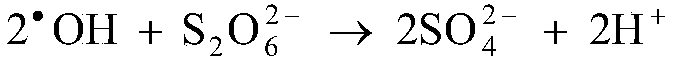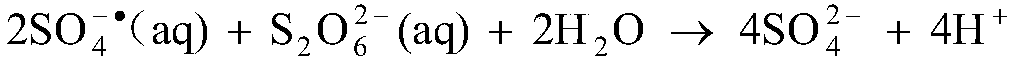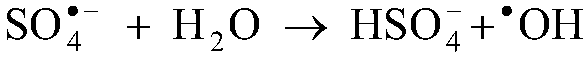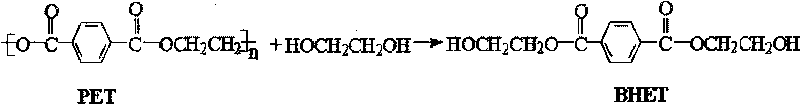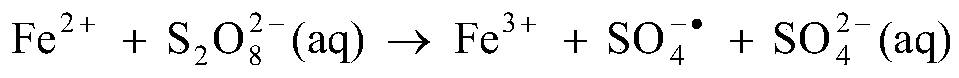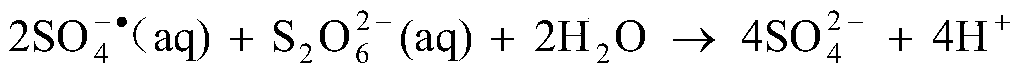Patents
Literature
43 results about "Dithionate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The dithionate (or metabisulfate) anion, S₂O²⁻₆, is a sulfur oxoanion derived from dithionic acid, H₂S₂O₆. Its chemical formula is sometimes written in a semistructural format, as [O₃SSO₃]²⁻. The sulfur atoms of the dithionate ion are in the +5 oxidation state due to the presence of the S–S bond. Generally dithionates form stable compounds that are not readily oxidised or reduced. Strong oxidants oxidise them to sulfates and strong reducing agents reduce them to sulfites and dithionites. Aqueous solutions of dithionates are quite stable and can be boiled without decomposition.
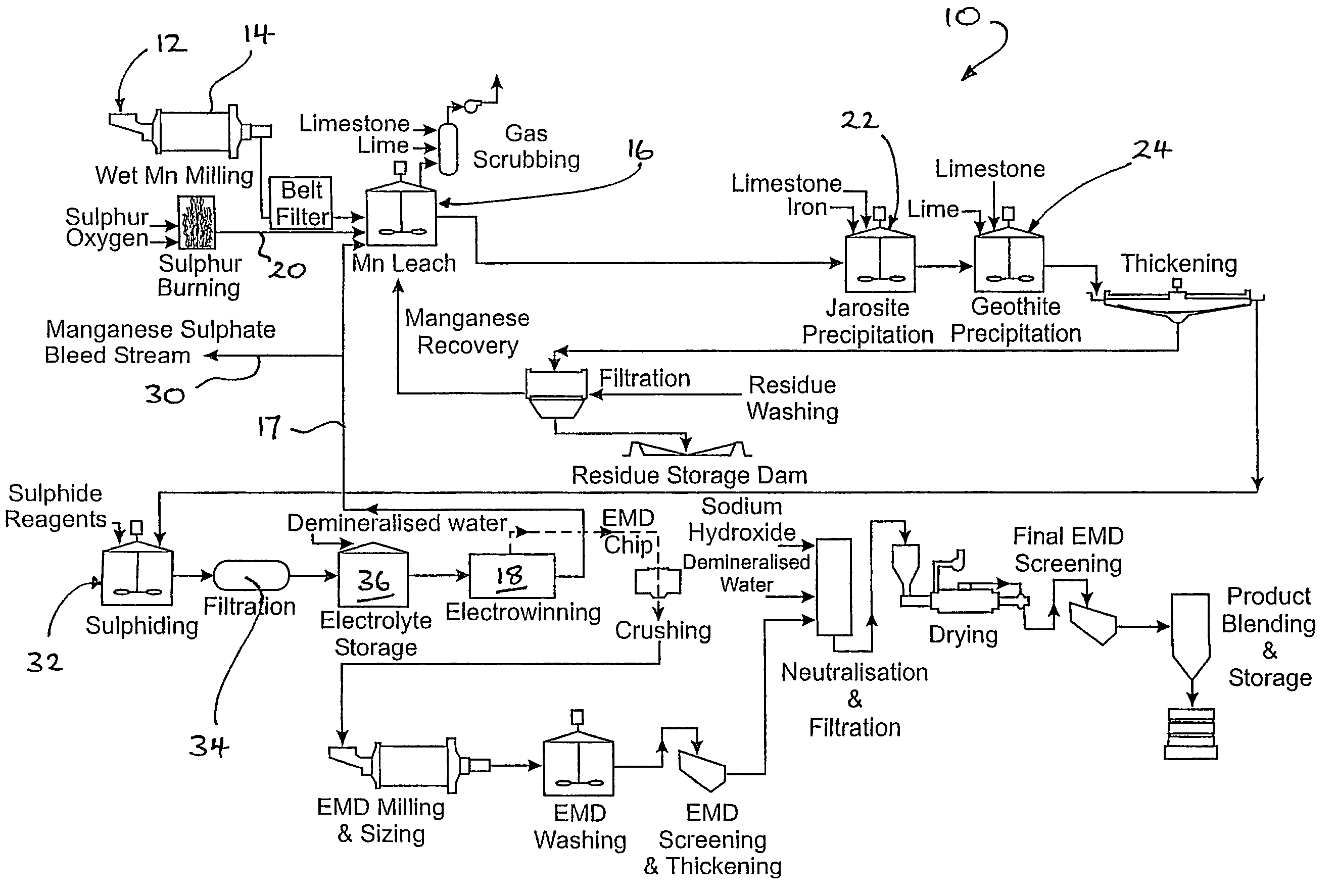
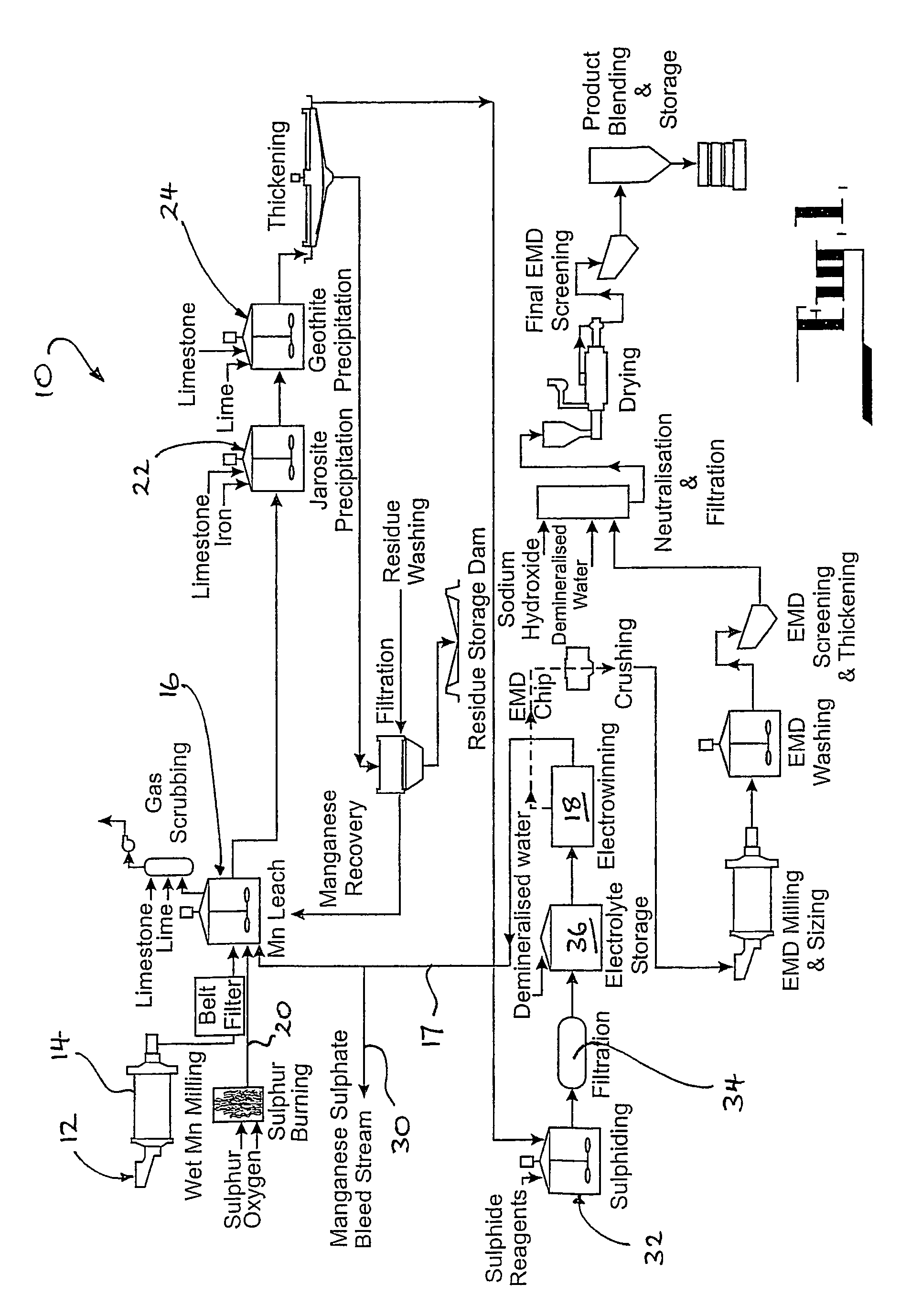
Method for restraining generation of manganous dithionate in process of leaching sulfur dioxide gas out of pyrolusite
The invention discloses a method for restraining the generation of manganous dithionate in the process of leaching sulfur dioxide gas out of pyrolusite, which comprises the following main technical measures: (1) pyrolusite pulp is prepared by stirring and mixing in the proportion that 100-500g of pyrolusite and 0.2-100g of granular active carbon are added into 1L of water; (2) in a synchronous reaction process of absorbing the sulfur dioxide gas by the pyrolusite pulp, desulphurizing and leaching manganese, the pH value of the pyrolusite pulp is controlled to be lower than 3.5 by blowing oxygen or air into the pyrolusite pulp. The method restraining the generation of the manganous dithionate does not need to extra consume sulphuric acid and guarantee an oxido-reduction potential by extra adjusting the concentration of Fe<3+> / Fe<2+>, is easy to meet the requirement of industrial process production and can guarantee the concentration of a manganese sulfate mother liquor.
Owner:成都合众新能源科技有限公司
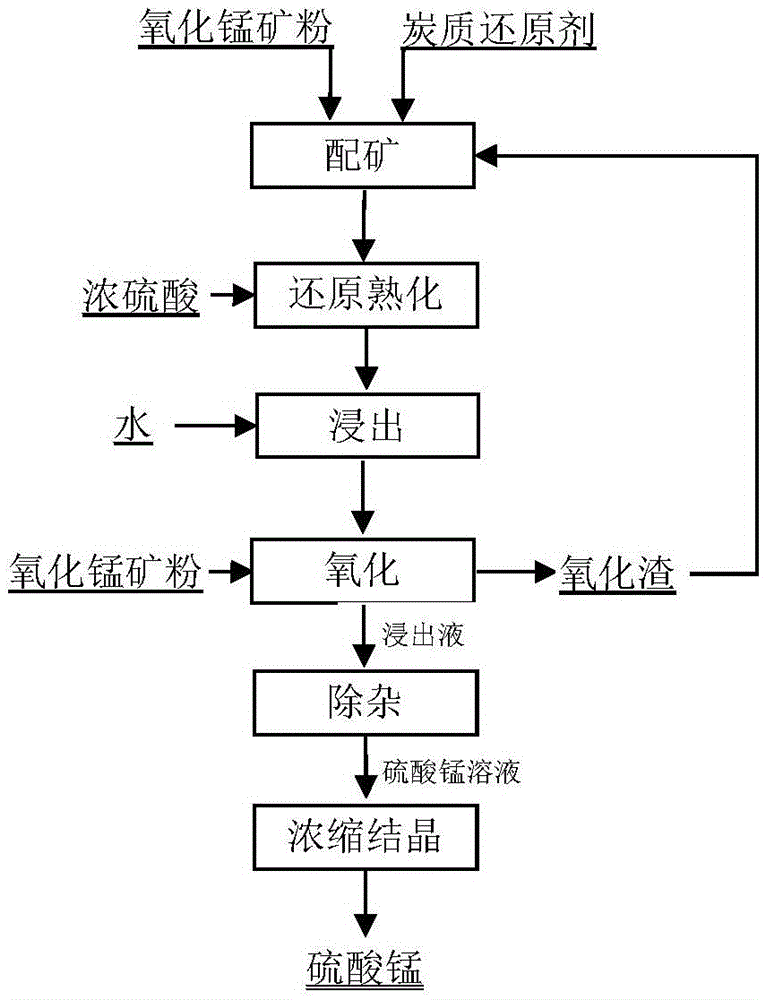
Method for producing manganese sulfate from manganese oxide ores
ActiveCN104817116ANo pollution in the processReduce energy consumptionManganese sulfatesDithionateDecomposition
