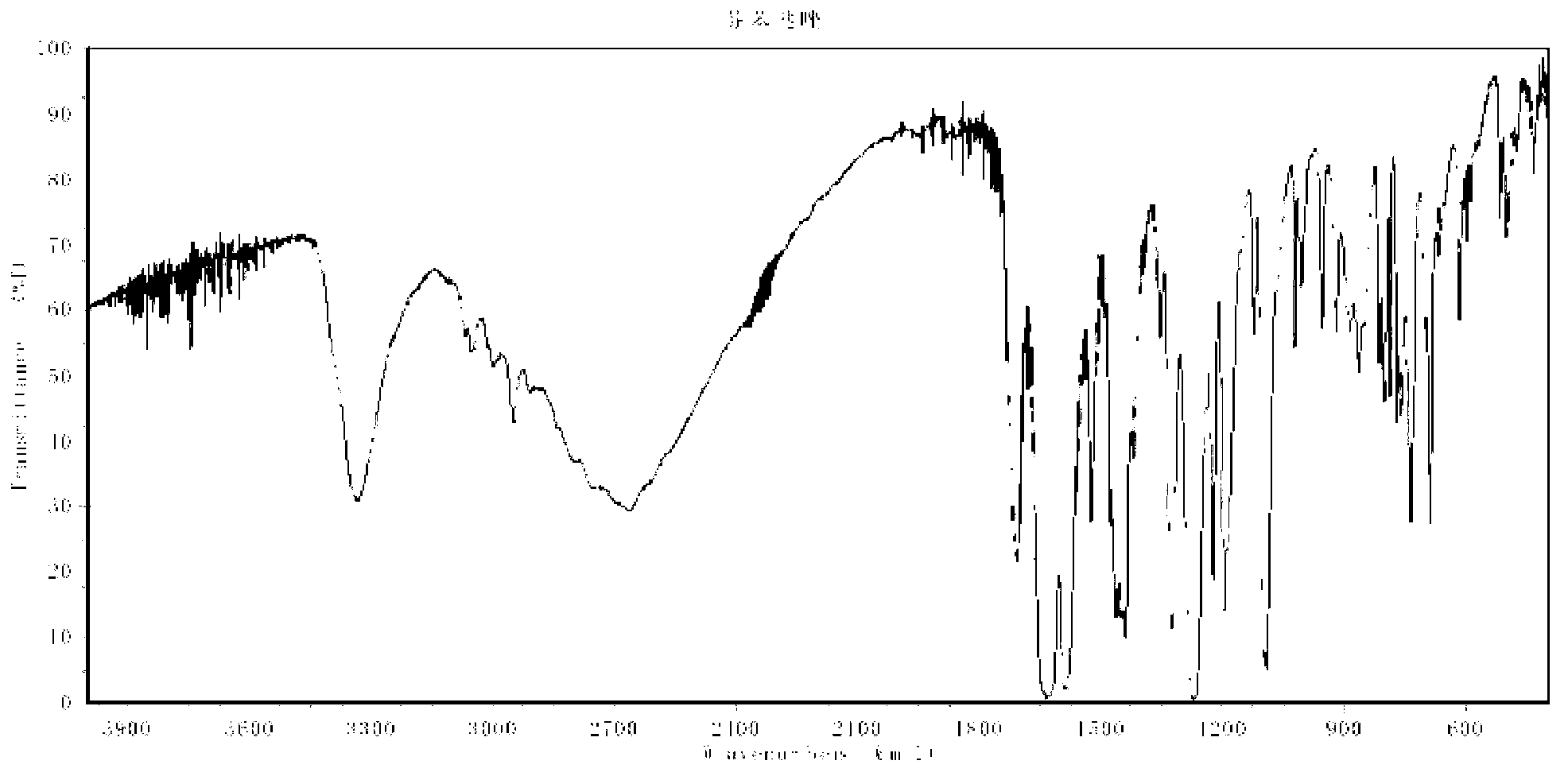
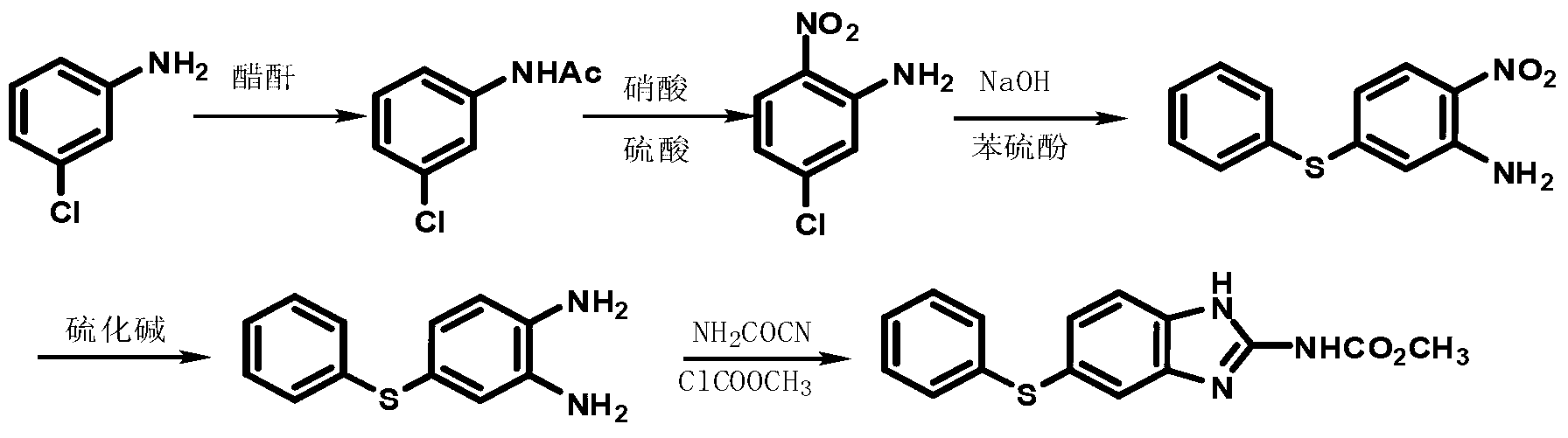
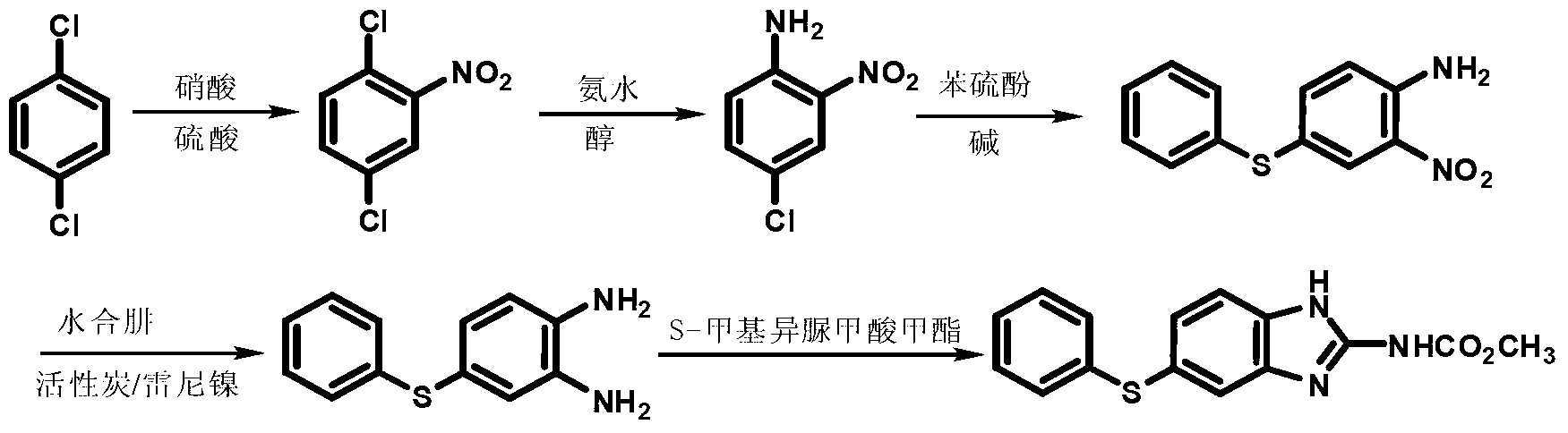
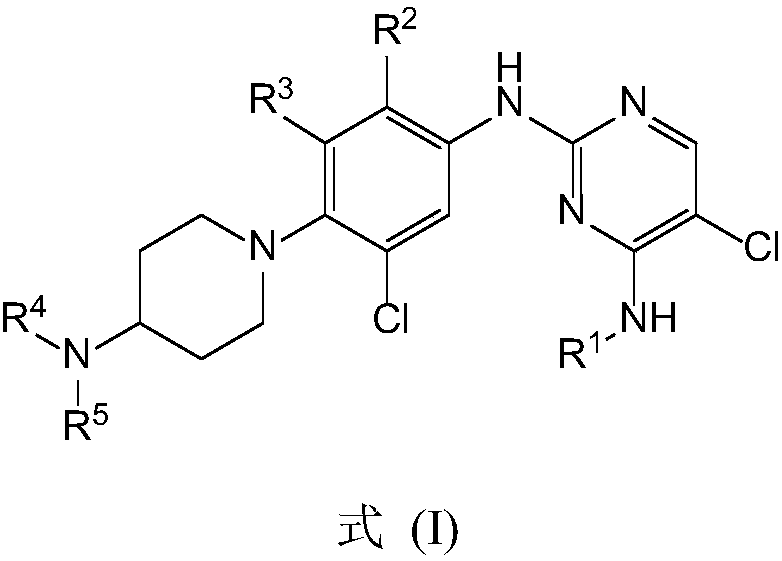
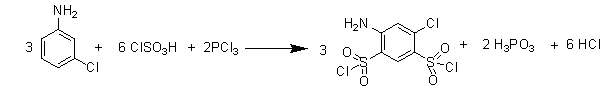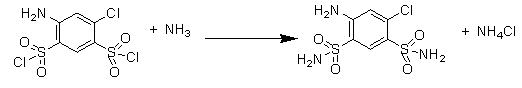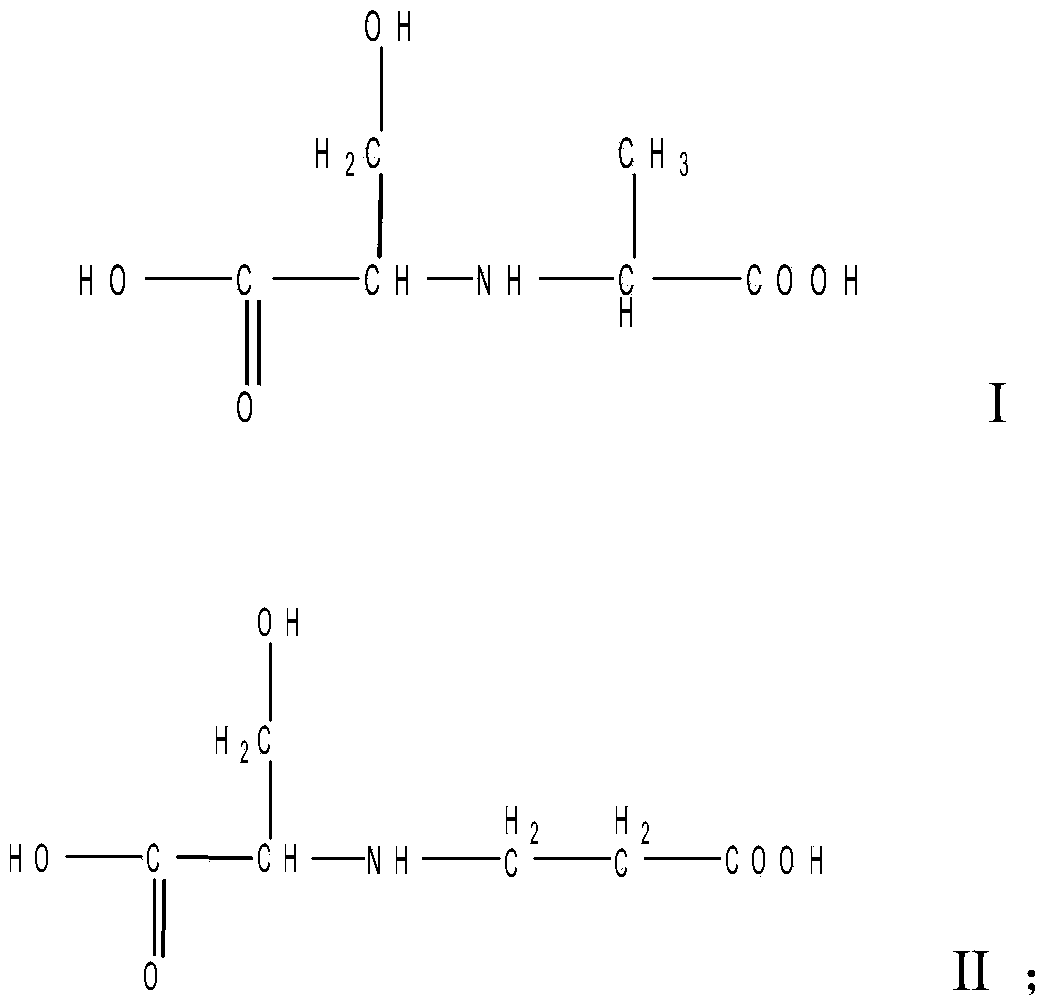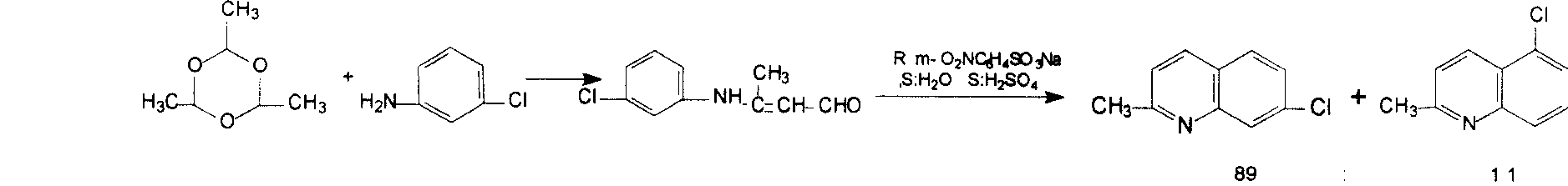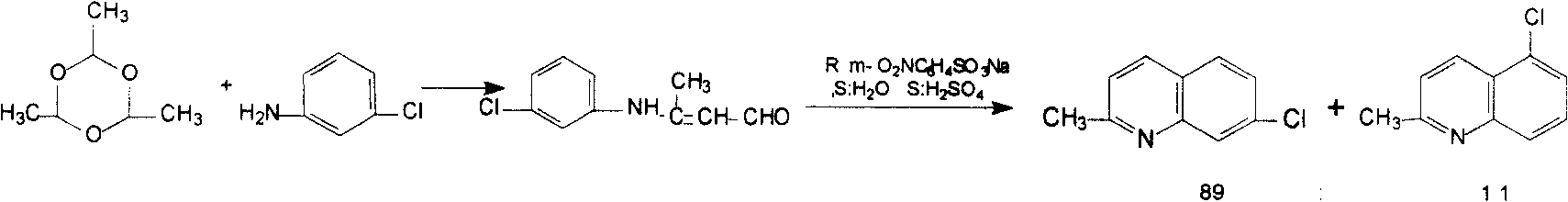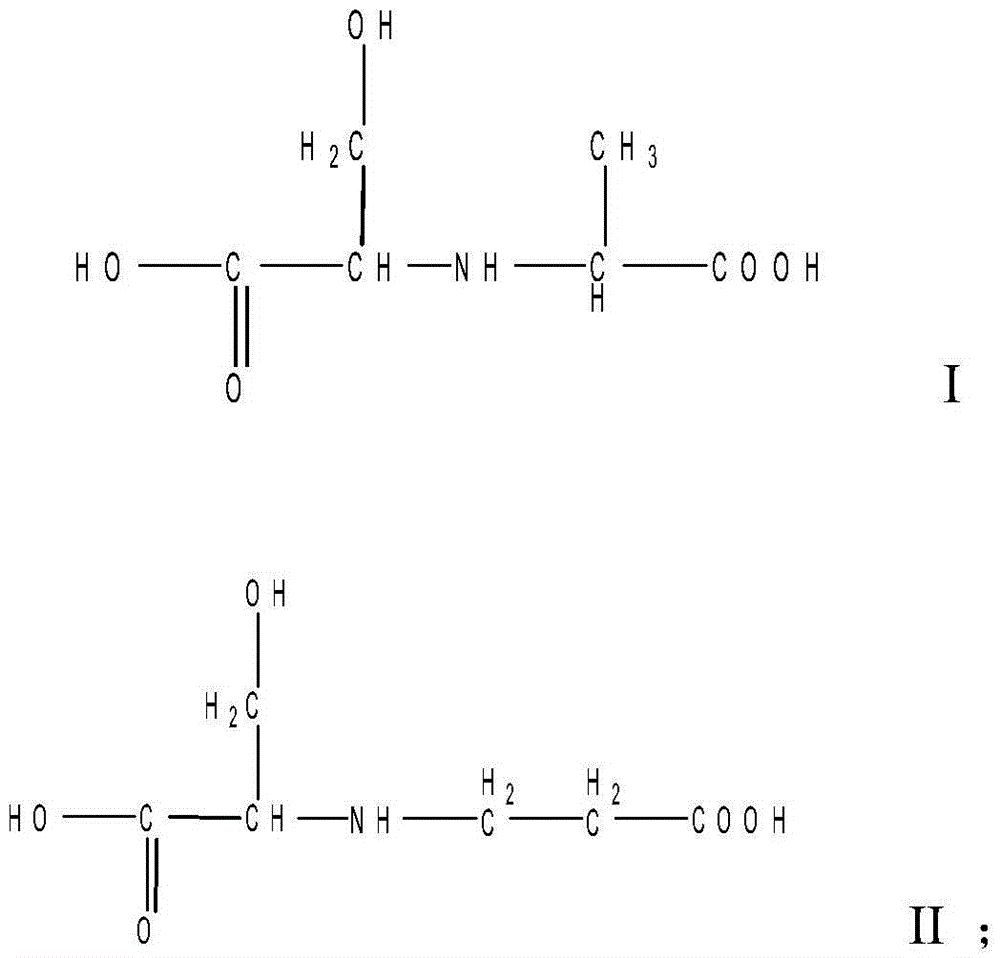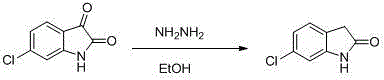Patents
Literature
41 results about "M-chloroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
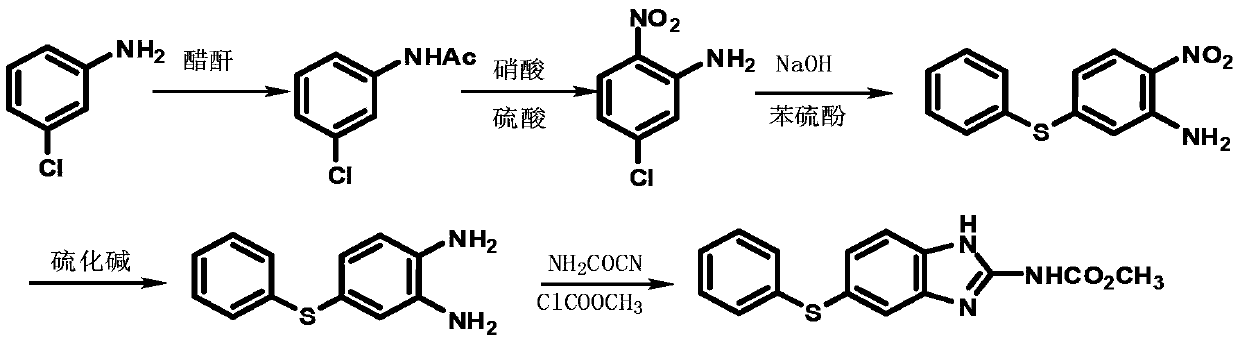
New preparation method for anthelmintic fenbendazole
InactiveCN103242237AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryFenbendazoleM-chloroaniline
The invention discloses a new preparation method for anthelmintic fenbendazole, and provides a new synthetic route for fenbendazole. Fenbendazole is obtained by taking m-dichlorobenzene as a starting raw material through the reactions of nitration, amination, condensation, reduction and cyclization. The new preparation method is characterized in that m-chloroaniline serving as the starting raw material in the conventional industrial route is changed into m-dichlorobenzene which is