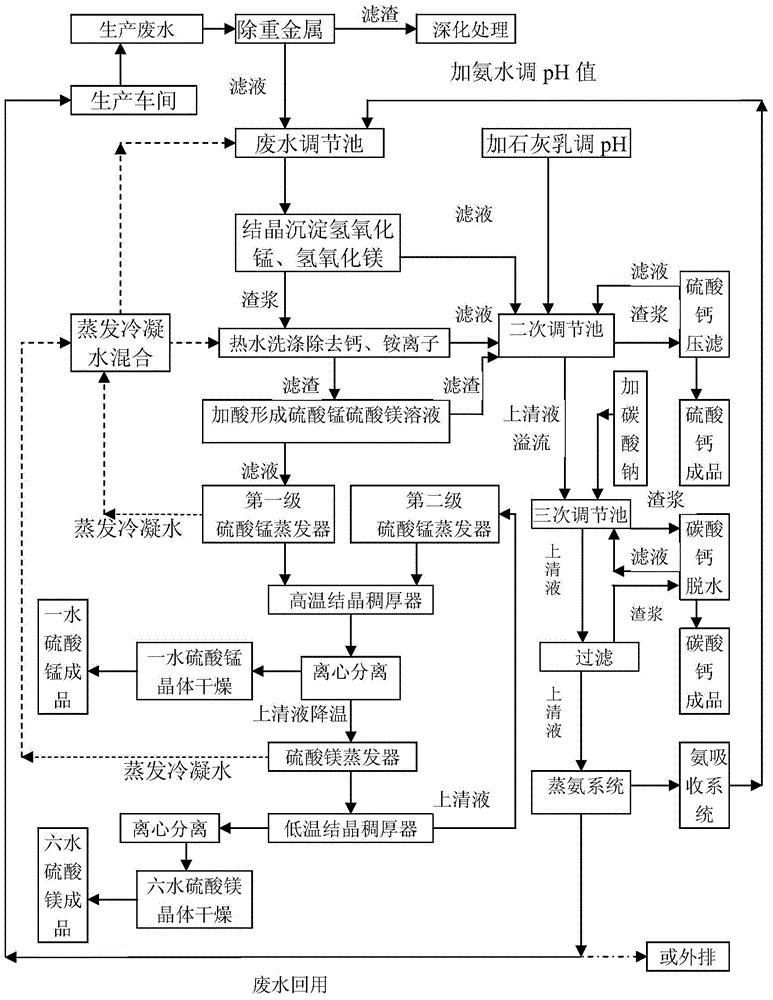
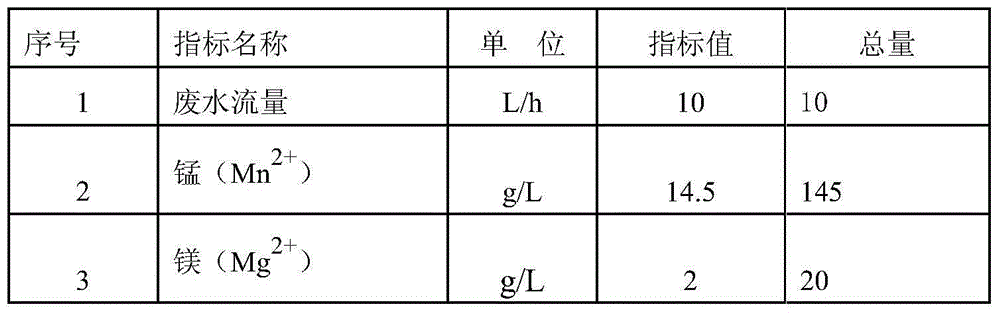
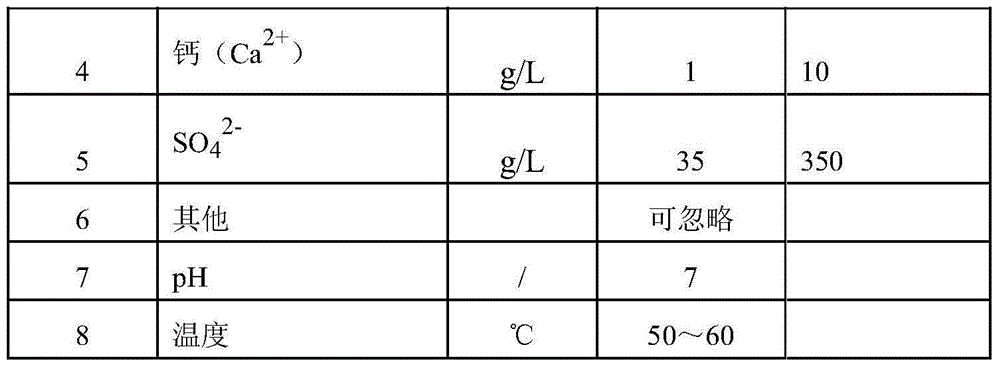
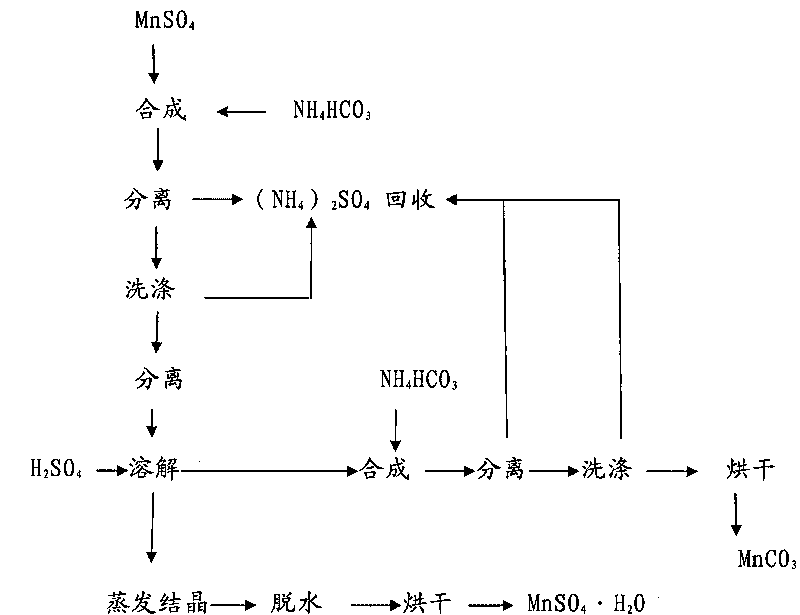
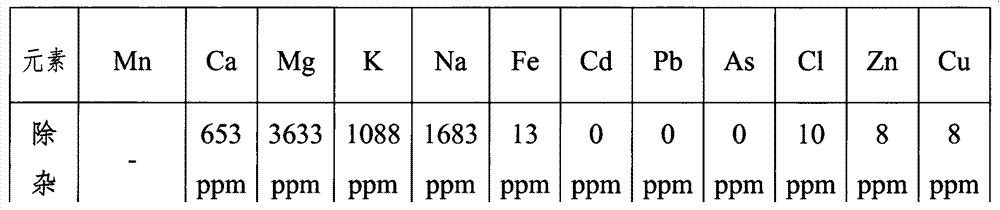
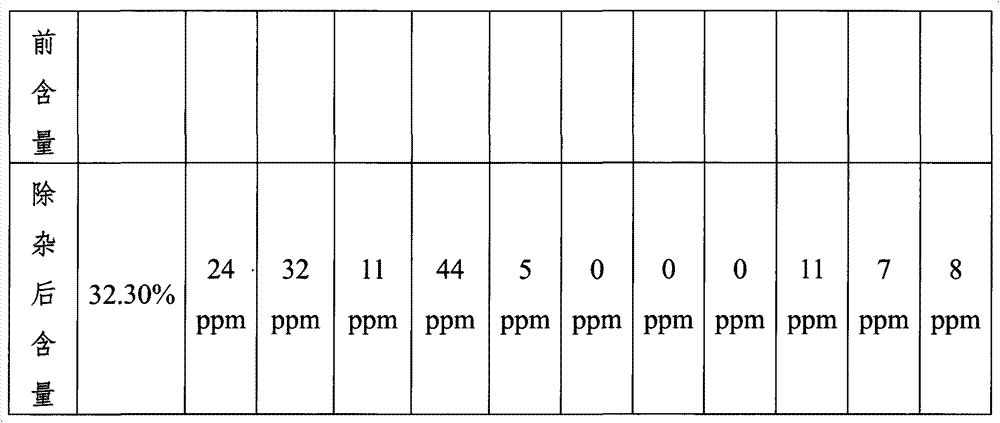
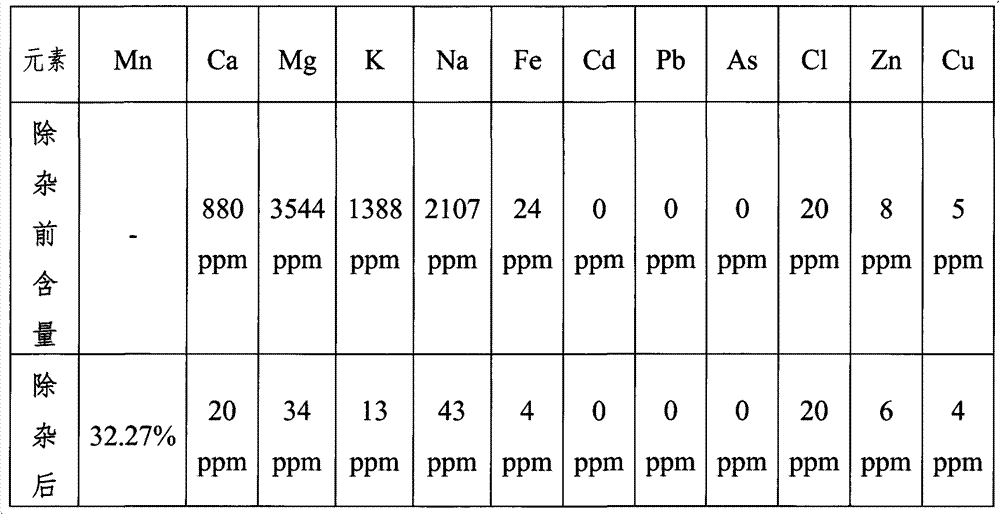
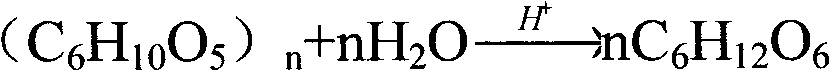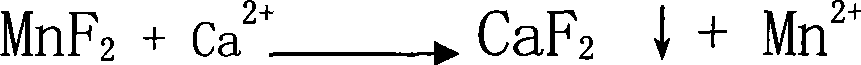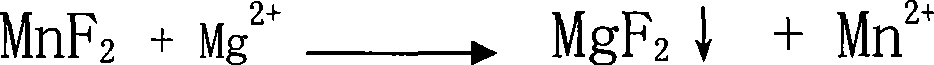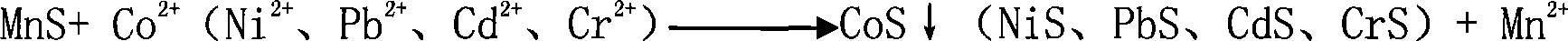Patents
Literature
504results about "Manganese sulfates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
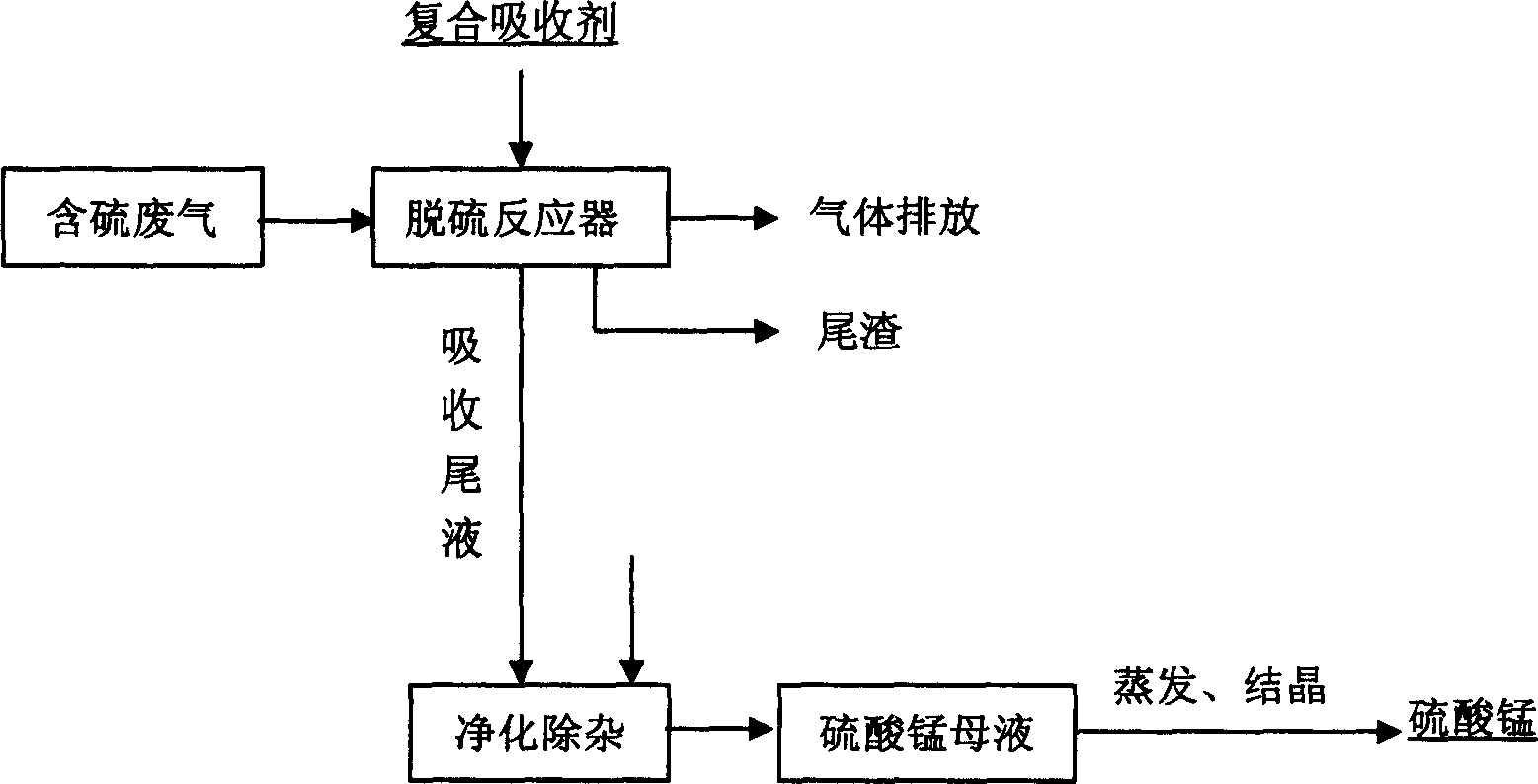
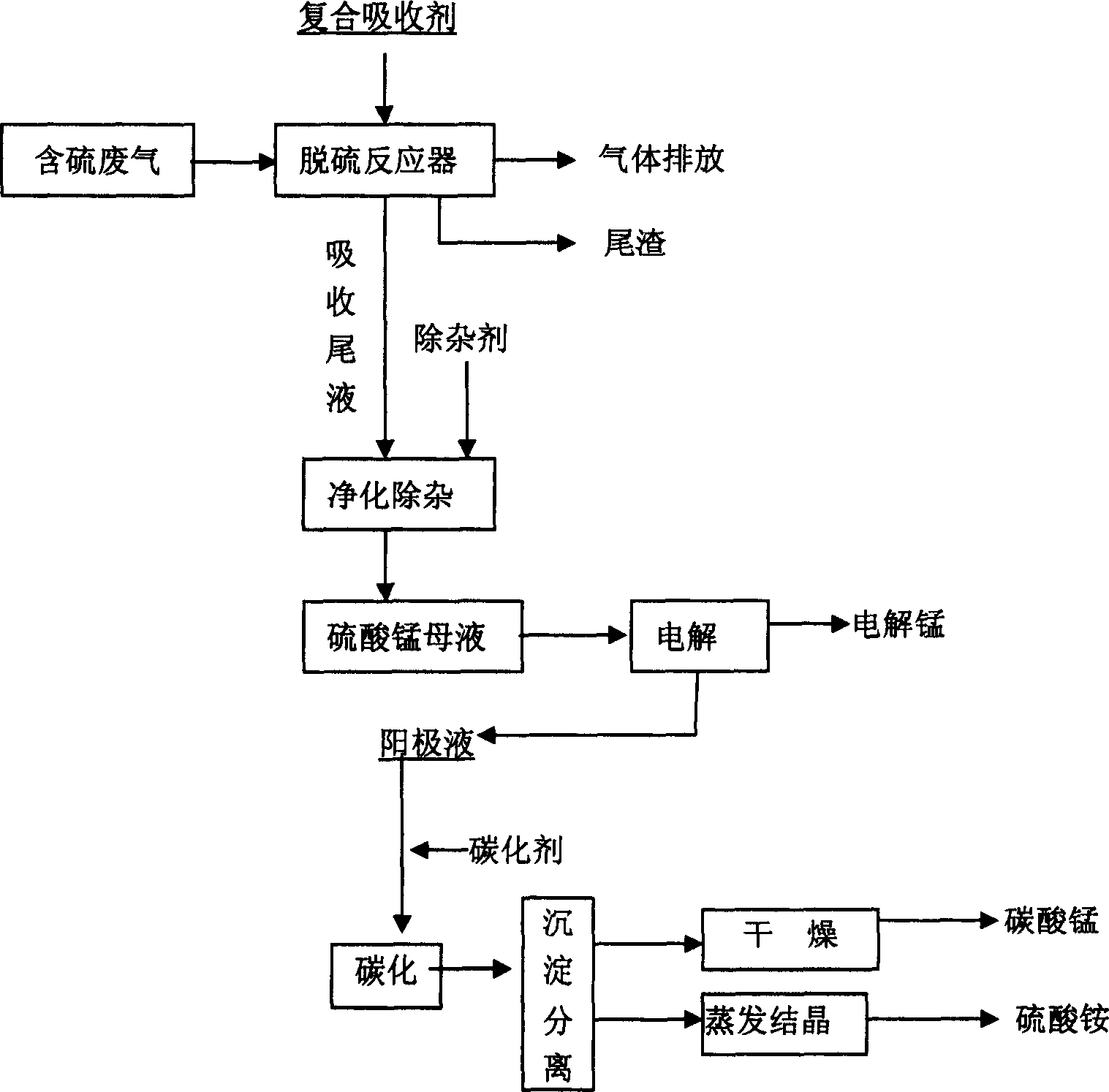
Waste gas desulfurizing method with composite absorbant comprising pyrolusite and pH buffering agent
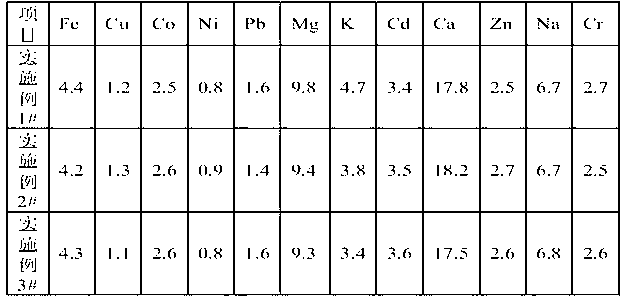
ActiveCN1772345ARich reservesLow pricePhotography auxillary processesDispersed particle separationPyrolusiteElectrolysis
The present invention is method of comprehensively utilizing waste SO2 gas resource and pyrolusite, and the method includes utilizing the composite absorbent comprising pyrolusite slurry and pH buffering agent to produce oxidation-reduction and neutralizing reaction with waste SO2 gas to eliminate SO2 from the waste gas, purifying the absorbed tail liquid to obtain manganese sulfate product through direct evaporating crystallization or metal manganese product through DC electrolysis, and treating the anode liquid to obtain high purity manganese carbonate product and ammonium sulfate product. The present invention realizes treatment of waste with waste to recover sulfur resource and comprehensively utilize pyrolusite. The method of the present invention is reasonable and has no secondary pollution.
Owner:SICHUAN UNIV
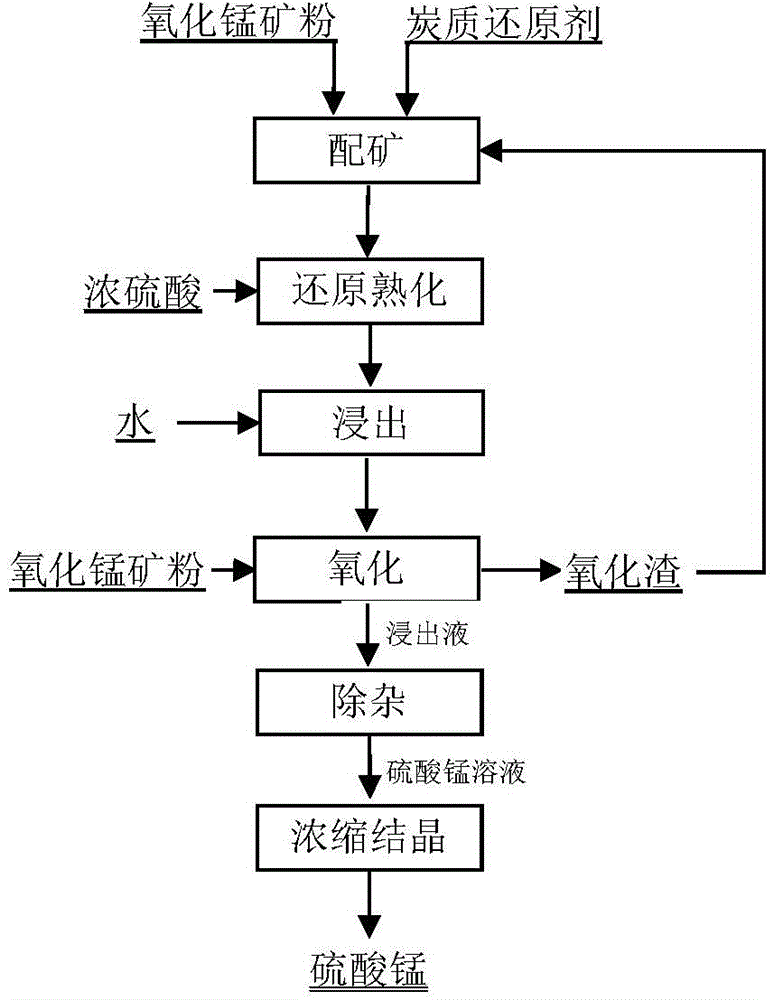
Production method for manganese sulfate by using biological cellulose and low-grade manganese ores
The invention discloses a production method for manganese sulfate by using low-grade manganese ores. According to the method, manganese sulfate with high purity can be produced by using waste low-grade manganese ores with manganese content of 10% to 20%, manganese tailing or manganese-containing solid waste residue. The method comprises the steps of preparation of raw materials, a slaking reaction, a leaching reaction, neutralization and purification of leachate, etc. According to the invention, production of manganese sulfate is not restricted by the grade of manganese ores, and low-grade manganese oxide ores with a grade greater