The invention provides a method for producing manganese sulfate from manganese oxide ores. The method comprises the steps of mixing the manganese oxide ore powder with an appropriate amount of carbon reducing agent such as pulverized coal evenly, adding concentrated sulfuric acid without adding water and mixing evenly to obtain a mixture, controlling the initial concentration of the sulfuric acid in the mixture to be greater than or equal to 70%, curing the mixture by use of reaction heat via self-heating reduction and reducing the quadrivalent manganese in the manganese ores into bivalent manganese, mixing the cured material with water for stirring and leaching, and filtering the leached ore pulp to obtain a leachate, adding an appropriate amount of manganese oxide ores to the leachate to realize the oxidative decomposition of manganese dithionate, and performing neutralization, purification and impurity removal and crystallization on the solution obtained by filtering to produce the manganese sulfate. The method has the advantages that the low-cost easily available carbon reducing agent such as the pulverized coal is utilized to directly reduce the manganese oxide ores at a low temperature, and therefore, the energy consumption and the cost are low and the smoke pollution problem of reducing roasting is avoided; the manganese oxide ore powder is utilized to realize the oxidative decomposition of the manganese dithionate in the manganese sulfate solution and therefore, the quality of the manganese sulfate product is improved.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Processing of manganous sulphate/dithionate liquors derived from manganese resource material
ActiveUS8460631B2Rubidium/caesium/francium compoundsProcess efficiency improvementManganese sulphateDithionate
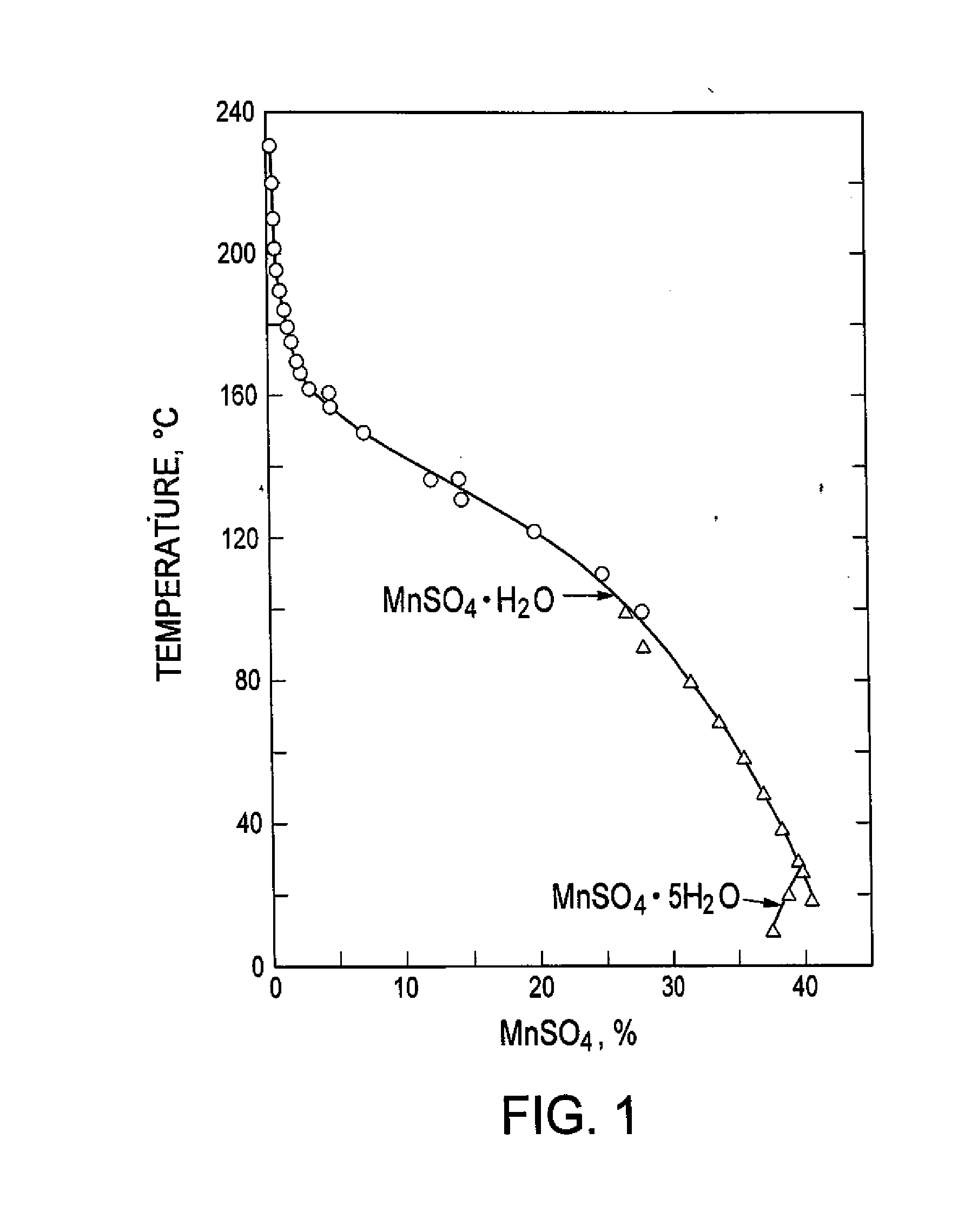
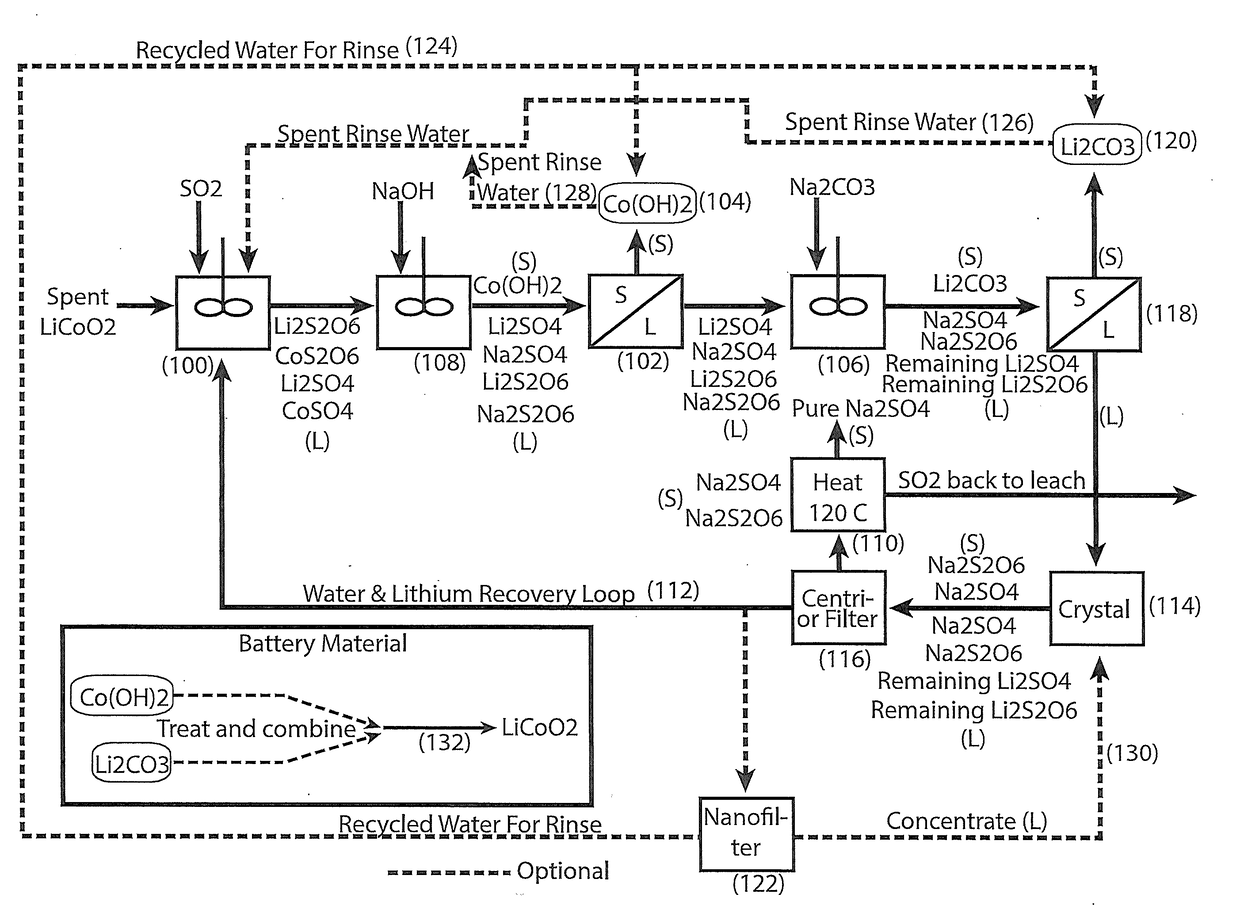
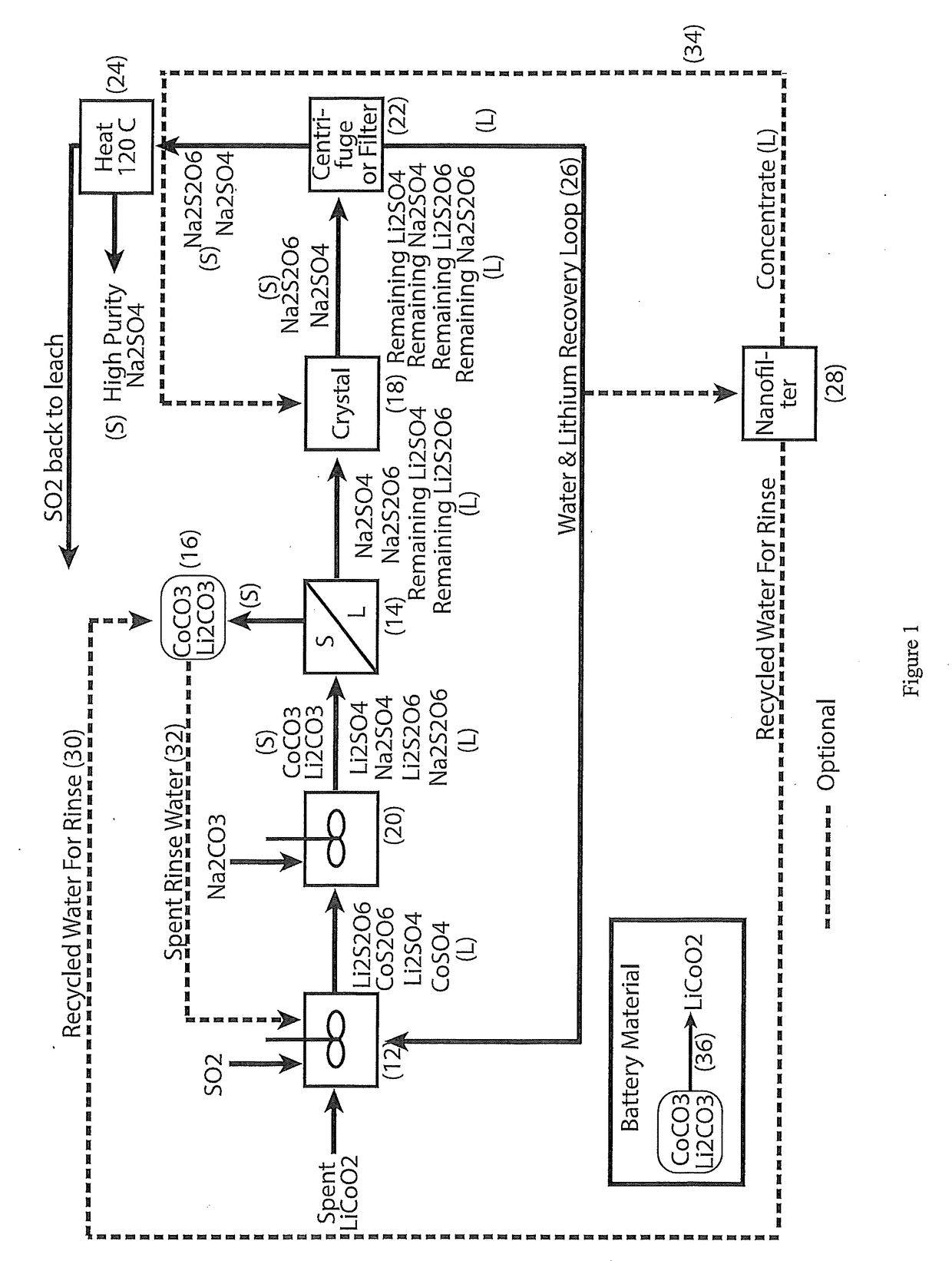
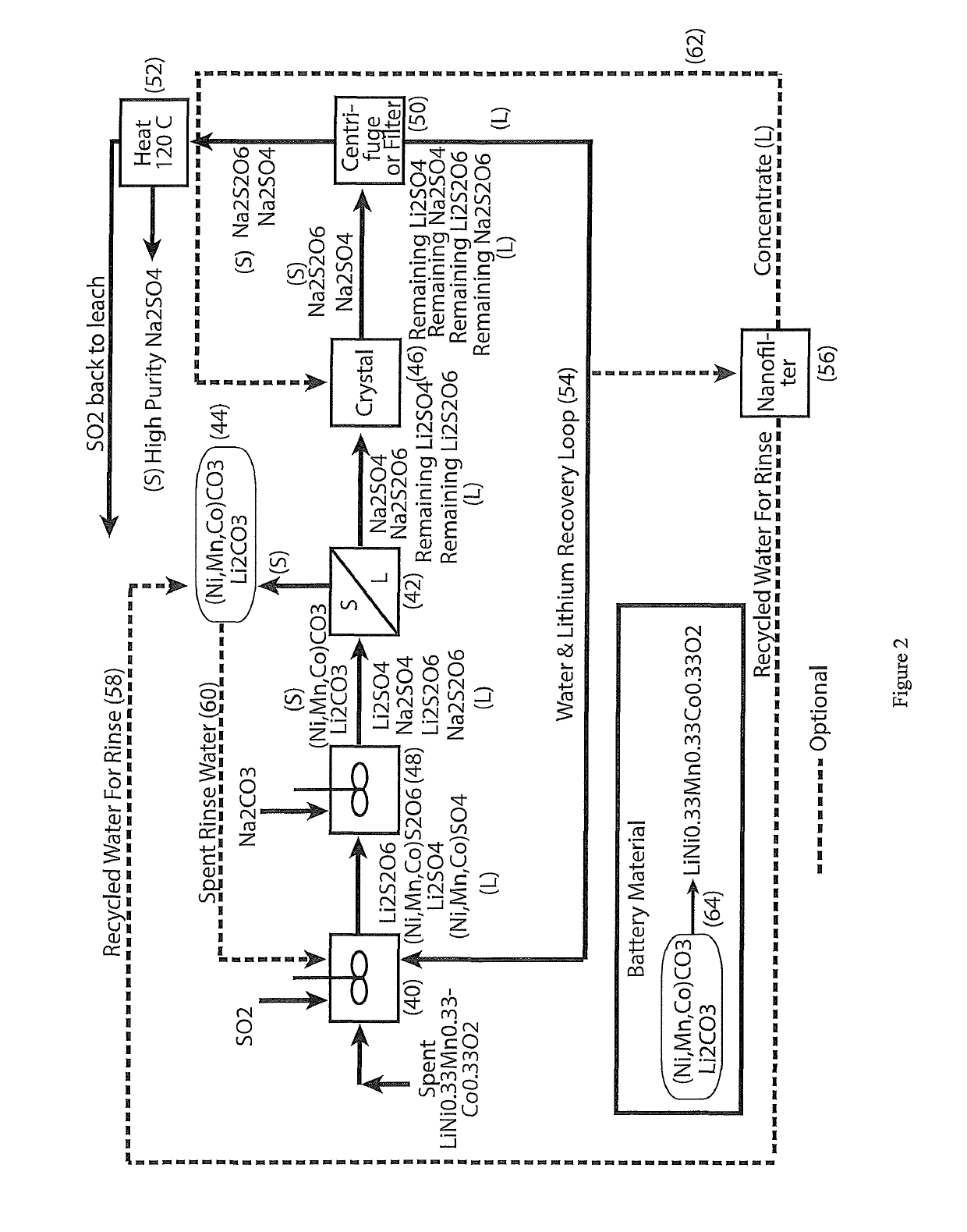
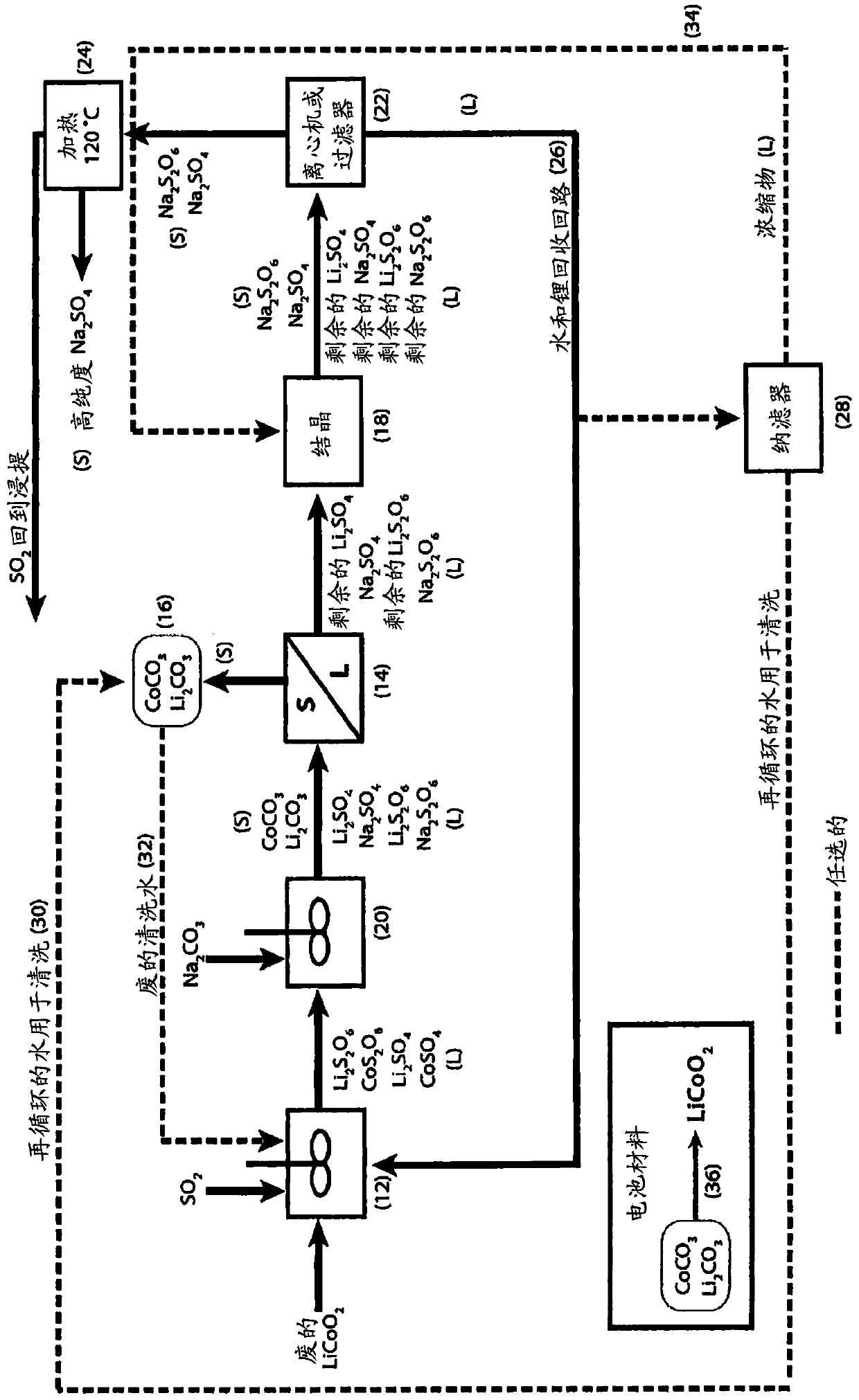
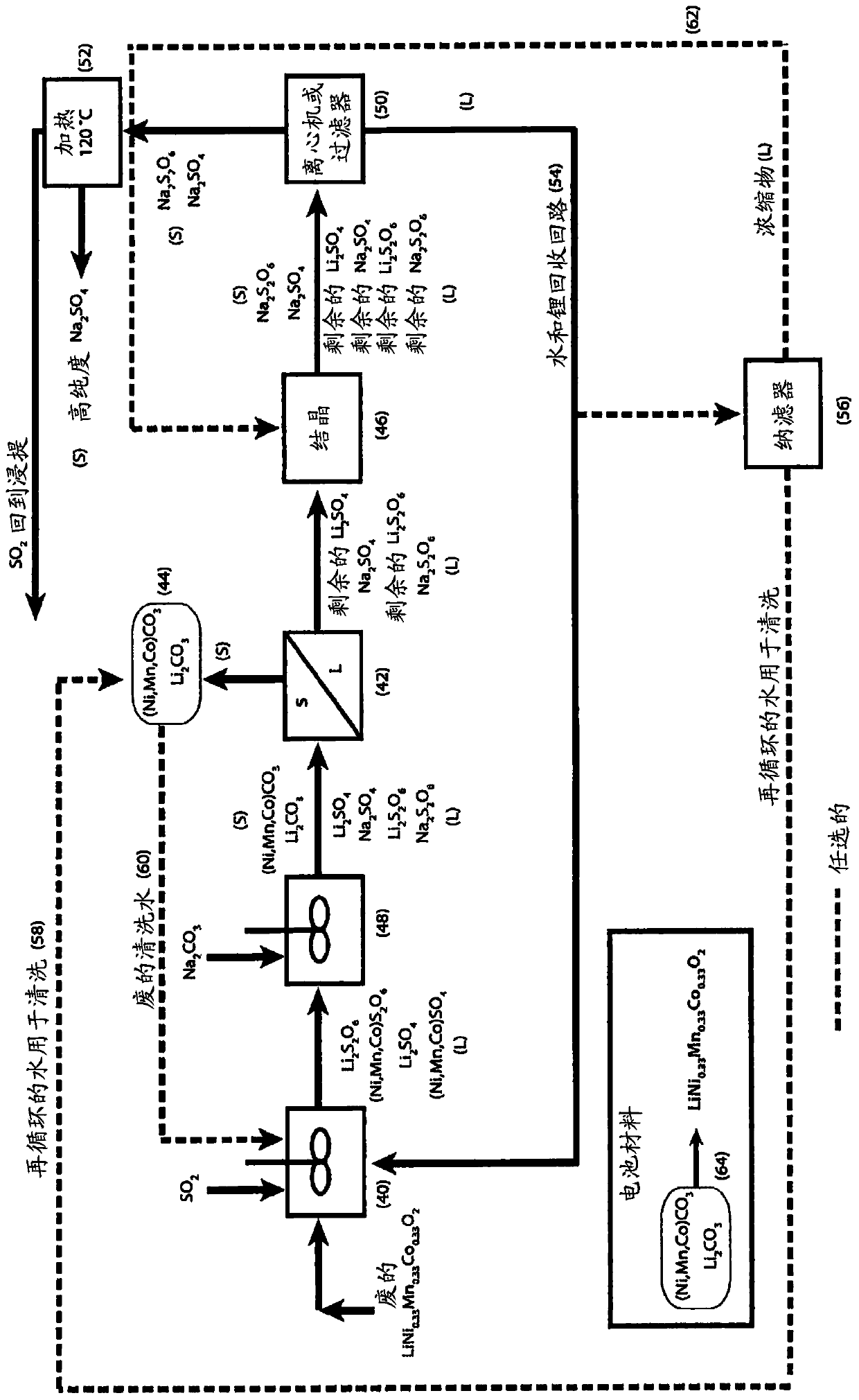
The process concerns hydrometallurgical processing of manganese sulphate and manganese dithionate containing liquors and recovery of water therefrom. Sodium sulphate and / or sodium dithionate containing liquors are derived from manganese sulphate and manganese dithionate containing liquids, which are then cooled to produce crystals of sodium sulphate decahydrate and sodium dithionate dehydrate. The sodium sulphate decahydrate and sodium dithionate dehydrate crystals are then heated to a temperature sufficient to decompose the sodium sulphate decahydrate crystals to form anhydrous sodium