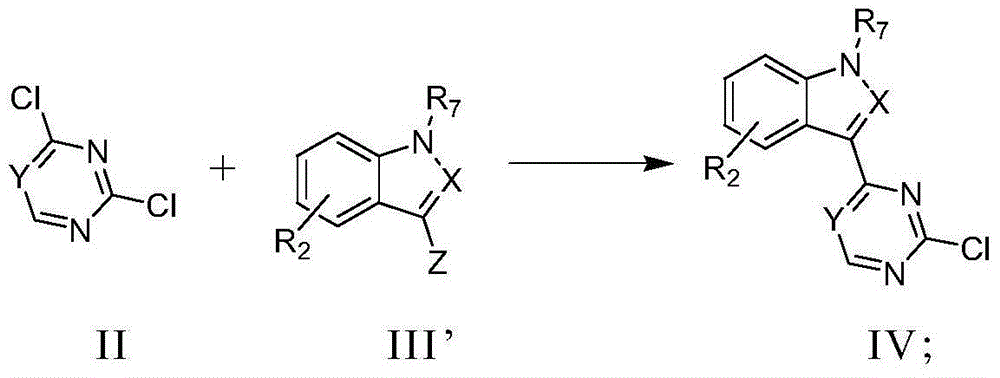
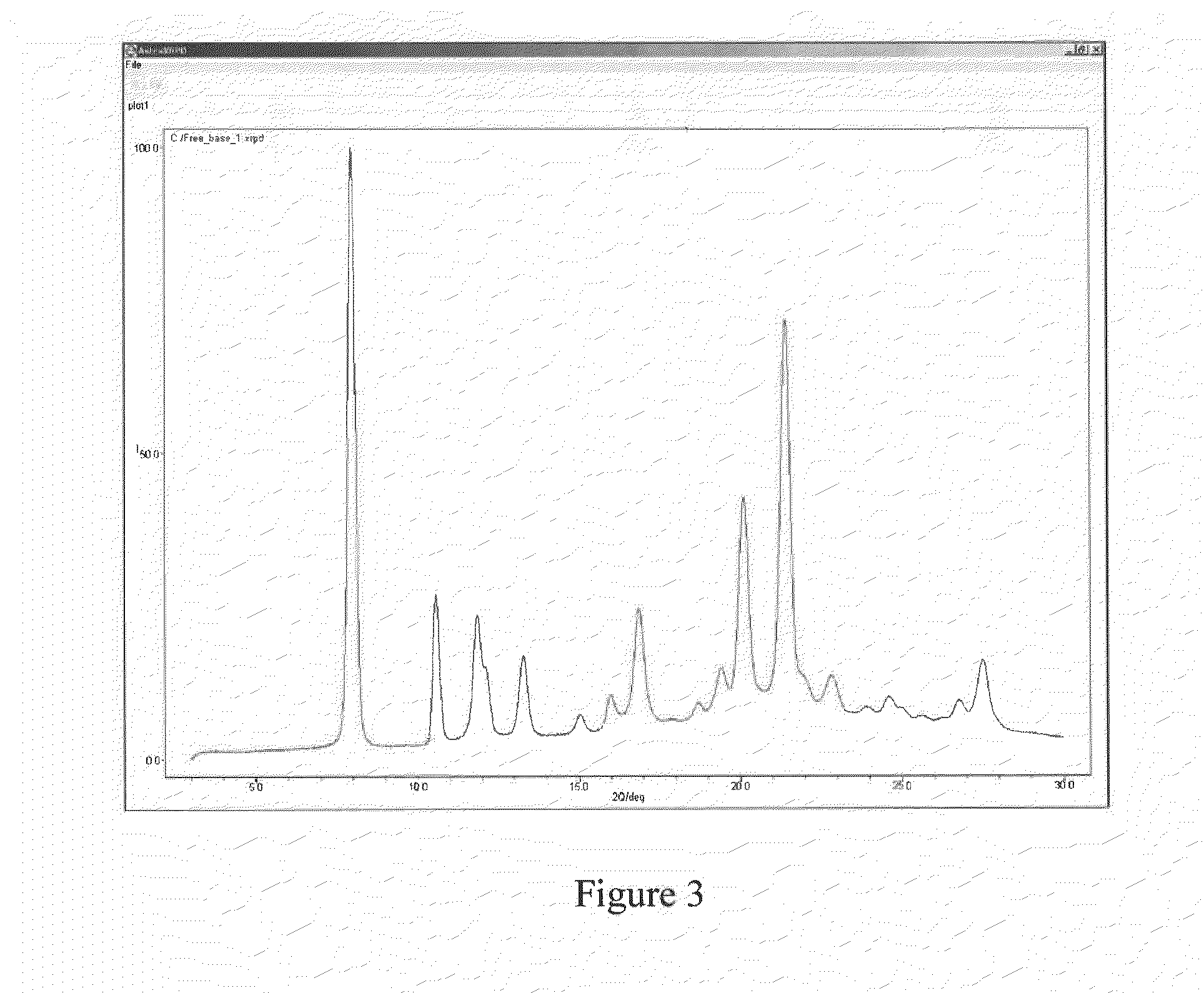
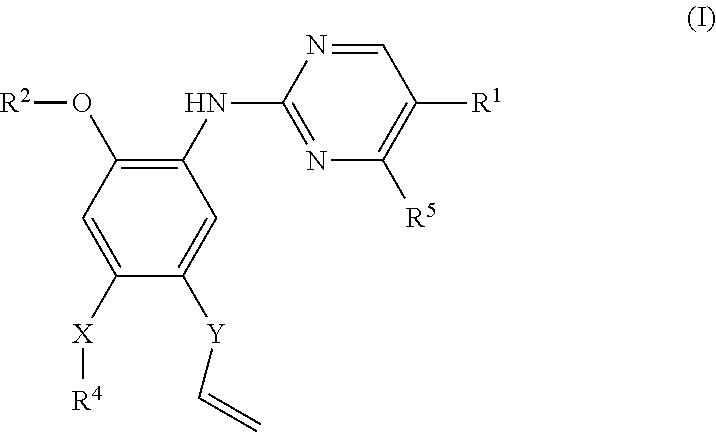
Pyrimidine or triazine derivative, and preparation method and use thereof
ActiveCN105461695AStrong inhibitory activityAvoid or reduce toxic side effectsOrganic chemistryAntineoplastic agentsTriazine derivativeInhibitory effect
The invention relates to an arylaminopyrimidine or triazine derivative, and a preparation method, a medicinal composition and a use thereof, and concretely relates to a compound represented by formula I, or a pharmaceutically acceptable salt or a solvate thereof. In the formula I, R1 to R7, X and Y are defined in the description and claims. The invention also relates to a preparation method of the compound of formula I, the medicinal composition containing the compound, and the pharmacy use of the compound and the medicinal composition. The compound of formula I is an effective tyrosine kinase irreversible inhibitor, and especially has a strong inhibition effect on EGFR-T790M drug-resistant tumors.
Owner:SHENZHEN FORWARD PHARMA LTD CO
Pentadeuteropyridine compounds, and preparation method, pharmaceutical compositions and uses thereof
ActiveCN105237515AImprove securityStrong inhibitory activityOrganic active ingredientsOrganic chemistry methodsDiseaseErlotinib
The invention relates to pentadeuteropyridine compounds represented by the following formula (I) and pharmaceutically acceptable salts, stereoisomers, prodrugs and solvates thereof, and a preparation method, pharmaceutical compositions and uses thereof. The compounds can generate an inhibitory effect on variation forms of epidermal growth factor receptor (EGFR) protein kinase, thereby effectively inhibiting the growth of a variety of tumor cells; the compounds can be used for preparation of antitumor drugs, are used for treatment or prevention of a plenty of different cancers, and moreover, can overcome the drug resistance induced by conventional drugs gefitinib, erlotinib and other first-generation EGFR inhibitors. More specifically, the compounds can be used for preparation of drugs for treatment or prevention of diseases, obstacles, disorders or illness conditions mediated by certain variation-form epidermal growth factor receptors (such as L858R activated mutants, Exon19 deletion activated mutants, and T790M resistance mutants).
Owner:INVENTISBIO CO LTD +1
2-arylaminopyridine, pyrimidine or triazine derivative and preparation method and application thereof
ActiveCN106132957AGrowth inhibitionPrevent proliferationOrganic chemistryAntineoplastic agentsDiseaseTriazene
The invention relates to a 2-arylaminopyridine, pyrimidine or triazine derivative and a preparation method and application thereof. The 2-arylaminopyridines, pyrimidines or triazine derivatives may act on certain mutant forms of epidermal growth factor receptors such as L858R activating mutants, delE746_A750 mutants, Exonl9 deletion activating mutants and T790M drug resistant mutants , for use in the treatment and prevention of diseases and conditions. The 2-arylaminopyridine, pyrimidine or triazine derivatives are useful for the treatment and prevention of cancer. The present invention also relates to pharmaceutical compositions comprising 2-arylaminopyridines, pyrimidines or triazine derivatives, intermediates useful in the preparation of 2-arylaminopyridines, pyrimidines or triazine derivatives, and the use of 2-arylamino Pyridine, pyrimidine or triazine derivatives in the treatment of diseases mediated by various forms of EGFR.
Owner:WUXI SHUANGLIANG BIOTECH CO LTD
EGFR inhibitor and preparing method and application thereof
InactiveCN105001208AStrong inhibitory activityGood inhibitory effectOrganic active ingredientsOrganic chemistryDiseaseProtein-Tyrosine Kinases
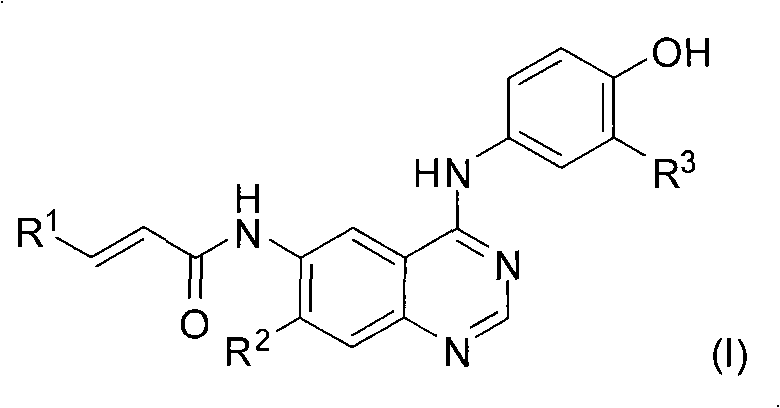
The invention discloses an EGFR inhibitor. The EGFR inhibitor is of the structure shown in the formula (I) and is a compound including alpha, beta-unsaturated carboxylic acid amides. Meanwhile, the invention discloses a preparing method of the compound and the application of the compound serving as a protein tyrosine kinase inhibitor, especially the inhibiting function on T790M variant EGFR as the EGFR inhibitor, and the application on treating diseases such as the kidney cancer, the ling cancer, the prostate cancer, the pancreatic cancer the breast cancer and the spongiocytoma which are related to EGFR over expression. The structure is shown in the specification.
Owner:NANJING LEIKEXING BIOTECH CO LTD
Pharmaceutical Compounds
InactiveUS20100004232A1Reduce morbidityPatient compliance is goodBiocideSenses disorderDiseaseImatinib resistant
The use of a compound for the manufacture of a medicament for the prophylaxis or treatment of: A. a disease state or condition mediated by a kinase which is BCR-abl, VEGFR, PDGFR, EGFR, Flt3, JAK (e.g. JAK2 or JAK3), C-abl, PDK1, Chk (e.g. Cbk1 or Chk2), FGFR (e.g. FGFR3), Ret, Eph (e.g. EphB2 or EphB4), or Src (e.g. cSrc); or B. a cancer in which the cancer cells thereof contain a drug resistant kinase mutation which is: (a) a threonine gatekeeper mutation; or (b) a drug-resistant gatekeeper mutation; or (c) an imatinib resistant mutation; or (d) a nilotinib resistant mutation; or (e) a dasatinib resistant mutation; or (f) a T670I mutation in KIT; or (g) a T674I mutation in PDGFR; or (h) T790M mutation in EGFR; or (i) a T315I mutation in abl; or C. a cancer which expresses a mutated molecular target which is a mutated form of BCRabl, c-kit, PDGF, EGF receptor or ErbB2; or D. a disease mediated by a kinase containing a mutation in a region of the protein that binds to or interacts with other cancer agents but does not bind to or interact with the compounds of formula (I) or (I′), for example a mutated kinase selected from c-abl, c-kit, PDGFR including PDGFR-beta and PDGFR-alpha, and ErbB family members such as EGFR (ErbB1), HER2 (ErbB2), ErbB3, and ErbB4, members of the Ephrin receptor family including EphA1, EphA2, EphA3, EphA4, EphA5, EphA8, EphA10, EphB1, EphB2, EphB3, EphB5, EphB6, c-Src and kinases of the JAK family such as TYK2; wherein the compound is a compound of the formula (I or I′): or a salt, solvate, tautomer or N-oxide thereof wherein R0′, R1, R1′, R2′, R3′, R4′, A′, X′, E, A and M are as defined in the claims.
