Patents
Literature
189 results about "Lithium perchlorate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
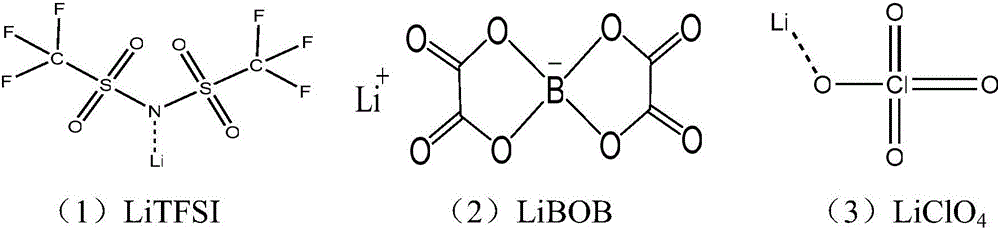
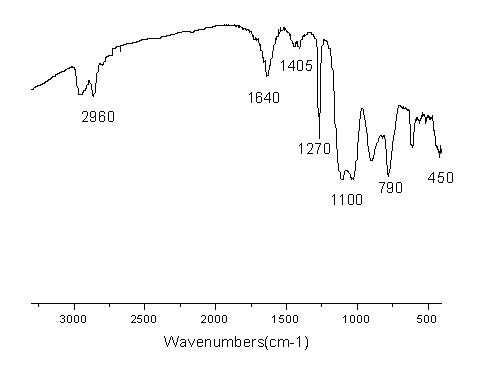
Lithium perchlorate is the inorganic compound with the formula LiClO₄. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
Grapheme electrode and preparation method and application thereof
ActiveCN103208373AFast wayEfficient methodHybrid capacitor electrodesHybrid/EDL manufactureInternal resistanceGraphene electrode
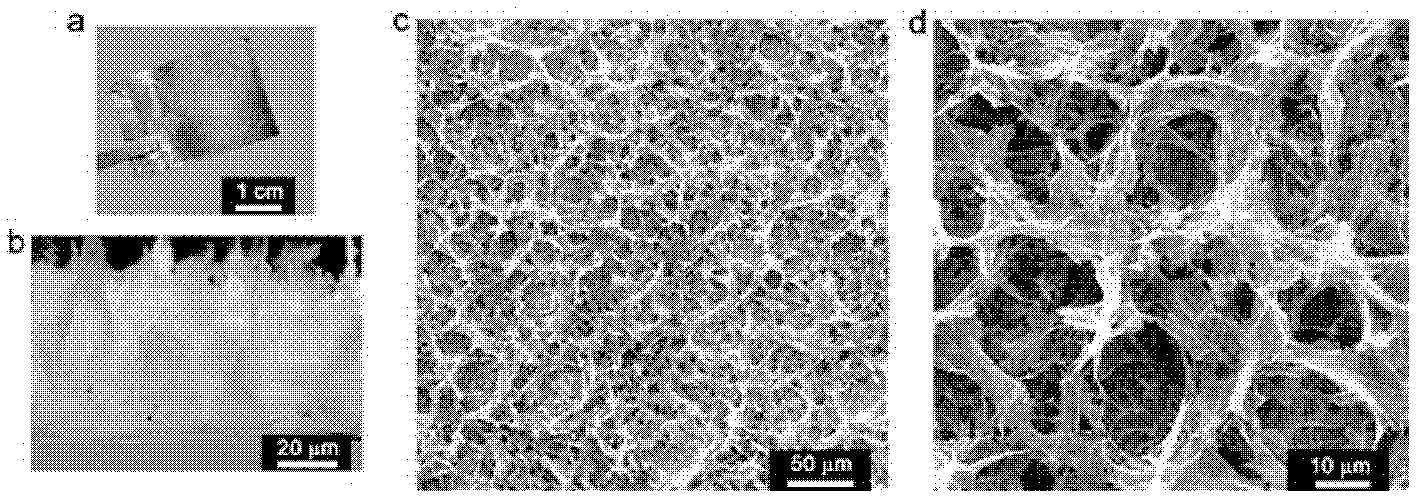
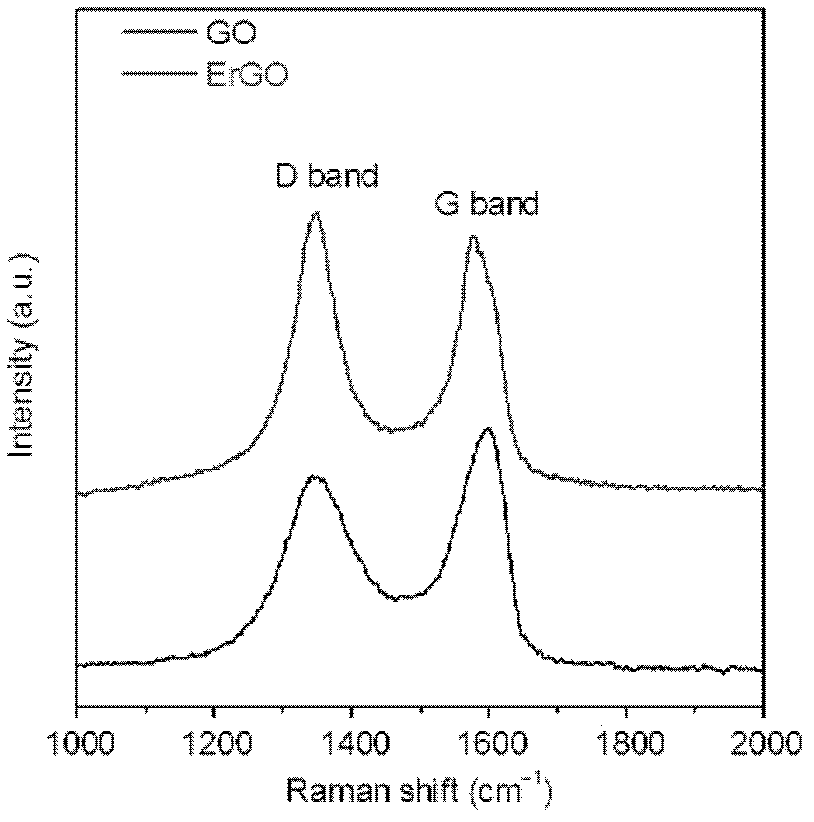
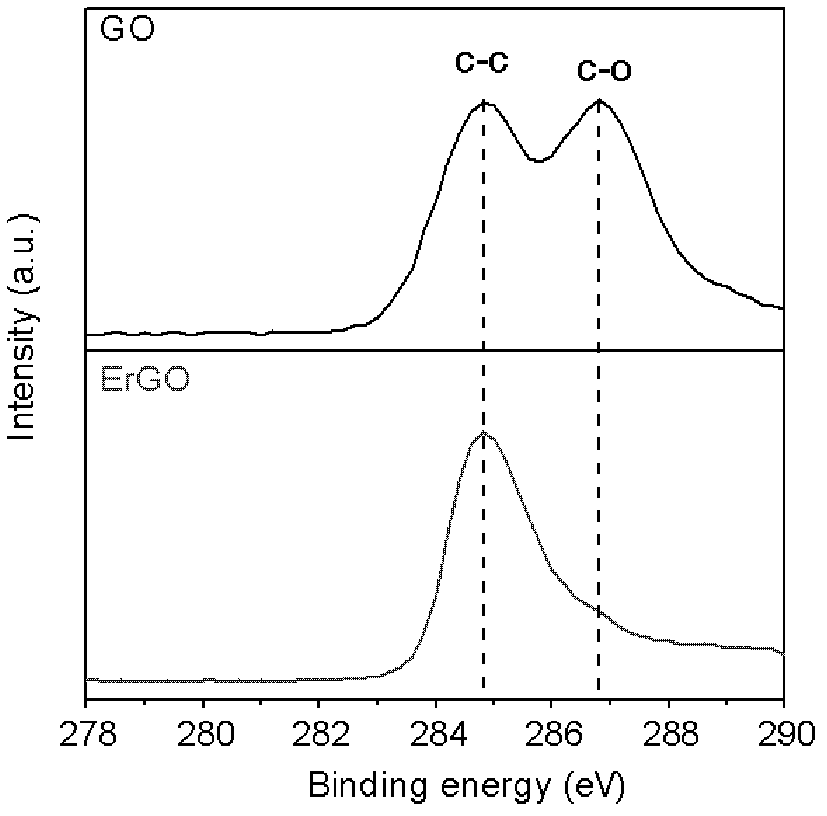
The invention discloses a grapheme electrode and a preparation method and application thereof. A substrate of the grapheme electrode is a polished metal substrate, the aperture is 5-20 micrometers, and the thickness is 10-60 micrometers. The preparation method of the grapheme electrode includes that an electrolytic water solution of oxidized grapheme is electrolyzed through constant potentials in a three electrode method, electrochemical reduction grapheme is deposited on the metal substrate, reduction is further performed in a lithium perchlorate electrolyte solution, and the three-dimensional porous meshed electrode is obtained after deionized water washing. The preparation method of the grapheme electrode is environment-friendly, simple, convenient and easy to do, and can be used for volume production of the three-dimensional porous meshed electrodes. The obtained grapheme electrodes can be assembled to a super-capacitor with charge-discharge time to be 0.8-4 milliseconds and internal resistance to be 0.09-0.14 ohm, and can be used for alternating current filter by replacing aluminum electrolytic capacitors.
Owner:TSINGHUA UNIV
Electrolyte for lithium secondary battery and lithium secondary battery comprising same
PURPOSE: Provided are a non-aqueous electrolyte capable of improving the electrochemical property, and a lithium secondary cell excellent in electrochemical property. CONSTITUTION: The electrolyte comprises a lithium salt; a non-aqueous, organic solvent; and at least one of organic compound selected from compounds of formula 1 to 4(wherein the formula 3 is R9-SO3-Si-(CmH2m+1)3 and formula 4 is CnX2n+1-SO3-Si(CmH2m+1)3). In the formulae, each of R1 to R9 independently represents primary, secondary, or tertiary alkyl group, alkenyl group or aryl group, X is hydrogen or halogen atom, and each of n and m is 0-3. The lithium salt is selected from the group consisting of lithium hexafluorophosphate(LiPF6), lithium tetrafluoroborate(LiBF4), lithium perchlorate(LiClO4), lithium trifluoromethanesulfonate(LiCF3SO3), lithium hexafluoroarsenate(LiAsF6), and mixture thereof.
Owner:SAMSUNG SDI CO LTD +1
Lithium cell electrolyte
InactiveCN101071863AImprove securityImprove flame retardant performanceOrganic electrolyte cellsSecondary cellsOxygenSolvent
A lithium battery electrolyte comprises lithium salt, organic solvent and the chemical additive. The lithium salt is one of lithium per chlorate, trifluoromethyl lithium sulfonate, six lithium flu phosphate, six fluorine arsenic acids lithium, the tetrachloride aluminum lithium, in the tetrachloride boron lithium one kind or its combination thing, the density is 0.5-2.0mol / L; The organic solvent is alkyl phosphate RPO3R ' R ';The chemical additive is the (volume compares) two oxygen link fifth heavenly stem Alkane DOL whose density is density is 0-5% . This invention has good anti- burn performance, the electrolyte conductivity maximum value can reach 4.0mS-1 also but not to affect the reaction nature of plurity of insertion electrode, may be used in -50 degree C-120 degree C temperature ranges, also causes the cathode surface to form a high flexible SEI membrane, thus enhance the safety of lithium battery in the production, storage and transportation and reduce the production cost.
