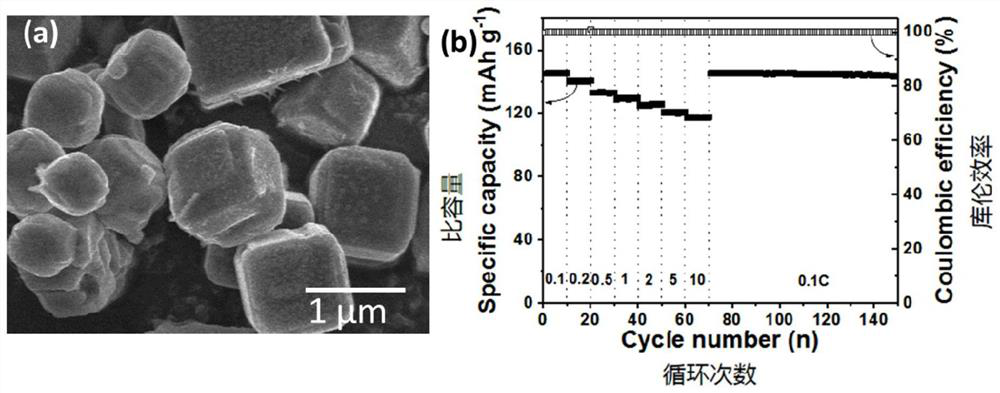
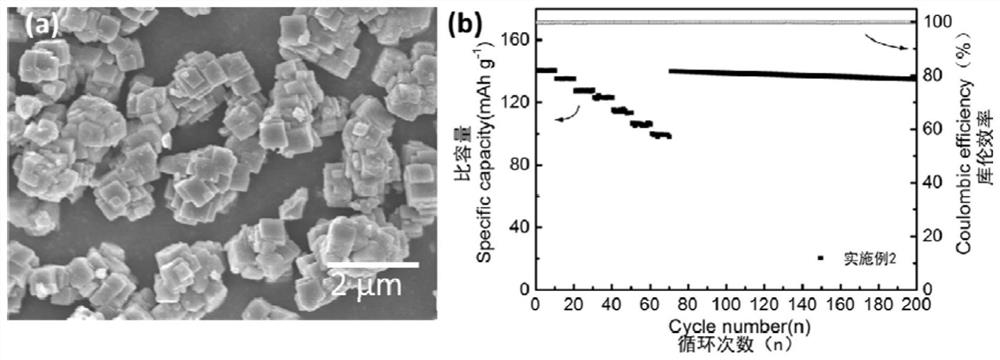
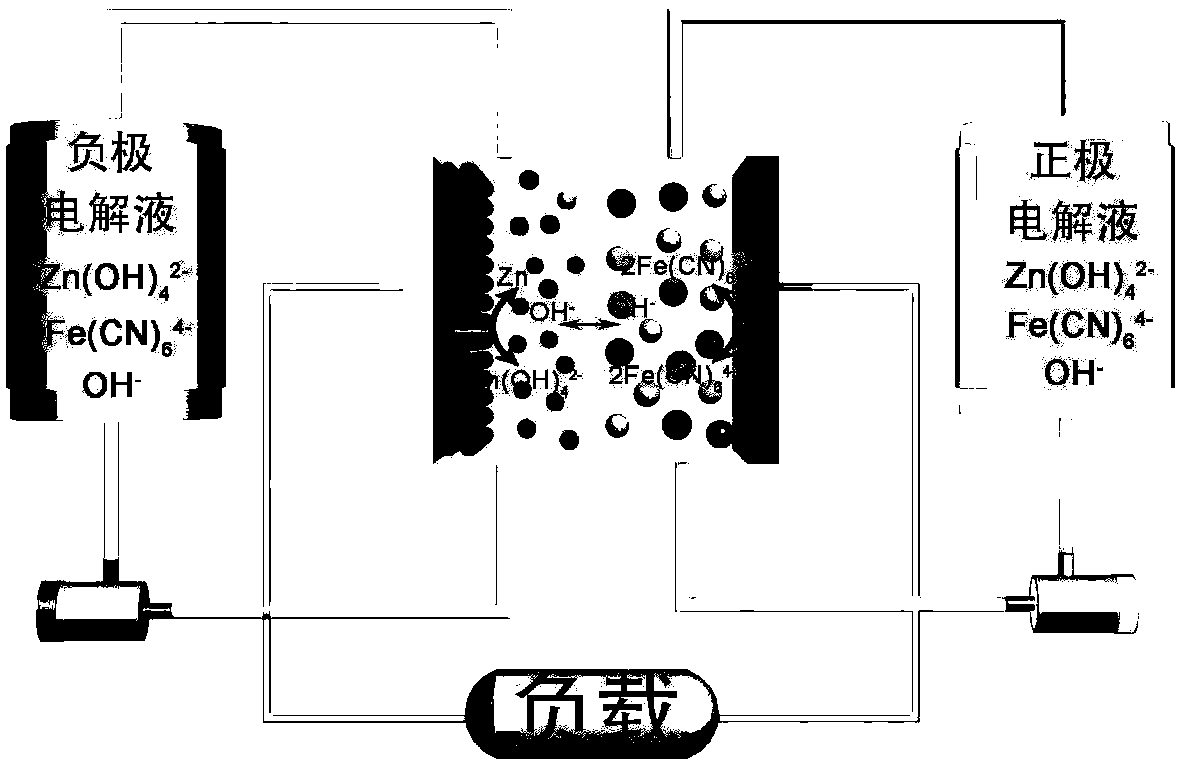
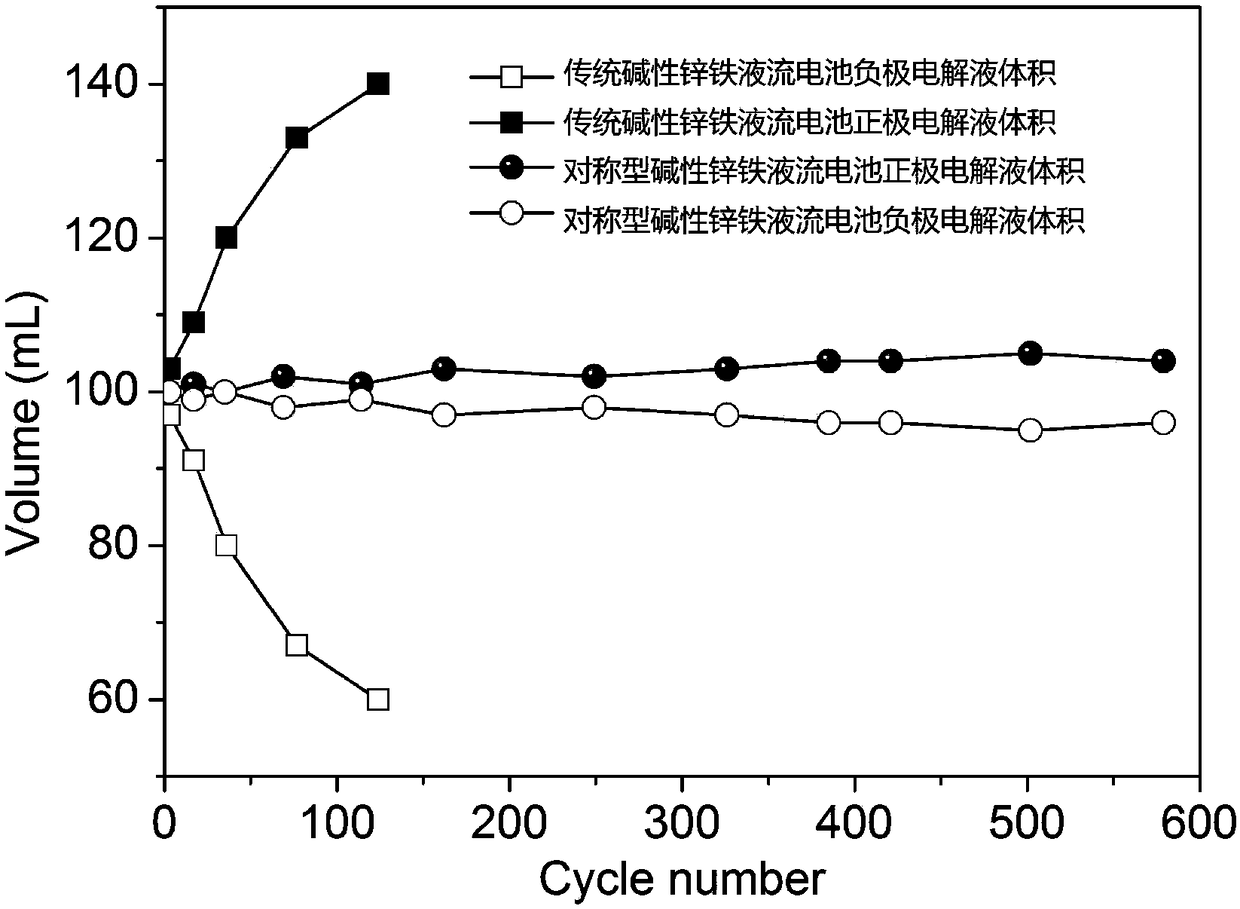
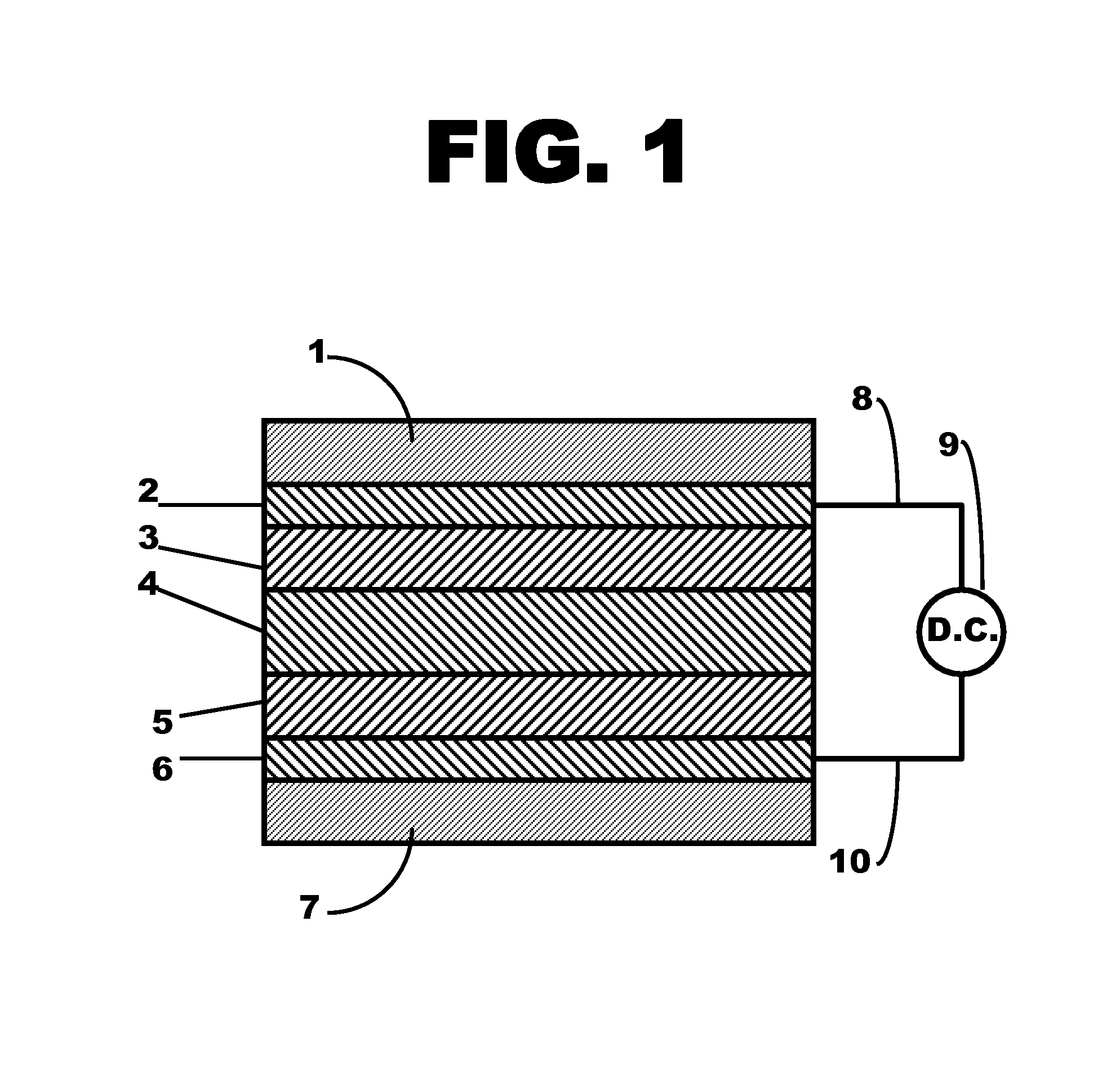
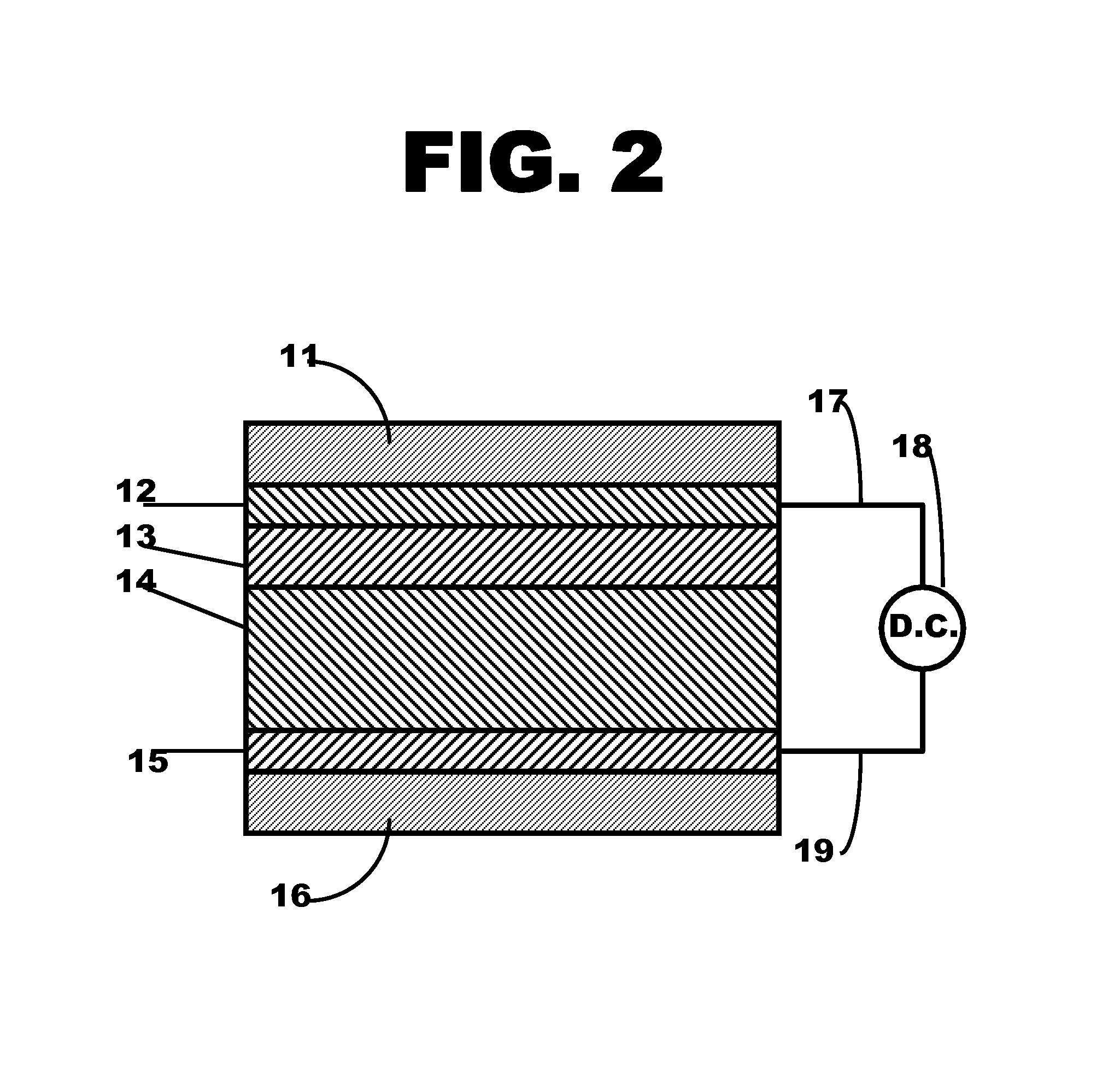
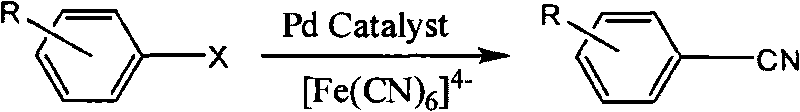Patents
Literature
110 results about "Ferrocyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
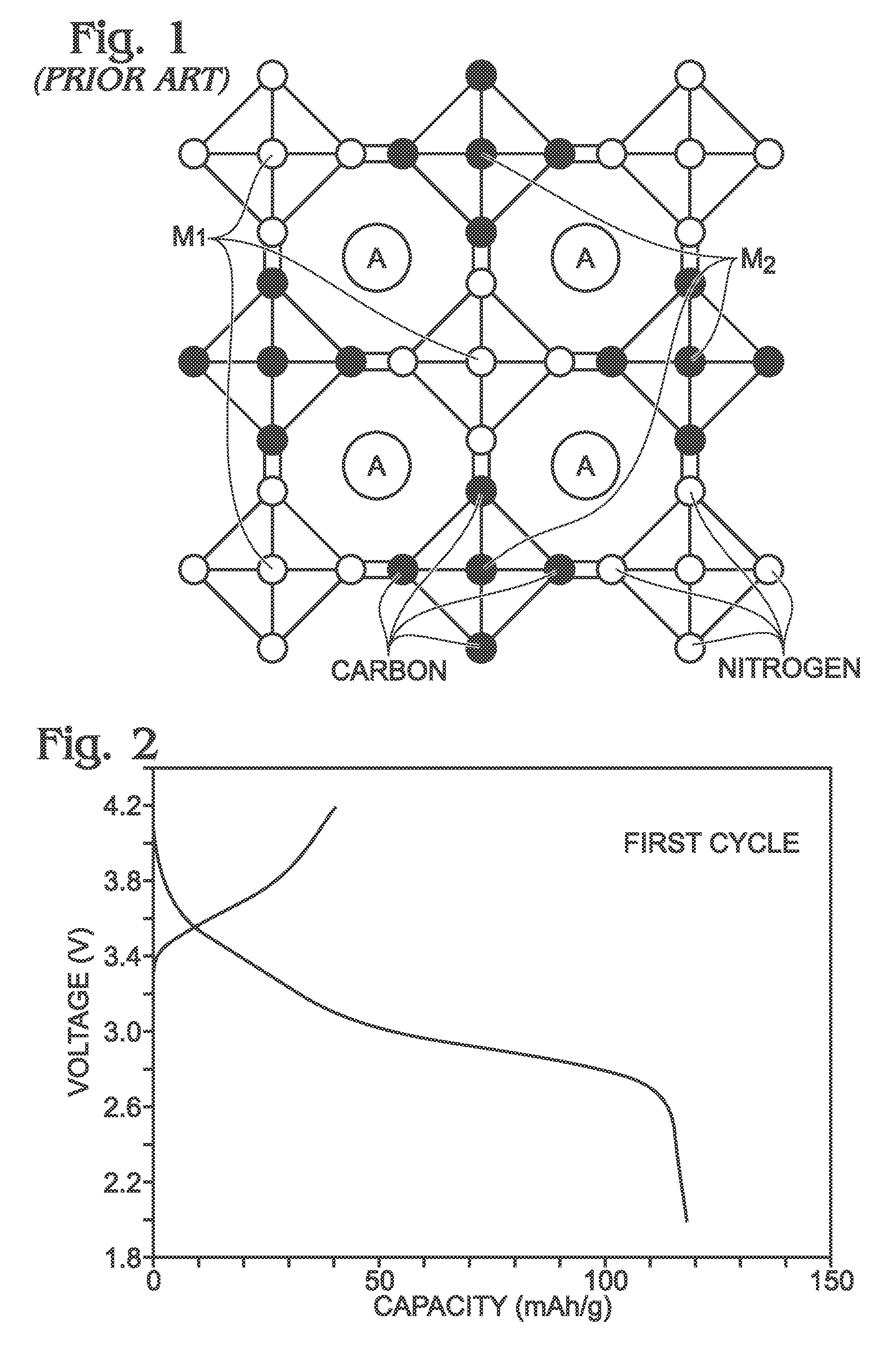
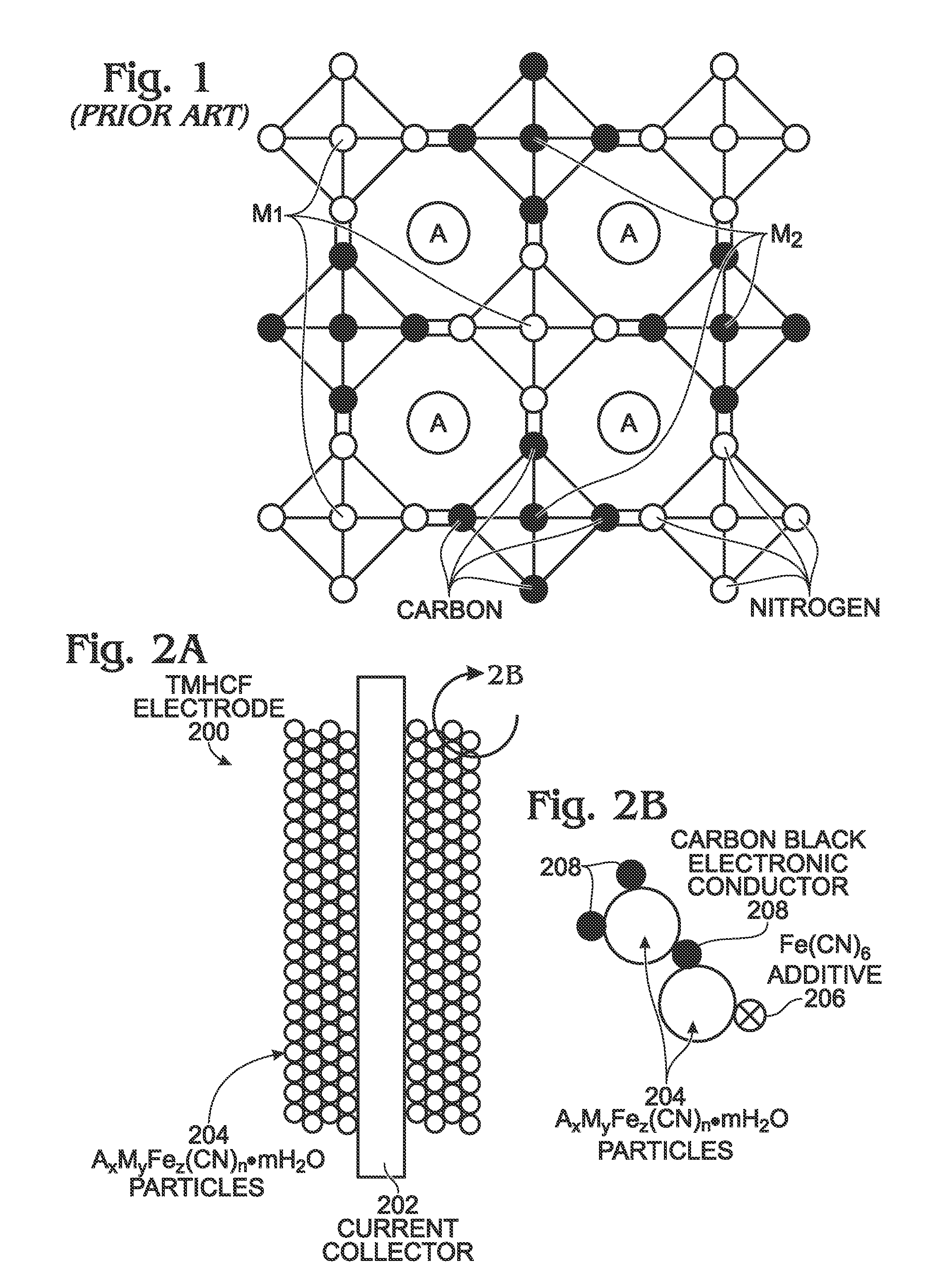
Ferrocyanide is the name of the anion [Fe(CN)₆]⁴⁻. Salts of this coordination complex give yellow solutions. It is usually available as the salt potassium ferrocyanide, which has the formula K₄Fe(CN)₆. [Fe(CN)₆]⁴⁻ is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide. It is of commercial interest as a precursor to the pigment Prussian blue and, as its potassium salt, an anticaking agent.
Method for preparing high-loading iron cyanide complex/silicon dioxide hybrid materials
ActiveCN101041123AIncrease loadFast adsorption rateSilicon compoundsRadioactive decontaminationPotassiumSilicon dioxide
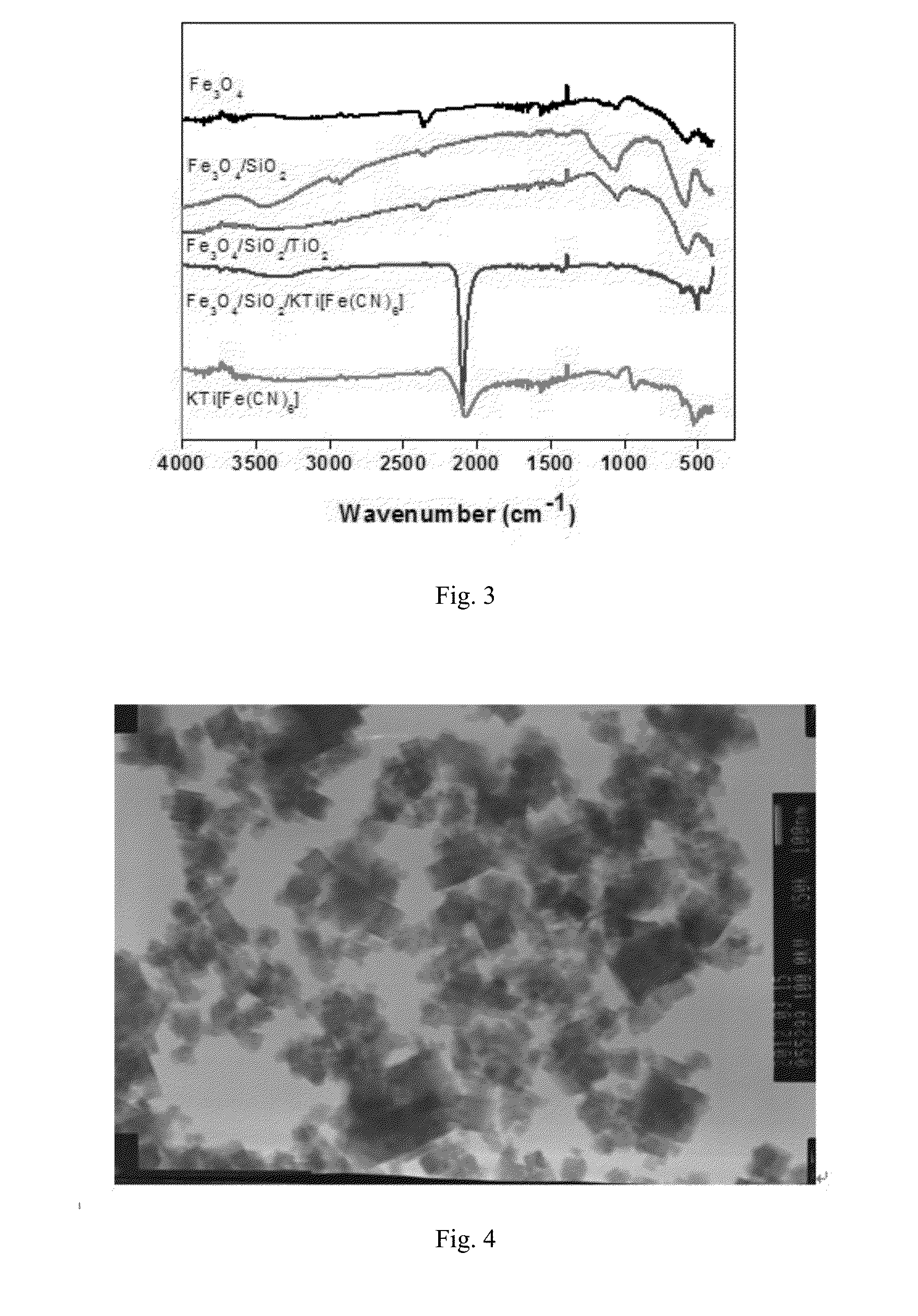
The invention relates to a preparing method for ferrocyanide with high load / silica hybrid material, belonging to a preparing method for radionuclide ion absorbing material. The method uses the salt solution with metal ion such as Mn, Sn, Ti, Fe, and Ni so on to react with potassium (sodium) ferrocyanide so as to obtain nanometer particle of ferrocyannide. The particle is fixed by silica solution in water system or is fixed by polymerization siloxane in organic solvent and proper inorganic acid, organic amine or amine water is added to obtain hybrid gel. The hybrid gel is dried, rubbed and screened to obtain ferrocyanide with high load / silica hybrid material. The high load of the material is high and the absorption to nuclide ion is strong. In addition, the material intensity can satisfy the demand of packed bed. The particle diameter can be controlled so as to avoid the problem that the water resistance of bed layer generated by ferrocyanide used alone is overlarge.
Owner:TSINGHUA UNIV
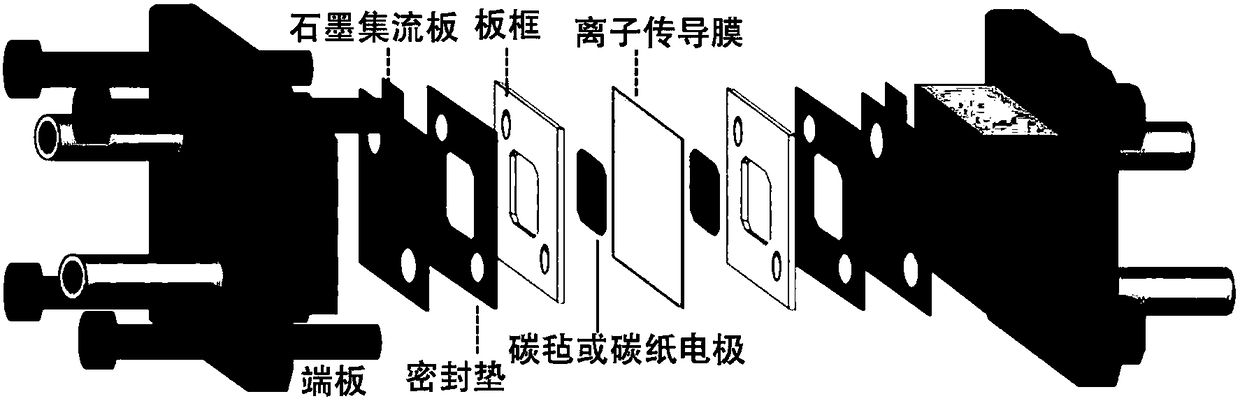
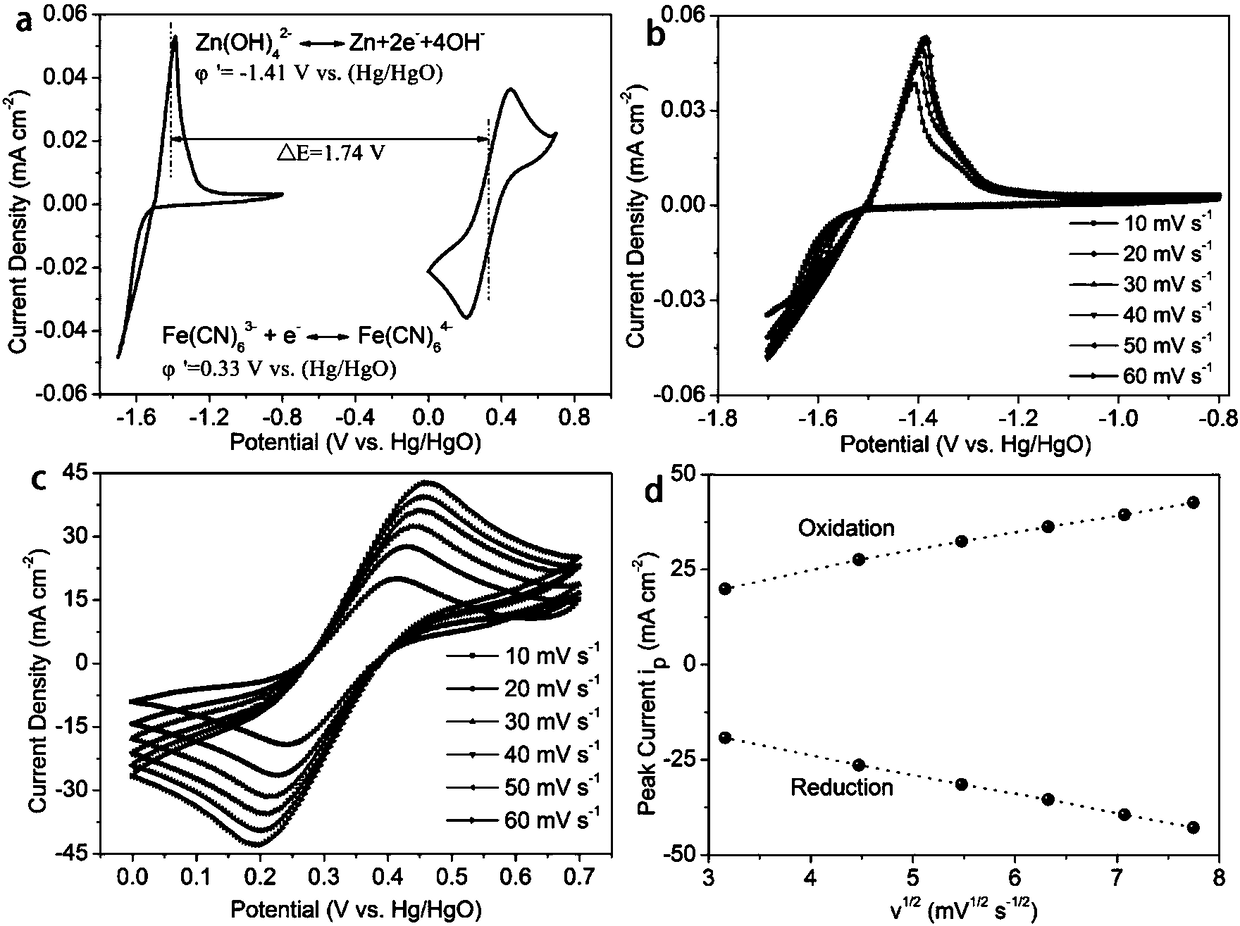
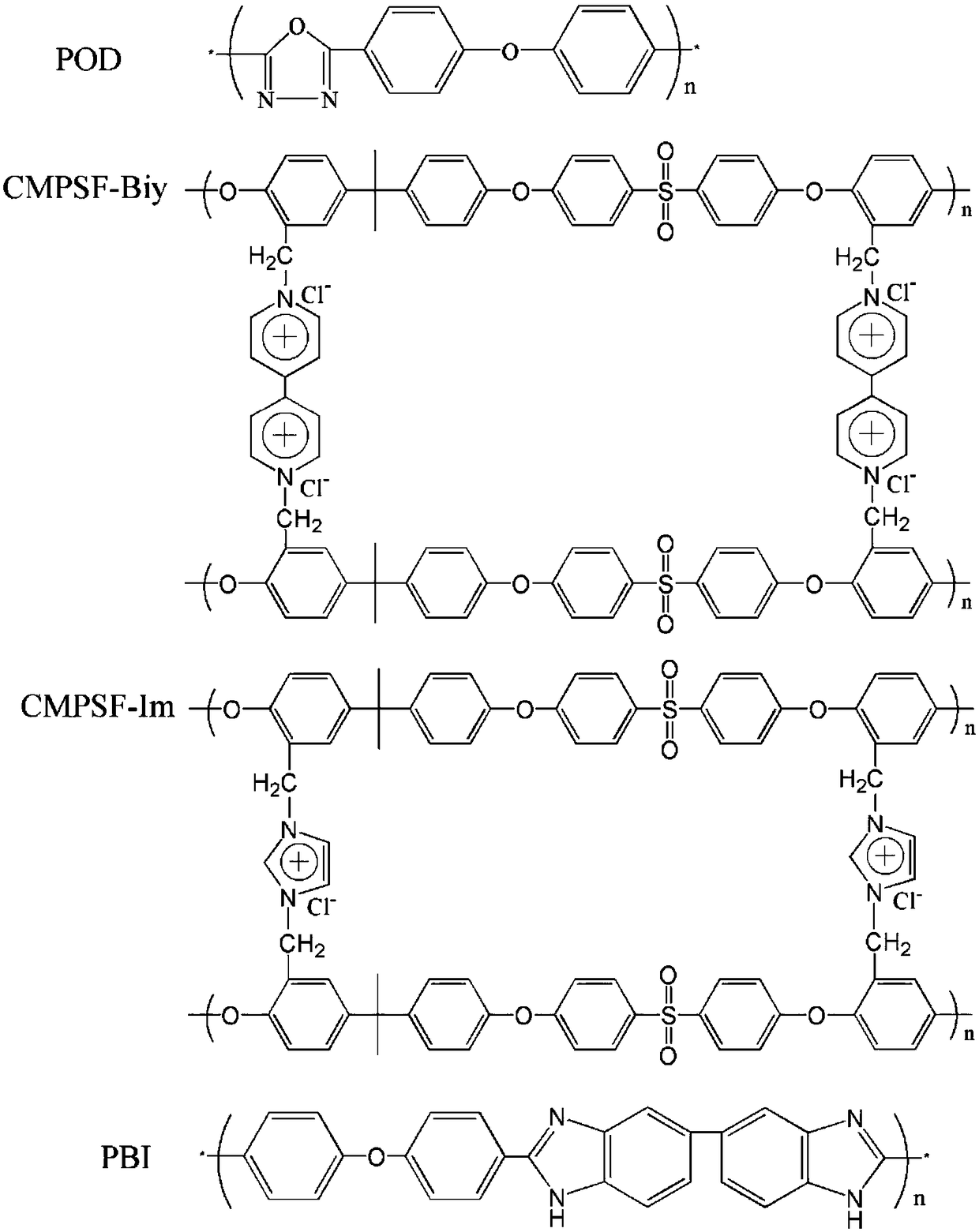
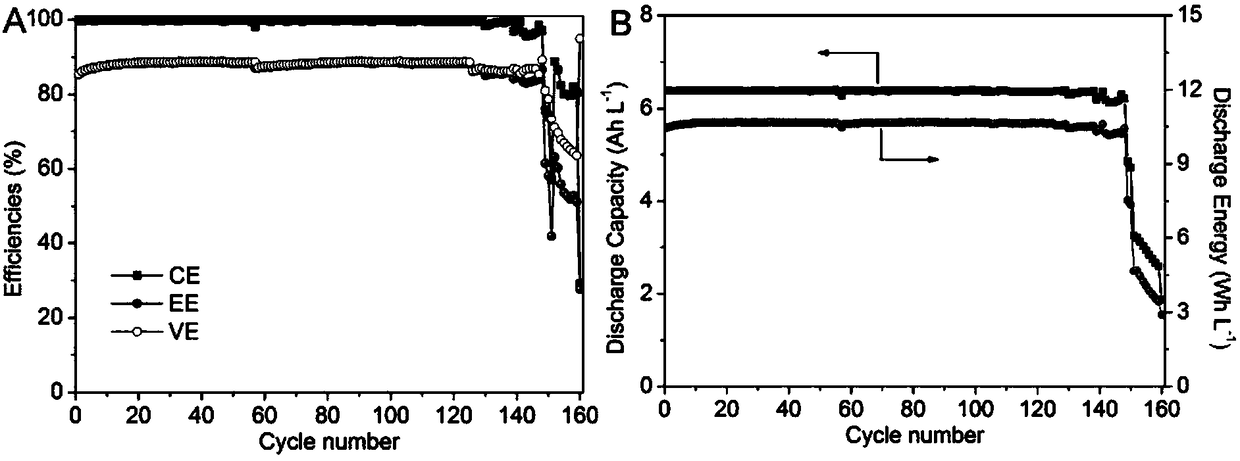
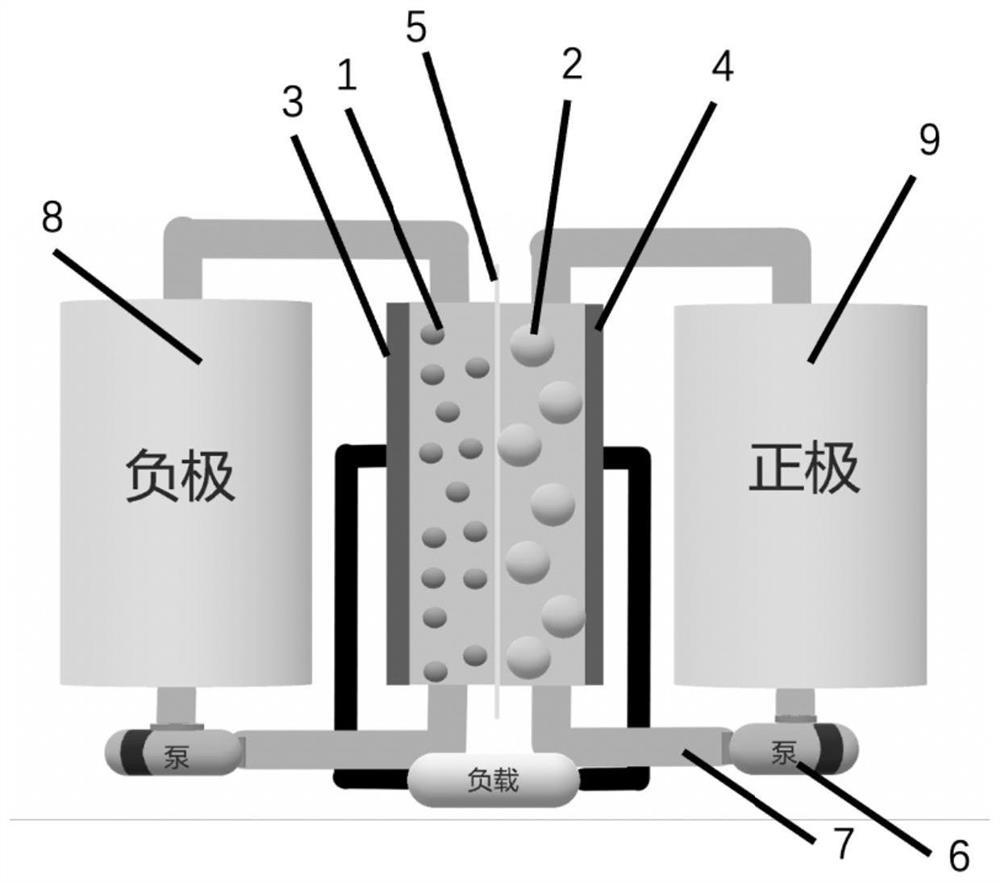
Alkaline zinc-iron flow battery
InactiveCN108461784ARich reservesLow costCell electrodesRegenerative fuel cellsHigh energyCarbon felt
The invention relates to an alkaline zinc-iron flow battery, wherein an ion conduction membrane is prepared from an aromatic polymer containing nitrogen heterocycle, electrodes are carbon felt or carbon paper, a positive electrode electrolytic solution is the mixed aqueous solution of ferrocyanide and strong alkali, a negative electrode electrolytic solution is the mixed aqueous solution of a zincsalt or / and a zinc oxide and strong alkali, the concentrations of the strong alkalis in the positive electrode electrolytic solution and the negative electrode electrolytic solution are 0.001-10 mol / L, and the concentrations of the active substances in the positive electrode electrolytic solution and the negative electrode electrolytic solution are 0.001-3 mol / L. According to the present invention, a class of the alkaline zinc-iron flow batteries with characteristics of high energy density, high power density and long service life are provided, wherein the performance of the batteries is comparable to or superior to the current mature all-vanadium flow batteries, and the batteries have good application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
MAGNETIC CORE COATED INORGANIC ION ADSORBENT FOR REMOVING Cs IONS IN RADIOACTIVE WASTEWATER AND PREPARATION METHOD THEREOF