sulphate crystals, sodium dithionate hydrate crystals and water, after which water is removed from the sodium sulphate and sodium dithionate hydrate crystal. The sodium sulphate and sodium dithionate dehydrate crystals are then heated to form anhydrous sodium sulphate, sulfur dioxide and water or steam. The anhydrous sodium sulphate is then separated from the sulfur dioxide and water.
Owner:AMERICAN MANGANESE
Processing of manganous sulphate/dithionate liquors derived from manganese resource material
ActiveUS20120207666A1Rubidium/caesium/francium compoundsAlkali metal sulfite/sulfate dehydrationManganese sulphateDithionate
The process concerns hydrometallurgical processing of manganese sulphate and manganese dithionate containing liquors and recovery of water therefrom. Sodium sulphate and / or sodium dithionate containing liquors are derived from manganese sulphate and manganese dithionate containing liquids, which are then cooled to produce crystals of sodium sulphate decahydrate and sodium dithionate dehydrate. The sodium sulphate decahydrate and sodium dithionate dehydrate crystals are then heated to a temperature sufficient to decompose the sodium sulphate decahydrate crystals to form anhydrous sodium sulphate crystals, sodium dithionate hydrate crystals and water, after which water is removed from the sodium sulphate and sodium dithionate hydrate crystal. The sodium sulphate and sodium dithionate dehydrate crystals are then heated to form anhydrous sodium sulphate, sulfur dioxide and water or steam. The anhydrous sodium sulphate is then separated from the sulfur dioxide and water.
Owner:AMERICAN MANGANESE
Processing of cobaltous sulpha/dithionate liquors derived from cobalt resource
ActiveUS20180155208A1Thiosulfates/dithionites/polythionitesManganese oxides/hydroxidesDithionateSulfide
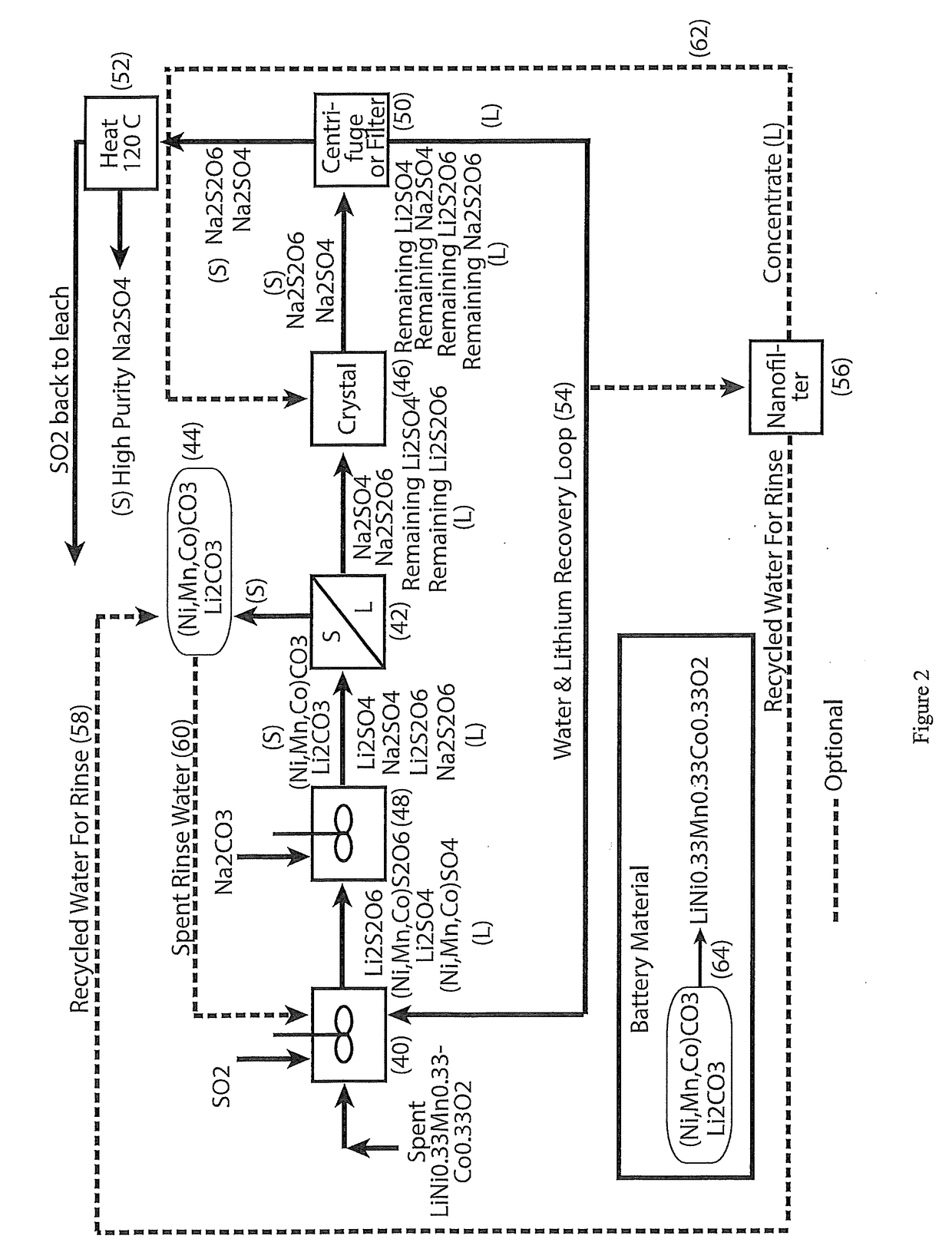
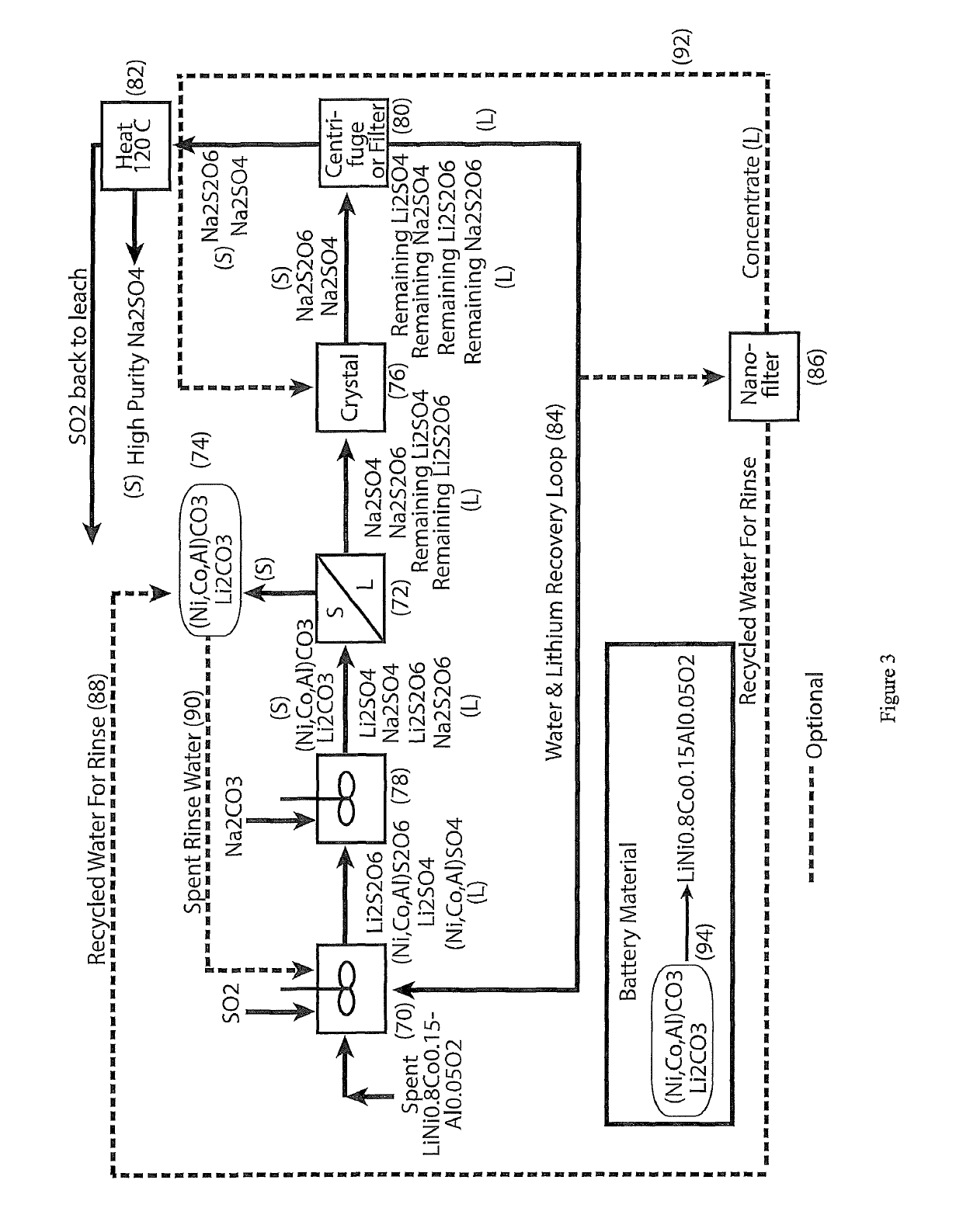
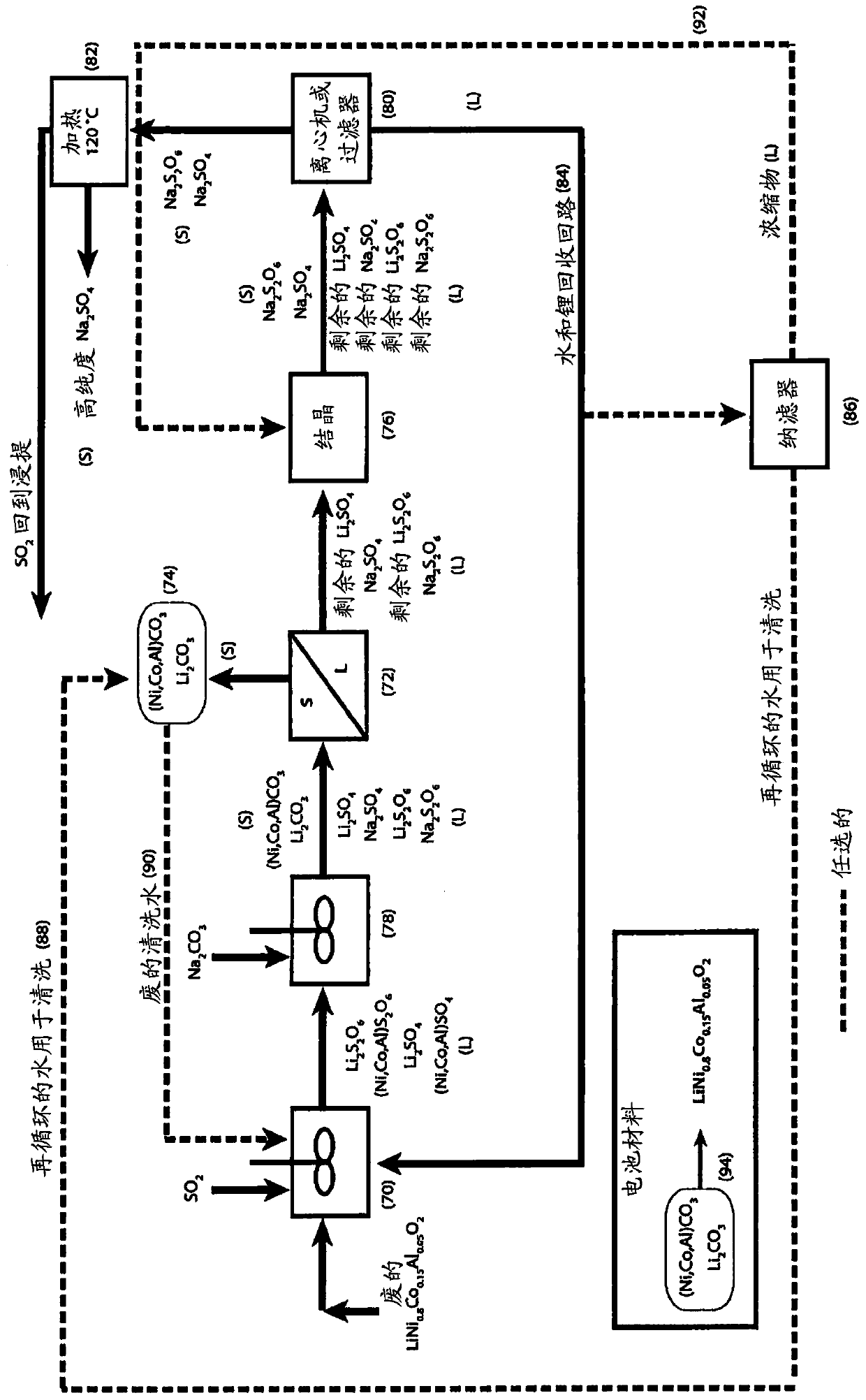
A process for water removal and / or recycling of sodium sulfate and / or sodium dithionate containing liquors derived from processing cobalt resource material essentially free of lithium comprising the steps of precipitation of cobalt as cobaltous carbonate or cobaltous hydroxide followed by removal thereof from the liquor, crystallization of sodium sulfate and sodium dithionate and removal of the crystals, followed by heating of the crystals to anhydrous sodium sulfate, sulphur dioxide and water and then separating the anhydrous sodium sulfate.
