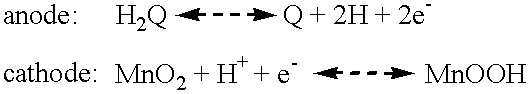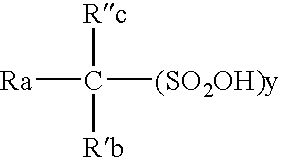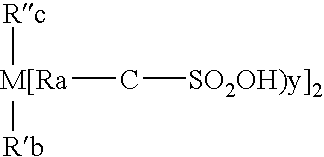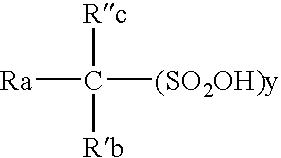Patents
Literature
606results about "Acid electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
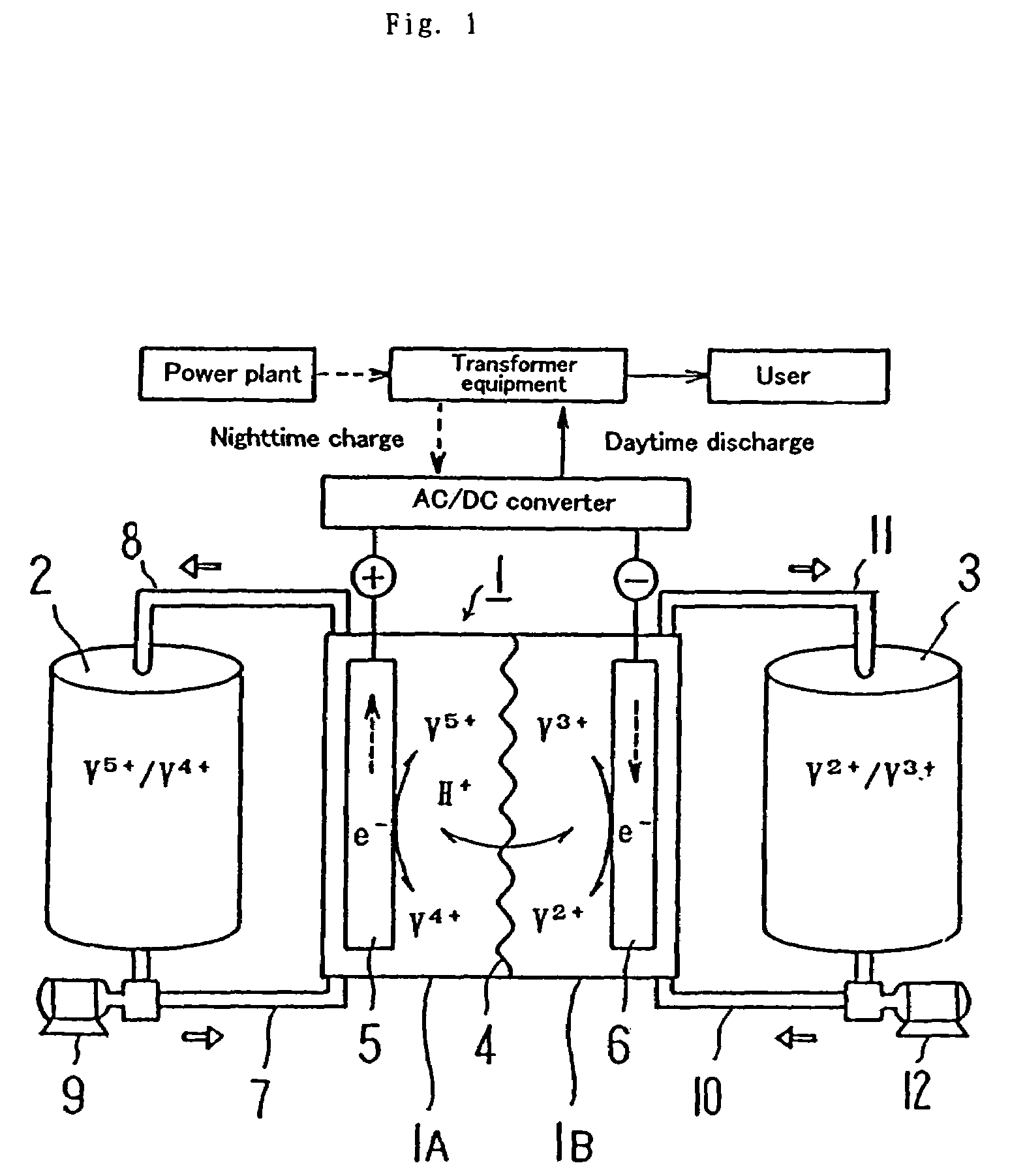
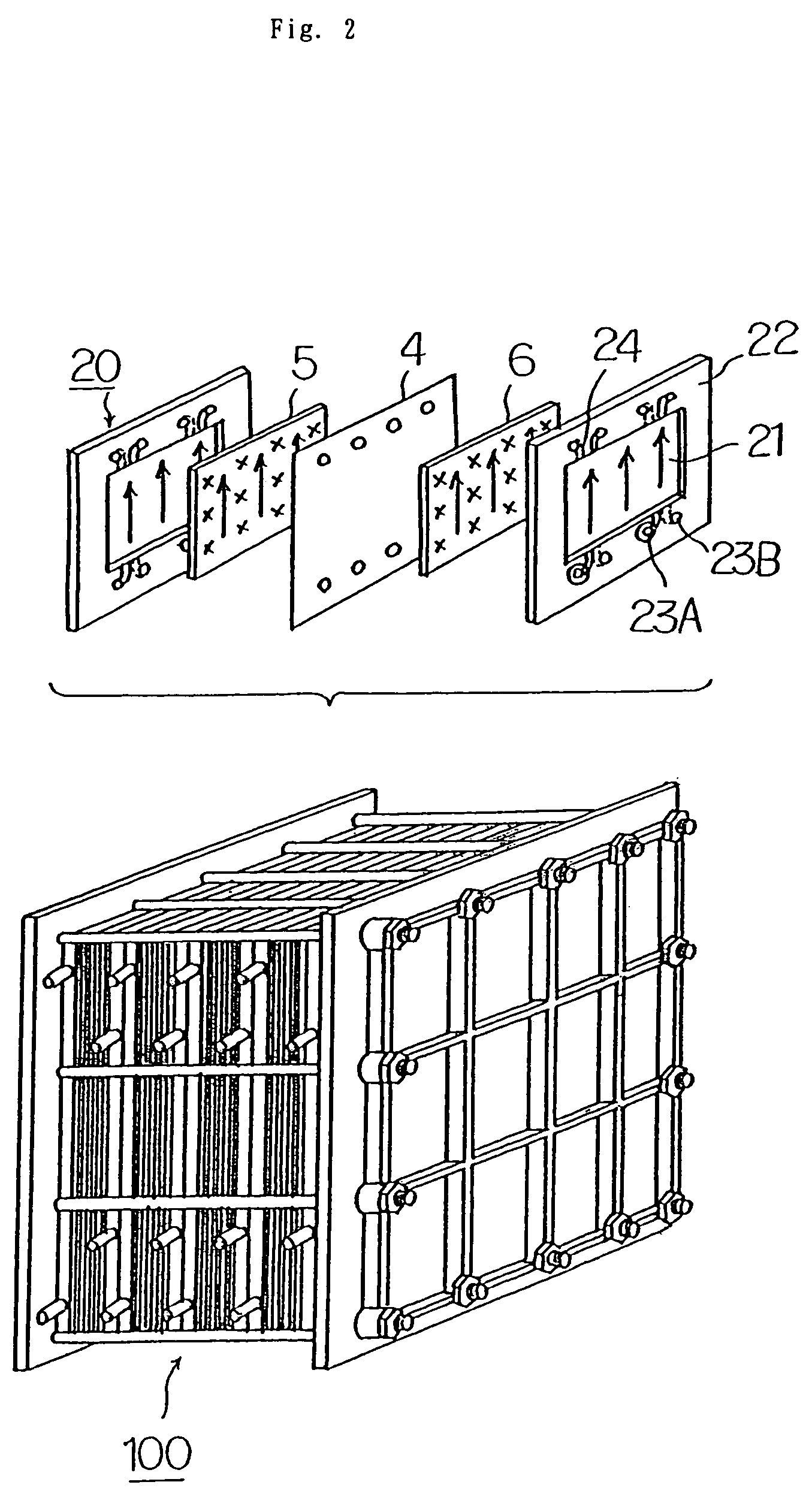
High performance energy storage devices
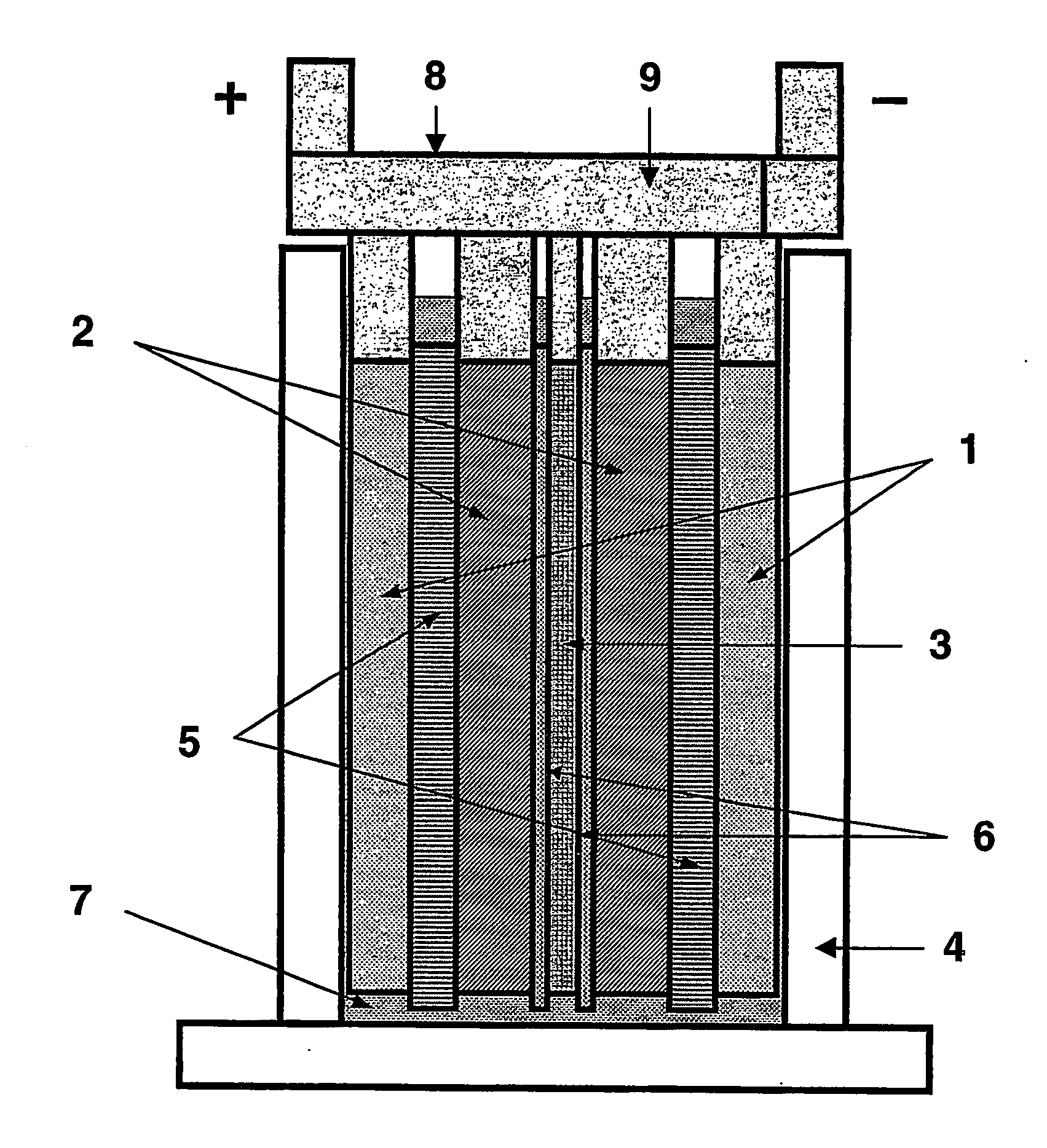
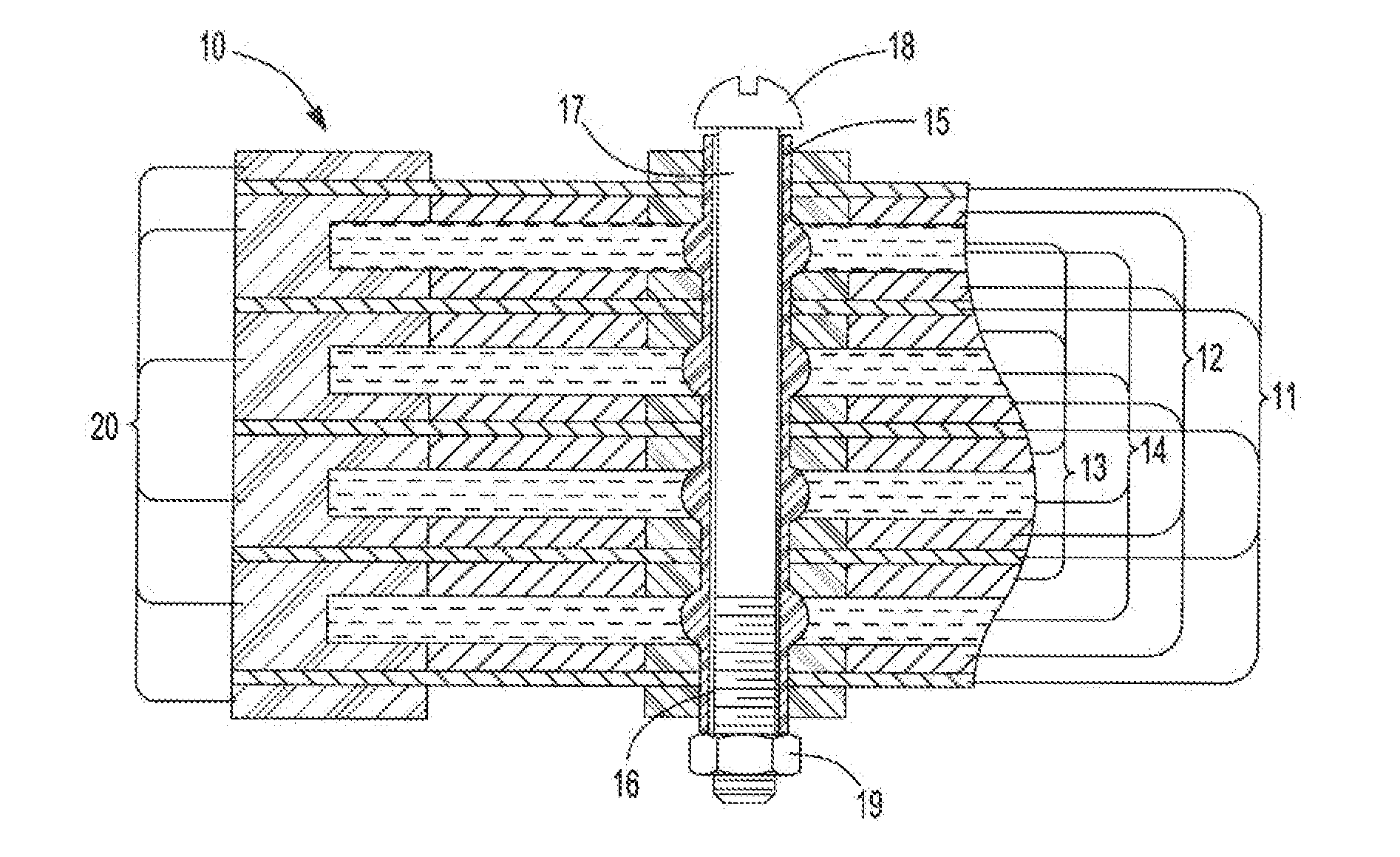
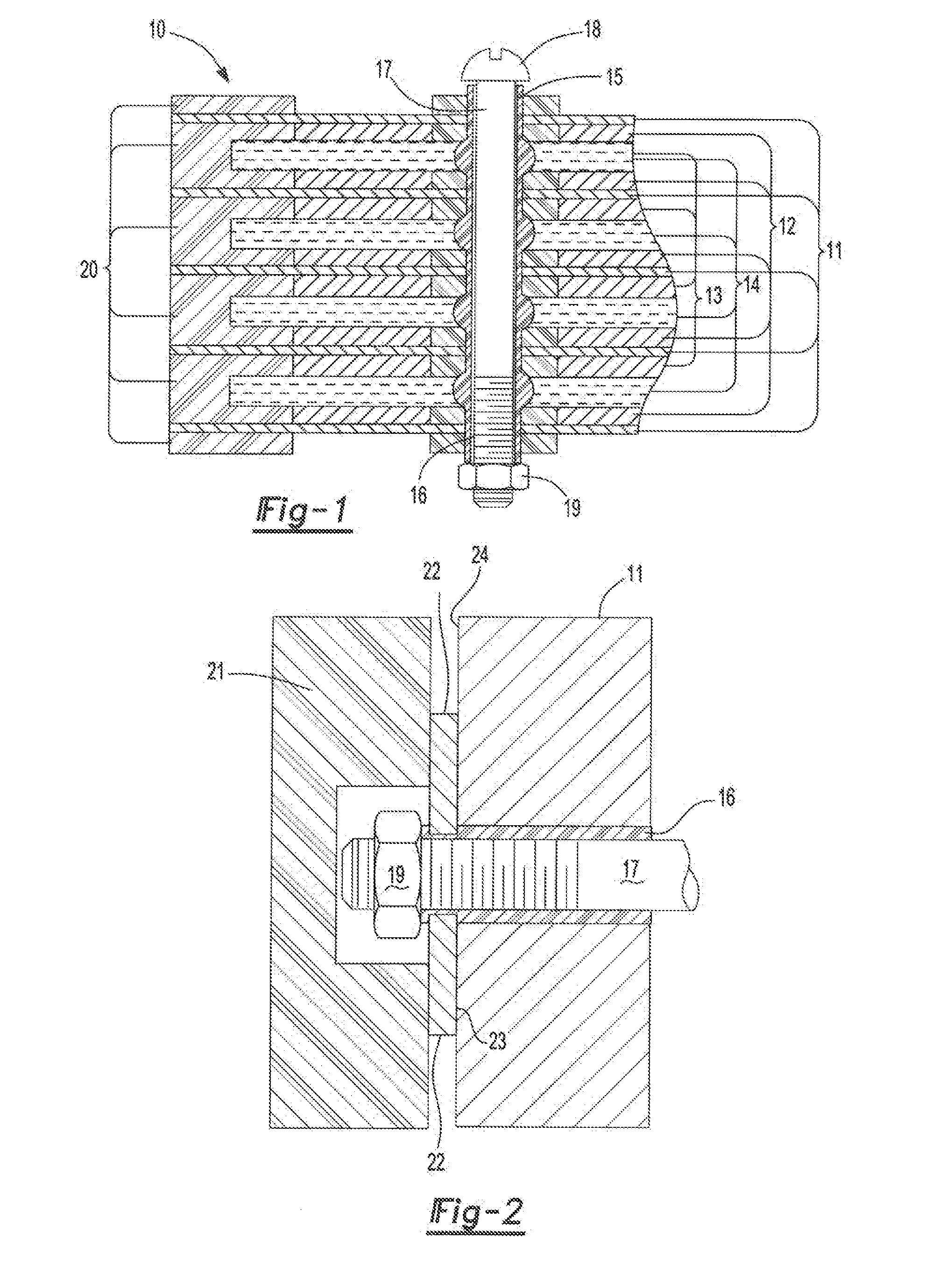
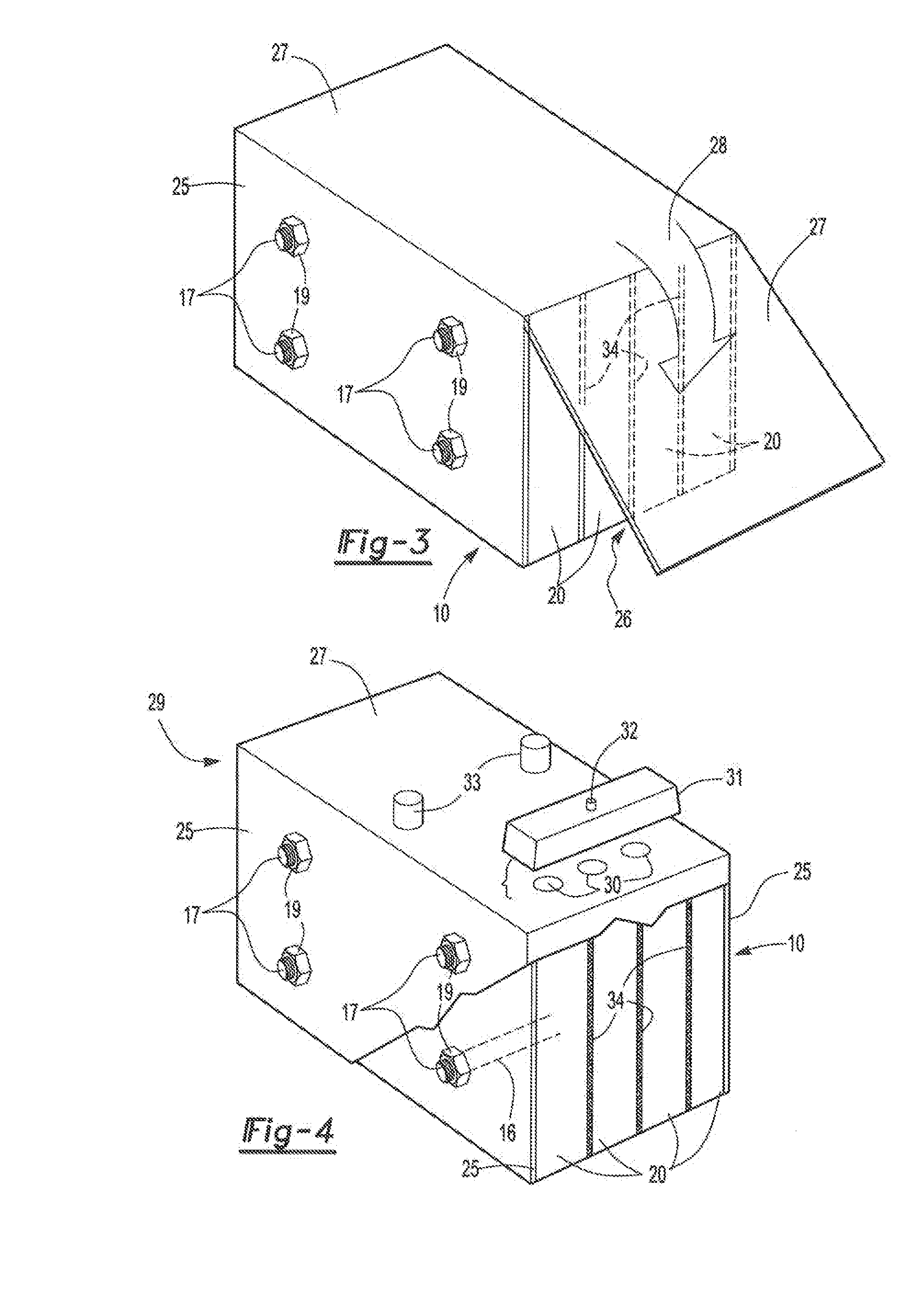
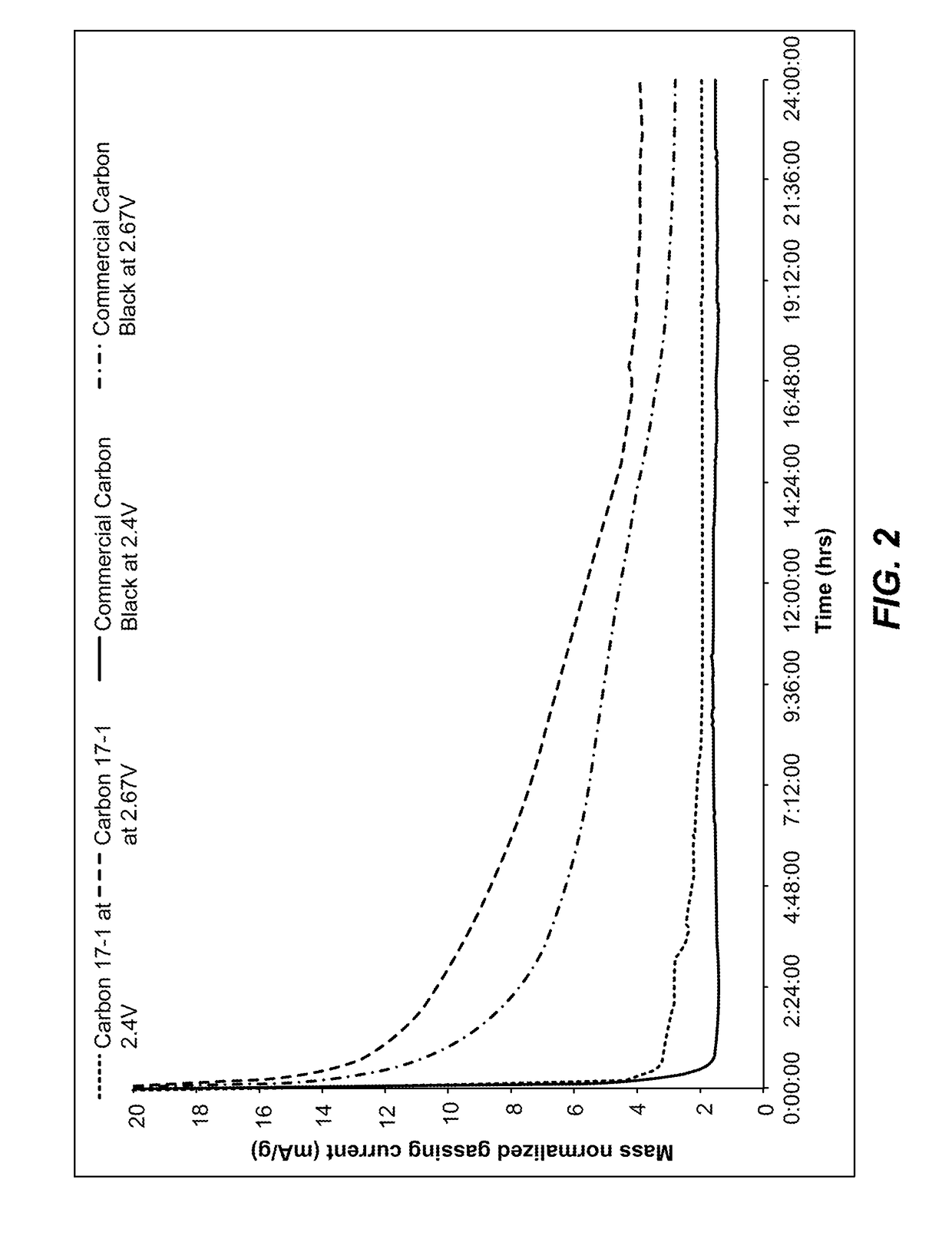
ActiveUS20070104981A1Avoid gasLower internal resistanceHybrid capacitor electrolytesCapacitor and primary/secondary cellsLead dioxideBusbar
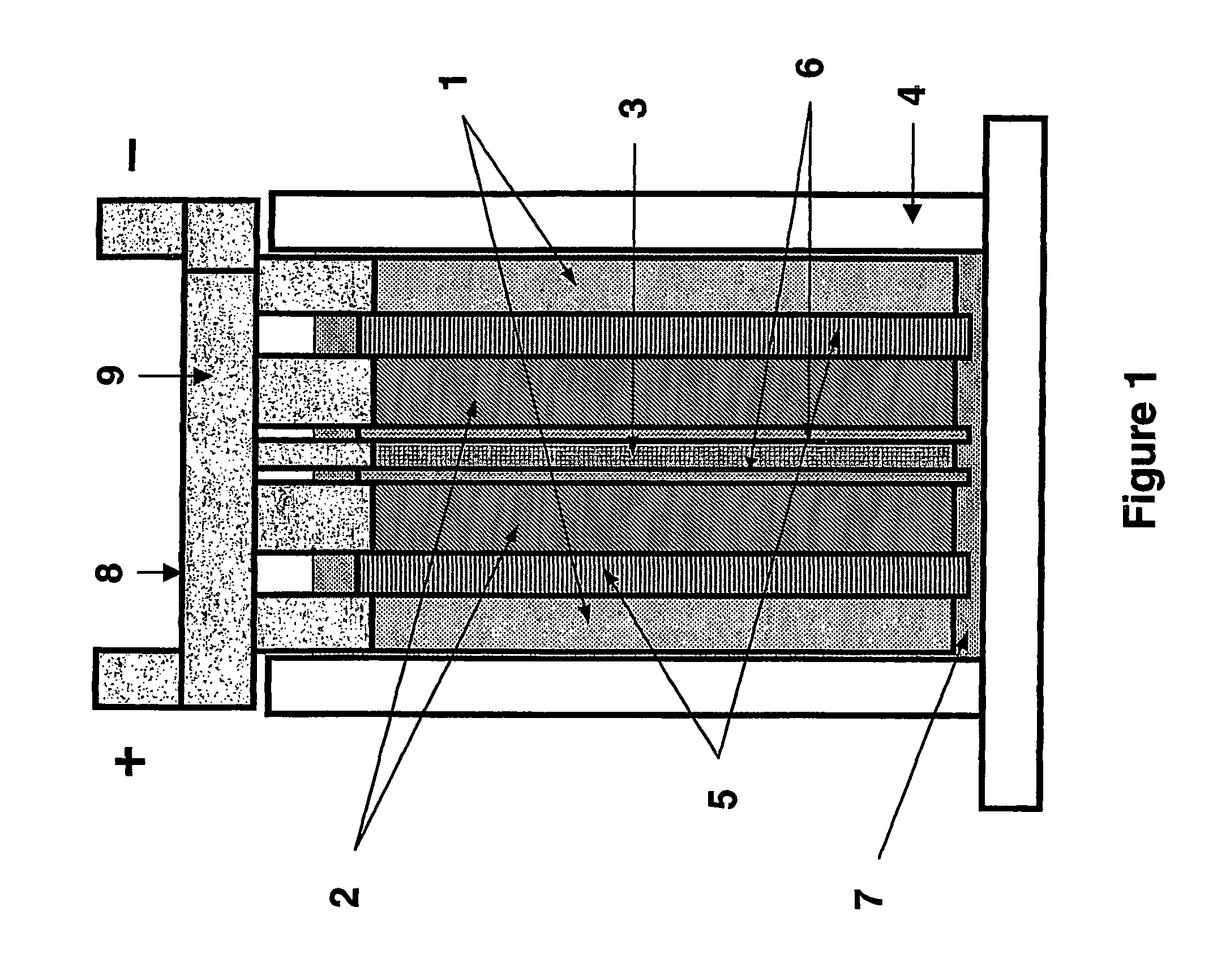
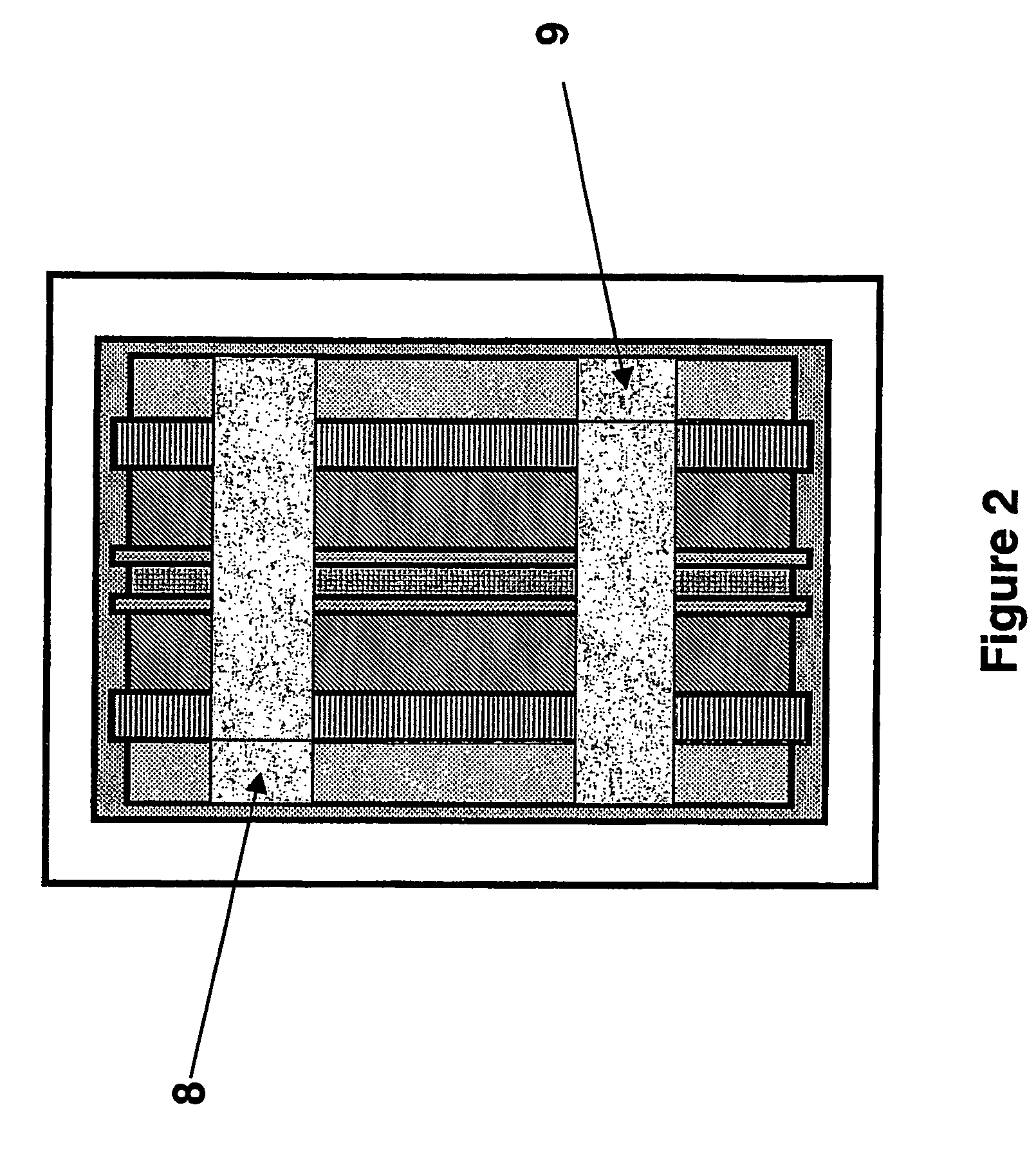
A lead-acid battery comprising: at least one lead-based negative electrode; at least one lead dioxide-based positive electrode; at least one capacitor electrode; and electrolyte in contact with the electrodes; wherein a battery part is formed by the lead based negative electrode and the lead dioxide-based positive electrode; and an asymmetric capacitor part is formed by the capacitor electrode and one electrode selected from the lead based negative electrode and the lead-dioxide based positive electrode; and wherein all negative electrodes are connected to a negative busbar, and all positive electrodes are connected to a positive busbar. The capacitor electrode may be a capacitor negative electrode comprising carbon and an additive mixture selected from oxides, hydroxides or sulfates of lead, zinc, cadmium, silver and bismuth, or a capacitor negative electrode comprising carbon, red lead, antimony in oxide, hydroxide or sulfate form, and optionally other additives. The capacitor electrode may be used in asymmetric capacitors and batteries of other types.
Owner:COMMONWEALTH SCI & IND RES ORG
Solid polymer fuel cell with improved voltage reversal tolerance
In a solid polymer fuel cell series, various circumstances can result in the fuel cell being driven into voltage reversal. For instance, cell voltage reversal can occur if that cell receives an inadequate supply of fuel (for example, fuel starvation). In order to pass current during fuel starvation, reactions other than fuel oxidation may take place at the fuel cell anode, including water electrolysis and oxidation of anode components. The latter may result in significant degradation of the anode. Such fuel cells can be made more tolerant to cell reversal by promoting water electrolysis over anode component oxidation at the anode. This can be accomplished by incorporating a catalyst composition at the anode to promote the water electrolysis reaction, in addition to the typical anode electrocatalyst for promoting fuel oxidation.
