Method for producing anhydrous lithium perchlorate
A technology of lithium perchlorate and production method, applied in perchloric acid, perchlorate and other directions, can solve the problems of high energy consumption, high water content, large equipment investment, etc., and achieves easy control, simple process and stable pH value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
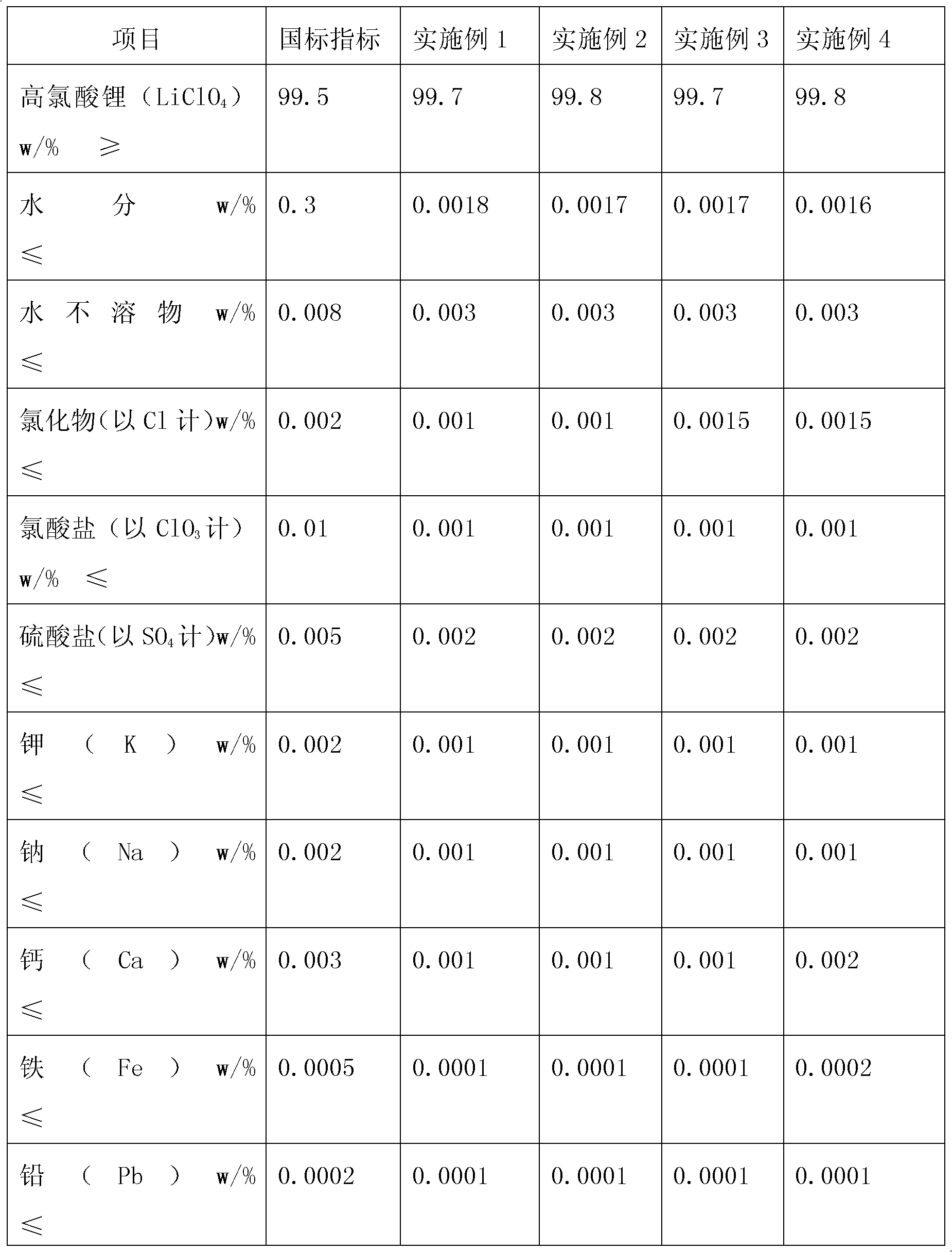
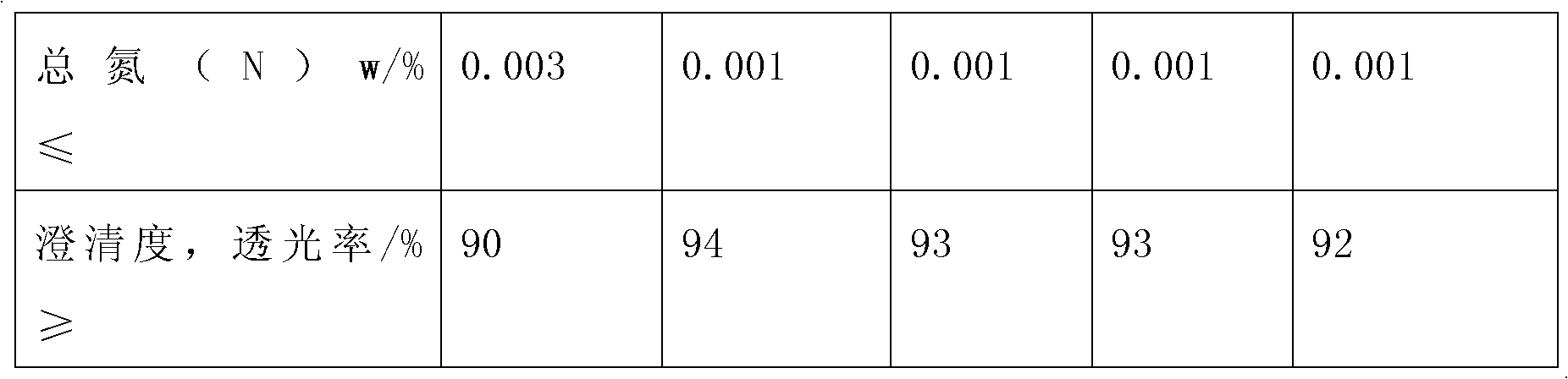
Examples
Embodiment 1
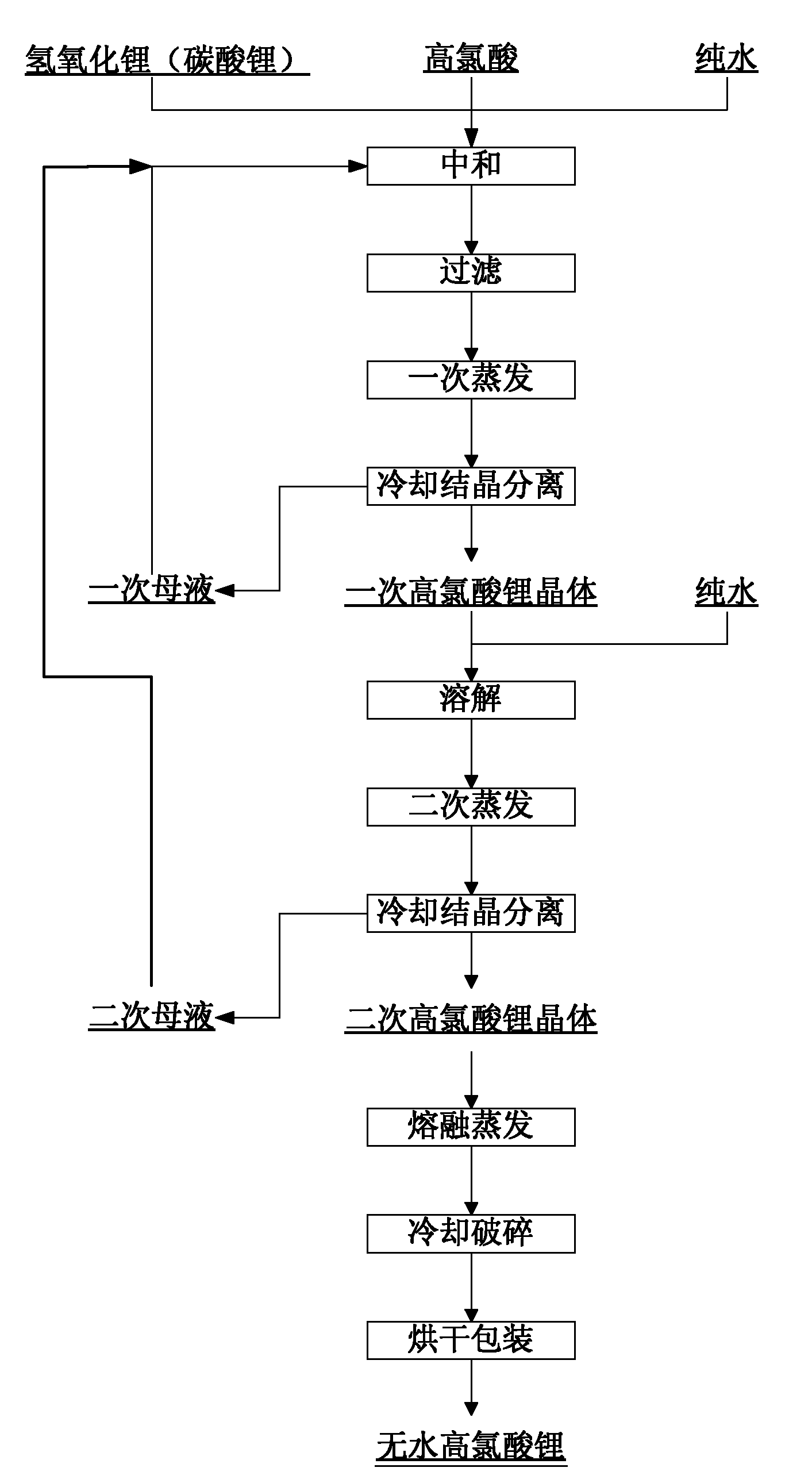
[0021] see figure 1 , a kind of production method of anhydrous lithium perchlorate, the steps are as follows:
[0022] 1. Add 75kg of industrial lithium hydroxide to 150L pure water and stir, then slowly add 252kg of perchloric acid under stirring, after the addition, adjust the pH value of the reaction system to 7 with lithium hydroxide or perchloric acid, wait for 5 Minutes, after detecting that the pH value has no change, filter while hot, remove insoluble impurities, and obtain lithium perchlorate clean solution;
