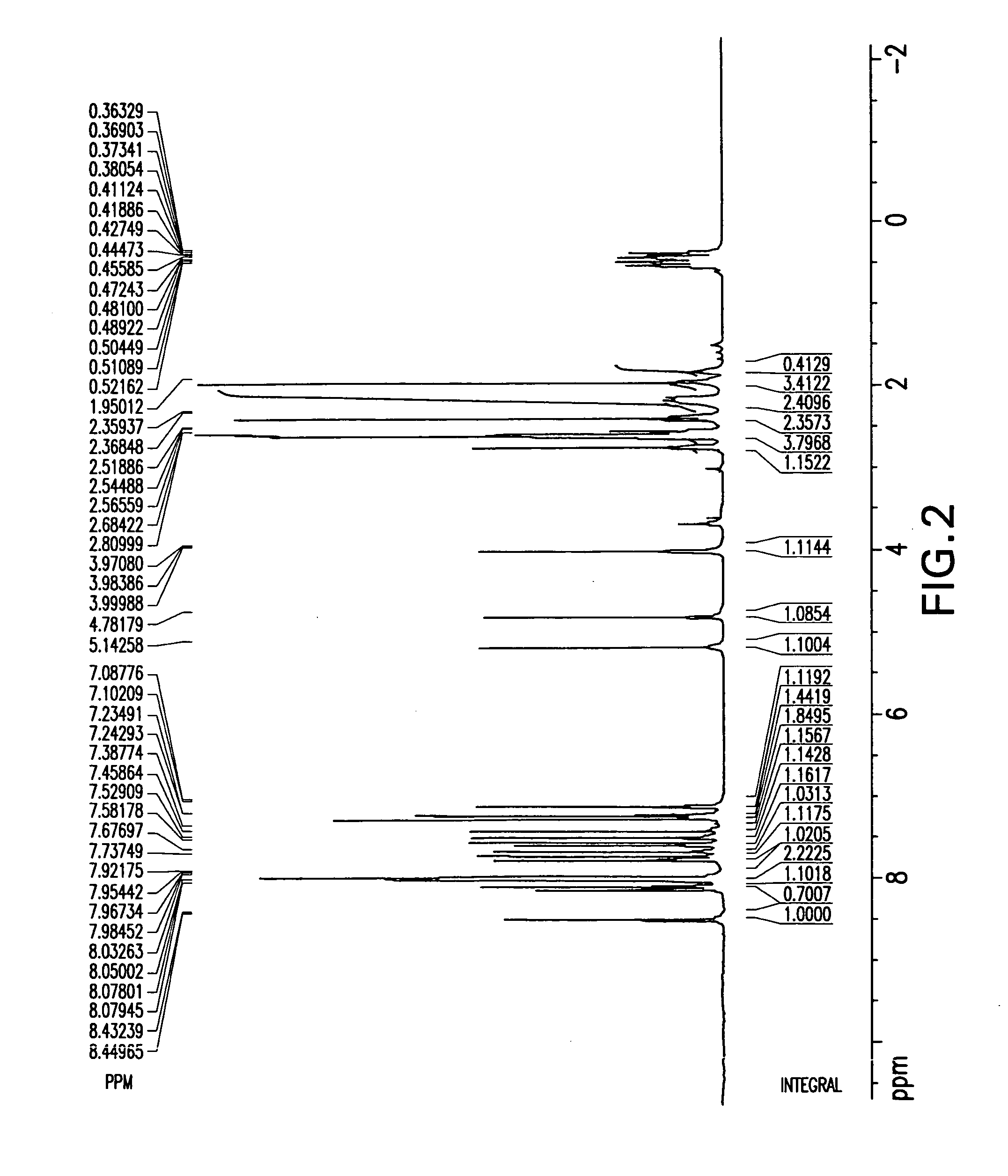
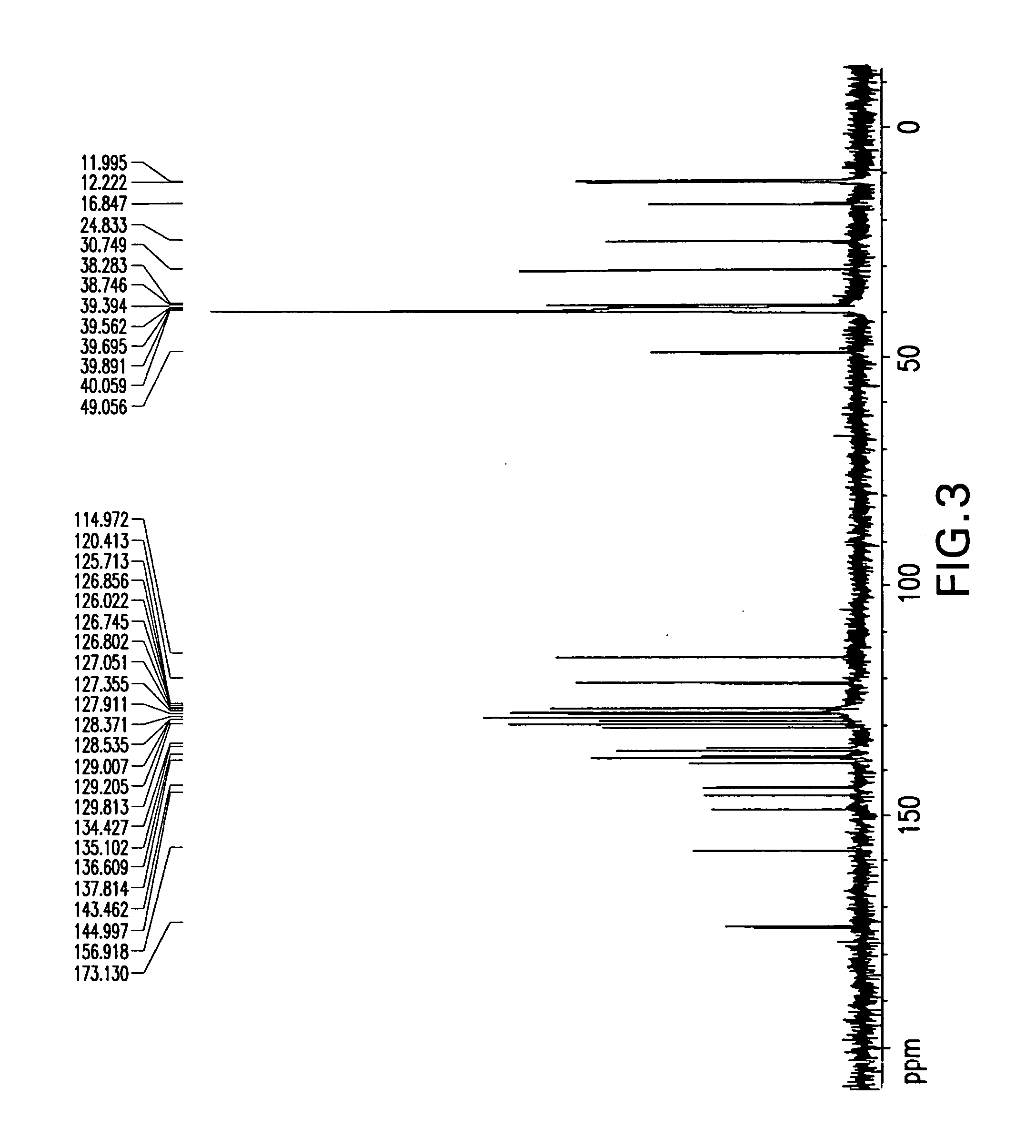
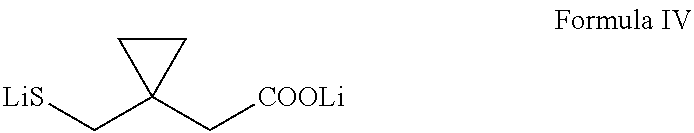Patents
Literature
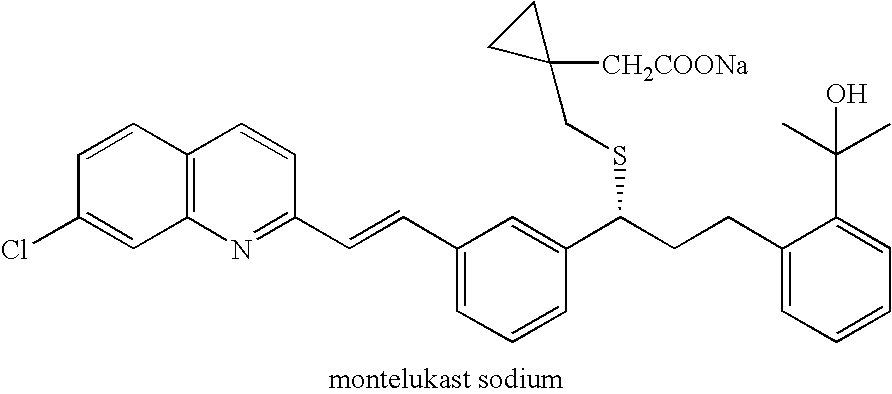
100 results about "Montelukast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Montelukast is used regularly to prevent the wheezing and shortness of breath caused by asthma and decrease the number of asthma attacks. Montelukast is also used before exercise to prevent breathing problems during exercise (bronchospasm).
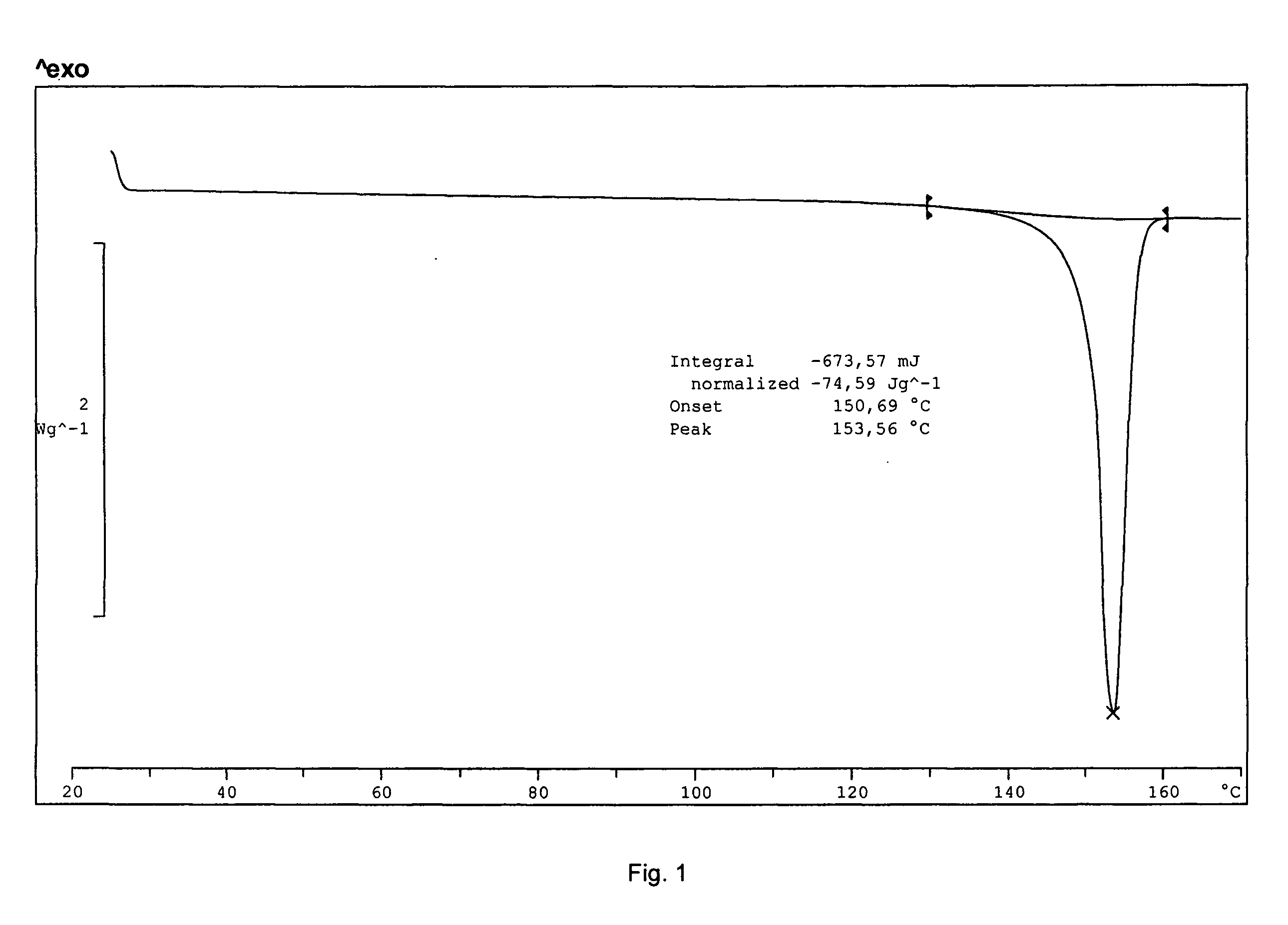
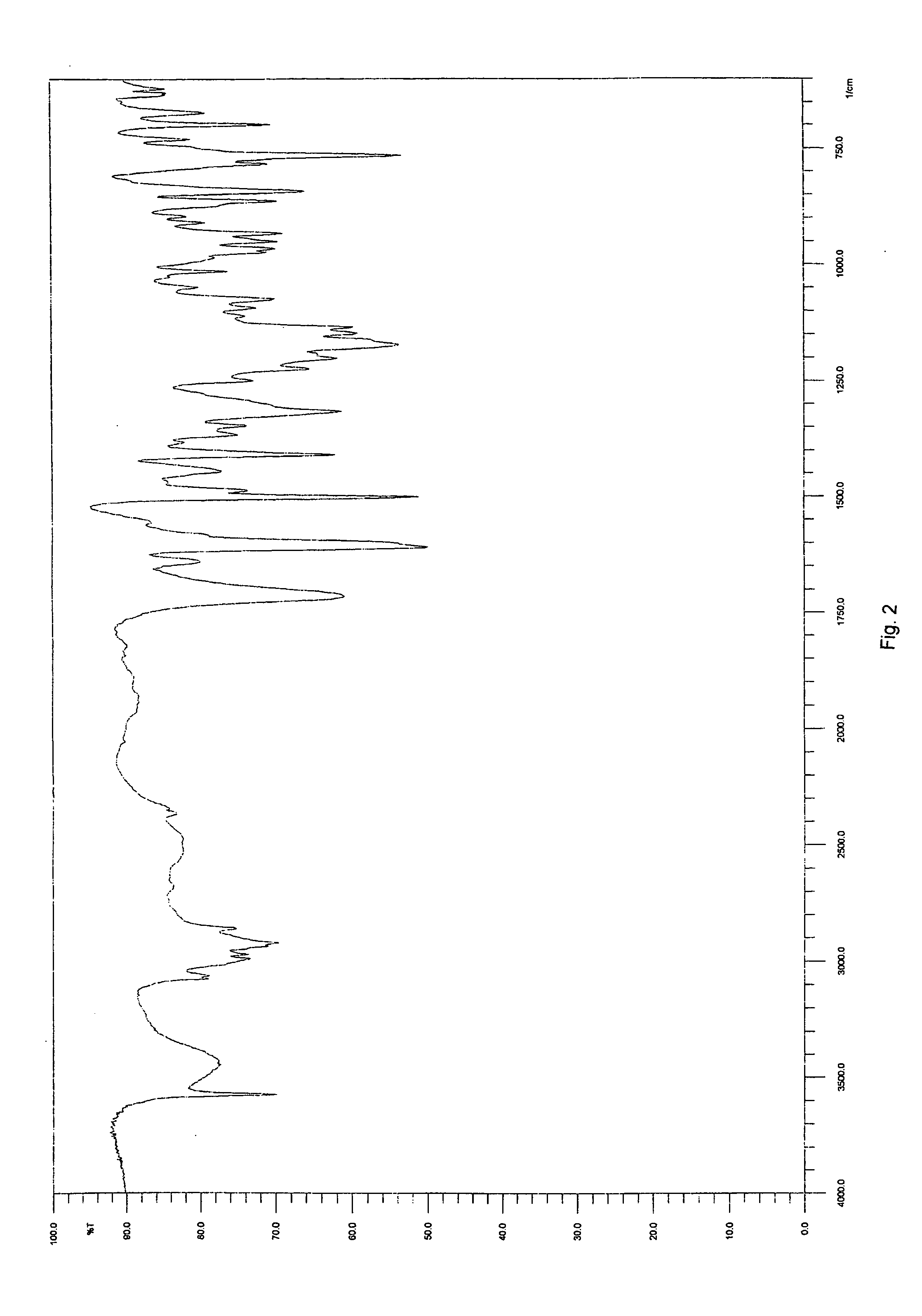
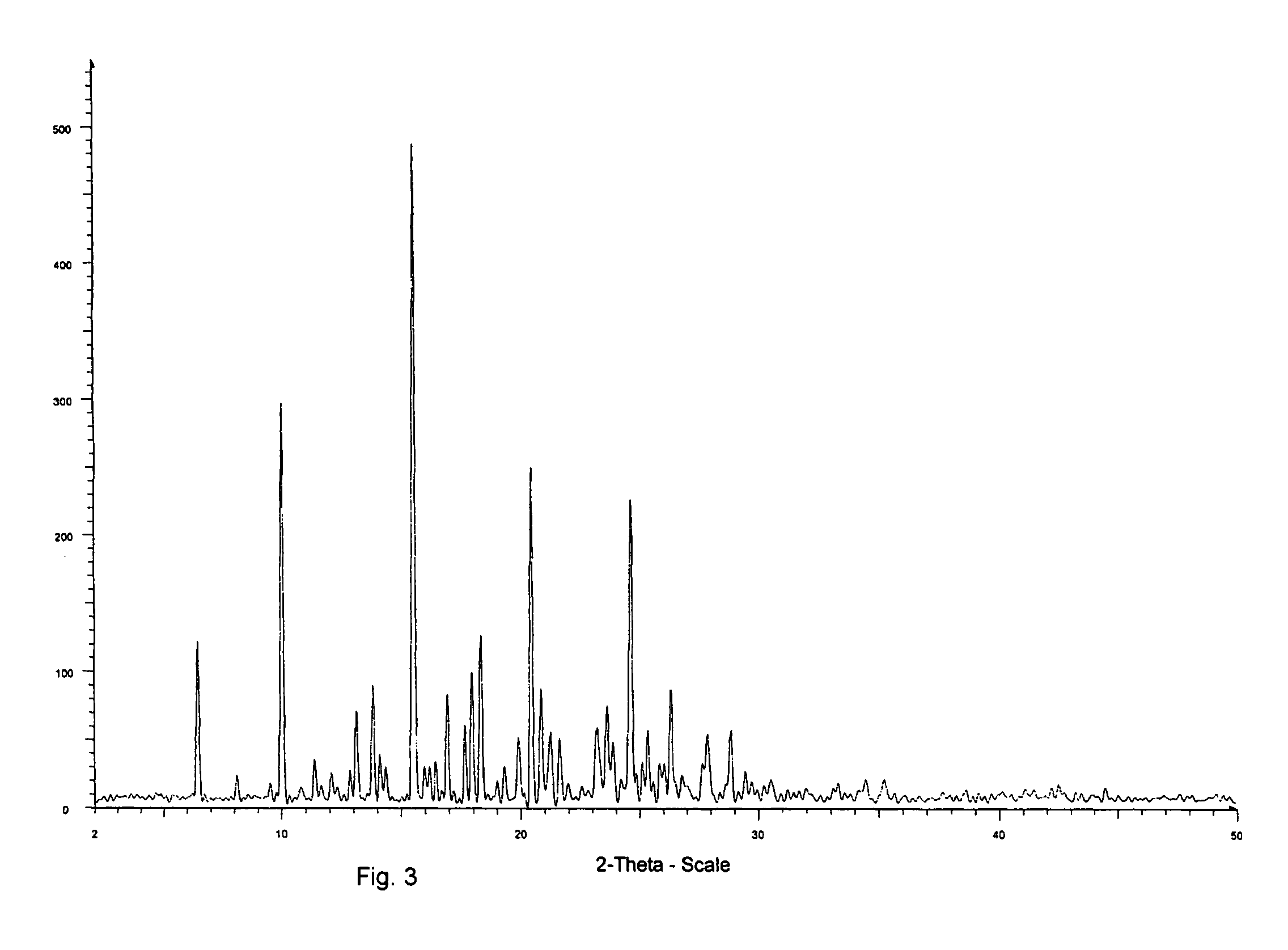
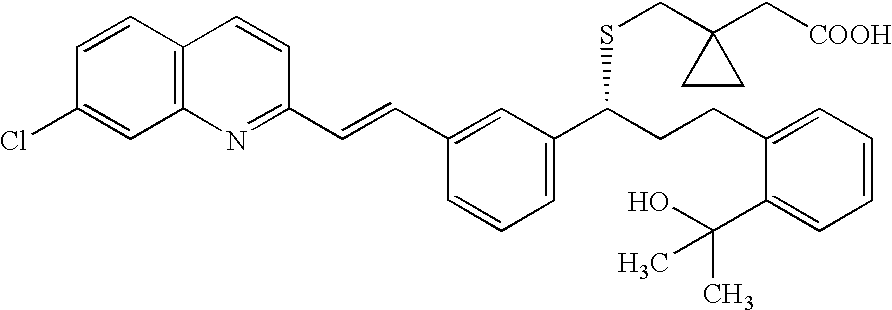
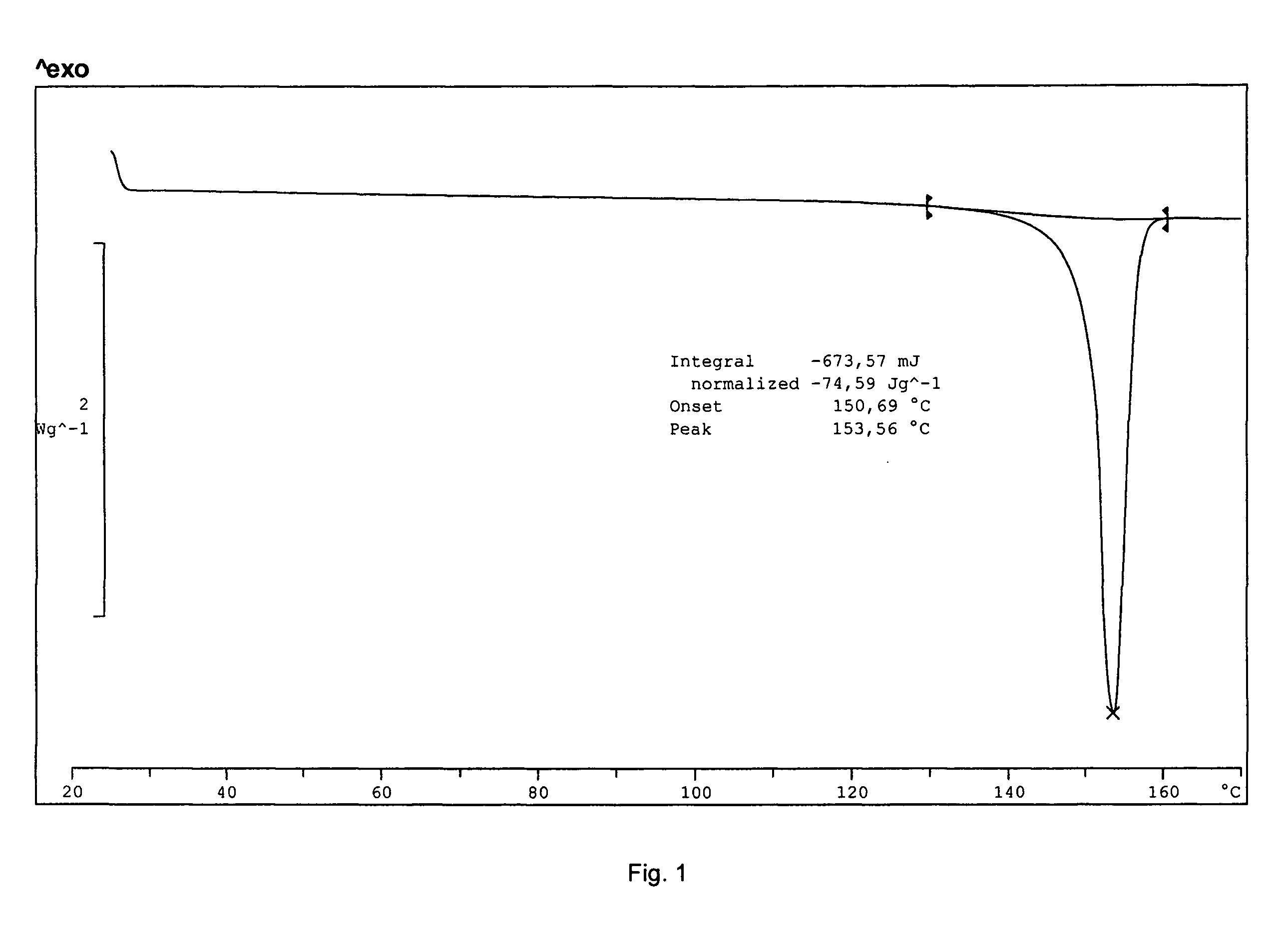
