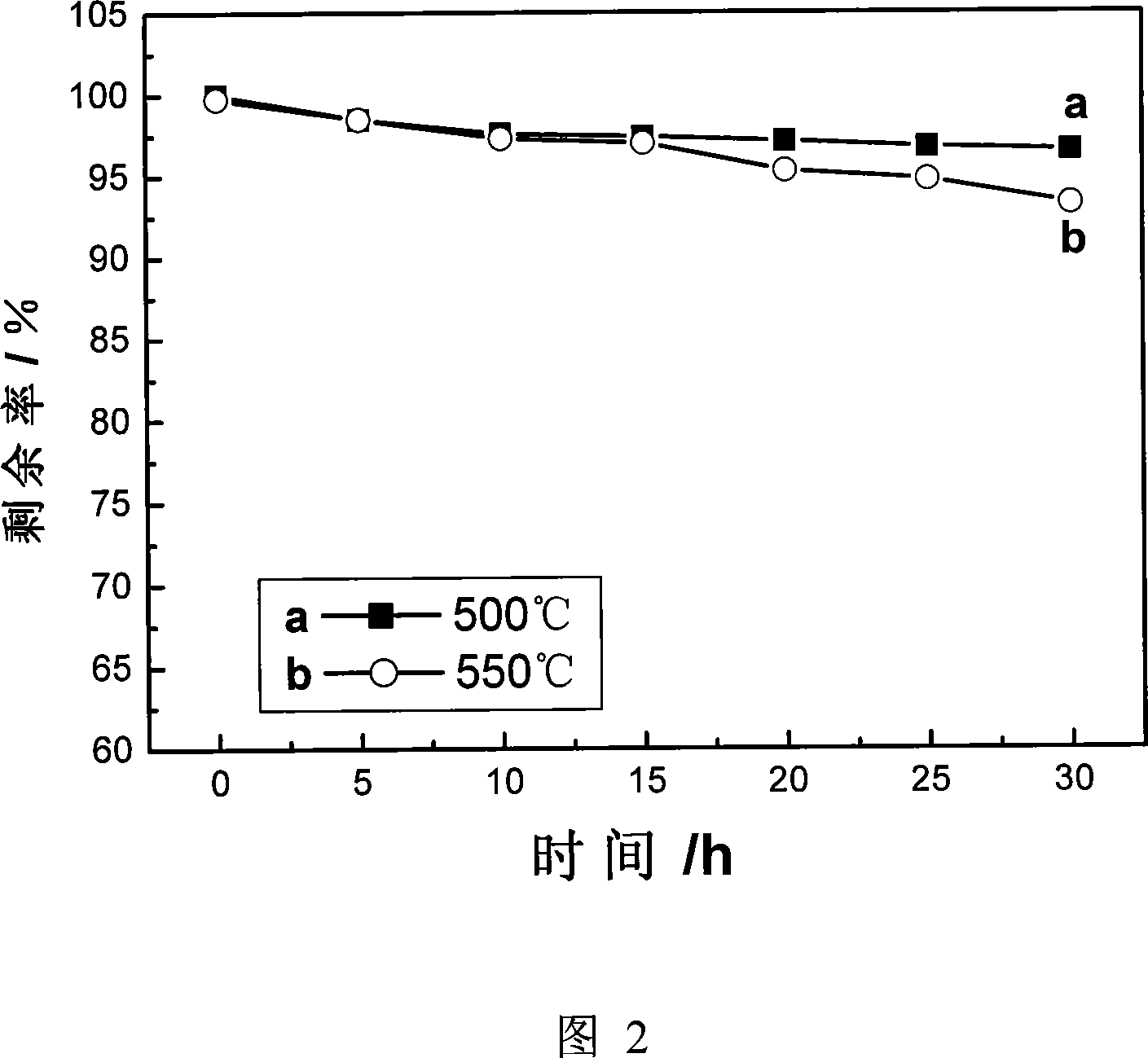
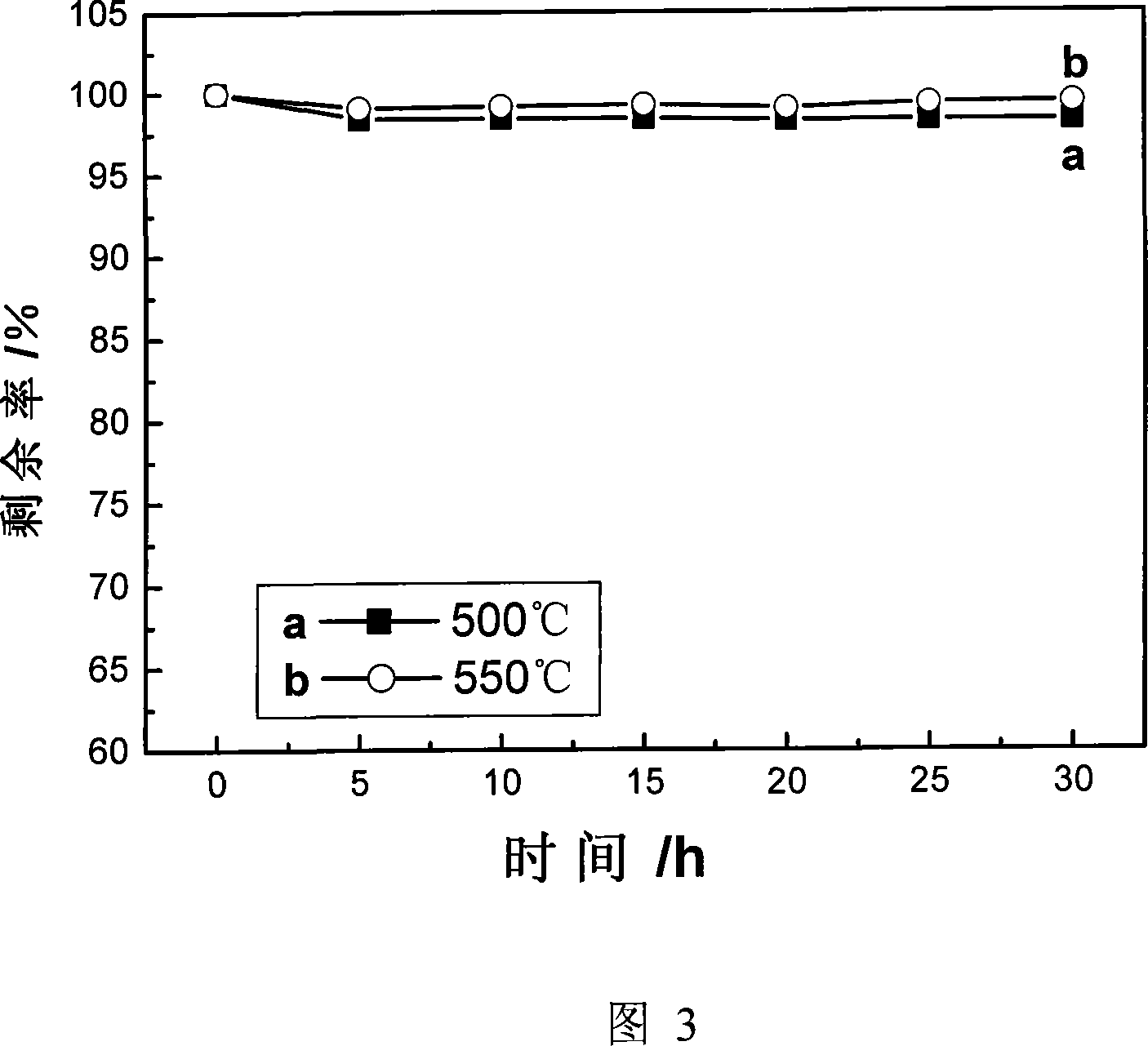
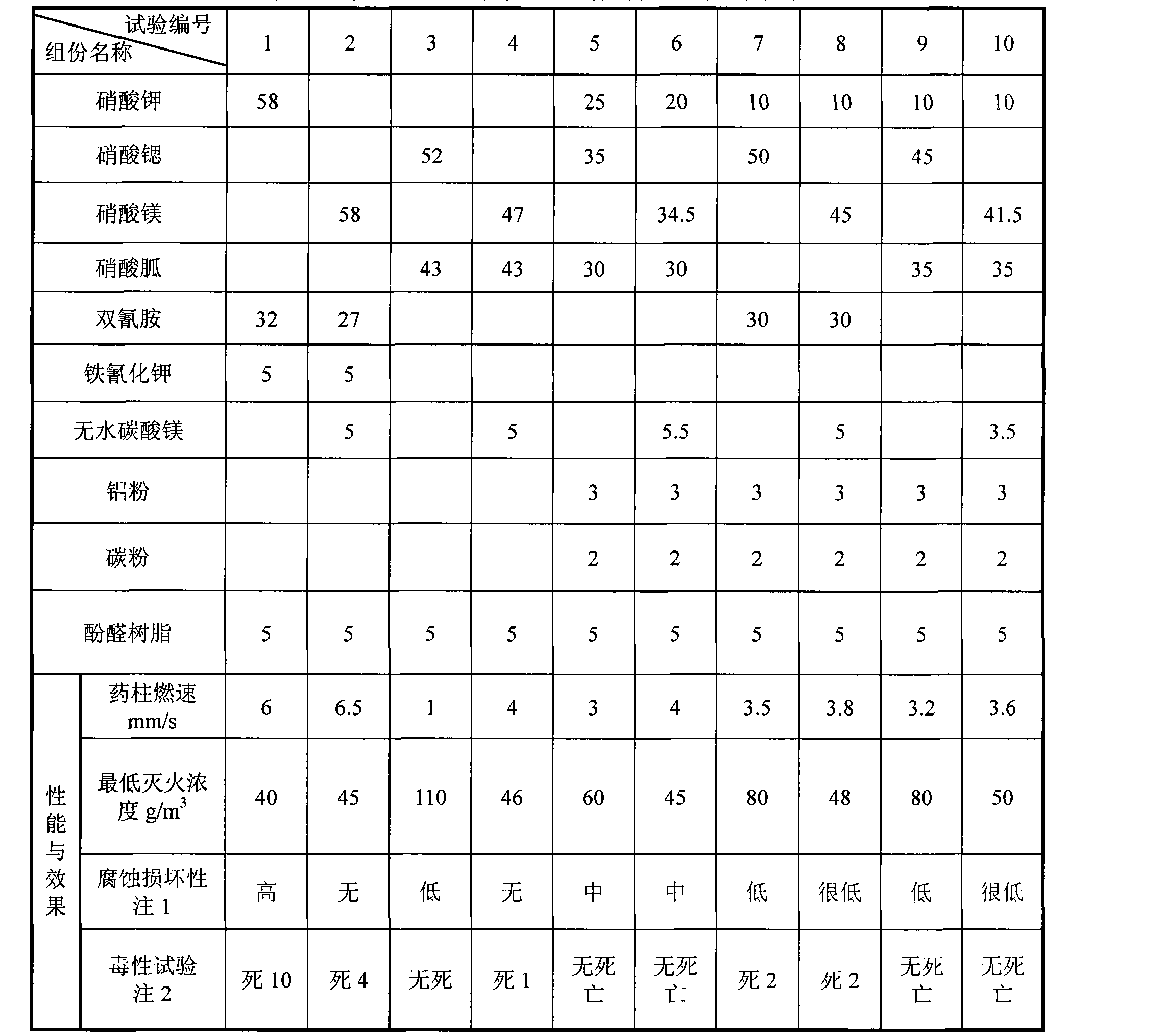
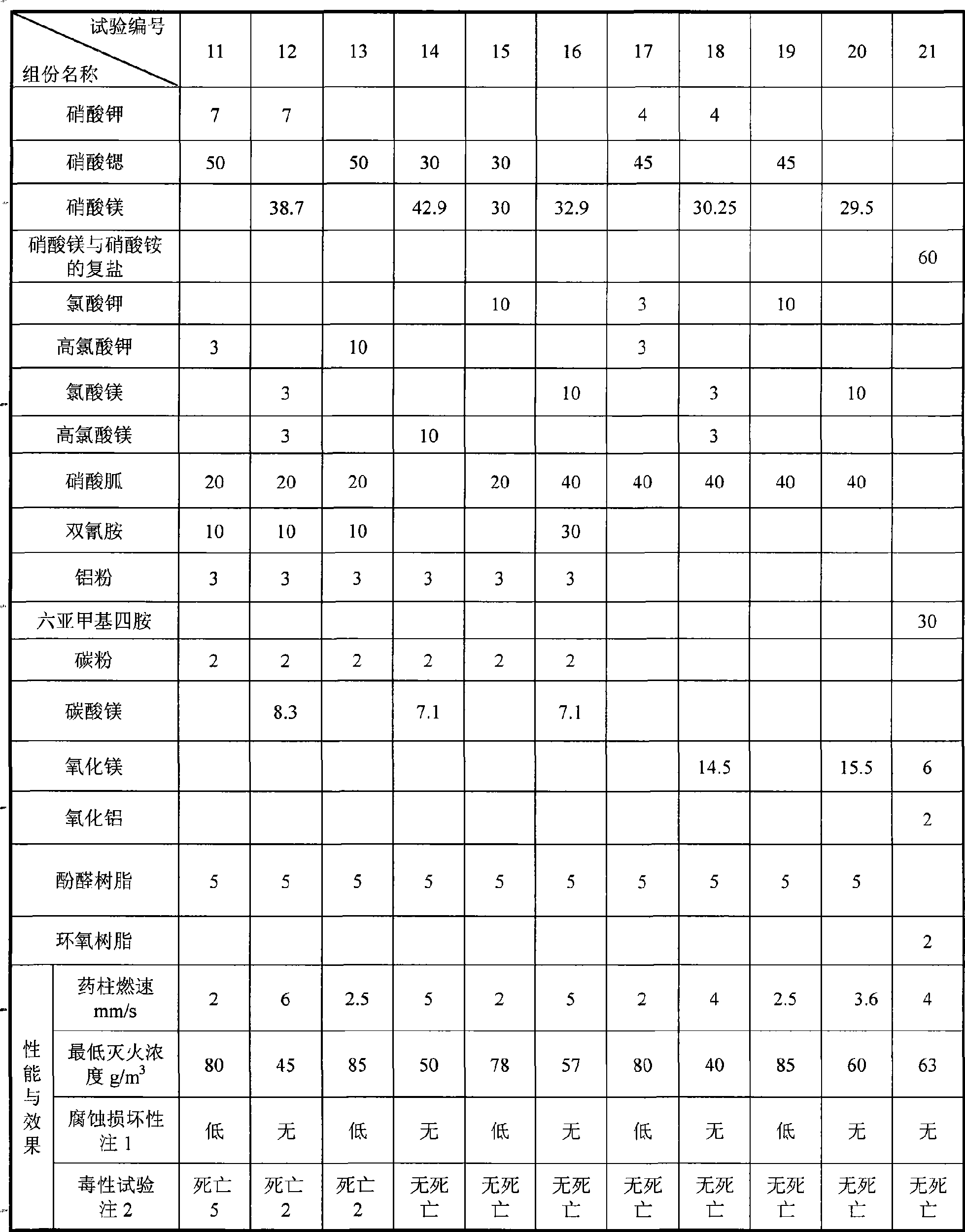
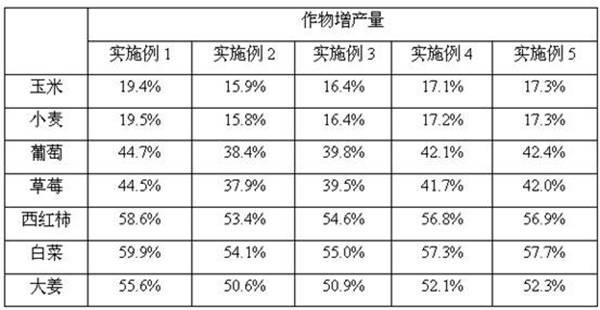
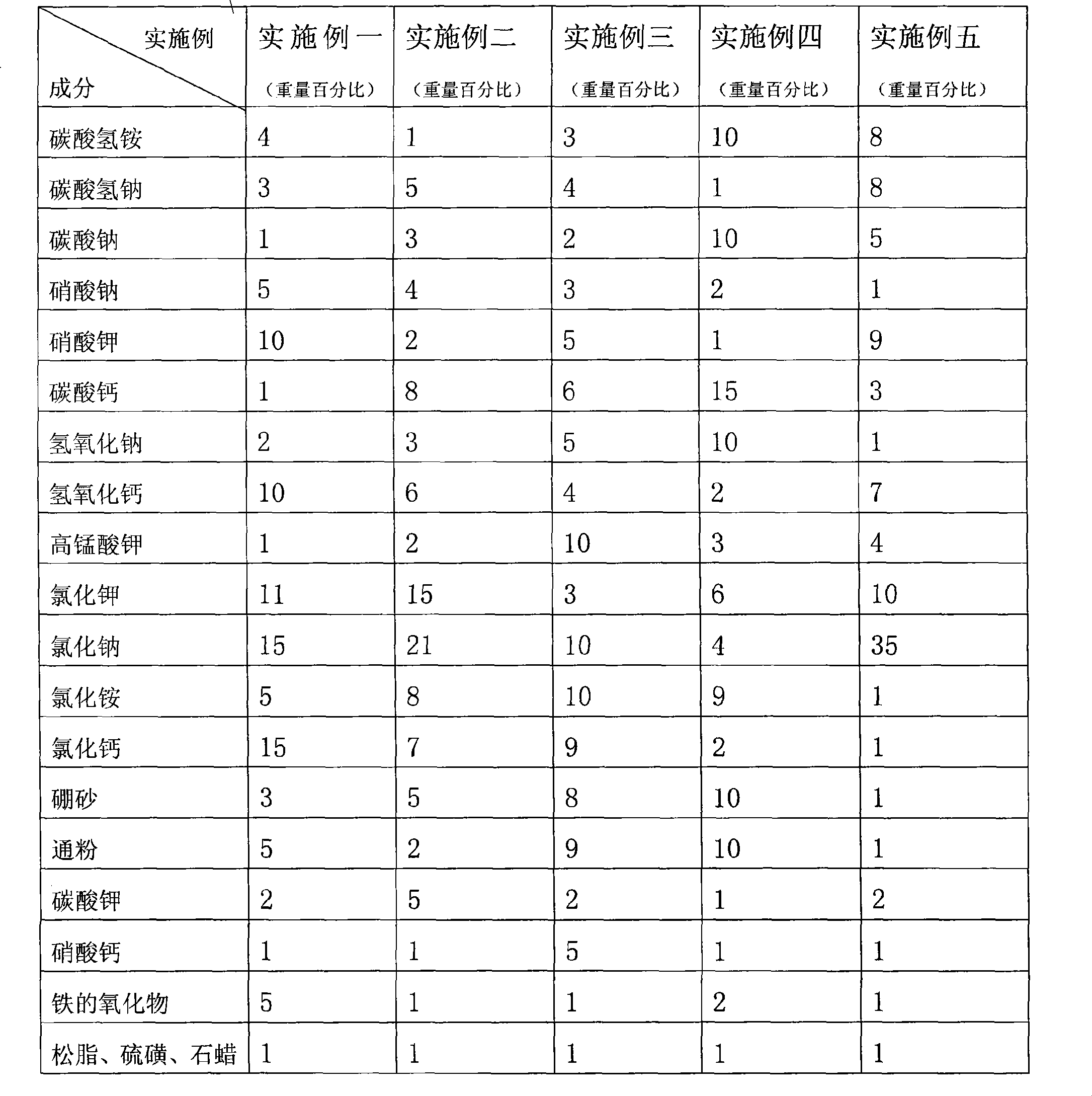
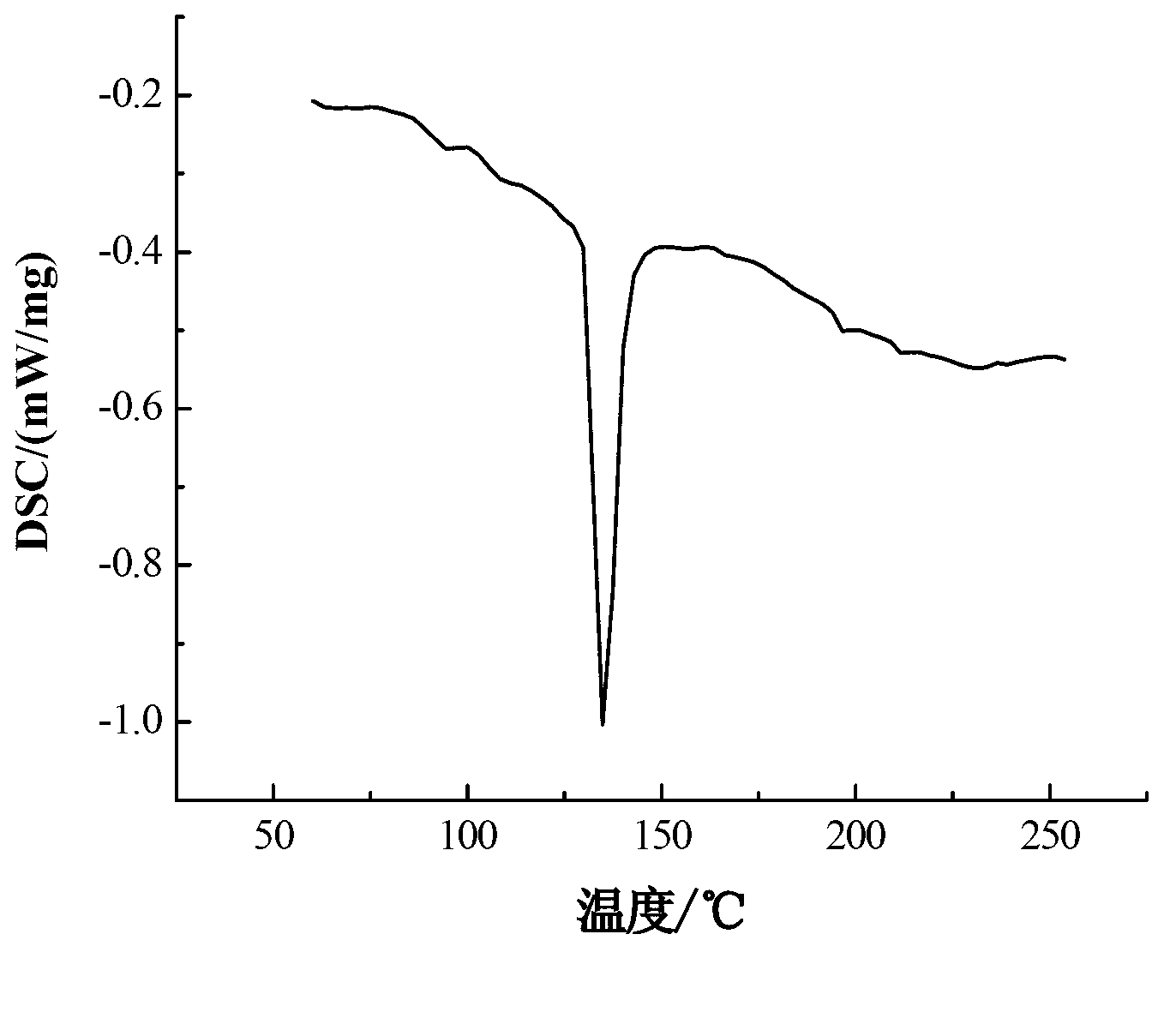
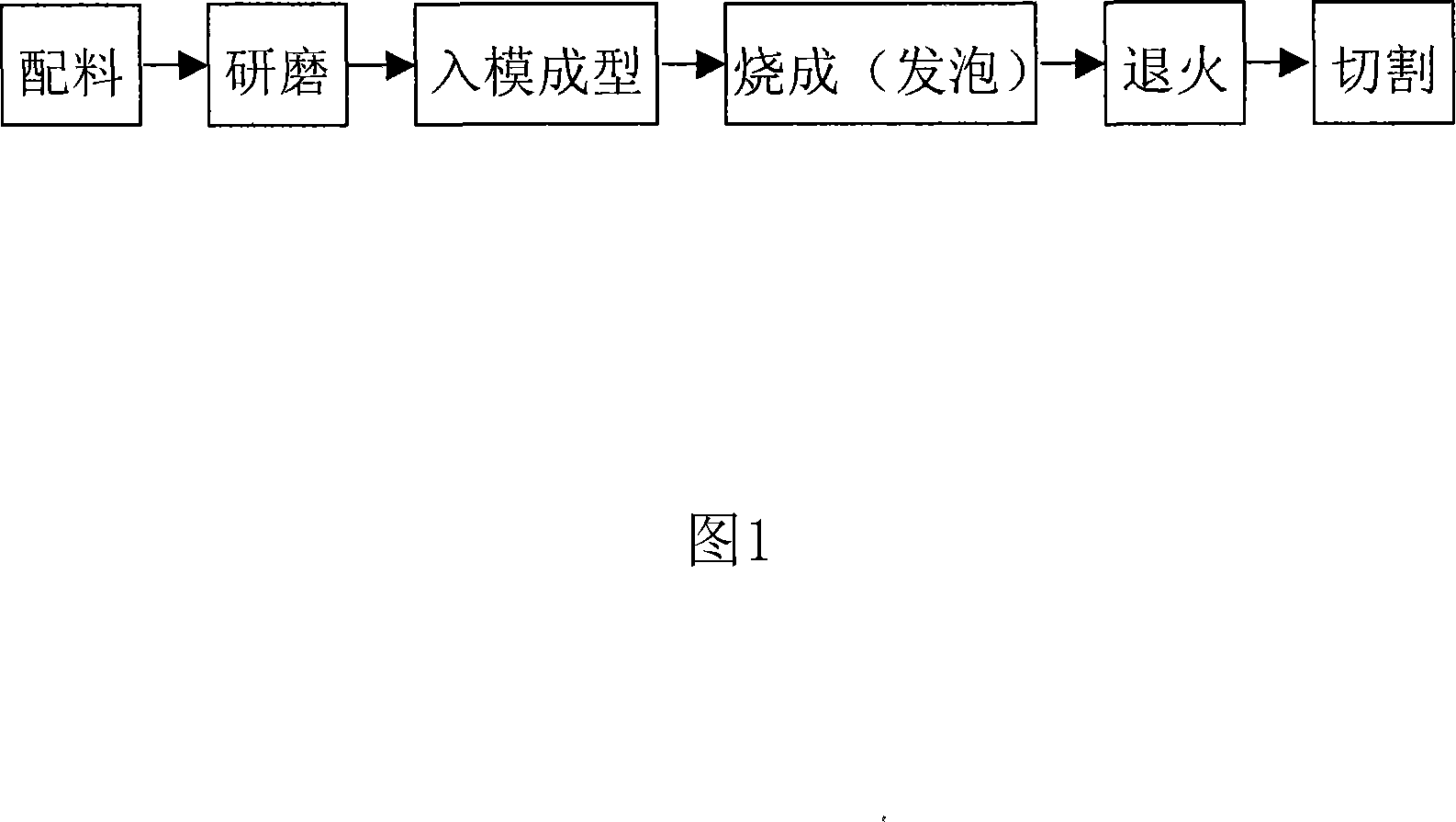
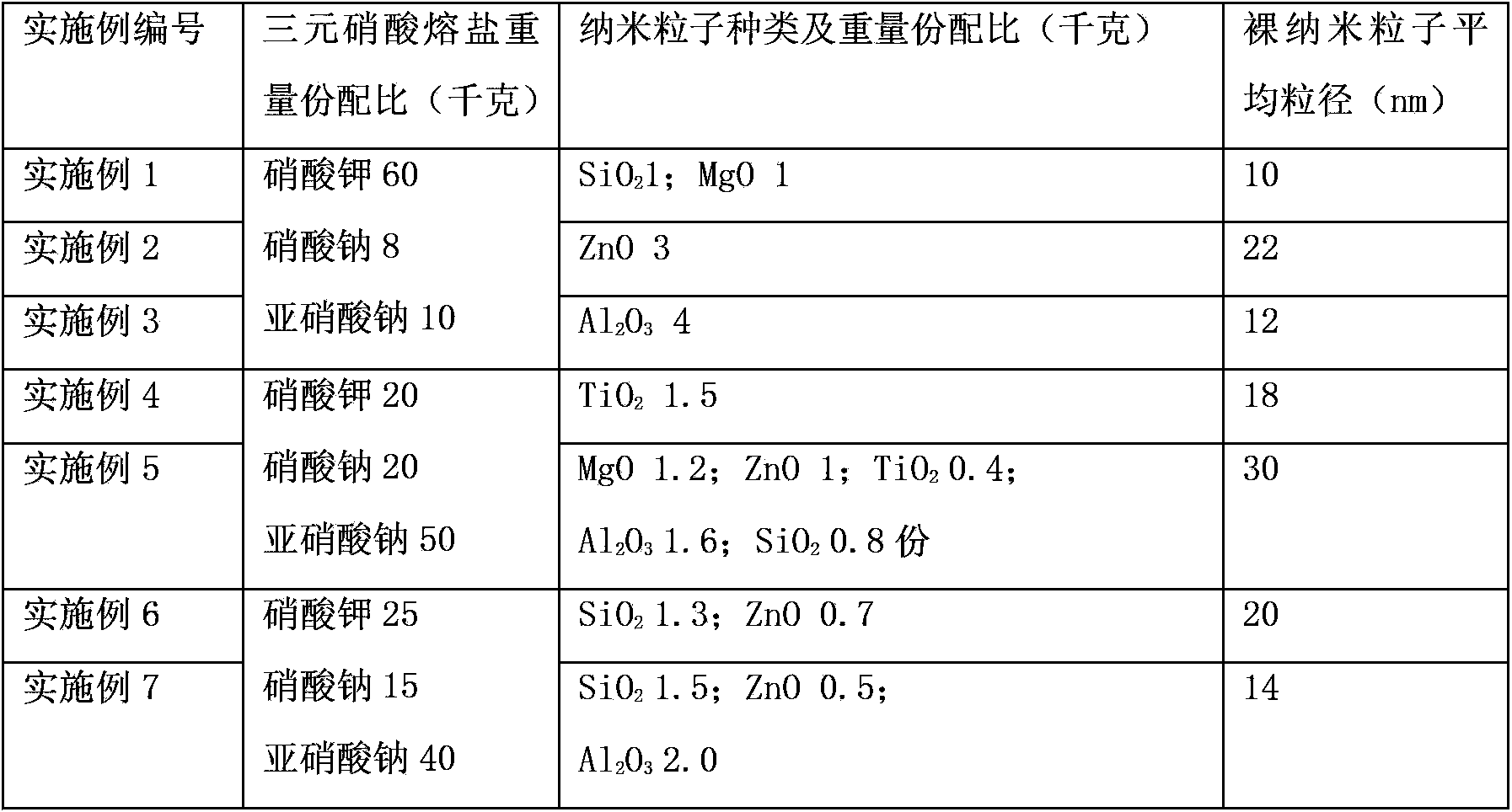
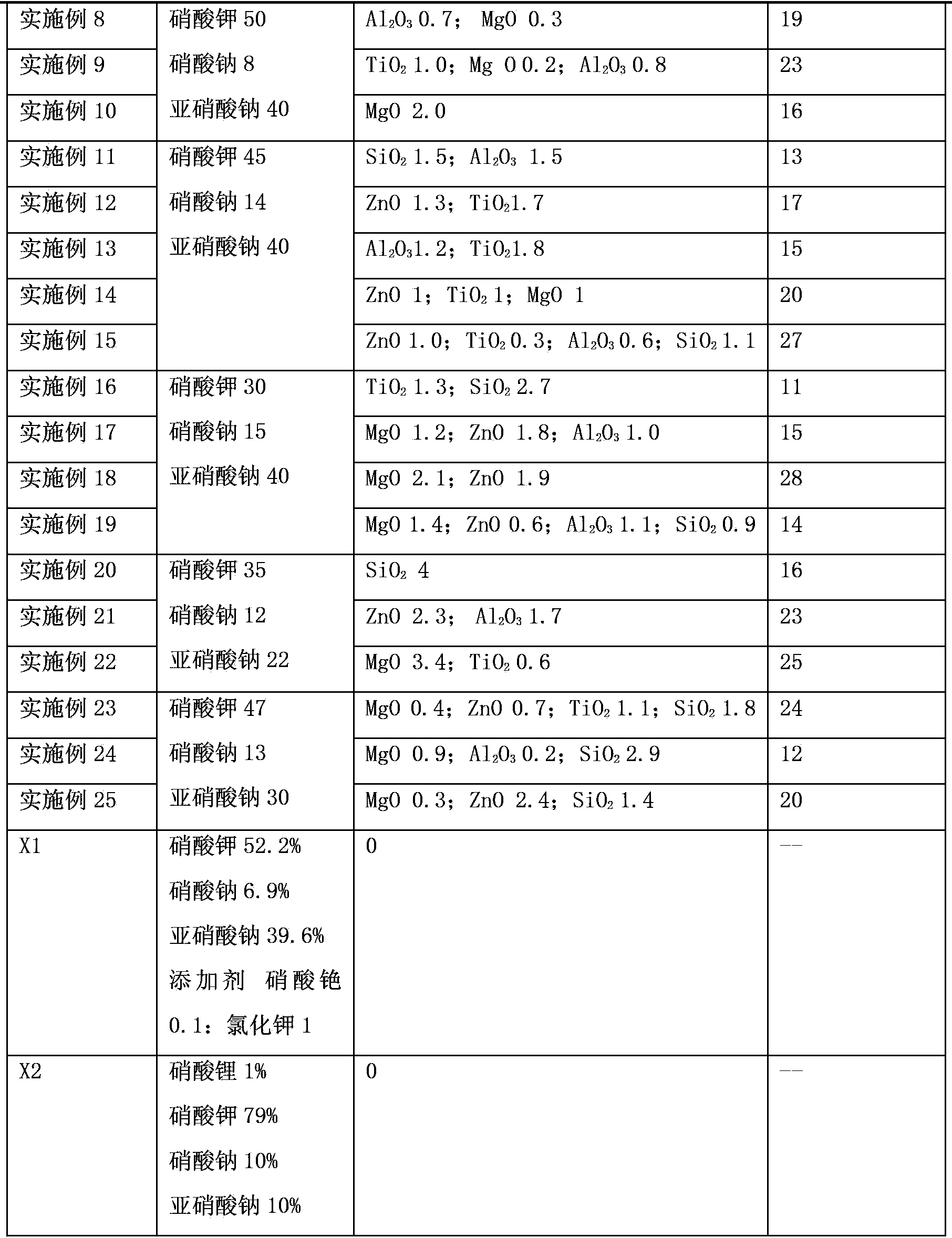
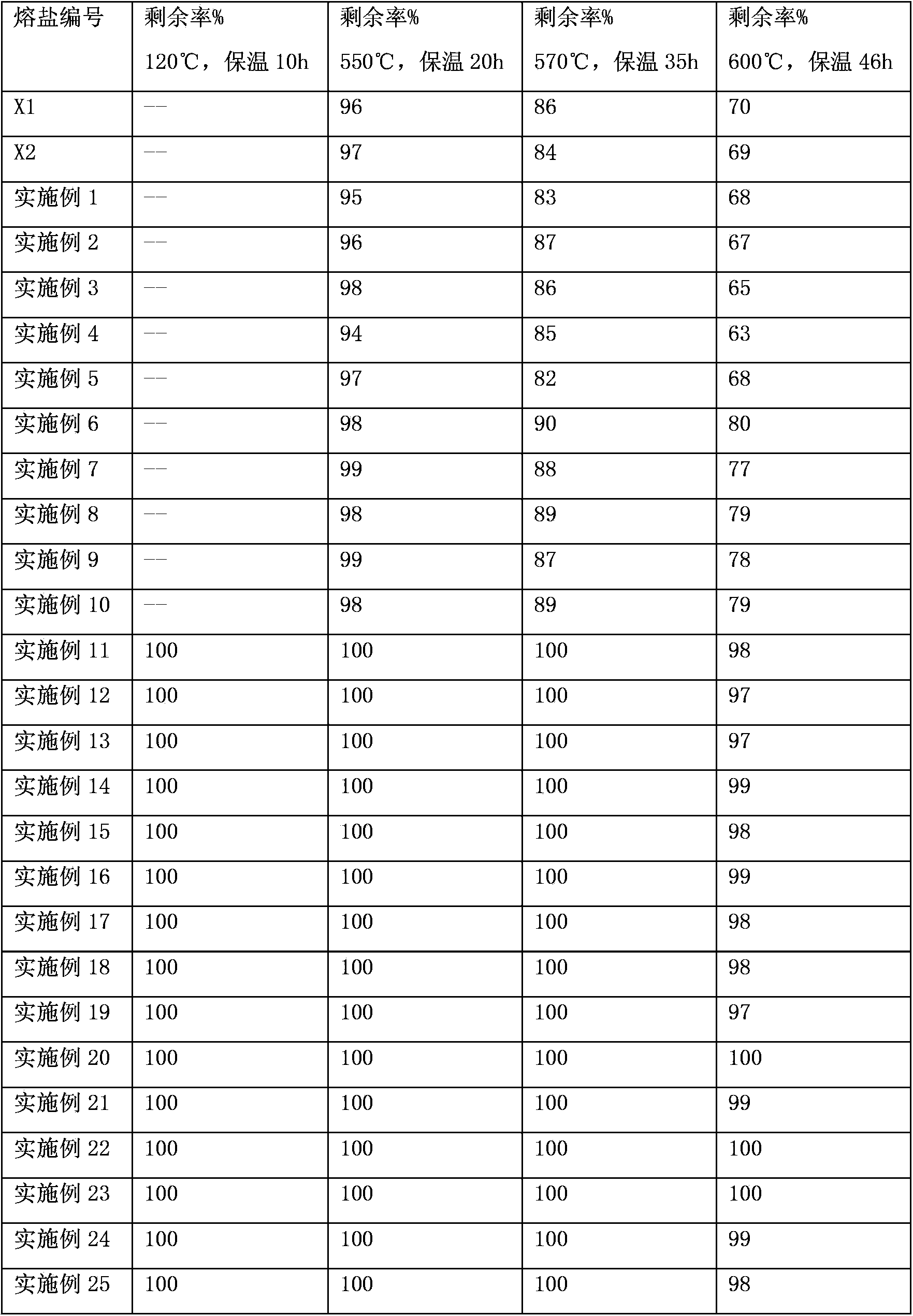
Patents
Literature
4974 results about "Potassium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potassium nitrate is a chemical compound with the chemical formula KNO₃. It is an ionic salt of potassium ions K⁺ and nitrate ions NO₃⁻, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter. It is a source of nitrogen, from which it derives its name. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter or saltpetre.