Owner:ASTEX THERAPEUTICS LTD
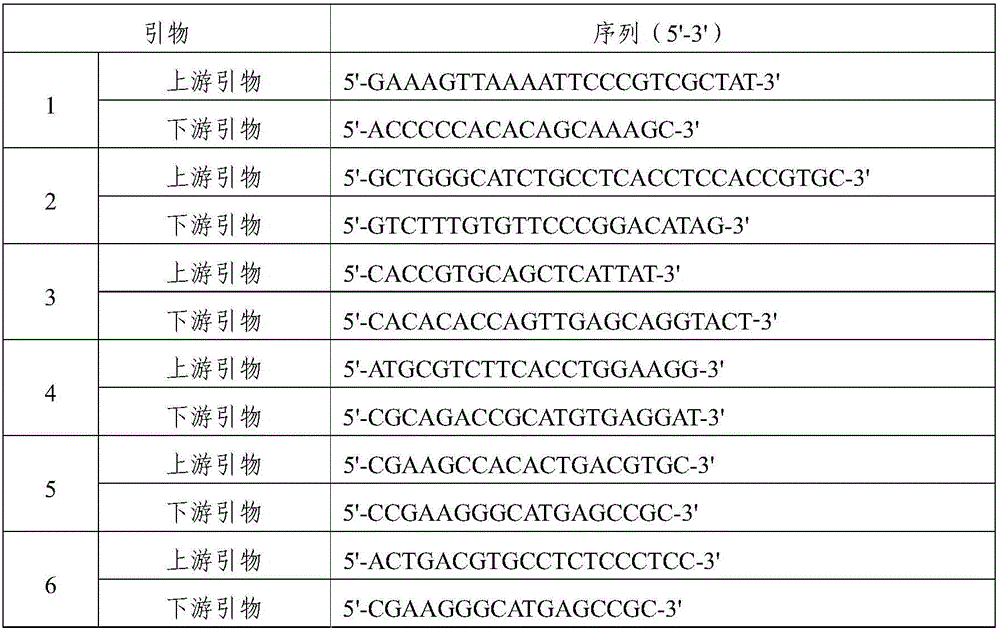
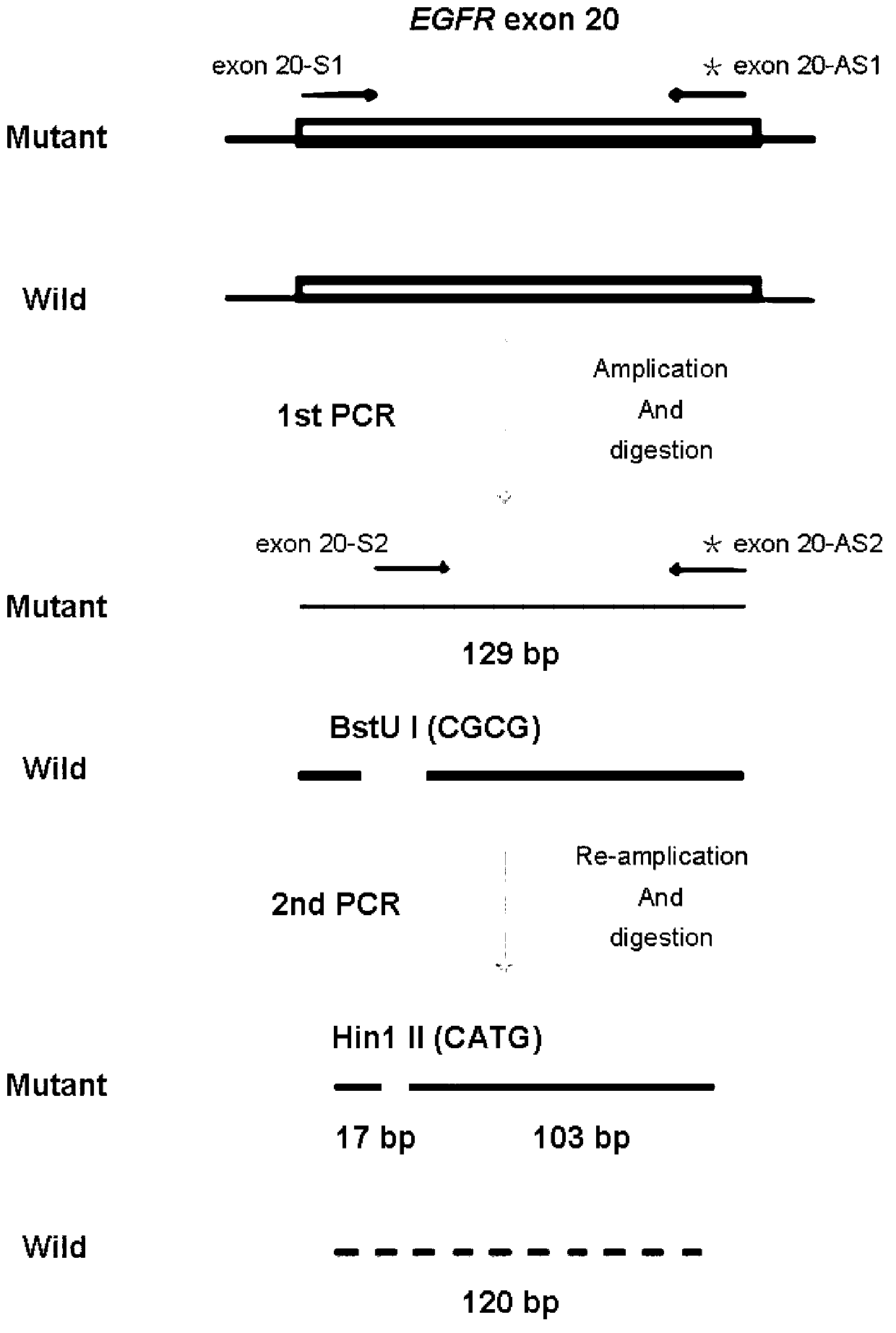
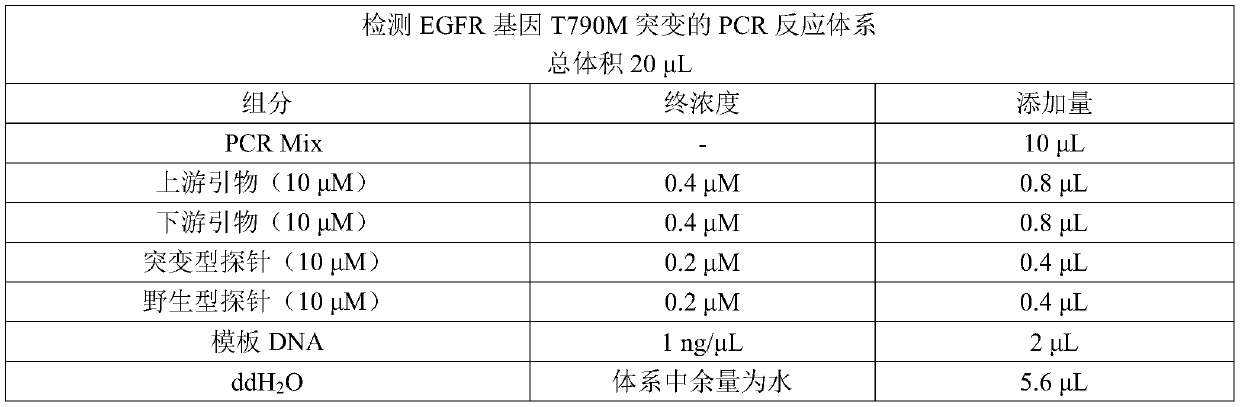
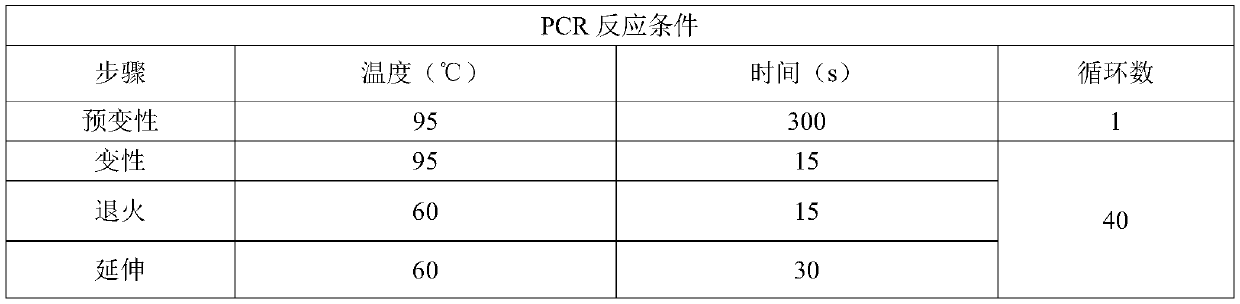
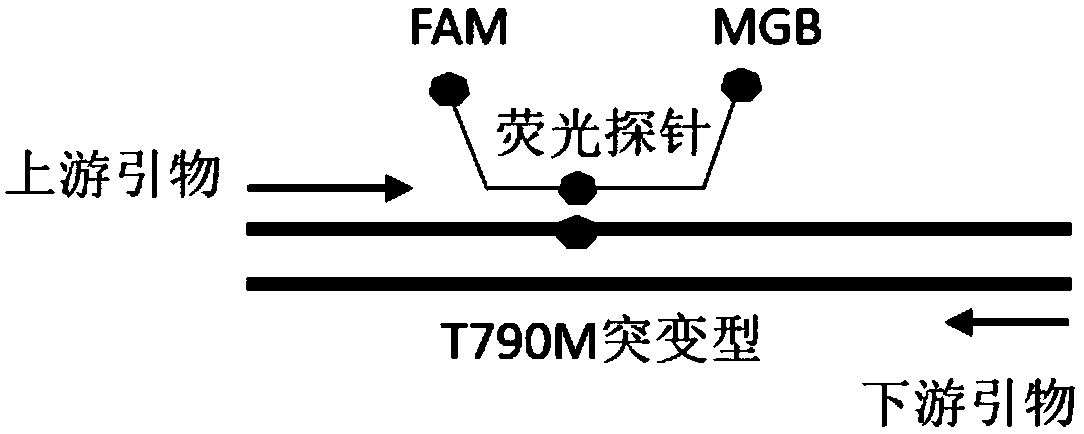
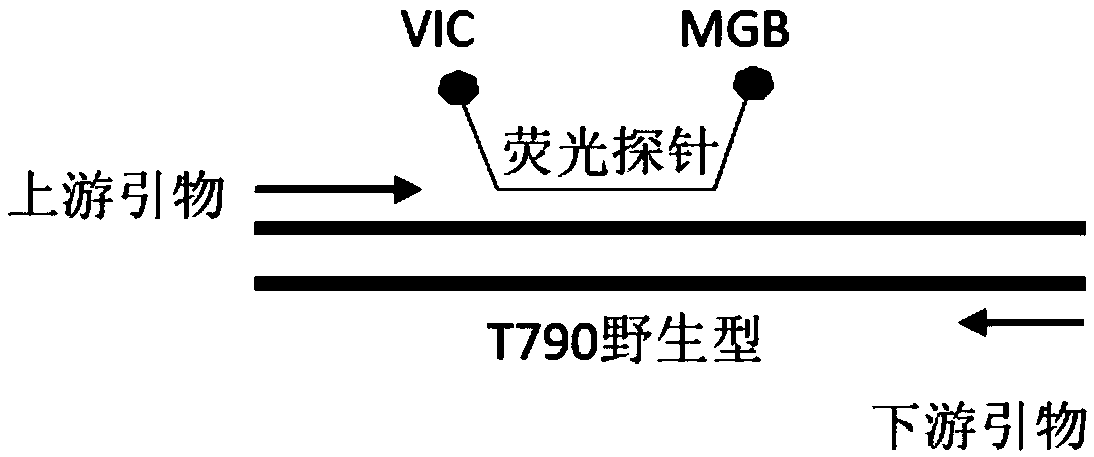
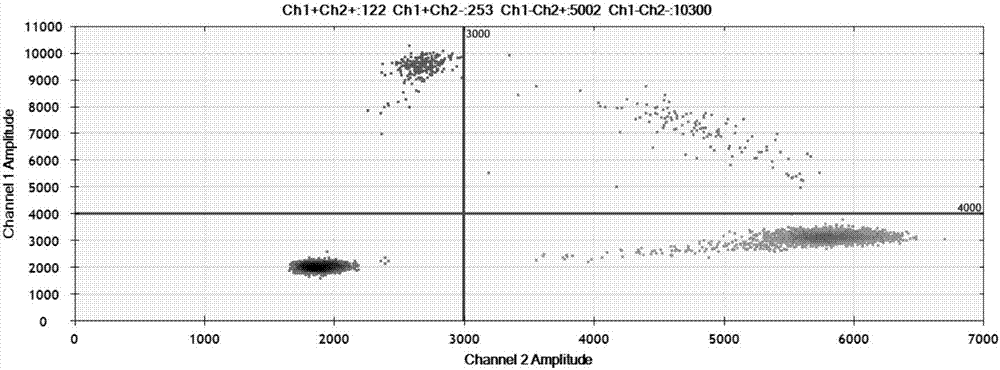
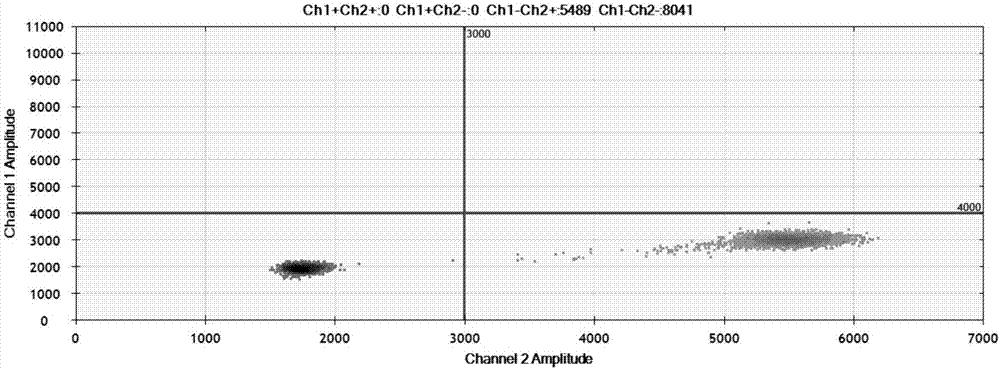
EGFR gene 20 exon T790M and C797S mutation detection primers, probes and method
InactiveCN107083438AReduce distractionsIncrease the Tm valueMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionMutation detection
The invention discloses a primer pair for specific amplification of EGFR gene 20 exon T790M and C797S loci. The sequence of the primer pair is shown in SEQ ID No:1-2. The invention further discloses a probe set for specific detection of the T790M and C797S loci. The probe set comprises a wild type probe and a mutant type probe, and the sequences are shown in SEQ ID No:3-7. The invention further discloses a reagent kit comprising the primer pair and the probe set, and a method for adopting the primer pair and the detection set for detecting EGFR gene 20 exon T790M and C797S mutation. By designing the specific primers and probes, the 3' end of the probes is connected with a minor groove binder and a non-fluorescent quenching group, the interference of background signals is lowered, hybridization of the probes and a template is stabilized, a Tm value of the probes is increased, and therefore the mutation detection sensitivity is improved.
Owner:上海捷易生物科技有限公司
EGFR inhibitor, preparation method and use thereof
ActiveUS20170313714A1Organic active ingredientsGroup 5/15 element organic compoundsDiseaseCancer prevention
Epidermal growth factor receptor (EGFR) inhibitors are provided. In particular, 4-substituted-2-(N-(5-substituted allyl amide)phenyl)amino)pyrimidine derivatives of formula (I), a preparation method and use thereof as an EGFR inhibitor are provided. The 4-substituted-2-(N-(5-substituted allyl amide)phenyl)amino)pyrimidine derivatives of formula (I) have inhibitory activity against the L858R EGFR mutant, the T790M EGFR mutant and the exon 19 deletion activating mutant, and can be used to treat diseases mediated alone or in part by EGFR mutant activity. The derivatives of formula (I) can be used to treat and / or prevent cancers, particularly non-small cell lung cancer.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Tricyclic compound and pharmaceutical compositions thereof and application thereof
The invention relates to a tricyclic compound of the formula (I) and the formula (II) and pharmaceutical acceptable salts of the tricyclic compound. The compounds of the kind can be applied to inhibit mutants, such as EGFR (L858R), EGFR (delE746-A750) and EGFR (T790M), of EGFR kinase, and can also be used for treating cancer, such as the non-small cell lung cancer, caused by the mutants of the EGFR. The invention further relates to pharmaceutical compositions containing the compounds of the kind, methods for preparing the compounds of the kind and application of the compounds of the kind or the pharmaceutical compositions for preparing medicines for treating the cancers caused by the mutants of the EGFR.
Owner:SHENZHEN BO LI JIAN MEDICINE CO LTD
Pyrimidine or pyridine compounds, preparation method therefor and pharmaceutical uses thereof
ActiveUS20170355696A1Strong inhibitory activityReduce inhibitionOrganic active ingredientsOrganic chemistryDiseaseActivating mutation
The present invention disclosed a class of pyrimidine or pyridine compounds, pharmaceutically acceptable salts, stereoisomers, prodrugs and solvates thereof, preparation method therefor and pharmaceutical compositions and pharmaceutical uses thereof. The compounds can inhibit the variants of EGFR (Epidermis Growth Factor Receptor) proteinases, and therefore can inhibit the growth of a variety of tumor cells effectively. The compounds can be used to prepare antitumor drugs, used for the treatment, combined therapy or prevention of various different cancers. The compounds can overcome the drug resistance induced by the existing first-generation EGFR inhibitors such as gefitinib, erlotinib and so on. Particularly, the compounds can be used to prepare drugs for treating or preventing diseases, disturbances, disorders or conditions mediated by epidermis growth factor receptor variants (such as L858R activated mutants, Exon19 deletion activated mutants and T790M resistant mutants).
Owner:INVENTISBIO CO LTD
Preparation method and application of compound or its pharmaceutically acceptable salt or composition
The present invention relates to a tricyclic compound of formula (I) and a pharmaceutically acceptable salt thereof. The compound can be used for inhibiting mutants of epidermal growth factor receptor(EGFR) kinases, such as EGFR (delE746-A750), EGFR (L858R), EGFR (delE746-A750 / T790M), EGFR (L858R / T790M), EGFR exon 20insertion and other mutants, so the compound can be used to treat cancer caused by EGFR mutants, such as non-small cell lung cancer. The invention also relates to a medicinal composition containing the compound, a method for preparing the compound, and an application of the compound or the medicinal composition in the preparation of medicines for treating the cancers caused by EGFR mutants.
Owner:SHENZHEN BO LI JIAN MEDICINE CO LTD
Pyrimidine, pyrimidone compound, and medical compound and application thereof
ActiveCN102816162AGrowth inhibitionOvercome drug resistanceOrganic active ingredientsOrganic chemistryCancer cellKetone
The invention provides 7-(substituted amino)-3, 4-dihydro-pyrimidine [4, 5-d] pyrimidone-(1H)-ketone compounds represented as general formula (I) or (II) and application thereof in preparing medicines for treating tumors. According to research, the compounds can restrain proliferation of various tumor cells, can be used for targeted restrain of epidermal growth factor receptor (EGFR) kinase, and particularly can effectively restrain single-point mutation or multipoint mutation cancer cells of an EGFR (T790M) mutant strain. Accordingly, the 7-(substituted amino)-3, 4-dihydro-pyrimidine [4, 5-d] pyrimidone-(1H)-ketone compounds can serve as EGFR inhibitors to be used for anti-cancer medicines and have high application values.
