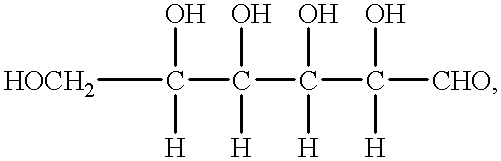
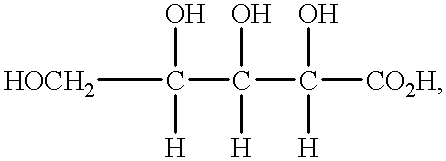
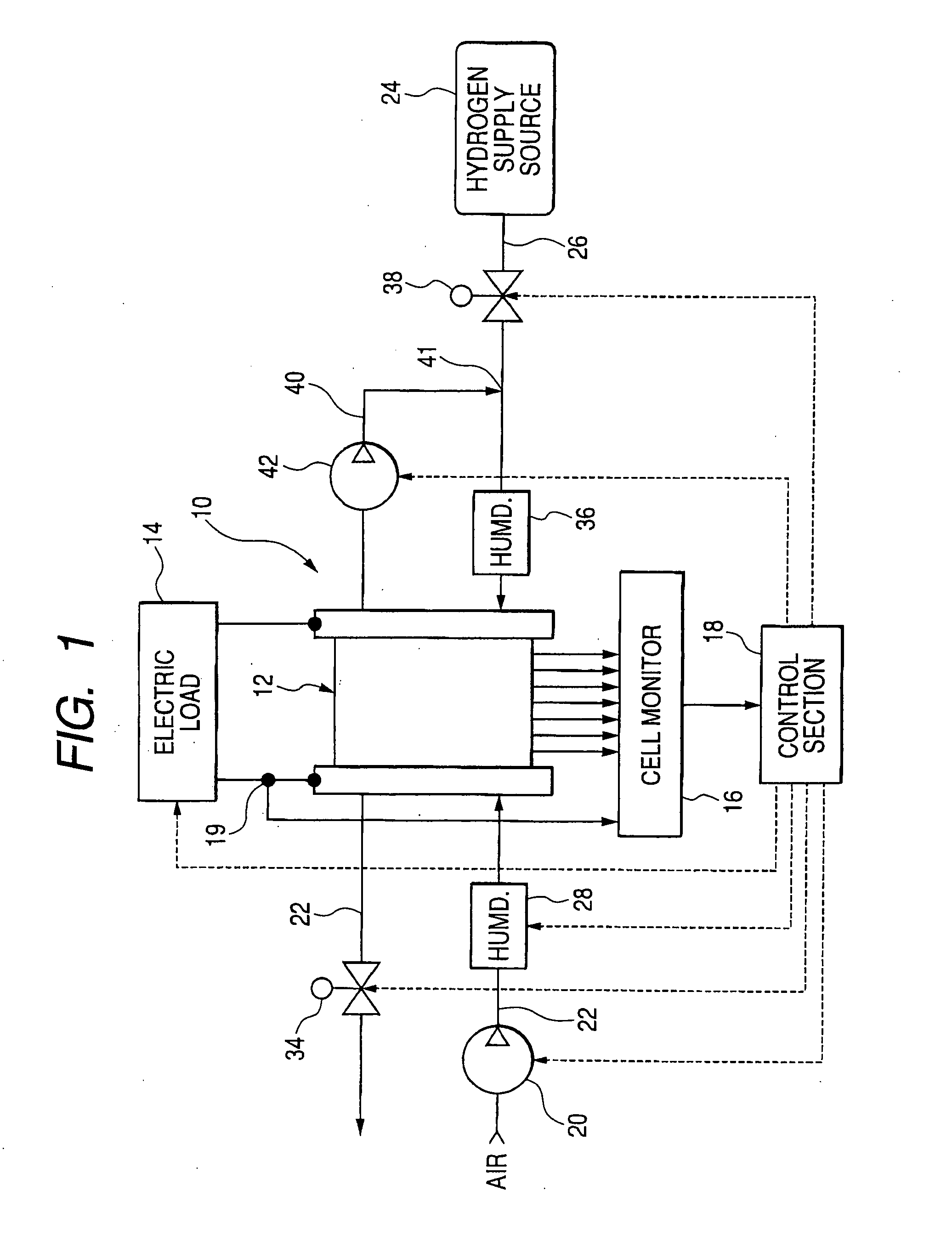
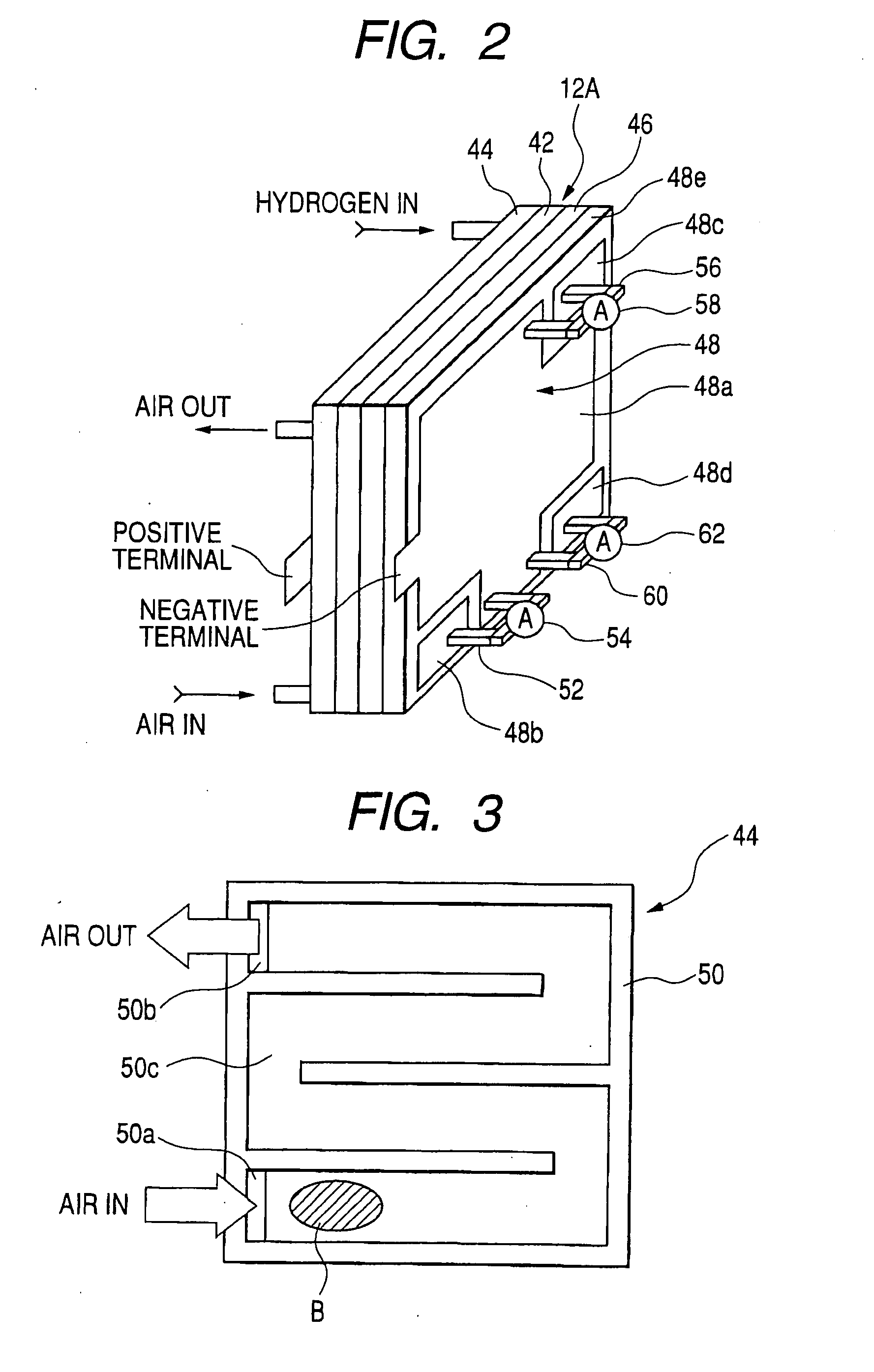
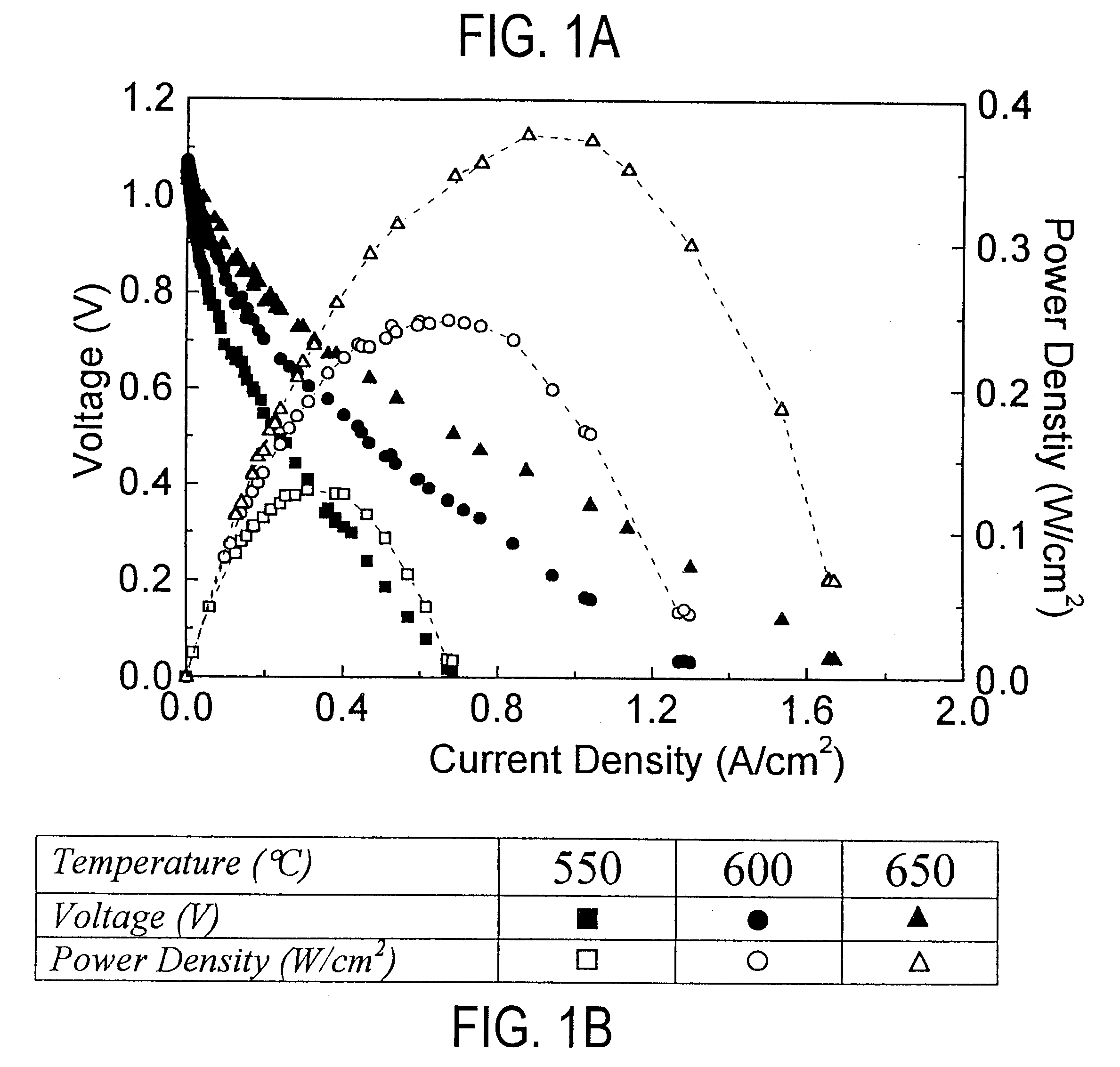
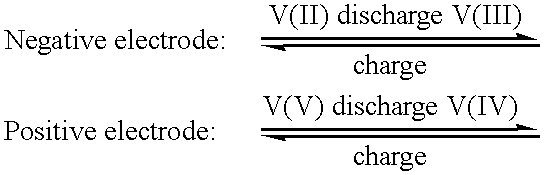Patents
Literature
4044results about "Regenerative fuel cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
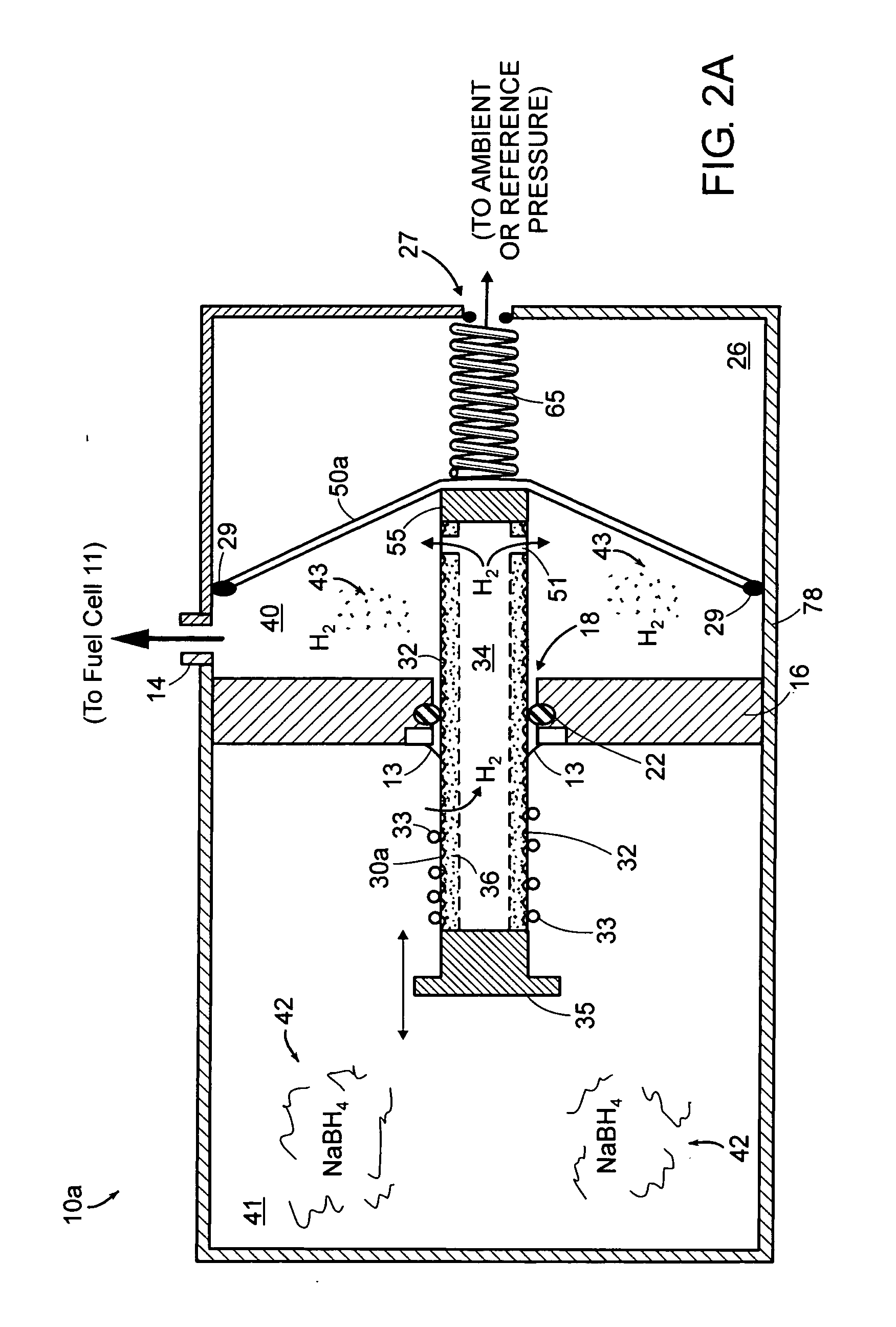
Method and system for supplying hydrogen for use in fuel cells
InactiveUS6348278B1Reduce hydrogen concentrationImprove concentrationElectricity cogenerationRegenerative fuel cellsHydrogenCombustor
The present invention provides a method and system for efficiently producing hydrogen that can be supplied to a fuel cell. The method and system of the present invention produces hydrogen in a reforming reactor using a hydrocarbon stream and water vapor stream as reactants. The hydrogen produced is purified in a hydrogen separating membrane to form a retentate stream and purified hydrogen stream. The purified hydrogen can then be fed to a fuel cell where electrical energy is produced and a fuel cell exhaust stream containing water vapor and oxygen depleted air is emitted. In one embodiment of the present invention, a means and method is provided for recycling a portion of the retentate stream to the reforming reactor for increased hydrogen yields. In another embodiment, a combustor is provided for combusting a second portion of the retentate stream to provide heat to the reforming reaction or other reactants. In a preferred embodiment, the combustion is carried out in the presence of at least a portion of the oxygen depleted air stream from the fuel cell. Thus, the system and method of the present invention advantageously uses products generated from the system to enhance the overall efficiency of the system.
Owner:MOBIL OIL CORP
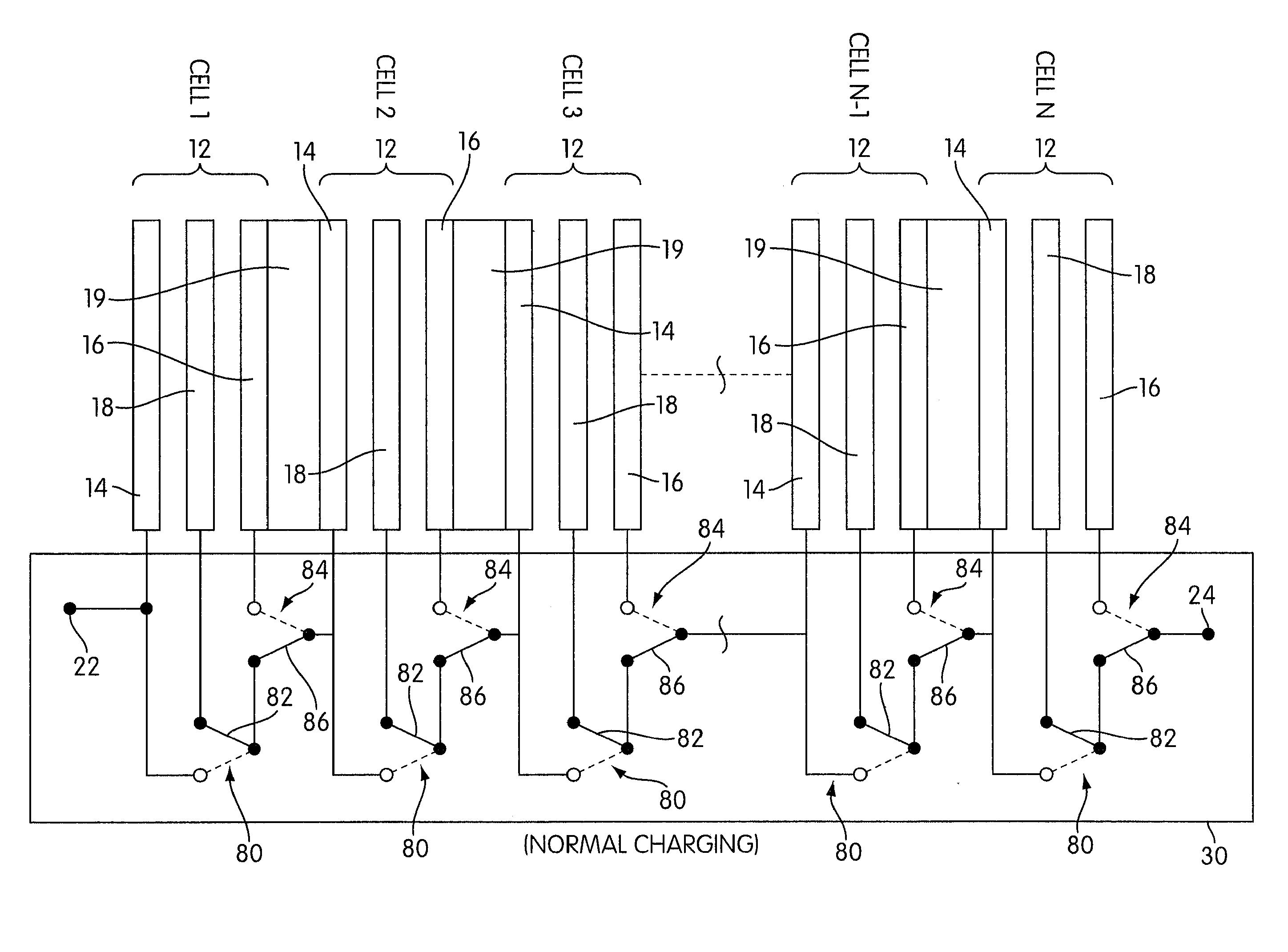
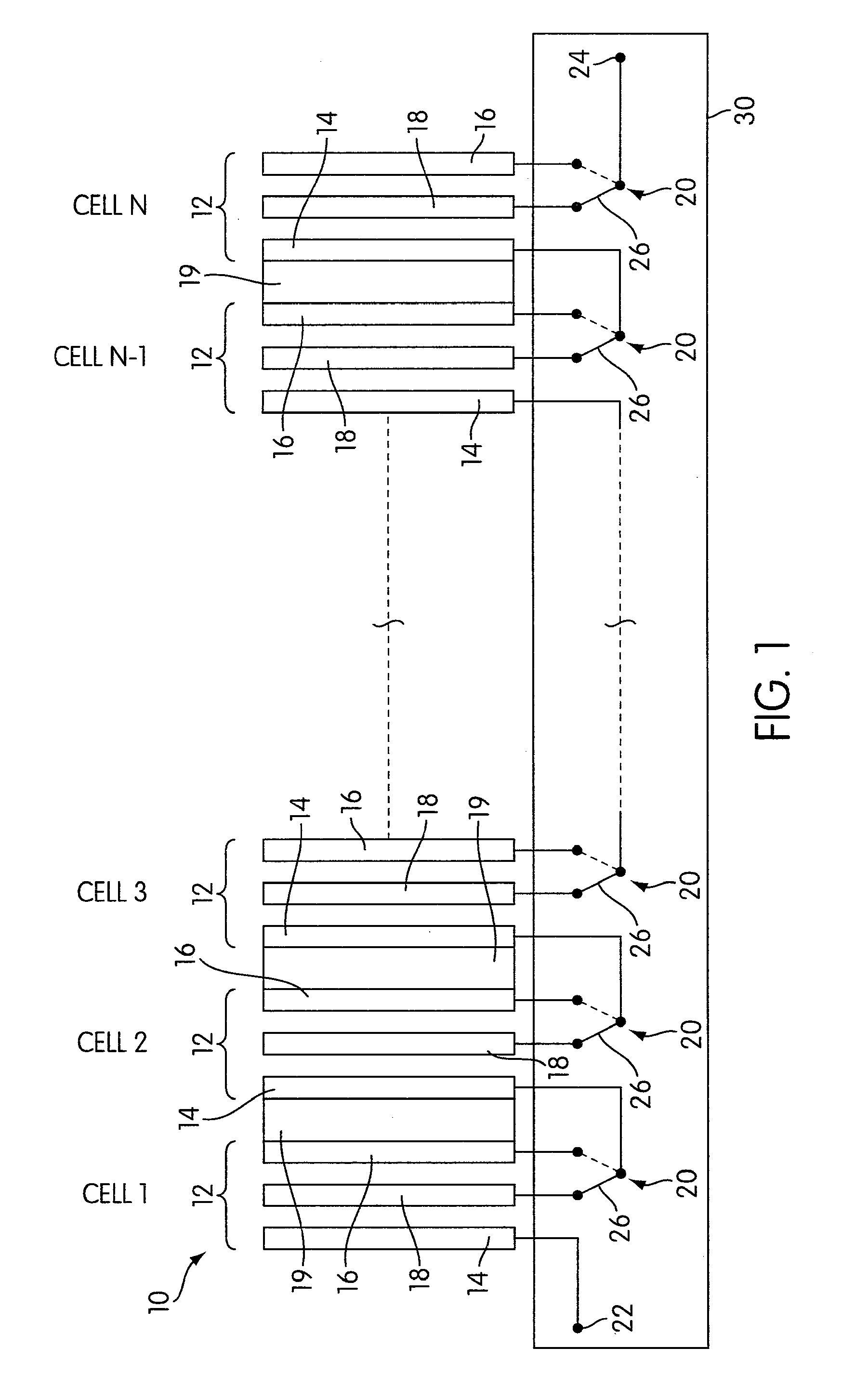
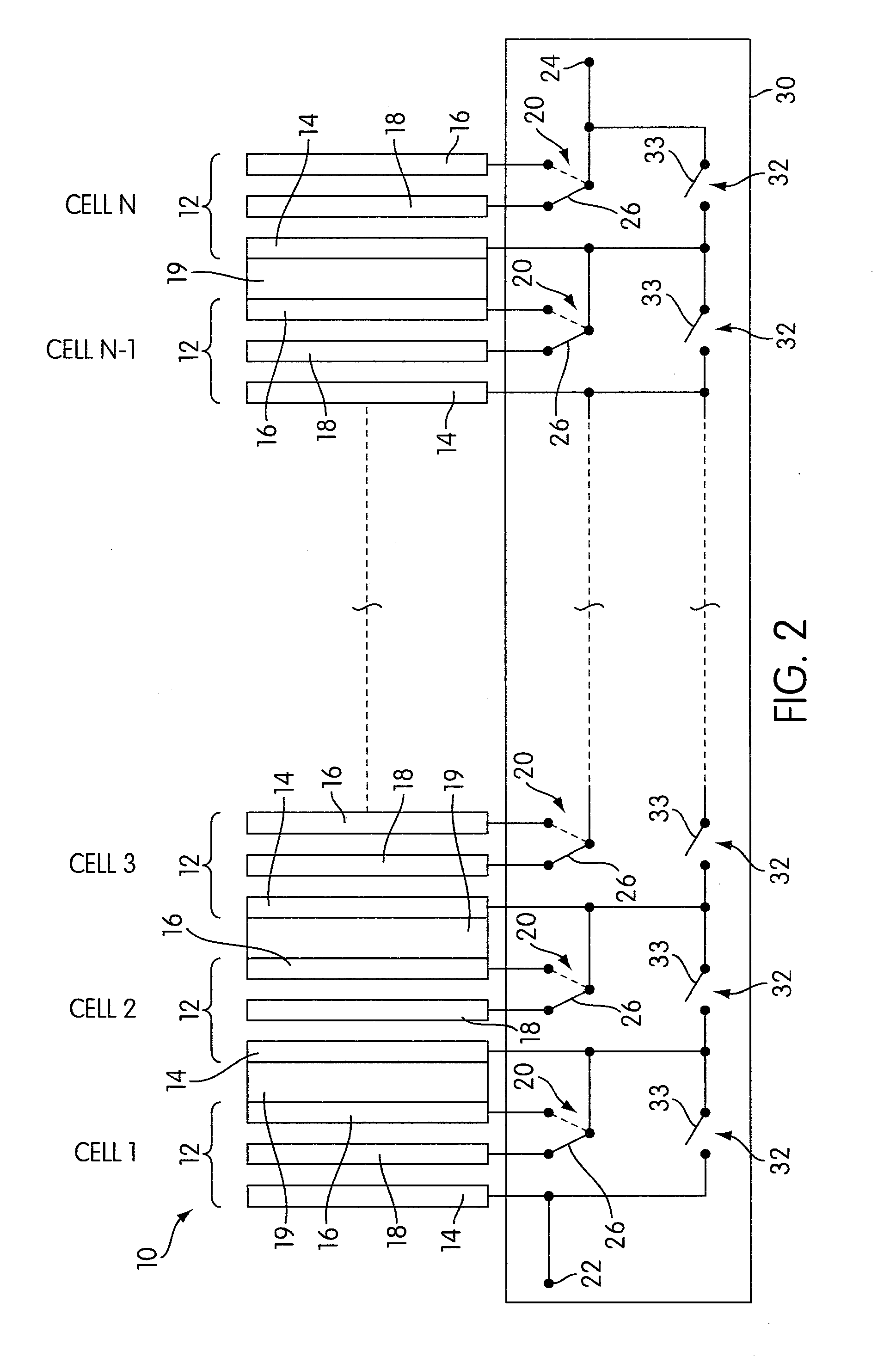
Rechargeable electrochemical cell system with a charging electrode charge/discharge mode switching in the cells
One aspect of the present invention provides a rechargeable electrochemical cell system for generating electrical current using a fuel and an oxidant. The cell system comprises N electrochemical cells each comprising a fuel electrode, an oxidant electrode, a charging electrode, and an ionically conductive medium communicating the electrodes, wherein N is an integer greater than or equal to two. Any number of cells may be used. The cell system includes a plurality of switches that are switcheable to a discharge mode coupling the oxidant electrode of each cell to the fuel electrode of the subsequent cell, a charge mode coupling the charging electrode of each cell to the fuel electrode of the subsequent cell, and a bypass mode coupling charging electrode or the oxidant electrode of a previous cell to the fuel electrode of a subsequent cell.
