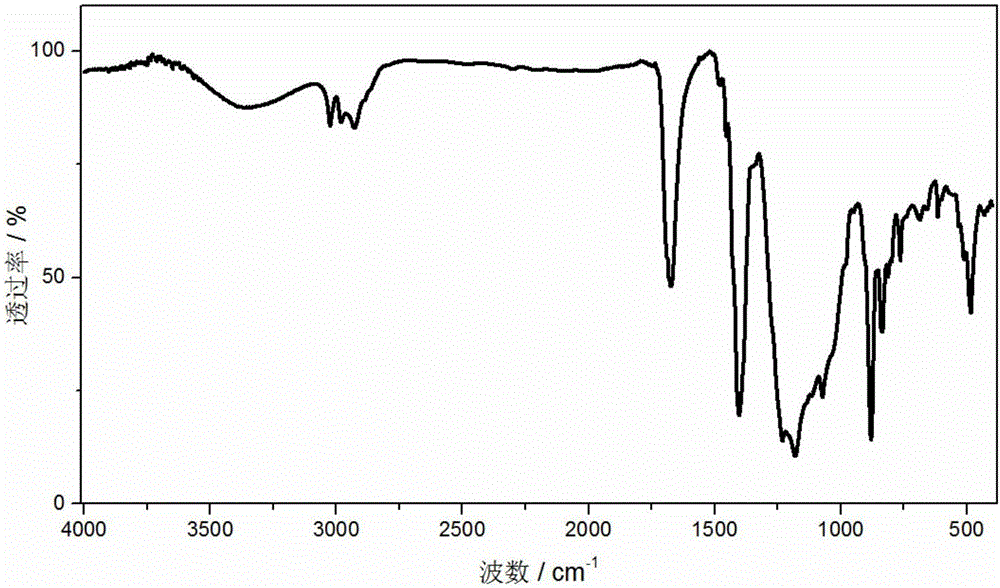
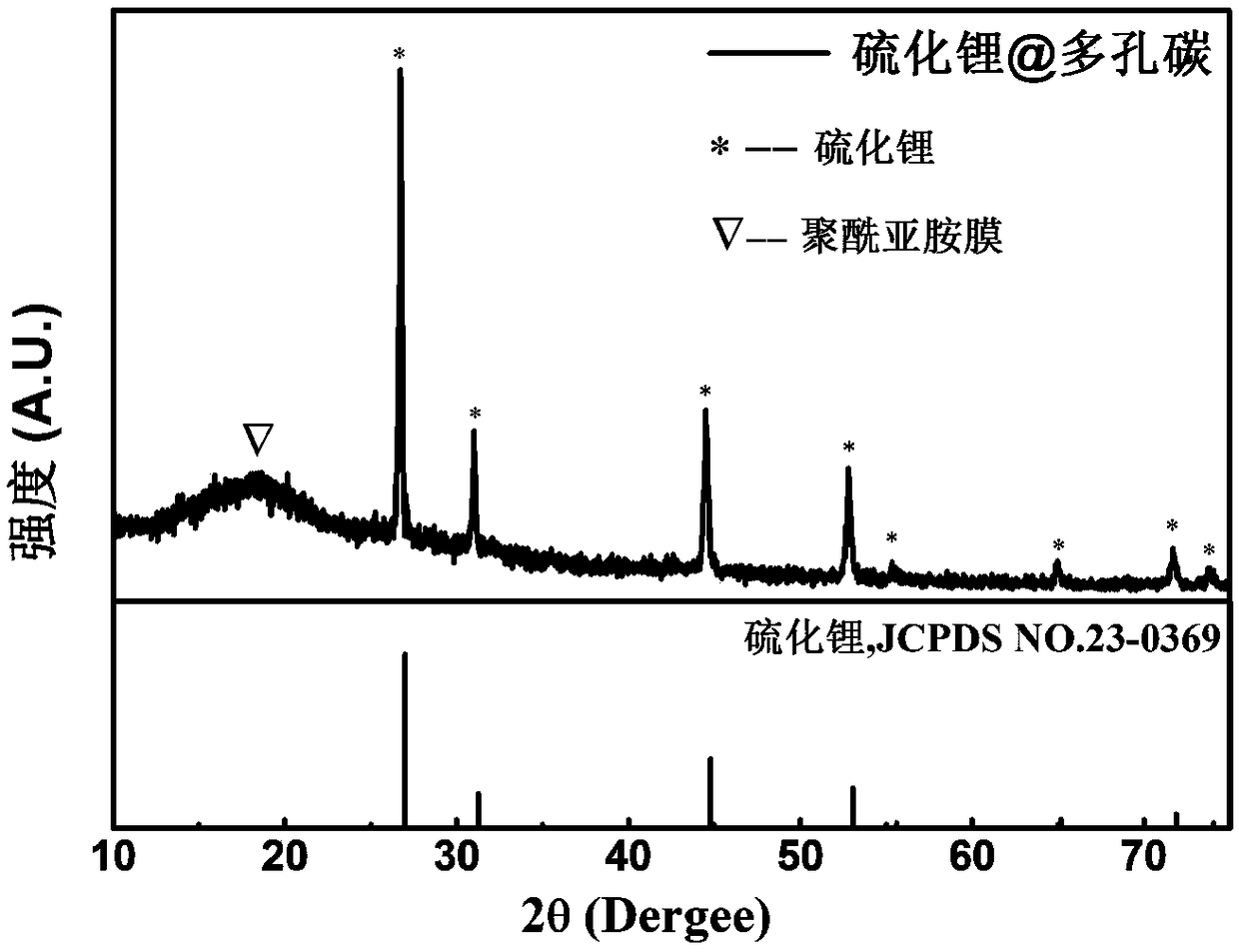
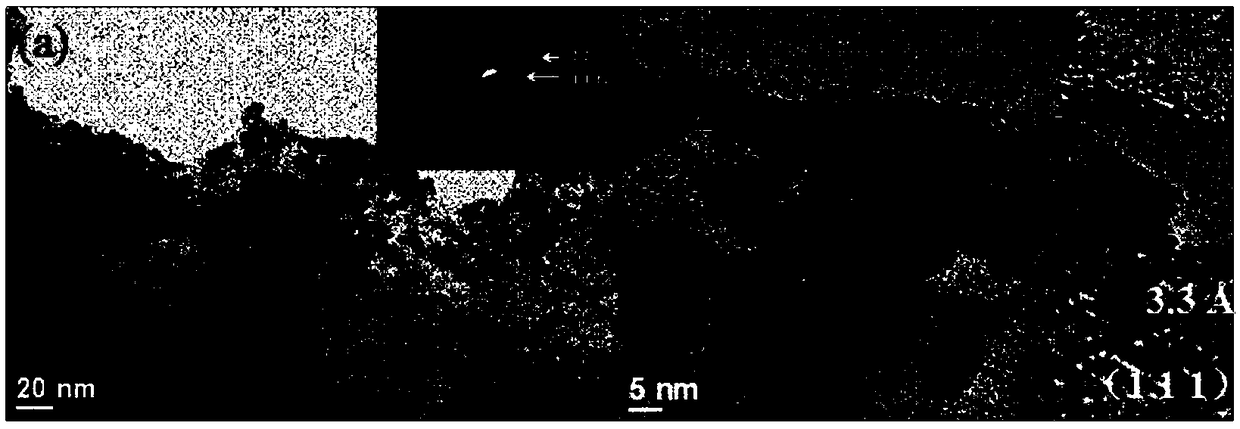
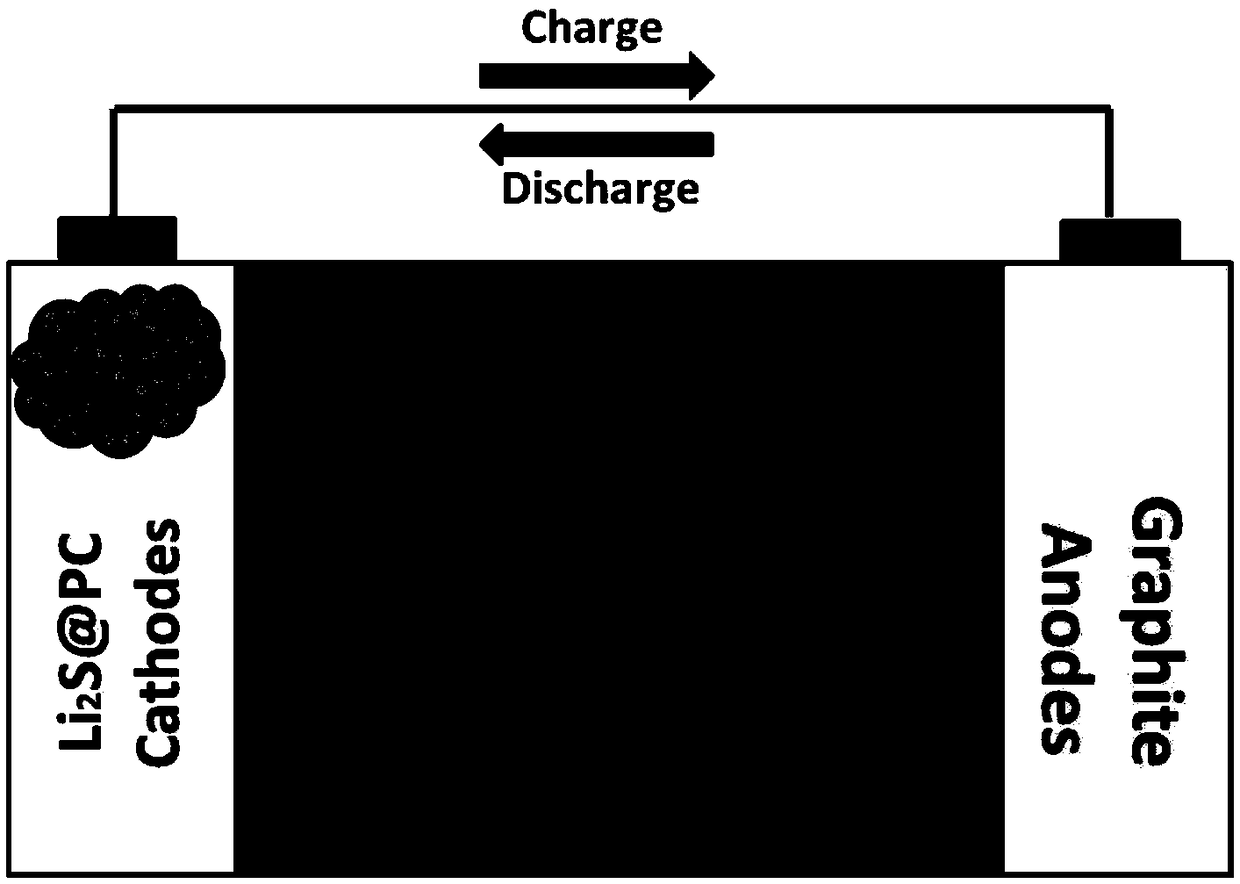
Patents
Literature
181 results about "Lithium sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium sulfate is a white inorganic salt with the formula Li₂SO₄. It is the lithium salt of sulfuric acid.
