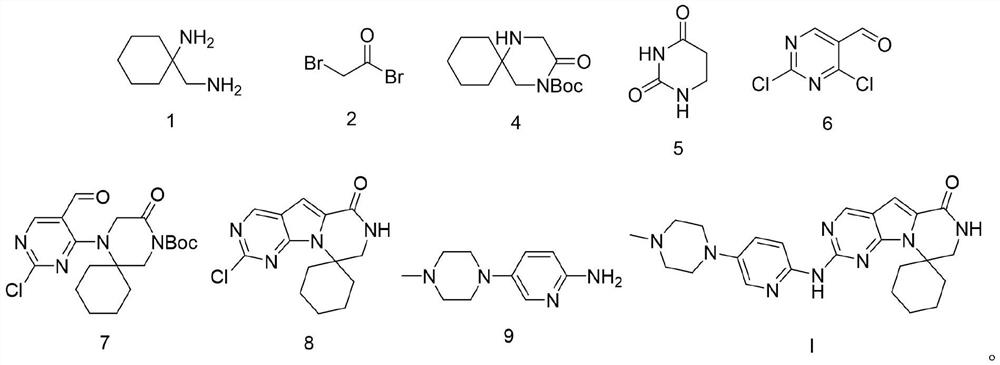
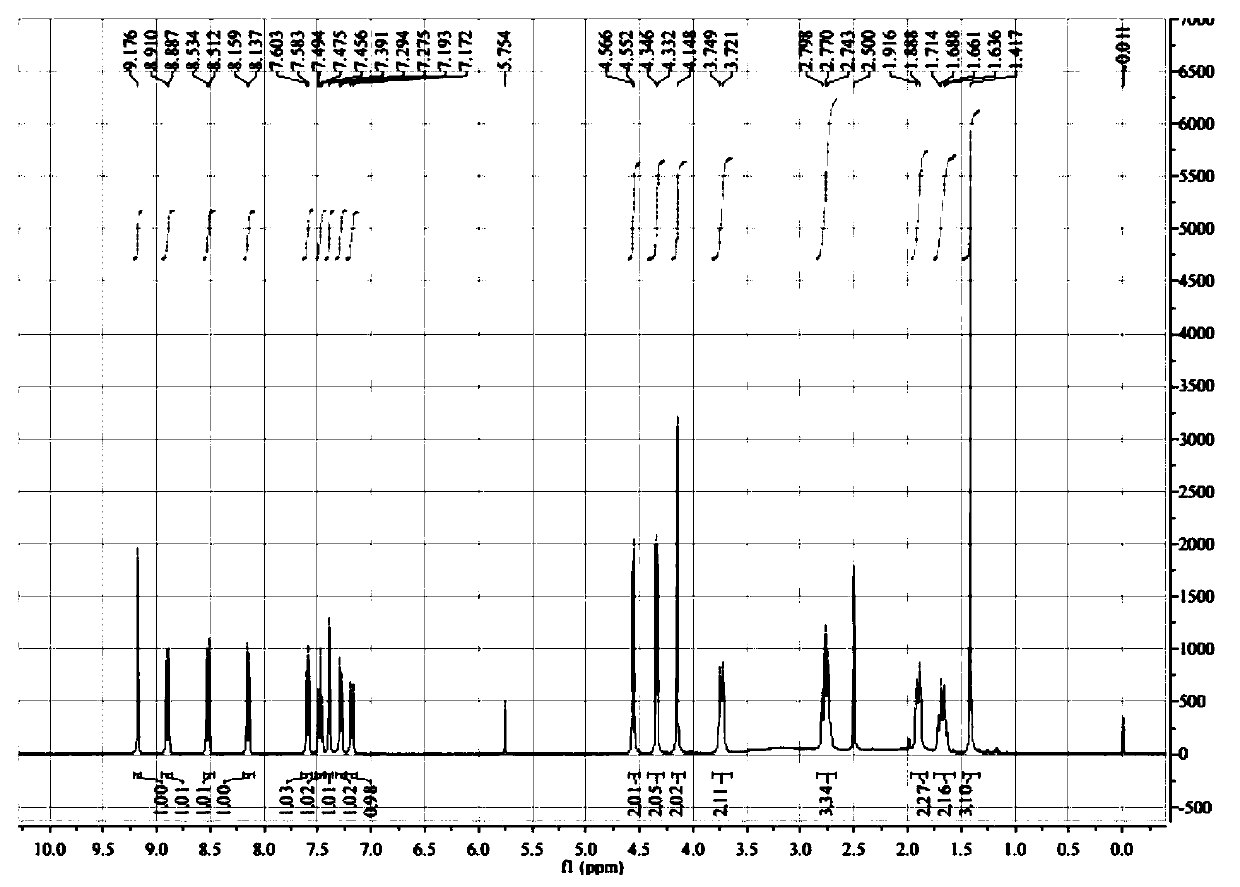
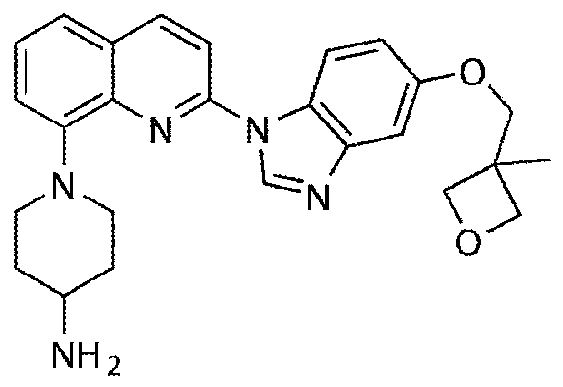
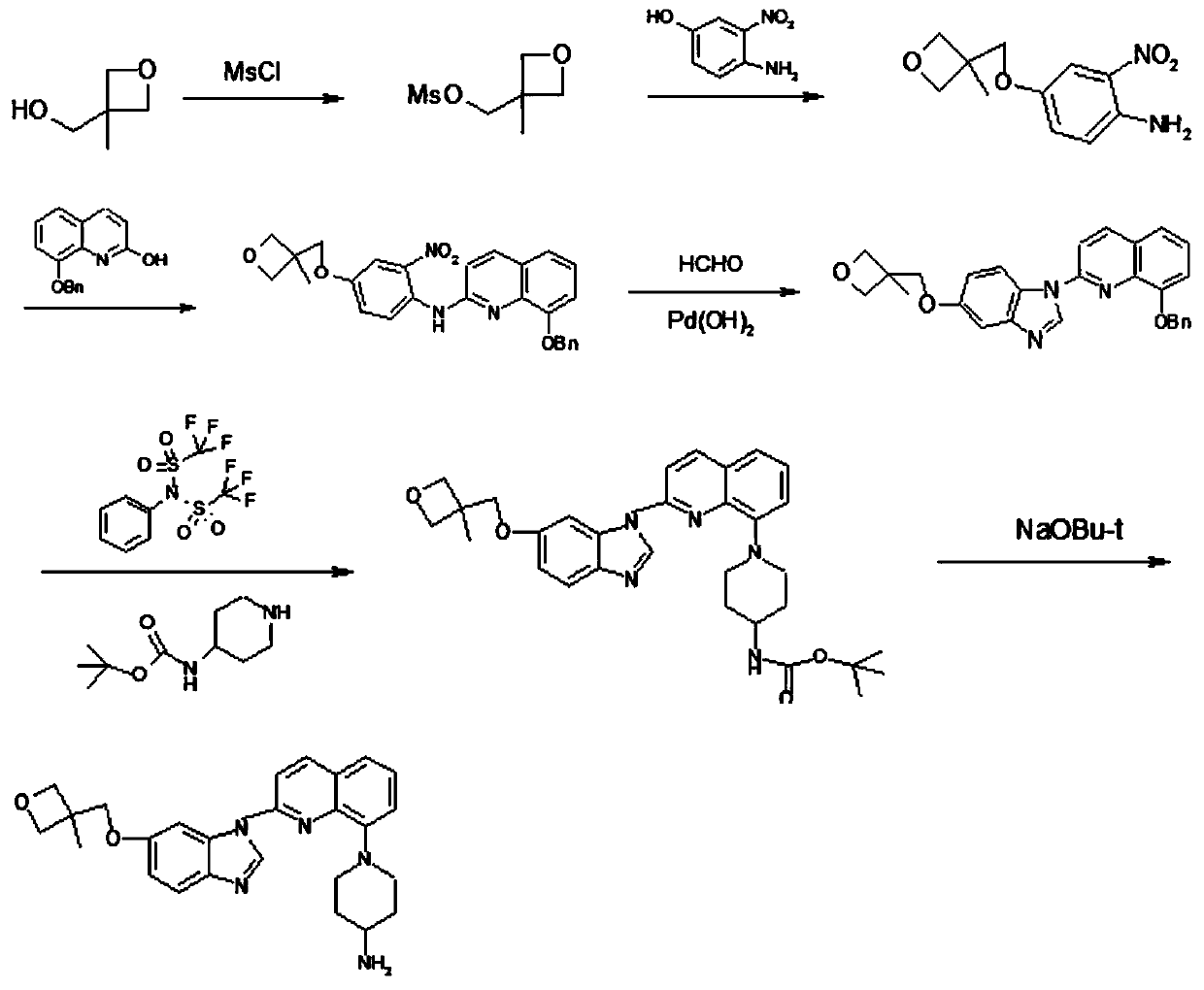
Patents
Literature
51 results about "BINAP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
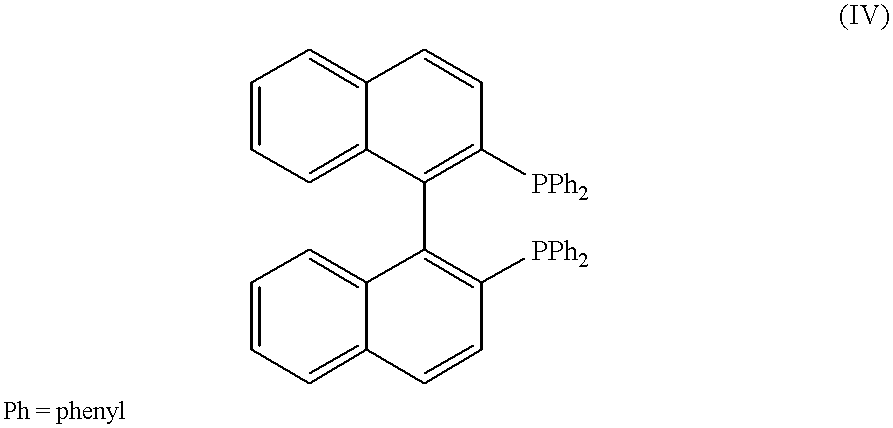
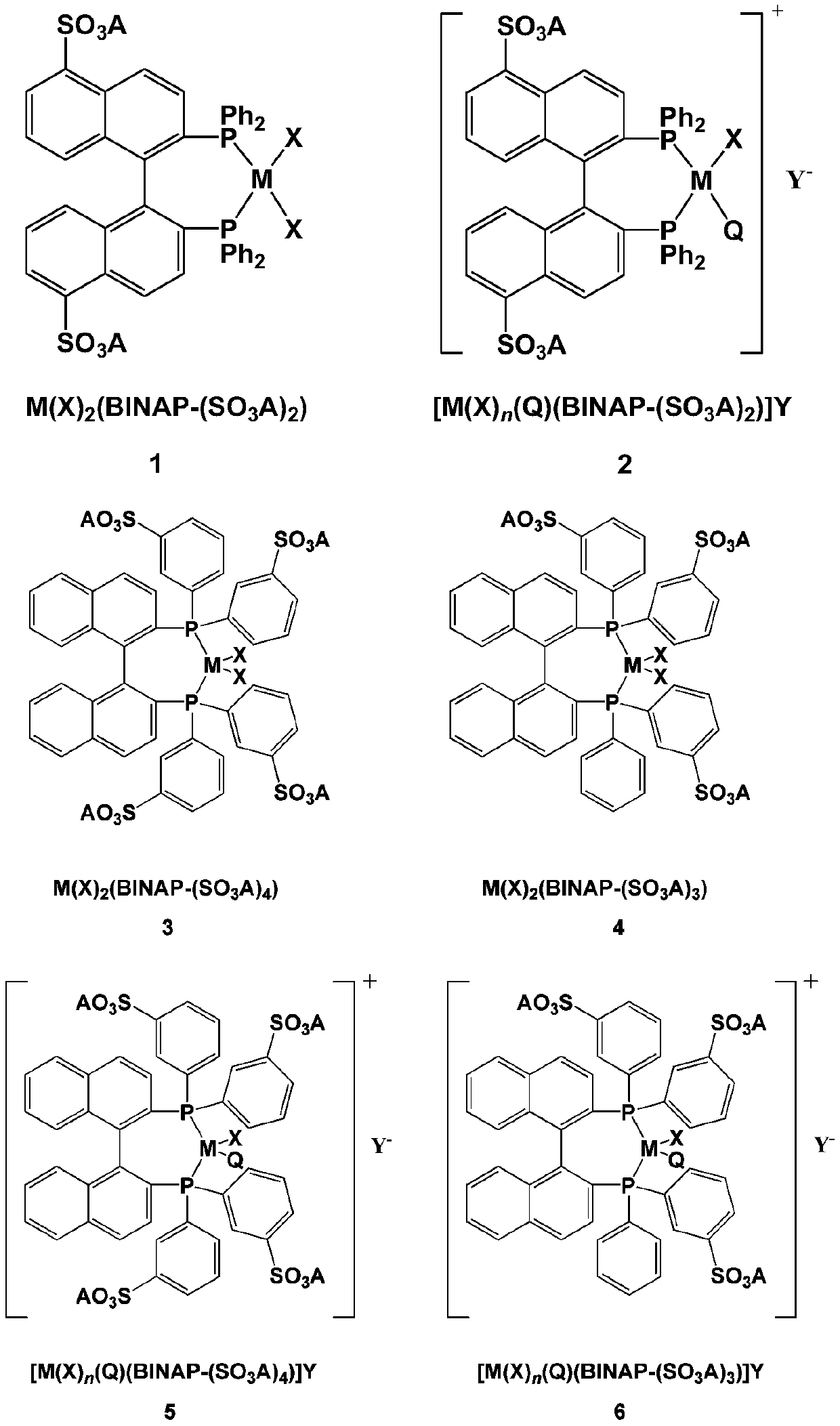
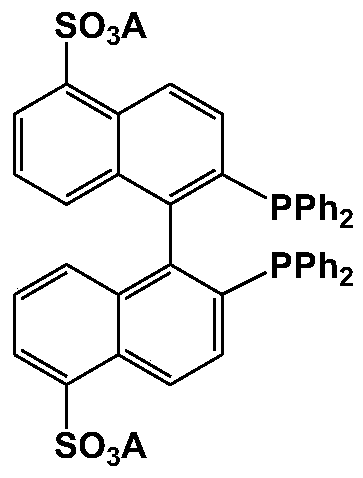
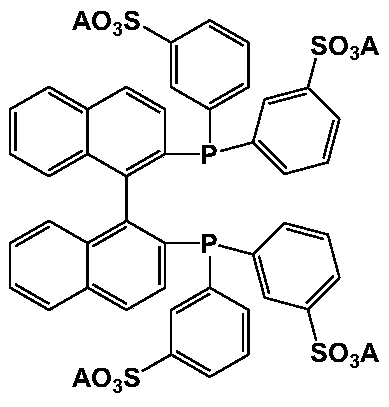
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1′ positions. This C₂-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle between the naphthyl groups is approximately 90°. The natural bite angle is 93°.