Owner:FLUIDIC
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
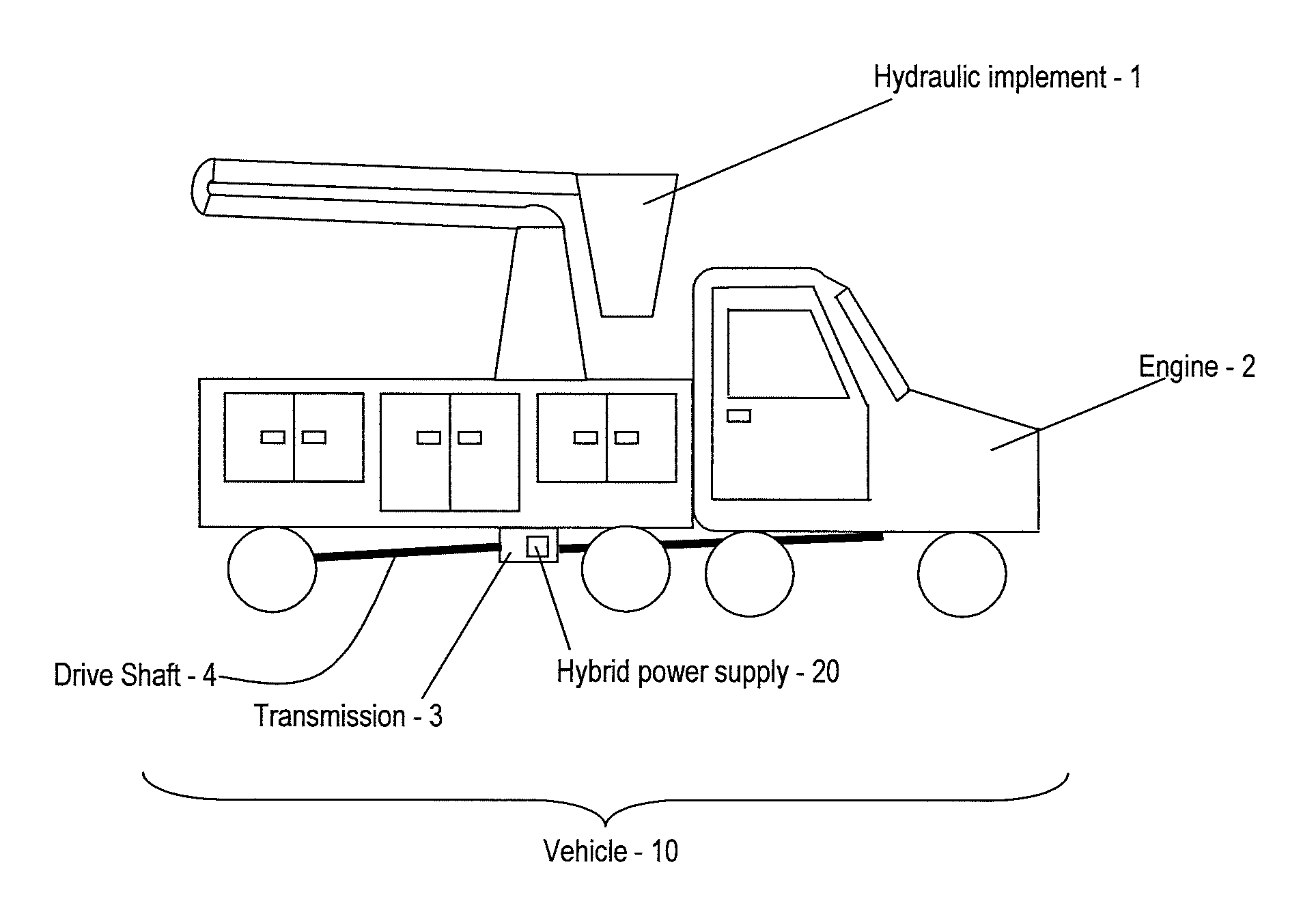
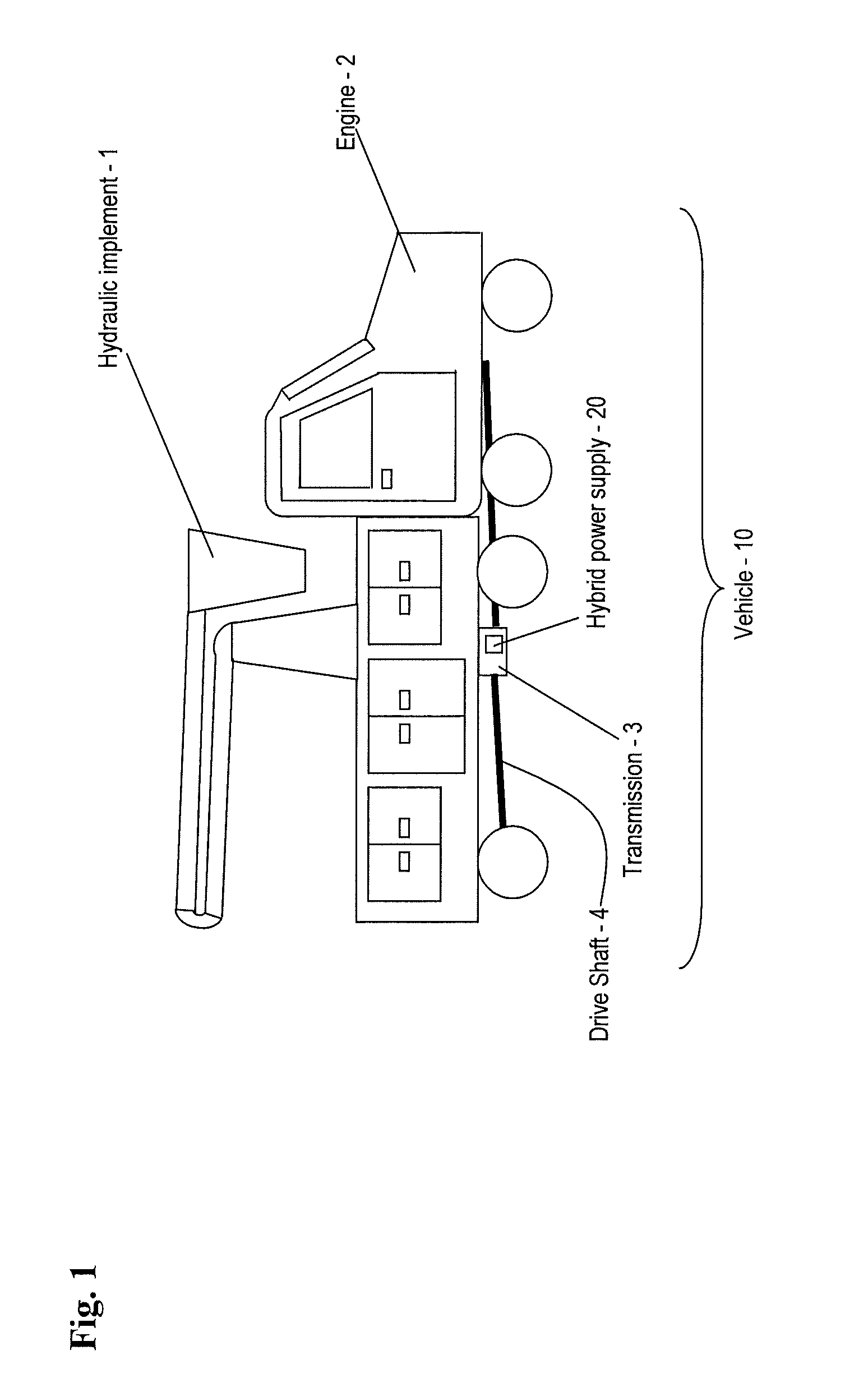
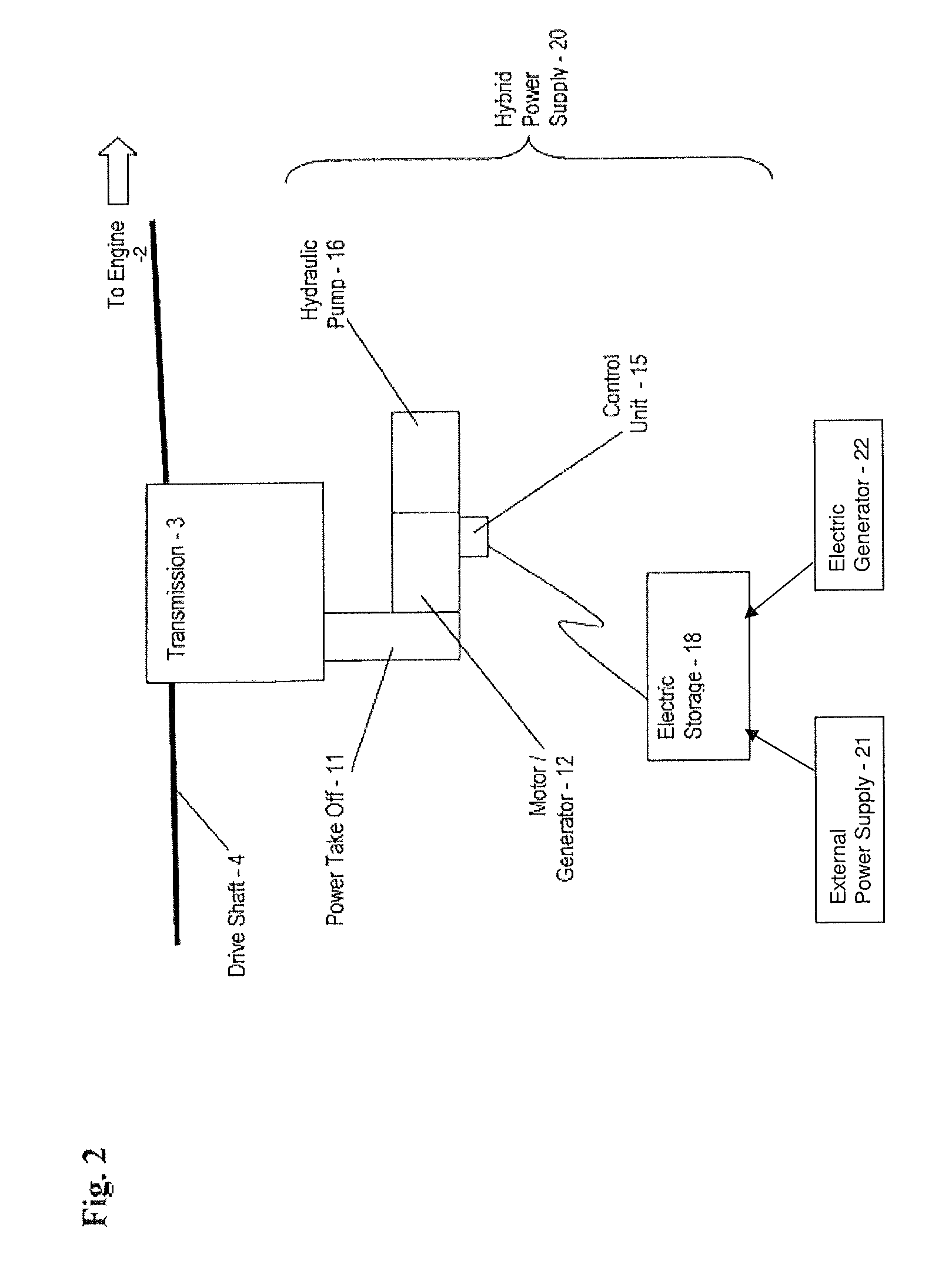
Hybrid drive for hydraulic power
A power supply for powering a hydraulic implement includes: an electric storage unit; an electric motor, an electric generator, a hydraulic pump and a control unit; the electric motor adapted for receiving electric power and driving the hydraulic pump to power the hydraulic implement; the electric generator adapted for translating mechanical energy into the electric power; the electric storage unit also being adapted for providing the electric power; and the control unit for selecting a source of the electric power from one of the generator and the electric storage unit. A method for operating the power supply and a vehicle are also provided.
Owner:TEREX SOUTH DAKOTA
Carbon electrode material for a vanadium-based redox-flow battery
The carbon electrode material of the present invention is used for a vanadium redox-flow cell. The carbon electrode material has quasi-graphite crystal structure in which <002> spacing obtained by X-ray wide angle analysis is 3.43 to 3.60 Å, size of a crystallite in c axial direction is 15 to 33 Å and size of crystallite in a axial direction is 30 to 70 Å. In addition, an amount of surface acidic functional groups obtained by XPS surface analysis is 0.1 to 1.2% and total number of surface bound-nitrogen atoms is 5% or smaller relative to total number of surface carbon atoms. The carbon electrode materials formed of a non-woven fabric of a carbonaceouss fiber is preferable.
Owner:TOYO TOYOBO CO LTD
Portable hydrogen generator-fuel cell apparatus
InactiveUS6653005B1Increase specific energy and overall energy efficiencyHydrogenFuel cell auxillariesKeroseneImpurity
A compact hydrogen generator is coupled to or integrated with a fuel cell for portable power applications. Hydrogen is produced via thermocatalytic decomposition (cracking, pyrolysis) of hydrocarbon fuels in oxidant-free environment. The apparatus can utilize a variety of hydrocarbon fuels, including natural gas, propane, gasoline, kerosene, diesel fuel, crude oil (including sulfurous fuels). The hydrogen-rich gas produced is free of carbon oxides or other reactive impurities, so it could be directly fed to any type of a fuel cell. The catalysts for hydrogen production in the apparatus are carbon-based or metal-based materials and doped, if necessary, with a sulfur-capturing agent. Additionally disclosed are two novel processes for the production of two types of carbon filaments, and a novel filamentous carbon product. The hydrogen generator can be conveniently integrated with high temperature fuel cells to produce an efficient and self-contained source of electrical power.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
Redox flow battery
InactiveUS6764789B1Reduce capacityReduce frequencyElectrolyte holding meansCell electrodesRedoxAqueous solution
The present invention provides a redox flow type battery which a liquid-circulating battery comprising a battery cell and storage tanks for positive and negative electrolytes, wherein the battery cell is separated by a membrane to provide a positive cell and a negative cell, each cell having a liquid-permeable porous electrode disposed therein, wherein the positive and negative electrolytes are sulfuric acid aqueous solutions with vanadium ion concentrations of 0.5 mol / l to 8 mol / l and the electrolyte which migrates through the membrane over cycles of charge and discharge is returned from the storage tank where the liquid increases to the storage tank where the liquid decreases in order to keep the change in the amounts of the positive and negative electrolytes in a certain range while charge and discharge are carried out.
Owner:SUMITOMO ELECTRIC IND LTD
Self-regulating gas generator and method
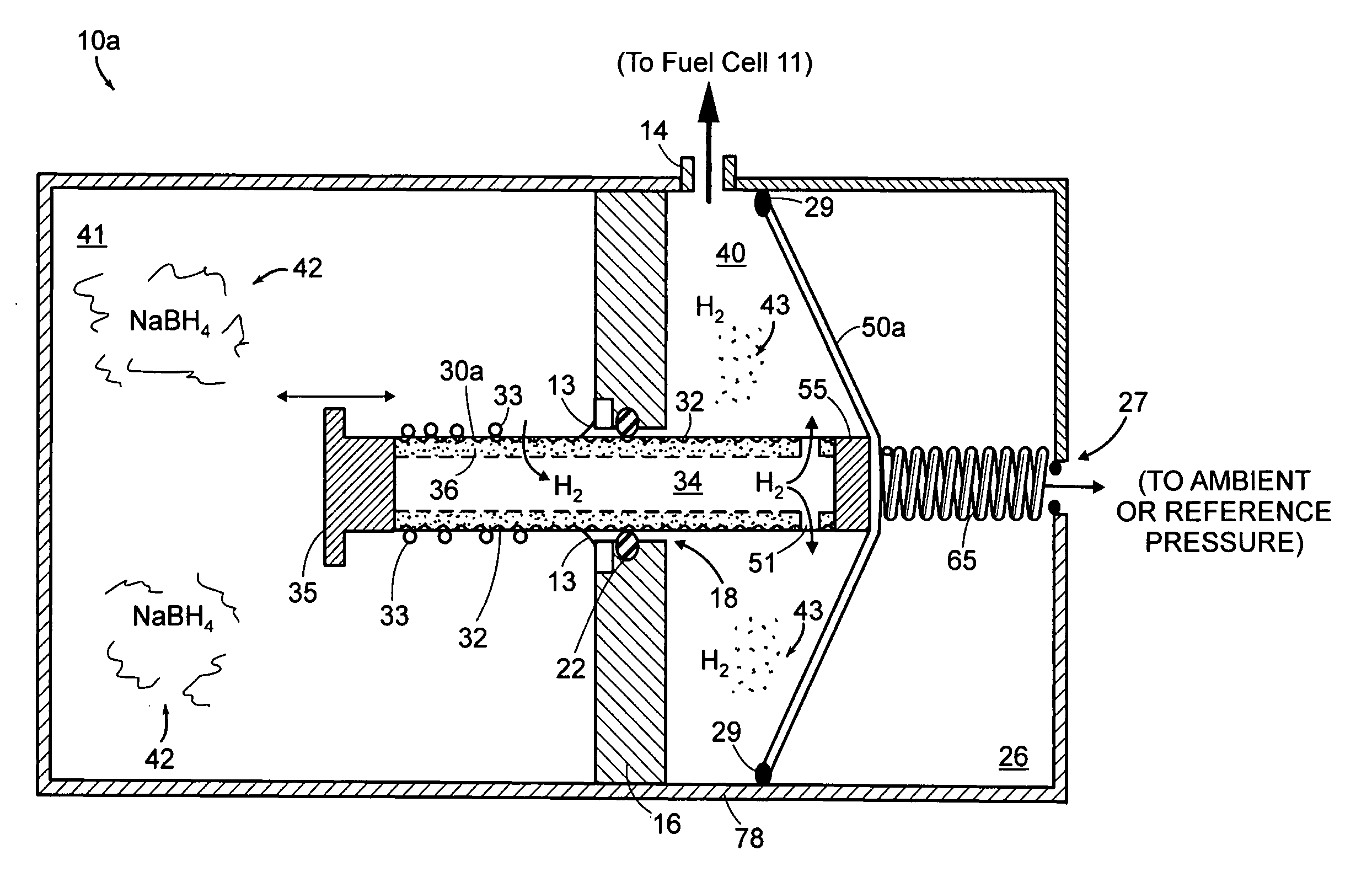
ActiveUS20050158595A1Increase and decreases gas production rateExtend your lifeReactant parameters controlMultiple metal hydridesHydrogenFuel cells
A self-regulating gas generator that, in response to gas demand, supplies and automatically adjusts the amount of gas (e.g., hydrogen or oxygen) catalytically generated in a chemical supply chamber from an appropriate chemical supply, such as a chemical solution, gas dissolved in liquid, or mixture. The gas generator may employ a piston, rotating rod, or other element(s) to expose the chemical supply to the catalyst in controlled amounts. The gas generator may be used to provide gas for various gas consuming devices, such as a fuel cell, torch, or oxygen respiratory devices.