Clean production process of plateau sulfate type boron-lithium salt lake brine
InactiveCN102910652AHigh purityReduce the ratio of magnesium to lithiumChemical industryAlkali metal halide purificationHydration reactionSylvinite
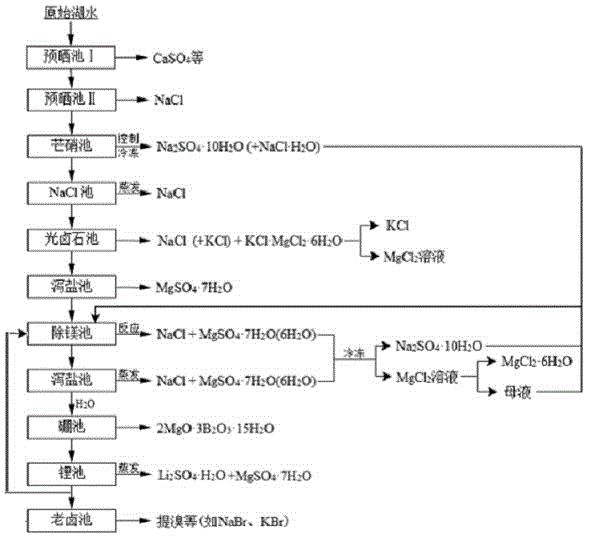
The invention relates to a clean production process of plateau sulfate type boron-lithium salt lake brine. The process comprises the following steps of: (1) arranging a pre-airing pond, a mirabilite pond, a NaCl pond, a carnallite pond, an epsom salt pond I, a magnesium removing pond, an epsom salt pond II, a boron pond, a lithium pond and an old brine pond; (2) controlling the sodium ion concentration in plateau sulfate type boron-lithium salt lake brine, precipitating mirabilite out in winter to obtain brine A, naturally evaporating the brine A, and salting out to obtain brine B; (3) naturally evaporating the brine B, and precipitating sylvine and carnallite out in sequence to obtain brine C; (4) naturally evaporating the brine C, precipitating an epsom salt out, and performing solid-liquid separation to obtain brine D and a solid A; (5) blending the brine D with mirabilite, removing magnesium to obtain brine E, and naturally evaporating brine E to obtain brine F and a solid B; (6) performing a hydration reaction on brine F, naturally evaporating, and precipitating reservoir water / inderite and brine G out; and (7) evaporating brine G or refrigerating for precipitating lithium sulfate, and processing the lithium sulfate into a corresponding product. The process has the advantages of comprehensive utilization of natural energy, saving in energy and environment friendliness.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Method for recovering lithium from lithium iron phosphate
ActiveCN108899601ADoes not affect the leaching rateHigh purityWaste accumulators reclaimingProcess efficiency improvementFerric hydroxideIron sulphate
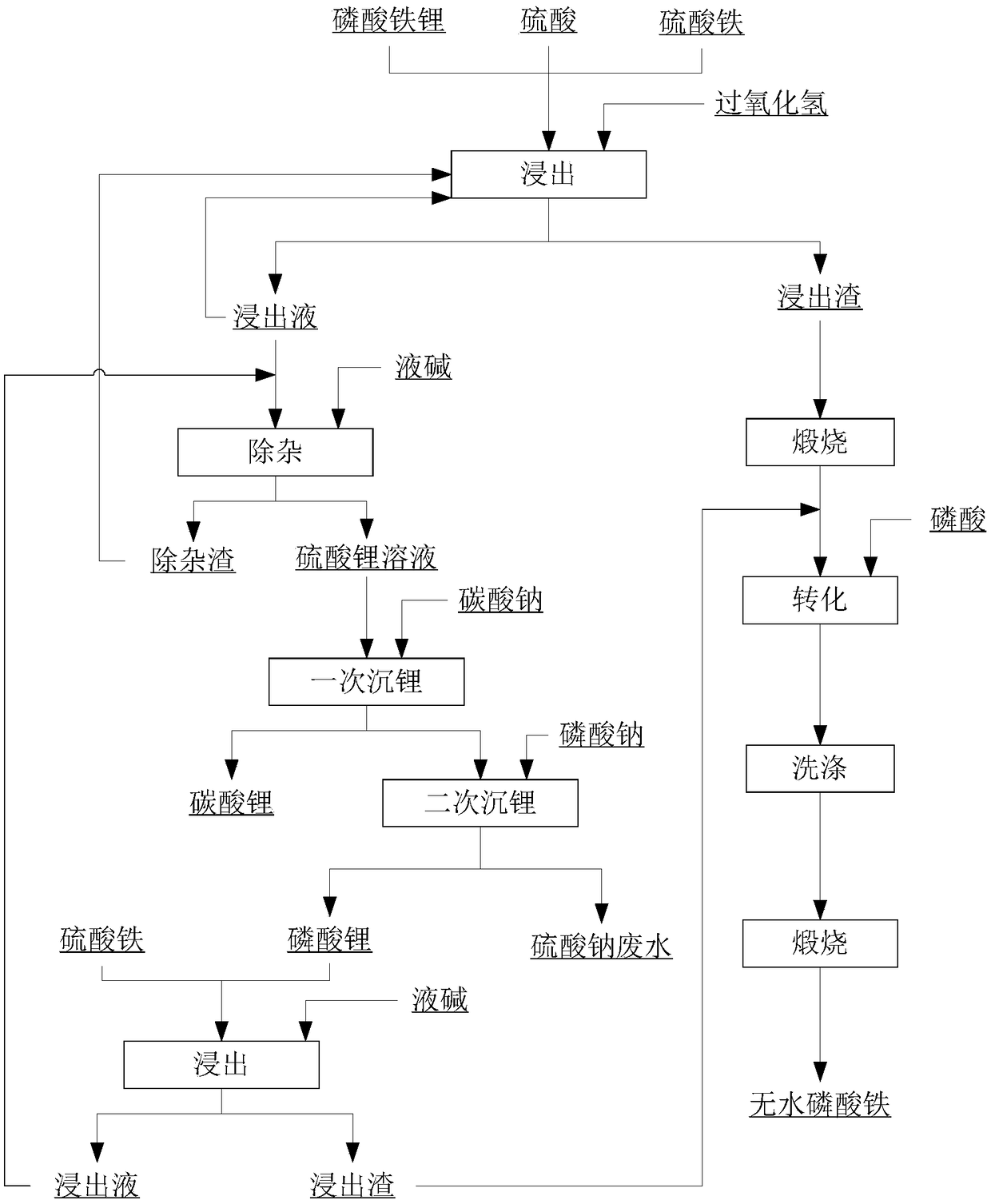
The invention discloses a method for recovering lithium from lithium iron phosphate. The method disclosed by the invention comprises the following steps: dissolving waste lithium iron phosphate slag with sulfuric acid and ferric sulfate, leaching iron, lithium and phosphorus, adding an oxidizing agent, reacting iron and phosphate radicals to produce an iron phosphate precipitate and a small amountof ferric hydroxide, converting lithium into a water-soluble lithium sulfate solution, filtering to obtain the lithium sulfate solution, adding sodium carbonate into the lithium sulfate solution to prepare a lithium carbonate product, and adding sodium phosphate or phosphoric acid to prepare lithium phosphate; dissolving the lithium phosphate with ferric sulfate again to obtain the lithium sulfate solution and a compound taking iron phosphate as a principle component, returning the lithium sulfate solution to the system to prepare lithium carbonate, and calcining the iron phosphate slag to remove organic matters and carbon in the slag; and slurrying to prepare cell grade iron phosphate. According to the method for recovering lithium from lithium iron phosphate disclosed by the invention,the lithium is totally converted into the product lithium carbonate in the method, the process flow is short, the cost is low, the lithium recovery rate is 97%, the metal lithium in the lithium iron phosphate can be effectively recovered, and all the slag is converted into the cell grade iron phosphate.
Owner:취저우화여우코발트뉴머터리얼컴퍼니리미티드 +1
Process for removing impurities from lepidolite leaching solution
The invention relates to a process for removing impurities from a lepidolite leaching solution. The process is completed by carrying out impurity removal on alumina twice and adjusting pH values for twice. Lithium salt precipitation products are obtained by adding additives to a lithium sulfate solution after impurity removal, and corresponding refined lithium salts can be prepared as required. The process has the advantages that the cost of impurity removal is low, by-products with high added values can be obtained, the extraction rate of lithium is high, various useful components of lepidolite ores can also be fully utilized when lithium salts are produced, and the recycling of resources is realized.
Owner:江西海汇龙洲锂业有限公司
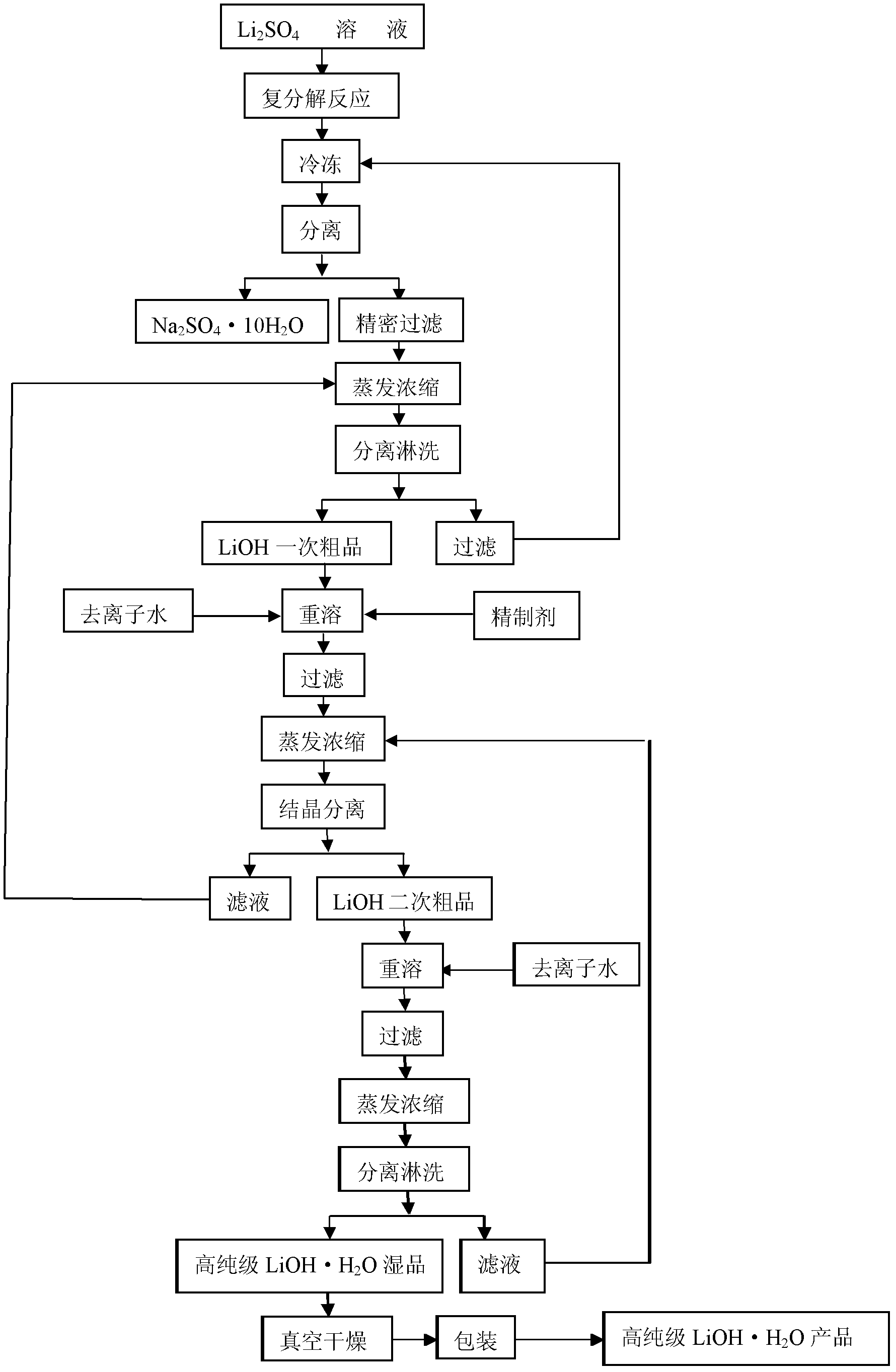
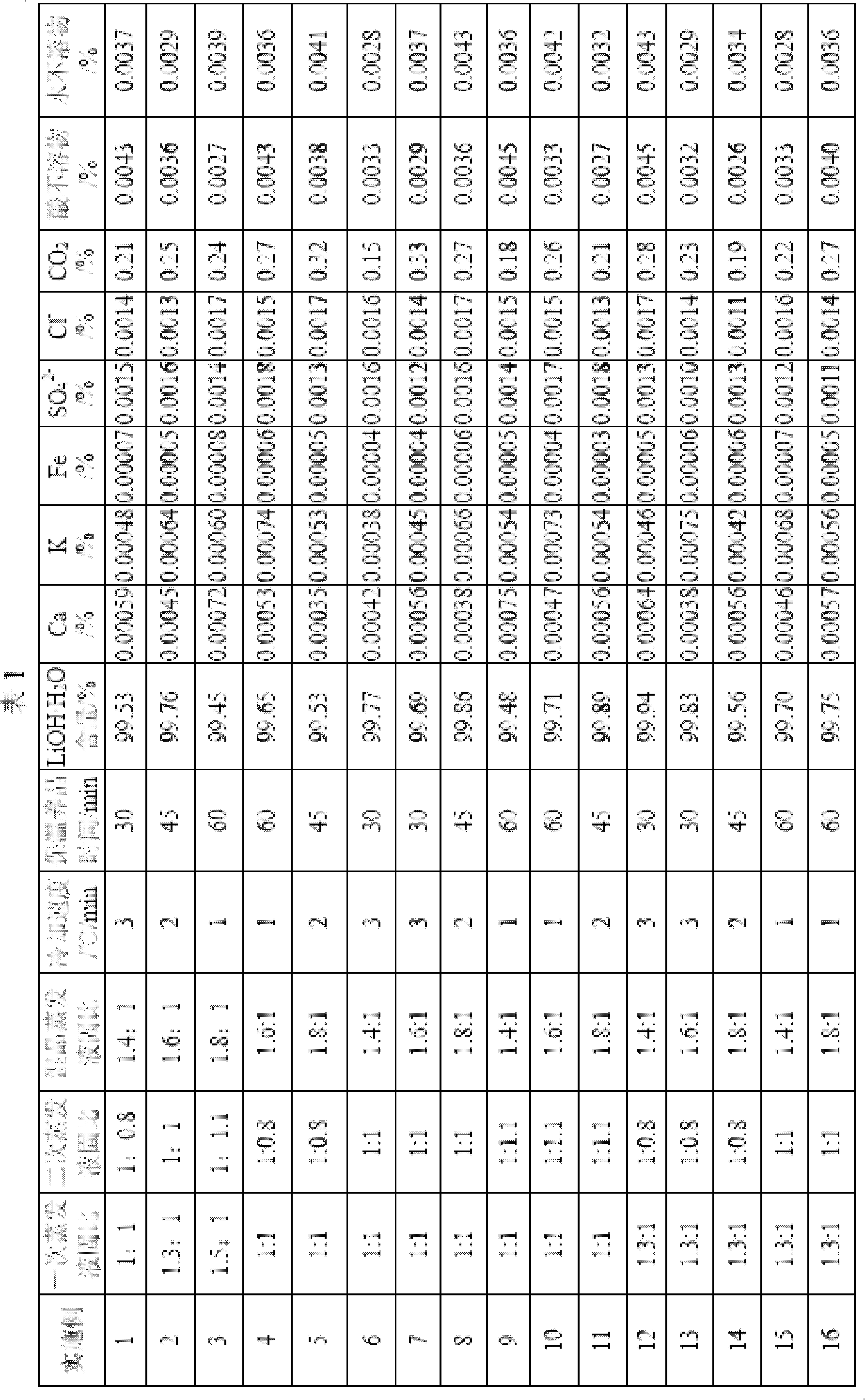
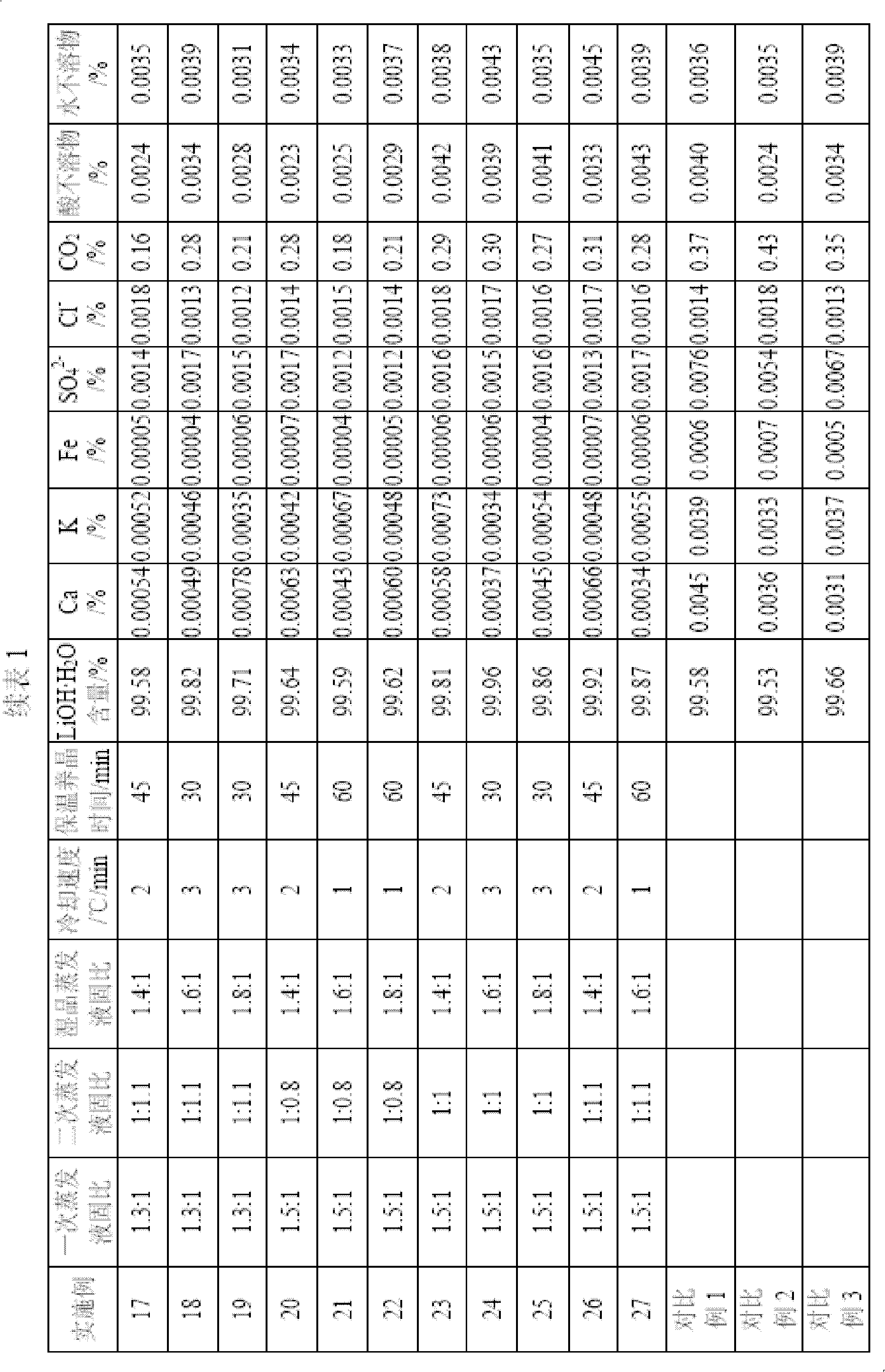
Method for preparing high purity level lithium hydroxide monohydrate
ActiveCN102659144AHigh recovery rate of lithiumSimple processLithium oxides/hydroxidesCalcium hydroxideHydrogen
The invention relates to a method for preparing a high purity level lithium hydroxide monohydrate and belongs to the technical field of lithium hydroxide preparations. The method includes the following steps of adding calcium hydroxide into a lithium sulfate leaching liquid to adjust the potential of hydrogen (pH) value, filtering to obtain a lithium sulfate primary cleaning liquid, adding sodium hydroxide, then filtering and removing impurities, crystallizing to obtain a sodium sulfate decahydrate solid and a lithium hydroxide refrigerating fluid, finely filtering the lithium hydroxide refrigerating fluid, then performing an evaporating separation to obtain a Lithium hydroxide monohydrate primary crude product, dissolving the crude product by adding water, adding a refining agent to remove sodium, then performing an evaporation concentration, separating and leaching to obtain a Lithium hydroxide monohydrate secondary crude product, adding water into the secondary crude product, performing the evaporation concentration, cooling to crystallize, and drying to obtain the high purity level lithium hydroxide monohydrate. By means of the method, the evaporation liquid-solid ratio is reasonably controlled, and processes of crystallizing and crystal growing are modified, so that the high purity level lithium hydroxide monohydrate product is obtained, the process is simple, the operation is easy, the equipment investment is few, the product cost is low, the lithium recovery rate is high, product qualities are stable, and the method is suitable for the industrialized production of the high purity level lithium hydroxide monohydrate.