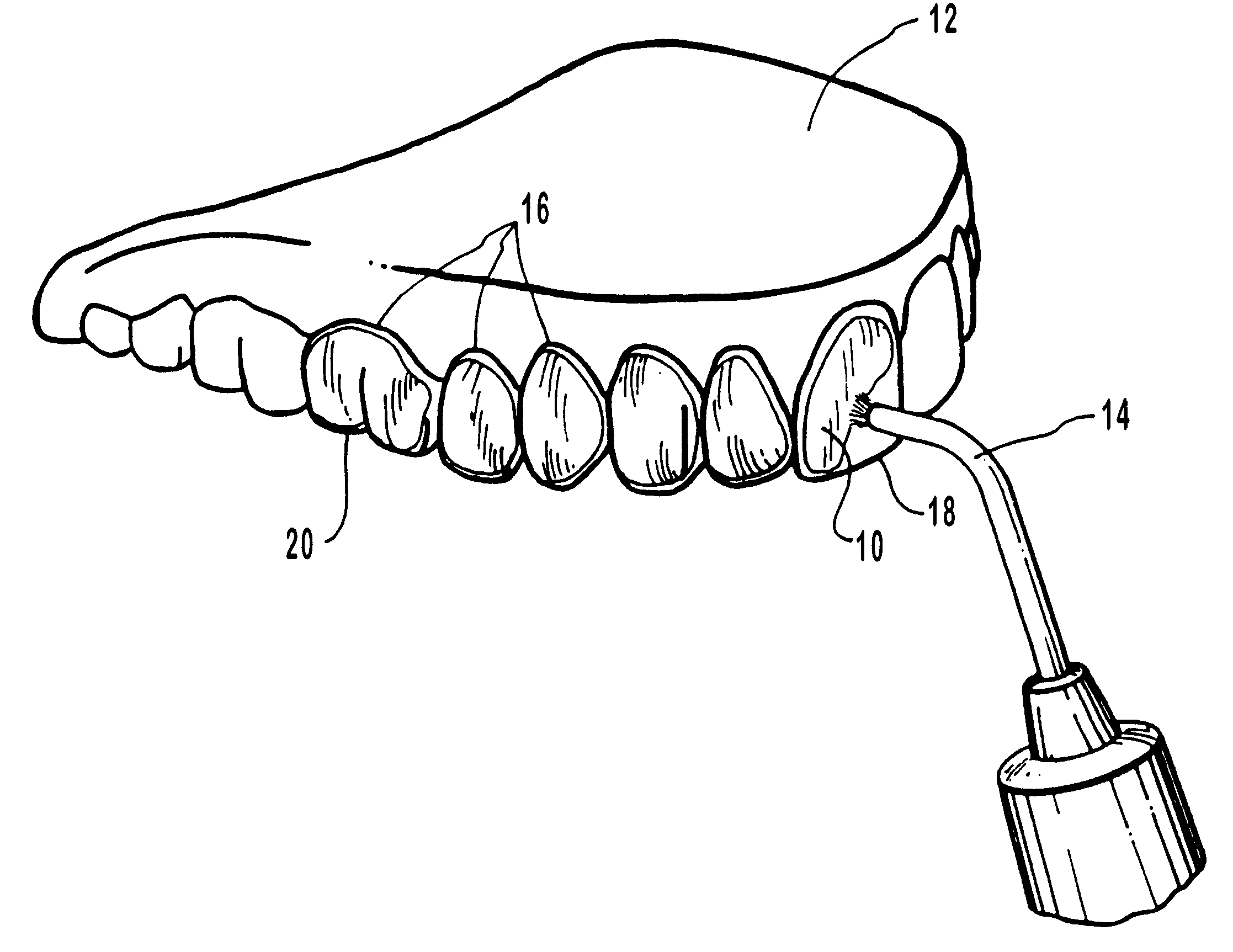
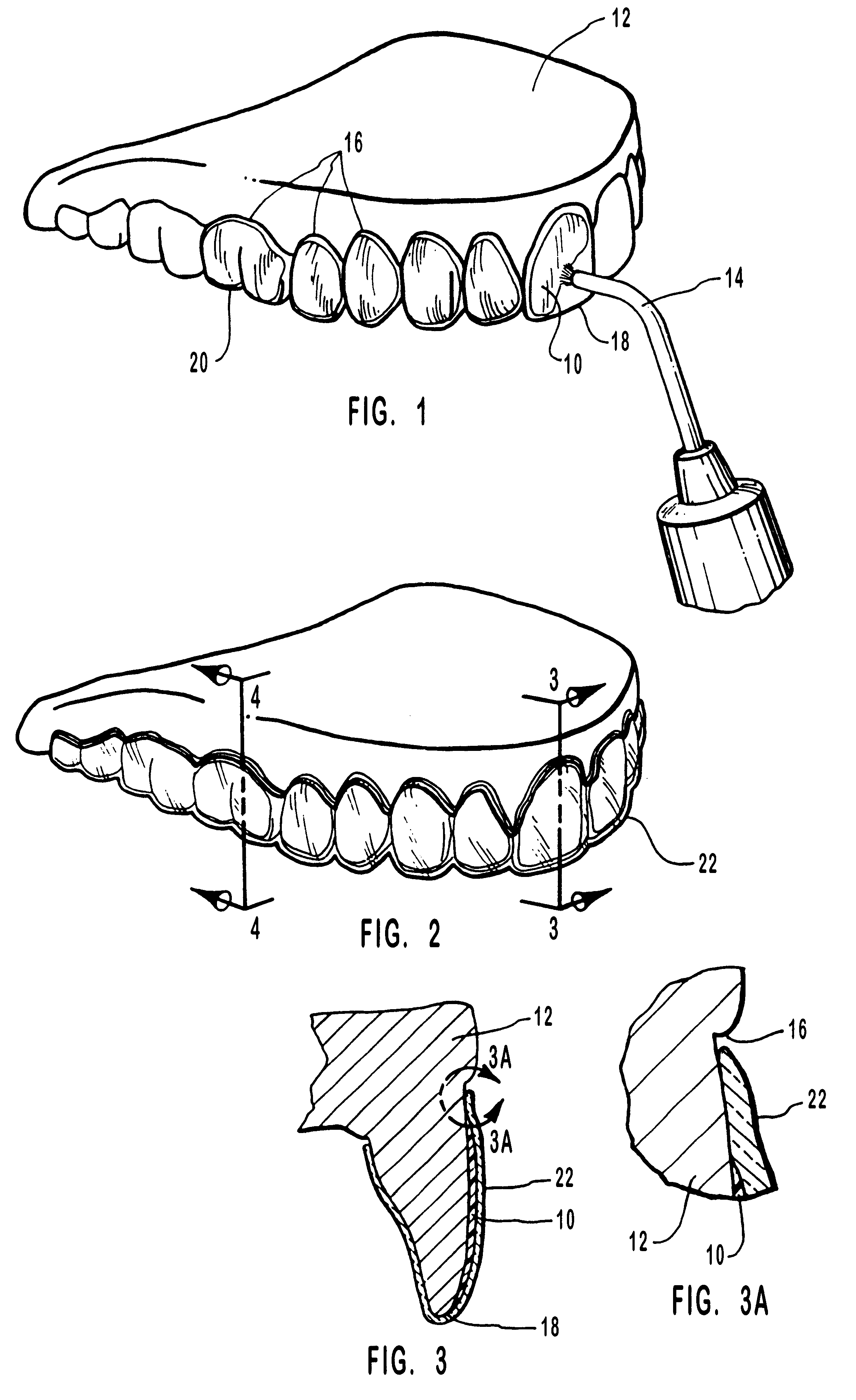
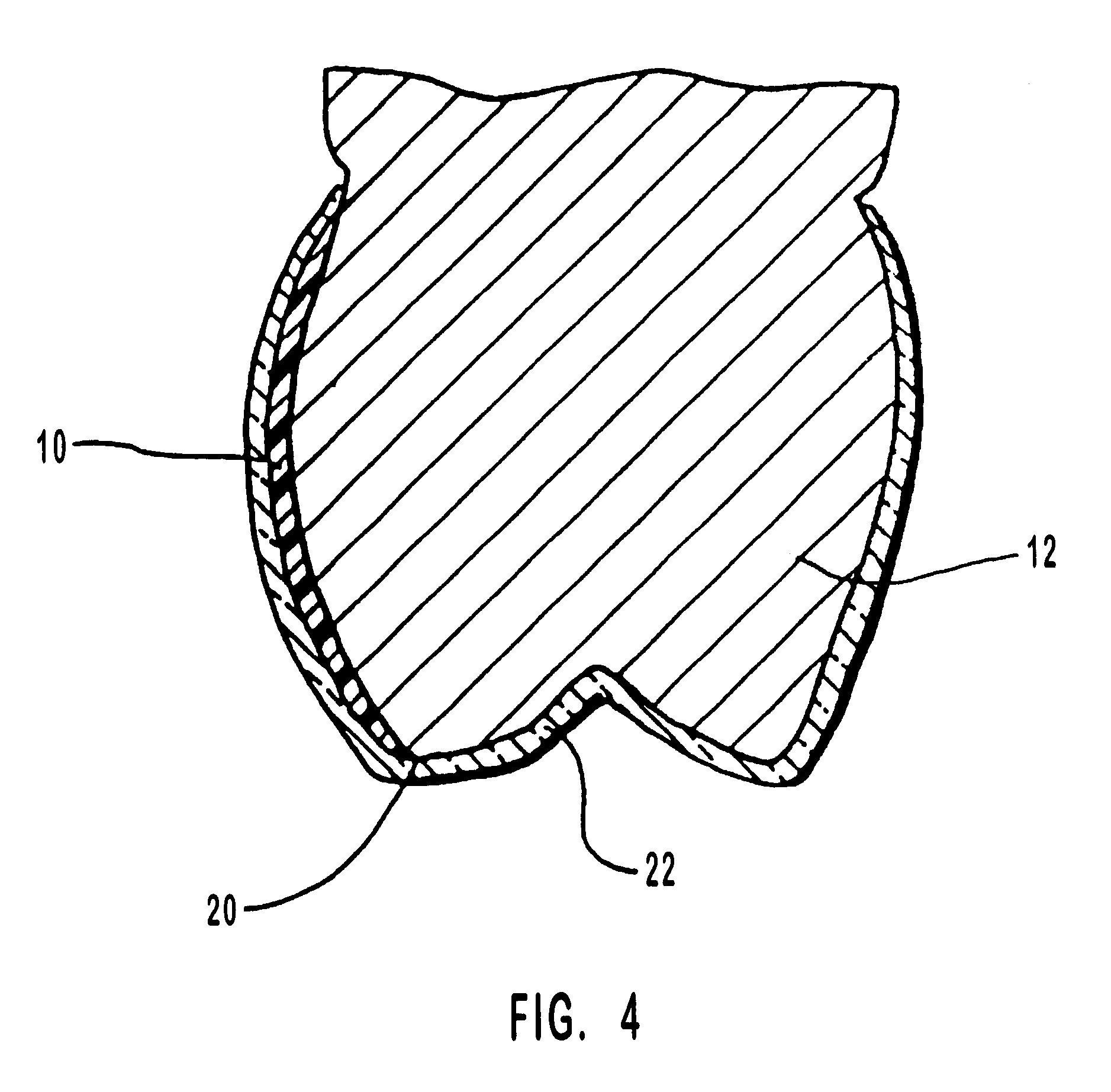
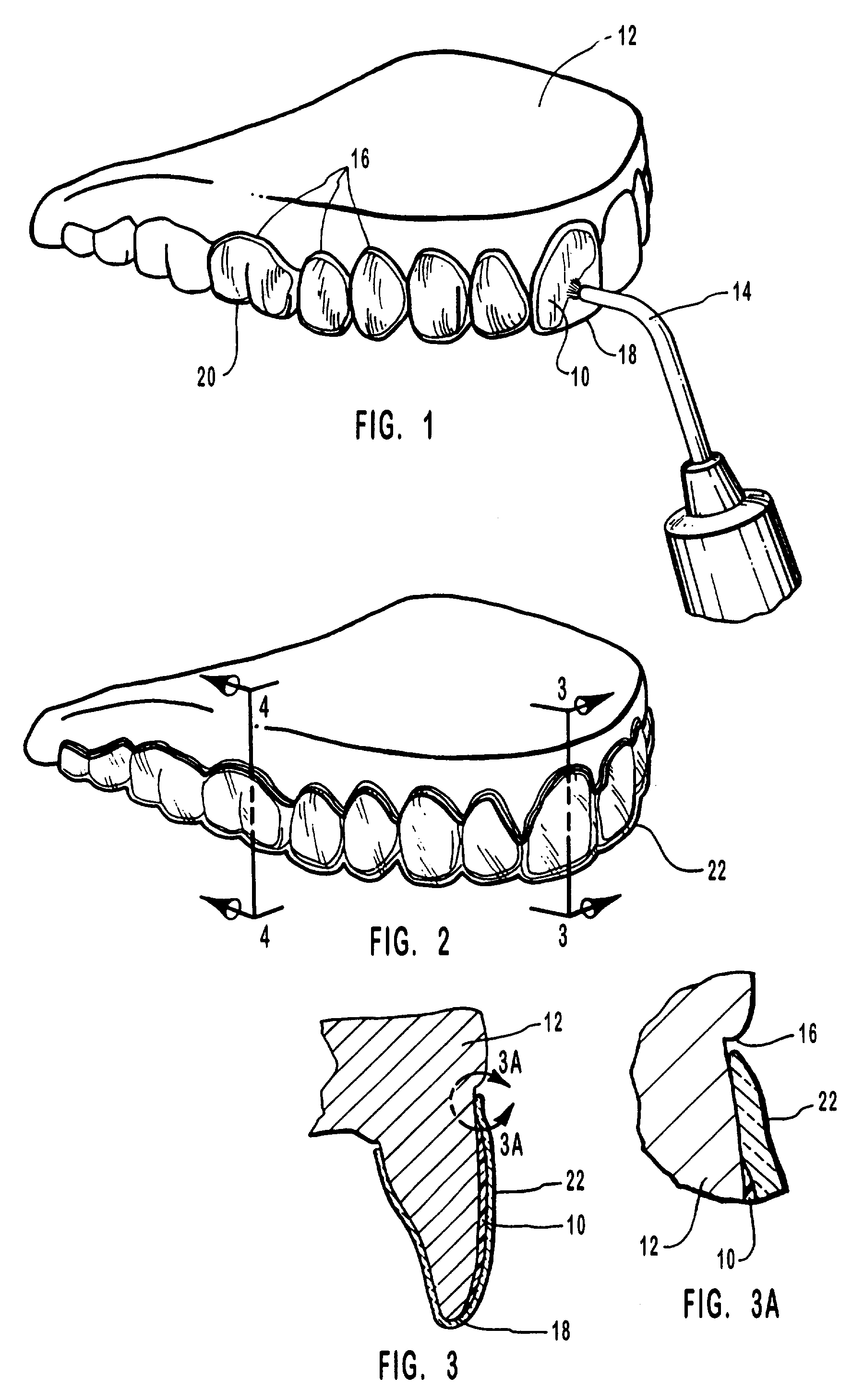
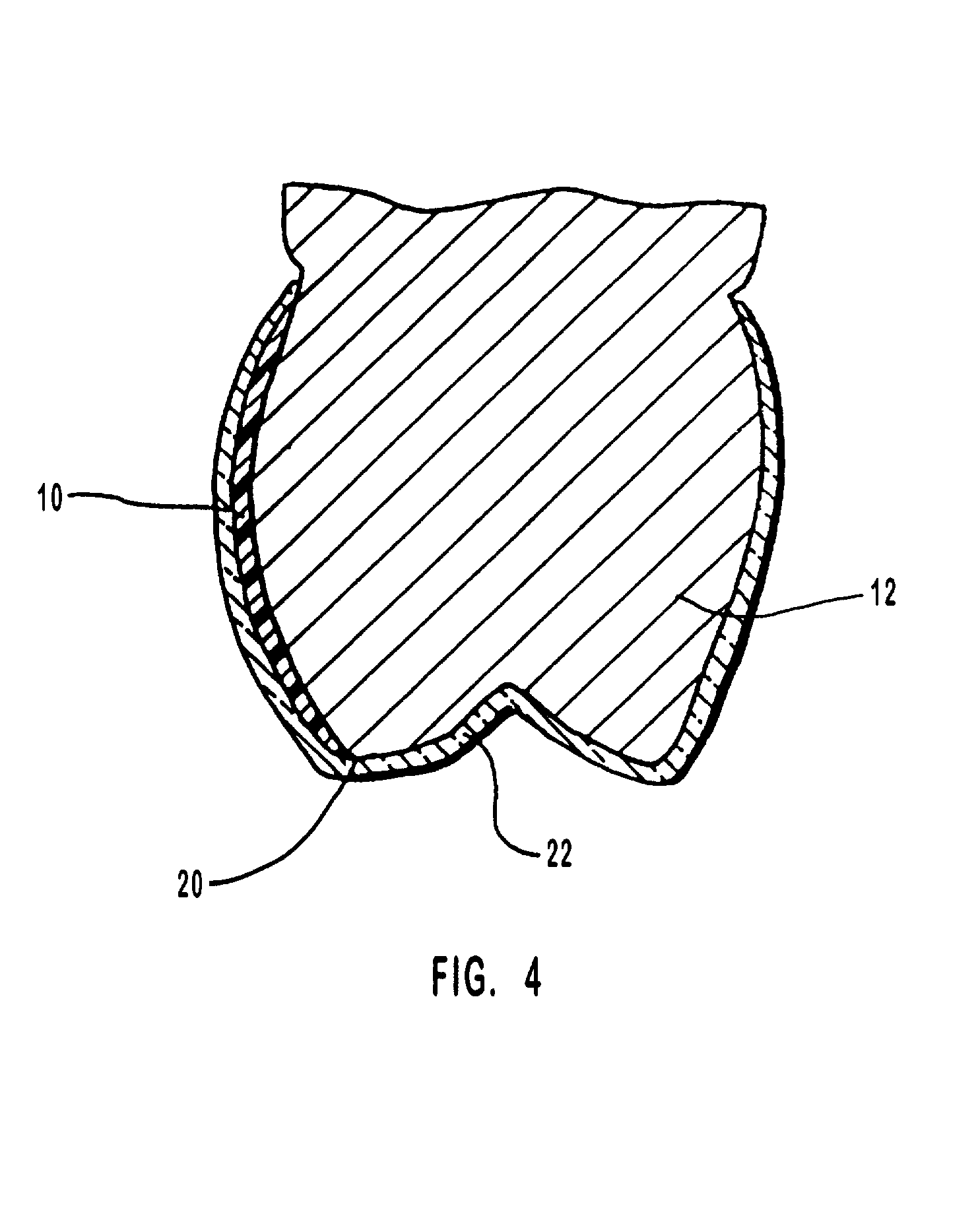
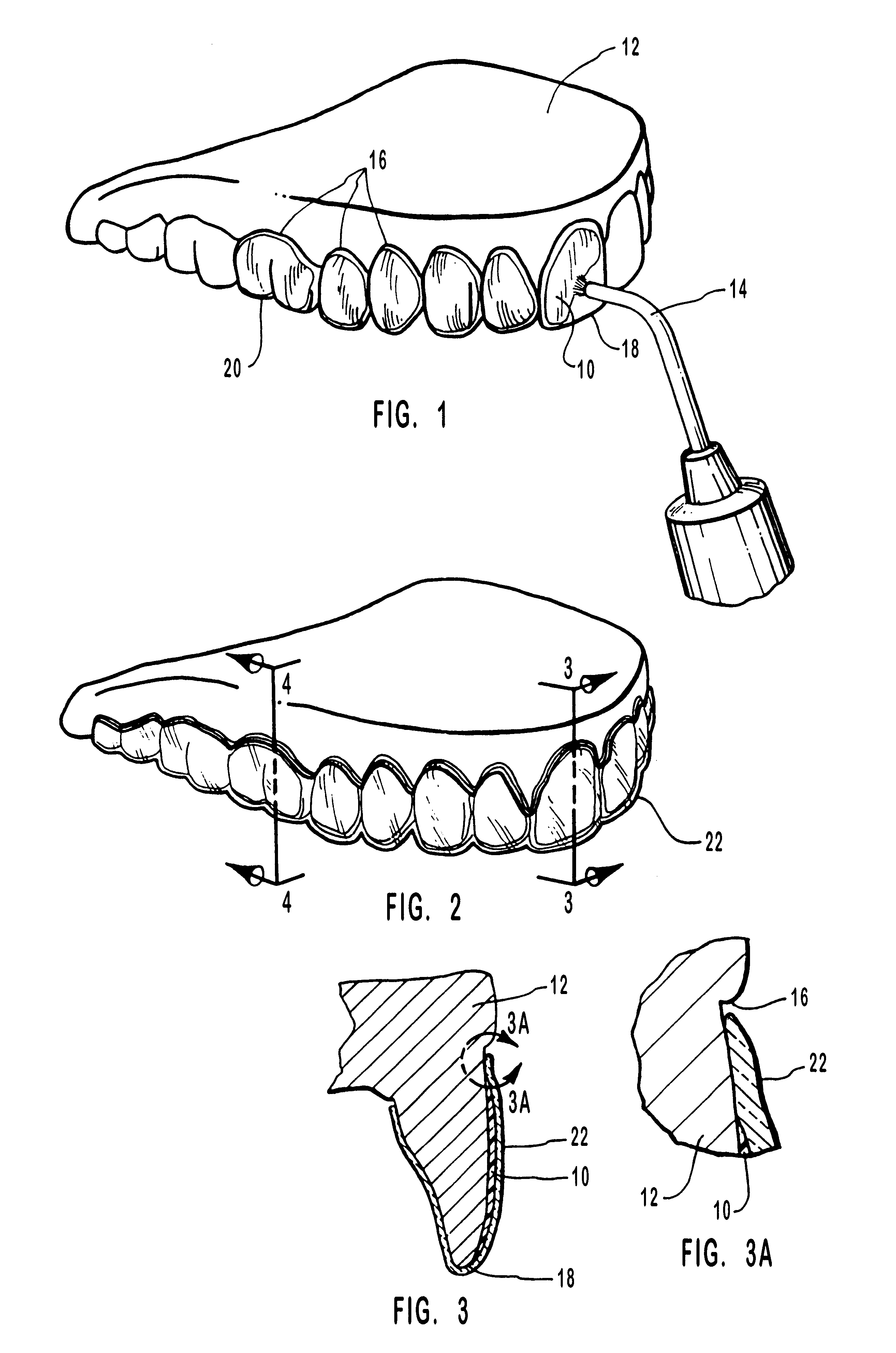
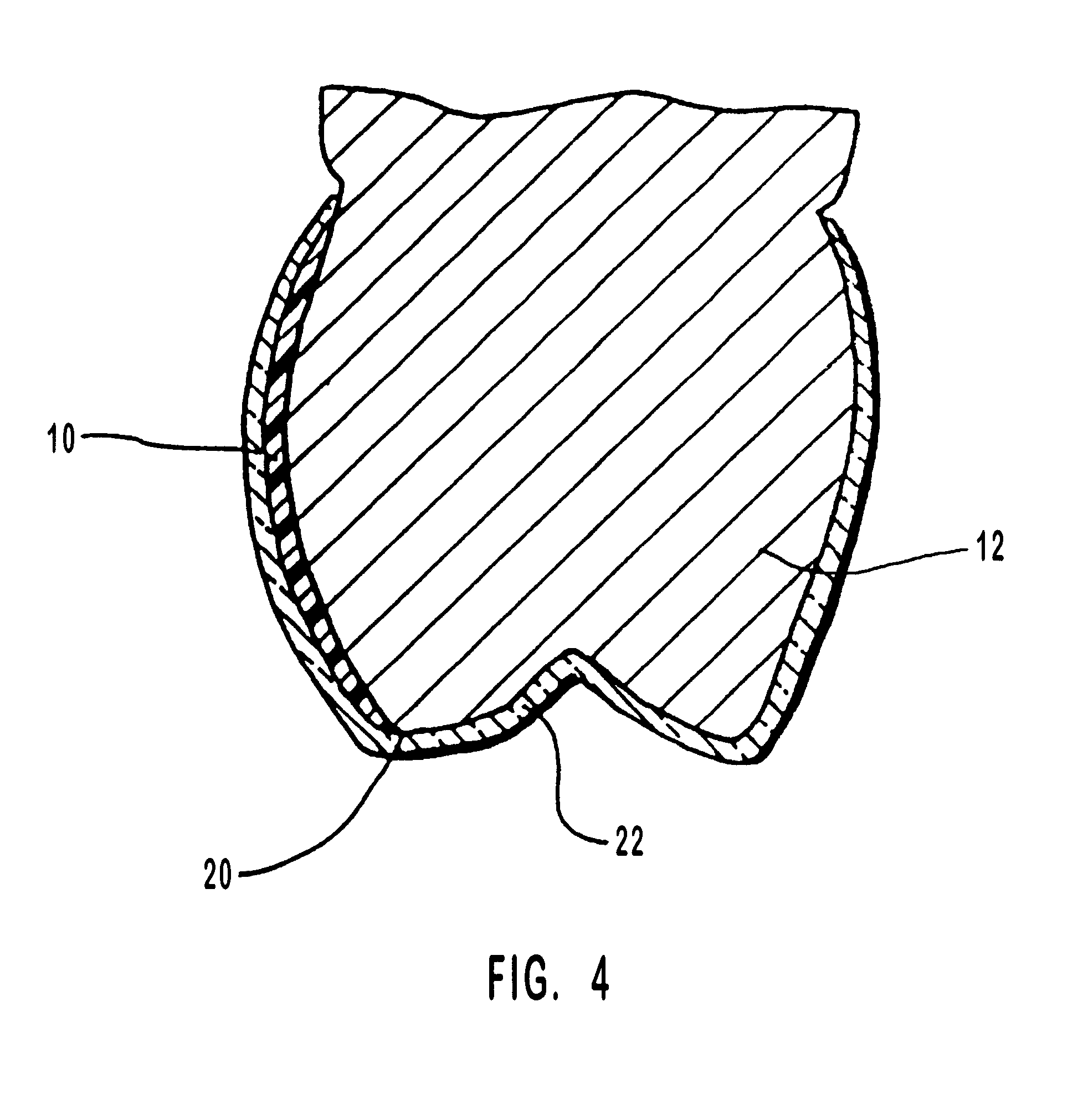
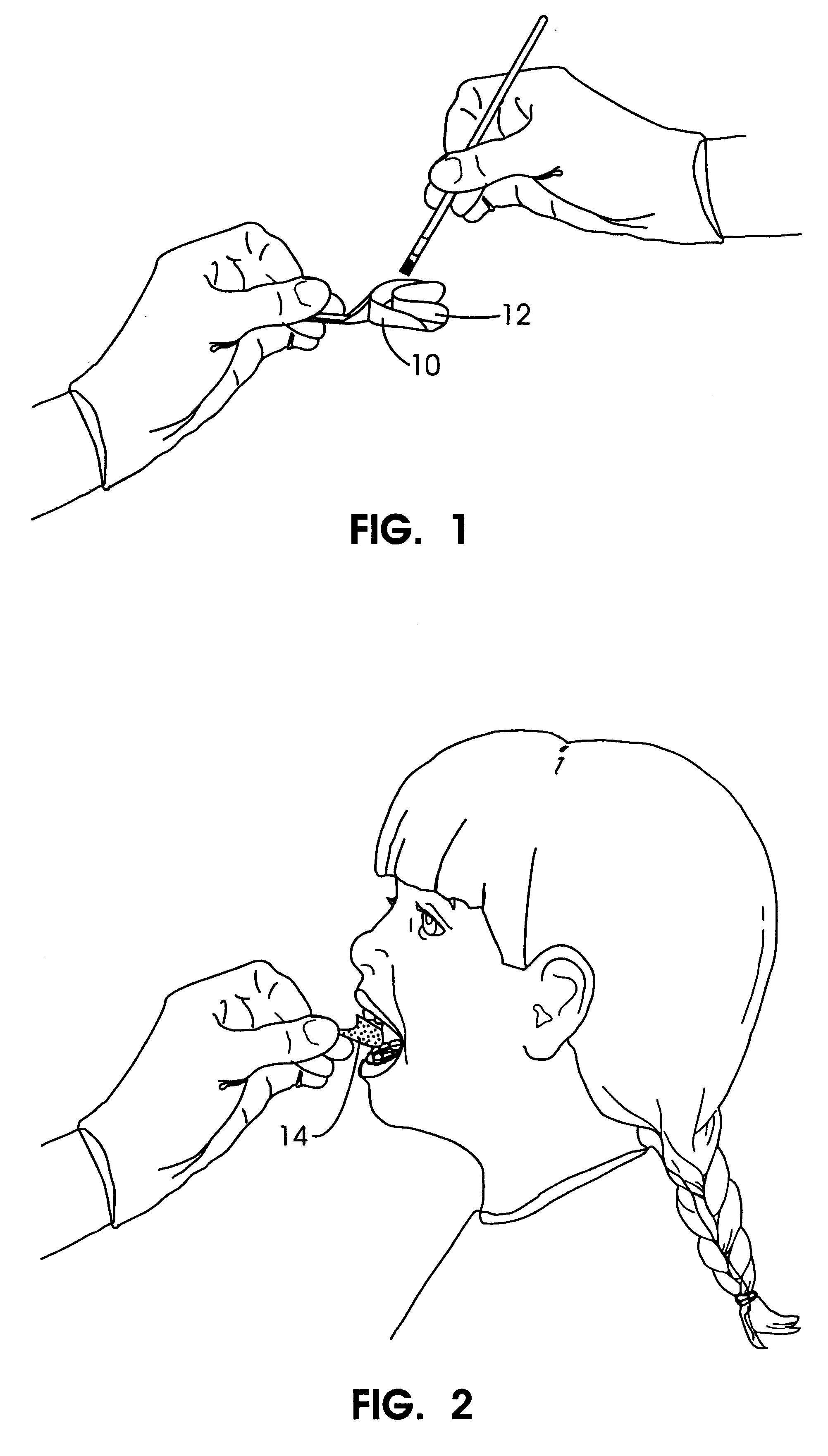
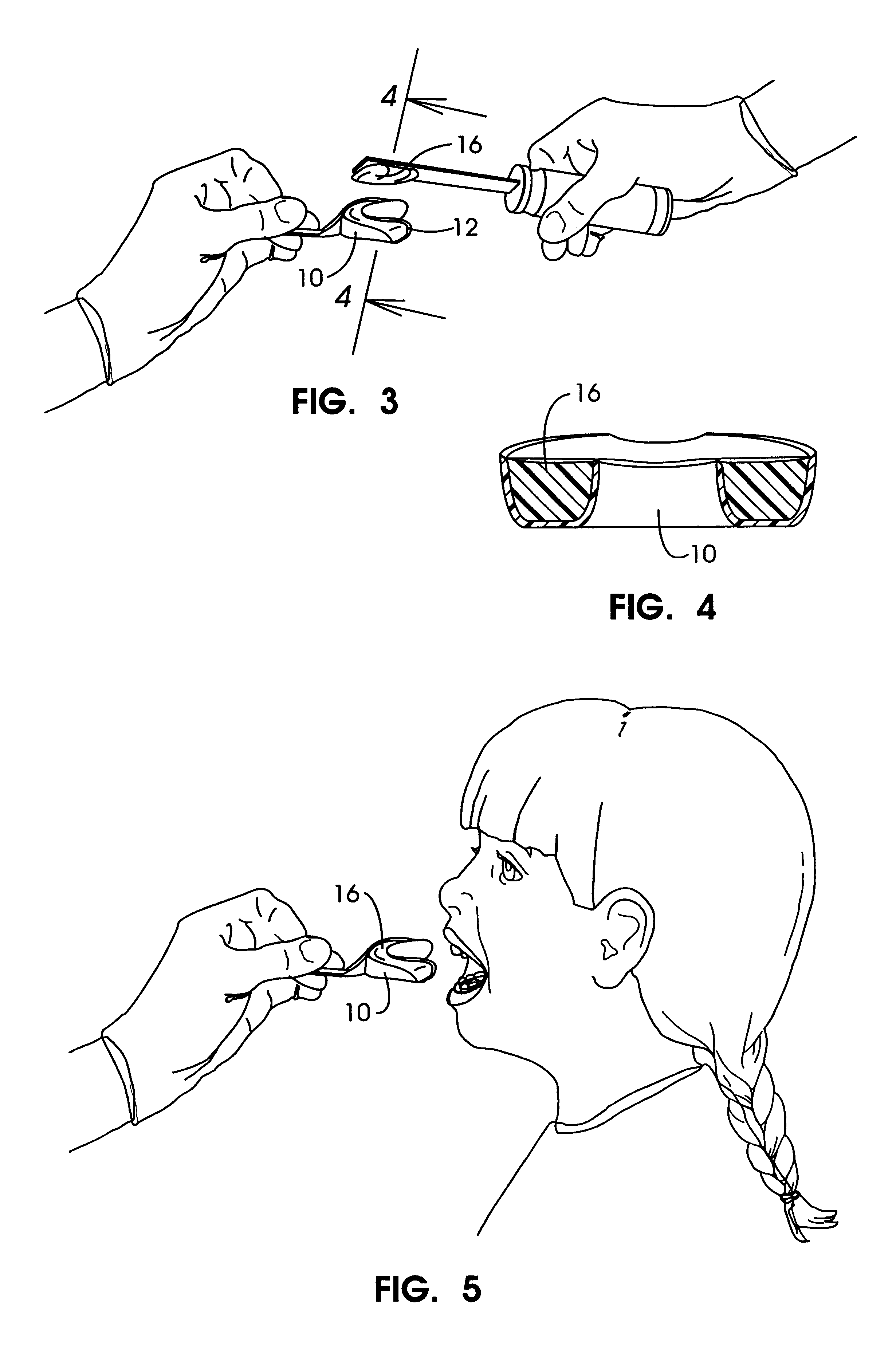
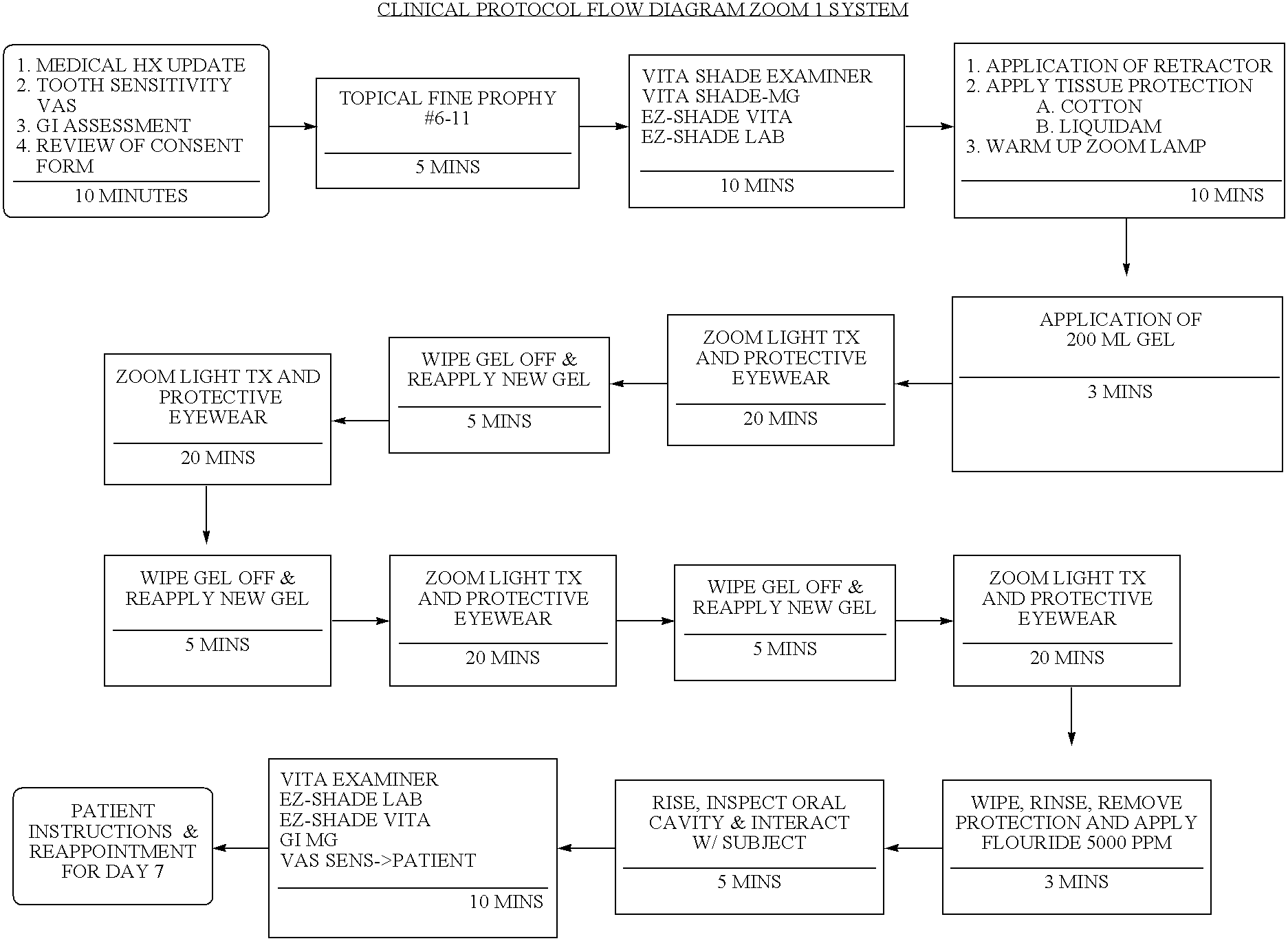
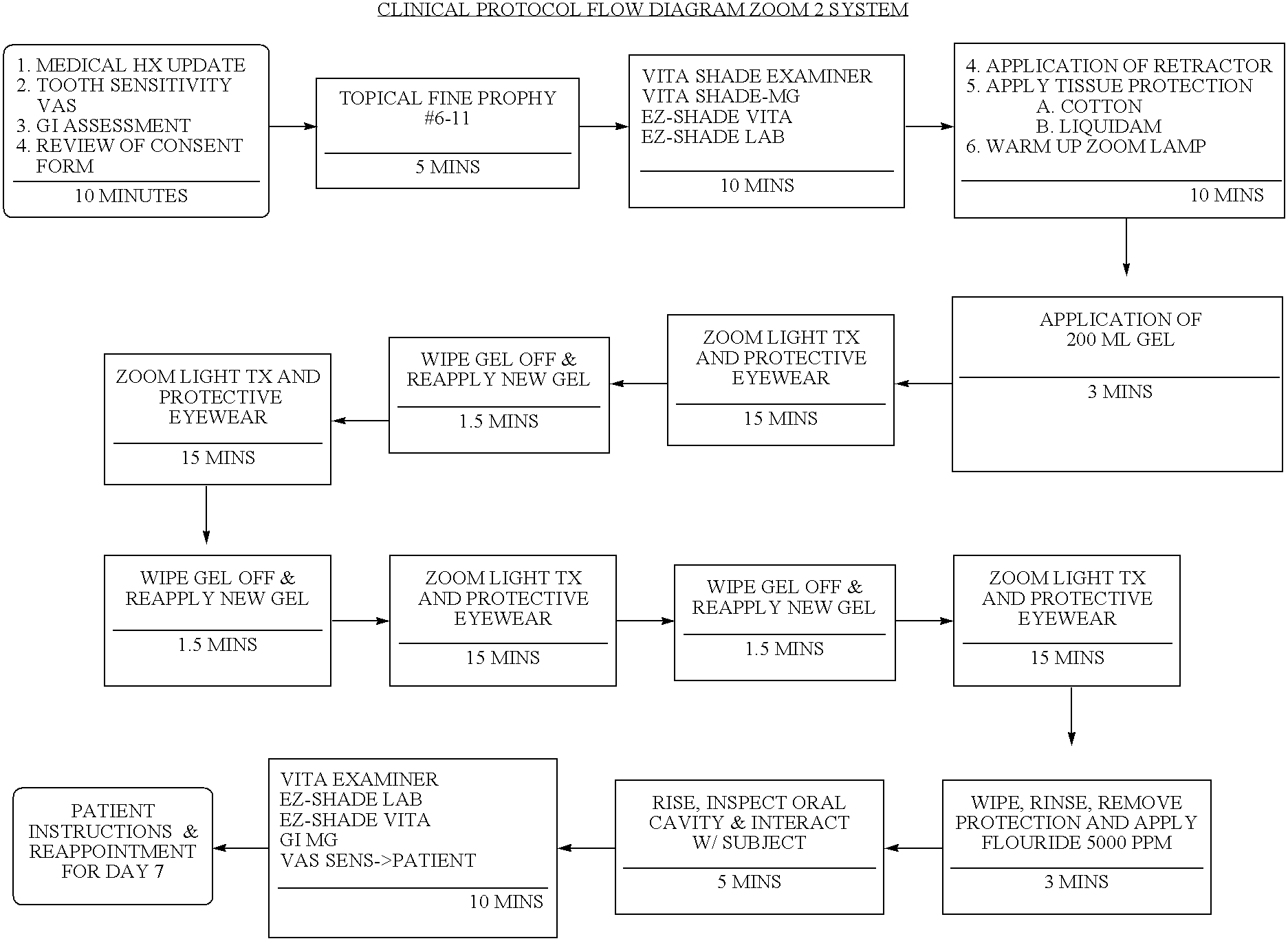
Methods for bleaching, opacifying and desensitizing teeth
InactiveUS6368576B1Trend downReduce sensitivityCosmetic preparationsTeeth fillingPotassium nitrateCarbamide peroxide
Owner:ULTRADENT PROD INC
One-part dental compositions and methods for bleaching and desensitizing teeth
InactiveUS6309625B1Reduce sensitivityTrend downCosmetic preparationsGum massagePotassium nitrateMedicine
Composition and methods that include potassium nitrate for whitening and / or reducing tooth sensitivity. The dental compositions may optionally include a dental bleaching agent, such as hydrogen peroxide or carbamide peroxide. The dental compositions may be applied directly to the person's teeth, or they may be loaded into a comfortable fitting, flexible, thin-walled dental tray and placed over the person's teeth. In that case, the dental compositions will include a tackifying agent, such as carboxypolymethylene, dispersed within a solvent, which assists the composition in retaining the dental tray over the person's teeth as a result of the adhesive properties of the dental composition rather than due to mechanical interlocking of the tray over the person's teeth. The dental compositions may further include anticariogenic and antimicrobial agents.