than 10%, manganese tailing or manganese-containing solid waste residue can be fully utilized; produced manganese sulfate has high yield and high purity and is a very important industrial fundamental product; almost no external heat supply is needed, low energy consumption is achieved, production cost is low, it does not need to turn over and mix materials in the process of the reactions, the reactions are smooth, no toxic gas is generated, and no environmental pollution is produced; discharge of three wastes (waste gas, waste water and industrial residue) reaches national discharge standards for environmental protection, and there is no dust pollution in a workshop.
Owner:陈昆先 +1
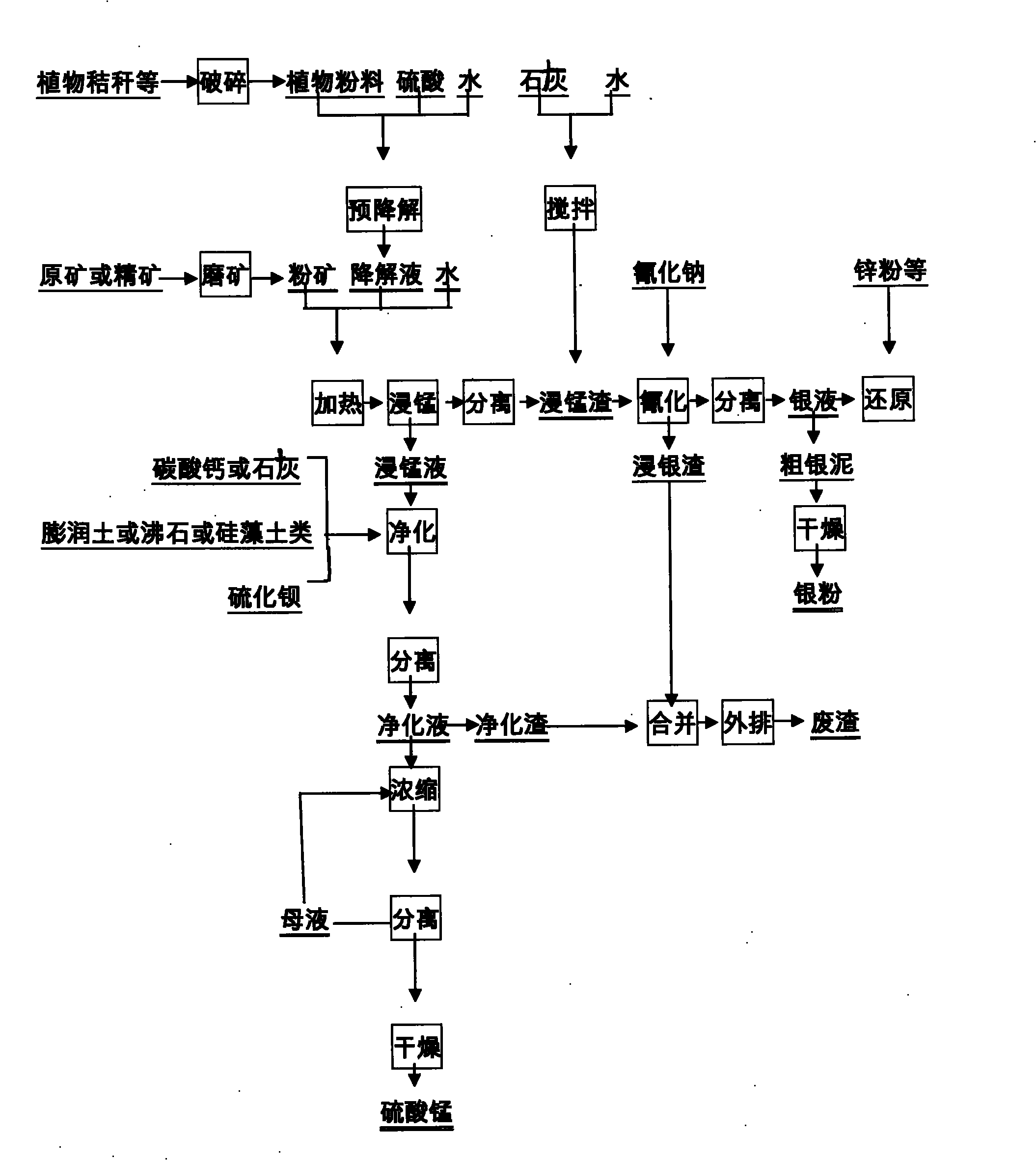
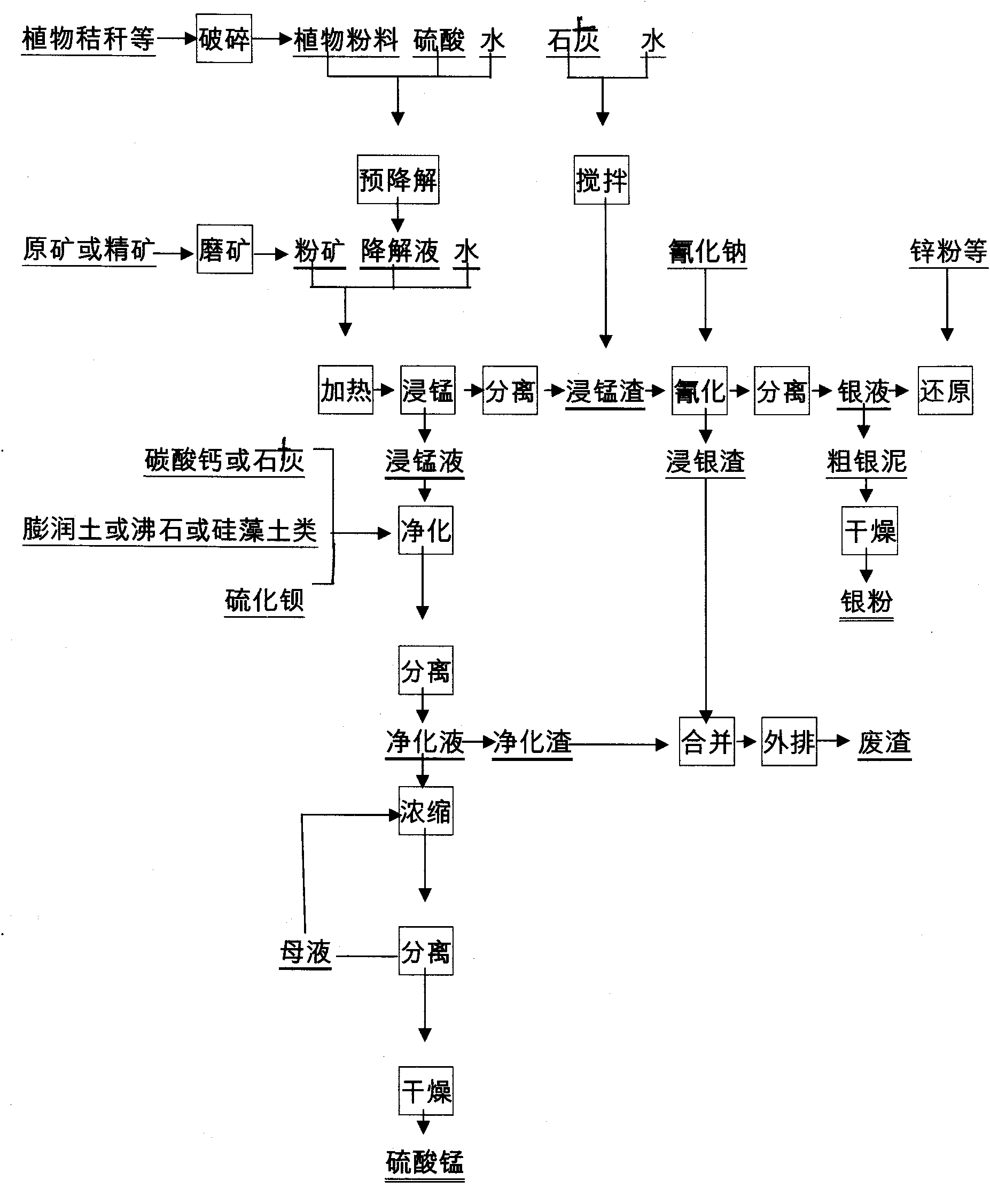
Method for separating manganese and silver of manganese-silver ore and extracting manganese sulfate by purifying manganese dipped solution
InactiveCN101831544AEasy to separateHigh recovery rateProcess efficiency improvementManganese sulfatesPyrolusiteSlag
The invention discloses a method for separating manganese and silver of manganese-silver ore and purifying manganese dipped solution thereof. The method comprises the following steps of: 1) pre-degrading and saccharifying plant byproducts comprising straws, hulls and slag; 2) reacting the product obtained in the step 1) with crude manganese-silver ore or enriched mixed concentrate dipped manganese; 3) separating, neutralizing and adsorbing the manganese dipped solution in the step 2) to obtain solution for preparing manganese sulfate and the like by further purification and crystallization; and 4) obtaining manganese dipped slag in the step 3), wherein the manganese dipped slag has high reaction activity, and the leaching time is short and the energy consumption is low during silver leaching treatment. The purifying method is also suitable for purifying the manganese dipped solution by adopting a reducing agent similar to rice bran and the like to treat pyrolusite or ferromanganese ore, and the treated manganese solution can be used for the production of manganese sulfate, electrolytic manganese and the like. The method has the characteristics of wide separation raw material source, reasonable process route, low equipment investment, low treatment cost, stable product performance and the like; and the manganese sulfate prepared by the method can meet the standard requirements of the industries such as chemical engineering, feed, agriculture and the like.