Owner:BALLARD POWER SYSTEMS +1
Laminate Including Active Material Layer and Solid Electrolyte Layer, and All Solid Lithium Secondary Battery Using the Same
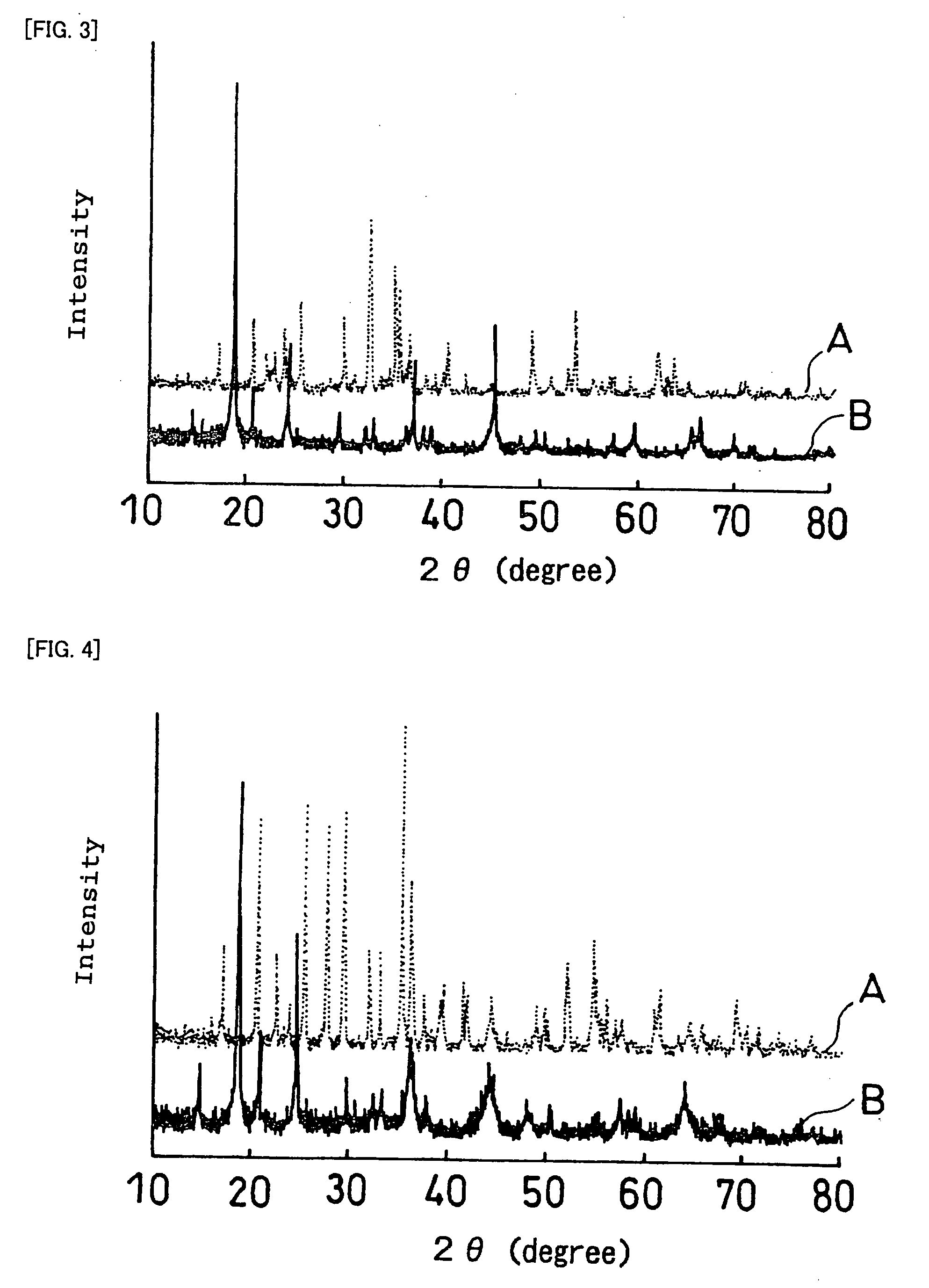
InactiveUS20070259271A1Improve life characteristicsSmall internal resistanceElectrode thermal treatmentFinal product manufactureLithiumX-ray
A laminate includes an active material layer and a solid electrolyte layer bonded to the active material layer by sintering. The active material layer includes a crystalline first substance capable of absorbing and desorbing lithium ions, and the solid electrolyte layer includes a crystalline second substance with lithium ion conductivity. An X-ray diffraction analysis of the laminate shows that there is no component other than constituent components of the active material layer and constituent components of the solid electrolyte layer. Also, an all solid lithium secondary battery includes such a laminate and a negative electrode active material layer.
Owner:PANASONIC CORP
Battery with bifunctional electrolyte
InactiveUS20060063065A1Improve solubilityCell electrodesRegenerative fuel cellsSolubilityOxidation-Reduction Agent
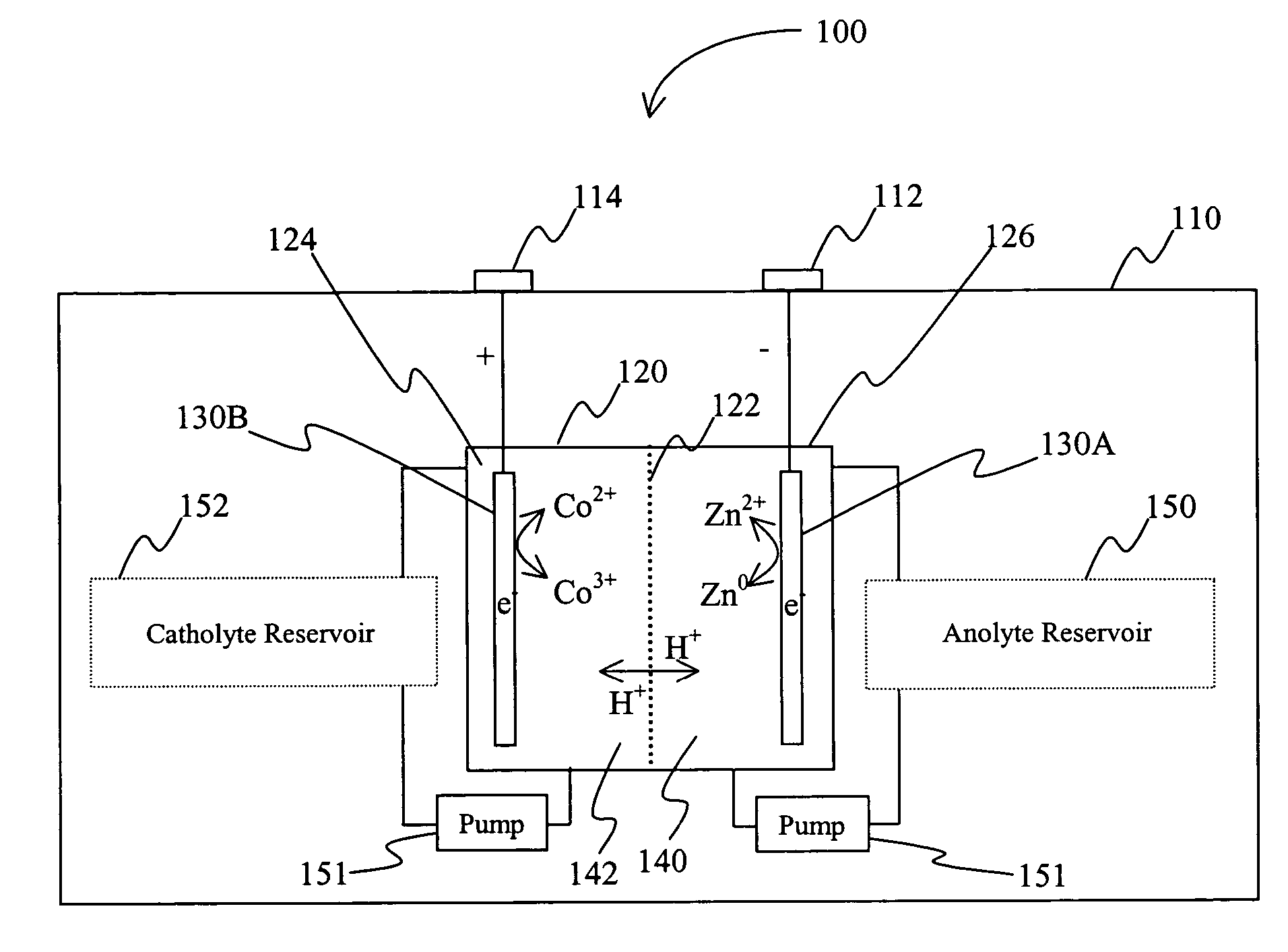
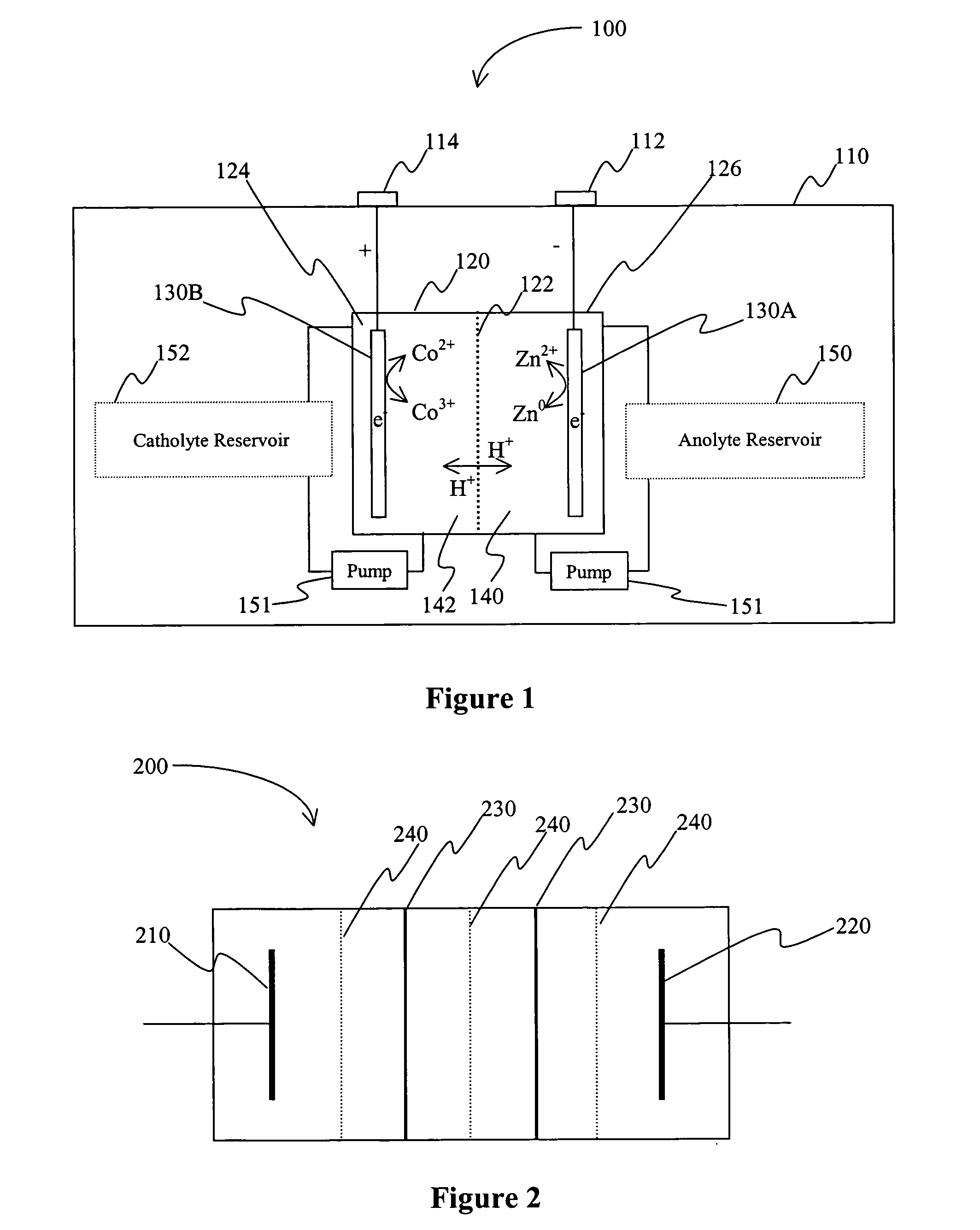
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids, amine sulfonic acids, and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:PLURION LTD
Metal-air cell with performance enhancing additive
ActiveUS20110281184A1Improves oxygen reduction thermodynamicsImproved kineticsFuel and primary cellsFuel and secondary cellsOxygenElectrochemical cell
Systems and methods drawn to an electrochemical cell comprising a low temperature ionic liquid comprising positive ions and negative ions and a performance enhancing additive added to the low temperature ionic liquid. The additive dissolves in the ionic liquid to form cations, which are coordinated with one or more negative ions forming ion complexes. The electrochemical cell also includes an air electrode configured to absorb and reduce oxygen. The ion complexes improve oxygen reduction thermodynamics and / or kinetics relative to the ionic liquid without the additive.
