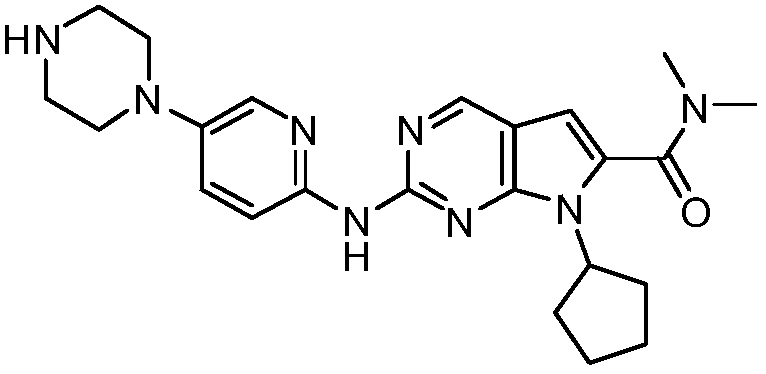
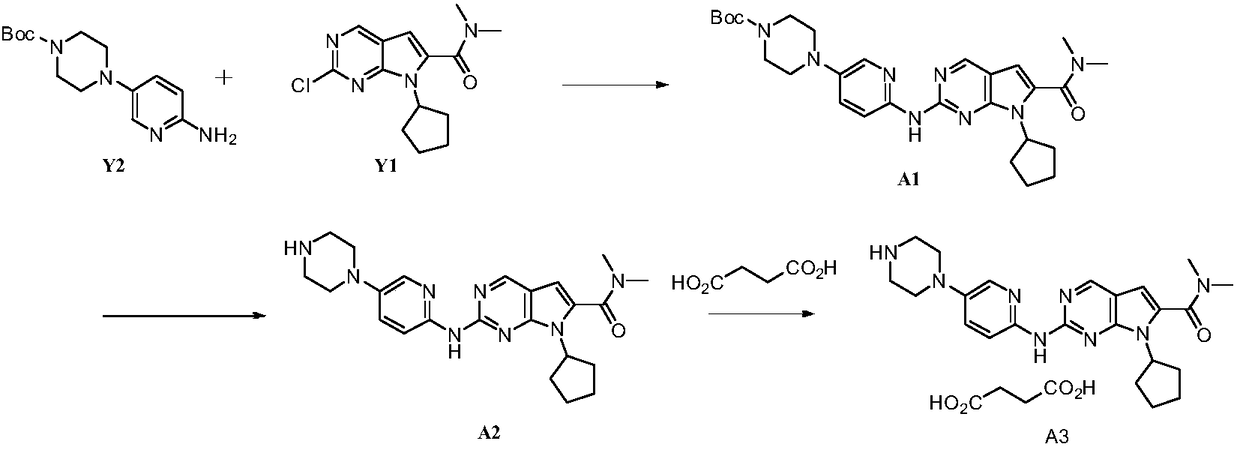
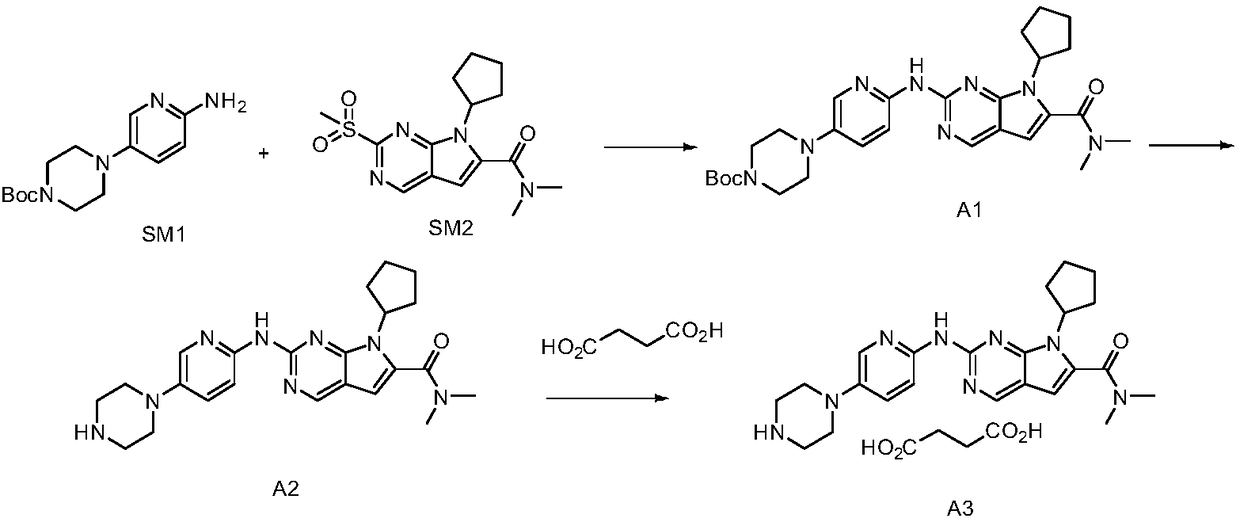
Preparation method of ribociclib and product and use thereof
A technology of ribociclib and aminopyridine, applied in the field of preparation of ribociclib, can solve the problems of high preparation difficulty, industrialization difficulty, separation and purification difficulty of SM2, etc., and is suitable for large-scale industrial application, low adsorption cost, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 4-(6-(7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)aminopyridin-3-yl) Piperazine-1-carboxylic acid tert-butyl ester) (formula 3) preparation, reaction formula is as follows:
[0033]
[0034] In a 2L four-neck round bottom flask, add 100g of 2-chloro-4-cyclopentyl-N,N-dimethyl-7H-pyrrole[2,3-d]pyrimidine-6-carboxamide (Formula 2), 104.6g4-(6-aminopyridin-3-yl)piperazine-1-carboxylate tert-butyl ester (formula 1), 155.8g cesium carbonate, after nitrogen replacement three times, add 800g4-methyl-2-pentanone, 1.534 g palladium acetate, 6.38g BINAP. After the feeding was completed, the temperature was raised to 90-100° C. under nitrogen protection environment, and the reaction was carried out for 3 hours. Cool down to 65°C, add 800g of water, 270g of n-heptane, 10ml of propylenediamine, cool down to room temperature, filter, soak the filter cake in 500g of water once, and use 160g of 4-methyl-2-pentanone / 270g of n-heptane mixed solvent ...