Owner:ZHENGZHOU MINERALS COMPOSITIVE UTILIZATION RES INST CHINESE GEOLOGICAL ACAD
Method for preparing manganese sulfate
ActiveCN101704554AOvercome the Difficulty of SeparationAvoid difficultiesManganese oxides/hydroxidesManganese sulfatesManganeseSulfide
The invention relates to a method for preparing manganese sulfate. The method comprises the following steps: quantitatively adding sulfide with reducibility into a manganese dioxide ore in a reaction molar ratio for sufficient reaction, determining a reaction end point, and separating and washing a solid-phase resultant; reacting the solid-phase resultant with 9 to 12mol / L H2SO4, controlling the reaction end point to the pH of between 3 and 5, controlling the concentration of MnSO4 in the reaction liquid in a range of between 300 and 400g / L, performing solid-liquid separation after the reaction is completed; acidizing the separated solution by H2SO4 until the pH value of the solution is between 2 and 3, adding hydrogen peroxide into the solution and heating the mixture, precisely filtering and removing the part of solid phase; and distilling, concentrating, crystallizing and dehydrating the filtrate to prepare the manganese sulfate. The method can prepare acid soluble manganous oxide and ensure that various heavy metals can generate insoluble sulfides so as to solve the problem of completely separating heavy metals and obtain the manganese sulfate with low calcium magnesium and low heavy metals.
Owner:贵州红星发展大龙锰业有限责任公司
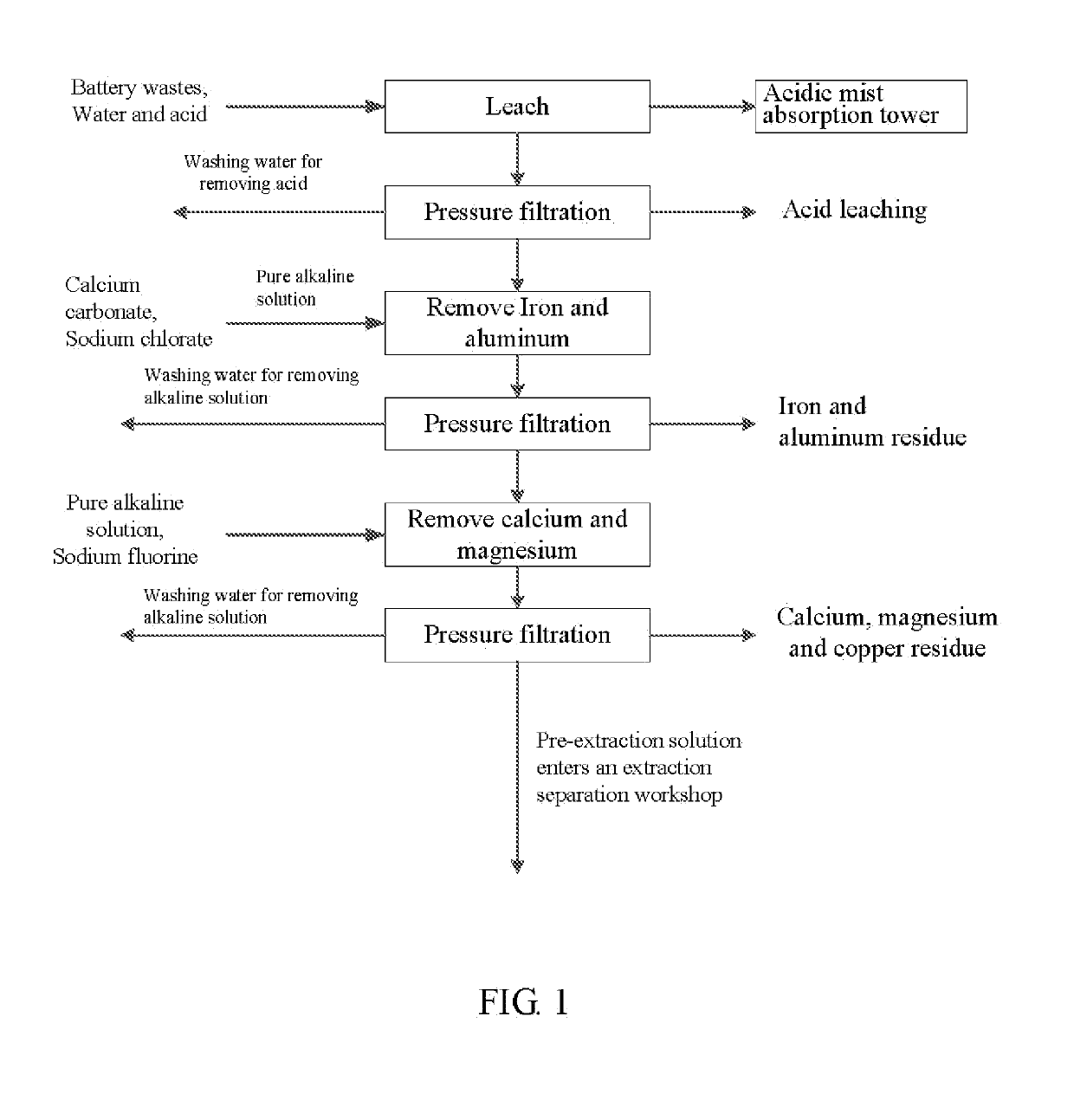
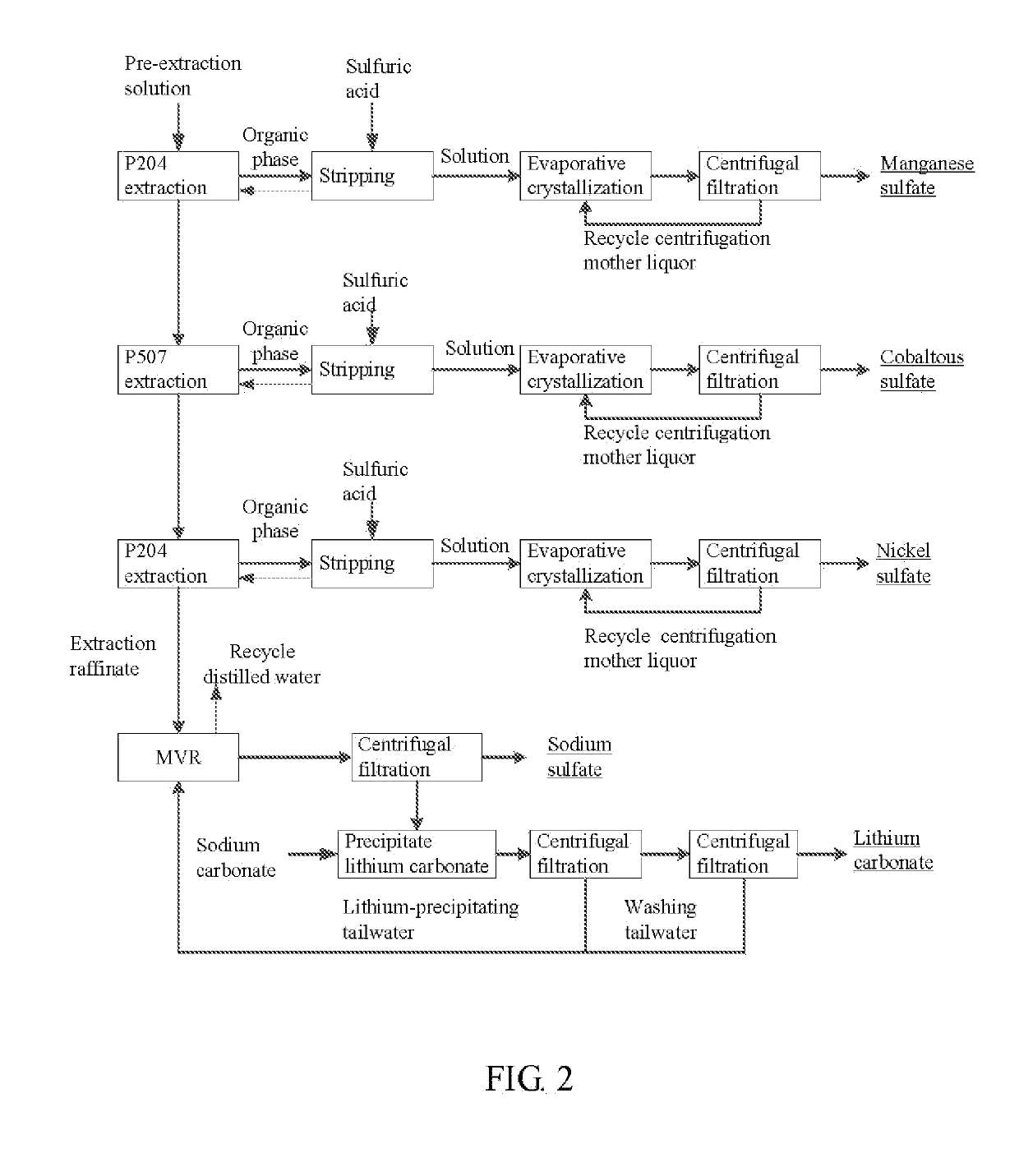
Method for preparing nickel/manganese/lithium/cobalt sulfate and tricobalt tetraoxide from battery wastes
ActiveUS20190152797A1Reduce productionHigh puritySolvent extractionCobalt sulfatesManganeseCobalt Sulfate
A method for preparing nickel / manganese / lithium / cobalt sulfate and tricobalt tetraoxide from battery wastes adopts the following process: dissolving battery wastes with acid, removing iron and aluminum, removing calcium, magnesium and copper, carrying extraction separation, and carrying out evaporative crystallization to prepare nickel sulfate, manganese sulfate, lithium sulfate, cobalt sulfate or / and tricobalt tetraoxide. By using the method, multiple metal elements, such as nickel, manganese, lithium and cobalt, can be simultaneously recovered from the battery wastes, the recovered products are high in purity and can reach battery grade, battery-grade tricobalt tetraoxide can also be directly produced. The method is simple in process, low in, energy consumption and free in exhaust gas pollution, and can realize zero release of wastewater.
Owner:HUNAN JINYUAN NEW MATERIALS CO LTD
Method for preparing manganese sulfate by reduction leaching of manganese ore using discard molasses and sulfuric acid
InactiveCN1884099AHighlight substantive featuresSignificant progressManganese sulfatesSulfateManganese oxide
The invention discloses a new preparing method of manganese sulfate through manganese oxide in the manganese ore in the leaching manganese ore extracting and manufacturing technological domain, which comprises the following steps: grinding manganese ore; moulding ore paste through water; adding sulfuric acid and waste molasses in the ore paste; stirring at 40-100 deg.c to obtain leaching liquid; neutralizing the leaching liquid through limestone; adding ammonia sulfate to remove heavy metal ion; adding ammonium fluoride solution to remove Ca2+, Mg2+; evaporating; condensing; crystallizing; drying to produce manganese sulfate with over 98 percent purity and 93 percent manganese collecting rate.