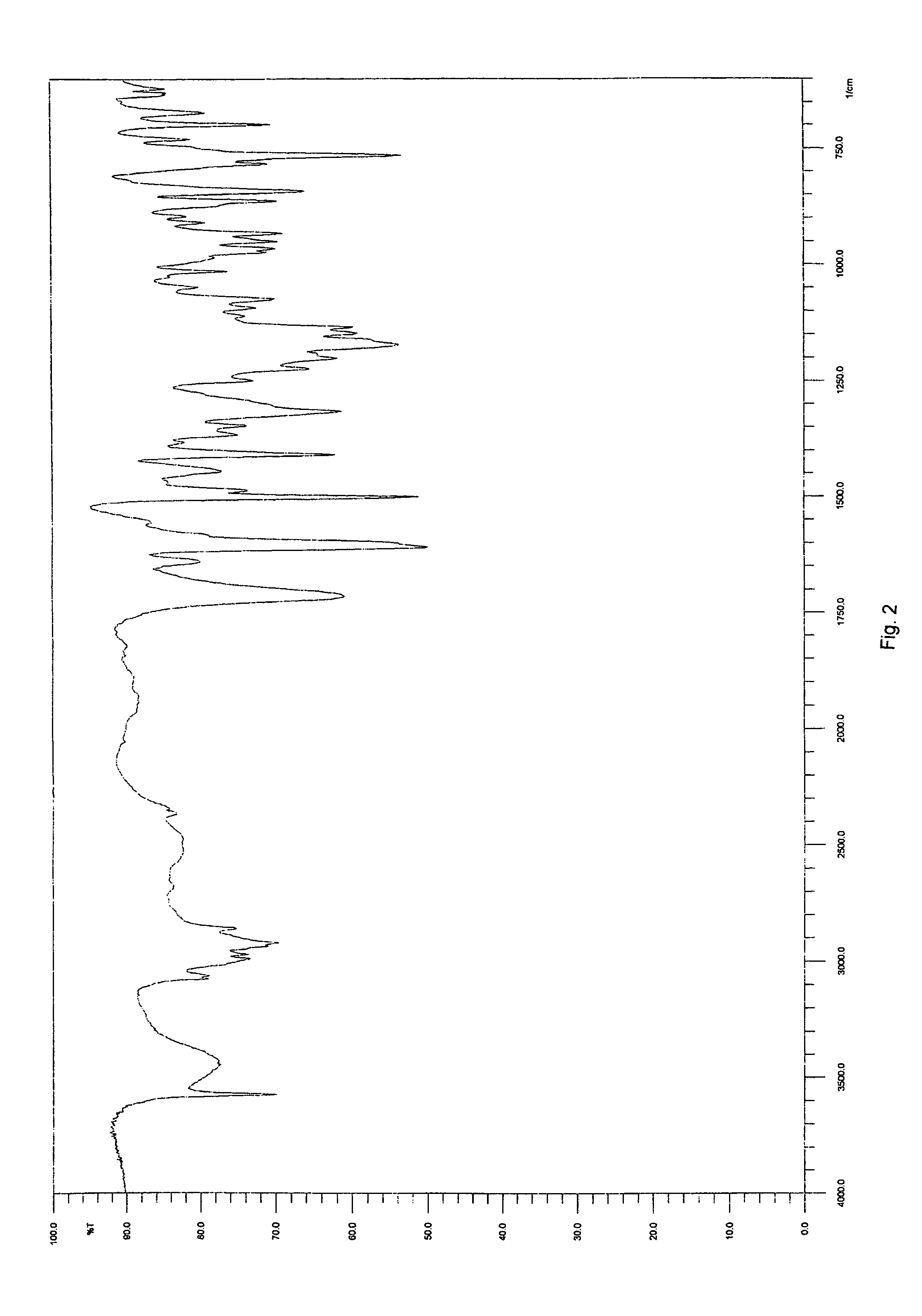
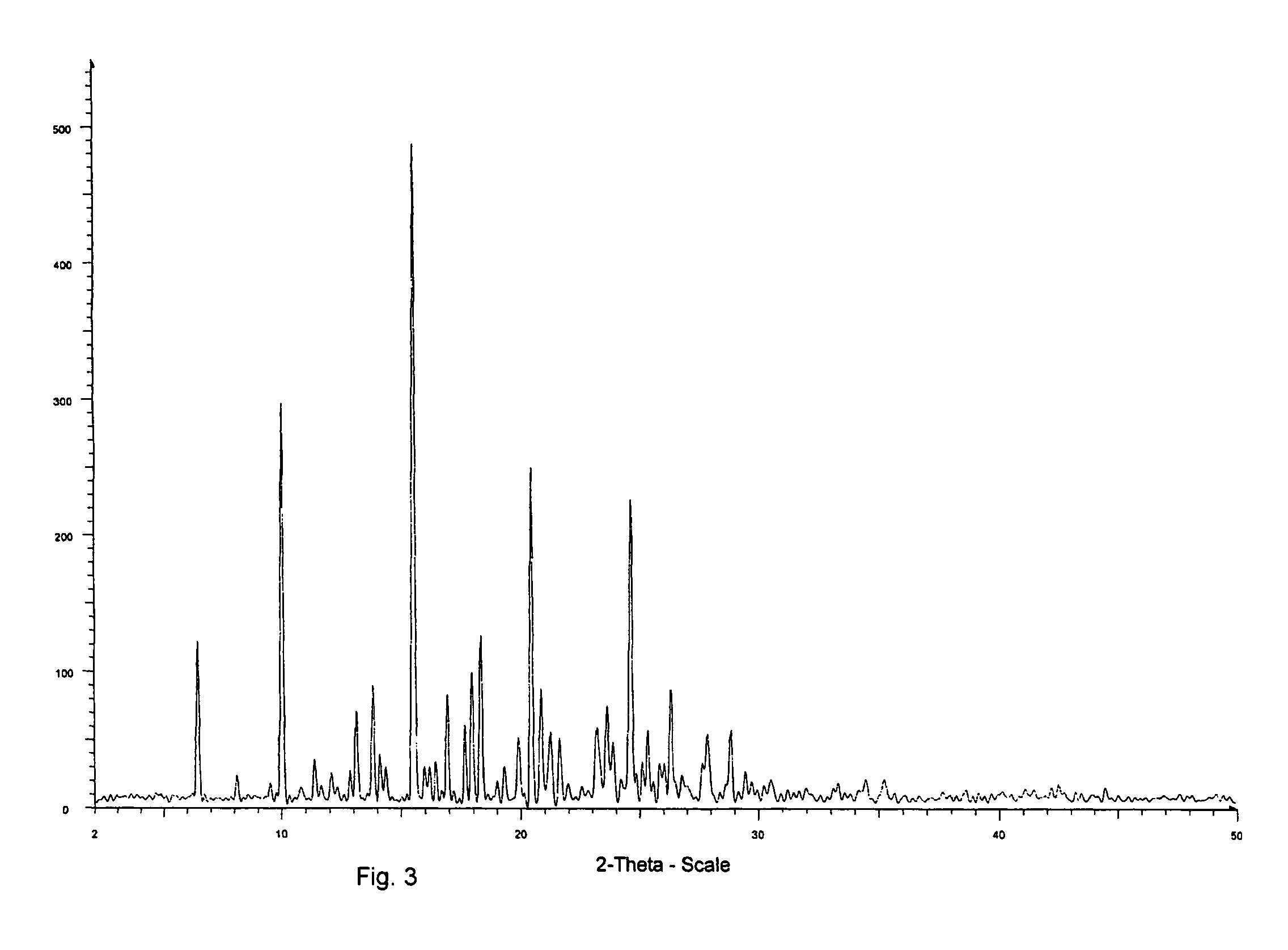
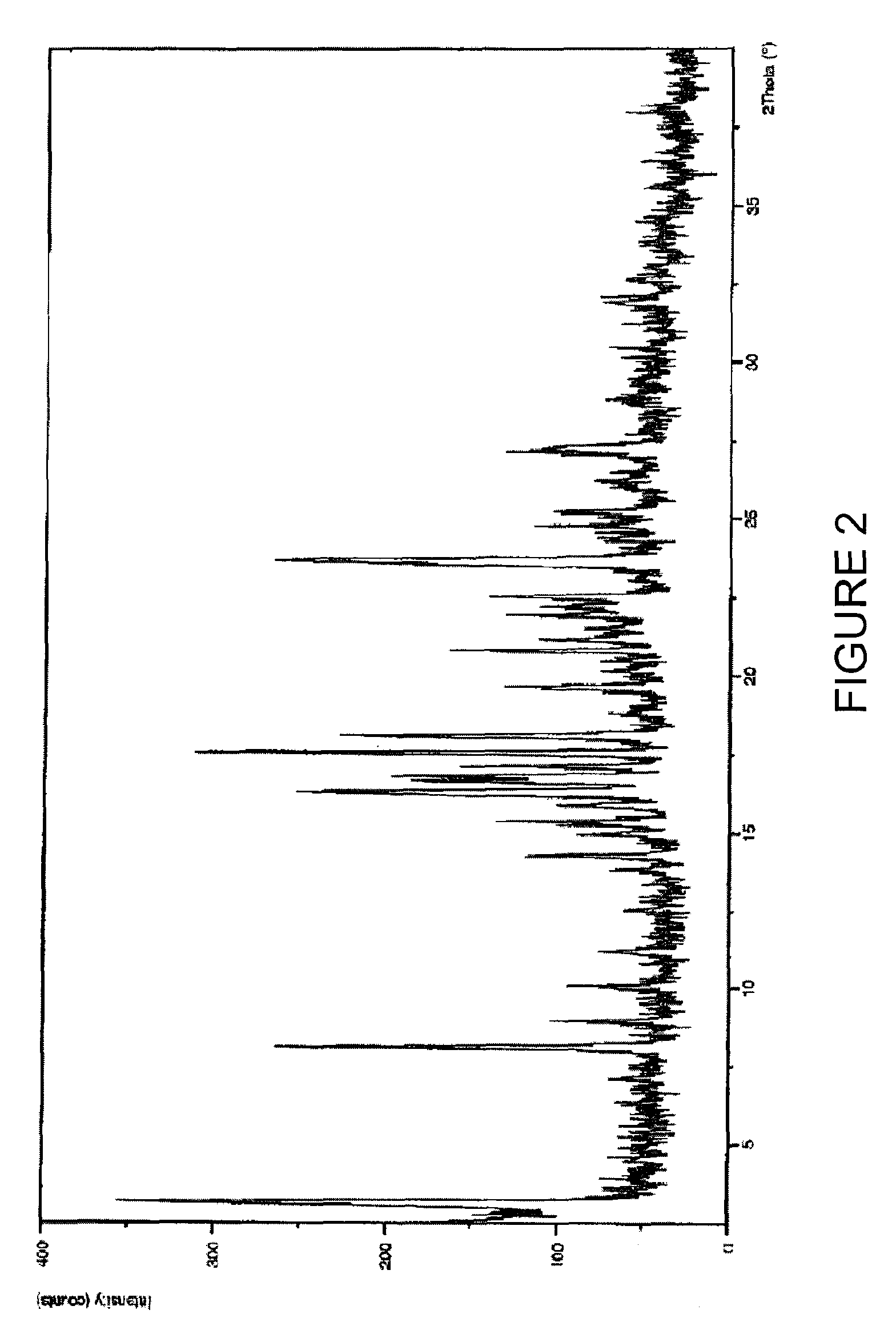
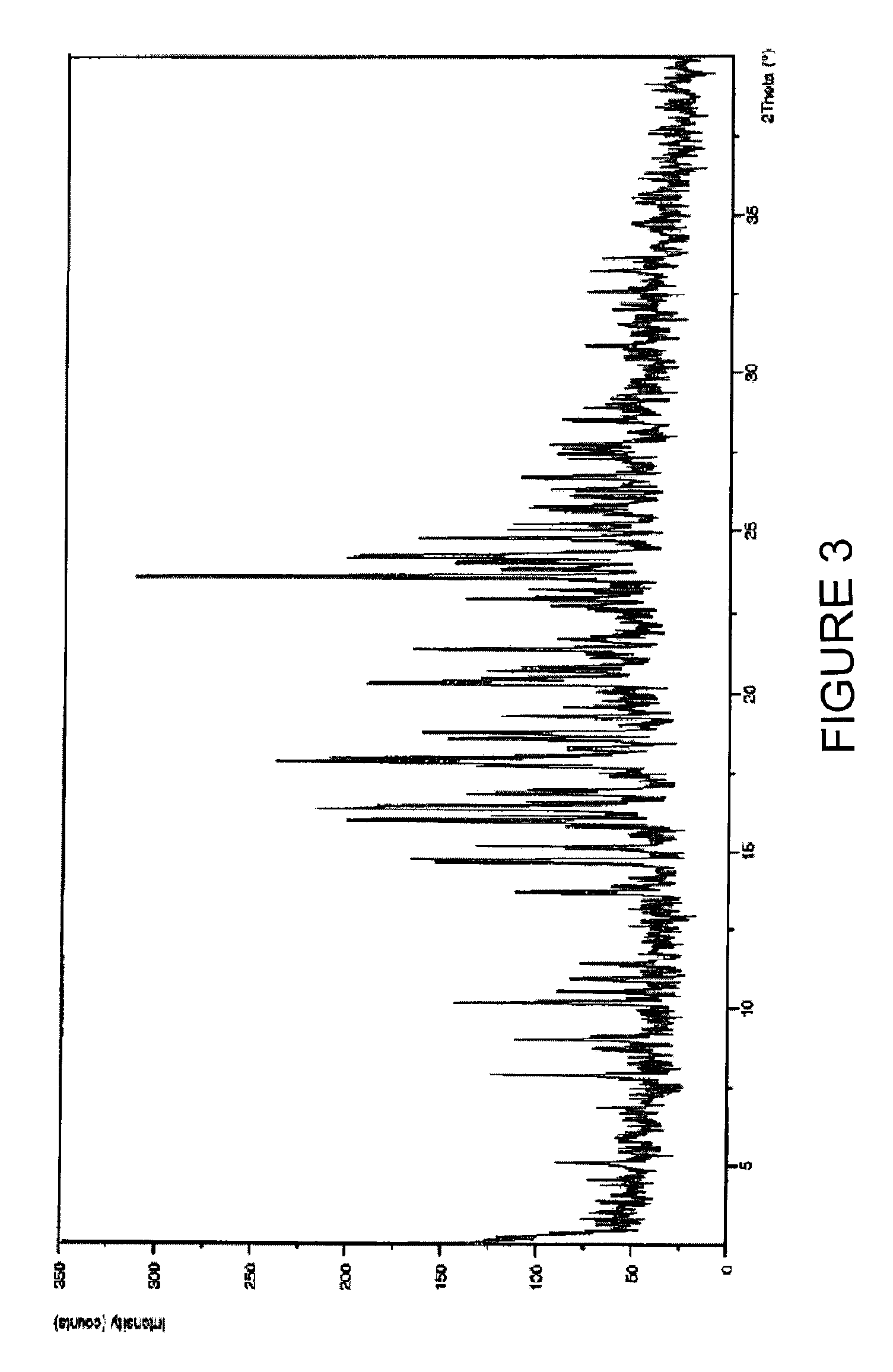
Solid-state montelukast
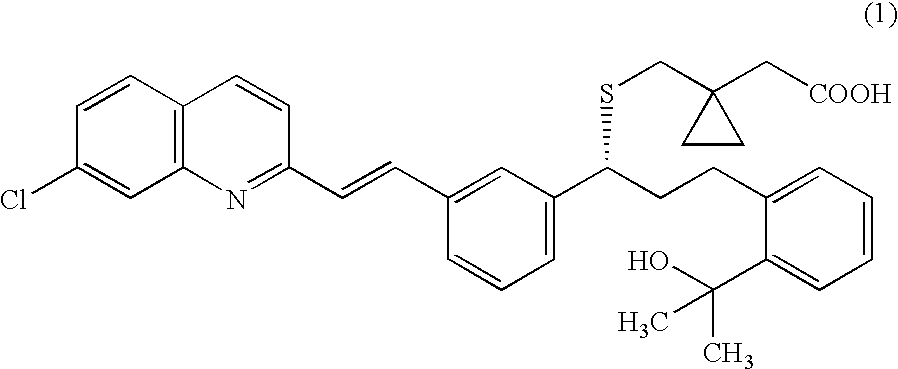
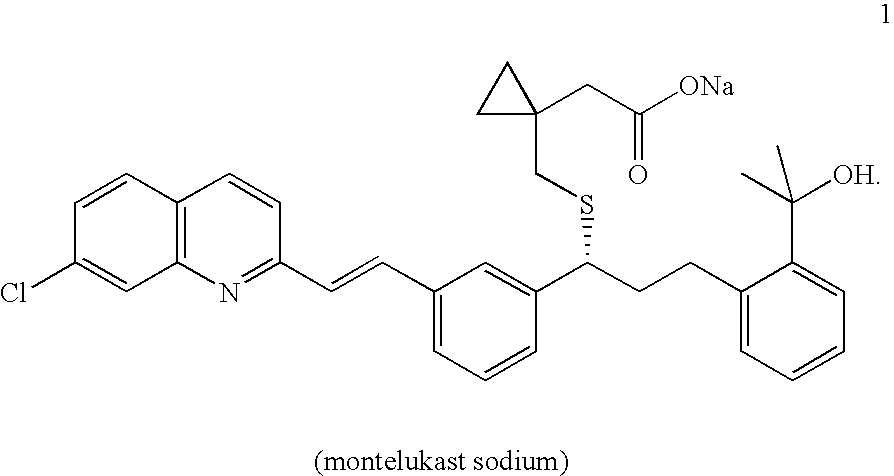
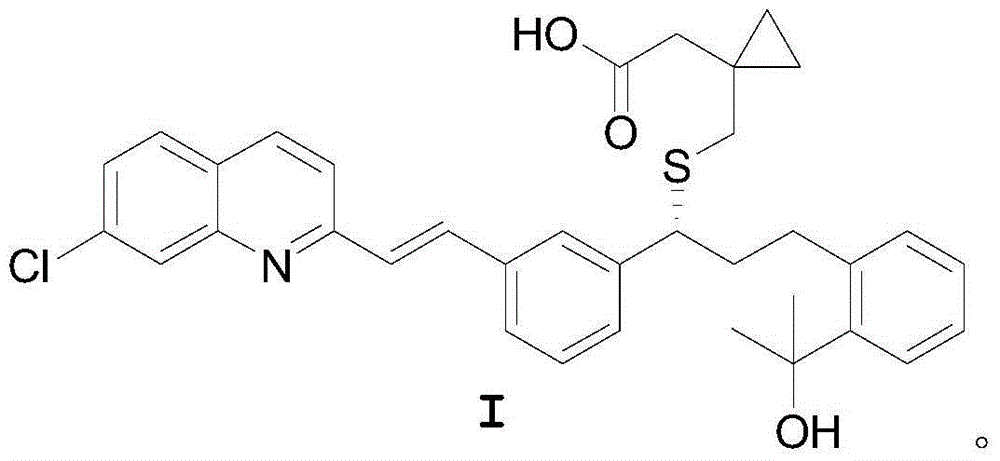
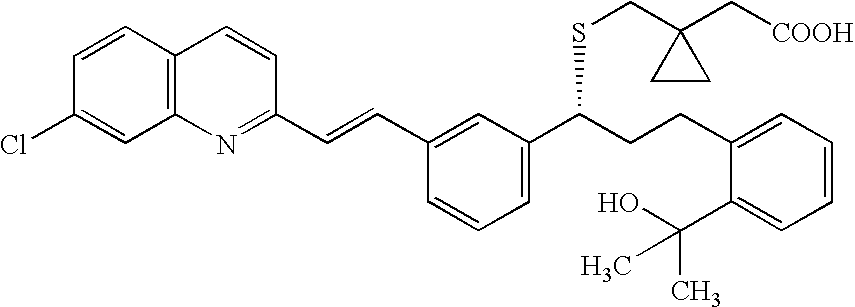
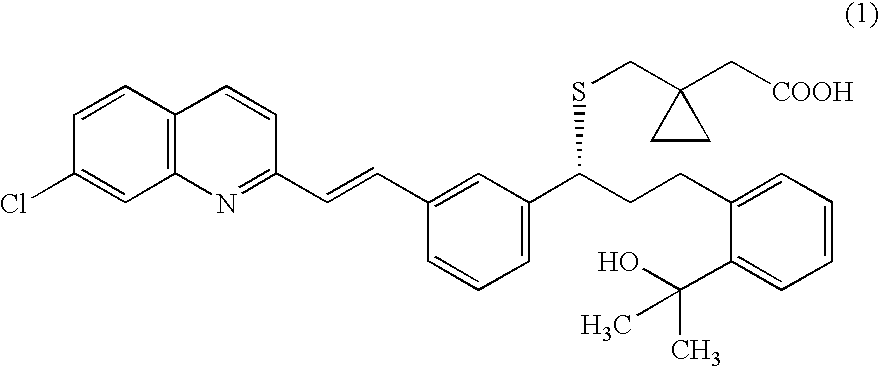
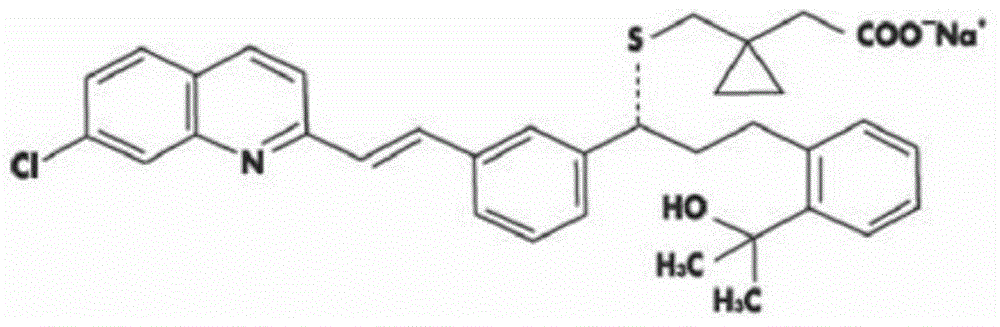
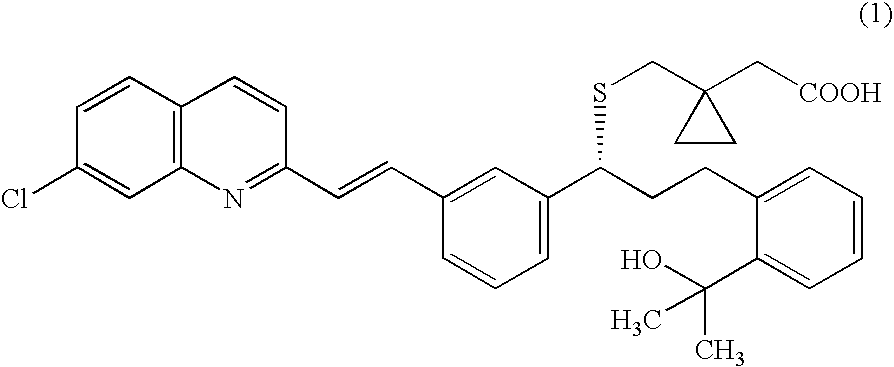
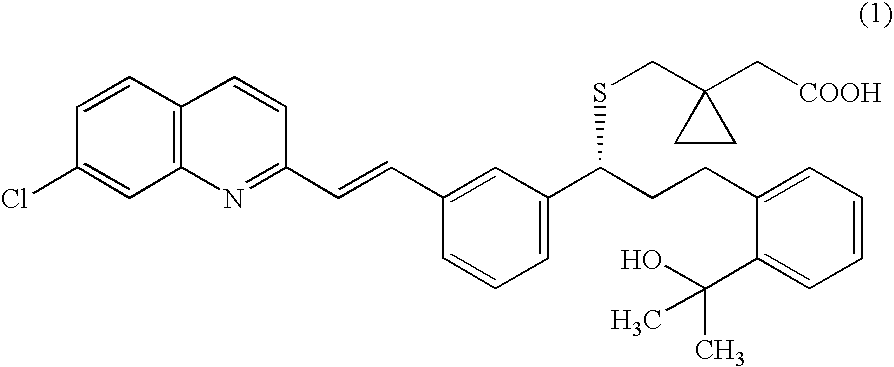
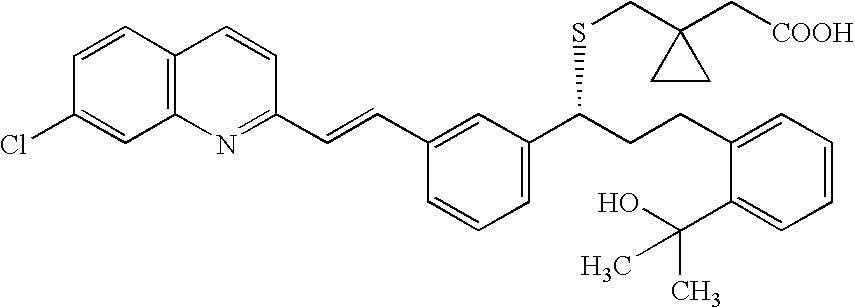
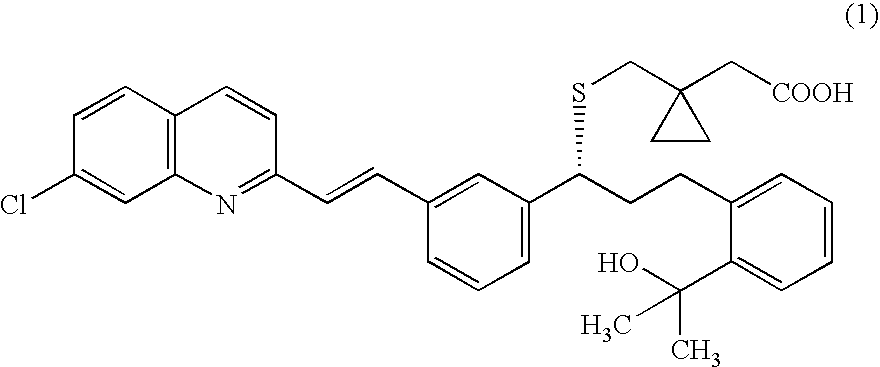
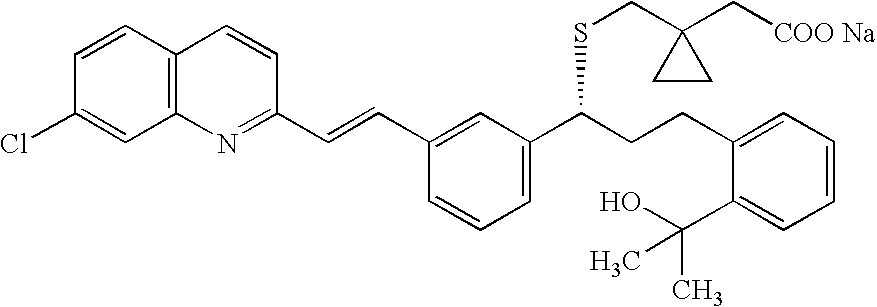
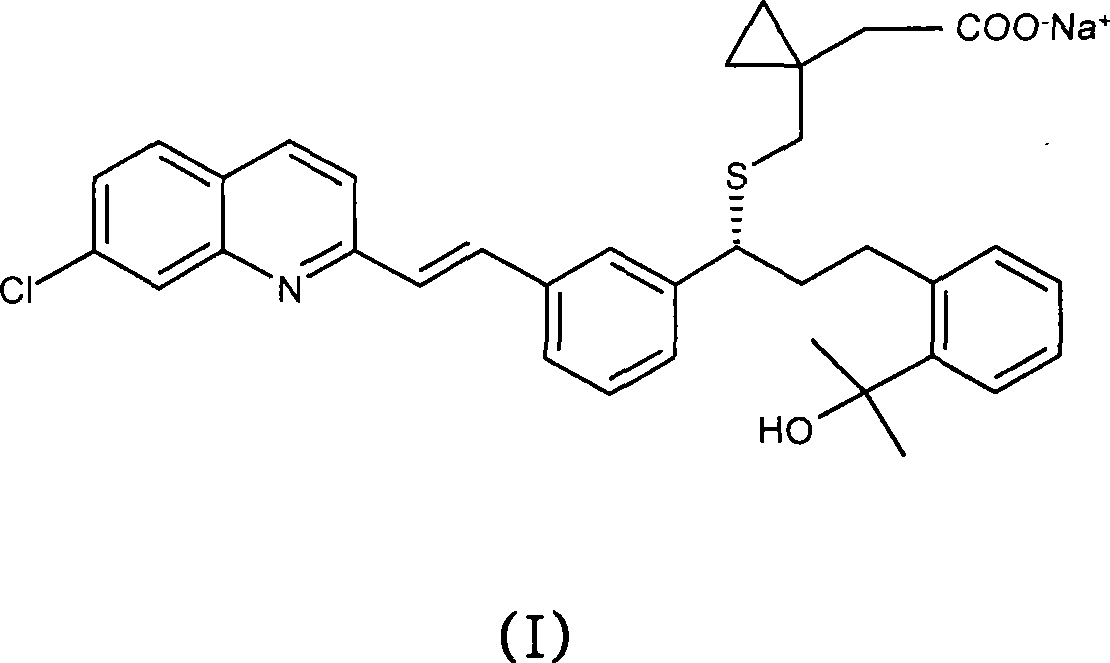