Owner:EVE ENERGY CO LTD
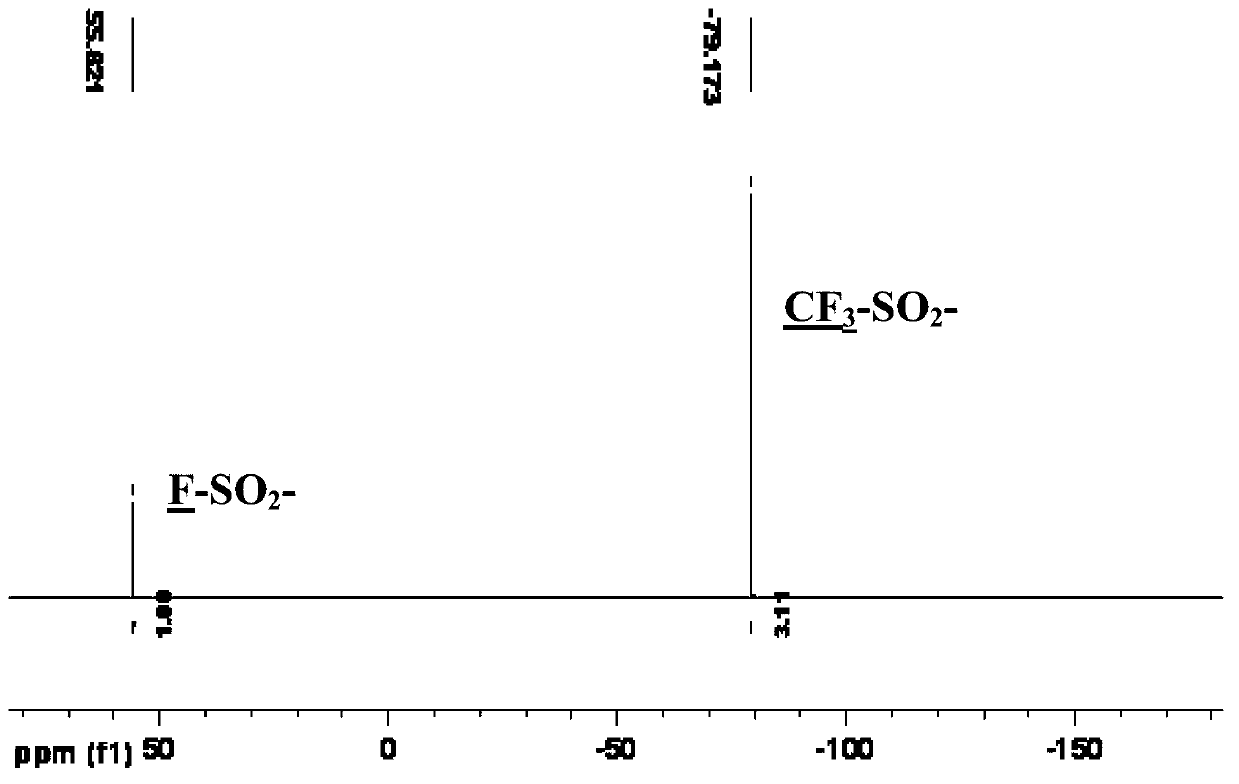
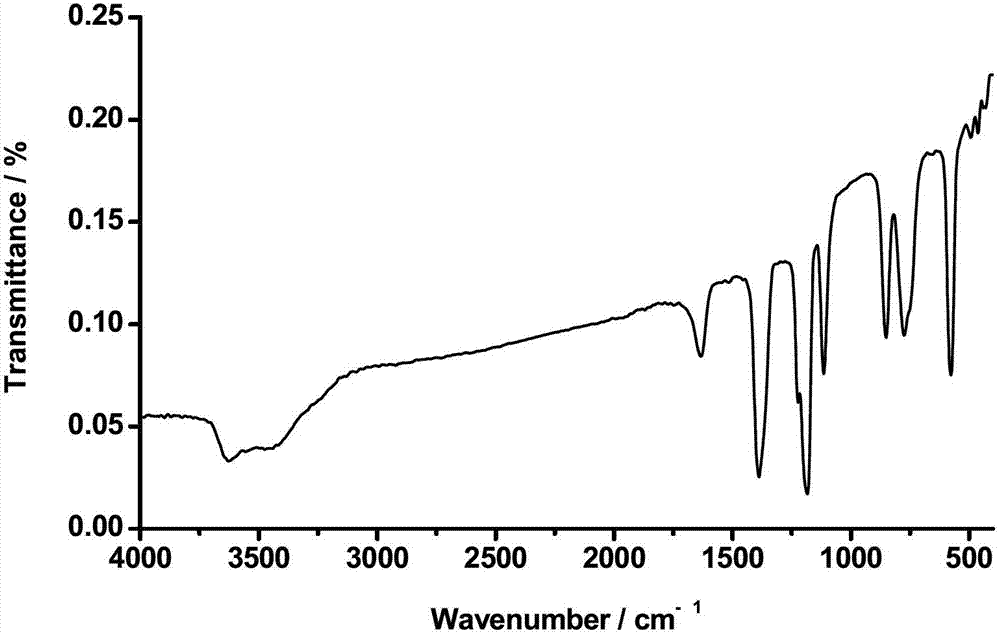
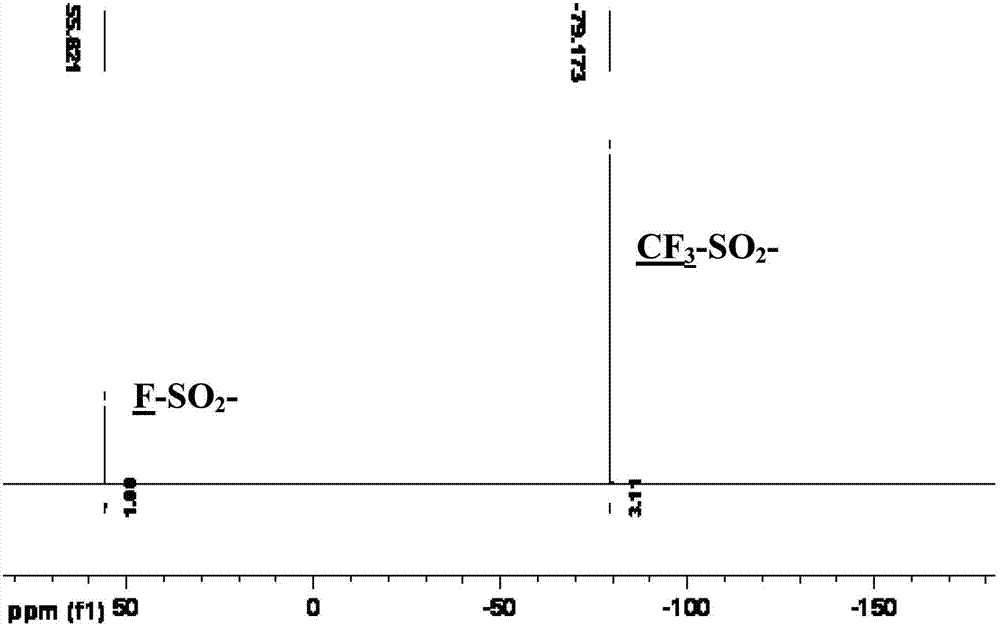
Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
ActiveCN102786452AOvercome operabilityOvercome fatal shortcomings such as difficult product purificationSulfonic acid amide preparationTetrafluoroborateDecomposition
The invention discloses a method for preparing bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. According to the method, sulfamide is utilized to take reaction with thionyl chloride and chlorosulfonic acid for preparing bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine, then, the bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine takes reaction with antimony trifluoride and potassium (rubidium or caesium and the like) carbonate, and corresponding high-purity bis(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt can be obtained; and the double decomposition exchange reaction of the potassium (rubidium or caesium) salt and lithium (or sodium) perchlorate or lithium (or sodium) tetrafluoroborate and the like in aprotic polar solvents is utilized to obtain corresponding high-purity lithium (or sodium) salt. The method provided by the invention has the characteristics that the operation step is simple, the products can be easily separated and purified, the purity and the yield are high, the environment pollution is avoided, the method is suitable for industrial mass production, and the like.
Owner:武汉市瑞华新能源科技有限公司
Lithium battery electrolyte and obtained lithium primary battery
ActiveCN106025307AGuaranteed electrochemical performanceImprove electrochemical performanceOrganic electrolyte cellsOrganic electrolytesOrganic solventPhysical chemistry
The invention belongs to the field of chemical batteries and particularly relates to a lithium battery electrolyte and a lithium primary battery containing the same. The lithium battery electrolyte comprises an organic solvent and an electrolyte. The electrolyte is mixed lithium salt, and the mixed lithium salt is composed of LiFSI and lithium perchlorate. A general formula of the mixed salt is xLiFSI-yLiClO4, wherein 45%<x<70%; 30%<y<55%; and x and y are mass percents. The primary battery obtained through the lithium battery electrolyte is suitable for lithium primary battery systems such as Li / MnO2, Li / FeS2, Li / CuO and Li / (CF)x. The obtained primary battery is low in production cost and excellent in safety performance and low temperature discharge effect.
Owner:HUIZHOU HUIDERUI LITHIUM BATTERY TECHNOLOGY CO LTD
Electrolyte additive and application thereof
ActiveCN105870502AImprove Coulombic efficiencyFree from corrosionFuel and secondary cellsSecondary cellsPhysical chemistrySulfite
The invention discloses an electrolyte additive and application of the electrolyte additive in prolonging the cycle lifetime of a lithium metal battery, and belongs to the technical field of a lithium metal battery. The electrolyte additive comprises Li2Sx (x=1-8) or is formed by coupling the Li2Sx (x=1-8) and other constituents, wherein the other constituents are one or more than one of lithium nitrate, lithium perchlorate, fluoroethylene carbonate, vinylene carbonate, propylene sulfite and Lithium bisimide. By the electrolyte additive, a layer of passivation film with a stable structure can be in-situ formed on the surface of a lithium metal negative electrode; by the passivation film, on one hand, lithium dendrites on the surface of the lithium metal negative electrode can be prevented, and the safety performance of the battery is improved; and on the other hand, the lithium metal also can be prevented from being corroded by the electrolyte, and the utilization ratio and the cycle stability of the battery are improved; and by matching a high-capacity positive electrode material, the industrial progress of the electrolyte additive can be promoted.
Owner:TSINGHUA UNIV
Variable resistance conductive polymer/polyelectrolyte solid composite or mixed film and its preparing method
InactiveCN101051697ADiffusion fastFast Adjustable ResistorElectrode manufacturing processesLi-accumulatorsPolyelectrolyteConductive polymer
Resistance quick adjustable conductive polymer (CP) / polymerized electrolyte solid (PES) composite membrane is CP / PES composite membrane by using CP solid membrane as anode material with polyelectrolyte membrane composed of polyvinylidene fluoride (PVDF), polycarbonate (PC), and lithium perchlorate (LiClO4) being compounded, or a kind of solid admixture membrane. The disclosed CP / PES composite membrane or admixture membrane can extend to be as anode layer of lithium ion cell of solid electrolyte thin film or polyelectrolyte layer to raise speed for charging / discharging cell effectively so as to possess wide foreground of application. After DC voltage is applied to the solid composite membrane, mutation of conductivity generates within short time so as to be able to apply to areas with special requirements. The invention also discloses preparation method.
Owner:NANJING UNIV
Electrolyte for inhibiting growth of lithium dendrites and lithium battery
PendingCN110416615AImprove deposition and dissolution efficiencyInhibition formationSecondary cells servicing/maintenanceSolid state electrolyteLithium chloride
The invention discloses an electrolyte for inhibiting the growth of lithium dendrites and a lithium battery. The electrolyte comprises an additive, a lithium salt and an organic solvent. The additiveincludes at least one in the group consisting of lithium hexafluorophosphate, lithium perchlorate, lithium bis(trifluoromethane) sulfonate, lithium trifluoromethanesulfonate, lithium fluoroborate, trilithium hexafluoroaluminate, lithium hexafluoroarsenate, lithium fluoride, lithium chloride, lithium bromide, lithium nitrate, lithium polysulfide, lithium nitride, lithium phosphide, lithium oxalateborate, lithium oxide, lithium sulfite, lithium sulfate, lithium acetate, lithium hydroxide and lithium oxalate, and the lithium salt is different from the additive. According to the lithium battery containing an additive, a layer of solid electrolyte membrane can be formed on a surface of the lithium metal negative electrode during charge and discharge, and the polymerization of the electrolyte can be induced to form an oligomer which covers a surface of a lithium negative electrode and a surface of a positive electrode material matched with the lithium negative electrode. The protective layer can effectively inhibit the growth of the lithium dendrites, and therefore, the safety performance of the battery is improved.
Owner:SOUTH CHINA UNIV OF TECH
Anticorrosive electrolyte of lithium battery and obtained lithium primary battery
InactiveCN106450365AImprove electrochemical performanceImprove securityOrganic electrolyte cellsCell preserving/storageElectrical batteryHeat stability
The invention belongs to the technical field of chemical batteries, and particularly relates to an anticorrosive electrolyte of a lithium battery and an obtained lithium primary battery. The anticorrosive electrolyte of the lithium battery comprises an organic solvent and an electrolyte, wherein the electrolyte is a mixed lithium salt; and the mixed lithium salt comprises lithium bis(trifluoromethanesulfonyl ) imide LiTFSI, lithium bis(oxalatoe ) borate LiBOB and lithium perchlorate. The lithium salt with a good anticorrosive effect and good heat stability is added to the electrolyte, so that the problems that aluminum foil is corroded and the battery is short in service life and poor in safety due to use of a high-conductivity fluorine-containing lithium salt are solved on the basis of not reducing the capacity of the battery and the protection effect on the aluminum foil. In addition, the electrolyte provided by the invention is suitable for the electrolytes, such as lithium / manganese dioxide (Li / MnO2), lithium / iron disulfide (Li / FeS2), lithium / copper oxide (Li / CuO), lithium / fluorinated carbon [Li / (CF)x], used for the lithium primary battery, and has the advantages of high applicability, a wide range and the like.
Owner:HUIZHOU HUIDERUI LITHIUM BATTERY TECHNOLOGY CO LTD
Preparation method of electrochromic intelligent fiber
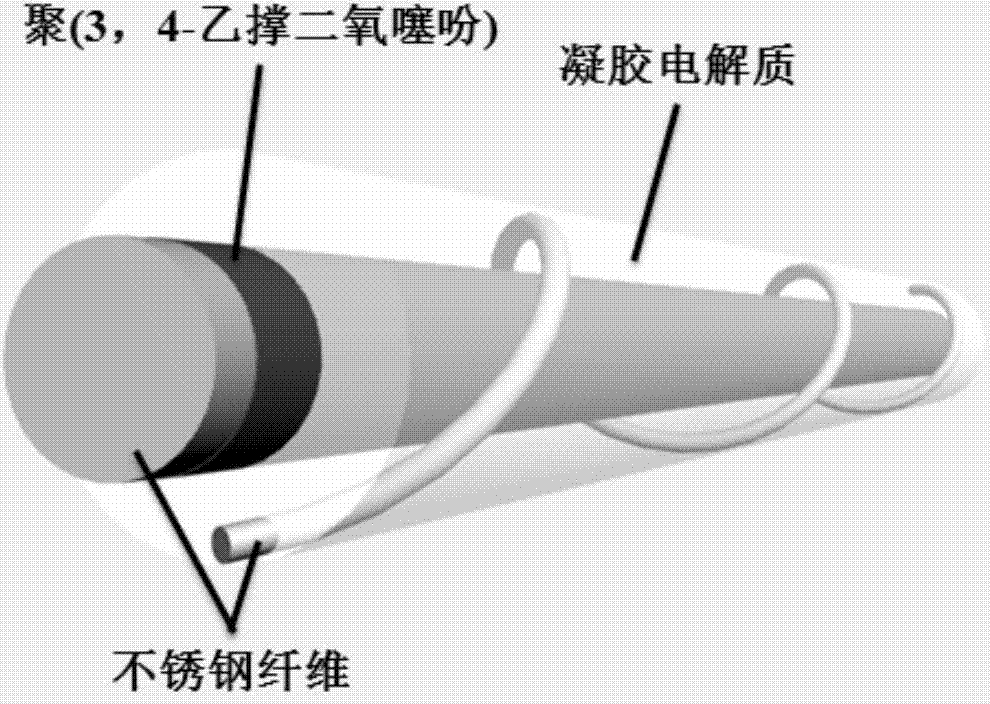
ActiveCN103898592AFast transition rateGood optical performanceElectrophoretic coatingsNon-linear opticsOptical propertyRoom temperature
The invention relates to a preparation method of an electrochromic intelligent fiber. The preparation method of the electrochromic intelligent fiber comprises the following steps: dissolving 3,4-ethylenedioxythiophene in lithium perchlorate propylene carbonate solution to obtain a deposition solution, then vertically placing a stainless steel fiber subjected to ultrasonic washing into the deposition solution for carrying out electrolytic deposition, performing vacuum drying, so that the stainless steel fiber coated with poly(3,4-ethylenedioxythiophene) (PEDOT) is obtained; dipping gel electrolyte on the stainless steel fibers coated with PEDOT, drying at room temperature, then winding another stainless steel fiber on the stainless steel fiber dipped with the gel electrolyte, dipping the gel electrolyte again, and drying at room temperature, so that the electrochromic intelligent fiber is obtained. The preparation method of the electrochromic intelligent fiber is simple, low in cost and applicable to large-scale production; the prepared electrochromic intelligent fiber has good optical property, high transformation speed, good flexibility and obvious gradient effect.
