Redox-flow cell electrolyte and redox-flow cell
An electrolyte and flow-type technology, which is applied in the field of redox flow batteries, can solve the problems of not fully considering the relationship between the absolute amount of impurities in the battery, the decline in battery efficiency, and insufficient removal of NH4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
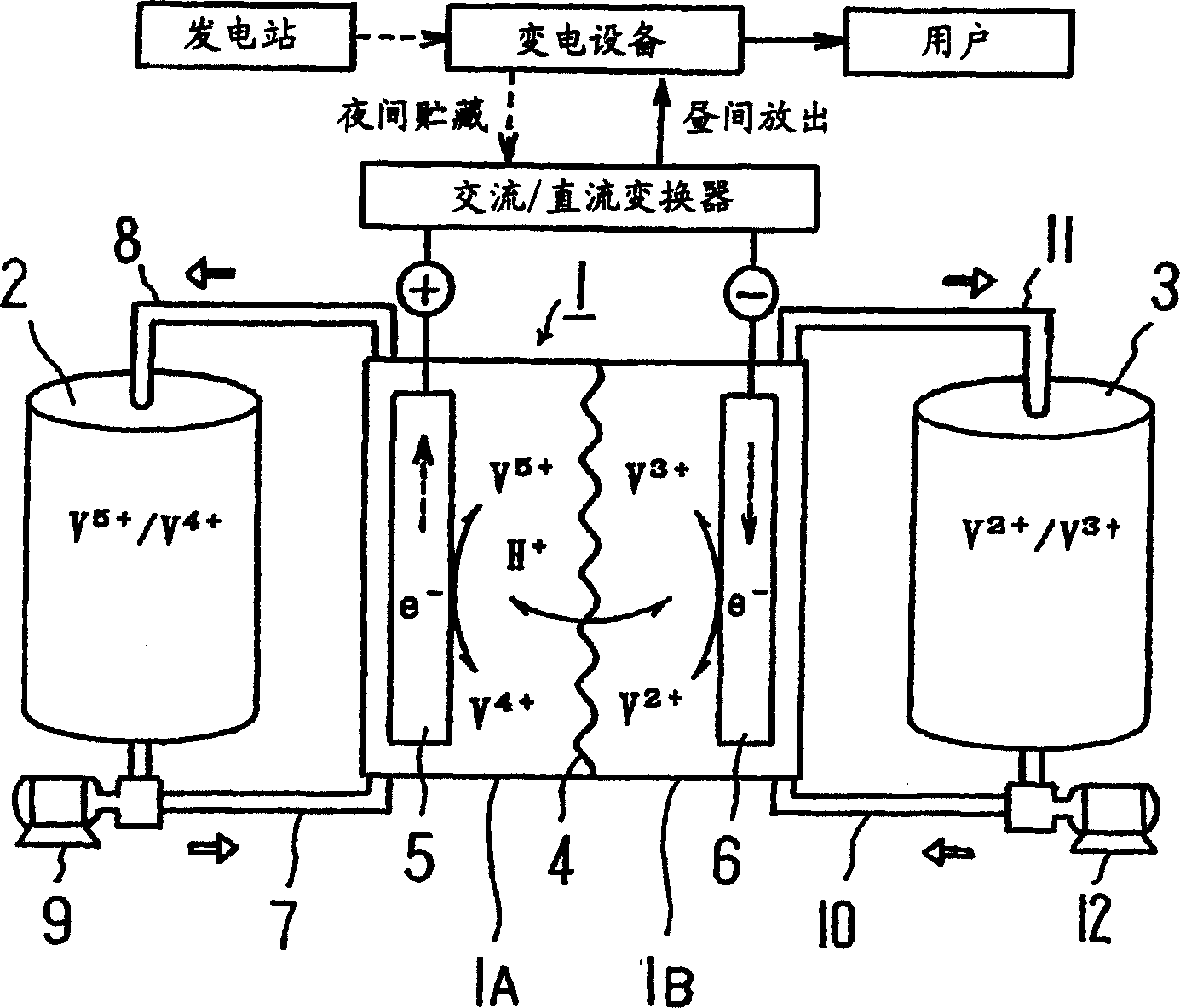
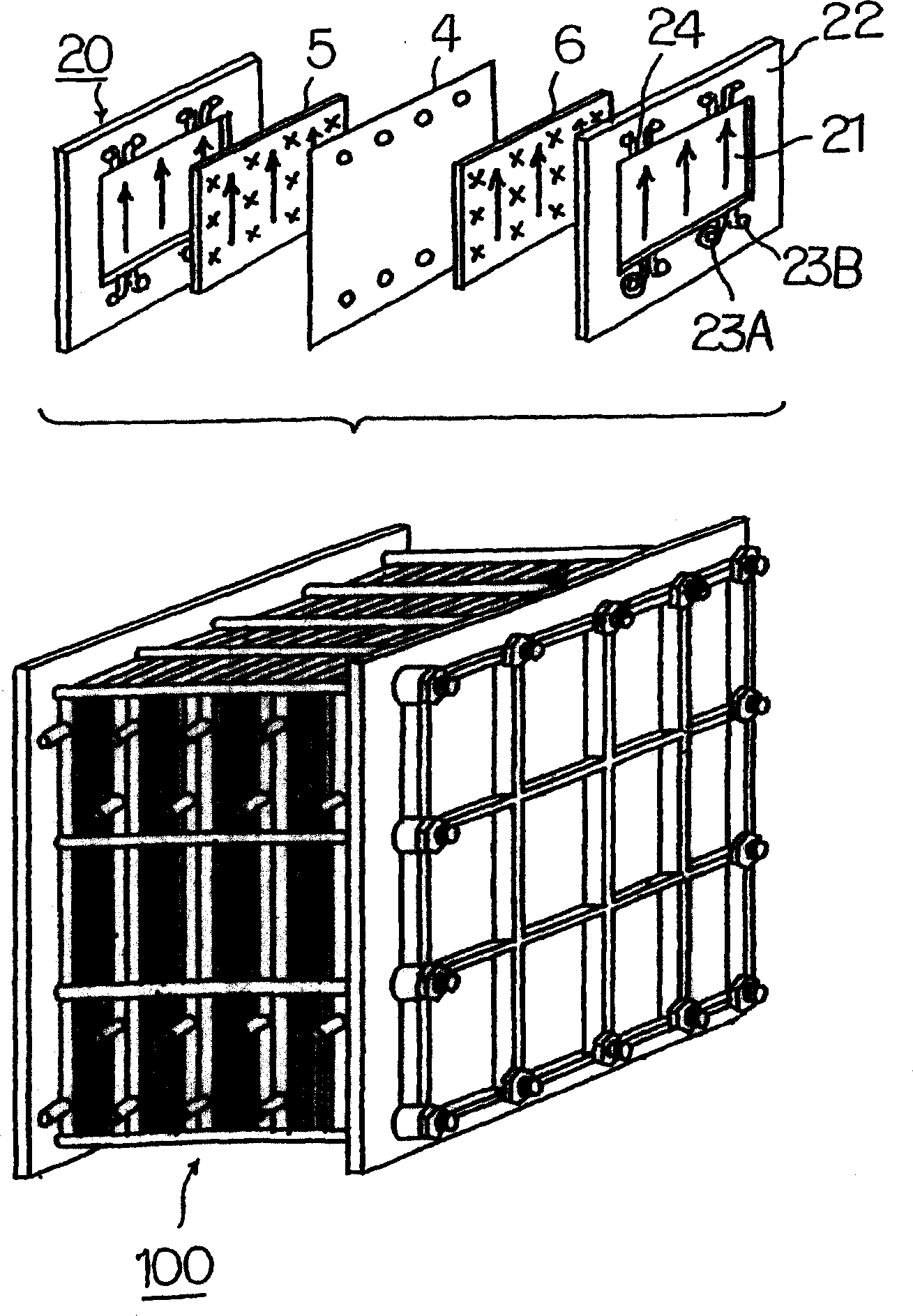
[0034] Use a 50kW-class battery pack to produce a capacity of 1 hour (electrolyte volume: plus and minus 1.5m each) 3 ) and 8-hour capacity (electrolyte volume: positive and negative 12m 3 )system. First, follow the figure 1 Explain how a redox flow battery works, then, follow figure 2 Describes the structure of the pool group.
[0035] This battery has a cell 1 partitioned into a positive electrode cell 1A and a negative electrode cell 1B using a separator 4 composed of an ion exchange membrane. A positive electrode 5 and a negative electrode 6 are placed in each positive electrode cell 1A and negative electrode cell 1B, respectively. In the positive electrode cell 1A, the positive electrode tank 2 for supplying and discharging the positive electrode electrolytic solution is connected through conduits 7 and 8 . In the negative electrode cell 1B, the negative electrode tank 3 that introduces and discharges the negative electrode electrolyte solution is connected through ...
Embodiment 2