Owner:ARIZONA STATE UNIVERSITY
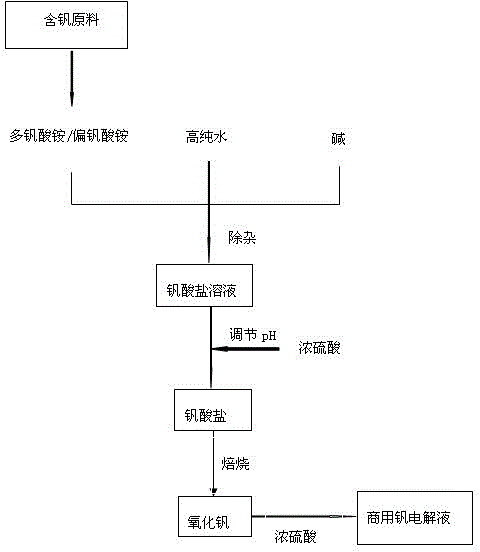
Preparation method for commercial vanadium battery electrolyte
ActiveCN103606694AHigh purityLimited service lifeIndirect fuel cellsAcid electrolytesSocial benefitsHydrogen
The invention discloses a preparation method for commercial vanadium battery electrolyte. According to the preparation method, vanadium electrolyte is prepared by one step through a chemical method; the preparation method comprises the following steps: dissolving raw materials containing vanadium by using an alkali; adjusting a pH (Potential of Hydrogen) value by acid-alkali regulation; repeatedly precipitating the vanadium to remove impurity elements; then roasting to obtain an oxide of the vanadium; finally, dissolving the oxide of the vanadium by using concentrated sulfuric acid to obtain the vanadium electrolyte. With the adoption of the method, impurities including iron, chrome, silicon, manganese and the like can be removed effectively and a process flow is shortened; the vanadium yield is improved, the impurity content is reduced and the process flow is shortened; the product purity can also be improved. The preparation method for the commercial vanadium battery electrolyte has important meanings on production technology levels and market competitiveness of the vanadium electrolyte; the method has good economic benefits and social benefits.
Owner:HEBEI IRON AND STEEL
Redox flow cell rebalancing
A redox cell rebalance system is provided. In some embodiments, the rebalance system includes electrochemical cell and a photochemical cell. In some embodiments, the photochemical cell contains a source of ultraviolet radiation for producing HCl from H2 and Cl2 generated by the system. The HCl product may be collected or circulated back through the system for the rebalancing of electrolytes. A rebalance cell for use in a rebalance system is also provided. In some embodiments, the rebalance cell is the combination of an electrochemical cell and a photochemical cell. In some embodiments, a source of ultraviolet radiation is housed in the cathode compartment of the rebalance cell. In some embodiments, the source of ultraviolet radiation is used to effect the formation of HCl from H2 and Cl2 present in the rebalance cell. The HCl is dissolved in aqueous electrolytes contained in the rebalance cell, which can subsequently be circulated through a rebalance system for the rebalancing of redox cells.
Owner:IMERGY POWER SYST
Bipolar battery assembly
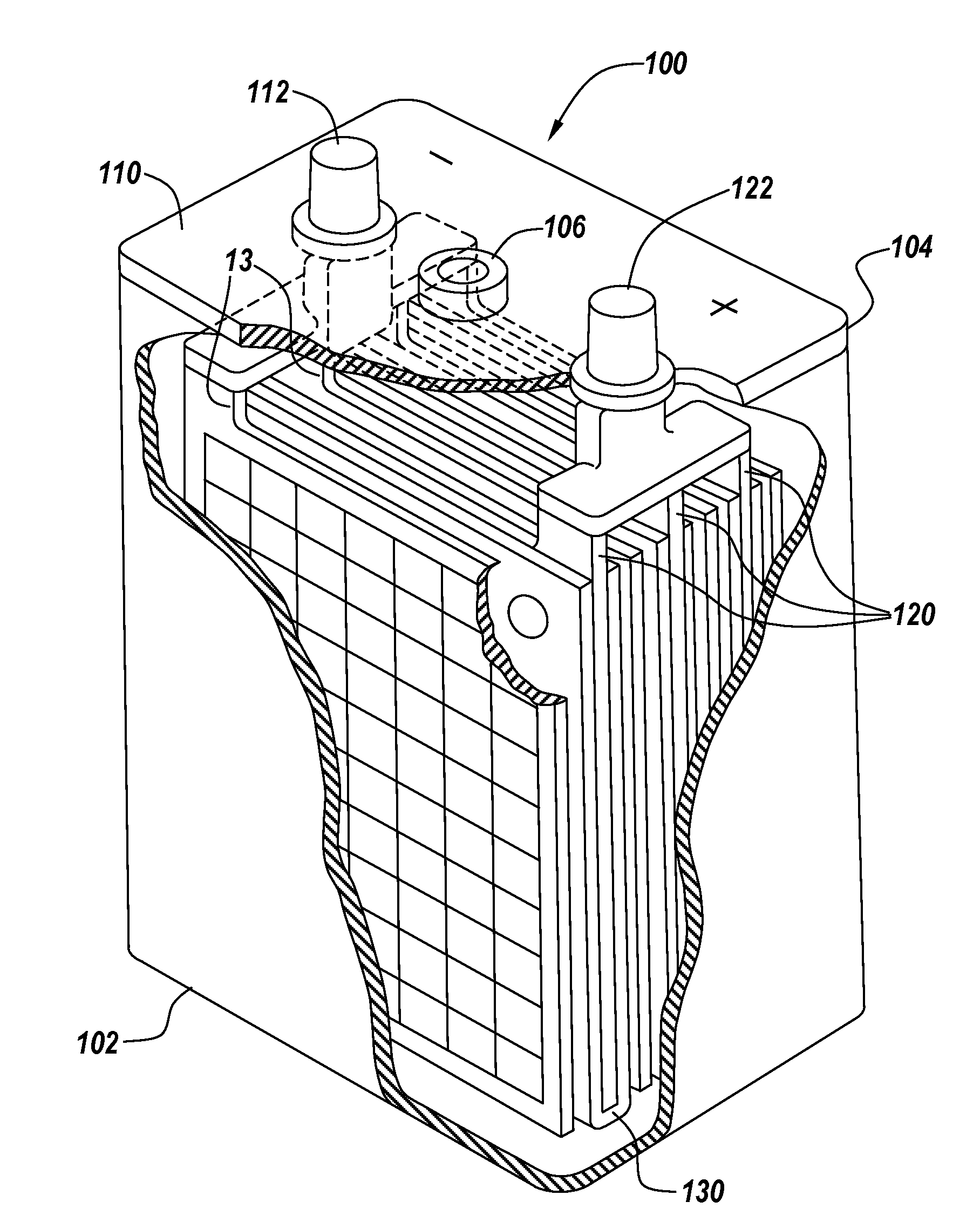
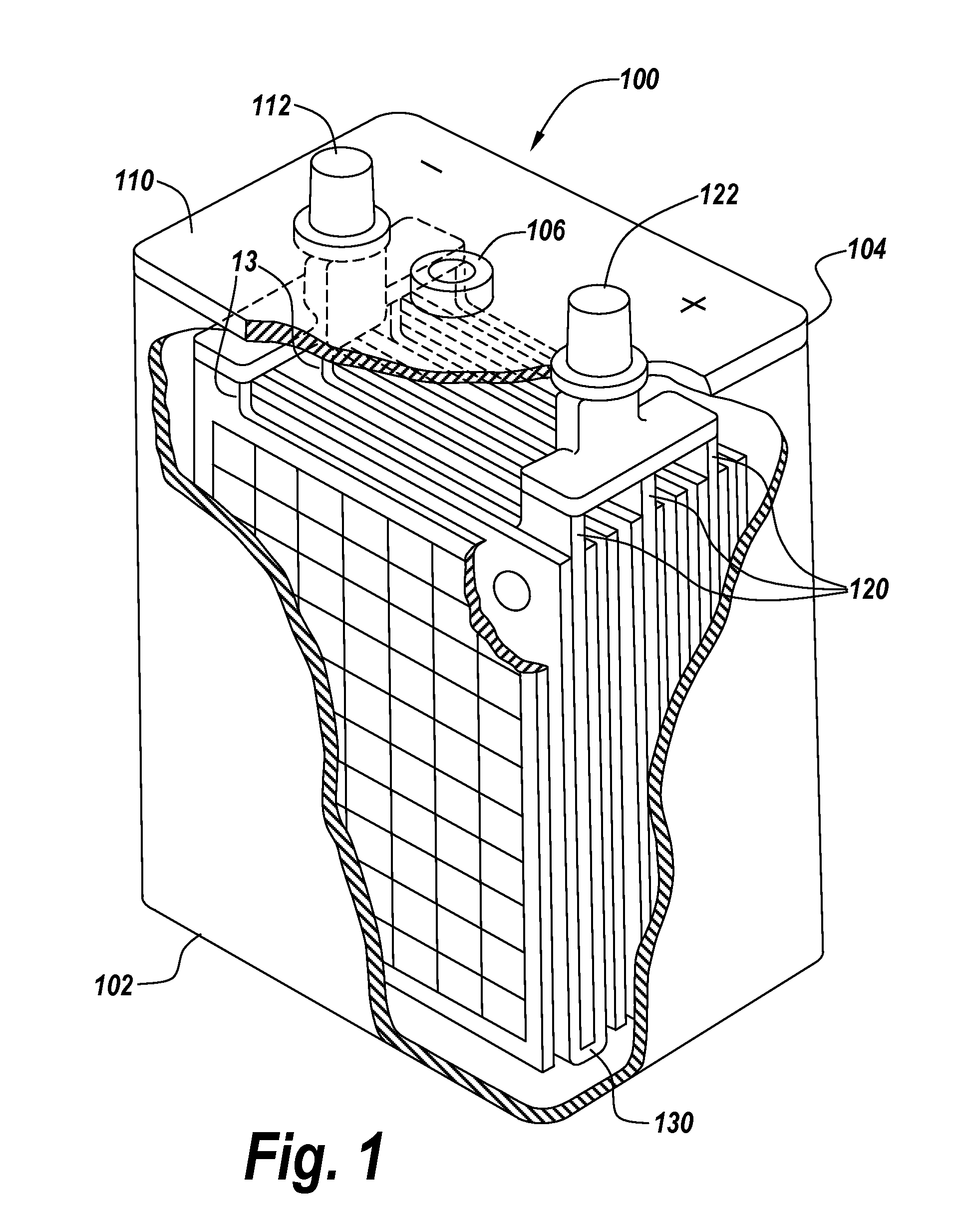
ActiveUS20140349147A1Sufficient structural integrityPreventing electrical shortingFinal product manufactureLead-acid accumulator electrodesElectrical batteryFluid electrolytes
The invention relates to an article comprising: a) one or more stacks of battery plates comprising one or more bipolar plates; b) located between each plate is a separator and a liquid electrolyte; further comprising one of more of the features: 1) c) the one or more stacks of battery plates having a plurality of channels passing transversely though the portion of the plates having the cathode and / or the anode deposited thereon; and d) i) one or more seals about the periphery of the channels which prevent the leakage of the liquid electrolyte into the channels, and / or posts located in one or more of the channels having on each end an overlapping portion that covers the channel and sealing surface on the outside of the monopolar plates adjacent to the holes for the transverse channels and applies pressure on the sealing surface of the monopolar plates wherein the pressure is sufficient to withstand pressures created during assembly and operation of electrochemical cells created by the stacks of battery plates; 2) c) a membrane comprising a thermoplastic polymer is disposed about the entire periphery of the edges of the stack of plates; 3) wherein the separator is in the form of a sheet having adhered to its periphery a frame; and 4) c) an integrated valve and integrated channel communicating with the valve.
Owner:ADVANCED BATTERY CONCEPTS
Chargeable zinc ion battery
ActiveCN101540417AImproved magnification performanceGood reversibilityAlkaline accumulatorsActive material electrodesZinc ionManganese
The invention discloses a chargeable zinc ion battery, which comprises an anode, a cathode, isolating membrane arranged between the anode and the cathode, and electrolyte which contains the anion and the cation and has ionicconductivity, wherein the cathode adopts active materials which are mainly zinc elements, the active materials of the anode are oxide materials of manganese which can occlude and release zinc ions, the electrolyte is liquid or gel materials with ionicconductivity which adopts the soluble salt of zinc as solute and water as solvent, and the pH value of the electrolyte lies between 3 and 7. The battery has the characteristics of high capacity, chargeable property, long cycle life and the like. Proven by experiments, the chargeable zinc ion battery has excellent rate performance, reversible performance and cycle performance.
Owner:SHENZHEN CITY THROUGH SCI & TECH OF NEW ENERGY CO LTD
Metal-air semi-fuel cell with an aqueous acid based cathode
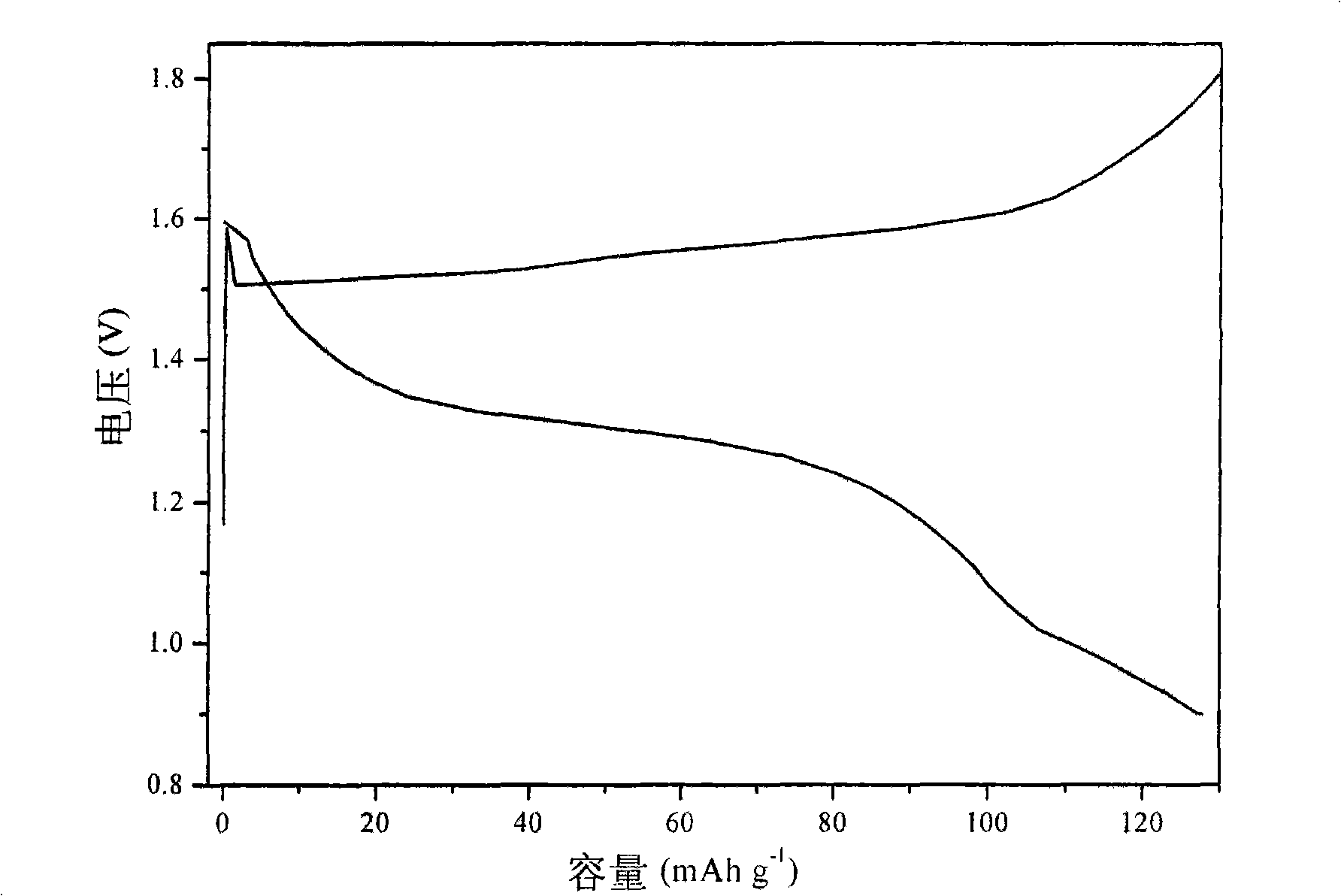
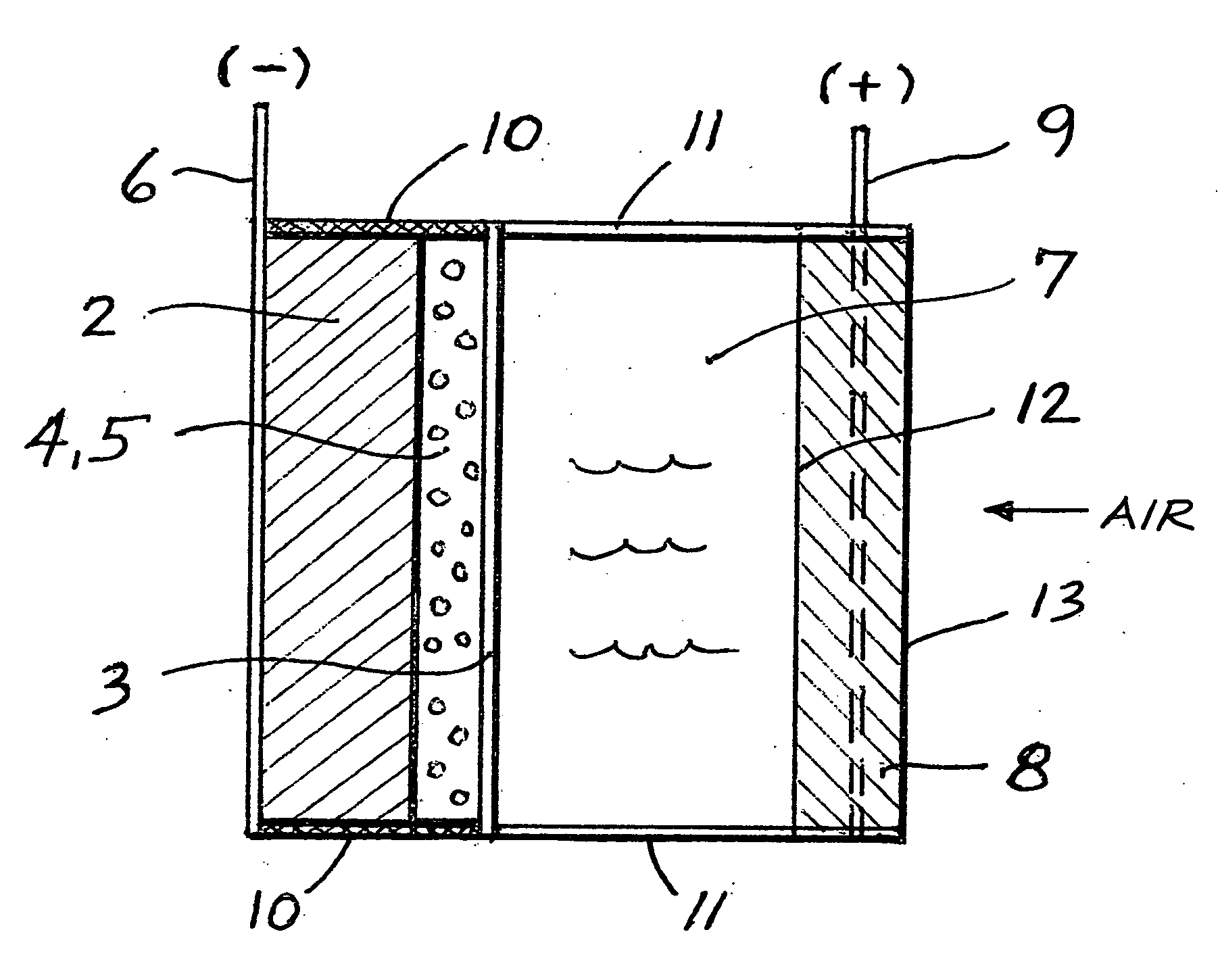
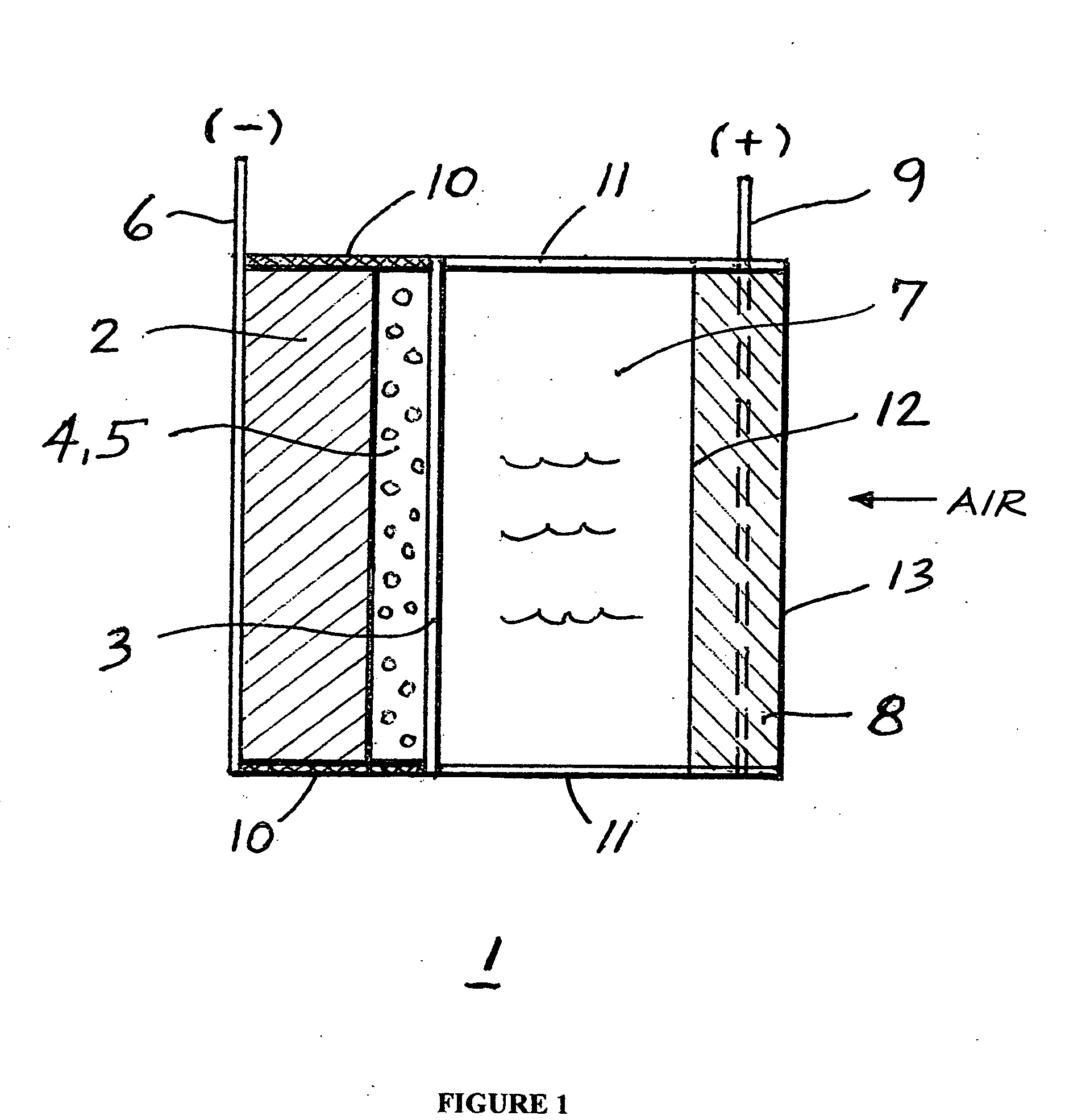
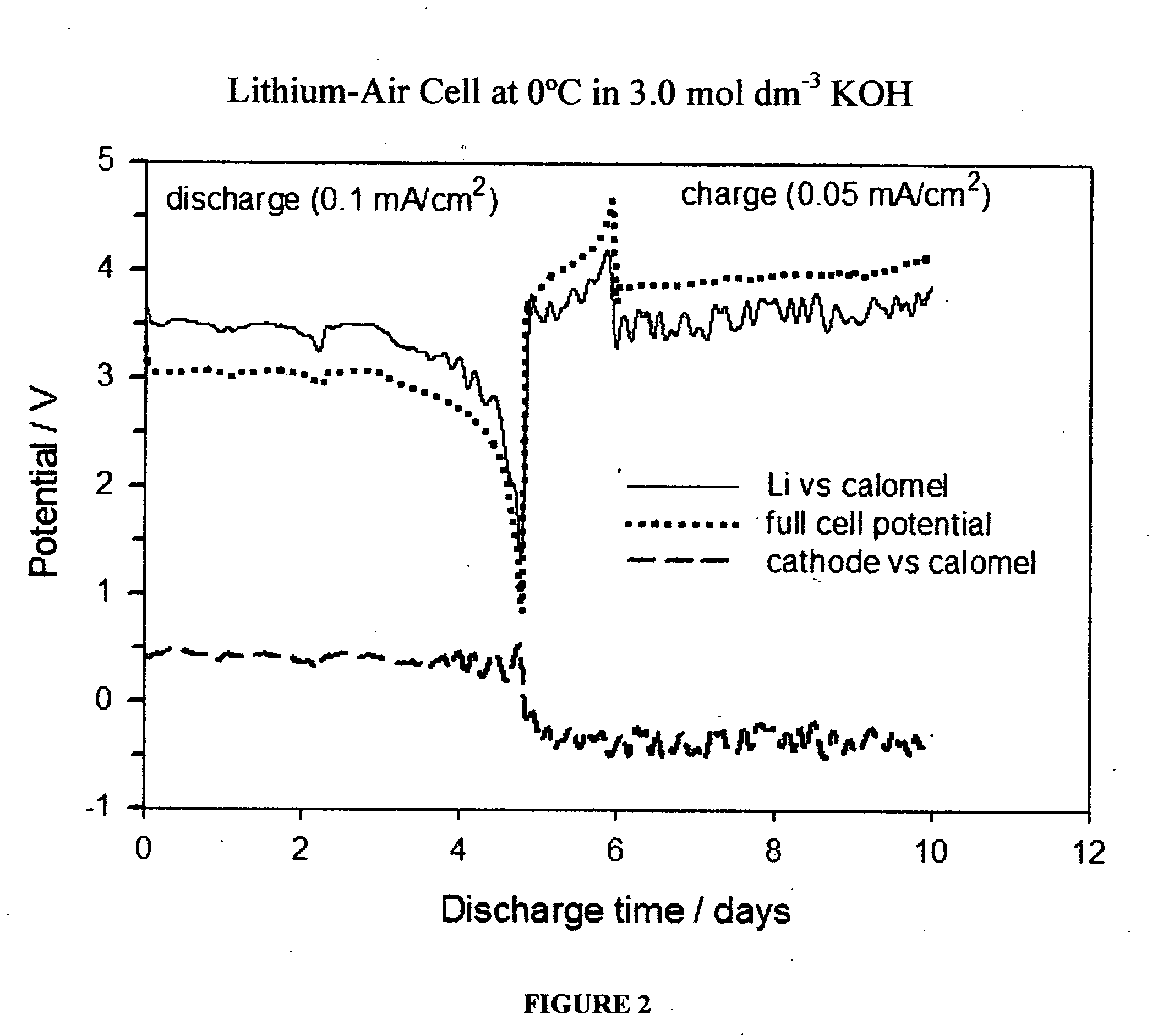
InactiveUS20070259234A1Long time operationCell energy increasedFuel and primary cellsFuel and secondary cellsFuel cellsOxygen
A metal-air semi-fuel cell is provided, preferably based on lithium anode and a fuel cell type air / oxygen electrode immersed in an aqueous neutral, alkali or acid solution. The lithium anode is comprised of the active metal and one or more separators protecting the anode from reacting with an aqueous solution. The outermost layer on the lithium electrode is a solid-state lithium-ion conducting glass-ceramic which is impervious to and stable towards aqueous solutions. The cathode is comprised of an air or oxygen fuel cell type electrode in contact with the aqueous solution. The lithium anode of this invention also can be replaced by other electroactive metals which react with water and acids, bases and neutral solutions, such as metals from Groups 1 and 2 of the Periodic Table of Elements in addition to Zn, Mg, and Al.