Owner:DONGHUA UNIV
Carbon fiber electrode used for diphenol microsensor
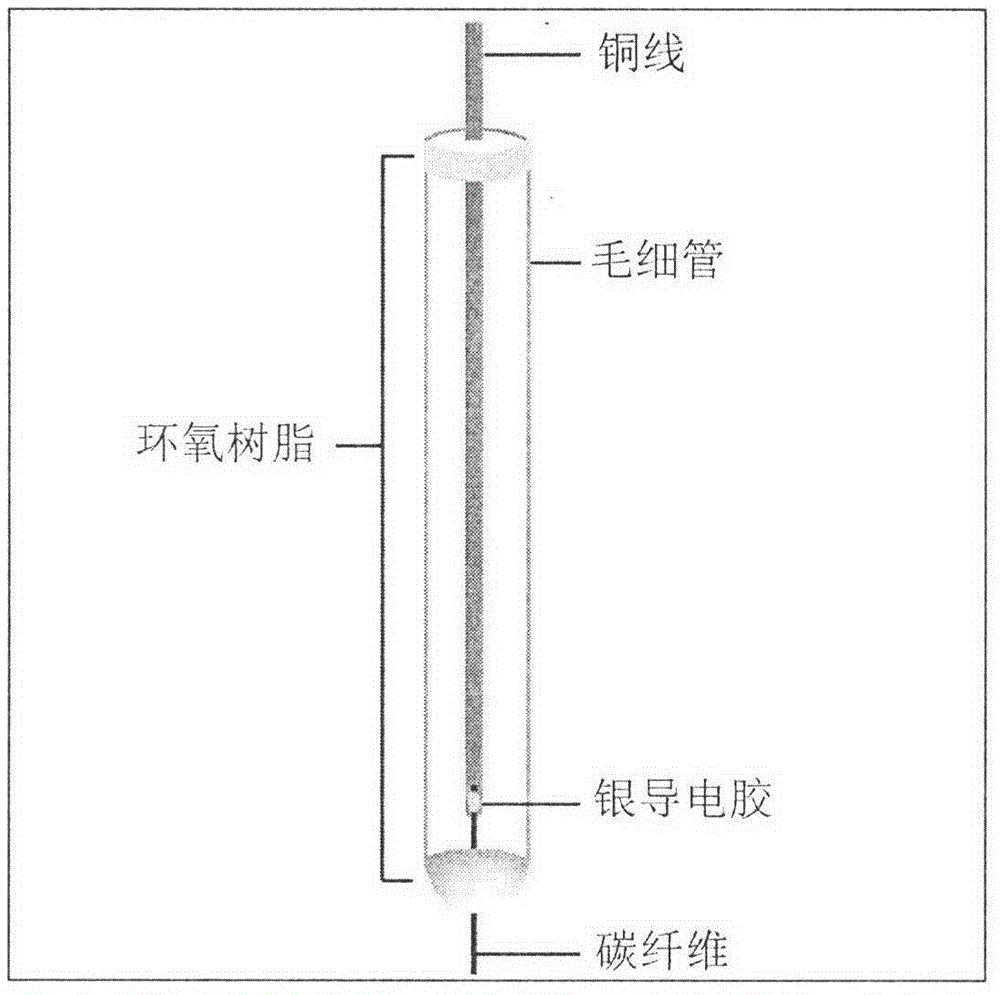
InactiveCN104914149AImprove electrocatalytic activityReduce manufacturing costMaterial electrochemical variablesFiberEpoxy
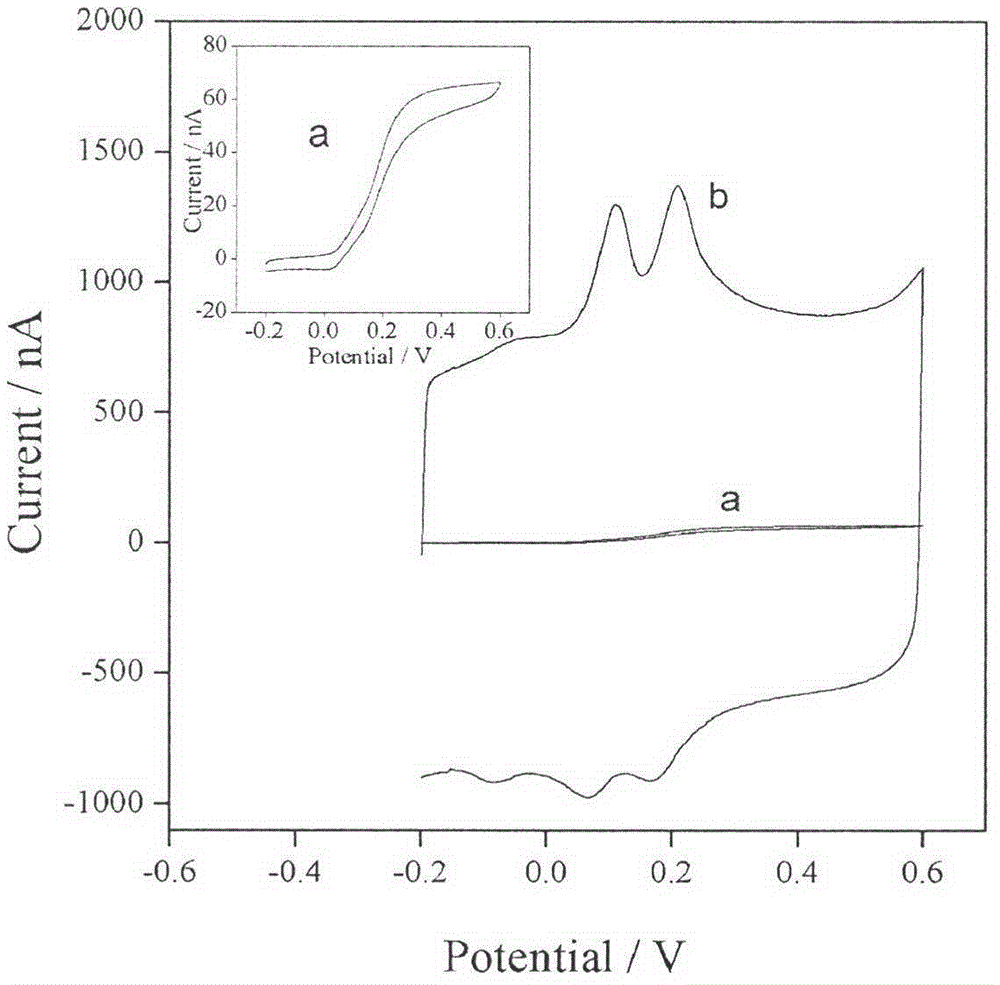
The invention relates to modification for an electrode of a sensor, in particular to the assembly of a carbon fiber electrode and modification for the surface of the carbon fiber electrode so that the sensitivity and the signal intensity of the carbon fiber electrode for a to-be-detected article are enhanced. A specific preparation process comprises the following steps: (1) performing ultrasonic treatment on carbon fibers for 2-3min by using acetone and distilled water, drying, connecting copper wires and carbon fibers together by using silver conductive glue; (2) encapsulating the copper wires into a capillary tube by adopting epoxy resin without electrochemical catalytic activity, and completely drying under room temperature; (3) cutting the carbon fibers at the top end of the capillary tube so that the carbon fibers have suitable lengths, and dipping the electrode into dilute H2SO4 for electrochemical activation; (4) dipping the electrode subjected to electrochemical activation into acetonitrile solution containing 3,4-ethylenedioxythiophene (EDOT) and lithium perchlorate for potentiostatic electropolymerization; and (5) testing the performances of the prepared electrode by adopting an electrochemical workstation. The preparation process is easy to operate, the cost of the electrode is greatly reduced, and the prepared electrode has extremely high catalytic oxidation capacity for diphenol substance and has a very high practical value in the field of microelectrodes.
Owner:TIANJIN POLYTECHNIC UNIV
Preparation method of high-voltage-resistant solid polymer electrolyte
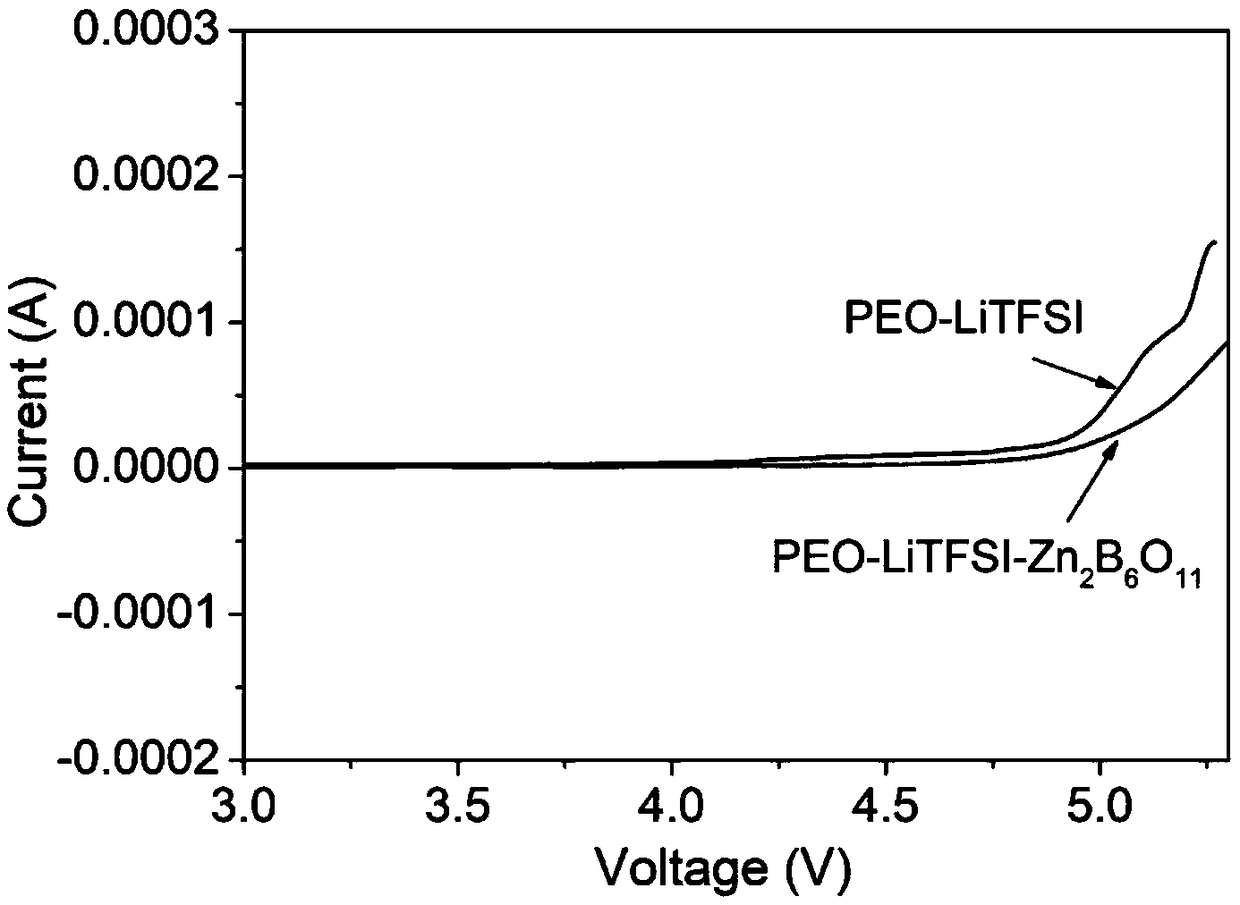
ActiveCN109301317AImprove high pressure performanceIncrease energy densitySolid electrolytesLi-accumulatorsPolymer scienceNanowire
A preparation method of a high-voltage-resistant solid polymer electrolyte includes the steps of: 1) according to certain ratio, dissolving a polymer substrate, lithium salt, and an inorganic additivein anhydrous acetonitrile and stirring the mixture at room temperature to obtain a uniform solution, wherein the polymer substrate is polyoxyethylene, the lithium salt is lithium bistrifluoromethylsulfonimide or lithium perchlorate; the inorganic additive is nano-wires or nano-particles of zinc borate, aluminum borate, sodium tetraborate, barium metaborate or calcium borate, the mass ratio of thepolyoxyethylene to the lithium salt EO : Li<+> is 10-20:1, and the mass of the inorganic additive is not more than 20% of the total mass of the polymer substrate and lithium salt; 2) pouring the solution into a polytetrafluoroethylene mold to volatilize the solution until completely dried, thus preparing the solid polymer electrolyte. The method improves the high-voltage resistance of the solid polymer electrolyte, so that the product fits a high-voltage ternary cathode material and energy density and safety of an all-solid-state battery are improved.