low in cost; and a reduction process which uses sodium sulfide is changed into a clean and efficient reduction process. The new synthetic process is simple and efficient, low in pollution, high in quality and applicable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM
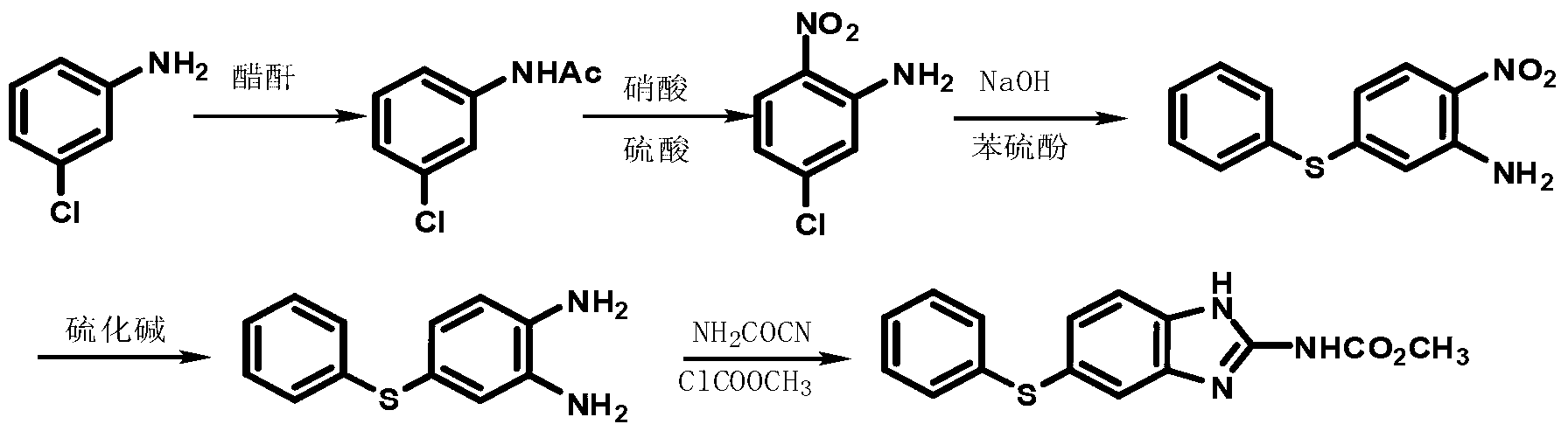
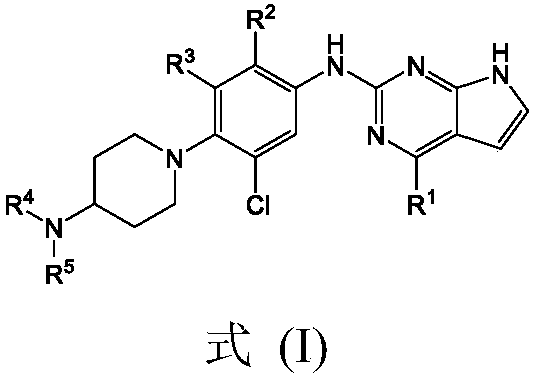
Biamino chloro pyrimidine compound containing m-chloroaniline substituent groups, and preparation method and applications thereof
InactiveCN110467638AOrganic active ingredientsGroup 5/15 element organic compoundsM-chloroanilineWild type
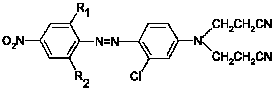
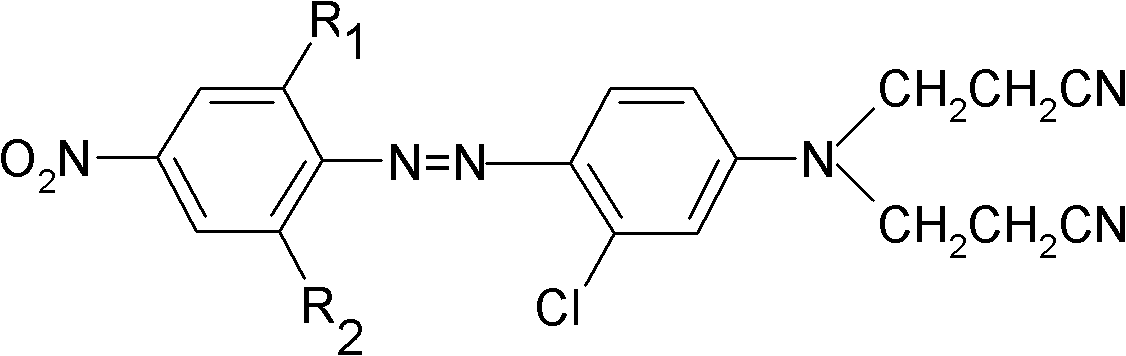
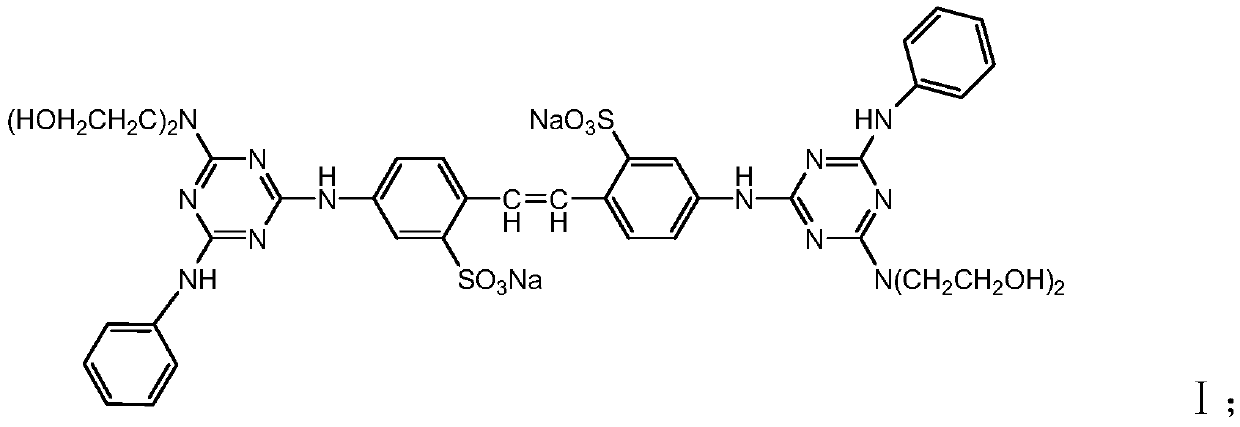
The invention provides a biamino chloro pyrimidine compound containing m-chloroaniline substituent groups, and a preparation method and applications thereof, and more specifically relates to a compound represented by formula I, and isomers, hydrates, solvates, pharmaceutically acceptable salts, and a prodrug thereof, a preparation method of the above compounds, and applications of the compounds inpreparation of drugs taken as kinases inhibitors. The preparation method is used for solving a problem in the