A solid form of a compound of formula 1: is provided. The compound of formula 1 can be obtained in solid state by precipitation from a solution containing the same. The compound is useful as leukotriene antagonist and can be formulated into a pharmaceutical composition that also includes a pharmaceutically acceptable excipient.
Owner:SYNTHON BV
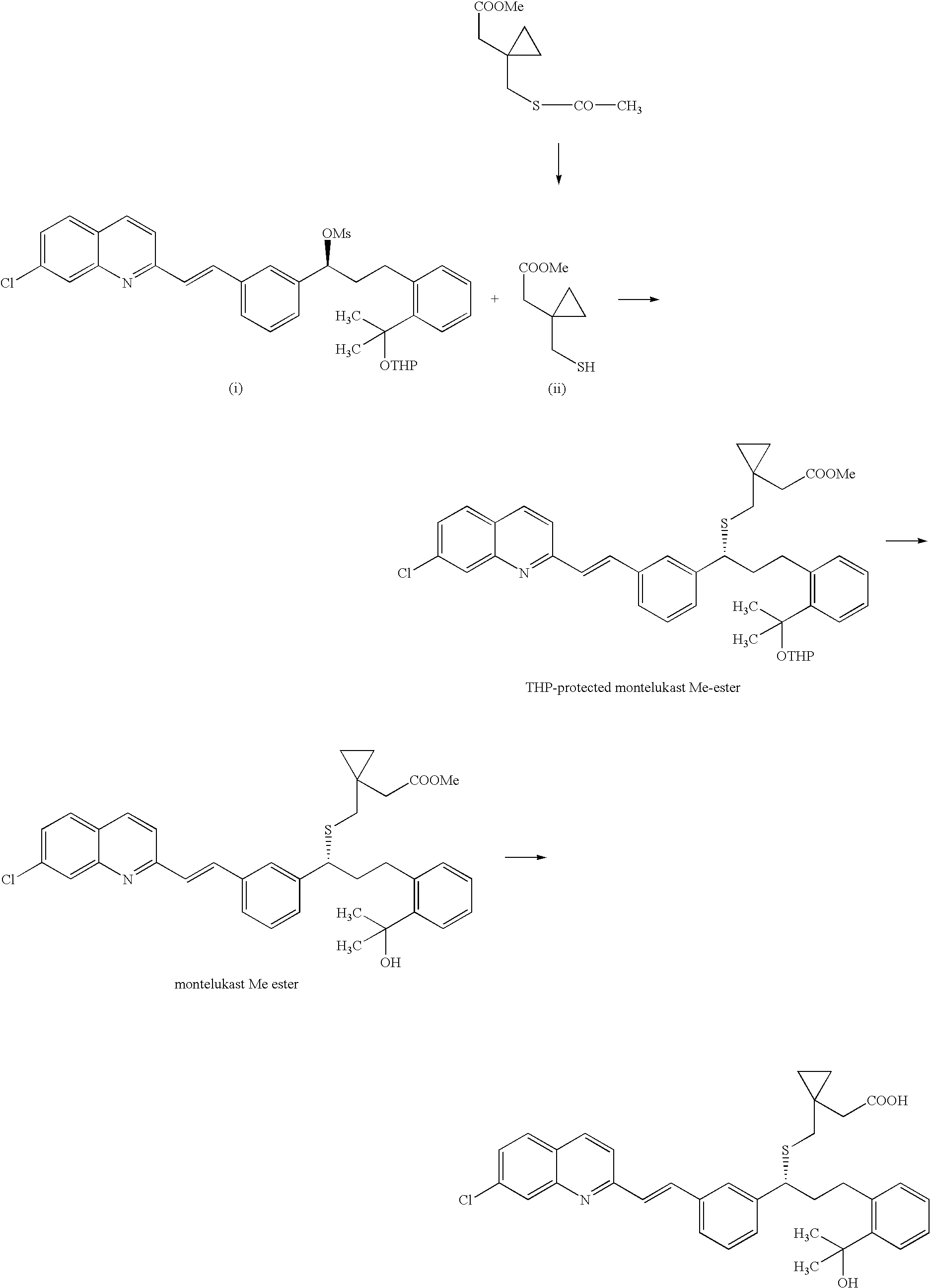
Process for making montelukast and intermediates therefor
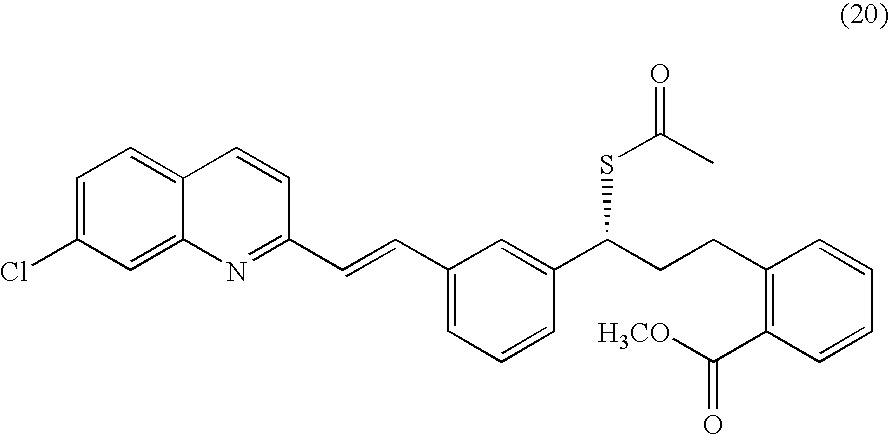
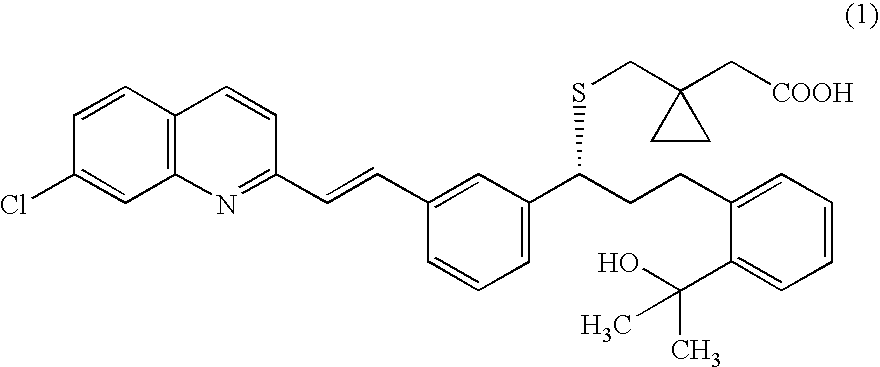
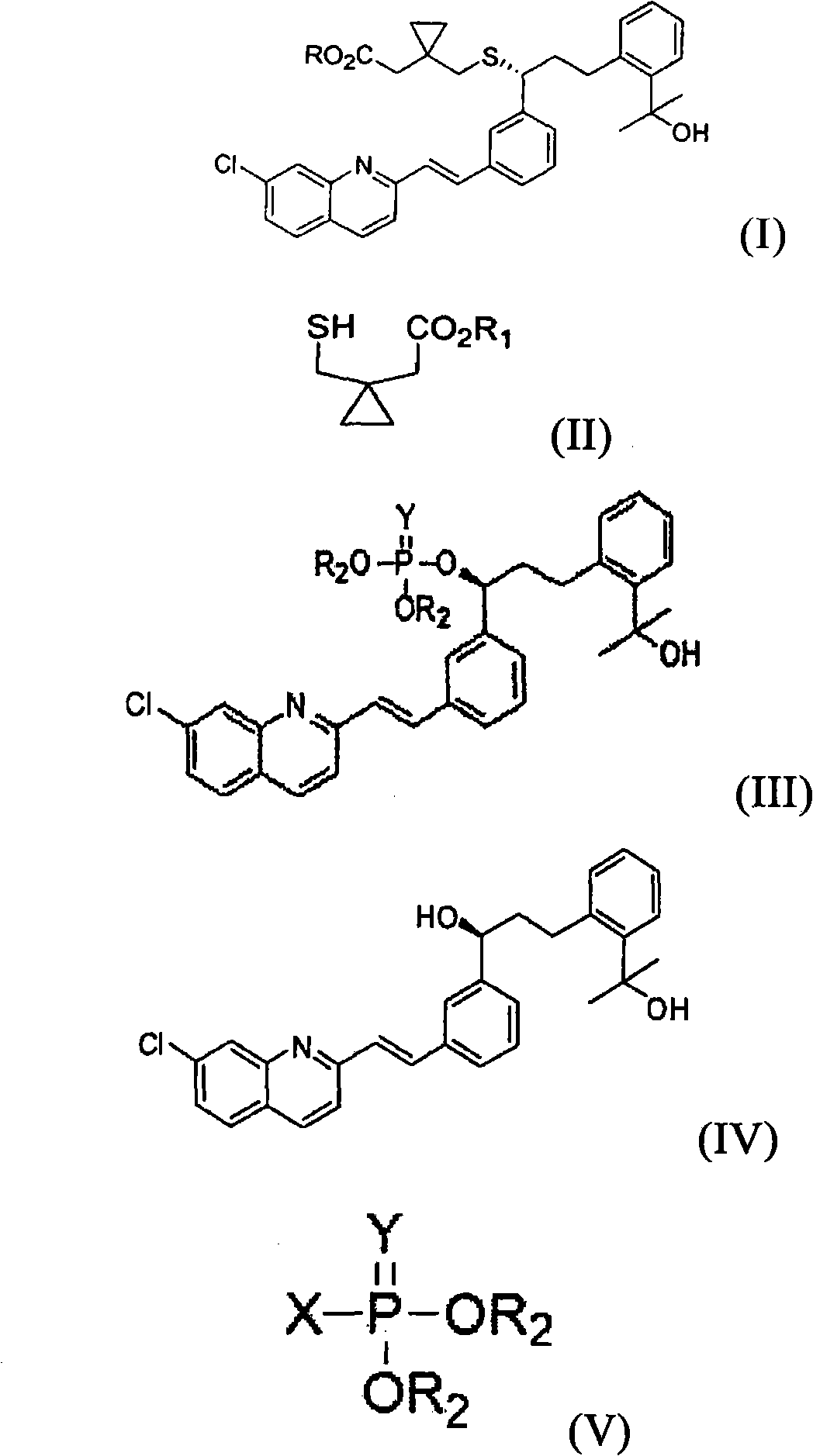
A process for making montelukast, a pharmaceutically useful compound of the following formula and salts thereof: using a compound of formula (20) is provided.