Owner:ZHEJIANG UNIV OF TECH
Method for inhibiting generation of lithium dendrite on surface of lithium metal
ActiveCN108063241AInhibitionEvenly distributedCell electrodesLi-accumulatorsMANGANESE ACETATENitrate
The invention relates to a method for inhibiting generation of a lithium dendrite on the surface of a lithium metal. The method comprises the following steps of dissolving manganese salt crystal intoan electrolyte containing a lithium salt and an organic solvent to obtain the electrolyte containing manganese ions, wherein the manganese salt crystal is one or more of manganese nitrate, manganese acetate and manganese sulfate, and the lithium salt is selected from lithium hexafluorophosphate, lithium perchlorate or LiTFSI; and making the smooth surface of the lithium metal in contact with the electrolyte containing the manganese ions until a bright black film is formed on the surface of the lithium metal. According to the method, generation of the lithium dendrite can be significantly inhibited; when the treated lithium metal is taken as an electrode, the cycle performance and the stability are greatly improved; and the method is simple in preparation technology and low in cost and hasrelatively high application value.
Owner:NANJING UNIV
Quasi-solid electrolyte film applied to electrochromism as well as preparation and application thereof
ActiveCN106633546ASimple preparation processLow costFlat articlesNon-linear opticsSolid state electrolyteSlurry
The invention relates to a quasi-solid electrolyte film applied to electrochromism as well as preparation and application thereof. A raw material mixed slurry comprises lithium perchlorate, a solvent, polyvinylidene fluoride and silicon dioxide. A preparation method of the quasi-solid electrolyte film comprises the following steps: carrying out vacuum drying on the lithium perchlorate to remove water, then preparing a lithium perchlorate solution, taking the polyvinylidene fluoride and the silicon dioxide to dissolve in the lithium perchlorate solution to prepare a slurry capable of being thermally pressed to form a film, taking a proper amount of the slurry, coating the slurry on a glass substrate, thermally pressing to form a film, cooling to room temperature to obtain the quasi-solid electrolyte film which is applied to electrochromic devices. The method is simple in preparation process and low in cost; the prepared quasi-solid electrolyte film is high in conductivity, can be applied to the electrochromic devices, is capable of effectively improving the electrochromism performance of the devices, can get rid of the limit that a polymer electrolyte film is difficult in large-area production, and can achieve the application prospect of industrialization of preparation of electrolyte films through a thermal pressing method.
Owner:DONGHUA UNIV
Electrochemical preparation method of MoS<2>/graphene composite counter electrode
ActiveCN105405663AImprove conversion efficiencySimple methodLight-sensitive devicesPhotovoltaic energy generationComposite filmPotassium
The invention discloses an electrochemical preparation method of an MoS<2> / graphene composite counter electrode. A three-electrode electrochemical deposition system is adopted firstly, a graphene oxide / lithium perchlorate mixed aqueous solution is taken as an electrolyte, and a graphene oxide thin film is directly formed on FTO conductive glass in an electro-deposition manner and forms a graphene thin film through electrochemical reduction at the same time; and the graphene thin film is taken as a substrate then, MoS<2> is continue to formed in an ammonium tetrathiomolybdate / potassium chloride aqueous solution in an electro-deposition manner and an MoS<2> / graphene composite thin film is obtained. The MoS<2> / graphene composite thin film prepared through the method can be directly used as a counter electrode of a dye-sensitized solar cell, and any postprocessing procedure does not needed.
Owner:SOUTHEAST UNIV
Polysilane containing transition metallic element and preparation method thereof
InactiveCN102134324ASolve the problem of low dispersionModerate viscosityElectrolysis componentsElectrolytic organic productionSupporting electrolyteDouble bond
The invention relates to a polysilane containing a transition metallic element and a preparation method thereof. In the invention, an electrochemical synthesis method is adopted, magnesium blocks are adopted as a cathode and an anode; methyl trichlorosilane, allyl chloride and cyclopentadiene are dissolved in tetrahydrofuran to prepare into an electrolytic solution, lithium perchlorate is added as a supporting electrolyte, transition metal chloride is added, and the electrolytic solution, lithium perchlorate and the transition metal chloride are polymerized to synthesize the polysilane containing double bonds and a metallic element, wherein the constitutional formula of the polysilane is shown in the specification. The result proves that the reaction condition in the invention is mild, and the operation is simple and safe; and the double bonds and the antioxidant metallic element titanium or zirconium or hafnium and the like are introduced into the polysilane, and the polysilane containing a transition metallic element is a ceramic precursor with excellent performance.
Owner:SHANGHAI UNIV
Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
InactiveCN102786451AOvercome operabilityOvercome fatal shortcomings such as difficult product purificationSulfonic acid amide preparationTetrafluoroborateRubidium
The invention discloses a method for preparing bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. According to the method, sulfamide is utilized to take reaction with thionyl chloride and chlorosulfonic acid for preparing bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine, then, the bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine takes reaction with antimony trifluoride and potassium (rubidium or caesium and the like) carbonate, and corresponding high-purity bis(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt can be obtained; and the double decomposition exchange reaction of the potassium (rubidium or caesium) salt and lithium (or sodium) perchlorate or lithium (or sodium) tetrafluoroborate and the like in aprotic polar solvents is utilized to obtain corresponding high-purity lithium (or sodium) salt. The method provided by the invention has the characteristics that the operation step is simple, the products can be easily separated and purified, the purity and the yield are high, the environment pollution is avoided, the method is suitable for industrial mass production, and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Process for manufacturing lithium sulfur battery
ActiveCN103985913ALow purityPrevent oxidationElectrode thermal treatmentFinal product manufactureSolid state electrolyteHigh rate
The invention discloses a process for manufacturing a lithium sulfur battery. The process comprises the following steps: adding polyacrylonitrile resin micro powder and diatomite into a high-speed mixing stirrer according to a mass ratio of 36-39 to 64-61, adding an NMP solvent which accounts for 6-24 weight percent of the diatomite to serve as a raw material, performing pretreatment, and performing repeated dipping and high-temperature evaporation in an NMP solution of lithium salt (namely a 6-9 weight percent of NMP solution containing lithium polysulfide, lithium methylaminobutyrate, lithium perchlorate and lithium phosphate), thereby preparing a positive material; preparing all-solid-state electrolyte of the lithium sulfur battery from lithium methylaminobutyrate, lithium perchlorate and lithium phosphate; and adsorbing molten metal lithium through pores of carbon-containing diatomite at 630-660 DEG C when metal lithium and the formed carbon-containing diatomite electrode are in a vacuum electric heating furnace, thereby finishing preparation of a negative material. The all-solid-state electrolyte of the lithium sulfur battery prepared by the process has high sulfur containing capacity, high ion transport capacity and high conductive performance, and the high rate performance and high cycle performance of the lithium sulfur battery can be improved.
Owner:威海区域创新中心有限责任公司
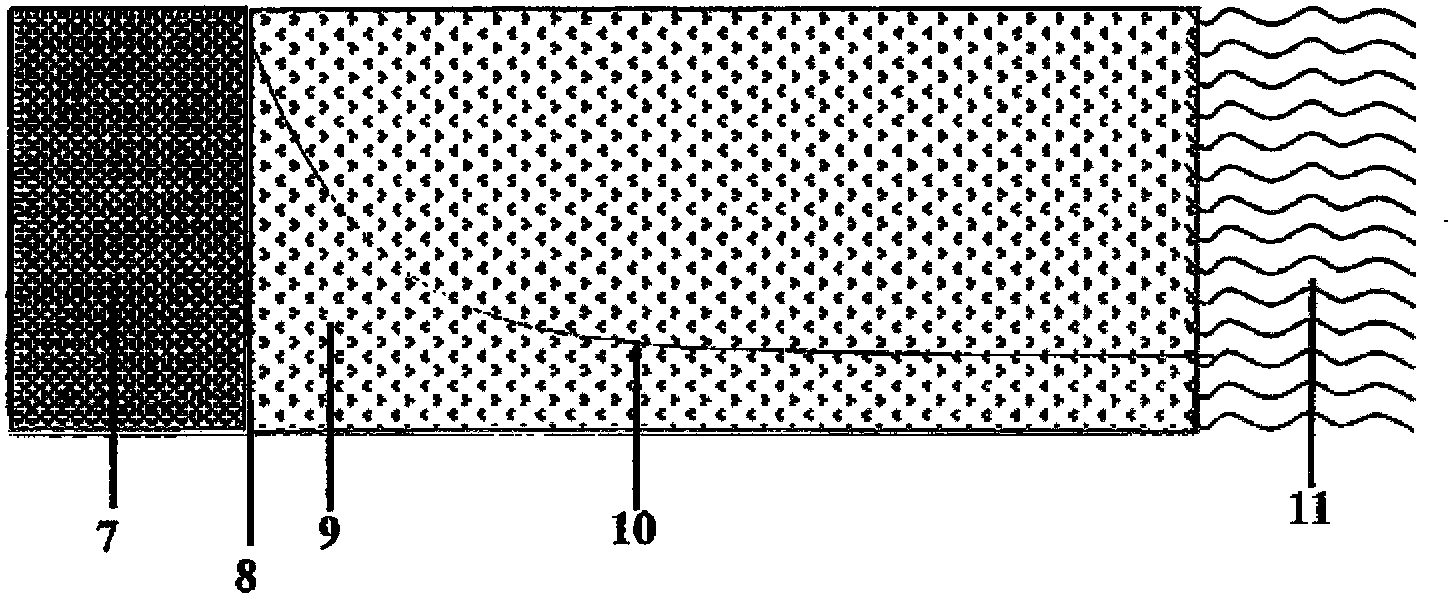
Chemical carbon dioxide gas generator
A chemical carbon dioxide gas generator comprising: - a charge housing; - a carbon dioxide gas penetrable charge, contained in the said housing, the charge comprising a) 40-60 wt. % of a substance which upon decomposition generates carbon dioxide, which substance is selected from the group of magnesium carbonate, other carbonates, magnesium oxalate and other oxalates, b) 20-50 wt. % of an oxidiser selected from the group of sodium chlorate, potassium chlorate, lithium chlorate, other metal chlorates, sodium perchlorate, potassium perchlorate, lithium perchlorate, and other metal perchlorates, c) 1-20 wt. % of carbon or another fuel, d) 1-10 wt. % binder, said components a), b), c) and d) together forming 90-100 wt. % of the total weight of the charge; - an ignition device for igniting the charge; - a carbon dioxide gas treatment unit for reducing the content of one or more side-products - which may have been formed by the charge - in the generated carbon dioxide, and / or for cooling carbon dioxide gas generated by the charge; and - an outlet for carbon dioxide gas generated by the charge.
Owner:荷兰应用自然科技研究组织TNO
Low-concentration lithium salt electrolyte and lithium secondary battery containing same
ActiveCN109935908AComplete structureProtect structural stabilityLi-accumulatorsSecondary cells servicing/maintenancePhosphatePhysical chemistry
The invention provides electrolyte solution containing low-concentration lithium salt and a lithium secondary battery containing the electrolyte solution. The electrolyte contains lithium salt (I), lithium salt (II) and a solvent, wherein the lithium salt (I) is selected from one of lithium bis(oxalato)borate, lithium tetrafluoxalato phosphate, 2-trifluoromethyl-4,5-dicyanoimidazole lithium, lithium difluorooxalatoborate, lithium chlorotrifluoroborate, and lithium trioxalatoborate or more; the lithium salt (II) is selected from one of lithium hexafluorophosphate, lithium hexafluoroarsenate, lithium tetrafluoroborate, lithium perchlorate, LiCF3SO3 and LiN (CxF2x + 1SO2) (CyF2y + 1SO2) or more, wherein x and y are each independently an integer of 0-5, and the total concentration of the lithium salt (I) and the lithium salt (II) is 0.3-0.6 mol / L.