[0044] A 50kW class battery pack was used to make a 1-hour capacity system, and the following three electrolytes were used for the test. Electrolyte volume, each positive and negative 1.5m 3 . Impurity amount, NH 4 is 18ppm. Samples with Si concentrations of 100, 50, and 40 ppm were used. For the adjustment of the impurity concentration, a polypropylene pleated filter manufactured by American Philta Co., Ltd. was used.
[0045] The main components of the electrolyte used here are: vanadium ion concentration 2.0 mol / liter, free sulfuric acid concentration 2.0 mol / liter, added phosphoric acid concentration 0.14 mol / liter. Cell resistance at various Si concentrations (Ω·cm 2 ) changes are shown in Figure 4 . The charging and discharging conditions are the same as in Example 1.
[0046] As a result, it was confirmed that when the Si concentration was greater than 40 ppm, the cell resistance increased, the capacity decreased, and the function as a battery was lost before 1...
Embodiment 3
[0048] 50kW level pool group is used to make an 8-hour capacity (plus and minus 12m 3 ) system to investigate the relationship between the number of operating cycles and cell resistance. The main components of the electrolyte used here are: vanadium ion concentration 1.7 mol / liter, free sulfuric acid concentration 2.6 mol / liter, added phosphoric acid concentration 0.12 mol / liter. Additionally, making NH 4 Samples with a concentration of 20ppm and a Si concentration of 4, 10, and 40ppm. Cell resistance at various Si concentrations (Ω·cm 2 ) changes are shown in Figure 5 . The charging and discharging conditions are the same as in Example 1.
[0049] From shown in Figure 5 The results show that the cell resistance increases for samples with a Si concentration of 40ppm, but hardly changes for samples below 10ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com