Owner:SYNTHON IP
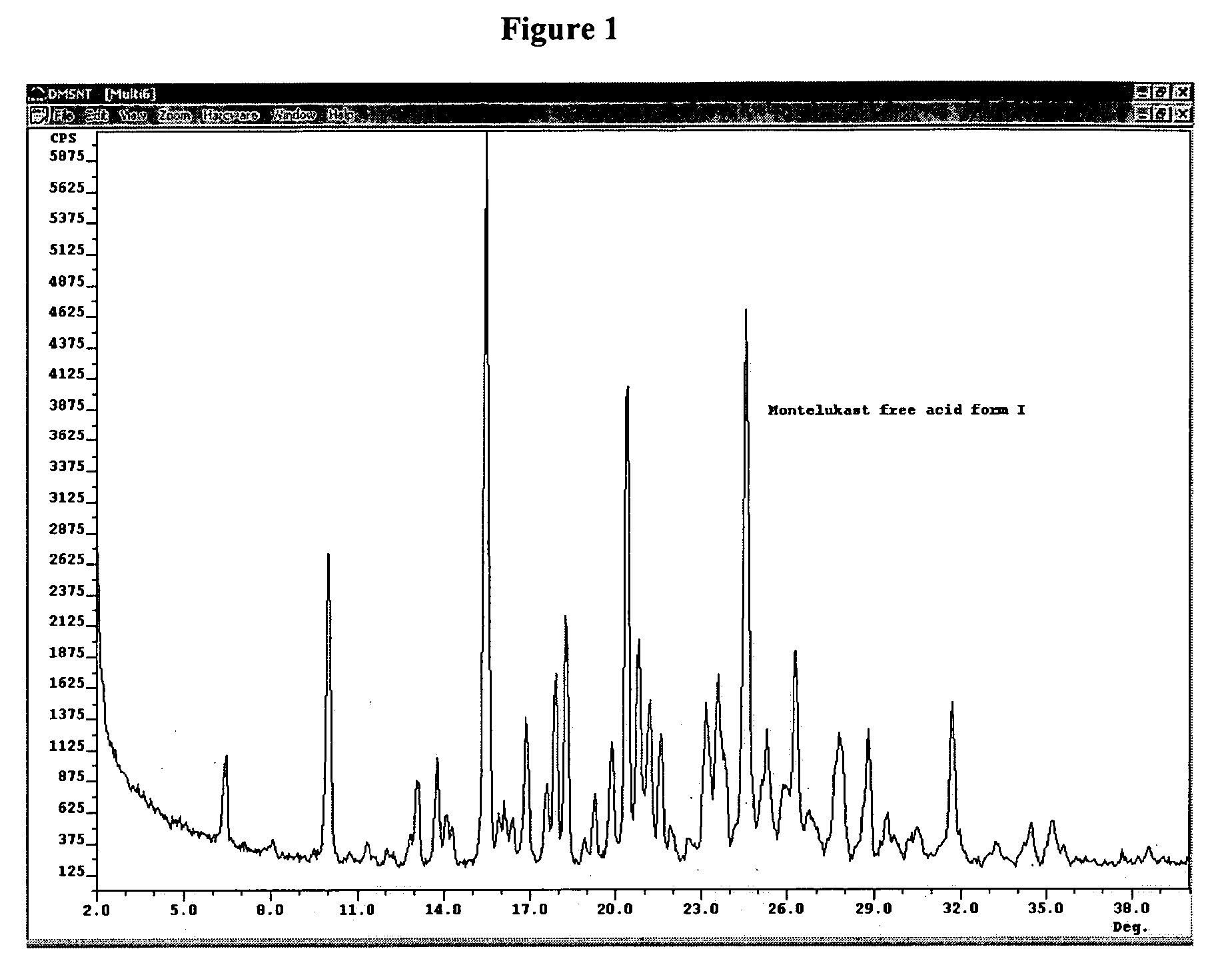
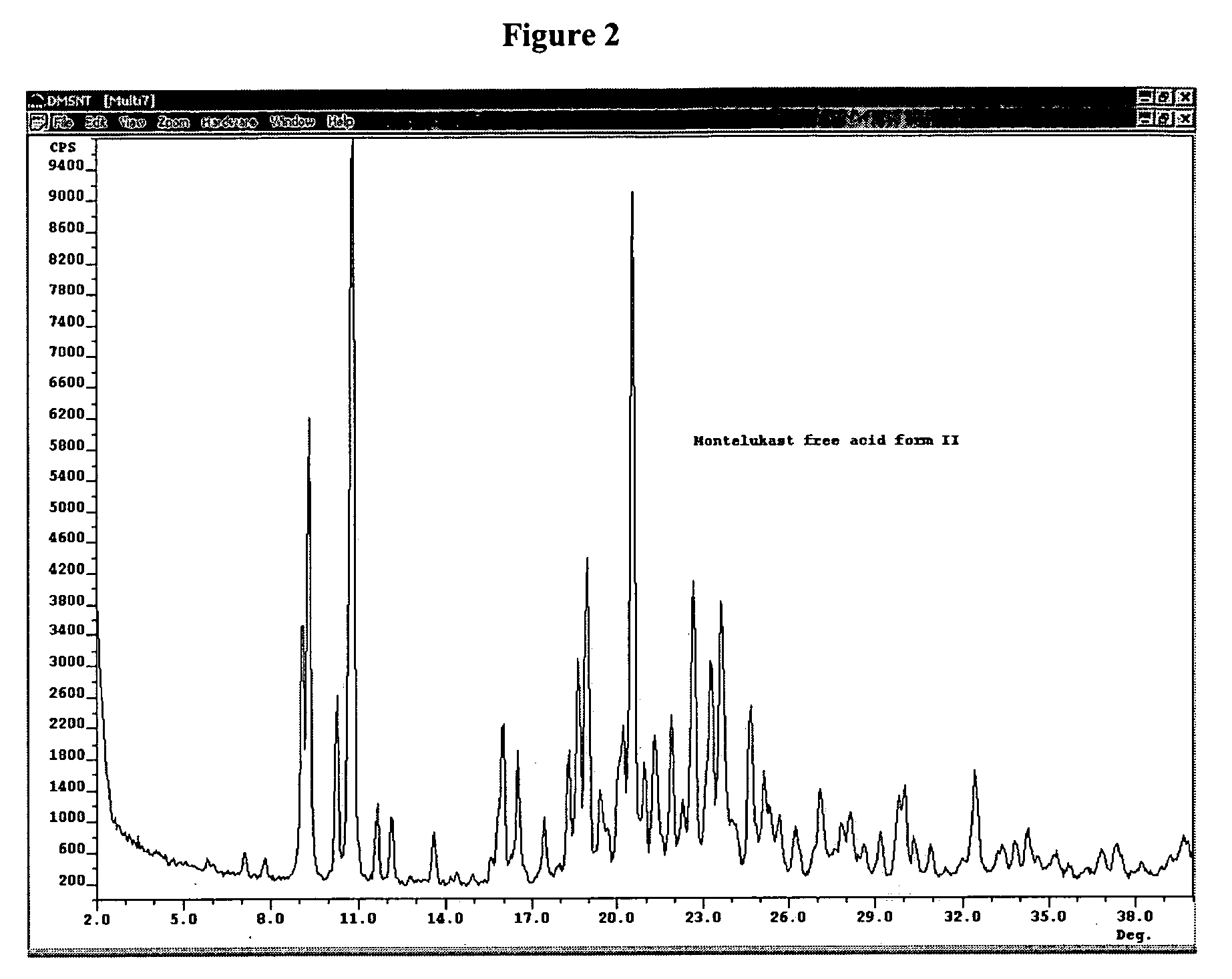
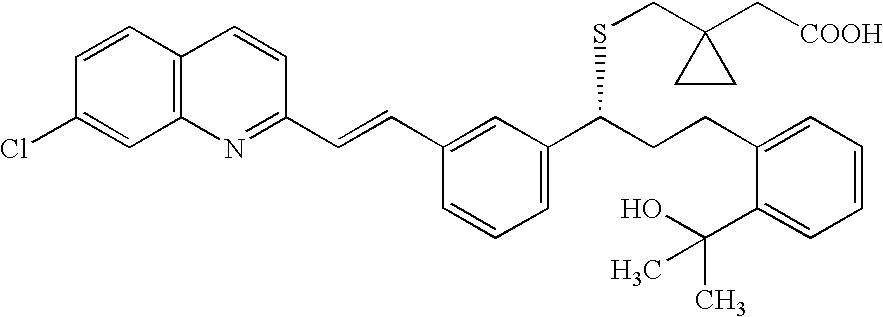
Montelukast free acid polymorphs
Owner:TEVA PHARM USA INC
Process for preparing montelukast and precursors thereof
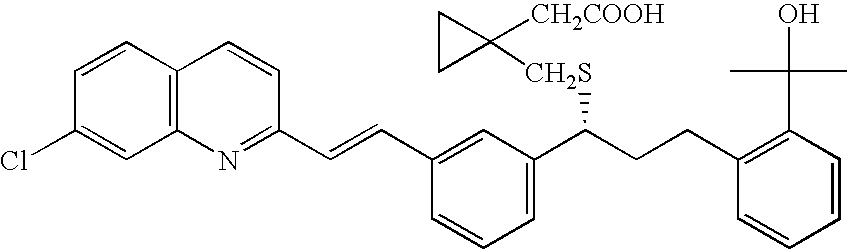
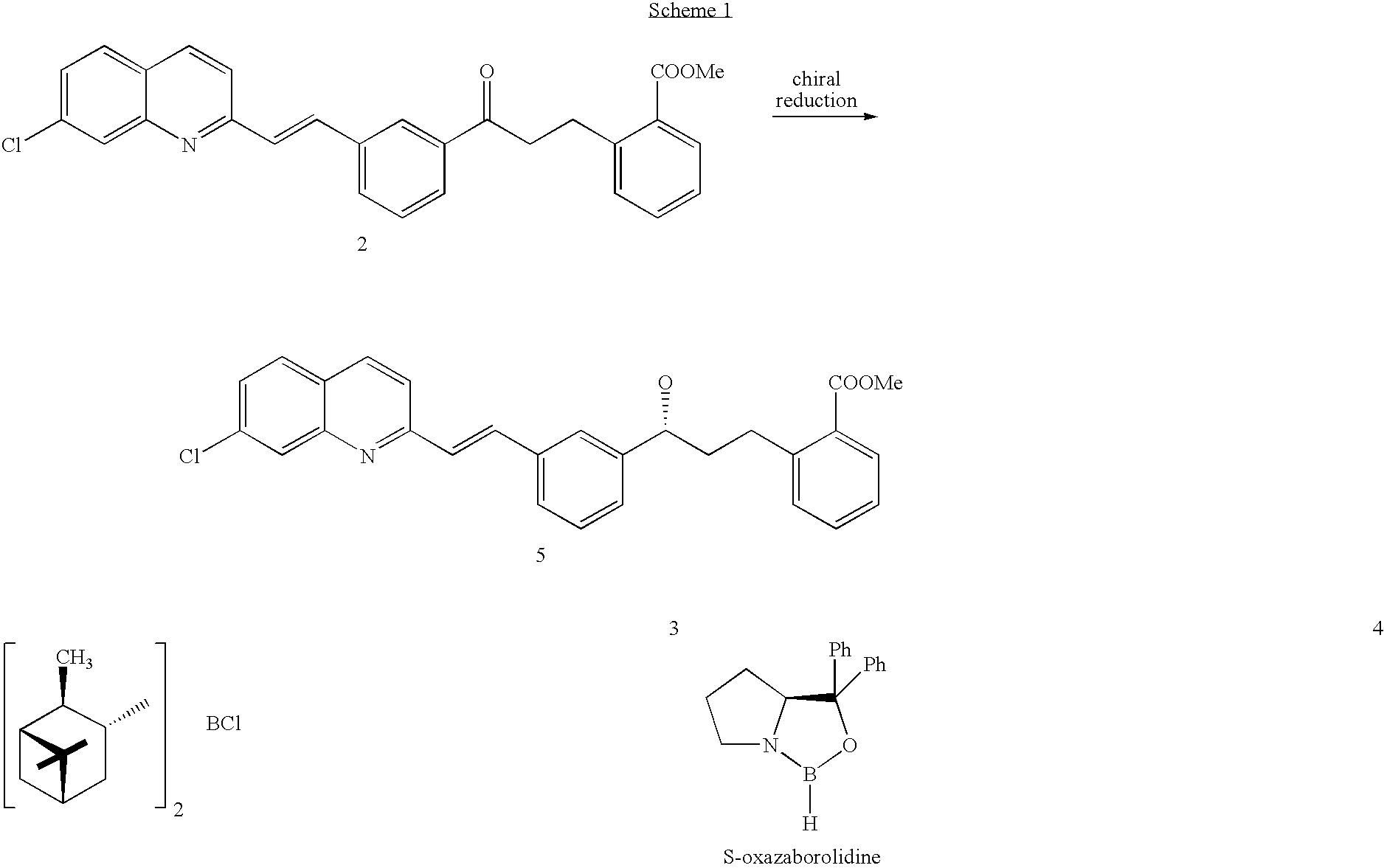
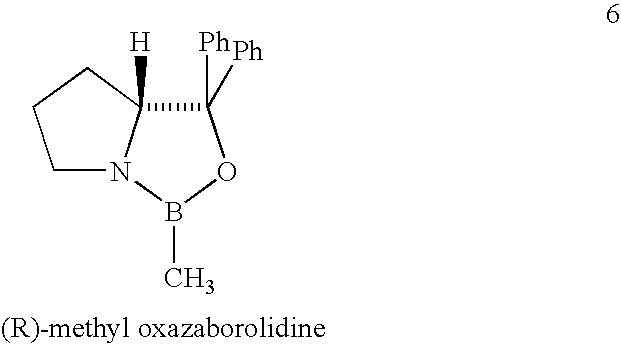
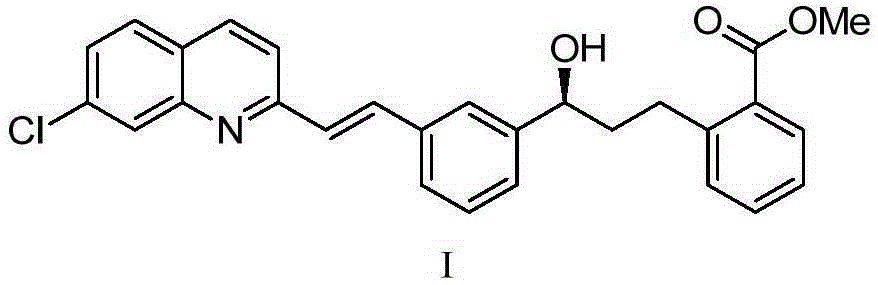
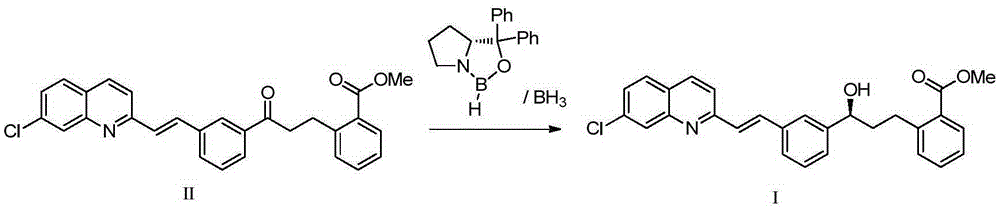
The present invention provides a process for stereoselectively reducing 2-[3-[3-[2-(7-chloro-2-quinolinyl)ethenyl]-phenyl]-3-oxopropyyl]benzoic-acid methyl ester, to produce to produce methyl 2-[3-(S)-[3-[2-(7-chloro-2-quinolinyl)-ethenyl]phenyl]-3-hydroxypropyl]benzoate, and a process for producing montelukast or a salt thereof. The present invention further provides a process for purifying methyl 2-[3-(S)-[3-[2-(7-chloro-2-quinolinyl)-ethenyl]phenyl]-3-hydroxypropyl]benzoate. The reduction process of the present invention uses a chiral reagent and can produce the desired reduction product in high enantiomeric excess (ee).