Owner:HEFEI UNIV OF TECH
Gel polymer electrolytes and preparation method thereof
InactiveCN101200568AImprove ionic conductivityGood chemical and electrochemical stabilitySecondary cell detailsPolymer sciencePolyethylene glycol
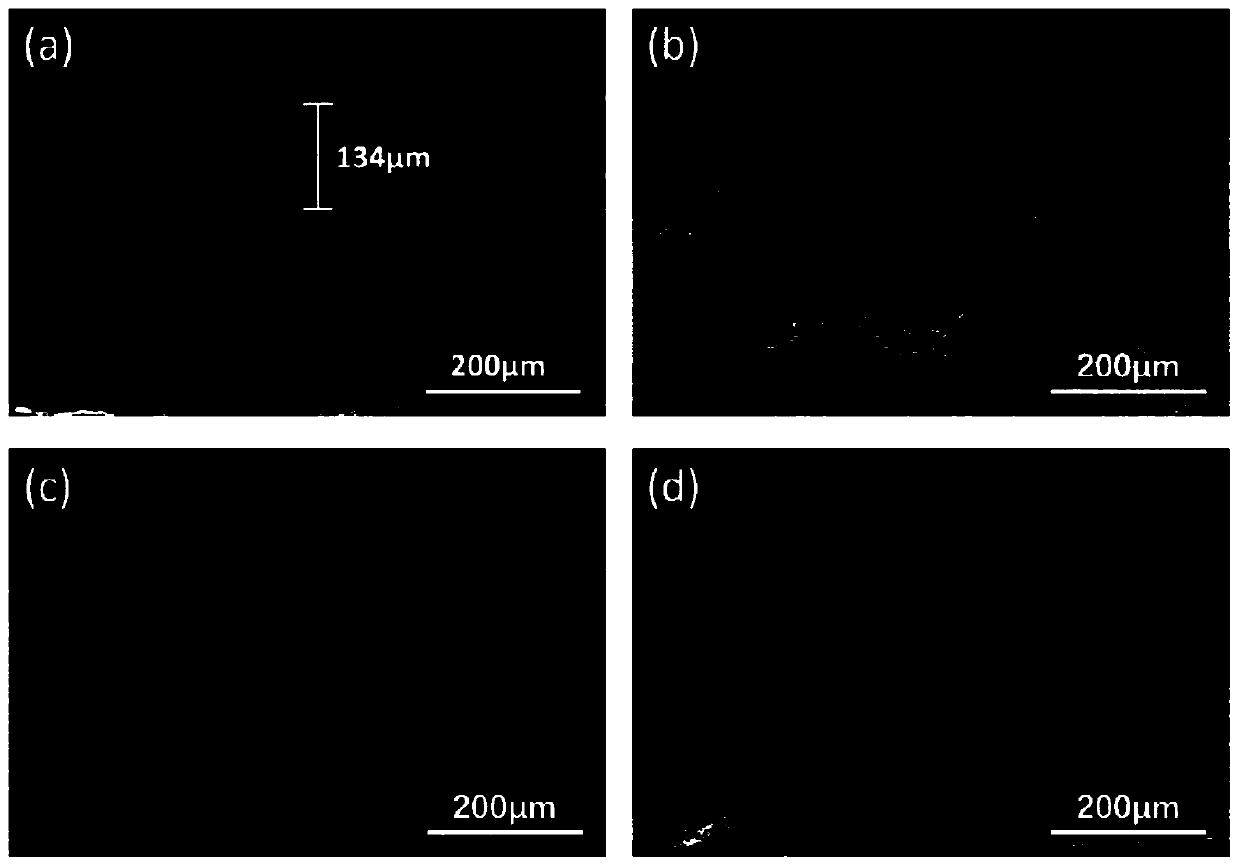
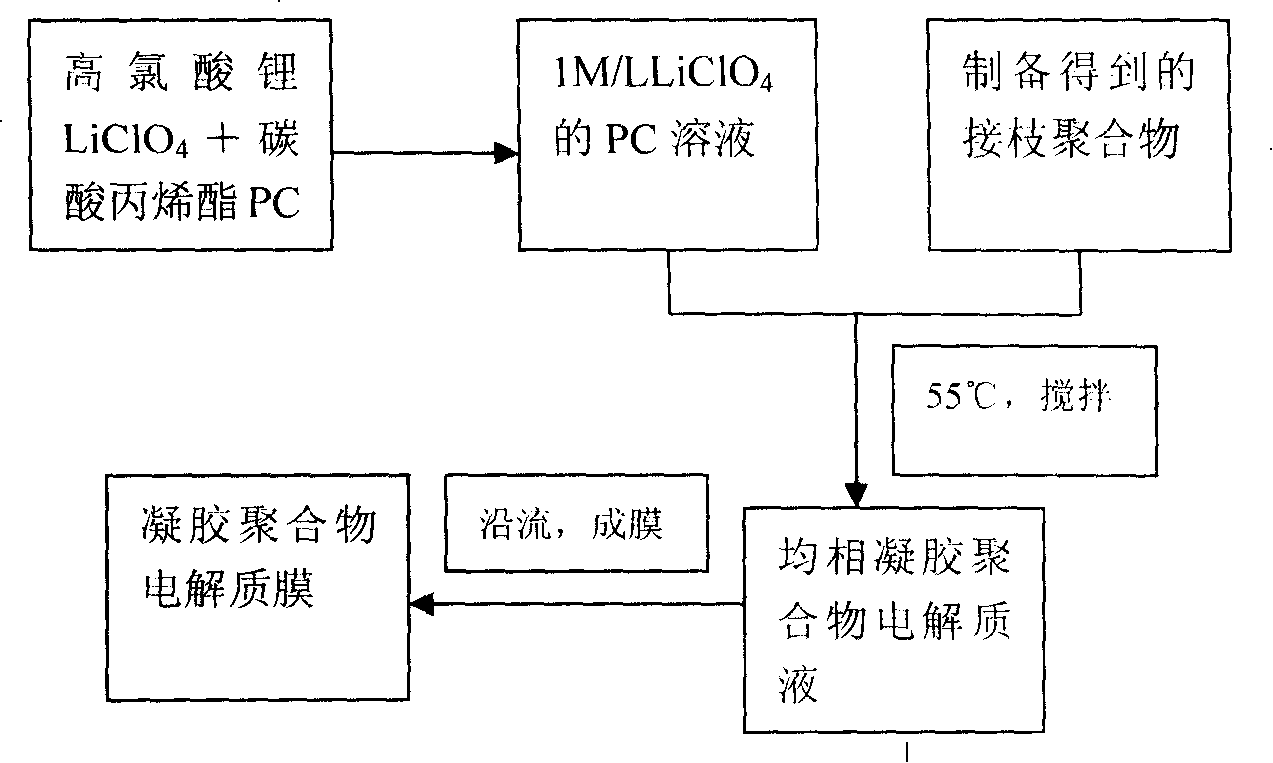
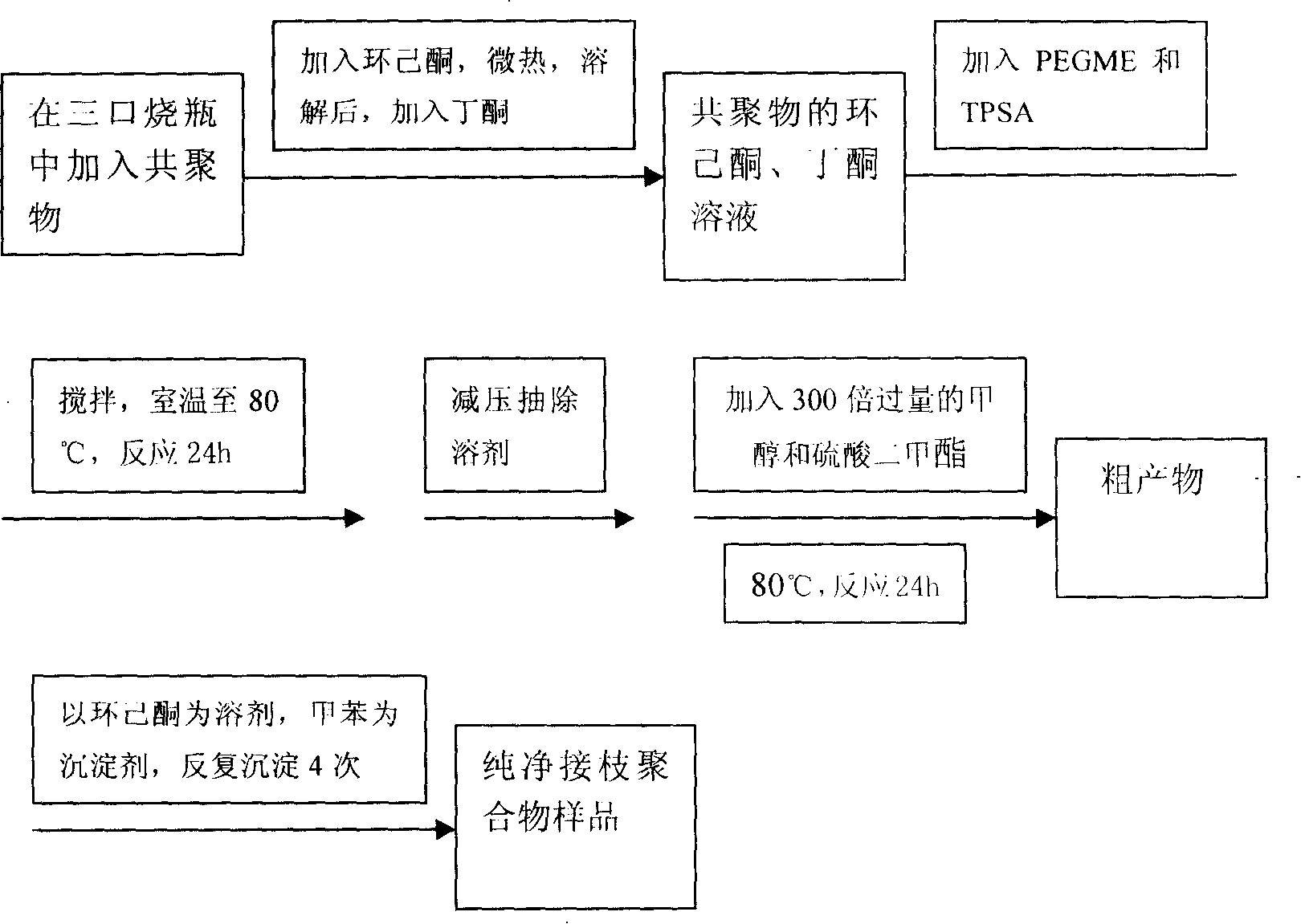
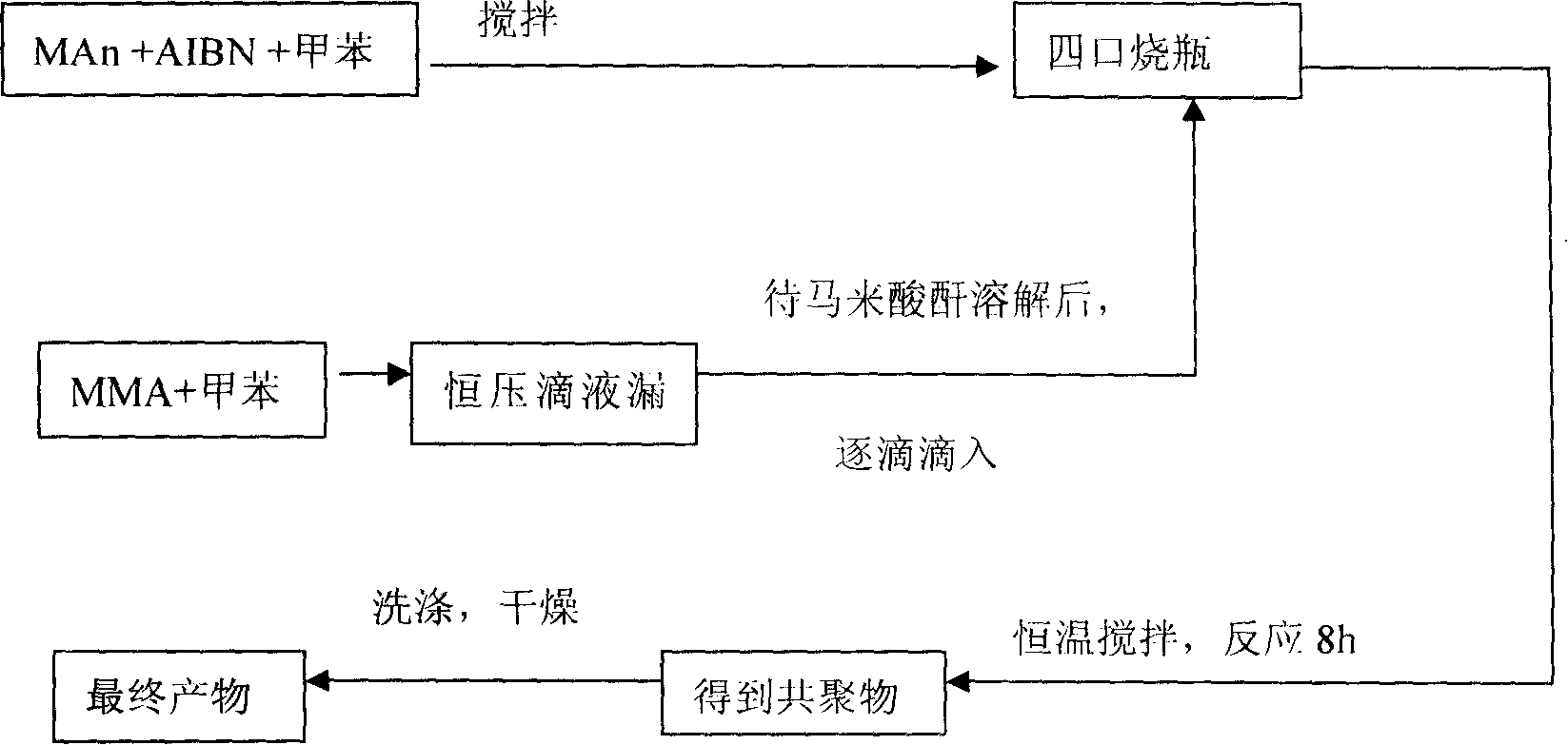
The present invention relates to a gel polymer electrolyte and the preparation method. The technical characteristics are that the proportion of the electrolyte is that the weight percentage of grafting polymer matrix is 30 to 60wt. percents and liquid electrolyte is 40 to 70wt. percents; the liquid electrolyte takes propylene carbonate ester (PC) as plasticizing agent and lithium perchlorate (LiClO4) is lithium salt organic solution with 1.0mol / L concentration. The synthetic recipe of thegrafting polymer includes 1.0mol of propylene type copolymer, 2.0mol of polyethylene monomethyl ether and 500ml of methanol. The components of the propylene type copolymer include 17g to 19g of methacrylate methyl ester, 16g to 18g of maleic anhydride, 350mL to 360mL of solvent and 0.0036g to 0.0040g of initiator; wherein, the mol ratio of methacrylate methyl ester and maleic anhydride is 1:1 to 1:1.5. The gel polymer electrolyte has the beneficial effects of high ion electric conductivity, good electrochemical stability, excellent electric performance and good size and space stability. The synthetic process is simple and workable.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
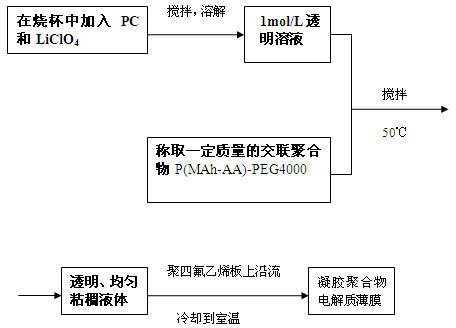
P(MAh-AA)-PEG4000-based gel polymer electrolyte and preparation method thereof
The invention relates to a polyacrylic ester cross linked polymer gel polymer electrolyte for a lithium ion battery. The cross linked polymer-based P(MAh-AA)-PEG4000 gel polymer electrolyte is technically characterized in that raw materials in a formula comprise 20-50g of polymer P(MAh-AA)-PEG4000 and 80-50g of liquid electrolyte; and the preparation steps are carried out in the following process conditions: lithium perchlorate (LiClO4) is dissolved in carbonic allyl ester (PC) to obtain 1mol / L liquid electrolyte; P(MAh-AA)-PEG4000 is dissolved in the liquid electrolyte at 50DEG C; and the electrolyte flows along a polytetrafluoroethylene sheet and is cooled to obtain condensed polymer electrolyte. The invention has the benefits that the polyacrylic ester cross linked polymer gel polymer electrolyte is prepared, has excellent comprehensive performance and meets the service requirement of the lithium ion battery. The invention has the advantages of low price of required raw materials, more direct and simpler operation process, wide applicability and certain practical popularization significance.