Owner:TIANQI LITHIUM CORP
Process for extracting lithium salt from spodumene by adopting sulfuric acid pressure boiling method
ActiveCN104071811AFully recycleShort process routeLithium carbonates/bicarbonatesLithium carbonatePhysical chemistry
The invention discloses a process for extracting lithium salt from spodumene by adopting a sulfuric acid pressure boiling method. The process comprises the following steps: transforming the spodumene at high temperature, and adding sulfuric acid for pressure boiling to obtain soluble lithium sulfate and insoluble high-silicon residues, and separating lithium from lithium concentrate to further prepare lithium carbonate and obtain a byproduct-high-silicon soil; adding lime for purifying leachate liquid containing a small quantity of aluminum, iron and other alkali metal sulfate, removing impurities in a multi-step way to obtain a high-purity lithium sulfate solution, and carbonizing to generate a lithium carbonate product and a sodium sulfate byproduct. According to the process, the yield is high, the wastewater discharge quantity is low, mother liquor can be recycled, and the cost is low.
Owner:江西雅保锂业有限公司
Positive electrode material for non-aqueous electrolyte lithium ion battery and battery using the same
PROBLEM TO BE SOLVED: To provide a positive electrode material for a nonaqueous electrolyte lithium ion battery, which can suppress a decomposition of an electrolyte even when moisture does not enter a cell and a battery is charged or discharged at high temperature.SOLUTION: The positive electrode material for a nonaqueous electrolyte lithium ion battery includes lithium-nickel oxide having a surface covered with an Li compound to be attached. The Li compound is at least one selected from the group consisting of lithium phosphate, an LiPON compound, an Li<SB POS="POST">2< / SB>O-B<SB POS="POST">2< / SB>O<SB POS="POST">3< / SB>compound, an Li<SB POS="POST">2< / SB>O-B<SB POS="POST">2< / SB>O<SB POS="POST">3< / SB>-LiI compound, an Li<SB POS="POST">2< / SB>S-SiS<SB POS="POST">2< / SB>compound, an Li<SB POS="POST">2< / SB>S-SiS<SB POS="POST">2< / SB>-Li<SB POS="POST">3< / SB>PO<SB POS="POST">4< / SB>compound, lithium hydroxide, lithium fluoride, lithium acetate, lithium acetylide ethylenediamine, lithium benzoate, lithium bromide, lithium carbonate, lithium nitrate, lithium oxalate, lithium pyruvate, lithium stearate, lithium tartrate and lithium sulfate.
Owner:ENVISION AESC JAPAN LTD
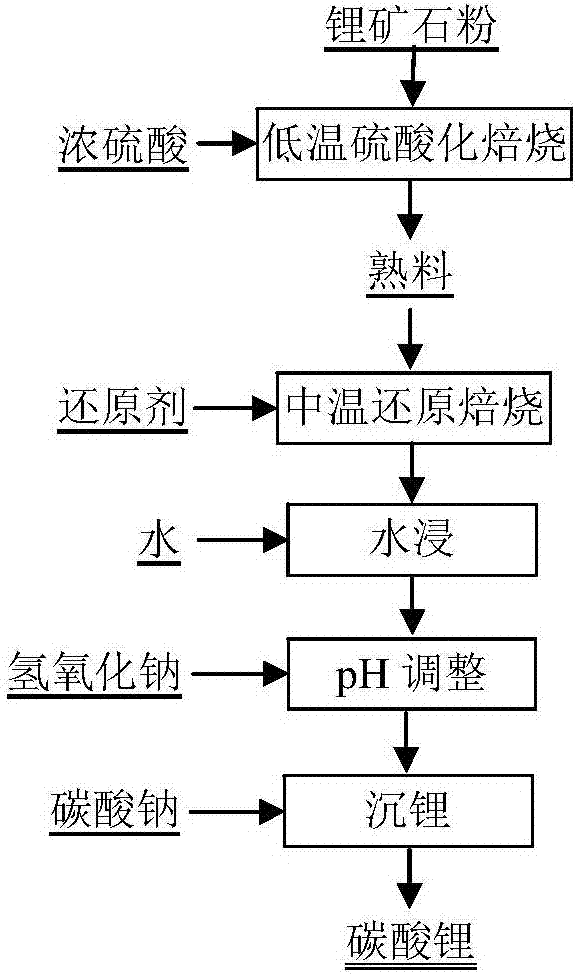
Method for preparing lithium carbonate through two-stage conversion of lithium ore
InactiveCN107089673AHigh recovery rateAchieve recyclingLithium carbonates/bicarbonatesSolubilityCalcination
The invention discloses a method for preparing lithium carbonate through two-stage conversion of lithium ore and belongs to the technical field of lithium metallurgy. The method comprises the steps: mixing lithium ore powder and concentrated sulfuric acid, performing low-temperature sulfation calcination under condition of 150 to 400 DEG C to convert lithium in the lithium ore into lithium sulfate with good water solubility and meanwhile converting fluorine into hydrogen fluoride gas to be removed to finish first-stage conversion; then performing medium-temperature reduction calcinations on a clinker under 550 to 900 DEG C to convert sulfate of iron, aluminum and the like into aluminum oxide and ferric oxide, which are difficult to dissolve in water, in the clinker to finish second-stage conversion, wherein generated sulfur dioxide flue gas can be used for preparing acid; soaking reduced calcine with water to extract lithium, leaving impurities of iron, aluminum and the like in leaching residues and filtering water-soaked ore pulp to directly obtain a purer lithium sulfate solution. The method not only achieves separation of the lithium from the aluminum and the iron in a leaching process, but also achieves regeneration cycle of sulfuric acid, reduces consumption of chemicals of sulfuric acid, neutralizing alkali and the like and has high lithium recovery rate.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Method for cyclically using lithium deposition for preparing sodium carbonate solution to produce lithium carbonate
ActiveCN101481125AReduce consumptionShort loss lessLithium carbonates/bicarbonatesLithium carbonateLithium sulfate
The invention discloses a method for producing lithium carbonate by circularly applying mother solution from which lithium is subsided to prepare a sodium carbonate solution, which comprises the sequential steps such as refrigerating the mother solution from which lithium is subsided, filtering the mother solution from which sodium is sought out, heating the purified liquid of the mother solution from which the sodium is sought out, sweating out the mother solution to prepare the sodium carbonate solution, and performing reaction of the sodium carbonate solution with the lithium sulfate solution so that lithium is subsided. The invention has the characteristics of high lithium sedimentation efficiency, simple technique, stable product, high production efficiency, high recovery rate, low consumption of sodium carbonate, low energy consumption and low cost.
Owner:JIANGSU RONGHUI GENERAL LITHIUM IND CO LTD
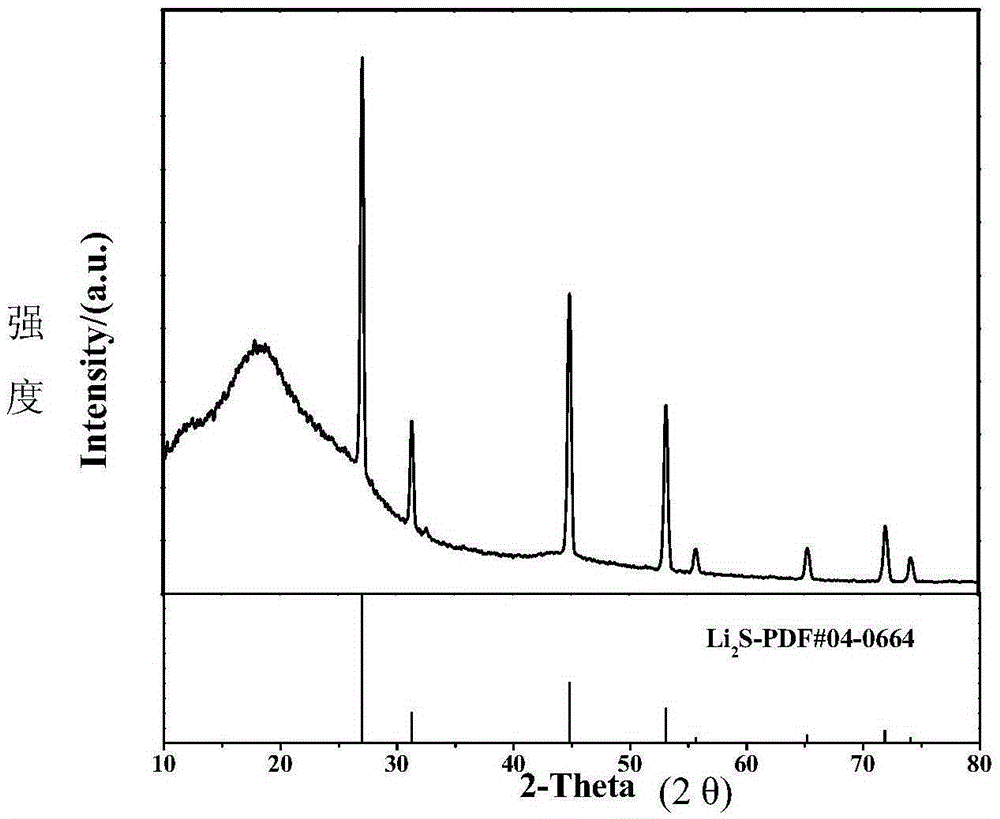
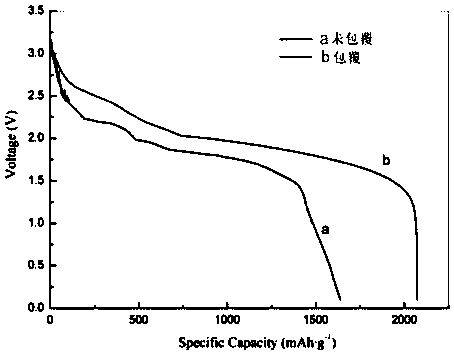
Method for preparing lithium carbide/lithium sulfide composite anode material of lithium-sulfur battery by performing carbon thermal reduction on lithium sulfate
InactiveCN106229487AReduce manufacturing costSimplify complex processesCell electrodesLi-accumulatorsMaterials preparationLithium–sulfur battery
The invention relates to a method for preparing a lithium carbide / lithium sulfide (Li2S) composite anode material of a lithium-sulfur battery by performing carbon thermal reduction on lithium sulfate (Li2SO4), and belongs to the technical field of lithium-sulfur batteries. The lithium carbide / lithium sulfide composite material is prepared by performing the carbon thermal reduction on a lithium sulfate-containing composite material serving as a precursor under a condition of inert atmosphere protection, and is applied to an anode of the lithium-sulfur battery. The lithium sulfide composite material preparation method disclosed by the invention is a novel method for in-situ synthesis of the anode material of the lithium-sulfur battery; and the prepared lithium carbide / lithium sulfide composite material is high in active substance dispersivity and stable in structure, and has the advantages of high specific capacity, excellent circulating and rate capability and the like when used as an anode material of the lithium-sulfur battery. The method is easy to operate, low in preparation cost and favorable for large-scale production.