Owner:CHEMAGIS
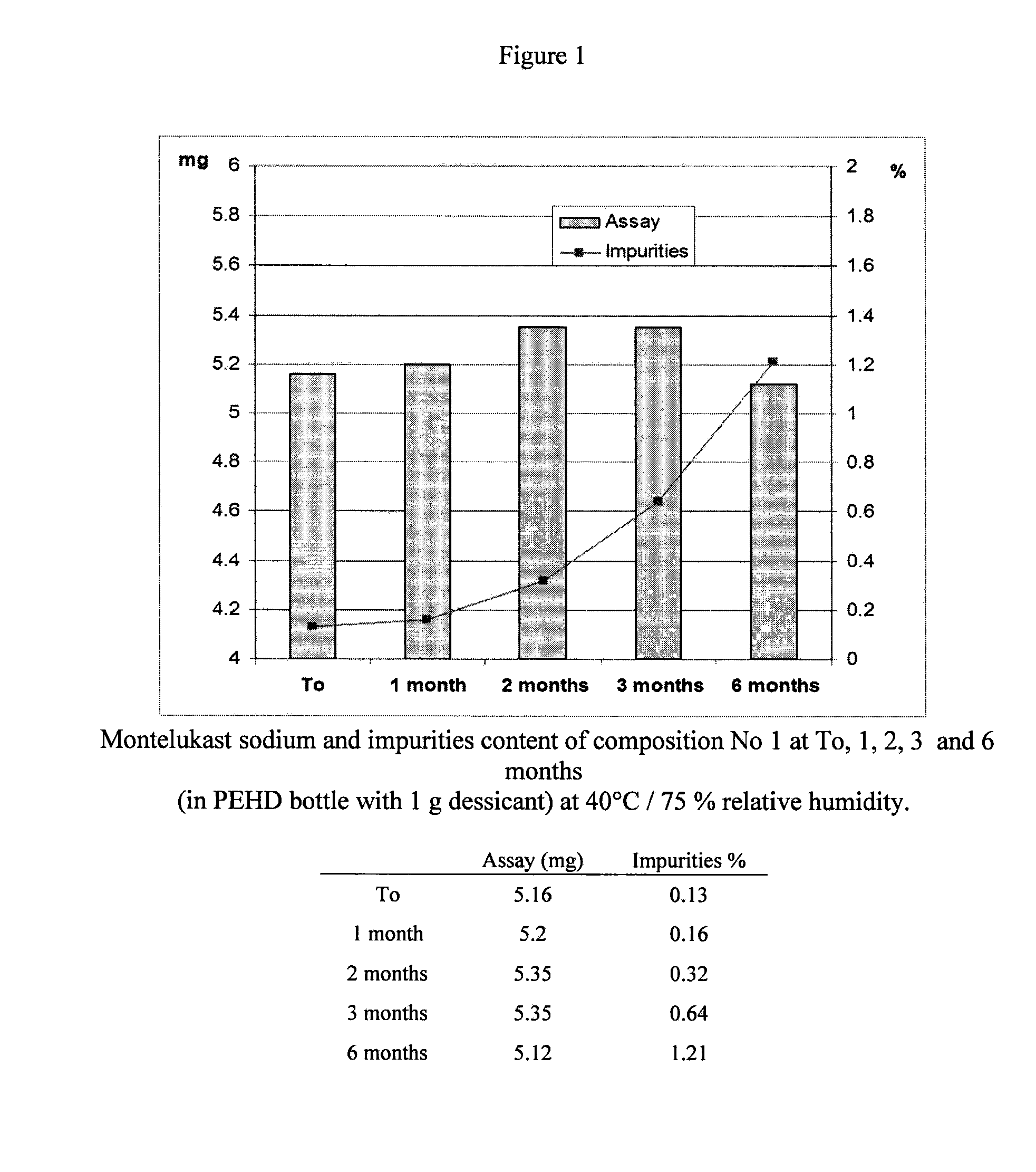
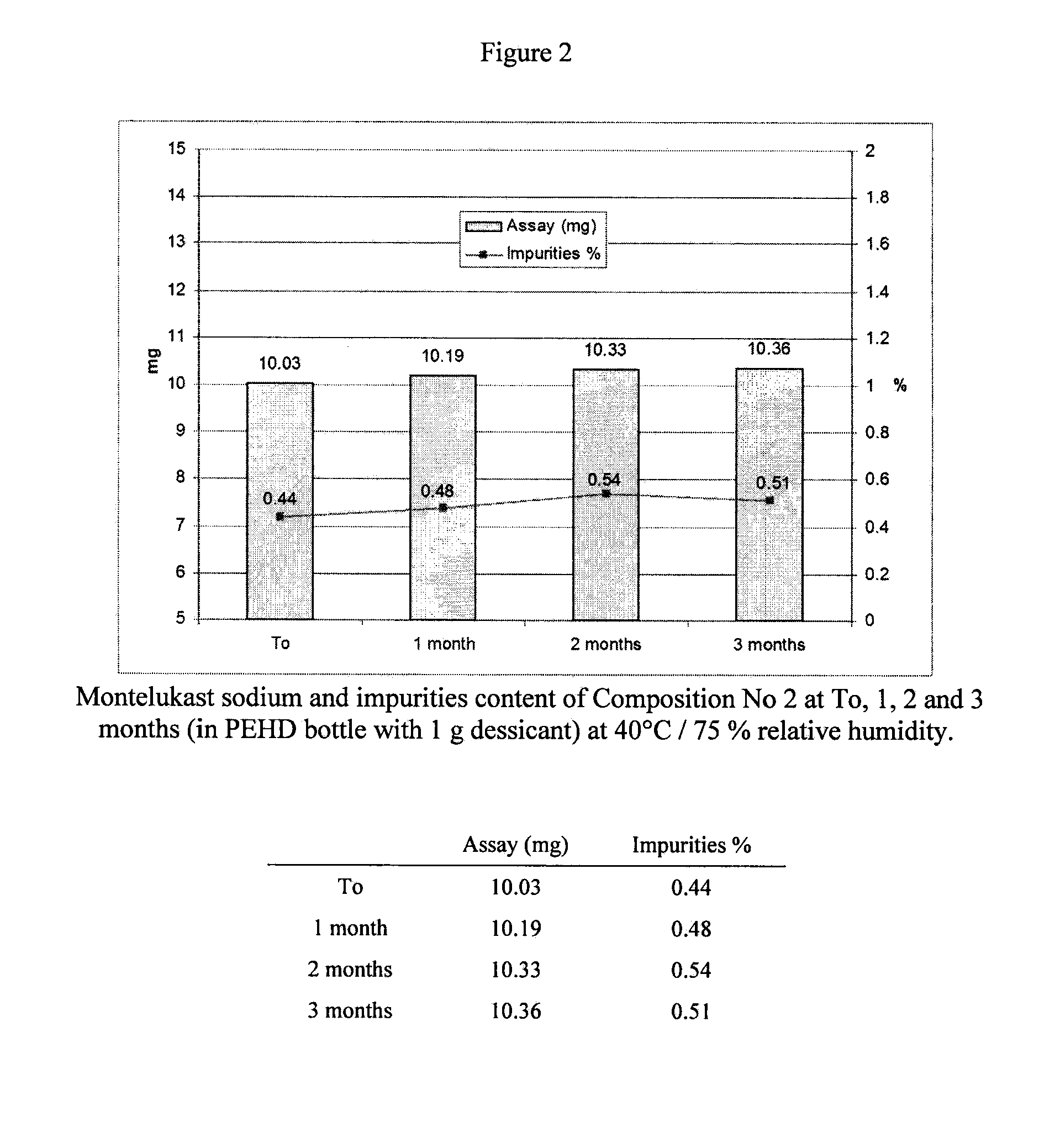
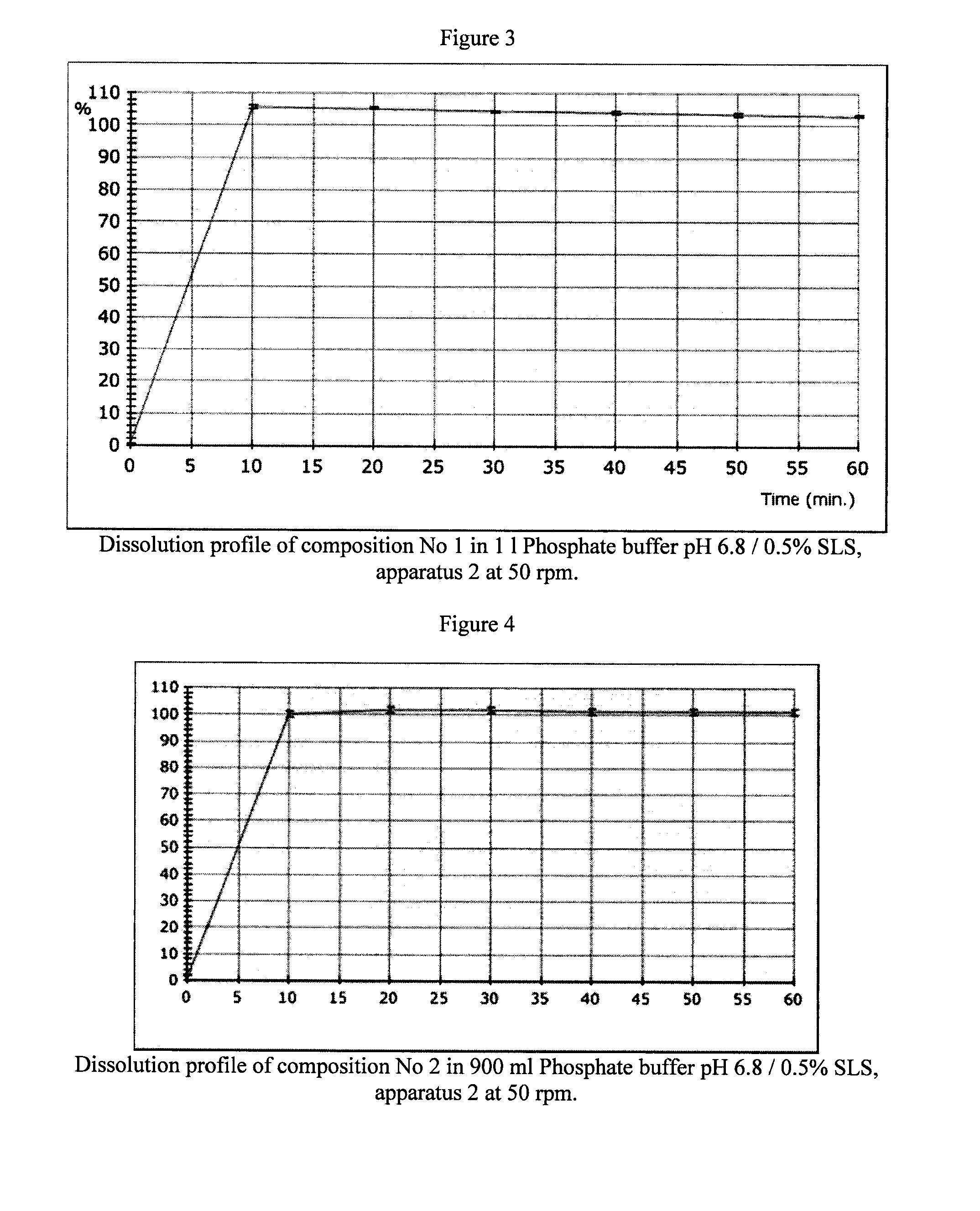
Pharmaceutical composition and process for montelukast tablets
The manufacture of compositions containing montelukast and to stable tablet compositions resulting thereof are disclosed, which include a first compaction step of a dry blend including, montelukast or a pharmaceutically acceptable salt thereof, and microcrystalline cellulose, and a further compression step into tablets.
Owner:KREMERS URBAN PHARMA
Purification of montelukast
The present invention provides methods of purifying montelukast, a new isolated impurity of montelukast, method for its isolation, and method of using montelukast impurity as a reference marker and a reference standard.
Owner:TEVA PHARM USA INC
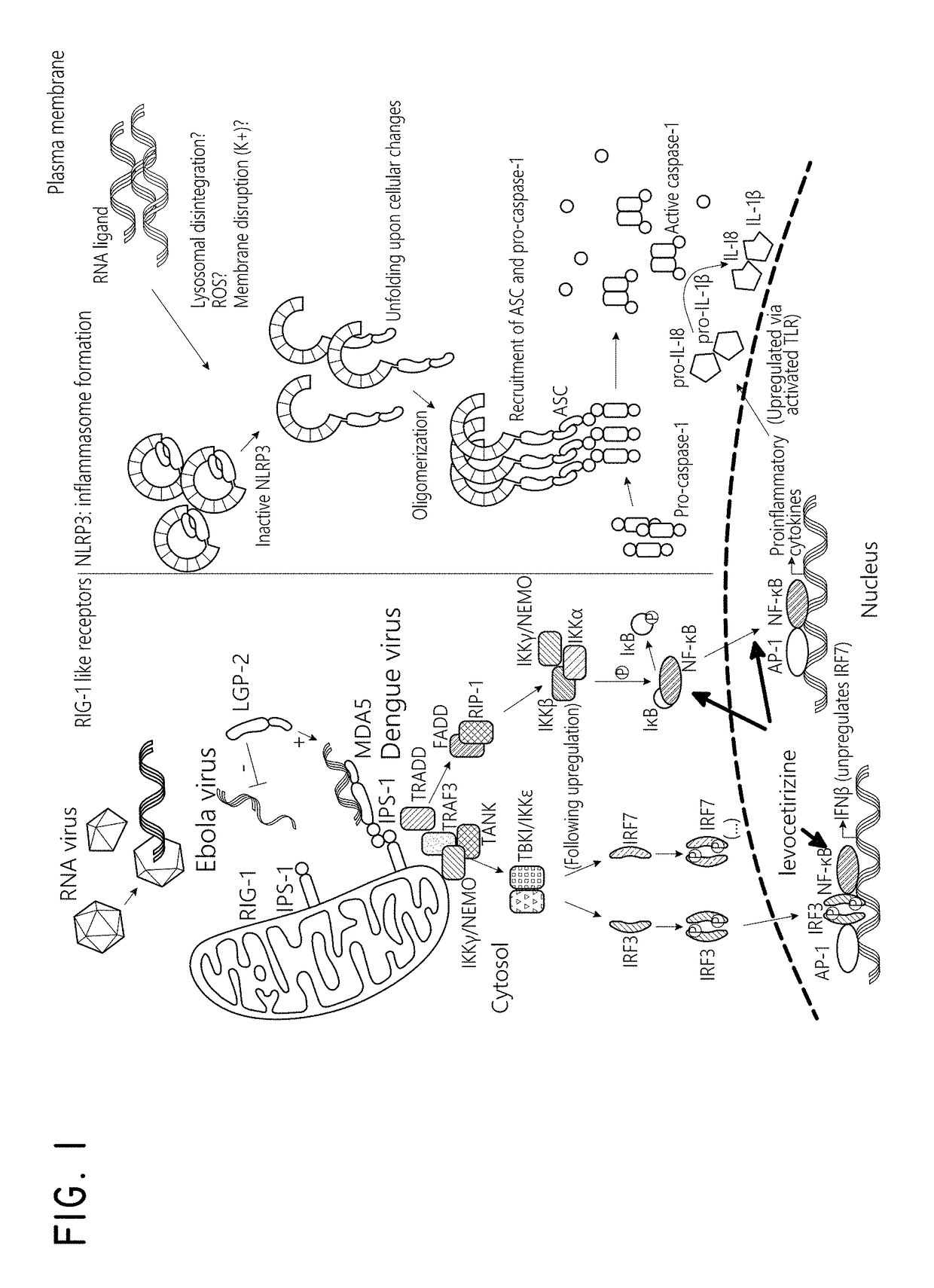
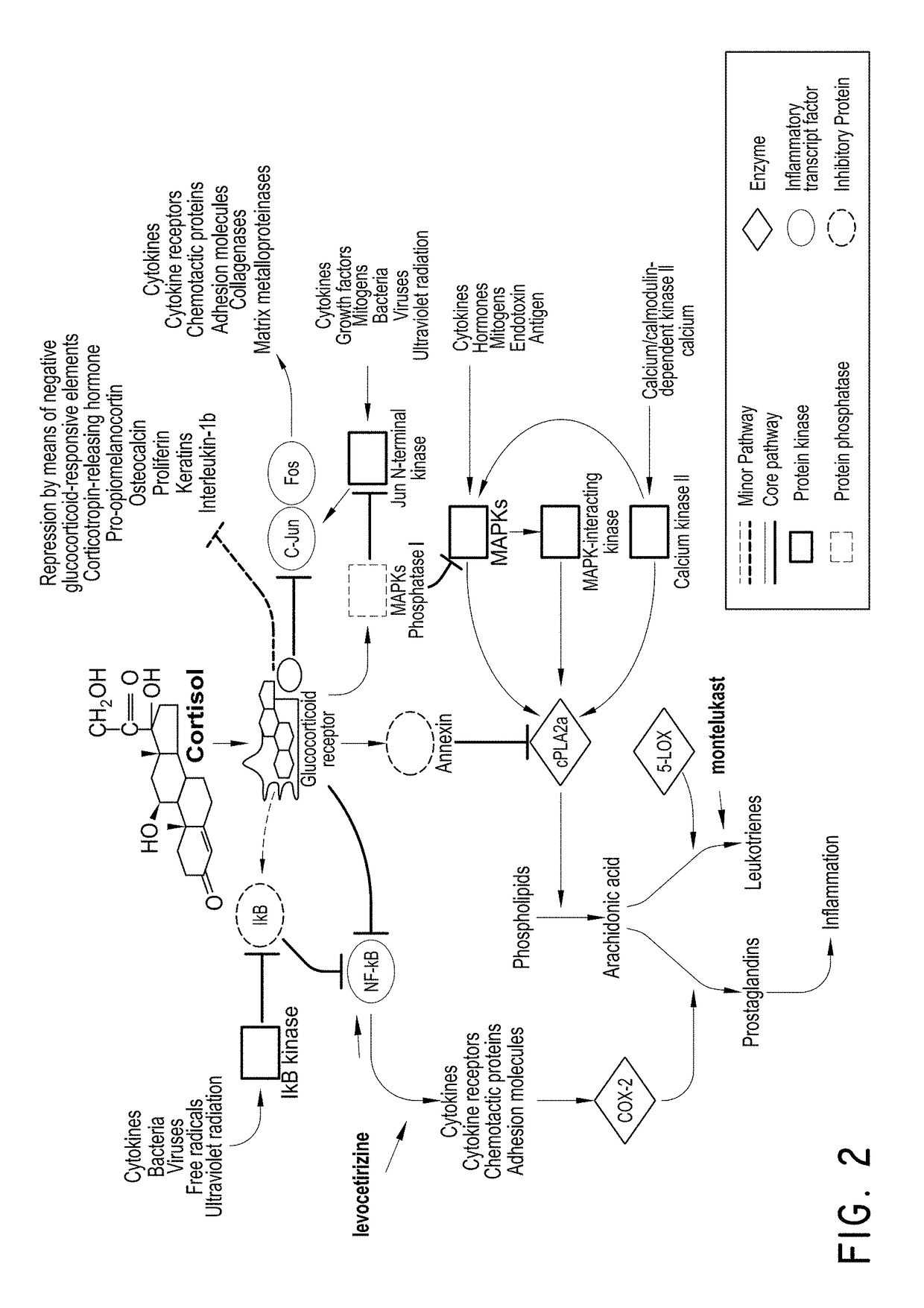
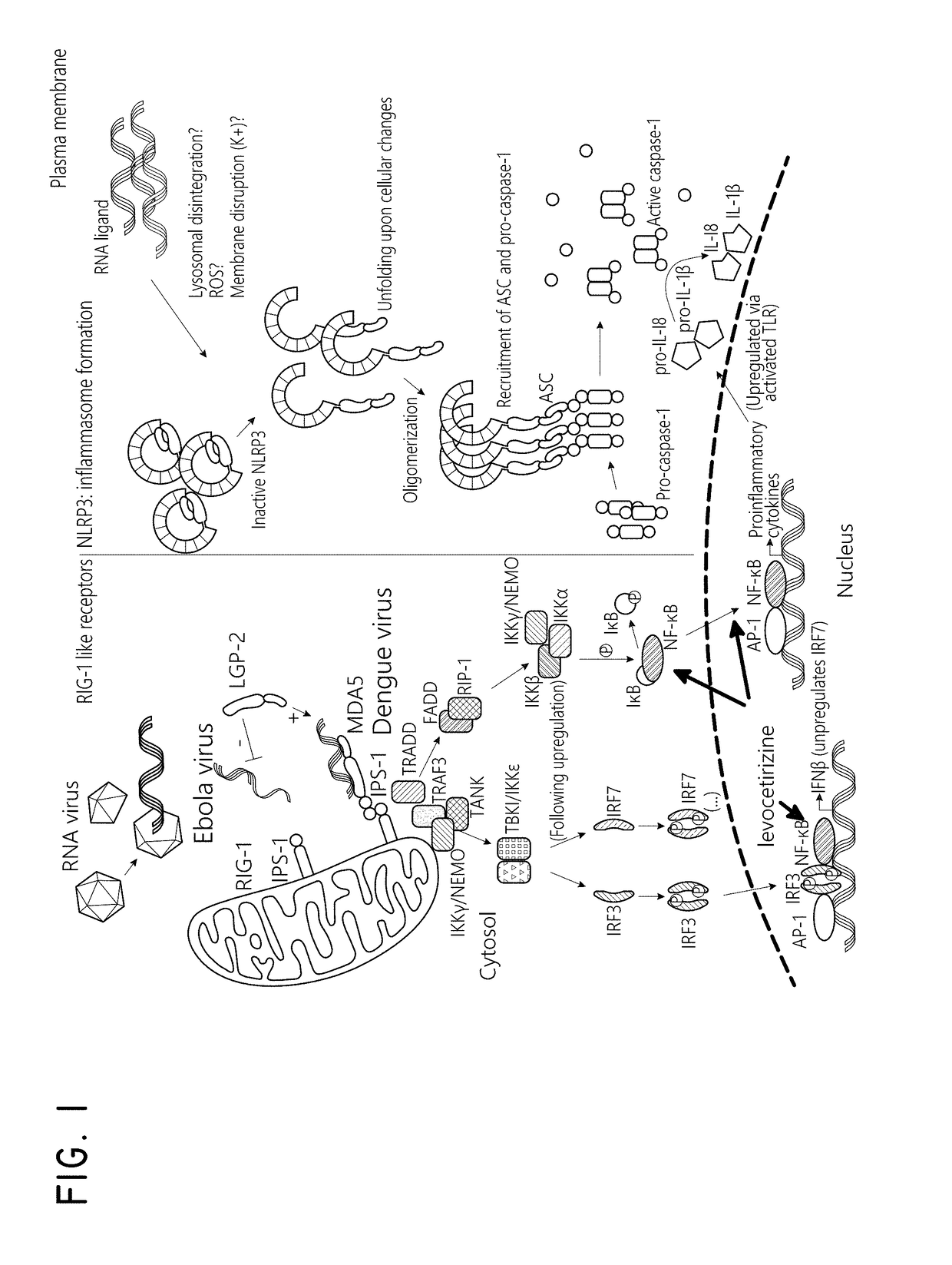
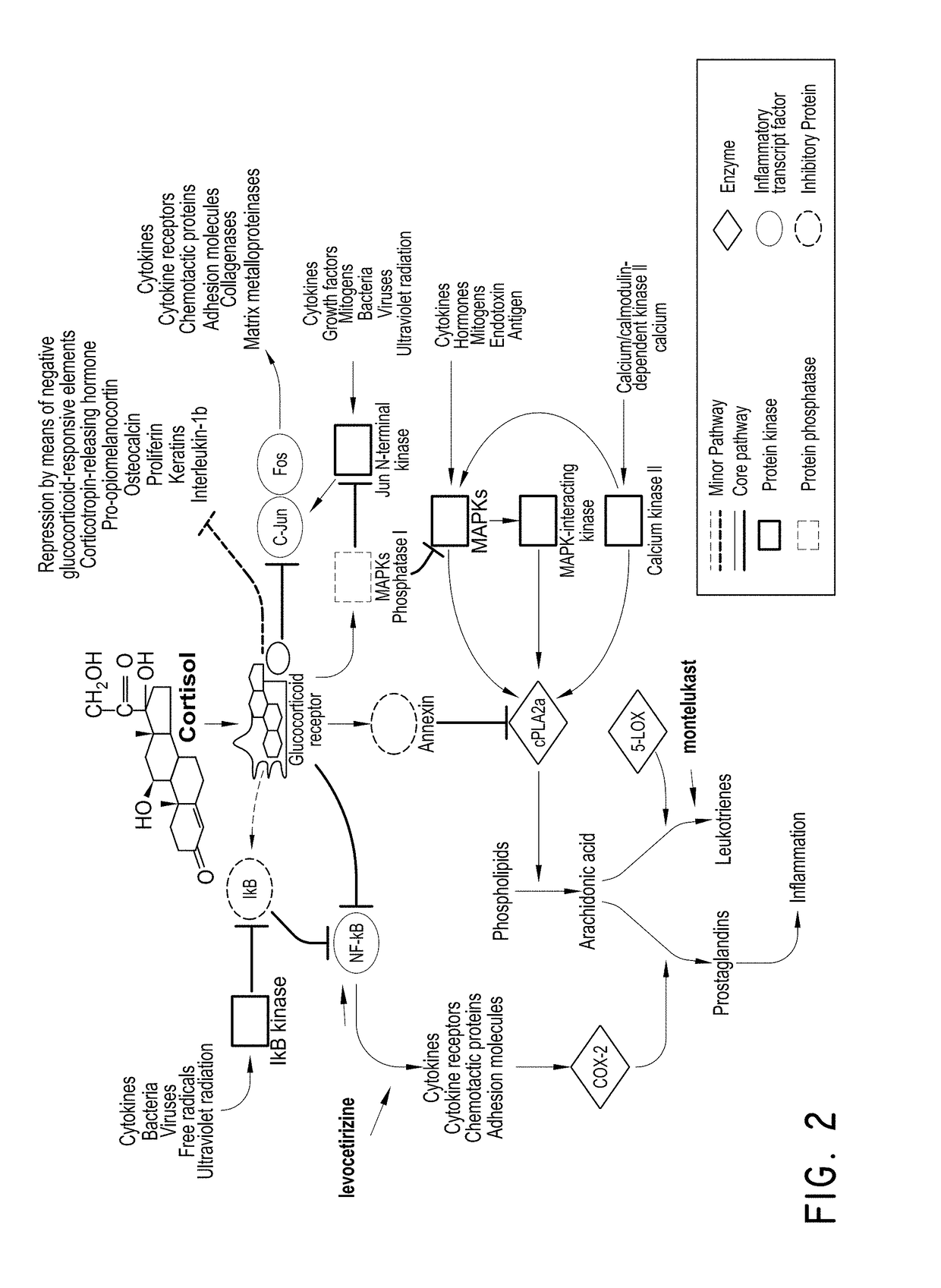
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
Method for preparing montelukast acid
The invention discloses a method for preparing montelukast acid. The method comprises a reaction b or a reaction a to a reaction b in the following synthetic route: the formula is shown in the description. The method disclosed by the invention can be used for preparing montelukast acid and salts thereof with low cost and high yield by means of simple operations by using low-cost and easily available raw materials, and is of significance and has practical value to prepare montelukast acid and salts thereof with low cost on a large scale.