Owner:东莞粤港澳干细胞生物科技有限公司
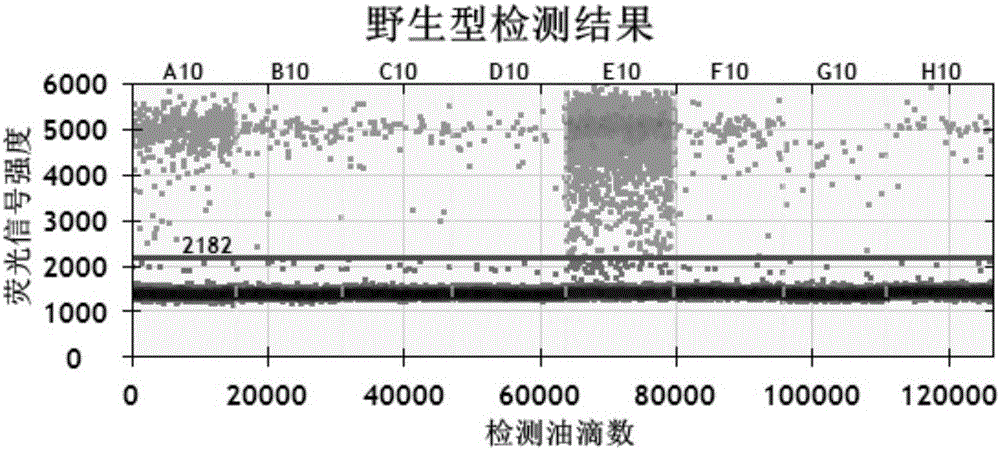
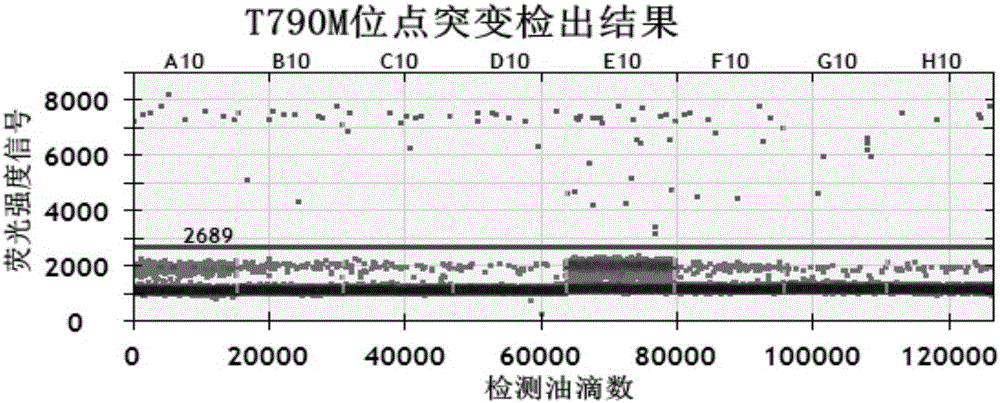
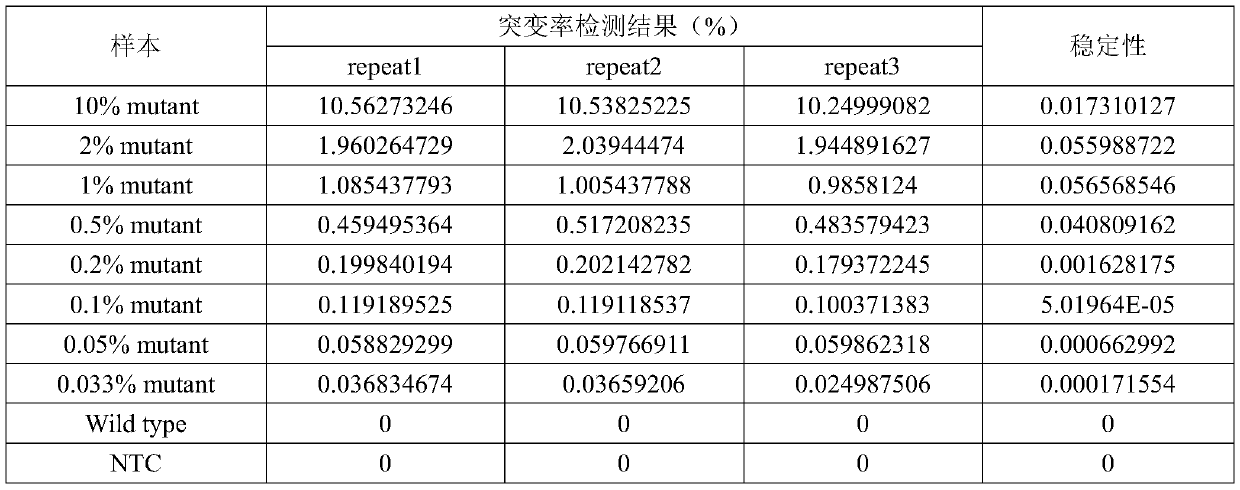
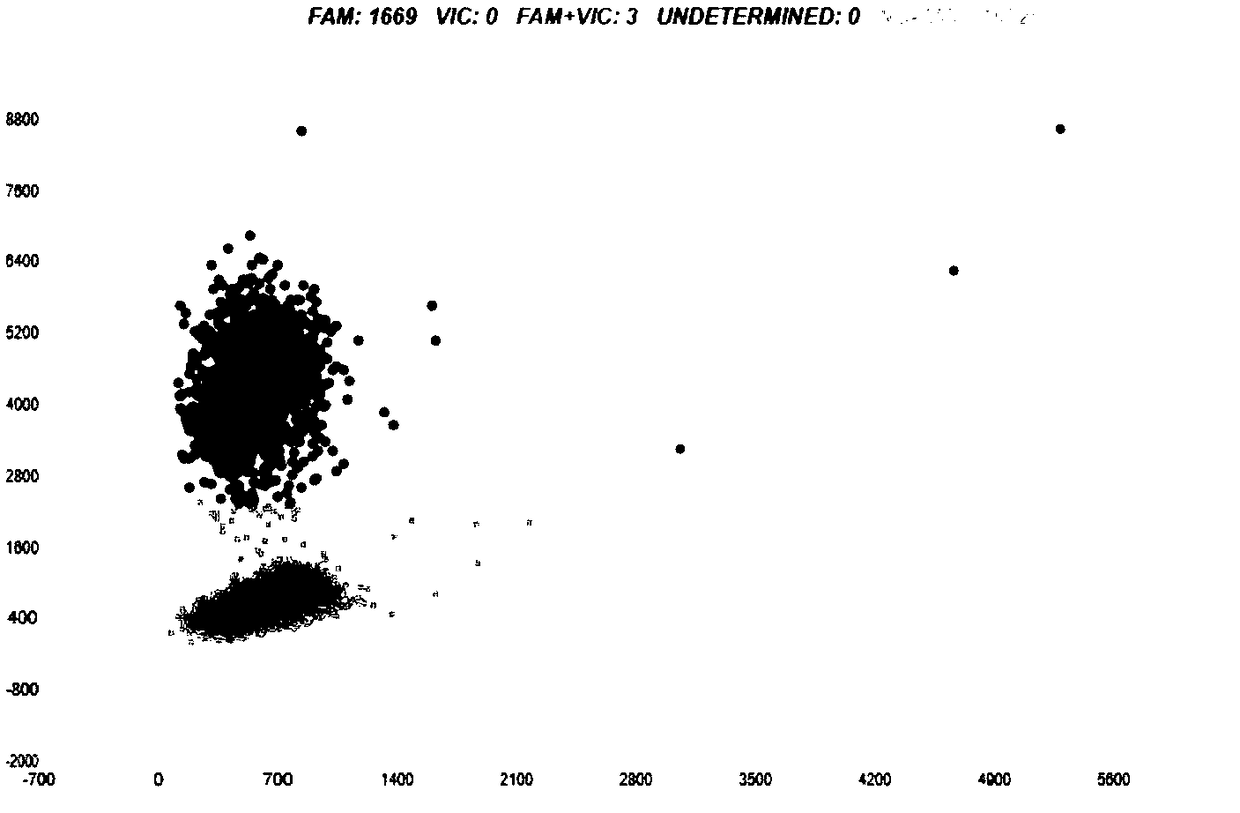
Specific primer probe composition, kit and method for detecting T790M site of EGFR gene
InactiveCN107868828AGood choiceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesWild type
The invention discloses a specific primer probe composition, a kit and a method for detecting a T790M site of an EGFR gene. The kit comprises an upstream primer, a downstream primer, a fluorescent probe I for detecting T790M mutation and a fluorescent probe II for detecting a wild type. For the kit, the primer pair with the specific sequences and the probes with the specific sequences are designedfor the human EGFR gene T790M mutation and reaction systems are optimized. According to the invention, through a method of a Raindropdigital PCR platform, high sensitivity detection on the T790M mutation of the EGFR gene is achieved and the abundance of the mutation is obtained simultaneously; the detection limit can be low to one in 100000. Furthermore, the invention can be used for detecting multi-source samples, comprising tumor tissue samples and ctDNAs, so that the application scope of the kit, reaction systems and method are expanded, and a medication guidance for treatment of patientswith lung cancer T790M mutation is provided.
Owner:中源协和基因科技有限公司
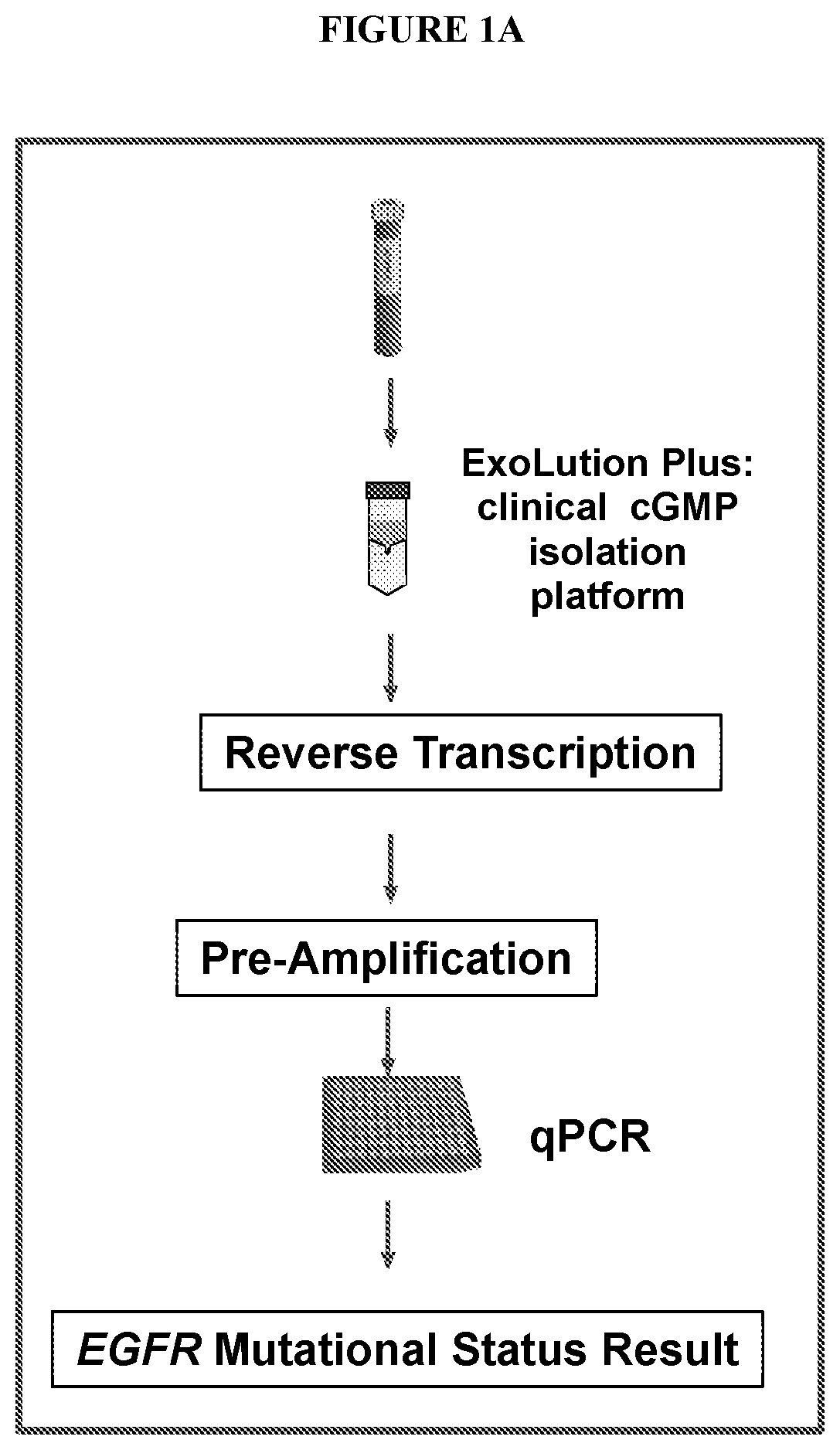
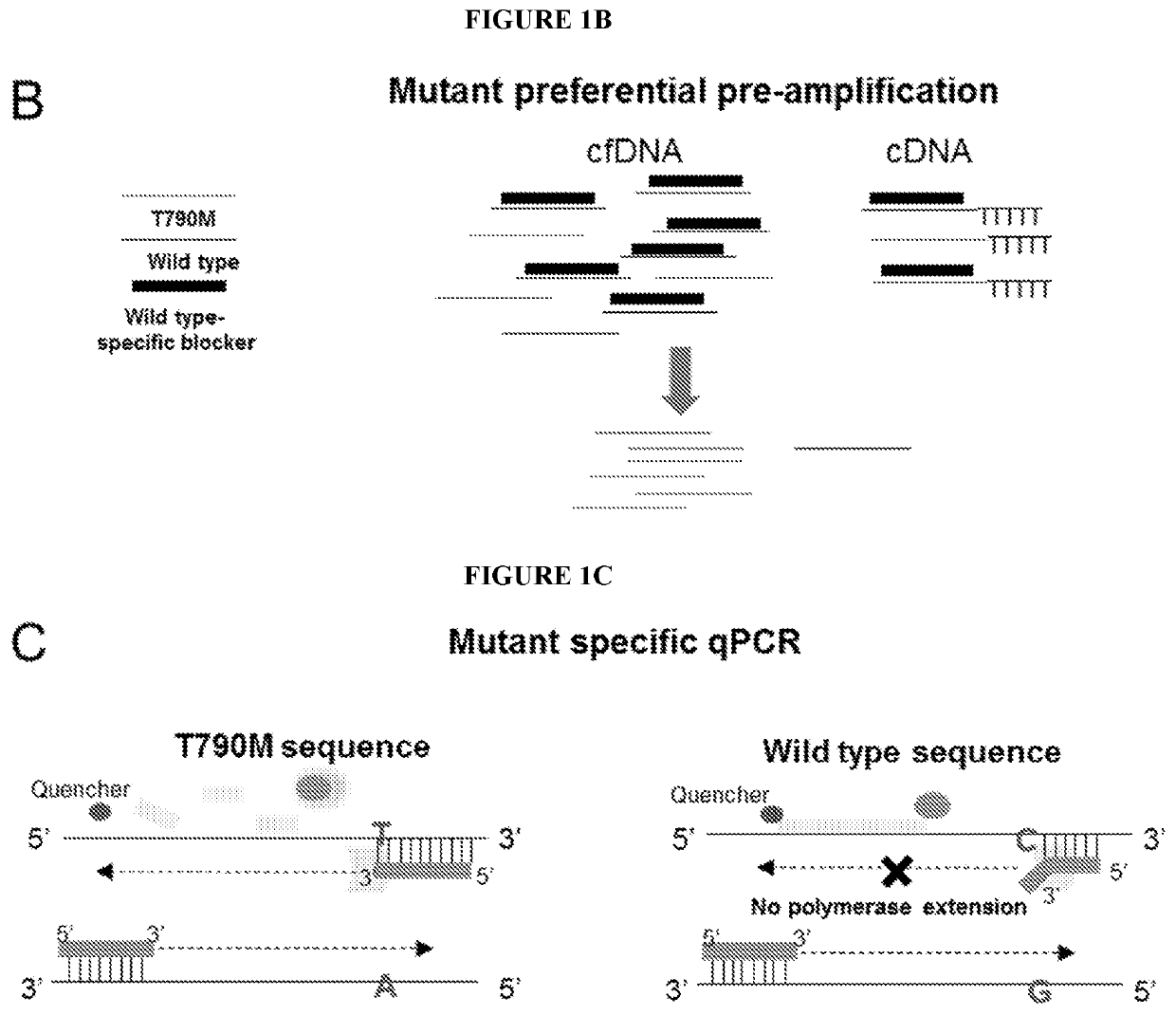
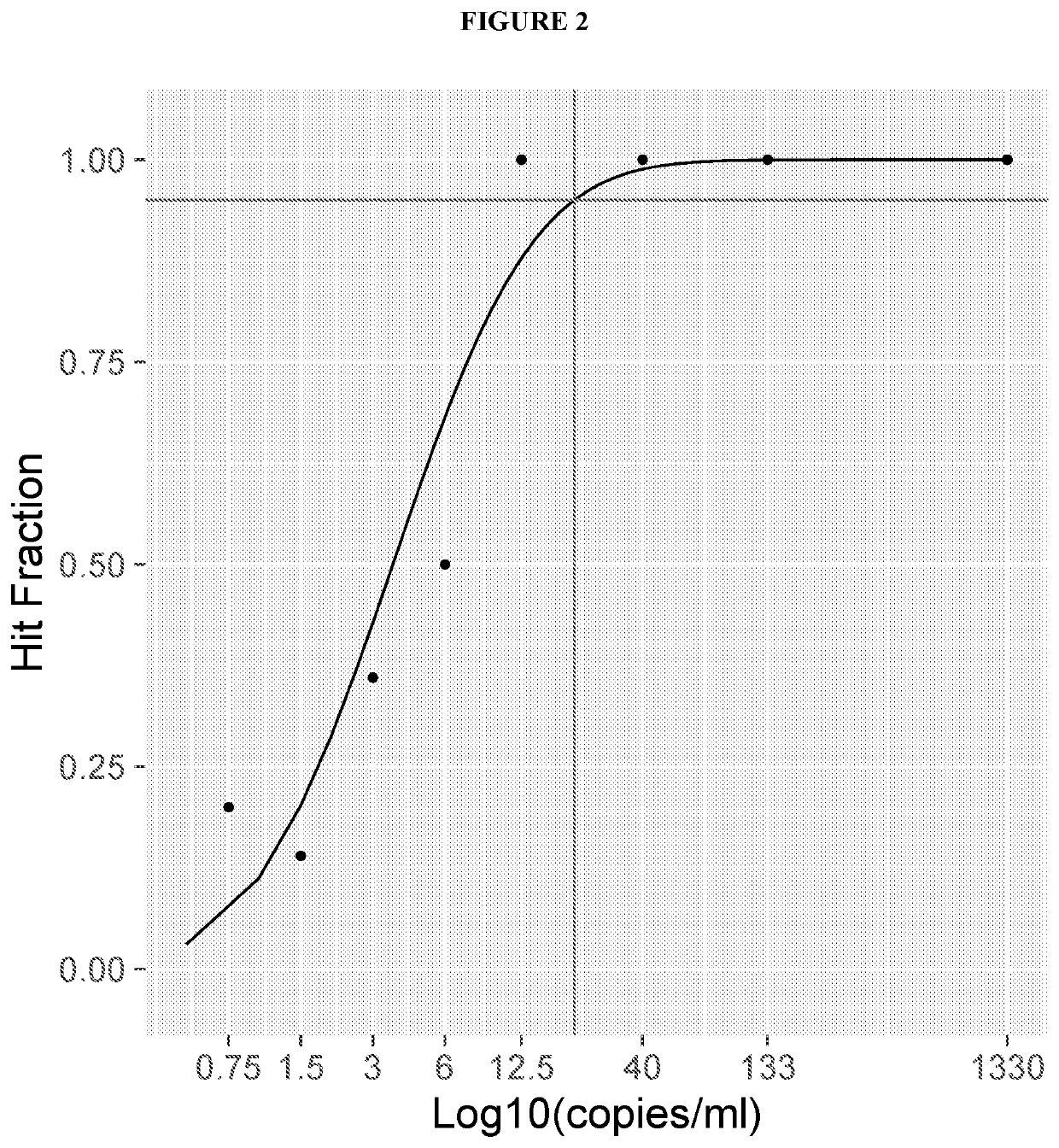
Methods and compositions to detect mutations in plasma using exosomal RNA and cell free DNA from non-small cell lung cancer patients
ActiveUS20190376128A1Stimulate immune responseHigh sensitivityMicrobiological testing/measurementDiseaseCell free
The present invention relates generally to methods and kits for detecting one or more biomarkers, such as an Epidermal Growth Factor Receptor (EGFR) mutation, e.g., T790M mutation, L858R mutation, one or more exon19 insertions and / or one or more exon19 deletions in the EGFR gene, in a biological sample to aid in diagnosis, prognosis, monitoring, or therapy selection for a disease such as, for example, cancer. The methods and kits are useful in aiding in diagnosis, prognosis, monitoring, or therapy selection for lung cancer, e.g., non-small cell lung cancer (NSCLC).