Owner:GUANGXI UNIV
Method for preparing high-purity manganese sulfate from manganese-containing waste liquid
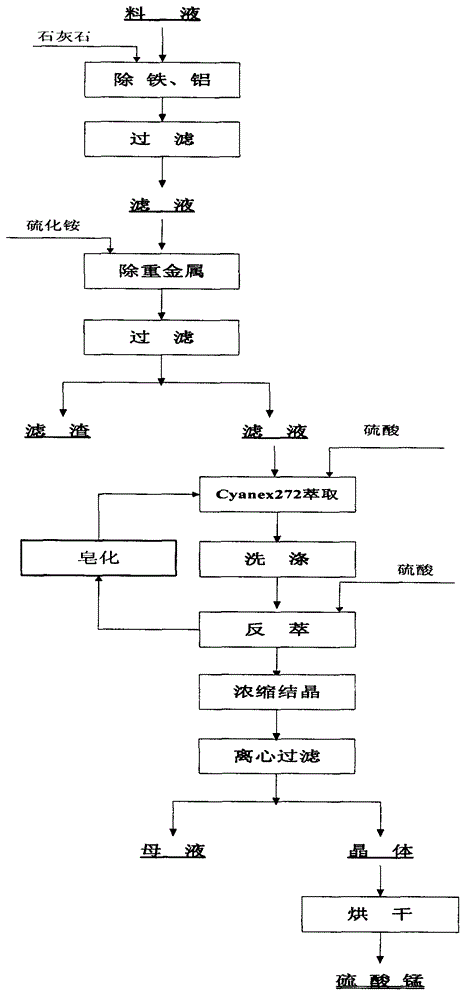
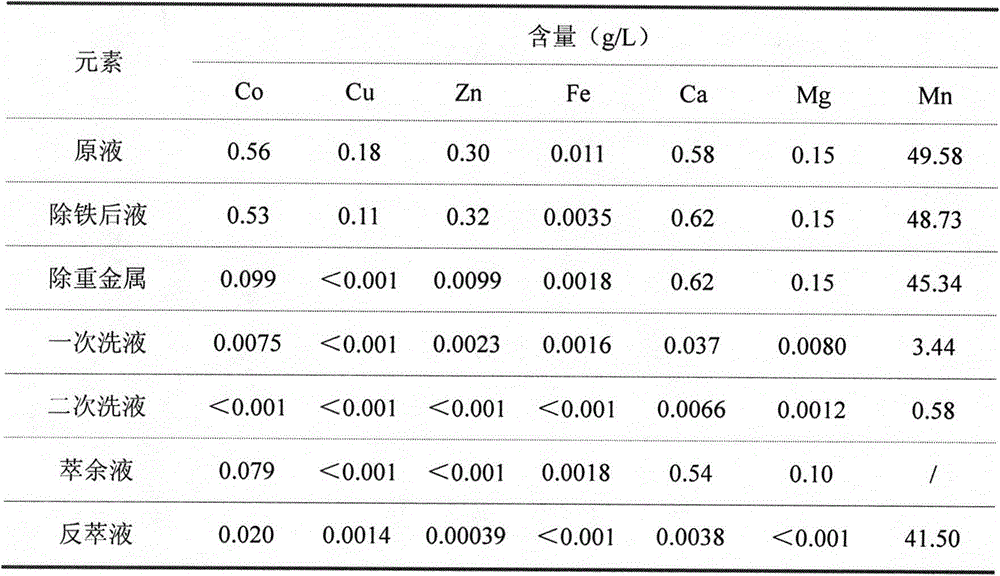
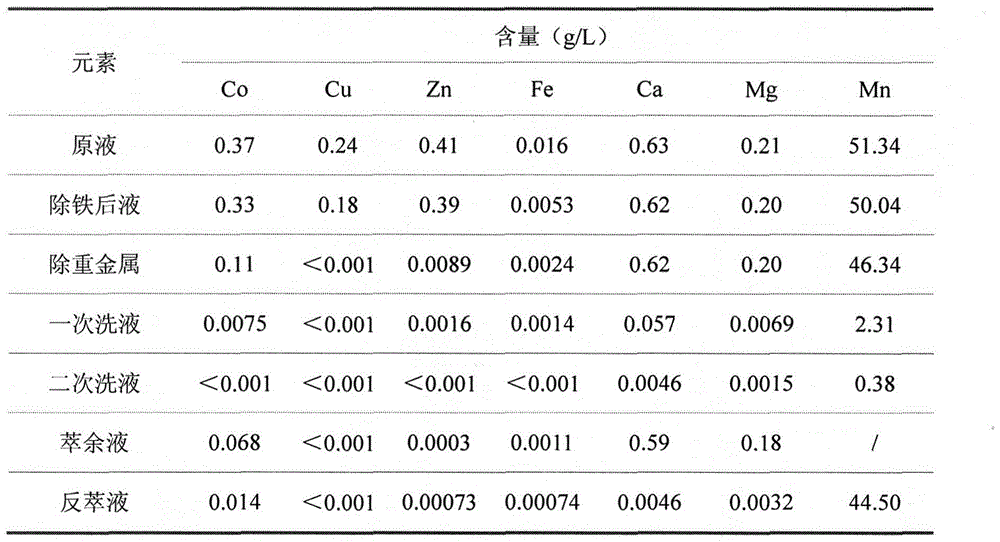
The invention relates to a method for preparing high-purity manganese sulfate from a manganese-containing waste liquid. The existing method for treating the high-manganese-containing waste liquid generated in the wet production of cobalt salts mainly comprises a lime precipitation method and a method for preparing manganese sulfate by removing calcium and magnesium via fluoride and adopting a P204 or P507 extracting agent and has the respective problems that the utilization value of prepared manganese hydroxide is low, the fluoride-containing wastewater is polluted and the like. According to the method, heavy metals are removed by virtue of sulfide, manganese is extracted through a Cyanex272 extracting agent, calcium and magnesium are thrown and the reverse extraction is performed to obtain the high-purity manganese sulfate product which can meet the requirement of a ternary cell material on manganese sulfate; and the method has the advantages of short process flow, no fluoride pollution and the like.
Owner:ZHEJIANG HUAYOU COBALT
Method for concentrating waste sulfuric acid by utilizing waste heat of titanium dioxide calcinator
InactiveCN102910594ASolve manySolve process problemsSulfur compoundsEnergy inputLiquid waterOperability
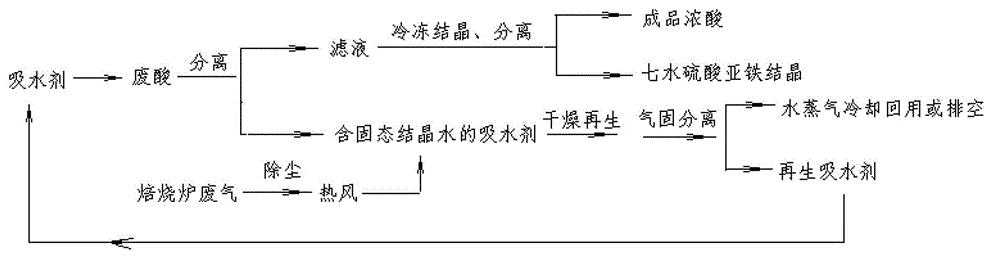
The invention discloses a method for concentrating waste sulfuric acid by utilizing waste heat of a titanium dioxide calcinator, comprising the following steps: at normal temperature and pressure, proper amount of water absorbent is added to titanium dioxide waste acid, liquid water is dissolved out in the form of solid crystal water through the water absorbent, filtrate after solid-liquid separation is cooled, iron vitriol in the solution is crystallized and dissolved out, and the acid liquid is further concentrated; the two steps are repeatedly carried out until the concentration of sulfuric acid in the final filtrate reaches more than 60%, and then the final filtrate is utilized in the acid hydrolysis procedure; and the water absorbent is dehydrated and dried to be regenerated by taking dustproof tail gas of the calcinator, and the steam generated in the drying and regeneration is cooled and flows back to technological process as washing water in titanic acid rinsing or is drained. The method provided by the invention solves the problems of more equipment, long flow and easy blockage in the existing technology, and the equipment investment and operating cost are reduced; and the water absorbent is recycled after being regenerated, so that the energy is saved, the consumption is reduced, and the efficiency is obvious; no wastewater, waste gases and residues are generated in the whole technology, the operability is strong, the productivity of equipment is high, the flow is simple, the investment is less, the cost is low, energy is saved, emission is reduced, and the benefit is obvious.
Owner:黄正源
Method for preparing manganese sulfate by biomass self-heating reduction of low grade manganese oxide ore
InactiveCN101439878AAchieving zero emissionsLow reduction temperatureEnergy inputManganese sulfatesManganese oxideCoal
The invention discloses a method for preparing manganese sulfate through the biomass self heating reduction of low-grade manganese oxide ore. The method comprises the following steps: 1) respectively crushing the low-grade manganese oxide ore and biomass material; 2) weighing the low-grade manganese oxide ore, the biomass material and an initiating additive according to the weight ratio of 100:10-30: 0.5-3; 3) mixing evenly and roasting the low-grade manganese oxide ore, the biomass material and the initiating additive; 4) leaching the roasted mass through dilute sulfuric acid at a temperature of between 40 and 80 DEG C, regulating the mixed liquor to make the pH value more than or equal to 6, carrying out solid-liquid separation, and keeping the obtained filtrate for standby; and 5) removing impurities from the filtrate to obtain the manganese sulfate solution. The method adopts renewable energy source of the biomass material to replace coal to reduce the manganese oxide ore and prepare manganese sulfate which can be the raw material for producing electrolytic manganese. The leaching efficiency of manganese can reach more than 95 percent. The method not only saves energy sources, but also reduces the production cost.
Owner:李学军 +2
Method for production of high purity manganese sulfate by using pyrolusite as raw material
ActiveCN103011297AImprove controllabilityGood reproducibilityManganese sulfatesPyrolusiteAcid dissolution
The invention relates to a production method for high purity manganese sulfate. The production method is characterized in that pyrolusite is used as a raw material and is reduced by using a carbon fire method, then an acid is added for leaching, a vulcanizing agent is used to remove heavy metals consisting of cobalt and nickel, a fluoride is employed to remove calcium and magnesium, then filtering is carried out, ammonium bicarbonate or ammonium carbonate is added into a filtrate for deposition of manganese to realize separation of potassium and sodium, an obtained filtrate is used for recovery of the by-product ammonia sulfate for usage as an additive for metal manganese, the filter residue manganese carbonate is washed and then subjected to acid dissolution so as to obtain a manganese sulfate solution, and then crystallization is carried out to obtain a high purity manganese sulfate product, wherein mother liquor can be returned to a high purity system for reutilization. With the method provided by the invention, the high purity manganese sulfate product in which MnSO4 content is more than 99.8% and respective content of impurities consisting of Ca, Mg, K and Na is lower than 10 ppm is produced.
Owner:DAXIN MANGANESE MINE BRANCH OF CITIC DAMENG MINING IND
High purity manganese sulfate monohydrate and preparation method thereof
ActiveCN101875507AReduce contentEasy to getManganese sulfatesManganese sulphate monohydrateHeavy metals
The invention relates to manganese sulfate and a preparation method thereof, in particular to high purity manganese sulfate monohydrate and a preparation method thereof. In the high purity manganese sulfate monohydrate, the content of manganese is more than 32%; the content of impurities of K, Na, Ca and Mg are lower than 50ppm; and contents of heavy metal and iron impurity are both lower than 10ppm. The preparation method comprises the steps of: firstly adjusting the pH value of solution to 3.5 to 5 for remove impurities for the first time by using iron sulfate as an impurity removing agent; then adjusting the pH value to 4 to 7 using manganese carbonate; adding an adsorbent to remove the impurities for the second time; removing the impurities for the third time by using manganese fluoride as an impurity removing agent under the condition that the pH value is 4 to 7; then removing the impurities for the fourth time by using barium sulfide as an impurity removing agent under the condition that the pH value is 4 to 7; and finally concentrating, crystallizing, washing and drying to obtain the high purity manganese sulfate monohydrate. In the manganese sulfate monohydrate of the invention, the contents of all main metal impurities are lower; the content of the manganese is high; and the requirement of the anode material of a lithium-ion battery can be satisfied.
Owner:贵州大龙汇成新材料有限公司
Method for producing manganese sulfate from manganese oxide ores
ActiveCN104817116ANo pollution in the processReduce energy consumptionManganese sulfatesDithionateDecomposition
The invention provides a method for producing manganese sulfate from manganese oxide ores. The method comprises the steps of mixing the manganese oxide ore powder with an appropriate amount of carbon reducing agent such as pulverized coal evenly, adding concentrated sulfuric acid without adding water and mixing evenly to obtain a mixture, controlling the initial concentration of the sulfuric acid in the mixture to be greater than or equal to 70%, curing the mixture by use of reaction heat via self-heating reduction and reducing the quadrivalent manganese in the manganese ores into bivalent manganese, mixing the cured material with water for stirring and leaching, and filtering the leached ore pulp to obtain a leachate, adding an appropriate amount of manganese oxide ores to the leachate to realize the oxidative decomposition of manganese dithionate, and performing neutralization, purification and impurity removal and crystallization on the solution obtained by filtering to produce the manganese sulfate. The method has the advantages that the low-cost easily available carbon reducing agent such as the pulverized coal is utilized to directly reduce the manganese oxide ores at a low temperature, and therefore, the energy consumption and the cost are low and the smoke pollution problem of reducing roasting is avoided; the manganese oxide ore powder is utilized to realize the oxidative decomposition of the manganese dithionate in the manganese sulfate solution and therefore, the quality of the manganese sulfate product is improved.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Preparation of high purity manganese sulfate
The invention provides a method for preparing high-purity manganese sulfate. The method comprises the following steps: preparing a manganese sulfate solid containing impurities into a manganese sulfate solution, or leaching manganese ore in sulfuric acid to obtain the manganese sulfate solution; adding an impurity removing agent MnF2 into the manganese sulfate solution to remove impurities which are Ca and Mg; using MnS or metal manganese powder as the impurity removing agent to remove heavy metal impurities so as to avoid the introduction of impurities and ensure the effect of impurity removal, and simultaneously controlling the pH value for reaction to between 4 and 10. The impurity removing agent MnF2 and MnS or the metal manganese powder can either be simultaneously added, or added one after another respectively to obtain the high-purity manganese sulfate through filtering, concentrating at normal pressure, crystallizing, dehydrating and drying. The method has the advantages that the method can obviously reduce the amount of Ca and Mg impurities and heavy metal impurities contained in a common manganese sulfate product or the manganese sulfate solution after the manganese ore is leached by sulfuric acid, and ensure that the content of Ca and Mg is lower than 100 ppm, and the content of the heavy metal impurities is lower than 50 ppm, and purity of the obtained manganese sulfate product can reach over 99 percent to meet the requirement of the manganese sulfate product used for ternary materials in the electronic industry.