prior art that drug resistance is caused by conventional kinases inhibitors. The compoundpossesses excellent inhibition activity on mutant EGFRT790M and EGFRC797S kinases, and moderate inhibition activity on wild type EGFR kinases.
Owner:BEIJING SCITECH MQ PHARMA LTD
Preparation method of 3-chloro-2,6-diethyl aniline
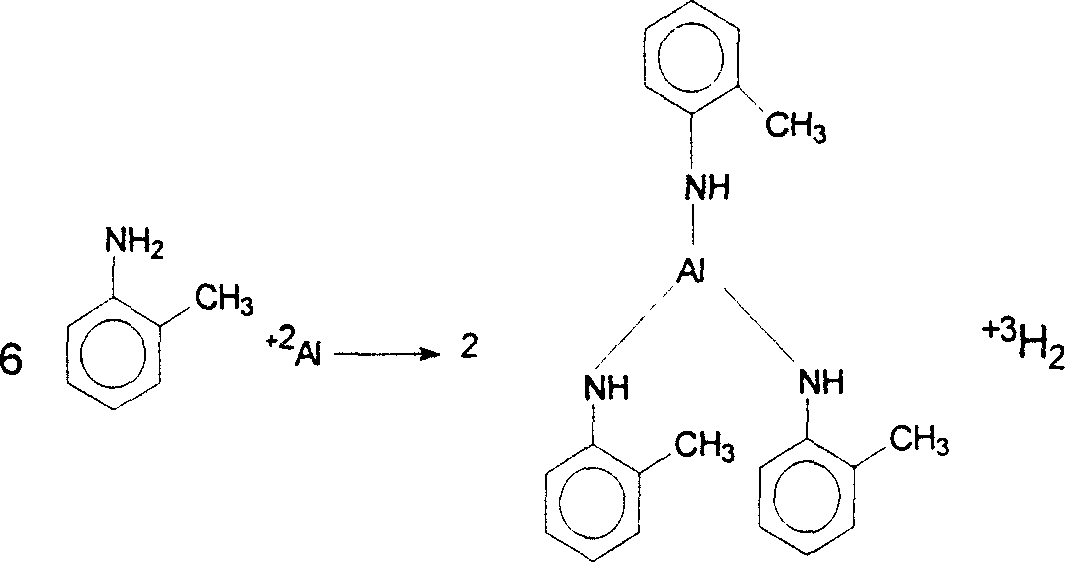
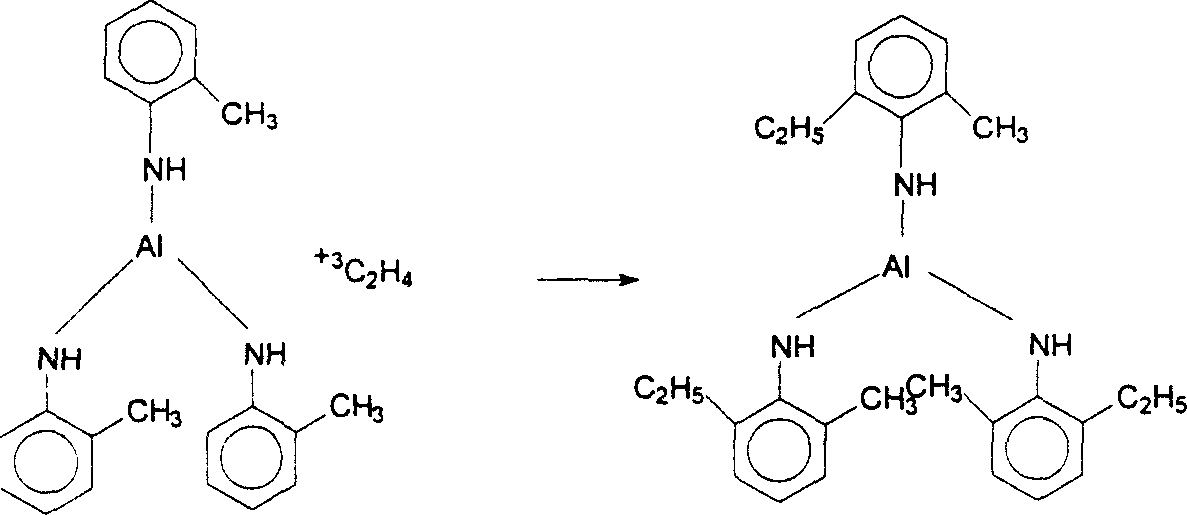
InactiveCN1903832ASmooth responseSuit one's needsOrganic chemistryChemical recyclingM-chloroanilineAniline
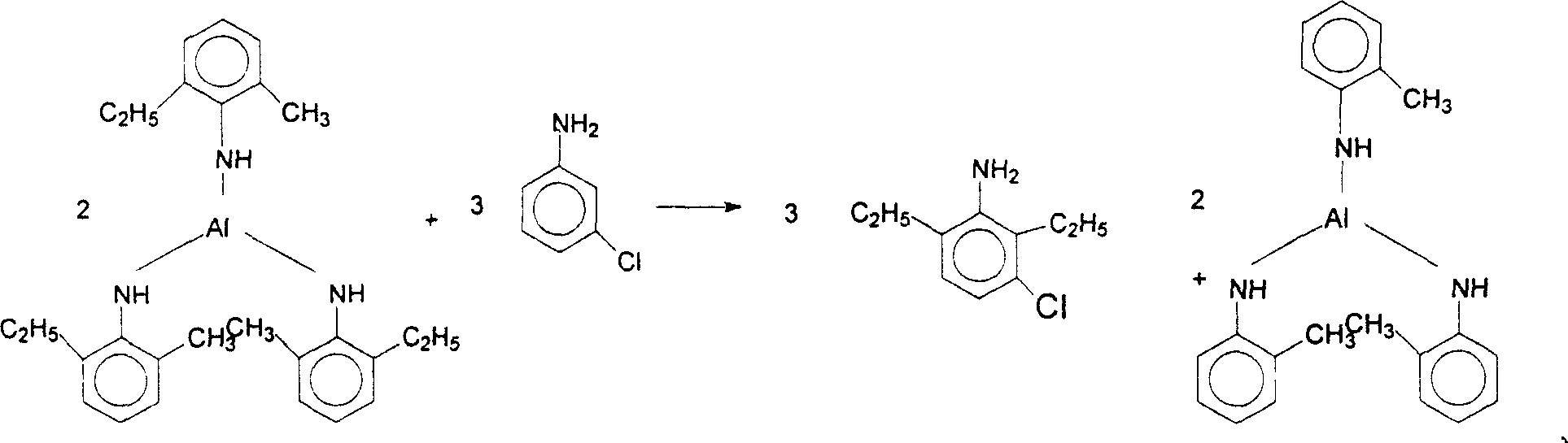
The present invention discloses a preparation method of 3-chloro-2,6-diethylaniline. Said method includes the following steps: heating metal or its salt in aniline or alkylaniline and making them produce reaction to obtain aniline or alkylaniline metal complex catalyst, adding m-chloroaniline into the obtained complex catalyst, under the condition of 150deg.C-400deg.C and 10MPa-25MPa introducing ethylene, under the action of aniline or alkylaniline metal complex catalyst producing ethylated complex, then making m-chloroailine and ehylated complex produce metathetical reaction so as to obtain 3-chloro-2,6-diethylaniline, and make the complex catalyst obtain regeneration.
