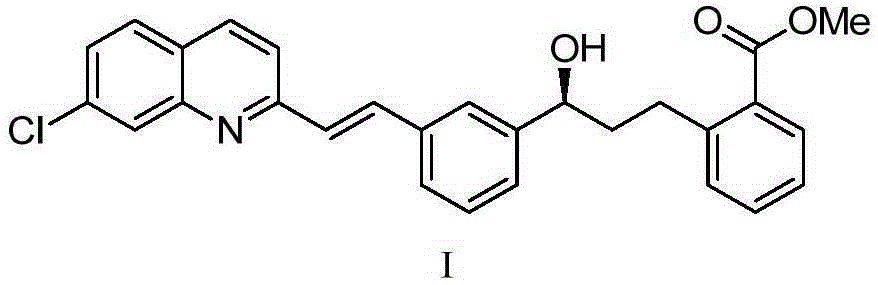
Method for preparing montelukast nano chiral alcohol intermediate
A technology of intermediates and chiral alcohols, which is applied in the field of preparation of Montelus Turner chiral alcohol intermediates, can solve the complex operation of chiral oxazolidine and borane reducing agent, and the cost of chiral oxazolidine High, unfavorable industrial production and other issues, to achieve the effect of favorable industrial production, good commercial value, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a kind of preparation method of Montelus Turner chiral alcohol intermediate, comprising the following steps:
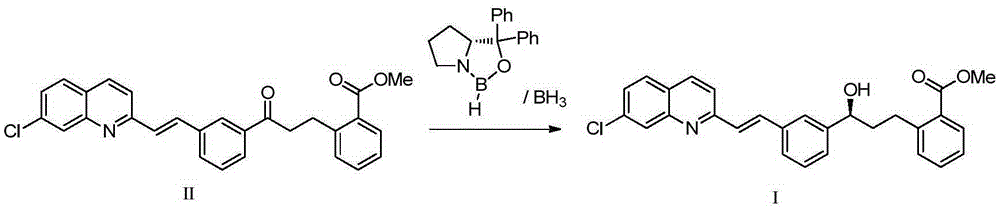
[0031] Provide reaction raw material 2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]methyl benzoate (II);
[0032] Provide transition metal complexes as catalysts, with NH2-N(sp 2 ) or nitrogen phosphine transition metals formed by coordination of ligands with NH2-NH2 structure characteristics and transition metals. The general formula of the transition metal complex is MLnL'XY, wherein M is Ru, X is chlorine, bromine, iodine or hydrogen, and Y is chlorine, bromine, iodine or BH 4 , L, L' are R configuration, S configuration or racemate, n=1 or 2, when n=1, L is BINAP, MeO-BIPHEP, DIOP or SegPhos; when n=2, L is P(C 6 h 5 ) 3 or P(C 6 h 4 -4-CH 3 ) 3 , L' is DPEN, DAIPEN,
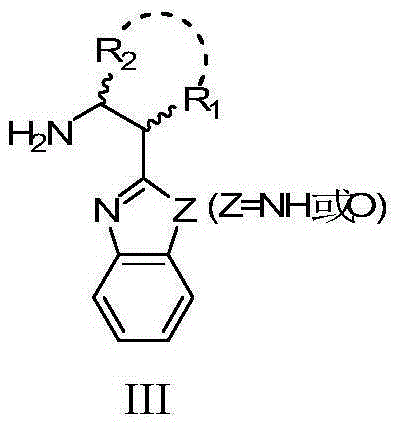
[0033] 1,2-Cyclohexanediamine, or L' has structure III:
[0034]
[0035] In the structure III, Z is NH or O, R 1 , R 2 It is hydrogen or a ...
Embodiment 1
[0044] The transition metal complex catalyst adopts structure IV; the base adopts alkoxy base, such as potassium tert-butoxide; the solvent adopts an aprotic solvent, such as toluene. Reaction materials 2-[3-[3-[2-(7-Chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]benzoic acid methyl ester (Ⅱ) and transition metal complexation The molar ratio of the substance is 20000. The reaction formula is as follows:
[0045]
[0046]
[0047]
[0048] In a 100L stainless steel autoclave, add 2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]methyl benzoate, toluene , at N 2 Under the atmosphere, add the catalyst [RuCl 2 ((S,S)-DIOP){(1H-benzo[d]imidazol-2-yl)ethanamine}] and potassium tert-butoxide; after hydrogen replacement, fill with H 2 To 5 atm, 100 ℃ of stirring reaction, when the hydrogen pressure is constant (about 4 hours), stop stirring, the H in the reactor 2 Empty, the reaction solution is sampled, carry out conventional post-treatment (filtration, centrifuga...
Embodiment 2
[0050] The transition metal complex catalyst adopts structure V; the base adopts triethylamine; the solvent adopts tetrahydrofuran. Reaction materials 2-[3-[3-[2-(7-Chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]benzoic acid methyl ester (Ⅱ) and transition metal complexation The molar ratio of the substance is 240000. The reaction formula is as follows:
[0051]
[0052]
[0053] In a 100L stainless steel autoclave, add 2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-oxopropyl]methyl benzoate and tetrahydrofuran , at N 2 Under the atmosphere, add the catalyst [RuCl 2 ((S)-SegPhos){(1H-benzo[d]imidazol-2-yl)ethanamine}] and triethylamine; after replacing hydrogen, fill with H 2 To 10atm, 30 ℃ of stirring reaction, when the hydrogen pressure is constant (about 8 hours), stop stirring, the H in the reactor 2 The reaction solution was vented, and the reaction solution was sampled for conventional post-treatment to obtain a yellow solid product, which was detected by liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com