Owner:湖北开元化工科技股份有限公司
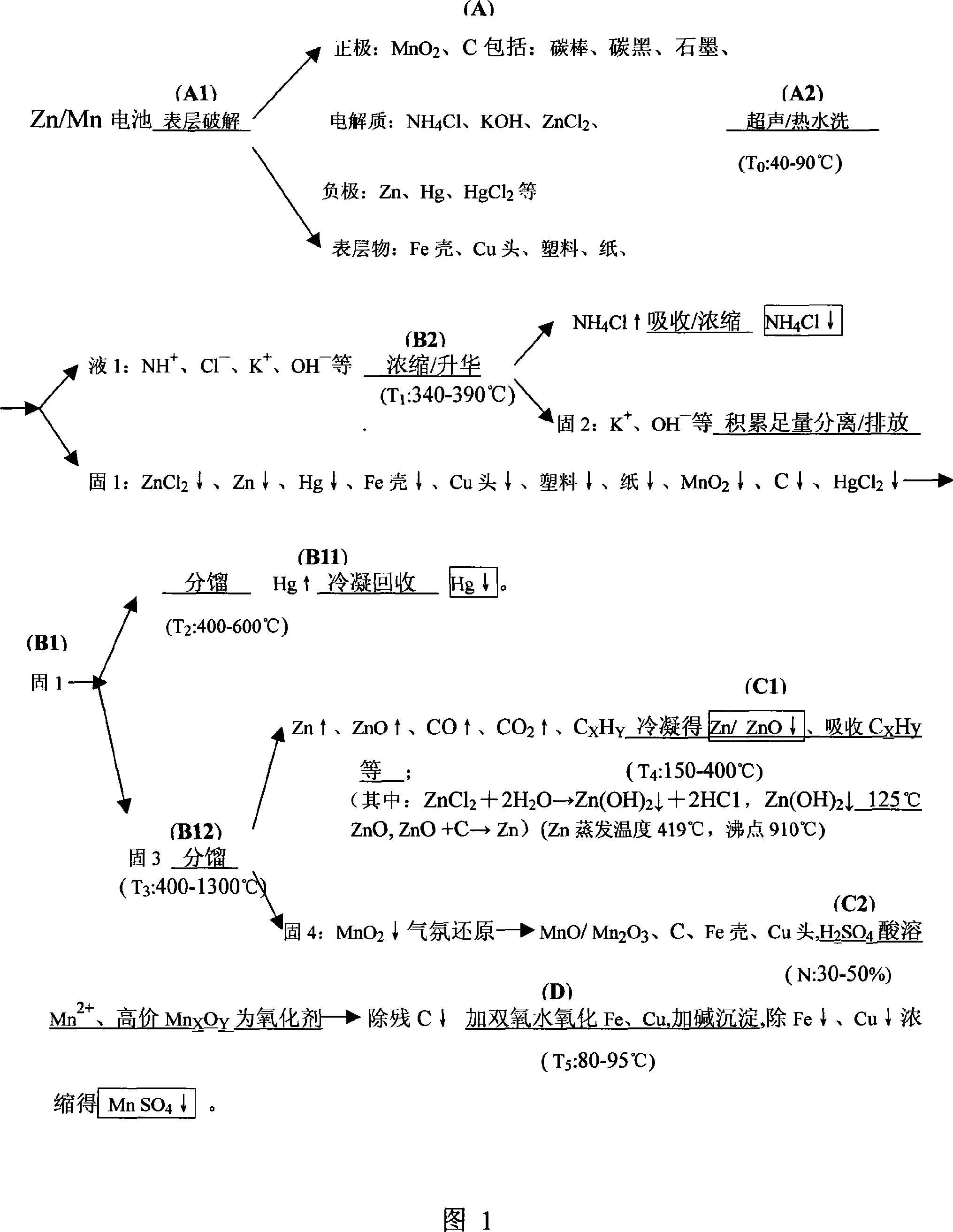
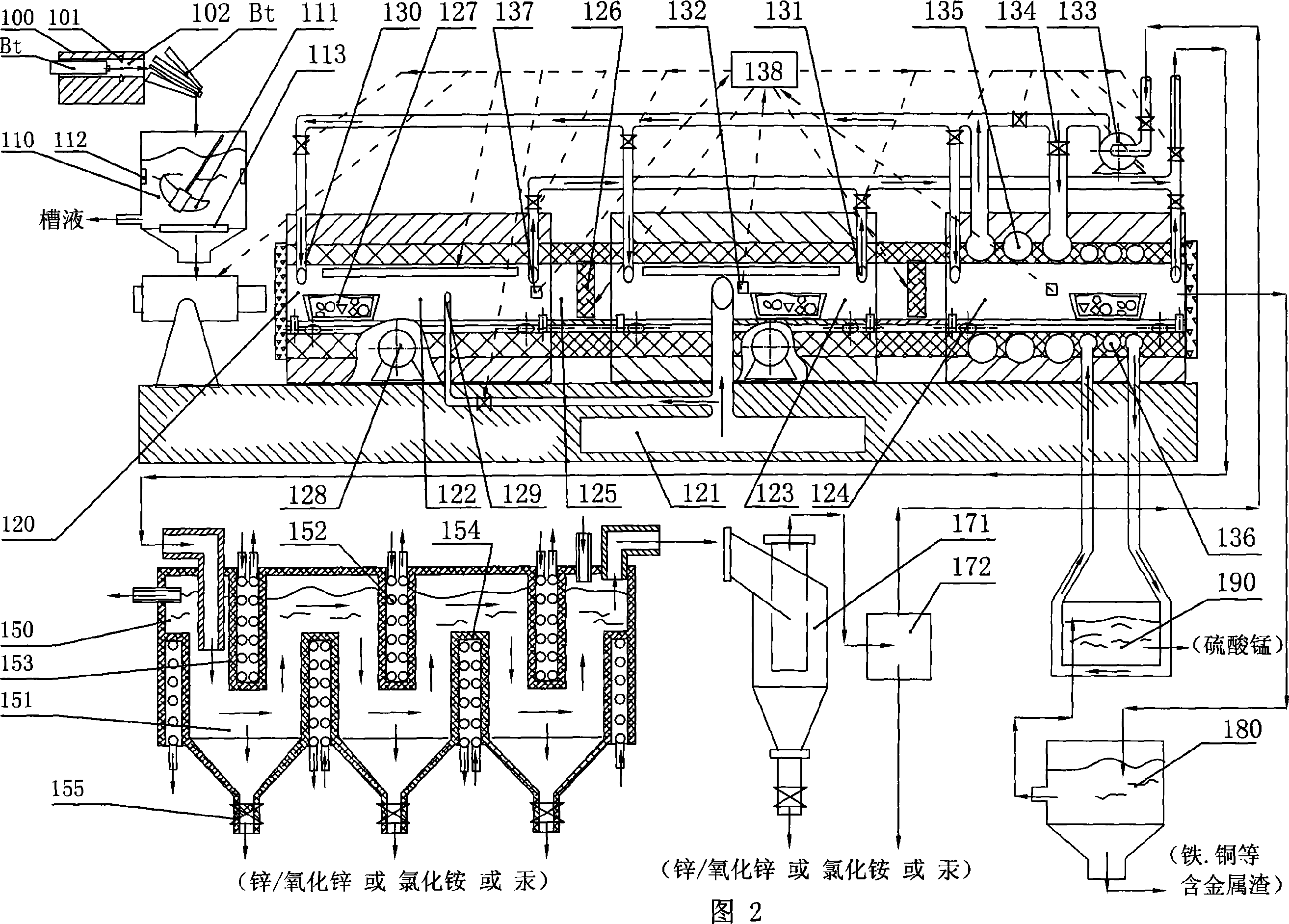
Selectivity volatilization reclaiming technique for waste zinc-manganese battery and reclaiming system thereof
ActiveCN101054187AGood for acid soluble recoveryFull recoveryZinc oxides/hydroxidesReclaiming serviceable partsSurface layerSulfate
The present invention relates to a selective volatilization recovery technology for abandoned zinc-manganese battery, which comprises the steps of that: the surface layer of the battery is broken and scrubbed, thereby the tank liquor, washed up surface layer matter and contained matter are obtained; then the remaining solid containing manganese / ferric oxide and high pure gas are obtained by high temperature carbonization in a properly closed roaster, wherein the temperature inside of the roaster is controlled; the multiple scrubbed tank liquor is heated and concentrated to obtain concentrate which is further heated and cooled to obtain ammonium chloride with a high purity; the distilled off hot gas is guided into a condensation and recovery container and is cooled to be separated and collected thereof, thereby the powder containing zinc and / or zinc oxide is obtained; the remaining solid is subjected to acid soluble to obtain the manganese sulfate primordial solution which is then subjected to oxidation, alkali-settling and separation so as to obtain the purified manganese sulfate solution. In the present invention, the bulk inclusion in the zinc-manganese battery can be completely recovered, the recovered product has a higher purity, the recovery operation and the equipment thereof is simple, and secondary pollution will not occur basically.
Owner:GEM CO LTD
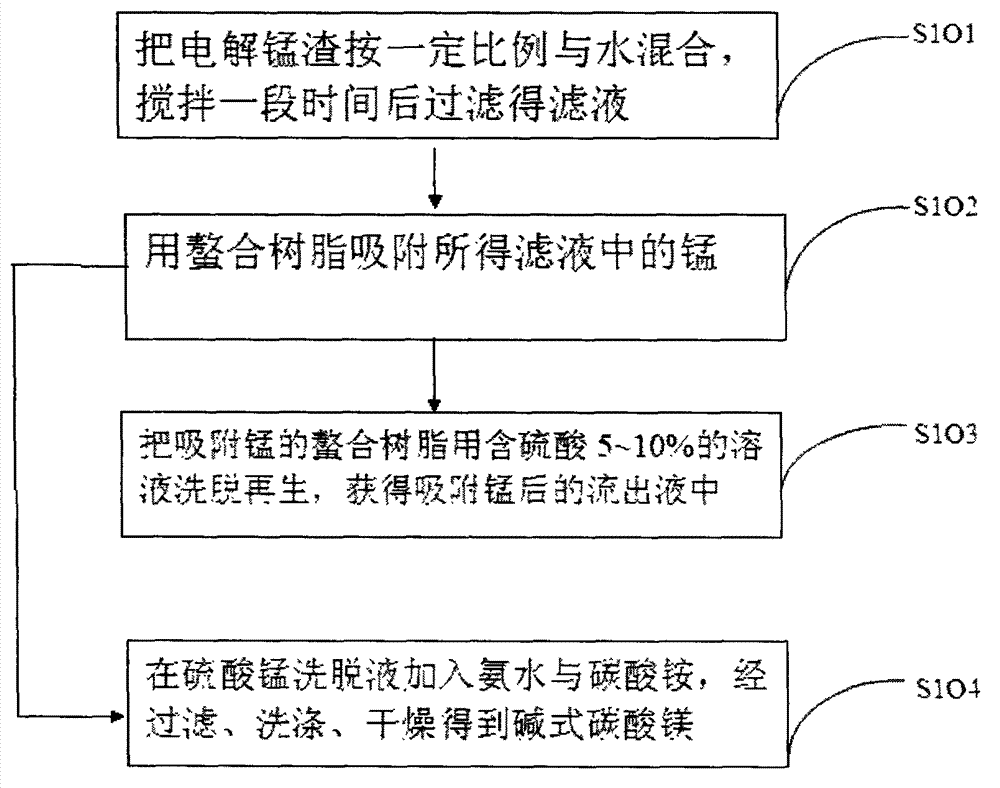
Method for recovering water soluble manganese and magnesium from electrolytic manganese residue
The invention discloses a method for recovering water soluble manganese and magnesium from electrolytic manganese residue. The method comprises the following steps: firstly, soluble salt in the electrolytic manganese residue is dissolved with water; secondly, by utilizing the manganese combining capability of chelating resin , which is far stronger than the magnesium combining capability of the chelating resin, a liquid is allowed to flow through a chelating resin column, manganese is absorbed and then flows out along with an outgoing liquid; and after absorbing the manganese, a resin-used sulfuric acid liquid is eluted to recover, thus the manganese is changed into a manganous sulfate liquid and enters into the liquid preparation procedures for an electrolytic manganese production system, and also can be used to prepare manganese salt. Liquid ammonia and ammonium carbonate are added into the outgoing liquid absorbing the manganese to enable the magnesium to deposit; alkali magnesium carbonate is obtained via filtering, washing and drying; and the deposited and filtered liquid is an ammonium sulfate liquid, which can be used to produce chemical fertilizers and electrolyte manganese production, and solves the problems that the electrolyte manganese residue contains a large amount of soluble manganese salt and magnesium salt and can enter into a soil environment, an underground water environment and a surface water to cause resource waste and environmental pollution.
Owner:JISHOU UNIVERSITY
Method for producing manganese sulfate by high-temperature crystallization process
InactiveCN101224908AReduce manufacturing costImprove product qualityManganese sulfatesManganese sulphateManganese(II) carbonate
The invention provides a method for producing high-purity manganese sulfate by adopting a high temperature crystallization method, which includes that the ore pulp of manganese ore and sulfurous iron ore is heated, removed of iron, removed of heavy metal, neutralized, pressure-filtered to obtain a manganese sulfate solution with the content of 160-200g / L, which is pumped to a manganese sulfate solution for carrying out crystallization and purification; the crystallization and purification includes crystallization, solid-liquid separation, dissolution, pressure filtration and other technologies; the pressure-filtered manganese sulfate solution can be crystallized and purified repeatedly as required. The invention can adopt a large amount of low-grade manganese ore with the manganese content of 10-20 percent as the raw material to produce the high-purity manganese sulfate solution and further deep process various high-purity manganese products, such as: electrolytic manganese dioxide, high-purity manganese carbonate, electronic grade mangano-manganic oxide and manganese monoxide, chemical manganese dioxide, industrial grade manganese sulfate monohydrate, chemical grade manganese sulfate monohydrate, medicine grade manganese sulfate monohydrate, food grade manganese sulfate monohydrate, analytically pure manganese sulfate monohydrate and so on.
Owner:广西双德锰业有限公司
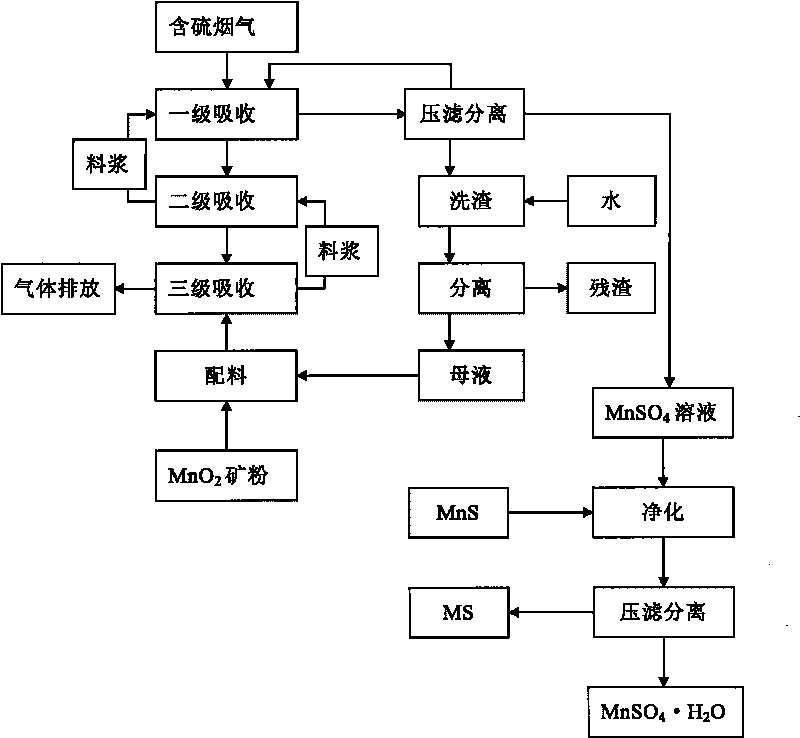
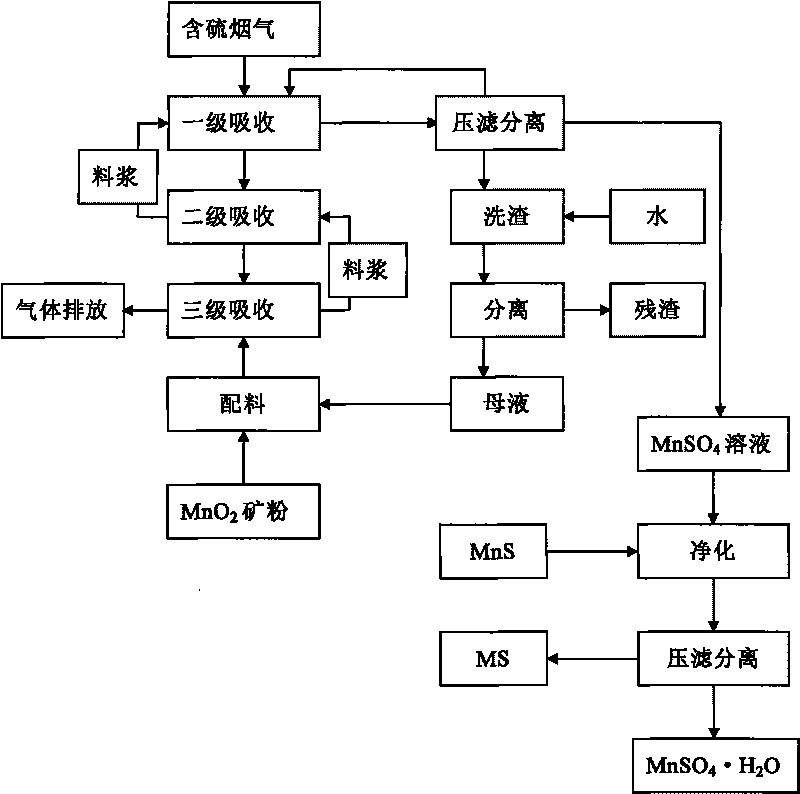
Method for preparing MnSO4.H2O by performing flue gas desulphurization on medium-and-low-grade MnO2 ore
ActiveCN101723466ASolve production costHigh recovery rateDispersed particle separationManganese sulfatesManganese sulphateFlue gas
Owner:GUIZHOU REDSTAR DEVING
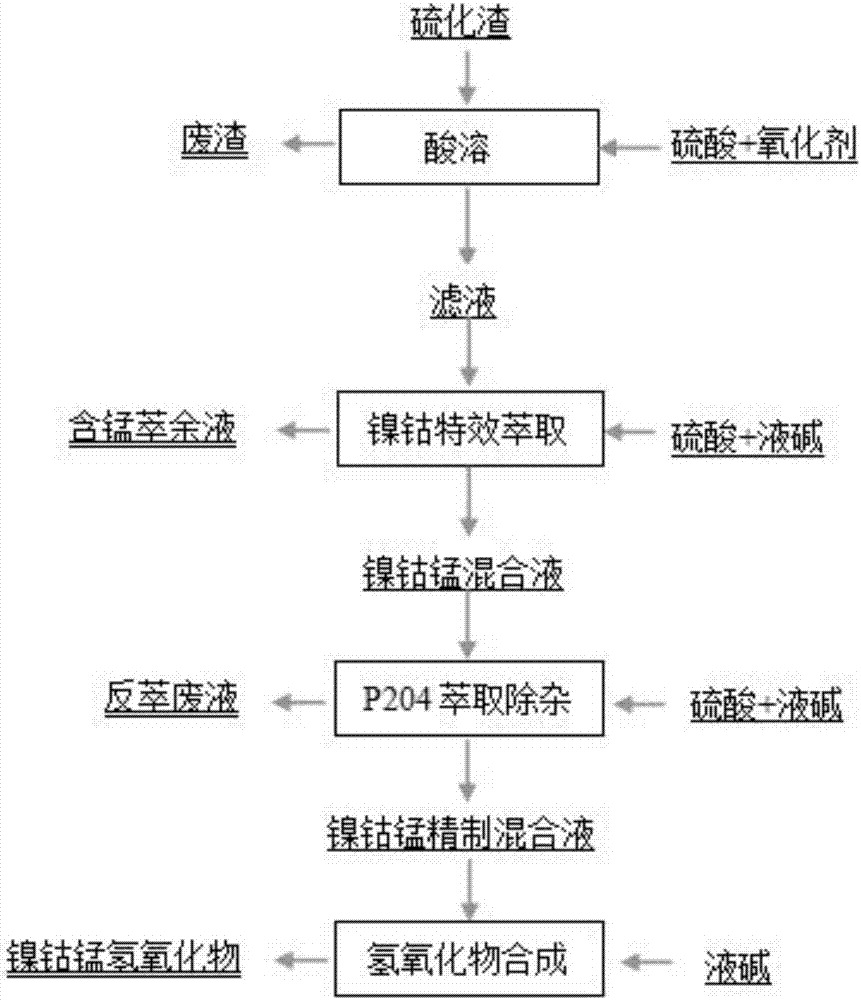
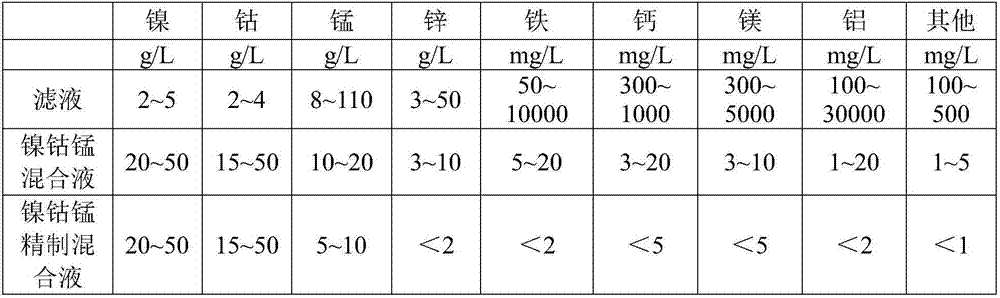
Method for recovering and purifying nickel cobalt from manganese-containing waste material
ActiveCN106904667AReduce security risksLow costNickel compounds preparationManganese sulfatesManganeseSulfide
The invention relates to a method for recovering and purifying nickel cobalt from a manganese-containing waste material. The method comprises the following steps: the manganese-containing waste sulfide residues is subjected to slurrying, an acid solution and oxidant are added to the sulfide residues for being dissolved, and the acid-soluble waste residue and a filtrate are obtained; the filtrate is subjected to saponification by liquid caustic soda and then extraction by a nickel cobalt specific extractant to obtain a raffinate containing manganese; a nickel and cobalt-organic phase is extracted by dilute sulfuric acid to obtain a nickel cobalt manganese mixture; the nickel cobalt manganese mixture is subjected to impurities extraction with a dodecyl alkyl phosphate extraction agent after saponification and then is subjected to purification to obtain a nickel cobalt manganese refining mixture; according to the stoichiometric ratio of nickel cobalt manganese metals, the corresponding sulfate is supplemented, and a precursor nickel cobalt manganese hydroxide of lithium cobalt nickel manganese is synthesized by adding the liquid caustic soda. The method has the advantages of effective utilization of waste resources, low cost, good impurity removing effect, and small environmental risk.