Owner:SHANGHAI DESANO CHEM PHARMA +1
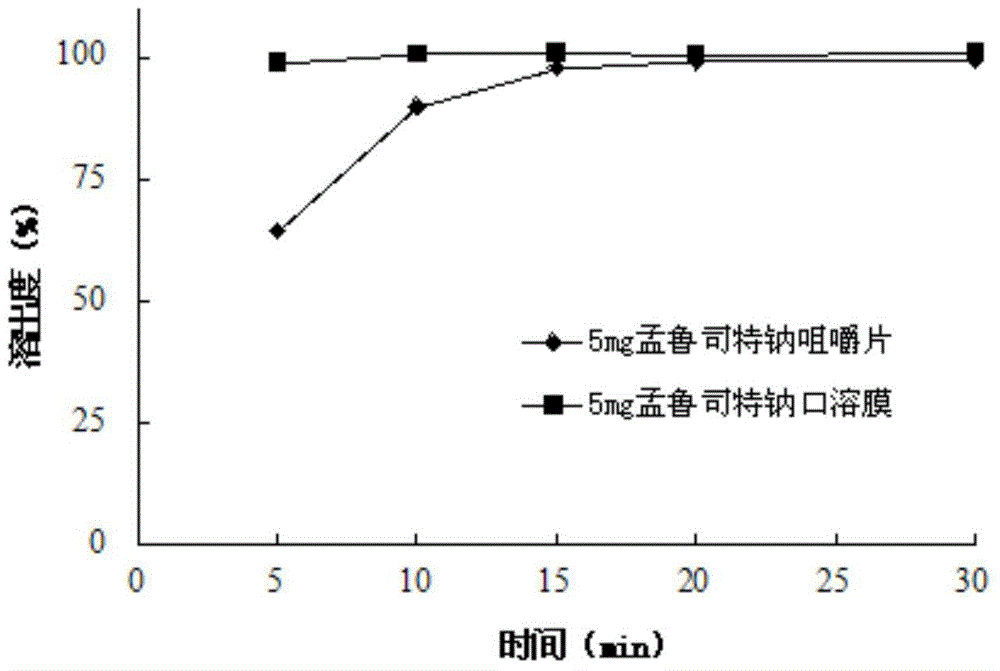
Montelukast transmucosal film
An oral film product in which a pharmaceutically active agent is stabilized in its partially-ionized form to better facilitate oral transmucosal delivery is provided. The film includes a bioadhesive layer including a pharmaceutically active agent having a logarithmic acid dissociation constant that is less than 4.5 and which is complexed with a cationic polymer.
Owner:INTELGENX CORP
Process for making montelukast and intermediates therefor
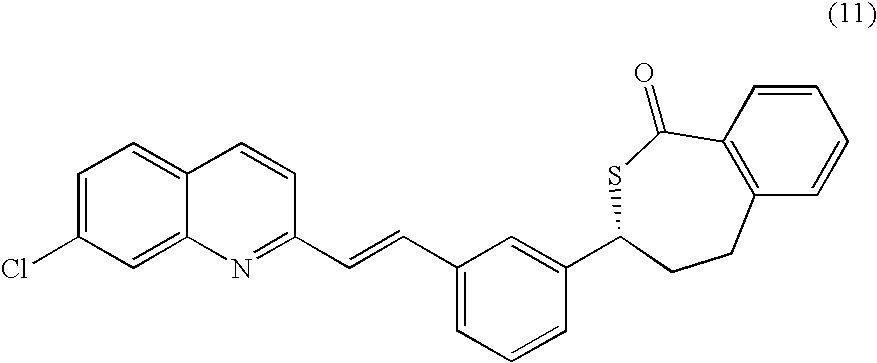
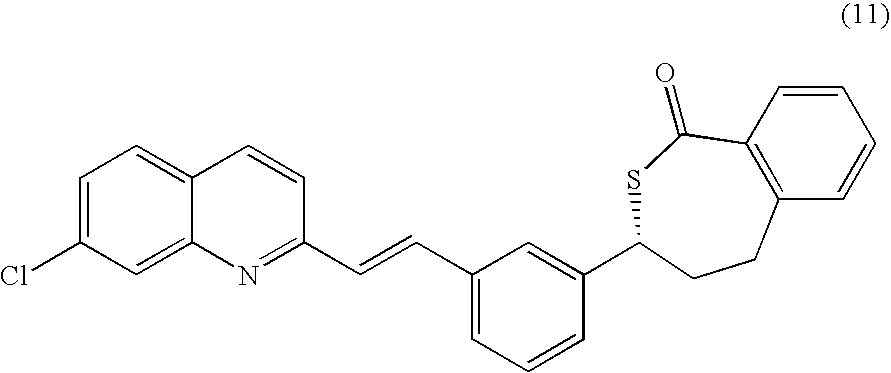
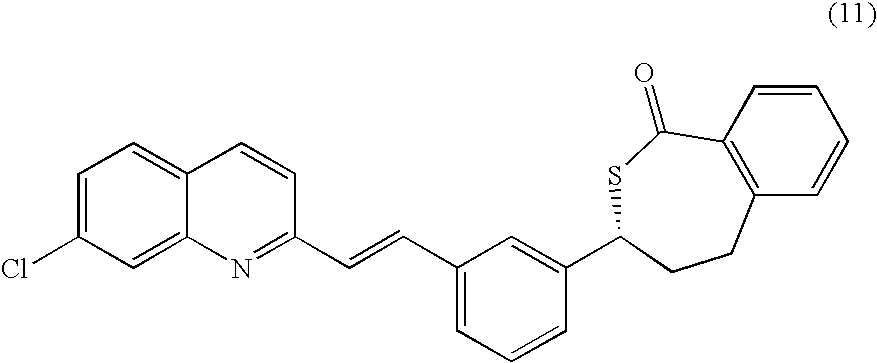
A process for making montelukast, a pharmaceutically useful compound of the following formula and salts thereof: using a compound of formula (11) is provided.
Owner:SYNTHON BV
Method of preparing montelukast and intermediates used therein
InactiveCN101558042AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsMontelukastOrganic chemistry
The present invention relates to a method for preparing montelukast, an inhibitor against leukotrienes, and an intermediate used therein. According to the inventive method, high-purity montelukast or its sodium salt can be prepared in a high yield.
Owner:HANMI SCI CO LTD
Preparation of montelukast and its salts
InactiveUS20110040095A1Reduce yieldQuality improvementOrganic chemistryMontelukastInorganic chemistry
Owner:DR REDDYS LAB LTD +1
Process for making montelukast and intermediates therefor
InactiveUS20070135643A1Lower Level RequirementsImprove the level ofBiocideOrganic chemistryMontelukastCe element
The reaction of methylmagnesium halide is improved by the presence of a cerium (III) salt such as cerium trichloride. The reactions are typically associated with the production of montelukast, a pharmaceutically useful compound of the following formula and salts thereof. Further enhancements can be derived from certain intermediates, salts thereof and / or purification thereof before and / or after the reaction involving methylmagnesium halide.
Owner:SYNTHON BV
Stable Montelukast oral film preparation
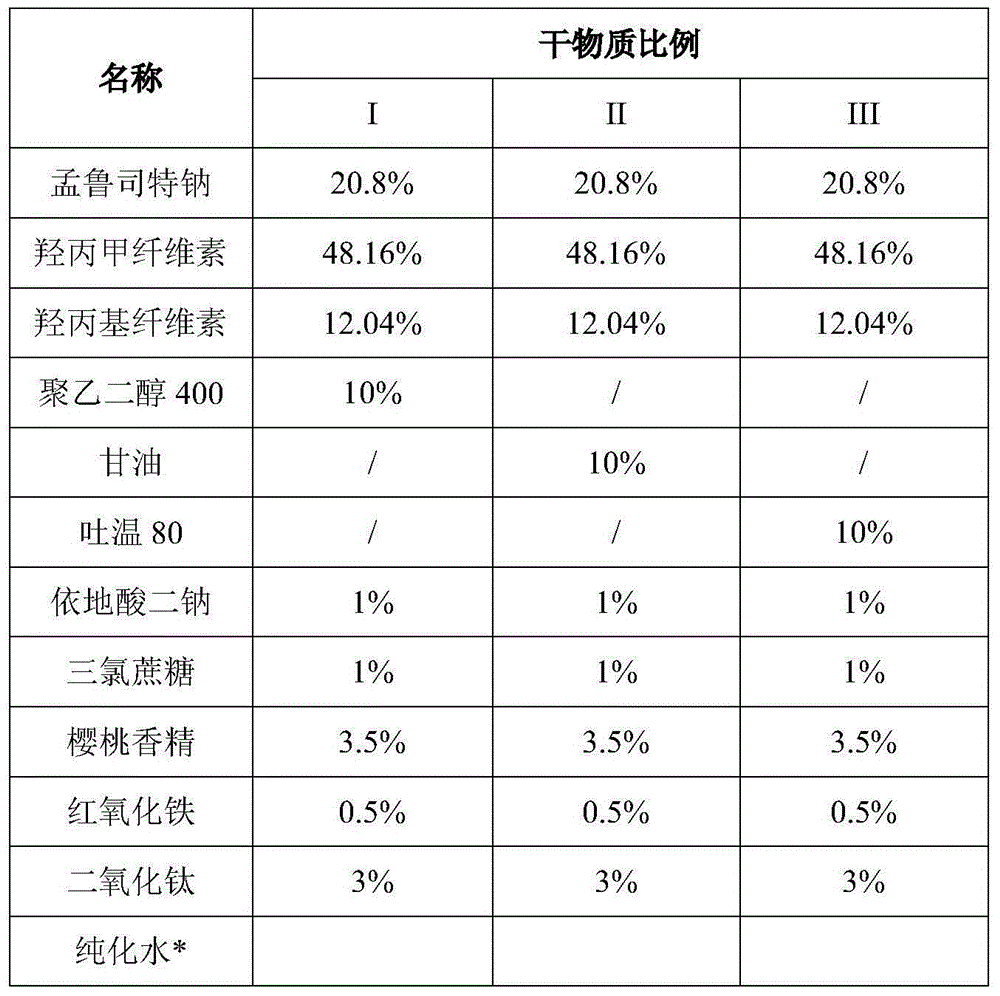
The invention relates to a stable Montelukast oral film preparation. The oral film preparation contains an effective amount of Montelukast or pharmaceutically acceptable salts of the Montelukast, a drug stabilizer at least comprising edetic acid and / or editate as well as pharmaceutically acceptable auxiliary materials. The Montelukast oral film preparation obtained according to the formula is accurate in dose, stable in quality and capable of effectively preventing oxidization and degradation of effective components, has the advantages of bright color, good taste, capability of being quickly dissolved in an oral cavity without water, improves the administration compliance of a patient and is particularly suitable for infants and patients suffering from dysphagia.
Owner:QILU PHARMA CO LTD
Method for preparing montelukast nano chiral alcohol intermediate
InactiveCN105294545AReduce dosageReduce manufacturing costOrganic chemistry methodsMethyl benzoateEthyl Chloride
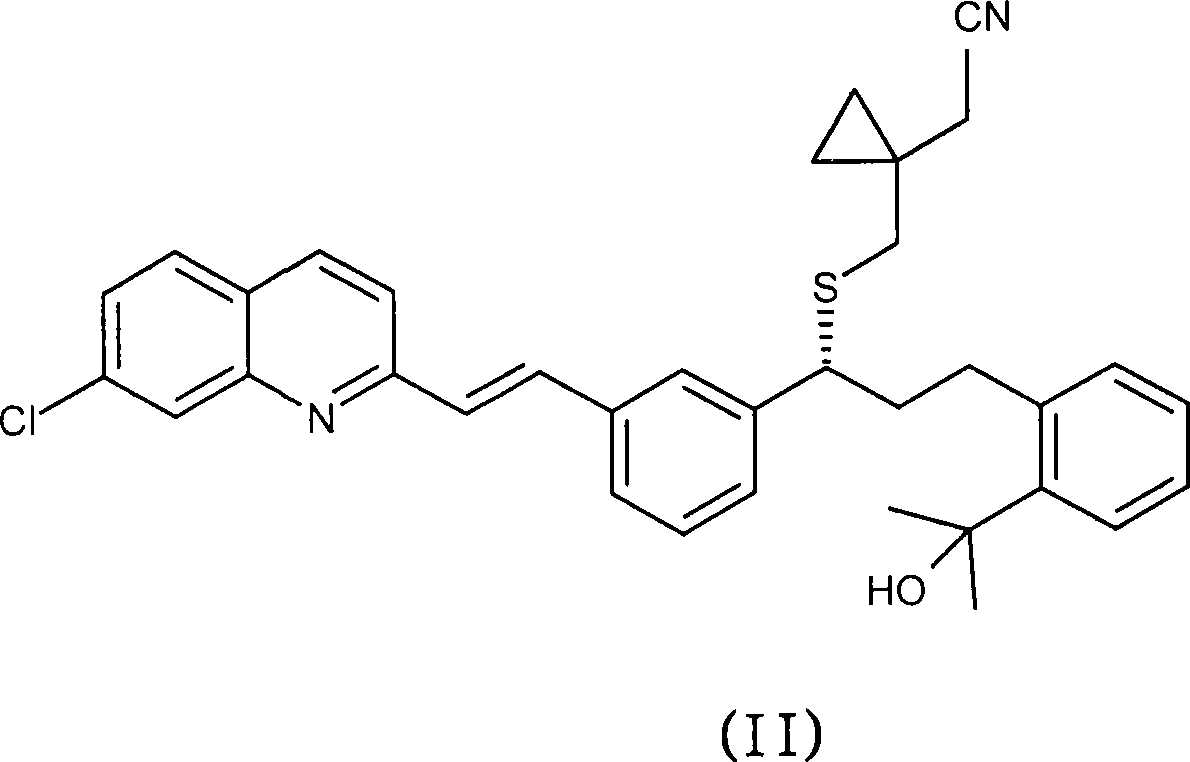
The invention discloses a method for preparing a montelukast nano chiral alcohol intermediate. In alkali and solvent environment, 2-[3-[3-[2-(7-chloro-2-quinolyl) vinyl] phenyl]-3-oxo propyl] methyl benzoate (II) is subjected to catalytic hydrogenation reaction by using a transition metal complex as a catalyst to generating 2-[3-(S)-[3-[2-(7-chloro-2-quinolyl)-phenyl] vinyl]-3-hydroxy propyl methyl benzoate (I), wherein the transition metal complex has a general formula of MLnL'XY, and is a nitrogen phosphine transition metal formed by coordination of a ligand with NH2-N(sp2) or NH2-NH2 structural characteristic and a transition metal complex. The preparation method has the advantages of small catalyst amount, mild reaction conditions, stable process, conversion rate of more than 98%, less pollution, and high yield and high purity of obtained product, and has the very good value of industrialization.