Owner:CHUA DAVID +2
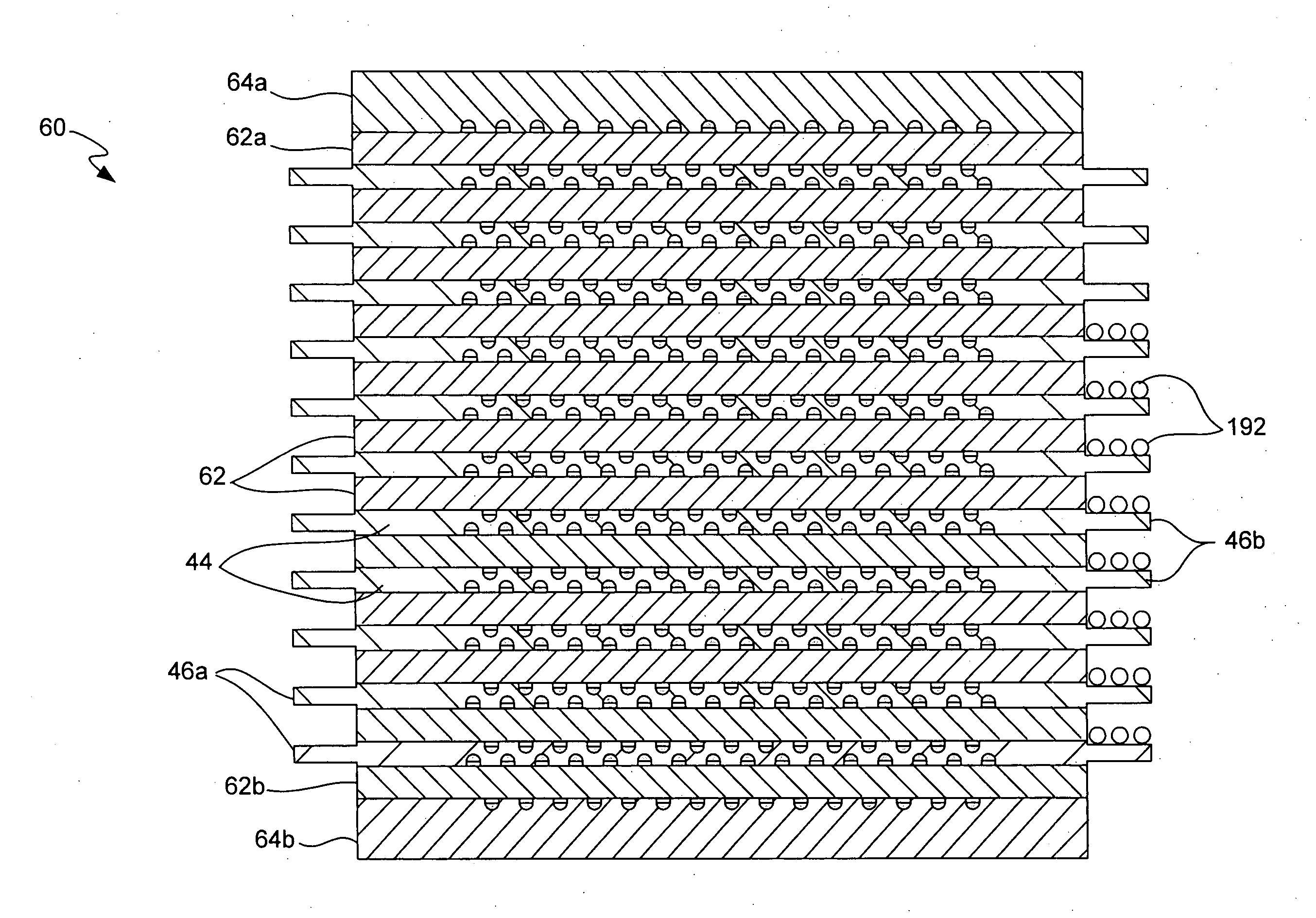
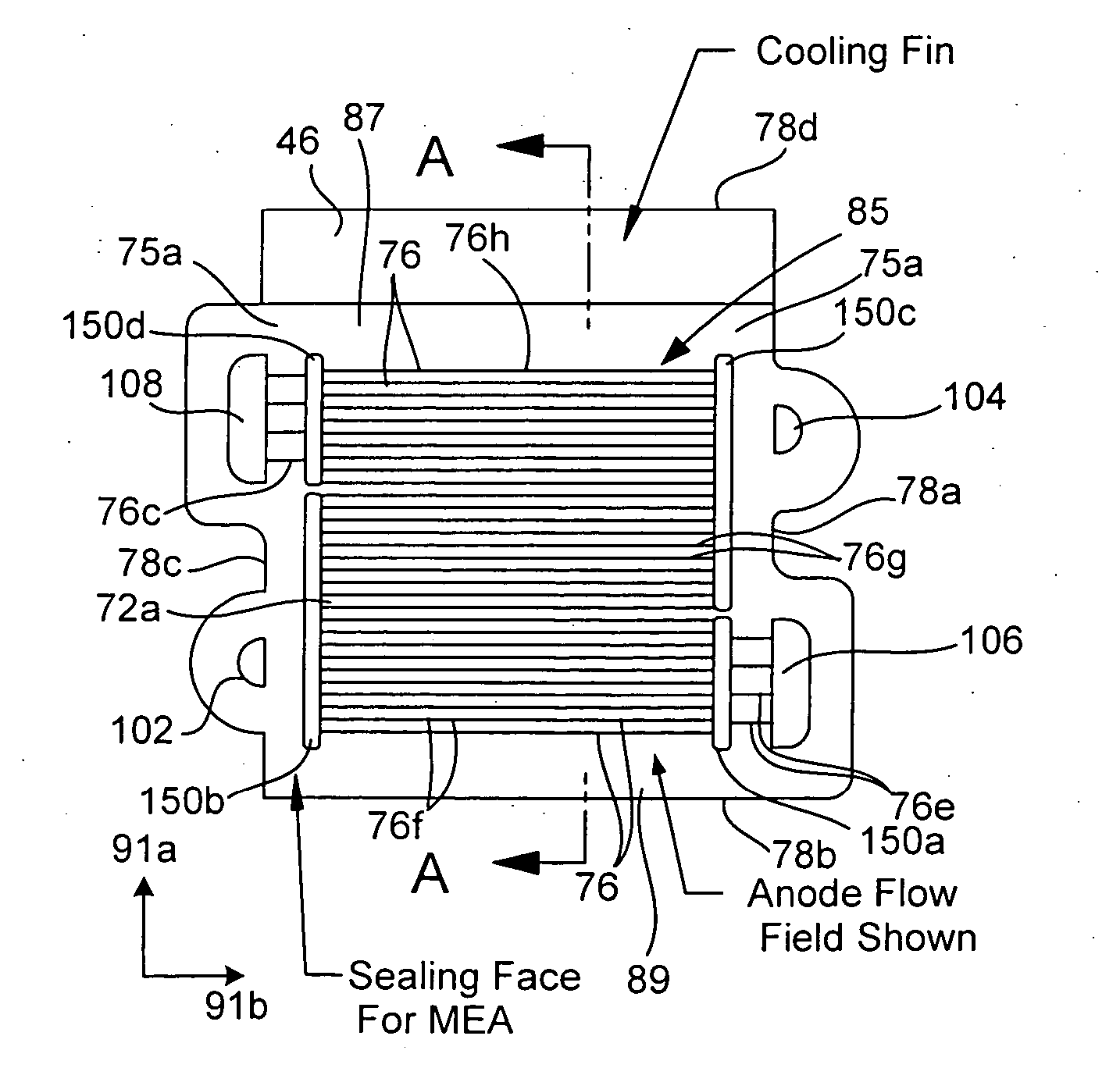
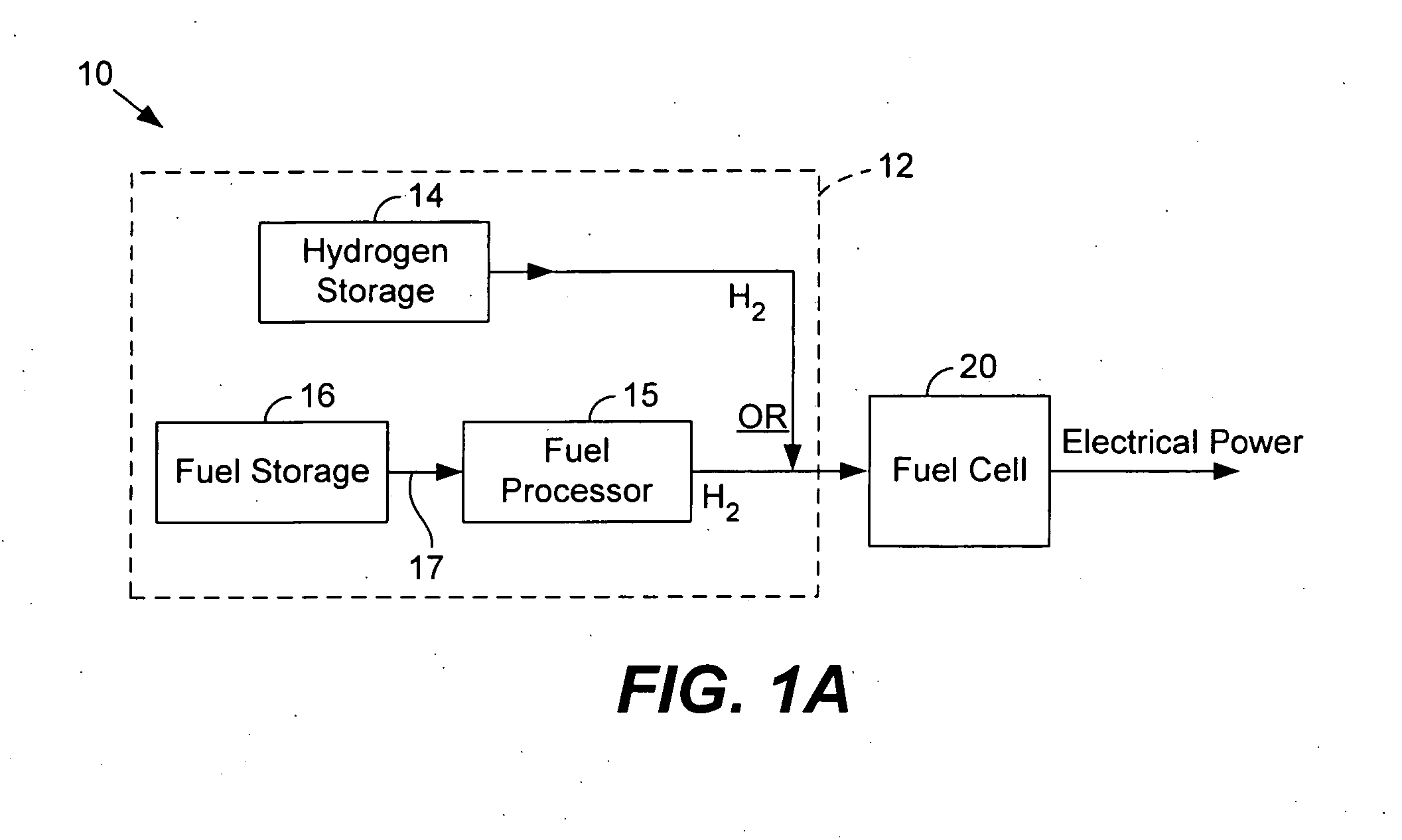
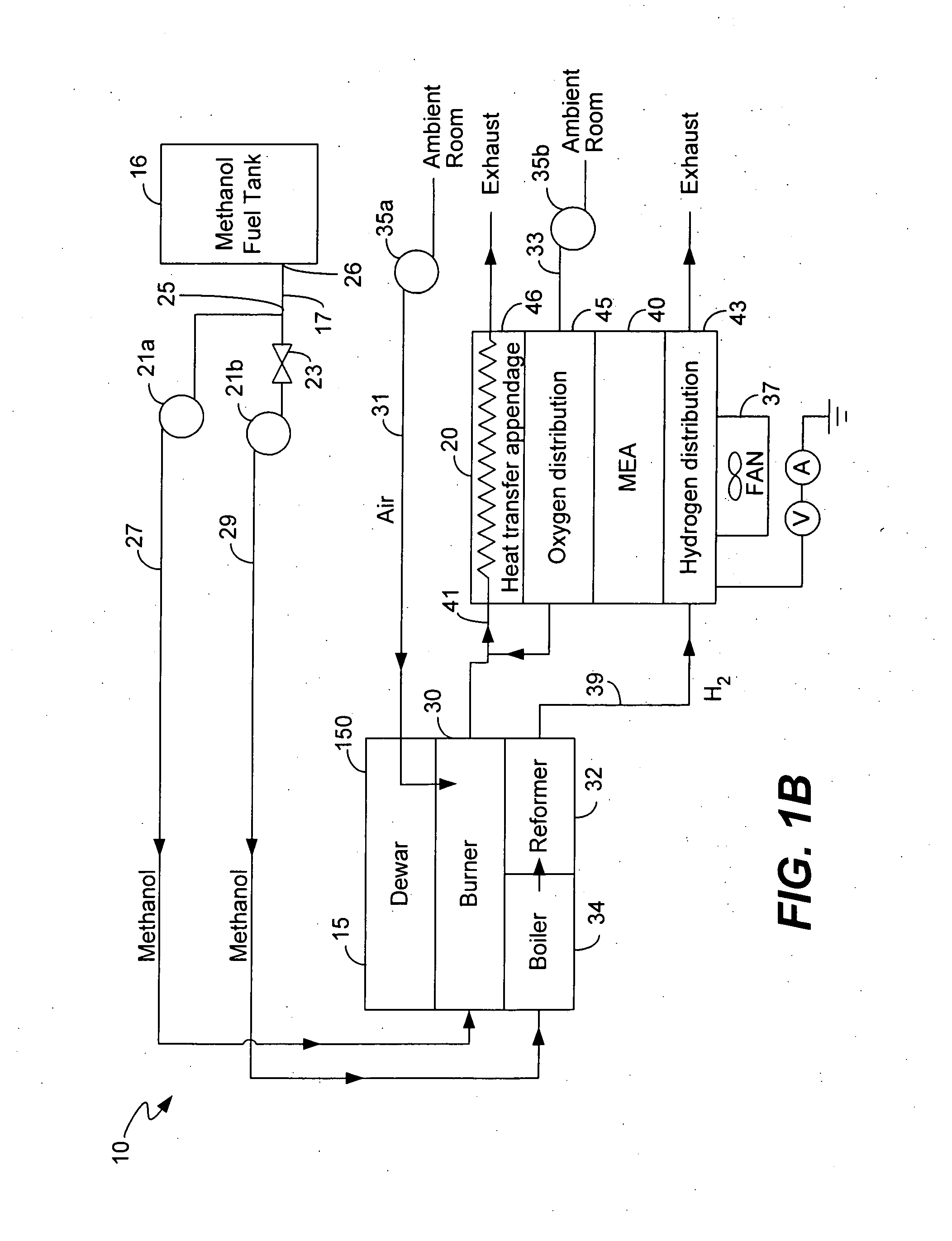
Micro fuel cell thermal management
ActiveUS20050008911A1Improves fuel cell thermal managementFacilitates thermal communicationFuel cell heat exchangeFuel cells groupingSingle plateEngineering
The present invention relates to fuel cells and components used within a fuel cell. Heat transfer appendages are described that improve fuel cell thermal management. Each heat transfer appendage is arranged on an external portion of a bi-polar plate and permits conductive heat transfer between inner portions of the bi-polar plate and outer portions of the bi-polar plate proximate to the appendage. The heat transfer appendage may be used for heating or cooling inner portions of a fuel cell stack. Improved thermal management provided by cooling the heat transfer appendages also permits new channel field designs that distribute the reactant gases to a membrane electrode assembly. Flow buffers are described that improve delivery of reactant gases and removal of reaction products. Single plate bi-polar plates may also include staggered channel designs that reduce the thickness of the single plate.
Owner:ULTRACELL LLC
Micro fuel cell architecture
InactiveUS20050014059A1Improved thermal managementFacilitate communicationFuel cell heat exchangeLayered productsSingle plateEngineering
The present invention relates to fuel cells and components used within a fuel cell. Heat transfer appendages are described that improve fuel cell thermal management. Each heat transfer appendage is arranged on an external portion of a bi-polar plate and permits conductive heat transfer between inner portions of the bi-polar plate and outer portions of the bi-polar plate proximate to the appendage. The heat transfer appendage may be used for heating or cooling inner portions of a fuel cell stack. Improved thermal management provided by cooling the heat transfer appendages also permits new channel field designs that distribute the reactant gases to a membrane electrode assembly. Flow buffers are described that improve delivery of reactant gases and removal of reaction products. Single plate bi-polar plates may also include staggered channel designs that reduce the thickness of the single plate.