Applications of chiral polymer catalyst in asymmetric reaction
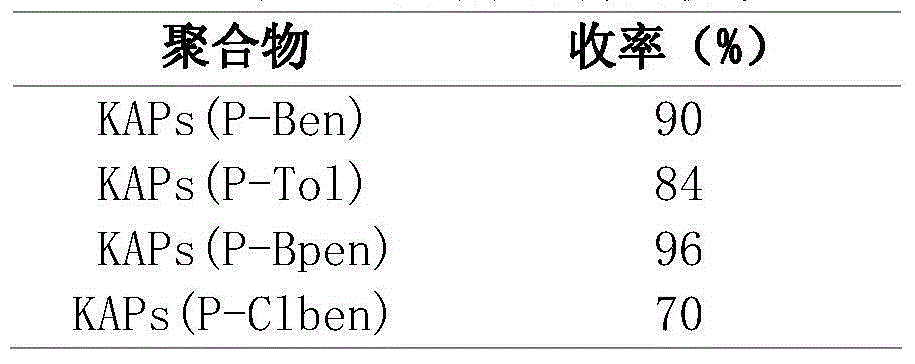
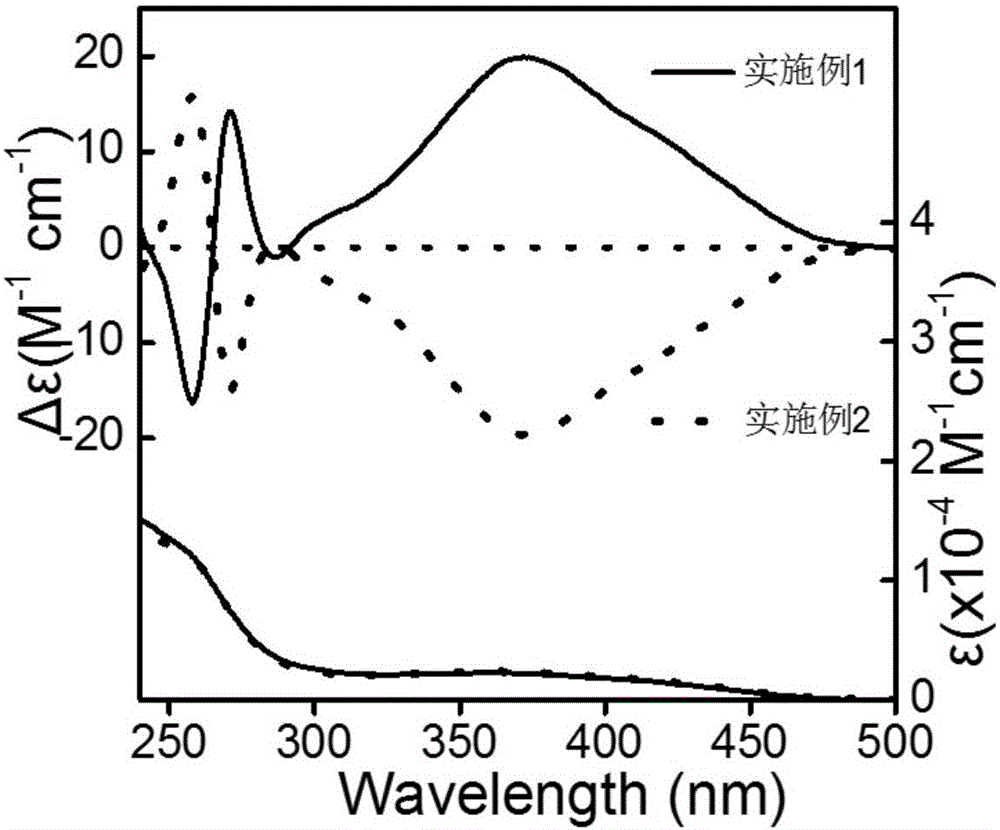
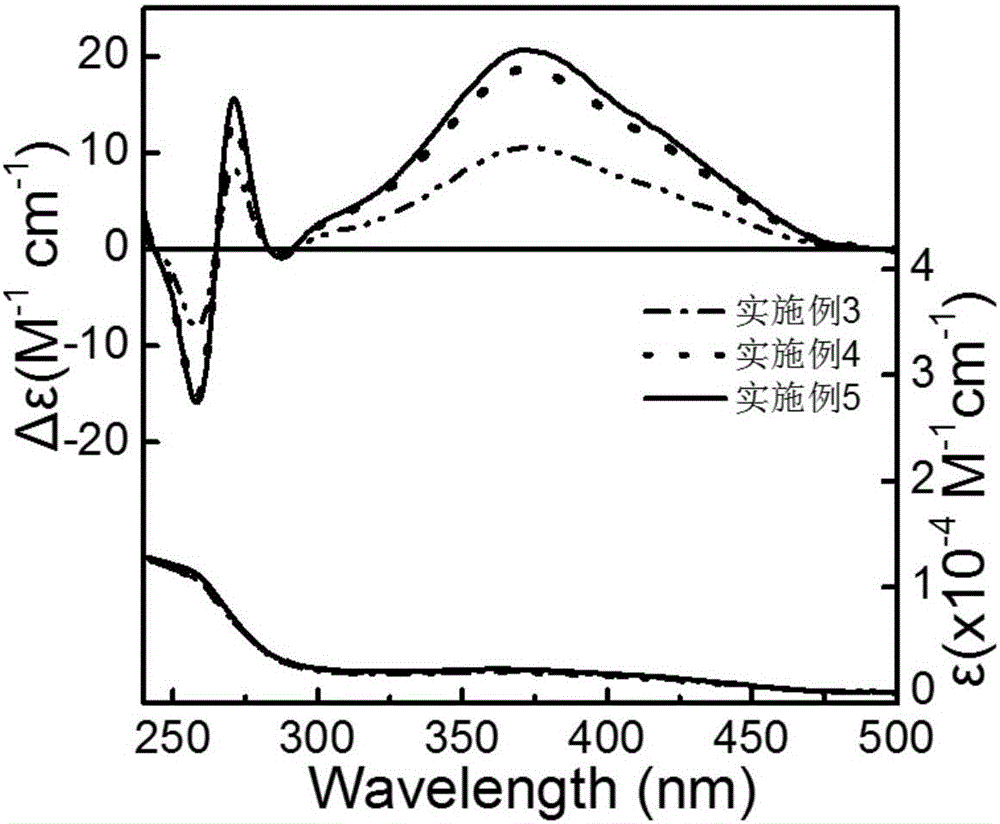
ActiveCN106853380AThe synthesis method is simpleShort reaction timeOrganic compound preparationOrganic chemistry methodsDispersityPorosity
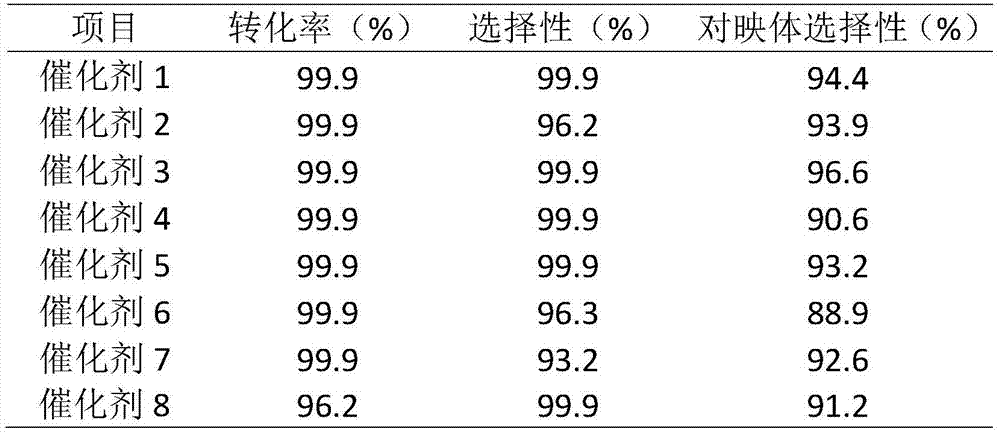
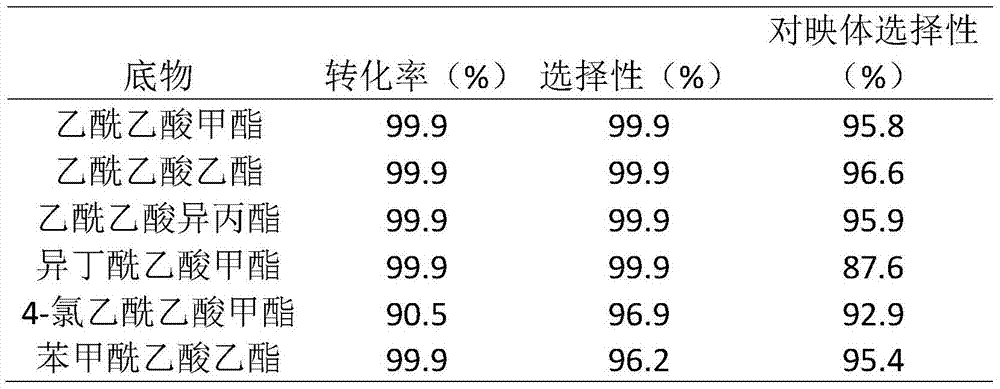
The invention discloses applications of a chiral polymer catalyst in asymmetric reaction, and belongs to the field of material synthesis and application. The phosphine-containing polymer is obtained via mixed polymerization of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) and derivative of vinyl-containing chiral bidentate phosphine ligand BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) with other vinyl comonomers. The vinyl polymer possesses relatively large specific surface area and porosity, and excellent thermal stability and chemical stability. In the heterogeneous catalyst, one or a plurality of elements selected from Ru, Rh, Ir, Pa, Au, and Cu are taken as active ingredients. The chiral ligand is uniformly embedded into and highly dispersed in a polymer skeleton, so that metal dispersion degree on the catalyst is relatively high, and relatively high catalytic activity is achieved. The heterogeneous catalyst is suitable for a plurality of reaction technology including intermittent still reaction, continuous fixed bed reaction, and trickle bed reaction. When the heterogeneous catalyst is used in catalytic kettle-type asymmetric hydrogenation, high target product yield is achieved, and enantioselectivity is higher than 96%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chiral diphosphine ligand and chiral catalyst, and preparation and application method thereof
ActiveCN103059064AHigh affinityReduced activityOrganic compound preparationGroup 5/15 element organic compoundsCyclic processDiphosphines
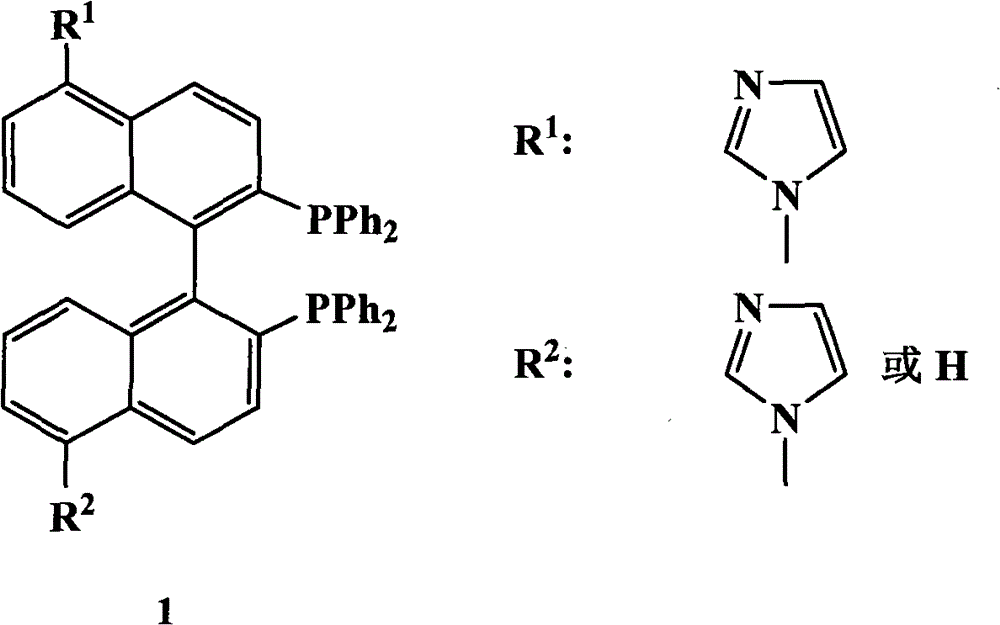
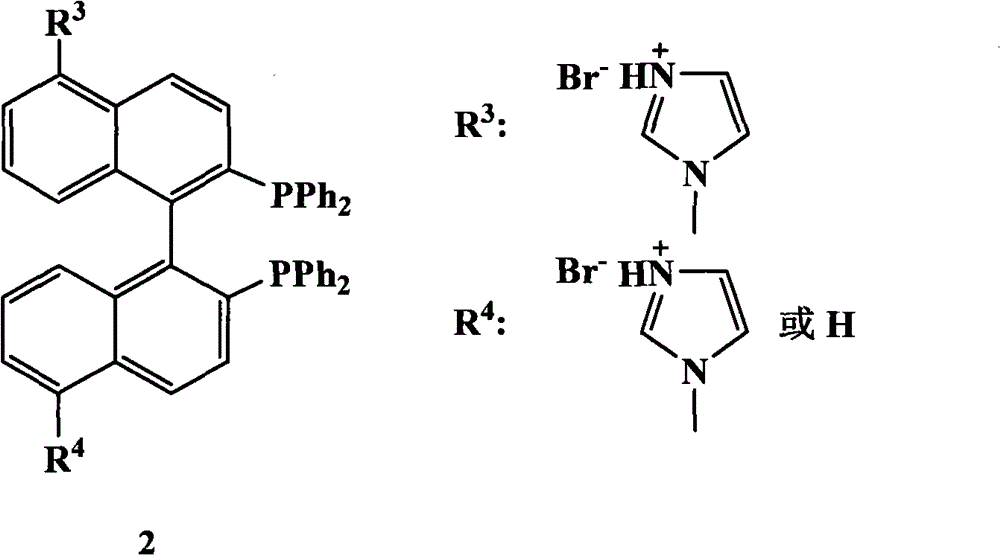
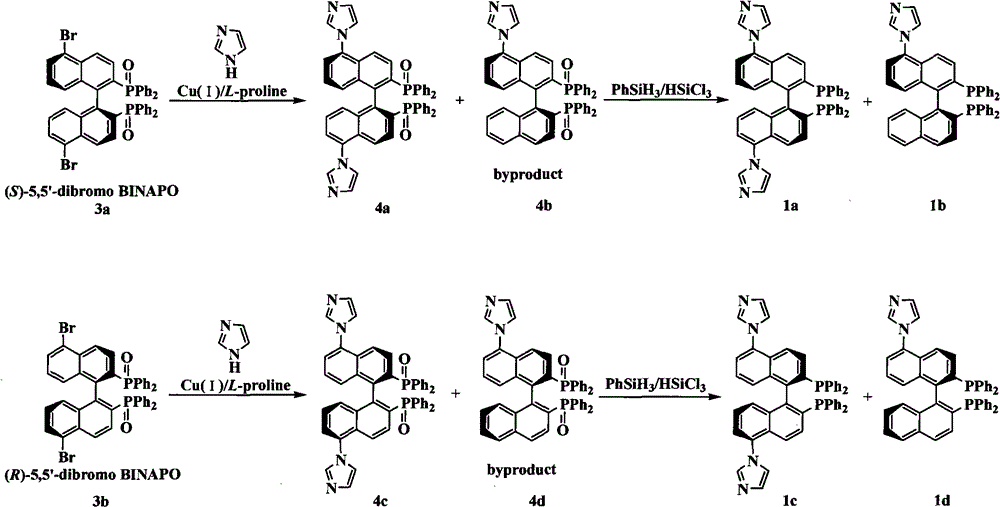
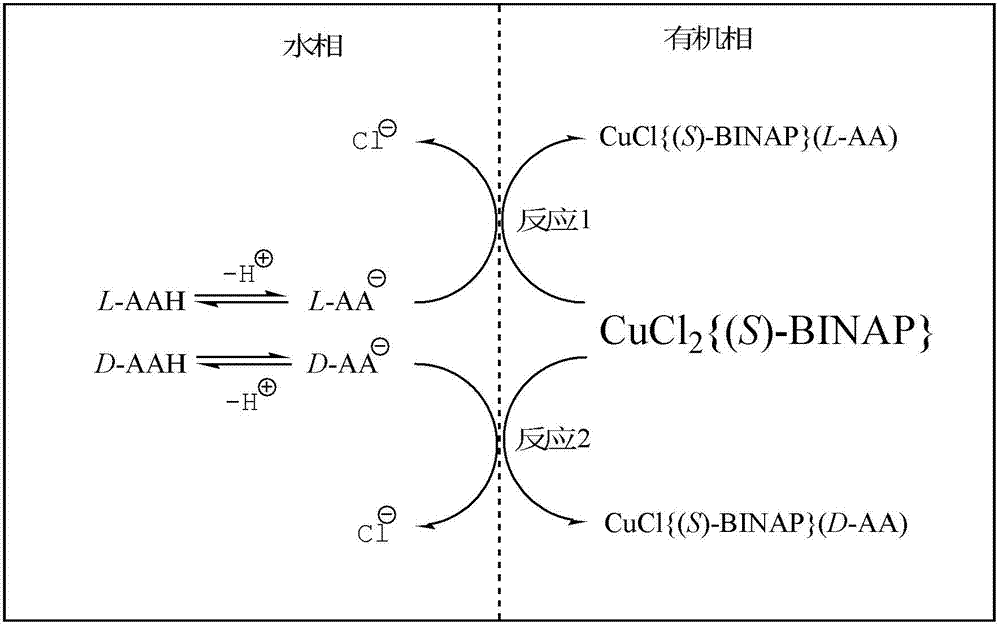
The invention relates to a chiral diphosphine ligand and a chiral catalyst, and a preparation and application method thereof. The preparation method of a BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) chiral diphosphine ligand based on novel imidazole and imidazole cation modification. Imidazole and imidazole cation are introduced to the 5,5'- position of the BINAP molecule framework to synthesize the chiral diphosphine ligand. The assembly of the imidazole and imidazole cation into the chiral diphosphine ligand molecule can effectively enhance the stability of the chiral catalyst in an ionic liquid and the affinity with the imidazole ionic liquid, and avoids loss of the catalyst in the cyclic process. The imidazole-modified chiral diphosphine ligand has the following chemical structural formula, wherein the spatial configuration of the imidazole-modified chiral diphosphine ligand is S type or R type.