Owner:ENANTIOTECH CORP
Process for the Preparation of Montelukast and Its Pharmaceutically Acceptable Salts
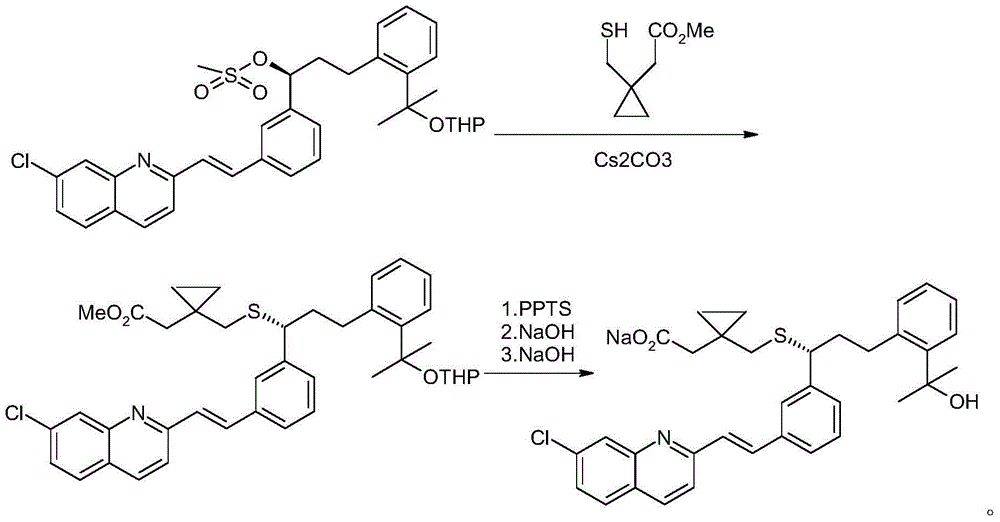
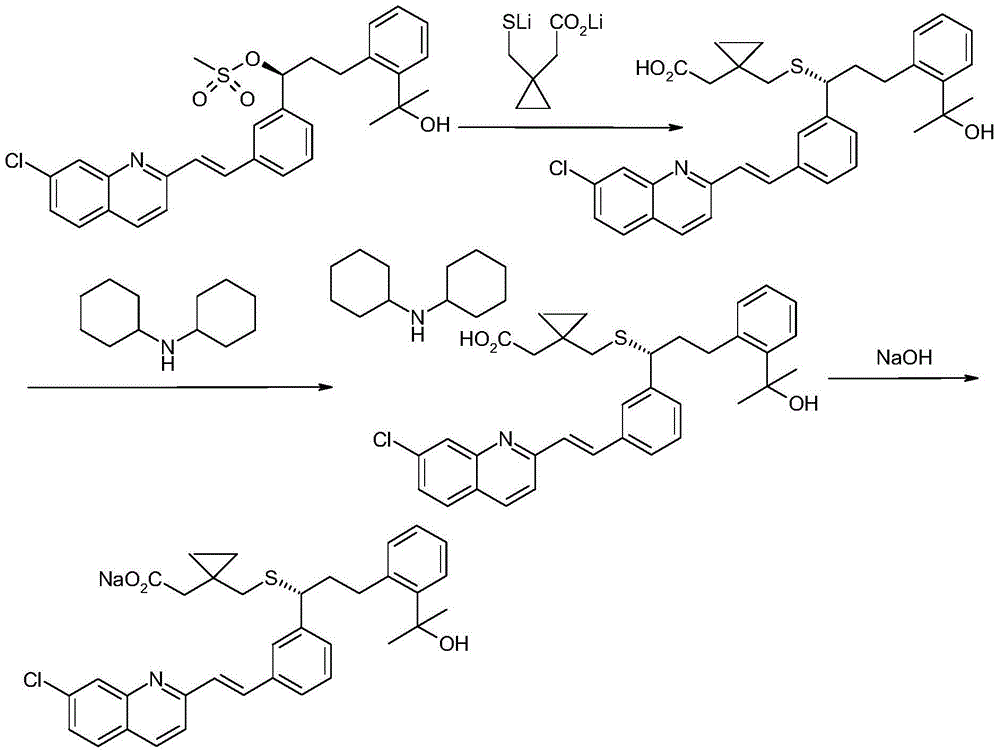
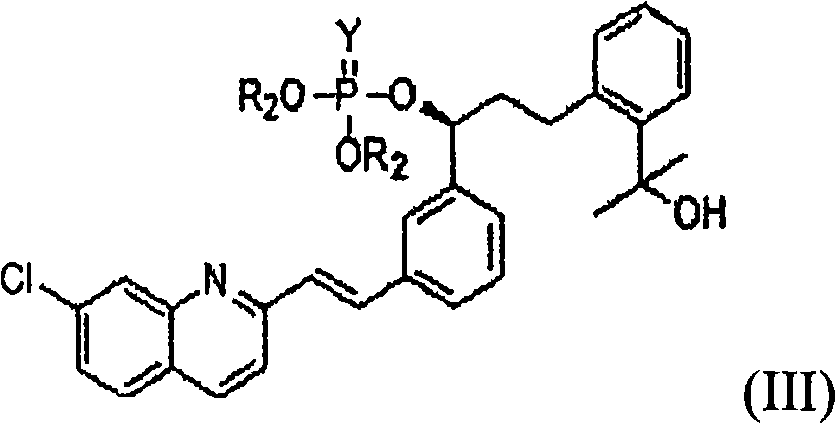
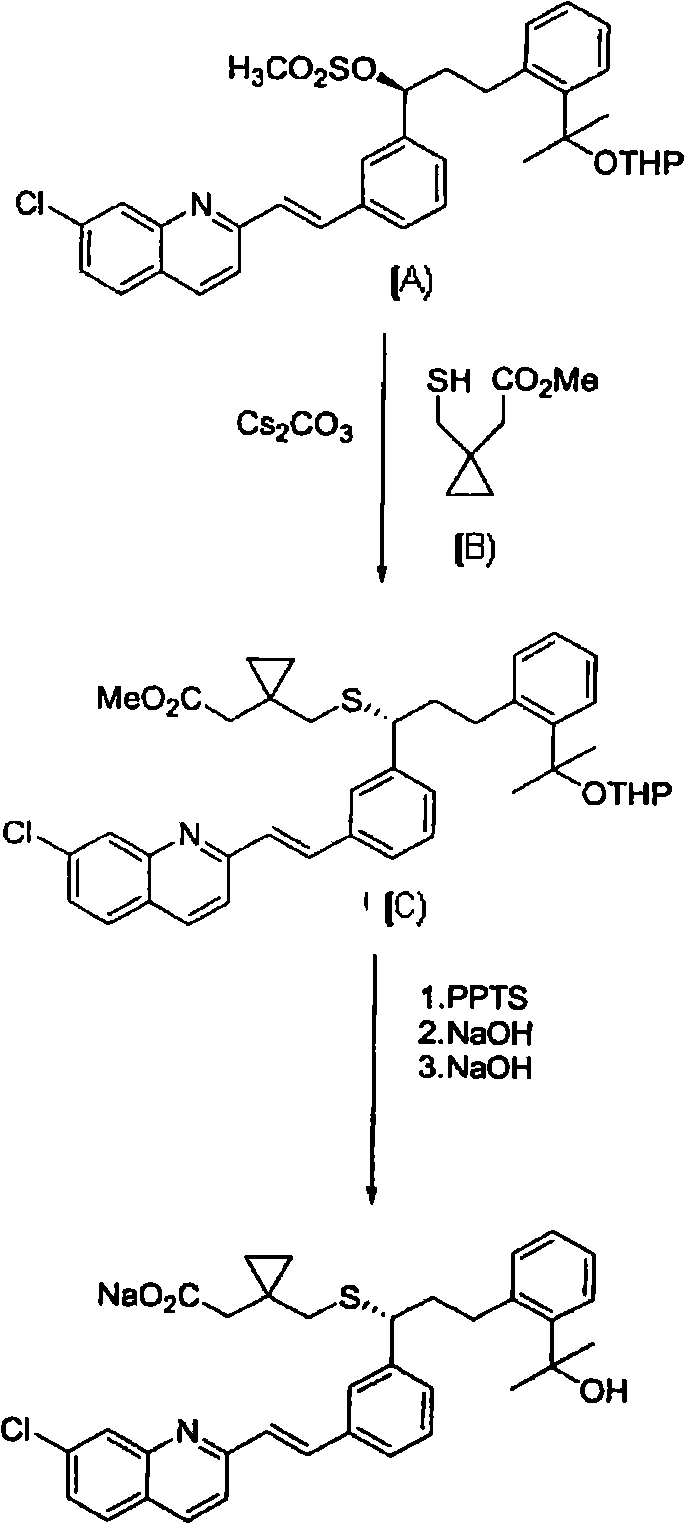
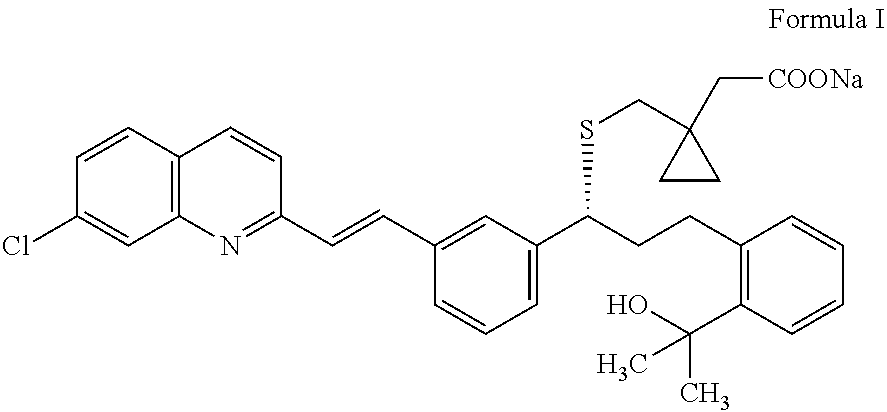
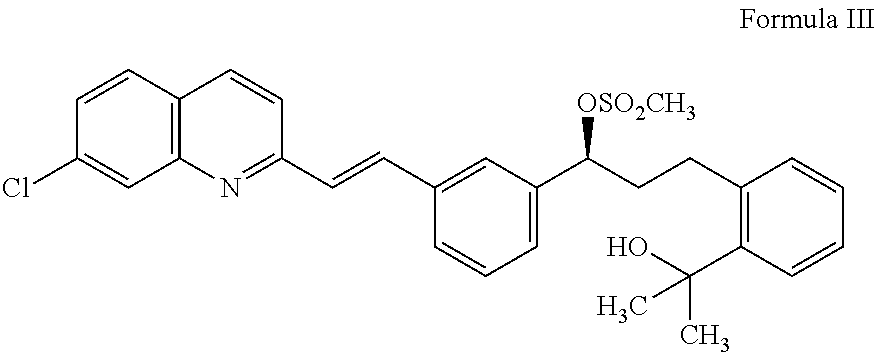
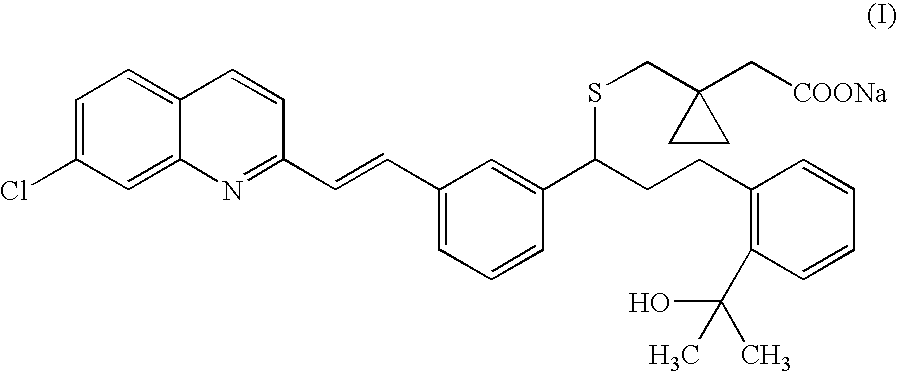
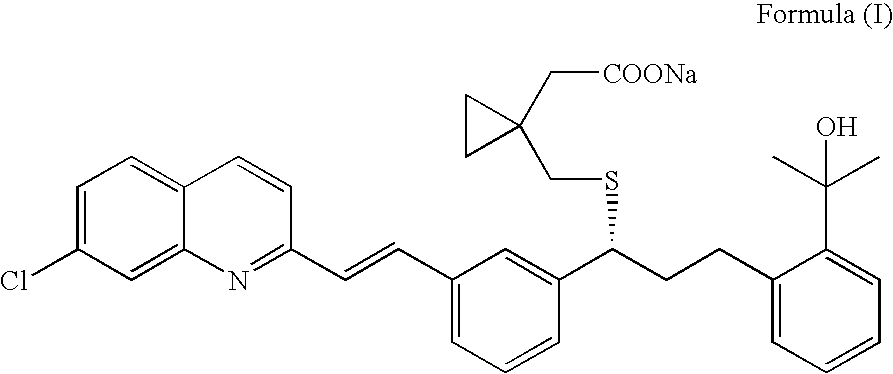
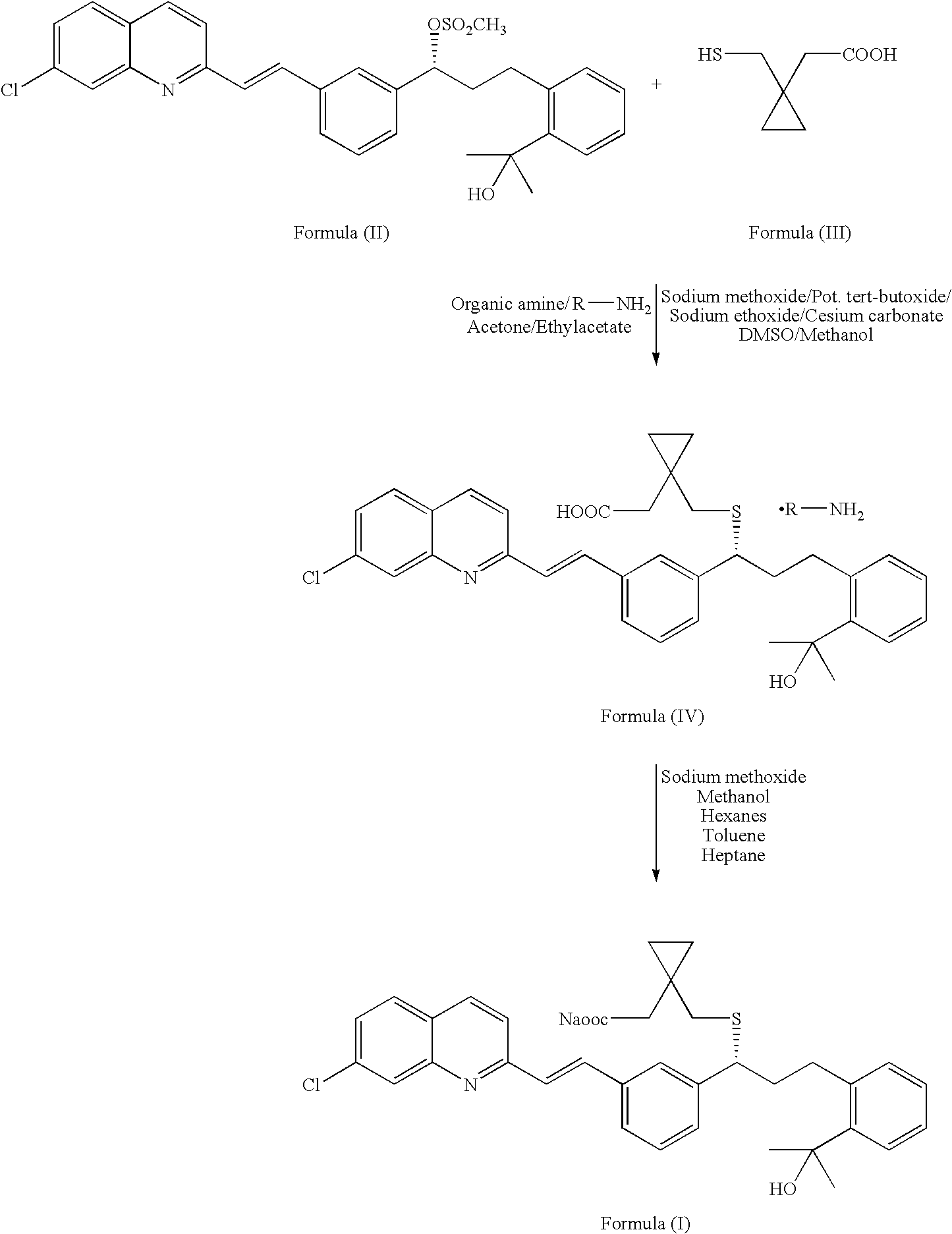
An improved process for the preparation of Montelukast and its pharmaceutically acceptable salts comprises of reacting (S) Benzenepropanol α-[3-[2-(7-chloro2-quinolinyl)ethenyl]phenyl]-2-(1-hydroxy-1-methyl ethyl)-α-methane sulfonate compound of formula (II) with 1-(mercapto methyl)cyclo propane acetic acid or its ester or nitrile in presence of alkali or alkaline carbonates and / or alkali or alkaline earth metal alkoxide in a suitable polar aprotic solvent with or without combination of C1-C4 alcoholic solvents and then treating with organic amine in a suitable ester and / or acetone and / or aliphatic or aromatic hydrocarbon solvents, and converting the corresponding amine salt compound of montelukast into its sodium salt compound of formula (I) using sodium ion source in methanol, without converting into montelukast free acid.
Owner:MSN LAB PTE LTD
Solid-state montelukast
A solid form of a compound of formula 1:is provided. The compound of formula 1 can be obtained in solid state by precipitation from a solution containing the same. The compound is useful as leukotriene antagonist and can be formulated into a pharmaceutical composition that also includes a pharmaceutically acceptable excipient.
Owner:SYNTHON BV
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
Leukotriene antagonist and antihistaminics composition
The invention relates to a pharmaceutical composition for treating asthma, allergic reaction and inflammation. The composition comprises a leukotriene antagonist, antihistaminics and medicinal carriers, wherein the leukotriene antagonist is Montelukast and salts thereof, and the antihistaminics is olopatadine hydrochloride and salts thereof. The composition is suitable for preventing and long-term treating of asthma for children and adults, and can treat asthma patients sensitive to aspirin, prevent bronchoconstriction induced by movement, treat pruritus concomitant with allergic rhinitis, urticaria and skin diseases, and the like. The composition has better curative effect compared with that the two medicines are used independently.
Owner:北京华禧联合科技发展有限公司
Process for making montelukast and intermediates therefor
A process for making montelukast, a pharmaceutically useful compound of the following formula and salts thereof:using a compound of formula (11)is provided.
Owner:BENOVSKY PETR +6
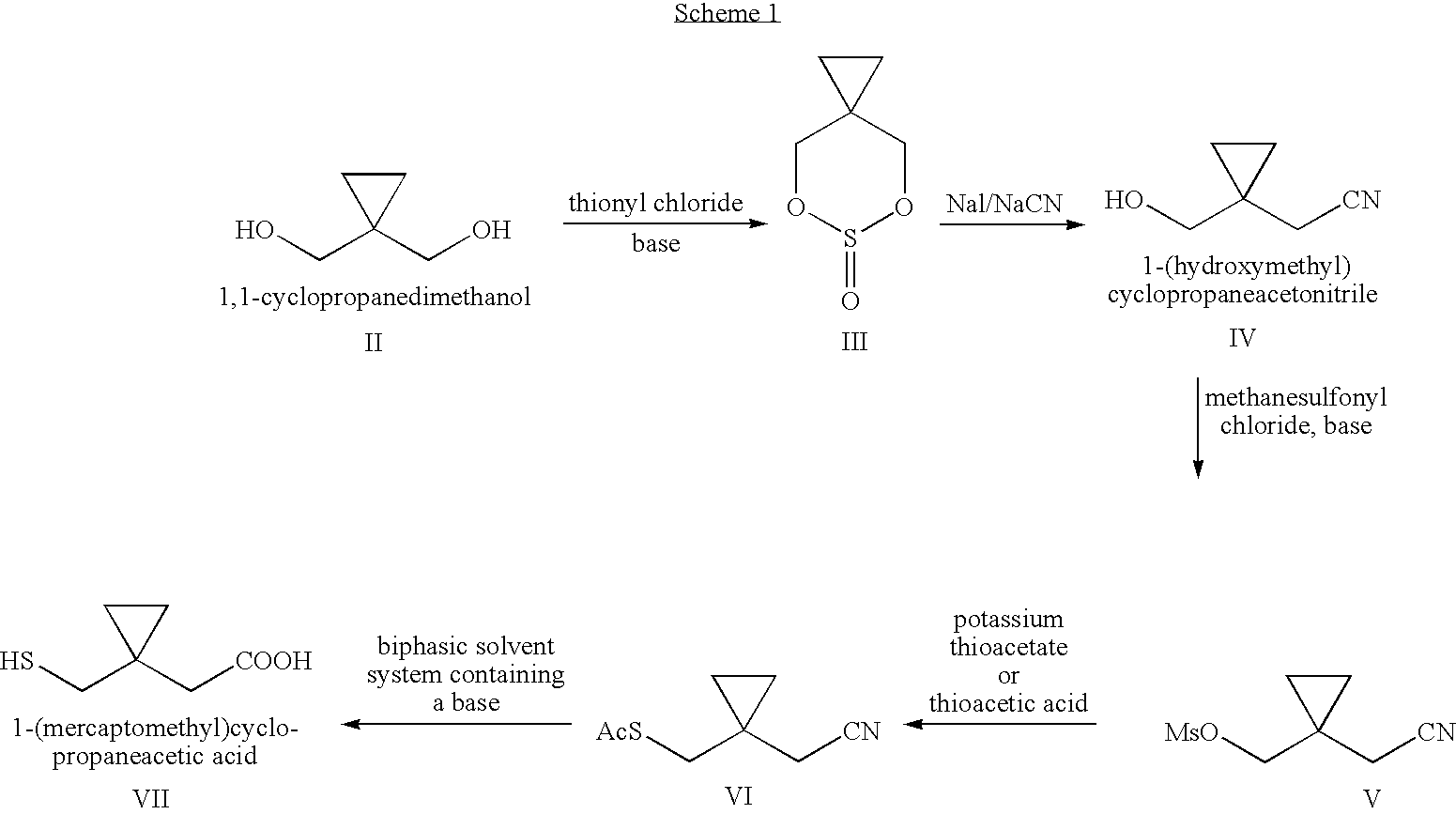
Process for preparing 1-(mercaptomethyl)cyclopropaneacetic acid, a useful intermediate in the preparation of montelukast and salts thereof
The present invention provides a novel montelukast intermediate and a simple and straightforward process for preparing it.According to the present invention, by using this intermediate and the process, essentially as described herein, montelukast acid and salts thereof are obtained.