Owner:BEIJING UNIV OF CHEM TECH
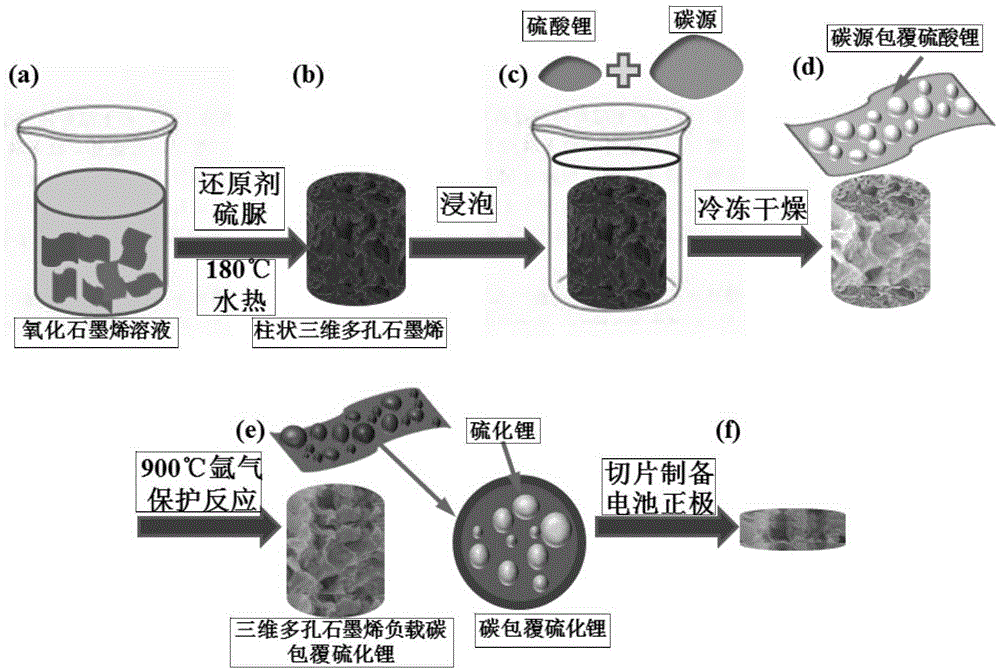
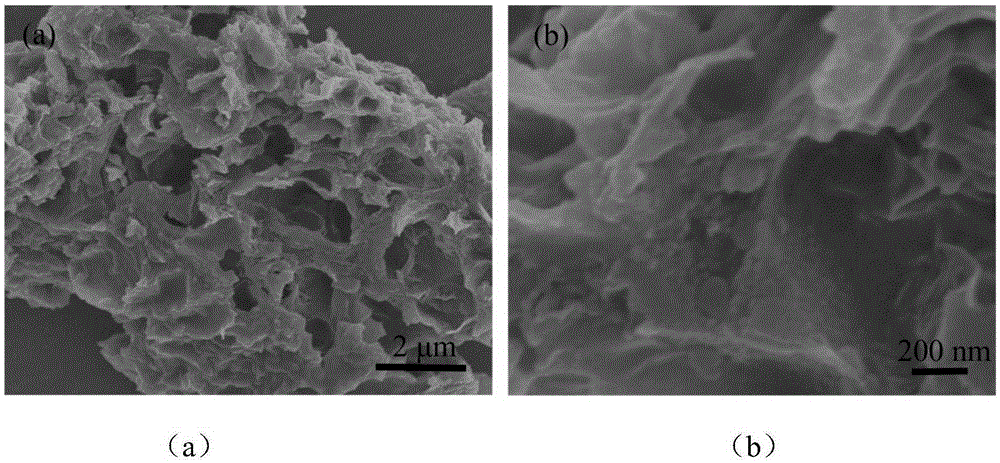
Three-dimensional porous graphene-supported carbon-coated lithium sulfide cathode material as well as preparation method and application thereof
InactiveCN105406034AImprove conductivityIncrease capacityCell electrodesSecondary cellsPorous grapheneFreeze-drying
The invention discloses a three-dimensional porous graphene-supported carbon-coated lithium sulfide cathode material as well as a preparation method and application thereof. The preparation method comprises the following steps: dispersing oxidized grapheme in water for magnetic stirring, then adding a reducing agent, stirring and dissolving to obtain a brown solution; putting the solution at the temperature of 120-200 DEG C to carry out a hydrothermal reaction for 4-12 h so as to obtain a columnar three-dimensional porous grapheme solution; adding lithium sulfate and a carbon source to the columnar three-dimensional porous grapheme solution to form a columnar three-dimensional porous grapheme soak solution; carrying out freeze drying on the soak solution to obtain a precursor; calcining the precursor for 2-12 h under a protective atmosphere at the temperature of 800-1000 DEG C so as to obtain the three-dimensional porous graphene-supported carbon-coated lithium sulfide material. A battery cathode can be prepared through direct slicing by using the prepared material, the steps of slurry preparation, coating and drying are saved, the technology is simpler, and the material is suitable for large-scale production and has excellent electrical properties.
Owner:ZHEJIANG UNIV
Sulfo group polymer electrolyte, as well as in-situ preparation method and application thereof
ActiveCN106785025AEvenly distributedPhase decomposition does not occurSecondary cellsPolymer dissolutionElectrical conductor
The invention provides a sulfo group polymer electrolyte, as well as an in-situ preparation method and an application thereof. The preparation method comprises the following steps: dissolving a polymer in a dissolvent; adding a silane coupling agent containing -SH; ultrasonically removing air bubbles from the system and forming a film; soaking into an oxidant solution; oxidizing the -SH terminal into a sulfo group, and meanwhile, hydrolyzing by using the silane coupling agent and generating SiO2; after cleaning, putting into an acid solution for acidizing; after taking out, washing with deionized water; boiling with water and removing the residual acid; putting into a lithium exchange solution for exchanging lithium, cleaning and drying, thereby acquiring a lithium sulfate-containing polymer electrolyte membrane. The thermal stability and the electrochemical properties of the prepared sulfo group polymer electrolyte are promoted; the sulfo group polymer electrolyte can be applied to an electrochemical power source system, such as, a lithium ion battery and can meet the requirement of large current charge and discharge of the lithium ion battery; an effect of a single ion conductor is achieved; and the safety performance of the battery is promoted.
Owner:XIAMEN UNIV
Method for synthesizing lithium sulfide/carbon composite material and lithium-sulfur battery using material
InactiveCN109360953AFast commercializationSave raw materialsPositive electrodesLi-accumulatorsCarbon compositesArgon atmosphere
The invention relates to a method for synthesizing lithium sulfide / carbon composite material by lithium sulfate and carbon sources in a heat treatment manner, and further relates to lithium-sulfur battery which takes the prepared lithium sulfide / carbon composite material as a positive-electrode material and graphite as a negative-electrode material. The method includes the steps: preparing water solution according to the mole ratio of the carbon sources to lithium sulphate monohydrate of (0.5-2):1, heating and stirring the water solution at the temperature of 60-100 DEG C, placing the water solution into a drying oven to heat and dry the water solution at the 60-100 DEG C, and grinding drying substances into powder; placing the powder prepared in the step (1) into the constant-temperaturearea of a tube furnace, heating the powder to reach the temperature of 500-700 DEG C in argon atmosphere, keeping the temperature for 0.5-4 hours, heating the powder to reach the temperature of 700-1000 DEG C at the speed of 2-10 DEG C / min, keeping the temperature for 0.5-4 hours, cooling the powder to reach the temperature of 300-350 DEG C at the speed of 5-10 DEG C / min in the protection atmosphere of argon, and cooling the powder to reach indoor temperature along with the tube furnace to obtain the lithium sulfide / carbon composite material synthesized by the lithium sulfate and the carbon sources.
Owner:TIANJIN UNIV
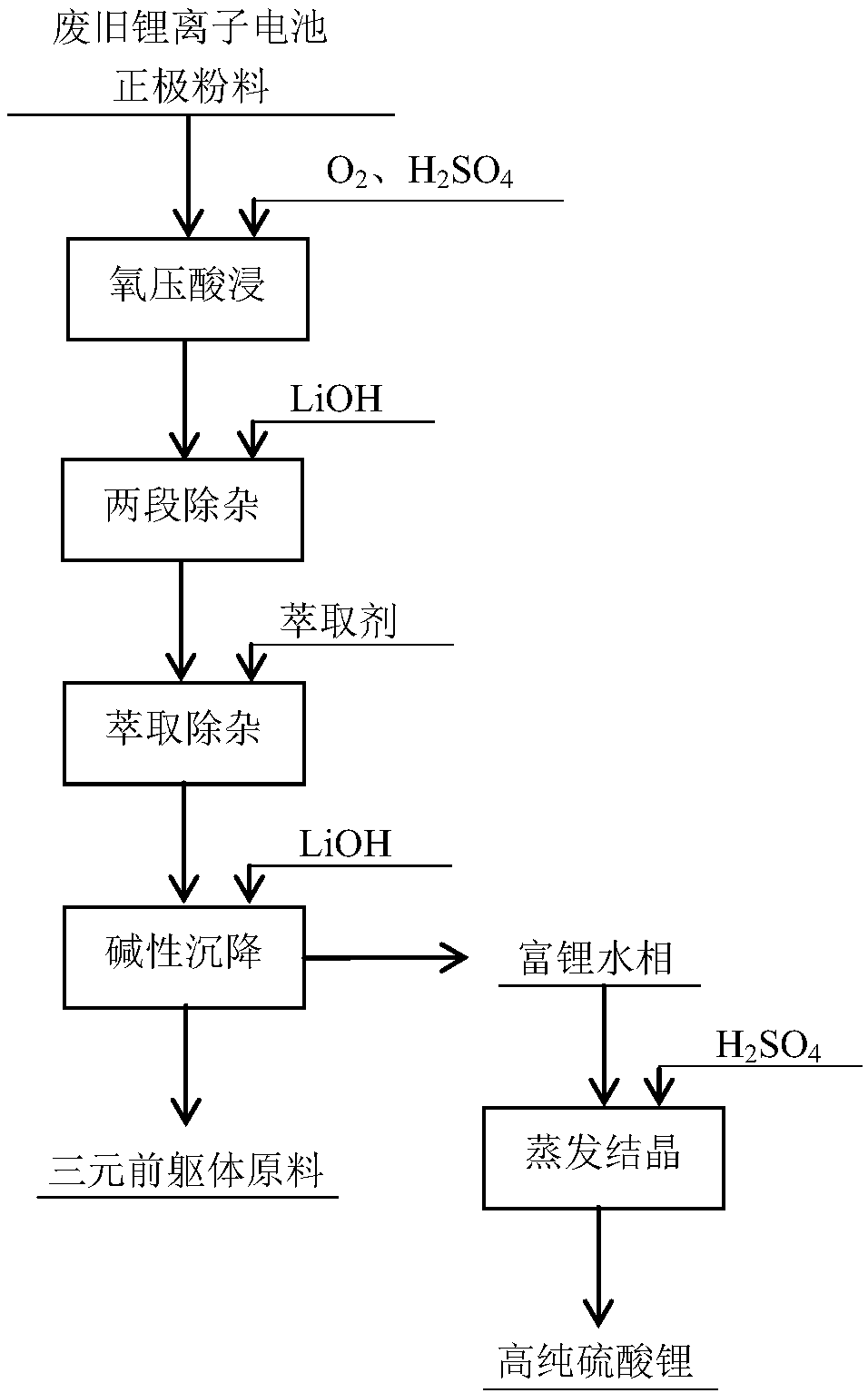
Recycling method of positive electrode powder of waste lithium ion battery
ActiveCN107591584AReduce dosageImprove leaching rateWaste accumulators reclaimingBattery recyclingLithium hydroxideEvaporation
The invention discloses a recycling method of positive electrode powder of a waste lithium ion battery. The recycling method comprises the following steps of acid leaching based on oxygen pressure: adding water to the positive electrode powder of the waste lithium ion battery, adding acid, pre-filling oxygen, performing a reaction and filtering to obtain an acid leached material; impurity removalin two sections: adjusting the pH value of the acid leached material to 1.5-2.5, adding lithium hydroxide to be reacted, and performing filtering, and adjusting the pH value of the filtering liquid to4-6, performing a reaction and filtering to obtain an impurity removal liquid; extraction and impurity removal: adjusting the pH value of the impurity removal liquid to be 2-5, and performing extraction to obtain a pure lithium nickel cobalt manganese water phase; alkali sedimentation: adding lithium hydroxide to adjust the pH value to be 9-11 to deposit nickel cobalt manganese to obtain a ternary precursor raw material; and regulating the lithium-rich water phase into a neutral state by sulfuric acid, and performing evaporation and crystallization to obtain high-purity lithium sulfate. Metalcontents and recycled values are taken into comprehensive consideration, and the ternary precursor raw material and high-purity lithium sulfate are separated and prepared from the positive electrodepowder of the waste lithium ion battery; and meanwhile, the recycling method is short in flow, simple in operation, and green and environment friendly.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Preparation method of lithium manganese phosphate nano-microsphere and product
InactiveCN104393289ASimple processEasy to controlMaterial nanotechnologyCell electrodesAfter treatmentMicrosphere
The invention discloses a preparation method of a lithium manganese phosphate nano-microsphere. The method comprises the steps of mixing ethylene glycol with water according to a volume ratio of 1:(1-2) to obtain a mixed solvent, and mixing part of ethylene glycol / water mixed solvent with manganese sulfate to obtain a mixed solution I of which the concentration is 0.1-0.2 M; mixing the part of ethylene glycol / water mixed solvent with lithium sulfate and ammonium dihydrogen phosphate, performing uniform stirring, adding potassium hydroxide, and continuously performing stirring to obtain a mixed solution II, wherein the concentration of the lithium sulfate in the mixed solution II is 0.125-0.25 M, the concentration of the ammonium dihydrogen phosphate is 0.112-0.1665 M, and the concentration of the potassium hydroxide is 0.25-0.3125 M; adding the mixed solution I into the mixed solution II, performing uniform stirring to obtain a precursor solution, performing hydrothermal reaction at the temperature of 160-240 DEG C, and then performing after-treatment to obtain the lithium manganese phosphate nano-microsphere. By accurately controlling a charging sequence and reaction conditions, the method for preparing the lithium manganese phosphate nano-microsphere is obtained.