Owner:EXOSOME DIAGNOSTICS
Probes, primers and kit for detecting T790M mutation of EGFR gene
InactiveCN105112544AMeet the actual needs of rapid detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPcr methodBlood plasma
The invention discloses probes, primers and a kit for detecting a T790M mutation of an EGFR gene. The probes and the primers have the following sequence: SEQ ID NO: 01 to SEQ ID NO. 07. The probes, the primers and the kit have the following benefits: (1) SNP sites on the primers are designed as G / A merged basic groups, so that all efficiencies are compatible, and the amplification efficiency is improved; (2) the sensitivity is high, that is, the detection sensitivity can reach 2 permillage; (3) compared with those adopting a digital PCR method, the operation is simple, the cost is reduced, and the clinical application range is wide; (4) through blood plasma sample detection with a large reaction volume, the DNA loading quantity of blood plasma samples is increased, the detection system is more stable, and the detection rate of the blood plasma samples is improved; (5) the detection speed is high, that is, the detection process can be completed within only 120 minutes, and the time consumed in the detection process is only a half of that consumed according to the digital PCR method; (6) the probes, the primers and the kit, provided by the invention, can be utilized for detecting peripheral blood samples, so that convenient sampling and dynamic detection can be realized.
Owner:AMOY DIAGNOSTICS CO LTD
Quinazoline, pyridopyrimidine or pyrimidopyrimidine derivative epidermal growth factor inhibitor and preparing method and application thereof
InactiveCN106496196AStrong inhibitory activityReduced inhibitory activityOrganic active ingredientsOrganic chemistryDiseaseProtein-Tyrosine Kinases
The invention discloses a selectivity inhibitor of a clinical mutant of EGFR protein tyrosine kinase. The selectivity inhibitor of the clinical mutant of EGFR protein tyrosine kinase has a structure which is shown in the formula (1) (The formula (1) is defined in the description), and is a double aromatic nucleus template compound containing quinazoline, pyridopyrimidine or pyrimidopyrimidine. The invention further discloses a preparing method of the compound and an application of the compound as the selectivity inhibitor of the clinical mutant of EGFR protein tyrosine kinase, especially an inhibiting effect of the compound in a T790M-variation EGFR and an application in treating diseases such as renal carcinoma, lung cancer, prostate gland cancer, pancreatic cancer, breast cancer and spongiocytoma, wherein the diseases are related to overexpression of the epidermal growth factor receptor EGFR.