Owner:黄秋耉
2-chloro-4-fluorobenzoic acid and preparation method thereof
InactiveCN105732357AReduce volatilityReduce manufacturing costSilicon organic compoundsOrganic compound preparationSilyleneCarboxylic acid
The invention discloses 2-chloro-4-fluorobenzoic acid and a preparation method thereof. In the method, m-chloroaniline is used as a raw material, and the protection of the amino group is realized through 2-(trimethylsilyl)ethoxymethyl chloride successively, and the Vilsmeier-Haack reaction is realized. Formylation, then oxidation to carboxylic acid, hydrogenation to reduce the nitro group, and then fluorination reaction to prepare 2-chloro-4-fluorobenzoic acid. The pesticide intermediate 2-chloro-4-fluorobenzoic acid provided by the present invention is simple to prepare, suitable for mass production, and uses cheap m-chloroaniline as a raw material, and uses a substance with low volatility and low toxicity as a reaction during the preparation process. Medium, reasonably control the type and amount of catalyst and oxidant, so that the yield of the product can reach more than 85%.
Owner:叶芳
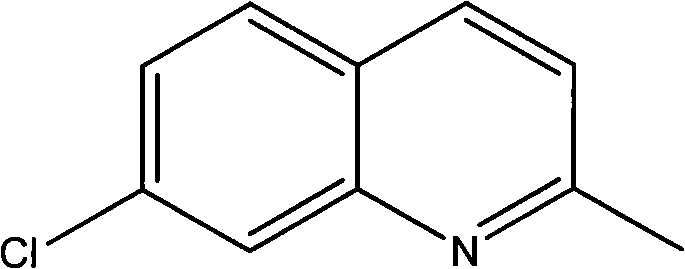
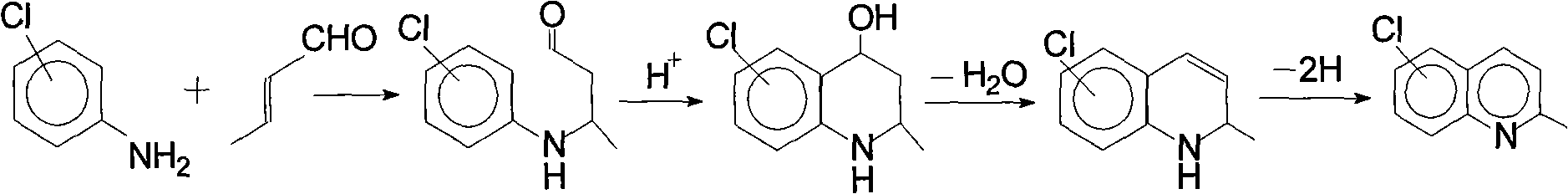
Method for preparing 7-chloroquinaldine by utilizing phase-transfer reaction
InactiveCN101638382AReduce polymerization side reactionsSettlement yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsM-chloroanilineOrganic solvent
The invention relates to a method for preparing 7-chloroquinaldine by utilizing a phase-transfer reaction. The method comprises the following steps: (1), dissolving m-chloroaniline to organic solvent;(2), carrying out a synthesis reaction: adding a phase-transfer catalyst and an oxidant to a diluted acid aqueous solution; stirring and dipping the m-chloroaniline solution obtained in the step (1)to the prepared diluted acid solution; heating to be 98-103 DEG C; fully stirring, dipping crotonic aldehyde, keeping temperature and stirring after dipping; and (3), purifying reaction products. Theinvention finishes the synthesis reaction by adopting a two-phase solvent system under a catalytic condition of strong Lewis acid, effectively reduces side reactions, lowers the purification difficulties, improves the yield and is suitable for industrialized production.
Owner:SHANGHAI YIMIN CHEM
Special material for protective casing for preventing pests and rats from biting communication cable and preparation method of special material
InactiveCN104371191AImprove the anti-pest performanceAvoid infringementPlastic/resin/waxes insulatorsInsulated cablesAdjuvantPhosphate
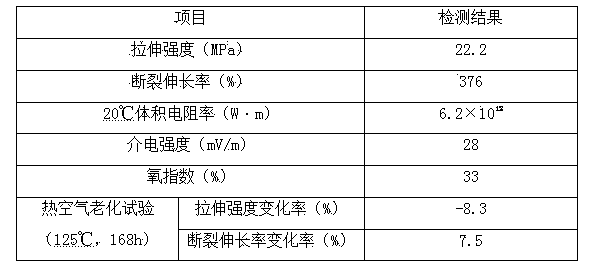
The invention discloses a special material for a protective casing for preventing pests and rats from biting a communication cable and a preparation method of the special material. The special material is prepared from the following raw materials in parts by weight: 52-76 parts of polypropylene, 31-43 parts of ethylene-trifluorochlor oethylene copolymer, 3-6 parts of benzyl benzoate, 4-6 parts of diethylene glycol bis(allylcarbonate), 2-4 parts of cycloheximide, 3-5 parts of tetrabasic lead sulfate, 10-15 parts of dicyclohexyl phthalate, 16-22 parts of spherical silica sand, 2-4 parts of triphenyltin acetate, 1.5-2.5 parts of m-chloroaniline, 5-10 parts of dibutyl azelate, 2-3 parts of phenyl diisodecyl phosphate, 4-8 parts of chlorinated paraffin, 8-14 parts of barium metatitanate, 5-10 parts of encapsulated red phosphorous, 1-2 parts of phenylethyl resorcinol and 4.5-6.5 parts of an adjuvant. The substances such as benzyl benzoate, triphenyltin acetate and m-chloroaniline are added to the special material for the protective casing disclosed by the invention, so that the pest and rat bite preventing property of the protective casing can be greatly improved; the damage to the protective casing caused by pests and rats is effectively prevented; the safety and normal operation of an optical cable are ensured; and meanwhile, the special material has excellent abrasive resistance, weather fastness, corrosion resistance and temperature tolerance, long service life and wide application range.
Owner:安徽电信器材贸易工业有限责任公司
N,N-diacetoxyethyl-m-chloroaniline series azo dyes
InactiveCN102086307AHigh color giving powerBright shadeMonoazo dyesDyeing processDisperse dyeM-chloroaniline
The invention provides N,N-diacetoxyethyl-m-chloroaniline series azo dyes. The general formula of the dyes is shown below, wherein R1 represents H, NO2, Cl, Br or COMMUNICATION and R2 represents H, NO2, Cl, Br or CN. The preparation method of the dyes comprises the following steps: using paranitroaniline or a derivative of paranitroaniline as diazo component to perform diazotization, and then reacting with N,N-diacetoxyethyl-m-chloroaniline to obtain orange or orange-red disperse dye which can have a single structure and be used to directly prepare dyes with mixed structures. The type of dyes are used to dye polyester fiber in dye liquor which is from weakly acidic to weakly alkaline; and the dyes have bright colors, good levelling property and redyeing property, high lifting force and excellent color fastness properties.
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
Method for preparing 7-chloroquinaldine by use of phase-transfer catalytic reaction
InactiveCN103524408AGood emulsifying effectSuppress generationOrganic chemistryPhosphomolybdic acidM-chloroaniline
The invention discloses a method for preparing 7-chloroquinaldine by use of a phase-transfer catalytic reaction. M-chloroaniline and crotonaldehyde are used as raw materials, lauryl sodium sulfate (serving as a surface active agent) and phosphomolybdic acid (serving as a oxidizing agent) are added in a water and 2-butanol reaction system for reaction to obtain the 7-chloroquinaldine with high yield. Compared with the prior art, the method has the remarkable advantages that (1) the lauryl sodium sulfate is added to enhance emulsibility of reaction liquid, the generation of byproducts (5-isomer) is inhibited, and the reaction efficiency is greatly improved; (2) the phosphomolybdic acid is also added, as the oxidizing agent has common gender of acid, the traditional acid solvent is avoided, and the property of the oxidizing agent can be realized; (3) the reaction can be performed in water, the aftertreatment is simple, and the reaction yield (up to 89%) is greatly improved.
Owner:LIANYUNGANG HUALUN CHEM
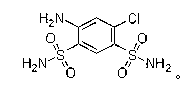
Preparation method of high-purity fine sulfanilamide
ActiveCN103319381AReduce dosageShort reaction timeSulfonic acid amide preparationSulfanilamideAniline
The invention relates to a preparation method of fine sulfanilamide and particularly relates to a preparation method of high-purity fine sulfanilamide. The preparation method sequentially comprises the following steps of: proportioning m-chloroaniline, chlorosulfonic acid and phosphorus trichloride, then, mixing chlorosulfonic acid, m-chloroaniline and phosphorus trichloride at the temperature of 30-45 DEG C, heating to 105-120 DEG C and carrying out heat preservation for 2-4hours; standing and cooling to obtain a chlorosulfonated substance; introducing ammonia gas to an amination reaction device for the first time, adding the obtained chlorosulfonated substance, then, introducing ammonia gas to the amination reaction device for the second time, carrying out amination reaction at the temperature of 40-45 DEG C and the pressure of 0.1-0.2MPa, and carrying out heat preservation for 1-3hours to obtain an amide; and carrying out dissolution, decoloration, crystallization and purification on the obtained amide: adding a sodium hydroxide solution to adjust the pH value to 10-11, then, adding active carbon, heating to 80-95 DEG C, carrying out heat preservation for 0.3-0.8h, filter pressing while the solution is hot, adding hydrochloric acid into the obtained filtrate to adjusting the pH to 2-4, separating out white crystal substances, and then, cooling, washing, centrifuging and drying to obtain a finished product of the fine sulfanilamide. The preparation method is few in reaction by-product and high in product purity.