ActiveUS20150231598A1Good alkali resistanceInhibiting oxidation of the magnetic core materialOther chemical processesRadioactive contaminantsHydration reactionSorbent
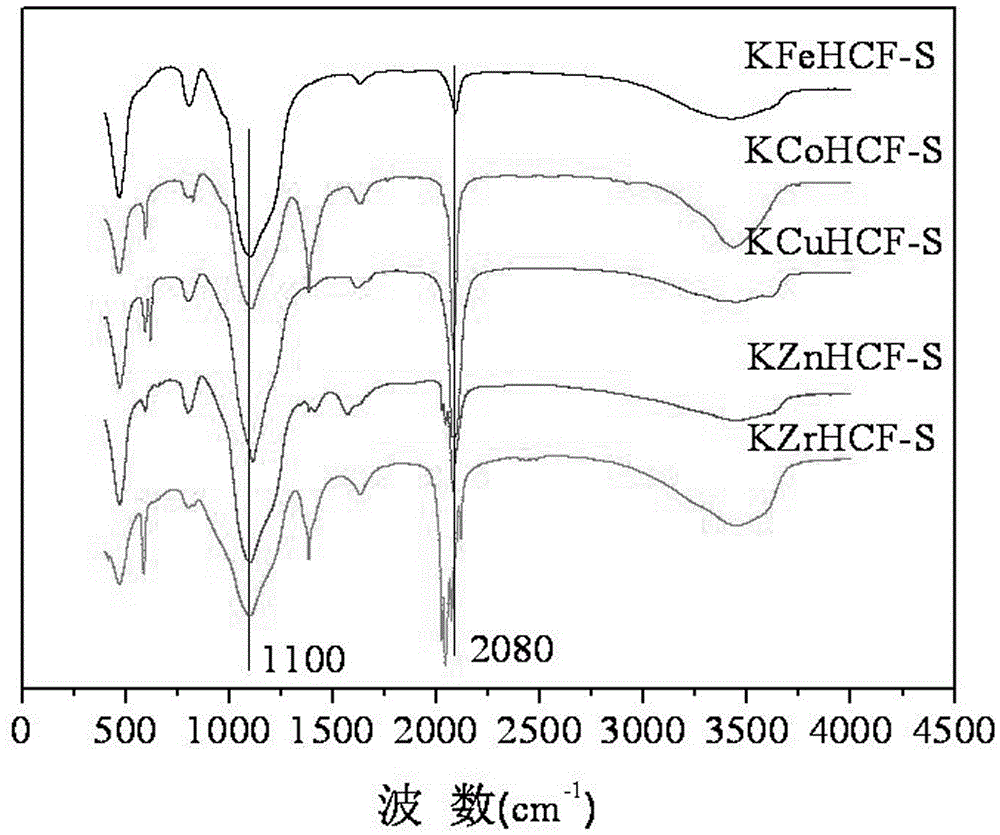
The invention discloses a micron-grade magnetic core coated ferrocyanide adsorbent for removing Cs ions in radioactive wastewater and a preparation method thereof. The adsorbent takes magnetic Fe3O4 as a core, the surface is coated with a dense SiO2 single layer serving as a protective layer, and an active component is metal ion stabilized potassium ferrocyanide coated on the outer layer, wherein stabilized metal ions comprise Ti, Zn, Cu, Ni, Co, and Zr. The particle size of the adsorbent is 0.2-5 μm, the adsorbent in the outermost layer is conductive to improving the adsorption efficiency for Cs+ ions, and an external magnetic field is adopted for realizing solid-liquid phase separation. The preparation method comprises the following steps: coating a hydrated metal oxide of Ti, Zr or Co, Ni, Cu or Zn on the surface of Fe3O4SiO2 to form a composite magnetic material, wherein the hydrated oxide performs hydroxyl polymerization reaction with the surface of SiO2 to produce M—O—Si bonds to improve the bonding strength between M and the surface of SiO2; and finally reacting the composite magnetic material with a potassium ferrocyanide solution to form the required composite adsorbent, wherein the metal ions M achieve the effects of stabilizing the ferrocyanide and also achieve a bridge effect for bonding the ferrocyanide and the composite magnetic material together.
Owner:TSINGHUA UNIV
A method for recycling coke oven gas desulfurization and decyanation waste liquid by vacuum potassium carbonate method
ActiveCN102267769AMeet the requirements of biochemical treatmentMultistage water/sewage treatmentMetal cyanidesHigh concentrationProcess equipment
The invention discloses a vacuum potassium carbonate method for utilizing coke oven coal gas desulphurization and decyanation waste liquid recycling as a resource. The method is also suitable to be used in other waste liquids containing high concentration cyanide and sulfide. According to the method, cyanide and sulfide are simultaneously precipitated; the precipitate is separated by centrifugation; the waste liquid processed from desulphurization and decyanation is processed through pH regulation and coagulant dosing; the processed waste liquid is discharged into a carbonization wastewater biochemical treatment system, and is treated; ferrocyanide precipitate is subject to a reaction with added lye in a high temperature, and is converted into potassium ferrocyanide; potassium ferrocyanide is disoluted, and is separated by centrifugation, such that potassium ferrocyanide mother liquor is obtained; potassium ferrocyanide mother liquor is cooled and crystallized, such that potassium ferrocyanide product is obtained. The method has advantages of simple equipment and convenient operation. With the method, simultaneous desulphurization and decyanation can be realized, requirements of subsequent treatments are satisfied, the wastes are utilized as resources, and economic benefits are brought in.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Prussian blue sodium ion battery positive electrode material and preparation method thereof
PendingCN111943228AIncrease contentImprove stabilityMaterial nanotechnologyIron cyanidesElectrical batteryPhysical chemistry
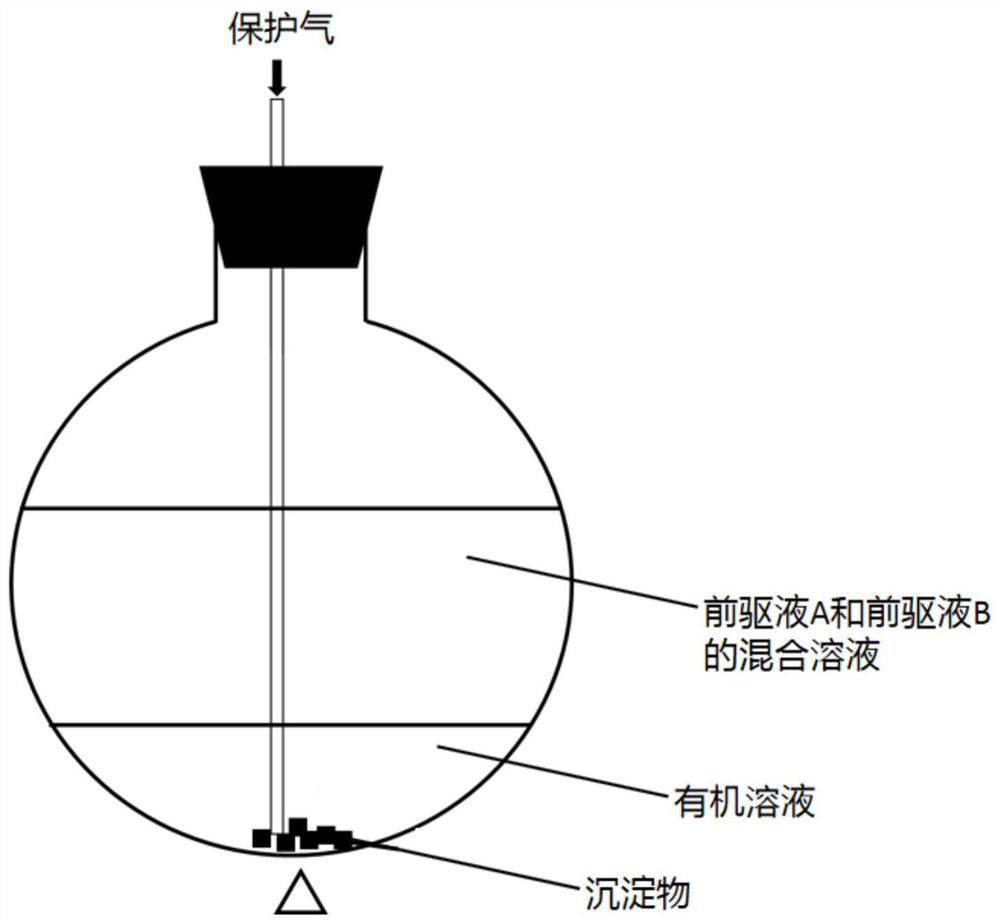