Owner:ROCHER MANGANESE INC
Preparation method of high-purity lithium sulfide
ActiveCN112678780AHigh purityShape is easy to controlAlkali metal sulfides/polysulfidesLithium sulphateDithionate
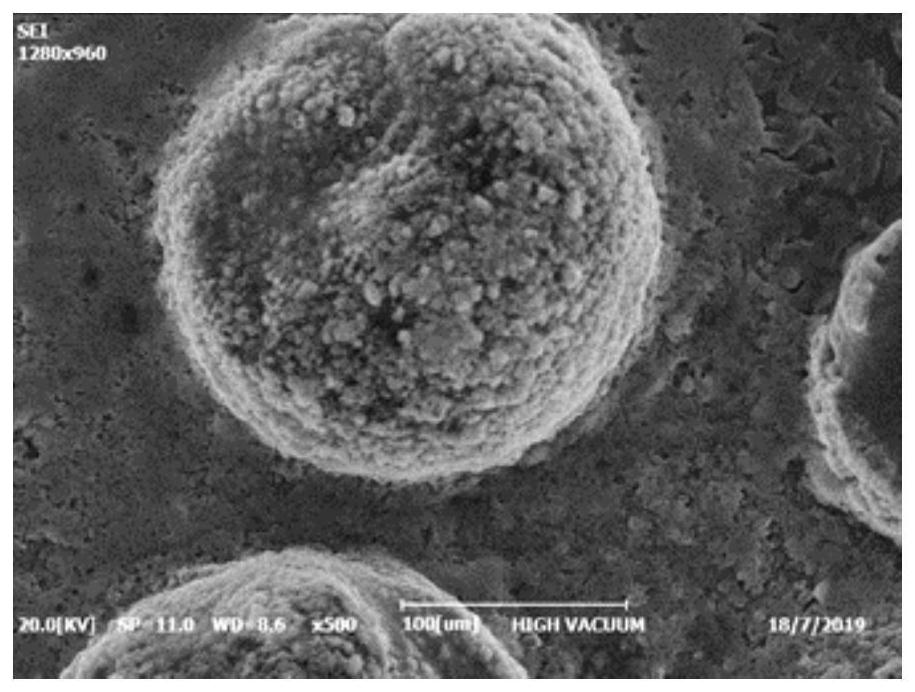
The invention relates to a preparation method of high-purity lithium sulfide, and belongs to the technical field of preparation of lithium sulfide. The technical problem to be solved by the invention is to provide the preparation method of the high-purity lithium sulfide. The method mainly comprises the following steps: reacting a lithium sulfide precursor (one or more of lithium sulfate, lithium sulfite, lithium bisulfate and lithium dithionate) with a related reducing agent at high temperature to prepare a lithium sulfide-containing substance, and leaching and purifying the lithium sulfide in the mixture; and finally, carrying out spray drying to obtain a lithium sulfide product with purity of 99.9% or above and controllable morphology. The method is simple and easy to operate, free of toxic organic solvents, safe and environmentally friendly, the purity of the obtained lithium sulfide is high and can reach 99.9% or above, the morphology is controllable, and the degree of sphericity is high.
Owner:天齐锂业(江苏)有限公司
Technology for producing electrolytic metal manganese from low-grade manganese mine
InactiveCN104232889ASolve the separation problemAcid balance problem solvingPhotography auxillary processesProcess efficiency improvementElectrolysisDecomposition
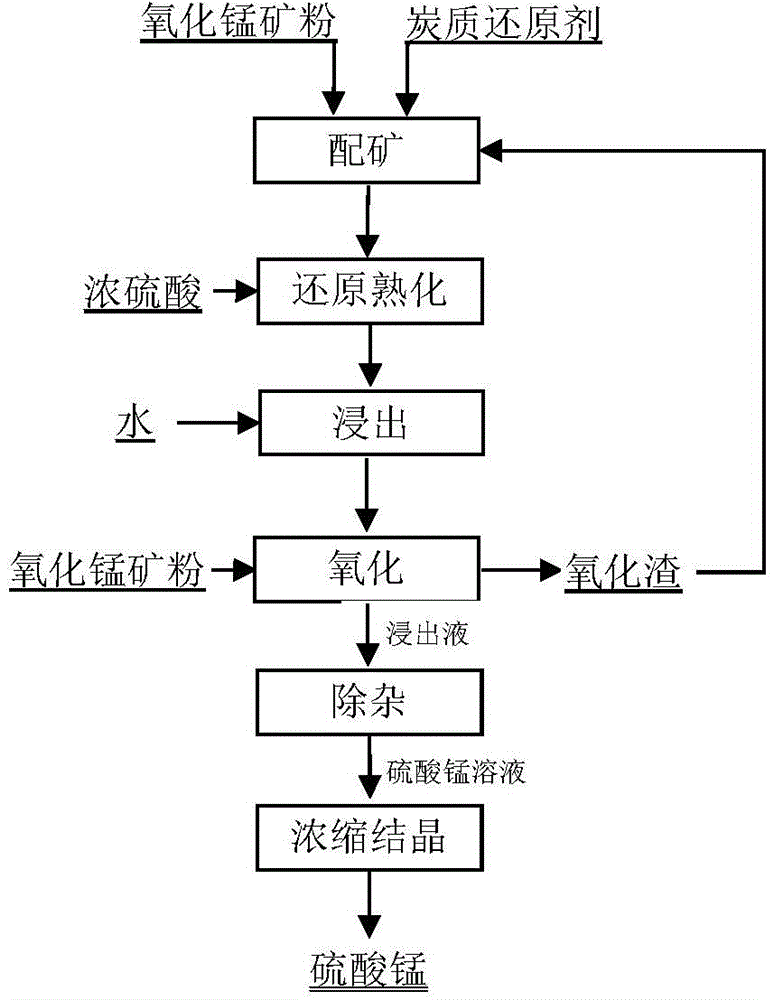
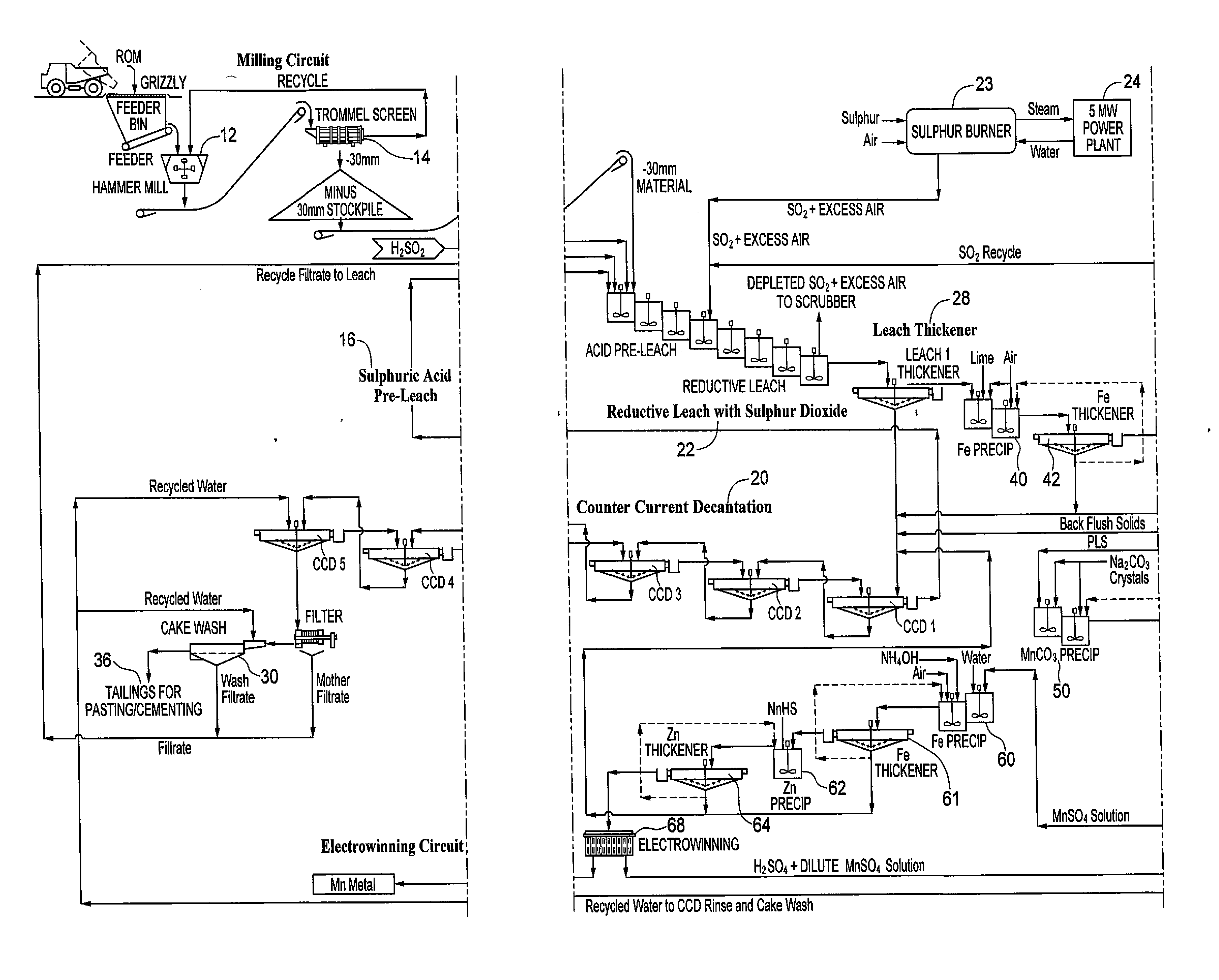
The invention relates to a technology for producing electrolytic metal manganese from low-grade manganese mine. The technological flow comprises ore grinding, sulfuric acid pre-leach, combusting sulfur for preparing sulfur dioxide, sulfur dioxide reduction leaching, solid-liquid separation, purifying of a leaching liquid, separation of dithionate radical, production of electrolytic metal manganese, decomposition of dithionate radical, production of anhydrous sodium sulfate and recovery of production water. The technology is simple, resource is cycled for utilization, the reduction leaching rate of manganese is even up to 98%, and the recovery utilization efficiency is high.
Owner:无锡市森信精密机械厂
Method for removing manganous dithionate in manganese ore desulfurizing liquid
The invention discloses a method for removing manganous dithionate in manganese ore desulfurizing liquid. Persulfate is added into the manganese oxide ore desulfurizing liquid, stirred and dissolved,ferrous sulfate is added, dithionate radical of the manganous dithionate in the manganese oxide ore desulfurizing liquid is oxidized into sulfate radical through sulfate radical and hydroxyl radical generated by high-order oxidation reaction between the persulfate and the ferrous iron, and thus the manganous dithionate in the manganese oxide ore desulfurizing liquid is removed. Through the method,purity of a manganese sulfate mother solution can be improved effectively, no energy needs to be consumed additionally to heat the manganese ore desulfurizing liquid, no acid or alkali needs to be consumed to adjust the pH of the desulfurizing liquid, technological conditions are simple and mild, operability is high, and industrialized application is easy to achieve.
Owner:SICHUAN UNIV
Method for preparing manganese sulfate solution with low manganous dithionate through desulfuration by using slurry prepared by anode liquid and manganese ore
ActiveCN108193047AImprove resource utilizationPhotography auxillary processesProcess efficiency improvementHigh concentrationElectrolysis
The invention discloses a method for preparing a manganese sulfate solution with low manganous dithionate through desulfuration by using the slurry prepared by an anode liquid in electrolytic manganese production and manganese ore, and belongs to the field of electrolytic manganese production. According to method, the slurry prepared by the anode liquid and the manganese ore is used as a desulfurizing agent, meanwhile, a multi-stage spray washing tower with a sieve plate is additionally arranged for flue gas desulfurization and manganese immersion, after the obtained slurry is filtered, an oxidizing agent in the liquid phase is added to reduce the manganous dithionate in the slurry, so that the production of the electrolytic manganese and / or manganese sulfate is facilitate; the solid-phasesubstance obtained by filtering undergoes secondary acidolysis , and then the leaching rate of the manganese can be further improved; by adopting the process and the method, the leaching rate of themanganese can reach more than 94.3%, meanwhile, the high-concentration sulfur dioxide in the flue gas is reduced to the value lower than 100mg / m<3>, and the desulfurization efficiency can reach more than 99%; the prepared desulfurized manganese leaching solution is sent to an electrolysis section for electrolytic manganese production, the average single-shift yield is increased from 2.5kg-2.6kg inthe prior art to 3.1kg-3.2kg; and when the manganese sulfate solution which is prepared by the method and provided with the low manganous dithionate is used for electrolytic manganese production, theyield of the manganese can be improved, the plate loading rate of the manganese can be increased, the quality and the yield of the manganese can be improved, and the energy consumption and the production cost can be reduced.
Owner:四川恒泰环境技术有限责任公司
Processing of cobaltous sulpha/dithionate liquors derived from cobalt resource
A process for water removal and / or recycling of sodium sulfate and / or sodium dithionate containing liquors derived from processing cobalt resource material essentially free of lithium comprising the steps of precipitation of cobalt as cobaltous carbonate or cobaltous hydroxide followed by removal thereof from the liquor, crystallization of sodium sulfate and sodium dithionate and removal of the crystals, followed by heating of the crystals to anhydrous sodium sulfate, sulphur dioxide and water and then separating the anhydrous sodium sulfate.