Owner:山东聚强绿洲生物科技有限公司
Method for preparing aryl ketone
ActiveCN102153434AMild reaction conditionsReduce usageCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystRuthenium Compounds
The invention relates to the field of catalysis, in particular to a method for preparing aryl ketone through reacting aldehyde with aryl boric acid under the catalysis of a ruthenium catalyst. In the method, an organic phosphide is used as a ligand, potassium phosphate is used as alkali, pinacolone or acetone is used as an additive, toluene or / and water is (are) used as a solvent(s), aldehyde and aryl boric acid which are used as reaction substrates react at 95-100 DEG C for 10-24h in the presence of a ruthenium compound used as a catalyst to prepare aryl ketone, wherein the catalyst is one of [Ru(cymene)Cl2]2, [Ru(CO)3Cl2]2, RuH2(CO)PPh3, Ru2(OAc)4, [Ru(benzene)Cl2]2, Ru(S-BINAP)Cl2 or Ru3(CO)12. In the invention, the used catalyst has relatively low price and low toxicity, thereby reducing the preparation cost and being more environmentally friendly.
Owner:铜陵市官作文化有限公司
New method for separation of phenylalanine enantiomer by reaction extraction
InactiveCN102702003ALow priceEasy to useOrganic compound preparationAmino-carboxyl compound preparationPhenylalanineOrganic chemistry
The invention discloses a new method for separation of phenylalanine enantiomer by reaction extraction, which uses (S)-BINAP-copper complex as chiral extractant in the organic phase to extract and separate phenylalanine enantiomer from the water phase. The invention discloses a preparation method for (S)-BINAP-copper complex. (S)-BINAP-copper complex has good complexing capability and high enantiomer selectivity to phenylalanine enantiomer, and the separation factor alpha is up to over 5.0.
Owner:唐课文 +1
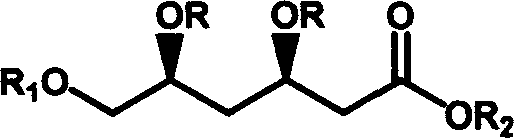
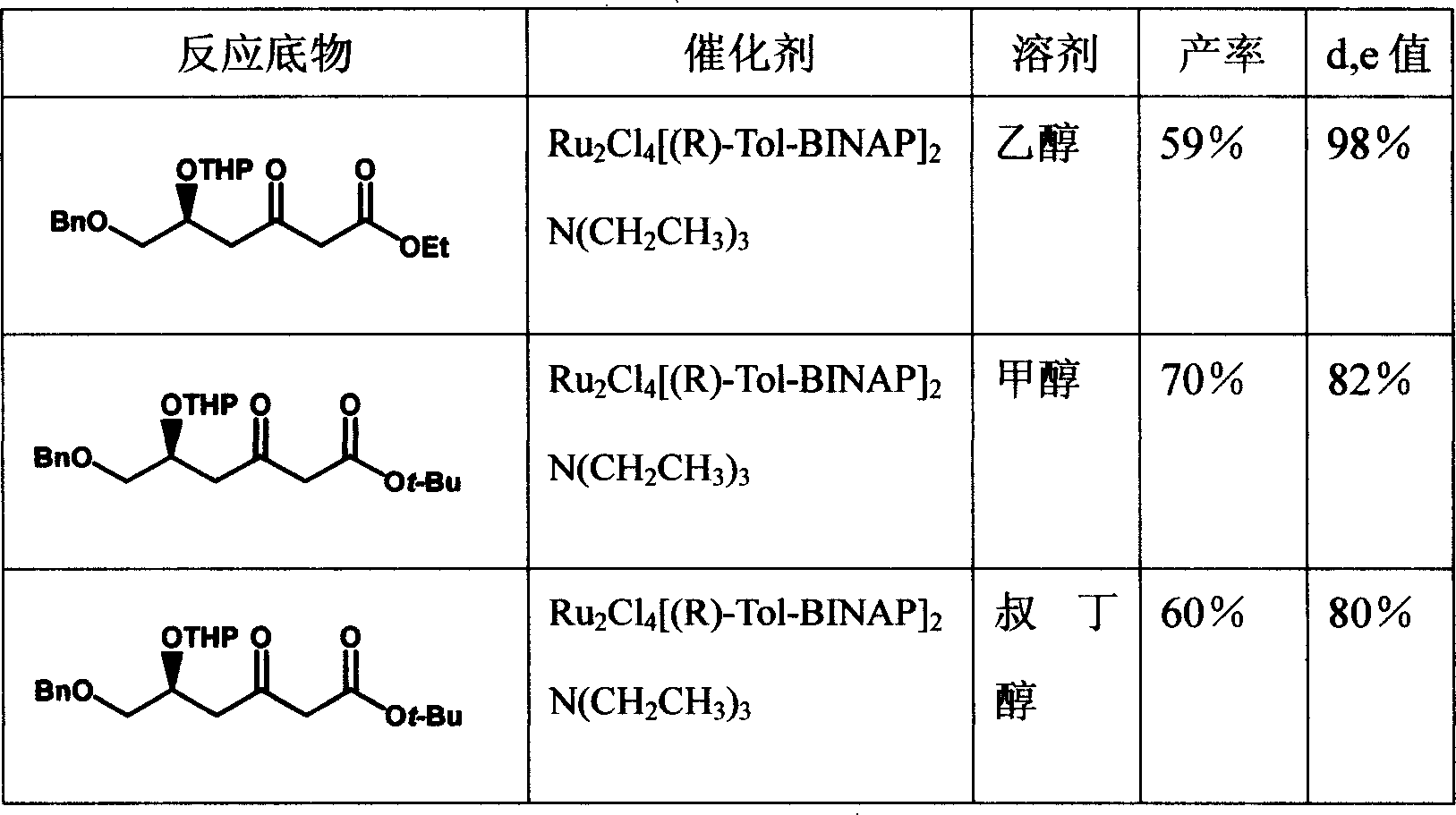
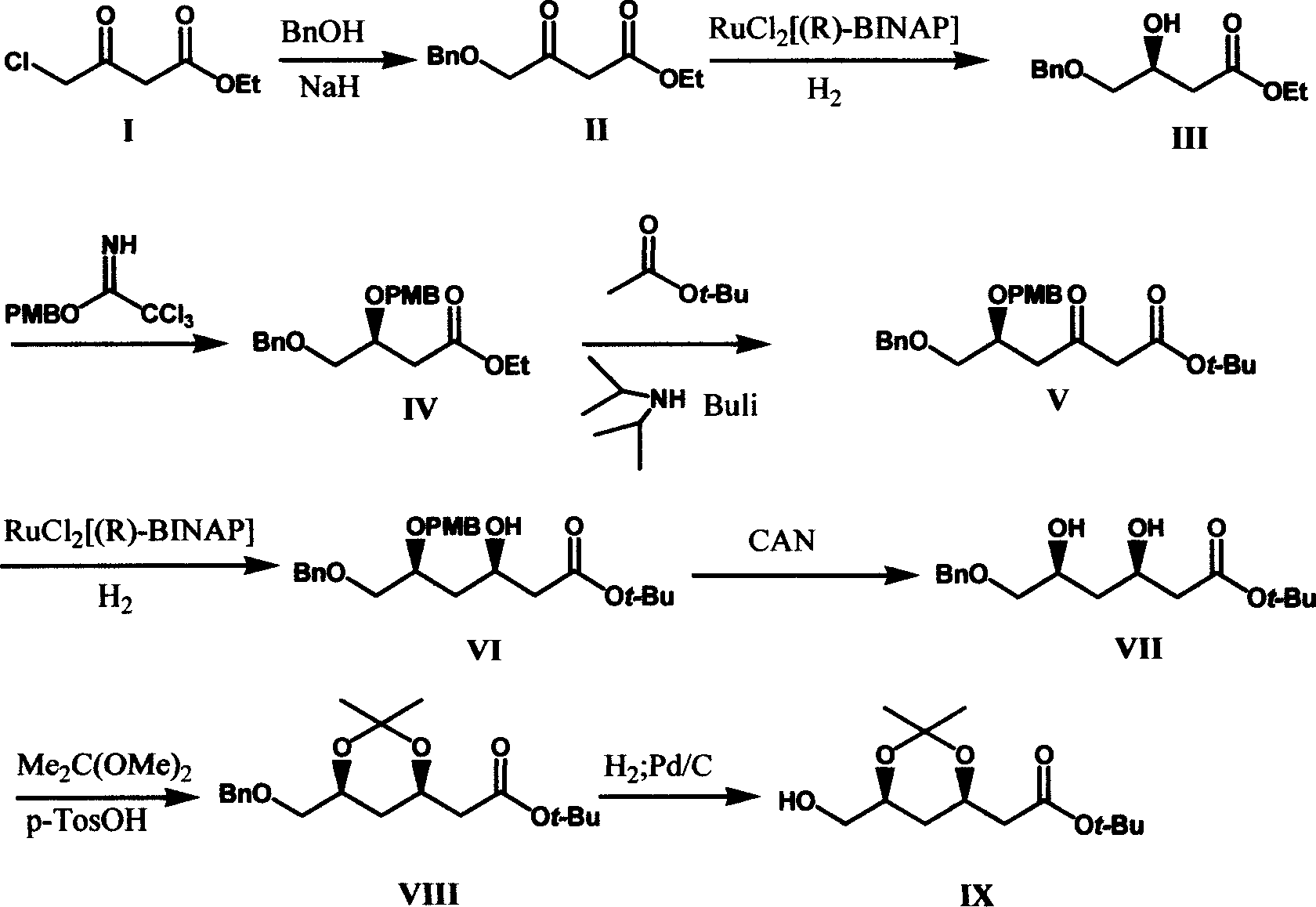
Method for preparing t-butyl (3R, 5S)-3,5,6-trihydroxy-hexanoate
The invention relates to a method for preparing (3R, 5S) - 3, 5, 6- trihydroxy- butylacetic acic tertbutyl ester, belonging to medical intermediate preparation technique. It comprises following steps: taking ethyl chloroacetate as raw material, oxides it with benzyl, catalyzing and hydrogenizing with RuCl2[(R)-BINAP] and getting(3S)- 3- hydroxy- 4- benzyloxy butyl acetate, employing methoxy benzyl to protect hydroxy, carryingout condensation reaction with Claisen ester to increase carbon chain, catalyzing and hydrogenizing the carbonyl at No. 3 carbon with RuCl2[(R)-BINAP] and getting (3R, 5S)- 5- p-methoxy benzyloxy- 6- benzyloxy- 3- hydroxy- butylacetic acic tertbutyl ester; removing p-methoxy benzyloxy with ammonium cerous nitrate, protecting with acetone, removing benzyl and getting (3R, 5S)- 6- hydroxy- 3, 5- 0- isopropylidene- 3, 5- dihydroxy butylacetic acic tertbutyl ester. The total productivity is 39.5%, d, e value is 99%. The cost is low and it is easy to industrialization.