Owner:ULTRACELL LLC
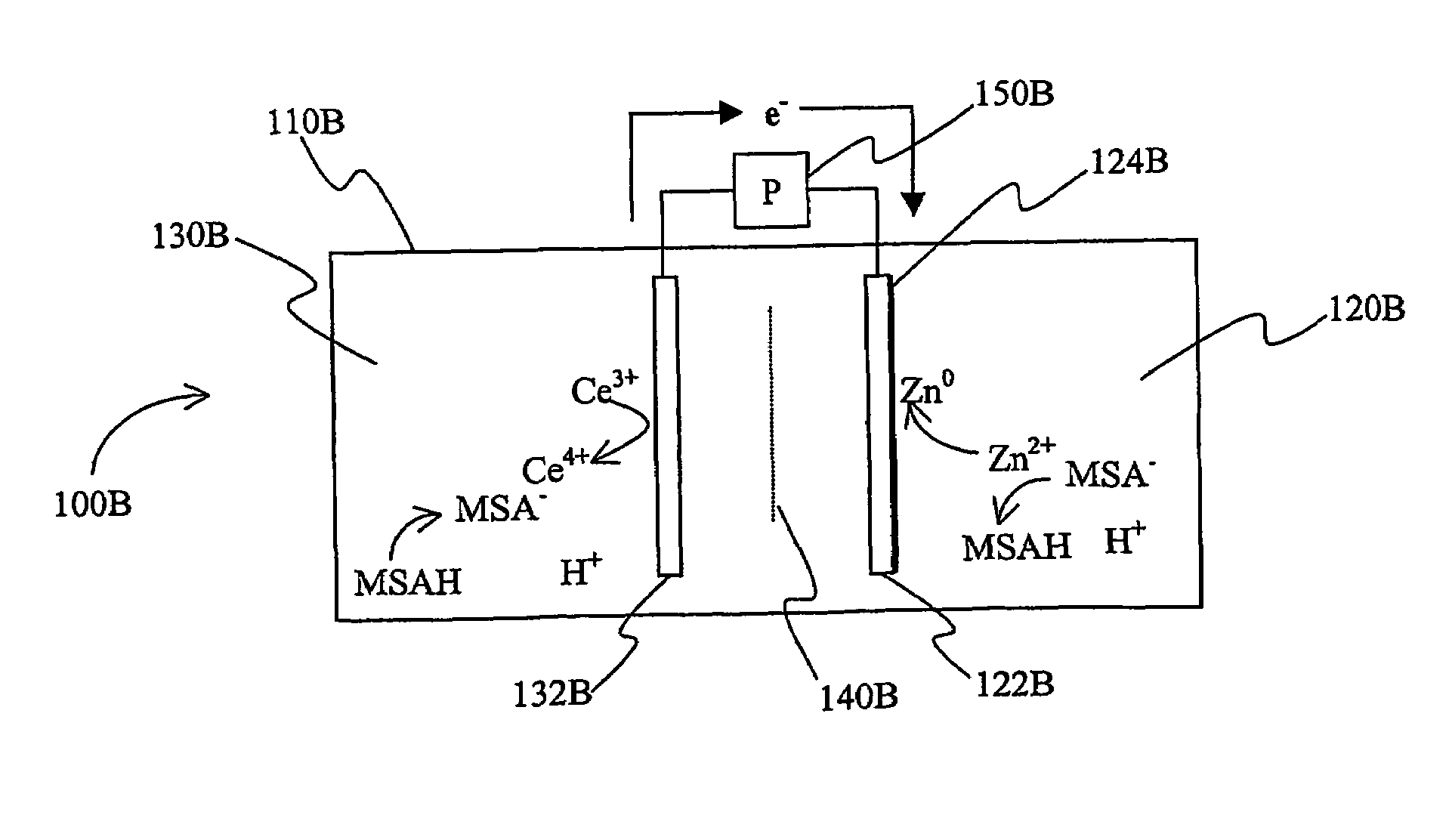
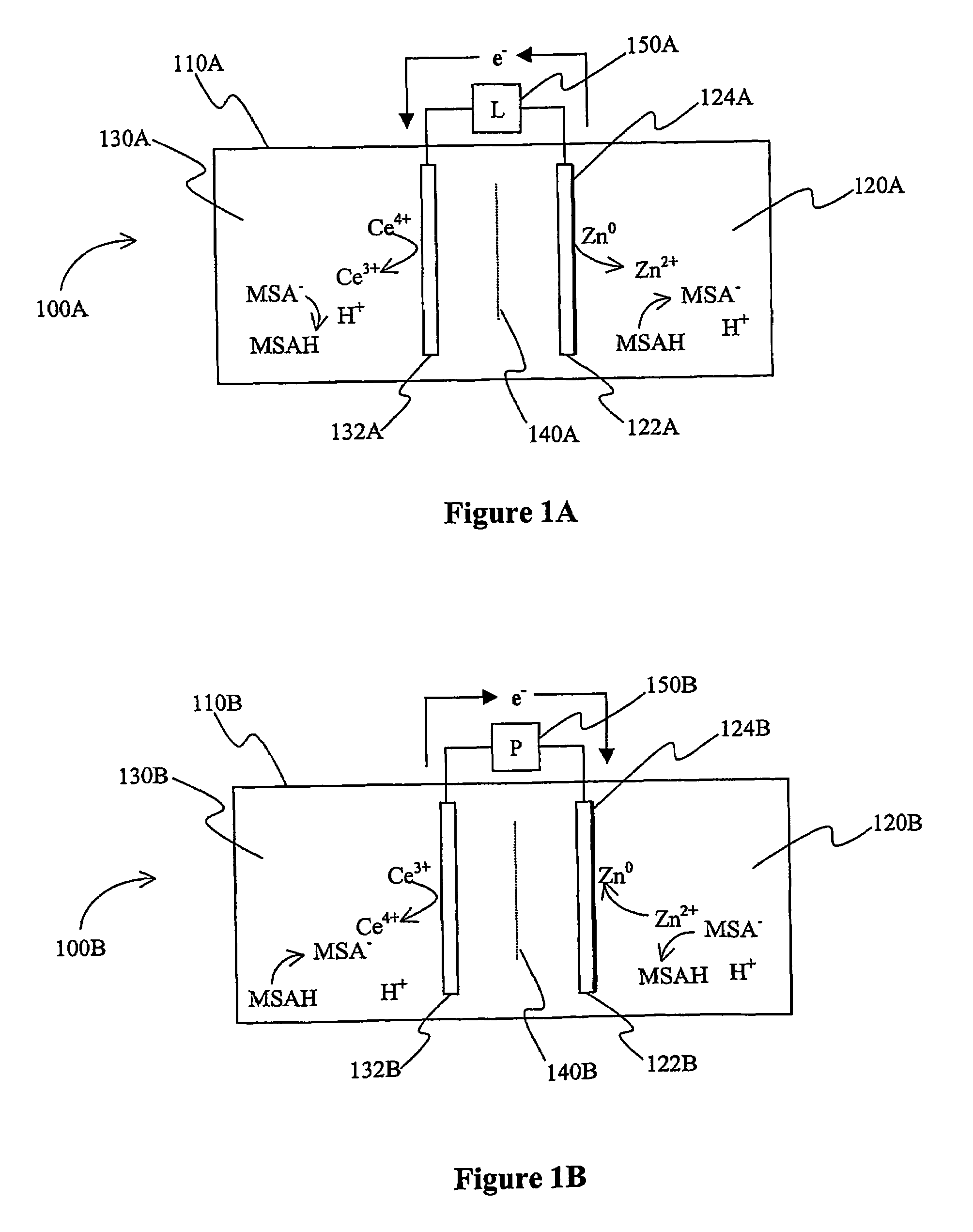
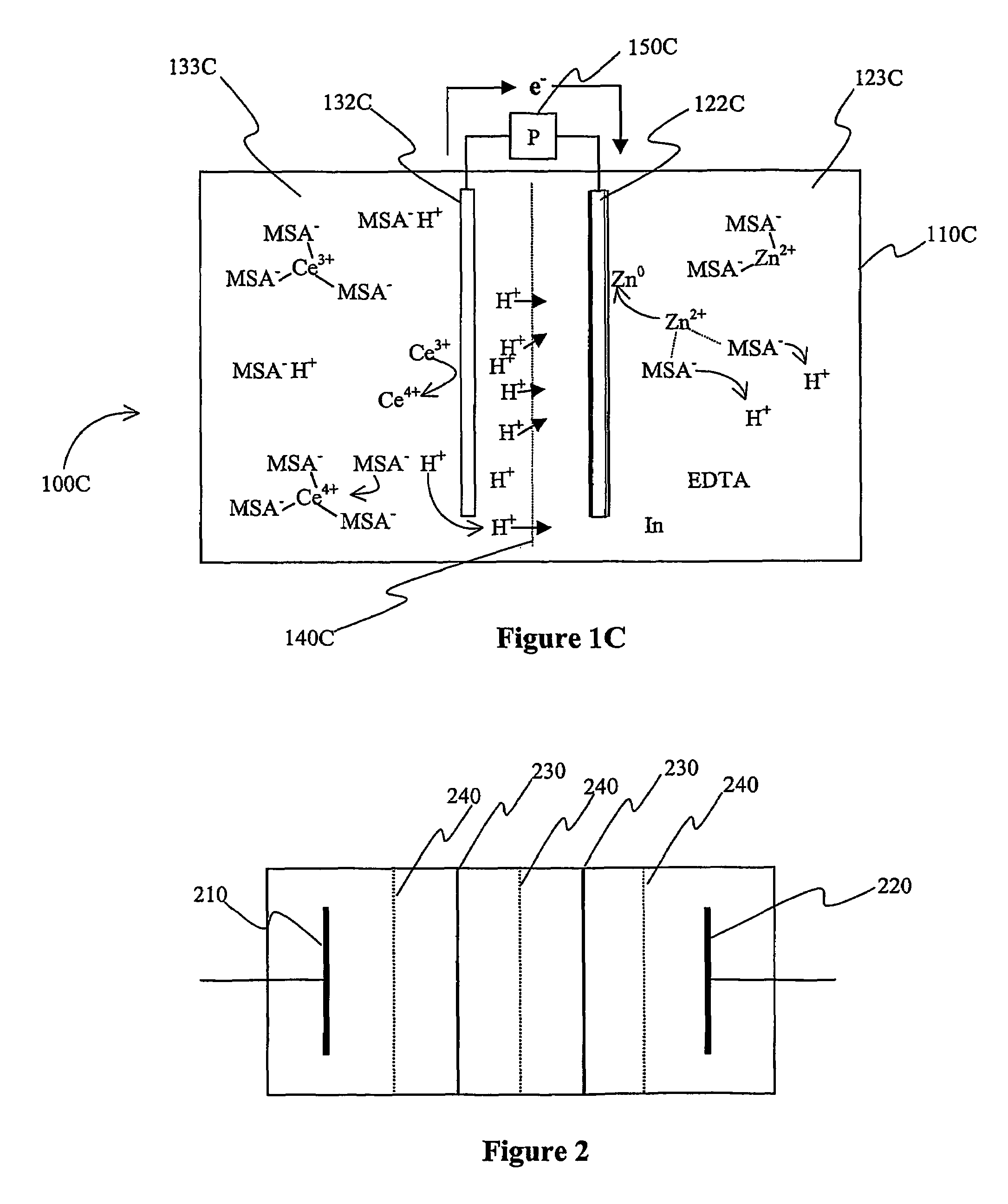
Cerium batteries
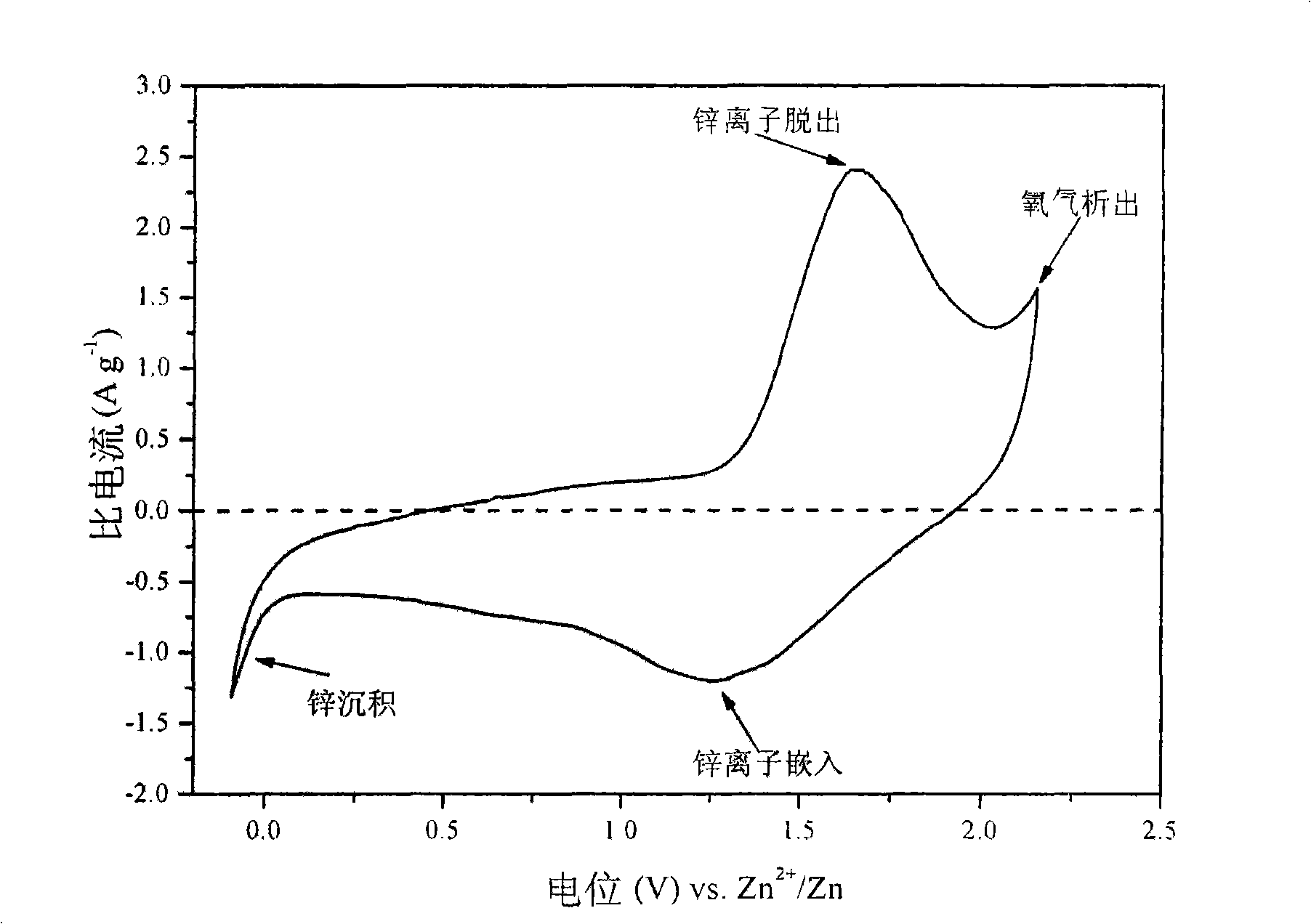
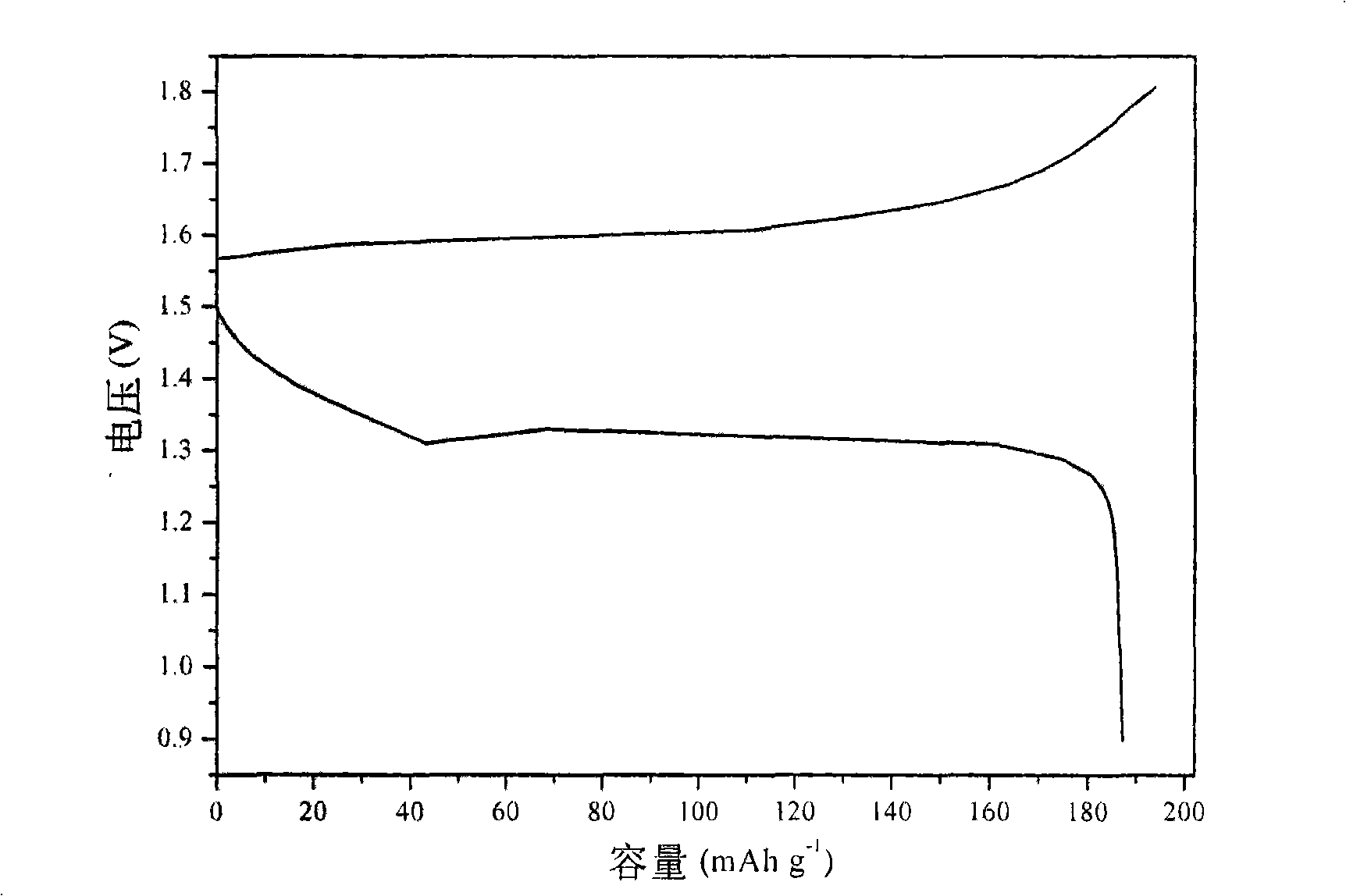
InactiveUS7625663B2Reduces hydrogen overpotentialCell electrodesOrganic electrolyte cellsOrganic acidHydrogen
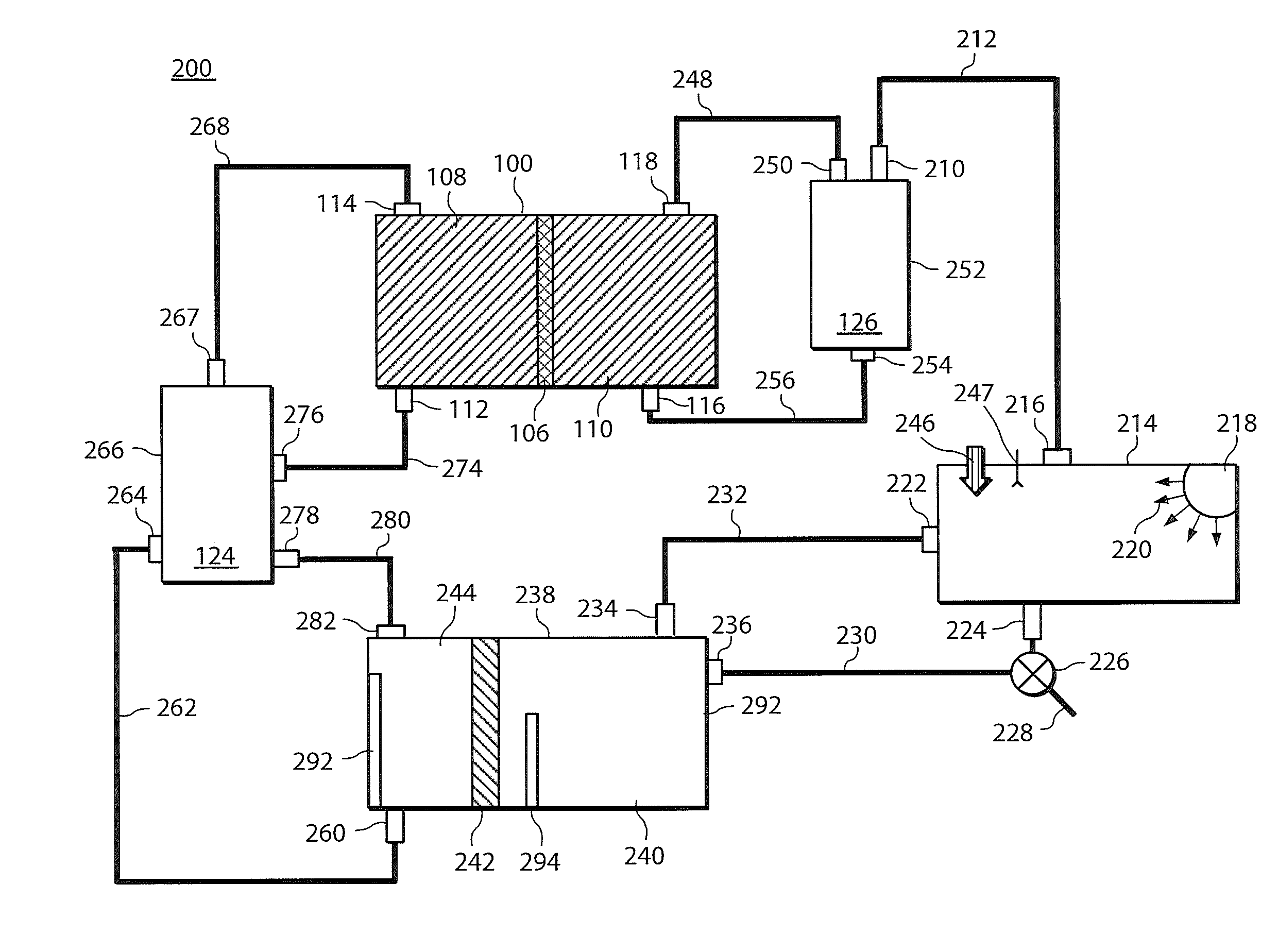
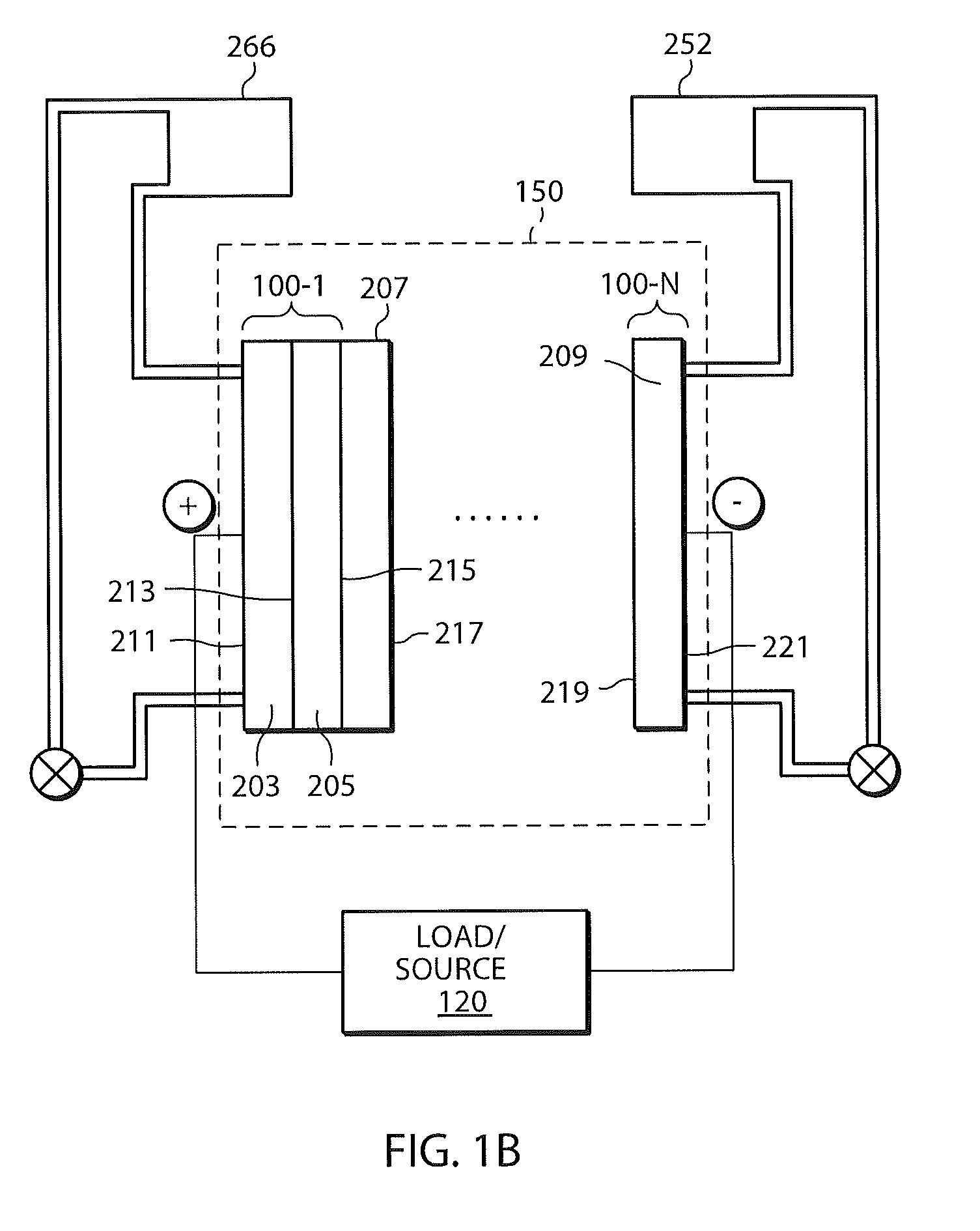
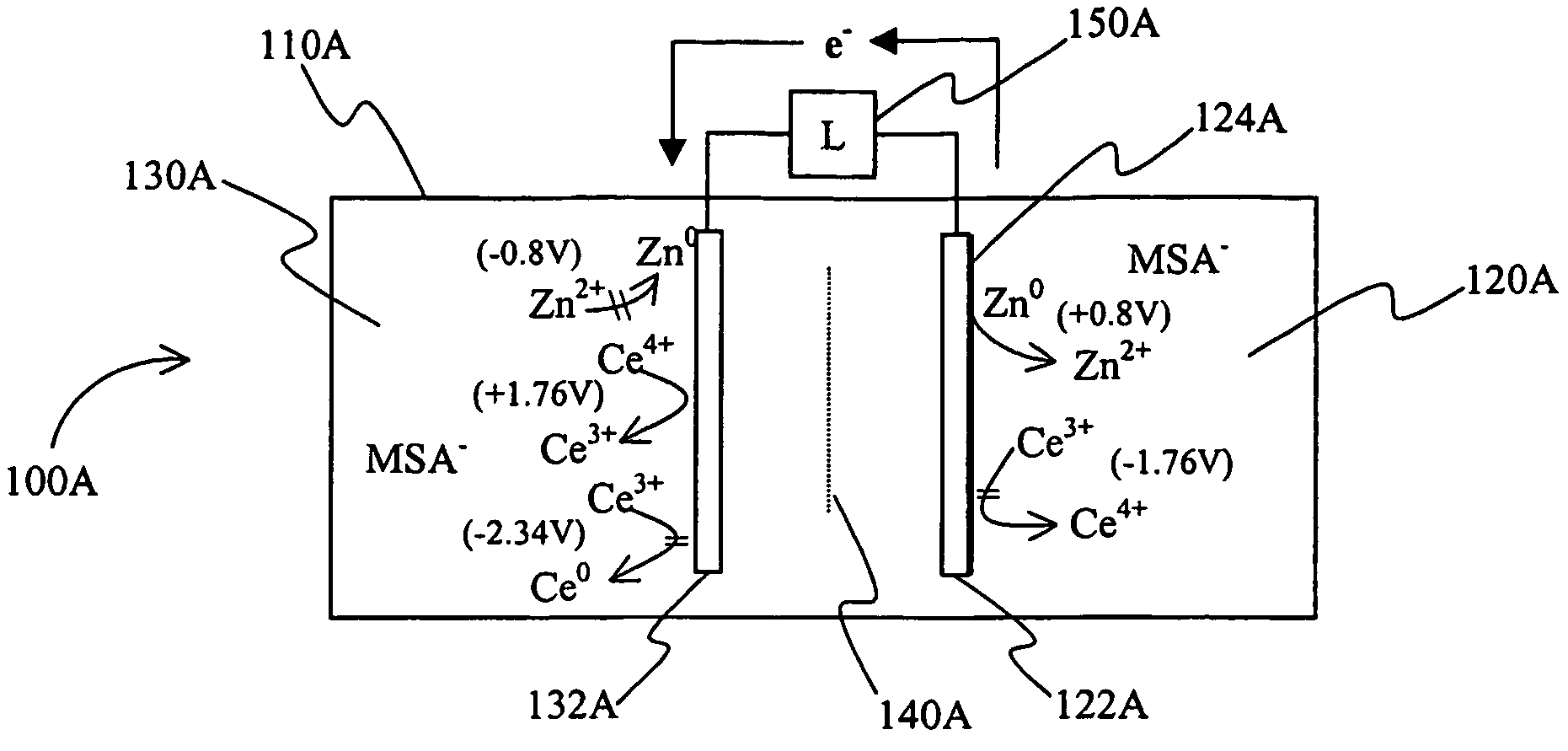
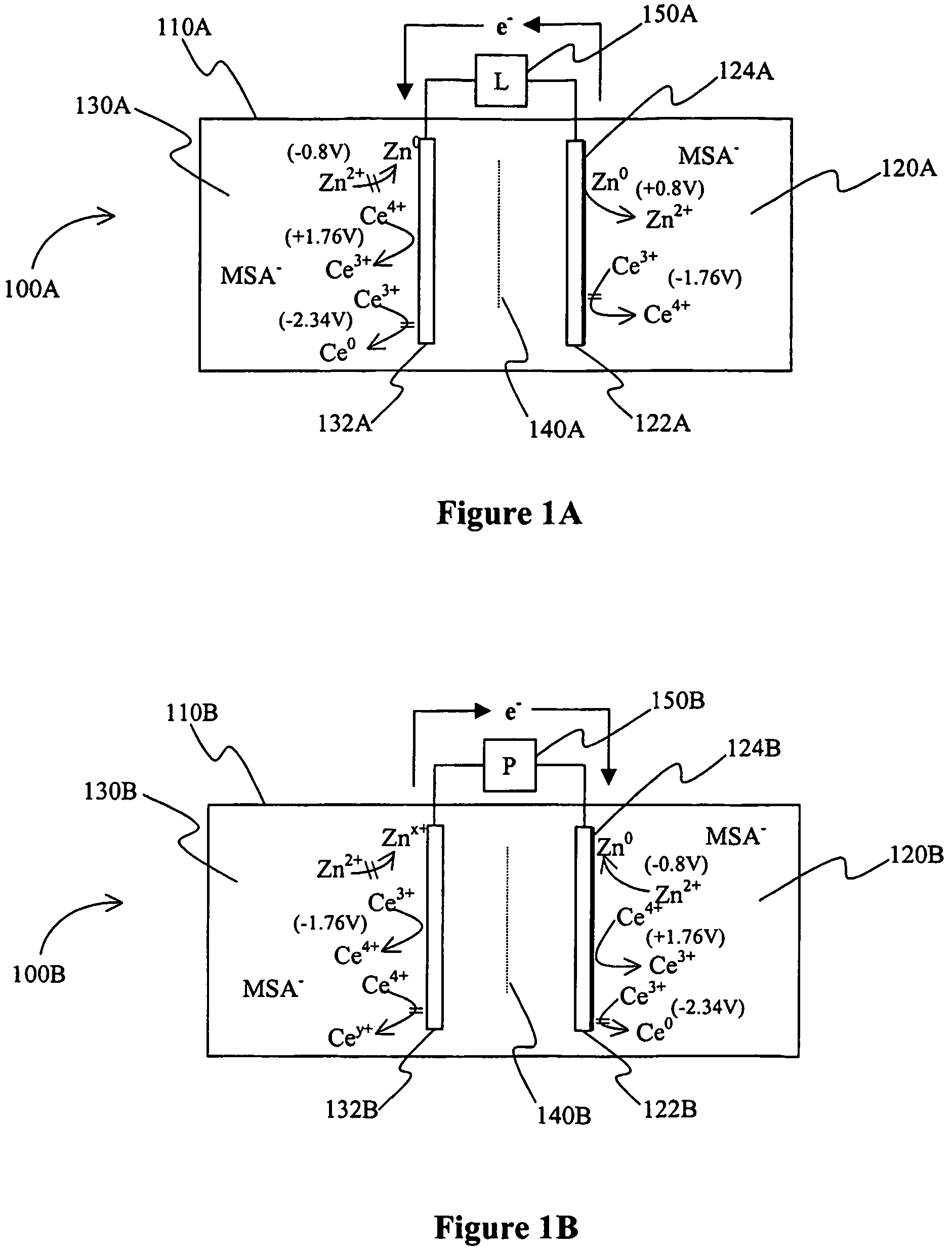
A power cell (100A) has an electrolyte that includes a reodox pair comprising cerium. The electrolyte in preferred cells is an acid electrolyte that comprises a element ion complexed by an organic acid or a chelating agent, and contemplated electrolytes may further include a compound that reduces the hydrogen overpotential. Where the power cell comprises a plurality of cells (100B), preferred configurations may include glassy carbon as bipolar electrolytes.
Owner:ITI SCOTLAND
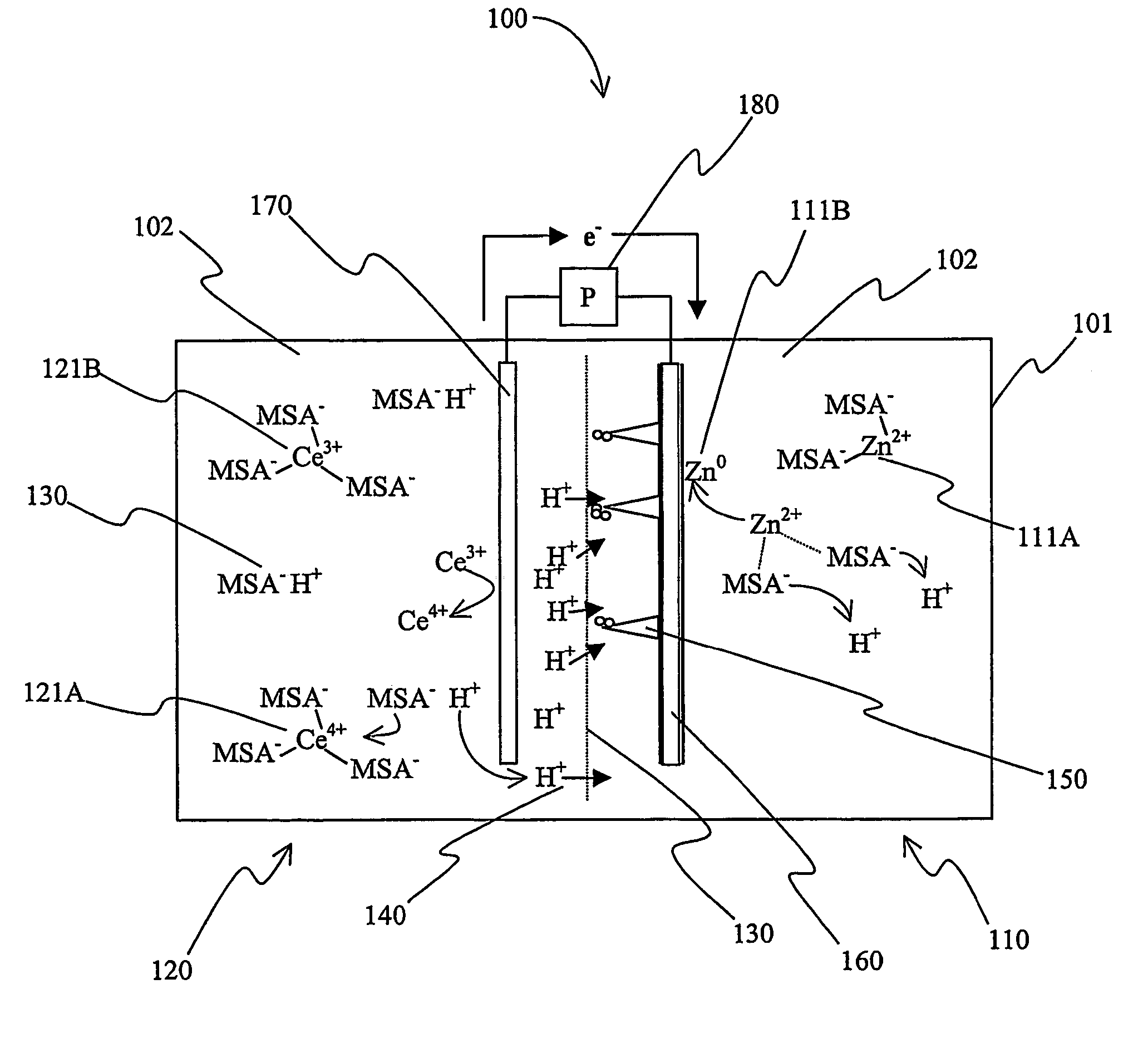
Secondary battery with autolytic dendrites
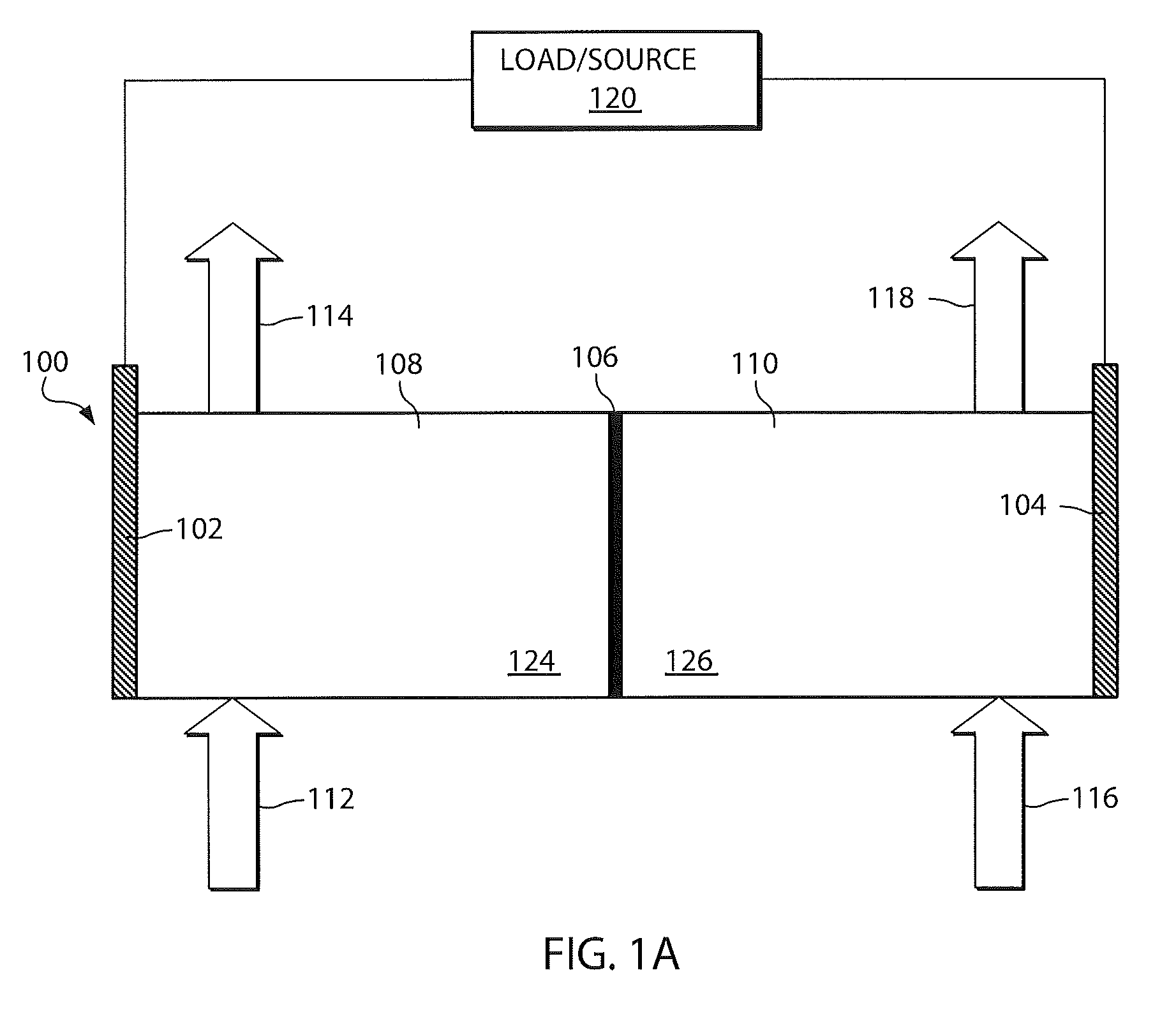
A battery (100) comprises a cell having a cathode compartment (120) that includes an element that is oxidized during charging of the battery (100), wherein the oxidized element forms a salt with an acid and thereby increases the H+ concentration in the cathode compartment (120) sufficient to promote an H+ flux into the anode compartment (110) across the separator (130), wherein the H+ flux across the separator (130) is sufficient to disintegrate a zinc dendrite proximal to the separator (130).
Owner:PLURION LTD
Electrochemical cell with a non-liquid electrolyte