Owner:NANJING LEIKEXING BIOTECH CO LTD
Quinazoline derivatives and preparation method and application thereof
InactiveCN102146059AOrganic active ingredientsOrganic chemistryAnticarcinogenHuman epidermal growth factor receptor
The invention discloses quinazoline derivatives serving as irreversible tyrosine kinase inhibitors, and use of the derivatives as inhibitors for human epidermal growth factor receptor (EGFR) mutant T790M and anticancer agents. The invention also relates to a preparation method for the quinazoline derivatives, and a medicinal composition containing the quinazoline derivatives.
Owner:SHANGHAI ALLIST PHARM CO LTD
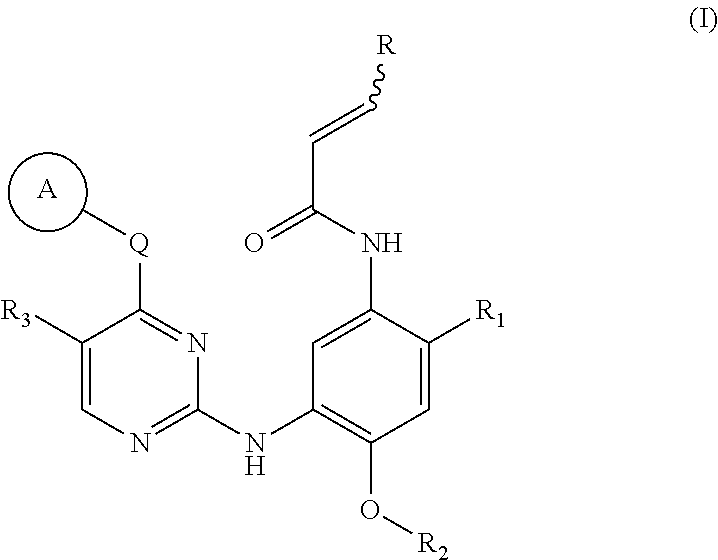
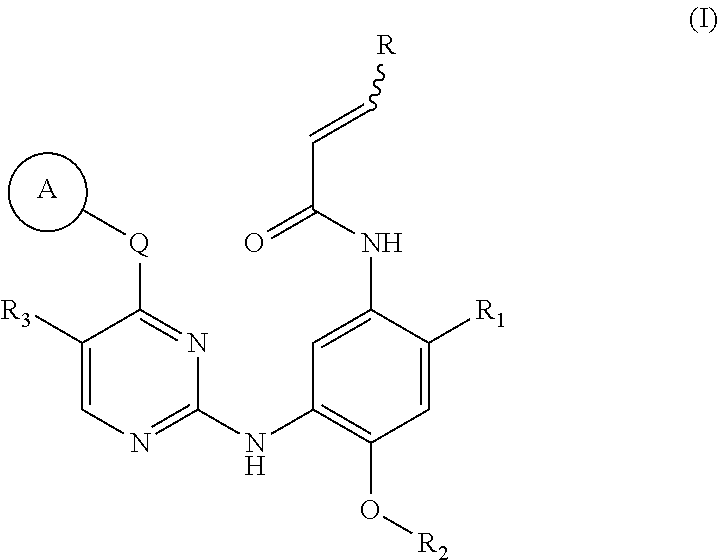
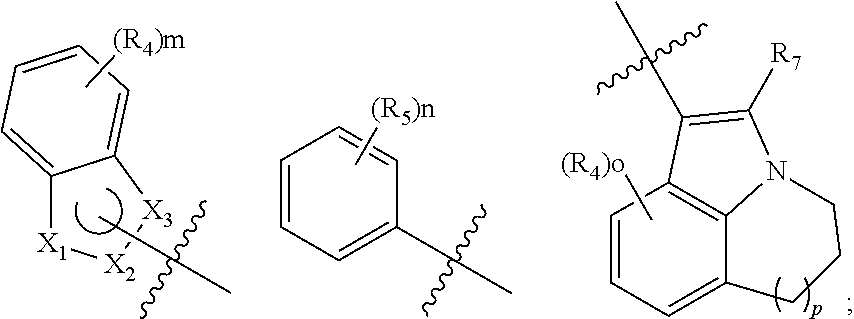
2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application
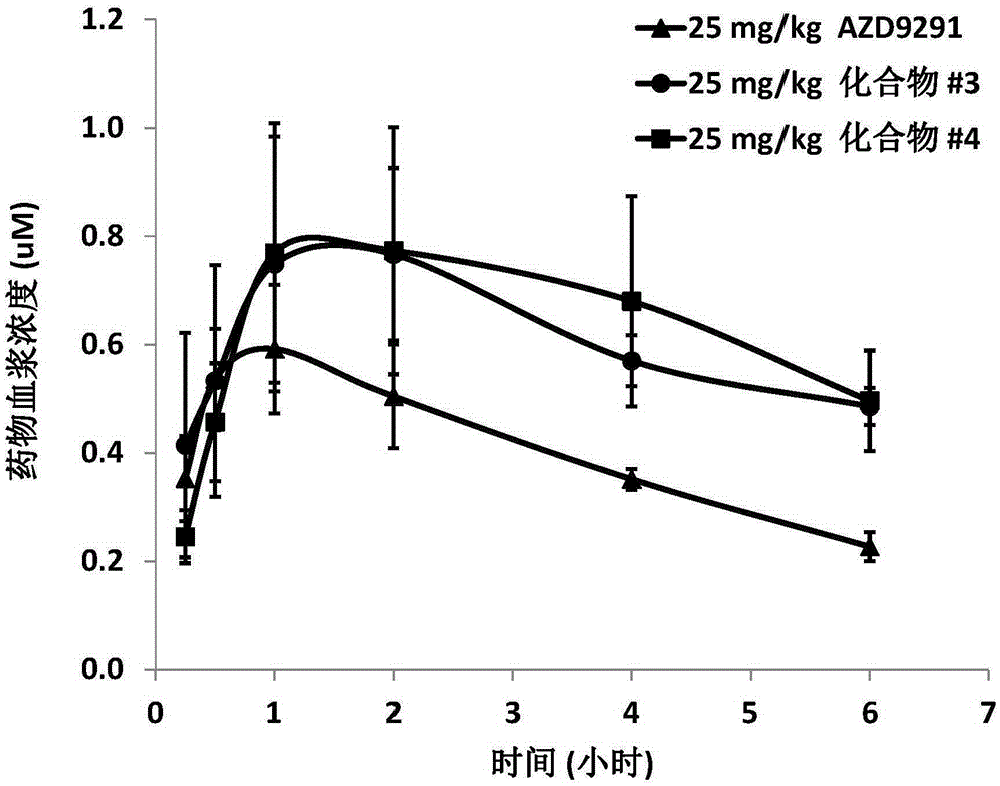
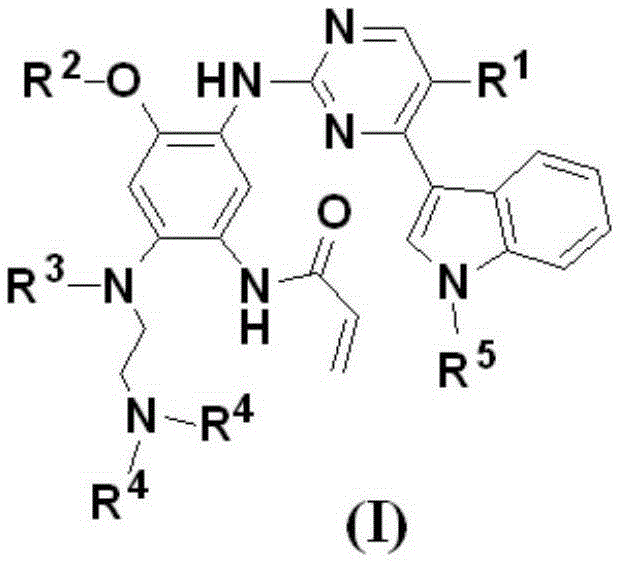
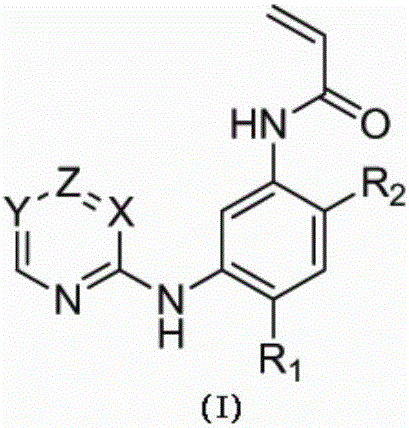
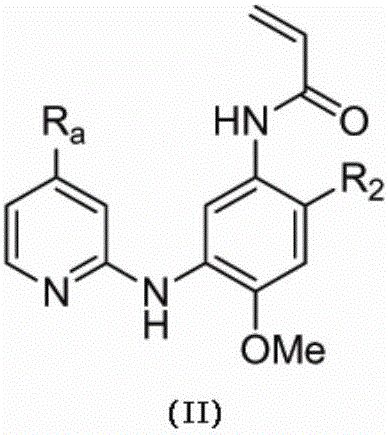
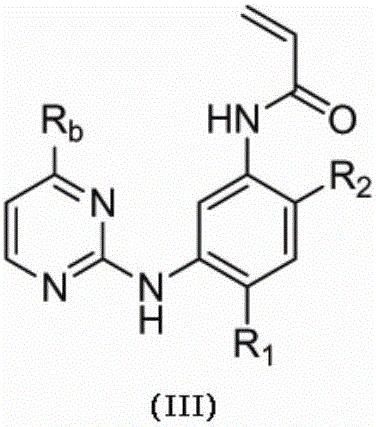
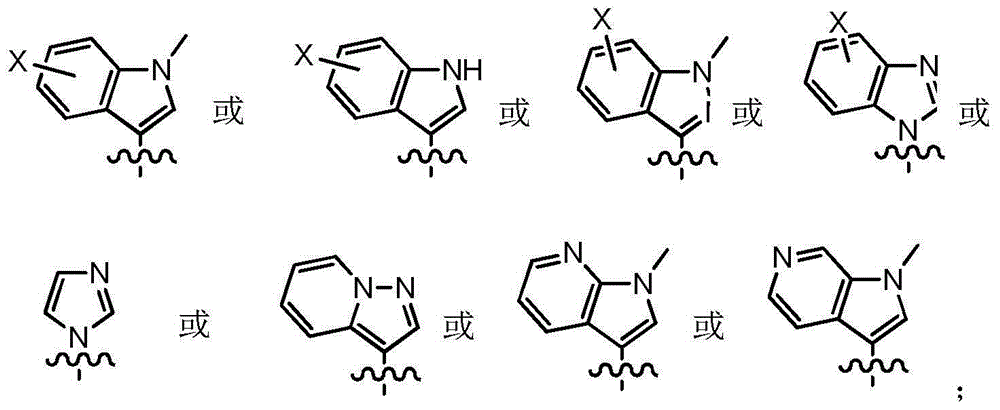
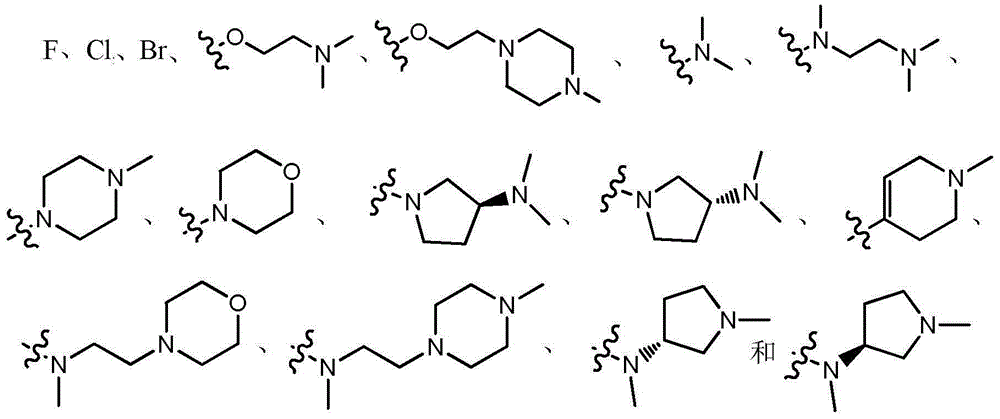
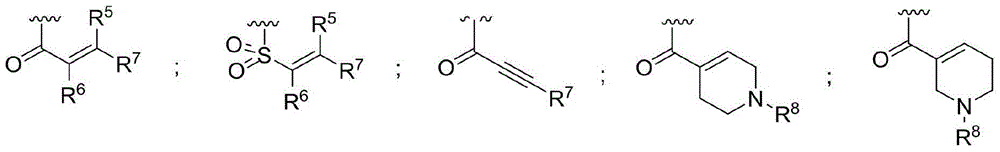
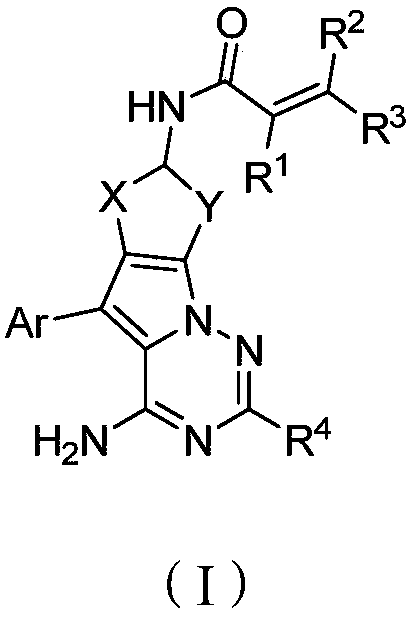
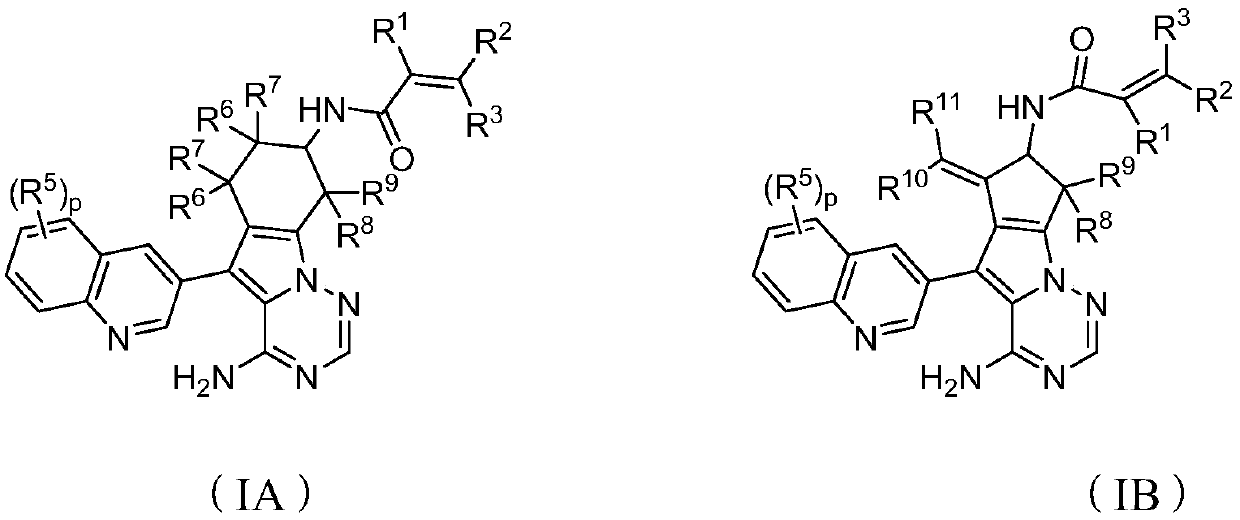
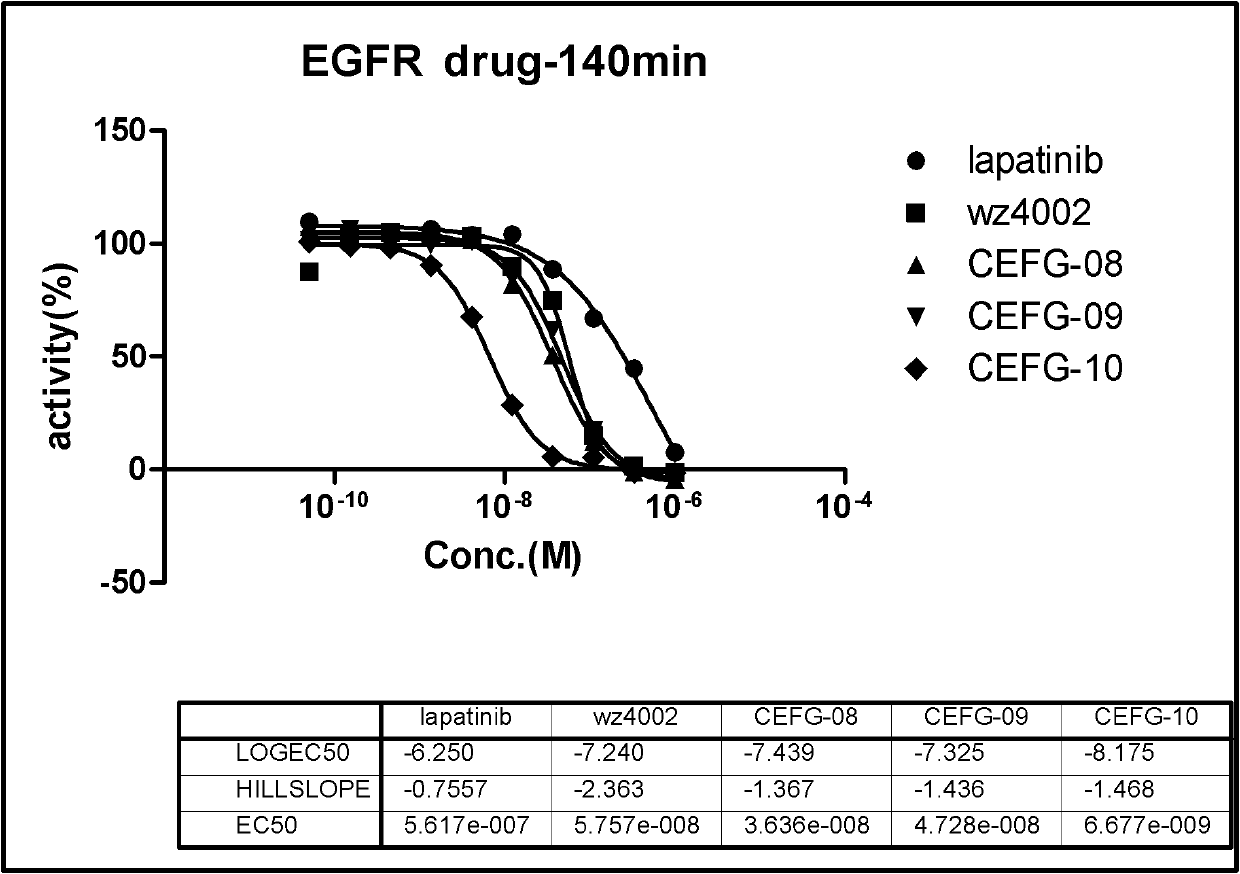
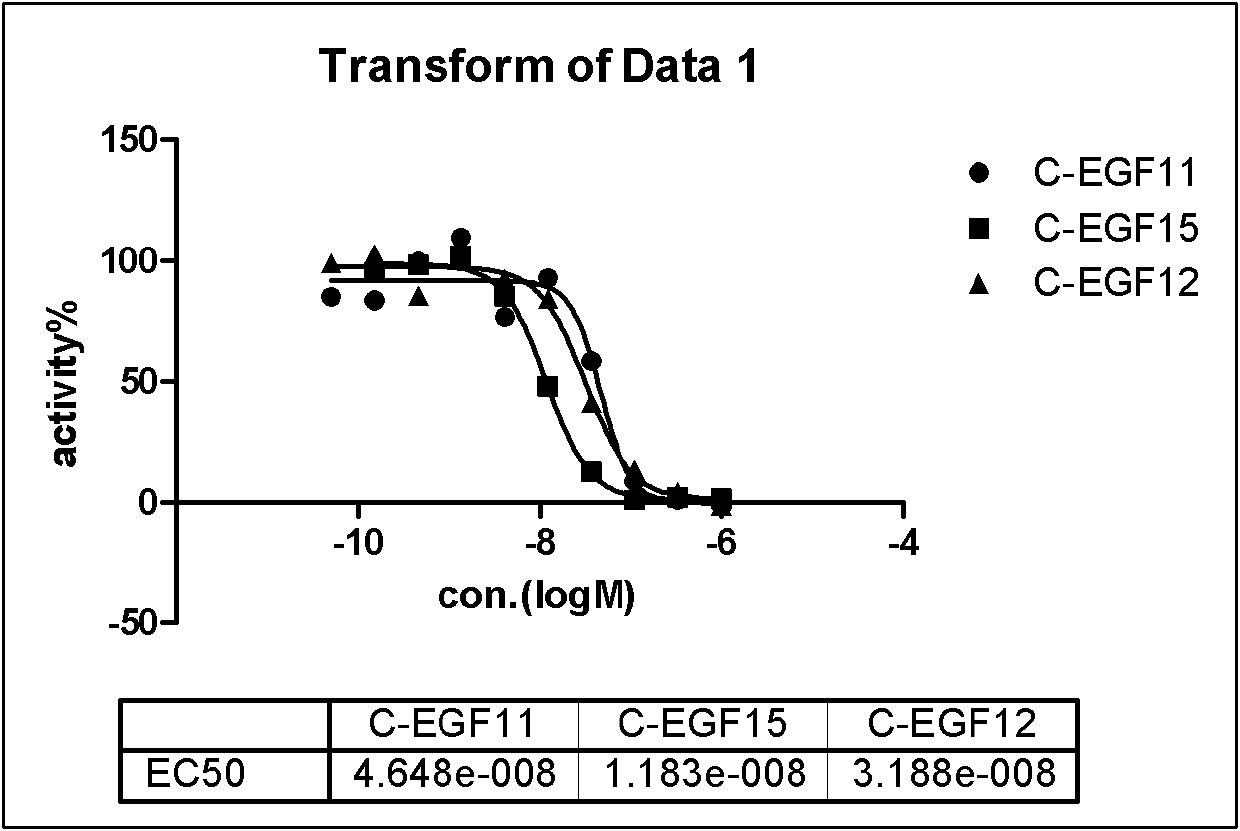
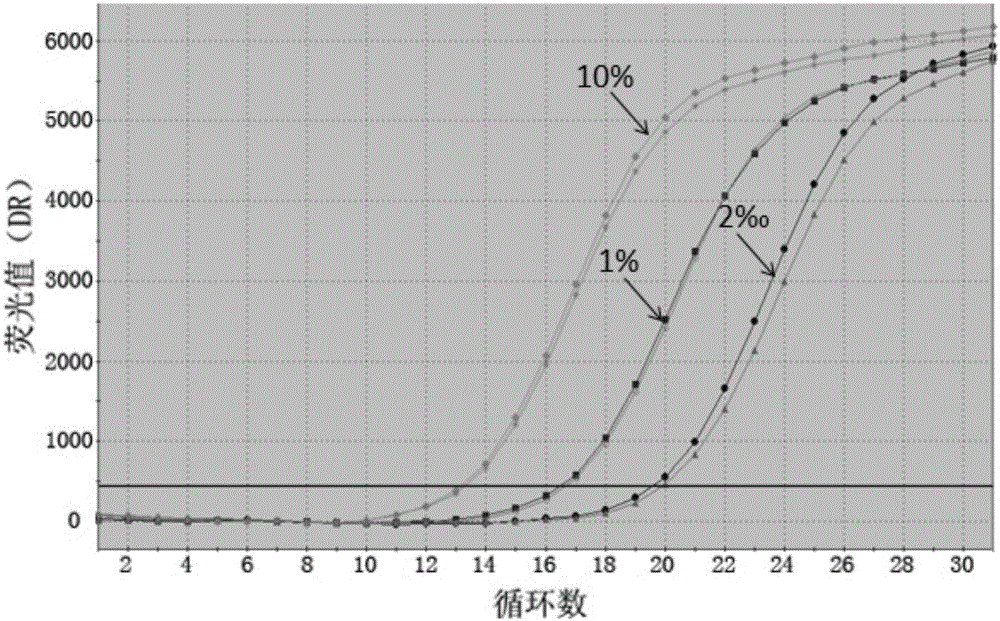
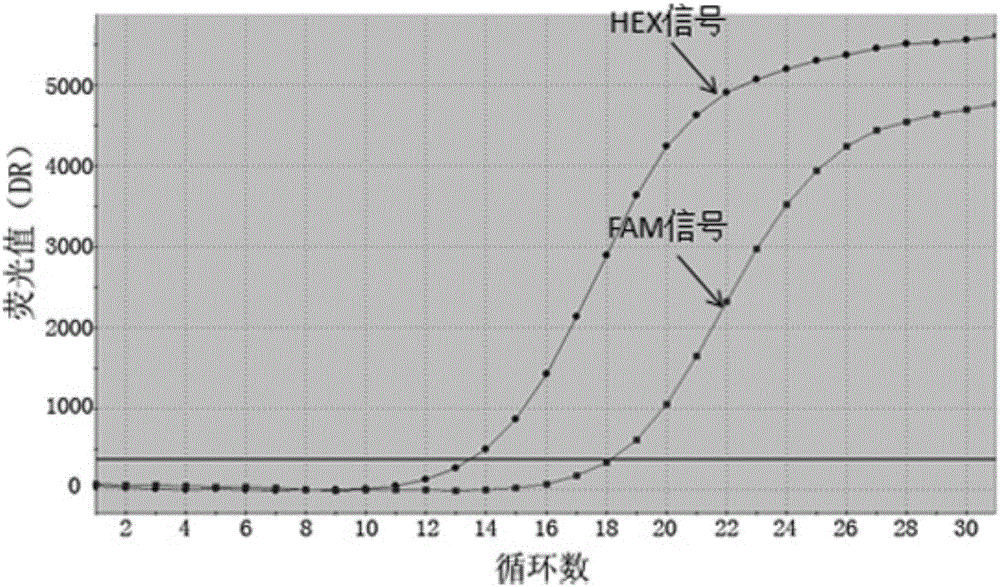
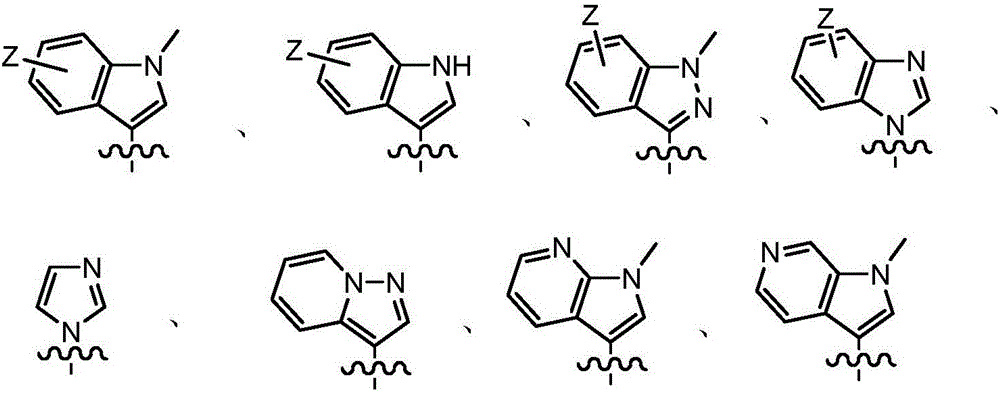
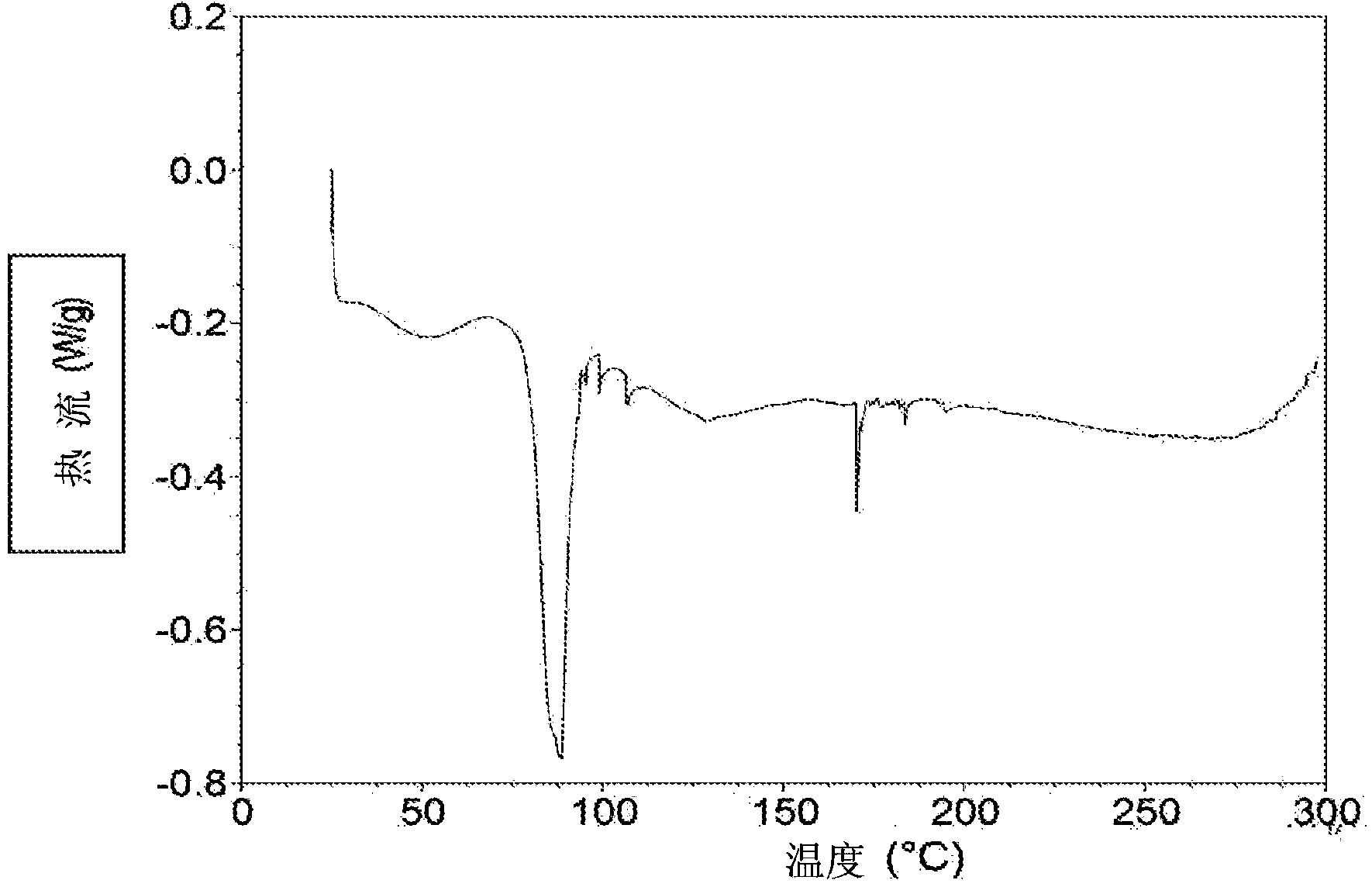
ActiveCN107245075ANovel structureEasy to synthesizeOrganic active ingredientsOrganic chemistrySynthesis methodsT790M
The invention provides 2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application and belongs to the technical field of anti-cancer drugs. The compounds are novel in structure and easy in synthesis, have inhibiting activity to epidermal growth factor receptor (EGFR) tyrosine kinase, have obvious inhibiting activity to EGFR of a single mutant (L858R) and double mutants (L858R / T790M), have obvious in-vivo and in-vitro anti-tumor activity and can be applied to the treatment of EGFR mutation related cancers, the synthesis raw materials are readily available, and the synthesis method is easy to achieve.