Owner:ULTRADENT PROD INC
Compositions and methods for whitening and desensitizing teeth
InactiveUS6306370B1Reduce sensitivityTrend downCosmetic preparationsImpression capsPotassium nitrateMedicine
Composition and methods that include potassium nitrate for whitening and / or reducing tooth sensitivity. The dental compositions may optionally include a dental bleaching agent, such as hydrogen peroxide or carbamide peroxide. The dental compositions may be applied directly to the person's teeth, or they may be loaded into a comfortable fitting, flexible, thin-walled dental tray and placed over the person's teeth. In that case, the dental compositions will include a tackifying agent, such as carboxypolymethylene, which assists the composition in retaining the dental tray over the person's teeth as a result of the adhesive properties of the dental composition rather than due to mechanical interlocking of the tray over the person's teeth. The dental compositions may further include anticariogenic and antimicrobial agents.
Owner:ULTRADENT PROD INC
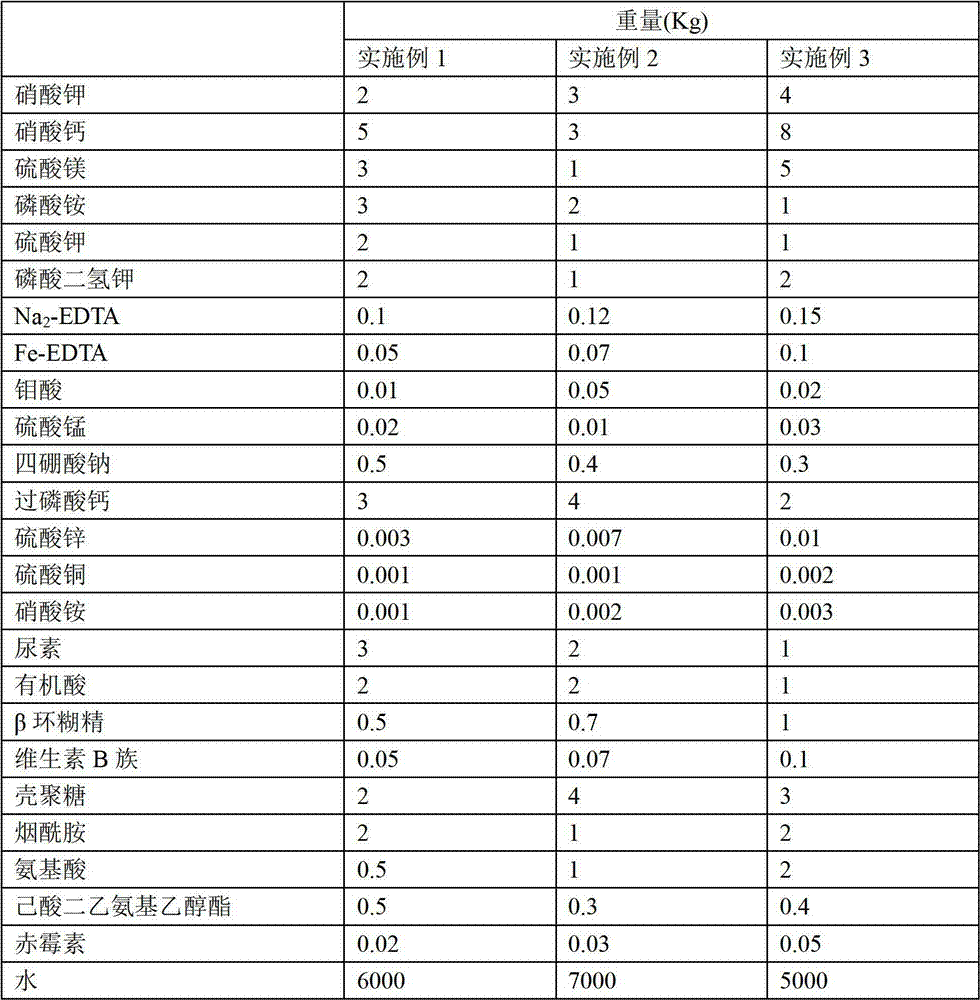
Plant nutrient solution for soilless culture of tomato
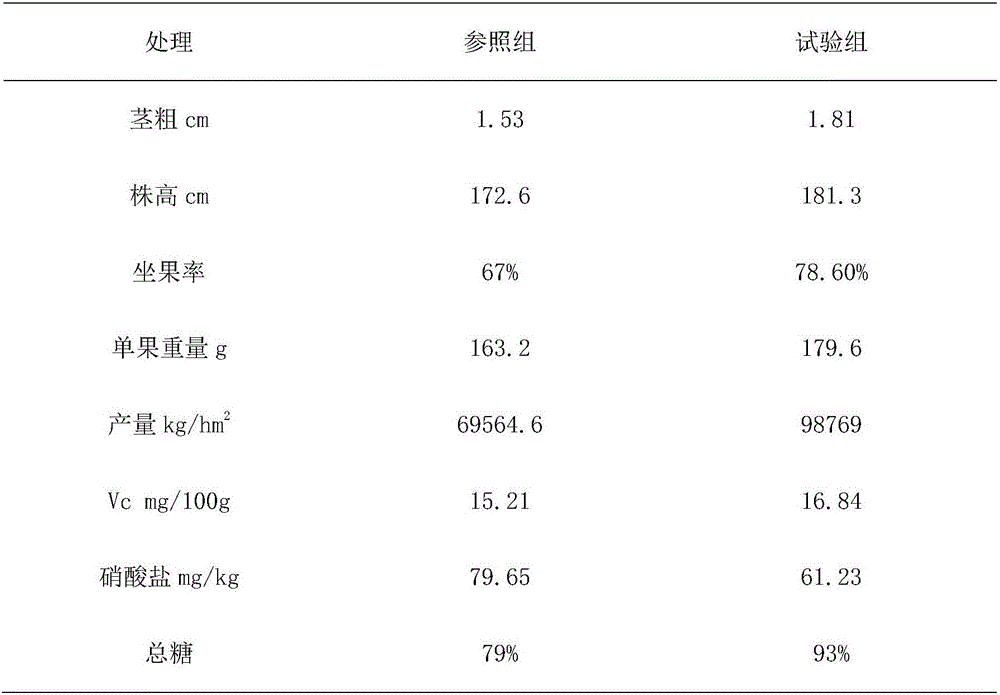
The invention provides a plant nutrient solution for soilless culture of tomato. The plant nutrient solution is prepared by the following raw materials in part by weight: 2 to 4 parts of potassium nitrate, 3 to 8 parts of calcium nitrate, 1 to 5 parts of magnesium sulfate, 1 to 3 parts of potassium phosphate, 1 to 2 parts of potassium sulphate, 1 to 2 parts of monopotassium phosphate, 0.1 to 0.15 part of Na2-EDTA, 0.05 to 0.1 part of Fe-EDTA, 0.01 to 0.05 part of molybdic acid, 0.01 to 0.03 part of manganese sulfate, 0.3 to 0.5 part of sodium tetraborate, 2 to 4 parts of superphosphate, 0.003 to 0.01 part of zinc sulfate, 0.001 to 0.002 part of copper sulfate, 0.001 to 0.003 part of ammonium nitrate, 1 to 3 parts of urea, 1 to 2 parts of organic acid, 0.5 to 1 part of beta cyclodextrin, 0.05 to 0.1 part of vitamin B, 2 to 4 parts of chitosan, 1 to 2 parts of nicotinamide, 0.5 to 2 parts of amino acid, 0.3 to 0.5 part of diethyl aminoethyl hexanoate, 0.02 to 0.5 part of gibberellin, and 5,000 to 7,000 parts of water. According to experiment, the plant nutrient solution provided by the invention can be used for carrying out soilless culture of tomato, and the tomato has high plant height, thick stem, high cluster and high output.
Owner:山西田森杜氏番茄科技有限公司
Liquid fertilizer with pyroligneous liquor and oxalacetic liquor and production thereof
InactiveCN1778773AMagnesium fertilisersFertilisers by pryogenic processesRare-earth elementPlant regulators
A liquid fertilizer containing wood vinegar liquid or oxalic vinegar liquid and its production are disclosed. The liquid fertilizer consists of urea 1í½20.0%, potassium phosphate 1í½20.0%, potassium nitrate 1í½20.0%, lime nitrate 0.1í½10.0%, magnesium sulfate 0.1í½5.0%, ferrous sulfate 0.1í½5.0%, manganous sulfate 0.1í½5.0%, zinc sulfate 0.1í½5.0%, cupric sulfate 0.1í½5.0%, boron sand or boric acid 0.1í½2.0%, sodium molybdate or ammonium molybdate 0.1í½1.0%, plant hormone or plant regulator, rare earth element or humus acid or pesticide auxiliaries etc. The process is carried out by proportioning and mixing. It can improve bacterium inhibiting and plant growth regulating functions.
Owner:ZHEJIANG JIANZHONG BAMBOO IND
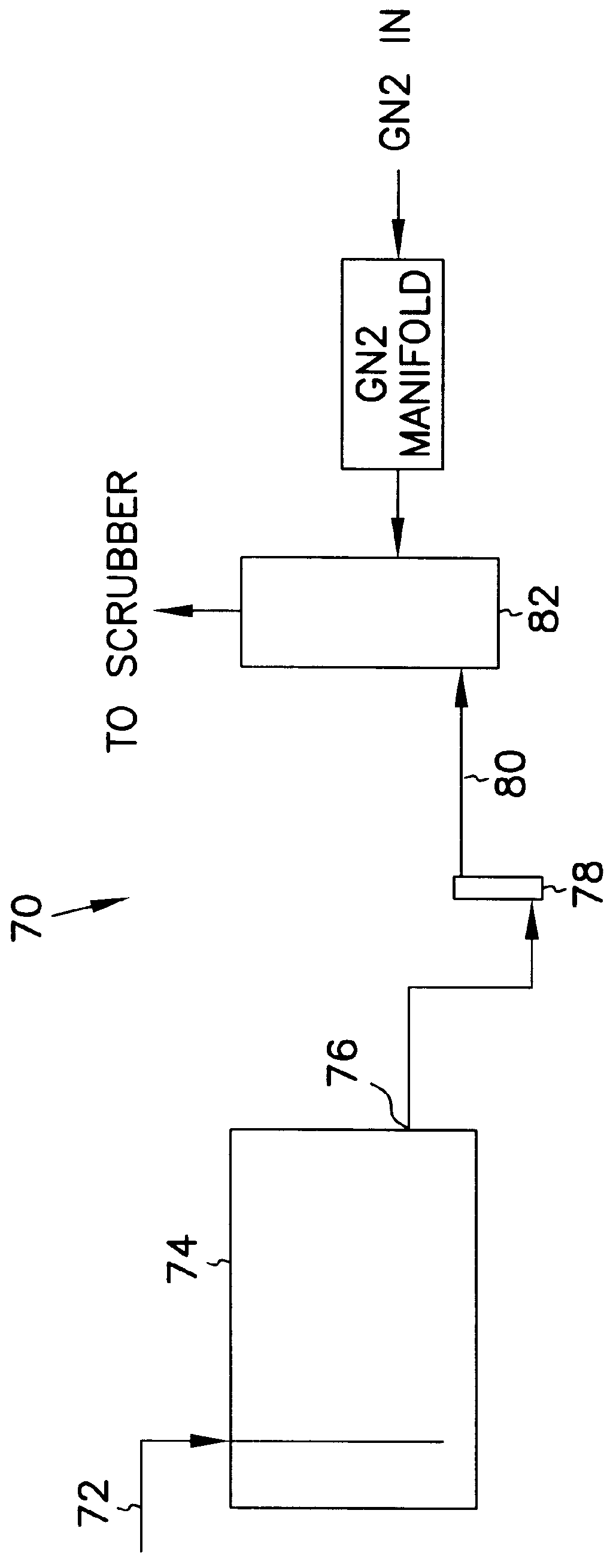
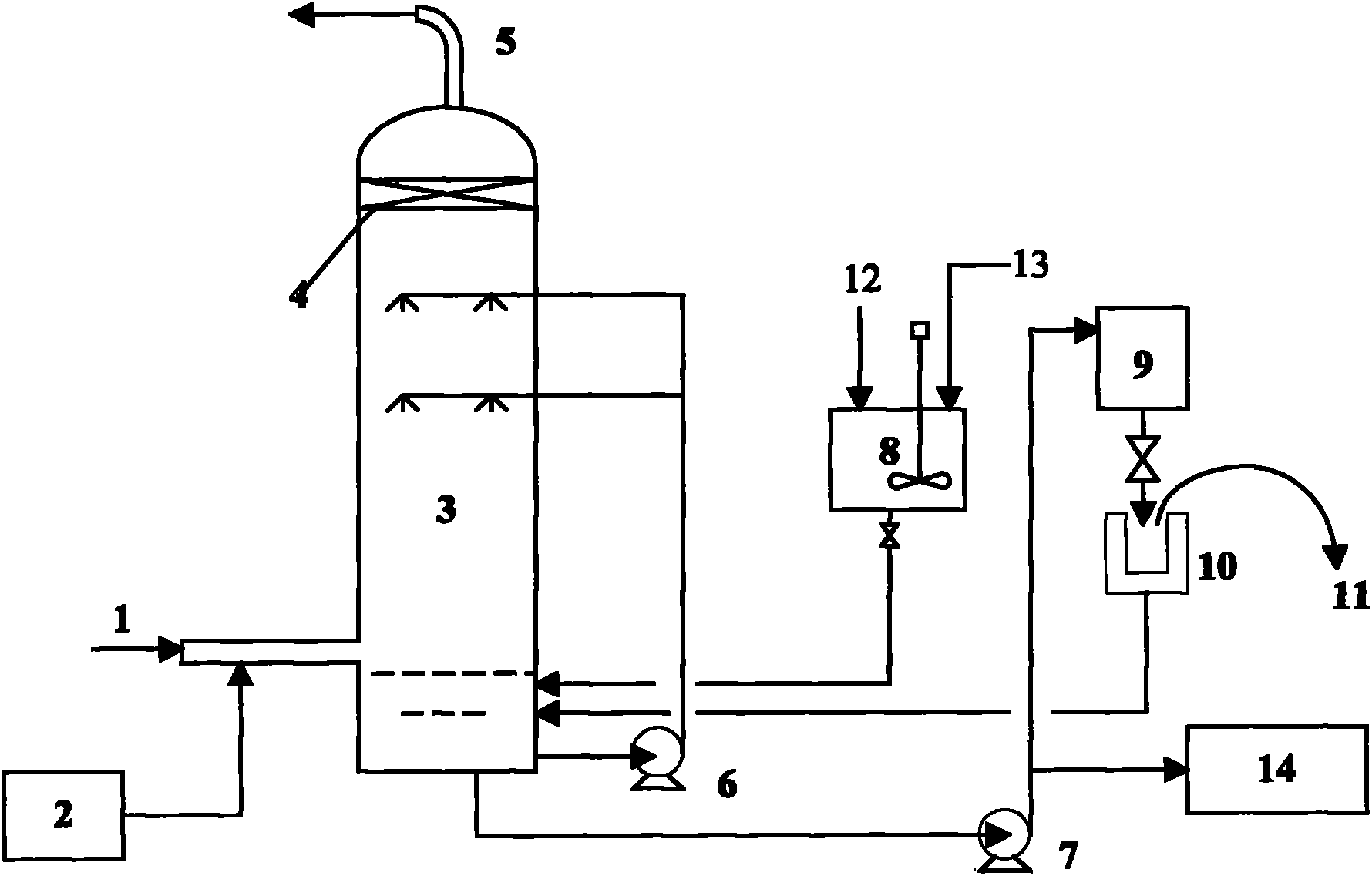
Process and equipment for nitrogen oxide waste conversion to fertilizer
The present invention describes a process for converting vapor streams from sources containing at least one nitrogen-containing oxidizing agent therein to a liquid fertilizer composition comprising the steps of: a) directing a vapor stream containing at least one nitrogen-containing oxidizing agent to a first contact zone, b) contacting said vapor stream with water to form nitrogen oxide(s) from said at least one nitrogen-containing oxidizing agent, c) directing said acid(s) as a second stream to a second contact zone, d) exposing said second stream to hydrogen peroxide which is present within said second contact zone in a relative amount of at least 0.1% by weight of said second stream within said second contact zone to convert at least some of any nitrogen oxide species or ions other than in the nitrate form present within said second stream to nitrate ion, e) sampling said stream within said second contact zone to determine the relative amount of hydrogen peroxide within said second contact zone, f) adding hydrogen peroxide to said second contact zone when a level of hydrogen peroxide less than 0.1 % by weight in said second stream is determined by said sampling, g) adding a solution comprising potassium hydroxide to said second stream to maintain a pH between 6.0 and 11.0 within said second stream within said second contact zone to form a solution of potassium nitrate, and h) removing said solution of potassium nitrate from said second contact zone.