Owner:SOUTHWEST PETROLEUM UNIV
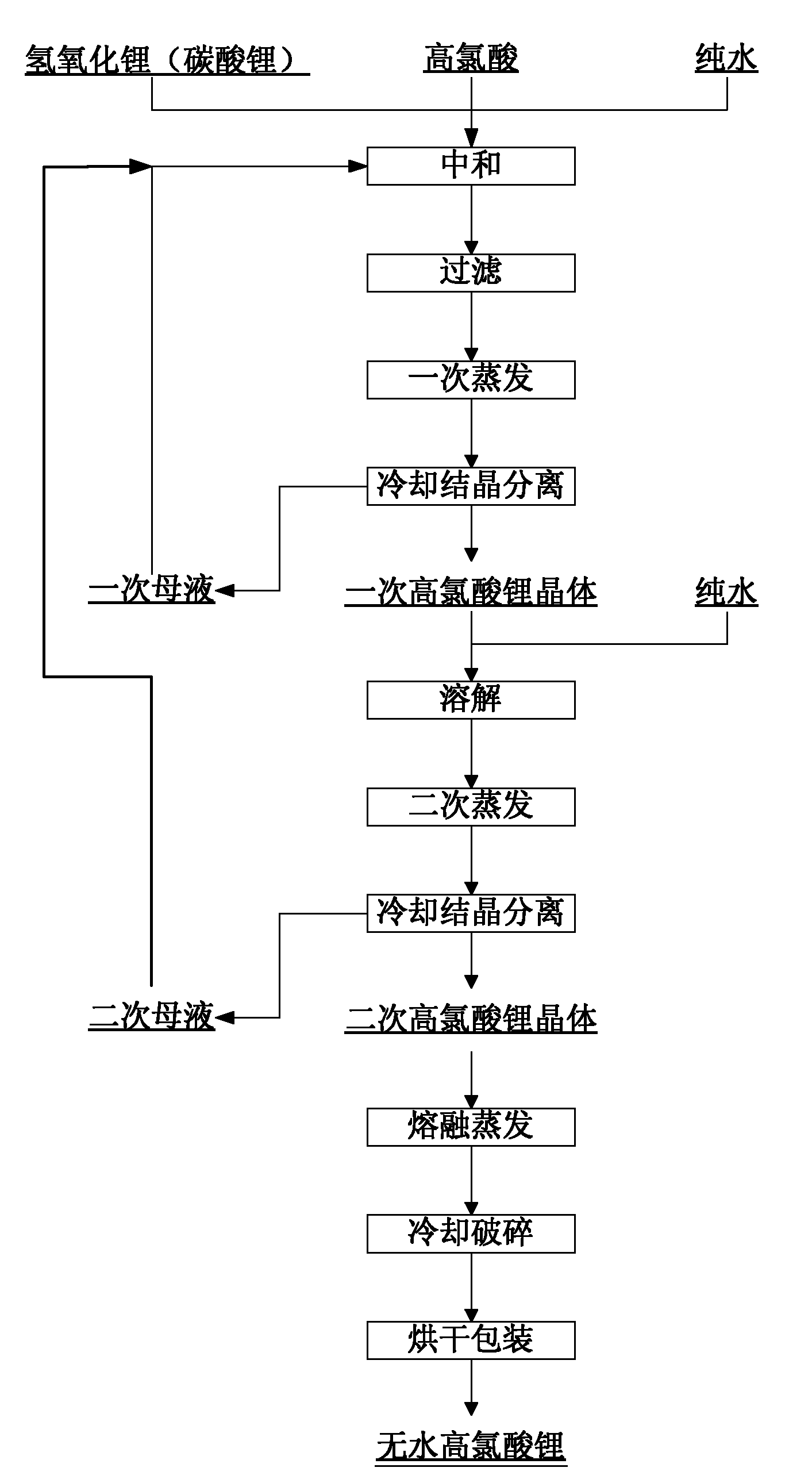
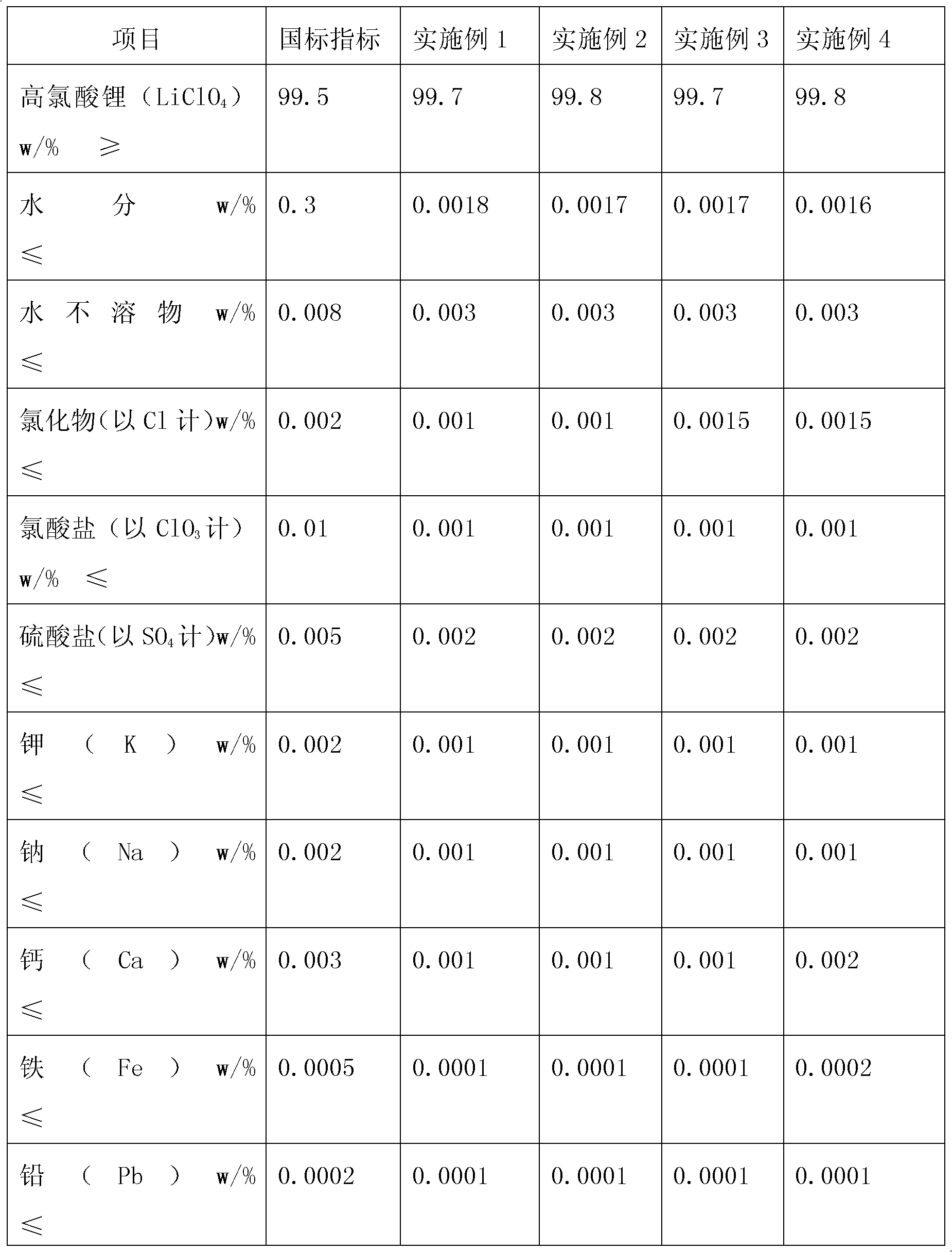
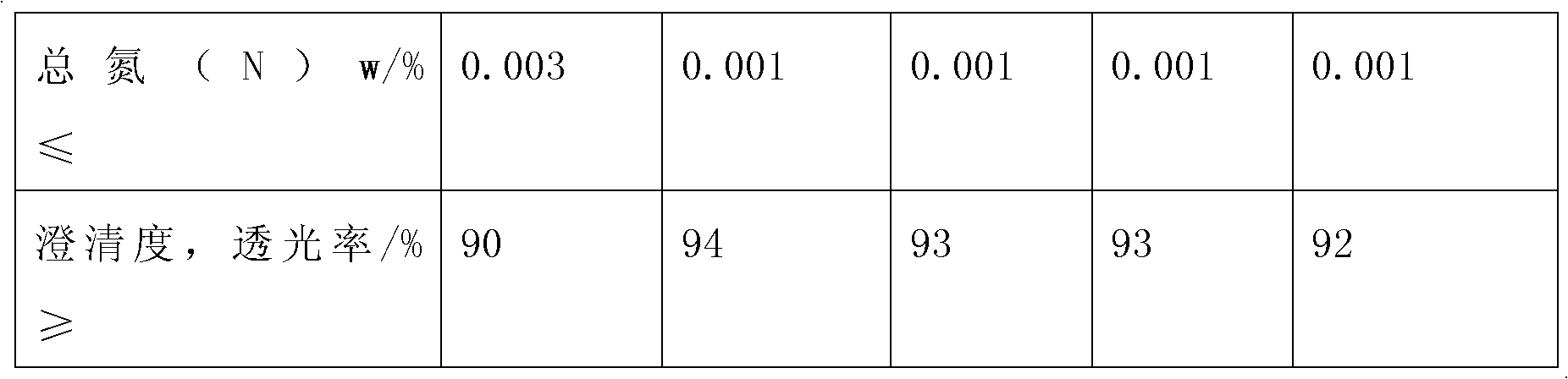
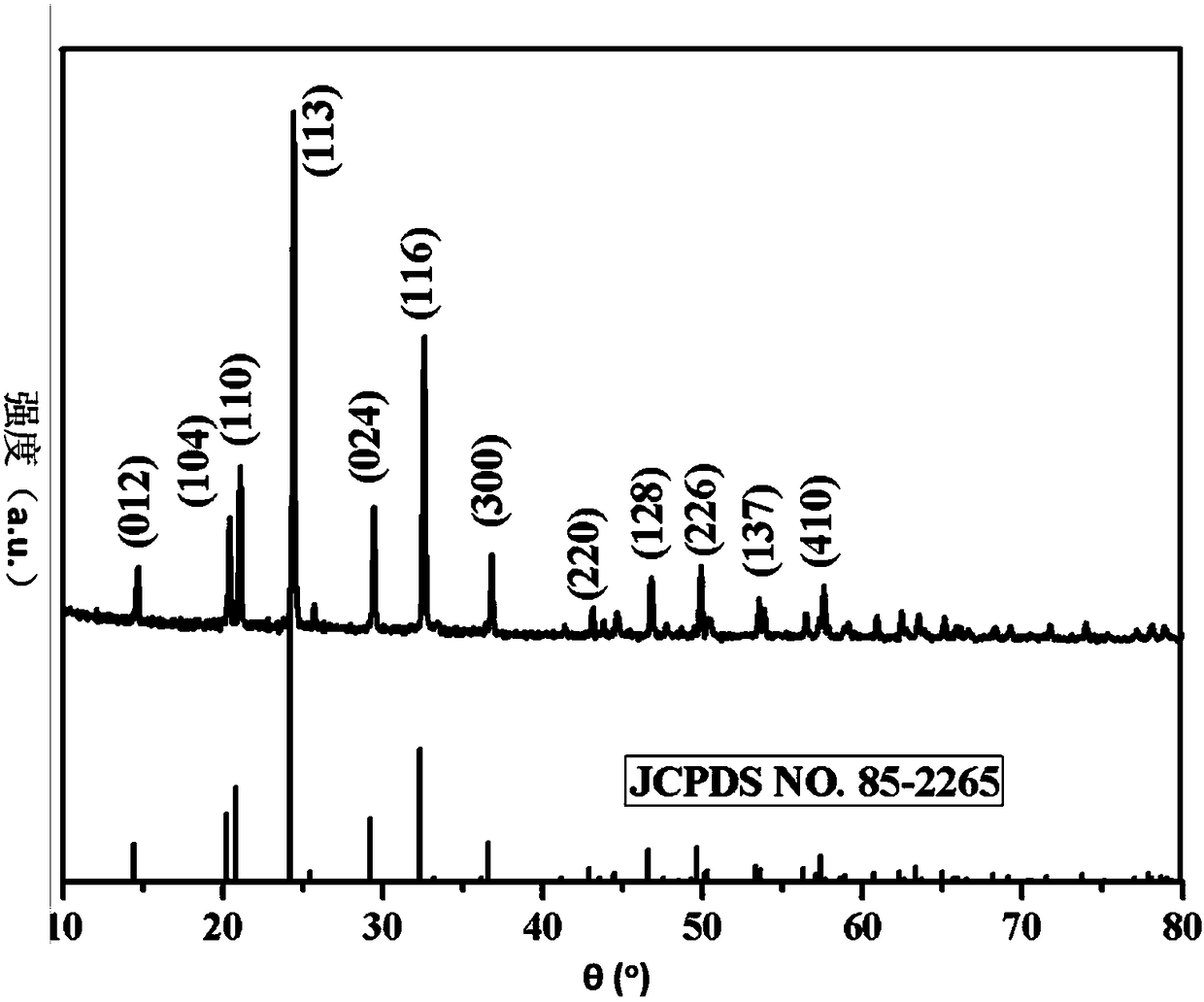
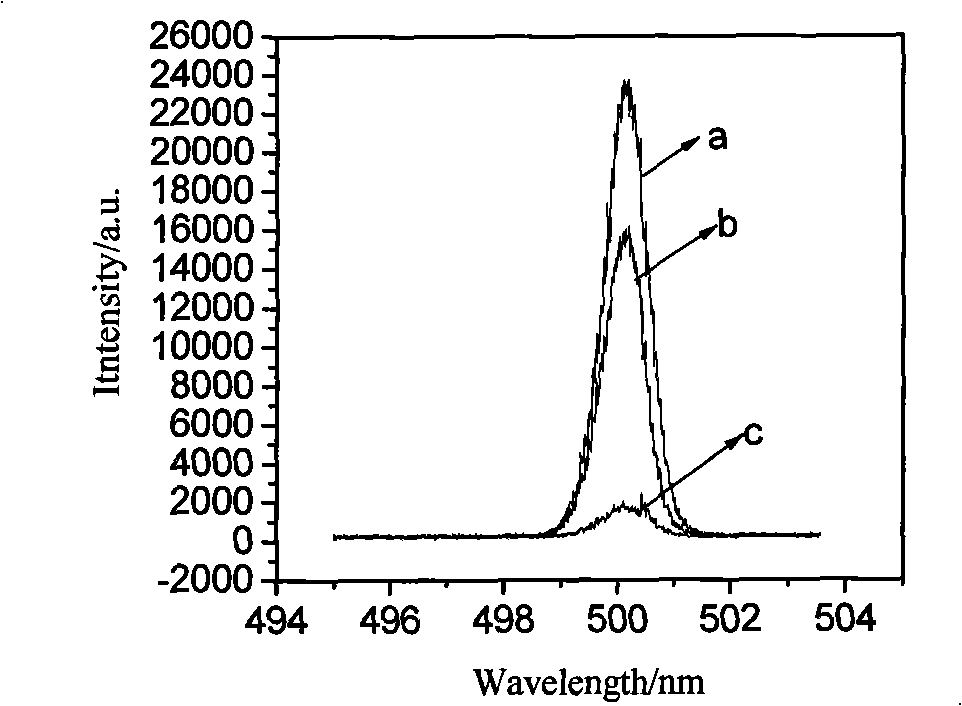
Method for producing anhydrous lithium perchlorate
The invention belongs to the technical field of electrolyte preparation of lithium battery electrolyte and discloses a method for producing anhydrous lithium perchlorate. The water content of anhydrous lithium perchlorate sold in the market is about 3,000ppm, so a factory using the anhydrous lithium perchlorate needs to remove water from the anhydrous lithium perchlorate before preparing electrolyte from the anhydrous lithium perchlorate. But the water is not completely removed and fluctuation frequently exists, the yield and the quality of the electrolyte are restricted. At the same time, with the development of lithium batteries, the requirements on the impurity content and the stability of a pH value of lithium perchlorate are higher and higher. The method, namely reaction between lithium hydroxide or lithium carbonate and perchloric acid, solves the problems. The method sequentially comprises the following steps of: neutralizing, filtering, evaporating for the first time, cooling for crystallization, performing centrifugal separation to obtain a solid, dissolving, evaporating for the second time, cooling for crystallization, performing centrifugal separation to obtain a solid,melting and evaporating, cooling and crushing, and drying and packing. The produced anhydrous lithium perchlorate has low impurity content and the water content of lower then 200ppm; the pH value of aqueous solution of the anhydrous lithium perchlorate is stable; and the anhydrous lithium perchlorate can be directly used for producing the electrolyte.
Owner:HUBEI BAIJIERUI ADVANCED MATERIALS
Mixed water system ionic battery and application thereof
ActiveCN108321442AIncrease energy densityImprove cycle stabilityCell electrodesSecondary cellsCarbon compositesElectrical battery
The invention provides a mixed water system ionic battery and application thereof. The battery comprises a positive electrode, a negative electrode, a diaphragm and quaternary mixed electrolyte, wherein the negative electrode takes a carbon composite NaTi2(PO4)3 material as a negative active substance; the diaphragm is arranged between the positive electrode and the negative electrode; the quaternary mixed electrolyte is prepared from water, a material selected from one or more of urea, N,N-dimethyl formamide, dimethyl sulfoxide, diethyl carbonate and dimethyl carbonate, a material selected from one or more of sodium perchlorate and sodium nitrate as well as a material selected from one or more of lithium perchlorate or lithium nitrate. The materials of the battery are non-toxic, pollution-free, non-combustible, non-explosible, safe and reliable, the output voltage of the total battery can reach 1.65 V, the energy density is up to 50 Wh / kg, and the service life is up to 1000 times or longer. Compared with the conventional water system ionic battery, the battery provided by the invention has high circulation stability; the manufacturing process is simple; the raw materials are cheapand have wide sources; the energy density is high; the problems that the conventional water system ionic battery has short circulation life and low energy density can be solved effectively.