Owner:中瑞(内蒙古)药业股份有限公司
Preparation method of fenbendazole
ActiveCN103242238AAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryM-chloroanilineFenbendazole
The invention discloses a preparation method of fenbendazole and provides a brand-new synthesis route of the fenbendazole. The fenbendazole is prepared from m-dichlorobenzene as a starting material through the steps of nitrification, condensation, amination, reduction and cyclization. The preparation method is characterized in that the starting material m-chloroaniline in the existing industrial route is changed to the cheap m-dichlorobenzene; the existing reduction technology with sodium sulfide dihydrate is changed to the clean and high-efficiency reduction technology; and the new synthesis technology is concise and simple, high in efficiency, slight in pollution, high in quality, and suitable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
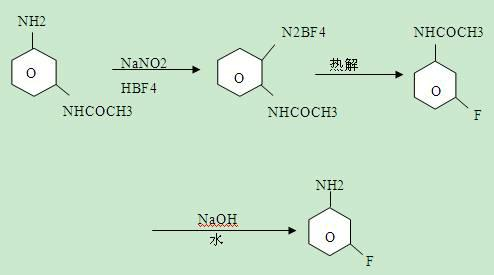
Synthesis method of m-fluoroaniline
InactiveCN102173995AHigh yieldOrganic compound preparationAmino compound preparationM-chloroanilineSynthesis methods
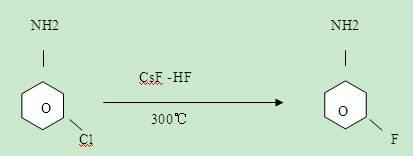
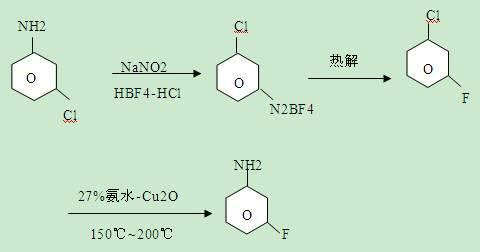
The invention relates to a synthesis method of the fluorine-containing aniline, in particular to a synthesis method of m-fluoroaniline. The synthesis method of m-fluoroaniline is characterized in that m-chloroaniline is used as the starting material to perform Schiemann reaction and obtain m-fluorochlorobenzene, m-fluorochlorobenzene is used to perform amination reaction and obtain m-fluoroaniline, the catalyst in the amination reaction is Cu2O or CuO; and the temperature of the amination reaction is 150-200 DEG C. The synthesis method of m-fluoroaniline has the following beneficial effects: m-chloroaniline is used as the starting material to perform ammonolysis and obtain m-fluoroaniline through the Schiemann reaction; and in the synthesis method, cheap m-chloroaniline is used as a raw material and the low-cost method is adopted to prepare m-fluoroaniline, and the m-fluoroaniline yield is high.
Owner:郓城县世炬商贸有限公司
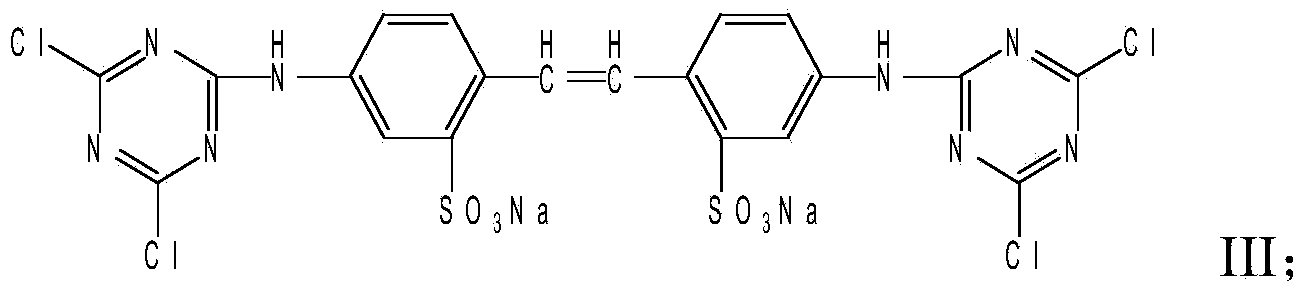
Liquid fluorescent whitening agent and preparation method thereof
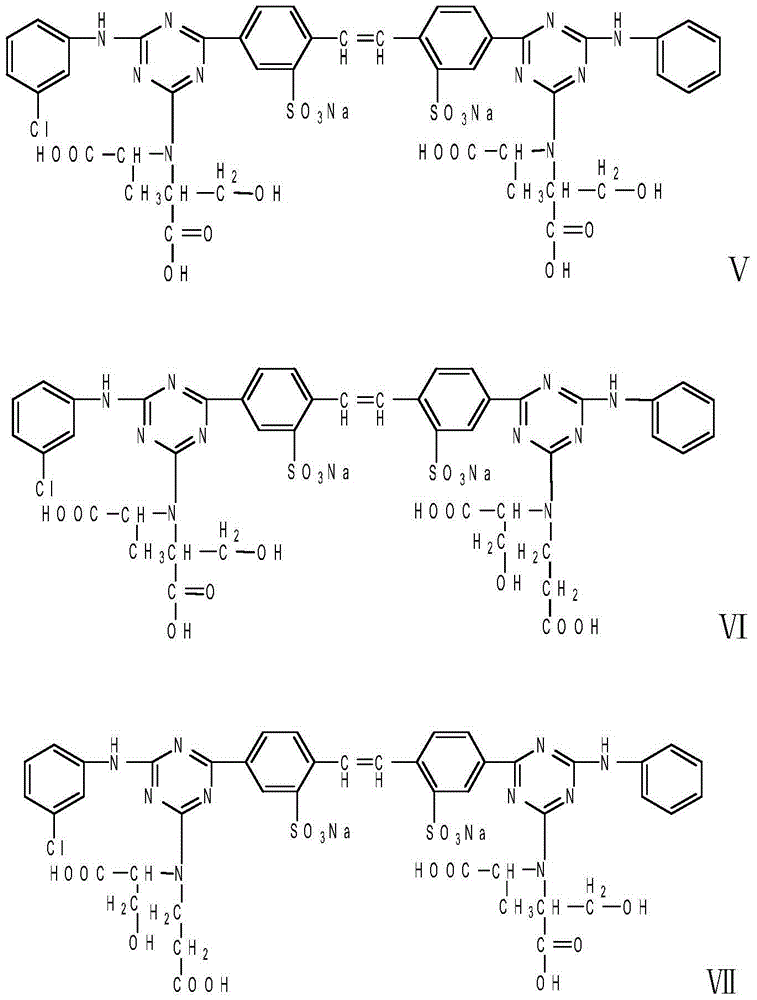
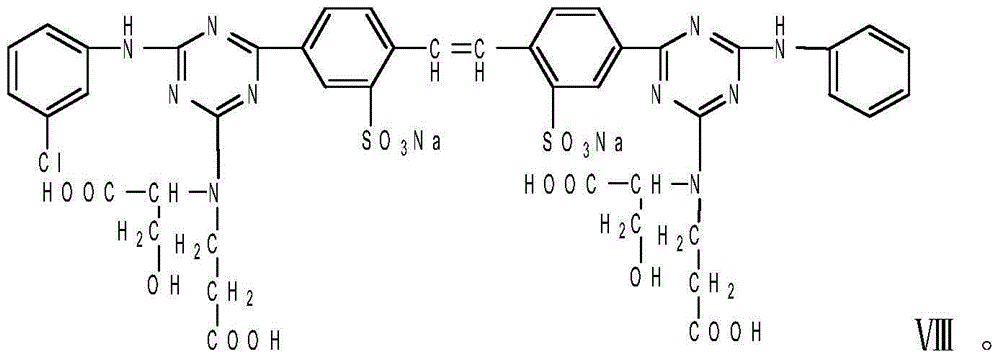
ActiveCN103436050AGood water solubilityStable storageStyryl dyesLuminescent compositionsIce waterFiltration
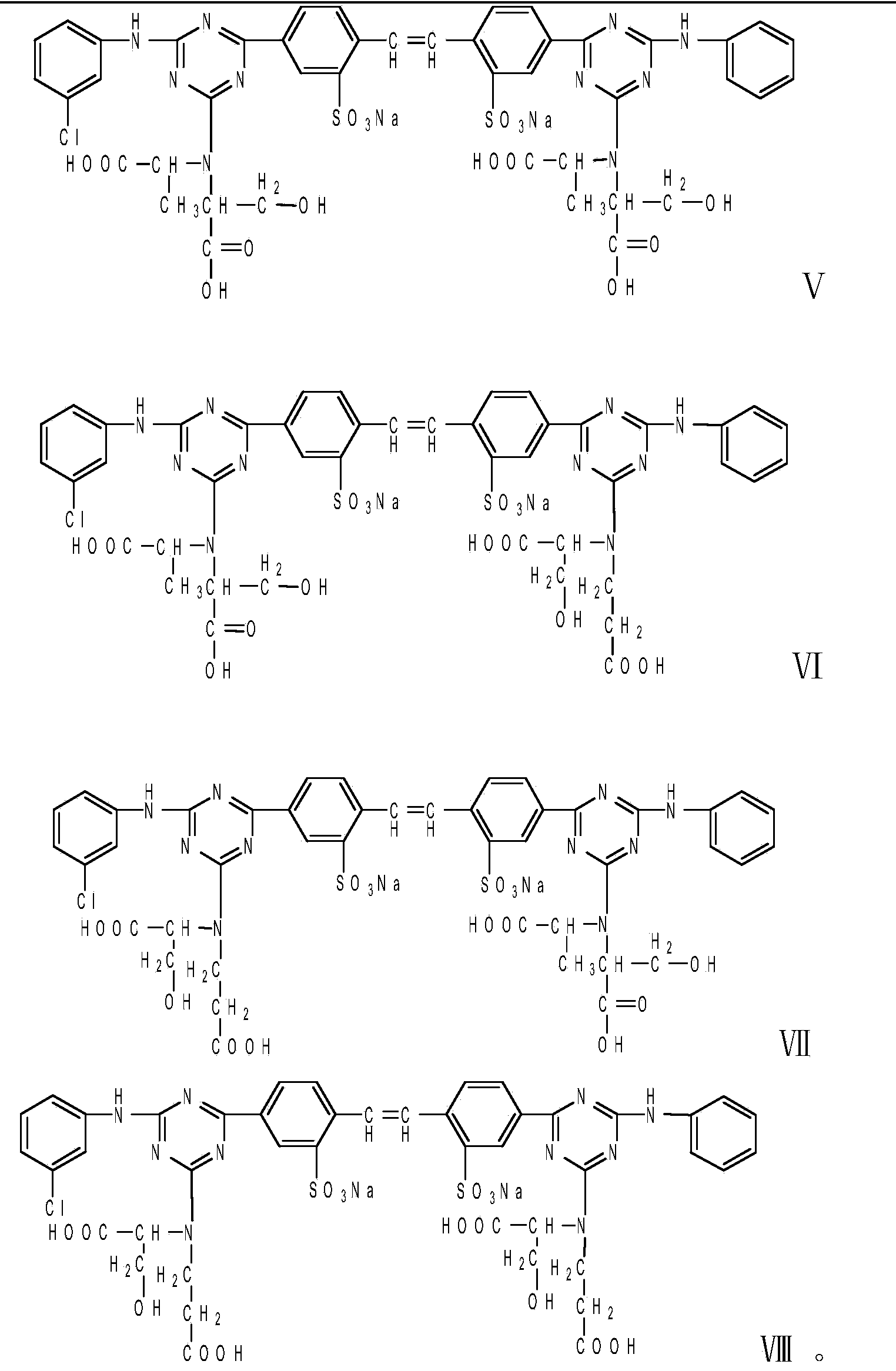
The invention relates to a liquid fluorescent whitening agent and a preparation method thereof and mainly aims at solving the technical problems that the whitening effect is not obvious and the stability is poor in an existing fluorescent whitening agent and a preparation method thereof. According to the technical scheme, the fluorescent whitening agent is mainly composed of V, VI, VII and VIII. The preparation method of the liquid fluorescent whitening agent comprises the following steps: with cyanuric chloride, 4,4'-diamino diphenylethene-2,2'-disulfonic acid, phenylamine, m-chloroaniline, acrylic acid and serine in ratio by amount of substance of 1.0:(0.45-0.55):(0.45-0.55):(0.45-0.55):1.0:(0.95-1.05) as raw materials, firstly dissolving acrylic acid and serine in water to generate a mixture of I and II, then mixing 4,4'-diamino diphenylethene-2,2'-disulfonic acid with an NaOH solution to prepare a sodium 4,4'-diamino diphenylethene-2,2'-disulfonate solution, adding the sodium 4,4'-diamino diphenylethene-2,2'-disulfonate solution, an emulsifier and cyanuric chloride into ice water, and carrying out reaction to generate a product III; adding phenylamine and m-chloroaniline into III to generate IV; and finally adding the mixture of I and II into IV to obtain a whitening agent crude product, and then carrying out suction filtration, desalination and concentration to obtain the liquid fluorescent whitening agent product.