InactiveUS6225009B1High ambient temperature proton conductivityNon-corrosive materialSolid electrolytesPrinted batteriesElectrochemical responseProton
A non-liquid electrolyte containing electrochemical cell which operates efficiently at room temperature. The cell includes (a) a non-liquid electrolyte in which protons are mobile, (b) an anode active material based on an organic compound which is a source of protons during cell discharge, or an anode active material including a metal whose cation can assume at least two different non-zero oxidation numbers and (c) a solid cathode including a compound which forms an electrochemical couple with the anode. Anode and cathode active materials can be chosen so that the cell has the feature that the electrochemical reactions at the anode and cathode are at least partially reversible. An important feature of the cell is that no thermal activation is required for its operation, therefore, the cell efficiently operates under ambient temperatures.
Owner:E C R ELECTRO CHEM RES
Load leveling battery and methods therefor
InactiveUS7270911B2Electrolyte moving arrangementsElectrolytic capacitorsMethane sulfonic acidCerium
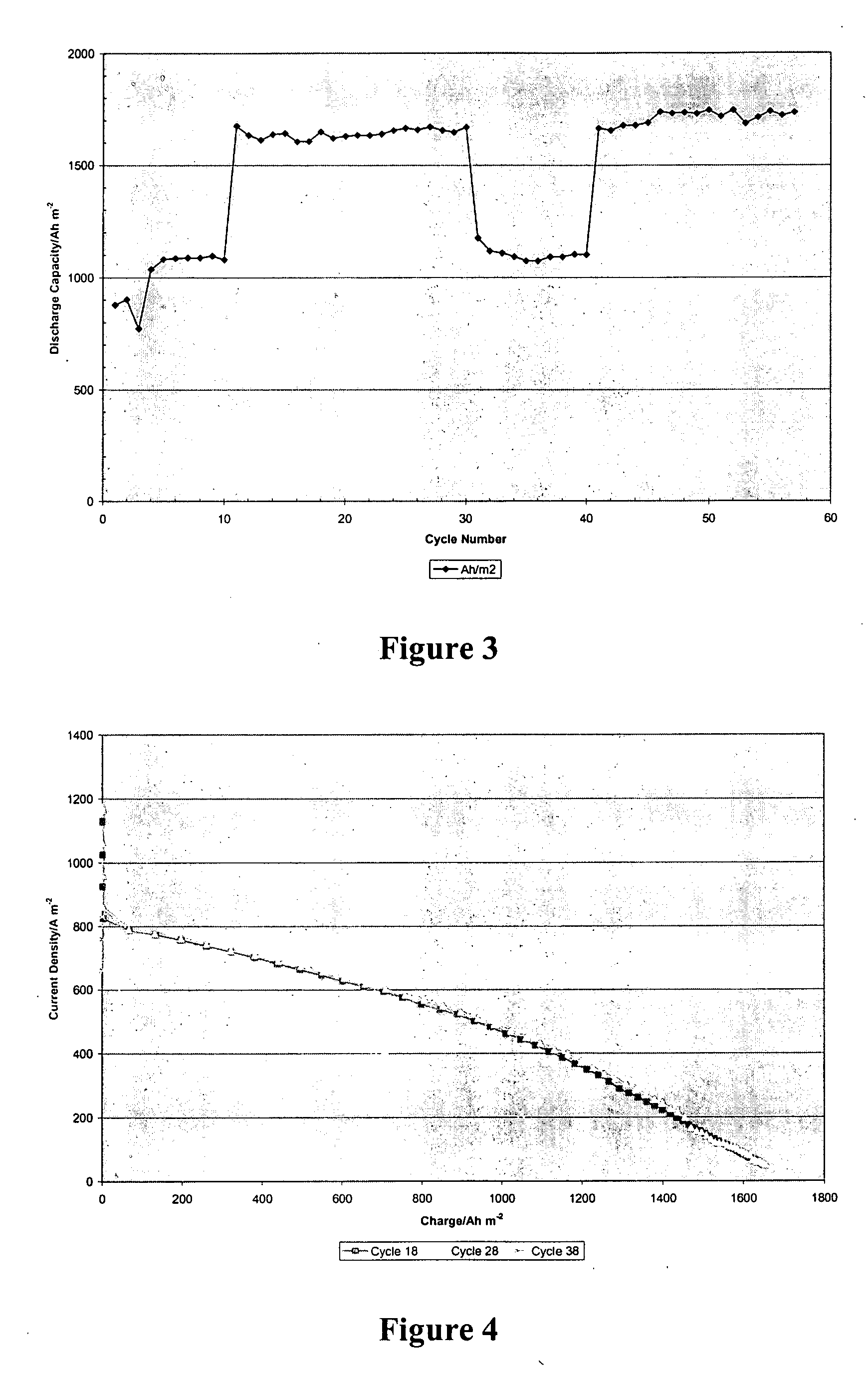
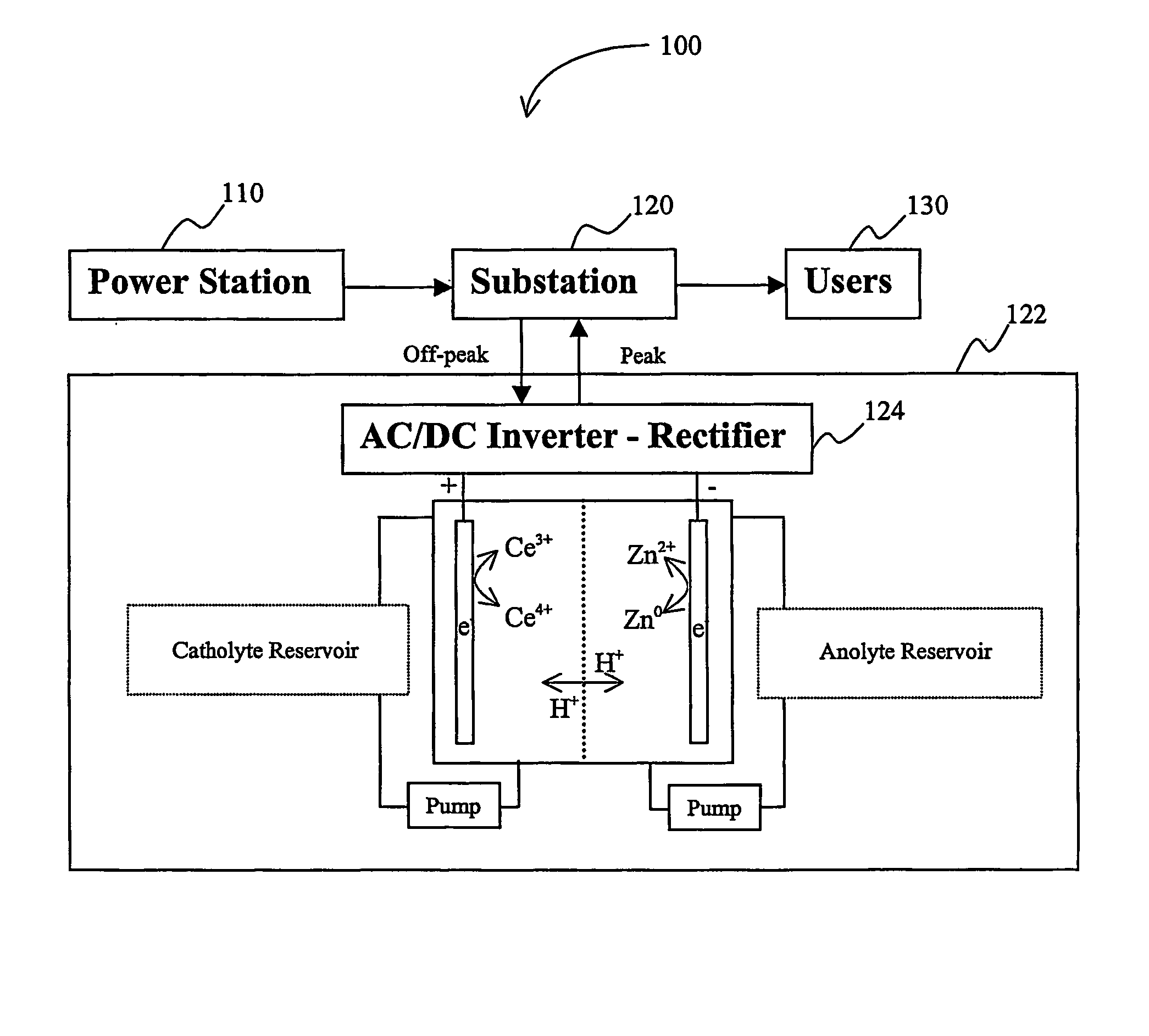
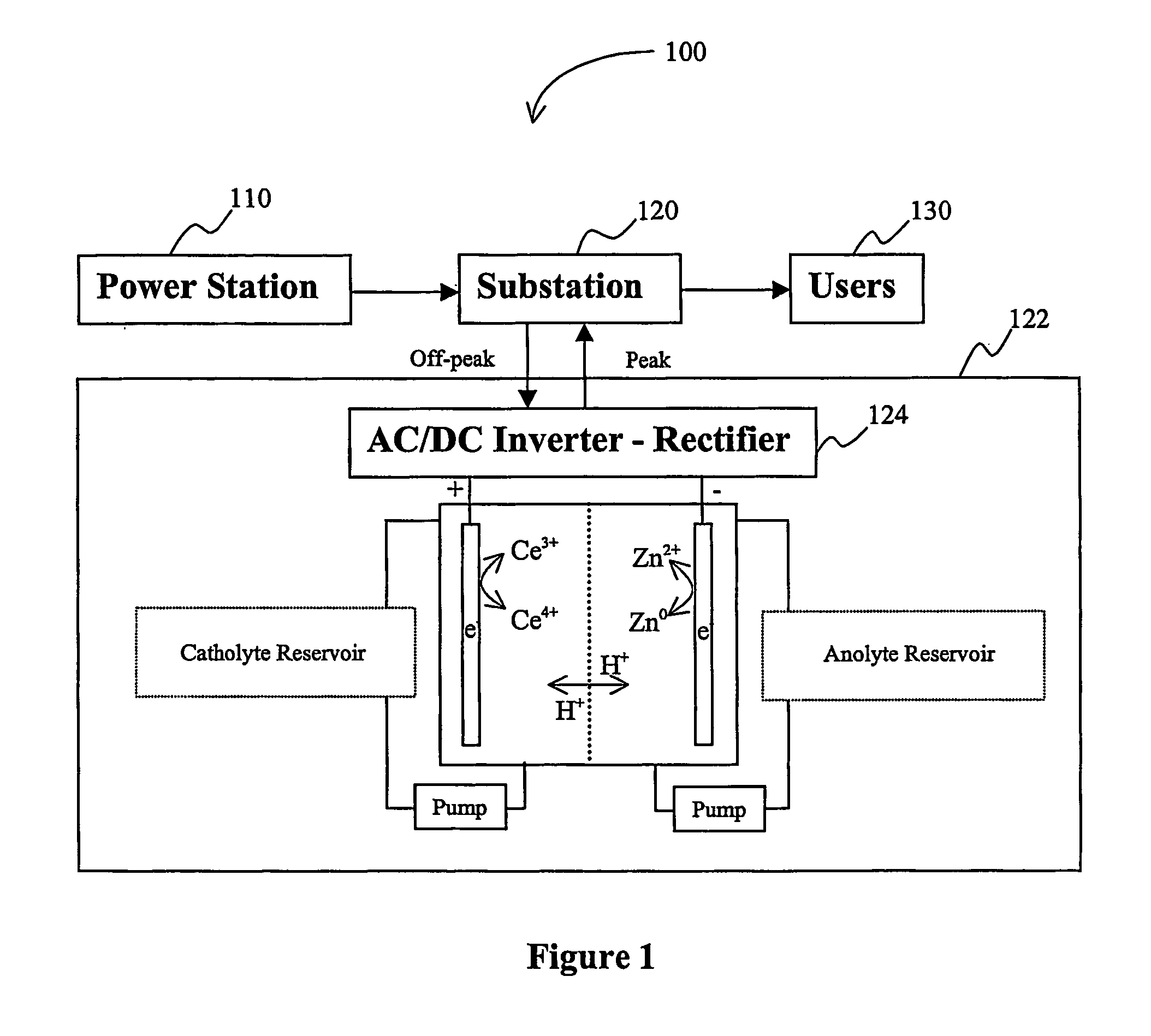
A load leveling battery (122) comprising an electrolyte that includes a cerium zinc redox pair wherein preferred electrolytes are acid electrolytes, and most preferably comprise methane sulfonic acid. Contemplated load leveling batteries (122) have an open circuit voltage of at least 2.4 Volt per cell. Such batteries are useful at power grid substations (120) and commercial and industrial applications were large amounts of power are used. Preferred capacity is at least 100,000 kWh, more preferably 250,000 kWh.