Owner:ROCHER MANGANESE INC
Cationic antiseptic compositions and methods of use
ActiveUS9028852B2Reduce usageUseful in treatmentAntibacterial agentsOrganic active ingredientsAmmonium compoundsCetylpyridinium
Owner:3M INNOVATIVE PROPERTIES CO
Method for catalytic-degrading polyethylene glycol terephthalate by dithionate
InactiveCN101747200APhysical/chemical process catalystsOrganic compound preparationPolyesterPolyethylene terephthalate
The invention relates to a method for catalytic-degrading polyethylene glycol terephthalate (PET) by dithionate; the method is characterized in that: solid superacid S2O82- / ZnO is used as catalyst, ethylene glycol is used as solvent, and the using amount of the catalyst is 0.01-5.00 percent of the solvent mass, and under the conditions that the reaction temperature is 150-220 DEG C, the pressure is 1atm, and the reaction time is 1-8h, alcoholysis is carried out to the PET polyester. The solid acid catalyst is used in the method, and the method has the advantages of easy separation of products, high selectivity and recycling usage and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
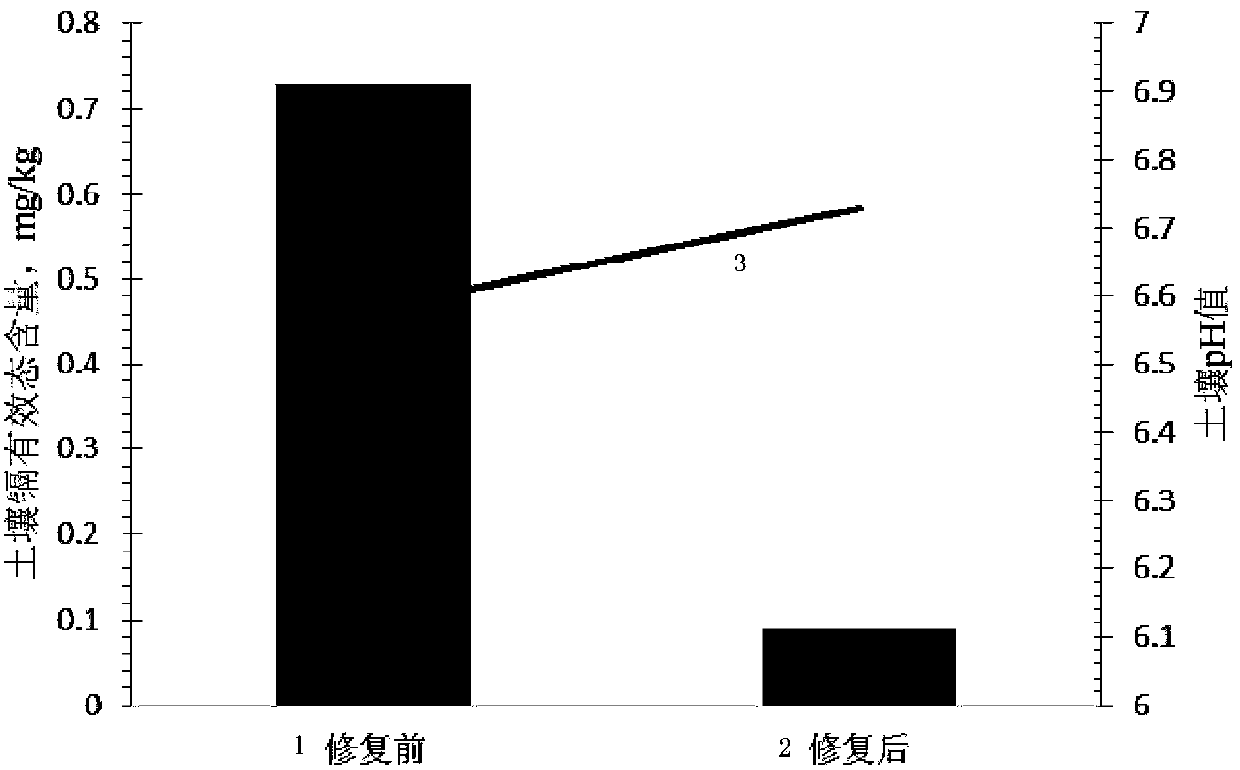
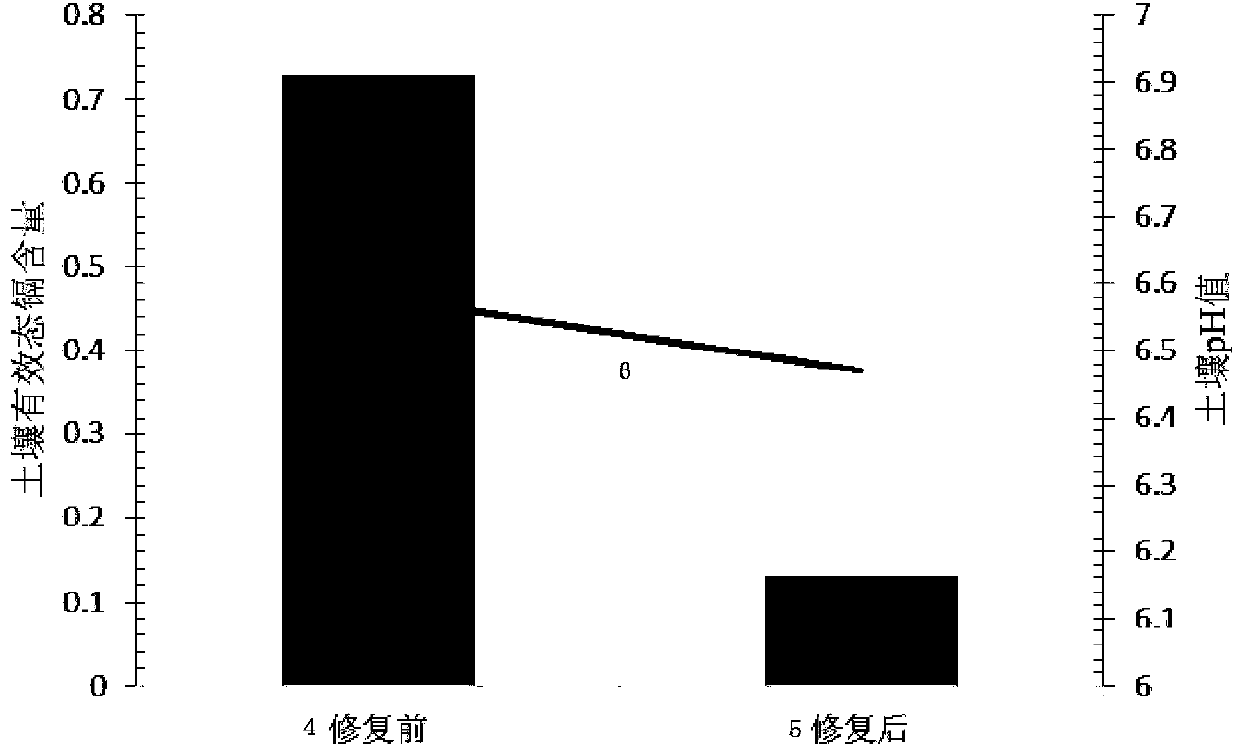
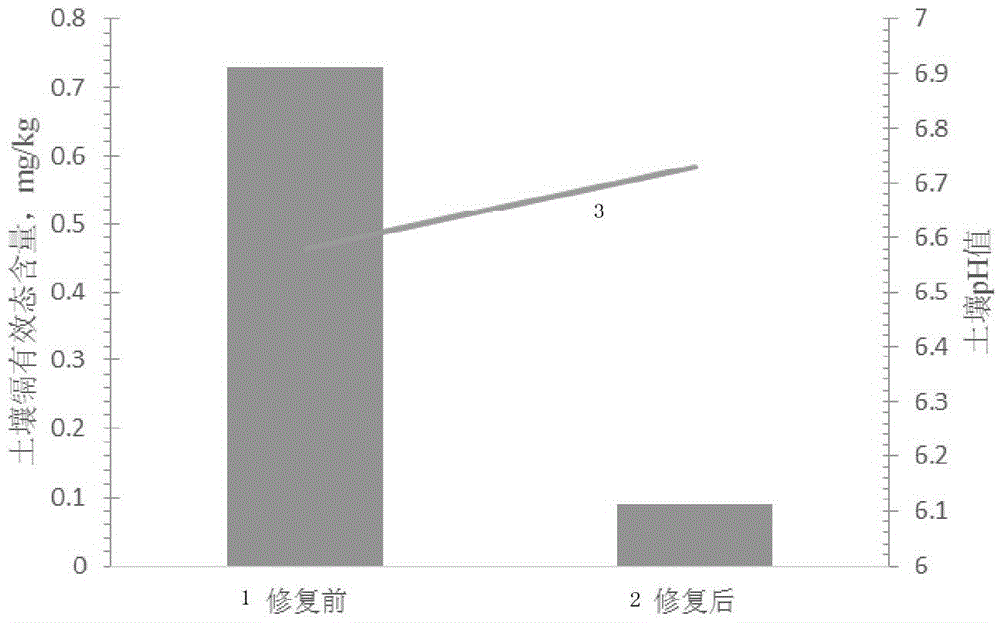
Passivator for cadmium in neutral soil and application method thereof
ActiveCN103805206ANo change in pHReach repairOrganic fertilisersSoil conditioning compositionsDithionateSediment
The invention provides a passivator for cadmium in neutral soil and an application method thereof. The passivator is composed of calcium oxide with a weight accounting for 0.3-2.0% of the weight of neutral soil, calcium dithionate monohydrate with a weight accounting for 0.3-2.5% of the weight of the neutral soil, and charcoal with a weight accounting for 0.3-1.34% of the weight of the neutral soil. The passivator for cadmium in neutral soil provided by the invention reacts with effective cadmium in the neutral soil by using calcium oxide and calcined gypsum (calcium dithionate monohydrate) so as to generate small-activity cadmium sediments such as cadmium hydroxide and the like, and charcoal and the like in the passivator can selectively adsorb cadmium ions with large activity in the soil so as to reduce the transportability of the cadmium ions, therefore, an effect of reducing the quantity of effective-state cadmium in the neutral soil is achieved, the restoration of contaminated soil is implemented, and a situation that the pH value of the soil before and after the soil is treated is not changed can be ensured.
Owner:HUNAN RES INST FOR NONFERROUS METALS
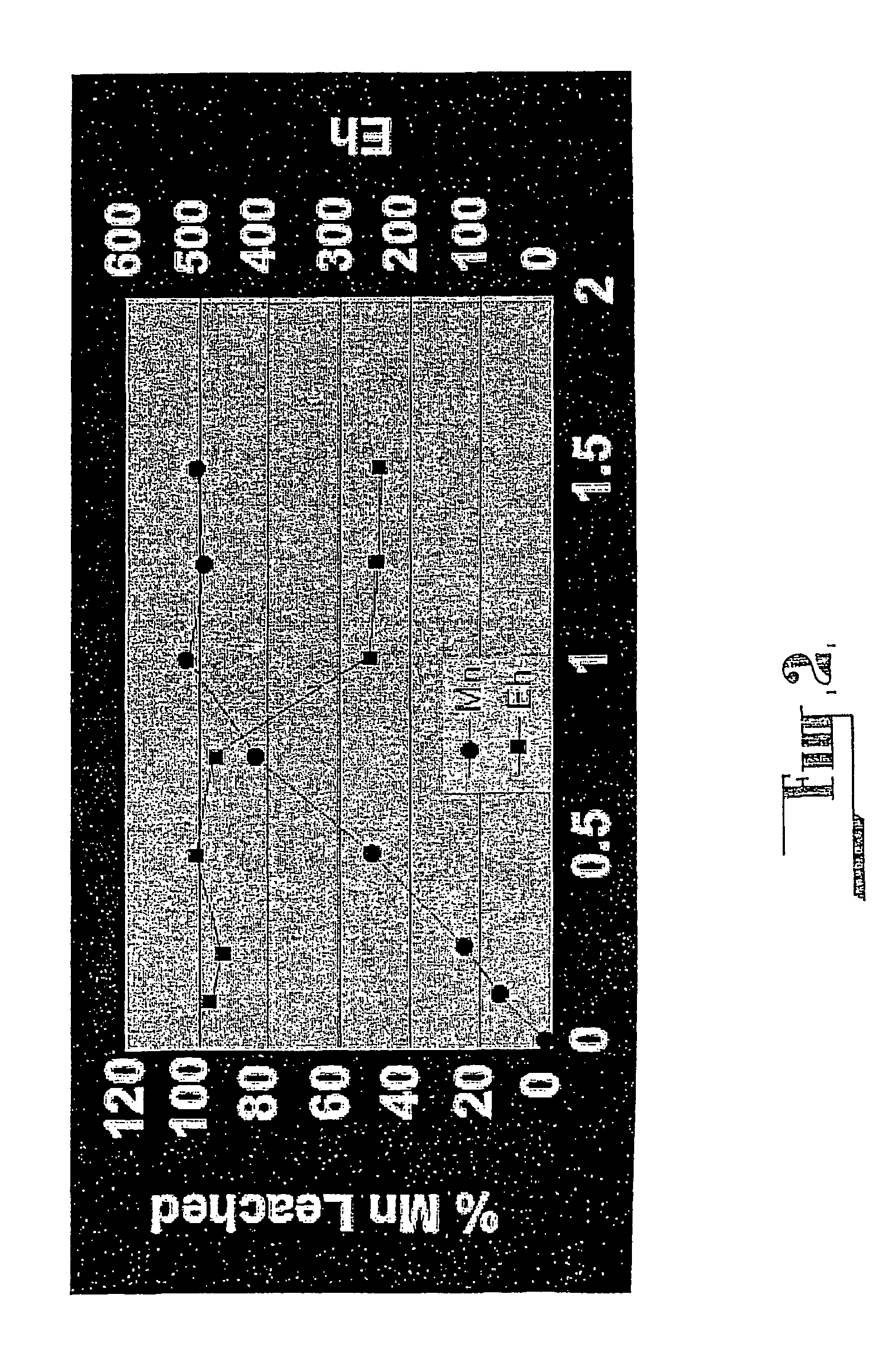
Efficient decomposition method for manganous dithionate in manganese ore sulfur dioxide lixivium
The invention discloses an efficient decomposition method for manganous dithionate in manganese ore sulfur dioxide lixivium. The method comprises the main steps that under the temperature condition ofthe sulfur dioxide lixivium of manganese ore pulp, sulfuric acid, manganese dioxide (or manganese bioxide ore) and ferric iron are sequentially added into the lixivium, and under the constant-temperature and acid conditions, through the catalytic activity of high-valent metal of ferric iron and manganese bioxide and the chain reaction process formed by coordination catalysis reduction and free radical oxidation formed by ferric iron and manganese bioxide, rapid oxidization conversion from sulfur dioxide to sulfate radicals is achieved. The process method has the advantages that cost is low, and large-scale industrialization production is easy. Manganese sulfate of main components in the lixivium serves as reaction products, no new impurity is introduced, and the lixivium can be used for producing electrolytic manganese metal or manganese sulfate easily later.
Owner:SICHUAN UNIV
PEG coupled heterocyclic compound and application thereof in acidic bright copper plating
Owner:WENZHOU INST UNIV OF CHINESE ACAD OF SCI
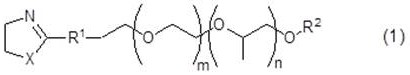
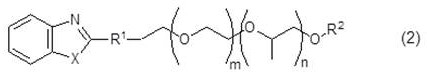
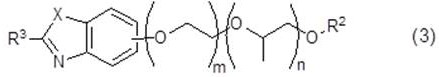
Aqueous solutions containing dithionic acid and/or metal dithionate
InactiveUS20020002128A1Inorganic/elemental detergent compounding agentsSurface-active detergent compositionsDithionic acidDithionate
This invention relates to solutions of dithionic acid and / or dithionate salts for use in metal finishing processes such as those used for the cleaning, activating, electroplating, electroless plating, conversion coating and / or other pre-treatment or post-treatment of a metallic surface. In particular the solutions are a useful electrolyte for the electroplating of metallic coatings, especially, Sn, Cu, Ni, Zn and precious metals, onto metal or plastic substrates and / or other surfaces.