Owner:山西晋光化工有限公司
Preparation method of midbody 7-chloroquinaldine
The present invention provides a preparation method of medicine intermediate 7-chlorine quinaldine. M-chloroaniline salt of inorganic acid and crotonaldehyde have a closed loop reaction in mixed solvent of alcohol and arene to prepare the salt of 7-chlorine quinaldine; alkaline is added for neutralization; and the 7-chlorine quinaldine can be prepared. The present invention has the advantages that the production of a large quantity of isomers is inhibited, the operation is simple, the production period is obviously shortened, no large amount of acid, alkaline or expensive solvent is used in the post-processing, and the product is more conducive to the environmental protection; a large amount of energy is saved; simultaneously, the reaction conditions are simplified, the reaction process is safer and more reliable, the quality and yield rate of the product are ensured, and the present invention is more suitable for industrial production.
Owner:中国科学院嘉兴应用化学工程中心
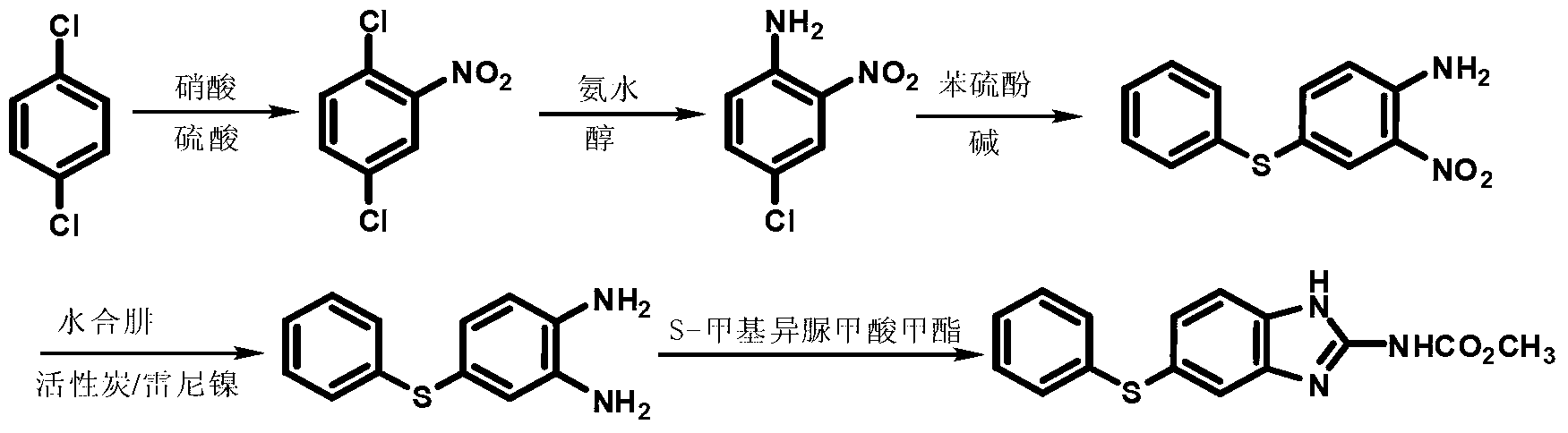
Pyrrolopyrimidine compounds containing m-chloroaniline substituents as well as preparation method and application of pyrrolopyrimidine compounds
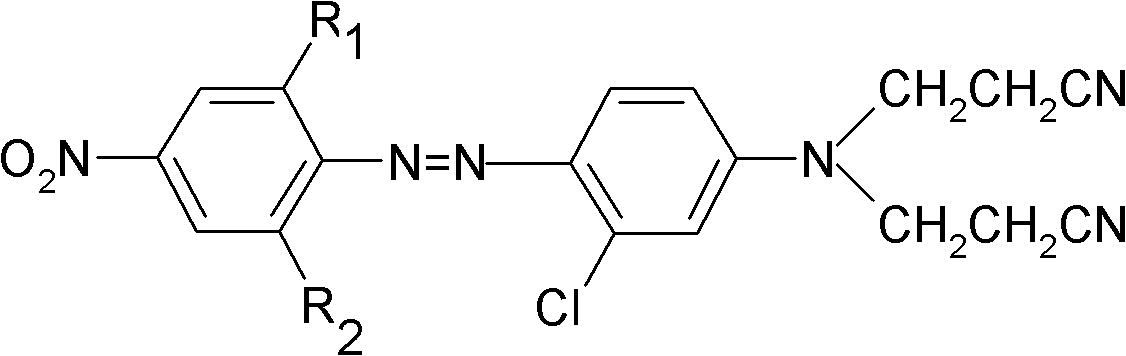
The invention provides pyrrolopyrimidine compounds containing m-chloroaniline substituents as well as a preparation method and application of the pyrrolopyrimidine compounds, and particularly relatesto compounds represented by a formula (I) shown in the specification, and isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds, a preparation method of the compounds, and the isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds and applications of the compounds, and the isomers, hydrates, solvates, pharmaceutically acceptable salts and prodrugs of the compounds in preparation of drugs used as a kinase inhibitor. The compounds provided by the invention have good inhibitory activity on mutant EGFR<T790M> and EGFRC<797S> kinases, and shows moderate inhibitory activity on wild EGFR kinase at the same time.
Owner:BEIJING SCITECH MQ PHARMA LTD
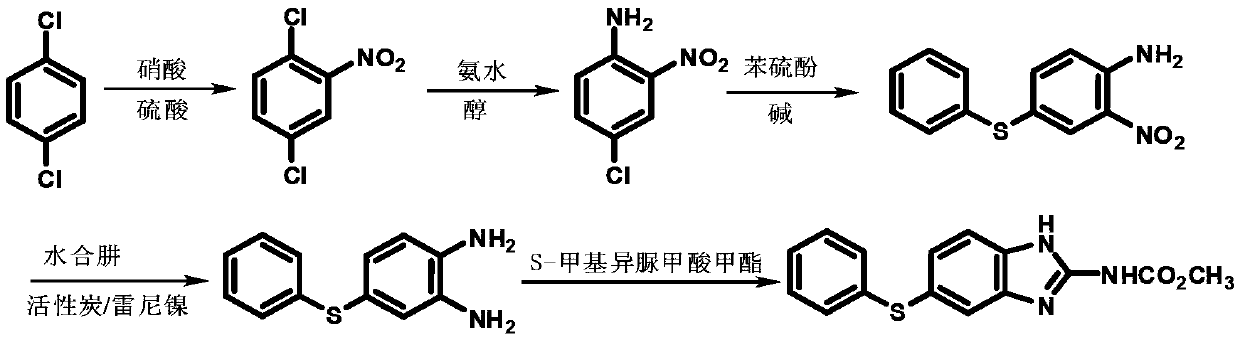
Method for preparing m-chloroaniline by using meta-position oil
ActiveCN106883129ALow costHigh outputOrganic compound preparationAmino compound preparationSodium hydrosulfideToxic industrial waste
The invention relates to a method for preparing m-chloroaniline by using meta-position oil and belongs to the technical field of preparation of m-chloroaniline. The method specifically comprises the steps of carrying out an ingredient reaction, carrying out water washing, carrying out drying, carrying out third-stage vacuum distillation, carrying out crystallization, carrying out reduction and carrying out re-distillation. According to the method, m-chloroaniline is prepared from waste, i.e., meta-position oil of production of p-nitrophenol and o-nitrophenol and industrial waste liquid, i.e., sodium hydrosulfide, which serve as raw materials, by using methoxylation, crystallization and reduction, and byproducts, i.e., o-aminoanisole and p-aminoanisole are produced, so that low cost and high yield are achieved, and the method has an obvious cost advantage and higher economic benefit compared with the existing production technologies.