Owner:SHANGHAI QINGSONG PHARMA
Optically-active helix chain poly(phenyl isocyanide) and polymerization method thereof
The invention discloses an optically-active helix chain poly(phenyl isocyanide) and a polymerization method thereof. The polymerization process is shown as the general formula in the description; in the formula, the structural formula of a chiral phosphine ligand is shown in the description. A palladium catalyst is adopted, the chiral phosphine ligand (S-or R-BINAP) is added to induce polymerization of achiral phenyl isocyanide, and the optically-active polymer with excessive one-handed helixes is obtained; obtained poly(phenyl isocyanide) has a high molecular weight and narrow molecular weight distribution.
Owner:HEFEI UNIV OF TECH
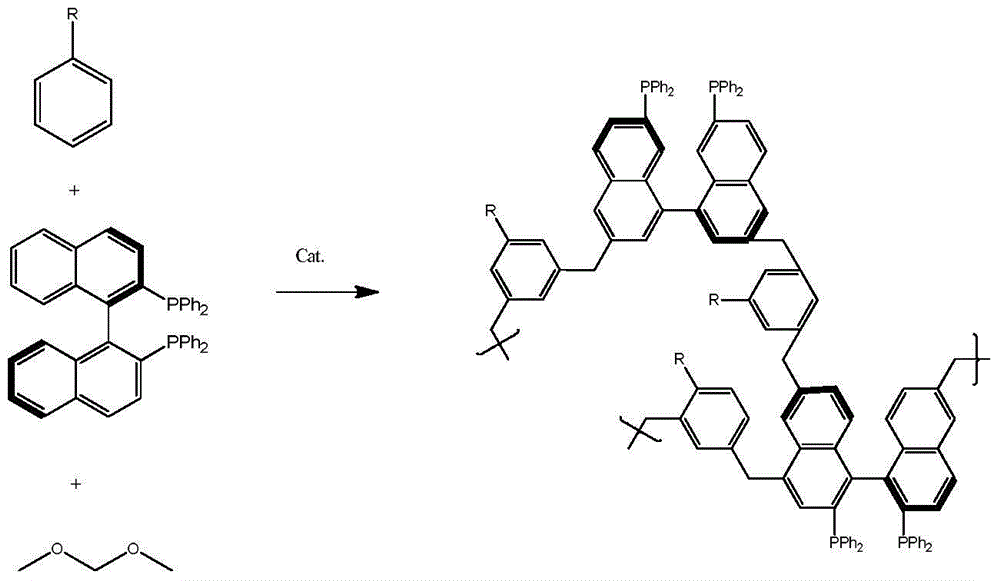
Preparation and application of multiphase asymmetric hydroformylation catalyst
ActiveCN108067307AThe synthesis method is simpleGood chemical stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHomogeneous catalysisPhosphine
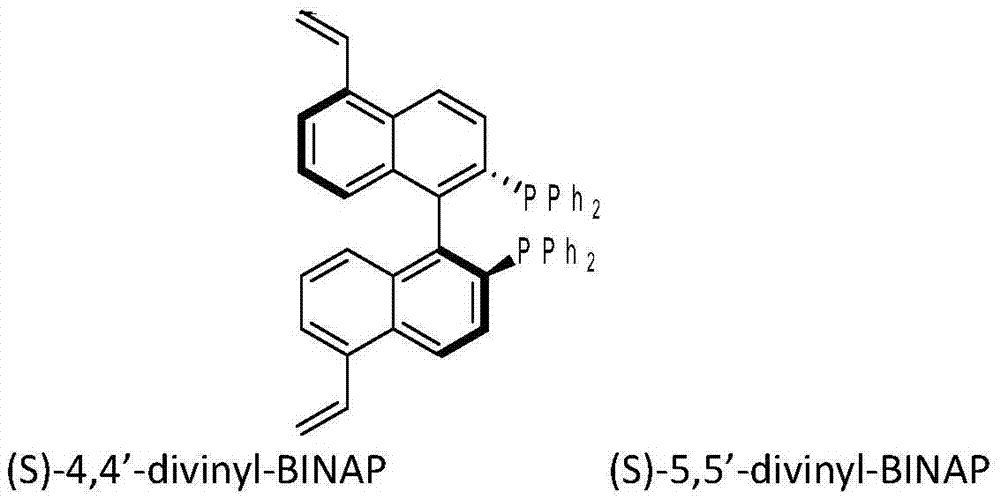
The invention discloses a preparation method of a multiphase asymmetric catalyst and belongs to the field of synthesis and application of functional materials. The catalyst is prepared from a metal component and a support. A preparation method of the catalyst support comprises the step as follows: a chiral bidentate phosphine ligand BINAP (5, 5'-divinyl-BIANP) containing vinyl is auto-polymerizedor is co-polymerized with other vinyl monomers with high steric hindrance; the metal component in the multiphase catalyst is Rh element. The periphery of the chiral phosphine ligand in a polymer skeleton is modified with other groups, a chiral pocket shown in the description is formed, so that catalytic performance of the multiphase catalyst in asymmetric hydroformylation can be improved, and enantio-selectivity of a product of the catalyst is increased by about 70% as compared with that of a homogeneous catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing polyimino-alcohol
InactiveCN101186680AImprove heat resistanceExcellent dynamic mechanical propertiesSynthesis methodsKetone
The invention relates to a synthesis method of polyuralcohol, belonging to polymer technical field, which comprises that adds dihalide aromatic ketone, sodium tert-butoxide NaOt-Bu, 1, 1'-dinaphthalene-2, 2'-diphenylphosphino BINAP, aromatic diamine and Pd2(dba)3 into a three-mouth flask with nitrogen gas feeding in turn, wherein the mole ratio is 1:1:2.8:0.03:0.01, adds N, N-dimethylacetamide until the monomer density is 0.5-0.65mol / L, increases temperature from 20DEG C to 100DEG C, keeps for 4-6h, increases temperature from 100DEG C to 150-200DEG C, keeps for 20-24h, cools to 20DEG C, adds the mixture of ammonia and methanol at the ratio of 1:4(L / L), mixes, stands for 1h, washes via the mixture of ammonia and methanol for three times, filters, dries at vacuum for 6h, mixes and dissolves poly(imino ketone) and benzoyl chloride in tetrahydrofuran for 2-12h at 1-3DEG C, to obtain polyuralcohol to be dried. The synthesized polyuralcohol Tg is higher than 190DEG C and TD is higher than 450DEG C, with better heat stability and dynamic mechanical stability.
Owner:QINGDAO UNIV OF SCI & TECH
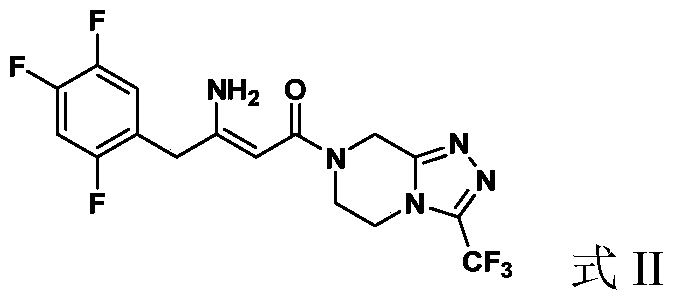
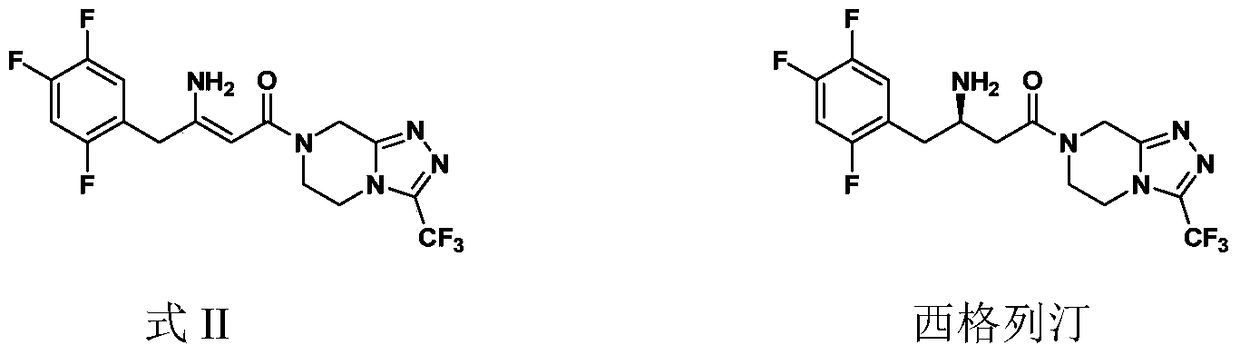
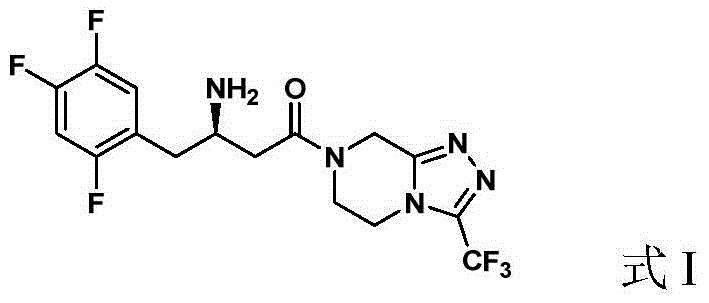
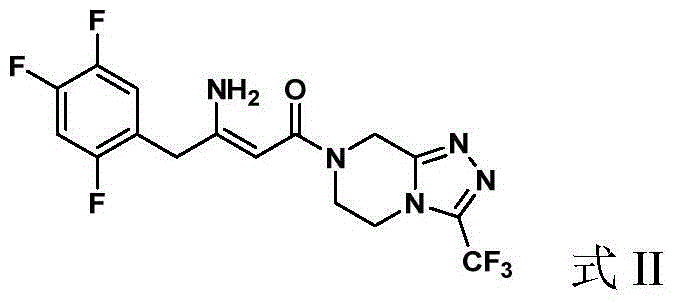
Preparation of Sitagliptin
ActiveCN105315286AHigh ee valueImprove recovery yieldOrganic chemistrySitagliptinAsymmetric hydrogenation
The invention belongs to the field of medicine synthesis and particularly provides a preparation method for Sitagliptin. According to the method, a cheap metal ruthenium complex and cheap R-BINAP serve as ligands, and the asymmetric hydrogenation reduction of an enamine intermediate is catalyzed, so that R-configuration Sitagliptin can be obtained in a high-selectivity manner; and the reaction time is short, and both the yield of reduction and the ee value of the product are relatively high, so that the method is applicable to industrialized amplified production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Method for synthesizing (R)-beta-hydroxytetradecanoate
InactiveCN102976940AReduce usageHigh stereoselectivityOrganic compound preparationCarboxylic acid esters preparationHydrogenNitrogen
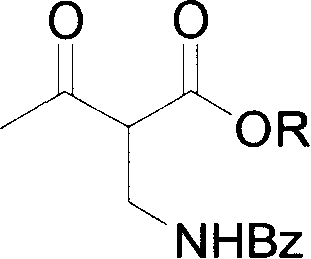
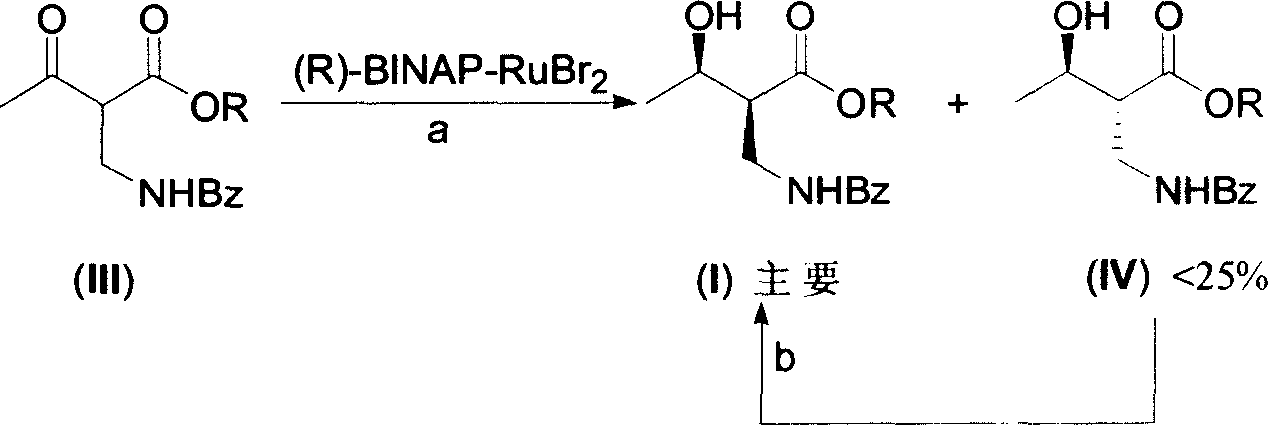
The invention discloses a method for synthesizing (R)-beta-hydroxytetradecanoate, which comprises the following steps of: (1) in the presence of an improved chiral catalyst (R)-BINAP-RuBr2, dissolving a compound of formula (III) in a solvent and performing catalytic hydrogenation; and (2) after the reaction ends, replacing nitrogen with hydrogen, and performing hydrogenation under a pressure of 0.1-0.2kg / cm<2>; and performing reduced-pressure concentration on the reaction solution, and recrystallizing to obtain white solid crystals which are (R)-beta-hydroxymethyltetradecanoate. According to the method, the improved chiral catalyst (R)-BINAP-RuBr2 is innovatively used for catalytic synthesis of (R)-beta-hydroxytetradecanoate compounds; and by use of the catalyst, the compound shown as the formula (III) can be converted into a high-stereoselectivity (greater than 98.5%ee) compound shown as the formula (I) under a relatively low pressure (0.01-6kg / cm<2>), and the use of high-pressure hydrogenation equipment and acid-resistant equipment is avoided. The method disclosed by the invention has the advantages of low cost, high yield, easy implementation of reaction conditions, adaptability to large-scale industrial production of compounds shown as the formula (I), and the like.