Owner:ENCITE LLC
Part solid, part fluid and flow electrochemical cells including metal-air and li-air battery systems
PendingUS20130189592A1Provide integrityAvoid shortingPrimary cell to battery groupingFuel and primary cellsLithium–air batteryEngineering
Owner:CALIFORNIA INST OF TECH
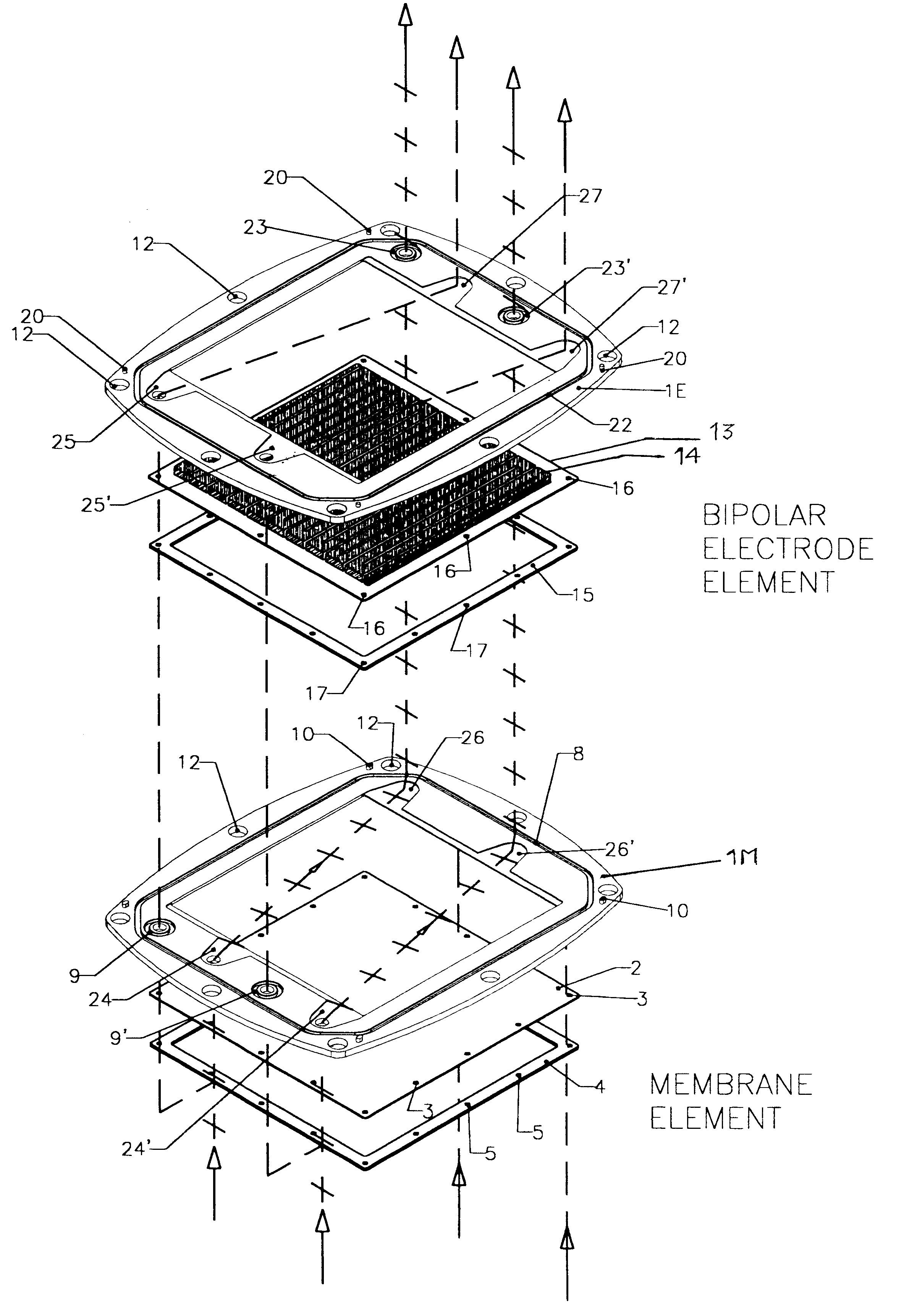
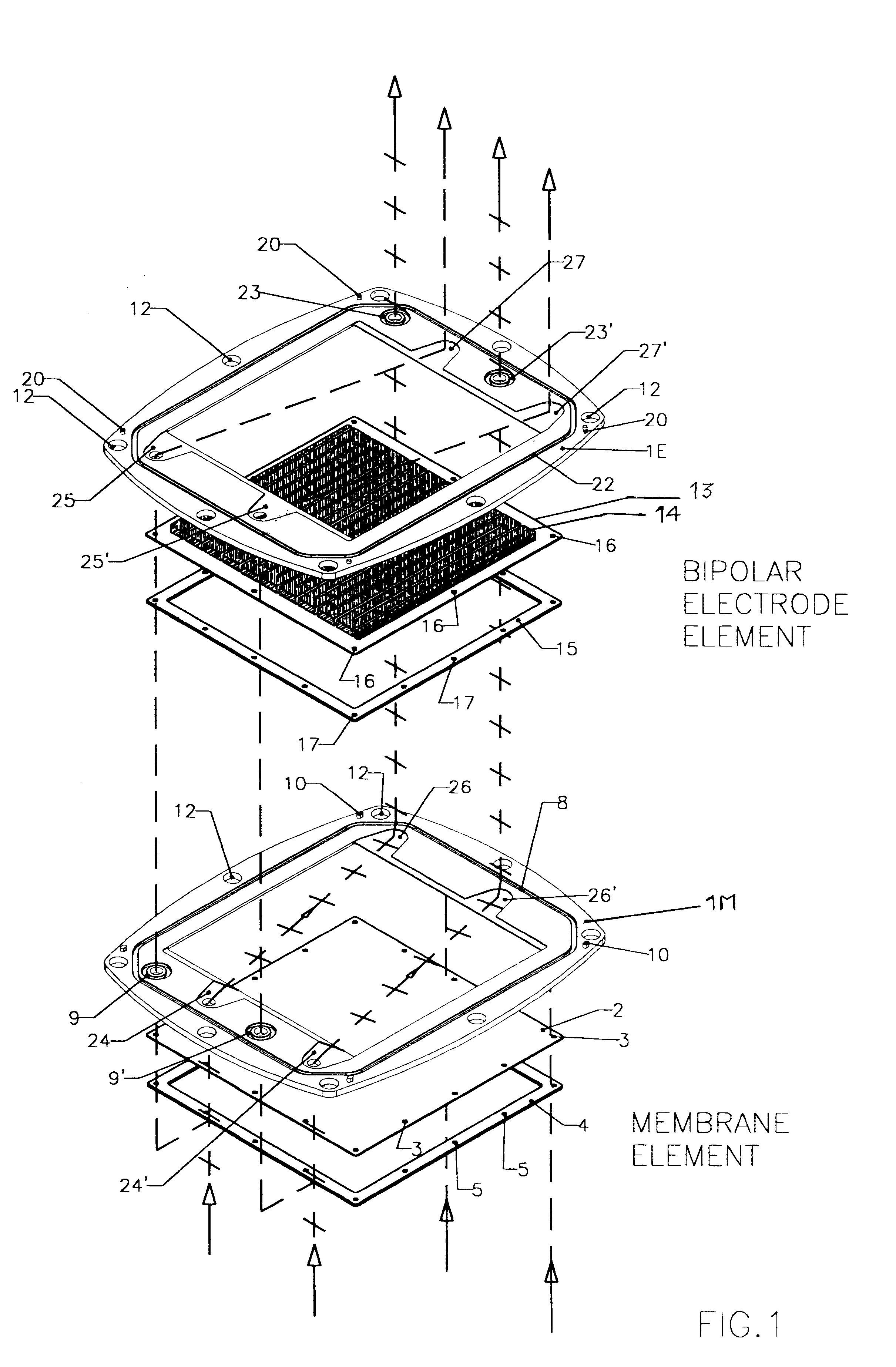
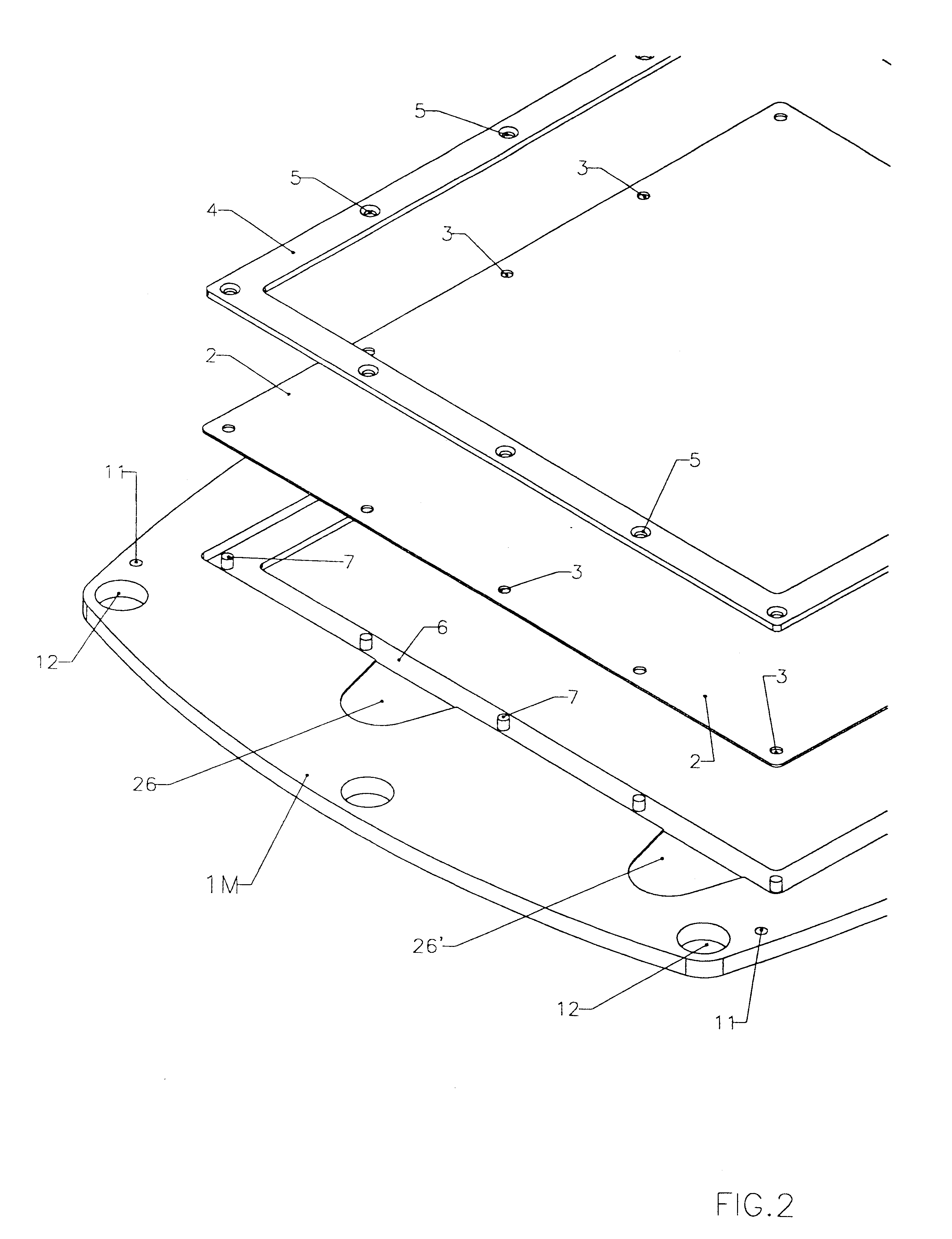
Membrane-separated, bipolar multicell electrochemical reactor
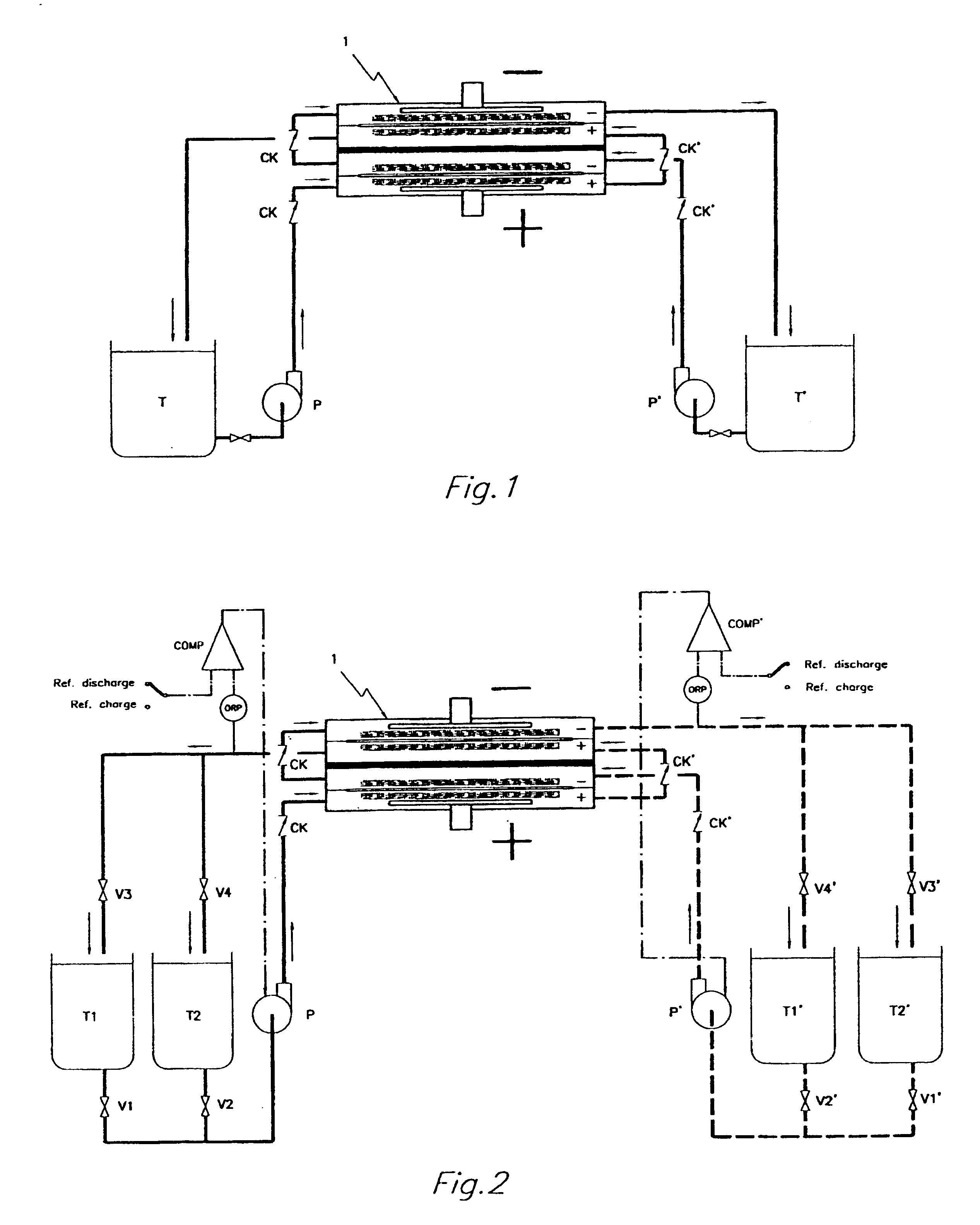
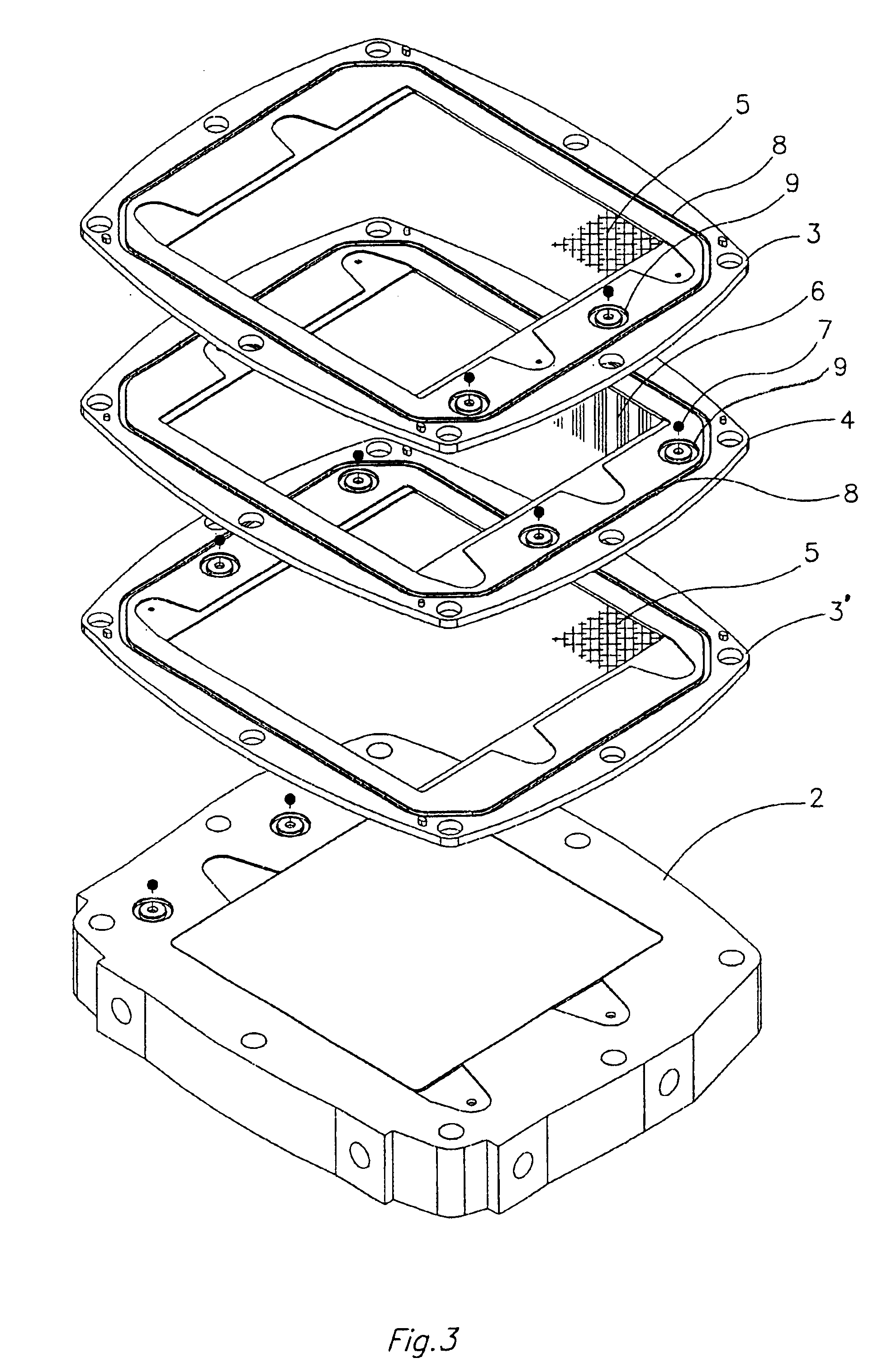
InactiveUS6555267B1Improve electrochemical performanceLower overall pressure dropFuel cells groupingFuel cell auxillariesPlastic materialsIon-exchange membranes
A multicell assembly is constituted by alternately stacking two types of pre-assembled elements: a bipolar electrode holding subassembly and a membrane holding subassembly. The alternate stack of elements is piled over a bottom end element and the stack is terminated by placing over the last membrane holding element a top end electrode element. Each bipolar plate electrode holding element and each ion exchange membrane separator holding element includes a substantially similar rectangular frame piece, made of an electrically nonconductive and chemically resistant material, typically of molded plastic material, having on its upper (assembly) face grooves for receiving O-ring type gasket means, and having through holes and recesses in coordinated locations disposed along two opposite sides of the rectangular frame forming, upon completion of the assembling, ducts for the separate circulation of the negative electrolyte and of the positive electrolyte through all the negative electrolyte flow chambers and all positive electrolyte flow chambers, respectively, in cascade.