Owner:ZHEJIANG UNIV
Production process of lithium hydroxide
ActiveCN104724729AHigh purityImprove qualityAlkali metal sulfite/sulfate purificationAlkali metal oxides/hydroxidesHigh concentrationSeparation technology
The invention discloses a production process of lithium hydroxide. According to the production process of lithium hydroxide, the crystallization mother liquor with high-concentration sodium sulfate and lithium hydroxide is used as the raw material, wherein the crystallization mother liquor with high-concentration sodium sulfate and lithium hydroxide is obtained after crystallization precipitation of sodium sulfate decahydrate in the production process of lithium hydroxide by the lithium sulfate method. Lithium hydroxide is prepared by the following steps: step A, introducing the crystallization mother liquor into a membrane separation system, wherein concentrated sodium sulfate solution is the intercept liquid and lithium hydroxide solution is the permeate liquid; step B, cooling (or cooling after further concentration) the sodium sulfate solution obtained in the step A, separating the sodium sulfate solution by centrifugation and drying the centrifuged sodium sulfate solution, so that crystal of sodium sulfate decahydrate is obtained; step C, concentrating the lithium hydroxide solution obtained in the step A by a steamer and further concentrating the concentrated lithium hydroxide solution in a crystallizing tank, then cooling, crystallizing and separating the concentrated lithium hydroxide solution by centrifugation and drying the centrifuged lithium hydroxide solution to obtain crystal of lithium hydroxide monohydrate. According to the production process, sodium sulfate and lithium hydroxide are effectively separated by the membrane separation technology, so that the purity of the lithium hydroxide solution is improved; thus, the quality of the lithium hydroxide product is effectively improved and production efficiency of the lithium hydroxide product is raised while the production cost is reduced.
Owner:SHANGHAI KAIXIN ISOLATION TECH CO LTD
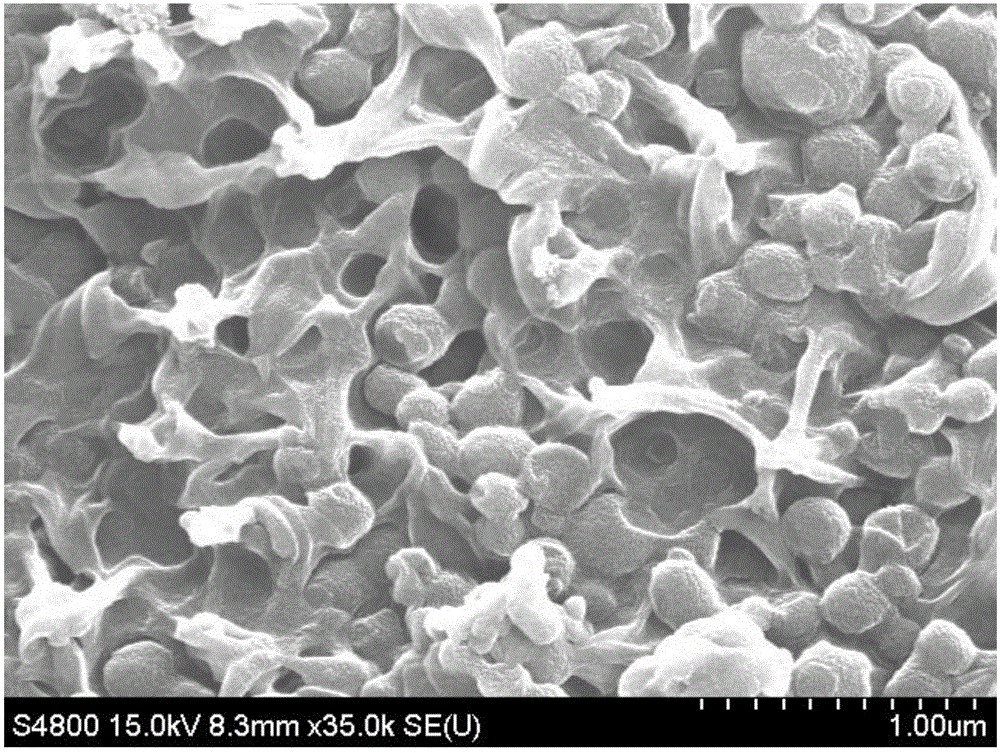
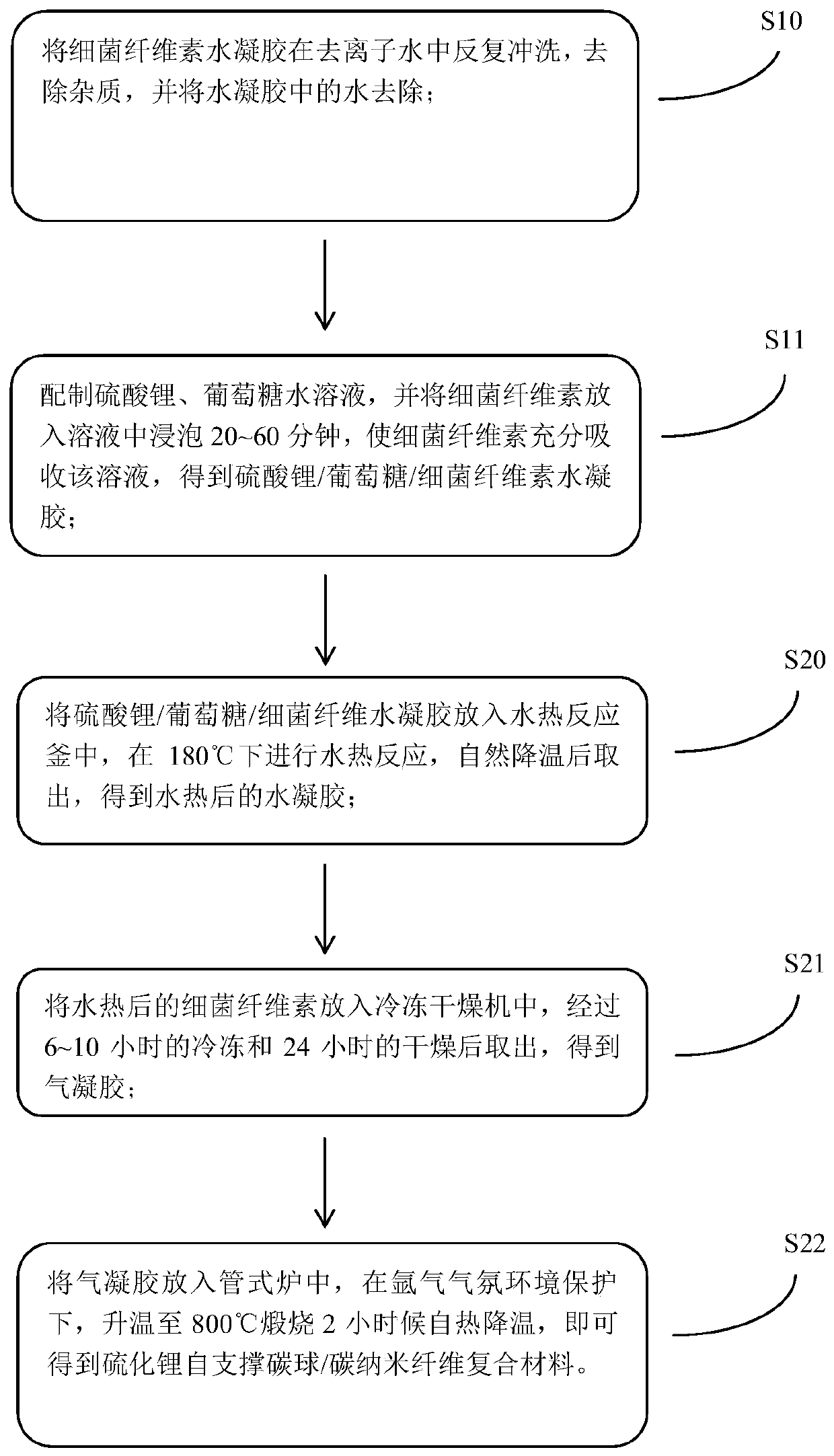
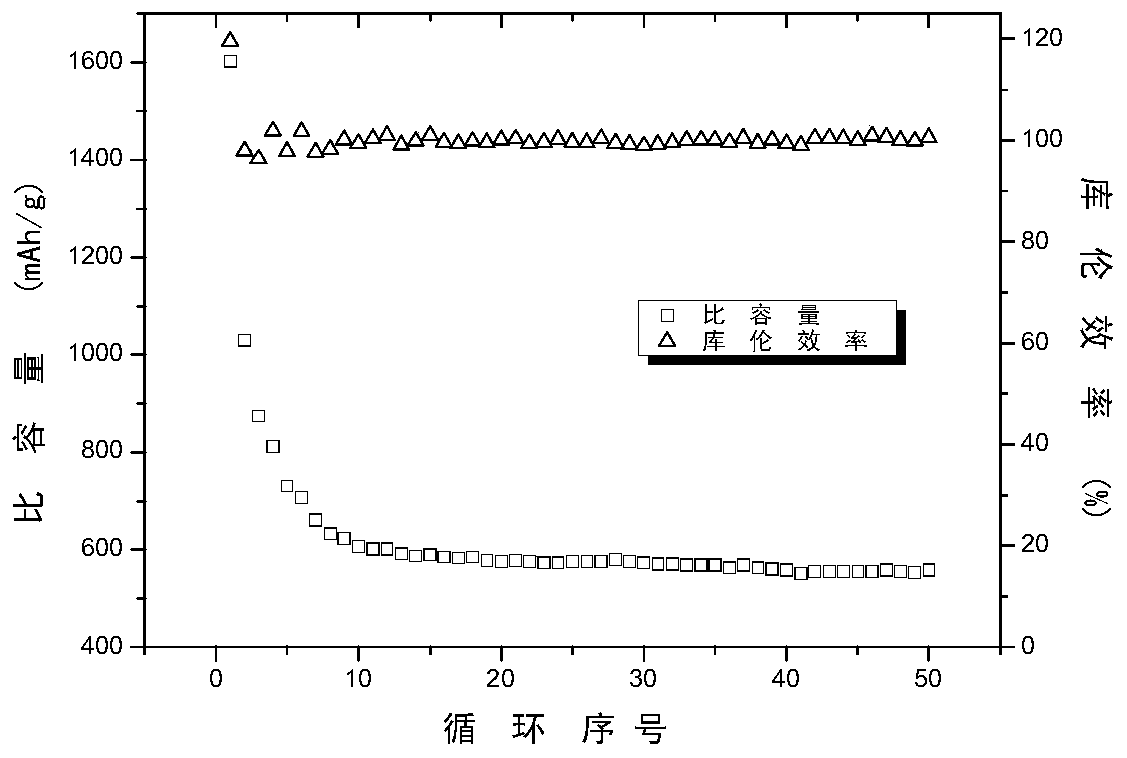
Preparation method for lithium sulfide self-supporting carbon sphere/carbon nanofiber composite material, and lithium-sulfur battery
ActiveCN110212180AEasy transferGuaranteed normal transmissionMaterial nanotechnologyCell electrodesCarbonizationCarbon nanofiber
The invention discloses a preparation method for a lithium sulfide self-supporting carbon sphere / carbon nanofiber composite material, and a lithium-sulfur battery. The preparation method comprises thesteps of S1, preparing lithium sulfate / glucose / bacterial cellulose hydrogel composite material; and S2, converting glucose in the lithium sulfate / glucose / bacterial cellulose hydrogel composite material into carbon spheres, enabling lithium sulfate to be better coated with a carbon material, slowing down diffusion of polysulfide lithium, and converting bacterial cellulose into carbon nanofibers, so that the carbon sphere / carbon nanofiber aerogel composite material is formed. According to the technical scheme, an adhesion agent does not need to be added, and then an electrode is formed by direct self-supporting after carbonization; and meanwhile, a carbon sphere structure and a carbon nanofiber network structure can be constructed, and lithium sulfide nanoparticles in the structure are effectively coated, so that the electron conductivity in the electrode can be improved, the transmission efficiency of electrons in the electrode is improved, the "shuttle effect" is inhibited, and the performance of the lithium-sulfur battery is improved.
Owner:HANGZHOU DIANZI UNIV
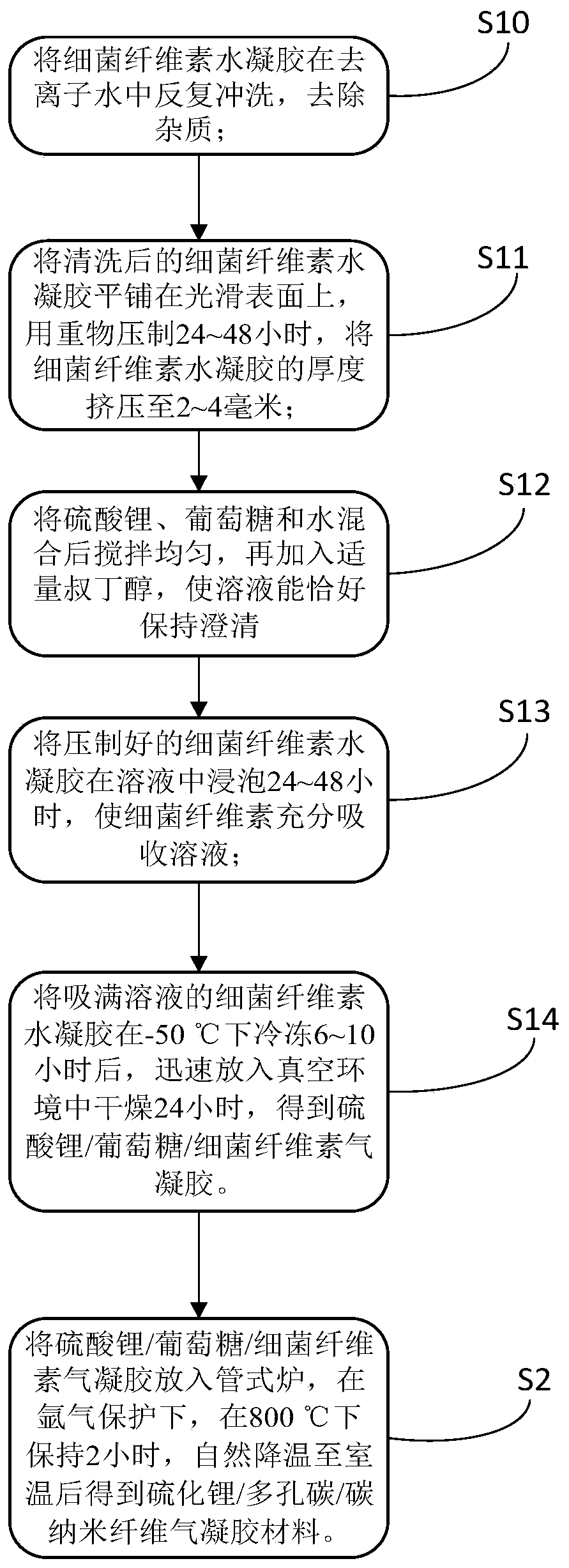
Method for preparing lithium-sulfur battery cathode material by using bacterial cellulose hydrogel
ActiveCN109962222AMitigates low electronic conductivityReduce diffuseCell electrodesPorous carbonCarbon nanofiber
The invention discloses a method for preparing a lithium-sulfur battery cathode material by using bacterial cellulose hydrogel, comprising the following steps of S1, preparing a lithium sulfate / glucose / bacterial cellulose aerogel composite material; and S2, converting the lithium sulfate in the lithium sulfate / glucose / bacterial cellulose aerogel composite material into lithium sulfide, convertingthe glucose into porous carbon, and converting the bacterial cellulose into carbon nanofibers. The method can construct a carbon nanofiber meshed structure, and the lithium sulfide nanoparticles in the structure are coated with the porous carbon, thereby improving the electron transmission efficiency in an electrode, suppressing a shuttling effect, and solving the problem of electrode collapse during lithium-sulfur battery charging and discharging so as to improve the performance of the lithium-sulfur battery.
