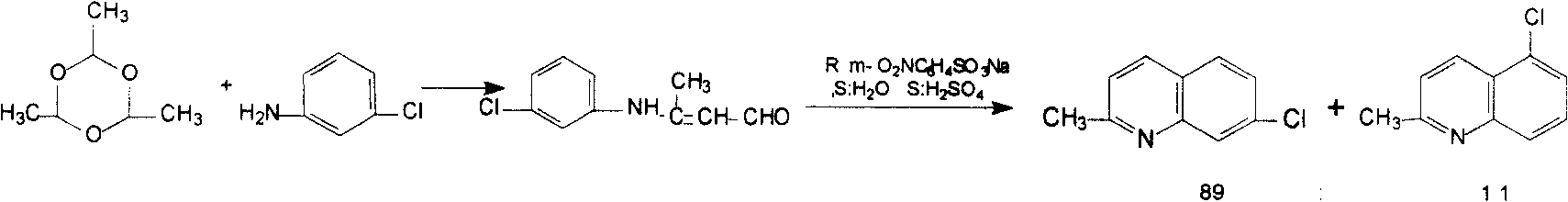Preparation method of midbody 7-chloroquinaldine
An intermediate, the technology of chloroquine, which is applied in the field of preparation of 7-chloroquinaldine, can solve the problems of high cost of raw materials, difficulty in separation, and inability to obtain purity, etc., so as to suppress the production of a large number of isomers and ensure product quality and yield , The effect of safe and reliable reaction process
Inactive Publication Date: 2010-12-01
中国科学院嘉兴应用化学工程中心
View PDF2 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In U.S. Patent Nos. 5,126,456 and 5,066,806, ZhiguoSong, Emmerich Pastorek and others use m-chloroaniline and crotonaldehyde to react under acidic conditions to obtain 7-chloro-2-methylquinoline, wherein tetrachloro-p-benzoquinone is used as an oxidant, Use zinc chloride, tartaric acid, etc. to complex with the product to separate from the isomer 5-chloro-2-methylquinoline. Tetrachloro-p-benzoquinone and tartaric acid are very expensive, and the post-treatment process is quite cumbersome. Complexation, filtration, the complex of the product is obtained, the product is separated from the complex, crystallized, filtered, and finally the product is obtained. Suitable for industrial production operations
In the Hungarian patent HU200401607, Salamon Zoltán Debrecen et al obtained 7-chloro-2-methylquinoline through the cyclization of m-chloroaniline and ethyl acetoacetate, N,N-dimethylformamide, chlorination, hydrolysis and decarboxylation. There are many reaction steps, the total yield is less than 25%, and the cost of raw materials is high
Document Chemical&PharmaceuticalBulletin, 34 (2), 463-70; In 1986, people such as MachikoOno adopts paraldehyde and m-chloroaniline to react and can obtain 7-chloroquinaldine (yield 34%) and 5-chloroquinaldine (yield 3.78%), but because this mixture is difficult to separate, cause can not obtain the higher 7-chloroquinaldine of purity
In document J.HeterocyclicChem.30,17(1993), people such as ZhiguoSong use m-chloroaniline and the reaction of crotonaldehyde and use p-chlorobenzoquinone as oxidant to improve the yield of product 7-chloro-2-methylquinoline by 81% , has also given the purification method of using tetrahydrofuran as a solvent and complexing with zinc chloride, but the process is cumbersome and the cost is higher
GanesabaskaranSivaprasad, people such as RengasamyRajeshandParamasivanT.perumal also use m-chloroaniline and crotonaldehyde to synthesize 7-chloroquinaldine under microwave conditions in TetrahedronLetters47 (2006) 1783-1785, and this method yield has improved, but has no practical application value, and There is no solution to the separation and purification of products
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention provides a preparation method of medicine intermediate 7-chlorine quinaldine. M-chloroaniline salt of inorganic acid and crotonaldehyde have a closed loop reaction in mixed solvent of alcohol and arene to prepare the salt of 7-chlorine quinaldine; alkaline is added for neutralization; and the 7-chlorine quinaldine can be prepared. The present invention has the advantages that the production of a large quantity of isomers is inhibited, the operation is simple, the production period is obviously shortened, no large amount of acid, alkaline or expensive solvent is used in the post-processing, and the product is more conducive to the environmental protection; a large amount of energy is saved; simultaneously, the reaction conditions are simplified, the reaction process is safer and more reliable, the quality and yield rate of the product are ensured, and the present invention is more suitable for industrial production.
Description
A kind of preparation method of medicine intermediate 7-chloroquinaldine technical field The invention relates to the field of pharmaceutical intermediates, more specifically to a preparation method of 7-chloroquinaldine. Background technique 7-Chloroquinaldine has another name called 7-chloro-2-methylquinoline, as an important intermediate for the synthesis of Montelukast sodium (Montelu-kast) and leukotriene receptor antagonist MK-0679, its synthetic method is prepared attention. In U.S. Patent Nos. 5,126,456 and 5,066,806, ZhiguoSong, Emmerich Pastorek and others use m-chloroaniline and crotonaldehyde to react under acidic conditions to obtain 7-chloro-2-methylquinoline, wherein tetrachloro-p-benzoquinone is used as an oxidant, Use zinc chloride, tartaric acid, etc. to complex with the product to separate from the isomer 5-chloro-2-methylquinoline. Tetrachloro-p-benzoquinone and tartaric acid are very expensive, and the post-treatment process is quite cumbersome. Com...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D215/18
Inventor 张振明吴长江张建荣左勇章
Owner 中国科学院嘉兴应用化学工程中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com