The invention provides a preparation method of a prussian blue sodium ion battery positive electrode material, which comprises the following steps: adding a slow-release agent into a precursor solution A and a precursor solution B, slowly releasing ferrous ions and transition metal ions in the process of mixing and reacting the precursor solution A and the precursor solution B, and forming a competitive relationship with the transition metal ions by the slow-release agent and ferrocyanide radicals. The reaction kinetics is greatly reduced, and the control on the reaction rate is realized. Theslow reaction greatly enhances the crystallinity of the material, can improve the morphology of the Prussian blue crystal, and reduces the generation of vacancies and the introduction of coordinationwater and crystal water. When the sodium-ion battery positive electrode material is used as a sodium-ion battery positive electrode material, a sodium-ion battery has relatively high initial Na<+> content and good stability and rate capability.
Owner:GLOBAL ENERGY INTERCONNECTION RES INST CO LTD +2
Alkaline zinc-iron electrochemical flow cell
ActiveCN109509901ASolve the problem of battery efficiency attenuationEfficiency attenuation problem solvingCell electrodesRegenerative fuel cellsSupporting electrolyteCarbon felt
The invention relates to an alkaline zinc-iron electrochemical flow cell. The alkaline zinc-iron electrochemical flow cell is composed of a battery module, an electrolyte storage tank, a circulating pump and a circulation pipeline, wherein the battery module is formed by connecting one single battery or more than two single batteries in series / in parallel; a positive electrode and a negative electrode adopt the same electrolyte, strong alkali serves as supporting electrolyte, carbon felts or carbon paper serves as the electrodes, a graphite plate serves as a current collecting plate to form the symmetric electrochemical flow cell; and the electrolyte of each of the positive electrode and the negative electrode is a mixed aqueous solution of ferrocyanide, zinc salt or / and zinc oxide and thestrong alkali. The symmetric alkaline zinc-iron electrochemical flow cell with the positive electrode and the negative electrode adopting the same electrolyte has the battery performance equivalent to that of a traditional alkaline zinc-iron electrochemical flow cell, meanwhile, due to the fact that the positive electrode and the negative electrode adopt the same electrolyte, the osmotic pressureof the electrolyte of the positive electrode and the electrolyte of the negative electrode is consistent, and therefore the problem of electrolyte migration caused by inconsistent osmotic pressure ofthe electrolyte of the positive electrode and the electrolyte of the negative electrode of the traditional alkaline zinc-iron electrochemical flow cell can be solved, and the maintenance cost of a system in the actual application process is effectively lowered.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Large-scale preparation method of high-stability caesium removing adsorbent, product of preparation method and application of product
PendingCN108160048AEfficient removalEmission reductionOther chemical processesRadioactive contaminantsParticulatesSorbent
The invention relates to a large-scale preparation method of high stability caesium removing adsorbent, a product of the preparation method and application of the product, in particular to transitionmetal-stabilized ferrocyanide adsorbent supported by particulate inorganic oxide or activated carbon. The transition metal-stabilized ferrocyanide adsorbent comprises a particulate inorganic oxide carrier or a particulate activated carbon carrier, a transition metal-stabilized ferrocyanide layer which coats the inorganic oxide carrier or activated carbon carrier and a polymer material layer coating the transition metal-stabilized ferrocyanide layer. The adsorbent has high crushing strength and a low ion leaching rate. The invention also relates to the preparation method of the adsorbent and the application of the adsorbent in removal of radioisotope Cs ions, stable-isotope Cs ions, radioisotope Rb ions and stable-isotopic Rb ions.