Owner:XI AN JIAOTONG UNIV
Primer, detection method and kit for detecting human EGFR (Epidermal Growth Factor Receptor) gene T790M mutation
InactiveCN109306379AEnable early detectionMicrobiological testing/measurementDNA/RNA fragmentationConserved sequenceFluorescence
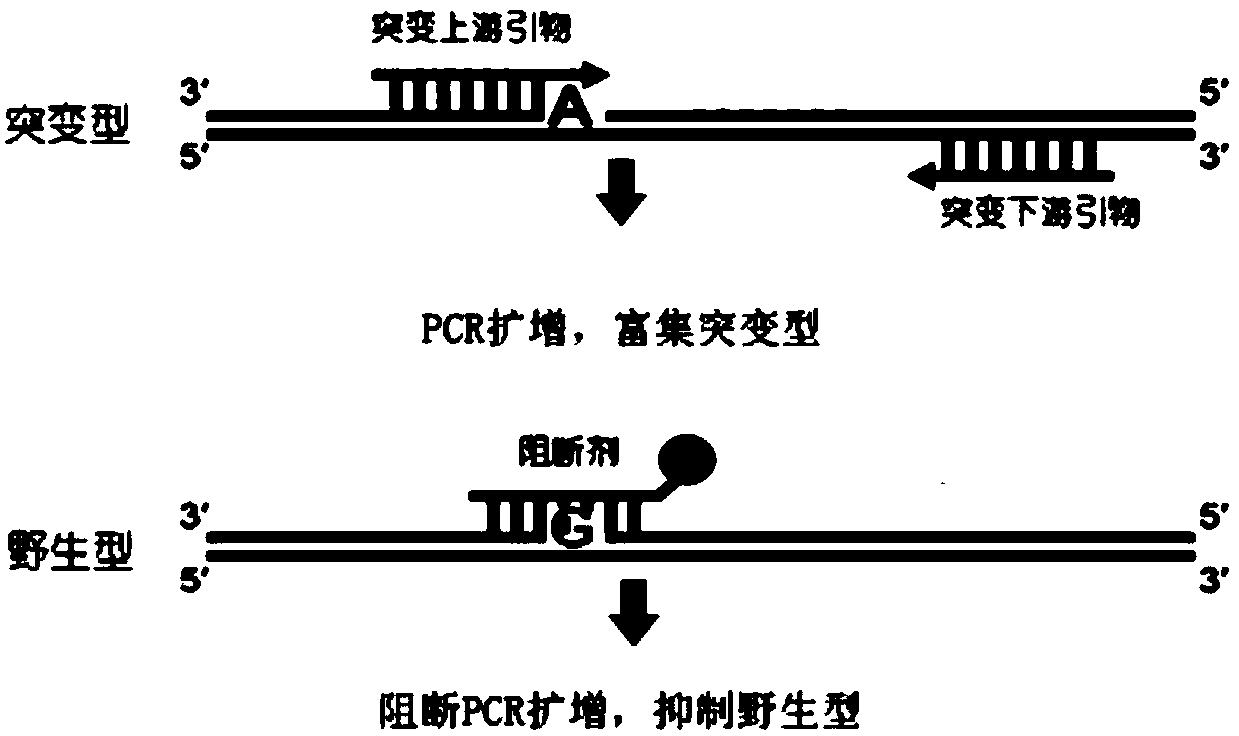
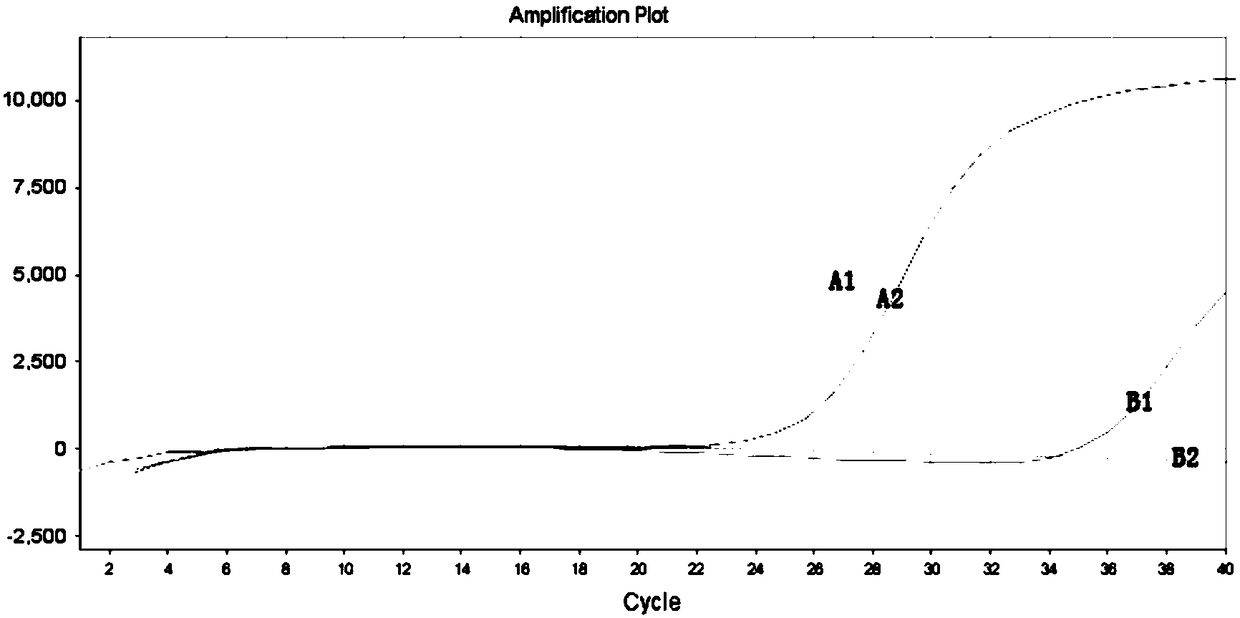
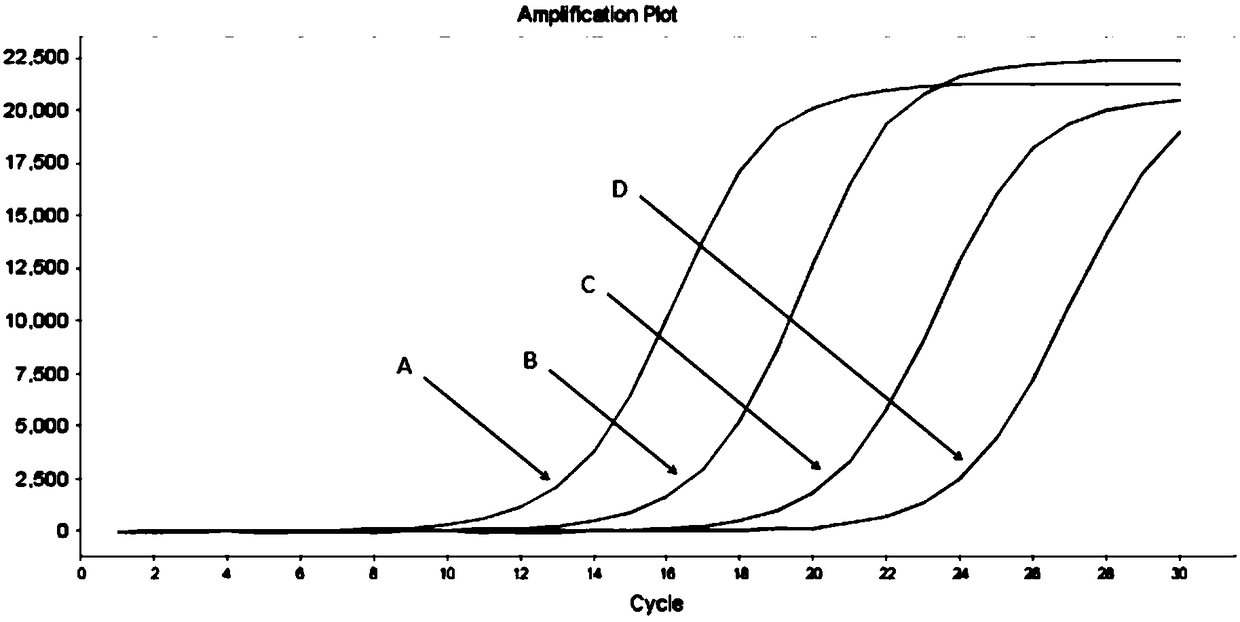
The invention provides a primer, detection method and kit for detecting human EGFR (Epidermal Growth Factor Receptor) gene T790M mutation. The detection primer provided by the invention can be combined with an EGFR gene conserved sequence to amplify a mutation sequence. The detection method provided by the invention can be combined with an amplified fragment by adopting a fluorescence signal detection group to send out a fluorescence signal; a blocking agent is specifically combined with a wild type sequence corresponding to a mutation site to inhibit wild type non-specific amplification. A competitive allele specific PCR (Polymerase Chain Reaction) amplification technology is adopted to establish the kit based on real-time fluorescence PCR and the EGFR gene T790M mutation can be accurately detected. The method provided by the invention has the advantages of simplicity in operation, easiness of reading results and high sensitivity, and can be used for detecting a sample containing 0.001 percent of the EGFR gene T790M mutation; ultrasensitive detection of the EGFR gene T790M mutation is realized.
Owner:广州健天医药科技有限公司
2 - (2, 4, 5 - substituted -anilino) pyrimidine derivatives as egfr modulators useful for treating cancer
The present invention relates to certain 2-(2,4,5-substituted-anilino)pyrimidine compounds and pharmaceutically acceptable salts thereof which may be useful in the treatment or prevention of a disease or medical condition mediated through certain mutated forms of epidermal growth factor receptor (for example the L858R activating mutant, the Exon19 deletion activating mutant and the T790M resistance mutant). Such compounds and salts thereof may be useful in the treatment or prevention of a number of different cancers. The invention also relates to pharmaceutical compositions comprising said compounds and salts thereof, especially useful polymorphic forms of these compounds and salts, intermediates useful in the manufacture of said compounds and to methods of treatment of diseases mediated by various different forms of EGFR using said compounds and salts thereof.
Owner:ASTRAZENECA AB
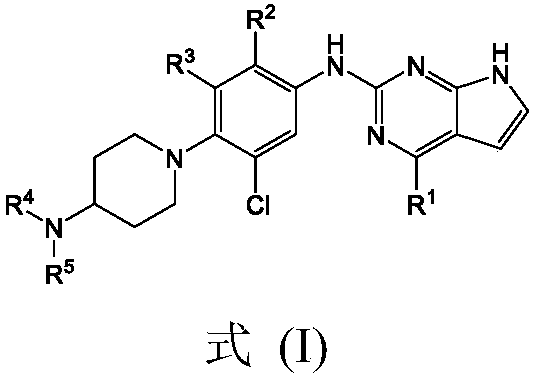
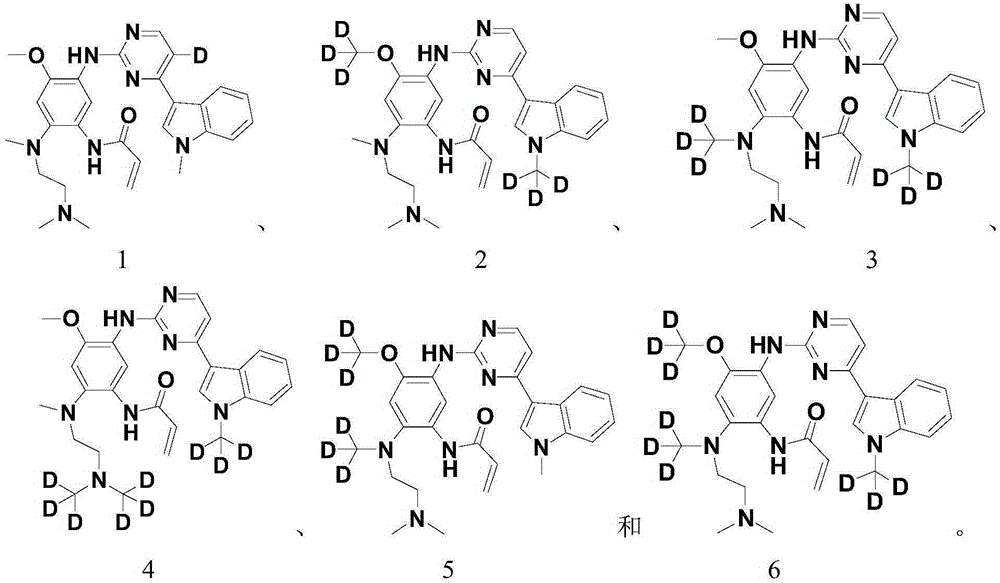
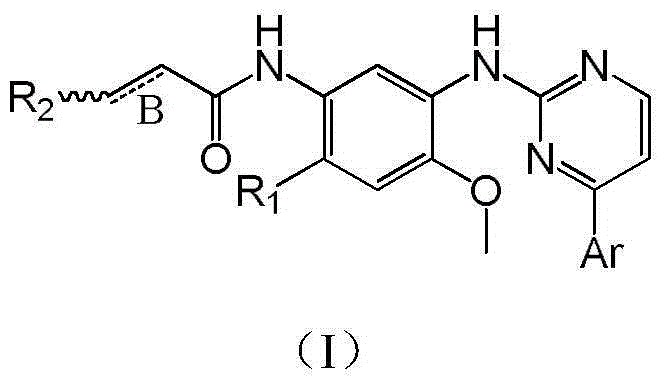
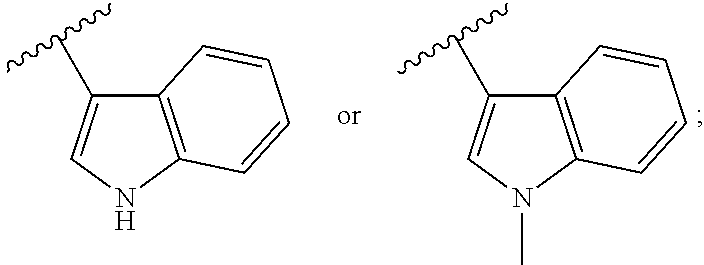
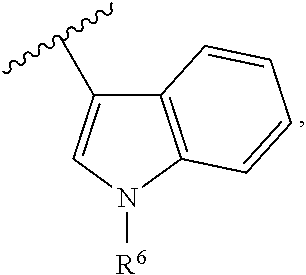
Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof
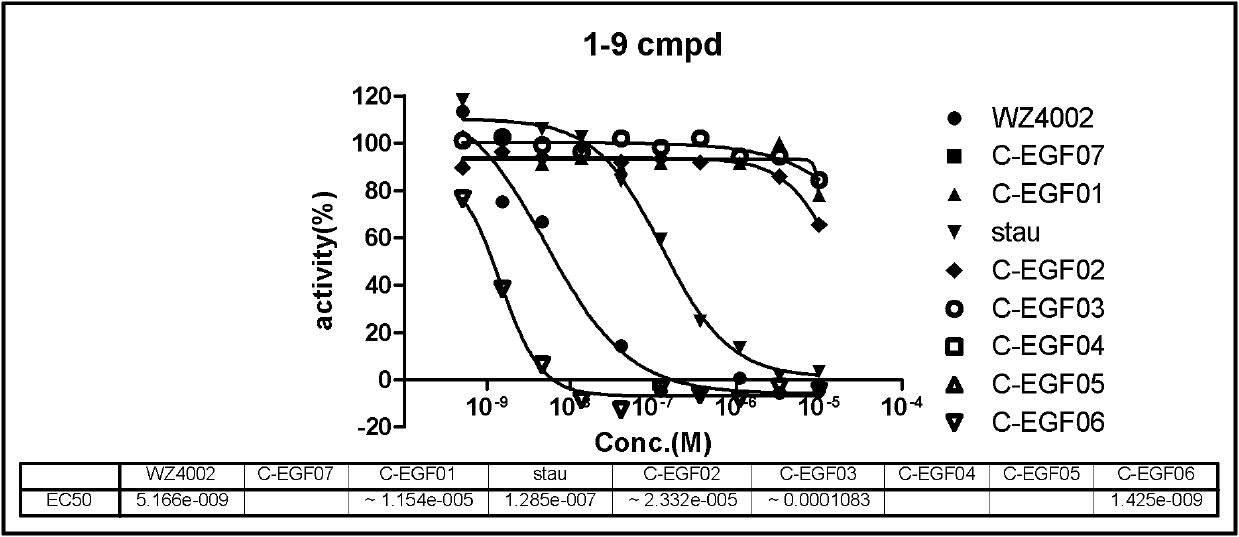
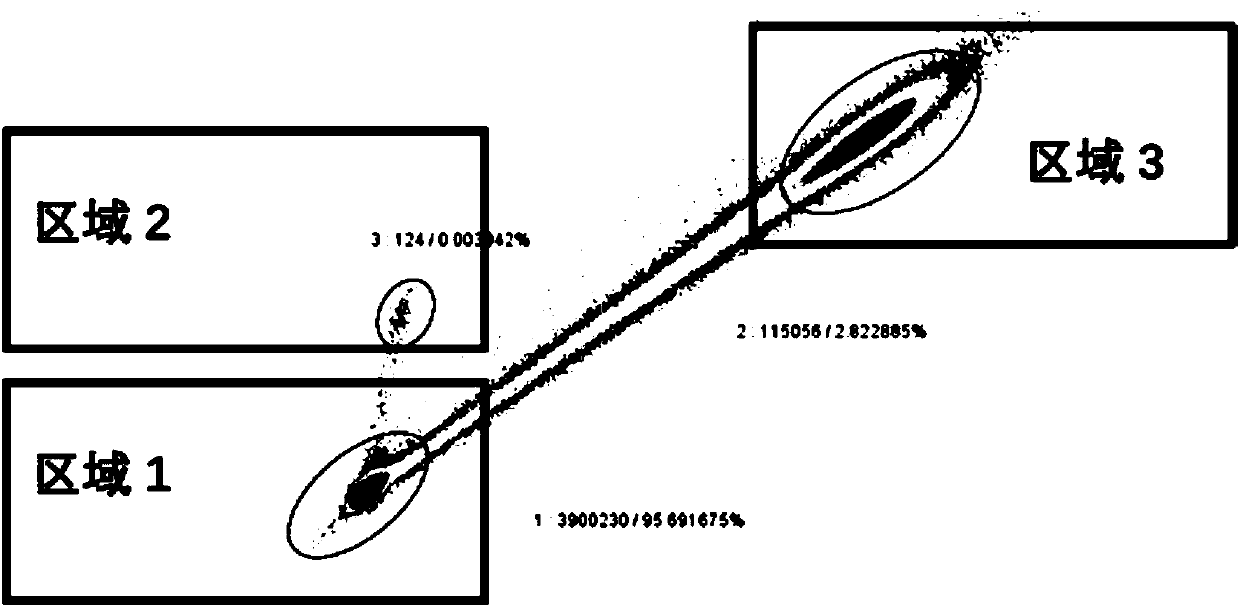
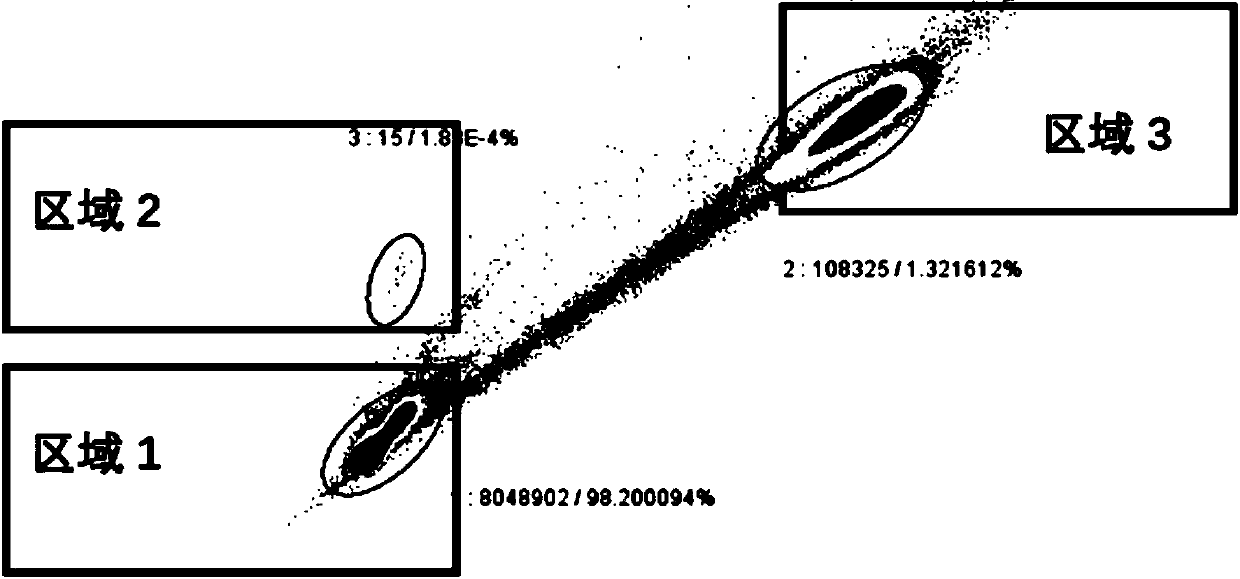
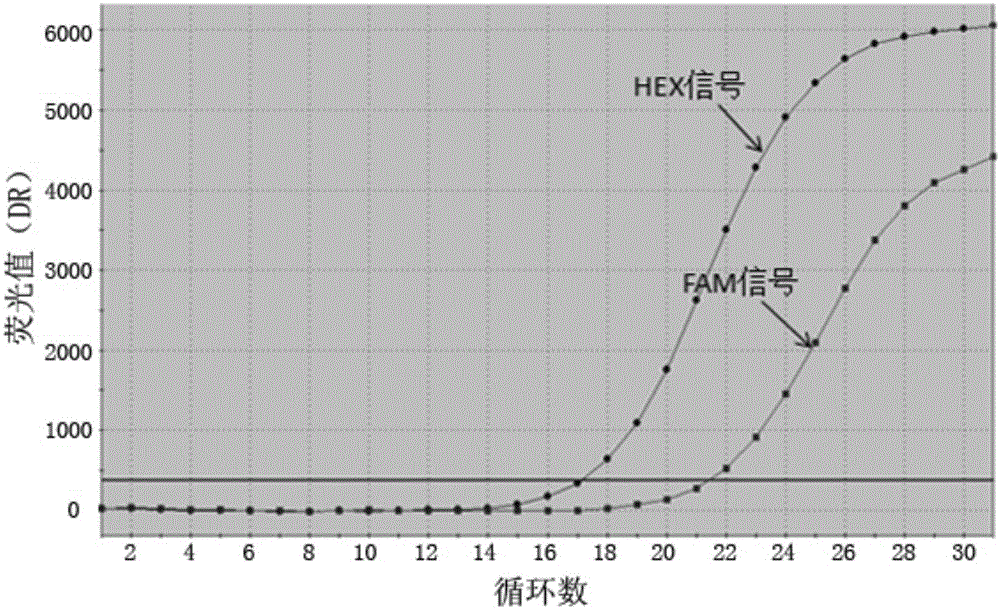
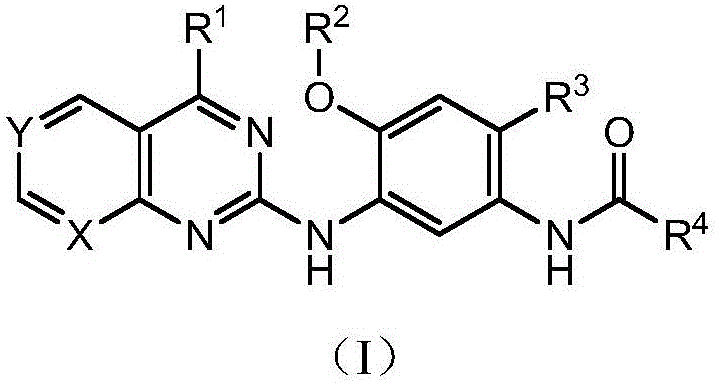
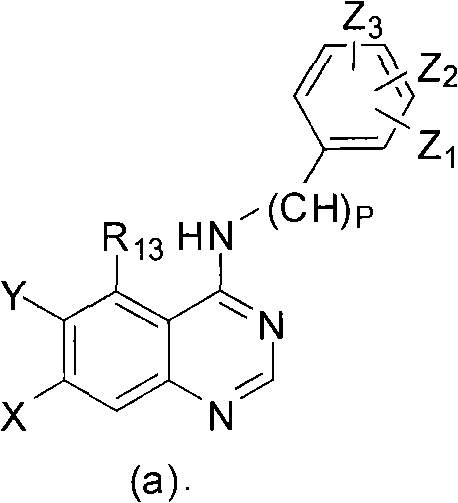
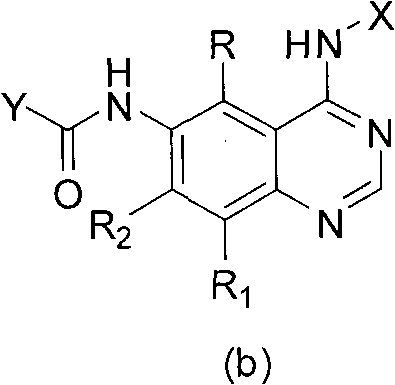
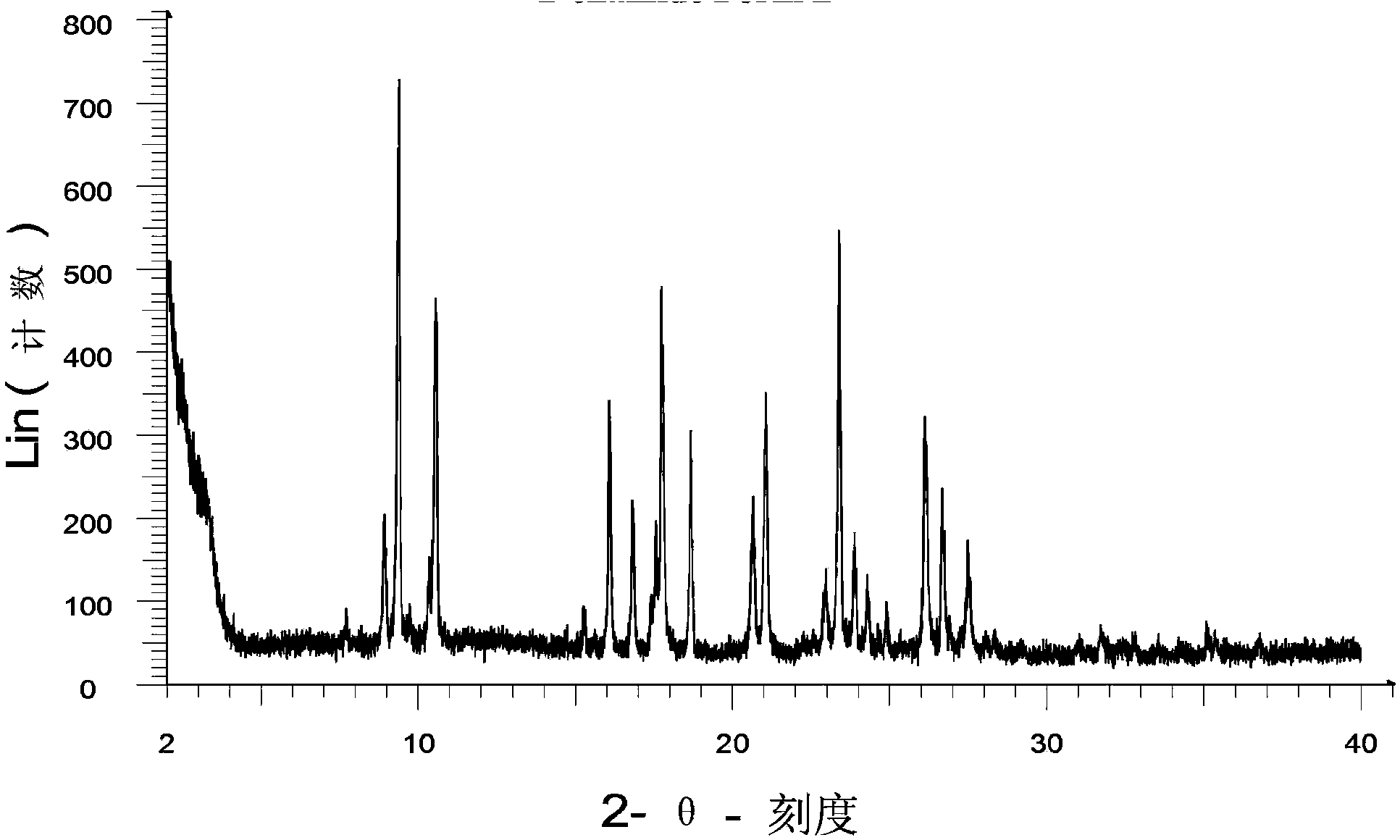
ActiveCN108558865AGood treatment effectOrganic active ingredientsOrganic chemistryTherapeutic effectPyridine
The invention discloses a derivative adopting pyridine-[2,3-d] pyridine as a mother nucleus as well as a preparation method and application thereof. The derivative of the invention is a compound having an inhibition effect for tumor cells mutated for EGFR tyrosine kinase and can be used for treating, jointly treating or preventing various cancers. Particularly, the effect of the compound for treating the mutation type of del19, L858R and T790M of EGFR is significant.
Owner:LIAONING UNIVERSITY