Owner:SHANDONG NORMAL UNIV
Method for preparing L-menthol intermediate d-citronellal
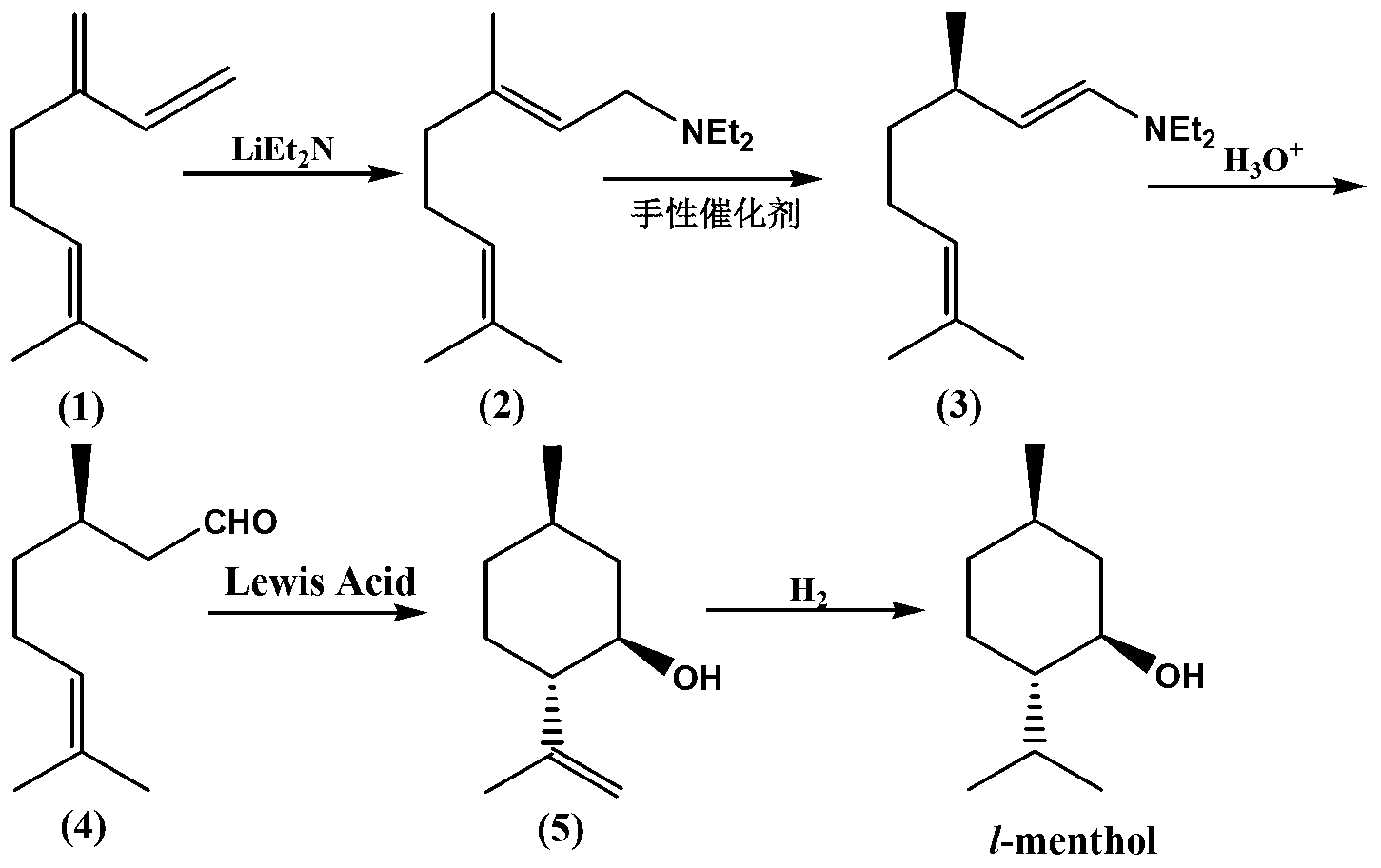
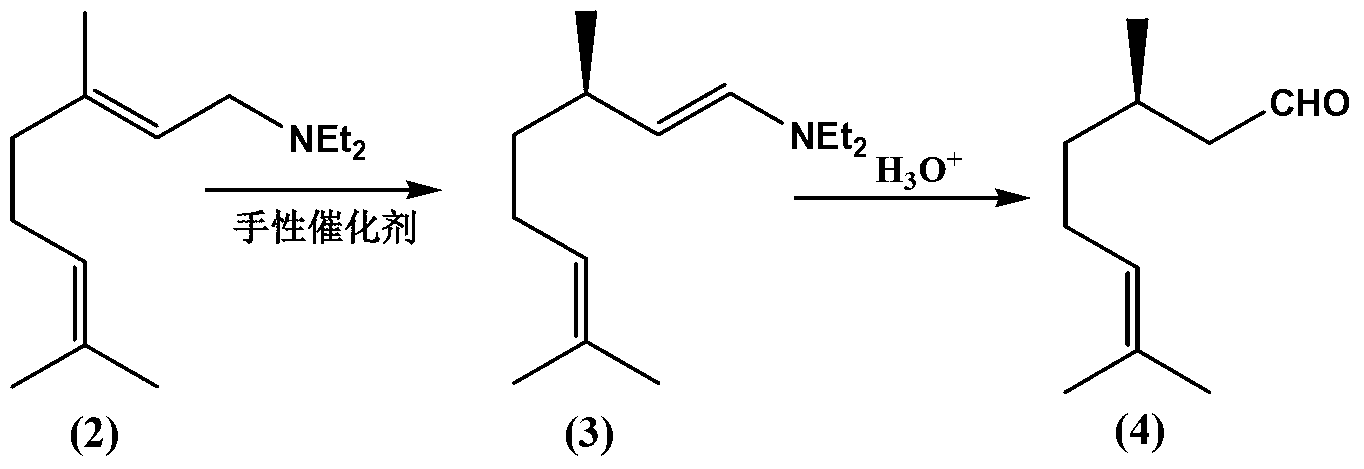
ActiveCN103254047AEasy to recycleAvoid recycling difficultiesAmino preparation from aminesCarbonyl compound preparation by hydrolysisL mentholSolvent
The invention discloses a method for preparing L-menthol intermediate d-citronellal. The method comprises the following steps of: (1) enabling geranyl amine (2) to react in a solvent A in the existence of a chiral catalyst, and then collecting myrcene amine (3) from a reaction product; and (2) carrying out acidic hydrolysis on the myrcene amine (3) to obtain d-citronellal (4). The structure of the chiral catalyst is as follows: [Rh(PS-Binap)2]<+>Y<->. The method has the characteristics of being mild in reaction condition, simple in operation, high in stereoselectivity, high in yield, simple in catalyst recovery and the like, and can be applied mechanically in cycle.
Owner:上海统益生物科技有限公司
Asymmetric catalytic hydrogenation process of synthesizing serial (2S,3R)-2 benzoyl aminomethyl-3-hydroxy butyrate compounds
ActiveCN1931831ALow costHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHigh pressureN-Butyrate
The present invention provides one process of preparing serial (2S, 3R)-2-benzoyl aminomethyl- 3-hydroxy butyrate compounds by means of improved chiral catalyst (R)-BINAP-RuBr2. The process of the present invention needs no use of high pressure hydrogenation apparatus, converts the catalytic side product into target product effectively, and has raised material converting rate and target product yield.
Owner:LUNAN PHARMA GROUP CORPORATION
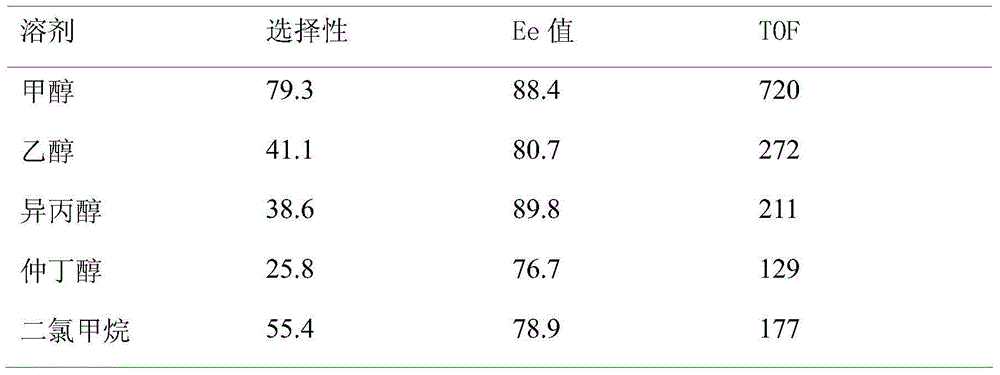
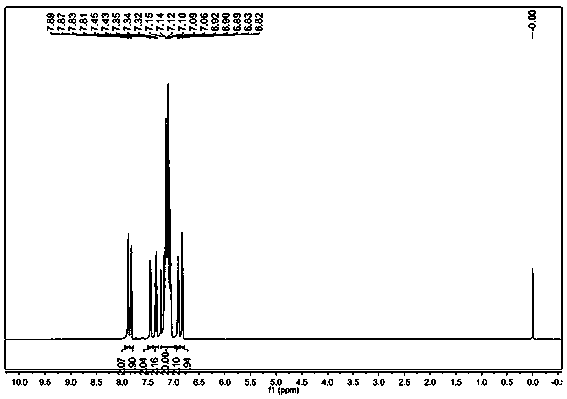
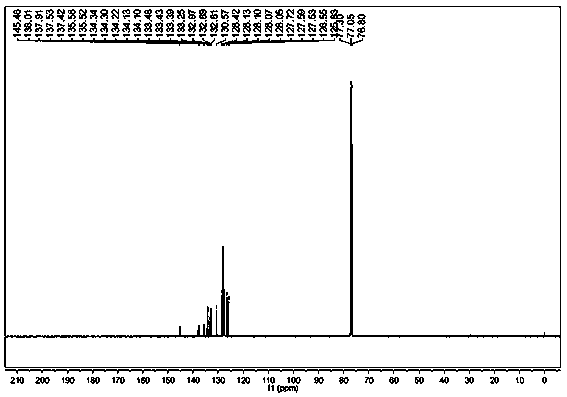
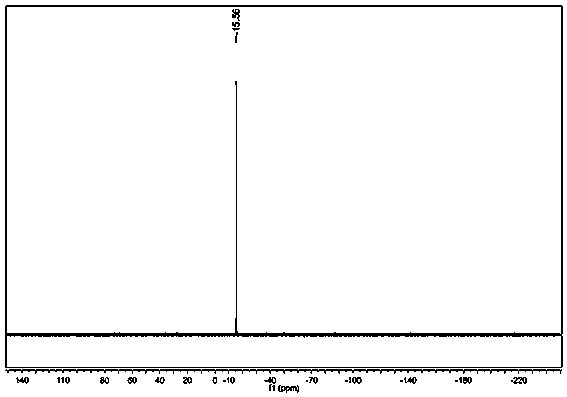
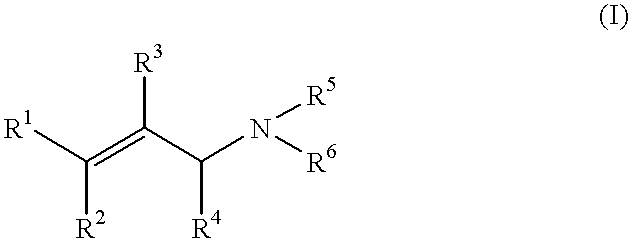
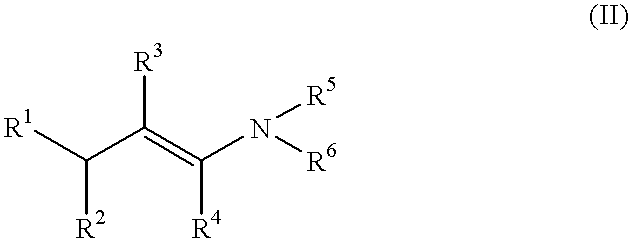
Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition
The invention relates to a new oxime-based method for synthesis of a pyridine derivative by [2+2+2] cycloaddition. According to the invention, corresponding oxime is synthesized from aldehyde, Rh(NBD)2BF4 is taken as a metal precursor, BINAP is adopted as a diphosphine ligand, DCE is employed as a solvent, a molecular sieve is used as an additive, and diyne is added to react with the materials for 48h at a temperature of 80DEG C, thus obtaining the corresponding pyridine derivative. Compared with previous nitrile raw materials, the starting material required by the method has the characteristics of simple synthesis, high yield, substantially reduced toxicity, stability in water and air, and strong operability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
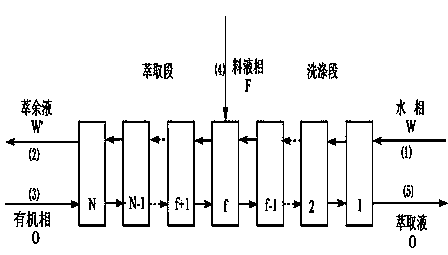
Method for extraction separation of 4-nitrobenzene glycine enantiomer by multistage centrifugal extractor
ActiveCN104163770AEnhanced mass transferImprove responseOrganic compound preparationAmino-carboxyl compound preparationGlycineEnantiomer