Owner:SQUIRREL HLDG
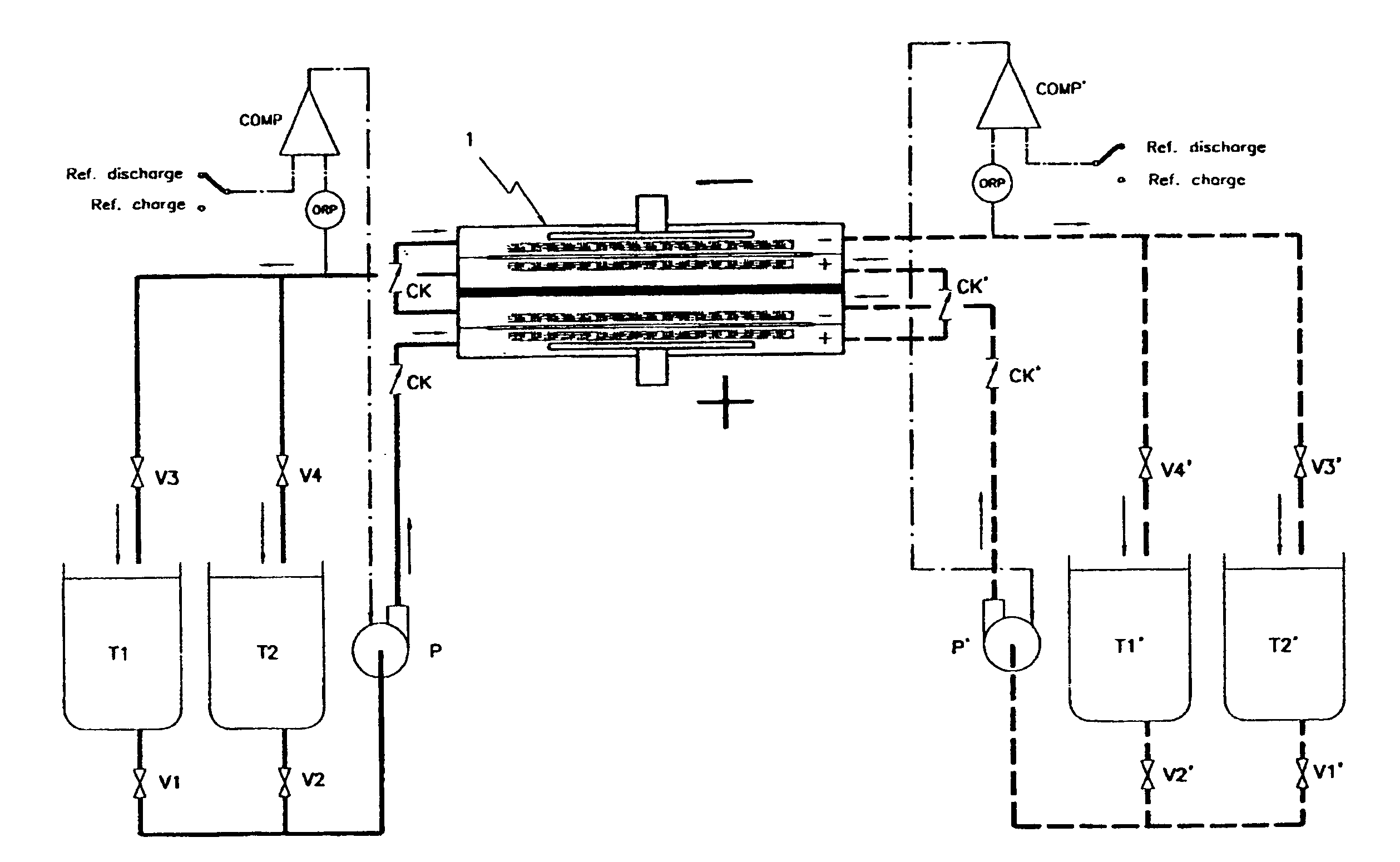
Redox flow battery and method of operating it
InactiveUS6692862B1Easy dischargeImprove efficiencyElectrolyte moving arrangementsFuel cells groupingWork cycleRedox
By realizing or installing check valve liquid vein interrupters in each compartment of the battery the phenomenon of slow discharge of the retained volumes of electrolytes during long periods of inactivity of a redox flow battery, with the electrolyte pumps stopped altogether, can be practically eliminated with the effect that the battery is perfectly ready to deliver electric power immediately upon request even after prolonged periods of inactivity. Moreover, the presence of liquid vein interrupters on each compartment in either an outlet or an inlet port substantially preventing by-pass current during a not pumping phase, permits to increase the by pumping the electrolytes through the compartments of a battery stack intermittently, in other words in a pulsed manner, with a certain duty-cycle. Relatively brief pumping phases at relatively high flow rate alternated to phases of not pumping provide for a volumetrically adequate refreshing of the electrolytes present in the battery compartments and contrast the formation of gradients in the bodies of electrolyte.
Owner:SQUIRREL HLDG
System and Method for Generating Hydrogen Gas
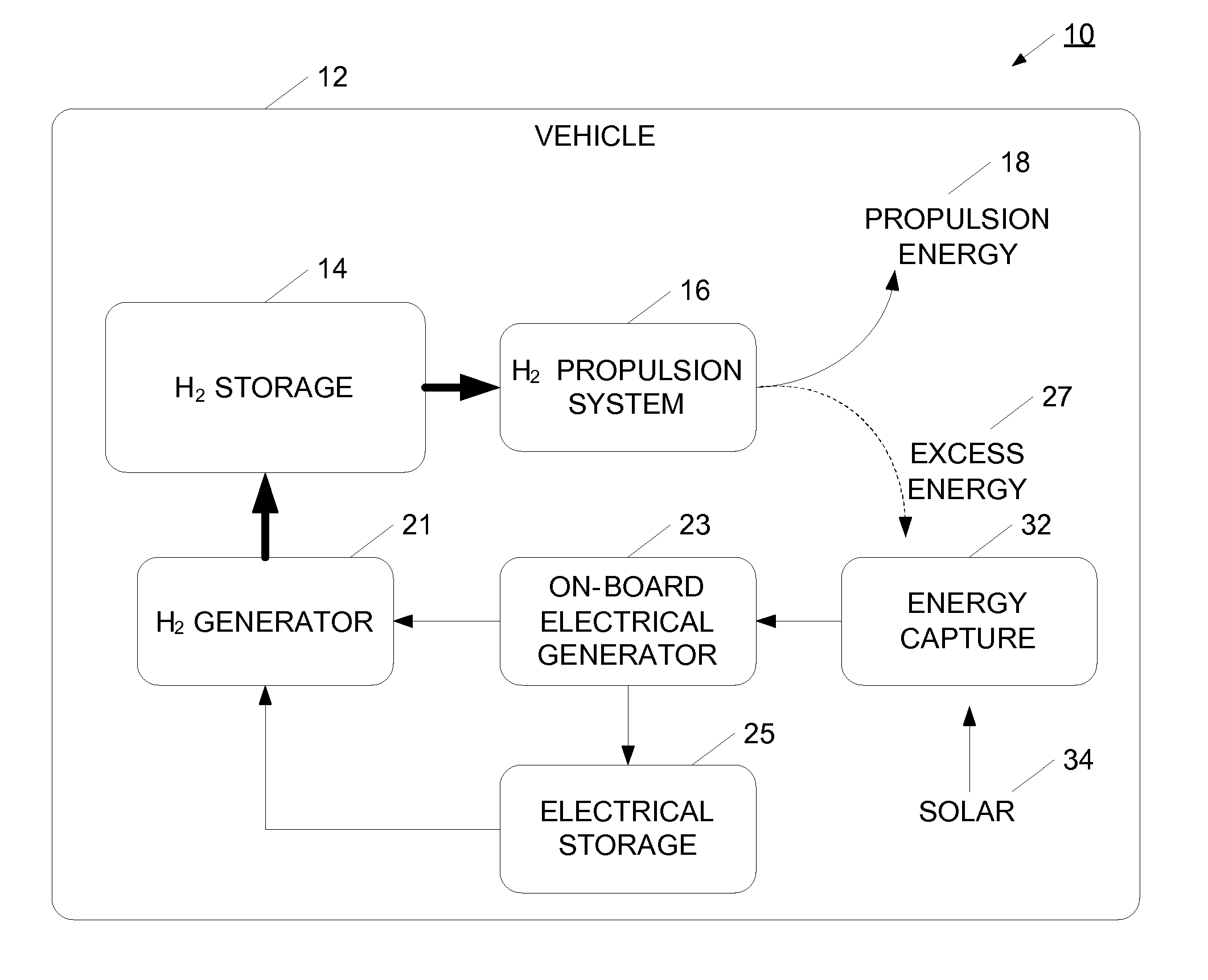
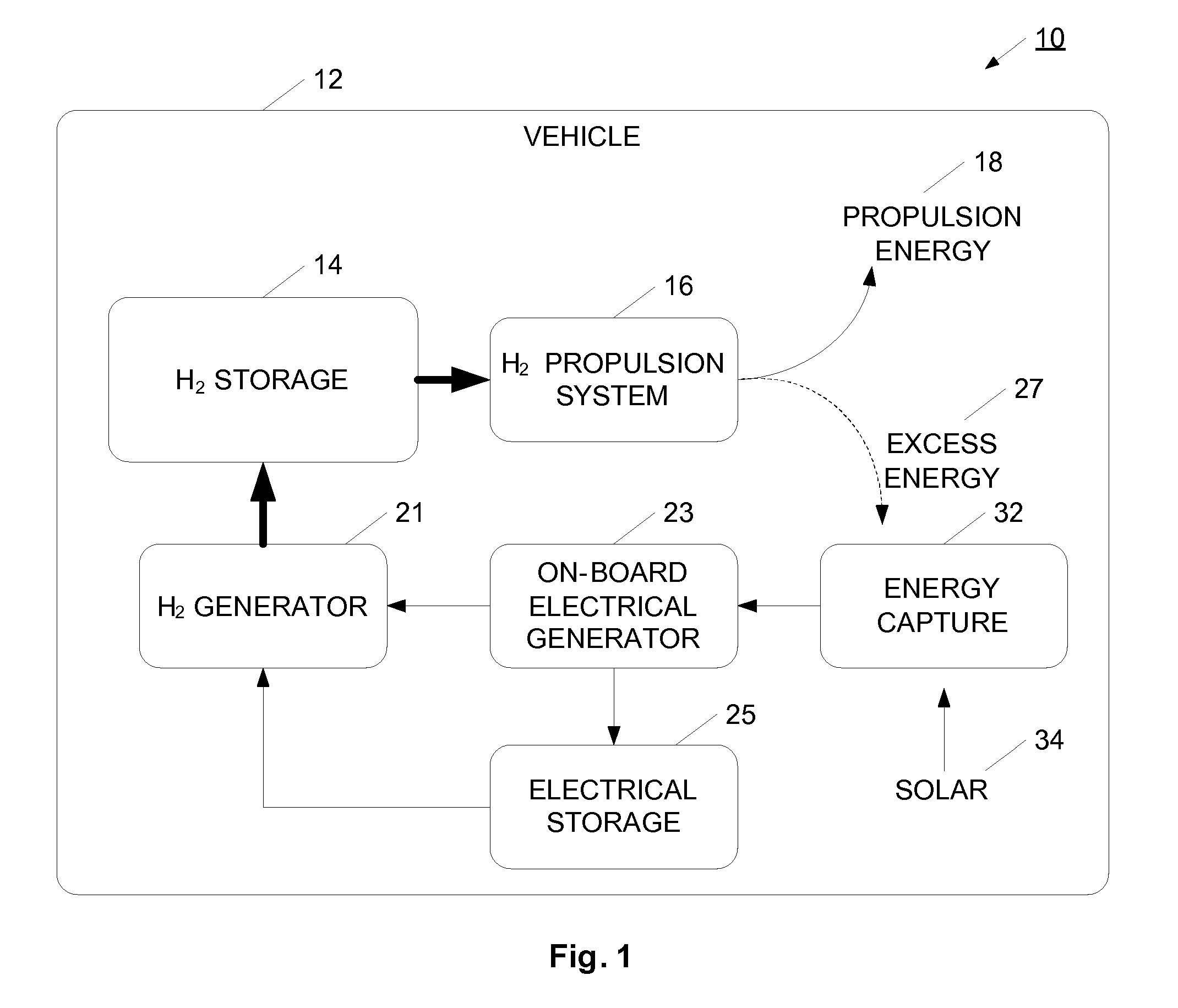
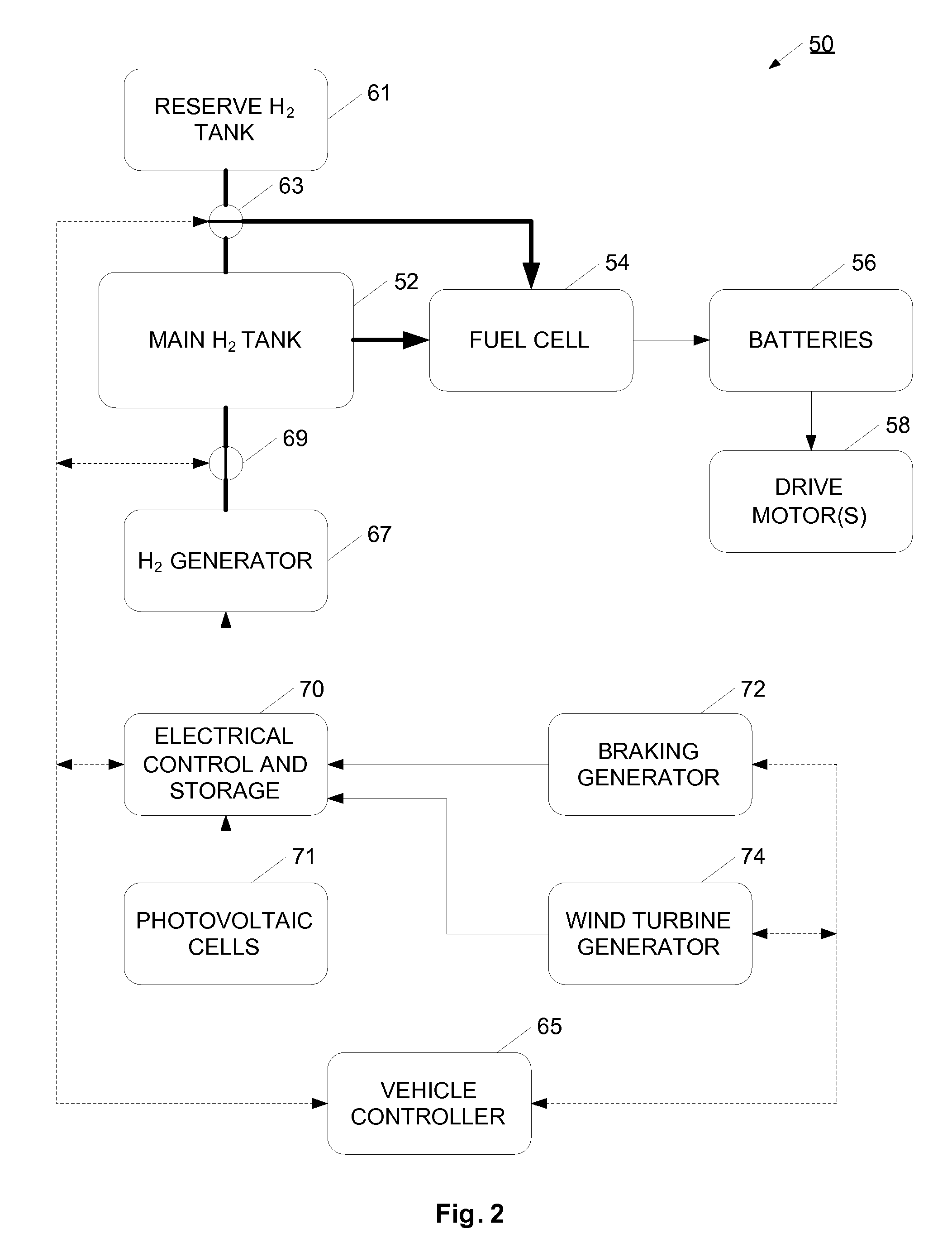
InactiveUS20070138006A1Reduce dependenceIncrease adoptionCellsRegenerative fuel cellsElectricityMobile vehicle
A hydrogen gas generation system is provided for use in a mobile vehicle. The mobile vehicle may be for example, a car or truck or other vehicle such as a balloon, dirigible, airship, ship, or boat. The vehicle has an on-board hydrogen generator for generating hydrogen gas, preferably using an electrolysis process. The hydrogen produced by the electrolysis process is stored in an on-board hydrogen storage tank. Hydrogen from the storage tank is flowed into a vehicle propulsion system where the hydrogen gas is consumed to provide power to propel the vehicle. An on-board electrical generation system provides at least some of the electricity for the electrolysis process. In one example, the vehicle has an on-board electrical generator for providing electricity for the electrolysis process. The on-board electric generation system may be, for example, a solar photovoltaic cell system, a wind turbine generator system, or a regenerative braking generator, for example. Depending on the particular electrical generation process or processes used, the vehicle may generate hydrogen gas when moving, when coasting or braking, or when long-term parked.
Owner:CENESTRA
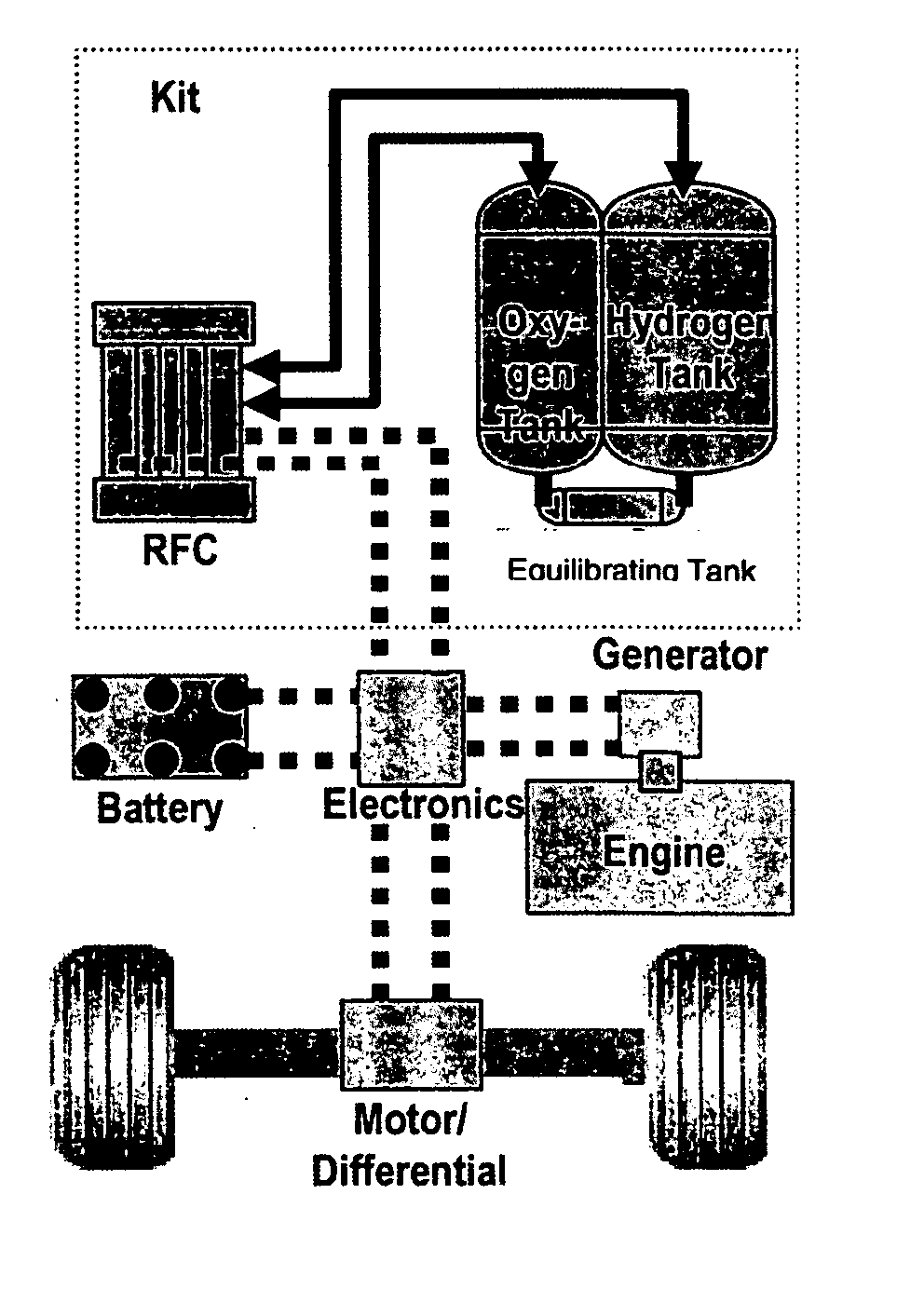
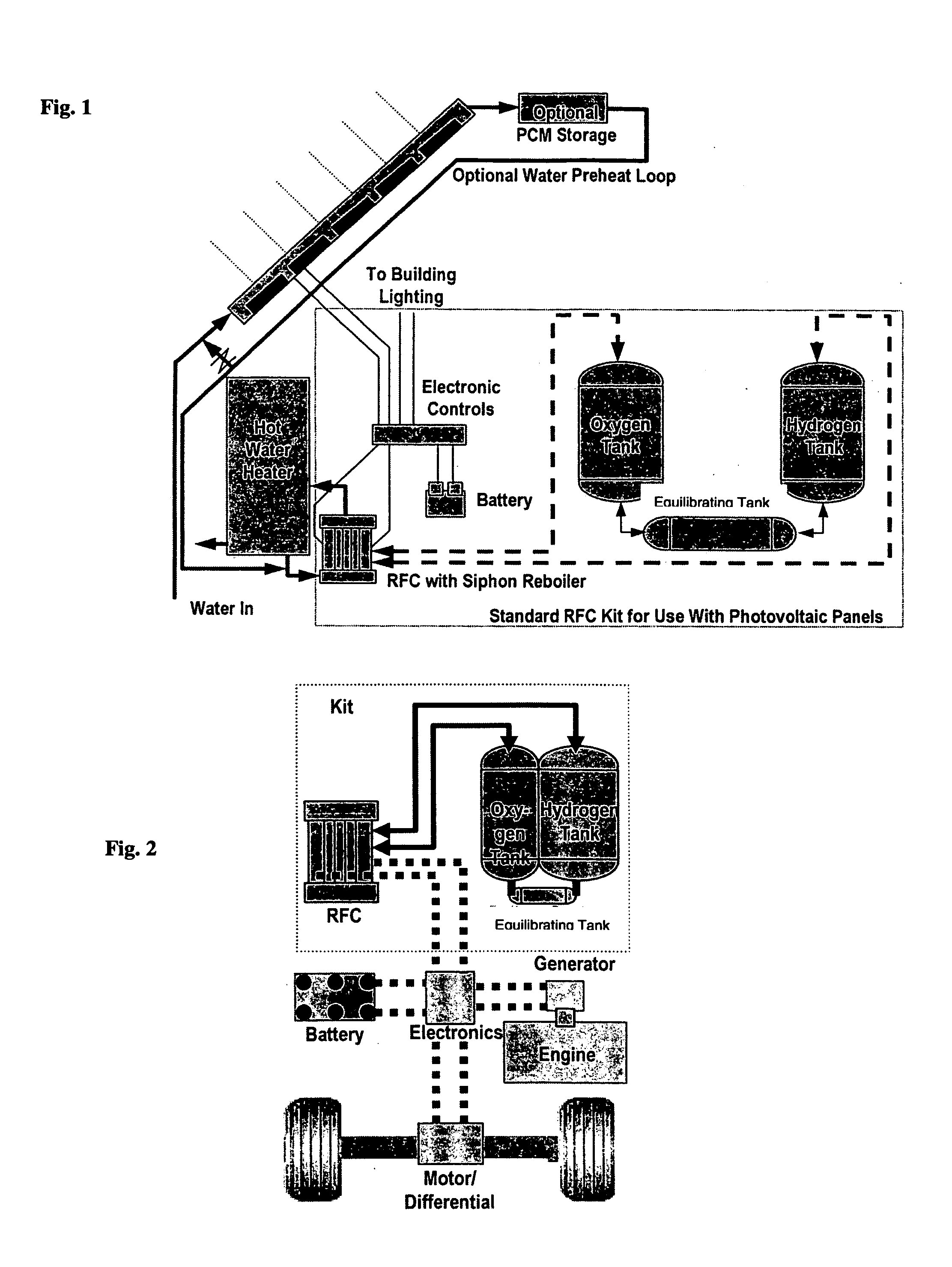
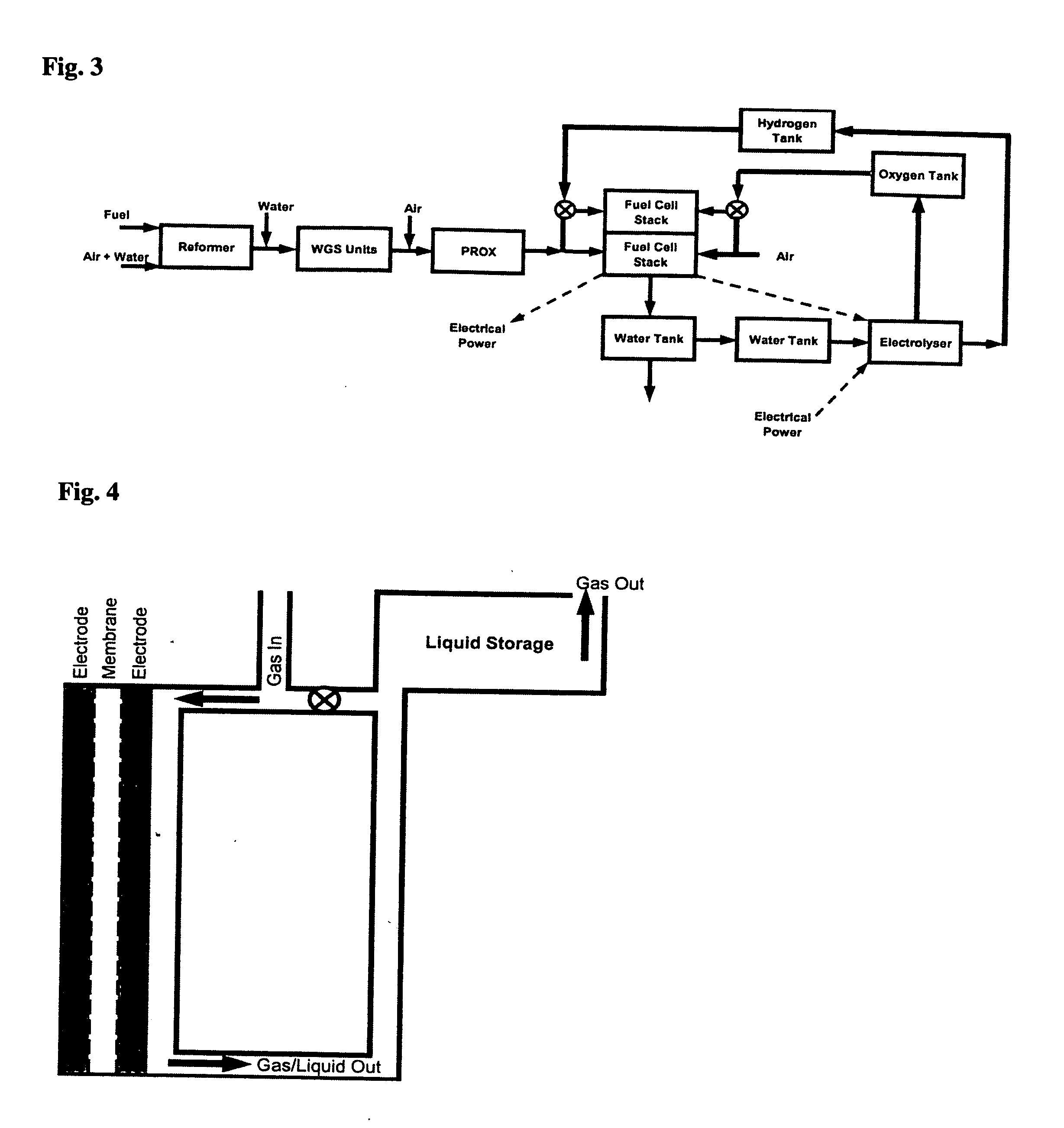
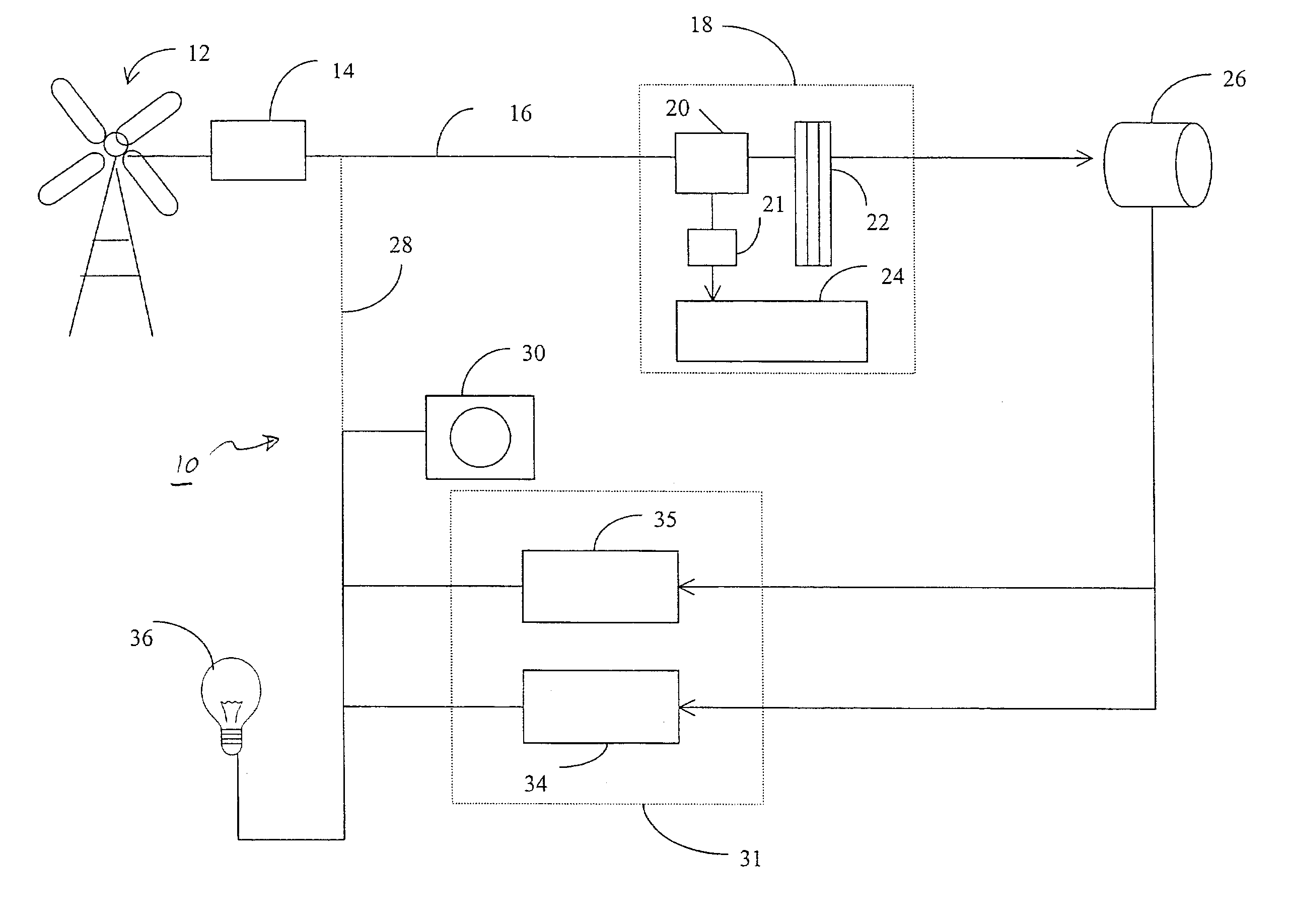
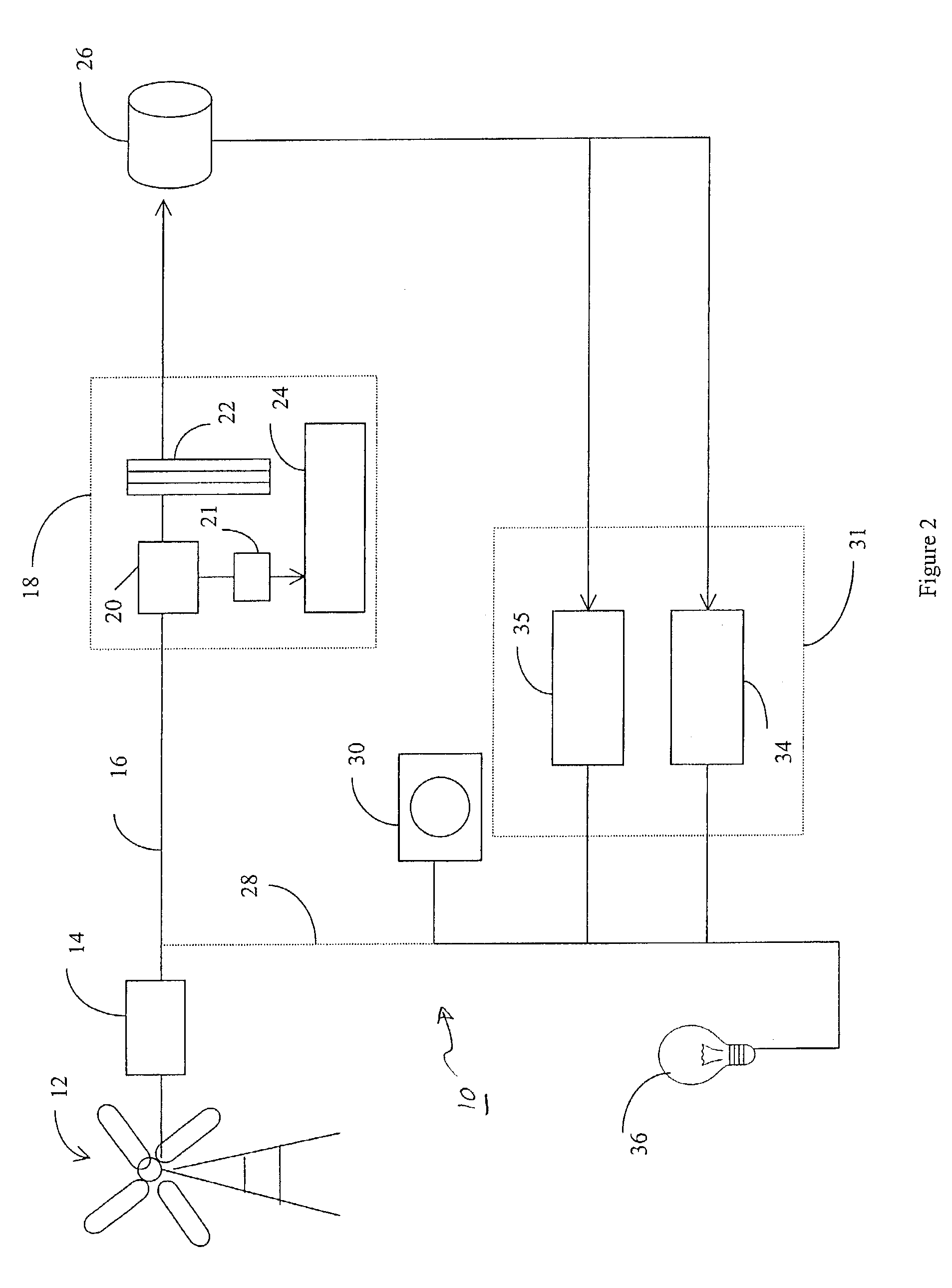
Regenerative fuel cell technology
InactiveUS20050008904A1Constant gainSmall fuel cellBatteries circuit arrangementsRegenerative fuel cellsElectrolysisOxygen
For a mobile fuel cell system a narrow-gap modular approach allows reforming to be performed in the same architecture as the fuel cell. A regenerative fuel cell operates much like a battery using electrical power to produce hydrogen and oxygen. The preferred mode of using the regenerative fuel cell is as a battery charger since this application is able to use a much smaller fuel cell than is required to power the vehicle. A novel equilibrating tank between the electrolysis oxygen and hydrogen tanks allows pressurized oxygen and hydrogen to be used without mechanical compression equipment.
Owner:SUPPES GALEN J
System for storing and recoving energy and method for use thereof
InactiveUS20040013923A1Decreases hydrogen gas pressureElectricity cogenerationElectrolysis componentsElectricityWorking pressure
An energy storage and recovery system includes a renewable power source, a hydrogen generation device in electrical communication with the renewable power source, a hydrogen storage device in fluid communication with the hydrogen generation device, a hydrogen fueled electricity generator in fluid communication with the hydrogen storage device, and a pressure regulator interposed between and in fluid communication with the hydrogen fueled electricity generator and the hydrogen storage device. The pressure regulator is set at an operating pressure of the hydrogen fueled electricity generator.
Owner:PROTON ENERGY SYST
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS6468688B2Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Fuel cell system, related method and current measuring device for fuel cell system