Owner:TSINGHUA UNIV
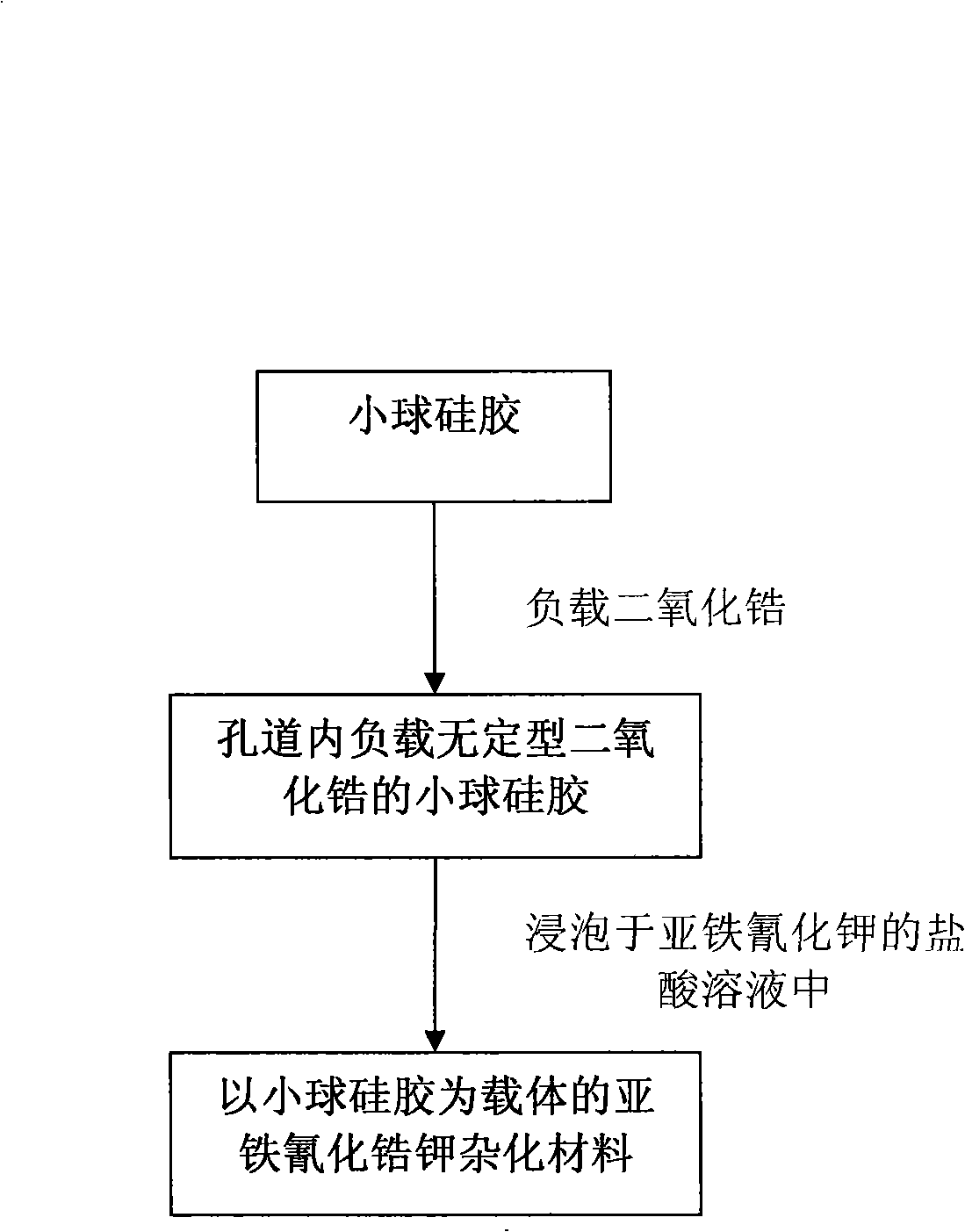
Preparation of potassium zirconium hexacyanoferrate using pellet silica-gel as carrier
ActiveCN101279249AThe load is easy to controlGood sphericityOther chemical processesWater/sewage treatment by sorptionPotassiumZirconium oxychloride
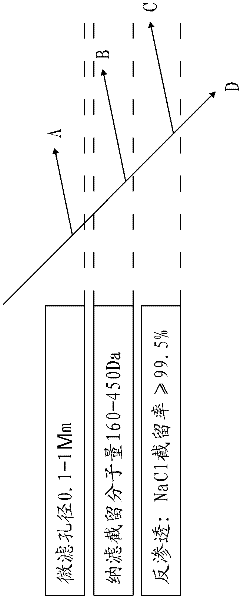
A preparation method for ferrocyanide zirconium Kalium taking bead silica gel as carrier relates to the preparation method for ferrocyanide zirconium Kalium with high specific surface and taking the bead silica gel as the carrier. In the method, fashioned porous bead silica gel react with the solution of zirconium oxychloride under the condition of heating and refluxing, acquired bead silica gel loaded with amorphous zirconium dioxide is dried and then dipped in the hydrochloric acid solution of yellow potassium ferrocyanide for reaction under stirring for 12-24 hours, thus the hybrid material of ferrocyanide zirconium Kalium with high specific surface and taking bead silica gel as the carrier is obtained. The load of ferrocyanide of the material can be adjusted and the material has strong adsorption capability for nuclide ion, high specific surface, good particle degree of sphericity, and being uneasy to break, which avoids the problem of oversized bed water resistance caused by using ferrocyanide zirconium Kalium particles separately; besides, owing to the existence of Zr-O-Si covalent bond, nano ferrocyanide zirconium Kalium particles can closely combine with the bead silica-gel, thus being uneasy to run away in the process of waste water treatment.
Owner:TSINGHUA UNIV
Composite anti-salt agent and applications thereof
ActiveCN107699209AImprove solubilityStrong complexationPipeline systemsDrilling compositionSolubilityFerrocyanide
The invention discloses a composite anti-salt agent, which mainly comprises a ferrocyanide solubilizer A and a complexone B, and can further comprise a polymer lattice modifier C and / or water. According to the present invention, the composite anti-salt agent has a synergistic effect, wherein the ferrocyanide solubilizer A in the composite anti-salt agent can substantially increase the solubility of the salt, and provides the main effect of the composite anti-salt agent, the complexone B in the composite anti-salt agent has good complexation effects on metal ions and can change the metal ion concentration of the salt crystal so as to reduce the solubility product of the salt crystal, and the polymer lattice modifier C can change the compact structure of the salt into the loose structure soas to make the salt be easily carried away by the flowing of the solution. According to the present invention, the composite anti-salt agent can provide excellent salt crystallization prevention effect on salt solutions with highly mineralized sodium chloride, and further can provide good salt release effects on salt solutions with highly mineralized sodium chloride, calcium chloride and magnesiumchloride.
Owner:CHINA PETROLEUM & CHEM CORP +1
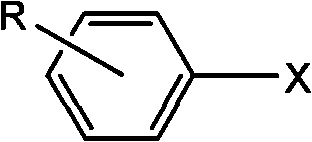
Synthetic method of aryl cyanide in water solution
InactiveCN101717350AEasy to separateReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by cyanide reactionSolubilityPalladium catalyst
The invention relates to a synthetic method of an aryl cyanide in a water solution, which comprises the following steps: adding an aryl compound, a ferrocyanide and a phase transfer agent into an alkaline water solution, or adding the aryl compound and the ferrocyanide into a mixed solution of alkaline water and an organic solution; controlling the reaction temperature at 30 DEG C-140 DEG C; and synthesizing the aryl cyanide by catalytic coupling of a metal palladium catalyst. In the invention, the non-toxic environment-friendly ferrocyanide is used as a cyanide source for synthesizing the aryl cyanide, and water is used as an environment-friendly solvent to substitute the virulent cyanide and the organic solvent in the prior art, thereby solving the problem of environment pollution in the process of synthesizing the aryl cyanide. In the invention, good solubility of the ferrocyanide in the water and the strong polarity of the ferrocyanide are used, thereby greatly promoting the generation of the aryl cyaniding reaction, the cyaniding reaction can be finished at lower temperature (30 DEG C-140 DEG C), the reaction yield is high, and the application range of the aryl cyaniding reaction is improved.
Owner:NANJING UNIV OF TECH
Process to separate the vanadium contained in inorganic acid solutions
A chemical process that recovers the vanadium contained in inorganic acid solutions, precipitating it as a solid compound of vanadium and alkali metal or monovalent cation ferrocyanide, is disclosed. Separation is carried out electrochemically, depositing the compound onto a metal immersed in the acid solution that contains vanadium as well as other dissolved metals, to which a ferrocyanide salt of an alkali metal or a monovalent cation has been previously added. If the inorganic acid present in solution is different than nitric acid, the vanadium can also be separated by direct addition of a ferrocyanide salt of an alkali metal or a monovalent cation to the acid solution containing vanadium. The method described allows recovery of vanadium without modifying the initial composition of the solution, except for the concentration of the vanadium dissolved.
Owner:UNIVE SIMON BOLIVAR
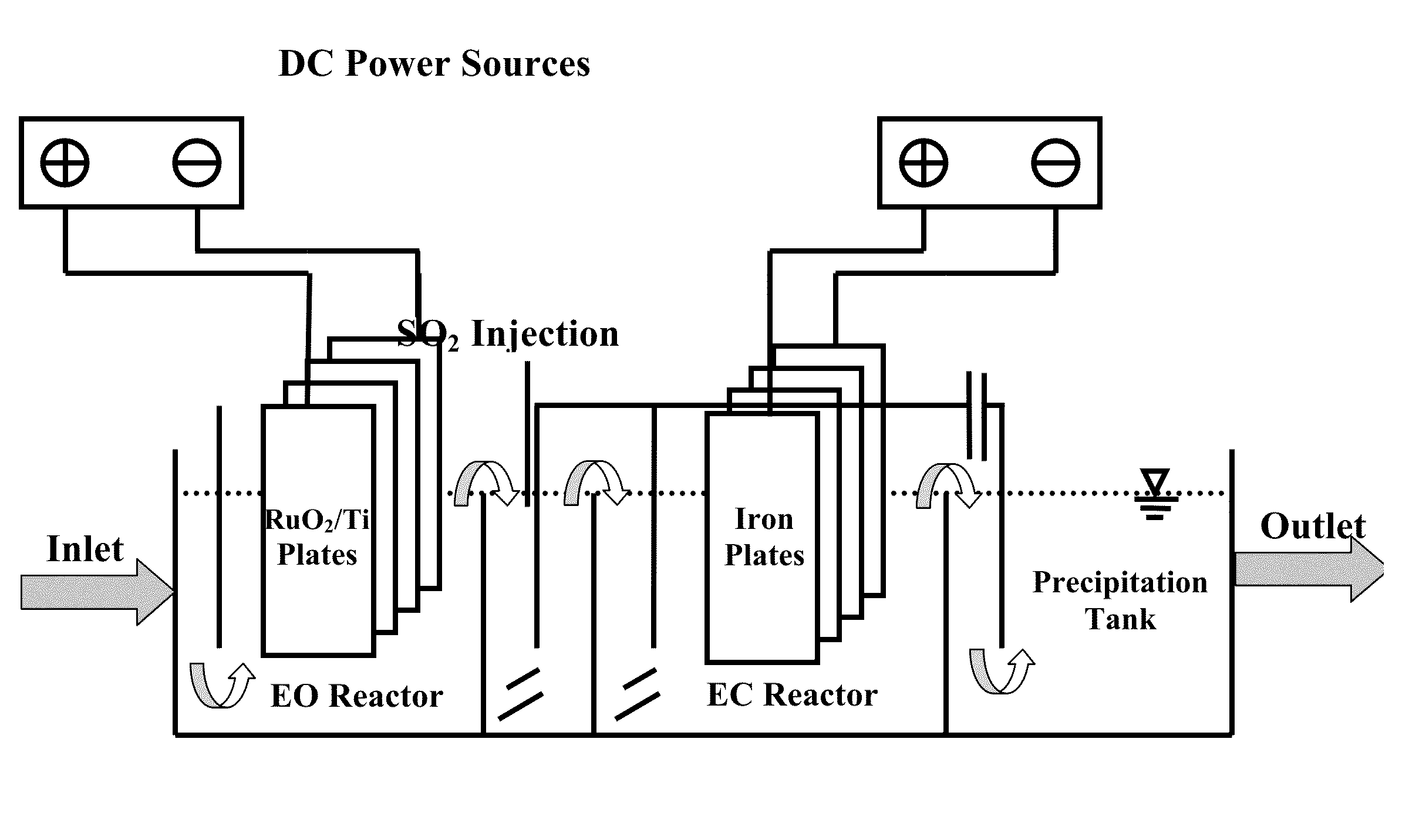
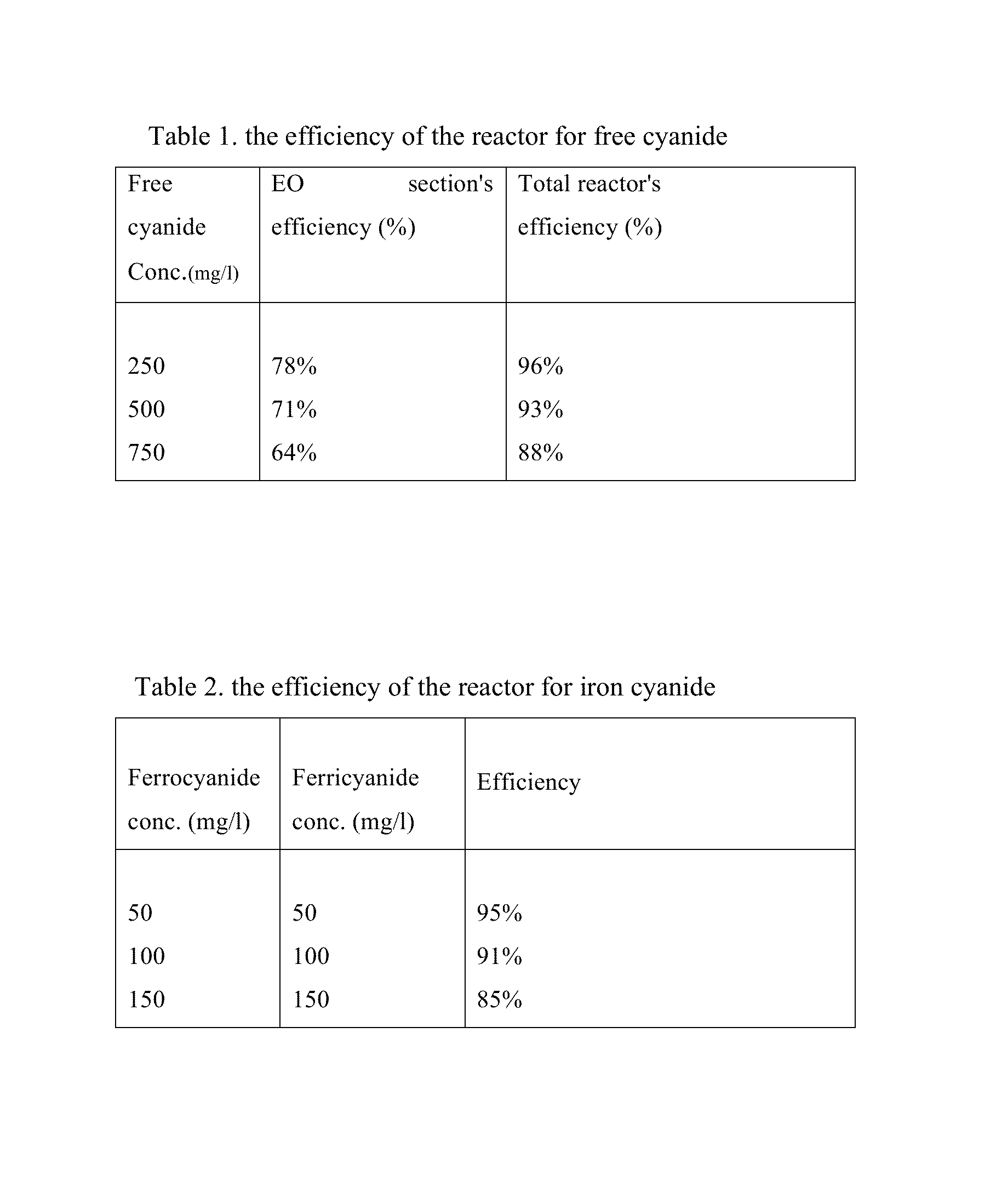
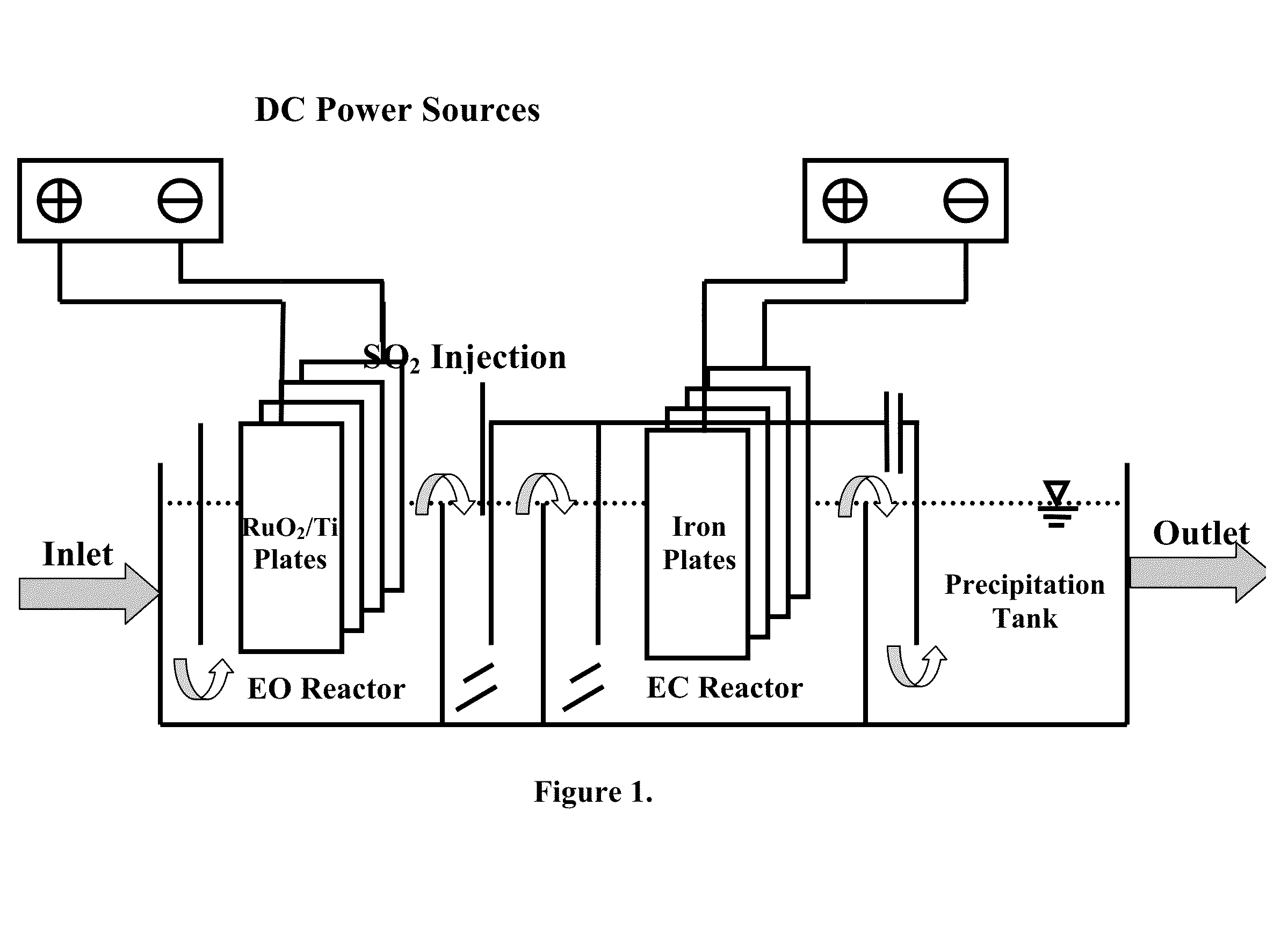
Electrochemical-based reactor for the removal of free cyanides and iron cyanide from industrial wastewater
InactiveUS20140246375A1Increase consumptionVolume of produced increaseWater contaminantsSedimentation separationElectrocoagulationIron cyanide
The present invention relates to a continuous bipolar electrochemically based reactor for the purpose of destroying free cyanide by direct and indirect oxidation and removing its strong complexes like ferrocyanide and ferricyanide by electrocoagulation. The designed reactor consists of four main sections, including electrooxidation, hydraulic mixing, electrocoagulation, and precipitation tank. The order of sections results in having a combination of reactions which separately remove cyanide and its compounds. The designed reactor shows a high flexibility in terms of handling highly variable kinds and concentrations of cyanide in the industrial wastewater effluents.