The invention introduces a method for extraction separation of a 4-nitrobenzene glycine enantiomer. The method provided by the invention makes use of the selective complexation of a BINAP-Cu metal complex extractant and a 4-nitrobenzene glycine enantiomer and the centrifugal action force of a centrifugal extractor to intensify the mass transfer efficiency and accelerate the mass transfer and reaction of 4-nitrobenzene glycine enantiomer in an aqueous phase and organic phase. At the same time, according to the invention, NaPF6 of certain concentration is added into an aqueous inlet solution so as to minimize the inhibition on the extraction effect of an extraction section while maximizing the washing effect of a washing section, thereby greatly improving the purity and yield at the extraction phase outlet and raffinate phase outlet. The technology overcomes the problems of low mass transfer efficiency and low purity and yield of single-stage extraction in common extraction technologies. The method can realize fast and high selective separation of 4-nitrobenzene glycine through multistage countercurrent extraction. Also the equipment is simple, and the operation is simple and convenient.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
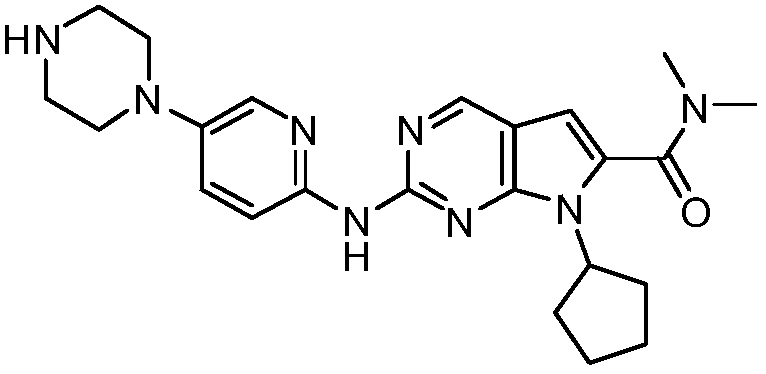
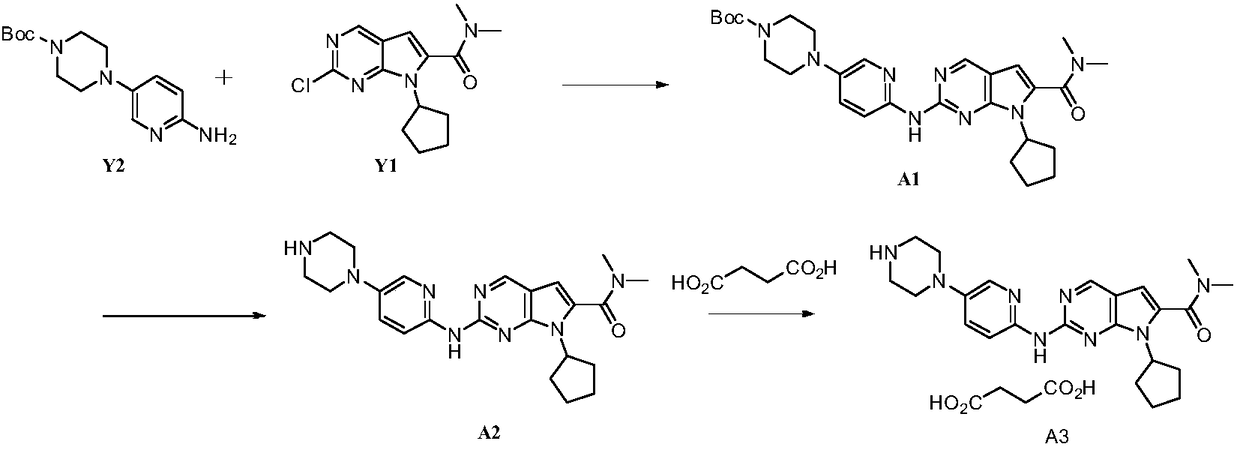
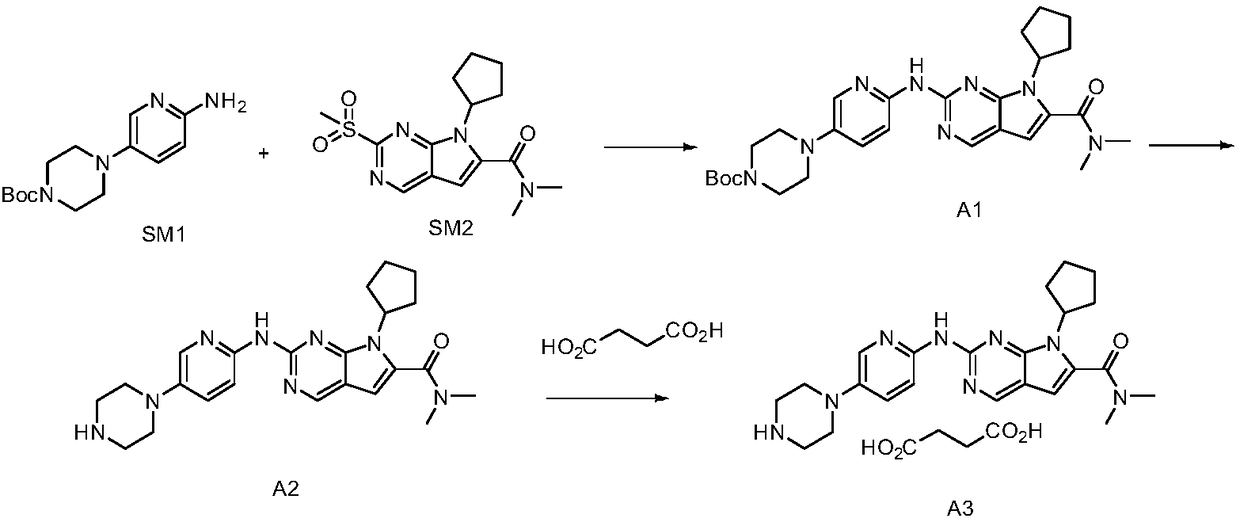
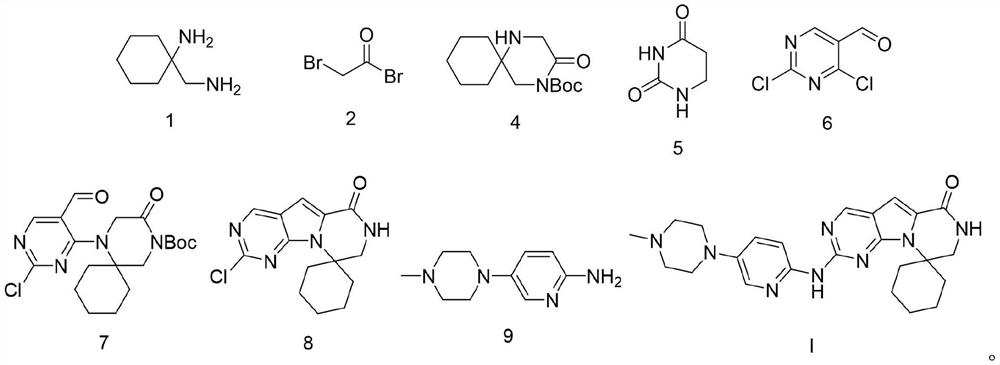
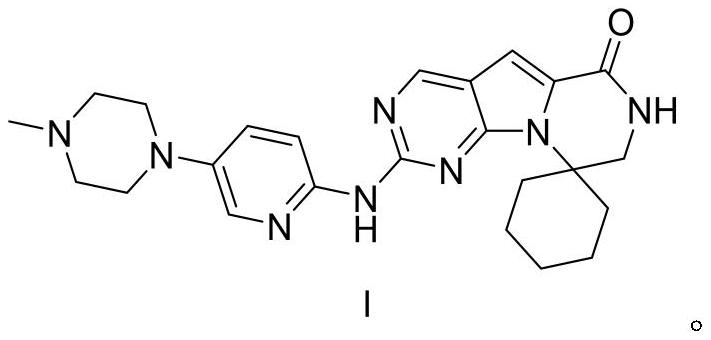
Preparation method of ribociclib and product and use thereof
InactiveCN109400612AAchieve primary separationMild responseOrganic chemistryCarboxylic acidRibociclib
A preparation method of ribociclib comprises the following steps: 1) utilizing 4-(6-aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester and 2-chloro-4-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-formamide as raw materials and conducting reaction in a protective atmosphere with palladium acetate / BINAP as a catalyst, cesium carbonate as an acid absorber and 4-methyl-2-pentanone as a solvent to obtain 4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester); 2) dissolving the4-(6-(7-cyclopentyl-6-(dimethylaminoformyl)-7H-pyrrolo[2,3-d]pyrimidine-2-yl)aminopyridine-3-yl)piperazine-1-carboxylic acid tert-butyl ester) obtained in step 1) in an organic solvent, adding acid dropwise at the room temperature to remove tert-butyl formate, conducting liquid separation, adding a water-soluble organic solvent to an aqueous layer, separating solids and filtering to obtain ribociclib acid salt; 3) adding water to the ribociclib acid salt for dissolving, adding an adsorbent, filtering and adding alkali to the filtrate to obtain the ribociclib.
Owner:CHONGQING SANSHENG IND CO LTD
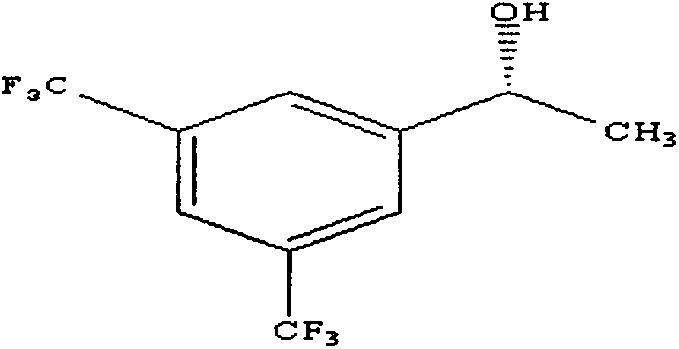
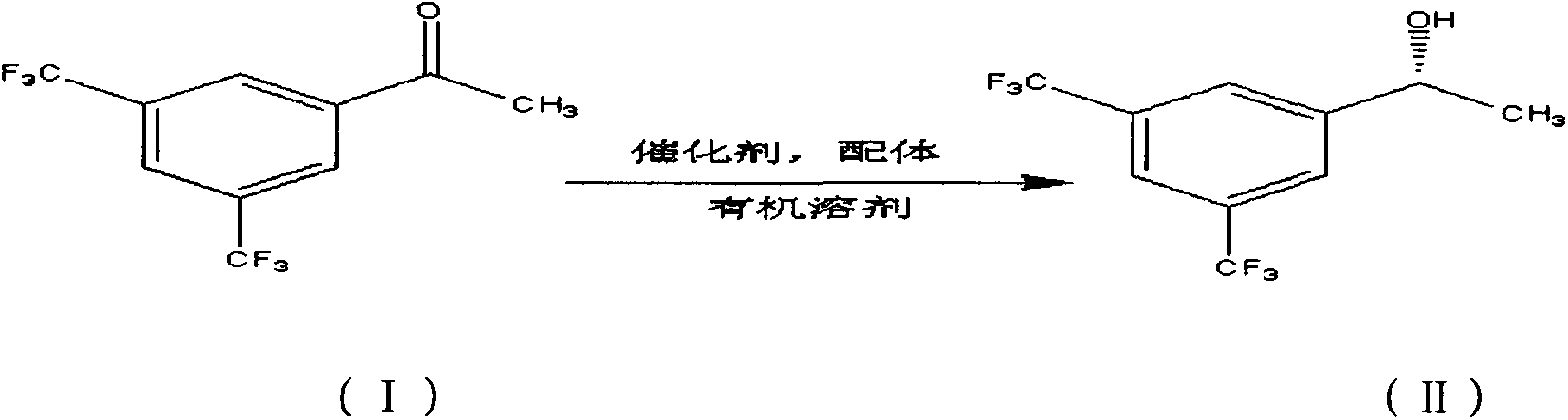
Method for preparing (R)-3,5-bis(trifluoromethyl)benzene-ethanol
ActiveCN103232324AHigh catalytic efficiencyShort reaction timeOrganic compound preparationHydroxy compound preparationPhenethyl alcoholAcetophenone
The present invention provides a method for preparing (R)-3,5-bis(trifluoromethyl)benzene-ethanol by using 3,5-bis(trifluoromethyl)acetophenone as a raw material, through asymmetric-hydrogenation catalytic reduction. The method of the invention belongs to the technical field of asymmetric catalytic hydrogenation synthesis of chiral pharmaceutical intermediates. In the method, [RuCl2(C10H14)2]2 or Ir[(COD)Cl]2 is used as a catalyst, and BINAP or (S, S)-C6P2 (NH) 2 is used as a chiral phosphine ligand to form a complex catalyst, in an oxygen-free sealed high-pressure hydrogenation reaction system, the substrate 3,5-bis(trifluoromethyl)acetophenone is catalyzed to prepare (R)-3,5-bis(trifluoromethyl)benzene-ethanol.