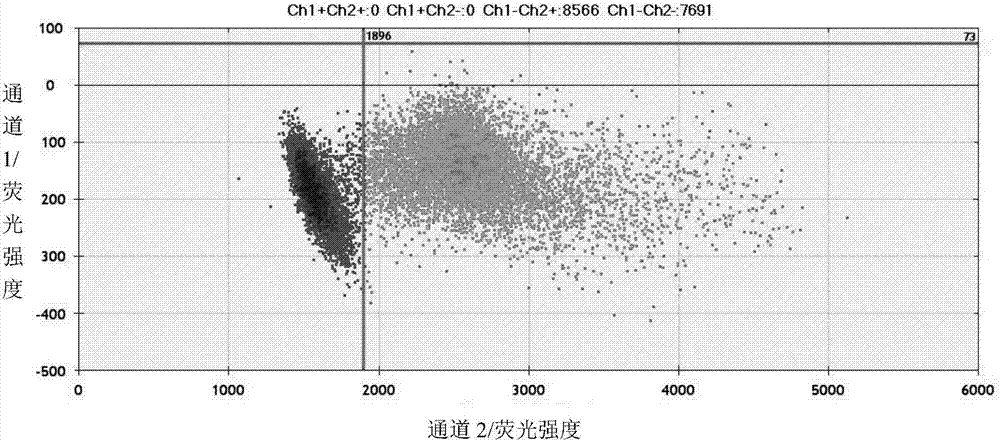
Specific primer and probe for detecting T790M locus of EGFR gene
InactiveCN105803076AIncrease the Tm valueAvoid difficultiesMicrobiological testing/measurementDNA/RNA fragmentationMutation frequencyMutation detection
The invention provides a specific primer and probe for detecting the T790M locus of the EGFR gene, a kit comprising the primer and the probe, and a method for detecting the T790M locus of the EGFR gene. By the adoption of the specific primer and the probe, blood free nucleic acids can be accurately detected, the mutation frequency of the genetic locus can be given directly while mutation detection is conducted, mutation detection rate can reach one five-thousandth under the assistance of a digital PCR system, and sensitivity is high.
Owner:广州漫瑞生物信息技术有限公司
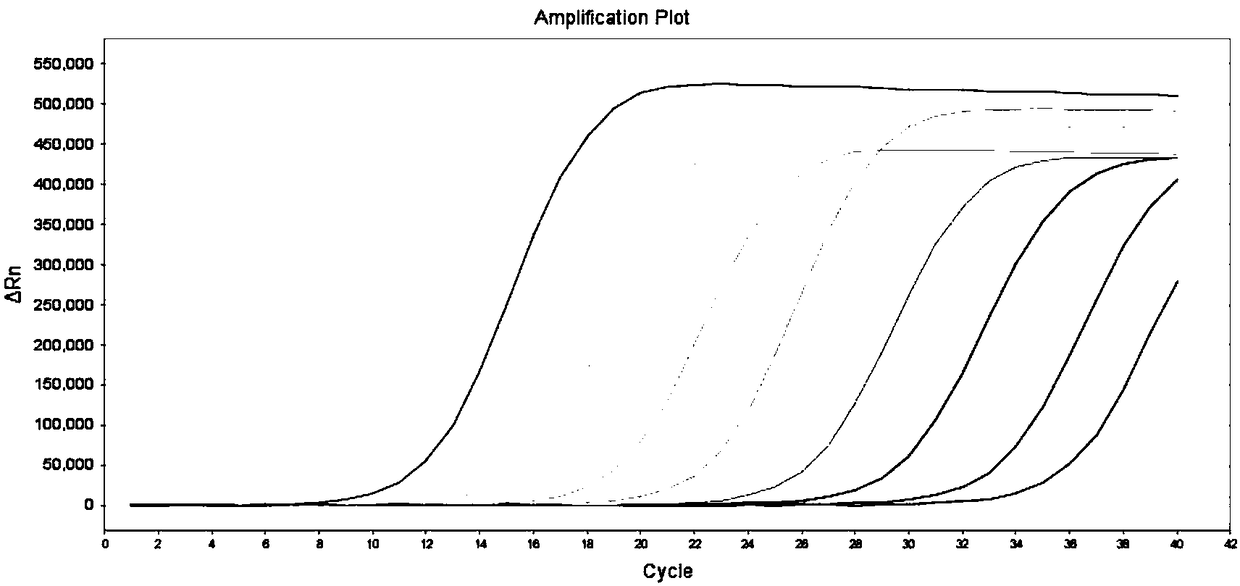
Enzyme digestion enriched PCR method used in noninvasive detection of epidermal growth factor receptor T790M mutation
InactiveCN103131779AAvoid interferenceThe detection method is simpleMicrobiological testing/measurementEnzyme digestionNucleotide
The invention relates to an enzyme digestion enriched PCR method used in noninvasive detection of epidermal growth factor receptor T790M mutation, and belongs to the field of molecular biology. The invention aims at solving a clinical application bottleneck problem of NSCLC patient EGFR gene T790M mutation detection. According to the nucleotide differences between EGFR gene T790M mutant and wild-type sequences, proper PCR primers are designed, and are introduced into specific restriction endonuclease site during a PCR amplification process, and the EGFR gene T790M mutation enzyme digestion enriched PCR method is established. The method provided by the invention is mainly advantages in that: time required by the detection method is greatly shorter than that of common sequencing technologies; target sequence PCR amplification is carried out by using an enzyme digestion enriching method, and is used in detection, such that the interference caused by a large amount of wild-type sequence in the product to the detection result is avoided; the detection method steps are simple; a second-round PCR product is analyzed through mutant gene fragment specific restriction endonuclease digestion, such that mutant gene sequence can be accurately detected, and detection accuracy can be greatly improved; sampling is convenient, patient pain is low, and dynamic detection can be carried out.
Owner:SHENZHEN BAOAN DISTRICT PEOPLES HOSPITAL
Kit for EGFR gene mutation detection and application
InactiveCN108130362AMeet the actual needs of rapid detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum igeEGFR Gene Mutation
The invention discloses a kit for EGFR gene mutation detection and application, and belongs to the fields of biotechnology and medicine. Compared with the prior art, the kit has the advantages that aprimer, a probe and the kit of an amplification system are high in detection sensitivity, and can detect specimens with the mutation content being smaller than 0.1 percent; the detection accuracy is high, a double control detection system and a locked nucleic acid technology are adopted, and the reliability of detection results is guaranteed; only 8 reactions are needed, including EGFR quality control reaction liquid, L858R detection reaction liquid, 19del detection reaction liquid, G719X detection reaction liquid, L861Q detection reaction liquid, 3Ins20 detection reaction liquid, T790M detection reaction liquid and S768I detection reaction liquid, to be used for detecting 29 common mutant types of EGFR gene in serum or plasma and tissue samples of non-small cell lung cancer patients.