Owner:NAT AERONAUTICS & SPACE ADMINISTATION U S GOVERNMENT AS REPRESENTED BY THE ADMINISTATOR
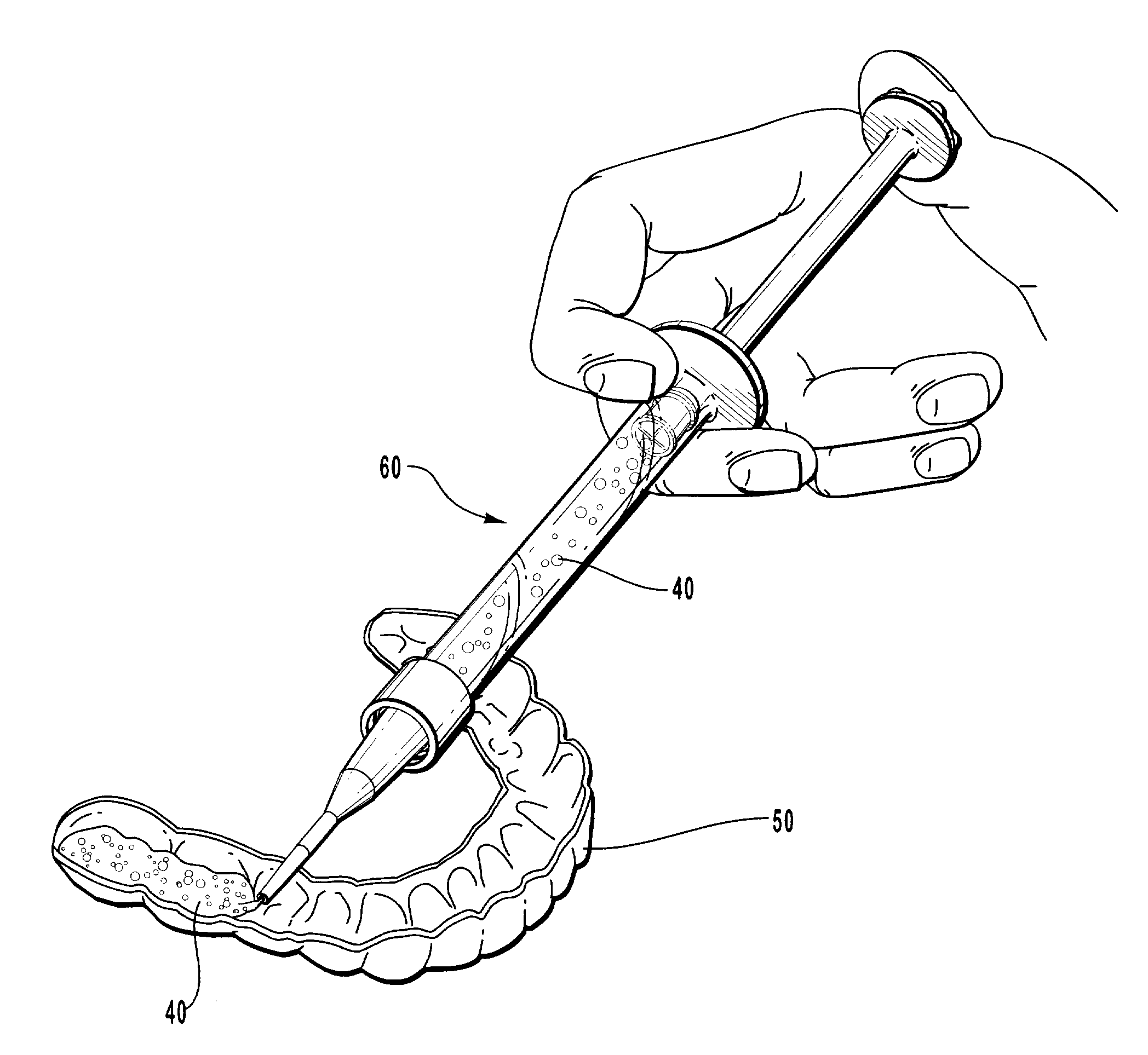
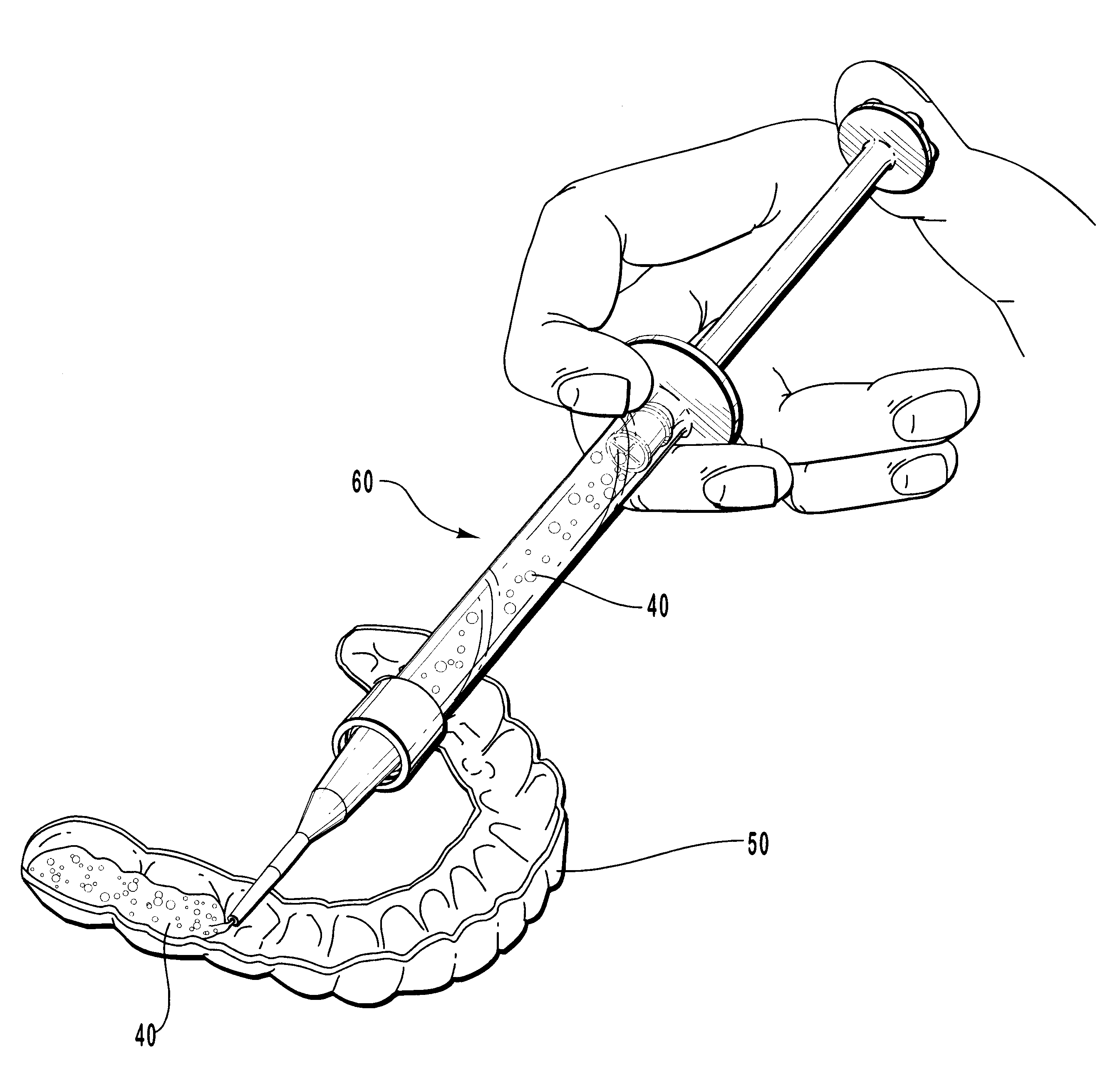
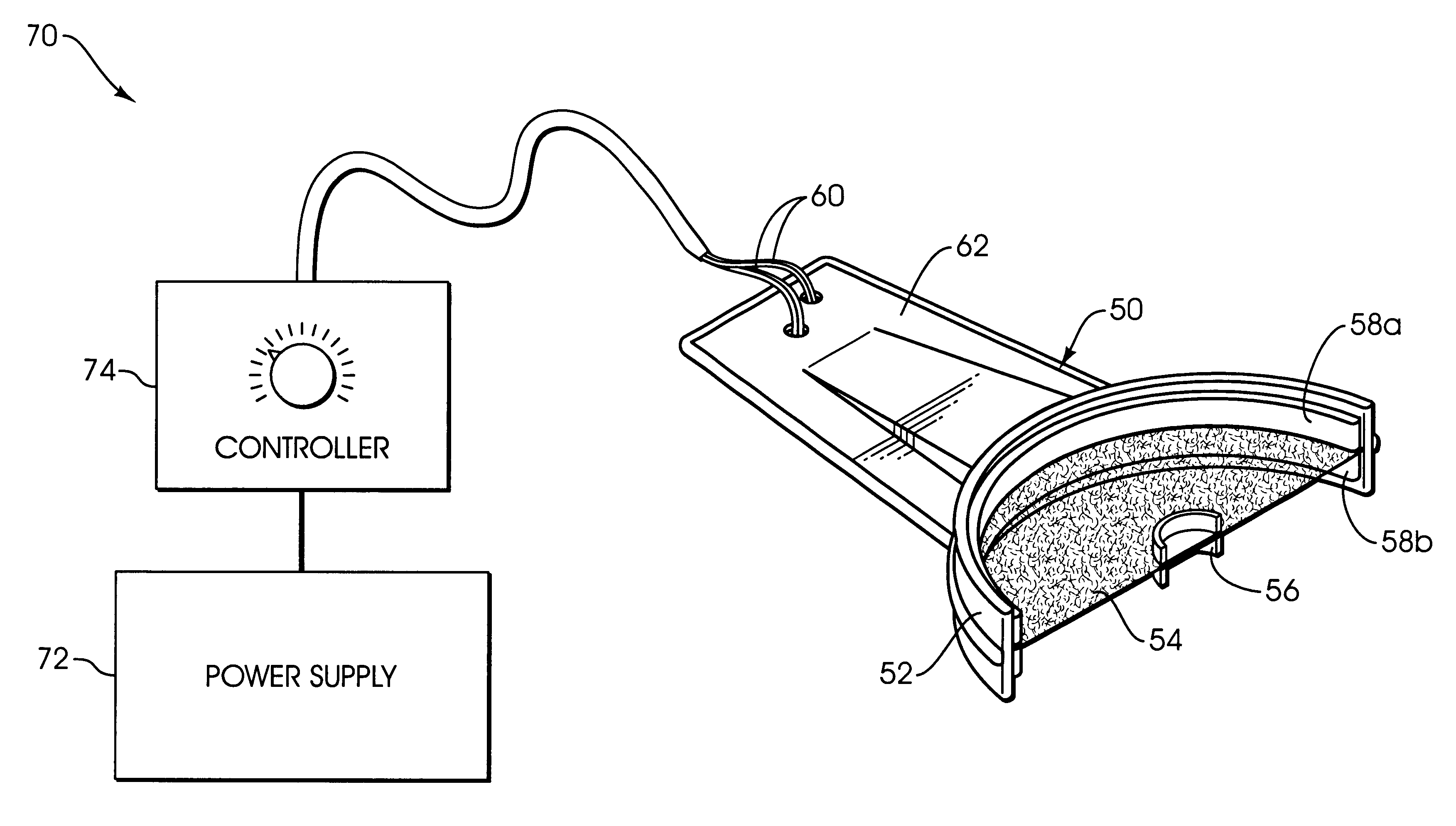
Apparatus and methods for accelerating dental treatments
InactiveUS6382979B2High activityIncrease ratingsImpression capsTeeth fillingElectrical resistance and conductancePotassium nitrate
Apparatus, kits and methods for providing accelerated treatment of a patient's teeth. The apparatus, kits and methods involve the use of a dental impression tray that includes a patient dentition impression formed from a dental impression material such as an alginate. The impression tray advantageously includes heating means for heating and maintaining the formed patient dentition impression at a temperature greater than about 105° F. The heated dental trays may be used to accelerate the activity of dental bleaching agents (e.g., peroxides), desensitizing agents (e.g., potassium nitrate), remineralizing agents (e.g., fluoride salts), and the like. A dental office procedure for treating teeth involves the basic steps of: (1) coating a specially-prepared patient dentition impression with a dental composition such as a bleaching, desensitizing or remineralizing composition; (2) placing the coated patient dentition impression into the patient's mouth; (3) heating the patient dentition impression and dental composition to a temperature of at least about 105° F.; and (4) retaining the patient dentition impression in position for period of time in a range of about 1 minute to about 60 minutes. The procedure can be adapted to microwave heating, electrical resistance heating, or hot fluid heating of the patient dentition impression.
Owner:LINDQUIST SHERRILL F DDS +1
Nitrate molten salt heat transferring and reserving medium and preparation method and application thereof
InactiveCN102533226AWide operating temperature rangeImprove thermal stabilityHeat-exchange elementsDecompositionInstability
The invention discloses a nitrate molten salt heat transferring and reserving medium and a preparation method and application thereof. The nitrate molten salt heat transferring and reserving medium is prepared with 5 to 40 percent of potassium nitrate, 5 to 25 percent of sodium nitrate, and 10 to 70 percent of calcium nitrate. The melting point of the nitrate molten salt heat transferring and reserving medium can be as low as 120 DEG C, and the upper limit of temperature for use can reach 550 DEG C, the nitrate molten salt heat transferring and reserving medium has a wide temperature scope of application, can work normally at the temperature scope of 180 DEG C to 550 DEG C, and has good thermal stability; the shortcoming of high melting point of binary nitrate molten salt system can be overcome, and the problem of instability caused by easy oxidative decomposition at high temperature of NaNO2 in the Chinese patent 200110027954.1 and ternary nitrate salt system can be solved, and the problems of corrosion and cost increase caused by the existing LiNO3 in the Chinese patent 00111406.9 and the American patent US007588694B1 can also be solved.
Owner:SUN YAT SEN UNIV +1
Method and composition for preventing tooth hypersensitivity when using passive bleaching agents
InactiveUS20060013778A1Avoid allergiesLonger contact/coating periodCosmetic preparationsGum massageZinc peroxidePotassium nitrate
Dental bleaching compositions, for example in the form of liquids, gels, creams, pastes and ointments, comprising a peroxide releasing compound and from 1% to 35% by weight of a potassium-containing compound such as potassium nitrate, wherein the potassium nitrate is present in a safe and effective amount to prevent tooth hypersensitivity in the patient during the bleaching process. The potassium nitrate contemplated by the invention is compatible with peroxide yielding bleaching compounds such as peroxide, carbamide peroxide, calcium peroxide, zinc peroxide, magnesium peroxide and sodium perborate. Potassium nitrate is complimentary and synergistic with the peroxide bleaching agents contemplated by the invention and enhances the release of oxygen to the tooth enamel. Also contemplated are methods of bleaching teeth comprising application of the dental bleaching compositions of the invention.
Owner:HODOSH MILTON
Desensitizing bleaching gel
InactiveUS6458340B1Easy and fast fashionImprove stabilityCosmetic preparationsBiocideAlkaline earth metalGlycerol
A substantially anhydrous gel useful for bleaching teeth comprising: (i) at least 25% by weight of organic polyol; (ii) less than 3% by weight polyacrylic acid thickening agent; (iii) at least 10% by weight carbamide peroxide (or a chemically equivalent amount of another bleaching agent, such as 3% by weight hydrogen peroxide); (iv) neutralizing agent; (v) chelating agents; (vi) desensitizing agent; and (vii) miscellaneous ingredients such as Cirtoxain(R) and flavorants. The organic polyol is preferably glycerin. The polyacrylic acid thickening agent is preferably a carbomer. The desensitizing agent is preferably potassium nitrate, strontium chloride, potassium citrate, strontium nitrate, or a similarly effective alkali or alkaline earth metal salt of an organic or inorganic acid.
Owner:RANIR LLC
Fusion tray of thermal transmission and storage medium, and preparation method
InactiveCN101050355AHigh Safe Operating Temperature Upper LimitSafe operating temperature upper limit expandedHeat-exchange elementsHigh energyWorking temperature
This invention discloses a method for preparing molten salt heat-transfer and heat-storage medium, which comprises potassium nitrate, sodium nitrate, sodium nitrite and additives. The additives are cesium nitrate and potassium chloride at a weight ratio of (0.1-0.8):1. The molten salt heat-transfer and heat-storage medium has such advantages as good heat transfer property, wide working temperature range, high heat stability, high upper limit of safe usage temperature, low melting point, high phase-change latent heat, low requirement for system size and energy, and high energy utility.
Owner:SUN YAT SEN UNIV +1
Extinguishment combination with hot gas sol
The invention provides ''a hot aerosol fire-extinguishing composition'' and relates to an oxidative magnesium salt hot aerosol fire-extinguishing composition represented by anhydrous magnesium nitrate. The composition is characterized in that the composition can be magnesium nitrate, magnesium carbonate, or other magnesium salt, and can also be a compound of magnesium nitrate, or other magnesium salt with potassium nitrate, strontium nitrate, or other potassium salt or strontium salt; a reducer can be one of or the combination of a plurality of ammonium carbamidine, dicyandiamide, red prussiate of potash, formamine, triazole, and tetrazole; a capability improver can be magnesium carbonate, manganous carbonate, aluminium powder, powdered carbon, magnesium hydrate, metal oxide, etc.; and the bond adopts phenolic resin, etc. The preparation of the magnesium salt comprises the continuous steps: medium temperature and low pressure dehydration, spray under the protection of nitrogen-oxygen flow or ultrafine grinding of grinded colloid, and microencapsulated hydrophobic treatment, etc. Compared with the prior art, the fire-extinguishing composition has the advantages of low price, extensive source, fire-extinguishing capability of K-type composition, and low causticity and toxicity of the ultimate product of combustion.
Owner:SHAANXI J&R FIRE FIGHTING CO LTD
Device and method for simultaneously desulfurizing and denitrifying flue gas by ozone catalytic oxidation process
InactiveCN102247750AEfficient oxidationDispersed particle separationSulfur-trioxide/sulfuric-acidCatalytic oxidationAbsorption of water
The invention relates to a flue gas pollutant treatment process and aims to provide a device and method for simultaneously desulfurizing and denitrifying a flue gas by ozone catalytic oxidation process. The device comprises a desulfurization and denitrification tower, an ozone generator, absorption liquid circulating equipment and desulfurization and denitrification by-product post-treatment equipment. Ozone enters from a flue or the lower part of the desulfurization and denitrification tower, a catalyst is added to an absorption liquid, and the absorption liquid is injected in from the upper part of the desulfurization and denitrification tower, so that SO2 and NO in the flue gas are oxidized by ozone with high efficiency under the action of the catalyst, and in combination with the absorption of water or alkaline substances, SO2 and NOx in the flue gas are recovered in the form of high value-added sulfuric acid and nitric acid products, or ammonium sulfate / ammonium nitrate mixed nitrogen fertilizers, potassium sulfate / potassium nitrate mixed potassium fertilizers or ammonium / potassium compound fertilizers respectively, thereby achieving resource recovery and value maximization of the desulfurization and denitrification process. The desulfurization and denitrification process provided by the invention has the advantages of simple structure, low investment and low operation cost. The desulfurization rate and the denitrification rate of the desulfurization and denitrification process provided by the invention can reach more than 96% and more than 90% respectively.
Owner:EAST CHINA UNIV OF SCI & TECH
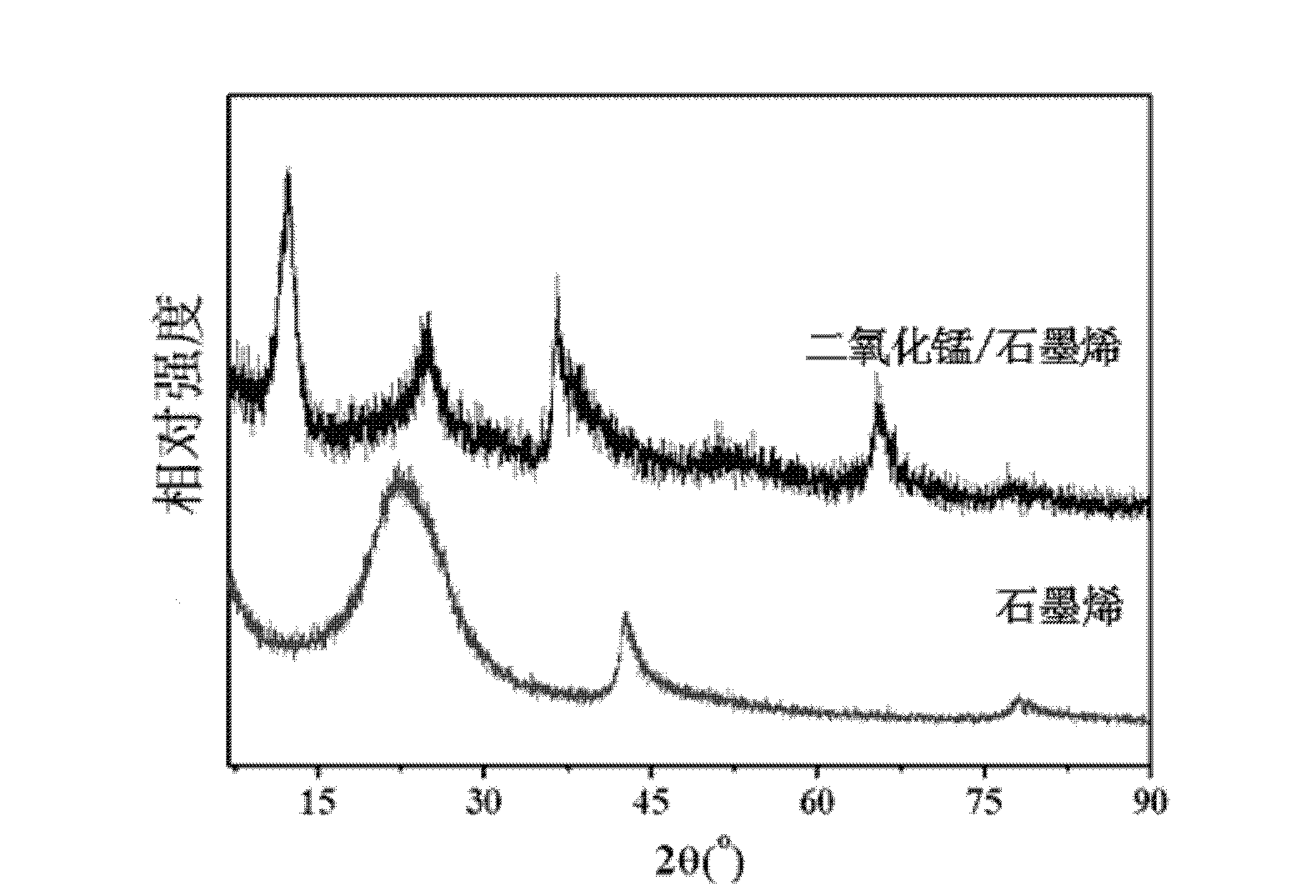
A kind of preparation method of graphene and manganese dioxide nanocomposite material
InactiveCN102275903AReduce typesLow costMaterial nanotechnologyManganese oxides/hydroxidesHydrazine compoundManganese
The invention relates to a preparation method of a graphene and manganese dioxide nanocomposite material, comprising: (1) stirring and mixing graphite, potassium nitrate and concentrated sulfur evenly, adding potassium permanganate, and reacting at 30-40° C. for 20-40 minutes , add deionized water at room temperature, add hydrogen peroxide after reacting for 15 to 30 minutes to obtain graphite oxide; (2) disperse the above graphite oxide in water, add hydrazine hydrate, and react at 95°C for 1 to 24 hours to obtain graphene (3) ultrasonically disperse the graphene in a saturated potassium permanganate solution, add acid, and react at 60-80° C. for 1-5 hours to obtain graphene and manganese dioxide nanocomposite material. The invention has the advantages of simple reaction, easy control, convenient operation and simple process; the obtained composite material has broad application prospects and can be used for catalysts, biosensing materials, electrode materials of lithium ion batteries and supercapacitor electrode materials, and the like.