Owner:GUIZHOU DALONG HUICHENG NEW MATERIAL CO LTD
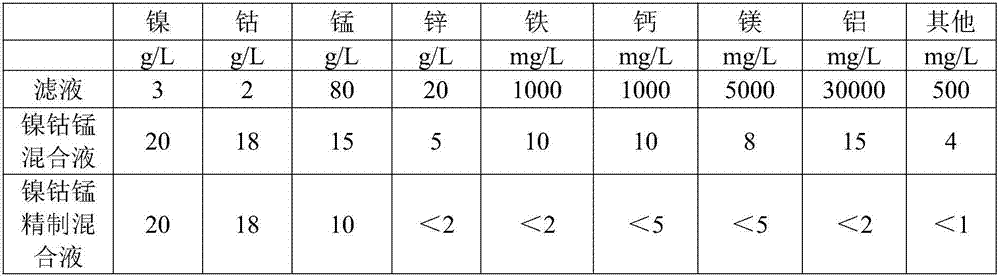
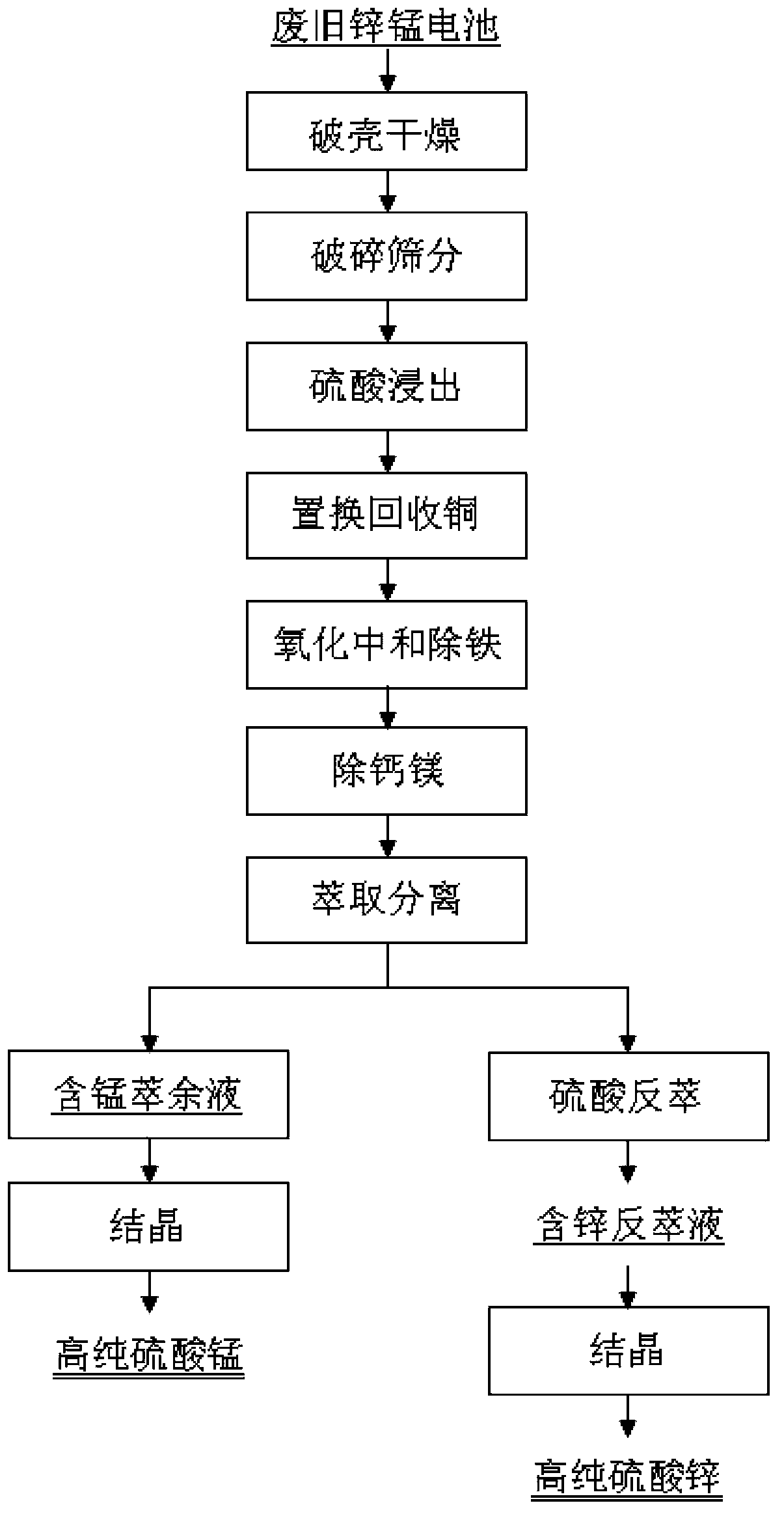
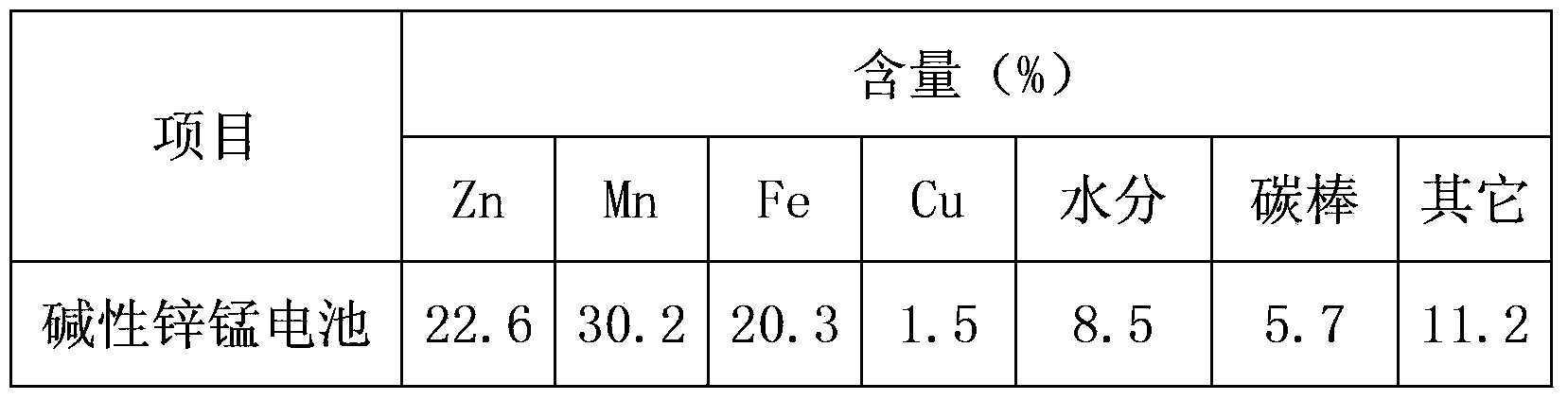
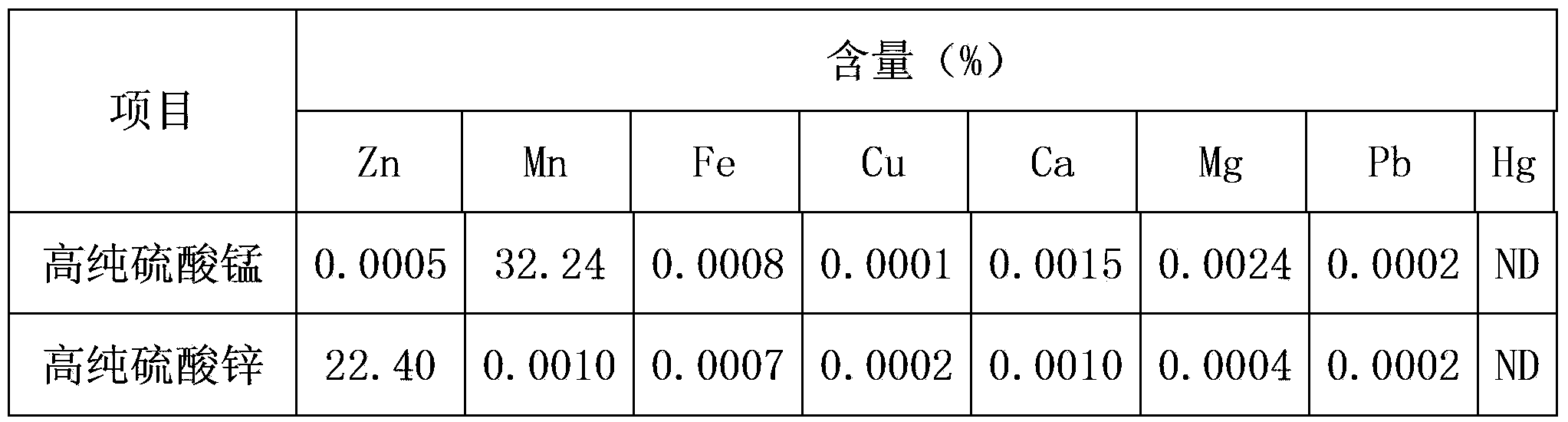
Method for preparation of high purity manganese sulfate and zinc sulfate from waste zinc-manganese batteries
ActiveCN104229898AStrong ability to useEfficient recyclingZinc sulatesManganese sulfatesChemical industryResource utilization
The invention discloses a method for preparation of high purity manganese sulfate and zinc sulfate from waste zinc-manganese batteries. Zinc-manganese batteries mainly contain manganese, zinc, iron, copper and other valuable metal components, and by means of sulfuric acid dissolution, iron powder replacement, oxidation neutralizing for iron removal, extraction purification and separation, crystallization and other processes, high purity manganese sulfate and zinc sulfate products can be prepared. The high purity manganese sulfate obtained by the invention can be used for preparation of battery materials, and the high purity zinc sulfate can be used for medicine, feed, food, chemical industry and other fields. The method provided by the invention has the characteristics of high resource utilization and recovery rate, and high product quality, etc.
Owner:HUNAN BRUNP RECYCLING TECH +1
Comprehensive utilization method for separating, concentrating and purifying manganese sulfate, magnesium sulfate and calcium sulfate in high-salt waste water
ActiveCN103553138AAchieving circular economy goalsImprove energy savingCalcium/strontium/barium carbonatesCalcium/strontium/barium sulfatesSulfateEvaporation
The invention relates to a comprehensive utilization method for separating, concentrating and purifying manganese sulfate, magnesium sulfate and calcium sulfate in high-salt waste water. The method is characterized by comprising the following steps of maintaining the temperature of mother liquor of waste water at 50DEG C to 60DEG C, utilizing ammonia water or liquid ammonia to adjust the pH value of the waste water to 10 to 1.5, collecting separating manganese ions and magnesium ions in the waste water in a form of manganese hydroxide and magnesium hydroxide, adding sulfuric acid into the separated manganese hydroxide and magnesium hydroxide to prepare a manganese sulfate solution and a magnesium sulfate solution, and adopting a segmented evaporation crystallization method to realize the separation to produce manganese sulfate monohydrate and magnesium sulfate hexahydrate; adding lime milk into the waste water after the manganese hydroxide and magnesium hydroxide are separated to adjust the pH value, crystallizing calcium sulfate, precipitating the calcium sulfate, and press filtering the calcium sulfate to produce calcium sulphate dehydrate. After being treated by utilizing the method, the waste water can reach the emission standard and can be externally discharged or recycled by the enterprise, and solid wastes in the waste water can be completely recycled. The investment is low, and the annual net investment return rate is more than 15 percent.