Owner:PLURION LTD
Compositions and delivery systems with leachable metal ions
InactiveUS20120070728A1Lead-acid accumulatorsLead-acid accumulator electrodesHydrogenPhysical chemistry
The disclosure describes compositions and methods for producing a change in the voltage at which hydrogen gas is produced in a lead acid battery. The compositions and methods relate to producing a concentration of one or more metal ions in the lead acid battery electrolyte.
Owner:HOLLINGSWORTH VOSE
Vanadium redox battery electrolyte
InactiveUS20040241552A1Loss in Coulombic efficiencyEvolution of hydrogen is thereby avoidedTantalum compoundsRegenerative fuel cellsVanadium redox batterySlurry
The present invention relates generally to the production of a vanadium electrolyte, including a mixture of trivalent and tetravalent vanadium ions in a sulphuric acid solution, by the reactive dissolution of vanadium trioxide and vanadium pentoxide powders, the surface area and particle size characteristics being controlled for complete reaction to produce the desired ratio of V(III) to V(IV) ions in the solution. The solution may be suitable for direct use in the vanadium redox battery, or the solution can provide an electrolyte concentrate or slurry which can be reconstituted by the addition of water or sulphuric acid prior to use in the vanadium redox battery.
Owner:UNISEARCH LTD
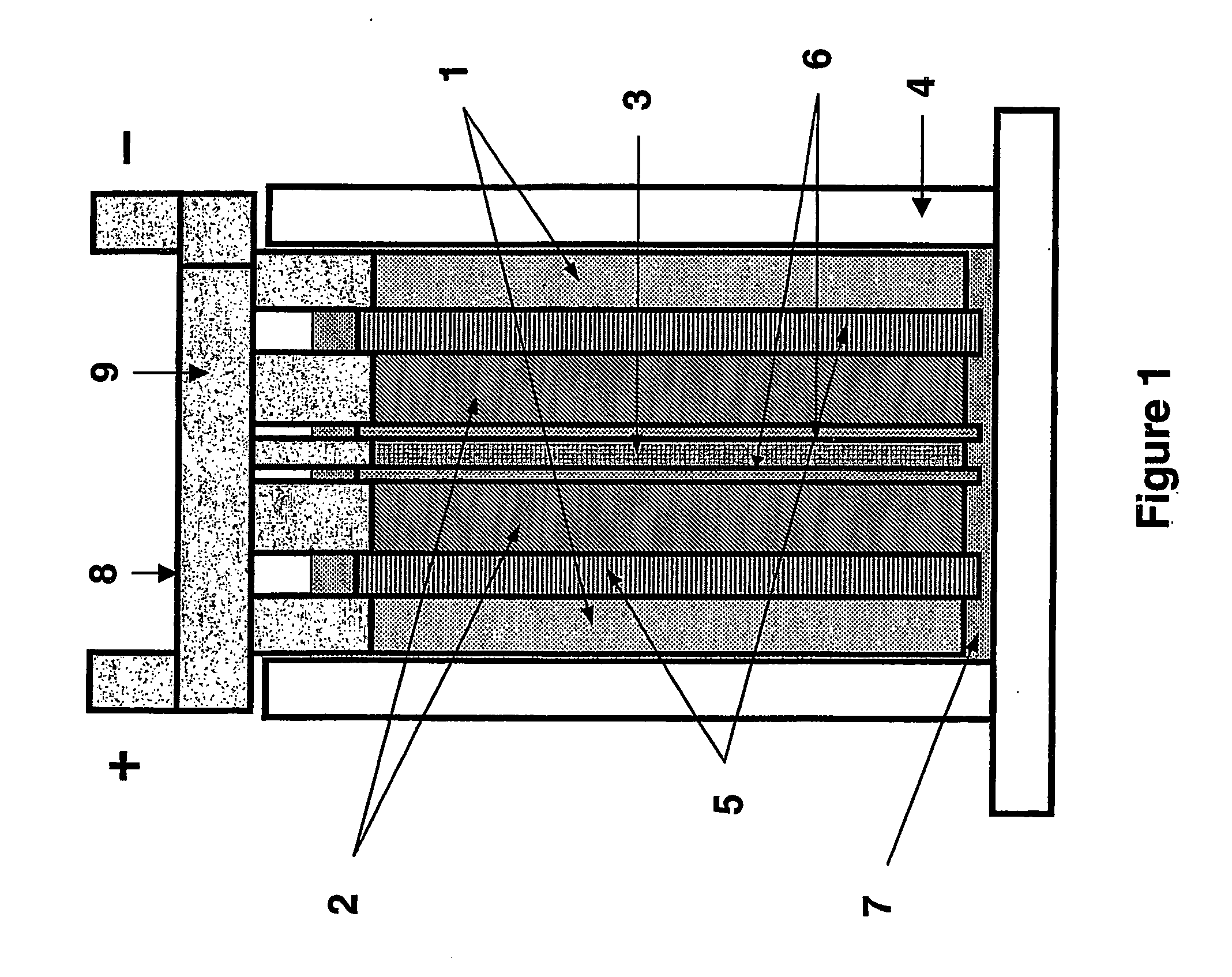
High performance energy storage devices
ActiveUS7923151B2Lower internal resistanceSolution to short lifeCapacitor and primary/secondary cellsElectrolytic capacitorsLead dioxideBusbar
A lead-acid battery comprising:at least one lead-based negative electrode;at least one lead dioxide-based positive electrode;at least one capacitor electrode; andelectrolyte in contact with the electrodes;wherein a battery part is formed by the lead based negative electrode and the lead dioxide-based positive electrode; and an asymmetric capacitor part is formed by the capacitor electrode and one electrode selected from the lead based negative electrode and the lead-dioxide based positive electrode; and wherein all negative electrodes are connected to a negative busbar, and all positive electrodes are connected to a positive busbar.The capacitor electrode may be a capacitor negative electrode comprising carbon and an additive mixture selected from oxides, hydroxides or sulfates of lead, zinc, cadmium, silver and bismuth, or a capacitor negative electrode comprising carbon, red lead, antimony in oxide, hydroxide or sulfate form, and optionally other additives. The capacitor electrode may be used in asymmetric capacitors and batteries of other types.
Owner:COMMONWEALTH SCI & IND RES ORG
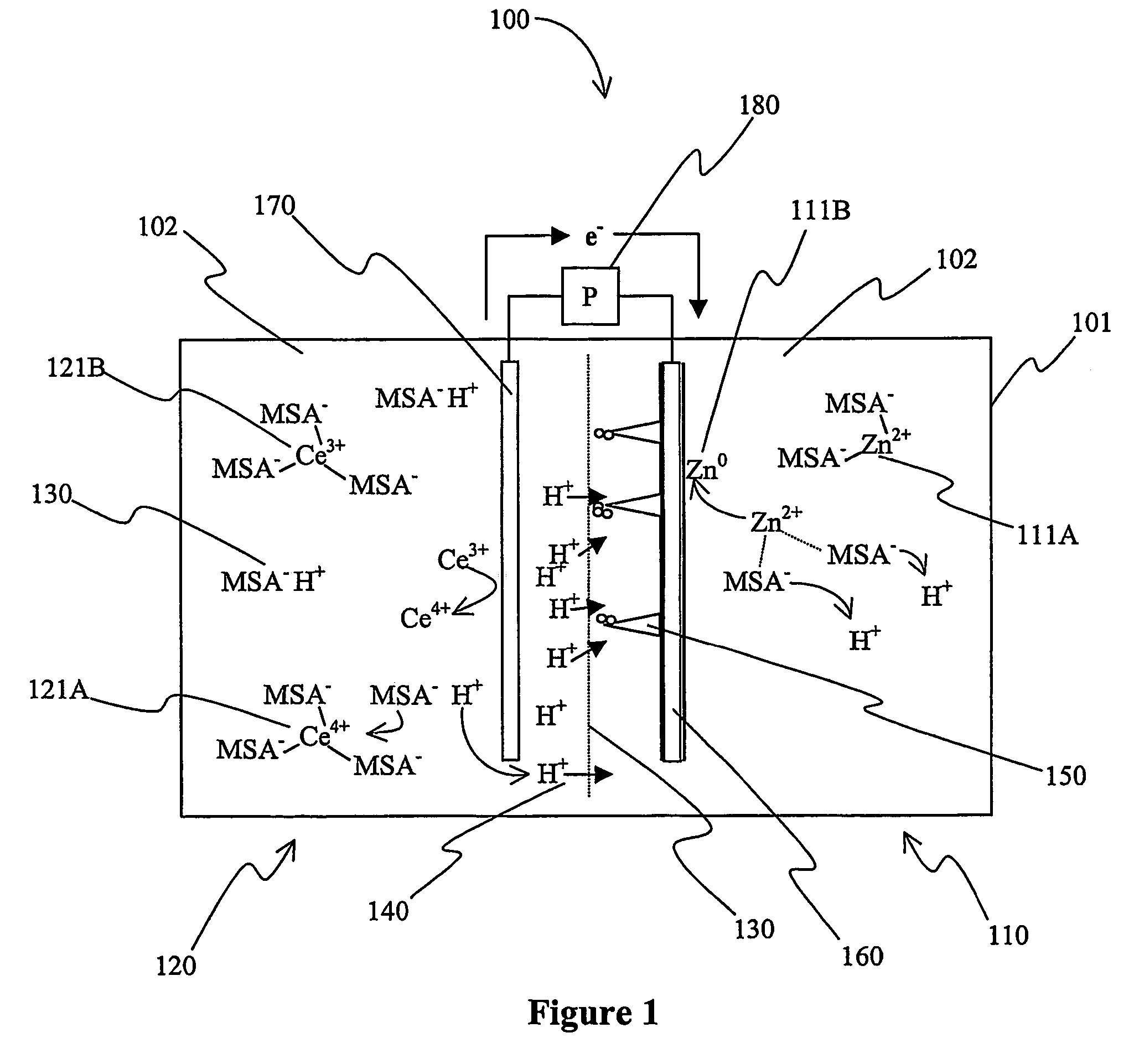
Mixed electrolyte battery
A battery (100B) comprises an electrolyte in which a first element forms a redox pair with a second element, wherein the battery (100B) is charged at a voltage sufficient to plate the first element at the anode (122B) and wherein the voltage is insufficient to plate the second element on the anode (122B). Preferred batteries include secondary batteries comprising a mixed electrolyte that includes a redox pair formed by a first element and a second element.
Owner:ITI SCOTLAND
High rate, long cycle life battery electrode materials with an open framework structure
InactiveUS8951673B2Extreme durabilityImprove rate performanceFinal product manufactureActive material electrodesHigh rateReference rate
A battery includes a cathode, an anode, and an aqueous electrolyte disposed between the cathode and the anode and including a cation A. At least one of the cathode and the anode includes an electrode material having an open framework crystal structure into which the cation A is reversibly inserted during operation of the battery. The battery has a reference specific capacity when cycled at a reference rate, and at least 75% of the reference specific capacity is retained when the battery is cycled at 10 times the reference rate.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Electrolyte for redox flow battery, and redox flow battery
InactiveUS7258947B2Develop battery performanceLower performance requirementsCell electrodesFinal product manufactureRedoxChemistry
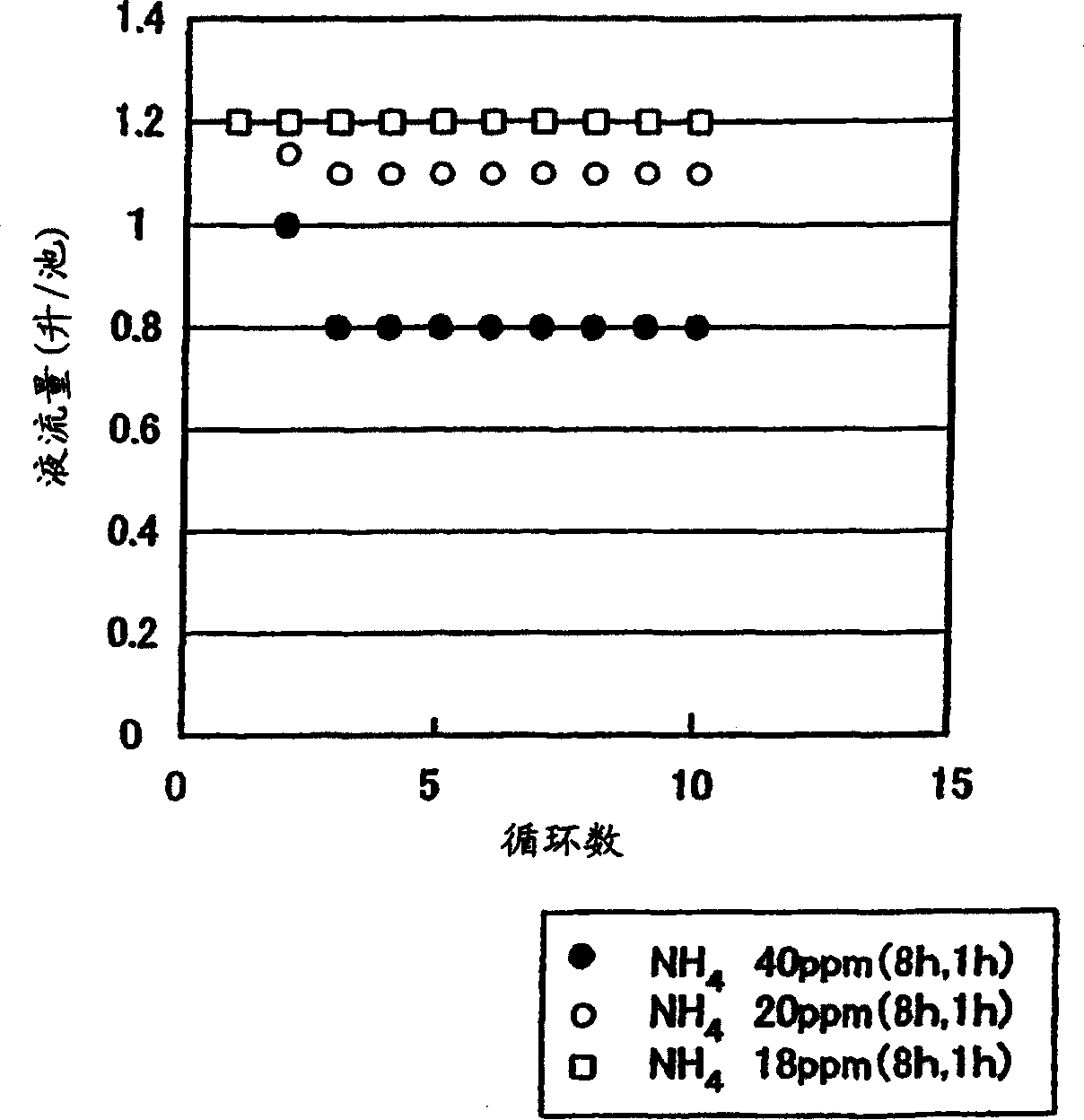
The present invention provides electrolyte that can suppress reduction of battery efficiencies and capacities with increased cycles of charge / discharge of the battery, a method for producing the same, and a redox flow battery using the same electrolyte. The redox flow battery uses the electrolyte having a NH4 content of not more than 20 ppm and a relation of Si concentration (ppm)×electrolyte quantity (m3) / electrode area (m2) of less than 5 ppm·m3 / m2. By limiting a quantity of contaminants in the electrolyte, a clogging of carbon electrodes to cause reduction of the battery performances with increased charge / discharge operations can be suppressed.
Owner:SUMITOMO ELECTRIC IND LTD +1
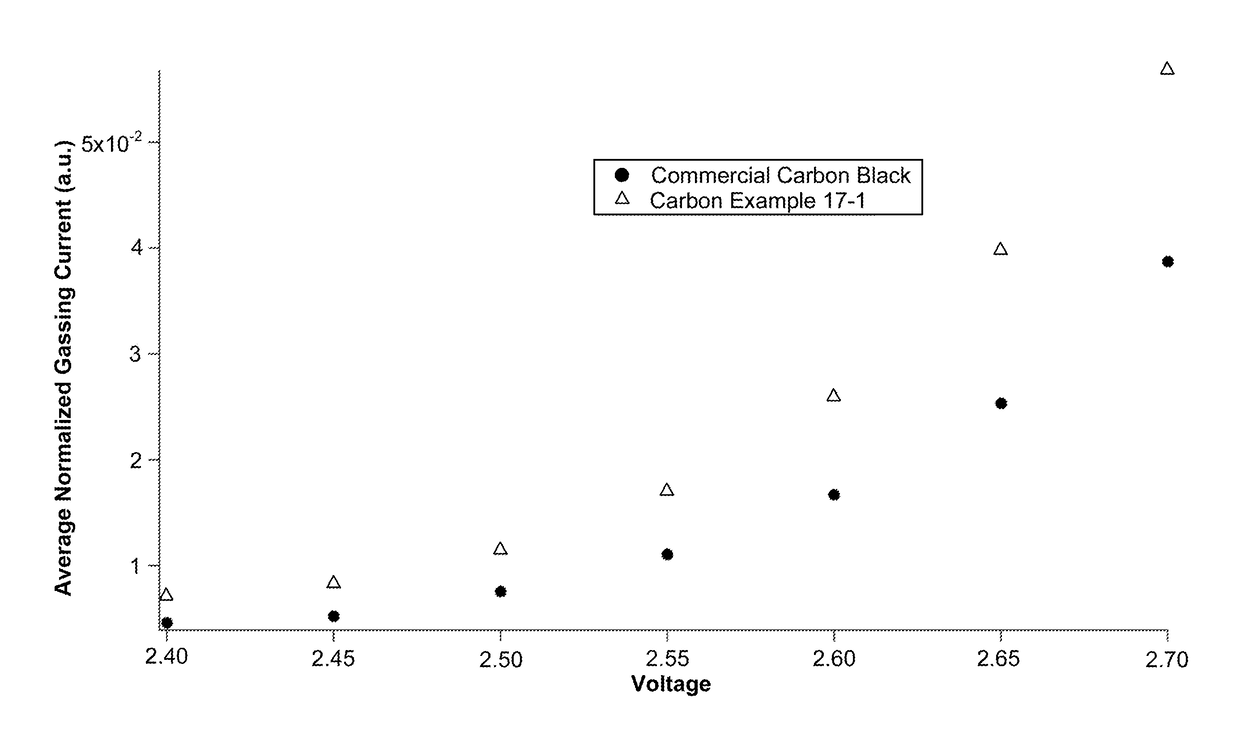
Low-gassing carbon materials for improving performance of lead acid batteries
InactiveUS20180294484A1Lead-acid accumulatorsFinal product manufactureMaterials scienceLead–acid battery
Carbon materials having low gassing properties and electrodes and electrical energy storage devices, especially lead-acid batteries, comprising the same are provided.