[0023] 2. Heating and evaporating the obtained lithium perchlorate clean liquid, the evaporation end temperature is 120°C, then stirring and cooling to 20°C, after the cooling is completed, use a centrifuge to separate, and after separation, 199kg of primary lithium perchlorate trihydrate crystals and 131L primary mother liquor, the primary mother liquor can be kept for returning to the neutralization process;
[0024] 3. Add 199kg of primary trihydrate lithiu...
Embodiment 2
[0028] see figure 1 , a kind of production method of anhydrous lithium perchlorate, the steps are as follows:
[0029] 1. Add 75kg of industrial lithium hydroxide to 150L of pure water and stir, then slowly add 252kg of perchloric acid under stirring, after the addition, adjust the pH value of the reaction system to 7.2 with lithium hydroxide or perchloric acid, wait for 5 Minutes, after detecting that the pH value has no change, filter while hot, remove insoluble impurities, and obtain lithium perchlorate clean solution;
[0030] 2. Heat and evaporate the obtained lithium perchlorate clean liquid, the evaporation end point temperature is 130°C, then stir and cool to 30°C, after the cooling is completed, separate with a centrifuge to obtain 206kg primary trihydrate lithium perchlorate crystals and 113L primary mother liquor, the primary mother liquor can be retained to be returned to the neutralization process;
[0031] 3. Add 206kg primary trihydrate lithium perchlorate cry...
Embodiment 3
[0035] see figure 1 , a kind of production method of anhydrous lithium perchlorate, the steps are as follows:
[0036] 1. Add 66kg of industrial lithium carbonate to 198L of pure water and stir, then slowly add 252kg of perchloric acid under stirring, after the addition, adjust the pH value of the reaction system to 7.2 with lithium hydroxide or perchloric acid, and wait for 5 minutes , filter while hot after detecting that the pH value has no change, remove insoluble impurities, and obtain lithium perchlorate clean solution;
[0037] 2. Heating and evaporating the obtained lithium perchlorate clean liquid, the evaporation end point temperature is 130°C, then stirring and cooling to 30°C, after the cooling is completed, use a centrifuge to separate, and after separation, 207kg of primary lithium perchlorate trihydrate crystals and 110L primary mother liquor, the primary mother liquor can be kept for returning to the neutralization process;
[0038] 3. Add 207kg primary trihy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com