Embodiment 2
[0035] The preparation of embodiment two Ribociclib hydrochloride
[0036] Add 20g of 4-(6-(7-cyclopentyl-6-(dimethylcarbamoyl)-7H-pyrrolo[2,3-d]pyrimidin-2-yl)aminopyridine- 3-base) piperazine-1-carboxylate tert-butyl ester) (formula 3), 100g n-hexane, stirred and dissolved, added dropwise 100g3mol / L hydrochloric acid at room temperature, reacted for 1 hour, separated liquids, took the water layer, and poured it into the water layer Add 200 g of acetone. Raise the temperature to reflux, then lower the temperature to below 10°C to crystallize, stir for 3 hours, and filter to obtain Ribociclib hydrochloride.
Embodiment 3
[0037] The preparation of embodiment three Ribociclib (formula 4)
[0038] Dissolve Ribociclib hydrochloride in 200g of water, add 1g of activated carbon, 0.5g of 100-200 mesh column chromatography silica gel, stir for adsorption and decolorization, filter, add saturated aqueous sodium bicarbonate solution to the filtrate to adjust the pH to 9-10, and precipitate solid, cooled to 10°C and stirred. After filtration, the filter cake was dried at 60° C., 14 g of Ribociclib (Formula 4) was obtained, and the yield was 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com