Owner:中诚和易(北京)国际科技有限公司
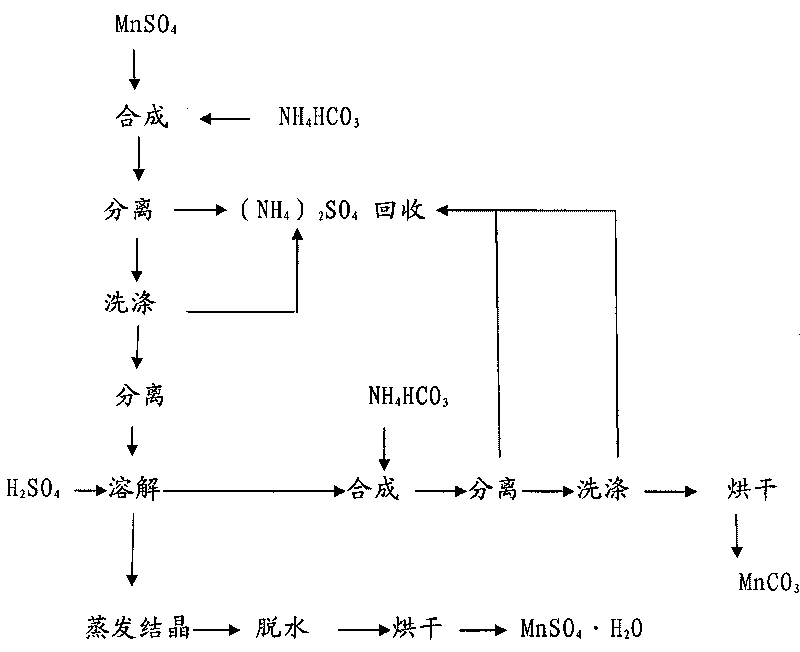
Method for circularly purifying manganese sulfate and manganese carbonate
The invention relates to a method for circularly purifying manganese sulfate and manganese carbonate. The method comprises the following steps: A, stirring manganese sulfate solution at the temperature of between 40 and 80 DEG C, and adding ammonium bicarbonate into the solution for synthesis, controlling the synthesis end point to be an equimolar reaction, separating solid, and washing the solid with hot water to prepare manganese carbonate; B, adding the prepared manganese carbonate into 6 to 12mol / L H2SO4 for reaction, controlling the pH value of the reaction solution to be between 1 and 2, and heating and boiling the solution; C, adding the manganese carbonate prepared in the step A into the reaction solution in the step B, and adjusting the pH value of the solution back to be 4 to 5; and D, performing solid-liquid separation on the reaction solution, distilling and crystallizing the prepared filtrate, and drying the crystal to prepare the manganese sulfate. The circularly purifying method can prepare high-purity manganese sulfate and manganese carbonate with low calcium magnesium and low potassium and sodium.
Owner:GUIZHOU REDSTAR DEVING +1
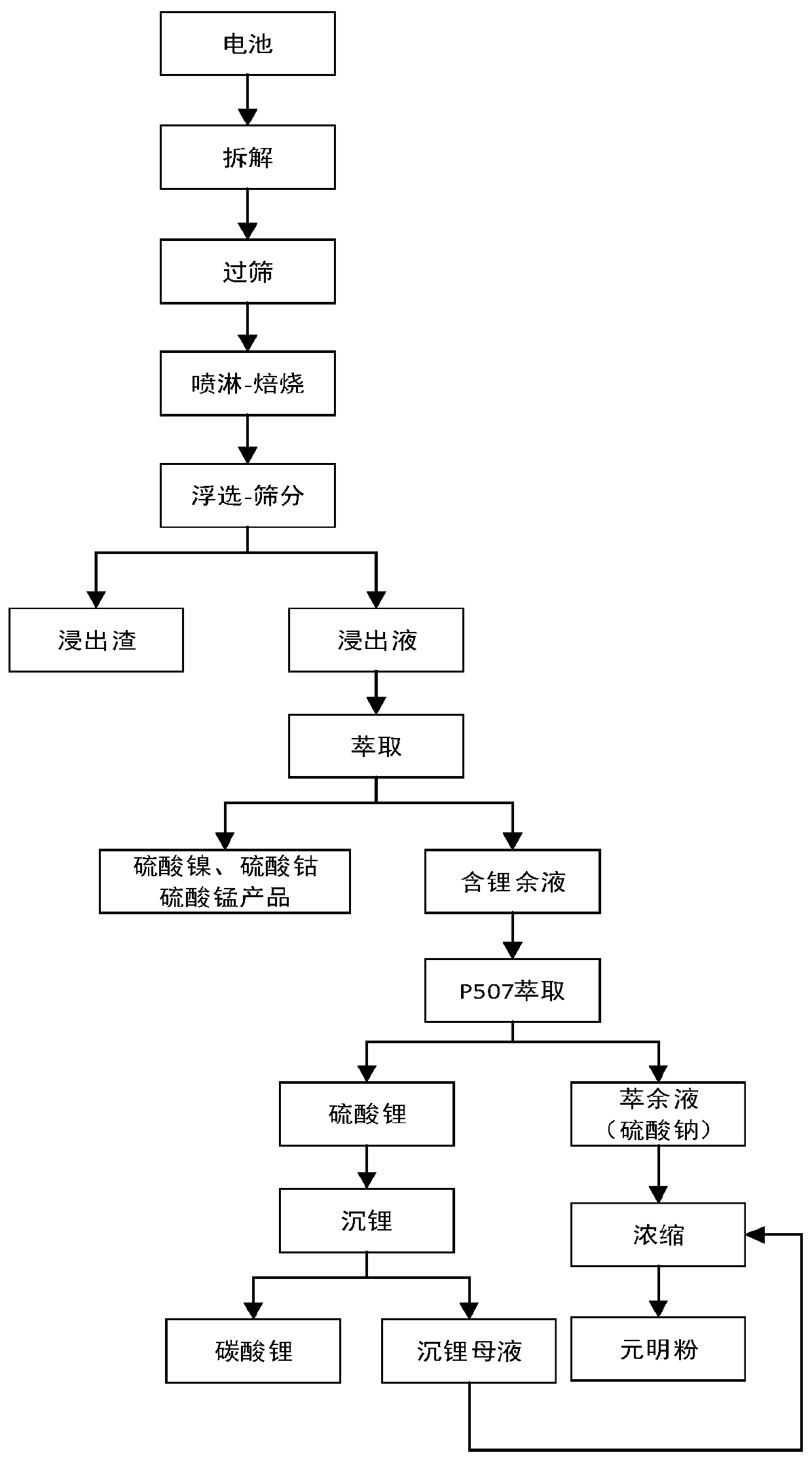
Power lithium ion battery all metal recycling and cyclic utilizing method
ActiveCN110616331AAchieve recyclingShort processCobalt sulfatesWaste accumulators reclaimingElectrical batteryLithium carbonate
The invention discloses a power lithium ion battery all metal recycling and cyclic utilizing method. Waste lithium ion batteries serve as raw materials, the disassembling-screening and roasting procedures are adopted for separating battery pole powder from other parts in the batteries, then, acid leaching is conducted, and leaching liquid containing cobalt, nickel, manganese and lithium is obtained; through extraction, separation and purification of cobalt, nickel, manganese and lithium are achieved, battery-level cobaltous sulfate, nickel sulfate and manganese sulfate are obtained, then, lithium-sodium separation is conducted, and lithium carbonate and sodium sulfate products are obtained through lithium deposition and concentration. The power lithium ion battery all metal recycling and cyclic utilizing method is green and efficient, danger waste generation is avoided, and large-scale production can be achieved. Various valuable metals are systematically recycled from the waste powerlithium batteries, according to the recycling rates, Co is larger than 95%, Ni is larger than 95%, Mn is larger than 98%, Li is larger than or equal to 94%, and the water cyclic utilization rate is larger than 95%. Nickel sulfate liquid, cobaltous sulfate liquid, manganese sulfate liquid and lithium carbonate obtained through the power lithium ion battery all metal recycling and cyclic utilizing method meet the battery level product standard.
Owner:衢州华友资源再生科技有限公司
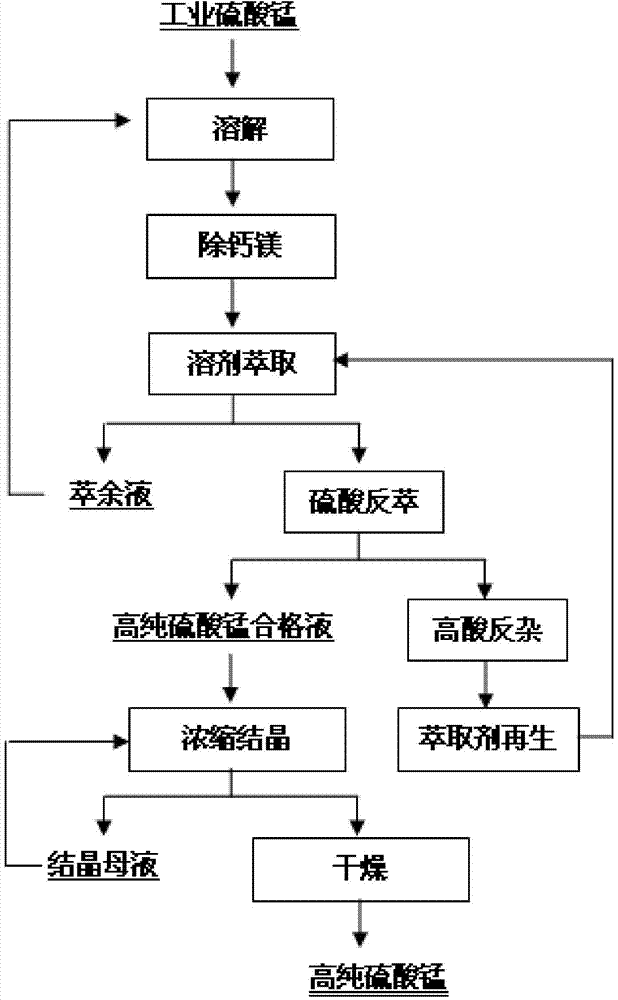
Method for preparing high-purity manganese sulfate with industrial manganese sulfate as raw material
The invention discloses a method for preparing high-purity manganese sulfate with industrial manganese sulfate as a raw material. The method is characterized in that the method in combination of chemical impurity removal and extraction impurity removal is carried out to remove calcium, magnesium, potassium, sodium, zinc, iron, halogen and other anions and cations from industrial manganese sulfate. The prepared high-purity manganese sulfate is high in purity and few in impurities; the method has the advantages of being simple in chemical impurity removal, less in villiaumite consumption, high in extraction impurity removal performance, capable of separating a plurality of anions and cations, free of alkali for organic regeneration, low in cost, etc.
Owner:HUNAN BRUNP RECYCLING TECH +1
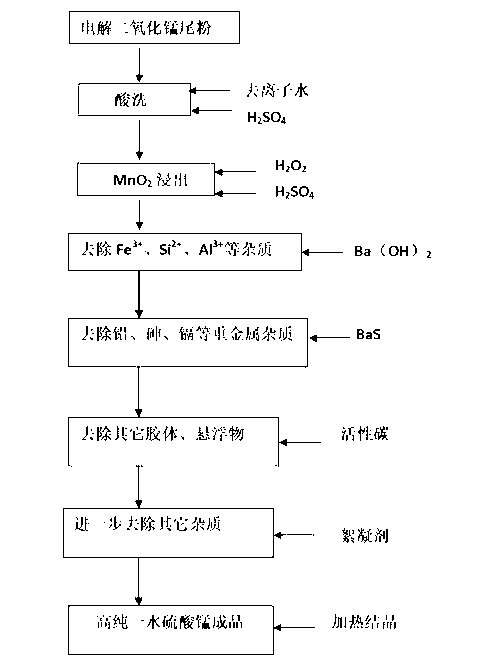
Method for high-purity manganese sulfate monohydrate
InactiveCN103342390ALess impuritiesQuality improvementManganese sulfatesElectrolysisChemical products
The invention relates to the preparation field of chemical products and particularly relates to a method for high-purity manganese sulfate monohydrate. The method specifically comprises the following steps of: taking tail powder of electrolytic manganese dioxide as a raw material, carrying out water washing and acid washing, then carrying out leaching and reducing on MnO2 by taking hydrogen peroxide as a reducing agent, adding barium hydroxide solution, removing Fe<3+>, Si<2+> and Al<3+>, adding barium sulfide solution into the obtained solution, heating the solution to 60-80 DEG C after uniform stirring, carrying out solid-liquid separation, and removing heavy metal elements such as Pb; adding activated carbon in the solution, carrying out stirring and solid-liquid separation at 60-80 DEG C, adding polyacrylamide as a flocculant into the solution after being subjected to activated carbon separation, standing for 24-36 hours after uniform stirring, and carrying out the solid-liquid separation; and carrying out high-temperature crystallization on the obtained solution so as to obtain a manganese sulfate monohydrate product. According to the method, the tail powder produced during the production of the electrolytic manganese dioxide is adequately utilized, the high-purity manganese sulfate monohydrate is produced at a relatively low cost, and then manganese sulfate for ternary materials applied to lithium battery industries is further obtained.
Owner:GUANGXI NANNING SHENGRUI METALLURGICAL & CHEM TECH
Method for reducing pyrolusite and co-producing sulfuric acid by utilizing sulfur in fluidized bed furnace
The invention discloses a method for reducing pyrolusite and co-producing sulfuric acid by utilizing sulfur in a fluidized bed furnace, which comprises the following steps of: blending the sulfur and the pyrolusite in a certain ratio, conveying the mixture into the fluidized bed furnace, reducing manganese dioxide in the pyrolusite into manganese oxide by using the sulfur and sulfur dioxide generated by combustion, discharging the manganese oxide into sulfuric acid solution or electrolytic manganese anode solution, slurrying and leaching manganese sulfate; and blowing air into the fluidized bed furnace from the upper section thereof, making sublimed sulfur fully combusted, performing temperature reduction and dedusting on sulfur dioxide generated by reaction, conveying the sulfur dioxide to a purification process, and producing the sulfuric acid by a sulfuric acid production process. By utilizing the conventional sulfuric acid producing fluidized bed furnace equipment, the production method can reduce the pyrolusite into the manganese sulfate, also can produce a great deal of sulfuric acid, greatly reduces equipment investment, saves investment, and reduces cost; meanwhile, in the reaction process, waste gas such as CO2, CO and SO2 and dust cannot be discharged, the operating conditions are good, and the environment is not influenced.