Owner:ATOCHEM OF NORTH AMERICA INC
A kind of method of manganese oxide ore producing manganese sulfate
ActiveCN104817116BNo pollution in the processReduce energy consumptionManganese sulfatesDithionateDecomposition
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
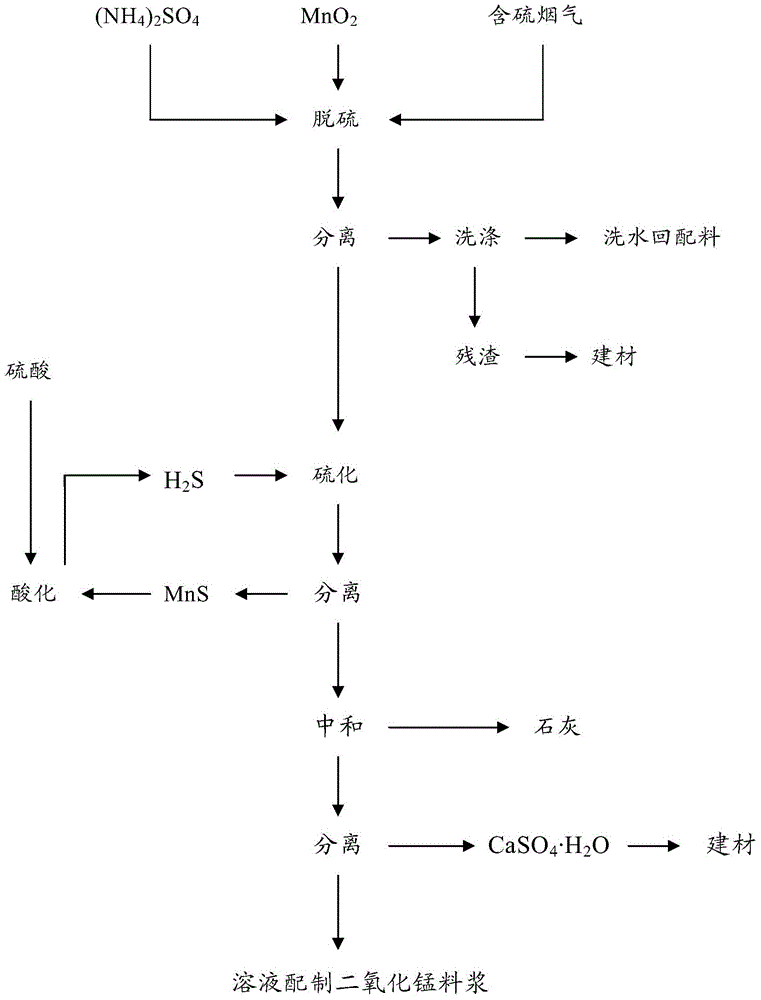
Cycling separation method for manganous dithionate in desulfurized liquid
ActiveCN104645815ASolve the problem of the influence of manganese dithionateReduce processing costsDispersed particle separationManganese sulfatesGas liquid reactionDithionate
The invention provides a cycling separation method for manganous dithionate in desulfurized liquid. The method comprises the following steps: (1) desulfurization: carrying out multi-stage countercurrent gas-liquid reaction between slurry containing manganese dioxide ore and ammonium sulfate with flue gas containing sulphur, and carrying out pressure filtration and separation; (2) sulfuration and acidification: introducing a filtrate obtained in step (1) into a hydrogen sulfide gas for sulfuration reaction, carrying out pressure filtration and separation, and acidifying a filter cake to obtain hydrogen sulfide and manganese sulfate products; (3) aftertreatment cycling working procedure: neutralizing the filtrate obtained in step (2) by sulfuration and acidification to reach pH of 7-7.0, carrying out pressure filtration and separation, returning the filtrate to prepare slurry by desulfurization for recycling, and using filter residue for preparing a building material. The method can separate 99.95%-99.96% of manganous dithionate in a manganese sulfate solution.
Owner:贵州红星发展大龙锰业有限责任公司
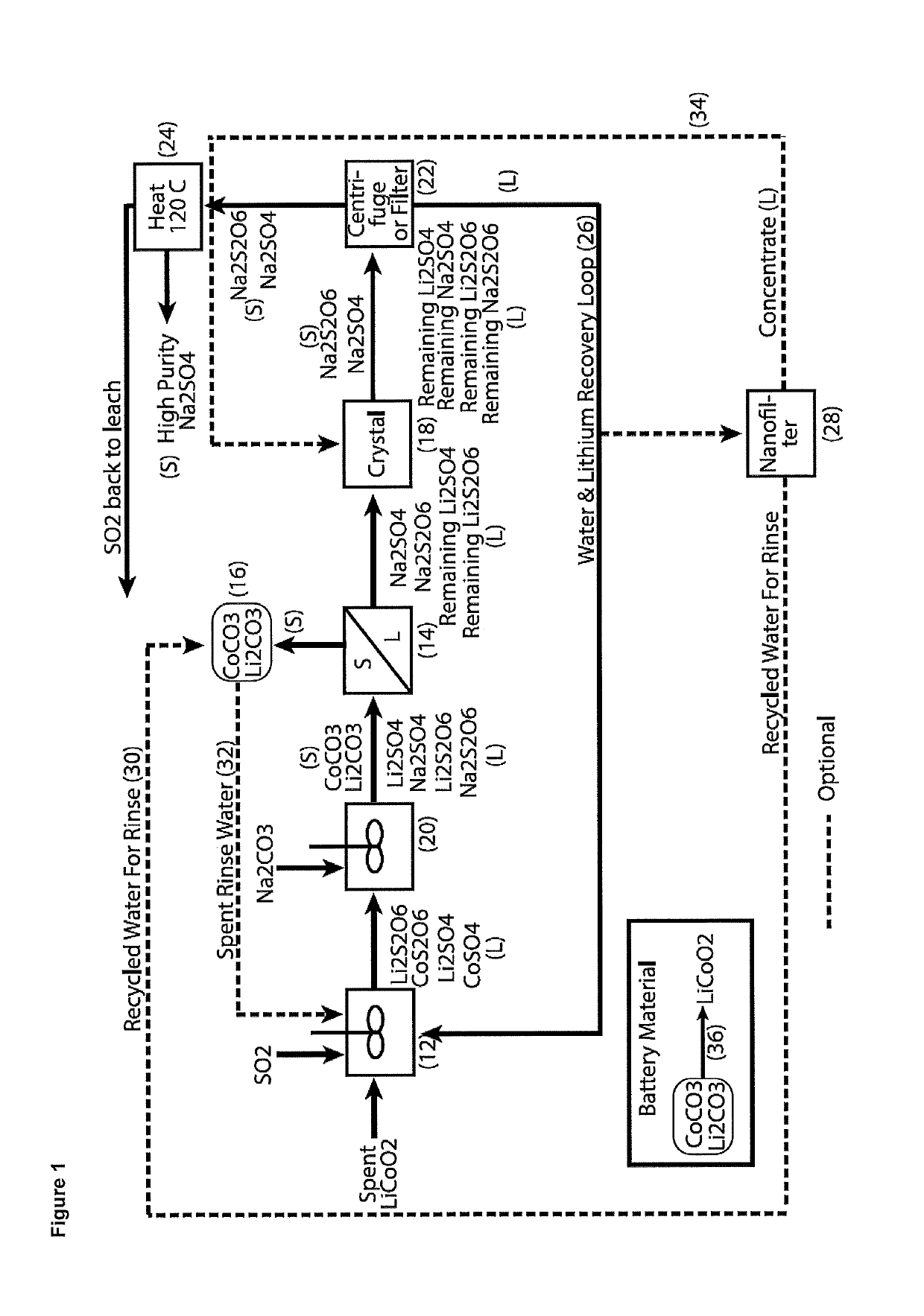
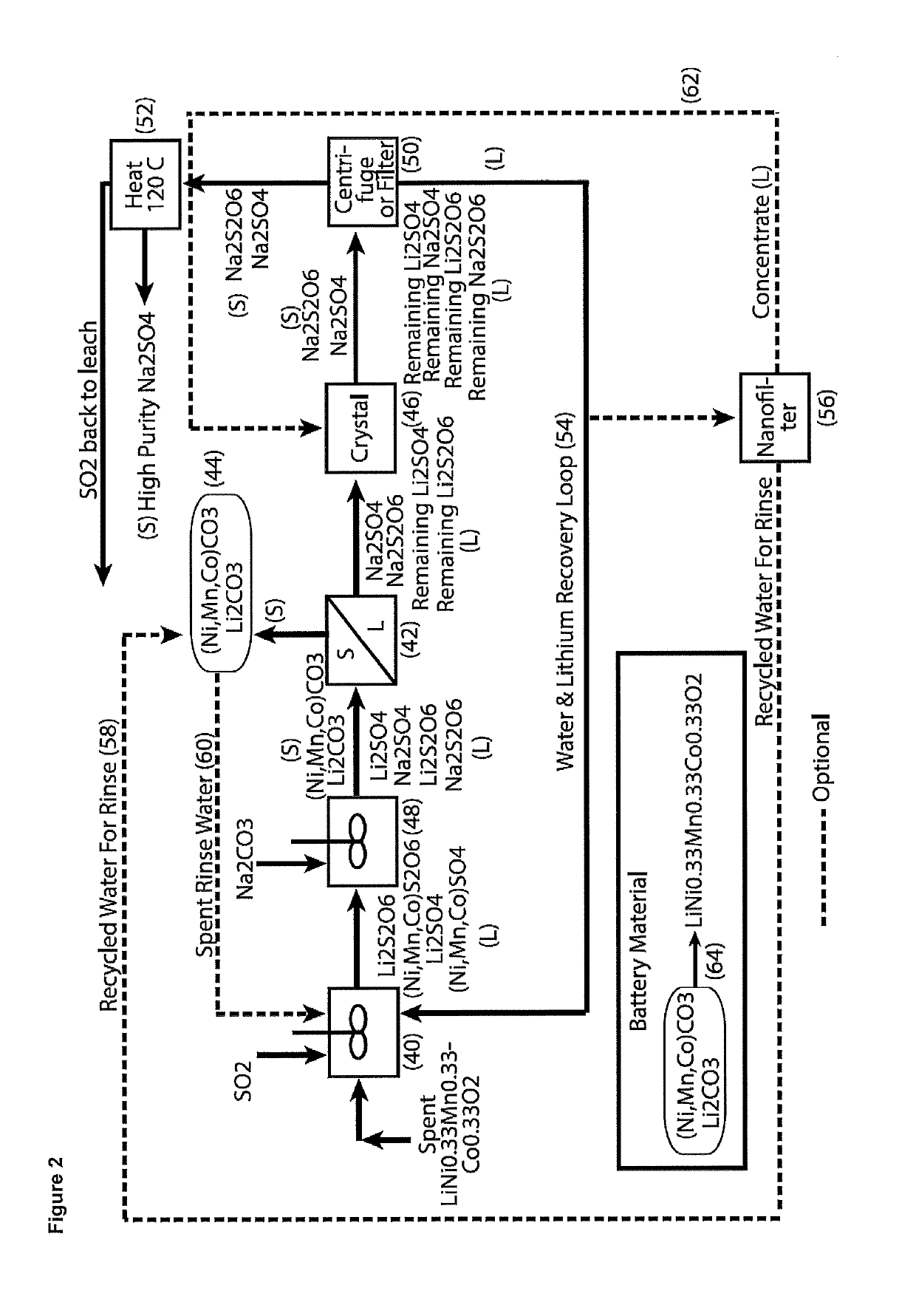
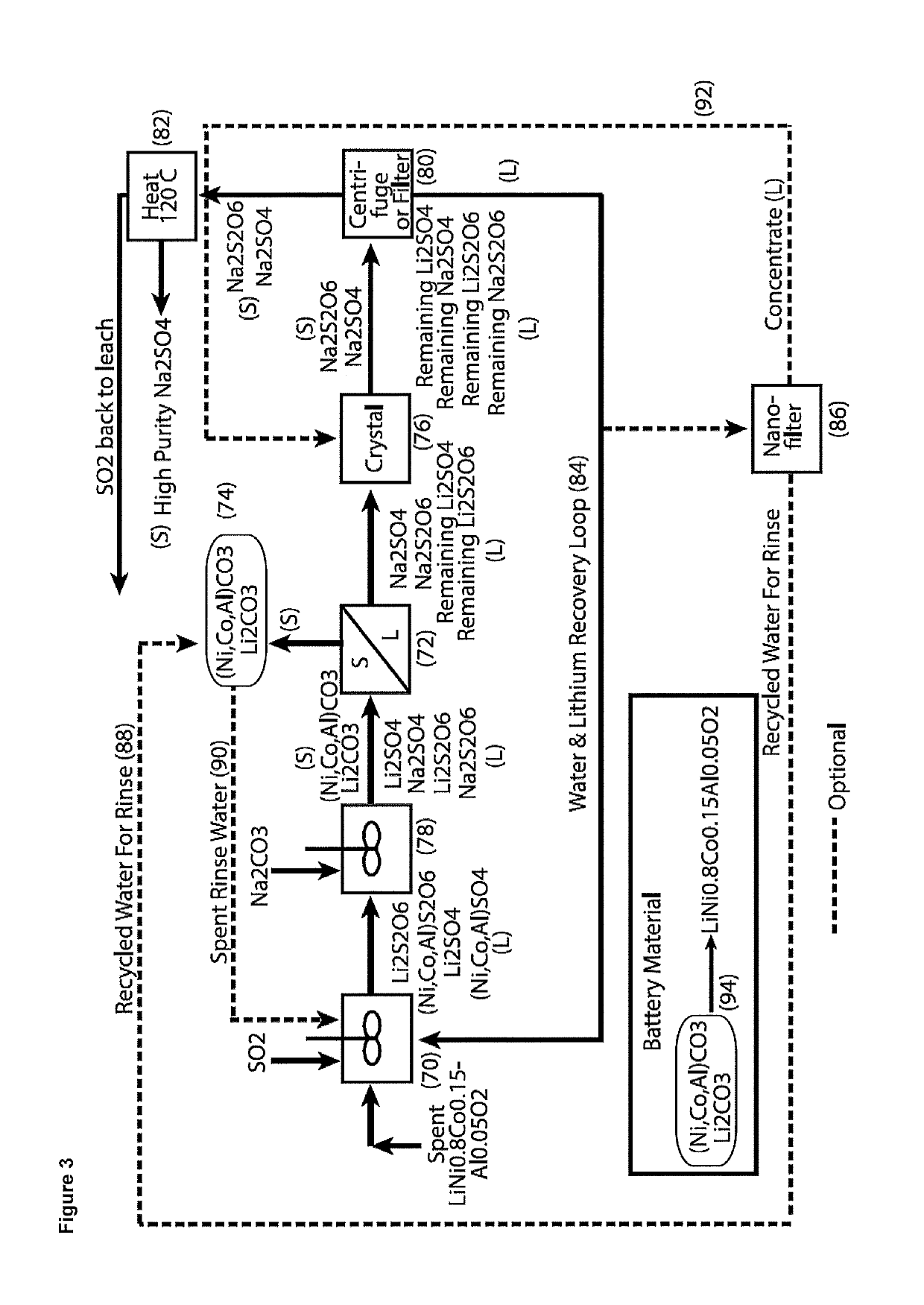
Processing of cobaltous sulphate/dithionate liquors derived from cobalt resource
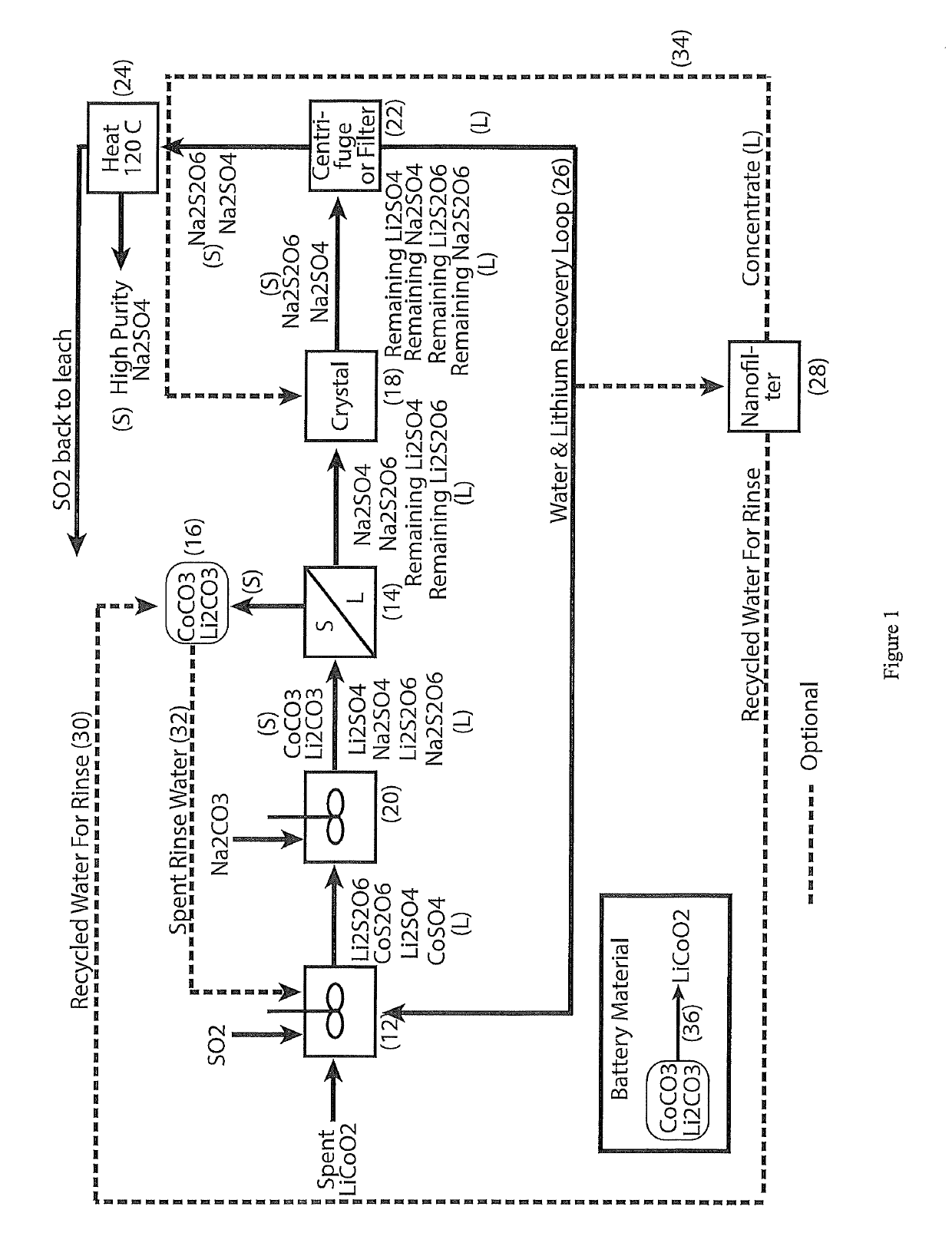
ActiveUS10308523B1Thiosulfates/dithionites/polythionitesWaste accumulators reclaimingDithionateCobalt(II) hydroxide
A process for water removal and / or recycling of sodium sulphate and / or sodium dithionate containing liquors derived from processing a cobalt resource derived from components of lithium ion batteries comprising steps of deriving from the cobalt resource a solution containing cobalt sulphate and cobalt dithionate, precipitation of cobalt as cobaltous carbonate or cobaltous hydroxide followed by removal thereof from the liquor, crystallization of sodium sulphate and sodium dithionate and removal of the resulting crystals, followed by heating of the crystals to anhydrous sodium sulphate, sulphur dioxide and water and then separating the anhydrous sodium sulphate.