Owner:ANHUI HAIHUA CHEM
A kind of preparation method of fenbendazole
ActiveCN103242238BAvoid pollutionThe new synthesis process is simple and efficientOrganic chemistryM-chloroanilineFenbendazole
The invention discloses a preparation method of fenbendazole and provides a brand-new synthesis route of the fenbendazole. The fenbendazole is prepared from m-dichlorobenzene as a starting material through the steps of nitrification, condensation, amination, reduction and cyclization. The preparation method is characterized in that the starting material m-chloroaniline in the existing industrial route is changed to the cheap m-dichlorobenzene; the existing reduction technology with sodium sulfide dihydrate is changed to the clean and high-efficiency reduction technology; and the new synthesis technology is concise and simple, high in efficiency, slight in pollution, high in quality, and suitable to industrial production.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
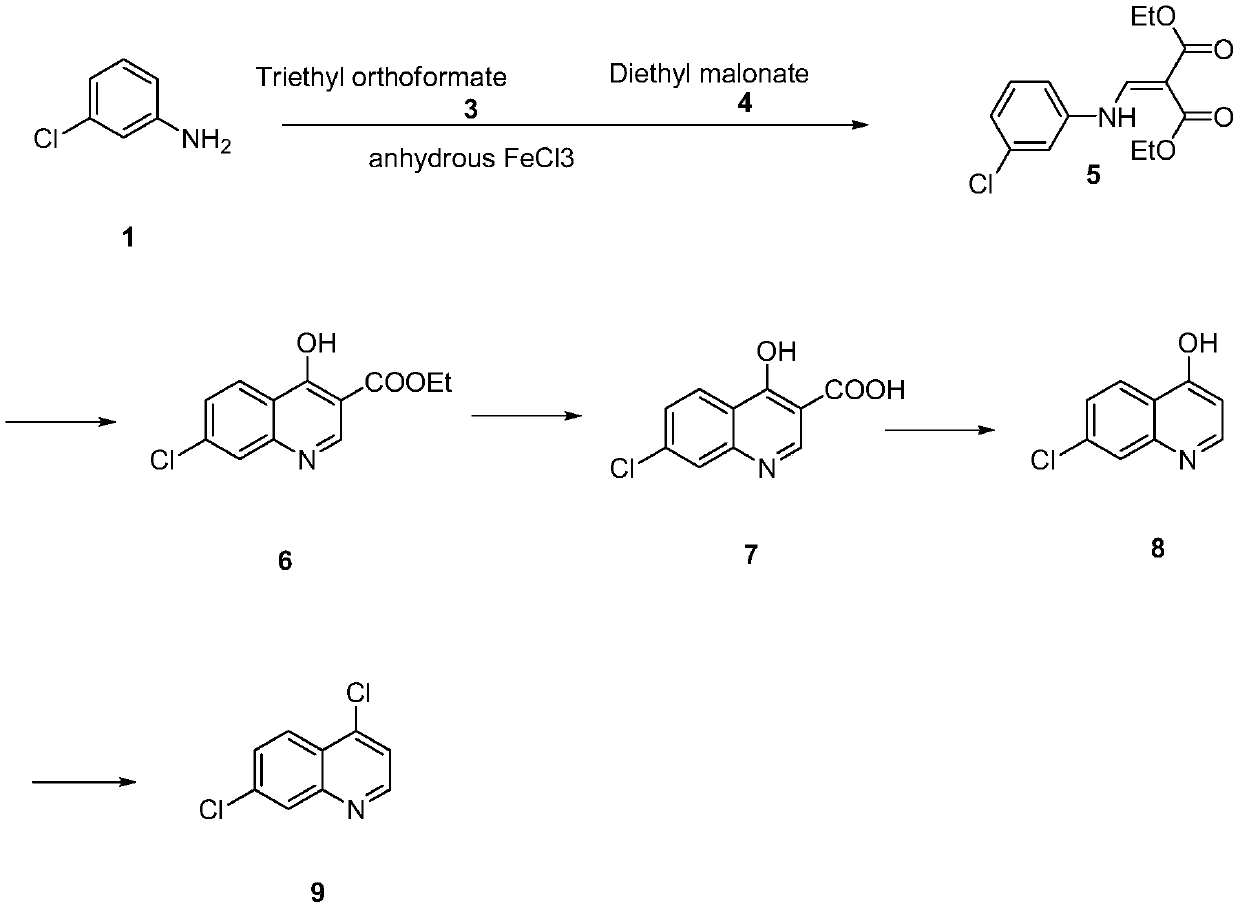
Synthesis method of 4,7-dichloroquinoline
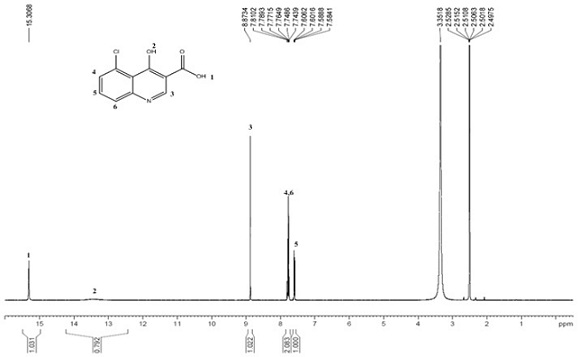
The invention discloses a synthesis method of 4,7-dichloroquinoline. The synthesis method is characterized by comprising the following steps: synthesizing 7-chloro-4-hydroxylquinoline-3-carboxylic acid by using a one-pot method, and carrying out decarboxylation and chlorination on the 7-chloro-4-hydroxylquinoline-3-carboxylic acid to obtain 4,7-dichloroquinoline. The step of synthesizing the 7-chloro-4-hydroxylquinoline-3-carboxylic acid by the one-pot method comprises the following sub-steps: with m-chloroaniline, triethyl orthoformate or trimethyl orthoformate and diethyl malonate as raw materials, carrying out condensation under the catalysis of anhydrous ferric trichloride to obtain diethyl 2-[[(3-chlorophenyl)amino]methylene]malonate, directly adding a condensation reaction solution into an organic solvent, carrying out heating cyclization to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid ethyl ester, and after the cyclization reaction is completed, adding sodium hydroxidefor hydrolysis to obtain 7-chloro-4-hydroxylquinoline-3-carboxylic acid. Although the whole process comprises five reactions, intermediate products are good enough in purity and can be directly synthesized into a target product without purification, so operation is easy and convenient and industrialization is facilitated; and raw materials are easily available, and pollution is small.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
A heterocycle azo yellow disperse dye and a preparing method thereof
A heterocycle azo yellow disperse dye is disclosed. The molecular structure of the disperse dye is shown as (I). A preparing method of the disperse dye is also disclosed. The method includes a step of adding water and m-chloroaniline or m-chloroaniline hydrochloride into a reaction pot, adding hydrochloric acid having a concentration of 30% under stirring, adding ice cubes, cooling to 0-5 DEG C, and adding a sodium nitrite solution having a concentration of 30% to obtain a diazonium salt solution; a step of adding water and sodium carbonate into a coupling pot, adding N-butyl pyridine under stirring, stirring, adding hydrochloric acid having a concentration of 8%, adding ice cubes, and cooling to 5-10 DEG C to obtain a coupling component; a step of adding the prepared diazonium salt solution into the coupling component, adjusting the pH value of a reaction product to 0.5-1.5, reacting for 4-5 h, heating to 50 DEG C, filtering, and rinsing until the product is neutral to obtain the disperse dye. The disperse dye is wide in dye solution pH range, almost same in colored light, and good in light fastness and soaping fastness. The formula (I) is shown in the specification.
Owner:沈金火
Application of rhodium monatomic catalyst in reaction for preparing m-chloroaniline through selective hydrogenation of m-chloronitrobenzene
InactiveCN112774670ALow costIncrease usageOrganic compound preparationAmino compound preparationM-chloroanilinePtru catalyst
The invention provides application of a rhodium monatomic catalyst in preparation of m-chloroaniline through selective hydrogenation of m-chloronitrobenzene, wherein the conversion rate and the selectivity of m-chloroaniline can reach 99% or above under mild conditions. The catalyst is composed of a carrier with MOF as a precursor and an active component loaded on the carrier, wherein the active component is precious metal rhodium. According to the invention, the reaction system is simple, the reaction conditions are mild, and the catalyst and the solvent are easy to separate and recover; and the rhodium monatomic catalyst provided by the invention is novel in structure, metal is uniformly dispersed on the carrier, and the rhodium monatomic catalyst has relatively high reaction rate and good stability when being applied to a reaction for preparing m-chloroaniline through selective hydrogenation of m-chloronitrobenzene, and has wide application in the field of utilization for preparing m-chloroaniline through selective hydrogenation of m-chloronitrobenzene.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method and application of impurities of 4-aminoquinoline compounds
The invention discloses a preparation method and application of impurities of 4-aminoquinoline compounds, and concretely discloses a preparation method of 5-chloro-4-hydroxyl-3-quinolinecarboxylic acid (LKL-5 isomer). The preparation method is characterized in that the 5-chloro-4-hydroxyl-3-quinolinecarboxylic acid is separated from a mixture 1 containing the 5-chloro-4-hydroxyl-3-quinolinecarboxylic acid and 7-chloro-4-hydroxyl-3-quinolinecarboxylic acid by adopting a liquid phase method. Preferably, the mixture 1 is obtained by reacting diethyl m-chloroaniline methylene malonate (LKL-3) under the conditions of mineral oil and concentrated sulfuric acid, post-treating after the reaction is completed to obtain a mixture 2, hydrolyzing the mixture 2 under an alkaline condition, and post-treating to obtain the mixture 1. The purity of the 5-chloro-4-hydroxy-3-quinolinecarboxylic acid is greater than 99%, and preferably greater than 99.5%, the 5-chloro-4-hydroxy-3-quinolinecarboxylic acidcan be used for synthesizing subsequent products, and the 5-chloro-4-hydroxy-3-quinolinecarboxylic acid and the synthesized subsequent products can be used for impurity reference substances of 4-aminoquinoline compounds and can be used for quality control of related raw material medicines.
Owner:珠海润都制药股份有限公司 +1
Method for preparing m-aminophenol through pressurization alkaline hydrolysis of m-chloroaniline
InactiveCN104045572AEasy to useEasy to prepareOrganic compound preparationAmino-hyroxy compound preparationChemical industryM-aminophenol
A method for preparing m-aminophenol through pressurization alkaline hydrolysis of m-chloroaniline relates to the technical field of the chemical industry. Raw materials used in the invention comprise 415-420g of m-chloroaniline, 710-730g of 40% sodium hydroxide and 45-55g of a catalyst. The method comprises the following steps: adding m-chloroaniline and a 40% sodium hydroxide solution and the catalyst into an autoclave; and introducing nitrogen to displace air in the autoclave, heating to 220DEG C, reacting under a pressure of 0.5MPa, collecting the obtained condensate liquid, pressurizing to 2MPa, continuously carrying out heat insulation for 12h, cooling, filtering to obtain an amino phenol sodium solution, adding 30% hydrochloric acid for a neutralization reaction until the pH value is 6, layering, rectifying, purifying to obtain an m-aminophenol finished product, packaging and warehousing. The method has the advantages of convenient and simple preparation, advanced technology, easily available materials, less equipment investment, high purity and low production cost, and the prepared m-aminophenol has the advantages of good use effect, safety and reliability.
Owner:ANHUI HUARUN PAINTS
Preparation method of midbody 7-chloroquinaldine
The present invention provides a preparation method of medicine intermediate 7-chlorine quinaldine. M-chloroaniline salt of inorganic acid and crotonaldehyde have a closed loop reaction in mixed solvent of alcohol and arene to prepare the salt of 7-chlorine quinaldine; alkaline is added for neutralization; and the 7-chlorine quinaldine can be prepared. The present invention has the advantages that the production of a large quantity of isomers is inhibited, the operation is simple, the production period is obviously shortened, no large amount of acid, alkaline or expensive solvent is used in the post-processing, and the product is more conducive to the environmental protection; a large amount of energy is saved; simultaneously, the reaction conditions are simplified, the reaction process is safer and more reliable, the quality and yield rate of the product are ensured, and the present invention is more suitable for industrial production.
Owner:中国科学院嘉兴应用化学工程中心
Synthesis method of 7-chloroquinaldine
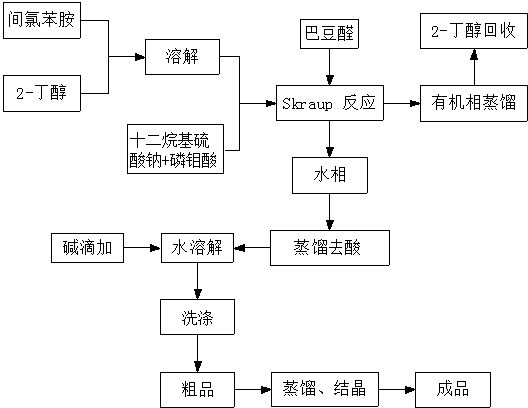
ActiveCN108822033AEasy to purifyLow priceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCrotonaldehydeSynthesis methods
The invention discloses a synthesis method of 7-chloroquinaldine. 2-nitrotoluene, SiO2-HEPIMBr, m-chloroaniline, crotonaldehyde, TEOS, 2-bromoethanol, imidazole and 3-chloropropyltriethoxysilane are taken as main raw materials. According to the synthesis process, m-chloroaniline and crotonaldehyde are subjected to Skraup reaction under action of an immobilized ion catalyst SiO2-HEPIMBr, and 7-chloroquinaldine is obtained. SiO2 immobilized hydroxyl ionic liquid HEPIMBr is prepared with a grafting method, in the reaction, few by-products are produced and no 5-isomer is produced, so that the separation and purification process is reduced, purity of a product is improved, yield of the product is increased, and higher market competitiveness is achieved.