Owner:HANGZHOU DIANZI UNIV
Method for preparing lithium carbonate through fluorinating and calcining spodumene and extracting lithium
InactiveCN107265485ASimple processLow reaction temperatureSilicaAlkali metal sulfite/sulfate purificationLithium carbonateLithium sulfate
The method for extracting lithium from spodumene by fluorination roasting provided by the present invention includes the following steps: 1) taking 50-200 mesh spodumene, adding a fluorine-containing compound in a molar ratio of 1:1-1:3, and mixing Grind to 100~200 meshes uniformly, roast at 200~600℃, and the reaction time is 2~5h to obtain slag; 2) Add 1~6mol / L at a liquid-solid ratio of 1:1~1:5 at 30~90℃ 3) Evaporating and concentrating the lithium sulfate mother liquor, adding hydrogen peroxide, basic compounds (one or more of NaOH, Na CO , ammonia water, etc.) in turn, precipitating the Al3+, Mg2+, Fe3+, etc., add soluble carbonate (one or more of Na2CO3, K2CO3, etc.) to the filtrate, heat to 50-100 ° C to precipitate lithium, filter and wash to obtain lithium carbonate with a purity of more than 96.4%. In addition, SiF4 can be absorbed and aged to obtain high-purity silica and NH4F during the roasting process. The invention has simple equipment requirements, low energy consumption, and is suitable for industrial production.
Owner:WUHAN UNIV OF TECH
Electrolyte for inhibiting growth of lithium dendrites and lithium battery
PendingCN110416615AImprove deposition and dissolution efficiencyInhibition formationSecondary cells servicing/maintenanceSolid state electrolyteLithium chloride
The invention discloses an electrolyte for inhibiting the growth of lithium dendrites and a lithium battery. The electrolyte comprises an additive, a lithium salt and an organic solvent. The additiveincludes at least one in the group consisting of lithium hexafluorophosphate, lithium perchlorate, lithium bis(trifluoromethane) sulfonate, lithium trifluoromethanesulfonate, lithium fluoroborate, trilithium hexafluoroaluminate, lithium hexafluoroarsenate, lithium fluoride, lithium chloride, lithium bromide, lithium nitrate, lithium polysulfide, lithium nitride, lithium phosphide, lithium oxalateborate, lithium oxide, lithium sulfite, lithium sulfate, lithium acetate, lithium hydroxide and lithium oxalate, and the lithium salt is different from the additive. According to the lithium battery containing an additive, a layer of solid electrolyte membrane can be formed on a surface of the lithium metal negative electrode during charge and discharge, and the polymerization of the electrolyte can be induced to form an oligomer which covers a surface of a lithium negative electrode and a surface of a positive electrode material matched with the lithium negative electrode. The protective layer can effectively inhibit the growth of the lithium dendrites, and therefore, the safety performance of the battery is improved.
Owner:SOUTH CHINA UNIV OF TECH
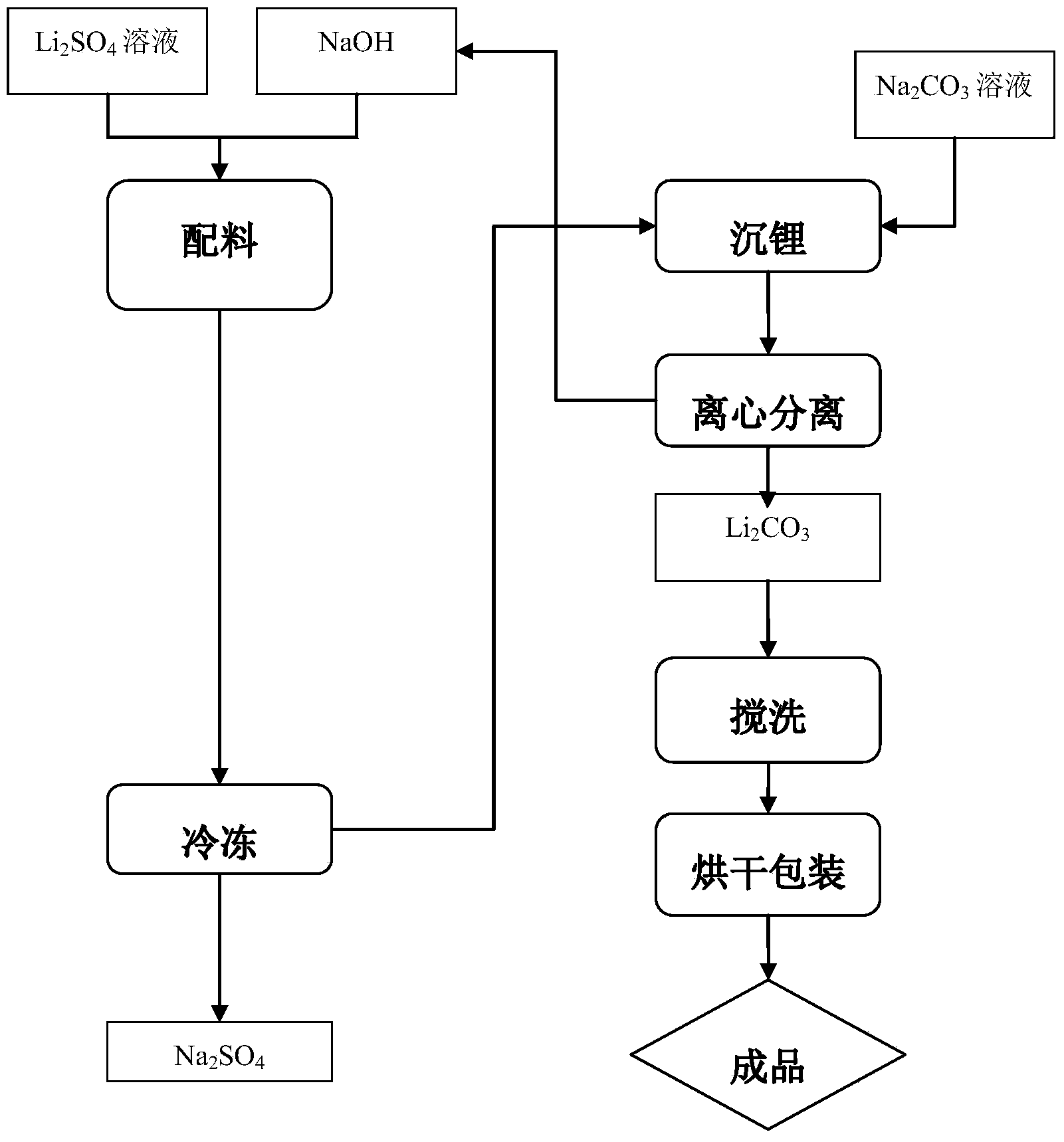
Lithium carbonate production technology
ActiveCN103408041ALow costHigh cost of solutionLithium carbonates/bicarbonatesLithium hydroxideLithium carbonate
The invention discloses a lithium carbonate production technology which comprises the following steps of: I, preparing a Li2SO4 and NaOH mixed solution, freezing, precipitating Na2SO4 crystals and then separating to obtain a LiOH solution; II, heating a saturated Na2CO3 solution to 90-95DEG C, adding the LiOH solution obtained from the step I into the saturated Na2CO3 solution to obtain a liquid-solid mixture of Li2CO3 precipitate generated from reaction and the NaOH solution; III, performing centrifugal separation on the liquid-solid mixture in the step II to obtain a crude Li2CO3 product and the NaOH solution; IV, washing off soluble impurity ions in the crude Li2CO3 product to obtain a purified wet Li2CO3 product; V, drying and packaging the wet Li2CO3 product. In the production technology, the LiOH solution can be prepared by preparing and freezing the mixed solution through taking Li2SO4 and NaOH as raw materials, the cost of the raw materials is low, and the problem that the cost is high by directly taking LiOH as a raw material can be solved; the by-product sodium hydroxide can be directly used in the technology for converting lithium sulfate into lithium hydroxide, so that the cost of the lithium hydroxide can be lowered, and the market competitiveness can be strengthened.
Owner:SICHUAN STATE LITHIUM MATERIALS
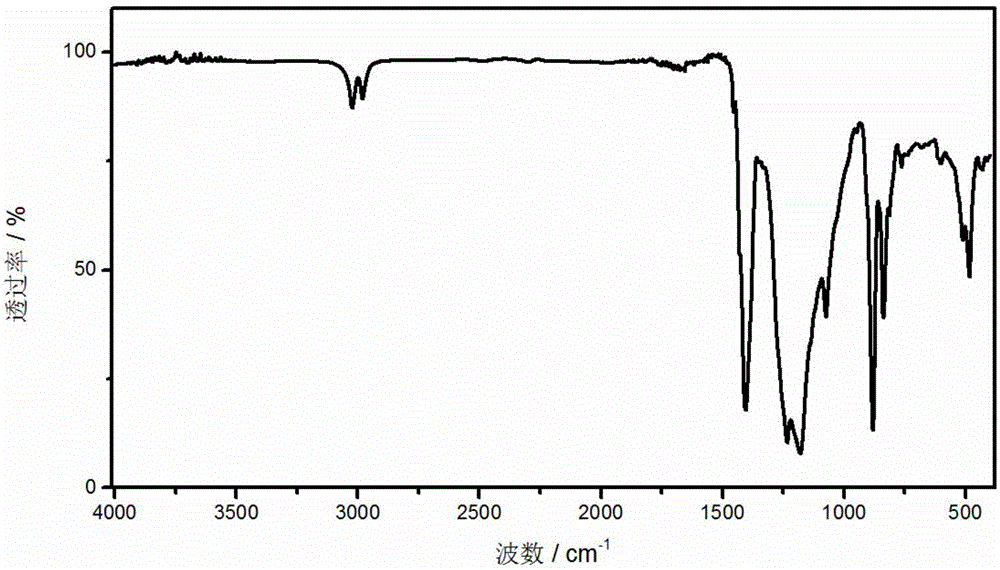
Preparation method of lithium sulfide
The invention discloses a preparation method of lithium sulfide. The preparation method comprises the following steps of 1) grinding, mixing and transferring anhydrous lithium sulfate, glucose and hard carbon into a quartz sintering tube of a vacuum tubular furnace; 2) per-heating the quartz sintering tube, vacuumizing the vacuum tubular furnace, and connecting a kipp gas generator to a pipe opening of the quartz sintering tube; 3) introducing hydrogen to the quartz sintering tube, heating the quartz sintering tube, and performing reaction to prepare the lithium sulfide, wherein the mass ratioof the anhydrous lithium sulfate, the hard carbon and the glucose is (1:2:5)-(10:1:5). The purity of the lithium sulfide prepared by the preparation method of the lithium sulfide reaches 99.9% or above, the lithium sulfide is low in production cost, simple in preparation method and process, short in preparation period and simple in preparation equipment and is easy to operate, the preparation process is high in safety, the raw material which is used is non-toxic, industrial production on a large scale is facilitated, the glucose is tightly combined with lithium sulfate after carbonization, and the conversion efficiency of the lithium sulfide can be improved.
Owner:北京天工新材科技发展有限公司
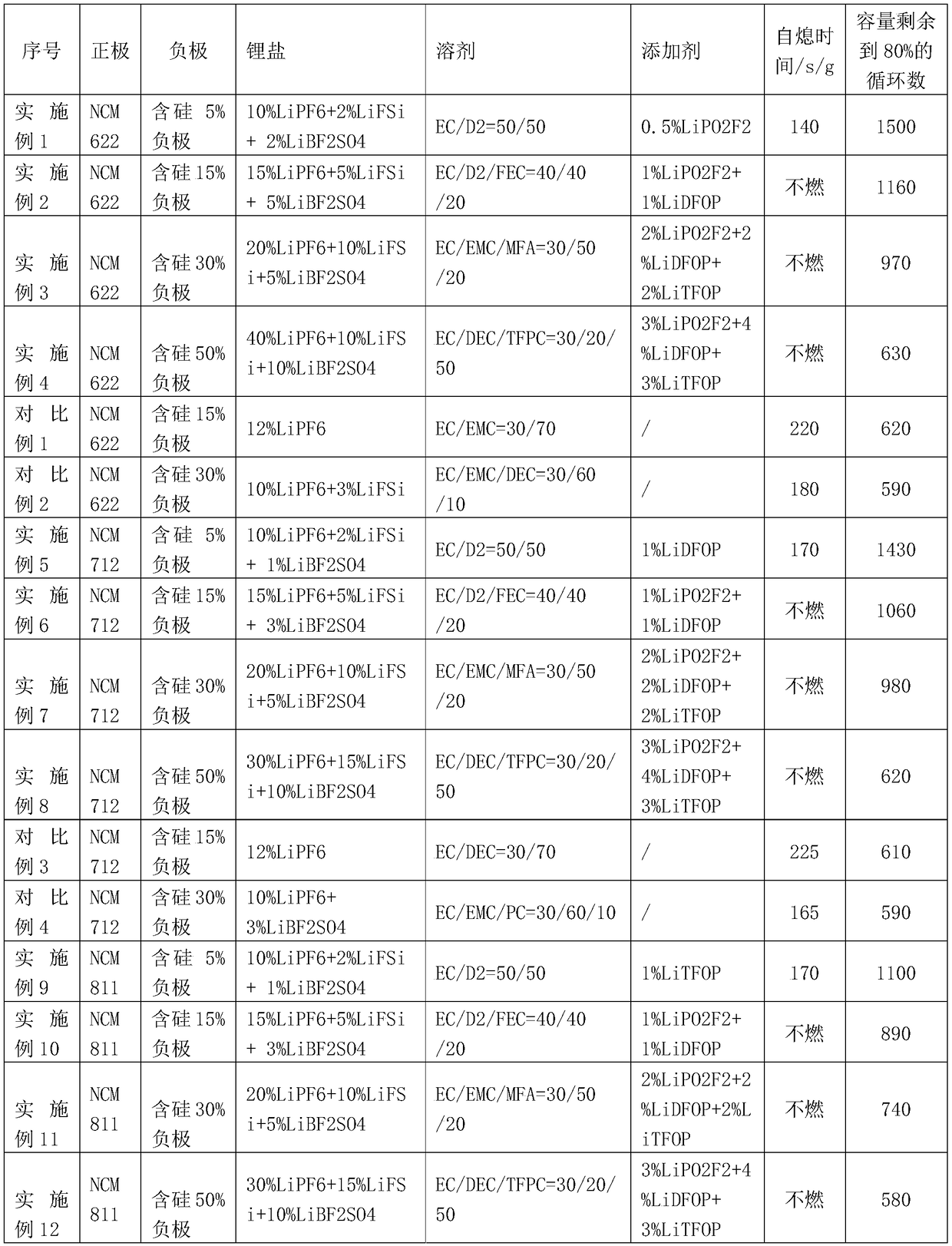
High-energy-density lithium-ion battery electrolyte
InactiveCN109301324AImproves Antioxidant PropertiesImprove compatibilityFinal product manufactureSecondary cells servicing/maintenanceOrganic solventHigh energy
The invention provides a high-energy-density lithium-ion battery electrolyte prepared from, by weight, 20-60% of lithium salt, 20-60% of a non-aqueous organic solvent and 0.5-10% of an additive, all the components adding to 100%. The lithium salt includes lithium hexafluorophosphate, lithium bifluorosulfonimide and lithium sulfate difluoroborate. The electrolyte is very short in self-extinguishingtime and is even incombustible, wherein the additive has excellent film-forming effect, so that an SEI film, having ultralow resistance and high thermo-stability, can be formed between cathode-anodeinterfaces.