Owner:DONGHUA UNIV
Dental whitening systems
InactiveUS20060099155A1Convenient whiteningFaster effective whiteningCosmetic preparationsToilet preparationsBiological activationTooth whitening
A system and method for tooth whitening are disclosed wherein at least one peroxide-containing gel and at least one transition metal compound-containing gel, particularly at least one lower atomic number transition metal compound, more particularly at least a ferrous compound including gluconate, sulfate, nitrate, acetate or mixtures thereof, are applied to a patient's mouth. Gelling agent is also included. The activation of the peroxide whitens the patient's teeth. The system may be used with or without the application of light. The system further provides an additional gel including a sensitivity reduction compound including potassium nitrate, sodium nitrate or mixtures thereof for possible sensitivity treatment if needed.
Owner:DISCUS DENTAL LLC
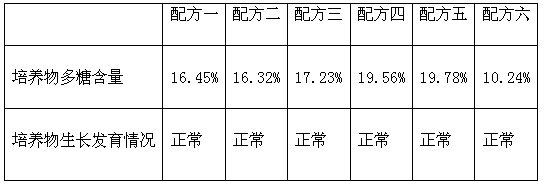
Dendrobium officinale culture solution
ActiveCN102976848AHigh in polysaccharidesHigh in amino acidsFertilizer mixturesEthylenediamineManganese
The invention discloses a Dendrobium officinale culture solution and belongs to the technical field of agricultural planting. The culture solution comprises the following ingredients: potassium nitrate, ammonium nitrate, magnesium sulfate, manganese sulfate, zinc sulfate, monopotassium phosphate, copper sulfate, potassium iodide, cobalt dichloride, boric acid, sodium molybdate, vitamin VB1, vitamin VB6, vitamin VB5, glycine, ferrous sulfate, disodium ethylenediamine tetraacetic acid, calcium chloride, naphthylacetic acid, mashed banana, mashed potato, white sugar, inositol, and powdered agar. The dendrobium officinale culture solution is reasonable in compatibility, can provide comprehensive nutrition for a Dendrobium officinale stem, fast promote the stem to grow out of a root, increase the polysaccharide content and the amino acid content in the Dendrobium officinale, shorten the time for culturing the Dendrobium officinale stem to a complete plant to about 25 days, and significantly improve the transplanting survival rate of the Dendrobium officinale.
Owner:杭州富阳文曲生态农业开发有限公司
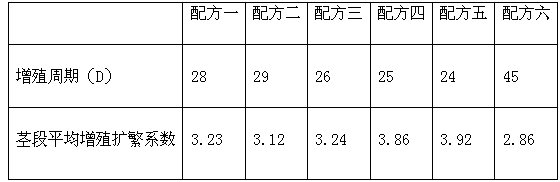
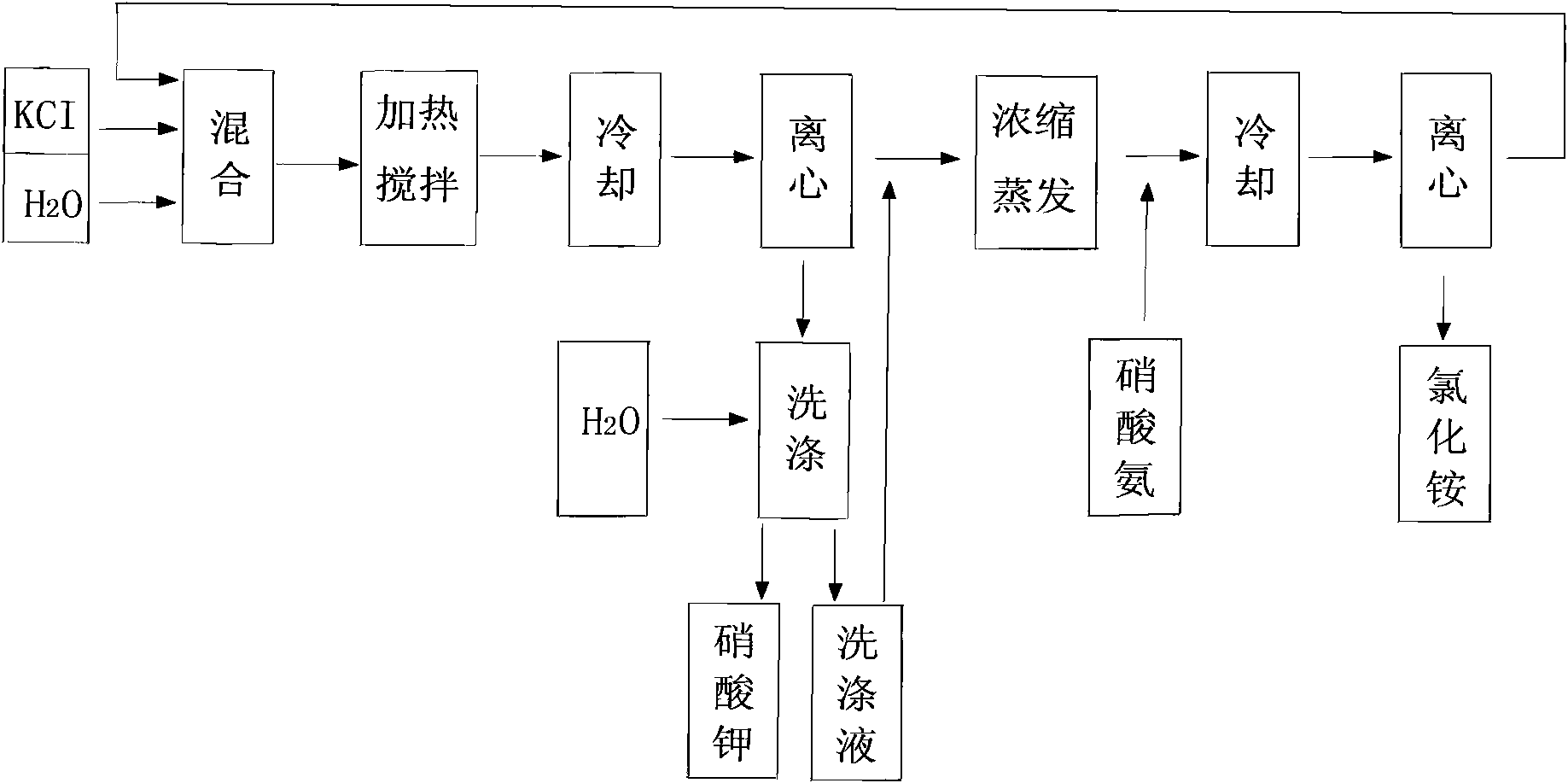
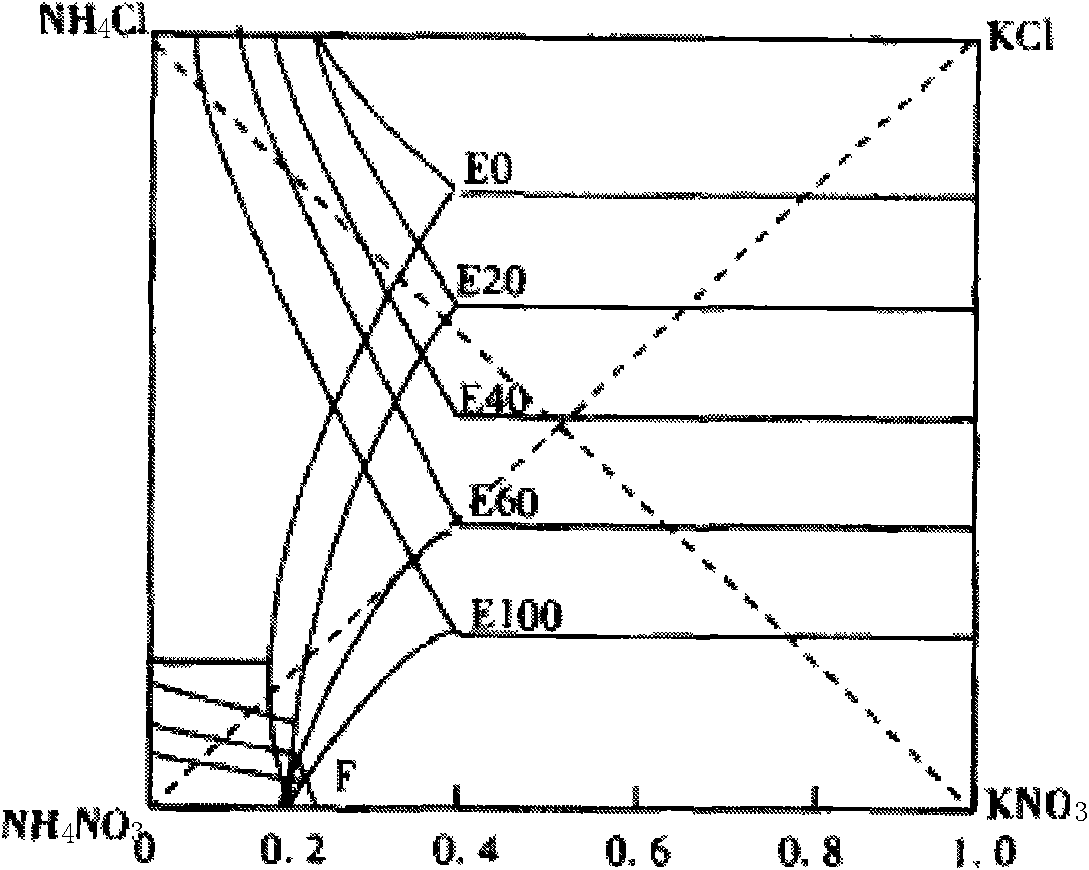
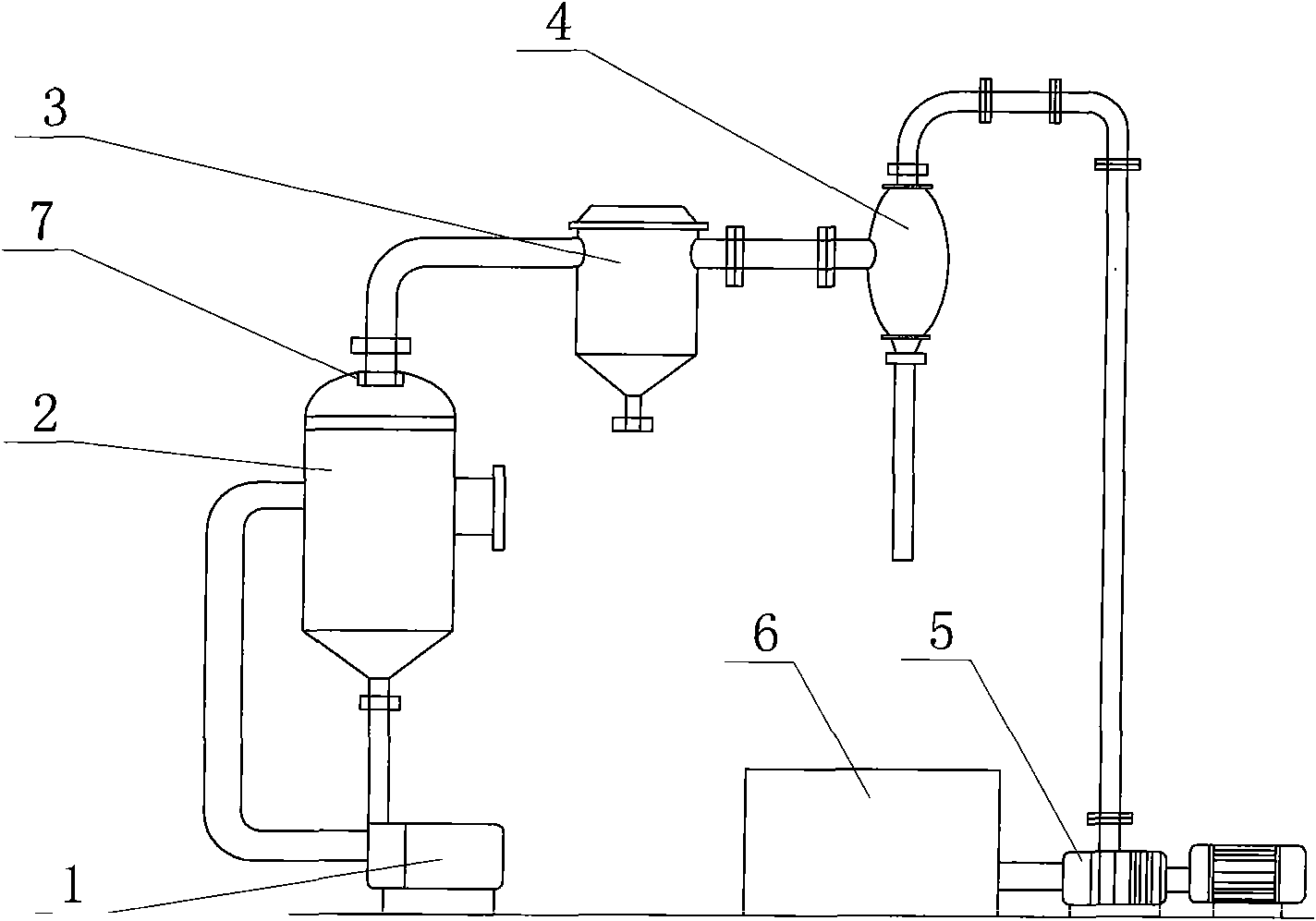
Method for preparing potassium nitrate and ammonium chloride employing double decomposition reaction
InactiveCN101628723ACreate pollutionRealize the concentrated evaporation processAlkali metal nitrate preparationAmmonium halidesDecompositionIon exchange
A method for preparing potassium nitrate and ammonium chloride employing double decomposition reaction comprises the following steps: dissolving ammonium nitrate and potassium chloride in water according to a defined ratio at 110 DEG C, continuously adding potassium chloride and water, heating while stirring to ensure that potassium nitrate is in supersaturation state, after stopping heating, cooling the solution in a vacuum cooling crystallizer to 36-40 DEG C to separate potassium nitrate crystal, placing the potassium nitrate crystal in a centrifugal machine with a filter cloth lining to obtain coarse potassium nitrate, then washing the potassium nitrate with cold water, drying to obtain the finished potassium nitrate; in addition, adding ammonium nitrate in mother solution I and cleaning solution to adjust solution concentration so that ammonium chloride can reach supersaturation state, using a vacuum concentration device to perform negative pressure evaporation, separating and precipitating ammonium chloride by centrifuging and obtaining a solid ammonium chloride product, wherein, when dissolving ammonium nitrate and potassium chloride, the ratio of ammonium ion to chlorine ion is 1:2 and when using the centrifugal machine to obtain the coarse potassium nitrate, the separated mother solution is another mother solution I sharing the same saturation point of potassium nitrate and ammonium chloride. The solution of feed liquid circular reaction overcomes the defects of the prior art that the price of potassium nitrate used in reaction is high, the resource of potassium nitrate is in short supply and the cost of devices used in ion-exchange method is high, thus being applicable to the production of potassium nitrate.
Owner:湖南丹化农资有限公司
Plant nutrient solution and application in plant cultivation
InactiveCN101062877AImprove survival rateDoes not affect life cycleMagnesium fertilisersAlkali orthophosphate fertiliserManganeseMonopotassium phosphate
The invention discloses a plant nutrition liquid, which is characterized by the following: comprising potassium nitrate, calcium nitrate, magnesium sulfate, ammonium phosphate, potassium sulfate, monopotassium phosphate and so on a good deal of element; comprising edetate disodium, ferrosi sulfas, boric acid, manganese sulfate, zinc sulfate, copper sulfate, ammonium molybdate and so on microelement; comprising azophoska fertilizer and fertilizer booster; including diverse nutritious element of vegetation development; providing nourishing element for large and medium scale bush and small arbor. This invention also discloses a utility scheme in large and medium scale woody plant soilless culture.
Owner:成都市第二农业科学研究所 +1
Soiless culture nutrient solution
InactiveCN103172438AInhibition of reproductionIncrease resistanceFertilizer mixturesCalcium nitrate tetrahydrateManganese
The invention relates to the soiless culture field and in particular relates to a soiless culture nutrient solution; every liter of water comprises the following components by weight: major constituents consisting of 900mg-2200mg of much EM (Effective Microorganisms) probiotics liquid, 900mg-2200mg of brown sugar, 900mg-1000mg of calcium nitrate tetrahydrate, 700mg-900mg of potassium nitrate, 150mg-220mg of ammonium dihydrogen phosphate and 300mg-500mg of magnesium sulfate heptahydrate; micro constituents consisting of 20mg-25mg of EDTA (Ethylene Diamine Tetraacetic Acid) chelated iron, 2mg-3mg of boric acid, 2mg-3mg of manganese sulfate monohydrate, 0.1mg-0.5mg of zinc sulfate heptahydrate, 0.01mg-0.1mg of copper sulfate and 0.01mg-0.08mg of ammonium molybdate tetrahydrate. The soiless culture nutrient solution provided by the invention can be used for improvising the immunity of the soiless culture nutrient solution for resisting plant diseases and insect pests of the crops.
Owner:北京天食和谷农业科技有限公司
Polypeptide-ammonium polyphosphate trace element liquid chelated fertilizer and preparation method thereof
The invention discloses a polypeptide-ammonium polyphosphate trace element liquid chelated fertilizer and a preparation method of the liquid chelated fertilizer. The polypeptide-ammonium polyphosphate trace element liquid chelated fertilizer comprises the components by weight percent: 5-25% of urea, 5-15% of ammonium polyphosphate, 5-25% of potassium nitrate, 0.2-3% of potassium silicate, 0.8-5% of potassium chloride, 0.008-0.1% of polyaspartic acid, 0.8-5% of ammonium sulfate, 0.8-5% of ethylene diamine tetraacetic acid (EDTA) calcium, 0.2-3% of EDTA magnesium, 0.02-1.5% of EDTA boron, 0.005-0.1% of EDTA zinc, 0.005-0.1% of EDTA iron, 0.005-0.05% of EDTA copper, 0.005-0.05% of EDTA manganese, 0.001-0.01% of nickel sulfate, 0.001-0.01% of ammonium molybdate, 0.0008-0.01% of cobaltous sulfate, 0.008-0.1% of polyacrylamide and the balance of water. The polypeptide-ammonium polyphosphate trace element liquid chelated fertilizer disclosed by the invention is simple in technology, scientific in proportion, balanced in fertilization, low in cost and good in effect.