Owner:GUANGXI XINJING TECH +1
New method for reactive extraction of 4-nitro-phenylglycine
InactiveCN104163765ASimple processEase of industrial productionOrganic compound preparationAmino-carboxyl compound preparationNitrobenzeneOrganic chemistry
The invention relates to a new method for reactive extraction of a 4-nitro-phenylglycine enantiomer. A (S)-BINAP-metal copper complex is adopted as an organic phase chiral extractant for extraction separation of the 4-nitro-phenylglycine enantiomer dissolved in an aqueous phase. The invention also discloses a preparation method of the (S)-BINAP-metal copper complex. The (S)-BINAP-metal copper complex has good complexing ability and high stereoselectivity on the 4-nitro-phenylglycine enantiomer, and the separation factor (alpha) reaches over 4.1.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Heterogeneous asymmetric hydrogenation catalyst and application thereof
ActiveCN105749968AHigh catalytic activityHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkHigh activity
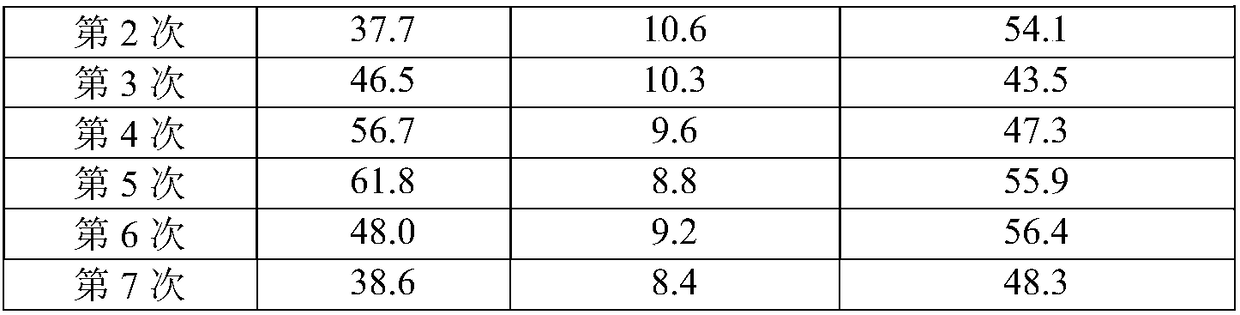
The invention provides a heterogeneous catalyst for Beta-ketoesters and application thereof and belongs to the field of material synthesis and catalyst application.The catalyst comprises a metal component and a carrier.A preparation method of the catalyst carrier comprises: subjecting commonly used chiral ligand BINAP(2,2'-bis(diphenylphosphine)-1,1'-binaphthyl) not modified to Friedel-Crafts reaction, copolymerizing with other monomers such as benzene through L-acid catalysis by using dimethoxymethane as a cross-linking agent, acquiring a chiral hyper-cross-linked material of various ducts, mainly micropores, through a one-step method.The metal component uses Ru2(C6H6)2Cl4 as a metal precursor.By using the catalyst provided herein in asymmetric hydrogenation of methyl acetoacetate under 2-6 Mpa at 20-100 DEG C, it is possible to acquire high-activity and about 95% of enantio-selectivity in methanol solution, and more than six times of reuse are available.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of synthetic method of 2,2'-bisdiphenylphosphino-1,1'-binaphthalene
ActiveCN108586528BHigh reaction yieldLow priceLithium organic compoundsGroup 5/15 element organic compoundsMetallic lithiumEthoxidine
The invention discloses a synthetic method of 2,2'-double diphenyl phosphine-1,1'-dinaphthalene. The method comprises the following steps: adding a lithium metal sheet, a ligand, 2,2'-diethoxy-1,1'-dinaphthalene and an ethers solvent are added in a reaction still, heating the materials to the temperature of 60-140 DEG C, reacting the materials for 6-12 hours, dropping chlorodiphenylphosphine at the temperature of 0 DEG C, heating the material to room temperature, and obtaining the product after the reaction is completed; equivalent water quenching is carried out, a product solution is concentrated and filtered, a filter cake is washed with methanol, and vacuum drying is carried out to obtain the product 2,2'-double diphenyl phosphine-1,1'-dinaphthalene (BINAP). The method has the advantages of high yield, low preparation cost, simple post-treatment, high product purity, and is suitable for process enlargement.
Owner:XINXIANG RUNYU NEW MATERIAL TECH CO LTD
Stereospecific isomerisation of allylamines with the aid of immobilized phosphorated chiral ligands
InactiveUS6350910B1Isocyanic acid derivatives preparationAmino preparation from aminesPhosphorylationEnamine
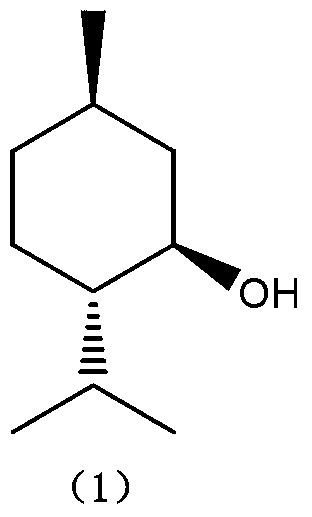
The present invention describes a method for stereospecific isomerisation of prochiral allylamines into enamines and chiral imines, by using catalysts of Rh, Ir and Ru having phosphine chiral ligands immobilised on a solid material. The immobilised ligands are derivatives of phosphines of the type bis(diphenylphosphino)biaryl such as, for example, the phosphine known by the name BINAP. The method is particularly suitable for the production of optically active citronellal which may be obtained in optical purities above 95%.
Owner:FIRMENICH SA
Synthesis method of (R)-5'-methoxyl laudanosine
PendingCN113480480AHigh yieldHigh optical purityOrganic chemistry methodsPtru catalystOrganic synthesis
The invention discloses a synthesis method of (R)-5'-methoxyl laudanosine, and belongs to the technical field of organic synthesis. The preparation method comprises the following steps: by taking 6,7-dimethoxy-1-(3,4,5-trimethoxybenzyl)-3,4-dihydroisoquinoline as a raw material, sequentially carrying out N-formylation, asymmetric hydrogenation reduction and formyl reduction to obtain (R)-5'-methoxy laudanosine. According to the technical scheme, the yield of (R)-5'-methoxyl laudanosine directly prepared is high, the total yield of the three-step reaction can reach up to 81.9%, the optical purity is 99.79% or above, and after refining, the optical purity is larger than 99.98%, and the chemical purity is larger than 99.93%. Besides, according to the technical scheme, the chiral selective catalyst Ru(CH3COO)2(R)-BINAP without genotoxicity is adopted, the defect that genotoxic impurities containing p-toluenesulfonyl and the like are prone to remaining is overcome, the Ru(CH3COO)2(R)-BINAP is an industrial mature catalyst, the technological operation process is not harsh, and large-scale production is easy to achieve.
Owner:GUANGDONG JIABO PHARM CO LTD
Preparation process of trirasilil compound
The invention relates to a novel preparation process of a trirasilil compound. According to the method disclosed by the invention, uracil and bromoacetyl bromide which are commercially easy to obtain and low in price are adopted as starting raw materials, wherein the trirasilil is obtained through five steps of chemical reactions in total; wherein tBuOK, Pd2(dba)3 and BINAP are used for halogenation reaction in the step (5), so that the yield of the product can be effectively improved, and generation of by-products is avoided. The reaction conditions of the whole synthesis route of the preparation method are mild; the used raw materials and reagents are cheap and simple, and the reaction yield of each step is high. In addition, post-treatment of the reaction is simple, pulping purification is mostly adopted for purification, column chromatography purification is avoided as much as possible, and the total yield and industrial operability of the reaction are effectively improved.
Owner:武汉九州钰民医药科技有限公司
Preparation process of hypotensive drug optically pure cilnidipine
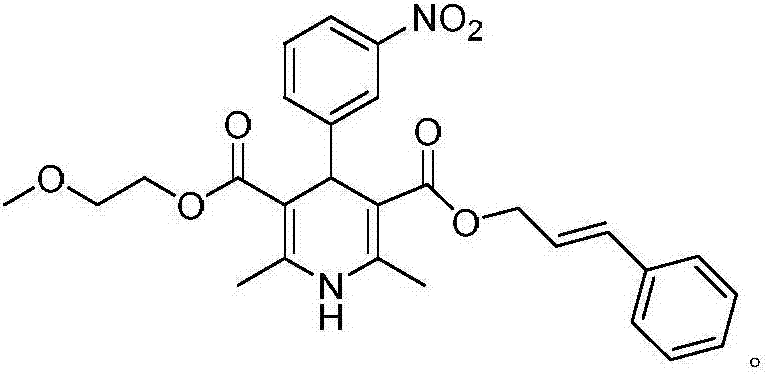
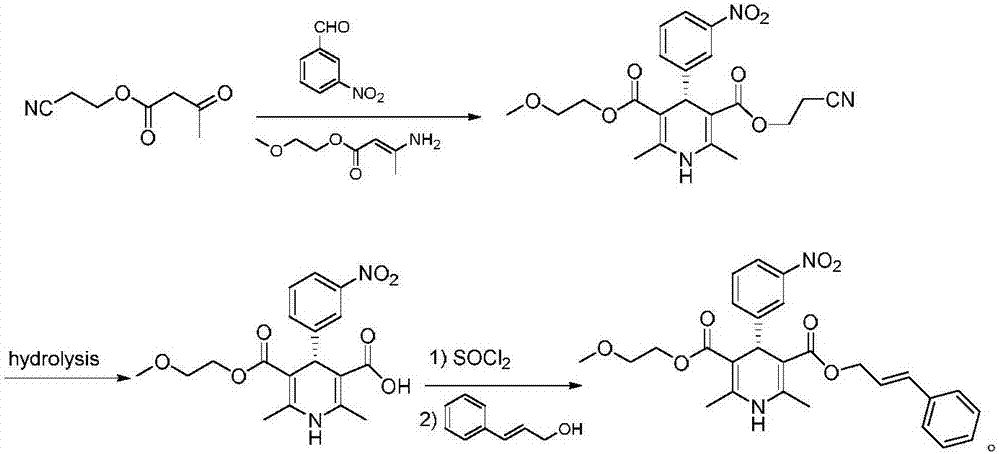
InactiveCN107879970AHigh yield in three stepsHigh chemoselectivityOrganic chemistry methodsHypotensive drugCinnamic alcohol
The invention discloses a preparation process of a hypotensive drug optically pure cilnidipine. The preparation process comprises the following steps: 1) in the presence of (S)-BINAP and a ferric ironcompound, adding ethyl cyano acetoacetate, m-nitrobenzaldehyde and 3-amino-2-methoxyethyl butenoate into isopropanol to react to obtain (R)-1,4-dihydro-2,6-dimethul-4-(3-nitryl phenyl)-3,5-dipicolinic acid-3-(2-cyano ethyl ester)-5-(2-methoxyl ethyl ester); 2) stirring the product in a tetrahydrofuran aqueous solution of alkali to react to obtain (R)-1,4-dihydro-2,6-dimethyl-4-(3-nitryl phenyl)-3,5-dipicolinic acid-5-(2-methoxyl ethyl ester); and 3) carrying out an esterification reaction with cinnamyl alcohol to obtain the optically pure (R)-cilnidipine. The three-step yield of the (R)-cilnidipine reaches up to 41%, ee% reaches up to 99.5%, and the preparation process is simple and suitable for industrial production.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Preparation method of L-carnitine
ActiveCN102633664BReduce pollutionRaw materials are easy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSpecific rotationSolvent
Owner:GUANGXI XINJING TECH +2
Application of sulfonated BINAP and polyether functionalized ionic liquid integrated chiral catalyst in asymmetric hydrogenation reaction
ActiveCN111389468AAdvantages and significant technological advancesHigh activityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAsymmetric hydrogenation
The invention relates to a phosphine ligand and polyether functionalized ionic liquid integrated chiral catalyst and application thereof in asymmetric catalytic hydrogenation reaction. In an asymmetric hydrogenation reaction, the dual functions of the chiral catalyst and the solvent ionic liquid can be exerted at the same time only by a catalytic amount of phosphine ligand and ionic liquid integrated catalyst; according to the method, the chiral catalyst is recycled and circulated, so that the solvent ionic liquid does not need to be additionally added, the negative effect of the solvent ionicliquid is reduced to the maximum extent, efficient, green and economical utilization of the ionic liquid is realized, and the problem that a large amount of solvent ionic liquid is needed for recycling the chiral catalyst in the prior art is solved.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation and application of a heterogeneous asymmetric hydroformylation catalyst
ActiveCN108067307BThe synthesis method is simpleGood chemical stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPolymer sciencePtru catalyst
The invention discloses a preparation method of a multiphase asymmetric catalyst and belongs to the field of synthesis and application of functional materials. The catalyst is prepared from a metal component and a support. A preparation method of the catalyst support comprises the step as follows: a chiral bidentate phosphine ligand BINAP (5, 5'-divinyl-BIANP) containing vinyl is auto-polymerizedor is co-polymerized with other vinyl monomers with high steric hindrance; the metal component in the multiphase catalyst is Rh element. The periphery of the chiral phosphine ligand in a polymer skeleton is modified with other groups, a chiral pocket shown in the description is formed, so that catalytic performance of the multiphase catalyst in asymmetric hydroformylation can be improved, and enantio-selectivity of a product of the catalyst is increased by about 70% as compared with that of a homogeneous catalyst.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hybrid inorganic/organic polymer catalytic membrane material including immobilized molecular catalyst and its preparation
InactiveCN102470356BOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationPolyvinyl alcoholSilicic acid
A low-cost, feasible and modular method for the preparation of new, highly selective "catalytic membranes" and their use in various types of reactors is disclosed. The membranes are versatile and reusable with negligible catalyst leaching, especially in the asymmetric hydrogenation of substituted α,β-unsaturated acids or esters. The membrane comprises a hybrid inorganic / polymer carrier material (i) and a molecular catalyst (ii) immobilized on the hybrid inorganic / polymer carrier material (i), wherein -(i) consists of an inorganic compound and an organic The hybrid inorganic / macromolecular compound composed of polymer chemical combination; -The inorganic compound is at least one selected from silicic acid compound, tungstic acid compound, molybdic acid compound and stannic acid compound; -The organic polymer Has a hydroxyl group, preferably polyvinyl alcohol (PVA); -(ii) is a pre-formed metal catalyst comprising at least one selected from Ru, Rh, Pd, Ir, Ni, Pt, Au A transition metal and at least one chiral ligand selected from phosphinos, aminos and / or amino-phosphines, preferably DIOP, BINAP monophosphorus or TMBTP.