Owner:GHARIBI HAMED +1
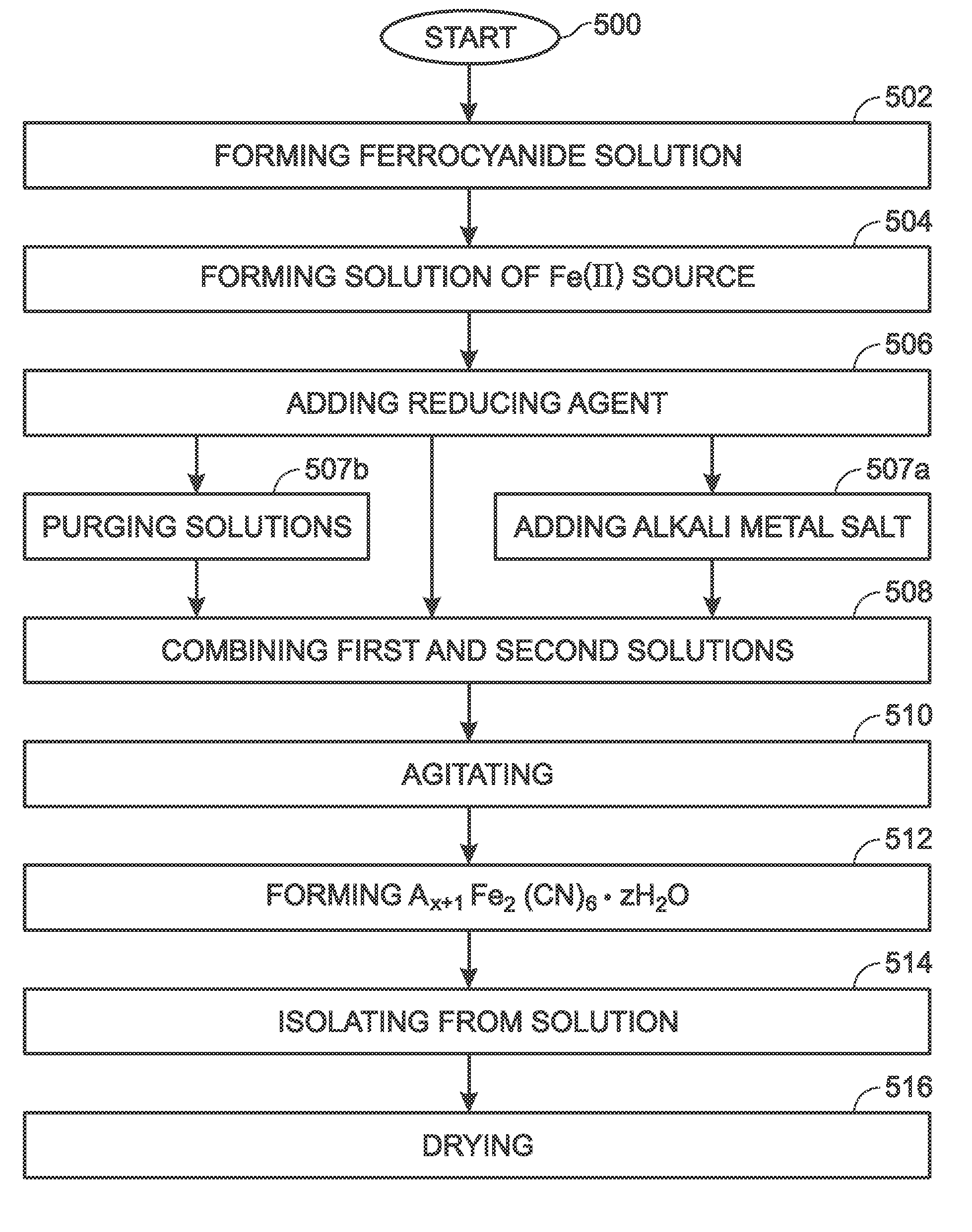
Precipitation Method for the Synthesis if Iron Hexacyaoferrate
ActiveUS20140370187A1High sodium contentGood flexibilityIron cyanidesElectrode thermal treatmentSolventFerrocyanide
A method is provided for synthesizing iron hexacyanoferrate (FeHCF). The method forms a first solution of a ferrocyanide source [A4Fe(CN)6.PH2O] material dissolved in a first solvent, where “A” is an alkali metal ion. A second solution is formed of a Fe(II) source dissolved in a second solvent. A reducing agent is added and, optionally, an alkali metal salt. The first and second solutions may be purged with an inert gas. The second solution is combined with the first solution to form a third solution in a low oxygen environment. The third solution is agitated in a low oxygen environment, and AX+1Fe2(CN)6.ZH2O is formed, where X is in the range of 0 to 1. The method isolates the AX+1Fe2(CN)6.ZH2O from the third solution, and dries the AX+1Fe2(CN)6.ZH2O under vacuum at a temperature greater than 60 degrees C.
Owner:SHARP KK
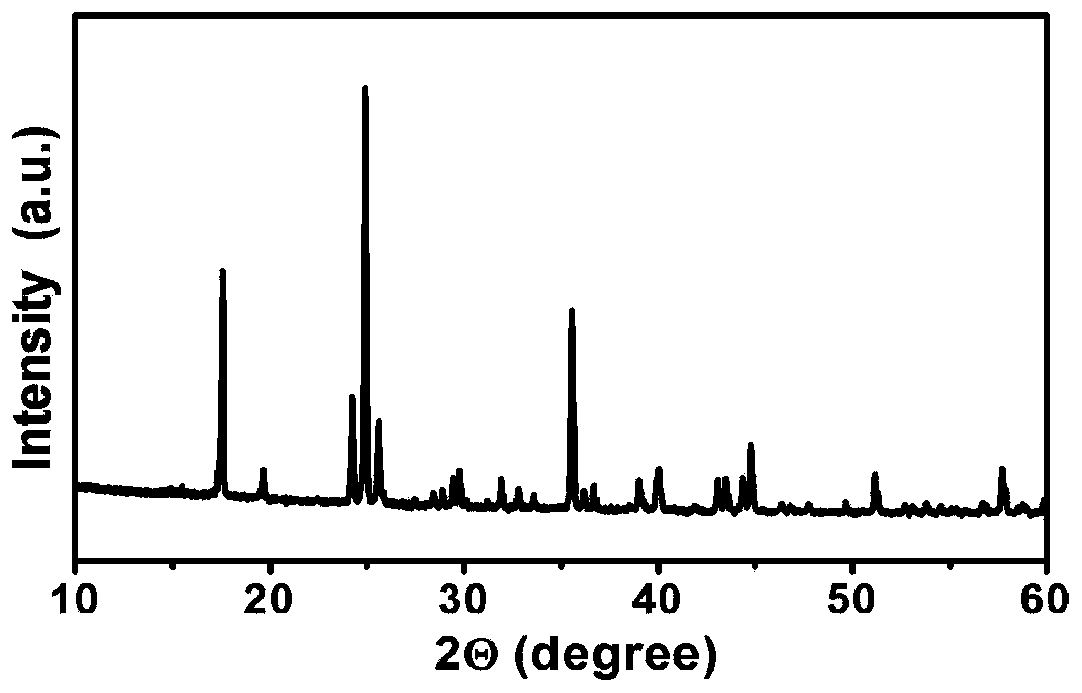
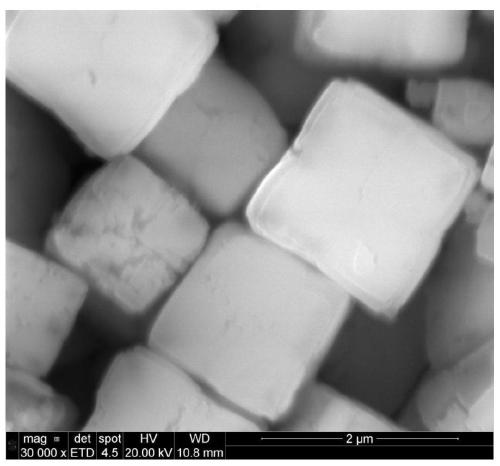
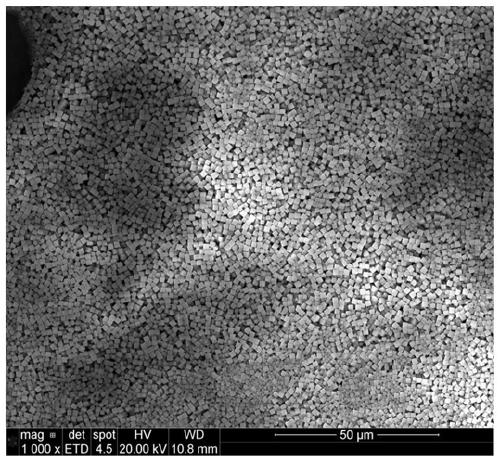
Low-water low-defect ferrocyanide manganese potassium Prussian blue cubic crystal and preparation method thereof
ActiveCN110002466AReduce the initial nucleation rateReduce defectsCyanic/isocyanic acidPotassiumManganese
The invention discloses a low-water low-defect ferrocyanide manganese potassium Prussian blue cubic crystal and a preparation method thereof, and belongs to the technical field of material synthesis.The invention aims to solve the problems of [Fe (CN) 6] 4-defect and high crystal water of ferrocyanide manganese potassium Prussian blue mateials, and provides the low-water low-defect ferrocyanide manganese potassium Prussian blue cubic crystal. A chemical general formula of the low-water low-defect ferrocyanide manganese potassium Prussian blue cubic crystal is shown in the description, wherein1.9<=x<=2.1, 0<=y<=0.03, 0<=z<=0.2, and a mass content of crystal water is less than 1%. By the adoption of the low-water low-defect ferrocyanide manganese potassium Prussian blue cubic crystal and the preparation method thereof, soluble manganese salt and potassium ferrocyanide are used as raw materials; and by optimizing a complexing agent and the reaction temperature, an excessively fast precipitation reaction speed is effectively reduced, and crystal growth time is prolonged, so that defects and crystal water in the Prussian blue crystal are reduced, and the Prussian blue cubic crystal with high quality and near stoichiometric ratio is obtained.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Metal ferrocyanide-polymer composite layer within a flexible electrochromic device
A flexible electrochromic device is disclosed, the device including a flexible substrate with at least one electrically conductive surface, and an electrochromic layer comprising particles of water-insoluble metal ferrocyanide with an average particle size less than about one micron, and polymer, the electrochromic layer deposited on a conductive surface of the flexible substrate, wherein the device is capable of being deformed and returned to “flatness” in an undamaged state.
Owner:CHAMELEON OPTICS
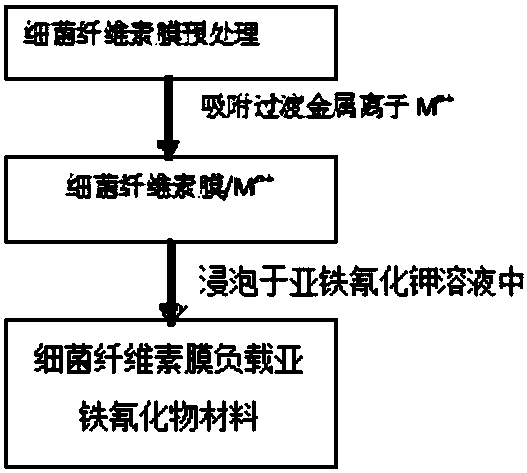
Method for preparing supported ferrocyanide bacterial cellulose membrane for adsorbing Cs+
InactiveCN108163923APreserve selective adsorptionEasy to operateRadioactive contaminantsCoatingsFiberPotassium ferrocyanide
The invention discloses a method for preparing a supported ferrocyanide bacterial cellulose membrane for adsorbing Cs+. The method is characterized by comprising the following steps: 1) pretreating abacterial cellulose membrane, and cutting the bacterial cellulose membrane into different sizes; 2) adsorbing transition metallic ions by using the bacterial cellulose membrane, soaking the bacterialcellulose membrane obtained in the step 1) into a transition metallic ion solution, and washing the surface of the bacterial cellulose membrane with distilled water; 3) supporting ferrocyanide on thebacterial cellulose membrane, soaking the bacterial cellulose membrane obtained in the step 2) into a potassium ferrocyanide solution, performing oscillation by using a table concentrator, and washingthe surface of the bacterial cellulose membrane with distilled water. The material prepared by using the method can be cut into different sizes according to demands, a ferrocyanide crystal is deeplyembedded into a fiber structure, and after treatment, the ferrocyanide is not liable to lose in the wastewater treatment process; the material has a relatively good adsorption property for radionuclide cesium ion, and meanwhile the problem that a bed layer has too large water resistance when the ferrocyanide is used independently is avoided.
Owner:TSINGHUA UNIV
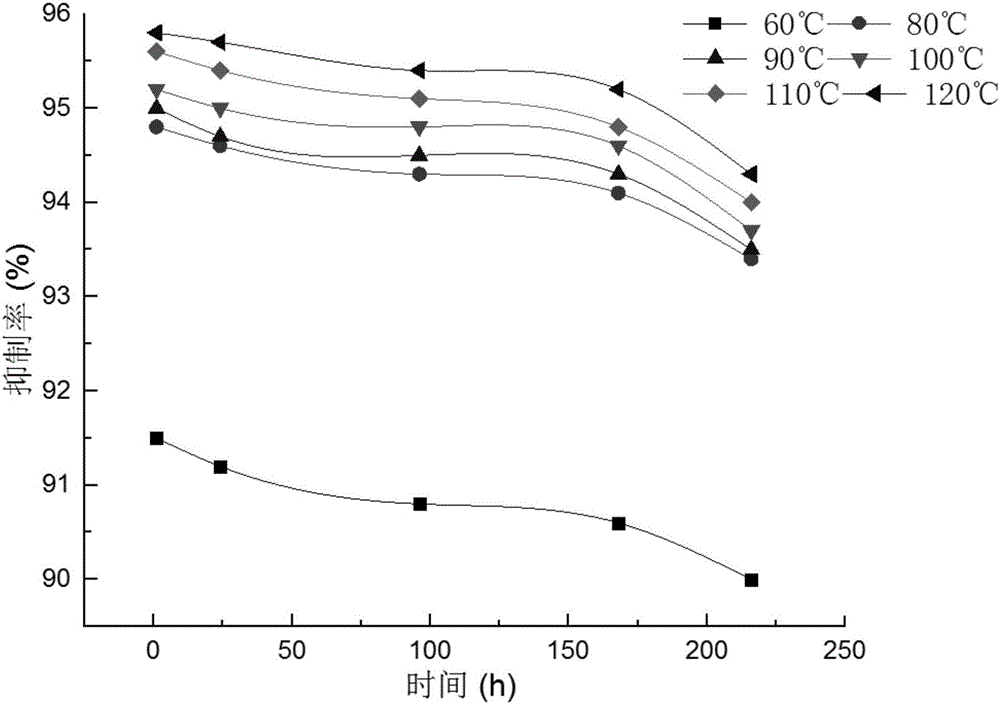
Composition for solid precipitation and inhibition of oil and gas well
The invention discloses a composition for solid precipitation and inhibition of an oil and gas well. The composition for solid precipitation and inhibition per 100 parts by weight is prepared from the following components: 0.08 to 0.13 part by weight of ferrocyanide, 0.005 to 0.02 part by weight of a polyether type surfactant, 0.005 to 0.02 part by weight of a hydrophobic modified cationic polymer and the balance of water. The composition can be put in the oil and gas well with hypersalinity; the ferrocyanide can be used for changing the charge distribution on the surface of metal cations, and the hydrophobic modified cationic polymer can be used for adsorbing anions in hydrops of the oil and gas well, so that the metal cations such as sodium ions can be prevented from being combined with the anions to form inorganic salts so as to be separated out from the hydrops of the oil and gas well, and the inhibition rate is 90 percent or more; the polyether type surfactant can be directionally adsorbed on the surface of hydrocarbon organisms such as paraffin, a polar surface can be formed, non-polar hydrocarbon organisms such as paraffin crystal can be prevented from being continuous crystallized and separated out, and the inhibition rate is 53 percent or more; the ferrocyanide, the hydrophobic modified cationic polymer and the polyether type surfactant can promote each other and are capable of commonly inhibiting the inorganic salts and the hydrocarbon organisms such as the paraffin from being separated out.