Owner:安徽安龙基因科技有限公司
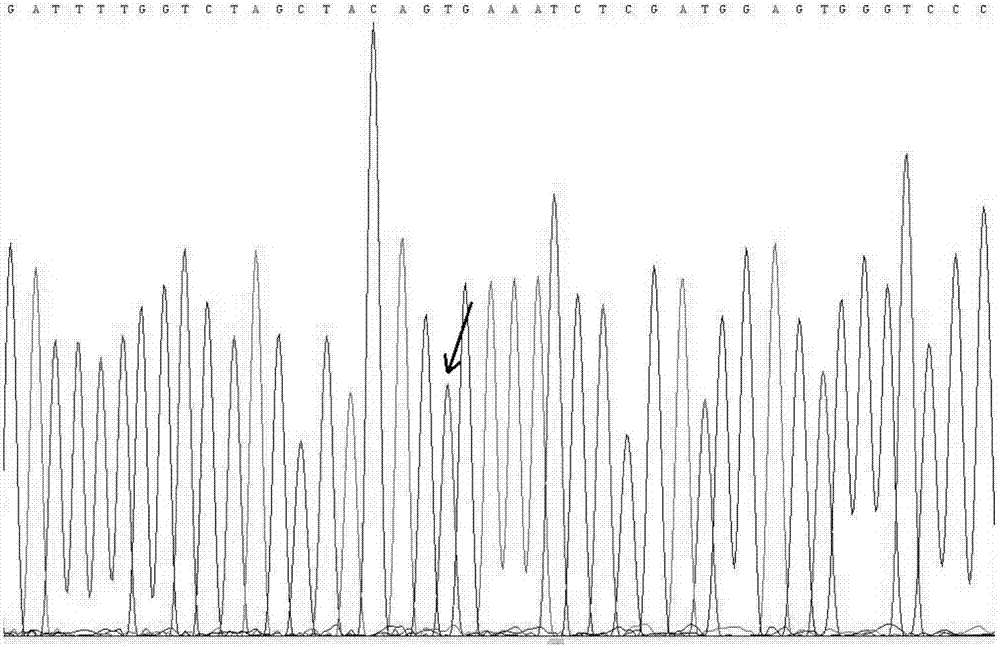
Quantitative standard substance for BRAF and EGFR gene mutation detection, preparation method and valuing method thereof
InactiveCN107365868AImprove efficiencyReduce testing quality control costsMicrobiological testing/measurementDeletion mutantSanger sequencing
The invention discloses a quantitative standard substance for BRAF and EGFR gene mutation detection, a preparation method and a valuing method thereof. The method comprises the following steps: respectively establishing plasmids containing BRAF gene V600E, EGFR gene T790M, L858R and deletion mutant E746_A750del mutation site, taking the plasmids as a template for performing PCR amplification, acquiring a PCR product and performing Sanger sequencing verification, and then using a scale for weighting ultrasonic fragmented wild type human genomic DNA and mixing at a certain ratio, thereby acquiring the quantitative standard substance with BRAF gene V600E mutant, EGFR gene T790M, L858R and deletion mutant E746_A750del mutant. The invention provides the preparation method for the quantitative standard substance. The fragmented wild type human genomic DNA is used as background DNA, so that the background for practically detecting a free DNA sample can be simulated. The valuing method for the quantitative standard substance is high in precision and accuracy and is independent from DNA standard substance and the measuring result can be traced to international basic unit (weight), so that the reliability and the traceability of the measuring result can be guaranteed.
Owner:NAT INST OF METROLOGY CHINA
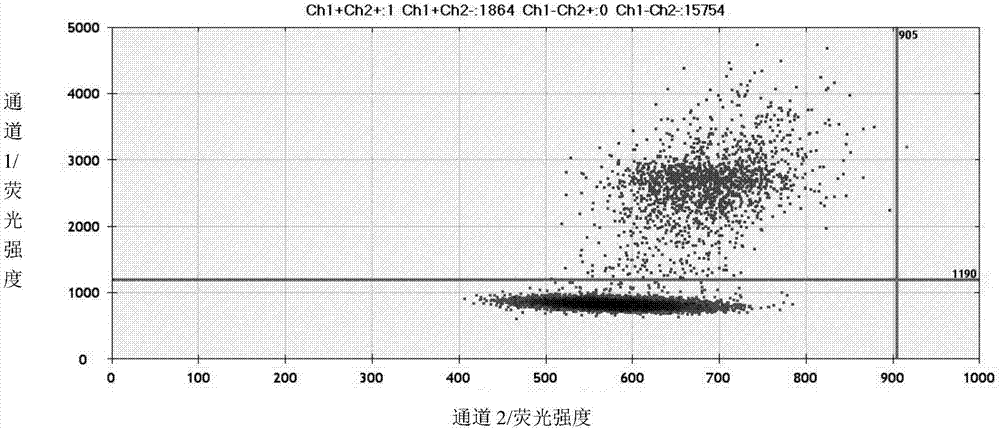
Kit and method for detection of mutation of T790M site of EGFR genes
InactiveCN111206100AAchieving High Sensitivity DetectionVarious sources of samplesMicrobiological testing/measurementFluoProbesNucleic acid detection
The invention provides a kit and method for detection of mutation of a T790M site of EGFR genes, and belongs to the field of nucleic acid detection. The kit includes an upstream primer and downstreamprimer which are used for detecting the T790M site and a mutant fluorescent probe, a wild-type fluorescent probe, a reaction premix, a positive quality control substance and a negative quality controlsubstance which are used for detecting the T790M site. According to the kit, the primers and probes with specific sequences are designed for the T790M site of the human EGFR genes, and the reaction system of the primers and probes are optimized; and through a digital PCR platform method, high-sensitivity detection of mutation of the T790M site of the EGFR genes is achieved, and the mutation abundance is obtained at the same time. The kit and method can be used for detecting nucleic acids in samples of multiple sources which include tumors, blood, saliva and serum, the application range of thekit and detection method is expanded, and medication guidance is provided for treatment of patients with lung-cancer T790M mutation.
Owner:宁波胤瑞生物医学仪器有限责任公司
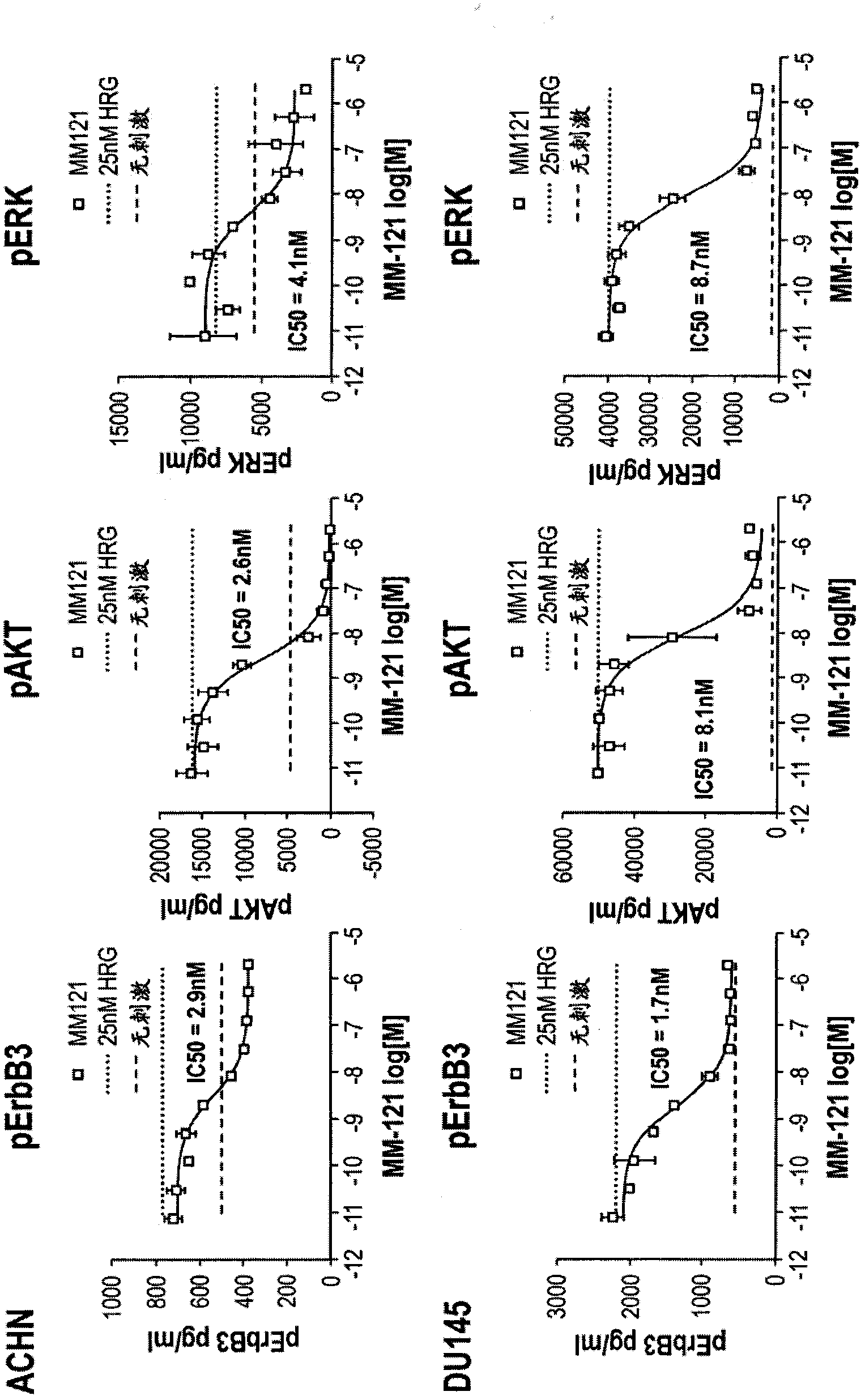
Overcoming resistance to ERBB pathway inhibitors
Provided are methods for overcoming resistance to an ErbB pathway inhibitor, such as an EGFR inhibitor or a HER2 inhibitor. The resistance may be acquired resistance to an EGFR inhibitor, such as acquired resistance to gefitinib. In the methods provided, a subject exhibiting resistance to an ErbB pathway inhibitor is selected and both an ErbB 3 inhibitor and a second ErbB pathway inhibitor are administered to the subject, such as an EGFR inhibitor or a HER2 inhibitor. Also provided are methods for inhibiting the growth of a tumor comprising a T790M EGFR mutation by contacting the tumor with an ErbB3 inhibitor and an EGFR inhibitor. Compositions for overcoming resistance to an ErbB pathway inhibitor, comprising both an ErbB 3 inhibitor and a second ErbB pathway inhibitor, such as an EGFR inhibitor or a HER2 inhibitor, are also provided.
Owner:MERRIMACK PHARMACEUTICALS INC
Methods for treating EGFR mutant cancers
Methods for the treatment of EGFR mutated cancer. For example, treatment of non-small cell lung cancer (NSCLC) with activating EGFR mutations (e.g., L858R and ex19del) the acquired or resistant “gatekeeper” T790M mutation, or any combination of these mutations.
Owner:NOVARTIS AG
2 -(2,4,5-substituted -anilino) Pyrimidine Derivatives As Egfr Modulators Useful For Treating Cancer
The present invention relates to certain 2-(2,4,5-substituted-anilino)pyrimidine compounds and pharmaceutically acceptable salts thereof which may be useful in the treatment or prevention of a disease or medical condition mediated through certain mutated forms of epidermal growth factor receptor (for example the L858R activating mutant, the Exon19 deletion activating mutant and the T790M resistance mutant). Such compounds and salts thereof may be useful in the treatment or prevention of a number of different cancers. The invention also relates to pharmaceutical compositions comprising said compounds and salts thereof, especially useful polymorphic forms of these compounds and salts, intermediates useful in the manufacture of said compounds and to methods of treatment of diseases mediated by various different forms of EGFR using said compounds and salts thereof.
Owner:ASTRAZENECA AB
Pyrrolopyrimidine compounds containing m-chloroaniline substituents as well as preparation method and application of pyrrolopyrimidine compounds
The invention provides pyrrolopyrimidine compounds containing m-chloroaniline substituents as well as a preparation method and application of the pyrrolopyrimidine compounds, and particularly relatesto compounds represented by a formula (I) shown in the specification, and isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds, a preparation method of the compounds, and the isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds and applications of the compounds, and the isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds in preparation of drugs used as a kinase inhibitor. The compounds provided by the invention have good inhibitory activity on mutant EGFR<T790M> and EGFRC<797S> kinases, and shows moderate inhibitory activity on wild EGFR kinase at the same time.
Owner:BEIJING SCITECH MQ PHARMA LTD
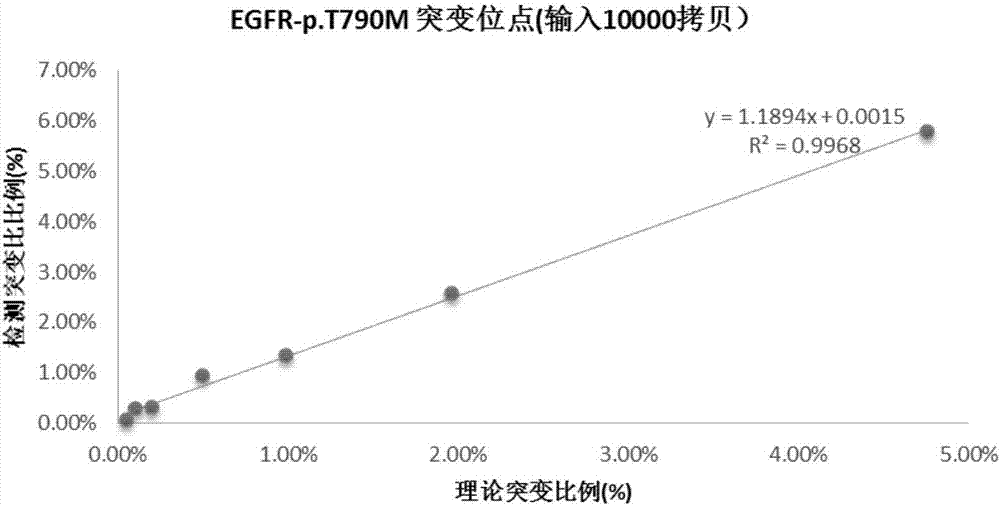
PCR kit for detecting EGFR gene T790M mutation and detection method thereof
InactiveCN109385476AHigh sensitivityQuantitatively accurateMicrobiological testing/measurementFluorescenceDrug treatment
The invention relates to the field of gene mutation detection, and discloses a PCR kit for detecting EGFR gene T790M mutation. The PCR kit comprises a nucleic acid amplification reagent and a reference substance, wherein the nucleic acid amplification reagent comprises EGFR T790M reaction solution which comprises a specific primer and a fluorescent probe for detecting the EGFR gene T790M mutation;the reaction solution further comprises a pair of upstream primer and downstream primer and an MGB fluorescent probe; the nucleic acid amplification reagent further comprises 2*QuantStudio 3D DigitalPCR Mix. The invention furthermore discloses a detection method of the PCR kit for detecting EGFR gene T790M mutation. The kit uses digital PCR technology to quantitatively detect T790M mutations intissues or plasma DNA samples of non-small cell lung cancer patients, thereby assisting the formulation of targeted drug treatment protocols for patients.
Owner:上海睿璟生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

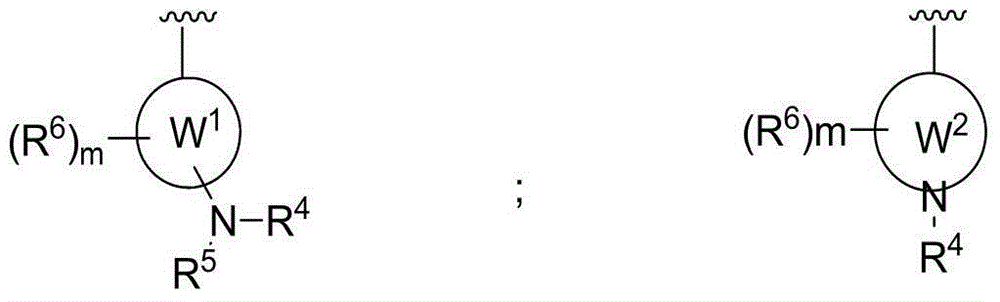

![2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application 2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application](https://images-eureka.patsnap.com/patent_img/5b54d3de-5d0b-4191-8119-8e704efb0283/BDA0001370603960000011.png)
![2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application 2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application](https://images-eureka.patsnap.com/patent_img/5b54d3de-5d0b-4191-8119-8e704efb0283/BDA0001370603960000031.png)
![2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application 2,4,6-trisubstituted pyridino[3, 4-d]pyrimidine compounds, salts thereof and application](https://images-eureka.patsnap.com/patent_img/5b54d3de-5d0b-4191-8119-8e704efb0283/BDA0001370603960000041.png)
![Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/935891c1-0fdb-4b44-a9a8-6386ce8d6850/BDA0001619614220000021.png)
![Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/935891c1-0fdb-4b44-a9a8-6386ce8d6850/BDA0001619614220000023.png)
![Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof Derivative adopting pyridino-[2,3-d]pyridine as mother nucleus as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/935891c1-0fdb-4b44-a9a8-6386ce8d6850/BDA0001619614220000031.png)