Owner:ROCHER MANGANESE INC
Method for quickly reaerating cells under anaerobic environment
The invention relates to a method for quickly reaerating cells under an anaerobic environment. The method comprises the following steps: preparing an oxygen carrier, culturing cells and detecting cells: sucking redundant oxygen carrier culture medium, adding 100 microliter of 10% 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-benezene dithionate)-2H-tetrazole monosodium (CCK-8) reagent, incubating for 2-4 hours in a constant temperature incubation box at 37 DEG C, using a microplate reader for detecting light absorbance thereof and determining the cell activity. The oxygen carrier can enrich a large amount of oxygen, is used as an oxygen supplier and can supply oxygen for the cells under the condition of insufficient air oxygen. According to the invention, no external matter is added, the biological safety is high, the toxicity is low, the method is simple and the operability is strong.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Processing of cobaltous sulphate/dithionate liquors derived from cobalt resource
ActiveCN110139832AThiosulfates/dithionites/polythionitesManganese oxides/hydroxidesDithionic acidDithionate
A process for water removal and / or recycling of sodium sulfate and / or sodium dithionate containing liquors derived from processing cobalt resource material essentially free of lithium comprising the steps of precipitation of cobalt as cobaltous carbonate or cobaltous hydroxide followed by removal thereof from the liquor, crystallization of sodium sulfate and sodium dithionate and removal of the crystals, followed by heating of the crystals to anhydrous sodium sulfate, sulphur dioxide and water and then separating the anhydrous sodium sulfate.
Owner:ROCHER MANGANESE INC
Methods of X-aptamer generation and compositions thereof
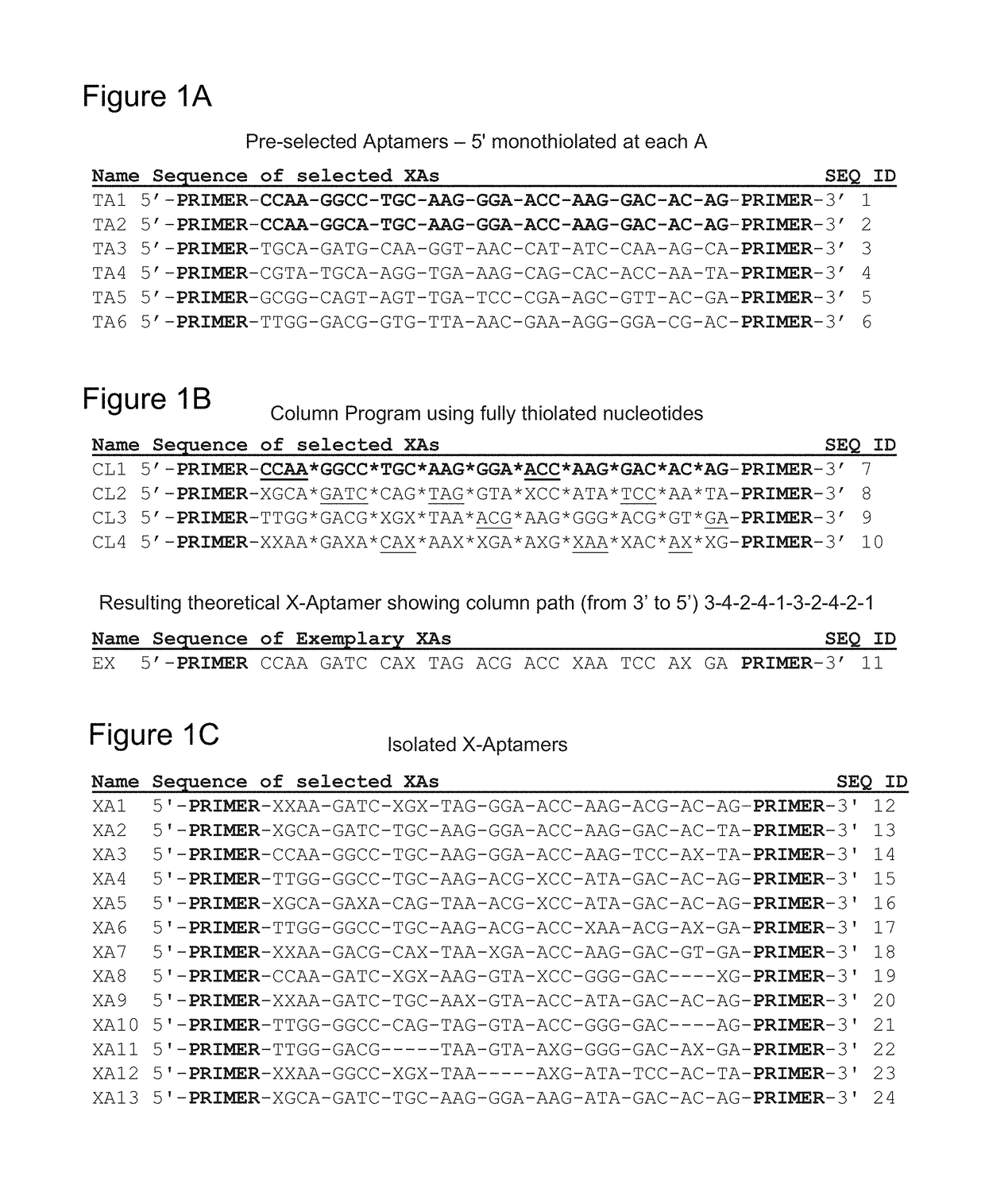
Provided herein are methods for a novel bead-based next-generation “X-aptamer” selection scheme that extends aptamer technology to include X-modified bases, thus resulting in X-aptamers, at any position along the sequence because the aptamers are chemically synthesized via a split-pool scheme on individual beads. Also provides are application to a wide range of commonly used DNA modifications, including, but not limited to, monothioate and dithioate backbone substitutions. This new class of aptamer allows chemical modifications introduced to any of the bases in the aptamer sequence as well as the phosphate backbones and can be extended to other carbohydrate-based systems.
Owner:AM BIOTECH +1
Tire tread based on a highly saturated diene elastomer
A tire tread comprising a rubber composition based on at least one highly saturated elastomer, a reinforcing filler and a vulcanization system is provided. The highly saturated diene elastomer contains 1,3-diene units and more than 50 mol % of ethylene units, the vulcanization system comprises a dithiosulfate salt of formula MO3S-S-A-S-SO3M in which the symbol A represents an alkanediyl group or a group comprising two or more alkanediyl units, which units are connected in pairs by means of an oxygen or sulfur atom, of a group of formula —SO2—, —NH—, —NH2+—, —N(C1—C16 alkyl)- or —COO—, or of an arylene or cycloalkylene group and the symbol M represents a metal atom. An improved compromise between the bubbling of the tread at the exit of curing presses and its hysteresis can be achieved.
Owner:MICHELIN & CO CIE GEN DES ESTAB MICHELIN
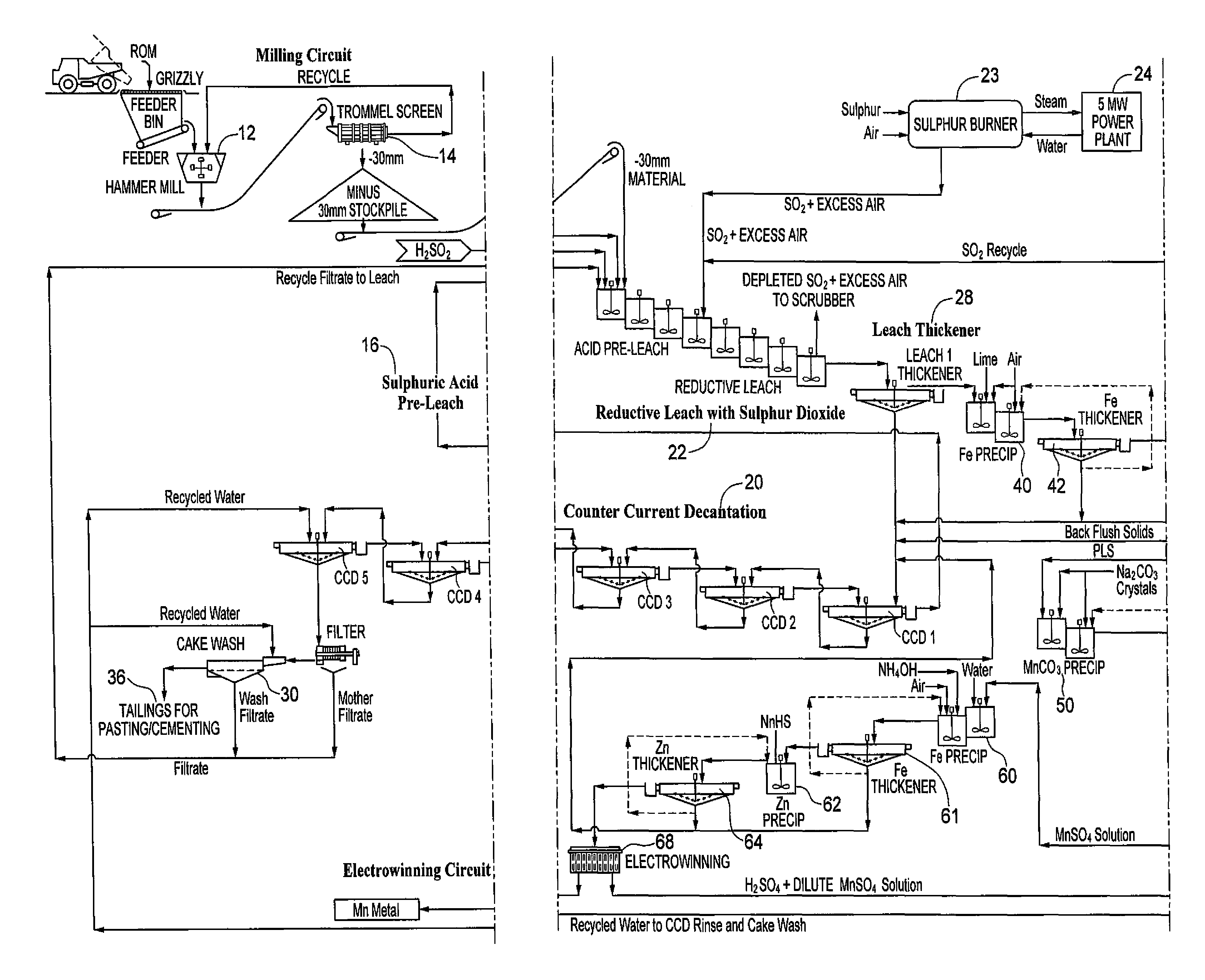
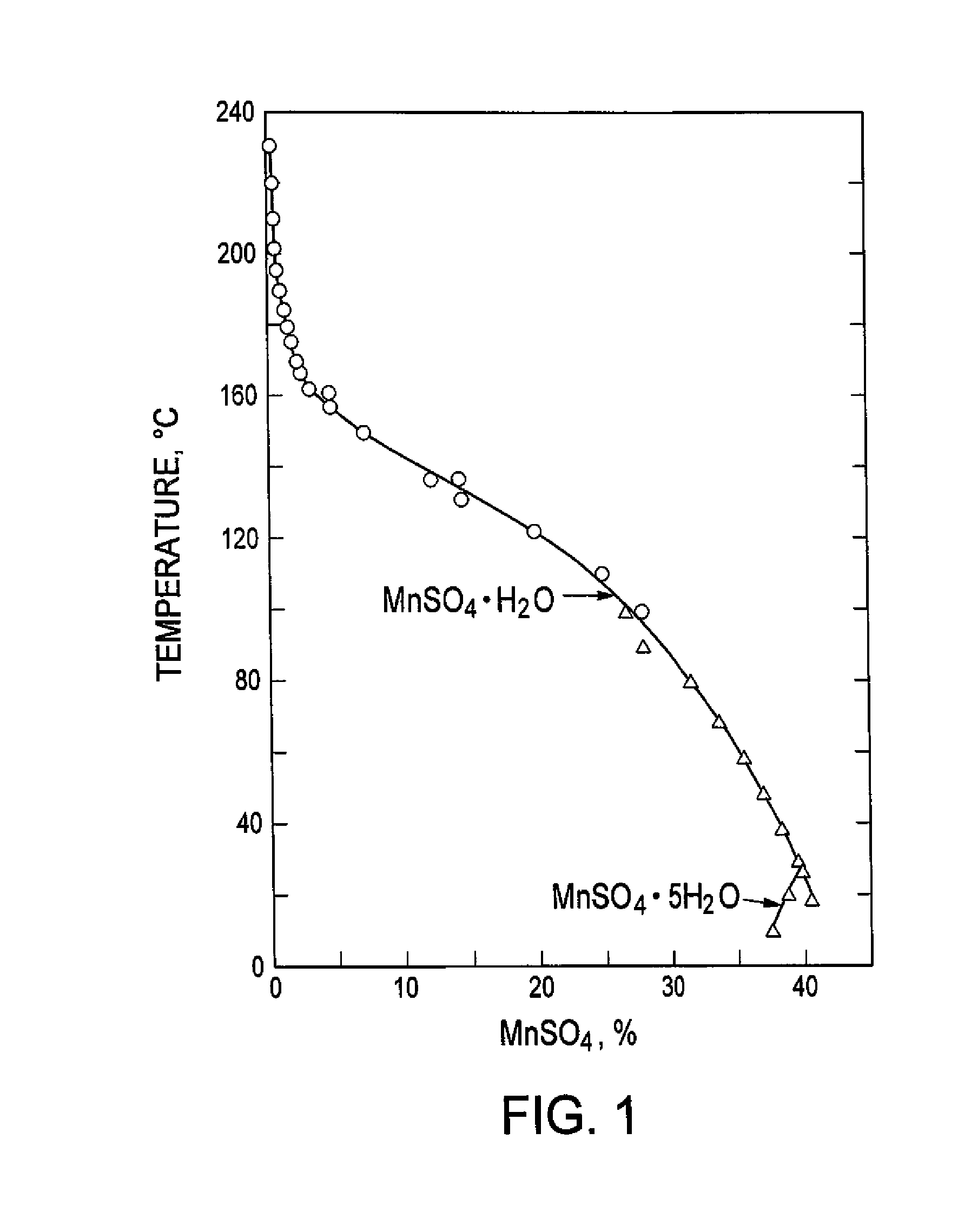
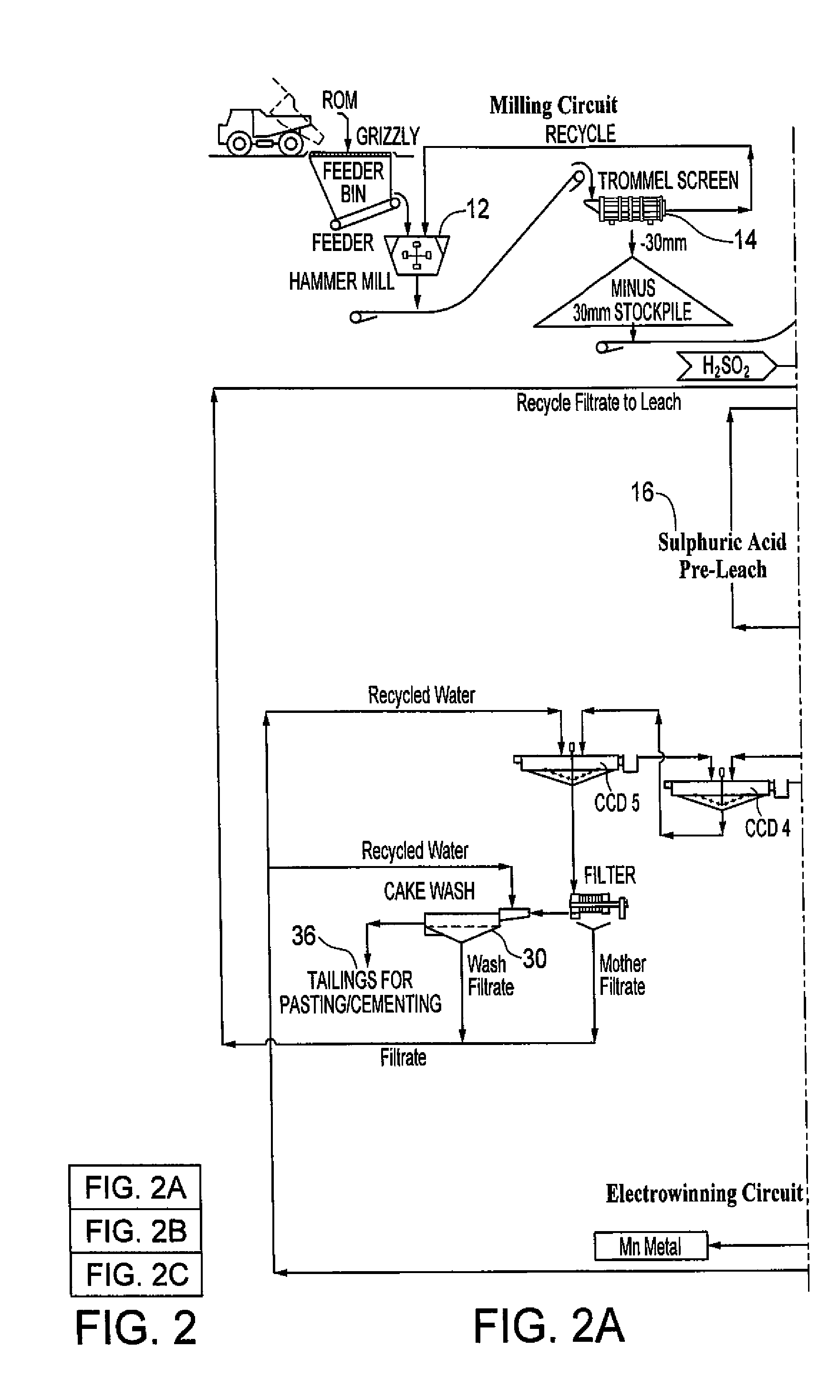
Hydrometallurgical processing of manganese containing materials
A process for the hydrometallurgical processing of manganese containing materials, the process characterized by the combination of a manganese dioxide containing feedstock and an acidic solution to form an acidic solution to be leached, and passing a volume of sulphur dioxide gas through that leach solution as the leaching agent, whereby no sintering or roasting pre-treatment step of the feedstock is undertaken and the levels of dithionate ion generated in the leach solution are less than about 5 g / l. Also described is a process for the production of electrolytic manganese dioxide.