Owner:PETROCHINA CO LTD
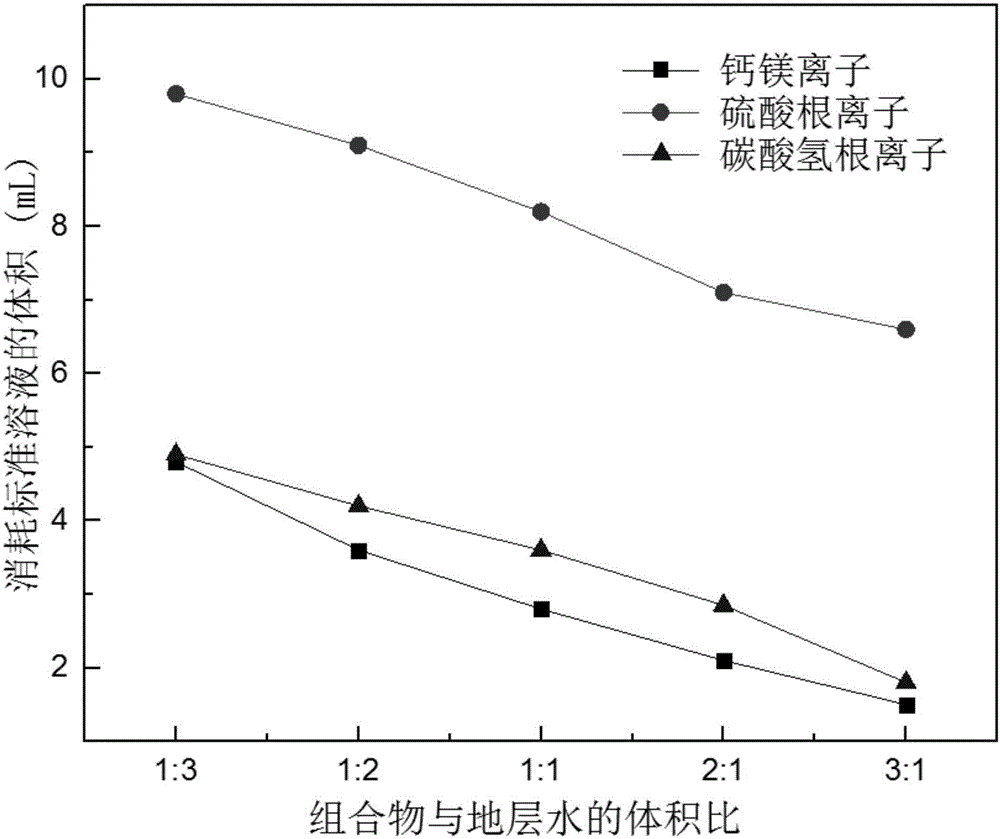
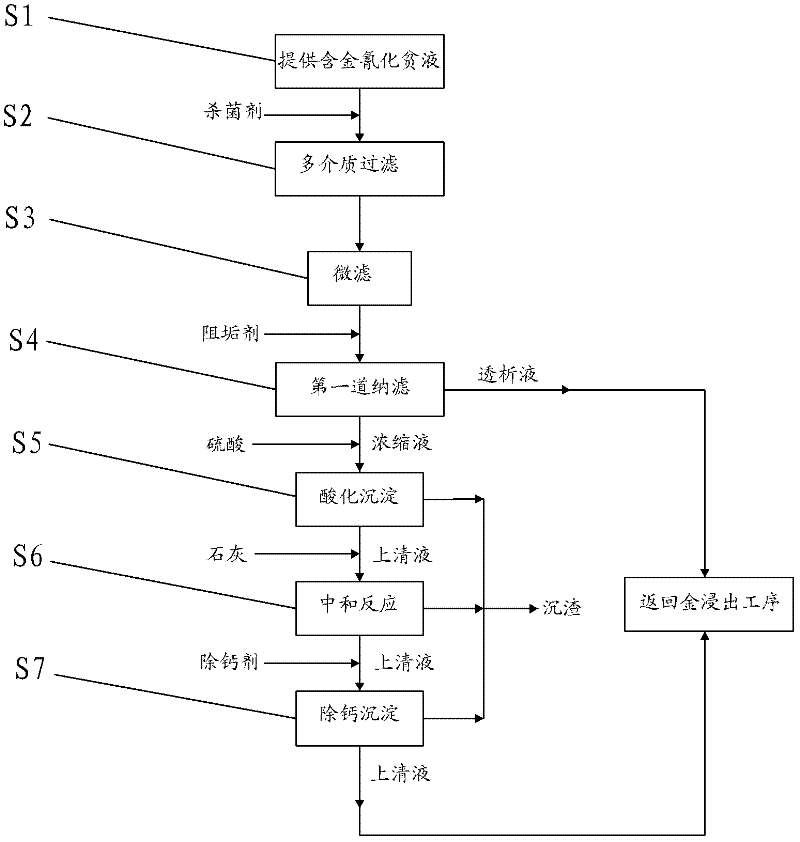
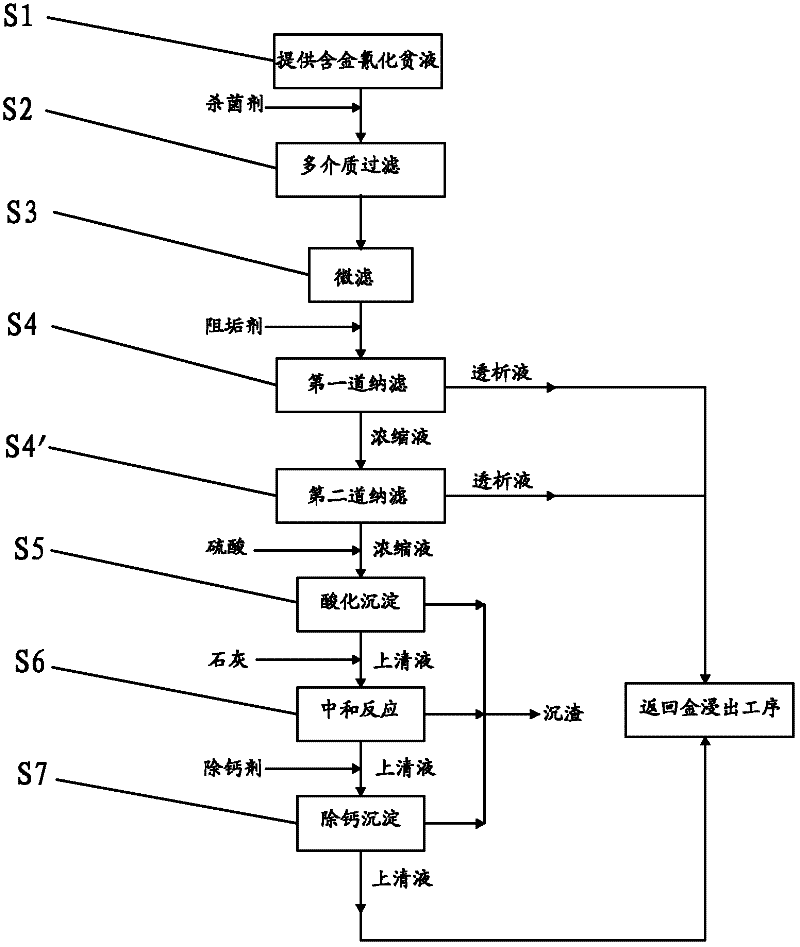
Method for recovering gold from gold-containing barren solution and purifying gold-containing barren solution
InactiveCN102329967AReduce dosageReduce recycling costsProcess efficiency improvementFiltrationSulfate
The invention discloses a method for recovering gold from gold-containing barren solution and purifying the barren solution. In the method, sulfuric acid is added into gold-containing cyanide barren solution to perform acidification precipitation, pH value of the solution is regulated to be less than or equal to 2, ferrocyanide, copper, zinc and lead in the gold-containing cyanide barren solution are separated and removed from the gold-containing cyanide barren solution in a precipitate mode; lime is added into the separated acidic supernatant for neutralizing until the pH value is between 10 and 12, and formed calcium sulfate precipitate is removed; and a calcium removing agent is added into the separated basic supernatant, the formed precipitate is removed, and the separated supernatant is returned to the gold leaching process, wherein before the acidification precipitation step, a bactericide is added to the gold-containing cyanide barren solution, and the gold-containing cyanide barren solution is subjected to multi-medium filtration, microfiltration and first nano-filtration in sequence. According to the method for recovering gold from the gold-containing barren solution and purifying the barren solution, the dosage of acid agent is greatly reduced, and the treatment cost for the barren solution is reduced.
Owner:FUJIAN SHUANGQISHAN MINING
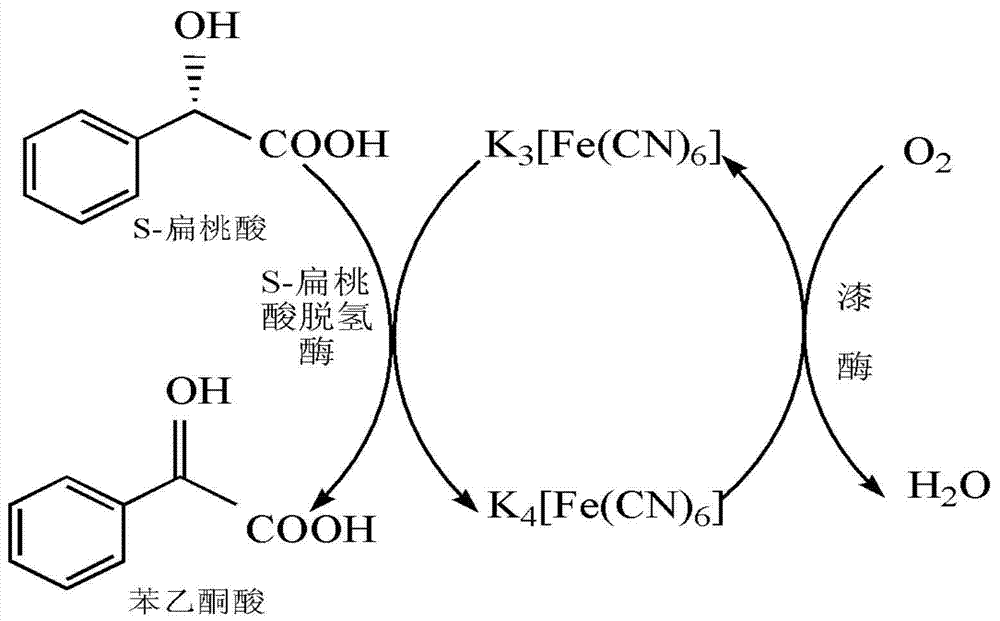
Method for preparing benzoyl formic acid and R-mandelic acid by coupling reaction of S- mandelic acid dehydrogenase and laccase
The invention provides a method for catalyzing S-mandelic acid to be converted into benzoyl formic acid and R-mandelic acid by coupling reaction of S-mandelic acid dehydrogenase and laccase. The method is characterized by continuously regenerating reaction media by laccase to continuously regenerate coenzyme FMN (Flavin Mononucleotide) of S-mandelic acid dehydrogenase, so that the S-mandelic acid dehydrogenase and the laccase are coupled to carry out catalytic reaction. Therefore, the conversion efficiency of independently using the S-mandelic acid dehydrogenase is improved by sufficiently utilizing the high efficiency of enzyme catalysis and continuously catalyzing and oxidizing the S- mandelic acid through less reaction media; and the reaction cost is reduced. According to the method disclosed by the invention, the S-mandelic acid dehydrogenase and laccase are coupled to catalyze the S-mandelic acid to be converted into benzoyl formic acid; and the enantiomer excess of the product R-mandelic acid can reach 99.8%. Moreover, only a small quantity of ferrocyanide or ferricyanide is needed as the reaction medium; the process is simple; the cost is low; and the environment pressure is small; and besides, the method is convenient for industrial production and of a great application value to chiral resolution of racemic mandelic acid.
Owner:NANJING UNIV OF SCI & TECH
Preparing method of particle caesium removing inorganic ion adsorbent and product and application
InactiveCN105597660AImprove adsorption capacityStable structureOther chemical processesWater/sewage treatmentSorbentX-ray
The invention comprises the specific steps: according to a principle of monolayer dispersion of salts on an oxide carrier surface, adopting an X ray diffraction method to determine a monolayer dispersion value of Mn <+> ion salt on a silica gel surface and taking the value as an optimal Mn <+> ion salt capacity. In the preparing of the adsorbent, silica gel particles are fully dipped with Mn <+> ion salt of certain concentration to load the Mn <+> ions on the silica gel surface to form a dispersion monolayer, and higher binding force exists between the Mn<+> ions and the silica gel; then an intermediate M / SiO2 is fully soaked with a potassium ferrocyanide solution to react potassium ferrocyanide with the Mn<+> ions on the surface, so as to generate a layer of ferrocyanide with stable M ions and higher binding force at the silica gel surface. Through static Cs<+> adsorptive property determining, fixed bed reactor cooling experiment determining and verification of a fixed bed reactor 137Cs radioactivity tracer test, the silica gel-carrying ferrocyanide adsorbent prepared by the method has well adsorptive property for Cs<+>.
Owner:TSINGHUA UNIV
Radionuclide adsorbent, method of preparing the same and method of removing radionuclide using the same
ActiveUS20210077980A1Effectively/selectively removeOther chemical processesAlkali metal oxides/hydroxidesCyanideFerrocyanide
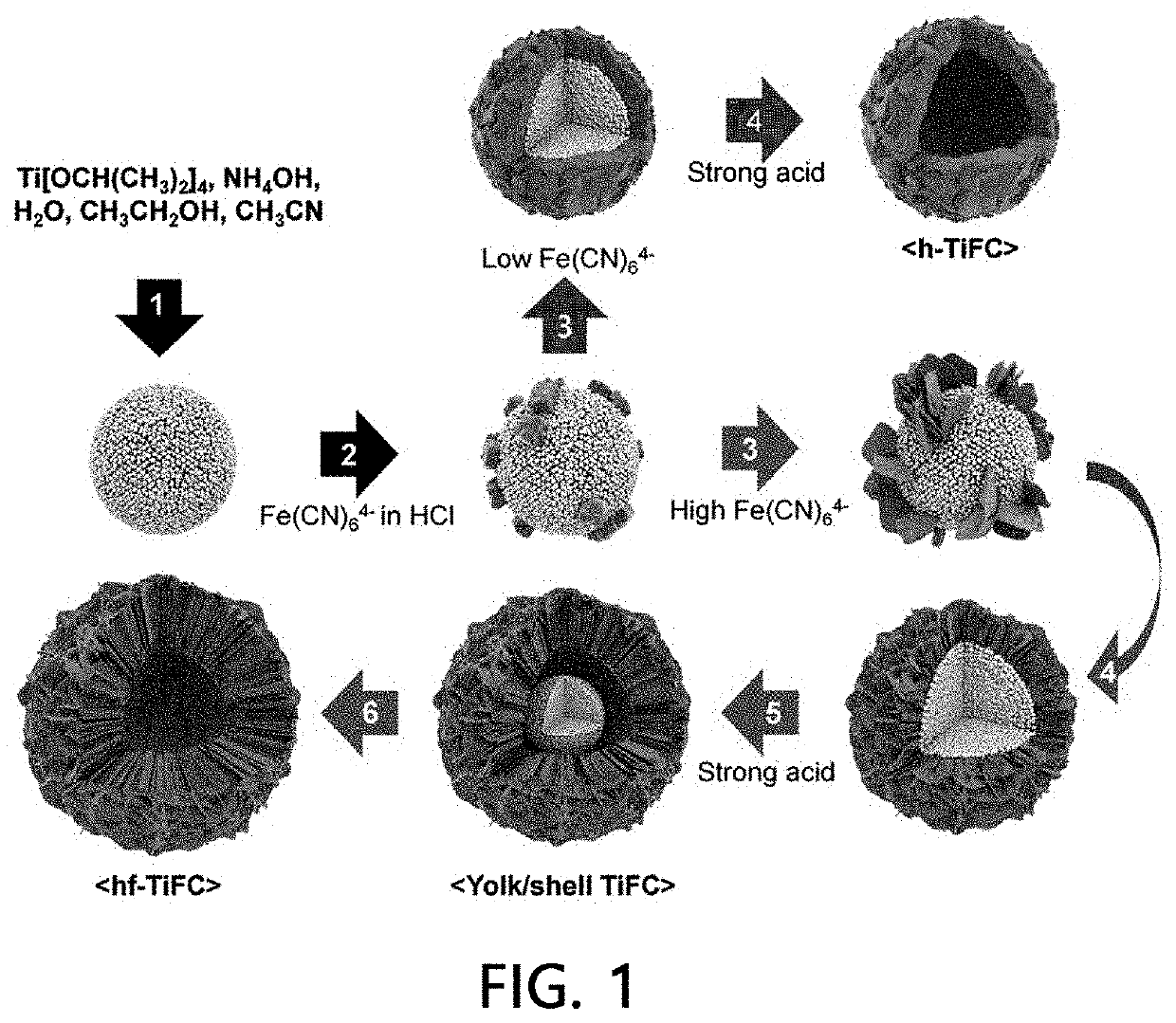
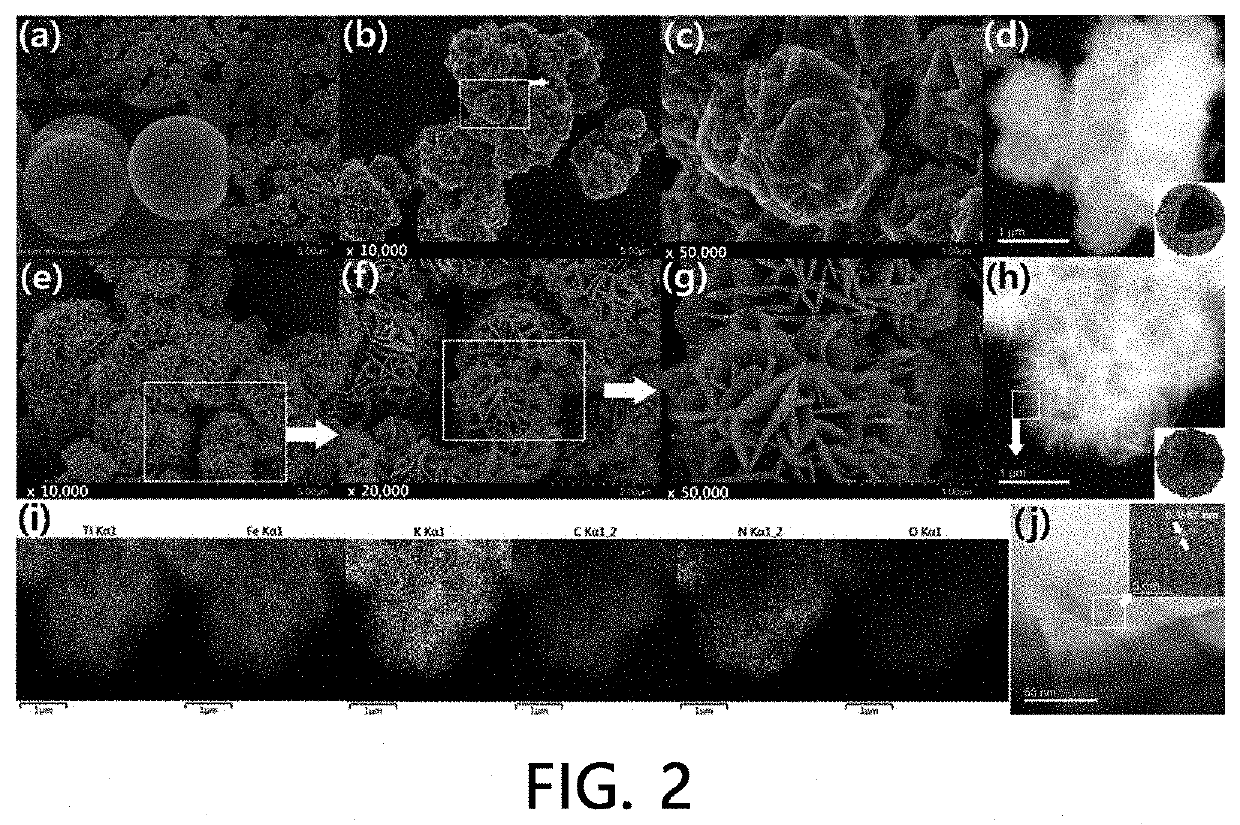
The present invention relates to a radionuclide adsorbent, which includes a hollow space (specifically, an area which is entirely empty or in which transition metal oxide particles are present); and a transition metal-ferrocyanide shell (specifically, a transition metal-ferrocyanide shell having a structure in which a plurality of two-dimensional nano flakes overlap or a transition metal-ferrocyanide shell having a structure in which a plurality of three-dimensional nano polyhedrons agglomerate) formed on the space surface, a preparation method thereof, and a method of removing a radionuclide using the same.