Owner:BASF AG
Bipolar battery assembly
ActiveUS20150140376A1Efficient assemblyLarge-sized flat cells/batteriesFinal product manufactureTransverse planeFluid electrolytes
The invention relates to an article comprising; a) one or more stacks of battery plates comprising one or more bipolar plates; b) located between each plate is a separator and a liquid electrolyte; further comprising one of more of the features: 1) c) the one or more stacks of battery plates having a plurality of channels passing transversely though the portion of the plates having the cathode and / or the anode deposited thereon; and d) i) one or more seals about the periphery of the channels which prevent the leakage of the liquid electrolyte into the channels, and / or posts located in one or more of the channels having on each end an overlapping portion that covers the channel and sealing surface on the outside of the monopolar plates adjacent to the holes for the transverse channels and applies pressure on the sealing surface of the monopolar plates.
Owner:ADVANCED BATTERY CONCEPTS
Redox-flow cell electrolyte and redox-flow cell
InactiveCN1515045AAvoid cloggingReduce performanceFinal product manufactureFuel cell auxillariesRedoxImpurity
The present invention provides electrolyte that can suppress reduction of battery efficiencies and capacities with increased cycles of charge / discharge of the battery, a method for producing the same, and a redox flow battery using the same electrolyte. The redox flow battery uses the electrolyte having a NH4 content of not more than 20 ppm and a relation of Si concentration (ppm)xelectrolyte quantity (m<3>) / electrode area (m<2>) of less than 5 ppm.m<3> / m<2>. By limiting a quantity of contaminants in the electrolyte, a clogging of carbon electrodes to cause reduction of the battery performances with increased charge / discharge operations can be suppressed.
Owner:SUMITOMO ELECTRIC IND LTD +1
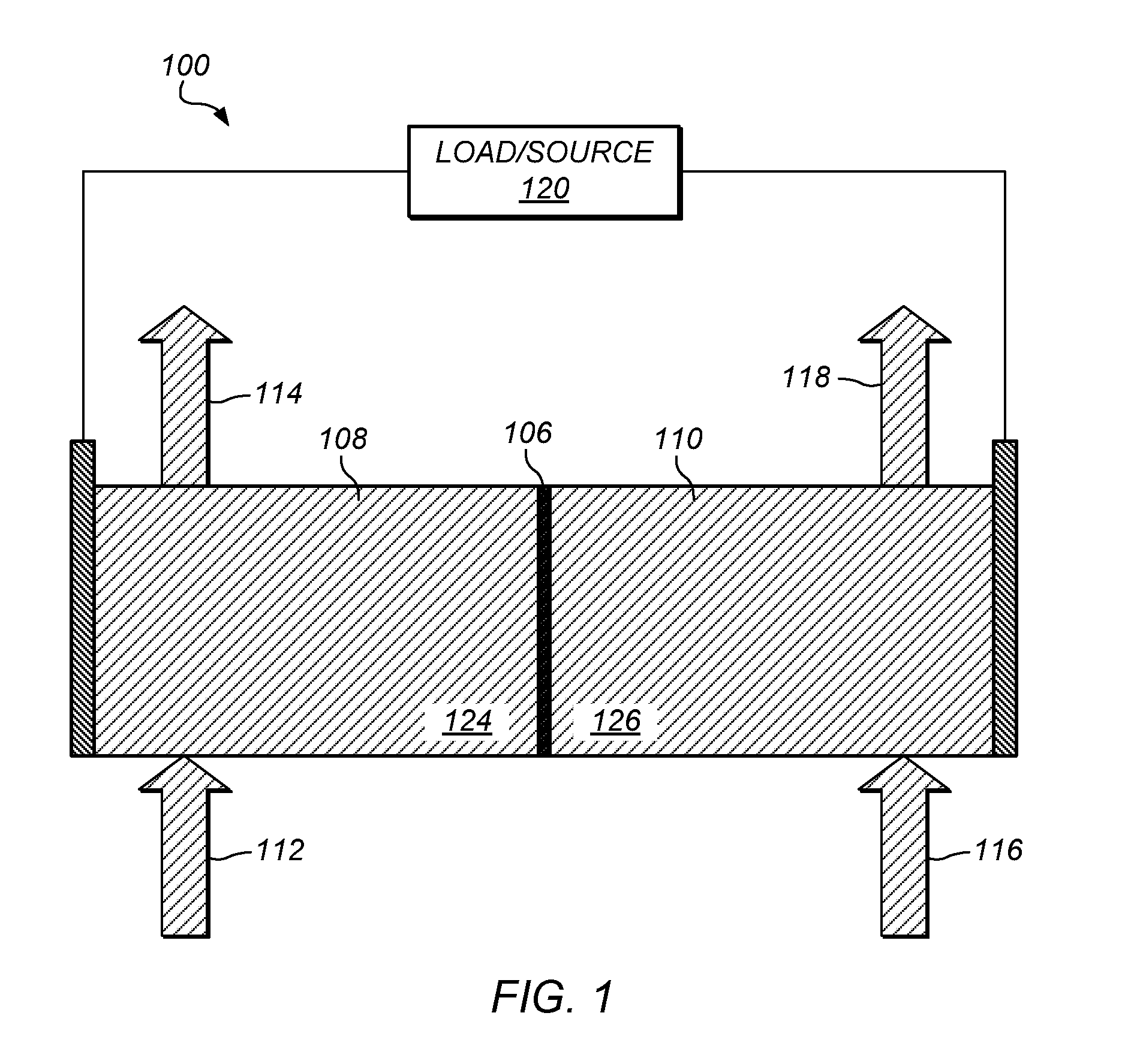
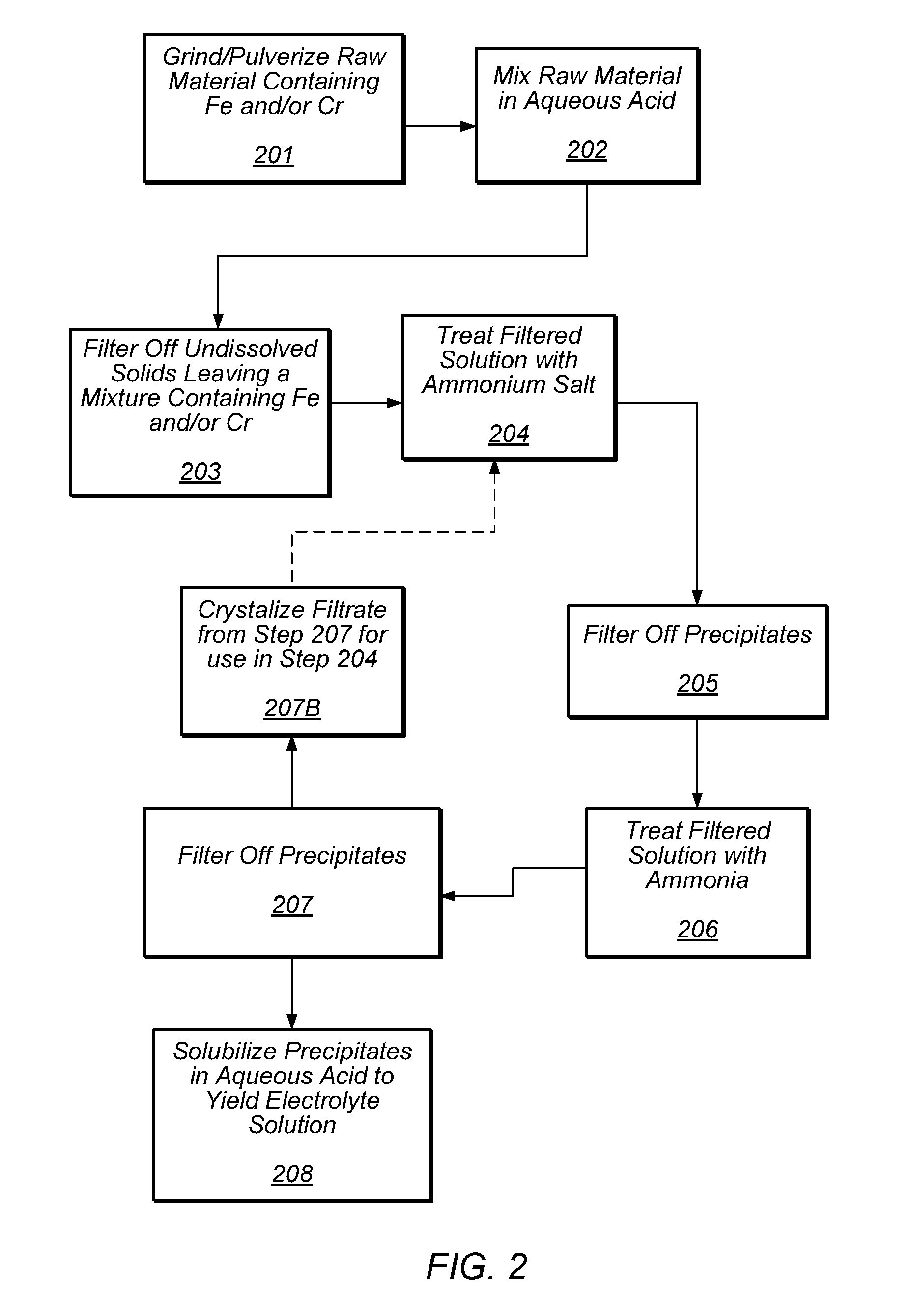
Preparation of flow cell battery electrolytes from raw materials
A method for preparing a redox flow battery electrolyte is provided. In some embodiments, the method includes the processing of raw materials that include sources of chromium ions and / or iron ions. The method further comprises the removal of impurities such as metal ions from those raw materials. In some embodiments, an ammonium salt may be used to remove metal impurities from an aqueous mixture of chromium ions and / or iron ions. Further provided is a redox flow battery comprising at least one electrolyte prepared from the above-identified methods.
Owner:IMERGY POWER SYST
Carbon materials for improving performance of lead acid batteries
InactiveUS20200144619A1Improve charge acceptanceImproved profileLead-acid accumulator electrodesAcid electrolytesLead oxideLead sulfate
A composition comprising a lead species (e.g., leady oxide, porous metallic lead, metallic lead, lead sulfate) a carbon material and an expander are described herein. Also disclosed are electrodes, devices (e.g., batteries) including the same. Methods for making and using the disclosed novel composition are also detailed herein.
Owner:BASF AG
Rechargeable zinc ion battery
ActiveUS20120034515A1Stable environmentNonoxidizableAlkaline accumulatorsCell electrodesZinc ionAqueous electrolyte
The present invitation discloses a rechargeable zinc ion battery, in which anodic zinc will be electrochemically dissolved as Zn2+ions, diffuses to the cathodic electrode / electrolyte interface through the electrolyte, and zinc ions are subsequently intercalated into manganese dioxide during discharging. In charging, above-mentioned process will be reverse. The rechargeable zinc ion battery comprises a cathode formed from a compressed mixture of alpha manganese dioxide particles, electrically conductive particles and a binder; a zinc anode separated from cathode; an aqueous electrolyte contains zinc ions in which the pH value may be controlled between 4 and 7.
Owner:SHENZHEN CITY THROUGH SCI & TECH OF NEW ENERGY CO LTD
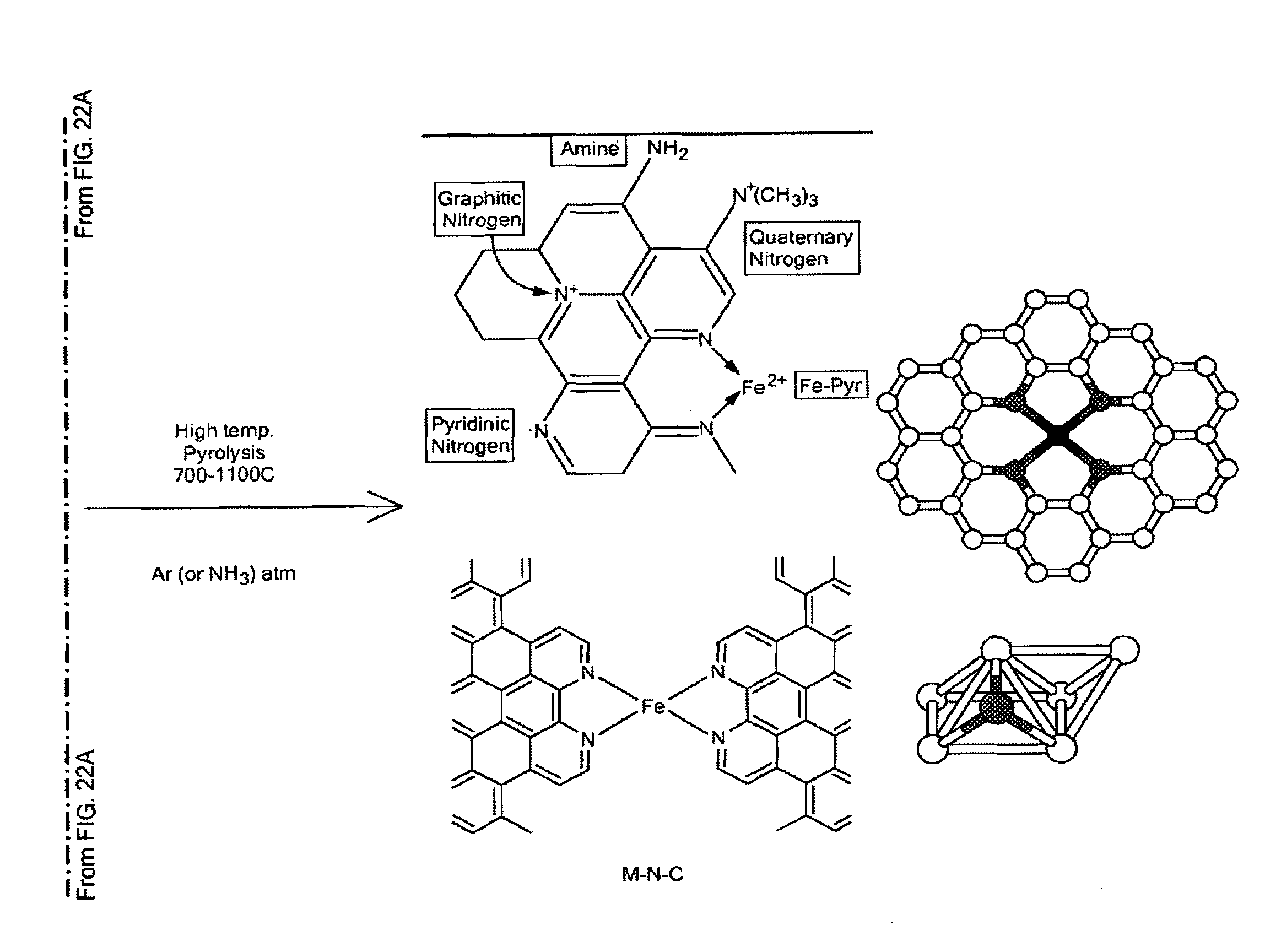
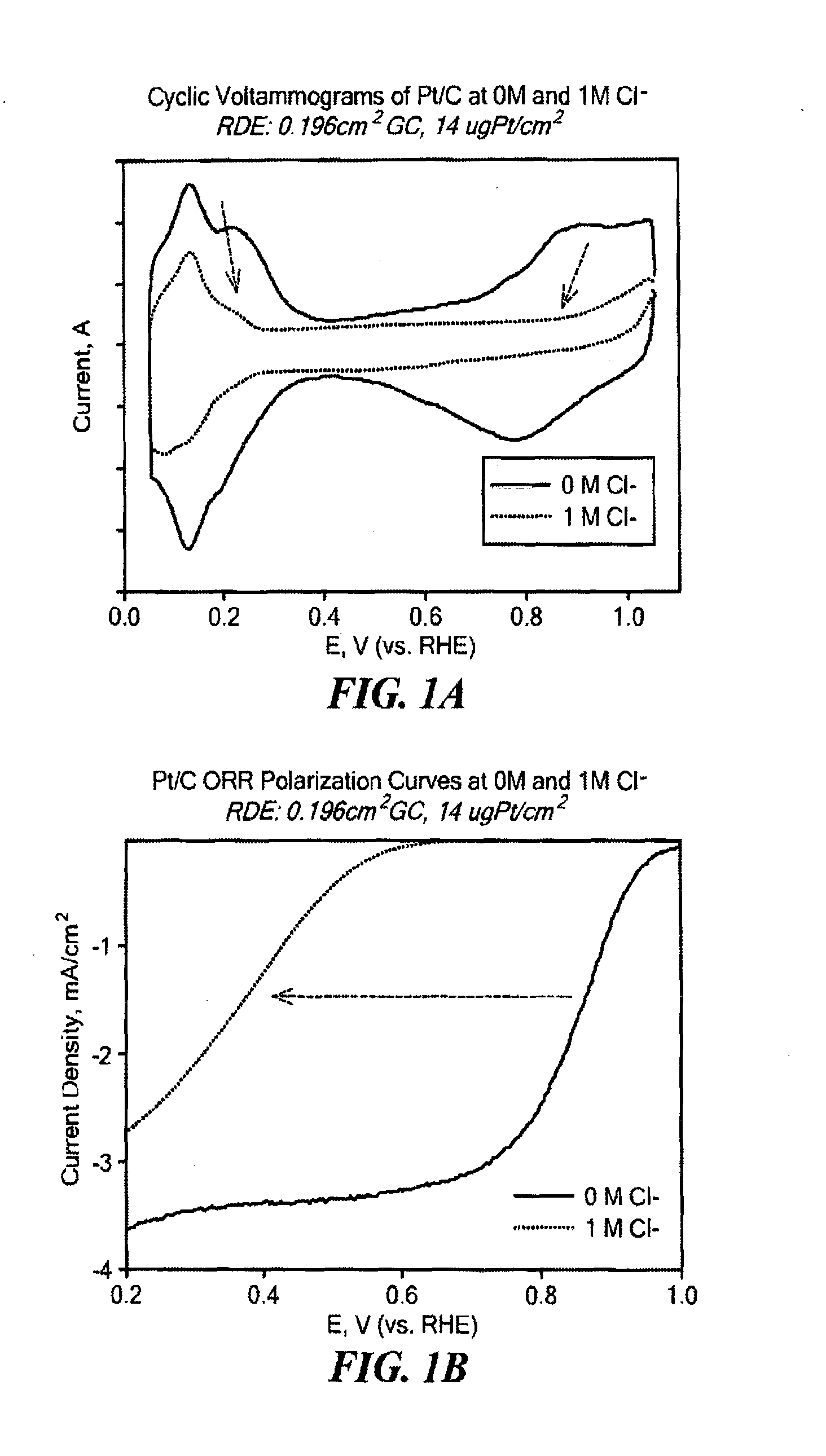
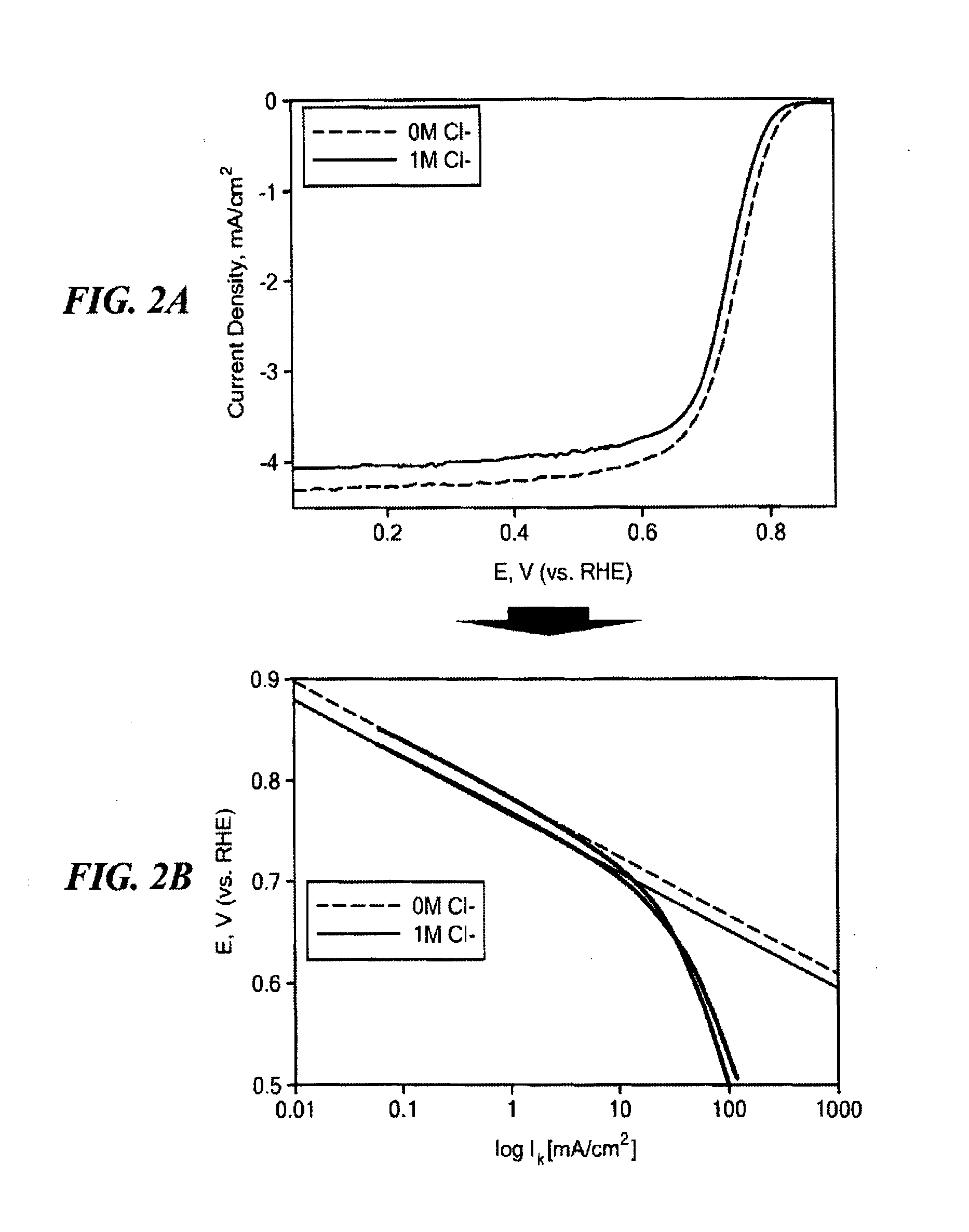
Non-Noble Metal Electrocatalysts for Oxygen Depolarized Cathodes and Their Uses
ActiveUS20150340705A1Machining electrodesPhotography auxillary processesMetal-organic frameworkPhosphoric-acid fuel cell
Highly anion resistant electrocatalysts suitable for catalyzing an oxygen reduction reaction (ORR) and methods of synthesizing the same are provided. The catalysts contain a transition metal, a heteroatom, and carbon. Preferred catalysts include N as the heteroatom and Fe as the transition metal, with active sites having Fe—N4 stoichiometry (FexNyCz) as part of a metal organic framework (MOF) or sequestered within a MOF. Electrocatalysts further including Fe nanoparticles (FeNPs) are also provided. The catalysts described herein are applicable in the preparation of oxygen decoupled cathodes (ODC) for chlorine evolution processes such as in chlor-alkali cells or HCl electrolyzers. The catalysts are also useful in preparing ODC for use in fuel cells, including phosphoric acid fuel cells.
Owner:NORTHEASTERN UNIV
High purity electrolytic sulfonic acid solutions
Disclosed is a solution for an electrochemical process, the solution containing a sulfonic acid and having a low concentration of sulfur compounds, either low or high valence, that are susceptible to reduction and which is intended for use in electrodeposition, batteries, conductive polymers and descaling processes.
Owner:ARKEMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com