Owner:NIPPON KODOSHI
A kind of synthesis method of medicine for treating leukemia
The invention relates to a synthesis method of a drug for treating leukemia. The synthesis method comprises that 1, a compound 3-methyl-3-oxetanemethanol and sodium metal undergo a reaction to produce 3-sodium methoxide-3-methyl-3-oxetane, 2, 4-amino-3-nitrochlorobenzene is added into the product obtained by the step 1 so that reddish-orange solids (A1) are obtained, a compound 8-benzyloxyquinolin-2-chlorine and A1 undergo a reaction to produce orange solids A2, 3, the compounds A2, 13g of palladium / carbon and formic acid undergo a reaction, formamidine acetate is added into the reaction product, and the mixture is subjected to reflux and drying so that white-like (or yellow) solids A3 are obtained, and 4, the compounds A3 and benzenesulfonyl chloride undergo a reaction, the product is dried to form maize-yellow solids A4, BINAP, A4, tert-butyl piperidin-4-yl-carbamate and potassium carbonate undergo a reaction, the reaction product is dried to form A5, and the A5 is refined through the step 5 so that a pure product is obtained. In the steps 1 and 2, a one-pot method is used.
Owner:扬州市三药制药有限公司
Preparation of sitagliptin
ActiveCN105315286BHigh purityPromote crystallizationOrganic chemistrySitagliptinAsymmetric hydrogenation
The invention belongs to the field of medicine synthesis and particularly provides a preparation method for Sitagliptin. According to the method, a cheap metal ruthenium complex and cheap R-BINAP serve as ligands, and the asymmetric hydrogenation reduction of an enamine intermediate is catalyzed, so that R-configuration Sitagliptin can be obtained in a high-selectivity manner; and the reaction time is short, and both the yield of reduction and the ee value of the product are relatively high, so that the method is applicable to industrialized amplified production.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Cuprous complex yellow phosphorescence material mixed by diphosphine and triazole
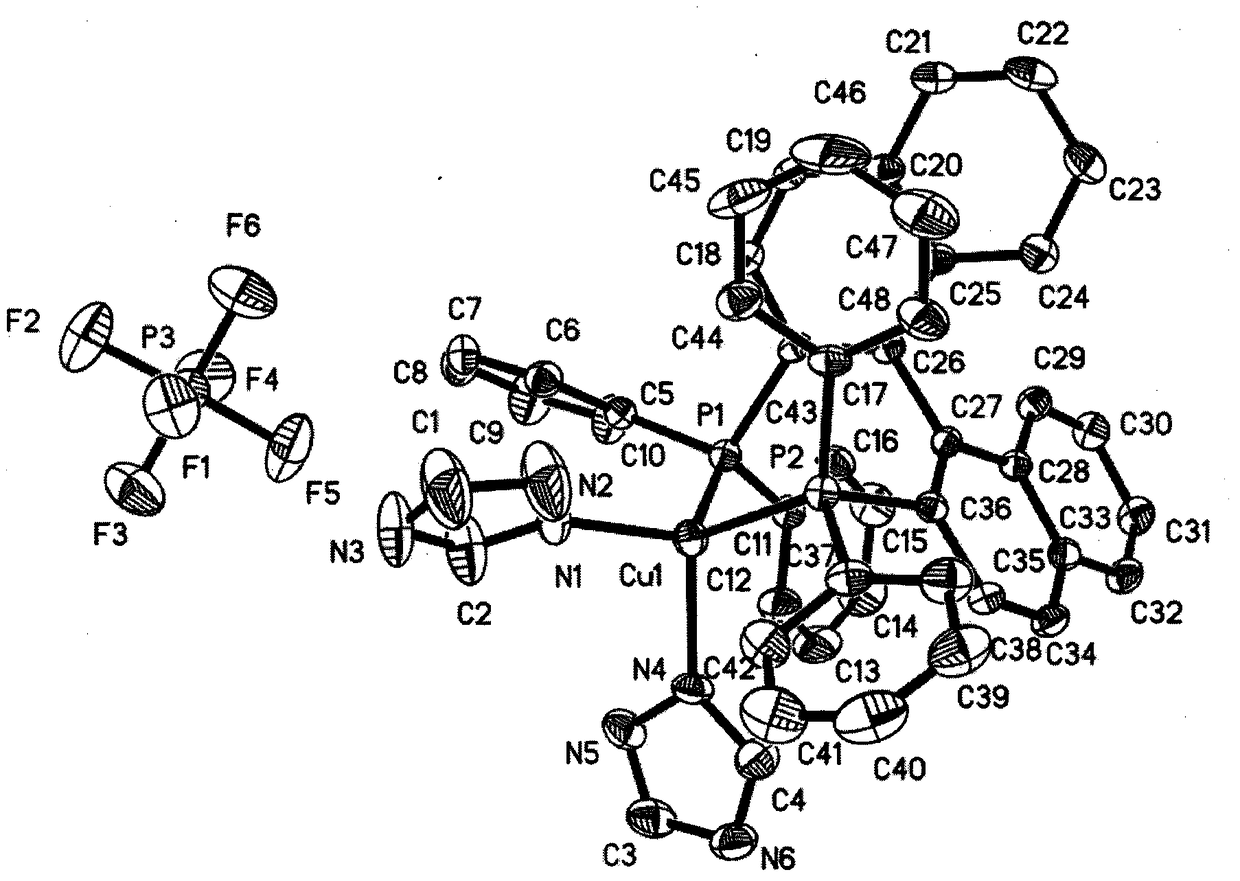
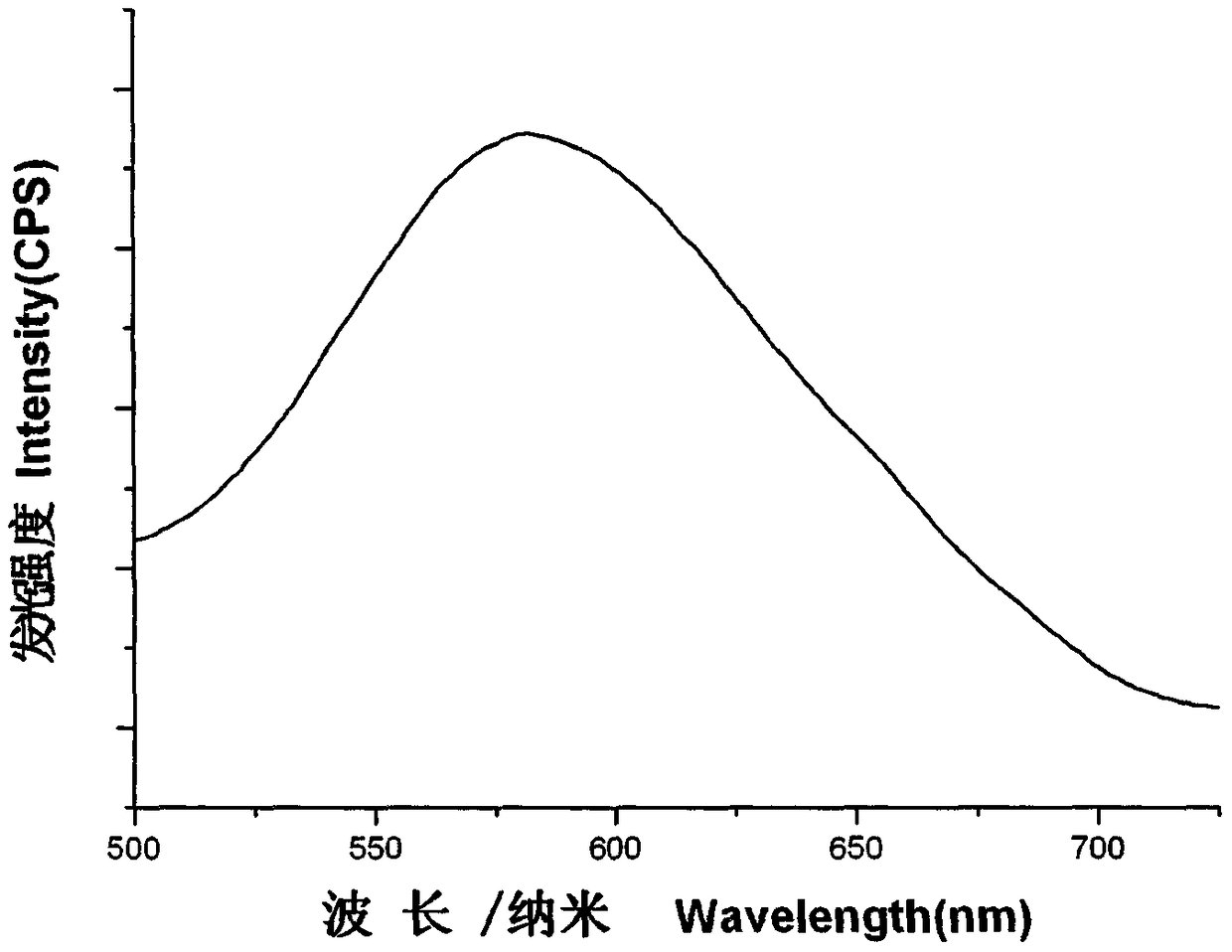
InactiveCN108794512AGood phosphorescent emission performanceEasy to purifyGroup 5/15 element organic compoundsCopper organic compoundsPhotoluminescenceDevice form
The invention discloses a crystal-form cuprous complex yellow phosphorescence material mixed based on a 1,2,4-triazole ligand without removing a proton and a preparation method thereof. The phosphorescence complex is obtained by complexing cuprous salt and the ligands. A molecular structure of the phosphorescence complex is [Cu(BINAP)(1Htri)2](PF6), wherein BINAP is the electric neutrality diphosphine ligand of 1,1'-binaphthyl-2, 2'-bis-diphenylphosphine, and 1Htri is the nitrogen ligand of 1,2,4-triazole. The material is obtained by a direct mixing reaction of Cu(CH3CN)4PF6 and dichloromethane solution of the ligand, and has the advantages of simple and convenient process, simple device, easy acquisition of raw materials and low cost and the like. The material can be used as a photoluminescence yellow phosphorescence material, or used as a luminous layer phosphorescence material in an electroluminescence device formed by multiple layers of organic materials.
Owner:CHINA JILIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




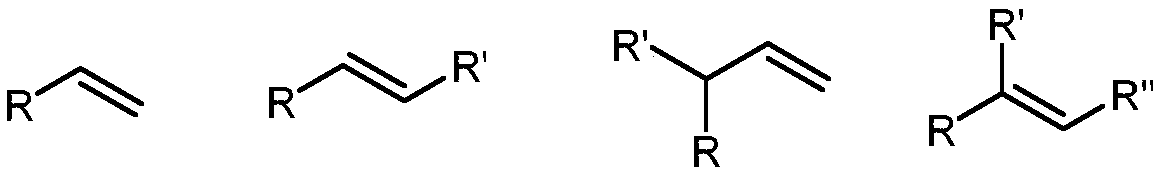
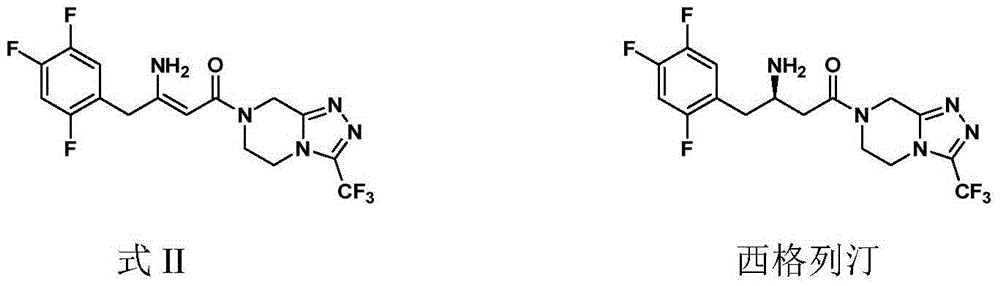
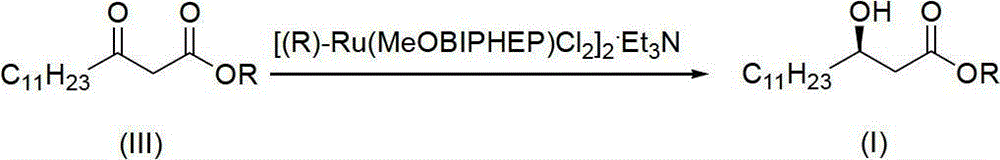
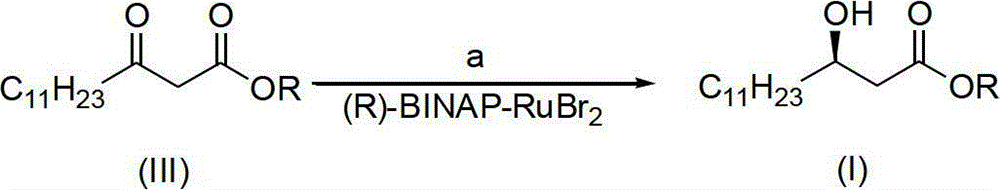
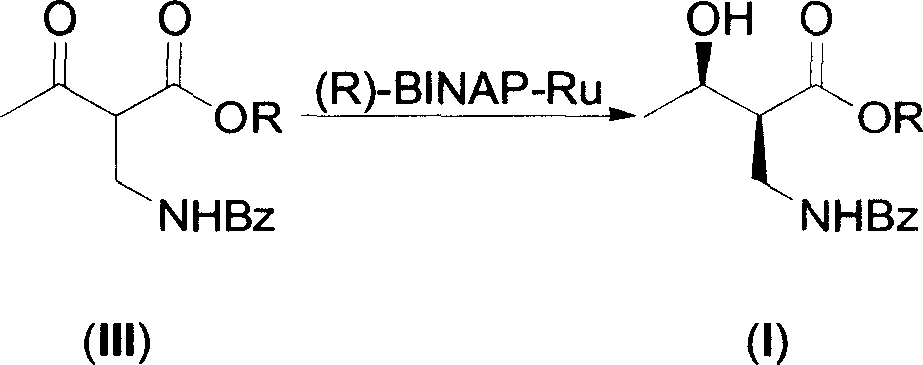
![Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition](https://images-eureka.patsnap.com/patent_img/5b699171-3120-4dba-8484-75e68a2fa39b/BDA0000075785650000011.PNG)
![Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition](https://images-eureka.patsnap.com/patent_img/5b699171-3120-4dba-8484-75e68a2fa39b/BDA0000075785650000021.PNG)
![Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition Oxime-based method for synthesis of pyridine derivative by [2+2+2] cycloaddition](https://images-eureka.patsnap.com/patent_img/5b699171-3120-4dba-8484-75e68a2fa39b/BDA0000075785650000031.PNG)