Owner:TIANJIN ENERGIES
Negative electrode material of high-voltage thermal battery, high-voltage thermal battery and preparation method thereof
InactiveCN108172757AIncreased diffusion rateImprove ionic conductivityDeferred-action cellsPrimary cell electrodesElectrical conductorSilicon alloy
The invention discloses a negative electrode material of a high-voltage thermal battery, and the high-voltage thermal battery. The thermal battery consists of a positive electrode material, a negativeelectrode material and an electrolyte diaphragm material; the positive electrode material is a manganese dioxide material; the negative electrode material is a lithium quick ion conductor lithium borate-lithium sulfate-coated lithium silicon alloy material; and the electrolyte diaphragm material is a lithium nitrate-potassium nitrate-magnesium oxide material. By coating the lithium silicon alloyby lithium quick ion conductor lithium borate-lithium sulfate, the interface composition of the negative electrode lithium silicon alloy and the electrolyte lithium nitrate-potassium nitrate and the transmission process of metal positive ions in the interface in the battery working process are changed, so that the diffusion rate and ion electrical conductivity of lithium ions in the interface passivation film layer are greatly improved, and the working voltage of the thermal battery is further improved.
Owner:INST OF ELECTRONICS ENG CHINA ACAD OF ENG PHYSICS
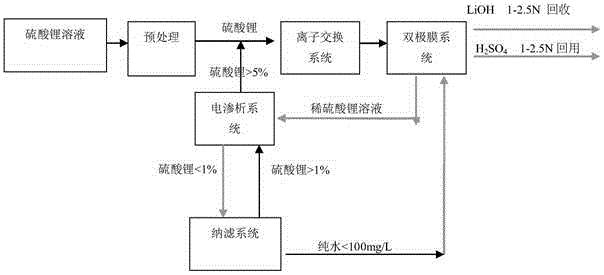
Technology for recycling lithium hydroxide from solution through bipolar membrane method
ActiveCN105154908AReduce consumptionSolve environmental problemsElectrolysis componentsLithium hydroxidePhysical chemistry
Owner:HANGZHOU BLUETEC ENVIRONMENTAL TECH
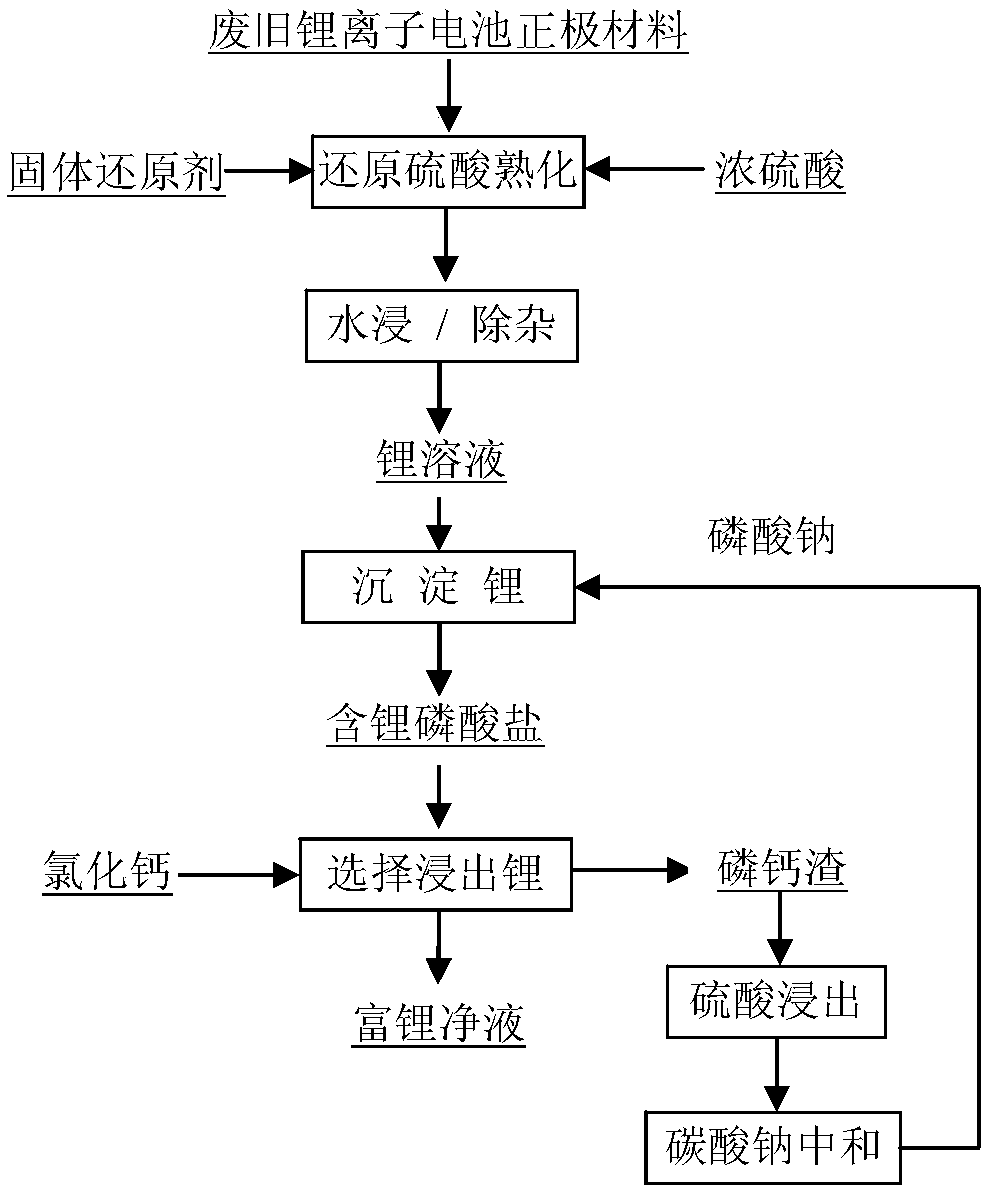
Method for producing lithium-rich pure solution from waste lithium ion battery positive electrode active material
ActiveCN108767353AHigh recovery rateAvoid pre-concentrationWaste accumulators reclaimingBattery recyclingHigh sodiumLithium chloride
The invention discloses a method for producing a lithium-rich pure solution from a waste lithium ion battery positive electrode active material, belongs to the technical field of waste lithium ion battery material recycling, and particularly provides a method for producing a lithium-rich pure solution from a reduced sulfuric acid leaching solution of a positive electrode active material. A high-sodium and low-lithium lithium sulfate solution obtained after the positive electrode active material is subjected to reduction leaching and leaching solution purification is subjected to lithium deposition with phosphoric acid or phosphate to obtain lithium-containing phosphate enrichment; lithium is selectively leached out from the lithium-containing phosphate enrichment by using less than a theoretically required amount of a calcium chloride solution to obtain the lithium-rich pure solution and lithium-containing phosphorus-calcium residues; and the lithium-containing pure solution is used for producing a product such as battery-grade lithium carbonate or lithium chloride through a conventional method such as deposition or crystallization, and a solution obtained after the lithium-containing phosphorus-calcium residues are subjected to sulfuric acid leaching and neutralization returns to the lithium deposition step for cyclic use. According to the method, the lithium-rich pure solution can be directly produced from the high-sodium and low-lithium solution without pre-concentration, the energy consumption and the cost are low, and the lithium recycling rate is high.
Owner:BEIJING MINING & METALLURGICAL TECH GRP CO LTD
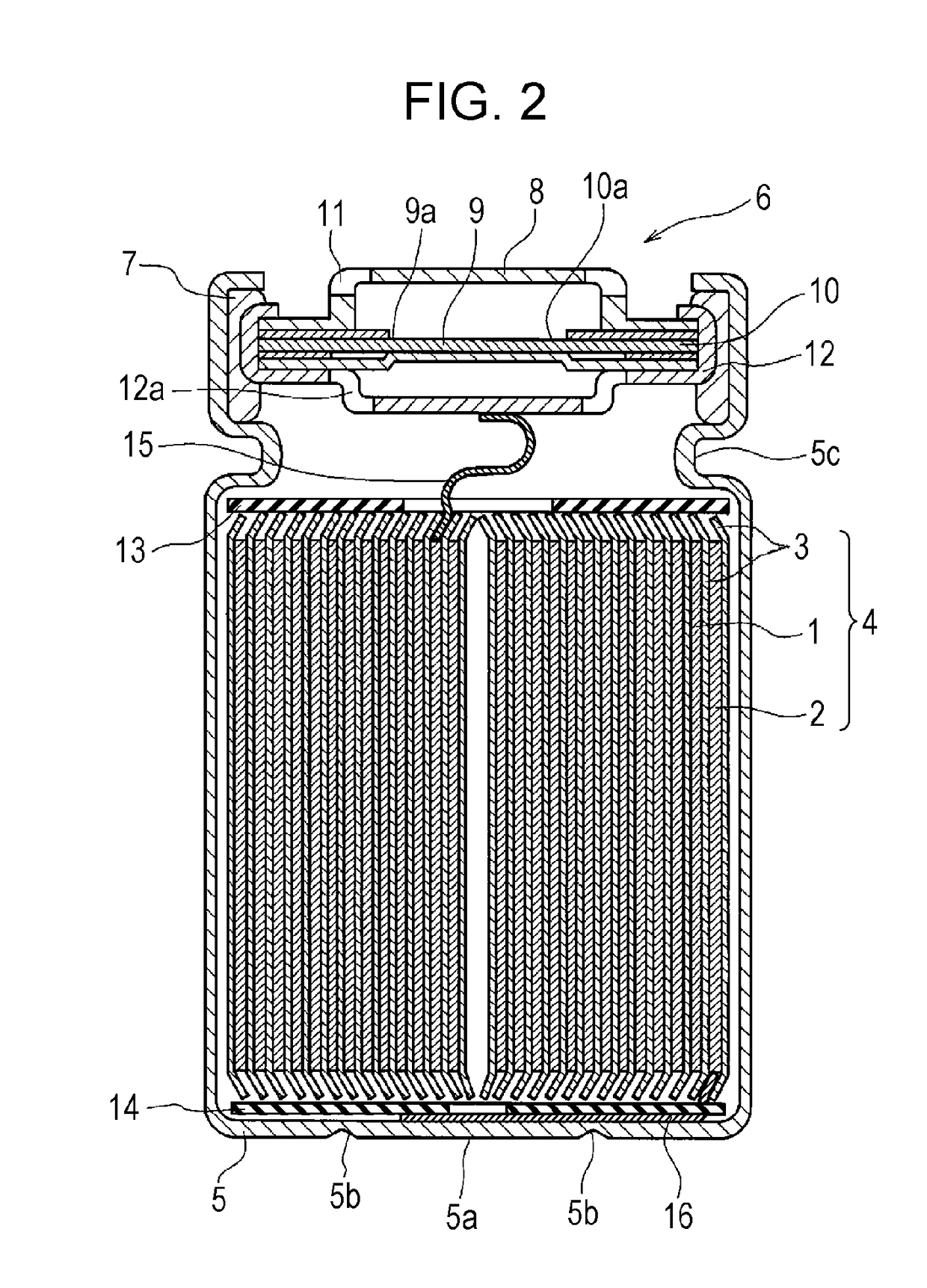
Nonaqueous electrolyte secondary batteries
ActiveUS20170092979A1Good low-temperature discharge characteristic and high-temperature storage characteristicLow-temperature discharging characteristicFinal product manufactureCell electrodesElectrolytic agentPhysical chemistry
A nonaqueous electrolytic solution for nonaqueous electrolyte secondary batteries includes a nonaqueous solvent and an electrolyte. The nonaqueous solvent includes a fluorinated carboxylate ester represented by the formula (1):where R1 and R2 are each any of H, F, CH3-xFx (x is 1, 2 or 3) and R3 is an optionally fluorinated alkyl group having 1 to 3 carbon atoms. The nonaqueous electrolytic solution further comprising lithium fluorosulfate salt (LiSO3F).