Owner:UNIV OF SCI & TECH OF CHINA
Galvano-chemistry preparation method for electrochromic magnesium-nickel alloy film
InactiveCN101270491AControl light transmissionControl reflexSupporting electrolyteN dimethylformamide
The invention relates to an electrochemistry preparation method for an electrochromism magnesium-nickel alloy film. Firstly, an organic solvent N,N-dimethylformamide is dried for 48 hours by an activated 4 molecular sieve, then is decompressed and distilled to remove the impurities; then the main salt dehydrate and nickelous chloride as well as the lithium perchlorate supporting electrolyte are respectively dried for four hours under the vacuum with the temperature of 150 DEG C and then placed in an vacuum drying box for spare; then the nicks and the oil stains on the surface of a copper sheet are removed; the main salt and the organic solvent N,N-dimethylformamide used for supporting electrolyte are prepared into liquid with the concentration of 0.2mol / L of the dehydrate, the concentration of 0.2mol / L of the lithium perchlorate and the concentration of 0.02mol / L of the nickelous chloride; argon is pumped into the liquid so as to expel the oxygen dissolved in the N,N-dimethylformamide; finally the magnesium-nickel alloy film is obtained by carrying out electrochemistry deposition under the constant temperature and constant potential condition by a three-electrode system. The method is simple, needs no complex post treatment working procedures like heat treatment and can effectively control the light transmittance and the reflectivity of the film.
Owner:OCEAN UNIV OF CHINA
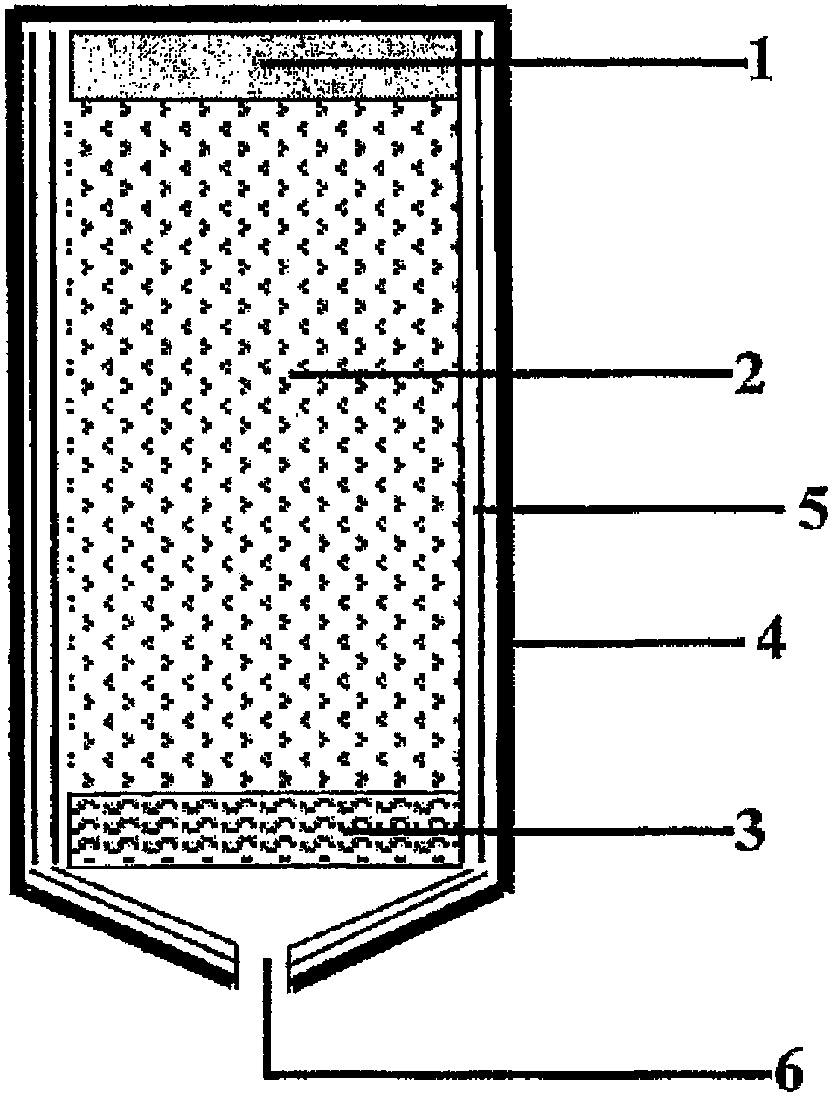
Hybrid minitype super capacitor based on organic electrolyte and manufacturing method thereof
InactiveCN101916664ALower internal resistanceImprove energy storage characteristicsCapacitor housing/encapsulationAdhesiveCapacitor voltage
The invention discloses a hybrid minitype super capacitor and a manufacturing method thereof, and belongs to the technical field of MEMS. The minitype super capacitor is composed of a support body, a positive electrode, an isolated body, a negative electrode and aluminum seal covers, wherein the minitype super capacitor is in a coiling structure; the positive electrode, the isolated body and the negative electrode are soaked in a lithium perchlorate organic electrolyte; the positive electrode comprises an active carbon energy storage material, an acetylene black conducting material and polyvinylidene fluoride adhesive, and is prepared by a screen printing method; the negative electrode comprises a lithium titanate energy storage material, acetylene black and polyvinylidene fluoride adhesive, and is prepared by the screen printing method; the isolated body is a copolymer in a polyvinylidene fluoride-hexafluoropropylene porous structure and is prepared by a spin coating method; and the ends of the positive electrode and the negative electrode are respectively provided with the aluminum seal cover prepared by a magnetron sputtering method, and the aluminum seal covers are used as electrode terminals to perform the function of a current collector. The large contact area of the aluminum seal covers and the electrodes can effectively reduce the resistance of the minitype super capacitor, thereby enhancing the energy storage characteristic of the minitype super capacitor; and the invention can increase the voltage of the minitype super capacitor to 3.6 V.