ActiveUS20050053814A1Accurate measurementHigh precisionPhotography auxillary processesElectrolysis componentsElectrical conductorFuel cells
A fuel cell system, control method and current measuring device for a power unit are disclosed. The fuel cell system includes a fuel cell having local areas, a current measuring device associated with at least one of the local areas to measure localized current related to a specified operating characteristic, and a control section for diagnosing an operating condition of the fuel cell in response to localized current to enable optimum control of the fuel cell depending upon a specified operating characteristic determined by localized current. The control method controls the operating condition of the fuel cell in response to localized current indicative of the specified operating characteristic of the fuel cell. The current measuring device includes an electrical conductor formed with a recessed portion, a localized current conductor received in the recessed portion, and a current sensor for detecting current flowing across the localized current conductor.
Owner:DENSO CORP
System and method for optimizing efficiency and power output from a vanadium redox battery energy storage system
An energy storage system includes a vanadium redox battery that interfaces with a control system to optimize performance and efficiency. The control system calculates optimal pump speeds, electrolyte temperature ranges, and charge and discharge rates. The control system instructs the vanadium redox battery to operate in accordance with the prescribed parameters. The control system further calculates optimal temperature ranges and charge and discharge rates for the vanadium redox battery.
Owner:VRB ENERGY INC
Battery assembly
InactiveUS20060177734A1Easy to produceImprove securityPrimary cell to battery groupingFuel cells groupingElectrical conductorEngineering
A battery pack includes multiple power bus lines connecting a quantity of cell strings in parallel, wherein each cell string includes multiple cells connected in series. The battery pack further has multiple conductors providing electrical communication between the cell strings such that a cell in one cell string is connected in parallel with a cell in other battery strings.
Owner:YAO LI HO
High-efficiency compound regenerative electrical energy device
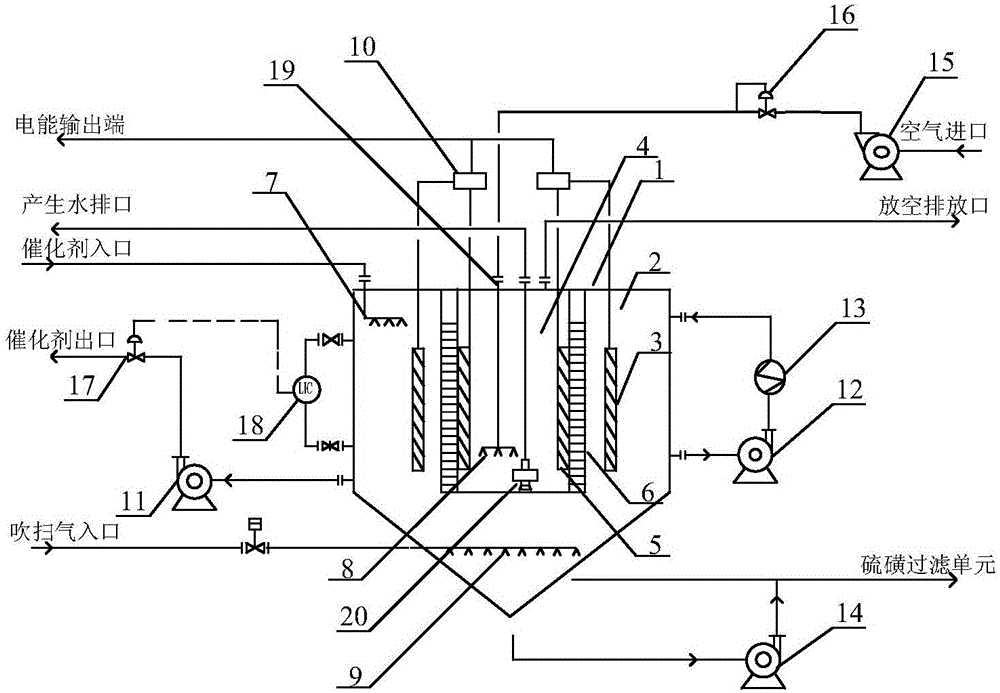
InactiveCN105958098AIncreased oxidation regeneration rateReduce degradation lossRegenerative fuel cellsFuel cellsCirculator pump
The invention provides a high-efficiency compound regenerative electrical energy device. The device comprises a reactor, an anode chamber, an anode plate, a cathode chamber, a cathode plate, a proton exchange membrane module, a liquid distributor, a gas distributor, a purge distributor, a battery load, an output pump, a circulating pump, a heating / cooling device, a slurry pump, an air blower, a purge gas stop valve, a flow control valve, a magnetic flap liquidometer, a self-operated pressure-regulating valve, and an immersed pump. The gas distributor is disposed at the bottom of the cathode chamber, and the circulating pump is disposed in the middle of the reactor barrel. The air blower communicates with the gas distributor by the self-operated pressure-regulating valve, and the immersed pump is disposed at the bottom of the cathode chamber. The device uses the coupling mode of fuel cell and catalyst reactivation by means of the arrangement of air cathode fuel cell, is beneficial to improve the oxidation regeneration rate of the catalyst, and realizes the rapid regeneration of the complex catalyst.
Owner:JEREH TIANJIN PETROLEUM ENG & TECH
Manclaw-Harrison fuel cell
The Manclaw-Harrison Fuel Cell is a new Environmentally SAFE Fuel Cell (Lead Free, Acid Free, Mercury Free and has No Heavy Metals), and as such, it sets the definition of a new Class of Fuel Cell device because it has been verified to be a Non-Faraday device, making it the first such device discovered in the past 160+ years. See the “Preamble” for a more technical explanation.
Owner:MANCLAW RONALD R +1
Direct hydrocarbon fuel cells
InactiveUS6214485B1Increase ratingsUseful power densityFuel cells groupingFinal product manufactureFuel cellsHydrogen
The direct electrochemical oxidation of hydrocarbons in solid oxide fuel cells, to generate greater power densities at lower temperatures without carbon deposition. The performance obtained is comparable to that of fuel cells used for hydrogen, and is achieved by using novel anode composites at low operating temperatures.