Owner:KOREA ATOMIC ENERGY RES INST
Modified water-quality stabilizing agent
InactiveCN104045171AFormulation ScienceReasonable formulaTreatment using complexing/solubilising chemicalsEnvironmental resistanceWater quality
A disclosed modified water-quality stabilizing agent is prepared from the following raw materials in parts by weight: 90-110 parts of water, 30-50 parts of polyaspartic acid, 5-10 parts of zinc sulfate, 20-35 parts of sodium polyacrylate, 4-12 parts of lignin, 25-35 parts of calcium gluconate, 6-10 parts of polyphosphate, 8-16 parts of sodium molybdate, 11-18 parts of benzotriazol, 15-25 parts of sodium silicate, 3-9 parts of ferrocyanide, 15-20 parts of isopropanol, 8-14 parts of calcium carbonate and 15-25 parts of glycol. The provided modified water-quality stabilizing agent is environment-friendly, less in usage amount, excellent in scale-inhibiting rust-inhibiting performances, and suitable for large-scale popularization.
Owner:QINGDAO BOHONG MARINE BIOTECH
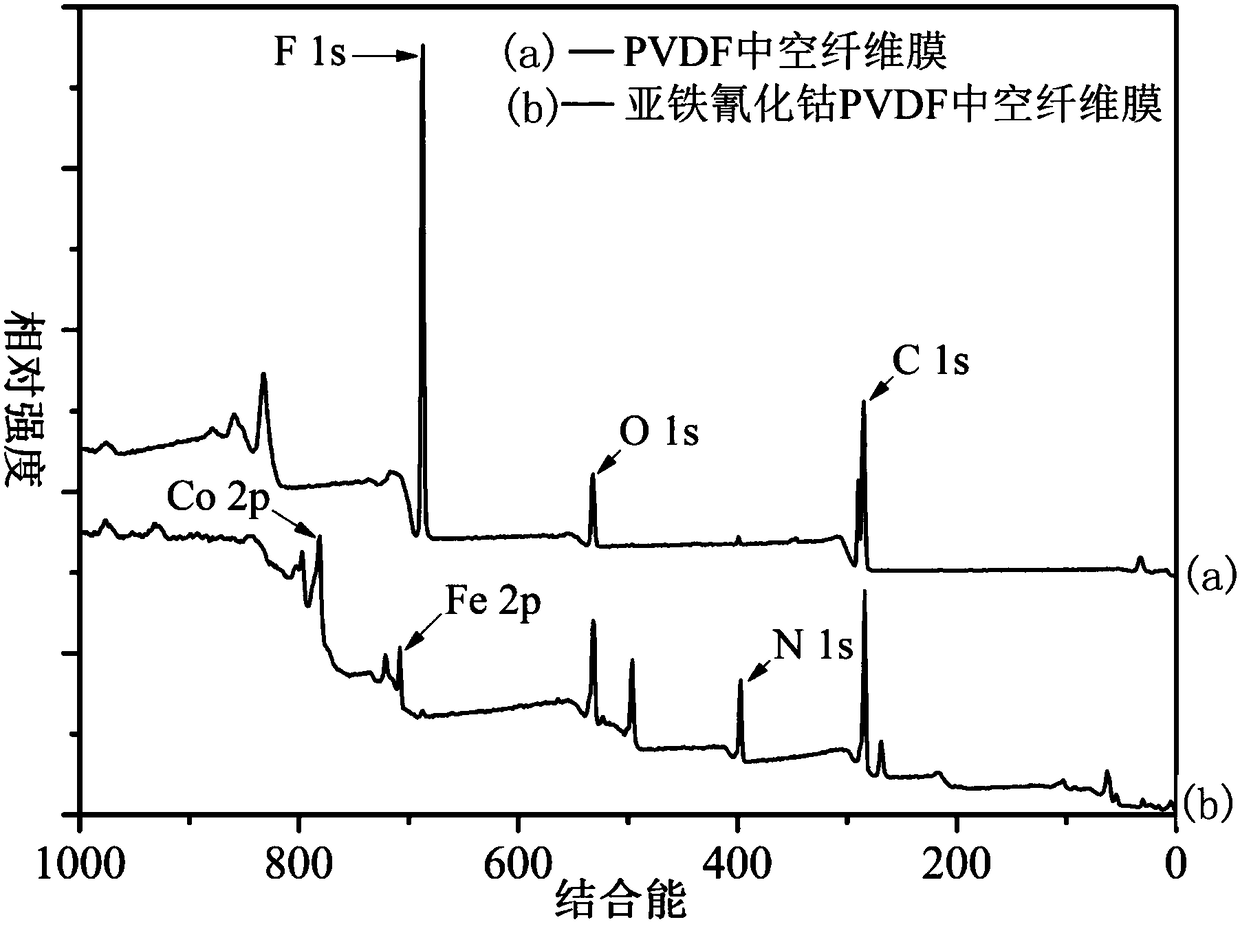
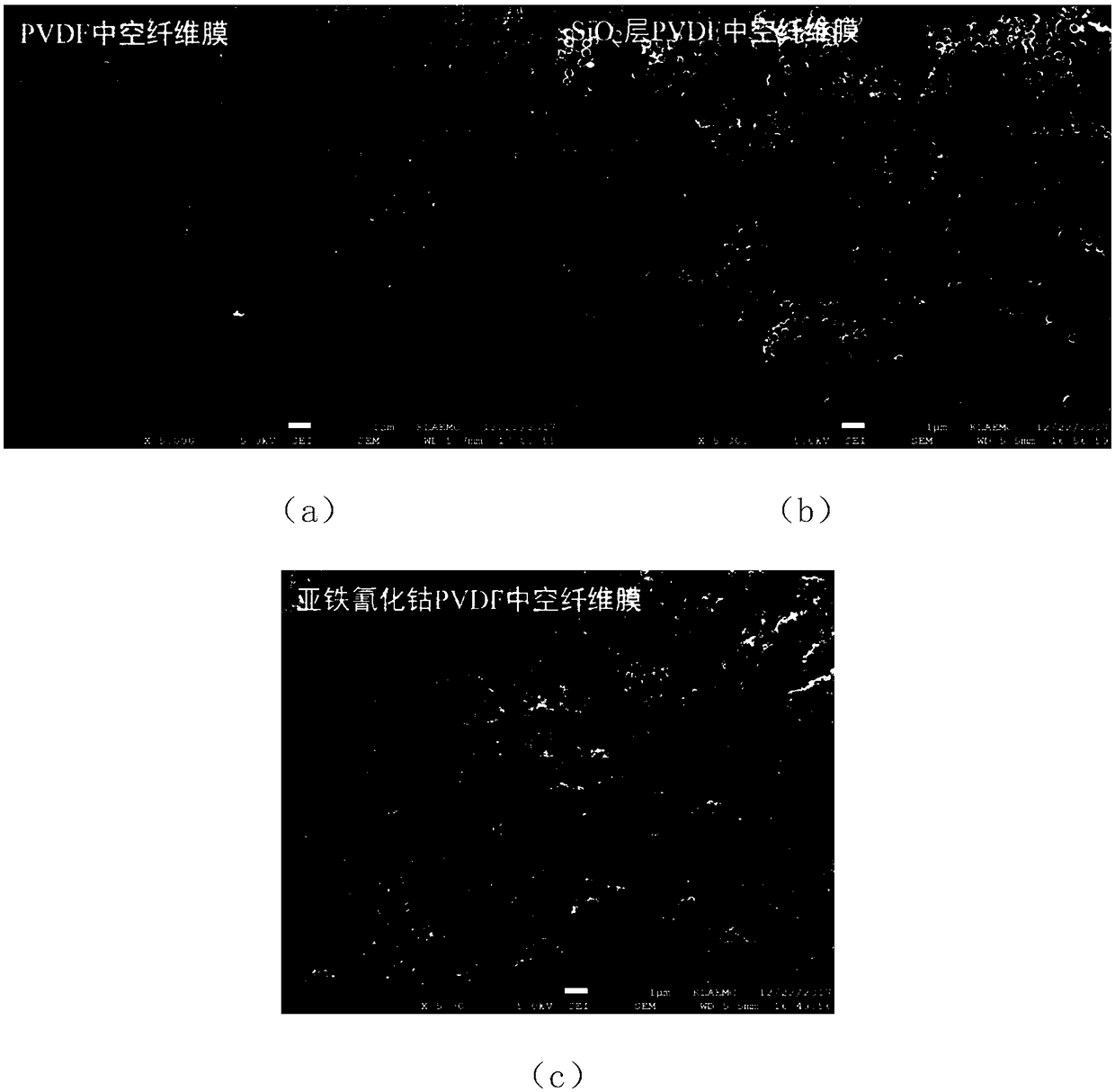
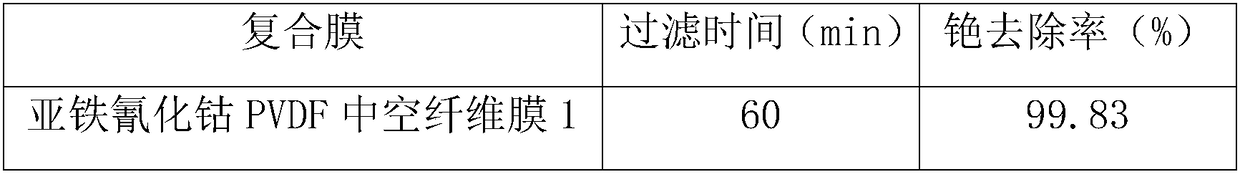
Cobaltous ferrocyanide PVDF (poly(vinylidene fluoride)) hollow fibrous membrane as well as preparation method and use thereof
ActiveCN108187509ASolve easy lossSolve problems such as difficult recyclingSemi-permeable membranesWater contaminantsFiberNanoparticle
The invention discloses a cobaltous ferrocyanide PVDF (poly(vinylidene fluoride)) hollow fibrous membrane as well as a preparation method and an use thereof. According to the preparation method, cobaltous ferrocyanide nanoparticles with high removal rate on caesium are loaded on the surface of the PVDF hollow fibrous membrane through intermediates of silicon dioxide by a chemical method. The aminated silicon dioxide used for preparation has the grain diameter being 300nm and the concentration being 0.05 percent to 0.5 percent (mass / volume); the loading layer number of the cobaltous ferrocyanide is three. The removal rate of the cobaltous ferrocyanide PVDF hollow fibrous membrane prepared by the method on the caesium within 6h can reach 99.5 percent or higher (the concentration of the silicon dioxide is 0.5 percent, and the caesium influent concentration is 100mu g / L). Meanwhile, the preparation process of the cobaltous ferrocyanide PVDF hollow fibrous membrane is simple; the cost is low; the caesium removal rate is high. Therefore wide application prospects are realized in caesium-containing water body treatment.
Owner:TIANJIN UNIV
Method for the synthesis of iron hexacyanoferrate
ActiveUS9546097B2Increase contentLarge capacityIron cyanidesElectrode thermal treatmentSolventFerrocyanide
A method is provided for synthesizing iron hexacyanoferrate (FeHCF). The method forms a first solution of a ferrocyanide source [A4Fe(CN)6.PH2O] material dissolved in a first solvent, where “A” is an alkali metal ion. A second solution is formed of a Fe(II) source dissolved in a second solvent. A reducing agent is added and, optionally, an alkali metal salt. The first and second solutions may be purged with an inert gas. The second solution is combined with the first solution to form a third solution in a low oxygen environment. The third solution is agitated in a low oxygen environment, and AX+1Fe2(CN)6.ZH2O is formed, where X is in the range of 0 to 1. The method isolates the AX+1Fe2(CN)6.ZH2O from the third solution, and dries the AX+1Fe2(CN)6.ZH2O under vacuum at a temperature greater than 60 degrees C.
Owner:SHARP KK
Method for preparing high-loading iron cyanide complex/silicon dioxide hybrid materials
ActiveCN100469435CIncrease loadFast adsorption rateRadioactive decontaminationSilicon compoundsPotassiumSilicon dioxide
The invention relates to a preparing method for ferrocyanide with high load / silica hybrid material, belonging to a preparing method for radionuclide ion absorbing material. The method uses the salt solution with metal ion such as Mn, Sn, Ti, Fe, and Ni so on to react with potassium (sodium) ferrocyanide so as to obtain nanometer particle of ferrocyannide. The particle is fixed by silica solution in water system or is fixed by polymerization siloxane in organic solvent and proper inorganic acid, organic amine or amine water is added to obtain hybrid gel. The hybrid gel is dried, rubbed and screened to obtain ferrocyanide with high load / silica hybrid material. The high load of the material is high and the absorption to nuclide ion is strong. In addition, the material intensity can satisfy the demand of packed bed. The particle diameter can be controlled so as to avoid the problem that the water resistance of bed layer generated by ferrocyanide used alone is overlarge.
Owner:TSINGHUA UNIV
Radioactive cesium decontaminator and method of producing the same, and method of removing the radioactive cesium
ActiveUS20150239758A1Easy to produceImprove productivityTreatment involving filtrationWater/sewage treatment by magnetic/electric fieldsCyanide compoundSlurry
There is provided a method of producing a radioactive cesium decontaminator, including: suspending magnetic particles in a solvent, and coating each magnetic particle with organic monomer or polymer, to thereby form a precursor; adding a ferrocyanide aqueous solution and an aqueous solution containing at least one kind of transition metal into a suspension liquid containing the precursor after coating while applying a strong shear force, to thereby generate a radioactive cesium decontaminator; and removing water content from a slurry containing the obtained radioactive cesium decontaminator.