Owner:马海红
Method for purifying 2,5-dichloroaniline by using decompression and continuous distillation
InactiveCN108033885ALow boiling pointReduce decompositionAmino compound purification/separationDecompositionInstability
The invention discloses a method for purifying 2,5-dichloroaniline by using decompression and continuous distillation. The method is characterized by comprising the following steps: enabling the pressure of a tower top to be 3*10 <-2> to 5*10 <-2> barometric pressure, controlling the temperature inside the tower to be between 50 to 55 DEG C, adding crude product 2,5-dichloroaniline, aniline, m-chloroaniline, 2,5-dichloronitrobenzene and mixed materials with low boiling point and high boiling point into the tower, and carrying out continuous distillation to purify 2,5-dichloroaniline. Accordingto the method, the boiling point of a system feed liquid is lowered, the decomposition of a thermosensitive material is reduced, the loss is reduced, and the byproducts and aftertreatment cost are reduced; by using continuous distillation, the automation level is increased, the manpower is saved, the instability of batch fractionating is reduced, the energy consumption is reduced, the equipment space is saved, the production efficiency is improved, and the method is applicable for green industrial mode. Above all, the method is high in yield, low in energy consumption, high in automation degree, labor-saving, stable in production and few in byproducts, the safety is improved, the production rate is improved, and the social benefit is increased.
Owner:杨向党
Method for producing chlorpropham
ActiveCN102167670BHigh spiritual contentReduce generationCarbamic acid derivatives preparationOrganic compound preparationM-chloroanilineChlorpropham
The invention discloses a method for producing chlorpropham, which comprises the following steps of: performing bubbling degassing treatment on isopropyl chlorocarbonate at the temperature of between 5 and 40DEG C under negative pressure condition; controlling content of isomerides of a raw material of m-chloroaniline to be within 0.2 percent, adding the m-chloroaniline into caustic soda liquid, and adding the treated isopropyl chlorocarbonate for reaction; regulating the PH value of reaction liquid to be 6-8 by using dilute acid, adding deionized water for washing and demixing, and obtaininga lower oil layer, namely a crude product of chlorpropham; and dehydrating the crude product to obtain the qualified product. By pretreating the raw material and acidifying synthetic liquid, the product quality is improved and production energy consumption is reduced.
Owner:CAC NANTONG CHEM
A kind of method utilizing meta-position oil to prepare m-chloroaniline
ActiveCN106883129BLow costHigh outputOrganic compound preparationAmino compound preparationSodium hydrosulfideAniline
The invention relates to a method for preparing m-chloroaniline by using meta-position oil, which belongs to the technical field of preparing m-chloroaniline, and specifically includes the steps of batching reaction, water washing, drying, three-stage vacuum distillation, crystallization, reduction, redistillation and the like. The present invention uses waste meta-oil produced by p- and o-nitrogen and industrial waste liquid sodium hydrosulfide as raw materials, utilizes methoxylation, crystallization and reduction to prepare m-chloroaniline, and generates by-products o-aminoanisole and p- Anisole, thereby realizing low cost, high output, compared with existing production technology, has obvious cost advantage, has higher economic benefit.
Owner:AZUREWAVE TEHNOLOGIES INC
Liquid fluorescent whitening agent and preparation method thereof
ActiveCN103436050BGood water solubilityStable storageStyryl dyesLuminescent compositionsIce waterWhitening Agents
The invention relates to a liquid fluorescent whitening agent and a preparation method thereof and mainly aims at solving the technical problems that the whitening effect is not obvious and the stability is poor in an existing fluorescent whitening agent and a preparation method thereof. According to the technical scheme, the fluorescent whitening agent is mainly composed of V, VI, VII and VIII. The preparation method of the liquid fluorescent whitening agent comprises the following steps: with cyanuric chloride, 4,4'-diamino diphenylethene-2,2'-disulfonic acid, phenylamine, m-chloroaniline, acrylic acid and serine in ratio by amount of substance of 1.0:(0.45-0.55):(0.45-0.55):(0.45-0.55):1.0:(0.95-1.05) as raw materials, firstly dissolving acrylic acid and serine in water to generate a mixture of I and II, then mixing 4,4'-diamino diphenylethene-2,2'-disulfonic acid with an NaOH solution to prepare a sodium 4,4'-diamino diphenylethene-2,2'-disulfonate solution, adding the sodium 4,4'-diamino diphenylethene-2,2'-disulfonate solution, an emulsifier and cyanuric chloride into ice water, and carrying out reaction to generate a product III; adding phenylamine and m-chloroaniline into III to generate IV; and finally adding the mixture of I and II into IV to obtain a whitening agent crude product, and then carrying out suction filtration, desalination and concentration to obtain the liquid fluorescent whitening agent product.
Owner:山西晋光化工有限公司
Method for producing chlorpropham
ActiveCN102167670AImprove product qualityReduce production energy consumptionCarbamic acid derivatives preparationOrganic compound preparationM-chloroanilineChlorpropham
The invention discloses a method for producing chlorpropham, which comprises the following steps of: performing bubbling degassing treatment on isopropyl chlorocarbonate at the temperature of between 5 and 40DEG C under negative pressure condition; controlling content of isomerides of a raw material of m-chloroaniline to be within 0.2 percent, adding the m-chloroaniline into caustic soda liquid, and adding the treated isopropyl chlorocarbonate for reaction; regulating the PH value of reaction liquid to be 6-8 by using dilute acid, adding deionized water for washing and demixing, and obtaininga lower oil layer, namely a crude product of chlorpropham; and dehydrating the crude product to obtain the qualified product. By pretreating the raw material and acidifying synthetic liquid, the product quality is improved and production energy consumption is reduced.
Owner:CAC NANTONG CHEM
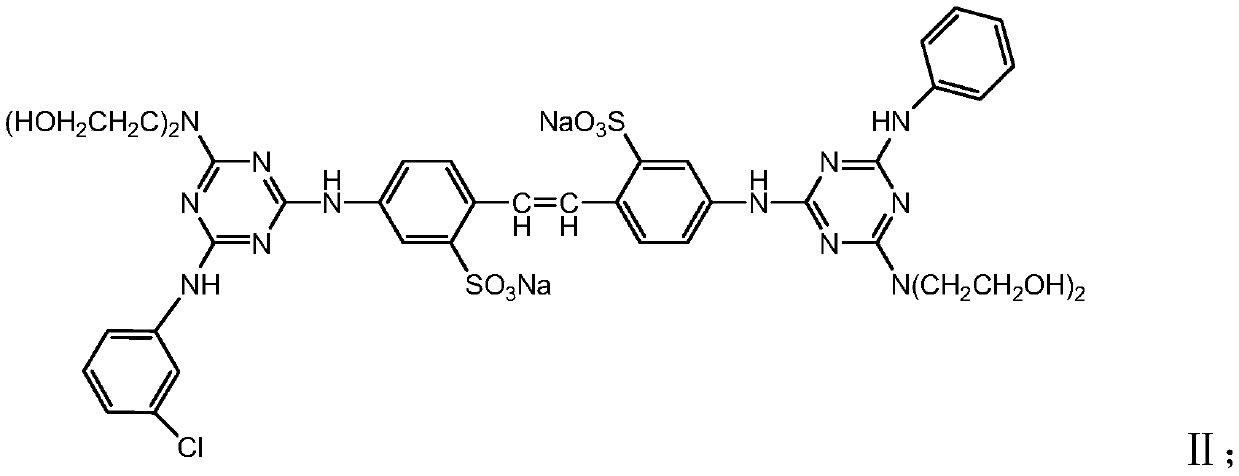
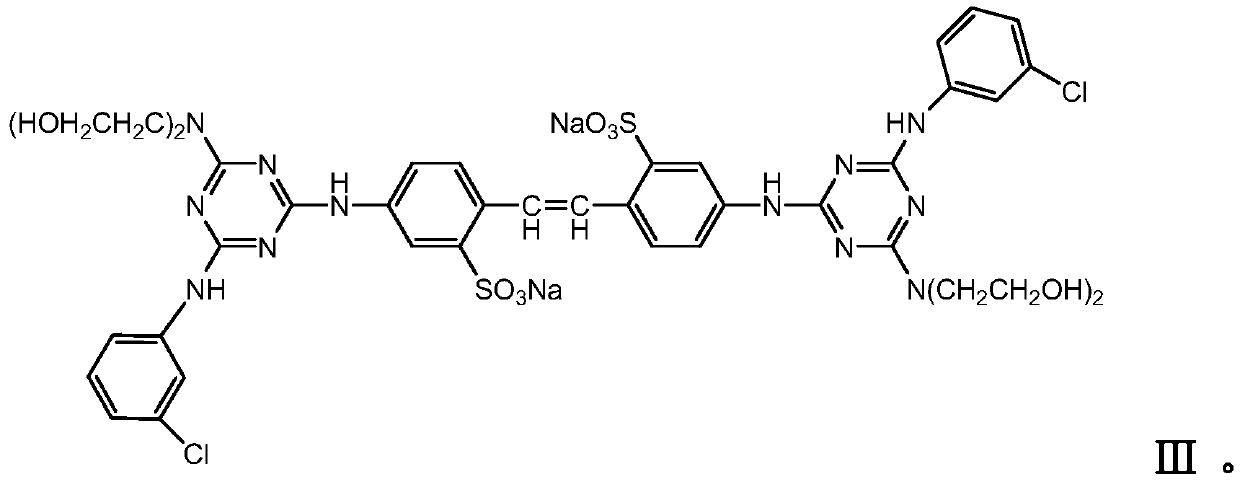
Stilbene triazine fluorescent whitening agent special for detergent and preparation method thereof
InactiveCN111088124AOmit traditional compounding process stepsAdd lessOrganic detergent compounding agentsDetergent dyesM-chloroanilineWhitening Agents
The invention relates to a stilbene triazine fluorescent whitening agent special for a detergent and a preparation method thereof, and aims at solving the technical problems that an existing fluorescent whitening agent for the detergent is not obvious in whitening effect, complex in preparation method and high in cost. According to the technical scheme, the stilbene triazine fluorescent whiteningagent special for the detergent is prepared from a component I, a component II and a component III according to the mass ratio of 6:3:1, wherein the component I is 4, 4'-bis[(4-anilino-6-hydroxydiethylamino-1, 3, 5-triazine-2-yl)amino]stilbene-2, 2'-disodium disulfonate, the component II is 4, 4'-bis[(4-anilino-4'-m-chloroanilino-6-hydroxydiethylamino-1, 3, 5-triazine-2-yl)amino]stilbene-2, and the component III is 4, 4'-bis[(4-m-chloroanilino-6-hydroxydiethylamino-1, 3, 5-triazine-2-yl)amino]stilbene-2. The fluorescent whitening agent prepared by the invention is low in cost and good in whitening effect, and can completely meet market requirements when being used alone.
Owner:山西晋光化工有限公司
The synthetic method of ziprasidone intermediate
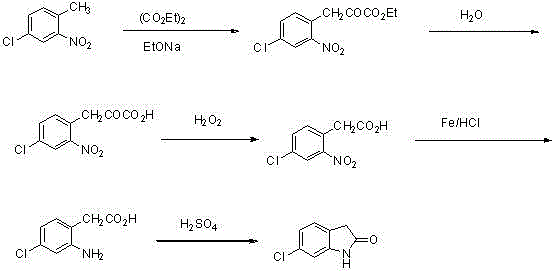
The invention discloses a novel synthetic method of a ziprasidone intermediate. The synthetic method comprises following steps: m-chloronitrobenzene is taken as an initial raw material, and is subjected to nitroreduction so as to obtain m-chloroaniline; m-chloroaniline is subjected to aminoacylation so as to obtain 3-chloroacetyl amino-chloro-benzene; and finally 3-chloroacetyl amino-chloro-benzene is subjected to Friedel-Crafts alkylation, and 6-chloro-2-indolone is obtained after cyclization. The raw materials of the synthetic method are cheap and easily available, synthetic route is short, yield is high, operation is convenient, cost is low, and the synthetic method is suitable for industrialized application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com