Owner:NORTHWESTERN UNIV
Battery with bifunctional electrolyte
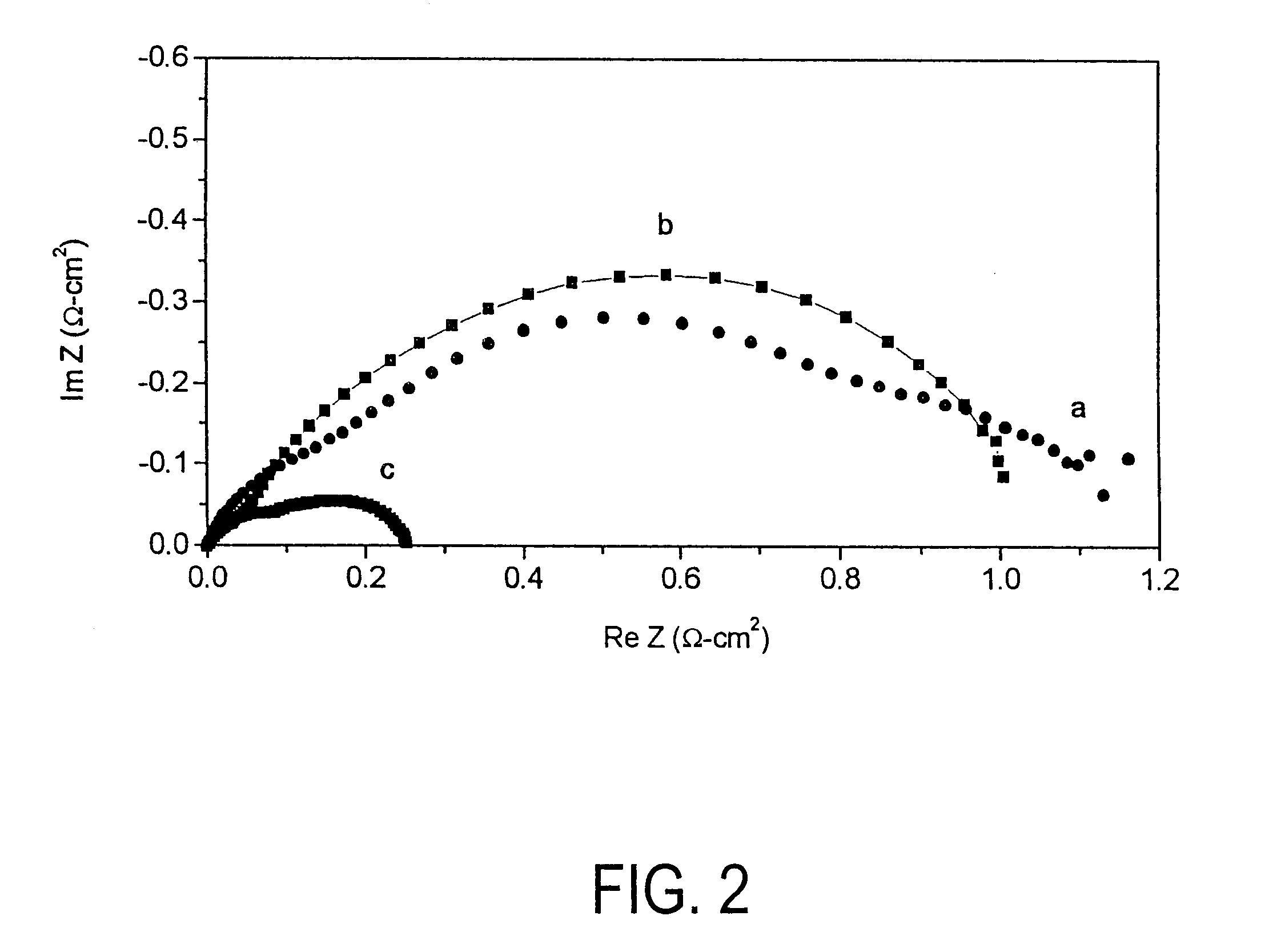
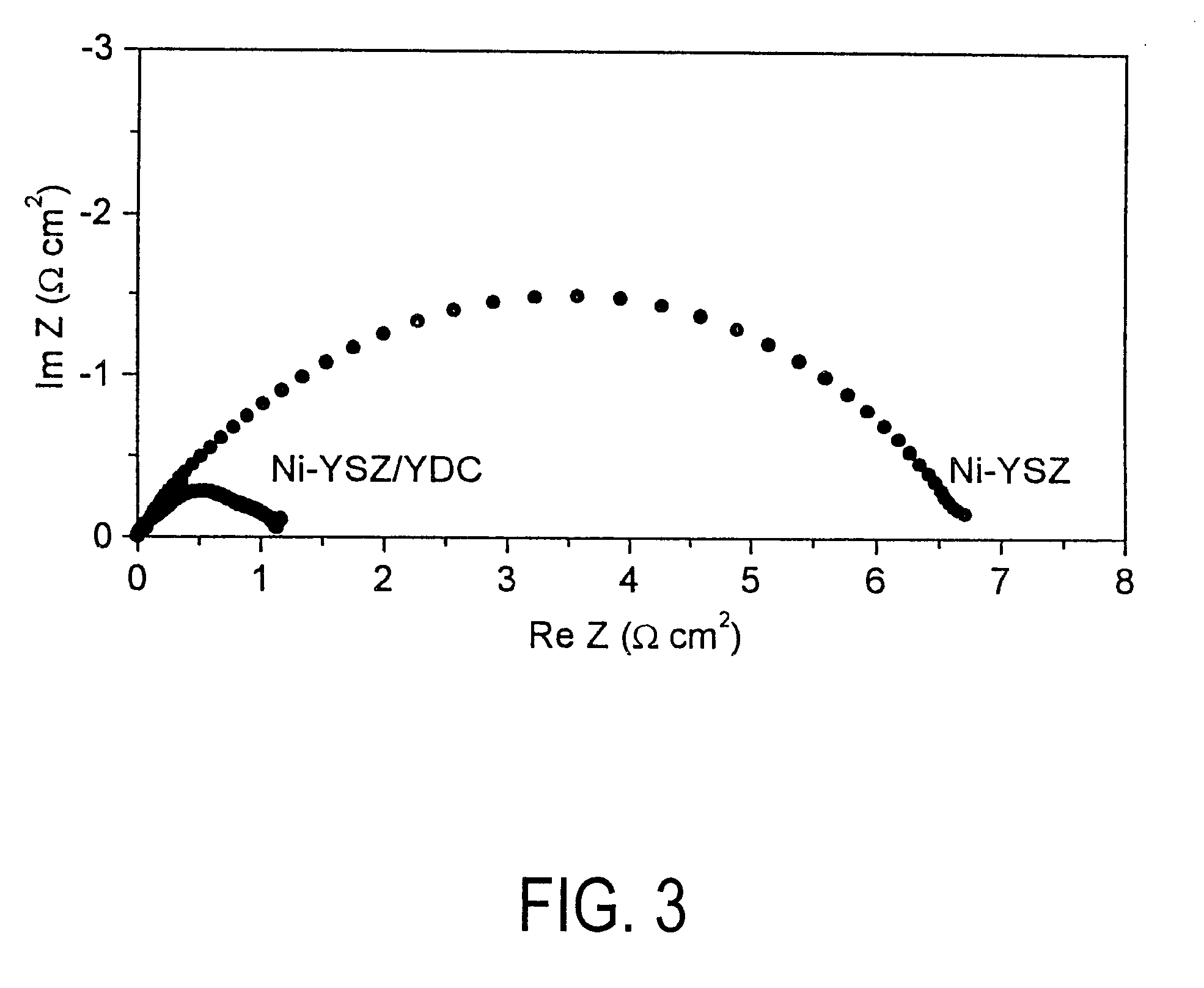
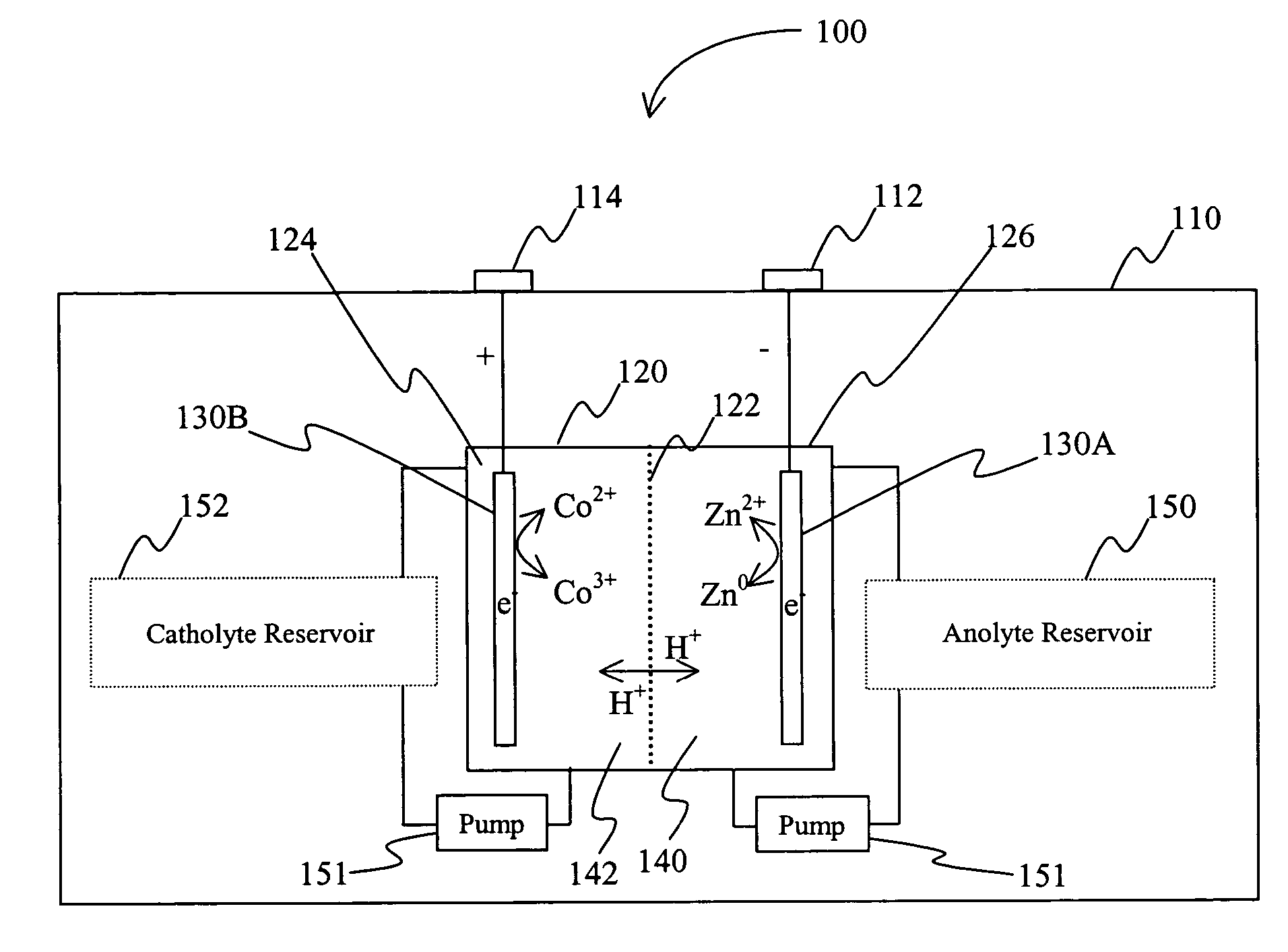
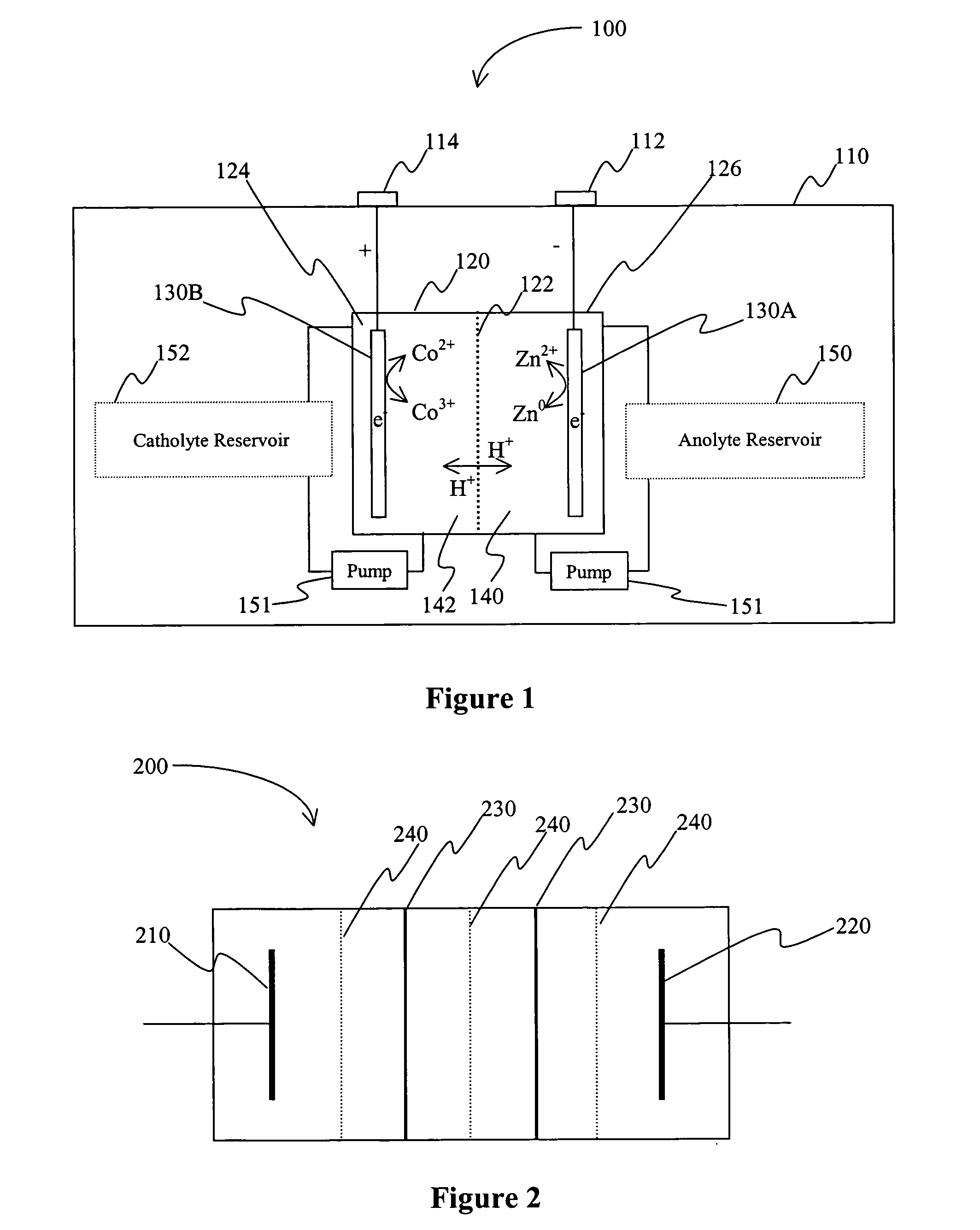
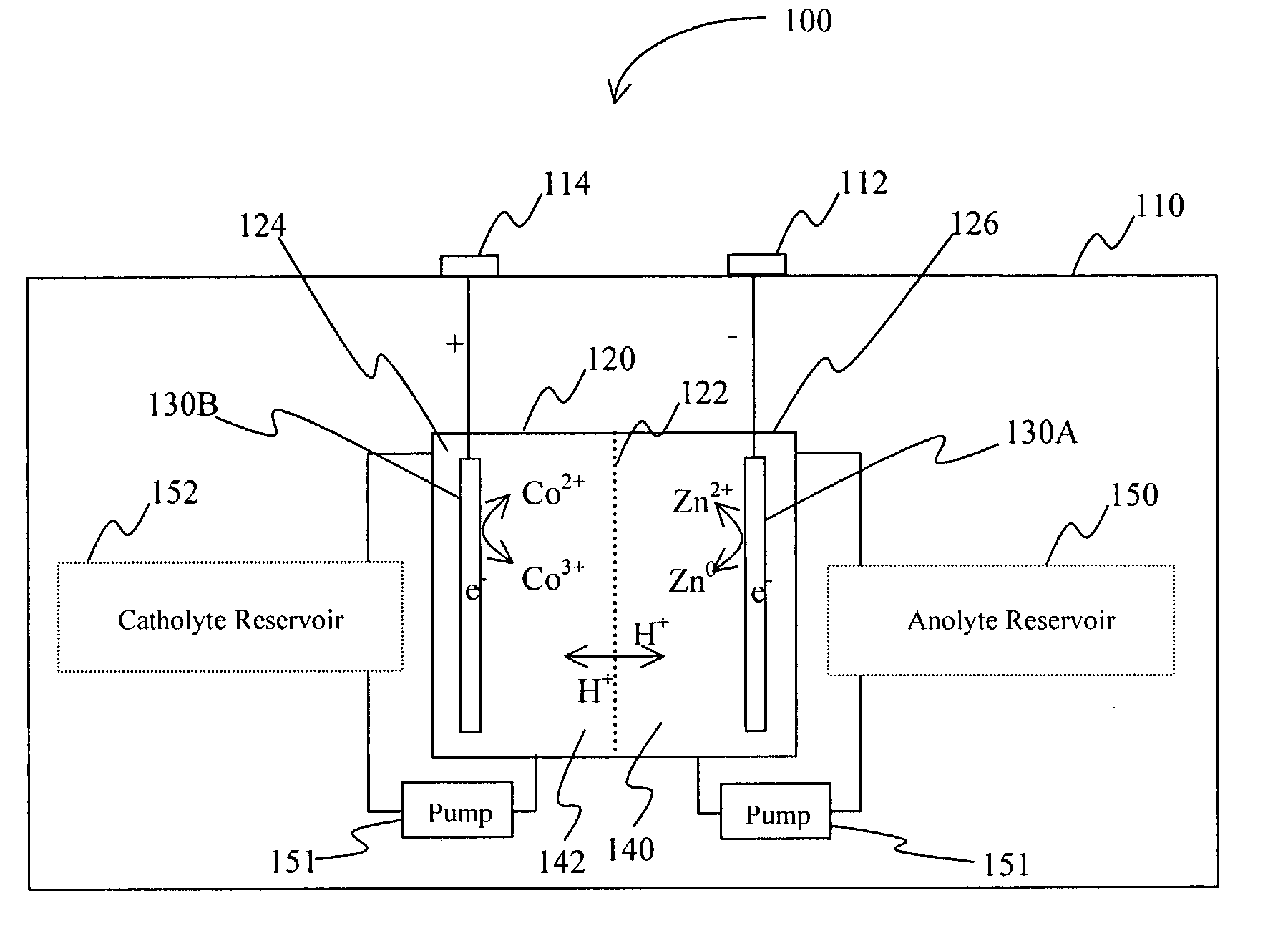
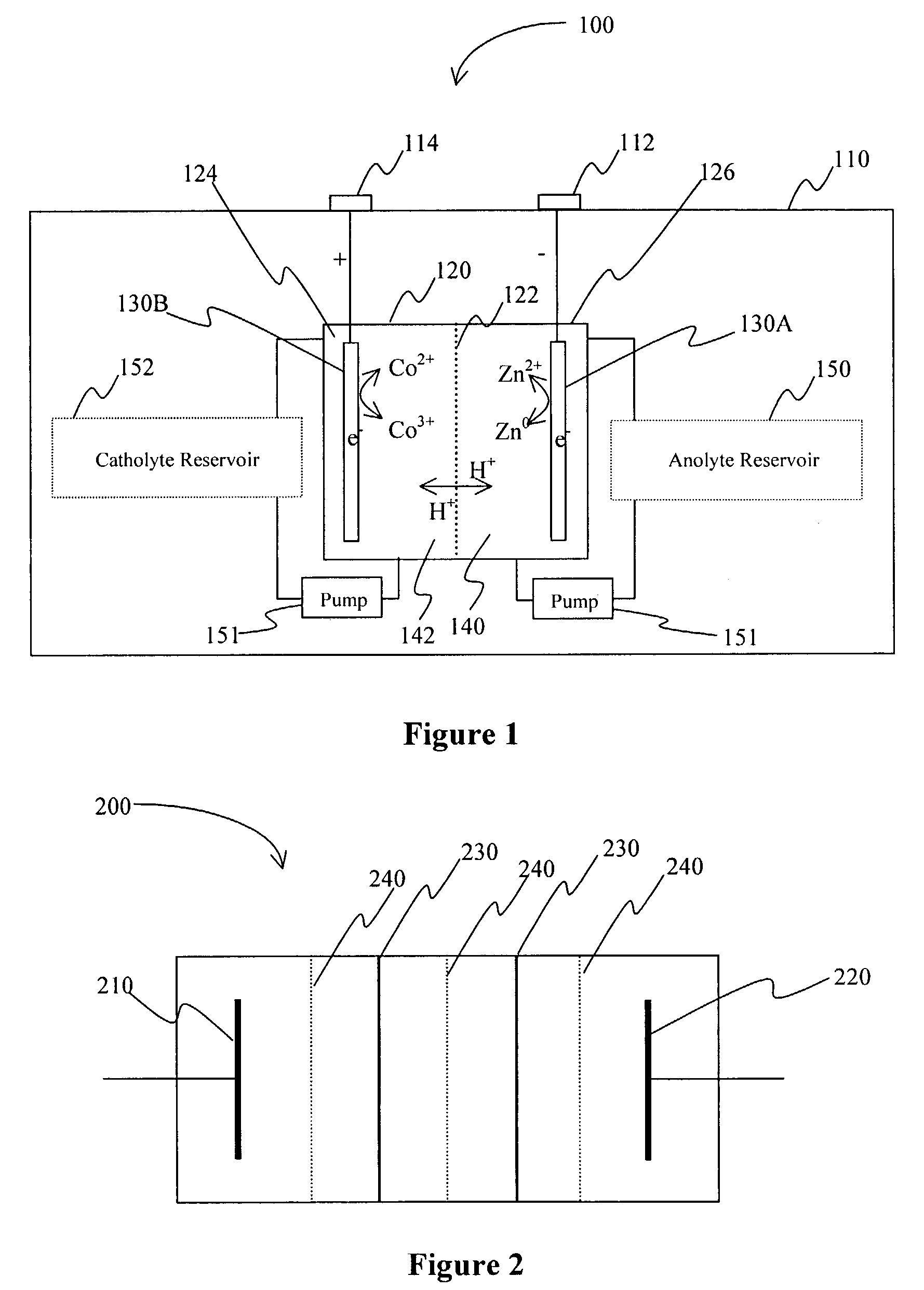
InactiveUS20060063065A1Improve solubilityCell electrodesRegenerative fuel cellsSolubilityOxidation-Reduction Agent
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids, amine sulfonic acids, and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:PLURION LTD
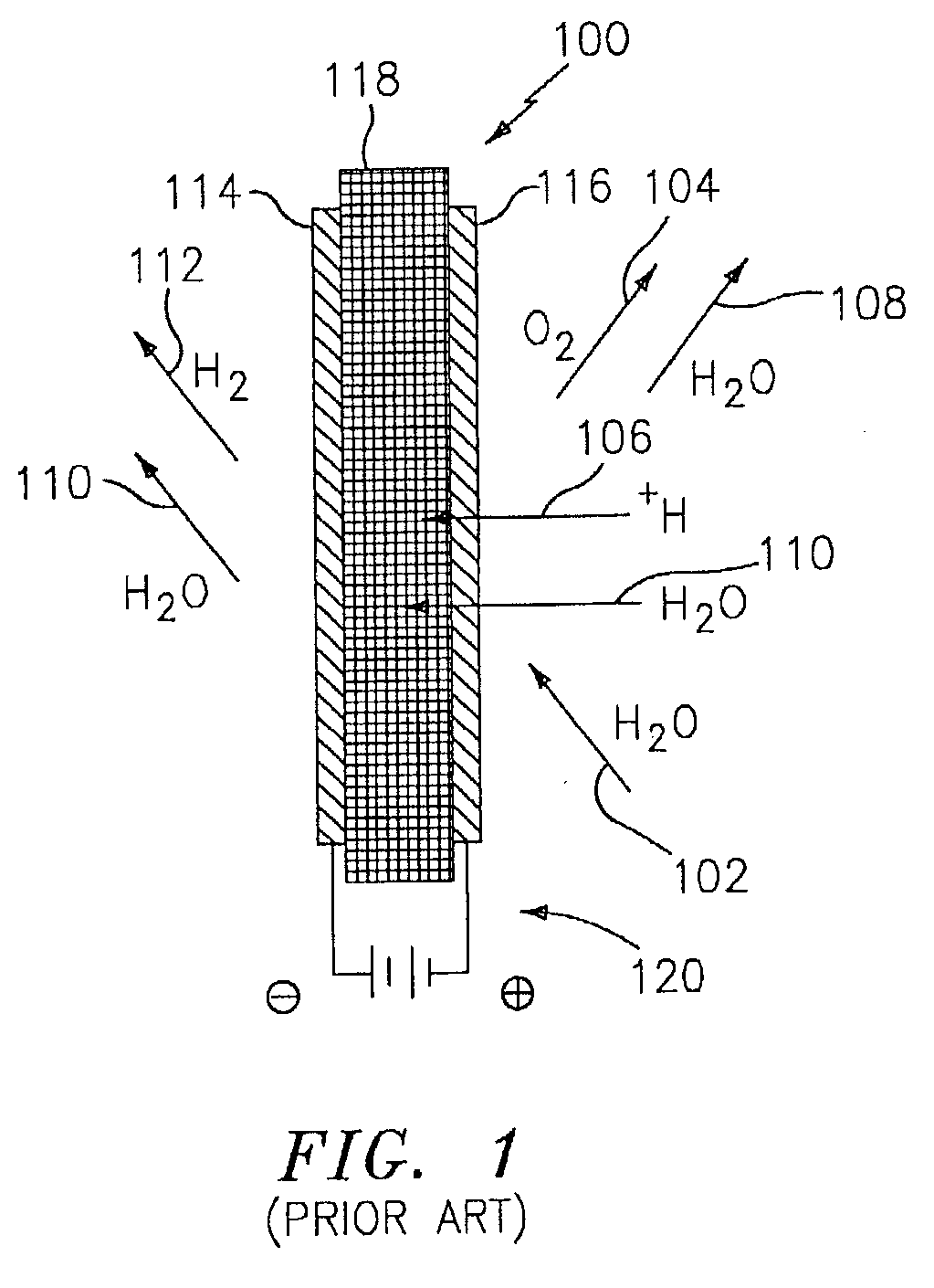
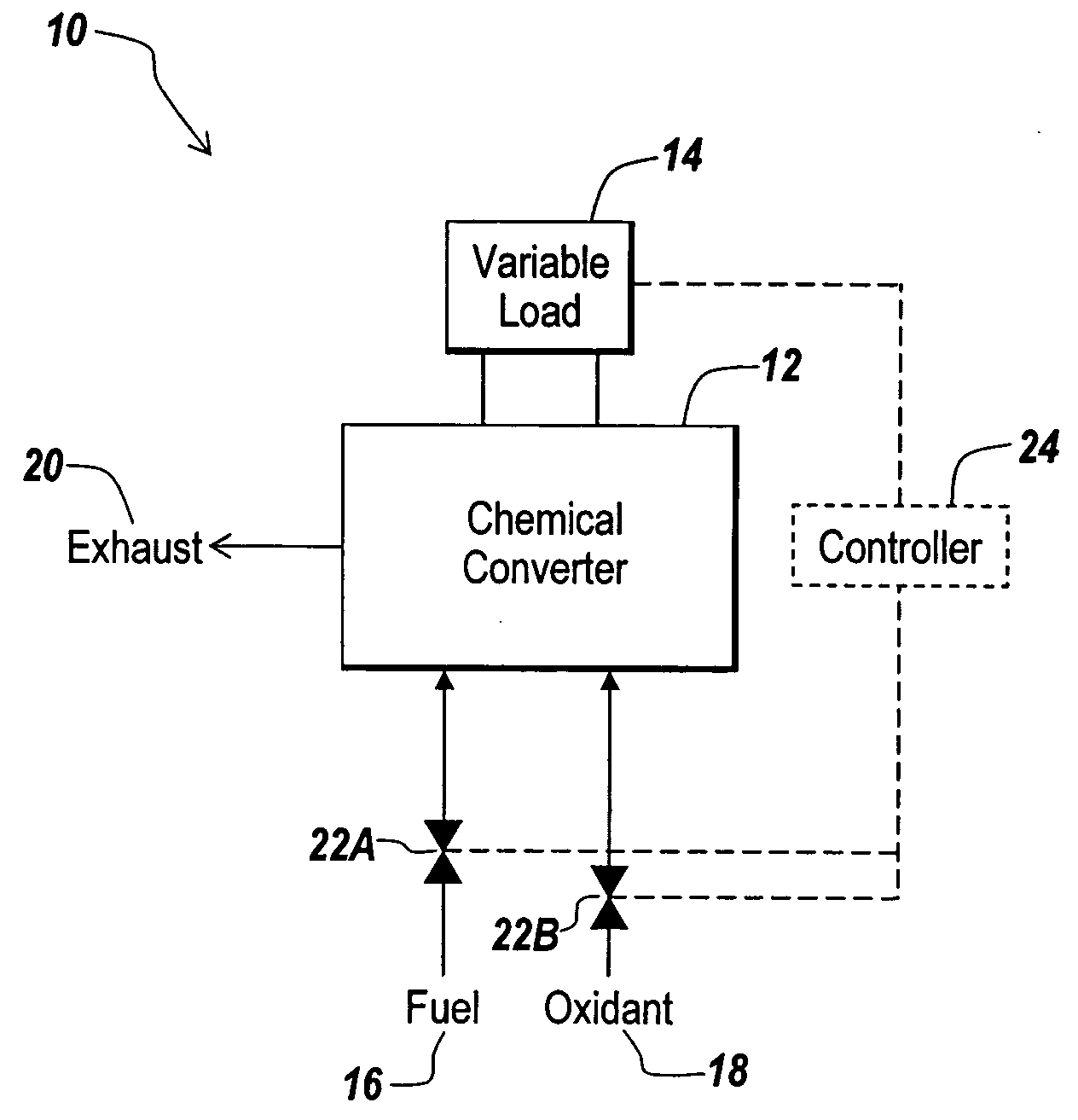
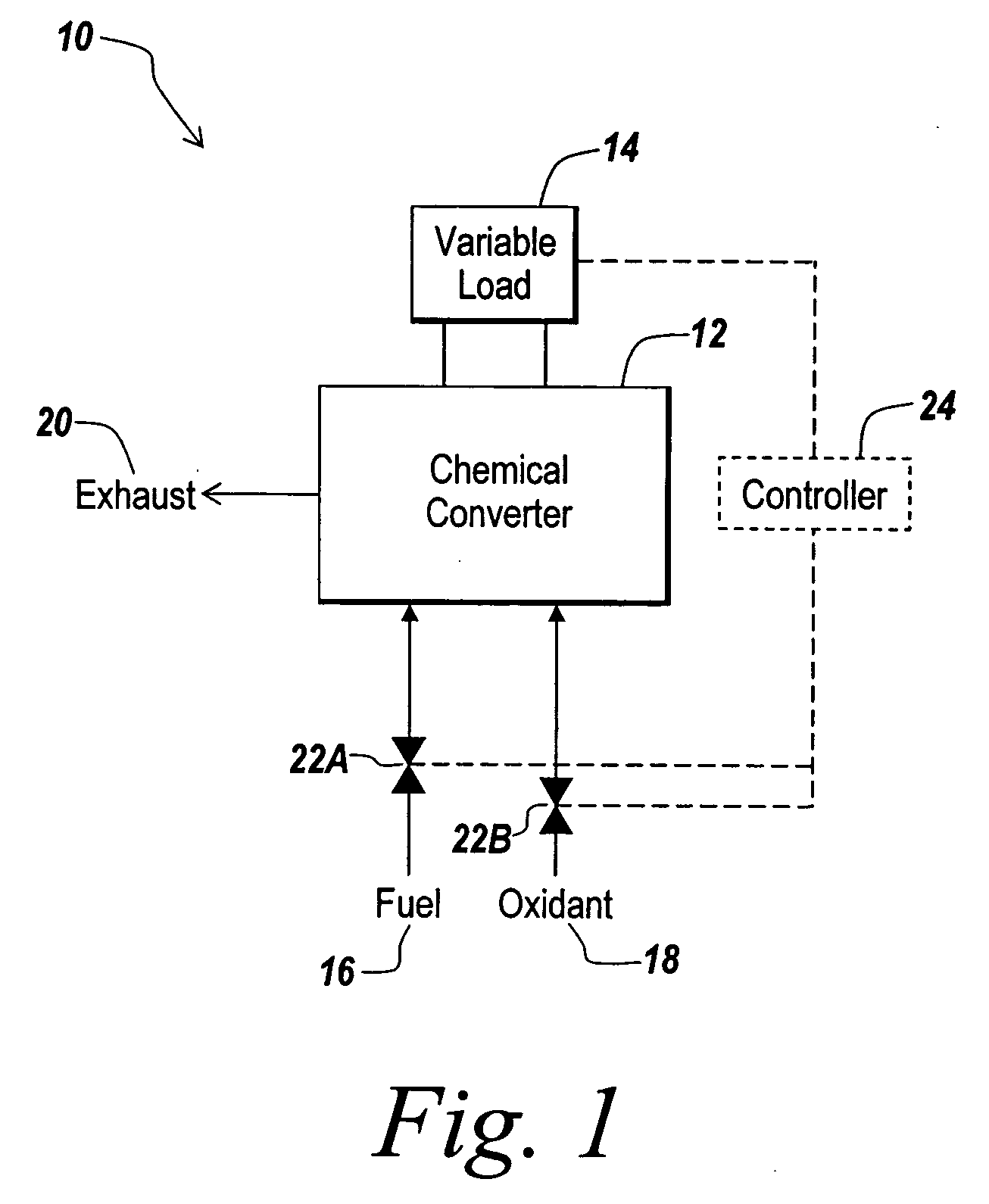
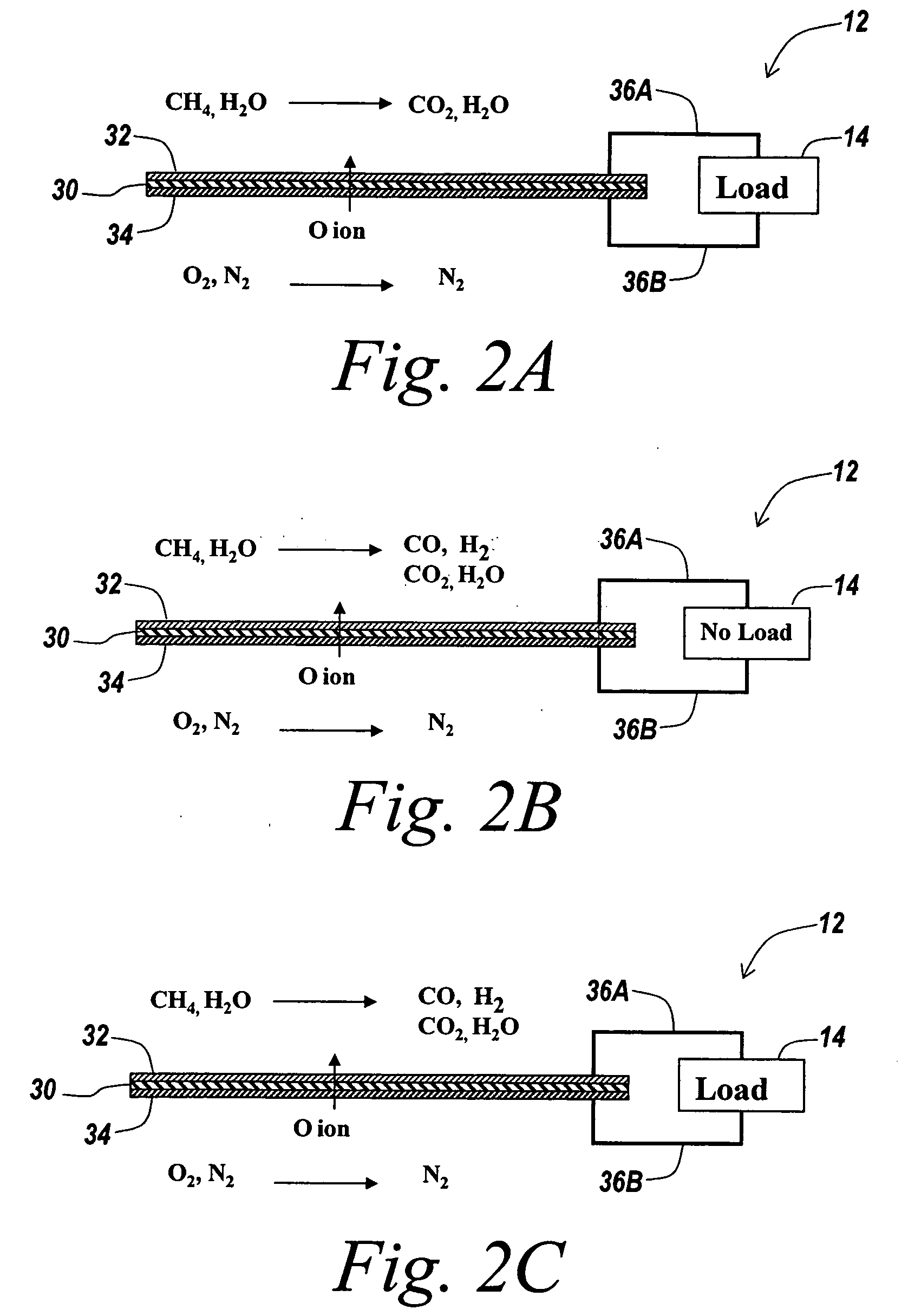
Hydrogen passivation shut down system for a fuel cell power plant
ActiveUS20050031917A1Move quicklyMinimize oxidation corrosionReactant parameters controlFuel cells groupingPower stationEngineering
The invention is a hydrogen passivation shut down system for a fuel cell power plant (10). An anode flow path (24) is in fluid communication with an anode catalyst (14) for directing hydrogen fuel to flow adjacent to the anode catalyst (14), and a cathode flow path (38) is in fluid communication with a cathode catalyst (16) for directing an oxidant to flow adjacent to the cathode catalyst (16) of a fuel cell (12). Hydrogen fuel is permitted to transfer between the anode flow path (24) and the cathode flow path (38). A hydrogen reservoir (66) is secured in fluid communication with the anode flow path (24) for receiving and storing hydrogen during fuel cell (12) operation, and for releasing the hydrogen into fuel cell (12) whenever the fuel cell (12) is shut down.
Owner:AUDI AG
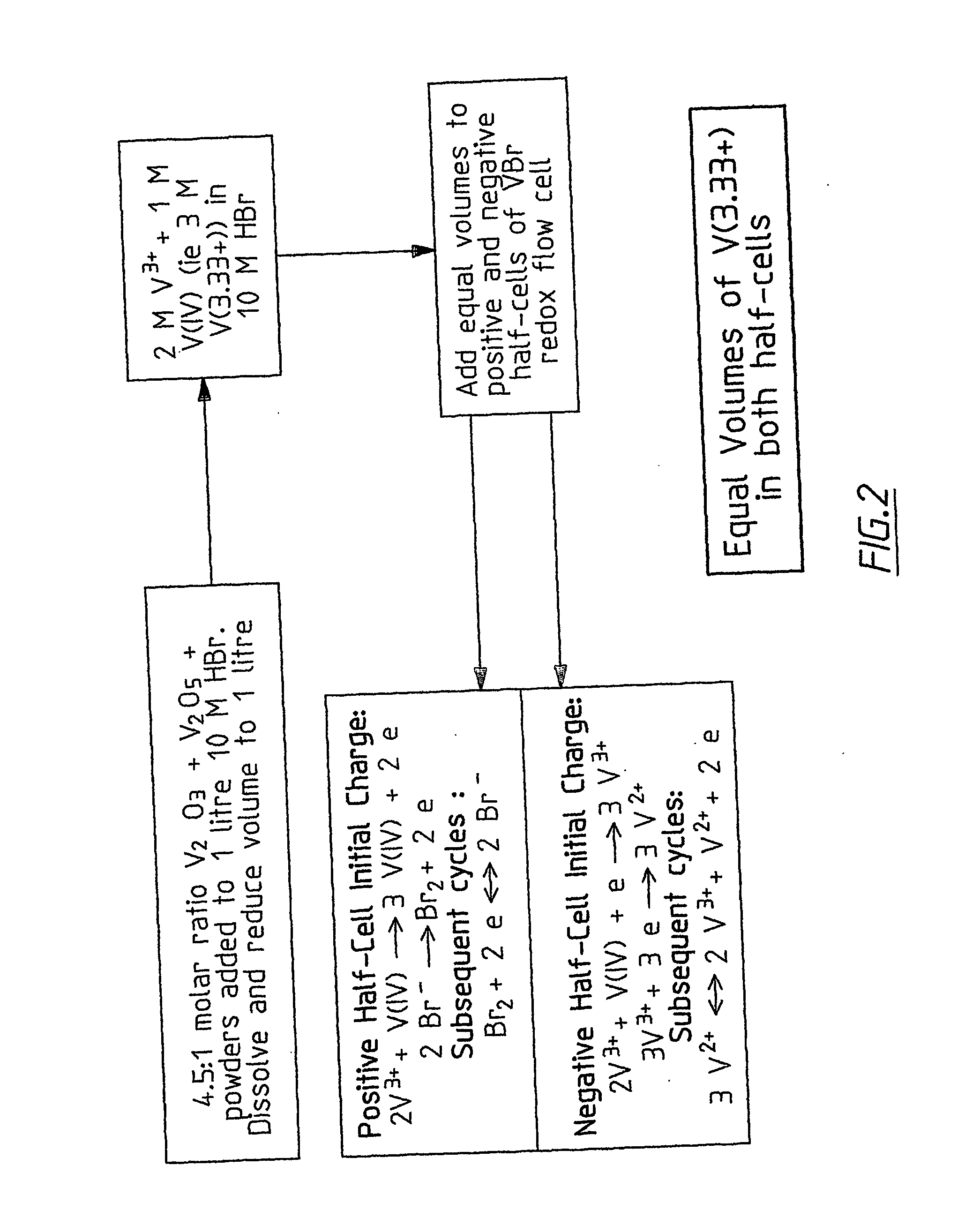
Novel vanadium halide redox flow battery
InactiveUS20060183016A1Avoid excessive bromine generationStabilise the bromine producedCharging stationsCell electrodesRedoxPhysical chemistry
A prior to charge vanadium halide redox cell, a vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge and vanadium halide redox cell which are fully charged and partially charged are described. The prior to charge vanadium halide redox cell comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (IV) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (N) halide wherein the amounts of vanadium (III) halide, vanadium (IV) halide and halide ions in the positive and negative half cell solutions are such that in a first charging step comprising charging the prior to charge vanadium halide redox cell, a vanadium halide redox cell having a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprising predominantly vanadium (N) halide in the positive half cell solution and predominantly V(III) halide in the negative half cell solution can be prepared. The vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (N) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (III) halide wherein the amount of vanadium (N) halide in the positive half cell solution and the amount of vanadium (III) halide in the negative half cell solution are such that the vanadium halide redox cell is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge. The vanadium halide redox cell which is fully charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and vanadium (II) halide wherein the molar concentration of vanadium (V) and polyhalide complex:molar concentration of vanadium (II) halide is about stoichiometrically balanced. The vanadium halide redox cell which is partially charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (II) halide and vanadium (III) halide wherein the number of moles of moles of polyhalide complex and vanadium (V): number of moles of vanadium (II) halide is about stoichiometrically balanced.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Fuel cell for hydrogen production, electricity generation and co-production
InactiveUS20050112425A1Amount be controlFuel cell combinationsHydrogenElectricityElectrochemical response
A hydrogen-electricity co-production (HECP) system utilizes a fuel cell to produce hydrogen, electricity, or a combination of both hydrogen and electricity. In a first mode, the fuel cell performs an electrochemical reaction by reacting a hydrogen-containing fuel with oxygen to produce electricity, water and heat. In a second mode, the fuel cell utilizes heat released by an electrochemical reaction of the fuel cell to reform a hydrogen-containing fuel to produce hydrogen rich gas. In a third mode, both hydrogen and electricity are co-produced by the fuel cell. The HECP system can control an amount of hydrogen and / or electricity produced and switch between modes by varying an electrical load on the system.
Owner:ZTEK
Battery with bifunctional electrolyte
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:ITI SCOTLAND +1
Self-regulating feedstock delivery systems and hydrogen-generating fuel processing assemblies and fuel cell systems incorporating the same
Feedstock delivery systems and hydrogen-producing fuel processing assemblies and fuel cell systems containing the same. The feedstock delivery systems include a liquid pump that draws at least one liquid feedstock from a supply and delivers at least one feed stream containing the feedstock(s) to a fuel processor, such as to the hydrogen-producing region thereof. The feedstock delivery system further includes a recycle conduit that establishes a fluid flow path for the liquid feedstock(s) from a location downstream of the pump back to a location upstream of the pump. In some embodiments, the feedstock delivery system further includes a flow restrictor associated with the recycle conduit and a pressure-actuated valve that selectively permits the recycled feedstock to bypass the flow restrictor. In some embodiments, the pump is configured to draw a greater flow rate of the feed stream from the supply than is delivered to the fuel processor.