Owner:AUVEX RESOURCES PTY LTD
Method for preparing manganese sulfate and battery-grade manganese sulfate by using manganese-containing ore
Owner:PANZHIHUA UNIV
A kind of method for removing manganese dithionate in manganese ore desulfurization liquid
The invention discloses a method for removing manganous dithionate in manganese ore desulfurizing liquid. Persulfate is added into the manganese oxide ore desulfurizing liquid, stirred and dissolved,ferrous sulfate is added, dithionate radical of the manganous dithionate in the manganese oxide ore desulfurizing liquid is oxidized into sulfate radical through sulfate radical and hydroxyl radical generated by high-order oxidation reaction between the persulfate and the ferrous iron, and thus the manganous dithionate in the manganese oxide ore desulfurizing liquid is removed. Through the method,purity of a manganese sulfate mother solution can be improved effectively, no energy needs to be consumed additionally to heat the manganese ore desulfurizing liquid, no acid or alkali needs to be consumed to adjust the pH of the desulfurizing liquid, technological conditions are simple and mild, operability is high, and industrialized application is easy to achieve.
Owner:SICHUAN UNIV
Efficient decomposition method of manganese dithionate in sulfur dioxide leaching solution of manganese ore
The invention discloses an efficient decomposition method for manganous dithionate in manganese ore sulfur dioxide lixivium. The method comprises the main steps that under the temperature condition ofthe sulfur dioxide lixivium of manganese ore pulp, sulfuric acid, manganese dioxide (or manganese bioxide ore) and ferric iron are sequentially added into the lixivium, and under the constant-temperature and acid conditions, through the catalytic activity of high-valent metal of ferric iron and manganese bioxide and the chain reaction process formed by coordination catalysis reduction and free radical oxidation formed by ferric iron and manganese bioxide, rapid oxidization conversion from sulfur dioxide to sulfate radicals is achieved. The process method has the advantages that cost is low, and large-scale industrialization production is easy. Manganese sulfate of main components in the lixivium serves as reaction products, no new impurity is introduced, and the lixivium can be used for producing electrolytic manganese metal or manganese sulfate easily later.
Owner:SICHUAN UNIV
Rubber composition made from highly saturated diene elastomer and dithiosulfate
Owner:MICHELIN & CO CIE GEN DES ESTAB MICHELIN
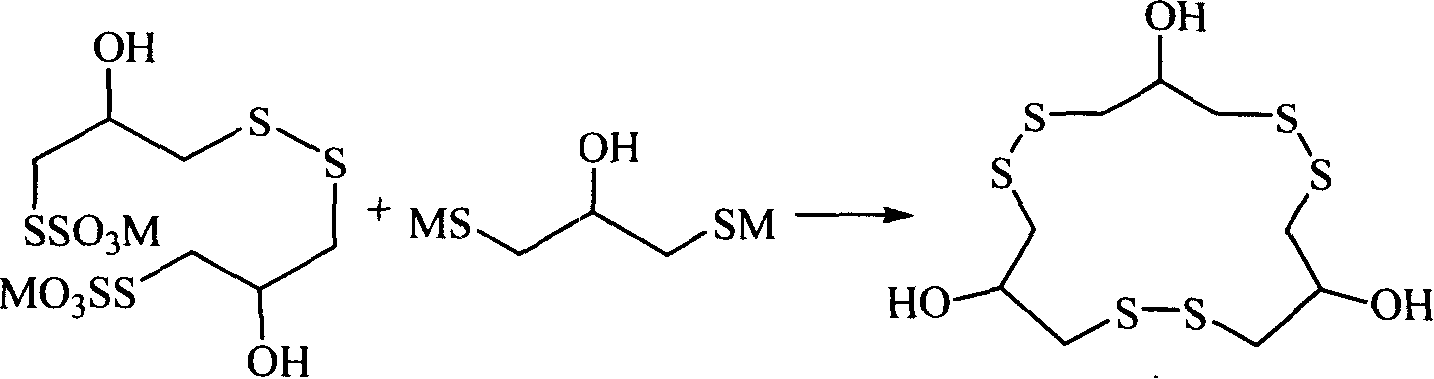
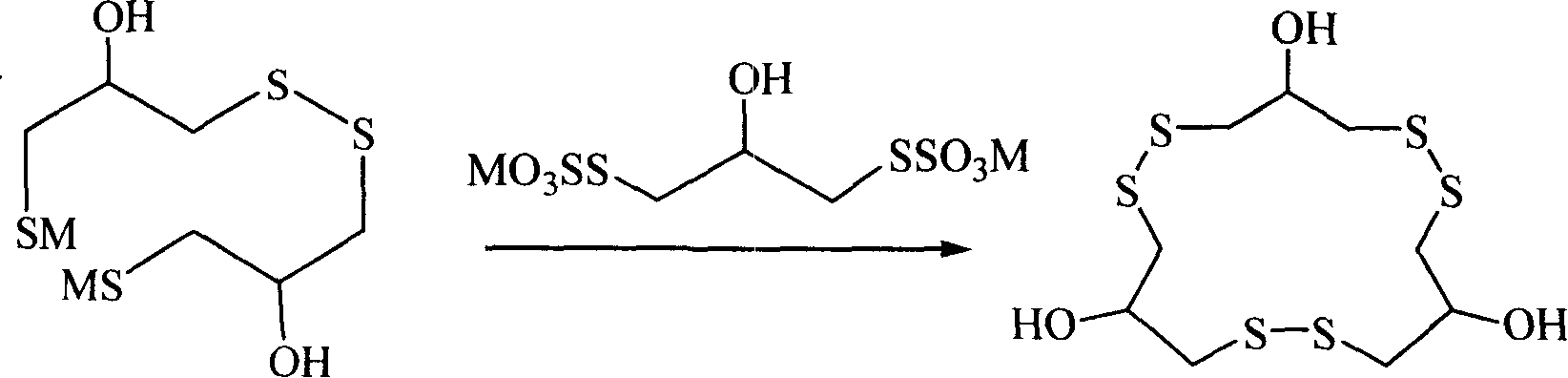
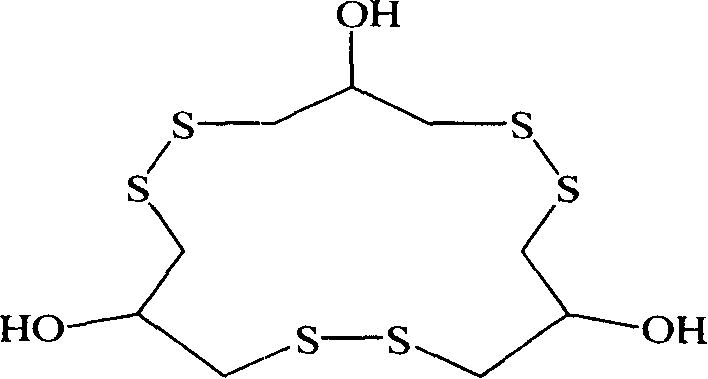
Method for synthesizing macrocyclic polydithioether compound gymnorrhizol
The invention relates to a method for synthesizing novel macrocyclic polydisulfide gymnorrhizol with an active constitutional formula as follows for resisting II-type diabetes. The method comprises: 3, 3'- disulfide group di (2- hydroxy propyl-1- thiosulfate) reacts with 2-hydroxide radical-1, 3- thioglycerin dibasic salt to obtain the gymnorrhizol, or the 3, 3'- disulfide group di (2- hydroxy propyl-1- thiosulfate) reacts with 2-hydroxide radical-1, 3-trimethylene thiosulfate to obtain the gymnorrhizol. The synthetic method of the gymnorrhizol provided by the invention has a new synthetic route, simple operation; compared with the prior art, the method has high yield and good reproduction quality.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
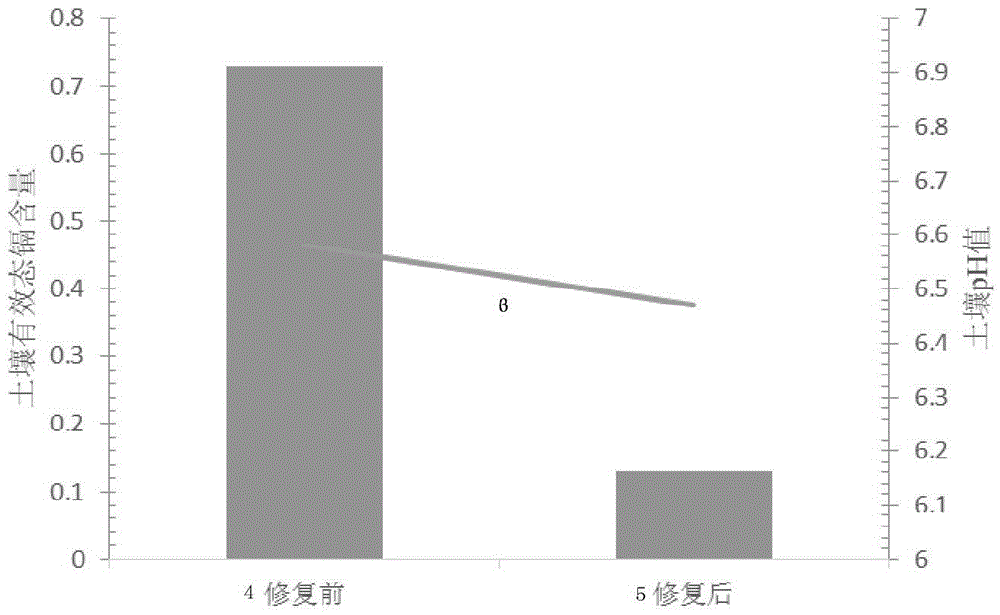
Neutral soil cadmium passivator and application method thereof
ActiveCN103805206BNo change in pHReach repairOrganic fertilisersSoil conditioning compositionsDithionateSediment
Owner:HUNAN RES INST FOR NONFERROUS METALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com