Owner:THE JIKEI UNIV +1
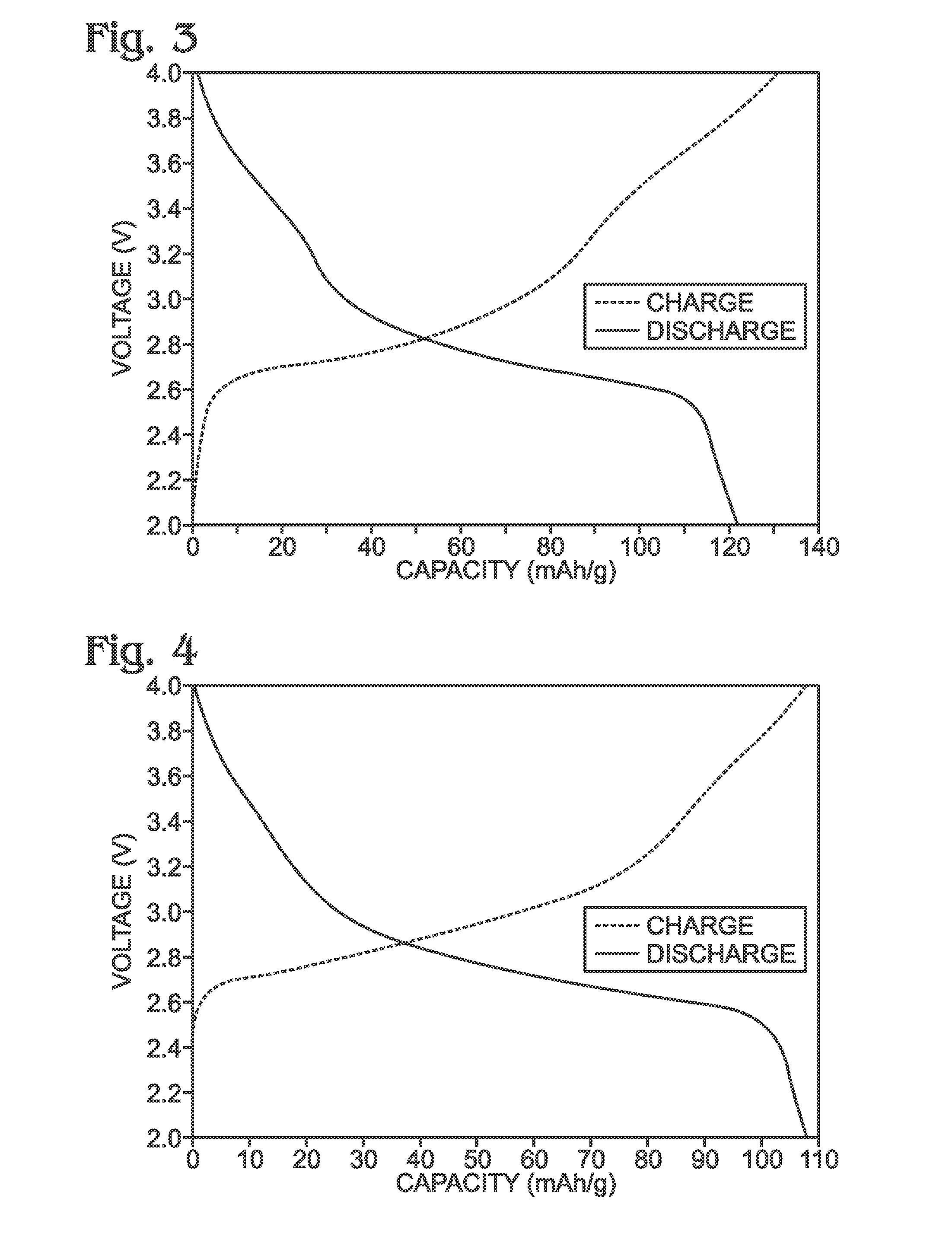
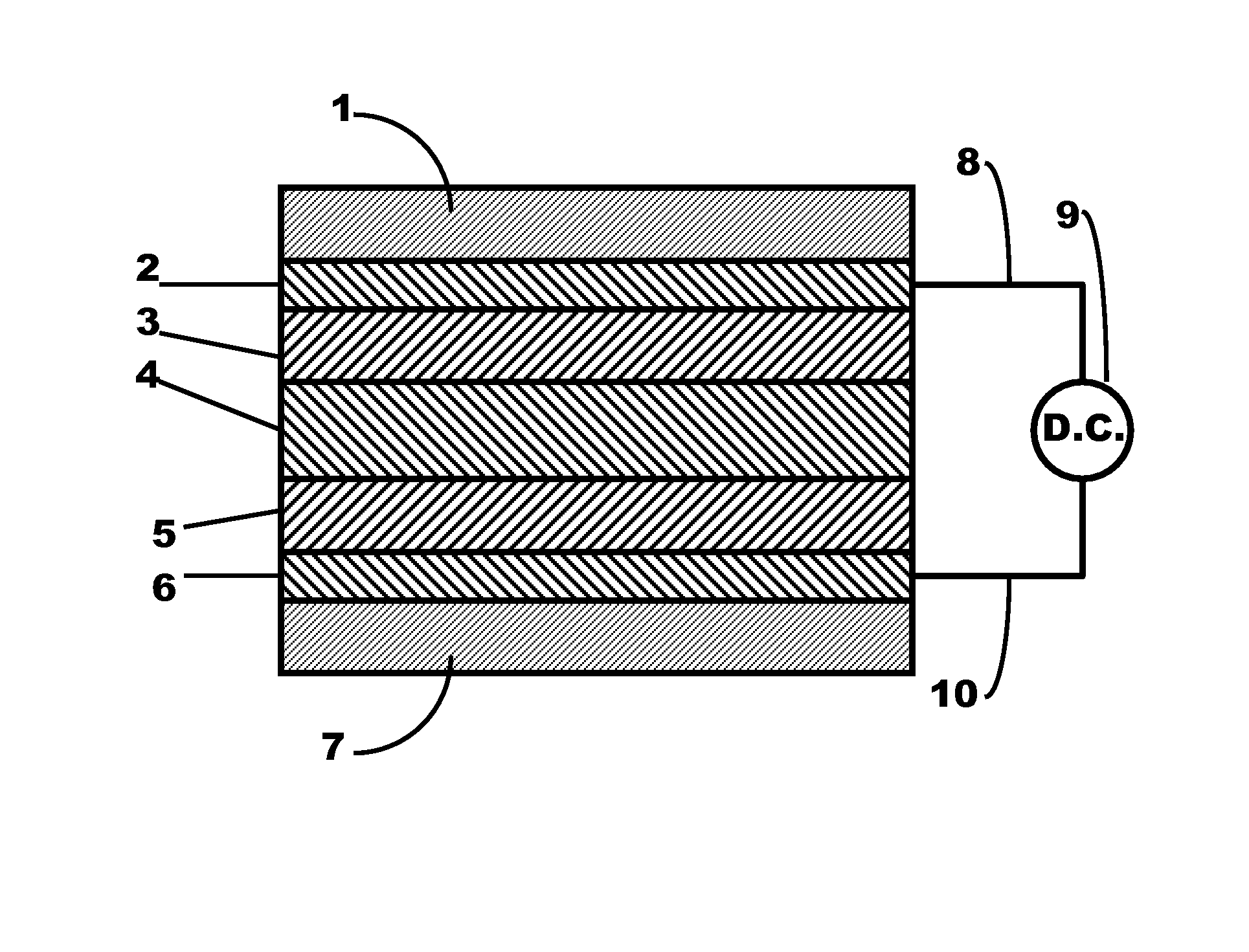
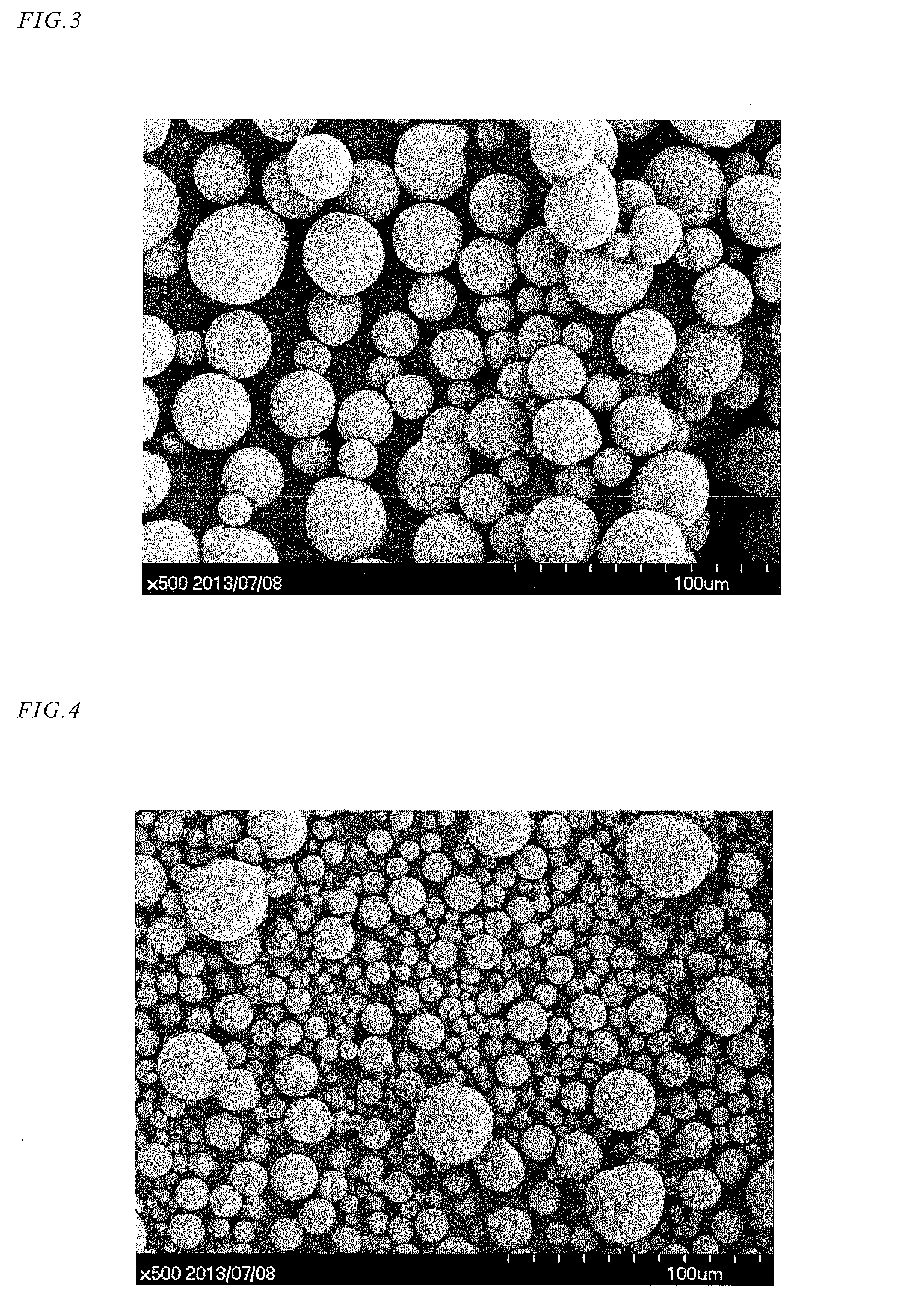
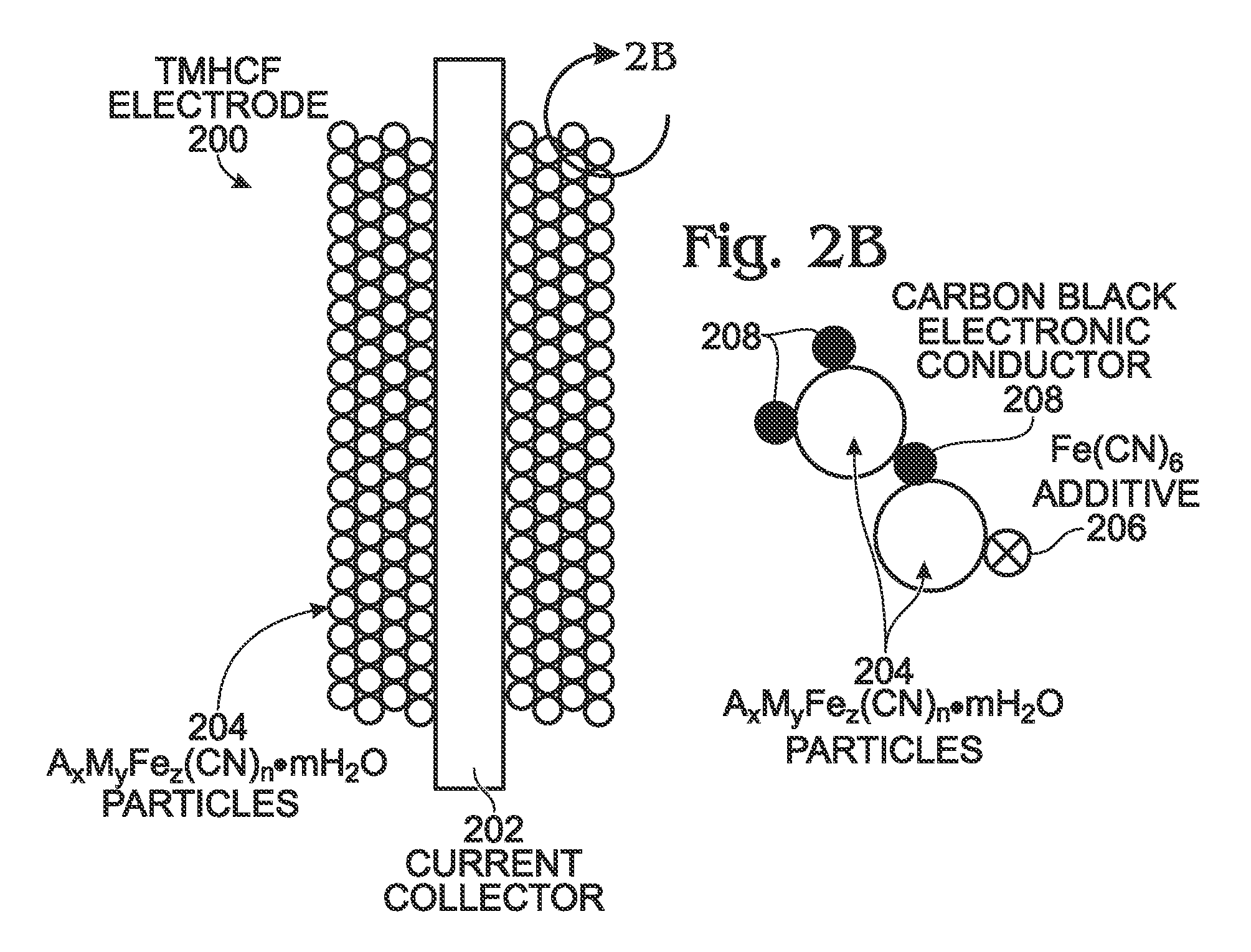
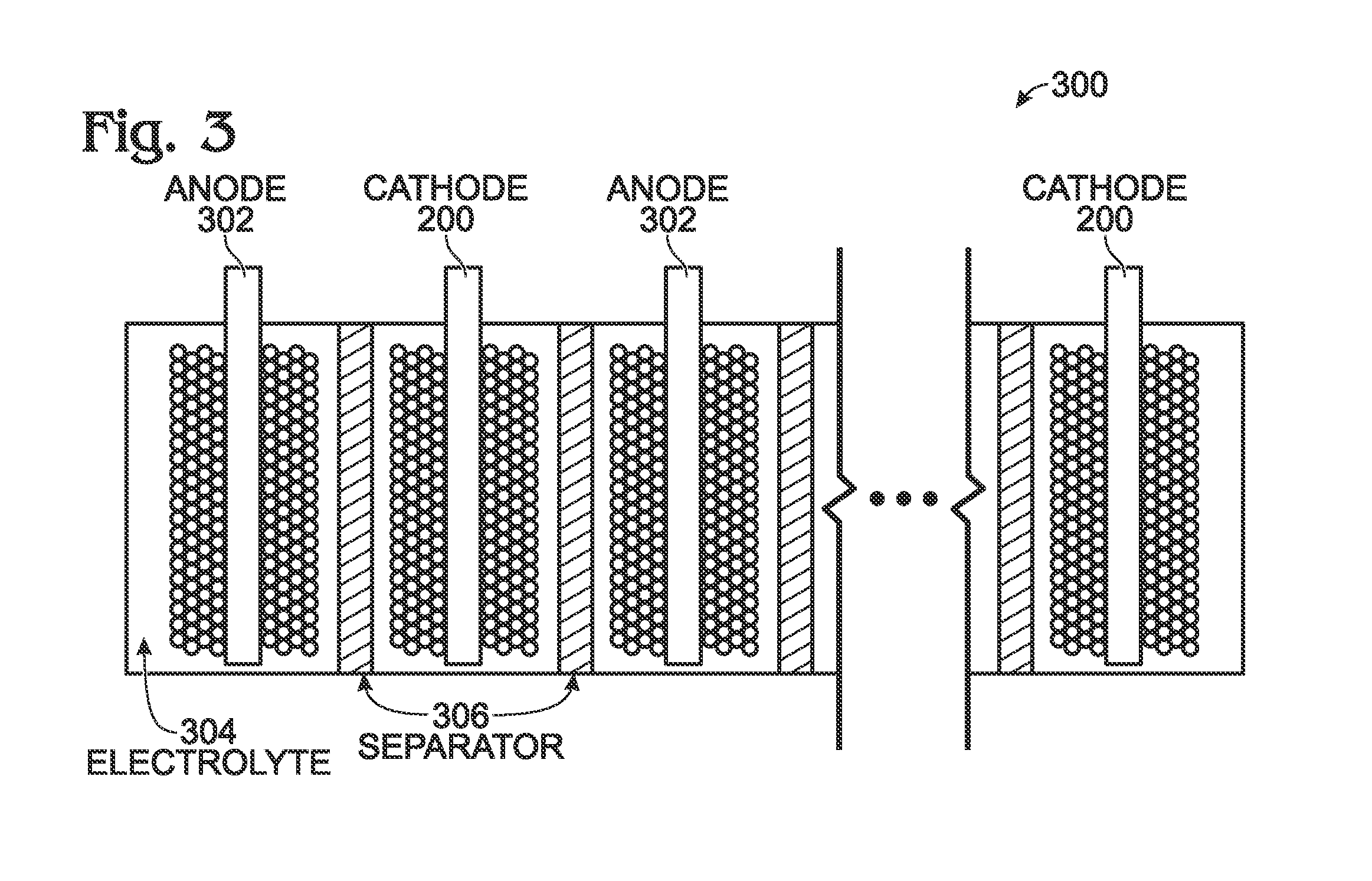
Hexacyanoferrate Battery Electrode Modified with Ferrocyanides or Ferricyanides
InactiveUS20130266860A1Improve electrode performanceHigh capacity retentionElectrode manufacturing processesPrimary cellsAlkaline earth metalCyanide
A transition metal hexacyanoferrate (TMHCF) battery electrode is provided with a Fe(CN)6 additive. The electrode is made from AxMyFez(CN)n.mH2O particles overlying a current collector, where the A cations are either alkali and alkaline-earth cations such as sodium (Na), potassium (K), calcium (Ca), or magnesium (Mg), and M is a transition metal. A Fe(CN)6 additive modifies the AxMyFez(CN)n.mH2O particles. The Fe(CN)6 additive may be ferrocyanide ([Fe(CN)6]4−) or ferricyanide ([Fe(CN)6]3−). Also provided are a related TMHCF battery with Fe(CN)6 additive, TMHCF fabrication process, and TMHCF battery fabrication process.
Owner:SHARP KK
Preparation method of particulate cesium removal inorganic ion adsorbent, and product and application of adsorbent
The invention relates to a preparation method of a particulate cesium removal inorganic ion adsorbent, and a product and application of the adsorbent. Concretely, firstly, according to the single-layer dispersion principle of salts on the oxide carrier surface, the single-layer dispersion threshold value of M<n+> ion salt on the silica gel surface is measured by an X ray diffraction method; the single-layer dispersion threshold value is used as the optimal M<n+> ion salt load quantity. During the adsorbent preparation, a certain concentration of M<n+> ion salt is firstly used for fully impregnating silica gel particles, so that M<n+> ions are loaded on the silica gel surface to form a dispersion single layer, and the strong bonding force is formed between the M<n+> ions and silica gel; then, a potassium ferrocyanide solution is used for fully soaking an intermediate M / SiO2, so that potassium ferrocyanide and the M<n+> ions at the surface take a reaction; a ferrocyanide with strong bonding force and stable M ions is generated on the silica gel surface. Through Cs<+> adsorption performance measurement, fixed bed reactor cold experimental determination and fixed bed reactor 137Cs radioactive tracing experiment verification, the silica gel load ferrocyanide prepared by the method has good adsorption performance on Cs<+>.
Owner:TSINGHUA UNIV
Electrolyte of water-based flow battery, all-iron water-based flow battery and application
ActiveCN113764714ANo capacity decayStable reversible cycleRegenerative fuel cellsAcid electrolytesElectrolytic agentElectrical battery
The invention belongs to the field of flow batteries, and particularly relates to an electrolyte of a water-based flow battery, an all-iron water-based flow battery and application of the all-iron water-based flow battery. According to the invention, ligands which are stably complexed with iron in an alkaline environment are respectively introduced into the positive electrode electrolyte and the negative electrode electrolyte, and the two ligands have different degrees of influence on the electron gaining and losing capabilities of iron ions or ferrous ions; a potential difference exists between a first complex contained in the positive electrode electrolyte and a second complex contained in the negative electrode electrolyte under the electrified condition, and the first complex is ferricyanide or ferrocyanide; the second complex is a complex formed by iron ions or ferrous ions and bis (2-ethoxyl) amino (trihydroxymethyl) methane or 3-[N-N-bis (2-ethoxyl) amino]-2-hydroxy propanesulfonic acid, so that the total-iron aqueous flow battery is constructed, and the flow battery realizes ion conduction through a cation exchange membrane Nafion series diaphragm and can stably and reversibly circulate for a long time.
Owner:HUAZHONG UNIV OF SCI & TECH
Cellulose gel electrolyte material and a preparation method thereof
ActiveCN108417410AConvenient sourceLow priceHybrid capacitor electrolytesLight-sensitive devicesCellulosePotassium iodine
A cellulose gel electrolyte material is prepared from natural cellulose wood pulp, paper pulp, bamboo pulp or degreased cotton, which are used as raw materials and react with sodium hydroxide and carbon disulfide to synthesize a cellulose viscose solution, and a gel-based cellulose gel electrolyte material is formed through hydration treatment. The preparation method comprises the steps of reacting paper pulp (wood pulp, bamboo pulp or degreasing cotton) with sodium hydroxide and carbon disulfide for 30-60 minute, preparing a tangerine viscose liquid, mixing the viscose liquid and deionized water according to the weight ratio of 1: 1-9, obtaining cellulose gel electrolyte material after gel curing, and preparing a composite cellulose gel electrolyte material if an acetonitrile solution ofpotassium ferrocyanide or potassium iodide and iodine is added. The method is simple, the product is easy to form, operation and control are simple and convenient, and industrial production is facilitated. The prepared cellulose gel electrolyte material is a porous gel polymer, has good liquid retention capability and ionic conductivity, and can be applied to a supercapacitor, a solar cell and a lithium battery.
Owner:YANSHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com