Owner:DCNS SA
Power device and oxygen generator
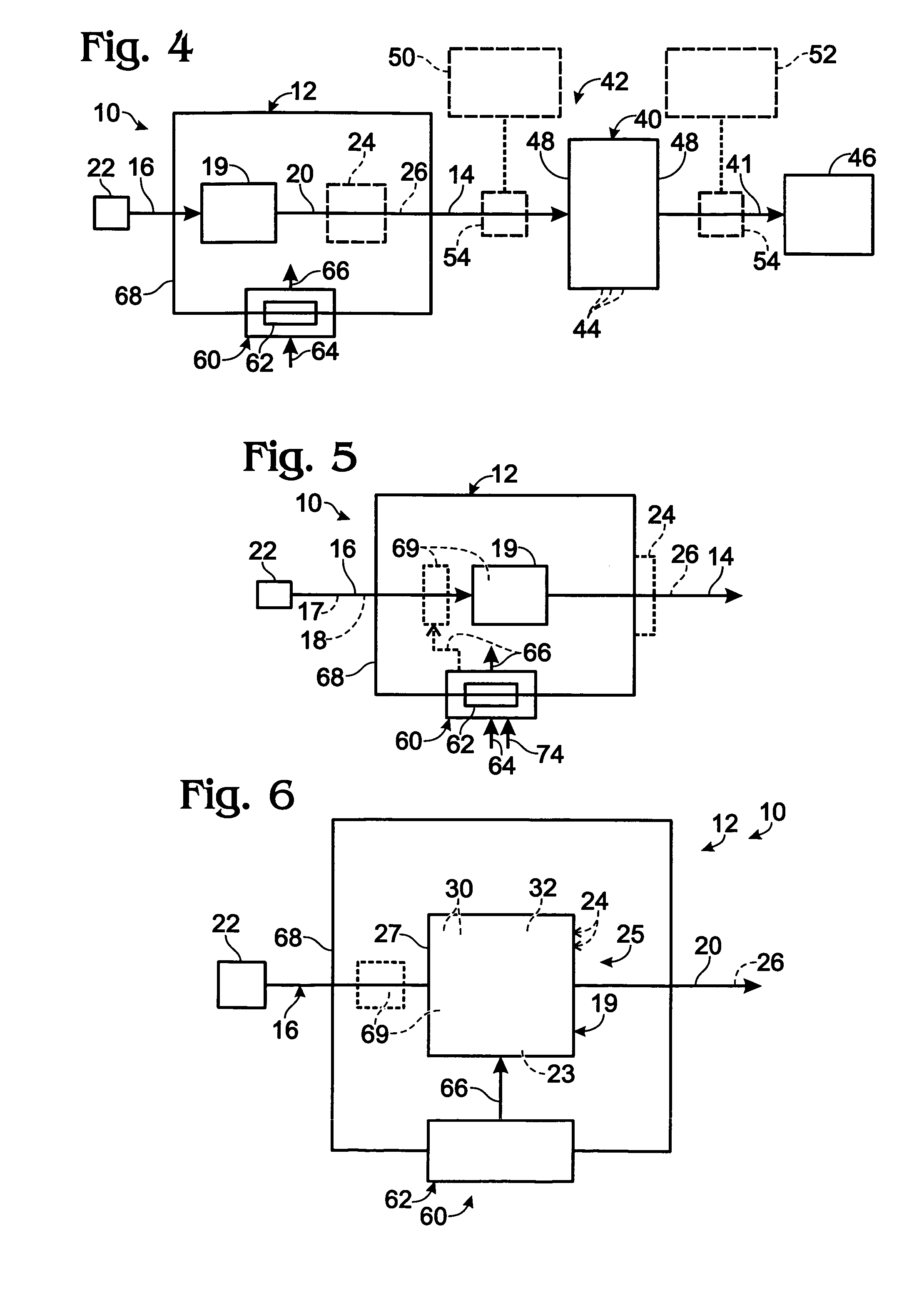
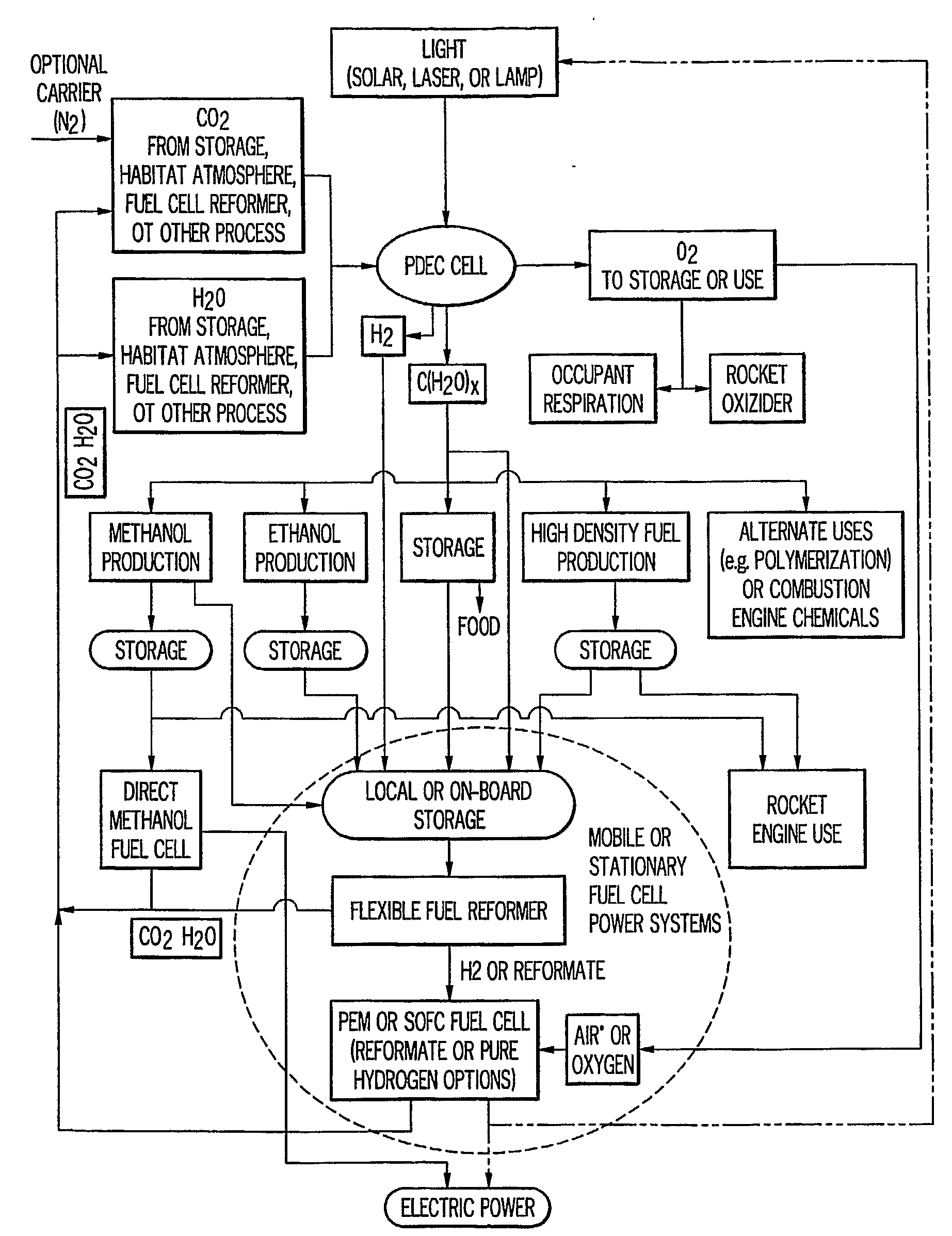
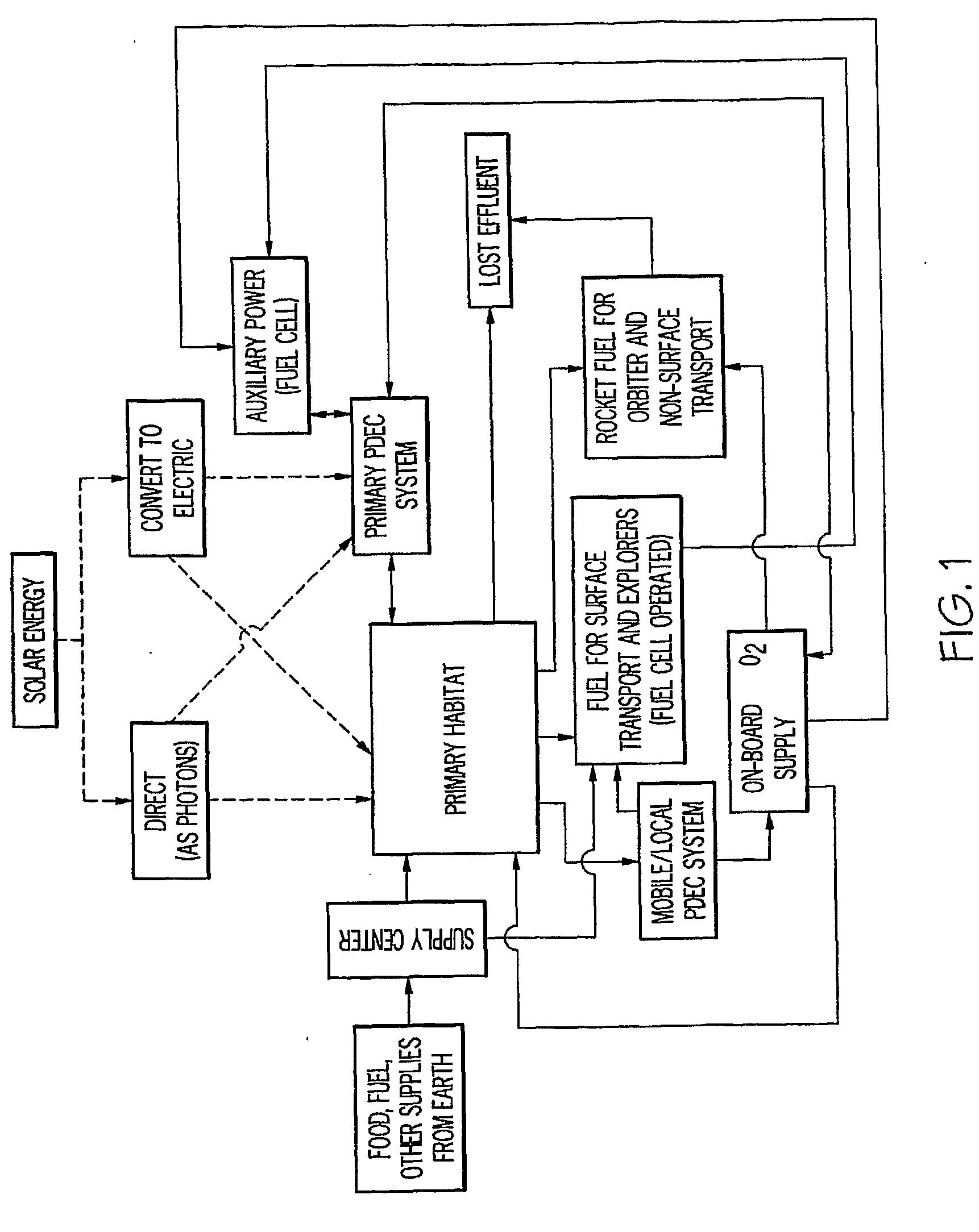
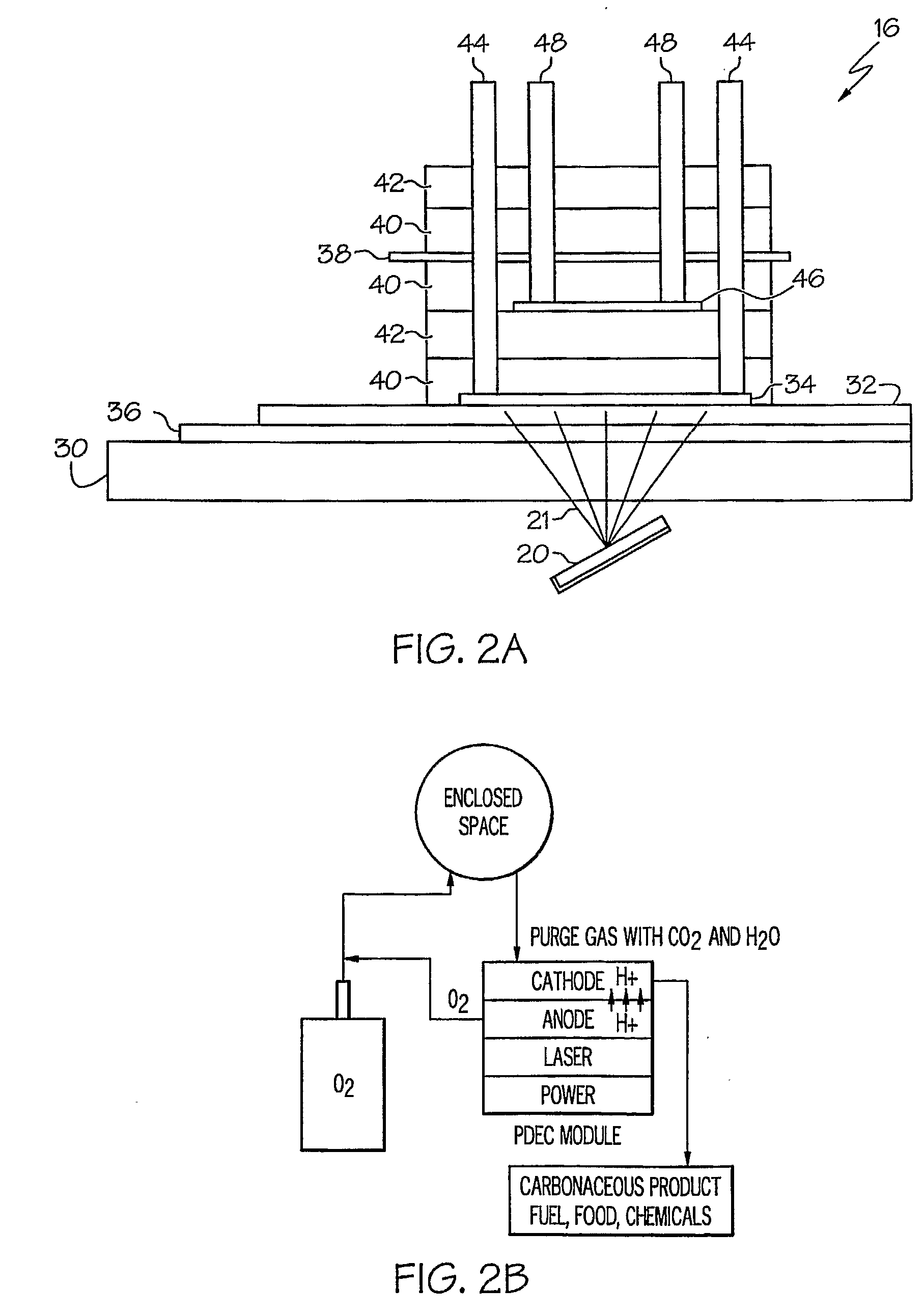
InactiveUS20090061267A1Improve quantum efficiencyLight weightSludge treatmentElectrostatic separatorsHydrogenFuel cells
A system for oxygen, hydrogen and carbon mass regeneration and recycling for breathing, and fuel / energy generation purposes, especially for fuel cells and rocket motors, by combination and integration of a photoelectrolytically powered electrochemical and gas handling system with one or more fuel cells.
Owner:BATTELLE MEMORIAL INST
Reforming unvaporized, atomized hydrocarbon fuel
InactiveUS20050274107A1Improved hydrogen generationLow costHydrogenExhaust apparatusKeroseneTurbocharger
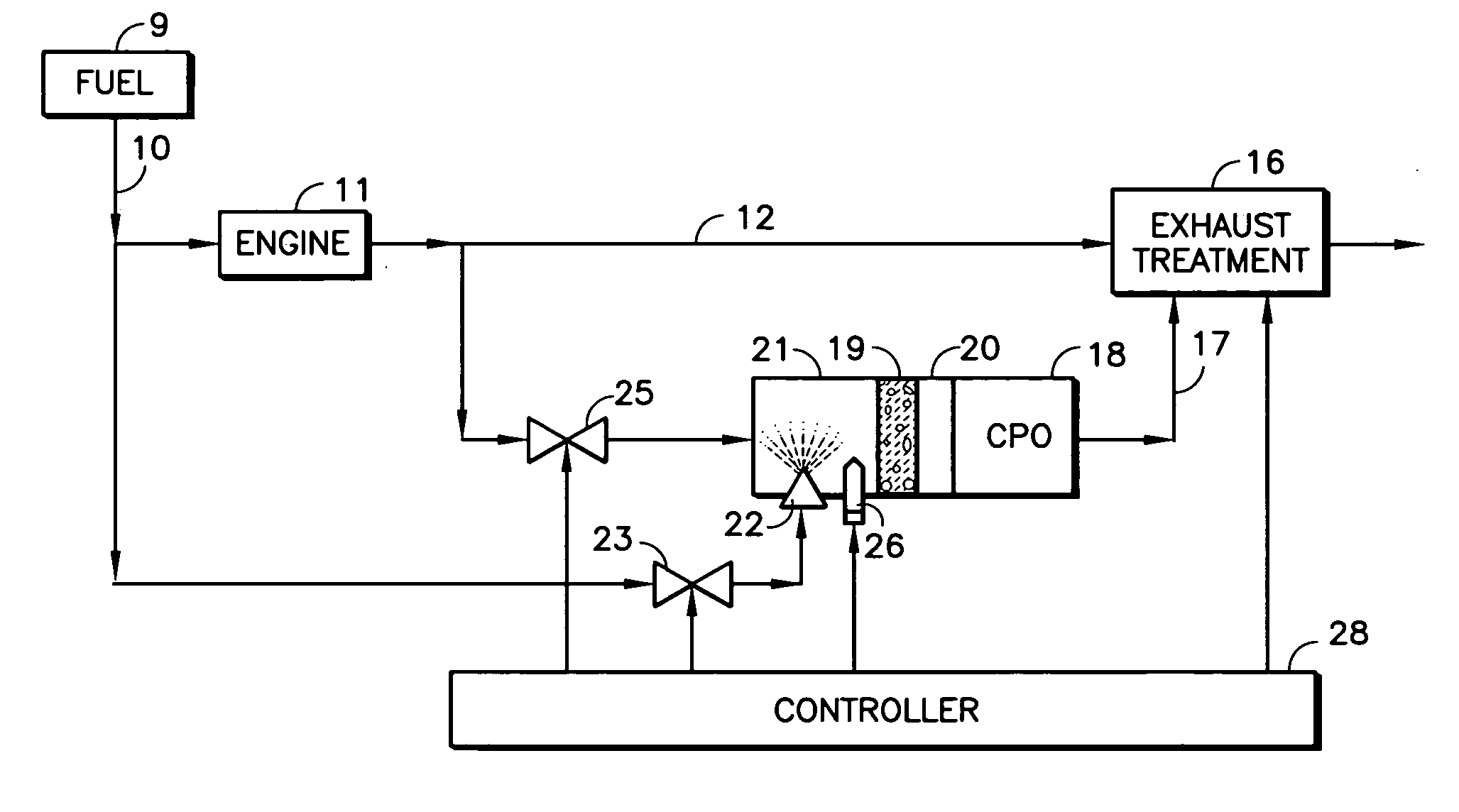
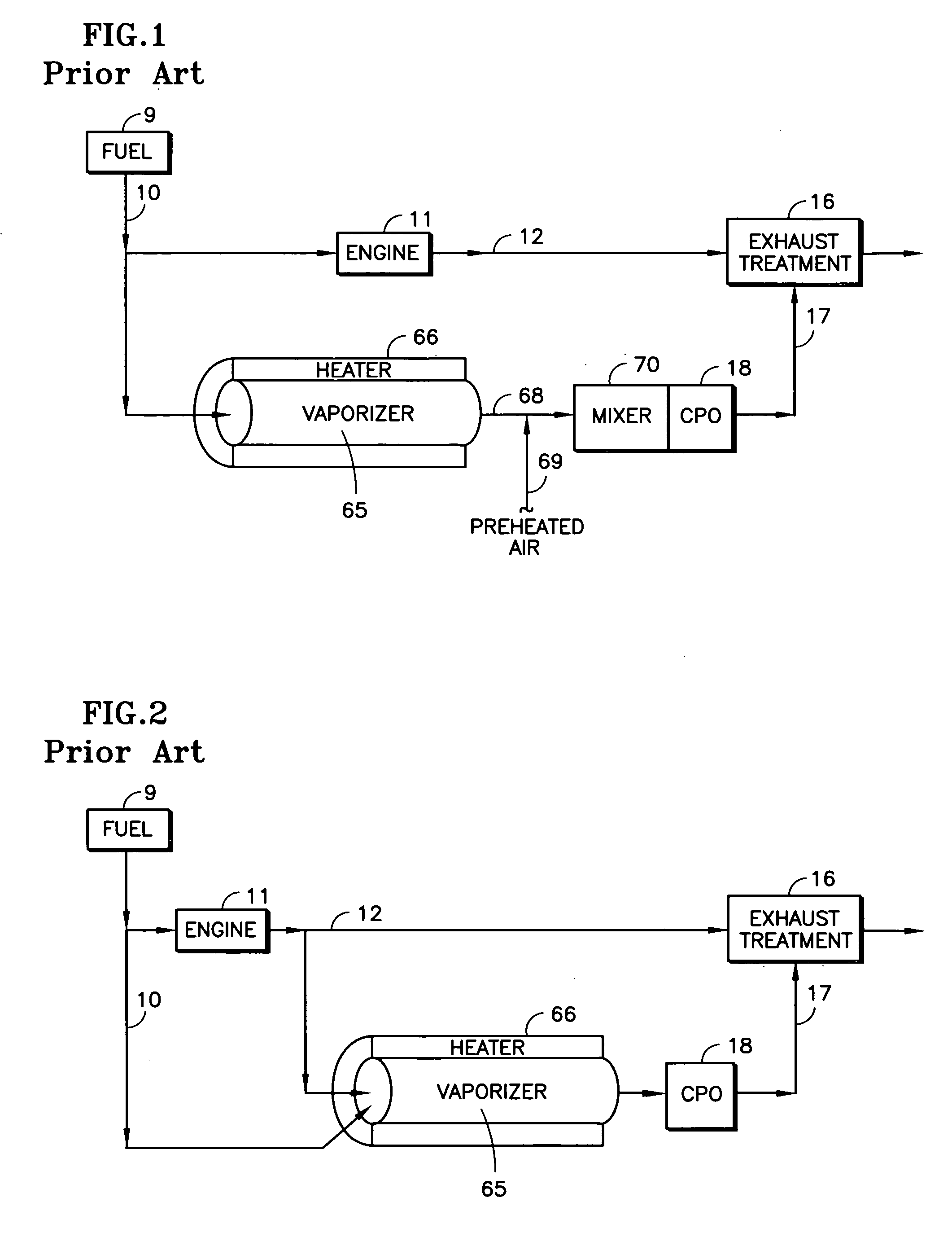
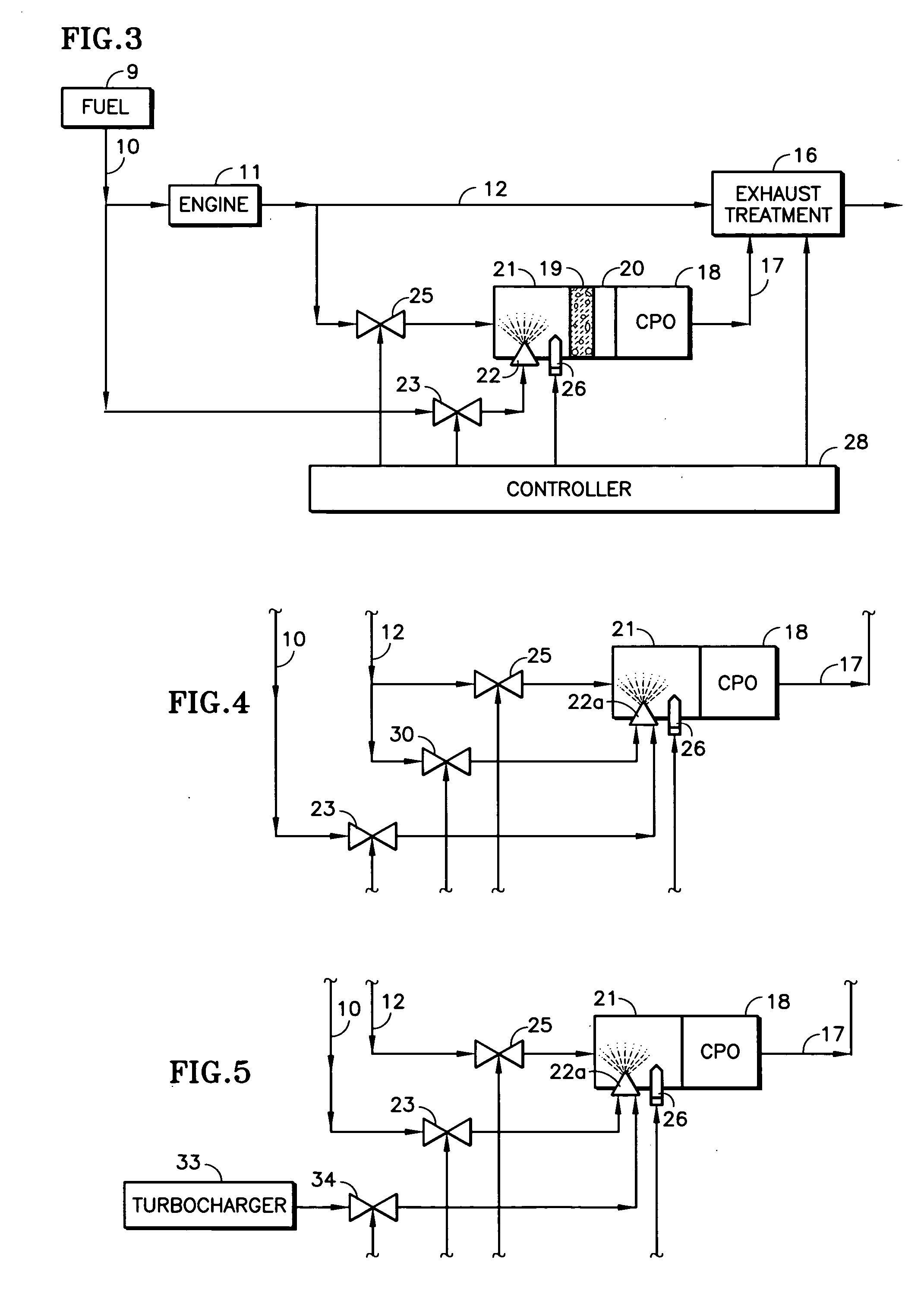
A reformer such as a CPO (18) receives a mix of fuel, moisture and oxygen from a mixing region (21) having an igniter (26, 66), which may include an inert ceramic foam (19), the fuel being provided by an atomizing nozzle (22), thereby avoiding the need for a vaporizer before use. The oxygen and moisture may comprise engine exhaust (11, 12). Fuel from a vehicle fuel tank (9), may be gasoline, diesel fuel, kerosene, jet fuel, or JP-8. The atomizing nozzle may be a gas-assist nozzle (22a), receiving the assisting gas from (a) engine exhaust (10), (b) a turbocharger (33), (c) an air pump (50) or (d) a steam generator (57). The oxygen and moisture may comprise moisturized air, which may be achieved by an ejector (41) which ingests water from a tank (43) in response to the flow of air from a pump (50) through a conduit (47). The air may be regeneratively heated (48) with the CPO exhaust. The igniter may be a glow plug (26) or a heater wire (66) coated with catalyst.
Owner:SHELL OIL CO
Popular searches
Hydrogen/synthetic gas production Solid electrolyte fuel cells Motive system fuel cells Hydrogen separation by diffusion Evaporation apparatus Semi-permeable membranes Iron oxides/hydroxides Hydrogen separation using solid contact Chemical industry Chemical/physical/physico-chemical stationary reactors
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com