Owner:TSINGHUA UNIV
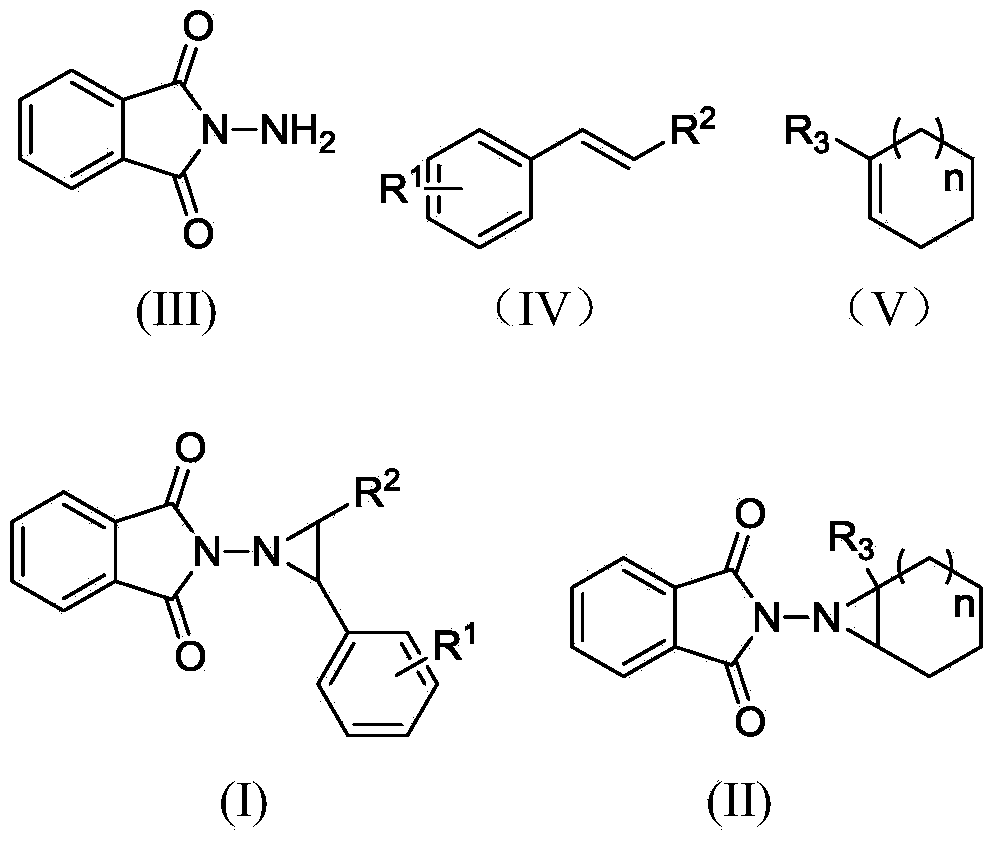
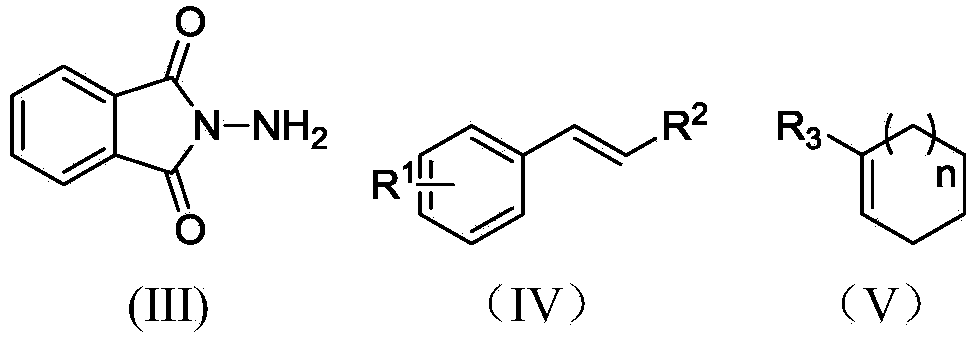
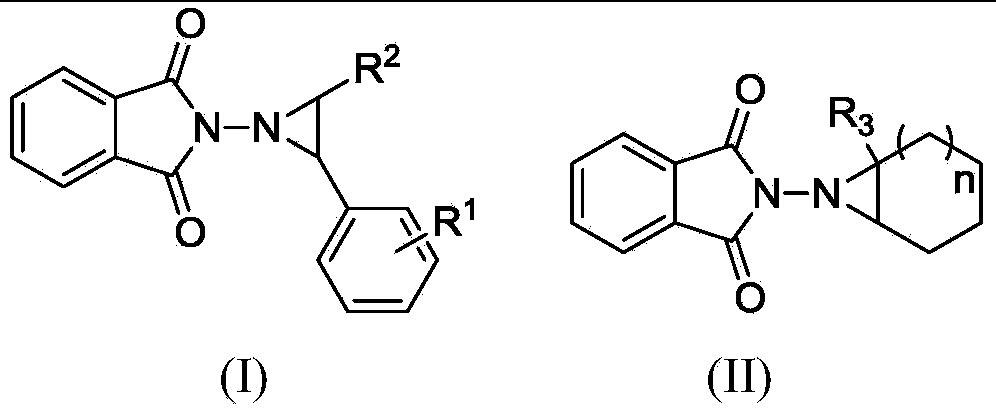
Electrochemical catalytic synthesis method of aziridine compounds
ActiveCN103436911ALower internal resistanceImproving electrochemical synthesis methodsElectrolysis componentsElectrolytic organic productionSupporting electrolyteN-aminophthalimide
The invention relates to an electrochemical catalytic synthesis method of aziridine compounds. The method comprises the following steps: by employing N-aminophthalimide and styrene or cycloolefin as raw materials in a single-room electrolytic tank, in electrolyte, employing halogenated tetra-alkylamine or alkali halide as an electrocatalyst, by employing lithium perchlorate or triethylamine / acetic acid as an supporting electrolyte, electrolyzing in the presence of alkali, wherein the reaction temperature is 0-40 DEG C, the current density is 4-12 mA / cm<2>, and thus obtaining the aziridine compounds after being electrified with the electric quantity of 2.5-3.5 F / mol. According to the electrochemical catalytic synthesis method of aziridine compounds, an indirect electrolysis method of electrochemical catalysis which is simple to operate is firstly employed to synthesize the aziridine compounds, the single-room electrolytic tank is used, constant-current electrolysis is employed, and glassy carbon electrodes are used as working electrodes, so that the conversion of the double-room electrolytic tank to the single-room electrolytic tank is achieved, and meanwhile, as the working electrodes are changed to be the glassy carbon electrodes in stead of previously used expensive platinum electrodes, the cost is greatly reduced, and the operation is much simpler, thus being more suitable for industrial production.
Owner:BEIJING UNIV OF TECH
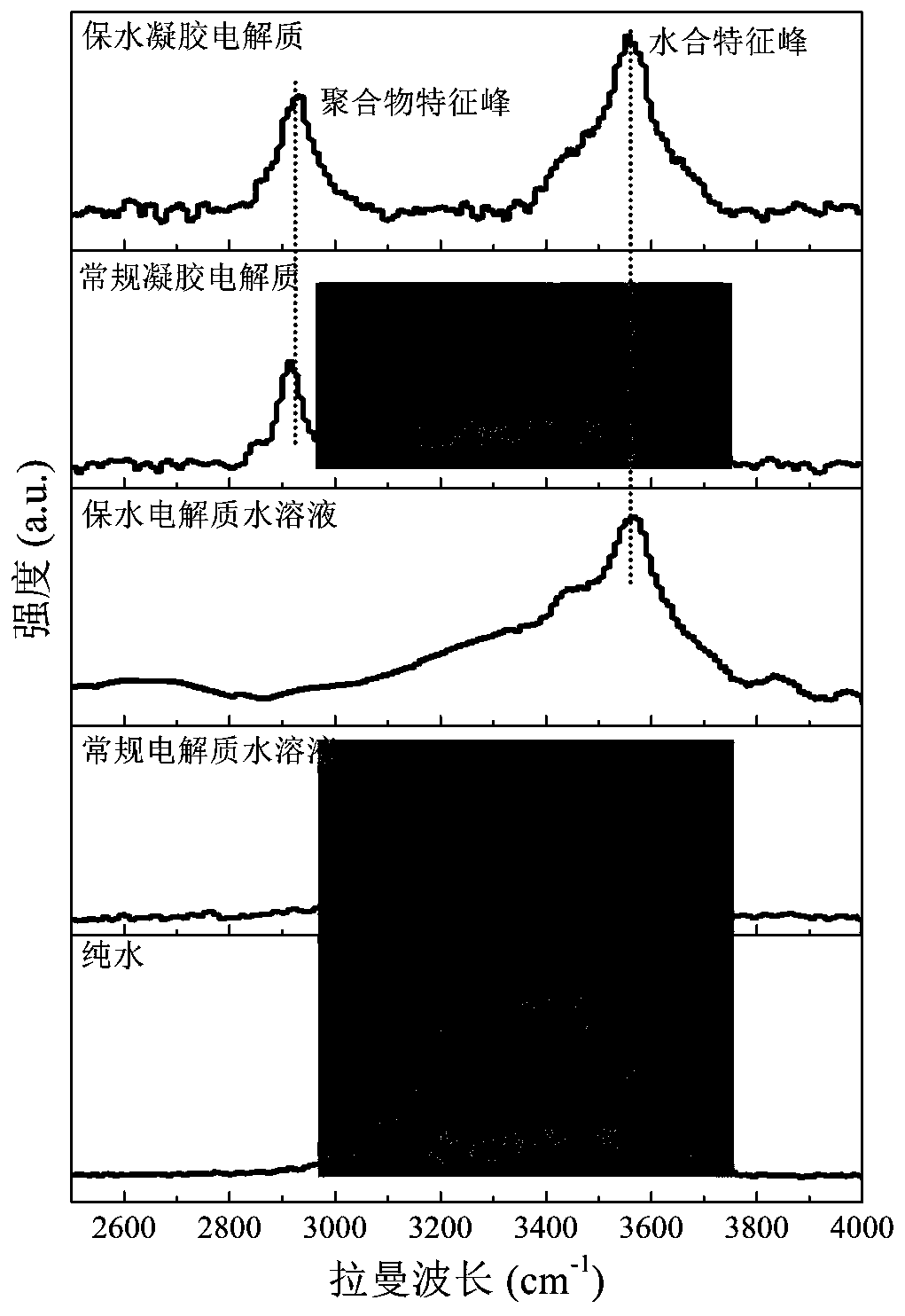
Water-retention gel electrolyte, preparation method thereof, and water-based supercapacitor and preparation method and application thereof
InactiveCN110246705AEasy to manufactureImprove water retentionHybrid capacitor electrolytesDouble layer capacitorsWater basedPotassium hexafluorophosphate
The invention provides a water-retention gel electrolyte , a preparation method thereof, and a water-based supercapacitor and a preparation method and application thereof, and relates to the field of gel electrolytes and supercapacitors. The water-retention gel electrolyte provided by the invention is prepared from an electrolyte aqueous solution and an oxygen-containing functional group polymer. The electrolyte in the electrolyte aqueous solution is one or more of lithium perchlorate, bis(trifluoromethane)sulfonimide lithium salt, lithium hexafluorophosphate, lithium nitrate, sodium chloride, sodium perchlorate, bis(trifluoromethane)sulfonimide sodium salt, sodium hexafluorophosphate, sodium nitrate, potassium chloride, potassium perchlorate, bis(trifluoromethane)sulfonimide potassium salt, potassium hexafluorophosphate and potassium nitrate. The oxygen-containing functional group polymer is one or more of polyvinylpyrrolidone, polyethylene glycol, polyvinyl alcohol, carboxymethyl cellulose and polyacrylamide. The water-based supercapacitor based on the water-retention gel electrolyte has ultra-long cycle life at the normal temperature, and can be normally charged and discharged at the high temperature of 120 DEG C and stably circulated.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
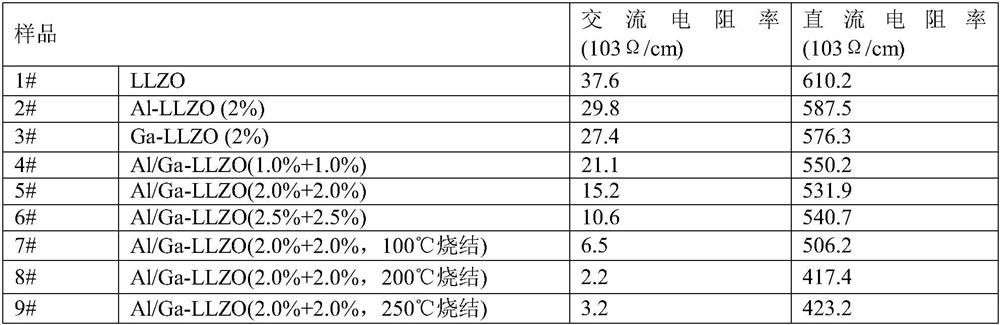
Preparation method of low-resistivity garnet type modified LLZO solid electrolyte
ActiveCN113363562AImprove mixing efficiencyImprove grain boundary conductivitySecondary cellsElectrolytesPhysical chemistryPolypropylene
The invention relates to the field of lithium batteries, and discloses a preparation method of a low-resistivity garnet type modified LLZO solid electrolyte. The method comprises the following steps: 1) adding raw materials into a three-dimensional high-energy vibration ball mill for ball milling after batching, sintering in air at 300-400 DEG C after ball milling, cooling, continuing ball milling, and performing anaerobic sintering under 300-500 Mpa and 900-1100 DEG C to prepare an Al / Ga doped modified LLZO solid electrolyte; and 2) uniformly mixing polypropylene carbonate and acetone, adding lithium perchlorate for fully dissolving, adding the Al / Ga doped modified LLZO solid electrolyte, continuously performing ultrasonic mixing uniformly, and sintering a finished product at a low temperature of 100-250 DEG C under 300-400 Mpa. The method disclosed by the invention can be used for remarkably reducing the actual resistivity of the LLZO solid electrolyte and is beneficial to application of the LLZO solid electrolyte in a solid battery.
Owner:WANXIANG 123 CO LTD
High-temperature-resistant high-voltage electrolyte of high-nickel lithium ion battery
PendingCN112216870AReduce exposureAvoid decompositionSecondary cellsOrganic electrolytesDifluorophosphateElectrolytic agent
A high-temperature-resistant high-voltage electrolyte of a high-nickel lithium ion battery is composed of a composite electrolyte lithium salt, an organic multi-component solvent and an additive, andthe composite electrolyte lithium salt is at least two of lithium hexafluorophosphate, lithium perchlorate, lithium difluorophosphate, lithium bis (fluorosulfonyl) imide, lithium bis (oxalato) borate,lithium difluoro (oxalato) borate, lithium difluoro (oxalato) phosphate and the like. The electrolyte lithium salt and the additive in the electrolyte participate in positive electrode film formationat the same time to hinder contact between the electrolyte and an electrode, so that decomposition reaction between the electrolyte in a high-potential region and an active substance is inhibited, and the storage and cycle performance of the lithium ion battery at high temperature and high voltage is improved.
Owner:HUNAN AEROSPACE MAGNET & MAGNETO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com