Owner:张朝晖
Environment-protecting synergistic agent for fuel coal
InactiveCN101440328AIncreased flammable contentIncreased space gapSolid fuelsFuel additivesSodium bicarbonateFurnace temperature
The invention discloses a bunker coal environment-friendly synergist, which comprises the following components by weight percent: 1 to 10 percent of ammonium bicarbonate, 1 to 8 percent of sodium bicarbonate, 1 to 10 percent of sodium carbonate, 1 to 5 percent of sodium nitrate, 1 to 10 percent of potassium nitrate, 1 to 15 percent of calcium carbonate, 1 to 10 percent of sodium hydroxide, 2 to 10 percent of calcium hydroxide, 1 to 10 percent of potassium permanganate, 3 to 15 percent of potassium chloride, 10 to 35 percent of sodium chloride, 1 to 10 percent of ammonium chloride, 1 to 15 percent of calcium chloride, 1 to 10 percent of borax, 1 to 10 percent of macaroni, 1 to 5 percent of potassium carbonate, 1 to 5 percent of calcium nitrate, 1 to 5 percent of iron oxide, and the balance being micro turpentine, sulfur and paraffin wax. The bunker coal environment-friendly synergist makes flame of combustion be more rampant and denser, the furnace temperature rise to a higher level, the hearth be brighter and cleaner and tail gas be cleaner.
Owner:李政 +1
Mixed molten salt as heat transfer and storage medium low in melting point
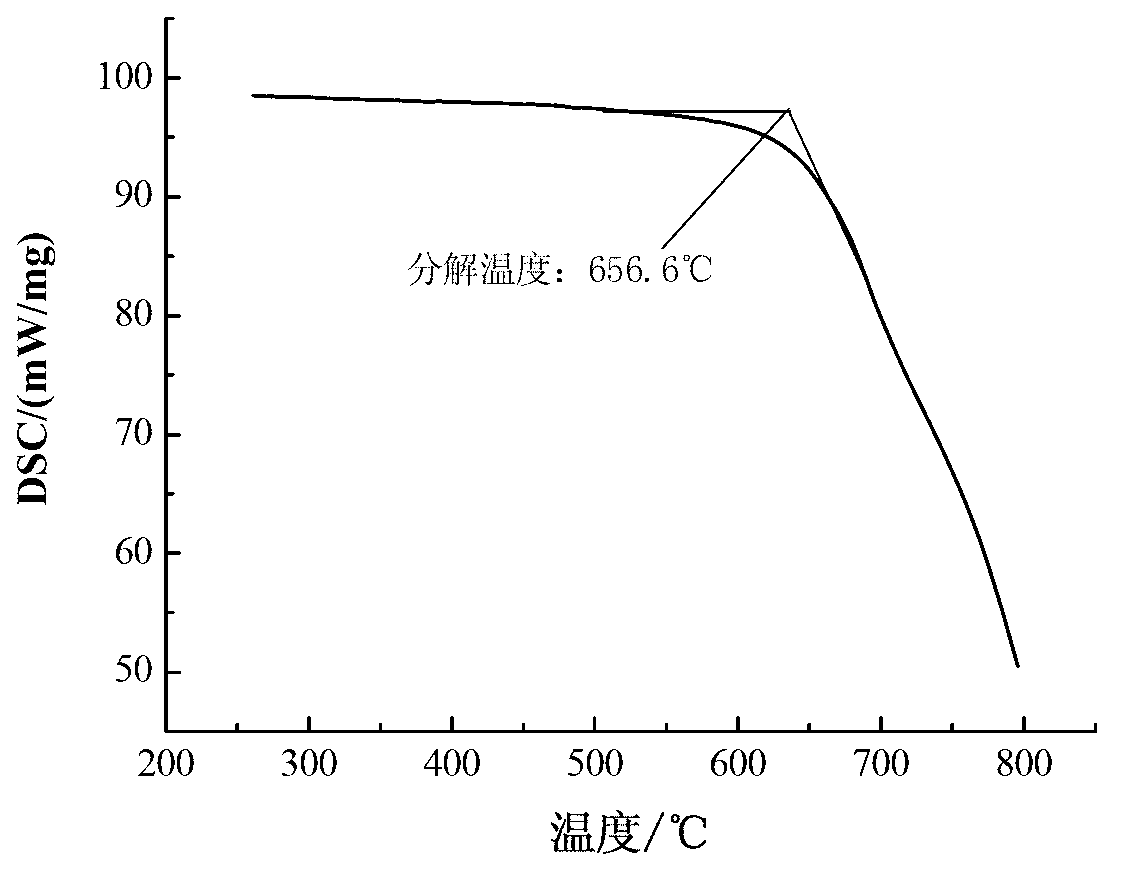
The invention relates to a formula of a mixed molten salt for medium-high temperature heat transfer and storage, and belongs to the physical heat transfer and energy storage technology in innovative and high technologies. The mixed molten salt comprises components in a ratio as follows: 10wt% of calcium nitrate, 60-70 wt% of potassium nitrate, 10-20 wt% sodium nitrate and 10wt% of sodium nitrite; the melting point of the mixed molten salt is about 130 DEG C, which is reduced by nearly 90 DEG C relative to solar salt and is reduced by about 15 DEG C relative to Hitec salt; and the thermal decomposition temperature thereof reaches above 650 DEG C. Sodium nitrate in the molten salt is changed into lithium nitrate, and the specific component ratio is as follows: 18-20wt% of calcium nitrate, 50-55 wt% of potassium nitrate, 9-10 wt% sodium nitrate and 18-20wt% of sodium lithium nitrate; after the component ratio is changed, the melting point of the mixed molten salt is about 90 DEG C, which is reduced by nearly 130 DEG C relative to the solar salt and is reduced by about 50 DEG C relative to the Hitec salt; and the thermal decomposition temperature thereof reaches above 600 DEG C. 10wt% of sodium carbonate is added continuously, then the melting point is raised to about 110 DEG C, while the decomposition temperature is raised by nearly 20 DEG C.
Owner:河北井矿新能源科技有限公司
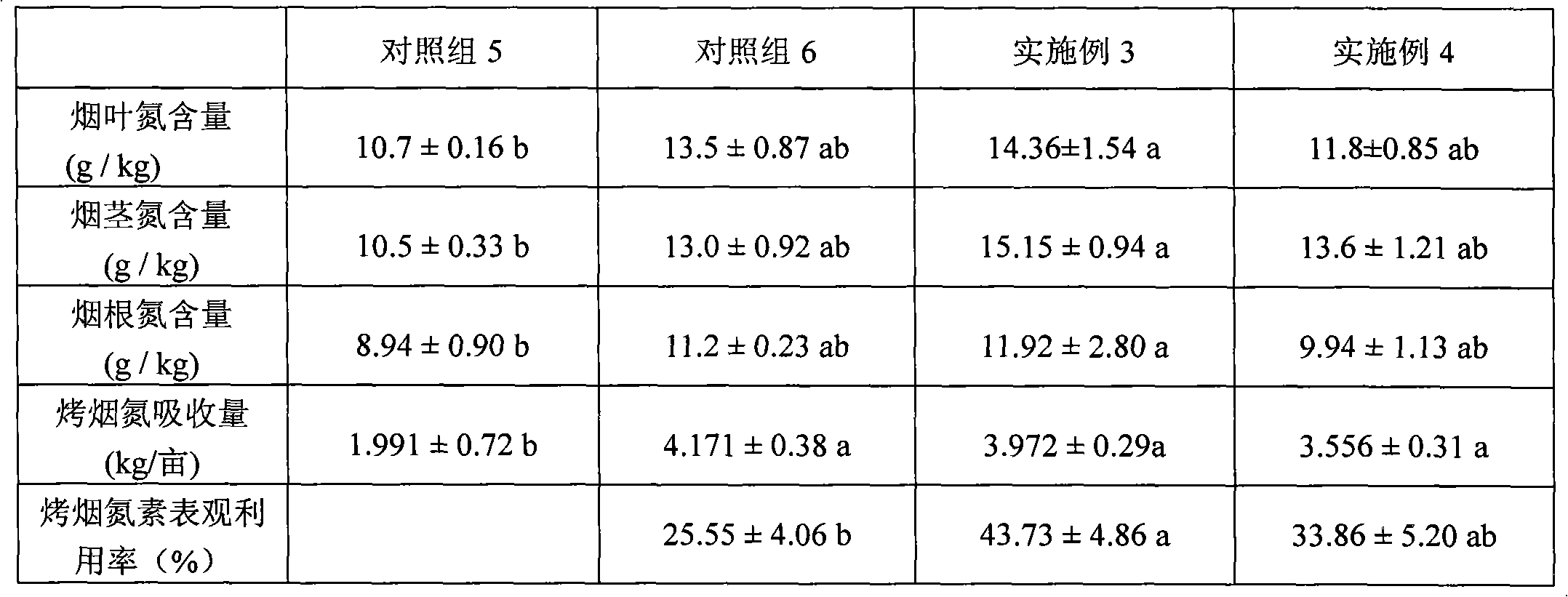
One-time nitrogen supply and fertilizer application method capable of improving applied nitrogen fertilizer effect of cured tobacco
InactiveCN102007843AStrong continuous nitrogen supply capacityEnhanced long-lasting nitrogen supply performanceFertilising methodsHorticultureRidgeHigh heat
The invention relates to a one-time nitrogen supply and fertilizer application method capable of improving applied nitrogen fertilizer effect of cured tobacco. The method is characterized in that: organic fertilizer decomposed cake fertilizer, decomposed pig manure, inorganic potassium nitrate fertilizer, special compound fertilizer for the cured tobacco and calcium superphosphate are used as base fertilizers; the fertilizers are applied in holes uniformly at the same day of transplanting the cured tobacco, tobacco ridges are covered by using a double-color plastic film, furrows of the tobacco ridges are connected with a drainage ditch, and the nitrogen amount of the organic fertilizer is 55 to 65 percent of the nitrogen amount of the base fertilizers; the nitrogen amount of the inorganic fertilizer is reduced by 45 to 55 percent according to the nitrogen amount of the inorganic fertilizers of the local base fertilizers and additional fertilizers, wherein the nitrogen of the potassium nitrate form accounts for 55 to 63 percent; in 25 to 35 days after the cured tobacco is transplanted, the film is uncovered, soil dressing is performed, and first top dressing is performed by using potassium sulfate and adopting a spraying mode; and second top dressing is performed in 40 to 45 days after transplanting, and third top dressing is performed after topping. In the method, the utilization rate of the cured tobacco nitrogen fertilizer is improved by 30 to 70 percent compared with the traditional fertilizer application, and the agricultural utilization rate of the cured tobacco nitrogen element is improved by 63 to 176 percent compared with the conventional fertilizer application; and the method is suitable for southern high-temperature rainy tobacco regions such as Guangdong and the like.
Owner:GUANGDONG INST OF ECO ENVIRONMENT & SOIL SCI +2
Water culture nutrient solution prescription of lettuce and preparation method of water culture nutrient solution prescription
InactiveCN103172431ASmooth changeMeet the changeFertilizer mixturesPhosphoric acidMonopotassium phosphate
The invention discloses a water culture nutrient solution prescription of a lettuce and a preparation method of the water culture nutrient solution prescription, and belongs to the technical field of plant soilless culture of the agricultural science. The nutrient solution prescription of the lettuce comprises the following components: a fertilizer A composed of 584.23g / T of calcium nitrate, 421.34g / T of potassium nitrate and 47.8g / T of ammonium nitrate; a fertilizer B composed of 182.4g / T of monopotassium phosphate and, 256.23g / T of magnesium sulfate; a fertilizer C composed of 16.8g / T of FeEDTA (Ethylene Diamine Tetraacetic Acid), 3g / T of boric acid, 2g / T of manganese sulfate, 0.25g / T of zinc sulfate, 0.85g / T of copper sulfate, and 0.38g / T of ammonium molybdate; hormone composed of 0.1g / T indolebutyric acid of or 0.1g / T of naphthylacetic acid; a bactericide composed of 15g / T of carbendazim; and nutrient solution pH (Potential Of Hydrogen) adjusting acid composed of 200ml / T of phosphoric acid or 150g / T of citric acid. The prescription is nutritionally balanced, so that the demand of the lettuce to nutrition can be met, the amount of used nutrient elements is decreased at the same time, the output of the lettuce is increased, and the fertilizers are saved; and the prescription effectively decreases the contents of nitrates in the lettuce under the water culture, improves the quality of the lettuce, enables the simplification of the complicated processes in preparation based on the demand of the actual operation, and is suitable for being used for meeting the requirements of industrialization production.
Owner:NORTHWEST A & F UNIV
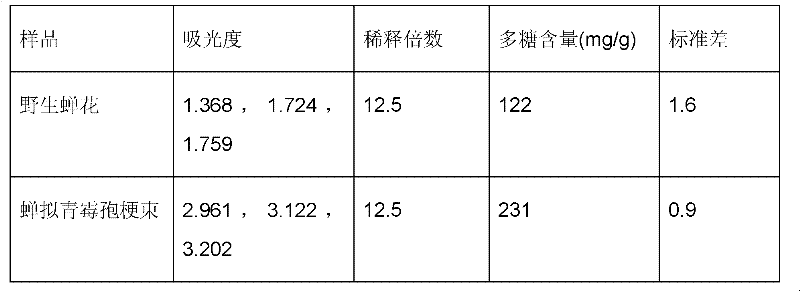
Method for artificially culturing paecilomyces cicadae and application of culturing product thereof
ActiveCN102242070AA Simple Method for Artificially Cultivating Paecilomyces cicadaeReduce manufacturing costCosmetic preparationsSenses disorderSucroseSaccharum
The invention discloses a method for artificially culturing paecilomyces cicadae and application of a culturing product thereof. The method for artificially culturing paecilomyces cicadae in large scales comprises the steps of: preparing strains, dosing and packing into a box, sterilizing, inoculating, solid-fermenting, collecting and the like. The paecilomyces cicadae is cultured by utilizing grains, such as corn flour, bran, wheat, barley, rice, millet, broomcorn and the like and culture mediums of bagasse, cane sugar, shell powder, silkworm chrysalis meal and potassium nitrate. The raw materials are obtained from local resources, a large amount of production cost is saved, the culturing period is shortened, and cordyceps sobolifera obtained by culturing has the advantages of high quality and favorable stability and the like. A culture obtained by the invention can be used for preparing foods, health-care products, drugs and cosmetics with functions of fighting tumor, regulating immunity, reducing blood sugar, blood fat and blood pressure, improving eye sight, resisting radiation, dispelling heat and easing pains, calming and hypnotizing, nourishing and strengthening, improving kidney function and the like.
Owner:ZHEJIANG BIOASIA PHARMA CO LTD
High-quality strawberry planting dedicated slow-release compound fertilizer
InactiveCN104030800AImprove the phenomenon of single fixationGood release effectFertilizer mixturesMicrobial agentGluconates
The invention relates to the compound fertilizer field, and particularly relates to a high-quality strawberry planting dedicated slow-release compound fertilizer. The fertilizer is prepared from the following raw materials in parts by weight: 10-15 parts of a 1250-2000 mesh diatomite, 4-5 parts of borax, 2-3 parts of ammonium molybdate heptahydrate, 10-12 parts of sodium humate, 20-25 parts of urea, 18-22 parts of potassium nitrate, 25-28 parts of organic compost, 2-4 parts of fish meal, 1-3 parts of sesame leaves, 8-10 parts of alfalfa meal, 15-18 parts of corn distiller grains, 2-4 parts of brine, 8-10 parts of an erythromycin fungi residue, 1-2 parts of an EM microbial agent, 4-6 parts of a fern root residue, 1-3 parts of nano silver, 1-2 parts of a semen ginkgo powder, 4-6 parts of 1,6-hexanediol diacrylate, 10-12 parts of an acrylic resin dispersion liquid, 1-2 parts of isocyanate, 1-2 parts of calcium stearoyl lactate, 2-4 parts of zinc gluconate, 6-8 parts of table vinegar, and 4-5 parts of an auxiliary agent. The compound fertilizer has multiple nutrients, is low in production cost and good in slow-release effect, allows double coating to be formed on the nutrients by utilizing diatomite and a coating agent in the process, also contains various trace elements, has no toxicity and no pollution, and is excellent in quality of planted strawberry and obvious in yield and income increasing effects.
Owner:ANHUI SUNSON CHEM
Preparation method of foam glass
The invention discloses a foam glass and a preparation method thereof. The main technique steps are that fly ash and waste glass are mixed and rubbed; the promoters composited by calcium carbonate, potassium nitrate, sodium nitrate, sodium carbonate, lithium carbonate, water glass and fibrin are added; the powder that has been rubbed into mould to mold is put; shaped product is sent to kiln to be dried and fired under high temperature, is annealed and finally cut, after the steps, the foam glass is made. The preparation technique is simple, and the production cost is reduced by choosing fly ash and waste glass instead of pearl rock, pumice, isinglass and other non-mine material, and the technique improves the use of solid wastes and is beneficial to environment protection.
Owner:内蒙古自治区建筑材料工业科学研究设计院
Fire-extinguishing aerosol without toxicity and corrosion for electric appliance
InactiveCN1386554AAvoid corrosionWon't happenFire extinguisherHazardous substancePotassium ferrocyanide
A fire-extinguishing aerosol not generating harmful substance for electric equipment (computer, communication equipment, electric generator, etc) is prepared from oxidant (strontium nitrate or strontium nitrate / potassium nitrate), reducer (guanidine nitrate or dicyandiamide) and modifier (potassium ferrocyanide, aluminium powder, carbon powder and phenolic resin). After it is ignited by sensor, alot of fire-extinguishing particles are generated to cover on the object to be protected while the inert gas is generated for extinguishing fire.
Owner:SHAANXI J&R FIRE FIGHTING CO LTD
Ternary nitric acid nano-molten salt heat transfer and storage medium, preparation method and application thereof
InactiveCN103881662AOvercome solubilityOvercoming thermal conductivityHeat-exchange elementsMetal oxide nanoparticlesNanoparticles dispersion
Belonging to the technical field of heat storage and transfer, the invention provides a ternary nitric acid nano-molten salt heat transfer and storage medium, a preparation method and application thereof. The ternary nitric acid nano-molten salt heat transfer and storage medium contains a ternary nitric acid molten salt system formed by potassium nitrate, sodium nitrate and sodium nitrite. The ternary nitric acid nano-molten salt heat transfer and storage medium is characterized in that it also includes metal oxide nanoparticles and / or non-metal oxide nanoparticles. The nanoparticles are dispersed into the ternary nitric acid molten salt system to undergo compounding so as to form the ternary nitric acid nano-molten salt heat transfer and storage medium. The ternary nitric acid nano-molten salt involved in the invention has a low melting point, an upper limit use temperature up to 600DEG C, good thermal stability, and high heat conductivity, thus being very suitable for the heat storage and transfer system of industrial energy storage and solar-thermal power generation.
Owner:QINGHAI ENESOON NEW MATERIAL TECH & SCI CO LTD
Soilless vegetable culture nutrient solution and preparation method
InactiveCN106699386AImprove stabilityHigh content of effective nutrientsCalcareous fertilisersMagnesium fertilisersAdditive ingredientNutrient solution
The invention relates to the field of nutrient solutions, in particular to a soilless vegetable culture nutrient solution and a preparation method. The soilless vegetable culture nutrient solution is prepared from sodium nitrate, urea phosphate, potassium nitrate, ammonium sulfate, magnesium chloride, Fe-EDTA, boric acid, manganese chloride, zinc sulfate, copper sulfate, sodium molybdate, mixed nitrogen-fixing bacterium solution and a nostoc extracting solution. The nutrient solution is simple and convenient to prepare, comprehensive in nutrient ingredient, high in biological activity and good in homogeneity, can decompose self-toxic materials produced by vegetables, remarkably improve the resistance, yield and the quality of the vegetables.
Owner:GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com