Owner:湖南省泸溪县金旭冶化有限责任公司
Preparation of high purity manganese sulfate
Owner:湖北开元化工科技股份有限公司
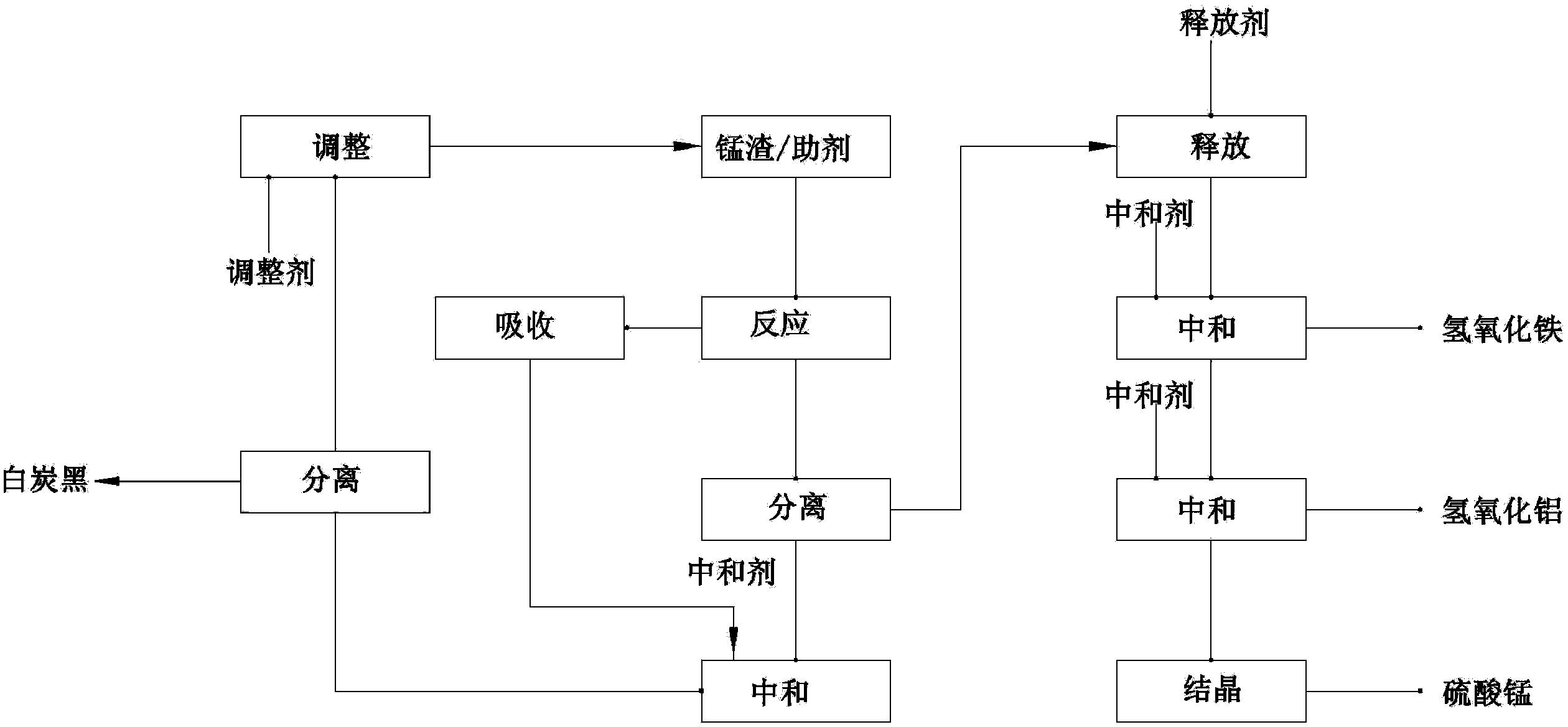
Method for producing chemical raw materials by comprehensively utilizing manganese slag
ActiveCN104016357AReduce secondary pollutionTurn waste into treasureSilicaIron oxides/hydroxidesSlagManganese
The invention relates to an industrial waste slag comprehensive utilization method, particularly a method for producing chemical raw materials by comprehensively utilizing manganese slag. A chemical process is adopted to decompose the manganese slag; reaction, absorption, neutralization, release, crystallization and other steps are utilized to effectively extract valuable elements in the manganese slag; and the valuable elements are comprehensively utilized to produce basic chemical raw materials, thereby changing wastes into valuable substances and greatly reducing the secondary environmental pollution caused by manganese slag.
Owner:山西升佳化工有限公司
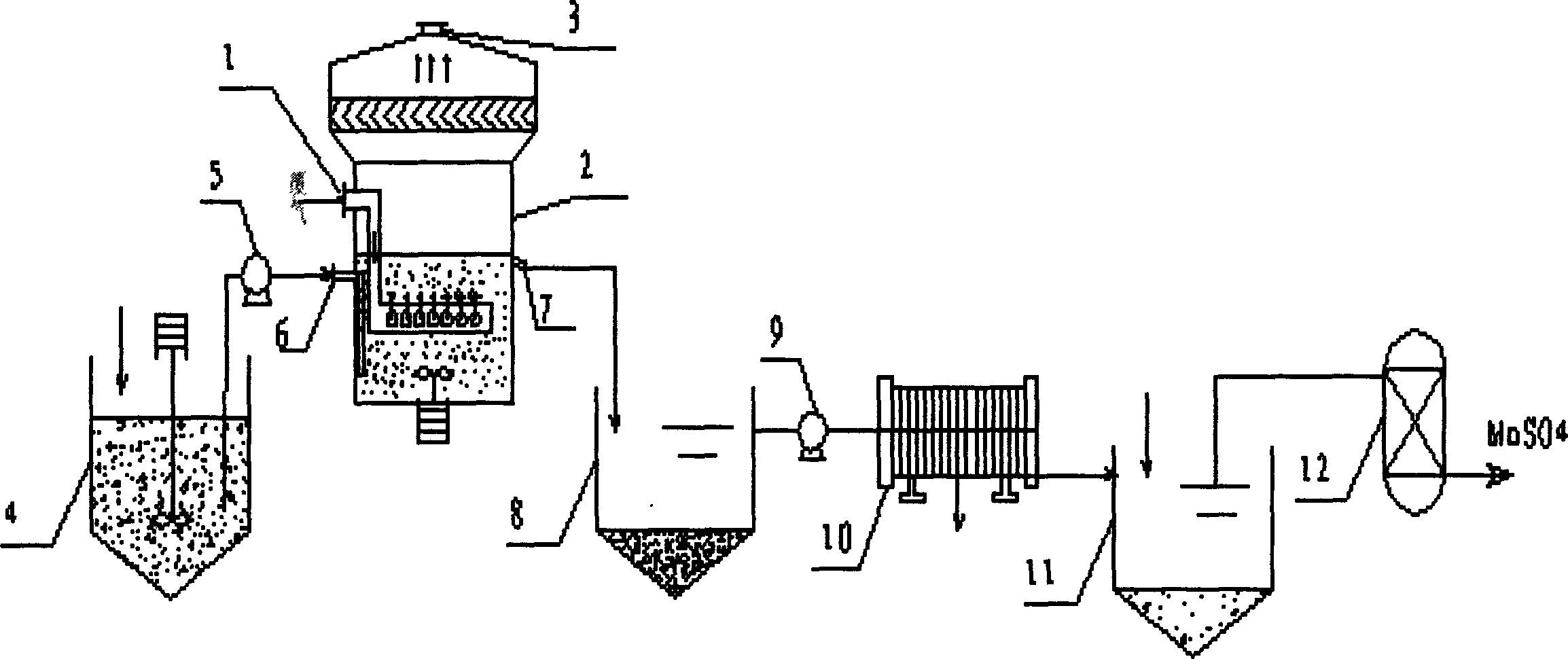
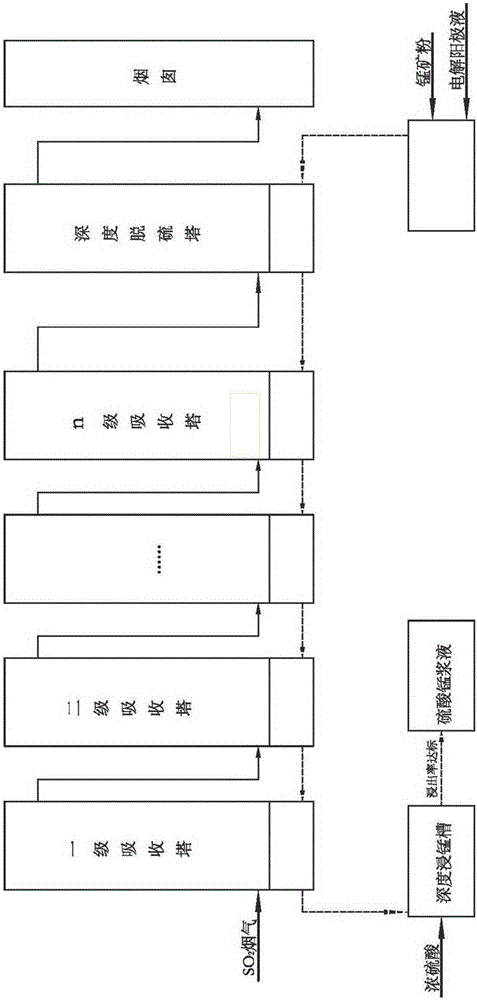
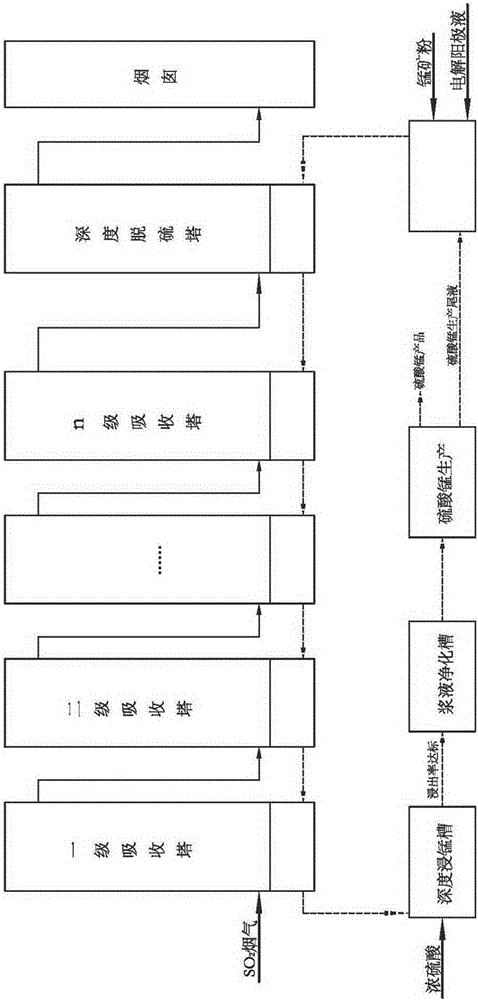
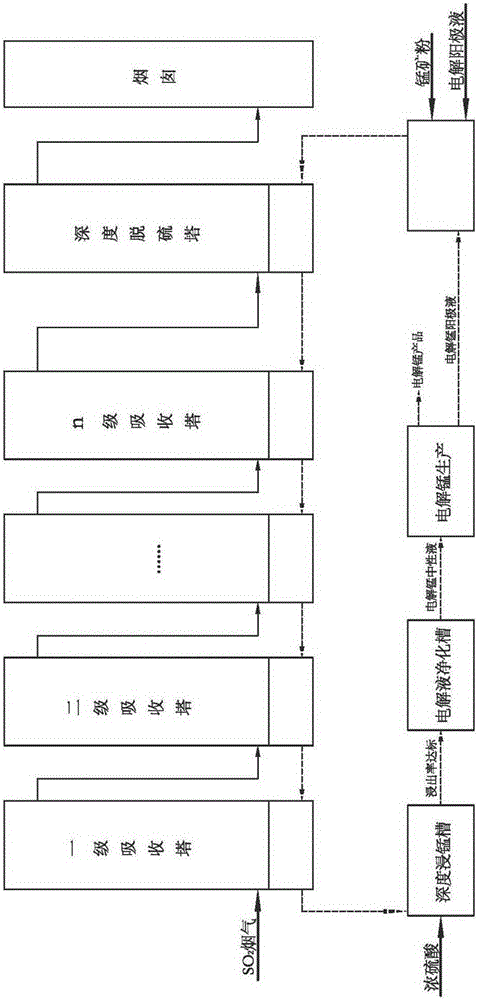
Process method for manufacturing manganese products through high-concentration SO2 smoke leaching manganese ore pulp
The invention discloses a process method for manufacturing manganese products through high-concentration SO2 smoke leaching manganese ore pulp. The process method mainly comprises the steps that high-concentration SO2 smoke enters from a first-stage tower segment of a multi-stage spray tower, manganese ore pulp liquid enters from a last-stage tower segment of the multi-stage spray tower, in each-stage tower segment, the smoke enters from the lower portion of the tower segment to be subjected to a contact reaction with the manganese ore pulp liquid sprayed down from the top, and a proper amount of air is blown into the tower bottom to reinforce desulfuration and leaching of manganese. The manganese ore pulp liquid subjected to full leaching is finally discharged out of the first-stage tower segment, a manganese sulfate solution product is obtained, the fully-desulfurated smoke is exhausted out of the last-stage tower segment, the content of SO2 reaches the standard, and the smoke is directly exhausted into air. The smoke with unqualified SO2 content enters a deep desulfurization tower, and SO2 in gas continues to be removed till the content of SO2 reaches the standard. The operating temperature of the contact reaction ranges from 70 DEG C to 85 DEG C, and pH ranges from 2 to 3. The method has the advantages of being high in resource utilization rate, high in desulfuration efficiency, high in manganese leaching rate, high in adaptability to manganese oxide ore, low in cost and the like.
Owner:SICHUAN UNIV
Method for reducing contents of calcium ions, magnesium ions, potassium ions and sodium ions in manganese sulfate
The invention discloses a method for reducing contents of calcium ions, magnesium ions, potassium ions and sodium ions in manganese sulfate. F<-1> in FeF3 can be used for precipitating the calcium ions and the magnesium ions in a solution, Fe<3+> can generate sulfate together with the potassium ions and the sodium ions so as to be precipitated, and Fe(OH)3 formed by hydrolyzing the superfluous Fe<3+> has a remarkable flocculation effect, so that the FeF3 is used as a novel manganese sulfate impurity removal agent; and the calcium ions, the magnesium ions, the potassium ions and the sodium ions are successively precipitated through adjusting the pH value, thus the calcium ions, the magnesium ions, the potassium ions and the sodium ions difficultly separated in common manganese sulfate are separated at one step, and the obtained product can meet the requirement of high-purity manganese sulfate for producing battery-grade or electronic-grade manganese-based raw materials on the contents of the calcium ions, the magnesium ions, the potassium ions and the sodium ions. The method has the advantages of fewer impurity removal agent adding times, simple process, low cost, remarkable impurity removal effect, high purity and obtained manganese sulfate yield and the like, and is easy to produce on large scale.
Owner:湘潭电化科技股份有限公司
Method for preparing manganese sulfate by manganese oxide ore
The invention provides a method for preparing manganese sulfate from manganese oxide ore. The method comprises the following processing steps: a, adding manganese oxide ore, ferrous sulfate or sulfur and water into a reactor; b, allowing for reaction; c, performing solid-liquid separation and slag washing to obtain iron concentrate and ready-to-use filtrate; d, removing impurities from the filtrate to get manganese sulfate solution. The extraction rate of manganese can exceed 85 percent. The manganese sulfate preparing method of the invention has the advantages of environment friendliness, short course and high manganese recovery, and can not only overcome problems of long course, low recovery, high cost, environmental pollution, etc. of traditional methods and significantly improve the recovery and extraction rate of manganese, but also utilize low grade manganese ore (iron-manganese ore in particular) and transform slag into products. The invention has prominent technological progress and outstanding substantial characteristics, and can produce excellent social and economic benefits.
Owner:汪云华 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com