Owner:PANASONIC CORP
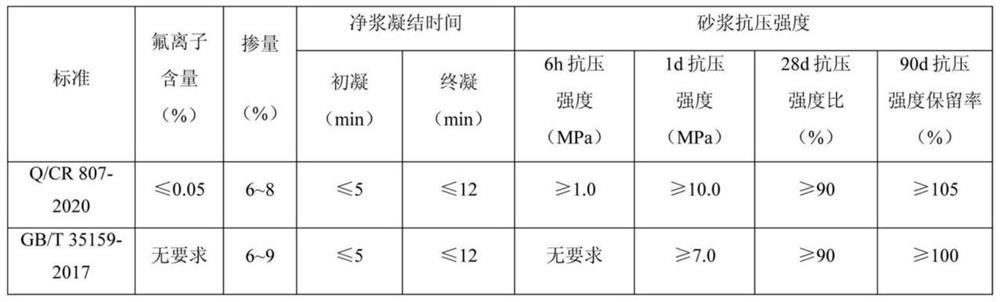
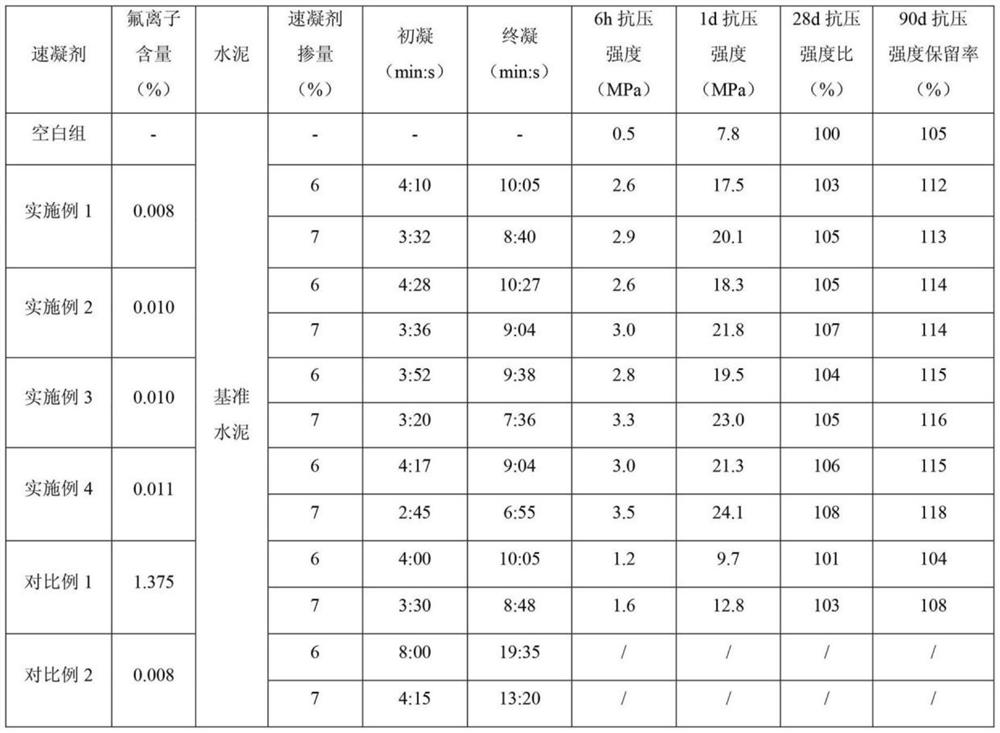
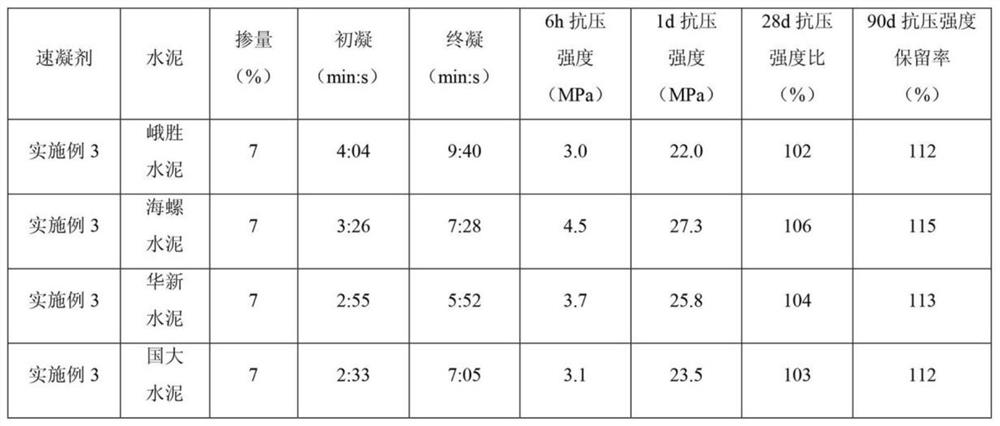
Super-early-strength fluoride-free alkali-free liquid accelerator and preparation method thereof
ActiveCN113603384AHigh aluminum contentAvoid hydrolysisSolid waste managementOXALIC ACID DIHYDRATEDiethylenetriamine
The invention relates to a super-early-strength fluorine-free alkali-free liquid accelerator and a preparation method thereof. The accelerator is prepared from the following raw materials: aluminum sulfate, an organic dissolution promoter, a stabilizer, an early strength agent, a pH regulator and water. The organic dissolution promoter is formed by mixing a component A, a component B and a component C, wherein the component A is monoethanolamine, diethanolamine or triethanolamine; the component B is N-methyldiethanolamine, N,N-dimethylethanolamine or N,N-dimethylformamide; and the component C is diethylenetriamine, triethylene tetramine or tetrahydroxypropyl ethylenediamine. The stabilizer is selected from superfine sepiolite and pseudo-boehmite. The pH regulator is selected from p-toluenesulfonic acid, oxalic acid and formic acid. The early strength agent is selected from lithium sulfate, magnesium sulfate, nanometer silica sol and nanometer aluminum sol. The super-early-strength fluorine-free alkali-free liquid accelerator is good in performance, simple in preparation process, free of fluorine, alkali and chlorine and capable of meeting the performance requirements of various setting accelerator standards for alkali-free accelerators; and according to the liquid accelerator, compressive strength within 6 hours is far larger than 2.5 MPa, compressive strength within 1 day is far larger than 15.0 MPa, a compressive strength retention rate within 90 days is larger than or equal to 110%, and stability exceeds 9 months.
Owner:SHIJIAZHUANG CHANGAN YUCAI BUILDING MATERIALS
Preparation method of cell-grade lithium hydroxide
The invention belongs to the field of materials, and particularly relates to a preparation method of cell-grade lithium hydroxide. The preparation method of cell-grade lithium hydroxide has the advantages of being high in product purity, few in production process, low in production energy consumption and the like. The preparation method comprises the following steps that 1, a pre-prepared mixed solution is prepared, wherein a lithium sulfate solution and a sodium hydroxide solution are mixed according to the mole ratio 2:(1-1.2) of lithium sulfate to sodium hydroxide; 2, the pre-prepared mixed solution is frozen, wherein the pre-prepared mixed solution is frozen and crystallized for 1-2 h at the temperature of minus 15 DEG C to minus 10 DEG C, and solid-liquid separation is conducted to obtain sodium sulfafe decahydrate crystals and lithium hydroxide freezing liquid; 3, the lithium hydroxide freezing liquid obtained in the step 2 is subjected to evaporation concentration and solid-liquid separation to obtain a crude lithium hydroxide product; 4, the crude lithium hydroxide product obtained in the step 3 is redissolved to remove impurities and then subjected to evaporation concentration and solid-liquid separation to obtain lithium hydroxide with the coarse grain cell grade quality. The product is high in purity, few in impurity, coarse in grain and good in appearance, and the operation method is simple, few in production process, low in cost and low in energy consumption.
Owner:SICHUAN STATE LITHIUM MATERIALS
Preparation method and application of ionic liquid for absorbing SO2
InactiveCN103071367AImprove gas efficiencyLow costDispersed particle separationN dimethylformamideLithium chloride
The invention provides a preparation method of an ionic liquid for absorbing SO2, and an application of the ionic liquid in absorbing SO2. The preparation method of the ionic liquid comprises the steps of uniformly mixing one of aluminium chloride, zinc chloride, lithium chloride, aluminum sulfate, zinc sulfate, lithium sulfate, aluminium nitrate, zinc nitrate and lithium nitrate with one or two of urea, propanamide, butyramide, caprolactam, N,N-dimethylformamide and N,N-dimethylacetamide at a molar ratio of 1:(1-10) or 1:(0.5-3):(1-7), conducting reaction for 1-10h at 70-150 DEG C, conducting vacuum drying on an obtained solution at 40-60 DEG C, and obtaining the ionic liquid. The ionic liquid is mainly used for quickly and efficiently absorbing SO2. With the adoption of the ionic liquid as an SO2 absorbent, requirements on equipment required for absorbing SO2 are simple, the operational conditions are mild, the absorption efficiency is high, the cost is low, the ionic liquid can be recycled, and waste liquid and waste water discharge and other problems do not exist, and the ionic liquid is suitable for industrial production, meets the requirements of green chemical development of the contemporary society, and has good economic and social benefits.
Owner:SHIJIAZHUANG UNIVERSITY
Method for extracting battery-grade iron phosphate from waste lithium iron phosphate battery
InactiveCN111285341AHigh purityIncrease compaction densityCell electrodesWaste accumulators reclaimingPhosphatePhosphoric acid
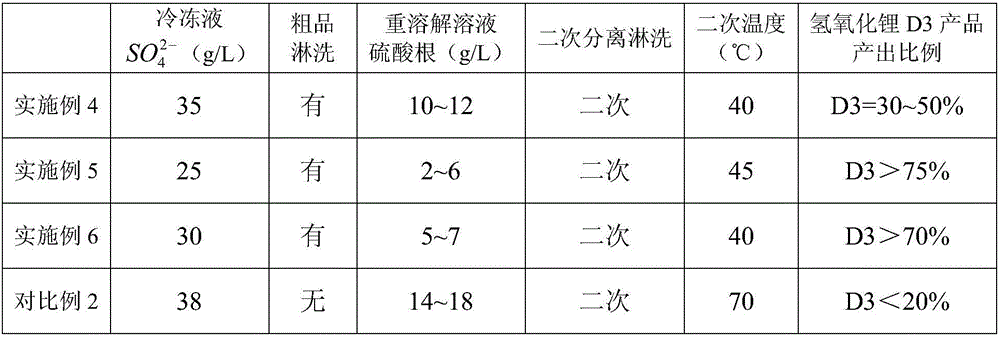
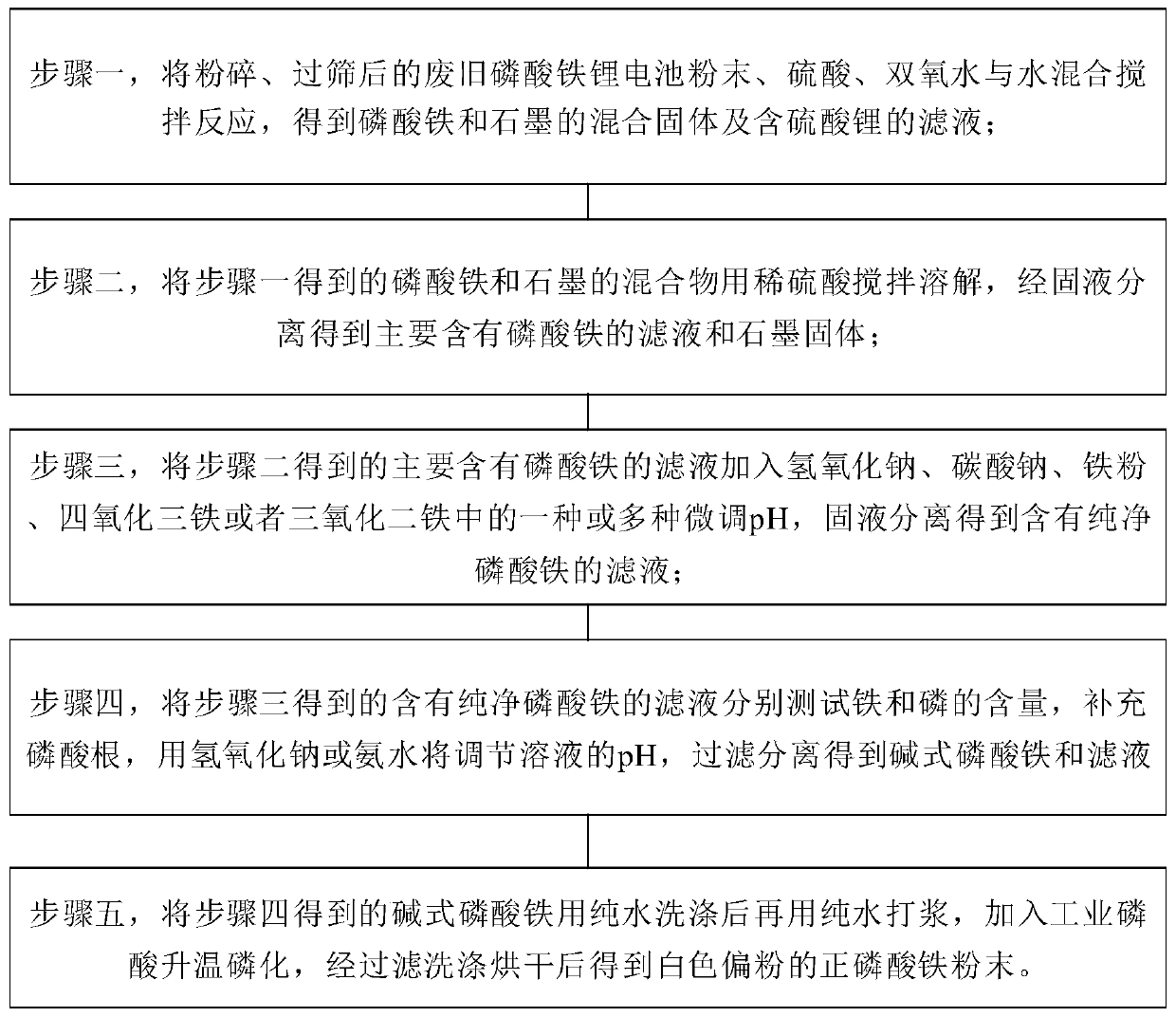
The invention relates to a method for extracting battery-grade iron phosphate from a waste lithium iron phosphate battery, which comprises the following steps: mixing crushed and sieved waste lithiumiron phosphate battery powder, sulfuric acid, hydrogen peroxide and water, and stirring for reaction to obtain a mixed solid of iron phosphate and graphite and a filtrate containing lithium sulfate; stirring and dissolving the obtained mixed solid of iron phosphate and graphite by using dilute sulfuric acid, and carrying out solid-liquid separation to obtain filtrate mainly containing iron phosphate and a graphite solid; adding one or more of sodium hydroxide, sodium carbonate, iron powder, ferroferric oxide or ferric oxide into the obtained filtrate to finely adjust the pH value, and carryingout solid-liquid separation to obtain filtrate containing pure iron phosphate; respectively testing the contents of iron and phosphorus in the obtained filtrate, supplementing phosphate radicals, adjusting the pH value of the solution by using sodium hydroxide or ammonia water, and performing filtering and separating to obtain basic iron phosphate and filtrate; washing the obtained basic iron phosphate with pure water, pulping with pure water, adding industrial phosphoric acid, heating for phosphorization, and performing filtering, washing and drying to obtain ferric orthophosphate powder.
Owner:北京蒙京石墨新材料科技研究院有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com