Owner:CHEMAGIS
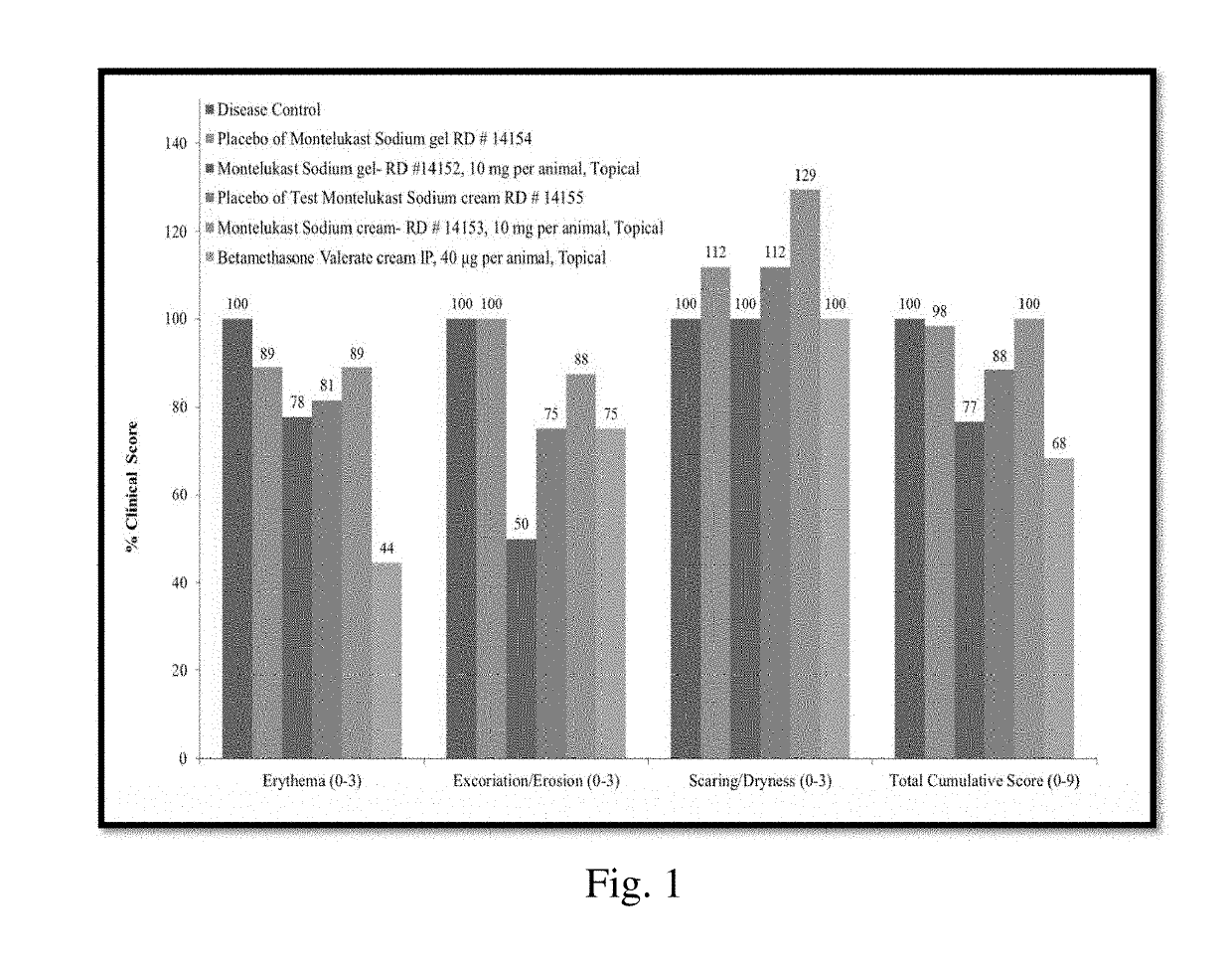
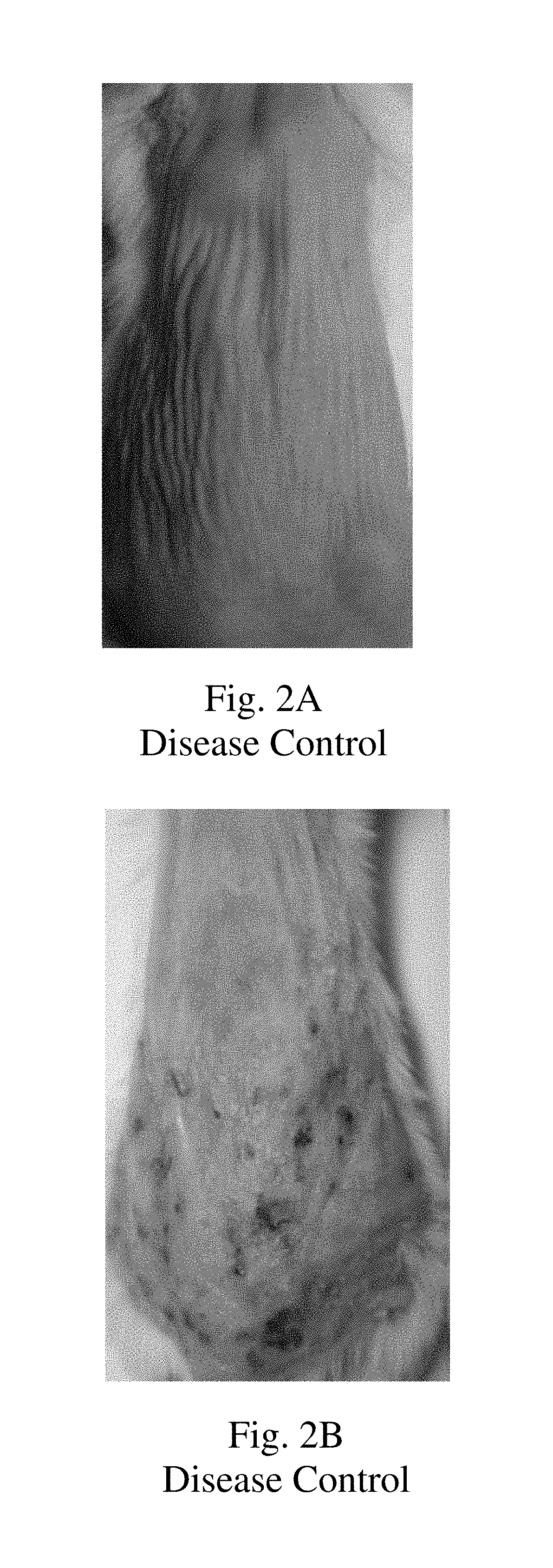
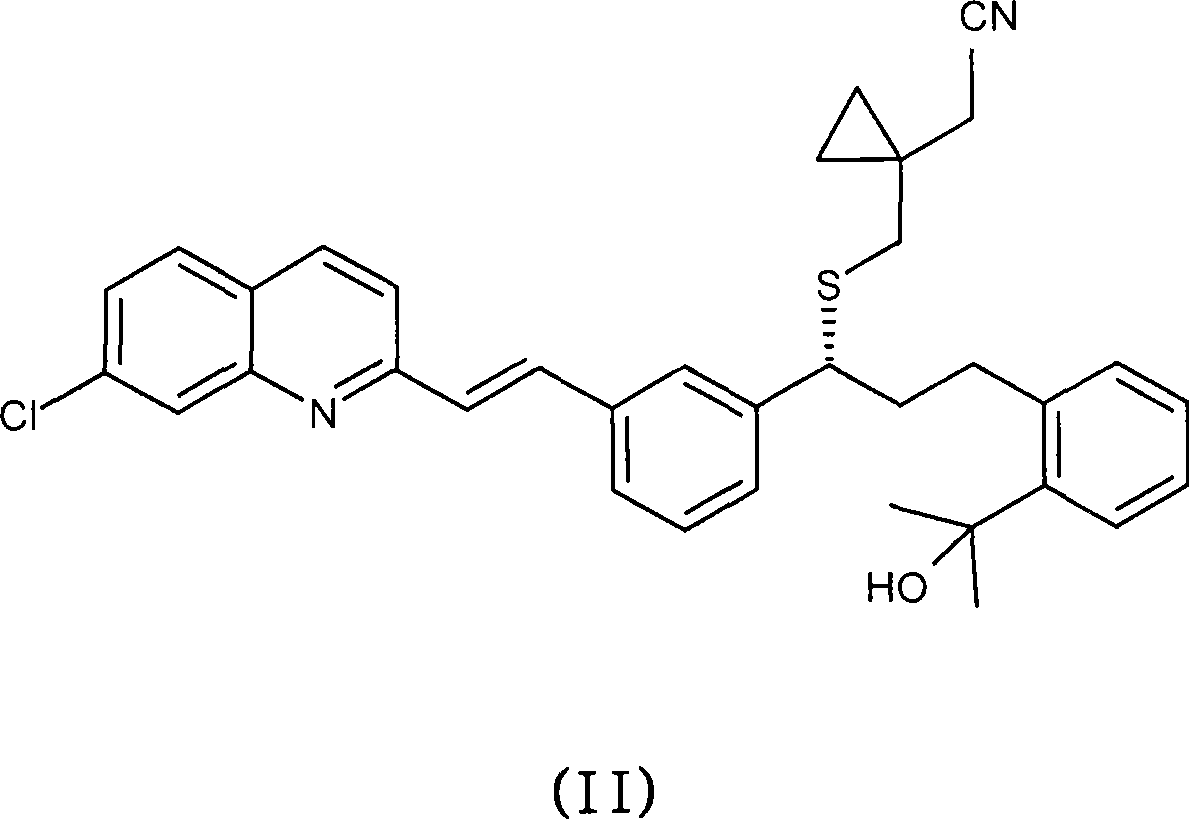
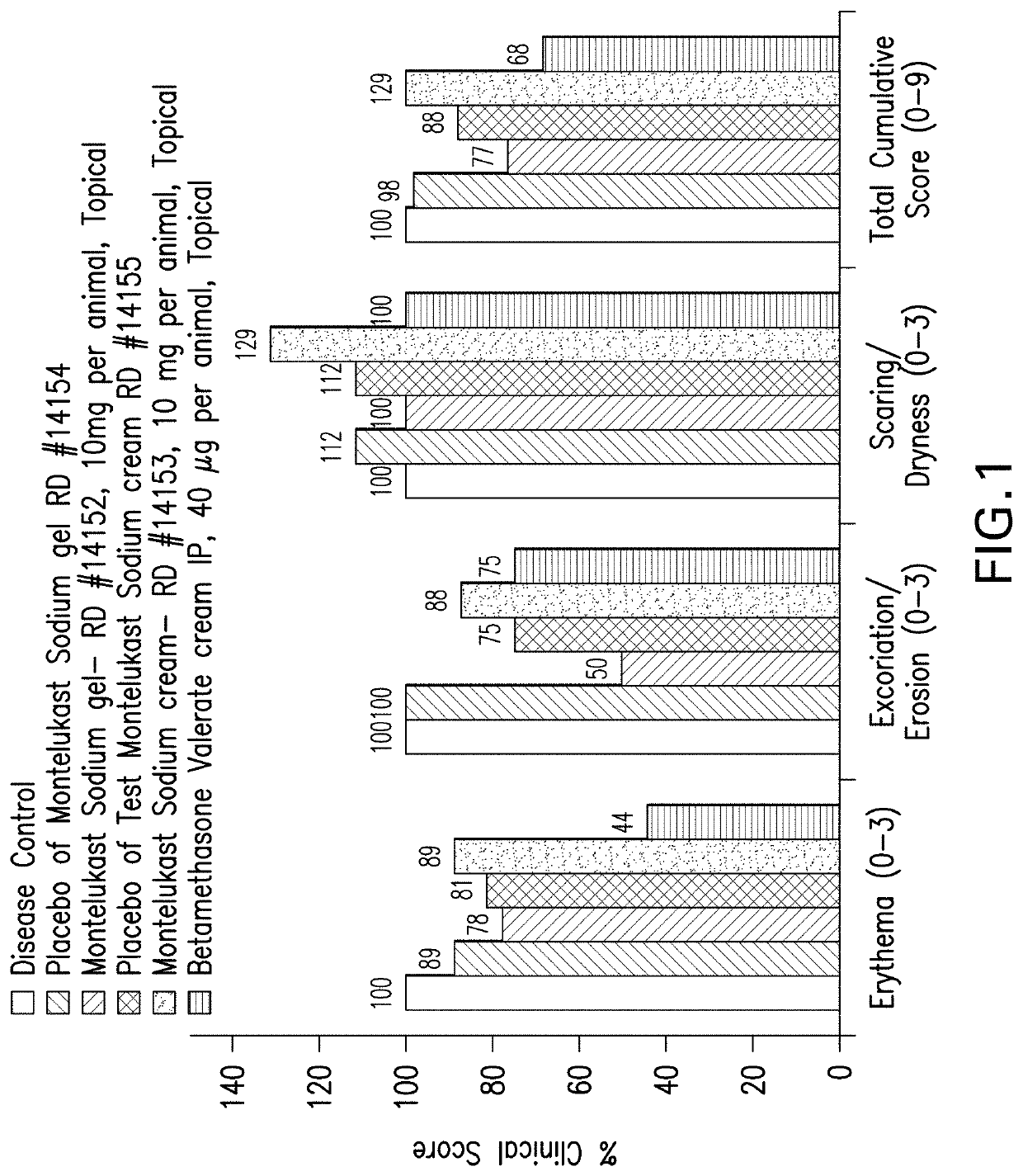
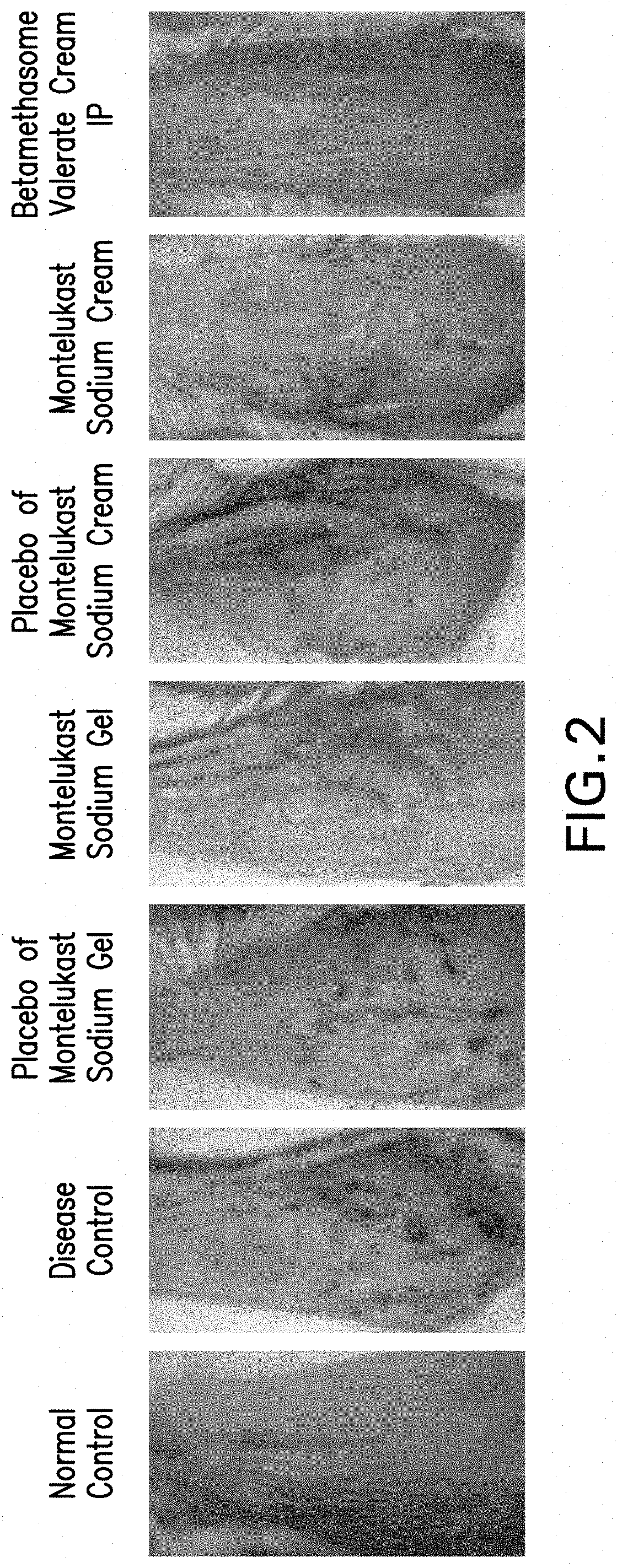
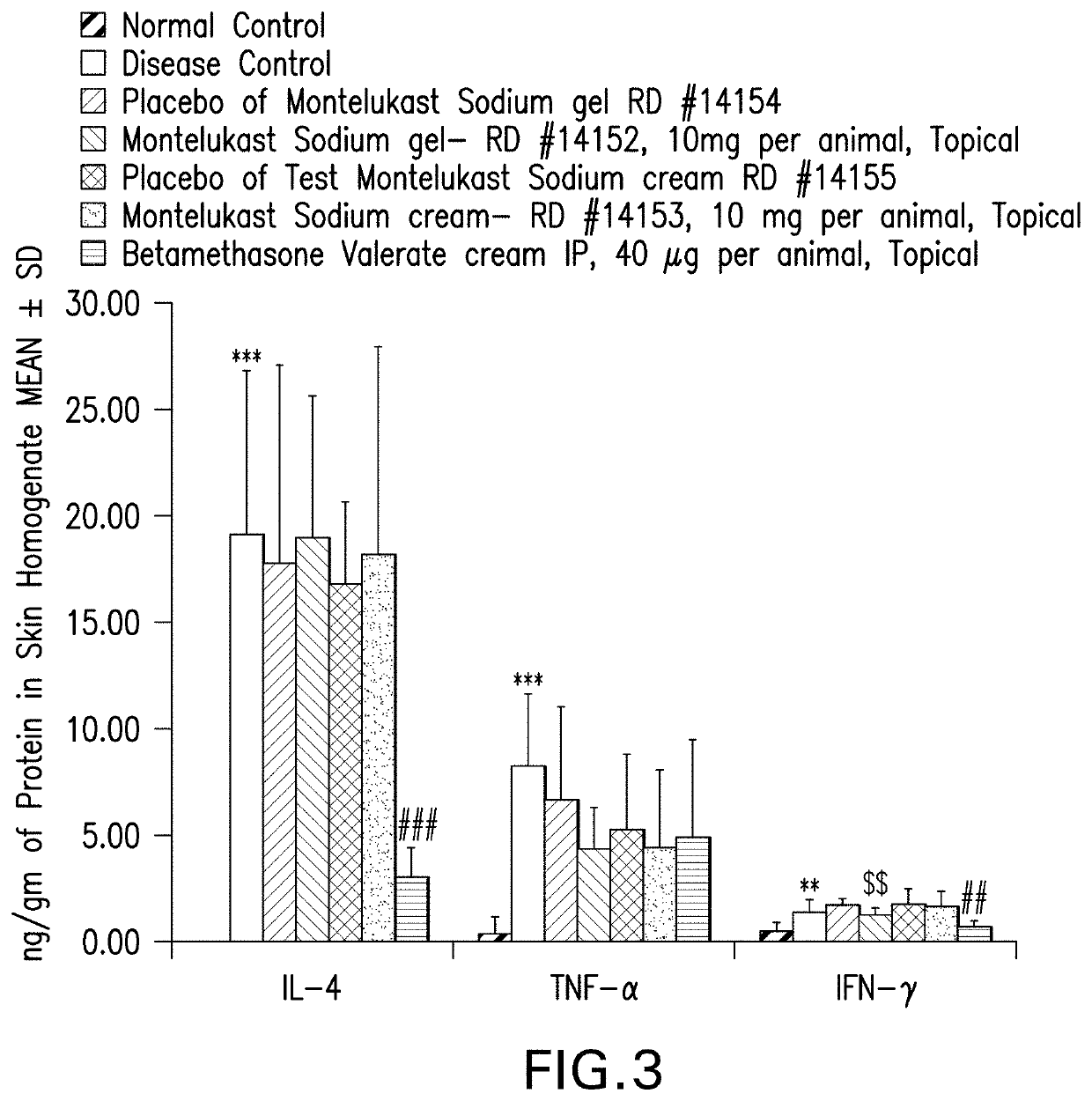
Topical montelukast for treatment of atopic dermatitis
A topical formulation comprising Montelukast or a pharmaceutically acceptable salt thereof, a gelling agent and water for the treatment of atopic dermatitis.
Owner:TARO PHARMA INDS
Process for making montelukast and intermediates therefor
A process for making montelukast, a pharmaceutically useful compound of the following formula and salts thereof:using a compound of formula (11)is provided.
Owner:SYNTHON BV
Stable montelukast oral rapidly disintegrating film as well as preparation method and application thereof
InactiveCN105878215AImprove stabilityOrganic active ingredientsRespiratory disorderMontelukastMedicine
The invention belongs to the technical field of medicine, and in particular relates to a stable oral instant montelukast film and its preparation method and application, wherein the film-forming material is a mixture of sodium hydroxypropyl methylcellulose and polyvinyl alcohol in a certain proportion . The product has high stability and is suitable for large-scale industrial production.
Owner:天津康鸿医药科技发展有限公司
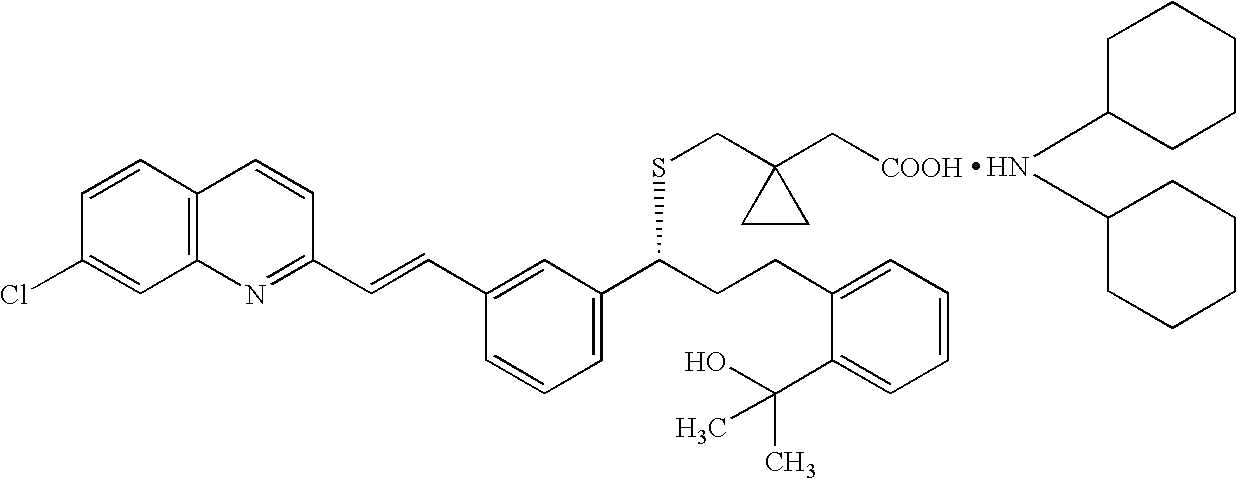
Montelukast amantadine salt
An amantadine salt of montelukast is useful in purification of montelukast or its salts and as a pharmaceutical active ingredient.
Owner:SYNTHON BV
Purification process of Montelukast and its amine salts
InactiveUS8188285B2High optical purityEasy to industrializeOrganic active ingredientsOrganic chemistryMontelukastCombinatorial chemistry
It comprises a process for the purification of Montelukast, or its salts or its solvates, including any stereoisomer or mixture thereof, which comprises converting Montelukast acid or a solvate thereof, including any stereoisomer or mixtures thereof, into an amine salt selected from the group consisting of tris-(hydroxymethyl)aminomethane, L-(+)-treo-2-amino-1-phenyl-1,3-propanediol, and L-(+)-α-phenylglycinol salt, in the presence of an appropriate solvent. It also comprises novel salts of Montelukast, in particular, tris-(hydroxymethyl)aminomethane, L-(+)-treo-2-amino-1-phenyl-1,3-propanediol, and L-(+)-α-phenylglycinol salts.
Owner:ESTEVE QUIMICA
Process for the purification of Montelukast
Owner:ESTEVE QUIMICA
Topical montelukast for treatment of atopic dermatitis
Owner:TARO PHARMA INDS
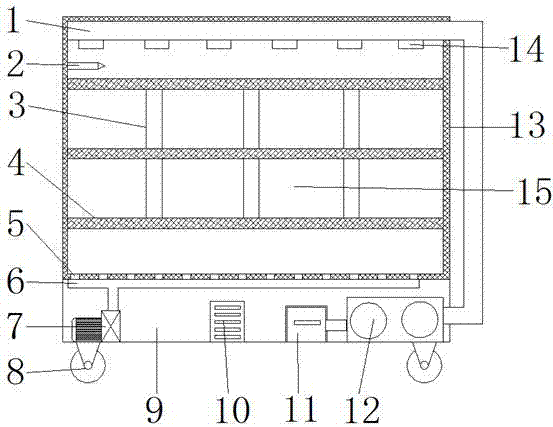
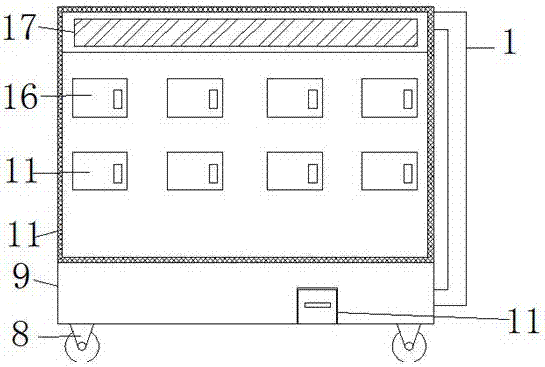
Montelukast storage cabinet with functions of dust removing and drying
PendingCN107455954AReduce humidityEasy to control humidityChestsDressing tablesMontelukastDust control
The invention discloses a Montelukast storage cabinet with functions of dust removing and drying. The Montelukast storage cabinet with the functions of dust removing and drying comprises a base, wherein universal wheels are fixed at all four corners of the outer wall of the bottom of the base through bolts, and a fan heater is fixed at one end of the inner wall of the bottom of the base through a bolt; the air outlet end of the fan heater is sleeved with an air outlet pipe, and an air outlet is formed in the outer wall of the top of the air outlet pipe; the inner wall of the air outlet is sleeved with an air outlet mouth, and a vacuum cleaner is fixed at the other end of the inner wall of the bottom of the base through a bolt; the air inlet of the vacuum cleaner is sleeved with a suction pipe, and an opening is formed in the outer wall of one side of the base. According to the Montelukast storage cabinet with the functions of dust removing and drying, the fan heater is controlled by adding a temperature humidity sensor and a controller, so that control over the humidity in the cabinet is facilitated, and a dry storage environment for Montelukast is ensured; the air in the cabinet can be purified by adding an air cleaner, so that the dry storage environment for Montelukast is ensured, and the storage cabinet has the functions of dust removing and drying, and is suitable for storing Montelukast.
Owner:盐城方正医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com